User login
Cost of Antibiotic Resistance Hits Private Payers
Major Finding: The cost of antimicrobial resistance is rising, but many patients now are younger and living longer, so the burden is shifting from Medicare to private insurance.
Data Source: Two studies assessing hospital data and one Internet survey.
Disclosures: The analyses were funded by an unrestricted educational grant from bioMeriéux.
BETHESDA, MD. — The overall cost burden of antimicrobial resistance—as high as $38 billion in one 2009 hospital estimate—has shifted sharply from Medicare to private payers over the last decade.
Medicare still pays the majority of the costs for excess length of stay, increased use of more expensive drugs, and poorer health attributable to treatment-resistant infections. But the rise in infections caused by methicillin-resistant Staphylococcus aureus (MRSA), which largely affects younger, healthier individuals, has meant that the overall cost per patient has declined but more is being borne by private HMOs and PPOs, Susan D. Foster, Ph.D., said at the 2010 Conference on Antimicrobial Resistance sponsored by the National Foundation for Infectious Diseases.
“There's been a major shift in who's actually paying. I don't think the insurance companies are quite aware of this,” said Dr. Foster, professor of international health at Boston University and director of public policy and education for the Alliance for the Prudent Use of Antibiotics at Tufts University, Boston.
Dr. Foster analyzed data from three studies. In an unpublished study, she and her associates reviewed Massachusetts hospital discharge data from 2000 to 2007 to look for ICD9 “VO9” codes, which are specific for drug-resistant infections. Although these codes are complex and difficult to use and therefore represent a study limitation, they do allow for analysis of trends over time, she explained.
Overall, the number of hospital discharges reporting antibiotic resistance in Massachusetts rose from 3,861 in 2000 to 11,218 in 2007. The inflation-adjusted total cost more than doubled over the 7 years, from $135 to $285 million. However, the length of stay (LOS) per patient for drug-resistant infections dropped by 4.5 days, and the cost per patient fell by nearly $10,000.
In contrast, the length of stay for drug-susceptible infections didn't change during the study period (just under 5 days), while the cost per patient with susceptible infections rose only slightly.
The drop in LOS and cost per patient with drug-resistant infections is largely explained by the dramatic shift in patient age, particularly among 19- to 64-year-olds: In 2000, that age group accounted for 30% of drug-resistant infection discharges, whereas in 2007 the proportion had risen to 45.5%. At the same time, the 65- to 80-year-old group dropped from 38% to 25%. While the proportion of infections due to drug-resistant organisms rose in all age groups, the greatest rise was among working-age adults, Dr. Foster noted.
Not surprisingly, then, was the concurrent payer shift: Medicare's proportion of the cost dropped from 73% in 2000 to 58% in 2007, while Medicaid's rose from 6% to 15%. The proportion paid by “Other,” including private insurance, rose from 20.5% to 28%, she said.
Also not surprising—but perhaps not considered previously—were declines in inpatient mortality due to drug-resistant infections (from 11% to 5%) and in discharge of patients to nursing homes (32% to 28%), and a rise in patients returning home from the hospital (33% to 48%).
The second study, conducted by Dr. Rebecca Roberts and her associates for the Chicago Antimicrobial Resistance Project, analyzed discharge data at Cook County Hospital for a random sample of 1,391 high-risk (more than five ICD-9 codes, excluding trauma, burn, or obstetric care) adult patients, of whom 13.5% (188) had an antibiotic-resistant infection (ARI). Societal costs for the study year 2000 were estimated at $10.7–$15 million, or $13.35 million in 2008 dollars (Clin. Infect. Dis. 2009;49:1175–84).
In that study, LOS was three times longer for patients with ARIs (24 vs. 8 days) and mortality 6 times higher (18% vs. 3%). Total inpatient costs were $58,029 vs. $13,210 for non-ARI patients. Even the daily cost was $517 greater for the ARI group, Dr. Foster noted.
The most common type of ARI was MRSA (43%), followed by vancomycin-resistant enterococci (VRE, 31%), Escherichia coli/Klebsiella species (16%), and multiple infections (6%). By cost, however, VRE accounted for the greatest proportion (36%), followed by MRSA (34%), and multiple infections (16%).
After publication of the study, Dr. Foster collaborated with Dr. Roberts in extrapolating the Chicago data to the entire United States: In 2000, there were 900,000 admissions with the same criteria the study used. Applying the costs found in that study gives $16.6–$26 billion in additional health care costs (the range reflects different inflation adjustments). Updating the figure to 2009 costs using the Consumer Price Index gives an estimated $21–$34 billion, while using medical inflation rates boosts the figures to as high as $24–$38 billion.
The third study, also unpublished work by Dr. Foster and her associates, was an Internet-based survey of more than 300 respondents recruited from MRSA chat rooms, listservs, and Google Adwords. Acknowledging the limitations of such surveys—particularly the bias toward younger, healthier Internet users with strong opinions—she described “some heart-rending responses,” including one from a 52-year-old woman who felt completely isolated from friends and family, a teacher who was fired when her MRSA diagnosis became known, and parents who felt they had to send their children away to prevent transmission.
Respondents reported a mean out-of-pocket expenditure of $2,251, including copays for office visits, prescription drugs, and hospital stays. Nearly 70% reported having private insurance (HMO or PPO), and 14% said they were uninsured, which is close to the national average, Dr. Foster said. “Individuals and households affected by drug resistance bear a large uncompensated burden in terms of out-of-pocket expenses and lost wages.”
A related video is at www.youtube.com/HospitalistNews
'Individuals and households affected by drug resistance bear a large uncompensated burden.'
Source DR. FOSTER
Major Finding: The cost of antimicrobial resistance is rising, but many patients now are younger and living longer, so the burden is shifting from Medicare to private insurance.
Data Source: Two studies assessing hospital data and one Internet survey.
Disclosures: The analyses were funded by an unrestricted educational grant from bioMeriéux.
BETHESDA, MD. — The overall cost burden of antimicrobial resistance—as high as $38 billion in one 2009 hospital estimate—has shifted sharply from Medicare to private payers over the last decade.
Medicare still pays the majority of the costs for excess length of stay, increased use of more expensive drugs, and poorer health attributable to treatment-resistant infections. But the rise in infections caused by methicillin-resistant Staphylococcus aureus (MRSA), which largely affects younger, healthier individuals, has meant that the overall cost per patient has declined but more is being borne by private HMOs and PPOs, Susan D. Foster, Ph.D., said at the 2010 Conference on Antimicrobial Resistance sponsored by the National Foundation for Infectious Diseases.
“There's been a major shift in who's actually paying. I don't think the insurance companies are quite aware of this,” said Dr. Foster, professor of international health at Boston University and director of public policy and education for the Alliance for the Prudent Use of Antibiotics at Tufts University, Boston.
Dr. Foster analyzed data from three studies. In an unpublished study, she and her associates reviewed Massachusetts hospital discharge data from 2000 to 2007 to look for ICD9 “VO9” codes, which are specific for drug-resistant infections. Although these codes are complex and difficult to use and therefore represent a study limitation, they do allow for analysis of trends over time, she explained.
Overall, the number of hospital discharges reporting antibiotic resistance in Massachusetts rose from 3,861 in 2000 to 11,218 in 2007. The inflation-adjusted total cost more than doubled over the 7 years, from $135 to $285 million. However, the length of stay (LOS) per patient for drug-resistant infections dropped by 4.5 days, and the cost per patient fell by nearly $10,000.
In contrast, the length of stay for drug-susceptible infections didn't change during the study period (just under 5 days), while the cost per patient with susceptible infections rose only slightly.
The drop in LOS and cost per patient with drug-resistant infections is largely explained by the dramatic shift in patient age, particularly among 19- to 64-year-olds: In 2000, that age group accounted for 30% of drug-resistant infection discharges, whereas in 2007 the proportion had risen to 45.5%. At the same time, the 65- to 80-year-old group dropped from 38% to 25%. While the proportion of infections due to drug-resistant organisms rose in all age groups, the greatest rise was among working-age adults, Dr. Foster noted.
Not surprisingly, then, was the concurrent payer shift: Medicare's proportion of the cost dropped from 73% in 2000 to 58% in 2007, while Medicaid's rose from 6% to 15%. The proportion paid by “Other,” including private insurance, rose from 20.5% to 28%, she said.
Also not surprising—but perhaps not considered previously—were declines in inpatient mortality due to drug-resistant infections (from 11% to 5%) and in discharge of patients to nursing homes (32% to 28%), and a rise in patients returning home from the hospital (33% to 48%).
The second study, conducted by Dr. Rebecca Roberts and her associates for the Chicago Antimicrobial Resistance Project, analyzed discharge data at Cook County Hospital for a random sample of 1,391 high-risk (more than five ICD-9 codes, excluding trauma, burn, or obstetric care) adult patients, of whom 13.5% (188) had an antibiotic-resistant infection (ARI). Societal costs for the study year 2000 were estimated at $10.7–$15 million, or $13.35 million in 2008 dollars (Clin. Infect. Dis. 2009;49:1175–84).
In that study, LOS was three times longer for patients with ARIs (24 vs. 8 days) and mortality 6 times higher (18% vs. 3%). Total inpatient costs were $58,029 vs. $13,210 for non-ARI patients. Even the daily cost was $517 greater for the ARI group, Dr. Foster noted.
The most common type of ARI was MRSA (43%), followed by vancomycin-resistant enterococci (VRE, 31%), Escherichia coli/Klebsiella species (16%), and multiple infections (6%). By cost, however, VRE accounted for the greatest proportion (36%), followed by MRSA (34%), and multiple infections (16%).
After publication of the study, Dr. Foster collaborated with Dr. Roberts in extrapolating the Chicago data to the entire United States: In 2000, there were 900,000 admissions with the same criteria the study used. Applying the costs found in that study gives $16.6–$26 billion in additional health care costs (the range reflects different inflation adjustments). Updating the figure to 2009 costs using the Consumer Price Index gives an estimated $21–$34 billion, while using medical inflation rates boosts the figures to as high as $24–$38 billion.
The third study, also unpublished work by Dr. Foster and her associates, was an Internet-based survey of more than 300 respondents recruited from MRSA chat rooms, listservs, and Google Adwords. Acknowledging the limitations of such surveys—particularly the bias toward younger, healthier Internet users with strong opinions—she described “some heart-rending responses,” including one from a 52-year-old woman who felt completely isolated from friends and family, a teacher who was fired when her MRSA diagnosis became known, and parents who felt they had to send their children away to prevent transmission.
Respondents reported a mean out-of-pocket expenditure of $2,251, including copays for office visits, prescription drugs, and hospital stays. Nearly 70% reported having private insurance (HMO or PPO), and 14% said they were uninsured, which is close to the national average, Dr. Foster said. “Individuals and households affected by drug resistance bear a large uncompensated burden in terms of out-of-pocket expenses and lost wages.”
A related video is at www.youtube.com/HospitalistNews
'Individuals and households affected by drug resistance bear a large uncompensated burden.'
Source DR. FOSTER
Major Finding: The cost of antimicrobial resistance is rising, but many patients now are younger and living longer, so the burden is shifting from Medicare to private insurance.
Data Source: Two studies assessing hospital data and one Internet survey.
Disclosures: The analyses were funded by an unrestricted educational grant from bioMeriéux.
BETHESDA, MD. — The overall cost burden of antimicrobial resistance—as high as $38 billion in one 2009 hospital estimate—has shifted sharply from Medicare to private payers over the last decade.
Medicare still pays the majority of the costs for excess length of stay, increased use of more expensive drugs, and poorer health attributable to treatment-resistant infections. But the rise in infections caused by methicillin-resistant Staphylococcus aureus (MRSA), which largely affects younger, healthier individuals, has meant that the overall cost per patient has declined but more is being borne by private HMOs and PPOs, Susan D. Foster, Ph.D., said at the 2010 Conference on Antimicrobial Resistance sponsored by the National Foundation for Infectious Diseases.
“There's been a major shift in who's actually paying. I don't think the insurance companies are quite aware of this,” said Dr. Foster, professor of international health at Boston University and director of public policy and education for the Alliance for the Prudent Use of Antibiotics at Tufts University, Boston.
Dr. Foster analyzed data from three studies. In an unpublished study, she and her associates reviewed Massachusetts hospital discharge data from 2000 to 2007 to look for ICD9 “VO9” codes, which are specific for drug-resistant infections. Although these codes are complex and difficult to use and therefore represent a study limitation, they do allow for analysis of trends over time, she explained.
Overall, the number of hospital discharges reporting antibiotic resistance in Massachusetts rose from 3,861 in 2000 to 11,218 in 2007. The inflation-adjusted total cost more than doubled over the 7 years, from $135 to $285 million. However, the length of stay (LOS) per patient for drug-resistant infections dropped by 4.5 days, and the cost per patient fell by nearly $10,000.
In contrast, the length of stay for drug-susceptible infections didn't change during the study period (just under 5 days), while the cost per patient with susceptible infections rose only slightly.
The drop in LOS and cost per patient with drug-resistant infections is largely explained by the dramatic shift in patient age, particularly among 19- to 64-year-olds: In 2000, that age group accounted for 30% of drug-resistant infection discharges, whereas in 2007 the proportion had risen to 45.5%. At the same time, the 65- to 80-year-old group dropped from 38% to 25%. While the proportion of infections due to drug-resistant organisms rose in all age groups, the greatest rise was among working-age adults, Dr. Foster noted.
Not surprisingly, then, was the concurrent payer shift: Medicare's proportion of the cost dropped from 73% in 2000 to 58% in 2007, while Medicaid's rose from 6% to 15%. The proportion paid by “Other,” including private insurance, rose from 20.5% to 28%, she said.
Also not surprising—but perhaps not considered previously—were declines in inpatient mortality due to drug-resistant infections (from 11% to 5%) and in discharge of patients to nursing homes (32% to 28%), and a rise in patients returning home from the hospital (33% to 48%).
The second study, conducted by Dr. Rebecca Roberts and her associates for the Chicago Antimicrobial Resistance Project, analyzed discharge data at Cook County Hospital for a random sample of 1,391 high-risk (more than five ICD-9 codes, excluding trauma, burn, or obstetric care) adult patients, of whom 13.5% (188) had an antibiotic-resistant infection (ARI). Societal costs for the study year 2000 were estimated at $10.7–$15 million, or $13.35 million in 2008 dollars (Clin. Infect. Dis. 2009;49:1175–84).
In that study, LOS was three times longer for patients with ARIs (24 vs. 8 days) and mortality 6 times higher (18% vs. 3%). Total inpatient costs were $58,029 vs. $13,210 for non-ARI patients. Even the daily cost was $517 greater for the ARI group, Dr. Foster noted.
The most common type of ARI was MRSA (43%), followed by vancomycin-resistant enterococci (VRE, 31%), Escherichia coli/Klebsiella species (16%), and multiple infections (6%). By cost, however, VRE accounted for the greatest proportion (36%), followed by MRSA (34%), and multiple infections (16%).
After publication of the study, Dr. Foster collaborated with Dr. Roberts in extrapolating the Chicago data to the entire United States: In 2000, there were 900,000 admissions with the same criteria the study used. Applying the costs found in that study gives $16.6–$26 billion in additional health care costs (the range reflects different inflation adjustments). Updating the figure to 2009 costs using the Consumer Price Index gives an estimated $21–$34 billion, while using medical inflation rates boosts the figures to as high as $24–$38 billion.
The third study, also unpublished work by Dr. Foster and her associates, was an Internet-based survey of more than 300 respondents recruited from MRSA chat rooms, listservs, and Google Adwords. Acknowledging the limitations of such surveys—particularly the bias toward younger, healthier Internet users with strong opinions—she described “some heart-rending responses,” including one from a 52-year-old woman who felt completely isolated from friends and family, a teacher who was fired when her MRSA diagnosis became known, and parents who felt they had to send their children away to prevent transmission.
Respondents reported a mean out-of-pocket expenditure of $2,251, including copays for office visits, prescription drugs, and hospital stays. Nearly 70% reported having private insurance (HMO or PPO), and 14% said they were uninsured, which is close to the national average, Dr. Foster said. “Individuals and households affected by drug resistance bear a large uncompensated burden in terms of out-of-pocket expenses and lost wages.”
A related video is at www.youtube.com/HospitalistNews
'Individuals and households affected by drug resistance bear a large uncompensated burden.'
Source DR. FOSTER
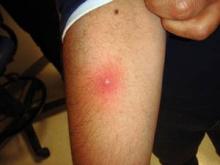
Drain Abscesses, Get Cultures in Pediatric Skin, Soft Tissue Infections
BETHESDA, Md. — Draining abscesses and obtaining cultures are now more important to the management of pediatric skin and soft tissue infections in the era of community-acquired methicillin-resistant Staphylococcus aureus infections.
"It's important to send cultures, which wasn't the case years ago. It helps to know what we're dealing with on a local level," said Dr. Kaplan, head of the pediatric infectious disease section at Baylor College of Medicine and chief of the infectious disease service at Texas Children's Hospital, both in Houston.
Although invasive CA-MRSA infections are increasingly a concern, skin and soft tissue infections continue to make up the majority of CA-MRSA infections. Among the 12,876 children with community-acquired S. aureus infections who were seen at Texas Children's between Aug. 1, 2001, and June 30, 2009, 73% had a MRSA infection. Of those, 97% were skin and soft tissue infections, compared with 93% of the methicillin-susceptible S. aureus (MSSA) infections.
Image above: Pustule with surrounding cellulitis (Photo courtesy Dr. Sheldon L. Kaplan)
BETHESDA, Md. — Draining abscesses and obtaining cultures are now more important to the management of pediatric skin and soft tissue infections in the era of community-acquired methicillin-resistant Staphylococcus aureus infections.
"It's important to send cultures, which wasn't the case years ago. It helps to know what we're dealing with on a local level," said Dr. Kaplan, head of the pediatric infectious disease section at Baylor College of Medicine and chief of the infectious disease service at Texas Children's Hospital, both in Houston.
Although invasive CA-MRSA infections are increasingly a concern, skin and soft tissue infections continue to make up the majority of CA-MRSA infections. Among the 12,876 children with community-acquired S. aureus infections who were seen at Texas Children's between Aug. 1, 2001, and June 30, 2009, 73% had a MRSA infection. Of those, 97% were skin and soft tissue infections, compared with 93% of the methicillin-susceptible S. aureus (MSSA) infections.
Image above: Pustule with surrounding cellulitis (Photo courtesy Dr. Sheldon L. Kaplan)
BETHESDA, Md. — Draining abscesses and obtaining cultures are now more important to the management of pediatric skin and soft tissue infections in the era of community-acquired methicillin-resistant Staphylococcus aureus infections.
"It's important to send cultures, which wasn't the case years ago. It helps to know what we're dealing with on a local level," said Dr. Kaplan, head of the pediatric infectious disease section at Baylor College of Medicine and chief of the infectious disease service at Texas Children's Hospital, both in Houston.
Although invasive CA-MRSA infections are increasingly a concern, skin and soft tissue infections continue to make up the majority of CA-MRSA infections. Among the 12,876 children with community-acquired S. aureus infections who were seen at Texas Children's between Aug. 1, 2001, and June 30, 2009, 73% had a MRSA infection. Of those, 97% were skin and soft tissue infections, compared with 93% of the methicillin-susceptible S. aureus (MSSA) infections.
Image above: Pustule with surrounding cellulitis (Photo courtesy Dr. Sheldon L. Kaplan)
Federal Panel Finds No Safety Signals With H1N1 Vaccine
The National Vaccine Advisory Committee has endorsed a working group report concluding that no safety signals have been identified so far with the 2009 pandemic influenza A(H1N1) vaccine.
In a public teleconference, working group chair Dr. Marie McCormick said the group reviewed data for a total of 74 million doses of inactivated (injected) vaccine and 19 million doses of live attenuated (intranasal) H1N1 vaccine distributed as of around Christmas. They concluded that the data do not suggest a safety signal between the outcomes examined and the vaccine, defining “signal” as an outcome occurring more than anticipated by chance alone.
No serious increases in adverse events have been seen to date in any of the pandemic H1N1 vaccine clinical trials, and a comparison of serious events reported to the Vaccine Adverse Events Reporting System have shown similar levels between the H1N1vaccine and the seasonal influenza vaccine, as well as other vaccines. In addition, active surveillance systems that are using rapid-cycle analysis of prespecified outcomes have also been within expected values, said Dr. McCormick, the Sumner and Esther Feldberg professor of Maternal and Child Health at Harvard University, Boston.
However, she cautioned, the size of the population captured under active surveillance is still somewhat limited. A new federal project called Post-Licensure Rapid Immunization Safety Monitoring (PRISM), designed to monitor H1N1 vaccine safety in real-time using data from large health plans covering approximately 10% of the U.S. population, was getting underway at the time of the teleconference.
“As more data are available through active surveillance, conclusions will be based on a larger accumulation of data. Larger samples may be needed to detect rare adverse events,” she said.
The NVAC members voted to endorse the report during the teleconference. It was then sent to the U.S. assistant secretary for health for review and formal implementation.
The National Vaccine Advisory Committee has endorsed a working group report concluding that no safety signals have been identified so far with the 2009 pandemic influenza A(H1N1) vaccine.
In a public teleconference, working group chair Dr. Marie McCormick said the group reviewed data for a total of 74 million doses of inactivated (injected) vaccine and 19 million doses of live attenuated (intranasal) H1N1 vaccine distributed as of around Christmas. They concluded that the data do not suggest a safety signal between the outcomes examined and the vaccine, defining “signal” as an outcome occurring more than anticipated by chance alone.
No serious increases in adverse events have been seen to date in any of the pandemic H1N1 vaccine clinical trials, and a comparison of serious events reported to the Vaccine Adverse Events Reporting System have shown similar levels between the H1N1vaccine and the seasonal influenza vaccine, as well as other vaccines. In addition, active surveillance systems that are using rapid-cycle analysis of prespecified outcomes have also been within expected values, said Dr. McCormick, the Sumner and Esther Feldberg professor of Maternal and Child Health at Harvard University, Boston.
However, she cautioned, the size of the population captured under active surveillance is still somewhat limited. A new federal project called Post-Licensure Rapid Immunization Safety Monitoring (PRISM), designed to monitor H1N1 vaccine safety in real-time using data from large health plans covering approximately 10% of the U.S. population, was getting underway at the time of the teleconference.
“As more data are available through active surveillance, conclusions will be based on a larger accumulation of data. Larger samples may be needed to detect rare adverse events,” she said.
The NVAC members voted to endorse the report during the teleconference. It was then sent to the U.S. assistant secretary for health for review and formal implementation.
The National Vaccine Advisory Committee has endorsed a working group report concluding that no safety signals have been identified so far with the 2009 pandemic influenza A(H1N1) vaccine.
In a public teleconference, working group chair Dr. Marie McCormick said the group reviewed data for a total of 74 million doses of inactivated (injected) vaccine and 19 million doses of live attenuated (intranasal) H1N1 vaccine distributed as of around Christmas. They concluded that the data do not suggest a safety signal between the outcomes examined and the vaccine, defining “signal” as an outcome occurring more than anticipated by chance alone.
No serious increases in adverse events have been seen to date in any of the pandemic H1N1 vaccine clinical trials, and a comparison of serious events reported to the Vaccine Adverse Events Reporting System have shown similar levels between the H1N1vaccine and the seasonal influenza vaccine, as well as other vaccines. In addition, active surveillance systems that are using rapid-cycle analysis of prespecified outcomes have also been within expected values, said Dr. McCormick, the Sumner and Esther Feldberg professor of Maternal and Child Health at Harvard University, Boston.
However, she cautioned, the size of the population captured under active surveillance is still somewhat limited. A new federal project called Post-Licensure Rapid Immunization Safety Monitoring (PRISM), designed to monitor H1N1 vaccine safety in real-time using data from large health plans covering approximately 10% of the U.S. population, was getting underway at the time of the teleconference.
“As more data are available through active surveillance, conclusions will be based on a larger accumulation of data. Larger samples may be needed to detect rare adverse events,” she said.
The NVAC members voted to endorse the report during the teleconference. It was then sent to the U.S. assistant secretary for health for review and formal implementation.
Interval Between H1N1 Symptom Onset, Antiviral Treatment Affects ICU Risk
Major Finding: Interval to treatment, First Nations ethnicity, and medical comorbidities independently predicted ICU admission for 2009 pandemic H1N1 influenza in Manitoba, Canada.
Data Source: Cumulative case-control study of 795 Manitoba residents with confirmed H1N1 infection.
Disclosures: None declared.
The time interval between the onset of symptoms and antiviral treatment was the strongest predictor of intensive care admission for severe infection with 2009 pandemic influenza A(H1N1) and increased the risk for ICU admission up to eightfold in a cumulative case-control study of 795 patients in Manitoba, Canada.
Other predictors of ICU admission, compared with more mild disease cared for in the community, included First Nations ethnicity and the presence of a medical comorbidity, said Dr. Ryan Zarychanski of the University of Manitoba, and his associates.
“These data may have implications for proactive public health and primary care using outreach models at the community level, whether for public health education, prioritization of vaccination efforts, or strategies for antiviral treatment,” the investigators said.
The 795 study patients came from a total 894 confirmed H1N1 cases among Manitoba residents for whom the location of care could be determined: 569 remained in the community, 181 were admitted to the hospital but not the ICU, and 45 were admitted to the ICU. The mean age of the 795 infected individuals analyzed was 25.3 years, and 52% were female. Of 588 for whom ethnicity was known, 37% described their ethnicity as “First Nations,” one of three officially recognized groups of Canadian aboriginal peoples.
First Nations residents accounted for 28% of the 410 community cases for whom ethnicity was known, compared with 54% of those admitted to the hospital and 60% of 42 admitted to the ICU. Females represented 52% of the total 569 community cases and 50% of the 181 hospital admissions, but 69% of the 45 ICU cases. The presence of an underlying medical condition—including heart or lung disease, diabetes, malignancy, and substance abuse as well as pregnancy—also increased with severity of disease, from 35% of the community cases to 57% of the hospital admissions to 76% of those admitted to the ICU, Dr. Zarychanski and his associates reported.
Two-thirds of the 34 adults with comorbid conditions admitted to the ICU were clinically obese (body mass index greater than 30), but height and weight were not recorded consistently enough for patients in the two control groups to allow analysis of obesity as a risk factor for severe outcomes, they noted.
Antiviral therapy was known to have been prescribed for 34% of patients in the community group, 54% admitted to the hospital, and 95% of those who ended up in the ICU. Severity of illness correlated with increasing interval from symptom onset to the start of antiviral therapy, with the median interval 2 days for the community patients, 4 days for those hospitalized but not in ICU, and 6 days for those admitted to the ICU (CMAJ 2010, [doi:10.1503/cmaj/.091884
In a multivariate analysis that accounted for age, sex, First Nations ethnicity, medical comorbidity, interval from symptom onset to antiviral initiation, urban vs. rural status, and income quintile, a treatment interval of greater than 2 days vs. 2 days or fewer increased the risk for ICU admission, compared with being treated in the community by more than eightfold, with an odds ratio of 8.24. First Nations ethnicity increased the ICU risk by an odds ratio of 6.52 and medical comorbidity by an odds ratio of 3.19.
Similarly, in an analysis comparing those admitted to the ICU with those admitted to the hospital but not the ICU, First Nations ethnicity increased the risk by more than threefold, with an odds ratio of 3.23. In this comparison, the odds ratio for treatment interval of more than 2 days vs. 2 or fewer was 2.44 but did not reach statistical significance, the investigators noted.
The finding of increased illness severity among First Nations people is consistent with historical records from the 1918 influenza pandemic and more recent studies. Some evidence suggests a genetic predisposition, Dr. Zarychanski and his associates commented.
The Public Health Agency of Canada provided salary support to help facilitate data collection and statistical consultation.
Major Finding: Interval to treatment, First Nations ethnicity, and medical comorbidities independently predicted ICU admission for 2009 pandemic H1N1 influenza in Manitoba, Canada.
Data Source: Cumulative case-control study of 795 Manitoba residents with confirmed H1N1 infection.
Disclosures: None declared.
The time interval between the onset of symptoms and antiviral treatment was the strongest predictor of intensive care admission for severe infection with 2009 pandemic influenza A(H1N1) and increased the risk for ICU admission up to eightfold in a cumulative case-control study of 795 patients in Manitoba, Canada.
Other predictors of ICU admission, compared with more mild disease cared for in the community, included First Nations ethnicity and the presence of a medical comorbidity, said Dr. Ryan Zarychanski of the University of Manitoba, and his associates.
“These data may have implications for proactive public health and primary care using outreach models at the community level, whether for public health education, prioritization of vaccination efforts, or strategies for antiviral treatment,” the investigators said.
The 795 study patients came from a total 894 confirmed H1N1 cases among Manitoba residents for whom the location of care could be determined: 569 remained in the community, 181 were admitted to the hospital but not the ICU, and 45 were admitted to the ICU. The mean age of the 795 infected individuals analyzed was 25.3 years, and 52% were female. Of 588 for whom ethnicity was known, 37% described their ethnicity as “First Nations,” one of three officially recognized groups of Canadian aboriginal peoples.
First Nations residents accounted for 28% of the 410 community cases for whom ethnicity was known, compared with 54% of those admitted to the hospital and 60% of 42 admitted to the ICU. Females represented 52% of the total 569 community cases and 50% of the 181 hospital admissions, but 69% of the 45 ICU cases. The presence of an underlying medical condition—including heart or lung disease, diabetes, malignancy, and substance abuse as well as pregnancy—also increased with severity of disease, from 35% of the community cases to 57% of the hospital admissions to 76% of those admitted to the ICU, Dr. Zarychanski and his associates reported.
Two-thirds of the 34 adults with comorbid conditions admitted to the ICU were clinically obese (body mass index greater than 30), but height and weight were not recorded consistently enough for patients in the two control groups to allow analysis of obesity as a risk factor for severe outcomes, they noted.
Antiviral therapy was known to have been prescribed for 34% of patients in the community group, 54% admitted to the hospital, and 95% of those who ended up in the ICU. Severity of illness correlated with increasing interval from symptom onset to the start of antiviral therapy, with the median interval 2 days for the community patients, 4 days for those hospitalized but not in ICU, and 6 days for those admitted to the ICU (CMAJ 2010, [doi:10.1503/cmaj/.091884
In a multivariate analysis that accounted for age, sex, First Nations ethnicity, medical comorbidity, interval from symptom onset to antiviral initiation, urban vs. rural status, and income quintile, a treatment interval of greater than 2 days vs. 2 days or fewer increased the risk for ICU admission, compared with being treated in the community by more than eightfold, with an odds ratio of 8.24. First Nations ethnicity increased the ICU risk by an odds ratio of 6.52 and medical comorbidity by an odds ratio of 3.19.
Similarly, in an analysis comparing those admitted to the ICU with those admitted to the hospital but not the ICU, First Nations ethnicity increased the risk by more than threefold, with an odds ratio of 3.23. In this comparison, the odds ratio for treatment interval of more than 2 days vs. 2 or fewer was 2.44 but did not reach statistical significance, the investigators noted.
The finding of increased illness severity among First Nations people is consistent with historical records from the 1918 influenza pandemic and more recent studies. Some evidence suggests a genetic predisposition, Dr. Zarychanski and his associates commented.
The Public Health Agency of Canada provided salary support to help facilitate data collection and statistical consultation.
Major Finding: Interval to treatment, First Nations ethnicity, and medical comorbidities independently predicted ICU admission for 2009 pandemic H1N1 influenza in Manitoba, Canada.
Data Source: Cumulative case-control study of 795 Manitoba residents with confirmed H1N1 infection.
Disclosures: None declared.
The time interval between the onset of symptoms and antiviral treatment was the strongest predictor of intensive care admission for severe infection with 2009 pandemic influenza A(H1N1) and increased the risk for ICU admission up to eightfold in a cumulative case-control study of 795 patients in Manitoba, Canada.
Other predictors of ICU admission, compared with more mild disease cared for in the community, included First Nations ethnicity and the presence of a medical comorbidity, said Dr. Ryan Zarychanski of the University of Manitoba, and his associates.
“These data may have implications for proactive public health and primary care using outreach models at the community level, whether for public health education, prioritization of vaccination efforts, or strategies for antiviral treatment,” the investigators said.
The 795 study patients came from a total 894 confirmed H1N1 cases among Manitoba residents for whom the location of care could be determined: 569 remained in the community, 181 were admitted to the hospital but not the ICU, and 45 were admitted to the ICU. The mean age of the 795 infected individuals analyzed was 25.3 years, and 52% were female. Of 588 for whom ethnicity was known, 37% described their ethnicity as “First Nations,” one of three officially recognized groups of Canadian aboriginal peoples.
First Nations residents accounted for 28% of the 410 community cases for whom ethnicity was known, compared with 54% of those admitted to the hospital and 60% of 42 admitted to the ICU. Females represented 52% of the total 569 community cases and 50% of the 181 hospital admissions, but 69% of the 45 ICU cases. The presence of an underlying medical condition—including heart or lung disease, diabetes, malignancy, and substance abuse as well as pregnancy—also increased with severity of disease, from 35% of the community cases to 57% of the hospital admissions to 76% of those admitted to the ICU, Dr. Zarychanski and his associates reported.
Two-thirds of the 34 adults with comorbid conditions admitted to the ICU were clinically obese (body mass index greater than 30), but height and weight were not recorded consistently enough for patients in the two control groups to allow analysis of obesity as a risk factor for severe outcomes, they noted.
Antiviral therapy was known to have been prescribed for 34% of patients in the community group, 54% admitted to the hospital, and 95% of those who ended up in the ICU. Severity of illness correlated with increasing interval from symptom onset to the start of antiviral therapy, with the median interval 2 days for the community patients, 4 days for those hospitalized but not in ICU, and 6 days for those admitted to the ICU (CMAJ 2010, [doi:10.1503/cmaj/.091884
In a multivariate analysis that accounted for age, sex, First Nations ethnicity, medical comorbidity, interval from symptom onset to antiviral initiation, urban vs. rural status, and income quintile, a treatment interval of greater than 2 days vs. 2 days or fewer increased the risk for ICU admission, compared with being treated in the community by more than eightfold, with an odds ratio of 8.24. First Nations ethnicity increased the ICU risk by an odds ratio of 6.52 and medical comorbidity by an odds ratio of 3.19.
Similarly, in an analysis comparing those admitted to the ICU with those admitted to the hospital but not the ICU, First Nations ethnicity increased the risk by more than threefold, with an odds ratio of 3.23. In this comparison, the odds ratio for treatment interval of more than 2 days vs. 2 or fewer was 2.44 but did not reach statistical significance, the investigators noted.
The finding of increased illness severity among First Nations people is consistent with historical records from the 1918 influenza pandemic and more recent studies. Some evidence suggests a genetic predisposition, Dr. Zarychanski and his associates commented.
The Public Health Agency of Canada provided salary support to help facilitate data collection and statistical consultation.
Partnership Forged to Design 'Closed-Loop' Insulin Pump
The Juvenile Diabetes Research Foundation has announced a partnership with Animas Corp. and DexCom Inc. to develop a first-generation automated system for managing type 1 diabetes.
The JDRF will provide $8 million over the next 3 years to Animas, a Johnson & Johnson company that manufactures insulin pumps. DexCom, a manufacturer of continuous glucose monitoring (CGM) devices, will supply that part of the technology for the system. The money will fund clinical trials of efficacy and safety, with the first-generation system expected to be ready for regulatory review within 4 years, Alan Lewis, Ph.D., JDRF president and CEO, said in a telephone briefing.
The ultimate goal is to develop a fully automated “closed-loop” system to regulate blood glucose levels, but the initial version would still require some input from the user and therefore would only be partially closed. It would consist of the insulin pump and the CGM—which are currently available but operate separately—with a computer program that would link the two. It would automatically increase insulin delivery upon detection of hyperglycemia and shut off delivery when hypoglycemia occurs, subsequently resuming delivery when glucose levels return to normal.
The patient would still need to manually instruct the pump to deliver insulin, but the system would improve overall control by minimizing the amount of time a patient spends out of target glucose range, said Aaron Kowalski, Ph.D., JDRF assistant vice president and director of glucose control research.
The insulin pump and glucose sensor will be linked via a computer program.
Source Courtesy JDRF (Juvenile Diabetes Research Foundation)
The Juvenile Diabetes Research Foundation has announced a partnership with Animas Corp. and DexCom Inc. to develop a first-generation automated system for managing type 1 diabetes.
The JDRF will provide $8 million over the next 3 years to Animas, a Johnson & Johnson company that manufactures insulin pumps. DexCom, a manufacturer of continuous glucose monitoring (CGM) devices, will supply that part of the technology for the system. The money will fund clinical trials of efficacy and safety, with the first-generation system expected to be ready for regulatory review within 4 years, Alan Lewis, Ph.D., JDRF president and CEO, said in a telephone briefing.
The ultimate goal is to develop a fully automated “closed-loop” system to regulate blood glucose levels, but the initial version would still require some input from the user and therefore would only be partially closed. It would consist of the insulin pump and the CGM—which are currently available but operate separately—with a computer program that would link the two. It would automatically increase insulin delivery upon detection of hyperglycemia and shut off delivery when hypoglycemia occurs, subsequently resuming delivery when glucose levels return to normal.
The patient would still need to manually instruct the pump to deliver insulin, but the system would improve overall control by minimizing the amount of time a patient spends out of target glucose range, said Aaron Kowalski, Ph.D., JDRF assistant vice president and director of glucose control research.
The insulin pump and glucose sensor will be linked via a computer program.
Source Courtesy JDRF (Juvenile Diabetes Research Foundation)
The Juvenile Diabetes Research Foundation has announced a partnership with Animas Corp. and DexCom Inc. to develop a first-generation automated system for managing type 1 diabetes.
The JDRF will provide $8 million over the next 3 years to Animas, a Johnson & Johnson company that manufactures insulin pumps. DexCom, a manufacturer of continuous glucose monitoring (CGM) devices, will supply that part of the technology for the system. The money will fund clinical trials of efficacy and safety, with the first-generation system expected to be ready for regulatory review within 4 years, Alan Lewis, Ph.D., JDRF president and CEO, said in a telephone briefing.
The ultimate goal is to develop a fully automated “closed-loop” system to regulate blood glucose levels, but the initial version would still require some input from the user and therefore would only be partially closed. It would consist of the insulin pump and the CGM—which are currently available but operate separately—with a computer program that would link the two. It would automatically increase insulin delivery upon detection of hyperglycemia and shut off delivery when hypoglycemia occurs, subsequently resuming delivery when glucose levels return to normal.
The patient would still need to manually instruct the pump to deliver insulin, but the system would improve overall control by minimizing the amount of time a patient spends out of target glucose range, said Aaron Kowalski, Ph.D., JDRF assistant vice president and director of glucose control research.
The insulin pump and glucose sensor will be linked via a computer program.
Source Courtesy JDRF (Juvenile Diabetes Research Foundation)
Increased Mortality Seen With HbA1c Below 7.5%
Major Finding: Hemoglobin A1c values below 7.5% were associated with increased all-cause mortality and cardiovascular events.
Data Source: A general practice database analysis of 47,970 type 2 diabetes patients with recently intensified glucose-lowering therapy.
Disclosures: The study was funded by Eli Lilly & Co. The investigators disclosed ties to numerous manufacturers of diabetes treatments, including Eli Lilly. The editorial authors reported relationships with several other pharmaceutical companies.
Hemoglobin A1cvalues below 7.5% were associated with increased all-cause mortality and cardiovascular events in patients with type 2 diabetes in an analysis of nearly 48,000 patients in a U.K. general practice database.
If confirmed, the findings suggest that diabetes guidelines might need revision to include a definition of a minimum HbA1c value, Dr. Craig J. Currie of Cardiff (Wales) University and his associates said (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)61969-3
The study also showed that insulin therapy was associated with higher mortality than combination oral therapy. Unadjusted mortality rates were 16.2 deaths per 1,000 person-years of follow-up for the oral combination therapy group and 27.2/1,000 for the insulin group. After exclusion of patients with high cardiovascular risk or renal impairment, insulin-based therapy remained associated with significantly greater all-cause mortality (HR 1.46) than did combination oral agents, the investigators reported.
The study, funded by Eli Lilly & Co., utilized data from November 1986 to November 2008 in the U.K. General Practice Research Database. Two cohorts of patients aged 50 years and older with type 2 diabetes were assessed: 27,965 whose treatment regimen had been intensified from oral glucose-lowering monotherapy to a combination oral regimen with a sulfonylurea plus metformin, and 20,005 on oral hypoglycemic agents alone who were initiated on insulin with or without concomitant oral agents.
All-cause mortality was the primary outcome. The secondary outcome was the occurrence of a major cardiovascular event among those who had no record of cardiovascular disease before the index date. The mean follow-up was 4.5 years in the oral medication group and 5.2 years in the insulin group.
Patients were divided by HbA1c decile, with the lowest decile (1) having a median HbA1c of 6.4% and the highest decile (10) at 10.5%. Mortality varied by decile in both treatment groups, with increased mortality seen in both the highest and lowest deciles. Patients in decile 4, who had a median HbA1c of 7.5%, had the lowest mortality across deciles, Dr. Currie and his associates reported.
In the combination oral therapy group, only those in deciles 1 and 10 had significantly increased mortality, compared with patients in HbA1c decile 4. However, in the insulin-treated cohort, deciles 1, 2, 3, 9, and 10 all had significantly greater mortality, compared with decile 4.
Progression to large-vessel disease events occurred in 8.2% of 20,817 patients who did not have large-vessel disease at baseline in the oral medication group, and in 11.9% of 13,475 patients in the insulin group. After adjustment for covariates, the adjusted risk of progression to large-vessel disease in both groups was higher for decile 1 (HR 1.54) and decile 10 (HR 1.36).
Compared with combination oral therapy, insulin treatment also was associated with an increased likelihood of progression to a first large-vessel disease event (HR 1.31).
The data suggest that for patients on oral combination therapy, a wide HbA1c range is safe with respect to all-cause mortality and large-vessel events, but a narrower range may be desirable for patients taking insulin, the investigators commented.
In an accompanying editorial, Dr. Beverley Balkau and Dr. Dominique Simon noted that although this study does lend support to earlier studies, epidemiologic studies cannot show causal relationships. Moreover, observational databases can't provide the detailed information available in a randomized clinical trial, such as the actual frequency of hypoglycemia.
However, this study does have the advantage of real-world observation, noted Dr. Balkau and Dr. Simon, who are with the CESP Centre for Research in Epidemiology and Population Health in Villejuif, France (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)62192-9
They recommended that priority be given to treatment with insulin sensitizers for as long as possible in patients with type 2 diabetes, because these drugs allow a low HbA1c to be achieved without risk of hypoglycemia. For patients with type 2 diabetes using insulin secretagogues or insulin itself, this study provides a rationale for an HbA1c threshold of 7.5%, which corresponds to the lowest threshold of death and lowest event rate for large-vessel disease, they said.
Major Finding: Hemoglobin A1c values below 7.5% were associated with increased all-cause mortality and cardiovascular events.
Data Source: A general practice database analysis of 47,970 type 2 diabetes patients with recently intensified glucose-lowering therapy.
Disclosures: The study was funded by Eli Lilly & Co. The investigators disclosed ties to numerous manufacturers of diabetes treatments, including Eli Lilly. The editorial authors reported relationships with several other pharmaceutical companies.
Hemoglobin A1cvalues below 7.5% were associated with increased all-cause mortality and cardiovascular events in patients with type 2 diabetes in an analysis of nearly 48,000 patients in a U.K. general practice database.
If confirmed, the findings suggest that diabetes guidelines might need revision to include a definition of a minimum HbA1c value, Dr. Craig J. Currie of Cardiff (Wales) University and his associates said (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)61969-3
The study also showed that insulin therapy was associated with higher mortality than combination oral therapy. Unadjusted mortality rates were 16.2 deaths per 1,000 person-years of follow-up for the oral combination therapy group and 27.2/1,000 for the insulin group. After exclusion of patients with high cardiovascular risk or renal impairment, insulin-based therapy remained associated with significantly greater all-cause mortality (HR 1.46) than did combination oral agents, the investigators reported.
The study, funded by Eli Lilly & Co., utilized data from November 1986 to November 2008 in the U.K. General Practice Research Database. Two cohorts of patients aged 50 years and older with type 2 diabetes were assessed: 27,965 whose treatment regimen had been intensified from oral glucose-lowering monotherapy to a combination oral regimen with a sulfonylurea plus metformin, and 20,005 on oral hypoglycemic agents alone who were initiated on insulin with or without concomitant oral agents.
All-cause mortality was the primary outcome. The secondary outcome was the occurrence of a major cardiovascular event among those who had no record of cardiovascular disease before the index date. The mean follow-up was 4.5 years in the oral medication group and 5.2 years in the insulin group.
Patients were divided by HbA1c decile, with the lowest decile (1) having a median HbA1c of 6.4% and the highest decile (10) at 10.5%. Mortality varied by decile in both treatment groups, with increased mortality seen in both the highest and lowest deciles. Patients in decile 4, who had a median HbA1c of 7.5%, had the lowest mortality across deciles, Dr. Currie and his associates reported.
In the combination oral therapy group, only those in deciles 1 and 10 had significantly increased mortality, compared with patients in HbA1c decile 4. However, in the insulin-treated cohort, deciles 1, 2, 3, 9, and 10 all had significantly greater mortality, compared with decile 4.
Progression to large-vessel disease events occurred in 8.2% of 20,817 patients who did not have large-vessel disease at baseline in the oral medication group, and in 11.9% of 13,475 patients in the insulin group. After adjustment for covariates, the adjusted risk of progression to large-vessel disease in both groups was higher for decile 1 (HR 1.54) and decile 10 (HR 1.36).
Compared with combination oral therapy, insulin treatment also was associated with an increased likelihood of progression to a first large-vessel disease event (HR 1.31).
The data suggest that for patients on oral combination therapy, a wide HbA1c range is safe with respect to all-cause mortality and large-vessel events, but a narrower range may be desirable for patients taking insulin, the investigators commented.
In an accompanying editorial, Dr. Beverley Balkau and Dr. Dominique Simon noted that although this study does lend support to earlier studies, epidemiologic studies cannot show causal relationships. Moreover, observational databases can't provide the detailed information available in a randomized clinical trial, such as the actual frequency of hypoglycemia.
However, this study does have the advantage of real-world observation, noted Dr. Balkau and Dr. Simon, who are with the CESP Centre for Research in Epidemiology and Population Health in Villejuif, France (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)62192-9
They recommended that priority be given to treatment with insulin sensitizers for as long as possible in patients with type 2 diabetes, because these drugs allow a low HbA1c to be achieved without risk of hypoglycemia. For patients with type 2 diabetes using insulin secretagogues or insulin itself, this study provides a rationale for an HbA1c threshold of 7.5%, which corresponds to the lowest threshold of death and lowest event rate for large-vessel disease, they said.
Major Finding: Hemoglobin A1c values below 7.5% were associated with increased all-cause mortality and cardiovascular events.
Data Source: A general practice database analysis of 47,970 type 2 diabetes patients with recently intensified glucose-lowering therapy.
Disclosures: The study was funded by Eli Lilly & Co. The investigators disclosed ties to numerous manufacturers of diabetes treatments, including Eli Lilly. The editorial authors reported relationships with several other pharmaceutical companies.
Hemoglobin A1cvalues below 7.5% were associated with increased all-cause mortality and cardiovascular events in patients with type 2 diabetes in an analysis of nearly 48,000 patients in a U.K. general practice database.
If confirmed, the findings suggest that diabetes guidelines might need revision to include a definition of a minimum HbA1c value, Dr. Craig J. Currie of Cardiff (Wales) University and his associates said (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)61969-3
The study also showed that insulin therapy was associated with higher mortality than combination oral therapy. Unadjusted mortality rates were 16.2 deaths per 1,000 person-years of follow-up for the oral combination therapy group and 27.2/1,000 for the insulin group. After exclusion of patients with high cardiovascular risk or renal impairment, insulin-based therapy remained associated with significantly greater all-cause mortality (HR 1.46) than did combination oral agents, the investigators reported.
The study, funded by Eli Lilly & Co., utilized data from November 1986 to November 2008 in the U.K. General Practice Research Database. Two cohorts of patients aged 50 years and older with type 2 diabetes were assessed: 27,965 whose treatment regimen had been intensified from oral glucose-lowering monotherapy to a combination oral regimen with a sulfonylurea plus metformin, and 20,005 on oral hypoglycemic agents alone who were initiated on insulin with or without concomitant oral agents.
All-cause mortality was the primary outcome. The secondary outcome was the occurrence of a major cardiovascular event among those who had no record of cardiovascular disease before the index date. The mean follow-up was 4.5 years in the oral medication group and 5.2 years in the insulin group.
Patients were divided by HbA1c decile, with the lowest decile (1) having a median HbA1c of 6.4% and the highest decile (10) at 10.5%. Mortality varied by decile in both treatment groups, with increased mortality seen in both the highest and lowest deciles. Patients in decile 4, who had a median HbA1c of 7.5%, had the lowest mortality across deciles, Dr. Currie and his associates reported.
In the combination oral therapy group, only those in deciles 1 and 10 had significantly increased mortality, compared with patients in HbA1c decile 4. However, in the insulin-treated cohort, deciles 1, 2, 3, 9, and 10 all had significantly greater mortality, compared with decile 4.
Progression to large-vessel disease events occurred in 8.2% of 20,817 patients who did not have large-vessel disease at baseline in the oral medication group, and in 11.9% of 13,475 patients in the insulin group. After adjustment for covariates, the adjusted risk of progression to large-vessel disease in both groups was higher for decile 1 (HR 1.54) and decile 10 (HR 1.36).
Compared with combination oral therapy, insulin treatment also was associated with an increased likelihood of progression to a first large-vessel disease event (HR 1.31).
The data suggest that for patients on oral combination therapy, a wide HbA1c range is safe with respect to all-cause mortality and large-vessel events, but a narrower range may be desirable for patients taking insulin, the investigators commented.
In an accompanying editorial, Dr. Beverley Balkau and Dr. Dominique Simon noted that although this study does lend support to earlier studies, epidemiologic studies cannot show causal relationships. Moreover, observational databases can't provide the detailed information available in a randomized clinical trial, such as the actual frequency of hypoglycemia.
However, this study does have the advantage of real-world observation, noted Dr. Balkau and Dr. Simon, who are with the CESP Centre for Research in Epidemiology and Population Health in Villejuif, France (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)62192-9
They recommended that priority be given to treatment with insulin sensitizers for as long as possible in patients with type 2 diabetes, because these drugs allow a low HbA1c to be achieved without risk of hypoglycemia. For patients with type 2 diabetes using insulin secretagogues or insulin itself, this study provides a rationale for an HbA1c threshold of 7.5%, which corresponds to the lowest threshold of death and lowest event rate for large-vessel disease, they said.
Partnerships Created to Develop Insulin Delivery Systems
The Juvenile Diabetes Research Foundation has announced a partnership with Animas Corp. and DexCom Inc. to develop a first-generation automated system for managing type 1 diabetes, as well as a collaboration with Becton, Dickinson and Co. to develop novel products that would work with insulin pumps to enhance insulin delivery.
The JDRF will provide $8 million over the next 3 years to Animas, a Johnson & Johnson company that manufactures insulin pumps. DexCom, a manufacturer of continuous glucose monitoring (CGM) devices, will supply that part of the technology for the system. The money will fund clinical trials of efficacy and safety, with the first-generation system expected to be ready for regulatory review within 4 years, Alan Lewis, Ph.D., JDRF president and CEO, said in a telephone briefing.
While the ultimate goal is to develop a fully automated “closed-loop” system to regulate blood glucose levels, the initial version of the system would still require some input from the user and therefore would only be partially “closed.” It would consist of the insulin pump and the CGM—which are currently available but operate separately—with a computer program that would wirelessly link the two components, enabling the device to automatically increase insulin delivery upon detection of hyperglycemia and shut off delivery when hypoglycemia occurs, subsequently resuming delivery when glucose levels return to normal.
Although the patient would still need to manually instruct the pump to deliver insulin at certain times—particularly at meals—the system would improve overall control by minimizing the amount of time a patient spends out of target glucose range, which is typically more than 70% even among the most sophisticated patients with type 1 diabetes, said Aaron Kowalski, Ph.D., the JDRF's assistant vice president and director of glucose control research.
In a follow-up interview, Dr. Kowalski said that this development partnership is in addition to JDRF's ongoing annual $4-$5 million funding of academic research in closed-loop insulin delivery systems involving devices made by all of the leading pump and CGM manufacturers.
The JDRF also announced that it will provide $4.3 million over the next several years for the nonexclusive collaboration with Becton Dickinson.
Becton Dickinson will use the foundation's funds for research and development of new products aimed at delivering insulin from pumps via either infusion sets or patch-pump configurations, with the goal of minimizing pain, kinking, occlusions, and site infections. The program will also aim to speed insulin action, a JDRF statement said.
One facet will utilize Becton Dickinson microneedles, which speed subcutaneous insulin uptake at the delivery site. Microdelivery technology development will initially focus on improved glucose control. Ultimately it targets the technology of a closed-loop artificial pancreas system utilizing insulin pumps and continuous glucose sensors, with communication between the two devices.
More information about all of JDRF's initiatives is available at www.artificialpancreasproject.com
A first-generation automated insulin delivery system would consist of an insulin pump and a continuous glucose monitor that are linked wirelessly with a computer program.
Source Courtesy JDRF (Juvenile Diabetes Research Foundation)
The Juvenile Diabetes Research Foundation has announced a partnership with Animas Corp. and DexCom Inc. to develop a first-generation automated system for managing type 1 diabetes, as well as a collaboration with Becton, Dickinson and Co. to develop novel products that would work with insulin pumps to enhance insulin delivery.
The JDRF will provide $8 million over the next 3 years to Animas, a Johnson & Johnson company that manufactures insulin pumps. DexCom, a manufacturer of continuous glucose monitoring (CGM) devices, will supply that part of the technology for the system. The money will fund clinical trials of efficacy and safety, with the first-generation system expected to be ready for regulatory review within 4 years, Alan Lewis, Ph.D., JDRF president and CEO, said in a telephone briefing.
While the ultimate goal is to develop a fully automated “closed-loop” system to regulate blood glucose levels, the initial version of the system would still require some input from the user and therefore would only be partially “closed.” It would consist of the insulin pump and the CGM—which are currently available but operate separately—with a computer program that would wirelessly link the two components, enabling the device to automatically increase insulin delivery upon detection of hyperglycemia and shut off delivery when hypoglycemia occurs, subsequently resuming delivery when glucose levels return to normal.
Although the patient would still need to manually instruct the pump to deliver insulin at certain times—particularly at meals—the system would improve overall control by minimizing the amount of time a patient spends out of target glucose range, which is typically more than 70% even among the most sophisticated patients with type 1 diabetes, said Aaron Kowalski, Ph.D., the JDRF's assistant vice president and director of glucose control research.
In a follow-up interview, Dr. Kowalski said that this development partnership is in addition to JDRF's ongoing annual $4-$5 million funding of academic research in closed-loop insulin delivery systems involving devices made by all of the leading pump and CGM manufacturers.
The JDRF also announced that it will provide $4.3 million over the next several years for the nonexclusive collaboration with Becton Dickinson.
Becton Dickinson will use the foundation's funds for research and development of new products aimed at delivering insulin from pumps via either infusion sets or patch-pump configurations, with the goal of minimizing pain, kinking, occlusions, and site infections. The program will also aim to speed insulin action, a JDRF statement said.
One facet will utilize Becton Dickinson microneedles, which speed subcutaneous insulin uptake at the delivery site. Microdelivery technology development will initially focus on improved glucose control. Ultimately it targets the technology of a closed-loop artificial pancreas system utilizing insulin pumps and continuous glucose sensors, with communication between the two devices.
More information about all of JDRF's initiatives is available at www.artificialpancreasproject.com
A first-generation automated insulin delivery system would consist of an insulin pump and a continuous glucose monitor that are linked wirelessly with a computer program.
Source Courtesy JDRF (Juvenile Diabetes Research Foundation)
The Juvenile Diabetes Research Foundation has announced a partnership with Animas Corp. and DexCom Inc. to develop a first-generation automated system for managing type 1 diabetes, as well as a collaboration with Becton, Dickinson and Co. to develop novel products that would work with insulin pumps to enhance insulin delivery.
The JDRF will provide $8 million over the next 3 years to Animas, a Johnson & Johnson company that manufactures insulin pumps. DexCom, a manufacturer of continuous glucose monitoring (CGM) devices, will supply that part of the technology for the system. The money will fund clinical trials of efficacy and safety, with the first-generation system expected to be ready for regulatory review within 4 years, Alan Lewis, Ph.D., JDRF president and CEO, said in a telephone briefing.
While the ultimate goal is to develop a fully automated “closed-loop” system to regulate blood glucose levels, the initial version of the system would still require some input from the user and therefore would only be partially “closed.” It would consist of the insulin pump and the CGM—which are currently available but operate separately—with a computer program that would wirelessly link the two components, enabling the device to automatically increase insulin delivery upon detection of hyperglycemia and shut off delivery when hypoglycemia occurs, subsequently resuming delivery when glucose levels return to normal.
Although the patient would still need to manually instruct the pump to deliver insulin at certain times—particularly at meals—the system would improve overall control by minimizing the amount of time a patient spends out of target glucose range, which is typically more than 70% even among the most sophisticated patients with type 1 diabetes, said Aaron Kowalski, Ph.D., the JDRF's assistant vice president and director of glucose control research.
In a follow-up interview, Dr. Kowalski said that this development partnership is in addition to JDRF's ongoing annual $4-$5 million funding of academic research in closed-loop insulin delivery systems involving devices made by all of the leading pump and CGM manufacturers.
The JDRF also announced that it will provide $4.3 million over the next several years for the nonexclusive collaboration with Becton Dickinson.
Becton Dickinson will use the foundation's funds for research and development of new products aimed at delivering insulin from pumps via either infusion sets or patch-pump configurations, with the goal of minimizing pain, kinking, occlusions, and site infections. The program will also aim to speed insulin action, a JDRF statement said.
One facet will utilize Becton Dickinson microneedles, which speed subcutaneous insulin uptake at the delivery site. Microdelivery technology development will initially focus on improved glucose control. Ultimately it targets the technology of a closed-loop artificial pancreas system utilizing insulin pumps and continuous glucose sensors, with communication between the two devices.
More information about all of JDRF's initiatives is available at www.artificialpancreasproject.com
A first-generation automated insulin delivery system would consist of an insulin pump and a continuous glucose monitor that are linked wirelessly with a computer program.
Source Courtesy JDRF (Juvenile Diabetes Research Foundation)
Mortality May Be Increased at HbA1c Below 7.5%
Hemoglobin A1c values below 7.5% were associated with increased all-cause mortality and cardiovascular events in patients with type 2 diabetes in an analysis of nearly 48,000 patients in a U.K. general practice database.
If confirmed, the findings suggest that diabetes guidelines might need revision to include a definition of a minimum HbA1c value, Dr. Craig J. Currie of Cardiff (Wales) University and his associates said (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)61969-3
The study also showed that insulin therapy was associated with higher mortality than combination oral therapy. Unadjusted mortality rates were 16.2 deaths per 1,000 person-years of follow-up for the oral combination therapy group and 27.2/1,000 for the insulin group. After exclusion of patients with high cardiovascular risk or renal impairment, insulin-based therapy remained associated with significantly greater all-cause mortality (HR 1.46) than did combination oral agents, the investigators reported.
The study utilized data from November 1986 to November 2008 in the U.K. General Practice Research Database. Two cohorts of patients aged 50 years and older with type 2 diabetes were assessed: 27,965 whose treatment regimen had been intensified from oral glucose-lowering monotherapy to a combination oral regimen with a sulfonylurea plus metformin, and 20,005 on oral hypoglycemic agents alone who were initiated on insulin with or without concomitant oral agents.
All-cause mortality was the primary outcome. The secondary outcome was the occurrence of a major cardiovascular event among those who had no record of cardiovascular disease before the index date. The mean follow-up was 4.5 years in the oral medication group and 5.2 years in the insulin group.
Patients were divided by HbA1c decile, with the lowest decile (1) having a median HbA1c of 6.4% and the highest decile (10) at 10.5%. Mortality varied by HbA1c decile in both treatment groups, with increased mortality seen in both the highest and lowest deciles. Patients in decile 4, who had a median HbA1c of 7.5%, had the lowest mortality across deciles, Dr. Currie and his associates reported.
In the combination oral therapy group, only those in deciles 1 and 10 had significantly increased mortality, compared with patients in HbA1c decile 4. However, in the insulin-treated cohort, deciles 1, 2, 3, 9, and 10 all had significantly greater mortality, compared with decile 4.
Progression to large-vessel disease events occurred in 8.2% of 20,817 patients who did not have large-vessel disease at baseline in the oral medication group, and in 11.9% of 13,475 patients in the insulin group. After adjustment for covariates, the adjusted risk of progression to large-vessel disease in both groups was higher for decile 1 (HR 1.54) and decile 10 (HR 1.36). Compared with combination oral therapy, insulin treatment also was associated with an increased likelihood of progression to a first large-vessel disease event (HR 1.31).
The data suggest that for patients on oral combination therapy, a wide HbA1c range is safe with respect to all-cause mortality and large-vessel events, but a narrower range may be desirable for patients taking insulin, the investigators commented.
Decreased survival in patients achieving low HbA1c levels might be related to hypoglycemia, a common complication of intensive blood glucose control. Postulated mechanisms include a link between the sympathomimetic or hypokalemic manifestations of hypoglycemia and the onset of cardiac arrhythmia.
Hypoglycemia might also potentiate glucose variability, contributing to increased oxidative stress and vascular inflammation and predisposing patients to atherosclerotic plaque destabilization and vascular function, Dr. Currie and his associates said.
In an accompanying editorial, Dr. Beverley Balkau and Dr. Dominique Simon noted that although this study does lend support to earlier studies, epidemiologic studies cannot show causal relationships. Moreover, observational databases can't provide the detailed information available in a randomized clinical trial, such as the actual frequency of hypoglycemia.
However, this study does have the advantage of real-world observation, said Dr. Balkau and Dr. Simon, of the CESP Centre for Research in Epidemiology and Population Health, Villejuif, France (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)62192-9
Dr. Balkau and Dr. Simon recommended that priority be given to treatment with insulin sensitizers for as long as possible in patients with type 2 diabetes, because these drugs allow a low HbA1c to be achieved without risk of hypoglycemia.
For patients with type 2 diabetes using insulin secretagogues or insulin itself, this study provides a rationale for an HbA1c threshold of 7.5%, which corresponds to the lowest threshold of death and lowest event rate for large-vessel disease, they said.
Disclosures: The study was funded by Eli Lilly & Co. Dr. Currie has received research grants from and consulted for various pharmaceutical companies, including Eli Lilly. Four of the study coauthors are employees of Eli Lilly. Dr. Balkau and Dr. Simon have served as speakers for and been on the advisory panels of several pharmaceutical companies.
Decreased survival in patients achieving low HbA1c levels might be related to hypoglycemia.
Source DR. CURRIE
Hemoglobin A1c values below 7.5% were associated with increased all-cause mortality and cardiovascular events in patients with type 2 diabetes in an analysis of nearly 48,000 patients in a U.K. general practice database.
If confirmed, the findings suggest that diabetes guidelines might need revision to include a definition of a minimum HbA1c value, Dr. Craig J. Currie of Cardiff (Wales) University and his associates said (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)61969-3
The study also showed that insulin therapy was associated with higher mortality than combination oral therapy. Unadjusted mortality rates were 16.2 deaths per 1,000 person-years of follow-up for the oral combination therapy group and 27.2/1,000 for the insulin group. After exclusion of patients with high cardiovascular risk or renal impairment, insulin-based therapy remained associated with significantly greater all-cause mortality (HR 1.46) than did combination oral agents, the investigators reported.
The study utilized data from November 1986 to November 2008 in the U.K. General Practice Research Database. Two cohorts of patients aged 50 years and older with type 2 diabetes were assessed: 27,965 whose treatment regimen had been intensified from oral glucose-lowering monotherapy to a combination oral regimen with a sulfonylurea plus metformin, and 20,005 on oral hypoglycemic agents alone who were initiated on insulin with or without concomitant oral agents.
All-cause mortality was the primary outcome. The secondary outcome was the occurrence of a major cardiovascular event among those who had no record of cardiovascular disease before the index date. The mean follow-up was 4.5 years in the oral medication group and 5.2 years in the insulin group.
Patients were divided by HbA1c decile, with the lowest decile (1) having a median HbA1c of 6.4% and the highest decile (10) at 10.5%. Mortality varied by HbA1c decile in both treatment groups, with increased mortality seen in both the highest and lowest deciles. Patients in decile 4, who had a median HbA1c of 7.5%, had the lowest mortality across deciles, Dr. Currie and his associates reported.
In the combination oral therapy group, only those in deciles 1 and 10 had significantly increased mortality, compared with patients in HbA1c decile 4. However, in the insulin-treated cohort, deciles 1, 2, 3, 9, and 10 all had significantly greater mortality, compared with decile 4.
Progression to large-vessel disease events occurred in 8.2% of 20,817 patients who did not have large-vessel disease at baseline in the oral medication group, and in 11.9% of 13,475 patients in the insulin group. After adjustment for covariates, the adjusted risk of progression to large-vessel disease in both groups was higher for decile 1 (HR 1.54) and decile 10 (HR 1.36). Compared with combination oral therapy, insulin treatment also was associated with an increased likelihood of progression to a first large-vessel disease event (HR 1.31).
The data suggest that for patients on oral combination therapy, a wide HbA1c range is safe with respect to all-cause mortality and large-vessel events, but a narrower range may be desirable for patients taking insulin, the investigators commented.
Decreased survival in patients achieving low HbA1c levels might be related to hypoglycemia, a common complication of intensive blood glucose control. Postulated mechanisms include a link between the sympathomimetic or hypokalemic manifestations of hypoglycemia and the onset of cardiac arrhythmia.
Hypoglycemia might also potentiate glucose variability, contributing to increased oxidative stress and vascular inflammation and predisposing patients to atherosclerotic plaque destabilization and vascular function, Dr. Currie and his associates said.
In an accompanying editorial, Dr. Beverley Balkau and Dr. Dominique Simon noted that although this study does lend support to earlier studies, epidemiologic studies cannot show causal relationships. Moreover, observational databases can't provide the detailed information available in a randomized clinical trial, such as the actual frequency of hypoglycemia.
However, this study does have the advantage of real-world observation, said Dr. Balkau and Dr. Simon, of the CESP Centre for Research in Epidemiology and Population Health, Villejuif, France (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)62192-9
Dr. Balkau and Dr. Simon recommended that priority be given to treatment with insulin sensitizers for as long as possible in patients with type 2 diabetes, because these drugs allow a low HbA1c to be achieved without risk of hypoglycemia.
For patients with type 2 diabetes using insulin secretagogues or insulin itself, this study provides a rationale for an HbA1c threshold of 7.5%, which corresponds to the lowest threshold of death and lowest event rate for large-vessel disease, they said.
Disclosures: The study was funded by Eli Lilly & Co. Dr. Currie has received research grants from and consulted for various pharmaceutical companies, including Eli Lilly. Four of the study coauthors are employees of Eli Lilly. Dr. Balkau and Dr. Simon have served as speakers for and been on the advisory panels of several pharmaceutical companies.
Decreased survival in patients achieving low HbA1c levels might be related to hypoglycemia.
Source DR. CURRIE
Hemoglobin A1c values below 7.5% were associated with increased all-cause mortality and cardiovascular events in patients with type 2 diabetes in an analysis of nearly 48,000 patients in a U.K. general practice database.
If confirmed, the findings suggest that diabetes guidelines might need revision to include a definition of a minimum HbA1c value, Dr. Craig J. Currie of Cardiff (Wales) University and his associates said (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)61969-3
The study also showed that insulin therapy was associated with higher mortality than combination oral therapy. Unadjusted mortality rates were 16.2 deaths per 1,000 person-years of follow-up for the oral combination therapy group and 27.2/1,000 for the insulin group. After exclusion of patients with high cardiovascular risk or renal impairment, insulin-based therapy remained associated with significantly greater all-cause mortality (HR 1.46) than did combination oral agents, the investigators reported.
The study utilized data from November 1986 to November 2008 in the U.K. General Practice Research Database. Two cohorts of patients aged 50 years and older with type 2 diabetes were assessed: 27,965 whose treatment regimen had been intensified from oral glucose-lowering monotherapy to a combination oral regimen with a sulfonylurea plus metformin, and 20,005 on oral hypoglycemic agents alone who were initiated on insulin with or without concomitant oral agents.
All-cause mortality was the primary outcome. The secondary outcome was the occurrence of a major cardiovascular event among those who had no record of cardiovascular disease before the index date. The mean follow-up was 4.5 years in the oral medication group and 5.2 years in the insulin group.
Patients were divided by HbA1c decile, with the lowest decile (1) having a median HbA1c of 6.4% and the highest decile (10) at 10.5%. Mortality varied by HbA1c decile in both treatment groups, with increased mortality seen in both the highest and lowest deciles. Patients in decile 4, who had a median HbA1c of 7.5%, had the lowest mortality across deciles, Dr. Currie and his associates reported.
In the combination oral therapy group, only those in deciles 1 and 10 had significantly increased mortality, compared with patients in HbA1c decile 4. However, in the insulin-treated cohort, deciles 1, 2, 3, 9, and 10 all had significantly greater mortality, compared with decile 4.
Progression to large-vessel disease events occurred in 8.2% of 20,817 patients who did not have large-vessel disease at baseline in the oral medication group, and in 11.9% of 13,475 patients in the insulin group. After adjustment for covariates, the adjusted risk of progression to large-vessel disease in both groups was higher for decile 1 (HR 1.54) and decile 10 (HR 1.36). Compared with combination oral therapy, insulin treatment also was associated with an increased likelihood of progression to a first large-vessel disease event (HR 1.31).
The data suggest that for patients on oral combination therapy, a wide HbA1c range is safe with respect to all-cause mortality and large-vessel events, but a narrower range may be desirable for patients taking insulin, the investigators commented.
Decreased survival in patients achieving low HbA1c levels might be related to hypoglycemia, a common complication of intensive blood glucose control. Postulated mechanisms include a link between the sympathomimetic or hypokalemic manifestations of hypoglycemia and the onset of cardiac arrhythmia.
Hypoglycemia might also potentiate glucose variability, contributing to increased oxidative stress and vascular inflammation and predisposing patients to atherosclerotic plaque destabilization and vascular function, Dr. Currie and his associates said.
In an accompanying editorial, Dr. Beverley Balkau and Dr. Dominique Simon noted that although this study does lend support to earlier studies, epidemiologic studies cannot show causal relationships. Moreover, observational databases can't provide the detailed information available in a randomized clinical trial, such as the actual frequency of hypoglycemia.
However, this study does have the advantage of real-world observation, said Dr. Balkau and Dr. Simon, of the CESP Centre for Research in Epidemiology and Population Health, Villejuif, France (Lancet 2010 Jan. 27 [doi:10.1016/S0140-6736(09)62192-9
Dr. Balkau and Dr. Simon recommended that priority be given to treatment with insulin sensitizers for as long as possible in patients with type 2 diabetes, because these drugs allow a low HbA1c to be achieved without risk of hypoglycemia.
For patients with type 2 diabetes using insulin secretagogues or insulin itself, this study provides a rationale for an HbA1c threshold of 7.5%, which corresponds to the lowest threshold of death and lowest event rate for large-vessel disease, they said.
Disclosures: The study was funded by Eli Lilly & Co. Dr. Currie has received research grants from and consulted for various pharmaceutical companies, including Eli Lilly. Four of the study coauthors are employees of Eli Lilly. Dr. Balkau and Dr. Simon have served as speakers for and been on the advisory panels of several pharmaceutical companies.
Decreased survival in patients achieving low HbA1c levels might be related to hypoglycemia.
Source DR. CURRIE
Complications Seen Early in Diabetic Teens
NEW YORK — Early diabetes complications were seen in a significant proportion of 821 adolescents with type 1 diabetes for just 2-5 years.
Up to 1 in 5 of these adolescents already had early indicators of eye, kidney, and/or nerve complications. The findings support early screening for diabetes complications as recommended by some—but not all—published consensus guidelines, Dr. Yoon Hi Cho said at a joint meeting of the Lawson Wilkins Pediatric Endocrine Society and the European Society for Pediatric Endocrinology.
All of the 821 patients were seen at the Children's Hospital at Westmead, Sydney, between 1990 and 2006. They were aged 11-17 years, with a type 1 diabetes duration of 2-5 years (median 3.8) and a median hemoglobin A1c level of 8.9%.
Early retinopathy, defined as one microaneurysm or hemorrhage on seven-field stereoscopic fundus photography, was detected in 9% of the patients. Albumin excretion rate (AER) was measured for overnight urine collections. Early nephropathy, defined as a borderline elevation of AER of 7.5 to less than 20 mcg/min, was seen in 22% of the adolescents.
Microalbuminuria, defined as an AER of 20 mcg/min or greater, was identified in 3%. Peripheral nerve abnormalities on thermal and vibration thresholds at the feet—measured by thermal threshold tester and biothesiometer—were found in 22% of the patients, said Dr. Cho, a clinical endocrinology fellow at the hospital.
The proportions who had borderline AER elevation rose from 16% of those aged 11-13 years to 23% of 13- to 15-year-olds to 25% of those aged 15-17 years.
There was no significant effect of age for retinopathy (seen in 6%, 10%, and 11% of the three groups) or for peripheral nerve abnormalities (seen in 27%, 19%, and 22%). The rates of microalbuminuria remained low (4% or below).
There was no significant difference in hemoglobin A1c levels between the three age groups (8.2%, 8.5%, and 8.6%), Dr. Cho said.
Retinopathy was significantly related to an elevated diastolic blood pressure level. A mean AER of 7.5 mcg/min or greater was associated with increased age, diabetes duration, and systolic blood pressure. Peripheral nerve abnormalities were correlated with a higher body mass index.
There was no significant relationship between any of the complications and hemoglobin A1c level, cholesterol level, sex, insulin dose, or insulin regimen, she said.
Guidelines from the International Society for Pediatric and Adolescent Diabetes and from the Australian Pediatric Endocrinology Group both advise screening children for diabetes complications after a diabetes duration of 5 years in prepubertal children and after 2 years in adolescents.
The American Diabetes Association recommends retinopathy screening within 5 years of the onset of diabetes and nephropathy screening after a diabetes duration of 5 years in children aged 10 years and older.
“Longitudinal analysis will help define predictors of early complications and the potential for modifying [their] natural history,” Dr. Cho said.
Disclosures: Dr. Cho stated that she had no relevant financial disclosures.
NEW YORK — Early diabetes complications were seen in a significant proportion of 821 adolescents with type 1 diabetes for just 2-5 years.
Up to 1 in 5 of these adolescents already had early indicators of eye, kidney, and/or nerve complications. The findings support early screening for diabetes complications as recommended by some—but not all—published consensus guidelines, Dr. Yoon Hi Cho said at a joint meeting of the Lawson Wilkins Pediatric Endocrine Society and the European Society for Pediatric Endocrinology.
All of the 821 patients were seen at the Children's Hospital at Westmead, Sydney, between 1990 and 2006. They were aged 11-17 years, with a type 1 diabetes duration of 2-5 years (median 3.8) and a median hemoglobin A1c level of 8.9%.
Early retinopathy, defined as one microaneurysm or hemorrhage on seven-field stereoscopic fundus photography, was detected in 9% of the patients. Albumin excretion rate (AER) was measured for overnight urine collections. Early nephropathy, defined as a borderline elevation of AER of 7.5 to less than 20 mcg/min, was seen in 22% of the adolescents.
Microalbuminuria, defined as an AER of 20 mcg/min or greater, was identified in 3%. Peripheral nerve abnormalities on thermal and vibration thresholds at the feet—measured by thermal threshold tester and biothesiometer—were found in 22% of the patients, said Dr. Cho, a clinical endocrinology fellow at the hospital.
The proportions who had borderline AER elevation rose from 16% of those aged 11-13 years to 23% of 13- to 15-year-olds to 25% of those aged 15-17 years.
There was no significant effect of age for retinopathy (seen in 6%, 10%, and 11% of the three groups) or for peripheral nerve abnormalities (seen in 27%, 19%, and 22%). The rates of microalbuminuria remained low (4% or below).
There was no significant difference in hemoglobin A1c levels between the three age groups (8.2%, 8.5%, and 8.6%), Dr. Cho said.
Retinopathy was significantly related to an elevated diastolic blood pressure level. A mean AER of 7.5 mcg/min or greater was associated with increased age, diabetes duration, and systolic blood pressure. Peripheral nerve abnormalities were correlated with a higher body mass index.
There was no significant relationship between any of the complications and hemoglobin A1c level, cholesterol level, sex, insulin dose, or insulin regimen, she said.
Guidelines from the International Society for Pediatric and Adolescent Diabetes and from the Australian Pediatric Endocrinology Group both advise screening children for diabetes complications after a diabetes duration of 5 years in prepubertal children and after 2 years in adolescents.
The American Diabetes Association recommends retinopathy screening within 5 years of the onset of diabetes and nephropathy screening after a diabetes duration of 5 years in children aged 10 years and older.
“Longitudinal analysis will help define predictors of early complications and the potential for modifying [their] natural history,” Dr. Cho said.
Disclosures: Dr. Cho stated that she had no relevant financial disclosures.
NEW YORK — Early diabetes complications were seen in a significant proportion of 821 adolescents with type 1 diabetes for just 2-5 years.
Up to 1 in 5 of these adolescents already had early indicators of eye, kidney, and/or nerve complications. The findings support early screening for diabetes complications as recommended by some—but not all—published consensus guidelines, Dr. Yoon Hi Cho said at a joint meeting of the Lawson Wilkins Pediatric Endocrine Society and the European Society for Pediatric Endocrinology.
All of the 821 patients were seen at the Children's Hospital at Westmead, Sydney, between 1990 and 2006. They were aged 11-17 years, with a type 1 diabetes duration of 2-5 years (median 3.8) and a median hemoglobin A1c level of 8.9%.
Early retinopathy, defined as one microaneurysm or hemorrhage on seven-field stereoscopic fundus photography, was detected in 9% of the patients. Albumin excretion rate (AER) was measured for overnight urine collections. Early nephropathy, defined as a borderline elevation of AER of 7.5 to less than 20 mcg/min, was seen in 22% of the adolescents.
Microalbuminuria, defined as an AER of 20 mcg/min or greater, was identified in 3%. Peripheral nerve abnormalities on thermal and vibration thresholds at the feet—measured by thermal threshold tester and biothesiometer—were found in 22% of the patients, said Dr. Cho, a clinical endocrinology fellow at the hospital.
The proportions who had borderline AER elevation rose from 16% of those aged 11-13 years to 23% of 13- to 15-year-olds to 25% of those aged 15-17 years.
There was no significant effect of age for retinopathy (seen in 6%, 10%, and 11% of the three groups) or for peripheral nerve abnormalities (seen in 27%, 19%, and 22%). The rates of microalbuminuria remained low (4% or below).
There was no significant difference in hemoglobin A1c levels between the three age groups (8.2%, 8.5%, and 8.6%), Dr. Cho said.
Retinopathy was significantly related to an elevated diastolic blood pressure level. A mean AER of 7.5 mcg/min or greater was associated with increased age, diabetes duration, and systolic blood pressure. Peripheral nerve abnormalities were correlated with a higher body mass index.
There was no significant relationship between any of the complications and hemoglobin A1c level, cholesterol level, sex, insulin dose, or insulin regimen, she said.
Guidelines from the International Society for Pediatric and Adolescent Diabetes and from the Australian Pediatric Endocrinology Group both advise screening children for diabetes complications after a diabetes duration of 5 years in prepubertal children and after 2 years in adolescents.
The American Diabetes Association recommends retinopathy screening within 5 years of the onset of diabetes and nephropathy screening after a diabetes duration of 5 years in children aged 10 years and older.
“Longitudinal analysis will help define predictors of early complications and the potential for modifying [their] natural history,” Dr. Cho said.
Disclosures: Dr. Cho stated that she had no relevant financial disclosures.
Eosinophilic GI Disorder Rates Vary Widely
NATIONAL HARBOR, MD. — Eosinophilic esophagitis affects an estimated 52/100,000 Americans, while eosinophilic gastritis/colitis affects about 28/100,000, according to the results of the first nationwide study to investigate the disease burden of eosinophilic gastrointestinal disorders.
The study, conducted via an e-mail survey of pediatric and adult gastroenterologists, demonstrated wide variations in the rates of both EoE and eosinophilic gastritis/eosinophilic colitis (EG/EC) in different parts of the country, with rates highest in the Northeast, followed by the South, the Midwest, and the West, Dr. Wendy M. Book said at the annual meeting of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Electronic surveys were e-mailed to 10,833 physicians. The total response rate was 17%, including 866 allergy-immunology specialists, 333 pediatric gastroenterologists, and 602 adult gastroenterologists.
Based on the survey responses and U.S. census data, Dr. Book said an estimated 158,705 patients have EoE, with a prevalence of 52.2 per 100,000 population. For EG/EC, the pediatric gastroenterologists reported seeing an average of 8.9 patients per year, while the adult gastroenterologists reported 5.9 per year, for an estimated 85,281, or 28.1/100,000, said Dr. Book, of the department of internal medicine at Emory University, Atlanta.
Disclosures: Dr. Book is president of the nonprofit organization American Partnership for Eosinophilic Disorders. She has no relevant conflicts of interest.
NATIONAL HARBOR, MD. — Eosinophilic esophagitis affects an estimated 52/100,000 Americans, while eosinophilic gastritis/colitis affects about 28/100,000, according to the results of the first nationwide study to investigate the disease burden of eosinophilic gastrointestinal disorders.
The study, conducted via an e-mail survey of pediatric and adult gastroenterologists, demonstrated wide variations in the rates of both EoE and eosinophilic gastritis/eosinophilic colitis (EG/EC) in different parts of the country, with rates highest in the Northeast, followed by the South, the Midwest, and the West, Dr. Wendy M. Book said at the annual meeting of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Electronic surveys were e-mailed to 10,833 physicians. The total response rate was 17%, including 866 allergy-immunology specialists, 333 pediatric gastroenterologists, and 602 adult gastroenterologists.
Based on the survey responses and U.S. census data, Dr. Book said an estimated 158,705 patients have EoE, with a prevalence of 52.2 per 100,000 population. For EG/EC, the pediatric gastroenterologists reported seeing an average of 8.9 patients per year, while the adult gastroenterologists reported 5.9 per year, for an estimated 85,281, or 28.1/100,000, said Dr. Book, of the department of internal medicine at Emory University, Atlanta.
Disclosures: Dr. Book is president of the nonprofit organization American Partnership for Eosinophilic Disorders. She has no relevant conflicts of interest.
NATIONAL HARBOR, MD. — Eosinophilic esophagitis affects an estimated 52/100,000 Americans, while eosinophilic gastritis/colitis affects about 28/100,000, according to the results of the first nationwide study to investigate the disease burden of eosinophilic gastrointestinal disorders.
The study, conducted via an e-mail survey of pediatric and adult gastroenterologists, demonstrated wide variations in the rates of both EoE and eosinophilic gastritis/eosinophilic colitis (EG/EC) in different parts of the country, with rates highest in the Northeast, followed by the South, the Midwest, and the West, Dr. Wendy M. Book said at the annual meeting of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Electronic surveys were e-mailed to 10,833 physicians. The total response rate was 17%, including 866 allergy-immunology specialists, 333 pediatric gastroenterologists, and 602 adult gastroenterologists.
Based on the survey responses and U.S. census data, Dr. Book said an estimated 158,705 patients have EoE, with a prevalence of 52.2 per 100,000 population. For EG/EC, the pediatric gastroenterologists reported seeing an average of 8.9 patients per year, while the adult gastroenterologists reported 5.9 per year, for an estimated 85,281, or 28.1/100,000, said Dr. Book, of the department of internal medicine at Emory University, Atlanta.
Disclosures: Dr. Book is president of the nonprofit organization American Partnership for Eosinophilic Disorders. She has no relevant conflicts of interest.