User login
Blood pressure smartphone app fails to beat standard self-monitoring
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
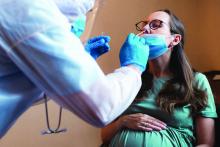
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
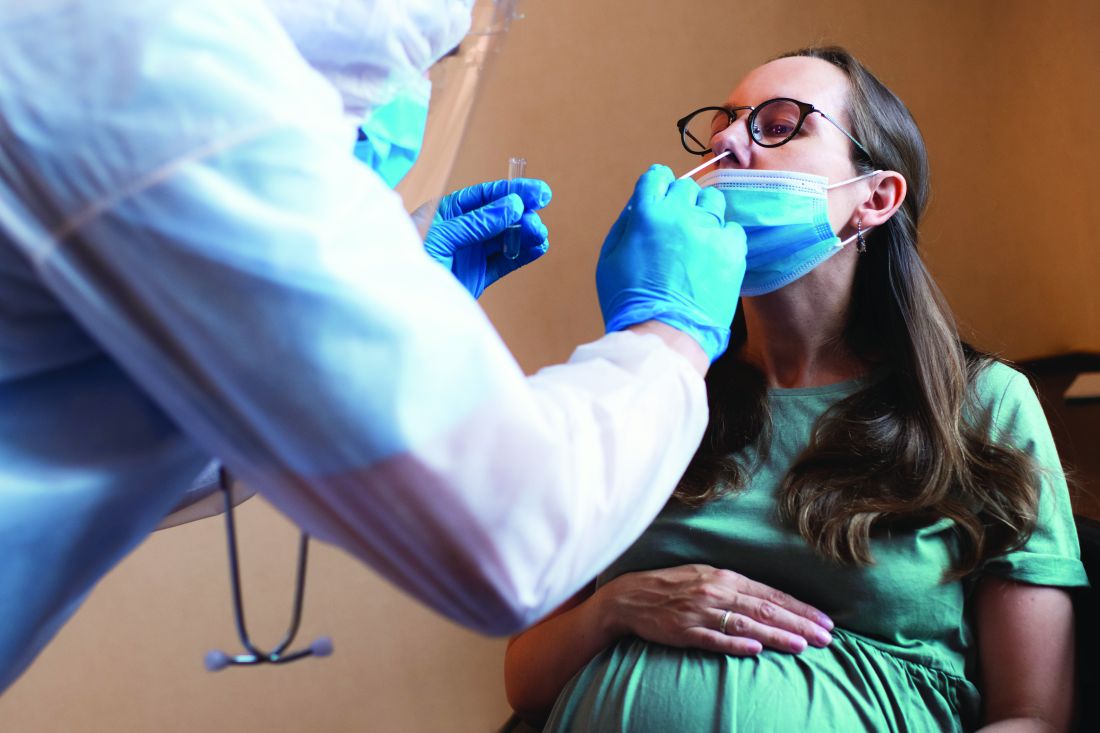
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
Here’s another vote for less screen time.
“By itself, standard self-measured blood pressure (SMBP) has minimal effect on BP control,” wrote lead author Mark J. Pletcher, MD, of the University of California, San Francisco, and colleagues in JAMA Internal Medicine. “To improve BP control, SMBP must be accompanied by patient feedback, counseling, or other cointerventions, and the BP-lowering effects of SMBP appear to be proportional to the intensity of the cointervention.”
While this is known, higher-intensity cointerventions demand both money and time, prompting development of new devices that link with smartphone apps, they continued.
In the prospective randomized trial, patients with hypertension were randomly assigned to self-measure their blood pressure using a standard device that paired with a connected smartphone application or to self-measure their blood pressure with a standard device alone. Both groups achieved about an 11 mm Hg reduction in systolic BP over 6 months, reported similar levels of satisfaction with the monitoring process, and shared their readings with their physicians with similar frequency.
Methods
Dr. Pletcher and colleagues enrolled 2,101 adults who self-reported a systolic BP greater than 145 mm Hg and expressed a commitment to reduce their BP by at least 10 points in their trial. The participants, who were generally middle-aged or older, were randomized in a 1:1 ratio to monitor their BP using standard SMBP or “enhanced” SMBP. The standard group used the OMRON BP monitor alone, while the enhanced group used the same BP monitor coupled with the OMRON Connect smartphone app.
After 6 months of follow-up for each patient, mean BP reduction from baseline in the standard group was 10.6 mm Hg, compared with 10.7 mm Hg in the enhanced group, a nonsignificant difference (P = .81). While slightly more patients in the enhanced group achieved a BP lower than 140/90 mm Hg (32% vs. 29%; odds ratio, 1.17; 95% confidence interval, 1.01-1.34), this trend did not extend below the 130/80 mm Hg threshold.
Other secondary outcomes were also similar between groups. For example, 70% of participants in the enhanced group said they would recommend their SMBP process to a friend, compared with 69% of participants who followed the standard monitoring approach. The smartphone app had little impact on sharing readings with physicians, either, based on a 44% share rate in the enhanced group versus 48% in the standard group (P = .22).
“Enhanced SMBP does not provide any additional reduction in BP,” the investigators concluded.
New devices that link with smartphone apps, like the one used in this trial, “transmit BP measurements via wireless connection to the patient’s smartphone, where they are processed in a smartphone application to support tracking, visualization, interpretation, reminders to measure BP and/or take medications; recommendations for lifestyle interventions, medication adherence, or to discuss their BP with their clinician; and communications (for example, emailing a summary to a family member or clinician),” the researchers explained. While these devices are “only slightly more expensive than standard SMBP devices,” their relative efficacy over standard SMBP is “unclear.”
Findings can likely be extrapolated to other apps
Although the trial evaluated just one smartphone app, Dr. Pletcher suggested that the findings can likely be extrapolated to other apps.
“Most basic BP-tracking apps have some version or subset of the same essential functionality,” he said, in an interview. “My guess is that apps that meet this description without some substantially different technology or feature would likely show the same basic results as we did.”
Making a similar remark, Matthew Jung, MD, of Keck Medicine of USC, Los Angeles, stated that the findings can be “reasonably extrapolated” to other BP-tracking apps with similar functionality “if we put aside the study’s issues with power.”
When it comes to smartphone apps, active engagement is needed to achieve greater impacts on blood pressure, Dr. Pletcher said, but “there is so much competition for people’s attention on their phone that it is hard to maintain active engagement with any health-related app for long.”
Still, Dr. Pletcher hasn’t given up on biometric apps, noting that “with the right technology and connectivity and user experience (for both patient and clinician), they still could be game-changing for managing chronic conditions like hypertension.”
To this end, he and his colleagues are exploring technologies to passively monitor health-related measurements like BP, potentially sidestepping the pitfall of active engagement.
Dr. Jung said the study is noteworthy for several reasons, including its large size, similar level of comfort with technology reported by both groups, and representation of Black and Hispanic participants, who accounted for almost one-third of the population.
Study limitations
Dr. Jung pointed out several study limitations, including the lack of standardized measurement of BP, which left more than one-third of patients unevaluated via chart review, as well as gaps in usage data, such as that one-third of the participants never confirmed receipt of a device, and less than half of the enhanced group reported using the smartphone application.
These limitations “may have detracted from its ability to identify the true efficacy of an enhanced app-based BP tracking device,” he said. “In contrast, each of these issues also helped us get a better picture for how well these devices may work in the real world.”
Dr. Jung also commented on the duration of the study, noting that only 10 weeks passed, on average, from baseline to follow-up BP measurement, which “may not have been sufficient for a possible difference between enhanced and standard BP monitoring to become noticeable.”
“This may be especially important when taking into consideration the time required to mail the devices out to patients, for patients to become familiar with usage of the devices, and for them to start using the devices in a meaningful way,” he added.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Jung, who was not involved in the study, disclosed no relevant conflicts of interest.
FROM JAMA INTERNAL MEDICINE
HCV reinfection uncommon among people who inject drugs
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Acute pancreatitis: Procalcitonin algorithm safely reduces antibiotic overuse
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY
High plasma IgE predicts COPD exacerbation, mortality
COPD patients with high plasma immunoglobulin E are more likely to have exacerbations and die from any cause, based on a Danish population-cohort study.
hinting at different subsets of patients with COPD, lead author Yunus Çolak MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“Additional biomarkers are necessary as blood eosinophils alone seem insufficient for risk stratification in COPD,” the investigators wrote in Annals of Allergy, Asthma & Immunology. “Since asthma and COPD share some pathophysiological mechanisms, a logical approach would be to investigate well-known biomarkers for asthma in COPD and vice versa.”
Dr. Çolak and colleagues cited previous research supporting this perspective. Specifically, IgE-targeting monoclonal antibodies have shown promise in patients with severe asthma and asthma-COPD overlap, whereas COPD with high IgE has been associated with a history of lung function decline and previous exacerbations.
The present study drew from a database of 46,598 adults enrolled in the Copenhagen General Population study. All participants underwent physical examination, completed a questionnaire, and provided blood for analysis. From this population, 1,559 individuals had COPD, among whom 446 had high plasma IgE (at least 76 IU/mL).
Over a median follow-up of 6.9 years in the COPD group, 224 severe exacerbations and 434 deaths of any cause occurred. Compared with COPD patients who had normal plasma IgE, those with high IgE were 43% more likely to have severe exacerbation (hazard ratio, 1.43; 95% confidence interval, 1.07-1.89) and 30% more likely to die of any cause (HR, 1.30; 95% CI, 1.06-1.62). These risks were similar when excluding patients with IgE of 700 IU/mL or higher.
“These findings suggest that plasma IgE concentration may be a potential prognostic biomarker and treatment target for a subset of COPD patient,” wrote Dr. Çolak and colleagues.
The above risks increased moderately when the high IgE group was trimmed to include only those with low eosinophils (less than 300 cells/mcL); in this subgroup, risk of exacerbation was increased 62% (HR, 1.62; 95% CI, 1.17-2.24), while risk of all-cause mortality was increased 47% (HR, 1.47; 95% CI, 1.14-1.88).
“We were not able to show that individuals with higher blood eosinophils further stratified by IgE had higher risk of severe exacerbation or all-cause mortality,” the investigators wrote, although they noted “the relatively low statistical power in stratified analysis,” considering the wide confidence intervals observed.
“Thus, we should be careful with interpreting the results in relation to blood eosinophils and IgE combined,” they suggested. “However, we believe that the mechanisms driving exacerbations through plasma IgE are different from those driving blood eosinophils, and we believe that plasma IgE may be a marker for a subset of COPD patients similar to blood eosinophils, which is compatible with the heterogeneity of patients with COPD.”
According to principal author Shoaib Afzal, MD, PhD, of Copenhagen University Hospital, the findings are “probably no surprise for practitioners that often observe overlap between asthma and COPD pathology.”
As smoking prevalence goes down in many countries, relatively more never-smokers are being diagnosed with COPD, Dr. Afzal said in a written comment, “which means that asthma as a risk factor for COPD is gaining importance.”
While patients with asthma can be treated with IgE-targeting omalizumab, a trial evaluating the same biologic for COPD patients with high IgE was withdrawn because of a lack of recruitment; however, Dr. Afzal suggested that this should not be the end of the story, since these new data imply that more patients could benefit than previously recognized.
“Our observational study has generated a hypothesis that needs to be tested by pulmonologists in randomized interventions trials designed with updated inclusion criteria,” he said.
Such trials are needed, Dr. Afzal went on, because they could help unlock the “huge” potential benefit that may come from characterizing COPD patients beyond “exposures, symptoms, and spirometry.”
“Sadly, the progress in establishing biomarkers in COPD for improving risk stratification and treatment allocation have been rather disappointing in the last decades, with the exception of small successes with eosinophils and perhaps FeNO,” Dr. Afzal said.
Nathaniel Marchetti, DO, professor of thoracic medicine and surgery at Temple University and medical director of the respiratory ICU at Temple University Hospital, both in Philadelphia, said the study by Dr. Afzal and colleagues is noteworthy because “biomarkers for COPD are desperately needed to help risk stratify patients for exacerbation risk and risk of disease progression and even mortality.”
In a written comment, Dr. Marchetti agreed with Dr. Afzal that the findings “open the possibility for interventional trials targeting IgE,” which could one day reshape the way patients with COPD are treated.
“I think that biomarkers will become vital in caring for patients with COPD in the future,” Dr. Marchetti said. “There will be medications that will be used to target different pathways of inflammation that drive disease progression and exacerbations. Biomarkers will be important in driving personalized medicine in COPD. We already know the disease seems to vary greatly from patient to patient.”
The study was supported by The Capital Region of Copenhagen, The Danish Lung Foundation, The Velux Foundation, and others. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Sanofi Genzyme, and others. Dr. Marchetti disclosed no conflicts of interest.
COPD patients with high plasma immunoglobulin E are more likely to have exacerbations and die from any cause, based on a Danish population-cohort study.
hinting at different subsets of patients with COPD, lead author Yunus Çolak MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“Additional biomarkers are necessary as blood eosinophils alone seem insufficient for risk stratification in COPD,” the investigators wrote in Annals of Allergy, Asthma & Immunology. “Since asthma and COPD share some pathophysiological mechanisms, a logical approach would be to investigate well-known biomarkers for asthma in COPD and vice versa.”
Dr. Çolak and colleagues cited previous research supporting this perspective. Specifically, IgE-targeting monoclonal antibodies have shown promise in patients with severe asthma and asthma-COPD overlap, whereas COPD with high IgE has been associated with a history of lung function decline and previous exacerbations.
The present study drew from a database of 46,598 adults enrolled in the Copenhagen General Population study. All participants underwent physical examination, completed a questionnaire, and provided blood for analysis. From this population, 1,559 individuals had COPD, among whom 446 had high plasma IgE (at least 76 IU/mL).
Over a median follow-up of 6.9 years in the COPD group, 224 severe exacerbations and 434 deaths of any cause occurred. Compared with COPD patients who had normal plasma IgE, those with high IgE were 43% more likely to have severe exacerbation (hazard ratio, 1.43; 95% confidence interval, 1.07-1.89) and 30% more likely to die of any cause (HR, 1.30; 95% CI, 1.06-1.62). These risks were similar when excluding patients with IgE of 700 IU/mL or higher.
“These findings suggest that plasma IgE concentration may be a potential prognostic biomarker and treatment target for a subset of COPD patient,” wrote Dr. Çolak and colleagues.
The above risks increased moderately when the high IgE group was trimmed to include only those with low eosinophils (less than 300 cells/mcL); in this subgroup, risk of exacerbation was increased 62% (HR, 1.62; 95% CI, 1.17-2.24), while risk of all-cause mortality was increased 47% (HR, 1.47; 95% CI, 1.14-1.88).
“We were not able to show that individuals with higher blood eosinophils further stratified by IgE had higher risk of severe exacerbation or all-cause mortality,” the investigators wrote, although they noted “the relatively low statistical power in stratified analysis,” considering the wide confidence intervals observed.
“Thus, we should be careful with interpreting the results in relation to blood eosinophils and IgE combined,” they suggested. “However, we believe that the mechanisms driving exacerbations through plasma IgE are different from those driving blood eosinophils, and we believe that plasma IgE may be a marker for a subset of COPD patients similar to blood eosinophils, which is compatible with the heterogeneity of patients with COPD.”
According to principal author Shoaib Afzal, MD, PhD, of Copenhagen University Hospital, the findings are “probably no surprise for practitioners that often observe overlap between asthma and COPD pathology.”
As smoking prevalence goes down in many countries, relatively more never-smokers are being diagnosed with COPD, Dr. Afzal said in a written comment, “which means that asthma as a risk factor for COPD is gaining importance.”
While patients with asthma can be treated with IgE-targeting omalizumab, a trial evaluating the same biologic for COPD patients with high IgE was withdrawn because of a lack of recruitment; however, Dr. Afzal suggested that this should not be the end of the story, since these new data imply that more patients could benefit than previously recognized.
“Our observational study has generated a hypothesis that needs to be tested by pulmonologists in randomized interventions trials designed with updated inclusion criteria,” he said.
Such trials are needed, Dr. Afzal went on, because they could help unlock the “huge” potential benefit that may come from characterizing COPD patients beyond “exposures, symptoms, and spirometry.”
“Sadly, the progress in establishing biomarkers in COPD for improving risk stratification and treatment allocation have been rather disappointing in the last decades, with the exception of small successes with eosinophils and perhaps FeNO,” Dr. Afzal said.
Nathaniel Marchetti, DO, professor of thoracic medicine and surgery at Temple University and medical director of the respiratory ICU at Temple University Hospital, both in Philadelphia, said the study by Dr. Afzal and colleagues is noteworthy because “biomarkers for COPD are desperately needed to help risk stratify patients for exacerbation risk and risk of disease progression and even mortality.”
In a written comment, Dr. Marchetti agreed with Dr. Afzal that the findings “open the possibility for interventional trials targeting IgE,” which could one day reshape the way patients with COPD are treated.
“I think that biomarkers will become vital in caring for patients with COPD in the future,” Dr. Marchetti said. “There will be medications that will be used to target different pathways of inflammation that drive disease progression and exacerbations. Biomarkers will be important in driving personalized medicine in COPD. We already know the disease seems to vary greatly from patient to patient.”
The study was supported by The Capital Region of Copenhagen, The Danish Lung Foundation, The Velux Foundation, and others. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Sanofi Genzyme, and others. Dr. Marchetti disclosed no conflicts of interest.
COPD patients with high plasma immunoglobulin E are more likely to have exacerbations and die from any cause, based on a Danish population-cohort study.
hinting at different subsets of patients with COPD, lead author Yunus Çolak MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“Additional biomarkers are necessary as blood eosinophils alone seem insufficient for risk stratification in COPD,” the investigators wrote in Annals of Allergy, Asthma & Immunology. “Since asthma and COPD share some pathophysiological mechanisms, a logical approach would be to investigate well-known biomarkers for asthma in COPD and vice versa.”
Dr. Çolak and colleagues cited previous research supporting this perspective. Specifically, IgE-targeting monoclonal antibodies have shown promise in patients with severe asthma and asthma-COPD overlap, whereas COPD with high IgE has been associated with a history of lung function decline and previous exacerbations.
The present study drew from a database of 46,598 adults enrolled in the Copenhagen General Population study. All participants underwent physical examination, completed a questionnaire, and provided blood for analysis. From this population, 1,559 individuals had COPD, among whom 446 had high plasma IgE (at least 76 IU/mL).
Over a median follow-up of 6.9 years in the COPD group, 224 severe exacerbations and 434 deaths of any cause occurred. Compared with COPD patients who had normal plasma IgE, those with high IgE were 43% more likely to have severe exacerbation (hazard ratio, 1.43; 95% confidence interval, 1.07-1.89) and 30% more likely to die of any cause (HR, 1.30; 95% CI, 1.06-1.62). These risks were similar when excluding patients with IgE of 700 IU/mL or higher.
“These findings suggest that plasma IgE concentration may be a potential prognostic biomarker and treatment target for a subset of COPD patient,” wrote Dr. Çolak and colleagues.
The above risks increased moderately when the high IgE group was trimmed to include only those with low eosinophils (less than 300 cells/mcL); in this subgroup, risk of exacerbation was increased 62% (HR, 1.62; 95% CI, 1.17-2.24), while risk of all-cause mortality was increased 47% (HR, 1.47; 95% CI, 1.14-1.88).
“We were not able to show that individuals with higher blood eosinophils further stratified by IgE had higher risk of severe exacerbation or all-cause mortality,” the investigators wrote, although they noted “the relatively low statistical power in stratified analysis,” considering the wide confidence intervals observed.
“Thus, we should be careful with interpreting the results in relation to blood eosinophils and IgE combined,” they suggested. “However, we believe that the mechanisms driving exacerbations through plasma IgE are different from those driving blood eosinophils, and we believe that plasma IgE may be a marker for a subset of COPD patients similar to blood eosinophils, which is compatible with the heterogeneity of patients with COPD.”
According to principal author Shoaib Afzal, MD, PhD, of Copenhagen University Hospital, the findings are “probably no surprise for practitioners that often observe overlap between asthma and COPD pathology.”
As smoking prevalence goes down in many countries, relatively more never-smokers are being diagnosed with COPD, Dr. Afzal said in a written comment, “which means that asthma as a risk factor for COPD is gaining importance.”
While patients with asthma can be treated with IgE-targeting omalizumab, a trial evaluating the same biologic for COPD patients with high IgE was withdrawn because of a lack of recruitment; however, Dr. Afzal suggested that this should not be the end of the story, since these new data imply that more patients could benefit than previously recognized.
“Our observational study has generated a hypothesis that needs to be tested by pulmonologists in randomized interventions trials designed with updated inclusion criteria,” he said.
Such trials are needed, Dr. Afzal went on, because they could help unlock the “huge” potential benefit that may come from characterizing COPD patients beyond “exposures, symptoms, and spirometry.”
“Sadly, the progress in establishing biomarkers in COPD for improving risk stratification and treatment allocation have been rather disappointing in the last decades, with the exception of small successes with eosinophils and perhaps FeNO,” Dr. Afzal said.
Nathaniel Marchetti, DO, professor of thoracic medicine and surgery at Temple University and medical director of the respiratory ICU at Temple University Hospital, both in Philadelphia, said the study by Dr. Afzal and colleagues is noteworthy because “biomarkers for COPD are desperately needed to help risk stratify patients for exacerbation risk and risk of disease progression and even mortality.”
In a written comment, Dr. Marchetti agreed with Dr. Afzal that the findings “open the possibility for interventional trials targeting IgE,” which could one day reshape the way patients with COPD are treated.
“I think that biomarkers will become vital in caring for patients with COPD in the future,” Dr. Marchetti said. “There will be medications that will be used to target different pathways of inflammation that drive disease progression and exacerbations. Biomarkers will be important in driving personalized medicine in COPD. We already know the disease seems to vary greatly from patient to patient.”
The study was supported by The Capital Region of Copenhagen, The Danish Lung Foundation, The Velux Foundation, and others. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Sanofi Genzyme, and others. Dr. Marchetti disclosed no conflicts of interest.
FROM ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Parkinson’s disease: Is copper culpable?
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.
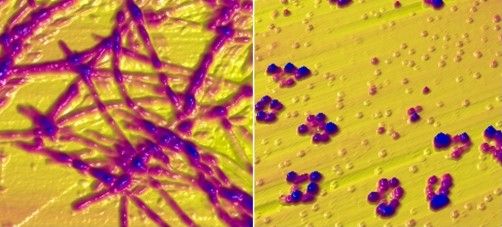
These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
FROM ACS CHEMICAL NEUROSCIENCE
COVID-19 infection late in pregnancy linked to sevenfold risk of preterm birth
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
FROM PLOS ONE
Strictures in Crohn’s: Balloon dilation avoids later surgery
Endoscopic balloon dilation (EBD) is an effective treatment option for strictures of the small bowel in patients with Crohn’s disease, based on a nationwide Danish cohort study.
Approximately three out of four patients who underwent an EBD were spared subsequent small bowel surgery. Similar outcomes were seen across primary and postsurgical strictures, reported lead author Mads Damsgaard Wewer, BSc, of the University of Copenhagen, and colleagues.
“Retrospective studies investigating EBD are available with variable follow-up periods; however, a nationwide study to demonstrate more precise durability of EBD in unselected patients is lacking,” the investigators wrote in European Journal of Gastroenterology & Hepatology. Their aim was to understand the use of EBD and the need for redilation and surgery. This retrospective study used a cohort of adult patients with Crohn’s disease who had strictures of the small bowel during a 19-year period.
The population comprised 9,737 patients with incident Crohn’s disease, among whom 90 (1%) underwent EBD during a median 8.2-year follow-up period. Of these 90 patients, 49 had primary strictures, while the remaining 41 had postsurgical strictures.
In the primary stricture group, 59% of patients had one EBD procedure and did not require subsequent small bowel surgery, 14% of patients required redilation but no further surgery, and 27% of patients required small bowel surgery after dilation. In this same group, the 1-, 3-, and 5-year cumulative incidence rates of EBD failure were 19%, 21%, and 25%, respectively. Of note, just 8% of patients with primary stricture who were treated with EBD ultimately required enterotomy, compared with 16% of patients with primary stricture who underwent small bowel resection without first attempting EBD.
In the postsurgical stricture group, 49% of patients underwent one EBD procedure without need for another small bowel surgery, 27% needed redilation but avoided surgery, and 24% required surgery after dilation. One-, three-, and five-year cumulative incidence rates of EBD failure in this group trended slightly higher than the primary stricture group over time, at 19%, 25%, and 29%, respectively.
The researchers noted that 25% of patients required small bowel surgery after EBD, which falls below rates of 29% to 33% reported by recent studies. They explained that this edge may be “partly explained by the careful selection of patients (with few and short strictures) receiving EBD,” as well as exclusion of patients with strictures outside the small intestine. They concluded that, “... small bowel-related EBD is an effective treatment option, and one that could be offered to more patients with Crohn’s disease in the future.”
‘Reassuring study’
David H. Bruining, MD, associate professor of medicine and section head of the inflammatory bowel disease interest group at Mayo Clinic, Rochester, Minn., called it a “reassuring study that confirms previous data regarding the efficacy of endoscopic balloon dilation of Crohn’s disease strictures.”
Dr. Bruining suggested in an interview that the findings, while drawn from Denmark, can be applied to a U.S. population; he also noted the “impressive” size of the study, as well the duration of follow-up, which extended up to 19 years.
EBD is “gaining more traction,” Dr. Bruining said, “as far as the belief among both referring physicians, and gastroenterologists, that it is effective, and it is safe. I think that body of literature is growing, and it’s more widely established at this point.”
Dr. Bruining noted that EBD should be reserved for patients who have short strictures no longer than 4-5 mm “without associated internal penetrating disease.”
In the future, such patients may have even more treatment options, Dr. Bruining predicted. New antifibrotic medications are “on the horizon,” which could one day be used with or without EBD to address fibrotic strictures in Crohn’s disease. Dr. Bruining is a part of the Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium, a group that aims to develop this emerging approach. He and his colleagues recently published a review of research into antifibrotic therapy to date.
Limiting factors
“This is an important study that really adds something to the literature,” noted Joseph Carmichael, MD, chief medical officer and chief of colon and rectal surgery at the University of California, Irvine. “The cohort is a little unusual in this area in that it encompasses a whole country. Yet this is exactly what makes the data stand apart from previous studies, since the patient population was unselected. That’s how you get a true incidence of the intervention.”
Beyond the generally favorable outcomes associated with EBD, Dr. Carmichael highlighted similar rates of success across both primary and postsurgical strictures. “Some of the previous data suggest postsurgical strictures don’t do as well with endoscopic dilation, and this [study] seems to go against that,” he said. “Which really deserves a closer look.”
Reflecting on the researchers’ call for more frequent use of EBD in patients with Crohn’s disease, Dr. Carmichael speculated that several factors may be limiting current utilization in Europe and the U.S. For one, there may not be enough interventional gastroenterologists. Also, the procedure “generally requires a high-volume provider who’s got surgical backup because these [procedures] have a 2% to 4% incidence of technical failure – including perforation or bleeding. These risks may deter patients from undergoing the procedure.
“Patients with Crohn’s disease can be pretty remarkable in their ability to endure obstruction,” he added. “If someone’s feeling their symptoms aren’t altering their quality of life, they may choose not to proceed with it.”
With all candidate patients, Dr. Carmichael recommended discussing the risks of EBD compared with the risks of declining the intervention, such as complete bowel obstruction, bowel perforation, or fistula. “That’s a question that needs to be included with every conversation when we discuss procedures,” he explained.
The researchers report that, among others, they have relationships with Janssen-Cilag, AbbVie A/S, and Celgene. Dr. Bruining and Dr. Carmichael reports no relevant conflicts of interest.
This article was updated 7/29/22.
Endoscopic balloon dilation (EBD) is an effective treatment option for strictures of the small bowel in patients with Crohn’s disease, based on a nationwide Danish cohort study.
Approximately three out of four patients who underwent an EBD were spared subsequent small bowel surgery. Similar outcomes were seen across primary and postsurgical strictures, reported lead author Mads Damsgaard Wewer, BSc, of the University of Copenhagen, and colleagues.
“Retrospective studies investigating EBD are available with variable follow-up periods; however, a nationwide study to demonstrate more precise durability of EBD in unselected patients is lacking,” the investigators wrote in European Journal of Gastroenterology & Hepatology. Their aim was to understand the use of EBD and the need for redilation and surgery. This retrospective study used a cohort of adult patients with Crohn’s disease who had strictures of the small bowel during a 19-year period.
The population comprised 9,737 patients with incident Crohn’s disease, among whom 90 (1%) underwent EBD during a median 8.2-year follow-up period. Of these 90 patients, 49 had primary strictures, while the remaining 41 had postsurgical strictures.
In the primary stricture group, 59% of patients had one EBD procedure and did not require subsequent small bowel surgery, 14% of patients required redilation but no further surgery, and 27% of patients required small bowel surgery after dilation. In this same group, the 1-, 3-, and 5-year cumulative incidence rates of EBD failure were 19%, 21%, and 25%, respectively. Of note, just 8% of patients with primary stricture who were treated with EBD ultimately required enterotomy, compared with 16% of patients with primary stricture who underwent small bowel resection without first attempting EBD.
In the postsurgical stricture group, 49% of patients underwent one EBD procedure without need for another small bowel surgery, 27% needed redilation but avoided surgery, and 24% required surgery after dilation. One-, three-, and five-year cumulative incidence rates of EBD failure in this group trended slightly higher than the primary stricture group over time, at 19%, 25%, and 29%, respectively.
The researchers noted that 25% of patients required small bowel surgery after EBD, which falls below rates of 29% to 33% reported by recent studies. They explained that this edge may be “partly explained by the careful selection of patients (with few and short strictures) receiving EBD,” as well as exclusion of patients with strictures outside the small intestine. They concluded that, “... small bowel-related EBD is an effective treatment option, and one that could be offered to more patients with Crohn’s disease in the future.”
‘Reassuring study’
David H. Bruining, MD, associate professor of medicine and section head of the inflammatory bowel disease interest group at Mayo Clinic, Rochester, Minn., called it a “reassuring study that confirms previous data regarding the efficacy of endoscopic balloon dilation of Crohn’s disease strictures.”
Dr. Bruining suggested in an interview that the findings, while drawn from Denmark, can be applied to a U.S. population; he also noted the “impressive” size of the study, as well the duration of follow-up, which extended up to 19 years.
EBD is “gaining more traction,” Dr. Bruining said, “as far as the belief among both referring physicians, and gastroenterologists, that it is effective, and it is safe. I think that body of literature is growing, and it’s more widely established at this point.”
Dr. Bruining noted that EBD should be reserved for patients who have short strictures no longer than 4-5 mm “without associated internal penetrating disease.”
In the future, such patients may have even more treatment options, Dr. Bruining predicted. New antifibrotic medications are “on the horizon,” which could one day be used with or without EBD to address fibrotic strictures in Crohn’s disease. Dr. Bruining is a part of the Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium, a group that aims to develop this emerging approach. He and his colleagues recently published a review of research into antifibrotic therapy to date.
Limiting factors
“This is an important study that really adds something to the literature,” noted Joseph Carmichael, MD, chief medical officer and chief of colon and rectal surgery at the University of California, Irvine. “The cohort is a little unusual in this area in that it encompasses a whole country. Yet this is exactly what makes the data stand apart from previous studies, since the patient population was unselected. That’s how you get a true incidence of the intervention.”
Beyond the generally favorable outcomes associated with EBD, Dr. Carmichael highlighted similar rates of success across both primary and postsurgical strictures. “Some of the previous data suggest postsurgical strictures don’t do as well with endoscopic dilation, and this [study] seems to go against that,” he said. “Which really deserves a closer look.”
Reflecting on the researchers’ call for more frequent use of EBD in patients with Crohn’s disease, Dr. Carmichael speculated that several factors may be limiting current utilization in Europe and the U.S. For one, there may not be enough interventional gastroenterologists. Also, the procedure “generally requires a high-volume provider who’s got surgical backup because these [procedures] have a 2% to 4% incidence of technical failure – including perforation or bleeding. These risks may deter patients from undergoing the procedure.
“Patients with Crohn’s disease can be pretty remarkable in their ability to endure obstruction,” he added. “If someone’s feeling their symptoms aren’t altering their quality of life, they may choose not to proceed with it.”
With all candidate patients, Dr. Carmichael recommended discussing the risks of EBD compared with the risks of declining the intervention, such as complete bowel obstruction, bowel perforation, or fistula. “That’s a question that needs to be included with every conversation when we discuss procedures,” he explained.
The researchers report that, among others, they have relationships with Janssen-Cilag, AbbVie A/S, and Celgene. Dr. Bruining and Dr. Carmichael reports no relevant conflicts of interest.
This article was updated 7/29/22.
Endoscopic balloon dilation (EBD) is an effective treatment option for strictures of the small bowel in patients with Crohn’s disease, based on a nationwide Danish cohort study.
Approximately three out of four patients who underwent an EBD were spared subsequent small bowel surgery. Similar outcomes were seen across primary and postsurgical strictures, reported lead author Mads Damsgaard Wewer, BSc, of the University of Copenhagen, and colleagues.
“Retrospective studies investigating EBD are available with variable follow-up periods; however, a nationwide study to demonstrate more precise durability of EBD in unselected patients is lacking,” the investigators wrote in European Journal of Gastroenterology & Hepatology. Their aim was to understand the use of EBD and the need for redilation and surgery. This retrospective study used a cohort of adult patients with Crohn’s disease who had strictures of the small bowel during a 19-year period.
The population comprised 9,737 patients with incident Crohn’s disease, among whom 90 (1%) underwent EBD during a median 8.2-year follow-up period. Of these 90 patients, 49 had primary strictures, while the remaining 41 had postsurgical strictures.
In the primary stricture group, 59% of patients had one EBD procedure and did not require subsequent small bowel surgery, 14% of patients required redilation but no further surgery, and 27% of patients required small bowel surgery after dilation. In this same group, the 1-, 3-, and 5-year cumulative incidence rates of EBD failure were 19%, 21%, and 25%, respectively. Of note, just 8% of patients with primary stricture who were treated with EBD ultimately required enterotomy, compared with 16% of patients with primary stricture who underwent small bowel resection without first attempting EBD.
In the postsurgical stricture group, 49% of patients underwent one EBD procedure without need for another small bowel surgery, 27% needed redilation but avoided surgery, and 24% required surgery after dilation. One-, three-, and five-year cumulative incidence rates of EBD failure in this group trended slightly higher than the primary stricture group over time, at 19%, 25%, and 29%, respectively.
The researchers noted that 25% of patients required small bowel surgery after EBD, which falls below rates of 29% to 33% reported by recent studies. They explained that this edge may be “partly explained by the careful selection of patients (with few and short strictures) receiving EBD,” as well as exclusion of patients with strictures outside the small intestine. They concluded that, “... small bowel-related EBD is an effective treatment option, and one that could be offered to more patients with Crohn’s disease in the future.”
‘Reassuring study’
David H. Bruining, MD, associate professor of medicine and section head of the inflammatory bowel disease interest group at Mayo Clinic, Rochester, Minn., called it a “reassuring study that confirms previous data regarding the efficacy of endoscopic balloon dilation of Crohn’s disease strictures.”
Dr. Bruining suggested in an interview that the findings, while drawn from Denmark, can be applied to a U.S. population; he also noted the “impressive” size of the study, as well the duration of follow-up, which extended up to 19 years.
EBD is “gaining more traction,” Dr. Bruining said, “as far as the belief among both referring physicians, and gastroenterologists, that it is effective, and it is safe. I think that body of literature is growing, and it’s more widely established at this point.”
Dr. Bruining noted that EBD should be reserved for patients who have short strictures no longer than 4-5 mm “without associated internal penetrating disease.”
In the future, such patients may have even more treatment options, Dr. Bruining predicted. New antifibrotic medications are “on the horizon,” which could one day be used with or without EBD to address fibrotic strictures in Crohn’s disease. Dr. Bruining is a part of the Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium, a group that aims to develop this emerging approach. He and his colleagues recently published a review of research into antifibrotic therapy to date.
Limiting factors
“This is an important study that really adds something to the literature,” noted Joseph Carmichael, MD, chief medical officer and chief of colon and rectal surgery at the University of California, Irvine. “The cohort is a little unusual in this area in that it encompasses a whole country. Yet this is exactly what makes the data stand apart from previous studies, since the patient population was unselected. That’s how you get a true incidence of the intervention.”
Beyond the generally favorable outcomes associated with EBD, Dr. Carmichael highlighted similar rates of success across both primary and postsurgical strictures. “Some of the previous data suggest postsurgical strictures don’t do as well with endoscopic dilation, and this [study] seems to go against that,” he said. “Which really deserves a closer look.”
Reflecting on the researchers’ call for more frequent use of EBD in patients with Crohn’s disease, Dr. Carmichael speculated that several factors may be limiting current utilization in Europe and the U.S. For one, there may not be enough interventional gastroenterologists. Also, the procedure “generally requires a high-volume provider who’s got surgical backup because these [procedures] have a 2% to 4% incidence of technical failure – including perforation or bleeding. These risks may deter patients from undergoing the procedure.
“Patients with Crohn’s disease can be pretty remarkable in their ability to endure obstruction,” he added. “If someone’s feeling their symptoms aren’t altering their quality of life, they may choose not to proceed with it.”
With all candidate patients, Dr. Carmichael recommended discussing the risks of EBD compared with the risks of declining the intervention, such as complete bowel obstruction, bowel perforation, or fistula. “That’s a question that needs to be included with every conversation when we discuss procedures,” he explained.
The researchers report that, among others, they have relationships with Janssen-Cilag, AbbVie A/S, and Celgene. Dr. Bruining and Dr. Carmichael reports no relevant conflicts of interest.
This article was updated 7/29/22.
FROM EUROPEAN JOURNAL OF GASTROENTEROLOGY & HEPATOLOGY
Does choice of biologic affect outcomes in perianal Crohn’s disease?
Choice of biologic therapy in the first line and later may impact long-term outcomes in patients with perianal Crohn’s disease (pCD), according to a retrospective study.
John Gubatan, MD, of Stanford (Calif.) University, and colleagues reported that, compared with no biologic therapy, first-line treatment with an anti–tumor necrosis factor (TNF) agent or ustekinumab significantly reduced risk of perianal abscess recurrence at 5 years, whereas vedolizumab offered no such benefit. After failure of the initial anti-TNF, switching to another anti-TNF agent is the most effective option.
“Although pCD is recognized to be an aggressive phenotype, data on whether escalating to a biologic at the time of perianal disease diagnosis may alter the natural history and long-term clinical outcomes of pCD is limited,” the researchers wrote in Journal of Clinical Gastroenterology. “This is the first study to explore how the type of biologic therapy at the time of perianal disease diagnosis and change in biologic therapy after first anti-TNF failure are associated with rates of long-term clinical outcomes.”
The study included 311 patients with pCD treated at Stanford University from 1998 to 2020. At the time of diagnosis, 168 of these patients started a biologic, most often an anti-TNF agent (n = 138), followed distantly by ustekinumab (n = 16) or vedolizumab (n = 14). Efficacy of these first-line biologics was compared with no biologic therapy in terms of five clinical outcomes at 5 years: surgical intervention, colectomy, permanent diversion, fistula closure, and perianal abscess recurrence.
Although both reduced risk of perianal abscess recurrence, it was still higher with anti-TNF therapy (hazard ratio, 0.48; 95% confidence interval, 0.32-0.74) than with ustekinumab (HR, 0.20; 95% CI, 0.07-0.56). Ustekinumab also increased the rate of perianal fistula closure by more than threefold (HR, 3.58; 95% CI, 1.04-12.35).
Vedolizumab, on the other hand, offered no significant benefit across any of the five outcomes.
None of the biologics had an impact on rates of surgical intervention, colectomy, or permanent diversion.
Further analyses explored the long-term effects of second-line biologic choice after initial failure with anti-TNF therapy. Switching to another anti-TNF agent was more effective than switching to ustekinumab at reducing risks of colectomy (HR, 0.20; 95% CI, 0.04-0.90) and permanent diversion (HR, 0.16; 95% CI, 0.03-0.94); switching to ustekinumab was more effective than switching to vedolizumab for perianal fistula closure (HR, 0.22; 95% CI, 0.05-0.96).
Switching to another anti-TNF biologic or ustekinumab may be associated with better 5-year outcomes, compared with switching to vedolizumab in patients with pCD, according to Dr. Gubatan. Other guidelines or data that might steer this sequencing decision are scant. However, the findings should be validated with prospective data, ideally from head-to-head trials.
Jordan E. Axelrad, MD, of NYU Langone Health, New York, said the present study is noteworthy for addressing a “very-difficult-to-treat condition that has limited data as well as very limited long-term outcome data for our currently available interventions.”
Dr. Axelrad appreciated how the study focused on the distribution of clinical manifestations of pCD, including ulcers (10%), fissures (23.2%), abscesses (76.1%), and fistulas (84.2%). According to Dr. Axelrad, the efficacy data provide really important insights for clinicians who choose biologic therapies. He noted that, in the absence of head-to-head clinical trials, “it’s absolutely important that we use these results to help us guide therapy” for patients with pCD.
While the biologics included in the study were efficacious to varying degrees, Dr. Axelrad pointed out that no choice was associated with a reduced risk of surgical intervention. “That really underscored for me how complex this patient population is,” he said. “Despite good medical therapies … we’re still not necessarily making a huge dent in the risk of surgical intervention requirements for this complex patient group.”
Dr. Gubatan disclosed support from a Chan Zuckerberg Biohub Physician Scientist Scholar Award, a National Institutes of Health NIDDK LRP Award, and a Doris Duke Physician Scientist Fellowship Award; his colleagues reported no conflicts of interest. Dr. Axelrad reports relationships with Janssen, AbbVie, Pfizer, and others.
Choice of biologic therapy in the first line and later may impact long-term outcomes in patients with perianal Crohn’s disease (pCD), according to a retrospective study.
John Gubatan, MD, of Stanford (Calif.) University, and colleagues reported that, compared with no biologic therapy, first-line treatment with an anti–tumor necrosis factor (TNF) agent or ustekinumab significantly reduced risk of perianal abscess recurrence at 5 years, whereas vedolizumab offered no such benefit. After failure of the initial anti-TNF, switching to another anti-TNF agent is the most effective option.
“Although pCD is recognized to be an aggressive phenotype, data on whether escalating to a biologic at the time of perianal disease diagnosis may alter the natural history and long-term clinical outcomes of pCD is limited,” the researchers wrote in Journal of Clinical Gastroenterology. “This is the first study to explore how the type of biologic therapy at the time of perianal disease diagnosis and change in biologic therapy after first anti-TNF failure are associated with rates of long-term clinical outcomes.”
The study included 311 patients with pCD treated at Stanford University from 1998 to 2020. At the time of diagnosis, 168 of these patients started a biologic, most often an anti-TNF agent (n = 138), followed distantly by ustekinumab (n = 16) or vedolizumab (n = 14). Efficacy of these first-line biologics was compared with no biologic therapy in terms of five clinical outcomes at 5 years: surgical intervention, colectomy, permanent diversion, fistula closure, and perianal abscess recurrence.
Although both reduced risk of perianal abscess recurrence, it was still higher with anti-TNF therapy (hazard ratio, 0.48; 95% confidence interval, 0.32-0.74) than with ustekinumab (HR, 0.20; 95% CI, 0.07-0.56). Ustekinumab also increased the rate of perianal fistula closure by more than threefold (HR, 3.58; 95% CI, 1.04-12.35).
Vedolizumab, on the other hand, offered no significant benefit across any of the five outcomes.
None of the biologics had an impact on rates of surgical intervention, colectomy, or permanent diversion.
Further analyses explored the long-term effects of second-line biologic choice after initial failure with anti-TNF therapy. Switching to another anti-TNF agent was more effective than switching to ustekinumab at reducing risks of colectomy (HR, 0.20; 95% CI, 0.04-0.90) and permanent diversion (HR, 0.16; 95% CI, 0.03-0.94); switching to ustekinumab was more effective than switching to vedolizumab for perianal fistula closure (HR, 0.22; 95% CI, 0.05-0.96).
Switching to another anti-TNF biologic or ustekinumab may be associated with better 5-year outcomes, compared with switching to vedolizumab in patients with pCD, according to Dr. Gubatan. Other guidelines or data that might steer this sequencing decision are scant. However, the findings should be validated with prospective data, ideally from head-to-head trials.
Jordan E. Axelrad, MD, of NYU Langone Health, New York, said the present study is noteworthy for addressing a “very-difficult-to-treat condition that has limited data as well as very limited long-term outcome data for our currently available interventions.”
Dr. Axelrad appreciated how the study focused on the distribution of clinical manifestations of pCD, including ulcers (10%), fissures (23.2%), abscesses (76.1%), and fistulas (84.2%). According to Dr. Axelrad, the efficacy data provide really important insights for clinicians who choose biologic therapies. He noted that, in the absence of head-to-head clinical trials, “it’s absolutely important that we use these results to help us guide therapy” for patients with pCD.
While the biologics included in the study were efficacious to varying degrees, Dr. Axelrad pointed out that no choice was associated with a reduced risk of surgical intervention. “That really underscored for me how complex this patient population is,” he said. “Despite good medical therapies … we’re still not necessarily making a huge dent in the risk of surgical intervention requirements for this complex patient group.”
Dr. Gubatan disclosed support from a Chan Zuckerberg Biohub Physician Scientist Scholar Award, a National Institutes of Health NIDDK LRP Award, and a Doris Duke Physician Scientist Fellowship Award; his colleagues reported no conflicts of interest. Dr. Axelrad reports relationships with Janssen, AbbVie, Pfizer, and others.
Choice of biologic therapy in the first line and later may impact long-term outcomes in patients with perianal Crohn’s disease (pCD), according to a retrospective study.
John Gubatan, MD, of Stanford (Calif.) University, and colleagues reported that, compared with no biologic therapy, first-line treatment with an anti–tumor necrosis factor (TNF) agent or ustekinumab significantly reduced risk of perianal abscess recurrence at 5 years, whereas vedolizumab offered no such benefit. After failure of the initial anti-TNF, switching to another anti-TNF agent is the most effective option.
“Although pCD is recognized to be an aggressive phenotype, data on whether escalating to a biologic at the time of perianal disease diagnosis may alter the natural history and long-term clinical outcomes of pCD is limited,” the researchers wrote in Journal of Clinical Gastroenterology. “This is the first study to explore how the type of biologic therapy at the time of perianal disease diagnosis and change in biologic therapy after first anti-TNF failure are associated with rates of long-term clinical outcomes.”
The study included 311 patients with pCD treated at Stanford University from 1998 to 2020. At the time of diagnosis, 168 of these patients started a biologic, most often an anti-TNF agent (n = 138), followed distantly by ustekinumab (n = 16) or vedolizumab (n = 14). Efficacy of these first-line biologics was compared with no biologic therapy in terms of five clinical outcomes at 5 years: surgical intervention, colectomy, permanent diversion, fistula closure, and perianal abscess recurrence.
Although both reduced risk of perianal abscess recurrence, it was still higher with anti-TNF therapy (hazard ratio, 0.48; 95% confidence interval, 0.32-0.74) than with ustekinumab (HR, 0.20; 95% CI, 0.07-0.56). Ustekinumab also increased the rate of perianal fistula closure by more than threefold (HR, 3.58; 95% CI, 1.04-12.35).
Vedolizumab, on the other hand, offered no significant benefit across any of the five outcomes.
None of the biologics had an impact on rates of surgical intervention, colectomy, or permanent diversion.
Further analyses explored the long-term effects of second-line biologic choice after initial failure with anti-TNF therapy. Switching to another anti-TNF agent was more effective than switching to ustekinumab at reducing risks of colectomy (HR, 0.20; 95% CI, 0.04-0.90) and permanent diversion (HR, 0.16; 95% CI, 0.03-0.94); switching to ustekinumab was more effective than switching to vedolizumab for perianal fistula closure (HR, 0.22; 95% CI, 0.05-0.96).
Switching to another anti-TNF biologic or ustekinumab may be associated with better 5-year outcomes, compared with switching to vedolizumab in patients with pCD, according to Dr. Gubatan. Other guidelines or data that might steer this sequencing decision are scant. However, the findings should be validated with prospective data, ideally from head-to-head trials.
Jordan E. Axelrad, MD, of NYU Langone Health, New York, said the present study is noteworthy for addressing a “very-difficult-to-treat condition that has limited data as well as very limited long-term outcome data for our currently available interventions.”
Dr. Axelrad appreciated how the study focused on the distribution of clinical manifestations of pCD, including ulcers (10%), fissures (23.2%), abscesses (76.1%), and fistulas (84.2%). According to Dr. Axelrad, the efficacy data provide really important insights for clinicians who choose biologic therapies. He noted that, in the absence of head-to-head clinical trials, “it’s absolutely important that we use these results to help us guide therapy” for patients with pCD.
While the biologics included in the study were efficacious to varying degrees, Dr. Axelrad pointed out that no choice was associated with a reduced risk of surgical intervention. “That really underscored for me how complex this patient population is,” he said. “Despite good medical therapies … we’re still not necessarily making a huge dent in the risk of surgical intervention requirements for this complex patient group.”
Dr. Gubatan disclosed support from a Chan Zuckerberg Biohub Physician Scientist Scholar Award, a National Institutes of Health NIDDK LRP Award, and a Doris Duke Physician Scientist Fellowship Award; his colleagues reported no conflicts of interest. Dr. Axelrad reports relationships with Janssen, AbbVie, Pfizer, and others.
FROM JOURNAL OF CLINICAL GASTROENTEROLOGY
Pulse oximeters lead to less oxygen supplementation for people of color
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Medicare to cover colonoscopy after positive fecal test
Medicare will cover the full cost of colonoscopy after a positive noninvasive fecal test beginning in 2023, largely in response to a year-long advocacy campaign.
The benefit expansion is a “huge win” for patients, according to the American Gastroenterological Association, because it represents the end of out-of-pocket costs for colorectal cancer (CRC) screening.
“The continuum is complete!” said John Inadomi, MD, AGAF, past president of the AGA and a champion of the initiative within the organization.
Colonoscopy after a positive fecal test was previously considered a diagnostic procedure and therefore not considered part of the screening process by the Affordable Care Act, allowing payers to charge patients. That is, until the AGA and partners, including the American Cancer Society Cancer Action Network and Fight Colorectal Cancer, pushed back. First, the organizations successfully campaigned to ensure that private payers would cover the follow-up procedure. Now, after multiple meetings with the United States Department of Health & Human Services and Centers for Medicare & Medicaid Services, their collaborative efforts will end screening costs for patients with Medicare, pending finalization of the rule this fall. If finalized, it will take effect Jan. 2, 2023.
The policy change will “directly advance health equity” the AGA said, particularly among “rural communities and communities of color,” which are disproportionally affected by CRC.
“Cost-sharing is a well-recognized barrier to screening and has resulted in disparities,” said David Lieberman, MD, AGAF, who met with the CMS multiple times on behalf of the AGA. “Patients can now engage in CRC screening programs and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test.”
AGA president John Carethers, MD, AGAF, who also met with the CMS, noted that reducing barriers to CRC screening will ultimately reduce CRC mortality.
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives,” he said.
Dr. Inadomi, Dr. Carethers, and Dr. Lieberman serve on the scientific advisory board of Geneoscopy; Dr. Lieberman is also on the scientific advisory board for ColoWrap.
Medicare will cover the full cost of colonoscopy after a positive noninvasive fecal test beginning in 2023, largely in response to a year-long advocacy campaign.
The benefit expansion is a “huge win” for patients, according to the American Gastroenterological Association, because it represents the end of out-of-pocket costs for colorectal cancer (CRC) screening.
“The continuum is complete!” said John Inadomi, MD, AGAF, past president of the AGA and a champion of the initiative within the organization.
Colonoscopy after a positive fecal test was previously considered a diagnostic procedure and therefore not considered part of the screening process by the Affordable Care Act, allowing payers to charge patients. That is, until the AGA and partners, including the American Cancer Society Cancer Action Network and Fight Colorectal Cancer, pushed back. First, the organizations successfully campaigned to ensure that private payers would cover the follow-up procedure. Now, after multiple meetings with the United States Department of Health & Human Services and Centers for Medicare & Medicaid Services, their collaborative efforts will end screening costs for patients with Medicare, pending finalization of the rule this fall. If finalized, it will take effect Jan. 2, 2023.
The policy change will “directly advance health equity” the AGA said, particularly among “rural communities and communities of color,” which are disproportionally affected by CRC.
“Cost-sharing is a well-recognized barrier to screening and has resulted in disparities,” said David Lieberman, MD, AGAF, who met with the CMS multiple times on behalf of the AGA. “Patients can now engage in CRC screening programs and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test.”
AGA president John Carethers, MD, AGAF, who also met with the CMS, noted that reducing barriers to CRC screening will ultimately reduce CRC mortality.
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives,” he said.
Dr. Inadomi, Dr. Carethers, and Dr. Lieberman serve on the scientific advisory board of Geneoscopy; Dr. Lieberman is also on the scientific advisory board for ColoWrap.
Medicare will cover the full cost of colonoscopy after a positive noninvasive fecal test beginning in 2023, largely in response to a year-long advocacy campaign.
The benefit expansion is a “huge win” for patients, according to the American Gastroenterological Association, because it represents the end of out-of-pocket costs for colorectal cancer (CRC) screening.
“The continuum is complete!” said John Inadomi, MD, AGAF, past president of the AGA and a champion of the initiative within the organization.
Colonoscopy after a positive fecal test was previously considered a diagnostic procedure and therefore not considered part of the screening process by the Affordable Care Act, allowing payers to charge patients. That is, until the AGA and partners, including the American Cancer Society Cancer Action Network and Fight Colorectal Cancer, pushed back. First, the organizations successfully campaigned to ensure that private payers would cover the follow-up procedure. Now, after multiple meetings with the United States Department of Health & Human Services and Centers for Medicare & Medicaid Services, their collaborative efforts will end screening costs for patients with Medicare, pending finalization of the rule this fall. If finalized, it will take effect Jan. 2, 2023.
The policy change will “directly advance health equity” the AGA said, particularly among “rural communities and communities of color,” which are disproportionally affected by CRC.
“Cost-sharing is a well-recognized barrier to screening and has resulted in disparities,” said David Lieberman, MD, AGAF, who met with the CMS multiple times on behalf of the AGA. “Patients can now engage in CRC screening programs and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test.”
AGA president John Carethers, MD, AGAF, who also met with the CMS, noted that reducing barriers to CRC screening will ultimately reduce CRC mortality.
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives,” he said.
Dr. Inadomi, Dr. Carethers, and Dr. Lieberman serve on the scientific advisory board of Geneoscopy; Dr. Lieberman is also on the scientific advisory board for ColoWrap.