User login
Advocacy and Compliance Issues Impacting Dermatology in 2025
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
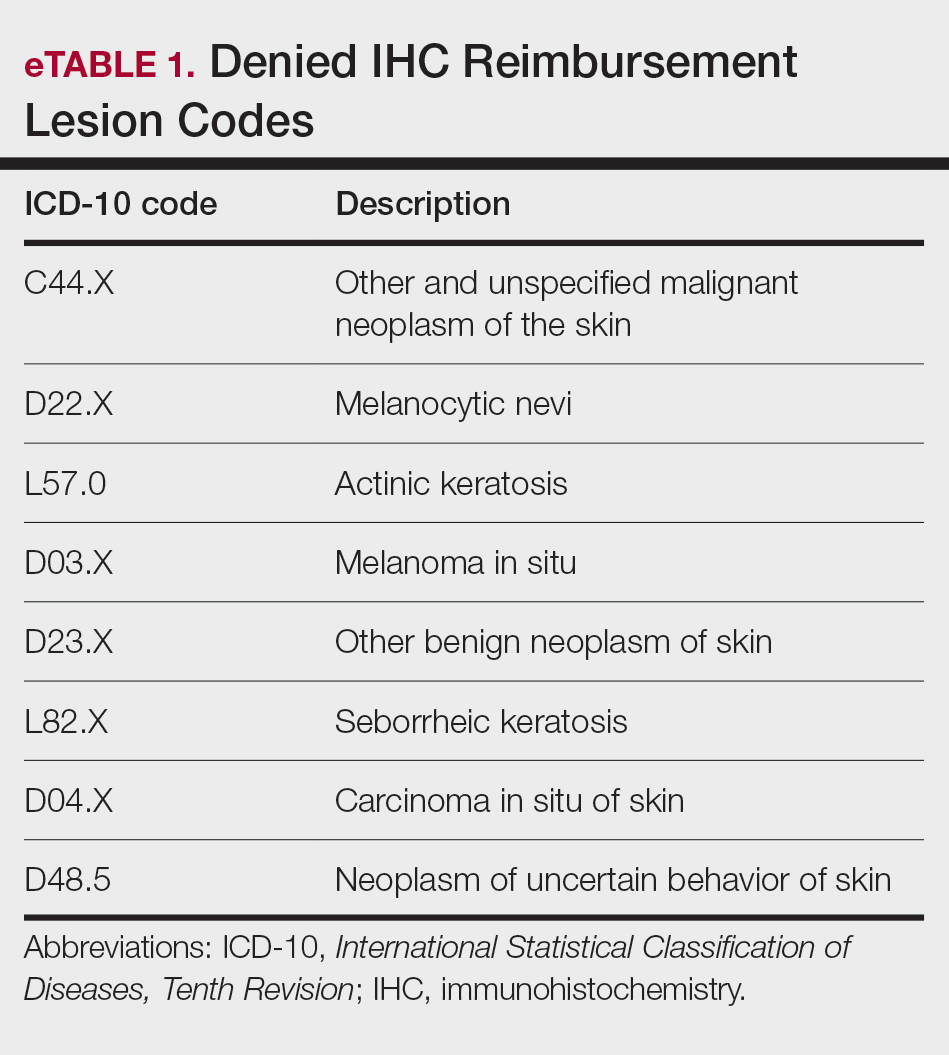
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6

Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6


Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6


Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
Advocacy and Compliance Issues Impacting Dermatology in 2025
Advocacy and Compliance Issues Impacting Dermatology in 2025
PRACTICE POINTS
- Recent advocacy efforts have led to the reversal of widespread insurer denials for immunohistochemistry stains; however, continued vigilance is necessary, as restrictive coverage policies may re-emerge.
- Laboratory directors must comply with updated Clinical Laboratory Improvement Amendments of 1988 and College of American Pathologists personnel requirements effective December 28, 2024, including stricter board certification and 2 years of laboratory training or experience and 20 hours of continuing education requirements.
- The American Society of Dermatopathology Appropriate Use Criteria mobile application provides physicians with evidence-based guidance for test selection in dermatopathology.