User login
Continuous no better than interrupted chest compressions
Continuous chest compressions during CPR failed to improve survival or neurologic function compared with standard chest compressions that are briefly interrupted for ventilation, based on findings in the first large randomized trial to compare the two strategies for out-of-hospital, nontraumatic cardiac arrest.
In a presentation at the American Heart Association scientific sessions, simultaneously published online Nov. 9 in the New England Journal of Medicine, Dr. Graham Nichol and his associates analyzed data from the Resuscitation Outcomes Consortium, a network of clinical centers and EMS agencies that have expertise in conducting research on out-of-hospital cardiac arrest.
Data were analyzed for 23,711 adults treated by 114 EMS agencies affiliated with eight clinical centers across the United States and Canada. These agencies were grouped into 47 clusters that were randomly assigned to perform CPR using either continuous chest compressions (100 per minute) with asynchronous positive-pressure ventilations (10 per minute) or standard chest compressions interrupted for ventilations (at a rate of 30 compressions per two ventilations) at every response to an out-of-hospital cardiac arrest. Twice per year, each cluster crossed over to the other resuscitation strategy, said Dr. Nichol of the University of Washington–Harborview Center for Prehospital Emergency Care and Clinical Trial Center in Seattle.
A total of 12,653 patients were assigned to continuous chest compressions (the intervention group) and 11,058 to interrupted chest compressions (the control group). The primary outcome – the rate of survival to hospital discharge – was 9.0% in the intervention group and 9.7% in the control group, a nonsignificant difference. Similarly, the rate of survival with favorable neurologic function did not differ significantly, at 7.0% and 7.7%, respectively, the investigators said (N Engl J Med. 2015 Nov 9. doi:10.1056/NEJMoa1509139).
The reason for these unexpected findings is not yet known. It is plausible that continuous chest compressions really don’t improve outcomes and that the previous positive results were actually due to improvements in the CPR process, such as more consistent rate and depth of compressions; concurrent improvements in the system of care; or Hawthorne effects, in which CPR providers altered their behavior during the studies because they were aware they were being observed.
However, it is also possible that three important limitations of this trial unduly influenced the results.
First, the per-protocol analysis, which used an automated algorithm to assess adherence to the compression assignments, could not classify many patients as having received either continuous or interrupted chest compressions. Second, the quality of postresuscitation care, which certainly influences outcomes, was not monitored. And third, actual oxygenation levels were not measured, nor were minutes of ventilation delivered. Thus, “we do not know whether there were important differences in oxygenation or ventilation between the two treatment strategies,” he said.
It is not yet clear why this large randomized trial showed no benefit from continuous chest compressions when previous observational research showed the opposite. One possibility is that many of the previous studies assessed not just chest compressions but an entire bundle of care related to CPR, so the benefits they reported may not be attributable to chest compressions alone.
In addition, in this study the mean chest-compression fraction – the proportion of each minute during which compressions are given, an important marker of interruptions in chest compressions – was already high in the control group (0.77) and not much different from that in the intervention group (0.83). Both of these are much higher than the target recommended by both American and European guidelines, which is only 0.60.
And of course a third reason may be that the interruptions for ventilation during CPR aren’t all that critical, and may be less detrimental to survival, than is currently believed.
Dr. Rudolph W. Koster is in the department of cardiology at Amsterdam Academic Medical Center. He reported having no relevant financial disclosures. Dr. Koster made these remarks in an editorial accompanying Dr. Nichol’s report (N Engl J Med. 2015 Nov 9. doi:10.1056/NEJMe1513415).
It is not yet clear why this large randomized trial showed no benefit from continuous chest compressions when previous observational research showed the opposite. One possibility is that many of the previous studies assessed not just chest compressions but an entire bundle of care related to CPR, so the benefits they reported may not be attributable to chest compressions alone.
In addition, in this study the mean chest-compression fraction – the proportion of each minute during which compressions are given, an important marker of interruptions in chest compressions – was already high in the control group (0.77) and not much different from that in the intervention group (0.83). Both of these are much higher than the target recommended by both American and European guidelines, which is only 0.60.
And of course a third reason may be that the interruptions for ventilation during CPR aren’t all that critical, and may be less detrimental to survival, than is currently believed.
Dr. Rudolph W. Koster is in the department of cardiology at Amsterdam Academic Medical Center. He reported having no relevant financial disclosures. Dr. Koster made these remarks in an editorial accompanying Dr. Nichol’s report (N Engl J Med. 2015 Nov 9. doi:10.1056/NEJMe1513415).
It is not yet clear why this large randomized trial showed no benefit from continuous chest compressions when previous observational research showed the opposite. One possibility is that many of the previous studies assessed not just chest compressions but an entire bundle of care related to CPR, so the benefits they reported may not be attributable to chest compressions alone.
In addition, in this study the mean chest-compression fraction – the proportion of each minute during which compressions are given, an important marker of interruptions in chest compressions – was already high in the control group (0.77) and not much different from that in the intervention group (0.83). Both of these are much higher than the target recommended by both American and European guidelines, which is only 0.60.
And of course a third reason may be that the interruptions for ventilation during CPR aren’t all that critical, and may be less detrimental to survival, than is currently believed.
Dr. Rudolph W. Koster is in the department of cardiology at Amsterdam Academic Medical Center. He reported having no relevant financial disclosures. Dr. Koster made these remarks in an editorial accompanying Dr. Nichol’s report (N Engl J Med. 2015 Nov 9. doi:10.1056/NEJMe1513415).
Continuous chest compressions during CPR failed to improve survival or neurologic function compared with standard chest compressions that are briefly interrupted for ventilation, based on findings in the first large randomized trial to compare the two strategies for out-of-hospital, nontraumatic cardiac arrest.
In a presentation at the American Heart Association scientific sessions, simultaneously published online Nov. 9 in the New England Journal of Medicine, Dr. Graham Nichol and his associates analyzed data from the Resuscitation Outcomes Consortium, a network of clinical centers and EMS agencies that have expertise in conducting research on out-of-hospital cardiac arrest.
Data were analyzed for 23,711 adults treated by 114 EMS agencies affiliated with eight clinical centers across the United States and Canada. These agencies were grouped into 47 clusters that were randomly assigned to perform CPR using either continuous chest compressions (100 per minute) with asynchronous positive-pressure ventilations (10 per minute) or standard chest compressions interrupted for ventilations (at a rate of 30 compressions per two ventilations) at every response to an out-of-hospital cardiac arrest. Twice per year, each cluster crossed over to the other resuscitation strategy, said Dr. Nichol of the University of Washington–Harborview Center for Prehospital Emergency Care and Clinical Trial Center in Seattle.
A total of 12,653 patients were assigned to continuous chest compressions (the intervention group) and 11,058 to interrupted chest compressions (the control group). The primary outcome – the rate of survival to hospital discharge – was 9.0% in the intervention group and 9.7% in the control group, a nonsignificant difference. Similarly, the rate of survival with favorable neurologic function did not differ significantly, at 7.0% and 7.7%, respectively, the investigators said (N Engl J Med. 2015 Nov 9. doi:10.1056/NEJMoa1509139).
The reason for these unexpected findings is not yet known. It is plausible that continuous chest compressions really don’t improve outcomes and that the previous positive results were actually due to improvements in the CPR process, such as more consistent rate and depth of compressions; concurrent improvements in the system of care; or Hawthorne effects, in which CPR providers altered their behavior during the studies because they were aware they were being observed.
However, it is also possible that three important limitations of this trial unduly influenced the results.
First, the per-protocol analysis, which used an automated algorithm to assess adherence to the compression assignments, could not classify many patients as having received either continuous or interrupted chest compressions. Second, the quality of postresuscitation care, which certainly influences outcomes, was not monitored. And third, actual oxygenation levels were not measured, nor were minutes of ventilation delivered. Thus, “we do not know whether there were important differences in oxygenation or ventilation between the two treatment strategies,” he said.
Continuous chest compressions during CPR failed to improve survival or neurologic function compared with standard chest compressions that are briefly interrupted for ventilation, based on findings in the first large randomized trial to compare the two strategies for out-of-hospital, nontraumatic cardiac arrest.
In a presentation at the American Heart Association scientific sessions, simultaneously published online Nov. 9 in the New England Journal of Medicine, Dr. Graham Nichol and his associates analyzed data from the Resuscitation Outcomes Consortium, a network of clinical centers and EMS agencies that have expertise in conducting research on out-of-hospital cardiac arrest.
Data were analyzed for 23,711 adults treated by 114 EMS agencies affiliated with eight clinical centers across the United States and Canada. These agencies were grouped into 47 clusters that were randomly assigned to perform CPR using either continuous chest compressions (100 per minute) with asynchronous positive-pressure ventilations (10 per minute) or standard chest compressions interrupted for ventilations (at a rate of 30 compressions per two ventilations) at every response to an out-of-hospital cardiac arrest. Twice per year, each cluster crossed over to the other resuscitation strategy, said Dr. Nichol of the University of Washington–Harborview Center for Prehospital Emergency Care and Clinical Trial Center in Seattle.
A total of 12,653 patients were assigned to continuous chest compressions (the intervention group) and 11,058 to interrupted chest compressions (the control group). The primary outcome – the rate of survival to hospital discharge – was 9.0% in the intervention group and 9.7% in the control group, a nonsignificant difference. Similarly, the rate of survival with favorable neurologic function did not differ significantly, at 7.0% and 7.7%, respectively, the investigators said (N Engl J Med. 2015 Nov 9. doi:10.1056/NEJMoa1509139).
The reason for these unexpected findings is not yet known. It is plausible that continuous chest compressions really don’t improve outcomes and that the previous positive results were actually due to improvements in the CPR process, such as more consistent rate and depth of compressions; concurrent improvements in the system of care; or Hawthorne effects, in which CPR providers altered their behavior during the studies because they were aware they were being observed.
However, it is also possible that three important limitations of this trial unduly influenced the results.
First, the per-protocol analysis, which used an automated algorithm to assess adherence to the compression assignments, could not classify many patients as having received either continuous or interrupted chest compressions. Second, the quality of postresuscitation care, which certainly influences outcomes, was not monitored. And third, actual oxygenation levels were not measured, nor were minutes of ventilation delivered. Thus, “we do not know whether there were important differences in oxygenation or ventilation between the two treatment strategies,” he said.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Continuous chest compressions during CPR didn’t improve survival or neurologic function compared with standard compressions briefly interrupted for ventilation.
Major finding: The primary outcome – the rate of survival to hospital discharge – was 9.0% for continuous chest compressions and 9.7% for interrupted compressions, a nonsignificant difference.
Data source: A cluster-randomized crossover trial involving 23,711 adults treated by 114 North American EMS agencies for nontraumatic out-of-hospital cardiac arrest.
Disclosures: This study was supported by the U.S. National Heart, Lung, and Blood Institute, the U.S. Army Medical Research and Materiel Command, the Canadian Institutes of Health Research, the Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, the American Heart Association, and the Medic One Foundation. Dr. Nichol and his associates reported ties to numerous industry sources.
AHA: Nonacute and inappropriate PCI drop substantially
Rates of both nonacute and inappropriate PCI dropped substantially in response to a concerted nationwide effort to curb overuse of the procedure, including the introduction of appropriate use criteria, Dr. Nihar R. Desai said in a presentation at the American Heart Association scientific sessions, which was simultaneously published online Nov. 9 in JAMA.
The American College of Cardiology and the American Heart Association, in conjunction with other professional societies, released appropriate use criteria for coronary revascularization in 2009 and revised them in 2011. Research indicated that the risks of the procedure outweighed the benefits in as many as 17% of patients, and that the proportion of inappropriate PCIs varied markedly across hospitals.
In what he described as the first comprehensive assessment of PCI appropriateness since that time, Dr. Desai and his associates examined national trends using information from the National Cardiovascular Data Registry’s CathPCI registry.
They focused on 2,685,683 PCIs performed at 766 U.S. hospitals during a 5-year period. A total of 76.3% of these were done for acute indications, and 14.8% were for nonacute indications. The indications could not be determined for the remaining 8.9%, usually because data were missing from the records, said Dr. Desai of the center for outcomes research and evaluation, Yale–New Haven (Conn.) Hospital (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13764).
Over time, substantial declines occurred in the number of nonacute PCIs (from 89,704 in 2010 to 59,375 in 2014) and of indeterminate PCIs (from 70,832 in 2010 to 22,589 in 2014), while the number of acute PCIs remained relatively stable (from 377,540 in 2010 to 374,543 in 2014). As a result, the percentage of PCIs performed for acute indications rose from 69.1% in 2009 to 82.0% in 2014.
Similarly, the number of nonacute PCIs classified as inappropriate dropped from 21,781 in 2010 to 7,921 in 2014. Accordingly, the percentage of nonacute PCIs deemed to be inappropriate was halved, decreasing from 26.2% in 2009 to 13.3% in 2014 of all nonacute PCIs.
The results reflect improvement in patient selection and clinical decision making, Dr. Desai said, and suggest that clinicians are doing a better job of identifying patients most likely to benefit from PCI.
In particular, “we observed significant declines in the proportions of patients undergoing nonacute PCI who were asymptomatic or had minimal symptoms, who were not receiving or were receiving only minimal antianginal therapy, and who had low- or intermediate-risk findings on noninvasive testing,” he noted.
“Collectively, these findings suggest that the practice of interventional cardiology has evolved since the introduction of appropriate use criteria,” Dr. Desai added.
The wide variation in inappropriate PCI across hospitals remains, however.
In the best-performing hospitals (those in the lowest quartile for inappropriate procedures), less than 6% of nonacute PCIs were classified as inappropriate in 2014, the most recent year for which data are available.
Conversely, in the worst-performing hospitals (those in the highest quartile for inappropriate procedures), more than 22% of nonacute PCIs were deemed inappropriate. This “suggests the need for ongoing performance improvement initiatives and hospital benchmarking,” Dr. Desai noted
It was encouraging that many of the worst-performing hospitals at baseline demonstrated immediate and steady improvement after the introduction of appropriate use criteria, he added, with a dramatic reduction in inappropriate PCIs from 70.6% in 2009-2010 to 9.4% in 2014.
However, a subset of 18 of the worst-performing hospitals showed minimal change during the first 2 years of the study. They have subsequently shown lower rates of inappropriate PCI during the final 2 years.
This study was supported by the National Cardiovascular Data Registry’s CathPCI registry, the Yale Center for Outcomes Research and Evaluation Data Analytic Center, the American College of Cardiology, the Agency for Healthcare Research and Quality, Veterans Affairs Health Services Research and Development, and the National Heart, Lung, and Blood Institute. Dr. Desai reported ties to Johnson & Johnson, and his associates reported ties to several industry sources.
Previous research has demonstrated that most practicing U.S. cardiologists typically respond to data, evidence, and guidelines in a positive manner of adoption and change. The findings of Dr. Desai and his colleagues confirm this.
Their study also demonstrates the vital importance of the National Cardiovascular Data Registry (NCDR), which collected the information on which their study was based. Some hospitals that perform PCI do not contribute data to the NCDR, which should be unacceptable. Hospitals and clinicians that perform technologically advanced procedures such as coronary revascularization should be required to contribute their data to a national registry as part of the compact for federal reimbursement.
Dr. Robert A. Harrington is in the department of medicine at Stanford (Calif.) University. He reported having no relevant potential conflicts of interest. Dr. Harrington made these remarks in an editorial accompanying Dr. Desai’s report (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.15436).
Previous research has demonstrated that most practicing U.S. cardiologists typically respond to data, evidence, and guidelines in a positive manner of adoption and change. The findings of Dr. Desai and his colleagues confirm this.
Their study also demonstrates the vital importance of the National Cardiovascular Data Registry (NCDR), which collected the information on which their study was based. Some hospitals that perform PCI do not contribute data to the NCDR, which should be unacceptable. Hospitals and clinicians that perform technologically advanced procedures such as coronary revascularization should be required to contribute their data to a national registry as part of the compact for federal reimbursement.
Dr. Robert A. Harrington is in the department of medicine at Stanford (Calif.) University. He reported having no relevant potential conflicts of interest. Dr. Harrington made these remarks in an editorial accompanying Dr. Desai’s report (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.15436).
Previous research has demonstrated that most practicing U.S. cardiologists typically respond to data, evidence, and guidelines in a positive manner of adoption and change. The findings of Dr. Desai and his colleagues confirm this.
Their study also demonstrates the vital importance of the National Cardiovascular Data Registry (NCDR), which collected the information on which their study was based. Some hospitals that perform PCI do not contribute data to the NCDR, which should be unacceptable. Hospitals and clinicians that perform technologically advanced procedures such as coronary revascularization should be required to contribute their data to a national registry as part of the compact for federal reimbursement.
Dr. Robert A. Harrington is in the department of medicine at Stanford (Calif.) University. He reported having no relevant potential conflicts of interest. Dr. Harrington made these remarks in an editorial accompanying Dr. Desai’s report (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.15436).
Rates of both nonacute and inappropriate PCI dropped substantially in response to a concerted nationwide effort to curb overuse of the procedure, including the introduction of appropriate use criteria, Dr. Nihar R. Desai said in a presentation at the American Heart Association scientific sessions, which was simultaneously published online Nov. 9 in JAMA.
The American College of Cardiology and the American Heart Association, in conjunction with other professional societies, released appropriate use criteria for coronary revascularization in 2009 and revised them in 2011. Research indicated that the risks of the procedure outweighed the benefits in as many as 17% of patients, and that the proportion of inappropriate PCIs varied markedly across hospitals.
In what he described as the first comprehensive assessment of PCI appropriateness since that time, Dr. Desai and his associates examined national trends using information from the National Cardiovascular Data Registry’s CathPCI registry.
They focused on 2,685,683 PCIs performed at 766 U.S. hospitals during a 5-year period. A total of 76.3% of these were done for acute indications, and 14.8% were for nonacute indications. The indications could not be determined for the remaining 8.9%, usually because data were missing from the records, said Dr. Desai of the center for outcomes research and evaluation, Yale–New Haven (Conn.) Hospital (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13764).
Over time, substantial declines occurred in the number of nonacute PCIs (from 89,704 in 2010 to 59,375 in 2014) and of indeterminate PCIs (from 70,832 in 2010 to 22,589 in 2014), while the number of acute PCIs remained relatively stable (from 377,540 in 2010 to 374,543 in 2014). As a result, the percentage of PCIs performed for acute indications rose from 69.1% in 2009 to 82.0% in 2014.
Similarly, the number of nonacute PCIs classified as inappropriate dropped from 21,781 in 2010 to 7,921 in 2014. Accordingly, the percentage of nonacute PCIs deemed to be inappropriate was halved, decreasing from 26.2% in 2009 to 13.3% in 2014 of all nonacute PCIs.
The results reflect improvement in patient selection and clinical decision making, Dr. Desai said, and suggest that clinicians are doing a better job of identifying patients most likely to benefit from PCI.
In particular, “we observed significant declines in the proportions of patients undergoing nonacute PCI who were asymptomatic or had minimal symptoms, who were not receiving or were receiving only minimal antianginal therapy, and who had low- or intermediate-risk findings on noninvasive testing,” he noted.
“Collectively, these findings suggest that the practice of interventional cardiology has evolved since the introduction of appropriate use criteria,” Dr. Desai added.
The wide variation in inappropriate PCI across hospitals remains, however.
In the best-performing hospitals (those in the lowest quartile for inappropriate procedures), less than 6% of nonacute PCIs were classified as inappropriate in 2014, the most recent year for which data are available.
Conversely, in the worst-performing hospitals (those in the highest quartile for inappropriate procedures), more than 22% of nonacute PCIs were deemed inappropriate. This “suggests the need for ongoing performance improvement initiatives and hospital benchmarking,” Dr. Desai noted
It was encouraging that many of the worst-performing hospitals at baseline demonstrated immediate and steady improvement after the introduction of appropriate use criteria, he added, with a dramatic reduction in inappropriate PCIs from 70.6% in 2009-2010 to 9.4% in 2014.
However, a subset of 18 of the worst-performing hospitals showed minimal change during the first 2 years of the study. They have subsequently shown lower rates of inappropriate PCI during the final 2 years.
This study was supported by the National Cardiovascular Data Registry’s CathPCI registry, the Yale Center for Outcomes Research and Evaluation Data Analytic Center, the American College of Cardiology, the Agency for Healthcare Research and Quality, Veterans Affairs Health Services Research and Development, and the National Heart, Lung, and Blood Institute. Dr. Desai reported ties to Johnson & Johnson, and his associates reported ties to several industry sources.
Rates of both nonacute and inappropriate PCI dropped substantially in response to a concerted nationwide effort to curb overuse of the procedure, including the introduction of appropriate use criteria, Dr. Nihar R. Desai said in a presentation at the American Heart Association scientific sessions, which was simultaneously published online Nov. 9 in JAMA.
The American College of Cardiology and the American Heart Association, in conjunction with other professional societies, released appropriate use criteria for coronary revascularization in 2009 and revised them in 2011. Research indicated that the risks of the procedure outweighed the benefits in as many as 17% of patients, and that the proportion of inappropriate PCIs varied markedly across hospitals.
In what he described as the first comprehensive assessment of PCI appropriateness since that time, Dr. Desai and his associates examined national trends using information from the National Cardiovascular Data Registry’s CathPCI registry.
They focused on 2,685,683 PCIs performed at 766 U.S. hospitals during a 5-year period. A total of 76.3% of these were done for acute indications, and 14.8% were for nonacute indications. The indications could not be determined for the remaining 8.9%, usually because data were missing from the records, said Dr. Desai of the center for outcomes research and evaluation, Yale–New Haven (Conn.) Hospital (JAMA 2015 Nov 9. doi: 10.1001/jama.2015.13764).
Over time, substantial declines occurred in the number of nonacute PCIs (from 89,704 in 2010 to 59,375 in 2014) and of indeterminate PCIs (from 70,832 in 2010 to 22,589 in 2014), while the number of acute PCIs remained relatively stable (from 377,540 in 2010 to 374,543 in 2014). As a result, the percentage of PCIs performed for acute indications rose from 69.1% in 2009 to 82.0% in 2014.
Similarly, the number of nonacute PCIs classified as inappropriate dropped from 21,781 in 2010 to 7,921 in 2014. Accordingly, the percentage of nonacute PCIs deemed to be inappropriate was halved, decreasing from 26.2% in 2009 to 13.3% in 2014 of all nonacute PCIs.
The results reflect improvement in patient selection and clinical decision making, Dr. Desai said, and suggest that clinicians are doing a better job of identifying patients most likely to benefit from PCI.
In particular, “we observed significant declines in the proportions of patients undergoing nonacute PCI who were asymptomatic or had minimal symptoms, who were not receiving or were receiving only minimal antianginal therapy, and who had low- or intermediate-risk findings on noninvasive testing,” he noted.
“Collectively, these findings suggest that the practice of interventional cardiology has evolved since the introduction of appropriate use criteria,” Dr. Desai added.
The wide variation in inappropriate PCI across hospitals remains, however.
In the best-performing hospitals (those in the lowest quartile for inappropriate procedures), less than 6% of nonacute PCIs were classified as inappropriate in 2014, the most recent year for which data are available.
Conversely, in the worst-performing hospitals (those in the highest quartile for inappropriate procedures), more than 22% of nonacute PCIs were deemed inappropriate. This “suggests the need for ongoing performance improvement initiatives and hospital benchmarking,” Dr. Desai noted
It was encouraging that many of the worst-performing hospitals at baseline demonstrated immediate and steady improvement after the introduction of appropriate use criteria, he added, with a dramatic reduction in inappropriate PCIs from 70.6% in 2009-2010 to 9.4% in 2014.
However, a subset of 18 of the worst-performing hospitals showed minimal change during the first 2 years of the study. They have subsequently shown lower rates of inappropriate PCI during the final 2 years.
This study was supported by the National Cardiovascular Data Registry’s CathPCI registry, the Yale Center for Outcomes Research and Evaluation Data Analytic Center, the American College of Cardiology, the Agency for Healthcare Research and Quality, Veterans Affairs Health Services Research and Development, and the National Heart, Lung, and Blood Institute. Dr. Desai reported ties to Johnson & Johnson, and his associates reported ties to several industry sources.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Rates of both nonacute and inappropriate PCI dropped substantially in response to a concerted nationwide effort to curb overuse of the procedure, including the introduction of appropriate use criteria.
Major finding: Substantial declines occurred in the number of nonacute PCIs (from 89,704 to 59,375) and of indeterminate PCIs (from 70,832 to 22,589), while the number of acute PCIs remained relatively stable (from 377,540 to 374,543).
Data source: A retrospective cohort study involving approximately 2.7 million PCIs conducted at 766 U.S. hospitals during a 5-year period.
Disclosures: This study was supported by the National Cardiovascular Data Registry’s CathPCI registry, the Yale Center for Outcomes Research and Evaluation Data Analytic Center, the American College of Cardiology, the Agency for Healthcare Research and Quality, Veterans Affairs Health Services Research and Development, and the National Heart, Lung, and Blood Institute. Dr. Desai reported ties to Johnson & Johnson, and his associates reported ties to several industry sources.
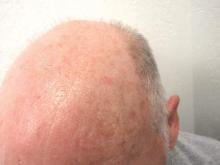
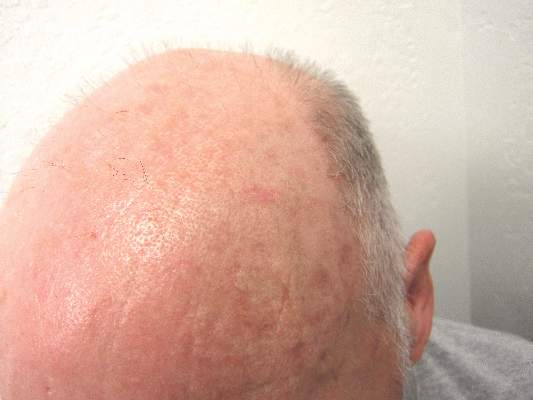
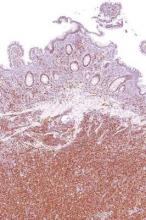
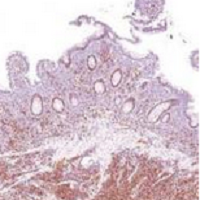
Actinic keratosis: RCM images accord with histopathology
Reflectance Confocal Microscopy (RCM) images of actinic keratoses (AKs) accord well with histopatholgy images, and trained dermatologists can readily distinguish different grades of cytological atypia in the lesions using RCM images alone, according to a report published in the November issue of Journal of the European Academy of Dermatology and Venereology.
RCM is a noninvasive optical technology that enhances clinical diagnostic accuracy, permitting clinicians to assess cytologic and architectural aspects of the epidermis in vivo. Researchers hypothesized that RCM could be used to grade AKs by allowing clinicians to assess “the irregularity of the honeycombed pattern reflecting the architectural disarray of the epidermal spinous layer,” said Dr. Giovanni Pellacani of the department of dermatology, University of Modena and Reggio Emilia, Modena, Italy, and his associates.
In a prospective study, two independent pathologists examined micrographs taken from punch biopsies and RCM images from 48 consecutive cases of facial/scalp AKs collected in a database; they also assessed two benign samples from one young patient with no sun-damaged skin and one elderly patient with severely sun-damaged skin, which served as the controls.
They graded the lesions/samples, classifying 38 as low- or moderate-grade AKs, 10 as high-grade AKs, and the two control samples as normal or lowest-grade sun-damaged skin.
Then three “raters” (two dermatopathologists and Dr. Pellacani) evaluated the RCM images, which showed a representative horizontal section of the epidermis at the stratum spinosum, while the two dermatopathologist raters evaluated photomicrographs taken at histopathology examination, grading the keratinocyte atypia they observed.
RCM grading of keratinocyte atypia strongly correlated among the three RCM raters, and the correlations were strongest for higher-grade lesions. Histopathologic grading also strongly correlated between two pathologist raters. Most important, grading of keratinocytic atypia strongly correlated between these two groups: Raters of the RCM images consistently distinguished different grades of atypia accurately.
These findings show that RCM can allow noninvasive in vivo assessment of keratinocyte morphology that is comparable to that obtained with invasive histopathology, the investigators concluded (J Eur Acad Dermatol Venereol. 2015;29[11]: 2216-21).
“RCM enables one to quickly explore several lesions and skin areas, offering a quasi-histological view of the epidermis useful for the study of AKs. This may lead, in the future, to the use of noninvasive technologies, such as RCM, for studying and monitoring of AKs and other diseases characterized by alterations of keratinocyte morphology thus avoiding the burden of invasive biopsies in clinical trials,” Dr. Pellacani and his associates said.
“Other advantages of noninvasive skin assessments are the possibility to evaluate a larger number of lesions and perilesional areas, and to collect dynamic information on quasi-histological changes occurring during the course of the disease, without alteration induced by the healing process following skin biopsy. This may also facilitate the evaluation of the efficacy of the treatments for AK and field cancerization at cellular level of keratinocytes,” they added.
Reflectance Confocal Microscopy (RCM) images of actinic keratoses (AKs) accord well with histopatholgy images, and trained dermatologists can readily distinguish different grades of cytological atypia in the lesions using RCM images alone, according to a report published in the November issue of Journal of the European Academy of Dermatology and Venereology.
RCM is a noninvasive optical technology that enhances clinical diagnostic accuracy, permitting clinicians to assess cytologic and architectural aspects of the epidermis in vivo. Researchers hypothesized that RCM could be used to grade AKs by allowing clinicians to assess “the irregularity of the honeycombed pattern reflecting the architectural disarray of the epidermal spinous layer,” said Dr. Giovanni Pellacani of the department of dermatology, University of Modena and Reggio Emilia, Modena, Italy, and his associates.
In a prospective study, two independent pathologists examined micrographs taken from punch biopsies and RCM images from 48 consecutive cases of facial/scalp AKs collected in a database; they also assessed two benign samples from one young patient with no sun-damaged skin and one elderly patient with severely sun-damaged skin, which served as the controls.
They graded the lesions/samples, classifying 38 as low- or moderate-grade AKs, 10 as high-grade AKs, and the two control samples as normal or lowest-grade sun-damaged skin.
Then three “raters” (two dermatopathologists and Dr. Pellacani) evaluated the RCM images, which showed a representative horizontal section of the epidermis at the stratum spinosum, while the two dermatopathologist raters evaluated photomicrographs taken at histopathology examination, grading the keratinocyte atypia they observed.
RCM grading of keratinocyte atypia strongly correlated among the three RCM raters, and the correlations were strongest for higher-grade lesions. Histopathologic grading also strongly correlated between two pathologist raters. Most important, grading of keratinocytic atypia strongly correlated between these two groups: Raters of the RCM images consistently distinguished different grades of atypia accurately.
These findings show that RCM can allow noninvasive in vivo assessment of keratinocyte morphology that is comparable to that obtained with invasive histopathology, the investigators concluded (J Eur Acad Dermatol Venereol. 2015;29[11]: 2216-21).
“RCM enables one to quickly explore several lesions and skin areas, offering a quasi-histological view of the epidermis useful for the study of AKs. This may lead, in the future, to the use of noninvasive technologies, such as RCM, for studying and monitoring of AKs and other diseases characterized by alterations of keratinocyte morphology thus avoiding the burden of invasive biopsies in clinical trials,” Dr. Pellacani and his associates said.
“Other advantages of noninvasive skin assessments are the possibility to evaluate a larger number of lesions and perilesional areas, and to collect dynamic information on quasi-histological changes occurring during the course of the disease, without alteration induced by the healing process following skin biopsy. This may also facilitate the evaluation of the efficacy of the treatments for AK and field cancerization at cellular level of keratinocytes,” they added.
Reflectance Confocal Microscopy (RCM) images of actinic keratoses (AKs) accord well with histopatholgy images, and trained dermatologists can readily distinguish different grades of cytological atypia in the lesions using RCM images alone, according to a report published in the November issue of Journal of the European Academy of Dermatology and Venereology.
RCM is a noninvasive optical technology that enhances clinical diagnostic accuracy, permitting clinicians to assess cytologic and architectural aspects of the epidermis in vivo. Researchers hypothesized that RCM could be used to grade AKs by allowing clinicians to assess “the irregularity of the honeycombed pattern reflecting the architectural disarray of the epidermal spinous layer,” said Dr. Giovanni Pellacani of the department of dermatology, University of Modena and Reggio Emilia, Modena, Italy, and his associates.
In a prospective study, two independent pathologists examined micrographs taken from punch biopsies and RCM images from 48 consecutive cases of facial/scalp AKs collected in a database; they also assessed two benign samples from one young patient with no sun-damaged skin and one elderly patient with severely sun-damaged skin, which served as the controls.
They graded the lesions/samples, classifying 38 as low- or moderate-grade AKs, 10 as high-grade AKs, and the two control samples as normal or lowest-grade sun-damaged skin.
Then three “raters” (two dermatopathologists and Dr. Pellacani) evaluated the RCM images, which showed a representative horizontal section of the epidermis at the stratum spinosum, while the two dermatopathologist raters evaluated photomicrographs taken at histopathology examination, grading the keratinocyte atypia they observed.
RCM grading of keratinocyte atypia strongly correlated among the three RCM raters, and the correlations were strongest for higher-grade lesions. Histopathologic grading also strongly correlated between two pathologist raters. Most important, grading of keratinocytic atypia strongly correlated between these two groups: Raters of the RCM images consistently distinguished different grades of atypia accurately.
These findings show that RCM can allow noninvasive in vivo assessment of keratinocyte morphology that is comparable to that obtained with invasive histopathology, the investigators concluded (J Eur Acad Dermatol Venereol. 2015;29[11]: 2216-21).
“RCM enables one to quickly explore several lesions and skin areas, offering a quasi-histological view of the epidermis useful for the study of AKs. This may lead, in the future, to the use of noninvasive technologies, such as RCM, for studying and monitoring of AKs and other diseases characterized by alterations of keratinocyte morphology thus avoiding the burden of invasive biopsies in clinical trials,” Dr. Pellacani and his associates said.
“Other advantages of noninvasive skin assessments are the possibility to evaluate a larger number of lesions and perilesional areas, and to collect dynamic information on quasi-histological changes occurring during the course of the disease, without alteration induced by the healing process following skin biopsy. This may also facilitate the evaluation of the efficacy of the treatments for AK and field cancerization at cellular level of keratinocytes,” they added.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Reflectance confocal microscopy images of actinic keratoses accord well with histopathology images, allowing noninvasive assessment of the lesions.
Major finding: The grading of keratinocytic atypia strongly correlated between three clinicians who rated the RCM images and two who rated the histopathology micrographs.
Data source: The study compared results of expert assessments of 48 actinic keratoses using either RCM images or histopathology slides.
Disclosures: This study had no sponsor or funding source. Dr. Pellacani and his associates reported having no relevant financial disclosures.
Lenalidomide plus rituximab achieves 87% response rate
First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3-4 mantle cell lymphoma, in an industry-sponsored, phase II clinical trial reported online Nov. 5 in the New England Journal of Medicine.
Mantle cell lymphoma is generally incurable, and patients have a median survival of 4-5 years. Initial therapy is usually very intensive, involving high-dose chemotherapy and hematopoietic cell transplantation. Since the malignancy primarily affects older adults who aren’t suitable candidates for intensive regimens, treatment “remains a clinical challenge,” said Dr. Jia Ruan of the Meyer Cancer Center and the division of biostatistics and epidemiology, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, and her associates.
Reasoning that biologic therapy might offer effective disease control with fewer and less intense adverse effects, the investigators performed the open-label, single-group trial over a 3-year period. They treated 38 patients whose mean age was 65 years (range, 42-86 years), most of whom were at intermediate or high risk for imminent progression. These participants received a 12-cycle induction phase of lenalidomide plus rituximab, followed by a maintenance phase until disease progressed, unacceptable adverse effects developed, or patients withdrew from the study. The median follow-up was 30 months (range, 10-42 months).
The primary endpoint – overall response rate – was 87% in the intention-to-treat population, and the complete response rate was 61%. The number of complete responses increased over time with continuing treatment: the median time to a partial response was 3 months, and the median time to a complete response was 11 months. Two-year progression-free survival was 85%, and 2-year overall survival was 97%, the investigators said (New Engl. J. Med. 2015 Nov 5. doi: 10.1056/NEJMoa1505237).
Only eight patients showed progression of mantle cell lymphoma while taking lenalidomide plus rituximab, two of whom died from their disease. The other six patients responded to second-line therapy and remain alive, indicating that this first-line combination biologic therapy doesn’t compromise outcomes after subsequent treatments, Dr. Ruan and her associates said.
Almost as important as these favorable survival results were the findings concerning adverse effects. Scores on several quality-of-life measures either remained stable or improved throughout the induction and maintenance phases of treatment. As expected, grade 3 or 4 hematologic adverse effects included neutropenia (50% of patients), thrombocytopenia (13%), and anemia (11%), all of which resolved; grade 3 or 4 nonhematologic adverse effects included rash (29%), tumor flare (11%), serum sickness (8%), and fatigue (8%), all of which also resolved. All the serious infections that developed during the maintenance phase of treatment, which included pneumonia, cholangitis, and West Nile viral encephalitis, also resolved with antibiotics and supportive care.
Secondary cancers that developed during follow-up included two squamous cell skin cancers, one basal cell skin cancer, two cases of melanoma in situ, one Merkel cell carcinoma, and one pancreatic cancer.
“Our data show that a lower-intensity approach for initial therapy than that usually used in the case of patients with this cancer can be highly active, with durable responses observed in most patients,” Dr. Ruan and her associates said.
This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3-4 mantle cell lymphoma, in an industry-sponsored, phase II clinical trial reported online Nov. 5 in the New England Journal of Medicine.
Mantle cell lymphoma is generally incurable, and patients have a median survival of 4-5 years. Initial therapy is usually very intensive, involving high-dose chemotherapy and hematopoietic cell transplantation. Since the malignancy primarily affects older adults who aren’t suitable candidates for intensive regimens, treatment “remains a clinical challenge,” said Dr. Jia Ruan of the Meyer Cancer Center and the division of biostatistics and epidemiology, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, and her associates.
Reasoning that biologic therapy might offer effective disease control with fewer and less intense adverse effects, the investigators performed the open-label, single-group trial over a 3-year period. They treated 38 patients whose mean age was 65 years (range, 42-86 years), most of whom were at intermediate or high risk for imminent progression. These participants received a 12-cycle induction phase of lenalidomide plus rituximab, followed by a maintenance phase until disease progressed, unacceptable adverse effects developed, or patients withdrew from the study. The median follow-up was 30 months (range, 10-42 months).
The primary endpoint – overall response rate – was 87% in the intention-to-treat population, and the complete response rate was 61%. The number of complete responses increased over time with continuing treatment: the median time to a partial response was 3 months, and the median time to a complete response was 11 months. Two-year progression-free survival was 85%, and 2-year overall survival was 97%, the investigators said (New Engl. J. Med. 2015 Nov 5. doi: 10.1056/NEJMoa1505237).
Only eight patients showed progression of mantle cell lymphoma while taking lenalidomide plus rituximab, two of whom died from their disease. The other six patients responded to second-line therapy and remain alive, indicating that this first-line combination biologic therapy doesn’t compromise outcomes after subsequent treatments, Dr. Ruan and her associates said.
Almost as important as these favorable survival results were the findings concerning adverse effects. Scores on several quality-of-life measures either remained stable or improved throughout the induction and maintenance phases of treatment. As expected, grade 3 or 4 hematologic adverse effects included neutropenia (50% of patients), thrombocytopenia (13%), and anemia (11%), all of which resolved; grade 3 or 4 nonhematologic adverse effects included rash (29%), tumor flare (11%), serum sickness (8%), and fatigue (8%), all of which also resolved. All the serious infections that developed during the maintenance phase of treatment, which included pneumonia, cholangitis, and West Nile viral encephalitis, also resolved with antibiotics and supportive care.
Secondary cancers that developed during follow-up included two squamous cell skin cancers, one basal cell skin cancer, two cases of melanoma in situ, one Merkel cell carcinoma, and one pancreatic cancer.
“Our data show that a lower-intensity approach for initial therapy than that usually used in the case of patients with this cancer can be highly active, with durable responses observed in most patients,” Dr. Ruan and her associates said.
This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3-4 mantle cell lymphoma, in an industry-sponsored, phase II clinical trial reported online Nov. 5 in the New England Journal of Medicine.
Mantle cell lymphoma is generally incurable, and patients have a median survival of 4-5 years. Initial therapy is usually very intensive, involving high-dose chemotherapy and hematopoietic cell transplantation. Since the malignancy primarily affects older adults who aren’t suitable candidates for intensive regimens, treatment “remains a clinical challenge,” said Dr. Jia Ruan of the Meyer Cancer Center and the division of biostatistics and epidemiology, Weill Cornell Medical College and New York-Presbyterian Hospital, New York, and her associates.
Reasoning that biologic therapy might offer effective disease control with fewer and less intense adverse effects, the investigators performed the open-label, single-group trial over a 3-year period. They treated 38 patients whose mean age was 65 years (range, 42-86 years), most of whom were at intermediate or high risk for imminent progression. These participants received a 12-cycle induction phase of lenalidomide plus rituximab, followed by a maintenance phase until disease progressed, unacceptable adverse effects developed, or patients withdrew from the study. The median follow-up was 30 months (range, 10-42 months).
The primary endpoint – overall response rate – was 87% in the intention-to-treat population, and the complete response rate was 61%. The number of complete responses increased over time with continuing treatment: the median time to a partial response was 3 months, and the median time to a complete response was 11 months. Two-year progression-free survival was 85%, and 2-year overall survival was 97%, the investigators said (New Engl. J. Med. 2015 Nov 5. doi: 10.1056/NEJMoa1505237).
Only eight patients showed progression of mantle cell lymphoma while taking lenalidomide plus rituximab, two of whom died from their disease. The other six patients responded to second-line therapy and remain alive, indicating that this first-line combination biologic therapy doesn’t compromise outcomes after subsequent treatments, Dr. Ruan and her associates said.
Almost as important as these favorable survival results were the findings concerning adverse effects. Scores on several quality-of-life measures either remained stable or improved throughout the induction and maintenance phases of treatment. As expected, grade 3 or 4 hematologic adverse effects included neutropenia (50% of patients), thrombocytopenia (13%), and anemia (11%), all of which resolved; grade 3 or 4 nonhematologic adverse effects included rash (29%), tumor flare (11%), serum sickness (8%), and fatigue (8%), all of which also resolved. All the serious infections that developed during the maintenance phase of treatment, which included pneumonia, cholangitis, and West Nile viral encephalitis, also resolved with antibiotics and supportive care.
Secondary cancers that developed during follow-up included two squamous cell skin cancers, one basal cell skin cancer, two cases of melanoma in situ, one Merkel cell carcinoma, and one pancreatic cancer.
“Our data show that a lower-intensity approach for initial therapy than that usually used in the case of patients with this cancer can be highly active, with durable responses observed in most patients,” Dr. Ruan and her associates said.
This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: First-line combination biologic therapy with lenalidomide plus rituximab produced an 87% overall response rate in stage 3 or 4 mantle cell lymphoma.
Major finding: The primary endpoint – overall response rate – was 87%, and the complete response rate was 61%.
Data source: A multicenter, industry-sponsored, open-label, phase II study involving 38 patients with mantle cell lymphoma followed for a median of 30 months.
Disclosures: This study was supported by Celgene, maker of lenalidomide, and a Weill Cornell Medical College Clinical Translational Science Center grant. Dr. Ruan reported ties to Celgene, and her associates reported ties to numerous industry sources.
AHA Releases First-ever Pediatric Pulmonary Hypertension Guideline
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
FROM CIRCULATION
AHA releases first-ever pediatric pulmonary hypertension guideline
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The pediatric pulmonary, pediatric cardiology, and neonatal and pediatric intensivists all have greatly anticipated directions for the care of pediatric pulmonary hypertension. The guidelines have excellent care maps for the diagnosis and evaluation of the various etiologies of pulmonary hypertension.
The new guidelines also should help also with insurance authorizations for the expensive medications for pulmonary hypertension! Dr. Robyn J. Barst, a renowned leader in pediatric pulmonary hypertension, who passed away in 2013, would be so proud of this AHA guideline!
Dr. Susan L. Millard is director of research, pediatric pulmonary & sleep medicine at Helen DeVos Children’s Hospital in Grand Rapids, MI.
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
The American Heart Association and the American Thoracic Society jointly released the first-ever clinical practice guideline for assessing and managing pulmonary hypertension (PH) in the pediatric population, which was published online Nov. 3 in Circulation.
The two organizations developed this guideline because the causes and treatments of PH in neonates, infants, and children are often different from those in adults. The literature for adult PH is “robust,” and there are several treatment guidelines available, whereas pediatric PH has not been well studied, “and little is understood about the natural history, fundamental mechanisms, and treatment of childhood PH,” said Dr. Steven H. Abman, cochair of the guideline committee and a pediatric pulmonologist at the University of Colorado and Children’s Hospital, both in Denver.
“It’s important to note that, although these guidelines provide a foundation for taking care of children with pulmonary hypertension, we still have a huge need for more specific data and research to further improve outcomes,” he said in a statement accompanying the guideline.
This guideline was developed by a working group of 27 clinicians and researchers with expertise in pediatric pulmonology, pediatric and adult cardiology, pediatric intensivism, neonatology, and translational science. They reviewed more than 600 articles in the literature, but given the paucity of high-quality data regarding pediatric PH, the guideline relies heavily on expert opinion and primarily describes “generally acceptable approaches” to diagnosis and management; more specific and detailed recommendations await the findings of future research, Dr. Abman and his associates said (Circulation. 2015 Oct 26. doi:10.1161/CIR.0000000000000329).
In the pediatric population, PH is defined as a resting mean pulmonary artery pressure greater than 25 mm Hg after the first few months of life and is usually related to cardiac, lung, or systemic diseases. Idiopathic PH, a pulmonary vasculopathy, is a diagnosis of exclusion after diseases of the left side of the heart, lung parenchyma, heart valves, thromboembolism, and other miscellaneous causes have been ruled out.
The guideline emphasizes that children thought to have PH should be evaluated and treated at comprehensive, multidisciplinary clinics at specialized pediatric centers. “When children are diagnosed, parents often feel helpless. However, it’s important that parents seek doctors and centers that see these children on a regular basis and can offer them access to new molecular diagnostics, new drug therapies, and new devices, as well as surgeries that have recently been developed,” Dr. Stephen L. Archer, cochair of the guideline committee and head of medicine at Queen’s University, Kingston, Ont., said in the statement.
“These children suffer with health issues throughout their lives or die prematurely, particularly if they’re not properly diagnosed and managed. But with the proper diagnosis and treatment at a specialized center for PH, the prognosis for many of these children is excellent,” he noted.
Properly classifying the type of PH is a key first step in determining treatment. The guideline addresses numerous methods for diagnosing and monitoring PH, including imaging studies, echocardiograms, cardiac catheterization, brain natriuretic peptide and other laboratory testing, 6-minute walk distance (at appropriate ages), sleep studies, and genetic testing. It specifically deals with persistent PH of the newborn and PH arising from congenital diaphragmatic hernia; bronchopulmonary dysplasia or other lung diseases; heart disease such as atrial-septal defect or patent ductus arteriosus; and systemic diseases such as hemolytic hemoglobinopathies and hepatic, renal, or metabolic illness; as well as idiopathic PH and PH related to high-altitude pulmonary edema.
Regarding ongoing outpatient care, the guideline recommends that children with PH receive influenza and pneumococcal vaccinations and prophylaxis for respiratory syncytial virus (if they are eligible), as well as antibiotic prophylaxis to prevent subacute bacterial endocarditis in those who are cyanotic or have indwelling central lines. Growth must be monitored rigorously, and infections and respiratory illnesses must be recognized and treated promptly. Any surgeries require careful preoperative planning and should be performed at hospitals with expertise in PH.
The guideline includes an extensive section on pharmacotherapy for childhood PH, including the use of digitalis, diuretics, long-term anticoagulation, oxygen therapy, calcium channel blockers, phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, intravenous and subcutaneous prostacyclin therapy, and the transition from parenteral to oral or inhaled treatment.
In addition, the guideline addresses exercise and sports participation, travel restrictions, and contraceptive counseling for adolescent patients. Finally, “given the impact of childhood PH on the entire family, [patients], siblings, and caregivers should be assessed for psychosocial stress and be readily provided support and referral as needed,” the guideline recommends.
A copy of the guideline is available at http://my.americanheart.org/statements.
FROM CIRCULATION
Efavirenz-based therapy an option for HIV-infected children
Switching HIV-infected children aged 3 and older from a recommended first-line antiretroviral treatment to efavirenz-based therapy may offer advantages, according to a new study in JAMA.
The study, based on a single-center open-label trial involving 298 South African children and published online Nov. 3, found that changing the HIV treatment regimen from ritonavir-boosted lopinavir-based therapy to efavirenz-based therapy was noninferior regarding rates of viral rebound and viral failure.
As a long-term maintenance treatment, efavirenz has several potential advantages over ritonavir-boosted lopinavir therapy. It requires once-daily rather than twice daily administration and doesn’t have the unpleasant taste of ritonavir-boosted lopinavir syrup, which would improve adherence and thus effectiveness. Efavirenz also may avoid some metabolic toxicities of the latter treatment and may simplify cotreatment for tuberculosis, a common coinfection among children with HIV in third-world countries. And it doesn’t require cold storage to maintain long-term stability, said Dr. Ashraf Coovadia of the University of the Witwatersrand and the Rahima Moosa Mother and Child Hospital, both in Johannesburg, and his associates.
There have been anecdotal reports of clinicians switching young pediatric patients to efavirenz, which is the recommended therapy for older children and adults, even though no data supported this practice until now. To shed light on the issue, the investigators compared the two treatment regimens in 298 children (average age, 4.3 years) who had been exposed to nevirapine for prevention of mother-to-child transmission and, after birth, had been maintained on ritonavir-boosted lopinavir-based therapy for an average of 3.5 years. A total of 148 of these children were randomly assigned to continue this recommended regimen and 150 were switched to efavirenz-based therapy; all were followed for 48 weeks.
Efavirenz-based therapy proved noninferior regarding both primary efficacy endpoints: viral rebound and viral failure. The probability of viral rebound, defined as more than 50 copies of HIV RNA per mL of plasma, was 17.6% for the efavirenz group and 28.4% for the ritonavir-boosted lopinavir group. The probability of viral failure, defined as more than 1,000 copies of HIV RNA per mL of plasma, was 2.7% and 2.0%, respectively. These data “support the safety and efficacy of switching to efavirenz,” Dr. Coovadia and his associates said (JAMA. 2015;314[17]:1808-17. doi:10.1001/jama.2015.13631).
The lipid profiles of children taking efavirenz were better, with lower levels of total cholesterol, LDL cholesterol, and triglycerides. CD4 cell counts and CD4 cell percentages remained in the normal range for both study groups, and there were no significant differences between the two in weight-for-age or height-for-age scores, rates of anemia or neutropenia, skin manifestations, or scores on a measure of emotional/behavioral problems. Alanine aminotransferase elevations were more common with efavirenz, as was nausea.
The adverse neuropsychiatric effects of efavirenz are well-known. After 4 weeks of treatment, 26% of children taking efavirenz had trouble sleeping or had nightmares, compared with no children in the other study group. However, this difference disappeared by 8 weeks. Two children developed seizure disorders presumed to be related to efavirenz. Both were found to have genetic mutations known to be associated with delayed drug clearance or abnormally high blood levels of efavirenz, and the seizures resolved after the drug was discontinued.
Even though Dr. Coovadia and his associates state that their study “provides evidence to support the safety and efficacy of switching to efavirenz,” many questions remain.
At least one report has linked current World Health Organization dosing guidelines for efavirenz to excessive blood levels of the drug, and it is possible that the high percentage of children in this study who had nightmares and sleeping problems reflect this toxicity. How many of these children also had impaired concentration and other, more subtle central nervous system toxic effects?
In addition, switching to efavirenz may not simplify cotreatment of tuberculosis, as the authors assert, because rifampicin may reduce the bioavailability of efavirenz. And other factors that can affect the pharmacokinetics and pharmacodynamics of efavirenz, such as nutritional status and developmental physiology, have not yet been adequately studied in children.
Dr. Ram Yogev is in the department of pediatrics at Lurie Children’s Hospital, Chicago. He reported having no relevant financial disclosures. Dr. Yogev made these remarks in an editorial accompanying Dr. Coovadia’s report (JAMA. 2015;314[17]:1801-2. doi:10.1001/jama.2015.13763).
Even though Dr. Coovadia and his associates state that their study “provides evidence to support the safety and efficacy of switching to efavirenz,” many questions remain.
At least one report has linked current World Health Organization dosing guidelines for efavirenz to excessive blood levels of the drug, and it is possible that the high percentage of children in this study who had nightmares and sleeping problems reflect this toxicity. How many of these children also had impaired concentration and other, more subtle central nervous system toxic effects?
In addition, switching to efavirenz may not simplify cotreatment of tuberculosis, as the authors assert, because rifampicin may reduce the bioavailability of efavirenz. And other factors that can affect the pharmacokinetics and pharmacodynamics of efavirenz, such as nutritional status and developmental physiology, have not yet been adequately studied in children.
Dr. Ram Yogev is in the department of pediatrics at Lurie Children’s Hospital, Chicago. He reported having no relevant financial disclosures. Dr. Yogev made these remarks in an editorial accompanying Dr. Coovadia’s report (JAMA. 2015;314[17]:1801-2. doi:10.1001/jama.2015.13763).
Even though Dr. Coovadia and his associates state that their study “provides evidence to support the safety and efficacy of switching to efavirenz,” many questions remain.
At least one report has linked current World Health Organization dosing guidelines for efavirenz to excessive blood levels of the drug, and it is possible that the high percentage of children in this study who had nightmares and sleeping problems reflect this toxicity. How many of these children also had impaired concentration and other, more subtle central nervous system toxic effects?
In addition, switching to efavirenz may not simplify cotreatment of tuberculosis, as the authors assert, because rifampicin may reduce the bioavailability of efavirenz. And other factors that can affect the pharmacokinetics and pharmacodynamics of efavirenz, such as nutritional status and developmental physiology, have not yet been adequately studied in children.
Dr. Ram Yogev is in the department of pediatrics at Lurie Children’s Hospital, Chicago. He reported having no relevant financial disclosures. Dr. Yogev made these remarks in an editorial accompanying Dr. Coovadia’s report (JAMA. 2015;314[17]:1801-2. doi:10.1001/jama.2015.13763).
Switching HIV-infected children aged 3 and older from a recommended first-line antiretroviral treatment to efavirenz-based therapy may offer advantages, according to a new study in JAMA.
The study, based on a single-center open-label trial involving 298 South African children and published online Nov. 3, found that changing the HIV treatment regimen from ritonavir-boosted lopinavir-based therapy to efavirenz-based therapy was noninferior regarding rates of viral rebound and viral failure.
As a long-term maintenance treatment, efavirenz has several potential advantages over ritonavir-boosted lopinavir therapy. It requires once-daily rather than twice daily administration and doesn’t have the unpleasant taste of ritonavir-boosted lopinavir syrup, which would improve adherence and thus effectiveness. Efavirenz also may avoid some metabolic toxicities of the latter treatment and may simplify cotreatment for tuberculosis, a common coinfection among children with HIV in third-world countries. And it doesn’t require cold storage to maintain long-term stability, said Dr. Ashraf Coovadia of the University of the Witwatersrand and the Rahima Moosa Mother and Child Hospital, both in Johannesburg, and his associates.
There have been anecdotal reports of clinicians switching young pediatric patients to efavirenz, which is the recommended therapy for older children and adults, even though no data supported this practice until now. To shed light on the issue, the investigators compared the two treatment regimens in 298 children (average age, 4.3 years) who had been exposed to nevirapine for prevention of mother-to-child transmission and, after birth, had been maintained on ritonavir-boosted lopinavir-based therapy for an average of 3.5 years. A total of 148 of these children were randomly assigned to continue this recommended regimen and 150 were switched to efavirenz-based therapy; all were followed for 48 weeks.
Efavirenz-based therapy proved noninferior regarding both primary efficacy endpoints: viral rebound and viral failure. The probability of viral rebound, defined as more than 50 copies of HIV RNA per mL of plasma, was 17.6% for the efavirenz group and 28.4% for the ritonavir-boosted lopinavir group. The probability of viral failure, defined as more than 1,000 copies of HIV RNA per mL of plasma, was 2.7% and 2.0%, respectively. These data “support the safety and efficacy of switching to efavirenz,” Dr. Coovadia and his associates said (JAMA. 2015;314[17]:1808-17. doi:10.1001/jama.2015.13631).
The lipid profiles of children taking efavirenz were better, with lower levels of total cholesterol, LDL cholesterol, and triglycerides. CD4 cell counts and CD4 cell percentages remained in the normal range for both study groups, and there were no significant differences between the two in weight-for-age or height-for-age scores, rates of anemia or neutropenia, skin manifestations, or scores on a measure of emotional/behavioral problems. Alanine aminotransferase elevations were more common with efavirenz, as was nausea.
The adverse neuropsychiatric effects of efavirenz are well-known. After 4 weeks of treatment, 26% of children taking efavirenz had trouble sleeping or had nightmares, compared with no children in the other study group. However, this difference disappeared by 8 weeks. Two children developed seizure disorders presumed to be related to efavirenz. Both were found to have genetic mutations known to be associated with delayed drug clearance or abnormally high blood levels of efavirenz, and the seizures resolved after the drug was discontinued.
Switching HIV-infected children aged 3 and older from a recommended first-line antiretroviral treatment to efavirenz-based therapy may offer advantages, according to a new study in JAMA.
The study, based on a single-center open-label trial involving 298 South African children and published online Nov. 3, found that changing the HIV treatment regimen from ritonavir-boosted lopinavir-based therapy to efavirenz-based therapy was noninferior regarding rates of viral rebound and viral failure.
As a long-term maintenance treatment, efavirenz has several potential advantages over ritonavir-boosted lopinavir therapy. It requires once-daily rather than twice daily administration and doesn’t have the unpleasant taste of ritonavir-boosted lopinavir syrup, which would improve adherence and thus effectiveness. Efavirenz also may avoid some metabolic toxicities of the latter treatment and may simplify cotreatment for tuberculosis, a common coinfection among children with HIV in third-world countries. And it doesn’t require cold storage to maintain long-term stability, said Dr. Ashraf Coovadia of the University of the Witwatersrand and the Rahima Moosa Mother and Child Hospital, both in Johannesburg, and his associates.
There have been anecdotal reports of clinicians switching young pediatric patients to efavirenz, which is the recommended therapy for older children and adults, even though no data supported this practice until now. To shed light on the issue, the investigators compared the two treatment regimens in 298 children (average age, 4.3 years) who had been exposed to nevirapine for prevention of mother-to-child transmission and, after birth, had been maintained on ritonavir-boosted lopinavir-based therapy for an average of 3.5 years. A total of 148 of these children were randomly assigned to continue this recommended regimen and 150 were switched to efavirenz-based therapy; all were followed for 48 weeks.
Efavirenz-based therapy proved noninferior regarding both primary efficacy endpoints: viral rebound and viral failure. The probability of viral rebound, defined as more than 50 copies of HIV RNA per mL of plasma, was 17.6% for the efavirenz group and 28.4% for the ritonavir-boosted lopinavir group. The probability of viral failure, defined as more than 1,000 copies of HIV RNA per mL of plasma, was 2.7% and 2.0%, respectively. These data “support the safety and efficacy of switching to efavirenz,” Dr. Coovadia and his associates said (JAMA. 2015;314[17]:1808-17. doi:10.1001/jama.2015.13631).
The lipid profiles of children taking efavirenz were better, with lower levels of total cholesterol, LDL cholesterol, and triglycerides. CD4 cell counts and CD4 cell percentages remained in the normal range for both study groups, and there were no significant differences between the two in weight-for-age or height-for-age scores, rates of anemia or neutropenia, skin manifestations, or scores on a measure of emotional/behavioral problems. Alanine aminotransferase elevations were more common with efavirenz, as was nausea.
The adverse neuropsychiatric effects of efavirenz are well-known. After 4 weeks of treatment, 26% of children taking efavirenz had trouble sleeping or had nightmares, compared with no children in the other study group. However, this difference disappeared by 8 weeks. Two children developed seizure disorders presumed to be related to efavirenz. Both were found to have genetic mutations known to be associated with delayed drug clearance or abnormally high blood levels of efavirenz, and the seizures resolved after the drug was discontinued.
FROM JAMA
Key clinical point: Switching to efavirenz was noninferior to maintaining ritonavir-boosted lopinavir-based therapy among HIV-infected children aged 3 years and older.
Major finding: The probability of viral rebound was 17.6% for efavirenz and 28.4% for the ritonavir-boosted lopinavir, and the probabilities of viral failure were 2.7% and 2.0%, respectively.
Data source: A single-center open-label randomized noninferiority trial involving 298 HIV-infected children in South Africa who were followed for 48 weeks.
Disclosures: This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Coovadia and his associates reported having no relevant financial disclosures.
Endovascular thrombectomy vs tPA: better function, same mortality
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
It is important to note some limitations with this well-conducted meta-analysis. First, functional outcomes showed significant heterogeneity, which the authors attributed to variations in patient-, treatment-, and study-related factors.
Second, the confidence intervals for mortality and intracranial hemorrhage were wide, indicating that more data are necessary to fully inform these outcomes.
Third, five of the eight trials were halted early because of the evident superiority of endovascular thrombectomy, which means they fell substantially short (by up to 74%) of their planned sample sizes. This tends to cause overestimation of treatment effects. Fourth, nearly all these strokes involved carotid territory, nearly all the patients were on the young end of the age spectrum, and very few participants had comorbidities such as AF or diabetes. Such favorable characteristics do not reflect real-world experience with ischemic stroke.
Dr. Joanna M. Wardlaw and Dr. Martin S. Dennis are at the Centre for Clinical Brain Sciences at the University of Edinburgh (Scotland). They reported having no relevant financial disclosures. Dr. Wardlaw and Dr. Dennis made these remarks in an editorial accompanying Dr. Badhiwala’s meta-analysis (JAMA 2015;314:1803-4).
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
Endovascular mechanical thrombectomy yielded better function and revascularization rates but similar mortality and intracranial hemorrhage rates as standard medical therapy using tissue plasminogen activator (tPA) in a meta-analysis of eight high-quality randomized clinical trials comparing the two approaches for acute ischemic stroke.
The results were published online Nov. 3 in JAMA.
This meta-analysis included only large multicenter trials published from 2013 to the present. Previous trials and meta-analyses “had several well-recognized limitations” including inconsistent use of vascular imaging to confirm vessel occlusion before randomization, variable use of tPA in patients who eventually were assigned to endovascular therapy, and reliance on less effective and now outdated mechanical devices, said Dr. Jetan H. Badhiwala of the division of neurosurgery, University of Toronto, and his associates.
The eight trials included 2,423 patients (mean age, 67.4 years); 46.7% were women. A total of 1,313 patients underwent endovascular therapy, defined as the intra-arterial use of a microcatheter or other device for mechanical thrombectomy, with or without the local use of a chemical thrombolytic agent. The remaining 1,110 received standard medical therapy (tPA). The interval between stroke onset and endovascular treatment varied from 5 to 12 hours across these studies, with a mean of 3.8 hours.
Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8. The rate of angiographic revascularization at 24 hours also was markedly higher for endovascular thrombectomy (75.8% vs 34.1%), for an OR of 6.49, the investigators said (JAMA 2015;314:1832-43).
However, there were no significant differences between the two study groups in rates of symptomatic intracranial hemorrhage at 90 days (5.7% vs 5.1%) or all-cause mortality at 90 days (15.8% vs 17.8%), and overall morbidity including in-hospital rates of deep venous thrombosis, MI, and pneumonia also were similar.
No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
FROM JAMA
Key clinical point: Endovascular mechanical thrombectomy yielded better function and angiographic revascularization but similar mortality and rate of intracranial hemorrhage compared with standard medical therapy (tPA) for acute ischemic stroke.
Major finding: Patients who had endovascular thrombectomy showed significantly higher rates of functional independence at 90 days (44.6%) than did those who had tPA (31.8%), for an OR of 1.71 and a number needed to treat of 8.
Data source: A meta-analysis of eight high-quality multicenter randomized clinical trials published during 2013-2015 involving 2,423 adults with acute ischemic stroke.
Disclosures: No sponsor or source of financial support was reported for this study. Dr. Badhiwala and his associates reported having no relevant financial disclosures.
Off-label prescriptions frequently cause adverse events
Off-label prescribing of drugs is common and very likely to cause adverse events, particularly when no strong scientific evidence supports the off-label use, according to a report published online Nov. 2 in JAMA Internal Medicine.
No systematic investigation of the off-label use of prescription drugs has been done to date, in part because physicians aren’t required to document intended indications. But recent innovations in electronic health records provided an opportunity to track off-label prescribing and its influence on adverse drug events in the Canadian province of Quebec, which provides health insurance for all 8.5 million residents. There, physicians must provide the indication(s) for every new prescription, the reason(s) for any dose changes or drug discontinuation, and the nature of any adverse events, said Dr. Tewodros Eguale of the department of epidemiology, biostatistics, and occupational health at McGill University, Montreal, and his associates.
In what they described as the first large study of its kind, the investigators analyzed the prescribing information in electronic medical records of 46,021 adults (mean age 58 years) given 151,305 new prescriptions during a 5-year period. Physicians reported off-label use in 17,847 (11.8%) of these prescriptions, and that off-label use lacked strong scientific evidence in the great majority of cases (80.9%). The median follow-up time for use of prescribed medications was 386 days (range, 1 day to 6 years).
Prescribed drugs were discontinued because of adverse events in 3,484 cases. The incidence of adverse events was 44% higher for off-label use (19.7 per 10,000 person-months) than for on-label use (12.5 per 10,000 person-months). Moreover, the incidence of adverse events was 54% higher for off-label use unsupported by strong scientific evidence (21.7 per 10,000 person-months) than for off-label use supported by strong scientific evidence (13.2 per 10,000 person-months), the investigators said (JAMA Intern Med. 2015 Nov 2. doi:10.1001/jamainternmed.2015.6058).
The class of drugs with the highest rate of adverse effects was anti-infective agents (66.2 per 10,000 person-months), followed by central nervous system drugs such as antidepressants, anxiolytics, and antimigraine medicine (18.1 per 10,000); cardiovascular drugs (15.9 per 10,000); hormonal agents (12.7); autonomic drugs including albuterol and terbutaline (8.4); gastrointestinal drugs (6.1); ear, nose, and throat medications (2.8); and “other” agents such as antihistamines, blood thinners, and antineoplastics (1.3).
“Selected examples of adverse events associated with the most frequently used off-label drugs include akathisia resulting from the use of gabapentin for neurogenic pain; agitation associated with the use of amitriptyline hydrochloride for migraine; hallucinations with the use of trazodone hydrochloride for insomnia; QT interval prolongation with the use of quetiapine fumarate for depression; and weight gain with the use of olanzapine for depression,” the authors added.
“Off-label use may be clinically appropriate given the complexity of the patient’s condition, the lack of alternative effective drugs, or after exhausting approved drugs.” However, previous research has shown that physicians’ lack of knowledge of approved treatment indications was one important factor contributing to off-label prescribing. And one study showed that physicians are finding it difficult to keep up with rapidly changing medication information, and this lack of knowledge is affecting treatment, Dr. Eguale and his associates said.
That knowledge gap could be filled by supplying clinicians with information regarding drug approval status and the quality of supporting scientific evidence at the point of care, when they write prescriptions into patients’ electronic health records, the investigators noted. This would have the added advantage of facilitating communication among physicians, pharmacists, and patients, and could reduce medication errors such as those caused by giving drugs to the wrong patients or by giving patients sound-alike or look-alike drugs.
No sponsors were identified for this study. Dr. Tewodros Eguale and his associates reported having no relevant financial disclosures.
This study, the most extensive and informative one to evaluate the safety of off-label drug use in adults to date, shows that clinicians often enter an arena of the unknown when they expand prescribing beyond the carefully devised confines of the labeled indication. It provides compelling evidence that off-label prescribing is frequently inappropriate and substantially raises the risk for an adverse event.
Even in cases in which an off-label indication has been studied, the pharmacokinetics, drug-disease interactions, drug-drug interactions, and other safety considerations weren’t studied to the degree required during the drug approval process. Moreover, how many clinicians have the time or motivation to review the evidence for those off-label indications, arriving at a balanced assessment of risks and benefits?
Dr. Chester B. Good is in pharmacy benefits management services at the U.S. Department of Veterans Affairs in Hines, Ill., and the department of pharmacy and therapeutics at the University of Pittsburgh. Dr. Good and Dr. Walid F. Gellad are in the department of medicine at the University of Pittsburgh and at the Center for Health Equity Research and Promotion in the Veterans Affairs Pittsburgh Healthcare System. Dr. Good and Dr. Gellad reported serving as unpaid advisers to the Food and Drug Administration’s Drug Safety Oversight Board. They made these remarks in an Invited Commentary accompanying Dr. Eguale’s report (JAMA Intern Med. 2015 Nov 2. doi:10.1001/jamainternmed.2015.6068).
This study, the most extensive and informative one to evaluate the safety of off-label drug use in adults to date, shows that clinicians often enter an arena of the unknown when they expand prescribing beyond the carefully devised confines of the labeled indication. It provides compelling evidence that off-label prescribing is frequently inappropriate and substantially raises the risk for an adverse event.
Even in cases in which an off-label indication has been studied, the pharmacokinetics, drug-disease interactions, drug-drug interactions, and other safety considerations weren’t studied to the degree required during the drug approval process. Moreover, how many clinicians have the time or motivation to review the evidence for those off-label indications, arriving at a balanced assessment of risks and benefits?
Dr. Chester B. Good is in pharmacy benefits management services at the U.S. Department of Veterans Affairs in Hines, Ill., and the department of pharmacy and therapeutics at the University of Pittsburgh. Dr. Good and Dr. Walid F. Gellad are in the department of medicine at the University of Pittsburgh and at the Center for Health Equity Research and Promotion in the Veterans Affairs Pittsburgh Healthcare System. Dr. Good and Dr. Gellad reported serving as unpaid advisers to the Food and Drug Administration’s Drug Safety Oversight Board. They made these remarks in an Invited Commentary accompanying Dr. Eguale’s report (JAMA Intern Med. 2015 Nov 2. doi:10.1001/jamainternmed.2015.6068).
This study, the most extensive and informative one to evaluate the safety of off-label drug use in adults to date, shows that clinicians often enter an arena of the unknown when they expand prescribing beyond the carefully devised confines of the labeled indication. It provides compelling evidence that off-label prescribing is frequently inappropriate and substantially raises the risk for an adverse event.
Even in cases in which an off-label indication has been studied, the pharmacokinetics, drug-disease interactions, drug-drug interactions, and other safety considerations weren’t studied to the degree required during the drug approval process. Moreover, how many clinicians have the time or motivation to review the evidence for those off-label indications, arriving at a balanced assessment of risks and benefits?
Dr. Chester B. Good is in pharmacy benefits management services at the U.S. Department of Veterans Affairs in Hines, Ill., and the department of pharmacy and therapeutics at the University of Pittsburgh. Dr. Good and Dr. Walid F. Gellad are in the department of medicine at the University of Pittsburgh and at the Center for Health Equity Research and Promotion in the Veterans Affairs Pittsburgh Healthcare System. Dr. Good and Dr. Gellad reported serving as unpaid advisers to the Food and Drug Administration’s Drug Safety Oversight Board. They made these remarks in an Invited Commentary accompanying Dr. Eguale’s report (JAMA Intern Med. 2015 Nov 2. doi:10.1001/jamainternmed.2015.6068).
Off-label prescribing of drugs is common and very likely to cause adverse events, particularly when no strong scientific evidence supports the off-label use, according to a report published online Nov. 2 in JAMA Internal Medicine.
No systematic investigation of the off-label use of prescription drugs has been done to date, in part because physicians aren’t required to document intended indications. But recent innovations in electronic health records provided an opportunity to track off-label prescribing and its influence on adverse drug events in the Canadian province of Quebec, which provides health insurance for all 8.5 million residents. There, physicians must provide the indication(s) for every new prescription, the reason(s) for any dose changes or drug discontinuation, and the nature of any adverse events, said Dr. Tewodros Eguale of the department of epidemiology, biostatistics, and occupational health at McGill University, Montreal, and his associates.
In what they described as the first large study of its kind, the investigators analyzed the prescribing information in electronic medical records of 46,021 adults (mean age 58 years) given 151,305 new prescriptions during a 5-year period. Physicians reported off-label use in 17,847 (11.8%) of these prescriptions, and that off-label use lacked strong scientific evidence in the great majority of cases (80.9%). The median follow-up time for use of prescribed medications was 386 days (range, 1 day to 6 years).
Prescribed drugs were discontinued because of adverse events in 3,484 cases. The incidence of adverse events was 44% higher for off-label use (19.7 per 10,000 person-months) than for on-label use (12.5 per 10,000 person-months). Moreover, the incidence of adverse events was 54% higher for off-label use unsupported by strong scientific evidence (21.7 per 10,000 person-months) than for off-label use supported by strong scientific evidence (13.2 per 10,000 person-months), the investigators said (JAMA Intern Med. 2015 Nov 2. doi:10.1001/jamainternmed.2015.6058).
The class of drugs with the highest rate of adverse effects was anti-infective agents (66.2 per 10,000 person-months), followed by central nervous system drugs such as antidepressants, anxiolytics, and antimigraine medicine (18.1 per 10,000); cardiovascular drugs (15.9 per 10,000); hormonal agents (12.7); autonomic drugs including albuterol and terbutaline (8.4); gastrointestinal drugs (6.1); ear, nose, and throat medications (2.8); and “other” agents such as antihistamines, blood thinners, and antineoplastics (1.3).
“Selected examples of adverse events associated with the most frequently used off-label drugs include akathisia resulting from the use of gabapentin for neurogenic pain; agitation associated with the use of amitriptyline hydrochloride for migraine; hallucinations with the use of trazodone hydrochloride for insomnia; QT interval prolongation with the use of quetiapine fumarate for depression; and weight gain with the use of olanzapine for depression,” the authors added.
“Off-label use may be clinically appropriate given the complexity of the patient’s condition, the lack of alternative effective drugs, or after exhausting approved drugs.” However, previous research has shown that physicians’ lack of knowledge of approved treatment indications was one important factor contributing to off-label prescribing. And one study showed that physicians are finding it difficult to keep up with rapidly changing medication information, and this lack of knowledge is affecting treatment, Dr. Eguale and his associates said.
That knowledge gap could be filled by supplying clinicians with information regarding drug approval status and the quality of supporting scientific evidence at the point of care, when they write prescriptions into patients’ electronic health records, the investigators noted. This would have the added advantage of facilitating communication among physicians, pharmacists, and patients, and could reduce medication errors such as those caused by giving drugs to the wrong patients or by giving patients sound-alike or look-alike drugs.
No sponsors were identified for this study. Dr. Tewodros Eguale and his associates reported having no relevant financial disclosures.
Off-label prescribing of drugs is common and very likely to cause adverse events, particularly when no strong scientific evidence supports the off-label use, according to a report published online Nov. 2 in JAMA Internal Medicine.
No systematic investigation of the off-label use of prescription drugs has been done to date, in part because physicians aren’t required to document intended indications. But recent innovations in electronic health records provided an opportunity to track off-label prescribing and its influence on adverse drug events in the Canadian province of Quebec, which provides health insurance for all 8.5 million residents. There, physicians must provide the indication(s) for every new prescription, the reason(s) for any dose changes or drug discontinuation, and the nature of any adverse events, said Dr. Tewodros Eguale of the department of epidemiology, biostatistics, and occupational health at McGill University, Montreal, and his associates.
In what they described as the first large study of its kind, the investigators analyzed the prescribing information in electronic medical records of 46,021 adults (mean age 58 years) given 151,305 new prescriptions during a 5-year period. Physicians reported off-label use in 17,847 (11.8%) of these prescriptions, and that off-label use lacked strong scientific evidence in the great majority of cases (80.9%). The median follow-up time for use of prescribed medications was 386 days (range, 1 day to 6 years).
Prescribed drugs were discontinued because of adverse events in 3,484 cases. The incidence of adverse events was 44% higher for off-label use (19.7 per 10,000 person-months) than for on-label use (12.5 per 10,000 person-months). Moreover, the incidence of adverse events was 54% higher for off-label use unsupported by strong scientific evidence (21.7 per 10,000 person-months) than for off-label use supported by strong scientific evidence (13.2 per 10,000 person-months), the investigators said (JAMA Intern Med. 2015 Nov 2. doi:10.1001/jamainternmed.2015.6058).
The class of drugs with the highest rate of adverse effects was anti-infective agents (66.2 per 10,000 person-months), followed by central nervous system drugs such as antidepressants, anxiolytics, and antimigraine medicine (18.1 per 10,000); cardiovascular drugs (15.9 per 10,000); hormonal agents (12.7); autonomic drugs including albuterol and terbutaline (8.4); gastrointestinal drugs (6.1); ear, nose, and throat medications (2.8); and “other” agents such as antihistamines, blood thinners, and antineoplastics (1.3).
“Selected examples of adverse events associated with the most frequently used off-label drugs include akathisia resulting from the use of gabapentin for neurogenic pain; agitation associated with the use of amitriptyline hydrochloride for migraine; hallucinations with the use of trazodone hydrochloride for insomnia; QT interval prolongation with the use of quetiapine fumarate for depression; and weight gain with the use of olanzapine for depression,” the authors added.
“Off-label use may be clinically appropriate given the complexity of the patient’s condition, the lack of alternative effective drugs, or after exhausting approved drugs.” However, previous research has shown that physicians’ lack of knowledge of approved treatment indications was one important factor contributing to off-label prescribing. And one study showed that physicians are finding it difficult to keep up with rapidly changing medication information, and this lack of knowledge is affecting treatment, Dr. Eguale and his associates said.
That knowledge gap could be filled by supplying clinicians with information regarding drug approval status and the quality of supporting scientific evidence at the point of care, when they write prescriptions into patients’ electronic health records, the investigators noted. This would have the added advantage of facilitating communication among physicians, pharmacists, and patients, and could reduce medication errors such as those caused by giving drugs to the wrong patients or by giving patients sound-alike or look-alike drugs.
No sponsors were identified for this study. Dr. Tewodros Eguale and his associates reported having no relevant financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Off-label prescribing in adults is common and very likely to cause adverse events.
Major finding: The incidence of adverse events was 44% higher for off-label use (19.7 per 10,000 person-months) than for on-label use (12.5 per 10,000 person-months) of prescription drugs.
Data source: A prospective cohort study of 46,021 adults who received 151,305 incident prescriptions from primary care clinicians in Quebec during a 5-year period.
Disclosures: No sponsors were identified for this study. Dr. Tewodros Eguale and his associates reported having no relevant financial disclosures.
Certain refractory prostate cancers respond to olaparib
Daily oral olaparib induced a 33% response rate in refractory, progressing prostate cancer in an international phase II clinical trial. The study was published online Oct. 29 in the New England Journal of Medicine.
Researchers hypothesized that olaparib, a poly-ADP-ribose polymerase (PARP) inhibitor recently approved for treatment of ovarian cancers with BRCA1/2 mutations, would also show antitumor activity in sporadic cases of metastatic, castration-resistant prostate cancer in which the tumors expressed DNA-repair defects. During a 2-year period, the researchers identified 49 patients at seven medical centers to participate in the study.
Fresh biopsy samples, obtained before treatment began, were used for germline whole-exome sequencing and transcriptome studies, said Dr. Joaquin Mateo of the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust, both in London, and his associates.
Participants took 400-mg tablets of olaparib twice daily until they showed radiologic progression, unequivocal clinical progression, or unacceptable side effects, or until they died. After a median follow-up of 14.4 months (range, 1.4-21.9 months), median overall survival was 10.1 months. Twelve patients survived more than 6 months on olaparib, and four survived more than 12 months on the drug.
The primary end point – treatment response rate – was 33%, with 16 of the 49 patients demonstrating a radiologic response on CT scanning, regression of bone metastases on serial MRI scanning, a reduction of 50% or more in PSA level, and/or “impressive” reductions in the number of circulating tumor cells to less than 5 per 7.5 mL (N Engl J Med. 2015 Oct 29. doi:10.1056/NEJMoa1506859).
Genetic testing of the tumor samples showed that 16 patients had tumor aberrations in DNA-repair genes, including BRCA2, ATM, BRCA1, PALB2, CHEK2, FANCA, and HDAC2. Fourteen of these 16 patients responded to olaparib. In contrast, 2 of the 33 patients whose tumors did not have aberrations in DNA-repair genes responded to olaparib. In addition, radiologic progression-free survival was significantly longer in the group with mutations in the DNA-repair genes (median, 9.8 months) than in the other group (2.7 months). Overall survival also was significantly longer (median 13.8 months vs. 7.5 months).
Three patients permanently discontinued olaparib because of adverse events, chiefly anemia. Another 13 patients required dose reductions. Drug-related toxicity included anemia (affecting 20% of patients), fatigue (12%), leukopenia (6%), thrombocytopenia (4%), and neutropenia (4%).
Prostate cancers characterized by defects in DNA repair are thought to comprise 25%-30% of all sporadic, castration-resistant prostate cancers. These study findings suggest that this commonly occurring subset of tumors can be molecularly stratified for treatment. Their results demonstrate that identifying predictive biomarkers from fresh tumor-biopsy samples is feasible, and that “next-generation sequencing analyses of tumor-biopsy samples can increase our understanding of treatment responses,” Dr. Mateo and his associates said.
They added that the DNA-repair defects found in these tumors may prove sensitive to platinum-based chemotherapies. This type of treatment usually is not used for metastatic, castration-resistant prostate cancer because phase III studies haven’t shown a survival benefit in unselected patients. However, anecdotal responses to platinum analogues have been reported, and emerging data show that PAPR-inhibitors show antitumor activity against similar (ovarian) cancers.
Cancer Research U.K., Royal Marsden NHS Foundation Trust, Stand Up To Cancer–Prostate Cancer Foundation, Prostate Cancer U.K., National Institute for Health Research, Medical Research Council–Prostate Cancer U.K., Swiss Cancer League, Marie Curie International Incoming Fellowship, and the Investigator-Sponsored Study Collaboration between AstraZeneca and the National Institute for Health Research Cancer Research Network funded the study. AstraZeneca provided the olaparib used in the trial. Dr. Mateo reported having no disclosures; his associates reported ties to numerous industry sources.
Daily oral olaparib induced a 33% response rate in refractory, progressing prostate cancer in an international phase II clinical trial. The study was published online Oct. 29 in the New England Journal of Medicine.
Researchers hypothesized that olaparib, a poly-ADP-ribose polymerase (PARP) inhibitor recently approved for treatment of ovarian cancers with BRCA1/2 mutations, would also show antitumor activity in sporadic cases of metastatic, castration-resistant prostate cancer in which the tumors expressed DNA-repair defects. During a 2-year period, the researchers identified 49 patients at seven medical centers to participate in the study.
Fresh biopsy samples, obtained before treatment began, were used for germline whole-exome sequencing and transcriptome studies, said Dr. Joaquin Mateo of the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust, both in London, and his associates.
Participants took 400-mg tablets of olaparib twice daily until they showed radiologic progression, unequivocal clinical progression, or unacceptable side effects, or until they died. After a median follow-up of 14.4 months (range, 1.4-21.9 months), median overall survival was 10.1 months. Twelve patients survived more than 6 months on olaparib, and four survived more than 12 months on the drug.
The primary end point – treatment response rate – was 33%, with 16 of the 49 patients demonstrating a radiologic response on CT scanning, regression of bone metastases on serial MRI scanning, a reduction of 50% or more in PSA level, and/or “impressive” reductions in the number of circulating tumor cells to less than 5 per 7.5 mL (N Engl J Med. 2015 Oct 29. doi:10.1056/NEJMoa1506859).
Genetic testing of the tumor samples showed that 16 patients had tumor aberrations in DNA-repair genes, including BRCA2, ATM, BRCA1, PALB2, CHEK2, FANCA, and HDAC2. Fourteen of these 16 patients responded to olaparib. In contrast, 2 of the 33 patients whose tumors did not have aberrations in DNA-repair genes responded to olaparib. In addition, radiologic progression-free survival was significantly longer in the group with mutations in the DNA-repair genes (median, 9.8 months) than in the other group (2.7 months). Overall survival also was significantly longer (median 13.8 months vs. 7.5 months).
Three patients permanently discontinued olaparib because of adverse events, chiefly anemia. Another 13 patients required dose reductions. Drug-related toxicity included anemia (affecting 20% of patients), fatigue (12%), leukopenia (6%), thrombocytopenia (4%), and neutropenia (4%).
Prostate cancers characterized by defects in DNA repair are thought to comprise 25%-30% of all sporadic, castration-resistant prostate cancers. These study findings suggest that this commonly occurring subset of tumors can be molecularly stratified for treatment. Their results demonstrate that identifying predictive biomarkers from fresh tumor-biopsy samples is feasible, and that “next-generation sequencing analyses of tumor-biopsy samples can increase our understanding of treatment responses,” Dr. Mateo and his associates said.
They added that the DNA-repair defects found in these tumors may prove sensitive to platinum-based chemotherapies. This type of treatment usually is not used for metastatic, castration-resistant prostate cancer because phase III studies haven’t shown a survival benefit in unselected patients. However, anecdotal responses to platinum analogues have been reported, and emerging data show that PAPR-inhibitors show antitumor activity against similar (ovarian) cancers.
Cancer Research U.K., Royal Marsden NHS Foundation Trust, Stand Up To Cancer–Prostate Cancer Foundation, Prostate Cancer U.K., National Institute for Health Research, Medical Research Council–Prostate Cancer U.K., Swiss Cancer League, Marie Curie International Incoming Fellowship, and the Investigator-Sponsored Study Collaboration between AstraZeneca and the National Institute for Health Research Cancer Research Network funded the study. AstraZeneca provided the olaparib used in the trial. Dr. Mateo reported having no disclosures; his associates reported ties to numerous industry sources.
Daily oral olaparib induced a 33% response rate in refractory, progressing prostate cancer in an international phase II clinical trial. The study was published online Oct. 29 in the New England Journal of Medicine.
Researchers hypothesized that olaparib, a poly-ADP-ribose polymerase (PARP) inhibitor recently approved for treatment of ovarian cancers with BRCA1/2 mutations, would also show antitumor activity in sporadic cases of metastatic, castration-resistant prostate cancer in which the tumors expressed DNA-repair defects. During a 2-year period, the researchers identified 49 patients at seven medical centers to participate in the study.
Fresh biopsy samples, obtained before treatment began, were used for germline whole-exome sequencing and transcriptome studies, said Dr. Joaquin Mateo of the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust, both in London, and his associates.
Participants took 400-mg tablets of olaparib twice daily until they showed radiologic progression, unequivocal clinical progression, or unacceptable side effects, or until they died. After a median follow-up of 14.4 months (range, 1.4-21.9 months), median overall survival was 10.1 months. Twelve patients survived more than 6 months on olaparib, and four survived more than 12 months on the drug.
The primary end point – treatment response rate – was 33%, with 16 of the 49 patients demonstrating a radiologic response on CT scanning, regression of bone metastases on serial MRI scanning, a reduction of 50% or more in PSA level, and/or “impressive” reductions in the number of circulating tumor cells to less than 5 per 7.5 mL (N Engl J Med. 2015 Oct 29. doi:10.1056/NEJMoa1506859).
Genetic testing of the tumor samples showed that 16 patients had tumor aberrations in DNA-repair genes, including BRCA2, ATM, BRCA1, PALB2, CHEK2, FANCA, and HDAC2. Fourteen of these 16 patients responded to olaparib. In contrast, 2 of the 33 patients whose tumors did not have aberrations in DNA-repair genes responded to olaparib. In addition, radiologic progression-free survival was significantly longer in the group with mutations in the DNA-repair genes (median, 9.8 months) than in the other group (2.7 months). Overall survival also was significantly longer (median 13.8 months vs. 7.5 months).
Three patients permanently discontinued olaparib because of adverse events, chiefly anemia. Another 13 patients required dose reductions. Drug-related toxicity included anemia (affecting 20% of patients), fatigue (12%), leukopenia (6%), thrombocytopenia (4%), and neutropenia (4%).
Prostate cancers characterized by defects in DNA repair are thought to comprise 25%-30% of all sporadic, castration-resistant prostate cancers. These study findings suggest that this commonly occurring subset of tumors can be molecularly stratified for treatment. Their results demonstrate that identifying predictive biomarkers from fresh tumor-biopsy samples is feasible, and that “next-generation sequencing analyses of tumor-biopsy samples can increase our understanding of treatment responses,” Dr. Mateo and his associates said.
They added that the DNA-repair defects found in these tumors may prove sensitive to platinum-based chemotherapies. This type of treatment usually is not used for metastatic, castration-resistant prostate cancer because phase III studies haven’t shown a survival benefit in unselected patients. However, anecdotal responses to platinum analogues have been reported, and emerging data show that PAPR-inhibitors show antitumor activity against similar (ovarian) cancers.
Cancer Research U.K., Royal Marsden NHS Foundation Trust, Stand Up To Cancer–Prostate Cancer Foundation, Prostate Cancer U.K., National Institute for Health Research, Medical Research Council–Prostate Cancer U.K., Swiss Cancer League, Marie Curie International Incoming Fellowship, and the Investigator-Sponsored Study Collaboration between AstraZeneca and the National Institute for Health Research Cancer Research Network funded the study. AstraZeneca provided the olaparib used in the trial. Dr. Mateo reported having no disclosures; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Olaparib induced a 33% response rate in refractory, progressing prostate cancers in a phase II trial.
Major finding: Fourteen of 16 patients with tumor aberrations in DNA-repair genes responded to olaparib, compared with 2 of the 33 patients whose tumors did not have such aberrations.
Data source: An international open-label phase II clinical trial involving 50 men with metastatic castration-resistant prostate cancer followed for a median of 14 months.
Disclosures: Cancer Research U.K., Royal Marsden NHS Foundation Trust, Stand Up To Cancer–Prostate Cancer Foundation, Prostate Cancer U.K., National Institute for Health Research, Medical Research Council–Prostate Cancer U.K., Swiss Cancer League, Marie Curie International Incoming Fellowship, and the Investigator-Sponsored Study Collaboration between AstraZeneca and the National Institute for Health Research Cancer Research Network funded the study. AstraZeneca provided the olaparib used in the trial. Dr. Mateo reported having no disclosures; his associates reported ties to numerous industry sources.