User login
Six factors predicted benefit from asthma triple therapy
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
REPORTING FROM ERS 2019
Multicenter trial backs pirfenidone for unclassifiable interstitial lung disease
MADRID – according to results of a late breaker, placebo-controlled, multinational trial presented at the annual congress of the European Respiratory Society.
For preservation of lung function as monitored with forced vital capacity (FVC), pirfenidone provided a large and highly statistically significant advantage over placebo in a phase 2 trial that randomized 253 uILD patients to 2,403 mg pirfenidone or placebo, according to Toby M. Maher, MD, head of the Fibrosis Research Group for the National Heart and Lung Institute, Imperial College, London.
At 24 weeks, FVC lung function declined by just 17.8 mL in the pirfenidone group vs. 113 mL in the placebo group (P = .002). The results, published simultaneously with Dr. Maher’s ERS presentation in The Lancet Respiratory Medicine, are particularly encouraging because there are no currently approved treatments for uILD, according to Dr. Maher.
However, the data from this study, even though it was double blind and involved 70 participating centers in 14 countries, come with an asterisk. The significant FVC advantage was documented with in-hospital measurements, but this was a secondary, not the primary, endpoint. Measurements with hand-held spirometry, which was the primary endpoint, proved to be uninterpretable due to intra-individual variability.
“We had hoped that daily home spirometry would give us more information of the patient’s trajectory over time,” said Dr. Maher, who blames himself for selecting hand-held device measurements as the primary endpoint. In the end, the variability in the home hand-held spirometry data prevented the planned statistical testing.
“There were issues with the hand-held devices we had not anticipated,” Dr. Maher reported. However, hospital-based measurement, which has long been the “regulatory standard” in ILD trials “supports the conclusion that pirfenidone was effective.”
The conclusion is also supported by other secondary outcomes and analyses. For example, the categorical declines in FVC of greater than 5% (37.0% vs. 58.7%; P = .001) and greater than 10% (14.2% vs. 27.9%; P = .011) both favored pirfenidone. There were no between-group differences in progression-free survival at 24 weeks, but events were low in both study arms over this time period.
There was evidence of functional benefit for pirfenidone relative to placebo, such as a smaller decline in the 6-minute walk test (–2 vs. –26.7 M, P = .04). Treatment favoring pirfenidone over placebo was observed across subgroups defined by age, gender, baseline lung function, and presence or absence of interstitial pneumonia with autoimmune features.
Pirfenidone was generally well tolerated with side effects similar to those reported in other studies. The rate of treatment-related discontinuation was 12.6% on pirfenidone versus 0.8% on placebo. The most frequent adverse events, all of which were more common in the pirfenidone group, were gastrointestinal complaints (47.2% vs. 25.8%), rash (10.2% vs. 7.3%), and dizziness (7.9% vs. 0.8%). Rates of photosensitivity were higher in the experimental arm (7.9% vs. 1.8%), but low relative to previous studies, potentially because of greater emphasis on sun protection, Dr. Maher reported.
About 10%-15% of patients with ILD have an unclassifiable type, he noted. Although it is possible for uILD to be a missed diagnosis of an established ILD type, Dr. Maher reported that participating centers for this study were specifically selected for their expertise in ILD. He noted that more than 45% of patients were deemed uILD on the basis of biopsy.
The ERS-invited discussant of this trial, Martin Kolb, MD, professor of respirology, McMaster University, Hamilton, Ont., called the data “strong.” He suggested the data are particularly encouraging in the context of the lack of approved therapies for uILD.
Despite the fact that benefit of pirfenidone was not established on the primary endpoint, Dr. Maher contended that this is a positive study that can be used to design future investigations. “When we use the normal standard endpoint for the study, we see a clear benefit of pirfenidone over placebo.”
Dr. Maher reported no potential conflicts of interest.
SOURCE: Maher TM et al. Lancet Respir Med. 2019 Sep 29. doi: 10.1016/S2213-2600(19)30341-8.
MADRID – according to results of a late breaker, placebo-controlled, multinational trial presented at the annual congress of the European Respiratory Society.
For preservation of lung function as monitored with forced vital capacity (FVC), pirfenidone provided a large and highly statistically significant advantage over placebo in a phase 2 trial that randomized 253 uILD patients to 2,403 mg pirfenidone or placebo, according to Toby M. Maher, MD, head of the Fibrosis Research Group for the National Heart and Lung Institute, Imperial College, London.
At 24 weeks, FVC lung function declined by just 17.8 mL in the pirfenidone group vs. 113 mL in the placebo group (P = .002). The results, published simultaneously with Dr. Maher’s ERS presentation in The Lancet Respiratory Medicine, are particularly encouraging because there are no currently approved treatments for uILD, according to Dr. Maher.
However, the data from this study, even though it was double blind and involved 70 participating centers in 14 countries, come with an asterisk. The significant FVC advantage was documented with in-hospital measurements, but this was a secondary, not the primary, endpoint. Measurements with hand-held spirometry, which was the primary endpoint, proved to be uninterpretable due to intra-individual variability.
“We had hoped that daily home spirometry would give us more information of the patient’s trajectory over time,” said Dr. Maher, who blames himself for selecting hand-held device measurements as the primary endpoint. In the end, the variability in the home hand-held spirometry data prevented the planned statistical testing.
“There were issues with the hand-held devices we had not anticipated,” Dr. Maher reported. However, hospital-based measurement, which has long been the “regulatory standard” in ILD trials “supports the conclusion that pirfenidone was effective.”
The conclusion is also supported by other secondary outcomes and analyses. For example, the categorical declines in FVC of greater than 5% (37.0% vs. 58.7%; P = .001) and greater than 10% (14.2% vs. 27.9%; P = .011) both favored pirfenidone. There were no between-group differences in progression-free survival at 24 weeks, but events were low in both study arms over this time period.
There was evidence of functional benefit for pirfenidone relative to placebo, such as a smaller decline in the 6-minute walk test (–2 vs. –26.7 M, P = .04). Treatment favoring pirfenidone over placebo was observed across subgroups defined by age, gender, baseline lung function, and presence or absence of interstitial pneumonia with autoimmune features.
Pirfenidone was generally well tolerated with side effects similar to those reported in other studies. The rate of treatment-related discontinuation was 12.6% on pirfenidone versus 0.8% on placebo. The most frequent adverse events, all of which were more common in the pirfenidone group, were gastrointestinal complaints (47.2% vs. 25.8%), rash (10.2% vs. 7.3%), and dizziness (7.9% vs. 0.8%). Rates of photosensitivity were higher in the experimental arm (7.9% vs. 1.8%), but low relative to previous studies, potentially because of greater emphasis on sun protection, Dr. Maher reported.
About 10%-15% of patients with ILD have an unclassifiable type, he noted. Although it is possible for uILD to be a missed diagnosis of an established ILD type, Dr. Maher reported that participating centers for this study were specifically selected for their expertise in ILD. He noted that more than 45% of patients were deemed uILD on the basis of biopsy.
The ERS-invited discussant of this trial, Martin Kolb, MD, professor of respirology, McMaster University, Hamilton, Ont., called the data “strong.” He suggested the data are particularly encouraging in the context of the lack of approved therapies for uILD.
Despite the fact that benefit of pirfenidone was not established on the primary endpoint, Dr. Maher contended that this is a positive study that can be used to design future investigations. “When we use the normal standard endpoint for the study, we see a clear benefit of pirfenidone over placebo.”
Dr. Maher reported no potential conflicts of interest.
SOURCE: Maher TM et al. Lancet Respir Med. 2019 Sep 29. doi: 10.1016/S2213-2600(19)30341-8.
MADRID – according to results of a late breaker, placebo-controlled, multinational trial presented at the annual congress of the European Respiratory Society.
For preservation of lung function as monitored with forced vital capacity (FVC), pirfenidone provided a large and highly statistically significant advantage over placebo in a phase 2 trial that randomized 253 uILD patients to 2,403 mg pirfenidone or placebo, according to Toby M. Maher, MD, head of the Fibrosis Research Group for the National Heart and Lung Institute, Imperial College, London.
At 24 weeks, FVC lung function declined by just 17.8 mL in the pirfenidone group vs. 113 mL in the placebo group (P = .002). The results, published simultaneously with Dr. Maher’s ERS presentation in The Lancet Respiratory Medicine, are particularly encouraging because there are no currently approved treatments for uILD, according to Dr. Maher.
However, the data from this study, even though it was double blind and involved 70 participating centers in 14 countries, come with an asterisk. The significant FVC advantage was documented with in-hospital measurements, but this was a secondary, not the primary, endpoint. Measurements with hand-held spirometry, which was the primary endpoint, proved to be uninterpretable due to intra-individual variability.
“We had hoped that daily home spirometry would give us more information of the patient’s trajectory over time,” said Dr. Maher, who blames himself for selecting hand-held device measurements as the primary endpoint. In the end, the variability in the home hand-held spirometry data prevented the planned statistical testing.
“There were issues with the hand-held devices we had not anticipated,” Dr. Maher reported. However, hospital-based measurement, which has long been the “regulatory standard” in ILD trials “supports the conclusion that pirfenidone was effective.”
The conclusion is also supported by other secondary outcomes and analyses. For example, the categorical declines in FVC of greater than 5% (37.0% vs. 58.7%; P = .001) and greater than 10% (14.2% vs. 27.9%; P = .011) both favored pirfenidone. There were no between-group differences in progression-free survival at 24 weeks, but events were low in both study arms over this time period.
There was evidence of functional benefit for pirfenidone relative to placebo, such as a smaller decline in the 6-minute walk test (–2 vs. –26.7 M, P = .04). Treatment favoring pirfenidone over placebo was observed across subgroups defined by age, gender, baseline lung function, and presence or absence of interstitial pneumonia with autoimmune features.
Pirfenidone was generally well tolerated with side effects similar to those reported in other studies. The rate of treatment-related discontinuation was 12.6% on pirfenidone versus 0.8% on placebo. The most frequent adverse events, all of which were more common in the pirfenidone group, were gastrointestinal complaints (47.2% vs. 25.8%), rash (10.2% vs. 7.3%), and dizziness (7.9% vs. 0.8%). Rates of photosensitivity were higher in the experimental arm (7.9% vs. 1.8%), but low relative to previous studies, potentially because of greater emphasis on sun protection, Dr. Maher reported.
About 10%-15% of patients with ILD have an unclassifiable type, he noted. Although it is possible for uILD to be a missed diagnosis of an established ILD type, Dr. Maher reported that participating centers for this study were specifically selected for their expertise in ILD. He noted that more than 45% of patients were deemed uILD on the basis of biopsy.
The ERS-invited discussant of this trial, Martin Kolb, MD, professor of respirology, McMaster University, Hamilton, Ont., called the data “strong.” He suggested the data are particularly encouraging in the context of the lack of approved therapies for uILD.
Despite the fact that benefit of pirfenidone was not established on the primary endpoint, Dr. Maher contended that this is a positive study that can be used to design future investigations. “When we use the normal standard endpoint for the study, we see a clear benefit of pirfenidone over placebo.”
Dr. Maher reported no potential conflicts of interest.
SOURCE: Maher TM et al. Lancet Respir Med. 2019 Sep 29. doi: 10.1016/S2213-2600(19)30341-8.
REPORTING FROM ERS 2019
TKI preserved lung function in patients with fibrosing interstitial disease
MADRID – according to findings from a phase 3, placebo-controlled trial presented at the annual congress of the European Respiratory Society.
The trial, called INBUILD, enrolled patients who had a progressive lung disease with a fibrosing phenotype, such as interstitial pneumonia with autoimmune features (IPAF) or noninterstitial pneumonia (NSIP), on the premise that these conditions might share a pathology responsive to a common therapy, explained Kevin R. Flaherty, MD, of National Jewish Health, Denver. The INBUILD trial was a randomized, double-blind, placebo-controlled, parallel-group trial conducted at 153 sites in 15 countries. A total of 663 patients underwent randomization and received at least one dose of nintedanib (332) or placebo (331).
Patients with fibrosing lung disease affecting more than 10% of lung volume were randomized to 150 mg twice daily of nintedanib, which inhibits intracellular growth factors implicated in fibrosis and is already indicated for IPF, or matching placebo.
On the primary endpoint of change in forced vital capacity (FVC) at 52 weeks, those in the nintedanib arm lost lung function at a rate that was less than half that of those randomized to placebo (–80.8 vs. –187.8 mL/year; P less than .001).
In a preplanned stratification, the protection from nintedanib against a decline in lung function was found to be at least as good in those with a usual interstitial pneumonia (UIP-like) pattern of fibrosis on baseline imaging (–82.9 vs. –211.1 mL/year), compared with those with other fibrotic patterns (–79.0 vs. –154.2 mL/year). The UIP-like subgroup represented about 60% of those enrolled.
“The relative protection from decline in lung function supports the hypothesis that progressive fibrosing interstitial lung diseases have a similar pathobiologic mechanism,” said Dr. Flaherty. Results from the INBUILD were published simultaneously with his ERS presentation (N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681).
The curves documenting change of lung function in favor of nintedanib relative to placebo separated within 12 weeks of treatment initiation, according to Dr. Flaherty. The ERS-invited discussant, Martin Kolb, MD, PhD, professor of respirology, McMaster University, Hamilton, Ont., called the reductions in loss of lung function “profound” and “very impactful.”
However, despite these reductions, there was no significant difference in quality of life as measured with the King’s Brief Interstitial Lung Disease (KBILD) questionnaire, which was a secondary outcome. The problem was that there was little change in KBILD in either group at 52 weeks, limiting the ability to show differences.
The rates of death were numerically lower at 52 weeks in the nintedanib arm for the study overall (4.8% vs. 5.1%) and for the UIP-like subgroup (5.3% vs. 7.8%), but the differences did not reach statistical significance.
A suggestion of benefit was derived from a design feature of INBUILD that called for patients to remain on blinded therapy until all enrolled patients completed the trial. When the effect of nintedanib was evaluated in this extended analysis, the event curves for the combined endpoint of interstitial lung disease or death separated and approached significance.
In this extended analysis, which suggests that clinical benefit is likely to accrue after longer periods of treatment, “we saw similar trends when we looked at mortality as an independent outcome,” Dr. Flaherty reported.
More patients in the nintedanib group discontinued therapy because of adverse events (19.6% vs. 10.3%), but Dr. Flaherty characterized the rate of serious adverse events as “similar.” He made this statement even though several adverse events, particularly those involving the gastrointestinal tract, such as diarrhea (66.9% vs. 23.9%), nausea (28.9% vs. 9.4%), vomiting (18.4% vs. 5.1%), and abdominal pain (10.2% vs. 2.4%), were higher in the nintedanib arm.
The INBUILD trial demonstrates that nintedanib preserves lung function in fibrosing lung diseases other than IPF. In his review of this paper, Dr. Kolb pointed out that non-IPF etiologies represent about 75% of interstitial lung diseases. For these patients “we have no drugs, so there is a big medical need.”
Dr. Flaherty reports no potential conflicts of interest. The study was funded by Boehringer-Ingelheim, which produces nintedanib.
SOURCE: Flaherty KR et al. N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681.
MADRID – according to findings from a phase 3, placebo-controlled trial presented at the annual congress of the European Respiratory Society.
The trial, called INBUILD, enrolled patients who had a progressive lung disease with a fibrosing phenotype, such as interstitial pneumonia with autoimmune features (IPAF) or noninterstitial pneumonia (NSIP), on the premise that these conditions might share a pathology responsive to a common therapy, explained Kevin R. Flaherty, MD, of National Jewish Health, Denver. The INBUILD trial was a randomized, double-blind, placebo-controlled, parallel-group trial conducted at 153 sites in 15 countries. A total of 663 patients underwent randomization and received at least one dose of nintedanib (332) or placebo (331).
Patients with fibrosing lung disease affecting more than 10% of lung volume were randomized to 150 mg twice daily of nintedanib, which inhibits intracellular growth factors implicated in fibrosis and is already indicated for IPF, or matching placebo.
On the primary endpoint of change in forced vital capacity (FVC) at 52 weeks, those in the nintedanib arm lost lung function at a rate that was less than half that of those randomized to placebo (–80.8 vs. –187.8 mL/year; P less than .001).
In a preplanned stratification, the protection from nintedanib against a decline in lung function was found to be at least as good in those with a usual interstitial pneumonia (UIP-like) pattern of fibrosis on baseline imaging (–82.9 vs. –211.1 mL/year), compared with those with other fibrotic patterns (–79.0 vs. –154.2 mL/year). The UIP-like subgroup represented about 60% of those enrolled.
“The relative protection from decline in lung function supports the hypothesis that progressive fibrosing interstitial lung diseases have a similar pathobiologic mechanism,” said Dr. Flaherty. Results from the INBUILD were published simultaneously with his ERS presentation (N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681).
The curves documenting change of lung function in favor of nintedanib relative to placebo separated within 12 weeks of treatment initiation, according to Dr. Flaherty. The ERS-invited discussant, Martin Kolb, MD, PhD, professor of respirology, McMaster University, Hamilton, Ont., called the reductions in loss of lung function “profound” and “very impactful.”
However, despite these reductions, there was no significant difference in quality of life as measured with the King’s Brief Interstitial Lung Disease (KBILD) questionnaire, which was a secondary outcome. The problem was that there was little change in KBILD in either group at 52 weeks, limiting the ability to show differences.
The rates of death were numerically lower at 52 weeks in the nintedanib arm for the study overall (4.8% vs. 5.1%) and for the UIP-like subgroup (5.3% vs. 7.8%), but the differences did not reach statistical significance.
A suggestion of benefit was derived from a design feature of INBUILD that called for patients to remain on blinded therapy until all enrolled patients completed the trial. When the effect of nintedanib was evaluated in this extended analysis, the event curves for the combined endpoint of interstitial lung disease or death separated and approached significance.
In this extended analysis, which suggests that clinical benefit is likely to accrue after longer periods of treatment, “we saw similar trends when we looked at mortality as an independent outcome,” Dr. Flaherty reported.
More patients in the nintedanib group discontinued therapy because of adverse events (19.6% vs. 10.3%), but Dr. Flaherty characterized the rate of serious adverse events as “similar.” He made this statement even though several adverse events, particularly those involving the gastrointestinal tract, such as diarrhea (66.9% vs. 23.9%), nausea (28.9% vs. 9.4%), vomiting (18.4% vs. 5.1%), and abdominal pain (10.2% vs. 2.4%), were higher in the nintedanib arm.
The INBUILD trial demonstrates that nintedanib preserves lung function in fibrosing lung diseases other than IPF. In his review of this paper, Dr. Kolb pointed out that non-IPF etiologies represent about 75% of interstitial lung diseases. For these patients “we have no drugs, so there is a big medical need.”
Dr. Flaherty reports no potential conflicts of interest. The study was funded by Boehringer-Ingelheim, which produces nintedanib.
SOURCE: Flaherty KR et al. N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681.
MADRID – according to findings from a phase 3, placebo-controlled trial presented at the annual congress of the European Respiratory Society.
The trial, called INBUILD, enrolled patients who had a progressive lung disease with a fibrosing phenotype, such as interstitial pneumonia with autoimmune features (IPAF) or noninterstitial pneumonia (NSIP), on the premise that these conditions might share a pathology responsive to a common therapy, explained Kevin R. Flaherty, MD, of National Jewish Health, Denver. The INBUILD trial was a randomized, double-blind, placebo-controlled, parallel-group trial conducted at 153 sites in 15 countries. A total of 663 patients underwent randomization and received at least one dose of nintedanib (332) or placebo (331).
Patients with fibrosing lung disease affecting more than 10% of lung volume were randomized to 150 mg twice daily of nintedanib, which inhibits intracellular growth factors implicated in fibrosis and is already indicated for IPF, or matching placebo.
On the primary endpoint of change in forced vital capacity (FVC) at 52 weeks, those in the nintedanib arm lost lung function at a rate that was less than half that of those randomized to placebo (–80.8 vs. –187.8 mL/year; P less than .001).
In a preplanned stratification, the protection from nintedanib against a decline in lung function was found to be at least as good in those with a usual interstitial pneumonia (UIP-like) pattern of fibrosis on baseline imaging (–82.9 vs. –211.1 mL/year), compared with those with other fibrotic patterns (–79.0 vs. –154.2 mL/year). The UIP-like subgroup represented about 60% of those enrolled.
“The relative protection from decline in lung function supports the hypothesis that progressive fibrosing interstitial lung diseases have a similar pathobiologic mechanism,” said Dr. Flaherty. Results from the INBUILD were published simultaneously with his ERS presentation (N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681).
The curves documenting change of lung function in favor of nintedanib relative to placebo separated within 12 weeks of treatment initiation, according to Dr. Flaherty. The ERS-invited discussant, Martin Kolb, MD, PhD, professor of respirology, McMaster University, Hamilton, Ont., called the reductions in loss of lung function “profound” and “very impactful.”
However, despite these reductions, there was no significant difference in quality of life as measured with the King’s Brief Interstitial Lung Disease (KBILD) questionnaire, which was a secondary outcome. The problem was that there was little change in KBILD in either group at 52 weeks, limiting the ability to show differences.
The rates of death were numerically lower at 52 weeks in the nintedanib arm for the study overall (4.8% vs. 5.1%) and for the UIP-like subgroup (5.3% vs. 7.8%), but the differences did not reach statistical significance.
A suggestion of benefit was derived from a design feature of INBUILD that called for patients to remain on blinded therapy until all enrolled patients completed the trial. When the effect of nintedanib was evaluated in this extended analysis, the event curves for the combined endpoint of interstitial lung disease or death separated and approached significance.
In this extended analysis, which suggests that clinical benefit is likely to accrue after longer periods of treatment, “we saw similar trends when we looked at mortality as an independent outcome,” Dr. Flaherty reported.
More patients in the nintedanib group discontinued therapy because of adverse events (19.6% vs. 10.3%), but Dr. Flaherty characterized the rate of serious adverse events as “similar.” He made this statement even though several adverse events, particularly those involving the gastrointestinal tract, such as diarrhea (66.9% vs. 23.9%), nausea (28.9% vs. 9.4%), vomiting (18.4% vs. 5.1%), and abdominal pain (10.2% vs. 2.4%), were higher in the nintedanib arm.
The INBUILD trial demonstrates that nintedanib preserves lung function in fibrosing lung diseases other than IPF. In his review of this paper, Dr. Kolb pointed out that non-IPF etiologies represent about 75% of interstitial lung diseases. For these patients “we have no drugs, so there is a big medical need.”
Dr. Flaherty reports no potential conflicts of interest. The study was funded by Boehringer-Ingelheim, which produces nintedanib.
SOURCE: Flaherty KR et al. N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681.
REPORTING FROM ERS 2019
Still no standard for HS, but new options increase chance of control
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
REPORTING FROM SOC 2019
Coming acne drugs might particularly benefit skin of color patients
NEW YORK – The most recently approved therapy for acne, sarecycline, as well as several agents in late stages of clinical testing, might represent a particular advance for treating black patients or others with darker skin tones due to a reduced risk of irritation, according to a review presented at the Skin of Color Update 2019.
a complication many consider worse than the acne itself, according to Andrew Alexis, MD, director of the Skin of Color Center and chair of the department of dermatology at Mount Sinai St. Luke’s, New York.
“The importance of PIH is that it alters our endpoint in patients of color. Not only are we treating the pustules, comedones, and other classic features of acne, but we have to treat all the way through to the resolution of the PIH if we want a satisfied patient,” he said.
There are data to back this up. In one of the surveys cited by Dr. Alexis, 42% of nonwhite patients identified resolution of PIH as the most important goal in the treatment of their acne.
As in those with light skin, acute acne lesions in darker skin can resolve relatively rapidly after initiating an effective regimen that includes established therapies such as retinoids or antibiotics. However, PIH, once it develops, might take 6-12 months to resolve, according to Dr. Alexis, who is a professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“You have to keep in mind the subclinical inflammation, which can be a slow burning process beneath the surface of the skin,” he said. He cited a biopsy study that demonstrated inflammation even in nonlesional skin of black patients with acne.
Because of the slow reversal of PIH, it is imperative in skin of color patients to employ therapies with the least risk of exacerbating PIH. While this includes judicious use of currently available agents, Dr. Alexis believes that newer agents might have a larger therapeutic window, reducing the potential for inflammation at effective doses.
This advantage has yet to be confirmed in head-to-head studies, but Dr. Alexis is optimistic. In the case of sarecycline, which became the first antibiotic approved specifically for acne when it was approved by the Food and Drug Administration in 2018, about 20% of those included in the phase 3 registration trial were nonwhite, he said.
The results were “impressive” regardless of skin color in the phase 3 study, according to Dr. Alexis. He conceded that this is not the only antibiotic with anti-inflammatory activity, but he suggested that a high degree of efficacy might be relevant for early acne control and a reduced risk of PIH.
The same can be said for trifarotene, a novel topical retinoid that was associated with highly significant reductions in both inflammatory and noninflammatory lesion counts in a recently published phase 3 trial (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9). According to Dr. Alexis, the impact of this therapy on PIH has not been specifically tested, but he expects those data to be forthcoming.
A new 0.045% lotion formulation of tazarotene might also widen the therapeutic window relative to current tazarotene formulations based on clinical trials he cited. Despite a concentration that is about half that of the currently available tazarotene cream, the efficacy of this product appeared to be at least as good “without the baggage of a greater potential for irritation,” he said.
After “a few years of drought” regarding new options for treatment of acne, these are not the only promising agents in clinical trials, according to Dr. Alexis. If these agents prove to offer greater efficacy with less irritation, their increased clinical value might prove most meaningful to patients with darker skin.
“There is a delicate balance between maximizing efficacy without causing irritation that leads to PIH in patients with skin of color,” he cautioned. He is hopeful that the newer agents will make this balance easier to achieve.
Dr. Alexis has financial relationships with many pharmaceutical companies, including many that market drugs for acne.
NEW YORK – The most recently approved therapy for acne, sarecycline, as well as several agents in late stages of clinical testing, might represent a particular advance for treating black patients or others with darker skin tones due to a reduced risk of irritation, according to a review presented at the Skin of Color Update 2019.
a complication many consider worse than the acne itself, according to Andrew Alexis, MD, director of the Skin of Color Center and chair of the department of dermatology at Mount Sinai St. Luke’s, New York.
“The importance of PIH is that it alters our endpoint in patients of color. Not only are we treating the pustules, comedones, and other classic features of acne, but we have to treat all the way through to the resolution of the PIH if we want a satisfied patient,” he said.
There are data to back this up. In one of the surveys cited by Dr. Alexis, 42% of nonwhite patients identified resolution of PIH as the most important goal in the treatment of their acne.
As in those with light skin, acute acne lesions in darker skin can resolve relatively rapidly after initiating an effective regimen that includes established therapies such as retinoids or antibiotics. However, PIH, once it develops, might take 6-12 months to resolve, according to Dr. Alexis, who is a professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“You have to keep in mind the subclinical inflammation, which can be a slow burning process beneath the surface of the skin,” he said. He cited a biopsy study that demonstrated inflammation even in nonlesional skin of black patients with acne.
Because of the slow reversal of PIH, it is imperative in skin of color patients to employ therapies with the least risk of exacerbating PIH. While this includes judicious use of currently available agents, Dr. Alexis believes that newer agents might have a larger therapeutic window, reducing the potential for inflammation at effective doses.
This advantage has yet to be confirmed in head-to-head studies, but Dr. Alexis is optimistic. In the case of sarecycline, which became the first antibiotic approved specifically for acne when it was approved by the Food and Drug Administration in 2018, about 20% of those included in the phase 3 registration trial were nonwhite, he said.
The results were “impressive” regardless of skin color in the phase 3 study, according to Dr. Alexis. He conceded that this is not the only antibiotic with anti-inflammatory activity, but he suggested that a high degree of efficacy might be relevant for early acne control and a reduced risk of PIH.
The same can be said for trifarotene, a novel topical retinoid that was associated with highly significant reductions in both inflammatory and noninflammatory lesion counts in a recently published phase 3 trial (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9). According to Dr. Alexis, the impact of this therapy on PIH has not been specifically tested, but he expects those data to be forthcoming.
A new 0.045% lotion formulation of tazarotene might also widen the therapeutic window relative to current tazarotene formulations based on clinical trials he cited. Despite a concentration that is about half that of the currently available tazarotene cream, the efficacy of this product appeared to be at least as good “without the baggage of a greater potential for irritation,” he said.
After “a few years of drought” regarding new options for treatment of acne, these are not the only promising agents in clinical trials, according to Dr. Alexis. If these agents prove to offer greater efficacy with less irritation, their increased clinical value might prove most meaningful to patients with darker skin.
“There is a delicate balance between maximizing efficacy without causing irritation that leads to PIH in patients with skin of color,” he cautioned. He is hopeful that the newer agents will make this balance easier to achieve.
Dr. Alexis has financial relationships with many pharmaceutical companies, including many that market drugs for acne.
NEW YORK – The most recently approved therapy for acne, sarecycline, as well as several agents in late stages of clinical testing, might represent a particular advance for treating black patients or others with darker skin tones due to a reduced risk of irritation, according to a review presented at the Skin of Color Update 2019.
a complication many consider worse than the acne itself, according to Andrew Alexis, MD, director of the Skin of Color Center and chair of the department of dermatology at Mount Sinai St. Luke’s, New York.
“The importance of PIH is that it alters our endpoint in patients of color. Not only are we treating the pustules, comedones, and other classic features of acne, but we have to treat all the way through to the resolution of the PIH if we want a satisfied patient,” he said.
There are data to back this up. In one of the surveys cited by Dr. Alexis, 42% of nonwhite patients identified resolution of PIH as the most important goal in the treatment of their acne.
As in those with light skin, acute acne lesions in darker skin can resolve relatively rapidly after initiating an effective regimen that includes established therapies such as retinoids or antibiotics. However, PIH, once it develops, might take 6-12 months to resolve, according to Dr. Alexis, who is a professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“You have to keep in mind the subclinical inflammation, which can be a slow burning process beneath the surface of the skin,” he said. He cited a biopsy study that demonstrated inflammation even in nonlesional skin of black patients with acne.
Because of the slow reversal of PIH, it is imperative in skin of color patients to employ therapies with the least risk of exacerbating PIH. While this includes judicious use of currently available agents, Dr. Alexis believes that newer agents might have a larger therapeutic window, reducing the potential for inflammation at effective doses.
This advantage has yet to be confirmed in head-to-head studies, but Dr. Alexis is optimistic. In the case of sarecycline, which became the first antibiotic approved specifically for acne when it was approved by the Food and Drug Administration in 2018, about 20% of those included in the phase 3 registration trial were nonwhite, he said.
The results were “impressive” regardless of skin color in the phase 3 study, according to Dr. Alexis. He conceded that this is not the only antibiotic with anti-inflammatory activity, but he suggested that a high degree of efficacy might be relevant for early acne control and a reduced risk of PIH.
The same can be said for trifarotene, a novel topical retinoid that was associated with highly significant reductions in both inflammatory and noninflammatory lesion counts in a recently published phase 3 trial (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9). According to Dr. Alexis, the impact of this therapy on PIH has not been specifically tested, but he expects those data to be forthcoming.
A new 0.045% lotion formulation of tazarotene might also widen the therapeutic window relative to current tazarotene formulations based on clinical trials he cited. Despite a concentration that is about half that of the currently available tazarotene cream, the efficacy of this product appeared to be at least as good “without the baggage of a greater potential for irritation,” he said.
After “a few years of drought” regarding new options for treatment of acne, these are not the only promising agents in clinical trials, according to Dr. Alexis. If these agents prove to offer greater efficacy with less irritation, their increased clinical value might prove most meaningful to patients with darker skin.
“There is a delicate balance between maximizing efficacy without causing irritation that leads to PIH in patients with skin of color,” he cautioned. He is hopeful that the newer agents will make this balance easier to achieve.
Dr. Alexis has financial relationships with many pharmaceutical companies, including many that market drugs for acne.
EXPERT ANALYSIS FROM SOC 2019
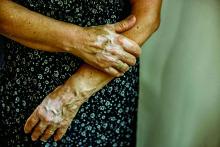
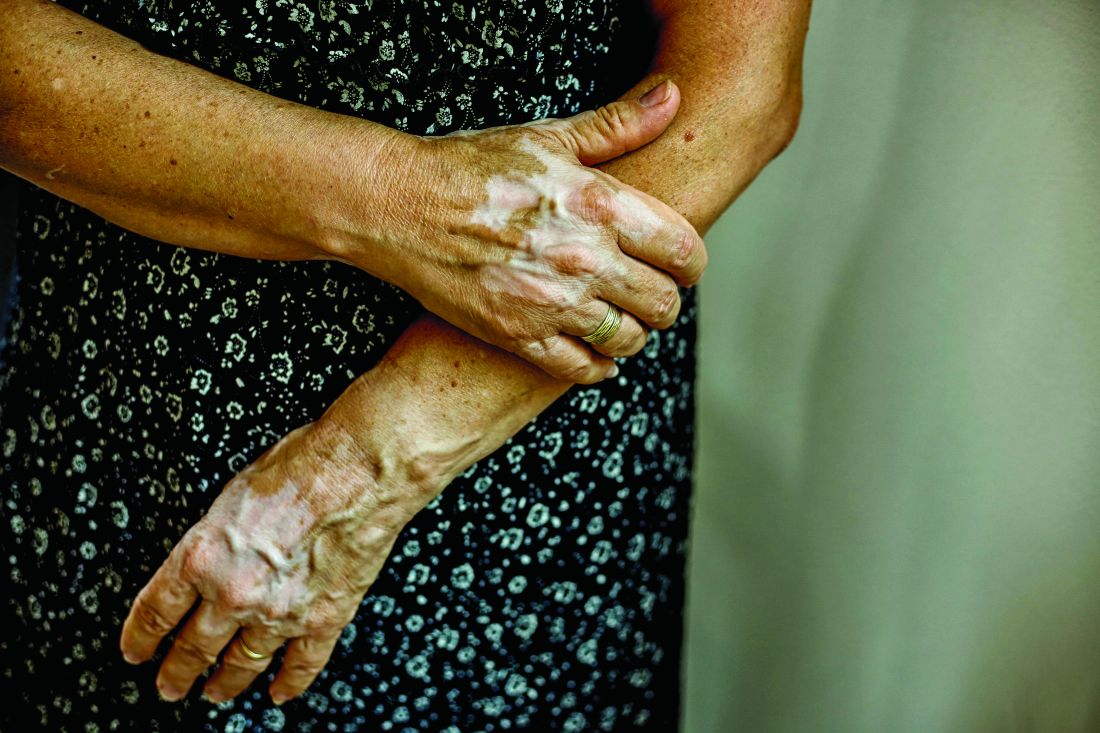
Recent progress in vitiligo treatment might be heading to vitiligo cure
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Dermatologists urged to take ownership of preventable AEs
NEW YORK – according to Galen T. Foulke, MD, who discussed avoidable clinical disasters in practice at the American Academy of Dermatology summer meeting.
Of a list of known and predictable risks that “fall under the purview of ‘You Should Have Known Better,’ ” Dr. Foulke focused on glucocorticoid-associated osteoporosis and infections associated with biologics.
Prior to delivering advice about preventing osteoporosis in patients taking glucocorticoids, he polled the audience about what they considered appropriate prophylaxis in this setting. The vast majority opted for vitamin D and calcium supplementation.
“This is a common answer, but it is the wrong answer,” said Dr. Foulke, a dermatologist affiliated with Penn State Milton S. Hershey Medical Center in Hershey, Pennsylvania.
The available data show that patients on vitamin D and calcium supplementation will continue to lose bone density on therapeutic doses of steroids, according to Dr. Foulke. This is true even when the daily doses for these supplements exceed 800 units and 900 mg, respectively. Moreover, calcium supplementation is associated with an increased risk of heart disease in patients without a calcium deficiency, he noted.
The correct answer is a bisphosphonate, said Dr. Foulke. Of the bisphosphonates, he recommended alendronate as one that is particularly well tolerated and readily reimbursed.
He believes that dermatologists prescribing glucocorticoids should not overlook the substantial risk of osteoporosis or their responsibility to discuss strategies for risk mitigation. Moreover, he believes dermatologists should consider prescribing alendronate in the appropriate candidates, not just refer to another specialist.
“In the first year of steroid use, substantial bone loss is a risk even at doses below 5 mg,” Dr. Foulke warned. At higher doses, patients can lose up to 25% of their bone density, he added.
Of preventable risks of biologics, Dr. Foulke focused on infection. In particular, he urged dermatologists who prescribe these drugs to routinely inform patients about the role and safety of vaccines in infection prevention.
“An immunosuppressant suppresses the immune system, increasing the risk of infection. It is our responsibility to protect patients from the known risks of the therapies we offer them,” he said.
Several organizations recommend the pneumococcal vaccine series and the annual influenza vaccine for patients taking biologics. Although Dr. Foulke was unable to find reliable data on vaccination rates among patients prescribed a biologic for a dermatologic indication, he cited rheumatology practice data to suggest that less than half of patients receive this protection.
A major reason for the low rate of vaccination was failure of the biologic prescriber to assume responsibility for this recommended step, according to Dr. Foulke. Although he does not believe that biologic prescribers need to administer the vaccine, and he acknowledged that he does not stock vaccines in his clinic, he does believe they should inform patients when vaccination is safe and appropriate.
Live attenuated vaccines, in his opinion, are not safe. Although he reported that this is an area of controversy, he takes a conservative approach, advising candidates for a live attenuated vaccine to undergo vaccination prior to starting the biologic or during a break from biologic therapy.
When he polled the audience about which vaccines are live attenuated vaccines, several failed to recognize that the MMR vaccine falls into this category. However, he also noted that the majority of vaccines are not live attenuated and should be considered. He specifically singled out the recombinant herpes zoster as a vaccine recommended by the American College of Rheumatology in patients on biologics.
“We do not have to give the shots, but we should be the ones who start the discussion,” said Dr. Foulke, referring to vaccines in patients on a biologic. He called these important steps “to protect patients from preventable disasters.”
Dr. Foulke reported no potential conflicts of interest.
SOURCE: Summer AAD 2019, Session F02.
NEW YORK – according to Galen T. Foulke, MD, who discussed avoidable clinical disasters in practice at the American Academy of Dermatology summer meeting.
Of a list of known and predictable risks that “fall under the purview of ‘You Should Have Known Better,’ ” Dr. Foulke focused on glucocorticoid-associated osteoporosis and infections associated with biologics.
Prior to delivering advice about preventing osteoporosis in patients taking glucocorticoids, he polled the audience about what they considered appropriate prophylaxis in this setting. The vast majority opted for vitamin D and calcium supplementation.
“This is a common answer, but it is the wrong answer,” said Dr. Foulke, a dermatologist affiliated with Penn State Milton S. Hershey Medical Center in Hershey, Pennsylvania.
The available data show that patients on vitamin D and calcium supplementation will continue to lose bone density on therapeutic doses of steroids, according to Dr. Foulke. This is true even when the daily doses for these supplements exceed 800 units and 900 mg, respectively. Moreover, calcium supplementation is associated with an increased risk of heart disease in patients without a calcium deficiency, he noted.
The correct answer is a bisphosphonate, said Dr. Foulke. Of the bisphosphonates, he recommended alendronate as one that is particularly well tolerated and readily reimbursed.
He believes that dermatologists prescribing glucocorticoids should not overlook the substantial risk of osteoporosis or their responsibility to discuss strategies for risk mitigation. Moreover, he believes dermatologists should consider prescribing alendronate in the appropriate candidates, not just refer to another specialist.
“In the first year of steroid use, substantial bone loss is a risk even at doses below 5 mg,” Dr. Foulke warned. At higher doses, patients can lose up to 25% of their bone density, he added.
Of preventable risks of biologics, Dr. Foulke focused on infection. In particular, he urged dermatologists who prescribe these drugs to routinely inform patients about the role and safety of vaccines in infection prevention.
“An immunosuppressant suppresses the immune system, increasing the risk of infection. It is our responsibility to protect patients from the known risks of the therapies we offer them,” he said.
Several organizations recommend the pneumococcal vaccine series and the annual influenza vaccine for patients taking biologics. Although Dr. Foulke was unable to find reliable data on vaccination rates among patients prescribed a biologic for a dermatologic indication, he cited rheumatology practice data to suggest that less than half of patients receive this protection.
A major reason for the low rate of vaccination was failure of the biologic prescriber to assume responsibility for this recommended step, according to Dr. Foulke. Although he does not believe that biologic prescribers need to administer the vaccine, and he acknowledged that he does not stock vaccines in his clinic, he does believe they should inform patients when vaccination is safe and appropriate.
Live attenuated vaccines, in his opinion, are not safe. Although he reported that this is an area of controversy, he takes a conservative approach, advising candidates for a live attenuated vaccine to undergo vaccination prior to starting the biologic or during a break from biologic therapy.
When he polled the audience about which vaccines are live attenuated vaccines, several failed to recognize that the MMR vaccine falls into this category. However, he also noted that the majority of vaccines are not live attenuated and should be considered. He specifically singled out the recombinant herpes zoster as a vaccine recommended by the American College of Rheumatology in patients on biologics.
“We do not have to give the shots, but we should be the ones who start the discussion,” said Dr. Foulke, referring to vaccines in patients on a biologic. He called these important steps “to protect patients from preventable disasters.”
Dr. Foulke reported no potential conflicts of interest.
SOURCE: Summer AAD 2019, Session F02.
NEW YORK – according to Galen T. Foulke, MD, who discussed avoidable clinical disasters in practice at the American Academy of Dermatology summer meeting.
Of a list of known and predictable risks that “fall under the purview of ‘You Should Have Known Better,’ ” Dr. Foulke focused on glucocorticoid-associated osteoporosis and infections associated with biologics.
Prior to delivering advice about preventing osteoporosis in patients taking glucocorticoids, he polled the audience about what they considered appropriate prophylaxis in this setting. The vast majority opted for vitamin D and calcium supplementation.
“This is a common answer, but it is the wrong answer,” said Dr. Foulke, a dermatologist affiliated with Penn State Milton S. Hershey Medical Center in Hershey, Pennsylvania.
The available data show that patients on vitamin D and calcium supplementation will continue to lose bone density on therapeutic doses of steroids, according to Dr. Foulke. This is true even when the daily doses for these supplements exceed 800 units and 900 mg, respectively. Moreover, calcium supplementation is associated with an increased risk of heart disease in patients without a calcium deficiency, he noted.
The correct answer is a bisphosphonate, said Dr. Foulke. Of the bisphosphonates, he recommended alendronate as one that is particularly well tolerated and readily reimbursed.
He believes that dermatologists prescribing glucocorticoids should not overlook the substantial risk of osteoporosis or their responsibility to discuss strategies for risk mitigation. Moreover, he believes dermatologists should consider prescribing alendronate in the appropriate candidates, not just refer to another specialist.
“In the first year of steroid use, substantial bone loss is a risk even at doses below 5 mg,” Dr. Foulke warned. At higher doses, patients can lose up to 25% of their bone density, he added.
Of preventable risks of biologics, Dr. Foulke focused on infection. In particular, he urged dermatologists who prescribe these drugs to routinely inform patients about the role and safety of vaccines in infection prevention.
“An immunosuppressant suppresses the immune system, increasing the risk of infection. It is our responsibility to protect patients from the known risks of the therapies we offer them,” he said.
Several organizations recommend the pneumococcal vaccine series and the annual influenza vaccine for patients taking biologics. Although Dr. Foulke was unable to find reliable data on vaccination rates among patients prescribed a biologic for a dermatologic indication, he cited rheumatology practice data to suggest that less than half of patients receive this protection.
A major reason for the low rate of vaccination was failure of the biologic prescriber to assume responsibility for this recommended step, according to Dr. Foulke. Although he does not believe that biologic prescribers need to administer the vaccine, and he acknowledged that he does not stock vaccines in his clinic, he does believe they should inform patients when vaccination is safe and appropriate.
Live attenuated vaccines, in his opinion, are not safe. Although he reported that this is an area of controversy, he takes a conservative approach, advising candidates for a live attenuated vaccine to undergo vaccination prior to starting the biologic or during a break from biologic therapy.
When he polled the audience about which vaccines are live attenuated vaccines, several failed to recognize that the MMR vaccine falls into this category. However, he also noted that the majority of vaccines are not live attenuated and should be considered. He specifically singled out the recombinant herpes zoster as a vaccine recommended by the American College of Rheumatology in patients on biologics.
“We do not have to give the shots, but we should be the ones who start the discussion,” said Dr. Foulke, referring to vaccines in patients on a biologic. He called these important steps “to protect patients from preventable disasters.”
Dr. Foulke reported no potential conflicts of interest.
SOURCE: Summer AAD 2019, Session F02.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Midlife hypertension is associated with subsequent risk of dementia
Uncontrolled hypertension among individuals aged 45-65 years of age is associated with an increased risk of subsequent dementia, according to a relatively large prospective population-based cohort study that followed patients for almost 30 years.
Even though previously published studies have not conclusively linked blood pressure control with a reduction in dementia risk, a second study, published simultaneously, did link blood pressure control with a smaller increase in white matter lesions, which are a marker of dementia risk. However, a reduction in total brain volume that accompanied this protection raised concern.
In the first of the two reports published Aug. 13 in JAMA, individuals 45-65 years of age participating in the Atherosclerosis Risk in Communities (ARIC) study were followed for cognitive function in relation to blood pressure. The baseline visit took place in 1987-1989. Cognitive function was also evaluated at the fifth visit, which took place in 2011-2013, and the sixth visit, which took place in 2016-2017.
At the sixth visit, the incidence of dementia among patients who were normotensive at baseline and also normotensive at the fifth visit was 1.31 per 100 person-years. For those with hypertension (greater than 140/90 mm Hg) at the fifth visit but normotensive at baseline, the incidence was 1.99 per 100 patient-years. For those with hypertension at both time points, the incidence was 4.26 per 100 patient-years.
When translated into hazard ratios, those with midlife and late-life hypertension were nearly 50% more likely to develop dementia (HR, 1.49) relative to those who remained normotensive. For those who had only midlife hypertension, the risk was also significantly increased (HR, 1.41) relative to those who remained normotensive at both time points.
Those with midlife hypertension but late-life hypotension were also found to be at greater risk of dementia (HR, 1.62) relative to those who remained normotensive.
These data support the premise that uncontrolled midlife hypertension increases risk of dementia but do not touch on whether blood pressure reductions reduce this risk. However, a second study published simultaneously provided at least some evidence that blood pressure control might offer some protection.
In this report, which is a substudy of the previously published Systolic Blood Pressure Intervention Trial (SPRINT) MIND trial, brain volume changes were evaluated via MRI in 449 of the more than 2,000 patients included in the previously published trial (Williamson JD et al. JAMA. 2019;321[6]:553-61).
After a median 3.4 years of follow-up, mean white matter lesion volume increased only 0.92 cm3 in patients receiving intensive systolic blood pressure control, defined as less than 120 mm Hg, versus 1.45 cm3 in those with higher systolic blood pressures.
These substudy data are encouraging, but it is important to recognize that the previously published and larger SPRINT MIND trial did not achieve its endpoint. In that study, the protection against dementia was nonsignificant (HR, 0.83; 95% confidence interval, 0.67-1.04).
In addition, the lower loss in white matter volume with intensive blood pressure lowering in the MRI substudy was accompanied with a greater loss in total brain volume (–30.6 vs. –26.9 cm3), which is considered a potentially negative effect.
As a result, the picture for risk management remains unclear, according to an editorial that accompanied publication of both studies.
“The important clinical question is whether changes of a few cubic millimeters in white matter hyperintensity volume or brain make a difference on brain function,” observed the author of the editorial, Shyam Prabhakaran, MD, of the department of neurology at the University of Chicago.
He believes that there are several findings from both studies that are “encouraging” in regard to blood pressure control for the prevention of dementia, but he also listed many unanswered questions, including why benefits observed to date have been so modest. He speculated that meaningful clinical benefits might depend on a multimodal approach that includes modification of other vascular risk factors, such as elevated lipids.
He also suggested that many issues regarding intensive blood pressure control for preventing dementia are unresolved, suggesting the need for more studies.
Not least, “later blood-pressure lowering interventions require careful monitoring for the potential cognitive harm associated with late-life hypotension,” Dr. Prabhakaran noted. Calling the effects of blood pressure control on brain health “nuanced,” he concluded that there is an opportunity for blood pressure modifications to prevent dementia, but stressed that optimal blood pressure targets for the purposes of preventing dementia are unknown.
The ARIC and SPRINT studies are supported by the National Institutes of Health. Several authors reported relationships with industry but no conflicts of interest relevant to this study.
SOURCES: Walker KA et al. JAMA. 2019;322(6):535-45; SPRINT MIND investigators. JAMA. 2019;322(6):524-34; Prabhakaran S. JAMA. 2019;322(6):512-3
Uncontrolled hypertension among individuals aged 45-65 years of age is associated with an increased risk of subsequent dementia, according to a relatively large prospective population-based cohort study that followed patients for almost 30 years.
Even though previously published studies have not conclusively linked blood pressure control with a reduction in dementia risk, a second study, published simultaneously, did link blood pressure control with a smaller increase in white matter lesions, which are a marker of dementia risk. However, a reduction in total brain volume that accompanied this protection raised concern.
In the first of the two reports published Aug. 13 in JAMA, individuals 45-65 years of age participating in the Atherosclerosis Risk in Communities (ARIC) study were followed for cognitive function in relation to blood pressure. The baseline visit took place in 1987-1989. Cognitive function was also evaluated at the fifth visit, which took place in 2011-2013, and the sixth visit, which took place in 2016-2017.
At the sixth visit, the incidence of dementia among patients who were normotensive at baseline and also normotensive at the fifth visit was 1.31 per 100 person-years. For those with hypertension (greater than 140/90 mm Hg) at the fifth visit but normotensive at baseline, the incidence was 1.99 per 100 patient-years. For those with hypertension at both time points, the incidence was 4.26 per 100 patient-years.
When translated into hazard ratios, those with midlife and late-life hypertension were nearly 50% more likely to develop dementia (HR, 1.49) relative to those who remained normotensive. For those who had only midlife hypertension, the risk was also significantly increased (HR, 1.41) relative to those who remained normotensive at both time points.
Those with midlife hypertension but late-life hypotension were also found to be at greater risk of dementia (HR, 1.62) relative to those who remained normotensive.
These data support the premise that uncontrolled midlife hypertension increases risk of dementia but do not touch on whether blood pressure reductions reduce this risk. However, a second study published simultaneously provided at least some evidence that blood pressure control might offer some protection.
In this report, which is a substudy of the previously published Systolic Blood Pressure Intervention Trial (SPRINT) MIND trial, brain volume changes were evaluated via MRI in 449 of the more than 2,000 patients included in the previously published trial (Williamson JD et al. JAMA. 2019;321[6]:553-61).
After a median 3.4 years of follow-up, mean white matter lesion volume increased only 0.92 cm3 in patients receiving intensive systolic blood pressure control, defined as less than 120 mm Hg, versus 1.45 cm3 in those with higher systolic blood pressures.
These substudy data are encouraging, but it is important to recognize that the previously published and larger SPRINT MIND trial did not achieve its endpoint. In that study, the protection against dementia was nonsignificant (HR, 0.83; 95% confidence interval, 0.67-1.04).
In addition, the lower loss in white matter volume with intensive blood pressure lowering in the MRI substudy was accompanied with a greater loss in total brain volume (–30.6 vs. –26.9 cm3), which is considered a potentially negative effect.
As a result, the picture for risk management remains unclear, according to an editorial that accompanied publication of both studies.
“The important clinical question is whether changes of a few cubic millimeters in white matter hyperintensity volume or brain make a difference on brain function,” observed the author of the editorial, Shyam Prabhakaran, MD, of the department of neurology at the University of Chicago.
He believes that there are several findings from both studies that are “encouraging” in regard to blood pressure control for the prevention of dementia, but he also listed many unanswered questions, including why benefits observed to date have been so modest. He speculated that meaningful clinical benefits might depend on a multimodal approach that includes modification of other vascular risk factors, such as elevated lipids.
He also suggested that many issues regarding intensive blood pressure control for preventing dementia are unresolved, suggesting the need for more studies.
Not least, “later blood-pressure lowering interventions require careful monitoring for the potential cognitive harm associated with late-life hypotension,” Dr. Prabhakaran noted. Calling the effects of blood pressure control on brain health “nuanced,” he concluded that there is an opportunity for blood pressure modifications to prevent dementia, but stressed that optimal blood pressure targets for the purposes of preventing dementia are unknown.
The ARIC and SPRINT studies are supported by the National Institutes of Health. Several authors reported relationships with industry but no conflicts of interest relevant to this study.
SOURCES: Walker KA et al. JAMA. 2019;322(6):535-45; SPRINT MIND investigators. JAMA. 2019;322(6):524-34; Prabhakaran S. JAMA. 2019;322(6):512-3
Uncontrolled hypertension among individuals aged 45-65 years of age is associated with an increased risk of subsequent dementia, according to a relatively large prospective population-based cohort study that followed patients for almost 30 years.
Even though previously published studies have not conclusively linked blood pressure control with a reduction in dementia risk, a second study, published simultaneously, did link blood pressure control with a smaller increase in white matter lesions, which are a marker of dementia risk. However, a reduction in total brain volume that accompanied this protection raised concern.
In the first of the two reports published Aug. 13 in JAMA, individuals 45-65 years of age participating in the Atherosclerosis Risk in Communities (ARIC) study were followed for cognitive function in relation to blood pressure. The baseline visit took place in 1987-1989. Cognitive function was also evaluated at the fifth visit, which took place in 2011-2013, and the sixth visit, which took place in 2016-2017.
At the sixth visit, the incidence of dementia among patients who were normotensive at baseline and also normotensive at the fifth visit was 1.31 per 100 person-years. For those with hypertension (greater than 140/90 mm Hg) at the fifth visit but normotensive at baseline, the incidence was 1.99 per 100 patient-years. For those with hypertension at both time points, the incidence was 4.26 per 100 patient-years.
When translated into hazard ratios, those with midlife and late-life hypertension were nearly 50% more likely to develop dementia (HR, 1.49) relative to those who remained normotensive. For those who had only midlife hypertension, the risk was also significantly increased (HR, 1.41) relative to those who remained normotensive at both time points.
Those with midlife hypertension but late-life hypotension were also found to be at greater risk of dementia (HR, 1.62) relative to those who remained normotensive.
These data support the premise that uncontrolled midlife hypertension increases risk of dementia but do not touch on whether blood pressure reductions reduce this risk. However, a second study published simultaneously provided at least some evidence that blood pressure control might offer some protection.
In this report, which is a substudy of the previously published Systolic Blood Pressure Intervention Trial (SPRINT) MIND trial, brain volume changes were evaluated via MRI in 449 of the more than 2,000 patients included in the previously published trial (Williamson JD et al. JAMA. 2019;321[6]:553-61).
After a median 3.4 years of follow-up, mean white matter lesion volume increased only 0.92 cm3 in patients receiving intensive systolic blood pressure control, defined as less than 120 mm Hg, versus 1.45 cm3 in those with higher systolic blood pressures.
These substudy data are encouraging, but it is important to recognize that the previously published and larger SPRINT MIND trial did not achieve its endpoint. In that study, the protection against dementia was nonsignificant (HR, 0.83; 95% confidence interval, 0.67-1.04).
In addition, the lower loss in white matter volume with intensive blood pressure lowering in the MRI substudy was accompanied with a greater loss in total brain volume (–30.6 vs. –26.9 cm3), which is considered a potentially negative effect.
As a result, the picture for risk management remains unclear, according to an editorial that accompanied publication of both studies.
“The important clinical question is whether changes of a few cubic millimeters in white matter hyperintensity volume or brain make a difference on brain function,” observed the author of the editorial, Shyam Prabhakaran, MD, of the department of neurology at the University of Chicago.
He believes that there are several findings from both studies that are “encouraging” in regard to blood pressure control for the prevention of dementia, but he also listed many unanswered questions, including why benefits observed to date have been so modest. He speculated that meaningful clinical benefits might depend on a multimodal approach that includes modification of other vascular risk factors, such as elevated lipids.
He also suggested that many issues regarding intensive blood pressure control for preventing dementia are unresolved, suggesting the need for more studies.
Not least, “later blood-pressure lowering interventions require careful monitoring for the potential cognitive harm associated with late-life hypotension,” Dr. Prabhakaran noted. Calling the effects of blood pressure control on brain health “nuanced,” he concluded that there is an opportunity for blood pressure modifications to prevent dementia, but stressed that optimal blood pressure targets for the purposes of preventing dementia are unknown.
The ARIC and SPRINT studies are supported by the National Institutes of Health. Several authors reported relationships with industry but no conflicts of interest relevant to this study.
SOURCES: Walker KA et al. JAMA. 2019;322(6):535-45; SPRINT MIND investigators. JAMA. 2019;322(6):524-34; Prabhakaran S. JAMA. 2019;322(6):512-3
FROM JAMA
Criteria found largely interchangeable for classifying radiographic axSpA
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
FROM ANNALS OF THE RHEUMATIC DISEASES
Nicotinamide-containing products gaining interest for aging, dermatologic disorders
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
EXPERT ANALYSIS FROM SUMMER AAD 2019