User login
Case suggests GSIs could treat Notch-mutated ALL
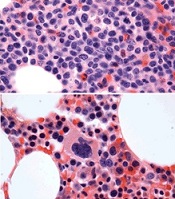
before (top) and after 7 weeks
of treatment (bottom)
© Knoechel et al.
Results of a case study suggest a gamma-secretase inhibitor (GSI) can be effective against Notch-mutated acute lymphoblastic leukemia (ALL).
The patient, who had early T-cell precursor ALL (ETP-ALL), achieved a complete hematologic response to treatment with BMS-906024, a GSI with anti-Notch
activity.
The patient was then able to proceed to hematopoietic stem cell transplant and was leukemia-free at last follow-up.
The researchers said this suggests that GSIs might hold promise for treating ALL and other cancers characterized by Notch mutations.
Birgit Knoechel, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues described this case study in Cold Spring Harbor Molecular Case Studies.
The patient was a 53-year-old male with ETP-ALL who had failed previous rounds of chemotherapy and was then enrolled in a clinical trial of BMS-906024.
The patient began to show immediate improvement after starting treatment with the GSI. After 3 cycles, he went on to transplant and has since been leukemia-free—for 19 months so far.
To determine the genetic basis for the patient’s response to BMS-906024, researchers performed targeted and whole-exome sequencing on his leukemic cells.
They identified 4 potential mutations driving disease progression, including a novel mutation in the NOTCH1 gene that resulted in hyperactive signaling. This mutated gene copy was also duplicated in the cancer genome, resulting in elevated expression.
However, the NOTCH1 mutation, along with 2 of the other mutations, were absent in the remission bone marrow.
The researchers also cultured the patient’s leukemic cells to determine the molecular response to treatment.
Cells treated with BMS-906024 had greatly reduced levels of mutated NOTCH1 protein. RNA sequencing demonstrated that Notch target genes were sensitive to the treatment.
The MYC oncogene, on the other hand, was not sensitive to BMS-906024.
Epigenetic analysis revealed that the enhancer driving MYC expression in the leukemic cells was not Notch-dependent, but rather BRD4-dependent, suggesting another possible therapeutic option for MYC-expressing tumors. ![]()

before (top) and after 7 weeks
of treatment (bottom)
© Knoechel et al.
Results of a case study suggest a gamma-secretase inhibitor (GSI) can be effective against Notch-mutated acute lymphoblastic leukemia (ALL).
The patient, who had early T-cell precursor ALL (ETP-ALL), achieved a complete hematologic response to treatment with BMS-906024, a GSI with anti-Notch
activity.
The patient was then able to proceed to hematopoietic stem cell transplant and was leukemia-free at last follow-up.
The researchers said this suggests that GSIs might hold promise for treating ALL and other cancers characterized by Notch mutations.
Birgit Knoechel, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues described this case study in Cold Spring Harbor Molecular Case Studies.
The patient was a 53-year-old male with ETP-ALL who had failed previous rounds of chemotherapy and was then enrolled in a clinical trial of BMS-906024.
The patient began to show immediate improvement after starting treatment with the GSI. After 3 cycles, he went on to transplant and has since been leukemia-free—for 19 months so far.
To determine the genetic basis for the patient’s response to BMS-906024, researchers performed targeted and whole-exome sequencing on his leukemic cells.
They identified 4 potential mutations driving disease progression, including a novel mutation in the NOTCH1 gene that resulted in hyperactive signaling. This mutated gene copy was also duplicated in the cancer genome, resulting in elevated expression.
However, the NOTCH1 mutation, along with 2 of the other mutations, were absent in the remission bone marrow.
The researchers also cultured the patient’s leukemic cells to determine the molecular response to treatment.
Cells treated with BMS-906024 had greatly reduced levels of mutated NOTCH1 protein. RNA sequencing demonstrated that Notch target genes were sensitive to the treatment.
The MYC oncogene, on the other hand, was not sensitive to BMS-906024.
Epigenetic analysis revealed that the enhancer driving MYC expression in the leukemic cells was not Notch-dependent, but rather BRD4-dependent, suggesting another possible therapeutic option for MYC-expressing tumors. ![]()

before (top) and after 7 weeks
of treatment (bottom)
© Knoechel et al.
Results of a case study suggest a gamma-secretase inhibitor (GSI) can be effective against Notch-mutated acute lymphoblastic leukemia (ALL).
The patient, who had early T-cell precursor ALL (ETP-ALL), achieved a complete hematologic response to treatment with BMS-906024, a GSI with anti-Notch
activity.
The patient was then able to proceed to hematopoietic stem cell transplant and was leukemia-free at last follow-up.
The researchers said this suggests that GSIs might hold promise for treating ALL and other cancers characterized by Notch mutations.
Birgit Knoechel, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues described this case study in Cold Spring Harbor Molecular Case Studies.
The patient was a 53-year-old male with ETP-ALL who had failed previous rounds of chemotherapy and was then enrolled in a clinical trial of BMS-906024.
The patient began to show immediate improvement after starting treatment with the GSI. After 3 cycles, he went on to transplant and has since been leukemia-free—for 19 months so far.
To determine the genetic basis for the patient’s response to BMS-906024, researchers performed targeted and whole-exome sequencing on his leukemic cells.
They identified 4 potential mutations driving disease progression, including a novel mutation in the NOTCH1 gene that resulted in hyperactive signaling. This mutated gene copy was also duplicated in the cancer genome, resulting in elevated expression.
However, the NOTCH1 mutation, along with 2 of the other mutations, were absent in the remission bone marrow.
The researchers also cultured the patient’s leukemic cells to determine the molecular response to treatment.
Cells treated with BMS-906024 had greatly reduced levels of mutated NOTCH1 protein. RNA sequencing demonstrated that Notch target genes were sensitive to the treatment.
The MYC oncogene, on the other hand, was not sensitive to BMS-906024.
Epigenetic analysis revealed that the enhancer driving MYC expression in the leukemic cells was not Notch-dependent, but rather BRD4-dependent, suggesting another possible therapeutic option for MYC-expressing tumors. ![]()
Word choice affects public perception of drugs

Photo courtesy of the FDA
Using the words “breakthrough” and “promising” to describe new drugs affects the public’s perception of the drugs’ effectiveness, according to a study published in JAMA Internal Medicine.
Investigators noted that, in everyday usage, the term “breakthrough” represents a highly significant or definitive advance.
However, the US Food and Drug Administration’s (FDA’s) “breakthrough therapy designation” has a different meaning.
Since the Safety and Innovation Act became law in 2012, the FDA can assign breakthrough designation to a drug that “treats a serious or life-threatening condition” and “may demonstrate a substantial improvement . . . over available therapies” based on preliminary evidence.
And since the creation of the Safety and Innovation Act, all FDA press releases announcing the approval of breakthrough-designated drugs have used the term “breakthrough,” while about half have used the term “promising.”
“Today, patients and their families can easily find FDA press releases on the Internet, or they often hear about them in the news,” said study author Steven Woloshin, MD, of The Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
“But the reality is that unless patients fully understand how the FDA is using the term ‘breakthrough,’ they may have unwarranted confidence in the evidence supporting drug claims. So we thought it was important to test how these terms affect the judgement of people without medical training.”
Survey details
The investigators conducted an online survey of 597 Americans. Participants were randomly given 1 of 5 short descriptions of a recently approved drug.
The descriptions were based on an FDA press release for a metastatic lung cancer breakthrough-designated drug that was conditionally approved based on the surrogate outcome of tumor shrinkage.
The first, “facts-only” description described the drug as meeting the criteria for breakthrough designation but did not actually use the term “breakthrough.”
A second and a third description included the facts and added the terms “breakthrough” and “promising,” respectively.
The fourth, “tentative” description included the facts, used the word “breakthrough,” and used the following FDA-required language for professional labeling:
The FDA pointed out that the drug was approved based on tumor shrinkage but that an improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
The fifth, “definitive” description included the same information as the tentative description, but “may be contingent” was changed to “is contingent.”
Participants were then asked to judge the drug’s benefit, harm, and strength of evidence.
Results
The investigators found that adding either “breakthrough” or “promising” in the description significantly increased the percentage of participants who rated the drug as “very” or “completely” effective compared with the facts-only description—23% and 25% vs 11%.
Adding “breakthrough” or “promising” to the description also significantly increased the number of people who reported believing that evidence supporting the drug is “strong” or “extremely strong”—59% and 63% vs 43%.
At the same time, adding either the tentative or definitive explanations significantly reduced the percentage of participants who believed (incorrectly) that the drug had been “proven to save lives”—16% tentative and 10% definitive vs 31% breakthrough.
Finally, when participants were asked which of 2 drugs—one described as “breakthrough,” the other as meeting the breakthrough criteria—they would take for a potentially deadly condition, 92% chose the “breakthrough” drug.
“Our findings clearly indicate that words like ‘breakthrough’ and ‘promising’ increase people’s beliefs in a drug’s effectiveness,” said Lisa Schwartz, MD, of The Dartmouth Institute for Health Policy and Clinical Practice.
“In light of [the findings], press releases with neutral terms and that clearly explain the limited evidence supporting what breakthrough designation and accelerated approval mean might help consumers make more accurate judgements about these drugs.” ![]()

Photo courtesy of the FDA
Using the words “breakthrough” and “promising” to describe new drugs affects the public’s perception of the drugs’ effectiveness, according to a study published in JAMA Internal Medicine.
Investigators noted that, in everyday usage, the term “breakthrough” represents a highly significant or definitive advance.
However, the US Food and Drug Administration’s (FDA’s) “breakthrough therapy designation” has a different meaning.
Since the Safety and Innovation Act became law in 2012, the FDA can assign breakthrough designation to a drug that “treats a serious or life-threatening condition” and “may demonstrate a substantial improvement . . . over available therapies” based on preliminary evidence.
And since the creation of the Safety and Innovation Act, all FDA press releases announcing the approval of breakthrough-designated drugs have used the term “breakthrough,” while about half have used the term “promising.”
“Today, patients and their families can easily find FDA press releases on the Internet, or they often hear about them in the news,” said study author Steven Woloshin, MD, of The Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
“But the reality is that unless patients fully understand how the FDA is using the term ‘breakthrough,’ they may have unwarranted confidence in the evidence supporting drug claims. So we thought it was important to test how these terms affect the judgement of people without medical training.”
Survey details
The investigators conducted an online survey of 597 Americans. Participants were randomly given 1 of 5 short descriptions of a recently approved drug.
The descriptions were based on an FDA press release for a metastatic lung cancer breakthrough-designated drug that was conditionally approved based on the surrogate outcome of tumor shrinkage.
The first, “facts-only” description described the drug as meeting the criteria for breakthrough designation but did not actually use the term “breakthrough.”
A second and a third description included the facts and added the terms “breakthrough” and “promising,” respectively.
The fourth, “tentative” description included the facts, used the word “breakthrough,” and used the following FDA-required language for professional labeling:
The FDA pointed out that the drug was approved based on tumor shrinkage but that an improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
The fifth, “definitive” description included the same information as the tentative description, but “may be contingent” was changed to “is contingent.”
Participants were then asked to judge the drug’s benefit, harm, and strength of evidence.
Results
The investigators found that adding either “breakthrough” or “promising” in the description significantly increased the percentage of participants who rated the drug as “very” or “completely” effective compared with the facts-only description—23% and 25% vs 11%.
Adding “breakthrough” or “promising” to the description also significantly increased the number of people who reported believing that evidence supporting the drug is “strong” or “extremely strong”—59% and 63% vs 43%.
At the same time, adding either the tentative or definitive explanations significantly reduced the percentage of participants who believed (incorrectly) that the drug had been “proven to save lives”—16% tentative and 10% definitive vs 31% breakthrough.
Finally, when participants were asked which of 2 drugs—one described as “breakthrough,” the other as meeting the breakthrough criteria—they would take for a potentially deadly condition, 92% chose the “breakthrough” drug.
“Our findings clearly indicate that words like ‘breakthrough’ and ‘promising’ increase people’s beliefs in a drug’s effectiveness,” said Lisa Schwartz, MD, of The Dartmouth Institute for Health Policy and Clinical Practice.
“In light of [the findings], press releases with neutral terms and that clearly explain the limited evidence supporting what breakthrough designation and accelerated approval mean might help consumers make more accurate judgements about these drugs.” ![]()

Photo courtesy of the FDA
Using the words “breakthrough” and “promising” to describe new drugs affects the public’s perception of the drugs’ effectiveness, according to a study published in JAMA Internal Medicine.
Investigators noted that, in everyday usage, the term “breakthrough” represents a highly significant or definitive advance.
However, the US Food and Drug Administration’s (FDA’s) “breakthrough therapy designation” has a different meaning.
Since the Safety and Innovation Act became law in 2012, the FDA can assign breakthrough designation to a drug that “treats a serious or life-threatening condition” and “may demonstrate a substantial improvement . . . over available therapies” based on preliminary evidence.
And since the creation of the Safety and Innovation Act, all FDA press releases announcing the approval of breakthrough-designated drugs have used the term “breakthrough,” while about half have used the term “promising.”
“Today, patients and their families can easily find FDA press releases on the Internet, or they often hear about them in the news,” said study author Steven Woloshin, MD, of The Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
“But the reality is that unless patients fully understand how the FDA is using the term ‘breakthrough,’ they may have unwarranted confidence in the evidence supporting drug claims. So we thought it was important to test how these terms affect the judgement of people without medical training.”
Survey details
The investigators conducted an online survey of 597 Americans. Participants were randomly given 1 of 5 short descriptions of a recently approved drug.
The descriptions were based on an FDA press release for a metastatic lung cancer breakthrough-designated drug that was conditionally approved based on the surrogate outcome of tumor shrinkage.
The first, “facts-only” description described the drug as meeting the criteria for breakthrough designation but did not actually use the term “breakthrough.”
A second and a third description included the facts and added the terms “breakthrough” and “promising,” respectively.
The fourth, “tentative” description included the facts, used the word “breakthrough,” and used the following FDA-required language for professional labeling:
The FDA pointed out that the drug was approved based on tumor shrinkage but that an improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
The fifth, “definitive” description included the same information as the tentative description, but “may be contingent” was changed to “is contingent.”
Participants were then asked to judge the drug’s benefit, harm, and strength of evidence.
Results
The investigators found that adding either “breakthrough” or “promising” in the description significantly increased the percentage of participants who rated the drug as “very” or “completely” effective compared with the facts-only description—23% and 25% vs 11%.
Adding “breakthrough” or “promising” to the description also significantly increased the number of people who reported believing that evidence supporting the drug is “strong” or “extremely strong”—59% and 63% vs 43%.
At the same time, adding either the tentative or definitive explanations significantly reduced the percentage of participants who believed (incorrectly) that the drug had been “proven to save lives”—16% tentative and 10% definitive vs 31% breakthrough.
Finally, when participants were asked which of 2 drugs—one described as “breakthrough,” the other as meeting the breakthrough criteria—they would take for a potentially deadly condition, 92% chose the “breakthrough” drug.
“Our findings clearly indicate that words like ‘breakthrough’ and ‘promising’ increase people’s beliefs in a drug’s effectiveness,” said Lisa Schwartz, MD, of The Dartmouth Institute for Health Policy and Clinical Practice.
“In light of [the findings], press releases with neutral terms and that clearly explain the limited evidence supporting what breakthrough designation and accelerated approval mean might help consumers make more accurate judgements about these drugs.” ![]()
Team identifies therapeutic target for HIT

Researchers believe they have identified a therapeutic target for heparin-induced thrombocytopenia (HIT).
The team noted that HIT is caused by antibodies to complexes that form between platelet factor 4 (PF4), which is released from activated platelets, and heparin or cellular glycosaminoglycans.
The researchers elucidated the crystal structure of 3 PF4 complexes and found evidence suggesting that tetramerization of PF4 is targetable.
Zheng Cai, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described this work in Nature Communications.
Previously, the researchers identified KKO, a murine monoclonal antibody to PF4/heparin complexes that causes HIT in a murine model. The team said human HIT antibodies compete with KKO for binding to PF4/heparin, and KKO augments the formation of pathogenic immune complexes.
The researchers also identified RTO, an isotype-matched, anti-PF4 antibody that binds to PF4 but does not generate pathogenic complexes.
For the current study, the team described and compared the crystal structures of PF4 in complex with Fabs derived from KKO and RTO to the structure of PF4 in complex with fondaparinux.
The researchers noted that PF4 molecules can exist singly as monomers, doubly as dimers, and as a 4-part complex called a tetramer, which have an “open” end and a “closed” end.
The crystal structure of PF4 in complex with fondaparinux showed that fondaparinux binds to the “closed” end of the PF4 tetramer, which stabilizes the tetramer.
The crystal structure of PF4 in complex with KKO showed that KKO binds to the “open” end of the stabilized tetramer, making contact with 3 of 4 monomers in the tetramer.
The researchers said this helps explain the requirement for heparin as a backbone for the complex. They also said this finding provides new insight into how a normal host protein such as PF4 can be converted into a target of the host immune system, which leads to an autoimmune disorder.
The crystal structure of PF4 in complex with RTO showed that RTO binds to PF4 monomers rather than tetramers. And RTO binds to the monomers in a way that prevents them from combining into tetramers.
Via cell experiments, the researchers confirmed that RTO prevents the formation of antigenic complexes, as well as the activation of platelets by KKO and human HIT antibodies. RTO also prevented clot formation caused by KKO in a mouse model of HIT.
These results suggest that binding of RTO to PF4 monomers prevents the formation of pathogenic complexes that are central to the pathology of HIT. So the researchers believe RTO can provide the basis for new diagnostics and may pave the way for a therapy to stop HIT early in its progression. ![]()

Researchers believe they have identified a therapeutic target for heparin-induced thrombocytopenia (HIT).
The team noted that HIT is caused by antibodies to complexes that form between platelet factor 4 (PF4), which is released from activated platelets, and heparin or cellular glycosaminoglycans.
The researchers elucidated the crystal structure of 3 PF4 complexes and found evidence suggesting that tetramerization of PF4 is targetable.
Zheng Cai, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described this work in Nature Communications.
Previously, the researchers identified KKO, a murine monoclonal antibody to PF4/heparin complexes that causes HIT in a murine model. The team said human HIT antibodies compete with KKO for binding to PF4/heparin, and KKO augments the formation of pathogenic immune complexes.
The researchers also identified RTO, an isotype-matched, anti-PF4 antibody that binds to PF4 but does not generate pathogenic complexes.
For the current study, the team described and compared the crystal structures of PF4 in complex with Fabs derived from KKO and RTO to the structure of PF4 in complex with fondaparinux.
The researchers noted that PF4 molecules can exist singly as monomers, doubly as dimers, and as a 4-part complex called a tetramer, which have an “open” end and a “closed” end.
The crystal structure of PF4 in complex with fondaparinux showed that fondaparinux binds to the “closed” end of the PF4 tetramer, which stabilizes the tetramer.
The crystal structure of PF4 in complex with KKO showed that KKO binds to the “open” end of the stabilized tetramer, making contact with 3 of 4 monomers in the tetramer.
The researchers said this helps explain the requirement for heparin as a backbone for the complex. They also said this finding provides new insight into how a normal host protein such as PF4 can be converted into a target of the host immune system, which leads to an autoimmune disorder.
The crystal structure of PF4 in complex with RTO showed that RTO binds to PF4 monomers rather than tetramers. And RTO binds to the monomers in a way that prevents them from combining into tetramers.
Via cell experiments, the researchers confirmed that RTO prevents the formation of antigenic complexes, as well as the activation of platelets by KKO and human HIT antibodies. RTO also prevented clot formation caused by KKO in a mouse model of HIT.
These results suggest that binding of RTO to PF4 monomers prevents the formation of pathogenic complexes that are central to the pathology of HIT. So the researchers believe RTO can provide the basis for new diagnostics and may pave the way for a therapy to stop HIT early in its progression. ![]()

Researchers believe they have identified a therapeutic target for heparin-induced thrombocytopenia (HIT).
The team noted that HIT is caused by antibodies to complexes that form between platelet factor 4 (PF4), which is released from activated platelets, and heparin or cellular glycosaminoglycans.
The researchers elucidated the crystal structure of 3 PF4 complexes and found evidence suggesting that tetramerization of PF4 is targetable.
Zheng Cai, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described this work in Nature Communications.
Previously, the researchers identified KKO, a murine monoclonal antibody to PF4/heparin complexes that causes HIT in a murine model. The team said human HIT antibodies compete with KKO for binding to PF4/heparin, and KKO augments the formation of pathogenic immune complexes.
The researchers also identified RTO, an isotype-matched, anti-PF4 antibody that binds to PF4 but does not generate pathogenic complexes.
For the current study, the team described and compared the crystal structures of PF4 in complex with Fabs derived from KKO and RTO to the structure of PF4 in complex with fondaparinux.
The researchers noted that PF4 molecules can exist singly as monomers, doubly as dimers, and as a 4-part complex called a tetramer, which have an “open” end and a “closed” end.
The crystal structure of PF4 in complex with fondaparinux showed that fondaparinux binds to the “closed” end of the PF4 tetramer, which stabilizes the tetramer.
The crystal structure of PF4 in complex with KKO showed that KKO binds to the “open” end of the stabilized tetramer, making contact with 3 of 4 monomers in the tetramer.
The researchers said this helps explain the requirement for heparin as a backbone for the complex. They also said this finding provides new insight into how a normal host protein such as PF4 can be converted into a target of the host immune system, which leads to an autoimmune disorder.
The crystal structure of PF4 in complex with RTO showed that RTO binds to PF4 monomers rather than tetramers. And RTO binds to the monomers in a way that prevents them from combining into tetramers.
Via cell experiments, the researchers confirmed that RTO prevents the formation of antigenic complexes, as well as the activation of platelets by KKO and human HIT antibodies. RTO also prevented clot formation caused by KKO in a mouse model of HIT.
These results suggest that binding of RTO to PF4 monomers prevents the formation of pathogenic complexes that are central to the pathology of HIT. So the researchers believe RTO can provide the basis for new diagnostics and may pave the way for a therapy to stop HIT early in its progression. ![]()
Hematocrit level may predict need for transfusion

Photo by Elise Amendola
A young trauma patient’s hematocrit level at hospital admission may predict the need for transfusion, new research suggests.
Results of this retrospective, single-center study indicate that children and adolescents with a hematocrit level of 35% or less at admission are more likely than their peers with higher hematocrit levels to require a transfusion after trauma.
The study was published in the Journal of Trauma and Acute Care Surgery.
“A quick and cost-effective measure, such as admission hematocrit, to identify pediatric patients who are at a high risk for bleeding could provide a critical improvement in optimizing care for children, while reducing costs,” said study author Christopher P. Gayer, MD, PhD, of Children's Hospital Los Angeles (CHLA) in California.
For this research, Dr Gayer and his colleagues examined the medical records of all patients, ages 0 to 17, who presented to the level 1 pediatric trauma center at CHLA between 2005 and 2013.
Of all the patients, 1341 had hematocrit measured at admission. The researchers divided this group into patients who required an intervention—transfusion or operation—for their bleeding (n=93) and those who did not (n=1248).
The mean hematocrit was 38.0 for patients who did not require an intervention and 34.3 for those who did (P<0.01). The mean hematocrit was 26.9 in patients who required a transfusion secondary to bleeding and 36.0 in those who required an operative intervention for nonhemostatic indications (P<0.01).
The researchers then analyzed a subset of patients who had an abdominal CT scan, as these patients had a definitive presence or absence of intra-abdominal injury. There was a significant decrease in admission hematocrit between patients who required a transfusion for an intra-abdominal injury and those who did not—29% and 37%, respectively (P<0.01).
Serial hematocrit values remained significantly lower in the patients who required a transfusion up to 67 hours after admission (P=0.04). The researchers said this suggests that hematocrit is a valuable predictor for requiring a transfusion at least 2 days after an injury, and it may be useful for patients presenting well after their initial injury.
The team then evaluated whether an admission hematocrit cutoff of 35% or less could predict the need for transfusion. For all patients, a cutoff of 35% or less had a sensitivity of 94%, specificity of 77%, positive-predictive value of 5%, and negative predictive value of 99.9%.
In patients who had an abdominal CT, a cutoff hematocrit of 35% or less had a sensitivity of 90%, specificity of 76%, positive predictive value of 21%, and negative predictive value of 99%.
These results led the researchers to conclude that this hematocrit cutoff may be a reliable screening tool.
“Admission hematocrit can be done rapidly in the trauma bay, is relatively inexpensive, causes minimal harm, and can aid in critical decision-making and rapid identification of occult bleeding,” said study author Jamie Golden, MD, of CHLA.
“Our results show that a hematocrit level of less than 35% on admission predicts a greater likelihood for the need of transfusion in pediatric blunt trauma patients.”
The researchers stressed that a doctor's concern in the face of clinical signs of hemorrhagic shock should always take priority over lab data. However, a repeat hematocrit can be quickly and easily performed if clinically indicated.
They added that the results of their study require validation in a prospective, multicenter study. ![]()

Photo by Elise Amendola
A young trauma patient’s hematocrit level at hospital admission may predict the need for transfusion, new research suggests.
Results of this retrospective, single-center study indicate that children and adolescents with a hematocrit level of 35% or less at admission are more likely than their peers with higher hematocrit levels to require a transfusion after trauma.
The study was published in the Journal of Trauma and Acute Care Surgery.
“A quick and cost-effective measure, such as admission hematocrit, to identify pediatric patients who are at a high risk for bleeding could provide a critical improvement in optimizing care for children, while reducing costs,” said study author Christopher P. Gayer, MD, PhD, of Children's Hospital Los Angeles (CHLA) in California.
For this research, Dr Gayer and his colleagues examined the medical records of all patients, ages 0 to 17, who presented to the level 1 pediatric trauma center at CHLA between 2005 and 2013.
Of all the patients, 1341 had hematocrit measured at admission. The researchers divided this group into patients who required an intervention—transfusion or operation—for their bleeding (n=93) and those who did not (n=1248).
The mean hematocrit was 38.0 for patients who did not require an intervention and 34.3 for those who did (P<0.01). The mean hematocrit was 26.9 in patients who required a transfusion secondary to bleeding and 36.0 in those who required an operative intervention for nonhemostatic indications (P<0.01).
The researchers then analyzed a subset of patients who had an abdominal CT scan, as these patients had a definitive presence or absence of intra-abdominal injury. There was a significant decrease in admission hematocrit between patients who required a transfusion for an intra-abdominal injury and those who did not—29% and 37%, respectively (P<0.01).
Serial hematocrit values remained significantly lower in the patients who required a transfusion up to 67 hours after admission (P=0.04). The researchers said this suggests that hematocrit is a valuable predictor for requiring a transfusion at least 2 days after an injury, and it may be useful for patients presenting well after their initial injury.
The team then evaluated whether an admission hematocrit cutoff of 35% or less could predict the need for transfusion. For all patients, a cutoff of 35% or less had a sensitivity of 94%, specificity of 77%, positive-predictive value of 5%, and negative predictive value of 99.9%.
In patients who had an abdominal CT, a cutoff hematocrit of 35% or less had a sensitivity of 90%, specificity of 76%, positive predictive value of 21%, and negative predictive value of 99%.
These results led the researchers to conclude that this hematocrit cutoff may be a reliable screening tool.
“Admission hematocrit can be done rapidly in the trauma bay, is relatively inexpensive, causes minimal harm, and can aid in critical decision-making and rapid identification of occult bleeding,” said study author Jamie Golden, MD, of CHLA.
“Our results show that a hematocrit level of less than 35% on admission predicts a greater likelihood for the need of transfusion in pediatric blunt trauma patients.”
The researchers stressed that a doctor's concern in the face of clinical signs of hemorrhagic shock should always take priority over lab data. However, a repeat hematocrit can be quickly and easily performed if clinically indicated.
They added that the results of their study require validation in a prospective, multicenter study. ![]()

Photo by Elise Amendola
A young trauma patient’s hematocrit level at hospital admission may predict the need for transfusion, new research suggests.
Results of this retrospective, single-center study indicate that children and adolescents with a hematocrit level of 35% or less at admission are more likely than their peers with higher hematocrit levels to require a transfusion after trauma.
The study was published in the Journal of Trauma and Acute Care Surgery.
“A quick and cost-effective measure, such as admission hematocrit, to identify pediatric patients who are at a high risk for bleeding could provide a critical improvement in optimizing care for children, while reducing costs,” said study author Christopher P. Gayer, MD, PhD, of Children's Hospital Los Angeles (CHLA) in California.
For this research, Dr Gayer and his colleagues examined the medical records of all patients, ages 0 to 17, who presented to the level 1 pediatric trauma center at CHLA between 2005 and 2013.
Of all the patients, 1341 had hematocrit measured at admission. The researchers divided this group into patients who required an intervention—transfusion or operation—for their bleeding (n=93) and those who did not (n=1248).
The mean hematocrit was 38.0 for patients who did not require an intervention and 34.3 for those who did (P<0.01). The mean hematocrit was 26.9 in patients who required a transfusion secondary to bleeding and 36.0 in those who required an operative intervention for nonhemostatic indications (P<0.01).
The researchers then analyzed a subset of patients who had an abdominal CT scan, as these patients had a definitive presence or absence of intra-abdominal injury. There was a significant decrease in admission hematocrit between patients who required a transfusion for an intra-abdominal injury and those who did not—29% and 37%, respectively (P<0.01).
Serial hematocrit values remained significantly lower in the patients who required a transfusion up to 67 hours after admission (P=0.04). The researchers said this suggests that hematocrit is a valuable predictor for requiring a transfusion at least 2 days after an injury, and it may be useful for patients presenting well after their initial injury.
The team then evaluated whether an admission hematocrit cutoff of 35% or less could predict the need for transfusion. For all patients, a cutoff of 35% or less had a sensitivity of 94%, specificity of 77%, positive-predictive value of 5%, and negative predictive value of 99.9%.
In patients who had an abdominal CT, a cutoff hematocrit of 35% or less had a sensitivity of 90%, specificity of 76%, positive predictive value of 21%, and negative predictive value of 99%.
These results led the researchers to conclude that this hematocrit cutoff may be a reliable screening tool.
“Admission hematocrit can be done rapidly in the trauma bay, is relatively inexpensive, causes minimal harm, and can aid in critical decision-making and rapid identification of occult bleeding,” said study author Jamie Golden, MD, of CHLA.
“Our results show that a hematocrit level of less than 35% on admission predicts a greater likelihood for the need of transfusion in pediatric blunt trauma patients.”
The researchers stressed that a doctor's concern in the face of clinical signs of hemorrhagic shock should always take priority over lab data. However, a repeat hematocrit can be quickly and easily performed if clinically indicated.
They added that the results of their study require validation in a prospective, multicenter study. ![]()
Study reveals tumor suppressor in AML

The protein-coding gene hnRNP K acts as a tumor suppressor in acute myeloid leukemia (AML), according to research published in Cancer Cell.
Investigators found that AML patients who carried a partial deletion of chromosome 9 also experienced a significant decrease in hnRNP K expression.
This deletion, 9q21.32, along with the decreased levels of hnRNP K, led to reduced survival and increased tumor formation.
“Our data implicates hnRNP K in the development of blood disorders and suggests it acts as a tumor suppressor,” said Sean Post, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Both in vivo and in vitro results indicate that hnRNP K achieves this through regulation of key genetic pathways. Our study found that hnRNP K expression must be maintained for proper cellular regulation and to prevent tumor formation.”
Dr Post and his colleagues examined hnRNP K’s role in tumorigenesis by generating a mouse model harboring an Hnrnpk knockout allele.
They found that Hnrnpk haploinsufficiency resulted in reduced survival, increased tumor formation, genomic instability, and the development of transplantable hematopoietic neoplasms with myeloproliferation.
“Our findings showed that Hnrnpk haploinsufficiency led to tumor development by deregulating cell proliferation and differentiation programs through control of key cellular pathways, which suggests these pathways may be exploited by targeted therapies,” Dr Post said.
Specifically, he and his colleagues found that reduced hnRNP K expression attenuated p21 activation, downregulated C/EBP levels, and activated STAT3 signaling.
The investigators also analyzed samples from AML patients who harbored 9q21.32 and found a significant decrease in hnRNP K expression.
“It was clear that these changes in AML patients with the 9q21.32 deletion resulted in a tumor suppressor gene involved in blood cancer development,” Dr Post said. ![]()

The protein-coding gene hnRNP K acts as a tumor suppressor in acute myeloid leukemia (AML), according to research published in Cancer Cell.
Investigators found that AML patients who carried a partial deletion of chromosome 9 also experienced a significant decrease in hnRNP K expression.
This deletion, 9q21.32, along with the decreased levels of hnRNP K, led to reduced survival and increased tumor formation.
“Our data implicates hnRNP K in the development of blood disorders and suggests it acts as a tumor suppressor,” said Sean Post, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Both in vivo and in vitro results indicate that hnRNP K achieves this through regulation of key genetic pathways. Our study found that hnRNP K expression must be maintained for proper cellular regulation and to prevent tumor formation.”
Dr Post and his colleagues examined hnRNP K’s role in tumorigenesis by generating a mouse model harboring an Hnrnpk knockout allele.
They found that Hnrnpk haploinsufficiency resulted in reduced survival, increased tumor formation, genomic instability, and the development of transplantable hematopoietic neoplasms with myeloproliferation.
“Our findings showed that Hnrnpk haploinsufficiency led to tumor development by deregulating cell proliferation and differentiation programs through control of key cellular pathways, which suggests these pathways may be exploited by targeted therapies,” Dr Post said.
Specifically, he and his colleagues found that reduced hnRNP K expression attenuated p21 activation, downregulated C/EBP levels, and activated STAT3 signaling.
The investigators also analyzed samples from AML patients who harbored 9q21.32 and found a significant decrease in hnRNP K expression.
“It was clear that these changes in AML patients with the 9q21.32 deletion resulted in a tumor suppressor gene involved in blood cancer development,” Dr Post said. ![]()

The protein-coding gene hnRNP K acts as a tumor suppressor in acute myeloid leukemia (AML), according to research published in Cancer Cell.
Investigators found that AML patients who carried a partial deletion of chromosome 9 also experienced a significant decrease in hnRNP K expression.
This deletion, 9q21.32, along with the decreased levels of hnRNP K, led to reduced survival and increased tumor formation.
“Our data implicates hnRNP K in the development of blood disorders and suggests it acts as a tumor suppressor,” said Sean Post, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Both in vivo and in vitro results indicate that hnRNP K achieves this through regulation of key genetic pathways. Our study found that hnRNP K expression must be maintained for proper cellular regulation and to prevent tumor formation.”
Dr Post and his colleagues examined hnRNP K’s role in tumorigenesis by generating a mouse model harboring an Hnrnpk knockout allele.
They found that Hnrnpk haploinsufficiency resulted in reduced survival, increased tumor formation, genomic instability, and the development of transplantable hematopoietic neoplasms with myeloproliferation.
“Our findings showed that Hnrnpk haploinsufficiency led to tumor development by deregulating cell proliferation and differentiation programs through control of key cellular pathways, which suggests these pathways may be exploited by targeted therapies,” Dr Post said.
Specifically, he and his colleagues found that reduced hnRNP K expression attenuated p21 activation, downregulated C/EBP levels, and activated STAT3 signaling.
The investigators also analyzed samples from AML patients who harbored 9q21.32 and found a significant decrease in hnRNP K expression.
“It was clear that these changes in AML patients with the 9q21.32 deletion resulted in a tumor suppressor gene involved in blood cancer development,” Dr Post said. ![]()
Patients may need anticoagulation

A new study suggests that certain patients with bioprosthetic aortic heart valves may require anticoagulant therapy.
In some patients, researchers observed reduced motion of the leaflets affecting the valve opening.
The team believes this may be a sign of subclinical leaflet thrombosis, particularly since anticoagulant therapy was able to restore leaflet motion.
The researchers described this study in NEJM.
“Transcatheter and surgically implantable tissue valves are life-saving devices in patients with aortic valve stenosis,” said study author Raj Makkar, MD, of the Cedars-Sinai Heart Institute in Los Angeles, California.
“These findings allow us a potentially valuable opportunity to further optimize the outcomes of these procedures. We are not recommending that all patients with these devices be on blood thinners, but, clearly, further studies need to be done to define best medication regimens.”
Dr Makkar and his colleagues began this research when a clinical trial participant with a bioprosthetic aortic heart valve had a stroke. High-resolution imaging revealed reduced motion of the leaflets that open and close to regulate the flow of blood.
“We wanted to find out if patients undergoing a tissue valve procedure are susceptible to blood clots on the leaflets and study the clinical consequences of the same,” Dr Makkar said.
“We also wanted to understand whether our aortic valve patients were more susceptible to having blood clots and whether those clots could indicate that the patient might experience a neurological complication—a mini-stroke.”
So Dr Makkar and his colleagues analyzed 187 patients who received a new valve via a transcatheter procedure or open-heart surgery—55 patients in a trial and 132 patients enrolled in registries.
All patients underwent high-resolution imaging—4-dimensional CT angiography—so the researchers could detect reduced leaflet motion.
Overall, 20% of patients (39/187) had reduced leaflet motion—40% in the trial (22/55) and 13% (17/132) in the registries. This included patients with multiple bioprosthesis types.
The researchers observed a lower incidence of reduced leaflet motion in patients receiving anticoagulant therapy.
The incidence was significantly lower in patients on warfarin than in those receiving dual antiplatelet therapy. It was 0% and 55%, respectively, in the clinical trial (P=0.01) and 0% and 29%, respectively, in the registry patients (P=0.04).
Dr Makkar and his colleagues also re-evaluated a handful of patients who underwent a follow-up CT—11 who were receiving anticoagulation and 10 who were not. Leaflet motion was restored in all 11 treated patients and 1 of the untreated patients (P<0.001).
Lastly, there was some suggestion that the incidence of stroke or transient ischemic attacks might be higher among patients with reduced valve motion. But the researchers said this finding was inconclusive and requires further study. ![]()

A new study suggests that certain patients with bioprosthetic aortic heart valves may require anticoagulant therapy.
In some patients, researchers observed reduced motion of the leaflets affecting the valve opening.
The team believes this may be a sign of subclinical leaflet thrombosis, particularly since anticoagulant therapy was able to restore leaflet motion.
The researchers described this study in NEJM.
“Transcatheter and surgically implantable tissue valves are life-saving devices in patients with aortic valve stenosis,” said study author Raj Makkar, MD, of the Cedars-Sinai Heart Institute in Los Angeles, California.
“These findings allow us a potentially valuable opportunity to further optimize the outcomes of these procedures. We are not recommending that all patients with these devices be on blood thinners, but, clearly, further studies need to be done to define best medication regimens.”
Dr Makkar and his colleagues began this research when a clinical trial participant with a bioprosthetic aortic heart valve had a stroke. High-resolution imaging revealed reduced motion of the leaflets that open and close to regulate the flow of blood.
“We wanted to find out if patients undergoing a tissue valve procedure are susceptible to blood clots on the leaflets and study the clinical consequences of the same,” Dr Makkar said.
“We also wanted to understand whether our aortic valve patients were more susceptible to having blood clots and whether those clots could indicate that the patient might experience a neurological complication—a mini-stroke.”
So Dr Makkar and his colleagues analyzed 187 patients who received a new valve via a transcatheter procedure or open-heart surgery—55 patients in a trial and 132 patients enrolled in registries.
All patients underwent high-resolution imaging—4-dimensional CT angiography—so the researchers could detect reduced leaflet motion.
Overall, 20% of patients (39/187) had reduced leaflet motion—40% in the trial (22/55) and 13% (17/132) in the registries. This included patients with multiple bioprosthesis types.
The researchers observed a lower incidence of reduced leaflet motion in patients receiving anticoagulant therapy.
The incidence was significantly lower in patients on warfarin than in those receiving dual antiplatelet therapy. It was 0% and 55%, respectively, in the clinical trial (P=0.01) and 0% and 29%, respectively, in the registry patients (P=0.04).
Dr Makkar and his colleagues also re-evaluated a handful of patients who underwent a follow-up CT—11 who were receiving anticoagulation and 10 who were not. Leaflet motion was restored in all 11 treated patients and 1 of the untreated patients (P<0.001).
Lastly, there was some suggestion that the incidence of stroke or transient ischemic attacks might be higher among patients with reduced valve motion. But the researchers said this finding was inconclusive and requires further study. ![]()

A new study suggests that certain patients with bioprosthetic aortic heart valves may require anticoagulant therapy.
In some patients, researchers observed reduced motion of the leaflets affecting the valve opening.
The team believes this may be a sign of subclinical leaflet thrombosis, particularly since anticoagulant therapy was able to restore leaflet motion.
The researchers described this study in NEJM.
“Transcatheter and surgically implantable tissue valves are life-saving devices in patients with aortic valve stenosis,” said study author Raj Makkar, MD, of the Cedars-Sinai Heart Institute in Los Angeles, California.
“These findings allow us a potentially valuable opportunity to further optimize the outcomes of these procedures. We are not recommending that all patients with these devices be on blood thinners, but, clearly, further studies need to be done to define best medication regimens.”
Dr Makkar and his colleagues began this research when a clinical trial participant with a bioprosthetic aortic heart valve had a stroke. High-resolution imaging revealed reduced motion of the leaflets that open and close to regulate the flow of blood.
“We wanted to find out if patients undergoing a tissue valve procedure are susceptible to blood clots on the leaflets and study the clinical consequences of the same,” Dr Makkar said.
“We also wanted to understand whether our aortic valve patients were more susceptible to having blood clots and whether those clots could indicate that the patient might experience a neurological complication—a mini-stroke.”
So Dr Makkar and his colleagues analyzed 187 patients who received a new valve via a transcatheter procedure or open-heart surgery—55 patients in a trial and 132 patients enrolled in registries.
All patients underwent high-resolution imaging—4-dimensional CT angiography—so the researchers could detect reduced leaflet motion.
Overall, 20% of patients (39/187) had reduced leaflet motion—40% in the trial (22/55) and 13% (17/132) in the registries. This included patients with multiple bioprosthesis types.
The researchers observed a lower incidence of reduced leaflet motion in patients receiving anticoagulant therapy.
The incidence was significantly lower in patients on warfarin than in those receiving dual antiplatelet therapy. It was 0% and 55%, respectively, in the clinical trial (P=0.01) and 0% and 29%, respectively, in the registry patients (P=0.04).
Dr Makkar and his colleagues also re-evaluated a handful of patients who underwent a follow-up CT—11 who were receiving anticoagulation and 10 who were not. Leaflet motion was restored in all 11 treated patients and 1 of the untreated patients (P<0.001).
Lastly, there was some suggestion that the incidence of stroke or transient ischemic attacks might be higher among patients with reduced valve motion. But the researchers said this finding was inconclusive and requires further study. ![]()
Trio wins Nobel Prize for parasite-related discoveries

Three researchers have won the 2015 Nobel Prize in Physiology or Medicine for discoveries related to parasitic diseases.
One half of the prize was awarded to Youyou Tu for discoveries concerning a novel therapy against malaria—artemisinin.
The other half of the prize was awarded to William C. Campbell, PhD, and Satoshi Ōmura, PhD, for their discoveries concerning a novel therapy against infections caused by roundworm parasites.
Drs Ōmura and Campbell discovered the drug avermectin. A derivative of this drug has lowered the incidence of river blindness and lymphatic filariasis and demonstrated efficacy against other parasitic diseases.
Artemisinin
Before artemisinin came into use, malaria was treated with chloroquine or quinine—with declining success. By the late 1960s, efforts to eradicate malaria had failed, and the disease was on the rise.
At that time, Tu turned to traditional herbal medicine to tackle the challenge of developing novel malaria therapies. From a large-scale screen of herbal remedies in malaria-infected animals, an extract from the plant Artemisia annua emerged as an interesting candidate.
However, the results were inconsistent. So Tu revisited the ancient literature and discovered clues that guided her in her quest to extract the active component from Artemisia annua. Tu was the first to show that this component, later called artemisinin, was effective against the malaria parasite in animals and humans.
Artemisinin is now used in all malaria-ridden parts of the world. When used in combination therapy, it is estimated to reduce mortality from malaria by more than 20% overall and by more than 30% in children.
Avermectin
The discovery of avermectin began with Streptomyces, bacteria that live in the soil and are known to produce agents with antibacterial activities.
Dr Ōmura isolated new strains of Streptomyces from soil samples and cultured them in the lab. He selected about 50 of the most promising cultures to analyze for their activity against harmful microorganisms. One of these cultures turned out to be Streptomyces avermitilis, the source of avermectin.
Dr Campbell acquired Dr Ōmura’s Streptomyces cultures and explored their efficacy. Dr Campbell showed that a component from one of the cultures could combat parasites in domestic and farm animals.
The bioactive agent was purified and named avermectin. It was subsequently modified to a more effective compound called ivermectin. Ivermectin turned out to be effective against a variety of parasites, including those that cause river blindness and lymphatic filariasis.
Today, ivermectin is used in all parts of the world that are plagued by parasitic diseases. The drug has proven effective against a range of parasites and has limited side effects. Thanks to ivermectin, river blindness and lymphatic filariasis are on the verge of eradication.
About the winners
Youyou Tu was born in 1930 in China. She graduated from Beijing Medical University in 1955. Tu has worked at the China Academy of Traditional Chinese Medicine since 1965. She has been chief professor there since 2000.
William C. Campbell was born in 1930 in Ramelton, Ireland. He received a BA from Trinity College, University of Dublin, in Ireland in 1952. He received a PhD from the University of Wisconsin in Madison, Wisconsin, in 1957.
From 1957 to 1990, Dr Campbell was with the Merck Institute for Therapeutic Research, from 1984 to 1990 as a senior scientist and director for assay research and development. Dr Campbell is currently a research fellow emeritus at Drew University in Madison, New Jersey.
Satoshi Ōmura was born in 1935 in the Yamanashi Prefecture, Japan. He received a PhD in pharmaceutical sciences in 1968 from the University of Tokyo and a PhD in chemistry in 1970 from Tokyo University of Science.
Dr Ōmura was a researcher at the Kitasato Institute in Japan from 1965 to 1971 and a professor at Kitasato University from 1975 to 2007. Since 2007, Dr Ōmura has been a professor emeritus at Kitasato University. ![]()

Three researchers have won the 2015 Nobel Prize in Physiology or Medicine for discoveries related to parasitic diseases.
One half of the prize was awarded to Youyou Tu for discoveries concerning a novel therapy against malaria—artemisinin.
The other half of the prize was awarded to William C. Campbell, PhD, and Satoshi Ōmura, PhD, for their discoveries concerning a novel therapy against infections caused by roundworm parasites.
Drs Ōmura and Campbell discovered the drug avermectin. A derivative of this drug has lowered the incidence of river blindness and lymphatic filariasis and demonstrated efficacy against other parasitic diseases.
Artemisinin
Before artemisinin came into use, malaria was treated with chloroquine or quinine—with declining success. By the late 1960s, efforts to eradicate malaria had failed, and the disease was on the rise.
At that time, Tu turned to traditional herbal medicine to tackle the challenge of developing novel malaria therapies. From a large-scale screen of herbal remedies in malaria-infected animals, an extract from the plant Artemisia annua emerged as an interesting candidate.
However, the results were inconsistent. So Tu revisited the ancient literature and discovered clues that guided her in her quest to extract the active component from Artemisia annua. Tu was the first to show that this component, later called artemisinin, was effective against the malaria parasite in animals and humans.
Artemisinin is now used in all malaria-ridden parts of the world. When used in combination therapy, it is estimated to reduce mortality from malaria by more than 20% overall and by more than 30% in children.
Avermectin
The discovery of avermectin began with Streptomyces, bacteria that live in the soil and are known to produce agents with antibacterial activities.
Dr Ōmura isolated new strains of Streptomyces from soil samples and cultured them in the lab. He selected about 50 of the most promising cultures to analyze for their activity against harmful microorganisms. One of these cultures turned out to be Streptomyces avermitilis, the source of avermectin.
Dr Campbell acquired Dr Ōmura’s Streptomyces cultures and explored their efficacy. Dr Campbell showed that a component from one of the cultures could combat parasites in domestic and farm animals.
The bioactive agent was purified and named avermectin. It was subsequently modified to a more effective compound called ivermectin. Ivermectin turned out to be effective against a variety of parasites, including those that cause river blindness and lymphatic filariasis.
Today, ivermectin is used in all parts of the world that are plagued by parasitic diseases. The drug has proven effective against a range of parasites and has limited side effects. Thanks to ivermectin, river blindness and lymphatic filariasis are on the verge of eradication.
About the winners
Youyou Tu was born in 1930 in China. She graduated from Beijing Medical University in 1955. Tu has worked at the China Academy of Traditional Chinese Medicine since 1965. She has been chief professor there since 2000.
William C. Campbell was born in 1930 in Ramelton, Ireland. He received a BA from Trinity College, University of Dublin, in Ireland in 1952. He received a PhD from the University of Wisconsin in Madison, Wisconsin, in 1957.
From 1957 to 1990, Dr Campbell was with the Merck Institute for Therapeutic Research, from 1984 to 1990 as a senior scientist and director for assay research and development. Dr Campbell is currently a research fellow emeritus at Drew University in Madison, New Jersey.
Satoshi Ōmura was born in 1935 in the Yamanashi Prefecture, Japan. He received a PhD in pharmaceutical sciences in 1968 from the University of Tokyo and a PhD in chemistry in 1970 from Tokyo University of Science.
Dr Ōmura was a researcher at the Kitasato Institute in Japan from 1965 to 1971 and a professor at Kitasato University from 1975 to 2007. Since 2007, Dr Ōmura has been a professor emeritus at Kitasato University. ![]()

Three researchers have won the 2015 Nobel Prize in Physiology or Medicine for discoveries related to parasitic diseases.
One half of the prize was awarded to Youyou Tu for discoveries concerning a novel therapy against malaria—artemisinin.
The other half of the prize was awarded to William C. Campbell, PhD, and Satoshi Ōmura, PhD, for their discoveries concerning a novel therapy against infections caused by roundworm parasites.
Drs Ōmura and Campbell discovered the drug avermectin. A derivative of this drug has lowered the incidence of river blindness and lymphatic filariasis and demonstrated efficacy against other parasitic diseases.
Artemisinin
Before artemisinin came into use, malaria was treated with chloroquine or quinine—with declining success. By the late 1960s, efforts to eradicate malaria had failed, and the disease was on the rise.
At that time, Tu turned to traditional herbal medicine to tackle the challenge of developing novel malaria therapies. From a large-scale screen of herbal remedies in malaria-infected animals, an extract from the plant Artemisia annua emerged as an interesting candidate.
However, the results were inconsistent. So Tu revisited the ancient literature and discovered clues that guided her in her quest to extract the active component from Artemisia annua. Tu was the first to show that this component, later called artemisinin, was effective against the malaria parasite in animals and humans.
Artemisinin is now used in all malaria-ridden parts of the world. When used in combination therapy, it is estimated to reduce mortality from malaria by more than 20% overall and by more than 30% in children.
Avermectin
The discovery of avermectin began with Streptomyces, bacteria that live in the soil and are known to produce agents with antibacterial activities.
Dr Ōmura isolated new strains of Streptomyces from soil samples and cultured them in the lab. He selected about 50 of the most promising cultures to analyze for their activity against harmful microorganisms. One of these cultures turned out to be Streptomyces avermitilis, the source of avermectin.
Dr Campbell acquired Dr Ōmura’s Streptomyces cultures and explored their efficacy. Dr Campbell showed that a component from one of the cultures could combat parasites in domestic and farm animals.
The bioactive agent was purified and named avermectin. It was subsequently modified to a more effective compound called ivermectin. Ivermectin turned out to be effective against a variety of parasites, including those that cause river blindness and lymphatic filariasis.
Today, ivermectin is used in all parts of the world that are plagued by parasitic diseases. The drug has proven effective against a range of parasites and has limited side effects. Thanks to ivermectin, river blindness and lymphatic filariasis are on the verge of eradication.
About the winners
Youyou Tu was born in 1930 in China. She graduated from Beijing Medical University in 1955. Tu has worked at the China Academy of Traditional Chinese Medicine since 1965. She has been chief professor there since 2000.
William C. Campbell was born in 1930 in Ramelton, Ireland. He received a BA from Trinity College, University of Dublin, in Ireland in 1952. He received a PhD from the University of Wisconsin in Madison, Wisconsin, in 1957.
From 1957 to 1990, Dr Campbell was with the Merck Institute for Therapeutic Research, from 1984 to 1990 as a senior scientist and director for assay research and development. Dr Campbell is currently a research fellow emeritus at Drew University in Madison, New Jersey.
Satoshi Ōmura was born in 1935 in the Yamanashi Prefecture, Japan. He received a PhD in pharmaceutical sciences in 1968 from the University of Tokyo and a PhD in chemistry in 1970 from Tokyo University of Science.
Dr Ōmura was a researcher at the Kitasato Institute in Japan from 1965 to 1971 and a professor at Kitasato University from 1975 to 2007. Since 2007, Dr Ōmura has been a professor emeritus at Kitasato University.
Self-propelled particles stop bleeding

carbonate microparticle
in acidic solution
Image by James Baylis
Researchers say they’ve created self-propelled particles that can travel against the flow of blood to treat severe bleeding.
These calcium carbonate microparticles, which are applied as a powder, release carbon dioxide gas to propel them toward the source of bleeding.
They can be loaded with thrombin and transport the clotting protein through wounds and into damaged tissue in animals.
The researchers described the particles in Science Advances.
“People have developed hundreds of agents that can clot blood, but the issue is that it’s hard to push these therapies against severe blood flow, especially far enough upstream to reach the leaking vessels,” said study author Christian Kastrup, PhD, of the University of British Columbia in Vancouver, Canada.
“Here, for the first time, we’ve come up with an agent that can do that.”
After studying and modeling the movement of their microparticles in vitro, the researchers loaded the particles with thrombin and tested them in mouse and pig models of hemorrhage.
The particles helped clot blood and stopped hemorrhaging in both models. In fact, the gas-generating, thrombin-loaded particles stopped bleeding better than topical thrombin or thrombin-loaded particles that did not produce gas.
The researchers believe that, after more testing and development, their microparticles could have a wide range of uses. And they would be particularly useful for treating bleeding that originates internally, such as in the uterus, sinus, gastrointestinal tract, or abdomen, where traditional topical drugs are less effective.
“The area we’re really focusing on is postpartum hemorrhage: in the uterus, after childbirth, where you can’t see the damaged vessels but you can put the powder into that area and the particles can propel and find those damaged vessels,” Dr Kastrup said.
The researchers also believe the microparticles could be used to deliver a range of therapeutics to wound and hemorrhage sites.

carbonate microparticle
in acidic solution
Image by James Baylis
Researchers say they’ve created self-propelled particles that can travel against the flow of blood to treat severe bleeding.
These calcium carbonate microparticles, which are applied as a powder, release carbon dioxide gas to propel them toward the source of bleeding.
They can be loaded with thrombin and transport the clotting protein through wounds and into damaged tissue in animals.
The researchers described the particles in Science Advances.
“People have developed hundreds of agents that can clot blood, but the issue is that it’s hard to push these therapies against severe blood flow, especially far enough upstream to reach the leaking vessels,” said study author Christian Kastrup, PhD, of the University of British Columbia in Vancouver, Canada.
“Here, for the first time, we’ve come up with an agent that can do that.”
After studying and modeling the movement of their microparticles in vitro, the researchers loaded the particles with thrombin and tested them in mouse and pig models of hemorrhage.
The particles helped clot blood and stopped hemorrhaging in both models. In fact, the gas-generating, thrombin-loaded particles stopped bleeding better than topical thrombin or thrombin-loaded particles that did not produce gas.
The researchers believe that, after more testing and development, their microparticles could have a wide range of uses. And they would be particularly useful for treating bleeding that originates internally, such as in the uterus, sinus, gastrointestinal tract, or abdomen, where traditional topical drugs are less effective.
“The area we’re really focusing on is postpartum hemorrhage: in the uterus, after childbirth, where you can’t see the damaged vessels but you can put the powder into that area and the particles can propel and find those damaged vessels,” Dr Kastrup said.
The researchers also believe the microparticles could be used to deliver a range of therapeutics to wound and hemorrhage sites.

carbonate microparticle
in acidic solution
Image by James Baylis
Researchers say they’ve created self-propelled particles that can travel against the flow of blood to treat severe bleeding.
These calcium carbonate microparticles, which are applied as a powder, release carbon dioxide gas to propel them toward the source of bleeding.
They can be loaded with thrombin and transport the clotting protein through wounds and into damaged tissue in animals.
The researchers described the particles in Science Advances.
“People have developed hundreds of agents that can clot blood, but the issue is that it’s hard to push these therapies against severe blood flow, especially far enough upstream to reach the leaking vessels,” said study author Christian Kastrup, PhD, of the University of British Columbia in Vancouver, Canada.
“Here, for the first time, we’ve come up with an agent that can do that.”
After studying and modeling the movement of their microparticles in vitro, the researchers loaded the particles with thrombin and tested them in mouse and pig models of hemorrhage.
The particles helped clot blood and stopped hemorrhaging in both models. In fact, the gas-generating, thrombin-loaded particles stopped bleeding better than topical thrombin or thrombin-loaded particles that did not produce gas.
The researchers believe that, after more testing and development, their microparticles could have a wide range of uses. And they would be particularly useful for treating bleeding that originates internally, such as in the uterus, sinus, gastrointestinal tract, or abdomen, where traditional topical drugs are less effective.
“The area we’re really focusing on is postpartum hemorrhage: in the uterus, after childbirth, where you can’t see the damaged vessels but you can put the powder into that area and the particles can propel and find those damaged vessels,” Dr Kastrup said.
The researchers also believe the microparticles could be used to deliver a range of therapeutics to wound and hemorrhage sites.
Group issues guideline for iliofemoral DVT

Image by Andre E.X. Brown
A new guideline aims to help physicians identify and manage blood clots, specifically iliofemoral deep vein thrombosis (DVT), in the groin and thigh.
The guideline states that anticoagulant therapy remains the cornerstone of management, but certain patients with iliofemoral DVT may benefit from alternative strategies, such as inferior vena cava filters, compression therapy, and clot removal or reduction strategies.
The guideline, which is based on the latest evidence, was published in CMAJ.
It was developed by a team of hematologists, interventional radiologists, vascular surgeons, emergency department physicians, and primary care physicians.
“We think this clinical practice guideline fills an important gap in knowledge for care providers by providing a practical approach to a common problem that can have serious implications for patients,” said author David Liu, MD, of Vancouver General Hospital in British Columbia, Canada.
“Complications associated with DVT can occur years after the presentation of DVT if it is not managed at onset. DVT is a life-threatening condition in the short term, with long-term implications to the patient and society if not managed properly.”
The guideline team has created a summary of recommendations and a decision tool to help physicians. Highlights include:
- All hospital staff must have the tools to diagnose and determine the severity of iliofemoral DVT.
- Anticoagulants are recommended for all patients with iliofemoral DVT, but the type and length of treatment will vary according to presentation.
- For patients not able to take anticoagulants, use of inferior vena cava filters is recommended with regular follow-up. The filters should be removed as soon as possible.
- Immediate intervention with clot removal is recommended in patients with phlegmasia cerulea dolens to reduce the associated risks of amputation and death.
- Clot removal intervention can also be considered for patients who are at low risk of bleeding to minimize possible long-term complications from iliofemoral DVT that may decrease quality of life (such as post-thrombotic syndrome).
- To manage post-thrombotic syndrome, the use of compression stockings is recommended, although the evidence that this intervention is effective is weak.
- Patient follow-up by the primary care physician is important.

Image by Andre E.X. Brown
A new guideline aims to help physicians identify and manage blood clots, specifically iliofemoral deep vein thrombosis (DVT), in the groin and thigh.
The guideline states that anticoagulant therapy remains the cornerstone of management, but certain patients with iliofemoral DVT may benefit from alternative strategies, such as inferior vena cava filters, compression therapy, and clot removal or reduction strategies.
The guideline, which is based on the latest evidence, was published in CMAJ.
It was developed by a team of hematologists, interventional radiologists, vascular surgeons, emergency department physicians, and primary care physicians.
“We think this clinical practice guideline fills an important gap in knowledge for care providers by providing a practical approach to a common problem that can have serious implications for patients,” said author David Liu, MD, of Vancouver General Hospital in British Columbia, Canada.
“Complications associated with DVT can occur years after the presentation of DVT if it is not managed at onset. DVT is a life-threatening condition in the short term, with long-term implications to the patient and society if not managed properly.”
The guideline team has created a summary of recommendations and a decision tool to help physicians. Highlights include:
- All hospital staff must have the tools to diagnose and determine the severity of iliofemoral DVT.
- Anticoagulants are recommended for all patients with iliofemoral DVT, but the type and length of treatment will vary according to presentation.
- For patients not able to take anticoagulants, use of inferior vena cava filters is recommended with regular follow-up. The filters should be removed as soon as possible.
- Immediate intervention with clot removal is recommended in patients with phlegmasia cerulea dolens to reduce the associated risks of amputation and death.
- Clot removal intervention can also be considered for patients who are at low risk of bleeding to minimize possible long-term complications from iliofemoral DVT that may decrease quality of life (such as post-thrombotic syndrome).
- To manage post-thrombotic syndrome, the use of compression stockings is recommended, although the evidence that this intervention is effective is weak.
- Patient follow-up by the primary care physician is important.

Image by Andre E.X. Brown
A new guideline aims to help physicians identify and manage blood clots, specifically iliofemoral deep vein thrombosis (DVT), in the groin and thigh.
The guideline states that anticoagulant therapy remains the cornerstone of management, but certain patients with iliofemoral DVT may benefit from alternative strategies, such as inferior vena cava filters, compression therapy, and clot removal or reduction strategies.
The guideline, which is based on the latest evidence, was published in CMAJ.
It was developed by a team of hematologists, interventional radiologists, vascular surgeons, emergency department physicians, and primary care physicians.
“We think this clinical practice guideline fills an important gap in knowledge for care providers by providing a practical approach to a common problem that can have serious implications for patients,” said author David Liu, MD, of Vancouver General Hospital in British Columbia, Canada.
“Complications associated with DVT can occur years after the presentation of DVT if it is not managed at onset. DVT is a life-threatening condition in the short term, with long-term implications to the patient and society if not managed properly.”
The guideline team has created a summary of recommendations and a decision tool to help physicians. Highlights include:
- All hospital staff must have the tools to diagnose and determine the severity of iliofemoral DVT.
- Anticoagulants are recommended for all patients with iliofemoral DVT, but the type and length of treatment will vary according to presentation.
- For patients not able to take anticoagulants, use of inferior vena cava filters is recommended with regular follow-up. The filters should be removed as soon as possible.
- Immediate intervention with clot removal is recommended in patients with phlegmasia cerulea dolens to reduce the associated risks of amputation and death.
- Clot removal intervention can also be considered for patients who are at low risk of bleeding to minimize possible long-term complications from iliofemoral DVT that may decrease quality of life (such as post-thrombotic syndrome).
- To manage post-thrombotic syndrome, the use of compression stockings is recommended, although the evidence that this intervention is effective is weak.
- Patient follow-up by the primary care physician is important.
NICE can’t recommend new sepsis tests

Photo by William Weinert
The UK’s National Institute for Health and Care Excellence (NICE) has said there is not enough evidence to recommend 3 new blood tests for routine use in the National Health Service.
The tests are designed to identify bacteria and fungi in the bloodstream more rapidly than current tests.
According to NICE, there is too much uncertainty regarding the accuracy of the new tests and the size of benefit they might confer for patients with suspected sepsis.
The tests in question are LightCycler SeptiFast Test MGRADE (Roche Diagnostics), SepsiTest (Molzym Molecular Diagnostics), and IRIDICA BAC BSI assay (Abbott Diagnostics).
They are used to analyze whole blood samples for bacterial and fungal DNA, which may identify pathogens earlier than microbiology techniques. Microbiology techniques require blood samples to be incubated and cultured before pathogens can be identified.
The new tests are designed to enable earlier targeted treatment for patients with sepsis and reduce the use of broad-spectrum antimicrobials, which could help reduce future antimicrobial resistance.
“Rapid molecular tests that can identify which pathogens are the cause of an infection in hours rather than the days typically needed for traditional microbiology tests could ensure the right antibiotics are used much earlier in treatment,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“This, in turn, could improve outcomes for patients with suspected sepsis as well as help to reduce the spread of resistant microbes. However, the committee [advising NICE] concluded that the tests may offer clinical benefit, but there is too much uncertainty in the size of the benefit to determine the effect of introducing the tests into clinical practice.”
“The committee also concluded that, although the rapid molecular tests might provide results more quickly, there was too much uncertainty in the accuracy of the tests for clinicians to be able to base a decision on whether to withdraw or continue antibiotics. The committee therefore decided that further research should be encouraged to determine the clinical scenarios in which the tests may offer most benefit.”
NICE’s draft diagnostics guidance on the LightCycler SeptiFast Test MGRADE, SepsiTest, and IRIDICA BAC BSI assay is available on the NICE website. The closing date for comments on this draft guidance is October 21, 2015.

Photo by William Weinert
The UK’s National Institute for Health and Care Excellence (NICE) has said there is not enough evidence to recommend 3 new blood tests for routine use in the National Health Service.
The tests are designed to identify bacteria and fungi in the bloodstream more rapidly than current tests.
According to NICE, there is too much uncertainty regarding the accuracy of the new tests and the size of benefit they might confer for patients with suspected sepsis.
The tests in question are LightCycler SeptiFast Test MGRADE (Roche Diagnostics), SepsiTest (Molzym Molecular Diagnostics), and IRIDICA BAC BSI assay (Abbott Diagnostics).
They are used to analyze whole blood samples for bacterial and fungal DNA, which may identify pathogens earlier than microbiology techniques. Microbiology techniques require blood samples to be incubated and cultured before pathogens can be identified.
The new tests are designed to enable earlier targeted treatment for patients with sepsis and reduce the use of broad-spectrum antimicrobials, which could help reduce future antimicrobial resistance.
“Rapid molecular tests that can identify which pathogens are the cause of an infection in hours rather than the days typically needed for traditional microbiology tests could ensure the right antibiotics are used much earlier in treatment,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“This, in turn, could improve outcomes for patients with suspected sepsis as well as help to reduce the spread of resistant microbes. However, the committee [advising NICE] concluded that the tests may offer clinical benefit, but there is too much uncertainty in the size of the benefit to determine the effect of introducing the tests into clinical practice.”
“The committee also concluded that, although the rapid molecular tests might provide results more quickly, there was too much uncertainty in the accuracy of the tests for clinicians to be able to base a decision on whether to withdraw or continue antibiotics. The committee therefore decided that further research should be encouraged to determine the clinical scenarios in which the tests may offer most benefit.”
NICE’s draft diagnostics guidance on the LightCycler SeptiFast Test MGRADE, SepsiTest, and IRIDICA BAC BSI assay is available on the NICE website. The closing date for comments on this draft guidance is October 21, 2015.

Photo by William Weinert
The UK’s National Institute for Health and Care Excellence (NICE) has said there is not enough evidence to recommend 3 new blood tests for routine use in the National Health Service.
The tests are designed to identify bacteria and fungi in the bloodstream more rapidly than current tests.
According to NICE, there is too much uncertainty regarding the accuracy of the new tests and the size of benefit they might confer for patients with suspected sepsis.
The tests in question are LightCycler SeptiFast Test MGRADE (Roche Diagnostics), SepsiTest (Molzym Molecular Diagnostics), and IRIDICA BAC BSI assay (Abbott Diagnostics).
They are used to analyze whole blood samples for bacterial and fungal DNA, which may identify pathogens earlier than microbiology techniques. Microbiology techniques require blood samples to be incubated and cultured before pathogens can be identified.
The new tests are designed to enable earlier targeted treatment for patients with sepsis and reduce the use of broad-spectrum antimicrobials, which could help reduce future antimicrobial resistance.
“Rapid molecular tests that can identify which pathogens are the cause of an infection in hours rather than the days typically needed for traditional microbiology tests could ensure the right antibiotics are used much earlier in treatment,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“This, in turn, could improve outcomes for patients with suspected sepsis as well as help to reduce the spread of resistant microbes. However, the committee [advising NICE] concluded that the tests may offer clinical benefit, but there is too much uncertainty in the size of the benefit to determine the effect of introducing the tests into clinical practice.”
“The committee also concluded that, although the rapid molecular tests might provide results more quickly, there was too much uncertainty in the accuracy of the tests for clinicians to be able to base a decision on whether to withdraw or continue antibiotics. The committee therefore decided that further research should be encouraged to determine the clinical scenarios in which the tests may offer most benefit.”
NICE’s draft diagnostics guidance on the LightCycler SeptiFast Test MGRADE, SepsiTest, and IRIDICA BAC BSI assay is available on the NICE website. The closing date for comments on this draft guidance is October 21, 2015.