User login
M.O.R.E. means less delirium in ICU
PHOENIX – Adding a protocol of bundled nonpharmacologic interventions to the currently recommended standard of care nearly halved the amount of time patients spent delirious in the intensive care unit, a study has shown.
The combination of soothing music, natural light, routine cognitive stimulation, and the use of any necessary patient vision or hearing aids, added to standard ICU mobility and sedation protocols, also reduced the odds of developing any delirium, when researchers controlled for age, dementia, APACHE II score, and mechanical ventilation.
The data are from a prospective pre- and post–quality improvement cohort evaluation of the percentage of time patients spent delirious during their total medical ICU stay, rather than just the prevalence of delirium.
“We think this study really brings something unique to the knowledge of ICU delirium,” Ryan Rivosecchi, Pharm.D., a second-year resident at the University of Pittsburgh, said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. “Delirium is a disease of a waxing and waning nature, and purely going on its prevalence may not tell the full story.”
Currently, the recommendation with the highest level of evidence for preventing ICU delirium, as noted in the 2013 updated SCCM guidelines for pain, agitation, and delirium, is early mobilization. There are scant other evidence-based recommendations for how to prevent and treat the condition, leaving “a lot of practitioners wondering how they should take care of their delirious patients,” Dr. Rivosecchi said.
Delirium experienced during ICU stays has been associated with some form of residual cognitive impairment in more than 70% of patients and increases in lengths of stay by as many as 15 days. Delirium in the ICU has also been associated with a nearly 20% increase in mortality rates 6 months after admission to the ICU.
Noting that his institution already adhered to the standard recommendations for early mobility and sedation algorithms, Dr. Rivosecchi said he and his colleagues conducted the study at the University of Pittsburgh’s 24-bed medical ICU, in hopes of providing an additional evidence-based intervention to reduce ICU delirium.
Among 729 adult patients screened between September 2013 and April 2014, 230 patients who had not been in the ICU prior to MICU admission, and who did not present with delirium or baseline cognitive impairment, were chosen for the pre–quality improvement phase.
The post–quality improvement arm of the study comprised 253 patients who met the same criteria as did those in the first arm. The median age for both groups was 59 years. Slightly more than half of all patients were male.
The primary outcome was the total time a patient spent delirious throughout the entire MICU stay, as measured six times daily, every 4 hours, via the Intensive Care Delirium Screening Checklist. “Patients evaluated as ‘delirious’ at time point A were considered delirious for the next 4 hours until they were evaluated again at time point B,” Dr. Rivosecchi said.
During the first 3 months of the study, nurses on the MICU floor were unaware that baseline data about their unit were being collected. In the fourth month, the investigators discussed their observations with the nursing staff, and with the nursing director and clinician on the unit.
Several unique interventions, distilled from these meetings and a review of protocols already in place, were combined with findings from a systematic review of the literature, into one preventive protocol named M.O.R.E.: Music; Opening of blinds; Reorientation and cognitive stimulation; Eye and ear protocol.
After the nurse meetings, another month passed before the post–quality improvement phase of the study began. The nurses were once again unaware data were being collected; however, according to Dr. Rivosecchi, not only did the entire medical staff, including the pharmacists, embrace the new protocol, but the nurses were so enthusiastic about it they collaborated on a poem to express their intentions to the patients and the patients’ families. Copies of the poem were placed in zippered pouches that included a sleep mask, ear plugs, and headphones, distributed to each MICU patient to help them block out unnecessary stimulation.
“The delirium prevention care bag was an idea the nurses came up with on their own to really help the patients out,” Dr. Rivosecchi said.
Signage detailing the specific actions included in the mnemonic M.O.R.E. was posted throughout the MICU to make it easier for the nursing staff to remember, although how the nurses implemented the protocol was left to them, Dr. Rivosecchi said.
The signs advised that MICU patients be exposed to at least 1 hour of “relaxing/soothing” music per nurse shift, and that if the patient were not actually viewing the television, it be kept off.
To help patients maintain a normal diurnal rhythm, nurses were advised to angle patients so that they could have a view of a window that was kept open from morning until evening to allow in as much natural light as possible.
Rather than employ the typical “alert and orient times three” protocol, the staff was instructed to create mental tasks for the patients, such as asking them their names and how they preferred to be addressed, and to reorient patients by discussing with them the status of their hospitalization.
If patients were accustomed to wearing visual or hearing aids, the nurses were asked to encourage them to use the aids while in the MICU.
The group of 230 studied before M.O.R.E. was in place had 36 patients who experienced delirium, compared with 24 of the 253 patients observed after the interventions were instituted.
The total reduction in the amount of time these patients spent in delirious states was 40.4%. The first arm’s total time spent delirious was 16.1% (1,088 out of 6,747 hours), compared with 9.6% (485 out of 5, 071 hours) of the total time in the second arm (P < .001).
The typical length of stay for the first arm was 58 hours, and 68 hours in the second.
At baseline, there was a statistically significant difference in illness severity as measured by APACHE II scores between the groups: The first had a score of 15, while the second had a score of 17 (P = .002), although according to Dr. Rivosecchi, both arms followed the same predictive value of mortality at 24 hours (7.5% vs. 11.1%, P = .21).
Also of note, Dr. Rivosecchi said, was that there was a higher use of the benzodiazepines lorazepam and midazolam, commonly associated with higher rates of delirium, in the second phase of the study.
In a subanalysis using risk factors reported in the literature, the investigators determined that age, severity of illness, the use of mechanical ventilation, home anticholinergic use, and home antipsychotic use increased the odds of delirium, as did baseline depression or respiratory disease. After the researchers controlled for these factors, the M.O.R.E. protocol reduced the risk of developing delirium by 57% (odds ratio, 0.43, 95% confidence interval 0.24 - 0.77).
Statistically significant predictors of delirium were mechanical ventilation (OR 2.09, CI 1.11-3.91, P = .022), APACHE II score (1.07, CI 1.02 -1.11, P = .002) and dementia (5.12, CI 1.8 -14.3, P = .91).
“I was extremely surprised by the results, particularly since we had greater benzodiazepine use and arguably a sicker patient population in the post phase,” said Dr. Rivosecchi. “I definitely did not expect a 40% reduction.”
Despite the nurses’ enthusiasm, the study did not actually track their adherence to the protocol, a weakness Dr. Rivosecchi said he and his colleagues hoped to address in future evaluations of the protocol.
Meanwhile, according to Pamela Smithburger, Pharm.D., Dr. Rivosecchi’s mentor and the senior author on the paper, use of the M.O.R.E. protocol is now being rolled out across all ICUs within the University of Pittsburgh Medical Center’s entire system, which includes 13 hospitals, and that as the implementation continues, she will be collecting data on patient outcomes systemwide.
“Each ICU will be able to modify the M.O.R.E. protocol to best fit their work flow, culture, and environment,” Dr. Smithburger said in an interview.
The cost to do so comes down to time and staff education. “We utilized tools and resources already available, but by combining them into one protocol, improved outcomes.”
On Twitter @whitneymcknight
PHOENIX – Adding a protocol of bundled nonpharmacologic interventions to the currently recommended standard of care nearly halved the amount of time patients spent delirious in the intensive care unit, a study has shown.
The combination of soothing music, natural light, routine cognitive stimulation, and the use of any necessary patient vision or hearing aids, added to standard ICU mobility and sedation protocols, also reduced the odds of developing any delirium, when researchers controlled for age, dementia, APACHE II score, and mechanical ventilation.
The data are from a prospective pre- and post–quality improvement cohort evaluation of the percentage of time patients spent delirious during their total medical ICU stay, rather than just the prevalence of delirium.
“We think this study really brings something unique to the knowledge of ICU delirium,” Ryan Rivosecchi, Pharm.D., a second-year resident at the University of Pittsburgh, said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. “Delirium is a disease of a waxing and waning nature, and purely going on its prevalence may not tell the full story.”
Currently, the recommendation with the highest level of evidence for preventing ICU delirium, as noted in the 2013 updated SCCM guidelines for pain, agitation, and delirium, is early mobilization. There are scant other evidence-based recommendations for how to prevent and treat the condition, leaving “a lot of practitioners wondering how they should take care of their delirious patients,” Dr. Rivosecchi said.
Delirium experienced during ICU stays has been associated with some form of residual cognitive impairment in more than 70% of patients and increases in lengths of stay by as many as 15 days. Delirium in the ICU has also been associated with a nearly 20% increase in mortality rates 6 months after admission to the ICU.
Noting that his institution already adhered to the standard recommendations for early mobility and sedation algorithms, Dr. Rivosecchi said he and his colleagues conducted the study at the University of Pittsburgh’s 24-bed medical ICU, in hopes of providing an additional evidence-based intervention to reduce ICU delirium.
Among 729 adult patients screened between September 2013 and April 2014, 230 patients who had not been in the ICU prior to MICU admission, and who did not present with delirium or baseline cognitive impairment, were chosen for the pre–quality improvement phase.
The post–quality improvement arm of the study comprised 253 patients who met the same criteria as did those in the first arm. The median age for both groups was 59 years. Slightly more than half of all patients were male.
The primary outcome was the total time a patient spent delirious throughout the entire MICU stay, as measured six times daily, every 4 hours, via the Intensive Care Delirium Screening Checklist. “Patients evaluated as ‘delirious’ at time point A were considered delirious for the next 4 hours until they were evaluated again at time point B,” Dr. Rivosecchi said.
During the first 3 months of the study, nurses on the MICU floor were unaware that baseline data about their unit were being collected. In the fourth month, the investigators discussed their observations with the nursing staff, and with the nursing director and clinician on the unit.
Several unique interventions, distilled from these meetings and a review of protocols already in place, were combined with findings from a systematic review of the literature, into one preventive protocol named M.O.R.E.: Music; Opening of blinds; Reorientation and cognitive stimulation; Eye and ear protocol.
After the nurse meetings, another month passed before the post–quality improvement phase of the study began. The nurses were once again unaware data were being collected; however, according to Dr. Rivosecchi, not only did the entire medical staff, including the pharmacists, embrace the new protocol, but the nurses were so enthusiastic about it they collaborated on a poem to express their intentions to the patients and the patients’ families. Copies of the poem were placed in zippered pouches that included a sleep mask, ear plugs, and headphones, distributed to each MICU patient to help them block out unnecessary stimulation.
“The delirium prevention care bag was an idea the nurses came up with on their own to really help the patients out,” Dr. Rivosecchi said.
Signage detailing the specific actions included in the mnemonic M.O.R.E. was posted throughout the MICU to make it easier for the nursing staff to remember, although how the nurses implemented the protocol was left to them, Dr. Rivosecchi said.
The signs advised that MICU patients be exposed to at least 1 hour of “relaxing/soothing” music per nurse shift, and that if the patient were not actually viewing the television, it be kept off.
To help patients maintain a normal diurnal rhythm, nurses were advised to angle patients so that they could have a view of a window that was kept open from morning until evening to allow in as much natural light as possible.
Rather than employ the typical “alert and orient times three” protocol, the staff was instructed to create mental tasks for the patients, such as asking them their names and how they preferred to be addressed, and to reorient patients by discussing with them the status of their hospitalization.
If patients were accustomed to wearing visual or hearing aids, the nurses were asked to encourage them to use the aids while in the MICU.
The group of 230 studied before M.O.R.E. was in place had 36 patients who experienced delirium, compared with 24 of the 253 patients observed after the interventions were instituted.
The total reduction in the amount of time these patients spent in delirious states was 40.4%. The first arm’s total time spent delirious was 16.1% (1,088 out of 6,747 hours), compared with 9.6% (485 out of 5, 071 hours) of the total time in the second arm (P < .001).
The typical length of stay for the first arm was 58 hours, and 68 hours in the second.
At baseline, there was a statistically significant difference in illness severity as measured by APACHE II scores between the groups: The first had a score of 15, while the second had a score of 17 (P = .002), although according to Dr. Rivosecchi, both arms followed the same predictive value of mortality at 24 hours (7.5% vs. 11.1%, P = .21).
Also of note, Dr. Rivosecchi said, was that there was a higher use of the benzodiazepines lorazepam and midazolam, commonly associated with higher rates of delirium, in the second phase of the study.
In a subanalysis using risk factors reported in the literature, the investigators determined that age, severity of illness, the use of mechanical ventilation, home anticholinergic use, and home antipsychotic use increased the odds of delirium, as did baseline depression or respiratory disease. After the researchers controlled for these factors, the M.O.R.E. protocol reduced the risk of developing delirium by 57% (odds ratio, 0.43, 95% confidence interval 0.24 - 0.77).
Statistically significant predictors of delirium were mechanical ventilation (OR 2.09, CI 1.11-3.91, P = .022), APACHE II score (1.07, CI 1.02 -1.11, P = .002) and dementia (5.12, CI 1.8 -14.3, P = .91).
“I was extremely surprised by the results, particularly since we had greater benzodiazepine use and arguably a sicker patient population in the post phase,” said Dr. Rivosecchi. “I definitely did not expect a 40% reduction.”
Despite the nurses’ enthusiasm, the study did not actually track their adherence to the protocol, a weakness Dr. Rivosecchi said he and his colleagues hoped to address in future evaluations of the protocol.
Meanwhile, according to Pamela Smithburger, Pharm.D., Dr. Rivosecchi’s mentor and the senior author on the paper, use of the M.O.R.E. protocol is now being rolled out across all ICUs within the University of Pittsburgh Medical Center’s entire system, which includes 13 hospitals, and that as the implementation continues, she will be collecting data on patient outcomes systemwide.
“Each ICU will be able to modify the M.O.R.E. protocol to best fit their work flow, culture, and environment,” Dr. Smithburger said in an interview.
The cost to do so comes down to time and staff education. “We utilized tools and resources already available, but by combining them into one protocol, improved outcomes.”
On Twitter @whitneymcknight
PHOENIX – Adding a protocol of bundled nonpharmacologic interventions to the currently recommended standard of care nearly halved the amount of time patients spent delirious in the intensive care unit, a study has shown.
The combination of soothing music, natural light, routine cognitive stimulation, and the use of any necessary patient vision or hearing aids, added to standard ICU mobility and sedation protocols, also reduced the odds of developing any delirium, when researchers controlled for age, dementia, APACHE II score, and mechanical ventilation.
The data are from a prospective pre- and post–quality improvement cohort evaluation of the percentage of time patients spent delirious during their total medical ICU stay, rather than just the prevalence of delirium.
“We think this study really brings something unique to the knowledge of ICU delirium,” Ryan Rivosecchi, Pharm.D., a second-year resident at the University of Pittsburgh, said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. “Delirium is a disease of a waxing and waning nature, and purely going on its prevalence may not tell the full story.”
Currently, the recommendation with the highest level of evidence for preventing ICU delirium, as noted in the 2013 updated SCCM guidelines for pain, agitation, and delirium, is early mobilization. There are scant other evidence-based recommendations for how to prevent and treat the condition, leaving “a lot of practitioners wondering how they should take care of their delirious patients,” Dr. Rivosecchi said.
Delirium experienced during ICU stays has been associated with some form of residual cognitive impairment in more than 70% of patients and increases in lengths of stay by as many as 15 days. Delirium in the ICU has also been associated with a nearly 20% increase in mortality rates 6 months after admission to the ICU.
Noting that his institution already adhered to the standard recommendations for early mobility and sedation algorithms, Dr. Rivosecchi said he and his colleagues conducted the study at the University of Pittsburgh’s 24-bed medical ICU, in hopes of providing an additional evidence-based intervention to reduce ICU delirium.
Among 729 adult patients screened between September 2013 and April 2014, 230 patients who had not been in the ICU prior to MICU admission, and who did not present with delirium or baseline cognitive impairment, were chosen for the pre–quality improvement phase.
The post–quality improvement arm of the study comprised 253 patients who met the same criteria as did those in the first arm. The median age for both groups was 59 years. Slightly more than half of all patients were male.
The primary outcome was the total time a patient spent delirious throughout the entire MICU stay, as measured six times daily, every 4 hours, via the Intensive Care Delirium Screening Checklist. “Patients evaluated as ‘delirious’ at time point A were considered delirious for the next 4 hours until they were evaluated again at time point B,” Dr. Rivosecchi said.
During the first 3 months of the study, nurses on the MICU floor were unaware that baseline data about their unit were being collected. In the fourth month, the investigators discussed their observations with the nursing staff, and with the nursing director and clinician on the unit.
Several unique interventions, distilled from these meetings and a review of protocols already in place, were combined with findings from a systematic review of the literature, into one preventive protocol named M.O.R.E.: Music; Opening of blinds; Reorientation and cognitive stimulation; Eye and ear protocol.
After the nurse meetings, another month passed before the post–quality improvement phase of the study began. The nurses were once again unaware data were being collected; however, according to Dr. Rivosecchi, not only did the entire medical staff, including the pharmacists, embrace the new protocol, but the nurses were so enthusiastic about it they collaborated on a poem to express their intentions to the patients and the patients’ families. Copies of the poem were placed in zippered pouches that included a sleep mask, ear plugs, and headphones, distributed to each MICU patient to help them block out unnecessary stimulation.
“The delirium prevention care bag was an idea the nurses came up with on their own to really help the patients out,” Dr. Rivosecchi said.
Signage detailing the specific actions included in the mnemonic M.O.R.E. was posted throughout the MICU to make it easier for the nursing staff to remember, although how the nurses implemented the protocol was left to them, Dr. Rivosecchi said.
The signs advised that MICU patients be exposed to at least 1 hour of “relaxing/soothing” music per nurse shift, and that if the patient were not actually viewing the television, it be kept off.
To help patients maintain a normal diurnal rhythm, nurses were advised to angle patients so that they could have a view of a window that was kept open from morning until evening to allow in as much natural light as possible.
Rather than employ the typical “alert and orient times three” protocol, the staff was instructed to create mental tasks for the patients, such as asking them their names and how they preferred to be addressed, and to reorient patients by discussing with them the status of their hospitalization.
If patients were accustomed to wearing visual or hearing aids, the nurses were asked to encourage them to use the aids while in the MICU.
The group of 230 studied before M.O.R.E. was in place had 36 patients who experienced delirium, compared with 24 of the 253 patients observed after the interventions were instituted.
The total reduction in the amount of time these patients spent in delirious states was 40.4%. The first arm’s total time spent delirious was 16.1% (1,088 out of 6,747 hours), compared with 9.6% (485 out of 5, 071 hours) of the total time in the second arm (P < .001).
The typical length of stay for the first arm was 58 hours, and 68 hours in the second.
At baseline, there was a statistically significant difference in illness severity as measured by APACHE II scores between the groups: The first had a score of 15, while the second had a score of 17 (P = .002), although according to Dr. Rivosecchi, both arms followed the same predictive value of mortality at 24 hours (7.5% vs. 11.1%, P = .21).
Also of note, Dr. Rivosecchi said, was that there was a higher use of the benzodiazepines lorazepam and midazolam, commonly associated with higher rates of delirium, in the second phase of the study.
In a subanalysis using risk factors reported in the literature, the investigators determined that age, severity of illness, the use of mechanical ventilation, home anticholinergic use, and home antipsychotic use increased the odds of delirium, as did baseline depression or respiratory disease. After the researchers controlled for these factors, the M.O.R.E. protocol reduced the risk of developing delirium by 57% (odds ratio, 0.43, 95% confidence interval 0.24 - 0.77).
Statistically significant predictors of delirium were mechanical ventilation (OR 2.09, CI 1.11-3.91, P = .022), APACHE II score (1.07, CI 1.02 -1.11, P = .002) and dementia (5.12, CI 1.8 -14.3, P = .91).
“I was extremely surprised by the results, particularly since we had greater benzodiazepine use and arguably a sicker patient population in the post phase,” said Dr. Rivosecchi. “I definitely did not expect a 40% reduction.”
Despite the nurses’ enthusiasm, the study did not actually track their adherence to the protocol, a weakness Dr. Rivosecchi said he and his colleagues hoped to address in future evaluations of the protocol.
Meanwhile, according to Pamela Smithburger, Pharm.D., Dr. Rivosecchi’s mentor and the senior author on the paper, use of the M.O.R.E. protocol is now being rolled out across all ICUs within the University of Pittsburgh Medical Center’s entire system, which includes 13 hospitals, and that as the implementation continues, she will be collecting data on patient outcomes systemwide.
“Each ICU will be able to modify the M.O.R.E. protocol to best fit their work flow, culture, and environment,” Dr. Smithburger said in an interview.
The cost to do so comes down to time and staff education. “We utilized tools and resources already available, but by combining them into one protocol, improved outcomes.”
On Twitter @whitneymcknight
AT THE CRITICAL CARE CONGRESS
Key clinical point: Certain nonpharmacologic interventions bundled into a single protocol could serve as a cost-effective way to cut ICU delirium rates.
Major finding: Time spent delirious by MICU patients was reduced by more than 40% (16.1% vs. 9.6%, P < .001) using the M.O.R.E protocol.
Data source: Prospective, pre- and post–quality improvement study of 729 MICU patients at a single academic medical center.
Disclosures: None of the study authors had relevant disclosures.
Sample size, patient selection keys to successful small studies
ORLANDO – Studies with small patient populations but large effect sizes are the backbone of an independent investigator’s success. Rigorous patient selection doesn’t hurt, either.
“We often hide behind the words ‘pilot and feasibility’ to justify what was not a very good study,” Dr. Joshua Korzenik, director of Harvard Medical School’s Crohn’s and Colitis Center, Boston, said at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America. “The term can indicate something was not statistically significant, and that can be legitimate, but ‘pilot’ should not be a substitute for not sizing the study appropriately.”
Sample size consideration is important with respect to data analysis and endpoints, said Dr. Korzenik, but disciplined selection criteria strictly applied sweetens the odds for a study’s impact. Cultivating a cohort that is the “most homogeneous, cleanest, and clearest ... will give you the best insight.” Consider choosing patients according to disease subtype, bio- and genetic markers, a history of at least 3 consecutive months of disease, and a history of certain medication failures.
Steer clear of the assumption that just because you already treat a certain number of patients, you will be able to recruit them. “Some patients won’t want to commit to a study,” warned Dr. Korzenik. “You need to think more carefully.”
And don’t forget the “tremendous” impact of standard deviation on sample size. Dr. Korzenik recommended the “usual” power of .8 with a P value less than .05 for early phase studies.
For the neophyte independent investigator, sweating over what to write in his or her hypothesis, and struggling against temptation to justify sample size by stretching how small the placebo response will be vs. how great the efficacy rate is only to find actual results are not nearly what was predicted, can be devastating. “Then you’ve shot yourself in the foot,” said Dr. Korzenik.
One problem is that few, if any, previous data exist for these kinds of studies. And preclinical data “tends not to be helpful at all,” Dr. Korzenik opined.
Even in trials for anti–tumor necrosis factor drugs, what Dr. Korzenik argued are the most revolutionary treatments to yet impact the field, the question of placebo effect on sample size was tricky. “For the most part, anti-TNFs are about 20% better than placebo for inducing remission. That’s a pretty high bar to set, and most investigator-sponsored studies set the bar even higher, making it very difficult.”
If, for example, an investigator hopes to achieve a 50% reduction in calprotectin, and so sets a “modest” rate of 20% for placebo and 35% for the test drug, that means the investigator must recruit 136 patients per arm. “Yikes!”
But estimating at 15% vs. 40% for the drug, with 47 in each arm, may push the benefit of the study drug “too much.” Using a placebo effect size of 10% vs. 50% for the drug, with 17 patients per arm, the investigator runs the risk of overestimating what’s possible. “You might need to look for another endpoint, or some other set of collaborators,” Dr. Korzenik said.
Open-label studies can be useful for helping with sample size, particularly if the study is to evaluate a novel approach to treatment, but things can still go wobbly. “Open-label trials have limitations we don’t fully understand,” Dr. Korzenik said.
To wit, open-label trials on the use of the helminth Trichuris suis to treat Crohn’s disease showed robust response remission rates, but a successive, placebo-controlled trial did not achieve these results. For independent investigators conducting a placebo-controlled trial using a comparator for the control group, Dr. Korzenik suggested ways to keep the placebo response lower. These included, among other strategies, recruiting patients with higher disease activity and keeping trials as short as possible. “When you do longer studies, the placebo response remission rates go up. Keep that in mind.”
And, don’t forget: Not all small studies with impact need focus on pharmaceuticals. Possibilities Dr. Korzenik suggested include alternative interventions such as marijuana, curcumin, and aloe vera. “These things have been done, but deserve further study,” he said, adding that nutritional interventions are “undervalued, and although difficult to study, are very important.”
The role of depression, fatigue, and other psychosocial impacts of inflammatory bowel disease are also worthy of study, as are the utility of telemedicine and social media for helping patients, he said.
Because investigators will want to protect their resources – namely, the goodwill of the patients they painstakingly recruited – Dr. Korzenik advised using telemedicine to interact with study participants whenever possible, and to consider using smartphone apps to record symptom data. “Remember that repeated evaluations become an enormous burden on the patient.”
Dr. Korzenik urged young investigators not to be intimidated, and to see their inexperience as liberation from having preconceived notions of what the correct approaches are to studying IBD. Still, finding a mentor “who can help shape your ideas and help develop techniques,” can be confidence building.
“You don’t necessarily need to have a final piece of work that can stand on its own,” Dr. Korzenik concluded. “You’re learning how to do a clinical trial and get your career moving forward.”
On Twitter @whitneymcknight
ORLANDO – Studies with small patient populations but large effect sizes are the backbone of an independent investigator’s success. Rigorous patient selection doesn’t hurt, either.
“We often hide behind the words ‘pilot and feasibility’ to justify what was not a very good study,” Dr. Joshua Korzenik, director of Harvard Medical School’s Crohn’s and Colitis Center, Boston, said at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America. “The term can indicate something was not statistically significant, and that can be legitimate, but ‘pilot’ should not be a substitute for not sizing the study appropriately.”
Sample size consideration is important with respect to data analysis and endpoints, said Dr. Korzenik, but disciplined selection criteria strictly applied sweetens the odds for a study’s impact. Cultivating a cohort that is the “most homogeneous, cleanest, and clearest ... will give you the best insight.” Consider choosing patients according to disease subtype, bio- and genetic markers, a history of at least 3 consecutive months of disease, and a history of certain medication failures.
Steer clear of the assumption that just because you already treat a certain number of patients, you will be able to recruit them. “Some patients won’t want to commit to a study,” warned Dr. Korzenik. “You need to think more carefully.”
And don’t forget the “tremendous” impact of standard deviation on sample size. Dr. Korzenik recommended the “usual” power of .8 with a P value less than .05 for early phase studies.
For the neophyte independent investigator, sweating over what to write in his or her hypothesis, and struggling against temptation to justify sample size by stretching how small the placebo response will be vs. how great the efficacy rate is only to find actual results are not nearly what was predicted, can be devastating. “Then you’ve shot yourself in the foot,” said Dr. Korzenik.
One problem is that few, if any, previous data exist for these kinds of studies. And preclinical data “tends not to be helpful at all,” Dr. Korzenik opined.
Even in trials for anti–tumor necrosis factor drugs, what Dr. Korzenik argued are the most revolutionary treatments to yet impact the field, the question of placebo effect on sample size was tricky. “For the most part, anti-TNFs are about 20% better than placebo for inducing remission. That’s a pretty high bar to set, and most investigator-sponsored studies set the bar even higher, making it very difficult.”
If, for example, an investigator hopes to achieve a 50% reduction in calprotectin, and so sets a “modest” rate of 20% for placebo and 35% for the test drug, that means the investigator must recruit 136 patients per arm. “Yikes!”
But estimating at 15% vs. 40% for the drug, with 47 in each arm, may push the benefit of the study drug “too much.” Using a placebo effect size of 10% vs. 50% for the drug, with 17 patients per arm, the investigator runs the risk of overestimating what’s possible. “You might need to look for another endpoint, or some other set of collaborators,” Dr. Korzenik said.
Open-label studies can be useful for helping with sample size, particularly if the study is to evaluate a novel approach to treatment, but things can still go wobbly. “Open-label trials have limitations we don’t fully understand,” Dr. Korzenik said.
To wit, open-label trials on the use of the helminth Trichuris suis to treat Crohn’s disease showed robust response remission rates, but a successive, placebo-controlled trial did not achieve these results. For independent investigators conducting a placebo-controlled trial using a comparator for the control group, Dr. Korzenik suggested ways to keep the placebo response lower. These included, among other strategies, recruiting patients with higher disease activity and keeping trials as short as possible. “When you do longer studies, the placebo response remission rates go up. Keep that in mind.”
And, don’t forget: Not all small studies with impact need focus on pharmaceuticals. Possibilities Dr. Korzenik suggested include alternative interventions such as marijuana, curcumin, and aloe vera. “These things have been done, but deserve further study,” he said, adding that nutritional interventions are “undervalued, and although difficult to study, are very important.”
The role of depression, fatigue, and other psychosocial impacts of inflammatory bowel disease are also worthy of study, as are the utility of telemedicine and social media for helping patients, he said.
Because investigators will want to protect their resources – namely, the goodwill of the patients they painstakingly recruited – Dr. Korzenik advised using telemedicine to interact with study participants whenever possible, and to consider using smartphone apps to record symptom data. “Remember that repeated evaluations become an enormous burden on the patient.”
Dr. Korzenik urged young investigators not to be intimidated, and to see their inexperience as liberation from having preconceived notions of what the correct approaches are to studying IBD. Still, finding a mentor “who can help shape your ideas and help develop techniques,” can be confidence building.
“You don’t necessarily need to have a final piece of work that can stand on its own,” Dr. Korzenik concluded. “You’re learning how to do a clinical trial and get your career moving forward.”
On Twitter @whitneymcknight
ORLANDO – Studies with small patient populations but large effect sizes are the backbone of an independent investigator’s success. Rigorous patient selection doesn’t hurt, either.
“We often hide behind the words ‘pilot and feasibility’ to justify what was not a very good study,” Dr. Joshua Korzenik, director of Harvard Medical School’s Crohn’s and Colitis Center, Boston, said at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America. “The term can indicate something was not statistically significant, and that can be legitimate, but ‘pilot’ should not be a substitute for not sizing the study appropriately.”
Sample size consideration is important with respect to data analysis and endpoints, said Dr. Korzenik, but disciplined selection criteria strictly applied sweetens the odds for a study’s impact. Cultivating a cohort that is the “most homogeneous, cleanest, and clearest ... will give you the best insight.” Consider choosing patients according to disease subtype, bio- and genetic markers, a history of at least 3 consecutive months of disease, and a history of certain medication failures.
Steer clear of the assumption that just because you already treat a certain number of patients, you will be able to recruit them. “Some patients won’t want to commit to a study,” warned Dr. Korzenik. “You need to think more carefully.”
And don’t forget the “tremendous” impact of standard deviation on sample size. Dr. Korzenik recommended the “usual” power of .8 with a P value less than .05 for early phase studies.
For the neophyte independent investigator, sweating over what to write in his or her hypothesis, and struggling against temptation to justify sample size by stretching how small the placebo response will be vs. how great the efficacy rate is only to find actual results are not nearly what was predicted, can be devastating. “Then you’ve shot yourself in the foot,” said Dr. Korzenik.
One problem is that few, if any, previous data exist for these kinds of studies. And preclinical data “tends not to be helpful at all,” Dr. Korzenik opined.
Even in trials for anti–tumor necrosis factor drugs, what Dr. Korzenik argued are the most revolutionary treatments to yet impact the field, the question of placebo effect on sample size was tricky. “For the most part, anti-TNFs are about 20% better than placebo for inducing remission. That’s a pretty high bar to set, and most investigator-sponsored studies set the bar even higher, making it very difficult.”
If, for example, an investigator hopes to achieve a 50% reduction in calprotectin, and so sets a “modest” rate of 20% for placebo and 35% for the test drug, that means the investigator must recruit 136 patients per arm. “Yikes!”
But estimating at 15% vs. 40% for the drug, with 47 in each arm, may push the benefit of the study drug “too much.” Using a placebo effect size of 10% vs. 50% for the drug, with 17 patients per arm, the investigator runs the risk of overestimating what’s possible. “You might need to look for another endpoint, or some other set of collaborators,” Dr. Korzenik said.
Open-label studies can be useful for helping with sample size, particularly if the study is to evaluate a novel approach to treatment, but things can still go wobbly. “Open-label trials have limitations we don’t fully understand,” Dr. Korzenik said.
To wit, open-label trials on the use of the helminth Trichuris suis to treat Crohn’s disease showed robust response remission rates, but a successive, placebo-controlled trial did not achieve these results. For independent investigators conducting a placebo-controlled trial using a comparator for the control group, Dr. Korzenik suggested ways to keep the placebo response lower. These included, among other strategies, recruiting patients with higher disease activity and keeping trials as short as possible. “When you do longer studies, the placebo response remission rates go up. Keep that in mind.”
And, don’t forget: Not all small studies with impact need focus on pharmaceuticals. Possibilities Dr. Korzenik suggested include alternative interventions such as marijuana, curcumin, and aloe vera. “These things have been done, but deserve further study,” he said, adding that nutritional interventions are “undervalued, and although difficult to study, are very important.”
The role of depression, fatigue, and other psychosocial impacts of inflammatory bowel disease are also worthy of study, as are the utility of telemedicine and social media for helping patients, he said.
Because investigators will want to protect their resources – namely, the goodwill of the patients they painstakingly recruited – Dr. Korzenik advised using telemedicine to interact with study participants whenever possible, and to consider using smartphone apps to record symptom data. “Remember that repeated evaluations become an enormous burden on the patient.”
Dr. Korzenik urged young investigators not to be intimidated, and to see their inexperience as liberation from having preconceived notions of what the correct approaches are to studying IBD. Still, finding a mentor “who can help shape your ideas and help develop techniques,” can be confidence building.
“You don’t necessarily need to have a final piece of work that can stand on its own,” Dr. Korzenik concluded. “You’re learning how to do a clinical trial and get your career moving forward.”
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM 2014 ADVANCES IN IBD
Burn injury risk doubles in HOT patients who smoke
AUSTIN, TEX. – Smokers offered home oxygen therapy were found to be at twice the risk for burn injuries, based on data from a retrospective study.
Even so, almost all the home oxygen therapy (HOT) burn victims were discharged with a prescription for oxygen, including the 15% of patients who had incurred similar injuries at least once, and in some cases, three times.
“I have a problem with this,” said Dr. Mary Baker, a critical care fellow at Indiana University and a medical ethics fellow at the university’s Richard M. Fairbanks Burn Center at Wishard-Eskenazi Health, both in Indianapolis. Dr. Baker presented the findings at the annual meeting of the American College of Chest Physicians.
“Should we be prescribing oxygen to patients who smoke? Maybe the bigger question is [whether] it is ever ethically defensible to take oxygen away once someone has sustained a combustion injury from smoking while using HOT,” she said.
Dr. Baker and her colleagues conducted a chart review of patients admitted to a single site for home oxygen–related burns between 2008 and 2013. They found that 55 of all such burn unit admissions were smokers, representing 4% of the center’s annual admissions rate and twice that of the national burn rate for smokers in general. Nearly all the patients, a balance of men and women with a median age of 61 years, were using HOT for chronic obstructive pulmonary disease.
“The location of the burns, probably not surprisingly, was the face. Probably the most common was the nasal cannula,” Dr. Baker said.
Although nearly three-quarters of the 55-member cohort had less than a 5% total body surface–area burn, Dr. Baker said that in a patient population with baseline respiratory compromise and respiratory failure, this was an alarming rate of morbidity, particularly since half of the injured were intubated, and bronchonscopic exam revealed a third of these patients also had inhalation injuries.
“And here’s the kicker,” said Dr. Baker. “Eight deaths over 5 years. This is huge. So when these [individuals] get burned, it’s often really bad. Several of them had house fires, and we were able to find in the chart where other people [in the home] were burned and admitted to the hospital.”
Still, after a median 5-day stay, almost all the patients who survived were discharged with prescriptions for HOT, including the so-called “repeat offenders.” Because nearly half of all surviving smoking-related HOT patients were discharged to a higher level of care, this cohort tended to have higher health care utilization rates as well, Dr. Baker noted.
A surprise finding was that more than a quarter of the cohort had either current or concomitant problems with substance abuse. “We were not expecting that, and it has not been previously reported,” Dr. Baker said.
The data demonstrate a need for the screening of HOT patients as to whether they smoke and whether they have substance use issues, she said. If either condition applies, then faster follow-up and, potentially, counseling could be offered, including better education about the risks of oxygen therapy. “Currently, we have no formalized way to educate patients on the dangers of those tanks in the home,” said Dr. Baker.
The data raise questions about the risk-benefit ratio of prescribing any breathing aid to COPD patients who are also smokers.
“I don’t know how much sense it makes to keep throwing these inhalers, which cost hundreds of dollars a month, at people who continue to smoke,” Dr. Baker said in an interview. “We take all comers, and we think oxygen therapy helps, and prolongs life, but when you factor in smoking, we don’t really know what the risks and benefits are.”
A large study population would be needed to determine the risks and benefits, she added.
On Twitter @whitneymcknight
AUSTIN, TEX. – Smokers offered home oxygen therapy were found to be at twice the risk for burn injuries, based on data from a retrospective study.
Even so, almost all the home oxygen therapy (HOT) burn victims were discharged with a prescription for oxygen, including the 15% of patients who had incurred similar injuries at least once, and in some cases, three times.
“I have a problem with this,” said Dr. Mary Baker, a critical care fellow at Indiana University and a medical ethics fellow at the university’s Richard M. Fairbanks Burn Center at Wishard-Eskenazi Health, both in Indianapolis. Dr. Baker presented the findings at the annual meeting of the American College of Chest Physicians.
“Should we be prescribing oxygen to patients who smoke? Maybe the bigger question is [whether] it is ever ethically defensible to take oxygen away once someone has sustained a combustion injury from smoking while using HOT,” she said.
Dr. Baker and her colleagues conducted a chart review of patients admitted to a single site for home oxygen–related burns between 2008 and 2013. They found that 55 of all such burn unit admissions were smokers, representing 4% of the center’s annual admissions rate and twice that of the national burn rate for smokers in general. Nearly all the patients, a balance of men and women with a median age of 61 years, were using HOT for chronic obstructive pulmonary disease.
“The location of the burns, probably not surprisingly, was the face. Probably the most common was the nasal cannula,” Dr. Baker said.
Although nearly three-quarters of the 55-member cohort had less than a 5% total body surface–area burn, Dr. Baker said that in a patient population with baseline respiratory compromise and respiratory failure, this was an alarming rate of morbidity, particularly since half of the injured were intubated, and bronchonscopic exam revealed a third of these patients also had inhalation injuries.
“And here’s the kicker,” said Dr. Baker. “Eight deaths over 5 years. This is huge. So when these [individuals] get burned, it’s often really bad. Several of them had house fires, and we were able to find in the chart where other people [in the home] were burned and admitted to the hospital.”
Still, after a median 5-day stay, almost all the patients who survived were discharged with prescriptions for HOT, including the so-called “repeat offenders.” Because nearly half of all surviving smoking-related HOT patients were discharged to a higher level of care, this cohort tended to have higher health care utilization rates as well, Dr. Baker noted.
A surprise finding was that more than a quarter of the cohort had either current or concomitant problems with substance abuse. “We were not expecting that, and it has not been previously reported,” Dr. Baker said.
The data demonstrate a need for the screening of HOT patients as to whether they smoke and whether they have substance use issues, she said. If either condition applies, then faster follow-up and, potentially, counseling could be offered, including better education about the risks of oxygen therapy. “Currently, we have no formalized way to educate patients on the dangers of those tanks in the home,” said Dr. Baker.
The data raise questions about the risk-benefit ratio of prescribing any breathing aid to COPD patients who are also smokers.
“I don’t know how much sense it makes to keep throwing these inhalers, which cost hundreds of dollars a month, at people who continue to smoke,” Dr. Baker said in an interview. “We take all comers, and we think oxygen therapy helps, and prolongs life, but when you factor in smoking, we don’t really know what the risks and benefits are.”
A large study population would be needed to determine the risks and benefits, she added.
On Twitter @whitneymcknight
AUSTIN, TEX. – Smokers offered home oxygen therapy were found to be at twice the risk for burn injuries, based on data from a retrospective study.
Even so, almost all the home oxygen therapy (HOT) burn victims were discharged with a prescription for oxygen, including the 15% of patients who had incurred similar injuries at least once, and in some cases, three times.
“I have a problem with this,” said Dr. Mary Baker, a critical care fellow at Indiana University and a medical ethics fellow at the university’s Richard M. Fairbanks Burn Center at Wishard-Eskenazi Health, both in Indianapolis. Dr. Baker presented the findings at the annual meeting of the American College of Chest Physicians.
“Should we be prescribing oxygen to patients who smoke? Maybe the bigger question is [whether] it is ever ethically defensible to take oxygen away once someone has sustained a combustion injury from smoking while using HOT,” she said.
Dr. Baker and her colleagues conducted a chart review of patients admitted to a single site for home oxygen–related burns between 2008 and 2013. They found that 55 of all such burn unit admissions were smokers, representing 4% of the center’s annual admissions rate and twice that of the national burn rate for smokers in general. Nearly all the patients, a balance of men and women with a median age of 61 years, were using HOT for chronic obstructive pulmonary disease.
“The location of the burns, probably not surprisingly, was the face. Probably the most common was the nasal cannula,” Dr. Baker said.
Although nearly three-quarters of the 55-member cohort had less than a 5% total body surface–area burn, Dr. Baker said that in a patient population with baseline respiratory compromise and respiratory failure, this was an alarming rate of morbidity, particularly since half of the injured were intubated, and bronchonscopic exam revealed a third of these patients also had inhalation injuries.
“And here’s the kicker,” said Dr. Baker. “Eight deaths over 5 years. This is huge. So when these [individuals] get burned, it’s often really bad. Several of them had house fires, and we were able to find in the chart where other people [in the home] were burned and admitted to the hospital.”
Still, after a median 5-day stay, almost all the patients who survived were discharged with prescriptions for HOT, including the so-called “repeat offenders.” Because nearly half of all surviving smoking-related HOT patients were discharged to a higher level of care, this cohort tended to have higher health care utilization rates as well, Dr. Baker noted.
A surprise finding was that more than a quarter of the cohort had either current or concomitant problems with substance abuse. “We were not expecting that, and it has not been previously reported,” Dr. Baker said.
The data demonstrate a need for the screening of HOT patients as to whether they smoke and whether they have substance use issues, she said. If either condition applies, then faster follow-up and, potentially, counseling could be offered, including better education about the risks of oxygen therapy. “Currently, we have no formalized way to educate patients on the dangers of those tanks in the home,” said Dr. Baker.
The data raise questions about the risk-benefit ratio of prescribing any breathing aid to COPD patients who are also smokers.
“I don’t know how much sense it makes to keep throwing these inhalers, which cost hundreds of dollars a month, at people who continue to smoke,” Dr. Baker said in an interview. “We take all comers, and we think oxygen therapy helps, and prolongs life, but when you factor in smoking, we don’t really know what the risks and benefits are.”
A large study population would be needed to determine the risks and benefits, she added.
On Twitter @whitneymcknight
AT CHEST 2014
Key clinical point: Counsel patients on the elevated risk of mortality and morbidity when HOT and smoking are combined.
Major finding: The burn injury rate for smokers with COPD using HOT was 4%, compared with 2% in smokers not using HOT.
Data source: Retrospective analysis of single site burn injury admissions beween 2008 and 2013.
Disclosures: Dr. Baker reported that she had no relevant disclosures.
New drugs are ‘nice’ but many IBD questions remain open for study
ORLANDO – Independent investigators eager to author studies that have notable impact on the field of inflammatory bowel disease have plenty of important paths to pursue, according to Dr. Jean-Frederic Colombel, a professor of gastroenterology at Mt. Sinai Hospital in New York.
“Over the next 3-5 years, we will have a lot of new drugs, which is nice, but we don’t yet know how to use them, or which ones to use,” Dr. Colombel said during a presentation on the future of the field at this year’s meeting of the annual Advances in Inflammatory Bowel Disease, sponsored by the Crohn’s and Colitis Foundation of America.
A host of biologics including vedolizumab, recently indicated by the U.S. Food and Drug Administration for ulcerative colitis and Crohn’s disease, and ustekinumab, currently in phase III studies for Crohn’s disease, are set to revolutionize treatment at a time when the field is already undergoing great change, according to Dr. Colombel. The result, he says, is that despite “formidable” challenges in recruitment and funding, “huge opportunities” exist for investigators willing to collaborate and be creative.
Studies that elucidate the natural history of Crohn’s disease and colitis offer insight into the efficacy of various treatment strategies, help determine whether to target symptoms or biomarkers, and answer whether combination therapies are safe and effective in certain patients, among others, are what Dr. Colombel says he hopes will help improve the field as the drug pipeline continues to grow.
He stipulated a caveat, however, “I strongly believe we need some new study designs.” Although he noted that trials needn’t be complicated, they should be long enough to collect sorely needed prospective data. “These can only be done in investigator-initiated trials because of the time frames,” Dr. Colombel urged the audience of young investigators and their colleagues.
A fieldwide shift in thinking about ulcerative colitis and Crohn’s disease as chronic, progressive diseases, rather than intermittent afflictions has already helped generate new study endpoints such as the Lémann Score, an index of progressive bowel damage that allows researchers to better track the history of IBD in patients, and thus provide a window of opportunity for interventions, said Dr. Colombel.
To wit, the CURE study, conducted by the French IBD society GITAID, is a 5-year, prospective study of patients whose early Crohn’s disease is treated with the biologic adalimumab. The Lémann Score is used to screen patients at the end of each successive year, in order to adjust their treatment to reach the final endpoint of deep remission by year 5 when several indicators are measured, including bowel image, level of disability, and whether there was the need for surgery. Dr. Colombel said the novel design of the study, which has already recruited 60 patients, with successive endpoints to evaluate new drugs and strategies over longer periods of time shows it is “feasible” to collect longitudinal data.
The field also has a responsibility to conduct randomly assigned controlled trials to demonstrate the comparative effectiveness, safety, immunogenicity, and cost benefits of the rapidly emerging spate of biosimilars, particularly since there is not global agreement about their use, according to Dr. Colombel. Their approval for IBD in Europe is “highly disputable ... I think this could be a very nice topic for an investigator-initiated trial, in Canada and the U.S.”
Head-to-head trials that are well designed will help answer “very important questions” about which treatment strategies, including the use of biosimilars, have the best outcomes, but doing so requires fortitude, according to Dr. Colombel. “The Dutch have the guts to conduct the LIRIC trial,” he said. In it, patients with Crohn’s disease in the terminal ileum, who have failed steroids or immune therapy, will be assigned randomly to either laparoscopic ileocolic resection or infliximab, the first available biosimilar. Sixty participants have enrolled to date, he said.
These types of studies could also help delineate how best to employ combination therapies. “What I propose for this kind of study is an intensive therapy combining biologics very early in patients with bad prognoses, and looking at the long-term outcome using bowel image,” Dr. Colombel said. These data, and others indicating the most appropriate length of treatment in patients with varying states of disease, are Dr. Colombel’s personal “top choice” for investigation.
The excitement that personalized medicine has engendered across the specialities has so far not resulted in specific, validated treatments for IBD patients. However, the question of how to use personal characteristics, serologic and genetic markers to create predictive models for which patients will need either step-up or top-down therapies, accounting for their individual risk of complication, is what he said is among the most “important [question] we need to answer because the choice of early therapy will depend on this predictor,” according to Dr. Colombel.
The question of which treatment targets are best remains unclear but is important to decipher, according to Dr. Colombel, who said simple studies comparing outcomes when patients are treated to symptoms vs. treated to biomarker measurements are needed. Whether to treat to certain medication trough levels vs. symptom relief is also a pressing need, as well as is the importance of mucosal healing vs. symptoms. “This is important because there is discordance between endoscopy and histology,” said Dr. Colombel. “Persistent histologic inflammation is frequently associated with bad outcomes.”
However, endoscopic scoring itself is another area Dr. Colombel said is worth investigating, especially when it comes to validating endpoints such as those in the Crohn’s Disease Index of Severity (CDEIS), and Simple Endoscopic Score for Crohn’s Disease (SES-CD), which will help determine remission cut offs. Also, using endoscopic scores in comparison with live video and imaging still need standardized approaches. “When you see how it is done in the U.S., it is generally very poor,” Dr. Colombel said.
The biggest obstacles of all when it comes to independent investigation in the United States, according to Frenchman Dr. Colombel, is heavy regulation and cost. “In France, [conducting trials] was considered part of my job. I was not compensated. It is different here.”
Despite that, he said successful studies are a matter of desire. “You have to have dedicated people who want to run these studies and who can meet often and drive the process, and be ready to recruit patients.”
Dr. Colombel has numerous financial ties to the pharmaceutical industry, including AB Science, Amgen, Baxter, Bristol-Meyers Squibb, Merck, Nutrition Science Partners, Teva, and Vertex, among several others.
On Twitter @whitneymcknight
ORLANDO – Independent investigators eager to author studies that have notable impact on the field of inflammatory bowel disease have plenty of important paths to pursue, according to Dr. Jean-Frederic Colombel, a professor of gastroenterology at Mt. Sinai Hospital in New York.
“Over the next 3-5 years, we will have a lot of new drugs, which is nice, but we don’t yet know how to use them, or which ones to use,” Dr. Colombel said during a presentation on the future of the field at this year’s meeting of the annual Advances in Inflammatory Bowel Disease, sponsored by the Crohn’s and Colitis Foundation of America.
A host of biologics including vedolizumab, recently indicated by the U.S. Food and Drug Administration for ulcerative colitis and Crohn’s disease, and ustekinumab, currently in phase III studies for Crohn’s disease, are set to revolutionize treatment at a time when the field is already undergoing great change, according to Dr. Colombel. The result, he says, is that despite “formidable” challenges in recruitment and funding, “huge opportunities” exist for investigators willing to collaborate and be creative.
Studies that elucidate the natural history of Crohn’s disease and colitis offer insight into the efficacy of various treatment strategies, help determine whether to target symptoms or biomarkers, and answer whether combination therapies are safe and effective in certain patients, among others, are what Dr. Colombel says he hopes will help improve the field as the drug pipeline continues to grow.
He stipulated a caveat, however, “I strongly believe we need some new study designs.” Although he noted that trials needn’t be complicated, they should be long enough to collect sorely needed prospective data. “These can only be done in investigator-initiated trials because of the time frames,” Dr. Colombel urged the audience of young investigators and their colleagues.
A fieldwide shift in thinking about ulcerative colitis and Crohn’s disease as chronic, progressive diseases, rather than intermittent afflictions has already helped generate new study endpoints such as the Lémann Score, an index of progressive bowel damage that allows researchers to better track the history of IBD in patients, and thus provide a window of opportunity for interventions, said Dr. Colombel.
To wit, the CURE study, conducted by the French IBD society GITAID, is a 5-year, prospective study of patients whose early Crohn’s disease is treated with the biologic adalimumab. The Lémann Score is used to screen patients at the end of each successive year, in order to adjust their treatment to reach the final endpoint of deep remission by year 5 when several indicators are measured, including bowel image, level of disability, and whether there was the need for surgery. Dr. Colombel said the novel design of the study, which has already recruited 60 patients, with successive endpoints to evaluate new drugs and strategies over longer periods of time shows it is “feasible” to collect longitudinal data.
The field also has a responsibility to conduct randomly assigned controlled trials to demonstrate the comparative effectiveness, safety, immunogenicity, and cost benefits of the rapidly emerging spate of biosimilars, particularly since there is not global agreement about their use, according to Dr. Colombel. Their approval for IBD in Europe is “highly disputable ... I think this could be a very nice topic for an investigator-initiated trial, in Canada and the U.S.”
Head-to-head trials that are well designed will help answer “very important questions” about which treatment strategies, including the use of biosimilars, have the best outcomes, but doing so requires fortitude, according to Dr. Colombel. “The Dutch have the guts to conduct the LIRIC trial,” he said. In it, patients with Crohn’s disease in the terminal ileum, who have failed steroids or immune therapy, will be assigned randomly to either laparoscopic ileocolic resection or infliximab, the first available biosimilar. Sixty participants have enrolled to date, he said.
These types of studies could also help delineate how best to employ combination therapies. “What I propose for this kind of study is an intensive therapy combining biologics very early in patients with bad prognoses, and looking at the long-term outcome using bowel image,” Dr. Colombel said. These data, and others indicating the most appropriate length of treatment in patients with varying states of disease, are Dr. Colombel’s personal “top choice” for investigation.
The excitement that personalized medicine has engendered across the specialities has so far not resulted in specific, validated treatments for IBD patients. However, the question of how to use personal characteristics, serologic and genetic markers to create predictive models for which patients will need either step-up or top-down therapies, accounting for their individual risk of complication, is what he said is among the most “important [question] we need to answer because the choice of early therapy will depend on this predictor,” according to Dr. Colombel.
The question of which treatment targets are best remains unclear but is important to decipher, according to Dr. Colombel, who said simple studies comparing outcomes when patients are treated to symptoms vs. treated to biomarker measurements are needed. Whether to treat to certain medication trough levels vs. symptom relief is also a pressing need, as well as is the importance of mucosal healing vs. symptoms. “This is important because there is discordance between endoscopy and histology,” said Dr. Colombel. “Persistent histologic inflammation is frequently associated with bad outcomes.”
However, endoscopic scoring itself is another area Dr. Colombel said is worth investigating, especially when it comes to validating endpoints such as those in the Crohn’s Disease Index of Severity (CDEIS), and Simple Endoscopic Score for Crohn’s Disease (SES-CD), which will help determine remission cut offs. Also, using endoscopic scores in comparison with live video and imaging still need standardized approaches. “When you see how it is done in the U.S., it is generally very poor,” Dr. Colombel said.
The biggest obstacles of all when it comes to independent investigation in the United States, according to Frenchman Dr. Colombel, is heavy regulation and cost. “In France, [conducting trials] was considered part of my job. I was not compensated. It is different here.”
Despite that, he said successful studies are a matter of desire. “You have to have dedicated people who want to run these studies and who can meet often and drive the process, and be ready to recruit patients.”
Dr. Colombel has numerous financial ties to the pharmaceutical industry, including AB Science, Amgen, Baxter, Bristol-Meyers Squibb, Merck, Nutrition Science Partners, Teva, and Vertex, among several others.
On Twitter @whitneymcknight
ORLANDO – Independent investigators eager to author studies that have notable impact on the field of inflammatory bowel disease have plenty of important paths to pursue, according to Dr. Jean-Frederic Colombel, a professor of gastroenterology at Mt. Sinai Hospital in New York.
“Over the next 3-5 years, we will have a lot of new drugs, which is nice, but we don’t yet know how to use them, or which ones to use,” Dr. Colombel said during a presentation on the future of the field at this year’s meeting of the annual Advances in Inflammatory Bowel Disease, sponsored by the Crohn’s and Colitis Foundation of America.
A host of biologics including vedolizumab, recently indicated by the U.S. Food and Drug Administration for ulcerative colitis and Crohn’s disease, and ustekinumab, currently in phase III studies for Crohn’s disease, are set to revolutionize treatment at a time when the field is already undergoing great change, according to Dr. Colombel. The result, he says, is that despite “formidable” challenges in recruitment and funding, “huge opportunities” exist for investigators willing to collaborate and be creative.
Studies that elucidate the natural history of Crohn’s disease and colitis offer insight into the efficacy of various treatment strategies, help determine whether to target symptoms or biomarkers, and answer whether combination therapies are safe and effective in certain patients, among others, are what Dr. Colombel says he hopes will help improve the field as the drug pipeline continues to grow.
He stipulated a caveat, however, “I strongly believe we need some new study designs.” Although he noted that trials needn’t be complicated, they should be long enough to collect sorely needed prospective data. “These can only be done in investigator-initiated trials because of the time frames,” Dr. Colombel urged the audience of young investigators and their colleagues.
A fieldwide shift in thinking about ulcerative colitis and Crohn’s disease as chronic, progressive diseases, rather than intermittent afflictions has already helped generate new study endpoints such as the Lémann Score, an index of progressive bowel damage that allows researchers to better track the history of IBD in patients, and thus provide a window of opportunity for interventions, said Dr. Colombel.
To wit, the CURE study, conducted by the French IBD society GITAID, is a 5-year, prospective study of patients whose early Crohn’s disease is treated with the biologic adalimumab. The Lémann Score is used to screen patients at the end of each successive year, in order to adjust their treatment to reach the final endpoint of deep remission by year 5 when several indicators are measured, including bowel image, level of disability, and whether there was the need for surgery. Dr. Colombel said the novel design of the study, which has already recruited 60 patients, with successive endpoints to evaluate new drugs and strategies over longer periods of time shows it is “feasible” to collect longitudinal data.
The field also has a responsibility to conduct randomly assigned controlled trials to demonstrate the comparative effectiveness, safety, immunogenicity, and cost benefits of the rapidly emerging spate of biosimilars, particularly since there is not global agreement about their use, according to Dr. Colombel. Their approval for IBD in Europe is “highly disputable ... I think this could be a very nice topic for an investigator-initiated trial, in Canada and the U.S.”
Head-to-head trials that are well designed will help answer “very important questions” about which treatment strategies, including the use of biosimilars, have the best outcomes, but doing so requires fortitude, according to Dr. Colombel. “The Dutch have the guts to conduct the LIRIC trial,” he said. In it, patients with Crohn’s disease in the terminal ileum, who have failed steroids or immune therapy, will be assigned randomly to either laparoscopic ileocolic resection or infliximab, the first available biosimilar. Sixty participants have enrolled to date, he said.
These types of studies could also help delineate how best to employ combination therapies. “What I propose for this kind of study is an intensive therapy combining biologics very early in patients with bad prognoses, and looking at the long-term outcome using bowel image,” Dr. Colombel said. These data, and others indicating the most appropriate length of treatment in patients with varying states of disease, are Dr. Colombel’s personal “top choice” for investigation.
The excitement that personalized medicine has engendered across the specialities has so far not resulted in specific, validated treatments for IBD patients. However, the question of how to use personal characteristics, serologic and genetic markers to create predictive models for which patients will need either step-up or top-down therapies, accounting for their individual risk of complication, is what he said is among the most “important [question] we need to answer because the choice of early therapy will depend on this predictor,” according to Dr. Colombel.
The question of which treatment targets are best remains unclear but is important to decipher, according to Dr. Colombel, who said simple studies comparing outcomes when patients are treated to symptoms vs. treated to biomarker measurements are needed. Whether to treat to certain medication trough levels vs. symptom relief is also a pressing need, as well as is the importance of mucosal healing vs. symptoms. “This is important because there is discordance between endoscopy and histology,” said Dr. Colombel. “Persistent histologic inflammation is frequently associated with bad outcomes.”
However, endoscopic scoring itself is another area Dr. Colombel said is worth investigating, especially when it comes to validating endpoints such as those in the Crohn’s Disease Index of Severity (CDEIS), and Simple Endoscopic Score for Crohn’s Disease (SES-CD), which will help determine remission cut offs. Also, using endoscopic scores in comparison with live video and imaging still need standardized approaches. “When you see how it is done in the U.S., it is generally very poor,” Dr. Colombel said.
The biggest obstacles of all when it comes to independent investigation in the United States, according to Frenchman Dr. Colombel, is heavy regulation and cost. “In France, [conducting trials] was considered part of my job. I was not compensated. It is different here.”
Despite that, he said successful studies are a matter of desire. “You have to have dedicated people who want to run these studies and who can meet often and drive the process, and be ready to recruit patients.”
Dr. Colombel has numerous financial ties to the pharmaceutical industry, including AB Science, Amgen, Baxter, Bristol-Meyers Squibb, Merck, Nutrition Science Partners, Teva, and Vertex, among several others.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM ADVANCES IN IBD 2014
New ICU sedation protocols linked to fewer ventilator days
AUSTIN, TEX. – Patients given propofol-based sedation in the intensive care unit were more likely to have daily dose optimization, stay at their target Richmond Agitation Sedation Scale rate, and be intubated for fewer days, compared with patients given benzodiazepine-based regimens, a retrospective study has shown. Being on propofol at the time of extubation, however, was associated with a significantly higher risk of being reintubated.
In 2013, the American College of Critical Care Medicine updated its guidelines for sedation in the ICU. The changes reflected what Dr. Steven J. Campbell, a presenter at this year’s annual meeting of the American College of Chest Physicians, said was the new paradigm in ICU sedation – namely, to use the least amount of sedation in patients and for the shortest amount of time possible. “We’ve also seen a shift away from benzodiazepines in recent years, and that when patients are given them, they stay in ICUs longer and have longer ventilation times,” Dr. Campbell said. The updated guidelines also suggest not relying on benzodiazepines as a first-line sedative.
To investigate how the updated sedation protocols have impacted the multiple ICUs at Ohio State University’s tertiary medical center, where Dr. Campbell is a third-year medical resident, and to assess the relationship of the changes with reintubation risk, Dr. Campbell and his colleagues retrospectively analyzed data on 988 intubated patients and 6,359 ventilator days recorded at the medical center during a 10-month period in 2013, after the new protocols were in place.
Considering either single sedation drips or combined sedation drips in the 988 unique intubations, about 69% of patients received at least 1 day of propofol, and roughly a third were given at least 1 day of a narcotic.
For 373 patients, the most commonly used drip was propofol only. Propofol combined with a narcotic was used in 141 patients, whereas the a combination of the two with a benzodiazepine was used in 140 patients.
A quarter of all intubated patients received at least 1 day of a continuous benzodiazepine drip, although only 7% of these received this sedation regimen as a first-line agent. Data were not presented on what previous benzodiazepine sedation rates were at the center before the protocol change.
The number of ventilator days for the propofol-only group was between 5.6 days plus or minus another 5.8 days, nearly half of the total propofol/narcotic/benzodiazepine ventilator days which came to 10.5 days give or take another 8 days.
“It intuitively makes sense that the more drips the patients were on, the more they would be on a ventilator,” Dr. Campbell said.
However, for patients given benzodiazepines only, the number of ventilator days was 4.3, plus or minus 4.2 days. Dr. Campbell theorized this was attributable to there being a number of patients withdrawing from alcohol and so needing to rely on an infusion of the benzodiazepine to help them through the process.
Propofol-based regimens were associated with improved dose optimization compliance if patients were eligible (P less than .0001).
Patients given narcotic drips were more likely to meet their targeted RASS levels of –1 to +1, compared with either benzodiazepines or propofol, although propofol patient RASS targets were higher than those of the benzodiazepine group (43% for narcotics, 22% for benzodiazepines, and 37% for propofol, P less than .0001).
The study also found a relationship between failed extubation rates and sedative use. There were 953 patients extubated in all. Seven percent of the extubated patients who had received continuous sedation on the day of extubation had to be reintubated within 48 hours. A significant risk of reintubation was found for patients who’d been given propofol alone since nearly half of that cohort were among those reintubated (P = .01).
Although Dr. Campbell and his colleagues wrote in their study that this could have been due to either lower levels of sedation to begin with, and so being more likely to have earlier extubation, or that the respiratory physiology of this group was altered by the propofol.
“The most important take-away here was that being on any sedative within 24 hours of extubation meant you had a high rate of failing that extubation,” Dr. Campbell said.
The investigators were not able to determine precisely when the sedation was terminated in each patient, only that it had occurred within a 24-hour period prior to extubation.
Dr. Campbell also noted that since dexmedetomidine was not widely used at the site ICUs as an alternative sedative, a future inquiry into the reasons why not would be worthwhile. “I suspect it’s because it’s still one of the newer agents,” he said.
On Twitter @whitneymcknight
AUSTIN, TEX. – Patients given propofol-based sedation in the intensive care unit were more likely to have daily dose optimization, stay at their target Richmond Agitation Sedation Scale rate, and be intubated for fewer days, compared with patients given benzodiazepine-based regimens, a retrospective study has shown. Being on propofol at the time of extubation, however, was associated with a significantly higher risk of being reintubated.
In 2013, the American College of Critical Care Medicine updated its guidelines for sedation in the ICU. The changes reflected what Dr. Steven J. Campbell, a presenter at this year’s annual meeting of the American College of Chest Physicians, said was the new paradigm in ICU sedation – namely, to use the least amount of sedation in patients and for the shortest amount of time possible. “We’ve also seen a shift away from benzodiazepines in recent years, and that when patients are given them, they stay in ICUs longer and have longer ventilation times,” Dr. Campbell said. The updated guidelines also suggest not relying on benzodiazepines as a first-line sedative.
To investigate how the updated sedation protocols have impacted the multiple ICUs at Ohio State University’s tertiary medical center, where Dr. Campbell is a third-year medical resident, and to assess the relationship of the changes with reintubation risk, Dr. Campbell and his colleagues retrospectively analyzed data on 988 intubated patients and 6,359 ventilator days recorded at the medical center during a 10-month period in 2013, after the new protocols were in place.
Considering either single sedation drips or combined sedation drips in the 988 unique intubations, about 69% of patients received at least 1 day of propofol, and roughly a third were given at least 1 day of a narcotic.
For 373 patients, the most commonly used drip was propofol only. Propofol combined with a narcotic was used in 141 patients, whereas the a combination of the two with a benzodiazepine was used in 140 patients.
A quarter of all intubated patients received at least 1 day of a continuous benzodiazepine drip, although only 7% of these received this sedation regimen as a first-line agent. Data were not presented on what previous benzodiazepine sedation rates were at the center before the protocol change.
The number of ventilator days for the propofol-only group was between 5.6 days plus or minus another 5.8 days, nearly half of the total propofol/narcotic/benzodiazepine ventilator days which came to 10.5 days give or take another 8 days.
“It intuitively makes sense that the more drips the patients were on, the more they would be on a ventilator,” Dr. Campbell said.
However, for patients given benzodiazepines only, the number of ventilator days was 4.3, plus or minus 4.2 days. Dr. Campbell theorized this was attributable to there being a number of patients withdrawing from alcohol and so needing to rely on an infusion of the benzodiazepine to help them through the process.
Propofol-based regimens were associated with improved dose optimization compliance if patients were eligible (P less than .0001).
Patients given narcotic drips were more likely to meet their targeted RASS levels of –1 to +1, compared with either benzodiazepines or propofol, although propofol patient RASS targets were higher than those of the benzodiazepine group (43% for narcotics, 22% for benzodiazepines, and 37% for propofol, P less than .0001).
The study also found a relationship between failed extubation rates and sedative use. There were 953 patients extubated in all. Seven percent of the extubated patients who had received continuous sedation on the day of extubation had to be reintubated within 48 hours. A significant risk of reintubation was found for patients who’d been given propofol alone since nearly half of that cohort were among those reintubated (P = .01).
Although Dr. Campbell and his colleagues wrote in their study that this could have been due to either lower levels of sedation to begin with, and so being more likely to have earlier extubation, or that the respiratory physiology of this group was altered by the propofol.
“The most important take-away here was that being on any sedative within 24 hours of extubation meant you had a high rate of failing that extubation,” Dr. Campbell said.
The investigators were not able to determine precisely when the sedation was terminated in each patient, only that it had occurred within a 24-hour period prior to extubation.
Dr. Campbell also noted that since dexmedetomidine was not widely used at the site ICUs as an alternative sedative, a future inquiry into the reasons why not would be worthwhile. “I suspect it’s because it’s still one of the newer agents,” he said.
On Twitter @whitneymcknight
AUSTIN, TEX. – Patients given propofol-based sedation in the intensive care unit were more likely to have daily dose optimization, stay at their target Richmond Agitation Sedation Scale rate, and be intubated for fewer days, compared with patients given benzodiazepine-based regimens, a retrospective study has shown. Being on propofol at the time of extubation, however, was associated with a significantly higher risk of being reintubated.
In 2013, the American College of Critical Care Medicine updated its guidelines for sedation in the ICU. The changes reflected what Dr. Steven J. Campbell, a presenter at this year’s annual meeting of the American College of Chest Physicians, said was the new paradigm in ICU sedation – namely, to use the least amount of sedation in patients and for the shortest amount of time possible. “We’ve also seen a shift away from benzodiazepines in recent years, and that when patients are given them, they stay in ICUs longer and have longer ventilation times,” Dr. Campbell said. The updated guidelines also suggest not relying on benzodiazepines as a first-line sedative.
To investigate how the updated sedation protocols have impacted the multiple ICUs at Ohio State University’s tertiary medical center, where Dr. Campbell is a third-year medical resident, and to assess the relationship of the changes with reintubation risk, Dr. Campbell and his colleagues retrospectively analyzed data on 988 intubated patients and 6,359 ventilator days recorded at the medical center during a 10-month period in 2013, after the new protocols were in place.
Considering either single sedation drips or combined sedation drips in the 988 unique intubations, about 69% of patients received at least 1 day of propofol, and roughly a third were given at least 1 day of a narcotic.
For 373 patients, the most commonly used drip was propofol only. Propofol combined with a narcotic was used in 141 patients, whereas the a combination of the two with a benzodiazepine was used in 140 patients.
A quarter of all intubated patients received at least 1 day of a continuous benzodiazepine drip, although only 7% of these received this sedation regimen as a first-line agent. Data were not presented on what previous benzodiazepine sedation rates were at the center before the protocol change.
The number of ventilator days for the propofol-only group was between 5.6 days plus or minus another 5.8 days, nearly half of the total propofol/narcotic/benzodiazepine ventilator days which came to 10.5 days give or take another 8 days.
“It intuitively makes sense that the more drips the patients were on, the more they would be on a ventilator,” Dr. Campbell said.
However, for patients given benzodiazepines only, the number of ventilator days was 4.3, plus or minus 4.2 days. Dr. Campbell theorized this was attributable to there being a number of patients withdrawing from alcohol and so needing to rely on an infusion of the benzodiazepine to help them through the process.
Propofol-based regimens were associated with improved dose optimization compliance if patients were eligible (P less than .0001).
Patients given narcotic drips were more likely to meet their targeted RASS levels of –1 to +1, compared with either benzodiazepines or propofol, although propofol patient RASS targets were higher than those of the benzodiazepine group (43% for narcotics, 22% for benzodiazepines, and 37% for propofol, P less than .0001).
The study also found a relationship between failed extubation rates and sedative use. There were 953 patients extubated in all. Seven percent of the extubated patients who had received continuous sedation on the day of extubation had to be reintubated within 48 hours. A significant risk of reintubation was found for patients who’d been given propofol alone since nearly half of that cohort were among those reintubated (P = .01).
Although Dr. Campbell and his colleagues wrote in their study that this could have been due to either lower levels of sedation to begin with, and so being more likely to have earlier extubation, or that the respiratory physiology of this group was altered by the propofol.
“The most important take-away here was that being on any sedative within 24 hours of extubation meant you had a high rate of failing that extubation,” Dr. Campbell said.
The investigators were not able to determine precisely when the sedation was terminated in each patient, only that it had occurred within a 24-hour period prior to extubation.
Dr. Campbell also noted that since dexmedetomidine was not widely used at the site ICUs as an alternative sedative, a future inquiry into the reasons why not would be worthwhile. “I suspect it’s because it’s still one of the newer agents,” he said.
On Twitter @whitneymcknight
AT CHEST 2014
Key clinical point: Physicians are preferentially avoiding benzodiazepines in the ICU with better results overall.
Major finding: ICU patients sedated with propofol-based regimens were more likely to undergo daily dose optimization, maintain target RASS, compared with benzodiazepines, and be intubated fewer days.
Data source: Retrospective study of 988 intubated patients and 6,359 ventilation days over 10 months at a single tertiary, academic medical center.
Disclosures: Dr. Campbell said neither he nor his coauthors had relevant disclosures.
Higher opioid doses associated with increase in depression
NEW YORK – Patients taking daily opioid medication for pain had double the risk of depression over time at dosages of 50 mg or more per day, a new study has shown.
“We already know that depressed patients, over time, take more opioids. But we’ve been questioning whether this high-dose opioid use is really one of the reasons pain patients have higher rates of depression,” Jeffrey Scherrer, Ph.D., said at the annual meeting of the North American Primary Care Research Group.
A previous analysis of Veterans Affairs data conducted by Dr. Scherrer and colleagues demonstrated a risk of new-onset depression over time as opioid exposure increased. However, the retrospective study did not include repeated measures of individual pain severity (J. Gen. Intern. Med. 2014;29:491-9).
So Dr. Scherrer of the department of family and community medicine at Saint Louis University, and his colleagues prospectively studied 355 primary care patients – primarily white, middle-aged women – being treated for chronic low back pain at nine clinics across Texas. Patient levels of depression, pain, anxiety, health-related quality of life, and levels of stress and social support were self-reported at baseline, and at 1 year and 2 years of follow-up.
Information on opioid type and dose, and on comorbid conditions, was collected from patient records and used to assess any association between changes in opioid dosage (0, 1-50 mg, and greater than 50 mg daily) and probability of depression over time, as well as the change in depression levels and the odds of increased opioid dosage.
The adjusted odds ratio of depression over time was 2.65 (1.17-5.98) when opioid dosages increased from 0 to less than 50 mg per day. Although the risk of increased depression with an increase from 1 mg up to 50 mg of opioid per day was marginal (odds ratio, 1.08), the unadjusted odds of developing depression more than doubled (OR, 2.13) when opioid dosage surpassed 50 mg daily. The final adjusted analysis placed the odds ratio of developing depression at 1.65.
But what if patients are depressed because they are in pain or are more susceptible to pain because they are depressed?
“The depression being caused by the pain may be the baseline situation, but the worsening of depression is not due to the pain, it is due to the opioid.” Dr. Scherrer said. “We know that because we have repeated measures of pain, and while the pain remains about the same, the depression keeps going up at each time point. We have an epidemic of new-onset depression being driven by the opioid use, not by the pain.”
Why would a person’s level of pain remain flat despite escalated opioid dosing? Dr. Scherrer said that a previous but unreported history of depression may be making the person more sensitive to pain, and therefore less sensitive to the analgesic effects of the opioids – thus creating a cycle in which they use higher doses of pain medications for longer periods of time.
“If you have a history of depression, you ought to keep your opioids below 50 mg a day,” Dr. Scherrer counseled. “Otherwise, you’re going to jack up your risk of depression.”
The study was supported by the Residency Research Network of Texas Investigators.
On Twitter @whitneymcknight
NEW YORK – Patients taking daily opioid medication for pain had double the risk of depression over time at dosages of 50 mg or more per day, a new study has shown.
“We already know that depressed patients, over time, take more opioids. But we’ve been questioning whether this high-dose opioid use is really one of the reasons pain patients have higher rates of depression,” Jeffrey Scherrer, Ph.D., said at the annual meeting of the North American Primary Care Research Group.
A previous analysis of Veterans Affairs data conducted by Dr. Scherrer and colleagues demonstrated a risk of new-onset depression over time as opioid exposure increased. However, the retrospective study did not include repeated measures of individual pain severity (J. Gen. Intern. Med. 2014;29:491-9).
So Dr. Scherrer of the department of family and community medicine at Saint Louis University, and his colleagues prospectively studied 355 primary care patients – primarily white, middle-aged women – being treated for chronic low back pain at nine clinics across Texas. Patient levels of depression, pain, anxiety, health-related quality of life, and levels of stress and social support were self-reported at baseline, and at 1 year and 2 years of follow-up.
Information on opioid type and dose, and on comorbid conditions, was collected from patient records and used to assess any association between changes in opioid dosage (0, 1-50 mg, and greater than 50 mg daily) and probability of depression over time, as well as the change in depression levels and the odds of increased opioid dosage.
The adjusted odds ratio of depression over time was 2.65 (1.17-5.98) when opioid dosages increased from 0 to less than 50 mg per day. Although the risk of increased depression with an increase from 1 mg up to 50 mg of opioid per day was marginal (odds ratio, 1.08), the unadjusted odds of developing depression more than doubled (OR, 2.13) when opioid dosage surpassed 50 mg daily. The final adjusted analysis placed the odds ratio of developing depression at 1.65.
But what if patients are depressed because they are in pain or are more susceptible to pain because they are depressed?
“The depression being caused by the pain may be the baseline situation, but the worsening of depression is not due to the pain, it is due to the opioid.” Dr. Scherrer said. “We know that because we have repeated measures of pain, and while the pain remains about the same, the depression keeps going up at each time point. We have an epidemic of new-onset depression being driven by the opioid use, not by the pain.”
Why would a person’s level of pain remain flat despite escalated opioid dosing? Dr. Scherrer said that a previous but unreported history of depression may be making the person more sensitive to pain, and therefore less sensitive to the analgesic effects of the opioids – thus creating a cycle in which they use higher doses of pain medications for longer periods of time.
“If you have a history of depression, you ought to keep your opioids below 50 mg a day,” Dr. Scherrer counseled. “Otherwise, you’re going to jack up your risk of depression.”
The study was supported by the Residency Research Network of Texas Investigators.
On Twitter @whitneymcknight
NEW YORK – Patients taking daily opioid medication for pain had double the risk of depression over time at dosages of 50 mg or more per day, a new study has shown.
“We already know that depressed patients, over time, take more opioids. But we’ve been questioning whether this high-dose opioid use is really one of the reasons pain patients have higher rates of depression,” Jeffrey Scherrer, Ph.D., said at the annual meeting of the North American Primary Care Research Group.
A previous analysis of Veterans Affairs data conducted by Dr. Scherrer and colleagues demonstrated a risk of new-onset depression over time as opioid exposure increased. However, the retrospective study did not include repeated measures of individual pain severity (J. Gen. Intern. Med. 2014;29:491-9).
So Dr. Scherrer of the department of family and community medicine at Saint Louis University, and his colleagues prospectively studied 355 primary care patients – primarily white, middle-aged women – being treated for chronic low back pain at nine clinics across Texas. Patient levels of depression, pain, anxiety, health-related quality of life, and levels of stress and social support were self-reported at baseline, and at 1 year and 2 years of follow-up.
Information on opioid type and dose, and on comorbid conditions, was collected from patient records and used to assess any association between changes in opioid dosage (0, 1-50 mg, and greater than 50 mg daily) and probability of depression over time, as well as the change in depression levels and the odds of increased opioid dosage.
The adjusted odds ratio of depression over time was 2.65 (1.17-5.98) when opioid dosages increased from 0 to less than 50 mg per day. Although the risk of increased depression with an increase from 1 mg up to 50 mg of opioid per day was marginal (odds ratio, 1.08), the unadjusted odds of developing depression more than doubled (OR, 2.13) when opioid dosage surpassed 50 mg daily. The final adjusted analysis placed the odds ratio of developing depression at 1.65.
But what if patients are depressed because they are in pain or are more susceptible to pain because they are depressed?
“The depression being caused by the pain may be the baseline situation, but the worsening of depression is not due to the pain, it is due to the opioid.” Dr. Scherrer said. “We know that because we have repeated measures of pain, and while the pain remains about the same, the depression keeps going up at each time point. We have an epidemic of new-onset depression being driven by the opioid use, not by the pain.”
Why would a person’s level of pain remain flat despite escalated opioid dosing? Dr. Scherrer said that a previous but unreported history of depression may be making the person more sensitive to pain, and therefore less sensitive to the analgesic effects of the opioids – thus creating a cycle in which they use higher doses of pain medications for longer periods of time.
“If you have a history of depression, you ought to keep your opioids below 50 mg a day,” Dr. Scherrer counseled. “Otherwise, you’re going to jack up your risk of depression.”
The study was supported by the Residency Research Network of Texas Investigators.
On Twitter @whitneymcknight
AT NAPCRG 2014
Key clinical point: Consider the risk of depression when prescribing opioid dosages greater than 50 mg daily.
Major finding: Daily opioid dosages of 50 mg or greater were associated with a nearly doubled rate of depression in chronic pain patients (odds ratio, 1.65).
Data source: A prospective cohort study of 355 primary care patients with chronic low back pain, assessed at three time points.
Disclosures: The study was supported by the Residency Research Network of Texas Investigators.
Rx for specialists: Know how ACA affects patients’ ability to pay for meds
ORLANDO – Despite recent, significant shifts in health care coverage thanks to the Affordable Care Act, many specialists are unaware of how patients pay for pricey prescriptions such as biologics.
One reason is that clinicians just haven’t been paying enough attention, according to Dr. Kim L. Isaacs, codirector of the multidiscipline treatment and research center for inflammatory bowel disease at the University of North Carolina.
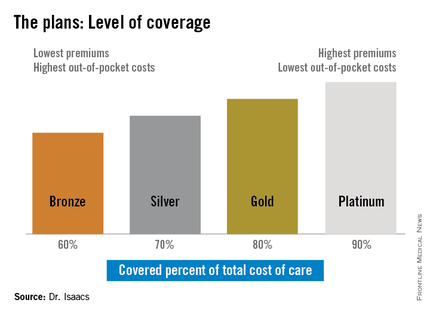
“It gets complicated because we’re taking care of patients, so it’s hard to think about the financial end of things as well, and it lands on the patient’s lap,” Dr. Isaacs said in an interview after her presentation, “Navigating the Affordable Care Act,” at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America.
But not knowing how and if chronically ill patients can afford to pay for their medications can impact their compliance, and even their disease states.
Dr. Isaacs compared the histories of two patients. The first one purchased through the ACA’s health insurance marketplace provision, and regardless of her preexisting condition, she was able to receive and afford treatment for her Crohn’s disease for the first time in a decade. The second patient purchased ACA-sanctioned insurance but it still wasn’t enough to cover the costs of her care.
“She told me she had thought the Silver Plan would work for her, but that she still couldn’t afford her medications, and I was thinking, ‘What’s a Silver Plan?’ ” Dr. Isaacs said. “I didn’t have a clue.”
The discrepancy between the patients’ plans prompted Dr. Isaacs to investigate whether what she was prescribing was practical under her patients’ various levels of coverage. She discovered that under the ACA, the individual mandate requiring all Americans to purchase some form of health insurance means many have turned to a variety of state- and federally-sponsored health insurance marketplaces that offer coverage plans ranging from Bronze to Platinum.
“If you ask [most specialists] what the Gold Plan is, most of them won’t have heard of it, they don’t know,” she said.
But she said that physicians need to be aware of whether the drugs they are prescribing are on the formulary used by their patients’ respective plans since the different insurance providers that back the various plans often don’t cover the same medications.
In addition, the so-called “donut hole,” the annually adjusted gap in prescription drug coverage for Medicare patients, is not scheduled to close until 2020. While the gap exists, Medicare patients have a set amount of annual drug coverage after which the patient shares a substantial portion of the cost until the following year when the process begins again.
“For our IBD patients, this is very important because for some of our more expensive drugs, there may be a period of time [annually] when the drugs are not covered,” Dr. Isaacs said.
The same could be true for other specialties such as oncology, rheumatology, and neurology, where disease states require high-cost specialty drugs; however, some patient advocacy groups will assist patients in paying for their treatment, she said.
Patients who purchase their health plans through the government-sponsored insurance marketplaces are usually eligible for subsidies, depending on their income and where they fall in relation to the federally set poverty level, said Dr. Isaacs.
Another important, basic point, she added, is that providers need to ensure that they are on their patients’ chosen plans. “If you’re not, then you need to be sure there is someone else who is on the plan who can take care of your patient.”
Dr. Isaacs reported she has affiliations with AbbVie, Janssen, Millennium, Takeda, UCB, and others.
On Twitter @whitneymcknight
ORLANDO – Despite recent, significant shifts in health care coverage thanks to the Affordable Care Act, many specialists are unaware of how patients pay for pricey prescriptions such as biologics.
One reason is that clinicians just haven’t been paying enough attention, according to Dr. Kim L. Isaacs, codirector of the multidiscipline treatment and research center for inflammatory bowel disease at the University of North Carolina.

“It gets complicated because we’re taking care of patients, so it’s hard to think about the financial end of things as well, and it lands on the patient’s lap,” Dr. Isaacs said in an interview after her presentation, “Navigating the Affordable Care Act,” at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America.
But not knowing how and if chronically ill patients can afford to pay for their medications can impact their compliance, and even their disease states.
Dr. Isaacs compared the histories of two patients. The first one purchased through the ACA’s health insurance marketplace provision, and regardless of her preexisting condition, she was able to receive and afford treatment for her Crohn’s disease for the first time in a decade. The second patient purchased ACA-sanctioned insurance but it still wasn’t enough to cover the costs of her care.
“She told me she had thought the Silver Plan would work for her, but that she still couldn’t afford her medications, and I was thinking, ‘What’s a Silver Plan?’ ” Dr. Isaacs said. “I didn’t have a clue.”
The discrepancy between the patients’ plans prompted Dr. Isaacs to investigate whether what she was prescribing was practical under her patients’ various levels of coverage. She discovered that under the ACA, the individual mandate requiring all Americans to purchase some form of health insurance means many have turned to a variety of state- and federally-sponsored health insurance marketplaces that offer coverage plans ranging from Bronze to Platinum.
“If you ask [most specialists] what the Gold Plan is, most of them won’t have heard of it, they don’t know,” she said.
But she said that physicians need to be aware of whether the drugs they are prescribing are on the formulary used by their patients’ respective plans since the different insurance providers that back the various plans often don’t cover the same medications.
In addition, the so-called “donut hole,” the annually adjusted gap in prescription drug coverage for Medicare patients, is not scheduled to close until 2020. While the gap exists, Medicare patients have a set amount of annual drug coverage after which the patient shares a substantial portion of the cost until the following year when the process begins again.
“For our IBD patients, this is very important because for some of our more expensive drugs, there may be a period of time [annually] when the drugs are not covered,” Dr. Isaacs said.
The same could be true for other specialties such as oncology, rheumatology, and neurology, where disease states require high-cost specialty drugs; however, some patient advocacy groups will assist patients in paying for their treatment, she said.
Patients who purchase their health plans through the government-sponsored insurance marketplaces are usually eligible for subsidies, depending on their income and where they fall in relation to the federally set poverty level, said Dr. Isaacs.
Another important, basic point, she added, is that providers need to ensure that they are on their patients’ chosen plans. “If you’re not, then you need to be sure there is someone else who is on the plan who can take care of your patient.”
Dr. Isaacs reported she has affiliations with AbbVie, Janssen, Millennium, Takeda, UCB, and others.
On Twitter @whitneymcknight
ORLANDO – Despite recent, significant shifts in health care coverage thanks to the Affordable Care Act, many specialists are unaware of how patients pay for pricey prescriptions such as biologics.
One reason is that clinicians just haven’t been paying enough attention, according to Dr. Kim L. Isaacs, codirector of the multidiscipline treatment and research center for inflammatory bowel disease at the University of North Carolina.

“It gets complicated because we’re taking care of patients, so it’s hard to think about the financial end of things as well, and it lands on the patient’s lap,” Dr. Isaacs said in an interview after her presentation, “Navigating the Affordable Care Act,” at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America.
But not knowing how and if chronically ill patients can afford to pay for their medications can impact their compliance, and even their disease states.
Dr. Isaacs compared the histories of two patients. The first one purchased through the ACA’s health insurance marketplace provision, and regardless of her preexisting condition, she was able to receive and afford treatment for her Crohn’s disease for the first time in a decade. The second patient purchased ACA-sanctioned insurance but it still wasn’t enough to cover the costs of her care.
“She told me she had thought the Silver Plan would work for her, but that she still couldn’t afford her medications, and I was thinking, ‘What’s a Silver Plan?’ ” Dr. Isaacs said. “I didn’t have a clue.”
The discrepancy between the patients’ plans prompted Dr. Isaacs to investigate whether what she was prescribing was practical under her patients’ various levels of coverage. She discovered that under the ACA, the individual mandate requiring all Americans to purchase some form of health insurance means many have turned to a variety of state- and federally-sponsored health insurance marketplaces that offer coverage plans ranging from Bronze to Platinum.
“If you ask [most specialists] what the Gold Plan is, most of them won’t have heard of it, they don’t know,” she said.
But she said that physicians need to be aware of whether the drugs they are prescribing are on the formulary used by their patients’ respective plans since the different insurance providers that back the various plans often don’t cover the same medications.
In addition, the so-called “donut hole,” the annually adjusted gap in prescription drug coverage for Medicare patients, is not scheduled to close until 2020. While the gap exists, Medicare patients have a set amount of annual drug coverage after which the patient shares a substantial portion of the cost until the following year when the process begins again.
“For our IBD patients, this is very important because for some of our more expensive drugs, there may be a period of time [annually] when the drugs are not covered,” Dr. Isaacs said.
The same could be true for other specialties such as oncology, rheumatology, and neurology, where disease states require high-cost specialty drugs; however, some patient advocacy groups will assist patients in paying for their treatment, she said.
Patients who purchase their health plans through the government-sponsored insurance marketplaces are usually eligible for subsidies, depending on their income and where they fall in relation to the federally set poverty level, said Dr. Isaacs.
Another important, basic point, she added, is that providers need to ensure that they are on their patients’ chosen plans. “If you’re not, then you need to be sure there is someone else who is on the plan who can take care of your patient.”
Dr. Isaacs reported she has affiliations with AbbVie, Janssen, Millennium, Takeda, UCB, and others.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM 2014 ADVANCES IN IBD
VIDEO: For family physicians, ‘Death is not a defeat’
NEW YORK– Family physicians are more comfortable with letting patients choose to die, and helping them do so comfortably, according to Dr. Richard Young, a speaker at this year’s annual meeting of the North American Primary Care Research Group.
“We’re more comfortable with death,” he said, comparing physicians accustomed to focusing on one part of the body rather than the whole. That can lead to unnecessary pain and agony for the patient and family, who would be better served being made comfortable, rather than having to endure the heroics of a specialist who “can’t let go,” noted Dr. Young, director of research at the John S. Peters Health System in Fort Worth, Tex.
This video interview is the third in a four-part series on the role family medicine plays in delivering humane, cost-effective health care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
NEW YORK– Family physicians are more comfortable with letting patients choose to die, and helping them do so comfortably, according to Dr. Richard Young, a speaker at this year’s annual meeting of the North American Primary Care Research Group.
“We’re more comfortable with death,” he said, comparing physicians accustomed to focusing on one part of the body rather than the whole. That can lead to unnecessary pain and agony for the patient and family, who would be better served being made comfortable, rather than having to endure the heroics of a specialist who “can’t let go,” noted Dr. Young, director of research at the John S. Peters Health System in Fort Worth, Tex.
This video interview is the third in a four-part series on the role family medicine plays in delivering humane, cost-effective health care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
NEW YORK– Family physicians are more comfortable with letting patients choose to die, and helping them do so comfortably, according to Dr. Richard Young, a speaker at this year’s annual meeting of the North American Primary Care Research Group.
“We’re more comfortable with death,” he said, comparing physicians accustomed to focusing on one part of the body rather than the whole. That can lead to unnecessary pain and agony for the patient and family, who would be better served being made comfortable, rather than having to endure the heroics of a specialist who “can’t let go,” noted Dr. Young, director of research at the John S. Peters Health System in Fort Worth, Tex.
This video interview is the third in a four-part series on the role family medicine plays in delivering humane, cost-effective health care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM NAPCRG 2014
VIDEO: Data support family physicians’ cost-effective care methods
NEW YORK – Family physicians are comfortable with uncertainty and view many tests as wastes of money.
These and other characteristics typical of family physicians are what Dr. Richard A. Young calls the foundation of the nation’s most practical and cost-effective care, despite what he calls “bigotry” toward the field from more specialized medicine and from those who created the current system of payment.
“The reason we add value to the world is because we don’t treat everybody the same,” said Dr. Young, the director of research and residency at the John Peter Smith Health System in Fort Worth, Tex.
In this video interview, the fourth and final in a series, Dr. Young makes the case for how, despite systematic overreliance on specialty care, evidence shows it is the primary care physician who is best suited to meet the majority of patients’ needs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
NEW YORK – Family physicians are comfortable with uncertainty and view many tests as wastes of money.
These and other characteristics typical of family physicians are what Dr. Richard A. Young calls the foundation of the nation’s most practical and cost-effective care, despite what he calls “bigotry” toward the field from more specialized medicine and from those who created the current system of payment.
“The reason we add value to the world is because we don’t treat everybody the same,” said Dr. Young, the director of research and residency at the John Peter Smith Health System in Fort Worth, Tex.
In this video interview, the fourth and final in a series, Dr. Young makes the case for how, despite systematic overreliance on specialty care, evidence shows it is the primary care physician who is best suited to meet the majority of patients’ needs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
NEW YORK – Family physicians are comfortable with uncertainty and view many tests as wastes of money.
These and other characteristics typical of family physicians are what Dr. Richard A. Young calls the foundation of the nation’s most practical and cost-effective care, despite what he calls “bigotry” toward the field from more specialized medicine and from those who created the current system of payment.
“The reason we add value to the world is because we don’t treat everybody the same,” said Dr. Young, the director of research and residency at the John Peter Smith Health System in Fort Worth, Tex.
In this video interview, the fourth and final in a series, Dr. Young makes the case for how, despite systematic overreliance on specialty care, evidence shows it is the primary care physician who is best suited to meet the majority of patients’ needs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM NAPCRG 2014
E-cigs surpass smoked tobacco in U.S. teens; marijuana use among ‘highest’ worldwide
Nearly a fifth of American high school seniors have used e-cigarettes recently, although tobacco smoking in this population is at an all-time low, according to the annual Monitoring the Future survey released Dec. 16.
“About 4% of 12th graders use e-cigarettes alone. They’ve never smoked a regular cigarette in their life,” Richard A. Miech, Ph.D., of the University of Michigan, Ann Arbor, one of the lead researchers on the study, said during a press conference held by the National Institute on Drug Abuse. This was the first year that data on e-cigarette use in teens were collected for the Monitoring the Future survey of drug, tobacco, and alcohol use in adolescents in 8th, 10th, and 12th grade. The survey has been conducted every year since 1975 by the University of Michigan and funded by NIDA.
Just over 17% of 12th graders reported past-month use of e-cigarettes. For 10th graders, the number was 16%, and for 8th graders it was nearly 9%. Daily cigarette smoking has decreased by half in the past 5 years across all age groups surveyed, with the largest decline being from just over 6% to about 3% in 10th graders.
Whether the decline in tobacco smoking correlates directly with the rise in e-cigarettes is unknown, NIDA director Dr. Nora Volkow said during the teleconference.
Particularly troubling, according to Dr. Miech, is that the 4% of 12th graders who reported using e-cigarettes exclusively tended to be college bound, a cohort he said typically does not use tobacco.
What actual harm e-cigarettes pose to youth, and whether they lead to other drug use are still open questions, according to Dr. Volkow.
“We can’t do a randomized, controlled trial of teens and give them e-cigarettes to see if they progress to harder drugs,” Dr. Miech said. “The best we can do is follow them as they age.”
Marijuana use among teens is steady and prevalent across all three age groups, according to the survey. More than a third of 12th graders (35%) reported past-year use of marijuana; nearly 6% reported daily use. “This constitutes one of the highest rates seen in any student population world wide,” said Dr. Volkow.
Similar to last year’s rates, 7% of eighth graders, 17% of 10th graders, and just over 21% of 12th graders reported smoking marijuana in the previous month.
The steady rates of marijuana use came as a welcome surprise, said Dr. Volkow, considering recent changes to marijuana laws at the state level and the way those changes might impact perception of marijuana use. In fact, there was a change across the entire study population in the perceived danger posed by marijuana, down from 27% 5 years ago to 16% this year.
In states with medical marijuana laws, 40% of 12th graders who reported using marijuana in the past year said they had used edible marijuana products, compared with 26% of seniors who lived in states without such legislation.
Whether digested versus smoked cannabinoids pose greater health risks to teens “is relatively new for us,” Dr. Volkow said, noting that there are little data on how quickly cannabinoids consumed orally may enter the bloodstream. “When you smoke a drug, it gets into the brain very rapidly, and that is associated with stronger rewarding affects and more addictiveness, but it also is associated with better control of how much you ingest.”
“It’s pretty obvious when people are smoking a joint – the feedback is quick,” said Dr. Lloyd D. Johnston, lead investigator for Monitoring the Future, also of the University of Michigan. Ingested marijuana is different and variable, he noted, both in the amount of marijuana consumed and in the time it takes for the drug to take effect. “I think it’s considerably more dangerous.”Understanding the bioavailability and the pharmacokinetics of edible marijuana is a pressing research concern at NIDA, Dr. Volkow said.
Nonmedical use of prescription drugs is down overall among survey respondents. Nonmedical use of Vicodin declined from 9.7% 5 years ago to 4.5% this year. For oxycontin, nonmedical use declined from 4.8% to 3.3%.
Alcohol use, particularly binge drinking in 12th graders, also is losing popularity. At its peak in 1998, 31.5% of 12th graders reported binge drinking; less than 20% did so in 2014. A decline in the use of synthetic marijuana (also known as K2/Spice) was also noted, down from nearly 8% in 12th graders to just under 6%. Heroin, crack cocaine, and methamphetamine use remained low and relatively unchanged from last year.
“Over all, this is good news,” said Dr. Volkow.
Monitoring the Future is unusual in that the data, gathered from nearly 42,000 U.S. students at 377 public and private schools across the country, are reported in the same year they are gathered.
On Twitter @whitneymcknight
Nearly a fifth of American high school seniors have used e-cigarettes recently, although tobacco smoking in this population is at an all-time low, according to the annual Monitoring the Future survey released Dec. 16.
“About 4% of 12th graders use e-cigarettes alone. They’ve never smoked a regular cigarette in their life,” Richard A. Miech, Ph.D., of the University of Michigan, Ann Arbor, one of the lead researchers on the study, said during a press conference held by the National Institute on Drug Abuse. This was the first year that data on e-cigarette use in teens were collected for the Monitoring the Future survey of drug, tobacco, and alcohol use in adolescents in 8th, 10th, and 12th grade. The survey has been conducted every year since 1975 by the University of Michigan and funded by NIDA.
Just over 17% of 12th graders reported past-month use of e-cigarettes. For 10th graders, the number was 16%, and for 8th graders it was nearly 9%. Daily cigarette smoking has decreased by half in the past 5 years across all age groups surveyed, with the largest decline being from just over 6% to about 3% in 10th graders.
Whether the decline in tobacco smoking correlates directly with the rise in e-cigarettes is unknown, NIDA director Dr. Nora Volkow said during the teleconference.
Particularly troubling, according to Dr. Miech, is that the 4% of 12th graders who reported using e-cigarettes exclusively tended to be college bound, a cohort he said typically does not use tobacco.
What actual harm e-cigarettes pose to youth, and whether they lead to other drug use are still open questions, according to Dr. Volkow.
“We can’t do a randomized, controlled trial of teens and give them e-cigarettes to see if they progress to harder drugs,” Dr. Miech said. “The best we can do is follow them as they age.”
Marijuana use among teens is steady and prevalent across all three age groups, according to the survey. More than a third of 12th graders (35%) reported past-year use of marijuana; nearly 6% reported daily use. “This constitutes one of the highest rates seen in any student population world wide,” said Dr. Volkow.
Similar to last year’s rates, 7% of eighth graders, 17% of 10th graders, and just over 21% of 12th graders reported smoking marijuana in the previous month.
The steady rates of marijuana use came as a welcome surprise, said Dr. Volkow, considering recent changes to marijuana laws at the state level and the way those changes might impact perception of marijuana use. In fact, there was a change across the entire study population in the perceived danger posed by marijuana, down from 27% 5 years ago to 16% this year.
In states with medical marijuana laws, 40% of 12th graders who reported using marijuana in the past year said they had used edible marijuana products, compared with 26% of seniors who lived in states without such legislation.
Whether digested versus smoked cannabinoids pose greater health risks to teens “is relatively new for us,” Dr. Volkow said, noting that there are little data on how quickly cannabinoids consumed orally may enter the bloodstream. “When you smoke a drug, it gets into the brain very rapidly, and that is associated with stronger rewarding affects and more addictiveness, but it also is associated with better control of how much you ingest.”
“It’s pretty obvious when people are smoking a joint – the feedback is quick,” said Dr. Lloyd D. Johnston, lead investigator for Monitoring the Future, also of the University of Michigan. Ingested marijuana is different and variable, he noted, both in the amount of marijuana consumed and in the time it takes for the drug to take effect. “I think it’s considerably more dangerous.”Understanding the bioavailability and the pharmacokinetics of edible marijuana is a pressing research concern at NIDA, Dr. Volkow said.
Nonmedical use of prescription drugs is down overall among survey respondents. Nonmedical use of Vicodin declined from 9.7% 5 years ago to 4.5% this year. For oxycontin, nonmedical use declined from 4.8% to 3.3%.
Alcohol use, particularly binge drinking in 12th graders, also is losing popularity. At its peak in 1998, 31.5% of 12th graders reported binge drinking; less than 20% did so in 2014. A decline in the use of synthetic marijuana (also known as K2/Spice) was also noted, down from nearly 8% in 12th graders to just under 6%. Heroin, crack cocaine, and methamphetamine use remained low and relatively unchanged from last year.
“Over all, this is good news,” said Dr. Volkow.
Monitoring the Future is unusual in that the data, gathered from nearly 42,000 U.S. students at 377 public and private schools across the country, are reported in the same year they are gathered.
On Twitter @whitneymcknight
Nearly a fifth of American high school seniors have used e-cigarettes recently, although tobacco smoking in this population is at an all-time low, according to the annual Monitoring the Future survey released Dec. 16.
“About 4% of 12th graders use e-cigarettes alone. They’ve never smoked a regular cigarette in their life,” Richard A. Miech, Ph.D., of the University of Michigan, Ann Arbor, one of the lead researchers on the study, said during a press conference held by the National Institute on Drug Abuse. This was the first year that data on e-cigarette use in teens were collected for the Monitoring the Future survey of drug, tobacco, and alcohol use in adolescents in 8th, 10th, and 12th grade. The survey has been conducted every year since 1975 by the University of Michigan and funded by NIDA.
Just over 17% of 12th graders reported past-month use of e-cigarettes. For 10th graders, the number was 16%, and for 8th graders it was nearly 9%. Daily cigarette smoking has decreased by half in the past 5 years across all age groups surveyed, with the largest decline being from just over 6% to about 3% in 10th graders.
Whether the decline in tobacco smoking correlates directly with the rise in e-cigarettes is unknown, NIDA director Dr. Nora Volkow said during the teleconference.
Particularly troubling, according to Dr. Miech, is that the 4% of 12th graders who reported using e-cigarettes exclusively tended to be college bound, a cohort he said typically does not use tobacco.
What actual harm e-cigarettes pose to youth, and whether they lead to other drug use are still open questions, according to Dr. Volkow.
“We can’t do a randomized, controlled trial of teens and give them e-cigarettes to see if they progress to harder drugs,” Dr. Miech said. “The best we can do is follow them as they age.”
Marijuana use among teens is steady and prevalent across all three age groups, according to the survey. More than a third of 12th graders (35%) reported past-year use of marijuana; nearly 6% reported daily use. “This constitutes one of the highest rates seen in any student population world wide,” said Dr. Volkow.
Similar to last year’s rates, 7% of eighth graders, 17% of 10th graders, and just over 21% of 12th graders reported smoking marijuana in the previous month.
The steady rates of marijuana use came as a welcome surprise, said Dr. Volkow, considering recent changes to marijuana laws at the state level and the way those changes might impact perception of marijuana use. In fact, there was a change across the entire study population in the perceived danger posed by marijuana, down from 27% 5 years ago to 16% this year.
In states with medical marijuana laws, 40% of 12th graders who reported using marijuana in the past year said they had used edible marijuana products, compared with 26% of seniors who lived in states without such legislation.
Whether digested versus smoked cannabinoids pose greater health risks to teens “is relatively new for us,” Dr. Volkow said, noting that there are little data on how quickly cannabinoids consumed orally may enter the bloodstream. “When you smoke a drug, it gets into the brain very rapidly, and that is associated with stronger rewarding affects and more addictiveness, but it also is associated with better control of how much you ingest.”
“It’s pretty obvious when people are smoking a joint – the feedback is quick,” said Dr. Lloyd D. Johnston, lead investigator for Monitoring the Future, also of the University of Michigan. Ingested marijuana is different and variable, he noted, both in the amount of marijuana consumed and in the time it takes for the drug to take effect. “I think it’s considerably more dangerous.”Understanding the bioavailability and the pharmacokinetics of edible marijuana is a pressing research concern at NIDA, Dr. Volkow said.
Nonmedical use of prescription drugs is down overall among survey respondents. Nonmedical use of Vicodin declined from 9.7% 5 years ago to 4.5% this year. For oxycontin, nonmedical use declined from 4.8% to 3.3%.
Alcohol use, particularly binge drinking in 12th graders, also is losing popularity. At its peak in 1998, 31.5% of 12th graders reported binge drinking; less than 20% did so in 2014. A decline in the use of synthetic marijuana (also known as K2/Spice) was also noted, down from nearly 8% in 12th graders to just under 6%. Heroin, crack cocaine, and methamphetamine use remained low and relatively unchanged from last year.
“Over all, this is good news,” said Dr. Volkow.
Monitoring the Future is unusual in that the data, gathered from nearly 42,000 U.S. students at 377 public and private schools across the country, are reported in the same year they are gathered.
On Twitter @whitneymcknight
FROM NIDA
Key clinical point: Drug use prevention efforts aimed at adolescents appear to have impact, but little is known about effects of e-cigarette use.
Major finding: Nearly one-fifth of U.S. high school seniors have used e-cigarettes this year, and 6% of high school seniors smoke marijuana daily.
Data source: Survey conducted in 2014 of 41,551 teens from 377 public and private schools across the U.S.
Disclosures: Monitoring the Future is supported by grant DA001411 from the National Institute on Drug Abuse. The investigators report no relevant conflicts of interest.