User login
Avoiding in-hospital acute kidney injury is a new imperative
NEW ORLEANS– Preventing acute kidney injury and its progression in hospitalized patients deserves to be a high priority – and now there is finally proof that it’s doable, Harold M. Szerlip, MD, declared at the annual meeting of the American College of Physicians.
The PrevAKI study, a recent randomized controlled clinical trial conducted by German investigators, has demonstrated that the use of renal biomarkers to identify patients at high risk for acute kidney injury (AKI) after major cardiac surgery and providing them with a range of internationally recommended supportive measures known as the KDIGO (Kidney Disease: Improving Global Outcomes) care bundle reduced the occurrence of moderate-to-severe AKI by 34% (Intensive Care Med. 2017 Nov;43[11]:1551-61).
The enthusiasm that greeted the PrevAKI trial findings is reflected in an editorial entitled, “AKI: the Myth of Inevitability is Finally Shattered,” by John A. Kellum, MD, professor of critical care medicine and director of the Center for Critical Care Nephrology at the University of Pittsburgh. Dr. Kellum noted that the renal biomarker-based approach to implementation of the KDIGO care bundle resulted in an attractively low number needed to treat (NNT) of only 6, whereas without biomarker-based enrichment of the target population, the NNT would have been more than 33.
“,” Dr. Kellum declared in the editorial (Nat Rev Nephrol. 2017 Mar;13[3]:140-1).
Indeed, another way to do it was recently demonstrated in the SALT-ED trial, in which 13,347 noncritically ill hospitalized patients requiring intravenous fluid administration were randomized to conventional saline or balanced crystalloids. The incidence of AKI and other major adverse kidney events was 4.7% in the balanced crystalloids group, for a significant 18% risk reduction relative to the 5.6% rate with saline (N Engl J Med. 2018 Mar 1;378[9]:819-28).
While that absolute 0.9% risk reduction might initially not sound like much, with 35 million people per year getting IV saline while in the hospital, it translates into 315,000 fewer major adverse kidney events as a result of a simple switch to balanced crystalloids, Dr. Szerlip observed.
The PrevAKI findings validate the concept of AKI ‘golden hours’ during which time potentially reversible early kidney injury detectable via renal biomarkers is occurring prior to the abrupt decline in kidney function measured by change in serum creatinine. “The problem with using change in creatinine to define AKI is the delay in diagnosis, which makes AKI more difficult to treat,” he explained.
The renal biomarkers utilized in PrevAKI were insulin-like growth factor binding protein-7 (IGFBP7) and tissue inhibitor of metalloproteinase-2 (TIMP-2), as incorporated in the commercially available urinary NephroCheck test, which was administered to study participants 4 hours after cardiopulmonary bypass. A test result of 0.3 or more identified a group at high risk for AKI for randomization to the KDIGO bundle or usual care. The KDIGO bundle consists of discontinuation of nephrotoxic agents when feasible, early optimization of fluid status, and maintenance of perfusion pressure.
Patients known to be at increased risk for in-hospital AKI include the elderly, those with diabetes, patients with heart failure or other conditions prone to volume contraction or overload, those undergoing major surgery, individuals with chronic kidney disease, and patients with sepsis.
Dr. Szerlip singled out as particularly nephrotoxic several drugs widely used in hospitalized patients, including the combination of vancomycin plus piperacillin-tazobactam, which in a recent metaanalysis was found to have a number needed to harm of 11 in terms of AKI in comparison to vancomycin monotherapy or vancomycin in combination with cefepime or carbapenem (Crit Care Med. 2018 Jan;46[1]:12-20). He was also critical of the American Society of Anesthesiologists practice parameter recommending that in-hospital pain management plans for surgical patients include continuous regimens of NSAIDs or COX-2 inhibitors as a means of combating the ongoing opioid epidemic.
“These are highly toxic drugs to the kidney and we shouldn’t be using them,” Dr. Szerlip said.
He reported receiving research grants from LaJolla, Bayer, Akebia, and BioPorto, serving on a speakers’ bureau for Astute Medical, and acting as a consultant to Zs Pharma, Amarin, and LaJolla.
NEW ORLEANS– Preventing acute kidney injury and its progression in hospitalized patients deserves to be a high priority – and now there is finally proof that it’s doable, Harold M. Szerlip, MD, declared at the annual meeting of the American College of Physicians.
The PrevAKI study, a recent randomized controlled clinical trial conducted by German investigators, has demonstrated that the use of renal biomarkers to identify patients at high risk for acute kidney injury (AKI) after major cardiac surgery and providing them with a range of internationally recommended supportive measures known as the KDIGO (Kidney Disease: Improving Global Outcomes) care bundle reduced the occurrence of moderate-to-severe AKI by 34% (Intensive Care Med. 2017 Nov;43[11]:1551-61).
The enthusiasm that greeted the PrevAKI trial findings is reflected in an editorial entitled, “AKI: the Myth of Inevitability is Finally Shattered,” by John A. Kellum, MD, professor of critical care medicine and director of the Center for Critical Care Nephrology at the University of Pittsburgh. Dr. Kellum noted that the renal biomarker-based approach to implementation of the KDIGO care bundle resulted in an attractively low number needed to treat (NNT) of only 6, whereas without biomarker-based enrichment of the target population, the NNT would have been more than 33.
“,” Dr. Kellum declared in the editorial (Nat Rev Nephrol. 2017 Mar;13[3]:140-1).
Indeed, another way to do it was recently demonstrated in the SALT-ED trial, in which 13,347 noncritically ill hospitalized patients requiring intravenous fluid administration were randomized to conventional saline or balanced crystalloids. The incidence of AKI and other major adverse kidney events was 4.7% in the balanced crystalloids group, for a significant 18% risk reduction relative to the 5.6% rate with saline (N Engl J Med. 2018 Mar 1;378[9]:819-28).
While that absolute 0.9% risk reduction might initially not sound like much, with 35 million people per year getting IV saline while in the hospital, it translates into 315,000 fewer major adverse kidney events as a result of a simple switch to balanced crystalloids, Dr. Szerlip observed.
The PrevAKI findings validate the concept of AKI ‘golden hours’ during which time potentially reversible early kidney injury detectable via renal biomarkers is occurring prior to the abrupt decline in kidney function measured by change in serum creatinine. “The problem with using change in creatinine to define AKI is the delay in diagnosis, which makes AKI more difficult to treat,” he explained.
The renal biomarkers utilized in PrevAKI were insulin-like growth factor binding protein-7 (IGFBP7) and tissue inhibitor of metalloproteinase-2 (TIMP-2), as incorporated in the commercially available urinary NephroCheck test, which was administered to study participants 4 hours after cardiopulmonary bypass. A test result of 0.3 or more identified a group at high risk for AKI for randomization to the KDIGO bundle or usual care. The KDIGO bundle consists of discontinuation of nephrotoxic agents when feasible, early optimization of fluid status, and maintenance of perfusion pressure.
Patients known to be at increased risk for in-hospital AKI include the elderly, those with diabetes, patients with heart failure or other conditions prone to volume contraction or overload, those undergoing major surgery, individuals with chronic kidney disease, and patients with sepsis.
Dr. Szerlip singled out as particularly nephrotoxic several drugs widely used in hospitalized patients, including the combination of vancomycin plus piperacillin-tazobactam, which in a recent metaanalysis was found to have a number needed to harm of 11 in terms of AKI in comparison to vancomycin monotherapy or vancomycin in combination with cefepime or carbapenem (Crit Care Med. 2018 Jan;46[1]:12-20). He was also critical of the American Society of Anesthesiologists practice parameter recommending that in-hospital pain management plans for surgical patients include continuous regimens of NSAIDs or COX-2 inhibitors as a means of combating the ongoing opioid epidemic.
“These are highly toxic drugs to the kidney and we shouldn’t be using them,” Dr. Szerlip said.
He reported receiving research grants from LaJolla, Bayer, Akebia, and BioPorto, serving on a speakers’ bureau for Astute Medical, and acting as a consultant to Zs Pharma, Amarin, and LaJolla.
NEW ORLEANS– Preventing acute kidney injury and its progression in hospitalized patients deserves to be a high priority – and now there is finally proof that it’s doable, Harold M. Szerlip, MD, declared at the annual meeting of the American College of Physicians.
The PrevAKI study, a recent randomized controlled clinical trial conducted by German investigators, has demonstrated that the use of renal biomarkers to identify patients at high risk for acute kidney injury (AKI) after major cardiac surgery and providing them with a range of internationally recommended supportive measures known as the KDIGO (Kidney Disease: Improving Global Outcomes) care bundle reduced the occurrence of moderate-to-severe AKI by 34% (Intensive Care Med. 2017 Nov;43[11]:1551-61).
The enthusiasm that greeted the PrevAKI trial findings is reflected in an editorial entitled, “AKI: the Myth of Inevitability is Finally Shattered,” by John A. Kellum, MD, professor of critical care medicine and director of the Center for Critical Care Nephrology at the University of Pittsburgh. Dr. Kellum noted that the renal biomarker-based approach to implementation of the KDIGO care bundle resulted in an attractively low number needed to treat (NNT) of only 6, whereas without biomarker-based enrichment of the target population, the NNT would have been more than 33.
“,” Dr. Kellum declared in the editorial (Nat Rev Nephrol. 2017 Mar;13[3]:140-1).
Indeed, another way to do it was recently demonstrated in the SALT-ED trial, in which 13,347 noncritically ill hospitalized patients requiring intravenous fluid administration were randomized to conventional saline or balanced crystalloids. The incidence of AKI and other major adverse kidney events was 4.7% in the balanced crystalloids group, for a significant 18% risk reduction relative to the 5.6% rate with saline (N Engl J Med. 2018 Mar 1;378[9]:819-28).
While that absolute 0.9% risk reduction might initially not sound like much, with 35 million people per year getting IV saline while in the hospital, it translates into 315,000 fewer major adverse kidney events as a result of a simple switch to balanced crystalloids, Dr. Szerlip observed.
The PrevAKI findings validate the concept of AKI ‘golden hours’ during which time potentially reversible early kidney injury detectable via renal biomarkers is occurring prior to the abrupt decline in kidney function measured by change in serum creatinine. “The problem with using change in creatinine to define AKI is the delay in diagnosis, which makes AKI more difficult to treat,” he explained.
The renal biomarkers utilized in PrevAKI were insulin-like growth factor binding protein-7 (IGFBP7) and tissue inhibitor of metalloproteinase-2 (TIMP-2), as incorporated in the commercially available urinary NephroCheck test, which was administered to study participants 4 hours after cardiopulmonary bypass. A test result of 0.3 or more identified a group at high risk for AKI for randomization to the KDIGO bundle or usual care. The KDIGO bundle consists of discontinuation of nephrotoxic agents when feasible, early optimization of fluid status, and maintenance of perfusion pressure.
Patients known to be at increased risk for in-hospital AKI include the elderly, those with diabetes, patients with heart failure or other conditions prone to volume contraction or overload, those undergoing major surgery, individuals with chronic kidney disease, and patients with sepsis.
Dr. Szerlip singled out as particularly nephrotoxic several drugs widely used in hospitalized patients, including the combination of vancomycin plus piperacillin-tazobactam, which in a recent metaanalysis was found to have a number needed to harm of 11 in terms of AKI in comparison to vancomycin monotherapy or vancomycin in combination with cefepime or carbapenem (Crit Care Med. 2018 Jan;46[1]:12-20). He was also critical of the American Society of Anesthesiologists practice parameter recommending that in-hospital pain management plans for surgical patients include continuous regimens of NSAIDs or COX-2 inhibitors as a means of combating the ongoing opioid epidemic.
“These are highly toxic drugs to the kidney and we shouldn’t be using them,” Dr. Szerlip said.
He reported receiving research grants from LaJolla, Bayer, Akebia, and BioPorto, serving on a speakers’ bureau for Astute Medical, and acting as a consultant to Zs Pharma, Amarin, and LaJolla.
EXPERT ANALYSIS FROM ACP INTERNAL MECICINE
Key to MGUS and myeloma may lie in Iceland
NEW ORLEANS – Everyone aged 40 years and older on the island nation of Iceland is being screened for monoclonal gammopathy of undetermined significance, smoldering myeloma, and full-blown multiple myeloma in an unprecedented project to identify the malignancy’s genetic roots, Joseph Mikhael, MD, said at the annual meeting of the American College of Physicians.
This effort to decode the underlying genetics of multiple myeloma is enormously facilitated by the fact that the DNA sequencing of the entire Icelandic population is already known, and everyone’s blood samples are stored in the national health care system.
The International Myeloma Foundation is funding the Icelandic project, called iStopMM (Iceland Screens Treats or Prevents Multiple Myeloma).
The results of iStopMM could be far reaching, in part because the findings will show whether screening an asymptomatic general population for MGUS – as for example, all American adults – is worthwhile.
In the interim, it’s important for primary care physicians to recognize when it is and isn’t appropriate to order a serum protein electrophoresis (SPE) study, on which the diagnosis of MGUS hinges. It’s also essential to recognize the difference between smoldering and full-blown multiple myeloma, because the distinction has implications for patient monitoring and treatment, added Dr. Mikhael, a hematologist at the City of Hope Cancer Center in Duarte, Calif.
Multiple myeloma accounts for 1% of all cancers and 10% of hematologic malignancies. MGUS is an obligate precursor of multiple myeloma. But MGUS is common, and therein lies a challenge for physicians – as well as a major source of anxiety for many MGUS-positive patients.
In a 12-year-old study, MGUS had a 3% prevalence in the general U.S. population older than age 50 years, and a greater than 5% prevalence after age 70. However, Dr. Mikhael thinks that more refined testing will show the true figure to be close to 10%. Thus, the great majority of patients with MGUS will never develop multiple myeloma.
Pending the potentially practice-changing outcome of iStopMM, Dr. Mikhael said SPE shouldn’t be ordered routinely in an asymptomatic patient, even one with a positive family history for multiple myeloma. The annual cost of monitoring the roughly 540,000 U.S. patients who now carry a diagnosis of MGUS – typically established as an incidental finding by primary care physicians while doing a work-up for another reason – is at least $110 million. And there’s no point in adding to that burden until the benefit of mass screening has been established.
An SPE is appropriate, however, in an older patient with unexplained anemia, known low immunoglobulin levels, unexplained renal insufficiency or neuropathy, or osteopenia or osteoporosis inconsistent with the patient’s age or gender – provided the patient doesn’t have a coexisting plasma cell dyscrasia or B-cell lymphoproliferative disorder, which would throw off the prognostic value of the test results, he continued.
When ordering an SPE to rule in/out MGUS, it’s essential to also order serum free light-chain testing, because it provides key prognostic information.
A landmark study led by investigators at the Mayo Clinic demonstrated that the risk of progression of MGUS to multiple myeloma or a related disorder is independently predicted by three key factors: a high serum M-protein spike of 15 g/L or more on the SPE; the presence of non-IgG MGUS; and an abnormal serum free light-chain ratio of less than 0.26 or more than 1.65. In this study, the 20-year risk of malignant transformation of MGUS ranged from 58% if all three risk factors were present, to just 5% if none were (Blood. 2005 Aug 1;106[3]:812-7).
CRAB vs. SLiM CRAB
Myeloma, smoldering myeloma, and MGUS were redefined a few years ago to reflect differences in prognosis. MGUS still requires the presence of a serum monoclonal protein in a concentration of 3 g/dL or less, less than 10% plasmacytosis in the bone marrow, asymptomatic status, and absence of end-organ damage as traditionally defined in the acronym CRAB (calcium elevation, renal insufficiency, anemia, or bony disease).
If CRAB is present in a patient with at least 10% plasma cells in bone marrow, that is by definition multiple myeloma warranting treatment. Smoldering myeloma requires at least 10% plasmacytosis in bone marrow and absence of the CRAB criteria. However, in a significant change, ultra–high-risk smoldering melanoma, defined by the acronym SLiM CRAB, is now considered active myeloma and should be treated (Lancet Oncol. 2014 Nov;15[12]:e538-48).
“Traditionally, we waited until CRAB [to define myeloma],” Dr. Mikhael explained. “But if you’re running toward a cliff, I don’t have to wait until you’re falling off to know you’re in trouble.”
The SLiM half of SLiM CRAB consists of 60% or more plasmacytosis in bone marrow, light chains in a kappa-to-lambda or lambda-to-kappa ratio of greater than 100, and MRI showing one or more focal lesions. If a patient is SLiM, with or without CRAB, that is now considered active myeloma warranting treatment.
Not all MGUS needs a bone marrow biopsy
A bone marrow biopsy and skeletal survey via whole-body CT or conventional radiographs can be deferred in patients with low-risk MGUS and no bony symptoms. Using the Mayo Clinic risk stratification model, low risk is defined as a serum M protein of 1.5 g/dL or less on SPE, an IgG isotype, and a normal free light-chain ratio.
The lifetime risk of progression in patients with MGUS who meet all three criteria is only about 2%. They can be followed at 6 months with an SPE, free light-chain testing, a CBC, and serum calcium and creatinine, then annually thereafter.
“For those who aren’t in this low-risk category, we actually do need to do a bone marrow test,” according to Dr. Mikhael. “Then, based on that, if they have malignancy, send them to a myeloma geek like me or to another hematologist. And if they don’t have a malignancy, they can be followed at 6 months and then subsequently at least every year.”
Dr. Mikhael has received research grants from AbbVie, Celgene, and Sanofi.
NEW ORLEANS – Everyone aged 40 years and older on the island nation of Iceland is being screened for monoclonal gammopathy of undetermined significance, smoldering myeloma, and full-blown multiple myeloma in an unprecedented project to identify the malignancy’s genetic roots, Joseph Mikhael, MD, said at the annual meeting of the American College of Physicians.
This effort to decode the underlying genetics of multiple myeloma is enormously facilitated by the fact that the DNA sequencing of the entire Icelandic population is already known, and everyone’s blood samples are stored in the national health care system.
The International Myeloma Foundation is funding the Icelandic project, called iStopMM (Iceland Screens Treats or Prevents Multiple Myeloma).
The results of iStopMM could be far reaching, in part because the findings will show whether screening an asymptomatic general population for MGUS – as for example, all American adults – is worthwhile.
In the interim, it’s important for primary care physicians to recognize when it is and isn’t appropriate to order a serum protein electrophoresis (SPE) study, on which the diagnosis of MGUS hinges. It’s also essential to recognize the difference between smoldering and full-blown multiple myeloma, because the distinction has implications for patient monitoring and treatment, added Dr. Mikhael, a hematologist at the City of Hope Cancer Center in Duarte, Calif.
Multiple myeloma accounts for 1% of all cancers and 10% of hematologic malignancies. MGUS is an obligate precursor of multiple myeloma. But MGUS is common, and therein lies a challenge for physicians – as well as a major source of anxiety for many MGUS-positive patients.
In a 12-year-old study, MGUS had a 3% prevalence in the general U.S. population older than age 50 years, and a greater than 5% prevalence after age 70. However, Dr. Mikhael thinks that more refined testing will show the true figure to be close to 10%. Thus, the great majority of patients with MGUS will never develop multiple myeloma.
Pending the potentially practice-changing outcome of iStopMM, Dr. Mikhael said SPE shouldn’t be ordered routinely in an asymptomatic patient, even one with a positive family history for multiple myeloma. The annual cost of monitoring the roughly 540,000 U.S. patients who now carry a diagnosis of MGUS – typically established as an incidental finding by primary care physicians while doing a work-up for another reason – is at least $110 million. And there’s no point in adding to that burden until the benefit of mass screening has been established.
An SPE is appropriate, however, in an older patient with unexplained anemia, known low immunoglobulin levels, unexplained renal insufficiency or neuropathy, or osteopenia or osteoporosis inconsistent with the patient’s age or gender – provided the patient doesn’t have a coexisting plasma cell dyscrasia or B-cell lymphoproliferative disorder, which would throw off the prognostic value of the test results, he continued.
When ordering an SPE to rule in/out MGUS, it’s essential to also order serum free light-chain testing, because it provides key prognostic information.
A landmark study led by investigators at the Mayo Clinic demonstrated that the risk of progression of MGUS to multiple myeloma or a related disorder is independently predicted by three key factors: a high serum M-protein spike of 15 g/L or more on the SPE; the presence of non-IgG MGUS; and an abnormal serum free light-chain ratio of less than 0.26 or more than 1.65. In this study, the 20-year risk of malignant transformation of MGUS ranged from 58% if all three risk factors were present, to just 5% if none were (Blood. 2005 Aug 1;106[3]:812-7).
CRAB vs. SLiM CRAB
Myeloma, smoldering myeloma, and MGUS were redefined a few years ago to reflect differences in prognosis. MGUS still requires the presence of a serum monoclonal protein in a concentration of 3 g/dL or less, less than 10% plasmacytosis in the bone marrow, asymptomatic status, and absence of end-organ damage as traditionally defined in the acronym CRAB (calcium elevation, renal insufficiency, anemia, or bony disease).
If CRAB is present in a patient with at least 10% plasma cells in bone marrow, that is by definition multiple myeloma warranting treatment. Smoldering myeloma requires at least 10% plasmacytosis in bone marrow and absence of the CRAB criteria. However, in a significant change, ultra–high-risk smoldering melanoma, defined by the acronym SLiM CRAB, is now considered active myeloma and should be treated (Lancet Oncol. 2014 Nov;15[12]:e538-48).
“Traditionally, we waited until CRAB [to define myeloma],” Dr. Mikhael explained. “But if you’re running toward a cliff, I don’t have to wait until you’re falling off to know you’re in trouble.”
The SLiM half of SLiM CRAB consists of 60% or more plasmacytosis in bone marrow, light chains in a kappa-to-lambda or lambda-to-kappa ratio of greater than 100, and MRI showing one or more focal lesions. If a patient is SLiM, with or without CRAB, that is now considered active myeloma warranting treatment.
Not all MGUS needs a bone marrow biopsy
A bone marrow biopsy and skeletal survey via whole-body CT or conventional radiographs can be deferred in patients with low-risk MGUS and no bony symptoms. Using the Mayo Clinic risk stratification model, low risk is defined as a serum M protein of 1.5 g/dL or less on SPE, an IgG isotype, and a normal free light-chain ratio.
The lifetime risk of progression in patients with MGUS who meet all three criteria is only about 2%. They can be followed at 6 months with an SPE, free light-chain testing, a CBC, and serum calcium and creatinine, then annually thereafter.
“For those who aren’t in this low-risk category, we actually do need to do a bone marrow test,” according to Dr. Mikhael. “Then, based on that, if they have malignancy, send them to a myeloma geek like me or to another hematologist. And if they don’t have a malignancy, they can be followed at 6 months and then subsequently at least every year.”
Dr. Mikhael has received research grants from AbbVie, Celgene, and Sanofi.
NEW ORLEANS – Everyone aged 40 years and older on the island nation of Iceland is being screened for monoclonal gammopathy of undetermined significance, smoldering myeloma, and full-blown multiple myeloma in an unprecedented project to identify the malignancy’s genetic roots, Joseph Mikhael, MD, said at the annual meeting of the American College of Physicians.
This effort to decode the underlying genetics of multiple myeloma is enormously facilitated by the fact that the DNA sequencing of the entire Icelandic population is already known, and everyone’s blood samples are stored in the national health care system.
The International Myeloma Foundation is funding the Icelandic project, called iStopMM (Iceland Screens Treats or Prevents Multiple Myeloma).
The results of iStopMM could be far reaching, in part because the findings will show whether screening an asymptomatic general population for MGUS – as for example, all American adults – is worthwhile.
In the interim, it’s important for primary care physicians to recognize when it is and isn’t appropriate to order a serum protein electrophoresis (SPE) study, on which the diagnosis of MGUS hinges. It’s also essential to recognize the difference between smoldering and full-blown multiple myeloma, because the distinction has implications for patient monitoring and treatment, added Dr. Mikhael, a hematologist at the City of Hope Cancer Center in Duarte, Calif.
Multiple myeloma accounts for 1% of all cancers and 10% of hematologic malignancies. MGUS is an obligate precursor of multiple myeloma. But MGUS is common, and therein lies a challenge for physicians – as well as a major source of anxiety for many MGUS-positive patients.
In a 12-year-old study, MGUS had a 3% prevalence in the general U.S. population older than age 50 years, and a greater than 5% prevalence after age 70. However, Dr. Mikhael thinks that more refined testing will show the true figure to be close to 10%. Thus, the great majority of patients with MGUS will never develop multiple myeloma.
Pending the potentially practice-changing outcome of iStopMM, Dr. Mikhael said SPE shouldn’t be ordered routinely in an asymptomatic patient, even one with a positive family history for multiple myeloma. The annual cost of monitoring the roughly 540,000 U.S. patients who now carry a diagnosis of MGUS – typically established as an incidental finding by primary care physicians while doing a work-up for another reason – is at least $110 million. And there’s no point in adding to that burden until the benefit of mass screening has been established.
An SPE is appropriate, however, in an older patient with unexplained anemia, known low immunoglobulin levels, unexplained renal insufficiency or neuropathy, or osteopenia or osteoporosis inconsistent with the patient’s age or gender – provided the patient doesn’t have a coexisting plasma cell dyscrasia or B-cell lymphoproliferative disorder, which would throw off the prognostic value of the test results, he continued.
When ordering an SPE to rule in/out MGUS, it’s essential to also order serum free light-chain testing, because it provides key prognostic information.
A landmark study led by investigators at the Mayo Clinic demonstrated that the risk of progression of MGUS to multiple myeloma or a related disorder is independently predicted by three key factors: a high serum M-protein spike of 15 g/L or more on the SPE; the presence of non-IgG MGUS; and an abnormal serum free light-chain ratio of less than 0.26 or more than 1.65. In this study, the 20-year risk of malignant transformation of MGUS ranged from 58% if all three risk factors were present, to just 5% if none were (Blood. 2005 Aug 1;106[3]:812-7).
CRAB vs. SLiM CRAB
Myeloma, smoldering myeloma, and MGUS were redefined a few years ago to reflect differences in prognosis. MGUS still requires the presence of a serum monoclonal protein in a concentration of 3 g/dL or less, less than 10% plasmacytosis in the bone marrow, asymptomatic status, and absence of end-organ damage as traditionally defined in the acronym CRAB (calcium elevation, renal insufficiency, anemia, or bony disease).
If CRAB is present in a patient with at least 10% plasma cells in bone marrow, that is by definition multiple myeloma warranting treatment. Smoldering myeloma requires at least 10% plasmacytosis in bone marrow and absence of the CRAB criteria. However, in a significant change, ultra–high-risk smoldering melanoma, defined by the acronym SLiM CRAB, is now considered active myeloma and should be treated (Lancet Oncol. 2014 Nov;15[12]:e538-48).
“Traditionally, we waited until CRAB [to define myeloma],” Dr. Mikhael explained. “But if you’re running toward a cliff, I don’t have to wait until you’re falling off to know you’re in trouble.”
The SLiM half of SLiM CRAB consists of 60% or more plasmacytosis in bone marrow, light chains in a kappa-to-lambda or lambda-to-kappa ratio of greater than 100, and MRI showing one or more focal lesions. If a patient is SLiM, with or without CRAB, that is now considered active myeloma warranting treatment.
Not all MGUS needs a bone marrow biopsy
A bone marrow biopsy and skeletal survey via whole-body CT or conventional radiographs can be deferred in patients with low-risk MGUS and no bony symptoms. Using the Mayo Clinic risk stratification model, low risk is defined as a serum M protein of 1.5 g/dL or less on SPE, an IgG isotype, and a normal free light-chain ratio.
The lifetime risk of progression in patients with MGUS who meet all three criteria is only about 2%. They can be followed at 6 months with an SPE, free light-chain testing, a CBC, and serum calcium and creatinine, then annually thereafter.
“For those who aren’t in this low-risk category, we actually do need to do a bone marrow test,” according to Dr. Mikhael. “Then, based on that, if they have malignancy, send them to a myeloma geek like me or to another hematologist. And if they don’t have a malignancy, they can be followed at 6 months and then subsequently at least every year.”
Dr. Mikhael has received research grants from AbbVie, Celgene, and Sanofi.
EXPERT ANALYSIS FROM ACP INTERNAL MEDICINE
MI before age 50? Think familial hypercholesterolemia, substance abuse
ORLANDO – Patients with an MI before age 50 commonly have familial hypercholesterolemia or a substance abuse issue, according to presentations at the annual meeting of the American College of Cardiology.
Not only is the prevalence of familial hypercholesterolemia (FH) increased in patients with an MI at a young age, but 1 year post MI, their LDL remains unacceptably high at 100 mg/dL or more in a high percentage of cases. For that matter, the same is true in patients with an MI before age 50 who don’t have FH, reported Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
FH patients after early MI
Dr. Blankstein presented a retrospective study of 1,996 adults with a first confirmed type 1 MI before at 50 who presented at Brigham and Women’s Hospital or Massachusetts General Hospital, of whom 9% met Dutch Lipid Clinic Network criteria for probable or definite FH.
Among patients with an MI before age 50 and a family history of premature CAD, the prevalence of FH was enriched, at 22%. Among those with an LDL of 160 mg/dL or more, the prevalence of FH rose further, to 36%. And by combining all three criteria – MI before age 50, a positive family history of early CAD, and an LDL of at least 160 mg/dL – the prevalence of FH shot up to 64%, Dr. Blankstein said.
Only 89% of patients with an MI prior to turning 50 years old were discharged on a statin. “That’s lower than I would have expected,” he said.
One year post MI, LDL levels had dropped by a mean of 79 mg/dL in the FH group and 39 mg/dL in the non-FH patients. This translated into a 45% reduction in the FH patients, a significantly greater decrease than the 34% drop in the non-FH group. Nonetheless, 43% of FH patients had an LDL of 100 mg/dL or greater at 1 year, as did 26% without FH. These are patients who are particularly likely to benefit from more aggressive lipid-lowering after an acute coronary syndrome. Given that almost 90% of patients with FH remain undiagnosed, assessment for the genetic disorder in young patients with MI is an important means of case finding, the cardiologist observed.
Session cochair Carl E. Orringer, MD, director of preventive cardiovascular medicine at the University of Miami, said he and his colleagues have just initiated a program at that medical center whereby patients with an LDL of 190 mg/dL or more are identified through their electronic medical records and referred to a lipid clinic or cardiovascular prevention program.
“I think this is certainly something to think about for other programs because you want to make sure that if you have lipid intervention services, they actually take care of patients who are at the highest risk,” he said.
Substance abuse plays role
Elsewhere at ACC 2018, Ersilia M. Defilippis, MD, reported on an expanded population of 2,097 patients with a first type 1 MI prior to age 50 at the same two hospitals. Their electronic medical records revealed that 6.0% of them used marijuana and 4.7% used cocaine. During a median 11.2 years of follow-up, the group that used cocaine or marijuana had a 2.2-fold increased risk of cardiovascular death and a 2.0-fold increase in all-cause mortality, compared with nonusers, in an analysis adjusted for baseline differences.
Among these differences, 46% of drug users and 61% of nonusers were hyperlipidemic, 70% of users and 49% of nonusers were smokers, 8% of users and 4% of nonusers presented in cardiac arrest, and the median normalized troponin level was 61 interquartile range (IQR) in users versus 39 IQR in nonusers.
“Given these findings, young patients with MI should be screened for substance abuse and counseled about behavioral change to prevent future adverse events,” concluded Dr. Defilippis of Brigham and Women’s Hospital and Harvard University. She and Dr. Blankstein were coinvestigators in this study. She reported having no financial conflicts of interest. Dr. Blankstein reported receiving research grants from Amgen, Astellas, and Sanofi, and serves as a consultant to Amgen.
Source: Blankstein R. Abstracts 1180M-03 and 1262-436/436.
ORLANDO – Patients with an MI before age 50 commonly have familial hypercholesterolemia or a substance abuse issue, according to presentations at the annual meeting of the American College of Cardiology.
Not only is the prevalence of familial hypercholesterolemia (FH) increased in patients with an MI at a young age, but 1 year post MI, their LDL remains unacceptably high at 100 mg/dL or more in a high percentage of cases. For that matter, the same is true in patients with an MI before age 50 who don’t have FH, reported Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
FH patients after early MI
Dr. Blankstein presented a retrospective study of 1,996 adults with a first confirmed type 1 MI before at 50 who presented at Brigham and Women’s Hospital or Massachusetts General Hospital, of whom 9% met Dutch Lipid Clinic Network criteria for probable or definite FH.
Among patients with an MI before age 50 and a family history of premature CAD, the prevalence of FH was enriched, at 22%. Among those with an LDL of 160 mg/dL or more, the prevalence of FH rose further, to 36%. And by combining all three criteria – MI before age 50, a positive family history of early CAD, and an LDL of at least 160 mg/dL – the prevalence of FH shot up to 64%, Dr. Blankstein said.
Only 89% of patients with an MI prior to turning 50 years old were discharged on a statin. “That’s lower than I would have expected,” he said.
One year post MI, LDL levels had dropped by a mean of 79 mg/dL in the FH group and 39 mg/dL in the non-FH patients. This translated into a 45% reduction in the FH patients, a significantly greater decrease than the 34% drop in the non-FH group. Nonetheless, 43% of FH patients had an LDL of 100 mg/dL or greater at 1 year, as did 26% without FH. These are patients who are particularly likely to benefit from more aggressive lipid-lowering after an acute coronary syndrome. Given that almost 90% of patients with FH remain undiagnosed, assessment for the genetic disorder in young patients with MI is an important means of case finding, the cardiologist observed.
Session cochair Carl E. Orringer, MD, director of preventive cardiovascular medicine at the University of Miami, said he and his colleagues have just initiated a program at that medical center whereby patients with an LDL of 190 mg/dL or more are identified through their electronic medical records and referred to a lipid clinic or cardiovascular prevention program.
“I think this is certainly something to think about for other programs because you want to make sure that if you have lipid intervention services, they actually take care of patients who are at the highest risk,” he said.
Substance abuse plays role
Elsewhere at ACC 2018, Ersilia M. Defilippis, MD, reported on an expanded population of 2,097 patients with a first type 1 MI prior to age 50 at the same two hospitals. Their electronic medical records revealed that 6.0% of them used marijuana and 4.7% used cocaine. During a median 11.2 years of follow-up, the group that used cocaine or marijuana had a 2.2-fold increased risk of cardiovascular death and a 2.0-fold increase in all-cause mortality, compared with nonusers, in an analysis adjusted for baseline differences.
Among these differences, 46% of drug users and 61% of nonusers were hyperlipidemic, 70% of users and 49% of nonusers were smokers, 8% of users and 4% of nonusers presented in cardiac arrest, and the median normalized troponin level was 61 interquartile range (IQR) in users versus 39 IQR in nonusers.
“Given these findings, young patients with MI should be screened for substance abuse and counseled about behavioral change to prevent future adverse events,” concluded Dr. Defilippis of Brigham and Women’s Hospital and Harvard University. She and Dr. Blankstein were coinvestigators in this study. She reported having no financial conflicts of interest. Dr. Blankstein reported receiving research grants from Amgen, Astellas, and Sanofi, and serves as a consultant to Amgen.
Source: Blankstein R. Abstracts 1180M-03 and 1262-436/436.
ORLANDO – Patients with an MI before age 50 commonly have familial hypercholesterolemia or a substance abuse issue, according to presentations at the annual meeting of the American College of Cardiology.
Not only is the prevalence of familial hypercholesterolemia (FH) increased in patients with an MI at a young age, but 1 year post MI, their LDL remains unacceptably high at 100 mg/dL or more in a high percentage of cases. For that matter, the same is true in patients with an MI before age 50 who don’t have FH, reported Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
FH patients after early MI
Dr. Blankstein presented a retrospective study of 1,996 adults with a first confirmed type 1 MI before at 50 who presented at Brigham and Women’s Hospital or Massachusetts General Hospital, of whom 9% met Dutch Lipid Clinic Network criteria for probable or definite FH.
Among patients with an MI before age 50 and a family history of premature CAD, the prevalence of FH was enriched, at 22%. Among those with an LDL of 160 mg/dL or more, the prevalence of FH rose further, to 36%. And by combining all three criteria – MI before age 50, a positive family history of early CAD, and an LDL of at least 160 mg/dL – the prevalence of FH shot up to 64%, Dr. Blankstein said.
Only 89% of patients with an MI prior to turning 50 years old were discharged on a statin. “That’s lower than I would have expected,” he said.
One year post MI, LDL levels had dropped by a mean of 79 mg/dL in the FH group and 39 mg/dL in the non-FH patients. This translated into a 45% reduction in the FH patients, a significantly greater decrease than the 34% drop in the non-FH group. Nonetheless, 43% of FH patients had an LDL of 100 mg/dL or greater at 1 year, as did 26% without FH. These are patients who are particularly likely to benefit from more aggressive lipid-lowering after an acute coronary syndrome. Given that almost 90% of patients with FH remain undiagnosed, assessment for the genetic disorder in young patients with MI is an important means of case finding, the cardiologist observed.
Session cochair Carl E. Orringer, MD, director of preventive cardiovascular medicine at the University of Miami, said he and his colleagues have just initiated a program at that medical center whereby patients with an LDL of 190 mg/dL or more are identified through their electronic medical records and referred to a lipid clinic or cardiovascular prevention program.
“I think this is certainly something to think about for other programs because you want to make sure that if you have lipid intervention services, they actually take care of patients who are at the highest risk,” he said.
Substance abuse plays role
Elsewhere at ACC 2018, Ersilia M. Defilippis, MD, reported on an expanded population of 2,097 patients with a first type 1 MI prior to age 50 at the same two hospitals. Their electronic medical records revealed that 6.0% of them used marijuana and 4.7% used cocaine. During a median 11.2 years of follow-up, the group that used cocaine or marijuana had a 2.2-fold increased risk of cardiovascular death and a 2.0-fold increase in all-cause mortality, compared with nonusers, in an analysis adjusted for baseline differences.
Among these differences, 46% of drug users and 61% of nonusers were hyperlipidemic, 70% of users and 49% of nonusers were smokers, 8% of users and 4% of nonusers presented in cardiac arrest, and the median normalized troponin level was 61 interquartile range (IQR) in users versus 39 IQR in nonusers.
“Given these findings, young patients with MI should be screened for substance abuse and counseled about behavioral change to prevent future adverse events,” concluded Dr. Defilippis of Brigham and Women’s Hospital and Harvard University. She and Dr. Blankstein were coinvestigators in this study. She reported having no financial conflicts of interest. Dr. Blankstein reported receiving research grants from Amgen, Astellas, and Sanofi, and serves as a consultant to Amgen.
Source: Blankstein R. Abstracts 1180M-03 and 1262-436/436.
REPORTING FROM ACC 18
Key clinical point: Familial hypercholesterolemia is common in young adults with MI.
Major finding: Among patients with MI before age 50, a family history of premature CAD, and an LDL of 160 mg/dL or more, the prevalence of familial hypercholesterolemia was 64%.
Study details: This retrospective study involved 1,996 adults diagnosed with a type 1 MI before age 50.
Disclosures: The study presenter reported receiving research grants from Amgen, Astellas, and Sanofi and serving as a consultant to Amgen.
Source: Blankstein R. Abstracts 1180M-03 and 1262-436/436.
Beware nonopiate meds with high street value
NEW ORLEANS – Gabapentin heads the short list of prescription drugs other than opioids and benzodiazepines with substantial black-market abuse potential, according to Alexander Y. Walley, MD.
“At least in Massachusetts, where I see patients, these are the pills that people are using and trading on the street. Your part of the country might have others,” noted Dr. Walley, director of the addiction medicine fellowship program at Boston Medical Center.
“I’m not telling you to never prescribe these medications – they are clinically indicated in certain cases and should certainly be used,” Dr. Walley said at the annual meeting of the American College of Physicians. “But now that you know that they might be misused, you should use safeguards.
“A lot of these medications – gabapentin is an example – are a problem primarily in people with other substance use disorders,” he added. “That’s really where I think you need to have the greatest caution.”
Gabapentinoids
Gabapentin and pregabalin are not addictive in the sense that it’s easy to get laboratory animals or healthy volunteers to self-administer them. However, gabapentinoid use disorder is extremely common among people with opioid use disorder, who report that the combination boosts the euphoric effects of opioids and reduces opioid withdrawal symptoms without causing side effects.
Indeed, a recent systematic review of 106 studies found that gabapentinoid use disorder was present in up to 26% of opioid users (Eur Neuropsychopharmacol. 2017 Dec;27[12]:1185-1215).
Overdoses involving gabapentinoids alone are uncommon because they require consumption at up to 25 times the maximum recommended dose. Moreover, these overdoses are rarely fatal.
“You almost can’t overdose on a gabapentinoid, because you have to take lots and lots of it to do so, and you’ll usually survive. But if you add it to an opioid or other sedative, then you’re really in dangerous territory. That’s where all the deaths are clustered,” Dr. Walley explained.
A recent Canadian/Dutch population-based, nested, case-control study concluded that concomitant prescription of opioids and gabapentin was associated with a 49% greater chance of fatal overdose, compared with opioid prescription alone, in an analysis extensively adjusted for potential confounders. A dose-response effect was noted, such that coprescription of high-dose gabapentin was linked to an adjusted 58% increased risk (PLoS Med. 2017 Oct 3;14[10]:e1002396).
Complicating the picture, however, is solid evidence from a randomized, placebo-controlled, crossover trial that gabapentin and opioids are synergistic for relief from neuropathic pain, which can be notoriously difficult to control (N Engl J Med. 2005 Mar 31;352[13]:1324-34).
“It’s tricky, because you can potentially spare having to use high-dose opioids by adding gabapentin,” Dr. Walley observed. “So, as prescribers, you’re in a difficult position, because there’s a mixed message here: When gabapentin is combined with opioids, that’s when it’s dangerous – but that’s also when they’re potentially more effective for pain.”
Promethazine
This drug has a host of neurobiologic actions, which collectively provide sedative and antiemetic effects. Promethazine jacks up opioid-induced euphoria and alleviates withdrawal symptoms. It’s commonly detected in toxicology testing of patients on prescription opioids for chronic pain or on methadone therapy for opioid use disorder.
National Poison Data System figures show an unwelcome trend: A sharp uptick in promethazine abuse/misuse beginning in 2008, even while the total number of poisoning events of all kinds reported to the system began a steady decline (J Addict Med. 2015 May-Jun;9[3]:233-7).
Clonidine
This centrally acting alpha2-adrenoreceptor and imidazoline-receptor agonist is indicated for treatment of hypertension. However, it’s also extensively used off-label to treat anxiety, as well as for alcohol and opioid withdrawal symptoms. The problem is, clonidine boosts opioid-induced euphoria.
A retrospective study of clonidine-overdose patients characterized the clonidine overdose syndrome as marked by sedation, hypotension, bradycardia, and excessive pupillary constriction (Clin Toxicol [Phila]. 2017 Mar;55[3]:187-92).
“The combination of clonidine and opioids is particularly dangerous,” according to Dr. Walley. “Even though it mimics what an opioid overdose looks like, a clonidine overdose is not responsive to naloxone.”
Stimulants
One-quarter of patients who are prescribed methylphenidate or amphetamine for ADHD report being asked to divert their medication, and 11%-29% sell or give it to others seeking to use it recreationally or as a performance aid.
It’s common for prescription seekers to misrepresent symptoms of ADHD, and this play-acting is often tough to detect. In contrast, the nonstimulant atomoxetine (Strattera) and the alpha-adrenergic agonists prescribed for ADHD aren’t linked to misuse or diversion (Postgrad Med. 2014 Sep;126[5]:64-81).
Bupropion
This norepinephrine and dopamine reuptake inhibitor is generally assumed to have low abuse potential. That’s usually true – except in jail and prisons.
“In my patient population, where I have a keen eye to what’s being used on the street, bupropion is not one of the medications that I see very often in my patients who are not incarcerated,” Dr. Walley said. “But in incarcerated settings, it does have a street value.”
Consider safeguards
None of the prescription drugs on Dr. Walley’s problem list is included in prescription monitoring programs, nor are they detectable with standard toxicology testing. This poses a challenge for prescribing physicians.
Before prescribing any of these potentially abusable medications for a given patient, therefore, Dr. Walley considers the underlying risks. For example, an addiction history is a big red flag. So is coprescription of an opioid or another drug that might have synergistic adverse effects. Dr. Walley makes sure there is a solid indication for the medication, and, having prescribed the drug, he wants to see and document clear functional benefit.
Drug-specific toxicology screening is worthy of consideration as a means of confirming the presence of the prescribed medication, along with the absence of opioids or other drugs that shouldn’t be on board.
“I do this with gabapentin, because I see a lot of diversion,” he explained. “If gabapentin doesn’t show up in the toxicology screen, I stop prescribing it. Or if I detect it and it hasn’t been prescribed, that allows me to have a safety discussion with the patient.”
Dr. Walley reported no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Gabapentin heads the short list of prescription drugs other than opioids and benzodiazepines with substantial black-market abuse potential, according to Alexander Y. Walley, MD.
“At least in Massachusetts, where I see patients, these are the pills that people are using and trading on the street. Your part of the country might have others,” noted Dr. Walley, director of the addiction medicine fellowship program at Boston Medical Center.
“I’m not telling you to never prescribe these medications – they are clinically indicated in certain cases and should certainly be used,” Dr. Walley said at the annual meeting of the American College of Physicians. “But now that you know that they might be misused, you should use safeguards.
“A lot of these medications – gabapentin is an example – are a problem primarily in people with other substance use disorders,” he added. “That’s really where I think you need to have the greatest caution.”
Gabapentinoids
Gabapentin and pregabalin are not addictive in the sense that it’s easy to get laboratory animals or healthy volunteers to self-administer them. However, gabapentinoid use disorder is extremely common among people with opioid use disorder, who report that the combination boosts the euphoric effects of opioids and reduces opioid withdrawal symptoms without causing side effects.
Indeed, a recent systematic review of 106 studies found that gabapentinoid use disorder was present in up to 26% of opioid users (Eur Neuropsychopharmacol. 2017 Dec;27[12]:1185-1215).
Overdoses involving gabapentinoids alone are uncommon because they require consumption at up to 25 times the maximum recommended dose. Moreover, these overdoses are rarely fatal.
“You almost can’t overdose on a gabapentinoid, because you have to take lots and lots of it to do so, and you’ll usually survive. But if you add it to an opioid or other sedative, then you’re really in dangerous territory. That’s where all the deaths are clustered,” Dr. Walley explained.
A recent Canadian/Dutch population-based, nested, case-control study concluded that concomitant prescription of opioids and gabapentin was associated with a 49% greater chance of fatal overdose, compared with opioid prescription alone, in an analysis extensively adjusted for potential confounders. A dose-response effect was noted, such that coprescription of high-dose gabapentin was linked to an adjusted 58% increased risk (PLoS Med. 2017 Oct 3;14[10]:e1002396).
Complicating the picture, however, is solid evidence from a randomized, placebo-controlled, crossover trial that gabapentin and opioids are synergistic for relief from neuropathic pain, which can be notoriously difficult to control (N Engl J Med. 2005 Mar 31;352[13]:1324-34).
“It’s tricky, because you can potentially spare having to use high-dose opioids by adding gabapentin,” Dr. Walley observed. “So, as prescribers, you’re in a difficult position, because there’s a mixed message here: When gabapentin is combined with opioids, that’s when it’s dangerous – but that’s also when they’re potentially more effective for pain.”
Promethazine
This drug has a host of neurobiologic actions, which collectively provide sedative and antiemetic effects. Promethazine jacks up opioid-induced euphoria and alleviates withdrawal symptoms. It’s commonly detected in toxicology testing of patients on prescription opioids for chronic pain or on methadone therapy for opioid use disorder.
National Poison Data System figures show an unwelcome trend: A sharp uptick in promethazine abuse/misuse beginning in 2008, even while the total number of poisoning events of all kinds reported to the system began a steady decline (J Addict Med. 2015 May-Jun;9[3]:233-7).
Clonidine
This centrally acting alpha2-adrenoreceptor and imidazoline-receptor agonist is indicated for treatment of hypertension. However, it’s also extensively used off-label to treat anxiety, as well as for alcohol and opioid withdrawal symptoms. The problem is, clonidine boosts opioid-induced euphoria.
A retrospective study of clonidine-overdose patients characterized the clonidine overdose syndrome as marked by sedation, hypotension, bradycardia, and excessive pupillary constriction (Clin Toxicol [Phila]. 2017 Mar;55[3]:187-92).
“The combination of clonidine and opioids is particularly dangerous,” according to Dr. Walley. “Even though it mimics what an opioid overdose looks like, a clonidine overdose is not responsive to naloxone.”
Stimulants
One-quarter of patients who are prescribed methylphenidate or amphetamine for ADHD report being asked to divert their medication, and 11%-29% sell or give it to others seeking to use it recreationally or as a performance aid.
It’s common for prescription seekers to misrepresent symptoms of ADHD, and this play-acting is often tough to detect. In contrast, the nonstimulant atomoxetine (Strattera) and the alpha-adrenergic agonists prescribed for ADHD aren’t linked to misuse or diversion (Postgrad Med. 2014 Sep;126[5]:64-81).
Bupropion
This norepinephrine and dopamine reuptake inhibitor is generally assumed to have low abuse potential. That’s usually true – except in jail and prisons.
“In my patient population, where I have a keen eye to what’s being used on the street, bupropion is not one of the medications that I see very often in my patients who are not incarcerated,” Dr. Walley said. “But in incarcerated settings, it does have a street value.”
Consider safeguards
None of the prescription drugs on Dr. Walley’s problem list is included in prescription monitoring programs, nor are they detectable with standard toxicology testing. This poses a challenge for prescribing physicians.
Before prescribing any of these potentially abusable medications for a given patient, therefore, Dr. Walley considers the underlying risks. For example, an addiction history is a big red flag. So is coprescription of an opioid or another drug that might have synergistic adverse effects. Dr. Walley makes sure there is a solid indication for the medication, and, having prescribed the drug, he wants to see and document clear functional benefit.
Drug-specific toxicology screening is worthy of consideration as a means of confirming the presence of the prescribed medication, along with the absence of opioids or other drugs that shouldn’t be on board.
“I do this with gabapentin, because I see a lot of diversion,” he explained. “If gabapentin doesn’t show up in the toxicology screen, I stop prescribing it. Or if I detect it and it hasn’t been prescribed, that allows me to have a safety discussion with the patient.”
Dr. Walley reported no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Gabapentin heads the short list of prescription drugs other than opioids and benzodiazepines with substantial black-market abuse potential, according to Alexander Y. Walley, MD.
“At least in Massachusetts, where I see patients, these are the pills that people are using and trading on the street. Your part of the country might have others,” noted Dr. Walley, director of the addiction medicine fellowship program at Boston Medical Center.
“I’m not telling you to never prescribe these medications – they are clinically indicated in certain cases and should certainly be used,” Dr. Walley said at the annual meeting of the American College of Physicians. “But now that you know that they might be misused, you should use safeguards.
“A lot of these medications – gabapentin is an example – are a problem primarily in people with other substance use disorders,” he added. “That’s really where I think you need to have the greatest caution.”
Gabapentinoids
Gabapentin and pregabalin are not addictive in the sense that it’s easy to get laboratory animals or healthy volunteers to self-administer them. However, gabapentinoid use disorder is extremely common among people with opioid use disorder, who report that the combination boosts the euphoric effects of opioids and reduces opioid withdrawal symptoms without causing side effects.
Indeed, a recent systematic review of 106 studies found that gabapentinoid use disorder was present in up to 26% of opioid users (Eur Neuropsychopharmacol. 2017 Dec;27[12]:1185-1215).
Overdoses involving gabapentinoids alone are uncommon because they require consumption at up to 25 times the maximum recommended dose. Moreover, these overdoses are rarely fatal.
“You almost can’t overdose on a gabapentinoid, because you have to take lots and lots of it to do so, and you’ll usually survive. But if you add it to an opioid or other sedative, then you’re really in dangerous territory. That’s where all the deaths are clustered,” Dr. Walley explained.
A recent Canadian/Dutch population-based, nested, case-control study concluded that concomitant prescription of opioids and gabapentin was associated with a 49% greater chance of fatal overdose, compared with opioid prescription alone, in an analysis extensively adjusted for potential confounders. A dose-response effect was noted, such that coprescription of high-dose gabapentin was linked to an adjusted 58% increased risk (PLoS Med. 2017 Oct 3;14[10]:e1002396).
Complicating the picture, however, is solid evidence from a randomized, placebo-controlled, crossover trial that gabapentin and opioids are synergistic for relief from neuropathic pain, which can be notoriously difficult to control (N Engl J Med. 2005 Mar 31;352[13]:1324-34).
“It’s tricky, because you can potentially spare having to use high-dose opioids by adding gabapentin,” Dr. Walley observed. “So, as prescribers, you’re in a difficult position, because there’s a mixed message here: When gabapentin is combined with opioids, that’s when it’s dangerous – but that’s also when they’re potentially more effective for pain.”
Promethazine
This drug has a host of neurobiologic actions, which collectively provide sedative and antiemetic effects. Promethazine jacks up opioid-induced euphoria and alleviates withdrawal symptoms. It’s commonly detected in toxicology testing of patients on prescription opioids for chronic pain or on methadone therapy for opioid use disorder.
National Poison Data System figures show an unwelcome trend: A sharp uptick in promethazine abuse/misuse beginning in 2008, even while the total number of poisoning events of all kinds reported to the system began a steady decline (J Addict Med. 2015 May-Jun;9[3]:233-7).
Clonidine
This centrally acting alpha2-adrenoreceptor and imidazoline-receptor agonist is indicated for treatment of hypertension. However, it’s also extensively used off-label to treat anxiety, as well as for alcohol and opioid withdrawal symptoms. The problem is, clonidine boosts opioid-induced euphoria.
A retrospective study of clonidine-overdose patients characterized the clonidine overdose syndrome as marked by sedation, hypotension, bradycardia, and excessive pupillary constriction (Clin Toxicol [Phila]. 2017 Mar;55[3]:187-92).
“The combination of clonidine and opioids is particularly dangerous,” according to Dr. Walley. “Even though it mimics what an opioid overdose looks like, a clonidine overdose is not responsive to naloxone.”
Stimulants
One-quarter of patients who are prescribed methylphenidate or amphetamine for ADHD report being asked to divert their medication, and 11%-29% sell or give it to others seeking to use it recreationally or as a performance aid.
It’s common for prescription seekers to misrepresent symptoms of ADHD, and this play-acting is often tough to detect. In contrast, the nonstimulant atomoxetine (Strattera) and the alpha-adrenergic agonists prescribed for ADHD aren’t linked to misuse or diversion (Postgrad Med. 2014 Sep;126[5]:64-81).
Bupropion
This norepinephrine and dopamine reuptake inhibitor is generally assumed to have low abuse potential. That’s usually true – except in jail and prisons.
“In my patient population, where I have a keen eye to what’s being used on the street, bupropion is not one of the medications that I see very often in my patients who are not incarcerated,” Dr. Walley said. “But in incarcerated settings, it does have a street value.”
Consider safeguards
None of the prescription drugs on Dr. Walley’s problem list is included in prescription monitoring programs, nor are they detectable with standard toxicology testing. This poses a challenge for prescribing physicians.
Before prescribing any of these potentially abusable medications for a given patient, therefore, Dr. Walley considers the underlying risks. For example, an addiction history is a big red flag. So is coprescription of an opioid or another drug that might have synergistic adverse effects. Dr. Walley makes sure there is a solid indication for the medication, and, having prescribed the drug, he wants to see and document clear functional benefit.
Drug-specific toxicology screening is worthy of consideration as a means of confirming the presence of the prescribed medication, along with the absence of opioids or other drugs that shouldn’t be on board.
“I do this with gabapentin, because I see a lot of diversion,” he explained. “If gabapentin doesn’t show up in the toxicology screen, I stop prescribing it. Or if I detect it and it hasn’t been prescribed, that allows me to have a safety discussion with the patient.”
Dr. Walley reported no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM ACP INTERNAL MEDICINE
Hidradenitis suppurativa packs mighty QOL impact
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).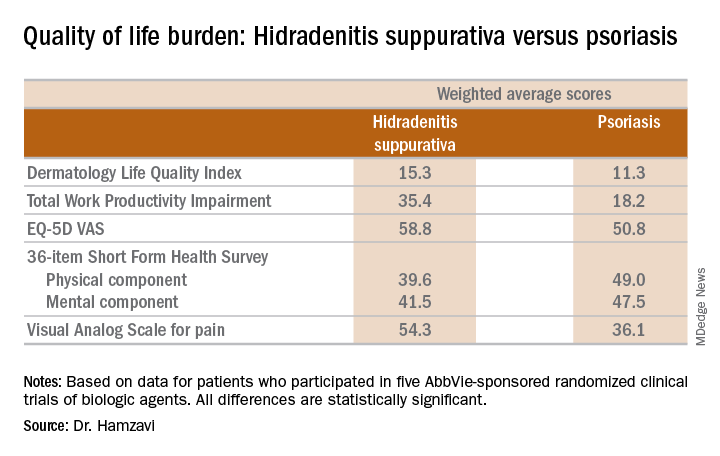
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Psoriasis duration reflects cardiovascular event risk
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Is PASI 100 the new benchmark in psoriasis?
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.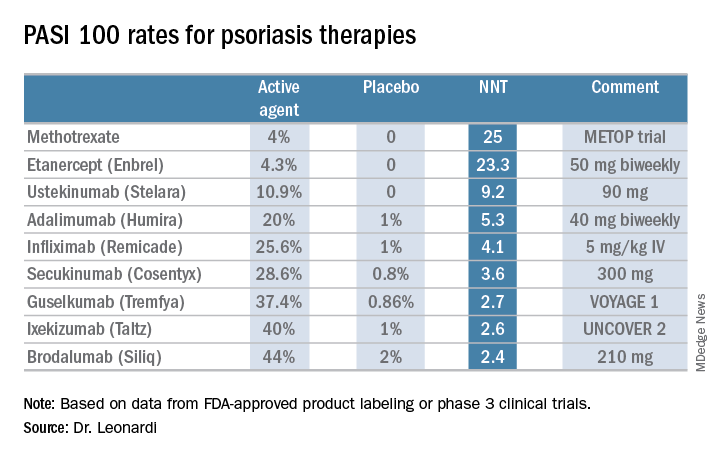
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Keloids: Cutting is never enough
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Statin-associated muscle symptoms? Rechallenge!
New Orleans – An effective and well-tolerated, evidence-based strategy for managing statin-associated muscle complaints is to rechallenge with the same statin, Douglas S. Paauw, MD, said at the annual meeting of the American College of Physicians.
That being said, it must be recognized that statin-associated muscle symptoms remain an enigma. This was underscored in a recent study by investigators at Hartford (Conn.) Hospital, which suggested a need to reassess the whole concept of statin-associated muscle symptoms, according to Dr. Paauw, professor of medicine at the University of Washington, Seattle.
“This study just blows my mind,” Dr. Paauw said. “The bottom line here is we just don’t know. A third of our patients might have true statin-associated muscle symptoms, but the other two-thirds would fall into these other groups, where they don’t have symptoms if we give them a statin again or they might get worse with placebo. So, we’ve got to rethink this.”
First things first
Before embarking on same-statin rechallenge, several things should first be done. Step 1 is to check the patient’s thyroid-stimulating hormone, creatine kinase, and serum vitamin D levels. Step 2 is to look for possible explanatory drug interactions, with verapamil and diltiazem being particularly common offenders in statin users.
If a patient with statin-associated muscle symptoms has a low serum vitamin D level, Dr. Paauw will give high-dose supplemental vitamin D. This strategy is supported by a University of Cincinnati prospective study of 146 patients with intolerable muscle symptoms on two or more statins, all of whom had a serum vitamin D below 32 ng/mL. They were placed on long-term vitamin D2 at 50,000-100,000 units per week and rechallenged with a statin. At 2 years of follow-up while still on supplemental vitamin D, 91% of patients had a normal serum vitamin D, and 95% of these previously statin-intolerant patients were on statin therapy without muscle complaints (N Am J Med Sci. 2015 Mar;7[3]:86-93).
Hypothyroidism increases statin toxicity. “I can’t overstate how important it is to check the thyroid-stimulating hormone to see if we’re dealing with something really simple,” Dr. Paauw said, “because trust me, treating hypothyroidism is much easier than treating statin-associated muscle symptoms.”
It has long been known that fibrates increase the risk of statin toxicity. Much less well recognized is that not all fibrates are the same in this regard: The risk is 15 times greater with gemfibrozil than with fenofibrate. The risk is also higher with verapamil or diltiazem than with nifedipine or amlodipine. Other important causes of drug interactions that increase statin toxicity include amiodarone, azole antifungals, protease inhibitors, erythromycin, and clarithromycin, but not azithromycin.
“Simvastatin and lovastatin are probably the worst as far as drug interactions,” Dr. Paauw cautioned. “They’re cheap, they’ve been generic for a long, long time, and a lot of times insurance companies want to move people to them. I try to move patients away from those two, because they are the dirtiest of the statins as far as side effects and drug interactions.”
When a drug interaction simply cannot be avoided, it’s best to have patients take the drugs 12 hours apart so peak levels don’t overlap, he added.
Same-statin rechallenge
If the checks of TSH, vitamin D, and possible drug interactions don’t turn up anything that needs fixing, it makes sense to launch same-statin rechallenge.
This management strategy was greenlighted by Canadian investigators in a retrospective study of 118 patients who presented to a tertiary lipid clinic with a history of muscle-related symptoms on at least two different statins.
At a median follow-up of 17 months, 71% of rechallenged patients were tolerating the same statin, compared with 53% of those who were switched to a different statin and 57% who were switched to ezetimibe or another nonstatin LDL-lowering drug. Moreover, 50% of patients in the same-statin rechallenge group achieved their target LDL, compared with only 15% switched to nonstatin therapy (Can J Cardiol. 2017 May;33[5]:666-73).
“I like this study; it helps a lot,” Dr. Paauw noted. “I think it’s worthwhile the first time they have muscle pain to get them off the statin and see if they feel better, so you become convinced that the statin may have had something to do with it. Then tell the patient, ‘Let’s just give it another try.’ ”
Alternative strategies
In the event of recurrent symptoms after same-statin rechallenge, it’s reasonable to switch to extended-release fluvastatin at 80 mg/day or low-dose rosuvastatin daily. Only if the patient remains symptomatic after trials of a couple of other statins does Dr. Paauw recommend turning to 5 mg of rosuvastatin twice weekly as a last resort.
Twice-weekly rosuvastatin is a popular treatment strategy in patients with intolerable muscle complaints on daily statin therapy. In a study of 40 such patients, twice-weekly rosuvastatin proved to be tolerated by 80% of them (Am J Cardiol. 2008 Jun 15;101[12]:1747-8). But the long-term effectiveness remains unknown.
“I think this is a really good option for our very statin-intolerant patients when nothing else works,” Dr. Paauw explained. “My only concern about this is we have no outcome data. Is twice-weekly rosuvastatin going to help reduce MI risk, especially if we give it for secondary prevention, as well as a daily statin? We know from this study that twice-weekly rosuvastatin can lower the lipids, but we also believe statins do more than just lower lipids. So, daily statin therapy is preferential.”
What about giving coenzyme Q10? It makes sense mechanistically: Coenzyme Q10 is an antioxidant, ubiquinone, and patients with statin-associated muscle symptoms are ubiquinone depleted.
But while oral coenzyme Q10 is a popular OTC treatment for statin-associated muscle complaints, it’s not supported by any good evidence. A meta-analysis of six randomized trials totaling 302 participants found coenzyme Q10 had no impact on symptoms (Mayo Clin Proc. 2015;90[1]:24-34).
In Dr. Paauw’s view, however, those trials didn’t address the proper question.
“The studies that have been done aren’t the right study,“ he said. “The study that needs to be done is for prevention: Do patients who start a statin and coenzyme Q10 at the same time stay on the statin and feel better?”
He reported having no financial conflicts of interest regarding his presentation.
New Orleans – An effective and well-tolerated, evidence-based strategy for managing statin-associated muscle complaints is to rechallenge with the same statin, Douglas S. Paauw, MD, said at the annual meeting of the American College of Physicians.
That being said, it must be recognized that statin-associated muscle symptoms remain an enigma. This was underscored in a recent study by investigators at Hartford (Conn.) Hospital, which suggested a need to reassess the whole concept of statin-associated muscle symptoms, according to Dr. Paauw, professor of medicine at the University of Washington, Seattle.
“This study just blows my mind,” Dr. Paauw said. “The bottom line here is we just don’t know. A third of our patients might have true statin-associated muscle symptoms, but the other two-thirds would fall into these other groups, where they don’t have symptoms if we give them a statin again or they might get worse with placebo. So, we’ve got to rethink this.”
First things first
Before embarking on same-statin rechallenge, several things should first be done. Step 1 is to check the patient’s thyroid-stimulating hormone, creatine kinase, and serum vitamin D levels. Step 2 is to look for possible explanatory drug interactions, with verapamil and diltiazem being particularly common offenders in statin users.
If a patient with statin-associated muscle symptoms has a low serum vitamin D level, Dr. Paauw will give high-dose supplemental vitamin D. This strategy is supported by a University of Cincinnati prospective study of 146 patients with intolerable muscle symptoms on two or more statins, all of whom had a serum vitamin D below 32 ng/mL. They were placed on long-term vitamin D2 at 50,000-100,000 units per week and rechallenged with a statin. At 2 years of follow-up while still on supplemental vitamin D, 91% of patients had a normal serum vitamin D, and 95% of these previously statin-intolerant patients were on statin therapy without muscle complaints (N Am J Med Sci. 2015 Mar;7[3]:86-93).
Hypothyroidism increases statin toxicity. “I can’t overstate how important it is to check the thyroid-stimulating hormone to see if we’re dealing with something really simple,” Dr. Paauw said, “because trust me, treating hypothyroidism is much easier than treating statin-associated muscle symptoms.”
It has long been known that fibrates increase the risk of statin toxicity. Much less well recognized is that not all fibrates are the same in this regard: The risk is 15 times greater with gemfibrozil than with fenofibrate. The risk is also higher with verapamil or diltiazem than with nifedipine or amlodipine. Other important causes of drug interactions that increase statin toxicity include amiodarone, azole antifungals, protease inhibitors, erythromycin, and clarithromycin, but not azithromycin.
“Simvastatin and lovastatin are probably the worst as far as drug interactions,” Dr. Paauw cautioned. “They’re cheap, they’ve been generic for a long, long time, and a lot of times insurance companies want to move people to them. I try to move patients away from those two, because they are the dirtiest of the statins as far as side effects and drug interactions.”
When a drug interaction simply cannot be avoided, it’s best to have patients take the drugs 12 hours apart so peak levels don’t overlap, he added.
Same-statin rechallenge
If the checks of TSH, vitamin D, and possible drug interactions don’t turn up anything that needs fixing, it makes sense to launch same-statin rechallenge.
This management strategy was greenlighted by Canadian investigators in a retrospective study of 118 patients who presented to a tertiary lipid clinic with a history of muscle-related symptoms on at least two different statins.
At a median follow-up of 17 months, 71% of rechallenged patients were tolerating the same statin, compared with 53% of those who were switched to a different statin and 57% who were switched to ezetimibe or another nonstatin LDL-lowering drug. Moreover, 50% of patients in the same-statin rechallenge group achieved their target LDL, compared with only 15% switched to nonstatin therapy (Can J Cardiol. 2017 May;33[5]:666-73).
“I like this study; it helps a lot,” Dr. Paauw noted. “I think it’s worthwhile the first time they have muscle pain to get them off the statin and see if they feel better, so you become convinced that the statin may have had something to do with it. Then tell the patient, ‘Let’s just give it another try.’ ”
Alternative strategies
In the event of recurrent symptoms after same-statin rechallenge, it’s reasonable to switch to extended-release fluvastatin at 80 mg/day or low-dose rosuvastatin daily. Only if the patient remains symptomatic after trials of a couple of other statins does Dr. Paauw recommend turning to 5 mg of rosuvastatin twice weekly as a last resort.
Twice-weekly rosuvastatin is a popular treatment strategy in patients with intolerable muscle complaints on daily statin therapy. In a study of 40 such patients, twice-weekly rosuvastatin proved to be tolerated by 80% of them (Am J Cardiol. 2008 Jun 15;101[12]:1747-8). But the long-term effectiveness remains unknown.
“I think this is a really good option for our very statin-intolerant patients when nothing else works,” Dr. Paauw explained. “My only concern about this is we have no outcome data. Is twice-weekly rosuvastatin going to help reduce MI risk, especially if we give it for secondary prevention, as well as a daily statin? We know from this study that twice-weekly rosuvastatin can lower the lipids, but we also believe statins do more than just lower lipids. So, daily statin therapy is preferential.”
What about giving coenzyme Q10? It makes sense mechanistically: Coenzyme Q10 is an antioxidant, ubiquinone, and patients with statin-associated muscle symptoms are ubiquinone depleted.
But while oral coenzyme Q10 is a popular OTC treatment for statin-associated muscle complaints, it’s not supported by any good evidence. A meta-analysis of six randomized trials totaling 302 participants found coenzyme Q10 had no impact on symptoms (Mayo Clin Proc. 2015;90[1]:24-34).
In Dr. Paauw’s view, however, those trials didn’t address the proper question.
“The studies that have been done aren’t the right study,“ he said. “The study that needs to be done is for prevention: Do patients who start a statin and coenzyme Q10 at the same time stay on the statin and feel better?”
He reported having no financial conflicts of interest regarding his presentation.
New Orleans – An effective and well-tolerated, evidence-based strategy for managing statin-associated muscle complaints is to rechallenge with the same statin, Douglas S. Paauw, MD, said at the annual meeting of the American College of Physicians.
That being said, it must be recognized that statin-associated muscle symptoms remain an enigma. This was underscored in a recent study by investigators at Hartford (Conn.) Hospital, which suggested a need to reassess the whole concept of statin-associated muscle symptoms, according to Dr. Paauw, professor of medicine at the University of Washington, Seattle.
“This study just blows my mind,” Dr. Paauw said. “The bottom line here is we just don’t know. A third of our patients might have true statin-associated muscle symptoms, but the other two-thirds would fall into these other groups, where they don’t have symptoms if we give them a statin again or they might get worse with placebo. So, we’ve got to rethink this.”
First things first
Before embarking on same-statin rechallenge, several things should first be done. Step 1 is to check the patient’s thyroid-stimulating hormone, creatine kinase, and serum vitamin D levels. Step 2 is to look for possible explanatory drug interactions, with verapamil and diltiazem being particularly common offenders in statin users.
If a patient with statin-associated muscle symptoms has a low serum vitamin D level, Dr. Paauw will give high-dose supplemental vitamin D. This strategy is supported by a University of Cincinnati prospective study of 146 patients with intolerable muscle symptoms on two or more statins, all of whom had a serum vitamin D below 32 ng/mL. They were placed on long-term vitamin D2 at 50,000-100,000 units per week and rechallenged with a statin. At 2 years of follow-up while still on supplemental vitamin D, 91% of patients had a normal serum vitamin D, and 95% of these previously statin-intolerant patients were on statin therapy without muscle complaints (N Am J Med Sci. 2015 Mar;7[3]:86-93).
Hypothyroidism increases statin toxicity. “I can’t overstate how important it is to check the thyroid-stimulating hormone to see if we’re dealing with something really simple,” Dr. Paauw said, “because trust me, treating hypothyroidism is much easier than treating statin-associated muscle symptoms.”
It has long been known that fibrates increase the risk of statin toxicity. Much less well recognized is that not all fibrates are the same in this regard: The risk is 15 times greater with gemfibrozil than with fenofibrate. The risk is also higher with verapamil or diltiazem than with nifedipine or amlodipine. Other important causes of drug interactions that increase statin toxicity include amiodarone, azole antifungals, protease inhibitors, erythromycin, and clarithromycin, but not azithromycin.
“Simvastatin and lovastatin are probably the worst as far as drug interactions,” Dr. Paauw cautioned. “They’re cheap, they’ve been generic for a long, long time, and a lot of times insurance companies want to move people to them. I try to move patients away from those two, because they are the dirtiest of the statins as far as side effects and drug interactions.”
When a drug interaction simply cannot be avoided, it’s best to have patients take the drugs 12 hours apart so peak levels don’t overlap, he added.
Same-statin rechallenge
If the checks of TSH, vitamin D, and possible drug interactions don’t turn up anything that needs fixing, it makes sense to launch same-statin rechallenge.
This management strategy was greenlighted by Canadian investigators in a retrospective study of 118 patients who presented to a tertiary lipid clinic with a history of muscle-related symptoms on at least two different statins.
At a median follow-up of 17 months, 71% of rechallenged patients were tolerating the same statin, compared with 53% of those who were switched to a different statin and 57% who were switched to ezetimibe or another nonstatin LDL-lowering drug. Moreover, 50% of patients in the same-statin rechallenge group achieved their target LDL, compared with only 15% switched to nonstatin therapy (Can J Cardiol. 2017 May;33[5]:666-73).
“I like this study; it helps a lot,” Dr. Paauw noted. “I think it’s worthwhile the first time they have muscle pain to get them off the statin and see if they feel better, so you become convinced that the statin may have had something to do with it. Then tell the patient, ‘Let’s just give it another try.’ ”
Alternative strategies
In the event of recurrent symptoms after same-statin rechallenge, it’s reasonable to switch to extended-release fluvastatin at 80 mg/day or low-dose rosuvastatin daily. Only if the patient remains symptomatic after trials of a couple of other statins does Dr. Paauw recommend turning to 5 mg of rosuvastatin twice weekly as a last resort.
Twice-weekly rosuvastatin is a popular treatment strategy in patients with intolerable muscle complaints on daily statin therapy. In a study of 40 such patients, twice-weekly rosuvastatin proved to be tolerated by 80% of them (Am J Cardiol. 2008 Jun 15;101[12]:1747-8). But the long-term effectiveness remains unknown.
“I think this is a really good option for our very statin-intolerant patients when nothing else works,” Dr. Paauw explained. “My only concern about this is we have no outcome data. Is twice-weekly rosuvastatin going to help reduce MI risk, especially if we give it for secondary prevention, as well as a daily statin? We know from this study that twice-weekly rosuvastatin can lower the lipids, but we also believe statins do more than just lower lipids. So, daily statin therapy is preferential.”
What about giving coenzyme Q10? It makes sense mechanistically: Coenzyme Q10 is an antioxidant, ubiquinone, and patients with statin-associated muscle symptoms are ubiquinone depleted.
But while oral coenzyme Q10 is a popular OTC treatment for statin-associated muscle complaints, it’s not supported by any good evidence. A meta-analysis of six randomized trials totaling 302 participants found coenzyme Q10 had no impact on symptoms (Mayo Clin Proc. 2015;90[1]:24-34).
In Dr. Paauw’s view, however, those trials didn’t address the proper question.
“The studies that have been done aren’t the right study,“ he said. “The study that needs to be done is for prevention: Do patients who start a statin and coenzyme Q10 at the same time stay on the statin and feel better?”
He reported having no financial conflicts of interest regarding his presentation.
REPORTING FROM ACP INTERNAL MEDICINE
Apixaban prevails in study of 163,000 DOAC users
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
REPORTING FROM ACC 2018
Key clinical point:
Major finding: The risk of stroke/systemic embolism in apixaban-treated patients was 31% lower than in those on dabigatran and 27% lower than with rivaroxaban.
Study details: This retrospective, observational study based upon claims data included 162,707 propensity score-matched patients with atrial fibrillation on a direct oral anticoagulant for stroke prevention.
Disclosures: The ongoing ARISTOPHANES study is sponsored by Bristol-Myers Squibb and Pfizer. The presenter reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
Source: Deitelzweig SB. ACC 2018.

















