User login
CMS's eHealth initiative takes aim at runaway digital demands
NEW ORLEANS – The continuing onslaught of digital demands is overwhelming many practices’ ability to keep up, driving efficiency down and costs up. The Centers for Medicare and Medicaid Services wants to show that it feels physicians’ pain, and has launched what it calls its "eHealth" initiative to better coordinate and streamline the requests that come out of its offices.
The agency unveiled the initiative at the Healthcare Information and Management Systems Society Annual Conference and exhibition here, with officials telling attendees in multiple sessions – and with an exhibit hall booth and full-page ad in the daily meeting newspaper – how they plan to start paring down demands.
The initiative is the result of at least 18 months of strategizing within the Department of Health and Human Services (HHS) and represents just the beginning of a transformation, Robert Tagalicod, director of the CMS’s Office of E-Health Standards and Services, said in an interview.
HHS officials wanted to curb what they acknowledge is a huge – and growing – regulatory burden for physicians and other health care providers in the information technology area. Much of the focus was on how to better align rules and policies that come out of the CMS with those issued by the Office of the National Coordinator (ONC), which is located within the Office of the Secretary of HHS.
Among other things, the CMS set up a dedicated website for the initiative, www.cms.gov/eHealth, and a listserv that will "act as a central hub of information on implementation, guidance, milestones, and critical steps so that providers and other stakeholders have a single source of information on coordinating efforts toward implementing ICD-10, EHRs and meaningful use, operating standards, electronic quality measurement, and payment models," according to a blog post by Mr. Tagalicod.
The initiative aims to get more physicians using ICD-10, with which they must comply by Oct. 14, 2014. Although the move to that new coding system has been delayed several times, it will not be again, according to CMS Acting Administrator Marilyn Tavenner. "ICD-10 will go forward as designed," Ms. Tavenner told HIMSS13 attendees.
To make it easier for clinicians, the CMS and the ONC are talking about making use of ICD-10 eligible for an incentive payment under stage 3 of the meaningful use program, said Mr. Tagalicod.
In the quality arena, the CMS is going to align measurements with the six core areas set out by the National Quality Strategy. Wherever possible, quality measures will be made similar across CMS programs, said Dr. Kate Goodrich, acting director of the quality measurement and health assessment group at CMS’s Center for Clinical Standards and Quality.
For physicians, that means aligning the measures in the Physician Quality Reporting System with those required for meaningful use, for the Physician Value Modifier (PVM), and for the Medicare Shared Savings Program, said Dr. Goodrich. "The goal is report once – that’s become our mantra," she said. Physicians would report once and get credit for all the programs.
Ms. Tavenner told attendees the agency understands that, over the years, the quality of care programs had become "not easy to understand and even more difficult to implement." Now, the agency has "tried to reduce measures, align measures across programs, and become more focused on outcomes, less on process," she said, adding, "We want your feedback on that."
The eHealth initiative is also working with the National Quality Forum on a task force that aims to come up with a core set of measures that can be used across all payers, not just federal payers.
Part of the eHealth rollout included a "request for information" from health information technology companies on how to accelerate the exchange of data. "Our focus has to be on interoperability this year and we need your help in understanding what is working and where we are going to need to put additional resources," Ms. Tavenner said.
The CMS and other health agencies also are looking ahead to the future, Mr. Tagalicod said – what might happen 10 years down the road in a very quickly developing area. Among the developments the agency is monitoring: mobile health, data exchange in real time, and predictive health.
In the meantime, a big focus will be on "outreach, education, and training to providers" to help them "adopt a whole slew of eHealth," he noted.
"We want to get all the stakeholders together and say: How can we make this all better in a way that we plan it with clear goals, with clear milestones, and with clear roles and responsibilities?" he said.
NEW ORLEANS – The continuing onslaught of digital demands is overwhelming many practices’ ability to keep up, driving efficiency down and costs up. The Centers for Medicare and Medicaid Services wants to show that it feels physicians’ pain, and has launched what it calls its "eHealth" initiative to better coordinate and streamline the requests that come out of its offices.
The agency unveiled the initiative at the Healthcare Information and Management Systems Society Annual Conference and exhibition here, with officials telling attendees in multiple sessions – and with an exhibit hall booth and full-page ad in the daily meeting newspaper – how they plan to start paring down demands.
The initiative is the result of at least 18 months of strategizing within the Department of Health and Human Services (HHS) and represents just the beginning of a transformation, Robert Tagalicod, director of the CMS’s Office of E-Health Standards and Services, said in an interview.
HHS officials wanted to curb what they acknowledge is a huge – and growing – regulatory burden for physicians and other health care providers in the information technology area. Much of the focus was on how to better align rules and policies that come out of the CMS with those issued by the Office of the National Coordinator (ONC), which is located within the Office of the Secretary of HHS.
Among other things, the CMS set up a dedicated website for the initiative, www.cms.gov/eHealth, and a listserv that will "act as a central hub of information on implementation, guidance, milestones, and critical steps so that providers and other stakeholders have a single source of information on coordinating efforts toward implementing ICD-10, EHRs and meaningful use, operating standards, electronic quality measurement, and payment models," according to a blog post by Mr. Tagalicod.
The initiative aims to get more physicians using ICD-10, with which they must comply by Oct. 14, 2014. Although the move to that new coding system has been delayed several times, it will not be again, according to CMS Acting Administrator Marilyn Tavenner. "ICD-10 will go forward as designed," Ms. Tavenner told HIMSS13 attendees.
To make it easier for clinicians, the CMS and the ONC are talking about making use of ICD-10 eligible for an incentive payment under stage 3 of the meaningful use program, said Mr. Tagalicod.
In the quality arena, the CMS is going to align measurements with the six core areas set out by the National Quality Strategy. Wherever possible, quality measures will be made similar across CMS programs, said Dr. Kate Goodrich, acting director of the quality measurement and health assessment group at CMS’s Center for Clinical Standards and Quality.
For physicians, that means aligning the measures in the Physician Quality Reporting System with those required for meaningful use, for the Physician Value Modifier (PVM), and for the Medicare Shared Savings Program, said Dr. Goodrich. "The goal is report once – that’s become our mantra," she said. Physicians would report once and get credit for all the programs.
Ms. Tavenner told attendees the agency understands that, over the years, the quality of care programs had become "not easy to understand and even more difficult to implement." Now, the agency has "tried to reduce measures, align measures across programs, and become more focused on outcomes, less on process," she said, adding, "We want your feedback on that."
The eHealth initiative is also working with the National Quality Forum on a task force that aims to come up with a core set of measures that can be used across all payers, not just federal payers.
Part of the eHealth rollout included a "request for information" from health information technology companies on how to accelerate the exchange of data. "Our focus has to be on interoperability this year and we need your help in understanding what is working and where we are going to need to put additional resources," Ms. Tavenner said.
The CMS and other health agencies also are looking ahead to the future, Mr. Tagalicod said – what might happen 10 years down the road in a very quickly developing area. Among the developments the agency is monitoring: mobile health, data exchange in real time, and predictive health.
In the meantime, a big focus will be on "outreach, education, and training to providers" to help them "adopt a whole slew of eHealth," he noted.
"We want to get all the stakeholders together and say: How can we make this all better in a way that we plan it with clear goals, with clear milestones, and with clear roles and responsibilities?" he said.
NEW ORLEANS – The continuing onslaught of digital demands is overwhelming many practices’ ability to keep up, driving efficiency down and costs up. The Centers for Medicare and Medicaid Services wants to show that it feels physicians’ pain, and has launched what it calls its "eHealth" initiative to better coordinate and streamline the requests that come out of its offices.
The agency unveiled the initiative at the Healthcare Information and Management Systems Society Annual Conference and exhibition here, with officials telling attendees in multiple sessions – and with an exhibit hall booth and full-page ad in the daily meeting newspaper – how they plan to start paring down demands.
The initiative is the result of at least 18 months of strategizing within the Department of Health and Human Services (HHS) and represents just the beginning of a transformation, Robert Tagalicod, director of the CMS’s Office of E-Health Standards and Services, said in an interview.
HHS officials wanted to curb what they acknowledge is a huge – and growing – regulatory burden for physicians and other health care providers in the information technology area. Much of the focus was on how to better align rules and policies that come out of the CMS with those issued by the Office of the National Coordinator (ONC), which is located within the Office of the Secretary of HHS.
Among other things, the CMS set up a dedicated website for the initiative, www.cms.gov/eHealth, and a listserv that will "act as a central hub of information on implementation, guidance, milestones, and critical steps so that providers and other stakeholders have a single source of information on coordinating efforts toward implementing ICD-10, EHRs and meaningful use, operating standards, electronic quality measurement, and payment models," according to a blog post by Mr. Tagalicod.
The initiative aims to get more physicians using ICD-10, with which they must comply by Oct. 14, 2014. Although the move to that new coding system has been delayed several times, it will not be again, according to CMS Acting Administrator Marilyn Tavenner. "ICD-10 will go forward as designed," Ms. Tavenner told HIMSS13 attendees.
To make it easier for clinicians, the CMS and the ONC are talking about making use of ICD-10 eligible for an incentive payment under stage 3 of the meaningful use program, said Mr. Tagalicod.
In the quality arena, the CMS is going to align measurements with the six core areas set out by the National Quality Strategy. Wherever possible, quality measures will be made similar across CMS programs, said Dr. Kate Goodrich, acting director of the quality measurement and health assessment group at CMS’s Center for Clinical Standards and Quality.
For physicians, that means aligning the measures in the Physician Quality Reporting System with those required for meaningful use, for the Physician Value Modifier (PVM), and for the Medicare Shared Savings Program, said Dr. Goodrich. "The goal is report once – that’s become our mantra," she said. Physicians would report once and get credit for all the programs.
Ms. Tavenner told attendees the agency understands that, over the years, the quality of care programs had become "not easy to understand and even more difficult to implement." Now, the agency has "tried to reduce measures, align measures across programs, and become more focused on outcomes, less on process," she said, adding, "We want your feedback on that."
The eHealth initiative is also working with the National Quality Forum on a task force that aims to come up with a core set of measures that can be used across all payers, not just federal payers.
Part of the eHealth rollout included a "request for information" from health information technology companies on how to accelerate the exchange of data. "Our focus has to be on interoperability this year and we need your help in understanding what is working and where we are going to need to put additional resources," Ms. Tavenner said.
The CMS and other health agencies also are looking ahead to the future, Mr. Tagalicod said – what might happen 10 years down the road in a very quickly developing area. Among the developments the agency is monitoring: mobile health, data exchange in real time, and predictive health.
In the meantime, a big focus will be on "outreach, education, and training to providers" to help them "adopt a whole slew of eHealth," he noted.
"We want to get all the stakeholders together and say: How can we make this all better in a way that we plan it with clear goals, with clear milestones, and with clear roles and responsibilities?" he said.
AT THE HIMSS13 ANNUAL MEETING
CMS sets aggressive 2013 meaningful use targets
NEW ORLEANS – The Centers for Medicare and Medicaid Services is expecting half of eligible physician offices and 80% of eligible hospitals to be meaningful users of electronic health records this year – a target that even the agency itself calls aggressive.
To date, 161,890 eligible professionals – out of 527,200 who are eligible – have attested to meaningful use, according to the most recent statistics from CMS. Most of those (161,677) did so successfully. About 200 were not successful. Of the 5,011 hospitals that are eligible, 2,653 have been successful. None failed.
So far, all of the eligible professionals and hospitals have had to prove only that they are meaningful users under stage 1 of the program. The rules outlining stage 2 criteria were published last year. The earliest physicians would have to attest to stage 2 criteria would be in 2014.
Even so, "2013 is going to be a busy year," said CMS Acting Administrator Marilyn Tavenner at the Healthcare Information and Management Systems Society annual conference. But the agency will try to take some heat off physicians and hospitals struggling to work within stage 1 and ramping up for stage 2, she said.
"We are going to spend 2013 focusing on education, focusing on learning, and focusing on what is working in stage 2 and what is not working in stage 2," said Ms. Tavenner.
She added that doctors would get a special focus this year. "We’re going to spend a lot more time with physicians – helping physicians learn, and helping physicians work with their current vendors to make things a little easier for them," Ms. Tavenner promised.
Under stage 2, physicians will have to meet a total of 20 core objectives. Among the new objectives: demonstrating the ability to use secure electronic messaging to communicate with patients; and providing patients the ability to view online, download, and transmit their health information within 4 days of those data being available to the doctor.
CMS still aims to have physicians begin attesting to stage 3 in 2016. However, the agency will hold off on issuing any stage 3 regulations for now, she said. "We are not going to issue additional rules in 2013," Ms. Tavenner said. "We are going to learn from stage 2 so it can help us design where we are going in stage 3."
Part of that learning curve will include what Ms. Tavenner called "tiny" audits. The idea is that CMS would work collaboratively with physicians and hospitals to figure out how to begin to audit electronic health records.
"This is a give and take – this is not like a payment audit," she explained. The goal is to understand what happens once a system is installed – that is, are physicians using it regularly and getting any benefit – and to determine what changes CMS might need to make, said Ms. Tavenner.
The agency will also convene a summit with physicians, hospitals, systems vendors, and government officials in May, she said. Again, the focus will be on understanding what needs to happen in stage 2.
On Twitter @aliciaault
NEW ORLEANS – The Centers for Medicare and Medicaid Services is expecting half of eligible physician offices and 80% of eligible hospitals to be meaningful users of electronic health records this year – a target that even the agency itself calls aggressive.
To date, 161,890 eligible professionals – out of 527,200 who are eligible – have attested to meaningful use, according to the most recent statistics from CMS. Most of those (161,677) did so successfully. About 200 were not successful. Of the 5,011 hospitals that are eligible, 2,653 have been successful. None failed.
So far, all of the eligible professionals and hospitals have had to prove only that they are meaningful users under stage 1 of the program. The rules outlining stage 2 criteria were published last year. The earliest physicians would have to attest to stage 2 criteria would be in 2014.
Even so, "2013 is going to be a busy year," said CMS Acting Administrator Marilyn Tavenner at the Healthcare Information and Management Systems Society annual conference. But the agency will try to take some heat off physicians and hospitals struggling to work within stage 1 and ramping up for stage 2, she said.
"We are going to spend 2013 focusing on education, focusing on learning, and focusing on what is working in stage 2 and what is not working in stage 2," said Ms. Tavenner.
She added that doctors would get a special focus this year. "We’re going to spend a lot more time with physicians – helping physicians learn, and helping physicians work with their current vendors to make things a little easier for them," Ms. Tavenner promised.
Under stage 2, physicians will have to meet a total of 20 core objectives. Among the new objectives: demonstrating the ability to use secure electronic messaging to communicate with patients; and providing patients the ability to view online, download, and transmit their health information within 4 days of those data being available to the doctor.
CMS still aims to have physicians begin attesting to stage 3 in 2016. However, the agency will hold off on issuing any stage 3 regulations for now, she said. "We are not going to issue additional rules in 2013," Ms. Tavenner said. "We are going to learn from stage 2 so it can help us design where we are going in stage 3."
Part of that learning curve will include what Ms. Tavenner called "tiny" audits. The idea is that CMS would work collaboratively with physicians and hospitals to figure out how to begin to audit electronic health records.
"This is a give and take – this is not like a payment audit," she explained. The goal is to understand what happens once a system is installed – that is, are physicians using it regularly and getting any benefit – and to determine what changes CMS might need to make, said Ms. Tavenner.
The agency will also convene a summit with physicians, hospitals, systems vendors, and government officials in May, she said. Again, the focus will be on understanding what needs to happen in stage 2.
On Twitter @aliciaault
NEW ORLEANS – The Centers for Medicare and Medicaid Services is expecting half of eligible physician offices and 80% of eligible hospitals to be meaningful users of electronic health records this year – a target that even the agency itself calls aggressive.
To date, 161,890 eligible professionals – out of 527,200 who are eligible – have attested to meaningful use, according to the most recent statistics from CMS. Most of those (161,677) did so successfully. About 200 were not successful. Of the 5,011 hospitals that are eligible, 2,653 have been successful. None failed.
So far, all of the eligible professionals and hospitals have had to prove only that they are meaningful users under stage 1 of the program. The rules outlining stage 2 criteria were published last year. The earliest physicians would have to attest to stage 2 criteria would be in 2014.
Even so, "2013 is going to be a busy year," said CMS Acting Administrator Marilyn Tavenner at the Healthcare Information and Management Systems Society annual conference. But the agency will try to take some heat off physicians and hospitals struggling to work within stage 1 and ramping up for stage 2, she said.
"We are going to spend 2013 focusing on education, focusing on learning, and focusing on what is working in stage 2 and what is not working in stage 2," said Ms. Tavenner.
She added that doctors would get a special focus this year. "We’re going to spend a lot more time with physicians – helping physicians learn, and helping physicians work with their current vendors to make things a little easier for them," Ms. Tavenner promised.
Under stage 2, physicians will have to meet a total of 20 core objectives. Among the new objectives: demonstrating the ability to use secure electronic messaging to communicate with patients; and providing patients the ability to view online, download, and transmit their health information within 4 days of those data being available to the doctor.
CMS still aims to have physicians begin attesting to stage 3 in 2016. However, the agency will hold off on issuing any stage 3 regulations for now, she said. "We are not going to issue additional rules in 2013," Ms. Tavenner said. "We are going to learn from stage 2 so it can help us design where we are going in stage 3."
Part of that learning curve will include what Ms. Tavenner called "tiny" audits. The idea is that CMS would work collaboratively with physicians and hospitals to figure out how to begin to audit electronic health records.
"This is a give and take – this is not like a payment audit," she explained. The goal is to understand what happens once a system is installed – that is, are physicians using it regularly and getting any benefit – and to determine what changes CMS might need to make, said Ms. Tavenner.
The agency will also convene a summit with physicians, hospitals, systems vendors, and government officials in May, she said. Again, the focus will be on understanding what needs to happen in stage 2.
On Twitter @aliciaault
AT THE HIMSS13 ANNUAL CONFERENCE
Survey exposes physician frustrations with EHRs
NEW ORLEANS – Frustrated with your electronic medical record system? Getting increasingly irritated? You most definitely are not alone.
A survey of thousands of physicians across multiple specialties shows that user satisfaction with electronic health records fell 12% from 2010 to 2012.
The survey was conducted by the American College of Physicians and AmericanEHR Partners, an online agent that helps physicians select and evaluate health information technology. It is supported by 16 medical societies and five health IT organizations.
"Dissatisfaction is increasing regardless of practice type or EHR system," said Dr. Michael S. Barr, who leads ACP\'s Medical Practice, Professionalism & Quality division. "These findings highlight the need for the Meaningful Use program and EHR manufacturers to focus on improving EHR features and usability to help reduce inefficient work flows, improve error rates and patient care, and for practices to recognize the importance of ongoing training at all stages of EHR adoption," said Dr. Barr, in a statement issued along with the survey results.
The survey was released at the Healthcare Information and Management Systems Society Annual Conference and exhibition.
Dr. Alan Brookstone, a cofounder of AmericanEHR Partners, said at the meeting that satisfaction rates may be dropping in part because there had been so much adoption of technology so quickly. Also, it’s not just the early adopters anymore, he said.
The largest number of respondents – almost 1,900 – was from primary care. Specialists, surgeons, hospital-based physicians, and psychiatrists were also represented.
The vast majority of respondents – 70% – were from practices with fewer than 10 physicians.
The number who said they intended to participate in meaningful use has grown over the past few years, with a full 82% saying they would apply for incentives paid by Medicare and Medicaid.
Satisfaction rates with current EHR systems were low across a spectrum of parameters. While 45% said they would recommend the product they use to a colleague, 39% said they would not. In 2010, more physicians said they’d recommend that system, while only 24% said they would urge against use.
Of those surveyed, 36% said that they had encountered unexpected events, problems, or costs after signing the initial contract for the system.
Physicians were especially frustrated with the systems’ promise to decrease their workload. Thirty-four percent said they were dissatisfied with that promised ability, up from only 19% in 2010. Some respondents said that the EHR had decreased productivity and increased the amount of time needed to complete documentation. Fully a third of respondents said they had not returned to the productivity they had before they began to use the system.
About half of respondents were satisfied with functionality and ease of use, but a third were dissatisfied with those measures. That level of dissatisfaction was higher than it had been in 2010.
For instance, thirty-six percent said that it was difficult to reconcile an imported medication list with medications listed in a patient record.
Overall, when compared with other specialties, primary care physicians were the most satisfied with their system’s ability to improve patient care. Surgeons, representing about 660 respondents, were the least satisfied.
Good customer support and training for the EHR systems was rated as crucial to satisfaction. There was an 11% increase in dissatisfaction with customer support from 2010 to 2012. Thirty-three percent of respondents said they weren’t happy with the customer support they received.
The number of practices using a patient portal increased by 20% from 2010 to 2012, rising to 40%. This is probably driven by the stage 2 meaningful use rules, which require physicians to be able to securely communicate with patients and for patients to be able to download and share their health information. Still, 50% of respondents did not have a portal.
Dr. Brookstone said they survey showed that vendors needed to better integrate functionality, improve training, and find ways to help physicians rebalance their workload.
If physicians’ concerns aren’t addressed, it will lead to a decline in willingness to use the systems, he said.
NEW ORLEANS – Frustrated with your electronic medical record system? Getting increasingly irritated? You most definitely are not alone.
A survey of thousands of physicians across multiple specialties shows that user satisfaction with electronic health records fell 12% from 2010 to 2012.
The survey was conducted by the American College of Physicians and AmericanEHR Partners, an online agent that helps physicians select and evaluate health information technology. It is supported by 16 medical societies and five health IT organizations.
"Dissatisfaction is increasing regardless of practice type or EHR system," said Dr. Michael S. Barr, who leads ACP\'s Medical Practice, Professionalism & Quality division. "These findings highlight the need for the Meaningful Use program and EHR manufacturers to focus on improving EHR features and usability to help reduce inefficient work flows, improve error rates and patient care, and for practices to recognize the importance of ongoing training at all stages of EHR adoption," said Dr. Barr, in a statement issued along with the survey results.
The survey was released at the Healthcare Information and Management Systems Society Annual Conference and exhibition.
Dr. Alan Brookstone, a cofounder of AmericanEHR Partners, said at the meeting that satisfaction rates may be dropping in part because there had been so much adoption of technology so quickly. Also, it’s not just the early adopters anymore, he said.
The largest number of respondents – almost 1,900 – was from primary care. Specialists, surgeons, hospital-based physicians, and psychiatrists were also represented.
The vast majority of respondents – 70% – were from practices with fewer than 10 physicians.
The number who said they intended to participate in meaningful use has grown over the past few years, with a full 82% saying they would apply for incentives paid by Medicare and Medicaid.
Satisfaction rates with current EHR systems were low across a spectrum of parameters. While 45% said they would recommend the product they use to a colleague, 39% said they would not. In 2010, more physicians said they’d recommend that system, while only 24% said they would urge against use.
Of those surveyed, 36% said that they had encountered unexpected events, problems, or costs after signing the initial contract for the system.
Physicians were especially frustrated with the systems’ promise to decrease their workload. Thirty-four percent said they were dissatisfied with that promised ability, up from only 19% in 2010. Some respondents said that the EHR had decreased productivity and increased the amount of time needed to complete documentation. Fully a third of respondents said they had not returned to the productivity they had before they began to use the system.
About half of respondents were satisfied with functionality and ease of use, but a third were dissatisfied with those measures. That level of dissatisfaction was higher than it had been in 2010.
For instance, thirty-six percent said that it was difficult to reconcile an imported medication list with medications listed in a patient record.
Overall, when compared with other specialties, primary care physicians were the most satisfied with their system’s ability to improve patient care. Surgeons, representing about 660 respondents, were the least satisfied.
Good customer support and training for the EHR systems was rated as crucial to satisfaction. There was an 11% increase in dissatisfaction with customer support from 2010 to 2012. Thirty-three percent of respondents said they weren’t happy with the customer support they received.
The number of practices using a patient portal increased by 20% from 2010 to 2012, rising to 40%. This is probably driven by the stage 2 meaningful use rules, which require physicians to be able to securely communicate with patients and for patients to be able to download and share their health information. Still, 50% of respondents did not have a portal.
Dr. Brookstone said they survey showed that vendors needed to better integrate functionality, improve training, and find ways to help physicians rebalance their workload.
If physicians’ concerns aren’t addressed, it will lead to a decline in willingness to use the systems, he said.
NEW ORLEANS – Frustrated with your electronic medical record system? Getting increasingly irritated? You most definitely are not alone.
A survey of thousands of physicians across multiple specialties shows that user satisfaction with electronic health records fell 12% from 2010 to 2012.
The survey was conducted by the American College of Physicians and AmericanEHR Partners, an online agent that helps physicians select and evaluate health information technology. It is supported by 16 medical societies and five health IT organizations.
"Dissatisfaction is increasing regardless of practice type or EHR system," said Dr. Michael S. Barr, who leads ACP\'s Medical Practice, Professionalism & Quality division. "These findings highlight the need for the Meaningful Use program and EHR manufacturers to focus on improving EHR features and usability to help reduce inefficient work flows, improve error rates and patient care, and for practices to recognize the importance of ongoing training at all stages of EHR adoption," said Dr. Barr, in a statement issued along with the survey results.
The survey was released at the Healthcare Information and Management Systems Society Annual Conference and exhibition.
Dr. Alan Brookstone, a cofounder of AmericanEHR Partners, said at the meeting that satisfaction rates may be dropping in part because there had been so much adoption of technology so quickly. Also, it’s not just the early adopters anymore, he said.
The largest number of respondents – almost 1,900 – was from primary care. Specialists, surgeons, hospital-based physicians, and psychiatrists were also represented.
The vast majority of respondents – 70% – were from practices with fewer than 10 physicians.
The number who said they intended to participate in meaningful use has grown over the past few years, with a full 82% saying they would apply for incentives paid by Medicare and Medicaid.
Satisfaction rates with current EHR systems were low across a spectrum of parameters. While 45% said they would recommend the product they use to a colleague, 39% said they would not. In 2010, more physicians said they’d recommend that system, while only 24% said they would urge against use.
Of those surveyed, 36% said that they had encountered unexpected events, problems, or costs after signing the initial contract for the system.
Physicians were especially frustrated with the systems’ promise to decrease their workload. Thirty-four percent said they were dissatisfied with that promised ability, up from only 19% in 2010. Some respondents said that the EHR had decreased productivity and increased the amount of time needed to complete documentation. Fully a third of respondents said they had not returned to the productivity they had before they began to use the system.
About half of respondents were satisfied with functionality and ease of use, but a third were dissatisfied with those measures. That level of dissatisfaction was higher than it had been in 2010.
For instance, thirty-six percent said that it was difficult to reconcile an imported medication list with medications listed in a patient record.
Overall, when compared with other specialties, primary care physicians were the most satisfied with their system’s ability to improve patient care. Surgeons, representing about 660 respondents, were the least satisfied.
Good customer support and training for the EHR systems was rated as crucial to satisfaction. There was an 11% increase in dissatisfaction with customer support from 2010 to 2012. Thirty-three percent of respondents said they weren’t happy with the customer support they received.
The number of practices using a patient portal increased by 20% from 2010 to 2012, rising to 40%. This is probably driven by the stage 2 meaningful use rules, which require physicians to be able to securely communicate with patients and for patients to be able to download and share their health information. Still, 50% of respondents did not have a portal.
Dr. Brookstone said they survey showed that vendors needed to better integrate functionality, improve training, and find ways to help physicians rebalance their workload.
If physicians’ concerns aren’t addressed, it will lead to a decline in willingness to use the systems, he said.
AT THE HIMSS13 ANNUAL CONFERENCE
CMS audits EHR incentives – before paying them
NEW ORLEANS – Haven’t received your meaningful use incentive? Check your mail for an audit letter.
If in January you submitted an attestation of meaningful use of your electronic health record – with an eye to reaping the federal health IT incentive – an audit letter may be on its way to you.
A contractor for the Centers for Medicare and Medicaid Services began sending audit letters this week to randomly selected Medicare-eligible professionals and hospitals, Elizabeth Holland, a director of the HIT Initiatives Group in the agency’s Office of E-Health Standards and Services, said March 5 at the Healthcare Information and Management Systems Society annual conference. The audits could result in delays or ultimately, non-payment, she said.
"We have a fiduciary responsibility to make sure that we are paying appropriately," Ms. Holland explained, adding that providers who were not selected for the audit have already received their payments.
Audit letters are being sent by Figliozzi & Co. to Medicare-eligible hospitals and physicians. If recipients do not respond, "their payment will be held up until they respond and provide the documentation" to back up their attestation, Ms. Holland said. "If a certain amount of time goes by and they still don’t respond, they will not be getting a payment."
These prepayment audits follow on the heels of postpayment audits that the CMS began in July 2012. Under that program, Figliozzi & Co. audited Medicare-eligible professionals and states audited Medicaid-eligible professionals.
Ms. Holland said that more than 2,000 postpayment audits are underway; some are random and some are targeted. The data generated by the audits is, and will be, used to modify the agency’s approach to meaningful use. For instance, one goal is to see whether providers are appropriately reporting measures, she said.
CMS also has found that professionals do not have the proper documentation to support what they are attesting to. In the next month, the CMS will issue guidance on what documentation is needed, Ms. Holland said.
She presented data showing that so far, 161,890 eligible professionals – out of 527,200 who are eligible – have attested to meaningful use. Most of those (161,677) did so successfully. About 200 were not successful.
Of the 5,011 hospitals that are eligible, 2,653 have been successful. None failed.
On Twitter @aliciaault
NEW ORLEANS – Haven’t received your meaningful use incentive? Check your mail for an audit letter.
If in January you submitted an attestation of meaningful use of your electronic health record – with an eye to reaping the federal health IT incentive – an audit letter may be on its way to you.
A contractor for the Centers for Medicare and Medicaid Services began sending audit letters this week to randomly selected Medicare-eligible professionals and hospitals, Elizabeth Holland, a director of the HIT Initiatives Group in the agency’s Office of E-Health Standards and Services, said March 5 at the Healthcare Information and Management Systems Society annual conference. The audits could result in delays or ultimately, non-payment, she said.
"We have a fiduciary responsibility to make sure that we are paying appropriately," Ms. Holland explained, adding that providers who were not selected for the audit have already received their payments.
Audit letters are being sent by Figliozzi & Co. to Medicare-eligible hospitals and physicians. If recipients do not respond, "their payment will be held up until they respond and provide the documentation" to back up their attestation, Ms. Holland said. "If a certain amount of time goes by and they still don’t respond, they will not be getting a payment."
These prepayment audits follow on the heels of postpayment audits that the CMS began in July 2012. Under that program, Figliozzi & Co. audited Medicare-eligible professionals and states audited Medicaid-eligible professionals.
Ms. Holland said that more than 2,000 postpayment audits are underway; some are random and some are targeted. The data generated by the audits is, and will be, used to modify the agency’s approach to meaningful use. For instance, one goal is to see whether providers are appropriately reporting measures, she said.
CMS also has found that professionals do not have the proper documentation to support what they are attesting to. In the next month, the CMS will issue guidance on what documentation is needed, Ms. Holland said.
She presented data showing that so far, 161,890 eligible professionals – out of 527,200 who are eligible – have attested to meaningful use. Most of those (161,677) did so successfully. About 200 were not successful.
Of the 5,011 hospitals that are eligible, 2,653 have been successful. None failed.
On Twitter @aliciaault
NEW ORLEANS – Haven’t received your meaningful use incentive? Check your mail for an audit letter.
If in January you submitted an attestation of meaningful use of your electronic health record – with an eye to reaping the federal health IT incentive – an audit letter may be on its way to you.
A contractor for the Centers for Medicare and Medicaid Services began sending audit letters this week to randomly selected Medicare-eligible professionals and hospitals, Elizabeth Holland, a director of the HIT Initiatives Group in the agency’s Office of E-Health Standards and Services, said March 5 at the Healthcare Information and Management Systems Society annual conference. The audits could result in delays or ultimately, non-payment, she said.
"We have a fiduciary responsibility to make sure that we are paying appropriately," Ms. Holland explained, adding that providers who were not selected for the audit have already received their payments.
Audit letters are being sent by Figliozzi & Co. to Medicare-eligible hospitals and physicians. If recipients do not respond, "their payment will be held up until they respond and provide the documentation" to back up their attestation, Ms. Holland said. "If a certain amount of time goes by and they still don’t respond, they will not be getting a payment."
These prepayment audits follow on the heels of postpayment audits that the CMS began in July 2012. Under that program, Figliozzi & Co. audited Medicare-eligible professionals and states audited Medicaid-eligible professionals.
Ms. Holland said that more than 2,000 postpayment audits are underway; some are random and some are targeted. The data generated by the audits is, and will be, used to modify the agency’s approach to meaningful use. For instance, one goal is to see whether providers are appropriately reporting measures, she said.
CMS also has found that professionals do not have the proper documentation to support what they are attesting to. In the next month, the CMS will issue guidance on what documentation is needed, Ms. Holland said.
She presented data showing that so far, 161,890 eligible professionals – out of 527,200 who are eligible – have attested to meaningful use. Most of those (161,677) did so successfully. About 200 were not successful.
Of the 5,011 hospitals that are eligible, 2,653 have been successful. None failed.
On Twitter @aliciaault
AT THE HIMSS13 ANNUAL CONFERENCE
HHS defines essential benefits under ACA
The Department of Health and Human Services has issued its final rule on what kinds of coverage must be offered by almost all health plans starting in 2014.
The rule outlines the parameters for the so-called essential health benefits that must be included in health plans offered through the health exchanges, and in the individual and small group markets, and also sets out guidelines for an expansion of mental health and substance use disorders.
As outlined in previous proposals and bulletins from the Administration, the rule requires plans to cover services in 10 categories: ambulatory patient services; emergency services; hospitalization; maternity and newborn care; mental health and substance use disorder services, including behavioral health treatment; prescription drugs; rehabilitative and habilitative services and devices; laboratory services; preventive and wellness services and chronic disease management; and pediatric services, including oral and vision care.
The final rule also spells out that health insurers must cover mental health and substance abuse services starting in 2014.
At that time, some 3.9 million Americans who currently have individual policies, and 1.2 million who are in small group plans will be covered for those benefits for the first time, according to the HHS.
The essential health benefit rule also broadens the parity requirement for mental health coverage, first established under the Mental Health Parity and Addiction Equity Act of 2008.
Health plans covered by the rule will also be categorized according to the value of the benefits they provide. The lowest-value plan will be bronze, in which patients will be responsible for about 40% of the costs of covered benefits. For the silver plans, policyholders will pay 30% of the costs; for a gold plan, 20%, and for a platinum plan, 10%.
As expected, the rule gives states a fair amount of flexibility in meeting the requirements for essential health benefits. But they must select a "benchmark" plan and offer benefits that are equivalent – either by matching benefits or providing the actuarial equivalent – to that benchmark.
The benchmark plan is required to include the services and benefits in the 10 categories. If the benchmark plan is missing any of those services, the final rule gives guidance on how the state or health plan can add the benefits.
Benchmark plans selected now will be used for coverage in 2014 and 2015.
Under the Affordable Care Act, the rule applies to all "non-grandfathered" health insurance plans offered through state health insurance exchanges, and also policies in the individual and small group market that are sold outside the exchanges.
Grandfathered plans are primarily those that were already in place when the ACA was signed into law on March 23, 2010.
HHS said that it received almost 6,000 comments on the various proposals it floated on the essential health benefits package since December 2011.
The final rule issued on Feb. 20 goes into effect on March 20.
On Twitter @aliciaault
The Department of Health and Human Services has issued its final rule on what kinds of coverage must be offered by almost all health plans starting in 2014.
The rule outlines the parameters for the so-called essential health benefits that must be included in health plans offered through the health exchanges, and in the individual and small group markets, and also sets out guidelines for an expansion of mental health and substance use disorders.
As outlined in previous proposals and bulletins from the Administration, the rule requires plans to cover services in 10 categories: ambulatory patient services; emergency services; hospitalization; maternity and newborn care; mental health and substance use disorder services, including behavioral health treatment; prescription drugs; rehabilitative and habilitative services and devices; laboratory services; preventive and wellness services and chronic disease management; and pediatric services, including oral and vision care.
The final rule also spells out that health insurers must cover mental health and substance abuse services starting in 2014.
At that time, some 3.9 million Americans who currently have individual policies, and 1.2 million who are in small group plans will be covered for those benefits for the first time, according to the HHS.
The essential health benefit rule also broadens the parity requirement for mental health coverage, first established under the Mental Health Parity and Addiction Equity Act of 2008.
Health plans covered by the rule will also be categorized according to the value of the benefits they provide. The lowest-value plan will be bronze, in which patients will be responsible for about 40% of the costs of covered benefits. For the silver plans, policyholders will pay 30% of the costs; for a gold plan, 20%, and for a platinum plan, 10%.
As expected, the rule gives states a fair amount of flexibility in meeting the requirements for essential health benefits. But they must select a "benchmark" plan and offer benefits that are equivalent – either by matching benefits or providing the actuarial equivalent – to that benchmark.
The benchmark plan is required to include the services and benefits in the 10 categories. If the benchmark plan is missing any of those services, the final rule gives guidance on how the state or health plan can add the benefits.
Benchmark plans selected now will be used for coverage in 2014 and 2015.
Under the Affordable Care Act, the rule applies to all "non-grandfathered" health insurance plans offered through state health insurance exchanges, and also policies in the individual and small group market that are sold outside the exchanges.
Grandfathered plans are primarily those that were already in place when the ACA was signed into law on March 23, 2010.
HHS said that it received almost 6,000 comments on the various proposals it floated on the essential health benefits package since December 2011.
The final rule issued on Feb. 20 goes into effect on March 20.
On Twitter @aliciaault
The Department of Health and Human Services has issued its final rule on what kinds of coverage must be offered by almost all health plans starting in 2014.
The rule outlines the parameters for the so-called essential health benefits that must be included in health plans offered through the health exchanges, and in the individual and small group markets, and also sets out guidelines for an expansion of mental health and substance use disorders.
As outlined in previous proposals and bulletins from the Administration, the rule requires plans to cover services in 10 categories: ambulatory patient services; emergency services; hospitalization; maternity and newborn care; mental health and substance use disorder services, including behavioral health treatment; prescription drugs; rehabilitative and habilitative services and devices; laboratory services; preventive and wellness services and chronic disease management; and pediatric services, including oral and vision care.
The final rule also spells out that health insurers must cover mental health and substance abuse services starting in 2014.
At that time, some 3.9 million Americans who currently have individual policies, and 1.2 million who are in small group plans will be covered for those benefits for the first time, according to the HHS.
The essential health benefit rule also broadens the parity requirement for mental health coverage, first established under the Mental Health Parity and Addiction Equity Act of 2008.
Health plans covered by the rule will also be categorized according to the value of the benefits they provide. The lowest-value plan will be bronze, in which patients will be responsible for about 40% of the costs of covered benefits. For the silver plans, policyholders will pay 30% of the costs; for a gold plan, 20%, and for a platinum plan, 10%.
As expected, the rule gives states a fair amount of flexibility in meeting the requirements for essential health benefits. But they must select a "benchmark" plan and offer benefits that are equivalent – either by matching benefits or providing the actuarial equivalent – to that benchmark.
The benchmark plan is required to include the services and benefits in the 10 categories. If the benchmark plan is missing any of those services, the final rule gives guidance on how the state or health plan can add the benefits.
Benchmark plans selected now will be used for coverage in 2014 and 2015.
Under the Affordable Care Act, the rule applies to all "non-grandfathered" health insurance plans offered through state health insurance exchanges, and also policies in the individual and small group market that are sold outside the exchanges.
Grandfathered plans are primarily those that were already in place when the ACA was signed into law on March 23, 2010.
HHS said that it received almost 6,000 comments on the various proposals it floated on the essential health benefits package since December 2011.
The final rule issued on Feb. 20 goes into effect on March 20.
On Twitter @aliciaault
Choosing Wisely: Second-round GI test list fine tuned
WASHINGTON – More than a dozen medical groups have issued new lists of tests and procedures that they say are often unnecessary and overused and should be questioned by both physicians and patients.
The lists – issued by 17 physician organizations on Feb. 21 – comprise the second iteration of the Choosing Wisely campaign, launched by the American Board of Internal Medicine Foundation in April 2012. The lists, compiled by each group as "Five Things Physicians and Patients Should Question," are evidence-based recommendations to help physicians and patients make decisions together.
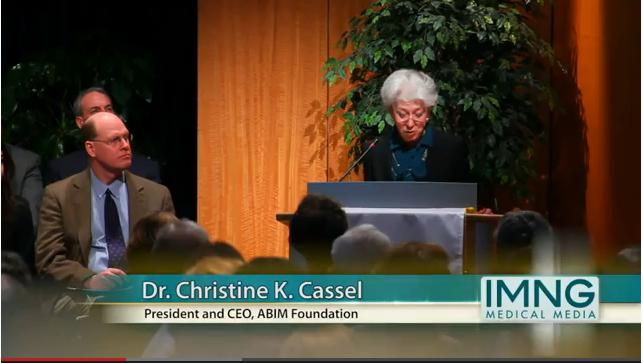
"Patient empowerment and appropriate care is what Choosing Wisely is all about," ABIM Foundation president and CEO Christine Cassel said at the press conference. The groups aim to change the perception that, "more is always better," she said.
Groups such as AARP, the National Business Group on Health, labor unions, and even Wikipedia have been brought into the campaign. Patient outreach is spearheaded by Consumer Reports, which is producing patient-friendly brochures based on the lists.
The first lists were issued in April 2012 and covered procedures and tests deemed overused by the American Academy of Family Physicians (AAFP), the American College of Physicians, the American Gastroenterological Association, and six other physician organizations.
"As part of our longstanding efforts to address the issues associated with health care utilization, the AGA is pleased to be a part of the Choosing Wisely campaign, which aims to build a more sustainable health care system that delivers high-quality, effective care," said Lawrence R. Kosinski, M.D., MBA, AGAF, chair of the AGA Institute Practice Management and Economics Committee. "AGA’s participation in Choosing Wisely is a natural extension of our years of work defining quality gastroenterological care and giving gastroenterologists tools for working with patients."
Each "Five Things" list is the result of a long process within the organization, which is explained at the end of the list. And each recommendation is accompanied by the reasoning and evidence for its selection.
The AGA’s list of Five Things makes the following recommendations:
• For pharmacological treatment of patients with gastroesophageal reflux disease (GERD), long-term acid suppression therapy (proton pump inhibitors or histamine2 receptor antagonists) should be titrated to the lowest effective dose needed to achieve therapeutic goals.
The main identifiable risk associated with reducing or discontinuing acid suppression therapy is an increased symptom burden. The decision regarding the need for (and dosage of) maintenance therapy is driven by the impact of those residual symptoms on the patient’s quality of life rather than as a disease control measure.
• Do not repeat colorectal cancer screening (by any method) for 10 years after a high-quality colonoscopy is negative in average-risk individuals.
A screening colonoscopy every 10 years is the recommended interval for adults without increased risk for colorectal cancer, beginning at age 50 years. Published studies indicate the risk of cancer is low for 10 years after a high-quality colonoscopy fails to detect neoplasia in this population.
• Do not repeat colonoscopy for at least 5 years for patients who have one or two small (less than 1 cm) adenomatous polyps, without high-grade dysplasia, completely removed via a high-quality colonoscopy.
The timing of a follow-up surveillance colonoscopy should be determined based on the results of a previous high-quality colonoscopy. Evidence-based (published) guidelines provide recommendations that patients with one or two small tubular adenomas with low-grade dysplasia have surveillance colonoscopy 5-10 years after initial polypectomy. "The precise timing within this interval should be based on other clinical factors (such as prior colonoscopy findings, family history, and the preferences of the patient and judgment of the physician)."
• For a patient who is diagnosed with Barrett’s esophagus who has undergone a second endoscopy that confirms the absence of dysplasia on biopsy, a follow-up surveillance examination should not be performed in less than 3 years as per published guidelines.
In patients with Barrett’s esophagus without dysplasia (cellular changes) the risk of cancer is very low. In these patients, it is appropriate and safe to examine the esophagus and check for dysplasia no more often than every 3 years because if these cellular changes occur, they do so very slowly.
• For a patient with functional abdominal pain syndrome (as per ROME III criteria) computed tomography (CT) scans should not be repeated unless there is a major change in clinical findings or symptoms.
There is a small but measurable increase in one’s cancer risk from x-ray exposure. An abdominal CT scan is one of the higher-radiation exposure x-rays – equivalent to 3 years of natural background radiation. Because of this risk and the high costs of this procedure, CT scans should be performed only when they are likely to provide useful information that changes patient management.
Click here to learn more about AGA’s involvement and download the patient reports.
Later this year, several groups will add to their lists and a new cohort of physician organizations will join the effort, ABIM’s Dr. Cassell noted.
"I don’t think we can overcommunicate on this issue – to patients, to providers, to employers," Dr. David L. Longworth, chairman of the Medicine Institute at the Cleveland Clinic, said at the briefing. "We need to reframe expectations and educate people about what is and is not appropriate."
All of the lists can be found at the Choosing Wisely website.
On Twitter @aliciaault
WASHINGTON – More than a dozen medical groups have issued new lists of tests and procedures that they say are often unnecessary and overused and should be questioned by both physicians and patients.
The lists – issued by 17 physician organizations on Feb. 21 – comprise the second iteration of the Choosing Wisely campaign, launched by the American Board of Internal Medicine Foundation in April 2012. The lists, compiled by each group as "Five Things Physicians and Patients Should Question," are evidence-based recommendations to help physicians and patients make decisions together.
"Patient empowerment and appropriate care is what Choosing Wisely is all about," ABIM Foundation president and CEO Christine Cassel said at the press conference. The groups aim to change the perception that, "more is always better," she said.
Groups such as AARP, the National Business Group on Health, labor unions, and even Wikipedia have been brought into the campaign. Patient outreach is spearheaded by Consumer Reports, which is producing patient-friendly brochures based on the lists.
The first lists were issued in April 2012 and covered procedures and tests deemed overused by the American Academy of Family Physicians (AAFP), the American College of Physicians, the American Gastroenterological Association, and six other physician organizations.
"As part of our longstanding efforts to address the issues associated with health care utilization, the AGA is pleased to be a part of the Choosing Wisely campaign, which aims to build a more sustainable health care system that delivers high-quality, effective care," said Lawrence R. Kosinski, M.D., MBA, AGAF, chair of the AGA Institute Practice Management and Economics Committee. "AGA’s participation in Choosing Wisely is a natural extension of our years of work defining quality gastroenterological care and giving gastroenterologists tools for working with patients."
Each "Five Things" list is the result of a long process within the organization, which is explained at the end of the list. And each recommendation is accompanied by the reasoning and evidence for its selection.
The AGA’s list of Five Things makes the following recommendations:
• For pharmacological treatment of patients with gastroesophageal reflux disease (GERD), long-term acid suppression therapy (proton pump inhibitors or histamine2 receptor antagonists) should be titrated to the lowest effective dose needed to achieve therapeutic goals.
The main identifiable risk associated with reducing or discontinuing acid suppression therapy is an increased symptom burden. The decision regarding the need for (and dosage of) maintenance therapy is driven by the impact of those residual symptoms on the patient’s quality of life rather than as a disease control measure.
• Do not repeat colorectal cancer screening (by any method) for 10 years after a high-quality colonoscopy is negative in average-risk individuals.
A screening colonoscopy every 10 years is the recommended interval for adults without increased risk for colorectal cancer, beginning at age 50 years. Published studies indicate the risk of cancer is low for 10 years after a high-quality colonoscopy fails to detect neoplasia in this population.
• Do not repeat colonoscopy for at least 5 years for patients who have one or two small (less than 1 cm) adenomatous polyps, without high-grade dysplasia, completely removed via a high-quality colonoscopy.
The timing of a follow-up surveillance colonoscopy should be determined based on the results of a previous high-quality colonoscopy. Evidence-based (published) guidelines provide recommendations that patients with one or two small tubular adenomas with low-grade dysplasia have surveillance colonoscopy 5-10 years after initial polypectomy. "The precise timing within this interval should be based on other clinical factors (such as prior colonoscopy findings, family history, and the preferences of the patient and judgment of the physician)."
• For a patient who is diagnosed with Barrett’s esophagus who has undergone a second endoscopy that confirms the absence of dysplasia on biopsy, a follow-up surveillance examination should not be performed in less than 3 years as per published guidelines.
In patients with Barrett’s esophagus without dysplasia (cellular changes) the risk of cancer is very low. In these patients, it is appropriate and safe to examine the esophagus and check for dysplasia no more often than every 3 years because if these cellular changes occur, they do so very slowly.
• For a patient with functional abdominal pain syndrome (as per ROME III criteria) computed tomography (CT) scans should not be repeated unless there is a major change in clinical findings or symptoms.
There is a small but measurable increase in one’s cancer risk from x-ray exposure. An abdominal CT scan is one of the higher-radiation exposure x-rays – equivalent to 3 years of natural background radiation. Because of this risk and the high costs of this procedure, CT scans should be performed only when they are likely to provide useful information that changes patient management.
Click here to learn more about AGA’s involvement and download the patient reports.
Later this year, several groups will add to their lists and a new cohort of physician organizations will join the effort, ABIM’s Dr. Cassell noted.
"I don’t think we can overcommunicate on this issue – to patients, to providers, to employers," Dr. David L. Longworth, chairman of the Medicine Institute at the Cleveland Clinic, said at the briefing. "We need to reframe expectations and educate people about what is and is not appropriate."
All of the lists can be found at the Choosing Wisely website.
On Twitter @aliciaault
WASHINGTON – More than a dozen medical groups have issued new lists of tests and procedures that they say are often unnecessary and overused and should be questioned by both physicians and patients.
The lists – issued by 17 physician organizations on Feb. 21 – comprise the second iteration of the Choosing Wisely campaign, launched by the American Board of Internal Medicine Foundation in April 2012. The lists, compiled by each group as "Five Things Physicians and Patients Should Question," are evidence-based recommendations to help physicians and patients make decisions together.
"Patient empowerment and appropriate care is what Choosing Wisely is all about," ABIM Foundation president and CEO Christine Cassel said at the press conference. The groups aim to change the perception that, "more is always better," she said.
Groups such as AARP, the National Business Group on Health, labor unions, and even Wikipedia have been brought into the campaign. Patient outreach is spearheaded by Consumer Reports, which is producing patient-friendly brochures based on the lists.
The first lists were issued in April 2012 and covered procedures and tests deemed overused by the American Academy of Family Physicians (AAFP), the American College of Physicians, the American Gastroenterological Association, and six other physician organizations.
"As part of our longstanding efforts to address the issues associated with health care utilization, the AGA is pleased to be a part of the Choosing Wisely campaign, which aims to build a more sustainable health care system that delivers high-quality, effective care," said Lawrence R. Kosinski, M.D., MBA, AGAF, chair of the AGA Institute Practice Management and Economics Committee. "AGA’s participation in Choosing Wisely is a natural extension of our years of work defining quality gastroenterological care and giving gastroenterologists tools for working with patients."
Each "Five Things" list is the result of a long process within the organization, which is explained at the end of the list. And each recommendation is accompanied by the reasoning and evidence for its selection.
The AGA’s list of Five Things makes the following recommendations:
• For pharmacological treatment of patients with gastroesophageal reflux disease (GERD), long-term acid suppression therapy (proton pump inhibitors or histamine2 receptor antagonists) should be titrated to the lowest effective dose needed to achieve therapeutic goals.
The main identifiable risk associated with reducing or discontinuing acid suppression therapy is an increased symptom burden. The decision regarding the need for (and dosage of) maintenance therapy is driven by the impact of those residual symptoms on the patient’s quality of life rather than as a disease control measure.
• Do not repeat colorectal cancer screening (by any method) for 10 years after a high-quality colonoscopy is negative in average-risk individuals.
A screening colonoscopy every 10 years is the recommended interval for adults without increased risk for colorectal cancer, beginning at age 50 years. Published studies indicate the risk of cancer is low for 10 years after a high-quality colonoscopy fails to detect neoplasia in this population.
• Do not repeat colonoscopy for at least 5 years for patients who have one or two small (less than 1 cm) adenomatous polyps, without high-grade dysplasia, completely removed via a high-quality colonoscopy.
The timing of a follow-up surveillance colonoscopy should be determined based on the results of a previous high-quality colonoscopy. Evidence-based (published) guidelines provide recommendations that patients with one or two small tubular adenomas with low-grade dysplasia have surveillance colonoscopy 5-10 years after initial polypectomy. "The precise timing within this interval should be based on other clinical factors (such as prior colonoscopy findings, family history, and the preferences of the patient and judgment of the physician)."
• For a patient who is diagnosed with Barrett’s esophagus who has undergone a second endoscopy that confirms the absence of dysplasia on biopsy, a follow-up surveillance examination should not be performed in less than 3 years as per published guidelines.
In patients with Barrett’s esophagus without dysplasia (cellular changes) the risk of cancer is very low. In these patients, it is appropriate and safe to examine the esophagus and check for dysplasia no more often than every 3 years because if these cellular changes occur, they do so very slowly.
• For a patient with functional abdominal pain syndrome (as per ROME III criteria) computed tomography (CT) scans should not be repeated unless there is a major change in clinical findings or symptoms.
There is a small but measurable increase in one’s cancer risk from x-ray exposure. An abdominal CT scan is one of the higher-radiation exposure x-rays – equivalent to 3 years of natural background radiation. Because of this risk and the high costs of this procedure, CT scans should be performed only when they are likely to provide useful information that changes patient management.
Click here to learn more about AGA’s involvement and download the patient reports.
Later this year, several groups will add to their lists and a new cohort of physician organizations will join the effort, ABIM’s Dr. Cassell noted.
"I don’t think we can overcommunicate on this issue – to patients, to providers, to employers," Dr. David L. Longworth, chairman of the Medicine Institute at the Cleveland Clinic, said at the briefing. "We need to reframe expectations and educate people about what is and is not appropriate."
All of the lists can be found at the Choosing Wisely website.
On Twitter @aliciaault
AT A PRESS CONFERENCE HELD BY THE AMERICAN BOARD OF INTERNAL MEDICINE
FDA approves novel oral estrogen for postmenopausal dyspareunia
The Food and Drug Administration has approved the novel selective estrogen receptor modulator ospemifene (Osphena) to treat moderate to severe dyspareunia due to vulvar and vaginal atrophy.
"Dyspareunia is among the problems most frequently reported by postmenopausal women," Dr. Victoria Kusiak, deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, said in a statement. "Osphena provides an additional treatment option for women seeking relief."
The once-daily pill acts like an estrogen on vaginal tissues, thickening them in the face of declining estrogen levels during menopause, according to the FDA. That may help alleviate pain during intercourse that occurs as a result of the thinned and fragile tissues.
According to ospemifene maker Shionogi Inc. of Florham Park, N.J., 32 million postmenopausal women in the United States experience symptoms of vulvar and vaginal atrophy (VVA). The company claims that only 7% or so are being treated with a prescription medication.
"While more than half of all women in the U.S. will experience symptoms of VVA at some time in their postmenopausal life, the vast majority of women with VVA are not being treated with a prescription medication because women and their health care professionals are not proactively discussing the condition, and its associated symptoms," Dr. David J. Portman, director of the Columbus (Ohio) Center for Women’s Health Research, said in a statement issued by Shionogi.
Most dyspareunia is addressed with over-the-counter moisturizers or creams, or with prescription tablets, creams, or rings, which are inserted vaginally.
Physicians and patients often seek alternatives because of concerns that those prescription estrogen-based treatments may also have effects on breast and endometrial tissue.
Ospemifene, however, will come with a boxed warning about the potential to act on endometrial tissue, which could lead to bleeding, precancerous, or cancerous conditions. The FDA said that ospemifene "should be prescribed for the shortest duration consistent with treatment goals and risks for the individual woman."
Even so, a 180-patient study presented at the American College of Obstetricians and Gynecologists annual meeting in June 2012 showed that ospemifene had minimal effects on the endometrial lining, compared with placebo.
The drug’s label also will contain a warning about increased rates of thrombotic and hemorrhagic strokes (pegging the incidence at 0.72 and 1.45 per 1,000 women, respectively). The label also will state the rate of deep vein thrombosis in ospemifene users (1.45 per 1,000 women). The FDA said that these elevated risks are lower than with therapies that contain only estrogen.
In three studies of 1,889 postmenopausal women with symptoms of vulvar and vaginal atrophy, women were randomly assigned to receive ospemifene or placebo. The first two studies showed a statistically significant improvement of dyspareunia in women treated with ospemifene after 12 weeks, compared with women treated with placebo. The third study showed that ospemifene was a safe drug for treating dyspareunia. Results from one of the first two trials were presented at the annual meeting of the North American Menopause Society in 2011.
The FDA reports that other common side effects reported during trials of ospemifene included hot flush/flashes, vaginal discharge, muscle spasms, genital discharge, and excessive sweating.
On Twitter @aliciaault
The Food and Drug Administration has approved the novel selective estrogen receptor modulator ospemifene (Osphena) to treat moderate to severe dyspareunia due to vulvar and vaginal atrophy.
"Dyspareunia is among the problems most frequently reported by postmenopausal women," Dr. Victoria Kusiak, deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, said in a statement. "Osphena provides an additional treatment option for women seeking relief."
The once-daily pill acts like an estrogen on vaginal tissues, thickening them in the face of declining estrogen levels during menopause, according to the FDA. That may help alleviate pain during intercourse that occurs as a result of the thinned and fragile tissues.
According to ospemifene maker Shionogi Inc. of Florham Park, N.J., 32 million postmenopausal women in the United States experience symptoms of vulvar and vaginal atrophy (VVA). The company claims that only 7% or so are being treated with a prescription medication.
"While more than half of all women in the U.S. will experience symptoms of VVA at some time in their postmenopausal life, the vast majority of women with VVA are not being treated with a prescription medication because women and their health care professionals are not proactively discussing the condition, and its associated symptoms," Dr. David J. Portman, director of the Columbus (Ohio) Center for Women’s Health Research, said in a statement issued by Shionogi.
Most dyspareunia is addressed with over-the-counter moisturizers or creams, or with prescription tablets, creams, or rings, which are inserted vaginally.
Physicians and patients often seek alternatives because of concerns that those prescription estrogen-based treatments may also have effects on breast and endometrial tissue.
Ospemifene, however, will come with a boxed warning about the potential to act on endometrial tissue, which could lead to bleeding, precancerous, or cancerous conditions. The FDA said that ospemifene "should be prescribed for the shortest duration consistent with treatment goals and risks for the individual woman."
Even so, a 180-patient study presented at the American College of Obstetricians and Gynecologists annual meeting in June 2012 showed that ospemifene had minimal effects on the endometrial lining, compared with placebo.
The drug’s label also will contain a warning about increased rates of thrombotic and hemorrhagic strokes (pegging the incidence at 0.72 and 1.45 per 1,000 women, respectively). The label also will state the rate of deep vein thrombosis in ospemifene users (1.45 per 1,000 women). The FDA said that these elevated risks are lower than with therapies that contain only estrogen.
In three studies of 1,889 postmenopausal women with symptoms of vulvar and vaginal atrophy, women were randomly assigned to receive ospemifene or placebo. The first two studies showed a statistically significant improvement of dyspareunia in women treated with ospemifene after 12 weeks, compared with women treated with placebo. The third study showed that ospemifene was a safe drug for treating dyspareunia. Results from one of the first two trials were presented at the annual meeting of the North American Menopause Society in 2011.
The FDA reports that other common side effects reported during trials of ospemifene included hot flush/flashes, vaginal discharge, muscle spasms, genital discharge, and excessive sweating.
On Twitter @aliciaault
The Food and Drug Administration has approved the novel selective estrogen receptor modulator ospemifene (Osphena) to treat moderate to severe dyspareunia due to vulvar and vaginal atrophy.
"Dyspareunia is among the problems most frequently reported by postmenopausal women," Dr. Victoria Kusiak, deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, said in a statement. "Osphena provides an additional treatment option for women seeking relief."
The once-daily pill acts like an estrogen on vaginal tissues, thickening them in the face of declining estrogen levels during menopause, according to the FDA. That may help alleviate pain during intercourse that occurs as a result of the thinned and fragile tissues.
According to ospemifene maker Shionogi Inc. of Florham Park, N.J., 32 million postmenopausal women in the United States experience symptoms of vulvar and vaginal atrophy (VVA). The company claims that only 7% or so are being treated with a prescription medication.
"While more than half of all women in the U.S. will experience symptoms of VVA at some time in their postmenopausal life, the vast majority of women with VVA are not being treated with a prescription medication because women and their health care professionals are not proactively discussing the condition, and its associated symptoms," Dr. David J. Portman, director of the Columbus (Ohio) Center for Women’s Health Research, said in a statement issued by Shionogi.
Most dyspareunia is addressed with over-the-counter moisturizers or creams, or with prescription tablets, creams, or rings, which are inserted vaginally.
Physicians and patients often seek alternatives because of concerns that those prescription estrogen-based treatments may also have effects on breast and endometrial tissue.
Ospemifene, however, will come with a boxed warning about the potential to act on endometrial tissue, which could lead to bleeding, precancerous, or cancerous conditions. The FDA said that ospemifene "should be prescribed for the shortest duration consistent with treatment goals and risks for the individual woman."
Even so, a 180-patient study presented at the American College of Obstetricians and Gynecologists annual meeting in June 2012 showed that ospemifene had minimal effects on the endometrial lining, compared with placebo.
The drug’s label also will contain a warning about increased rates of thrombotic and hemorrhagic strokes (pegging the incidence at 0.72 and 1.45 per 1,000 women, respectively). The label also will state the rate of deep vein thrombosis in ospemifene users (1.45 per 1,000 women). The FDA said that these elevated risks are lower than with therapies that contain only estrogen.
In three studies of 1,889 postmenopausal women with symptoms of vulvar and vaginal atrophy, women were randomly assigned to receive ospemifene or placebo. The first two studies showed a statistically significant improvement of dyspareunia in women treated with ospemifene after 12 weeks, compared with women treated with placebo. The third study showed that ospemifene was a safe drug for treating dyspareunia. Results from one of the first two trials were presented at the annual meeting of the North American Menopause Society in 2011.
The FDA reports that other common side effects reported during trials of ospemifene included hot flush/flashes, vaginal discharge, muscle spasms, genital discharge, and excessive sweating.
On Twitter @aliciaault
Outcomes no better at bariatric centers of excellence
Centers of Excellence for bariatric surgery – the only locations where the procedures are covered by Medicare – do not yield fewer complications or better outcomes for patients.
The Centers for Medicare and Medicaid Services established the Centers of Excellence in 2006 with an eye on increasing safety and decreasing negative outcomes.
Dr. Justin B. Dimick of the University of Michigan, Ann Arbor, and his colleagues, reviewed bariatric surgeries performed before and after that policy went into effect and found that there were no statistically significant improvement in complications, serious complications, or reoperations. Their findings were published Feb. 26 in JAMA.
The overall safety of bariatric surgery has increased over the years, Dr. Dimick noted, as surgeons have increasingly chosen less-invasive, lower-risk procedures such as laparoscopic gastric banding, rather than higher-risk, open procedures. Surgeons’ experience has increased and technology has improved as well; both trends have made the operations easier and less dangerous.
"Our study found large improvements in bariatric surgery outcomes over time even after adjusting for changes in procedure use," he wrote.
Taking into account patient factors such as age and comorbidities, procedure type, and year of operation, 5.5% of patients at a COE hospital had any complication, compared with 6% for those at a nondesignated facility. For serious complications, the rate was 2.2% at COEs vs. 2.5% at non-COEs. The reoperation rate was 0.83% for patients in COE hospitals, compared with 0.96% at nondesignated facilities (JAMA 2013;309:792-9).
The results were drawn from comparisons of discharge data from 2004-2009 in 12 states, chosen for geographic diversity. The discharges were for Medicare and non-Medicare patients, and the researchers examined outcomes for the 2 years before the CMS policy change and about 3 years after. Overall, there were 6,723 Medicare patients who had bariatric surgery before 2006, and 15,684 who had it afterward. For non-Medicare patients, the data covered 95,558 procedures before the change, and 155,117 afterward.
Facilities can gain the Centers of Excellence designation if they meet three primary criteria: provide accommodations for obese patients, and other structural elements; perform a minimum volume of 125 cases per year; and, submit data to either the American College of Surgeons or the American Society of Metabolic and Bariatric Surgery registry. Facilities are certified by the ACS or the ASMBS. Since 2006, the CMS has designated almost 600 facilities as a Bariatric Center of Excellence.
The structural resources required by the CMS do not differ much from what the Joint Commission requires, Dr. Dimick wrote, and volume standards don’t necessarily correlate with quality. He added that the registry data are not used in any kind of continuous quality improvement process, and thus probably do not have much of an impact.
The ACS and the ASMBS have been developing new outcomes measures and standards for that registry, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). Public comment closed on Jan. 15. The organizations said they expected to incorporate any comments into a final draft.
The CMS policy restricting coverage to Centers of Excellence should be revisited, Dr. Dimick advised. The policy does not appear to improve outcomes and may even have the unintended consequence of "sacrificing long-term effectiveness for improved short-term safety."
The study was supported by grants from the National Institute on Aging and the Agency for Healthcare Research and Quality. The authors had no relevant disclosures.
On Twitter @aliciaault
Since the Medicare policy decision 7 years ago, the Centers of Excellence have contributed to notable improvements in bariatric surgery, primarily by increasing awareness of variation between centers and focusing the attention of physicians, hospital health care personnel and administrators, and payers on the need to improve quality and safety. However, the limitations of COEs are now recognized by the American Society for Metabolic and Bariatric Surgery and the American College of Surgeons, which have joined forces and have an initiative underway to develop new standards, with an increased focus on more robust outcome measures.
As the CMS and the surgical societies reexamine the COE policy in bariatric surgery, there is an opportunity for them to be creative; to catapult surgical outcomes science forward through scalable approaches to data sharing, measurement, collaborative networks and comparative effectiveness research; and to design a program that can not only identify high-quality hospitals, but also provide a sustained mechanism for quality improvement.
Dr. Caprice C. Greenberg is associate professor of surgery at the University of Wisconsin, Madison, and director of the Wisconsin Surgical Outcomes Research Program. Her remarks were made in an editorial accompanying Dr. Dimick’s report (JAMA 2013;309:827-8).
The Centers for Medicare and Medicaid Services,
Dr. Justin B. Dimick
Since the Medicare policy decision 7 years ago, the Centers of Excellence have contributed to notable improvements in bariatric surgery, primarily by increasing awareness of variation between centers and focusing the attention of physicians, hospital health care personnel and administrators, and payers on the need to improve quality and safety. However, the limitations of COEs are now recognized by the American Society for Metabolic and Bariatric Surgery and the American College of Surgeons, which have joined forces and have an initiative underway to develop new standards, with an increased focus on more robust outcome measures.
As the CMS and the surgical societies reexamine the COE policy in bariatric surgery, there is an opportunity for them to be creative; to catapult surgical outcomes science forward through scalable approaches to data sharing, measurement, collaborative networks and comparative effectiveness research; and to design a program that can not only identify high-quality hospitals, but also provide a sustained mechanism for quality improvement.
Dr. Caprice C. Greenberg is associate professor of surgery at the University of Wisconsin, Madison, and director of the Wisconsin Surgical Outcomes Research Program. Her remarks were made in an editorial accompanying Dr. Dimick’s report (JAMA 2013;309:827-8).
Since the Medicare policy decision 7 years ago, the Centers of Excellence have contributed to notable improvements in bariatric surgery, primarily by increasing awareness of variation between centers and focusing the attention of physicians, hospital health care personnel and administrators, and payers on the need to improve quality and safety. However, the limitations of COEs are now recognized by the American Society for Metabolic and Bariatric Surgery and the American College of Surgeons, which have joined forces and have an initiative underway to develop new standards, with an increased focus on more robust outcome measures.
As the CMS and the surgical societies reexamine the COE policy in bariatric surgery, there is an opportunity for them to be creative; to catapult surgical outcomes science forward through scalable approaches to data sharing, measurement, collaborative networks and comparative effectiveness research; and to design a program that can not only identify high-quality hospitals, but also provide a sustained mechanism for quality improvement.
Dr. Caprice C. Greenberg is associate professor of surgery at the University of Wisconsin, Madison, and director of the Wisconsin Surgical Outcomes Research Program. Her remarks were made in an editorial accompanying Dr. Dimick’s report (JAMA 2013;309:827-8).
Centers of Excellence for bariatric surgery – the only locations where the procedures are covered by Medicare – do not yield fewer complications or better outcomes for patients.
The Centers for Medicare and Medicaid Services established the Centers of Excellence in 2006 with an eye on increasing safety and decreasing negative outcomes.
Dr. Justin B. Dimick of the University of Michigan, Ann Arbor, and his colleagues, reviewed bariatric surgeries performed before and after that policy went into effect and found that there were no statistically significant improvement in complications, serious complications, or reoperations. Their findings were published Feb. 26 in JAMA.
The overall safety of bariatric surgery has increased over the years, Dr. Dimick noted, as surgeons have increasingly chosen less-invasive, lower-risk procedures such as laparoscopic gastric banding, rather than higher-risk, open procedures. Surgeons’ experience has increased and technology has improved as well; both trends have made the operations easier and less dangerous.
"Our study found large improvements in bariatric surgery outcomes over time even after adjusting for changes in procedure use," he wrote.
Taking into account patient factors such as age and comorbidities, procedure type, and year of operation, 5.5% of patients at a COE hospital had any complication, compared with 6% for those at a nondesignated facility. For serious complications, the rate was 2.2% at COEs vs. 2.5% at non-COEs. The reoperation rate was 0.83% for patients in COE hospitals, compared with 0.96% at nondesignated facilities (JAMA 2013;309:792-9).
The results were drawn from comparisons of discharge data from 2004-2009 in 12 states, chosen for geographic diversity. The discharges were for Medicare and non-Medicare patients, and the researchers examined outcomes for the 2 years before the CMS policy change and about 3 years after. Overall, there were 6,723 Medicare patients who had bariatric surgery before 2006, and 15,684 who had it afterward. For non-Medicare patients, the data covered 95,558 procedures before the change, and 155,117 afterward.
Facilities can gain the Centers of Excellence designation if they meet three primary criteria: provide accommodations for obese patients, and other structural elements; perform a minimum volume of 125 cases per year; and, submit data to either the American College of Surgeons or the American Society of Metabolic and Bariatric Surgery registry. Facilities are certified by the ACS or the ASMBS. Since 2006, the CMS has designated almost 600 facilities as a Bariatric Center of Excellence.
The structural resources required by the CMS do not differ much from what the Joint Commission requires, Dr. Dimick wrote, and volume standards don’t necessarily correlate with quality. He added that the registry data are not used in any kind of continuous quality improvement process, and thus probably do not have much of an impact.
The ACS and the ASMBS have been developing new outcomes measures and standards for that registry, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). Public comment closed on Jan. 15. The organizations said they expected to incorporate any comments into a final draft.
The CMS policy restricting coverage to Centers of Excellence should be revisited, Dr. Dimick advised. The policy does not appear to improve outcomes and may even have the unintended consequence of "sacrificing long-term effectiveness for improved short-term safety."
The study was supported by grants from the National Institute on Aging and the Agency for Healthcare Research and Quality. The authors had no relevant disclosures.
On Twitter @aliciaault
Centers of Excellence for bariatric surgery – the only locations where the procedures are covered by Medicare – do not yield fewer complications or better outcomes for patients.
The Centers for Medicare and Medicaid Services established the Centers of Excellence in 2006 with an eye on increasing safety and decreasing negative outcomes.
Dr. Justin B. Dimick of the University of Michigan, Ann Arbor, and his colleagues, reviewed bariatric surgeries performed before and after that policy went into effect and found that there were no statistically significant improvement in complications, serious complications, or reoperations. Their findings were published Feb. 26 in JAMA.
The overall safety of bariatric surgery has increased over the years, Dr. Dimick noted, as surgeons have increasingly chosen less-invasive, lower-risk procedures such as laparoscopic gastric banding, rather than higher-risk, open procedures. Surgeons’ experience has increased and technology has improved as well; both trends have made the operations easier and less dangerous.
"Our study found large improvements in bariatric surgery outcomes over time even after adjusting for changes in procedure use," he wrote.
Taking into account patient factors such as age and comorbidities, procedure type, and year of operation, 5.5% of patients at a COE hospital had any complication, compared with 6% for those at a nondesignated facility. For serious complications, the rate was 2.2% at COEs vs. 2.5% at non-COEs. The reoperation rate was 0.83% for patients in COE hospitals, compared with 0.96% at nondesignated facilities (JAMA 2013;309:792-9).
The results were drawn from comparisons of discharge data from 2004-2009 in 12 states, chosen for geographic diversity. The discharges were for Medicare and non-Medicare patients, and the researchers examined outcomes for the 2 years before the CMS policy change and about 3 years after. Overall, there were 6,723 Medicare patients who had bariatric surgery before 2006, and 15,684 who had it afterward. For non-Medicare patients, the data covered 95,558 procedures before the change, and 155,117 afterward.
Facilities can gain the Centers of Excellence designation if they meet three primary criteria: provide accommodations for obese patients, and other structural elements; perform a minimum volume of 125 cases per year; and, submit data to either the American College of Surgeons or the American Society of Metabolic and Bariatric Surgery registry. Facilities are certified by the ACS or the ASMBS. Since 2006, the CMS has designated almost 600 facilities as a Bariatric Center of Excellence.
The structural resources required by the CMS do not differ much from what the Joint Commission requires, Dr. Dimick wrote, and volume standards don’t necessarily correlate with quality. He added that the registry data are not used in any kind of continuous quality improvement process, and thus probably do not have much of an impact.
The ACS and the ASMBS have been developing new outcomes measures and standards for that registry, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). Public comment closed on Jan. 15. The organizations said they expected to incorporate any comments into a final draft.
The CMS policy restricting coverage to Centers of Excellence should be revisited, Dr. Dimick advised. The policy does not appear to improve outcomes and may even have the unintended consequence of "sacrificing long-term effectiveness for improved short-term safety."
The study was supported by grants from the National Institute on Aging and the Agency for Healthcare Research and Quality. The authors had no relevant disclosures.
On Twitter @aliciaault
The Centers for Medicare and Medicaid Services,
Dr. Justin B. Dimick
The Centers for Medicare and Medicaid Services,
Dr. Justin B. Dimick
FROM JAMA
Major finding: Complication rates were 5.5% at Centers of Excellence, compared with 6% at nondesignated facilities. Reoperation rates were 0.83% for COEs, compared with 0.96% at nondesignated facilities.
Data source: A retrospective, longitudinal study using Medicare and non-Medicare hospital discharge data for 321,464 patients from 12 states for 2004-2009.
Disclosures: The study was supported by grants from the National Institute on Aging and the Agency for Healthcare Research and Quality. The authors had no relevant disclosures.
Cancer deaths declined 20% since 1991
Cancer deaths have declined 20% since 1991, which means there were 1.2 million fewer deaths from cancer in 2009, according to the American Cancer Society.
The overall cancer death rate decreased from 215 per 100,000 in 1991 to 173 per 100,000 in 2009. Death rates declined more sharply for colon and rectal cancers in both men and women, for cancers of the lung and prostate in men, and for breast cancers in women. The ACS attributes the drops to decreases in smoking and improvements in early detection and treatment.
The most common causes of cancer death in Americans, accounting for 50% of cancer deaths, are cancers of the colorectum, lung and bronchus, and prostate in men; and cancers of the colorectum, lung and bronchus, breast in women. This year, there will be 1.6 million cancer cases and 580,350 cancer deaths; lung cancers will account for 25% of cancer deaths in men and women this year, according to estimates from the ACS.
In men, colorectal cancer deaths declined annually by 2% during 1992-2002, 4% during 2002-2005, and 2.4% during 2005-2009; the rates in women were -1.7% during 1992-2001 and -3.1% during 2001-2009.
The mortality figures and incidence data are contained in two reports: Cancer Facts & Figures 2013 and Cancer Statistics 2013 (CA Cancer J. Clin. 2013;63:11-30).
Incidence rates are on the decline for most cancers with the exception of pancreatic, liver, and thyroid cancer, and melanoma.
Death rates from pancreatic cancer have increased over the last decade, due to what the ACS called "a lack progress in primary prevention, early diagnosis, and treatment of this cancer." The ACS report included a special section devoted to updated information on the occurrence and treatment of pancreatic cancer. Most patients with pancreatic cancer die within a year of diagnosis; the overall 5-year survival rate was 6% during 2002-2008.
During 1992-2009, deaths from liver and intrahepatic bile duct cancers increased by 2.3% in men and by 1.3% in women. The incidence of those cancers increased by 3.7% and 3%, respectively, over the same time period.
The ACS also noted that the disparity in cancer outcomes based on ethnicity and income, "particularly [among] those diagnosed with colorectal or breast cancer where earlier detection and better treatments are credited for the improving trends," said John R. Seffrin, Ph.D., chief executive officer of the ACS, in a statement. "We can and must close this gap so that people are not punished for having the misfortune of being born poor and disadvantaged."
The 5-year survival rate in 2002-2008 for white women with breast cancer, for instance, was 92%, whereas for black women, it was 78%. For colon cancer, the 5-year survival rate was 66% for whites, but 55% for blacks.
On Twitter @aliciaault
Cancer deaths have declined 20% since 1991, which means there were 1.2 million fewer deaths from cancer in 2009, according to the American Cancer Society.
The overall cancer death rate decreased from 215 per 100,000 in 1991 to 173 per 100,000 in 2009. Death rates declined more sharply for colon and rectal cancers in both men and women, for cancers of the lung and prostate in men, and for breast cancers in women. The ACS attributes the drops to decreases in smoking and improvements in early detection and treatment.
The most common causes of cancer death in Americans, accounting for 50% of cancer deaths, are cancers of the colorectum, lung and bronchus, and prostate in men; and cancers of the colorectum, lung and bronchus, breast in women. This year, there will be 1.6 million cancer cases and 580,350 cancer deaths; lung cancers will account for 25% of cancer deaths in men and women this year, according to estimates from the ACS.
In men, colorectal cancer deaths declined annually by 2% during 1992-2002, 4% during 2002-2005, and 2.4% during 2005-2009; the rates in women were -1.7% during 1992-2001 and -3.1% during 2001-2009.
The mortality figures and incidence data are contained in two reports: Cancer Facts & Figures 2013 and Cancer Statistics 2013 (CA Cancer J. Clin. 2013;63:11-30).
Incidence rates are on the decline for most cancers with the exception of pancreatic, liver, and thyroid cancer, and melanoma.
Death rates from pancreatic cancer have increased over the last decade, due to what the ACS called "a lack progress in primary prevention, early diagnosis, and treatment of this cancer." The ACS report included a special section devoted to updated information on the occurrence and treatment of pancreatic cancer. Most patients with pancreatic cancer die within a year of diagnosis; the overall 5-year survival rate was 6% during 2002-2008.
During 1992-2009, deaths from liver and intrahepatic bile duct cancers increased by 2.3% in men and by 1.3% in women. The incidence of those cancers increased by 3.7% and 3%, respectively, over the same time period.
The ACS also noted that the disparity in cancer outcomes based on ethnicity and income, "particularly [among] those diagnosed with colorectal or breast cancer where earlier detection and better treatments are credited for the improving trends," said John R. Seffrin, Ph.D., chief executive officer of the ACS, in a statement. "We can and must close this gap so that people are not punished for having the misfortune of being born poor and disadvantaged."
The 5-year survival rate in 2002-2008 for white women with breast cancer, for instance, was 92%, whereas for black women, it was 78%. For colon cancer, the 5-year survival rate was 66% for whites, but 55% for blacks.
On Twitter @aliciaault
Cancer deaths have declined 20% since 1991, which means there were 1.2 million fewer deaths from cancer in 2009, according to the American Cancer Society.
The overall cancer death rate decreased from 215 per 100,000 in 1991 to 173 per 100,000 in 2009. Death rates declined more sharply for colon and rectal cancers in both men and women, for cancers of the lung and prostate in men, and for breast cancers in women. The ACS attributes the drops to decreases in smoking and improvements in early detection and treatment.
The most common causes of cancer death in Americans, accounting for 50% of cancer deaths, are cancers of the colorectum, lung and bronchus, and prostate in men; and cancers of the colorectum, lung and bronchus, breast in women. This year, there will be 1.6 million cancer cases and 580,350 cancer deaths; lung cancers will account for 25% of cancer deaths in men and women this year, according to estimates from the ACS.
In men, colorectal cancer deaths declined annually by 2% during 1992-2002, 4% during 2002-2005, and 2.4% during 2005-2009; the rates in women were -1.7% during 1992-2001 and -3.1% during 2001-2009.
The mortality figures and incidence data are contained in two reports: Cancer Facts & Figures 2013 and Cancer Statistics 2013 (CA Cancer J. Clin. 2013;63:11-30).
Incidence rates are on the decline for most cancers with the exception of pancreatic, liver, and thyroid cancer, and melanoma.
Death rates from pancreatic cancer have increased over the last decade, due to what the ACS called "a lack progress in primary prevention, early diagnosis, and treatment of this cancer." The ACS report included a special section devoted to updated information on the occurrence and treatment of pancreatic cancer. Most patients with pancreatic cancer die within a year of diagnosis; the overall 5-year survival rate was 6% during 2002-2008.
During 1992-2009, deaths from liver and intrahepatic bile duct cancers increased by 2.3% in men and by 1.3% in women. The incidence of those cancers increased by 3.7% and 3%, respectively, over the same time period.
The ACS also noted that the disparity in cancer outcomes based on ethnicity and income, "particularly [among] those diagnosed with colorectal or breast cancer where earlier detection and better treatments are credited for the improving trends," said John R. Seffrin, Ph.D., chief executive officer of the ACS, in a statement. "We can and must close this gap so that people are not punished for having the misfortune of being born poor and disadvantaged."
The 5-year survival rate in 2002-2008 for white women with breast cancer, for instance, was 92%, whereas for black women, it was 78%. For colon cancer, the 5-year survival rate was 66% for whites, but 55% for blacks.
On Twitter @aliciaault
Choosing Wisely: More tests questioned in second round
More than a dozen medical groups have issued new lists of tests and procedures that they say are often unnecessary and overused and should be questioned by both physicians and patients.
The lists – issued by 17 physician organizations on Feb. 21 – comprise the second iteration of the Choosing Wisely campaign, launched by the American Board of Internal Medicine Foundation in April 2012. The lists, compiled by each group as "Five Things Physicians and Patients Should Question," are evidence-based recommendations to help physicians and patients make decisions together.
"Patient empowerment and appropriate care is what Choosing Wisely is all about," ABIM Foundation President and CEO Christine Cassel said at a press conference held by the ABIM. The groups aim to change the perception that "more is always better," she said.
Groups such as AARP, the National Business Group on Health, labor unions, and even Wikipedia have been brought into the campaign. Patient outreach is spearheaded by Consumer Reports, which is producing patient-friendly brochures based on the lists.
The first lists were issued in April 2012 and covered procedures and tests deemed overused by the American Academy of Family Physicians and the American College of Physicians.
Each "Five Things" list is the result of a long process within the organization, which is explained at the end of the list. And each recommendation is accompanied by the reasoning and evidence for its selection.
Later this year, several groups will add to their lists and a new cohort of physician organizations will join the effort, ABIM’s Dr. Cassell noted.
"I don’t think we can overcommunicate on this issue – to patients, to providers, to employers," Dr. David L. Longworth, chairman of the Medicine Institute at the Cleveland Clinic, said at the briefing. "We need to reframe expectations and educate people about what is and is not appropriate."
All of the lists can be found at the Choosing Wisely website.
On Twitter @aliciaault
More than a dozen medical groups have issued new lists of tests and procedures that they say are often unnecessary and overused and should be questioned by both physicians and patients.
The lists – issued by 17 physician organizations on Feb. 21 – comprise the second iteration of the Choosing Wisely campaign, launched by the American Board of Internal Medicine Foundation in April 2012. The lists, compiled by each group as "Five Things Physicians and Patients Should Question," are evidence-based recommendations to help physicians and patients make decisions together.
"Patient empowerment and appropriate care is what Choosing Wisely is all about," ABIM Foundation President and CEO Christine Cassel said at a press conference held by the ABIM. The groups aim to change the perception that "more is always better," she said.
Groups such as AARP, the National Business Group on Health, labor unions, and even Wikipedia have been brought into the campaign. Patient outreach is spearheaded by Consumer Reports, which is producing patient-friendly brochures based on the lists.
The first lists were issued in April 2012 and covered procedures and tests deemed overused by the American Academy of Family Physicians and the American College of Physicians.
Each "Five Things" list is the result of a long process within the organization, which is explained at the end of the list. And each recommendation is accompanied by the reasoning and evidence for its selection.
Later this year, several groups will add to their lists and a new cohort of physician organizations will join the effort, ABIM’s Dr. Cassell noted.
"I don’t think we can overcommunicate on this issue – to patients, to providers, to employers," Dr. David L. Longworth, chairman of the Medicine Institute at the Cleveland Clinic, said at the briefing. "We need to reframe expectations and educate people about what is and is not appropriate."
All of the lists can be found at the Choosing Wisely website.
On Twitter @aliciaault
More than a dozen medical groups have issued new lists of tests and procedures that they say are often unnecessary and overused and should be questioned by both physicians and patients.
The lists – issued by 17 physician organizations on Feb. 21 – comprise the second iteration of the Choosing Wisely campaign, launched by the American Board of Internal Medicine Foundation in April 2012. The lists, compiled by each group as "Five Things Physicians and Patients Should Question," are evidence-based recommendations to help physicians and patients make decisions together.
"Patient empowerment and appropriate care is what Choosing Wisely is all about," ABIM Foundation President and CEO Christine Cassel said at a press conference held by the ABIM. The groups aim to change the perception that "more is always better," she said.
Groups such as AARP, the National Business Group on Health, labor unions, and even Wikipedia have been brought into the campaign. Patient outreach is spearheaded by Consumer Reports, which is producing patient-friendly brochures based on the lists.
The first lists were issued in April 2012 and covered procedures and tests deemed overused by the American Academy of Family Physicians and the American College of Physicians.
Each "Five Things" list is the result of a long process within the organization, which is explained at the end of the list. And each recommendation is accompanied by the reasoning and evidence for its selection.
Later this year, several groups will add to their lists and a new cohort of physician organizations will join the effort, ABIM’s Dr. Cassell noted.
"I don’t think we can overcommunicate on this issue – to patients, to providers, to employers," Dr. David L. Longworth, chairman of the Medicine Institute at the Cleveland Clinic, said at the briefing. "We need to reframe expectations and educate people about what is and is not appropriate."
All of the lists can be found at the Choosing Wisely website.
On Twitter @aliciaault
AT A PRESS CONFERENCE HELD BY THE AMERICAN BOARD OF INTERNAL MEDICINE