User login
Migraine takes toll on intimate relationships
SAN FRANCISCO – Nearly 1 in 10 chronic migraine patients say they’ve delayed having children or had fewer children because of their headaches, according to a new analysis from the CaMEO study.
“I think this is the most heartbreaking of the survey responses; we asked, ‘Have you delayed having children or had fewer children because of your headaches?’ and 2.6% of patients with episodic migraine and 9.6% with chronic migraine said yes,” Dawn C. Buse, PhD, said at the annual meeting of the American Headache Society.
CaMEO (the Chronic Migraine Epidemiology and Outcomes study) is a longitudinal, prospective, Internet-based survey whose aim is to flesh out the full impact of migraine. Dr. Buse presented an analysis of 13,064 migraineur participants, which focused on the impact of migraine on intimate relationships and parenting, an aspect of the disease burden that hasn’t been closely examined. All subjects completed the in-depth Family Burden Module, which is concerned with the emotional consequences of migraine.
The bottom line is that “migraine has significant negative impact on the most important relationships in our life: with our spouses, partners, and children,” declared Dr. Buse, a clinical psychologist at the Albert Einstein College of Medicine and director of behavioral medicine for the Montefiore Headache Center in New York.
The extent to which migraineurs perceived their headaches to be a problem increased stepwise with their number of headache days per month. For example, when the 8,127 CaMEO participants in a relationship with a live-in partner were asked to respond to the statement, “If I did not have headaches, I would be a better partner,” somewhat or complete agreement was endorsed by 38% of those with low-frequency episodic migraine at a rate of up to 4 headache days per month, 68% of those with 5-9 headache days per month, 73% of those with high-frequency episodic migraine with 10-14 headache days per month, and 78% of those with chronic migraine, defined as 15 or more headache days per month.
“Not surprising, certainly, but something to keep in mind as we care for our patients; that just because someone has episodic migraine they may have a range of expressions of how much those migraines have impacted their life,” Dr. Buse observed.
Another example: The proportion of subjects who reported delaying having children or having fewer kids because of their headaches was 1.6%, 5.5%, and 6.5% in low-, moderate-, and high-frequency episodic migraineurs, respectively, before topping out at 9.6% among those with chronic migraine.
Although she and her coinvestigators broke down the data by gender, there were no significant gender differences in the impact of migraine on significant relationships. The major differences were between patients with episodic as compared with chronic migraine.
Among the more than 3,500 CaMEO participants not currently in a relationship, 37% of those with chronic migraine and 15% of those with episodic migraine indicated that their headaches had contributed to relationship problems.
Of those in a relationship but not living together, 44% of individuals with chronic migraine reported that their headaches were preventing a closer relationship, such as moving in together or getting married, as did 16% of those with episodic migraine.
About 47%of respondents with chronic migraine reported that their headaches had caused at least one previous break-up, as did 18% of those with episodic migraine.
“Health care professionals should consider the overall burden of disease when managing migraine, particularly for those with chronic migraine,” Dr. Buse concluded. “Personalized comprehensive treatment plans may include both acute and preventive pharmacologic treatments as well as behavioral treatment for the proband, marital dyad, and family members as appropriate.”
She reported receiving research support and honoraria from Allergan, the study sponsor, as well as from Avenir, Eli Lilly, and Promius.
SOURCE: Buse DC et al. AHS 2018, Abstract OR06.
SAN FRANCISCO – Nearly 1 in 10 chronic migraine patients say they’ve delayed having children or had fewer children because of their headaches, according to a new analysis from the CaMEO study.
“I think this is the most heartbreaking of the survey responses; we asked, ‘Have you delayed having children or had fewer children because of your headaches?’ and 2.6% of patients with episodic migraine and 9.6% with chronic migraine said yes,” Dawn C. Buse, PhD, said at the annual meeting of the American Headache Society.
CaMEO (the Chronic Migraine Epidemiology and Outcomes study) is a longitudinal, prospective, Internet-based survey whose aim is to flesh out the full impact of migraine. Dr. Buse presented an analysis of 13,064 migraineur participants, which focused on the impact of migraine on intimate relationships and parenting, an aspect of the disease burden that hasn’t been closely examined. All subjects completed the in-depth Family Burden Module, which is concerned with the emotional consequences of migraine.
The bottom line is that “migraine has significant negative impact on the most important relationships in our life: with our spouses, partners, and children,” declared Dr. Buse, a clinical psychologist at the Albert Einstein College of Medicine and director of behavioral medicine for the Montefiore Headache Center in New York.
The extent to which migraineurs perceived their headaches to be a problem increased stepwise with their number of headache days per month. For example, when the 8,127 CaMEO participants in a relationship with a live-in partner were asked to respond to the statement, “If I did not have headaches, I would be a better partner,” somewhat or complete agreement was endorsed by 38% of those with low-frequency episodic migraine at a rate of up to 4 headache days per month, 68% of those with 5-9 headache days per month, 73% of those with high-frequency episodic migraine with 10-14 headache days per month, and 78% of those with chronic migraine, defined as 15 or more headache days per month.
“Not surprising, certainly, but something to keep in mind as we care for our patients; that just because someone has episodic migraine they may have a range of expressions of how much those migraines have impacted their life,” Dr. Buse observed.
Another example: The proportion of subjects who reported delaying having children or having fewer kids because of their headaches was 1.6%, 5.5%, and 6.5% in low-, moderate-, and high-frequency episodic migraineurs, respectively, before topping out at 9.6% among those with chronic migraine.
Although she and her coinvestigators broke down the data by gender, there were no significant gender differences in the impact of migraine on significant relationships. The major differences were between patients with episodic as compared with chronic migraine.
Among the more than 3,500 CaMEO participants not currently in a relationship, 37% of those with chronic migraine and 15% of those with episodic migraine indicated that their headaches had contributed to relationship problems.
Of those in a relationship but not living together, 44% of individuals with chronic migraine reported that their headaches were preventing a closer relationship, such as moving in together or getting married, as did 16% of those with episodic migraine.
About 47%of respondents with chronic migraine reported that their headaches had caused at least one previous break-up, as did 18% of those with episodic migraine.
“Health care professionals should consider the overall burden of disease when managing migraine, particularly for those with chronic migraine,” Dr. Buse concluded. “Personalized comprehensive treatment plans may include both acute and preventive pharmacologic treatments as well as behavioral treatment for the proband, marital dyad, and family members as appropriate.”
She reported receiving research support and honoraria from Allergan, the study sponsor, as well as from Avenir, Eli Lilly, and Promius.
SOURCE: Buse DC et al. AHS 2018, Abstract OR06.
SAN FRANCISCO – Nearly 1 in 10 chronic migraine patients say they’ve delayed having children or had fewer children because of their headaches, according to a new analysis from the CaMEO study.
“I think this is the most heartbreaking of the survey responses; we asked, ‘Have you delayed having children or had fewer children because of your headaches?’ and 2.6% of patients with episodic migraine and 9.6% with chronic migraine said yes,” Dawn C. Buse, PhD, said at the annual meeting of the American Headache Society.
CaMEO (the Chronic Migraine Epidemiology and Outcomes study) is a longitudinal, prospective, Internet-based survey whose aim is to flesh out the full impact of migraine. Dr. Buse presented an analysis of 13,064 migraineur participants, which focused on the impact of migraine on intimate relationships and parenting, an aspect of the disease burden that hasn’t been closely examined. All subjects completed the in-depth Family Burden Module, which is concerned with the emotional consequences of migraine.
The bottom line is that “migraine has significant negative impact on the most important relationships in our life: with our spouses, partners, and children,” declared Dr. Buse, a clinical psychologist at the Albert Einstein College of Medicine and director of behavioral medicine for the Montefiore Headache Center in New York.
The extent to which migraineurs perceived their headaches to be a problem increased stepwise with their number of headache days per month. For example, when the 8,127 CaMEO participants in a relationship with a live-in partner were asked to respond to the statement, “If I did not have headaches, I would be a better partner,” somewhat or complete agreement was endorsed by 38% of those with low-frequency episodic migraine at a rate of up to 4 headache days per month, 68% of those with 5-9 headache days per month, 73% of those with high-frequency episodic migraine with 10-14 headache days per month, and 78% of those with chronic migraine, defined as 15 or more headache days per month.
“Not surprising, certainly, but something to keep in mind as we care for our patients; that just because someone has episodic migraine they may have a range of expressions of how much those migraines have impacted their life,” Dr. Buse observed.
Another example: The proportion of subjects who reported delaying having children or having fewer kids because of their headaches was 1.6%, 5.5%, and 6.5% in low-, moderate-, and high-frequency episodic migraineurs, respectively, before topping out at 9.6% among those with chronic migraine.
Although she and her coinvestigators broke down the data by gender, there were no significant gender differences in the impact of migraine on significant relationships. The major differences were between patients with episodic as compared with chronic migraine.
Among the more than 3,500 CaMEO participants not currently in a relationship, 37% of those with chronic migraine and 15% of those with episodic migraine indicated that their headaches had contributed to relationship problems.
Of those in a relationship but not living together, 44% of individuals with chronic migraine reported that their headaches were preventing a closer relationship, such as moving in together or getting married, as did 16% of those with episodic migraine.
About 47%of respondents with chronic migraine reported that their headaches had caused at least one previous break-up, as did 18% of those with episodic migraine.
“Health care professionals should consider the overall burden of disease when managing migraine, particularly for those with chronic migraine,” Dr. Buse concluded. “Personalized comprehensive treatment plans may include both acute and preventive pharmacologic treatments as well as behavioral treatment for the proband, marital dyad, and family members as appropriate.”
She reported receiving research support and honoraria from Allergan, the study sponsor, as well as from Avenir, Eli Lilly, and Promius.
SOURCE: Buse DC et al. AHS 2018, Abstract OR06.
REPORTING FROM THE AHS ANNUAL MEETING
Key clinical point: Migraine is a major contributor to relationship problems.
Major finding: Nearly 10% of chronic migraineurs say they’ve delayed having children or had fewer of them because of their headaches.
Study details: This analysis included more than 13,000 participants in the CaMEO study, a prospective, longitudinal, Internet-based survey.
Disclosures: The presenter reported receiving research support and honoraria from Allergan, the study sponsor, as well as from Avenir, Eli Lilly, and Promius.
Source: Buse DC et al. AHS 2018, Abstract OR06.
Prepare for ‘the coming tsunami’ of NAFLD
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
REPORTING FROM ACP INTERNAL MEDICINE
Spontaneous intracranial hypotension: a triple misnomer
SAN FRANCISCO – Spontaneous intracranial hypotension couldn’t have a more misleading moniker.
First of all, spontaneous intracranial hypotension (SIH) is not always spontaneous; often there is an antecedent causal event. Second, the main problem isn’t intracranial, it’s a leak in the spinal column, most often in the low cervical or thoracic zone. And cerebrospinal fluid (CSF) pressure is usually normal in these patients, Deborah I. Friedman, MD, said in the annual Seymour Solomon Award Lecture at the annual meeting of the American Headache Society.
Moreover, although SIH is usually labeled a rare disorder, with a published annual incidence of 5 cases per 100,000, that’s doubtless a gross underestimate stemming from the absence of an ICD-9 or -10 code for the condition, according to Dr. Friedman, chief of the division of headache medicine, professor of neurology, and professor of ophthalmology at the University of Texas Southwestern Medical Center, Dallas.
“[SIH is] much more common than we think,” she asserted. “These people are out there. They’re in your offices. I can guarantee you, there are patients you’ve been seeing for years in your practice that have [SIH]. I’ve missed it. I bet you have, too.”
She focused her award lecture on the diagnosis of SIH from the perspective of a headache specialist.
“Most of the literature that’s out there – and it is good literature – wasn’t written by headache medicine specialists, it was written by very famous and prominent neurosurgeons and neuroradiologists. But the people we see are not necessarily the people they see,” Dr. Friedman explained.
SIH can be a challenging diagnosis because of its myriad presentations.
“You need to be a detective,” she emphasized.
Headache is the most common symptom of SIH and the reason patients with the disorder seek out a headache specialist. It’s time to consider the diagnosis in a patient with a new daily persistent headache, or in the patient running around with a diagnosis of chronic migraine for which nothing works.
“The people who come in with a huge list of medications they’ve tried and nothing works? That’s unusual for migraine. Usually something works for migraine,” she observed.
The headache of SIH can be thunderclap in onset, although not necessarily so. The most common location is posterior, but the pain can be centered anywhere in the head or face. Bilateral pain is more common than unilateral.
The most typical headache pattern is one that’s orthostatic or gets worse at the end of the day. The longer a patient has SIH, though, the less likely that they’ll have a postural component. The majority of patients are awakened by their headache in the middle of the night. The headache is often exertional and usually worsens with Valsalva maneuvers, including coughing, sneezing, lifting, bending forward, straining, singing, and/or sexual activity. People with SIH often find that caffeine works very well for them. Ask nonleading questions about these issues, she suggested.
Besides headache, other common symptoms of SIH include tinnitus, abnormal hearing as if underwater, neck pain, imbalance, pain between the shoulder blades, and blurred or double vision.
Risk factors that are a tipoff include joint hypermobility, previous lumbar puncture, epidural or spinal anesthesia, known disc disease, or a personal or family history of retinal detachment at a young age, aneurysm, dissection, or valvular heart disease.
Joint hypermobility is really common in patients with SIH. These are often headache patients who enjoy yoga and were superflexible as children, participating in gymnastics, ballet, or cheerleading.
“We’re looking for Cirque du Soleil here, so you have to ask the Cirque du Soleil questions,” Dr. Friedman said.
On physical examination, she looks for joint hypermobility, makes sure that spontaneous retinal venous pulsations indicative of normal CSF pressure are present when she looks in the eyes, and puts the patient in 5 degrees of Trendelenburg position for 5-10 minutes to see if that improves the headache and other symptoms.
One of the first things Dr. Friedman does when she suspects the diagnosis is to send a patient to the recommended website of the Spinal CSF Leak Foundation. She asks them to take a look around the site and tell her if the descriptions sound like them.
There is broad agreement that the first-line diagnostic test is brain imaging via MRI with gadolinium enhancement. The diagnostic challenge posed by SIH is that the test is normal in 30% of affected patients.
At present there is no consensus as to the next move when the brain MRI is negative. Some favor CT with or without MR myelography, others a T2-weighted spine MRI. But despite a thorough search, no leak is found in about half of individuals with SIH.
Although Dr. Friedman focused on diagnosis, she turned briefly to treatment. She has found that conservative measures don’t work very well. A reasonable strategy, even if a leak site hasn’t been identified, is to treat with a high-volume epidural CT-guided targeted blood patch with fibrin sealant.
“It gives relief about a third of the time each time you do it,” according to Dr. Friedman.
SAN FRANCISCO – Spontaneous intracranial hypotension couldn’t have a more misleading moniker.
First of all, spontaneous intracranial hypotension (SIH) is not always spontaneous; often there is an antecedent causal event. Second, the main problem isn’t intracranial, it’s a leak in the spinal column, most often in the low cervical or thoracic zone. And cerebrospinal fluid (CSF) pressure is usually normal in these patients, Deborah I. Friedman, MD, said in the annual Seymour Solomon Award Lecture at the annual meeting of the American Headache Society.
Moreover, although SIH is usually labeled a rare disorder, with a published annual incidence of 5 cases per 100,000, that’s doubtless a gross underestimate stemming from the absence of an ICD-9 or -10 code for the condition, according to Dr. Friedman, chief of the division of headache medicine, professor of neurology, and professor of ophthalmology at the University of Texas Southwestern Medical Center, Dallas.
“[SIH is] much more common than we think,” she asserted. “These people are out there. They’re in your offices. I can guarantee you, there are patients you’ve been seeing for years in your practice that have [SIH]. I’ve missed it. I bet you have, too.”
She focused her award lecture on the diagnosis of SIH from the perspective of a headache specialist.
“Most of the literature that’s out there – and it is good literature – wasn’t written by headache medicine specialists, it was written by very famous and prominent neurosurgeons and neuroradiologists. But the people we see are not necessarily the people they see,” Dr. Friedman explained.
SIH can be a challenging diagnosis because of its myriad presentations.
“You need to be a detective,” she emphasized.
Headache is the most common symptom of SIH and the reason patients with the disorder seek out a headache specialist. It’s time to consider the diagnosis in a patient with a new daily persistent headache, or in the patient running around with a diagnosis of chronic migraine for which nothing works.
“The people who come in with a huge list of medications they’ve tried and nothing works? That’s unusual for migraine. Usually something works for migraine,” she observed.
The headache of SIH can be thunderclap in onset, although not necessarily so. The most common location is posterior, but the pain can be centered anywhere in the head or face. Bilateral pain is more common than unilateral.
The most typical headache pattern is one that’s orthostatic or gets worse at the end of the day. The longer a patient has SIH, though, the less likely that they’ll have a postural component. The majority of patients are awakened by their headache in the middle of the night. The headache is often exertional and usually worsens with Valsalva maneuvers, including coughing, sneezing, lifting, bending forward, straining, singing, and/or sexual activity. People with SIH often find that caffeine works very well for them. Ask nonleading questions about these issues, she suggested.
Besides headache, other common symptoms of SIH include tinnitus, abnormal hearing as if underwater, neck pain, imbalance, pain between the shoulder blades, and blurred or double vision.
Risk factors that are a tipoff include joint hypermobility, previous lumbar puncture, epidural or spinal anesthesia, known disc disease, or a personal or family history of retinal detachment at a young age, aneurysm, dissection, or valvular heart disease.
Joint hypermobility is really common in patients with SIH. These are often headache patients who enjoy yoga and were superflexible as children, participating in gymnastics, ballet, or cheerleading.
“We’re looking for Cirque du Soleil here, so you have to ask the Cirque du Soleil questions,” Dr. Friedman said.
On physical examination, she looks for joint hypermobility, makes sure that spontaneous retinal venous pulsations indicative of normal CSF pressure are present when she looks in the eyes, and puts the patient in 5 degrees of Trendelenburg position for 5-10 minutes to see if that improves the headache and other symptoms.
One of the first things Dr. Friedman does when she suspects the diagnosis is to send a patient to the recommended website of the Spinal CSF Leak Foundation. She asks them to take a look around the site and tell her if the descriptions sound like them.
There is broad agreement that the first-line diagnostic test is brain imaging via MRI with gadolinium enhancement. The diagnostic challenge posed by SIH is that the test is normal in 30% of affected patients.
At present there is no consensus as to the next move when the brain MRI is negative. Some favor CT with or without MR myelography, others a T2-weighted spine MRI. But despite a thorough search, no leak is found in about half of individuals with SIH.
Although Dr. Friedman focused on diagnosis, she turned briefly to treatment. She has found that conservative measures don’t work very well. A reasonable strategy, even if a leak site hasn’t been identified, is to treat with a high-volume epidural CT-guided targeted blood patch with fibrin sealant.
“It gives relief about a third of the time each time you do it,” according to Dr. Friedman.
SAN FRANCISCO – Spontaneous intracranial hypotension couldn’t have a more misleading moniker.
First of all, spontaneous intracranial hypotension (SIH) is not always spontaneous; often there is an antecedent causal event. Second, the main problem isn’t intracranial, it’s a leak in the spinal column, most often in the low cervical or thoracic zone. And cerebrospinal fluid (CSF) pressure is usually normal in these patients, Deborah I. Friedman, MD, said in the annual Seymour Solomon Award Lecture at the annual meeting of the American Headache Society.
Moreover, although SIH is usually labeled a rare disorder, with a published annual incidence of 5 cases per 100,000, that’s doubtless a gross underestimate stemming from the absence of an ICD-9 or -10 code for the condition, according to Dr. Friedman, chief of the division of headache medicine, professor of neurology, and professor of ophthalmology at the University of Texas Southwestern Medical Center, Dallas.
“[SIH is] much more common than we think,” she asserted. “These people are out there. They’re in your offices. I can guarantee you, there are patients you’ve been seeing for years in your practice that have [SIH]. I’ve missed it. I bet you have, too.”
She focused her award lecture on the diagnosis of SIH from the perspective of a headache specialist.
“Most of the literature that’s out there – and it is good literature – wasn’t written by headache medicine specialists, it was written by very famous and prominent neurosurgeons and neuroradiologists. But the people we see are not necessarily the people they see,” Dr. Friedman explained.
SIH can be a challenging diagnosis because of its myriad presentations.
“You need to be a detective,” she emphasized.
Headache is the most common symptom of SIH and the reason patients with the disorder seek out a headache specialist. It’s time to consider the diagnosis in a patient with a new daily persistent headache, or in the patient running around with a diagnosis of chronic migraine for which nothing works.
“The people who come in with a huge list of medications they’ve tried and nothing works? That’s unusual for migraine. Usually something works for migraine,” she observed.
The headache of SIH can be thunderclap in onset, although not necessarily so. The most common location is posterior, but the pain can be centered anywhere in the head or face. Bilateral pain is more common than unilateral.
The most typical headache pattern is one that’s orthostatic or gets worse at the end of the day. The longer a patient has SIH, though, the less likely that they’ll have a postural component. The majority of patients are awakened by their headache in the middle of the night. The headache is often exertional and usually worsens with Valsalva maneuvers, including coughing, sneezing, lifting, bending forward, straining, singing, and/or sexual activity. People with SIH often find that caffeine works very well for them. Ask nonleading questions about these issues, she suggested.
Besides headache, other common symptoms of SIH include tinnitus, abnormal hearing as if underwater, neck pain, imbalance, pain between the shoulder blades, and blurred or double vision.
Risk factors that are a tipoff include joint hypermobility, previous lumbar puncture, epidural or spinal anesthesia, known disc disease, or a personal or family history of retinal detachment at a young age, aneurysm, dissection, or valvular heart disease.
Joint hypermobility is really common in patients with SIH. These are often headache patients who enjoy yoga and were superflexible as children, participating in gymnastics, ballet, or cheerleading.
“We’re looking for Cirque du Soleil here, so you have to ask the Cirque du Soleil questions,” Dr. Friedman said.
On physical examination, she looks for joint hypermobility, makes sure that spontaneous retinal venous pulsations indicative of normal CSF pressure are present when she looks in the eyes, and puts the patient in 5 degrees of Trendelenburg position for 5-10 minutes to see if that improves the headache and other symptoms.
One of the first things Dr. Friedman does when she suspects the diagnosis is to send a patient to the recommended website of the Spinal CSF Leak Foundation. She asks them to take a look around the site and tell her if the descriptions sound like them.
There is broad agreement that the first-line diagnostic test is brain imaging via MRI with gadolinium enhancement. The diagnostic challenge posed by SIH is that the test is normal in 30% of affected patients.
At present there is no consensus as to the next move when the brain MRI is negative. Some favor CT with or without MR myelography, others a T2-weighted spine MRI. But despite a thorough search, no leak is found in about half of individuals with SIH.
Although Dr. Friedman focused on diagnosis, she turned briefly to treatment. She has found that conservative measures don’t work very well. A reasonable strategy, even if a leak site hasn’t been identified, is to treat with a high-volume epidural CT-guided targeted blood patch with fibrin sealant.
“It gives relief about a third of the time each time you do it,” according to Dr. Friedman.
REPORTING FROM THE AHS ANNUAL MEETING
Hybrid PCI strategy rules for complex CTO
PARIS – The so-called hybrid strategy is now the preferred approach to percutaneous coronary intervention (PCI) for chronic total occlusions (CTO), 12-month outcomes of the multicenter observational CONSISTENT CTO trial suggest, according to Simon J. Walsh, MD, an interventional cardiologist at Belfast Health and Social Care Trust.
With use of the hybrid strategy, a CTO’s anatomic complexity is no longer a barrier to performing PCI with a success rate that by former standards would be considered astronomical, he said in presenting the CONSISTENT CTO results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, the key take-home messages from CONSISTENT CTO were that technical success rates were dramatic at 98.6%, the target vessel failure rate at 12 months was impressively low at 5.24%, and quality of life scores showed clinically meaningful gains maintained through 1 year.
“Opening a CTO makes people better, reduces their symptom burden, and is something worth doing,” Dr. Walsh concluded.
That hasn’t always been the prevailing view. Historically, most interventional cardiologists had a pessimistic attitude regarding PCI for CTOs. The procedures had a low technical success rate, frequent complications, and lousy durability. Moreover, these subpar results came at a considerable price in terms of extensive radiation exposure and catheterization laboratory time. These various shortcomings became particularly prominent when traditional wire-based PCI strategies were applied to long, complex occlusions.
All that has changed as a result of improved stent technologies and procedural techniques, along with the development of the hybrid PCI algorithm by U.S. cardiologists. Using these tools, an interventionalist skilled in the hybrid strategy now selects cases on the basis of clinical indications, such as disabling angina, rather than on anatomic considerations. This point was emphatically brought home in CONSISTENT CTO (Conventional antegrade vs. sub-intimal synergy stenting in chronic total occlusions).
“This is the most complex set of treated CTO lesions ever investigated in this rigorous way,” according to Dr. Walsh.
This assertion was bolstered by the fact that the average Japan CTO (JCTO) score in the 210 study participants was 2.4, climbing to 2.9 in the 101 patients with dissection present. Further adding to the lesion complexity was the fact that roughly one in five subjects had a degenerated coronary artery graft in their target vessel. The average lesion length as measured at the study core lab was 29.1 mm. The impetus for the CONSISTENT CTO Study was a recognition that, even though a growing number of skilled operators are embracing the hybrid PCI strategy with heretofore unprecedented favorable results, there remains an evidence gap, with little in the way of long-term studies featuring rigorous follow-up. Participants in CONSISTENT CTO therefore underwent baseline formal quality of life assessment, repeated at 12 months of follow-up together with angiography and/or optical coherence tomography. Further follow-up is planned at 2, 3, and 5 years.
Of the 231 patients who consented to participate in the study, 210, or 90%, were able to have their CTO opened with a Synergy everolimus-eluting stent. Those 210 constituted the study population.
The hybrid algorithm-based PCI strategy utilizes an individual’s CTO anatomy to dictate the initial choice of approach. The goal is to pick the strategy that is most likely to achieve successful outcome efficiently, with minimal contrast and radiation doses for that particular type of CTO. The hybrid algorithm begins with dual-catheter injection and intravascular ultrasound aimed at determining whether four key anatomic features are present: an ambiguous proximal cap, a poor distal target, good interventional collaterals, and a major side branch at the distal cap.
If those four features are absent and the CTO lesion is less than 20-mm long, the algorithm dictates that the initial approach is antegrade wire escalation; if the lesion is 20 mm or more, the first approach is antegrade dissection reentry. However, if those anatomic features are present, the initial strategy is retrograde wire escalation if the lesion is less than 20 mm, and retrograde dissection reentry for longer lesions. When the initial approach fails, structured protocols dictate the selection of second and third approaches (JACC Cardiovasc Interv. 2012 Apr;5[4]:367-79).
The right coronary artery was the site of the CTO in 70% of study participants. Dual-catheter access was utilized in 79% of patients; only 10% had exclusively radial access. An average of 2.8 Synergy stents were deployed, with a whopping mean total stent length of 96.6 mm in the 48% of patients with dissection and 75.4 mm in those without. Mean procedure time was 122 minutes, with a fluoroscopy time of 44.6 minutes.
The primary efficacy endpoint was the rate of target vessel failure (TVF) at 12 months, defined as cardiac death, MI related to the target vessel, or any ischemia-driven revascularization of the target vessel. The rate was 5.24%.
“We were pleasantly surprised by that,” Dr. Walsh admitted.
Indeed, based upon studies of PCI for CTO published by other investigators, he and his colleagues had initially projected a 13% TVF rate, then bumped it up to 15% because of the high degree of lesion complexity.
Diabetes was the main predictor of target vessel revascularization.
At the time of the index PCI, 19% of patients were scheduled to return for an early optimization procedure within 3 months. This was arranged when operators anticipated significant positive remodeling would occur as the target vessel readjusted to blood flow or distal disease of borderline severity was noted beyond the CTO segment. These were not counted as adverse events. Half of the returnees underwent angiography and no further action was undertaken. The others underwent postdilation of their Synergy stents or new stenting of distal disease.
Complete revascularization of the target territory was achieved in 98.6% of patients. Key complications consisted of 5 perforations, 10 hematomas at vascular access sites, 2 cases of pericardiocentesis, and 4 instances of bleeding requiring transfusion.
The final successful CTO revascularization strategy was antegrade dissection reentry in 18% of patients, retrograde dissection reentry in 30%, antegrade wire escalation in 34%, and retrograde wire escalation in 18%. A switch from the initial algorithm-based strategy to a second strategy occurred in 41% of patients, and a third strategy was employed in 9%.
“I think the key message is you need to have more than one option to treat complicated disease. Half of patients had a switch in strategy,” Dr. Walsh observed.
Intravascular ultrasound (IVUS) adjudication in the central core lab showed a humbling rate of discordance between the operators’ impression of how their procedures were proceeding and what was really going on.
“There’s a bit of a mantra [that says] if you’re wiring stuff you’re always in the lumen, and if you’re using dissection techniques you’re always not in the lumen. In fact, that’s nonsense. You get it wrong one in six times. When you use IVUS adjudication to see if you’re outside the lumen or not, with wire-based retrograde escalation you’re out of the lumen and in the subintimal space 27% of the time. And with dissection strategies you’re wrong in about 15%,” according to the cardiologist.
He described the study participants as “extremely limited” at baseline as evidenced by their scores on the Seattle Angina Questionnaire Physical Limitation, Angina Stability, and Angina Frequency domains. At 12 months of follow-up, patients averaged 20- to 40-point improvements across all three domains.
One member of the discussion panel expressed a wish that the study had included a sham PCI arm. He raised the possibility that PCI had exerted an enormous placebo effect that could conceivably account for the substantial quality of life benefits documented in the study. But another panelist scoffed at this notion. This wasn’t a modest improvement in quality of life, nor was it measured after a mere 6 weeks, as was the case in the sham-controlled ORBITA trial.
“It’s really difficult to imagine a sham effect that persists out to a year,” he argued.
Dr. Walsh reported receiving research grants from and serving as a consultant to Boston Scientific, which funded the CONSISTENT CTO Study.
PARIS – The so-called hybrid strategy is now the preferred approach to percutaneous coronary intervention (PCI) for chronic total occlusions (CTO), 12-month outcomes of the multicenter observational CONSISTENT CTO trial suggest, according to Simon J. Walsh, MD, an interventional cardiologist at Belfast Health and Social Care Trust.
With use of the hybrid strategy, a CTO’s anatomic complexity is no longer a barrier to performing PCI with a success rate that by former standards would be considered astronomical, he said in presenting the CONSISTENT CTO results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, the key take-home messages from CONSISTENT CTO were that technical success rates were dramatic at 98.6%, the target vessel failure rate at 12 months was impressively low at 5.24%, and quality of life scores showed clinically meaningful gains maintained through 1 year.
“Opening a CTO makes people better, reduces their symptom burden, and is something worth doing,” Dr. Walsh concluded.
That hasn’t always been the prevailing view. Historically, most interventional cardiologists had a pessimistic attitude regarding PCI for CTOs. The procedures had a low technical success rate, frequent complications, and lousy durability. Moreover, these subpar results came at a considerable price in terms of extensive radiation exposure and catheterization laboratory time. These various shortcomings became particularly prominent when traditional wire-based PCI strategies were applied to long, complex occlusions.
All that has changed as a result of improved stent technologies and procedural techniques, along with the development of the hybrid PCI algorithm by U.S. cardiologists. Using these tools, an interventionalist skilled in the hybrid strategy now selects cases on the basis of clinical indications, such as disabling angina, rather than on anatomic considerations. This point was emphatically brought home in CONSISTENT CTO (Conventional antegrade vs. sub-intimal synergy stenting in chronic total occlusions).
“This is the most complex set of treated CTO lesions ever investigated in this rigorous way,” according to Dr. Walsh.
This assertion was bolstered by the fact that the average Japan CTO (JCTO) score in the 210 study participants was 2.4, climbing to 2.9 in the 101 patients with dissection present. Further adding to the lesion complexity was the fact that roughly one in five subjects had a degenerated coronary artery graft in their target vessel. The average lesion length as measured at the study core lab was 29.1 mm. The impetus for the CONSISTENT CTO Study was a recognition that, even though a growing number of skilled operators are embracing the hybrid PCI strategy with heretofore unprecedented favorable results, there remains an evidence gap, with little in the way of long-term studies featuring rigorous follow-up. Participants in CONSISTENT CTO therefore underwent baseline formal quality of life assessment, repeated at 12 months of follow-up together with angiography and/or optical coherence tomography. Further follow-up is planned at 2, 3, and 5 years.
Of the 231 patients who consented to participate in the study, 210, or 90%, were able to have their CTO opened with a Synergy everolimus-eluting stent. Those 210 constituted the study population.
The hybrid algorithm-based PCI strategy utilizes an individual’s CTO anatomy to dictate the initial choice of approach. The goal is to pick the strategy that is most likely to achieve successful outcome efficiently, with minimal contrast and radiation doses for that particular type of CTO. The hybrid algorithm begins with dual-catheter injection and intravascular ultrasound aimed at determining whether four key anatomic features are present: an ambiguous proximal cap, a poor distal target, good interventional collaterals, and a major side branch at the distal cap.
If those four features are absent and the CTO lesion is less than 20-mm long, the algorithm dictates that the initial approach is antegrade wire escalation; if the lesion is 20 mm or more, the first approach is antegrade dissection reentry. However, if those anatomic features are present, the initial strategy is retrograde wire escalation if the lesion is less than 20 mm, and retrograde dissection reentry for longer lesions. When the initial approach fails, structured protocols dictate the selection of second and third approaches (JACC Cardiovasc Interv. 2012 Apr;5[4]:367-79).
The right coronary artery was the site of the CTO in 70% of study participants. Dual-catheter access was utilized in 79% of patients; only 10% had exclusively radial access. An average of 2.8 Synergy stents were deployed, with a whopping mean total stent length of 96.6 mm in the 48% of patients with dissection and 75.4 mm in those without. Mean procedure time was 122 minutes, with a fluoroscopy time of 44.6 minutes.
The primary efficacy endpoint was the rate of target vessel failure (TVF) at 12 months, defined as cardiac death, MI related to the target vessel, or any ischemia-driven revascularization of the target vessel. The rate was 5.24%.
“We were pleasantly surprised by that,” Dr. Walsh admitted.
Indeed, based upon studies of PCI for CTO published by other investigators, he and his colleagues had initially projected a 13% TVF rate, then bumped it up to 15% because of the high degree of lesion complexity.
Diabetes was the main predictor of target vessel revascularization.
At the time of the index PCI, 19% of patients were scheduled to return for an early optimization procedure within 3 months. This was arranged when operators anticipated significant positive remodeling would occur as the target vessel readjusted to blood flow or distal disease of borderline severity was noted beyond the CTO segment. These were not counted as adverse events. Half of the returnees underwent angiography and no further action was undertaken. The others underwent postdilation of their Synergy stents or new stenting of distal disease.
Complete revascularization of the target territory was achieved in 98.6% of patients. Key complications consisted of 5 perforations, 10 hematomas at vascular access sites, 2 cases of pericardiocentesis, and 4 instances of bleeding requiring transfusion.
The final successful CTO revascularization strategy was antegrade dissection reentry in 18% of patients, retrograde dissection reentry in 30%, antegrade wire escalation in 34%, and retrograde wire escalation in 18%. A switch from the initial algorithm-based strategy to a second strategy occurred in 41% of patients, and a third strategy was employed in 9%.
“I think the key message is you need to have more than one option to treat complicated disease. Half of patients had a switch in strategy,” Dr. Walsh observed.
Intravascular ultrasound (IVUS) adjudication in the central core lab showed a humbling rate of discordance between the operators’ impression of how their procedures were proceeding and what was really going on.
“There’s a bit of a mantra [that says] if you’re wiring stuff you’re always in the lumen, and if you’re using dissection techniques you’re always not in the lumen. In fact, that’s nonsense. You get it wrong one in six times. When you use IVUS adjudication to see if you’re outside the lumen or not, with wire-based retrograde escalation you’re out of the lumen and in the subintimal space 27% of the time. And with dissection strategies you’re wrong in about 15%,” according to the cardiologist.
He described the study participants as “extremely limited” at baseline as evidenced by their scores on the Seattle Angina Questionnaire Physical Limitation, Angina Stability, and Angina Frequency domains. At 12 months of follow-up, patients averaged 20- to 40-point improvements across all three domains.
One member of the discussion panel expressed a wish that the study had included a sham PCI arm. He raised the possibility that PCI had exerted an enormous placebo effect that could conceivably account for the substantial quality of life benefits documented in the study. But another panelist scoffed at this notion. This wasn’t a modest improvement in quality of life, nor was it measured after a mere 6 weeks, as was the case in the sham-controlled ORBITA trial.
“It’s really difficult to imagine a sham effect that persists out to a year,” he argued.
Dr. Walsh reported receiving research grants from and serving as a consultant to Boston Scientific, which funded the CONSISTENT CTO Study.
PARIS – The so-called hybrid strategy is now the preferred approach to percutaneous coronary intervention (PCI) for chronic total occlusions (CTO), 12-month outcomes of the multicenter observational CONSISTENT CTO trial suggest, according to Simon J. Walsh, MD, an interventional cardiologist at Belfast Health and Social Care Trust.
With use of the hybrid strategy, a CTO’s anatomic complexity is no longer a barrier to performing PCI with a success rate that by former standards would be considered astronomical, he said in presenting the CONSISTENT CTO results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, the key take-home messages from CONSISTENT CTO were that technical success rates were dramatic at 98.6%, the target vessel failure rate at 12 months was impressively low at 5.24%, and quality of life scores showed clinically meaningful gains maintained through 1 year.
“Opening a CTO makes people better, reduces their symptom burden, and is something worth doing,” Dr. Walsh concluded.
That hasn’t always been the prevailing view. Historically, most interventional cardiologists had a pessimistic attitude regarding PCI for CTOs. The procedures had a low technical success rate, frequent complications, and lousy durability. Moreover, these subpar results came at a considerable price in terms of extensive radiation exposure and catheterization laboratory time. These various shortcomings became particularly prominent when traditional wire-based PCI strategies were applied to long, complex occlusions.
All that has changed as a result of improved stent technologies and procedural techniques, along with the development of the hybrid PCI algorithm by U.S. cardiologists. Using these tools, an interventionalist skilled in the hybrid strategy now selects cases on the basis of clinical indications, such as disabling angina, rather than on anatomic considerations. This point was emphatically brought home in CONSISTENT CTO (Conventional antegrade vs. sub-intimal synergy stenting in chronic total occlusions).
“This is the most complex set of treated CTO lesions ever investigated in this rigorous way,” according to Dr. Walsh.
This assertion was bolstered by the fact that the average Japan CTO (JCTO) score in the 210 study participants was 2.4, climbing to 2.9 in the 101 patients with dissection present. Further adding to the lesion complexity was the fact that roughly one in five subjects had a degenerated coronary artery graft in their target vessel. The average lesion length as measured at the study core lab was 29.1 mm. The impetus for the CONSISTENT CTO Study was a recognition that, even though a growing number of skilled operators are embracing the hybrid PCI strategy with heretofore unprecedented favorable results, there remains an evidence gap, with little in the way of long-term studies featuring rigorous follow-up. Participants in CONSISTENT CTO therefore underwent baseline formal quality of life assessment, repeated at 12 months of follow-up together with angiography and/or optical coherence tomography. Further follow-up is planned at 2, 3, and 5 years.
Of the 231 patients who consented to participate in the study, 210, or 90%, were able to have their CTO opened with a Synergy everolimus-eluting stent. Those 210 constituted the study population.
The hybrid algorithm-based PCI strategy utilizes an individual’s CTO anatomy to dictate the initial choice of approach. The goal is to pick the strategy that is most likely to achieve successful outcome efficiently, with minimal contrast and radiation doses for that particular type of CTO. The hybrid algorithm begins with dual-catheter injection and intravascular ultrasound aimed at determining whether four key anatomic features are present: an ambiguous proximal cap, a poor distal target, good interventional collaterals, and a major side branch at the distal cap.
If those four features are absent and the CTO lesion is less than 20-mm long, the algorithm dictates that the initial approach is antegrade wire escalation; if the lesion is 20 mm or more, the first approach is antegrade dissection reentry. However, if those anatomic features are present, the initial strategy is retrograde wire escalation if the lesion is less than 20 mm, and retrograde dissection reentry for longer lesions. When the initial approach fails, structured protocols dictate the selection of second and third approaches (JACC Cardiovasc Interv. 2012 Apr;5[4]:367-79).
The right coronary artery was the site of the CTO in 70% of study participants. Dual-catheter access was utilized in 79% of patients; only 10% had exclusively radial access. An average of 2.8 Synergy stents were deployed, with a whopping mean total stent length of 96.6 mm in the 48% of patients with dissection and 75.4 mm in those without. Mean procedure time was 122 minutes, with a fluoroscopy time of 44.6 minutes.
The primary efficacy endpoint was the rate of target vessel failure (TVF) at 12 months, defined as cardiac death, MI related to the target vessel, or any ischemia-driven revascularization of the target vessel. The rate was 5.24%.
“We were pleasantly surprised by that,” Dr. Walsh admitted.
Indeed, based upon studies of PCI for CTO published by other investigators, he and his colleagues had initially projected a 13% TVF rate, then bumped it up to 15% because of the high degree of lesion complexity.
Diabetes was the main predictor of target vessel revascularization.
At the time of the index PCI, 19% of patients were scheduled to return for an early optimization procedure within 3 months. This was arranged when operators anticipated significant positive remodeling would occur as the target vessel readjusted to blood flow or distal disease of borderline severity was noted beyond the CTO segment. These were not counted as adverse events. Half of the returnees underwent angiography and no further action was undertaken. The others underwent postdilation of their Synergy stents or new stenting of distal disease.
Complete revascularization of the target territory was achieved in 98.6% of patients. Key complications consisted of 5 perforations, 10 hematomas at vascular access sites, 2 cases of pericardiocentesis, and 4 instances of bleeding requiring transfusion.
The final successful CTO revascularization strategy was antegrade dissection reentry in 18% of patients, retrograde dissection reentry in 30%, antegrade wire escalation in 34%, and retrograde wire escalation in 18%. A switch from the initial algorithm-based strategy to a second strategy occurred in 41% of patients, and a third strategy was employed in 9%.
“I think the key message is you need to have more than one option to treat complicated disease. Half of patients had a switch in strategy,” Dr. Walsh observed.
Intravascular ultrasound (IVUS) adjudication in the central core lab showed a humbling rate of discordance between the operators’ impression of how their procedures were proceeding and what was really going on.
“There’s a bit of a mantra [that says] if you’re wiring stuff you’re always in the lumen, and if you’re using dissection techniques you’re always not in the lumen. In fact, that’s nonsense. You get it wrong one in six times. When you use IVUS adjudication to see if you’re outside the lumen or not, with wire-based retrograde escalation you’re out of the lumen and in the subintimal space 27% of the time. And with dissection strategies you’re wrong in about 15%,” according to the cardiologist.
He described the study participants as “extremely limited” at baseline as evidenced by their scores on the Seattle Angina Questionnaire Physical Limitation, Angina Stability, and Angina Frequency domains. At 12 months of follow-up, patients averaged 20- to 40-point improvements across all three domains.
One member of the discussion panel expressed a wish that the study had included a sham PCI arm. He raised the possibility that PCI had exerted an enormous placebo effect that could conceivably account for the substantial quality of life benefits documented in the study. But another panelist scoffed at this notion. This wasn’t a modest improvement in quality of life, nor was it measured after a mere 6 weeks, as was the case in the sham-controlled ORBITA trial.
“It’s really difficult to imagine a sham effect that persists out to a year,” he argued.
Dr. Walsh reported receiving research grants from and serving as a consultant to Boston Scientific, which funded the CONSISTENT CTO Study.
REPORTING FROM EUROPCR 2018
Key clinical point: The hybrid PCI strategy is now the preferred approach to treatment of CTOs.
Major finding: The 1-year target vessel failure rate following PCI for complex CTOs was 5.24%, with durable major quality of life improvements.
Study details: This prospective multicenter study included 210 patients with highly complex CTOs treated using Synergy stents according to the hybrid algorithm.
Disclosures: The presenter reported receiving research grants from and serving as a consultant to Boston Scientific, which sponsored the CONSISTENT CTO Study.
French warn of upsurge in pneumococcal meningitis
MALMO, SWEDEN – A French national study has documented a sharp increase in pneumococcal meningitis since 2015 in children under age 15 years.
The culprit has been identified as serotype 24F, which is not covered by the infant 13-valent conjugate pneumococcal vaccine (PCV13), Naim Ouldali, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The rapid emergence of serotype 24F has been accompanied by a disturbing change in its penicillin susceptibility. Indeed, penicillin resistance was present in only 18% of serotype 24F isolates in France during 2000-2014, then jumped to 74% during 2015-2016, according to Dr. Ouldali of René Descartes University in Paris.
“PCV13 has strongly reduced the pneumococcal meningitis burden in children, but its benefit now seems to be jeopardized, at least in France. So serum 24F could become a major concern in the coming years because of its characteristics. And now the question is, is this emergence an epidemic phenomenon or not? And if it’s confirmed in future studies and in other countries, probably it should drive the development of next-generation PCV formulations,” he said.
Dr. Ouldali presented a population-based interrupted time-series analysis of a nationwide prospective survey conducted in France during 2001-2016. He noted that the Cochrane Collaboration has deemed this study design second only to the randomized controlled trial in terms of quality of evidence.
The study, which included 227 French pediatric wards and 168 microbiology departments, identified 1,778 children under age 15 years with pneumococcal meningitis. This is believed to be more than 60% of all cases that occurred in the country during the study years.
The purpose of the study was to determine the impact of implementation of routine PCV13 as part of the national vaccine strategy. Rates of PCV13 coverage in French children are very high: in excess of 90% during 2015 to 2016.
Implementation of PCV13 led to a dramatic 38% reduction in the monthly incidence of pneumococcal meningitis, from 0.12 cases per 100,000 children before PCV13 to a low of 0.07 cases per 100,000 in December 2014. But after that the rate rebounded sharply, by 2.3% per month during 2015-2016, to a high of 0.13 cases per 100,000 per month by the end of 2016. Drilling down into the data, Dr. Ouldali and his coinvestigators learned that the resurgence of pneumococcal meningitis was due largely to the emergence of serotype 24F.
“This serotype is of particular concern because of two characteristics: First, it is already known to have a high disease potential – one of the highest, along with serotype 12F – and second, this rapid emergence was accompanied by a change in its penicillin susceptibility,” he noted.
Most of the French rebound in pneumococcal meningitis has occurred in children under 2 years of age. Of note, German investigators also have recently reported a rebound in invasive pneumococcal disease in German children under 16 years of age. Non-PCV13 serotypes accounted for 84% of all invasive pneumococcal disease during 2015-2016, with serotypes 10A and 24F leading the way. As in France, most of the resurgence has involved children less than 2 years old. However, unlike in France, most of the German increase has been in nonmeningitis forms of invasive pneumococcal disease (Vaccine. 2018 Jan 25;36[4]:572-7).
In response to a question from a concerned audience member, Dr. Ouldali said that while the penicillin susceptibility of serotype 24F has taken a sharp turn for the worse, cephalosporin susceptibility has not.
“To date, we have not seen any cephalosporin-resistant strains. To date, there is no need to use vancomycin,” he said.
Dr. Ouldali said the next step he and his colleagues plan to take is to see if there is a clonal expansion or a particular underlying genetic pattern which could explain the explosive emergence of 24F.
The study was funded by a research grant from Pfizer and by the French Pediatric Infectious Diseases Group.
MALMO, SWEDEN – A French national study has documented a sharp increase in pneumococcal meningitis since 2015 in children under age 15 years.
The culprit has been identified as serotype 24F, which is not covered by the infant 13-valent conjugate pneumococcal vaccine (PCV13), Naim Ouldali, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The rapid emergence of serotype 24F has been accompanied by a disturbing change in its penicillin susceptibility. Indeed, penicillin resistance was present in only 18% of serotype 24F isolates in France during 2000-2014, then jumped to 74% during 2015-2016, according to Dr. Ouldali of René Descartes University in Paris.
“PCV13 has strongly reduced the pneumococcal meningitis burden in children, but its benefit now seems to be jeopardized, at least in France. So serum 24F could become a major concern in the coming years because of its characteristics. And now the question is, is this emergence an epidemic phenomenon or not? And if it’s confirmed in future studies and in other countries, probably it should drive the development of next-generation PCV formulations,” he said.
Dr. Ouldali presented a population-based interrupted time-series analysis of a nationwide prospective survey conducted in France during 2001-2016. He noted that the Cochrane Collaboration has deemed this study design second only to the randomized controlled trial in terms of quality of evidence.
The study, which included 227 French pediatric wards and 168 microbiology departments, identified 1,778 children under age 15 years with pneumococcal meningitis. This is believed to be more than 60% of all cases that occurred in the country during the study years.
The purpose of the study was to determine the impact of implementation of routine PCV13 as part of the national vaccine strategy. Rates of PCV13 coverage in French children are very high: in excess of 90% during 2015 to 2016.
Implementation of PCV13 led to a dramatic 38% reduction in the monthly incidence of pneumococcal meningitis, from 0.12 cases per 100,000 children before PCV13 to a low of 0.07 cases per 100,000 in December 2014. But after that the rate rebounded sharply, by 2.3% per month during 2015-2016, to a high of 0.13 cases per 100,000 per month by the end of 2016. Drilling down into the data, Dr. Ouldali and his coinvestigators learned that the resurgence of pneumococcal meningitis was due largely to the emergence of serotype 24F.
“This serotype is of particular concern because of two characteristics: First, it is already known to have a high disease potential – one of the highest, along with serotype 12F – and second, this rapid emergence was accompanied by a change in its penicillin susceptibility,” he noted.
Most of the French rebound in pneumococcal meningitis has occurred in children under 2 years of age. Of note, German investigators also have recently reported a rebound in invasive pneumococcal disease in German children under 16 years of age. Non-PCV13 serotypes accounted for 84% of all invasive pneumococcal disease during 2015-2016, with serotypes 10A and 24F leading the way. As in France, most of the resurgence has involved children less than 2 years old. However, unlike in France, most of the German increase has been in nonmeningitis forms of invasive pneumococcal disease (Vaccine. 2018 Jan 25;36[4]:572-7).
In response to a question from a concerned audience member, Dr. Ouldali said that while the penicillin susceptibility of serotype 24F has taken a sharp turn for the worse, cephalosporin susceptibility has not.
“To date, we have not seen any cephalosporin-resistant strains. To date, there is no need to use vancomycin,” he said.
Dr. Ouldali said the next step he and his colleagues plan to take is to see if there is a clonal expansion or a particular underlying genetic pattern which could explain the explosive emergence of 24F.
The study was funded by a research grant from Pfizer and by the French Pediatric Infectious Diseases Group.
MALMO, SWEDEN – A French national study has documented a sharp increase in pneumococcal meningitis since 2015 in children under age 15 years.
The culprit has been identified as serotype 24F, which is not covered by the infant 13-valent conjugate pneumococcal vaccine (PCV13), Naim Ouldali, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The rapid emergence of serotype 24F has been accompanied by a disturbing change in its penicillin susceptibility. Indeed, penicillin resistance was present in only 18% of serotype 24F isolates in France during 2000-2014, then jumped to 74% during 2015-2016, according to Dr. Ouldali of René Descartes University in Paris.
“PCV13 has strongly reduced the pneumococcal meningitis burden in children, but its benefit now seems to be jeopardized, at least in France. So serum 24F could become a major concern in the coming years because of its characteristics. And now the question is, is this emergence an epidemic phenomenon or not? And if it’s confirmed in future studies and in other countries, probably it should drive the development of next-generation PCV formulations,” he said.
Dr. Ouldali presented a population-based interrupted time-series analysis of a nationwide prospective survey conducted in France during 2001-2016. He noted that the Cochrane Collaboration has deemed this study design second only to the randomized controlled trial in terms of quality of evidence.
The study, which included 227 French pediatric wards and 168 microbiology departments, identified 1,778 children under age 15 years with pneumococcal meningitis. This is believed to be more than 60% of all cases that occurred in the country during the study years.
The purpose of the study was to determine the impact of implementation of routine PCV13 as part of the national vaccine strategy. Rates of PCV13 coverage in French children are very high: in excess of 90% during 2015 to 2016.
Implementation of PCV13 led to a dramatic 38% reduction in the monthly incidence of pneumococcal meningitis, from 0.12 cases per 100,000 children before PCV13 to a low of 0.07 cases per 100,000 in December 2014. But after that the rate rebounded sharply, by 2.3% per month during 2015-2016, to a high of 0.13 cases per 100,000 per month by the end of 2016. Drilling down into the data, Dr. Ouldali and his coinvestigators learned that the resurgence of pneumococcal meningitis was due largely to the emergence of serotype 24F.
“This serotype is of particular concern because of two characteristics: First, it is already known to have a high disease potential – one of the highest, along with serotype 12F – and second, this rapid emergence was accompanied by a change in its penicillin susceptibility,” he noted.
Most of the French rebound in pneumococcal meningitis has occurred in children under 2 years of age. Of note, German investigators also have recently reported a rebound in invasive pneumococcal disease in German children under 16 years of age. Non-PCV13 serotypes accounted for 84% of all invasive pneumococcal disease during 2015-2016, with serotypes 10A and 24F leading the way. As in France, most of the resurgence has involved children less than 2 years old. However, unlike in France, most of the German increase has been in nonmeningitis forms of invasive pneumococcal disease (Vaccine. 2018 Jan 25;36[4]:572-7).
In response to a question from a concerned audience member, Dr. Ouldali said that while the penicillin susceptibility of serotype 24F has taken a sharp turn for the worse, cephalosporin susceptibility has not.
“To date, we have not seen any cephalosporin-resistant strains. To date, there is no need to use vancomycin,” he said.
Dr. Ouldali said the next step he and his colleagues plan to take is to see if there is a clonal expansion or a particular underlying genetic pattern which could explain the explosive emergence of 24F.
The study was funded by a research grant from Pfizer and by the French Pediatric Infectious Diseases Group.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: The incidence of pneumococcal meningitis in French children jumped by 2.3% per month during 2015-2016.
Study details: This population-based interrupted time-series analysis included all 1,778 cases of pneumococcal meningitis in children under age 15 years during 2001-2016 in 227 French pediatric wards.
Disclosures: The study was funded by a grant from Pfizer and by the French Pediatric Infectious Diseases Group.
Next-gen sputum PCR panel boosts CAP diagnostics
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point: A new CAP diagnostic panel represents a significant advance in clinical care.
Major finding: The investigational BioFire Pneumonia Panel identified specific pathogens in 100% of patients hospitalized for CAP, compared with 84% using the hospital’s standard test bundle.
Study details: This was a prospective head-to-head study comparing two approaches to identification of specific pathogens in 63 patients hospitalized for CAP.
Disclosures: The study was supported by BioFire Diagnostics. The presenter reported having no financial conflicts.
ACP roadmap to raise adult immunization rates
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
bjancin@mdedge.com
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
bjancin@mdedge.com
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
bjancin@mdedge.com
REPORTING FROM ACP INTERNAL MEDICINE
Sapien 3 performs well (mostly) in bicuspid aortic stenosis
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.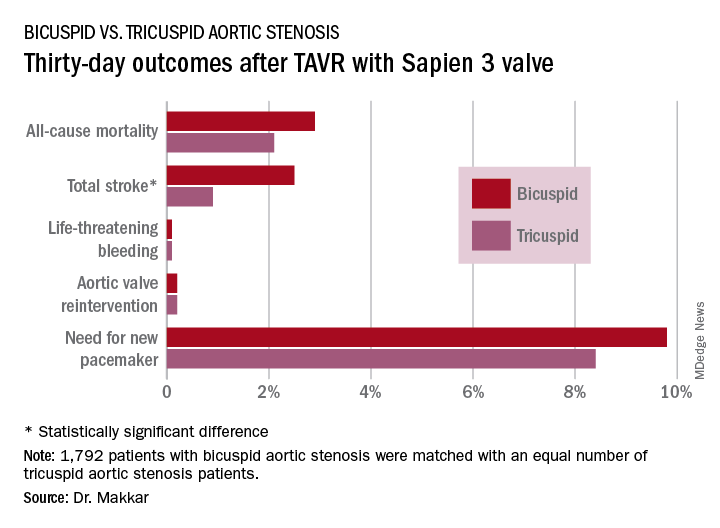
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
REPORTING FROM EUROPCR 2018
Key clinical point: Overall, outcomes were similarly favorable between patients with bicuspid aortic stenosis and those with tricuspid aortic stenosis.
Major finding: The 1-year all-cause mortality and total stroke rates in 1,792 TAVR patients who got the Sapien 3 valve for bicuspid aortic stenosis were 10.4% and 3.4%.
Study details: This was a propensity-matched comparison of TAVR outcomes using the Sapien 3 valve in 1,792 patients with bicuspid aortic stenosis and in an equal number with tricuspid aortic stenosis in the STS/ACC TVT Registry.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
What to expect from next-gen CGRP inhibitors for migraine, cluster headache
SAN FRANCISCO – Galcanezumab, the investigational calcitonin gene-related peptide inhibitor under development as preventive therapy for migraine, looks like a triple threat: Multiple phase 3 randomized trials have not only demonstrated safety and efficacy of the monoclonal antibody for prevention of both episodic and chronic migraine but for prevention of episodic cluster headache as well.
Galcanezumab’s demonstrated preventive benefit in patients with episodic cluster headache, if confirmed, would be a big deal. This type of headache is far less common than migraine, but often characterized as not merely debilitating but devastating. Phase 3 evidence of galcanezumab’s safety and efficacy for prevention of episodic cluster headache constitutes a unique advantage over fremanezumab and eptinezumab, the other two monoclonal antibodies directed against calcitonin gene-related peptide (CGRP) for which new phase 3 data were presented at the annual meeting of the American Headache Society. But the latter two novel agents showed advantages of their own.
While the efficacy of the CGRP inhibitors isn’t light-years ahead of the long-standard preventive therapies for migraine, such as topiramate, beta-blockers, antidepressants, and valproate, the new agents offer far greater tolerability and safety – side effect profiles are essentially the same as for placebo except for a modest increase in mild injection site reactions – along with documented substantial quality of life improvements using validated measures. And onset of benefit is far more rapid than with the long-time standard oral preventive medications.
Here are the highlights from the AHS annual meeting:
Galcanezumab
Sheena K. Aurora, MD, presented the results of the GCAL study, a phase 3 randomized, double-blind, 8-week study of subcutaneous galcanezumab at 300 mg once monthly or placebo administered for 2 months in 106 patients with ongoing episodic cluster headache attacks at baseline.
“This is really uncharted territory. The American Headache Society, in its 2016 cluster headache guidelines, reported that there was no high-quality evidence for prophylactic therapy,” noted Dr. Aurora of Eli Lilly in Indianapolis.
After the GCAL study, that’s no longer true. Study participants were a typical population of episodic cluster headache patients: that is, mostly middle-aged men. They averaged more than 17 cluster headache attacks per week during the baseline period, or 2-3 daily, with roughly a 16-year history of episodic cluster headaches, which typically occur in bursts lasting 6-8 weeks before temporarily fading. Of note, 14 of the 106 patients reported a history of prior suicidal ideation; while disturbing, that’s actually a lower rate than in some epidemiologic studies examining patients with this excruciating form of head pain.
The primary endpoint was the mean change in weekly cluster headache attack frequency during weeks 1-3. The difference was significant: a reduction of 8.7 attacks per week in the galcanezumab group, compared with 5.2 fewer in controls. The key secondary endpoint was the proportion of patients with at least a 50% reduction in weekly cluster attack frequency from baseline at week 3: This was achieved in 76% in the galcanezumab arm, versus 57% for placebo, again a significant difference.
At week 4, 73% of patients on galcanezumab rated themselves as very much or much better on the Patient Global Impression of Improvement, significantly greater than the 46% rate in controls. By week 8, this difference – while trending favorably – was no longer statistically significant, but it must be noted that by then 8 of the original 57 patients randomized to placebo had discontinued treatment because of the lack of efficacy, compared with just 1 of 49 in the galcanezumab group.
Adverse events in the two treatment arms were essentially the same as placebo except for an 8% rate of mild injection site reactions in the active treatment group.
Another double-blind, placebo-controlled phase 3 trial known as the CGAM study was conducted in 237 patients with chronic rather than episodic cluster headache. However, it proved negative for the same endpoints, Dr. Aurora said.
Separately, she presented a new post hoc analysis of the recently published EVOLVE-1 (JAMA Neurol. 2018 May 29. doi: 10.1001/jamaneurol.2018.1212) and EVOLVE-2 (Cephalalgia. 2018 Jul;38[8]:1442-54) trials. These two mirror-image pivotal phase 3, double-blind, placebo-controlled, 6-month studies of galcanezumab at 120 or 240 mg once monthly included a collective 1,773 episodic migraine patients with a baseline mean of 9.1 migraine headache days per month. Her analysis focused on the rapidity of onset of the humanized monoclonal antibody’s preventive effect.
“As most clinicians are aware, the current oral preventive treatments that we use require titration, and we don’t really see a good effect for maybe 2-3 months,” she observed.
In an ordinal logistic regression analysis, she and her coinvestigators first determined that the galcanezumab group separated from placebo in terms of reduced migraine headache days as early as 1 month in both studies. Next, they drilled down deeper into the participants’ daily headache diaries and determined that the onset of effect was seen at week 1, at which point the odds of a significant reduction in weekly migraine headache days were 2.71-fold greater with galcanezumab than placebo in EVOLVE-1 and 2.88-fold greater in EVOLVE-2.
“Patients would like to know how quickly they might see an effect, so the fact that we start seeing it for patients who’ve had migraine for 20 years with an average of 9.1 migraine headache days per month as early as week 1 is gratifying, even though the absolute numbers who benefit at that point are small,” Dr. Aurora said.
Fremanezumab
Fremanezumab is a fully humanized monoclonal antibody selectively targeting CGRP. Like galcanezumab and eptinezumab, it is now supported by phase 3 data from short-term, double-blind, placebo-controlled trials backed up by reassuring results from long-term, open-label studies.
During the 28 days prior to the start of the long-term, double-blind study, the chronic migraine patients averaged 16.4 migraine days, while the episodic migraine patients averaged 9.2. After 6 months on their assigned dosing regimen, the episodic migraine patients in the 225 mg monthly group had a mean 4.9 fewer monthly migraine days than at baseline, and the 675 mg quarterly group averaged 5.0 fewer days. The chronic migraine patients on 225 mg monthly had a mean 7.9-day reduction, while the 675 mg quarterly group averaged a 6.5-day reduction in monthly migraine days.
The key point in this interim analysis is that these clinically meaningful reductions in monthly number of migraine days at 6 months were of the same magnitude as at month 1, demonstrating solid maintenance of efficacy over time, noted Dr. Goadsby, professor of neurology at the University of California, San Francisco, and AHS president-elect.
Jessica Ailani, MD, presented evidence that fremanezumab is not only an effective migraine preventive therapy, it also shows potential as a medication for reversion from chronic migraine to episodic migraine.
She presented a post hoc analysis of the 1,130 chronic migraine patients who participated in the 12-week, placebo-controlled HALO CM trial. Thirty-two percent of them who were randomized to 675 mg of fremanezumab quarterly reverted from chronic to episodic migraine, meaning they had fewer than 15 headache days per month during all 3 months of the treatment period. So did 35% of patients who received 225 mg monthly. Both results were significantly better than the 23% placebo reversion rate, which is consistent with the effect of placebo in other studies.
The mean number of monthly headache days of at least moderate severity fell by 4.3 days from baseline in the fremanezumab quarterly group and by 4.6 with monthly therapy, significantly outperforming the 2.5-day reduction with placebo. And the patients who did well did very well indeed: those who reverted from chronic to episodic migraine went from a mean of 18-19 headache days per month at baseline to about 7 days during any month in the treatment period, she reported.
Two patients on 675 mg of fremanezumab quarterly developed antidrug antibodies.
Patients with MOH at baseline experienced a mean reduction of 4.7 headache days of at least moderate severity per month on fremanezumab at 675 mg quarterly and a 5.2-day reduction with monthly fremanezumab, both significantly better than the 2.5-day reduction with placebo. A total of 35% of the fremanezumab quarterly group achieved a 50% or greater reduction in the monthly average number of at least moderately severe headaches, as did 39% of patients on monthly fremanezumab and 14% on placebo.
Moreover, during the 12-week study period, 55% of patients with MOH at baseline who were on fremanezumab quarterly reported no medication overuse, as did 61% of those on 225 mg of fremanezumab once monthly and 46% of controls, according to Dr. Silberstein, professor of neurology and director of the headache center at Thomas Jefferson University in Philadelphia.
For the 1,034 participants who completed testing, the average baseline HIT-6 score was 64 points on a test that can range from 36-78, with higher scores indicating greater adverse impact. At repeat testing 4 weeks after the last dose, the quarterly dosing group showed a statistically significant and clinically meaningful 6.4-point reduction from baseline, and the monthly fremanezumab group had a 6.8-point reduction. Both of those outcomes were significantly better than the placebo group’s 4.5-point decrease, reported Dr. Winner, director of Palm Beach Neurology in West Palm Beach, Fla.
Those HIT-6 changes in fremanezumab-treated patients moved them from the severe disability category to moderate disability, he noted.
“We also evaluated other disability components in this study. The MSQ – the Migraine Specific Quality-of-Life Questionnaire – also showed significant improvement by patient assessment, and on a patient global assessment measure, over 50% of patients who got fremanezumab noted improvement in that 12-week study, getting back to work, functioning normally, etc.,” the neurologist added.
Eptinezumab
Unlike other anti-CGRP antibodies, eptinezumab is administered intravenously, once every 3 months, rather than by subcutaneous injection. This IgG1 anti-CGRP monoclonal antibody was engineered for reduced immune activation and a 30-day half-life. It’s onset of action is extremely rapid, with 100% of the agent being bioavailable within a few hours after administration.
The positive 12-week outcomes of the phase 3 randomized, double-blind, placebo-controlled PROMISE-2 trial of eptinezumab for prophylaxis against chronic migraine have previously been presented, as have the 1-year results in episodic migraine patients in PROMISE-1.
Of those on the higher dose of eptinezumab, 33% had at least a 75% reduction in monthly migraine days from baseline, a figure that climbed to 43% after the second infusion; and of those who received eptinezumab at the 300-mg dose, 61% experienced a 50% or greater reduction in monthly migraine days from baseline during weeks 1-12, as did 64% during weeks 13-24 following a second infusion.
A key secondary endpoint in PROMISE-2 was the proportion of patients having a migraine on any given day. During the 28-day baseline period, that figure was 58%. But the day after the first infusion that proportion dropped to 28% in both the eptinezumab 100- and 300-mg groups, and that rate held through the next 12 weeks. In contrast, roughly 45% of placebo-treated controls had a migraine on any given day during the 12 weeks.
“The key thing is on the day following infusion you have established pretty much the full preventive value of eptinezumab, and it is maintained stable all the way through 12 weeks,” according to Dr. Cady, vice president of neurology at Alder BioPharmaceuticals, which is developing the biologic.
When an audience member remarked on the placebo response rates in the CRGP inhibitor studies and indeed in clinical trials in migraine generally, Dr. Cady responded with one of the meeting’s more memorable comments: “If placebo brought you to the dance, you’re still at the party.”
Dr. Winner reported serving as a consultant to Alder BioPharmaceuticals, which sponsored the HALO trials and is developing eptinezumab, as well as to numerous other pharmaceutical companies. Similarly, Dr. Goadsby, Dr. Ailani, and Dr. Silberstein are consultants to many companies, including Teva Pharmaceuticals, which is developing fremanezumab.
SAN FRANCISCO – Galcanezumab, the investigational calcitonin gene-related peptide inhibitor under development as preventive therapy for migraine, looks like a triple threat: Multiple phase 3 randomized trials have not only demonstrated safety and efficacy of the monoclonal antibody for prevention of both episodic and chronic migraine but for prevention of episodic cluster headache as well.
Galcanezumab’s demonstrated preventive benefit in patients with episodic cluster headache, if confirmed, would be a big deal. This type of headache is far less common than migraine, but often characterized as not merely debilitating but devastating. Phase 3 evidence of galcanezumab’s safety and efficacy for prevention of episodic cluster headache constitutes a unique advantage over fremanezumab and eptinezumab, the other two monoclonal antibodies directed against calcitonin gene-related peptide (CGRP) for which new phase 3 data were presented at the annual meeting of the American Headache Society. But the latter two novel agents showed advantages of their own.
While the efficacy of the CGRP inhibitors isn’t light-years ahead of the long-standard preventive therapies for migraine, such as topiramate, beta-blockers, antidepressants, and valproate, the new agents offer far greater tolerability and safety – side effect profiles are essentially the same as for placebo except for a modest increase in mild injection site reactions – along with documented substantial quality of life improvements using validated measures. And onset of benefit is far more rapid than with the long-time standard oral preventive medications.
Here are the highlights from the AHS annual meeting:
Galcanezumab
Sheena K. Aurora, MD, presented the results of the GCAL study, a phase 3 randomized, double-blind, 8-week study of subcutaneous galcanezumab at 300 mg once monthly or placebo administered for 2 months in 106 patients with ongoing episodic cluster headache attacks at baseline.
“This is really uncharted territory. The American Headache Society, in its 2016 cluster headache guidelines, reported that there was no high-quality evidence for prophylactic therapy,” noted Dr. Aurora of Eli Lilly in Indianapolis.
After the GCAL study, that’s no longer true. Study participants were a typical population of episodic cluster headache patients: that is, mostly middle-aged men. They averaged more than 17 cluster headache attacks per week during the baseline period, or 2-3 daily, with roughly a 16-year history of episodic cluster headaches, which typically occur in bursts lasting 6-8 weeks before temporarily fading. Of note, 14 of the 106 patients reported a history of prior suicidal ideation; while disturbing, that’s actually a lower rate than in some epidemiologic studies examining patients with this excruciating form of head pain.
The primary endpoint was the mean change in weekly cluster headache attack frequency during weeks 1-3. The difference was significant: a reduction of 8.7 attacks per week in the galcanezumab group, compared with 5.2 fewer in controls. The key secondary endpoint was the proportion of patients with at least a 50% reduction in weekly cluster attack frequency from baseline at week 3: This was achieved in 76% in the galcanezumab arm, versus 57% for placebo, again a significant difference.
At week 4, 73% of patients on galcanezumab rated themselves as very much or much better on the Patient Global Impression of Improvement, significantly greater than the 46% rate in controls. By week 8, this difference – while trending favorably – was no longer statistically significant, but it must be noted that by then 8 of the original 57 patients randomized to placebo had discontinued treatment because of the lack of efficacy, compared with just 1 of 49 in the galcanezumab group.
Adverse events in the two treatment arms were essentially the same as placebo except for an 8% rate of mild injection site reactions in the active treatment group.
Another double-blind, placebo-controlled phase 3 trial known as the CGAM study was conducted in 237 patients with chronic rather than episodic cluster headache. However, it proved negative for the same endpoints, Dr. Aurora said.
Separately, she presented a new post hoc analysis of the recently published EVOLVE-1 (JAMA Neurol. 2018 May 29. doi: 10.1001/jamaneurol.2018.1212) and EVOLVE-2 (Cephalalgia. 2018 Jul;38[8]:1442-54) trials. These two mirror-image pivotal phase 3, double-blind, placebo-controlled, 6-month studies of galcanezumab at 120 or 240 mg once monthly included a collective 1,773 episodic migraine patients with a baseline mean of 9.1 migraine headache days per month. Her analysis focused on the rapidity of onset of the humanized monoclonal antibody’s preventive effect.
“As most clinicians are aware, the current oral preventive treatments that we use require titration, and we don’t really see a good effect for maybe 2-3 months,” she observed.
In an ordinal logistic regression analysis, she and her coinvestigators first determined that the galcanezumab group separated from placebo in terms of reduced migraine headache days as early as 1 month in both studies. Next, they drilled down deeper into the participants’ daily headache diaries and determined that the onset of effect was seen at week 1, at which point the odds of a significant reduction in weekly migraine headache days were 2.71-fold greater with galcanezumab than placebo in EVOLVE-1 and 2.88-fold greater in EVOLVE-2.
“Patients would like to know how quickly they might see an effect, so the fact that we start seeing it for patients who’ve had migraine for 20 years with an average of 9.1 migraine headache days per month as early as week 1 is gratifying, even though the absolute numbers who benefit at that point are small,” Dr. Aurora said.
Fremanezumab
Fremanezumab is a fully humanized monoclonal antibody selectively targeting CGRP. Like galcanezumab and eptinezumab, it is now supported by phase 3 data from short-term, double-blind, placebo-controlled trials backed up by reassuring results from long-term, open-label studies.
During the 28 days prior to the start of the long-term, double-blind study, the chronic migraine patients averaged 16.4 migraine days, while the episodic migraine patients averaged 9.2. After 6 months on their assigned dosing regimen, the episodic migraine patients in the 225 mg monthly group had a mean 4.9 fewer monthly migraine days than at baseline, and the 675 mg quarterly group averaged 5.0 fewer days. The chronic migraine patients on 225 mg monthly had a mean 7.9-day reduction, while the 675 mg quarterly group averaged a 6.5-day reduction in monthly migraine days.
The key point in this interim analysis is that these clinically meaningful reductions in monthly number of migraine days at 6 months were of the same magnitude as at month 1, demonstrating solid maintenance of efficacy over time, noted Dr. Goadsby, professor of neurology at the University of California, San Francisco, and AHS president-elect.
Jessica Ailani, MD, presented evidence that fremanezumab is not only an effective migraine preventive therapy, it also shows potential as a medication for reversion from chronic migraine to episodic migraine.
She presented a post hoc analysis of the 1,130 chronic migraine patients who participated in the 12-week, placebo-controlled HALO CM trial. Thirty-two percent of them who were randomized to 675 mg of fremanezumab quarterly reverted from chronic to episodic migraine, meaning they had fewer than 15 headache days per month during all 3 months of the treatment period. So did 35% of patients who received 225 mg monthly. Both results were significantly better than the 23% placebo reversion rate, which is consistent with the effect of placebo in other studies.
The mean number of monthly headache days of at least moderate severity fell by 4.3 days from baseline in the fremanezumab quarterly group and by 4.6 with monthly therapy, significantly outperforming the 2.5-day reduction with placebo. And the patients who did well did very well indeed: those who reverted from chronic to episodic migraine went from a mean of 18-19 headache days per month at baseline to about 7 days during any month in the treatment period, she reported.
Two patients on 675 mg of fremanezumab quarterly developed antidrug antibodies.
Patients with MOH at baseline experienced a mean reduction of 4.7 headache days of at least moderate severity per month on fremanezumab at 675 mg quarterly and a 5.2-day reduction with monthly fremanezumab, both significantly better than the 2.5-day reduction with placebo. A total of 35% of the fremanezumab quarterly group achieved a 50% or greater reduction in the monthly average number of at least moderately severe headaches, as did 39% of patients on monthly fremanezumab and 14% on placebo.
Moreover, during the 12-week study period, 55% of patients with MOH at baseline who were on fremanezumab quarterly reported no medication overuse, as did 61% of those on 225 mg of fremanezumab once monthly and 46% of controls, according to Dr. Silberstein, professor of neurology and director of the headache center at Thomas Jefferson University in Philadelphia.
For the 1,034 participants who completed testing, the average baseline HIT-6 score was 64 points on a test that can range from 36-78, with higher scores indicating greater adverse impact. At repeat testing 4 weeks after the last dose, the quarterly dosing group showed a statistically significant and clinically meaningful 6.4-point reduction from baseline, and the monthly fremanezumab group had a 6.8-point reduction. Both of those outcomes were significantly better than the placebo group’s 4.5-point decrease, reported Dr. Winner, director of Palm Beach Neurology in West Palm Beach, Fla.
Those HIT-6 changes in fremanezumab-treated patients moved them from the severe disability category to moderate disability, he noted.
“We also evaluated other disability components in this study. The MSQ – the Migraine Specific Quality-of-Life Questionnaire – also showed significant improvement by patient assessment, and on a patient global assessment measure, over 50% of patients who got fremanezumab noted improvement in that 12-week study, getting back to work, functioning normally, etc.,” the neurologist added.
Eptinezumab
Unlike other anti-CGRP antibodies, eptinezumab is administered intravenously, once every 3 months, rather than by subcutaneous injection. This IgG1 anti-CGRP monoclonal antibody was engineered for reduced immune activation and a 30-day half-life. It’s onset of action is extremely rapid, with 100% of the agent being bioavailable within a few hours after administration.
The positive 12-week outcomes of the phase 3 randomized, double-blind, placebo-controlled PROMISE-2 trial of eptinezumab for prophylaxis against chronic migraine have previously been presented, as have the 1-year results in episodic migraine patients in PROMISE-1.
Of those on the higher dose of eptinezumab, 33% had at least a 75% reduction in monthly migraine days from baseline, a figure that climbed to 43% after the second infusion; and of those who received eptinezumab at the 300-mg dose, 61% experienced a 50% or greater reduction in monthly migraine days from baseline during weeks 1-12, as did 64% during weeks 13-24 following a second infusion.
A key secondary endpoint in PROMISE-2 was the proportion of patients having a migraine on any given day. During the 28-day baseline period, that figure was 58%. But the day after the first infusion that proportion dropped to 28% in both the eptinezumab 100- and 300-mg groups, and that rate held through the next 12 weeks. In contrast, roughly 45% of placebo-treated controls had a migraine on any given day during the 12 weeks.
“The key thing is on the day following infusion you have established pretty much the full preventive value of eptinezumab, and it is maintained stable all the way through 12 weeks,” according to Dr. Cady, vice president of neurology at Alder BioPharmaceuticals, which is developing the biologic.
When an audience member remarked on the placebo response rates in the CRGP inhibitor studies and indeed in clinical trials in migraine generally, Dr. Cady responded with one of the meeting’s more memorable comments: “If placebo brought you to the dance, you’re still at the party.”
Dr. Winner reported serving as a consultant to Alder BioPharmaceuticals, which sponsored the HALO trials and is developing eptinezumab, as well as to numerous other pharmaceutical companies. Similarly, Dr. Goadsby, Dr. Ailani, and Dr. Silberstein are consultants to many companies, including Teva Pharmaceuticals, which is developing fremanezumab.
SAN FRANCISCO – Galcanezumab, the investigational calcitonin gene-related peptide inhibitor under development as preventive therapy for migraine, looks like a triple threat: Multiple phase 3 randomized trials have not only demonstrated safety and efficacy of the monoclonal antibody for prevention of both episodic and chronic migraine but for prevention of episodic cluster headache as well.
Galcanezumab’s demonstrated preventive benefit in patients with episodic cluster headache, if confirmed, would be a big deal. This type of headache is far less common than migraine, but often characterized as not merely debilitating but devastating. Phase 3 evidence of galcanezumab’s safety and efficacy for prevention of episodic cluster headache constitutes a unique advantage over fremanezumab and eptinezumab, the other two monoclonal antibodies directed against calcitonin gene-related peptide (CGRP) for which new phase 3 data were presented at the annual meeting of the American Headache Society. But the latter two novel agents showed advantages of their own.
While the efficacy of the CGRP inhibitors isn’t light-years ahead of the long-standard preventive therapies for migraine, such as topiramate, beta-blockers, antidepressants, and valproate, the new agents offer far greater tolerability and safety – side effect profiles are essentially the same as for placebo except for a modest increase in mild injection site reactions – along with documented substantial quality of life improvements using validated measures. And onset of benefit is far more rapid than with the long-time standard oral preventive medications.
Here are the highlights from the AHS annual meeting:
Galcanezumab
Sheena K. Aurora, MD, presented the results of the GCAL study, a phase 3 randomized, double-blind, 8-week study of subcutaneous galcanezumab at 300 mg once monthly or placebo administered for 2 months in 106 patients with ongoing episodic cluster headache attacks at baseline.
“This is really uncharted territory. The American Headache Society, in its 2016 cluster headache guidelines, reported that there was no high-quality evidence for prophylactic therapy,” noted Dr. Aurora of Eli Lilly in Indianapolis.
After the GCAL study, that’s no longer true. Study participants were a typical population of episodic cluster headache patients: that is, mostly middle-aged men. They averaged more than 17 cluster headache attacks per week during the baseline period, or 2-3 daily, with roughly a 16-year history of episodic cluster headaches, which typically occur in bursts lasting 6-8 weeks before temporarily fading. Of note, 14 of the 106 patients reported a history of prior suicidal ideation; while disturbing, that’s actually a lower rate than in some epidemiologic studies examining patients with this excruciating form of head pain.
The primary endpoint was the mean change in weekly cluster headache attack frequency during weeks 1-3. The difference was significant: a reduction of 8.7 attacks per week in the galcanezumab group, compared with 5.2 fewer in controls. The key secondary endpoint was the proportion of patients with at least a 50% reduction in weekly cluster attack frequency from baseline at week 3: This was achieved in 76% in the galcanezumab arm, versus 57% for placebo, again a significant difference.
At week 4, 73% of patients on galcanezumab rated themselves as very much or much better on the Patient Global Impression of Improvement, significantly greater than the 46% rate in controls. By week 8, this difference – while trending favorably – was no longer statistically significant, but it must be noted that by then 8 of the original 57 patients randomized to placebo had discontinued treatment because of the lack of efficacy, compared with just 1 of 49 in the galcanezumab group.
Adverse events in the two treatment arms were essentially the same as placebo except for an 8% rate of mild injection site reactions in the active treatment group.
Another double-blind, placebo-controlled phase 3 trial known as the CGAM study was conducted in 237 patients with chronic rather than episodic cluster headache. However, it proved negative for the same endpoints, Dr. Aurora said.
Separately, she presented a new post hoc analysis of the recently published EVOLVE-1 (JAMA Neurol. 2018 May 29. doi: 10.1001/jamaneurol.2018.1212) and EVOLVE-2 (Cephalalgia. 2018 Jul;38[8]:1442-54) trials. These two mirror-image pivotal phase 3, double-blind, placebo-controlled, 6-month studies of galcanezumab at 120 or 240 mg once monthly included a collective 1,773 episodic migraine patients with a baseline mean of 9.1 migraine headache days per month. Her analysis focused on the rapidity of onset of the humanized monoclonal antibody’s preventive effect.
“As most clinicians are aware, the current oral preventive treatments that we use require titration, and we don’t really see a good effect for maybe 2-3 months,” she observed.
In an ordinal logistic regression analysis, she and her coinvestigators first determined that the galcanezumab group separated from placebo in terms of reduced migraine headache days as early as 1 month in both studies. Next, they drilled down deeper into the participants’ daily headache diaries and determined that the onset of effect was seen at week 1, at which point the odds of a significant reduction in weekly migraine headache days were 2.71-fold greater with galcanezumab than placebo in EVOLVE-1 and 2.88-fold greater in EVOLVE-2.
“Patients would like to know how quickly they might see an effect, so the fact that we start seeing it for patients who’ve had migraine for 20 years with an average of 9.1 migraine headache days per month as early as week 1 is gratifying, even though the absolute numbers who benefit at that point are small,” Dr. Aurora said.
Fremanezumab
Fremanezumab is a fully humanized monoclonal antibody selectively targeting CGRP. Like galcanezumab and eptinezumab, it is now supported by phase 3 data from short-term, double-blind, placebo-controlled trials backed up by reassuring results from long-term, open-label studies.
During the 28 days prior to the start of the long-term, double-blind study, the chronic migraine patients averaged 16.4 migraine days, while the episodic migraine patients averaged 9.2. After 6 months on their assigned dosing regimen, the episodic migraine patients in the 225 mg monthly group had a mean 4.9 fewer monthly migraine days than at baseline, and the 675 mg quarterly group averaged 5.0 fewer days. The chronic migraine patients on 225 mg monthly had a mean 7.9-day reduction, while the 675 mg quarterly group averaged a 6.5-day reduction in monthly migraine days.
The key point in this interim analysis is that these clinically meaningful reductions in monthly number of migraine days at 6 months were of the same magnitude as at month 1, demonstrating solid maintenance of efficacy over time, noted Dr. Goadsby, professor of neurology at the University of California, San Francisco, and AHS president-elect.
Jessica Ailani, MD, presented evidence that fremanezumab is not only an effective migraine preventive therapy, it also shows potential as a medication for reversion from chronic migraine to episodic migraine.
She presented a post hoc analysis of the 1,130 chronic migraine patients who participated in the 12-week, placebo-controlled HALO CM trial. Thirty-two percent of them who were randomized to 675 mg of fremanezumab quarterly reverted from chronic to episodic migraine, meaning they had fewer than 15 headache days per month during all 3 months of the treatment period. So did 35% of patients who received 225 mg monthly. Both results were significantly better than the 23% placebo reversion rate, which is consistent with the effect of placebo in other studies.
The mean number of monthly headache days of at least moderate severity fell by 4.3 days from baseline in the fremanezumab quarterly group and by 4.6 with monthly therapy, significantly outperforming the 2.5-day reduction with placebo. And the patients who did well did very well indeed: those who reverted from chronic to episodic migraine went from a mean of 18-19 headache days per month at baseline to about 7 days during any month in the treatment period, she reported.
Two patients on 675 mg of fremanezumab quarterly developed antidrug antibodies.
Patients with MOH at baseline experienced a mean reduction of 4.7 headache days of at least moderate severity per month on fremanezumab at 675 mg quarterly and a 5.2-day reduction with monthly fremanezumab, both significantly better than the 2.5-day reduction with placebo. A total of 35% of the fremanezumab quarterly group achieved a 50% or greater reduction in the monthly average number of at least moderately severe headaches, as did 39% of patients on monthly fremanezumab and 14% on placebo.
Moreover, during the 12-week study period, 55% of patients with MOH at baseline who were on fremanezumab quarterly reported no medication overuse, as did 61% of those on 225 mg of fremanezumab once monthly and 46% of controls, according to Dr. Silberstein, professor of neurology and director of the headache center at Thomas Jefferson University in Philadelphia.
For the 1,034 participants who completed testing, the average baseline HIT-6 score was 64 points on a test that can range from 36-78, with higher scores indicating greater adverse impact. At repeat testing 4 weeks after the last dose, the quarterly dosing group showed a statistically significant and clinically meaningful 6.4-point reduction from baseline, and the monthly fremanezumab group had a 6.8-point reduction. Both of those outcomes were significantly better than the placebo group’s 4.5-point decrease, reported Dr. Winner, director of Palm Beach Neurology in West Palm Beach, Fla.
Those HIT-6 changes in fremanezumab-treated patients moved them from the severe disability category to moderate disability, he noted.
“We also evaluated other disability components in this study. The MSQ – the Migraine Specific Quality-of-Life Questionnaire – also showed significant improvement by patient assessment, and on a patient global assessment measure, over 50% of patients who got fremanezumab noted improvement in that 12-week study, getting back to work, functioning normally, etc.,” the neurologist added.
Eptinezumab
Unlike other anti-CGRP antibodies, eptinezumab is administered intravenously, once every 3 months, rather than by subcutaneous injection. This IgG1 anti-CGRP monoclonal antibody was engineered for reduced immune activation and a 30-day half-life. It’s onset of action is extremely rapid, with 100% of the agent being bioavailable within a few hours after administration.
The positive 12-week outcomes of the phase 3 randomized, double-blind, placebo-controlled PROMISE-2 trial of eptinezumab for prophylaxis against chronic migraine have previously been presented, as have the 1-year results in episodic migraine patients in PROMISE-1.
Of those on the higher dose of eptinezumab, 33% had at least a 75% reduction in monthly migraine days from baseline, a figure that climbed to 43% after the second infusion; and of those who received eptinezumab at the 300-mg dose, 61% experienced a 50% or greater reduction in monthly migraine days from baseline during weeks 1-12, as did 64% during weeks 13-24 following a second infusion.
A key secondary endpoint in PROMISE-2 was the proportion of patients having a migraine on any given day. During the 28-day baseline period, that figure was 58%. But the day after the first infusion that proportion dropped to 28% in both the eptinezumab 100- and 300-mg groups, and that rate held through the next 12 weeks. In contrast, roughly 45% of placebo-treated controls had a migraine on any given day during the 12 weeks.
“The key thing is on the day following infusion you have established pretty much the full preventive value of eptinezumab, and it is maintained stable all the way through 12 weeks,” according to Dr. Cady, vice president of neurology at Alder BioPharmaceuticals, which is developing the biologic.
When an audience member remarked on the placebo response rates in the CRGP inhibitor studies and indeed in clinical trials in migraine generally, Dr. Cady responded with one of the meeting’s more memorable comments: “If placebo brought you to the dance, you’re still at the party.”
Dr. Winner reported serving as a consultant to Alder BioPharmaceuticals, which sponsored the HALO trials and is developing eptinezumab, as well as to numerous other pharmaceutical companies. Similarly, Dr. Goadsby, Dr. Ailani, and Dr. Silberstein are consultants to many companies, including Teva Pharmaceuticals, which is developing fremanezumab.
REPORTING FROM THE AHS ANNUAL MEETING
Four syndromes suggest life-threatening PVL-positive S. aureus infection
MALMO, SWEDEN – Methicillin-resistant Staphylococcus aureus gets the blame in the Americas as the main cause of a great wave of community-acquired severe invasive staphylococcal infections in children and adolescents during the past nearly 2 decades, but many European pediatric infectious disease specialists believe that Panton-Valentine leukocidin (PVL), a frequent co-traveler with MRSA, is the true bad actor.
“The American literature focused first on MRSA, but we’ve seen very similar, very severe cases with MSSA [methicillin-susceptible S. aureus] PVL-positive and MRSA PVL-positive infections,” Pablo Rojo, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“It is only because at the beginning there were so many MRSA cases in the States that they thought that was the driver of the disease. It is still unclear. There is still a discussion. But I wanted to bring you my opinion and that of many other authors that it’s mostly PVL-associated,” added Dr. Rojo of Complutense University in Madrid.
He was senior author of a multinational European and Israeli prospective study of risk factors associated with the severity of invasive community-acquired S. aureus infections in children, with invasive infection being defined as hospitalization for an infection with S. aureus isolated from a normally sterile body site such as blood, bone, or cerebrospinal fluid, or S. aureus pneumonia. They identified 152 affected children, 17% of whom had severe community-acquired invasive S. aureus, defined by death or admission to a pediatric intensive care unit due to respiratory failure or hemodynamic instability.
The prevalence of PVL-positive S. aureus infection in the overall invasive infection group was 19%, while 8% of the isolates were MRSA. while MRSA was not associated with a significantly increased risk. The other independent risk factors for severe outcome were pneumonia, with an adjusted 13-fold increased risk, and leukopenia at admission, with an associated 18-fold risk (Clin Microbiol Infect. 2016 Jul;22[7]:643.e1-6.).
Of note, the virulence of PVL stems from the pore-forming toxin’s ability to lyse white blood cells. Because a leukocyte count is always available once a patient reaches the ED, severe leukopenia as defined by a count of less than 3,000 cells/mm3 at admission becomes a useful early marker of the likely severity of any case of S. aureus invasive disease, according to Dr. Rojo.
He highlighted four key syndromes involving severe invasive S. aureus infection in previously healthy children and adolescents that entail a high likelihood of being PVL positive and should cause physicians to run – not walk – to start appropriate empiric therapy. He also described the treatment regimen that he and other European thought leaders recommend for severe PVL-positive S. aureus invasive infections.
The microbiologic diagnosis of PVL can be made by ELISA (enzyme-linked immunoassay) to detect the toxin in an S. aureus isolate, by a rapid monoclonal antibody test, or by polymerase chain reaction to detect PVL genes in an S. aureus isolate. But don’t wait for test results to initiate treatment because these are high-mortality syndromes, he advised.
“Many people tell me, ‘My lab doesn’t have a way to diagnose PVL.’ And it’s true, it’s not available in real life at many hospitals. My message to you is that you don’t need to wait for a microbiological diagnosis or the results to come back from a sample you have sent to the reference lab in the main referral center. We can base our diagnosis and decision to treat on clinical grounds if we focus on these four very uncommon syndromes involving invasive S. aureus infection. I think if you have any child with these symptoms you have to manage them on the assumption that PVL is present,” said Dr. Rojo, principal investigator of the European Project on Invasive S. aureus Pediatric Infections.
The four key syndromes
The four syndromes are severe S. aureus pneumonia, S. aureus bone and joint infections with multiple foci, S. aureus osteomyelitis complicated by deep vein thrombosis, and invasive S. aureus infection plus shock.
- Severe S. aureus pneumonia. Investigators at Claude Bernard University in Lyon, France, have done extensive pioneering work on severe PVL-positive S. aureus invasive infections in children. In an early paper, they highlighted the characteristics that distinguish severe PVL-positive pneumonia: it typically occurs in previously healthy children and adolescents without underlying comorbid conditions, and it is often preceded by a influenza-like syndrome followed by an acute severe pneumonia with hemoptysis. Mortality was very high in this early series, with nearly half of the patients being dead within the first several days after admission (Lancet. 2002 Mar 2;359[9308]:753-9).
- Severe osteomyelitis. Investigators at Baylor College of Medicine, Houston, were among the first to observe that osteomyelitis caused by PVL-positive strains of S. aureus are associated with more severe local disease, with multiple affected areas, bigger abscesses, a greater systemic inflammatory response, and more surgeries required compared with osteomyelitis caused by PVL-negative S. aureus (Pediatrics. 2006 Feb;117[2]:433-40).
- Osteomyelitis with deep vein thrombosis. When a child hospitalized for acute hematogenous osteomyelitis due to S. aureus develops difficulty breathing, that’s a red flag for a severe PVL-positive infection involving deep vein thrombosis. Indeed, investigators at the Leeds (England) General Infirmary have reported that deep vein thrombosis in the setting of S. aureus osteomyelitis is associated with a greater than eightfold increased likelihood of a PVL-positive infection (Br J Hosp Med [Lond]. 2015 Jan;76[1]:18-24). Also, patients with PVL-positive osteomyelitis and deep vein thrombosis are prone to formation of septic emboli.
- Osteomyelitis with septic shock. The Lyon group compared outcomes in 14 pediatric patients with PVL-positive S. aureus osteomyelitis and a control group of 17 patients with PVL-negative disease. All 14 PVL-positive patients had severe sepsis and 6 of them had septic shock. In contrast, none of the controls did. Median duration of hospitalization was 46 days in the PVL-positive group, compared with 13 days in controls (Pediatr Infect Dis J. 2007 Nov;26[11]:1042-8).
Treatment
No randomized trials exist to guide treatment, but Dr. Rojo recommends the protocol utilized by the Lyon group: a bactericidal antibiotic – vancomycin or a beta-lactam – to take on the S. aureus, coupled with a ribosomally active antibiotic – clindamycin or linezolid – to suppress the PVL toxin’s virulence expression. The French group cites both in vitro and in vivo evidence that clindamycin and linezolid in their standard dosing have such an antitoxin effect (Clin Microbiol Rev. 2017 Oct;30[4]:887-917).
In addition, Dr. Rojo recommends utilizing any of the commercially available intravenous immunoglobulin (IVIG) products on the basis of work by investigators at Vanderbilt University in Nashville, Tenn., who have demonstrated that these products contain functional neutralizing antibodies against S. aureus leukocidins. This observation provides a likely explanation for anecdotal reports of improved outcomes in IVIG-treated patients with toxin-associated staphylococcal disease (Antimicrob Agents Chemother. 2017 Oct 24;61[11]. pii: e00968-17).
Challenged as to when specifically he would use IVIG in light of the global shortage of immunoglobulins, Dr. Rojo replied: “Not in every invasive S. aureus infection, but in serious infections that are PVL positive. I think if you have a child with one of these four syndromes who is in a pediatric ICU, you should use it. I mean, the mortality is around 30% in healthy children, so you would not stop from giving it. The risk of giving IVIG is very low, no side effects, so I highly recommend it for these severe cases.”
He reported having no financial conflicts.
MALMO, SWEDEN – Methicillin-resistant Staphylococcus aureus gets the blame in the Americas as the main cause of a great wave of community-acquired severe invasive staphylococcal infections in children and adolescents during the past nearly 2 decades, but many European pediatric infectious disease specialists believe that Panton-Valentine leukocidin (PVL), a frequent co-traveler with MRSA, is the true bad actor.
“The American literature focused first on MRSA, but we’ve seen very similar, very severe cases with MSSA [methicillin-susceptible S. aureus] PVL-positive and MRSA PVL-positive infections,” Pablo Rojo, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“It is only because at the beginning there were so many MRSA cases in the States that they thought that was the driver of the disease. It is still unclear. There is still a discussion. But I wanted to bring you my opinion and that of many other authors that it’s mostly PVL-associated,” added Dr. Rojo of Complutense University in Madrid.
He was senior author of a multinational European and Israeli prospective study of risk factors associated with the severity of invasive community-acquired S. aureus infections in children, with invasive infection being defined as hospitalization for an infection with S. aureus isolated from a normally sterile body site such as blood, bone, or cerebrospinal fluid, or S. aureus pneumonia. They identified 152 affected children, 17% of whom had severe community-acquired invasive S. aureus, defined by death or admission to a pediatric intensive care unit due to respiratory failure or hemodynamic instability.
The prevalence of PVL-positive S. aureus infection in the overall invasive infection group was 19%, while 8% of the isolates were MRSA. while MRSA was not associated with a significantly increased risk. The other independent risk factors for severe outcome were pneumonia, with an adjusted 13-fold increased risk, and leukopenia at admission, with an associated 18-fold risk (Clin Microbiol Infect. 2016 Jul;22[7]:643.e1-6.).
Of note, the virulence of PVL stems from the pore-forming toxin’s ability to lyse white blood cells. Because a leukocyte count is always available once a patient reaches the ED, severe leukopenia as defined by a count of less than 3,000 cells/mm3 at admission becomes a useful early marker of the likely severity of any case of S. aureus invasive disease, according to Dr. Rojo.
He highlighted four key syndromes involving severe invasive S. aureus infection in previously healthy children and adolescents that entail a high likelihood of being PVL positive and should cause physicians to run – not walk – to start appropriate empiric therapy. He also described the treatment regimen that he and other European thought leaders recommend for severe PVL-positive S. aureus invasive infections.
The microbiologic diagnosis of PVL can be made by ELISA (enzyme-linked immunoassay) to detect the toxin in an S. aureus isolate, by a rapid monoclonal antibody test, or by polymerase chain reaction to detect PVL genes in an S. aureus isolate. But don’t wait for test results to initiate treatment because these are high-mortality syndromes, he advised.
“Many people tell me, ‘My lab doesn’t have a way to diagnose PVL.’ And it’s true, it’s not available in real life at many hospitals. My message to you is that you don’t need to wait for a microbiological diagnosis or the results to come back from a sample you have sent to the reference lab in the main referral center. We can base our diagnosis and decision to treat on clinical grounds if we focus on these four very uncommon syndromes involving invasive S. aureus infection. I think if you have any child with these symptoms you have to manage them on the assumption that PVL is present,” said Dr. Rojo, principal investigator of the European Project on Invasive S. aureus Pediatric Infections.
The four key syndromes
The four syndromes are severe S. aureus pneumonia, S. aureus bone and joint infections with multiple foci, S. aureus osteomyelitis complicated by deep vein thrombosis, and invasive S. aureus infection plus shock.
- Severe S. aureus pneumonia. Investigators at Claude Bernard University in Lyon, France, have done extensive pioneering work on severe PVL-positive S. aureus invasive infections in children. In an early paper, they highlighted the characteristics that distinguish severe PVL-positive pneumonia: it typically occurs in previously healthy children and adolescents without underlying comorbid conditions, and it is often preceded by a influenza-like syndrome followed by an acute severe pneumonia with hemoptysis. Mortality was very high in this early series, with nearly half of the patients being dead within the first several days after admission (Lancet. 2002 Mar 2;359[9308]:753-9).
- Severe osteomyelitis. Investigators at Baylor College of Medicine, Houston, were among the first to observe that osteomyelitis caused by PVL-positive strains of S. aureus are associated with more severe local disease, with multiple affected areas, bigger abscesses, a greater systemic inflammatory response, and more surgeries required compared with osteomyelitis caused by PVL-negative S. aureus (Pediatrics. 2006 Feb;117[2]:433-40).
- Osteomyelitis with deep vein thrombosis. When a child hospitalized for acute hematogenous osteomyelitis due to S. aureus develops difficulty breathing, that’s a red flag for a severe PVL-positive infection involving deep vein thrombosis. Indeed, investigators at the Leeds (England) General Infirmary have reported that deep vein thrombosis in the setting of S. aureus osteomyelitis is associated with a greater than eightfold increased likelihood of a PVL-positive infection (Br J Hosp Med [Lond]. 2015 Jan;76[1]:18-24). Also, patients with PVL-positive osteomyelitis and deep vein thrombosis are prone to formation of septic emboli.
- Osteomyelitis with septic shock. The Lyon group compared outcomes in 14 pediatric patients with PVL-positive S. aureus osteomyelitis and a control group of 17 patients with PVL-negative disease. All 14 PVL-positive patients had severe sepsis and 6 of them had septic shock. In contrast, none of the controls did. Median duration of hospitalization was 46 days in the PVL-positive group, compared with 13 days in controls (Pediatr Infect Dis J. 2007 Nov;26[11]:1042-8).
Treatment
No randomized trials exist to guide treatment, but Dr. Rojo recommends the protocol utilized by the Lyon group: a bactericidal antibiotic – vancomycin or a beta-lactam – to take on the S. aureus, coupled with a ribosomally active antibiotic – clindamycin or linezolid – to suppress the PVL toxin’s virulence expression. The French group cites both in vitro and in vivo evidence that clindamycin and linezolid in their standard dosing have such an antitoxin effect (Clin Microbiol Rev. 2017 Oct;30[4]:887-917).
In addition, Dr. Rojo recommends utilizing any of the commercially available intravenous immunoglobulin (IVIG) products on the basis of work by investigators at Vanderbilt University in Nashville, Tenn., who have demonstrated that these products contain functional neutralizing antibodies against S. aureus leukocidins. This observation provides a likely explanation for anecdotal reports of improved outcomes in IVIG-treated patients with toxin-associated staphylococcal disease (Antimicrob Agents Chemother. 2017 Oct 24;61[11]. pii: e00968-17).
Challenged as to when specifically he would use IVIG in light of the global shortage of immunoglobulins, Dr. Rojo replied: “Not in every invasive S. aureus infection, but in serious infections that are PVL positive. I think if you have a child with one of these four syndromes who is in a pediatric ICU, you should use it. I mean, the mortality is around 30% in healthy children, so you would not stop from giving it. The risk of giving IVIG is very low, no side effects, so I highly recommend it for these severe cases.”
He reported having no financial conflicts.
MALMO, SWEDEN – Methicillin-resistant Staphylococcus aureus gets the blame in the Americas as the main cause of a great wave of community-acquired severe invasive staphylococcal infections in children and adolescents during the past nearly 2 decades, but many European pediatric infectious disease specialists believe that Panton-Valentine leukocidin (PVL), a frequent co-traveler with MRSA, is the true bad actor.
“The American literature focused first on MRSA, but we’ve seen very similar, very severe cases with MSSA [methicillin-susceptible S. aureus] PVL-positive and MRSA PVL-positive infections,” Pablo Rojo, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“It is only because at the beginning there were so many MRSA cases in the States that they thought that was the driver of the disease. It is still unclear. There is still a discussion. But I wanted to bring you my opinion and that of many other authors that it’s mostly PVL-associated,” added Dr. Rojo of Complutense University in Madrid.
He was senior author of a multinational European and Israeli prospective study of risk factors associated with the severity of invasive community-acquired S. aureus infections in children, with invasive infection being defined as hospitalization for an infection with S. aureus isolated from a normally sterile body site such as blood, bone, or cerebrospinal fluid, or S. aureus pneumonia. They identified 152 affected children, 17% of whom had severe community-acquired invasive S. aureus, defined by death or admission to a pediatric intensive care unit due to respiratory failure or hemodynamic instability.
The prevalence of PVL-positive S. aureus infection in the overall invasive infection group was 19%, while 8% of the isolates were MRSA. while MRSA was not associated with a significantly increased risk. The other independent risk factors for severe outcome were pneumonia, with an adjusted 13-fold increased risk, and leukopenia at admission, with an associated 18-fold risk (Clin Microbiol Infect. 2016 Jul;22[7]:643.e1-6.).
Of note, the virulence of PVL stems from the pore-forming toxin’s ability to lyse white blood cells. Because a leukocyte count is always available once a patient reaches the ED, severe leukopenia as defined by a count of less than 3,000 cells/mm3 at admission becomes a useful early marker of the likely severity of any case of S. aureus invasive disease, according to Dr. Rojo.
He highlighted four key syndromes involving severe invasive S. aureus infection in previously healthy children and adolescents that entail a high likelihood of being PVL positive and should cause physicians to run – not walk – to start appropriate empiric therapy. He also described the treatment regimen that he and other European thought leaders recommend for severe PVL-positive S. aureus invasive infections.
The microbiologic diagnosis of PVL can be made by ELISA (enzyme-linked immunoassay) to detect the toxin in an S. aureus isolate, by a rapid monoclonal antibody test, or by polymerase chain reaction to detect PVL genes in an S. aureus isolate. But don’t wait for test results to initiate treatment because these are high-mortality syndromes, he advised.
“Many people tell me, ‘My lab doesn’t have a way to diagnose PVL.’ And it’s true, it’s not available in real life at many hospitals. My message to you is that you don’t need to wait for a microbiological diagnosis or the results to come back from a sample you have sent to the reference lab in the main referral center. We can base our diagnosis and decision to treat on clinical grounds if we focus on these four very uncommon syndromes involving invasive S. aureus infection. I think if you have any child with these symptoms you have to manage them on the assumption that PVL is present,” said Dr. Rojo, principal investigator of the European Project on Invasive S. aureus Pediatric Infections.
The four key syndromes
The four syndromes are severe S. aureus pneumonia, S. aureus bone and joint infections with multiple foci, S. aureus osteomyelitis complicated by deep vein thrombosis, and invasive S. aureus infection plus shock.
- Severe S. aureus pneumonia. Investigators at Claude Bernard University in Lyon, France, have done extensive pioneering work on severe PVL-positive S. aureus invasive infections in children. In an early paper, they highlighted the characteristics that distinguish severe PVL-positive pneumonia: it typically occurs in previously healthy children and adolescents without underlying comorbid conditions, and it is often preceded by a influenza-like syndrome followed by an acute severe pneumonia with hemoptysis. Mortality was very high in this early series, with nearly half of the patients being dead within the first several days after admission (Lancet. 2002 Mar 2;359[9308]:753-9).
- Severe osteomyelitis. Investigators at Baylor College of Medicine, Houston, were among the first to observe that osteomyelitis caused by PVL-positive strains of S. aureus are associated with more severe local disease, with multiple affected areas, bigger abscesses, a greater systemic inflammatory response, and more surgeries required compared with osteomyelitis caused by PVL-negative S. aureus (Pediatrics. 2006 Feb;117[2]:433-40).
- Osteomyelitis with deep vein thrombosis. When a child hospitalized for acute hematogenous osteomyelitis due to S. aureus develops difficulty breathing, that’s a red flag for a severe PVL-positive infection involving deep vein thrombosis. Indeed, investigators at the Leeds (England) General Infirmary have reported that deep vein thrombosis in the setting of S. aureus osteomyelitis is associated with a greater than eightfold increased likelihood of a PVL-positive infection (Br J Hosp Med [Lond]. 2015 Jan;76[1]:18-24). Also, patients with PVL-positive osteomyelitis and deep vein thrombosis are prone to formation of septic emboli.
- Osteomyelitis with septic shock. The Lyon group compared outcomes in 14 pediatric patients with PVL-positive S. aureus osteomyelitis and a control group of 17 patients with PVL-negative disease. All 14 PVL-positive patients had severe sepsis and 6 of them had septic shock. In contrast, none of the controls did. Median duration of hospitalization was 46 days in the PVL-positive group, compared with 13 days in controls (Pediatr Infect Dis J. 2007 Nov;26[11]:1042-8).
Treatment
No randomized trials exist to guide treatment, but Dr. Rojo recommends the protocol utilized by the Lyon group: a bactericidal antibiotic – vancomycin or a beta-lactam – to take on the S. aureus, coupled with a ribosomally active antibiotic – clindamycin or linezolid – to suppress the PVL toxin’s virulence expression. The French group cites both in vitro and in vivo evidence that clindamycin and linezolid in their standard dosing have such an antitoxin effect (Clin Microbiol Rev. 2017 Oct;30[4]:887-917).
In addition, Dr. Rojo recommends utilizing any of the commercially available intravenous immunoglobulin (IVIG) products on the basis of work by investigators at Vanderbilt University in Nashville, Tenn., who have demonstrated that these products contain functional neutralizing antibodies against S. aureus leukocidins. This observation provides a likely explanation for anecdotal reports of improved outcomes in IVIG-treated patients with toxin-associated staphylococcal disease (Antimicrob Agents Chemother. 2017 Oct 24;61[11]. pii: e00968-17).
Challenged as to when specifically he would use IVIG in light of the global shortage of immunoglobulins, Dr. Rojo replied: “Not in every invasive S. aureus infection, but in serious infections that are PVL positive. I think if you have a child with one of these four syndromes who is in a pediatric ICU, you should use it. I mean, the mortality is around 30% in healthy children, so you would not stop from giving it. The risk of giving IVIG is very low, no side effects, so I highly recommend it for these severe cases.”
He reported having no financial conflicts.
EXPERT ANALYSIS FROM ESPID 2018