User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
Transcatheter Aortic Valves Boost Quality of Life
CHICAGO – Transcatheter aortic valve implantation produced a dramatic improvement in quality of life scores, compared with standard medical management, in patients with inoperable, severe aortic stenosis in a pivotal trial with 358 randomized patients.
The primary end point of the trial, which was first reported in September and then published in October, showed that transcatheter aortic valve implantation (TAVI) significantly improved the rate of all-cause death, compared with medical care, in patients who were judged to be unable to undergo conventional surgical aortic valve replacement (N. Engl. J. Med. 2010;363:1597-607). Additional results in the new report showed a sharp improvement in the quality of life of these patients, with 62% having a 20-point or greater rise in their KCCQ (Kansas City Cardiomyopathy Questionnaire) summary scores, compared with baseline. That increase translates into an improvement of two classes on the New York Heart Association heart failure scale, Dr. David J. Cohen said at the annual scientific sessions of the American Heart Association.
"These findings add further support to the concept that TAVI should be considered an emerging standard of care for patients with severe aortic stenosis who are not candidates for surgical aortic valve replacement," said Dr. Cohen, professor and director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City.
The substantial quality of life improvement seen in most patients who are treated with TAVI adds an important dimension to the study’s outcome because "these patients care far more about how they feel than how long they live," Dr. Cohen said in an interview. "If patients lived longer but didn’t feel any better than they felt at baseline – which was lousy – it wouldn’t be much of an accomplishment." Patients who seek care for severe aortic stenosis usually put a high value on treatment that produces an improved quality of life, he said.
"The impact of TAVI on health status [quality of life] is as important as any other outcome," including survival, commented Dr. John S. Rumsfeld, a cardiologist at the Denver VA Medical Center and the University of Colorado, Denver. It is an efficacy outcome that is very meaningful to patients, he said.
Based on the benefits for both survival and quality of life now reported from the PARTNER (Placement of Aortic Transcatheter Valves) study, staffers from Edwards Lifesciences Corp. announced in November that the company submitted an application to the Food and Drug Administration for marketing approval of the Sapien valve and delivery system used in the study. A report on the results from the second portion of the study, which randomized patients to surgical valve replacement or TAVI, is expected during the first half of 2011, a company spokeswoman said in an interview.
The PARTNER trial enrolled 358 patients with severe aortic stenosis who were judged inoperable by two independent surgeons. Their average age was 83 years, and slightly more than half the patients were women. Assessment using the KCCQ occurred in 81%-91% of the participants at baseline and at 1, 6, and 12 months following the start of the study. At baseline, the average KCCQ summary score among patients in both treatment arms was 35, and 70% of the patients in both arms had a score of 45 or less. A KCCQ score at that level indicates that the patient’s health status is comparable to someone with class IV New York Heart Association heart failure.
At 12 months after entry, patients who underwent TAVI had a KCCQ summary score that averaged 25 points higher than the score of those on medical therapy. The TAVI patients also had higher scores in four elements of the KCCQ assessment: symptoms, physical limitations, social limitations, and quality of life. The TAVI patients also showed substantial improvements at 12 months in three secondary measures of health status: the SF-12 physical scale, the SF-12 mental scale, and the Euro quality of life measure. For example, on the SF-12 physical scale, the average improvement with TAVI was 5 points better than with medical management. On this scale, an increase of at least 2 points reflects a clinically important difference, Dr. Cohen said.
Further analysis tallied the percentage of patients who had an "excellent" outcome, defined as those who survived to a particular follow-up interval and had a 20-point or better rise in their KCCQ summary score, compared with baseline. At all follow-up intervals, substantially more patients who were treated with TAVI met the excellent outcome criteria (see box), with a number-needed-to-treat of 3.5 to achieve one excellent outcome at 12 months.
A series of subgroup analyses showed no interaction of these effects by TAVI with age, sex, surgical risk score, aortic valve gradient pressure, or the severity of comorbid chronic obstructive pulmonary disease.
Inoperable patients make up a small portion – about 5%-10% – of all patients with severe aortic stenosis, Dr. Cohen said. Other patients with severe aortic stenosis don’t undergo surgical valve replacement for other, unknown reasons; thus, about one-fifth to one-quarter of U.S. aortic stenosis patients with disease that is severe enough to warrant valve replacement surgery don’t get it, he added. Although truly inoperable patients are uncommon, they stand to gain markedly when the transcatheter valve system becomes routinely available. They have "the biggest unmet need," Dr. Cohen said.
The PARTNER study was funded by Edwards Lifesciences. Dr. Cohen said that he has received research funding from Edwards Lifesciences.
It is pretty clear that TAVI is a potential paradigm shift in the treatment of patients with aortic stenosis. The design of the PARTNER trial appropriately emphasized patient health status outcomes, which include three elements: symptom burden, functional limitation, and health-related quality of life. The availability of validated questionnaires allows researchers to assess and interpret these outcomes with high reliability. These outcomes are sensitive to changes in clinical status, and they predict mortality, rehospitalization, and the cost of care. The impact that TAVI had on health status was as important as any other outcome measured.
The size of the effect of TAVI on quality of life in the PARTNER trial was astounding. I have no doubt that the results for both quality of life and survival will lead to tremendous excitement about moving TAVI into routine clinical practice. When that happens, we must be vigilant about safety. So far, TAVI has been used in selected centers, and on patients with severe baseline symptoms who were at very high risk. Will the same benefits occur in patients with less functional impairment? We need registries to longitudinally monitor patients who receive TAVI once it is on the market.
The PARTNER results also reinforce the role of health status outcomes in clinical trials. These outcomes are clinically important and very meaningful to patients. The results of this study solidify the essential role that health status outcomes play in evaluating the efficacy of clinical therapeutics.
Dr. John S. Rumsfeld is a cardiologist at the Denver VA Medical Center and the University of Colorado, Denver. He said that he had no disclosures. He made these comments as a discussant of Dr. Cohen’s report at the meeting.
It is pretty clear that TAVI is a potential paradigm shift in the treatment of patients with aortic stenosis. The design of the PARTNER trial appropriately emphasized patient health status outcomes, which include three elements: symptom burden, functional limitation, and health-related quality of life. The availability of validated questionnaires allows researchers to assess and interpret these outcomes with high reliability. These outcomes are sensitive to changes in clinical status, and they predict mortality, rehospitalization, and the cost of care. The impact that TAVI had on health status was as important as any other outcome measured.
The size of the effect of TAVI on quality of life in the PARTNER trial was astounding. I have no doubt that the results for both quality of life and survival will lead to tremendous excitement about moving TAVI into routine clinical practice. When that happens, we must be vigilant about safety. So far, TAVI has been used in selected centers, and on patients with severe baseline symptoms who were at very high risk. Will the same benefits occur in patients with less functional impairment? We need registries to longitudinally monitor patients who receive TAVI once it is on the market.
The PARTNER results also reinforce the role of health status outcomes in clinical trials. These outcomes are clinically important and very meaningful to patients. The results of this study solidify the essential role that health status outcomes play in evaluating the efficacy of clinical therapeutics.
Dr. John S. Rumsfeld is a cardiologist at the Denver VA Medical Center and the University of Colorado, Denver. He said that he had no disclosures. He made these comments as a discussant of Dr. Cohen’s report at the meeting.
It is pretty clear that TAVI is a potential paradigm shift in the treatment of patients with aortic stenosis. The design of the PARTNER trial appropriately emphasized patient health status outcomes, which include three elements: symptom burden, functional limitation, and health-related quality of life. The availability of validated questionnaires allows researchers to assess and interpret these outcomes with high reliability. These outcomes are sensitive to changes in clinical status, and they predict mortality, rehospitalization, and the cost of care. The impact that TAVI had on health status was as important as any other outcome measured.
The size of the effect of TAVI on quality of life in the PARTNER trial was astounding. I have no doubt that the results for both quality of life and survival will lead to tremendous excitement about moving TAVI into routine clinical practice. When that happens, we must be vigilant about safety. So far, TAVI has been used in selected centers, and on patients with severe baseline symptoms who were at very high risk. Will the same benefits occur in patients with less functional impairment? We need registries to longitudinally monitor patients who receive TAVI once it is on the market.
The PARTNER results also reinforce the role of health status outcomes in clinical trials. These outcomes are clinically important and very meaningful to patients. The results of this study solidify the essential role that health status outcomes play in evaluating the efficacy of clinical therapeutics.
Dr. John S. Rumsfeld is a cardiologist at the Denver VA Medical Center and the University of Colorado, Denver. He said that he had no disclosures. He made these comments as a discussant of Dr. Cohen’s report at the meeting.
CHICAGO – Transcatheter aortic valve implantation produced a dramatic improvement in quality of life scores, compared with standard medical management, in patients with inoperable, severe aortic stenosis in a pivotal trial with 358 randomized patients.
The primary end point of the trial, which was first reported in September and then published in October, showed that transcatheter aortic valve implantation (TAVI) significantly improved the rate of all-cause death, compared with medical care, in patients who were judged to be unable to undergo conventional surgical aortic valve replacement (N. Engl. J. Med. 2010;363:1597-607). Additional results in the new report showed a sharp improvement in the quality of life of these patients, with 62% having a 20-point or greater rise in their KCCQ (Kansas City Cardiomyopathy Questionnaire) summary scores, compared with baseline. That increase translates into an improvement of two classes on the New York Heart Association heart failure scale, Dr. David J. Cohen said at the annual scientific sessions of the American Heart Association.
"These findings add further support to the concept that TAVI should be considered an emerging standard of care for patients with severe aortic stenosis who are not candidates for surgical aortic valve replacement," said Dr. Cohen, professor and director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City.
The substantial quality of life improvement seen in most patients who are treated with TAVI adds an important dimension to the study’s outcome because "these patients care far more about how they feel than how long they live," Dr. Cohen said in an interview. "If patients lived longer but didn’t feel any better than they felt at baseline – which was lousy – it wouldn’t be much of an accomplishment." Patients who seek care for severe aortic stenosis usually put a high value on treatment that produces an improved quality of life, he said.
"The impact of TAVI on health status [quality of life] is as important as any other outcome," including survival, commented Dr. John S. Rumsfeld, a cardiologist at the Denver VA Medical Center and the University of Colorado, Denver. It is an efficacy outcome that is very meaningful to patients, he said.
Based on the benefits for both survival and quality of life now reported from the PARTNER (Placement of Aortic Transcatheter Valves) study, staffers from Edwards Lifesciences Corp. announced in November that the company submitted an application to the Food and Drug Administration for marketing approval of the Sapien valve and delivery system used in the study. A report on the results from the second portion of the study, which randomized patients to surgical valve replacement or TAVI, is expected during the first half of 2011, a company spokeswoman said in an interview.
The PARTNER trial enrolled 358 patients with severe aortic stenosis who were judged inoperable by two independent surgeons. Their average age was 83 years, and slightly more than half the patients were women. Assessment using the KCCQ occurred in 81%-91% of the participants at baseline and at 1, 6, and 12 months following the start of the study. At baseline, the average KCCQ summary score among patients in both treatment arms was 35, and 70% of the patients in both arms had a score of 45 or less. A KCCQ score at that level indicates that the patient’s health status is comparable to someone with class IV New York Heart Association heart failure.
At 12 months after entry, patients who underwent TAVI had a KCCQ summary score that averaged 25 points higher than the score of those on medical therapy. The TAVI patients also had higher scores in four elements of the KCCQ assessment: symptoms, physical limitations, social limitations, and quality of life. The TAVI patients also showed substantial improvements at 12 months in three secondary measures of health status: the SF-12 physical scale, the SF-12 mental scale, and the Euro quality of life measure. For example, on the SF-12 physical scale, the average improvement with TAVI was 5 points better than with medical management. On this scale, an increase of at least 2 points reflects a clinically important difference, Dr. Cohen said.
Further analysis tallied the percentage of patients who had an "excellent" outcome, defined as those who survived to a particular follow-up interval and had a 20-point or better rise in their KCCQ summary score, compared with baseline. At all follow-up intervals, substantially more patients who were treated with TAVI met the excellent outcome criteria (see box), with a number-needed-to-treat of 3.5 to achieve one excellent outcome at 12 months.
A series of subgroup analyses showed no interaction of these effects by TAVI with age, sex, surgical risk score, aortic valve gradient pressure, or the severity of comorbid chronic obstructive pulmonary disease.
Inoperable patients make up a small portion – about 5%-10% – of all patients with severe aortic stenosis, Dr. Cohen said. Other patients with severe aortic stenosis don’t undergo surgical valve replacement for other, unknown reasons; thus, about one-fifth to one-quarter of U.S. aortic stenosis patients with disease that is severe enough to warrant valve replacement surgery don’t get it, he added. Although truly inoperable patients are uncommon, they stand to gain markedly when the transcatheter valve system becomes routinely available. They have "the biggest unmet need," Dr. Cohen said.
The PARTNER study was funded by Edwards Lifesciences. Dr. Cohen said that he has received research funding from Edwards Lifesciences.
CHICAGO – Transcatheter aortic valve implantation produced a dramatic improvement in quality of life scores, compared with standard medical management, in patients with inoperable, severe aortic stenosis in a pivotal trial with 358 randomized patients.
The primary end point of the trial, which was first reported in September and then published in October, showed that transcatheter aortic valve implantation (TAVI) significantly improved the rate of all-cause death, compared with medical care, in patients who were judged to be unable to undergo conventional surgical aortic valve replacement (N. Engl. J. Med. 2010;363:1597-607). Additional results in the new report showed a sharp improvement in the quality of life of these patients, with 62% having a 20-point or greater rise in their KCCQ (Kansas City Cardiomyopathy Questionnaire) summary scores, compared with baseline. That increase translates into an improvement of two classes on the New York Heart Association heart failure scale, Dr. David J. Cohen said at the annual scientific sessions of the American Heart Association.
"These findings add further support to the concept that TAVI should be considered an emerging standard of care for patients with severe aortic stenosis who are not candidates for surgical aortic valve replacement," said Dr. Cohen, professor and director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City.
The substantial quality of life improvement seen in most patients who are treated with TAVI adds an important dimension to the study’s outcome because "these patients care far more about how they feel than how long they live," Dr. Cohen said in an interview. "If patients lived longer but didn’t feel any better than they felt at baseline – which was lousy – it wouldn’t be much of an accomplishment." Patients who seek care for severe aortic stenosis usually put a high value on treatment that produces an improved quality of life, he said.
"The impact of TAVI on health status [quality of life] is as important as any other outcome," including survival, commented Dr. John S. Rumsfeld, a cardiologist at the Denver VA Medical Center and the University of Colorado, Denver. It is an efficacy outcome that is very meaningful to patients, he said.
Based on the benefits for both survival and quality of life now reported from the PARTNER (Placement of Aortic Transcatheter Valves) study, staffers from Edwards Lifesciences Corp. announced in November that the company submitted an application to the Food and Drug Administration for marketing approval of the Sapien valve and delivery system used in the study. A report on the results from the second portion of the study, which randomized patients to surgical valve replacement or TAVI, is expected during the first half of 2011, a company spokeswoman said in an interview.
The PARTNER trial enrolled 358 patients with severe aortic stenosis who were judged inoperable by two independent surgeons. Their average age was 83 years, and slightly more than half the patients were women. Assessment using the KCCQ occurred in 81%-91% of the participants at baseline and at 1, 6, and 12 months following the start of the study. At baseline, the average KCCQ summary score among patients in both treatment arms was 35, and 70% of the patients in both arms had a score of 45 or less. A KCCQ score at that level indicates that the patient’s health status is comparable to someone with class IV New York Heart Association heart failure.
At 12 months after entry, patients who underwent TAVI had a KCCQ summary score that averaged 25 points higher than the score of those on medical therapy. The TAVI patients also had higher scores in four elements of the KCCQ assessment: symptoms, physical limitations, social limitations, and quality of life. The TAVI patients also showed substantial improvements at 12 months in three secondary measures of health status: the SF-12 physical scale, the SF-12 mental scale, and the Euro quality of life measure. For example, on the SF-12 physical scale, the average improvement with TAVI was 5 points better than with medical management. On this scale, an increase of at least 2 points reflects a clinically important difference, Dr. Cohen said.
Further analysis tallied the percentage of patients who had an "excellent" outcome, defined as those who survived to a particular follow-up interval and had a 20-point or better rise in their KCCQ summary score, compared with baseline. At all follow-up intervals, substantially more patients who were treated with TAVI met the excellent outcome criteria (see box), with a number-needed-to-treat of 3.5 to achieve one excellent outcome at 12 months.
A series of subgroup analyses showed no interaction of these effects by TAVI with age, sex, surgical risk score, aortic valve gradient pressure, or the severity of comorbid chronic obstructive pulmonary disease.
Inoperable patients make up a small portion – about 5%-10% – of all patients with severe aortic stenosis, Dr. Cohen said. Other patients with severe aortic stenosis don’t undergo surgical valve replacement for other, unknown reasons; thus, about one-fifth to one-quarter of U.S. aortic stenosis patients with disease that is severe enough to warrant valve replacement surgery don’t get it, he added. Although truly inoperable patients are uncommon, they stand to gain markedly when the transcatheter valve system becomes routinely available. They have "the biggest unmet need," Dr. Cohen said.
The PARTNER study was funded by Edwards Lifesciences. Dr. Cohen said that he has received research funding from Edwards Lifesciences.
Stenting Works When Clot Treatments Fail in Large-Vessel Strokes
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
FROM THE INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Stenting Works When Clot Treatments Fail in Large-Vessel Strokes
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
FROM THE INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: In a series of patients with large-vessel strokes who failed conventional treatment, deployment of either a Wingspan or Enterprise stent resulted in TIMI grade 2 or 3 blood flow in 95% of treated patients. At 90 days after treatment, 42% of patients had a modified Rankin score of 2 or less.
Data Source: Single-center series of 19 patients with large-vessel strokes who failed initial treatment with a combination of intra-arterial tissue plasminogen activator and attempted clot removal using either the Merci or Penumbra devices.
Disclosures: Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
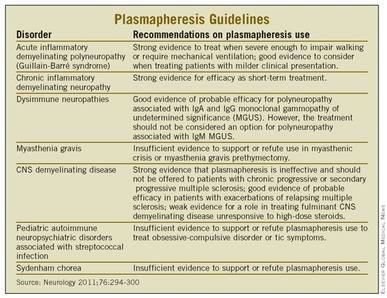
Plasmapheresis Guidelines Revised for Neurologic Diseases
Despite widespread use of plasmapheresis for treating several different neurologic diseases, it has clearly proven efficacy for only acute inflammatory demyelinating polyneuropathy and chronic inflammatory demyelinating polyneuropathy, according to revised guidelines being released on Jan. 18 by the American Academy of Neurology.
An expert subcommittee of the academy also determined that plasmapheresis is probably effective for two other indications: polyneuropathy associated with immunoglobulin A and immunoglobulin G, and for managing exacerbations in relapsing forms of multiple sclerosis. The treatment also might be effective for fulminant demyelinating central nervous system disease, but for all other current neurologic applications of plasmapheresis the committee determined that either the evidence base was insufficient to judge its efficacy or the treatment is probably ineffective or proven ineffective (Neurology 2011;76:294-300).
"Plasmapheresis is one of the key, major treatments used in a variety of neurologic diseases, but it is relatively expensive, labor intensive, and intrusive with some risk to patients. That’s why it needs to be fully evaluated in a critical way," said Dr. Alexander Rae-Grant, a neurologist at the Mellen Center for Multiple Sclerosis of the Cleveland Clinic and a member of the AAN Therapeutics and Technology Assessment subcommittee that wrote the new guidelines.
"We need to look hard at the data and advise people and warn them when there is not good evidence of efficacy. It may help prevent having patients treated with something that has really not been shown effective," he said.
The subcommittee’s recommendations form the AAN’s first revision of its plasmapheresis recommendations since 1996 (Neurology 1996;47:840-3). For certain indications the intervening years produced new data, and in other cases the subcommittee produced a more contemporary assessment of the existing data.
"We look at the information more stringently over time. We’ve upgraded the quality of evidence that we expect from studies. We sometimes reclassified older studies and downgraded them because we now have a higher level of expectation," Dr. Rae-Grant said in an interview.
Despite this, "not many differences exist" between the new revision and the prior guidelines, he noted. In particular, the two most well-documented applications of plasmapheresis in neurology remain the same as 15 years ago: treatment of acute inflammatory demyelinating polyneuropathy (Guillain-Barr? syndrome), and short-term treatment of chronic inflammatory demyelinating neuropathy.
Perhaps the most controversial application of plasmapheresis in neurology is for myasthenia gravis, an indication that the subcommittee judged had insufficient evidence to either support or refute its efficacy when used for myasthenic crisis or myasthenia gravis prethymectomy. Despite the equivocal evidence base, "plasmapheresis is used at many medical centers for this indication," the guidelines noted.
"We tried very hard to stick with the evidence despite the practice pattern. We felt that, because plasmapheresis is so widely used [for these indications] we should comment on that use. Of all the indications, myasthenia gravis is the place where it has become common practice, even though when you look at the data it’s not strong. This is what people do, but it needs further evaluation.
"Experts in myasthenia gravis feel there are anecdotal data [in favor of its efficacy]. We tried to balance the expert concept and what the data show. Because our assessment was not in line with active practice, we tried to show [in the wording of the guidelines] that we were aware of this and thought about it," Dr. Rae-Grant said.
The subcommittee also found insufficient evidence for a role of plasmapheresis in treating Sydenham chorea and acute obsessive-compulsive disorder and tics in patients with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). It determined that good evidence exists to show that plasmapheresis is ineffective for treating polyneuropathy associated with immunoglobulin M, a monoclonal gammopathy of undetermined significance, and hence should not be used in these patients. The subcommittee also found strong evidence that plasmapheresis does not work in patients with chronic progressive or secondary progressive multiple sclerosis and should definitely not be used in these patients.
A final section of the guidelines highlighted seven recommendations for future research: the optimal plasma exchange protocol; the role for plasmapheresis in patients with mild acute inflammatory demyelinating polyneuropathy who have preserved ambulation and in those with this disorder who fail to respond to initial plasmapheresis or relapse after an initial response; the role for long-term plasmapheresis in patients with chronic inflammatory demyelinating neuropathy; adequately powered studies to assess the duration of benefit from plasmapheresis in patients with neuropathies associated with IgA or IgG gammopathy and in neuropathies associated with IgM gammopathy; the best way to use plasmapheresis in patients in myasthenic crisis and for myasthenia gravis prethymectomy; the treatment’s role in patients with fulminant demyelinating central nervous system disease that has not responded to corticosteroid treatment; and the role for plasmapheresis in patients with infectious complications following natalizumab treatment.
 |
Plasmapherisis guidelines. |
These recommendations "give people encouragement and set the groundwork" for future actions on these studies, Dr. Rae-Grant said.
Dr. Rae-Grant said that he has received speaker honoraria from Biogen Idec, Teva, and EMG Serono. He receives publishing royalties for "Handbook of Multiple Sclerosis" and has served on the speaker’s bureau for Biogen Idec.
Despite widespread use of plasmapheresis for treating several different neurologic diseases, it has clearly proven efficacy for only acute inflammatory demyelinating polyneuropathy and chronic inflammatory demyelinating polyneuropathy, according to revised guidelines being released on Jan. 18 by the American Academy of Neurology.
An expert subcommittee of the academy also determined that plasmapheresis is probably effective for two other indications: polyneuropathy associated with immunoglobulin A and immunoglobulin G, and for managing exacerbations in relapsing forms of multiple sclerosis. The treatment also might be effective for fulminant demyelinating central nervous system disease, but for all other current neurologic applications of plasmapheresis the committee determined that either the evidence base was insufficient to judge its efficacy or the treatment is probably ineffective or proven ineffective (Neurology 2011;76:294-300).
"Plasmapheresis is one of the key, major treatments used in a variety of neurologic diseases, but it is relatively expensive, labor intensive, and intrusive with some risk to patients. That’s why it needs to be fully evaluated in a critical way," said Dr. Alexander Rae-Grant, a neurologist at the Mellen Center for Multiple Sclerosis of the Cleveland Clinic and a member of the AAN Therapeutics and Technology Assessment subcommittee that wrote the new guidelines.
"We need to look hard at the data and advise people and warn them when there is not good evidence of efficacy. It may help prevent having patients treated with something that has really not been shown effective," he said.
The subcommittee’s recommendations form the AAN’s first revision of its plasmapheresis recommendations since 1996 (Neurology 1996;47:840-3). For certain indications the intervening years produced new data, and in other cases the subcommittee produced a more contemporary assessment of the existing data.
"We look at the information more stringently over time. We’ve upgraded the quality of evidence that we expect from studies. We sometimes reclassified older studies and downgraded them because we now have a higher level of expectation," Dr. Rae-Grant said in an interview.
Despite this, "not many differences exist" between the new revision and the prior guidelines, he noted. In particular, the two most well-documented applications of plasmapheresis in neurology remain the same as 15 years ago: treatment of acute inflammatory demyelinating polyneuropathy (Guillain-Barr? syndrome), and short-term treatment of chronic inflammatory demyelinating neuropathy.
Perhaps the most controversial application of plasmapheresis in neurology is for myasthenia gravis, an indication that the subcommittee judged had insufficient evidence to either support or refute its efficacy when used for myasthenic crisis or myasthenia gravis prethymectomy. Despite the equivocal evidence base, "plasmapheresis is used at many medical centers for this indication," the guidelines noted.
"We tried very hard to stick with the evidence despite the practice pattern. We felt that, because plasmapheresis is so widely used [for these indications] we should comment on that use. Of all the indications, myasthenia gravis is the place where it has become common practice, even though when you look at the data it’s not strong. This is what people do, but it needs further evaluation.
"Experts in myasthenia gravis feel there are anecdotal data [in favor of its efficacy]. We tried to balance the expert concept and what the data show. Because our assessment was not in line with active practice, we tried to show [in the wording of the guidelines] that we were aware of this and thought about it," Dr. Rae-Grant said.
The subcommittee also found insufficient evidence for a role of plasmapheresis in treating Sydenham chorea and acute obsessive-compulsive disorder and tics in patients with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). It determined that good evidence exists to show that plasmapheresis is ineffective for treating polyneuropathy associated with immunoglobulin M, a monoclonal gammopathy of undetermined significance, and hence should not be used in these patients. The subcommittee also found strong evidence that plasmapheresis does not work in patients with chronic progressive or secondary progressive multiple sclerosis and should definitely not be used in these patients.
A final section of the guidelines highlighted seven recommendations for future research: the optimal plasma exchange protocol; the role for plasmapheresis in patients with mild acute inflammatory demyelinating polyneuropathy who have preserved ambulation and in those with this disorder who fail to respond to initial plasmapheresis or relapse after an initial response; the role for long-term plasmapheresis in patients with chronic inflammatory demyelinating neuropathy; adequately powered studies to assess the duration of benefit from plasmapheresis in patients with neuropathies associated with IgA or IgG gammopathy and in neuropathies associated with IgM gammopathy; the best way to use plasmapheresis in patients in myasthenic crisis and for myasthenia gravis prethymectomy; the treatment’s role in patients with fulminant demyelinating central nervous system disease that has not responded to corticosteroid treatment; and the role for plasmapheresis in patients with infectious complications following natalizumab treatment.
 |
Plasmapherisis guidelines. |
These recommendations "give people encouragement and set the groundwork" for future actions on these studies, Dr. Rae-Grant said.
Dr. Rae-Grant said that he has received speaker honoraria from Biogen Idec, Teva, and EMG Serono. He receives publishing royalties for "Handbook of Multiple Sclerosis" and has served on the speaker’s bureau for Biogen Idec.
Despite widespread use of plasmapheresis for treating several different neurologic diseases, it has clearly proven efficacy for only acute inflammatory demyelinating polyneuropathy and chronic inflammatory demyelinating polyneuropathy, according to revised guidelines being released on Jan. 18 by the American Academy of Neurology.
An expert subcommittee of the academy also determined that plasmapheresis is probably effective for two other indications: polyneuropathy associated with immunoglobulin A and immunoglobulin G, and for managing exacerbations in relapsing forms of multiple sclerosis. The treatment also might be effective for fulminant demyelinating central nervous system disease, but for all other current neurologic applications of plasmapheresis the committee determined that either the evidence base was insufficient to judge its efficacy or the treatment is probably ineffective or proven ineffective (Neurology 2011;76:294-300).
"Plasmapheresis is one of the key, major treatments used in a variety of neurologic diseases, but it is relatively expensive, labor intensive, and intrusive with some risk to patients. That’s why it needs to be fully evaluated in a critical way," said Dr. Alexander Rae-Grant, a neurologist at the Mellen Center for Multiple Sclerosis of the Cleveland Clinic and a member of the AAN Therapeutics and Technology Assessment subcommittee that wrote the new guidelines.
"We need to look hard at the data and advise people and warn them when there is not good evidence of efficacy. It may help prevent having patients treated with something that has really not been shown effective," he said.
The subcommittee’s recommendations form the AAN’s first revision of its plasmapheresis recommendations since 1996 (Neurology 1996;47:840-3). For certain indications the intervening years produced new data, and in other cases the subcommittee produced a more contemporary assessment of the existing data.
"We look at the information more stringently over time. We’ve upgraded the quality of evidence that we expect from studies. We sometimes reclassified older studies and downgraded them because we now have a higher level of expectation," Dr. Rae-Grant said in an interview.
Despite this, "not many differences exist" between the new revision and the prior guidelines, he noted. In particular, the two most well-documented applications of plasmapheresis in neurology remain the same as 15 years ago: treatment of acute inflammatory demyelinating polyneuropathy (Guillain-Barr? syndrome), and short-term treatment of chronic inflammatory demyelinating neuropathy.
Perhaps the most controversial application of plasmapheresis in neurology is for myasthenia gravis, an indication that the subcommittee judged had insufficient evidence to either support or refute its efficacy when used for myasthenic crisis or myasthenia gravis prethymectomy. Despite the equivocal evidence base, "plasmapheresis is used at many medical centers for this indication," the guidelines noted.
"We tried very hard to stick with the evidence despite the practice pattern. We felt that, because plasmapheresis is so widely used [for these indications] we should comment on that use. Of all the indications, myasthenia gravis is the place where it has become common practice, even though when you look at the data it’s not strong. This is what people do, but it needs further evaluation.
"Experts in myasthenia gravis feel there are anecdotal data [in favor of its efficacy]. We tried to balance the expert concept and what the data show. Because our assessment was not in line with active practice, we tried to show [in the wording of the guidelines] that we were aware of this and thought about it," Dr. Rae-Grant said.
The subcommittee also found insufficient evidence for a role of plasmapheresis in treating Sydenham chorea and acute obsessive-compulsive disorder and tics in patients with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). It determined that good evidence exists to show that plasmapheresis is ineffective for treating polyneuropathy associated with immunoglobulin M, a monoclonal gammopathy of undetermined significance, and hence should not be used in these patients. The subcommittee also found strong evidence that plasmapheresis does not work in patients with chronic progressive or secondary progressive multiple sclerosis and should definitely not be used in these patients.
A final section of the guidelines highlighted seven recommendations for future research: the optimal plasma exchange protocol; the role for plasmapheresis in patients with mild acute inflammatory demyelinating polyneuropathy who have preserved ambulation and in those with this disorder who fail to respond to initial plasmapheresis or relapse after an initial response; the role for long-term plasmapheresis in patients with chronic inflammatory demyelinating neuropathy; adequately powered studies to assess the duration of benefit from plasmapheresis in patients with neuropathies associated with IgA or IgG gammopathy and in neuropathies associated with IgM gammopathy; the best way to use plasmapheresis in patients in myasthenic crisis and for myasthenia gravis prethymectomy; the treatment’s role in patients with fulminant demyelinating central nervous system disease that has not responded to corticosteroid treatment; and the role for plasmapheresis in patients with infectious complications following natalizumab treatment.
 |
Plasmapherisis guidelines. |
These recommendations "give people encouragement and set the groundwork" for future actions on these studies, Dr. Rae-Grant said.
Dr. Rae-Grant said that he has received speaker honoraria from Biogen Idec, Teva, and EMG Serono. He receives publishing royalties for "Handbook of Multiple Sclerosis" and has served on the speaker’s bureau for Biogen Idec.
Evidence Mounts for Vitamin E Benefit in Diabetic Subgroup
CHICAGO – Evidence continues to build that vitamin E, an antioxidant supplement that became discredited and discarded for preventing cardiovascular disease events through the accumulated results from several large, negative trials, may actually have a substantial benefit for a select subgroup of patients with diabetes.
The key appears to be targeting vitamin E to patients with diabetes who also have a haptoglobin 2-2 genotype, which means they lack a robust antioxidant effect from this blood protein. Roughly a third of people in Western populations carry this genotype. Everyone else has a 1-1 or 2-1 genotype, both of which produce haptoglobin with adequate antioxidant activity.
The latest results to back up this paradigm came from a post hoc analysis of the 1,027 women with diabetes enrolled in the Women’s Health Study (WHS). In this analysis, women with diabetes and the haptoglobin 2-2 genotype who received an every-other-day supplement of 600 IU of vitamin E had a 15% reduced rate of cardiovascular disease events during an average 10 years of follow-up compared with similar women randomized to placebo, Dr. Shany Blum said at the annual scientific sessions of the American Heart Association. In contrast, post hoc analysis of WHS women with diabetes and the 2-1 genotype who received vitamin E showed a 20%-25% increased rate of cardiovascular disease events during follow-up compared with similar women who received placebo.
The stroke rate in women with the 2-1 haptoglobin genotype totaled 5.7% in those who received vitamin E and 1.2% in the placebo arm, a 4.5-fold increased rate of stroke after adjustment, said Dr. Blum, a researcher at the Technion-Israel Institute of Technology in Haifa, Israel.
These findings follow similar observations about the interaction of vitamin E and the haptoglobin genotype in both women and men with diabetes in a post hoc analysis of data from the Heart Outcomes Prevention Evaluation (HOPE) study (New Engl. J. Med. 2000;342:154-60), and from a prospective test of vitamin E compared with placebo in 1,434 patients with diabetes and the haptoglobin 2-2 genotype in the Israel Cardiovascular Events Reduction With Vitamin E (ICARE) study (Arterioscler. Thromb. Vasc. Biol. 2008;28:341-7).
Both studies used a daily supplement with 400 IU of vitamin E. The Haifa researchers published a meta-analysis of the results from HOPE and ICARE in patients with diabetes analyzed by their haptoglobin genotype last May (Pharmacogenomics 2010;11:675-84).
"We have now addressed, in three independent studies, the ability to target specifically haptoglobin 2-2 individuals with antioxidant treatment, particularly vitamin E," said Dr. Andrew P. Levy, professor of anatomy and cell biology at the Technion-Israel Institute and the lead investigator of these analyses and the ICARE study.
"We’ve now shown in the HOPE study, ICARE, and WHS that patients with the 2-2 genotype [and diabetes] benefited from receiving vitamin E. In HOPE, they had about a 30% reduction in cardiovascular events [compared with patients who received placebo], in ICARE about a 45% reduction in cardiovascular events, and in the WHS about a 15% reduction in cardiovascular events," Dr. Levy said in an interview. The HOPE and WHS studies "had previously both shown no overall benefit from vitamin E" compared with placebo when the analysis included all participants, regardless of their diabetes status and haptoglobin genotype status. "But when we specifically targeted people with 2-2, they benefited from vitamin E treatment."
Dr. Levy stressed that in his opinion this paradigm needs additional testing in a large, prospective trial before physicians start routinely prescribing vitamin E to patients with diabetes and a haptoglobin 2-2 genotype. He conceded, however, that such a study may be difficult to fund, as no drug company stands to reap a financial benefit from vitamin E, an inexpensive generic agent.
Dr. Blum said he had no disclosures. Dr Levy has served as a consultant for Synvista Therapeutics.
The analyses done by Dr. Levy and his associates are fascinating, intriguing, and suggestive. The cardiology community discarded vitamin E as a beneficial supplement based on the results of several large clinical trials. But the Haifa group went back, phenotyped the study participants using stored blood specimens, and found that vitamin E produced a benefit that was hiding in plain sight.
Based on the evidence the group has reported so far, I think it is unlikely that the committee currently writing the Adult Treatment Panel IV for the National Heart, Lung, and Blood Institute will include an official recommendation on targeting a vitamin E supplement to patients with diabetes who carry the haptoglobin 2-2 genotype. But the evidence is now compelling enough for individual physicians to present the case to patients with diabetes and ask if they would like to have their haptoglobin genotype checked and go on vitamin E if they have the 2-2 profile. The 400-IU/day dose of vitamin E that Dr. Levy and his associates tested in their ICARE study is cheap and innocuous, with an adverse effect profile that is very nearly zero but which could produce a significant benefit.
Testing for the haptoglobin profile need only be done once, and can be done as either a genotype test, or as a phenotype test based on the haptoglobin forms in a patient’s blood. Testing is critical because patients who carry a haptoglobin genotype that is not 2-2 appear to be harmed by vitamin E treatment. Vitamin E cannot simply be given to everyone with diabetes.
Unfortunately, vitamin E is so cheap that no drug company will be motivated to fund the large, prospective study needed to confirm the benefits that Dr. Levy’s analyses and study results have suggested. Companies that offer haptoglobin testing stand to gain the most financially from large-scale adoption of this approach, but those companies are generally too small to have the resources to fund a large trial. That leaves it to a public health agency, such as the National Institutes of Health, but the NIH is currently so strapped for funds that it is also unlikely to sponsor this study.
Dr. Eliot A. Brinton is director of the metabolism section in the division of cardiovascular genetics at the University of Utah in Salt Lake City. He said that he has served as a consultant to, or a speaker for, Abbott, Amarin, AstraZeneca, Atherotech, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Kaneka, Kowa, Kronos Longevity Research Institute, Merck, and Takeda. He has received research grants from Abbott, the Aurora Foundation, and GlaxoSmithKline. He has been an expert witness for the law firm Reilly Pozner.
The analyses done by Dr. Levy and his associates are fascinating, intriguing, and suggestive. The cardiology community discarded vitamin E as a beneficial supplement based on the results of several large clinical trials. But the Haifa group went back, phenotyped the study participants using stored blood specimens, and found that vitamin E produced a benefit that was hiding in plain sight.
Based on the evidence the group has reported so far, I think it is unlikely that the committee currently writing the Adult Treatment Panel IV for the National Heart, Lung, and Blood Institute will include an official recommendation on targeting a vitamin E supplement to patients with diabetes who carry the haptoglobin 2-2 genotype. But the evidence is now compelling enough for individual physicians to present the case to patients with diabetes and ask if they would like to have their haptoglobin genotype checked and go on vitamin E if they have the 2-2 profile. The 400-IU/day dose of vitamin E that Dr. Levy and his associates tested in their ICARE study is cheap and innocuous, with an adverse effect profile that is very nearly zero but which could produce a significant benefit.
Testing for the haptoglobin profile need only be done once, and can be done as either a genotype test, or as a phenotype test based on the haptoglobin forms in a patient’s blood. Testing is critical because patients who carry a haptoglobin genotype that is not 2-2 appear to be harmed by vitamin E treatment. Vitamin E cannot simply be given to everyone with diabetes.
Unfortunately, vitamin E is so cheap that no drug company will be motivated to fund the large, prospective study needed to confirm the benefits that Dr. Levy’s analyses and study results have suggested. Companies that offer haptoglobin testing stand to gain the most financially from large-scale adoption of this approach, but those companies are generally too small to have the resources to fund a large trial. That leaves it to a public health agency, such as the National Institutes of Health, but the NIH is currently so strapped for funds that it is also unlikely to sponsor this study.
Dr. Eliot A. Brinton is director of the metabolism section in the division of cardiovascular genetics at the University of Utah in Salt Lake City. He said that he has served as a consultant to, or a speaker for, Abbott, Amarin, AstraZeneca, Atherotech, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Kaneka, Kowa, Kronos Longevity Research Institute, Merck, and Takeda. He has received research grants from Abbott, the Aurora Foundation, and GlaxoSmithKline. He has been an expert witness for the law firm Reilly Pozner.
The analyses done by Dr. Levy and his associates are fascinating, intriguing, and suggestive. The cardiology community discarded vitamin E as a beneficial supplement based on the results of several large clinical trials. But the Haifa group went back, phenotyped the study participants using stored blood specimens, and found that vitamin E produced a benefit that was hiding in plain sight.
Based on the evidence the group has reported so far, I think it is unlikely that the committee currently writing the Adult Treatment Panel IV for the National Heart, Lung, and Blood Institute will include an official recommendation on targeting a vitamin E supplement to patients with diabetes who carry the haptoglobin 2-2 genotype. But the evidence is now compelling enough for individual physicians to present the case to patients with diabetes and ask if they would like to have their haptoglobin genotype checked and go on vitamin E if they have the 2-2 profile. The 400-IU/day dose of vitamin E that Dr. Levy and his associates tested in their ICARE study is cheap and innocuous, with an adverse effect profile that is very nearly zero but which could produce a significant benefit.
Testing for the haptoglobin profile need only be done once, and can be done as either a genotype test, or as a phenotype test based on the haptoglobin forms in a patient’s blood. Testing is critical because patients who carry a haptoglobin genotype that is not 2-2 appear to be harmed by vitamin E treatment. Vitamin E cannot simply be given to everyone with diabetes.
Unfortunately, vitamin E is so cheap that no drug company will be motivated to fund the large, prospective study needed to confirm the benefits that Dr. Levy’s analyses and study results have suggested. Companies that offer haptoglobin testing stand to gain the most financially from large-scale adoption of this approach, but those companies are generally too small to have the resources to fund a large trial. That leaves it to a public health agency, such as the National Institutes of Health, but the NIH is currently so strapped for funds that it is also unlikely to sponsor this study.
Dr. Eliot A. Brinton is director of the metabolism section in the division of cardiovascular genetics at the University of Utah in Salt Lake City. He said that he has served as a consultant to, or a speaker for, Abbott, Amarin, AstraZeneca, Atherotech, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Kaneka, Kowa, Kronos Longevity Research Institute, Merck, and Takeda. He has received research grants from Abbott, the Aurora Foundation, and GlaxoSmithKline. He has been an expert witness for the law firm Reilly Pozner.
CHICAGO – Evidence continues to build that vitamin E, an antioxidant supplement that became discredited and discarded for preventing cardiovascular disease events through the accumulated results from several large, negative trials, may actually have a substantial benefit for a select subgroup of patients with diabetes.
The key appears to be targeting vitamin E to patients with diabetes who also have a haptoglobin 2-2 genotype, which means they lack a robust antioxidant effect from this blood protein. Roughly a third of people in Western populations carry this genotype. Everyone else has a 1-1 or 2-1 genotype, both of which produce haptoglobin with adequate antioxidant activity.
The latest results to back up this paradigm came from a post hoc analysis of the 1,027 women with diabetes enrolled in the Women’s Health Study (WHS). In this analysis, women with diabetes and the haptoglobin 2-2 genotype who received an every-other-day supplement of 600 IU of vitamin E had a 15% reduced rate of cardiovascular disease events during an average 10 years of follow-up compared with similar women randomized to placebo, Dr. Shany Blum said at the annual scientific sessions of the American Heart Association. In contrast, post hoc analysis of WHS women with diabetes and the 2-1 genotype who received vitamin E showed a 20%-25% increased rate of cardiovascular disease events during follow-up compared with similar women who received placebo.
The stroke rate in women with the 2-1 haptoglobin genotype totaled 5.7% in those who received vitamin E and 1.2% in the placebo arm, a 4.5-fold increased rate of stroke after adjustment, said Dr. Blum, a researcher at the Technion-Israel Institute of Technology in Haifa, Israel.
These findings follow similar observations about the interaction of vitamin E and the haptoglobin genotype in both women and men with diabetes in a post hoc analysis of data from the Heart Outcomes Prevention Evaluation (HOPE) study (New Engl. J. Med. 2000;342:154-60), and from a prospective test of vitamin E compared with placebo in 1,434 patients with diabetes and the haptoglobin 2-2 genotype in the Israel Cardiovascular Events Reduction With Vitamin E (ICARE) study (Arterioscler. Thromb. Vasc. Biol. 2008;28:341-7).
Both studies used a daily supplement with 400 IU of vitamin E. The Haifa researchers published a meta-analysis of the results from HOPE and ICARE in patients with diabetes analyzed by their haptoglobin genotype last May (Pharmacogenomics 2010;11:675-84).
"We have now addressed, in three independent studies, the ability to target specifically haptoglobin 2-2 individuals with antioxidant treatment, particularly vitamin E," said Dr. Andrew P. Levy, professor of anatomy and cell biology at the Technion-Israel Institute and the lead investigator of these analyses and the ICARE study.
"We’ve now shown in the HOPE study, ICARE, and WHS that patients with the 2-2 genotype [and diabetes] benefited from receiving vitamin E. In HOPE, they had about a 30% reduction in cardiovascular events [compared with patients who received placebo], in ICARE about a 45% reduction in cardiovascular events, and in the WHS about a 15% reduction in cardiovascular events," Dr. Levy said in an interview. The HOPE and WHS studies "had previously both shown no overall benefit from vitamin E" compared with placebo when the analysis included all participants, regardless of their diabetes status and haptoglobin genotype status. "But when we specifically targeted people with 2-2, they benefited from vitamin E treatment."
Dr. Levy stressed that in his opinion this paradigm needs additional testing in a large, prospective trial before physicians start routinely prescribing vitamin E to patients with diabetes and a haptoglobin 2-2 genotype. He conceded, however, that such a study may be difficult to fund, as no drug company stands to reap a financial benefit from vitamin E, an inexpensive generic agent.
Dr. Blum said he had no disclosures. Dr Levy has served as a consultant for Synvista Therapeutics.
CHICAGO – Evidence continues to build that vitamin E, an antioxidant supplement that became discredited and discarded for preventing cardiovascular disease events through the accumulated results from several large, negative trials, may actually have a substantial benefit for a select subgroup of patients with diabetes.
The key appears to be targeting vitamin E to patients with diabetes who also have a haptoglobin 2-2 genotype, which means they lack a robust antioxidant effect from this blood protein. Roughly a third of people in Western populations carry this genotype. Everyone else has a 1-1 or 2-1 genotype, both of which produce haptoglobin with adequate antioxidant activity.
The latest results to back up this paradigm came from a post hoc analysis of the 1,027 women with diabetes enrolled in the Women’s Health Study (WHS). In this analysis, women with diabetes and the haptoglobin 2-2 genotype who received an every-other-day supplement of 600 IU of vitamin E had a 15% reduced rate of cardiovascular disease events during an average 10 years of follow-up compared with similar women randomized to placebo, Dr. Shany Blum said at the annual scientific sessions of the American Heart Association. In contrast, post hoc analysis of WHS women with diabetes and the 2-1 genotype who received vitamin E showed a 20%-25% increased rate of cardiovascular disease events during follow-up compared with similar women who received placebo.
The stroke rate in women with the 2-1 haptoglobin genotype totaled 5.7% in those who received vitamin E and 1.2% in the placebo arm, a 4.5-fold increased rate of stroke after adjustment, said Dr. Blum, a researcher at the Technion-Israel Institute of Technology in Haifa, Israel.
These findings follow similar observations about the interaction of vitamin E and the haptoglobin genotype in both women and men with diabetes in a post hoc analysis of data from the Heart Outcomes Prevention Evaluation (HOPE) study (New Engl. J. Med. 2000;342:154-60), and from a prospective test of vitamin E compared with placebo in 1,434 patients with diabetes and the haptoglobin 2-2 genotype in the Israel Cardiovascular Events Reduction With Vitamin E (ICARE) study (Arterioscler. Thromb. Vasc. Biol. 2008;28:341-7).
Both studies used a daily supplement with 400 IU of vitamin E. The Haifa researchers published a meta-analysis of the results from HOPE and ICARE in patients with diabetes analyzed by their haptoglobin genotype last May (Pharmacogenomics 2010;11:675-84).
"We have now addressed, in three independent studies, the ability to target specifically haptoglobin 2-2 individuals with antioxidant treatment, particularly vitamin E," said Dr. Andrew P. Levy, professor of anatomy and cell biology at the Technion-Israel Institute and the lead investigator of these analyses and the ICARE study.
"We’ve now shown in the HOPE study, ICARE, and WHS that patients with the 2-2 genotype [and diabetes] benefited from receiving vitamin E. In HOPE, they had about a 30% reduction in cardiovascular events [compared with patients who received placebo], in ICARE about a 45% reduction in cardiovascular events, and in the WHS about a 15% reduction in cardiovascular events," Dr. Levy said in an interview. The HOPE and WHS studies "had previously both shown no overall benefit from vitamin E" compared with placebo when the analysis included all participants, regardless of their diabetes status and haptoglobin genotype status. "But when we specifically targeted people with 2-2, they benefited from vitamin E treatment."
Dr. Levy stressed that in his opinion this paradigm needs additional testing in a large, prospective trial before physicians start routinely prescribing vitamin E to patients with diabetes and a haptoglobin 2-2 genotype. He conceded, however, that such a study may be difficult to fund, as no drug company stands to reap a financial benefit from vitamin E, an inexpensive generic agent.
Dr. Blum said he had no disclosures. Dr Levy has served as a consultant for Synvista Therapeutics.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Among women with diabetes who had the haptoglobin 2-2 genotype, every-other-day supplementation with 600 IU of vitamin E led to a 15% reduction in all cardiovascular events during 10 years of follow-up and a 4.5-fold reduction in stroke compared with similar women who were randomized to placebo.
Data Source: Post hoc analysis of 1,027 women with diabetes who were enrolled in the Women’s Health Study.
Disclosures: Dr. Blum said he had no disclosures. Dr Levy has served as a consultant for Synvista Therapeutics. Dr. Brinton said that he has served as a consultant to, or as a speaker for, Abbott, Amarin, AstraZeneca, Atherotech, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Kaneka, Kowa, Kronos Longevity Research Institute, Merck, and Takeda. He has received research grants from Abbott, the Aurora Foundation, and GlaxoSmithKline, and has been an expert witness for the law firm of Reilly Pozner.
Fixed AV Delay in CRT Devices Works for Most Patients
CHICAGO – The standard, atrioventricular delay built into many cardiac resynchronization devices worked as effectively as did an automated patient-tailored delay or a delay based on a time-consuming echocardiography assessment in a randomized trial that involved nearly 1,000 patients.
"The routine use of AV [atrioventricular] optimization techniques assessed in this trial is not warranted," Dr. Kenneth A. Ellenbogen said at the annual scientific sessions of the American Heart Association. But it may help patients who don’t initially respond to cardiac resynchronization therapy (CRT), he added, although the results he reported did not assess this possibility.
The findings refuted a recommendation made in 2008 by a panel of the American Society of Echocardiography to routinely use an echo assessment to set the AV delay in patients receiving a CRT device (J. Am. Soc. Echocardiogr. 2008;21:191-213).
"The algorithms [for determining an optimal AV delay] made excellent sense hemodynamically, but you need a clinical trial to look at clinical outcomes," said Dr. Ellenbogen, vice-chairman of cardiology and director of clinical cardiac electrophysiology and pacing at Virginia Commonwealth University in Richmond, Va.
"The bottom line is we can save patients an expensive and time-consuming study that really doesn’t benefit the majority of patients. The out-of-the-box settings seem to work in most patients," he added.
A detailed echo study "is about an hour long and uses resources," and was no better than the alternatives, commented Dr. Gordon F. Tomaselli, professor and chief of cardiology at Johns Hopkins University in Baltimore. "The key comparison of the study was do we need to do a detailed echo study on everyone to optimize the hemodynamics, and the answer was no," he said in an interview.
The results also called into question the usefulness of a feature in all CRT devices on the U.S. market that automatically attempts to optimize the AV delay based on what the device detects as the patient’s intrinsic AV interval and baseline QRS width. In the Boston Scientific CRT unit used in the new study, this built-in programming algorithm is called the "SmartDelay" feature. The study results showed no statistically significant benefit from the SmartDelay feature compared with a fixed AV delay of 120 msec for all patients.
"All the companies have their algorithms" for setting the AV delay, said Dr. Tomaselli. He also noted that while the new results showed no statistically significant difference between the fixed delay of 120 msec and the variable delays applied by the device’s built-in programming function, several of the outcomes examined showed trends toward superiority using the built-in program for setting the delay. For example, in the study’s primary outcome – the median change in left ventricular end systolic volume at 6 months after placement of the CRT device – the results showed a median 21-mL reduction in patients whose delay got set by the device’s internal program (which produced an average delay of 48 msec) and a median 15-mL reduction in patients who received a device set to the fixed, 120-msec delay. Patients who underwent an echo-guided procedure to set the delay had a median reduction of 19 mL.
The SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMART-AV) trial randomized and implanted 980 patients at 100 centers in the United States and Europe during May 2008–December 2009. The researchers were able to perform follow-up assessments on 86% of the randomized patients. The patient’s average age was 66, two-thirds were men, and more than 90% had New York Heart Association class III heart failure.
The study’s secondary outcomes analyses also showed no significant differences between the three methods used to set the AV delay for changes in left ventricular end diastolic volume, ejection fraction, 6-minute walk, quality of life, or New York Heart Association heart failure class. A series of post hoc subgroup analyses also generally showed no difference in the performance of the three methods. These included patients with ischemic or nonischemic heart failure etiology, patients with either greater or less than 30% atrial pacing, patients with or without bundle branch block, and those with a QRS duration of less than 150 msec and those with a duration of at least 150 msec.
The only subgroup that showed a differential effect was in women, who had a significantly better response to either the device-selected AV delay or an echo-guided delay compared with the fixed, 120-msec delay. In men, the three approaches had identical effects. The implications of this finding for selecting a CRT delay in women will need further study, Dr. Ellenbogen said. He also stressed the need to compare the efficacy of the three delay-setting approaches in the roughly 25% of patients who don’t initially respond to their CRT device.
Concurrently with Dr. Ellenbogen’s report at the meeting, the results appeared in an article published online (Circulation 2010 Nov. 15 [doi:10.1161/circulationaha.110.992552]).
The study was sponsored by Boston Scientific. Dr. Ellenbogen said that he has served as a consultant or an advisory board member to, lectured on behalf of, and/or received research grants from, Boston Scientific, Biotronik, Medtronic, St. Jude Medical, Sorin Group, Cardionet, Atricare, EBR, Sanofi-Aventis, and Biosense Webster. Dr. Tomaselli said that he had no disclosures.
CHICAGO – The standard, atrioventricular delay built into many cardiac resynchronization devices worked as effectively as did an automated patient-tailored delay or a delay based on a time-consuming echocardiography assessment in a randomized trial that involved nearly 1,000 patients.
"The routine use of AV [atrioventricular] optimization techniques assessed in this trial is not warranted," Dr. Kenneth A. Ellenbogen said at the annual scientific sessions of the American Heart Association. But it may help patients who don’t initially respond to cardiac resynchronization therapy (CRT), he added, although the results he reported did not assess this possibility.
The findings refuted a recommendation made in 2008 by a panel of the American Society of Echocardiography to routinely use an echo assessment to set the AV delay in patients receiving a CRT device (J. Am. Soc. Echocardiogr. 2008;21:191-213).
"The algorithms [for determining an optimal AV delay] made excellent sense hemodynamically, but you need a clinical trial to look at clinical outcomes," said Dr. Ellenbogen, vice-chairman of cardiology and director of clinical cardiac electrophysiology and pacing at Virginia Commonwealth University in Richmond, Va.
"The bottom line is we can save patients an expensive and time-consuming study that really doesn’t benefit the majority of patients. The out-of-the-box settings seem to work in most patients," he added.
A detailed echo study "is about an hour long and uses resources," and was no better than the alternatives, commented Dr. Gordon F. Tomaselli, professor and chief of cardiology at Johns Hopkins University in Baltimore. "The key comparison of the study was do we need to do a detailed echo study on everyone to optimize the hemodynamics, and the answer was no," he said in an interview.
The results also called into question the usefulness of a feature in all CRT devices on the U.S. market that automatically attempts to optimize the AV delay based on what the device detects as the patient’s intrinsic AV interval and baseline QRS width. In the Boston Scientific CRT unit used in the new study, this built-in programming algorithm is called the "SmartDelay" feature. The study results showed no statistically significant benefit from the SmartDelay feature compared with a fixed AV delay of 120 msec for all patients.
"All the companies have their algorithms" for setting the AV delay, said Dr. Tomaselli. He also noted that while the new results showed no statistically significant difference between the fixed delay of 120 msec and the variable delays applied by the device’s built-in programming function, several of the outcomes examined showed trends toward superiority using the built-in program for setting the delay. For example, in the study’s primary outcome – the median change in left ventricular end systolic volume at 6 months after placement of the CRT device – the results showed a median 21-mL reduction in patients whose delay got set by the device’s internal program (which produced an average delay of 48 msec) and a median 15-mL reduction in patients who received a device set to the fixed, 120-msec delay. Patients who underwent an echo-guided procedure to set the delay had a median reduction of 19 mL.
The SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMART-AV) trial randomized and implanted 980 patients at 100 centers in the United States and Europe during May 2008–December 2009. The researchers were able to perform follow-up assessments on 86% of the randomized patients. The patient’s average age was 66, two-thirds were men, and more than 90% had New York Heart Association class III heart failure.
The study’s secondary outcomes analyses also showed no significant differences between the three methods used to set the AV delay for changes in left ventricular end diastolic volume, ejection fraction, 6-minute walk, quality of life, or New York Heart Association heart failure class. A series of post hoc subgroup analyses also generally showed no difference in the performance of the three methods. These included patients with ischemic or nonischemic heart failure etiology, patients with either greater or less than 30% atrial pacing, patients with or without bundle branch block, and those with a QRS duration of less than 150 msec and those with a duration of at least 150 msec.
The only subgroup that showed a differential effect was in women, who had a significantly better response to either the device-selected AV delay or an echo-guided delay compared with the fixed, 120-msec delay. In men, the three approaches had identical effects. The implications of this finding for selecting a CRT delay in women will need further study, Dr. Ellenbogen said. He also stressed the need to compare the efficacy of the three delay-setting approaches in the roughly 25% of patients who don’t initially respond to their CRT device.
Concurrently with Dr. Ellenbogen’s report at the meeting, the results appeared in an article published online (Circulation 2010 Nov. 15 [doi:10.1161/circulationaha.110.992552]).
The study was sponsored by Boston Scientific. Dr. Ellenbogen said that he has served as a consultant or an advisory board member to, lectured on behalf of, and/or received research grants from, Boston Scientific, Biotronik, Medtronic, St. Jude Medical, Sorin Group, Cardionet, Atricare, EBR, Sanofi-Aventis, and Biosense Webster. Dr. Tomaselli said that he had no disclosures.
CHICAGO – The standard, atrioventricular delay built into many cardiac resynchronization devices worked as effectively as did an automated patient-tailored delay or a delay based on a time-consuming echocardiography assessment in a randomized trial that involved nearly 1,000 patients.
"The routine use of AV [atrioventricular] optimization techniques assessed in this trial is not warranted," Dr. Kenneth A. Ellenbogen said at the annual scientific sessions of the American Heart Association. But it may help patients who don’t initially respond to cardiac resynchronization therapy (CRT), he added, although the results he reported did not assess this possibility.
The findings refuted a recommendation made in 2008 by a panel of the American Society of Echocardiography to routinely use an echo assessment to set the AV delay in patients receiving a CRT device (J. Am. Soc. Echocardiogr. 2008;21:191-213).
"The algorithms [for determining an optimal AV delay] made excellent sense hemodynamically, but you need a clinical trial to look at clinical outcomes," said Dr. Ellenbogen, vice-chairman of cardiology and director of clinical cardiac electrophysiology and pacing at Virginia Commonwealth University in Richmond, Va.
"The bottom line is we can save patients an expensive and time-consuming study that really doesn’t benefit the majority of patients. The out-of-the-box settings seem to work in most patients," he added.
A detailed echo study "is about an hour long and uses resources," and was no better than the alternatives, commented Dr. Gordon F. Tomaselli, professor and chief of cardiology at Johns Hopkins University in Baltimore. "The key comparison of the study was do we need to do a detailed echo study on everyone to optimize the hemodynamics, and the answer was no," he said in an interview.
The results also called into question the usefulness of a feature in all CRT devices on the U.S. market that automatically attempts to optimize the AV delay based on what the device detects as the patient’s intrinsic AV interval and baseline QRS width. In the Boston Scientific CRT unit used in the new study, this built-in programming algorithm is called the "SmartDelay" feature. The study results showed no statistically significant benefit from the SmartDelay feature compared with a fixed AV delay of 120 msec for all patients.
"All the companies have their algorithms" for setting the AV delay, said Dr. Tomaselli. He also noted that while the new results showed no statistically significant difference between the fixed delay of 120 msec and the variable delays applied by the device’s built-in programming function, several of the outcomes examined showed trends toward superiority using the built-in program for setting the delay. For example, in the study’s primary outcome – the median change in left ventricular end systolic volume at 6 months after placement of the CRT device – the results showed a median 21-mL reduction in patients whose delay got set by the device’s internal program (which produced an average delay of 48 msec) and a median 15-mL reduction in patients who received a device set to the fixed, 120-msec delay. Patients who underwent an echo-guided procedure to set the delay had a median reduction of 19 mL.
The SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMART-AV) trial randomized and implanted 980 patients at 100 centers in the United States and Europe during May 2008–December 2009. The researchers were able to perform follow-up assessments on 86% of the randomized patients. The patient’s average age was 66, two-thirds were men, and more than 90% had New York Heart Association class III heart failure.
The study’s secondary outcomes analyses also showed no significant differences between the three methods used to set the AV delay for changes in left ventricular end diastolic volume, ejection fraction, 6-minute walk, quality of life, or New York Heart Association heart failure class. A series of post hoc subgroup analyses also generally showed no difference in the performance of the three methods. These included patients with ischemic or nonischemic heart failure etiology, patients with either greater or less than 30% atrial pacing, patients with or without bundle branch block, and those with a QRS duration of less than 150 msec and those with a duration of at least 150 msec.
The only subgroup that showed a differential effect was in women, who had a significantly better response to either the device-selected AV delay or an echo-guided delay compared with the fixed, 120-msec delay. In men, the three approaches had identical effects. The implications of this finding for selecting a CRT delay in women will need further study, Dr. Ellenbogen said. He also stressed the need to compare the efficacy of the three delay-setting approaches in the roughly 25% of patients who don’t initially respond to their CRT device.
Concurrently with Dr. Ellenbogen’s report at the meeting, the results appeared in an article published online (Circulation 2010 Nov. 15 [doi:10.1161/circulationaha.110.992552]).
The study was sponsored by Boston Scientific. Dr. Ellenbogen said that he has served as a consultant or an advisory board member to, lectured on behalf of, and/or received research grants from, Boston Scientific, Biotronik, Medtronic, St. Jude Medical, Sorin Group, Cardionet, Atricare, EBR, Sanofi-Aventis, and Biosense Webster. Dr. Tomaselli said that he had no disclosures.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Stenting Works When Clot Treatments Fail in Large-Vessel Strokes
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
MIAMI BEACH – Deployed stents produced unexpectedly good outcomes in a series of 19 stroke patients with large-vessel occlusions that resisted recanalization by more conventional treatments.
"Stenting is a safe and very effective option," Dr. Italo Linfante said at the annual International Symposium on Endovascular Therapy. In the series of 19 patients he reported, 8 (42%) had a modified Rankin score of 2 (slight disability) or less at 90 days after stent placement after failing recanalization with intra-arterial tissue plasminogen activator (tPA) as well as treatment with either the Merci clot retriever or the Penumbra clot suction device. Without stent treatment as a last resort, expected mortality in the series would have been about 90%, said Dr. Linfante, director of interventional neuroradiology at the Baptist Cardiac & Vascular Institute in Miami Beach. This series included five deaths – a 26.3% mortality.
"These are desperate cases." When clot lysis or removal fails "there is nothing else to do" but try stent deployment or stent placement and retrieval, Dr. Linfante said in an interview. For patients with large-vessel occlusions "We usually try one or two passes with a device," either Merci or Penumbra, but this fails in about 40% of patients, who then immediately become candidates for stenting.
Stenting was also relatively safe, with no device-related complications. The five deaths comprised three patients who died from hemorrhagic transformations and two who died from large ischemic infarctions. Dr. Linfante attributed the hemorrhages to delayed recanalization rather than to any stenting-related problems.
"Some patients had huge strokes. You can open their arteries beautifully, but it’s too late because by the time you have tried everything else and then go to a stent you’re already 2 hours into the procedure. Then when you open the artery it’s either too late or the patient bleeds," he said.
Selected patients with large-vessel strokes are likely good candidates for immediate stenting, an approach that would avoid delaying treatment with failed attempts to remove the clot, he added. But currently, no evidence-based method exists for identifying which stroke patients with large-vessel occlusions are likely to fail conventional clot-removal treatments. In Dr. Linfante’s experience, clot removal typically fails in patients with occlusions that also involve a substantial amount of plaque. As experience with acute stroke stenting grows and more reports appear in the literature it will become easier to skip an attempt at clot removal in such patients and proceed directly to stenting, he said.
Dr. Linfante treated the 19 patients in his series during August 2008-September 2010. They ranged in age from 28-91 years, with an average age of 65 years. Their average NIH stroke scale score was 18, with one patient having a score as high as 28. All had complete obstructions with no blood flow in their affected vessel. Ten patients had obstructions at the M1 level of the middle cerebral artery; in four, the block occurred at the terminus of the internal carotid, three had occlusions of their basilar artery, and two had tandem occlusions in both the middle and internal carotid arteries. Despite the time needed for the initial, failed attempts at clot removal, 14 patients underwent stenting within 8 hours of symptom onset. Thirteen patients received a Wingspan stent (Boston Scientific), the only stent with approval from the Food and Drug Administration for use in stroke occlusions, Dr. Linfante said. In six patients, vessel tortuosity prevented deployment of a Wingspan stent and so he used an Enterprise stent (Codman).
In 13 patients (68%), stenting resulted in TIMI 3-level blood flow through the affected vessel, and in another five patients it produced TIMI 2 flow. In the final patient from the series stenting led to TIMI 1 flow. In addition to the eight patients who had a modified Rankin score of 2 or less 90 days after treatment, another four patients had a modified Rankin score of 3 (moderate disability) at follow-up.
Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
FROM THE INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: In a series of patients with large-vessel strokes who failed conventional treatment, deployment of either a Wingspan or Enterprise stent resulted in TIMI grade 2 or 3 blood flow in 95% of treated patients. At 90 days after treatment, 42% of patients had a modified Rankin score of 2 or less.
Data Source: Single-center series of 19 patients with large-vessel strokes who failed initial treatment with a combination of intra-arterial tissue plasminogen activator and attempted clot removal using either the Merci or Penumbra devices.
Disclosures: Dr. Linfante said that he has served as a speaker for or as a consultant to Codman (the company that markets the Enterprise stent), Micrus Endovascular, and Surpass Medical.
High Cardiac Troponin T Doubles Event Risk
Major Finding: Community-dwelling older adults in the highest quartile for their serum cardiac troponin T level, as measured with a high-sensitivity assay, had a two- to threefold increased risk for new-onset heart failure and for cardiovascular death during a median follow-up of 12 years.
Data Source: The 4,221 unselected U.S. residents age 65 or older (median age of 71) enrolled in the Cardiovascular Health Study.
Disclosures: The study was partially funded by Roche Diagnostics, which markets a high-sensitivity cardiac troponin T assay. Dr. deFilippi said that he has served as a consultant to and has received honoraria and grant support from Roche Diagnostics and from Siemens Healthcare Diagnostics. He has also been a consultant to and received grant support from Critical Diagnostics and BG Medicine.
CHICAGO — Higher serum concentrations of cardiac troponin T independently predicted an increased rate of new-onset heart failure and cardiovascular death in a longitudinal study of more than 4,000 elderly, community-dwelling Americans with a median follow-up of 12 years.
“Measurement of cTnT [cardiac troponin T] may be useful in cardiovascular risk stratification in older adults,” Dr. Christopher R. deFilippi said at the scientific sessions.
Assessing cTnT's role as a risk predictor became possible with the recent availability of high-sensitivity assays. Previous studies using conventional cTnT assays found roughly 4% of the general elderly population had detectable levels; in Dr. deFilippi's new study, 66% of community-dwelling U.S. adults with a median age of 71 had detectable cTnT levels. The high-sensitivity test produces “about a 10-fold increase in the number of people with detectable cTnT; that's what gives us a dynamic range,” said Dr. deFilippi, a cardiologist at the University of Maryland in Baltimore.
Results from two other studies presented at the meeting and a third study published in early December showed similar links between high levels of cTnT and cardiovascular events, cardiac structure, and death (see box).
The consistent findings from all these studies show that cTnT “is a pretty good risk predictor. Cardiac troponin offers a very easy way for a physician to say that a person is at high risk” for new-onset heart failure, cardiovascular death, or other cardiovascular disease events, Dr. deFilippi said in an interview.
“I look at cardiac troponin T as early biochemical evidence of pathology. Finding a high level in a person could be a wake-up call. It gives some of the earliest, direct evidence with a cardiac-specific molecule that pathology is taking place,” independent of traditional risk markers, he said.
“Cardiac troponin T could be the summation of all other risk factors. We use cholesterol level as a motivator, even though it is much less effective for measuring risk,” Dr. deFilippi explained.
Another attractive feature of measuring cTnT is that the evidence collected by Dr. deFilippi and his associates suggest that in some people high levels are reversible, and when levels drop a person's risk drops.
In the analyses so far, the strongest correlation with a lowered serum level of cTnT has been a person's level of activity and exercise, he said.
The high-sensitivity cardiac troponin T test has not yet received marketing approval from the Food and Drug Administration, but is commercially available in Europe.
To examine the prognostic ability of cTnT, Dr. deFilippi and his associates used serum specimens collected from 4,221 community-dwelling Americans aged 65 or older enrolled in the Cardiovascular Health Study.
At baseline, 2,794 (66%) of the participants had a detectable level of cTnT, at least 3 pg/mL, and their median age was 71.
During a median follow-up of almost 12 years, the incidence of heart failure and cardiovascular death tracked along with baseline levels of cTnT. Among the one-third of patients with an undetectable level at baseline the rate of new-onset heart failure during follow-up averaged 1.6% per year. Among people in the highest quintile of cTnT level, greater than 12.9 pg/mL, the incident heart failure rate averaged 6.4% per year. “It's a huge difference,” he said.
In an analysis that adjusted for demographic differences and traditional risk factors including systolic blood pressure, smoking status, serum creatinine, and left ventricular size, people with baseline cTnT levels above the median all had a significantly increased risk for both end points.
The quintile of people with the highest cardiac troponin T level had a 2.5-fold increased risk of new-onset heart failure and a threefold increased risk of cardiovascular death compared with those who had an undetectable level at baseline.
Even when also adjusted for baseline levels of NT-pro brain natriuretic peptide and C-reactive protein, people in the highest quintile for baseline level had about a twofold higher rate of heart failure and cardiovascular death during follow-up, Dr. deFilippi reported.
Records on follow-up cTnT levels, measured 2-3 years after baseline in 86% of the study participants, showed that among those with a detectable cTnT level at baseline, nearly two-thirds stayed at about the same level, 22% increased by more than 50%, and 14% decreased by more than 50%.
The high increasers had their subsequent heart failure and cardiovascular death rates rise by about 50% compared with people with more moderate changes.
In contrast, among those whose levels fell by more than 50% during follow-up subsequent event rates dropped by about 25% compared with those with less change in their cTnT level.
Concurrent with Dr. deFilippi's talk at the meeting the findings also appeared in an article published online in JAMA (2010; 304:doi:10.1001/jama.2010.1708).
The results also identified some people with very high levels at baseline that then fell to an undetectable level at their second cTnT measurement. Few people showed this kind of change, but it occurred often enough for Dr. deFilippi to speculate that certain actions can effectively lower serum cardiac troponin T levels.
The source of the cTnT isn't clear. Dr. deFilippi believes it's caused by a chronic process, although the specifics remain unknown. “It's unlikely an ischemic cause,” he said. “The issue is, once you see [a high level] can you intervene? Right now, that's an open question.”
Over 12 years, the rates of heart failure and cardiac death tracked along with baseline cTnT levels.
Source DR. DEFILIPPI
Other Studies Using High-Sensitivity Test Support cTnT's Risk-Marker Role
CHICAGO – In addition to the report by Dr. deFilippi, three other research groups recently reported finding significant links between elevated serum levels of cardiac troponin T and an increased risk for cardiovascular events:
▸ Researchers measured cardiac troponin T (cTnT) using a high-sensitivity assay in 10,820 Americans aged 53-75 without prevalent cardiovascular disease enrolled in the Atherosclerosis Risk in Communities (ARIC) study. In these people, with an average age of 63 years, 61% had a detectable level of serum cTnT at baseline using the high-sensitivity test. During an average follow-up of 10 years, Researchers found a significant link between detectable levels at baseline and death and hospitalization for heart failure during follow-up, Dr. Justin T. Saunders from Baylor College of Medicine in Houston reported at the scientific sessions.
▸ In a second, independent study, researchers measured serum cTnT with the high-sensitivity assay in women enrolled in the Women's Health Study. The current analysis focused on 512 women with diabetes at the time of their serum sampling and 564 women without diabetes. Among the women with diabetes, detectable levels of cTnT excisted in 56% of those who had cardiovascular disease events during follow-up and in 42% of the women who did not later have an event, a statistically significant difference. The event that seemed most responsible for this difference was cardiovascular disease death. Among the women without diabetes, detectable levels of cTnT appeared to have no significant relationship to subsequent cardiovascular disease events. Detectable cTnT appeared in 34% of the women with a subsequent event and in 30% of those without a later event, Dr. Brendan M. Everett from Brigham and Women's Hospital in Boston reported at the scientific sessions.
▸ In the third study, researchers ran high-sensitivity cTnT measures on 3,546 people aged 30-65 enrolled in the Dallas Heart Study. They found detectable levels in 25% of the participants. The prevalence of detectable levels depended on age and gender. People younger than 40 had a prevalence rate of 14% compared with a prevalence of 58% in people aged 65 or older. Men had a prevalence rate of 37%, compared with a rate of 13% in women. During a median follow-up of 6.4 years, in an analysis that adjusted for a series of baseline variables and risk factors, people in the highest quintile for serum cTnT level had a statistically significant, greater than fourfold increased risk for both all-cause death and cardiovascular death, said Dr. James A. de Lemos, from the University of Texas Southwestern Medical Center, and his associates in a report published in the Dec. 8, 2010, issue of JAMA (2010;304:2503-12).
Dr. Saunders had no disclosures.
Dr. Everett said he had received research grants from Roche Diagnostics.
Dr. de Lemos said that he has received research grants from Roche Diagnostics and Biosite, and consulting fees, lecture honoraria, or both from Tethys Biomedical, Johnson & Johnson, Roche Diagnostics, Biosite/Invemess, Siemens, AstraZeneca, Pfizer, Bristol Myers Squibb/Sanofi Aventis, and Merck.
– Mitchel L. Zoler
Major Finding: Community-dwelling older adults in the highest quartile for their serum cardiac troponin T level, as measured with a high-sensitivity assay, had a two- to threefold increased risk for new-onset heart failure and for cardiovascular death during a median follow-up of 12 years.
Data Source: The 4,221 unselected U.S. residents age 65 or older (median age of 71) enrolled in the Cardiovascular Health Study.
Disclosures: The study was partially funded by Roche Diagnostics, which markets a high-sensitivity cardiac troponin T assay. Dr. deFilippi said that he has served as a consultant to and has received honoraria and grant support from Roche Diagnostics and from Siemens Healthcare Diagnostics. He has also been a consultant to and received grant support from Critical Diagnostics and BG Medicine.
CHICAGO — Higher serum concentrations of cardiac troponin T independently predicted an increased rate of new-onset heart failure and cardiovascular death in a longitudinal study of more than 4,000 elderly, community-dwelling Americans with a median follow-up of 12 years.
“Measurement of cTnT [cardiac troponin T] may be useful in cardiovascular risk stratification in older adults,” Dr. Christopher R. deFilippi said at the scientific sessions.
Assessing cTnT's role as a risk predictor became possible with the recent availability of high-sensitivity assays. Previous studies using conventional cTnT assays found roughly 4% of the general elderly population had detectable levels; in Dr. deFilippi's new study, 66% of community-dwelling U.S. adults with a median age of 71 had detectable cTnT levels. The high-sensitivity test produces “about a 10-fold increase in the number of people with detectable cTnT; that's what gives us a dynamic range,” said Dr. deFilippi, a cardiologist at the University of Maryland in Baltimore.
Results from two other studies presented at the meeting and a third study published in early December showed similar links between high levels of cTnT and cardiovascular events, cardiac structure, and death (see box).
The consistent findings from all these studies show that cTnT “is a pretty good risk predictor. Cardiac troponin offers a very easy way for a physician to say that a person is at high risk” for new-onset heart failure, cardiovascular death, or other cardiovascular disease events, Dr. deFilippi said in an interview.
“I look at cardiac troponin T as early biochemical evidence of pathology. Finding a high level in a person could be a wake-up call. It gives some of the earliest, direct evidence with a cardiac-specific molecule that pathology is taking place,” independent of traditional risk markers, he said.
“Cardiac troponin T could be the summation of all other risk factors. We use cholesterol level as a motivator, even though it is much less effective for measuring risk,” Dr. deFilippi explained.
Another attractive feature of measuring cTnT is that the evidence collected by Dr. deFilippi and his associates suggest that in some people high levels are reversible, and when levels drop a person's risk drops.
In the analyses so far, the strongest correlation with a lowered serum level of cTnT has been a person's level of activity and exercise, he said.
The high-sensitivity cardiac troponin T test has not yet received marketing approval from the Food and Drug Administration, but is commercially available in Europe.
To examine the prognostic ability of cTnT, Dr. deFilippi and his associates used serum specimens collected from 4,221 community-dwelling Americans aged 65 or older enrolled in the Cardiovascular Health Study.
At baseline, 2,794 (66%) of the participants had a detectable level of cTnT, at least 3 pg/mL, and their median age was 71.
During a median follow-up of almost 12 years, the incidence of heart failure and cardiovascular death tracked along with baseline levels of cTnT. Among the one-third of patients with an undetectable level at baseline the rate of new-onset heart failure during follow-up averaged 1.6% per year. Among people in the highest quintile of cTnT level, greater than 12.9 pg/mL, the incident heart failure rate averaged 6.4% per year. “It's a huge difference,” he said.
In an analysis that adjusted for demographic differences and traditional risk factors including systolic blood pressure, smoking status, serum creatinine, and left ventricular size, people with baseline cTnT levels above the median all had a significantly increased risk for both end points.
The quintile of people with the highest cardiac troponin T level had a 2.5-fold increased risk of new-onset heart failure and a threefold increased risk of cardiovascular death compared with those who had an undetectable level at baseline.
Even when also adjusted for baseline levels of NT-pro brain natriuretic peptide and C-reactive protein, people in the highest quintile for baseline level had about a twofold higher rate of heart failure and cardiovascular death during follow-up, Dr. deFilippi reported.
Records on follow-up cTnT levels, measured 2-3 years after baseline in 86% of the study participants, showed that among those with a detectable cTnT level at baseline, nearly two-thirds stayed at about the same level, 22% increased by more than 50%, and 14% decreased by more than 50%.
The high increasers had their subsequent heart failure and cardiovascular death rates rise by about 50% compared with people with more moderate changes.
In contrast, among those whose levels fell by more than 50% during follow-up subsequent event rates dropped by about 25% compared with those with less change in their cTnT level.
Concurrent with Dr. deFilippi's talk at the meeting the findings also appeared in an article published online in JAMA (2010; 304:doi:10.1001/jama.2010.1708).
The results also identified some people with very high levels at baseline that then fell to an undetectable level at their second cTnT measurement. Few people showed this kind of change, but it occurred often enough for Dr. deFilippi to speculate that certain actions can effectively lower serum cardiac troponin T levels.
The source of the cTnT isn't clear. Dr. deFilippi believes it's caused by a chronic process, although the specifics remain unknown. “It's unlikely an ischemic cause,” he said. “The issue is, once you see [a high level] can you intervene? Right now, that's an open question.”
Over 12 years, the rates of heart failure and cardiac death tracked along with baseline cTnT levels.
Source DR. DEFILIPPI
Other Studies Using High-Sensitivity Test Support cTnT's Risk-Marker Role
CHICAGO – In addition to the report by Dr. deFilippi, three other research groups recently reported finding significant links between elevated serum levels of cardiac troponin T and an increased risk for cardiovascular events:
▸ Researchers measured cardiac troponin T (cTnT) using a high-sensitivity assay in 10,820 Americans aged 53-75 without prevalent cardiovascular disease enrolled in the Atherosclerosis Risk in Communities (ARIC) study. In these people, with an average age of 63 years, 61% had a detectable level of serum cTnT at baseline using the high-sensitivity test. During an average follow-up of 10 years, Researchers found a significant link between detectable levels at baseline and death and hospitalization for heart failure during follow-up, Dr. Justin T. Saunders from Baylor College of Medicine in Houston reported at the scientific sessions.
▸ In a second, independent study, researchers measured serum cTnT with the high-sensitivity assay in women enrolled in the Women's Health Study. The current analysis focused on 512 women with diabetes at the time of their serum sampling and 564 women without diabetes. Among the women with diabetes, detectable levels of cTnT excisted in 56% of those who had cardiovascular disease events during follow-up and in 42% of the women who did not later have an event, a statistically significant difference. The event that seemed most responsible for this difference was cardiovascular disease death. Among the women without diabetes, detectable levels of cTnT appeared to have no significant relationship to subsequent cardiovascular disease events. Detectable cTnT appeared in 34% of the women with a subsequent event and in 30% of those without a later event, Dr. Brendan M. Everett from Brigham and Women's Hospital in Boston reported at the scientific sessions.
▸ In the third study, researchers ran high-sensitivity cTnT measures on 3,546 people aged 30-65 enrolled in the Dallas Heart Study. They found detectable levels in 25% of the participants. The prevalence of detectable levels depended on age and gender. People younger than 40 had a prevalence rate of 14% compared with a prevalence of 58% in people aged 65 or older. Men had a prevalence rate of 37%, compared with a rate of 13% in women. During a median follow-up of 6.4 years, in an analysis that adjusted for a series of baseline variables and risk factors, people in the highest quintile for serum cTnT level had a statistically significant, greater than fourfold increased risk for both all-cause death and cardiovascular death, said Dr. James A. de Lemos, from the University of Texas Southwestern Medical Center, and his associates in a report published in the Dec. 8, 2010, issue of JAMA (2010;304:2503-12).
Dr. Saunders had no disclosures.
Dr. Everett said he had received research grants from Roche Diagnostics.
Dr. de Lemos said that he has received research grants from Roche Diagnostics and Biosite, and consulting fees, lecture honoraria, or both from Tethys Biomedical, Johnson & Johnson, Roche Diagnostics, Biosite/Invemess, Siemens, AstraZeneca, Pfizer, Bristol Myers Squibb/Sanofi Aventis, and Merck.
– Mitchel L. Zoler
Major Finding: Community-dwelling older adults in the highest quartile for their serum cardiac troponin T level, as measured with a high-sensitivity assay, had a two- to threefold increased risk for new-onset heart failure and for cardiovascular death during a median follow-up of 12 years.
Data Source: The 4,221 unselected U.S. residents age 65 or older (median age of 71) enrolled in the Cardiovascular Health Study.
Disclosures: The study was partially funded by Roche Diagnostics, which markets a high-sensitivity cardiac troponin T assay. Dr. deFilippi said that he has served as a consultant to and has received honoraria and grant support from Roche Diagnostics and from Siemens Healthcare Diagnostics. He has also been a consultant to and received grant support from Critical Diagnostics and BG Medicine.
CHICAGO — Higher serum concentrations of cardiac troponin T independently predicted an increased rate of new-onset heart failure and cardiovascular death in a longitudinal study of more than 4,000 elderly, community-dwelling Americans with a median follow-up of 12 years.
“Measurement of cTnT [cardiac troponin T] may be useful in cardiovascular risk stratification in older adults,” Dr. Christopher R. deFilippi said at the scientific sessions.
Assessing cTnT's role as a risk predictor became possible with the recent availability of high-sensitivity assays. Previous studies using conventional cTnT assays found roughly 4% of the general elderly population had detectable levels; in Dr. deFilippi's new study, 66% of community-dwelling U.S. adults with a median age of 71 had detectable cTnT levels. The high-sensitivity test produces “about a 10-fold increase in the number of people with detectable cTnT; that's what gives us a dynamic range,” said Dr. deFilippi, a cardiologist at the University of Maryland in Baltimore.
Results from two other studies presented at the meeting and a third study published in early December showed similar links between high levels of cTnT and cardiovascular events, cardiac structure, and death (see box).
The consistent findings from all these studies show that cTnT “is a pretty good risk predictor. Cardiac troponin offers a very easy way for a physician to say that a person is at high risk” for new-onset heart failure, cardiovascular death, or other cardiovascular disease events, Dr. deFilippi said in an interview.
“I look at cardiac troponin T as early biochemical evidence of pathology. Finding a high level in a person could be a wake-up call. It gives some of the earliest, direct evidence with a cardiac-specific molecule that pathology is taking place,” independent of traditional risk markers, he said.
“Cardiac troponin T could be the summation of all other risk factors. We use cholesterol level as a motivator, even though it is much less effective for measuring risk,” Dr. deFilippi explained.
Another attractive feature of measuring cTnT is that the evidence collected by Dr. deFilippi and his associates suggest that in some people high levels are reversible, and when levels drop a person's risk drops.
In the analyses so far, the strongest correlation with a lowered serum level of cTnT has been a person's level of activity and exercise, he said.
The high-sensitivity cardiac troponin T test has not yet received marketing approval from the Food and Drug Administration, but is commercially available in Europe.
To examine the prognostic ability of cTnT, Dr. deFilippi and his associates used serum specimens collected from 4,221 community-dwelling Americans aged 65 or older enrolled in the Cardiovascular Health Study.
At baseline, 2,794 (66%) of the participants had a detectable level of cTnT, at least 3 pg/mL, and their median age was 71.
During a median follow-up of almost 12 years, the incidence of heart failure and cardiovascular death tracked along with baseline levels of cTnT. Among the one-third of patients with an undetectable level at baseline the rate of new-onset heart failure during follow-up averaged 1.6% per year. Among people in the highest quintile of cTnT level, greater than 12.9 pg/mL, the incident heart failure rate averaged 6.4% per year. “It's a huge difference,” he said.
In an analysis that adjusted for demographic differences and traditional risk factors including systolic blood pressure, smoking status, serum creatinine, and left ventricular size, people with baseline cTnT levels above the median all had a significantly increased risk for both end points.
The quintile of people with the highest cardiac troponin T level had a 2.5-fold increased risk of new-onset heart failure and a threefold increased risk of cardiovascular death compared with those who had an undetectable level at baseline.
Even when also adjusted for baseline levels of NT-pro brain natriuretic peptide and C-reactive protein, people in the highest quintile for baseline level had about a twofold higher rate of heart failure and cardiovascular death during follow-up, Dr. deFilippi reported.
Records on follow-up cTnT levels, measured 2-3 years after baseline in 86% of the study participants, showed that among those with a detectable cTnT level at baseline, nearly two-thirds stayed at about the same level, 22% increased by more than 50%, and 14% decreased by more than 50%.
The high increasers had their subsequent heart failure and cardiovascular death rates rise by about 50% compared with people with more moderate changes.
In contrast, among those whose levels fell by more than 50% during follow-up subsequent event rates dropped by about 25% compared with those with less change in their cTnT level.
Concurrent with Dr. deFilippi's talk at the meeting the findings also appeared in an article published online in JAMA (2010; 304:doi:10.1001/jama.2010.1708).
The results also identified some people with very high levels at baseline that then fell to an undetectable level at their second cTnT measurement. Few people showed this kind of change, but it occurred often enough for Dr. deFilippi to speculate that certain actions can effectively lower serum cardiac troponin T levels.
The source of the cTnT isn't clear. Dr. deFilippi believes it's caused by a chronic process, although the specifics remain unknown. “It's unlikely an ischemic cause,” he said. “The issue is, once you see [a high level] can you intervene? Right now, that's an open question.”
Over 12 years, the rates of heart failure and cardiac death tracked along with baseline cTnT levels.
Source DR. DEFILIPPI
Other Studies Using High-Sensitivity Test Support cTnT's Risk-Marker Role
CHICAGO – In addition to the report by Dr. deFilippi, three other research groups recently reported finding significant links between elevated serum levels of cardiac troponin T and an increased risk for cardiovascular events:
▸ Researchers measured cardiac troponin T (cTnT) using a high-sensitivity assay in 10,820 Americans aged 53-75 without prevalent cardiovascular disease enrolled in the Atherosclerosis Risk in Communities (ARIC) study. In these people, with an average age of 63 years, 61% had a detectable level of serum cTnT at baseline using the high-sensitivity test. During an average follow-up of 10 years, Researchers found a significant link between detectable levels at baseline and death and hospitalization for heart failure during follow-up, Dr. Justin T. Saunders from Baylor College of Medicine in Houston reported at the scientific sessions.
▸ In a second, independent study, researchers measured serum cTnT with the high-sensitivity assay in women enrolled in the Women's Health Study. The current analysis focused on 512 women with diabetes at the time of their serum sampling and 564 women without diabetes. Among the women with diabetes, detectable levels of cTnT excisted in 56% of those who had cardiovascular disease events during follow-up and in 42% of the women who did not later have an event, a statistically significant difference. The event that seemed most responsible for this difference was cardiovascular disease death. Among the women without diabetes, detectable levels of cTnT appeared to have no significant relationship to subsequent cardiovascular disease events. Detectable cTnT appeared in 34% of the women with a subsequent event and in 30% of those without a later event, Dr. Brendan M. Everett from Brigham and Women's Hospital in Boston reported at the scientific sessions.
▸ In the third study, researchers ran high-sensitivity cTnT measures on 3,546 people aged 30-65 enrolled in the Dallas Heart Study. They found detectable levels in 25% of the participants. The prevalence of detectable levels depended on age and gender. People younger than 40 had a prevalence rate of 14% compared with a prevalence of 58% in people aged 65 or older. Men had a prevalence rate of 37%, compared with a rate of 13% in women. During a median follow-up of 6.4 years, in an analysis that adjusted for a series of baseline variables and risk factors, people in the highest quintile for serum cTnT level had a statistically significant, greater than fourfold increased risk for both all-cause death and cardiovascular death, said Dr. James A. de Lemos, from the University of Texas Southwestern Medical Center, and his associates in a report published in the Dec. 8, 2010, issue of JAMA (2010;304:2503-12).
Dr. Saunders had no disclosures.
Dr. Everett said he had received research grants from Roche Diagnostics.
Dr. de Lemos said that he has received research grants from Roche Diagnostics and Biosite, and consulting fees, lecture honoraria, or both from Tethys Biomedical, Johnson & Johnson, Roche Diagnostics, Biosite/Invemess, Siemens, AstraZeneca, Pfizer, Bristol Myers Squibb/Sanofi Aventis, and Merck.
– Mitchel L. Zoler
High Cardiac Troponin T Doubled Event Risk
Major Finding: Community-dwelling older adults in the highest quartile for their serum cardiac troponin T level, as measured with a high-sensitivity assay, had a two- to threefold increased risk for new-onset heart failure and for cardiovascular death during a median follow-up of 12 years.
Data Source: The 4,221 unselected U.S. residents age 65 or older (median age of 71) enrolled in the Cardiovascular Health Study.
Disclosures: The study was partially funded by Roche Diagnostics, which markets a high-sensitivity cardiac troponin T assay. Dr. deFilippi said that he has served as a consultant to and has received honoraria and grant support from Roche Diagnostics and from Siemens Healthcare Diagnostics. He has also been a consultant to and received grant support from Critical Diagnostics and BG Medicine.
CHICAGO – Higher serum levels of cardiac troponin T independently predicted an increased rate of new-onset heart failure and cardiovascular death in a longitudinal study of more than 4,000 elderly, community-dwelling Americans.
“Measurement of cTnT [cardiac troponin T] may be useful in cardiovascular risk stratification in older adults,” Dr. Christopher R. deFilippi explained at the meeting.
Assessing cTnT's role as a risk predictor became possible with the recent availability of high-sensitivity assays. Previous studies using conventional cTnT assays found roughly 4% of the general elderly population had detectable levels; in Dr. deFilippi's new study, 66% of community-dwelling U.S. adults with a median age of 71 had detectable cTnT levels.
The high-sensitivity test produces “about a 10-fold increase in the number of people with detectable cTnT; that's what gives us a dynamic range,” said Dr. deFilippi, a cardiologist at the University of Maryland in Baltimore.
Results from two other studies presented at the meeting and a third study published in early December showed similar links between high levels of cTnT and cardiovascular events, cardiac structure, and death.
The consistent findings from all these studies show that cTnT “is a pretty good risk predictor. Cardiac troponin offers a very easy way for a physician to say that a person is at high risk” for new-onset heart failure, cardiovascular death, or other cardiovascular disease events, Dr. deFillipi said in an interview.
“I look at [cTnT] as early biochemical evidence of pathology. Finding a high level in a person could be a wake-up call. It gives some of the earliest, direct evidence with a cardiac-specific molecule that pathology is taking place,” independent of traditional risk markers.
“Cardiac troponin T could be the summation of all other risk factors. We use cholesterol level as a motivator, even though it is much less effective for measuring risk,” Dr. deFillipi noted.
Another attractive feature of measuring cTnT is that the evidence collected by Dr. deFilippi and his associates suggest that in some people high levels are reversible, and when levels drop a person's risk drops. In the analyses so far, the strongest correlation with a lowered serum level of cTnT has been a person's level of activity and exercise, he said.
The high-sensitivity cTnT test has not yet received marketing approval from the Food and Drug Administration, but is commercially available in Europe.
To examine the prognostic capability of cTnT, Dr. deFilippi and his associates used serum specimens collected from 4,221 community-dwelling Americans aged 65 or older enrolled in the Cardiovascular Health Study. At baseline, 2,794 (66%) of the participants had a detectable level of cTnT, at least 3 pg/mL, and their median age was 71.
During a median follow-up of almost 12 years, the incidence of heart failure and cardiovascular death tracked along with baseline levels of cTnT. Among the one-third of patients with an undetectable level at baseline the rate of new-onset heart failure during follow-up averaged 1.6% per year. Among people in the highest quintile of cTnT level, greater than 12.9 pg/mL, the incident heart failure rate averaged 6.4% per year. “It's a huge difference,” he said.
In an analysis that adjusted for demographic differences and traditional risk factors, including systolic blood pressure, smoking status, serum creatinine, and left ventricular size, people with baseline cTnT levels above the median all had a significantly increased risk for both new-onset heart failure and cardiovascular death. The quintile of people with the highest cTnT level had a 2.5-fold increased risk of new-onset heart failure and a threefold increased risk of cardiovascular death compared with those who had an undetectable level at baseline.
Even when the investigators adjusted the anaysis for baseline levels of NT-pro brain natriuretic peptide and C-reactive protein, people in the highest quintile for baseline level had about a twofold higher rate of heart failure and cardiovascular death during follow-up.
Records on follow-up cTnT levels, measured 2-3 years after baseline in 86% of the study participants, showed that among those with a detectable cTnT level at baseline, nearly two-thirds stayed at about the same level, 22% increased by more than 50%, and 14% decreased by more than 50%.
The high increasers had their subsequent heart failure and cardiovascular death rates rise by about 50% compared with people with more moderate changes. In contrast, among those whose levels fell by more than 50% during follow-up subsequent event rates dropped by about 25% compared with those with less change in their cTnT level. Concurrent with Dr. deFilippi's talk at the meeting the findings also appeared in an article published on-line (JAMA 2010; 304:doi:10.1001/jama.2010.1708).
The results of the study also identified a number of people with very high levels at baseline that then fell to an undetectable level at their second cTnT measurement. Few people showed this kind of change, but it occurred often enough for Dr. deFilippi to speculate that certain actions can effectively lower serum cTnT levels.
The source of the cTnT isn't clear. Dr. deFilippi said that he believes it's caused by a chronic process, although the specifics remain unknown. “It's unlikely an ischemic cause,” he said. “The issue is, once you see [a high level,] can you intervene? Right now, that's an open question.
Over 12 years, the rates of heart failure and cardiac death tracked along with baseline cTnT levels.
Source DR. DeFILIPPI
Major Finding: Community-dwelling older adults in the highest quartile for their serum cardiac troponin T level, as measured with a high-sensitivity assay, had a two- to threefold increased risk for new-onset heart failure and for cardiovascular death during a median follow-up of 12 years.
Data Source: The 4,221 unselected U.S. residents age 65 or older (median age of 71) enrolled in the Cardiovascular Health Study.
Disclosures: The study was partially funded by Roche Diagnostics, which markets a high-sensitivity cardiac troponin T assay. Dr. deFilippi said that he has served as a consultant to and has received honoraria and grant support from Roche Diagnostics and from Siemens Healthcare Diagnostics. He has also been a consultant to and received grant support from Critical Diagnostics and BG Medicine.
CHICAGO – Higher serum levels of cardiac troponin T independently predicted an increased rate of new-onset heart failure and cardiovascular death in a longitudinal study of more than 4,000 elderly, community-dwelling Americans.
“Measurement of cTnT [cardiac troponin T] may be useful in cardiovascular risk stratification in older adults,” Dr. Christopher R. deFilippi explained at the meeting.
Assessing cTnT's role as a risk predictor became possible with the recent availability of high-sensitivity assays. Previous studies using conventional cTnT assays found roughly 4% of the general elderly population had detectable levels; in Dr. deFilippi's new study, 66% of community-dwelling U.S. adults with a median age of 71 had detectable cTnT levels.
The high-sensitivity test produces “about a 10-fold increase in the number of people with detectable cTnT; that's what gives us a dynamic range,” said Dr. deFilippi, a cardiologist at the University of Maryland in Baltimore.
Results from two other studies presented at the meeting and a third study published in early December showed similar links between high levels of cTnT and cardiovascular events, cardiac structure, and death.
The consistent findings from all these studies show that cTnT “is a pretty good risk predictor. Cardiac troponin offers a very easy way for a physician to say that a person is at high risk” for new-onset heart failure, cardiovascular death, or other cardiovascular disease events, Dr. deFillipi said in an interview.
“I look at [cTnT] as early biochemical evidence of pathology. Finding a high level in a person could be a wake-up call. It gives some of the earliest, direct evidence with a cardiac-specific molecule that pathology is taking place,” independent of traditional risk markers.
“Cardiac troponin T could be the summation of all other risk factors. We use cholesterol level as a motivator, even though it is much less effective for measuring risk,” Dr. deFillipi noted.
Another attractive feature of measuring cTnT is that the evidence collected by Dr. deFilippi and his associates suggest that in some people high levels are reversible, and when levels drop a person's risk drops. In the analyses so far, the strongest correlation with a lowered serum level of cTnT has been a person's level of activity and exercise, he said.
The high-sensitivity cTnT test has not yet received marketing approval from the Food and Drug Administration, but is commercially available in Europe.
To examine the prognostic capability of cTnT, Dr. deFilippi and his associates used serum specimens collected from 4,221 community-dwelling Americans aged 65 or older enrolled in the Cardiovascular Health Study. At baseline, 2,794 (66%) of the participants had a detectable level of cTnT, at least 3 pg/mL, and their median age was 71.
During a median follow-up of almost 12 years, the incidence of heart failure and cardiovascular death tracked along with baseline levels of cTnT. Among the one-third of patients with an undetectable level at baseline the rate of new-onset heart failure during follow-up averaged 1.6% per year. Among people in the highest quintile of cTnT level, greater than 12.9 pg/mL, the incident heart failure rate averaged 6.4% per year. “It's a huge difference,” he said.
In an analysis that adjusted for demographic differences and traditional risk factors, including systolic blood pressure, smoking status, serum creatinine, and left ventricular size, people with baseline cTnT levels above the median all had a significantly increased risk for both new-onset heart failure and cardiovascular death. The quintile of people with the highest cTnT level had a 2.5-fold increased risk of new-onset heart failure and a threefold increased risk of cardiovascular death compared with those who had an undetectable level at baseline.
Even when the investigators adjusted the anaysis for baseline levels of NT-pro brain natriuretic peptide and C-reactive protein, people in the highest quintile for baseline level had about a twofold higher rate of heart failure and cardiovascular death during follow-up.
Records on follow-up cTnT levels, measured 2-3 years after baseline in 86% of the study participants, showed that among those with a detectable cTnT level at baseline, nearly two-thirds stayed at about the same level, 22% increased by more than 50%, and 14% decreased by more than 50%.
The high increasers had their subsequent heart failure and cardiovascular death rates rise by about 50% compared with people with more moderate changes. In contrast, among those whose levels fell by more than 50% during follow-up subsequent event rates dropped by about 25% compared with those with less change in their cTnT level. Concurrent with Dr. deFilippi's talk at the meeting the findings also appeared in an article published on-line (JAMA 2010; 304:doi:10.1001/jama.2010.1708).
The results of the study also identified a number of people with very high levels at baseline that then fell to an undetectable level at their second cTnT measurement. Few people showed this kind of change, but it occurred often enough for Dr. deFilippi to speculate that certain actions can effectively lower serum cTnT levels.
The source of the cTnT isn't clear. Dr. deFilippi said that he believes it's caused by a chronic process, although the specifics remain unknown. “It's unlikely an ischemic cause,” he said. “The issue is, once you see [a high level,] can you intervene? Right now, that's an open question.
Over 12 years, the rates of heart failure and cardiac death tracked along with baseline cTnT levels.
Source DR. DeFILIPPI
Major Finding: Community-dwelling older adults in the highest quartile for their serum cardiac troponin T level, as measured with a high-sensitivity assay, had a two- to threefold increased risk for new-onset heart failure and for cardiovascular death during a median follow-up of 12 years.
Data Source: The 4,221 unselected U.S. residents age 65 or older (median age of 71) enrolled in the Cardiovascular Health Study.
Disclosures: The study was partially funded by Roche Diagnostics, which markets a high-sensitivity cardiac troponin T assay. Dr. deFilippi said that he has served as a consultant to and has received honoraria and grant support from Roche Diagnostics and from Siemens Healthcare Diagnostics. He has also been a consultant to and received grant support from Critical Diagnostics and BG Medicine.
CHICAGO – Higher serum levels of cardiac troponin T independently predicted an increased rate of new-onset heart failure and cardiovascular death in a longitudinal study of more than 4,000 elderly, community-dwelling Americans.
“Measurement of cTnT [cardiac troponin T] may be useful in cardiovascular risk stratification in older adults,” Dr. Christopher R. deFilippi explained at the meeting.
Assessing cTnT's role as a risk predictor became possible with the recent availability of high-sensitivity assays. Previous studies using conventional cTnT assays found roughly 4% of the general elderly population had detectable levels; in Dr. deFilippi's new study, 66% of community-dwelling U.S. adults with a median age of 71 had detectable cTnT levels.
The high-sensitivity test produces “about a 10-fold increase in the number of people with detectable cTnT; that's what gives us a dynamic range,” said Dr. deFilippi, a cardiologist at the University of Maryland in Baltimore.
Results from two other studies presented at the meeting and a third study published in early December showed similar links between high levels of cTnT and cardiovascular events, cardiac structure, and death.
The consistent findings from all these studies show that cTnT “is a pretty good risk predictor. Cardiac troponin offers a very easy way for a physician to say that a person is at high risk” for new-onset heart failure, cardiovascular death, or other cardiovascular disease events, Dr. deFillipi said in an interview.
“I look at [cTnT] as early biochemical evidence of pathology. Finding a high level in a person could be a wake-up call. It gives some of the earliest, direct evidence with a cardiac-specific molecule that pathology is taking place,” independent of traditional risk markers.
“Cardiac troponin T could be the summation of all other risk factors. We use cholesterol level as a motivator, even though it is much less effective for measuring risk,” Dr. deFillipi noted.
Another attractive feature of measuring cTnT is that the evidence collected by Dr. deFilippi and his associates suggest that in some people high levels are reversible, and when levels drop a person's risk drops. In the analyses so far, the strongest correlation with a lowered serum level of cTnT has been a person's level of activity and exercise, he said.
The high-sensitivity cTnT test has not yet received marketing approval from the Food and Drug Administration, but is commercially available in Europe.
To examine the prognostic capability of cTnT, Dr. deFilippi and his associates used serum specimens collected from 4,221 community-dwelling Americans aged 65 or older enrolled in the Cardiovascular Health Study. At baseline, 2,794 (66%) of the participants had a detectable level of cTnT, at least 3 pg/mL, and their median age was 71.
During a median follow-up of almost 12 years, the incidence of heart failure and cardiovascular death tracked along with baseline levels of cTnT. Among the one-third of patients with an undetectable level at baseline the rate of new-onset heart failure during follow-up averaged 1.6% per year. Among people in the highest quintile of cTnT level, greater than 12.9 pg/mL, the incident heart failure rate averaged 6.4% per year. “It's a huge difference,” he said.
In an analysis that adjusted for demographic differences and traditional risk factors, including systolic blood pressure, smoking status, serum creatinine, and left ventricular size, people with baseline cTnT levels above the median all had a significantly increased risk for both new-onset heart failure and cardiovascular death. The quintile of people with the highest cTnT level had a 2.5-fold increased risk of new-onset heart failure and a threefold increased risk of cardiovascular death compared with those who had an undetectable level at baseline.
Even when the investigators adjusted the anaysis for baseline levels of NT-pro brain natriuretic peptide and C-reactive protein, people in the highest quintile for baseline level had about a twofold higher rate of heart failure and cardiovascular death during follow-up.
Records on follow-up cTnT levels, measured 2-3 years after baseline in 86% of the study participants, showed that among those with a detectable cTnT level at baseline, nearly two-thirds stayed at about the same level, 22% increased by more than 50%, and 14% decreased by more than 50%.
The high increasers had their subsequent heart failure and cardiovascular death rates rise by about 50% compared with people with more moderate changes. In contrast, among those whose levels fell by more than 50% during follow-up subsequent event rates dropped by about 25% compared with those with less change in their cTnT level. Concurrent with Dr. deFilippi's talk at the meeting the findings also appeared in an article published on-line (JAMA 2010; 304:doi:10.1001/jama.2010.1708).
The results of the study also identified a number of people with very high levels at baseline that then fell to an undetectable level at their second cTnT measurement. Few people showed this kind of change, but it occurred often enough for Dr. deFilippi to speculate that certain actions can effectively lower serum cTnT levels.
The source of the cTnT isn't clear. Dr. deFilippi said that he believes it's caused by a chronic process, although the specifics remain unknown. “It's unlikely an ischemic cause,” he said. “The issue is, once you see [a high level,] can you intervene? Right now, that's an open question.
Over 12 years, the rates of heart failure and cardiac death tracked along with baseline cTnT levels.
Source DR. DeFILIPPI
Dabigatran, Rivaroxaban Vie as Warfarin Alternatives : Both drugs eliminate the need for regular dose monitoring and adjustment required with warfarin.
CHICAGO – Rivaroxaban, the first in class, oral factor Xa inhibitor, showed noninferiority to warfarin for preventing stroke and other embolic events in a pivotal trial with more than 14,000 atrial fibrillation patients.
But with the report of these results coming less than 2 weeks after the release of dabigatran, the rival, new anticoagulant that came onto the U.S. market on Nov. 3, the question by many people who heard the results was not just how rivaroxaban compared with warfarin but how to weigh its clinical role versus dabigatran.
Cardiologists had no firm answer, and expect none until rivaroxaban and dabigatran go head to head in a trial. But talk at the meeting about both drugs made it clear that warfarin's time as the go-to anticoagulant for atrial fibrillation patients had passed. Both of the new drugs eliminate the regular dose monitoring and adjustment required for patients on warfarin.
Comparing the Competitors
How rivaroxaban compares with dabigatran “is the inevitable question,” said Dr. Kenneth W. Mahaffey, a cardiologist at Duke University in Durham, N.C., who reported the results from Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF).
He noted the pitfalls in comparing the outcomes of ROCKET AF with results from the pivotal trial for dabigatran, the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial (N. Engl. J. Med. 2009;361:1139-51). ROCKET AF enrolled substantially sicker patients, with an average CHADS score of 3.5 compared with an average 2.1 score in RE-LY, and ROCKET AF was run in a double-blind, double-dummy fashion while in RE-LY patients and physicians knew who received dabigatran or warfarin.
Dr. Mahaffey's coinvestigator in ROCKET AF, Dr. Robert M. Califf, agreed. The researchers who ran ROCKET AF “have been discussing [the comparison of rivaroxaban and dabigatran] incessantly, but there is no scientifically valid way to compare the two, so we'll be left with our feelings.”
Dr. Califf cited his 84-year-old mother, on warfarin and in remission from multiple myeloma, who has been unhappy with her monitoring regimen. “I can assure you that she will go on one of these two new drugs just because of convenience, but she can afford it,” said Dr. Califf, professor of cardiology at Duke. “Cost will ultimately have to be a factor that we need to be sensitive about.”
What Role Will Price Play?
Price will likely be a major factor in deciding the role for both alternatives to warfarin, and the cost calculation is not simple.
No price yet exists for rivaroxaban, an agent not yet approved for sale in any country. But on Nov. 3, Boehringer-Ingelheim, the company that markets dabigatran (Pradaxa) announced that the direct thrombin inhibitor was available for U.S. sale at a wholesale price of $6.75/day for either two 150-mg pills or two 75-mg pills, the two dosages approved for U.S. use by the Food and Drug Administration. A recent, informal survey of several large, U.S. retail pharmacies found the 150-mg dose often selling for just under $8 per day. That compares with a drug cost for generic warfarin of usually less than $1 a day. But the price of warfarin therapy also includes the substantial cost for laboratory monitoring of patients on warfarin and the cost of the complications patients have when they are either over- or under-anticoagulated with warfarin.
An analysis that was published online Nov. 1 presented a cost-effectiveness analysis of dabigatran compared with warfarin (Ann. Intern. Med. 2010, Nov. 1 [epub ahead of print 0003-4819-154-1-201101040-00289v2]). According to the analysis, at a daily cost of $8, treatment of patients with atrial fibrillation with dabigatran had an incremental cost-effectiveness ratio of about $12,500 per quality adjusted life-year compared with warfarin, said Dr. James V. Freeman, a cardiologist at Stanford (Calif.) University and lead author of the cost-effectiveness analysis. Although this figure is still subject to adjustment based on newly updated clinical-event data in RE-LY, an incremental cost-effectiveness ratio of “roughly $10,000-$20,000 is likely in the ballpark” based on current dabigatran pricing, Dr. Freeman said in an interview.
“This is in a range generally considered very cost effective. By comparison, implantable cardioverter defibrillators have been estimated to have an incremental cost-effectiveness ratio of $34,000-$70,000 per quality adjusted life-year, compared with control therapy,” he said.
Even if calculations show that dabigatran is cost effective, a monthly prescription could still deliver a patient an unexpectedly high drug bill. “We've been very careful, in this early phase, that patients don't get whacked with a $200 bill they can't pay,” said Dr. Peter R. Kowey, a cardiologist and professor of medicine at Thomas Jefferson University in Philadelphia. “We call each insurance company to make sure the patient will have some kind of compensation. That's our biggest concern with dabigatran.”
In most other respects, dabigatran is a winner so far, Dr. Kowey said. The RE-LY results showed the 150-mg b.i.d. dosage had superior efficacy compared with warfarin, while in ROCKET AF, rivaroxaban proved noninferior but failed to show significant superiority in an intention-to-treat analysis (rivaroxaban showed significant superiority to warfarin in the on-treatment analysis.
“That's where I get stuck, on the superiority thing,” Dr. Kowey said in an interview. “One drug proved itself better than warfarin in a gigantic trial, the other didn't.” Based on what he called the “pristine” RE-LY results, “if you need to pick one of these anticoagulants for your patient it would have to be dabigatran,” he said.
Switch With Caution
Despite dabigatran's advantages, he warned physicians against precipitously switching patients who are well controlled on warfarin to dabigatran.
“You can't pull the rug out” from patients. “I hope physicians won't make that mistake.” He noted that some patients maintain a “rock stable” anticoagulated state on warfarin, the drug itself is inexpensive, and some patients enjoy the social contact they have by regularly returning to their anticoagulation clinic.
On the other hand, patients should know that dabigatran is an option, with its superior stroke prevention and reduced cerebral hemorrhage rate compared with warfarin, he said.
Other experts weren't as sure about dabigatran's edge over rivaroxaban, citing rivaroxaban's performance in ROCKET AF in patients with a high comorbidity profile based on their average CHADS score of 3.5. Rivaroxaban's performance in very sick patients in ROCKET AF was “very impressive,” commented Dr. Christine M. Albert, a cardiologist and director of the Center for Arrhythmia Prevention at Brigham and Women's Hospital in Boston. “We may put a patient on rivaroxaban rather than dabigatran because RE-LY was not done in such a sick population. We'll probably individualize [use of each drug] based on which patients were studied in each trial,” Dr. Albert said in an interview.
Others cited additional considerations needed when prescribing dabigatran, warfarin, and eventually rivaroxaban. “Dabigatran is 80% renally cleared. That will pose problems for some patients, and there is some gastrointestinal bleeding and some dyspepsia with dabigatran,” said Dr. Elaine M. Hylek, a cardiologist and warfarin specialist at Boston University. I can't say that warfarin will disappear. There will be some compelling reasons why warfarin will remain.”
A limitation for rivaroxaban is that it is hepatically metabolized, which may pose difficulties or at least require dose adjustment for patients with liver disease who are prescribed rivaroxaban, noted Dr. Gordon F. Tomaselli, professor of medicine and chief of cardiology at Johns Hopkins University in Baltimore. But availability of dabigatran, and the promise from the ROCKET HF results that rivaroxaban will soon join it on the market, spells the end of warfarin treatment for the vast majority of atrial fibrillation patients, Dr. Tomaselli predicted.
“Over the course of the next year, a lot of my patients will change from warfarin to one of these two [new] drugs,” he said in an interview. “What I hear from patients now who are on warfarin is, 'When can I start with the new drug so that I can stop the rat poison?'”
Disclosures: ROCKET AF was sponsored by Bayer, the company developing rivaroxaban (Xarelto). RE-LY was sponsored by Boehringer-Ingelheim, the company marketing dabigatran (Pradaxa). Dr. Mahaffey has received consulting fees and research grants from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novartis, and Sanofi Aventis. He has also received research grants from Portola, Regado, and The Medicines Company. Dr. Califf has been a consultant to AstraZeneca, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi Sankyo, GlaxoSmithKline,
'There is no scientifically valid way to compare [dabigatran and rivaroxaban], so we'll be left with our feelings.'
Source DR. CALIFF
CHICAGO – Rivaroxaban, the first in class, oral factor Xa inhibitor, showed noninferiority to warfarin for preventing stroke and other embolic events in a pivotal trial with more than 14,000 atrial fibrillation patients.
But with the report of these results coming less than 2 weeks after the release of dabigatran, the rival, new anticoagulant that came onto the U.S. market on Nov. 3, the question by many people who heard the results was not just how rivaroxaban compared with warfarin but how to weigh its clinical role versus dabigatran.
Cardiologists had no firm answer, and expect none until rivaroxaban and dabigatran go head to head in a trial. But talk at the meeting about both drugs made it clear that warfarin's time as the go-to anticoagulant for atrial fibrillation patients had passed. Both of the new drugs eliminate the regular dose monitoring and adjustment required for patients on warfarin.
Comparing the Competitors
How rivaroxaban compares with dabigatran “is the inevitable question,” said Dr. Kenneth W. Mahaffey, a cardiologist at Duke University in Durham, N.C., who reported the results from Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF).
He noted the pitfalls in comparing the outcomes of ROCKET AF with results from the pivotal trial for dabigatran, the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial (N. Engl. J. Med. 2009;361:1139-51). ROCKET AF enrolled substantially sicker patients, with an average CHADS score of 3.5 compared with an average 2.1 score in RE-LY, and ROCKET AF was run in a double-blind, double-dummy fashion while in RE-LY patients and physicians knew who received dabigatran or warfarin.
Dr. Mahaffey's coinvestigator in ROCKET AF, Dr. Robert M. Califf, agreed. The researchers who ran ROCKET AF “have been discussing [the comparison of rivaroxaban and dabigatran] incessantly, but there is no scientifically valid way to compare the two, so we'll be left with our feelings.”
Dr. Califf cited his 84-year-old mother, on warfarin and in remission from multiple myeloma, who has been unhappy with her monitoring regimen. “I can assure you that she will go on one of these two new drugs just because of convenience, but she can afford it,” said Dr. Califf, professor of cardiology at Duke. “Cost will ultimately have to be a factor that we need to be sensitive about.”
What Role Will Price Play?
Price will likely be a major factor in deciding the role for both alternatives to warfarin, and the cost calculation is not simple.
No price yet exists for rivaroxaban, an agent not yet approved for sale in any country. But on Nov. 3, Boehringer-Ingelheim, the company that markets dabigatran (Pradaxa) announced that the direct thrombin inhibitor was available for U.S. sale at a wholesale price of $6.75/day for either two 150-mg pills or two 75-mg pills, the two dosages approved for U.S. use by the Food and Drug Administration. A recent, informal survey of several large, U.S. retail pharmacies found the 150-mg dose often selling for just under $8 per day. That compares with a drug cost for generic warfarin of usually less than $1 a day. But the price of warfarin therapy also includes the substantial cost for laboratory monitoring of patients on warfarin and the cost of the complications patients have when they are either over- or under-anticoagulated with warfarin.
An analysis that was published online Nov. 1 presented a cost-effectiveness analysis of dabigatran compared with warfarin (Ann. Intern. Med. 2010, Nov. 1 [epub ahead of print 0003-4819-154-1-201101040-00289v2]). According to the analysis, at a daily cost of $8, treatment of patients with atrial fibrillation with dabigatran had an incremental cost-effectiveness ratio of about $12,500 per quality adjusted life-year compared with warfarin, said Dr. James V. Freeman, a cardiologist at Stanford (Calif.) University and lead author of the cost-effectiveness analysis. Although this figure is still subject to adjustment based on newly updated clinical-event data in RE-LY, an incremental cost-effectiveness ratio of “roughly $10,000-$20,000 is likely in the ballpark” based on current dabigatran pricing, Dr. Freeman said in an interview.
“This is in a range generally considered very cost effective. By comparison, implantable cardioverter defibrillators have been estimated to have an incremental cost-effectiveness ratio of $34,000-$70,000 per quality adjusted life-year, compared with control therapy,” he said.
Even if calculations show that dabigatran is cost effective, a monthly prescription could still deliver a patient an unexpectedly high drug bill. “We've been very careful, in this early phase, that patients don't get whacked with a $200 bill they can't pay,” said Dr. Peter R. Kowey, a cardiologist and professor of medicine at Thomas Jefferson University in Philadelphia. “We call each insurance company to make sure the patient will have some kind of compensation. That's our biggest concern with dabigatran.”
In most other respects, dabigatran is a winner so far, Dr. Kowey said. The RE-LY results showed the 150-mg b.i.d. dosage had superior efficacy compared with warfarin, while in ROCKET AF, rivaroxaban proved noninferior but failed to show significant superiority in an intention-to-treat analysis (rivaroxaban showed significant superiority to warfarin in the on-treatment analysis.
“That's where I get stuck, on the superiority thing,” Dr. Kowey said in an interview. “One drug proved itself better than warfarin in a gigantic trial, the other didn't.” Based on what he called the “pristine” RE-LY results, “if you need to pick one of these anticoagulants for your patient it would have to be dabigatran,” he said.
Switch With Caution
Despite dabigatran's advantages, he warned physicians against precipitously switching patients who are well controlled on warfarin to dabigatran.
“You can't pull the rug out” from patients. “I hope physicians won't make that mistake.” He noted that some patients maintain a “rock stable” anticoagulated state on warfarin, the drug itself is inexpensive, and some patients enjoy the social contact they have by regularly returning to their anticoagulation clinic.
On the other hand, patients should know that dabigatran is an option, with its superior stroke prevention and reduced cerebral hemorrhage rate compared with warfarin, he said.
Other experts weren't as sure about dabigatran's edge over rivaroxaban, citing rivaroxaban's performance in ROCKET AF in patients with a high comorbidity profile based on their average CHADS score of 3.5. Rivaroxaban's performance in very sick patients in ROCKET AF was “very impressive,” commented Dr. Christine M. Albert, a cardiologist and director of the Center for Arrhythmia Prevention at Brigham and Women's Hospital in Boston. “We may put a patient on rivaroxaban rather than dabigatran because RE-LY was not done in such a sick population. We'll probably individualize [use of each drug] based on which patients were studied in each trial,” Dr. Albert said in an interview.
Others cited additional considerations needed when prescribing dabigatran, warfarin, and eventually rivaroxaban. “Dabigatran is 80% renally cleared. That will pose problems for some patients, and there is some gastrointestinal bleeding and some dyspepsia with dabigatran,” said Dr. Elaine M. Hylek, a cardiologist and warfarin specialist at Boston University. I can't say that warfarin will disappear. There will be some compelling reasons why warfarin will remain.”
A limitation for rivaroxaban is that it is hepatically metabolized, which may pose difficulties or at least require dose adjustment for patients with liver disease who are prescribed rivaroxaban, noted Dr. Gordon F. Tomaselli, professor of medicine and chief of cardiology at Johns Hopkins University in Baltimore. But availability of dabigatran, and the promise from the ROCKET HF results that rivaroxaban will soon join it on the market, spells the end of warfarin treatment for the vast majority of atrial fibrillation patients, Dr. Tomaselli predicted.
“Over the course of the next year, a lot of my patients will change from warfarin to one of these two [new] drugs,” he said in an interview. “What I hear from patients now who are on warfarin is, 'When can I start with the new drug so that I can stop the rat poison?'”
Disclosures: ROCKET AF was sponsored by Bayer, the company developing rivaroxaban (Xarelto). RE-LY was sponsored by Boehringer-Ingelheim, the company marketing dabigatran (Pradaxa). Dr. Mahaffey has received consulting fees and research grants from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novartis, and Sanofi Aventis. He has also received research grants from Portola, Regado, and The Medicines Company. Dr. Califf has been a consultant to AstraZeneca, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi Sankyo, GlaxoSmithKline,
'There is no scientifically valid way to compare [dabigatran and rivaroxaban], so we'll be left with our feelings.'
Source DR. CALIFF
CHICAGO – Rivaroxaban, the first in class, oral factor Xa inhibitor, showed noninferiority to warfarin for preventing stroke and other embolic events in a pivotal trial with more than 14,000 atrial fibrillation patients.
But with the report of these results coming less than 2 weeks after the release of dabigatran, the rival, new anticoagulant that came onto the U.S. market on Nov. 3, the question by many people who heard the results was not just how rivaroxaban compared with warfarin but how to weigh its clinical role versus dabigatran.
Cardiologists had no firm answer, and expect none until rivaroxaban and dabigatran go head to head in a trial. But talk at the meeting about both drugs made it clear that warfarin's time as the go-to anticoagulant for atrial fibrillation patients had passed. Both of the new drugs eliminate the regular dose monitoring and adjustment required for patients on warfarin.
Comparing the Competitors
How rivaroxaban compares with dabigatran “is the inevitable question,” said Dr. Kenneth W. Mahaffey, a cardiologist at Duke University in Durham, N.C., who reported the results from Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF).
He noted the pitfalls in comparing the outcomes of ROCKET AF with results from the pivotal trial for dabigatran, the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial (N. Engl. J. Med. 2009;361:1139-51). ROCKET AF enrolled substantially sicker patients, with an average CHADS score of 3.5 compared with an average 2.1 score in RE-LY, and ROCKET AF was run in a double-blind, double-dummy fashion while in RE-LY patients and physicians knew who received dabigatran or warfarin.
Dr. Mahaffey's coinvestigator in ROCKET AF, Dr. Robert M. Califf, agreed. The researchers who ran ROCKET AF “have been discussing [the comparison of rivaroxaban and dabigatran] incessantly, but there is no scientifically valid way to compare the two, so we'll be left with our feelings.”
Dr. Califf cited his 84-year-old mother, on warfarin and in remission from multiple myeloma, who has been unhappy with her monitoring regimen. “I can assure you that she will go on one of these two new drugs just because of convenience, but she can afford it,” said Dr. Califf, professor of cardiology at Duke. “Cost will ultimately have to be a factor that we need to be sensitive about.”
What Role Will Price Play?
Price will likely be a major factor in deciding the role for both alternatives to warfarin, and the cost calculation is not simple.
No price yet exists for rivaroxaban, an agent not yet approved for sale in any country. But on Nov. 3, Boehringer-Ingelheim, the company that markets dabigatran (Pradaxa) announced that the direct thrombin inhibitor was available for U.S. sale at a wholesale price of $6.75/day for either two 150-mg pills or two 75-mg pills, the two dosages approved for U.S. use by the Food and Drug Administration. A recent, informal survey of several large, U.S. retail pharmacies found the 150-mg dose often selling for just under $8 per day. That compares with a drug cost for generic warfarin of usually less than $1 a day. But the price of warfarin therapy also includes the substantial cost for laboratory monitoring of patients on warfarin and the cost of the complications patients have when they are either over- or under-anticoagulated with warfarin.
An analysis that was published online Nov. 1 presented a cost-effectiveness analysis of dabigatran compared with warfarin (Ann. Intern. Med. 2010, Nov. 1 [epub ahead of print 0003-4819-154-1-201101040-00289v2]). According to the analysis, at a daily cost of $8, treatment of patients with atrial fibrillation with dabigatran had an incremental cost-effectiveness ratio of about $12,500 per quality adjusted life-year compared with warfarin, said Dr. James V. Freeman, a cardiologist at Stanford (Calif.) University and lead author of the cost-effectiveness analysis. Although this figure is still subject to adjustment based on newly updated clinical-event data in RE-LY, an incremental cost-effectiveness ratio of “roughly $10,000-$20,000 is likely in the ballpark” based on current dabigatran pricing, Dr. Freeman said in an interview.
“This is in a range generally considered very cost effective. By comparison, implantable cardioverter defibrillators have been estimated to have an incremental cost-effectiveness ratio of $34,000-$70,000 per quality adjusted life-year, compared with control therapy,” he said.
Even if calculations show that dabigatran is cost effective, a monthly prescription could still deliver a patient an unexpectedly high drug bill. “We've been very careful, in this early phase, that patients don't get whacked with a $200 bill they can't pay,” said Dr. Peter R. Kowey, a cardiologist and professor of medicine at Thomas Jefferson University in Philadelphia. “We call each insurance company to make sure the patient will have some kind of compensation. That's our biggest concern with dabigatran.”
In most other respects, dabigatran is a winner so far, Dr. Kowey said. The RE-LY results showed the 150-mg b.i.d. dosage had superior efficacy compared with warfarin, while in ROCKET AF, rivaroxaban proved noninferior but failed to show significant superiority in an intention-to-treat analysis (rivaroxaban showed significant superiority to warfarin in the on-treatment analysis.
“That's where I get stuck, on the superiority thing,” Dr. Kowey said in an interview. “One drug proved itself better than warfarin in a gigantic trial, the other didn't.” Based on what he called the “pristine” RE-LY results, “if you need to pick one of these anticoagulants for your patient it would have to be dabigatran,” he said.
Switch With Caution
Despite dabigatran's advantages, he warned physicians against precipitously switching patients who are well controlled on warfarin to dabigatran.
“You can't pull the rug out” from patients. “I hope physicians won't make that mistake.” He noted that some patients maintain a “rock stable” anticoagulated state on warfarin, the drug itself is inexpensive, and some patients enjoy the social contact they have by regularly returning to their anticoagulation clinic.
On the other hand, patients should know that dabigatran is an option, with its superior stroke prevention and reduced cerebral hemorrhage rate compared with warfarin, he said.
Other experts weren't as sure about dabigatran's edge over rivaroxaban, citing rivaroxaban's performance in ROCKET AF in patients with a high comorbidity profile based on their average CHADS score of 3.5. Rivaroxaban's performance in very sick patients in ROCKET AF was “very impressive,” commented Dr. Christine M. Albert, a cardiologist and director of the Center for Arrhythmia Prevention at Brigham and Women's Hospital in Boston. “We may put a patient on rivaroxaban rather than dabigatran because RE-LY was not done in such a sick population. We'll probably individualize [use of each drug] based on which patients were studied in each trial,” Dr. Albert said in an interview.
Others cited additional considerations needed when prescribing dabigatran, warfarin, and eventually rivaroxaban. “Dabigatran is 80% renally cleared. That will pose problems for some patients, and there is some gastrointestinal bleeding and some dyspepsia with dabigatran,” said Dr. Elaine M. Hylek, a cardiologist and warfarin specialist at Boston University. I can't say that warfarin will disappear. There will be some compelling reasons why warfarin will remain.”
A limitation for rivaroxaban is that it is hepatically metabolized, which may pose difficulties or at least require dose adjustment for patients with liver disease who are prescribed rivaroxaban, noted Dr. Gordon F. Tomaselli, professor of medicine and chief of cardiology at Johns Hopkins University in Baltimore. But availability of dabigatran, and the promise from the ROCKET HF results that rivaroxaban will soon join it on the market, spells the end of warfarin treatment for the vast majority of atrial fibrillation patients, Dr. Tomaselli predicted.
“Over the course of the next year, a lot of my patients will change from warfarin to one of these two [new] drugs,” he said in an interview. “What I hear from patients now who are on warfarin is, 'When can I start with the new drug so that I can stop the rat poison?'”
Disclosures: ROCKET AF was sponsored by Bayer, the company developing rivaroxaban (Xarelto). RE-LY was sponsored by Boehringer-Ingelheim, the company marketing dabigatran (Pradaxa). Dr. Mahaffey has received consulting fees and research grants from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novartis, and Sanofi Aventis. He has also received research grants from Portola, Regado, and The Medicines Company. Dr. Califf has been a consultant to AstraZeneca, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi Sankyo, GlaxoSmithKline,
'There is no scientifically valid way to compare [dabigatran and rivaroxaban], so we'll be left with our feelings.'
Source DR. CALIFF

