User login
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
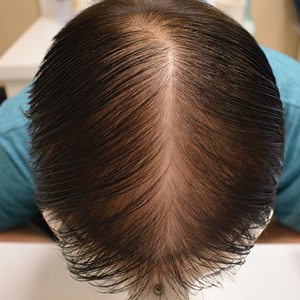
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
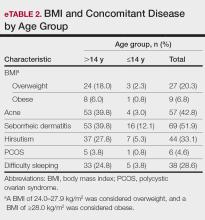
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
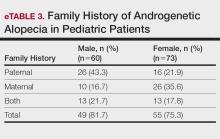
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
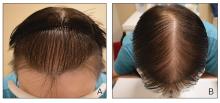
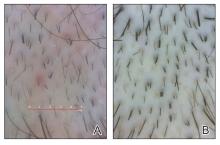
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
PRACTICE POINTS
- Early identification of androgenetic alopecia (AGA) is key in pediatric patients, especially in those with a family history of AGA and comorbidities such as seborrheic dermatitis, acne, or sleep disturbances.
- It is important to evaluate pediatric patients with AGA for hormonal imbalances and deficiencies in vitamin D and iron to guide treatment.
- Use targeted therapies such as topical minoxidil to treat pediatric AGA while also monitoring for adverse effects. For optimal outcomes, encourage consistent medication use and regular follow-up.