User login
ADA/EASD draft guidance aims to bring adults with type 1 diabetes out of shadows
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
Not so crazy: Pancreas transplants in type 2 diabetes rising
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
FDA rejects teplizumab for type 1 diabetes delay
The U.S. , despite narrow endorsement in a 10-7 vote in favor of approval by one of its advisory panels in May.
According to the company, the FDA did not cite any clinical deficiencies related to the efficacy and safety data packages submitted as part of the biologics license application for teplizumab.
Rather, the sticking point appears to be a study in healthy volunteers that had been raised as an issue with Provention Bio in April.
That study was designed to compare the planned commercial product with the product originally manufactured for clinical trials, but the former was not pharmacologically comparable to the latter, the FDA said in its complete response letter, issued on July 2.
The company expects, later this quarter, to obtain data from a substudy in patients receiving 12 days of therapy in the ongoing PROTECT trial of newly diagnosed patients with type 1 diabetes, which it hopes will help alleviate the FDA’s concerns.
“Upon review of the results from this substudy, the company will determine whether to submit these data to the FDA for its review ... to support pharmacokinetic comparability or otherwise justify why pharmacokinetic comparability is not necessary,” it said in its statement.
The FDA’s complete response letter had also mentioned additional issues related to product quality that Provention believes it has or will be able to address in the short term.
Teplizumab delays type 1 diabetes onset by years
Phase 2 data showing that a 14-day teplizumab infusion delayed the onset of type 1 diabetes by 2 years in high-risk relatives of people with the condition were called “game-changing” when presented at the American Diabetes Association 2019 Scientific Sessions and simultaneously published in the New England Journal of Medicine. These were the data considered by the FDA advisory panel in May.
In response to the FDA decision, the type 1 diabetes research and advocacy organization JDRF said: “It is unfortunate that the FDA has not approved teplizumab at this time and instead has requested additional information from the sponsor. We look forward to Provention Bio addressing the issues outlined in the Complete Response Letter and working with the FDA to bring this option to market safely.”
Teplizumab is one of several potential disease-modifying therapies being studied for type 1 diabetes administered either soon after diagnosis or to asymptomatic individuals with high-risk autoantibodies.
“Disease-modifying therapies such as teplizumab will help address the unmet needs of people with type 1 diabetes and those at risk for developing the disease. In the meantime, our organization will continue to support the research of other disease-modifying therapies that put us on the critical pathway to preventing and ultimately curing type 1 diabetes,” JDRF said in a statement.
A version of this article first appeared on Medscape.com.
The U.S. , despite narrow endorsement in a 10-7 vote in favor of approval by one of its advisory panels in May.
According to the company, the FDA did not cite any clinical deficiencies related to the efficacy and safety data packages submitted as part of the biologics license application for teplizumab.
Rather, the sticking point appears to be a study in healthy volunteers that had been raised as an issue with Provention Bio in April.
That study was designed to compare the planned commercial product with the product originally manufactured for clinical trials, but the former was not pharmacologically comparable to the latter, the FDA said in its complete response letter, issued on July 2.
The company expects, later this quarter, to obtain data from a substudy in patients receiving 12 days of therapy in the ongoing PROTECT trial of newly diagnosed patients with type 1 diabetes, which it hopes will help alleviate the FDA’s concerns.
“Upon review of the results from this substudy, the company will determine whether to submit these data to the FDA for its review ... to support pharmacokinetic comparability or otherwise justify why pharmacokinetic comparability is not necessary,” it said in its statement.
The FDA’s complete response letter had also mentioned additional issues related to product quality that Provention believes it has or will be able to address in the short term.
Teplizumab delays type 1 diabetes onset by years
Phase 2 data showing that a 14-day teplizumab infusion delayed the onset of type 1 diabetes by 2 years in high-risk relatives of people with the condition were called “game-changing” when presented at the American Diabetes Association 2019 Scientific Sessions and simultaneously published in the New England Journal of Medicine. These were the data considered by the FDA advisory panel in May.
In response to the FDA decision, the type 1 diabetes research and advocacy organization JDRF said: “It is unfortunate that the FDA has not approved teplizumab at this time and instead has requested additional information from the sponsor. We look forward to Provention Bio addressing the issues outlined in the Complete Response Letter and working with the FDA to bring this option to market safely.”
Teplizumab is one of several potential disease-modifying therapies being studied for type 1 diabetes administered either soon after diagnosis or to asymptomatic individuals with high-risk autoantibodies.
“Disease-modifying therapies such as teplizumab will help address the unmet needs of people with type 1 diabetes and those at risk for developing the disease. In the meantime, our organization will continue to support the research of other disease-modifying therapies that put us on the critical pathway to preventing and ultimately curing type 1 diabetes,” JDRF said in a statement.
A version of this article first appeared on Medscape.com.
The U.S. , despite narrow endorsement in a 10-7 vote in favor of approval by one of its advisory panels in May.
According to the company, the FDA did not cite any clinical deficiencies related to the efficacy and safety data packages submitted as part of the biologics license application for teplizumab.
Rather, the sticking point appears to be a study in healthy volunteers that had been raised as an issue with Provention Bio in April.
That study was designed to compare the planned commercial product with the product originally manufactured for clinical trials, but the former was not pharmacologically comparable to the latter, the FDA said in its complete response letter, issued on July 2.
The company expects, later this quarter, to obtain data from a substudy in patients receiving 12 days of therapy in the ongoing PROTECT trial of newly diagnosed patients with type 1 diabetes, which it hopes will help alleviate the FDA’s concerns.
“Upon review of the results from this substudy, the company will determine whether to submit these data to the FDA for its review ... to support pharmacokinetic comparability or otherwise justify why pharmacokinetic comparability is not necessary,” it said in its statement.
The FDA’s complete response letter had also mentioned additional issues related to product quality that Provention believes it has or will be able to address in the short term.
Teplizumab delays type 1 diabetes onset by years
Phase 2 data showing that a 14-day teplizumab infusion delayed the onset of type 1 diabetes by 2 years in high-risk relatives of people with the condition were called “game-changing” when presented at the American Diabetes Association 2019 Scientific Sessions and simultaneously published in the New England Journal of Medicine. These were the data considered by the FDA advisory panel in May.
In response to the FDA decision, the type 1 diabetes research and advocacy organization JDRF said: “It is unfortunate that the FDA has not approved teplizumab at this time and instead has requested additional information from the sponsor. We look forward to Provention Bio addressing the issues outlined in the Complete Response Letter and working with the FDA to bring this option to market safely.”
Teplizumab is one of several potential disease-modifying therapies being studied for type 1 diabetes administered either soon after diagnosis or to asymptomatic individuals with high-risk autoantibodies.
“Disease-modifying therapies such as teplizumab will help address the unmet needs of people with type 1 diabetes and those at risk for developing the disease. In the meantime, our organization will continue to support the research of other disease-modifying therapies that put us on the critical pathway to preventing and ultimately curing type 1 diabetes,” JDRF said in a statement.
A version of this article first appeared on Medscape.com.
Omnipod 5 ‘artificial pancreas’ shows benefit in type 1 diabetes
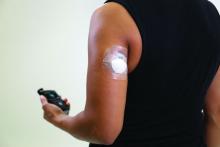
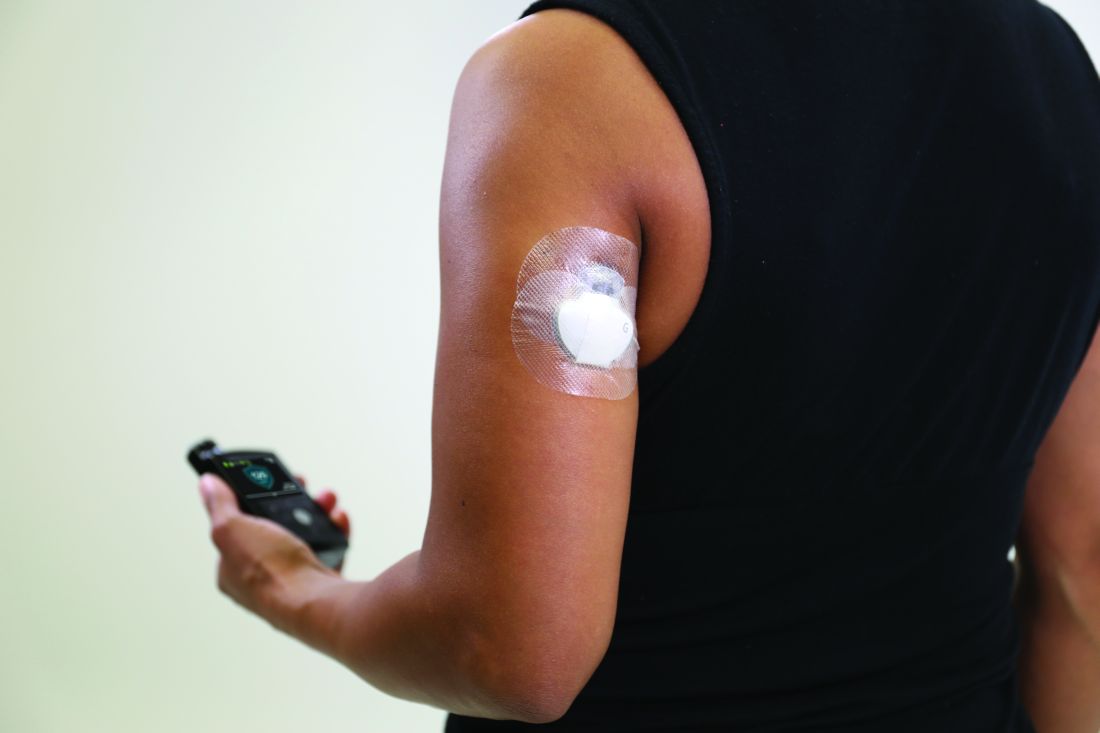
Insulet’s investigational Omnipod 5 automated insulin delivery system improves glycemic control in people with type 1 diabetes aged as young as 2 years, new data suggest.
The Omnipod 5 system combines a tubing-free insulin-filled delivery “Pod” with the Dexcom G6 continuous glucose monitor and an algorithm built into the Pod connecting the two devices via a smartphone app to semiautomate insulin delivery. It is currently under review by the Food and Drug Administration. The company expects to launch it in limited release during the second half of 2021.
Results from a pivotal trial of the system in children aged 2-5.9 years with type 1 diabetes were presented during the annual scientific sessions of the American Diabetes Association.
Follow-up data at 6 months were also presented for another pivotal study of 112 children aged 6-13.9 years and 129 adults aged 14-70 years. Those primary 3-month data were reported earlier this year at the Endocrine Society’s annual meeting and subsequently published online June 7, 2021, in Diabetes Care. Another study presented at ADA looked at quality of life in children using Omnipod 5 and their caregivers.
If approved by the FDA, the Omnipod 5 would be the third commercially available automated insulin delivery system – also called hybrid closed-loop or artificial pancreas systems – in the United States. It would be the second approved for children as young as 2 years of age and the first to deliver insulin subcutaneously without tubing.
‘No-tubing’ feature will be a draw for parents of young children
Asked to comment, pediatric endocrinologist Laura M. Jacobsen, MD, of the University of Florida, Gainesville, said in an interview: “I think the big advantage for the Omnipod 5 is that [if approved it will be] the only tubeless automated insulin delivery system in the U.S.”
“The automated delivery systems have just been wonderful for helping patients achieve time in range, especially overnight. And the fact that this goes down to such a young age where that can be very difficult is wonderful.”
Another difference between the Omnipod 5 and other systems is the ability to adjust glucose targets (from 110 to 150 mg/dL), although newer versions of the currently available hybrid closed-loop systems are expected to include that feature as well. “They’re all slightly different in the way the algorithms work, but I think the end result is similar,” Dr. Jacobsen said.
But, she said, the no-tubing feature might be particularly helpful for some very active young kids. “A lot of small kids do use the tubed pumps, and you can make it work with a lot of kids, but with some kids it just won’t ... the tubing gets caught. I think this really helps parents make the step. A lot of them don’t want to try the tubing whereas they see the Omnipod and might feel a little more confidence to try a pump.”
Overall, said Dr. Jacobsen, who has no financial disclosures with Insulet, Dexcom, or any of their competitors, “I think any addition to the technology field to improve quality of life for people with type 1 diabetes is important and people need choices.”
Pivotal data show benefit in ‘difficult-to-manage’ preschool children
Pivotal 3-month data for the Omnipod 5 in children aged 2-5.9 years with type 1 diabetes were presented on June 26 by pediatric endocrinologist Jennifer Sherr, MD, PhD, Yale University, New Haven, Conn.
“As a pediatric endocrinologist, I can attest to the difficulty of managing this age group, due to grazing eating patterns and erratic physical activity. Oftentimes, care providers may fear hypoglycemia as these youth can not verbalize or self-treat lows,” she remarked.
A total of 80 children were enrolled at 10 institutions across the United Sates. There was a single 14-day standard therapy phase (baseline), followed by 3 months of automated insulin delivery during which the children’s eating and exercise were unrestricted.
At 3 months, average hemoglobin A1c had fallen from 7.4% at baseline to 6.9%, a significant difference (P < .05). The proportions achieving the target A1c of less than 7% were 54% at 3 months versus 31% at baseline. The reduction was even greater among the 25 with baseline A1c of 8% or greater, although it was significant even among the 55 who started with a lower A1c (–1.06 vs. –0.31 percentage points; both P < .05).
Time in range rose from 57.2% at baseline to 68.1% at 3 months (P < .05).
“These youngsters are spending an average of 2.6 more hours/day in range,” Dr. Sherr commented, noting that the difference became apparent shortly after study start and was maintained during the 3 months.
Dr. Sherr noted that this 10.9% improvement in time in range with Omnipod 5 was similar to improvements in the previously reported pivotal study of older children and adults. Data from that study showed improvement in time in range from a gain of 15.6% for the 6 to 13.9 year olds to 8.0% for those aged 26-49 years. Interestingly, improvements in time in range were seen even in the oldest group, aged 50-70, who increased from an already high baseline of 69.9% to 79.1% with Omnipod 5 after 3 months.
In her current study, in the youngest age group, the improvement in time in range was achieved primarily by a reduction of time above range, from 2.4 fewer hours/day above 180 mg/dL, while time below 70 mg/dL was reduced by 4 minutes/day. Overnight time in range improved by 1.4 hours/night, with most of the improvements in reduction of hyperglycemia.
The proportions meeting the combined goals of less than 4% time below range and greater than 60% time in range rose from 29% to 65%.
There were no episodes of severe hypoglycemia or diabetic ketoacidosis during the 3-month study phase.
Another important related metric, sleep quality for parents/caregivers, also improved. The percentage reporting overall sleep quality of “very good” or “fairly good” increased from 65% at baseline to 90% with Omnipod 5, while “very bad” sleep quality fell from 8.8% to 0%.
All 80 patients completed the study and elected to continue in a 12-month extension phase.
Ongoing benefit seen in older children and adults
In a late-breaking poster presented on June 25, Anders L. Carlson, MD, medical director at the International Diabetes Center at Park Nicollet, Minneapolis, presented more follow-up data to the previously reported 3-month pivotal study, including 108 older children and 109 adults from the original study.
A1c remained lower after 6 months than at baseline for both children and adults (P < .001). In the children, A1c levels weren’t significantly different at the end of 6 versus 3 months, while in the adults there was an additional 0.1 percentage point decrease (P < .01).
There was one episode of diabetic ketoacidosis and no severe hypoglycemic episodes in the 3-month extension. “Sustained reduction of A1c indicates the potential long-term benefit of the Omnipod 5 System,” Dr. Carlson and colleagues concluded.
Reduced diabetes distress, don’t forget parents’ quality of life
Meanwhile, psychologist Korey K. Hood, PhD, of Stanford (Calif.) University, presented quality of life data at the meeting for 83 children aged 6-11.9 years and 42 teens aged 12-17.9 years using the Omnipod 5 from the larger study population and their parents.
Significant improvements were seen for both the youth and their caregivers in the Problem Areas in Diabetes score, a measure of diabetes-related emotional distress. Changes were less dramatic on the Hypoglycemic Confidence Scale, although improvements were significant for the caregivers of the younger children.
“We know this is a group that is really worried about hypoglycemia across a lot of situations, not just sleep but also school and outside of the home. So, to increase their confidence to this extent I think is a pretty important finding,” Dr. Hood commented.
There were nonsignificant trends in improvement across groups on the Pittsburgh Sleep Quality Index, but overall sleep quality did significantly improve among parents of the younger children. And on the World Health Organization–5 quality of life survey, significant improvements again were seen among the caregivers of young children.
“Reduced diabetes distress and improved quality of life are key benefits of using the Omnipod 5 [automated insulin delivery] system that are complementary to the glycemic benefits achieved,” Dr. Hood said.
Dr. Jacobsen has reported no relevant financial relationships. Dr. Sherr has reported being an adviser for, consultant for, and/or grant recipient from Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic Diabetes, Eli Lilly, Lexicon, Sanofi, and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Hood has reported being a consultant for Cecelia Health, Havas, and Cercacor.
A version of this article first appeared on Medscape.com.
Insulet’s investigational Omnipod 5 automated insulin delivery system improves glycemic control in people with type 1 diabetes aged as young as 2 years, new data suggest.
The Omnipod 5 system combines a tubing-free insulin-filled delivery “Pod” with the Dexcom G6 continuous glucose monitor and an algorithm built into the Pod connecting the two devices via a smartphone app to semiautomate insulin delivery. It is currently under review by the Food and Drug Administration. The company expects to launch it in limited release during the second half of 2021.
Results from a pivotal trial of the system in children aged 2-5.9 years with type 1 diabetes were presented during the annual scientific sessions of the American Diabetes Association.
Follow-up data at 6 months were also presented for another pivotal study of 112 children aged 6-13.9 years and 129 adults aged 14-70 years. Those primary 3-month data were reported earlier this year at the Endocrine Society’s annual meeting and subsequently published online June 7, 2021, in Diabetes Care. Another study presented at ADA looked at quality of life in children using Omnipod 5 and their caregivers.
If approved by the FDA, the Omnipod 5 would be the third commercially available automated insulin delivery system – also called hybrid closed-loop or artificial pancreas systems – in the United States. It would be the second approved for children as young as 2 years of age and the first to deliver insulin subcutaneously without tubing.
‘No-tubing’ feature will be a draw for parents of young children
Asked to comment, pediatric endocrinologist Laura M. Jacobsen, MD, of the University of Florida, Gainesville, said in an interview: “I think the big advantage for the Omnipod 5 is that [if approved it will be] the only tubeless automated insulin delivery system in the U.S.”
“The automated delivery systems have just been wonderful for helping patients achieve time in range, especially overnight. And the fact that this goes down to such a young age where that can be very difficult is wonderful.”
Another difference between the Omnipod 5 and other systems is the ability to adjust glucose targets (from 110 to 150 mg/dL), although newer versions of the currently available hybrid closed-loop systems are expected to include that feature as well. “They’re all slightly different in the way the algorithms work, but I think the end result is similar,” Dr. Jacobsen said.
But, she said, the no-tubing feature might be particularly helpful for some very active young kids. “A lot of small kids do use the tubed pumps, and you can make it work with a lot of kids, but with some kids it just won’t ... the tubing gets caught. I think this really helps parents make the step. A lot of them don’t want to try the tubing whereas they see the Omnipod and might feel a little more confidence to try a pump.”
Overall, said Dr. Jacobsen, who has no financial disclosures with Insulet, Dexcom, or any of their competitors, “I think any addition to the technology field to improve quality of life for people with type 1 diabetes is important and people need choices.”
Pivotal data show benefit in ‘difficult-to-manage’ preschool children
Pivotal 3-month data for the Omnipod 5 in children aged 2-5.9 years with type 1 diabetes were presented on June 26 by pediatric endocrinologist Jennifer Sherr, MD, PhD, Yale University, New Haven, Conn.
“As a pediatric endocrinologist, I can attest to the difficulty of managing this age group, due to grazing eating patterns and erratic physical activity. Oftentimes, care providers may fear hypoglycemia as these youth can not verbalize or self-treat lows,” she remarked.
A total of 80 children were enrolled at 10 institutions across the United Sates. There was a single 14-day standard therapy phase (baseline), followed by 3 months of automated insulin delivery during which the children’s eating and exercise were unrestricted.
At 3 months, average hemoglobin A1c had fallen from 7.4% at baseline to 6.9%, a significant difference (P < .05). The proportions achieving the target A1c of less than 7% were 54% at 3 months versus 31% at baseline. The reduction was even greater among the 25 with baseline A1c of 8% or greater, although it was significant even among the 55 who started with a lower A1c (–1.06 vs. –0.31 percentage points; both P < .05).
Time in range rose from 57.2% at baseline to 68.1% at 3 months (P < .05).
“These youngsters are spending an average of 2.6 more hours/day in range,” Dr. Sherr commented, noting that the difference became apparent shortly after study start and was maintained during the 3 months.
Dr. Sherr noted that this 10.9% improvement in time in range with Omnipod 5 was similar to improvements in the previously reported pivotal study of older children and adults. Data from that study showed improvement in time in range from a gain of 15.6% for the 6 to 13.9 year olds to 8.0% for those aged 26-49 years. Interestingly, improvements in time in range were seen even in the oldest group, aged 50-70, who increased from an already high baseline of 69.9% to 79.1% with Omnipod 5 after 3 months.
In her current study, in the youngest age group, the improvement in time in range was achieved primarily by a reduction of time above range, from 2.4 fewer hours/day above 180 mg/dL, while time below 70 mg/dL was reduced by 4 minutes/day. Overnight time in range improved by 1.4 hours/night, with most of the improvements in reduction of hyperglycemia.
The proportions meeting the combined goals of less than 4% time below range and greater than 60% time in range rose from 29% to 65%.
There were no episodes of severe hypoglycemia or diabetic ketoacidosis during the 3-month study phase.
Another important related metric, sleep quality for parents/caregivers, also improved. The percentage reporting overall sleep quality of “very good” or “fairly good” increased from 65% at baseline to 90% with Omnipod 5, while “very bad” sleep quality fell from 8.8% to 0%.
All 80 patients completed the study and elected to continue in a 12-month extension phase.
Ongoing benefit seen in older children and adults
In a late-breaking poster presented on June 25, Anders L. Carlson, MD, medical director at the International Diabetes Center at Park Nicollet, Minneapolis, presented more follow-up data to the previously reported 3-month pivotal study, including 108 older children and 109 adults from the original study.
A1c remained lower after 6 months than at baseline for both children and adults (P < .001). In the children, A1c levels weren’t significantly different at the end of 6 versus 3 months, while in the adults there was an additional 0.1 percentage point decrease (P < .01).
There was one episode of diabetic ketoacidosis and no severe hypoglycemic episodes in the 3-month extension. “Sustained reduction of A1c indicates the potential long-term benefit of the Omnipod 5 System,” Dr. Carlson and colleagues concluded.
Reduced diabetes distress, don’t forget parents’ quality of life
Meanwhile, psychologist Korey K. Hood, PhD, of Stanford (Calif.) University, presented quality of life data at the meeting for 83 children aged 6-11.9 years and 42 teens aged 12-17.9 years using the Omnipod 5 from the larger study population and their parents.
Significant improvements were seen for both the youth and their caregivers in the Problem Areas in Diabetes score, a measure of diabetes-related emotional distress. Changes were less dramatic on the Hypoglycemic Confidence Scale, although improvements were significant for the caregivers of the younger children.
“We know this is a group that is really worried about hypoglycemia across a lot of situations, not just sleep but also school and outside of the home. So, to increase their confidence to this extent I think is a pretty important finding,” Dr. Hood commented.
There were nonsignificant trends in improvement across groups on the Pittsburgh Sleep Quality Index, but overall sleep quality did significantly improve among parents of the younger children. And on the World Health Organization–5 quality of life survey, significant improvements again were seen among the caregivers of young children.
“Reduced diabetes distress and improved quality of life are key benefits of using the Omnipod 5 [automated insulin delivery] system that are complementary to the glycemic benefits achieved,” Dr. Hood said.
Dr. Jacobsen has reported no relevant financial relationships. Dr. Sherr has reported being an adviser for, consultant for, and/or grant recipient from Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic Diabetes, Eli Lilly, Lexicon, Sanofi, and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Hood has reported being a consultant for Cecelia Health, Havas, and Cercacor.
A version of this article first appeared on Medscape.com.
Insulet’s investigational Omnipod 5 automated insulin delivery system improves glycemic control in people with type 1 diabetes aged as young as 2 years, new data suggest.
The Omnipod 5 system combines a tubing-free insulin-filled delivery “Pod” with the Dexcom G6 continuous glucose monitor and an algorithm built into the Pod connecting the two devices via a smartphone app to semiautomate insulin delivery. It is currently under review by the Food and Drug Administration. The company expects to launch it in limited release during the second half of 2021.
Results from a pivotal trial of the system in children aged 2-5.9 years with type 1 diabetes were presented during the annual scientific sessions of the American Diabetes Association.
Follow-up data at 6 months were also presented for another pivotal study of 112 children aged 6-13.9 years and 129 adults aged 14-70 years. Those primary 3-month data were reported earlier this year at the Endocrine Society’s annual meeting and subsequently published online June 7, 2021, in Diabetes Care. Another study presented at ADA looked at quality of life in children using Omnipod 5 and their caregivers.
If approved by the FDA, the Omnipod 5 would be the third commercially available automated insulin delivery system – also called hybrid closed-loop or artificial pancreas systems – in the United States. It would be the second approved for children as young as 2 years of age and the first to deliver insulin subcutaneously without tubing.
‘No-tubing’ feature will be a draw for parents of young children
Asked to comment, pediatric endocrinologist Laura M. Jacobsen, MD, of the University of Florida, Gainesville, said in an interview: “I think the big advantage for the Omnipod 5 is that [if approved it will be] the only tubeless automated insulin delivery system in the U.S.”
“The automated delivery systems have just been wonderful for helping patients achieve time in range, especially overnight. And the fact that this goes down to such a young age where that can be very difficult is wonderful.”
Another difference between the Omnipod 5 and other systems is the ability to adjust glucose targets (from 110 to 150 mg/dL), although newer versions of the currently available hybrid closed-loop systems are expected to include that feature as well. “They’re all slightly different in the way the algorithms work, but I think the end result is similar,” Dr. Jacobsen said.
But, she said, the no-tubing feature might be particularly helpful for some very active young kids. “A lot of small kids do use the tubed pumps, and you can make it work with a lot of kids, but with some kids it just won’t ... the tubing gets caught. I think this really helps parents make the step. A lot of them don’t want to try the tubing whereas they see the Omnipod and might feel a little more confidence to try a pump.”
Overall, said Dr. Jacobsen, who has no financial disclosures with Insulet, Dexcom, or any of their competitors, “I think any addition to the technology field to improve quality of life for people with type 1 diabetes is important and people need choices.”
Pivotal data show benefit in ‘difficult-to-manage’ preschool children
Pivotal 3-month data for the Omnipod 5 in children aged 2-5.9 years with type 1 diabetes were presented on June 26 by pediatric endocrinologist Jennifer Sherr, MD, PhD, Yale University, New Haven, Conn.
“As a pediatric endocrinologist, I can attest to the difficulty of managing this age group, due to grazing eating patterns and erratic physical activity. Oftentimes, care providers may fear hypoglycemia as these youth can not verbalize or self-treat lows,” she remarked.
A total of 80 children were enrolled at 10 institutions across the United Sates. There was a single 14-day standard therapy phase (baseline), followed by 3 months of automated insulin delivery during which the children’s eating and exercise were unrestricted.
At 3 months, average hemoglobin A1c had fallen from 7.4% at baseline to 6.9%, a significant difference (P < .05). The proportions achieving the target A1c of less than 7% were 54% at 3 months versus 31% at baseline. The reduction was even greater among the 25 with baseline A1c of 8% or greater, although it was significant even among the 55 who started with a lower A1c (–1.06 vs. –0.31 percentage points; both P < .05).
Time in range rose from 57.2% at baseline to 68.1% at 3 months (P < .05).
“These youngsters are spending an average of 2.6 more hours/day in range,” Dr. Sherr commented, noting that the difference became apparent shortly after study start and was maintained during the 3 months.
Dr. Sherr noted that this 10.9% improvement in time in range with Omnipod 5 was similar to improvements in the previously reported pivotal study of older children and adults. Data from that study showed improvement in time in range from a gain of 15.6% for the 6 to 13.9 year olds to 8.0% for those aged 26-49 years. Interestingly, improvements in time in range were seen even in the oldest group, aged 50-70, who increased from an already high baseline of 69.9% to 79.1% with Omnipod 5 after 3 months.
In her current study, in the youngest age group, the improvement in time in range was achieved primarily by a reduction of time above range, from 2.4 fewer hours/day above 180 mg/dL, while time below 70 mg/dL was reduced by 4 minutes/day. Overnight time in range improved by 1.4 hours/night, with most of the improvements in reduction of hyperglycemia.
The proportions meeting the combined goals of less than 4% time below range and greater than 60% time in range rose from 29% to 65%.
There were no episodes of severe hypoglycemia or diabetic ketoacidosis during the 3-month study phase.
Another important related metric, sleep quality for parents/caregivers, also improved. The percentage reporting overall sleep quality of “very good” or “fairly good” increased from 65% at baseline to 90% with Omnipod 5, while “very bad” sleep quality fell from 8.8% to 0%.
All 80 patients completed the study and elected to continue in a 12-month extension phase.
Ongoing benefit seen in older children and adults
In a late-breaking poster presented on June 25, Anders L. Carlson, MD, medical director at the International Diabetes Center at Park Nicollet, Minneapolis, presented more follow-up data to the previously reported 3-month pivotal study, including 108 older children and 109 adults from the original study.
A1c remained lower after 6 months than at baseline for both children and adults (P < .001). In the children, A1c levels weren’t significantly different at the end of 6 versus 3 months, while in the adults there was an additional 0.1 percentage point decrease (P < .01).
There was one episode of diabetic ketoacidosis and no severe hypoglycemic episodes in the 3-month extension. “Sustained reduction of A1c indicates the potential long-term benefit of the Omnipod 5 System,” Dr. Carlson and colleagues concluded.
Reduced diabetes distress, don’t forget parents’ quality of life
Meanwhile, psychologist Korey K. Hood, PhD, of Stanford (Calif.) University, presented quality of life data at the meeting for 83 children aged 6-11.9 years and 42 teens aged 12-17.9 years using the Omnipod 5 from the larger study population and their parents.
Significant improvements were seen for both the youth and their caregivers in the Problem Areas in Diabetes score, a measure of diabetes-related emotional distress. Changes were less dramatic on the Hypoglycemic Confidence Scale, although improvements were significant for the caregivers of the younger children.
“We know this is a group that is really worried about hypoglycemia across a lot of situations, not just sleep but also school and outside of the home. So, to increase their confidence to this extent I think is a pretty important finding,” Dr. Hood commented.
There were nonsignificant trends in improvement across groups on the Pittsburgh Sleep Quality Index, but overall sleep quality did significantly improve among parents of the younger children. And on the World Health Organization–5 quality of life survey, significant improvements again were seen among the caregivers of young children.
“Reduced diabetes distress and improved quality of life are key benefits of using the Omnipod 5 [automated insulin delivery] system that are complementary to the glycemic benefits achieved,” Dr. Hood said.
Dr. Jacobsen has reported no relevant financial relationships. Dr. Sherr has reported being an adviser for, consultant for, and/or grant recipient from Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic Diabetes, Eli Lilly, Lexicon, Sanofi, and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Hood has reported being a consultant for Cecelia Health, Havas, and Cercacor.
A version of this article first appeared on Medscape.com.
‘Staggering’ doubling of type 2 diabetes in children during pandemic
The incidence of type 2 diabetes in children appears to have doubled during the COVID-19 pandemic, data from two new U.S. studies suggest, with the lead investigator of one saying she was “surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Findings from the two separate retrospective chart reviews – one conducted in Washington, D.C., and the other in Baton Rouge, La. – were presented June 25 at the annual scientific sessions of the American Diabetes Association.
Although the two studies differed somewhat in the clinical parameters examined, both revealed a similar doubling of the rates of hospitalizations for type 2 diabetes among youth during 2020, compared with the same time period in 2019, as well as greater severity of metabolic disturbance.
And, as has been previously described with type 2 diabetes in youth, African American ethnicity predominated in both cohorts.
“Although we could not assess the cause of the increases in type 2 diabetes from our data, these disparities suggest that indirect effects of social distancing measures, including school closure and unemployment, are placing undue burden on underserved communities. Decreases in well-child care and fears of seeking medical care during the pandemic may have also contributed,” lead investigator of one of the studies, pediatric endocrinologist Brynn E. Marks, MD, Children’s National Hospital, Washington, said in an interview.
More hospitalizations, racial disparities aggravated by COVID-19
Lead author of the other study, Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, said in an interview: “Since the pandemic, our data suggest that more children may be diagnosed with type 2 diabetes and may require hospitalization when they are diagnosed. Looking at both datasets, there appears to be a racial disparity in type 2 diabetes diagnoses that has only been exacerbated by the COVID-19 pandemic.”
Of concern, Dr. Hsia said, “The incidence rate of type 2 diabetes in children was already on the rise before the pandemic. While there may be a brief leveling off now that children are getting regular health care and going back to school in person, I believe these rates will continue to rise especially in light of the childhood obesity rates not improving.”
Their dataset captured all youth who were newly diagnosed with type 2 diabetes during the first full year of the COVID-19 pandemic, from March 11, 2020, to March 10, 2021, and compared those data with the time period from March 11, 2019, to March 10, 2020.
During the pandemic, the number of cases of type 2 diabetes increased by 182%, from 50 in 2019 to 141 in 2020. The average age at diagnosis was about 14 years in both time periods.
In the prepandemic period, 18 (36%) diagnosed with type 2 diabetes required inpatient admission, compared with 85 (60.3%) during the pandemic. At Children’s National, youth with suspected new-onset type 2 diabetes aren’t typically hospitalized unless they have severe hyperglycemia, ketosis, or are unable to schedule urgent outpatient follow-up, Dr. Marks noted.
The proportions of youth with new-onset type 2 diabetes who presented in diabetic ketoacidosis (DKA) rose from 2 (4%) prepandemic to 33 (23.4%) during the pandemic. Presentation with hyperosmolar DKA rose from 0 to 13 (9.2%).
However, during the pandemic only five youth were actively infected with SARS-CoV-2 at the time of type 2 diabetes diagnosis among the 90 tested.
Dr. Marks said: “We believe the increase in inpatient admissions was due to more severe presentation during the pandemic. ...We were surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Shift in diagnoses to type 2 diabetes
The pandemic also appears to have shifted the proportion of youth diagnosed with type 2 diabetes, compared with type 1 diabetes. Whereas 24% of youth with new-onset diabetes prepandemic had type 2 diabetes and the rest had type 1 diabetes, during pandemic the proportion with type 2 diabetes rose to 44%.
“Rates of type 2 diabetes rose steadily at a rate of 1.45 cases per month throughout the course of the pandemic, suggesting a cumulative effect of the indirect effects of social distancing measures,” Dr. Marks said.
Furthermore, she added, whereas 60% of youth diagnosed with type 2 diabetes before the pandemic were female, the rate fell to 40% during the pandemic. This trend might be because of activity levels in that, while male adolescents are typically more active, rates of exercise fell in both sexes during the pandemic but declined more sharply in males such that activity levels between the sexes became equal.
Although type 2 diabetes in youth has always been more common in ethnic minorities, the pandemic appears to have exacerbated these disparities.
While 58% of youth diagnosed with type 2 diabetes prepandemic identified as non-Hispanic Black, that proportion rose to 76.7% during the pandemic. Among Black youth with new-onset type 2 diabetes, 31 of 33 presented in DKA, and 12 of the 13 who presented in hyperosmolar DKA during the pandemic were Black.
“Strategies to promote health equity and address the undue burden of the COVID-19 pandemic on underserved communities must be developed to avoid worsening disparities and long-term health outcomes,” Dr. Marks said.
‘A microcosm’: Similar findings in a smaller population
Dr. Hsia and colleagues looked at a smaller number of patients in a shorter time period. In March–December 2019, the hospitalization rate for new-onset type 2 diabetes was 0.27% (8 out of 2,964 hospitalizations), compared with 0.62% (17 out of 2,729 hospitalizations) during the same period in 2020 (P < .048) – also more than a doubling. Age at admission, sex, and body mass index didn’t differ between the two groups.
Criteria for DKA were met by three children in 2019 versus eight in 2020, and hyperosmolar hyperglycemic syndrome in zero versus two, respectively. Mean hemoglobin A1c on admission was 12.4% in 2019 versus 13.1% in 2020 (P = .59), and mean serum glucose was 441 mg/dL versus 669 mg/dL (P = .14), respectively. Serum osmolality on admission was 314 mmol/kg in 2019 versus 335 mmol/kg in 2020 (P = .19).
“Clinically speaking the differences in the lab values were significant, but we did not have enough numbers ... to see a statistically significant difference. I think by looking at more centers, our site likely represents a microcosm of what is happening across the country,” Dr. Hsia said.
In 2019, 7 of the 8 children were African American, as were 16 of the 17 children in 2020. The other single child in each group was White.
Dr. Hsia said: “Larger studies that include more patients are needed to confirm our initial findings. More research is needed to understand why this increasing trend of type 2 diabetes diagnoses in children may be occurring [and] to better understand how stay-at-home orders and other restrictions due to COVID-19 have worsened risk factors for type 2 diabetes.”
“These include decreased physical activity, more screen time, disturbed sleep, and increased intake of processed foods, which can all lead to weight gain,” he concluded.
Dr. Marks reported receiving research support from Tandem, Dexcom, and the Cystic Fibrosis Foundation. Dr. Hsia reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of type 2 diabetes in children appears to have doubled during the COVID-19 pandemic, data from two new U.S. studies suggest, with the lead investigator of one saying she was “surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Findings from the two separate retrospective chart reviews – one conducted in Washington, D.C., and the other in Baton Rouge, La. – were presented June 25 at the annual scientific sessions of the American Diabetes Association.
Although the two studies differed somewhat in the clinical parameters examined, both revealed a similar doubling of the rates of hospitalizations for type 2 diabetes among youth during 2020, compared with the same time period in 2019, as well as greater severity of metabolic disturbance.
And, as has been previously described with type 2 diabetes in youth, African American ethnicity predominated in both cohorts.
“Although we could not assess the cause of the increases in type 2 diabetes from our data, these disparities suggest that indirect effects of social distancing measures, including school closure and unemployment, are placing undue burden on underserved communities. Decreases in well-child care and fears of seeking medical care during the pandemic may have also contributed,” lead investigator of one of the studies, pediatric endocrinologist Brynn E. Marks, MD, Children’s National Hospital, Washington, said in an interview.
More hospitalizations, racial disparities aggravated by COVID-19
Lead author of the other study, Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, said in an interview: “Since the pandemic, our data suggest that more children may be diagnosed with type 2 diabetes and may require hospitalization when they are diagnosed. Looking at both datasets, there appears to be a racial disparity in type 2 diabetes diagnoses that has only been exacerbated by the COVID-19 pandemic.”
Of concern, Dr. Hsia said, “The incidence rate of type 2 diabetes in children was already on the rise before the pandemic. While there may be a brief leveling off now that children are getting regular health care and going back to school in person, I believe these rates will continue to rise especially in light of the childhood obesity rates not improving.”
Their dataset captured all youth who were newly diagnosed with type 2 diabetes during the first full year of the COVID-19 pandemic, from March 11, 2020, to March 10, 2021, and compared those data with the time period from March 11, 2019, to March 10, 2020.
During the pandemic, the number of cases of type 2 diabetes increased by 182%, from 50 in 2019 to 141 in 2020. The average age at diagnosis was about 14 years in both time periods.
In the prepandemic period, 18 (36%) diagnosed with type 2 diabetes required inpatient admission, compared with 85 (60.3%) during the pandemic. At Children’s National, youth with suspected new-onset type 2 diabetes aren’t typically hospitalized unless they have severe hyperglycemia, ketosis, or are unable to schedule urgent outpatient follow-up, Dr. Marks noted.
The proportions of youth with new-onset type 2 diabetes who presented in diabetic ketoacidosis (DKA) rose from 2 (4%) prepandemic to 33 (23.4%) during the pandemic. Presentation with hyperosmolar DKA rose from 0 to 13 (9.2%).
However, during the pandemic only five youth were actively infected with SARS-CoV-2 at the time of type 2 diabetes diagnosis among the 90 tested.
Dr. Marks said: “We believe the increase in inpatient admissions was due to more severe presentation during the pandemic. ...We were surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Shift in diagnoses to type 2 diabetes
The pandemic also appears to have shifted the proportion of youth diagnosed with type 2 diabetes, compared with type 1 diabetes. Whereas 24% of youth with new-onset diabetes prepandemic had type 2 diabetes and the rest had type 1 diabetes, during pandemic the proportion with type 2 diabetes rose to 44%.
“Rates of type 2 diabetes rose steadily at a rate of 1.45 cases per month throughout the course of the pandemic, suggesting a cumulative effect of the indirect effects of social distancing measures,” Dr. Marks said.
Furthermore, she added, whereas 60% of youth diagnosed with type 2 diabetes before the pandemic were female, the rate fell to 40% during the pandemic. This trend might be because of activity levels in that, while male adolescents are typically more active, rates of exercise fell in both sexes during the pandemic but declined more sharply in males such that activity levels between the sexes became equal.
Although type 2 diabetes in youth has always been more common in ethnic minorities, the pandemic appears to have exacerbated these disparities.
While 58% of youth diagnosed with type 2 diabetes prepandemic identified as non-Hispanic Black, that proportion rose to 76.7% during the pandemic. Among Black youth with new-onset type 2 diabetes, 31 of 33 presented in DKA, and 12 of the 13 who presented in hyperosmolar DKA during the pandemic were Black.
“Strategies to promote health equity and address the undue burden of the COVID-19 pandemic on underserved communities must be developed to avoid worsening disparities and long-term health outcomes,” Dr. Marks said.
‘A microcosm’: Similar findings in a smaller population
Dr. Hsia and colleagues looked at a smaller number of patients in a shorter time period. In March–December 2019, the hospitalization rate for new-onset type 2 diabetes was 0.27% (8 out of 2,964 hospitalizations), compared with 0.62% (17 out of 2,729 hospitalizations) during the same period in 2020 (P < .048) – also more than a doubling. Age at admission, sex, and body mass index didn’t differ between the two groups.
Criteria for DKA were met by three children in 2019 versus eight in 2020, and hyperosmolar hyperglycemic syndrome in zero versus two, respectively. Mean hemoglobin A1c on admission was 12.4% in 2019 versus 13.1% in 2020 (P = .59), and mean serum glucose was 441 mg/dL versus 669 mg/dL (P = .14), respectively. Serum osmolality on admission was 314 mmol/kg in 2019 versus 335 mmol/kg in 2020 (P = .19).
“Clinically speaking the differences in the lab values were significant, but we did not have enough numbers ... to see a statistically significant difference. I think by looking at more centers, our site likely represents a microcosm of what is happening across the country,” Dr. Hsia said.
In 2019, 7 of the 8 children were African American, as were 16 of the 17 children in 2020. The other single child in each group was White.
Dr. Hsia said: “Larger studies that include more patients are needed to confirm our initial findings. More research is needed to understand why this increasing trend of type 2 diabetes diagnoses in children may be occurring [and] to better understand how stay-at-home orders and other restrictions due to COVID-19 have worsened risk factors for type 2 diabetes.”
“These include decreased physical activity, more screen time, disturbed sleep, and increased intake of processed foods, which can all lead to weight gain,” he concluded.
Dr. Marks reported receiving research support from Tandem, Dexcom, and the Cystic Fibrosis Foundation. Dr. Hsia reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of type 2 diabetes in children appears to have doubled during the COVID-19 pandemic, data from two new U.S. studies suggest, with the lead investigator of one saying she was “surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Findings from the two separate retrospective chart reviews – one conducted in Washington, D.C., and the other in Baton Rouge, La. – were presented June 25 at the annual scientific sessions of the American Diabetes Association.
Although the two studies differed somewhat in the clinical parameters examined, both revealed a similar doubling of the rates of hospitalizations for type 2 diabetes among youth during 2020, compared with the same time period in 2019, as well as greater severity of metabolic disturbance.
And, as has been previously described with type 2 diabetes in youth, African American ethnicity predominated in both cohorts.
“Although we could not assess the cause of the increases in type 2 diabetes from our data, these disparities suggest that indirect effects of social distancing measures, including school closure and unemployment, are placing undue burden on underserved communities. Decreases in well-child care and fears of seeking medical care during the pandemic may have also contributed,” lead investigator of one of the studies, pediatric endocrinologist Brynn E. Marks, MD, Children’s National Hospital, Washington, said in an interview.
More hospitalizations, racial disparities aggravated by COVID-19
Lead author of the other study, Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, said in an interview: “Since the pandemic, our data suggest that more children may be diagnosed with type 2 diabetes and may require hospitalization when they are diagnosed. Looking at both datasets, there appears to be a racial disparity in type 2 diabetes diagnoses that has only been exacerbated by the COVID-19 pandemic.”
Of concern, Dr. Hsia said, “The incidence rate of type 2 diabetes in children was already on the rise before the pandemic. While there may be a brief leveling off now that children are getting regular health care and going back to school in person, I believe these rates will continue to rise especially in light of the childhood obesity rates not improving.”
Their dataset captured all youth who were newly diagnosed with type 2 diabetes during the first full year of the COVID-19 pandemic, from March 11, 2020, to March 10, 2021, and compared those data with the time period from March 11, 2019, to March 10, 2020.
During the pandemic, the number of cases of type 2 diabetes increased by 182%, from 50 in 2019 to 141 in 2020. The average age at diagnosis was about 14 years in both time periods.
In the prepandemic period, 18 (36%) diagnosed with type 2 diabetes required inpatient admission, compared with 85 (60.3%) during the pandemic. At Children’s National, youth with suspected new-onset type 2 diabetes aren’t typically hospitalized unless they have severe hyperglycemia, ketosis, or are unable to schedule urgent outpatient follow-up, Dr. Marks noted.
The proportions of youth with new-onset type 2 diabetes who presented in diabetic ketoacidosis (DKA) rose from 2 (4%) prepandemic to 33 (23.4%) during the pandemic. Presentation with hyperosmolar DKA rose from 0 to 13 (9.2%).
However, during the pandemic only five youth were actively infected with SARS-CoV-2 at the time of type 2 diabetes diagnosis among the 90 tested.
Dr. Marks said: “We believe the increase in inpatient admissions was due to more severe presentation during the pandemic. ...We were surprised by the staggering increase in cases of type 2 diabetes ... and the increase in severity of presentation.”
Shift in diagnoses to type 2 diabetes
The pandemic also appears to have shifted the proportion of youth diagnosed with type 2 diabetes, compared with type 1 diabetes. Whereas 24% of youth with new-onset diabetes prepandemic had type 2 diabetes and the rest had type 1 diabetes, during pandemic the proportion with type 2 diabetes rose to 44%.
“Rates of type 2 diabetes rose steadily at a rate of 1.45 cases per month throughout the course of the pandemic, suggesting a cumulative effect of the indirect effects of social distancing measures,” Dr. Marks said.
Furthermore, she added, whereas 60% of youth diagnosed with type 2 diabetes before the pandemic were female, the rate fell to 40% during the pandemic. This trend might be because of activity levels in that, while male adolescents are typically more active, rates of exercise fell in both sexes during the pandemic but declined more sharply in males such that activity levels between the sexes became equal.
Although type 2 diabetes in youth has always been more common in ethnic minorities, the pandemic appears to have exacerbated these disparities.
While 58% of youth diagnosed with type 2 diabetes prepandemic identified as non-Hispanic Black, that proportion rose to 76.7% during the pandemic. Among Black youth with new-onset type 2 diabetes, 31 of 33 presented in DKA, and 12 of the 13 who presented in hyperosmolar DKA during the pandemic were Black.
“Strategies to promote health equity and address the undue burden of the COVID-19 pandemic on underserved communities must be developed to avoid worsening disparities and long-term health outcomes,” Dr. Marks said.
‘A microcosm’: Similar findings in a smaller population
Dr. Hsia and colleagues looked at a smaller number of patients in a shorter time period. In March–December 2019, the hospitalization rate for new-onset type 2 diabetes was 0.27% (8 out of 2,964 hospitalizations), compared with 0.62% (17 out of 2,729 hospitalizations) during the same period in 2020 (P < .048) – also more than a doubling. Age at admission, sex, and body mass index didn’t differ between the two groups.
Criteria for DKA were met by three children in 2019 versus eight in 2020, and hyperosmolar hyperglycemic syndrome in zero versus two, respectively. Mean hemoglobin A1c on admission was 12.4% in 2019 versus 13.1% in 2020 (P = .59), and mean serum glucose was 441 mg/dL versus 669 mg/dL (P = .14), respectively. Serum osmolality on admission was 314 mmol/kg in 2019 versus 335 mmol/kg in 2020 (P = .19).
“Clinically speaking the differences in the lab values were significant, but we did not have enough numbers ... to see a statistically significant difference. I think by looking at more centers, our site likely represents a microcosm of what is happening across the country,” Dr. Hsia said.
In 2019, 7 of the 8 children were African American, as were 16 of the 17 children in 2020. The other single child in each group was White.
Dr. Hsia said: “Larger studies that include more patients are needed to confirm our initial findings. More research is needed to understand why this increasing trend of type 2 diabetes diagnoses in children may be occurring [and] to better understand how stay-at-home orders and other restrictions due to COVID-19 have worsened risk factors for type 2 diabetes.”
“These include decreased physical activity, more screen time, disturbed sleep, and increased intake of processed foods, which can all lead to weight gain,” he concluded.
Dr. Marks reported receiving research support from Tandem, Dexcom, and the Cystic Fibrosis Foundation. Dr. Hsia reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADA scientific sessions address the old and the new
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What’s behind brain fog in treated hypothyroidism?
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medicare rule changes allow for broader CGM use
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Healthy with obesity? The latest study casts doubt
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
FROM DIABETOLOGIA
‘A better picture’: First AACE guidelines on diabetes technology
The American Association of Clinical Endocrinology (AACE) has issued its first-ever official guidelines addressing the use of advanced technologies in the management of people with diabetes.
The guidelines cover use of continuous glucose monitoring (CGM), insulin pumps, connected pens, automated insulin delivery systems, telemedicine technologies, and smartphone apps. They also address safety considerations, special situations such as hospitalization, and implementation in clinical practice.
They were presented on May 28 at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists and simultaneously published in Endocrine Practice.
Previous AACE guidance on the clinical use of insulin pumps and CGM over the past decade has been published in the form of consensus or position statements rather than official evidence-based guidelines, task force cochair George Grunberger, MD, of the Grunberger Diabetes Institute, Bloomfield Hills, Mich., explained.
“There’s never really been, until now, hardcore evidence, [with] peer-reviewed, quality trials published in the literature to go after the evidence that is required for guidelines. ... This is not an opinion piece or position statement.”
The problem with that strict approach to “guidelines” is how quickly the diabetes technology field is evolving, he acknowledged. “It’s frustrating because we know what’s [coming up], but we can’t put it in a guideline because it hasn’t been published yet.”
In an AACE podcast, Dr. Grunberger said the guidelines will likely become a “living” document, along the lines of the American Diabetes Association’s annual Standards of Care, as “any cutoff date is arbitrary. More and more papers will be published on these technologies. ... This is certainly not a static field.”
In the meantime, task force cochair and author Jennifer Sherr, MD, PhD, a pediatric endocrinologist, said she hopes the guidelines will help to reduce insurance company barriers to use of the currently available technologies.
“I am very hopeful that these guidelines will also encourage payers to change their stance. And I think that we as a community can continue to advocate and inform them of these guidelines so they can appropriately change their coverage practices,” added Dr. Sherr, of Yale University, New Haven, Conn.
Recommendations address CGM, pumps, and connected systems
In the guidelines, CGM is “strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as three or more injections of insulin per day or the use of an insulin pump.” For those with diabetes who use CGM, “priority metrics” include a “time in range” of greater than 70% from 14 days of active use. Targets for mean glucose should be individualized, with glycemic variability 36% or lower.
Further specific CGM target metrics are given for people with type 1 diabetes, older/high risk individuals, and for pregnant women. The recommendations align with those issued in a 2019 joint consensus statement on CGM time-in-range endorsed by several organizations, including AACE.
In response to an audience question about whether AACE is advising that time-in-range replace A1c for glycemia assessment, Dr. Sherr responded: “I think currently we’re not in a position where we can completely replace A1c with time in range. However, I’m hopeful that in future years we’ll see further data gathered ... to allow for that recommendation to occur.”
For now, she said, “What we really want to hone in on in the guidelines is that time-in-range and use of CGM truly allow clinicians to better understand how to optimize care for their persons with diabetes. It gives us a better picture. It’s not just a number of whether we’re hitting target. It tells us whether we need to attack time above range or time below range. So we really think it’s critical for clinical care.”
The document also provides specifics about real-time versus intermittently scanned CGM and use of diagnostic/professional CGM.
The “insulin delivery technologies” section covers use of connected pens, insulin pumps without CGM, insulin pumps with separate CGM, and the more advanced combined insulin pump-CGM systems including those with low-glucose suspend, predictive low-glucose suspend, and hybrid closed-loops (sometimes called the artificial pancreas).
In general, these automated insulin delivery systems (artificial pancreas), “are strongly recommended for all persons with [type 1 diabetes], since their use has been shown to increase time in range, especially in the overnight period, without causing an increased risk of hypoglycemia,” Dr. Sherr observed.
Other tech topics: Apps, telemedicine, and safety
The new guidelines say that “clinically validated” smartphone apps should be recommended to help teach or reinforce diabetes self-management skills and provide support and encouragement for healthy behaviors around food and exercise.
Dr. Grunberger pointed out: “As we know, there are tons of apps out there, and patients are using them. The problem is that very few of them have actually been validated in clinical trials in published peer-reviewed [journals].”
He recommended a joint statement on diabetes apps from the American Diabetes Association and the European Association for the Study of Diabetes that was initially discussed at the 2019 EASD meeting, as reported by this news organization, and subsequently published in January 2020 in Diabetes Care and Diabetologia.
“Telemedicine, including periodic phone calls, smartphone-web interactions ... by health care professionals ... is strongly recommended to treat persons with diabetes, provide diabetes education, remotely monitor glucose and/or insulin data to indicate the need for therapy adjustments, and improve diabetes-related outcomes/control with better engagement,” the document says.
Safety concerns addressed include the issue of certain medications interfering with CGM [readings] ... including acetaminophen, high-dose vitamin C, and hydroxyurea, as well as cautions about what to do in the event of device malfunction and assessing that the patient is sufficiently trained in proper device use. Criteria for insulin pump discontinuation are also given.
Implementation: Who will be prescribing? ‘This is not for amateurs’
A final section on implementation recommends that “initiation and use of diabetes technology should be implemented by health care professionals who are trained, committed, and experienced to prescribe and direct the use of these tools. Clinicians should have the infrastructure to support the needs of persons with diabetes using the technology.”
Dr. Grunberger commented: “I think the key is going to be who should be doing this? What is the role of a clinical endocrinologist in the future? What is our responsibility, [since] we don’t have the manpower and womanpower to take care of all these people as these technologies advance? It’s our responsibility to provide these hopefully valued recommendations as a resource for those who want to know more about it.”
However, he noted, “This is not for amateurs. If you want to actually use this in your practice, you need the infrastructure, the expertise, the training, the dedication, and the energy to be there for the patients all the time ... This clinical practice guideline is a foundation.”
Dr. Sherr added: “To me, it’s really thinking about ... changing our mindset from who is an appropriate candidate to who can benefit and how vast a group that entails ... I’m hopeful that we will see more technology use through continued conversations with our patients with diabetes, and hopefully through more clinicians being excited to be part of this revolution.”
Dr. Grunberger has reported being on speakers bureaus for Eli Lilly, Novo Nordisk, and Abbott. Dr. Sherr has reported being a consultant and speaker for Lilly and Medtronic Diabetes, a consultant for Insulet and Sanofi, and on advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, JDRF T1D fund, and Medtronic.
A version of this article first appeared on Medscape.com.
The American Association of Clinical Endocrinology (AACE) has issued its first-ever official guidelines addressing the use of advanced technologies in the management of people with diabetes.
The guidelines cover use of continuous glucose monitoring (CGM), insulin pumps, connected pens, automated insulin delivery systems, telemedicine technologies, and smartphone apps. They also address safety considerations, special situations such as hospitalization, and implementation in clinical practice.
They were presented on May 28 at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists and simultaneously published in Endocrine Practice.
Previous AACE guidance on the clinical use of insulin pumps and CGM over the past decade has been published in the form of consensus or position statements rather than official evidence-based guidelines, task force cochair George Grunberger, MD, of the Grunberger Diabetes Institute, Bloomfield Hills, Mich., explained.
“There’s never really been, until now, hardcore evidence, [with] peer-reviewed, quality trials published in the literature to go after the evidence that is required for guidelines. ... This is not an opinion piece or position statement.”
The problem with that strict approach to “guidelines” is how quickly the diabetes technology field is evolving, he acknowledged. “It’s frustrating because we know what’s [coming up], but we can’t put it in a guideline because it hasn’t been published yet.”
In an AACE podcast, Dr. Grunberger said the guidelines will likely become a “living” document, along the lines of the American Diabetes Association’s annual Standards of Care, as “any cutoff date is arbitrary. More and more papers will be published on these technologies. ... This is certainly not a static field.”
In the meantime, task force cochair and author Jennifer Sherr, MD, PhD, a pediatric endocrinologist, said she hopes the guidelines will help to reduce insurance company barriers to use of the currently available technologies.
“I am very hopeful that these guidelines will also encourage payers to change their stance. And I think that we as a community can continue to advocate and inform them of these guidelines so they can appropriately change their coverage practices,” added Dr. Sherr, of Yale University, New Haven, Conn.
Recommendations address CGM, pumps, and connected systems
In the guidelines, CGM is “strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as three or more injections of insulin per day or the use of an insulin pump.” For those with diabetes who use CGM, “priority metrics” include a “time in range” of greater than 70% from 14 days of active use. Targets for mean glucose should be individualized, with glycemic variability 36% or lower.
Further specific CGM target metrics are given for people with type 1 diabetes, older/high risk individuals, and for pregnant women. The recommendations align with those issued in a 2019 joint consensus statement on CGM time-in-range endorsed by several organizations, including AACE.
In response to an audience question about whether AACE is advising that time-in-range replace A1c for glycemia assessment, Dr. Sherr responded: “I think currently we’re not in a position where we can completely replace A1c with time in range. However, I’m hopeful that in future years we’ll see further data gathered ... to allow for that recommendation to occur.”
For now, she said, “What we really want to hone in on in the guidelines is that time-in-range and use of CGM truly allow clinicians to better understand how to optimize care for their persons with diabetes. It gives us a better picture. It’s not just a number of whether we’re hitting target. It tells us whether we need to attack time above range or time below range. So we really think it’s critical for clinical care.”
The document also provides specifics about real-time versus intermittently scanned CGM and use of diagnostic/professional CGM.
The “insulin delivery technologies” section covers use of connected pens, insulin pumps without CGM, insulin pumps with separate CGM, and the more advanced combined insulin pump-CGM systems including those with low-glucose suspend, predictive low-glucose suspend, and hybrid closed-loops (sometimes called the artificial pancreas).
In general, these automated insulin delivery systems (artificial pancreas), “are strongly recommended for all persons with [type 1 diabetes], since their use has been shown to increase time in range, especially in the overnight period, without causing an increased risk of hypoglycemia,” Dr. Sherr observed.
Other tech topics: Apps, telemedicine, and safety
The new guidelines say that “clinically validated” smartphone apps should be recommended to help teach or reinforce diabetes self-management skills and provide support and encouragement for healthy behaviors around food and exercise.
Dr. Grunberger pointed out: “As we know, there are tons of apps out there, and patients are using them. The problem is that very few of them have actually been validated in clinical trials in published peer-reviewed [journals].”
He recommended a joint statement on diabetes apps from the American Diabetes Association and the European Association for the Study of Diabetes that was initially discussed at the 2019 EASD meeting, as reported by this news organization, and subsequently published in January 2020 in Diabetes Care and Diabetologia.
“Telemedicine, including periodic phone calls, smartphone-web interactions ... by health care professionals ... is strongly recommended to treat persons with diabetes, provide diabetes education, remotely monitor glucose and/or insulin data to indicate the need for therapy adjustments, and improve diabetes-related outcomes/control with better engagement,” the document says.
Safety concerns addressed include the issue of certain medications interfering with CGM [readings] ... including acetaminophen, high-dose vitamin C, and hydroxyurea, as well as cautions about what to do in the event of device malfunction and assessing that the patient is sufficiently trained in proper device use. Criteria for insulin pump discontinuation are also given.
Implementation: Who will be prescribing? ‘This is not for amateurs’
A final section on implementation recommends that “initiation and use of diabetes technology should be implemented by health care professionals who are trained, committed, and experienced to prescribe and direct the use of these tools. Clinicians should have the infrastructure to support the needs of persons with diabetes using the technology.”
Dr. Grunberger commented: “I think the key is going to be who should be doing this? What is the role of a clinical endocrinologist in the future? What is our responsibility, [since] we don’t have the manpower and womanpower to take care of all these people as these technologies advance? It’s our responsibility to provide these hopefully valued recommendations as a resource for those who want to know more about it.”
However, he noted, “This is not for amateurs. If you want to actually use this in your practice, you need the infrastructure, the expertise, the training, the dedication, and the energy to be there for the patients all the time ... This clinical practice guideline is a foundation.”
Dr. Sherr added: “To me, it’s really thinking about ... changing our mindset from who is an appropriate candidate to who can benefit and how vast a group that entails ... I’m hopeful that we will see more technology use through continued conversations with our patients with diabetes, and hopefully through more clinicians being excited to be part of this revolution.”
Dr. Grunberger has reported being on speakers bureaus for Eli Lilly, Novo Nordisk, and Abbott. Dr. Sherr has reported being a consultant and speaker for Lilly and Medtronic Diabetes, a consultant for Insulet and Sanofi, and on advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, JDRF T1D fund, and Medtronic.
A version of this article first appeared on Medscape.com.
The American Association of Clinical Endocrinology (AACE) has issued its first-ever official guidelines addressing the use of advanced technologies in the management of people with diabetes.
The guidelines cover use of continuous glucose monitoring (CGM), insulin pumps, connected pens, automated insulin delivery systems, telemedicine technologies, and smartphone apps. They also address safety considerations, special situations such as hospitalization, and implementation in clinical practice.
They were presented on May 28 at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists and simultaneously published in Endocrine Practice.
Previous AACE guidance on the clinical use of insulin pumps and CGM over the past decade has been published in the form of consensus or position statements rather than official evidence-based guidelines, task force cochair George Grunberger, MD, of the Grunberger Diabetes Institute, Bloomfield Hills, Mich., explained.
“There’s never really been, until now, hardcore evidence, [with] peer-reviewed, quality trials published in the literature to go after the evidence that is required for guidelines. ... This is not an opinion piece or position statement.”
The problem with that strict approach to “guidelines” is how quickly the diabetes technology field is evolving, he acknowledged. “It’s frustrating because we know what’s [coming up], but we can’t put it in a guideline because it hasn’t been published yet.”
In an AACE podcast, Dr. Grunberger said the guidelines will likely become a “living” document, along the lines of the American Diabetes Association’s annual Standards of Care, as “any cutoff date is arbitrary. More and more papers will be published on these technologies. ... This is certainly not a static field.”
In the meantime, task force cochair and author Jennifer Sherr, MD, PhD, a pediatric endocrinologist, said she hopes the guidelines will help to reduce insurance company barriers to use of the currently available technologies.
“I am very hopeful that these guidelines will also encourage payers to change their stance. And I think that we as a community can continue to advocate and inform them of these guidelines so they can appropriately change their coverage practices,” added Dr. Sherr, of Yale University, New Haven, Conn.
Recommendations address CGM, pumps, and connected systems
In the guidelines, CGM is “strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as three or more injections of insulin per day or the use of an insulin pump.” For those with diabetes who use CGM, “priority metrics” include a “time in range” of greater than 70% from 14 days of active use. Targets for mean glucose should be individualized, with glycemic variability 36% or lower.
Further specific CGM target metrics are given for people with type 1 diabetes, older/high risk individuals, and for pregnant women. The recommendations align with those issued in a 2019 joint consensus statement on CGM time-in-range endorsed by several organizations, including AACE.
In response to an audience question about whether AACE is advising that time-in-range replace A1c for glycemia assessment, Dr. Sherr responded: “I think currently we’re not in a position where we can completely replace A1c with time in range. However, I’m hopeful that in future years we’ll see further data gathered ... to allow for that recommendation to occur.”
For now, she said, “What we really want to hone in on in the guidelines is that time-in-range and use of CGM truly allow clinicians to better understand how to optimize care for their persons with diabetes. It gives us a better picture. It’s not just a number of whether we’re hitting target. It tells us whether we need to attack time above range or time below range. So we really think it’s critical for clinical care.”
The document also provides specifics about real-time versus intermittently scanned CGM and use of diagnostic/professional CGM.
The “insulin delivery technologies” section covers use of connected pens, insulin pumps without CGM, insulin pumps with separate CGM, and the more advanced combined insulin pump-CGM systems including those with low-glucose suspend, predictive low-glucose suspend, and hybrid closed-loops (sometimes called the artificial pancreas).
In general, these automated insulin delivery systems (artificial pancreas), “are strongly recommended for all persons with [type 1 diabetes], since their use has been shown to increase time in range, especially in the overnight period, without causing an increased risk of hypoglycemia,” Dr. Sherr observed.
Other tech topics: Apps, telemedicine, and safety
The new guidelines say that “clinically validated” smartphone apps should be recommended to help teach or reinforce diabetes self-management skills and provide support and encouragement for healthy behaviors around food and exercise.
Dr. Grunberger pointed out: “As we know, there are tons of apps out there, and patients are using them. The problem is that very few of them have actually been validated in clinical trials in published peer-reviewed [journals].”
He recommended a joint statement on diabetes apps from the American Diabetes Association and the European Association for the Study of Diabetes that was initially discussed at the 2019 EASD meeting, as reported by this news organization, and subsequently published in January 2020 in Diabetes Care and Diabetologia.
“Telemedicine, including periodic phone calls, smartphone-web interactions ... by health care professionals ... is strongly recommended to treat persons with diabetes, provide diabetes education, remotely monitor glucose and/or insulin data to indicate the need for therapy adjustments, and improve diabetes-related outcomes/control with better engagement,” the document says.
Safety concerns addressed include the issue of certain medications interfering with CGM [readings] ... including acetaminophen, high-dose vitamin C, and hydroxyurea, as well as cautions about what to do in the event of device malfunction and assessing that the patient is sufficiently trained in proper device use. Criteria for insulin pump discontinuation are also given.
Implementation: Who will be prescribing? ‘This is not for amateurs’
A final section on implementation recommends that “initiation and use of diabetes technology should be implemented by health care professionals who are trained, committed, and experienced to prescribe and direct the use of these tools. Clinicians should have the infrastructure to support the needs of persons with diabetes using the technology.”
Dr. Grunberger commented: “I think the key is going to be who should be doing this? What is the role of a clinical endocrinologist in the future? What is our responsibility, [since] we don’t have the manpower and womanpower to take care of all these people as these technologies advance? It’s our responsibility to provide these hopefully valued recommendations as a resource for those who want to know more about it.”
However, he noted, “This is not for amateurs. If you want to actually use this in your practice, you need the infrastructure, the expertise, the training, the dedication, and the energy to be there for the patients all the time ... This clinical practice guideline is a foundation.”
Dr. Sherr added: “To me, it’s really thinking about ... changing our mindset from who is an appropriate candidate to who can benefit and how vast a group that entails ... I’m hopeful that we will see more technology use through continued conversations with our patients with diabetes, and hopefully through more clinicians being excited to be part of this revolution.”
Dr. Grunberger has reported being on speakers bureaus for Eli Lilly, Novo Nordisk, and Abbott. Dr. Sherr has reported being a consultant and speaker for Lilly and Medtronic Diabetes, a consultant for Insulet and Sanofi, and on advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, JDRF T1D fund, and Medtronic.
A version of this article first appeared on Medscape.com.