User login
Long-acting injectable antiretroviral combo as good as HIV oral therapy
A long-acting injectable formulation combining two antiretroviral drugs – cabotegravir and rilpivirine – is as effective as a daily oral regimen of cabotegravir plus abacavir and lamivudine, according to interim results from the LATTE-2 trial.
Writing in the July 24 online edition of The Lancet, researchers have reported the 96-week results of the ongoing open-label phase 2b trial involving 286 treatment-naive adults infected with HIV-1.
At 32 weeks, 95% of patients in 8-week group, 94% of patients in the 4-week group, and 91% of patients in the oral therapy group had maintained viral suppression, defined as plasma HIV-1 RNA less than 50 copies per mL (Lancet. 2017 Jul 24. doi: 10.1016/S0140-6736[17]31917-7).
By week 96, viral suppression was seen in 94% of patients in the 8-week group, 87% of patients in the 4-week group and 84% of patients on oral treatment.
A nonresponse at 96 weeks was seen in five patients in the 8-week group and one patient in the oral therapy group. There were also two patients in the 8-week group and one in the oral treatment group who met the criteria for virologic failure.
“Viral genotyping analysis for the patient in the oral treatment group had no treatment-emergent resistance mutations in the genes encoding viral reverse transcriptase, protease, or integrase,” wrote David A. Margolis, MD, from ViiV Healthcare, and his coauthors.
Injection-site pain was the most common adverse event in the injectable groups, experienced by 96%-97% of patients, but most reactions were mild or moderate and lasted a median of 3 days.
There was only one drug-related serious adverse event, which was a migraine that occurred in one patient during the oral induction period. None of the other serious adverse events were deemed to be drug-related.
Patients reported high levels of treatment satisfaction across all study groups, and more than 99% of patients in the injectable treatment arms said they would be highly satisfied to continue with the regimen, compared with 78% of patients in the oral treatment arm.
“The acceptability and tolerability of injectable dosing options will be an important component of long-term treatment success, and a high degree of treatment satisfaction will avail this option for patients burdened by life-long daily oral medication compliance,” the authors wrote.
However they did note that the study population was mostly white men with a CD4+ cell count at entry of at least 200 cells per mm.
“Therefore, the efficacy, safety, and pharmacokinetic outcomes of long-acting cabotegravir plus rilpivirine in different subpopulations infected with HIV-1 needs further evaluation,” the investigators concluded.
The study was funded by ViiV Healthcare and Janssen R&D. Nine authors are employees of ViiV Healthcare and shareholders in GlaxoSmithKline. Nine authors declared funding, grants, and honoraria from a range of pharmaceutical companies including ViiV Healthcare. Two authors are employees and shareholders of GlaxoSmithKline. Two authors are employees of Janssen, and stockholders of Johnson & Johnson, AstraZeneca, or GlaxoSmithKline. One author had no conflicts to disclose.
A long-acting injectable formulation combining two antiretroviral drugs – cabotegravir and rilpivirine – is as effective as a daily oral regimen of cabotegravir plus abacavir and lamivudine, according to interim results from the LATTE-2 trial.
Writing in the July 24 online edition of The Lancet, researchers have reported the 96-week results of the ongoing open-label phase 2b trial involving 286 treatment-naive adults infected with HIV-1.
At 32 weeks, 95% of patients in 8-week group, 94% of patients in the 4-week group, and 91% of patients in the oral therapy group had maintained viral suppression, defined as plasma HIV-1 RNA less than 50 copies per mL (Lancet. 2017 Jul 24. doi: 10.1016/S0140-6736[17]31917-7).
By week 96, viral suppression was seen in 94% of patients in the 8-week group, 87% of patients in the 4-week group and 84% of patients on oral treatment.
A nonresponse at 96 weeks was seen in five patients in the 8-week group and one patient in the oral therapy group. There were also two patients in the 8-week group and one in the oral treatment group who met the criteria for virologic failure.
“Viral genotyping analysis for the patient in the oral treatment group had no treatment-emergent resistance mutations in the genes encoding viral reverse transcriptase, protease, or integrase,” wrote David A. Margolis, MD, from ViiV Healthcare, and his coauthors.
Injection-site pain was the most common adverse event in the injectable groups, experienced by 96%-97% of patients, but most reactions were mild or moderate and lasted a median of 3 days.
There was only one drug-related serious adverse event, which was a migraine that occurred in one patient during the oral induction period. None of the other serious adverse events were deemed to be drug-related.
Patients reported high levels of treatment satisfaction across all study groups, and more than 99% of patients in the injectable treatment arms said they would be highly satisfied to continue with the regimen, compared with 78% of patients in the oral treatment arm.
“The acceptability and tolerability of injectable dosing options will be an important component of long-term treatment success, and a high degree of treatment satisfaction will avail this option for patients burdened by life-long daily oral medication compliance,” the authors wrote.
However they did note that the study population was mostly white men with a CD4+ cell count at entry of at least 200 cells per mm.
“Therefore, the efficacy, safety, and pharmacokinetic outcomes of long-acting cabotegravir plus rilpivirine in different subpopulations infected with HIV-1 needs further evaluation,” the investigators concluded.
The study was funded by ViiV Healthcare and Janssen R&D. Nine authors are employees of ViiV Healthcare and shareholders in GlaxoSmithKline. Nine authors declared funding, grants, and honoraria from a range of pharmaceutical companies including ViiV Healthcare. Two authors are employees and shareholders of GlaxoSmithKline. Two authors are employees of Janssen, and stockholders of Johnson & Johnson, AstraZeneca, or GlaxoSmithKline. One author had no conflicts to disclose.
A long-acting injectable formulation combining two antiretroviral drugs – cabotegravir and rilpivirine – is as effective as a daily oral regimen of cabotegravir plus abacavir and lamivudine, according to interim results from the LATTE-2 trial.
Writing in the July 24 online edition of The Lancet, researchers have reported the 96-week results of the ongoing open-label phase 2b trial involving 286 treatment-naive adults infected with HIV-1.
At 32 weeks, 95% of patients in 8-week group, 94% of patients in the 4-week group, and 91% of patients in the oral therapy group had maintained viral suppression, defined as plasma HIV-1 RNA less than 50 copies per mL (Lancet. 2017 Jul 24. doi: 10.1016/S0140-6736[17]31917-7).
By week 96, viral suppression was seen in 94% of patients in the 8-week group, 87% of patients in the 4-week group and 84% of patients on oral treatment.
A nonresponse at 96 weeks was seen in five patients in the 8-week group and one patient in the oral therapy group. There were also two patients in the 8-week group and one in the oral treatment group who met the criteria for virologic failure.
“Viral genotyping analysis for the patient in the oral treatment group had no treatment-emergent resistance mutations in the genes encoding viral reverse transcriptase, protease, or integrase,” wrote David A. Margolis, MD, from ViiV Healthcare, and his coauthors.
Injection-site pain was the most common adverse event in the injectable groups, experienced by 96%-97% of patients, but most reactions were mild or moderate and lasted a median of 3 days.
There was only one drug-related serious adverse event, which was a migraine that occurred in one patient during the oral induction period. None of the other serious adverse events were deemed to be drug-related.
Patients reported high levels of treatment satisfaction across all study groups, and more than 99% of patients in the injectable treatment arms said they would be highly satisfied to continue with the regimen, compared with 78% of patients in the oral treatment arm.
“The acceptability and tolerability of injectable dosing options will be an important component of long-term treatment success, and a high degree of treatment satisfaction will avail this option for patients burdened by life-long daily oral medication compliance,” the authors wrote.
However they did note that the study population was mostly white men with a CD4+ cell count at entry of at least 200 cells per mm.
“Therefore, the efficacy, safety, and pharmacokinetic outcomes of long-acting cabotegravir plus rilpivirine in different subpopulations infected with HIV-1 needs further evaluation,” the investigators concluded.
The study was funded by ViiV Healthcare and Janssen R&D. Nine authors are employees of ViiV Healthcare and shareholders in GlaxoSmithKline. Nine authors declared funding, grants, and honoraria from a range of pharmaceutical companies including ViiV Healthcare. Two authors are employees and shareholders of GlaxoSmithKline. Two authors are employees of Janssen, and stockholders of Johnson & Johnson, AstraZeneca, or GlaxoSmithKline. One author had no conflicts to disclose.
FROM THE LANCET
Key clinical point: The long-acting injectable formulation of cabotegravir and rilpivirine is as effective as a daily oral regimen of cabotegravir plus abacavir and lamivudine.
Major finding: A long-acting injectable formulation of cabotegravir and rilpivirine, given either every 4 or 8 weeks, achieved similar levels of viral suppression to daily oral therapy with cabotegravir plus abacavir and lamivudine.
Data source: Randomized, open-label phase 2b study in 286 adults with HIV-1 infection.
Disclosures: This study was funded by ViiV Healthcare and Janssen R&D. Nine authors are employees of ViiV Healthcare and shareholders in GlaxoSmithKline. Nine authors declared funding, grants, and honoraria from a range of pharmaceutical companies including ViiV Healthcare. Two authors are employees and shareholders of GlaxoSmithKline. Two authors are employees of Janssen, and stockholders of Johnson & Johnson, AstraZeneca, or GlaxoSmithKline. One author had no conflicts to disclose.
Increased risk of death seen in PPI users
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2-receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, MPH, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.
Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2-receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2-receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total had only a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
The authors declared no relevant financial conflicts of interest.
Review AGA’s “Guide to Conversations About the Latest PPI Research Results” for tips on talking with your patients about this research study.
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2-receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, MPH, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.
Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2-receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2-receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total had only a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
The authors declared no relevant financial conflicts of interest.
Review AGA’s “Guide to Conversations About the Latest PPI Research Results” for tips on talking with your patients about this research study.
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2-receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, MPH, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.
Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2-receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2-receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total had only a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
The authors declared no relevant financial conflicts of interest.
Review AGA’s “Guide to Conversations About the Latest PPI Research Results” for tips on talking with your patients about this research study.
Study finds family history, chocolate intake increases acne risk
Having two parents with a history of acne was associated with an eightfold higher risk of acne during adolescence and young adulthood, in a European study that surveyed people aged 15-24 years in seven European countries.
Researchers conducted an online population-based survey of 10,521 individuals aged 15-24 years in Belgium, Czech and Slovak republics, France, Italy, Poland, and Spain, with questions about the presence or absence of acne, sociodemographic characteristics, and lifestyle factors (such as diet, tobacco, cannabis or alcohol use, and family history). The results were published online on July 14.
The overall prevalence of self-reported acne was 57.8%, with the highest prevalence – 65.8% – found in the 15- to 17-years age group, and the lowest being 52.6% in the 21- to 24-years age group. Individuals who reported having one parent with a history of acne had a threefold greater incidence of acne compared to those without a history of paternal or maternal acne (P less than .0001 for both). Individuals whose parents both had acne had a nearly eightfold higher risk, which the authors noted was consistent with other studies showing a strong hereditary component of acne (J Eur Acad Dermatol Venereol. 2017 Jul 14. doi: 10.1111/jdv.14475).
“Previous studies have demonstrated an association between high glycemic index foods and acne, although in our study, only chocolate, and not pasta or sweets, was independently associated in multivariate analysis,” wrote Pierre Wolkenstein, MD, of the department of dermatology, Hôpital Henri Mondor, Créteil, France, and his coauthors.
“The relationship between smoking and acne is not clear. Some observational studies have found that smoking increases the prevalence of acne, others have found a negative association, and some have found no relationship,” they added.
The study also showed significant variation in the incidence of acne across different countries. Using Spain, which had a median prevalence of acne, as a reference point, the researchers found that respondents in the Czech and Slovak republics had a 96% higher incidence of acne, while those in Poland had a 55% lower incidence.
The authors cautioned that their results were based on self-report, rather than a physician diagnosis, but they noted that since acne is so common, false positive or false negative reports were unlikely. “An association between self-reported acne and chocolate consumption, and an apparent inverse relationship with smoking, need to be confirmed by additional studies,” they noted.
The survey was funded and supported by Pierre Fabre Dermatologie. Five authors declared fees as members of the European Severe Acne Board, supported by Pierre Fabre Dermatologie, and one author is an employee of the company.
Having two parents with a history of acne was associated with an eightfold higher risk of acne during adolescence and young adulthood, in a European study that surveyed people aged 15-24 years in seven European countries.
Researchers conducted an online population-based survey of 10,521 individuals aged 15-24 years in Belgium, Czech and Slovak republics, France, Italy, Poland, and Spain, with questions about the presence or absence of acne, sociodemographic characteristics, and lifestyle factors (such as diet, tobacco, cannabis or alcohol use, and family history). The results were published online on July 14.
The overall prevalence of self-reported acne was 57.8%, with the highest prevalence – 65.8% – found in the 15- to 17-years age group, and the lowest being 52.6% in the 21- to 24-years age group. Individuals who reported having one parent with a history of acne had a threefold greater incidence of acne compared to those without a history of paternal or maternal acne (P less than .0001 for both). Individuals whose parents both had acne had a nearly eightfold higher risk, which the authors noted was consistent with other studies showing a strong hereditary component of acne (J Eur Acad Dermatol Venereol. 2017 Jul 14. doi: 10.1111/jdv.14475).
“Previous studies have demonstrated an association between high glycemic index foods and acne, although in our study, only chocolate, and not pasta or sweets, was independently associated in multivariate analysis,” wrote Pierre Wolkenstein, MD, of the department of dermatology, Hôpital Henri Mondor, Créteil, France, and his coauthors.
“The relationship between smoking and acne is not clear. Some observational studies have found that smoking increases the prevalence of acne, others have found a negative association, and some have found no relationship,” they added.
The study also showed significant variation in the incidence of acne across different countries. Using Spain, which had a median prevalence of acne, as a reference point, the researchers found that respondents in the Czech and Slovak republics had a 96% higher incidence of acne, while those in Poland had a 55% lower incidence.
The authors cautioned that their results were based on self-report, rather than a physician diagnosis, but they noted that since acne is so common, false positive or false negative reports were unlikely. “An association between self-reported acne and chocolate consumption, and an apparent inverse relationship with smoking, need to be confirmed by additional studies,” they noted.
The survey was funded and supported by Pierre Fabre Dermatologie. Five authors declared fees as members of the European Severe Acne Board, supported by Pierre Fabre Dermatologie, and one author is an employee of the company.
Having two parents with a history of acne was associated with an eightfold higher risk of acne during adolescence and young adulthood, in a European study that surveyed people aged 15-24 years in seven European countries.
Researchers conducted an online population-based survey of 10,521 individuals aged 15-24 years in Belgium, Czech and Slovak republics, France, Italy, Poland, and Spain, with questions about the presence or absence of acne, sociodemographic characteristics, and lifestyle factors (such as diet, tobacco, cannabis or alcohol use, and family history). The results were published online on July 14.
The overall prevalence of self-reported acne was 57.8%, with the highest prevalence – 65.8% – found in the 15- to 17-years age group, and the lowest being 52.6% in the 21- to 24-years age group. Individuals who reported having one parent with a history of acne had a threefold greater incidence of acne compared to those without a history of paternal or maternal acne (P less than .0001 for both). Individuals whose parents both had acne had a nearly eightfold higher risk, which the authors noted was consistent with other studies showing a strong hereditary component of acne (J Eur Acad Dermatol Venereol. 2017 Jul 14. doi: 10.1111/jdv.14475).
“Previous studies have demonstrated an association between high glycemic index foods and acne, although in our study, only chocolate, and not pasta or sweets, was independently associated in multivariate analysis,” wrote Pierre Wolkenstein, MD, of the department of dermatology, Hôpital Henri Mondor, Créteil, France, and his coauthors.
“The relationship between smoking and acne is not clear. Some observational studies have found that smoking increases the prevalence of acne, others have found a negative association, and some have found no relationship,” they added.
The study also showed significant variation in the incidence of acne across different countries. Using Spain, which had a median prevalence of acne, as a reference point, the researchers found that respondents in the Czech and Slovak republics had a 96% higher incidence of acne, while those in Poland had a 55% lower incidence.
The authors cautioned that their results were based on self-report, rather than a physician diagnosis, but they noted that since acne is so common, false positive or false negative reports were unlikely. “An association between self-reported acne and chocolate consumption, and an apparent inverse relationship with smoking, need to be confirmed by additional studies,” they noted.
The survey was funded and supported by Pierre Fabre Dermatologie. Five authors declared fees as members of the European Severe Acne Board, supported by Pierre Fabre Dermatologie, and one author is an employee of the company.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: A parental history of acne, higher levels of personal chocolate consumption, and younger age were significantly associated with an increased risk of acne.
Major finding: Adolescents and young adults with two parents with a history of acne have a nearly eightfold higher risk of the condition.
Data source: A population-based survey of 10,521 individuals aged 15-24 years in seven European countries.
Disclosures: Five authors declared fees as members of the European Severe Acne Board – supported by Pierre Fabre Dermatologie, which funded the survey – and one author is an employee of the company.
Liver cancer risk lower after sustained response to DAAs
Individuals with hepatitis C infection who achieved a sustained virologic response (SVR) to treatment with direct-acting antivirals had a significantly lower risk of hepatocellular carcinoma (HCC), a new study suggests.
A retrospective cohort study of 22,500 U.S. veterans with hepatitis C who had been treated with direct-acting antivirals (DAAs) found those with an SVR had a 72% lower risk of HCC, compared with those who did not achieve that response (hazard ratio, 0.28; 95% confidence interval, 0.22-0.36; P less than .0001), even after adjusting for demographics as well as clinical and health utilization factors.
“These data show that successful eradication of HCV [hepatitis C virus] confers a benefit in DAA-treated patients,” wrote Fasiha Kanwal, MD, from the Michael E. DeBakey Veterans Affairs Medical Center in Houston and her coauthors. “Although a few recent studies have raised concerns that DAA might accelerate the risk of HCC in some patients early in the course of treatment, we did not find any factors that differentiated patients with HCC that developed during DAA treatment.”
The results highlighted the importance of early treatment with antivirals, beginning well before the patients showed signs of progressing to advanced fibrosis or cirrhosis, the investigators noted.
“Delaying treatment until patients progress to cirrhosis might be associated with substantial downstream costs incurred as part of lifelong HCC surveillance and/or management of HCC,” they wrote.
Sustained virologic response to DAAs also was associated with a longer time to diagnosis, and patients who didn’t achieve it showed higher rates of cancer much earlier. The most common antivirals used were sofosbuvir (75.2%; 51.1% in combination with ledipasvir), the combination of paritaprevir/ritonavir (23.3%), daclatasvir-based treatments (0.8%), and simeprevir (0.7%).
While the patients achieved SVR that showed similarly beneficial effects on HCC risk in patients with or without cirrhosis, the authors also noted that patients with cirrhosis had a nearly fivefold greater risk of developing cancer than did those without (HR, 4.73; 95% CI, 3.34-6.68). Similarly, patients with a fibrosis score (FIB-4) greater than 3.25 had a sixfold higher risk of HCC, compared with those with a value of 1.45 or lower.
Researchers commented that, at this level of risk, surveillance for HCC in these patients may be cost effective.
“Based on these data, HCC surveillance or risk modification may be needed for all patients who have progressed to cirrhosis or advanced fibrosis (as indicated by high FIB-4) at the time of SVR,” they wrote.
Alcohol use was also associated with a significantly higher annual incidence of HCC (HR, 1.56; 95% CI, 1.11-2.18).
Among the study cohort, 39% had cirrhosis, 29.7% had advanced fibrosis, and nearly one-quarter had previously been treated for hepatitis C infection. More than 40% also had diabetes, 61.4% reported alcohol use, and 54.2% had a history of drug use.
“DAAs offer a chance of cure for all patients with HCV, including patients with advanced cirrhosis, older patients, and those with alcohol use – all characteristics independently associated with risk of HCC in HCV,” the authors explained. “These data show the treated population has changed significantly in the DAA era to include many patients with other HCC risk factors; these differences likely explain why the newer cohorts of DAA-treated patients face higher absolute HCC risk than expected, based on historic data.”
The study was partly supported by the Department of Veteran Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
The availability of direct-acting antivirals (DAAs) has revolutionized treatment of hepatitis C. Sustained virologic response (SVR) can be routinely achieved in more than 95% of patients – except in those with decompensated cirrhosis – with a 12-week course of these oral drugs, which have minimal adverse effects. Thus, guidelines recommend that all patients with hepatitis C should be treated with DAAs.1 It was a shock to the medical community when the recent Cochrane review concluded there was insufficient evidence to confirm or reject an effect of DAA therapy on HCV-related morbidity or all-cause mortality.2 The authors cautioned that the lack of valid evidence for DAAs’ effectiveness and the possibility of potential harm should be considered before treating people with hepatitis C with DAAs. Their conclusion was in part based on their rejection of SVR as a valid surrogate for clinical outcome. Previous studies of interferon-based therapies showed that SVR was associated with improvement in liver histology, decreased risk of hepatocellular carcinoma (HCC), and mortality.
Treatment of hepatitis C with DAAs represents one out of a handful of cases in which we can claim that a cure for a chronic disease is possible; however, treatment must be initiated early before advanced fibrosis or cirrhosis to prevent a persistent, though greatly reduced, risk of HCC. Physicians managing patients with hepatitis C should make treatment decisions based on evidence from the entire literature – which supports claims of the DAA treatment’s benefits and refutes allegations of its harmfulness – and should not be swayed by the misguided conclusions of the Cochrane review.
References
1. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed on July 2, 2017.
2. Jakobsen J.C., Nielsen E.E., Feinberg J., et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017 Jun 6;6:CD012143.
3. Curry M.P., O’Leary J.G., Bzowej N., et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618-28.
4. Kanwal F., Kramer J., Asch S.M., et al. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology. 2017 Jun 19. pii: S0016-5085(17)35797.
Anna S. Lok, MD, AGAF, FAASLD, is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine at the University of Michigan Health System in Ann Arbor. She has received research grants from Bristol-Myers Squibb and Gilead through the University of Michigan.
The availability of direct-acting antivirals (DAAs) has revolutionized treatment of hepatitis C. Sustained virologic response (SVR) can be routinely achieved in more than 95% of patients – except in those with decompensated cirrhosis – with a 12-week course of these oral drugs, which have minimal adverse effects. Thus, guidelines recommend that all patients with hepatitis C should be treated with DAAs.1 It was a shock to the medical community when the recent Cochrane review concluded there was insufficient evidence to confirm or reject an effect of DAA therapy on HCV-related morbidity or all-cause mortality.2 The authors cautioned that the lack of valid evidence for DAAs’ effectiveness and the possibility of potential harm should be considered before treating people with hepatitis C with DAAs. Their conclusion was in part based on their rejection of SVR as a valid surrogate for clinical outcome. Previous studies of interferon-based therapies showed that SVR was associated with improvement in liver histology, decreased risk of hepatocellular carcinoma (HCC), and mortality.
Treatment of hepatitis C with DAAs represents one out of a handful of cases in which we can claim that a cure for a chronic disease is possible; however, treatment must be initiated early before advanced fibrosis or cirrhosis to prevent a persistent, though greatly reduced, risk of HCC. Physicians managing patients with hepatitis C should make treatment decisions based on evidence from the entire literature – which supports claims of the DAA treatment’s benefits and refutes allegations of its harmfulness – and should not be swayed by the misguided conclusions of the Cochrane review.
References
1. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed on July 2, 2017.
2. Jakobsen J.C., Nielsen E.E., Feinberg J., et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017 Jun 6;6:CD012143.
3. Curry M.P., O’Leary J.G., Bzowej N., et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618-28.
4. Kanwal F., Kramer J., Asch S.M., et al. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology. 2017 Jun 19. pii: S0016-5085(17)35797.
Anna S. Lok, MD, AGAF, FAASLD, is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine at the University of Michigan Health System in Ann Arbor. She has received research grants from Bristol-Myers Squibb and Gilead through the University of Michigan.
The availability of direct-acting antivirals (DAAs) has revolutionized treatment of hepatitis C. Sustained virologic response (SVR) can be routinely achieved in more than 95% of patients – except in those with decompensated cirrhosis – with a 12-week course of these oral drugs, which have minimal adverse effects. Thus, guidelines recommend that all patients with hepatitis C should be treated with DAAs.1 It was a shock to the medical community when the recent Cochrane review concluded there was insufficient evidence to confirm or reject an effect of DAA therapy on HCV-related morbidity or all-cause mortality.2 The authors cautioned that the lack of valid evidence for DAAs’ effectiveness and the possibility of potential harm should be considered before treating people with hepatitis C with DAAs. Their conclusion was in part based on their rejection of SVR as a valid surrogate for clinical outcome. Previous studies of interferon-based therapies showed that SVR was associated with improvement in liver histology, decreased risk of hepatocellular carcinoma (HCC), and mortality.
Treatment of hepatitis C with DAAs represents one out of a handful of cases in which we can claim that a cure for a chronic disease is possible; however, treatment must be initiated early before advanced fibrosis or cirrhosis to prevent a persistent, though greatly reduced, risk of HCC. Physicians managing patients with hepatitis C should make treatment decisions based on evidence from the entire literature – which supports claims of the DAA treatment’s benefits and refutes allegations of its harmfulness – and should not be swayed by the misguided conclusions of the Cochrane review.
References
1. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed on July 2, 2017.
2. Jakobsen J.C., Nielsen E.E., Feinberg J., et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017 Jun 6;6:CD012143.
3. Curry M.P., O’Leary J.G., Bzowej N., et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618-28.
4. Kanwal F., Kramer J., Asch S.M., et al. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology. 2017 Jun 19. pii: S0016-5085(17)35797.
Anna S. Lok, MD, AGAF, FAASLD, is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine at the University of Michigan Health System in Ann Arbor. She has received research grants from Bristol-Myers Squibb and Gilead through the University of Michigan.
Individuals with hepatitis C infection who achieved a sustained virologic response (SVR) to treatment with direct-acting antivirals had a significantly lower risk of hepatocellular carcinoma (HCC), a new study suggests.
A retrospective cohort study of 22,500 U.S. veterans with hepatitis C who had been treated with direct-acting antivirals (DAAs) found those with an SVR had a 72% lower risk of HCC, compared with those who did not achieve that response (hazard ratio, 0.28; 95% confidence interval, 0.22-0.36; P less than .0001), even after adjusting for demographics as well as clinical and health utilization factors.
“These data show that successful eradication of HCV [hepatitis C virus] confers a benefit in DAA-treated patients,” wrote Fasiha Kanwal, MD, from the Michael E. DeBakey Veterans Affairs Medical Center in Houston and her coauthors. “Although a few recent studies have raised concerns that DAA might accelerate the risk of HCC in some patients early in the course of treatment, we did not find any factors that differentiated patients with HCC that developed during DAA treatment.”
The results highlighted the importance of early treatment with antivirals, beginning well before the patients showed signs of progressing to advanced fibrosis or cirrhosis, the investigators noted.
“Delaying treatment until patients progress to cirrhosis might be associated with substantial downstream costs incurred as part of lifelong HCC surveillance and/or management of HCC,” they wrote.
Sustained virologic response to DAAs also was associated with a longer time to diagnosis, and patients who didn’t achieve it showed higher rates of cancer much earlier. The most common antivirals used were sofosbuvir (75.2%; 51.1% in combination with ledipasvir), the combination of paritaprevir/ritonavir (23.3%), daclatasvir-based treatments (0.8%), and simeprevir (0.7%).
While the patients achieved SVR that showed similarly beneficial effects on HCC risk in patients with or without cirrhosis, the authors also noted that patients with cirrhosis had a nearly fivefold greater risk of developing cancer than did those without (HR, 4.73; 95% CI, 3.34-6.68). Similarly, patients with a fibrosis score (FIB-4) greater than 3.25 had a sixfold higher risk of HCC, compared with those with a value of 1.45 or lower.
Researchers commented that, at this level of risk, surveillance for HCC in these patients may be cost effective.
“Based on these data, HCC surveillance or risk modification may be needed for all patients who have progressed to cirrhosis or advanced fibrosis (as indicated by high FIB-4) at the time of SVR,” they wrote.
Alcohol use was also associated with a significantly higher annual incidence of HCC (HR, 1.56; 95% CI, 1.11-2.18).
Among the study cohort, 39% had cirrhosis, 29.7% had advanced fibrosis, and nearly one-quarter had previously been treated for hepatitis C infection. More than 40% also had diabetes, 61.4% reported alcohol use, and 54.2% had a history of drug use.
“DAAs offer a chance of cure for all patients with HCV, including patients with advanced cirrhosis, older patients, and those with alcohol use – all characteristics independently associated with risk of HCC in HCV,” the authors explained. “These data show the treated population has changed significantly in the DAA era to include many patients with other HCC risk factors; these differences likely explain why the newer cohorts of DAA-treated patients face higher absolute HCC risk than expected, based on historic data.”
The study was partly supported by the Department of Veteran Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
Individuals with hepatitis C infection who achieved a sustained virologic response (SVR) to treatment with direct-acting antivirals had a significantly lower risk of hepatocellular carcinoma (HCC), a new study suggests.
A retrospective cohort study of 22,500 U.S. veterans with hepatitis C who had been treated with direct-acting antivirals (DAAs) found those with an SVR had a 72% lower risk of HCC, compared with those who did not achieve that response (hazard ratio, 0.28; 95% confidence interval, 0.22-0.36; P less than .0001), even after adjusting for demographics as well as clinical and health utilization factors.
“These data show that successful eradication of HCV [hepatitis C virus] confers a benefit in DAA-treated patients,” wrote Fasiha Kanwal, MD, from the Michael E. DeBakey Veterans Affairs Medical Center in Houston and her coauthors. “Although a few recent studies have raised concerns that DAA might accelerate the risk of HCC in some patients early in the course of treatment, we did not find any factors that differentiated patients with HCC that developed during DAA treatment.”
The results highlighted the importance of early treatment with antivirals, beginning well before the patients showed signs of progressing to advanced fibrosis or cirrhosis, the investigators noted.
“Delaying treatment until patients progress to cirrhosis might be associated with substantial downstream costs incurred as part of lifelong HCC surveillance and/or management of HCC,” they wrote.
Sustained virologic response to DAAs also was associated with a longer time to diagnosis, and patients who didn’t achieve it showed higher rates of cancer much earlier. The most common antivirals used were sofosbuvir (75.2%; 51.1% in combination with ledipasvir), the combination of paritaprevir/ritonavir (23.3%), daclatasvir-based treatments (0.8%), and simeprevir (0.7%).
While the patients achieved SVR that showed similarly beneficial effects on HCC risk in patients with or without cirrhosis, the authors also noted that patients with cirrhosis had a nearly fivefold greater risk of developing cancer than did those without (HR, 4.73; 95% CI, 3.34-6.68). Similarly, patients with a fibrosis score (FIB-4) greater than 3.25 had a sixfold higher risk of HCC, compared with those with a value of 1.45 or lower.
Researchers commented that, at this level of risk, surveillance for HCC in these patients may be cost effective.
“Based on these data, HCC surveillance or risk modification may be needed for all patients who have progressed to cirrhosis or advanced fibrosis (as indicated by high FIB-4) at the time of SVR,” they wrote.
Alcohol use was also associated with a significantly higher annual incidence of HCC (HR, 1.56; 95% CI, 1.11-2.18).
Among the study cohort, 39% had cirrhosis, 29.7% had advanced fibrosis, and nearly one-quarter had previously been treated for hepatitis C infection. More than 40% also had diabetes, 61.4% reported alcohol use, and 54.2% had a history of drug use.
“DAAs offer a chance of cure for all patients with HCV, including patients with advanced cirrhosis, older patients, and those with alcohol use – all characteristics independently associated with risk of HCC in HCV,” the authors explained. “These data show the treated population has changed significantly in the DAA era to include many patients with other HCC risk factors; these differences likely explain why the newer cohorts of DAA-treated patients face higher absolute HCC risk than expected, based on historic data.”
The study was partly supported by the Department of Veteran Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: Individuals who achieved an SVR to antiviral treatment for hepatitis C infection had a 72% lower risk of hepatocellular carcinoma than those who do not show a sustained response.
Data source: Retrospective cohort study in 22,500 U.S. veterans with hepatitis C.
Disclosures: The study was partly supported by the Department of Veterans Affairs’ Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center. No conflicts of interest were declared.
Subsequent squamous cell carcinoma risk higher in HIV patients with low CD4 count
HIV-infected individuals who have experienced a nonmelanoma skin cancer may be at significantly greater risk of subsequent new squamous cell carcinoma if they have a lower CD4 cell count, a new study suggests.
In a study published online July 12 in JAMA Dermatology, researchers reported the results of a retrospective cohort study using medical record data from 455 HIV-infected and 1,952 HIV-uninfected patients who had previously been diagnosed with at least one nonmelanoma skin cancer.
Patients with CD4 cell counts below 200 cells/mcL had a 44% greater risk of a subsequent nonmelanoma skin cancer, compared with uninfected individuals (95% confidence interval, 1.10-1.88), while those with a viral load greater than 10,000 copies/mL had a 31% greater risk (95% CI, 1.00-1.72), after adjusting for age, sex, smoking status, and obesity.
The increase in nonmelanoma skin cancer risk was largely accounted for by an increased risk of squamous cell carcinoma (SCC). Among patients with lower CD4 cell counts and those with higher viral loads, the risk of SCC was more than twofold greater than among uninfected individuals. In contrast, while there was a trend toward a higher risk of basal cell carcinoma in those two groups, it did not reach significance (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1716).
Overall, HIV-infected individuals had a significant 15% increase in the risk of subsequent nonmelanoma skin cancer over an average follow-up period of 5 years, compared with uninfected individuals.
“This study addresses key questions regarding subsequent NMSC [nonmelanoma skin cancer] risk among a high-risk subgroup of HIV-infected population who, by virtue of having had a pathologically validated skin cancer, are at increased risk of subsequent NMSCs,” wrote Maryam M. Asgari, MD, of Massachusetts General Hospital, Boston, and her coauthors. “Specifically, it was previously not known precisely which NMSC subtype is increased in high-risk persons with HIV and whether biomarkers of HIV infections, such as degree of immune dysfunction, are associated with subsequent skin cancer risk.”
While the study wasn’t able to control for known skin cancer risk factors such as skin type, the patients were all non-Hispanic white, which the authors said selected for individuals with fair skin and some sun-exposure history.
The findings could have implications for skin cancer screening among individuals with HIV infection, with the authors suggesting more targeted monitoring for SCC among individuals with low CD4 counts or high viral loads.
“Our findings support immune dysfunction as a risk factor for SCCs and dovetail with SCC risk data from iatrogenic immunodeficiency states, such as organ transplantation and autoimmune diseases.”
The study was partly supported by Kaiser Permanente Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
HIV-infected individuals who have experienced a nonmelanoma skin cancer may be at significantly greater risk of subsequent new squamous cell carcinoma if they have a lower CD4 cell count, a new study suggests.
In a study published online July 12 in JAMA Dermatology, researchers reported the results of a retrospective cohort study using medical record data from 455 HIV-infected and 1,952 HIV-uninfected patients who had previously been diagnosed with at least one nonmelanoma skin cancer.
Patients with CD4 cell counts below 200 cells/mcL had a 44% greater risk of a subsequent nonmelanoma skin cancer, compared with uninfected individuals (95% confidence interval, 1.10-1.88), while those with a viral load greater than 10,000 copies/mL had a 31% greater risk (95% CI, 1.00-1.72), after adjusting for age, sex, smoking status, and obesity.
The increase in nonmelanoma skin cancer risk was largely accounted for by an increased risk of squamous cell carcinoma (SCC). Among patients with lower CD4 cell counts and those with higher viral loads, the risk of SCC was more than twofold greater than among uninfected individuals. In contrast, while there was a trend toward a higher risk of basal cell carcinoma in those two groups, it did not reach significance (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1716).
Overall, HIV-infected individuals had a significant 15% increase in the risk of subsequent nonmelanoma skin cancer over an average follow-up period of 5 years, compared with uninfected individuals.
“This study addresses key questions regarding subsequent NMSC [nonmelanoma skin cancer] risk among a high-risk subgroup of HIV-infected population who, by virtue of having had a pathologically validated skin cancer, are at increased risk of subsequent NMSCs,” wrote Maryam M. Asgari, MD, of Massachusetts General Hospital, Boston, and her coauthors. “Specifically, it was previously not known precisely which NMSC subtype is increased in high-risk persons with HIV and whether biomarkers of HIV infections, such as degree of immune dysfunction, are associated with subsequent skin cancer risk.”
While the study wasn’t able to control for known skin cancer risk factors such as skin type, the patients were all non-Hispanic white, which the authors said selected for individuals with fair skin and some sun-exposure history.
The findings could have implications for skin cancer screening among individuals with HIV infection, with the authors suggesting more targeted monitoring for SCC among individuals with low CD4 counts or high viral loads.
“Our findings support immune dysfunction as a risk factor for SCCs and dovetail with SCC risk data from iatrogenic immunodeficiency states, such as organ transplantation and autoimmune diseases.”
The study was partly supported by Kaiser Permanente Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
HIV-infected individuals who have experienced a nonmelanoma skin cancer may be at significantly greater risk of subsequent new squamous cell carcinoma if they have a lower CD4 cell count, a new study suggests.
In a study published online July 12 in JAMA Dermatology, researchers reported the results of a retrospective cohort study using medical record data from 455 HIV-infected and 1,952 HIV-uninfected patients who had previously been diagnosed with at least one nonmelanoma skin cancer.
Patients with CD4 cell counts below 200 cells/mcL had a 44% greater risk of a subsequent nonmelanoma skin cancer, compared with uninfected individuals (95% confidence interval, 1.10-1.88), while those with a viral load greater than 10,000 copies/mL had a 31% greater risk (95% CI, 1.00-1.72), after adjusting for age, sex, smoking status, and obesity.
The increase in nonmelanoma skin cancer risk was largely accounted for by an increased risk of squamous cell carcinoma (SCC). Among patients with lower CD4 cell counts and those with higher viral loads, the risk of SCC was more than twofold greater than among uninfected individuals. In contrast, while there was a trend toward a higher risk of basal cell carcinoma in those two groups, it did not reach significance (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1716).
Overall, HIV-infected individuals had a significant 15% increase in the risk of subsequent nonmelanoma skin cancer over an average follow-up period of 5 years, compared with uninfected individuals.
“This study addresses key questions regarding subsequent NMSC [nonmelanoma skin cancer] risk among a high-risk subgroup of HIV-infected population who, by virtue of having had a pathologically validated skin cancer, are at increased risk of subsequent NMSCs,” wrote Maryam M. Asgari, MD, of Massachusetts General Hospital, Boston, and her coauthors. “Specifically, it was previously not known precisely which NMSC subtype is increased in high-risk persons with HIV and whether biomarkers of HIV infections, such as degree of immune dysfunction, are associated with subsequent skin cancer risk.”
While the study wasn’t able to control for known skin cancer risk factors such as skin type, the patients were all non-Hispanic white, which the authors said selected for individuals with fair skin and some sun-exposure history.
The findings could have implications for skin cancer screening among individuals with HIV infection, with the authors suggesting more targeted monitoring for SCC among individuals with low CD4 counts or high viral loads.
“Our findings support immune dysfunction as a risk factor for SCCs and dovetail with SCC risk data from iatrogenic immunodeficiency states, such as organ transplantation and autoimmune diseases.”
The study was partly supported by Kaiser Permanente Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
FROM JAMA DERMATOLOGY
Key clinical point: HIV-infected people who have had a previous nonmelanoma skin cancer are at significantly higher risk of subsequent SCC if they have a lower CD4 count or higher viral load.
Major finding: HIV-infected people with a low CD4 cell count or high viral load have a greater than twofold increased risk of subsequent SCC after a primary nonmelanoma skin cancer than do uninfected people who have had a previous nonmelanoma skin cancer.
Data source: A retrospective cohort study in 455 HIV-infected and 1,945 HIV-uninfected patients.
Disclosures: The study was partly supported by Kaiser Permanente, Northern California, and one author was supported by a grant from the National Cancer Institute. Two authors had previously served as investigators on studies funded by the pharmaceutical industry, one author declared research funding from the pharmaceutical industry, and one declared shares in two medical companies.
Increased risk of death even in lower-risk PPI users
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2 receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, PhD, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.
“Whether PPI use is associated with excess risk of death is not known and has not been examined in large epidemiological studies spanning a sufficiently long duration of follow-up.”
In this study, a cohort of 349,312 veterans initiated on acid-suppression therapy was followed for a mean duration of 5.71 years (BMJ Open 2017. July 4;7:e015735. doi: 10.1136/bmjopen-2016-015735).
Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2 receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.
When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2 receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total only had a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.
“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
No conflicts of interest were declared.
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2 receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, PhD, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.
“Whether PPI use is associated with excess risk of death is not known and has not been examined in large epidemiological studies spanning a sufficiently long duration of follow-up.”
In this study, a cohort of 349,312 veterans initiated on acid-suppression therapy was followed for a mean duration of 5.71 years (BMJ Open 2017. July 4;7:e015735. doi: 10.1136/bmjopen-2016-015735).
Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2 receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.
When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2 receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total only had a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.
“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
No conflicts of interest were declared.
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2 receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, PhD, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.
“Whether PPI use is associated with excess risk of death is not known and has not been examined in large epidemiological studies spanning a sufficiently long duration of follow-up.”
In this study, a cohort of 349,312 veterans initiated on acid-suppression therapy was followed for a mean duration of 5.71 years (BMJ Open 2017. July 4;7:e015735. doi: 10.1136/bmjopen-2016-015735).
Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2 receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.
When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2 receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total only had a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.
“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
No conflicts of interest were declared.
FROM BMJ OPEN
Key clinical point: Proton pump inhibitors are associated with a significantly higher risk of death, even among people without the gastrointestinal conditions for which the drugs are normally prescribed.
Major finding: People taking PPIs have a 25% higher risk of death, compared with those taking H2 receptor antagonists.
Data source: A longitudinal cohort study in 349,312 veterans.
Disclosures: No conflicts of interest were declared.
July 2017: Click for Credit
Here are 6 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. High-dose Oral Vitamin D3 Significantly Reduced Effects of Sunburn
To take the posttest, go to: http://bit.ly/2tmDiKc
Expires May 23, 2018
2. Women Less Likely to Be Diagnosed With Sleep Disorders
To take the posttest, go to: http://bit.ly/2rgLdne
Expires May 30, 2018
3. RA Treatment Delays Raise Risk for Long-term Disability
To take the posttest, go to: http://bit.ly/2tC0IGF
Expires May 30, 2018
4. Target Self-medication of Mood and Anxiety Symptoms
To take the posttest, go to: http://bit.ly/2vy5jel
Expires May 2, 2018
5. Two New Biomarkers for Breast Cancer Show Validity
To take the posttest, go to: http://bit.ly/2ve9H2L
Expires May 2, 2018
6. Time to Therapy for Gram-positive Bacteremia Reduced From 60 Hours to 4 Hours
To take the posttest, go to: http://bit.ly/2ssacIf
Expires May 25, 2018
Here are 6 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. High-dose Oral Vitamin D3 Significantly Reduced Effects of Sunburn
To take the posttest, go to: http://bit.ly/2tmDiKc
Expires May 23, 2018
2. Women Less Likely to Be Diagnosed With Sleep Disorders
To take the posttest, go to: http://bit.ly/2rgLdne
Expires May 30, 2018
3. RA Treatment Delays Raise Risk for Long-term Disability
To take the posttest, go to: http://bit.ly/2tC0IGF
Expires May 30, 2018
4. Target Self-medication of Mood and Anxiety Symptoms
To take the posttest, go to: http://bit.ly/2vy5jel
Expires May 2, 2018
5. Two New Biomarkers for Breast Cancer Show Validity
To take the posttest, go to: http://bit.ly/2ve9H2L
Expires May 2, 2018
6. Time to Therapy for Gram-positive Bacteremia Reduced From 60 Hours to 4 Hours
To take the posttest, go to: http://bit.ly/2ssacIf
Expires May 25, 2018
Here are 6 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. High-dose Oral Vitamin D3 Significantly Reduced Effects of Sunburn
To take the posttest, go to: http://bit.ly/2tmDiKc
Expires May 23, 2018
2. Women Less Likely to Be Diagnosed With Sleep Disorders
To take the posttest, go to: http://bit.ly/2rgLdne
Expires May 30, 2018
3. RA Treatment Delays Raise Risk for Long-term Disability
To take the posttest, go to: http://bit.ly/2tC0IGF
Expires May 30, 2018
4. Target Self-medication of Mood and Anxiety Symptoms
To take the posttest, go to: http://bit.ly/2vy5jel
Expires May 2, 2018
5. Two New Biomarkers for Breast Cancer Show Validity
To take the posttest, go to: http://bit.ly/2ve9H2L
Expires May 2, 2018
6. Time to Therapy for Gram-positive Bacteremia Reduced From 60 Hours to 4 Hours
To take the posttest, go to: http://bit.ly/2ssacIf
Expires May 25, 2018
Guidelines on classification, diagnosis, and treatment of acne fulminans
The incidence of acne fulminans may be decreasing but the isotretinoin-induced form is on the rise, say the authors of new guidelines on this severe variant of inflammatory acne.
Writing in the July issue of the Journal of the American Academy of Dermatology, Tanya Greywal, MD, from the University of California, San Diego, and coauthors, presented guidelines that aimed to better characterize this relatively rare condition and provide guidance on classification, treatment, and prevention.
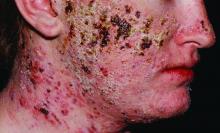
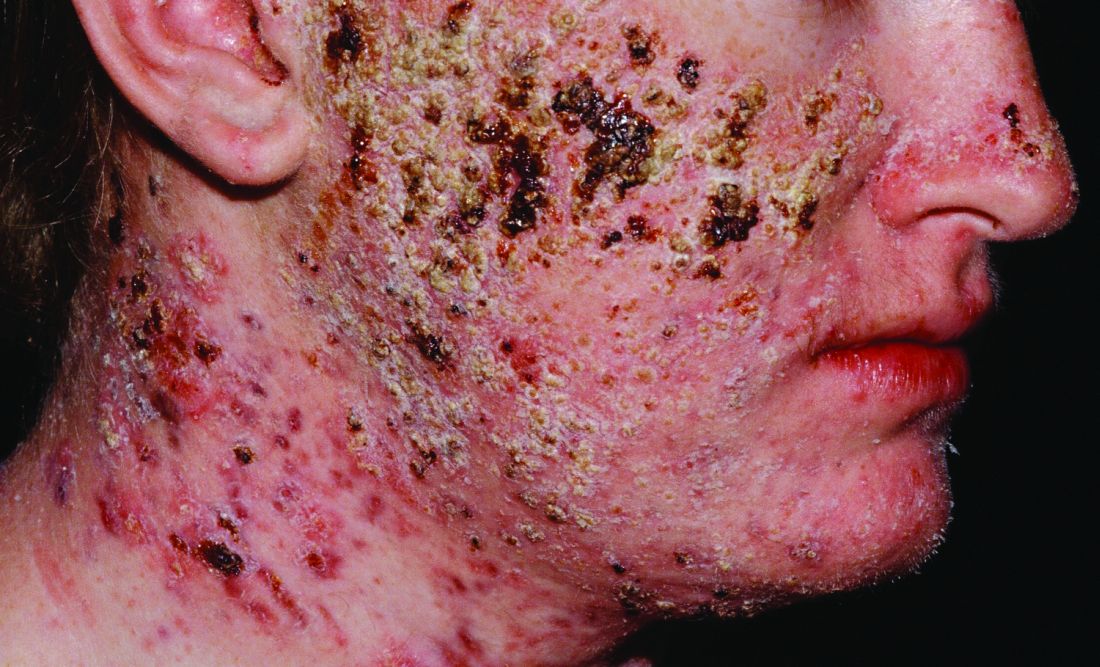
Acne fulminans presents with rapid onset of painful erosions and hemorrhagic crusts – usually on the trunk – that can lead to severe, disfiguring scars. A more extreme form of the disorder also features systemic inflammation including fever, arthralgia, osteolytic bony lesions, and can even require hospitalization.
It is most commonly seen in white adolescent males, many of whom have a prior history of acne (J Am Acad Dermatol. 2017;77:109-17).
While alterations in innate immunity, autoimmunity, adaptive immunity, and autoinflammation have all been suggested as playing a role in the pathogenesis of acne fulminans, it can also be associated with isotretinoin use.
The overall incidence of the disorder has been decreasing over the last decade, which some suggest may be because the disease is being recognized and treated earlier. However, isotretinoin-induced acne fulminans is on the rise, perhaps because of more widespread use of the drug.
Acne fulminans previously has been described with a range of terms, including acne maligna, acute febrile ulcerative acne conglobota, and pseudo-acne fulminans, which the authors said has led to some confusion.
However, the expert panel behind the guidelines proposed that acne fulminans should be classified as being either with or without systemic symptoms, and either isotretinoin-induced or not.
They also recognized a range of associated disorders, including SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), and PASH (pyoderma gangrenosum, acne, and hidradenitis suppurativa).
In the absence of large-scale randomized controlled trials of treatments for acne fulminans, the authors said case series and individual reports supported the use of systemic corticosteroids in combination with isotretinoin when treating all forms of the disorder.
The expert group recommended starting patients on prednisone 0.5 to 1 mg/kg per day as monotherapy for at least 4 weeks for acne fulminans with systemic symptoms, and for at least 2 weeks in the absence of systemic symptoms.
They proposed a typical isotretinoin cumulative goal dose of 120-150 mg/kg, starting at a lower dose and gradually increasing, and overlapping with prednisone.
Case studies suggest that tetracyclines are minimally effective against acne fulminans, but the authors said there was a need for studies to examine whether the use of antibiotics overlapping with isotretinoin might reduce the development of isotretinoin-induced acne fulminans.
No funding or conflicts of interest were declared.
The incidence of acne fulminans may be decreasing but the isotretinoin-induced form is on the rise, say the authors of new guidelines on this severe variant of inflammatory acne.
Writing in the July issue of the Journal of the American Academy of Dermatology, Tanya Greywal, MD, from the University of California, San Diego, and coauthors, presented guidelines that aimed to better characterize this relatively rare condition and provide guidance on classification, treatment, and prevention.
Acne fulminans presents with rapid onset of painful erosions and hemorrhagic crusts – usually on the trunk – that can lead to severe, disfiguring scars. A more extreme form of the disorder also features systemic inflammation including fever, arthralgia, osteolytic bony lesions, and can even require hospitalization.
It is most commonly seen in white adolescent males, many of whom have a prior history of acne (J Am Acad Dermatol. 2017;77:109-17).
While alterations in innate immunity, autoimmunity, adaptive immunity, and autoinflammation have all been suggested as playing a role in the pathogenesis of acne fulminans, it can also be associated with isotretinoin use.
The overall incidence of the disorder has been decreasing over the last decade, which some suggest may be because the disease is being recognized and treated earlier. However, isotretinoin-induced acne fulminans is on the rise, perhaps because of more widespread use of the drug.
Acne fulminans previously has been described with a range of terms, including acne maligna, acute febrile ulcerative acne conglobota, and pseudo-acne fulminans, which the authors said has led to some confusion.
However, the expert panel behind the guidelines proposed that acne fulminans should be classified as being either with or without systemic symptoms, and either isotretinoin-induced or not.
They also recognized a range of associated disorders, including SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), and PASH (pyoderma gangrenosum, acne, and hidradenitis suppurativa).
In the absence of large-scale randomized controlled trials of treatments for acne fulminans, the authors said case series and individual reports supported the use of systemic corticosteroids in combination with isotretinoin when treating all forms of the disorder.
The expert group recommended starting patients on prednisone 0.5 to 1 mg/kg per day as monotherapy for at least 4 weeks for acne fulminans with systemic symptoms, and for at least 2 weeks in the absence of systemic symptoms.
They proposed a typical isotretinoin cumulative goal dose of 120-150 mg/kg, starting at a lower dose and gradually increasing, and overlapping with prednisone.
Case studies suggest that tetracyclines are minimally effective against acne fulminans, but the authors said there was a need for studies to examine whether the use of antibiotics overlapping with isotretinoin might reduce the development of isotretinoin-induced acne fulminans.
No funding or conflicts of interest were declared.
The incidence of acne fulminans may be decreasing but the isotretinoin-induced form is on the rise, say the authors of new guidelines on this severe variant of inflammatory acne.
Writing in the July issue of the Journal of the American Academy of Dermatology, Tanya Greywal, MD, from the University of California, San Diego, and coauthors, presented guidelines that aimed to better characterize this relatively rare condition and provide guidance on classification, treatment, and prevention.
Acne fulminans presents with rapid onset of painful erosions and hemorrhagic crusts – usually on the trunk – that can lead to severe, disfiguring scars. A more extreme form of the disorder also features systemic inflammation including fever, arthralgia, osteolytic bony lesions, and can even require hospitalization.
It is most commonly seen in white adolescent males, many of whom have a prior history of acne (J Am Acad Dermatol. 2017;77:109-17).
While alterations in innate immunity, autoimmunity, adaptive immunity, and autoinflammation have all been suggested as playing a role in the pathogenesis of acne fulminans, it can also be associated with isotretinoin use.
The overall incidence of the disorder has been decreasing over the last decade, which some suggest may be because the disease is being recognized and treated earlier. However, isotretinoin-induced acne fulminans is on the rise, perhaps because of more widespread use of the drug.
Acne fulminans previously has been described with a range of terms, including acne maligna, acute febrile ulcerative acne conglobota, and pseudo-acne fulminans, which the authors said has led to some confusion.
However, the expert panel behind the guidelines proposed that acne fulminans should be classified as being either with or without systemic symptoms, and either isotretinoin-induced or not.
They also recognized a range of associated disorders, including SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), and PASH (pyoderma gangrenosum, acne, and hidradenitis suppurativa).
In the absence of large-scale randomized controlled trials of treatments for acne fulminans, the authors said case series and individual reports supported the use of systemic corticosteroids in combination with isotretinoin when treating all forms of the disorder.
The expert group recommended starting patients on prednisone 0.5 to 1 mg/kg per day as monotherapy for at least 4 weeks for acne fulminans with systemic symptoms, and for at least 2 weeks in the absence of systemic symptoms.
They proposed a typical isotretinoin cumulative goal dose of 120-150 mg/kg, starting at a lower dose and gradually increasing, and overlapping with prednisone.
Case studies suggest that tetracyclines are minimally effective against acne fulminans, but the authors said there was a need for studies to examine whether the use of antibiotics overlapping with isotretinoin might reduce the development of isotretinoin-induced acne fulminans.
No funding or conflicts of interest were declared.
FROM THE JOURNAL OF AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Experts have presented evidence-based guidelines on the diagnosis, classification, and treatment of acne fulminans.
Major finding: Acne fulminans is classified as either presenting with or without systemic symptoms, and being either isotretinoin-induced or not.
Data source: Review.
Disclosures: No conflicts of interest were declared.
Weekly buprenorphine depot effective for opioid use disorder
A weekly subcutaneous buprenorphine depot could improve adherence and reduce the potential for misuse, according to a study presented at the annual meeting of the College on Problems of Drug Dependence and published simultaneously June 22 in JAMA Psychiatry.
In a phase II, double-blind, randomized study, 47 adults with moderate to severe opioid use disorder were randomized to a weekly dose of the subcutaneous buprenorphine depot CAM2038 – either 24 mg or 32 mg – for 2 weeks. They also underwent five 3-day test sessions to evaluate their response to 6 mg and 18 mg doses of intramuscular hydromorphone and a 0 mg control. One qualification session was held before randomization and the remaining four sessions after.
The primary outcome of the study was drug liking, and both doses of CAM2038 were associated with immediate, sustained, and dose-related suppression of participants’ response to hydromorphone (JAMA Psychiatry. 2017 June 22. doi:10.1001/jamapsychiatry.2017.1874).
The depot also appeared to block the dose-dependent increase in drowsiness that was seen in response to hydromorphone in the qualification session.
However, there was a reversal of the dose-dependent response in participants’ desire to use opioids, with greater suppression seen at the lower depot dose.
Buprenorphine is known to be safer than methadone for the treatment of opioid use disorders, but Sharon L. Walsh, PhD, of the Center on Drug and Alcohol Research at the University of Kentucky, Lexington, and her coauthors said sublingual buprenorphine itself has become an abuse liability in some countries.
“Sublingual formulations of buprenorphine can be injected or snorted to enhance euphoric effects,” they wrote. “Unintentional overdose with buprenorphine has been reported, leading to toxicity and fatality in children and those who coingest buprenorphine with benzodiazepines or alcohol.”
The results mean the formulation meets the U.S. Food and Drug Administration criteria for complete opioid blockade.
CAM2038 achieved complete suppression of opioid withdrawal at both doses, and Clinical Opiate Withdrawal Scale (COWS) remained suppressed for the entire duration of the study.
“During treatment initiation, it is important that withdrawal symptoms are well-controlled,” the authors wrote. “The COWS and [Objective Opioid Withdrawal Scale] scores were reduced to near zero on the first dosing day with suppression thereafter.”
The mean COWS preinjection score was 11, which did not include five participants who accidentally were inducted with CAM2038 before they had achieved the prespecified criteria of a COWS score of 8 or above.
They also noted that the pharmacokinetic profile of CAM2038 showed gradually increasing buprenorphine concentrations, reaching maximum at around 24 hours after the dose was given. Perhaps because of this effect – which they likened to a de factor induction procedure mimicking the recommended induction with sublingual buprenorphine – the authors suggested that patients using CAM2038 could likely be inducted directly using the depot.
Researchers also looked at some key physiological outcomes before and after treatment with the depot. They found that, while patients showed significant dose-dependent reductions in oxygen saturation with hydromorphone before receiving depot treatment, these were significantly attenuated after treatment with CAM2038.
One subject withdrew from the study because of ventricular extrasystoles, and one showed abnormal liver function at discharge that was later attributed a hepatitis C diagnosis. Neither event was linked to the study drug.
However, 81% of patients experienced at least one adverse event, with significantly more events reported in the higher dose group. The most common reported events that were possibly related to the study drug were constipation, injection site pain and erythema, and headache. Most were rated as mild.
The College on Problems of Drug Dependence, a nonprofit corporation, is holding its annual meeting in Montreal. The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus, and other pharmaceutical companies.
A weekly subcutaneous buprenorphine depot could improve adherence and reduce the potential for misuse, according to a study presented at the annual meeting of the College on Problems of Drug Dependence and published simultaneously June 22 in JAMA Psychiatry.
In a phase II, double-blind, randomized study, 47 adults with moderate to severe opioid use disorder were randomized to a weekly dose of the subcutaneous buprenorphine depot CAM2038 – either 24 mg or 32 mg – for 2 weeks. They also underwent five 3-day test sessions to evaluate their response to 6 mg and 18 mg doses of intramuscular hydromorphone and a 0 mg control. One qualification session was held before randomization and the remaining four sessions after.
The primary outcome of the study was drug liking, and both doses of CAM2038 were associated with immediate, sustained, and dose-related suppression of participants’ response to hydromorphone (JAMA Psychiatry. 2017 June 22. doi:10.1001/jamapsychiatry.2017.1874).
The depot also appeared to block the dose-dependent increase in drowsiness that was seen in response to hydromorphone in the qualification session.
However, there was a reversal of the dose-dependent response in participants’ desire to use opioids, with greater suppression seen at the lower depot dose.
Buprenorphine is known to be safer than methadone for the treatment of opioid use disorders, but Sharon L. Walsh, PhD, of the Center on Drug and Alcohol Research at the University of Kentucky, Lexington, and her coauthors said sublingual buprenorphine itself has become an abuse liability in some countries.
“Sublingual formulations of buprenorphine can be injected or snorted to enhance euphoric effects,” they wrote. “Unintentional overdose with buprenorphine has been reported, leading to toxicity and fatality in children and those who coingest buprenorphine with benzodiazepines or alcohol.”
The results mean the formulation meets the U.S. Food and Drug Administration criteria for complete opioid blockade.
CAM2038 achieved complete suppression of opioid withdrawal at both doses, and Clinical Opiate Withdrawal Scale (COWS) remained suppressed for the entire duration of the study.
“During treatment initiation, it is important that withdrawal symptoms are well-controlled,” the authors wrote. “The COWS and [Objective Opioid Withdrawal Scale] scores were reduced to near zero on the first dosing day with suppression thereafter.”
The mean COWS preinjection score was 11, which did not include five participants who accidentally were inducted with CAM2038 before they had achieved the prespecified criteria of a COWS score of 8 or above.
They also noted that the pharmacokinetic profile of CAM2038 showed gradually increasing buprenorphine concentrations, reaching maximum at around 24 hours after the dose was given. Perhaps because of this effect – which they likened to a de factor induction procedure mimicking the recommended induction with sublingual buprenorphine – the authors suggested that patients using CAM2038 could likely be inducted directly using the depot.
Researchers also looked at some key physiological outcomes before and after treatment with the depot. They found that, while patients showed significant dose-dependent reductions in oxygen saturation with hydromorphone before receiving depot treatment, these were significantly attenuated after treatment with CAM2038.
One subject withdrew from the study because of ventricular extrasystoles, and one showed abnormal liver function at discharge that was later attributed a hepatitis C diagnosis. Neither event was linked to the study drug.
However, 81% of patients experienced at least one adverse event, with significantly more events reported in the higher dose group. The most common reported events that were possibly related to the study drug were constipation, injection site pain and erythema, and headache. Most were rated as mild.
The College on Problems of Drug Dependence, a nonprofit corporation, is holding its annual meeting in Montreal. The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus, and other pharmaceutical companies.
A weekly subcutaneous buprenorphine depot could improve adherence and reduce the potential for misuse, according to a study presented at the annual meeting of the College on Problems of Drug Dependence and published simultaneously June 22 in JAMA Psychiatry.
In a phase II, double-blind, randomized study, 47 adults with moderate to severe opioid use disorder were randomized to a weekly dose of the subcutaneous buprenorphine depot CAM2038 – either 24 mg or 32 mg – for 2 weeks. They also underwent five 3-day test sessions to evaluate their response to 6 mg and 18 mg doses of intramuscular hydromorphone and a 0 mg control. One qualification session was held before randomization and the remaining four sessions after.
The primary outcome of the study was drug liking, and both doses of CAM2038 were associated with immediate, sustained, and dose-related suppression of participants’ response to hydromorphone (JAMA Psychiatry. 2017 June 22. doi:10.1001/jamapsychiatry.2017.1874).
The depot also appeared to block the dose-dependent increase in drowsiness that was seen in response to hydromorphone in the qualification session.
However, there was a reversal of the dose-dependent response in participants’ desire to use opioids, with greater suppression seen at the lower depot dose.
Buprenorphine is known to be safer than methadone for the treatment of opioid use disorders, but Sharon L. Walsh, PhD, of the Center on Drug and Alcohol Research at the University of Kentucky, Lexington, and her coauthors said sublingual buprenorphine itself has become an abuse liability in some countries.
“Sublingual formulations of buprenorphine can be injected or snorted to enhance euphoric effects,” they wrote. “Unintentional overdose with buprenorphine has been reported, leading to toxicity and fatality in children and those who coingest buprenorphine with benzodiazepines or alcohol.”
The results mean the formulation meets the U.S. Food and Drug Administration criteria for complete opioid blockade.
CAM2038 achieved complete suppression of opioid withdrawal at both doses, and Clinical Opiate Withdrawal Scale (COWS) remained suppressed for the entire duration of the study.
“During treatment initiation, it is important that withdrawal symptoms are well-controlled,” the authors wrote. “The COWS and [Objective Opioid Withdrawal Scale] scores were reduced to near zero on the first dosing day with suppression thereafter.”
The mean COWS preinjection score was 11, which did not include five participants who accidentally were inducted with CAM2038 before they had achieved the prespecified criteria of a COWS score of 8 or above.
They also noted that the pharmacokinetic profile of CAM2038 showed gradually increasing buprenorphine concentrations, reaching maximum at around 24 hours after the dose was given. Perhaps because of this effect – which they likened to a de factor induction procedure mimicking the recommended induction with sublingual buprenorphine – the authors suggested that patients using CAM2038 could likely be inducted directly using the depot.
Researchers also looked at some key physiological outcomes before and after treatment with the depot. They found that, while patients showed significant dose-dependent reductions in oxygen saturation with hydromorphone before receiving depot treatment, these were significantly attenuated after treatment with CAM2038.
One subject withdrew from the study because of ventricular extrasystoles, and one showed abnormal liver function at discharge that was later attributed a hepatitis C diagnosis. Neither event was linked to the study drug.
However, 81% of patients experienced at least one adverse event, with significantly more events reported in the higher dose group. The most common reported events that were possibly related to the study drug were constipation, injection site pain and erythema, and headache. Most were rated as mild.
The College on Problems of Drug Dependence, a nonprofit corporation, is holding its annual meeting in Montreal. The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus, and other pharmaceutical companies.
FROM JAMA Psychiatry
Key clinical point: A weekly subcutaneous buprenorphine depot is effective and could reduce the potential for misuse of buprenorphine in patients with opioid use disorder.
Major finding: Buprenorphine depot CAM2038 achieves immediate and sustained opioid blockade and suppression of opioid withdrawal.
Data source: A double-blind, randomized phase II study in 47 patients with opioid use disorder.
Disclosures: The study was supported by research contracts from Braeburn Pharmaceuticals, with additional support from the National Institute on Drug Abuse, the National Center for Research Resources, and the National Center for Advancing of Translational Sciences. Two authors are employees of study sponsor Braeburn Pharmaceuticals, and one is an employee of Camurus – a partner to the sponsor. Three authors also received research contract support from Braeburn, four recieved research consultant fees from Braeburn, and two received consulting fees or partial salary support from Camurus and other pharmaceutical companies.
Cancer, heart disease increase MRSA mortality
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Key clinical point:
Major finding: Cancer is associated with a more than twofold increase in 30-day mortality from MRSA.
Data source: A 9-year retrospective study of 1,168 patients with MRSA infection.
Disclosures: Two authors declared research grants from the pharmaceutical industry.