User login
Poor weight loss after bariatric surgery? Liraglutide may help
Up to one in four patients who undergo metabolic/bariatric surgery have less than 20% weight loss and patients need additional strategies to help them reach their goals.
In the new BARI-OPTIMISE trial, patients with poor weight loss after such surgery were randomized to the glucagonlike peptide–1 (GLP-1) agonist liraglutide 3.0 mg/day or placebo. , report Jessica Mok, BMBS, MPhil, University College London and colleagues, in their study published online in JAMA Surgery.
Weight loss in BARI-OPTIMISE (–9.2 kg or –20 lb) was greater than the weight loss in the earlier GRAVITAS trial of 80 patients with persistent or recurrent type 2 diabetes randomized to liraglutide 1.8 mg/day or placebo, Dr. Mok and colleagues note. And more patients in BARI-OPTIMISE than in GRAVITAS lost 5% or more of their baseline weight (72% vs. 46%).
“Our findings therefore suggest that liraglutide, 3.0 mg, may have a role in the treatment of people with poor weight loss following metabolic surgery,” they write.
However, newer gut hormone–based therapies with greater efficacy than liraglutide 3.0 mg (for example, semaglutide and tirzepatide) are emerging, they add.
Therefore, “randomized clinical trials investigating the efficacy of novel pharmaceutical agents will be needed to generate the evidence required to deliver individualized precision-medicine approaches to patients with obesity and suboptimal weight loss following metabolic surgery,” the researchers urge.
‘Extremely welcome tools for severe obesity’
“The additional weight loss with associated favorable metabolic changes achieved with liraglutide reported in [the BARI-OPTIMISE and GRAVITAS trials] is extremely welcomed with the new antiobesity medications ... adding another effective tool in the toolbox for the treatment of severe obesity,” Paulina Salminen, MD, PhD, Turku (Finland) University Hospital, and Ali Aminian, MD, Cleveland Clinic, write in an accompanying editorial.
However, they also point to limitations of the current trial.
Almost all patients (65 of 70 [93%]) underwent laparoscopic sleeve gastrectomy in BARI-OPTIMISE. However, “there are safe and more effective surgical options that can be considered in patients with suboptimal initial clinical response or recurrent weight gain” after laparoscopic sleeve gastrectomy, such as “conversion to Roux-en-Y gastric bypass (RYGB), duodenal switch, or single-anastomosis duodeno-ileal bypass,” they note.
The small number of patients and low follow-up rate of 81% (57 of 70 patients) for the short intervention are other limitations.
“In treating a patient with ischemic heart disease, a combination of lifestyle intervention, risk factor modification, pharmacotherapy, coronary stenting, and open-heart surgery may be needed,” note the editorialists. “A very similar concept would be applicable in the management of severe obesity.”
In the past, they add, there was not much progress with combination therapies for obesity because of a lack of effective antiobesity medications.
However, “with the better availability of potent [antiobesity medications] now and in the near future, the practice of combination therapy will grow as [metabolic and bariatric surgery] and [antiobesity medications] work in synergy in both treating severe obesity and hopefully also in enabling increased access to effective obesity treatment,” Dr. Salminen and Dr. Aminian speculate.
“Hopefully, based on findings of future studies and the use of global uniform criteria for evaluating treatment outcomes,” the editorialists conclude, “we can develop practice guidelines to assist and optimize phenotype-tailored multimodal treatment of this heterogeneous chronic disease of severe obesity.”
Most patients had severe obesity, sleeve gastrectomy
Individuals with poor weight loss after surgery have increased appetite coupled with an unfavorable gut hormone profile, including lower circulating GLP-1 levels, Dr. Mok and colleagues note.
In 2018 and 2019, they recruited and randomized 70 adults who had had metabolic surgery at two hospitals in London at least a year earlier and had 20% or less weight loss, compared with the day of surgery, as well as a suboptimal nutrient-stimulated GLP-1 response.
Patients were excluded if they had type 1 diabetes or were taking a GLP-1 agonist, insulin, or other medications that can affect weight, among other criteria.
The mean age of patients was 48 years, and 74% were women; 13% had type 2 diabetes.
Participants had a mean weight of 120 kg, and a mean body mass index of 43 kg/m2 (57% had a BMI ≥ 40 kg/m2). Almost all patients (93%) had had sleeve gastrectomy and 7% had RYGB.
On average, they had surgery 4.3 years earlier and had lost 7% of their initial weight.
Patients were randomized 1:1 to receive liraglutide 3.0 mg or placebo daily for 24 weeks. All patients received dietary counseling and aimed for a 500 kcal/day energy deficit. They were encouraged to do a minimum of 150 minutes of moderate to vigorous exercise each week.
The primary endpoint, percentage change in body weight from baseline to week 24, was –8.8% with liraglutide versus –0.53% with placebo.
Adverse effects were predominantly gastrointestinal in nature and were more frequent with liraglutide (80%) than placebo (57%). There were no serious adverse events.
This study was funded by the Sir Jules Thorn Charitable Trust and the National Institute for Health and Care Research. Novo Nordisk provided the liraglutide and placebo pens. Author disclosures are listed with the article. Dr. Salminen has reported receiving personal fees from Novo Nordisk. Dr. Aminian has reported receiving received grants and personal fees from Medtronic and Ethicon.
A version of this article appeared on Medscape.com.
Up to one in four patients who undergo metabolic/bariatric surgery have less than 20% weight loss and patients need additional strategies to help them reach their goals.
In the new BARI-OPTIMISE trial, patients with poor weight loss after such surgery were randomized to the glucagonlike peptide–1 (GLP-1) agonist liraglutide 3.0 mg/day or placebo. , report Jessica Mok, BMBS, MPhil, University College London and colleagues, in their study published online in JAMA Surgery.
Weight loss in BARI-OPTIMISE (–9.2 kg or –20 lb) was greater than the weight loss in the earlier GRAVITAS trial of 80 patients with persistent or recurrent type 2 diabetes randomized to liraglutide 1.8 mg/day or placebo, Dr. Mok and colleagues note. And more patients in BARI-OPTIMISE than in GRAVITAS lost 5% or more of their baseline weight (72% vs. 46%).
“Our findings therefore suggest that liraglutide, 3.0 mg, may have a role in the treatment of people with poor weight loss following metabolic surgery,” they write.
However, newer gut hormone–based therapies with greater efficacy than liraglutide 3.0 mg (for example, semaglutide and tirzepatide) are emerging, they add.
Therefore, “randomized clinical trials investigating the efficacy of novel pharmaceutical agents will be needed to generate the evidence required to deliver individualized precision-medicine approaches to patients with obesity and suboptimal weight loss following metabolic surgery,” the researchers urge.
‘Extremely welcome tools for severe obesity’
“The additional weight loss with associated favorable metabolic changes achieved with liraglutide reported in [the BARI-OPTIMISE and GRAVITAS trials] is extremely welcomed with the new antiobesity medications ... adding another effective tool in the toolbox for the treatment of severe obesity,” Paulina Salminen, MD, PhD, Turku (Finland) University Hospital, and Ali Aminian, MD, Cleveland Clinic, write in an accompanying editorial.
However, they also point to limitations of the current trial.
Almost all patients (65 of 70 [93%]) underwent laparoscopic sleeve gastrectomy in BARI-OPTIMISE. However, “there are safe and more effective surgical options that can be considered in patients with suboptimal initial clinical response or recurrent weight gain” after laparoscopic sleeve gastrectomy, such as “conversion to Roux-en-Y gastric bypass (RYGB), duodenal switch, or single-anastomosis duodeno-ileal bypass,” they note.
The small number of patients and low follow-up rate of 81% (57 of 70 patients) for the short intervention are other limitations.
“In treating a patient with ischemic heart disease, a combination of lifestyle intervention, risk factor modification, pharmacotherapy, coronary stenting, and open-heart surgery may be needed,” note the editorialists. “A very similar concept would be applicable in the management of severe obesity.”
In the past, they add, there was not much progress with combination therapies for obesity because of a lack of effective antiobesity medications.
However, “with the better availability of potent [antiobesity medications] now and in the near future, the practice of combination therapy will grow as [metabolic and bariatric surgery] and [antiobesity medications] work in synergy in both treating severe obesity and hopefully also in enabling increased access to effective obesity treatment,” Dr. Salminen and Dr. Aminian speculate.
“Hopefully, based on findings of future studies and the use of global uniform criteria for evaluating treatment outcomes,” the editorialists conclude, “we can develop practice guidelines to assist and optimize phenotype-tailored multimodal treatment of this heterogeneous chronic disease of severe obesity.”
Most patients had severe obesity, sleeve gastrectomy
Individuals with poor weight loss after surgery have increased appetite coupled with an unfavorable gut hormone profile, including lower circulating GLP-1 levels, Dr. Mok and colleagues note.
In 2018 and 2019, they recruited and randomized 70 adults who had had metabolic surgery at two hospitals in London at least a year earlier and had 20% or less weight loss, compared with the day of surgery, as well as a suboptimal nutrient-stimulated GLP-1 response.
Patients were excluded if they had type 1 diabetes or were taking a GLP-1 agonist, insulin, or other medications that can affect weight, among other criteria.
The mean age of patients was 48 years, and 74% were women; 13% had type 2 diabetes.
Participants had a mean weight of 120 kg, and a mean body mass index of 43 kg/m2 (57% had a BMI ≥ 40 kg/m2). Almost all patients (93%) had had sleeve gastrectomy and 7% had RYGB.
On average, they had surgery 4.3 years earlier and had lost 7% of their initial weight.
Patients were randomized 1:1 to receive liraglutide 3.0 mg or placebo daily for 24 weeks. All patients received dietary counseling and aimed for a 500 kcal/day energy deficit. They were encouraged to do a minimum of 150 minutes of moderate to vigorous exercise each week.
The primary endpoint, percentage change in body weight from baseline to week 24, was –8.8% with liraglutide versus –0.53% with placebo.
Adverse effects were predominantly gastrointestinal in nature and were more frequent with liraglutide (80%) than placebo (57%). There were no serious adverse events.
This study was funded by the Sir Jules Thorn Charitable Trust and the National Institute for Health and Care Research. Novo Nordisk provided the liraglutide and placebo pens. Author disclosures are listed with the article. Dr. Salminen has reported receiving personal fees from Novo Nordisk. Dr. Aminian has reported receiving received grants and personal fees from Medtronic and Ethicon.
A version of this article appeared on Medscape.com.
Up to one in four patients who undergo metabolic/bariatric surgery have less than 20% weight loss and patients need additional strategies to help them reach their goals.
In the new BARI-OPTIMISE trial, patients with poor weight loss after such surgery were randomized to the glucagonlike peptide–1 (GLP-1) agonist liraglutide 3.0 mg/day or placebo. , report Jessica Mok, BMBS, MPhil, University College London and colleagues, in their study published online in JAMA Surgery.
Weight loss in BARI-OPTIMISE (–9.2 kg or –20 lb) was greater than the weight loss in the earlier GRAVITAS trial of 80 patients with persistent or recurrent type 2 diabetes randomized to liraglutide 1.8 mg/day or placebo, Dr. Mok and colleagues note. And more patients in BARI-OPTIMISE than in GRAVITAS lost 5% or more of their baseline weight (72% vs. 46%).
“Our findings therefore suggest that liraglutide, 3.0 mg, may have a role in the treatment of people with poor weight loss following metabolic surgery,” they write.
However, newer gut hormone–based therapies with greater efficacy than liraglutide 3.0 mg (for example, semaglutide and tirzepatide) are emerging, they add.
Therefore, “randomized clinical trials investigating the efficacy of novel pharmaceutical agents will be needed to generate the evidence required to deliver individualized precision-medicine approaches to patients with obesity and suboptimal weight loss following metabolic surgery,” the researchers urge.
‘Extremely welcome tools for severe obesity’
“The additional weight loss with associated favorable metabolic changes achieved with liraglutide reported in [the BARI-OPTIMISE and GRAVITAS trials] is extremely welcomed with the new antiobesity medications ... adding another effective tool in the toolbox for the treatment of severe obesity,” Paulina Salminen, MD, PhD, Turku (Finland) University Hospital, and Ali Aminian, MD, Cleveland Clinic, write in an accompanying editorial.
However, they also point to limitations of the current trial.
Almost all patients (65 of 70 [93%]) underwent laparoscopic sleeve gastrectomy in BARI-OPTIMISE. However, “there are safe and more effective surgical options that can be considered in patients with suboptimal initial clinical response or recurrent weight gain” after laparoscopic sleeve gastrectomy, such as “conversion to Roux-en-Y gastric bypass (RYGB), duodenal switch, or single-anastomosis duodeno-ileal bypass,” they note.
The small number of patients and low follow-up rate of 81% (57 of 70 patients) for the short intervention are other limitations.
“In treating a patient with ischemic heart disease, a combination of lifestyle intervention, risk factor modification, pharmacotherapy, coronary stenting, and open-heart surgery may be needed,” note the editorialists. “A very similar concept would be applicable in the management of severe obesity.”
In the past, they add, there was not much progress with combination therapies for obesity because of a lack of effective antiobesity medications.
However, “with the better availability of potent [antiobesity medications] now and in the near future, the practice of combination therapy will grow as [metabolic and bariatric surgery] and [antiobesity medications] work in synergy in both treating severe obesity and hopefully also in enabling increased access to effective obesity treatment,” Dr. Salminen and Dr. Aminian speculate.
“Hopefully, based on findings of future studies and the use of global uniform criteria for evaluating treatment outcomes,” the editorialists conclude, “we can develop practice guidelines to assist and optimize phenotype-tailored multimodal treatment of this heterogeneous chronic disease of severe obesity.”
Most patients had severe obesity, sleeve gastrectomy
Individuals with poor weight loss after surgery have increased appetite coupled with an unfavorable gut hormone profile, including lower circulating GLP-1 levels, Dr. Mok and colleagues note.
In 2018 and 2019, they recruited and randomized 70 adults who had had metabolic surgery at two hospitals in London at least a year earlier and had 20% or less weight loss, compared with the day of surgery, as well as a suboptimal nutrient-stimulated GLP-1 response.
Patients were excluded if they had type 1 diabetes or were taking a GLP-1 agonist, insulin, or other medications that can affect weight, among other criteria.
The mean age of patients was 48 years, and 74% were women; 13% had type 2 diabetes.
Participants had a mean weight of 120 kg, and a mean body mass index of 43 kg/m2 (57% had a BMI ≥ 40 kg/m2). Almost all patients (93%) had had sleeve gastrectomy and 7% had RYGB.
On average, they had surgery 4.3 years earlier and had lost 7% of their initial weight.
Patients were randomized 1:1 to receive liraglutide 3.0 mg or placebo daily for 24 weeks. All patients received dietary counseling and aimed for a 500 kcal/day energy deficit. They were encouraged to do a minimum of 150 minutes of moderate to vigorous exercise each week.
The primary endpoint, percentage change in body weight from baseline to week 24, was –8.8% with liraglutide versus –0.53% with placebo.
Adverse effects were predominantly gastrointestinal in nature and were more frequent with liraglutide (80%) than placebo (57%). There were no serious adverse events.
This study was funded by the Sir Jules Thorn Charitable Trust and the National Institute for Health and Care Research. Novo Nordisk provided the liraglutide and placebo pens. Author disclosures are listed with the article. Dr. Salminen has reported receiving personal fees from Novo Nordisk. Dr. Aminian has reported receiving received grants and personal fees from Medtronic and Ethicon.
A version of this article appeared on Medscape.com.
FROM JAMA SURGERY
Few patients with BMI of 30-35 get bariatric surgery
Although multiple international medical societies over recent years have recommended lowering the threshold for bariatric surgery to a body mass index (BMI) of 30-35 (class 1 obesity) in certain patients, very few patients in this weight category have had such surgery, according to a new study.
On the basis of data from a large U.S. national registry, during 2015 through 2021,
Most surgeries (96.5%) were in patients with a BMI greater than 35. This reflects advice from a 1991 consensus statement by the National Institutes of Health stating that bariatric surgery can be offered to patients with BMI greater than or equal to 40, or greater than or equal to 35 with comorbidities.
However, medical societies have recommended lower cutoffs in position statements in 2016, 2018, and 2022.
Paul Wisniowski, MD, a surgical resident at Keck School of Medicine of University of Southern California, Los Angeles, presented the study findings in an e-poster at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
“Professional guidelines and increasing data support bariatric surgery for patients beginning at BMI 30, which is a tipping point for disease progression. Now it needs to happen in the real world,” outgoing ASMBS president Teresa LaMasters, MD, who was not involved with this research, said in an ASMBS press release.
“We encourage greater consideration of this important treatment option earlier in the disease process,” stressed Dr. LaMasters, a bariatric surgeon and Medical Director, Unity Point Clinic Weight Loss Specialists, West Des Moines, IA.
‘Not unexpected,’ ‘need to expand eligibility’
“We expected that there had been little widespread adoption of the new BMI criteria/cutoffs,” senior study author Matthew J. Martin, MD, said in an interview.
“We know that bariatric surgery is already underutilized, as only about 1%-2% of eligible patients who would benefit end up getting surgery,” added Dr. Martin, Chief, Emergency General Surgery, and Director, Acute Care Surgery Research, USC Medical Center and Keck School of Medicine.
He suggests that the main reason that more patients with lower BMIs are not being offered surgery is related to insurance coverage and reimbursement.
“Even though the professional society guidelines have changed, based on the scientific evidence, most insurers are still using the very outdated (1990s) NIH consensus criteria of BMI greater than 35 with comorbidities, or BMI greater than 40.”
Another potential reason is “the lack of awareness of the changing guidelines and recommendation among primary care physicians who refer patients for a bariatric surgery evaluation.”
“I think it is too early in the experience with the new, more effective antiobesity medications to say which group will benefit the most or will prefer them over surgery,” he said.
“There is still only a small minority of patients who end up getting the [newer antiobesity] medications or surgery.”
“The takeaway,” Dr. Martin summarized, “is that bariatric surgery remains the only intervention with a high success rate for patients with class 1 or higher obesity in terms of weight loss, comorbidity improvement or resolution, and sustained health benefits.”
“We need to expand the availability of bariatric surgery for all eligible patients, particularly the class 1 obesity population who are currently the most underserved,” he said.
“This will take continued lobbying and working with the insurance companies to update their guidelines/criteria, education of patients, and education of primary care physicians so that patients can be appropriately referred for a surgical evaluation.”
Surgery vs. pharmacotherapy
Invited to comment on this study, Neil Skolnik, MD, who was not involved with this research, noted that data from patients with a lower BMI “has continued to accumulate, showing much greater safety than earlier studies and giving further support of efficacy.”
However, “[new] recommendations take time to take hold,” noted Dr. Skolnik, a family physician and professor in the department of family medicine, Thomas Jefferson University, Philadelphia.
“And from March of 2020 through 2021, surgery referrals were likely influenced by the COVID pandemic,” he added in an email.
Dr. Skolnik authored a commentary sharing his reservations about ASMBS recommendations issued in 2022 for lower BMI thresholds for this surgery.
“Medications are a safe, effective option for patients with a BMI from 30 to 35,” he said, “and [they] achieve approximately a 15%-20% average weight loss, which is enough to markedly improved both metabolic parameters and biomechanical issues such as knee pain, hip pain, and back pain.”
However, “bariatric surgery remains an excellent option for patients who do not respond sufficiently to pharmacotherapy,” he acknowledged.
National registry study, 2015-2021
Dr. Wisniowski and colleagues analyzed data from around 900 U.S. centers that are currently part of the Metabolic Bariatric Surgery Accreditation Quality Improvement Program.
They found that from 2015 to 2021, 38,669 patients (3.5%) with type 1 obesity and 1,1067,094 patients (96.5%) with a higher BMI had metabolic and bariatric surgery.
Compared with patients with BMI greater than 35, those with class 1 obesity had shorter operating times and hospital stays, but they lost less weight on short-term evaluation, after multivariable adjustment.
There were no significant differences between the two patient groups in rates of postoperative complications (< 5%) or mortality (< 0.1%).
Sleeve gastrectomy was the most common procedure and increased from 70% to 76% of all procedures during the study period.
Single-center study
In a second e-poster presented at the meeting, Tina T. Thomas, MD, New Jersey Bariatric Center, analyzed data from 23 patients with BMI less than 35 or less than 30 with comorbidities who had sleeve gastrectomy or Roux-en-Y gastric bypass at their center during 2017 to 2021 and who had 6 months of follow-up data.
At study entry, the patients had a mean BMI of 33.5. At 6 months after the surgery, they had a mean BMI of 25.6, and on average, they had lost 55% of their excess weight.
Nearly 60% of the patients had lost at least 50% of their excess weight, and 9 of 16 patients (56%) with comorbidities had improved or resolved comorbidities. None of the patients died or had surgery-related complications.
“Our study shows significant weight loss and health benefits, as well as the safety and efficacy of the gastric bypass and gastric sleeve procedures, for this patient population,” Ajay Goyal, MD, senior author, and bariatric surgeon at New Jersey Bariatric Center, said in an ASMBS press release.
“Often by the time a patient qualifies for bariatric surgery, their weight-related medical conditions such as [type 2] diabetes and hypertension are severe. By expanding access to bariatric surgery to patients with a lower BMI with obesity-related illnesses, patients can halt the progression, and in some cases resolve, significant and uncontrolled weight-related chronic diseases through weight loss.”
Societies call for lower BMI thresholds
Providers, hospitals, and insurers currently use BMI thresholds greater than or equal to 40, or greater than or equal to 35 with an obesity-related comorbidity, to define patients eligible for metabolic and bariatric surgery, based on criteria established in a 1991 consensus statement by NIH.
As more data accumulated, in 2016, a position statement from 45 societies recommended that bariatric surgery should be “considered for patients with [type 2 diabetes] and BMI 30.0-34.9 kg/m2 if hyperglycemia is inadequately controlled” despite optimal medical treatment.
Similarly, in 2018, the ASMBS issued a position statement saying that “for patients with BMI 30-35 kg/m2 and obesity-related comorbidities who do not achieve substantial, durable weight loss and comorbidity improvement with reasonable nonsurgical methods, bariatric surgery should be offered” to suitable individuals.
Then in October 2022, the ASMBS and International Federation for the Surgery of Obesity and Metabolic Disorders issued a joint statement that recommended lowering the thresholds for bariatric surgery to a BMI greater than or equal to 35 or greater than or equal to 30 with weight-related comorbidities.
A version of this article appeared on Medscape.com.
Although multiple international medical societies over recent years have recommended lowering the threshold for bariatric surgery to a body mass index (BMI) of 30-35 (class 1 obesity) in certain patients, very few patients in this weight category have had such surgery, according to a new study.
On the basis of data from a large U.S. national registry, during 2015 through 2021,
Most surgeries (96.5%) were in patients with a BMI greater than 35. This reflects advice from a 1991 consensus statement by the National Institutes of Health stating that bariatric surgery can be offered to patients with BMI greater than or equal to 40, or greater than or equal to 35 with comorbidities.
However, medical societies have recommended lower cutoffs in position statements in 2016, 2018, and 2022.
Paul Wisniowski, MD, a surgical resident at Keck School of Medicine of University of Southern California, Los Angeles, presented the study findings in an e-poster at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
“Professional guidelines and increasing data support bariatric surgery for patients beginning at BMI 30, which is a tipping point for disease progression. Now it needs to happen in the real world,” outgoing ASMBS president Teresa LaMasters, MD, who was not involved with this research, said in an ASMBS press release.
“We encourage greater consideration of this important treatment option earlier in the disease process,” stressed Dr. LaMasters, a bariatric surgeon and Medical Director, Unity Point Clinic Weight Loss Specialists, West Des Moines, IA.
‘Not unexpected,’ ‘need to expand eligibility’
“We expected that there had been little widespread adoption of the new BMI criteria/cutoffs,” senior study author Matthew J. Martin, MD, said in an interview.
“We know that bariatric surgery is already underutilized, as only about 1%-2% of eligible patients who would benefit end up getting surgery,” added Dr. Martin, Chief, Emergency General Surgery, and Director, Acute Care Surgery Research, USC Medical Center and Keck School of Medicine.
He suggests that the main reason that more patients with lower BMIs are not being offered surgery is related to insurance coverage and reimbursement.
“Even though the professional society guidelines have changed, based on the scientific evidence, most insurers are still using the very outdated (1990s) NIH consensus criteria of BMI greater than 35 with comorbidities, or BMI greater than 40.”
Another potential reason is “the lack of awareness of the changing guidelines and recommendation among primary care physicians who refer patients for a bariatric surgery evaluation.”
“I think it is too early in the experience with the new, more effective antiobesity medications to say which group will benefit the most or will prefer them over surgery,” he said.
“There is still only a small minority of patients who end up getting the [newer antiobesity] medications or surgery.”
“The takeaway,” Dr. Martin summarized, “is that bariatric surgery remains the only intervention with a high success rate for patients with class 1 or higher obesity in terms of weight loss, comorbidity improvement or resolution, and sustained health benefits.”
“We need to expand the availability of bariatric surgery for all eligible patients, particularly the class 1 obesity population who are currently the most underserved,” he said.
“This will take continued lobbying and working with the insurance companies to update their guidelines/criteria, education of patients, and education of primary care physicians so that patients can be appropriately referred for a surgical evaluation.”
Surgery vs. pharmacotherapy
Invited to comment on this study, Neil Skolnik, MD, who was not involved with this research, noted that data from patients with a lower BMI “has continued to accumulate, showing much greater safety than earlier studies and giving further support of efficacy.”
However, “[new] recommendations take time to take hold,” noted Dr. Skolnik, a family physician and professor in the department of family medicine, Thomas Jefferson University, Philadelphia.
“And from March of 2020 through 2021, surgery referrals were likely influenced by the COVID pandemic,” he added in an email.
Dr. Skolnik authored a commentary sharing his reservations about ASMBS recommendations issued in 2022 for lower BMI thresholds for this surgery.
“Medications are a safe, effective option for patients with a BMI from 30 to 35,” he said, “and [they] achieve approximately a 15%-20% average weight loss, which is enough to markedly improved both metabolic parameters and biomechanical issues such as knee pain, hip pain, and back pain.”
However, “bariatric surgery remains an excellent option for patients who do not respond sufficiently to pharmacotherapy,” he acknowledged.
National registry study, 2015-2021
Dr. Wisniowski and colleagues analyzed data from around 900 U.S. centers that are currently part of the Metabolic Bariatric Surgery Accreditation Quality Improvement Program.
They found that from 2015 to 2021, 38,669 patients (3.5%) with type 1 obesity and 1,1067,094 patients (96.5%) with a higher BMI had metabolic and bariatric surgery.
Compared with patients with BMI greater than 35, those with class 1 obesity had shorter operating times and hospital stays, but they lost less weight on short-term evaluation, after multivariable adjustment.
There were no significant differences between the two patient groups in rates of postoperative complications (< 5%) or mortality (< 0.1%).
Sleeve gastrectomy was the most common procedure and increased from 70% to 76% of all procedures during the study period.
Single-center study
In a second e-poster presented at the meeting, Tina T. Thomas, MD, New Jersey Bariatric Center, analyzed data from 23 patients with BMI less than 35 or less than 30 with comorbidities who had sleeve gastrectomy or Roux-en-Y gastric bypass at their center during 2017 to 2021 and who had 6 months of follow-up data.
At study entry, the patients had a mean BMI of 33.5. At 6 months after the surgery, they had a mean BMI of 25.6, and on average, they had lost 55% of their excess weight.
Nearly 60% of the patients had lost at least 50% of their excess weight, and 9 of 16 patients (56%) with comorbidities had improved or resolved comorbidities. None of the patients died or had surgery-related complications.
“Our study shows significant weight loss and health benefits, as well as the safety and efficacy of the gastric bypass and gastric sleeve procedures, for this patient population,” Ajay Goyal, MD, senior author, and bariatric surgeon at New Jersey Bariatric Center, said in an ASMBS press release.
“Often by the time a patient qualifies for bariatric surgery, their weight-related medical conditions such as [type 2] diabetes and hypertension are severe. By expanding access to bariatric surgery to patients with a lower BMI with obesity-related illnesses, patients can halt the progression, and in some cases resolve, significant and uncontrolled weight-related chronic diseases through weight loss.”
Societies call for lower BMI thresholds
Providers, hospitals, and insurers currently use BMI thresholds greater than or equal to 40, or greater than or equal to 35 with an obesity-related comorbidity, to define patients eligible for metabolic and bariatric surgery, based on criteria established in a 1991 consensus statement by NIH.
As more data accumulated, in 2016, a position statement from 45 societies recommended that bariatric surgery should be “considered for patients with [type 2 diabetes] and BMI 30.0-34.9 kg/m2 if hyperglycemia is inadequately controlled” despite optimal medical treatment.
Similarly, in 2018, the ASMBS issued a position statement saying that “for patients with BMI 30-35 kg/m2 and obesity-related comorbidities who do not achieve substantial, durable weight loss and comorbidity improvement with reasonable nonsurgical methods, bariatric surgery should be offered” to suitable individuals.
Then in October 2022, the ASMBS and International Federation for the Surgery of Obesity and Metabolic Disorders issued a joint statement that recommended lowering the thresholds for bariatric surgery to a BMI greater than or equal to 35 or greater than or equal to 30 with weight-related comorbidities.
A version of this article appeared on Medscape.com.
Although multiple international medical societies over recent years have recommended lowering the threshold for bariatric surgery to a body mass index (BMI) of 30-35 (class 1 obesity) in certain patients, very few patients in this weight category have had such surgery, according to a new study.
On the basis of data from a large U.S. national registry, during 2015 through 2021,
Most surgeries (96.5%) were in patients with a BMI greater than 35. This reflects advice from a 1991 consensus statement by the National Institutes of Health stating that bariatric surgery can be offered to patients with BMI greater than or equal to 40, or greater than or equal to 35 with comorbidities.
However, medical societies have recommended lower cutoffs in position statements in 2016, 2018, and 2022.
Paul Wisniowski, MD, a surgical resident at Keck School of Medicine of University of Southern California, Los Angeles, presented the study findings in an e-poster at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
“Professional guidelines and increasing data support bariatric surgery for patients beginning at BMI 30, which is a tipping point for disease progression. Now it needs to happen in the real world,” outgoing ASMBS president Teresa LaMasters, MD, who was not involved with this research, said in an ASMBS press release.
“We encourage greater consideration of this important treatment option earlier in the disease process,” stressed Dr. LaMasters, a bariatric surgeon and Medical Director, Unity Point Clinic Weight Loss Specialists, West Des Moines, IA.
‘Not unexpected,’ ‘need to expand eligibility’
“We expected that there had been little widespread adoption of the new BMI criteria/cutoffs,” senior study author Matthew J. Martin, MD, said in an interview.
“We know that bariatric surgery is already underutilized, as only about 1%-2% of eligible patients who would benefit end up getting surgery,” added Dr. Martin, Chief, Emergency General Surgery, and Director, Acute Care Surgery Research, USC Medical Center and Keck School of Medicine.
He suggests that the main reason that more patients with lower BMIs are not being offered surgery is related to insurance coverage and reimbursement.
“Even though the professional society guidelines have changed, based on the scientific evidence, most insurers are still using the very outdated (1990s) NIH consensus criteria of BMI greater than 35 with comorbidities, or BMI greater than 40.”
Another potential reason is “the lack of awareness of the changing guidelines and recommendation among primary care physicians who refer patients for a bariatric surgery evaluation.”
“I think it is too early in the experience with the new, more effective antiobesity medications to say which group will benefit the most or will prefer them over surgery,” he said.
“There is still only a small minority of patients who end up getting the [newer antiobesity] medications or surgery.”
“The takeaway,” Dr. Martin summarized, “is that bariatric surgery remains the only intervention with a high success rate for patients with class 1 or higher obesity in terms of weight loss, comorbidity improvement or resolution, and sustained health benefits.”
“We need to expand the availability of bariatric surgery for all eligible patients, particularly the class 1 obesity population who are currently the most underserved,” he said.
“This will take continued lobbying and working with the insurance companies to update their guidelines/criteria, education of patients, and education of primary care physicians so that patients can be appropriately referred for a surgical evaluation.”
Surgery vs. pharmacotherapy
Invited to comment on this study, Neil Skolnik, MD, who was not involved with this research, noted that data from patients with a lower BMI “has continued to accumulate, showing much greater safety than earlier studies and giving further support of efficacy.”
However, “[new] recommendations take time to take hold,” noted Dr. Skolnik, a family physician and professor in the department of family medicine, Thomas Jefferson University, Philadelphia.
“And from March of 2020 through 2021, surgery referrals were likely influenced by the COVID pandemic,” he added in an email.
Dr. Skolnik authored a commentary sharing his reservations about ASMBS recommendations issued in 2022 for lower BMI thresholds for this surgery.
“Medications are a safe, effective option for patients with a BMI from 30 to 35,” he said, “and [they] achieve approximately a 15%-20% average weight loss, which is enough to markedly improved both metabolic parameters and biomechanical issues such as knee pain, hip pain, and back pain.”
However, “bariatric surgery remains an excellent option for patients who do not respond sufficiently to pharmacotherapy,” he acknowledged.
National registry study, 2015-2021
Dr. Wisniowski and colleagues analyzed data from around 900 U.S. centers that are currently part of the Metabolic Bariatric Surgery Accreditation Quality Improvement Program.
They found that from 2015 to 2021, 38,669 patients (3.5%) with type 1 obesity and 1,1067,094 patients (96.5%) with a higher BMI had metabolic and bariatric surgery.
Compared with patients with BMI greater than 35, those with class 1 obesity had shorter operating times and hospital stays, but they lost less weight on short-term evaluation, after multivariable adjustment.
There were no significant differences between the two patient groups in rates of postoperative complications (< 5%) or mortality (< 0.1%).
Sleeve gastrectomy was the most common procedure and increased from 70% to 76% of all procedures during the study period.
Single-center study
In a second e-poster presented at the meeting, Tina T. Thomas, MD, New Jersey Bariatric Center, analyzed data from 23 patients with BMI less than 35 or less than 30 with comorbidities who had sleeve gastrectomy or Roux-en-Y gastric bypass at their center during 2017 to 2021 and who had 6 months of follow-up data.
At study entry, the patients had a mean BMI of 33.5. At 6 months after the surgery, they had a mean BMI of 25.6, and on average, they had lost 55% of their excess weight.
Nearly 60% of the patients had lost at least 50% of their excess weight, and 9 of 16 patients (56%) with comorbidities had improved or resolved comorbidities. None of the patients died or had surgery-related complications.
“Our study shows significant weight loss and health benefits, as well as the safety and efficacy of the gastric bypass and gastric sleeve procedures, for this patient population,” Ajay Goyal, MD, senior author, and bariatric surgeon at New Jersey Bariatric Center, said in an ASMBS press release.
“Often by the time a patient qualifies for bariatric surgery, their weight-related medical conditions such as [type 2] diabetes and hypertension are severe. By expanding access to bariatric surgery to patients with a lower BMI with obesity-related illnesses, patients can halt the progression, and in some cases resolve, significant and uncontrolled weight-related chronic diseases through weight loss.”
Societies call for lower BMI thresholds
Providers, hospitals, and insurers currently use BMI thresholds greater than or equal to 40, or greater than or equal to 35 with an obesity-related comorbidity, to define patients eligible for metabolic and bariatric surgery, based on criteria established in a 1991 consensus statement by NIH.
As more data accumulated, in 2016, a position statement from 45 societies recommended that bariatric surgery should be “considered for patients with [type 2 diabetes] and BMI 30.0-34.9 kg/m2 if hyperglycemia is inadequately controlled” despite optimal medical treatment.
Similarly, in 2018, the ASMBS issued a position statement saying that “for patients with BMI 30-35 kg/m2 and obesity-related comorbidities who do not achieve substantial, durable weight loss and comorbidity improvement with reasonable nonsurgical methods, bariatric surgery should be offered” to suitable individuals.
Then in October 2022, the ASMBS and International Federation for the Surgery of Obesity and Metabolic Disorders issued a joint statement that recommended lowering the thresholds for bariatric surgery to a BMI greater than or equal to 35 or greater than or equal to 30 with weight-related comorbidities.
A version of this article appeared on Medscape.com.
Diabetes, cholesterol meds use drops after bariatric surgery
compared with patients with obesity who did not have such an operation. However, these declines didn’t extend to cardiovascular medication use.
“In this study, undergoing bariatric surgery was associated with a substantial and long-lasting reduction in the use of lipid-lowering and antidiabetic medications, compared with no surgery for obesity, while for cardiovascular medications this reduction was only transient,” the authors report in research published in JAMA Surgery.
“The results can aid in informed decision-making when considering bariatric surgery for patients with morbid obesity and inform patients and professionals about the expected long-term effects of medication use for obesity-related comorbidities,” they write.
The study “highlights the benefits of mandated databases that report metabolic bariatric surgery, obesity-related comorbidities, and medications,” writes Paulina Salminen, MD, in an accompanying editorial.
However, key limitations include a lack of weight data, which is important in light of previous studies showing that suboptimal weight loss after bariatric surgery is linked to a higher incidence of type 2 diabetes, dyslipidemia, and hypertension, note Dr. Salminen, of the department of digestive surgery, University Hospital, Turku, Finland, and colleagues.
Swedish, Finnish obesity data probed
When significant weight loss is achieved, bariatric surgery has been well documented to be associated with improvements in a variety of comorbidities, quality of life, and even life expectancy.
Key comorbidities shown to improve with the surgery include hyperlipidemia, cardiovascular disease, and type 2 diabetes.
However, data are lacking on the association between bariatric surgery and the use of medications for those conditions, particularly compared with people with obesity who don’t have bariatric surgery.
To investigate, first author Joonas H. Kauppila, MD, PhD, of Upper Gastrointestinal Surgery, Karolinska University Hospital, Stockholm, and colleagues conducted a population-based cohort study, evaluating data on 26,396 patients who underwent bariatric surgery with gastric bypass or sleeve gastrectomy in Sweden between 2005 and 2020 or Finland between 1995 and 2018.
Overall, 66.4% of patients were women and their median age was 50.
They were compared with five times as many matched controls with obesity who had not had bariatric surgery from the same population databases, representing a total of 131,980 patients who were matched based on age, country, sex, calendar year, and medication use.
In terms of lipid-lowering medication, rates of use after bariatric surgery decreased from 20.3% at baseline to 12.9% after 2 years and bounced back somewhat to 17.6% after 15 years. Comparatively, in the no surgery group, baseline lipid-lowering medication use of 21.0% increased to 44.6% after 15 years, more than twice the rate of usage in the bariatric surgery group in the same period.
Antidiabetic medications were used by 27.7% of patients in the bariatric surgery group at baseline, with a drop to 10.0% after 2 years, followed by an increase to 23.5% after 15 years. In the no surgery group, the rate of antidiabetic medication use steadily increased from 27.7% at baseline to 54.2% after 15 years, which again was nearly double the rate of antidiabetic medication use in the bariatric surgery group at 15 years.
Meanwhile, cardiovascular medications were used by 60.2% of patients receiving bariatric surgery at baseline, with the rate decreasing to 43.2% after 2 years but increasing to 74.6% after 15 years. Among the nonbariatric surgery patients, use of cardiovascular medications increased from 54.4% at baseline to 83.3% after 15 years.
Causes?
As for the cause of the lack of any decline in use of cardiovascular medications versus other medications in the surgery patients, the authors speculate that the effect “may be related to aging and regain of weight over time after bariatric surgery, a phenomenon caused by hormonal, dietary, physical, and behavioral factors.”
“In contrast, as expected, a gradual increase in the use of all three medication groups was observed over time among the patients treated with no surgery for obesity,” they note.
The lower medication use with bariatric surgery can also translate to economic benefits, the authors add.
“Economically, the long-lasting reductions in medication use for hyperlipidemia, cardiovascular morbidity, and diabetes suggest that surgical treatment of morbid obesity may infer savings in medication expenses for patients, health care, and society,” they report.
“Future research may focus on subgroups that are most likely to benefit from bariatric surgery, including resolution and severity of comorbidities,” they continue.
In their editorial, Dr. Salminen and colleagues note that previous research has shown remission of dyslipidemia in up to 70% of patients after bariatric surgery that was independent of weight loss, which appears to support the sustained reduction in lipid-lowering medications following surgery observed in the current study, suggesting some benefits on lipids beyond weight loss.
Other limitations, however, include that the bariatric surgery group in the study was older and had more comorbidities than those in previous bariatric surgery studies.
“Future studies should assess this in a younger cohort with less disease at baseline and differentiation within cardiovascular disease regarding at least hypertension, ischemic heart disease, and heart failure,” the authors conclude.
The authors have reported no relevant financial relationships. Dr. Salminen has reported receiving grants from the Sigrid Jusélius Foundation, Academy of Finland, Government Research Grant Foundation, and the University of Turku (Finland).
A version of this article first appeared on Medscape.com.
compared with patients with obesity who did not have such an operation. However, these declines didn’t extend to cardiovascular medication use.
“In this study, undergoing bariatric surgery was associated with a substantial and long-lasting reduction in the use of lipid-lowering and antidiabetic medications, compared with no surgery for obesity, while for cardiovascular medications this reduction was only transient,” the authors report in research published in JAMA Surgery.
“The results can aid in informed decision-making when considering bariatric surgery for patients with morbid obesity and inform patients and professionals about the expected long-term effects of medication use for obesity-related comorbidities,” they write.
The study “highlights the benefits of mandated databases that report metabolic bariatric surgery, obesity-related comorbidities, and medications,” writes Paulina Salminen, MD, in an accompanying editorial.
However, key limitations include a lack of weight data, which is important in light of previous studies showing that suboptimal weight loss after bariatric surgery is linked to a higher incidence of type 2 diabetes, dyslipidemia, and hypertension, note Dr. Salminen, of the department of digestive surgery, University Hospital, Turku, Finland, and colleagues.
Swedish, Finnish obesity data probed
When significant weight loss is achieved, bariatric surgery has been well documented to be associated with improvements in a variety of comorbidities, quality of life, and even life expectancy.
Key comorbidities shown to improve with the surgery include hyperlipidemia, cardiovascular disease, and type 2 diabetes.
However, data are lacking on the association between bariatric surgery and the use of medications for those conditions, particularly compared with people with obesity who don’t have bariatric surgery.
To investigate, first author Joonas H. Kauppila, MD, PhD, of Upper Gastrointestinal Surgery, Karolinska University Hospital, Stockholm, and colleagues conducted a population-based cohort study, evaluating data on 26,396 patients who underwent bariatric surgery with gastric bypass or sleeve gastrectomy in Sweden between 2005 and 2020 or Finland between 1995 and 2018.
Overall, 66.4% of patients were women and their median age was 50.
They were compared with five times as many matched controls with obesity who had not had bariatric surgery from the same population databases, representing a total of 131,980 patients who were matched based on age, country, sex, calendar year, and medication use.
In terms of lipid-lowering medication, rates of use after bariatric surgery decreased from 20.3% at baseline to 12.9% after 2 years and bounced back somewhat to 17.6% after 15 years. Comparatively, in the no surgery group, baseline lipid-lowering medication use of 21.0% increased to 44.6% after 15 years, more than twice the rate of usage in the bariatric surgery group in the same period.
Antidiabetic medications were used by 27.7% of patients in the bariatric surgery group at baseline, with a drop to 10.0% after 2 years, followed by an increase to 23.5% after 15 years. In the no surgery group, the rate of antidiabetic medication use steadily increased from 27.7% at baseline to 54.2% after 15 years, which again was nearly double the rate of antidiabetic medication use in the bariatric surgery group at 15 years.
Meanwhile, cardiovascular medications were used by 60.2% of patients receiving bariatric surgery at baseline, with the rate decreasing to 43.2% after 2 years but increasing to 74.6% after 15 years. Among the nonbariatric surgery patients, use of cardiovascular medications increased from 54.4% at baseline to 83.3% after 15 years.
Causes?
As for the cause of the lack of any decline in use of cardiovascular medications versus other medications in the surgery patients, the authors speculate that the effect “may be related to aging and regain of weight over time after bariatric surgery, a phenomenon caused by hormonal, dietary, physical, and behavioral factors.”
“In contrast, as expected, a gradual increase in the use of all three medication groups was observed over time among the patients treated with no surgery for obesity,” they note.
The lower medication use with bariatric surgery can also translate to economic benefits, the authors add.
“Economically, the long-lasting reductions in medication use for hyperlipidemia, cardiovascular morbidity, and diabetes suggest that surgical treatment of morbid obesity may infer savings in medication expenses for patients, health care, and society,” they report.
“Future research may focus on subgroups that are most likely to benefit from bariatric surgery, including resolution and severity of comorbidities,” they continue.
In their editorial, Dr. Salminen and colleagues note that previous research has shown remission of dyslipidemia in up to 70% of patients after bariatric surgery that was independent of weight loss, which appears to support the sustained reduction in lipid-lowering medications following surgery observed in the current study, suggesting some benefits on lipids beyond weight loss.
Other limitations, however, include that the bariatric surgery group in the study was older and had more comorbidities than those in previous bariatric surgery studies.
“Future studies should assess this in a younger cohort with less disease at baseline and differentiation within cardiovascular disease regarding at least hypertension, ischemic heart disease, and heart failure,” the authors conclude.
The authors have reported no relevant financial relationships. Dr. Salminen has reported receiving grants from the Sigrid Jusélius Foundation, Academy of Finland, Government Research Grant Foundation, and the University of Turku (Finland).
A version of this article first appeared on Medscape.com.
compared with patients with obesity who did not have such an operation. However, these declines didn’t extend to cardiovascular medication use.
“In this study, undergoing bariatric surgery was associated with a substantial and long-lasting reduction in the use of lipid-lowering and antidiabetic medications, compared with no surgery for obesity, while for cardiovascular medications this reduction was only transient,” the authors report in research published in JAMA Surgery.
“The results can aid in informed decision-making when considering bariatric surgery for patients with morbid obesity and inform patients and professionals about the expected long-term effects of medication use for obesity-related comorbidities,” they write.
The study “highlights the benefits of mandated databases that report metabolic bariatric surgery, obesity-related comorbidities, and medications,” writes Paulina Salminen, MD, in an accompanying editorial.
However, key limitations include a lack of weight data, which is important in light of previous studies showing that suboptimal weight loss after bariatric surgery is linked to a higher incidence of type 2 diabetes, dyslipidemia, and hypertension, note Dr. Salminen, of the department of digestive surgery, University Hospital, Turku, Finland, and colleagues.
Swedish, Finnish obesity data probed
When significant weight loss is achieved, bariatric surgery has been well documented to be associated with improvements in a variety of comorbidities, quality of life, and even life expectancy.
Key comorbidities shown to improve with the surgery include hyperlipidemia, cardiovascular disease, and type 2 diabetes.
However, data are lacking on the association between bariatric surgery and the use of medications for those conditions, particularly compared with people with obesity who don’t have bariatric surgery.
To investigate, first author Joonas H. Kauppila, MD, PhD, of Upper Gastrointestinal Surgery, Karolinska University Hospital, Stockholm, and colleagues conducted a population-based cohort study, evaluating data on 26,396 patients who underwent bariatric surgery with gastric bypass or sleeve gastrectomy in Sweden between 2005 and 2020 or Finland between 1995 and 2018.
Overall, 66.4% of patients were women and their median age was 50.
They were compared with five times as many matched controls with obesity who had not had bariatric surgery from the same population databases, representing a total of 131,980 patients who were matched based on age, country, sex, calendar year, and medication use.
In terms of lipid-lowering medication, rates of use after bariatric surgery decreased from 20.3% at baseline to 12.9% after 2 years and bounced back somewhat to 17.6% after 15 years. Comparatively, in the no surgery group, baseline lipid-lowering medication use of 21.0% increased to 44.6% after 15 years, more than twice the rate of usage in the bariatric surgery group in the same period.
Antidiabetic medications were used by 27.7% of patients in the bariatric surgery group at baseline, with a drop to 10.0% after 2 years, followed by an increase to 23.5% after 15 years. In the no surgery group, the rate of antidiabetic medication use steadily increased from 27.7% at baseline to 54.2% after 15 years, which again was nearly double the rate of antidiabetic medication use in the bariatric surgery group at 15 years.
Meanwhile, cardiovascular medications were used by 60.2% of patients receiving bariatric surgery at baseline, with the rate decreasing to 43.2% after 2 years but increasing to 74.6% after 15 years. Among the nonbariatric surgery patients, use of cardiovascular medications increased from 54.4% at baseline to 83.3% after 15 years.
Causes?
As for the cause of the lack of any decline in use of cardiovascular medications versus other medications in the surgery patients, the authors speculate that the effect “may be related to aging and regain of weight over time after bariatric surgery, a phenomenon caused by hormonal, dietary, physical, and behavioral factors.”
“In contrast, as expected, a gradual increase in the use of all three medication groups was observed over time among the patients treated with no surgery for obesity,” they note.
The lower medication use with bariatric surgery can also translate to economic benefits, the authors add.
“Economically, the long-lasting reductions in medication use for hyperlipidemia, cardiovascular morbidity, and diabetes suggest that surgical treatment of morbid obesity may infer savings in medication expenses for patients, health care, and society,” they report.
“Future research may focus on subgroups that are most likely to benefit from bariatric surgery, including resolution and severity of comorbidities,” they continue.
In their editorial, Dr. Salminen and colleagues note that previous research has shown remission of dyslipidemia in up to 70% of patients after bariatric surgery that was independent of weight loss, which appears to support the sustained reduction in lipid-lowering medications following surgery observed in the current study, suggesting some benefits on lipids beyond weight loss.
Other limitations, however, include that the bariatric surgery group in the study was older and had more comorbidities than those in previous bariatric surgery studies.
“Future studies should assess this in a younger cohort with less disease at baseline and differentiation within cardiovascular disease regarding at least hypertension, ischemic heart disease, and heart failure,” the authors conclude.
The authors have reported no relevant financial relationships. Dr. Salminen has reported receiving grants from the Sigrid Jusélius Foundation, Academy of Finland, Government Research Grant Foundation, and the University of Turku (Finland).
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Endoscopic sleeve gastroplasty plus obesity drugs add up to more weight loss
CHICAGO – Antiobesity medications and endoscopic sleeve gastroplasty (ESG) are popular strategies for weight loss on their own. Now researchers are looking at what happens when you combine them.
In a study presented at the annual Digestive Disease Week® (DDW), they found
Starting medication within 6 months of ESG was more ideal than other timing intervals. Initiating medical therapy more than 6 months before ESG was associated with less weight loss.
In the single-center, retrospective study, 224 patients were enrolled, of whom 34% were on monotherapy (ESG alone), 31% had combination therapy (medication prescribed within 6 months prior to or after ESG), and 35% had sequential therapy (medication more than 6 months prior to or after ESG).
Most patients were female, ranging from 74% to 95% of each group, and baseline BMI ranged from a mean 37.5 kg/m2 to 40.1 kg/m2.
The medications involved in the study were phentermine, phentermine/topiramate extended release (Qsymia), orlistat (Xenical, Alli), bupropion/naltrexone ER (Contrave), or the glucagonlike peptide–1 receptor agonist (GLP-1RA) liraglutide (Saxenda, Victoza) or semaglutide (Ozempic, Wegovy, Rybelsus). Of the patients who underwent combination therapy, 30% were prescribed a regimen that included a GLP-1RA. Of the patients who underwent sequential therapy, 81% were prescribed a medication first and 19% underwent ESG first.
At 1 year, the greatest total weight loss was a mean 23.7% with the combination of ESG and a GLP-1RA. Total weight loss was 18% with ESG plus a non–GLP-1RA medication. ESG alone led to 17.3%. Sequential therapy that began with ESG yielded 14.7% total weight loss, whereas sequential therapy that began with medication first resulted in 12% weight loss.
It’s possible that gastroplasty performed second was less impressive because the medications were very effective, and there was not as much weight to lose, said Pichamol Jirapinyo, MD, MPH, a bariatric endoscopist at Brigham and Women’s Hospital, Boston, and lead author of the study.
Researchers stopped medication therapy if people did not experience at least 5% total weight loss after 3 months on a maintenance dose.
Waiting for weight loss to start to plateau after gastroplasty might be an ideal time to add weight loss medication, said Dr. Jirapinyo. “Usually when I see them at 3 months, I plot how fast their weight loss has been. If it’s been going down [steadily], we do not offer an antiobesity medication until I see them again at 6 months.”
The serious adverse event (SAE) rate associated with ESG was similar among the three cohorts: 2.6% with monotherapy group, 1.4% with combination therapy, and 1.3% with sequential therapy. SAEs associated with antiobesity medication occurred in 1.3% of the sequential therapy group and was not reported in either of the other two groups.
“I certainly think combination therapy should be more effective than just gastroplasty alone and is probably better,” said Gregory L. Austin, MD, session comoderator and a gastroenterologist at the UCHealth Digestive Health Center, Denver.
“Whether you start immediately or wait 3 months afterwards is a question that still needs to be answered,” he added.
Dr. Austin agreed that taking an antiobesity medicine more than 6 months before gastroplasty might be associated with enough weight loss to make the gastroplasty look less effective.
He also noted that the study “doesn’t really address the question of whether you should offer gastroplasty to somebody who’s been on [medication] for more than 6 months because you probably still should if they haven’t achieved an appropriate weight loss that’s associated with reduced comorbidity risk going forward.”
Different study, similar result
In a second study, also presented at DDW 2023, investigators looked at timing of liraglutide for weight loss in a randomized controlled trial. They found that administration of GLP-1RA right after transoral outlet reduction endoscopy (TORe) in people with a history of Roux-en-Y gastric bypass extended weight loss longer than a placebo injection. This strategy was also favorable versus waiting to give liraglutide 1 year later.
The researchers randomly assigned 51 people to get weekly subcutaneous liraglutide injections following TORe for 12 months, then placebo injections for 12 months. They assigned 58 patients to receive weekly placebo injections following TORe for 12 months, then liraglutide injections for 12 months.
At 12 months following the procedure, total body weight loss (TBWL) among participants receiving liraglutide was about 22%, compared with about 14% among patients receiving placebo. At 24 months following the procedure (12 months after crossover), TBWL among patients in the liraglutide-first group was almost 35%, compared with about 24% in the placebo-first/liraglutide-second group.
There was a durable effect associated with liraglutide even after switching to placebo, said Ali Lahooti, lead study author and second-year medical student at Weill Cornell Medicine, New York.
“There did seem to be a better benefit of starting on it for the first year and then stopping it,” Dr. Austin noted.
These two studies come at a time when the debate over the timing of different obesity interventions continues. Some experts believe weight loss medications can help with the rebound in weight that some people experience months after bariatric surgery, for example.
‘Wave of the future’
The study by Dr. Jirapinyo and colleagues is “really exciting and interesting,” said Linda S. Lee, MD, medical director of endoscopy, Brigham and Women’s Hospital, Boston, when asked to comment.
Medication begun within 6 months of the endoscopic procedure “led to superior outcomes, compared to just endoscopy alone,” Dr. Lee said. “I think that’s really the wave of the future as far as treating patients with obesity issues. We clearly know that diet and exercise alone for most people is not good enough. Of course, we have surgery, but we also realize that with surgery sometimes the weight starts to creep back up over time.”
Dr. Lee noted that the study was limited because it was retrospective. Ideally, it would be good if future, prospective research randomly assigns people to endoscopy alone or endoscopy plus medication.
Dr. Lee also noted there is a limited number of bariatric endoscopists. By the time people with obesity get to a specialist, they’ve likely tried diet and exercise and “probably have seen all the commercials for these different medications. I think the reality is that most people will ask their primary care physicians about antiobesity medication.
“From my point of view, as long as the medicine is safe and not harming them, then let’s do both of them together,” Dr. Lee added.
Dr. Lee also mentioned another study (Abstract Mo1898) presented at DDW 2023 that showed total weight loss with endoscopic sleeve gastroplasty was durable over 10 years. Follow-up was with only seven patients, however.
Larger numbers are needed to confirm the finding, but it’s “exciting,” she said.
Dr. Jirapinyo receives grant/research support from Apollo Endosurgery, Fractyl, and USGI Medical, and is a consultant for ERBE, GI Dynamics, and Spatz Medical. Dr. Lahooti, Dr. Austin, and Dr. Lee reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – Antiobesity medications and endoscopic sleeve gastroplasty (ESG) are popular strategies for weight loss on their own. Now researchers are looking at what happens when you combine them.
In a study presented at the annual Digestive Disease Week® (DDW), they found
Starting medication within 6 months of ESG was more ideal than other timing intervals. Initiating medical therapy more than 6 months before ESG was associated with less weight loss.
In the single-center, retrospective study, 224 patients were enrolled, of whom 34% were on monotherapy (ESG alone), 31% had combination therapy (medication prescribed within 6 months prior to or after ESG), and 35% had sequential therapy (medication more than 6 months prior to or after ESG).
Most patients were female, ranging from 74% to 95% of each group, and baseline BMI ranged from a mean 37.5 kg/m2 to 40.1 kg/m2.
The medications involved in the study were phentermine, phentermine/topiramate extended release (Qsymia), orlistat (Xenical, Alli), bupropion/naltrexone ER (Contrave), or the glucagonlike peptide–1 receptor agonist (GLP-1RA) liraglutide (Saxenda, Victoza) or semaglutide (Ozempic, Wegovy, Rybelsus). Of the patients who underwent combination therapy, 30% were prescribed a regimen that included a GLP-1RA. Of the patients who underwent sequential therapy, 81% were prescribed a medication first and 19% underwent ESG first.
At 1 year, the greatest total weight loss was a mean 23.7% with the combination of ESG and a GLP-1RA. Total weight loss was 18% with ESG plus a non–GLP-1RA medication. ESG alone led to 17.3%. Sequential therapy that began with ESG yielded 14.7% total weight loss, whereas sequential therapy that began with medication first resulted in 12% weight loss.
It’s possible that gastroplasty performed second was less impressive because the medications were very effective, and there was not as much weight to lose, said Pichamol Jirapinyo, MD, MPH, a bariatric endoscopist at Brigham and Women’s Hospital, Boston, and lead author of the study.
Researchers stopped medication therapy if people did not experience at least 5% total weight loss after 3 months on a maintenance dose.
Waiting for weight loss to start to plateau after gastroplasty might be an ideal time to add weight loss medication, said Dr. Jirapinyo. “Usually when I see them at 3 months, I plot how fast their weight loss has been. If it’s been going down [steadily], we do not offer an antiobesity medication until I see them again at 6 months.”
The serious adverse event (SAE) rate associated with ESG was similar among the three cohorts: 2.6% with monotherapy group, 1.4% with combination therapy, and 1.3% with sequential therapy. SAEs associated with antiobesity medication occurred in 1.3% of the sequential therapy group and was not reported in either of the other two groups.
“I certainly think combination therapy should be more effective than just gastroplasty alone and is probably better,” said Gregory L. Austin, MD, session comoderator and a gastroenterologist at the UCHealth Digestive Health Center, Denver.
“Whether you start immediately or wait 3 months afterwards is a question that still needs to be answered,” he added.
Dr. Austin agreed that taking an antiobesity medicine more than 6 months before gastroplasty might be associated with enough weight loss to make the gastroplasty look less effective.
He also noted that the study “doesn’t really address the question of whether you should offer gastroplasty to somebody who’s been on [medication] for more than 6 months because you probably still should if they haven’t achieved an appropriate weight loss that’s associated with reduced comorbidity risk going forward.”
Different study, similar result
In a second study, also presented at DDW 2023, investigators looked at timing of liraglutide for weight loss in a randomized controlled trial. They found that administration of GLP-1RA right after transoral outlet reduction endoscopy (TORe) in people with a history of Roux-en-Y gastric bypass extended weight loss longer than a placebo injection. This strategy was also favorable versus waiting to give liraglutide 1 year later.
The researchers randomly assigned 51 people to get weekly subcutaneous liraglutide injections following TORe for 12 months, then placebo injections for 12 months. They assigned 58 patients to receive weekly placebo injections following TORe for 12 months, then liraglutide injections for 12 months.
At 12 months following the procedure, total body weight loss (TBWL) among participants receiving liraglutide was about 22%, compared with about 14% among patients receiving placebo. At 24 months following the procedure (12 months after crossover), TBWL among patients in the liraglutide-first group was almost 35%, compared with about 24% in the placebo-first/liraglutide-second group.
There was a durable effect associated with liraglutide even after switching to placebo, said Ali Lahooti, lead study author and second-year medical student at Weill Cornell Medicine, New York.
“There did seem to be a better benefit of starting on it for the first year and then stopping it,” Dr. Austin noted.
These two studies come at a time when the debate over the timing of different obesity interventions continues. Some experts believe weight loss medications can help with the rebound in weight that some people experience months after bariatric surgery, for example.
‘Wave of the future’
The study by Dr. Jirapinyo and colleagues is “really exciting and interesting,” said Linda S. Lee, MD, medical director of endoscopy, Brigham and Women’s Hospital, Boston, when asked to comment.
Medication begun within 6 months of the endoscopic procedure “led to superior outcomes, compared to just endoscopy alone,” Dr. Lee said. “I think that’s really the wave of the future as far as treating patients with obesity issues. We clearly know that diet and exercise alone for most people is not good enough. Of course, we have surgery, but we also realize that with surgery sometimes the weight starts to creep back up over time.”
Dr. Lee noted that the study was limited because it was retrospective. Ideally, it would be good if future, prospective research randomly assigns people to endoscopy alone or endoscopy plus medication.
Dr. Lee also noted there is a limited number of bariatric endoscopists. By the time people with obesity get to a specialist, they’ve likely tried diet and exercise and “probably have seen all the commercials for these different medications. I think the reality is that most people will ask their primary care physicians about antiobesity medication.
“From my point of view, as long as the medicine is safe and not harming them, then let’s do both of them together,” Dr. Lee added.
Dr. Lee also mentioned another study (Abstract Mo1898) presented at DDW 2023 that showed total weight loss with endoscopic sleeve gastroplasty was durable over 10 years. Follow-up was with only seven patients, however.
Larger numbers are needed to confirm the finding, but it’s “exciting,” she said.
Dr. Jirapinyo receives grant/research support from Apollo Endosurgery, Fractyl, and USGI Medical, and is a consultant for ERBE, GI Dynamics, and Spatz Medical. Dr. Lahooti, Dr. Austin, and Dr. Lee reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – Antiobesity medications and endoscopic sleeve gastroplasty (ESG) are popular strategies for weight loss on their own. Now researchers are looking at what happens when you combine them.
In a study presented at the annual Digestive Disease Week® (DDW), they found
Starting medication within 6 months of ESG was more ideal than other timing intervals. Initiating medical therapy more than 6 months before ESG was associated with less weight loss.
In the single-center, retrospective study, 224 patients were enrolled, of whom 34% were on monotherapy (ESG alone), 31% had combination therapy (medication prescribed within 6 months prior to or after ESG), and 35% had sequential therapy (medication more than 6 months prior to or after ESG).
Most patients were female, ranging from 74% to 95% of each group, and baseline BMI ranged from a mean 37.5 kg/m2 to 40.1 kg/m2.
The medications involved in the study were phentermine, phentermine/topiramate extended release (Qsymia), orlistat (Xenical, Alli), bupropion/naltrexone ER (Contrave), or the glucagonlike peptide–1 receptor agonist (GLP-1RA) liraglutide (Saxenda, Victoza) or semaglutide (Ozempic, Wegovy, Rybelsus). Of the patients who underwent combination therapy, 30% were prescribed a regimen that included a GLP-1RA. Of the patients who underwent sequential therapy, 81% were prescribed a medication first and 19% underwent ESG first.
At 1 year, the greatest total weight loss was a mean 23.7% with the combination of ESG and a GLP-1RA. Total weight loss was 18% with ESG plus a non–GLP-1RA medication. ESG alone led to 17.3%. Sequential therapy that began with ESG yielded 14.7% total weight loss, whereas sequential therapy that began with medication first resulted in 12% weight loss.
It’s possible that gastroplasty performed second was less impressive because the medications were very effective, and there was not as much weight to lose, said Pichamol Jirapinyo, MD, MPH, a bariatric endoscopist at Brigham and Women’s Hospital, Boston, and lead author of the study.
Researchers stopped medication therapy if people did not experience at least 5% total weight loss after 3 months on a maintenance dose.
Waiting for weight loss to start to plateau after gastroplasty might be an ideal time to add weight loss medication, said Dr. Jirapinyo. “Usually when I see them at 3 months, I plot how fast their weight loss has been. If it’s been going down [steadily], we do not offer an antiobesity medication until I see them again at 6 months.”
The serious adverse event (SAE) rate associated with ESG was similar among the three cohorts: 2.6% with monotherapy group, 1.4% with combination therapy, and 1.3% with sequential therapy. SAEs associated with antiobesity medication occurred in 1.3% of the sequential therapy group and was not reported in either of the other two groups.
“I certainly think combination therapy should be more effective than just gastroplasty alone and is probably better,” said Gregory L. Austin, MD, session comoderator and a gastroenterologist at the UCHealth Digestive Health Center, Denver.
“Whether you start immediately or wait 3 months afterwards is a question that still needs to be answered,” he added.
Dr. Austin agreed that taking an antiobesity medicine more than 6 months before gastroplasty might be associated with enough weight loss to make the gastroplasty look less effective.
He also noted that the study “doesn’t really address the question of whether you should offer gastroplasty to somebody who’s been on [medication] for more than 6 months because you probably still should if they haven’t achieved an appropriate weight loss that’s associated with reduced comorbidity risk going forward.”
Different study, similar result
In a second study, also presented at DDW 2023, investigators looked at timing of liraglutide for weight loss in a randomized controlled trial. They found that administration of GLP-1RA right after transoral outlet reduction endoscopy (TORe) in people with a history of Roux-en-Y gastric bypass extended weight loss longer than a placebo injection. This strategy was also favorable versus waiting to give liraglutide 1 year later.
The researchers randomly assigned 51 people to get weekly subcutaneous liraglutide injections following TORe for 12 months, then placebo injections for 12 months. They assigned 58 patients to receive weekly placebo injections following TORe for 12 months, then liraglutide injections for 12 months.
At 12 months following the procedure, total body weight loss (TBWL) among participants receiving liraglutide was about 22%, compared with about 14% among patients receiving placebo. At 24 months following the procedure (12 months after crossover), TBWL among patients in the liraglutide-first group was almost 35%, compared with about 24% in the placebo-first/liraglutide-second group.
There was a durable effect associated with liraglutide even after switching to placebo, said Ali Lahooti, lead study author and second-year medical student at Weill Cornell Medicine, New York.
“There did seem to be a better benefit of starting on it for the first year and then stopping it,” Dr. Austin noted.
These two studies come at a time when the debate over the timing of different obesity interventions continues. Some experts believe weight loss medications can help with the rebound in weight that some people experience months after bariatric surgery, for example.
‘Wave of the future’
The study by Dr. Jirapinyo and colleagues is “really exciting and interesting,” said Linda S. Lee, MD, medical director of endoscopy, Brigham and Women’s Hospital, Boston, when asked to comment.
Medication begun within 6 months of the endoscopic procedure “led to superior outcomes, compared to just endoscopy alone,” Dr. Lee said. “I think that’s really the wave of the future as far as treating patients with obesity issues. We clearly know that diet and exercise alone for most people is not good enough. Of course, we have surgery, but we also realize that with surgery sometimes the weight starts to creep back up over time.”
Dr. Lee noted that the study was limited because it was retrospective. Ideally, it would be good if future, prospective research randomly assigns people to endoscopy alone or endoscopy plus medication.
Dr. Lee also noted there is a limited number of bariatric endoscopists. By the time people with obesity get to a specialist, they’ve likely tried diet and exercise and “probably have seen all the commercials for these different medications. I think the reality is that most people will ask their primary care physicians about antiobesity medication.
“From my point of view, as long as the medicine is safe and not harming them, then let’s do both of them together,” Dr. Lee added.
Dr. Lee also mentioned another study (Abstract Mo1898) presented at DDW 2023 that showed total weight loss with endoscopic sleeve gastroplasty was durable over 10 years. Follow-up was with only seven patients, however.
Larger numbers are needed to confirm the finding, but it’s “exciting,” she said.
Dr. Jirapinyo receives grant/research support from Apollo Endosurgery, Fractyl, and USGI Medical, and is a consultant for ERBE, GI Dynamics, and Spatz Medical. Dr. Lahooti, Dr. Austin, and Dr. Lee reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
Medications provide best risk-to-benefit ratio for weight loss, says expert
Lifestyle changes result in the least weight loss and may be safest, while surgery provides the most weight loss and has the greatest risk. Antiobesity medications, especially the newer ones used in combination with lifestyle changes, can provide significant and sustained weight loss with manageable side effects, said Daniel Bessesen, MD, a professor in the endocrinology, diabetes, and metabolism at University of Colorado at Denver, Aurora.
New and more effective antiobesity medications have given internists more potential options to discuss with their patients, Dr. Bessesen said. He reviewed the pros and cons of the different options.
Medications are indicated for patients with a body mass index greater than 30, including those with a weight-related comorbidity, Dr. Bessesen said. The average weight loss is 5%-15% over 3-6 months but may vary greatly. Insurance often does not cover the medication costs.
Older FDA-approved antiobesity medications
Phentermine is the most widely prescribed antiobesity medication, partly because it is the only option most people can afford out of pocket. Dr. Bessesen presented recent data showing that long-term use of phentermine was associated with greater weight loss and that patients continuously taking phentermine for 24 months lost 7.5% of their weight.
Phentermine suppresses appetite by increasing norepinephrine production. Dr. Bessesen warned that internists should be careful when prescribing it to patients with mental conditions, because it acts as a stimulant. Early studies raised concerns about the risk of cardiovascular disease (CVD) in patients taking phentermine. However, analysis of data from over 13,000 individuals showed no evidence of a relationship between phentermine exposure and CVD events.
“These data provide some reassurance that it could be used in patients with CVD risk,” he noted. Phentermine can also be combined with topiramate extended release, a combination that provides greater efficacy (up to 10% weight loss) with fewer side effects. However, this combination is less effective in patients with diabetes than in those without.
Additional treatment options included orlistat and naltrexone sustained release/bupropion SR. Orlistat is a good treatment alternative for patients with constipation and is the safest option among older anti-obesity medications, whereas naltrexone SR/bupropion SR may be useful in patients with food cravings. However, there is more variability in the individual-level benefit from these agents compared to phentermine and phentermine/topiramate ER, Dr. Bessesen said.
Newer anti‐obesity medications
Liraglutide, an agent used for the management of type 2 diabetes, has recently been approved for weight loss. Liraglutide causes moderate weight loss, and it may reduce the risk of CVD. However, there are tolerability issues, such as nausea and other risks, and Dr. Bessesen advises internists to “start at low doses and increase slowly.”
Semaglutide is the newest and most effective antiobesity drug approved by the Food and Drug Administration, providing sustained weight loss of 8% for up to 48 weeks after starting treatment. Although its efficacy is lower in patients with diabetes, Dr. Bessesen noted that “this is common for antiobesity agents, and clinicians should not refrain from prescribing it in this population.”
Setmelanotide is another new medication approved for chronic weight management in patients with monogenic obesity. This medication can be considered for patients with early-onset severe obesity with abnormal feeding behavior.
Commenting on barriers to access to new antiobesity medications, Dr. Bessesen said that “the high cost of these medications is a substantial problem, but as more companies become involved and products are on the market for a longer period of time, I am hopeful that prices will come down.”
Emerging antiobesity medications
Dr. Bessesen presented recent phase 3 data showing that treatment with tirzepatide provided sustained chronic loss and improved cardiometabolic measures with no diet. Tirzepatide, which targets receptors for glucagonlike peptide–1 and glucose-dependent insulinotropic polypeptide, is used for the management of type 2 diabetes and is expected to be reviewed soon by the FDA for its use in weight management.
A semaglutide/cagrilintide combination may also provide a new treatment option for patients with obesity. In a phase 1b trial, semaglutide/cagrilintide treatment resulted in up to 17% weight loss in patients with obesity who were otherwise healthy; however, phase 2 and 3 data are needed to confirm its efficacy.
A ‘holistic approach’
When deciding whether to prescribe antiobesity medications, Dr. Bessesen noted that medications are better than exercise alone. Factors to consider when deciding whether to prescribe drugs, as well as which ones, include costs, local regulatory guidelines, requirement for long-term use, and patient comorbidities.
He also stated that lifestyle changes, such as adopting healthy nutrition and exercising regularly, are also important and can enhance weight loss when combined with medications.
Richele Corrado, DO, MPH, agreed that lifestyle management in combination with medications may provide greater weight loss than each of these interventions alone.
“If you look at the data, exercise doesn’t help you lose much weight,” said Dr. Corrado, a staff internist and obesity medicine specialist at Walter Reed National Military Medical Center in Bethesda, Md., who spoke at the same session. She added that she has many patients who struggle to lose weight despite having a healthy lifestyle. “It’s important to discuss with these patients about medications and surgery.”
Dr. Bessesen noted that management of mental health and emotional well-being should also be an integral part of obesity management. “Treatment for obesity may be more successful when underlying psychological conditions such as depression, childhood sexual trauma, or anxiety are addressed and treated,” he said.
Dr. Bessesen was involved in the study of the efficacy of semaglutide/cagrilintide. He does not have any financial conflicts with the companies that make other mentioned medications. He has received research grants or contracts from Novo Nordisk, honoraria from Novo Nordisk, and consultantship from Eli Lilly. Dr. Corrado reported no relevant financial conflicts.
Lifestyle changes result in the least weight loss and may be safest, while surgery provides the most weight loss and has the greatest risk. Antiobesity medications, especially the newer ones used in combination with lifestyle changes, can provide significant and sustained weight loss with manageable side effects, said Daniel Bessesen, MD, a professor in the endocrinology, diabetes, and metabolism at University of Colorado at Denver, Aurora.
New and more effective antiobesity medications have given internists more potential options to discuss with their patients, Dr. Bessesen said. He reviewed the pros and cons of the different options.
Medications are indicated for patients with a body mass index greater than 30, including those with a weight-related comorbidity, Dr. Bessesen said. The average weight loss is 5%-15% over 3-6 months but may vary greatly. Insurance often does not cover the medication costs.
Older FDA-approved antiobesity medications
Phentermine is the most widely prescribed antiobesity medication, partly because it is the only option most people can afford out of pocket. Dr. Bessesen presented recent data showing that long-term use of phentermine was associated with greater weight loss and that patients continuously taking phentermine for 24 months lost 7.5% of their weight.
Phentermine suppresses appetite by increasing norepinephrine production. Dr. Bessesen warned that internists should be careful when prescribing it to patients with mental conditions, because it acts as a stimulant. Early studies raised concerns about the risk of cardiovascular disease (CVD) in patients taking phentermine. However, analysis of data from over 13,000 individuals showed no evidence of a relationship between phentermine exposure and CVD events.
“These data provide some reassurance that it could be used in patients with CVD risk,” he noted. Phentermine can also be combined with topiramate extended release, a combination that provides greater efficacy (up to 10% weight loss) with fewer side effects. However, this combination is less effective in patients with diabetes than in those without.
Additional treatment options included orlistat and naltrexone sustained release/bupropion SR. Orlistat is a good treatment alternative for patients with constipation and is the safest option among older anti-obesity medications, whereas naltrexone SR/bupropion SR may be useful in patients with food cravings. However, there is more variability in the individual-level benefit from these agents compared to phentermine and phentermine/topiramate ER, Dr. Bessesen said.
Newer anti‐obesity medications
Liraglutide, an agent used for the management of type 2 diabetes, has recently been approved for weight loss. Liraglutide causes moderate weight loss, and it may reduce the risk of CVD. However, there are tolerability issues, such as nausea and other risks, and Dr. Bessesen advises internists to “start at low doses and increase slowly.”
Semaglutide is the newest and most effective antiobesity drug approved by the Food and Drug Administration, providing sustained weight loss of 8% for up to 48 weeks after starting treatment. Although its efficacy is lower in patients with diabetes, Dr. Bessesen noted that “this is common for antiobesity agents, and clinicians should not refrain from prescribing it in this population.”
Setmelanotide is another new medication approved for chronic weight management in patients with monogenic obesity. This medication can be considered for patients with early-onset severe obesity with abnormal feeding behavior.
Commenting on barriers to access to new antiobesity medications, Dr. Bessesen said that “the high cost of these medications is a substantial problem, but as more companies become involved and products are on the market for a longer period of time, I am hopeful that prices will come down.”
Emerging antiobesity medications
Dr. Bessesen presented recent phase 3 data showing that treatment with tirzepatide provided sustained chronic loss and improved cardiometabolic measures with no diet. Tirzepatide, which targets receptors for glucagonlike peptide–1 and glucose-dependent insulinotropic polypeptide, is used for the management of type 2 diabetes and is expected to be reviewed soon by the FDA for its use in weight management.
A semaglutide/cagrilintide combination may also provide a new treatment option for patients with obesity. In a phase 1b trial, semaglutide/cagrilintide treatment resulted in up to 17% weight loss in patients with obesity who were otherwise healthy; however, phase 2 and 3 data are needed to confirm its efficacy.
A ‘holistic approach’
When deciding whether to prescribe antiobesity medications, Dr. Bessesen noted that medications are better than exercise alone. Factors to consider when deciding whether to prescribe drugs, as well as which ones, include costs, local regulatory guidelines, requirement for long-term use, and patient comorbidities.
He also stated that lifestyle changes, such as adopting healthy nutrition and exercising regularly, are also important and can enhance weight loss when combined with medications.
Richele Corrado, DO, MPH, agreed that lifestyle management in combination with medications may provide greater weight loss than each of these interventions alone.
“If you look at the data, exercise doesn’t help you lose much weight,” said Dr. Corrado, a staff internist and obesity medicine specialist at Walter Reed National Military Medical Center in Bethesda, Md., who spoke at the same session. She added that she has many patients who struggle to lose weight despite having a healthy lifestyle. “It’s important to discuss with these patients about medications and surgery.”
Dr. Bessesen noted that management of mental health and emotional well-being should also be an integral part of obesity management. “Treatment for obesity may be more successful when underlying psychological conditions such as depression, childhood sexual trauma, or anxiety are addressed and treated,” he said.
Dr. Bessesen was involved in the study of the efficacy of semaglutide/cagrilintide. He does not have any financial conflicts with the companies that make other mentioned medications. He has received research grants or contracts from Novo Nordisk, honoraria from Novo Nordisk, and consultantship from Eli Lilly. Dr. Corrado reported no relevant financial conflicts.
Lifestyle changes result in the least weight loss and may be safest, while surgery provides the most weight loss and has the greatest risk. Antiobesity medications, especially the newer ones used in combination with lifestyle changes, can provide significant and sustained weight loss with manageable side effects, said Daniel Bessesen, MD, a professor in the endocrinology, diabetes, and metabolism at University of Colorado at Denver, Aurora.
New and more effective antiobesity medications have given internists more potential options to discuss with their patients, Dr. Bessesen said. He reviewed the pros and cons of the different options.
Medications are indicated for patients with a body mass index greater than 30, including those with a weight-related comorbidity, Dr. Bessesen said. The average weight loss is 5%-15% over 3-6 months but may vary greatly. Insurance often does not cover the medication costs.
Older FDA-approved antiobesity medications
Phentermine is the most widely prescribed antiobesity medication, partly because it is the only option most people can afford out of pocket. Dr. Bessesen presented recent data showing that long-term use of phentermine was associated with greater weight loss and that patients continuously taking phentermine for 24 months lost 7.5% of their weight.
Phentermine suppresses appetite by increasing norepinephrine production. Dr. Bessesen warned that internists should be careful when prescribing it to patients with mental conditions, because it acts as a stimulant. Early studies raised concerns about the risk of cardiovascular disease (CVD) in patients taking phentermine. However, analysis of data from over 13,000 individuals showed no evidence of a relationship between phentermine exposure and CVD events.
“These data provide some reassurance that it could be used in patients with CVD risk,” he noted. Phentermine can also be combined with topiramate extended release, a combination that provides greater efficacy (up to 10% weight loss) with fewer side effects. However, this combination is less effective in patients with diabetes than in those without.
Additional treatment options included orlistat and naltrexone sustained release/bupropion SR. Orlistat is a good treatment alternative for patients with constipation and is the safest option among older anti-obesity medications, whereas naltrexone SR/bupropion SR may be useful in patients with food cravings. However, there is more variability in the individual-level benefit from these agents compared to phentermine and phentermine/topiramate ER, Dr. Bessesen said.
Newer anti‐obesity medications
Liraglutide, an agent used for the management of type 2 diabetes, has recently been approved for weight loss. Liraglutide causes moderate weight loss, and it may reduce the risk of CVD. However, there are tolerability issues, such as nausea and other risks, and Dr. Bessesen advises internists to “start at low doses and increase slowly.”
Semaglutide is the newest and most effective antiobesity drug approved by the Food and Drug Administration, providing sustained weight loss of 8% for up to 48 weeks after starting treatment. Although its efficacy is lower in patients with diabetes, Dr. Bessesen noted that “this is common for antiobesity agents, and clinicians should not refrain from prescribing it in this population.”
Setmelanotide is another new medication approved for chronic weight management in patients with monogenic obesity. This medication can be considered for patients with early-onset severe obesity with abnormal feeding behavior.
Commenting on barriers to access to new antiobesity medications, Dr. Bessesen said that “the high cost of these medications is a substantial problem, but as more companies become involved and products are on the market for a longer period of time, I am hopeful that prices will come down.”
Emerging antiobesity medications
Dr. Bessesen presented recent phase 3 data showing that treatment with tirzepatide provided sustained chronic loss and improved cardiometabolic measures with no diet. Tirzepatide, which targets receptors for glucagonlike peptide–1 and glucose-dependent insulinotropic polypeptide, is used for the management of type 2 diabetes and is expected to be reviewed soon by the FDA for its use in weight management.
A semaglutide/cagrilintide combination may also provide a new treatment option for patients with obesity. In a phase 1b trial, semaglutide/cagrilintide treatment resulted in up to 17% weight loss in patients with obesity who were otherwise healthy; however, phase 2 and 3 data are needed to confirm its efficacy.
A ‘holistic approach’
When deciding whether to prescribe antiobesity medications, Dr. Bessesen noted that medications are better than exercise alone. Factors to consider when deciding whether to prescribe drugs, as well as which ones, include costs, local regulatory guidelines, requirement for long-term use, and patient comorbidities.
He also stated that lifestyle changes, such as adopting healthy nutrition and exercising regularly, are also important and can enhance weight loss when combined with medications.
Richele Corrado, DO, MPH, agreed that lifestyle management in combination with medications may provide greater weight loss than each of these interventions alone.
“If you look at the data, exercise doesn’t help you lose much weight,” said Dr. Corrado, a staff internist and obesity medicine specialist at Walter Reed National Military Medical Center in Bethesda, Md., who spoke at the same session. She added that she has many patients who struggle to lose weight despite having a healthy lifestyle. “It’s important to discuss with these patients about medications and surgery.”
Dr. Bessesen noted that management of mental health and emotional well-being should also be an integral part of obesity management. “Treatment for obesity may be more successful when underlying psychological conditions such as depression, childhood sexual trauma, or anxiety are addressed and treated,” he said.
Dr. Bessesen was involved in the study of the efficacy of semaglutide/cagrilintide. He does not have any financial conflicts with the companies that make other mentioned medications. He has received research grants or contracts from Novo Nordisk, honoraria from Novo Nordisk, and consultantship from Eli Lilly. Dr. Corrado reported no relevant financial conflicts.
AT INTERNAL MEDICINE 2023
What’s it like to take Ozempic? A doctor’s own story
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Longer life after bariatric surgery, but suicide risk in young
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM OBESITY
‘Clinical paradox’? Bariatric surgery may protect from GI cancers
In fact, an analysis of close to 1 million French adults suggests that the weight-loss surgery may offer some protection against these cancers.
The study results present a “clinical paradox,” according to authors of a commentary published this week along with the study in JAMA Surgery. A procedure known to increase the risk of gastroesophageal reflux disease (GERD), and potentially adenocarcinoma of the distal esophagus and gastroesophageal junction, may help shield patients from esophagogastric cancer.
The study marks “an important step toward improving the understanding of potential lifetime risks of bariatric surgery and overall major health benefits of surgically induced weight loss,” commentary authors Piotr Gorecki, MD, and Michael Zenilman, MD, with Weill Cornell Medicine in New York, write.
Recent data indicate that excess body weight is associated with nearly 8% of cancer cases and 6.5% of cancer deaths. Studies also show that bariatric surgery can reduce the risk of some cancers, but whether this extends to esophageal and gastric cancer remains unclear.
To investigate, the researchers used French national data to compare the incidence of esophageal and gastric cancer in 303,709 mostly female patients with obesity who underwent bariatric surgery and a matched group of 605,140 patients with obesity who did not undergo the surgery.
The mean age of the cohort was about 40 years. The mean period of follow-up was 6 years for the surgery group and 5.6 years for the control arm. A total of 337 patients underwent esophagogastric cancer – 83 in the surgical group and 254 in the control group. Gastric cancer was about two times more common than esophageal cancer (225 vs. 112 patients).
The incidence rate of esophagogastric cancer was higher in the control group than in the surgery group – 6.9 vs. 4.9 cases per 100,000 population per year, for an incidence rate ratio of 1.42 (P = .005).
Bariatric surgery was associated with a significant 24% lower risk of esophagogastric cancer (hazard ratio, 0.76; P = .03) and a 40% lower risk of overall in-hospital mortality, defined as “any death occurring during a hospital stay regardless of the cause” (HR, 0.60; P < .001).
The authors also found no significant difference in cancer outcomes and type of bariatric procedure, which included sleeve gastrectomy, gastric bypass, and adjustable gastric banding.
They note that key study limitations include the retrospective design, limited follow-up period, and lack of histologic data on the specific cancers. In addition, the study population was relatively young, whereas esophageal cancer is more common in older people.
But overall, the findings suggest that bariatric surgery can be performed to treat severe obesity without increasing the risk of esophageal and gastric cancer, the authors conclude.
“It seems that the balance between protective factors (weight loss, metabolic effects, and eradication of H. pylori infection) and risk factors (GERD and bile reflux) for cancer after bariatric surgery is in favor of protective factors,” the authors, led by Andrea Lazzati, MD, PhD, of Centre Hospitalier Intercommunal de Créteil, France, explain.
Although the potential protective mechanisms remain unclear, in their commentary, Dr. Gorecki and Dr. Zenilman suggest that a reduction in chronic inflammation and immunosuppression following bariatric surgery could help explain the results.
Although the study provides “reassurance of the protective clinical benefits of weight loss surgery,” more large-scale studies are needed to “better identify, elucidate, and address the pathophysiological processes of bariatric procedure,” Dr. Gorecki and Dr. Zenilman conclude.
No specific funding for the study was reported. Dr. Lazzati has received personal fees from Johnson & Johnson, Medtronic, and Gore. Dr. Zenilman has received personal fees from Academic Medical Professionals Insurance and Mohamed & Obaid Almulla Group.
A version of this article first appeared on Medscape.com.
In fact, an analysis of close to 1 million French adults suggests that the weight-loss surgery may offer some protection against these cancers.
The study results present a “clinical paradox,” according to authors of a commentary published this week along with the study in JAMA Surgery. A procedure known to increase the risk of gastroesophageal reflux disease (GERD), and potentially adenocarcinoma of the distal esophagus and gastroesophageal junction, may help shield patients from esophagogastric cancer.
The study marks “an important step toward improving the understanding of potential lifetime risks of bariatric surgery and overall major health benefits of surgically induced weight loss,” commentary authors Piotr Gorecki, MD, and Michael Zenilman, MD, with Weill Cornell Medicine in New York, write.
Recent data indicate that excess body weight is associated with nearly 8% of cancer cases and 6.5% of cancer deaths. Studies also show that bariatric surgery can reduce the risk of some cancers, but whether this extends to esophageal and gastric cancer remains unclear.
To investigate, the researchers used French national data to compare the incidence of esophageal and gastric cancer in 303,709 mostly female patients with obesity who underwent bariatric surgery and a matched group of 605,140 patients with obesity who did not undergo the surgery.
The mean age of the cohort was about 40 years. The mean period of follow-up was 6 years for the surgery group and 5.6 years for the control arm. A total of 337 patients underwent esophagogastric cancer – 83 in the surgical group and 254 in the control group. Gastric cancer was about two times more common than esophageal cancer (225 vs. 112 patients).
The incidence rate of esophagogastric cancer was higher in the control group than in the surgery group – 6.9 vs. 4.9 cases per 100,000 population per year, for an incidence rate ratio of 1.42 (P = .005).
Bariatric surgery was associated with a significant 24% lower risk of esophagogastric cancer (hazard ratio, 0.76; P = .03) and a 40% lower risk of overall in-hospital mortality, defined as “any death occurring during a hospital stay regardless of the cause” (HR, 0.60; P < .001).
The authors also found no significant difference in cancer outcomes and type of bariatric procedure, which included sleeve gastrectomy, gastric bypass, and adjustable gastric banding.
They note that key study limitations include the retrospective design, limited follow-up period, and lack of histologic data on the specific cancers. In addition, the study population was relatively young, whereas esophageal cancer is more common in older people.
But overall, the findings suggest that bariatric surgery can be performed to treat severe obesity without increasing the risk of esophageal and gastric cancer, the authors conclude.
“It seems that the balance between protective factors (weight loss, metabolic effects, and eradication of H. pylori infection) and risk factors (GERD and bile reflux) for cancer after bariatric surgery is in favor of protective factors,” the authors, led by Andrea Lazzati, MD, PhD, of Centre Hospitalier Intercommunal de Créteil, France, explain.
Although the potential protective mechanisms remain unclear, in their commentary, Dr. Gorecki and Dr. Zenilman suggest that a reduction in chronic inflammation and immunosuppression following bariatric surgery could help explain the results.
Although the study provides “reassurance of the protective clinical benefits of weight loss surgery,” more large-scale studies are needed to “better identify, elucidate, and address the pathophysiological processes of bariatric procedure,” Dr. Gorecki and Dr. Zenilman conclude.
No specific funding for the study was reported. Dr. Lazzati has received personal fees from Johnson & Johnson, Medtronic, and Gore. Dr. Zenilman has received personal fees from Academic Medical Professionals Insurance and Mohamed & Obaid Almulla Group.
A version of this article first appeared on Medscape.com.
In fact, an analysis of close to 1 million French adults suggests that the weight-loss surgery may offer some protection against these cancers.
The study results present a “clinical paradox,” according to authors of a commentary published this week along with the study in JAMA Surgery. A procedure known to increase the risk of gastroesophageal reflux disease (GERD), and potentially adenocarcinoma of the distal esophagus and gastroesophageal junction, may help shield patients from esophagogastric cancer.
The study marks “an important step toward improving the understanding of potential lifetime risks of bariatric surgery and overall major health benefits of surgically induced weight loss,” commentary authors Piotr Gorecki, MD, and Michael Zenilman, MD, with Weill Cornell Medicine in New York, write.
Recent data indicate that excess body weight is associated with nearly 8% of cancer cases and 6.5% of cancer deaths. Studies also show that bariatric surgery can reduce the risk of some cancers, but whether this extends to esophageal and gastric cancer remains unclear.
To investigate, the researchers used French national data to compare the incidence of esophageal and gastric cancer in 303,709 mostly female patients with obesity who underwent bariatric surgery and a matched group of 605,140 patients with obesity who did not undergo the surgery.
The mean age of the cohort was about 40 years. The mean period of follow-up was 6 years for the surgery group and 5.6 years for the control arm. A total of 337 patients underwent esophagogastric cancer – 83 in the surgical group and 254 in the control group. Gastric cancer was about two times more common than esophageal cancer (225 vs. 112 patients).
The incidence rate of esophagogastric cancer was higher in the control group than in the surgery group – 6.9 vs. 4.9 cases per 100,000 population per year, for an incidence rate ratio of 1.42 (P = .005).
Bariatric surgery was associated with a significant 24% lower risk of esophagogastric cancer (hazard ratio, 0.76; P = .03) and a 40% lower risk of overall in-hospital mortality, defined as “any death occurring during a hospital stay regardless of the cause” (HR, 0.60; P < .001).
The authors also found no significant difference in cancer outcomes and type of bariatric procedure, which included sleeve gastrectomy, gastric bypass, and adjustable gastric banding.
They note that key study limitations include the retrospective design, limited follow-up period, and lack of histologic data on the specific cancers. In addition, the study population was relatively young, whereas esophageal cancer is more common in older people.
But overall, the findings suggest that bariatric surgery can be performed to treat severe obesity without increasing the risk of esophageal and gastric cancer, the authors conclude.
“It seems that the balance between protective factors (weight loss, metabolic effects, and eradication of H. pylori infection) and risk factors (GERD and bile reflux) for cancer after bariatric surgery is in favor of protective factors,” the authors, led by Andrea Lazzati, MD, PhD, of Centre Hospitalier Intercommunal de Créteil, France, explain.
Although the potential protective mechanisms remain unclear, in their commentary, Dr. Gorecki and Dr. Zenilman suggest that a reduction in chronic inflammation and immunosuppression following bariatric surgery could help explain the results.
Although the study provides “reassurance of the protective clinical benefits of weight loss surgery,” more large-scale studies are needed to “better identify, elucidate, and address the pathophysiological processes of bariatric procedure,” Dr. Gorecki and Dr. Zenilman conclude.
No specific funding for the study was reported. Dr. Lazzati has received personal fees from Johnson & Johnson, Medtronic, and Gore. Dr. Zenilman has received personal fees from Academic Medical Professionals Insurance and Mohamed & Obaid Almulla Group.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
New guidelines on peds obesity call for aggressive treatment
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Rates of health care use after bariatric surgery in teens
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.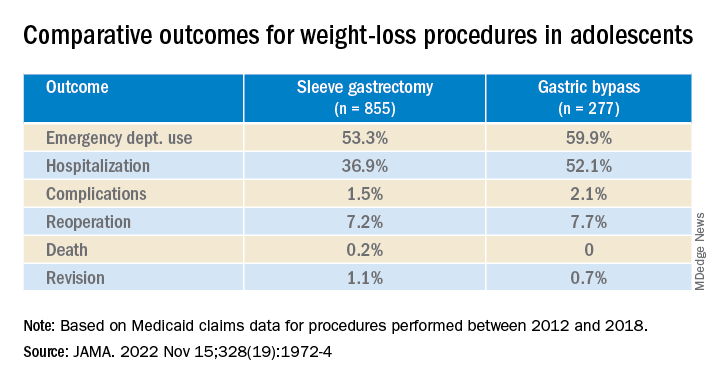
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA




