User login
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
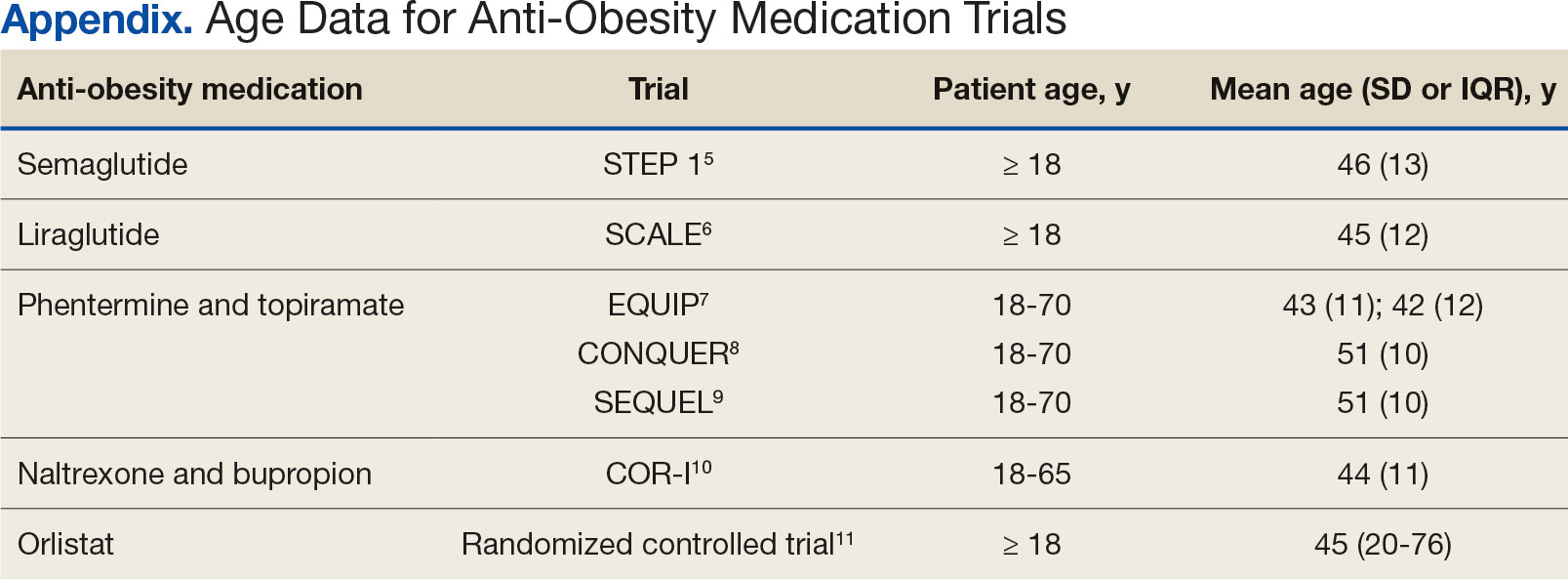
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.
Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
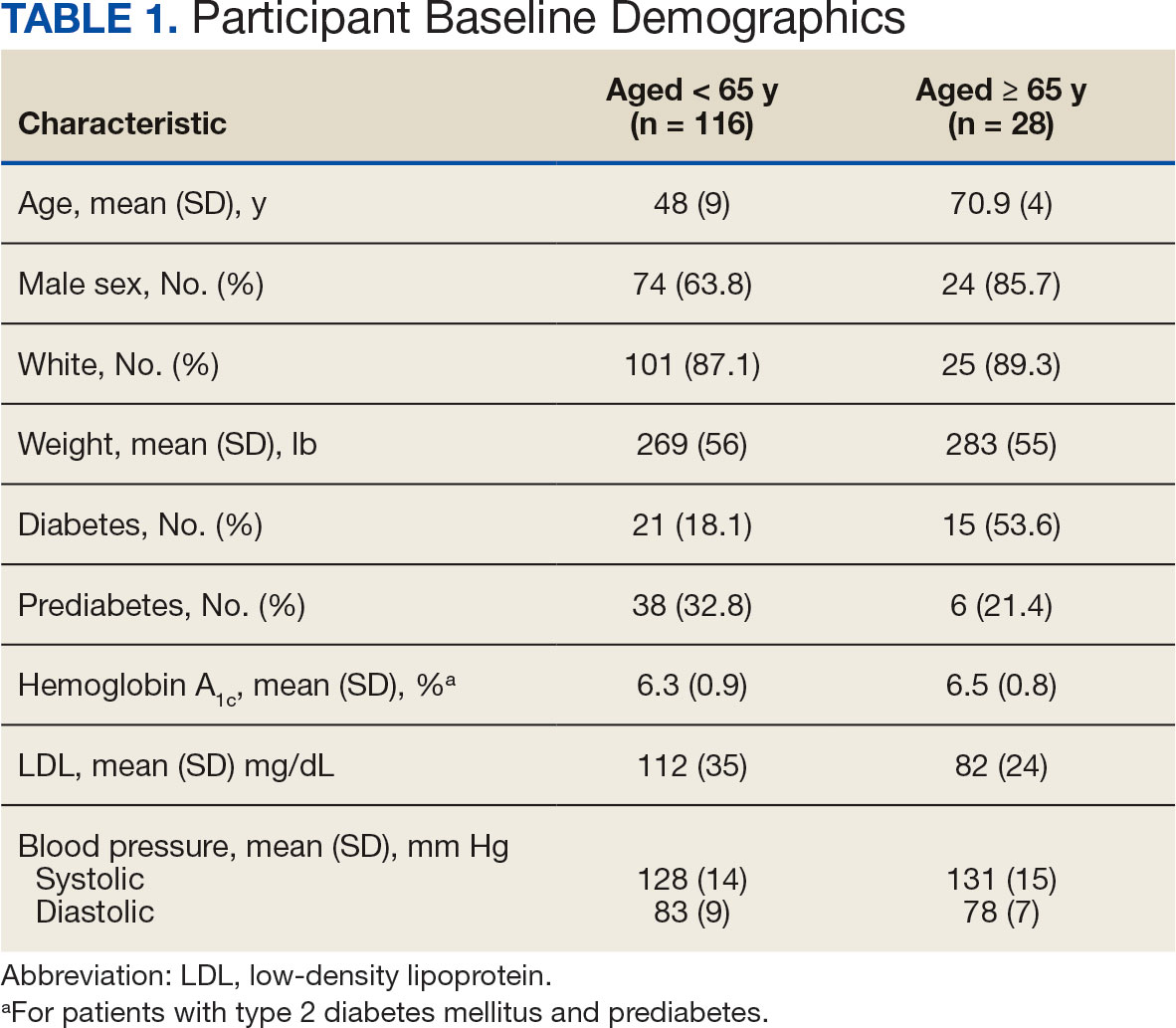
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).
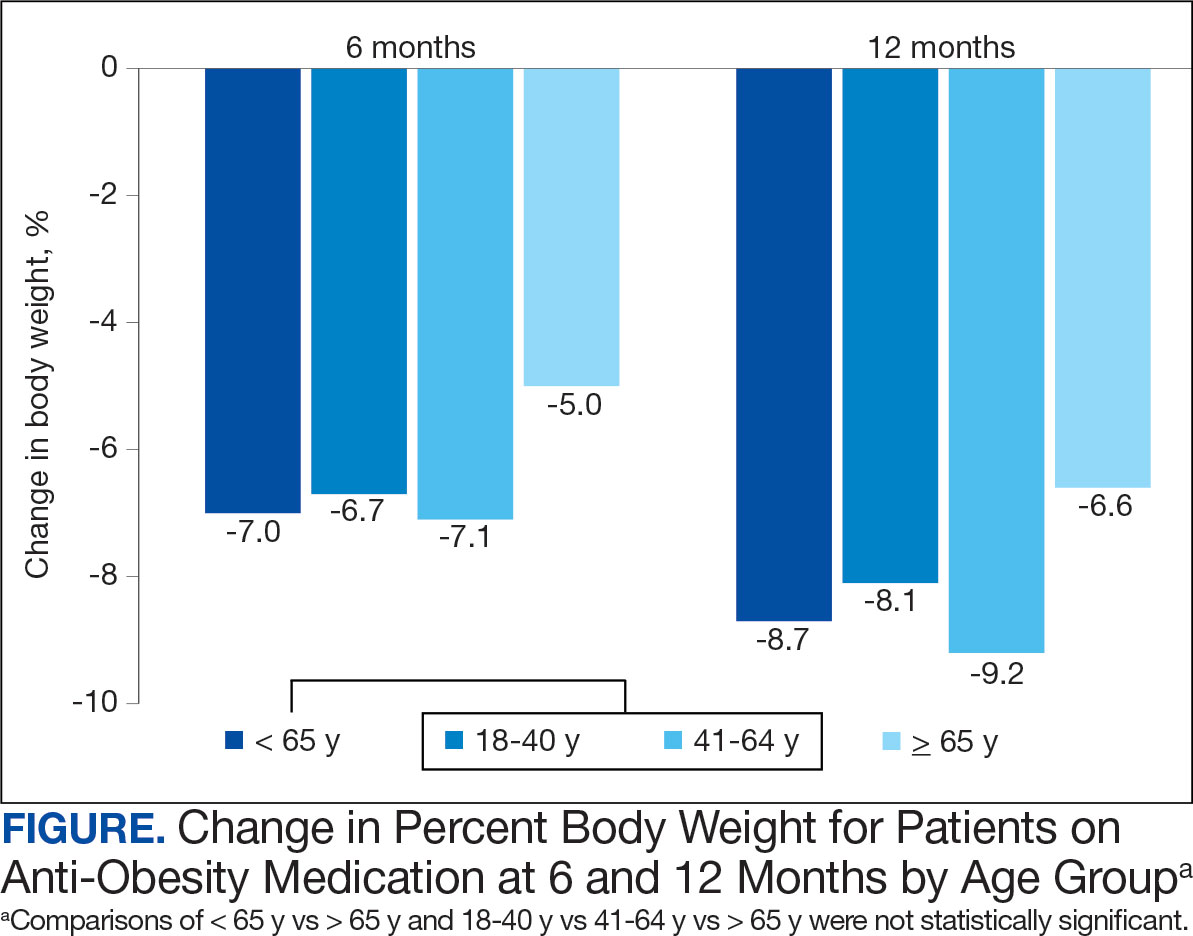
Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).
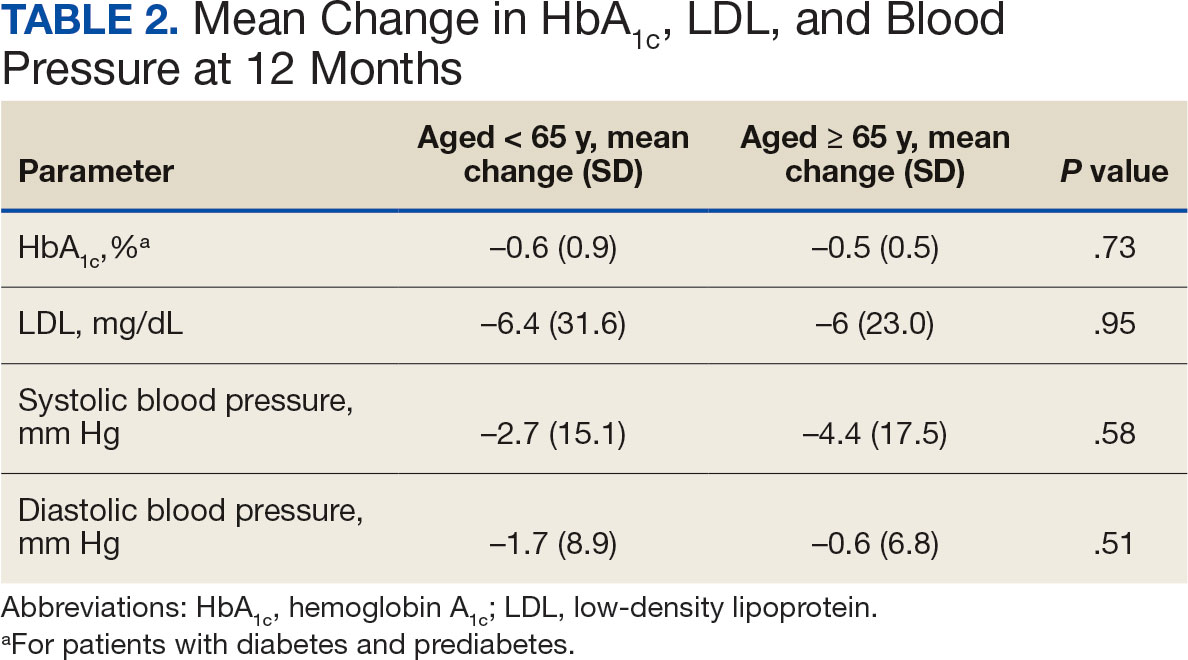
At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).
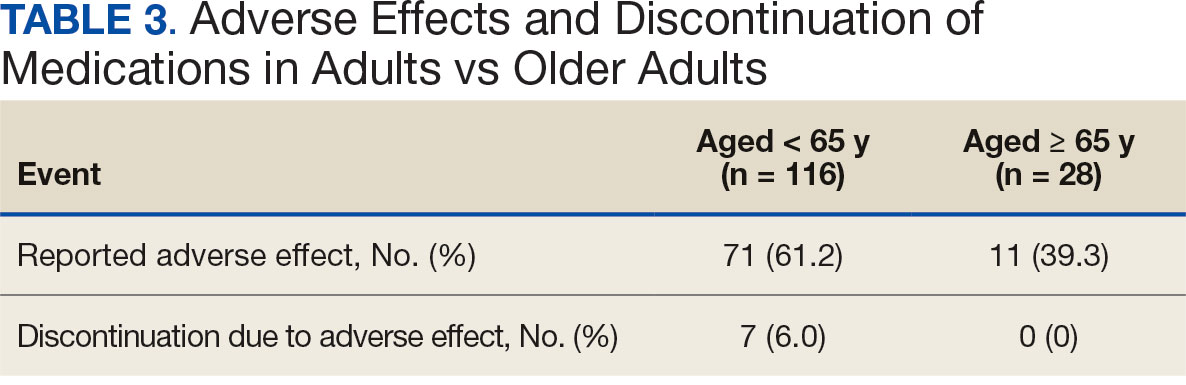
For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).
Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations