User login
Analysis finds 28.8% prevalence of depression in residents
The estimated prevalence of depression or depressive symptoms was 28.8% among residents and interns worldwide in a meta-analysis of 54 studies of the issue, according to a report published online December 8 in JAMA.
The depression rate ranged from 20.9% to 43.2%, depending on the instrument used to assess symptoms. Eleven studies used the Beck Depression Inventory (BDI), 11 used the Center for Epidemiological Studies Depression Scale (CES-D), 8 used the two-item Primary Care Evaluation of Mental Disorders questionnaire (PRIME-MD), 7 used the nine-item Patient Health Questionnaire (PHQ-9), 4 used the Zung Self-Rating Depression Scale (SDS), 3 used the Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS), and 11 used other validated methods, said Dr. Douglas A. Mata of the department of pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“It is important to note that the vast majority of participants were assessed through self-report inventories that measured depressive symptoms, rather than gold-standard diagnostic clinical interviews for major depressive disorder,” they said.
The meta-analysis included 31 cross-sectional and 23 longitudinal studies published in peer-reviewed journals since 1963 and involving 17,560 residents or interns in North America (35 studies), Asia (9 studies), Europe (5 studies), South America (4 studies), and Africa (1 study). When the results were pooled, the overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants).
In a sensitivity analysis, no individual study affected the overall prevalence estimate by more than 1%. Further analyses showed no significant differences in the prevalence of depression between cross-sectional and longitudinal studies, between U.S. studies and those performed in other countries, between studies of nonsurgical residents only vs. studies of all types of residents, or between studies of interns only vs. studies of upper level residents only. This suggests that the underlying causes of depressive symptoms “are common to the residency experience,” Dr. Mata and his associates said (JAMA. 2015 Dec 8. doi: 10.1001/jama.2015.15845).
The prevalence of depression increased over time. Although this rise was characterized as modest, “it is notable, given efforts by the Accreditation Council for Graduate Medical Education, European Working Time Directive, and others to limit trainee duty hours and improve work conditions. [This] trend may reflect the medical community’s increased awareness of depression or developments external to medical education. Future studies should explore specific factors that may explain this trend,” the investigators said.
The study findings indicate that the long-term health of physicians may be affected, since depression has been linked to a higher risk of future depressive episodes and greater long-term morbidity. Patient care may also be affected, given the established association between physician depression and lower-quality care, they added.
 |
Dr. Thomas L. Schwenk |
The meta-analysis by Mata et al. makes it clear that the extent of significant depressive symptomatology, if not overt clinical depression, among physicians-in-training is extraordinarily and unacceptably high. Relieving the burden of depression in these individuals is an issue of professional performance in addition to one of human compassion.
A national conversation about the fundamental structure and function of the graduate medical education system is long overdue, not unlike the discussion that reformed undergraduate medical education after the Flexner Report. The high burden of depressive symptoms among residents and interns has reached a crisis level. It is a marker for deeper and more profound problems in the medical education system, which require equally profound solutions.
Dr. Thomas L. Schwenk is at the University of Nevada, Reno. He reported having no relevant financial disclosures. Dr. Schwenk made these remarks in an editorial accompanying Dr. Mata’s report (JAMA 2015;314:2357-8).
 |
Dr. Thomas L. Schwenk |
The meta-analysis by Mata et al. makes it clear that the extent of significant depressive symptomatology, if not overt clinical depression, among physicians-in-training is extraordinarily and unacceptably high. Relieving the burden of depression in these individuals is an issue of professional performance in addition to one of human compassion.
A national conversation about the fundamental structure and function of the graduate medical education system is long overdue, not unlike the discussion that reformed undergraduate medical education after the Flexner Report. The high burden of depressive symptoms among residents and interns has reached a crisis level. It is a marker for deeper and more profound problems in the medical education system, which require equally profound solutions.
Dr. Thomas L. Schwenk is at the University of Nevada, Reno. He reported having no relevant financial disclosures. Dr. Schwenk made these remarks in an editorial accompanying Dr. Mata’s report (JAMA 2015;314:2357-8).
 |
Dr. Thomas L. Schwenk |
The meta-analysis by Mata et al. makes it clear that the extent of significant depressive symptomatology, if not overt clinical depression, among physicians-in-training is extraordinarily and unacceptably high. Relieving the burden of depression in these individuals is an issue of professional performance in addition to one of human compassion.
A national conversation about the fundamental structure and function of the graduate medical education system is long overdue, not unlike the discussion that reformed undergraduate medical education after the Flexner Report. The high burden of depressive symptoms among residents and interns has reached a crisis level. It is a marker for deeper and more profound problems in the medical education system, which require equally profound solutions.
Dr. Thomas L. Schwenk is at the University of Nevada, Reno. He reported having no relevant financial disclosures. Dr. Schwenk made these remarks in an editorial accompanying Dr. Mata’s report (JAMA 2015;314:2357-8).
The estimated prevalence of depression or depressive symptoms was 28.8% among residents and interns worldwide in a meta-analysis of 54 studies of the issue, according to a report published online December 8 in JAMA.
The depression rate ranged from 20.9% to 43.2%, depending on the instrument used to assess symptoms. Eleven studies used the Beck Depression Inventory (BDI), 11 used the Center for Epidemiological Studies Depression Scale (CES-D), 8 used the two-item Primary Care Evaluation of Mental Disorders questionnaire (PRIME-MD), 7 used the nine-item Patient Health Questionnaire (PHQ-9), 4 used the Zung Self-Rating Depression Scale (SDS), 3 used the Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS), and 11 used other validated methods, said Dr. Douglas A. Mata of the department of pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“It is important to note that the vast majority of participants were assessed through self-report inventories that measured depressive symptoms, rather than gold-standard diagnostic clinical interviews for major depressive disorder,” they said.
The meta-analysis included 31 cross-sectional and 23 longitudinal studies published in peer-reviewed journals since 1963 and involving 17,560 residents or interns in North America (35 studies), Asia (9 studies), Europe (5 studies), South America (4 studies), and Africa (1 study). When the results were pooled, the overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants).
In a sensitivity analysis, no individual study affected the overall prevalence estimate by more than 1%. Further analyses showed no significant differences in the prevalence of depression between cross-sectional and longitudinal studies, between U.S. studies and those performed in other countries, between studies of nonsurgical residents only vs. studies of all types of residents, or between studies of interns only vs. studies of upper level residents only. This suggests that the underlying causes of depressive symptoms “are common to the residency experience,” Dr. Mata and his associates said (JAMA. 2015 Dec 8. doi: 10.1001/jama.2015.15845).
The prevalence of depression increased over time. Although this rise was characterized as modest, “it is notable, given efforts by the Accreditation Council for Graduate Medical Education, European Working Time Directive, and others to limit trainee duty hours and improve work conditions. [This] trend may reflect the medical community’s increased awareness of depression or developments external to medical education. Future studies should explore specific factors that may explain this trend,” the investigators said.
The study findings indicate that the long-term health of physicians may be affected, since depression has been linked to a higher risk of future depressive episodes and greater long-term morbidity. Patient care may also be affected, given the established association between physician depression and lower-quality care, they added.
The estimated prevalence of depression or depressive symptoms was 28.8% among residents and interns worldwide in a meta-analysis of 54 studies of the issue, according to a report published online December 8 in JAMA.
The depression rate ranged from 20.9% to 43.2%, depending on the instrument used to assess symptoms. Eleven studies used the Beck Depression Inventory (BDI), 11 used the Center for Epidemiological Studies Depression Scale (CES-D), 8 used the two-item Primary Care Evaluation of Mental Disorders questionnaire (PRIME-MD), 7 used the nine-item Patient Health Questionnaire (PHQ-9), 4 used the Zung Self-Rating Depression Scale (SDS), 3 used the Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS), and 11 used other validated methods, said Dr. Douglas A. Mata of the department of pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“It is important to note that the vast majority of participants were assessed through self-report inventories that measured depressive symptoms, rather than gold-standard diagnostic clinical interviews for major depressive disorder,” they said.
The meta-analysis included 31 cross-sectional and 23 longitudinal studies published in peer-reviewed journals since 1963 and involving 17,560 residents or interns in North America (35 studies), Asia (9 studies), Europe (5 studies), South America (4 studies), and Africa (1 study). When the results were pooled, the overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants).
In a sensitivity analysis, no individual study affected the overall prevalence estimate by more than 1%. Further analyses showed no significant differences in the prevalence of depression between cross-sectional and longitudinal studies, between U.S. studies and those performed in other countries, between studies of nonsurgical residents only vs. studies of all types of residents, or between studies of interns only vs. studies of upper level residents only. This suggests that the underlying causes of depressive symptoms “are common to the residency experience,” Dr. Mata and his associates said (JAMA. 2015 Dec 8. doi: 10.1001/jama.2015.15845).
The prevalence of depression increased over time. Although this rise was characterized as modest, “it is notable, given efforts by the Accreditation Council for Graduate Medical Education, European Working Time Directive, and others to limit trainee duty hours and improve work conditions. [This] trend may reflect the medical community’s increased awareness of depression or developments external to medical education. Future studies should explore specific factors that may explain this trend,” the investigators said.
The study findings indicate that the long-term health of physicians may be affected, since depression has been linked to a higher risk of future depressive episodes and greater long-term morbidity. Patient care may also be affected, given the established association between physician depression and lower-quality care, they added.
FROM JAMA
Key clinical point: The prevalence of depression or depressive symptoms was 28.8% (range, 20.9%-43.2%) among residents in a meta-analysis of 54 studies.
Major finding: The overall prevalence of depression or depressive symptoms was 28.8% (4,969 of 17,560 participants) across all countries, all types of studies, and all types of graduate medical education programs.
Data source: A meta-analysis of 31 cross-sectional and 23 longitudinal studies involving 17,560 residents and interns worldwide.
Disclosures: This study was supported by the U.S. Department of State Fulbright Scholarship program, the National Institutes of Health, and the NIH Medical Scientist Training Program. Dr. Mata and his associates reported having no relevant financial disclosures.
Comparing once-weekly GLP-1RAs
When compared head-to-head in patients with type 2 diabetes mellitus, five once-weekly glucagonlike peptide–1 receptor agonists yield appreciably different effects on hemoglonin A1c (HbA1c) fasting plasma glucose, body weight, and nausea but very similar effects on hypoglycemia, blood pressure, lipids, and C-reactive protein levels, according to a report published online Dec. 7 in Annals of Internal Medicine.
Until now, no studies have directly compared the efficacy and safety of various glucagonlike peptide–1 receptor agonists (GLP-1RAs) for patients with type 2 diabetes. To do so, researchers performed a network meta-analysis of 34 randomized controlled trials of at least 6 months’ duration involving a total of 21,126 patients and comparing albiglutide, dulaglutide, exenatide, semaglutide, or taspoglutide against either placebo or another glucose-lowering agent. All the trials were published between 2008 and 2015, said Dr. Francesco Zaccardi of the Diabetes Research Centre, Leicester (England) General Hospital and his associates.
Taken together, the five once-weekly GLP-1RAs reduced the primary outcome measure, hemoglobin A1c, compared with placebo. Dulaglutide produced the greatest reduction (1.4%). All the agents also significantly reduced fasting plasma glucose, compared with placebo, with exenatide producing the greatest reduction. Exenatide, semaglutide, and higher doses of dulaglutide and taspoglutide also significantly reduced body weight by between 0.7 kg and 1.3 kg, compared with placebo. “Our results would therefore suggest clinically significant differences on three key indicators of metabolic control,” Dr. Zaccardi and his associates said (Ann. Intern. Med. 2015 Dec. 7. doi: 10.7326/M15-1432).
The risk for nausea also differed among the five agents. Taspoglutide carried the highest risk, which ranged from two- to ninefold higher than that of the other GLP-1RAs. With taspoglutide the risk for nausea was more than eight times higher than that with albiglutide.
Conversely, the risks for symptomatic hypoglycemia and rates of beneficial changes in blood pressure, lipid profiles, and C-reactive protein levels were similar across the five GLP-1RAs.
This meta-analysis was supported by an unrestricted grant from Sanofi-Aventis to the University of Leicester, and by the National Institute for Health Research and University Hospitals of Leicester and Loughborough University. Dr. Zaccardi reported having no relevant financial disclosures other than funding from Sanofi-Aventis; his associates reported ties to numerous industry sources, including the manufacturers of the five GLP-1RAs analyzed here.
Be skeptical of any claims that the 5 GLP-1RAs assessed by Zaccardi et al. differ in efficacy or safety measures, because of the low quality of the evidence summarized in this network meta-analysis.
The trials that were reviewed had inadequate blinding and substantial loss to follow-up, and data on outcomes that are most important to patients—quality of life, treatment burden, morbidity, and mortality – were sparse or nonexistent. The trials were primarily designed and reported by the manufacturers, whose goal is to enter the drug market and position their product favorably, not to facilitate clinical decision making. That’s why reliable head-to-head comparison studies of agents made by competitors are so rare, and why researchers have to rely on questionable strategies like pooling data from studies with very different patient populations and methodologies.
Perhaps the top priority in the effort to find the antihyperglycemic agent that best addresses a patient’s situation should be finding the best drug class. Tools are available to facilitate this task (Arch Intern Med. 2009;169:1560-8. doi: 10.1001/archinternmed.2009.293).
Dr. Victor M. Montori is in the Knowledge and Evaluation Research Unit in the divisions of endocrinology, diabetes, metabolism, and nutrition research at the Mayo Clinic in Rochester, Minn. His financial disclosures are available at www.annals.orgDr. Montori made these remarks in an editorial accompanying Dr. Zaccardi’s report (Ann. Intern. Med. 2015 Dec. 7. doi: 10.7326/M15-2610).
Be skeptical of any claims that the 5 GLP-1RAs assessed by Zaccardi et al. differ in efficacy or safety measures, because of the low quality of the evidence summarized in this network meta-analysis.
The trials that were reviewed had inadequate blinding and substantial loss to follow-up, and data on outcomes that are most important to patients—quality of life, treatment burden, morbidity, and mortality – were sparse or nonexistent. The trials were primarily designed and reported by the manufacturers, whose goal is to enter the drug market and position their product favorably, not to facilitate clinical decision making. That’s why reliable head-to-head comparison studies of agents made by competitors are so rare, and why researchers have to rely on questionable strategies like pooling data from studies with very different patient populations and methodologies.
Perhaps the top priority in the effort to find the antihyperglycemic agent that best addresses a patient’s situation should be finding the best drug class. Tools are available to facilitate this task (Arch Intern Med. 2009;169:1560-8. doi: 10.1001/archinternmed.2009.293).
Dr. Victor M. Montori is in the Knowledge and Evaluation Research Unit in the divisions of endocrinology, diabetes, metabolism, and nutrition research at the Mayo Clinic in Rochester, Minn. His financial disclosures are available at www.annals.orgDr. Montori made these remarks in an editorial accompanying Dr. Zaccardi’s report (Ann. Intern. Med. 2015 Dec. 7. doi: 10.7326/M15-2610).
Be skeptical of any claims that the 5 GLP-1RAs assessed by Zaccardi et al. differ in efficacy or safety measures, because of the low quality of the evidence summarized in this network meta-analysis.
The trials that were reviewed had inadequate blinding and substantial loss to follow-up, and data on outcomes that are most important to patients—quality of life, treatment burden, morbidity, and mortality – were sparse or nonexistent. The trials were primarily designed and reported by the manufacturers, whose goal is to enter the drug market and position their product favorably, not to facilitate clinical decision making. That’s why reliable head-to-head comparison studies of agents made by competitors are so rare, and why researchers have to rely on questionable strategies like pooling data from studies with very different patient populations and methodologies.
Perhaps the top priority in the effort to find the antihyperglycemic agent that best addresses a patient’s situation should be finding the best drug class. Tools are available to facilitate this task (Arch Intern Med. 2009;169:1560-8. doi: 10.1001/archinternmed.2009.293).
Dr. Victor M. Montori is in the Knowledge and Evaluation Research Unit in the divisions of endocrinology, diabetes, metabolism, and nutrition research at the Mayo Clinic in Rochester, Minn. His financial disclosures are available at www.annals.orgDr. Montori made these remarks in an editorial accompanying Dr. Zaccardi’s report (Ann. Intern. Med. 2015 Dec. 7. doi: 10.7326/M15-2610).
When compared head-to-head in patients with type 2 diabetes mellitus, five once-weekly glucagonlike peptide–1 receptor agonists yield appreciably different effects on hemoglonin A1c (HbA1c) fasting plasma glucose, body weight, and nausea but very similar effects on hypoglycemia, blood pressure, lipids, and C-reactive protein levels, according to a report published online Dec. 7 in Annals of Internal Medicine.
Until now, no studies have directly compared the efficacy and safety of various glucagonlike peptide–1 receptor agonists (GLP-1RAs) for patients with type 2 diabetes. To do so, researchers performed a network meta-analysis of 34 randomized controlled trials of at least 6 months’ duration involving a total of 21,126 patients and comparing albiglutide, dulaglutide, exenatide, semaglutide, or taspoglutide against either placebo or another glucose-lowering agent. All the trials were published between 2008 and 2015, said Dr. Francesco Zaccardi of the Diabetes Research Centre, Leicester (England) General Hospital and his associates.
Taken together, the five once-weekly GLP-1RAs reduced the primary outcome measure, hemoglobin A1c, compared with placebo. Dulaglutide produced the greatest reduction (1.4%). All the agents also significantly reduced fasting plasma glucose, compared with placebo, with exenatide producing the greatest reduction. Exenatide, semaglutide, and higher doses of dulaglutide and taspoglutide also significantly reduced body weight by between 0.7 kg and 1.3 kg, compared with placebo. “Our results would therefore suggest clinically significant differences on three key indicators of metabolic control,” Dr. Zaccardi and his associates said (Ann. Intern. Med. 2015 Dec. 7. doi: 10.7326/M15-1432).
The risk for nausea also differed among the five agents. Taspoglutide carried the highest risk, which ranged from two- to ninefold higher than that of the other GLP-1RAs. With taspoglutide the risk for nausea was more than eight times higher than that with albiglutide.
Conversely, the risks for symptomatic hypoglycemia and rates of beneficial changes in blood pressure, lipid profiles, and C-reactive protein levels were similar across the five GLP-1RAs.
This meta-analysis was supported by an unrestricted grant from Sanofi-Aventis to the University of Leicester, and by the National Institute for Health Research and University Hospitals of Leicester and Loughborough University. Dr. Zaccardi reported having no relevant financial disclosures other than funding from Sanofi-Aventis; his associates reported ties to numerous industry sources, including the manufacturers of the five GLP-1RAs analyzed here.
When compared head-to-head in patients with type 2 diabetes mellitus, five once-weekly glucagonlike peptide–1 receptor agonists yield appreciably different effects on hemoglonin A1c (HbA1c) fasting plasma glucose, body weight, and nausea but very similar effects on hypoglycemia, blood pressure, lipids, and C-reactive protein levels, according to a report published online Dec. 7 in Annals of Internal Medicine.
Until now, no studies have directly compared the efficacy and safety of various glucagonlike peptide–1 receptor agonists (GLP-1RAs) for patients with type 2 diabetes. To do so, researchers performed a network meta-analysis of 34 randomized controlled trials of at least 6 months’ duration involving a total of 21,126 patients and comparing albiglutide, dulaglutide, exenatide, semaglutide, or taspoglutide against either placebo or another glucose-lowering agent. All the trials were published between 2008 and 2015, said Dr. Francesco Zaccardi of the Diabetes Research Centre, Leicester (England) General Hospital and his associates.
Taken together, the five once-weekly GLP-1RAs reduced the primary outcome measure, hemoglobin A1c, compared with placebo. Dulaglutide produced the greatest reduction (1.4%). All the agents also significantly reduced fasting plasma glucose, compared with placebo, with exenatide producing the greatest reduction. Exenatide, semaglutide, and higher doses of dulaglutide and taspoglutide also significantly reduced body weight by between 0.7 kg and 1.3 kg, compared with placebo. “Our results would therefore suggest clinically significant differences on three key indicators of metabolic control,” Dr. Zaccardi and his associates said (Ann. Intern. Med. 2015 Dec. 7. doi: 10.7326/M15-1432).
The risk for nausea also differed among the five agents. Taspoglutide carried the highest risk, which ranged from two- to ninefold higher than that of the other GLP-1RAs. With taspoglutide the risk for nausea was more than eight times higher than that with albiglutide.
Conversely, the risks for symptomatic hypoglycemia and rates of beneficial changes in blood pressure, lipid profiles, and C-reactive protein levels were similar across the five GLP-1RAs.
This meta-analysis was supported by an unrestricted grant from Sanofi-Aventis to the University of Leicester, and by the National Institute for Health Research and University Hospitals of Leicester and Loughborough University. Dr. Zaccardi reported having no relevant financial disclosures other than funding from Sanofi-Aventis; his associates reported ties to numerous industry sources, including the manufacturers of the five GLP-1RAs analyzed here.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Different once-weekly GLP-1RAs yield appreciably different effects on HbA1c, fasting plasma glucose, body weight, and nausea but very similar effects on hypoglycemia, blood pressure, lipids, and C-reactive protein levels.
Major finding: The 5 GLP-1RAs reduced the primary outcome measure, HbA1c, compared with placebo; dulaglutide produced the greatest reduction (1.4%).
Data source: A network meta-analysis of 34 phase III, randomized clinical trials published in 2008-2015 involving 21,126 participants with type 2 diabetes.
Disclosures: This meta-analysis was supported by an unrestricted grant from Sanofi-Aventis to the University of Leicester, and by the National Institute for Health Research and University Hospitals of Leicester and Loughborough University. Dr. Zaccardi reported having no relevant financial disclosures other than funding from Sanofi-Aventis; his associates reported ties to numerous industry sources, including the manufacturers of the five GLP-1RAs analyzed here.
Study: Behavioral strategies needed for HIV+ young gay men
Behavioral, not just medical, strategies are needed to curb transmission from HIV-positive adolescent boys and young men who have sex with men, according to a report published online Dec. 7 in JAMA Pediatrics.
Condom use, together with treatment to suppress viral load, drastically reduce the likelihood that HIV-positive teens and men will transmit the virus to their sexual partners. But it is “challenging” to keep this patient group engaged in health care and adherent to antiretroviral treatment (ART), said Patrick A. Wilson, Ph.D., of the department of sociomedical sciences, Mailman School of Public Health, Columbia University, New York, and his associates.
They performed a cross-sectional survey involving 991 HIV-positive males who reported having recent oral or anal intercourse with other males. They were aged 12-26 years and received at least some care at 20 specialty clinics in major cities across the United States. They completed 45- to 90-minute computer-assisted self-interviews assessing substance use, mental health, and sexual behavior. Their medical records provided information regarding treatment and viral load.
A little more than half (52.1%) of the respondents said they were currently prescribed ART, and a majority – 69.4% – had a detectable viral load. “It was particularly troubling” that fewer than 3 in 10 (29.1%) of these sexually active young men had an undetectable viral load. Clinicians should be aware of the stark disparities in viral suppression among men who have sex with men, Dr. Wilson and his associates said.
Just as alarming was the high incidence of engaging in anal intercourse without a condom: 46.2%. Moreover, fully 31.3% of the respondents said they had condomless anal intercourse with male partners who were not HIV positive.
Several factors were strongly and independently linked with both unprotected anal sex and unprotected anal sex with an HIV-negative partner, including problematic substance use, unemployment, previous incarceration, and nonblack race. Previous research has found that substance use both escalates high-risk sexual behavior and disrupts adherence to ART, so this study result demonstrates that “substance use must continue to be a target of health promotion interventions [aimed at] HIV-infected youth,” the investigators said (JAMA Pediatrics 2015 Dec 7. doi: 10.1001/jamapediatrics.2015.3333).
Previous research has revealed a paradox: Among young men who have sex with men, those who are black are less likely to engage in high-risk sexual behavior than are those of other races, yet black men are the population group most affected by HIV in the United States. “This finding underscores the role that social network and structural factors [such as unemployment and incarceration] play in explaining enhanced HIV vulnerability among young black men who have sex with men,” Dr. Wilson and his associates wrote.
One factor in particular correlated with safe sexual practice in this study population: transgender gender identity. This constitutes another paradox that deserves further research, since HIV-infected transgender individuals are at uniquely high risk for poor health outcomes, the researchers added.
Overall, the study findings clearly demonstrate that behavioral interventions are critical to addressing HIV transmission in this patient population, they said.
The National Institute of Child Health and Human Development, the National Institute on Drug Abuse, and the National Institute on Mental Health funded the study. Dr. Wilson and his associates reported having no financial conflicts.
Behavioral, not just medical, strategies are needed to curb transmission from HIV-positive adolescent boys and young men who have sex with men, according to a report published online Dec. 7 in JAMA Pediatrics.
Condom use, together with treatment to suppress viral load, drastically reduce the likelihood that HIV-positive teens and men will transmit the virus to their sexual partners. But it is “challenging” to keep this patient group engaged in health care and adherent to antiretroviral treatment (ART), said Patrick A. Wilson, Ph.D., of the department of sociomedical sciences, Mailman School of Public Health, Columbia University, New York, and his associates.
They performed a cross-sectional survey involving 991 HIV-positive males who reported having recent oral or anal intercourse with other males. They were aged 12-26 years and received at least some care at 20 specialty clinics in major cities across the United States. They completed 45- to 90-minute computer-assisted self-interviews assessing substance use, mental health, and sexual behavior. Their medical records provided information regarding treatment and viral load.
A little more than half (52.1%) of the respondents said they were currently prescribed ART, and a majority – 69.4% – had a detectable viral load. “It was particularly troubling” that fewer than 3 in 10 (29.1%) of these sexually active young men had an undetectable viral load. Clinicians should be aware of the stark disparities in viral suppression among men who have sex with men, Dr. Wilson and his associates said.
Just as alarming was the high incidence of engaging in anal intercourse without a condom: 46.2%. Moreover, fully 31.3% of the respondents said they had condomless anal intercourse with male partners who were not HIV positive.
Several factors were strongly and independently linked with both unprotected anal sex and unprotected anal sex with an HIV-negative partner, including problematic substance use, unemployment, previous incarceration, and nonblack race. Previous research has found that substance use both escalates high-risk sexual behavior and disrupts adherence to ART, so this study result demonstrates that “substance use must continue to be a target of health promotion interventions [aimed at] HIV-infected youth,” the investigators said (JAMA Pediatrics 2015 Dec 7. doi: 10.1001/jamapediatrics.2015.3333).
Previous research has revealed a paradox: Among young men who have sex with men, those who are black are less likely to engage in high-risk sexual behavior than are those of other races, yet black men are the population group most affected by HIV in the United States. “This finding underscores the role that social network and structural factors [such as unemployment and incarceration] play in explaining enhanced HIV vulnerability among young black men who have sex with men,” Dr. Wilson and his associates wrote.
One factor in particular correlated with safe sexual practice in this study population: transgender gender identity. This constitutes another paradox that deserves further research, since HIV-infected transgender individuals are at uniquely high risk for poor health outcomes, the researchers added.
Overall, the study findings clearly demonstrate that behavioral interventions are critical to addressing HIV transmission in this patient population, they said.
The National Institute of Child Health and Human Development, the National Institute on Drug Abuse, and the National Institute on Mental Health funded the study. Dr. Wilson and his associates reported having no financial conflicts.
Behavioral, not just medical, strategies are needed to curb transmission from HIV-positive adolescent boys and young men who have sex with men, according to a report published online Dec. 7 in JAMA Pediatrics.
Condom use, together with treatment to suppress viral load, drastically reduce the likelihood that HIV-positive teens and men will transmit the virus to their sexual partners. But it is “challenging” to keep this patient group engaged in health care and adherent to antiretroviral treatment (ART), said Patrick A. Wilson, Ph.D., of the department of sociomedical sciences, Mailman School of Public Health, Columbia University, New York, and his associates.
They performed a cross-sectional survey involving 991 HIV-positive males who reported having recent oral or anal intercourse with other males. They were aged 12-26 years and received at least some care at 20 specialty clinics in major cities across the United States. They completed 45- to 90-minute computer-assisted self-interviews assessing substance use, mental health, and sexual behavior. Their medical records provided information regarding treatment and viral load.
A little more than half (52.1%) of the respondents said they were currently prescribed ART, and a majority – 69.4% – had a detectable viral load. “It was particularly troubling” that fewer than 3 in 10 (29.1%) of these sexually active young men had an undetectable viral load. Clinicians should be aware of the stark disparities in viral suppression among men who have sex with men, Dr. Wilson and his associates said.
Just as alarming was the high incidence of engaging in anal intercourse without a condom: 46.2%. Moreover, fully 31.3% of the respondents said they had condomless anal intercourse with male partners who were not HIV positive.
Several factors were strongly and independently linked with both unprotected anal sex and unprotected anal sex with an HIV-negative partner, including problematic substance use, unemployment, previous incarceration, and nonblack race. Previous research has found that substance use both escalates high-risk sexual behavior and disrupts adherence to ART, so this study result demonstrates that “substance use must continue to be a target of health promotion interventions [aimed at] HIV-infected youth,” the investigators said (JAMA Pediatrics 2015 Dec 7. doi: 10.1001/jamapediatrics.2015.3333).
Previous research has revealed a paradox: Among young men who have sex with men, those who are black are less likely to engage in high-risk sexual behavior than are those of other races, yet black men are the population group most affected by HIV in the United States. “This finding underscores the role that social network and structural factors [such as unemployment and incarceration] play in explaining enhanced HIV vulnerability among young black men who have sex with men,” Dr. Wilson and his associates wrote.
One factor in particular correlated with safe sexual practice in this study population: transgender gender identity. This constitutes another paradox that deserves further research, since HIV-infected transgender individuals are at uniquely high risk for poor health outcomes, the researchers added.
Overall, the study findings clearly demonstrate that behavioral interventions are critical to addressing HIV transmission in this patient population, they said.
The National Institute of Child Health and Human Development, the National Institute on Drug Abuse, and the National Institute on Mental Health funded the study. Dr. Wilson and his associates reported having no financial conflicts.
FROM JAMA PEDIATRICS
Key clinical point: Behavioral, not just medical, strategies are needed to curb transmission in HIV-positive young gay men.
Major finding: Among respondents, 69.4% had a detectable viral load, 46.2% engaged in anal intercourse without a condom, and 31.3% said they had condomless anal intercourse with male partners who were not HIV positive.
Data source: A cross-sectional survey of 991 HIV+ gay males aged 12-26 years treated at 20 U.S. clinics.
Disclosures: The National Institute of Child Health and Human Development, the National Institute on Drug Abuse, and the National Institute on Mental Health funded the study. Dr. Wilson and his associates reported having no financial conflicts.
Postop C. diff infection associated with presurgical antibiotics
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.
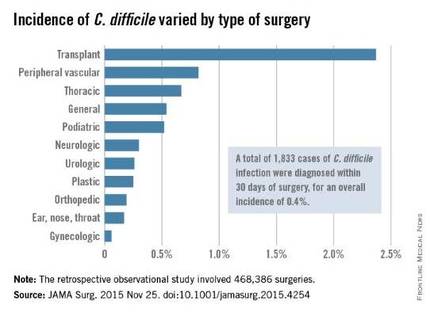
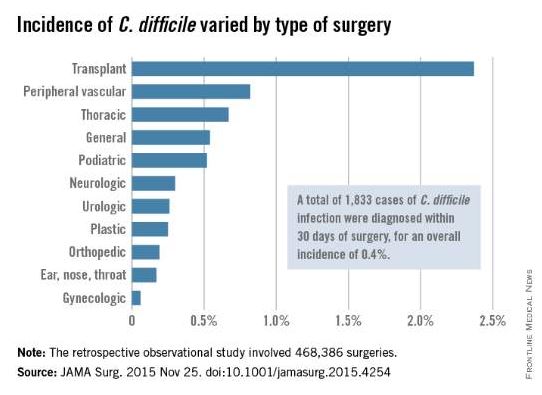
The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.

The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.

The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: A hospital’s rate of postoperative C. difficile infection is related to the number of preoperative antibiotics patients took and the complexity of their surgeries.
Major finding: Patients who had taken three or more preoperative antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics.
Data source: A retrospective observational study involving 468,386 surgeries at 134 VA medical centers during a 4-year period.
Disclosures: This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
Lixisenatide not cardioprotective in type 2 diabetes
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding lixisenatide to usual care failed to prevent major cardiovascular events in patients with type 2 diabetes who had a recent ACS.
Major finding: Death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina occurred in 13.4% of participants receiving lixisenatide and 13.2% of those receiving placebo.
Data source: An international randomized double-blind placebo-controlled trial involving 6,068 patients followed for a median of 2 years.
Disclosures: Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
C-section target rates may be set too low
Countries with a cesarean delivery rate as high as 19% have lower maternal and neonatal mortality, compared with countries that have higher c-section rates, according to a report published online Dec. 1 in JAMA.
The findings suggest that the current World Health Organization (WHO) recommendation that national rates of cesarean delivery shouldn’t exceed 10%-15% of live births may be setting the target rate too low, according to Dr. George Molina of Ariadne Labs at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, Boston, and his associates.
“The WHO recommendation that population level cesarean delivery rates should not exceed 10%-15% was a consensus opinion based on the observation that some countries with the lowest perinatal mortality rates had cesarean delivery rates that were less than 10/100 live births,” the researchers wrote. “Prior studies suggesting that lower cesarean delivery rate thresholds were optimal for maternal and neonatal mortality were incomplete because they examined data from limited sets of countries and often examined outcomes in wealthier countries.”
The researchers estimated that globally, the number of cesarean deliveries was 22.9 million in 2012 for a global cesarean delivery rate of 19.4/100 live births.
Cesarean delivery rates of up to 19.1/100 live births were inversely correlated with the maternal mortality ratio at the country level, and cesarean delivery rates of up to 19.4/100 live births were inversely correlated with the neonatal mortality ratio.
Dr. Molina and his colleagues analyzed the most recent information concerning modes of delivery, per capita health expenditures, life expectancy, and numerous other related factors using several multinational health databases. The goal was to estimate national rates of cesarean delivery and relate those figures to maternal and neonatal mortality for as many countries as possible, focusing on the most recent single year (2012) for which adequate data were available.
A sensitivity analysis of the 76 countries with the highest-quality cesarean delivery rate information showed that cesarean delivery rates greater than 6.9 to 20.1 per 100 live births were inversely correlated with the maternal mortality ratio. Cesarean delivery rates of 12.6 to 24.0 per 100 live births were inversely correlated with neonatal mortality (JAMA. 2015 Dec 1;314[21]:2263-70. doi: 10.1001/jama.2015.15553).
The researchers noted that they focused solely on mortality and did not assess other health outcomes. This means that they could not address the many possible benefits of cesarean delivery, such as reduced morbidity from complicated vaginal delivery or from prolonged obstructed labor.
In a separate report, a different research group found that children born by planned cesarean delivery, as compared with vaginal delivery, had a slightly increased risk of asthma requiring hospital admission, of needing a prescription for a salbutamol inhaler at age 5 years, and of all-cause mortality by age 21 years.
Dr. Mairead Blackof the University of Aberdeen (England) and her associates examined the relationship between planned cesarean delivery and chronic illness and death among the offspring. They assessed outcomes in 321,287 singleton live births to first-time mothers that occurred in Scotland in 1993-2007; the children were followed until January 2015, for a mean follow-up of 14.8 years.
In this cohort, 12,355 children (3.8%) were born by planned cesarean delivery, 56,015 (17.5%) by unscheduled cesarean, and 252,917 (78.7%) by vaginal delivery.
Children born by planned C-sections were slightly, but significantly more likely than were those born vaginally to develop asthma requiring hospital admission (3.73% vs. 3.41%; hazard ratio, 1.22).
Compared with children delivered vaginally, those delivered by planned cesarean section also were slightly, but significantly more likely to require a prescription for a salbutamol inhaler at age 5 years (10.34% vs. 9.62%; HR, 1.13) and to die before the age of 21 years(0.40% vs. 0.32%; HR, 1.41).
However, there were no significant differences between the two study groups in risk of developing inflammatory bowel disease (0.11% vs. 0.13%) or cancer (0.23% vs 0.21%), they noted (JAMA. 2015 Dec 1;314[21]:2271-9. doi: 10.1001/jama.2015.16176).
These findings “suggest that avoidance of vaginal birth may be an important early-life factor in the growing global burden of asthma, although absolute increase in risk to individuals is low,” Dr. Black and her associates wrote.
The researchers in both studies reported having no relevant financial disclosures. Dr. Black’s study was supported in part by the Wellcome Trust.
The findings of Molina et al. challenge a 30-year-old recommendation that a cesarean delivery rate of less than 15% should be a target for all health care institutions.
The study results also highlight the enormous variation in cesarean rates around the world and the need for the international obstetric community to evaluate this important health care issue. There is no one-size-fits-all optimal level of cesarean delivery that applies to all institutions, all health care systems, or all countries. The goal should be to identify meaningful ranges of risk-adjusted rates of cesarean deliveries for different populations and practices.
Dr. Mary E. D’Alton and Dr. Mark P. Hehir are in the department of obstetrics and gynecology at New York Presbyterian Hospital and Columbia University, New York. They reported having no relevant financial disclosures. These comments are adapted from an editorial accompanying the reports by Dr. Molina and Dr. Black (JAMA. 2015 Dec 1;314[21]:2238-40).
The findings of Molina et al. challenge a 30-year-old recommendation that a cesarean delivery rate of less than 15% should be a target for all health care institutions.
The study results also highlight the enormous variation in cesarean rates around the world and the need for the international obstetric community to evaluate this important health care issue. There is no one-size-fits-all optimal level of cesarean delivery that applies to all institutions, all health care systems, or all countries. The goal should be to identify meaningful ranges of risk-adjusted rates of cesarean deliveries for different populations and practices.
Dr. Mary E. D’Alton and Dr. Mark P. Hehir are in the department of obstetrics and gynecology at New York Presbyterian Hospital and Columbia University, New York. They reported having no relevant financial disclosures. These comments are adapted from an editorial accompanying the reports by Dr. Molina and Dr. Black (JAMA. 2015 Dec 1;314[21]:2238-40).
The findings of Molina et al. challenge a 30-year-old recommendation that a cesarean delivery rate of less than 15% should be a target for all health care institutions.
The study results also highlight the enormous variation in cesarean rates around the world and the need for the international obstetric community to evaluate this important health care issue. There is no one-size-fits-all optimal level of cesarean delivery that applies to all institutions, all health care systems, or all countries. The goal should be to identify meaningful ranges of risk-adjusted rates of cesarean deliveries for different populations and practices.
Dr. Mary E. D’Alton and Dr. Mark P. Hehir are in the department of obstetrics and gynecology at New York Presbyterian Hospital and Columbia University, New York. They reported having no relevant financial disclosures. These comments are adapted from an editorial accompanying the reports by Dr. Molina and Dr. Black (JAMA. 2015 Dec 1;314[21]:2238-40).
Countries with a cesarean delivery rate as high as 19% have lower maternal and neonatal mortality, compared with countries that have higher c-section rates, according to a report published online Dec. 1 in JAMA.
The findings suggest that the current World Health Organization (WHO) recommendation that national rates of cesarean delivery shouldn’t exceed 10%-15% of live births may be setting the target rate too low, according to Dr. George Molina of Ariadne Labs at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, Boston, and his associates.
“The WHO recommendation that population level cesarean delivery rates should not exceed 10%-15% was a consensus opinion based on the observation that some countries with the lowest perinatal mortality rates had cesarean delivery rates that were less than 10/100 live births,” the researchers wrote. “Prior studies suggesting that lower cesarean delivery rate thresholds were optimal for maternal and neonatal mortality were incomplete because they examined data from limited sets of countries and often examined outcomes in wealthier countries.”
The researchers estimated that globally, the number of cesarean deliveries was 22.9 million in 2012 for a global cesarean delivery rate of 19.4/100 live births.
Cesarean delivery rates of up to 19.1/100 live births were inversely correlated with the maternal mortality ratio at the country level, and cesarean delivery rates of up to 19.4/100 live births were inversely correlated with the neonatal mortality ratio.
Dr. Molina and his colleagues analyzed the most recent information concerning modes of delivery, per capita health expenditures, life expectancy, and numerous other related factors using several multinational health databases. The goal was to estimate national rates of cesarean delivery and relate those figures to maternal and neonatal mortality for as many countries as possible, focusing on the most recent single year (2012) for which adequate data were available.
A sensitivity analysis of the 76 countries with the highest-quality cesarean delivery rate information showed that cesarean delivery rates greater than 6.9 to 20.1 per 100 live births were inversely correlated with the maternal mortality ratio. Cesarean delivery rates of 12.6 to 24.0 per 100 live births were inversely correlated with neonatal mortality (JAMA. 2015 Dec 1;314[21]:2263-70. doi: 10.1001/jama.2015.15553).
The researchers noted that they focused solely on mortality and did not assess other health outcomes. This means that they could not address the many possible benefits of cesarean delivery, such as reduced morbidity from complicated vaginal delivery or from prolonged obstructed labor.
In a separate report, a different research group found that children born by planned cesarean delivery, as compared with vaginal delivery, had a slightly increased risk of asthma requiring hospital admission, of needing a prescription for a salbutamol inhaler at age 5 years, and of all-cause mortality by age 21 years.
Dr. Mairead Blackof the University of Aberdeen (England) and her associates examined the relationship between planned cesarean delivery and chronic illness and death among the offspring. They assessed outcomes in 321,287 singleton live births to first-time mothers that occurred in Scotland in 1993-2007; the children were followed until January 2015, for a mean follow-up of 14.8 years.
In this cohort, 12,355 children (3.8%) were born by planned cesarean delivery, 56,015 (17.5%) by unscheduled cesarean, and 252,917 (78.7%) by vaginal delivery.
Children born by planned C-sections were slightly, but significantly more likely than were those born vaginally to develop asthma requiring hospital admission (3.73% vs. 3.41%; hazard ratio, 1.22).
Compared with children delivered vaginally, those delivered by planned cesarean section also were slightly, but significantly more likely to require a prescription for a salbutamol inhaler at age 5 years (10.34% vs. 9.62%; HR, 1.13) and to die before the age of 21 years(0.40% vs. 0.32%; HR, 1.41).
However, there were no significant differences between the two study groups in risk of developing inflammatory bowel disease (0.11% vs. 0.13%) or cancer (0.23% vs 0.21%), they noted (JAMA. 2015 Dec 1;314[21]:2271-9. doi: 10.1001/jama.2015.16176).
These findings “suggest that avoidance of vaginal birth may be an important early-life factor in the growing global burden of asthma, although absolute increase in risk to individuals is low,” Dr. Black and her associates wrote.
The researchers in both studies reported having no relevant financial disclosures. Dr. Black’s study was supported in part by the Wellcome Trust.
Countries with a cesarean delivery rate as high as 19% have lower maternal and neonatal mortality, compared with countries that have higher c-section rates, according to a report published online Dec. 1 in JAMA.
The findings suggest that the current World Health Organization (WHO) recommendation that national rates of cesarean delivery shouldn’t exceed 10%-15% of live births may be setting the target rate too low, according to Dr. George Molina of Ariadne Labs at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, Boston, and his associates.
“The WHO recommendation that population level cesarean delivery rates should not exceed 10%-15% was a consensus opinion based on the observation that some countries with the lowest perinatal mortality rates had cesarean delivery rates that were less than 10/100 live births,” the researchers wrote. “Prior studies suggesting that lower cesarean delivery rate thresholds were optimal for maternal and neonatal mortality were incomplete because they examined data from limited sets of countries and often examined outcomes in wealthier countries.”
The researchers estimated that globally, the number of cesarean deliveries was 22.9 million in 2012 for a global cesarean delivery rate of 19.4/100 live births.
Cesarean delivery rates of up to 19.1/100 live births were inversely correlated with the maternal mortality ratio at the country level, and cesarean delivery rates of up to 19.4/100 live births were inversely correlated with the neonatal mortality ratio.
Dr. Molina and his colleagues analyzed the most recent information concerning modes of delivery, per capita health expenditures, life expectancy, and numerous other related factors using several multinational health databases. The goal was to estimate national rates of cesarean delivery and relate those figures to maternal and neonatal mortality for as many countries as possible, focusing on the most recent single year (2012) for which adequate data were available.
A sensitivity analysis of the 76 countries with the highest-quality cesarean delivery rate information showed that cesarean delivery rates greater than 6.9 to 20.1 per 100 live births were inversely correlated with the maternal mortality ratio. Cesarean delivery rates of 12.6 to 24.0 per 100 live births were inversely correlated with neonatal mortality (JAMA. 2015 Dec 1;314[21]:2263-70. doi: 10.1001/jama.2015.15553).
The researchers noted that they focused solely on mortality and did not assess other health outcomes. This means that they could not address the many possible benefits of cesarean delivery, such as reduced morbidity from complicated vaginal delivery or from prolonged obstructed labor.
In a separate report, a different research group found that children born by planned cesarean delivery, as compared with vaginal delivery, had a slightly increased risk of asthma requiring hospital admission, of needing a prescription for a salbutamol inhaler at age 5 years, and of all-cause mortality by age 21 years.
Dr. Mairead Blackof the University of Aberdeen (England) and her associates examined the relationship between planned cesarean delivery and chronic illness and death among the offspring. They assessed outcomes in 321,287 singleton live births to first-time mothers that occurred in Scotland in 1993-2007; the children were followed until January 2015, for a mean follow-up of 14.8 years.
In this cohort, 12,355 children (3.8%) were born by planned cesarean delivery, 56,015 (17.5%) by unscheduled cesarean, and 252,917 (78.7%) by vaginal delivery.
Children born by planned C-sections were slightly, but significantly more likely than were those born vaginally to develop asthma requiring hospital admission (3.73% vs. 3.41%; hazard ratio, 1.22).
Compared with children delivered vaginally, those delivered by planned cesarean section also were slightly, but significantly more likely to require a prescription for a salbutamol inhaler at age 5 years (10.34% vs. 9.62%; HR, 1.13) and to die before the age of 21 years(0.40% vs. 0.32%; HR, 1.41).
However, there were no significant differences between the two study groups in risk of developing inflammatory bowel disease (0.11% vs. 0.13%) or cancer (0.23% vs 0.21%), they noted (JAMA. 2015 Dec 1;314[21]:2271-9. doi: 10.1001/jama.2015.16176).
These findings “suggest that avoidance of vaginal birth may be an important early-life factor in the growing global burden of asthma, although absolute increase in risk to individuals is low,” Dr. Black and her associates wrote.
The researchers in both studies reported having no relevant financial disclosures. Dr. Black’s study was supported in part by the Wellcome Trust.
FROM JAMA
Key clinical point: Countries in which the national rate of cesarean deliveries is up to 19% have lower maternal and neonatal mortality.
Major finding: Cesarean delivery rates of up to 19.1/100 live births were inversely correlated with the maternal mortality ratio at the country level, and cesarean delivery rates of up 19.4/100 live births were inversely correlated with the neonatal mortality ratio.
Data source: A cross-sectional ecologic study estimating the 2012 rate of cesarean delivery in 194 World Health Organization member nations.
Disclosures: The researchers reported having no relevant financial disclosures.
Metformin did not improve glycemic control in type 1 diabetes
Add-on metformin therapy did not improve glycemic control in overweight adolescents with type 1 diabetes, according to a report published online Dec. 1 in JAMA.
Even though patients with type 1 diabetes are traditionally thought of as being underweight or of normal weight, the percentage who are overweight or obese is increasing in this patient population as it is in the general population. Being overweight or obese has serious metabolic consequences for patients with diabetes, particularly during adolescence, said Dr. Ingrid M. Libman, a pediatric endocrinologist at the Children’s Hospital of Pittsburgh, and her associates.
They assessed the effect of adding oral metformin (2,000 mg/day) to insulin therapy in a double-blind placebo-controlled trial involving 140 patients aged 12-19 years (mean age, 15.3 years) at 26 U.S. clinics. The study participants were overweight or obese and had had type 1 diabetes for a mean of 7 years.
The primary efficacy outcome – change in HbA1c level after 6 months of adjunctive metformin (71 patients) or matching placebo (69 patients) – was exactly the same, with an increase of 0.2% in both study groups. Continuous glucose monitoring confirmed that there were no significant differences between the two study groups in glycemic control.
Compared with patients taking placebo, those taking metformin showed a slight reduction in total daily insulin dose and a slightly lower weight gain. “The clinical relevance of these treatment group differences is uncertain,” Dr. Libman and her associates wrote (JAMA 2015 Dec 1. doi: 10.1001/jama.2015.16174). There were no differences between the two groups in blood pressure, lipid profiles, or inflammatory markers.
Patients taking metformin reported significantly more adverse gastrointestinal events than those taking placebo. And severe hypoglycemia developed in five patients taking metformin, compared with none who were taking placebo.
“These results do not support prescribing metformin to adolescents to improve glycemic control,” the investigators wrote.
Add-on metformin therapy did not improve glycemic control in overweight adolescents with type 1 diabetes, according to a report published online Dec. 1 in JAMA.
Even though patients with type 1 diabetes are traditionally thought of as being underweight or of normal weight, the percentage who are overweight or obese is increasing in this patient population as it is in the general population. Being overweight or obese has serious metabolic consequences for patients with diabetes, particularly during adolescence, said Dr. Ingrid M. Libman, a pediatric endocrinologist at the Children’s Hospital of Pittsburgh, and her associates.
They assessed the effect of adding oral metformin (2,000 mg/day) to insulin therapy in a double-blind placebo-controlled trial involving 140 patients aged 12-19 years (mean age, 15.3 years) at 26 U.S. clinics. The study participants were overweight or obese and had had type 1 diabetes for a mean of 7 years.
The primary efficacy outcome – change in HbA1c level after 6 months of adjunctive metformin (71 patients) or matching placebo (69 patients) – was exactly the same, with an increase of 0.2% in both study groups. Continuous glucose monitoring confirmed that there were no significant differences between the two study groups in glycemic control.
Compared with patients taking placebo, those taking metformin showed a slight reduction in total daily insulin dose and a slightly lower weight gain. “The clinical relevance of these treatment group differences is uncertain,” Dr. Libman and her associates wrote (JAMA 2015 Dec 1. doi: 10.1001/jama.2015.16174). There were no differences between the two groups in blood pressure, lipid profiles, or inflammatory markers.
Patients taking metformin reported significantly more adverse gastrointestinal events than those taking placebo. And severe hypoglycemia developed in five patients taking metformin, compared with none who were taking placebo.
“These results do not support prescribing metformin to adolescents to improve glycemic control,” the investigators wrote.
Add-on metformin therapy did not improve glycemic control in overweight adolescents with type 1 diabetes, according to a report published online Dec. 1 in JAMA.
Even though patients with type 1 diabetes are traditionally thought of as being underweight or of normal weight, the percentage who are overweight or obese is increasing in this patient population as it is in the general population. Being overweight or obese has serious metabolic consequences for patients with diabetes, particularly during adolescence, said Dr. Ingrid M. Libman, a pediatric endocrinologist at the Children’s Hospital of Pittsburgh, and her associates.
They assessed the effect of adding oral metformin (2,000 mg/day) to insulin therapy in a double-blind placebo-controlled trial involving 140 patients aged 12-19 years (mean age, 15.3 years) at 26 U.S. clinics. The study participants were overweight or obese and had had type 1 diabetes for a mean of 7 years.
The primary efficacy outcome – change in HbA1c level after 6 months of adjunctive metformin (71 patients) or matching placebo (69 patients) – was exactly the same, with an increase of 0.2% in both study groups. Continuous glucose monitoring confirmed that there were no significant differences between the two study groups in glycemic control.
Compared with patients taking placebo, those taking metformin showed a slight reduction in total daily insulin dose and a slightly lower weight gain. “The clinical relevance of these treatment group differences is uncertain,” Dr. Libman and her associates wrote (JAMA 2015 Dec 1. doi: 10.1001/jama.2015.16174). There were no differences between the two groups in blood pressure, lipid profiles, or inflammatory markers.
Patients taking metformin reported significantly more adverse gastrointestinal events than those taking placebo. And severe hypoglycemia developed in five patients taking metformin, compared with none who were taking placebo.
“These results do not support prescribing metformin to adolescents to improve glycemic control,” the investigators wrote.
FROM JAMA
Key clinical point: Adjunctive metformin did not improve glycemic control in overweight adolescents with type 1 diabetes.
Major finding: The primary efficacy outcome – change in HbA1c level after 6 months of adjunctive metformin (71 patients) or matching placebo (69 patients) – increased 0.2% in both study groups.
Data source: A multicenter, double-blind placebo-controlled clinical trial involving 140 patients aged 12-19 years.
Disclosures: This study was supported by the Juvenile Diabetes Research Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Libman reported that her institution receives grants from Novo Nordisk; her associates reported ties to Novo Nordisk, Medtronic, and the Helmsley Foundation.
Unsupervised, at-home closed-loop insulin delivery system
Unsupervised home use of a closed-loop insulin delivery system, also known as an artificial beta cell, is feasible and safe, and more effective than a sensor-augmented insulin pump for adults and children with type 1 diabetes, according to a report published in the New England Journal of Medicine.
The closed-loop insulin delivery system differs from conventional pump therapy “in that it uses a control algorithm that autonomously and continually increases and decreases the subcutaneous delivery of insulin” based on real-time glucose levels monitored by sensors. These devices have been studied extensively under controlled laboratory conditions and in transitional outpatient settings (including diabetes camps) that included monitoring and supervision by nearby research staff. But until now, only two studies have assessed the devices in at-home, free-living conditions, and those were limited to 1 week of daytime and nighttime use among adults and 3-6 weeks of overnight use in adolescents and adults, said Dr. Hood Thabit of the Wellcome Trust–Medical Research Council Institute of Metabolic Science and the department of diabetes and endocrinology, Cambridge University Hospitals National Health Service Foundation Trust, and his associates.
They performed two multicenter open-label, randomized crossover trials assessing 12 weeks of a closed-loop insulin delivery system (intervention period) and 12 weeks of conventional insulin-pump therapy (control period) in study participants who performed their usual daily activities and were free to consume foods of their choice. In one study, 33 adults (mean age, 40 years) used the closed-loop system during day and at night while awake, both at home and at work. In the other study, 25 children and adolescents aged 6-18 years (mean age, 12 years) used the closed-loop system overnight while asleep.
The primary endpoint of both studies was the proportion of time that the glucose level remained within the target range, which was 70-180 mg/dL in the adults and 70-145 mg/dL for the children and adolescents. This was significantly greater during the intervention period than during the control period for both the adults (by a mean of 11 percentage points) and the children and adolescents (by a mean of 25 percentage points).
In addition, in both studies the mean glucose level was significantly lower, the time spent in a hypoglycemic range was significantly less, the glycated hemoglobin level was significantly lower, and glucose variability was significantly lower during the intervention period than in the control period.
“The advantage of a closed-loop system is the responsive, graduated modulation of insulin delivery, both below and above the preset pump regimen, which allows for improvements in the proportion of time spent in target glucose range and the lowering of the mean glucose level without increasing the risk of hypoglycemia,” Dr. Thabit and his associates wrote (New Engl J Med. 2015;373:2129-40).
There were two serious adverse events. One adult had an episode of severe hypoglycemia when the closed-loop system was not in use because of low battery power and the patient was receiving insulin through a conventional sensor-augmented pump. And one adolescent had two episodes of severe hypoglycemia, also when the closed-loop system was not turned on and the patient was receiving insulin through a sensor-augmented pump. Both participants recovered without clinical sequelae.
This study was supported by the European Union, the U.K. National Institute for Health Research Cambridge Biomedical Research Centre, and Wellcome Trust. Abbott Diabetes Care provided continuous glucose-monitoring devices and sensors at a discount, and Diasend provided hardware and software platforms at a discount, but neither had any role in the study. Two of the authors reported holding patents related to insulin-delivery systems. Dr. Thabit reported having no relevant financial disclosures; several of his associates reported ties to numerous industry sources.
Unsupervised home use of a closed-loop insulin delivery system, also known as an artificial beta cell, is feasible and safe, and more effective than a sensor-augmented insulin pump for adults and children with type 1 diabetes, according to a report published in the New England Journal of Medicine.
The closed-loop insulin delivery system differs from conventional pump therapy “in that it uses a control algorithm that autonomously and continually increases and decreases the subcutaneous delivery of insulin” based on real-time glucose levels monitored by sensors. These devices have been studied extensively under controlled laboratory conditions and in transitional outpatient settings (including diabetes camps) that included monitoring and supervision by nearby research staff. But until now, only two studies have assessed the devices in at-home, free-living conditions, and those were limited to 1 week of daytime and nighttime use among adults and 3-6 weeks of overnight use in adolescents and adults, said Dr. Hood Thabit of the Wellcome Trust–Medical Research Council Institute of Metabolic Science and the department of diabetes and endocrinology, Cambridge University Hospitals National Health Service Foundation Trust, and his associates.
They performed two multicenter open-label, randomized crossover trials assessing 12 weeks of a closed-loop insulin delivery system (intervention period) and 12 weeks of conventional insulin-pump therapy (control period) in study participants who performed their usual daily activities and were free to consume foods of their choice. In one study, 33 adults (mean age, 40 years) used the closed-loop system during day and at night while awake, both at home and at work. In the other study, 25 children and adolescents aged 6-18 years (mean age, 12 years) used the closed-loop system overnight while asleep.
The primary endpoint of both studies was the proportion of time that the glucose level remained within the target range, which was 70-180 mg/dL in the adults and 70-145 mg/dL for the children and adolescents. This was significantly greater during the intervention period than during the control period for both the adults (by a mean of 11 percentage points) and the children and adolescents (by a mean of 25 percentage points).
In addition, in both studies the mean glucose level was significantly lower, the time spent in a hypoglycemic range was significantly less, the glycated hemoglobin level was significantly lower, and glucose variability was significantly lower during the intervention period than in the control period.
“The advantage of a closed-loop system is the responsive, graduated modulation of insulin delivery, both below and above the preset pump regimen, which allows for improvements in the proportion of time spent in target glucose range and the lowering of the mean glucose level without increasing the risk of hypoglycemia,” Dr. Thabit and his associates wrote (New Engl J Med. 2015;373:2129-40).
There were two serious adverse events. One adult had an episode of severe hypoglycemia when the closed-loop system was not in use because of low battery power and the patient was receiving insulin through a conventional sensor-augmented pump. And one adolescent had two episodes of severe hypoglycemia, also when the closed-loop system was not turned on and the patient was receiving insulin through a sensor-augmented pump. Both participants recovered without clinical sequelae.
This study was supported by the European Union, the U.K. National Institute for Health Research Cambridge Biomedical Research Centre, and Wellcome Trust. Abbott Diabetes Care provided continuous glucose-monitoring devices and sensors at a discount, and Diasend provided hardware and software platforms at a discount, but neither had any role in the study. Two of the authors reported holding patents related to insulin-delivery systems. Dr. Thabit reported having no relevant financial disclosures; several of his associates reported ties to numerous industry sources.
Unsupervised home use of a closed-loop insulin delivery system, also known as an artificial beta cell, is feasible and safe, and more effective than a sensor-augmented insulin pump for adults and children with type 1 diabetes, according to a report published in the New England Journal of Medicine.
The closed-loop insulin delivery system differs from conventional pump therapy “in that it uses a control algorithm that autonomously and continually increases and decreases the subcutaneous delivery of insulin” based on real-time glucose levels monitored by sensors. These devices have been studied extensively under controlled laboratory conditions and in transitional outpatient settings (including diabetes camps) that included monitoring and supervision by nearby research staff. But until now, only two studies have assessed the devices in at-home, free-living conditions, and those were limited to 1 week of daytime and nighttime use among adults and 3-6 weeks of overnight use in adolescents and adults, said Dr. Hood Thabit of the Wellcome Trust–Medical Research Council Institute of Metabolic Science and the department of diabetes and endocrinology, Cambridge University Hospitals National Health Service Foundation Trust, and his associates.
They performed two multicenter open-label, randomized crossover trials assessing 12 weeks of a closed-loop insulin delivery system (intervention period) and 12 weeks of conventional insulin-pump therapy (control period) in study participants who performed their usual daily activities and were free to consume foods of their choice. In one study, 33 adults (mean age, 40 years) used the closed-loop system during day and at night while awake, both at home and at work. In the other study, 25 children and adolescents aged 6-18 years (mean age, 12 years) used the closed-loop system overnight while asleep.
The primary endpoint of both studies was the proportion of time that the glucose level remained within the target range, which was 70-180 mg/dL in the adults and 70-145 mg/dL for the children and adolescents. This was significantly greater during the intervention period than during the control period for both the adults (by a mean of 11 percentage points) and the children and adolescents (by a mean of 25 percentage points).
In addition, in both studies the mean glucose level was significantly lower, the time spent in a hypoglycemic range was significantly less, the glycated hemoglobin level was significantly lower, and glucose variability was significantly lower during the intervention period than in the control period.
“The advantage of a closed-loop system is the responsive, graduated modulation of insulin delivery, both below and above the preset pump regimen, which allows for improvements in the proportion of time spent in target glucose range and the lowering of the mean glucose level without increasing the risk of hypoglycemia,” Dr. Thabit and his associates wrote (New Engl J Med. 2015;373:2129-40).
There were two serious adverse events. One adult had an episode of severe hypoglycemia when the closed-loop system was not in use because of low battery power and the patient was receiving insulin through a conventional sensor-augmented pump. And one adolescent had two episodes of severe hypoglycemia, also when the closed-loop system was not turned on and the patient was receiving insulin through a sensor-augmented pump. Both participants recovered without clinical sequelae.
This study was supported by the European Union, the U.K. National Institute for Health Research Cambridge Biomedical Research Centre, and Wellcome Trust. Abbott Diabetes Care provided continuous glucose-monitoring devices and sensors at a discount, and Diasend provided hardware and software platforms at a discount, but neither had any role in the study. Two of the authors reported holding patents related to insulin-delivery systems. Dr. Thabit reported having no relevant financial disclosures; several of his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Unsupervised home use of a closed-loop insulin delivery system is feasible and safe, and more effective than sensor-augmented pump therapy for adults and children with type 1 diabetes.
Major finding: The primary endpoint of both studies – the proportion of time that the glucose level remained within the target range – was significantly greater during the intervention period than during the control period for both the adults (by a mean of 11%) and the children and adolescents (by a mean of 25%).
Data source: Two multicenter open-label, randomized crossover trials involving 33 adults and 25 children and adolescents treated for 12 weeks.
Disclosures: This study was supported by the European Union, the U.K. National Institute for Health Research Cambridge Biomedical Research Centre, and Wellcome Trust. Abbott Diabetes Care provided continuous glucose-monitoring devices and sensors at a discount, and Diasend provided hardware and software platforms at a discount, but neither had any role in the study. Two of the authors reported holding patents related to insulin-delivery systems. Dr. Thabit reported having no relevant financial disclosures; several of his associates reported ties to numerous industry sources.
Prime-boost flu vaccination strategy effective in children
A prime-boost flu vaccination strategy proved effective in children and adolescents in an open-label phase III trial conducted by the vaccine manufacturer and reported online in the Pediatric Infectious Disease Journal.
This proof-of-concept result suggests that at the outbreak of the next flu pandemic, immediate priming with two doses of a subtype-matched flu vaccine, followed by boosting once a strain-matched vaccine becomes available, will offer the pediatric population better protection than simply waiting for adequate quantities of the strain-matched vaccine to be developed and marketed.
It is impossible to predict which specific viral strain will cause the next flu pandemic, but H5N1 is considered a likely candidate virus, “to the extent that vaccine manufacturers and regulatory authorities are actively developing H5N1 vaccines” for all age groups, said Dr. Patricia Izurieta of GlaxoSmithKline Vaccines, Belgium, and her associates.
Waiting for a pandemic to begin and then producing strain-specific vaccines could take as long as 6 months, and even then supplies probably would be limited. Experts have theorized that preemptive “priming” with already stockpiled H5N1 vaccines immediately after a pandemic begins may provide broad cross-protection that could mitigate the intensity of the subsequent pandemic, potentially reducing morbidity, mortality, and viral transmission.
To test this theory, Dr. Izurieta and her associates mimicked a potential flu pandemic by priming children and adolescents with two doses of an H5N1-AS03 vaccine, giving a booster dose of a specific H5N1 strain at 6 months, and assessing antibody response to all the vaccinations through 12 months. (Assessing actual vaccine efficacy was impossible because it would be unethical to determine this by exposing children to a flu virus.)
A total of 520 participants at a single medical center in the Philippines who were aged 3-18 years (mean age, 9 years) were assigned to two intervention groups and two control groups. Approximately half of these children and adolescents received the intervention – two priming doses of A/Indonesia/05/2005/H5N1-AS03B vaccine – while the control subjects received a single dose of hepatitis A vaccine. At day 182, half of the primed and half of the unprimed participants then received a booster dose of A/turkey/Turkey/01/2005-H5N1-As03B, while the remainder received the hepatitis A vaccine.
Compared with no priming, priming afforded superior seroconversion and putative seroprotection against the second, specific strain of H5N1. This robust protective effect was seen within 10 days after vaccination, occurred across all ages, and persisted through 12 months of follow-up, the investigators said (Ped Infect Dis J. 2015 Nov 6. [doi: 10.1097/INF.0000000000000968]).
Adverse events within 7 days of the priming vaccinations developed in 68%-76% of the intervention group, compared with only 37%-64% of the control group. Adverse events within 7 days of the booster vaccinations developed in 69% of the intervention group, compared with only 40% of the control group. The most common adverse events were upper respiratory tract infection (20% vs 14%), nasopharyngitis (4% vs 3%), and rhinitis (3% in both study groups). The most serious adverse events affected two children and were classified as grade 3.
The study results suggest that a prime-boost strategy can induce broad and long-lasting immunity and could be effectively employed in this age group in the prepandemic setting, Dr. Izurieta and her associates reported.
This trial was funded by GlaxoSmithKline, which also was involved in the design and conduct of the study, the data analysis, and the writing and publishing of the report. Dr. Izurieta and two of her associates are employed by GSK.
A prime-boost flu vaccination strategy proved effective in children and adolescents in an open-label phase III trial conducted by the vaccine manufacturer and reported online in the Pediatric Infectious Disease Journal.
This proof-of-concept result suggests that at the outbreak of the next flu pandemic, immediate priming with two doses of a subtype-matched flu vaccine, followed by boosting once a strain-matched vaccine becomes available, will offer the pediatric population better protection than simply waiting for adequate quantities of the strain-matched vaccine to be developed and marketed.
It is impossible to predict which specific viral strain will cause the next flu pandemic, but H5N1 is considered a likely candidate virus, “to the extent that vaccine manufacturers and regulatory authorities are actively developing H5N1 vaccines” for all age groups, said Dr. Patricia Izurieta of GlaxoSmithKline Vaccines, Belgium, and her associates.
Waiting for a pandemic to begin and then producing strain-specific vaccines could take as long as 6 months, and even then supplies probably would be limited. Experts have theorized that preemptive “priming” with already stockpiled H5N1 vaccines immediately after a pandemic begins may provide broad cross-protection that could mitigate the intensity of the subsequent pandemic, potentially reducing morbidity, mortality, and viral transmission.
To test this theory, Dr. Izurieta and her associates mimicked a potential flu pandemic by priming children and adolescents with two doses of an H5N1-AS03 vaccine, giving a booster dose of a specific H5N1 strain at 6 months, and assessing antibody response to all the vaccinations through 12 months. (Assessing actual vaccine efficacy was impossible because it would be unethical to determine this by exposing children to a flu virus.)
A total of 520 participants at a single medical center in the Philippines who were aged 3-18 years (mean age, 9 years) were assigned to two intervention groups and two control groups. Approximately half of these children and adolescents received the intervention – two priming doses of A/Indonesia/05/2005/H5N1-AS03B vaccine – while the control subjects received a single dose of hepatitis A vaccine. At day 182, half of the primed and half of the unprimed participants then received a booster dose of A/turkey/Turkey/01/2005-H5N1-As03B, while the remainder received the hepatitis A vaccine.
Compared with no priming, priming afforded superior seroconversion and putative seroprotection against the second, specific strain of H5N1. This robust protective effect was seen within 10 days after vaccination, occurred across all ages, and persisted through 12 months of follow-up, the investigators said (Ped Infect Dis J. 2015 Nov 6. [doi: 10.1097/INF.0000000000000968]).
Adverse events within 7 days of the priming vaccinations developed in 68%-76% of the intervention group, compared with only 37%-64% of the control group. Adverse events within 7 days of the booster vaccinations developed in 69% of the intervention group, compared with only 40% of the control group. The most common adverse events were upper respiratory tract infection (20% vs 14%), nasopharyngitis (4% vs 3%), and rhinitis (3% in both study groups). The most serious adverse events affected two children and were classified as grade 3.
The study results suggest that a prime-boost strategy can induce broad and long-lasting immunity and could be effectively employed in this age group in the prepandemic setting, Dr. Izurieta and her associates reported.
This trial was funded by GlaxoSmithKline, which also was involved in the design and conduct of the study, the data analysis, and the writing and publishing of the report. Dr. Izurieta and two of her associates are employed by GSK.
A prime-boost flu vaccination strategy proved effective in children and adolescents in an open-label phase III trial conducted by the vaccine manufacturer and reported online in the Pediatric Infectious Disease Journal.
This proof-of-concept result suggests that at the outbreak of the next flu pandemic, immediate priming with two doses of a subtype-matched flu vaccine, followed by boosting once a strain-matched vaccine becomes available, will offer the pediatric population better protection than simply waiting for adequate quantities of the strain-matched vaccine to be developed and marketed.
It is impossible to predict which specific viral strain will cause the next flu pandemic, but H5N1 is considered a likely candidate virus, “to the extent that vaccine manufacturers and regulatory authorities are actively developing H5N1 vaccines” for all age groups, said Dr. Patricia Izurieta of GlaxoSmithKline Vaccines, Belgium, and her associates.
Waiting for a pandemic to begin and then producing strain-specific vaccines could take as long as 6 months, and even then supplies probably would be limited. Experts have theorized that preemptive “priming” with already stockpiled H5N1 vaccines immediately after a pandemic begins may provide broad cross-protection that could mitigate the intensity of the subsequent pandemic, potentially reducing morbidity, mortality, and viral transmission.
To test this theory, Dr. Izurieta and her associates mimicked a potential flu pandemic by priming children and adolescents with two doses of an H5N1-AS03 vaccine, giving a booster dose of a specific H5N1 strain at 6 months, and assessing antibody response to all the vaccinations through 12 months. (Assessing actual vaccine efficacy was impossible because it would be unethical to determine this by exposing children to a flu virus.)
A total of 520 participants at a single medical center in the Philippines who were aged 3-18 years (mean age, 9 years) were assigned to two intervention groups and two control groups. Approximately half of these children and adolescents received the intervention – two priming doses of A/Indonesia/05/2005/H5N1-AS03B vaccine – while the control subjects received a single dose of hepatitis A vaccine. At day 182, half of the primed and half of the unprimed participants then received a booster dose of A/turkey/Turkey/01/2005-H5N1-As03B, while the remainder received the hepatitis A vaccine.
Compared with no priming, priming afforded superior seroconversion and putative seroprotection against the second, specific strain of H5N1. This robust protective effect was seen within 10 days after vaccination, occurred across all ages, and persisted through 12 months of follow-up, the investigators said (Ped Infect Dis J. 2015 Nov 6. [doi: 10.1097/INF.0000000000000968]).
Adverse events within 7 days of the priming vaccinations developed in 68%-76% of the intervention group, compared with only 37%-64% of the control group. Adverse events within 7 days of the booster vaccinations developed in 69% of the intervention group, compared with only 40% of the control group. The most common adverse events were upper respiratory tract infection (20% vs 14%), nasopharyngitis (4% vs 3%), and rhinitis (3% in both study groups). The most serious adverse events affected two children and were classified as grade 3.
The study results suggest that a prime-boost strategy can induce broad and long-lasting immunity and could be effectively employed in this age group in the prepandemic setting, Dr. Izurieta and her associates reported.
This trial was funded by GlaxoSmithKline, which also was involved in the design and conduct of the study, the data analysis, and the writing and publishing of the report. Dr. Izurieta and two of her associates are employed by GSK.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Key clinical point: A prime-boost flu vaccination strategy was found effective in children and adolescents.
Major finding: Compared with no priming, priming afforded superior seroconversion and putative seroprotection against the second, specific strain of H5N1.
Data source: An industry-sponsored, single-center, open-label phase III trial involving 520 participants aged 3-18 years followed for 1 year.
Disclosures: This trial was funded by GlaxoSmithKline, which also was involved in the design and conduct of the study, the data analysis, and the writing and publishing of the report. Dr. Izurieta and two of her associates are employed by GSK.
Procalcitonin assay best for detecting invasive bacterial infection
The procalcitonin assay is superior to C-reactive protein, neutrophil, and white blood cell measurements at identifying invasive bacterial infection in very young febrile infants, according to a study published Nov. 23 in JAMA Pediatrics.
Procalcitonin assays allow earlier detection of certain infections compared with other biomarker assays in older children, and a few small studies have hinted at their usefulness in infants, but to date no large prospective studies have assessed procalcitonin assays in the youngest infants. So investigators evaluated the diagnostic accuracy of procalcitonin and other biomarkers in a prospective study involving 2,047 febrile infants aged 7-91 days who presented to 15 pediatric emergency departments in France during a 30-month period.
“We did not include infants 6 days or younger because they are likely to have early-onset sepsis related to perinatal factors and because physiologic procalcitonin concentrations during the first [few] days of life are higher than thereafter,” said Dr. Karen Milcent of Hôpital Antoine Béclère, Clamart (France), and her associates.
Serum samples were collected from the infants at the initial clinical examination, but procalcitonin assays were not performed at that time. Attending physicians diagnosed the infants as having either bacterial or nonbacterial infections without knowing the procalcitonin results. Then procalcitonin tests were done retrospectively on frozen serum samples by lab personnel who were blinded to the infants’ clinical features. Thirteen (1.0%) infants had bacteremia and 8 (0.6%) had bacterial meningitis.
The procalcitonin assay was significantly more accurate at identifying invasive bacterial infections than was C-reactive protein level, absolute neutrophil count, or white blood cell count. At a threshold of 0.3 ng/mL or more, procalcitonin level had a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. In addition, the procalcitonin assay was the most accurate in a subgroup analysis restricted to patients whose fever duration was less than 6 hours and another subgroup analysis restricted to patients younger than 1 month of age, the investigators said (JAMA Ped. 2015 Nov 23. doi:10.1001/jamapediatrics.2015.3210).
“Our results suggest that it may be possible to improve clinical practice for the treatment of young febrile infants” by combining procalcitonin assay results with a careful case history, a thorough physical examination, and other appropriate testing. Among other benefits, this approach offers the potential of avoiding lumbar punctures, they added.
These study findings “should encourage the development of decision-making rules that incorporate procalcitonin,” Dr. Milcent and her associates said.
The findings by Milcent et al. are an important step forward in managing very young febrile infants, which remains a vexing and crucial problem in pediatrics.
A vital next step is to find alternatives to culture-based testing of blood, urine, and CSF. Genomic technologies that reliably detect molecular signatures in small amounts of biologic samples may be one such alternative. They may offer the additional benefit of identifying the pathogen and the host’s response to the presence of the pathogen.
Dr. Nathan Kuppermann is in the departments of emergency medicine and pediatrics at the University of California–Davis. Dr. Prashant Mahajan is in the departments of pediatrics and emergency medicine at Children’s Hospital of Michigan and Wayne State University, Detroit. They having no relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Milcent’s report (JAMA Ped. 2015 Nov 23. doi:10.1001/jamapediatrics.2015.3267).
The findings by Milcent et al. are an important step forward in managing very young febrile infants, which remains a vexing and crucial problem in pediatrics.
A vital next step is to find alternatives to culture-based testing of blood, urine, and CSF. Genomic technologies that reliably detect molecular signatures in small amounts of biologic samples may be one such alternative. They may offer the additional benefit of identifying the pathogen and the host’s response to the presence of the pathogen.
Dr. Nathan Kuppermann is in the departments of emergency medicine and pediatrics at the University of California–Davis. Dr. Prashant Mahajan is in the departments of pediatrics and emergency medicine at Children’s Hospital of Michigan and Wayne State University, Detroit. They having no relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Milcent’s report (JAMA Ped. 2015 Nov 23. doi:10.1001/jamapediatrics.2015.3267).
The findings by Milcent et al. are an important step forward in managing very young febrile infants, which remains a vexing and crucial problem in pediatrics.
A vital next step is to find alternatives to culture-based testing of blood, urine, and CSF. Genomic technologies that reliably detect molecular signatures in small amounts of biologic samples may be one such alternative. They may offer the additional benefit of identifying the pathogen and the host’s response to the presence of the pathogen.
Dr. Nathan Kuppermann is in the departments of emergency medicine and pediatrics at the University of California–Davis. Dr. Prashant Mahajan is in the departments of pediatrics and emergency medicine at Children’s Hospital of Michigan and Wayne State University, Detroit. They having no relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Milcent’s report (JAMA Ped. 2015 Nov 23. doi:10.1001/jamapediatrics.2015.3267).
The procalcitonin assay is superior to C-reactive protein, neutrophil, and white blood cell measurements at identifying invasive bacterial infection in very young febrile infants, according to a study published Nov. 23 in JAMA Pediatrics.
Procalcitonin assays allow earlier detection of certain infections compared with other biomarker assays in older children, and a few small studies have hinted at their usefulness in infants, but to date no large prospective studies have assessed procalcitonin assays in the youngest infants. So investigators evaluated the diagnostic accuracy of procalcitonin and other biomarkers in a prospective study involving 2,047 febrile infants aged 7-91 days who presented to 15 pediatric emergency departments in France during a 30-month period.
“We did not include infants 6 days or younger because they are likely to have early-onset sepsis related to perinatal factors and because physiologic procalcitonin concentrations during the first [few] days of life are higher than thereafter,” said Dr. Karen Milcent of Hôpital Antoine Béclère, Clamart (France), and her associates.
Serum samples were collected from the infants at the initial clinical examination, but procalcitonin assays were not performed at that time. Attending physicians diagnosed the infants as having either bacterial or nonbacterial infections without knowing the procalcitonin results. Then procalcitonin tests were done retrospectively on frozen serum samples by lab personnel who were blinded to the infants’ clinical features. Thirteen (1.0%) infants had bacteremia and 8 (0.6%) had bacterial meningitis.
The procalcitonin assay was significantly more accurate at identifying invasive bacterial infections than was C-reactive protein level, absolute neutrophil count, or white blood cell count. At a threshold of 0.3 ng/mL or more, procalcitonin level had a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. In addition, the procalcitonin assay was the most accurate in a subgroup analysis restricted to patients whose fever duration was less than 6 hours and another subgroup analysis restricted to patients younger than 1 month of age, the investigators said (JAMA Ped. 2015 Nov 23. doi:10.1001/jamapediatrics.2015.3210).
“Our results suggest that it may be possible to improve clinical practice for the treatment of young febrile infants” by combining procalcitonin assay results with a careful case history, a thorough physical examination, and other appropriate testing. Among other benefits, this approach offers the potential of avoiding lumbar punctures, they added.
These study findings “should encourage the development of decision-making rules that incorporate procalcitonin,” Dr. Milcent and her associates said.
The procalcitonin assay is superior to C-reactive protein, neutrophil, and white blood cell measurements at identifying invasive bacterial infection in very young febrile infants, according to a study published Nov. 23 in JAMA Pediatrics.
Procalcitonin assays allow earlier detection of certain infections compared with other biomarker assays in older children, and a few small studies have hinted at their usefulness in infants, but to date no large prospective studies have assessed procalcitonin assays in the youngest infants. So investigators evaluated the diagnostic accuracy of procalcitonin and other biomarkers in a prospective study involving 2,047 febrile infants aged 7-91 days who presented to 15 pediatric emergency departments in France during a 30-month period.
“We did not include infants 6 days or younger because they are likely to have early-onset sepsis related to perinatal factors and because physiologic procalcitonin concentrations during the first [few] days of life are higher than thereafter,” said Dr. Karen Milcent of Hôpital Antoine Béclère, Clamart (France), and her associates.
Serum samples were collected from the infants at the initial clinical examination, but procalcitonin assays were not performed at that time. Attending physicians diagnosed the infants as having either bacterial or nonbacterial infections without knowing the procalcitonin results. Then procalcitonin tests were done retrospectively on frozen serum samples by lab personnel who were blinded to the infants’ clinical features. Thirteen (1.0%) infants had bacteremia and 8 (0.6%) had bacterial meningitis.
The procalcitonin assay was significantly more accurate at identifying invasive bacterial infections than was C-reactive protein level, absolute neutrophil count, or white blood cell count. At a threshold of 0.3 ng/mL or more, procalcitonin level had a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. In addition, the procalcitonin assay was the most accurate in a subgroup analysis restricted to patients whose fever duration was less than 6 hours and another subgroup analysis restricted to patients younger than 1 month of age, the investigators said (JAMA Ped. 2015 Nov 23. doi:10.1001/jamapediatrics.2015.3210).
“Our results suggest that it may be possible to improve clinical practice for the treatment of young febrile infants” by combining procalcitonin assay results with a careful case history, a thorough physical examination, and other appropriate testing. Among other benefits, this approach offers the potential of avoiding lumbar punctures, they added.
These study findings “should encourage the development of decision-making rules that incorporate procalcitonin,” Dr. Milcent and her associates said.
FROM JAMA PEDIATRICS
Key clinical point: The procalcitonin assay is superior to three other tests at detecting invasive bacterial infection in febrile infants aged 7-91 days.
Major finding: At a threshold of 0.3 ng/mL or more, procalcitonin level detected invasive bacterial infections with a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1.
Data source: A multicenter prospective cohort study involving 2,047 infants treated at pediatric EDs in France during a 30-month period.
Disclosures: The French Health Ministry funded the study. Dr. Milcent and her associates reported having no financial disclosures.