User login
Ezetimibe found effective for primary prevention in elderly
CHICAGO – has been provided by the Japanese EWTOPIA 75 trial.

Ezetimibe (Zetia) at 10 mg/day reduced the risk of the primary endpoint, a composite of atherosclerotic cardiovascular events, by 34% compared with a dietary counseling control group over the course of 5 years of followup. Yasuyoshi Ouchi, MD, PhD, reported the findings of the 3,796-patient study at the American Heart Association scientific sessions.
There was also a 40% relative risk reduction for cardiac events in the ezetimibe group. The 22% reduction in cerebrovascular events, however, didn’t achieve statistical significance, and there was no between-group difference in all-cause mortality, said Dr. Ouchi, principal investigator in EWTOPIA 75 and professor emeritus of geriatric medicine at the University of Tokyo.
The landmark randomized clinical trials of lipid-lowering for primary cardiovascular prevention included too few elderly participants to permit assessment of its merits and possible harms in that population. This has left a major evidence gap at a time when in many parts of the world, including the United States, Europe, and Japan, the population over age 75 is growing explosively.
“Along with this population change, the number of patients age 75 and older with hypercholesterolemia has dramatically increased,” Dr. Ouchi continued.
Eligibility for EWTOPIA was restricted to patients who were at least 75 years old, had an LDL of at least 140 mg/dL, no history of CAD, and had at least one high-risk factor, such as diabetes or hypertension. Their mean age at enrollment was 80.7 years. Seventy-four percent of them were women, reflecting the significantly longer life expectancy of Japanese women compared to men.
The study design was open-label with no placebo arm. Dr. Ouchi argued that this was appropriate, given that the components of the primary composite endpoint were “entirely objective”: fatal and nonfatal MI, fatal and nonfatal stroke, sudden cardiac death, and coronary revascularization.
The mean LDL in the ezetimibe group dropped from 162 mg/dL at baseline to 120 mg/dL at 5 years, versus 131 mg/dL in the control group.
Ezetimibe was the lipid-lowering agent selected for EWTOPIA because it has an excellent safety record in older patients. There were no important differences between the two study arms in terms of adverse events, according to Dr. Ouchi.

Discussant Jennifer G. Robinson, MD, said that for a decade she has tried without success to get backing for a primary prevention statin trial in elderly U.S. patients, so congratulations to the Japanese investigators are in order.
She expressed doubts as to the generalizability of the EWTOPIA results to non-Japanese populations, however.
“Frankly, I was very surprised to see the large effect size. EWTOPIA had far more effect than we expected based on other trials of LDL-lowering agents to date,” said Dr. Robinson, professor of epidemiology and medicine and director of the Prevention Intervention Center at the University of Iowa, Iowa City.
“It’s a little better performance than we expected from that magnitude of LDL lowering, which was quite modest,” she added.
Among the possible explanations she cited for the greater magnitude of reduction in major vascular events seen in EWTOPIA as compared, for example, to the IMPROVE-IT trial, which also utilized ezetimibe, are genetic differences in the Japanese population. It’s known that the Japanese have different genetic polymorphisms of Niemann-Pick C1 Like 1 (NPC1L1), which is what ezetimibe binds to in order to inhibit small intestinal enterocyte uptake and absorption of cholesterol. Or it might just be that older adults, regardless of their ethnicity, have a more robust response to LDL lowering than the younger ones who’ve been the focus of previous trials.
“I think the LDL lowering from ezetimibe was very effective in Japanese older adults without cardiovascular disease, and I think that’s a very appropriate therapy for primary prevention moving forward in that population,” Dr. Robinson said.
As for herself, she’s awaiting confirmation in other populations. She’s particularly eager to see the outcome of the ongoing double-blind, randomized STAREE trial of atorvastatin (Lipitor) at 40 mg/day or placebo for primary prevention in 18,000 Australians age 70 and up. Results are expected in 2022.
Dr. Ouchi reported having no financial conflicts regarding the EWTOPIA study, funded by the Japanese government.
SOURCE: Ouchi Y. AHA Late Breaker 02.
CHICAGO – has been provided by the Japanese EWTOPIA 75 trial.

Ezetimibe (Zetia) at 10 mg/day reduced the risk of the primary endpoint, a composite of atherosclerotic cardiovascular events, by 34% compared with a dietary counseling control group over the course of 5 years of followup. Yasuyoshi Ouchi, MD, PhD, reported the findings of the 3,796-patient study at the American Heart Association scientific sessions.
There was also a 40% relative risk reduction for cardiac events in the ezetimibe group. The 22% reduction in cerebrovascular events, however, didn’t achieve statistical significance, and there was no between-group difference in all-cause mortality, said Dr. Ouchi, principal investigator in EWTOPIA 75 and professor emeritus of geriatric medicine at the University of Tokyo.
The landmark randomized clinical trials of lipid-lowering for primary cardiovascular prevention included too few elderly participants to permit assessment of its merits and possible harms in that population. This has left a major evidence gap at a time when in many parts of the world, including the United States, Europe, and Japan, the population over age 75 is growing explosively.
“Along with this population change, the number of patients age 75 and older with hypercholesterolemia has dramatically increased,” Dr. Ouchi continued.
Eligibility for EWTOPIA was restricted to patients who were at least 75 years old, had an LDL of at least 140 mg/dL, no history of CAD, and had at least one high-risk factor, such as diabetes or hypertension. Their mean age at enrollment was 80.7 years. Seventy-four percent of them were women, reflecting the significantly longer life expectancy of Japanese women compared to men.
The study design was open-label with no placebo arm. Dr. Ouchi argued that this was appropriate, given that the components of the primary composite endpoint were “entirely objective”: fatal and nonfatal MI, fatal and nonfatal stroke, sudden cardiac death, and coronary revascularization.
The mean LDL in the ezetimibe group dropped from 162 mg/dL at baseline to 120 mg/dL at 5 years, versus 131 mg/dL in the control group.
Ezetimibe was the lipid-lowering agent selected for EWTOPIA because it has an excellent safety record in older patients. There were no important differences between the two study arms in terms of adverse events, according to Dr. Ouchi.

Discussant Jennifer G. Robinson, MD, said that for a decade she has tried without success to get backing for a primary prevention statin trial in elderly U.S. patients, so congratulations to the Japanese investigators are in order.
She expressed doubts as to the generalizability of the EWTOPIA results to non-Japanese populations, however.
“Frankly, I was very surprised to see the large effect size. EWTOPIA had far more effect than we expected based on other trials of LDL-lowering agents to date,” said Dr. Robinson, professor of epidemiology and medicine and director of the Prevention Intervention Center at the University of Iowa, Iowa City.
“It’s a little better performance than we expected from that magnitude of LDL lowering, which was quite modest,” she added.
Among the possible explanations she cited for the greater magnitude of reduction in major vascular events seen in EWTOPIA as compared, for example, to the IMPROVE-IT trial, which also utilized ezetimibe, are genetic differences in the Japanese population. It’s known that the Japanese have different genetic polymorphisms of Niemann-Pick C1 Like 1 (NPC1L1), which is what ezetimibe binds to in order to inhibit small intestinal enterocyte uptake and absorption of cholesterol. Or it might just be that older adults, regardless of their ethnicity, have a more robust response to LDL lowering than the younger ones who’ve been the focus of previous trials.
“I think the LDL lowering from ezetimibe was very effective in Japanese older adults without cardiovascular disease, and I think that’s a very appropriate therapy for primary prevention moving forward in that population,” Dr. Robinson said.
As for herself, she’s awaiting confirmation in other populations. She’s particularly eager to see the outcome of the ongoing double-blind, randomized STAREE trial of atorvastatin (Lipitor) at 40 mg/day or placebo for primary prevention in 18,000 Australians age 70 and up. Results are expected in 2022.
Dr. Ouchi reported having no financial conflicts regarding the EWTOPIA study, funded by the Japanese government.
SOURCE: Ouchi Y. AHA Late Breaker 02.
CHICAGO – has been provided by the Japanese EWTOPIA 75 trial.

Ezetimibe (Zetia) at 10 mg/day reduced the risk of the primary endpoint, a composite of atherosclerotic cardiovascular events, by 34% compared with a dietary counseling control group over the course of 5 years of followup. Yasuyoshi Ouchi, MD, PhD, reported the findings of the 3,796-patient study at the American Heart Association scientific sessions.
There was also a 40% relative risk reduction for cardiac events in the ezetimibe group. The 22% reduction in cerebrovascular events, however, didn’t achieve statistical significance, and there was no between-group difference in all-cause mortality, said Dr. Ouchi, principal investigator in EWTOPIA 75 and professor emeritus of geriatric medicine at the University of Tokyo.
The landmark randomized clinical trials of lipid-lowering for primary cardiovascular prevention included too few elderly participants to permit assessment of its merits and possible harms in that population. This has left a major evidence gap at a time when in many parts of the world, including the United States, Europe, and Japan, the population over age 75 is growing explosively.
“Along with this population change, the number of patients age 75 and older with hypercholesterolemia has dramatically increased,” Dr. Ouchi continued.
Eligibility for EWTOPIA was restricted to patients who were at least 75 years old, had an LDL of at least 140 mg/dL, no history of CAD, and had at least one high-risk factor, such as diabetes or hypertension. Their mean age at enrollment was 80.7 years. Seventy-four percent of them were women, reflecting the significantly longer life expectancy of Japanese women compared to men.
The study design was open-label with no placebo arm. Dr. Ouchi argued that this was appropriate, given that the components of the primary composite endpoint were “entirely objective”: fatal and nonfatal MI, fatal and nonfatal stroke, sudden cardiac death, and coronary revascularization.
The mean LDL in the ezetimibe group dropped from 162 mg/dL at baseline to 120 mg/dL at 5 years, versus 131 mg/dL in the control group.
Ezetimibe was the lipid-lowering agent selected for EWTOPIA because it has an excellent safety record in older patients. There were no important differences between the two study arms in terms of adverse events, according to Dr. Ouchi.

Discussant Jennifer G. Robinson, MD, said that for a decade she has tried without success to get backing for a primary prevention statin trial in elderly U.S. patients, so congratulations to the Japanese investigators are in order.
She expressed doubts as to the generalizability of the EWTOPIA results to non-Japanese populations, however.
“Frankly, I was very surprised to see the large effect size. EWTOPIA had far more effect than we expected based on other trials of LDL-lowering agents to date,” said Dr. Robinson, professor of epidemiology and medicine and director of the Prevention Intervention Center at the University of Iowa, Iowa City.
“It’s a little better performance than we expected from that magnitude of LDL lowering, which was quite modest,” she added.
Among the possible explanations she cited for the greater magnitude of reduction in major vascular events seen in EWTOPIA as compared, for example, to the IMPROVE-IT trial, which also utilized ezetimibe, are genetic differences in the Japanese population. It’s known that the Japanese have different genetic polymorphisms of Niemann-Pick C1 Like 1 (NPC1L1), which is what ezetimibe binds to in order to inhibit small intestinal enterocyte uptake and absorption of cholesterol. Or it might just be that older adults, regardless of their ethnicity, have a more robust response to LDL lowering than the younger ones who’ve been the focus of previous trials.
“I think the LDL lowering from ezetimibe was very effective in Japanese older adults without cardiovascular disease, and I think that’s a very appropriate therapy for primary prevention moving forward in that population,” Dr. Robinson said.
As for herself, she’s awaiting confirmation in other populations. She’s particularly eager to see the outcome of the ongoing double-blind, randomized STAREE trial of atorvastatin (Lipitor) at 40 mg/day or placebo for primary prevention in 18,000 Australians age 70 and up. Results are expected in 2022.
Dr. Ouchi reported having no financial conflicts regarding the EWTOPIA study, funded by the Japanese government.
SOURCE: Ouchi Y. AHA Late Breaker 02.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: LDL-lowering for primary cardiovascular prevention in elderly patients has been shown for the first time to impart significant net benefit.
Major finding: The incidence of atherosclerotic cardiovascular events was reduced by 34% in elderly patients on ezetimibe at 10 mg/day, compared with usual care.
Study details: The 5-year prospective randomized EWTOPIA 75 trial included 3,796 Japanese patients age 75 and older with elevated LDL and no history of CAD.
Disclosures: The presenter reported having no financial conflicts regarding the study, sponsored by the Japanese government.
Source: Ouchi Y. AHA Late Breaker 02.
Obesity paradox applies to post-stroke mortality
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.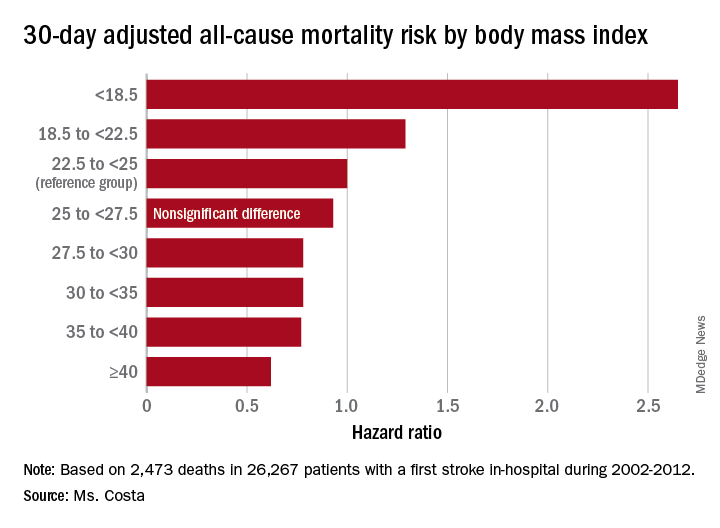
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Heavier stroke patients have lower 30-day and 1-year all-cause mortality.
Major finding: The 30-day stroke-related mortality rate after in-hospital stroke was reduced by 45% in VA patients with Class III obesity.
Study details: This retrospective study looked at the relationship between body mass index and post-stroke mortality in more than 26,000 veterans who had an inpatient stroke, with extensive adjustments made for potential confounders.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was sponsored by the Department of Veterans Affairs.
Source: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
Getting serious about post-EVAR aortic neck dilation
CHICAGO – Clinically significant aortic neck dilation occurs in one-quarter of patients after endovascular abdominal aortic aneurysm repair and is associated with sharply increased risk for type Ia endoleak, stent migration at the proximal seal zone, continued aneurysmal sack enlargement, and even aneurysm rupture, Jason T. Lee, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
He cited what he considers to be the best-quality systematic review of the literature regarding proximal neck dilation after endovascular aneurysm repair (EVAR), which was conducted by investigators at Paracelsus Medical University in Nuremberg, Germany. The data, he said, speak to the importance of trying to minimize the chances of aortic neck dilation (AND).
The review, which included 26 published studies through 2015 and nearly 10,000 EVAR patients, concluded that AND occurred in 24.6% of these patients. The investigators defined AND as more than 3 mm of dilation, which with an average neck diameter of about 30 mm in all comers, would represent a 10% aortic neck-diameter expansion. The incidence of the composite adverse outcome of type I endoleak, stent migration, and reintervention during a maximum of 9 years of follow-up was 29-fold greater in the AND group than in AND-free patients (J Endovasc Ther. 2017;24[1]:59-67).
Continuing controversy exists regarding the extent to which post-EVAR AND is a manifestation of the underlying aneurysmal process as opposed to an adverse effect caused by the outward radial force applied by the stent. That being said, there are several factors related to AND that are potentially within the vascular interventionalist’s control. Topping the list is aggressive oversizing of self-expanding stent grafts in an effort to obtain an excellent seal, according to Dr. Lee, a professor of surgery at Stanford (Calif.) University.
“The data from multiple series definitely suggests that more than 20% oversizing is correlated with a higher incidence of AND. So maybe the answer is oversizing by more like 10%-15%,” the vascular surgeon said.
Another factor contributing to AND is the overwhelming popularity of self-expanding aortic stent grafts, now utilized in 95% of all EVARS done in the United States. Balloon-expandable stent grafts require much less oversizing – less than 5% – to maintain their diameter post deployment, but they are rarely used. Indeed, the only commercially available balloon-expandable device in the United States is the TriVascular Ovation stent system, which seals the stent graft to the wall of the aorta via polyethylene glycol rather than by radial force.
Intriguingly, Italian investigators have reported that, in a multicenter series of 161 patients who underwent EVAR with the Ovation endograft, no AND occurred at CT scanning done after a minimum of 24 months of follow-up (J Vasc Surg. 2016;63[1]:8-15).
Previous studies of the implications of AND after EVAR have been limited to comparisons of early-generation devices, so Dr. Lee and his coinvestigators conducted a retrospective review of a prospective Stanford database that included 86 patients who underwent elective, uncomplicated infrarenal endovascular abdominal aortic aneurysm repairs using a variety of contemporary stent grafts. Eighty-six percent of patients experienced AND during a median radiologic follow-up of 21.9 months, with a mean 1.3-mm increase at 30 days and 3.3 mm at most recent follow-up. The degree of AND correlated with the amount of oversizing. However, AND didn’t vary significantly by device type, which included the Cook Zenith, Gore Excluder, Medtronic Endurant, and Endologix Powerlink self-expanding stent grafts (Ann Vasc Surg. 2017;43:115-20).
Aortic anatomy also plays a role in AND. Shorter necks with severe angulation have been found to pose a higher risk. Open repair, which entails much less AND than does EVAR, may make more sense in that challenging anatomic situation, according to Dr. Lee.
He reported current research funding from Cook, Gore, and Medtronic.
CHICAGO – Clinically significant aortic neck dilation occurs in one-quarter of patients after endovascular abdominal aortic aneurysm repair and is associated with sharply increased risk for type Ia endoleak, stent migration at the proximal seal zone, continued aneurysmal sack enlargement, and even aneurysm rupture, Jason T. Lee, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
He cited what he considers to be the best-quality systematic review of the literature regarding proximal neck dilation after endovascular aneurysm repair (EVAR), which was conducted by investigators at Paracelsus Medical University in Nuremberg, Germany. The data, he said, speak to the importance of trying to minimize the chances of aortic neck dilation (AND).
The review, which included 26 published studies through 2015 and nearly 10,000 EVAR patients, concluded that AND occurred in 24.6% of these patients. The investigators defined AND as more than 3 mm of dilation, which with an average neck diameter of about 30 mm in all comers, would represent a 10% aortic neck-diameter expansion. The incidence of the composite adverse outcome of type I endoleak, stent migration, and reintervention during a maximum of 9 years of follow-up was 29-fold greater in the AND group than in AND-free patients (J Endovasc Ther. 2017;24[1]:59-67).
Continuing controversy exists regarding the extent to which post-EVAR AND is a manifestation of the underlying aneurysmal process as opposed to an adverse effect caused by the outward radial force applied by the stent. That being said, there are several factors related to AND that are potentially within the vascular interventionalist’s control. Topping the list is aggressive oversizing of self-expanding stent grafts in an effort to obtain an excellent seal, according to Dr. Lee, a professor of surgery at Stanford (Calif.) University.
“The data from multiple series definitely suggests that more than 20% oversizing is correlated with a higher incidence of AND. So maybe the answer is oversizing by more like 10%-15%,” the vascular surgeon said.
Another factor contributing to AND is the overwhelming popularity of self-expanding aortic stent grafts, now utilized in 95% of all EVARS done in the United States. Balloon-expandable stent grafts require much less oversizing – less than 5% – to maintain their diameter post deployment, but they are rarely used. Indeed, the only commercially available balloon-expandable device in the United States is the TriVascular Ovation stent system, which seals the stent graft to the wall of the aorta via polyethylene glycol rather than by radial force.
Intriguingly, Italian investigators have reported that, in a multicenter series of 161 patients who underwent EVAR with the Ovation endograft, no AND occurred at CT scanning done after a minimum of 24 months of follow-up (J Vasc Surg. 2016;63[1]:8-15).
Previous studies of the implications of AND after EVAR have been limited to comparisons of early-generation devices, so Dr. Lee and his coinvestigators conducted a retrospective review of a prospective Stanford database that included 86 patients who underwent elective, uncomplicated infrarenal endovascular abdominal aortic aneurysm repairs using a variety of contemporary stent grafts. Eighty-six percent of patients experienced AND during a median radiologic follow-up of 21.9 months, with a mean 1.3-mm increase at 30 days and 3.3 mm at most recent follow-up. The degree of AND correlated with the amount of oversizing. However, AND didn’t vary significantly by device type, which included the Cook Zenith, Gore Excluder, Medtronic Endurant, and Endologix Powerlink self-expanding stent grafts (Ann Vasc Surg. 2017;43:115-20).
Aortic anatomy also plays a role in AND. Shorter necks with severe angulation have been found to pose a higher risk. Open repair, which entails much less AND than does EVAR, may make more sense in that challenging anatomic situation, according to Dr. Lee.
He reported current research funding from Cook, Gore, and Medtronic.
CHICAGO – Clinically significant aortic neck dilation occurs in one-quarter of patients after endovascular abdominal aortic aneurysm repair and is associated with sharply increased risk for type Ia endoleak, stent migration at the proximal seal zone, continued aneurysmal sack enlargement, and even aneurysm rupture, Jason T. Lee, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
He cited what he considers to be the best-quality systematic review of the literature regarding proximal neck dilation after endovascular aneurysm repair (EVAR), which was conducted by investigators at Paracelsus Medical University in Nuremberg, Germany. The data, he said, speak to the importance of trying to minimize the chances of aortic neck dilation (AND).
The review, which included 26 published studies through 2015 and nearly 10,000 EVAR patients, concluded that AND occurred in 24.6% of these patients. The investigators defined AND as more than 3 mm of dilation, which with an average neck diameter of about 30 mm in all comers, would represent a 10% aortic neck-diameter expansion. The incidence of the composite adverse outcome of type I endoleak, stent migration, and reintervention during a maximum of 9 years of follow-up was 29-fold greater in the AND group than in AND-free patients (J Endovasc Ther. 2017;24[1]:59-67).
Continuing controversy exists regarding the extent to which post-EVAR AND is a manifestation of the underlying aneurysmal process as opposed to an adverse effect caused by the outward radial force applied by the stent. That being said, there are several factors related to AND that are potentially within the vascular interventionalist’s control. Topping the list is aggressive oversizing of self-expanding stent grafts in an effort to obtain an excellent seal, according to Dr. Lee, a professor of surgery at Stanford (Calif.) University.
“The data from multiple series definitely suggests that more than 20% oversizing is correlated with a higher incidence of AND. So maybe the answer is oversizing by more like 10%-15%,” the vascular surgeon said.
Another factor contributing to AND is the overwhelming popularity of self-expanding aortic stent grafts, now utilized in 95% of all EVARS done in the United States. Balloon-expandable stent grafts require much less oversizing – less than 5% – to maintain their diameter post deployment, but they are rarely used. Indeed, the only commercially available balloon-expandable device in the United States is the TriVascular Ovation stent system, which seals the stent graft to the wall of the aorta via polyethylene glycol rather than by radial force.
Intriguingly, Italian investigators have reported that, in a multicenter series of 161 patients who underwent EVAR with the Ovation endograft, no AND occurred at CT scanning done after a minimum of 24 months of follow-up (J Vasc Surg. 2016;63[1]:8-15).
Previous studies of the implications of AND after EVAR have been limited to comparisons of early-generation devices, so Dr. Lee and his coinvestigators conducted a retrospective review of a prospective Stanford database that included 86 patients who underwent elective, uncomplicated infrarenal endovascular abdominal aortic aneurysm repairs using a variety of contemporary stent grafts. Eighty-six percent of patients experienced AND during a median radiologic follow-up of 21.9 months, with a mean 1.3-mm increase at 30 days and 3.3 mm at most recent follow-up. The degree of AND correlated with the amount of oversizing. However, AND didn’t vary significantly by device type, which included the Cook Zenith, Gore Excluder, Medtronic Endurant, and Endologix Powerlink self-expanding stent grafts (Ann Vasc Surg. 2017;43:115-20).
Aortic anatomy also plays a role in AND. Shorter necks with severe angulation have been found to pose a higher risk. Open repair, which entails much less AND than does EVAR, may make more sense in that challenging anatomic situation, according to Dr. Lee.
He reported current research funding from Cook, Gore, and Medtronic.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Novel agent cut LDL in statin-intolerant patients
CHICAGO – Bempedoic acid, a novel oral lipid-lowering agent, reduced LDL cholesterol by 21% and high-sensitivity C-reactive protein by 24% with a side-effect profile similar to placebo in statin-intolerant hypercholesterolemic patients with or at high risk for atherosclerotic cardiovascular disease in the pivotal phase 3 CLEAR Serenity trial, Ulrich Laufs, MD, PhD, reported at the American Heart Association scientific sessions.
CLEAR Serenity is one of five pivotal phase 3 trials of bempedoic acid. The others evaluated bempedoic acid as add-on therapy to obtain additional lipid lowering in patients already on a maximum-dose statin, and in combination with ezetimibe (Zetia) as a single pill, also in patients on full-dose statin therapy. All of these trials met their efficacy and safety endpoints. The drug’s developer, Esperion Therapeutics, has announced plans to file for marketing approval of bempedoic acid and for the bempedoic acid/ezetimibe combo pill with the Food and Drug Administration and the European Medicines Agency in the first months of 2019.
CLEAR Serenity was a 24-week, double-blind, placebo-controlled trial conducted at 67 North American sites. The 345 statin-intolerant participants were randomized 2:1 to bempedoic acid at 180 mg once daily or placebo.
“I think the specific contribution of this study is, importantly, that myalgia and other muscle-related symptoms were not increased with bempedoic acid versus placebo in this population that’s statin intolerant, more than 90% of whom complained of statin-related muscle symptoms,” said Dr. Laufs, professor and chair of the department of cardiology at the University of Leipzig (Germany).
Rates of major adverse cardiovascular events were too low in this relatively short-term, modest-size trial to be informative, but the results of the previously presented CLEAR Harmony trial are reassuring in this regard, according to the cardiologist. CLEAR Harmony was a 52-week study that included 2,230 patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia whose LDL was inadequately controlled despite high-intensity statin therapy. They were randomized to add-on bempedoic acid or placebo. The adjudicated major adverse cardiovascular event rate was 4.6% in the bempedoic acid group and not significantly different at 5.7% in controls.
Definitive data on the effect of bempedoic acid on cardiovascular morbidity and mortality event rates will eventually be provided by an ongoing global randomized, double-blind, placebo-controlled trial expected to enroll more than 12,000 patients.
Rates of various types of adverse events were closely similar in the bempedoic acid and placebo groups in CLEAR Serenity, with a couple of intriguing exceptions, according to Dr. Laufs. For example, the rate of new-onset or worsening diabetes was 2.1% in the bempedoic acid group, compared with 4.5% in controls.
“This is consistent with results in the other studies in the overall bempedoic acid program. It will be something of great interest to follow up in the ongoing outcomes trial,” he said. “At this point I would feel comfortable in saying that there is no deterioration of glucose tolerance, unlike with statins. Whether there is an actual improvement or not needs to be characterized a little better.”
The other difference in the safety profile between the two study arms in CLEAR Serenity was a trend for higher uric acid levels and an increased risk of developing gout in the bempedoic acid group. Gout occurred in 1.7% of the bempedoic acid group and 0.9% of placebo-treated controls. A similar signal has been seen in the other pivotal trials, but a definitive answer as to gout risk must await the large ongoing outcomes trial, Dr. Laufs continued.
Bempedoic acid is a first-in-class oral inhibitor of ATP citrate lyase, an enzyme that is inactive in skeletal muscle – thus, the lack of myalgia complaints – and lies upstream of HMG-CoA reductase in cholesterol synthesis. When combined in a single pill with ezetimibe, which lowers LDL by stimulating the LDL receptor, the lipid-lowering impact is magnified over that of either drug alone. In the phase 3 program, the combination pill resulted in a further 35% reduction in LDL when added to a maximally tolerated statin and a 43% reduction in LDL when used as monotherapy.
Session cochair Robert H. Eckel, MD, commented that bempedoic acid appears to be poised to address a significant unmet need in preventive cardiology.
“We clearly need alternative therapies in patients with statin intolerance. We see a lot of these patients in referral centers. This drug looks safe and very effective at modifying LDL, about as much so as ezetimibe and maybe a little bit more,” said Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
Dr. Laufs reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
CHICAGO – Bempedoic acid, a novel oral lipid-lowering agent, reduced LDL cholesterol by 21% and high-sensitivity C-reactive protein by 24% with a side-effect profile similar to placebo in statin-intolerant hypercholesterolemic patients with or at high risk for atherosclerotic cardiovascular disease in the pivotal phase 3 CLEAR Serenity trial, Ulrich Laufs, MD, PhD, reported at the American Heart Association scientific sessions.
CLEAR Serenity is one of five pivotal phase 3 trials of bempedoic acid. The others evaluated bempedoic acid as add-on therapy to obtain additional lipid lowering in patients already on a maximum-dose statin, and in combination with ezetimibe (Zetia) as a single pill, also in patients on full-dose statin therapy. All of these trials met their efficacy and safety endpoints. The drug’s developer, Esperion Therapeutics, has announced plans to file for marketing approval of bempedoic acid and for the bempedoic acid/ezetimibe combo pill with the Food and Drug Administration and the European Medicines Agency in the first months of 2019.
CLEAR Serenity was a 24-week, double-blind, placebo-controlled trial conducted at 67 North American sites. The 345 statin-intolerant participants were randomized 2:1 to bempedoic acid at 180 mg once daily or placebo.
“I think the specific contribution of this study is, importantly, that myalgia and other muscle-related symptoms were not increased with bempedoic acid versus placebo in this population that’s statin intolerant, more than 90% of whom complained of statin-related muscle symptoms,” said Dr. Laufs, professor and chair of the department of cardiology at the University of Leipzig (Germany).
Rates of major adverse cardiovascular events were too low in this relatively short-term, modest-size trial to be informative, but the results of the previously presented CLEAR Harmony trial are reassuring in this regard, according to the cardiologist. CLEAR Harmony was a 52-week study that included 2,230 patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia whose LDL was inadequately controlled despite high-intensity statin therapy. They were randomized to add-on bempedoic acid or placebo. The adjudicated major adverse cardiovascular event rate was 4.6% in the bempedoic acid group and not significantly different at 5.7% in controls.
Definitive data on the effect of bempedoic acid on cardiovascular morbidity and mortality event rates will eventually be provided by an ongoing global randomized, double-blind, placebo-controlled trial expected to enroll more than 12,000 patients.
Rates of various types of adverse events were closely similar in the bempedoic acid and placebo groups in CLEAR Serenity, with a couple of intriguing exceptions, according to Dr. Laufs. For example, the rate of new-onset or worsening diabetes was 2.1% in the bempedoic acid group, compared with 4.5% in controls.
“This is consistent with results in the other studies in the overall bempedoic acid program. It will be something of great interest to follow up in the ongoing outcomes trial,” he said. “At this point I would feel comfortable in saying that there is no deterioration of glucose tolerance, unlike with statins. Whether there is an actual improvement or not needs to be characterized a little better.”
The other difference in the safety profile between the two study arms in CLEAR Serenity was a trend for higher uric acid levels and an increased risk of developing gout in the bempedoic acid group. Gout occurred in 1.7% of the bempedoic acid group and 0.9% of placebo-treated controls. A similar signal has been seen in the other pivotal trials, but a definitive answer as to gout risk must await the large ongoing outcomes trial, Dr. Laufs continued.
Bempedoic acid is a first-in-class oral inhibitor of ATP citrate lyase, an enzyme that is inactive in skeletal muscle – thus, the lack of myalgia complaints – and lies upstream of HMG-CoA reductase in cholesterol synthesis. When combined in a single pill with ezetimibe, which lowers LDL by stimulating the LDL receptor, the lipid-lowering impact is magnified over that of either drug alone. In the phase 3 program, the combination pill resulted in a further 35% reduction in LDL when added to a maximally tolerated statin and a 43% reduction in LDL when used as monotherapy.
Session cochair Robert H. Eckel, MD, commented that bempedoic acid appears to be poised to address a significant unmet need in preventive cardiology.
“We clearly need alternative therapies in patients with statin intolerance. We see a lot of these patients in referral centers. This drug looks safe and very effective at modifying LDL, about as much so as ezetimibe and maybe a little bit more,” said Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
Dr. Laufs reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
CHICAGO – Bempedoic acid, a novel oral lipid-lowering agent, reduced LDL cholesterol by 21% and high-sensitivity C-reactive protein by 24% with a side-effect profile similar to placebo in statin-intolerant hypercholesterolemic patients with or at high risk for atherosclerotic cardiovascular disease in the pivotal phase 3 CLEAR Serenity trial, Ulrich Laufs, MD, PhD, reported at the American Heart Association scientific sessions.
CLEAR Serenity is one of five pivotal phase 3 trials of bempedoic acid. The others evaluated bempedoic acid as add-on therapy to obtain additional lipid lowering in patients already on a maximum-dose statin, and in combination with ezetimibe (Zetia) as a single pill, also in patients on full-dose statin therapy. All of these trials met their efficacy and safety endpoints. The drug’s developer, Esperion Therapeutics, has announced plans to file for marketing approval of bempedoic acid and for the bempedoic acid/ezetimibe combo pill with the Food and Drug Administration and the European Medicines Agency in the first months of 2019.
CLEAR Serenity was a 24-week, double-blind, placebo-controlled trial conducted at 67 North American sites. The 345 statin-intolerant participants were randomized 2:1 to bempedoic acid at 180 mg once daily or placebo.
“I think the specific contribution of this study is, importantly, that myalgia and other muscle-related symptoms were not increased with bempedoic acid versus placebo in this population that’s statin intolerant, more than 90% of whom complained of statin-related muscle symptoms,” said Dr. Laufs, professor and chair of the department of cardiology at the University of Leipzig (Germany).
Rates of major adverse cardiovascular events were too low in this relatively short-term, modest-size trial to be informative, but the results of the previously presented CLEAR Harmony trial are reassuring in this regard, according to the cardiologist. CLEAR Harmony was a 52-week study that included 2,230 patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia whose LDL was inadequately controlled despite high-intensity statin therapy. They were randomized to add-on bempedoic acid or placebo. The adjudicated major adverse cardiovascular event rate was 4.6% in the bempedoic acid group and not significantly different at 5.7% in controls.
Definitive data on the effect of bempedoic acid on cardiovascular morbidity and mortality event rates will eventually be provided by an ongoing global randomized, double-blind, placebo-controlled trial expected to enroll more than 12,000 patients.
Rates of various types of adverse events were closely similar in the bempedoic acid and placebo groups in CLEAR Serenity, with a couple of intriguing exceptions, according to Dr. Laufs. For example, the rate of new-onset or worsening diabetes was 2.1% in the bempedoic acid group, compared with 4.5% in controls.
“This is consistent with results in the other studies in the overall bempedoic acid program. It will be something of great interest to follow up in the ongoing outcomes trial,” he said. “At this point I would feel comfortable in saying that there is no deterioration of glucose tolerance, unlike with statins. Whether there is an actual improvement or not needs to be characterized a little better.”
The other difference in the safety profile between the two study arms in CLEAR Serenity was a trend for higher uric acid levels and an increased risk of developing gout in the bempedoic acid group. Gout occurred in 1.7% of the bempedoic acid group and 0.9% of placebo-treated controls. A similar signal has been seen in the other pivotal trials, but a definitive answer as to gout risk must await the large ongoing outcomes trial, Dr. Laufs continued.
Bempedoic acid is a first-in-class oral inhibitor of ATP citrate lyase, an enzyme that is inactive in skeletal muscle – thus, the lack of myalgia complaints – and lies upstream of HMG-CoA reductase in cholesterol synthesis. When combined in a single pill with ezetimibe, which lowers LDL by stimulating the LDL receptor, the lipid-lowering impact is magnified over that of either drug alone. In the phase 3 program, the combination pill resulted in a further 35% reduction in LDL when added to a maximally tolerated statin and a 43% reduction in LDL when used as monotherapy.
Session cochair Robert H. Eckel, MD, commented that bempedoic acid appears to be poised to address a significant unmet need in preventive cardiology.
“We clearly need alternative therapies in patients with statin intolerance. We see a lot of these patients in referral centers. This drug looks safe and very effective at modifying LDL, about as much so as ezetimibe and maybe a little bit more,” said Dr. Eckel, professor of medicine and director of the lipid clinic at University of Colorado Hospital, Aurora.
Dr. Laufs reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Oral once-daily bempedoic acid may be an attractive option for statin-intolerant patients.
Major finding: Bempedoic acid reduced elevated LDL cholesterol by 21% in statin-intolerant patients at high cardiovascular risk.
Study details: CLEAR Serenity was a 24-week, double-blind, placebo-controlled, multicenter, pivotal phase 3 trial including 345 statin-intolerant patients at high risk for cardiovascular events.
Disclosures: The presenter reported serving as a paid consultant to Esperion Therapeutics, the study sponsor, as well as to Amgen and Sanofi.
Know the red flags for synaptic autoimmune psychosis
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 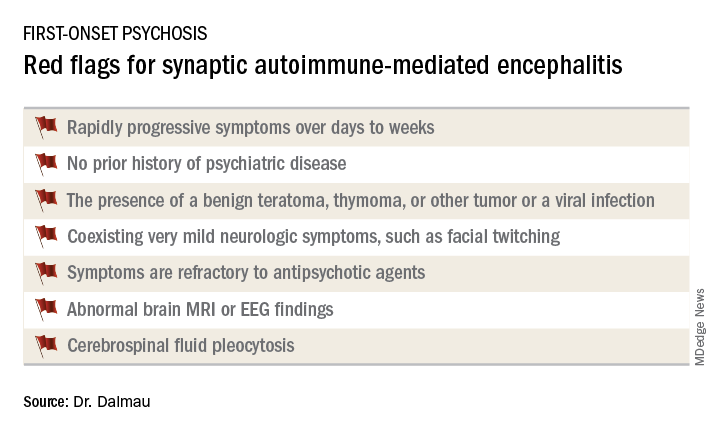
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
REPORTING FROM THE ECNP CONGRESS
ATTRACT trial shouldn’t detract from pharmacomechanical thrombolysis
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Brodalumab raced past ustekinumab to PASI 100
PARIS – The interleukin-17 receptor inhibitor
That’s according to a post hoc pooled analysis of the phase 3 randomized AMAGINE-1 and -3 trials that Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Other interleukin-17 inhibitors have also outperformed ustekinumab (Stelara) in head-to-head, randomized trials. What’s unique about this new secondary analysis of the AMAGINE trials is the demonstration that the complete clearance rate – that is, 100% improvement in Psoriasis Area and Severity Index (PASI) – with brodalumab (Siliq) was consistent, regardless of a psoriasis patient’s prior treatment history, according to Dr. Reich, professor of dermatology at Georg-August-University in Göttingen, Germany, and a partner at the Dermatologikum Hamburg.
“I don’t want to niche brodalumab as a rescue drug; but if you need a response in a patient who has failed a biologic, then obviously, this is a pretty good choice,” he said.
Typically, psoriasis patients who have previously failed to respond favorably to a biologic agent have a substantially lower complete clearance rate when placed on another biologic than do those who are biologic naive or haven’t been on a nonbiologic systemic therapy.
“I think it’s interesting that there is very little impact of previous treatment response with regard to this analysis when it comes to brodalumab,” the dermatologist observed. “It goes down a little bit, but if you compare it to ustekinumab, you see a very good robustness despite previous therapy.”
His presentation focused on the 339 AMAGINE-2 or AMAGINE-3 participants randomized to brodalumab at the approved dose of 210 mg by subcutaneous injection every 2 weeks, or to subcutaneous ustekinumab at the approved dose of 45 mg or 90 mg, depending upon body weight, on day 1, week 4, and then every 12 weeks in the 52-week trials.
It took 14 weeks for 50% of patients assigned to brodalumab to achieve a PASI 100 response, and 44 weeks to accomplish the same in the ustekinumab group. At 52 weeks, the PASI 100 response rate was 76% for brodalumab and 52% for ustekinumab.
This was a competing-risk analysis – a methodology relatively new to dermatology – in which the coprimary endpoint was inadequate response to treatment, as defined by a static Physician’s Global Assessment score of 3 or more or two consecutive sPGAs of at least 2 over a 4-week interval at any point from week 16 on. The inadequate response rate was 20% in the brodalumab group and 40% with ustekinumab.
Looking in the brodalumab group at PASI 100 response rates in relation to prior treatments, the complete clearance rate at week 52 was 76% in those with no prior systemic treatment at study entry, 78% in those with a history of nonbiologic systemic treatment, 75% in patients who hadn’t experienced treatment failure when previously on another biologic agent, and 70% in those with a baseline history of failure on a different biologic.
The corresponding PASI 100 rates in the ustekinumab group were strikingly lower, at 58%, 55%, 41%, and 30%.
Leo Pharma funded Dr. Reich’s post hoc analysis; Leo markets brodalumab in Europe. Dr. Reich reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
SOURCE: Reich K. EADV Congress, Abstract #FC03.06.
PARIS – The interleukin-17 receptor inhibitor
That’s according to a post hoc pooled analysis of the phase 3 randomized AMAGINE-1 and -3 trials that Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Other interleukin-17 inhibitors have also outperformed ustekinumab (Stelara) in head-to-head, randomized trials. What’s unique about this new secondary analysis of the AMAGINE trials is the demonstration that the complete clearance rate – that is, 100% improvement in Psoriasis Area and Severity Index (PASI) – with brodalumab (Siliq) was consistent, regardless of a psoriasis patient’s prior treatment history, according to Dr. Reich, professor of dermatology at Georg-August-University in Göttingen, Germany, and a partner at the Dermatologikum Hamburg.
“I don’t want to niche brodalumab as a rescue drug; but if you need a response in a patient who has failed a biologic, then obviously, this is a pretty good choice,” he said.
Typically, psoriasis patients who have previously failed to respond favorably to a biologic agent have a substantially lower complete clearance rate when placed on another biologic than do those who are biologic naive or haven’t been on a nonbiologic systemic therapy.
“I think it’s interesting that there is very little impact of previous treatment response with regard to this analysis when it comes to brodalumab,” the dermatologist observed. “It goes down a little bit, but if you compare it to ustekinumab, you see a very good robustness despite previous therapy.”
His presentation focused on the 339 AMAGINE-2 or AMAGINE-3 participants randomized to brodalumab at the approved dose of 210 mg by subcutaneous injection every 2 weeks, or to subcutaneous ustekinumab at the approved dose of 45 mg or 90 mg, depending upon body weight, on day 1, week 4, and then every 12 weeks in the 52-week trials.
It took 14 weeks for 50% of patients assigned to brodalumab to achieve a PASI 100 response, and 44 weeks to accomplish the same in the ustekinumab group. At 52 weeks, the PASI 100 response rate was 76% for brodalumab and 52% for ustekinumab.
This was a competing-risk analysis – a methodology relatively new to dermatology – in which the coprimary endpoint was inadequate response to treatment, as defined by a static Physician’s Global Assessment score of 3 or more or two consecutive sPGAs of at least 2 over a 4-week interval at any point from week 16 on. The inadequate response rate was 20% in the brodalumab group and 40% with ustekinumab.
Looking in the brodalumab group at PASI 100 response rates in relation to prior treatments, the complete clearance rate at week 52 was 76% in those with no prior systemic treatment at study entry, 78% in those with a history of nonbiologic systemic treatment, 75% in patients who hadn’t experienced treatment failure when previously on another biologic agent, and 70% in those with a baseline history of failure on a different biologic.
The corresponding PASI 100 rates in the ustekinumab group were strikingly lower, at 58%, 55%, 41%, and 30%.
Leo Pharma funded Dr. Reich’s post hoc analysis; Leo markets brodalumab in Europe. Dr. Reich reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
SOURCE: Reich K. EADV Congress, Abstract #FC03.06.
PARIS – The interleukin-17 receptor inhibitor
That’s according to a post hoc pooled analysis of the phase 3 randomized AMAGINE-1 and -3 trials that Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Other interleukin-17 inhibitors have also outperformed ustekinumab (Stelara) in head-to-head, randomized trials. What’s unique about this new secondary analysis of the AMAGINE trials is the demonstration that the complete clearance rate – that is, 100% improvement in Psoriasis Area and Severity Index (PASI) – with brodalumab (Siliq) was consistent, regardless of a psoriasis patient’s prior treatment history, according to Dr. Reich, professor of dermatology at Georg-August-University in Göttingen, Germany, and a partner at the Dermatologikum Hamburg.
“I don’t want to niche brodalumab as a rescue drug; but if you need a response in a patient who has failed a biologic, then obviously, this is a pretty good choice,” he said.
Typically, psoriasis patients who have previously failed to respond favorably to a biologic agent have a substantially lower complete clearance rate when placed on another biologic than do those who are biologic naive or haven’t been on a nonbiologic systemic therapy.
“I think it’s interesting that there is very little impact of previous treatment response with regard to this analysis when it comes to brodalumab,” the dermatologist observed. “It goes down a little bit, but if you compare it to ustekinumab, you see a very good robustness despite previous therapy.”
His presentation focused on the 339 AMAGINE-2 or AMAGINE-3 participants randomized to brodalumab at the approved dose of 210 mg by subcutaneous injection every 2 weeks, or to subcutaneous ustekinumab at the approved dose of 45 mg or 90 mg, depending upon body weight, on day 1, week 4, and then every 12 weeks in the 52-week trials.
It took 14 weeks for 50% of patients assigned to brodalumab to achieve a PASI 100 response, and 44 weeks to accomplish the same in the ustekinumab group. At 52 weeks, the PASI 100 response rate was 76% for brodalumab and 52% for ustekinumab.
This was a competing-risk analysis – a methodology relatively new to dermatology – in which the coprimary endpoint was inadequate response to treatment, as defined by a static Physician’s Global Assessment score of 3 or more or two consecutive sPGAs of at least 2 over a 4-week interval at any point from week 16 on. The inadequate response rate was 20% in the brodalumab group and 40% with ustekinumab.
Looking in the brodalumab group at PASI 100 response rates in relation to prior treatments, the complete clearance rate at week 52 was 76% in those with no prior systemic treatment at study entry, 78% in those with a history of nonbiologic systemic treatment, 75% in patients who hadn’t experienced treatment failure when previously on another biologic agent, and 70% in those with a baseline history of failure on a different biologic.
The corresponding PASI 100 rates in the ustekinumab group were strikingly lower, at 58%, 55%, 41%, and 30%.
Leo Pharma funded Dr. Reich’s post hoc analysis; Leo markets brodalumab in Europe. Dr. Reich reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
SOURCE: Reich K. EADV Congress, Abstract #FC03.06.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Complete clearance rates in psoriasis patients on brodalumab were similar regardless of treatment history.
Major finding: Half of brodalumab-treated patients with moderate-to-severe psoriasis experienced complete clearance at 14 weeks; it took 44 weeks in patients assigned to ustekinumab.
Study details: This was a post hoc analysis of 52-week outcomes in more than 900 participants in the phase 3 AMAGINE-2 and AMAGINE-3 randomized head-to-head comparisons of brodalumab and ustekinumab.
Disclosures: Leo Pharma funded the post hoc analysis. The presenter reported receiving research funding from and serving as a consultant to that pharmaceutical company and numerous others involved in developing new drugs for psoriasis and atopic dermatitis.
Source: Reich K. EADV Congress, Abstract #FC03.06.
Adult atopic dermatitis is fraught with dermatologic comorbidities
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Adult atopic dermatitis patients have higher prevalence of psoriasis, alopecia areata, vitiligo, and other dermatologic disorders.
Major finding: The risk of contact dermatitis is increased 3.4- to 5.6-fold.
Study details: This German population-based study used both a medical claims database including more than 1.3 million adults and dermatologist-conducted occupational skin screening in more than 162,000 workers.
Disclosures: The study presenter reported having no financial conflicts regarding her study, conducted without commercial support.
Danish study finds reassuring data on pregnancy outcomes in atopic dermatitis patients
PARIS – over an 18-year period, which showed no increased risk of pregnancy and birth problems other than modestly increased risks of premature rupture of membranes and neonatal staphylococcal septicemia, according to Jacob P. Thyssen, MD, PhD.
At a session of the European Task Force of Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology, he presented a case control study of 10,668 births to Danish women with atopic dermatitis (AD) during 1997-2014. They were matched 1:10 by age, parity, and birth year to mothers without AD.
The risk of premature rupture of membranes was 15% higher in mothers with AD. And while the increased relative risk of neonatal staphylococcal septicemia was more substantial – a 145% increase – this was in fact a rare complication, observed Dr. Thyssen, a dermatologist at the University of Copenhagen.
There was no significant difference between women with or without AD in rates of preeclampsia, prematurity, pregnancy-induced hypertension, placenta previa, placental abruption, neonatal nonstaphylococcal septicemia, or other complications. The two groups had a similar number of visits to physicians and midwives during pregnancy.
Moreover, although the body mass index was similar in women with or without AD, the risk of gestational diabetes in women with the disease was significantly reduced by 21%; their risk of having a large-for-gestational-age baby with a birth weight of 4,500 g or more was also significantly lower than in controls.
Women received less treatment for AD during their pregnancy than they did beforehand. While pregnant, their disease was managed predominantly with topical corticosteroids and UV therapy. There was very little use of superpotent topical steroids, topical calcineurin inhibitors, or immunosuppressants, although 10% of pregnant women received systemic corticosteroids for their AD.
Dr. Thyssen reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
PARIS – over an 18-year period, which showed no increased risk of pregnancy and birth problems other than modestly increased risks of premature rupture of membranes and neonatal staphylococcal septicemia, according to Jacob P. Thyssen, MD, PhD.
At a session of the European Task Force of Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology, he presented a case control study of 10,668 births to Danish women with atopic dermatitis (AD) during 1997-2014. They were matched 1:10 by age, parity, and birth year to mothers without AD.
The risk of premature rupture of membranes was 15% higher in mothers with AD. And while the increased relative risk of neonatal staphylococcal septicemia was more substantial – a 145% increase – this was in fact a rare complication, observed Dr. Thyssen, a dermatologist at the University of Copenhagen.
There was no significant difference between women with or without AD in rates of preeclampsia, prematurity, pregnancy-induced hypertension, placenta previa, placental abruption, neonatal nonstaphylococcal septicemia, or other complications. The two groups had a similar number of visits to physicians and midwives during pregnancy.
Moreover, although the body mass index was similar in women with or without AD, the risk of gestational diabetes in women with the disease was significantly reduced by 21%; their risk of having a large-for-gestational-age baby with a birth weight of 4,500 g or more was also significantly lower than in controls.
Women received less treatment for AD during their pregnancy than they did beforehand. While pregnant, their disease was managed predominantly with topical corticosteroids and UV therapy. There was very little use of superpotent topical steroids, topical calcineurin inhibitors, or immunosuppressants, although 10% of pregnant women received systemic corticosteroids for their AD.
Dr. Thyssen reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
PARIS – over an 18-year period, which showed no increased risk of pregnancy and birth problems other than modestly increased risks of premature rupture of membranes and neonatal staphylococcal septicemia, according to Jacob P. Thyssen, MD, PhD.
At a session of the European Task Force of Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology, he presented a case control study of 10,668 births to Danish women with atopic dermatitis (AD) during 1997-2014. They were matched 1:10 by age, parity, and birth year to mothers without AD.
The risk of premature rupture of membranes was 15% higher in mothers with AD. And while the increased relative risk of neonatal staphylococcal septicemia was more substantial – a 145% increase – this was in fact a rare complication, observed Dr. Thyssen, a dermatologist at the University of Copenhagen.
There was no significant difference between women with or without AD in rates of preeclampsia, prematurity, pregnancy-induced hypertension, placenta previa, placental abruption, neonatal nonstaphylococcal septicemia, or other complications. The two groups had a similar number of visits to physicians and midwives during pregnancy.
Moreover, although the body mass index was similar in women with or without AD, the risk of gestational diabetes in women with the disease was significantly reduced by 21%; their risk of having a large-for-gestational-age baby with a birth weight of 4,500 g or more was also significantly lower than in controls.
Women received less treatment for AD during their pregnancy than they did beforehand. While pregnant, their disease was managed predominantly with topical corticosteroids and UV therapy. There was very little use of superpotent topical steroids, topical calcineurin inhibitors, or immunosuppressants, although 10% of pregnant women received systemic corticosteroids for their AD.
Dr. Thyssen reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Birth complications are uncommon for women with atopic dermatitis in pregnancy.
Major finding: The risk of premature rupture of membranes was increased by 15% in women with atopic dermatitis in pregnancy, but their risk of gestational diabetes was reduced by 21%.
Study details: This case control study included 10,668 births to Danish women with atopic dermatitis and 10 times as many matched controls without the disease.
Disclosures: The study presenter reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
Difelikefalin shows promise for hemodialysis-associated itch
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Moderate to severe chronic itching associated with chronic kidney disease is a common and underrecognized problem with a huge quality of life impact.
Major finding: Sixty-four percent of hemodialysis patients on difelikefalin 0.5 mcg/kg experienced at least a 3-point reduction on a 0-10 worst daily itch numeric rating scale, compared with 29% of placebo-treated controls.
Study details: This phase 2, multicenter, 8-week, double-blind study comprised 174 patients with moderate to severe hemodialysis-related itching.
Disclosures: The study was sponsored by Cara Therapeutics and presented by a company officer.
Source: Menzaghi F. EADV Congress, Abstract FC0.4.7.