User login
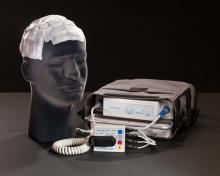
FDA Panel Supports Device That Targets Brain Tumors
GAITHERSBURG, MD. – A portable, battery-powered device that delivers electrical fields to the brain has a favorable risk-benefit profile in patients who have used up other treatment options for recurrent glioblastoma multiforme, according to a Food and Drug Administration advisory committee.
The FDA’s Neurological Devices Advisory Panel voted 7-3 with two abstentions on March 17 that the benefits of the NovoTTF-100A system exceed its risks when used as monotherapy in adults who have exhausted surgical and radiation treatment options for histologically or radiologically confirmed recurrent glioblastoma multiforme (GBM).
The device’s manufacturer, Israel-based Novocure Ltd., is seeking an indication in this population based on the results of a company-sponsored prospective randomized trial that compared treatment with the device to best standard of care (BSC) chemotherapy in 237 patients with recurrent GBM.
Panelists emphasized that the indication should reflect that patients have also failed chemotherapy options, and that while the treatment appears to be safe short term, the potential for chronic adverse effects should be monitored closely.
The device weighs about 6 pounds and is intended to be worn about 20 hours per day. It delivers "tumor treating fields" (TTF) via four electrodes placed on the scalp. This creates "a low intensity, alternating electric field within the tumor that exerts physical forces on electrically charged cellular components, preventing the normal mitotic process and causing cancer cell death prior to division," according to the company. Normal cells are not affected, it said.
In the pivotal study, median overall survival, the primary end point, was 6.3 months among those treated with the device and 6.4 months among those treated with the best available chemotherapy. Investigators have presented subgroup analyses showing better survival in some populations.
Both the FDA and the panelists had concerns about methodologic problems in the study, which was designed to show that TTF therapy is superior to the best standard of care chemotherapy. But those voting positively cited the safety of the device, and evidence that it benefited some patients with few treatment options. Median survival of patients diagnosed with GBM is 15 months, and the 5-year overall survival rate is less than 10%, according to data cited by the company and the FDA.
NovoCure is conducting a phase III study of the device in patients with newly diagnosed GBM, in combination with temozolomide. The device has been approved in the European Union as a treatment for newly diagnosed and recurrent GBM and for the treatment of non–small cell lung cancer.
The FDA usually follows the recommendations of its advisory committees. Panel members have been cleared of potential conflicts of interest by the FDA before meetings.
GAITHERSBURG, MD. – A portable, battery-powered device that delivers electrical fields to the brain has a favorable risk-benefit profile in patients who have used up other treatment options for recurrent glioblastoma multiforme, according to a Food and Drug Administration advisory committee.
The FDA’s Neurological Devices Advisory Panel voted 7-3 with two abstentions on March 17 that the benefits of the NovoTTF-100A system exceed its risks when used as monotherapy in adults who have exhausted surgical and radiation treatment options for histologically or radiologically confirmed recurrent glioblastoma multiforme (GBM).
The device’s manufacturer, Israel-based Novocure Ltd., is seeking an indication in this population based on the results of a company-sponsored prospective randomized trial that compared treatment with the device to best standard of care (BSC) chemotherapy in 237 patients with recurrent GBM.
Panelists emphasized that the indication should reflect that patients have also failed chemotherapy options, and that while the treatment appears to be safe short term, the potential for chronic adverse effects should be monitored closely.
The device weighs about 6 pounds and is intended to be worn about 20 hours per day. It delivers "tumor treating fields" (TTF) via four electrodes placed on the scalp. This creates "a low intensity, alternating electric field within the tumor that exerts physical forces on electrically charged cellular components, preventing the normal mitotic process and causing cancer cell death prior to division," according to the company. Normal cells are not affected, it said.
In the pivotal study, median overall survival, the primary end point, was 6.3 months among those treated with the device and 6.4 months among those treated with the best available chemotherapy. Investigators have presented subgroup analyses showing better survival in some populations.
Both the FDA and the panelists had concerns about methodologic problems in the study, which was designed to show that TTF therapy is superior to the best standard of care chemotherapy. But those voting positively cited the safety of the device, and evidence that it benefited some patients with few treatment options. Median survival of patients diagnosed with GBM is 15 months, and the 5-year overall survival rate is less than 10%, according to data cited by the company and the FDA.
NovoCure is conducting a phase III study of the device in patients with newly diagnosed GBM, in combination with temozolomide. The device has been approved in the European Union as a treatment for newly diagnosed and recurrent GBM and for the treatment of non–small cell lung cancer.
The FDA usually follows the recommendations of its advisory committees. Panel members have been cleared of potential conflicts of interest by the FDA before meetings.
GAITHERSBURG, MD. – A portable, battery-powered device that delivers electrical fields to the brain has a favorable risk-benefit profile in patients who have used up other treatment options for recurrent glioblastoma multiforme, according to a Food and Drug Administration advisory committee.
The FDA’s Neurological Devices Advisory Panel voted 7-3 with two abstentions on March 17 that the benefits of the NovoTTF-100A system exceed its risks when used as monotherapy in adults who have exhausted surgical and radiation treatment options for histologically or radiologically confirmed recurrent glioblastoma multiforme (GBM).
The device’s manufacturer, Israel-based Novocure Ltd., is seeking an indication in this population based on the results of a company-sponsored prospective randomized trial that compared treatment with the device to best standard of care (BSC) chemotherapy in 237 patients with recurrent GBM.
Panelists emphasized that the indication should reflect that patients have also failed chemotherapy options, and that while the treatment appears to be safe short term, the potential for chronic adverse effects should be monitored closely.
The device weighs about 6 pounds and is intended to be worn about 20 hours per day. It delivers "tumor treating fields" (TTF) via four electrodes placed on the scalp. This creates "a low intensity, alternating electric field within the tumor that exerts physical forces on electrically charged cellular components, preventing the normal mitotic process and causing cancer cell death prior to division," according to the company. Normal cells are not affected, it said.
In the pivotal study, median overall survival, the primary end point, was 6.3 months among those treated with the device and 6.4 months among those treated with the best available chemotherapy. Investigators have presented subgroup analyses showing better survival in some populations.
Both the FDA and the panelists had concerns about methodologic problems in the study, which was designed to show that TTF therapy is superior to the best standard of care chemotherapy. But those voting positively cited the safety of the device, and evidence that it benefited some patients with few treatment options. Median survival of patients diagnosed with GBM is 15 months, and the 5-year overall survival rate is less than 10%, according to data cited by the company and the FDA.
NovoCure is conducting a phase III study of the device in patients with newly diagnosed GBM, in combination with temozolomide. The device has been approved in the European Union as a treatment for newly diagnosed and recurrent GBM and for the treatment of non–small cell lung cancer.
The FDA usually follows the recommendations of its advisory committees. Panel members have been cleared of potential conflicts of interest by the FDA before meetings.
FROM THE FDA'S NEUROLOGICAL DEVICES ADVISORY PANEL
SDEF: Consider Potential Risks of Newly Approved Therapeutics
The limitations of the drug approval process in fully characterizing a new drug's safety profile are clearly illustrated in the dermatologic therapeutics arena and clinicians should keep these limitations in mind when prescribing and counseling patients about a relatively new therapeutic agent, according to Dr. Joel M. Gelfand.
Previously unknown risks of new drugs are routinely identified after Food and Drug Administration approval, when "rare" adverse events are more likely to be detected, said Dr. Gelfand at the Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF). Examples of rare adverse events – those that occur at a rate of less than 1 per 1,000 – include fatal arrhythmias associated with terfenadine and astemizole, lymphomas associated with biologics, and progressive multifocal leukoencephalopathy (PML) associated with efalizumab.
The clinical trials that are the basis of drug approvals evaluate short-term safety only and lack the statistical power to detect rare events, he said, pointing out that a study of 3,000 patients can only detect adverse events that occur at a rate of more than 1 per 1,000.
The types of adverse effects detected pre-approval are pharmacologic side effects, which are common and dose dependent, such as isotretinoin-induced cheilitis, said Dr. Gelfand, of the department of dermatology at the University of Pennsylvania in Philadelphia.
Adverse effects that are detected after approval fall into two categories: idiosyncratic or allergic reactions, which are rare and "occur in close proximity to exposure," he said, such as dapsone-induced agranulocytosis, and new morbidities, which are delayed and uncommon. An example of the latter is skin cancers associated with psoralen and ultraviolet A (PUVA) treatment, which was associated with melanoma in 1997, more than 2 decades after it was found to be effective for treating psoriasis.
There is a need for ongoing risk assessment throughout the life cycle of a drug, Dr. Gelfand said, which includes MedWatch, the FDA's voluntary adverse event reporting program that relies on spontaneous reporting of adverse events. MedWatch's advantages include that it is inexpensive and can identify safety signals; however, it is limited by under-reporting and MedWatch reports usually cannot be used to determine causality, he noted.
The association between efalizumab (Raptiva) and PML, an untreatable and often fatal central nervous system infection that occurs primarily in the setting of immunosuppression, is an example of "signal detection in action," Dr. Gelfand said. Efalizumab was approved in 2003 for psoriasis, based on studies of about 2,700 people, including 218 treated for more than 1 year. By 2008, after 46,000 people had been treated, including 3,000 for at least 2 years, there had been 3 confirmed cases and one suspected case of PML. All four were spontaneous reports, which "can be useful for very rare diseases such as PML," he said.
With efalizumab treatment, the overall estimated risk of PML is 1 in 15,000 patients per year and one in 1,000 patients treated for more than 2 years. These are likely underestimates because of incomplete reporting, and is a relationship that is "likely causal," he said. In the case of efalizumab, the risk was "judged unacceptable given treatment alternatives and disease indication" and the drug was withdrawn from the market in 2009.
A causal association between isotretinoin and inflammatory bowel disease (IBD) has not been established, but the drug's prescribing information includes a warning about the association.
The data on isotretinoin and IBD include a large administrative claims database of over 8,000 cases of IBD, and 21,832 controls published in 2010 (Am. J. Gastroenterol. 2010;105:1986-93). Analysis of the database found that the risk of IBD within 12 months of being treated with isotretinoin was 1.7; for ulcerative colitis, the risk was increased fourfold; for Crohn's disease the risk was slightly reduced.
In that study, which controlled for age, sex, and geographic region, the dose response was evident for ulcerative colitis only, Dr. Gelfand said.
In a review of 85 spontaneous reports of IBD in isotretinoin patients to the FDA between 1997-2002, isotretinoin was considered "highly probable" as the cause in 4 cases (5%), "probable" in 58 cases (68%), and "possible" in 23 cases (27%). These included three cases with a positive dechallenge and rechallenge.
When in comes to ulcerative colitis, the overall data available "suggest a specific association" between isotretinoin and the disease, but the data are conflicting, he said. Studies have not addressed the possibility that patients with severe acne are at an increased risk of ulcerative colitis, or of confounding variables including oral antibiotics and smoking, he said. If the risk is real, it is small, with a number needed to harm that exceeds 3,300.
With these examples in mind, Dr. Gelfand advised clinicians to "use the science of medicine in judging safety... [and] the art of medicine" when communicating the risk of therapies to their patients.
"The benefits of treatments are well-characterized relative to the long-term safety and the risk of rare but serious medical events," he said. "Sir William Osler was the first to recognize the need to be cautious when prescribing new medications advising physicians 'Do not be the first to prescribe a new drug and do not be the last to stop prescribing an old drug.' "
Dr. Gelfand disclosed that he has been an investigator and/or consultant to Amgen, Abbott, Centocor, Pfizer, Celegene, Novartis, and Genentech, and that his presentation was his work only.
SDEF and this news organization are owned by Elsevier.
The limitations of the drug approval process in fully characterizing a new drug's safety profile are clearly illustrated in the dermatologic therapeutics arena and clinicians should keep these limitations in mind when prescribing and counseling patients about a relatively new therapeutic agent, according to Dr. Joel M. Gelfand.
Previously unknown risks of new drugs are routinely identified after Food and Drug Administration approval, when "rare" adverse events are more likely to be detected, said Dr. Gelfand at the Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF). Examples of rare adverse events – those that occur at a rate of less than 1 per 1,000 – include fatal arrhythmias associated with terfenadine and astemizole, lymphomas associated with biologics, and progressive multifocal leukoencephalopathy (PML) associated with efalizumab.
The clinical trials that are the basis of drug approvals evaluate short-term safety only and lack the statistical power to detect rare events, he said, pointing out that a study of 3,000 patients can only detect adverse events that occur at a rate of more than 1 per 1,000.
The types of adverse effects detected pre-approval are pharmacologic side effects, which are common and dose dependent, such as isotretinoin-induced cheilitis, said Dr. Gelfand, of the department of dermatology at the University of Pennsylvania in Philadelphia.
Adverse effects that are detected after approval fall into two categories: idiosyncratic or allergic reactions, which are rare and "occur in close proximity to exposure," he said, such as dapsone-induced agranulocytosis, and new morbidities, which are delayed and uncommon. An example of the latter is skin cancers associated with psoralen and ultraviolet A (PUVA) treatment, which was associated with melanoma in 1997, more than 2 decades after it was found to be effective for treating psoriasis.
There is a need for ongoing risk assessment throughout the life cycle of a drug, Dr. Gelfand said, which includes MedWatch, the FDA's voluntary adverse event reporting program that relies on spontaneous reporting of adverse events. MedWatch's advantages include that it is inexpensive and can identify safety signals; however, it is limited by under-reporting and MedWatch reports usually cannot be used to determine causality, he noted.
The association between efalizumab (Raptiva) and PML, an untreatable and often fatal central nervous system infection that occurs primarily in the setting of immunosuppression, is an example of "signal detection in action," Dr. Gelfand said. Efalizumab was approved in 2003 for psoriasis, based on studies of about 2,700 people, including 218 treated for more than 1 year. By 2008, after 46,000 people had been treated, including 3,000 for at least 2 years, there had been 3 confirmed cases and one suspected case of PML. All four were spontaneous reports, which "can be useful for very rare diseases such as PML," he said.
With efalizumab treatment, the overall estimated risk of PML is 1 in 15,000 patients per year and one in 1,000 patients treated for more than 2 years. These are likely underestimates because of incomplete reporting, and is a relationship that is "likely causal," he said. In the case of efalizumab, the risk was "judged unacceptable given treatment alternatives and disease indication" and the drug was withdrawn from the market in 2009.
A causal association between isotretinoin and inflammatory bowel disease (IBD) has not been established, but the drug's prescribing information includes a warning about the association.
The data on isotretinoin and IBD include a large administrative claims database of over 8,000 cases of IBD, and 21,832 controls published in 2010 (Am. J. Gastroenterol. 2010;105:1986-93). Analysis of the database found that the risk of IBD within 12 months of being treated with isotretinoin was 1.7; for ulcerative colitis, the risk was increased fourfold; for Crohn's disease the risk was slightly reduced.
In that study, which controlled for age, sex, and geographic region, the dose response was evident for ulcerative colitis only, Dr. Gelfand said.
In a review of 85 spontaneous reports of IBD in isotretinoin patients to the FDA between 1997-2002, isotretinoin was considered "highly probable" as the cause in 4 cases (5%), "probable" in 58 cases (68%), and "possible" in 23 cases (27%). These included three cases with a positive dechallenge and rechallenge.
When in comes to ulcerative colitis, the overall data available "suggest a specific association" between isotretinoin and the disease, but the data are conflicting, he said. Studies have not addressed the possibility that patients with severe acne are at an increased risk of ulcerative colitis, or of confounding variables including oral antibiotics and smoking, he said. If the risk is real, it is small, with a number needed to harm that exceeds 3,300.
With these examples in mind, Dr. Gelfand advised clinicians to "use the science of medicine in judging safety... [and] the art of medicine" when communicating the risk of therapies to their patients.
"The benefits of treatments are well-characterized relative to the long-term safety and the risk of rare but serious medical events," he said. "Sir William Osler was the first to recognize the need to be cautious when prescribing new medications advising physicians 'Do not be the first to prescribe a new drug and do not be the last to stop prescribing an old drug.' "
Dr. Gelfand disclosed that he has been an investigator and/or consultant to Amgen, Abbott, Centocor, Pfizer, Celegene, Novartis, and Genentech, and that his presentation was his work only.
SDEF and this news organization are owned by Elsevier.
The limitations of the drug approval process in fully characterizing a new drug's safety profile are clearly illustrated in the dermatologic therapeutics arena and clinicians should keep these limitations in mind when prescribing and counseling patients about a relatively new therapeutic agent, according to Dr. Joel M. Gelfand.
Previously unknown risks of new drugs are routinely identified after Food and Drug Administration approval, when "rare" adverse events are more likely to be detected, said Dr. Gelfand at the Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF). Examples of rare adverse events – those that occur at a rate of less than 1 per 1,000 – include fatal arrhythmias associated with terfenadine and astemizole, lymphomas associated with biologics, and progressive multifocal leukoencephalopathy (PML) associated with efalizumab.
The clinical trials that are the basis of drug approvals evaluate short-term safety only and lack the statistical power to detect rare events, he said, pointing out that a study of 3,000 patients can only detect adverse events that occur at a rate of more than 1 per 1,000.
The types of adverse effects detected pre-approval are pharmacologic side effects, which are common and dose dependent, such as isotretinoin-induced cheilitis, said Dr. Gelfand, of the department of dermatology at the University of Pennsylvania in Philadelphia.
Adverse effects that are detected after approval fall into two categories: idiosyncratic or allergic reactions, which are rare and "occur in close proximity to exposure," he said, such as dapsone-induced agranulocytosis, and new morbidities, which are delayed and uncommon. An example of the latter is skin cancers associated with psoralen and ultraviolet A (PUVA) treatment, which was associated with melanoma in 1997, more than 2 decades after it was found to be effective for treating psoriasis.
There is a need for ongoing risk assessment throughout the life cycle of a drug, Dr. Gelfand said, which includes MedWatch, the FDA's voluntary adverse event reporting program that relies on spontaneous reporting of adverse events. MedWatch's advantages include that it is inexpensive and can identify safety signals; however, it is limited by under-reporting and MedWatch reports usually cannot be used to determine causality, he noted.
The association between efalizumab (Raptiva) and PML, an untreatable and often fatal central nervous system infection that occurs primarily in the setting of immunosuppression, is an example of "signal detection in action," Dr. Gelfand said. Efalizumab was approved in 2003 for psoriasis, based on studies of about 2,700 people, including 218 treated for more than 1 year. By 2008, after 46,000 people had been treated, including 3,000 for at least 2 years, there had been 3 confirmed cases and one suspected case of PML. All four were spontaneous reports, which "can be useful for very rare diseases such as PML," he said.
With efalizumab treatment, the overall estimated risk of PML is 1 in 15,000 patients per year and one in 1,000 patients treated for more than 2 years. These are likely underestimates because of incomplete reporting, and is a relationship that is "likely causal," he said. In the case of efalizumab, the risk was "judged unacceptable given treatment alternatives and disease indication" and the drug was withdrawn from the market in 2009.
A causal association between isotretinoin and inflammatory bowel disease (IBD) has not been established, but the drug's prescribing information includes a warning about the association.
The data on isotretinoin and IBD include a large administrative claims database of over 8,000 cases of IBD, and 21,832 controls published in 2010 (Am. J. Gastroenterol. 2010;105:1986-93). Analysis of the database found that the risk of IBD within 12 months of being treated with isotretinoin was 1.7; for ulcerative colitis, the risk was increased fourfold; for Crohn's disease the risk was slightly reduced.
In that study, which controlled for age, sex, and geographic region, the dose response was evident for ulcerative colitis only, Dr. Gelfand said.
In a review of 85 spontaneous reports of IBD in isotretinoin patients to the FDA between 1997-2002, isotretinoin was considered "highly probable" as the cause in 4 cases (5%), "probable" in 58 cases (68%), and "possible" in 23 cases (27%). These included three cases with a positive dechallenge and rechallenge.
When in comes to ulcerative colitis, the overall data available "suggest a specific association" between isotretinoin and the disease, but the data are conflicting, he said. Studies have not addressed the possibility that patients with severe acne are at an increased risk of ulcerative colitis, or of confounding variables including oral antibiotics and smoking, he said. If the risk is real, it is small, with a number needed to harm that exceeds 3,300.
With these examples in mind, Dr. Gelfand advised clinicians to "use the science of medicine in judging safety... [and] the art of medicine" when communicating the risk of therapies to their patients.
"The benefits of treatments are well-characterized relative to the long-term safety and the risk of rare but serious medical events," he said. "Sir William Osler was the first to recognize the need to be cautious when prescribing new medications advising physicians 'Do not be the first to prescribe a new drug and do not be the last to stop prescribing an old drug.' "
Dr. Gelfand disclosed that he has been an investigator and/or consultant to Amgen, Abbott, Centocor, Pfizer, Celegene, Novartis, and Genentech, and that his presentation was his work only.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dearth of Drugs Leaves Few Options for Obesity
As studies documenting the toll that obesity and excess weight have on health and mortality continue to be reported, access to multidisciplinary weight-loss programs remain poor – and the approved weight-loss drugs have dwindled to just orlistat and phentermine.
With the absence of effective pharmaceutical options and inconsistent insurance coverage of multidisciplinary weight-loss programs, the options that clinicians have available to address the treatment gap between basic lifestyle changes and referral for surgical treatment of obesity now include improved in-office counseling, setting modest weight-loss goals, the use of medications with weight loss as a side effect for indicated conditions, and online weight-loss programs.
A recent study of national health survey data suggests that frank conversations are far more motivating than previously thought. Researchers found that fewer than half of overweight people surveyed and fewer than two-thirds of the obese people surveyed had been told by their physicians that they were overweight. The study also found that if their physicians had told them they were overweight, the odds that the patient would see themselves as overweight and would try to lose weight would increase considerably.
Dr. Adrienne Youdim, medical director of the center for weight loss at Cedars-Sinai Medical Center, Los Angeles, said in an interview that the study shows that "physician advice goes a long way towards patients recognizing the condition [and] is a first step in addressing the condition." Although there are multiple barriers to effective weight-loss treatment that are often cited (including lack of resources, lack of reimbursement, personal physician bias, or lack of comfort in addressing this disease), "from a preventive medicine standpoint [and] as internists and primary care physicians, we need to take this challenge not only in identification and diagnosis but also [in] providing guidance and counseling on how to address the problem," said Dr. Youdim, who was not involved in the study. Without intervening at the front lines, "we cannot sway the tide of what has become the No. 1 public health crisis in the United States."
She also recommended helping patients keep modest weight-loss goals that are achievable with lifestyle changes. "When you’re talking about disease burden, a lot can be gained from modest weight loss and small lifestyle changes – an important point that is often understated," she said, referring to a study indicating that patients would reach their desired weight if they lost more than 100 pounds, and that they would be disappointed if they lost 17% of their body weight. But that is a substantial amount of weight, she pointed out, particularly because a 5%-10% weight loss results in significant cardiometabolic improvements.
Many studies show "that physician-guided advice goes a long way, and that it doesn’t have to be intensive," Dr. Youdim said. Physicians can use outside resources for behavioral modification if they don’t have a dietician on staff. She recommends programs like Weight Watchers, Overeaters Anonymous, and TOPS Club, which can be augmented with in-office counseling and even applications that are available for smart phones and can used by a motivated patient to help monitor food intake, she added.
"Be forceful, direct, and blunt in your discussions with patients about the need for the interventions in terms of diet and exercise," as advocated by the American Heart Association, the American College of Cardiology, the American Diabetes Association, the American Association of Clinical Endocrinologists, and other organizations, said Dr. Helena Rodbard, an endocrinologist in Gaithersburg, Md. She recommended that clinicians calculate the body mass index of their overweight and obese patients "and let people know where they stand in terms of the percentiles of their body weight compared to the norms set before the current epidemic." She advises clinicians to screen patients for metabolic syndrome, prediabetes, and diabetes, and to inform them about their risk factors, using calculations from available programs about patients’ risks of developing heart disease or diabetes within the next 5-10 years. She emphasized paying special attention to obesity in youth and adolescents, who "will be obese for life and have diabetes early and diabetic complications early."
In an interview, Dr. Rodbard said that because of logistical issues and costs, she rarely refers patients to a weight-loss program; instead, she counsels them and arranges for them to see a dietician in her office 1 or 2 days a week. "Frequent return visits and motivational sessions within those visits are helpful," she said, advising clinicians to use the services of a dietician when possible, and to "fight for better reimbursement for those kinds of services."
The prospects for any new obesity drugs’ becoming available in the near future dimmed considerably after the majority of the Food and Drug Administration’s Endocrinologic Drugs Advisory Panel recommended against approval of the phentermine-topiramate (Qnexa) combination and the serotonergic drug lorcaserin (Lorqess) at meetings last summer and fall. Then in February, the FDA put its decision on another drug combination – the antidepressant bupropion and the opioid antagonist naltrexone (Contrave) – on hold when it requested a large cardiovascular safety trial in overweight and obese people before the agency would consider approval.
Dr. Rodbard, who is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology, said that although these drugs had potential side effects, she considers their side effects to be lower than the risk of remaining obese, adding that "it is a tragedy that we do not have more and better drugs available for treatment of obesity, and that there has not been a larger and more effective and sustained program to change the lifestyle habits of Americans with regard to diet and exercise."
Although Dr. Youdim acknowledged that concerns over the safety of the drugs are legitimate, "there is a lot of bias around drugs for obesity, because the thought is that if patients would just stop eating, they’d lose weight. ... The rigor placed on these drugs is a lot greater because of that bias." But obesity is a disease, she added, "and there should be treatment options available to us," just as there are options available for other diseases.
Although weight-loss drugs have not been effective on their own, they have been useful adjuncts to lifestyle modification, and "the cornerstone of treatment in a select group of patients who are having trouble adhering to lifestyle recommendations."
Dr. Youdim said that certain drugs are associated with some weight loss and can be used – not "completely without indication" – to help patients lose weight. For example, a patient who is both overweight and clinically depressed could be prescribed bupropion, someone with insulin resistance can benefit from metformin, and a patient with type 2 diabetes who requires insulin may benefit from exenatide (Byetta). All three of these drugs are associated with weight loss.
Dr. Rodbard said that in patients with diabetes in whom exenatide is indicated, about a third of those receiving it "have very dramatic weight loss ... especially in some patients with a very massive degree of obesity to begin with," and that this loss is "very nicely sustained." Published data support the notion that the combination of the glucagonlike peptide–1 receptor agonists with insulin will help to reduce the weight gain associated with insulin therapy, she added.
Metformin has a very small effect on weight, but appears to have beneficial effects on the risk of heart disease and cancer; "hence, it is one of the backbones of therapy for type 2 diabetes and used in combination with nearly all other medications," she added.
As for the FDA-approved weight-loss drugs, Dr. Rodbard said that she rarely uses phentermine, and – when it was available – she rarely used sibutramine, which was used mainly because there were not many other options available. The side effects of orlistat, she said, are unacceptable "to all but a very few patients."
Dr. Louis Aronne, director of the comprehensive weight control program at New York–Presbyterian Hospital, said that there clearly is a need for more medical treatments for obesity – as well as improved access to comprehensive weight-loss programs that are affordable and effective – before the clinicians opt for surgery. "Right now, a patient can go from Weight Watchers to the operating room" for bariatric surgery because of the gap in treatment and dearth of medical options, he said.
Dr. Aronne is a developer of an online program designed to address the treatment gap and poor access to comprehensive weight-loss programs. The "BMIQ" program is a 16-session, online, comprehensive program run by a dietician in a group setting. It includes dietary and exercise counseling that is aimed at helping obese people with type 2 diabetes to lose weight and manage their diabetes by using a "weight-centric approach" efficiently and without extra costs. Primary care physicians refer their patients to the program; after the patient fills out an evaluation form, the physician receives information on the patient along with recommendations on how to manage that patient. For example, the clinician is alerted if the patient is taking over-the-counter sleep medications that contain strong antihistamines, which can both cause weight gain and make it difficult to lose weight; eliminating them is "like giving someone an appetite suppressant," he said.
In a pilot program, a large New York State health insurer is providing the BMIQ program to 2,000 of its beneficiaries who are overweight and have type 2 diabetes. Data indicate that programs like this help patients lose weight, reduce medication costs, and can save over $1,000 per person, and "we’re hopeful that we will be able to show the insurer that they can save money," said Dr. Aronne, who believes that insurers and employers will pay for the program once they see that it’s an efficient model of delivering this service. The program is available to people now, at a cost of $300, at BMIQ.com.
Dr. Youdim had no disclosures. Dr. Aronne said he has been involved in studies of more than 20 weight-loss drugs, including the three reviewed by the FDA panel last year, and has served as a consultant to Vivus and Orexigen; he also developed the BMIQ program. Dr. Rodbard has received research grants and served as a consultant to, adviser to, and/or speaker for Amylin, Astra-Zeneca, Biodel, Bristol-Myers Squibb, Eli Lilly, Mannkind, Merck, Roche, and Sanofi-Aventis.
As studies documenting the toll that obesity and excess weight have on health and mortality continue to be reported, access to multidisciplinary weight-loss programs remain poor – and the approved weight-loss drugs have dwindled to just orlistat and phentermine.
With the absence of effective pharmaceutical options and inconsistent insurance coverage of multidisciplinary weight-loss programs, the options that clinicians have available to address the treatment gap between basic lifestyle changes and referral for surgical treatment of obesity now include improved in-office counseling, setting modest weight-loss goals, the use of medications with weight loss as a side effect for indicated conditions, and online weight-loss programs.
A recent study of national health survey data suggests that frank conversations are far more motivating than previously thought. Researchers found that fewer than half of overweight people surveyed and fewer than two-thirds of the obese people surveyed had been told by their physicians that they were overweight. The study also found that if their physicians had told them they were overweight, the odds that the patient would see themselves as overweight and would try to lose weight would increase considerably.
Dr. Adrienne Youdim, medical director of the center for weight loss at Cedars-Sinai Medical Center, Los Angeles, said in an interview that the study shows that "physician advice goes a long way towards patients recognizing the condition [and] is a first step in addressing the condition." Although there are multiple barriers to effective weight-loss treatment that are often cited (including lack of resources, lack of reimbursement, personal physician bias, or lack of comfort in addressing this disease), "from a preventive medicine standpoint [and] as internists and primary care physicians, we need to take this challenge not only in identification and diagnosis but also [in] providing guidance and counseling on how to address the problem," said Dr. Youdim, who was not involved in the study. Without intervening at the front lines, "we cannot sway the tide of what has become the No. 1 public health crisis in the United States."
She also recommended helping patients keep modest weight-loss goals that are achievable with lifestyle changes. "When you’re talking about disease burden, a lot can be gained from modest weight loss and small lifestyle changes – an important point that is often understated," she said, referring to a study indicating that patients would reach their desired weight if they lost more than 100 pounds, and that they would be disappointed if they lost 17% of their body weight. But that is a substantial amount of weight, she pointed out, particularly because a 5%-10% weight loss results in significant cardiometabolic improvements.
Many studies show "that physician-guided advice goes a long way, and that it doesn’t have to be intensive," Dr. Youdim said. Physicians can use outside resources for behavioral modification if they don’t have a dietician on staff. She recommends programs like Weight Watchers, Overeaters Anonymous, and TOPS Club, which can be augmented with in-office counseling and even applications that are available for smart phones and can used by a motivated patient to help monitor food intake, she added.
"Be forceful, direct, and blunt in your discussions with patients about the need for the interventions in terms of diet and exercise," as advocated by the American Heart Association, the American College of Cardiology, the American Diabetes Association, the American Association of Clinical Endocrinologists, and other organizations, said Dr. Helena Rodbard, an endocrinologist in Gaithersburg, Md. She recommended that clinicians calculate the body mass index of their overweight and obese patients "and let people know where they stand in terms of the percentiles of their body weight compared to the norms set before the current epidemic." She advises clinicians to screen patients for metabolic syndrome, prediabetes, and diabetes, and to inform them about their risk factors, using calculations from available programs about patients’ risks of developing heart disease or diabetes within the next 5-10 years. She emphasized paying special attention to obesity in youth and adolescents, who "will be obese for life and have diabetes early and diabetic complications early."
In an interview, Dr. Rodbard said that because of logistical issues and costs, she rarely refers patients to a weight-loss program; instead, she counsels them and arranges for them to see a dietician in her office 1 or 2 days a week. "Frequent return visits and motivational sessions within those visits are helpful," she said, advising clinicians to use the services of a dietician when possible, and to "fight for better reimbursement for those kinds of services."
The prospects for any new obesity drugs’ becoming available in the near future dimmed considerably after the majority of the Food and Drug Administration’s Endocrinologic Drugs Advisory Panel recommended against approval of the phentermine-topiramate (Qnexa) combination and the serotonergic drug lorcaserin (Lorqess) at meetings last summer and fall. Then in February, the FDA put its decision on another drug combination – the antidepressant bupropion and the opioid antagonist naltrexone (Contrave) – on hold when it requested a large cardiovascular safety trial in overweight and obese people before the agency would consider approval.
Dr. Rodbard, who is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology, said that although these drugs had potential side effects, she considers their side effects to be lower than the risk of remaining obese, adding that "it is a tragedy that we do not have more and better drugs available for treatment of obesity, and that there has not been a larger and more effective and sustained program to change the lifestyle habits of Americans with regard to diet and exercise."
Although Dr. Youdim acknowledged that concerns over the safety of the drugs are legitimate, "there is a lot of bias around drugs for obesity, because the thought is that if patients would just stop eating, they’d lose weight. ... The rigor placed on these drugs is a lot greater because of that bias." But obesity is a disease, she added, "and there should be treatment options available to us," just as there are options available for other diseases.
Although weight-loss drugs have not been effective on their own, they have been useful adjuncts to lifestyle modification, and "the cornerstone of treatment in a select group of patients who are having trouble adhering to lifestyle recommendations."
Dr. Youdim said that certain drugs are associated with some weight loss and can be used – not "completely without indication" – to help patients lose weight. For example, a patient who is both overweight and clinically depressed could be prescribed bupropion, someone with insulin resistance can benefit from metformin, and a patient with type 2 diabetes who requires insulin may benefit from exenatide (Byetta). All three of these drugs are associated with weight loss.
Dr. Rodbard said that in patients with diabetes in whom exenatide is indicated, about a third of those receiving it "have very dramatic weight loss ... especially in some patients with a very massive degree of obesity to begin with," and that this loss is "very nicely sustained." Published data support the notion that the combination of the glucagonlike peptide–1 receptor agonists with insulin will help to reduce the weight gain associated with insulin therapy, she added.
Metformin has a very small effect on weight, but appears to have beneficial effects on the risk of heart disease and cancer; "hence, it is one of the backbones of therapy for type 2 diabetes and used in combination with nearly all other medications," she added.
As for the FDA-approved weight-loss drugs, Dr. Rodbard said that she rarely uses phentermine, and – when it was available – she rarely used sibutramine, which was used mainly because there were not many other options available. The side effects of orlistat, she said, are unacceptable "to all but a very few patients."
Dr. Louis Aronne, director of the comprehensive weight control program at New York–Presbyterian Hospital, said that there clearly is a need for more medical treatments for obesity – as well as improved access to comprehensive weight-loss programs that are affordable and effective – before the clinicians opt for surgery. "Right now, a patient can go from Weight Watchers to the operating room" for bariatric surgery because of the gap in treatment and dearth of medical options, he said.
Dr. Aronne is a developer of an online program designed to address the treatment gap and poor access to comprehensive weight-loss programs. The "BMIQ" program is a 16-session, online, comprehensive program run by a dietician in a group setting. It includes dietary and exercise counseling that is aimed at helping obese people with type 2 diabetes to lose weight and manage their diabetes by using a "weight-centric approach" efficiently and without extra costs. Primary care physicians refer their patients to the program; after the patient fills out an evaluation form, the physician receives information on the patient along with recommendations on how to manage that patient. For example, the clinician is alerted if the patient is taking over-the-counter sleep medications that contain strong antihistamines, which can both cause weight gain and make it difficult to lose weight; eliminating them is "like giving someone an appetite suppressant," he said.
In a pilot program, a large New York State health insurer is providing the BMIQ program to 2,000 of its beneficiaries who are overweight and have type 2 diabetes. Data indicate that programs like this help patients lose weight, reduce medication costs, and can save over $1,000 per person, and "we’re hopeful that we will be able to show the insurer that they can save money," said Dr. Aronne, who believes that insurers and employers will pay for the program once they see that it’s an efficient model of delivering this service. The program is available to people now, at a cost of $300, at BMIQ.com.
Dr. Youdim had no disclosures. Dr. Aronne said he has been involved in studies of more than 20 weight-loss drugs, including the three reviewed by the FDA panel last year, and has served as a consultant to Vivus and Orexigen; he also developed the BMIQ program. Dr. Rodbard has received research grants and served as a consultant to, adviser to, and/or speaker for Amylin, Astra-Zeneca, Biodel, Bristol-Myers Squibb, Eli Lilly, Mannkind, Merck, Roche, and Sanofi-Aventis.
As studies documenting the toll that obesity and excess weight have on health and mortality continue to be reported, access to multidisciplinary weight-loss programs remain poor – and the approved weight-loss drugs have dwindled to just orlistat and phentermine.
With the absence of effective pharmaceutical options and inconsistent insurance coverage of multidisciplinary weight-loss programs, the options that clinicians have available to address the treatment gap between basic lifestyle changes and referral for surgical treatment of obesity now include improved in-office counseling, setting modest weight-loss goals, the use of medications with weight loss as a side effect for indicated conditions, and online weight-loss programs.
A recent study of national health survey data suggests that frank conversations are far more motivating than previously thought. Researchers found that fewer than half of overweight people surveyed and fewer than two-thirds of the obese people surveyed had been told by their physicians that they were overweight. The study also found that if their physicians had told them they were overweight, the odds that the patient would see themselves as overweight and would try to lose weight would increase considerably.
Dr. Adrienne Youdim, medical director of the center for weight loss at Cedars-Sinai Medical Center, Los Angeles, said in an interview that the study shows that "physician advice goes a long way towards patients recognizing the condition [and] is a first step in addressing the condition." Although there are multiple barriers to effective weight-loss treatment that are often cited (including lack of resources, lack of reimbursement, personal physician bias, or lack of comfort in addressing this disease), "from a preventive medicine standpoint [and] as internists and primary care physicians, we need to take this challenge not only in identification and diagnosis but also [in] providing guidance and counseling on how to address the problem," said Dr. Youdim, who was not involved in the study. Without intervening at the front lines, "we cannot sway the tide of what has become the No. 1 public health crisis in the United States."
She also recommended helping patients keep modest weight-loss goals that are achievable with lifestyle changes. "When you’re talking about disease burden, a lot can be gained from modest weight loss and small lifestyle changes – an important point that is often understated," she said, referring to a study indicating that patients would reach their desired weight if they lost more than 100 pounds, and that they would be disappointed if they lost 17% of their body weight. But that is a substantial amount of weight, she pointed out, particularly because a 5%-10% weight loss results in significant cardiometabolic improvements.
Many studies show "that physician-guided advice goes a long way, and that it doesn’t have to be intensive," Dr. Youdim said. Physicians can use outside resources for behavioral modification if they don’t have a dietician on staff. She recommends programs like Weight Watchers, Overeaters Anonymous, and TOPS Club, which can be augmented with in-office counseling and even applications that are available for smart phones and can used by a motivated patient to help monitor food intake, she added.
"Be forceful, direct, and blunt in your discussions with patients about the need for the interventions in terms of diet and exercise," as advocated by the American Heart Association, the American College of Cardiology, the American Diabetes Association, the American Association of Clinical Endocrinologists, and other organizations, said Dr. Helena Rodbard, an endocrinologist in Gaithersburg, Md. She recommended that clinicians calculate the body mass index of their overweight and obese patients "and let people know where they stand in terms of the percentiles of their body weight compared to the norms set before the current epidemic." She advises clinicians to screen patients for metabolic syndrome, prediabetes, and diabetes, and to inform them about their risk factors, using calculations from available programs about patients’ risks of developing heart disease or diabetes within the next 5-10 years. She emphasized paying special attention to obesity in youth and adolescents, who "will be obese for life and have diabetes early and diabetic complications early."
In an interview, Dr. Rodbard said that because of logistical issues and costs, she rarely refers patients to a weight-loss program; instead, she counsels them and arranges for them to see a dietician in her office 1 or 2 days a week. "Frequent return visits and motivational sessions within those visits are helpful," she said, advising clinicians to use the services of a dietician when possible, and to "fight for better reimbursement for those kinds of services."
The prospects for any new obesity drugs’ becoming available in the near future dimmed considerably after the majority of the Food and Drug Administration’s Endocrinologic Drugs Advisory Panel recommended against approval of the phentermine-topiramate (Qnexa) combination and the serotonergic drug lorcaserin (Lorqess) at meetings last summer and fall. Then in February, the FDA put its decision on another drug combination – the antidepressant bupropion and the opioid antagonist naltrexone (Contrave) – on hold when it requested a large cardiovascular safety trial in overweight and obese people before the agency would consider approval.
Dr. Rodbard, who is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology, said that although these drugs had potential side effects, she considers their side effects to be lower than the risk of remaining obese, adding that "it is a tragedy that we do not have more and better drugs available for treatment of obesity, and that there has not been a larger and more effective and sustained program to change the lifestyle habits of Americans with regard to diet and exercise."
Although Dr. Youdim acknowledged that concerns over the safety of the drugs are legitimate, "there is a lot of bias around drugs for obesity, because the thought is that if patients would just stop eating, they’d lose weight. ... The rigor placed on these drugs is a lot greater because of that bias." But obesity is a disease, she added, "and there should be treatment options available to us," just as there are options available for other diseases.
Although weight-loss drugs have not been effective on their own, they have been useful adjuncts to lifestyle modification, and "the cornerstone of treatment in a select group of patients who are having trouble adhering to lifestyle recommendations."
Dr. Youdim said that certain drugs are associated with some weight loss and can be used – not "completely without indication" – to help patients lose weight. For example, a patient who is both overweight and clinically depressed could be prescribed bupropion, someone with insulin resistance can benefit from metformin, and a patient with type 2 diabetes who requires insulin may benefit from exenatide (Byetta). All three of these drugs are associated with weight loss.
Dr. Rodbard said that in patients with diabetes in whom exenatide is indicated, about a third of those receiving it "have very dramatic weight loss ... especially in some patients with a very massive degree of obesity to begin with," and that this loss is "very nicely sustained." Published data support the notion that the combination of the glucagonlike peptide–1 receptor agonists with insulin will help to reduce the weight gain associated with insulin therapy, she added.
Metformin has a very small effect on weight, but appears to have beneficial effects on the risk of heart disease and cancer; "hence, it is one of the backbones of therapy for type 2 diabetes and used in combination with nearly all other medications," she added.
As for the FDA-approved weight-loss drugs, Dr. Rodbard said that she rarely uses phentermine, and – when it was available – she rarely used sibutramine, which was used mainly because there were not many other options available. The side effects of orlistat, she said, are unacceptable "to all but a very few patients."
Dr. Louis Aronne, director of the comprehensive weight control program at New York–Presbyterian Hospital, said that there clearly is a need for more medical treatments for obesity – as well as improved access to comprehensive weight-loss programs that are affordable and effective – before the clinicians opt for surgery. "Right now, a patient can go from Weight Watchers to the operating room" for bariatric surgery because of the gap in treatment and dearth of medical options, he said.
Dr. Aronne is a developer of an online program designed to address the treatment gap and poor access to comprehensive weight-loss programs. The "BMIQ" program is a 16-session, online, comprehensive program run by a dietician in a group setting. It includes dietary and exercise counseling that is aimed at helping obese people with type 2 diabetes to lose weight and manage their diabetes by using a "weight-centric approach" efficiently and without extra costs. Primary care physicians refer their patients to the program; after the patient fills out an evaluation form, the physician receives information on the patient along with recommendations on how to manage that patient. For example, the clinician is alerted if the patient is taking over-the-counter sleep medications that contain strong antihistamines, which can both cause weight gain and make it difficult to lose weight; eliminating them is "like giving someone an appetite suppressant," he said.
In a pilot program, a large New York State health insurer is providing the BMIQ program to 2,000 of its beneficiaries who are overweight and have type 2 diabetes. Data indicate that programs like this help patients lose weight, reduce medication costs, and can save over $1,000 per person, and "we’re hopeful that we will be able to show the insurer that they can save money," said Dr. Aronne, who believes that insurers and employers will pay for the program once they see that it’s an efficient model of delivering this service. The program is available to people now, at a cost of $300, at BMIQ.com.
Dr. Youdim had no disclosures. Dr. Aronne said he has been involved in studies of more than 20 weight-loss drugs, including the three reviewed by the FDA panel last year, and has served as a consultant to Vivus and Orexigen; he also developed the BMIQ program. Dr. Rodbard has received research grants and served as a consultant to, adviser to, and/or speaker for Amylin, Astra-Zeneca, Biodel, Bristol-Myers Squibb, Eli Lilly, Mannkind, Merck, Roche, and Sanofi-Aventis.
Dearth of Drugs Leaves Few Options for Obesity
As studies documenting the toll that obesity and excess weight have on health and mortality continue to be reported, access to multidisciplinary weight-loss programs remain poor – and the approved weight-loss drugs have dwindled to just orlistat and phentermine.
With the absence of effective pharmaceutical options and inconsistent insurance coverage of multidisciplinary weight-loss programs, the options that clinicians have available to address the treatment gap between basic lifestyle changes and referral for surgical treatment of obesity now include improved in-office counseling, setting modest weight-loss goals, the use of medications with weight loss as a side effect for indicated conditions, and online weight-loss programs.
A recent study of national health survey data suggests that frank conversations are far more motivating than previously thought. Researchers found that fewer than half of overweight people surveyed and fewer than two-thirds of the obese people surveyed had been told by their physicians that they were overweight. The study also found that if their physicians had told them they were overweight, the odds that the patient would see themselves as overweight and would try to lose weight would increase considerably.
Dr. Adrienne Youdim, medical director of the center for weight loss at Cedars-Sinai Medical Center, Los Angeles, said in an interview that the study shows that "physician advice goes a long way towards patients recognizing the condition [and] is a first step in addressing the condition." Although there are multiple barriers to effective weight-loss treatment that are often cited (including lack of resources, lack of reimbursement, personal physician bias, or lack of comfort in addressing this disease), "from a preventive medicine standpoint [and] as internists and primary care physicians, we need to take this challenge not only in identification and diagnosis but also [in] providing guidance and counseling on how to address the problem," said Dr. Youdim, who was not involved in the study. Without intervening at the front lines, "we cannot sway the tide of what has become the No. 1 public health crisis in the United States."
She also recommended helping patients keep modest weight-loss goals that are achievable with lifestyle changes. "When you’re talking about disease burden, a lot can be gained from modest weight loss and small lifestyle changes – an important point that is often understated," she said, referring to a study indicating that patients would reach their desired weight if they lost more than 100 pounds, and that they would be disappointed if they lost 17% of their body weight. But that is a substantial amount of weight, she pointed out, particularly because a 5%-10% weight loss results in significant cardiometabolic improvements.
Many studies show "that physician-guided advice goes a long way, and that it doesn’t have to be intensive," Dr. Youdim said. Physicians can use outside resources for behavioral modification if they don’t have a dietician on staff. She recommends programs like Weight Watchers, Overeaters Anonymous, and TOPS Club, which can be augmented with in-office counseling and even applications that are available for smart phones and can used by a motivated patient to help monitor food intake, she added.
"Be forceful, direct, and blunt in your discussions with patients about the need for the interventions in terms of diet and exercise," as advocated by the American Heart Association, the American College of Cardiology, the American Diabetes Association, the American Association of Clinical Endocrinologists, and other organizations, said Dr. Helena Rodbard, an endocrinologist in Gaithersburg, Md. She recommended that clinicians calculate the body mass index of their overweight and obese patients "and let people know where they stand in terms of the percentiles of their body weight compared to the norms set before the current epidemic." She advises clinicians to screen patients for metabolic syndrome, prediabetes, and diabetes, and to inform them about their risk factors, using calculations from available programs about patients’ risks of developing heart disease or diabetes within the next 5-10 years. She emphasized paying special attention to obesity in youth and adolescents, who "will be obese for life and have diabetes early and diabetic complications early."
In an interview, Dr. Rodbard said that because of logistical issues and costs, she rarely refers patients to a weight-loss program; instead, she counsels them and arranges for them to see a dietician in her office 1 or 2 days a week. "Frequent return visits and motivational sessions within those visits are helpful," she said, advising clinicians to use the services of a dietician when possible, and to "fight for better reimbursement for those kinds of services."
The prospects for any new obesity drugs’ becoming available in the near future dimmed considerably after the majority of the Food and Drug Administration’s Endocrinologic Drugs Advisory Panel recommended against approval of the phentermine-topiramate (Qnexa) combination and the serotonergic drug lorcaserin (Lorqess) at meetings last summer and fall. Then in February, the FDA put its decision on another drug combination – the antidepressant bupropion and the opioid antagonist naltrexone (Contrave) – on hold when it requested a large cardiovascular safety trial in overweight and obese people before the agency would consider approval.
Dr. Rodbard, who is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology, said that although these drugs had potential side effects, she considers their side effects to be lower than the risk of remaining obese, adding that "it is a tragedy that we do not have more and better drugs available for treatment of obesity, and that there has not been a larger and more effective and sustained program to change the lifestyle habits of Americans with regard to diet and exercise."
Although Dr. Youdim acknowledged that concerns over the safety of the drugs are legitimate, "there is a lot of bias around drugs for obesity, because the thought is that if patients would just stop eating, they’d lose weight. ... The rigor placed on these drugs is a lot greater because of that bias." But obesity is a disease, she added, "and there should be treatment options available to us," just as there are options available for other diseases.
Although weight-loss drugs have not been effective on their own, they have been useful adjuncts to lifestyle modification, and "the cornerstone of treatment in a select group of patients who are having trouble adhering to lifestyle recommendations."
Dr. Youdim said that certain drugs are associated with some weight loss and can be used – not "completely without indication" – to help patients lose weight. For example, a patient who is both overweight and clinically depressed could be prescribed bupropion, someone with insulin resistance can benefit from metformin, and a patient with type 2 diabetes who requires insulin may benefit from exenatide (Byetta). All three of these drugs are associated with weight loss.
Dr. Rodbard said that in patients with diabetes in whom exenatide is indicated, about a third of those receiving it "have very dramatic weight loss ... especially in some patients with a very massive degree of obesity to begin with," and that this loss is "very nicely sustained." Published data support the notion that the combination of the glucagonlike peptide–1 receptor agonists with insulin will help to reduce the weight gain associated with insulin therapy, she added.
Metformin has a very small effect on weight, but appears to have beneficial effects on the risk of heart disease and cancer; "hence, it is one of the backbones of therapy for type 2 diabetes and used in combination with nearly all other medications," she added.
As for the FDA-approved weight-loss drugs, Dr. Rodbard said that she rarely uses phentermine, and – when it was available – she rarely used sibutramine, which was used mainly because there were not many other options available. The side effects of orlistat, she said, are unacceptable "to all but a very few patients."
Dr. Louis Aronne, director of the comprehensive weight control program at New York–Presbyterian Hospital, said that there clearly is a need for more medical treatments for obesity – as well as improved access to comprehensive weight-loss programs that are affordable and effective – before the clinicians opt for surgery. "Right now, a patient can go from Weight Watchers to the operating room" for bariatric surgery because of the gap in treatment and dearth of medical options, he said.
Dr. Aronne is a developer of an online program designed to address the treatment gap and poor access to comprehensive weight-loss programs. The "BMIQ" program is a 16-session, online, comprehensive program run by a dietician in a group setting. It includes dietary and exercise counseling that is aimed at helping obese people with type 2 diabetes to lose weight and manage their diabetes by using a "weight-centric approach" efficiently and without extra costs. Primary care physicians refer their patients to the program; after the patient fills out an evaluation form, the physician receives information on the patient along with recommendations on how to manage that patient. For example, the clinician is alerted if the patient is taking over-the-counter sleep medications that contain strong antihistamines, which can both cause weight gain and make it difficult to lose weight; eliminating them is "like giving someone an appetite suppressant," he said.
In a pilot program, a large New York State health insurer is providing the BMIQ program to 2,000 of its beneficiaries who are overweight and have type 2 diabetes. Data indicate that programs like this help patients lose weight, reduce medication costs, and can save over $1,000 per person, and "we’re hopeful that we will be able to show the insurer that they can save money," said Dr. Aronne, who believes that insurers and employers will pay for the program once they see that it’s an efficient model of delivering this service. The program is available to people now, at a cost of $300, at BMIQ.com.
Dr. Youdim had no disclosures. Dr. Aronne said he has been involved in studies of more than 20 weight-loss drugs, including the three reviewed by the FDA panel last year, and has served as a consultant to Vivus and Orexigen; he also developed the BMIQ program. Dr. Rodbard has received research grants and served as a consultant to, adviser to, and/or speaker for Amylin, Astra-Zeneca, Biodel, Bristol-Myers Squibb, Eli Lilly, Mannkind, Merck, Roche, and Sanofi-Aventis.
As studies documenting the toll that obesity and excess weight have on health and mortality continue to be reported, access to multidisciplinary weight-loss programs remain poor – and the approved weight-loss drugs have dwindled to just orlistat and phentermine.
With the absence of effective pharmaceutical options and inconsistent insurance coverage of multidisciplinary weight-loss programs, the options that clinicians have available to address the treatment gap between basic lifestyle changes and referral for surgical treatment of obesity now include improved in-office counseling, setting modest weight-loss goals, the use of medications with weight loss as a side effect for indicated conditions, and online weight-loss programs.
A recent study of national health survey data suggests that frank conversations are far more motivating than previously thought. Researchers found that fewer than half of overweight people surveyed and fewer than two-thirds of the obese people surveyed had been told by their physicians that they were overweight. The study also found that if their physicians had told them they were overweight, the odds that the patient would see themselves as overweight and would try to lose weight would increase considerably.
Dr. Adrienne Youdim, medical director of the center for weight loss at Cedars-Sinai Medical Center, Los Angeles, said in an interview that the study shows that "physician advice goes a long way towards patients recognizing the condition [and] is a first step in addressing the condition." Although there are multiple barriers to effective weight-loss treatment that are often cited (including lack of resources, lack of reimbursement, personal physician bias, or lack of comfort in addressing this disease), "from a preventive medicine standpoint [and] as internists and primary care physicians, we need to take this challenge not only in identification and diagnosis but also [in] providing guidance and counseling on how to address the problem," said Dr. Youdim, who was not involved in the study. Without intervening at the front lines, "we cannot sway the tide of what has become the No. 1 public health crisis in the United States."
She also recommended helping patients keep modest weight-loss goals that are achievable with lifestyle changes. "When you’re talking about disease burden, a lot can be gained from modest weight loss and small lifestyle changes – an important point that is often understated," she said, referring to a study indicating that patients would reach their desired weight if they lost more than 100 pounds, and that they would be disappointed if they lost 17% of their body weight. But that is a substantial amount of weight, she pointed out, particularly because a 5%-10% weight loss results in significant cardiometabolic improvements.
Many studies show "that physician-guided advice goes a long way, and that it doesn’t have to be intensive," Dr. Youdim said. Physicians can use outside resources for behavioral modification if they don’t have a dietician on staff. She recommends programs like Weight Watchers, Overeaters Anonymous, and TOPS Club, which can be augmented with in-office counseling and even applications that are available for smart phones and can used by a motivated patient to help monitor food intake, she added.
"Be forceful, direct, and blunt in your discussions with patients about the need for the interventions in terms of diet and exercise," as advocated by the American Heart Association, the American College of Cardiology, the American Diabetes Association, the American Association of Clinical Endocrinologists, and other organizations, said Dr. Helena Rodbard, an endocrinologist in Gaithersburg, Md. She recommended that clinicians calculate the body mass index of their overweight and obese patients "and let people know where they stand in terms of the percentiles of their body weight compared to the norms set before the current epidemic." She advises clinicians to screen patients for metabolic syndrome, prediabetes, and diabetes, and to inform them about their risk factors, using calculations from available programs about patients’ risks of developing heart disease or diabetes within the next 5-10 years. She emphasized paying special attention to obesity in youth and adolescents, who "will be obese for life and have diabetes early and diabetic complications early."
In an interview, Dr. Rodbard said that because of logistical issues and costs, she rarely refers patients to a weight-loss program; instead, she counsels them and arranges for them to see a dietician in her office 1 or 2 days a week. "Frequent return visits and motivational sessions within those visits are helpful," she said, advising clinicians to use the services of a dietician when possible, and to "fight for better reimbursement for those kinds of services."
The prospects for any new obesity drugs’ becoming available in the near future dimmed considerably after the majority of the Food and Drug Administration’s Endocrinologic Drugs Advisory Panel recommended against approval of the phentermine-topiramate (Qnexa) combination and the serotonergic drug lorcaserin (Lorqess) at meetings last summer and fall. Then in February, the FDA put its decision on another drug combination – the antidepressant bupropion and the opioid antagonist naltrexone (Contrave) – on hold when it requested a large cardiovascular safety trial in overweight and obese people before the agency would consider approval.
Dr. Rodbard, who is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology, said that although these drugs had potential side effects, she considers their side effects to be lower than the risk of remaining obese, adding that "it is a tragedy that we do not have more and better drugs available for treatment of obesity, and that there has not been a larger and more effective and sustained program to change the lifestyle habits of Americans with regard to diet and exercise."
Although Dr. Youdim acknowledged that concerns over the safety of the drugs are legitimate, "there is a lot of bias around drugs for obesity, because the thought is that if patients would just stop eating, they’d lose weight. ... The rigor placed on these drugs is a lot greater because of that bias." But obesity is a disease, she added, "and there should be treatment options available to us," just as there are options available for other diseases.
Although weight-loss drugs have not been effective on their own, they have been useful adjuncts to lifestyle modification, and "the cornerstone of treatment in a select group of patients who are having trouble adhering to lifestyle recommendations."
Dr. Youdim said that certain drugs are associated with some weight loss and can be used – not "completely without indication" – to help patients lose weight. For example, a patient who is both overweight and clinically depressed could be prescribed bupropion, someone with insulin resistance can benefit from metformin, and a patient with type 2 diabetes who requires insulin may benefit from exenatide (Byetta). All three of these drugs are associated with weight loss.
Dr. Rodbard said that in patients with diabetes in whom exenatide is indicated, about a third of those receiving it "have very dramatic weight loss ... especially in some patients with a very massive degree of obesity to begin with," and that this loss is "very nicely sustained." Published data support the notion that the combination of the glucagonlike peptide–1 receptor agonists with insulin will help to reduce the weight gain associated with insulin therapy, she added.
Metformin has a very small effect on weight, but appears to have beneficial effects on the risk of heart disease and cancer; "hence, it is one of the backbones of therapy for type 2 diabetes and used in combination with nearly all other medications," she added.
As for the FDA-approved weight-loss drugs, Dr. Rodbard said that she rarely uses phentermine, and – when it was available – she rarely used sibutramine, which was used mainly because there were not many other options available. The side effects of orlistat, she said, are unacceptable "to all but a very few patients."
Dr. Louis Aronne, director of the comprehensive weight control program at New York–Presbyterian Hospital, said that there clearly is a need for more medical treatments for obesity – as well as improved access to comprehensive weight-loss programs that are affordable and effective – before the clinicians opt for surgery. "Right now, a patient can go from Weight Watchers to the operating room" for bariatric surgery because of the gap in treatment and dearth of medical options, he said.
Dr. Aronne is a developer of an online program designed to address the treatment gap and poor access to comprehensive weight-loss programs. The "BMIQ" program is a 16-session, online, comprehensive program run by a dietician in a group setting. It includes dietary and exercise counseling that is aimed at helping obese people with type 2 diabetes to lose weight and manage their diabetes by using a "weight-centric approach" efficiently and without extra costs. Primary care physicians refer their patients to the program; after the patient fills out an evaluation form, the physician receives information on the patient along with recommendations on how to manage that patient. For example, the clinician is alerted if the patient is taking over-the-counter sleep medications that contain strong antihistamines, which can both cause weight gain and make it difficult to lose weight; eliminating them is "like giving someone an appetite suppressant," he said.
In a pilot program, a large New York State health insurer is providing the BMIQ program to 2,000 of its beneficiaries who are overweight and have type 2 diabetes. Data indicate that programs like this help patients lose weight, reduce medication costs, and can save over $1,000 per person, and "we’re hopeful that we will be able to show the insurer that they can save money," said Dr. Aronne, who believes that insurers and employers will pay for the program once they see that it’s an efficient model of delivering this service. The program is available to people now, at a cost of $300, at BMIQ.com.
Dr. Youdim had no disclosures. Dr. Aronne said he has been involved in studies of more than 20 weight-loss drugs, including the three reviewed by the FDA panel last year, and has served as a consultant to Vivus and Orexigen; he also developed the BMIQ program. Dr. Rodbard has received research grants and served as a consultant to, adviser to, and/or speaker for Amylin, Astra-Zeneca, Biodel, Bristol-Myers Squibb, Eli Lilly, Mannkind, Merck, Roche, and Sanofi-Aventis.
As studies documenting the toll that obesity and excess weight have on health and mortality continue to be reported, access to multidisciplinary weight-loss programs remain poor – and the approved weight-loss drugs have dwindled to just orlistat and phentermine.
With the absence of effective pharmaceutical options and inconsistent insurance coverage of multidisciplinary weight-loss programs, the options that clinicians have available to address the treatment gap between basic lifestyle changes and referral for surgical treatment of obesity now include improved in-office counseling, setting modest weight-loss goals, the use of medications with weight loss as a side effect for indicated conditions, and online weight-loss programs.
A recent study of national health survey data suggests that frank conversations are far more motivating than previously thought. Researchers found that fewer than half of overweight people surveyed and fewer than two-thirds of the obese people surveyed had been told by their physicians that they were overweight. The study also found that if their physicians had told them they were overweight, the odds that the patient would see themselves as overweight and would try to lose weight would increase considerably.
Dr. Adrienne Youdim, medical director of the center for weight loss at Cedars-Sinai Medical Center, Los Angeles, said in an interview that the study shows that "physician advice goes a long way towards patients recognizing the condition [and] is a first step in addressing the condition." Although there are multiple barriers to effective weight-loss treatment that are often cited (including lack of resources, lack of reimbursement, personal physician bias, or lack of comfort in addressing this disease), "from a preventive medicine standpoint [and] as internists and primary care physicians, we need to take this challenge not only in identification and diagnosis but also [in] providing guidance and counseling on how to address the problem," said Dr. Youdim, who was not involved in the study. Without intervening at the front lines, "we cannot sway the tide of what has become the No. 1 public health crisis in the United States."
She also recommended helping patients keep modest weight-loss goals that are achievable with lifestyle changes. "When you’re talking about disease burden, a lot can be gained from modest weight loss and small lifestyle changes – an important point that is often understated," she said, referring to a study indicating that patients would reach their desired weight if they lost more than 100 pounds, and that they would be disappointed if they lost 17% of their body weight. But that is a substantial amount of weight, she pointed out, particularly because a 5%-10% weight loss results in significant cardiometabolic improvements.
Many studies show "that physician-guided advice goes a long way, and that it doesn’t have to be intensive," Dr. Youdim said. Physicians can use outside resources for behavioral modification if they don’t have a dietician on staff. She recommends programs like Weight Watchers, Overeaters Anonymous, and TOPS Club, which can be augmented with in-office counseling and even applications that are available for smart phones and can used by a motivated patient to help monitor food intake, she added.
"Be forceful, direct, and blunt in your discussions with patients about the need for the interventions in terms of diet and exercise," as advocated by the American Heart Association, the American College of Cardiology, the American Diabetes Association, the American Association of Clinical Endocrinologists, and other organizations, said Dr. Helena Rodbard, an endocrinologist in Gaithersburg, Md. She recommended that clinicians calculate the body mass index of their overweight and obese patients "and let people know where they stand in terms of the percentiles of their body weight compared to the norms set before the current epidemic." She advises clinicians to screen patients for metabolic syndrome, prediabetes, and diabetes, and to inform them about their risk factors, using calculations from available programs about patients’ risks of developing heart disease or diabetes within the next 5-10 years. She emphasized paying special attention to obesity in youth and adolescents, who "will be obese for life and have diabetes early and diabetic complications early."
In an interview, Dr. Rodbard said that because of logistical issues and costs, she rarely refers patients to a weight-loss program; instead, she counsels them and arranges for them to see a dietician in her office 1 or 2 days a week. "Frequent return visits and motivational sessions within those visits are helpful," she said, advising clinicians to use the services of a dietician when possible, and to "fight for better reimbursement for those kinds of services."
The prospects for any new obesity drugs’ becoming available in the near future dimmed considerably after the majority of the Food and Drug Administration’s Endocrinologic Drugs Advisory Panel recommended against approval of the phentermine-topiramate (Qnexa) combination and the serotonergic drug lorcaserin (Lorqess) at meetings last summer and fall. Then in February, the FDA put its decision on another drug combination – the antidepressant bupropion and the opioid antagonist naltrexone (Contrave) – on hold when it requested a large cardiovascular safety trial in overweight and obese people before the agency would consider approval.
Dr. Rodbard, who is past president of the American Association of Clinical Endocrinologists and the American College of Endocrinology, said that although these drugs had potential side effects, she considers their side effects to be lower than the risk of remaining obese, adding that "it is a tragedy that we do not have more and better drugs available for treatment of obesity, and that there has not been a larger and more effective and sustained program to change the lifestyle habits of Americans with regard to diet and exercise."
Although Dr. Youdim acknowledged that concerns over the safety of the drugs are legitimate, "there is a lot of bias around drugs for obesity, because the thought is that if patients would just stop eating, they’d lose weight. ... The rigor placed on these drugs is a lot greater because of that bias." But obesity is a disease, she added, "and there should be treatment options available to us," just as there are options available for other diseases.
Although weight-loss drugs have not been effective on their own, they have been useful adjuncts to lifestyle modification, and "the cornerstone of treatment in a select group of patients who are having trouble adhering to lifestyle recommendations."
Dr. Youdim said that certain drugs are associated with some weight loss and can be used – not "completely without indication" – to help patients lose weight. For example, a patient who is both overweight and clinically depressed could be prescribed bupropion, someone with insulin resistance can benefit from metformin, and a patient with type 2 diabetes who requires insulin may benefit from exenatide (Byetta). All three of these drugs are associated with weight loss.
Dr. Rodbard said that in patients with diabetes in whom exenatide is indicated, about a third of those receiving it "have very dramatic weight loss ... especially in some patients with a very massive degree of obesity to begin with," and that this loss is "very nicely sustained." Published data support the notion that the combination of the glucagonlike peptide–1 receptor agonists with insulin will help to reduce the weight gain associated with insulin therapy, she added.
Metformin has a very small effect on weight, but appears to have beneficial effects on the risk of heart disease and cancer; "hence, it is one of the backbones of therapy for type 2 diabetes and used in combination with nearly all other medications," she added.
As for the FDA-approved weight-loss drugs, Dr. Rodbard said that she rarely uses phentermine, and – when it was available – she rarely used sibutramine, which was used mainly because there were not many other options available. The side effects of orlistat, she said, are unacceptable "to all but a very few patients."
Dr. Louis Aronne, director of the comprehensive weight control program at New York–Presbyterian Hospital, said that there clearly is a need for more medical treatments for obesity – as well as improved access to comprehensive weight-loss programs that are affordable and effective – before the clinicians opt for surgery. "Right now, a patient can go from Weight Watchers to the operating room" for bariatric surgery because of the gap in treatment and dearth of medical options, he said.
Dr. Aronne is a developer of an online program designed to address the treatment gap and poor access to comprehensive weight-loss programs. The "BMIQ" program is a 16-session, online, comprehensive program run by a dietician in a group setting. It includes dietary and exercise counseling that is aimed at helping obese people with type 2 diabetes to lose weight and manage their diabetes by using a "weight-centric approach" efficiently and without extra costs. Primary care physicians refer their patients to the program; after the patient fills out an evaluation form, the physician receives information on the patient along with recommendations on how to manage that patient. For example, the clinician is alerted if the patient is taking over-the-counter sleep medications that contain strong antihistamines, which can both cause weight gain and make it difficult to lose weight; eliminating them is "like giving someone an appetite suppressant," he said.
In a pilot program, a large New York State health insurer is providing the BMIQ program to 2,000 of its beneficiaries who are overweight and have type 2 diabetes. Data indicate that programs like this help patients lose weight, reduce medication costs, and can save over $1,000 per person, and "we’re hopeful that we will be able to show the insurer that they can save money," said Dr. Aronne, who believes that insurers and employers will pay for the program once they see that it’s an efficient model of delivering this service. The program is available to people now, at a cost of $300, at BMIQ.com.
Dr. Youdim had no disclosures. Dr. Aronne said he has been involved in studies of more than 20 weight-loss drugs, including the three reviewed by the FDA panel last year, and has served as a consultant to Vivus and Orexigen; he also developed the BMIQ program. Dr. Rodbard has received research grants and served as a consultant to, adviser to, and/or speaker for Amylin, Astra-Zeneca, Biodel, Bristol-Myers Squibb, Eli Lilly, Mannkind, Merck, Roche, and Sanofi-Aventis.
FDA Panel: Approve Long-Acting Bronchodilator for COPD
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
FROM THE FDA'S PULMONARY-ALLERGY DRUGS ADVISORY COMMITTEE
FDA Panel: Approve Long-Acting Bronchodilator for COPD
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
FROM THE FDA'S PULMONARY-ALLERGY DRUGS ADVISORY COMMITTEE
FDA Panel: Approve Long-Acting Bronchodilator for COPD
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel on March 8 recommended that the inhaled bronchodilator indacaterol should be approved for patients with chronic obstructive pulmonary disease.
The FDA’s Pulmonary-Allergy Drugs Advisory Committee voted 13-4 that the data on the safety and efficacy of the 75-mcg dose of indacaterol, as a maintenance treatment, provided substantial evidence to support the drug’s approval at this dose, for the proposed indication: the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Indacaterol, a long-acting beta-2 adrenergic agonist (LABA) that is administered in a dry-powder inhaler, has a rapid onset of effect sustained over 24 hours, according to Novartis, which filed for approval of two once-daily doses: 75 mcg and 150 mcg. But in a 12-5 vote, the panel recommended against approval of the higher dose, largely because of the paucity of data directly comparing the two doses and, as the panel chair said, "No compelling difference" in efficacy that was evident between the two doses.
The 150-mcg once-daily dose and a higher dose (300 mcg once daily) of indacaterol were approved to treat COPD in the European Union in September 2009, where it is marketed as the Onbrez Breezhaler; the drug at those doses is now approved in more than 50 countries.
Initially, Novartis had applied for approval of these two doses in December 2008, but the FDA requested that the company study lower doses of the drug, after the agency review concluded that no clinically meaningful advantage had been shown for the 300-mcg dose over the 150-mcg dose and because of safety concerns. There were more cardiovascular and cerebrovascular adverse events among patients with COPD treated with indacaterol, when compared with those on placebo and the active compactor, formoterol; and in studies of indacaterol in patients with asthma, there were some deaths, possibly related to indacaterol. In addition, treatment with inhaled LABAs has been linked to severe asthma exacerbations and asthma-related deaths in patients with asthma.
At the meeting, Novartis presented the results of five phase III studies of the 75-mcg, 150-mcg, and 300-mcg doses, compared with placebo or active controls in approximately 4,000 patients with COPD. After 12 weeks, there were significant improvements in lung function associated with each dose, when compared with placebo. In the safety database of patients with COPD, the risk of serious cardiovascular events (including MI, stroke, or cardiac death) was not increased, and there was no increase in acute respiratory events associated with any dose of indacaterol studied, according to Novartis.
If both doses were approved, indacaterol would be the first bronchodilator in the United States to be approved at two doses for the treatment of COPD; only one dose of formoterol and salmeterol, which are also LABAs, are approved for COPD treatment
Pending approval, Novartis plans to market indacaterol in the United States as the Arcapta Neohaler.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest by the FDA prior to meetings; occasionally, the FDA grants a waiver to a panelist with a conflict but not at this meeting.
FROM THE FDA'S PULMONARY-ALLERGY DRUGS ADVISORY COMMITTEE
Data Link High Oral Cleft Risk to Topiramate
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
FROM THE FDA
Data Link High Oral Cleft Risk to Topiramate
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
FROM THE FDA
Data Link High Oral Cleft Risk to Topiramate
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
New data from a national pregnancy registry indicate an increased risk of oral clefts among infants exposed to topiramate monotherapy during the first trimester of pregnancy, which should be considered when prescribing the anticonvulsant to women of childbearing age, the Food and Drug Administration announced on March 4.
In the North American Antiepileptic Drugs Pregnancy Registry (NAAED), the prevalence of oral clefts (cleft lip or palate) was 1.4% among infants exposed in utero to topiramate monotherapy during the first trimester, compared with 0.38%-0.55% among infants exposed to other antiepileptic drugs (AEDs). The rate was 0.07% among infants whose mothers did not have epilepsy or were not treated with other AEDs, according to an FDA statement.
In the NAAED registry, the relative risk of oral clefts among infants exposed to topiramate was 21.3 times that of the background risk among untreated women. The prevalence of oral clefts was also increased among infants exposed to topiramate monotherapy in a U.K. Epilepsy and Pregnancy Register: 3.2% among the infants exposed to topiramate monotherapy, compared with a background prevalence of 0.2% – a 16-fold increase in risk.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," and should consider alternative medications with a lower risk of birth defects, Dr. Russell Katz, director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. The agency’s announcement also notes that if the decision is made to prescribe topiramate to a woman of childbearing age, effective birth control should be used, "keeping in mind the potential for a decrease in hormonal exposure and a possible decrease in contraceptive efficacy when using estrogen-containing birth control with topiramate."
The new information is being added to the prescribing information of topiramate, which is also being switched from a pregnancy risk category C (applied to drugs for which animal data suggest potential fetal risks, with no adequate data from human clinical trials or studies) to the pregnancy risk category D (applied to drugs for which there is evidence for human fetal risk based on human data, but potential benefits may be acceptable in certain situations, despite the risks.)
Topiramate, which is marketed as Topamax and is also available in generic formulations, is approved as a treatment for epilepsy and for preventing migraine headaches. It is also used off label for some unapproved indications, including some that may not be considered serious, the FDA said.
Adverse events associated with topiramate should be reported to the FDA’s MedWatch program or at 800-332-1088. Women who are on topiramate and become pregnant should be encouraged to register in the North American AED pregnancy registry by calling 888-233-2334.
FROM THE FDA