User login
Cutaneous Cold Weather Injuries in the US Military
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
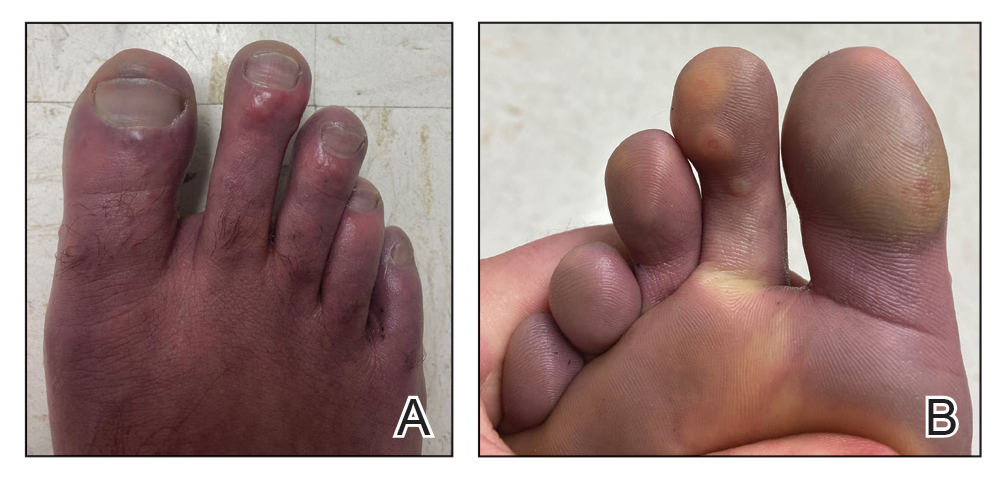
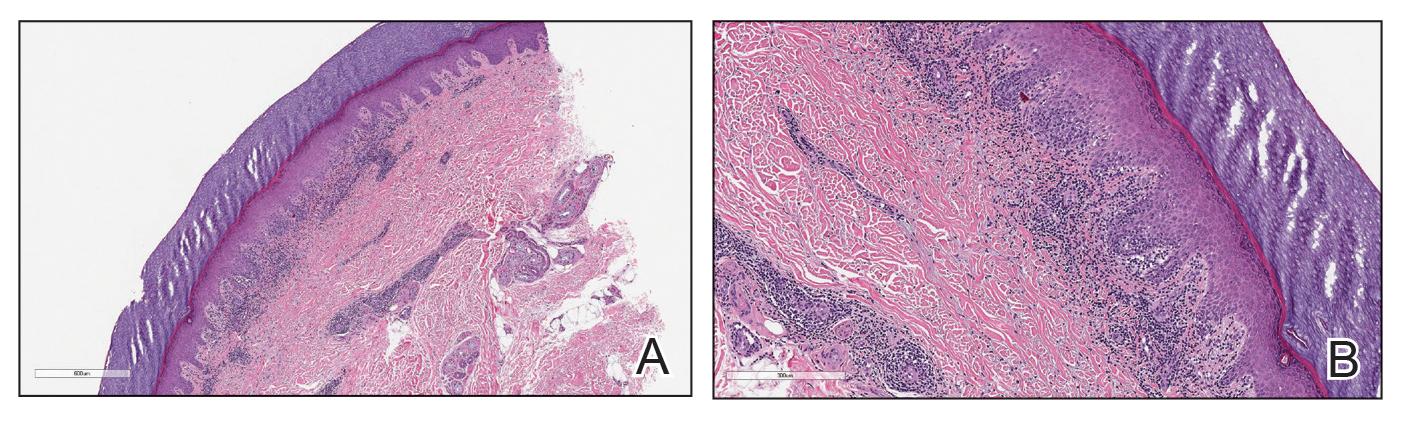
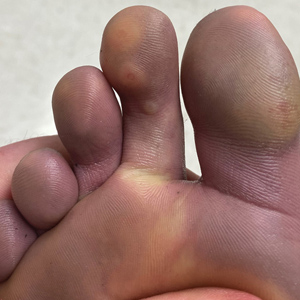
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
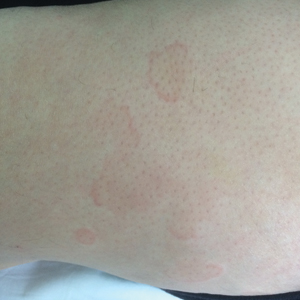
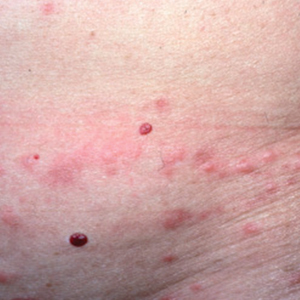
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
Practice Points
- Military service members are at an increased risk for cutaneous cold weather injuries in certain circumstances due to the demands of military training and combat operations.
- Cold weather may cause injury by directly damaging tissues, leading to neurovascular disruption, and by exacerbating existing medical conditions.
Rashes in Pregnancy
Rashes that develop during pregnancy often result in considerable anxiety or concern for patients and their families. Recognizing these pregnancy-specific dermatoses is important in identifying fetal risks as well as providing appropriate management and expert guidance for patients regarding future pregnancies. Managing cutaneous manifestations of pregnancy-related disorders is challenging and requires knowledge of potential side effects of therapy for both the mother and fetus. It also is important to appreciate the physiologic cutaneous changes of pregnancy along with their clinical significance and management.
In 2006, Ambrose-Rudolph et al1 proposed reclassification of pregnancy-specific dermatoses, which has since been widely accepted by the academic dermatology community. The 4 most prominent disorders include intrahepatic cholestasis of pregnancy (ICP); pemphigoid gestationis (PG); polymorphic eruption of pregnancy (PEP), also known as pruritic urticarial papules and plaques of pregnancy; and atopic eruption of pregnancy.2 It is important to recognize these pregnancy-specific disorders and to understand their clinical significance. The morphology of the eruption as well as the location and timing of the onset of the rash are important clues in making an accurate diagnosis.3
Clinical Presentation
Intrahepatic cholestasis of pregnancy presents with severe generalized pruritus, usually with involvement of the palms and soles, in the late second or third trimester. Pemphigoid gestationis presents with urticarial papules and/or bullae, often in the second or third trimester or postpartum. An important diagnostic clue for PG is involvement near the umbilicus. Polymorphic eruption of pregnancy presents with urticarial papules and plaques; onset occurs in the third trimester or postpartum and initially involves the striae while sparing the umbilicus, unlike in PG. Atopic eruption of pregnancy has an earlier onset than the other pregnancy-specific dermatoses, often in the first or second trimester, and presents with widespread eczematous lesions.3
Diagnosis
The pregnancy dermatoses with the greatest potential for fetal risks are ICP and PG; therefore, it is critical for health care providers to diagnose these dermatoses in a timely manner and initiate appropriate management. Intrahepatic cholestasis of pregnancy is confirmed by elevated serum bile acids (ie, >10 µmol/L), often during the third trimester. The risk of fetal morbidity is high in ICP with increased bile acids crossing the placenta causing placental anoxia and impaired cardiomyocyte function.4 Fetal risks, including preterm delivery, meconium-stained amniotic fluid, and stillbirth, correlate with the level of bile acids in the serum.5 Maternal prognosis is favorable, but there is an increased association with hepatitis C and hepatobiliary disease.6
Diagnosis of PG is confirmed by classic biopsy results and direct immunofluorescence revealing C3 with or without IgG in a linear band along the basement membrane zone. Additionally, complement indirect immunofluorescence reveals circulating IgG anti–basement membrane zone antibodies. Pemphigoid gestationis is associated with increased fetal risks of preterm labor and intrauterine growth retardation.7 Clinical findings of PG may present in the fetus upon delivery due to transmission of autoantibodies across the placenta. The symptoms usually are mild.8 An increased risk of Graves disease has been reported in mothers with PG.
In most cases, diagnosis of PEP is based on history and morphology, but if the presentation is not classic, skin biopsy must be used to differentiate it from PG as well as more common dermatologic conditions such as contact dermatitis, drug and viral eruptions, and urticaria.
Atopic eruption of pregnancy manifests as widespread eczematous excoriated papules and plaques. Lesions of prurigo nodularis are common.
Comorbidities
It is important to be aware of specific clinical associations related to pregnancy-specific dermatoses. Pemphigoid gestationis has been associated with gestational trophoblastic tumors including hydatiform mole and choriocarcinoma.4 An increased risk for Graves disease has been reported in patients with PG.9 Patients who develop ICP have a higher incidence of hepatitis C, postpartum cholecystitis, gallstones, and nonalcoholic cirrhosis.8 Polymorphic eruption of pregnancy is associated with a notably higher incidence in multiple gestation pregnancies.2
Treatment and Management
Management of ICP requires an accurate and timely diagnosis, and advanced neonatal-obstetric management is critical.3 Ursodeoxycholic acid is the treatment of choice and reduces pruritus, prolongs pregnancy, and reduces fetal risk.4 Most stillbirths cluster at the 38th week of pregnancy, and patients with ICP and highly elevated serum bile acids (>40 µmol/L) should be considered for delivery at 37 weeks or earlier.5
Management of the other cutaneous disorders of pregnancy can be challenging for health care providers based on safety concerns for the fetus. Although it is important to minimize risks to the fetus, it also is important to adequately treat the mother’s cutaneous disease, which requires a solid knowledge of drug safety during pregnancy. The former US Food and Drug Administration classification system using A, B, C, D, and X pregnancy categories was replaced by the Pregnancy Lactation Label Final Rule, which provides counseling on medication safety during pregnancy.10 In 2014, Murase et al11 published a review of dermatologic medication safety during pregnancy, which serves as an excellent guide.
Before instituting treatment, the therapeutic plan should be discussed with the physician managing the patient’s pregnancy. In general, topical steroids are considered safe during pregnancy, and low-potency to moderate-potency topical steroids are preferred. If possible, use of topical steroids should be limited to less than 300 g for the duration of the pregnancy. Fluticasone propionate should be avoided during pregnancy because it is not metabolized by the placenta. When systemic steroids are considered appropriate for management during pregnancy, nonhalogenated corticosteroids such as prednisone and prednisolone are preferred because they are enzymatically inactivated by the placenta, which results in a favorable maternal-fetal gradient.12 There has been concern expressed in the medical literature that systemic steroids during the first trimester may increase the risk of cleft lip and cleft palate.3,12 When managing pregnancy dermatoses, consideration should be given to keep prednisone exposure below 20 mg/d, and try to limit prolonged use to 7.5 mg/d. However, this may not be possible in PG.3 Vitamin D and calcium supplementation may be appropriate when patients are on prolonged systemic steroids to control disease.
Antihistamines can be used to control pruritus complicating pregnancy-associated dermatoses. First-generation antihistamines such as chlorpheniramine and diphenhydramine are preferred due to long-term safety data.3,11,12 Loratadine is the first choice and cetirizine is the second choice if a second-generation antihistamine is preferred.3 Loratadine is preferred during breastfeeding due to less sedation.12 High-dose antihistamines prior to delivery may cause concerns for potential side effects in the newborn, including tremulousness, irritability, and poor feeding.
Recurrence
Women with pregnancy dermatoses often are concerned about recurrence with future pregnancies. Pemphigoid gestationis may flare with subsequent pregnancies, subsequent menses, or with oral contraceptive use.3 Recurrence of PEP in subsequent pregnancies is rare and usually is less severe than the primary eruption.8 Often, the rare recurrent eruption of PEP is associated with multigestational pregnancies.2 Mothers can anticipate a recurrence of ICP in up to 60% to 70% of future pregnancies. Patients with AEP have an underlying atopic diathesis, and recurrence in future pregnancies is not uncommon.8
Final Thoughts
In summary, it is important for health care providers to recognize the specific cutaneous disorders of pregnancy and their potential fetal complications. The anatomical location of onset of the dermatosis and timing of onset during pregnancy can give important clues. Appropriate management, especially with ICP, can minimize fetal complications. A fundamental knowledge of medication safety and management during pregnancy is essential. Rashes during pregnancy can cause anxiety in the mother and family and require support, comfort, and guidance.
- Ambrose-Rudolph CM, Müllegger RR, Vaughn-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
- Bechtel M, Plotner A. Dermatoses of pregnancy. Clin Obstet Gynecol. 2015;58:104-111.
- Bechtel M. Pruritus in pregnancy and its management. Dermatol Clin. 2018;36:259-265.
- Ambrose-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk, and therapy. Ann Dermatol. 2011;23:265-275.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-controlled study. Hepatology. 2014;59:1482-1491.
- Bergman H, Melamed N, Koven G. Pruritus in pregnancy: treatment of dermatoses unique to pregnancy. Can Fam Physician. 2013;59:1290-1294.
- Beard MP, Millington GW. Recent developments in the specific dermatoses of pregnancy. Clin Exp Dermatol. 2012;37:1-14.
- Shears S, Blaszczak A, Kaffenberger J. Pregnancy dermatosis. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. 1st ed. Springer Nature; 2020:13-39.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2015;26:274-284.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Registr. 2014;79:72064-72103. To be codified at 21 CFR § 201.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part 1. pregnancy. J Am Acad Dermatol. 2014;401:E1-E14.
- Friedman B, Bercovitch L. Atopic dermatitis in pregnancy. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. Springer Nature; 2020:59-74.
Rashes that develop during pregnancy often result in considerable anxiety or concern for patients and their families. Recognizing these pregnancy-specific dermatoses is important in identifying fetal risks as well as providing appropriate management and expert guidance for patients regarding future pregnancies. Managing cutaneous manifestations of pregnancy-related disorders is challenging and requires knowledge of potential side effects of therapy for both the mother and fetus. It also is important to appreciate the physiologic cutaneous changes of pregnancy along with their clinical significance and management.
In 2006, Ambrose-Rudolph et al1 proposed reclassification of pregnancy-specific dermatoses, which has since been widely accepted by the academic dermatology community. The 4 most prominent disorders include intrahepatic cholestasis of pregnancy (ICP); pemphigoid gestationis (PG); polymorphic eruption of pregnancy (PEP), also known as pruritic urticarial papules and plaques of pregnancy; and atopic eruption of pregnancy.2 It is important to recognize these pregnancy-specific disorders and to understand their clinical significance. The morphology of the eruption as well as the location and timing of the onset of the rash are important clues in making an accurate diagnosis.3
Clinical Presentation
Intrahepatic cholestasis of pregnancy presents with severe generalized pruritus, usually with involvement of the palms and soles, in the late second or third trimester. Pemphigoid gestationis presents with urticarial papules and/or bullae, often in the second or third trimester or postpartum. An important diagnostic clue for PG is involvement near the umbilicus. Polymorphic eruption of pregnancy presents with urticarial papules and plaques; onset occurs in the third trimester or postpartum and initially involves the striae while sparing the umbilicus, unlike in PG. Atopic eruption of pregnancy has an earlier onset than the other pregnancy-specific dermatoses, often in the first or second trimester, and presents with widespread eczematous lesions.3
Diagnosis
The pregnancy dermatoses with the greatest potential for fetal risks are ICP and PG; therefore, it is critical for health care providers to diagnose these dermatoses in a timely manner and initiate appropriate management. Intrahepatic cholestasis of pregnancy is confirmed by elevated serum bile acids (ie, >10 µmol/L), often during the third trimester. The risk of fetal morbidity is high in ICP with increased bile acids crossing the placenta causing placental anoxia and impaired cardiomyocyte function.4 Fetal risks, including preterm delivery, meconium-stained amniotic fluid, and stillbirth, correlate with the level of bile acids in the serum.5 Maternal prognosis is favorable, but there is an increased association with hepatitis C and hepatobiliary disease.6
Diagnosis of PG is confirmed by classic biopsy results and direct immunofluorescence revealing C3 with or without IgG in a linear band along the basement membrane zone. Additionally, complement indirect immunofluorescence reveals circulating IgG anti–basement membrane zone antibodies. Pemphigoid gestationis is associated with increased fetal risks of preterm labor and intrauterine growth retardation.7 Clinical findings of PG may present in the fetus upon delivery due to transmission of autoantibodies across the placenta. The symptoms usually are mild.8 An increased risk of Graves disease has been reported in mothers with PG.
In most cases, diagnosis of PEP is based on history and morphology, but if the presentation is not classic, skin biopsy must be used to differentiate it from PG as well as more common dermatologic conditions such as contact dermatitis, drug and viral eruptions, and urticaria.
Atopic eruption of pregnancy manifests as widespread eczematous excoriated papules and plaques. Lesions of prurigo nodularis are common.
Comorbidities
It is important to be aware of specific clinical associations related to pregnancy-specific dermatoses. Pemphigoid gestationis has been associated with gestational trophoblastic tumors including hydatiform mole and choriocarcinoma.4 An increased risk for Graves disease has been reported in patients with PG.9 Patients who develop ICP have a higher incidence of hepatitis C, postpartum cholecystitis, gallstones, and nonalcoholic cirrhosis.8 Polymorphic eruption of pregnancy is associated with a notably higher incidence in multiple gestation pregnancies.2
Treatment and Management
Management of ICP requires an accurate and timely diagnosis, and advanced neonatal-obstetric management is critical.3 Ursodeoxycholic acid is the treatment of choice and reduces pruritus, prolongs pregnancy, and reduces fetal risk.4 Most stillbirths cluster at the 38th week of pregnancy, and patients with ICP and highly elevated serum bile acids (>40 µmol/L) should be considered for delivery at 37 weeks or earlier.5
Management of the other cutaneous disorders of pregnancy can be challenging for health care providers based on safety concerns for the fetus. Although it is important to minimize risks to the fetus, it also is important to adequately treat the mother’s cutaneous disease, which requires a solid knowledge of drug safety during pregnancy. The former US Food and Drug Administration classification system using A, B, C, D, and X pregnancy categories was replaced by the Pregnancy Lactation Label Final Rule, which provides counseling on medication safety during pregnancy.10 In 2014, Murase et al11 published a review of dermatologic medication safety during pregnancy, which serves as an excellent guide.
Before instituting treatment, the therapeutic plan should be discussed with the physician managing the patient’s pregnancy. In general, topical steroids are considered safe during pregnancy, and low-potency to moderate-potency topical steroids are preferred. If possible, use of topical steroids should be limited to less than 300 g for the duration of the pregnancy. Fluticasone propionate should be avoided during pregnancy because it is not metabolized by the placenta. When systemic steroids are considered appropriate for management during pregnancy, nonhalogenated corticosteroids such as prednisone and prednisolone are preferred because they are enzymatically inactivated by the placenta, which results in a favorable maternal-fetal gradient.12 There has been concern expressed in the medical literature that systemic steroids during the first trimester may increase the risk of cleft lip and cleft palate.3,12 When managing pregnancy dermatoses, consideration should be given to keep prednisone exposure below 20 mg/d, and try to limit prolonged use to 7.5 mg/d. However, this may not be possible in PG.3 Vitamin D and calcium supplementation may be appropriate when patients are on prolonged systemic steroids to control disease.
Antihistamines can be used to control pruritus complicating pregnancy-associated dermatoses. First-generation antihistamines such as chlorpheniramine and diphenhydramine are preferred due to long-term safety data.3,11,12 Loratadine is the first choice and cetirizine is the second choice if a second-generation antihistamine is preferred.3 Loratadine is preferred during breastfeeding due to less sedation.12 High-dose antihistamines prior to delivery may cause concerns for potential side effects in the newborn, including tremulousness, irritability, and poor feeding.
Recurrence
Women with pregnancy dermatoses often are concerned about recurrence with future pregnancies. Pemphigoid gestationis may flare with subsequent pregnancies, subsequent menses, or with oral contraceptive use.3 Recurrence of PEP in subsequent pregnancies is rare and usually is less severe than the primary eruption.8 Often, the rare recurrent eruption of PEP is associated with multigestational pregnancies.2 Mothers can anticipate a recurrence of ICP in up to 60% to 70% of future pregnancies. Patients with AEP have an underlying atopic diathesis, and recurrence in future pregnancies is not uncommon.8
Final Thoughts
In summary, it is important for health care providers to recognize the specific cutaneous disorders of pregnancy and their potential fetal complications. The anatomical location of onset of the dermatosis and timing of onset during pregnancy can give important clues. Appropriate management, especially with ICP, can minimize fetal complications. A fundamental knowledge of medication safety and management during pregnancy is essential. Rashes during pregnancy can cause anxiety in the mother and family and require support, comfort, and guidance.
Rashes that develop during pregnancy often result in considerable anxiety or concern for patients and their families. Recognizing these pregnancy-specific dermatoses is important in identifying fetal risks as well as providing appropriate management and expert guidance for patients regarding future pregnancies. Managing cutaneous manifestations of pregnancy-related disorders is challenging and requires knowledge of potential side effects of therapy for both the mother and fetus. It also is important to appreciate the physiologic cutaneous changes of pregnancy along with their clinical significance and management.
In 2006, Ambrose-Rudolph et al1 proposed reclassification of pregnancy-specific dermatoses, which has since been widely accepted by the academic dermatology community. The 4 most prominent disorders include intrahepatic cholestasis of pregnancy (ICP); pemphigoid gestationis (PG); polymorphic eruption of pregnancy (PEP), also known as pruritic urticarial papules and plaques of pregnancy; and atopic eruption of pregnancy.2 It is important to recognize these pregnancy-specific disorders and to understand their clinical significance. The morphology of the eruption as well as the location and timing of the onset of the rash are important clues in making an accurate diagnosis.3
Clinical Presentation
Intrahepatic cholestasis of pregnancy presents with severe generalized pruritus, usually with involvement of the palms and soles, in the late second or third trimester. Pemphigoid gestationis presents with urticarial papules and/or bullae, often in the second or third trimester or postpartum. An important diagnostic clue for PG is involvement near the umbilicus. Polymorphic eruption of pregnancy presents with urticarial papules and plaques; onset occurs in the third trimester or postpartum and initially involves the striae while sparing the umbilicus, unlike in PG. Atopic eruption of pregnancy has an earlier onset than the other pregnancy-specific dermatoses, often in the first or second trimester, and presents with widespread eczematous lesions.3
Diagnosis
The pregnancy dermatoses with the greatest potential for fetal risks are ICP and PG; therefore, it is critical for health care providers to diagnose these dermatoses in a timely manner and initiate appropriate management. Intrahepatic cholestasis of pregnancy is confirmed by elevated serum bile acids (ie, >10 µmol/L), often during the third trimester. The risk of fetal morbidity is high in ICP with increased bile acids crossing the placenta causing placental anoxia and impaired cardiomyocyte function.4 Fetal risks, including preterm delivery, meconium-stained amniotic fluid, and stillbirth, correlate with the level of bile acids in the serum.5 Maternal prognosis is favorable, but there is an increased association with hepatitis C and hepatobiliary disease.6
Diagnosis of PG is confirmed by classic biopsy results and direct immunofluorescence revealing C3 with or without IgG in a linear band along the basement membrane zone. Additionally, complement indirect immunofluorescence reveals circulating IgG anti–basement membrane zone antibodies. Pemphigoid gestationis is associated with increased fetal risks of preterm labor and intrauterine growth retardation.7 Clinical findings of PG may present in the fetus upon delivery due to transmission of autoantibodies across the placenta. The symptoms usually are mild.8 An increased risk of Graves disease has been reported in mothers with PG.
In most cases, diagnosis of PEP is based on history and morphology, but if the presentation is not classic, skin biopsy must be used to differentiate it from PG as well as more common dermatologic conditions such as contact dermatitis, drug and viral eruptions, and urticaria.
Atopic eruption of pregnancy manifests as widespread eczematous excoriated papules and plaques. Lesions of prurigo nodularis are common.
Comorbidities
It is important to be aware of specific clinical associations related to pregnancy-specific dermatoses. Pemphigoid gestationis has been associated with gestational trophoblastic tumors including hydatiform mole and choriocarcinoma.4 An increased risk for Graves disease has been reported in patients with PG.9 Patients who develop ICP have a higher incidence of hepatitis C, postpartum cholecystitis, gallstones, and nonalcoholic cirrhosis.8 Polymorphic eruption of pregnancy is associated with a notably higher incidence in multiple gestation pregnancies.2
Treatment and Management
Management of ICP requires an accurate and timely diagnosis, and advanced neonatal-obstetric management is critical.3 Ursodeoxycholic acid is the treatment of choice and reduces pruritus, prolongs pregnancy, and reduces fetal risk.4 Most stillbirths cluster at the 38th week of pregnancy, and patients with ICP and highly elevated serum bile acids (>40 µmol/L) should be considered for delivery at 37 weeks or earlier.5
Management of the other cutaneous disorders of pregnancy can be challenging for health care providers based on safety concerns for the fetus. Although it is important to minimize risks to the fetus, it also is important to adequately treat the mother’s cutaneous disease, which requires a solid knowledge of drug safety during pregnancy. The former US Food and Drug Administration classification system using A, B, C, D, and X pregnancy categories was replaced by the Pregnancy Lactation Label Final Rule, which provides counseling on medication safety during pregnancy.10 In 2014, Murase et al11 published a review of dermatologic medication safety during pregnancy, which serves as an excellent guide.
Before instituting treatment, the therapeutic plan should be discussed with the physician managing the patient’s pregnancy. In general, topical steroids are considered safe during pregnancy, and low-potency to moderate-potency topical steroids are preferred. If possible, use of topical steroids should be limited to less than 300 g for the duration of the pregnancy. Fluticasone propionate should be avoided during pregnancy because it is not metabolized by the placenta. When systemic steroids are considered appropriate for management during pregnancy, nonhalogenated corticosteroids such as prednisone and prednisolone are preferred because they are enzymatically inactivated by the placenta, which results in a favorable maternal-fetal gradient.12 There has been concern expressed in the medical literature that systemic steroids during the first trimester may increase the risk of cleft lip and cleft palate.3,12 When managing pregnancy dermatoses, consideration should be given to keep prednisone exposure below 20 mg/d, and try to limit prolonged use to 7.5 mg/d. However, this may not be possible in PG.3 Vitamin D and calcium supplementation may be appropriate when patients are on prolonged systemic steroids to control disease.
Antihistamines can be used to control pruritus complicating pregnancy-associated dermatoses. First-generation antihistamines such as chlorpheniramine and diphenhydramine are preferred due to long-term safety data.3,11,12 Loratadine is the first choice and cetirizine is the second choice if a second-generation antihistamine is preferred.3 Loratadine is preferred during breastfeeding due to less sedation.12 High-dose antihistamines prior to delivery may cause concerns for potential side effects in the newborn, including tremulousness, irritability, and poor feeding.
Recurrence
Women with pregnancy dermatoses often are concerned about recurrence with future pregnancies. Pemphigoid gestationis may flare with subsequent pregnancies, subsequent menses, or with oral contraceptive use.3 Recurrence of PEP in subsequent pregnancies is rare and usually is less severe than the primary eruption.8 Often, the rare recurrent eruption of PEP is associated with multigestational pregnancies.2 Mothers can anticipate a recurrence of ICP in up to 60% to 70% of future pregnancies. Patients with AEP have an underlying atopic diathesis, and recurrence in future pregnancies is not uncommon.8
Final Thoughts
In summary, it is important for health care providers to recognize the specific cutaneous disorders of pregnancy and their potential fetal complications. The anatomical location of onset of the dermatosis and timing of onset during pregnancy can give important clues. Appropriate management, especially with ICP, can minimize fetal complications. A fundamental knowledge of medication safety and management during pregnancy is essential. Rashes during pregnancy can cause anxiety in the mother and family and require support, comfort, and guidance.
- Ambrose-Rudolph CM, Müllegger RR, Vaughn-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
- Bechtel M, Plotner A. Dermatoses of pregnancy. Clin Obstet Gynecol. 2015;58:104-111.
- Bechtel M. Pruritus in pregnancy and its management. Dermatol Clin. 2018;36:259-265.
- Ambrose-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk, and therapy. Ann Dermatol. 2011;23:265-275.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-controlled study. Hepatology. 2014;59:1482-1491.
- Bergman H, Melamed N, Koven G. Pruritus in pregnancy: treatment of dermatoses unique to pregnancy. Can Fam Physician. 2013;59:1290-1294.
- Beard MP, Millington GW. Recent developments in the specific dermatoses of pregnancy. Clin Exp Dermatol. 2012;37:1-14.
- Shears S, Blaszczak A, Kaffenberger J. Pregnancy dermatosis. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. 1st ed. Springer Nature; 2020:13-39.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2015;26:274-284.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Registr. 2014;79:72064-72103. To be codified at 21 CFR § 201.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part 1. pregnancy. J Am Acad Dermatol. 2014;401:E1-E14.
- Friedman B, Bercovitch L. Atopic dermatitis in pregnancy. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. Springer Nature; 2020:59-74.
- Ambrose-Rudolph CM, Müllegger RR, Vaughn-Jones SA, et al. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395-404.
- Bechtel M, Plotner A. Dermatoses of pregnancy. Clin Obstet Gynecol. 2015;58:104-111.
- Bechtel M. Pruritus in pregnancy and its management. Dermatol Clin. 2018;36:259-265.
- Ambrose-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk, and therapy. Ann Dermatol. 2011;23:265-275.
- Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-controlled study. Hepatology. 2014;59:1482-1491.
- Bergman H, Melamed N, Koven G. Pruritus in pregnancy: treatment of dermatoses unique to pregnancy. Can Fam Physician. 2013;59:1290-1294.
- Beard MP, Millington GW. Recent developments in the specific dermatoses of pregnancy. Clin Exp Dermatol. 2012;37:1-14.
- Shears S, Blaszczak A, Kaffenberger J. Pregnancy dermatosis. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. 1st ed. Springer Nature; 2020:13-39.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2015;26:274-284.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Registr. 2014;79:72064-72103. To be codified at 21 CFR § 201.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part 1. pregnancy. J Am Acad Dermatol. 2014;401:E1-E14.
- Friedman B, Bercovitch L. Atopic dermatitis in pregnancy. In: Tyler KH, ed. Cutaneous Disorders of Pregnancy. Springer Nature; 2020:59-74.
Urticarial Vasculitis Successfully Treated With Omalizumab
To the Editor:
Urticarial vasculitis (UV) is a clinicopathologic entity. It manifests as an eruption of erythematous wheals that clinically resemble urticaria, but the lesions of UV last longer, may leave residual hyperpigmentation, and may or may not be pruritic.1 Therapies most often employed include oral antihistamines and systemic immunosuppressant drugs such as corticosteroids, dapsone, colchicine, or hydroxychloroquine.2 We present a woman with UV who successfully was treated with omalizumab.
A 49-year-old woman presented to our outpatient clinic with generalized pruritic skin rashes of 2 years’ duration. She also described swelling on the upper eyelids 2 times monthly. She used several antihistamines (up to 4 times daily) and was taking systemic corticosteroids and antidepressants. Physical examination revealed generalized erythematous and edematous papules and plaques on the trunk and extremities (Figure 1). At follow-up a few days later, we observed that the lesions were lasting for more than 24 hours, but there was no residual pigmentation. According to clinical concerns and the association with angioedema, we initially thought the diagnosis was chronic urticaria and angioedema. The patient had no extracutaneous manifestations such as fever, arthralgia, or lymphadenopathy. Routine laboratory examinations including antinuclear antibodies were within reference range. She had normal C3 and C4 levels and an elevated total IgE level (344 IU/mL [reference range, 0–170 IU/mL]). Because the IgE level was elevated and she had no response to the highest dosages of antihistamines, we decided to start omalizumab therapy. Prior to starting omalizumab, we performed a skin biopsy for histopathologic and direct immunofluorescence examinations for UV, as the duration of the lesions was more than 24 hours. Histopathologic examination revealed lymphocytes within the vessel wall and perivascular lymphocytic infiltration with eosinophils (Figure 2). On direct immunofluorescence, perivascular IgA deposition was observed (Figure 3). Histopathologic findings were associated with lymphocytic vasculitis. Systemic involvement was not detected on detailed laboratory and radiologic examinations.
After the first application of omalizumab, the lesions disappeared within a few days. She was treated with subcutaneous omalizumab 300 mg every 4 weeks for 6 months, and we did not observe any adverse effects related to the drug. There was no relapse after therapy cessation.
Omalizumab is a recombinant humanized anti-IgE monoclonal antibody that is approved by the US Food and Drug Administration for treatment of chronic idiopathic urticaria.3-5 Studies have suggested that omalizumab might play an important role in the treatment of other potentially IgE-mediated disease processes including allergic asthma, atopic dermatitis, allergic rhinitis, nasal polyposis, and severe ocular allergies.6 The proposed mechanism of action of omalizumab includes reduction of free IgE through the reversible formation of tiny, biologically inert complexes; targeting IgE-expressing B cells; and inhibiting production of IgE. Because it reduces free IgE, omalizumab has been used in normal IgE or hyper-IgE situations. Omalizumab also induces eosinophil apoptosis; increases IL-2, IL-3, tumor necrosis factor α, and IFN-γ; and reduces IL-4.7 A number of off-label uses have been described such as atopic dermatitis, bullous pemphigoid, hyper-IgE syndrome, cutaneous mastocytosis, toxic epidermal necrolysis, and eosinophilic granulomatosis with polyangitis.8 There are no clinical studies of omalizumab for UV, and only a few case reports have shown that omalizumab also might be beneficial for this condition.2-4 Diez et al4 reported 3 cases of women aged 28, 51, and 54 years with spontaneous chronic urticaria with autoimmune and pressure components as well as vasculitis whose symptoms completely improved after starting omalizumab. Kai et al3 successfully treated a patient with normocomplementemic UV with omalizumab and suggested that omalizumab markedly improved the patient’s quality of life with chronic urticaria and UV. Ghazanfar and Thomsen2 reported the case of a 68-year-old man diagnosed with histopathologically confirmed leukocytoclastic vasculitis. He had used systemic corticosteroid therapy and dapsone without notable improvement. The patient was switched to subcutaneous omalizumab 300 mg once every 4 weeks; after 1 month, he observed complete remission of the UV and symptoms.2
Our case suggests that omalizumab has a beneficial effect on patients with UV. Omalizumab may be effective in UV through its reduction of IgE, as in chronic urticaria, and through downstream effects on cellular activation mechanisms (possibly a reduction in chemotaxis or immune complex formation). However, the mechanism of action of omalizumab for UV remains, in part, unresolved. It is not known whether omalizumab is efficacious against both normocomplementemic and hypocomplementemic UV. Further studies with a greater number of patients are needed to confirm the effects of omalizumab for vasculitic patients.
- Chang S, Carr W. Urticarial vasculitis. Allergy Asthma Proc. 2007;28:97-100.
- Ghazanfar MN, Thomsen SF. Omalizumab for urticarial vasculitis: case report and review of the literature. Case Rep Dermatol Med. 2015:576893.
- Kai AC, Flohr C, Grattan CE. Improvement in quality of life impairment followed by relapse with 6-monthly periodic administration of omalizumab for severe treatment-refractory chronic urticaria and urticarial vasculitis. Clin Exp Dermatol. 2014;39:651-652.
- Diez LS, Tamayo LM, Cardona R. Omalizumab: therapeutic option in chronic spontaneous urticaria difficult to control with associated vasculitis, report of three cases. Biomedica. 2013;33:503-512.
- Maurer M, Rosen K, Hsieh HJ. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2530.
- Ben Shoshan M. Omalizumab: not only for asthma. Recent Pat Inflamm Allergy Drug Discov. 2008;2:191-201.
- Fueyo-Casado A, Campos-Munoz L, Gonzalez-Guerra E, et al. Effectiveness of omalizumab in a case of urticarial vasculitis. Clin Exp Dermatol. Published March 1, 2017. doi:10.1111/ced.13076
- Chia JC, Mydlarski PR. Dermatologic uses of omalizumab. J Dermatol Treat. Published November 7, 2016. doi:10.1080/09546634.2016.1249819
To the Editor:
Urticarial vasculitis (UV) is a clinicopathologic entity. It manifests as an eruption of erythematous wheals that clinically resemble urticaria, but the lesions of UV last longer, may leave residual hyperpigmentation, and may or may not be pruritic.1 Therapies most often employed include oral antihistamines and systemic immunosuppressant drugs such as corticosteroids, dapsone, colchicine, or hydroxychloroquine.2 We present a woman with UV who successfully was treated with omalizumab.
A 49-year-old woman presented to our outpatient clinic with generalized pruritic skin rashes of 2 years’ duration. She also described swelling on the upper eyelids 2 times monthly. She used several antihistamines (up to 4 times daily) and was taking systemic corticosteroids and antidepressants. Physical examination revealed generalized erythematous and edematous papules and plaques on the trunk and extremities (Figure 1). At follow-up a few days later, we observed that the lesions were lasting for more than 24 hours, but there was no residual pigmentation. According to clinical concerns and the association with angioedema, we initially thought the diagnosis was chronic urticaria and angioedema. The patient had no extracutaneous manifestations such as fever, arthralgia, or lymphadenopathy. Routine laboratory examinations including antinuclear antibodies were within reference range. She had normal C3 and C4 levels and an elevated total IgE level (344 IU/mL [reference range, 0–170 IU/mL]). Because the IgE level was elevated and she had no response to the highest dosages of antihistamines, we decided to start omalizumab therapy. Prior to starting omalizumab, we performed a skin biopsy for histopathologic and direct immunofluorescence examinations for UV, as the duration of the lesions was more than 24 hours. Histopathologic examination revealed lymphocytes within the vessel wall and perivascular lymphocytic infiltration with eosinophils (Figure 2). On direct immunofluorescence, perivascular IgA deposition was observed (Figure 3). Histopathologic findings were associated with lymphocytic vasculitis. Systemic involvement was not detected on detailed laboratory and radiologic examinations.
After the first application of omalizumab, the lesions disappeared within a few days. She was treated with subcutaneous omalizumab 300 mg every 4 weeks for 6 months, and we did not observe any adverse effects related to the drug. There was no relapse after therapy cessation.
Omalizumab is a recombinant humanized anti-IgE monoclonal antibody that is approved by the US Food and Drug Administration for treatment of chronic idiopathic urticaria.3-5 Studies have suggested that omalizumab might play an important role in the treatment of other potentially IgE-mediated disease processes including allergic asthma, atopic dermatitis, allergic rhinitis, nasal polyposis, and severe ocular allergies.6 The proposed mechanism of action of omalizumab includes reduction of free IgE through the reversible formation of tiny, biologically inert complexes; targeting IgE-expressing B cells; and inhibiting production of IgE. Because it reduces free IgE, omalizumab has been used in normal IgE or hyper-IgE situations. Omalizumab also induces eosinophil apoptosis; increases IL-2, IL-3, tumor necrosis factor α, and IFN-γ; and reduces IL-4.7 A number of off-label uses have been described such as atopic dermatitis, bullous pemphigoid, hyper-IgE syndrome, cutaneous mastocytosis, toxic epidermal necrolysis, and eosinophilic granulomatosis with polyangitis.8 There are no clinical studies of omalizumab for UV, and only a few case reports have shown that omalizumab also might be beneficial for this condition.2-4 Diez et al4 reported 3 cases of women aged 28, 51, and 54 years with spontaneous chronic urticaria with autoimmune and pressure components as well as vasculitis whose symptoms completely improved after starting omalizumab. Kai et al3 successfully treated a patient with normocomplementemic UV with omalizumab and suggested that omalizumab markedly improved the patient’s quality of life with chronic urticaria and UV. Ghazanfar and Thomsen2 reported the case of a 68-year-old man diagnosed with histopathologically confirmed leukocytoclastic vasculitis. He had used systemic corticosteroid therapy and dapsone without notable improvement. The patient was switched to subcutaneous omalizumab 300 mg once every 4 weeks; after 1 month, he observed complete remission of the UV and symptoms.2
Our case suggests that omalizumab has a beneficial effect on patients with UV. Omalizumab may be effective in UV through its reduction of IgE, as in chronic urticaria, and through downstream effects on cellular activation mechanisms (possibly a reduction in chemotaxis or immune complex formation). However, the mechanism of action of omalizumab for UV remains, in part, unresolved. It is not known whether omalizumab is efficacious against both normocomplementemic and hypocomplementemic UV. Further studies with a greater number of patients are needed to confirm the effects of omalizumab for vasculitic patients.
To the Editor:
Urticarial vasculitis (UV) is a clinicopathologic entity. It manifests as an eruption of erythematous wheals that clinically resemble urticaria, but the lesions of UV last longer, may leave residual hyperpigmentation, and may or may not be pruritic.1 Therapies most often employed include oral antihistamines and systemic immunosuppressant drugs such as corticosteroids, dapsone, colchicine, or hydroxychloroquine.2 We present a woman with UV who successfully was treated with omalizumab.
A 49-year-old woman presented to our outpatient clinic with generalized pruritic skin rashes of 2 years’ duration. She also described swelling on the upper eyelids 2 times monthly. She used several antihistamines (up to 4 times daily) and was taking systemic corticosteroids and antidepressants. Physical examination revealed generalized erythematous and edematous papules and plaques on the trunk and extremities (Figure 1). At follow-up a few days later, we observed that the lesions were lasting for more than 24 hours, but there was no residual pigmentation. According to clinical concerns and the association with angioedema, we initially thought the diagnosis was chronic urticaria and angioedema. The patient had no extracutaneous manifestations such as fever, arthralgia, or lymphadenopathy. Routine laboratory examinations including antinuclear antibodies were within reference range. She had normal C3 and C4 levels and an elevated total IgE level (344 IU/mL [reference range, 0–170 IU/mL]). Because the IgE level was elevated and she had no response to the highest dosages of antihistamines, we decided to start omalizumab therapy. Prior to starting omalizumab, we performed a skin biopsy for histopathologic and direct immunofluorescence examinations for UV, as the duration of the lesions was more than 24 hours. Histopathologic examination revealed lymphocytes within the vessel wall and perivascular lymphocytic infiltration with eosinophils (Figure 2). On direct immunofluorescence, perivascular IgA deposition was observed (Figure 3). Histopathologic findings were associated with lymphocytic vasculitis. Systemic involvement was not detected on detailed laboratory and radiologic examinations.
After the first application of omalizumab, the lesions disappeared within a few days. She was treated with subcutaneous omalizumab 300 mg every 4 weeks for 6 months, and we did not observe any adverse effects related to the drug. There was no relapse after therapy cessation.
Omalizumab is a recombinant humanized anti-IgE monoclonal antibody that is approved by the US Food and Drug Administration for treatment of chronic idiopathic urticaria.3-5 Studies have suggested that omalizumab might play an important role in the treatment of other potentially IgE-mediated disease processes including allergic asthma, atopic dermatitis, allergic rhinitis, nasal polyposis, and severe ocular allergies.6 The proposed mechanism of action of omalizumab includes reduction of free IgE through the reversible formation of tiny, biologically inert complexes; targeting IgE-expressing B cells; and inhibiting production of IgE. Because it reduces free IgE, omalizumab has been used in normal IgE or hyper-IgE situations. Omalizumab also induces eosinophil apoptosis; increases IL-2, IL-3, tumor necrosis factor α, and IFN-γ; and reduces IL-4.7 A number of off-label uses have been described such as atopic dermatitis, bullous pemphigoid, hyper-IgE syndrome, cutaneous mastocytosis, toxic epidermal necrolysis, and eosinophilic granulomatosis with polyangitis.8 There are no clinical studies of omalizumab for UV, and only a few case reports have shown that omalizumab also might be beneficial for this condition.2-4 Diez et al4 reported 3 cases of women aged 28, 51, and 54 years with spontaneous chronic urticaria with autoimmune and pressure components as well as vasculitis whose symptoms completely improved after starting omalizumab. Kai et al3 successfully treated a patient with normocomplementemic UV with omalizumab and suggested that omalizumab markedly improved the patient’s quality of life with chronic urticaria and UV. Ghazanfar and Thomsen2 reported the case of a 68-year-old man diagnosed with histopathologically confirmed leukocytoclastic vasculitis. He had used systemic corticosteroid therapy and dapsone without notable improvement. The patient was switched to subcutaneous omalizumab 300 mg once every 4 weeks; after 1 month, he observed complete remission of the UV and symptoms.2
Our case suggests that omalizumab has a beneficial effect on patients with UV. Omalizumab may be effective in UV through its reduction of IgE, as in chronic urticaria, and through downstream effects on cellular activation mechanisms (possibly a reduction in chemotaxis or immune complex formation). However, the mechanism of action of omalizumab for UV remains, in part, unresolved. It is not known whether omalizumab is efficacious against both normocomplementemic and hypocomplementemic UV. Further studies with a greater number of patients are needed to confirm the effects of omalizumab for vasculitic patients.
- Chang S, Carr W. Urticarial vasculitis. Allergy Asthma Proc. 2007;28:97-100.
- Ghazanfar MN, Thomsen SF. Omalizumab for urticarial vasculitis: case report and review of the literature. Case Rep Dermatol Med. 2015:576893.
- Kai AC, Flohr C, Grattan CE. Improvement in quality of life impairment followed by relapse with 6-monthly periodic administration of omalizumab for severe treatment-refractory chronic urticaria and urticarial vasculitis. Clin Exp Dermatol. 2014;39:651-652.
- Diez LS, Tamayo LM, Cardona R. Omalizumab: therapeutic option in chronic spontaneous urticaria difficult to control with associated vasculitis, report of three cases. Biomedica. 2013;33:503-512.
- Maurer M, Rosen K, Hsieh HJ. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2530.
- Ben Shoshan M. Omalizumab: not only for asthma. Recent Pat Inflamm Allergy Drug Discov. 2008;2:191-201.
- Fueyo-Casado A, Campos-Munoz L, Gonzalez-Guerra E, et al. Effectiveness of omalizumab in a case of urticarial vasculitis. Clin Exp Dermatol. Published March 1, 2017. doi:10.1111/ced.13076
- Chia JC, Mydlarski PR. Dermatologic uses of omalizumab. J Dermatol Treat. Published November 7, 2016. doi:10.1080/09546634.2016.1249819
- Chang S, Carr W. Urticarial vasculitis. Allergy Asthma Proc. 2007;28:97-100.
- Ghazanfar MN, Thomsen SF. Omalizumab for urticarial vasculitis: case report and review of the literature. Case Rep Dermatol Med. 2015:576893.
- Kai AC, Flohr C, Grattan CE. Improvement in quality of life impairment followed by relapse with 6-monthly periodic administration of omalizumab for severe treatment-refractory chronic urticaria and urticarial vasculitis. Clin Exp Dermatol. 2014;39:651-652.
- Diez LS, Tamayo LM, Cardona R. Omalizumab: therapeutic option in chronic spontaneous urticaria difficult to control with associated vasculitis, report of three cases. Biomedica. 2013;33:503-512.
- Maurer M, Rosen K, Hsieh HJ. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2530.
- Ben Shoshan M. Omalizumab: not only for asthma. Recent Pat Inflamm Allergy Drug Discov. 2008;2:191-201.
- Fueyo-Casado A, Campos-Munoz L, Gonzalez-Guerra E, et al. Effectiveness of omalizumab in a case of urticarial vasculitis. Clin Exp Dermatol. Published March 1, 2017. doi:10.1111/ced.13076
- Chia JC, Mydlarski PR. Dermatologic uses of omalizumab. J Dermatol Treat. Published November 7, 2016. doi:10.1080/09546634.2016.1249819
Practice Points
- The differential diagnosis of urticaria and urticarial vasculitis may be complicated.
- Omalizumab is an effective urticaria treatment and also can be an alternative treatment choice in resistant urticarial vasculitis.
Hospitalizations for food anaphylaxis triple, but deaths down in United Kingdom
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com
Oral difelikefalin quells severe chronic kidney disease–associated itch
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
FROM AAD 2020
Raynaud Phenomenon of the Nipple Successfully Treated With Nifedipine and Gabapentin
To the Editor:
Raynaud phenomenon is characterized by vasospasm of arterioles causing intermittent ischemia of the digits. The characteristic triphasic color change presents first as a dramatic change in skin color from normal to white, as the vasoconstriction causes pallor secondary to ischemia. This change is followed by a blue appearance, as cyanosis results from the deoxygenated venous blood. Finally, reflex vasodilation and reperfusion manifest as a red color from erythema. Several cases have been reported describing Raynaud phenomenon affecting the nipples of breastfeeding women.1-5 This vasospasm results in episodic nipple pain manifesting from breastfeeding and exposure to cold. If it is not appropriately treated, the pain’s severity causes affected women to stop breastfeeding. We report a case of vasospasm of the nipple in which the patient experienced nipple pain and a separate lancinating pain that radiated through the breasts.
A 36-year-old woman presented with excruciating nipple and breast pain 3 weeks after delivering her first child. She had no history of smoking or Raynaud phenomenon. The nipple pain was triggered upon breastfeeding and exposure to cold. During these episodes, the nipples would initially blanch white, then turn purple and finally a deep red. The patient also experienced an episodic excruciating lancinating pain of the breast that would randomly and spontaneously radiate through either breast several times per day for 15 to 30 seconds. A workup including an antinuclear antibody test, complete blood cell count with differential, and comprehensive metabolic panel all were within reference range.
The patient was diagnosed with nipple vasospasm. Partial relief of nipple pain occurred after treatment with 30 mg daily of nifedipine; 60 mg daily resulted in complete control, allowing the patient to breastfeed without discomfort, but the lancinating pain continued unabated. The patient could not discontinue breastfeeding because her child was intolerant to formula. She became despondent, as she could find no relief from the pain that she found to be intolerable. Because the patient’s description was reminiscent of the lancinating pain seen in postherpetic neuralgia, a trial of pregabalin was prescribed. A dosage of 75 mg twice daily resulted in near-complete resolution of the pain. After 3 months, the patient successfully weaned her child from breast milk to formula, and the nipple and breast pain promptly resolved. The baby experienced no adverse effects from the patient’s use of pregabalin.
This condition was first described by Gunther1 in 1970 as initial blanching of the nipple followed by a mulberry color. It was termed psychosomatic sore nipples.1 Lawlor-Smith and Lawlor-Smith2 described the condition in 1997 and termed it vasospasm of the nipple. They reported 5 patients who experienced debilitating nipple pain as well as the triphasic color change of Raynaud phenomenon or a biphasic color change (white and blue). Two patients had a history of Raynaud phenomenon affecting the digits before their first pregnancy.2 Anderson et al3 presented 12 breastfeeding women with Raynaud phenomenon of the nipple; only 1 patient had a history of Raynaud phenomenon. In this series, all 6 women who chose to try nifedipine responded well to the drug.3
Raynaud phenomenon of the nipple also has been reported to be associated with the use of labetalol.4 In this case, the patient had a history of Raynaud phenomenon affecting the toes and nipples on cold days. In 2 subsequent pregnancies she was treated with labetalol for pregnancy-induced hypertension, which resulted in severe nipple pain with each pregnancy unrelated to cold weather. Unlike other cases, this patient experienced antenatal symptoms in addition to the typical postnatal symptoms. The nipple pain resolved with discontinuation of the labetalol.4
Barrett et al5 conducted a retrospective review of medical records of 88 breastfeeding mothers who presented with nipple pain and dermatitis. They defined the criteria for Raynaud phenomenon of the nipple as chronic deep breast pain (in general lasting >4 weeks) that responded to therapy for the condition and had at least 2 of the following characteristics: (1) observed or self-reported color changes of the nipple, especially with cold exposure (white, blue, or red); (2) cold sensitivity or color changes of the hands or feet with cold exposure; or (3) failed therapy with oral antifungals. Using these criteria, they diagnosed 22 women (25%) with Raynaud phenomenon of the nipple; 20 (91%) reported a history of cold sensitivity or color change of acral surfaces. Of 12 patients who received and tolerated nifedipine use, 10 (83%) reported decreased pain or complete resolution. This series described breast or nipple pain, whereas other reported cases only described nipple pain. The authors described a sharp, shooting, or stabbing pain—qualifications not previously noted.5 Our patient experienced both nipple pain and a lancinating breast pain consistent with the cases reported by Barrett et al.5
The nipple pain and treatment response in our patient was typical of previously reported cases of vasospasm of the nipple in breastfeeding women; however, Barrett et al5 did not describe individual patients who exhibited the dual nature of the pain described in our patient. The nipple pain experienced during breastfeeding in our patient was successfully treated with nifedipine. We report the successful treatment of the separate lancinating pain with pregabalin.
- Gunther M. Infant Feeding. London, United Kingdom: Methuen; 1970.
- Lawlor-Smith L, Lawlor-Smith C. Vasospasm of the nipple—a manifestation of Raynaud’s phenomenon: case reports. BMJ. 1997;314:644-645.
- Anderson JE, Held N, Wright K. Raynaud phenomenon of the nipple: a treatable cause of painful breastfeeding. Pediatrics. 2004;113:360-364.
- McGuinness N, Cording V. Raynaud’s phenomenon of the nipple associated with labetalol use. J Hum Lact. 2013;29:17-19.
- Barrett ME, Heller MM, Stone HF, et al. Raynaud phenomenon of the nipple in breastfeeding mothers: an underdiagnosed cause of nipple pain. JAMA Dermatol. 2013;149:300-306.
To the Editor:
Raynaud phenomenon is characterized by vasospasm of arterioles causing intermittent ischemia of the digits. The characteristic triphasic color change presents first as a dramatic change in skin color from normal to white, as the vasoconstriction causes pallor secondary to ischemia. This change is followed by a blue appearance, as cyanosis results from the deoxygenated venous blood. Finally, reflex vasodilation and reperfusion manifest as a red color from erythema. Several cases have been reported describing Raynaud phenomenon affecting the nipples of breastfeeding women.1-5 This vasospasm results in episodic nipple pain manifesting from breastfeeding and exposure to cold. If it is not appropriately treated, the pain’s severity causes affected women to stop breastfeeding. We report a case of vasospasm of the nipple in which the patient experienced nipple pain and a separate lancinating pain that radiated through the breasts.
A 36-year-old woman presented with excruciating nipple and breast pain 3 weeks after delivering her first child. She had no history of smoking or Raynaud phenomenon. The nipple pain was triggered upon breastfeeding and exposure to cold. During these episodes, the nipples would initially blanch white, then turn purple and finally a deep red. The patient also experienced an episodic excruciating lancinating pain of the breast that would randomly and spontaneously radiate through either breast several times per day for 15 to 30 seconds. A workup including an antinuclear antibody test, complete blood cell count with differential, and comprehensive metabolic panel all were within reference range.
The patient was diagnosed with nipple vasospasm. Partial relief of nipple pain occurred after treatment with 30 mg daily of nifedipine; 60 mg daily resulted in complete control, allowing the patient to breastfeed without discomfort, but the lancinating pain continued unabated. The patient could not discontinue breastfeeding because her child was intolerant to formula. She became despondent, as she could find no relief from the pain that she found to be intolerable. Because the patient’s description was reminiscent of the lancinating pain seen in postherpetic neuralgia, a trial of pregabalin was prescribed. A dosage of 75 mg twice daily resulted in near-complete resolution of the pain. After 3 months, the patient successfully weaned her child from breast milk to formula, and the nipple and breast pain promptly resolved. The baby experienced no adverse effects from the patient’s use of pregabalin.
This condition was first described by Gunther1 in 1970 as initial blanching of the nipple followed by a mulberry color. It was termed psychosomatic sore nipples.1 Lawlor-Smith and Lawlor-Smith2 described the condition in 1997 and termed it vasospasm of the nipple. They reported 5 patients who experienced debilitating nipple pain as well as the triphasic color change of Raynaud phenomenon or a biphasic color change (white and blue). Two patients had a history of Raynaud phenomenon affecting the digits before their first pregnancy.2 Anderson et al3 presented 12 breastfeeding women with Raynaud phenomenon of the nipple; only 1 patient had a history of Raynaud phenomenon. In this series, all 6 women who chose to try nifedipine responded well to the drug.3
Raynaud phenomenon of the nipple also has been reported to be associated with the use of labetalol.4 In this case, the patient had a history of Raynaud phenomenon affecting the toes and nipples on cold days. In 2 subsequent pregnancies she was treated with labetalol for pregnancy-induced hypertension, which resulted in severe nipple pain with each pregnancy unrelated to cold weather. Unlike other cases, this patient experienced antenatal symptoms in addition to the typical postnatal symptoms. The nipple pain resolved with discontinuation of the labetalol.4
Barrett et al5 conducted a retrospective review of medical records of 88 breastfeeding mothers who presented with nipple pain and dermatitis. They defined the criteria for Raynaud phenomenon of the nipple as chronic deep breast pain (in general lasting >4 weeks) that responded to therapy for the condition and had at least 2 of the following characteristics: (1) observed or self-reported color changes of the nipple, especially with cold exposure (white, blue, or red); (2) cold sensitivity or color changes of the hands or feet with cold exposure; or (3) failed therapy with oral antifungals. Using these criteria, they diagnosed 22 women (25%) with Raynaud phenomenon of the nipple; 20 (91%) reported a history of cold sensitivity or color change of acral surfaces. Of 12 patients who received and tolerated nifedipine use, 10 (83%) reported decreased pain or complete resolution. This series described breast or nipple pain, whereas other reported cases only described nipple pain. The authors described a sharp, shooting, or stabbing pain—qualifications not previously noted.5 Our patient experienced both nipple pain and a lancinating breast pain consistent with the cases reported by Barrett et al.5
The nipple pain and treatment response in our patient was typical of previously reported cases of vasospasm of the nipple in breastfeeding women; however, Barrett et al5 did not describe individual patients who exhibited the dual nature of the pain described in our patient. The nipple pain experienced during breastfeeding in our patient was successfully treated with nifedipine. We report the successful treatment of the separate lancinating pain with pregabalin.
To the Editor:
Raynaud phenomenon is characterized by vasospasm of arterioles causing intermittent ischemia of the digits. The characteristic triphasic color change presents first as a dramatic change in skin color from normal to white, as the vasoconstriction causes pallor secondary to ischemia. This change is followed by a blue appearance, as cyanosis results from the deoxygenated venous blood. Finally, reflex vasodilation and reperfusion manifest as a red color from erythema. Several cases have been reported describing Raynaud phenomenon affecting the nipples of breastfeeding women.1-5 This vasospasm results in episodic nipple pain manifesting from breastfeeding and exposure to cold. If it is not appropriately treated, the pain’s severity causes affected women to stop breastfeeding. We report a case of vasospasm of the nipple in which the patient experienced nipple pain and a separate lancinating pain that radiated through the breasts.
A 36-year-old woman presented with excruciating nipple and breast pain 3 weeks after delivering her first child. She had no history of smoking or Raynaud phenomenon. The nipple pain was triggered upon breastfeeding and exposure to cold. During these episodes, the nipples would initially blanch white, then turn purple and finally a deep red. The patient also experienced an episodic excruciating lancinating pain of the breast that would randomly and spontaneously radiate through either breast several times per day for 15 to 30 seconds. A workup including an antinuclear antibody test, complete blood cell count with differential, and comprehensive metabolic panel all were within reference range.
The patient was diagnosed with nipple vasospasm. Partial relief of nipple pain occurred after treatment with 30 mg daily of nifedipine; 60 mg daily resulted in complete control, allowing the patient to breastfeed without discomfort, but the lancinating pain continued unabated. The patient could not discontinue breastfeeding because her child was intolerant to formula. She became despondent, as she could find no relief from the pain that she found to be intolerable. Because the patient’s description was reminiscent of the lancinating pain seen in postherpetic neuralgia, a trial of pregabalin was prescribed. A dosage of 75 mg twice daily resulted in near-complete resolution of the pain. After 3 months, the patient successfully weaned her child from breast milk to formula, and the nipple and breast pain promptly resolved. The baby experienced no adverse effects from the patient’s use of pregabalin.
This condition was first described by Gunther1 in 1970 as initial blanching of the nipple followed by a mulberry color. It was termed psychosomatic sore nipples.1 Lawlor-Smith and Lawlor-Smith2 described the condition in 1997 and termed it vasospasm of the nipple. They reported 5 patients who experienced debilitating nipple pain as well as the triphasic color change of Raynaud phenomenon or a biphasic color change (white and blue). Two patients had a history of Raynaud phenomenon affecting the digits before their first pregnancy.2 Anderson et al3 presented 12 breastfeeding women with Raynaud phenomenon of the nipple; only 1 patient had a history of Raynaud phenomenon. In this series, all 6 women who chose to try nifedipine responded well to the drug.3
Raynaud phenomenon of the nipple also has been reported to be associated with the use of labetalol.4 In this case, the patient had a history of Raynaud phenomenon affecting the toes and nipples on cold days. In 2 subsequent pregnancies she was treated with labetalol for pregnancy-induced hypertension, which resulted in severe nipple pain with each pregnancy unrelated to cold weather. Unlike other cases, this patient experienced antenatal symptoms in addition to the typical postnatal symptoms. The nipple pain resolved with discontinuation of the labetalol.4
Barrett et al5 conducted a retrospective review of medical records of 88 breastfeeding mothers who presented with nipple pain and dermatitis. They defined the criteria for Raynaud phenomenon of the nipple as chronic deep breast pain (in general lasting >4 weeks) that responded to therapy for the condition and had at least 2 of the following characteristics: (1) observed or self-reported color changes of the nipple, especially with cold exposure (white, blue, or red); (2) cold sensitivity or color changes of the hands or feet with cold exposure; or (3) failed therapy with oral antifungals. Using these criteria, they diagnosed 22 women (25%) with Raynaud phenomenon of the nipple; 20 (91%) reported a history of cold sensitivity or color change of acral surfaces. Of 12 patients who received and tolerated nifedipine use, 10 (83%) reported decreased pain or complete resolution. This series described breast or nipple pain, whereas other reported cases only described nipple pain. The authors described a sharp, shooting, or stabbing pain—qualifications not previously noted.5 Our patient experienced both nipple pain and a lancinating breast pain consistent with the cases reported by Barrett et al.5
The nipple pain and treatment response in our patient was typical of previously reported cases of vasospasm of the nipple in breastfeeding women; however, Barrett et al5 did not describe individual patients who exhibited the dual nature of the pain described in our patient. The nipple pain experienced during breastfeeding in our patient was successfully treated with nifedipine. We report the successful treatment of the separate lancinating pain with pregabalin.
- Gunther M. Infant Feeding. London, United Kingdom: Methuen; 1970.
- Lawlor-Smith L, Lawlor-Smith C. Vasospasm of the nipple—a manifestation of Raynaud’s phenomenon: case reports. BMJ. 1997;314:644-645.
- Anderson JE, Held N, Wright K. Raynaud phenomenon of the nipple: a treatable cause of painful breastfeeding. Pediatrics. 2004;113:360-364.
- McGuinness N, Cording V. Raynaud’s phenomenon of the nipple associated with labetalol use. J Hum Lact. 2013;29:17-19.
- Barrett ME, Heller MM, Stone HF, et al. Raynaud phenomenon of the nipple in breastfeeding mothers: an underdiagnosed cause of nipple pain. JAMA Dermatol. 2013;149:300-306.
- Gunther M. Infant Feeding. London, United Kingdom: Methuen; 1970.
- Lawlor-Smith L, Lawlor-Smith C. Vasospasm of the nipple—a manifestation of Raynaud’s phenomenon: case reports. BMJ. 1997;314:644-645.
- Anderson JE, Held N, Wright K. Raynaud phenomenon of the nipple: a treatable cause of painful breastfeeding. Pediatrics. 2004;113:360-364.
- McGuinness N, Cording V. Raynaud’s phenomenon of the nipple associated with labetalol use. J Hum Lact. 2013;29:17-19.
- Barrett ME, Heller MM, Stone HF, et al. Raynaud phenomenon of the nipple in breastfeeding mothers: an underdiagnosed cause of nipple pain. JAMA Dermatol. 2013;149:300-306.
Practice Points
- Raynaud phenomenon of the nipple may be accompanied by lancinating pain of the breast in addition to nipple pain reminiscent of postherpetic neuralgia.
- Associated breast pain is particularly distressing for breastfeeding women, particularly primiparous mothers with children intolerant to formula.
- In women with Raynaud phenomenon accompanied by lancinating breast pain, consider a trial of pregabalin.
Follicular Traction Urticaria Induced by Electric Epilation
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.
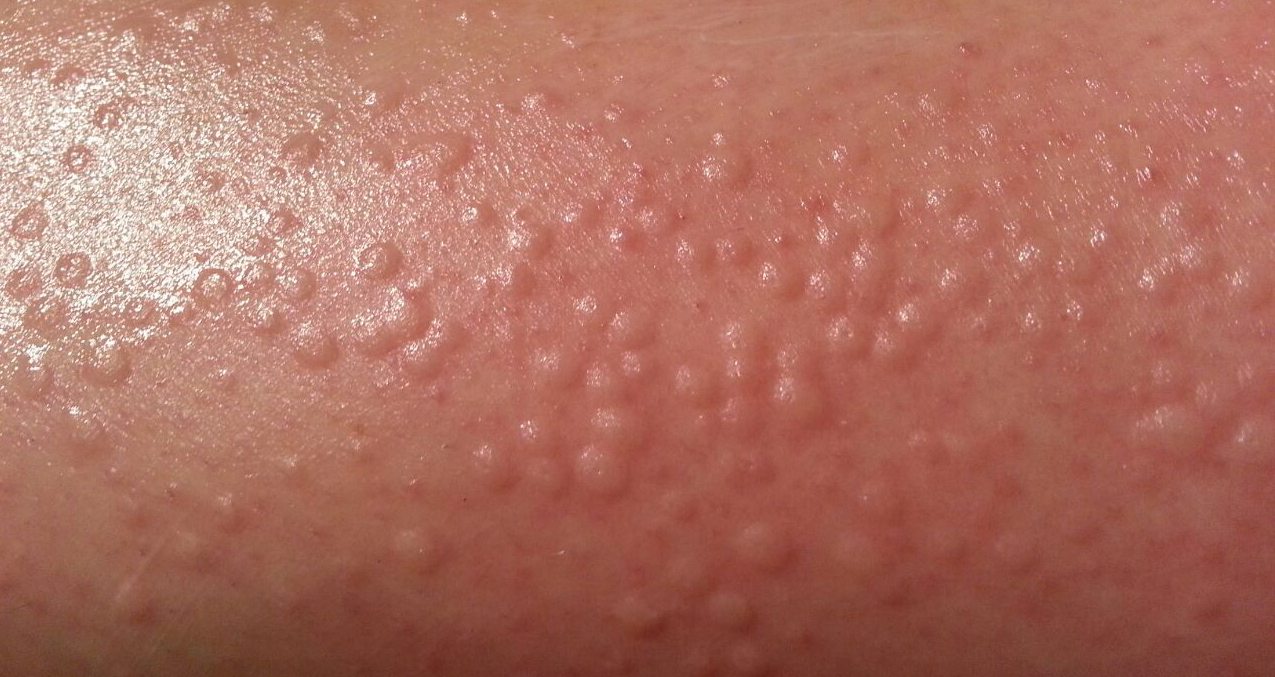
Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.

Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.

Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
Practice Points
- Follicular traction urticaria is an unusual form of chronic inducible urticaria.
- Follicular traction urticaria consists of follicular hives that develop after being triggered by hair traction.
Study: Delays filling biologic prescriptions have consequences
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
REPORTING FROM AAAAI
What’s Eating You? Human Body Lice (Pediculus humanus corporis)
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Practice Points
- Body lice reside in clothing, particularly folds and seams, and migrate to the host for blood meals. To evaluate for infestation, the clinician should not only look at the skin but also closely examine the patient’s clothing. Clothes also are a target for treatment via washing in hot water.
- Due to observed and theoretical adverse effects of other chemical treatments, benzyl alcohol is the authors’ choice for treatment of head lice.
- Oral ivermectin is a promising future treatment for body lice.
Rapid improvement seen with nemolizumab for prurigo nodularis in phase 2b study
MADRID – Nemolizumab, an investigational humanized monoclonal antibody targeting the interleukin-31 receptor alpha subunit, achieved rapid and clinically meaningful improvement in both itch and skin lesions of severe prurigo nodularis in a phase 2b, randomized trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We saw onset of pruritus improvement in week 1 and onset of lesion healing at week 4,” reported Dr. Stander, professor of dermatology and neurodermatology and head of the Center for Chronic Pruritus at the University of Münster (Germany).
The study results confirm IL-31 signaling as an important therapeutic target in prurigo nodularis and herald the arrival of nemolizumab as a promising potential therapy for severely affected patients, she added.
Prurigo nodularis is a chronic, highly pruritic disease that is difficult to treat and carries a high disease burden. While the disease’s pathogenesis is not completely understood, IL-31 is up-regulated in affected patients. And IL-31, a proinflammatory and immunomodulatory cytokine, is known to have a broad range of actions, including serving as a link between the immune and neural systems, as well as induction of itch and skin lesions.
Dr. Stander presented the results of a 20-center, double-blind, at weeks 0, 4, and 8, then followed off therapy out to week 18. These were severely affected patients: their mean weekly peak pruritus score on a 0-10 numeric rating scale was 8.4, with 7 being the accepted threshold for severe itch. The group had a mean Dermatologic Life Quality Index score of 16.4; 40% of patients had more than 100 nodules on their body, and the rest had 20-100.
The primary endpoint was the percentage decrease in the peak pruritus score from baseline to week 4, at which point they had only received one dose. The nemolizumab group averaged a 53.4% reduction, compared with 15.3% in placebo-treated controls. At week 12, a full month after the final injection, the split was 63.2% versus 20.2%. And at week 18, the nemolizumab group maintained a mean 58.2% reduction from baseline versus 20.9% in controls.
“The effect starts at week 1, with a 26% reduction in itch intensity in the nemolizumab group, compared to 6.7% with placebo,” the dermatologist observed.
The absolute decrease in weekly peak pruritus score at week 12 was 5.2 points with nemolizumab and 1.7 points with placebo.
Among the secondary endpoints was achievement of an Investigator Global Assessment score of 0-1, meaning clear or almost clear of skin lesions. The rate in the nemolizumab group climbed steadily from week 4 on, reaching 38.2% and still rising without a plateau at week 18, versus 5.6% in controls.
Another secondary endpoint was 75% or greater healing on the 7-item Prurigo Activity Scale. By week 4 there was already a statistically significant between-group difference: 23.5% versus 11.2%. Once again, in the nemolizumab group, this rate climbed without a plateau through the study’s end at week 18, by which point it was 44.1%, compared with 8.4% among those on placebo.
Scores on the Dermatologic Life Quality Index improved by an average of 10.2 points at week 4 in patients on nemolizumab, compared with 6 points among controls.
Self-reported sleep disturbance scores improved by 56% at week 4 in the nemolizumab group and 22.9% with placebo.
The safety profile of nemolizumab was similar to that of placebo, with roughly 5.7% of patients in each study arm withdrawing because of treatment-emergent adverse events. Unlike in the positive studies of the IL-31 inhibitor in patients with atopic dermatitis – another potential indication under active investigation – there was no signal of an increased risk of staphylococcal skin infections, conjunctivitis, or head and neck dermatitis in patients on nemolizumab for prurigo nodularis. Patients with comorbid atopic dermatitis were excluded from the prurigo nodularis trial in order to get a clearer picture of the biologic’s efficacy and safety specifically for that condition.
Dr. Stander reported serving as a consultant to Galderma, the study sponsor, as well as numerous other pharmaceutical companies.
MADRID – Nemolizumab, an investigational humanized monoclonal antibody targeting the interleukin-31 receptor alpha subunit, achieved rapid and clinically meaningful improvement in both itch and skin lesions of severe prurigo nodularis in a phase 2b, randomized trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We saw onset of pruritus improvement in week 1 and onset of lesion healing at week 4,” reported Dr. Stander, professor of dermatology and neurodermatology and head of the Center for Chronic Pruritus at the University of Münster (Germany).
The study results confirm IL-31 signaling as an important therapeutic target in prurigo nodularis and herald the arrival of nemolizumab as a promising potential therapy for severely affected patients, she added.
Prurigo nodularis is a chronic, highly pruritic disease that is difficult to treat and carries a high disease burden. While the disease’s pathogenesis is not completely understood, IL-31 is up-regulated in affected patients. And IL-31, a proinflammatory and immunomodulatory cytokine, is known to have a broad range of actions, including serving as a link between the immune and neural systems, as well as induction of itch and skin lesions.
Dr. Stander presented the results of a 20-center, double-blind, at weeks 0, 4, and 8, then followed off therapy out to week 18. These were severely affected patients: their mean weekly peak pruritus score on a 0-10 numeric rating scale was 8.4, with 7 being the accepted threshold for severe itch. The group had a mean Dermatologic Life Quality Index score of 16.4; 40% of patients had more than 100 nodules on their body, and the rest had 20-100.
The primary endpoint was the percentage decrease in the peak pruritus score from baseline to week 4, at which point they had only received one dose. The nemolizumab group averaged a 53.4% reduction, compared with 15.3% in placebo-treated controls. At week 12, a full month after the final injection, the split was 63.2% versus 20.2%. And at week 18, the nemolizumab group maintained a mean 58.2% reduction from baseline versus 20.9% in controls.
“The effect starts at week 1, with a 26% reduction in itch intensity in the nemolizumab group, compared to 6.7% with placebo,” the dermatologist observed.
The absolute decrease in weekly peak pruritus score at week 12 was 5.2 points with nemolizumab and 1.7 points with placebo.
Among the secondary endpoints was achievement of an Investigator Global Assessment score of 0-1, meaning clear or almost clear of skin lesions. The rate in the nemolizumab group climbed steadily from week 4 on, reaching 38.2% and still rising without a plateau at week 18, versus 5.6% in controls.
Another secondary endpoint was 75% or greater healing on the 7-item Prurigo Activity Scale. By week 4 there was already a statistically significant between-group difference: 23.5% versus 11.2%. Once again, in the nemolizumab group, this rate climbed without a plateau through the study’s end at week 18, by which point it was 44.1%, compared with 8.4% among those on placebo.
Scores on the Dermatologic Life Quality Index improved by an average of 10.2 points at week 4 in patients on nemolizumab, compared with 6 points among controls.
Self-reported sleep disturbance scores improved by 56% at week 4 in the nemolizumab group and 22.9% with placebo.
The safety profile of nemolizumab was similar to that of placebo, with roughly 5.7% of patients in each study arm withdrawing because of treatment-emergent adverse events. Unlike in the positive studies of the IL-31 inhibitor in patients with atopic dermatitis – another potential indication under active investigation – there was no signal of an increased risk of staphylococcal skin infections, conjunctivitis, or head and neck dermatitis in patients on nemolizumab for prurigo nodularis. Patients with comorbid atopic dermatitis were excluded from the prurigo nodularis trial in order to get a clearer picture of the biologic’s efficacy and safety specifically for that condition.
Dr. Stander reported serving as a consultant to Galderma, the study sponsor, as well as numerous other pharmaceutical companies.
MADRID – Nemolizumab, an investigational humanized monoclonal antibody targeting the interleukin-31 receptor alpha subunit, achieved rapid and clinically meaningful improvement in both itch and skin lesions of severe prurigo nodularis in a phase 2b, randomized trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We saw onset of pruritus improvement in week 1 and onset of lesion healing at week 4,” reported Dr. Stander, professor of dermatology and neurodermatology and head of the Center for Chronic Pruritus at the University of Münster (Germany).
The study results confirm IL-31 signaling as an important therapeutic target in prurigo nodularis and herald the arrival of nemolizumab as a promising potential therapy for severely affected patients, she added.
Prurigo nodularis is a chronic, highly pruritic disease that is difficult to treat and carries a high disease burden. While the disease’s pathogenesis is not completely understood, IL-31 is up-regulated in affected patients. And IL-31, a proinflammatory and immunomodulatory cytokine, is known to have a broad range of actions, including serving as a link between the immune and neural systems, as well as induction of itch and skin lesions.
Dr. Stander presented the results of a 20-center, double-blind, at weeks 0, 4, and 8, then followed off therapy out to week 18. These were severely affected patients: their mean weekly peak pruritus score on a 0-10 numeric rating scale was 8.4, with 7 being the accepted threshold for severe itch. The group had a mean Dermatologic Life Quality Index score of 16.4; 40% of patients had more than 100 nodules on their body, and the rest had 20-100.
The primary endpoint was the percentage decrease in the peak pruritus score from baseline to week 4, at which point they had only received one dose. The nemolizumab group averaged a 53.4% reduction, compared with 15.3% in placebo-treated controls. At week 12, a full month after the final injection, the split was 63.2% versus 20.2%. And at week 18, the nemolizumab group maintained a mean 58.2% reduction from baseline versus 20.9% in controls.
“The effect starts at week 1, with a 26% reduction in itch intensity in the nemolizumab group, compared to 6.7% with placebo,” the dermatologist observed.
The absolute decrease in weekly peak pruritus score at week 12 was 5.2 points with nemolizumab and 1.7 points with placebo.
Among the secondary endpoints was achievement of an Investigator Global Assessment score of 0-1, meaning clear or almost clear of skin lesions. The rate in the nemolizumab group climbed steadily from week 4 on, reaching 38.2% and still rising without a plateau at week 18, versus 5.6% in controls.
Another secondary endpoint was 75% or greater healing on the 7-item Prurigo Activity Scale. By week 4 there was already a statistically significant between-group difference: 23.5% versus 11.2%. Once again, in the nemolizumab group, this rate climbed without a plateau through the study’s end at week 18, by which point it was 44.1%, compared with 8.4% among those on placebo.
Scores on the Dermatologic Life Quality Index improved by an average of 10.2 points at week 4 in patients on nemolizumab, compared with 6 points among controls.
Self-reported sleep disturbance scores improved by 56% at week 4 in the nemolizumab group and 22.9% with placebo.
The safety profile of nemolizumab was similar to that of placebo, with roughly 5.7% of patients in each study arm withdrawing because of treatment-emergent adverse events. Unlike in the positive studies of the IL-31 inhibitor in patients with atopic dermatitis – another potential indication under active investigation – there was no signal of an increased risk of staphylococcal skin infections, conjunctivitis, or head and neck dermatitis in patients on nemolizumab for prurigo nodularis. Patients with comorbid atopic dermatitis were excluded from the prurigo nodularis trial in order to get a clearer picture of the biologic’s efficacy and safety specifically for that condition.
Dr. Stander reported serving as a consultant to Galderma, the study sponsor, as well as numerous other pharmaceutical companies.
REPORTING FROM THE EADV CONGRESS