User login
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
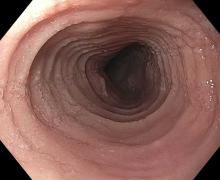
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.
Eosinophilic esophagitis (EoE) can be considered a “young” disease, with initial case series reported only about 30 years ago. Since that time, it has become a commonly encountered condition in both emergency and clinic settings. The most recent prevalence study estimates that 1 in 700 people in the U.S. have EoE,1 the volume of EoE-associated ED visits tripped between 2009 and 2019 and is projected to double again by 2030,2 and “new” gastroenterologists undoubtedly have learned about and seen this condition. As a chronic disease, EoE necessitates longitudinal follow-up and optimization of care to prevent complications. With increasing diagnostic delay, EoE progresses in most, but not all, patients from an inflammatory- to fibrostenotic-predominant condition.3
Diagnosis of EoE
The most likely area that you will encounter EoE is during an emergent middle-of-the-night endoscopy for food impaction. If called in for this, EoE will be the cause in more than 50% of patients.4 However, the diagnosis can only be made if esophageal biopsies are obtained at the time of the procedure. This is a critical time to decrease diagnostic delay, as half of patients are lost to follow-up after a food impaction.5 Unfortunately, although taking biopsies during index food impaction is guideline-recommended, a quality metric, and safe to obtain after the food bolus is cleared, this is infrequently done in practice.6, 7
The next most likely area for EoE detection is in the endoscopy suite where 15-23% of patients with dysphagia and 5-7% of patients undergoing upper endoscopy for any indication will have EoE.4 Sometimes EoE will be detected “incidentally” during an open-access case (for example, in a patient with diarrhea undergoing evaluation for celiac). In these cases, it is important to perform a careful history (as noted below) as subtle EoE symptoms can frequently be identified. Finally, when patients are seen in clinic for solid food dysphagia, EoE is clearly on the differential. A few percent of patients with refractory heartburn or chest pain will have EoE causing the symptoms rather than reflux,4 and all patients under consideration for antireflux surgery should have an endoscopy to assess for EoE.
When talking to patients with known or suspected EoE, the history must go beyond general questions about dysphagia or trouble swallowing. Many patients with EoE have overtly or subconsciously modified their eating behaviors over many years to minimize symptoms, may have adapted to chronic dysphagia, and will answer “no” when asked if they have trouble swallowing. Instead, use the acronym “IMPACT” to delve deeper into possible symptoms.8 Do they “Imbibe” fluids or liquids between each bite to help get food down? Do they “Modify” the way they eat (cut food into small bites; puree foods)? Do they “Prolong” mealtimes? Do they “Avoid” certain foods that stick? Do they “Chew’ until their food is a mush to get it down? And do they “Turn away” tablets or pills? Pill dysphagia is often a subtle symptom, and sometimes the only symptom elicited.
Additionally, it may be important to ask a partner or family member (if present) about their observations. They may provide insight (e.g. “yes – he chokes with every bite but never says it bothers him”) that the patient might not otherwise provide. The suspicion for EoE should also be increased in patients with concomitant atopic diseases and in those with a family history of dysphagia or who have family members needing esophageal dilation. It is important to remember that EoE can be seen across all ages, sexes, and races/ethnicities.
Diagnosis of EoE is based on the AGREE consensus,9 which is also echoed in the recently updated American College of Gastroenterology (ACG) guidelines.10 Diagnosis requires three steps. First, symptoms of esophageal dysfunction must be present. This will most typically be dysphagia in adolescents and adults, but symptoms are non-specific in children (e.g. poor growth and feeding, abdominal pain, vomiting, regurgitation, heartburn).
Second, at least 15 eosinophils per high-power field (eos/hpf) are required on esophageal biopsy, which implies that an endoscopy be performed. A high-quality endoscopic exam in EoE is of the utmost importance. The approach has been described elsewhere,11 but enough time on insertion should be taken to fully insufflate and examine the esophagus, including the areas of the gastroesophageal junction and upper esophageal sphincter where strictures can be missed, to gently wash debris, and to assess the endoscopic findings of EoE. Endoscopic findings should be reported using the validated EoE Endoscopy Reference Score (EREFS),12 which grades five key features. EREFS is reproducible, is responsive to treatment, and is guideline-recommended (see Figure 1).6, 10 The features are edema (present=1), rings (mild=1; moderate=2; severe=3), exudates (mild=1; severe=2), furrows (mild=1; severe=2), and stricture (present=1; also estimate diameter in mm) and are incorporated into many endoscopic reporting programs. Additionally, diffuse luminal narrowing and mucosal fragility (“crepe-paper” mucosa) should be assessed.
After this, biopsies should be obtained with at least 6 biopsy fragments from different locations in the esophagus. Any visible endoscopic abnormalities should be targeted (the highest yield is in exudates and furrows). The rationale is that EoE is patchy and at least 6 biopsies will maximize diagnostic yield.10 Ideally the initial endoscopy for EoE should be done off of treatments (like PPI or diet restriction) as these could mask the diagnosis. If a patient with suspected EoE has an endoscopy while on PPI, and the endoscopy is normal, a diagnosis of EoE cannot be made. In this case, consideration should be given as to stopping the PPI, allowing a wash out period (at least 1-2 months), and then repeating the endoscopy to confirm the diagnosis. This is important as EoE is a chronic condition necessitating life-long treatment and monitoring, so a definitive diagnosis is critical.
The third and final step in diagnosis is assessing for other conditions that could cause esophageal eosinophilia.9 The most common differential diagnosis is gastroesophageal reflux disease (GERD). In some cases, EoE and GERD overlap or can have a complex relationship.13 Unfortunately the location of the eosinophilia (i.e. distal only) and the level of the eosinophil counts are not useful in making this distinction, so all clinical features (symptoms, presence of erosive esophagitis, or a hiatal hernia endoscopically), and ancillary reflex testing when indicated may be required prior to a formal EoE diagnosis. After the diagnosis is established, there should be direct communication with the patient to review the diagnosis and select treatments. While it is possible to convey results electronically in a messaging portal or with a letter, a more formal interaction, such as a clinic visit, is recommended because this is a new diagnosis of a chronic condition. Similarly, a new diagnosis of inflammatory bowel disease would never be made in a pathology follow-up letter alone.
Treatment of EoE
When it comes to treatment, the new guidelines emphasize several points.10 First, there is the concept that anti-inflammatory treatment should be paired with assessment of fibrostenosis and esophageal dilation; to do either in isolation is incomplete treatment. It is safe to perform dilation both prior to anti-inflammatory treatment (for example, with a critical stricture in a patient with dysphagia) and after anti-inflammatory treatment has been prescribed (for example, during an endoscopy to assess treatment response).
Second, proton pump inhibitors (PPIs), swallowed topical corticosteroids (tCS), or dietary elimination are all acceptable first-line treatment options for EoE. A shared decision-making framework should be used for this discussion. If dietary elimination is selected,14 based on new clinical trial data, guidelines recommend using empiric elimination and starting with a less restrictive diet (either a one-food elimination diet with dairy alone or a two-food elimination with dairy and wheat elimination). If PPIs are selected, the dose should be double the standard reflux dose. Data are mixed as to whether to use twice daily dosing (i.e., omeprazole 20 mg twice daily) or once a day dosing (i.e., omeprazole 40 mg daily), but total dose and adherence may be more important than frequency.10
For tCS use, either budesonide or fluticasone can be selected, but budesonide oral suspension is the only FDA-approved tCS for EoE.15 Initial treatment length is usually 6-8 weeks for diet elimination and, 12 weeks for PPI and tCS. In general, it is best to pick a single treatment to start, and reserve combining therapies for patients who do not have a complete response to a single modality as there are few data to support combination therapy.
After initial treatment, it is critical to assess for treatment response.16 Goals of EoE treatment include improvement in symptoms, but also improvement in endoscopic and histologic features to prevent complications. Symptoms in EoE do not always correlate with underlying biologic disease activity: patients can minimize symptoms with careful eating; they may perceive no difference in symptoms despite histologic improvement if a stricture persists; and they may have minimal symptoms after esophageal dilation despite ongoing inflammation. Because of this, performing a follow-up endoscopy after initial treatment is guideline-recommended.10, 17 This allows assessing for endoscopic improvement, re-assessing for fibrostenosis and performing dilation if indicated, and obtaining esophageal biopsies. If there is non-response, options include switching between other first line treatments or considering “stepping-up” to dupilumab which is also an FDA-approved option for EoE that is recommended in the guidelines.10, 18 In some cases where patients have multiple severe atopic conditions such as asthma or eczema that would warrant dupilumab use, or if patients are intolerant to PPIs or tCS, dupilumab could be considered as an earlier treatment for EoE.
Long-Term Maintenance
If a patient has a good response (for example, improved symptoms, improved endoscopic features, and <15 eos/hpf on biopsy), treatment can be maintained long-term. In almost all cases, if treatment is stopped, EoE disease activity recurs.19 Patients could be seen back in clinic in 6-12 months, and then a discussion can be conducted about a follow-up endoscopy, with timing to be determined based on their individual disease features and severity.17
Patients with more severe strictures, however, may have to be seen in endoscopy for serial dilations. Continued follow-up is essential for optimal care. Just as patients can progress in their disease course with diagnostic delay, there are data that show they can also progress after diagnosis when there are gaps in care without regular follow-up.20 Unlike other chronic esophageal disorders such as GERD and Barrett’s esophagus and other chronic GI inflammatory conditions like inflammatory bowel disease, however, EoE is not associated with an increased risk of esophageal cancer.21, 22
Given its increasing frequency, EoE will be commonly encountered by gastroenterologists both new and established. Having a systematic approach for diagnosis, understanding how to elicit subtle symptoms, implementing a shared decision-making framework for treatment with a structured algorithm for assessing response, performing follow-up, maintaining treatment, and monitoring patients long-term will allow the large majority of EoE patients to be successfully managed.
Dr. Dellon is based at the Center for Esophageal Diseases and Swallowing, Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill. He disclosed research funding, consultant fees, and educational grants from multiple companies.
References
1. Thel HL, et al. Prevalence and Costs of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.09.031.
2. Lam AY, et al. Epidemiologic Burden and Projections for Eosinophilic Esophagitis-Associated Emergency Department Visits in the United States: 2009-2030. Clin Gastroenterol Hepatol. 2023 Nov. doi: 10.1016/j.cgh.2023.04.028.
3. Schoepfer AM, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec. doi: 10.1053/j.gastro.2013.08.015.
4. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018 Jan. doi: 10.1053/j.gastro.2017.06.067.
5. Chang JW, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus. 2019 Dec. doi: 10.1093/dote/doz056.
6. Aceves SS, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Aug. doi: 10.1016/j.gie.2022.05.013.
7. Leiman DA, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2023 Jun. doi: 10.14309/ajg.0000000000002138.
8. Hirano I, Furuta GT. Approaches and Challenges to Management of Pediatric and Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2020 Mar. doi: 10.1053/j.gastro.2019.09.052.
9. Dellon ES, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018 Oct. doi: 10.1053/j.gastro.2018.07.009.
10. Dellon ES, et al. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol. 2025 Jan. doi: 10.14309/ajg.0000000000003194.
11. Dellon ES. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2021 Dec. doi: 10.1016/j.cgh.2021.07.011.
12. Hirano I, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 May. doi: 10.1136/gutjnl-2011-301817.
13. Spechler SJ, et al. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007 Jun. doi: 10.1111/j.1572-0241.2007.01179.x.
14. Chang JW, et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul. doi: 10.1016/j.cgh.2023.03.006.
15. Hirano I, et al. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin Gastroenterol Hepatol. 2022 Mar. doi: 10.1016/j.cgh.2021.04.022.
16. Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019 Oct. doi: 10.1016/j.cgh.2019.01.030.
17. von Arnim U, et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep. doi: 10.1016/j.cgh.2022.12.018.
18. Dellon ES, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med. 2022 Dec. doi: 10.1056/NEJMoa220598.
19. Dellon ES, et al. Rapid Recurrence of Eosinophilic Esophagitis Activity After Successful Treatment in the Observation Phase of a Randomized, Double-Blind, Double-Dummy Trial. Clin Gastroenterol Hepatol. 2020 Jun. doi: 10.1016/j.cgh.2019.08.050.
20. Chang NC, et al. A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients. Clin Gastroenterol Hepatol. 2022 Aug. doi: 10.1016/j.cgh.2021.10.028.
21. Syed A, et al. The relationship between eosinophilic esophagitis and esophageal cancer. Dis Esophagus. 2017 Jul. doi: 10.1093/dote/dox050.
22. Albaneze N, et al. No Association Between Eosinophilic Oesophagitis and Oesophageal Cancer in US Adults: A Case-Control Study. Aliment Pharmacol Ther. 2025 Jan. doi: 10.1111/apt.18431.