User login
Trifarotene cream for acne meets all endpoints in twin phase 3 trials
PARIS – A novel kinder, gentler topical retinoid met all of its primary and secondary endpoints in two identical phase 3 randomized trials totaling 2,420 patients with moderate acne vulgaris on both the face and trunk.
Trifarotene cream 50 mcg/g selectively targets the gamma retinoic acid receptor. This unique selectivity for just one of the three retinoic acid receptors results in less of the classic retinoid side effects – redness, scaling, dryness, stinging, burning – that can limit the clinical utility of existing retinoids. This was borne out by the high completion rates in trifarotene-treated participants in the two 12-week trials: 88.2% in the PERFECT 1 trial and 92.7% in PERFECT 2, compared with rates of 89.8% and 93.9% in vehicle-treated controls, Jerry K.L. Tan, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Most adverse events involved local intolerance occurring at application sites and were mild and transient,” Dr. Tan, a dermatologist at the University of Western Ontario, Windsor, and head of Windsor Clinical Research, reported.
PERFECT 1 and 2 were multicenter, double-blind, randomized, vehicle-controlled, 12-week phase 3 trials. Of note, these were the first-ever large-scale randomized trials to simultaneously evaluate a topical therapy for treatment of both facial and truncal acne. This ambitious goal created some unique challenges, which Dr. Tan described.
Participants, all of whom had moderate acne vulgaris on the face and trunk, ranged in age from 9 to 58 years, with a mean age of 19 years. They were randomized to once-daily application of trifarotene cream 50 mcg/g or its vehicle in the evening.
Primary and secondary outcomes
One major efficacy endpoint on the face was achievement of Investigator Global Assessment success as defined by a score of 0 or 1, meaning clear or almost clear, coupled with at least a 2-grade improvement from baseline to week 12. In PERFECT 1 and 2 this was achieved by 29.7% and 42.8% of trifarotene cream-treated patients, response rates significantly better than the 20% and 25.8% in vehicle-treated controls.
Another endpoint for facial therapy were the absolute reductions from baseline in facial inflammatory and noninflammatory acne lesions. The mean reduction in inflammatory lesion count in trifarotene-treated patients was 19.6% in PERFECT 1 and 24.6% in PERFECT 2, both significantly better than the mean 15.8% and 19.6% decreases in controls. Noninflammatory facial lesion counts dropped by 26.7% and 30.4% with trifarotene, versus 18.9% and 22.3% with vehicle.
The efficacy yardsticks utilized on the trunk were the same as on the face except that Physician Global Assessment was the terminology utilized in lieu of Investigator Global Assessment. Physician Global Assessment success on the trunk was achieved by 35.8% and 41.1% of trifarotene-treated patients in the two trials, as compared with 25.7% and 30.1% of controls.
The mean reductions in truncal inflammatory lesion count obtained with trifarotene cream were 22% and 26.1%, both significantly better than the 18.8% and 20.3% rates with vehicle.
Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene-treated patients in one trial and 1% in the other.
One audience member commented that the vehicle response rates looked too strong for that compound to be inert. Dr. Tan agreed. “The vehicle looks really good. One of the issues with vehicles is that many of them have to contain products to prevent decay, fermentation, and proliferation of yeast and bacteria. So I quite agree: I think many of our vehicles do have active ingredients,” he replied. “If you look at the topical dapsone trials, the vehicles look amazing.”
“The other possibility is that there’s what we call ‘investigator creep,’” the dermatologist continued. “It’s the notion that you have no idea what the patients are getting, but they look like maybe they’re getting better, so you grade it as better.”
Dr. Tan reported serving as an advisor and consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials. The company is also developing trifarotene for the treatment of lamellar ichthyosis.
PARIS – A novel kinder, gentler topical retinoid met all of its primary and secondary endpoints in two identical phase 3 randomized trials totaling 2,420 patients with moderate acne vulgaris on both the face and trunk.
Trifarotene cream 50 mcg/g selectively targets the gamma retinoic acid receptor. This unique selectivity for just one of the three retinoic acid receptors results in less of the classic retinoid side effects – redness, scaling, dryness, stinging, burning – that can limit the clinical utility of existing retinoids. This was borne out by the high completion rates in trifarotene-treated participants in the two 12-week trials: 88.2% in the PERFECT 1 trial and 92.7% in PERFECT 2, compared with rates of 89.8% and 93.9% in vehicle-treated controls, Jerry K.L. Tan, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Most adverse events involved local intolerance occurring at application sites and were mild and transient,” Dr. Tan, a dermatologist at the University of Western Ontario, Windsor, and head of Windsor Clinical Research, reported.
PERFECT 1 and 2 were multicenter, double-blind, randomized, vehicle-controlled, 12-week phase 3 trials. Of note, these were the first-ever large-scale randomized trials to simultaneously evaluate a topical therapy for treatment of both facial and truncal acne. This ambitious goal created some unique challenges, which Dr. Tan described.
Participants, all of whom had moderate acne vulgaris on the face and trunk, ranged in age from 9 to 58 years, with a mean age of 19 years. They were randomized to once-daily application of trifarotene cream 50 mcg/g or its vehicle in the evening.
Primary and secondary outcomes
One major efficacy endpoint on the face was achievement of Investigator Global Assessment success as defined by a score of 0 or 1, meaning clear or almost clear, coupled with at least a 2-grade improvement from baseline to week 12. In PERFECT 1 and 2 this was achieved by 29.7% and 42.8% of trifarotene cream-treated patients, response rates significantly better than the 20% and 25.8% in vehicle-treated controls.
Another endpoint for facial therapy were the absolute reductions from baseline in facial inflammatory and noninflammatory acne lesions. The mean reduction in inflammatory lesion count in trifarotene-treated patients was 19.6% in PERFECT 1 and 24.6% in PERFECT 2, both significantly better than the mean 15.8% and 19.6% decreases in controls. Noninflammatory facial lesion counts dropped by 26.7% and 30.4% with trifarotene, versus 18.9% and 22.3% with vehicle.
The efficacy yardsticks utilized on the trunk were the same as on the face except that Physician Global Assessment was the terminology utilized in lieu of Investigator Global Assessment. Physician Global Assessment success on the trunk was achieved by 35.8% and 41.1% of trifarotene-treated patients in the two trials, as compared with 25.7% and 30.1% of controls.
The mean reductions in truncal inflammatory lesion count obtained with trifarotene cream were 22% and 26.1%, both significantly better than the 18.8% and 20.3% rates with vehicle.
Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene-treated patients in one trial and 1% in the other.
One audience member commented that the vehicle response rates looked too strong for that compound to be inert. Dr. Tan agreed. “The vehicle looks really good. One of the issues with vehicles is that many of them have to contain products to prevent decay, fermentation, and proliferation of yeast and bacteria. So I quite agree: I think many of our vehicles do have active ingredients,” he replied. “If you look at the topical dapsone trials, the vehicles look amazing.”
“The other possibility is that there’s what we call ‘investigator creep,’” the dermatologist continued. “It’s the notion that you have no idea what the patients are getting, but they look like maybe they’re getting better, so you grade it as better.”
Dr. Tan reported serving as an advisor and consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials. The company is also developing trifarotene for the treatment of lamellar ichthyosis.
PARIS – A novel kinder, gentler topical retinoid met all of its primary and secondary endpoints in two identical phase 3 randomized trials totaling 2,420 patients with moderate acne vulgaris on both the face and trunk.
Trifarotene cream 50 mcg/g selectively targets the gamma retinoic acid receptor. This unique selectivity for just one of the three retinoic acid receptors results in less of the classic retinoid side effects – redness, scaling, dryness, stinging, burning – that can limit the clinical utility of existing retinoids. This was borne out by the high completion rates in trifarotene-treated participants in the two 12-week trials: 88.2% in the PERFECT 1 trial and 92.7% in PERFECT 2, compared with rates of 89.8% and 93.9% in vehicle-treated controls, Jerry K.L. Tan, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Most adverse events involved local intolerance occurring at application sites and were mild and transient,” Dr. Tan, a dermatologist at the University of Western Ontario, Windsor, and head of Windsor Clinical Research, reported.
PERFECT 1 and 2 were multicenter, double-blind, randomized, vehicle-controlled, 12-week phase 3 trials. Of note, these were the first-ever large-scale randomized trials to simultaneously evaluate a topical therapy for treatment of both facial and truncal acne. This ambitious goal created some unique challenges, which Dr. Tan described.
Participants, all of whom had moderate acne vulgaris on the face and trunk, ranged in age from 9 to 58 years, with a mean age of 19 years. They were randomized to once-daily application of trifarotene cream 50 mcg/g or its vehicle in the evening.
Primary and secondary outcomes
One major efficacy endpoint on the face was achievement of Investigator Global Assessment success as defined by a score of 0 or 1, meaning clear or almost clear, coupled with at least a 2-grade improvement from baseline to week 12. In PERFECT 1 and 2 this was achieved by 29.7% and 42.8% of trifarotene cream-treated patients, response rates significantly better than the 20% and 25.8% in vehicle-treated controls.
Another endpoint for facial therapy were the absolute reductions from baseline in facial inflammatory and noninflammatory acne lesions. The mean reduction in inflammatory lesion count in trifarotene-treated patients was 19.6% in PERFECT 1 and 24.6% in PERFECT 2, both significantly better than the mean 15.8% and 19.6% decreases in controls. Noninflammatory facial lesion counts dropped by 26.7% and 30.4% with trifarotene, versus 18.9% and 22.3% with vehicle.
The efficacy yardsticks utilized on the trunk were the same as on the face except that Physician Global Assessment was the terminology utilized in lieu of Investigator Global Assessment. Physician Global Assessment success on the trunk was achieved by 35.8% and 41.1% of trifarotene-treated patients in the two trials, as compared with 25.7% and 30.1% of controls.
The mean reductions in truncal inflammatory lesion count obtained with trifarotene cream were 22% and 26.1%, both significantly better than the 18.8% and 20.3% rates with vehicle.
Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene-treated patients in one trial and 1% in the other.
One audience member commented that the vehicle response rates looked too strong for that compound to be inert. Dr. Tan agreed. “The vehicle looks really good. One of the issues with vehicles is that many of them have to contain products to prevent decay, fermentation, and proliferation of yeast and bacteria. So I quite agree: I think many of our vehicles do have active ingredients,” he replied. “If you look at the topical dapsone trials, the vehicles look amazing.”
“The other possibility is that there’s what we call ‘investigator creep,’” the dermatologist continued. “It’s the notion that you have no idea what the patients are getting, but they look like maybe they’re getting better, so you grade it as better.”
Dr. Tan reported serving as an advisor and consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials. The company is also developing trifarotene for the treatment of lamellar ichthyosis.
REPORTING FROM THE EADV CONGRESS
Key clinical point:
Major finding: Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene cream–treated patients in one trial and 1% in the other.
Study details: PERFECT 1 and PERFECT 2 were identically designed 12-week phase 3 randomized trials including 2,420 patients with moderate facial and truncal acne.
Disclosures: The study presenter reported serving as an advisor, consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials.
Novel oral agent shows unprecedented efficacy in psoriasis
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.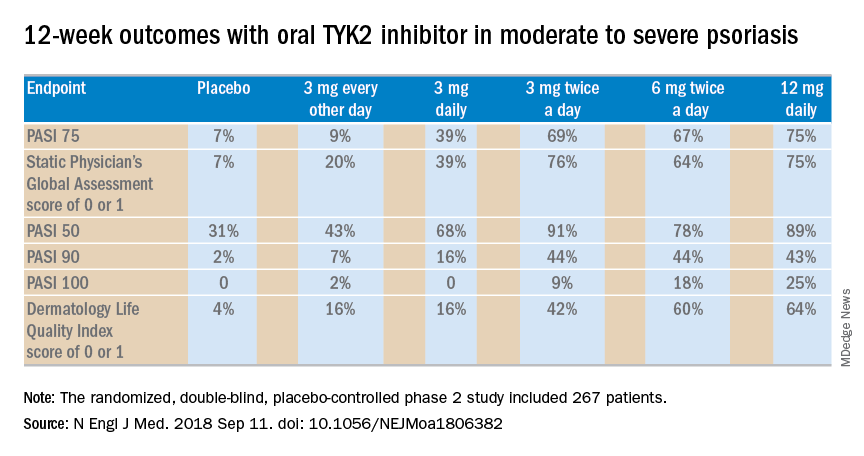
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A novel selective tyrosine kinase 2 inhibitor achieves response rates previously unheard of in oral therapy for moderate to severe psoriasis.
Major finding: At the top dose of oral BMS-986165 studied to date, the PASI 75 rate at 12 weeks was 75%.
Study details: This eight-country, randomized, double-blind, placebo-controlled phase 2 study included 267 patients with moderate to severe psoriasis.
Disclosures: The study was sponsored by Bristol-Myers Squibb. The presenter reported receiving personal fees and institutional research grants from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
Two red flags spell trouble ahead in spontaneous coronary artery dissection
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: SCAD occurring during the peripartum period or in patients with connective tissue disease identifies subgroups at high risk for major adverse cardiovascular events within 30 days.
Major finding: Patients with spontaneous coronary artery dissection and comorbid connective tissue disease were at 8.7-fold increased risk of major adverse cardiovascular events within 30 days.
Study details: CanSCAD is an ongoing, prospective, multicenter, observational study in 750 patients with confirmed spontaneous coronary artery dissection.
Disclosures: CanSCAD is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier.
Pantothenic acid enhances doxycycline’s antiacne effects
PARIS – in a randomized, double-blind, placebo-controlled trial, Maria A. Santos, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The beauty of this treatment strategy is that it enables acne patients to obtain the therapeutic benefits of oral antibiotic therapy while minimizing exposure in accord with the current push to curb the growing problem of antibiotic resistance. Indeed, adjunctive oral pantothenic acid (also known as vitamin B5) appears to offer a solution to the high rate of clinical deterioration that often follows antibiotic discontinuation, observed Dr. Santos, a dermatologist at University of Santo Tomas Hospital in Manila.
“The use of pantothenic acid as an adjunct may enhance acne therapy and possibly prolong control despite antibiotic discontinuation,” she said.
Dr. Santos reported on 40 patients aged 16-45 years with moderate to severe acne who were randomized to 2 g/day of pantothenic acid or placebo for 16 weeks on top of 6 weeks of oral doxycycline at 100 mg once daily, plus ongoing topical adapalene and benzoyl peroxide gel.
After 8 weeks – that is, 2 weeks after everyone completed 6 weeks on doxycycline – the mean reduction in noninflammatory and total lesion counts was virtually identical in the two groups. For example, there was a 57.7% decrease in total lesion count compared with baseline in the pantothenic acid group and a 55% reduction in placebo-treated controls. Thereafter, however, the response curves diverged. Backsliding occurred in the control group such that their mean reduction in total lesions was 48.4% at 14 weeks and 40.4% at 16 weeks, compared with baseline. In contrast, patients on daily pantothenic acid had a 78.7% reduction in total lesion count at 14 weeks and an 80% decrease from baseline at 16 weeks.
Similarly, at 16 weeks, the mean reduction in noninflammatory lesions was 49% in the pantothenic acid group versus 19.2% in controls, compared with 34%-36% reductions at 10 weeks, even though all patients remained on topical adapalene and benzoyl peroxide throughout.
Mean reduction in inflammatory lesions followed the same trend; however, the numeric advantage in the pantothenic acid group didn’t achieve statistical significance.
Median Dermatology Life Quality Index scores improved over the course of the study in both groups, although significantly more patients in the pantothenic acid group achieved at least a 4-point improvement over baseline, which is considered the minimum clinically important difference. Moreover, while modified Global Severity Scores and subjective self-assessment scores improved in both groups, from week 12 onwards, the improvements were significantly greater in the pantothenic acid group.
Receiving adjunctive pantothenic acid for 10 weeks was safe. Adverse events were the same in both study arms, consisting chiefly of mild erythema, scaling, dryness, and nausea during the initial weeks on doxycycline.
Pantothenic acid is a water-soluble essential nutrient. Dr. Santos said that while the precise mechanism of the benefit seen in this randomized trial isn’t known, other investigators have generated evidence suggestive of enhanced epidermal barrier function through normalization of keratinocyte proliferation and differentiation in acne patients, coupled with antioxidant and anti-inflammatory effects.
Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.
PARIS – in a randomized, double-blind, placebo-controlled trial, Maria A. Santos, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The beauty of this treatment strategy is that it enables acne patients to obtain the therapeutic benefits of oral antibiotic therapy while minimizing exposure in accord with the current push to curb the growing problem of antibiotic resistance. Indeed, adjunctive oral pantothenic acid (also known as vitamin B5) appears to offer a solution to the high rate of clinical deterioration that often follows antibiotic discontinuation, observed Dr. Santos, a dermatologist at University of Santo Tomas Hospital in Manila.
“The use of pantothenic acid as an adjunct may enhance acne therapy and possibly prolong control despite antibiotic discontinuation,” she said.
Dr. Santos reported on 40 patients aged 16-45 years with moderate to severe acne who were randomized to 2 g/day of pantothenic acid or placebo for 16 weeks on top of 6 weeks of oral doxycycline at 100 mg once daily, plus ongoing topical adapalene and benzoyl peroxide gel.
After 8 weeks – that is, 2 weeks after everyone completed 6 weeks on doxycycline – the mean reduction in noninflammatory and total lesion counts was virtually identical in the two groups. For example, there was a 57.7% decrease in total lesion count compared with baseline in the pantothenic acid group and a 55% reduction in placebo-treated controls. Thereafter, however, the response curves diverged. Backsliding occurred in the control group such that their mean reduction in total lesions was 48.4% at 14 weeks and 40.4% at 16 weeks, compared with baseline. In contrast, patients on daily pantothenic acid had a 78.7% reduction in total lesion count at 14 weeks and an 80% decrease from baseline at 16 weeks.
Similarly, at 16 weeks, the mean reduction in noninflammatory lesions was 49% in the pantothenic acid group versus 19.2% in controls, compared with 34%-36% reductions at 10 weeks, even though all patients remained on topical adapalene and benzoyl peroxide throughout.
Mean reduction in inflammatory lesions followed the same trend; however, the numeric advantage in the pantothenic acid group didn’t achieve statistical significance.
Median Dermatology Life Quality Index scores improved over the course of the study in both groups, although significantly more patients in the pantothenic acid group achieved at least a 4-point improvement over baseline, which is considered the minimum clinically important difference. Moreover, while modified Global Severity Scores and subjective self-assessment scores improved in both groups, from week 12 onwards, the improvements were significantly greater in the pantothenic acid group.
Receiving adjunctive pantothenic acid for 10 weeks was safe. Adverse events were the same in both study arms, consisting chiefly of mild erythema, scaling, dryness, and nausea during the initial weeks on doxycycline.
Pantothenic acid is a water-soluble essential nutrient. Dr. Santos said that while the precise mechanism of the benefit seen in this randomized trial isn’t known, other investigators have generated evidence suggestive of enhanced epidermal barrier function through normalization of keratinocyte proliferation and differentiation in acne patients, coupled with antioxidant and anti-inflammatory effects.
Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.
PARIS – in a randomized, double-blind, placebo-controlled trial, Maria A. Santos, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The beauty of this treatment strategy is that it enables acne patients to obtain the therapeutic benefits of oral antibiotic therapy while minimizing exposure in accord with the current push to curb the growing problem of antibiotic resistance. Indeed, adjunctive oral pantothenic acid (also known as vitamin B5) appears to offer a solution to the high rate of clinical deterioration that often follows antibiotic discontinuation, observed Dr. Santos, a dermatologist at University of Santo Tomas Hospital in Manila.
“The use of pantothenic acid as an adjunct may enhance acne therapy and possibly prolong control despite antibiotic discontinuation,” she said.
Dr. Santos reported on 40 patients aged 16-45 years with moderate to severe acne who were randomized to 2 g/day of pantothenic acid or placebo for 16 weeks on top of 6 weeks of oral doxycycline at 100 mg once daily, plus ongoing topical adapalene and benzoyl peroxide gel.
After 8 weeks – that is, 2 weeks after everyone completed 6 weeks on doxycycline – the mean reduction in noninflammatory and total lesion counts was virtually identical in the two groups. For example, there was a 57.7% decrease in total lesion count compared with baseline in the pantothenic acid group and a 55% reduction in placebo-treated controls. Thereafter, however, the response curves diverged. Backsliding occurred in the control group such that their mean reduction in total lesions was 48.4% at 14 weeks and 40.4% at 16 weeks, compared with baseline. In contrast, patients on daily pantothenic acid had a 78.7% reduction in total lesion count at 14 weeks and an 80% decrease from baseline at 16 weeks.
Similarly, at 16 weeks, the mean reduction in noninflammatory lesions was 49% in the pantothenic acid group versus 19.2% in controls, compared with 34%-36% reductions at 10 weeks, even though all patients remained on topical adapalene and benzoyl peroxide throughout.
Mean reduction in inflammatory lesions followed the same trend; however, the numeric advantage in the pantothenic acid group didn’t achieve statistical significance.
Median Dermatology Life Quality Index scores improved over the course of the study in both groups, although significantly more patients in the pantothenic acid group achieved at least a 4-point improvement over baseline, which is considered the minimum clinically important difference. Moreover, while modified Global Severity Scores and subjective self-assessment scores improved in both groups, from week 12 onwards, the improvements were significantly greater in the pantothenic acid group.
Receiving adjunctive pantothenic acid for 10 weeks was safe. Adverse events were the same in both study arms, consisting chiefly of mild erythema, scaling, dryness, and nausea during the initial weeks on doxycycline.
Pantothenic acid is a water-soluble essential nutrient. Dr. Santos said that while the precise mechanism of the benefit seen in this randomized trial isn’t known, other investigators have generated evidence suggestive of enhanced epidermal barrier function through normalization of keratinocyte proliferation and differentiation in acne patients, coupled with antioxidant and anti-inflammatory effects.
Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Oral pantothenic acid at 2 g/day appears to be a safe and effective adjunct to oral doxycycline for moderate to severe acne.
Major finding: Acne patients on oral pantothenic acid had a mean 80% reduction in total lesion count 10 weeks after completing 6 weeks on oral doxycycline, a rate twice that of placebo-treated controls.
Study details: This 16-week, prospective, randomized, double-blind, placebo-controlled study included 40 patients with moderate to severe acne.
Disclosures: Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.
Think DEB, not BMS, with high bleeding risk
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
REPORTING FROM EUROPCR 2018
Key clinical point:
Major finding: The 9-month MACE rate was 1.9% in the drug-eluting balloon group versus 12.4% with a bare-metal stent.
Study details: This prospective, multicenter, single-blind trial randomized 208 high–bleeding risk patients with de novo lesions in large coronary vessels to PCI with a drug-eluting balloon-only or a bare-metal stent.
Disclosures: The presenter reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
Reflectance confocal microscopy: The future looks bright
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point: The future looks bright for reflectance confocal microscopy in dermatology.
Major finding: The sensitivity and specificity of reflectance confocal microscopy for diagnosis of skin cancer in patients with equivocal dermoscopic findings was 98.2% and 99.8%, respectively.
Study details: This retrospective single center study included 1,189 clinically suspicious skin lesions with equivocal dermoscopy findings, which were then evaluated using reflectance confocal microscopy.
Disclosures: The presenter reported having no financial conflicts regarding her study.
Mohs underutilized for melanoma of head and neck
CHICAGO – Contemporary national guidelines undervalue the benefits of Mohs micrographic surgery for patients with melanoma of the head and neck, William C. Fix asserted at the annual meeting of the American College of Mohs Surgery.
Mr. Fix, a medical student at the University of Pennsylvania, Philadelphia, presented a single-center retrospective study of 13,644 cases of head and neck skin cancer treated with Mohs micrographic surgery (MMS) for margin control. The cohort included 1,065 melanomas in situ, 410 invasive melanomas, more than 8,700 basal cell carcinomas, and 3,343 squamous cell carcinomas.
Mr. Fix and his coinvestigators undertook this observational study because they identified a gap in current guidelines for treatment of skin cancers of the head and neck. For example, the National Comprehensive Cancer Network recommends margin control at the time of primary surgery for BCCs and SCCs deemed at high risk for local recurrence and defines what those high-risk features are. For melanomas, however, the guidelines recommend wide local excision, even though that approach has roughly a 10% recurrence rate, compared with less than 1% for MMS.
Moreover, the 2012 appropriate use criteria for MMS put forth by the American Academy of Dermatology in concert with several other medical societies are unclear about invasive melanoma. As a result of this lack of guidance, the use of margin control in primary surgery for melanoma is applied in less than 4% of cases, according to Mr. Fix.
The University of Pennsylvania data he presented showed that melanomas of the head and neck were significantly more likely to be large in size, to be poorly defined, and to have other high-risk features for local recurrence than were the BCCs and SCCs. In a multivariate logistic regression analysis controlling for high-risk characteristics, melanomas were independently associated with a twofold increased likelihood of requiring flap reconstruction compared with BCCs and SCCs of the head and neck.
“We’ve shown that melanomas have high-risk features for local recurrence, possibly to a greater extent than BCCs and SCCs. These features help us triage resource use for BCC and SCC. Could these same features help us make decisions for melanomas?” he asked rhetorically.
Mr. Fix reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
CHICAGO – Contemporary national guidelines undervalue the benefits of Mohs micrographic surgery for patients with melanoma of the head and neck, William C. Fix asserted at the annual meeting of the American College of Mohs Surgery.

Mr. Fix, a medical student at the University of Pennsylvania, Philadelphia, presented a single-center retrospective study of 13,644 cases of head and neck skin cancer treated with Mohs micrographic surgery (MMS) for margin control. The cohort included 1,065 melanomas in situ, 410 invasive melanomas, more than 8,700 basal cell carcinomas, and 3,343 squamous cell carcinomas.
Mr. Fix and his coinvestigators undertook this observational study because they identified a gap in current guidelines for treatment of skin cancers of the head and neck. For example, the National Comprehensive Cancer Network recommends margin control at the time of primary surgery for BCCs and SCCs deemed at high risk for local recurrence and defines what those high-risk features are. For melanomas, however, the guidelines recommend wide local excision, even though that approach has roughly a 10% recurrence rate, compared with less than 1% for MMS.
Moreover, the 2012 appropriate use criteria for MMS put forth by the American Academy of Dermatology in concert with several other medical societies are unclear about invasive melanoma. As a result of this lack of guidance, the use of margin control in primary surgery for melanoma is applied in less than 4% of cases, according to Mr. Fix.
The University of Pennsylvania data he presented showed that melanomas of the head and neck were significantly more likely to be large in size, to be poorly defined, and to have other high-risk features for local recurrence than were the BCCs and SCCs. In a multivariate logistic regression analysis controlling for high-risk characteristics, melanomas were independently associated with a twofold increased likelihood of requiring flap reconstruction compared with BCCs and SCCs of the head and neck.
“We’ve shown that melanomas have high-risk features for local recurrence, possibly to a greater extent than BCCs and SCCs. These features help us triage resource use for BCC and SCC. Could these same features help us make decisions for melanomas?” he asked rhetorically.
Mr. Fix reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
CHICAGO – Contemporary national guidelines undervalue the benefits of Mohs micrographic surgery for patients with melanoma of the head and neck, William C. Fix asserted at the annual meeting of the American College of Mohs Surgery.

Mr. Fix, a medical student at the University of Pennsylvania, Philadelphia, presented a single-center retrospective study of 13,644 cases of head and neck skin cancer treated with Mohs micrographic surgery (MMS) for margin control. The cohort included 1,065 melanomas in situ, 410 invasive melanomas, more than 8,700 basal cell carcinomas, and 3,343 squamous cell carcinomas.
Mr. Fix and his coinvestigators undertook this observational study because they identified a gap in current guidelines for treatment of skin cancers of the head and neck. For example, the National Comprehensive Cancer Network recommends margin control at the time of primary surgery for BCCs and SCCs deemed at high risk for local recurrence and defines what those high-risk features are. For melanomas, however, the guidelines recommend wide local excision, even though that approach has roughly a 10% recurrence rate, compared with less than 1% for MMS.
Moreover, the 2012 appropriate use criteria for MMS put forth by the American Academy of Dermatology in concert with several other medical societies are unclear about invasive melanoma. As a result of this lack of guidance, the use of margin control in primary surgery for melanoma is applied in less than 4% of cases, according to Mr. Fix.
The University of Pennsylvania data he presented showed that melanomas of the head and neck were significantly more likely to be large in size, to be poorly defined, and to have other high-risk features for local recurrence than were the BCCs and SCCs. In a multivariate logistic regression analysis controlling for high-risk characteristics, melanomas were independently associated with a twofold increased likelihood of requiring flap reconstruction compared with BCCs and SCCs of the head and neck.
“We’ve shown that melanomas have high-risk features for local recurrence, possibly to a greater extent than BCCs and SCCs. These features help us triage resource use for BCC and SCC. Could these same features help us make decisions for melanomas?” he asked rhetorically.
Mr. Fix reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point: Margin control at the time of primary surgery for melanoma of the head and neck makes sense.
Major finding: Patients with a melanoma of the head and neck were twice as likely to require secondary flap reconstruction compared with patients with a basal cell carcinoma or squamous cell carcinoma of the head and neck.
Study details: A retrospective single-center study of 13,644 cases of skin cancer of the head and neck treated with Mohs surgery.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was conducted free of commercial support.
Danish endocarditis strategy halved hospital days
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
MUNICH – Patients with left-sided endocarditis who are clinically stable after a couple weeks of inpatient intravenous antibiotics may at that point become candidates for discharge on oral antibiotics for the remainder of their treatment course, according to the findings of the randomized, multicenter, Danish POET trial.
“Shifting to oral antibiotic treatment in stabilized patients with endocarditis was as effective and safe as continued intravenous antibiotic treatment and was given during half the antibiotic treatment period. These novel findings may have a significant impact on future clinical practice for the management of patients who are stable,” Henning Bundgård, MD, said at the annual congress of the European Society of Cardiology.
Both ESC and American Heart Association/American College of Cardiology guidelines now recommend treatment of infective endocarditis with intravenous antibiotics for up to 6 weeks. Safely cutting the duration of in-hospital intravenous antibiotics in half is likely to generate major cost savings while improving patient quality of life and avoiding prolonged exposure to the iatrogenic risks inherent to the hospital environment, noted Dr. Bundgård, a cardiologist at Copenhagen University Hospital.
The rationale for the POET (Partial Oral Treatment of Endocarditis) trial was the investigators’ recognition that, even though infectious endocarditis is a feared disease with an in-hospital mortality of 15% or more, the great majority of serious complications occur in the early critical phase of therapy; that is, during the first 10 days or so of inpatient intravenous antibiotic therapy.
“After stabilization, the main reason for staying in the hospital is just to receive IV antibiotics,” Dr. Bundgård noted.
POET included 400 patients with left-sided endocarditis hospitalized at multiple cardiac centers across Denmark, 35% of whom had at least one major comorbid condition. When this reporter observed that this was the smallest study he’d ever seen reported from Denmark, where researchers famously like to utilize interconnected national databases to conduct nationwide observational studies incorporating the country’s entire population, the cardiologist replied, “Denmark is a small country, but we like to make big trials. And this is actually the largest-ever clinical trial in endocarditis, so we are still going big.”
Important to the generalizability of the POET results was the requirement that all 400 participants had to be infected with streptococcus, Staphylococcus aureus, Enterococcus faecalis, or coagulase-negative staphylococci – the major pathogens responsible for three-quarters of all cases of infectious endocarditis.
Once participants were clinically stable after a median of 17 days of intravenous antibiotics, they were randomized to continued in-hospital intravenous antibiotic therapy for a median of another 19 days or to discharge on two oral antibiotics from different classes with different mechanisms of action administered for a median of 17 days, with selection of the oral agents being guided by the results of bacterial susceptibility testing.
The primary outcome was a composite of all-cause mortality, embolic events, unplanned cardiac surgery, and relapse of bacteremia from randomization through 6 months after completion of antibiotic therapy. This occurred in 9.0% of the orally treated group and 12.1% of patients on full-course intravenous therapy for a 28% relative risk reduction, which statistically established the noninferioritiy of the partial oral regimen. The results were similar in patients with native as compared with prosthetic valves, with or without major comorbidities, and in surgically as opposed to conservatively treated patients.
Rates of three of the four components of the composite endpoint were similar in both groups. However, all-cause mortality occurred in 3.5% of the oral therapy group, compared with 6.5% of those on intravenous therapy. Dr. Bundgård said he and his coinvestigators think the disparity in mortality was probably caused by play of chance, although he added that they were struck that four sudden deaths occurred in the intravenous group and none in patients who got oral antibiotics.
Side effects were similarly mild and low frequency in both study arms.
Audience members were eager for details on how the Danish investigators decided patients were clinically stable on intravenous antibiotics and thus ready for randomization, as well as the outpatient follow-up procedures employed in those discharged on oral therapy.
Dr. Bundgård explained that clinical stability required that a patient be afebrile, have C-reactive protein and leukocyte levels less than 35% of their peaks, and needed to have been on intravenous antibiotics for a minimum of 10 days. Moreover, patients who underwent valve surgery during their hospitalization, as did 38% of POET participants, had to wait a minimum of 7 days afterwards before they could be declared clinically stable. Lastly, just prior to randomization all participants underwent transesophageal echocardiography to rule out abscess formation or other valve abnormalities requiring surgery.
Outpatient follow-up required that patients drop in two or three times per week to be checked by a familiar physician or nurse at the hospital ward where they had stayed. Compliance was very good, although it should be noted that only five patients in the POET study were intravenous drug abusers.
Asked why investigators discharged patients on oral therapy rather than on home intravenous antibiotics, Dr. Bundgård explained that home intravenous antibiotic therapy isn’t utilized in Denmark because of the expense and logistic complexity.
Discussant Chris P. Gale, MD, urged care in generalizing the study findings.
“The ‘O’ in POET does not stand for ‘outpatient.’ Outpatients were only selected for oral therapy if they had no heart failure, no emboli, no arrhythmia, no complicating comorbidities, and they were strictly monitored – and frequently. Should we elect to adopt POET into practice, I would recommend strict adherence to the study’s patient selection and monitoring criteria,” said Dr. Gale, a cardiologist at the University of Leeds (England).
The POET results clearly swayed the full-house audience attending the late-breaking Hot Line session in the conference main arena. Immediately before Dr. Bundgård’s presentation, 66% of the audience indicated electronically that they would continue intravenous antibiotics for another 2-4 weeks in a patient with infectious endocarditis who had responded well to 2 weeks of such therapy. After seeing the study results, however, only 19% would still follow that course of action, while 59% of the audience would switch to oral antibiotics and discharge the patient.
Dr. Bundgård reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Simultaneous with Dr. Bundgård’s presentation in Munich, the POET results were published online by the New England Journal of Medicine (2018 Aug 28. doi: 10.1056/NEJMoa1808312).
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Clinically stable patients with left-sided infectious endocarditis can safely and effectively be discharged on oral antibiotics after completing half of a full course of intravenous antibiotics.
Major finding: Key 6-month outcomes were similar in patients with left-sided infectious endocarditis regardless of whether they were discharged early on carefully selected oral antibiotics or remained in hospital to complete a full course of intravenous antibiotics.
Study details: This prospective, multicenter, Danish randomized trial included 400 patients with left-sided infectious endocarditis.
Disclosures: The presenter reported having no financial conflicts regarding the POET study, which was funded by the Danish Heart Foundation and other research foundations.
Consider dosing as well as drug choice for anxiety disorders
ESTES PARK, COLO. – The first-line medications for anxiety disorders are the same ones used for depression – the SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs) – but the dosing strategies are quite different, Elizabeth L. Lowdermilk, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“We know that folks with anxiety disorders tend to need the higher doses. They just need to get there a little more slowly,” according to Dr. Lowdermilk, a psychiatrist at the university and medical director of outpatient psychiatry at Denver Health.
“The trick is not what drug you use, it’s what dose you started at. For example, when I start sertraline for depression I start it at 50 mg per day. When I start it for anxiety I start it at 25. I just give it a week or 2 at 25 and then I double it to 50. And then I keep going, titrating over 2-3 months to a high or maximum dose,” she explained.
The rapidity of response to the SSRIs and SNRIs is quite different, too. When patients are started on one of these agents as treatment for depression, they can expect that it will take at least 4-6 weeks and maybe as long as 12 weeks before they experience the full therapeutic effect. Not so when the same drugs are used to treat anxiety disorders.
“The anxiolytic effect seems to be more robust sooner. Really, people start noticing something within the first couple days to a week. And they will keep getting better, which people love,” Dr. Lowdermilk said.
One of the early positive effects of SSRI/SNRI therapy that patients can be advised to be on the lookout for is “the Teflon mind,” she said.
“It’s not that the worried thoughts won’t arise, but they’re going to slide out faster and patients are going to be able to shift their focus back to where they want it to be and move forward,” the psychiatrist explained.
Other positive treatment effects include reductions in irritability, anger, perseverative thoughts, restlessness, and physical tension, along with improved sleep.
How to monitor patient response
The most practical anxiety rating scale for busy primary care physicians with tight office visit scheduling is the seven-item Generalized Anxiety Disorder Assessment, or GAD 7. It can be used repeatedly to follow a patient’s treatment response.
“While technically it is picking up the symptoms of GAD, because there are so many overlapping symptoms in all of the anxiety disorders this is going to give you a sense of whether someone is getting better. It won’t pick up their nightmares if they have PTSD, and it won’t necessarily pick up their checking behaviors if they have obsessive-compulsive disorder – you can ask them quickly about that,” she said.
Augmenting an SSRI/SNRI
Be advised: While some patients with an anxiety disorder will experience complete resolution of symptoms on the maximum approved dose of an SSRI/SNRI, such as 200 mg/day of sertraline (Zoloft), lots of patients will have only a partial response. The next move is not to add a second antidepressant, it’s to augment the high-dose antidepressant the patient is already on with something else. Dr. Lowdermilk highlighted the best and worst strategies:
- Hydroxyzine: This drug, approved by the Food and Drug Administration for treatment of panic attacks at 25-50 mg up to four times per day, has sedating side effects that help with sleep issues.
“We use a lot of this,” Dr. Lowdermilk said.
Hydroxyzine (Vistaril) can be dosed on a fixed schedule or as needed. She strongly favors scheduled dosing. “It’s better to use drugs to reduce anxiety tone, then teach skills to cope with anxiety. As needed treatment subtly teaches patients to take a pill if they have breakthrough symptoms.”
- Buspirone: It takes high doses of this medication to get a robust anxiolytic response. Dr. Lowdermilk recommends starting at 5 mg three times daily and increasing the total daily dose by 15 mg every 2 weeks up to a maximum of 60 mg.
“Buspirone isn’t the strongest medication out there, but it can help. I personally don’t stop until I’m at least at 30 mg per day,” she said.
The drug is especially handy as monotherapy in patients with mild to moderate anxiety who can’t tolerate serotonergic medications well. Also, at higher doses buspirone (Buspar) may reduce the sexual side effects of a concomitant serotonergic agent.
- Gabapentin: Dr. Lowdermilk often turns to this drug off label as an SSRI/SNRI augmentation strategy, starting at 100-300 mg three times daily and increasing over the same time frame as for neuropathic pain up to a maximum total daily dose of 3,600 mg. Like hydroxyzine, it helps with sleep.
- Atypical antipsychotics: Reserve these for patients with an inadequate response to maximum-dose SSRI/SNRI. There is some evidence of efficacy for low-dose risperidone (Risperdal) at 1-2 mg/day, quetiapine(Seroquel) at 50-100 mg, and aripiprazole (Abilify) at 2-5 mg. Because of the risks of metabolic side effects and tardive dyskinesia, it’s best to evaluate the drug’s effectiveness after 2-4 weeks and consider stopping if there is no clinical improvement.
- Benzodiazepines: Yes, they are approved for treatment of anxiety disorders. Nevertheless, Dr. Lowdermilk advises against their as routine practice.
“I want to acknowledge that they really do work for anxiety and there are times you might want to consider them. But we are almost never initiating benzodiazepines anymore. We are learning that they cause more problems than not. What I’ve found over the years is short bursts of use are not short. Patients tend to come back and want more,” according to the psychiatrist.
She is not convinced about the claimed link to dementia, but she does believe long-term use of benzodiazepines is associated with memory and balance problems as well as slowed reaction time. If they are going to be used to treat anxiety disorders, it’s best to turn to a longer-acting agent such as clonazepam (Klonopin) or extended-release alprazolam (Xanax XR), which keep the anxiety tone down without producing the euphoria of short-acting benzodiazepines.
- Behavioral therapies: “I really do think that the combination of medication and behavioral therapy is the best approach,” Dr. Lowdermilk said.
Consider referring patients with a problematic anxiety disorder to a behavioral therapist skilled in cognitive behavioral therapy, exposure therapy, response prevention therapy, or eye movement desensitization and reprocessing.
She reported having no financial conflicts regarding her presentation.
ESTES PARK, COLO. – The first-line medications for anxiety disorders are the same ones used for depression – the SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs) – but the dosing strategies are quite different, Elizabeth L. Lowdermilk, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“We know that folks with anxiety disorders tend to need the higher doses. They just need to get there a little more slowly,” according to Dr. Lowdermilk, a psychiatrist at the university and medical director of outpatient psychiatry at Denver Health.
“The trick is not what drug you use, it’s what dose you started at. For example, when I start sertraline for depression I start it at 50 mg per day. When I start it for anxiety I start it at 25. I just give it a week or 2 at 25 and then I double it to 50. And then I keep going, titrating over 2-3 months to a high or maximum dose,” she explained.
The rapidity of response to the SSRIs and SNRIs is quite different, too. When patients are started on one of these agents as treatment for depression, they can expect that it will take at least 4-6 weeks and maybe as long as 12 weeks before they experience the full therapeutic effect. Not so when the same drugs are used to treat anxiety disorders.
“The anxiolytic effect seems to be more robust sooner. Really, people start noticing something within the first couple days to a week. And they will keep getting better, which people love,” Dr. Lowdermilk said.
One of the early positive effects of SSRI/SNRI therapy that patients can be advised to be on the lookout for is “the Teflon mind,” she said.
“It’s not that the worried thoughts won’t arise, but they’re going to slide out faster and patients are going to be able to shift their focus back to where they want it to be and move forward,” the psychiatrist explained.
Other positive treatment effects include reductions in irritability, anger, perseverative thoughts, restlessness, and physical tension, along with improved sleep.
How to monitor patient response
The most practical anxiety rating scale for busy primary care physicians with tight office visit scheduling is the seven-item Generalized Anxiety Disorder Assessment, or GAD 7. It can be used repeatedly to follow a patient’s treatment response.
“While technically it is picking up the symptoms of GAD, because there are so many overlapping symptoms in all of the anxiety disorders this is going to give you a sense of whether someone is getting better. It won’t pick up their nightmares if they have PTSD, and it won’t necessarily pick up their checking behaviors if they have obsessive-compulsive disorder – you can ask them quickly about that,” she said.
Augmenting an SSRI/SNRI
Be advised: While some patients with an anxiety disorder will experience complete resolution of symptoms on the maximum approved dose of an SSRI/SNRI, such as 200 mg/day of sertraline (Zoloft), lots of patients will have only a partial response. The next move is not to add a second antidepressant, it’s to augment the high-dose antidepressant the patient is already on with something else. Dr. Lowdermilk highlighted the best and worst strategies:
- Hydroxyzine: This drug, approved by the Food and Drug Administration for treatment of panic attacks at 25-50 mg up to four times per day, has sedating side effects that help with sleep issues.
“We use a lot of this,” Dr. Lowdermilk said.
Hydroxyzine (Vistaril) can be dosed on a fixed schedule or as needed. She strongly favors scheduled dosing. “It’s better to use drugs to reduce anxiety tone, then teach skills to cope with anxiety. As needed treatment subtly teaches patients to take a pill if they have breakthrough symptoms.”
- Buspirone: It takes high doses of this medication to get a robust anxiolytic response. Dr. Lowdermilk recommends starting at 5 mg three times daily and increasing the total daily dose by 15 mg every 2 weeks up to a maximum of 60 mg.
“Buspirone isn’t the strongest medication out there, but it can help. I personally don’t stop until I’m at least at 30 mg per day,” she said.
The drug is especially handy as monotherapy in patients with mild to moderate anxiety who can’t tolerate serotonergic medications well. Also, at higher doses buspirone (Buspar) may reduce the sexual side effects of a concomitant serotonergic agent.
- Gabapentin: Dr. Lowdermilk often turns to this drug off label as an SSRI/SNRI augmentation strategy, starting at 100-300 mg three times daily and increasing over the same time frame as for neuropathic pain up to a maximum total daily dose of 3,600 mg. Like hydroxyzine, it helps with sleep.
- Atypical antipsychotics: Reserve these for patients with an inadequate response to maximum-dose SSRI/SNRI. There is some evidence of efficacy for low-dose risperidone (Risperdal) at 1-2 mg/day, quetiapine(Seroquel) at 50-100 mg, and aripiprazole (Abilify) at 2-5 mg. Because of the risks of metabolic side effects and tardive dyskinesia, it’s best to evaluate the drug’s effectiveness after 2-4 weeks and consider stopping if there is no clinical improvement.
- Benzodiazepines: Yes, they are approved for treatment of anxiety disorders. Nevertheless, Dr. Lowdermilk advises against their as routine practice.
“I want to acknowledge that they really do work for anxiety and there are times you might want to consider them. But we are almost never initiating benzodiazepines anymore. We are learning that they cause more problems than not. What I’ve found over the years is short bursts of use are not short. Patients tend to come back and want more,” according to the psychiatrist.
She is not convinced about the claimed link to dementia, but she does believe long-term use of benzodiazepines is associated with memory and balance problems as well as slowed reaction time. If they are going to be used to treat anxiety disorders, it’s best to turn to a longer-acting agent such as clonazepam (Klonopin) or extended-release alprazolam (Xanax XR), which keep the anxiety tone down without producing the euphoria of short-acting benzodiazepines.
- Behavioral therapies: “I really do think that the combination of medication and behavioral therapy is the best approach,” Dr. Lowdermilk said.
Consider referring patients with a problematic anxiety disorder to a behavioral therapist skilled in cognitive behavioral therapy, exposure therapy, response prevention therapy, or eye movement desensitization and reprocessing.
She reported having no financial conflicts regarding her presentation.
ESTES PARK, COLO. – The first-line medications for anxiety disorders are the same ones used for depression – the SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs) – but the dosing strategies are quite different, Elizabeth L. Lowdermilk, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“We know that folks with anxiety disorders tend to need the higher doses. They just need to get there a little more slowly,” according to Dr. Lowdermilk, a psychiatrist at the university and medical director of outpatient psychiatry at Denver Health.
“The trick is not what drug you use, it’s what dose you started at. For example, when I start sertraline for depression I start it at 50 mg per day. When I start it for anxiety I start it at 25. I just give it a week or 2 at 25 and then I double it to 50. And then I keep going, titrating over 2-3 months to a high or maximum dose,” she explained.
The rapidity of response to the SSRIs and SNRIs is quite different, too. When patients are started on one of these agents as treatment for depression, they can expect that it will take at least 4-6 weeks and maybe as long as 12 weeks before they experience the full therapeutic effect. Not so when the same drugs are used to treat anxiety disorders.
“The anxiolytic effect seems to be more robust sooner. Really, people start noticing something within the first couple days to a week. And they will keep getting better, which people love,” Dr. Lowdermilk said.
One of the early positive effects of SSRI/SNRI therapy that patients can be advised to be on the lookout for is “the Teflon mind,” she said.
“It’s not that the worried thoughts won’t arise, but they’re going to slide out faster and patients are going to be able to shift their focus back to where they want it to be and move forward,” the psychiatrist explained.
Other positive treatment effects include reductions in irritability, anger, perseverative thoughts, restlessness, and physical tension, along with improved sleep.
How to monitor patient response
The most practical anxiety rating scale for busy primary care physicians with tight office visit scheduling is the seven-item Generalized Anxiety Disorder Assessment, or GAD 7. It can be used repeatedly to follow a patient’s treatment response.
“While technically it is picking up the symptoms of GAD, because there are so many overlapping symptoms in all of the anxiety disorders this is going to give you a sense of whether someone is getting better. It won’t pick up their nightmares if they have PTSD, and it won’t necessarily pick up their checking behaviors if they have obsessive-compulsive disorder – you can ask them quickly about that,” she said.
Augmenting an SSRI/SNRI
Be advised: While some patients with an anxiety disorder will experience complete resolution of symptoms on the maximum approved dose of an SSRI/SNRI, such as 200 mg/day of sertraline (Zoloft), lots of patients will have only a partial response. The next move is not to add a second antidepressant, it’s to augment the high-dose antidepressant the patient is already on with something else. Dr. Lowdermilk highlighted the best and worst strategies:
- Hydroxyzine: This drug, approved by the Food and Drug Administration for treatment of panic attacks at 25-50 mg up to four times per day, has sedating side effects that help with sleep issues.
“We use a lot of this,” Dr. Lowdermilk said.
Hydroxyzine (Vistaril) can be dosed on a fixed schedule or as needed. She strongly favors scheduled dosing. “It’s better to use drugs to reduce anxiety tone, then teach skills to cope with anxiety. As needed treatment subtly teaches patients to take a pill if they have breakthrough symptoms.”
- Buspirone: It takes high doses of this medication to get a robust anxiolytic response. Dr. Lowdermilk recommends starting at 5 mg three times daily and increasing the total daily dose by 15 mg every 2 weeks up to a maximum of 60 mg.
“Buspirone isn’t the strongest medication out there, but it can help. I personally don’t stop until I’m at least at 30 mg per day,” she said.
The drug is especially handy as monotherapy in patients with mild to moderate anxiety who can’t tolerate serotonergic medications well. Also, at higher doses buspirone (Buspar) may reduce the sexual side effects of a concomitant serotonergic agent.
- Gabapentin: Dr. Lowdermilk often turns to this drug off label as an SSRI/SNRI augmentation strategy, starting at 100-300 mg three times daily and increasing over the same time frame as for neuropathic pain up to a maximum total daily dose of 3,600 mg. Like hydroxyzine, it helps with sleep.
- Atypical antipsychotics: Reserve these for patients with an inadequate response to maximum-dose SSRI/SNRI. There is some evidence of efficacy for low-dose risperidone (Risperdal) at 1-2 mg/day, quetiapine(Seroquel) at 50-100 mg, and aripiprazole (Abilify) at 2-5 mg. Because of the risks of metabolic side effects and tardive dyskinesia, it’s best to evaluate the drug’s effectiveness after 2-4 weeks and consider stopping if there is no clinical improvement.
- Benzodiazepines: Yes, they are approved for treatment of anxiety disorders. Nevertheless, Dr. Lowdermilk advises against their as routine practice.
“I want to acknowledge that they really do work for anxiety and there are times you might want to consider them. But we are almost never initiating benzodiazepines anymore. We are learning that they cause more problems than not. What I’ve found over the years is short bursts of use are not short. Patients tend to come back and want more,” according to the psychiatrist.
She is not convinced about the claimed link to dementia, but she does believe long-term use of benzodiazepines is associated with memory and balance problems as well as slowed reaction time. If they are going to be used to treat anxiety disorders, it’s best to turn to a longer-acting agent such as clonazepam (Klonopin) or extended-release alprazolam (Xanax XR), which keep the anxiety tone down without producing the euphoria of short-acting benzodiazepines.
- Behavioral therapies: “I really do think that the combination of medication and behavioral therapy is the best approach,” Dr. Lowdermilk said.
Consider referring patients with a problematic anxiety disorder to a behavioral therapist skilled in cognitive behavioral therapy, exposure therapy, response prevention therapy, or eye movement desensitization and reprocessing.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM COLORADO IM
ATTR-ACT shows treatment breakthrough in amyloid cardiomyopathy
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Tafamidis is the first-ever proven disease-modifying therapy for patients with a rapidly progressive form of cardiomyopathy.
Major finding: .
Study details: This 13-country, randomized, phase 3, double-blind trial included 441 patients with transthyretin amyloid cardiomyopathy.
Disclosures: The presenter reported receiving research grants, speaker honoraria, and consultant fees from Pfizer, which sponsored the ATTR-ACT trial.