User login
Sharon Worcester is an award-winning medical journalist for MDedge News. She has been with the company since 1996, first as the Southeast Bureau Chief (1996-2009) when the company was known as International Medical News Group, then as a freelance writer (2010-2015) before returning as a reporter in 2015. She previously worked as a daily newspaper reporter covering health and local government. Sharon currently reports primarily on oncology and hematology. She has a BA from Eckerd College and an MA in Mass Communication/Print Journalism from the University of Florida. Connect with her via LinkedIn and follow her on twitter @SW_MedReporter.
Treatment Does Not Explain Slightly Increased Opportunistic Infections in JIA
WASHINGTON – Opportunistic infections in children with juvenile idiopathic arthritis are rare, but they occur at a significantly higher rate in JIA patients than in unaffected children, according to findings from an analysis of national Medicaid claims data.
However, the association between these infections and the use of immunosuppressive treatments for JIA appears weak.
The findings, based on data from 2000 through 2005, show that 42 infections occurred in 8,503 children with JIA, and 584 infections occurred in 360,362 non-JIA comparators, for incidence rates of 300 and 125 per 100,000 person years, respectively, and an incident rate ratio (IRR) of 2.4, Dr. Timothy Beukelman reported at the annual meeting of the American College of Rheumatology.
After excluding herpes zoster, which was by far the most common infection among those with JIA, the rate of opportunistic infections was fourfold higher in the JIA cohort compared with the non-JIA cohort.
The infections that occurred at a higher rate in the JIA cohort were Coccidioides (3 infections, for an incidence rate of 21 per 100,000 and an IRR of 101.0), Salmonella (5 infections, for an incidence rate of 35 per 100,000 and an IRR of 3.8), and herpes zoster infections (32 infections for an incidence rate of 225 per 100,000 and an IRR of 2.1), said Dr. Beukelman of the University of Alabama at Birmingham.
One infection each with Nocardia, nontuberculosis mycobateria, Toxoplasma, and Pneumocystis occurred, for an incidence rate of 7 per 100,000 for each of these infections. Incidence rate ratios were not calculated.
No infections with Aspergillus, Blastomyces, Histoplasma, Cryptococcus, Legionella, Listeria, John Cunningham virus, or tuberculosis were identified in the JIA cohort.
These findings differ from those in adults with rheumatoid arthritis (RA).
"We know that adults with RA who are treated with certain biologics have an increased incidence of some opportunistic infections, in particular tuberculosis, endemic mycoses, Listeria, and Legionella," Dr. Beukelman said.
Among the children with JIA, 1,392 used tumor necrosis factor (TNF) inhibitors, and 3,491 used methotrexate during follow-up. An analysis of the association between these treatments and herpes zoster infection found only weak associations with respect to current use of methotrexate (IRR, 1.4 vs. no current methotrexate or TNF inhibitor use), TNF inhibitors (IRR, 2.2 vs. current methotrexate use without current TNF inhibitor use), or glucocorticoids (IRR, 1.8 vs. no current glucocorticoid use), Dr. Beukelman said.
With respect to the Coccidioides infections, the rate was unexpectedly high in the JIA cohort, but two of the patients had no current exposure to TNF inhibitors, methotrexate, or glucocorticoids, and one had exposure only to methotrexate at the time of the infection. None had been treated by TNF inhibitors at any time during the study.
The findings are the first from a controlled trial to address and characterize the incidence of opportunistic infections in JIA patients, Dr. Beukelman said, noting that very little is known about the incidence of opportunistic infections in children with JIA.
Prior studies have, however, demonstrated a twofold increase in the rate of hospitalized infections associated with JIA vs. without JIA in the absence of immunosuppressive infection, so it appears that the JIA disease process itself may predispose to serious bacterial infections.
"We wondered if there might be a similar situation with opportunistic infections," he said.
The findings of the current study suggest that is, indeed, the case.
JIA in this study was based on physician diagnosis codes and dispensed medication. The comparator cohort was composed of children diagnosed with attention deficit hyperactivity disorder. The cohorts had 14,370 and 490,939 person-years of follow-up, respectively.
All subjects had a 3-month baseline period prior to study follow-up during which they were assessed for prevalent opportunistic infections and medication exposures. Infections were identified using physician diagnosis or hospital discharge codes, with the exception of mycoses, tuberculosis, and herpes zoster, which also required evidence of treatment with specific antimicrobials.
"Future studies of the association between opportunistic infections and JIA medications must consider there’s likely an increased rate of opportunistic infections associated with JIA itself, and not just with the medications we use to treat it," Dr. Beukelman said.
This study was supported by the Agency for Healthcare Research and Quality. Dr. Beukelman disclosed that he has received a research grant from Pfizer, and consulting fees and/or other remuneration from Novartis Pharmaceutical Corporation, and Genentech and Biogen IDEC Inc.
WASHINGTON – Opportunistic infections in children with juvenile idiopathic arthritis are rare, but they occur at a significantly higher rate in JIA patients than in unaffected children, according to findings from an analysis of national Medicaid claims data.
However, the association between these infections and the use of immunosuppressive treatments for JIA appears weak.
The findings, based on data from 2000 through 2005, show that 42 infections occurred in 8,503 children with JIA, and 584 infections occurred in 360,362 non-JIA comparators, for incidence rates of 300 and 125 per 100,000 person years, respectively, and an incident rate ratio (IRR) of 2.4, Dr. Timothy Beukelman reported at the annual meeting of the American College of Rheumatology.
After excluding herpes zoster, which was by far the most common infection among those with JIA, the rate of opportunistic infections was fourfold higher in the JIA cohort compared with the non-JIA cohort.
The infections that occurred at a higher rate in the JIA cohort were Coccidioides (3 infections, for an incidence rate of 21 per 100,000 and an IRR of 101.0), Salmonella (5 infections, for an incidence rate of 35 per 100,000 and an IRR of 3.8), and herpes zoster infections (32 infections for an incidence rate of 225 per 100,000 and an IRR of 2.1), said Dr. Beukelman of the University of Alabama at Birmingham.
One infection each with Nocardia, nontuberculosis mycobateria, Toxoplasma, and Pneumocystis occurred, for an incidence rate of 7 per 100,000 for each of these infections. Incidence rate ratios were not calculated.
No infections with Aspergillus, Blastomyces, Histoplasma, Cryptococcus, Legionella, Listeria, John Cunningham virus, or tuberculosis were identified in the JIA cohort.
These findings differ from those in adults with rheumatoid arthritis (RA).
"We know that adults with RA who are treated with certain biologics have an increased incidence of some opportunistic infections, in particular tuberculosis, endemic mycoses, Listeria, and Legionella," Dr. Beukelman said.
Among the children with JIA, 1,392 used tumor necrosis factor (TNF) inhibitors, and 3,491 used methotrexate during follow-up. An analysis of the association between these treatments and herpes zoster infection found only weak associations with respect to current use of methotrexate (IRR, 1.4 vs. no current methotrexate or TNF inhibitor use), TNF inhibitors (IRR, 2.2 vs. current methotrexate use without current TNF inhibitor use), or glucocorticoids (IRR, 1.8 vs. no current glucocorticoid use), Dr. Beukelman said.
With respect to the Coccidioides infections, the rate was unexpectedly high in the JIA cohort, but two of the patients had no current exposure to TNF inhibitors, methotrexate, or glucocorticoids, and one had exposure only to methotrexate at the time of the infection. None had been treated by TNF inhibitors at any time during the study.
The findings are the first from a controlled trial to address and characterize the incidence of opportunistic infections in JIA patients, Dr. Beukelman said, noting that very little is known about the incidence of opportunistic infections in children with JIA.
Prior studies have, however, demonstrated a twofold increase in the rate of hospitalized infections associated with JIA vs. without JIA in the absence of immunosuppressive infection, so it appears that the JIA disease process itself may predispose to serious bacterial infections.
"We wondered if there might be a similar situation with opportunistic infections," he said.
The findings of the current study suggest that is, indeed, the case.
JIA in this study was based on physician diagnosis codes and dispensed medication. The comparator cohort was composed of children diagnosed with attention deficit hyperactivity disorder. The cohorts had 14,370 and 490,939 person-years of follow-up, respectively.
All subjects had a 3-month baseline period prior to study follow-up during which they were assessed for prevalent opportunistic infections and medication exposures. Infections were identified using physician diagnosis or hospital discharge codes, with the exception of mycoses, tuberculosis, and herpes zoster, which also required evidence of treatment with specific antimicrobials.
"Future studies of the association between opportunistic infections and JIA medications must consider there’s likely an increased rate of opportunistic infections associated with JIA itself, and not just with the medications we use to treat it," Dr. Beukelman said.
This study was supported by the Agency for Healthcare Research and Quality. Dr. Beukelman disclosed that he has received a research grant from Pfizer, and consulting fees and/or other remuneration from Novartis Pharmaceutical Corporation, and Genentech and Biogen IDEC Inc.
WASHINGTON – Opportunistic infections in children with juvenile idiopathic arthritis are rare, but they occur at a significantly higher rate in JIA patients than in unaffected children, according to findings from an analysis of national Medicaid claims data.
However, the association between these infections and the use of immunosuppressive treatments for JIA appears weak.
The findings, based on data from 2000 through 2005, show that 42 infections occurred in 8,503 children with JIA, and 584 infections occurred in 360,362 non-JIA comparators, for incidence rates of 300 and 125 per 100,000 person years, respectively, and an incident rate ratio (IRR) of 2.4, Dr. Timothy Beukelman reported at the annual meeting of the American College of Rheumatology.
After excluding herpes zoster, which was by far the most common infection among those with JIA, the rate of opportunistic infections was fourfold higher in the JIA cohort compared with the non-JIA cohort.
The infections that occurred at a higher rate in the JIA cohort were Coccidioides (3 infections, for an incidence rate of 21 per 100,000 and an IRR of 101.0), Salmonella (5 infections, for an incidence rate of 35 per 100,000 and an IRR of 3.8), and herpes zoster infections (32 infections for an incidence rate of 225 per 100,000 and an IRR of 2.1), said Dr. Beukelman of the University of Alabama at Birmingham.
One infection each with Nocardia, nontuberculosis mycobateria, Toxoplasma, and Pneumocystis occurred, for an incidence rate of 7 per 100,000 for each of these infections. Incidence rate ratios were not calculated.
No infections with Aspergillus, Blastomyces, Histoplasma, Cryptococcus, Legionella, Listeria, John Cunningham virus, or tuberculosis were identified in the JIA cohort.
These findings differ from those in adults with rheumatoid arthritis (RA).
"We know that adults with RA who are treated with certain biologics have an increased incidence of some opportunistic infections, in particular tuberculosis, endemic mycoses, Listeria, and Legionella," Dr. Beukelman said.
Among the children with JIA, 1,392 used tumor necrosis factor (TNF) inhibitors, and 3,491 used methotrexate during follow-up. An analysis of the association between these treatments and herpes zoster infection found only weak associations with respect to current use of methotrexate (IRR, 1.4 vs. no current methotrexate or TNF inhibitor use), TNF inhibitors (IRR, 2.2 vs. current methotrexate use without current TNF inhibitor use), or glucocorticoids (IRR, 1.8 vs. no current glucocorticoid use), Dr. Beukelman said.
With respect to the Coccidioides infections, the rate was unexpectedly high in the JIA cohort, but two of the patients had no current exposure to TNF inhibitors, methotrexate, or glucocorticoids, and one had exposure only to methotrexate at the time of the infection. None had been treated by TNF inhibitors at any time during the study.
The findings are the first from a controlled trial to address and characterize the incidence of opportunistic infections in JIA patients, Dr. Beukelman said, noting that very little is known about the incidence of opportunistic infections in children with JIA.
Prior studies have, however, demonstrated a twofold increase in the rate of hospitalized infections associated with JIA vs. without JIA in the absence of immunosuppressive infection, so it appears that the JIA disease process itself may predispose to serious bacterial infections.
"We wondered if there might be a similar situation with opportunistic infections," he said.
The findings of the current study suggest that is, indeed, the case.
JIA in this study was based on physician diagnosis codes and dispensed medication. The comparator cohort was composed of children diagnosed with attention deficit hyperactivity disorder. The cohorts had 14,370 and 490,939 person-years of follow-up, respectively.
All subjects had a 3-month baseline period prior to study follow-up during which they were assessed for prevalent opportunistic infections and medication exposures. Infections were identified using physician diagnosis or hospital discharge codes, with the exception of mycoses, tuberculosis, and herpes zoster, which also required evidence of treatment with specific antimicrobials.
"Future studies of the association between opportunistic infections and JIA medications must consider there’s likely an increased rate of opportunistic infections associated with JIA itself, and not just with the medications we use to treat it," Dr. Beukelman said.
This study was supported by the Agency for Healthcare Research and Quality. Dr. Beukelman disclosed that he has received a research grant from Pfizer, and consulting fees and/or other remuneration from Novartis Pharmaceutical Corporation, and Genentech and Biogen IDEC Inc.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: Infections that occurred at a higher rate in the JIA cohort were Coccidioides (3 infections, for an incidence rate of 21 per 100,000 and an IRR of 101.0), Salmonella (5 infections, for an incidence rate of 35 per 100,000 and an IRR of 3.8), and herpes zoster infections (32 infections for an incidence rate of 225 per 100,000 and an IRR of 2.1). The association between these infections and use of immunosuppressive treatments for JIA appears weak
Data Source: These findings come from an analysis of national Medicaid claims data.
Disclosures: This study was supported by the Agency for Healthcare Research and Quality. Dr. Beukelman disclosed that he has received a research grant from Pfizer, and consulting fees and/or other remuneration from Novartis Pharmaceutical Corporation, and Genentech and Biogen IDEC Inc.
Subcutaneous Nodules Linked to Cardiovascular Disease Risk in RA
WASHINGTON – Subcutaneous nodules in patients with rheumatoid arthritis signal a significantly increased risk of cardiovascular disease, based on findings from the CORRONA database.
Although subcutaneous nodules are associated with more severe rheumatoid arthritis (RA), and RA is known to be associated with an increased risk of cardiovascular disease, the findings are the first to demonstrate a link between these "conspicuous, accessible, and assessable clinical markers" and cardiovascular morbidity in RA, Dr. Prashant Kaushik reported at the annual meeting of the American College of Rheumatology.
In 23,327 RA patients included in the CORRONA (Consortium of Rheumatology Researchers of North America) database who were evaluated at 182,201 individual visits between October 2001 and September 2011, the presence of subcutaneous nodules was found to confer a 44% increase in the risk of cardiovascular disease (hazard ratio, 1.44). The association remained significant after the investigators adjusted for age, sex, age of onset of RA, presence of diabetes mellitus, hypertension, smoking, alcohol consumption, and the use of a lipid-lowering agent (adjusted hazard ratio, 1.25), said Dr. Kaushik, who is rheumatology section chief at the Stratton VA Medical Center, Albany, N.Y.
Additionally, an age-sex interaction was found for women, who had a steeper increase in risk with age, compared with men. The hazard ratios per year of age for men and women were 1.01 and 1.03, but the hazard ratio for women vs. men at age 40 was 0.39, and at age 70 was 0.59, he noted.
Patients included in the CORRONA database had an average follow-up of 3 years and 70,455 patient-years. Nearly a third had subcutaneous nodules, and 795 had a cardiovascular event including a myocardial infarction, stroke or transient ischemic attack, heart failure, and/or cardiovascular death.
While the precise mechanism of the biologic association still needs to be ascertained, these findings indicate that subcutaneous nodules, which are the most common extra-articular manifestation of RA, should serve as a red flag for clinicians, Dr. Kaushik said.
"Subcutaneous nodules may be thought of as a clinical indicator of cardiovascular disease in RA," he concluded.
Dr. Kaushik reported having no relevant financial disclosures.
WASHINGTON – Subcutaneous nodules in patients with rheumatoid arthritis signal a significantly increased risk of cardiovascular disease, based on findings from the CORRONA database.
Although subcutaneous nodules are associated with more severe rheumatoid arthritis (RA), and RA is known to be associated with an increased risk of cardiovascular disease, the findings are the first to demonstrate a link between these "conspicuous, accessible, and assessable clinical markers" and cardiovascular morbidity in RA, Dr. Prashant Kaushik reported at the annual meeting of the American College of Rheumatology.
In 23,327 RA patients included in the CORRONA (Consortium of Rheumatology Researchers of North America) database who were evaluated at 182,201 individual visits between October 2001 and September 2011, the presence of subcutaneous nodules was found to confer a 44% increase in the risk of cardiovascular disease (hazard ratio, 1.44). The association remained significant after the investigators adjusted for age, sex, age of onset of RA, presence of diabetes mellitus, hypertension, smoking, alcohol consumption, and the use of a lipid-lowering agent (adjusted hazard ratio, 1.25), said Dr. Kaushik, who is rheumatology section chief at the Stratton VA Medical Center, Albany, N.Y.
Additionally, an age-sex interaction was found for women, who had a steeper increase in risk with age, compared with men. The hazard ratios per year of age for men and women were 1.01 and 1.03, but the hazard ratio for women vs. men at age 40 was 0.39, and at age 70 was 0.59, he noted.
Patients included in the CORRONA database had an average follow-up of 3 years and 70,455 patient-years. Nearly a third had subcutaneous nodules, and 795 had a cardiovascular event including a myocardial infarction, stroke or transient ischemic attack, heart failure, and/or cardiovascular death.
While the precise mechanism of the biologic association still needs to be ascertained, these findings indicate that subcutaneous nodules, which are the most common extra-articular manifestation of RA, should serve as a red flag for clinicians, Dr. Kaushik said.
"Subcutaneous nodules may be thought of as a clinical indicator of cardiovascular disease in RA," he concluded.
Dr. Kaushik reported having no relevant financial disclosures.
WASHINGTON – Subcutaneous nodules in patients with rheumatoid arthritis signal a significantly increased risk of cardiovascular disease, based on findings from the CORRONA database.
Although subcutaneous nodules are associated with more severe rheumatoid arthritis (RA), and RA is known to be associated with an increased risk of cardiovascular disease, the findings are the first to demonstrate a link between these "conspicuous, accessible, and assessable clinical markers" and cardiovascular morbidity in RA, Dr. Prashant Kaushik reported at the annual meeting of the American College of Rheumatology.
In 23,327 RA patients included in the CORRONA (Consortium of Rheumatology Researchers of North America) database who were evaluated at 182,201 individual visits between October 2001 and September 2011, the presence of subcutaneous nodules was found to confer a 44% increase in the risk of cardiovascular disease (hazard ratio, 1.44). The association remained significant after the investigators adjusted for age, sex, age of onset of RA, presence of diabetes mellitus, hypertension, smoking, alcohol consumption, and the use of a lipid-lowering agent (adjusted hazard ratio, 1.25), said Dr. Kaushik, who is rheumatology section chief at the Stratton VA Medical Center, Albany, N.Y.
Additionally, an age-sex interaction was found for women, who had a steeper increase in risk with age, compared with men. The hazard ratios per year of age for men and women were 1.01 and 1.03, but the hazard ratio for women vs. men at age 40 was 0.39, and at age 70 was 0.59, he noted.
Patients included in the CORRONA database had an average follow-up of 3 years and 70,455 patient-years. Nearly a third had subcutaneous nodules, and 795 had a cardiovascular event including a myocardial infarction, stroke or transient ischemic attack, heart failure, and/or cardiovascular death.
While the precise mechanism of the biologic association still needs to be ascertained, these findings indicate that subcutaneous nodules, which are the most common extra-articular manifestation of RA, should serve as a red flag for clinicians, Dr. Kaushik said.
"Subcutaneous nodules may be thought of as a clinical indicator of cardiovascular disease in RA," he concluded.
Dr. Kaushik reported having no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: In 23,327 patients with RA, the presence of subcutaneous nodules was found to confer a 44% increase in the risk of cardiovascular disease (hazard ratio, 1.44). The association remained significant after adjusting for age, sex, age of onset of RA, presence of diabetes mellitus, hypertension, smoking, alcohol consumption, and use of a lipid-lowering agent (adjusted hazard ratio, 1.25).
Data Source: The findings come from the CORRONA database.
Disclosures: Dr. Kaushik said he had no relevant financial disclosures.
Anti-TNFs Have Not Leveled Cardiovascular Risk in RA
WASHINGTON – The advent of effective anti-inflammatory therapies and the increased use of cardioprotective therapies in recent years have failed to reduce the risk of incident cardiovascular disease in patients with rheumatoid arthritis, according to findings from the prospective longitudinal CARRE study.
During the 10-year Dutch study, 58 cardiovascular events occurred in 353 patients with more than 2,361 patient-years of follow-up, for an incidence rate of 25.3/1,000 patient-years. This did not differ significantly from the incidence rate of 22.8/1,000 patient-years between 3 years and 10 years of follow-up, Dr. Alper M. van Sijl reported at the annual meeting of the American College of Rheumatology.
Nonetheless, the findings do suggest that improving disease activity and inflammatory markers over time might mitigate cardiovascular risk, said Dr. van Sijl of the Jan van Breemen Research Institute, Reade, Amsterdam.
Patients with RA have a twofold increase in the risk of mortality compared with the general population, and this is largely attributable to cardiovascular disease, he said, noting that it has been unclear what role cardiovascular risk factors vs. the underlying inflammatory processes of RA play in this.
"Most research only accounts for cardiovascular risk factors at baseline, and does not look at longitudinal data," he said.
To evaluate whether the increased use of anti-inflammatory and cardioprotective medications has reduced the risk, Dr. van Sijl and his colleagues compared changes in cardiovascular risk factors, RA-related factors, and medication use over time based on whether study participants did or did not develop cardiovascular disease during follow-up. The investigators found that general cardiovascular risk increased over time, as did the use of antihypertensives, statins, and tumor necrosis factor inhibitors, while RA-related factors improved significantly over time.
For example, with respect to cardiovascular disease risk, changes in markers such as intima-media thickness and cardiovascular risk score were seen. Intima-media thickness increased by 0.030 mm between baseline and 3-year follow-up, and decreased by 0.006 mm between 3 and 10 years. Ten-year CV risk (SCORE) increased by 0.5 points between baseline and 3-year follow-up, and by 4.4 points between 3 and 10 years. Antihypertensive use increased by 4% and 11% during the same periods (from 24% at baseline), respectively; statin use increased by 3% and 5% (from 9% at baseline) during the same periods; and use of biologic agents increased 9% and 14% (from 2% at baseline).
As for RA-related factors, improvements were seen in erythrocyte sedimentation rate in those who did not develop cardiovascular disease, while the rate increased in those who did develop cardiovascular disease. Similarly, disease activity scores (DAS28) improved in both groups between baseline and 3 years, then increased between the assessment done at 3 and that done at 10 years in those who developed cardiovascular disease, while they continued to decrease in those who did not.
The use of biologics increased significantly more over time in those without cardiovascular disease, compared with those with cardiovascular disease (to about 35% vs. 15%).
Generalized estimating equation analysis demonstrated a positive association between the use of tumor necrosis factor inhibitors and a reduction in incident cardiovascular disease, Dr. van Sijl said.
The findings suggest that more aggressive cardioprotective and anti-inflammatory treatment might mitigate the burden of cardiovascular disease in patients with RA, he said.
CARRE study participants were enrolled beginning in 2000. All fulfilled ACR 1987 criteria for RA, and had a mean disease duration of 7 years. Most were women in their 60s with high levels of disease activity, Dr. van Sijl said.
"In summary, the risk of incident cardiovascular disease persists in RA despite incremental use of cardioprotective medication and/or TNF blocking agents. At baseline, cardiovascular risk factors such as blood pressure and renal function were more associated with incident cardiovascular disease than RA-related factors, and changes in RA-related factors during follow-up were associated with an increased risk of incident cardiovascular disease," he said. Although traditional cardiovascular risk factors remain relevant in the risk of cardiovascular disease in RA patients, improving disease activity and inflammatory markers over time might positively influence this risk, he added.
Dr. van Sijl reported having no disclosures.
WASHINGTON – The advent of effective anti-inflammatory therapies and the increased use of cardioprotective therapies in recent years have failed to reduce the risk of incident cardiovascular disease in patients with rheumatoid arthritis, according to findings from the prospective longitudinal CARRE study.
During the 10-year Dutch study, 58 cardiovascular events occurred in 353 patients with more than 2,361 patient-years of follow-up, for an incidence rate of 25.3/1,000 patient-years. This did not differ significantly from the incidence rate of 22.8/1,000 patient-years between 3 years and 10 years of follow-up, Dr. Alper M. van Sijl reported at the annual meeting of the American College of Rheumatology.
Nonetheless, the findings do suggest that improving disease activity and inflammatory markers over time might mitigate cardiovascular risk, said Dr. van Sijl of the Jan van Breemen Research Institute, Reade, Amsterdam.
Patients with RA have a twofold increase in the risk of mortality compared with the general population, and this is largely attributable to cardiovascular disease, he said, noting that it has been unclear what role cardiovascular risk factors vs. the underlying inflammatory processes of RA play in this.
"Most research only accounts for cardiovascular risk factors at baseline, and does not look at longitudinal data," he said.
To evaluate whether the increased use of anti-inflammatory and cardioprotective medications has reduced the risk, Dr. van Sijl and his colleagues compared changes in cardiovascular risk factors, RA-related factors, and medication use over time based on whether study participants did or did not develop cardiovascular disease during follow-up. The investigators found that general cardiovascular risk increased over time, as did the use of antihypertensives, statins, and tumor necrosis factor inhibitors, while RA-related factors improved significantly over time.
For example, with respect to cardiovascular disease risk, changes in markers such as intima-media thickness and cardiovascular risk score were seen. Intima-media thickness increased by 0.030 mm between baseline and 3-year follow-up, and decreased by 0.006 mm between 3 and 10 years. Ten-year CV risk (SCORE) increased by 0.5 points between baseline and 3-year follow-up, and by 4.4 points between 3 and 10 years. Antihypertensive use increased by 4% and 11% during the same periods (from 24% at baseline), respectively; statin use increased by 3% and 5% (from 9% at baseline) during the same periods; and use of biologic agents increased 9% and 14% (from 2% at baseline).
As for RA-related factors, improvements were seen in erythrocyte sedimentation rate in those who did not develop cardiovascular disease, while the rate increased in those who did develop cardiovascular disease. Similarly, disease activity scores (DAS28) improved in both groups between baseline and 3 years, then increased between the assessment done at 3 and that done at 10 years in those who developed cardiovascular disease, while they continued to decrease in those who did not.
The use of biologics increased significantly more over time in those without cardiovascular disease, compared with those with cardiovascular disease (to about 35% vs. 15%).
Generalized estimating equation analysis demonstrated a positive association between the use of tumor necrosis factor inhibitors and a reduction in incident cardiovascular disease, Dr. van Sijl said.
The findings suggest that more aggressive cardioprotective and anti-inflammatory treatment might mitigate the burden of cardiovascular disease in patients with RA, he said.
CARRE study participants were enrolled beginning in 2000. All fulfilled ACR 1987 criteria for RA, and had a mean disease duration of 7 years. Most were women in their 60s with high levels of disease activity, Dr. van Sijl said.
"In summary, the risk of incident cardiovascular disease persists in RA despite incremental use of cardioprotective medication and/or TNF blocking agents. At baseline, cardiovascular risk factors such as blood pressure and renal function were more associated with incident cardiovascular disease than RA-related factors, and changes in RA-related factors during follow-up were associated with an increased risk of incident cardiovascular disease," he said. Although traditional cardiovascular risk factors remain relevant in the risk of cardiovascular disease in RA patients, improving disease activity and inflammatory markers over time might positively influence this risk, he added.
Dr. van Sijl reported having no disclosures.
WASHINGTON – The advent of effective anti-inflammatory therapies and the increased use of cardioprotective therapies in recent years have failed to reduce the risk of incident cardiovascular disease in patients with rheumatoid arthritis, according to findings from the prospective longitudinal CARRE study.
During the 10-year Dutch study, 58 cardiovascular events occurred in 353 patients with more than 2,361 patient-years of follow-up, for an incidence rate of 25.3/1,000 patient-years. This did not differ significantly from the incidence rate of 22.8/1,000 patient-years between 3 years and 10 years of follow-up, Dr. Alper M. van Sijl reported at the annual meeting of the American College of Rheumatology.
Nonetheless, the findings do suggest that improving disease activity and inflammatory markers over time might mitigate cardiovascular risk, said Dr. van Sijl of the Jan van Breemen Research Institute, Reade, Amsterdam.
Patients with RA have a twofold increase in the risk of mortality compared with the general population, and this is largely attributable to cardiovascular disease, he said, noting that it has been unclear what role cardiovascular risk factors vs. the underlying inflammatory processes of RA play in this.
"Most research only accounts for cardiovascular risk factors at baseline, and does not look at longitudinal data," he said.
To evaluate whether the increased use of anti-inflammatory and cardioprotective medications has reduced the risk, Dr. van Sijl and his colleagues compared changes in cardiovascular risk factors, RA-related factors, and medication use over time based on whether study participants did or did not develop cardiovascular disease during follow-up. The investigators found that general cardiovascular risk increased over time, as did the use of antihypertensives, statins, and tumor necrosis factor inhibitors, while RA-related factors improved significantly over time.
For example, with respect to cardiovascular disease risk, changes in markers such as intima-media thickness and cardiovascular risk score were seen. Intima-media thickness increased by 0.030 mm between baseline and 3-year follow-up, and decreased by 0.006 mm between 3 and 10 years. Ten-year CV risk (SCORE) increased by 0.5 points between baseline and 3-year follow-up, and by 4.4 points between 3 and 10 years. Antihypertensive use increased by 4% and 11% during the same periods (from 24% at baseline), respectively; statin use increased by 3% and 5% (from 9% at baseline) during the same periods; and use of biologic agents increased 9% and 14% (from 2% at baseline).
As for RA-related factors, improvements were seen in erythrocyte sedimentation rate in those who did not develop cardiovascular disease, while the rate increased in those who did develop cardiovascular disease. Similarly, disease activity scores (DAS28) improved in both groups between baseline and 3 years, then increased between the assessment done at 3 and that done at 10 years in those who developed cardiovascular disease, while they continued to decrease in those who did not.
The use of biologics increased significantly more over time in those without cardiovascular disease, compared with those with cardiovascular disease (to about 35% vs. 15%).
Generalized estimating equation analysis demonstrated a positive association between the use of tumor necrosis factor inhibitors and a reduction in incident cardiovascular disease, Dr. van Sijl said.
The findings suggest that more aggressive cardioprotective and anti-inflammatory treatment might mitigate the burden of cardiovascular disease in patients with RA, he said.
CARRE study participants were enrolled beginning in 2000. All fulfilled ACR 1987 criteria for RA, and had a mean disease duration of 7 years. Most were women in their 60s with high levels of disease activity, Dr. van Sijl said.
"In summary, the risk of incident cardiovascular disease persists in RA despite incremental use of cardioprotective medication and/or TNF blocking agents. At baseline, cardiovascular risk factors such as blood pressure and renal function were more associated with incident cardiovascular disease than RA-related factors, and changes in RA-related factors during follow-up were associated with an increased risk of incident cardiovascular disease," he said. Although traditional cardiovascular risk factors remain relevant in the risk of cardiovascular disease in RA patients, improving disease activity and inflammatory markers over time might positively influence this risk, he added.
Dr. van Sijl reported having no disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: During the 10-year Dutch study, 58 cardiovascular events occurred in 353 patients with more than 2,361 patient-years of follow-up, for an incidence rate of 25.3/1,000 patient-years. This did not differ significantly from the incidence rate of 22.8/1,000 patient-years between 3 years and 10 years of follow-up.
Data Source: This finding comes from the prospective longitudinal CARRE study.
Disclosures: Dr. van Sijl reported having no disclosures.
Tocilizumab Helped Kids With sJIA Catch Up on Growth
WASHINGTON – Tocilizumab for the treatment of systemic juvenile idiopathic arthritis was associated with significant catch-up growth at 2-year follow-up in the vast majority of children who participated in the pivotal phase III TENDER study.
Treatment with the interleukin-6 (IL-6) receptor inhibitor, which is marketed as Actemra, also significantly increased insulinlike growth factor-1 (IGF-1) levels and osteocalcin/C-telopeptide of type 1 collagen (OC/CTX-1) ratios, suggesting that the therapy has beneficial effects on growth hormone axis and bone metabolism, reported Dr. Fabrizio De Benedetti, speaking at the annual meeting of the American College of Rheumatology.
The findings are important because systemic juvenile idiopathic arthritis (sJIA) has a significant impact on the growing skeleton and is associated with impaired linear growth and systemic osteoporosis, he explained.
Height measurements at baseline in 112 children aged 2-17 years who were enrolled in the study showed profound growth failure, with most having height velocity well below normal for age and gender. However, during treatment, most patients had above normal height velocities: 85% of girls and 73% of boys experienced catch-up growth, said Dr. De Benedetti of Ospedale Pediatrico Bambino Gesu, Rome.
In 86 children from the study who were below Tanner stage 4 development at baseline, the 0.61 mean improvement in height standard deviation score at 2-year follow-up was statistically significant, and although mean corticosteroid dose was higher in the first year of treatment (0.13 mg/kg per day vs. 0.05 mg/kg per day), mean height velocities were comparable in both years, at 5.8 cm and 6.3 cm, respectively, he noted.
IGF-1 standard deviation score increased from a mean of –1.1 at baseline to 0.0 at 2 years, and OC/CTX-1 ratio increased significantly, suggesting an increase in osteoblast activity relative to osteoclast activity.
Improvements in the Juvenile Arthritis Disease Activity Score–71 (JADAS-71) during the first year correlated with increases in height velocities during that year, as did younger age at baseline.
Tocilizumab, which was approved by the U.S. Food and Drugs Administration in 2010 for the treatment of rheumatoid arthritis, received an expanded approval for the treatment of sJIA in April 2011, based on 12-week results from the randomized, placebo-controlled TENDER study. Treatment was shown in that phase, as well as in an open-label long-term extension phase, to be safe and effective for the management of sJIA. Two-year findings of the study, which included children with active refractory disease, were reported at the 2011 annual meeting of the American College of Rheumatology.
Data collection will continue through 5 years of follow-up, allowing a more comprehensive analysis of growth outcomes, said Dr. De Benedetti, the lead investigator for the TENDER study.
Dr. De Benedetti disclosed that he has received research grants and/or consulting fees from Abbott, BMS, Novartis, NovImmune, Pfizer, Roche Pharmaceuticals, and Sobi. The TENDER study was sponsored by Roche, the maker of tocilizumab.
WASHINGTON – Tocilizumab for the treatment of systemic juvenile idiopathic arthritis was associated with significant catch-up growth at 2-year follow-up in the vast majority of children who participated in the pivotal phase III TENDER study.
Treatment with the interleukin-6 (IL-6) receptor inhibitor, which is marketed as Actemra, also significantly increased insulinlike growth factor-1 (IGF-1) levels and osteocalcin/C-telopeptide of type 1 collagen (OC/CTX-1) ratios, suggesting that the therapy has beneficial effects on growth hormone axis and bone metabolism, reported Dr. Fabrizio De Benedetti, speaking at the annual meeting of the American College of Rheumatology.
The findings are important because systemic juvenile idiopathic arthritis (sJIA) has a significant impact on the growing skeleton and is associated with impaired linear growth and systemic osteoporosis, he explained.
Height measurements at baseline in 112 children aged 2-17 years who were enrolled in the study showed profound growth failure, with most having height velocity well below normal for age and gender. However, during treatment, most patients had above normal height velocities: 85% of girls and 73% of boys experienced catch-up growth, said Dr. De Benedetti of Ospedale Pediatrico Bambino Gesu, Rome.
In 86 children from the study who were below Tanner stage 4 development at baseline, the 0.61 mean improvement in height standard deviation score at 2-year follow-up was statistically significant, and although mean corticosteroid dose was higher in the first year of treatment (0.13 mg/kg per day vs. 0.05 mg/kg per day), mean height velocities were comparable in both years, at 5.8 cm and 6.3 cm, respectively, he noted.
IGF-1 standard deviation score increased from a mean of –1.1 at baseline to 0.0 at 2 years, and OC/CTX-1 ratio increased significantly, suggesting an increase in osteoblast activity relative to osteoclast activity.
Improvements in the Juvenile Arthritis Disease Activity Score–71 (JADAS-71) during the first year correlated with increases in height velocities during that year, as did younger age at baseline.
Tocilizumab, which was approved by the U.S. Food and Drugs Administration in 2010 for the treatment of rheumatoid arthritis, received an expanded approval for the treatment of sJIA in April 2011, based on 12-week results from the randomized, placebo-controlled TENDER study. Treatment was shown in that phase, as well as in an open-label long-term extension phase, to be safe and effective for the management of sJIA. Two-year findings of the study, which included children with active refractory disease, were reported at the 2011 annual meeting of the American College of Rheumatology.
Data collection will continue through 5 years of follow-up, allowing a more comprehensive analysis of growth outcomes, said Dr. De Benedetti, the lead investigator for the TENDER study.
Dr. De Benedetti disclosed that he has received research grants and/or consulting fees from Abbott, BMS, Novartis, NovImmune, Pfizer, Roche Pharmaceuticals, and Sobi. The TENDER study was sponsored by Roche, the maker of tocilizumab.
WASHINGTON – Tocilizumab for the treatment of systemic juvenile idiopathic arthritis was associated with significant catch-up growth at 2-year follow-up in the vast majority of children who participated in the pivotal phase III TENDER study.
Treatment with the interleukin-6 (IL-6) receptor inhibitor, which is marketed as Actemra, also significantly increased insulinlike growth factor-1 (IGF-1) levels and osteocalcin/C-telopeptide of type 1 collagen (OC/CTX-1) ratios, suggesting that the therapy has beneficial effects on growth hormone axis and bone metabolism, reported Dr. Fabrizio De Benedetti, speaking at the annual meeting of the American College of Rheumatology.
The findings are important because systemic juvenile idiopathic arthritis (sJIA) has a significant impact on the growing skeleton and is associated with impaired linear growth and systemic osteoporosis, he explained.
Height measurements at baseline in 112 children aged 2-17 years who were enrolled in the study showed profound growth failure, with most having height velocity well below normal for age and gender. However, during treatment, most patients had above normal height velocities: 85% of girls and 73% of boys experienced catch-up growth, said Dr. De Benedetti of Ospedale Pediatrico Bambino Gesu, Rome.
In 86 children from the study who were below Tanner stage 4 development at baseline, the 0.61 mean improvement in height standard deviation score at 2-year follow-up was statistically significant, and although mean corticosteroid dose was higher in the first year of treatment (0.13 mg/kg per day vs. 0.05 mg/kg per day), mean height velocities were comparable in both years, at 5.8 cm and 6.3 cm, respectively, he noted.
IGF-1 standard deviation score increased from a mean of –1.1 at baseline to 0.0 at 2 years, and OC/CTX-1 ratio increased significantly, suggesting an increase in osteoblast activity relative to osteoclast activity.
Improvements in the Juvenile Arthritis Disease Activity Score–71 (JADAS-71) during the first year correlated with increases in height velocities during that year, as did younger age at baseline.
Tocilizumab, which was approved by the U.S. Food and Drugs Administration in 2010 for the treatment of rheumatoid arthritis, received an expanded approval for the treatment of sJIA in April 2011, based on 12-week results from the randomized, placebo-controlled TENDER study. Treatment was shown in that phase, as well as in an open-label long-term extension phase, to be safe and effective for the management of sJIA. Two-year findings of the study, which included children with active refractory disease, were reported at the 2011 annual meeting of the American College of Rheumatology.
Data collection will continue through 5 years of follow-up, allowing a more comprehensive analysis of growth outcomes, said Dr. De Benedetti, the lead investigator for the TENDER study.
Dr. De Benedetti disclosed that he has received research grants and/or consulting fees from Abbott, BMS, Novartis, NovImmune, Pfizer, Roche Pharmaceuticals, and Sobi. The TENDER study was sponsored by Roche, the maker of tocilizumab.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: Height measurements at baseline in 112 children aged 2-17 years who were enrolled in the study showed profound growth failure, but during treatment, most patients had above-normal height velocities: 85% of girls and 73% of boys experienced catch-up growth.
Data Source: The findings come from the phase III TENDER study.
Disclosures: Dr. De Benedetti disclosed that he has received research grants and/or consulting fees from Abbott, BMS, Novartis, Novimmune, Pfizer, Roche Pharmaceuticals, and SOBI. The TENDER study was sponsored by Roche, the maker of tocilizumab.
ACE Inhibitors Up Mortality Risk When Given Prior to Scleroderma Renal Crisis
WASHINGTON – Exposure to angiotensin-converting enzyme inhibitors prior to the onset of renal crisis in patients with scleroderma increases the risk of death, according to 1-year findings from the prospective observational International Scleroderma Renal Crisis Survey.
The findings, which contrast with those from a preliminary analysis reported last year, suggest that clinicians caring for patients with systemic scleroderma should exercise caution when prescribing angiotensin-converting enzyme (ACE) inhibitors, Dr. Marie Hudson said at the annual meeting of the American College of Rheumatology, where she reported the survey results.
Of 75 patients who experienced scleroderma renal crisis (SRC) in the course of the study, 16 were taking an ACE inhibitor prior to onset of SRC. At the 1-year follow-up, 27 (36%) of the patients had died and 25% remained on dialysis. After adjusting for differences in prednisone exposure and history of systemic hypertension, ACE inhibitor exposure prior to SRC, compared with no such exposure, was associated with significantly increased risk of death (adjusted hazard ratio, 2.52), said Dr. Hudson of the division of rheumatology at McGill University, Montreal.
SRC is a rare but life-threatening complication of systemic sclerosis that typically presents with malignant hypertension and acute renal failure.
"Prior to the advent of ACE inhibitors, it was almost a universally deadly complication of scleroderma, but since the advent of ACE inhibitors, the outcomes of patients with scleroderma renal crisis have improved tremendously," Dr. Hudson said, noting also that some evidence suggest that the incidence of SRC has decreased over the past two decades as well – due, perhaps, to more liberal use of ACE inhibitors.
"So, given the benefits of ACE inhibitors to treat SRC along with this perceived decrease in the incidence of SRC, the prophylactic use of ACE inhibitors to prevent SRC has been considered. However, some would argue that there’s no clear physiologic rationale for this," she said, explaining that most patients with SRC are not hyperreninemic prior to renal crisis and that prophylactic treatment could mask hypertension and delay diagnosis, thus leading to worse outcomes in those who develop SRC.
In fact, recent retrospective data support the idea that those exposed prior to SRC may have worse outcomes, she noted.
The findings of this survey, which are important given the widespread availability of ACE inhibitors, confirm that, she said.
Patients included in the study were identified by physicians from numerous practices around the world who had agreed to participate in the survey. A total of 589 physicians were asked biweekly if they had made a diagnosis of SRC, and if so, they filled out a short case report form at that time and then submitted another 1 year later.
The patients had a mean age of 52 years, and 67% were women. Most (76%) had diffuse systemic scleroderma with a median disease duration of 1.5 years. SRC was hypertensive in 71 patients; only 5 patients had normotensive SRC, she noted.
The rate of prednisone use was surprisingly high at nearly 50% overall, but, more importantly, the mean daily dose was twice as high in the group of patients who were not exposed to ACE inhibitors prior to SRC (mean dose of 18 mg/day vs. 9 mg/day in the exposed group), Dr. Hudson said.
However, even after adjusting for prednisone use, the risk of death in exposed patients was more than twice that of nonexposed patients, and the difference was statistically significant, she said.
Although the precise risk of death after SRC remains uncertain, it does appear that caution when using ACE inhibitors in these patients is warranted, especially early in disease when the risk of SRC is the greatest, she said.
Dr. Hudson had no disclosures to report.
WASHINGTON – Exposure to angiotensin-converting enzyme inhibitors prior to the onset of renal crisis in patients with scleroderma increases the risk of death, according to 1-year findings from the prospective observational International Scleroderma Renal Crisis Survey.
The findings, which contrast with those from a preliminary analysis reported last year, suggest that clinicians caring for patients with systemic scleroderma should exercise caution when prescribing angiotensin-converting enzyme (ACE) inhibitors, Dr. Marie Hudson said at the annual meeting of the American College of Rheumatology, where she reported the survey results.
Of 75 patients who experienced scleroderma renal crisis (SRC) in the course of the study, 16 were taking an ACE inhibitor prior to onset of SRC. At the 1-year follow-up, 27 (36%) of the patients had died and 25% remained on dialysis. After adjusting for differences in prednisone exposure and history of systemic hypertension, ACE inhibitor exposure prior to SRC, compared with no such exposure, was associated with significantly increased risk of death (adjusted hazard ratio, 2.52), said Dr. Hudson of the division of rheumatology at McGill University, Montreal.
SRC is a rare but life-threatening complication of systemic sclerosis that typically presents with malignant hypertension and acute renal failure.
"Prior to the advent of ACE inhibitors, it was almost a universally deadly complication of scleroderma, but since the advent of ACE inhibitors, the outcomes of patients with scleroderma renal crisis have improved tremendously," Dr. Hudson said, noting also that some evidence suggest that the incidence of SRC has decreased over the past two decades as well – due, perhaps, to more liberal use of ACE inhibitors.
"So, given the benefits of ACE inhibitors to treat SRC along with this perceived decrease in the incidence of SRC, the prophylactic use of ACE inhibitors to prevent SRC has been considered. However, some would argue that there’s no clear physiologic rationale for this," she said, explaining that most patients with SRC are not hyperreninemic prior to renal crisis and that prophylactic treatment could mask hypertension and delay diagnosis, thus leading to worse outcomes in those who develop SRC.
In fact, recent retrospective data support the idea that those exposed prior to SRC may have worse outcomes, she noted.
The findings of this survey, which are important given the widespread availability of ACE inhibitors, confirm that, she said.
Patients included in the study were identified by physicians from numerous practices around the world who had agreed to participate in the survey. A total of 589 physicians were asked biweekly if they had made a diagnosis of SRC, and if so, they filled out a short case report form at that time and then submitted another 1 year later.
The patients had a mean age of 52 years, and 67% were women. Most (76%) had diffuse systemic scleroderma with a median disease duration of 1.5 years. SRC was hypertensive in 71 patients; only 5 patients had normotensive SRC, she noted.
The rate of prednisone use was surprisingly high at nearly 50% overall, but, more importantly, the mean daily dose was twice as high in the group of patients who were not exposed to ACE inhibitors prior to SRC (mean dose of 18 mg/day vs. 9 mg/day in the exposed group), Dr. Hudson said.
However, even after adjusting for prednisone use, the risk of death in exposed patients was more than twice that of nonexposed patients, and the difference was statistically significant, she said.
Although the precise risk of death after SRC remains uncertain, it does appear that caution when using ACE inhibitors in these patients is warranted, especially early in disease when the risk of SRC is the greatest, she said.
Dr. Hudson had no disclosures to report.
WASHINGTON – Exposure to angiotensin-converting enzyme inhibitors prior to the onset of renal crisis in patients with scleroderma increases the risk of death, according to 1-year findings from the prospective observational International Scleroderma Renal Crisis Survey.
The findings, which contrast with those from a preliminary analysis reported last year, suggest that clinicians caring for patients with systemic scleroderma should exercise caution when prescribing angiotensin-converting enzyme (ACE) inhibitors, Dr. Marie Hudson said at the annual meeting of the American College of Rheumatology, where she reported the survey results.
Of 75 patients who experienced scleroderma renal crisis (SRC) in the course of the study, 16 were taking an ACE inhibitor prior to onset of SRC. At the 1-year follow-up, 27 (36%) of the patients had died and 25% remained on dialysis. After adjusting for differences in prednisone exposure and history of systemic hypertension, ACE inhibitor exposure prior to SRC, compared with no such exposure, was associated with significantly increased risk of death (adjusted hazard ratio, 2.52), said Dr. Hudson of the division of rheumatology at McGill University, Montreal.
SRC is a rare but life-threatening complication of systemic sclerosis that typically presents with malignant hypertension and acute renal failure.
"Prior to the advent of ACE inhibitors, it was almost a universally deadly complication of scleroderma, but since the advent of ACE inhibitors, the outcomes of patients with scleroderma renal crisis have improved tremendously," Dr. Hudson said, noting also that some evidence suggest that the incidence of SRC has decreased over the past two decades as well – due, perhaps, to more liberal use of ACE inhibitors.
"So, given the benefits of ACE inhibitors to treat SRC along with this perceived decrease in the incidence of SRC, the prophylactic use of ACE inhibitors to prevent SRC has been considered. However, some would argue that there’s no clear physiologic rationale for this," she said, explaining that most patients with SRC are not hyperreninemic prior to renal crisis and that prophylactic treatment could mask hypertension and delay diagnosis, thus leading to worse outcomes in those who develop SRC.
In fact, recent retrospective data support the idea that those exposed prior to SRC may have worse outcomes, she noted.
The findings of this survey, which are important given the widespread availability of ACE inhibitors, confirm that, she said.
Patients included in the study were identified by physicians from numerous practices around the world who had agreed to participate in the survey. A total of 589 physicians were asked biweekly if they had made a diagnosis of SRC, and if so, they filled out a short case report form at that time and then submitted another 1 year later.
The patients had a mean age of 52 years, and 67% were women. Most (76%) had diffuse systemic scleroderma with a median disease duration of 1.5 years. SRC was hypertensive in 71 patients; only 5 patients had normotensive SRC, she noted.
The rate of prednisone use was surprisingly high at nearly 50% overall, but, more importantly, the mean daily dose was twice as high in the group of patients who were not exposed to ACE inhibitors prior to SRC (mean dose of 18 mg/day vs. 9 mg/day in the exposed group), Dr. Hudson said.
However, even after adjusting for prednisone use, the risk of death in exposed patients was more than twice that of nonexposed patients, and the difference was statistically significant, she said.
Although the precise risk of death after SRC remains uncertain, it does appear that caution when using ACE inhibitors in these patients is warranted, especially early in disease when the risk of SRC is the greatest, she said.
Dr. Hudson had no disclosures to report.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: ACE inhibitor exposure prior to SRC, compared with no such exposure, was found to be associated with significantly increased risk of death (adjusted hazard ratio, 2.52).
Data Source: This finding comes from a prospective international cohort study (International Scleroderma Renal Crisis Survey).
Disclosures: Dr. Hudson had no disclosures to report.
NIPPV Benefits Seen in Severe Stable COPD
ATLANTA – Long-term nocturnal use of noninvasive positive pressure ventilation significantly reduced the likelihood of intensive care unit admission in patients with severe stable chronic obstructive pulmonary disease, according to findings from a systematic review of 582 patients in 13 randomized, controlled clinical trials.
After 1 year, noninvasive positive pressure ventilation (NIPPV) was associated with a significant decrease in ICU admissions (odds ratio, 0.41) compared with standard medical therapy. Patients using NIPPV for more than 3 months also had improvements in oxygenation (mean difference of -2.43 mm Hg), reduction in PCO2 (mean difference, -2.96 mm Hg), and an improvement in 6-minute walk distance (mean difference 45.15 m), Dr. Monali Patil reported at the annual meeting of the American College of Chest Physicians.
A trend toward improved mortality at 1 year did not reach statistical significance, and no significant improvements in lung function were noted, said Dr. Patil of the University at Buffalo (N.Y.).
Dr. Patil selected the 13 trials from a review of more than 700 studies conducted between 1991 and 2011. The analysis included only randomized, controlled trials of COPD patients who had an FEV1 less than 50% of predicted and a PCO2 greater than 45 mm Hg and were receiving bilevel positive airway pressure (BIPAP). The patients in the studies were aged 18-75 years, and had no COPD exacerbations within 2 weeks prior to study enrollment.
The long-term use of NIPPV in patients with severe stable COPD has been controversial, but these findings demonstrate significant benefits.
"So NIPPV can be used as adjuvant treatment for management of severe stable COPD patients," she concluded.
Dr Patil reported having no financial disclosures.
ATLANTA – Long-term nocturnal use of noninvasive positive pressure ventilation significantly reduced the likelihood of intensive care unit admission in patients with severe stable chronic obstructive pulmonary disease, according to findings from a systematic review of 582 patients in 13 randomized, controlled clinical trials.
After 1 year, noninvasive positive pressure ventilation (NIPPV) was associated with a significant decrease in ICU admissions (odds ratio, 0.41) compared with standard medical therapy. Patients using NIPPV for more than 3 months also had improvements in oxygenation (mean difference of -2.43 mm Hg), reduction in PCO2 (mean difference, -2.96 mm Hg), and an improvement in 6-minute walk distance (mean difference 45.15 m), Dr. Monali Patil reported at the annual meeting of the American College of Chest Physicians.
A trend toward improved mortality at 1 year did not reach statistical significance, and no significant improvements in lung function were noted, said Dr. Patil of the University at Buffalo (N.Y.).
Dr. Patil selected the 13 trials from a review of more than 700 studies conducted between 1991 and 2011. The analysis included only randomized, controlled trials of COPD patients who had an FEV1 less than 50% of predicted and a PCO2 greater than 45 mm Hg and were receiving bilevel positive airway pressure (BIPAP). The patients in the studies were aged 18-75 years, and had no COPD exacerbations within 2 weeks prior to study enrollment.
The long-term use of NIPPV in patients with severe stable COPD has been controversial, but these findings demonstrate significant benefits.
"So NIPPV can be used as adjuvant treatment for management of severe stable COPD patients," she concluded.
Dr Patil reported having no financial disclosures.
ATLANTA – Long-term nocturnal use of noninvasive positive pressure ventilation significantly reduced the likelihood of intensive care unit admission in patients with severe stable chronic obstructive pulmonary disease, according to findings from a systematic review of 582 patients in 13 randomized, controlled clinical trials.
After 1 year, noninvasive positive pressure ventilation (NIPPV) was associated with a significant decrease in ICU admissions (odds ratio, 0.41) compared with standard medical therapy. Patients using NIPPV for more than 3 months also had improvements in oxygenation (mean difference of -2.43 mm Hg), reduction in PCO2 (mean difference, -2.96 mm Hg), and an improvement in 6-minute walk distance (mean difference 45.15 m), Dr. Monali Patil reported at the annual meeting of the American College of Chest Physicians.
A trend toward improved mortality at 1 year did not reach statistical significance, and no significant improvements in lung function were noted, said Dr. Patil of the University at Buffalo (N.Y.).
Dr. Patil selected the 13 trials from a review of more than 700 studies conducted between 1991 and 2011. The analysis included only randomized, controlled trials of COPD patients who had an FEV1 less than 50% of predicted and a PCO2 greater than 45 mm Hg and were receiving bilevel positive airway pressure (BIPAP). The patients in the studies were aged 18-75 years, and had no COPD exacerbations within 2 weeks prior to study enrollment.
The long-term use of NIPPV in patients with severe stable COPD has been controversial, but these findings demonstrate significant benefits.
"So NIPPV can be used as adjuvant treatment for management of severe stable COPD patients," she concluded.
Dr Patil reported having no financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CHEST PHYSICIANS
Major Finding: Noninvasive positive pressure ventilation (NIPPV) was associated with a significant decrease in ICU admissions at 1 year (odds ratio, 0.41).
Data Source: Findings were based on a review of 582 patients in 13 randomized, controlled clinical trials.
Disclosures: Dr. Patil reported having no disclosures.
Botulinum Toxin Threading Yields More Uniform Result
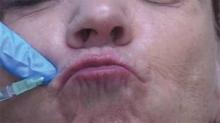
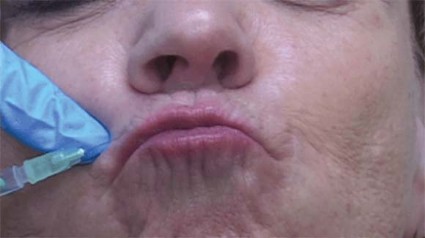
ATLANTA – The use of a threading technique, rather than the standard depot injection technique, when using botulinum toxin A to treat perioral and glabellar rhytides provides a more uniform and natural cosmetic result, according to Dr. H. William Higgins II.
Threading involves injecting the muscle along its normal anatomic course to paralyze the related muscle more evenly, he explained at the annual meeting of the American Society for Dermatologic Surgery.
For upper and lower lip treatment, for example, injections are made at a 20- to 30-degree angle, entering the skin at a location just lateral to the targeted rhytid. The toxin is dispensed while withdrawing, thereby threading the injection along the length of the orbicularis oris. This differs from the typical approach, which often involves a depot injection at an angle more perpendicular to the skin, said Dr. Higgins of Brown University in Providence, R.I.
For the glabellar lines, the threading technique involves four symmetrical injection points, with two points targeting each corrugator. Injections at the more medial points are made directly above the inner canthus, with intramuscular injections made perpendicularly to the skin in the traditional depot manner.
At the two lateral injection points, however, the needle is inserted in most cases just medial to the mid-pupillary lines, thereby targeting the "tail" of the corrugators, he explained.
"Similar to our approach at the orbicularis oris, rather than injecting at an angle more perpendicular to the skin, we inject at an angle of roughly 20-30 degrees, entering the skin at a location just medial to the glabellar rhytid we intend to treat. The needle is then directed laterally and slightly superiorly in order to follow the anatomy of the corrugator supercilii, and the injection is threaded along the muscle’s length while withdrawing," he explained.
This approach corrects for the inadequate responses sometimes seen when using the typical method of placing subepidermal blebs to produce localized microparesis of the targeted muscle, and could reduce the need for touch-up injections.
Cosmetic outcomes have been excellent and patient satisfaction high with the use of this technique, he said. In his experience, the technique has dramatically reduced the incidence of adverse effects.
"It has been documented that, even with conservative dosing, neuromodulator treatment of perioral rhytides can affect mouth function by weakening the lip sphincter, but this has not been the case in our patient population when using this technique," he said.
Similarly, when treating glabellar rhytides, the injection of the toxin at a more precise depth – and more evenly along the tail of the corrugators, has resulted in a reduced incidence of brow ptosis as well as more natural smoothing.
"This approach helps prevent the undesirable appearance of a 'forehead freeze,' " he said.
The threading technique also results in fewer needle sticks, which means less pain and bruising for the patients.
The use of a longer 1- or 1.5-inch needle could potentially allow for even fewer injections without compromising the result, Dr. Higgins noted.
"Furthermore, this technique could conceivably be applied on other areas of the face. Crow's feet, for example, could be treated with fewer threading injections rather than with multiple depot injections," he said.
Dr. Higgins reported having no relevant financial disclosures.
ATLANTA – The use of a threading technique, rather than the standard depot injection technique, when using botulinum toxin A to treat perioral and glabellar rhytides provides a more uniform and natural cosmetic result, according to Dr. H. William Higgins II.
Threading involves injecting the muscle along its normal anatomic course to paralyze the related muscle more evenly, he explained at the annual meeting of the American Society for Dermatologic Surgery.
For upper and lower lip treatment, for example, injections are made at a 20- to 30-degree angle, entering the skin at a location just lateral to the targeted rhytid. The toxin is dispensed while withdrawing, thereby threading the injection along the length of the orbicularis oris. This differs from the typical approach, which often involves a depot injection at an angle more perpendicular to the skin, said Dr. Higgins of Brown University in Providence, R.I.
For the glabellar lines, the threading technique involves four symmetrical injection points, with two points targeting each corrugator. Injections at the more medial points are made directly above the inner canthus, with intramuscular injections made perpendicularly to the skin in the traditional depot manner.
At the two lateral injection points, however, the needle is inserted in most cases just medial to the mid-pupillary lines, thereby targeting the "tail" of the corrugators, he explained.
"Similar to our approach at the orbicularis oris, rather than injecting at an angle more perpendicular to the skin, we inject at an angle of roughly 20-30 degrees, entering the skin at a location just medial to the glabellar rhytid we intend to treat. The needle is then directed laterally and slightly superiorly in order to follow the anatomy of the corrugator supercilii, and the injection is threaded along the muscle’s length while withdrawing," he explained.
This approach corrects for the inadequate responses sometimes seen when using the typical method of placing subepidermal blebs to produce localized microparesis of the targeted muscle, and could reduce the need for touch-up injections.
Cosmetic outcomes have been excellent and patient satisfaction high with the use of this technique, he said. In his experience, the technique has dramatically reduced the incidence of adverse effects.
"It has been documented that, even with conservative dosing, neuromodulator treatment of perioral rhytides can affect mouth function by weakening the lip sphincter, but this has not been the case in our patient population when using this technique," he said.
Similarly, when treating glabellar rhytides, the injection of the toxin at a more precise depth – and more evenly along the tail of the corrugators, has resulted in a reduced incidence of brow ptosis as well as more natural smoothing.
"This approach helps prevent the undesirable appearance of a 'forehead freeze,' " he said.
The threading technique also results in fewer needle sticks, which means less pain and bruising for the patients.
The use of a longer 1- or 1.5-inch needle could potentially allow for even fewer injections without compromising the result, Dr. Higgins noted.
"Furthermore, this technique could conceivably be applied on other areas of the face. Crow's feet, for example, could be treated with fewer threading injections rather than with multiple depot injections," he said.
Dr. Higgins reported having no relevant financial disclosures.
ATLANTA – The use of a threading technique, rather than the standard depot injection technique, when using botulinum toxin A to treat perioral and glabellar rhytides provides a more uniform and natural cosmetic result, according to Dr. H. William Higgins II.
Threading involves injecting the muscle along its normal anatomic course to paralyze the related muscle more evenly, he explained at the annual meeting of the American Society for Dermatologic Surgery.
For upper and lower lip treatment, for example, injections are made at a 20- to 30-degree angle, entering the skin at a location just lateral to the targeted rhytid. The toxin is dispensed while withdrawing, thereby threading the injection along the length of the orbicularis oris. This differs from the typical approach, which often involves a depot injection at an angle more perpendicular to the skin, said Dr. Higgins of Brown University in Providence, R.I.
For the glabellar lines, the threading technique involves four symmetrical injection points, with two points targeting each corrugator. Injections at the more medial points are made directly above the inner canthus, with intramuscular injections made perpendicularly to the skin in the traditional depot manner.
At the two lateral injection points, however, the needle is inserted in most cases just medial to the mid-pupillary lines, thereby targeting the "tail" of the corrugators, he explained.
"Similar to our approach at the orbicularis oris, rather than injecting at an angle more perpendicular to the skin, we inject at an angle of roughly 20-30 degrees, entering the skin at a location just medial to the glabellar rhytid we intend to treat. The needle is then directed laterally and slightly superiorly in order to follow the anatomy of the corrugator supercilii, and the injection is threaded along the muscle’s length while withdrawing," he explained.
This approach corrects for the inadequate responses sometimes seen when using the typical method of placing subepidermal blebs to produce localized microparesis of the targeted muscle, and could reduce the need for touch-up injections.
Cosmetic outcomes have been excellent and patient satisfaction high with the use of this technique, he said. In his experience, the technique has dramatically reduced the incidence of adverse effects.
"It has been documented that, even with conservative dosing, neuromodulator treatment of perioral rhytides can affect mouth function by weakening the lip sphincter, but this has not been the case in our patient population when using this technique," he said.
Similarly, when treating glabellar rhytides, the injection of the toxin at a more precise depth – and more evenly along the tail of the corrugators, has resulted in a reduced incidence of brow ptosis as well as more natural smoothing.
"This approach helps prevent the undesirable appearance of a 'forehead freeze,' " he said.
The threading technique also results in fewer needle sticks, which means less pain and bruising for the patients.
The use of a longer 1- or 1.5-inch needle could potentially allow for even fewer injections without compromising the result, Dr. Higgins noted.
"Furthermore, this technique could conceivably be applied on other areas of the face. Crow's feet, for example, could be treated with fewer threading injections rather than with multiple depot injections," he said.
Dr. Higgins reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR DERMATOLOGIC SURGERY
Cryolipolysis Appears Safe for All Skin Types
ATLANTA – Cryolipolysis appears as safe and effective in dark skin types as in lighter skin types, according to a review of outcomes in 396 patients.
The primary goal of the study was to assess whether this technology is safe in patients with dark skin types, given their increased risk for developing postinflammatory hyperpigmentation with cold exposure and other treatments such as laser treatments, Dr. Ava Shamban said at the annual meeting of the American Society for Dermatologic Surgery.
However, no differences were seen in efficacy or safety based on skin type or ethnicity in the patients who participated in the multicenter study, she said.
"Cryolipolysis is, indeed, a color-blind technology."
In fact, patients with darker skin types had slightly higher satisfaction with the procedure, raising the question of whether the treatment might even be more effective in these patients, said Dr. Shamban, a dermatologist in private practice in Santa Monica, Calif.
The patients were treated on the back, flank, and/or abdomen, with most having multiple areas treated. Fitzpatrick skin types II-VI were represented: 85 had type II, 185 had type III, 104 had type IV, 18 had type V, and 4 had type VI.
Numerous ethnicities also were represented: 7 patients were African American, 38 were Asian, 295 were white, 37 were Latino, 5 were Mediterranean, and 14 were of Middle Eastern descent.
Women comprised 84% of the study population, and patients ranged in age from 24 to 74 years, with most in their late 40s to early 50s, Dr. Shamban noted.
No major adverse events occurred. Some patients did, however, experience minor effects, including bruising in 16%, swelling in 20%, and both bruising and swelling in 4%, she said, adding that the incidence of these effects did not differ between those with Fitzpatrick skin types II-III and those with type IV-VI.
Satisfaction with the procedure and outcomes also did not differ between those groups in 201 patients who completed a patient-satisfaction assessment. Only 7% of 122 patients with skin types II-III and 4% of 79 patients with skin types IV-VI were unsatisfied with the results, Dr. Shamban said.
"Cryolipolysis is, indeed, a color-blind technology, and it is a safe and effective method to reduce fat and thickening in patients of all skin types and ethnicities," she concluded.
Dr. Shamban had no disclosures to report. Her coauthor, Dr. Vic Narurkar, reported serving as a consultant for Zeltiq, the maker of the CoolSculpting cryolipolysis system used in this study.
ATLANTA – Cryolipolysis appears as safe and effective in dark skin types as in lighter skin types, according to a review of outcomes in 396 patients.
The primary goal of the study was to assess whether this technology is safe in patients with dark skin types, given their increased risk for developing postinflammatory hyperpigmentation with cold exposure and other treatments such as laser treatments, Dr. Ava Shamban said at the annual meeting of the American Society for Dermatologic Surgery.
However, no differences were seen in efficacy or safety based on skin type or ethnicity in the patients who participated in the multicenter study, she said.
"Cryolipolysis is, indeed, a color-blind technology."
In fact, patients with darker skin types had slightly higher satisfaction with the procedure, raising the question of whether the treatment might even be more effective in these patients, said Dr. Shamban, a dermatologist in private practice in Santa Monica, Calif.
The patients were treated on the back, flank, and/or abdomen, with most having multiple areas treated. Fitzpatrick skin types II-VI were represented: 85 had type II, 185 had type III, 104 had type IV, 18 had type V, and 4 had type VI.
Numerous ethnicities also were represented: 7 patients were African American, 38 were Asian, 295 were white, 37 were Latino, 5 were Mediterranean, and 14 were of Middle Eastern descent.
Women comprised 84% of the study population, and patients ranged in age from 24 to 74 years, with most in their late 40s to early 50s, Dr. Shamban noted.
No major adverse events occurred. Some patients did, however, experience minor effects, including bruising in 16%, swelling in 20%, and both bruising and swelling in 4%, she said, adding that the incidence of these effects did not differ between those with Fitzpatrick skin types II-III and those with type IV-VI.
Satisfaction with the procedure and outcomes also did not differ between those groups in 201 patients who completed a patient-satisfaction assessment. Only 7% of 122 patients with skin types II-III and 4% of 79 patients with skin types IV-VI were unsatisfied with the results, Dr. Shamban said.
"Cryolipolysis is, indeed, a color-blind technology, and it is a safe and effective method to reduce fat and thickening in patients of all skin types and ethnicities," she concluded.
Dr. Shamban had no disclosures to report. Her coauthor, Dr. Vic Narurkar, reported serving as a consultant for Zeltiq, the maker of the CoolSculpting cryolipolysis system used in this study.
ATLANTA – Cryolipolysis appears as safe and effective in dark skin types as in lighter skin types, according to a review of outcomes in 396 patients.
The primary goal of the study was to assess whether this technology is safe in patients with dark skin types, given their increased risk for developing postinflammatory hyperpigmentation with cold exposure and other treatments such as laser treatments, Dr. Ava Shamban said at the annual meeting of the American Society for Dermatologic Surgery.
However, no differences were seen in efficacy or safety based on skin type or ethnicity in the patients who participated in the multicenter study, she said.
"Cryolipolysis is, indeed, a color-blind technology."
In fact, patients with darker skin types had slightly higher satisfaction with the procedure, raising the question of whether the treatment might even be more effective in these patients, said Dr. Shamban, a dermatologist in private practice in Santa Monica, Calif.
The patients were treated on the back, flank, and/or abdomen, with most having multiple areas treated. Fitzpatrick skin types II-VI were represented: 85 had type II, 185 had type III, 104 had type IV, 18 had type V, and 4 had type VI.
Numerous ethnicities also were represented: 7 patients were African American, 38 were Asian, 295 were white, 37 were Latino, 5 were Mediterranean, and 14 were of Middle Eastern descent.
Women comprised 84% of the study population, and patients ranged in age from 24 to 74 years, with most in their late 40s to early 50s, Dr. Shamban noted.
No major adverse events occurred. Some patients did, however, experience minor effects, including bruising in 16%, swelling in 20%, and both bruising and swelling in 4%, she said, adding that the incidence of these effects did not differ between those with Fitzpatrick skin types II-III and those with type IV-VI.
Satisfaction with the procedure and outcomes also did not differ between those groups in 201 patients who completed a patient-satisfaction assessment. Only 7% of 122 patients with skin types II-III and 4% of 79 patients with skin types IV-VI were unsatisfied with the results, Dr. Shamban said.
"Cryolipolysis is, indeed, a color-blind technology, and it is a safe and effective method to reduce fat and thickening in patients of all skin types and ethnicities," she concluded.
Dr. Shamban had no disclosures to report. Her coauthor, Dr. Vic Narurkar, reported serving as a consultant for Zeltiq, the maker of the CoolSculpting cryolipolysis system used in this study.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR DERMATOLOGIC SURGERY
Major Finding: No differences were seen in efficacy or safety based on skin type or ethnicity.
Data Source: This review of outcomes involved 396 patients.
Disclosures: Dr. Shamban had no disclosures to report. Her coauthor, Dr. Vic Narurkar, reported serving as a consultant for Zeltiq, the maker of the CoolSculpting cryolipolysis system used in this study.
Macitentan Promising for Long-Term Outcomes in PAH
ATLANTA – Macitentan, a novel dual endothelin receptor antagonist with enhanced tissue penetration, significantly improves morbidity and mortality in patients with pulmonary arterial hypertension, according to findings from an industry-sponsored, randomized controlled phase III SERAPHIN study.
Macitentan treatment reduced the risk of occurrence of combined morbidity and mortality events by 30% in 250 patients randomized to receive 3 mg once daily and by 45% in 242 patients randomized to receive 10 mg once daily, compared with 250 patients who received placebo, Dr. Lewis Rubin reported at the annual meeting of the American College of Chest Physicians.
The differences were highly significant for both macitentan doses, and the effect of treatment on this novel primary end point was observed irrespective of background therapy, which consisted mainly of phosphodiesterase type-5 (PDE-5) inhibitors.
Among patients using background therapy, risk was reduced by 17% and 38% for the 3 mg and 10 mg groups, respectively; in treatment-naive patients, the risk was reduced by 47% and 55% in the dosage groups, respectively, said Dr. Rubin of the University of California, San Diego.
The findings hold promise for improved long-term outcomes in patients with pulmonary arterial hypertension (PAH), Dr. Rubin said. "This primary morbidity/mortality end point captures clinically relevant events that reflect true disease progression," he noted, explaining that the end point included time to death, atrial septostomy, lung transplantation, initiation of intravenous/subcutaneous prostanoids, or worsening of PAH.
To meet the criteria for PAH worsening, participants had to experience a confirmed 15% or greater decrease in 6-minute walk distance and worsening of symptoms as defined by either a worsening in functional class, worsening symptoms of right heart failure, need for a new PAH treatment, or need for an intravenous diuretic. The majority of events contributing to achievement of the primary end point were associated with worsening of PAH, rather than death, he noted.
In addition to improvements with respect to the primary end point, macitentan treatment also was associated with improvement on the secondary end point of the composite of mortality or hospitalization due to PAH, with risk reduction of 33% and 50% in the 3-mg and 10-mg groups, respectively, compared with placebo, Dr. Rubin said.
Macitentan was well tolerated, with both the treatment group and the placebo group experiencing similar incidences of elevated liver aminotransferases and peripheral edema, although headache, nasopharyngitis, and anemia all occurred more frequently in the treatment groups.
Participants in the double-blind, event-driven SERAPHIN study (Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome) were individuals aged 12 years or older with PAH. Randomization began in May of 2008, and study end was predefined as the occurrence of 285 morbidity/mortality events, which occurred as of March 2012.
The findings are notable because existing PAH therapies, including bosentan and ambrisentan, have been approved based only on short-term trials with exercise capacity as the primary end point, and have potential for adverse events that can limit tolerability, Dr. Rubin said.
"So an endothelin receptor antagonist that has a better tolerability profile would be potentially desirable," he said.
Indeed, macitentan, which is a product of a tailored discovery program, has not only been shown to have enhanced tissue penetration and "superior in vivo efficacy in a number of animal models," but also to have no effect on bile salts. It, therefore, has diminished adverse effects on the rate of hepatic dysfunction that is seen, with varying degrees, as a manifestation of endothelin receptor antagonism, he explained.
"In addition, it has demonstrated unique sustained receptor binding, which also may be beneficial in long-term therapy," he said.
The SERAPHIN study was sponsored by Actelion, the maker of macitentan. Dr. Rubin disclosed that he has received payment for consulting and/or serving on speaker bureaus or advisory committees for Actelion, Pfizer, United Therapeutics, Lung LLC, Gilead, GlaxoSmithKline, Bayer, and GeNo.
ATLANTA – Macitentan, a novel dual endothelin receptor antagonist with enhanced tissue penetration, significantly improves morbidity and mortality in patients with pulmonary arterial hypertension, according to findings from an industry-sponsored, randomized controlled phase III SERAPHIN study.
Macitentan treatment reduced the risk of occurrence of combined morbidity and mortality events by 30% in 250 patients randomized to receive 3 mg once daily and by 45% in 242 patients randomized to receive 10 mg once daily, compared with 250 patients who received placebo, Dr. Lewis Rubin reported at the annual meeting of the American College of Chest Physicians.
The differences were highly significant for both macitentan doses, and the effect of treatment on this novel primary end point was observed irrespective of background therapy, which consisted mainly of phosphodiesterase type-5 (PDE-5) inhibitors.
Among patients using background therapy, risk was reduced by 17% and 38% for the 3 mg and 10 mg groups, respectively; in treatment-naive patients, the risk was reduced by 47% and 55% in the dosage groups, respectively, said Dr. Rubin of the University of California, San Diego.
The findings hold promise for improved long-term outcomes in patients with pulmonary arterial hypertension (PAH), Dr. Rubin said. "This primary morbidity/mortality end point captures clinically relevant events that reflect true disease progression," he noted, explaining that the end point included time to death, atrial septostomy, lung transplantation, initiation of intravenous/subcutaneous prostanoids, or worsening of PAH.
To meet the criteria for PAH worsening, participants had to experience a confirmed 15% or greater decrease in 6-minute walk distance and worsening of symptoms as defined by either a worsening in functional class, worsening symptoms of right heart failure, need for a new PAH treatment, or need for an intravenous diuretic. The majority of events contributing to achievement of the primary end point were associated with worsening of PAH, rather than death, he noted.
In addition to improvements with respect to the primary end point, macitentan treatment also was associated with improvement on the secondary end point of the composite of mortality or hospitalization due to PAH, with risk reduction of 33% and 50% in the 3-mg and 10-mg groups, respectively, compared with placebo, Dr. Rubin said.
Macitentan was well tolerated, with both the treatment group and the placebo group experiencing similar incidences of elevated liver aminotransferases and peripheral edema, although headache, nasopharyngitis, and anemia all occurred more frequently in the treatment groups.
Participants in the double-blind, event-driven SERAPHIN study (Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome) were individuals aged 12 years or older with PAH. Randomization began in May of 2008, and study end was predefined as the occurrence of 285 morbidity/mortality events, which occurred as of March 2012.
The findings are notable because existing PAH therapies, including bosentan and ambrisentan, have been approved based only on short-term trials with exercise capacity as the primary end point, and have potential for adverse events that can limit tolerability, Dr. Rubin said.
"So an endothelin receptor antagonist that has a better tolerability profile would be potentially desirable," he said.
Indeed, macitentan, which is a product of a tailored discovery program, has not only been shown to have enhanced tissue penetration and "superior in vivo efficacy in a number of animal models," but also to have no effect on bile salts. It, therefore, has diminished adverse effects on the rate of hepatic dysfunction that is seen, with varying degrees, as a manifestation of endothelin receptor antagonism, he explained.
"In addition, it has demonstrated unique sustained receptor binding, which also may be beneficial in long-term therapy," he said.
The SERAPHIN study was sponsored by Actelion, the maker of macitentan. Dr. Rubin disclosed that he has received payment for consulting and/or serving on speaker bureaus or advisory committees for Actelion, Pfizer, United Therapeutics, Lung LLC, Gilead, GlaxoSmithKline, Bayer, and GeNo.
ATLANTA – Macitentan, a novel dual endothelin receptor antagonist with enhanced tissue penetration, significantly improves morbidity and mortality in patients with pulmonary arterial hypertension, according to findings from an industry-sponsored, randomized controlled phase III SERAPHIN study.
Macitentan treatment reduced the risk of occurrence of combined morbidity and mortality events by 30% in 250 patients randomized to receive 3 mg once daily and by 45% in 242 patients randomized to receive 10 mg once daily, compared with 250 patients who received placebo, Dr. Lewis Rubin reported at the annual meeting of the American College of Chest Physicians.
The differences were highly significant for both macitentan doses, and the effect of treatment on this novel primary end point was observed irrespective of background therapy, which consisted mainly of phosphodiesterase type-5 (PDE-5) inhibitors.
Among patients using background therapy, risk was reduced by 17% and 38% for the 3 mg and 10 mg groups, respectively; in treatment-naive patients, the risk was reduced by 47% and 55% in the dosage groups, respectively, said Dr. Rubin of the University of California, San Diego.
The findings hold promise for improved long-term outcomes in patients with pulmonary arterial hypertension (PAH), Dr. Rubin said. "This primary morbidity/mortality end point captures clinically relevant events that reflect true disease progression," he noted, explaining that the end point included time to death, atrial septostomy, lung transplantation, initiation of intravenous/subcutaneous prostanoids, or worsening of PAH.
To meet the criteria for PAH worsening, participants had to experience a confirmed 15% or greater decrease in 6-minute walk distance and worsening of symptoms as defined by either a worsening in functional class, worsening symptoms of right heart failure, need for a new PAH treatment, or need for an intravenous diuretic. The majority of events contributing to achievement of the primary end point were associated with worsening of PAH, rather than death, he noted.
In addition to improvements with respect to the primary end point, macitentan treatment also was associated with improvement on the secondary end point of the composite of mortality or hospitalization due to PAH, with risk reduction of 33% and 50% in the 3-mg and 10-mg groups, respectively, compared with placebo, Dr. Rubin said.
Macitentan was well tolerated, with both the treatment group and the placebo group experiencing similar incidences of elevated liver aminotransferases and peripheral edema, although headache, nasopharyngitis, and anemia all occurred more frequently in the treatment groups.
Participants in the double-blind, event-driven SERAPHIN study (Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome) were individuals aged 12 years or older with PAH. Randomization began in May of 2008, and study end was predefined as the occurrence of 285 morbidity/mortality events, which occurred as of March 2012.
The findings are notable because existing PAH therapies, including bosentan and ambrisentan, have been approved based only on short-term trials with exercise capacity as the primary end point, and have potential for adverse events that can limit tolerability, Dr. Rubin said.
"So an endothelin receptor antagonist that has a better tolerability profile would be potentially desirable," he said.
Indeed, macitentan, which is a product of a tailored discovery program, has not only been shown to have enhanced tissue penetration and "superior in vivo efficacy in a number of animal models," but also to have no effect on bile salts. It, therefore, has diminished adverse effects on the rate of hepatic dysfunction that is seen, with varying degrees, as a manifestation of endothelin receptor antagonism, he explained.
"In addition, it has demonstrated unique sustained receptor binding, which also may be beneficial in long-term therapy," he said.
The SERAPHIN study was sponsored by Actelion, the maker of macitentan. Dr. Rubin disclosed that he has received payment for consulting and/or serving on speaker bureaus or advisory committees for Actelion, Pfizer, United Therapeutics, Lung LLC, Gilead, GlaxoSmithKline, Bayer, and GeNo.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CHEST PHYSICIANS
Major Finding: Macitentan treatment reduced the risk of morbidity and mortality by 30% in patients who received 3 mg once daily and by 45% in those who received 10 mg once daily, compared patients who received placebo.
Data Source: The randomized controlled phase III SERAPHIN study included 250 and 242 patients in the 3-mg and 10-mg treatment groups, respectively, and 250 patients in the placebo group.
Disclosures: The SERAPHIN study was sponsored by Actelion, the maker of macitentan. Dr. Rubin has received payment for consulting and/or serving on speaker bureaus or advisory committees for Actelion, Pfizer, United Therapeutics, Lung LLC, Gilead, GlaxoSmithKline, Bayer, and GeNo.
New Gene-Expression Test Improves Diagnostic Yield of Bronchoscopy
ATLANTA – The combined use of a novel bronchial airway gene-expression test and bronchoscopy improves the ability to rule out lung cancer in patients with benign disease, compared with bronchoscopy alone, according to findings from the prospective case-controlled AEGIS-1 trial.
Because bronchoscopy, which plays a central role in lung cancer diagnosis, has varying diagnostic yield based on factors such as the size and location of the lesion, the method used to collect cells, and pathological processing methods, the findings suggest that the test (BronchoGen) could minimize the need for additional invasive procedures in these patients, Duncan Whitney, Ph.D., said at the annual meeting of the American College of Chest Physicians.
The AEGIS-1 (Airway Epithelium Gene Expression in the Diagnosis of Lung Cancer 1) trial included more than 700 current or former smokers undergoing bronchoscopy for suspicion of lung cancer. It was designed to evaluate the diagnostic accuracy of the genomic test, which detects gene expression of cytologically normal bronchial airway epithelial cells.
The investigators collected mainstem bronchial airway brushings from 330 patients, including 240 with confirmed primary lung cancer and 90 controls, and performed microarray analysis. The sample set was then split into an independent training sample of 220 cases and a test set of 110 cases, and the gene-expression prediction model was optimized.
Next, reverse transcription polymerase chain reaction (RT-PCR) assays were developed for candidate biomarker genes, and a multivariate test algorithm was reoptimized using the RT-PCR data generated by a reanalysis of 153 of the samples from cancer patients and 64 of the samples from controls, explained Dr. Whitney.
The test, which ultimately focused on 30 genes, yielded a sensitivity of 77%, compared with 74% for bronchoscopy alone. The combined use of the test and bronchoscopy yielded a sensitivity of 96% and a specificity of 73%.
The negative predictive value of the combined test also was better than that of bronchoscopy alone (0.85 vs. 0.65, respectively), said Dr. Whitney.
"So in essence, we’re reducing the false negative rate from 26% down to 4%," he said.
The BronchoGen test was developed based on the airway "field of injury" principle, which refers to the common molecular response that occurs throughout the respiratory tract in current and former smokers with lung cancer, Dr. Whitney explained.
These changes are detected in a gene-expression signature from normal airway cells, even decades after smoking cessation, he noted.
Despite tremendous work done in this area, which has dramatically improved the diagnostic yield and sensitivity of bronchoscopy in the past few years, the procedure is either inconclusive or not diagnostic in up to 50% of cases, he said.
Thus, a "fairly large need" exists in the medical community, given that about 300,000 bronchoscopy procedures are performed each year for suspicion of lung cancer, he added.
Complete results from AEGIS-1, as well as clinical validation of the BronchoGen test, are expected to be released later this year. An additional 1,300 patients have been enrolled in the ongoing AEGIS-II trial.
Allegro Diagnostics, which developed the BronchoGen test, reports that it plans to commercialize it for use with bronchoscopy beginning in 2013.
This study was sponsored by Allegro Diagnostics. Dr. Whitney is an employee of the company, and had no other disclosures to report.
ATLANTA – The combined use of a novel bronchial airway gene-expression test and bronchoscopy improves the ability to rule out lung cancer in patients with benign disease, compared with bronchoscopy alone, according to findings from the prospective case-controlled AEGIS-1 trial.
Because bronchoscopy, which plays a central role in lung cancer diagnosis, has varying diagnostic yield based on factors such as the size and location of the lesion, the method used to collect cells, and pathological processing methods, the findings suggest that the test (BronchoGen) could minimize the need for additional invasive procedures in these patients, Duncan Whitney, Ph.D., said at the annual meeting of the American College of Chest Physicians.
The AEGIS-1 (Airway Epithelium Gene Expression in the Diagnosis of Lung Cancer 1) trial included more than 700 current or former smokers undergoing bronchoscopy for suspicion of lung cancer. It was designed to evaluate the diagnostic accuracy of the genomic test, which detects gene expression of cytologically normal bronchial airway epithelial cells.
The investigators collected mainstem bronchial airway brushings from 330 patients, including 240 with confirmed primary lung cancer and 90 controls, and performed microarray analysis. The sample set was then split into an independent training sample of 220 cases and a test set of 110 cases, and the gene-expression prediction model was optimized.
Next, reverse transcription polymerase chain reaction (RT-PCR) assays were developed for candidate biomarker genes, and a multivariate test algorithm was reoptimized using the RT-PCR data generated by a reanalysis of 153 of the samples from cancer patients and 64 of the samples from controls, explained Dr. Whitney.
The test, which ultimately focused on 30 genes, yielded a sensitivity of 77%, compared with 74% for bronchoscopy alone. The combined use of the test and bronchoscopy yielded a sensitivity of 96% and a specificity of 73%.
The negative predictive value of the combined test also was better than that of bronchoscopy alone (0.85 vs. 0.65, respectively), said Dr. Whitney.
"So in essence, we’re reducing the false negative rate from 26% down to 4%," he said.
The BronchoGen test was developed based on the airway "field of injury" principle, which refers to the common molecular response that occurs throughout the respiratory tract in current and former smokers with lung cancer, Dr. Whitney explained.
These changes are detected in a gene-expression signature from normal airway cells, even decades after smoking cessation, he noted.
Despite tremendous work done in this area, which has dramatically improved the diagnostic yield and sensitivity of bronchoscopy in the past few years, the procedure is either inconclusive or not diagnostic in up to 50% of cases, he said.
Thus, a "fairly large need" exists in the medical community, given that about 300,000 bronchoscopy procedures are performed each year for suspicion of lung cancer, he added.
Complete results from AEGIS-1, as well as clinical validation of the BronchoGen test, are expected to be released later this year. An additional 1,300 patients have been enrolled in the ongoing AEGIS-II trial.
Allegro Diagnostics, which developed the BronchoGen test, reports that it plans to commercialize it for use with bronchoscopy beginning in 2013.
This study was sponsored by Allegro Diagnostics. Dr. Whitney is an employee of the company, and had no other disclosures to report.
ATLANTA – The combined use of a novel bronchial airway gene-expression test and bronchoscopy improves the ability to rule out lung cancer in patients with benign disease, compared with bronchoscopy alone, according to findings from the prospective case-controlled AEGIS-1 trial.
Because bronchoscopy, which plays a central role in lung cancer diagnosis, has varying diagnostic yield based on factors such as the size and location of the lesion, the method used to collect cells, and pathological processing methods, the findings suggest that the test (BronchoGen) could minimize the need for additional invasive procedures in these patients, Duncan Whitney, Ph.D., said at the annual meeting of the American College of Chest Physicians.
The AEGIS-1 (Airway Epithelium Gene Expression in the Diagnosis of Lung Cancer 1) trial included more than 700 current or former smokers undergoing bronchoscopy for suspicion of lung cancer. It was designed to evaluate the diagnostic accuracy of the genomic test, which detects gene expression of cytologically normal bronchial airway epithelial cells.
The investigators collected mainstem bronchial airway brushings from 330 patients, including 240 with confirmed primary lung cancer and 90 controls, and performed microarray analysis. The sample set was then split into an independent training sample of 220 cases and a test set of 110 cases, and the gene-expression prediction model was optimized.
Next, reverse transcription polymerase chain reaction (RT-PCR) assays were developed for candidate biomarker genes, and a multivariate test algorithm was reoptimized using the RT-PCR data generated by a reanalysis of 153 of the samples from cancer patients and 64 of the samples from controls, explained Dr. Whitney.
The test, which ultimately focused on 30 genes, yielded a sensitivity of 77%, compared with 74% for bronchoscopy alone. The combined use of the test and bronchoscopy yielded a sensitivity of 96% and a specificity of 73%.
The negative predictive value of the combined test also was better than that of bronchoscopy alone (0.85 vs. 0.65, respectively), said Dr. Whitney.
"So in essence, we’re reducing the false negative rate from 26% down to 4%," he said.
The BronchoGen test was developed based on the airway "field of injury" principle, which refers to the common molecular response that occurs throughout the respiratory tract in current and former smokers with lung cancer, Dr. Whitney explained.
These changes are detected in a gene-expression signature from normal airway cells, even decades after smoking cessation, he noted.
Despite tremendous work done in this area, which has dramatically improved the diagnostic yield and sensitivity of bronchoscopy in the past few years, the procedure is either inconclusive or not diagnostic in up to 50% of cases, he said.
Thus, a "fairly large need" exists in the medical community, given that about 300,000 bronchoscopy procedures are performed each year for suspicion of lung cancer, he added.
Complete results from AEGIS-1, as well as clinical validation of the BronchoGen test, are expected to be released later this year. An additional 1,300 patients have been enrolled in the ongoing AEGIS-II trial.
Allegro Diagnostics, which developed the BronchoGen test, reports that it plans to commercialize it for use with bronchoscopy beginning in 2013.
This study was sponsored by Allegro Diagnostics. Dr. Whitney is an employee of the company, and had no other disclosures to report.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CHEST PHYSICIANS
Major Finding: The test, which focused on 30 genes, yielded a sensitivity of 77% for lung cancer, compared with 74% for bronchoscopy alone. The combined use of the test and bronchoscopy yielded a sensitivity of 96% and a specificity of 73%.
Data Source: This was a prospective case-controlled trial (AEGIS-1).
Disclosures: This study was sponsored by Allegro Diagnostics. Dr. Whitney is an employee of the company, and had no other disclosures to report.