User login
Antiamyloid solanezumab fails to slow decline in mild Alzheimer’s
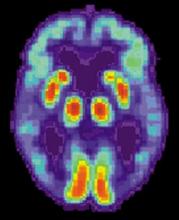
Solanezumab, a monoclonal antibody that targets amyloid plaques, did not slow cognitive decline in patients with mild Alzheimer’s disease.
Although the drug did reduce soluble amyloid beta by 40%, compared with placebo, that change did not translate into a clinically meaningful cognitive benefit, Eric Siemers, MD, said in a press briefing called by Eli Lilly & Co., which manufactures solanezumab. The company did not release specific cognitive data from the highly anticipated Expedition 3 trial, except to say that changes on the ADAS-Cog14 (Alzheimer’s Disease Assessment Scale–cognitive subscale-14), the main cognitive endpoint, had a nonsignificant P value of 0.095 [Corection: Changed from 0.95 in original posting]. Functional data were also withheld.
The entire clinical picture won’t emerge until Dec. 8, when Lilly presents full data at the annual Clinical Trials in Alzheimer’s Disease meeting in San Diego. But, in the meantime, Dr. Siemers did say that Lilly won’t be pursuing a Food and Drug Administration New Drug Application for solanezumab as a disease-modifying therapy for mild Alzheimer’s. However, it’s not the end of the road for solanezumab. Lilly is still investigating it in ExpeditionPro, a trial which will employ the antibody in patients with prodromal AD.
Solanezumab is also part of two very important public/private partnership studies: The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study), investigating its effect in cognitively healthy elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
It’s unclear what impact the failed Expedition 3 could have on those, Dr. Siemers said. “These studies are also funded by the National Institute on Aging in collaboration with academic institutions, so we will need to sort through the data very carefully and speak with our academic colleagues before making any decisions on this.”
If solanezumab was successful in either one of those trials – if they continue – Lilly could still apply for approval in those patient groups, Dr. Siemers noted.
The results are disappointing, but not entirely unexpected in the research community. Many felt that Expedition 3 was founded on shaky clinical ground from the start. The study was based on subgroup analyses of Expedition 1 and Expedition 2, both of which failed to meet their primary endpoints in patients with mild-moderate AD. But when researchers pooled both groups of mild patients, they found that solanezumab conferred a 34% slowing of cognitive decline in one cognitive measure, the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted Expedition 3 to come as close to recreating those findings as possible, said Lon Schneider, MD, of the University of Southern California, Los Angeles.
“By taking the ideal patients from two studies and combining them as if they were from one study, Lilly predicted that they could find a 1.5-2.0 point [Correction: Changed from 1.5 in original posting] difference in one particular outcome, the ADAS-Cog14, [Correction: the ADAS-Cog14 not included in previous posting] in a study of more than 2,000 patients,” Dr. Schneider said in an interview.
This is the research equivalent of Heraclitus’ adage that one can never step in the same river twice: These could never be the same patients, from the same places, at the same time, with the same clinical picture, Dr. Schneider said – especially since Expedition 3 had a purified cohort of only amyloid-positive patients, while close to 25% of patients in the earlier studies probably had no brain amyloid at all.
“This is what happens when we rely on subanalysis to create drug trials. We slice it and dice it until we find some patients for whom the drug is better than placebo, and then say, ‘We have a winner.’ ” Even in the best-case scenario, he said, Expedition 3 would have found only the same modest benefit as its predecessors. “Then we would be in the difficult situation of saying we have a disease-modifying therapy that is associated with only very small clinical changes.”
Dr. Siemers rejected the idea that Expedition 3’s failure is another nail in the coffin of the amyloid hypothesis, or at least in the idea that taming amyloid can prevent dementia or rescue cognition. “You can’t disprove a hypothesis based on one study done in patients with mild dementia,” he said.
Dennis Selkoe, PhD, the Vincent and Stella Coates Professor of Neurologic Diseases at Harvard Medical School, Boston, said Expedition’s failure does not negate the amyloid hypothesis or the appropriateness of amyloid as a therapeutic target.
“Like others, I am disappointed for our patients that solanezumab failed to meet its clinical endpoint by just a little bit. But it is not truly surprising, as this drug was always known to be a ‘weak’ antibody in terms of its ability to mobilize amyloid from the brain,” he said in an interview.
Research into antiamyloid therapies should continue, he said.
“This outcome does nothing to alter the overwhelming genetic, neuropathological and biomarker evidence that amyloid beta protein buildup acts to precipitate the AD process, and it also does not alter the potential promise of current agents in trials: Merck’s beta secretase inhibitor and Biogen’s aducanumab antibody, which appears to be much more effective in mobilizing amyloid beta. Other approaches, in addition to antiamyloid agents, are very much desired and are gradually moving forward, which is good news. But for the time being, antiamyloid treatments will be those most examined in trials, for scientifically and technically sound reasons.”
Dr. Schneider has served as a consultant for Eli Lilly & Co in the past. Dr. Selkoe is a founding scientist and director of Elan Pharmaceuticals.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
Solenezumab’s failure in patients with mild-stage Alzheimer’s disease is disappointing, but is it really surprising?
Post hoc analyses of subsets in clinical trials have been notoriously misleading, and this, sadly, is one more example. Overinterpretation of ongoing trials should likewise be kept in check until phase III data are in.
The signals from every putative disease-modifying therapy to date have been weak at best, and one might worry more if such a weak signal actually hit the magical statistical P value of .05. Then we might launch an expensive new phase of AD therapy that not only falls short of halting disease progression, but simply prolongs the course, adds to the cost, further burdens an already overburdened system, and leads family members to ask providers whether the drug is “still working” (a common question I hear in patients taking any one of the current symptomatic medications).
We need a major breakthrough, and reducing disease progression a little bit is not a major breakthrough. It may even be something worse.
Dr. Richard J. Caselli is associate director and clinical core director of the Alzheimer’s Disease Center at Mayo Clinic, Scottsdale, Ariz.
Solenezumab’s failure in patients with mild-stage Alzheimer’s disease is disappointing, but is it really surprising?
Post hoc analyses of subsets in clinical trials have been notoriously misleading, and this, sadly, is one more example. Overinterpretation of ongoing trials should likewise be kept in check until phase III data are in.
The signals from every putative disease-modifying therapy to date have been weak at best, and one might worry more if such a weak signal actually hit the magical statistical P value of .05. Then we might launch an expensive new phase of AD therapy that not only falls short of halting disease progression, but simply prolongs the course, adds to the cost, further burdens an already overburdened system, and leads family members to ask providers whether the drug is “still working” (a common question I hear in patients taking any one of the current symptomatic medications).
We need a major breakthrough, and reducing disease progression a little bit is not a major breakthrough. It may even be something worse.
Dr. Richard J. Caselli is associate director and clinical core director of the Alzheimer’s Disease Center at Mayo Clinic, Scottsdale, Ariz.
Solenezumab’s failure in patients with mild-stage Alzheimer’s disease is disappointing, but is it really surprising?
Post hoc analyses of subsets in clinical trials have been notoriously misleading, and this, sadly, is one more example. Overinterpretation of ongoing trials should likewise be kept in check until phase III data are in.
The signals from every putative disease-modifying therapy to date have been weak at best, and one might worry more if such a weak signal actually hit the magical statistical P value of .05. Then we might launch an expensive new phase of AD therapy that not only falls short of halting disease progression, but simply prolongs the course, adds to the cost, further burdens an already overburdened system, and leads family members to ask providers whether the drug is “still working” (a common question I hear in patients taking any one of the current symptomatic medications).
We need a major breakthrough, and reducing disease progression a little bit is not a major breakthrough. It may even be something worse.
Dr. Richard J. Caselli is associate director and clinical core director of the Alzheimer’s Disease Center at Mayo Clinic, Scottsdale, Ariz.
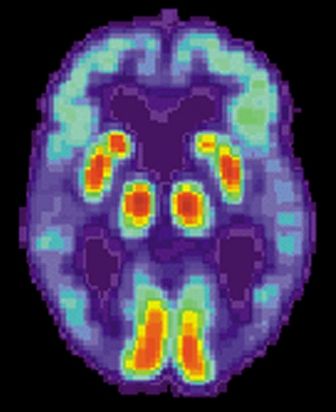
Solanezumab, a monoclonal antibody that targets amyloid plaques, did not slow cognitive decline in patients with mild Alzheimer’s disease.
Although the drug did reduce soluble amyloid beta by 40%, compared with placebo, that change did not translate into a clinically meaningful cognitive benefit, Eric Siemers, MD, said in a press briefing called by Eli Lilly & Co., which manufactures solanezumab. The company did not release specific cognitive data from the highly anticipated Expedition 3 trial, except to say that changes on the ADAS-Cog14 (Alzheimer’s Disease Assessment Scale–cognitive subscale-14), the main cognitive endpoint, had a nonsignificant P value of 0.095 [Corection: Changed from 0.95 in original posting]. Functional data were also withheld.
The entire clinical picture won’t emerge until Dec. 8, when Lilly presents full data at the annual Clinical Trials in Alzheimer’s Disease meeting in San Diego. But, in the meantime, Dr. Siemers did say that Lilly won’t be pursuing a Food and Drug Administration New Drug Application for solanezumab as a disease-modifying therapy for mild Alzheimer’s. However, it’s not the end of the road for solanezumab. Lilly is still investigating it in ExpeditionPro, a trial which will employ the antibody in patients with prodromal AD.
Solanezumab is also part of two very important public/private partnership studies: The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study), investigating its effect in cognitively healthy elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
It’s unclear what impact the failed Expedition 3 could have on those, Dr. Siemers said. “These studies are also funded by the National Institute on Aging in collaboration with academic institutions, so we will need to sort through the data very carefully and speak with our academic colleagues before making any decisions on this.”
If solanezumab was successful in either one of those trials – if they continue – Lilly could still apply for approval in those patient groups, Dr. Siemers noted.
The results are disappointing, but not entirely unexpected in the research community. Many felt that Expedition 3 was founded on shaky clinical ground from the start. The study was based on subgroup analyses of Expedition 1 and Expedition 2, both of which failed to meet their primary endpoints in patients with mild-moderate AD. But when researchers pooled both groups of mild patients, they found that solanezumab conferred a 34% slowing of cognitive decline in one cognitive measure, the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted Expedition 3 to come as close to recreating those findings as possible, said Lon Schneider, MD, of the University of Southern California, Los Angeles.
“By taking the ideal patients from two studies and combining them as if they were from one study, Lilly predicted that they could find a 1.5-2.0 point [Correction: Changed from 1.5 in original posting] difference in one particular outcome, the ADAS-Cog14, [Correction: the ADAS-Cog14 not included in previous posting] in a study of more than 2,000 patients,” Dr. Schneider said in an interview.
This is the research equivalent of Heraclitus’ adage that one can never step in the same river twice: These could never be the same patients, from the same places, at the same time, with the same clinical picture, Dr. Schneider said – especially since Expedition 3 had a purified cohort of only amyloid-positive patients, while close to 25% of patients in the earlier studies probably had no brain amyloid at all.
“This is what happens when we rely on subanalysis to create drug trials. We slice it and dice it until we find some patients for whom the drug is better than placebo, and then say, ‘We have a winner.’ ” Even in the best-case scenario, he said, Expedition 3 would have found only the same modest benefit as its predecessors. “Then we would be in the difficult situation of saying we have a disease-modifying therapy that is associated with only very small clinical changes.”
Dr. Siemers rejected the idea that Expedition 3’s failure is another nail in the coffin of the amyloid hypothesis, or at least in the idea that taming amyloid can prevent dementia or rescue cognition. “You can’t disprove a hypothesis based on one study done in patients with mild dementia,” he said.
Dennis Selkoe, PhD, the Vincent and Stella Coates Professor of Neurologic Diseases at Harvard Medical School, Boston, said Expedition’s failure does not negate the amyloid hypothesis or the appropriateness of amyloid as a therapeutic target.
“Like others, I am disappointed for our patients that solanezumab failed to meet its clinical endpoint by just a little bit. But it is not truly surprising, as this drug was always known to be a ‘weak’ antibody in terms of its ability to mobilize amyloid from the brain,” he said in an interview.
Research into antiamyloid therapies should continue, he said.
“This outcome does nothing to alter the overwhelming genetic, neuropathological and biomarker evidence that amyloid beta protein buildup acts to precipitate the AD process, and it also does not alter the potential promise of current agents in trials: Merck’s beta secretase inhibitor and Biogen’s aducanumab antibody, which appears to be much more effective in mobilizing amyloid beta. Other approaches, in addition to antiamyloid agents, are very much desired and are gradually moving forward, which is good news. But for the time being, antiamyloid treatments will be those most examined in trials, for scientifically and technically sound reasons.”
Dr. Schneider has served as a consultant for Eli Lilly & Co in the past. Dr. Selkoe is a founding scientist and director of Elan Pharmaceuticals.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
Solanezumab, a monoclonal antibody that targets amyloid plaques, did not slow cognitive decline in patients with mild Alzheimer’s disease.
Although the drug did reduce soluble amyloid beta by 40%, compared with placebo, that change did not translate into a clinically meaningful cognitive benefit, Eric Siemers, MD, said in a press briefing called by Eli Lilly & Co., which manufactures solanezumab. The company did not release specific cognitive data from the highly anticipated Expedition 3 trial, except to say that changes on the ADAS-Cog14 (Alzheimer’s Disease Assessment Scale–cognitive subscale-14), the main cognitive endpoint, had a nonsignificant P value of 0.095 [Corection: Changed from 0.95 in original posting]. Functional data were also withheld.
The entire clinical picture won’t emerge until Dec. 8, when Lilly presents full data at the annual Clinical Trials in Alzheimer’s Disease meeting in San Diego. But, in the meantime, Dr. Siemers did say that Lilly won’t be pursuing a Food and Drug Administration New Drug Application for solanezumab as a disease-modifying therapy for mild Alzheimer’s. However, it’s not the end of the road for solanezumab. Lilly is still investigating it in ExpeditionPro, a trial which will employ the antibody in patients with prodromal AD.
Solanezumab is also part of two very important public/private partnership studies: The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study), investigating its effect in cognitively healthy elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
It’s unclear what impact the failed Expedition 3 could have on those, Dr. Siemers said. “These studies are also funded by the National Institute on Aging in collaboration with academic institutions, so we will need to sort through the data very carefully and speak with our academic colleagues before making any decisions on this.”
If solanezumab was successful in either one of those trials – if they continue – Lilly could still apply for approval in those patient groups, Dr. Siemers noted.
The results are disappointing, but not entirely unexpected in the research community. Many felt that Expedition 3 was founded on shaky clinical ground from the start. The study was based on subgroup analyses of Expedition 1 and Expedition 2, both of which failed to meet their primary endpoints in patients with mild-moderate AD. But when researchers pooled both groups of mild patients, they found that solanezumab conferred a 34% slowing of cognitive decline in one cognitive measure, the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted Expedition 3 to come as close to recreating those findings as possible, said Lon Schneider, MD, of the University of Southern California, Los Angeles.
“By taking the ideal patients from two studies and combining them as if they were from one study, Lilly predicted that they could find a 1.5-2.0 point [Correction: Changed from 1.5 in original posting] difference in one particular outcome, the ADAS-Cog14, [Correction: the ADAS-Cog14 not included in previous posting] in a study of more than 2,000 patients,” Dr. Schneider said in an interview.
This is the research equivalent of Heraclitus’ adage that one can never step in the same river twice: These could never be the same patients, from the same places, at the same time, with the same clinical picture, Dr. Schneider said – especially since Expedition 3 had a purified cohort of only amyloid-positive patients, while close to 25% of patients in the earlier studies probably had no brain amyloid at all.
“This is what happens when we rely on subanalysis to create drug trials. We slice it and dice it until we find some patients for whom the drug is better than placebo, and then say, ‘We have a winner.’ ” Even in the best-case scenario, he said, Expedition 3 would have found only the same modest benefit as its predecessors. “Then we would be in the difficult situation of saying we have a disease-modifying therapy that is associated with only very small clinical changes.”
Dr. Siemers rejected the idea that Expedition 3’s failure is another nail in the coffin of the amyloid hypothesis, or at least in the idea that taming amyloid can prevent dementia or rescue cognition. “You can’t disprove a hypothesis based on one study done in patients with mild dementia,” he said.
Dennis Selkoe, PhD, the Vincent and Stella Coates Professor of Neurologic Diseases at Harvard Medical School, Boston, said Expedition’s failure does not negate the amyloid hypothesis or the appropriateness of amyloid as a therapeutic target.
“Like others, I am disappointed for our patients that solanezumab failed to meet its clinical endpoint by just a little bit. But it is not truly surprising, as this drug was always known to be a ‘weak’ antibody in terms of its ability to mobilize amyloid from the brain,” he said in an interview.
Research into antiamyloid therapies should continue, he said.
“This outcome does nothing to alter the overwhelming genetic, neuropathological and biomarker evidence that amyloid beta protein buildup acts to precipitate the AD process, and it also does not alter the potential promise of current agents in trials: Merck’s beta secretase inhibitor and Biogen’s aducanumab antibody, which appears to be much more effective in mobilizing amyloid beta. Other approaches, in addition to antiamyloid agents, are very much desired and are gradually moving forward, which is good news. But for the time being, antiamyloid treatments will be those most examined in trials, for scientifically and technically sound reasons.”
Dr. Schneider has served as a consultant for Eli Lilly & Co in the past. Dr. Selkoe is a founding scientist and director of Elan Pharmaceuticals.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
Key clinical point:
Major finding: Specific clinical data were not released.
Data source: The 18-month–long Expedition 3 study randomized 2,100 patients with imaging-proven amyloid plaques to either placebo or 400-mg solanezumab every 4 weeks.
Disclosures: Eli Lilly & Co. manufactures solanezumab. Dr. Siemers is an employee of the company.
Breast milk doesn’t contain meaningful levels of certolizumab pegol
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: None of the 137 samples contained more than 0.076 mcg/mL of the drug.
Data source: The 4-week postmarketing study comprised 17 breastfeeding women.
Disclosures: UCB sponsored the study. Dr. Clowse is a consultant for the company.
ACR 2015 Workforce Study: Fewer rheumatologists, more patients, and the struggle to bridge the gap
WASHINGTON – The mass retirement of Baby Boomer workhorses, the changing face of a Millennial workforce, and the graying of America will deliver a triple-whammy to rheumatology over the next 15 years: By 2030, the United States will be short 4,700 full-time rheumatologists – just about as many as are currently in practice.
“This is drastic,” Marcy Bolster, MD, said at the annual meeting of the American College of Rheumatology. “We need to take action now or we will not be able to meet the expected increases in patient demand.”
Dr. Bolster, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston, was one of 10 clinicians who discussed the ACR’s massive new project, “The 2015 Workforce Study of Rheumatology Specialists in the United States,” a comprehensive assessment of the current supply of rheumatologists in this country, and a sobering prediction of how many will be needed in the future.
Released at the ACR meeting in Washington, the 2015 survey is the first that ACR has conducted since 2005. The project drew on a number of sources: questionnaires sent out to the entire ACR membership; published research, position papers, and government reports; the Institute of Medicine; and state licensure and National Resident Matching Program data. Four online questionnaires surveyed not only rheumatology clinicians and fellows, but other health professionals, adult and young patients with rheumatic diseases, and the parents of pediatric patients.
The survey response rate of 31% among practicing rheumatologists was not as high as the Committee on Rheumatology Training and Workforce Issues would have liked, said Daniel Battafarano, DO, a study cochair. But fellows had a 95% response rate, offering the study a very solid look at the near future of the specialty.
The 2015 results were a striking contrast to the 2005 report, which examined supply and demand up to 2025. It came to a brighter conclusion: that a relative balance would be maintained. Not so now.
The reasons for this projected imbalance are not overly complicated, and are actually recapitulated in other areas of medicine, said Dr. Battafarano, chief of rheumatology at San Antonio (Tex.) Military Medical Center. Baby Boomer senior physicians are tired of working 70 hours a week, and moving toward retirement in unprecedented numbers. In fact, according to the report, 50% of today’s full-time rheumatologists will retire within the next 15 years, and 80% of those plan to cut back their workload by about 25%.
It’s not that new blood isn’t coming into the profession. In fact, Dr. Bolster said during her presentation, the percentage of internal medicine residents moving into rheumatology will stay stable, at about 4%. But the number isn’t projected to increase as patient demand does, and these new doctors will look and practice very differently than the new doctors of 30 or 40 years ago.
“The majority of medical school graduates now – about 60% – are women,” Dr. Battafarano said. “And they are entering the workforce at a very challenging time of life, simultaneously building careers and families.”
Studies have also demonstrated that women have different practice patterns than men. They tend to spend more time with patients, so they sometimes see fewer in a day. In fact, in its supply assessment, the ACR study characterized women rheumatologists as 0.7 of a full-time equivalent position – an important factor in the projected supply gap.
But the projected workforce shortage does not hinge on the practice patterns of women rheumatologists, Dr. Battafarano cautioned. Part of it is based on his own generation of work-a-holism. In general, young physicians now place more importance on a healthy work-life balance than did his generation, and this he sees as a healthy way to approach a new career.
“I’ll admit it: Baby Boomers are dysfunctionally working too hard, and they miss the importance of a health work/life balance. That’s changing, and that’s a good thing.”
His protégé, Katrina Lawrence-Wolff, DO, agrees. A second-year rheumatology fellow, Dr. Lawrence-Wolff is also expecting her first child. She presented the breakout results focusing on the adult rheumatologist supply/demand picture.
“None of us think working 80 hours a week is a good idea for us personally, or a good way to give good medical care to patients,” she said in an interview. “We would rather have more time with family, more time for volunteering in our community, or to do advocacy work. We want to be flexible, and we do understand that this attitude may change the level of our reimbursement. But we are willing to forgo some salary to become a happier, better-balanced person.”
International medical graduates also affect this snapshot of the future, Dr. Bolster said during her lecture. More than half of new medical graduates are international students, and 17% of those intend to leave the United States after their education is complete.
Shortages will affect all regions, but some more than others
All of these issues are now converging at a time when patient demand is expected to soar. In the United States, about 22.5 million adults and 300,000 children have a rheumatic disease. According to the study, that number will increase by 61% by 2030.
Dr. Lawrence-Wolff used population statistics to really put the numbers needed to care for these patients into perspective. Studies in the United States, Canada, and Europe generally agree that the ideal rheumatologist:patient ratio is somewhere around 2 per 100,000 adults. “In 2005, when we were felt to be balanced in this way, our ratio was 1.67/100,000 patients.”
This ratio will look very different by 2025, she said, and the regional imbalances already seen will be magnified. These regional differences aren’t a surprise, Dr. Lawrence-Wolff noted. They directly reflect the density of academic rheumatology training centers. “Most practicing rheumatologists tend to stay in the region where they received their training,” she said, “so we have more clinicians in areas with more academic centers.”
For example, the Northeast U.S. hosts a highly enriched rheumatologist population, with a rheumatologist:patient ratio of 3.7/100,000. By 2025, this will be reduced to 1.6/100,000 – still acceptable, but more than a 50% decrease.
That same decrease will be much more drastically felt in regions that already have a paucity of rheumatologists. In the Southwest, the current ratio is 1.2/100,000; in 10 years, this will be 0.64/100,000. Even areas that are moderately well supplied now will suffer. Both the Northwest and North Central regions have a ratio of about 1.6/100,000. Both those regions will sink into the range of 0.6-0.5/100,000.
“All regions are going to decline below the 1.67 threshold by 2030,” she said. “Every single one.”
Troubles for pediatric rheumatology workforce
If things are concerning in the world of adult rheumatology, they’re downright alarming in the world of pediatric rheumatology, said Dr. Battafarano, who broke out the pediatric subspecialty results.
These clinicians are already scarce, with only 287 currently practicing full-time in 2015. By 2030, 461 will be needed, but the supply is expected to drop to 231.
The pediatric supply problem starts much earlier in the academic process, he said. For years, about 50% of the slots of pediatric rheumatology fellows have gone unfilled. In this world, recruitment woes will drive supply problems more than retirement. “As a whole, pediatric rheumatologists are younger,” Dr. Battafarano said, with only 32% planning to retire by 2030.
“Recruitment into adult rheumatology isn’t a problem, but on the pediatric side, it’s been very hard to recruit. This isn’t unique to rheumatology; it’s being seen in other pediatric subspecialties as well.”
Reimbursement and work overload are at the root of it, he believes.
“It translates pretty well to income. Reimbursement for chronic diseases, like what we see, is predominately Medicaid. The reimbursement rate and income for subspecialty pediatrics is definitely lower than it is in other academic subspecialties. And I have to think that time spent observing an exhausted, overworked physician isn’t helpful either.”
The mandatory 3-year pediatric rheumatology fellowship may also be a deal killer for some potential recruits, Dr. Battafarano said. The prevailing thought has always been to include a year of academic research in the pediatric track, which extends it beyond the 2-year fellowship that adult rheumatologists experience.
“So you marry the 3-year fellowship with workload, quality of life, student debt, and income and you get a combination that’s just less appealing than some other areas.”
Adjusting that fellowship track is one way to potentially improve the pediatric supply picture, he said. “One of the things I recommended is adding a 2-year clinical track. There are ways to do research that can be folded into a clinical setting that wouldn’t require an entire year. The majority of adult fellowships are 2 years with concurrent research, so there is already a precedent.”
In fact, Dr. Lawrence-Wolff is doing just that, he said. “She will do clinical research that’s integrated into her practice, but her primary role is to learn to be a clinical rheumatologist.”
Ideas for stretching rheumatologic care
Dr. Battafarano had some other practical suggestions for improving recruitment into the field. “We have to offer some incentives. I’d like to see us exploring the potential of loan repayment and visa programs.”
Overall, expanding the musculoskeletal expertise of primary care providers should also be on the table, Dr. Lawrence-Wolff said. “It’s possible that we can help our primary care colleagues extend rheumatology care by treating things like osteoarthritis and gout.”
Rheumatologists also can’t afford to ignore the expanding-role of mid-level providers, she said. “We would like to recruit more nurse practitioners and physician assistants into the specialty. The numbers we hope will go up, even if the percentage remains the same as it is, about 2%-5%.”
Skillfully leveraging these clinicians’ strengths will be the key to successfully employing them.
“We see different ways to utilize them to stretch our care. One suggestion is having them in the clinic to see more patients per day, but also to use them to see lower-level patients so the rheumatologist can take care of the more complex cases.”
They could also serve patients who have multiple regularly scheduled checkups for chronic illness. “If you have a patient who needs to be seen four times a year, the NP or PA can see that person, check the labs and determine if the patent is stable, and doesn’t really need to see the rheumatologist.”
Technology will invariably come into play as well, Dr. Battafarano said.
“We envision this as a multipronged approach that includes telehealth and ‘E-consults,’ although we don’t precisely know what that will look like. But other specialties – and primary care as well – are going through very similar trends here. We are all talking about working with other providers to reach more patients, and telemedicine is a key area of investigation. We really are all in the same boat.”
Finally, Dr. Battafarano urges his fellow senior clinicians to consider severing professional ties gradually. “It’s not just a dearth of bodies we’re facing, but a sudden depletion of valuable experience and clinical wisdom. In my practice, for example, the three of us each have more than 30 years’ experience. That’s close to a century of experience, and two of them want to retire. It’s such a brain-drain on a terrific practice to lose our colleagues overnight.”
For some reason, he said, the locum tenens model has never really caught on in rheumatology, and he’d like to see that idea explored and embraced. It’s a perfect way to keep experienced hands in the mix, both seeing patients and mentoring young rheumatologists, he added.
“Even if we’re in our 60s and 70s, we’re not brain-dead yet. A lot of us want to keep contributing, just not full-time.”
None of the clinicians quoted in this article had any relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The mass retirement of Baby Boomer workhorses, the changing face of a Millennial workforce, and the graying of America will deliver a triple-whammy to rheumatology over the next 15 years: By 2030, the United States will be short 4,700 full-time rheumatologists – just about as many as are currently in practice.
“This is drastic,” Marcy Bolster, MD, said at the annual meeting of the American College of Rheumatology. “We need to take action now or we will not be able to meet the expected increases in patient demand.”
Dr. Bolster, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston, was one of 10 clinicians who discussed the ACR’s massive new project, “The 2015 Workforce Study of Rheumatology Specialists in the United States,” a comprehensive assessment of the current supply of rheumatologists in this country, and a sobering prediction of how many will be needed in the future.
Released at the ACR meeting in Washington, the 2015 survey is the first that ACR has conducted since 2005. The project drew on a number of sources: questionnaires sent out to the entire ACR membership; published research, position papers, and government reports; the Institute of Medicine; and state licensure and National Resident Matching Program data. Four online questionnaires surveyed not only rheumatology clinicians and fellows, but other health professionals, adult and young patients with rheumatic diseases, and the parents of pediatric patients.
The survey response rate of 31% among practicing rheumatologists was not as high as the Committee on Rheumatology Training and Workforce Issues would have liked, said Daniel Battafarano, DO, a study cochair. But fellows had a 95% response rate, offering the study a very solid look at the near future of the specialty.
The 2015 results were a striking contrast to the 2005 report, which examined supply and demand up to 2025. It came to a brighter conclusion: that a relative balance would be maintained. Not so now.
The reasons for this projected imbalance are not overly complicated, and are actually recapitulated in other areas of medicine, said Dr. Battafarano, chief of rheumatology at San Antonio (Tex.) Military Medical Center. Baby Boomer senior physicians are tired of working 70 hours a week, and moving toward retirement in unprecedented numbers. In fact, according to the report, 50% of today’s full-time rheumatologists will retire within the next 15 years, and 80% of those plan to cut back their workload by about 25%.
It’s not that new blood isn’t coming into the profession. In fact, Dr. Bolster said during her presentation, the percentage of internal medicine residents moving into rheumatology will stay stable, at about 4%. But the number isn’t projected to increase as patient demand does, and these new doctors will look and practice very differently than the new doctors of 30 or 40 years ago.
“The majority of medical school graduates now – about 60% – are women,” Dr. Battafarano said. “And they are entering the workforce at a very challenging time of life, simultaneously building careers and families.”
Studies have also demonstrated that women have different practice patterns than men. They tend to spend more time with patients, so they sometimes see fewer in a day. In fact, in its supply assessment, the ACR study characterized women rheumatologists as 0.7 of a full-time equivalent position – an important factor in the projected supply gap.
But the projected workforce shortage does not hinge on the practice patterns of women rheumatologists, Dr. Battafarano cautioned. Part of it is based on his own generation of work-a-holism. In general, young physicians now place more importance on a healthy work-life balance than did his generation, and this he sees as a healthy way to approach a new career.
“I’ll admit it: Baby Boomers are dysfunctionally working too hard, and they miss the importance of a health work/life balance. That’s changing, and that’s a good thing.”
His protégé, Katrina Lawrence-Wolff, DO, agrees. A second-year rheumatology fellow, Dr. Lawrence-Wolff is also expecting her first child. She presented the breakout results focusing on the adult rheumatologist supply/demand picture.
“None of us think working 80 hours a week is a good idea for us personally, or a good way to give good medical care to patients,” she said in an interview. “We would rather have more time with family, more time for volunteering in our community, or to do advocacy work. We want to be flexible, and we do understand that this attitude may change the level of our reimbursement. But we are willing to forgo some salary to become a happier, better-balanced person.”
International medical graduates also affect this snapshot of the future, Dr. Bolster said during her lecture. More than half of new medical graduates are international students, and 17% of those intend to leave the United States after their education is complete.
Shortages will affect all regions, but some more than others
All of these issues are now converging at a time when patient demand is expected to soar. In the United States, about 22.5 million adults and 300,000 children have a rheumatic disease. According to the study, that number will increase by 61% by 2030.
Dr. Lawrence-Wolff used population statistics to really put the numbers needed to care for these patients into perspective. Studies in the United States, Canada, and Europe generally agree that the ideal rheumatologist:patient ratio is somewhere around 2 per 100,000 adults. “In 2005, when we were felt to be balanced in this way, our ratio was 1.67/100,000 patients.”
This ratio will look very different by 2025, she said, and the regional imbalances already seen will be magnified. These regional differences aren’t a surprise, Dr. Lawrence-Wolff noted. They directly reflect the density of academic rheumatology training centers. “Most practicing rheumatologists tend to stay in the region where they received their training,” she said, “so we have more clinicians in areas with more academic centers.”
For example, the Northeast U.S. hosts a highly enriched rheumatologist population, with a rheumatologist:patient ratio of 3.7/100,000. By 2025, this will be reduced to 1.6/100,000 – still acceptable, but more than a 50% decrease.
That same decrease will be much more drastically felt in regions that already have a paucity of rheumatologists. In the Southwest, the current ratio is 1.2/100,000; in 10 years, this will be 0.64/100,000. Even areas that are moderately well supplied now will suffer. Both the Northwest and North Central regions have a ratio of about 1.6/100,000. Both those regions will sink into the range of 0.6-0.5/100,000.
“All regions are going to decline below the 1.67 threshold by 2030,” she said. “Every single one.”
Troubles for pediatric rheumatology workforce
If things are concerning in the world of adult rheumatology, they’re downright alarming in the world of pediatric rheumatology, said Dr. Battafarano, who broke out the pediatric subspecialty results.
These clinicians are already scarce, with only 287 currently practicing full-time in 2015. By 2030, 461 will be needed, but the supply is expected to drop to 231.
The pediatric supply problem starts much earlier in the academic process, he said. For years, about 50% of the slots of pediatric rheumatology fellows have gone unfilled. In this world, recruitment woes will drive supply problems more than retirement. “As a whole, pediatric rheumatologists are younger,” Dr. Battafarano said, with only 32% planning to retire by 2030.
“Recruitment into adult rheumatology isn’t a problem, but on the pediatric side, it’s been very hard to recruit. This isn’t unique to rheumatology; it’s being seen in other pediatric subspecialties as well.”
Reimbursement and work overload are at the root of it, he believes.
“It translates pretty well to income. Reimbursement for chronic diseases, like what we see, is predominately Medicaid. The reimbursement rate and income for subspecialty pediatrics is definitely lower than it is in other academic subspecialties. And I have to think that time spent observing an exhausted, overworked physician isn’t helpful either.”
The mandatory 3-year pediatric rheumatology fellowship may also be a deal killer for some potential recruits, Dr. Battafarano said. The prevailing thought has always been to include a year of academic research in the pediatric track, which extends it beyond the 2-year fellowship that adult rheumatologists experience.
“So you marry the 3-year fellowship with workload, quality of life, student debt, and income and you get a combination that’s just less appealing than some other areas.”
Adjusting that fellowship track is one way to potentially improve the pediatric supply picture, he said. “One of the things I recommended is adding a 2-year clinical track. There are ways to do research that can be folded into a clinical setting that wouldn’t require an entire year. The majority of adult fellowships are 2 years with concurrent research, so there is already a precedent.”
In fact, Dr. Lawrence-Wolff is doing just that, he said. “She will do clinical research that’s integrated into her practice, but her primary role is to learn to be a clinical rheumatologist.”
Ideas for stretching rheumatologic care
Dr. Battafarano had some other practical suggestions for improving recruitment into the field. “We have to offer some incentives. I’d like to see us exploring the potential of loan repayment and visa programs.”
Overall, expanding the musculoskeletal expertise of primary care providers should also be on the table, Dr. Lawrence-Wolff said. “It’s possible that we can help our primary care colleagues extend rheumatology care by treating things like osteoarthritis and gout.”
Rheumatologists also can’t afford to ignore the expanding-role of mid-level providers, she said. “We would like to recruit more nurse practitioners and physician assistants into the specialty. The numbers we hope will go up, even if the percentage remains the same as it is, about 2%-5%.”
Skillfully leveraging these clinicians’ strengths will be the key to successfully employing them.
“We see different ways to utilize them to stretch our care. One suggestion is having them in the clinic to see more patients per day, but also to use them to see lower-level patients so the rheumatologist can take care of the more complex cases.”
They could also serve patients who have multiple regularly scheduled checkups for chronic illness. “If you have a patient who needs to be seen four times a year, the NP or PA can see that person, check the labs and determine if the patent is stable, and doesn’t really need to see the rheumatologist.”
Technology will invariably come into play as well, Dr. Battafarano said.
“We envision this as a multipronged approach that includes telehealth and ‘E-consults,’ although we don’t precisely know what that will look like. But other specialties – and primary care as well – are going through very similar trends here. We are all talking about working with other providers to reach more patients, and telemedicine is a key area of investigation. We really are all in the same boat.”
Finally, Dr. Battafarano urges his fellow senior clinicians to consider severing professional ties gradually. “It’s not just a dearth of bodies we’re facing, but a sudden depletion of valuable experience and clinical wisdom. In my practice, for example, the three of us each have more than 30 years’ experience. That’s close to a century of experience, and two of them want to retire. It’s such a brain-drain on a terrific practice to lose our colleagues overnight.”
For some reason, he said, the locum tenens model has never really caught on in rheumatology, and he’d like to see that idea explored and embraced. It’s a perfect way to keep experienced hands in the mix, both seeing patients and mentoring young rheumatologists, he added.
“Even if we’re in our 60s and 70s, we’re not brain-dead yet. A lot of us want to keep contributing, just not full-time.”
None of the clinicians quoted in this article had any relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The mass retirement of Baby Boomer workhorses, the changing face of a Millennial workforce, and the graying of America will deliver a triple-whammy to rheumatology over the next 15 years: By 2030, the United States will be short 4,700 full-time rheumatologists – just about as many as are currently in practice.
“This is drastic,” Marcy Bolster, MD, said at the annual meeting of the American College of Rheumatology. “We need to take action now or we will not be able to meet the expected increases in patient demand.”
Dr. Bolster, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston, was one of 10 clinicians who discussed the ACR’s massive new project, “The 2015 Workforce Study of Rheumatology Specialists in the United States,” a comprehensive assessment of the current supply of rheumatologists in this country, and a sobering prediction of how many will be needed in the future.
Released at the ACR meeting in Washington, the 2015 survey is the first that ACR has conducted since 2005. The project drew on a number of sources: questionnaires sent out to the entire ACR membership; published research, position papers, and government reports; the Institute of Medicine; and state licensure and National Resident Matching Program data. Four online questionnaires surveyed not only rheumatology clinicians and fellows, but other health professionals, adult and young patients with rheumatic diseases, and the parents of pediatric patients.
The survey response rate of 31% among practicing rheumatologists was not as high as the Committee on Rheumatology Training and Workforce Issues would have liked, said Daniel Battafarano, DO, a study cochair. But fellows had a 95% response rate, offering the study a very solid look at the near future of the specialty.
The 2015 results were a striking contrast to the 2005 report, which examined supply and demand up to 2025. It came to a brighter conclusion: that a relative balance would be maintained. Not so now.
The reasons for this projected imbalance are not overly complicated, and are actually recapitulated in other areas of medicine, said Dr. Battafarano, chief of rheumatology at San Antonio (Tex.) Military Medical Center. Baby Boomer senior physicians are tired of working 70 hours a week, and moving toward retirement in unprecedented numbers. In fact, according to the report, 50% of today’s full-time rheumatologists will retire within the next 15 years, and 80% of those plan to cut back their workload by about 25%.
It’s not that new blood isn’t coming into the profession. In fact, Dr. Bolster said during her presentation, the percentage of internal medicine residents moving into rheumatology will stay stable, at about 4%. But the number isn’t projected to increase as patient demand does, and these new doctors will look and practice very differently than the new doctors of 30 or 40 years ago.
“The majority of medical school graduates now – about 60% – are women,” Dr. Battafarano said. “And they are entering the workforce at a very challenging time of life, simultaneously building careers and families.”
Studies have also demonstrated that women have different practice patterns than men. They tend to spend more time with patients, so they sometimes see fewer in a day. In fact, in its supply assessment, the ACR study characterized women rheumatologists as 0.7 of a full-time equivalent position – an important factor in the projected supply gap.
But the projected workforce shortage does not hinge on the practice patterns of women rheumatologists, Dr. Battafarano cautioned. Part of it is based on his own generation of work-a-holism. In general, young physicians now place more importance on a healthy work-life balance than did his generation, and this he sees as a healthy way to approach a new career.
“I’ll admit it: Baby Boomers are dysfunctionally working too hard, and they miss the importance of a health work/life balance. That’s changing, and that’s a good thing.”
His protégé, Katrina Lawrence-Wolff, DO, agrees. A second-year rheumatology fellow, Dr. Lawrence-Wolff is also expecting her first child. She presented the breakout results focusing on the adult rheumatologist supply/demand picture.
“None of us think working 80 hours a week is a good idea for us personally, or a good way to give good medical care to patients,” she said in an interview. “We would rather have more time with family, more time for volunteering in our community, or to do advocacy work. We want to be flexible, and we do understand that this attitude may change the level of our reimbursement. But we are willing to forgo some salary to become a happier, better-balanced person.”
International medical graduates also affect this snapshot of the future, Dr. Bolster said during her lecture. More than half of new medical graduates are international students, and 17% of those intend to leave the United States after their education is complete.
Shortages will affect all regions, but some more than others
All of these issues are now converging at a time when patient demand is expected to soar. In the United States, about 22.5 million adults and 300,000 children have a rheumatic disease. According to the study, that number will increase by 61% by 2030.
Dr. Lawrence-Wolff used population statistics to really put the numbers needed to care for these patients into perspective. Studies in the United States, Canada, and Europe generally agree that the ideal rheumatologist:patient ratio is somewhere around 2 per 100,000 adults. “In 2005, when we were felt to be balanced in this way, our ratio was 1.67/100,000 patients.”
This ratio will look very different by 2025, she said, and the regional imbalances already seen will be magnified. These regional differences aren’t a surprise, Dr. Lawrence-Wolff noted. They directly reflect the density of academic rheumatology training centers. “Most practicing rheumatologists tend to stay in the region where they received their training,” she said, “so we have more clinicians in areas with more academic centers.”
For example, the Northeast U.S. hosts a highly enriched rheumatologist population, with a rheumatologist:patient ratio of 3.7/100,000. By 2025, this will be reduced to 1.6/100,000 – still acceptable, but more than a 50% decrease.
That same decrease will be much more drastically felt in regions that already have a paucity of rheumatologists. In the Southwest, the current ratio is 1.2/100,000; in 10 years, this will be 0.64/100,000. Even areas that are moderately well supplied now will suffer. Both the Northwest and North Central regions have a ratio of about 1.6/100,000. Both those regions will sink into the range of 0.6-0.5/100,000.
“All regions are going to decline below the 1.67 threshold by 2030,” she said. “Every single one.”
Troubles for pediatric rheumatology workforce
If things are concerning in the world of adult rheumatology, they’re downright alarming in the world of pediatric rheumatology, said Dr. Battafarano, who broke out the pediatric subspecialty results.
These clinicians are already scarce, with only 287 currently practicing full-time in 2015. By 2030, 461 will be needed, but the supply is expected to drop to 231.
The pediatric supply problem starts much earlier in the academic process, he said. For years, about 50% of the slots of pediatric rheumatology fellows have gone unfilled. In this world, recruitment woes will drive supply problems more than retirement. “As a whole, pediatric rheumatologists are younger,” Dr. Battafarano said, with only 32% planning to retire by 2030.
“Recruitment into adult rheumatology isn’t a problem, but on the pediatric side, it’s been very hard to recruit. This isn’t unique to rheumatology; it’s being seen in other pediatric subspecialties as well.”
Reimbursement and work overload are at the root of it, he believes.
“It translates pretty well to income. Reimbursement for chronic diseases, like what we see, is predominately Medicaid. The reimbursement rate and income for subspecialty pediatrics is definitely lower than it is in other academic subspecialties. And I have to think that time spent observing an exhausted, overworked physician isn’t helpful either.”
The mandatory 3-year pediatric rheumatology fellowship may also be a deal killer for some potential recruits, Dr. Battafarano said. The prevailing thought has always been to include a year of academic research in the pediatric track, which extends it beyond the 2-year fellowship that adult rheumatologists experience.
“So you marry the 3-year fellowship with workload, quality of life, student debt, and income and you get a combination that’s just less appealing than some other areas.”
Adjusting that fellowship track is one way to potentially improve the pediatric supply picture, he said. “One of the things I recommended is adding a 2-year clinical track. There are ways to do research that can be folded into a clinical setting that wouldn’t require an entire year. The majority of adult fellowships are 2 years with concurrent research, so there is already a precedent.”
In fact, Dr. Lawrence-Wolff is doing just that, he said. “She will do clinical research that’s integrated into her practice, but her primary role is to learn to be a clinical rheumatologist.”
Ideas for stretching rheumatologic care
Dr. Battafarano had some other practical suggestions for improving recruitment into the field. “We have to offer some incentives. I’d like to see us exploring the potential of loan repayment and visa programs.”
Overall, expanding the musculoskeletal expertise of primary care providers should also be on the table, Dr. Lawrence-Wolff said. “It’s possible that we can help our primary care colleagues extend rheumatology care by treating things like osteoarthritis and gout.”
Rheumatologists also can’t afford to ignore the expanding-role of mid-level providers, she said. “We would like to recruit more nurse practitioners and physician assistants into the specialty. The numbers we hope will go up, even if the percentage remains the same as it is, about 2%-5%.”
Skillfully leveraging these clinicians’ strengths will be the key to successfully employing them.
“We see different ways to utilize them to stretch our care. One suggestion is having them in the clinic to see more patients per day, but also to use them to see lower-level patients so the rheumatologist can take care of the more complex cases.”
They could also serve patients who have multiple regularly scheduled checkups for chronic illness. “If you have a patient who needs to be seen four times a year, the NP or PA can see that person, check the labs and determine if the patent is stable, and doesn’t really need to see the rheumatologist.”
Technology will invariably come into play as well, Dr. Battafarano said.
“We envision this as a multipronged approach that includes telehealth and ‘E-consults,’ although we don’t precisely know what that will look like. But other specialties – and primary care as well – are going through very similar trends here. We are all talking about working with other providers to reach more patients, and telemedicine is a key area of investigation. We really are all in the same boat.”
Finally, Dr. Battafarano urges his fellow senior clinicians to consider severing professional ties gradually. “It’s not just a dearth of bodies we’re facing, but a sudden depletion of valuable experience and clinical wisdom. In my practice, for example, the three of us each have more than 30 years’ experience. That’s close to a century of experience, and two of them want to retire. It’s such a brain-drain on a terrific practice to lose our colleagues overnight.”
For some reason, he said, the locum tenens model has never really caught on in rheumatology, and he’d like to see that idea explored and embraced. It’s a perfect way to keep experienced hands in the mix, both seeing patients and mentoring young rheumatologists, he added.
“Even if we’re in our 60s and 70s, we’re not brain-dead yet. A lot of us want to keep contributing, just not full-time.”
None of the clinicians quoted in this article had any relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
VIDEO: TNF inhibitors don’t boost cancer risk in JIA
WASHINGTON – Tumor necrosis factor inhibitors don’t appear to confer any additional cancer risk upon children with juvenile idiopathic arthritis above the increased incidence of cancer that comes hand in hand with the disease itself.
In 2009, the drugs came under suspicion of boosting the already-known increased cancer risk in these patients, Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology. But the large database review that he conducted with his colleagues doesn’t validate those fears.
“I feel fairly confident now that I can stand in front of parents and say that we can treat their child effectively without putting that child at an even higher risk of a malignancy,” he said in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Thomas J.A. Lehman, MD, chief of pediatric rheumatology at the Hospital for Special Surgery, New York, and professor of clinical pediatrics at Weill Cornell Medical College in New York, agreed.
“This study again indicates that anti-TNF therapy does not increase the risk of cancer for children with arthritis,” he said in an interview. “Although children with rheumatic diseases have a small increased background risk of malignancies, this is independent of the use of anti-TNF therapies. For physicians who have cared for children in the era when we did not have anti-TNF therapies available, it is clear that any minor risks associated with these medications are far outweighed by their dramatic benefits.”
In the last few years, five large studies have found that children with juvenile idiopathic arthritis (JIA) have a two- to sixfold increased malignancy risk, compared with the general pediatric population. However, only two of those studies included children taking TNF inhibitors, who comprised just 2% and 9% of those study populations.
In 2009, based on voluntary adverse event reporting, the Food and Drug Administration issued a black box warning on TNF inhibitors, citing a possibly increased risk of cancer in children and adolescents who received the drugs for JIA, inflammatory bowel diseases, and other inflammatory diseases.
Shortly thereafter, a report identified a fivefold increase in the risk of childhood lymphoma associated with the medications (Arthritis Rheum. 2010 Aug;62[8]:2517-24). Other studies have not borne this out, but the boxed warning stands.
To further explore the association, Dr. Beukelman of the University of Alabama, Birmingham, and his associates examined billing data from two large national billing databases: the National U.S. Truven MarketScan claims database and Medicaid billing records. Together, the databases contained information on 27,000 children with JIA who received a prescription for a TNF inhibitor any time during 2000-2014. Cancer rates in this population were compared with those seen in a cohort of 2.64 million children with attention-deficit/hyperactivity disorder who were included in the national Surveillance, Epidemiology, and End Results (SEER) database. The investigators chose individuals with ADHD as a control group because of ADHD’s chronicity and lack of any association with cancer risk.
Dr. Beukelman also performed a within-group analysis on the JIA patients, comparing cancer rates among those treated with a TNF inhibitor and with methotrexate. The mean follow-up for patients who took TNF inhibitors was 4 years (median of 1.4 years), but there were a full 14 years of data for some patients.
Among the controls, with more than 4 million person-years of follow-up, there were 727 cases of any malignancy – a standardized incident rate (SIR) of 1.03. Among all children with JIA, with more than 52,000 person-years of follow-up, there were 20 malignancies. The SEER database predicted eight among a sex- and age-matched cohort of healthy children. This translated to an SIR of 2.4. This represents the baseline increased risk of cancer conferred by JIA alone.
Nine malignancies occurred in the subgroup of children with JIA who took no medications. The SEER expectation among this group was 3.8 cancers, also translating to an SIR of 2.4
One malignancy occurred in the group treated with methotrexate only. Among these children, the SEER expected number was 1.9; the SIR in this group was 0.53.
Seven malignancies occurred among children who took TNF inhibitors, translating to an SIR of 2.9. Six occurred in children who took a TNF inhibitor in combination with or without methotrexate – an SIR of 3.0.
A final group consisted of children who took a wide range of other medications used in JIA (abatacept, anakinra, canakinumab, rilonacept, rituximab, tocilizumab, ustekinumab, tofacitinib, azathioprine, cyclosporine, gold, leflunomide, mycophenolate mofetil, tacrolimus, thalidomide, lenalidomide). This group also included patients who may or may not have taken methotrexate or a TNF inhibitor. Among these, there were four cancers when the SEER expected number was 0.7. This translated to an SIR of almost 6 – a surprising finding, Dr. Beukelman said. But since there were only four cancers and the group was exposed to so many different medications, it’s tough to know what that means, if anything, Dr. Beukelman said.
“There’s a lot to unpack here. The treatment paradigm for JIA is methotrexate followed by a TNF inhibitor if that’s ineffective. So these kids were on all of these more uncommon drugs,” suggesting that neither TNF inhibition nor methotrexate worked. “Some of these patients might actually have had systemic arthritis, Still’s disease, which is a completely separate thing, and we don’t know anything about the risk of malignancy in that. They might have an even higher rate of malignancies at baseline due to having worse disease, or uncontrolled inflammation. It is concerning, but I think it probably speaks to the fact that these patients are difficult to treat and probably at higher risk.”
Dr. Beukelman didn’t specifically break out the types and numbers of cancer, except to say that 3 of the 20 were lymphomas. The rest were leukemias and brain cancers – a finding that reflects the general pattern of childhood malignancies.
“Unfortunately, the most common childhood cancers are lymphomas, leukemias, and brain cancers, and that is what we saw in this study as well,” he said.
The study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Beukelman noted that he has received consulting fees from Novartis, Genetech/Roche, and UCB.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Tumor necrosis factor inhibitors don’t appear to confer any additional cancer risk upon children with juvenile idiopathic arthritis above the increased incidence of cancer that comes hand in hand with the disease itself.
In 2009, the drugs came under suspicion of boosting the already-known increased cancer risk in these patients, Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology. But the large database review that he conducted with his colleagues doesn’t validate those fears.
“I feel fairly confident now that I can stand in front of parents and say that we can treat their child effectively without putting that child at an even higher risk of a malignancy,” he said in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Thomas J.A. Lehman, MD, chief of pediatric rheumatology at the Hospital for Special Surgery, New York, and professor of clinical pediatrics at Weill Cornell Medical College in New York, agreed.
“This study again indicates that anti-TNF therapy does not increase the risk of cancer for children with arthritis,” he said in an interview. “Although children with rheumatic diseases have a small increased background risk of malignancies, this is independent of the use of anti-TNF therapies. For physicians who have cared for children in the era when we did not have anti-TNF therapies available, it is clear that any minor risks associated with these medications are far outweighed by their dramatic benefits.”
In the last few years, five large studies have found that children with juvenile idiopathic arthritis (JIA) have a two- to sixfold increased malignancy risk, compared with the general pediatric population. However, only two of those studies included children taking TNF inhibitors, who comprised just 2% and 9% of those study populations.
In 2009, based on voluntary adverse event reporting, the Food and Drug Administration issued a black box warning on TNF inhibitors, citing a possibly increased risk of cancer in children and adolescents who received the drugs for JIA, inflammatory bowel diseases, and other inflammatory diseases.
Shortly thereafter, a report identified a fivefold increase in the risk of childhood lymphoma associated with the medications (Arthritis Rheum. 2010 Aug;62[8]:2517-24). Other studies have not borne this out, but the boxed warning stands.
To further explore the association, Dr. Beukelman of the University of Alabama, Birmingham, and his associates examined billing data from two large national billing databases: the National U.S. Truven MarketScan claims database and Medicaid billing records. Together, the databases contained information on 27,000 children with JIA who received a prescription for a TNF inhibitor any time during 2000-2014. Cancer rates in this population were compared with those seen in a cohort of 2.64 million children with attention-deficit/hyperactivity disorder who were included in the national Surveillance, Epidemiology, and End Results (SEER) database. The investigators chose individuals with ADHD as a control group because of ADHD’s chronicity and lack of any association with cancer risk.
Dr. Beukelman also performed a within-group analysis on the JIA patients, comparing cancer rates among those treated with a TNF inhibitor and with methotrexate. The mean follow-up for patients who took TNF inhibitors was 4 years (median of 1.4 years), but there were a full 14 years of data for some patients.
Among the controls, with more than 4 million person-years of follow-up, there were 727 cases of any malignancy – a standardized incident rate (SIR) of 1.03. Among all children with JIA, with more than 52,000 person-years of follow-up, there were 20 malignancies. The SEER database predicted eight among a sex- and age-matched cohort of healthy children. This translated to an SIR of 2.4. This represents the baseline increased risk of cancer conferred by JIA alone.
Nine malignancies occurred in the subgroup of children with JIA who took no medications. The SEER expectation among this group was 3.8 cancers, also translating to an SIR of 2.4
One malignancy occurred in the group treated with methotrexate only. Among these children, the SEER expected number was 1.9; the SIR in this group was 0.53.
Seven malignancies occurred among children who took TNF inhibitors, translating to an SIR of 2.9. Six occurred in children who took a TNF inhibitor in combination with or without methotrexate – an SIR of 3.0.
A final group consisted of children who took a wide range of other medications used in JIA (abatacept, anakinra, canakinumab, rilonacept, rituximab, tocilizumab, ustekinumab, tofacitinib, azathioprine, cyclosporine, gold, leflunomide, mycophenolate mofetil, tacrolimus, thalidomide, lenalidomide). This group also included patients who may or may not have taken methotrexate or a TNF inhibitor. Among these, there were four cancers when the SEER expected number was 0.7. This translated to an SIR of almost 6 – a surprising finding, Dr. Beukelman said. But since there were only four cancers and the group was exposed to so many different medications, it’s tough to know what that means, if anything, Dr. Beukelman said.
“There’s a lot to unpack here. The treatment paradigm for JIA is methotrexate followed by a TNF inhibitor if that’s ineffective. So these kids were on all of these more uncommon drugs,” suggesting that neither TNF inhibition nor methotrexate worked. “Some of these patients might actually have had systemic arthritis, Still’s disease, which is a completely separate thing, and we don’t know anything about the risk of malignancy in that. They might have an even higher rate of malignancies at baseline due to having worse disease, or uncontrolled inflammation. It is concerning, but I think it probably speaks to the fact that these patients are difficult to treat and probably at higher risk.”
Dr. Beukelman didn’t specifically break out the types and numbers of cancer, except to say that 3 of the 20 were lymphomas. The rest were leukemias and brain cancers – a finding that reflects the general pattern of childhood malignancies.
“Unfortunately, the most common childhood cancers are lymphomas, leukemias, and brain cancers, and that is what we saw in this study as well,” he said.
The study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Beukelman noted that he has received consulting fees from Novartis, Genetech/Roche, and UCB.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Tumor necrosis factor inhibitors don’t appear to confer any additional cancer risk upon children with juvenile idiopathic arthritis above the increased incidence of cancer that comes hand in hand with the disease itself.
In 2009, the drugs came under suspicion of boosting the already-known increased cancer risk in these patients, Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology. But the large database review that he conducted with his colleagues doesn’t validate those fears.
“I feel fairly confident now that I can stand in front of parents and say that we can treat their child effectively without putting that child at an even higher risk of a malignancy,” he said in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Thomas J.A. Lehman, MD, chief of pediatric rheumatology at the Hospital for Special Surgery, New York, and professor of clinical pediatrics at Weill Cornell Medical College in New York, agreed.
“This study again indicates that anti-TNF therapy does not increase the risk of cancer for children with arthritis,” he said in an interview. “Although children with rheumatic diseases have a small increased background risk of malignancies, this is independent of the use of anti-TNF therapies. For physicians who have cared for children in the era when we did not have anti-TNF therapies available, it is clear that any minor risks associated with these medications are far outweighed by their dramatic benefits.”
In the last few years, five large studies have found that children with juvenile idiopathic arthritis (JIA) have a two- to sixfold increased malignancy risk, compared with the general pediatric population. However, only two of those studies included children taking TNF inhibitors, who comprised just 2% and 9% of those study populations.
In 2009, based on voluntary adverse event reporting, the Food and Drug Administration issued a black box warning on TNF inhibitors, citing a possibly increased risk of cancer in children and adolescents who received the drugs for JIA, inflammatory bowel diseases, and other inflammatory diseases.
Shortly thereafter, a report identified a fivefold increase in the risk of childhood lymphoma associated with the medications (Arthritis Rheum. 2010 Aug;62[8]:2517-24). Other studies have not borne this out, but the boxed warning stands.
To further explore the association, Dr. Beukelman of the University of Alabama, Birmingham, and his associates examined billing data from two large national billing databases: the National U.S. Truven MarketScan claims database and Medicaid billing records. Together, the databases contained information on 27,000 children with JIA who received a prescription for a TNF inhibitor any time during 2000-2014. Cancer rates in this population were compared with those seen in a cohort of 2.64 million children with attention-deficit/hyperactivity disorder who were included in the national Surveillance, Epidemiology, and End Results (SEER) database. The investigators chose individuals with ADHD as a control group because of ADHD’s chronicity and lack of any association with cancer risk.
Dr. Beukelman also performed a within-group analysis on the JIA patients, comparing cancer rates among those treated with a TNF inhibitor and with methotrexate. The mean follow-up for patients who took TNF inhibitors was 4 years (median of 1.4 years), but there were a full 14 years of data for some patients.
Among the controls, with more than 4 million person-years of follow-up, there were 727 cases of any malignancy – a standardized incident rate (SIR) of 1.03. Among all children with JIA, with more than 52,000 person-years of follow-up, there were 20 malignancies. The SEER database predicted eight among a sex- and age-matched cohort of healthy children. This translated to an SIR of 2.4. This represents the baseline increased risk of cancer conferred by JIA alone.
Nine malignancies occurred in the subgroup of children with JIA who took no medications. The SEER expectation among this group was 3.8 cancers, also translating to an SIR of 2.4
One malignancy occurred in the group treated with methotrexate only. Among these children, the SEER expected number was 1.9; the SIR in this group was 0.53.
Seven malignancies occurred among children who took TNF inhibitors, translating to an SIR of 2.9. Six occurred in children who took a TNF inhibitor in combination with or without methotrexate – an SIR of 3.0.
A final group consisted of children who took a wide range of other medications used in JIA (abatacept, anakinra, canakinumab, rilonacept, rituximab, tocilizumab, ustekinumab, tofacitinib, azathioprine, cyclosporine, gold, leflunomide, mycophenolate mofetil, tacrolimus, thalidomide, lenalidomide). This group also included patients who may or may not have taken methotrexate or a TNF inhibitor. Among these, there were four cancers when the SEER expected number was 0.7. This translated to an SIR of almost 6 – a surprising finding, Dr. Beukelman said. But since there were only four cancers and the group was exposed to so many different medications, it’s tough to know what that means, if anything, Dr. Beukelman said.
“There’s a lot to unpack here. The treatment paradigm for JIA is methotrexate followed by a TNF inhibitor if that’s ineffective. So these kids were on all of these more uncommon drugs,” suggesting that neither TNF inhibition nor methotrexate worked. “Some of these patients might actually have had systemic arthritis, Still’s disease, which is a completely separate thing, and we don’t know anything about the risk of malignancy in that. They might have an even higher rate of malignancies at baseline due to having worse disease, or uncontrolled inflammation. It is concerning, but I think it probably speaks to the fact that these patients are difficult to treat and probably at higher risk.”
Dr. Beukelman didn’t specifically break out the types and numbers of cancer, except to say that 3 of the 20 were lymphomas. The rest were leukemias and brain cancers – a finding that reflects the general pattern of childhood malignancies.
“Unfortunately, the most common childhood cancers are lymphomas, leukemias, and brain cancers, and that is what we saw in this study as well,” he said.
The study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Beukelman noted that he has received consulting fees from Novartis, Genetech/Roche, and UCB.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Children with JIA were about twice as likely to get cancer as the general population, regardless of whether they took a TNF inhibitor.
Data source: A database review comprising 27,000 patients and 2.5 million controls.
Disclosures: The study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Beukelman noted that he has received consulting fees from Novartis, Genetech/Roche, and UCB.
Hypervirulent Mycobacterium clone infecting cystic fibrosis patients worldwide
A recently evolved strain of Mycobacterium is circulating in hospitals worldwide, causing nearly impossible-to-treat lung infections among patients with cystic fibrosis.
A genome-wide study has determined that Mycobacterium abscessus is not transmitted through soil and water, as once thought, but is a nosocomial infection transmitted person to person through droplet and surface contamination, Andres Floto, MD, reported in Science (2016 Nov 11;354[6313]:751-7).
“The bug initially seems to have entered the patient population from the environment, but we think it has recently evolved to become capable of jumping from patient to patient, getting more virulent as it does so,” Dr. Floto of the University of Cambridge, England, wrote in a press statement.
The path of global transmission is not yet entirely clear, the authors noted. But since it first appeared, around 1978, M. abscessus has spread globally, strongly suggesting that asymptomatic carriers may be one source of transmission.
“We found no evidence of cystic fibrosis patients or of equipment moving between centers in different countries, indicating that the global spread of M. abscessus may be driven by alternative human, zoonotic, or environmental vectors of transmission,” the researchers wrote.
The team conducted whole-genome sequencing on 1,080 samples of M. abscessus obtained from 517 cystic fibrosis patients in clinics and hospitals within the United States, the United Kingdom, Europe, and Australia. They identified three subspecies, some of which contained nearly genetically identical strains, “suggesting widespread transmission of circulating clones within the global cystic fibrosis patient community.”
Most patients (74%) were infected with these genetically identical strains despite their diverse geographic locations. The isolates were amazingly similar, the authors noted: 90% differed by less than 20 single nucleotide polymorphisms.
Using these strains, the researchers were able to construct several possible transmission chains in most of the cystic fibrosis centers included in the study. The three dominant circulating clones were all observed in the United States, European, and Australian samples, indicating transcontinental transmission.
“We also detected numerous examples of identical or near-identical isolates infecting groups of patients in different cystic fibrosis centers and, indeed, across different countries, indicating the recent global spread of M. abscessus clones throughout the international cystic fibrosis patient community.”
The team also determined that the common ancestor of these strains probably emerged around 1978.
Another sequencing series tracked specific isolates among individual patients. This strongly suggests person-to-person transmission. Adding this to their previous work on M. abscessus transmission, the authors postulated that spread was probably by surface contamination by droplet contamination and by cough aerosol from infected patients.
The team then looked at clinical outcomes associated with the bacteria and treatment with amikacin and macrolides – antibiotics typically used to fight this very-challenging infection. “We did observe increased rates of chronic infection in individuals,” infected with the clones, which were resistant to both those medications, they said.
In immunodeficient mice, the strains were more likely to cause granulomatous and inflammatory lung changes. And the bacteria tended to survive even after being consumed by macrophages, “suggesting that the establishment of transmission chains may have permitted multiple rounds of within-host genetic adaptation to allow M. abscessus to evolve from an environmental organism to a true lung pathogen.”
The research was funded by the Wellcome Trust and the Cystic Fibrosis Trust in the United Kingdom. There were no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
Approximately 30,000 American adults, children, and infants have cystic fibrosis. Nontuberculous mycobacteria (NTM) are ubiquitous environmental microorganisms, and it has been known for some time that these infections can be transmitted person to person. Any patient, actually, who has preexisting lung disease – and especially those with poor mucociliary clearance – are at risk for a nontuberculous mycobacterial infection. This type of lung infection also can be difficult to diagnose and hard to treat. The U.S. Cystic Fibrosis Foundation in conjunction with the European Cystic Fibrosis Society has developed consensus guidelines for infection control, evaluation, and treatment of this problem. This executive summary was published last January (Floto et al. Thorax.2016;71:i1-i22).
At our center, in addition to the standard contact precautions we use for every CF patient, patients with confirmed NTM infections are seen at every clinic visit in an airborne infection isolation room. We also require all CF patients to wear an isolation mask when entering the hospital or clinic facility, when going to a laboratory, or even when going to the bathroom down the hall. Finally, we stress the significant importance of good hand hygiene.
Susan Millard, MD, FCCP, is a pediatric pulmonologist with Spectrum Health/Butterworth Hospital in Grand Rapids, Mich.
Approximately 30,000 American adults, children, and infants have cystic fibrosis. Nontuberculous mycobacteria (NTM) are ubiquitous environmental microorganisms, and it has been known for some time that these infections can be transmitted person to person. Any patient, actually, who has preexisting lung disease – and especially those with poor mucociliary clearance – are at risk for a nontuberculous mycobacterial infection. This type of lung infection also can be difficult to diagnose and hard to treat. The U.S. Cystic Fibrosis Foundation in conjunction with the European Cystic Fibrosis Society has developed consensus guidelines for infection control, evaluation, and treatment of this problem. This executive summary was published last January (Floto et al. Thorax.2016;71:i1-i22).
At our center, in addition to the standard contact precautions we use for every CF patient, patients with confirmed NTM infections are seen at every clinic visit in an airborne infection isolation room. We also require all CF patients to wear an isolation mask when entering the hospital or clinic facility, when going to a laboratory, or even when going to the bathroom down the hall. Finally, we stress the significant importance of good hand hygiene.
Susan Millard, MD, FCCP, is a pediatric pulmonologist with Spectrum Health/Butterworth Hospital in Grand Rapids, Mich.
Approximately 30,000 American adults, children, and infants have cystic fibrosis. Nontuberculous mycobacteria (NTM) are ubiquitous environmental microorganisms, and it has been known for some time that these infections can be transmitted person to person. Any patient, actually, who has preexisting lung disease – and especially those with poor mucociliary clearance – are at risk for a nontuberculous mycobacterial infection. This type of lung infection also can be difficult to diagnose and hard to treat. The U.S. Cystic Fibrosis Foundation in conjunction with the European Cystic Fibrosis Society has developed consensus guidelines for infection control, evaluation, and treatment of this problem. This executive summary was published last January (Floto et al. Thorax.2016;71:i1-i22).
At our center, in addition to the standard contact precautions we use for every CF patient, patients with confirmed NTM infections are seen at every clinic visit in an airborne infection isolation room. We also require all CF patients to wear an isolation mask when entering the hospital or clinic facility, when going to a laboratory, or even when going to the bathroom down the hall. Finally, we stress the significant importance of good hand hygiene.
Susan Millard, MD, FCCP, is a pediatric pulmonologist with Spectrum Health/Butterworth Hospital in Grand Rapids, Mich.
A recently evolved strain of Mycobacterium is circulating in hospitals worldwide, causing nearly impossible-to-treat lung infections among patients with cystic fibrosis.
A genome-wide study has determined that Mycobacterium abscessus is not transmitted through soil and water, as once thought, but is a nosocomial infection transmitted person to person through droplet and surface contamination, Andres Floto, MD, reported in Science (2016 Nov 11;354[6313]:751-7).
“The bug initially seems to have entered the patient population from the environment, but we think it has recently evolved to become capable of jumping from patient to patient, getting more virulent as it does so,” Dr. Floto of the University of Cambridge, England, wrote in a press statement.
The path of global transmission is not yet entirely clear, the authors noted. But since it first appeared, around 1978, M. abscessus has spread globally, strongly suggesting that asymptomatic carriers may be one source of transmission.
“We found no evidence of cystic fibrosis patients or of equipment moving between centers in different countries, indicating that the global spread of M. abscessus may be driven by alternative human, zoonotic, or environmental vectors of transmission,” the researchers wrote.
The team conducted whole-genome sequencing on 1,080 samples of M. abscessus obtained from 517 cystic fibrosis patients in clinics and hospitals within the United States, the United Kingdom, Europe, and Australia. They identified three subspecies, some of which contained nearly genetically identical strains, “suggesting widespread transmission of circulating clones within the global cystic fibrosis patient community.”
Most patients (74%) were infected with these genetically identical strains despite their diverse geographic locations. The isolates were amazingly similar, the authors noted: 90% differed by less than 20 single nucleotide polymorphisms.
Using these strains, the researchers were able to construct several possible transmission chains in most of the cystic fibrosis centers included in the study. The three dominant circulating clones were all observed in the United States, European, and Australian samples, indicating transcontinental transmission.
“We also detected numerous examples of identical or near-identical isolates infecting groups of patients in different cystic fibrosis centers and, indeed, across different countries, indicating the recent global spread of M. abscessus clones throughout the international cystic fibrosis patient community.”
The team also determined that the common ancestor of these strains probably emerged around 1978.
Another sequencing series tracked specific isolates among individual patients. This strongly suggests person-to-person transmission. Adding this to their previous work on M. abscessus transmission, the authors postulated that spread was probably by surface contamination by droplet contamination and by cough aerosol from infected patients.
The team then looked at clinical outcomes associated with the bacteria and treatment with amikacin and macrolides – antibiotics typically used to fight this very-challenging infection. “We did observe increased rates of chronic infection in individuals,” infected with the clones, which were resistant to both those medications, they said.
In immunodeficient mice, the strains were more likely to cause granulomatous and inflammatory lung changes. And the bacteria tended to survive even after being consumed by macrophages, “suggesting that the establishment of transmission chains may have permitted multiple rounds of within-host genetic adaptation to allow M. abscessus to evolve from an environmental organism to a true lung pathogen.”
The research was funded by the Wellcome Trust and the Cystic Fibrosis Trust in the United Kingdom. There were no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
A recently evolved strain of Mycobacterium is circulating in hospitals worldwide, causing nearly impossible-to-treat lung infections among patients with cystic fibrosis.
A genome-wide study has determined that Mycobacterium abscessus is not transmitted through soil and water, as once thought, but is a nosocomial infection transmitted person to person through droplet and surface contamination, Andres Floto, MD, reported in Science (2016 Nov 11;354[6313]:751-7).
“The bug initially seems to have entered the patient population from the environment, but we think it has recently evolved to become capable of jumping from patient to patient, getting more virulent as it does so,” Dr. Floto of the University of Cambridge, England, wrote in a press statement.
The path of global transmission is not yet entirely clear, the authors noted. But since it first appeared, around 1978, M. abscessus has spread globally, strongly suggesting that asymptomatic carriers may be one source of transmission.
“We found no evidence of cystic fibrosis patients or of equipment moving between centers in different countries, indicating that the global spread of M. abscessus may be driven by alternative human, zoonotic, or environmental vectors of transmission,” the researchers wrote.
The team conducted whole-genome sequencing on 1,080 samples of M. abscessus obtained from 517 cystic fibrosis patients in clinics and hospitals within the United States, the United Kingdom, Europe, and Australia. They identified three subspecies, some of which contained nearly genetically identical strains, “suggesting widespread transmission of circulating clones within the global cystic fibrosis patient community.”
Most patients (74%) were infected with these genetically identical strains despite their diverse geographic locations. The isolates were amazingly similar, the authors noted: 90% differed by less than 20 single nucleotide polymorphisms.
Using these strains, the researchers were able to construct several possible transmission chains in most of the cystic fibrosis centers included in the study. The three dominant circulating clones were all observed in the United States, European, and Australian samples, indicating transcontinental transmission.
“We also detected numerous examples of identical or near-identical isolates infecting groups of patients in different cystic fibrosis centers and, indeed, across different countries, indicating the recent global spread of M. abscessus clones throughout the international cystic fibrosis patient community.”
The team also determined that the common ancestor of these strains probably emerged around 1978.
Another sequencing series tracked specific isolates among individual patients. This strongly suggests person-to-person transmission. Adding this to their previous work on M. abscessus transmission, the authors postulated that spread was probably by surface contamination by droplet contamination and by cough aerosol from infected patients.
The team then looked at clinical outcomes associated with the bacteria and treatment with amikacin and macrolides – antibiotics typically used to fight this very-challenging infection. “We did observe increased rates of chronic infection in individuals,” infected with the clones, which were resistant to both those medications, they said.
In immunodeficient mice, the strains were more likely to cause granulomatous and inflammatory lung changes. And the bacteria tended to survive even after being consumed by macrophages, “suggesting that the establishment of transmission chains may have permitted multiple rounds of within-host genetic adaptation to allow M. abscessus to evolve from an environmental organism to a true lung pathogen.”
The research was funded by the Wellcome Trust and the Cystic Fibrosis Trust in the United Kingdom. There were no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
FROM SCIENCE
Key clinical point:
Major finding: Hypervirulent clones of Mycobacterium abscessus with apparent person-to-person transmission, are appearing in cystic fibrosis centers worldwide.
Data source: Gene sequencing was performed on 1,080 samples of M. abscessus from the United States, the United Kingdom, Europe, and Australia.
Disclosures: The research was funded by the Wellcome Trust and the Cystic Fibrosis Trust in the United Kingdom.
VIDEO: Celecoxib just as safe as naproxen or ibuprofen in OA and RA
WASHINGTON – Celecoxib conferred a 53% decreased risk of overall mortality upon patients with rheumatoid arthritis when compared with naproxen – a surprise finding in a subanalysis of the newly released PRECISION study of anti-inflammatory drugs in arthritis.
But it’s tough to know what to make of the difference, according to the investigators at the annual meeting of the American College of Rheumatology. Although statistically significant, the mortality finding was based on just 45 events: 30 among those taking naproxen and 15 among those taking celecoxib.
“While I would say we were surprised to see this, we really can’t say what it means – or if it means anything,” primary investigator Daniel Solomon, MD, said in an interview. “This is a finding we will continue to investigate and look at, but at this point it’s not enough to base any prescribing decisions on.”
PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen) enrolled more than 24,000 patients with osteoarthritis or rheumatoid arthritis. They were randomized to celecoxib, naproxen, or ibuprofen for a mean of 20 months, with an additional mean follow-up of 34 months.
The primary outcome was the first occurrence of an adverse event from the Antiplatelet Trialists Collaboration criteria (APTC; death from cardiovascular causes, nonfatal heart attack, or nonfatal stroke). Secondary outcomes included:
• Major cardiovascular events (heart attack, stroke, cardiovascular death, revascularization, and hospitalization for unstable angina or transient ischemic attack).
• Renal events (acute kidney injury, including hospitalization for renal failure).
• Gastrointestinal events (hemorrhage, perforation, gastroduodenal ulcer, anemia of gastrointestinal origin, and gastric outlet obstruction.
The main PRECISION findings were released Nov. 13 at the annual meeting of the American Heart Association and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2016 Nov 13. doi: 10.1056/NEJMoa1611593).
Overall, celecoxib was just as safe as were the other drugs on the APTC endpoint, which occurred in about 2% of each groups. The study found significantly decreased risks of both GI and renal events with celecoxib, compared with either naproxen or ibuprofen. Celecoxib and naproxen were equivalent with regard to GI and renal events.
The subanalysis, released at the ACR meeting, adds important disease-specific context to the overall PRECISION results.
“We wanted to really delve into the subtle differences in how these two patient groups responded to these medications,” said PRECISION coinvestigator Elaine Husni, MD, vice chair of the department of rheumatic and immunologic diseases at the Cleveland Clinic.
“We learned that each of these NSAIDs has a unique safety profile that can be different in different groups. And in general, celecoxib seems less risky than the others.”
The cohort subanalysis broke down these endpoints among 21,600 patients with osteoarthritis and 2,400 with rheumatoid arthritis.
Among patients with osteoarthritis, celecoxib was also associated with a 16% decreased risk of a major adverse cardiovascular event, compared with ibuprofen. GI events were also less likely in this group: Celecoxib was associated with a 32% decreased risk, compared with ibuprofen, and a 27% decreased risk, compared with naproxen.
Renal outcomes were almost identical in all of the medications in both groups, Dr. Husni said.
In addition to the adverse event outcomes, the subanalysis examined how well patients responded to their assigned NSAID. There were also some subtle differences seen here, Dr. Solomon and Dr. Husni said.
For patients with RA, ibuprofen was slightly, but significantly, more effective than either celecoxib or ibuprofen at controlling pain as measured by a visual analog pain scale. Patients with osteoarthritis responded equally well to all of the drugs.
RA patients also responded best to ibuprofen as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI). Again, patients with osteoarthritis responded equally well to all of the medications.
The overall findings, as well as the subanalysis, should be reassuring to both physicians and patients, said Dr. Solomon, chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, Boston.
“I can now stand in front of patients and say with confidence, ‘Each of these drugs is fairly safe, and together we can choose the one that will be best for you, based on your own individual history and your own individual risk factors.’ I feel good about that.”
Pfizer funded the trial. Dr. Husni has research grants from Genzyme/Sanofi on a knee osteoarthritis trial related to hyaluronic acid injections. Dr. Solomon has research grants from Pfizer on non-NSAID related topics. He also receives royalties from UpToDate on chapters related to NSAIDs and selective COX-2 inhibitors.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Celecoxib conferred a 53% decreased risk of overall mortality upon patients with rheumatoid arthritis when compared with naproxen – a surprise finding in a subanalysis of the newly released PRECISION study of anti-inflammatory drugs in arthritis.
But it’s tough to know what to make of the difference, according to the investigators at the annual meeting of the American College of Rheumatology. Although statistically significant, the mortality finding was based on just 45 events: 30 among those taking naproxen and 15 among those taking celecoxib.
“While I would say we were surprised to see this, we really can’t say what it means – or if it means anything,” primary investigator Daniel Solomon, MD, said in an interview. “This is a finding we will continue to investigate and look at, but at this point it’s not enough to base any prescribing decisions on.”
PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen) enrolled more than 24,000 patients with osteoarthritis or rheumatoid arthritis. They were randomized to celecoxib, naproxen, or ibuprofen for a mean of 20 months, with an additional mean follow-up of 34 months.
The primary outcome was the first occurrence of an adverse event from the Antiplatelet Trialists Collaboration criteria (APTC; death from cardiovascular causes, nonfatal heart attack, or nonfatal stroke). Secondary outcomes included:
• Major cardiovascular events (heart attack, stroke, cardiovascular death, revascularization, and hospitalization for unstable angina or transient ischemic attack).
• Renal events (acute kidney injury, including hospitalization for renal failure).
• Gastrointestinal events (hemorrhage, perforation, gastroduodenal ulcer, anemia of gastrointestinal origin, and gastric outlet obstruction.
The main PRECISION findings were released Nov. 13 at the annual meeting of the American Heart Association and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2016 Nov 13. doi: 10.1056/NEJMoa1611593).
Overall, celecoxib was just as safe as were the other drugs on the APTC endpoint, which occurred in about 2% of each groups. The study found significantly decreased risks of both GI and renal events with celecoxib, compared with either naproxen or ibuprofen. Celecoxib and naproxen were equivalent with regard to GI and renal events.
The subanalysis, released at the ACR meeting, adds important disease-specific context to the overall PRECISION results.
“We wanted to really delve into the subtle differences in how these two patient groups responded to these medications,” said PRECISION coinvestigator Elaine Husni, MD, vice chair of the department of rheumatic and immunologic diseases at the Cleveland Clinic.
“We learned that each of these NSAIDs has a unique safety profile that can be different in different groups. And in general, celecoxib seems less risky than the others.”
The cohort subanalysis broke down these endpoints among 21,600 patients with osteoarthritis and 2,400 with rheumatoid arthritis.
Among patients with osteoarthritis, celecoxib was also associated with a 16% decreased risk of a major adverse cardiovascular event, compared with ibuprofen. GI events were also less likely in this group: Celecoxib was associated with a 32% decreased risk, compared with ibuprofen, and a 27% decreased risk, compared with naproxen.
Renal outcomes were almost identical in all of the medications in both groups, Dr. Husni said.
In addition to the adverse event outcomes, the subanalysis examined how well patients responded to their assigned NSAID. There were also some subtle differences seen here, Dr. Solomon and Dr. Husni said.
For patients with RA, ibuprofen was slightly, but significantly, more effective than either celecoxib or ibuprofen at controlling pain as measured by a visual analog pain scale. Patients with osteoarthritis responded equally well to all of the drugs.
RA patients also responded best to ibuprofen as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI). Again, patients with osteoarthritis responded equally well to all of the medications.
The overall findings, as well as the subanalysis, should be reassuring to both physicians and patients, said Dr. Solomon, chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, Boston.
“I can now stand in front of patients and say with confidence, ‘Each of these drugs is fairly safe, and together we can choose the one that will be best for you, based on your own individual history and your own individual risk factors.’ I feel good about that.”
Pfizer funded the trial. Dr. Husni has research grants from Genzyme/Sanofi on a knee osteoarthritis trial related to hyaluronic acid injections. Dr. Solomon has research grants from Pfizer on non-NSAID related topics. He also receives royalties from UpToDate on chapters related to NSAIDs and selective COX-2 inhibitors.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Celecoxib conferred a 53% decreased risk of overall mortality upon patients with rheumatoid arthritis when compared with naproxen – a surprise finding in a subanalysis of the newly released PRECISION study of anti-inflammatory drugs in arthritis.
But it’s tough to know what to make of the difference, according to the investigators at the annual meeting of the American College of Rheumatology. Although statistically significant, the mortality finding was based on just 45 events: 30 among those taking naproxen and 15 among those taking celecoxib.
“While I would say we were surprised to see this, we really can’t say what it means – or if it means anything,” primary investigator Daniel Solomon, MD, said in an interview. “This is a finding we will continue to investigate and look at, but at this point it’s not enough to base any prescribing decisions on.”
PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen) enrolled more than 24,000 patients with osteoarthritis or rheumatoid arthritis. They were randomized to celecoxib, naproxen, or ibuprofen for a mean of 20 months, with an additional mean follow-up of 34 months.
The primary outcome was the first occurrence of an adverse event from the Antiplatelet Trialists Collaboration criteria (APTC; death from cardiovascular causes, nonfatal heart attack, or nonfatal stroke). Secondary outcomes included:
• Major cardiovascular events (heart attack, stroke, cardiovascular death, revascularization, and hospitalization for unstable angina or transient ischemic attack).
• Renal events (acute kidney injury, including hospitalization for renal failure).
• Gastrointestinal events (hemorrhage, perforation, gastroduodenal ulcer, anemia of gastrointestinal origin, and gastric outlet obstruction.
The main PRECISION findings were released Nov. 13 at the annual meeting of the American Heart Association and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2016 Nov 13. doi: 10.1056/NEJMoa1611593).
Overall, celecoxib was just as safe as were the other drugs on the APTC endpoint, which occurred in about 2% of each groups. The study found significantly decreased risks of both GI and renal events with celecoxib, compared with either naproxen or ibuprofen. Celecoxib and naproxen were equivalent with regard to GI and renal events.
The subanalysis, released at the ACR meeting, adds important disease-specific context to the overall PRECISION results.
“We wanted to really delve into the subtle differences in how these two patient groups responded to these medications,” said PRECISION coinvestigator Elaine Husni, MD, vice chair of the department of rheumatic and immunologic diseases at the Cleveland Clinic.
“We learned that each of these NSAIDs has a unique safety profile that can be different in different groups. And in general, celecoxib seems less risky than the others.”
The cohort subanalysis broke down these endpoints among 21,600 patients with osteoarthritis and 2,400 with rheumatoid arthritis.
Among patients with osteoarthritis, celecoxib was also associated with a 16% decreased risk of a major adverse cardiovascular event, compared with ibuprofen. GI events were also less likely in this group: Celecoxib was associated with a 32% decreased risk, compared with ibuprofen, and a 27% decreased risk, compared with naproxen.
Renal outcomes were almost identical in all of the medications in both groups, Dr. Husni said.
In addition to the adverse event outcomes, the subanalysis examined how well patients responded to their assigned NSAID. There were also some subtle differences seen here, Dr. Solomon and Dr. Husni said.
For patients with RA, ibuprofen was slightly, but significantly, more effective than either celecoxib or ibuprofen at controlling pain as measured by a visual analog pain scale. Patients with osteoarthritis responded equally well to all of the drugs.
RA patients also responded best to ibuprofen as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI). Again, patients with osteoarthritis responded equally well to all of the medications.
The overall findings, as well as the subanalysis, should be reassuring to both physicians and patients, said Dr. Solomon, chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, Boston.
“I can now stand in front of patients and say with confidence, ‘Each of these drugs is fairly safe, and together we can choose the one that will be best for you, based on your own individual history and your own individual risk factors.’ I feel good about that.”
Pfizer funded the trial. Dr. Husni has research grants from Genzyme/Sanofi on a knee osteoarthritis trial related to hyaluronic acid injections. Dr. Solomon has research grants from Pfizer on non-NSAID related topics. He also receives royalties from UpToDate on chapters related to NSAIDs and selective COX-2 inhibitors.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Compared with naproxen, celecoxib was associated with a 53% lower risk of overall mortality, although the clinical impact of that finding remains unknown.
Data source: PRECISION, which randomized more than 24,000 patients to celecoxib, ibuprofen, or naproxen.
Disclosures: Pfizer funded the trial. Dr. Husni has research grants from Genzyme/Sanofi on a knee osteoarthritis trial related to hyaluronic acid injections. Dr. Solomon has research grants from Pfizer on non-NSAID related topics. He also receives royalties from UpToDate on chapters related to NSAIDs and selective COX-2 inhibitors.
Travel barriers can impede patient choice for pancreatectomy
AT THE ACS CLINICAL CONGRESS
WASHINGTON – Patients who have the resources to travel to higher-volume hospitals for pancreatectomy have better outcomes than do those who opt to have the surgery in local, small-volume hospitals.
A 10-year review of travel patterns associated with pancreatectomy in California found those who didn’t travel were often elderly, black or Hispanic, and were either self-pay or had public insurance.
“It’s probably because these patients don’t have the resources [finances and transportation] to travel far to the best hospitals for their surgery and have to rely on the closest hospitals for better or for worse – and it seems more like the latter,” he said in an interview at the annual clinical congress of the American College of Surgeons. “So what you see is there is an aspatial barrier to access [limited insurance], as well as spatial barriers to access.”
He and his mentor, David C. Chang, PhD, examined about 13,000 pancreatectomy records included in the California Office of Statewide Health Planning and Development database from 2005 to 2014. The research was conducted at Harvard University and the Massachusetts General Hospital, Boston.
The majority of these patients (11,000) bypassed at least one hospital that offered pancreatectomy to reach their ultimate choice. After bypassing a median of seven facilities, they ended up traveling about 16 miles from home to reach their chosen hospital. Patients who stuck to the closest hospital traveled only about 3 miles.
Generally, bypassers tended to end up in higher-volume hospitals with better outcomes. About half had their pancreatectomy at a hospital that performed more than 20 per year; 40% were at a facility that performed in excess of 40 pancreatectomies annually. Almost 50% of the bypassers also had their surgery at a teaching hospital. The median length of stay at these facilities was 10 days, and they had a median pancreatectomy mortality rate of 3%.
In contrast, patients who didn’t travel ended up at lower-volume hospitals; 60% had their pancreatectomy at a hospital that performed fewer than 10 per year and 22% at a hospital that performed 10-20 per year. Only 18% were treated at an academic center. These hospitals had a significantly longer pancreatectomy length of stay (12 days) and significantly higher pancreatectomy mortality rate (6%).
Older patients were less likely to travel. The age difference came into play beginning at age 50 and grew stronger as patients aged.
Insurance status was highly associated with hospital destination. Privately insured patients were the most likely to travel to better hospitals, followed by those on Medicare. Patients on Medicaid and those who identified as self-pay were significantly less likely to travel. Minorities traveled far less as well; blacks were the least likely to travel from their home base.
In this time of value-based surgical outcomes, the study has some interesting implications, Dr. Fong said. Many health care systems are undertaking a volume pledge, which aims to funnel patients who need high-risk procedures to centers that perform a large number of them annually. But there is a flip side to that coin, which could, in essence, make things even tougher on patients who find travel challenging.
“The Volume Pledge aims to stop hospitals that are low volume in certain procedures from continuing to do them, on the basis that high-volume hospitals often do better in terms of outcomes. But there are very much unintended consequences of the pledge, such as hampering access to surgery. Because inevitably, you’ll increase distance needed to travel for each patient to get care if low-volume hospitals stopped offering their services.”
In his study, Dr. Fong found that some California counties had only one hospital that offered pancreatectomy, and that was a low-volume facility. If that hospital was forced to stop offering the procedure, patients with less resources could face even more obstacles to getting the treatment they need.
“Eliminating [low-volume hospital procedures] will have dire consequences. Our study showed that the elderly, racial/ethnic minorities, uninsured and those on Medicaid generally don’t travel for care, and this pledge may compound on that and widen disparity or even worse, some may not even get care as a result.”
Dr. Fong had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
WASHINGTON – Patients who have the resources to travel to higher-volume hospitals for pancreatectomy have better outcomes than do those who opt to have the surgery in local, small-volume hospitals.
A 10-year review of travel patterns associated with pancreatectomy in California found those who didn’t travel were often elderly, black or Hispanic, and were either self-pay or had public insurance.
“It’s probably because these patients don’t have the resources [finances and transportation] to travel far to the best hospitals for their surgery and have to rely on the closest hospitals for better or for worse – and it seems more like the latter,” he said in an interview at the annual clinical congress of the American College of Surgeons. “So what you see is there is an aspatial barrier to access [limited insurance], as well as spatial barriers to access.”
He and his mentor, David C. Chang, PhD, examined about 13,000 pancreatectomy records included in the California Office of Statewide Health Planning and Development database from 2005 to 2014. The research was conducted at Harvard University and the Massachusetts General Hospital, Boston.
The majority of these patients (11,000) bypassed at least one hospital that offered pancreatectomy to reach their ultimate choice. After bypassing a median of seven facilities, they ended up traveling about 16 miles from home to reach their chosen hospital. Patients who stuck to the closest hospital traveled only about 3 miles.
Generally, bypassers tended to end up in higher-volume hospitals with better outcomes. About half had their pancreatectomy at a hospital that performed more than 20 per year; 40% were at a facility that performed in excess of 40 pancreatectomies annually. Almost 50% of the bypassers also had their surgery at a teaching hospital. The median length of stay at these facilities was 10 days, and they had a median pancreatectomy mortality rate of 3%.
In contrast, patients who didn’t travel ended up at lower-volume hospitals; 60% had their pancreatectomy at a hospital that performed fewer than 10 per year and 22% at a hospital that performed 10-20 per year. Only 18% were treated at an academic center. These hospitals had a significantly longer pancreatectomy length of stay (12 days) and significantly higher pancreatectomy mortality rate (6%).
Older patients were less likely to travel. The age difference came into play beginning at age 50 and grew stronger as patients aged.
Insurance status was highly associated with hospital destination. Privately insured patients were the most likely to travel to better hospitals, followed by those on Medicare. Patients on Medicaid and those who identified as self-pay were significantly less likely to travel. Minorities traveled far less as well; blacks were the least likely to travel from their home base.
In this time of value-based surgical outcomes, the study has some interesting implications, Dr. Fong said. Many health care systems are undertaking a volume pledge, which aims to funnel patients who need high-risk procedures to centers that perform a large number of them annually. But there is a flip side to that coin, which could, in essence, make things even tougher on patients who find travel challenging.
“The Volume Pledge aims to stop hospitals that are low volume in certain procedures from continuing to do them, on the basis that high-volume hospitals often do better in terms of outcomes. But there are very much unintended consequences of the pledge, such as hampering access to surgery. Because inevitably, you’ll increase distance needed to travel for each patient to get care if low-volume hospitals stopped offering their services.”
In his study, Dr. Fong found that some California counties had only one hospital that offered pancreatectomy, and that was a low-volume facility. If that hospital was forced to stop offering the procedure, patients with less resources could face even more obstacles to getting the treatment they need.
“Eliminating [low-volume hospital procedures] will have dire consequences. Our study showed that the elderly, racial/ethnic minorities, uninsured and those on Medicaid generally don’t travel for care, and this pledge may compound on that and widen disparity or even worse, some may not even get care as a result.”
Dr. Fong had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
WASHINGTON – Patients who have the resources to travel to higher-volume hospitals for pancreatectomy have better outcomes than do those who opt to have the surgery in local, small-volume hospitals.
A 10-year review of travel patterns associated with pancreatectomy in California found those who didn’t travel were often elderly, black or Hispanic, and were either self-pay or had public insurance.
“It’s probably because these patients don’t have the resources [finances and transportation] to travel far to the best hospitals for their surgery and have to rely on the closest hospitals for better or for worse – and it seems more like the latter,” he said in an interview at the annual clinical congress of the American College of Surgeons. “So what you see is there is an aspatial barrier to access [limited insurance], as well as spatial barriers to access.”
He and his mentor, David C. Chang, PhD, examined about 13,000 pancreatectomy records included in the California Office of Statewide Health Planning and Development database from 2005 to 2014. The research was conducted at Harvard University and the Massachusetts General Hospital, Boston.
The majority of these patients (11,000) bypassed at least one hospital that offered pancreatectomy to reach their ultimate choice. After bypassing a median of seven facilities, they ended up traveling about 16 miles from home to reach their chosen hospital. Patients who stuck to the closest hospital traveled only about 3 miles.
Generally, bypassers tended to end up in higher-volume hospitals with better outcomes. About half had their pancreatectomy at a hospital that performed more than 20 per year; 40% were at a facility that performed in excess of 40 pancreatectomies annually. Almost 50% of the bypassers also had their surgery at a teaching hospital. The median length of stay at these facilities was 10 days, and they had a median pancreatectomy mortality rate of 3%.
In contrast, patients who didn’t travel ended up at lower-volume hospitals; 60% had their pancreatectomy at a hospital that performed fewer than 10 per year and 22% at a hospital that performed 10-20 per year. Only 18% were treated at an academic center. These hospitals had a significantly longer pancreatectomy length of stay (12 days) and significantly higher pancreatectomy mortality rate (6%).
Older patients were less likely to travel. The age difference came into play beginning at age 50 and grew stronger as patients aged.
Insurance status was highly associated with hospital destination. Privately insured patients were the most likely to travel to better hospitals, followed by those on Medicare. Patients on Medicaid and those who identified as self-pay were significantly less likely to travel. Minorities traveled far less as well; blacks were the least likely to travel from their home base.
In this time of value-based surgical outcomes, the study has some interesting implications, Dr. Fong said. Many health care systems are undertaking a volume pledge, which aims to funnel patients who need high-risk procedures to centers that perform a large number of them annually. But there is a flip side to that coin, which could, in essence, make things even tougher on patients who find travel challenging.
“The Volume Pledge aims to stop hospitals that are low volume in certain procedures from continuing to do them, on the basis that high-volume hospitals often do better in terms of outcomes. But there are very much unintended consequences of the pledge, such as hampering access to surgery. Because inevitably, you’ll increase distance needed to travel for each patient to get care if low-volume hospitals stopped offering their services.”
In his study, Dr. Fong found that some California counties had only one hospital that offered pancreatectomy, and that was a low-volume facility. If that hospital was forced to stop offering the procedure, patients with less resources could face even more obstacles to getting the treatment they need.
“Eliminating [low-volume hospital procedures] will have dire consequences. Our study showed that the elderly, racial/ethnic minorities, uninsured and those on Medicaid generally don’t travel for care, and this pledge may compound on that and widen disparity or even worse, some may not even get care as a result.”
Dr. Fong had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
Key clinical point:
Major finding: About 60% of patients who didn’t travel had surgery at a low-volume hospital with a higher mortality rate and longer length of stay.
Data source: The 10-year California database review comprised 13,000 patients.
Disclosures: Dr. Fong had no financial disclosures.
Readmissions after bariatric surgery more common among black patients
WASHINGTON – Readmissions after bariatric surgery are significantly higher among black patients than among whites.
The reasons aren’t entirely clear, but since long-term morbidity and mortality are equivalent, they are probably more related to socioeconomics than clinical factors, Matthew Whealon, MD, said at the annual clinical congress of the American College of Surgeons.
“I think they are multifactorial,” said Dr. Whealon of the University of California, Irvine. “Some of it may be related to comorbidities, but other factors could be socioeconomic status, insurance status, access to primary care and follow-up care, even home support systems and patient expectations after surgery. I think it’s incumbent upon us to try and identify some of these risk factors and address them before surgery to reduce this disparity in readmissions.”
Black patients undergoing Roux-en-Y bypass were significantly younger (43 vs. 45 years), and more often women (86% vs. 78%). They also had significantly higher body mass index than did white patients (48 vs. 46 kg/m2). More black individuals had a BMI of 50 kg/m2 or higher.
There were no significant differences in the severity of comorbidities. About 70% of each group had severe comorbidities as classified by the American Anesthesiologists Society risk assessment profile.
However, those comorbidities were different. Among black patients, steroid use, heart failure, hypertension, and end-stage renal disease were significantly more common. Among white patients, chronic obstructive pulmonary disease and bleeding disorders were more common.
There were no differences in 30-day mortality (less than 1% of each group); serious morbidity (3%) or any morbidity (5%); length of stay (2.4 days); or reoperation (2.6%).
However, readmissions were significantly more likely among black patients (8% vs. 5.6%). This translated to a 29% increased risk of readmission (OR 1.29).
Compared to whites, blacks who had a laparoscopic vertical sleeve gastrectomy were also significantly younger (42 vs. 45 years); more often women (87% vs. 76%); and heavier (BMI 47 vs. 45 kg/m2). Again, they were more likely to have a BMI of more than 50 kg/m2 (28% vs. 21%).
Significantly more were in the ASA class 3 of severe comorbidities (70% vs.66%). There were also differences in the comorbidities, with blacks more likely to have heart failure, hypertension, and end-stage renal disease, and whites more likely to have diabetes, smoking, dyspnea, and chronic obstructive pulmonary disease.
Among these patients, 30-day mortality was not different (less than 1%). Serious morbidity was also similar (about 2%), as was any morbidity (about 3%). The reoperation rate was the same (1.2%).
Length of stay was longer among black patients but this was not clinically significant, Dr. Whealon said: It still hovered right around 2 days.
But readmissions were significantly more common among blacks (5% vs. 3%). This difference translated to a 35% increased risk of readmission (odds ratio 1.35).
The nature of the NSQIP data makes it impossible to tease out any other factors that might have contributed to this finding. However, Dr. Whealon said, the equivalent findings on morbidity and mortality are very encouraging and represent a big improvement.
“We have done very well in driving down morbidity and mortality among these patients. Mortality rates are one tenth of what we were seeing a decade ago.”
This change hasn’t been well documented yet because many of the large studies showing racial and ethnic mortality disparities include data drawn from open bariatric surgery, which has been almost completely abandoned in favor of the much safer laparoscopic approaches.
Dr. Whealon had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
WASHINGTON – Readmissions after bariatric surgery are significantly higher among black patients than among whites.
The reasons aren’t entirely clear, but since long-term morbidity and mortality are equivalent, they are probably more related to socioeconomics than clinical factors, Matthew Whealon, MD, said at the annual clinical congress of the American College of Surgeons.
“I think they are multifactorial,” said Dr. Whealon of the University of California, Irvine. “Some of it may be related to comorbidities, but other factors could be socioeconomic status, insurance status, access to primary care and follow-up care, even home support systems and patient expectations after surgery. I think it’s incumbent upon us to try and identify some of these risk factors and address them before surgery to reduce this disparity in readmissions.”
Black patients undergoing Roux-en-Y bypass were significantly younger (43 vs. 45 years), and more often women (86% vs. 78%). They also had significantly higher body mass index than did white patients (48 vs. 46 kg/m2). More black individuals had a BMI of 50 kg/m2 or higher.
There were no significant differences in the severity of comorbidities. About 70% of each group had severe comorbidities as classified by the American Anesthesiologists Society risk assessment profile.
However, those comorbidities were different. Among black patients, steroid use, heart failure, hypertension, and end-stage renal disease were significantly more common. Among white patients, chronic obstructive pulmonary disease and bleeding disorders were more common.
There were no differences in 30-day mortality (less than 1% of each group); serious morbidity (3%) or any morbidity (5%); length of stay (2.4 days); or reoperation (2.6%).
However, readmissions were significantly more likely among black patients (8% vs. 5.6%). This translated to a 29% increased risk of readmission (OR 1.29).
Compared to whites, blacks who had a laparoscopic vertical sleeve gastrectomy were also significantly younger (42 vs. 45 years); more often women (87% vs. 76%); and heavier (BMI 47 vs. 45 kg/m2). Again, they were more likely to have a BMI of more than 50 kg/m2 (28% vs. 21%).
Significantly more were in the ASA class 3 of severe comorbidities (70% vs.66%). There were also differences in the comorbidities, with blacks more likely to have heart failure, hypertension, and end-stage renal disease, and whites more likely to have diabetes, smoking, dyspnea, and chronic obstructive pulmonary disease.
Among these patients, 30-day mortality was not different (less than 1%). Serious morbidity was also similar (about 2%), as was any morbidity (about 3%). The reoperation rate was the same (1.2%).
Length of stay was longer among black patients but this was not clinically significant, Dr. Whealon said: It still hovered right around 2 days.
But readmissions were significantly more common among blacks (5% vs. 3%). This difference translated to a 35% increased risk of readmission (odds ratio 1.35).
The nature of the NSQIP data makes it impossible to tease out any other factors that might have contributed to this finding. However, Dr. Whealon said, the equivalent findings on morbidity and mortality are very encouraging and represent a big improvement.
“We have done very well in driving down morbidity and mortality among these patients. Mortality rates are one tenth of what we were seeing a decade ago.”
This change hasn’t been well documented yet because many of the large studies showing racial and ethnic mortality disparities include data drawn from open bariatric surgery, which has been almost completely abandoned in favor of the much safer laparoscopic approaches.
Dr. Whealon had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
WASHINGTON – Readmissions after bariatric surgery are significantly higher among black patients than among whites.
The reasons aren’t entirely clear, but since long-term morbidity and mortality are equivalent, they are probably more related to socioeconomics than clinical factors, Matthew Whealon, MD, said at the annual clinical congress of the American College of Surgeons.
“I think they are multifactorial,” said Dr. Whealon of the University of California, Irvine. “Some of it may be related to comorbidities, but other factors could be socioeconomic status, insurance status, access to primary care and follow-up care, even home support systems and patient expectations after surgery. I think it’s incumbent upon us to try and identify some of these risk factors and address them before surgery to reduce this disparity in readmissions.”
Black patients undergoing Roux-en-Y bypass were significantly younger (43 vs. 45 years), and more often women (86% vs. 78%). They also had significantly higher body mass index than did white patients (48 vs. 46 kg/m2). More black individuals had a BMI of 50 kg/m2 or higher.
There were no significant differences in the severity of comorbidities. About 70% of each group had severe comorbidities as classified by the American Anesthesiologists Society risk assessment profile.
However, those comorbidities were different. Among black patients, steroid use, heart failure, hypertension, and end-stage renal disease were significantly more common. Among white patients, chronic obstructive pulmonary disease and bleeding disorders were more common.
There were no differences in 30-day mortality (less than 1% of each group); serious morbidity (3%) or any morbidity (5%); length of stay (2.4 days); or reoperation (2.6%).
However, readmissions were significantly more likely among black patients (8% vs. 5.6%). This translated to a 29% increased risk of readmission (OR 1.29).
Compared to whites, blacks who had a laparoscopic vertical sleeve gastrectomy were also significantly younger (42 vs. 45 years); more often women (87% vs. 76%); and heavier (BMI 47 vs. 45 kg/m2). Again, they were more likely to have a BMI of more than 50 kg/m2 (28% vs. 21%).
Significantly more were in the ASA class 3 of severe comorbidities (70% vs.66%). There were also differences in the comorbidities, with blacks more likely to have heart failure, hypertension, and end-stage renal disease, and whites more likely to have diabetes, smoking, dyspnea, and chronic obstructive pulmonary disease.
Among these patients, 30-day mortality was not different (less than 1%). Serious morbidity was also similar (about 2%), as was any morbidity (about 3%). The reoperation rate was the same (1.2%).
Length of stay was longer among black patients but this was not clinically significant, Dr. Whealon said: It still hovered right around 2 days.
But readmissions were significantly more common among blacks (5% vs. 3%). This difference translated to a 35% increased risk of readmission (odds ratio 1.35).
The nature of the NSQIP data makes it impossible to tease out any other factors that might have contributed to this finding. However, Dr. Whealon said, the equivalent findings on morbidity and mortality are very encouraging and represent a big improvement.
“We have done very well in driving down morbidity and mortality among these patients. Mortality rates are one tenth of what we were seeing a decade ago.”
This change hasn’t been well documented yet because many of the large studies showing racial and ethnic mortality disparities include data drawn from open bariatric surgery, which has been almost completely abandoned in favor of the much safer laparoscopic approaches.
Dr. Whealon had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: Black patients were 29% more likely to be readmitted after Roux-en-Y and 35% more likely to be readmitted after sleeve gastrectomy.
Data source: The NSQIP database review comprised about 62,000 surgeries.
Disclosures: Dr. Whealon had no financial disclosures.
VIDEO: Cardiac inflammation present in RA patients, but resolves with RA therapy
WASHINGTON – Chronic cardiac inflammation is present in rheumatoid arthritis patients and appears to resolve when they achieve a good clinical response to medical therapy.
The findings of two cardiac imaging studies offer a tantalizing clue to the link between RA and heart failure, said Isabelle Amigues, MD, who reported the findings at the annual meeting of the American College of Rheumatology.
“We know that the inflammation of RA is not just restricted to joints,” said Dr. Amigues, a rheumatology fellow at Columbia University, New York. “We see it throughout the body, so of course it makes sense that we would see it in the heart as well. And now we see that we may be able to manage this with good disease management.
The study that found improvement of myocarditis with RA therapy was very small – just 8 patients and 12 controls – but the results were surprising and encouraging, Dr. Amigues said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The initial study examined chronic myocarditis in 119 patients with active RA; most (76%) were positive for anti-cyclic citrullinated peptides. They had a mean disease duration of 7 years. The mean Disease Activity Score was 3.8; 28% had low disease activity. About a third of the patients were taking a tumor necrosis factor inhibitor.
These patients were age- and gender-matched with 13 controls who did not have RA. All underwent cardiac FDG-PET scans as a marker of inflammation, as well as 3-D echocardiography to assess left ventricular mass and volume, and both systolic and diastolic function.
The maximum standard uptake value (SUVmax) in the scans was 12% higher in study patients than in the controls. This finding was associated with a higher body mass index and moderate to severe disease activity. After adjustment for BMI and RA treatment, the mean SUVmax was 30% higher for the patients with moderate to severe disease activity than in those who had low disease activity. Patients taking non-TNF biologics had 35% less cardiac inflammation than did those taking a TNF inhibitor or those not taking a biologic.
There were no significant associations with age, gender, race, diabetes, C-reactive protein or IL-6, coronary flow reserve, or coronary calcium. Inflammation was not related to any measure of cardiac structure or function on echocardiography.
“This finding makes sense, because we know that RA causes systemic inflammation, so it’s not surprising to see inflammation at the level of the heart,” Dr. Amigues said in an interview. “We do need longitudinal studies though to determine if structural or functional changes occur later on in the disease process.”
While the initial study didn’t look at cardiac changes in the disease process, Dr. Amigues’ subsequent study did find these associated with successful RA treatment. This follow-up study comprised eight patients who underwent PET scans and 3-D echocardiography at baseline and 6 months after initiation of a step-up treatment regimen. These were compared to 12 age- and gender-matched controls without RA, who had one baseline scan.
Most patients (87%) were women; their mean age was 62 years, and mean disease duration, 5 months. Most of the patients received TNF inhibitors with methotrexate as their step-up therapy; the rest were given triple therapy.
At the baseline scan, mean SUVmax was significantly higher in the patients than in controls (7.2 vs. 3.4 units). After 6 months of treatment, the mean DAS28 score among patients had decreased more than 1 point (4.57-3.51). Their mean SUVmax had also decreased to the level seen in controls. This was a significant improvement, Dr. Amigues said.
Again, there were no structural or functional differences between the two groups at baseline or follow-up, suggesting that cardiac inflammation is not inducing structural change – at least early in the RA disease process, Dr. Amigues said.
She reported having no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
WASHINGTON – Chronic cardiac inflammation is present in rheumatoid arthritis patients and appears to resolve when they achieve a good clinical response to medical therapy.
The findings of two cardiac imaging studies offer a tantalizing clue to the link between RA and heart failure, said Isabelle Amigues, MD, who reported the findings at the annual meeting of the American College of Rheumatology.
“We know that the inflammation of RA is not just restricted to joints,” said Dr. Amigues, a rheumatology fellow at Columbia University, New York. “We see it throughout the body, so of course it makes sense that we would see it in the heart as well. And now we see that we may be able to manage this with good disease management.
The study that found improvement of myocarditis with RA therapy was very small – just 8 patients and 12 controls – but the results were surprising and encouraging, Dr. Amigues said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The initial study examined chronic myocarditis in 119 patients with active RA; most (76%) were positive for anti-cyclic citrullinated peptides. They had a mean disease duration of 7 years. The mean Disease Activity Score was 3.8; 28% had low disease activity. About a third of the patients were taking a tumor necrosis factor inhibitor.
These patients were age- and gender-matched with 13 controls who did not have RA. All underwent cardiac FDG-PET scans as a marker of inflammation, as well as 3-D echocardiography to assess left ventricular mass and volume, and both systolic and diastolic function.
The maximum standard uptake value (SUVmax) in the scans was 12% higher in study patients than in the controls. This finding was associated with a higher body mass index and moderate to severe disease activity. After adjustment for BMI and RA treatment, the mean SUVmax was 30% higher for the patients with moderate to severe disease activity than in those who had low disease activity. Patients taking non-TNF biologics had 35% less cardiac inflammation than did those taking a TNF inhibitor or those not taking a biologic.
There were no significant associations with age, gender, race, diabetes, C-reactive protein or IL-6, coronary flow reserve, or coronary calcium. Inflammation was not related to any measure of cardiac structure or function on echocardiography.
“This finding makes sense, because we know that RA causes systemic inflammation, so it’s not surprising to see inflammation at the level of the heart,” Dr. Amigues said in an interview. “We do need longitudinal studies though to determine if structural or functional changes occur later on in the disease process.”
While the initial study didn’t look at cardiac changes in the disease process, Dr. Amigues’ subsequent study did find these associated with successful RA treatment. This follow-up study comprised eight patients who underwent PET scans and 3-D echocardiography at baseline and 6 months after initiation of a step-up treatment regimen. These were compared to 12 age- and gender-matched controls without RA, who had one baseline scan.
Most patients (87%) were women; their mean age was 62 years, and mean disease duration, 5 months. Most of the patients received TNF inhibitors with methotrexate as their step-up therapy; the rest were given triple therapy.
At the baseline scan, mean SUVmax was significantly higher in the patients than in controls (7.2 vs. 3.4 units). After 6 months of treatment, the mean DAS28 score among patients had decreased more than 1 point (4.57-3.51). Their mean SUVmax had also decreased to the level seen in controls. This was a significant improvement, Dr. Amigues said.
Again, there were no structural or functional differences between the two groups at baseline or follow-up, suggesting that cardiac inflammation is not inducing structural change – at least early in the RA disease process, Dr. Amigues said.
She reported having no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
WASHINGTON – Chronic cardiac inflammation is present in rheumatoid arthritis patients and appears to resolve when they achieve a good clinical response to medical therapy.
The findings of two cardiac imaging studies offer a tantalizing clue to the link between RA and heart failure, said Isabelle Amigues, MD, who reported the findings at the annual meeting of the American College of Rheumatology.
“We know that the inflammation of RA is not just restricted to joints,” said Dr. Amigues, a rheumatology fellow at Columbia University, New York. “We see it throughout the body, so of course it makes sense that we would see it in the heart as well. And now we see that we may be able to manage this with good disease management.
The study that found improvement of myocarditis with RA therapy was very small – just 8 patients and 12 controls – but the results were surprising and encouraging, Dr. Amigues said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The initial study examined chronic myocarditis in 119 patients with active RA; most (76%) were positive for anti-cyclic citrullinated peptides. They had a mean disease duration of 7 years. The mean Disease Activity Score was 3.8; 28% had low disease activity. About a third of the patients were taking a tumor necrosis factor inhibitor.
These patients were age- and gender-matched with 13 controls who did not have RA. All underwent cardiac FDG-PET scans as a marker of inflammation, as well as 3-D echocardiography to assess left ventricular mass and volume, and both systolic and diastolic function.
The maximum standard uptake value (SUVmax) in the scans was 12% higher in study patients than in the controls. This finding was associated with a higher body mass index and moderate to severe disease activity. After adjustment for BMI and RA treatment, the mean SUVmax was 30% higher for the patients with moderate to severe disease activity than in those who had low disease activity. Patients taking non-TNF biologics had 35% less cardiac inflammation than did those taking a TNF inhibitor or those not taking a biologic.
There were no significant associations with age, gender, race, diabetes, C-reactive protein or IL-6, coronary flow reserve, or coronary calcium. Inflammation was not related to any measure of cardiac structure or function on echocardiography.
“This finding makes sense, because we know that RA causes systemic inflammation, so it’s not surprising to see inflammation at the level of the heart,” Dr. Amigues said in an interview. “We do need longitudinal studies though to determine if structural or functional changes occur later on in the disease process.”
While the initial study didn’t look at cardiac changes in the disease process, Dr. Amigues’ subsequent study did find these associated with successful RA treatment. This follow-up study comprised eight patients who underwent PET scans and 3-D echocardiography at baseline and 6 months after initiation of a step-up treatment regimen. These were compared to 12 age- and gender-matched controls without RA, who had one baseline scan.
Most patients (87%) were women; their mean age was 62 years, and mean disease duration, 5 months. Most of the patients received TNF inhibitors with methotrexate as their step-up therapy; the rest were given triple therapy.
At the baseline scan, mean SUVmax was significantly higher in the patients than in controls (7.2 vs. 3.4 units). After 6 months of treatment, the mean DAS28 score among patients had decreased more than 1 point (4.57-3.51). Their mean SUVmax had also decreased to the level seen in controls. This was a significant improvement, Dr. Amigues said.
Again, there were no structural or functional differences between the two groups at baseline or follow-up, suggesting that cardiac inflammation is not inducing structural change – at least early in the RA disease process, Dr. Amigues said.
She reported having no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: PET imaging showed 12% more inflammatory activity in the hearts of patients than in those of controls; this decreased significantly as disease activity remitted with treatment.
Data source: The myocarditis study comprised 119 patients; the therapeutic response study comprised 8 patients and 12 controls.
Disclosures: Dr. Amigues reported having no relevant financial disclosures.
Long-term opioid use uncommon among trauma patients
WASHINGTON – Patients with traumatic injuries don’t appear to be at undue risk of sustained opioid use, a large database review has demonstrated.
More than half of the 13,000 patients in the study were discharged on opioids, but they were able to discontinue them fairly rapidly, Muhammad Chaudhary, MD, said at the annual clinical congress of the American College of Surgeons. Within 3 months, less than one-third were still using the drugs, and 1 year later, only 1% were still taking an opioid pain medication.
Dr. Chaudhary examined opioid use among 13,624 patients included in the Tricare military insurance database. The patients were treated for traumatic injuries they received during 2007-2013. Most of the patients were men (82%), and the largest age group was 18- to 24-year-olds (39%). Military rank was used as a proxy for socioeconomic status in this study: 15% of the cohort had an officer rank, while the rest were junior or senior enlisted personnel.
The group was very healthy, with a median Charlson Comorbidity Index score of 0. They were somewhat seriously injured, however. The median Injury Severity Score was 13, and the range was 9-17. Anxiety and depression were uncommon (9% and 7%, respectively).
More than half the patients (54%) were discharged on an opioid medication. That percentage dropped very rapidly. By 90 days after discharge, just 9% of patients were still taking the drugs. By 1 year, only 1% were using opioids.
Dr. Chaudhary conducted a multivariate analysis that controlled for a number of factors, including age, gender, marital status, rank, mental health status, injury severity, comorbidities, and treatment environment. Two factors – black race and younger age (18-24 years) – significantly increased the likelihood of early opioid discontinuation (8% and 11%, respectively). There were no significant interactions with anxiety or depression.
Junior enlisted personnel – the proxy group for lower socioeconomic status – and those with a prolonged length of stay were significantly less likely to get off the medications, Dr. Chaudhary said.
“While we strongly believe that these factors should not be used to determine who can get opioids, it might make sense to enhance perioperative surveillance and engage pain management services early on in patients with risk factors, to reduce the risk of sustained opioid use,” he concluded.
Dr. Chaudhary had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Patients with traumatic injuries don’t appear to be at undue risk of sustained opioid use, a large database review has demonstrated.
More than half of the 13,000 patients in the study were discharged on opioids, but they were able to discontinue them fairly rapidly, Muhammad Chaudhary, MD, said at the annual clinical congress of the American College of Surgeons. Within 3 months, less than one-third were still using the drugs, and 1 year later, only 1% were still taking an opioid pain medication.
Dr. Chaudhary examined opioid use among 13,624 patients included in the Tricare military insurance database. The patients were treated for traumatic injuries they received during 2007-2013. Most of the patients were men (82%), and the largest age group was 18- to 24-year-olds (39%). Military rank was used as a proxy for socioeconomic status in this study: 15% of the cohort had an officer rank, while the rest were junior or senior enlisted personnel.
The group was very healthy, with a median Charlson Comorbidity Index score of 0. They were somewhat seriously injured, however. The median Injury Severity Score was 13, and the range was 9-17. Anxiety and depression were uncommon (9% and 7%, respectively).
More than half the patients (54%) were discharged on an opioid medication. That percentage dropped very rapidly. By 90 days after discharge, just 9% of patients were still taking the drugs. By 1 year, only 1% were using opioids.
Dr. Chaudhary conducted a multivariate analysis that controlled for a number of factors, including age, gender, marital status, rank, mental health status, injury severity, comorbidities, and treatment environment. Two factors – black race and younger age (18-24 years) – significantly increased the likelihood of early opioid discontinuation (8% and 11%, respectively). There were no significant interactions with anxiety or depression.
Junior enlisted personnel – the proxy group for lower socioeconomic status – and those with a prolonged length of stay were significantly less likely to get off the medications, Dr. Chaudhary said.
“While we strongly believe that these factors should not be used to determine who can get opioids, it might make sense to enhance perioperative surveillance and engage pain management services early on in patients with risk factors, to reduce the risk of sustained opioid use,” he concluded.
Dr. Chaudhary had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Patients with traumatic injuries don’t appear to be at undue risk of sustained opioid use, a large database review has demonstrated.
More than half of the 13,000 patients in the study were discharged on opioids, but they were able to discontinue them fairly rapidly, Muhammad Chaudhary, MD, said at the annual clinical congress of the American College of Surgeons. Within 3 months, less than one-third were still using the drugs, and 1 year later, only 1% were still taking an opioid pain medication.
Dr. Chaudhary examined opioid use among 13,624 patients included in the Tricare military insurance database. The patients were treated for traumatic injuries they received during 2007-2013. Most of the patients were men (82%), and the largest age group was 18- to 24-year-olds (39%). Military rank was used as a proxy for socioeconomic status in this study: 15% of the cohort had an officer rank, while the rest were junior or senior enlisted personnel.
The group was very healthy, with a median Charlson Comorbidity Index score of 0. They were somewhat seriously injured, however. The median Injury Severity Score was 13, and the range was 9-17. Anxiety and depression were uncommon (9% and 7%, respectively).
More than half the patients (54%) were discharged on an opioid medication. That percentage dropped very rapidly. By 90 days after discharge, just 9% of patients were still taking the drugs. By 1 year, only 1% were using opioids.
Dr. Chaudhary conducted a multivariate analysis that controlled for a number of factors, including age, gender, marital status, rank, mental health status, injury severity, comorbidities, and treatment environment. Two factors – black race and younger age (18-24 years) – significantly increased the likelihood of early opioid discontinuation (8% and 11%, respectively). There were no significant interactions with anxiety or depression.
Junior enlisted personnel – the proxy group for lower socioeconomic status – and those with a prolonged length of stay were significantly less likely to get off the medications, Dr. Chaudhary said.
“While we strongly believe that these factors should not be used to determine who can get opioids, it might make sense to enhance perioperative surveillance and engage pain management services early on in patients with risk factors, to reduce the risk of sustained opioid use,” he concluded.
Dr. Chaudhary had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: A year after discharge, only 1% of the patients were still using a prescription opioid pain medication.
Data source: A database review including 13,642 patients.
Disclosures: Dr. Chaudhary had no financial disclosures.