User login
Tonsillectomy Reduces Strep-Triggered Psoriasis
GOTHENBURG, SWEDEN - Tonsillectomy has beneficial clinical and immunologic effects on chronic plaque psoriasis in patients who get exacerbations associated with streptococcal throat infections, according to a small, prospective, randomized trial.
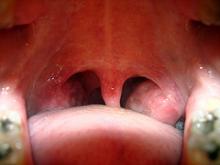
The proposed mechanism of benefit from the surgery is that it removes an important source of circulating pathogenic T-cells generated in the palatine tonsils. The T-cells respond to antigenic short peptides common to streptococcal M-protein and skin keratins, Dr. Ragna Hlin Thorleifsdottir explained at the annual congress of the European Academy of Dermatology and Venereology.
"Streptococcal M-protein contains a number of amino acid sequences also found in keratins that are upregulated in psoriatic lesions. It’s possible that T-cells that recognize such M-protein sequences in the tonsils also recognize corresponding sequences in the skin. We have identified such cross-reactive T-cells in the blood of psoriasis patients," said Dr. Thorleifsdottir of Landspitali University Hospital, Reykjavik, Iceland.
She reported on 29 patients with chronic plaque psoriasis and a history of exacerbations linked to strep throat randomized to tonsillectomy or a nonsurgical control group. Participants were followed prospectively for 2 years by blinded observers who assessed them every 6 months by Psoriasis Area and Severity Index (PASI) scores and quality of life instruments. Patients remained on their pre-enrollment psoriasis therapies.
Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up. The reduction in PASI scores peaked a mean of 18 months post-surgery, at which point 9 of 15 patients (60%) reached PASI 50 (50% improvement), compared with baseline. At 24 months, the tonsillectomy group still had a mean 32% reduction in PASI scores. In contrast, PASI scores did not change significantly over time in the control group.
The improvement in PASI scores in the tonsillectomy group was strongly correlated with a reduction in circulating, cross-reactive, skin-homing CLA+ CD8+ T-cells responsive to 16 homologous M-protein and skin keratin peptides. Skin-homing CLA+ CD4+ T-cells also declined with decreasing PASI scores in the tonsillectomy patients, although this association, while significant, was not as strong as for CD8+ T-cells.
Other investigators have anecdotally reported in uncontrolled series that tonsillectomy appears to have a beneficial effect on psoriasis, said Dr. Thorleifsdottir. What distinguishes the Iceland study is that it was randomized, prospective, and blinded, and it evaluated a specific proposed mechanism involving cross-reactive T-cells. Whether the observed clinical improvement will be maintained beyond 2 years remains to be seen.
Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
GOTHENBURG, SWEDEN - Tonsillectomy has beneficial clinical and immunologic effects on chronic plaque psoriasis in patients who get exacerbations associated with streptococcal throat infections, according to a small, prospective, randomized trial.
The proposed mechanism of benefit from the surgery is that it removes an important source of circulating pathogenic T-cells generated in the palatine tonsils. The T-cells respond to antigenic short peptides common to streptococcal M-protein and skin keratins, Dr. Ragna Hlin Thorleifsdottir explained at the annual congress of the European Academy of Dermatology and Venereology.
"Streptococcal M-protein contains a number of amino acid sequences also found in keratins that are upregulated in psoriatic lesions. It’s possible that T-cells that recognize such M-protein sequences in the tonsils also recognize corresponding sequences in the skin. We have identified such cross-reactive T-cells in the blood of psoriasis patients," said Dr. Thorleifsdottir of Landspitali University Hospital, Reykjavik, Iceland.
She reported on 29 patients with chronic plaque psoriasis and a history of exacerbations linked to strep throat randomized to tonsillectomy or a nonsurgical control group. Participants were followed prospectively for 2 years by blinded observers who assessed them every 6 months by Psoriasis Area and Severity Index (PASI) scores and quality of life instruments. Patients remained on their pre-enrollment psoriasis therapies.
Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up. The reduction in PASI scores peaked a mean of 18 months post-surgery, at which point 9 of 15 patients (60%) reached PASI 50 (50% improvement), compared with baseline. At 24 months, the tonsillectomy group still had a mean 32% reduction in PASI scores. In contrast, PASI scores did not change significantly over time in the control group.
The improvement in PASI scores in the tonsillectomy group was strongly correlated with a reduction in circulating, cross-reactive, skin-homing CLA+ CD8+ T-cells responsive to 16 homologous M-protein and skin keratin peptides. Skin-homing CLA+ CD4+ T-cells also declined with decreasing PASI scores in the tonsillectomy patients, although this association, while significant, was not as strong as for CD8+ T-cells.
Other investigators have anecdotally reported in uncontrolled series that tonsillectomy appears to have a beneficial effect on psoriasis, said Dr. Thorleifsdottir. What distinguishes the Iceland study is that it was randomized, prospective, and blinded, and it evaluated a specific proposed mechanism involving cross-reactive T-cells. Whether the observed clinical improvement will be maintained beyond 2 years remains to be seen.
Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
GOTHENBURG, SWEDEN - Tonsillectomy has beneficial clinical and immunologic effects on chronic plaque psoriasis in patients who get exacerbations associated with streptococcal throat infections, according to a small, prospective, randomized trial.
The proposed mechanism of benefit from the surgery is that it removes an important source of circulating pathogenic T-cells generated in the palatine tonsils. The T-cells respond to antigenic short peptides common to streptococcal M-protein and skin keratins, Dr. Ragna Hlin Thorleifsdottir explained at the annual congress of the European Academy of Dermatology and Venereology.
"Streptococcal M-protein contains a number of amino acid sequences also found in keratins that are upregulated in psoriatic lesions. It’s possible that T-cells that recognize such M-protein sequences in the tonsils also recognize corresponding sequences in the skin. We have identified such cross-reactive T-cells in the blood of psoriasis patients," said Dr. Thorleifsdottir of Landspitali University Hospital, Reykjavik, Iceland.
She reported on 29 patients with chronic plaque psoriasis and a history of exacerbations linked to strep throat randomized to tonsillectomy or a nonsurgical control group. Participants were followed prospectively for 2 years by blinded observers who assessed them every 6 months by Psoriasis Area and Severity Index (PASI) scores and quality of life instruments. Patients remained on their pre-enrollment psoriasis therapies.
Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up. The reduction in PASI scores peaked a mean of 18 months post-surgery, at which point 9 of 15 patients (60%) reached PASI 50 (50% improvement), compared with baseline. At 24 months, the tonsillectomy group still had a mean 32% reduction in PASI scores. In contrast, PASI scores did not change significantly over time in the control group.
The improvement in PASI scores in the tonsillectomy group was strongly correlated with a reduction in circulating, cross-reactive, skin-homing CLA+ CD8+ T-cells responsive to 16 homologous M-protein and skin keratin peptides. Skin-homing CLA+ CD4+ T-cells also declined with decreasing PASI scores in the tonsillectomy patients, although this association, while significant, was not as strong as for CD8+ T-cells.
Other investigators have anecdotally reported in uncontrolled series that tonsillectomy appears to have a beneficial effect on psoriasis, said Dr. Thorleifsdottir. What distinguishes the Iceland study is that it was randomized, prospective, and blinded, and it evaluated a specific proposed mechanism involving cross-reactive T-cells. Whether the observed clinical improvement will be maintained beyond 2 years remains to be seen.
Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up.
Data Source: Patients with chronic plaque psoriasis (n = 29) and a history of exacerbations linked to strep throat were randomized to tonsillectomy or a nonsurgical control group.
Disclosures: Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
Tonsillectomy Reduces Strep-Triggered Psoriasis
GOTHENBURG, SWEDEN - Tonsillectomy has beneficial clinical and immunologic effects on chronic plaque psoriasis in patients who get exacerbations associated with streptococcal throat infections, according to a small, prospective, randomized trial.
The proposed mechanism of benefit from the surgery is that it removes an important source of circulating pathogenic T-cells generated in the palatine tonsils. The T-cells respond to antigenic short peptides common to streptococcal M-protein and skin keratins, Dr. Ragna Hlin Thorleifsdottir explained at the annual congress of the European Academy of Dermatology and Venereology.
"Streptococcal M-protein contains a number of amino acid sequences also found in keratins that are upregulated in psoriatic lesions. It’s possible that T-cells that recognize such M-protein sequences in the tonsils also recognize corresponding sequences in the skin. We have identified such cross-reactive T-cells in the blood of psoriasis patients," said Dr. Thorleifsdottir of Landspitali University Hospital, Reykjavik, Iceland.
She reported on 29 patients with chronic plaque psoriasis and a history of exacerbations linked to strep throat randomized to tonsillectomy or a nonsurgical control group. Participants were followed prospectively for 2 years by blinded observers who assessed them every 6 months by Psoriasis Area and Severity Index (PASI) scores and quality of life instruments. Patients remained on their pre-enrollment psoriasis therapies.
Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up. The reduction in PASI scores peaked a mean of 18 months post-surgery, at which point 9 of 15 patients (60%) reached PASI 50 (50% improvement), compared with baseline. At 24 months, the tonsillectomy group still had a mean 32% reduction in PASI scores. In contrast, PASI scores did not change significantly over time in the control group.
The improvement in PASI scores in the tonsillectomy group was strongly correlated with a reduction in circulating, cross-reactive, skin-homing CLA+ CD8+ T-cells responsive to 16 homologous M-protein and skin keratin peptides. Skin-homing CLA+ CD4+ T-cells also declined with decreasing PASI scores in the tonsillectomy patients, although this association, while significant, was not as strong as for CD8+ T-cells.
Other investigators have anecdotally reported in uncontrolled series that tonsillectomy appears to have a beneficial effect on psoriasis, said Dr. Thorleifsdottir. What distinguishes the Iceland study is that it was randomized, prospective, and blinded, and it evaluated a specific proposed mechanism involving cross-reactive T-cells. Whether the observed clinical improvement will be maintained beyond 2 years remains to be seen.
Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
GOTHENBURG, SWEDEN - Tonsillectomy has beneficial clinical and immunologic effects on chronic plaque psoriasis in patients who get exacerbations associated with streptococcal throat infections, according to a small, prospective, randomized trial.
The proposed mechanism of benefit from the surgery is that it removes an important source of circulating pathogenic T-cells generated in the palatine tonsils. The T-cells respond to antigenic short peptides common to streptococcal M-protein and skin keratins, Dr. Ragna Hlin Thorleifsdottir explained at the annual congress of the European Academy of Dermatology and Venereology.
"Streptococcal M-protein contains a number of amino acid sequences also found in keratins that are upregulated in psoriatic lesions. It’s possible that T-cells that recognize such M-protein sequences in the tonsils also recognize corresponding sequences in the skin. We have identified such cross-reactive T-cells in the blood of psoriasis patients," said Dr. Thorleifsdottir of Landspitali University Hospital, Reykjavik, Iceland.
She reported on 29 patients with chronic plaque psoriasis and a history of exacerbations linked to strep throat randomized to tonsillectomy or a nonsurgical control group. Participants were followed prospectively for 2 years by blinded observers who assessed them every 6 months by Psoriasis Area and Severity Index (PASI) scores and quality of life instruments. Patients remained on their pre-enrollment psoriasis therapies.
Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up. The reduction in PASI scores peaked a mean of 18 months post-surgery, at which point 9 of 15 patients (60%) reached PASI 50 (50% improvement), compared with baseline. At 24 months, the tonsillectomy group still had a mean 32% reduction in PASI scores. In contrast, PASI scores did not change significantly over time in the control group.
The improvement in PASI scores in the tonsillectomy group was strongly correlated with a reduction in circulating, cross-reactive, skin-homing CLA+ CD8+ T-cells responsive to 16 homologous M-protein and skin keratin peptides. Skin-homing CLA+ CD4+ T-cells also declined with decreasing PASI scores in the tonsillectomy patients, although this association, while significant, was not as strong as for CD8+ T-cells.
Other investigators have anecdotally reported in uncontrolled series that tonsillectomy appears to have a beneficial effect on psoriasis, said Dr. Thorleifsdottir. What distinguishes the Iceland study is that it was randomized, prospective, and blinded, and it evaluated a specific proposed mechanism involving cross-reactive T-cells. Whether the observed clinical improvement will be maintained beyond 2 years remains to be seen.
Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
GOTHENBURG, SWEDEN - Tonsillectomy has beneficial clinical and immunologic effects on chronic plaque psoriasis in patients who get exacerbations associated with streptococcal throat infections, according to a small, prospective, randomized trial.
The proposed mechanism of benefit from the surgery is that it removes an important source of circulating pathogenic T-cells generated in the palatine tonsils. The T-cells respond to antigenic short peptides common to streptococcal M-protein and skin keratins, Dr. Ragna Hlin Thorleifsdottir explained at the annual congress of the European Academy of Dermatology and Venereology.
"Streptococcal M-protein contains a number of amino acid sequences also found in keratins that are upregulated in psoriatic lesions. It’s possible that T-cells that recognize such M-protein sequences in the tonsils also recognize corresponding sequences in the skin. We have identified such cross-reactive T-cells in the blood of psoriasis patients," said Dr. Thorleifsdottir of Landspitali University Hospital, Reykjavik, Iceland.
She reported on 29 patients with chronic plaque psoriasis and a history of exacerbations linked to strep throat randomized to tonsillectomy or a nonsurgical control group. Participants were followed prospectively for 2 years by blinded observers who assessed them every 6 months by Psoriasis Area and Severity Index (PASI) scores and quality of life instruments. Patients remained on their pre-enrollment psoriasis therapies.
Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up. The reduction in PASI scores peaked a mean of 18 months post-surgery, at which point 9 of 15 patients (60%) reached PASI 50 (50% improvement), compared with baseline. At 24 months, the tonsillectomy group still had a mean 32% reduction in PASI scores. In contrast, PASI scores did not change significantly over time in the control group.
The improvement in PASI scores in the tonsillectomy group was strongly correlated with a reduction in circulating, cross-reactive, skin-homing CLA+ CD8+ T-cells responsive to 16 homologous M-protein and skin keratin peptides. Skin-homing CLA+ CD4+ T-cells also declined with decreasing PASI scores in the tonsillectomy patients, although this association, while significant, was not as strong as for CD8+ T-cells.
Other investigators have anecdotally reported in uncontrolled series that tonsillectomy appears to have a beneficial effect on psoriasis, said Dr. Thorleifsdottir. What distinguishes the Iceland study is that it was randomized, prospective, and blinded, and it evaluated a specific proposed mechanism involving cross-reactive T-cells. Whether the observed clinical improvement will be maintained beyond 2 years remains to be seen.
Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Thirteen of 15 tonsillectomy patients showed significant improvement, with reductions of 30%-90% in PASI scores during 2 years of follow-up.
Data Source: Patients with chronic plaque psoriasis (n = 29) and a history of exacerbations linked to strep throat were randomized to tonsillectomy or a nonsurgical control group.
Disclosures: Dr. Thorleifsdottir said she had no financial conflicts regarding the tonsillectomy study, which was funded by the University of Iceland Research Fund.
Tissue-Selective Estrogen Complex Shows Metabolic Benefits in Phase III Trial
DENVER – A novel oral, once-daily, tissue-selective estrogen complex designed to treat postmenopausal symptoms more safely than traditional estrogen/progestin hormone therapy achieved an overall favorable effect on metabolic parameters in a large phase III clinical trial.
The combination of the selective estrogen receptor modulator (SERM) bazedoxifene and conjugated estrogens produced "very favorable" changes in lipid profiles and no clinically meaningful effects on coagulation parameters, fibrinolytic activity, or carbohydrate metabolism in the phase III SMART-1 trial, Dr. Hugh S. Taylor reported at the annual meeting of the American Society for Reproductive Medicine.
The concept underlying the tissue-selective estrogen complex (TSEC) is that the SERM will serve as an antagonist to estrogen’s adverse effects on the uterus and breast without interfering with its favorable CNS effects on vasomotor symptoms, explained Dr. Taylor of Yale University, New Haven, Conn.
The Selective Estrogens, Menopause, and Response to Therapy (SMART-1) trial was a 2-year, double-blind, multicenter, placebo- and active comparator-controlled study involving 7,492 postmenopausal women aged 40-72 years with an intact uterus and acceptable baseline endometrial biopsy results. They were randomized to one of eight treatment arms: bazedoxifene at 10, 20, or 40 mg, each combined with conjugated estrogens at 0.45 or 0.625 mg; raloxifene at 60 mg/day; or placebo.
There are three large randomized SMART trials in the bazedoxifene/conjugated estrogens development program. Prior reports from SMART-1 showed that the TSEC significantly increased bone mineral density while reducing hot flashes and vulvar/vaginal symptoms compared with placebo (Fertil. Steril. 2009;92:1025-38, 1045-52). TSEC also was associated with low rates of endometrial hyperplasia and cumulative amenorrhea rates comparable to those with placebo (Fertil. Steril. 2009;92:1018-24, 1039-44). In the overall SMART series, the thrombotic event rate in TSEC-treated patients was similar to that of estrogen alone. In other words, there was no synergistic effect on clotting events with the combined therapy. The TSEC had no effect on blood pressure, Dr. Taylor continued.
At the meeting, he presented for the first time the results of the SMART-1 metabolic substudy. Metabolic end points take on particular importance because menopause is often associated with unfavorable effects on the metabolic profile. Dr. Taylor focused on the subset of women treated with bazedoxifene 20 mg/conjugated estrogens 0.45 mg because that’s the regimen going forward in development under the trade name Aprela.
The metabolic substudy included 111 women randomized to Aprela and 108 on placebo. All were 1-5 years postmenopausal, with an average of 3 years since their last menstrual period.
Patients who took Aprela had a mean 11% reduction in LDL cholesterol over a 2-year period compared with baseline, a significantly greater change than with placebo. Other significant lipid changes in the Aprela group included a 4% decrease in total cholesterol, an 11% increase in HDL cholesterol level, a 28% rise in the cardioprotective HDL2 subfraction, an 11% increase in apolipoprotein A-I, and a 19% drop in lipoprotein (a). Most of these favorable changes were significantly greater than with placebo at all time points in the study, with testing done at 6, 12, 18, and 24 months.
The fly in the lipid ointment was the 23% increase in triglyceride levels in the Aprela group, which was significantly greater than the 6% increase in the placebo arm. Most hormonal therapies have this unwelcome effect of boosting triglycerides, Dr. Taylor observed.
In terms of coagulation parameters, the Aprela group showed favorable changes in fibrinogen, antithrombin III activity, and plasminogen activator inhibitor–1 activity that were statistically significantly greater than with placebo, but small in size and not clinically meaningful. Fasting insulin, fasting glucose, plasma homocysteine, C-reactive protein, and thyroid-stimulating hormone levels were unaffected by Aprela, he said.
The SMART-1 trial was funded by Wyeth, which has been acquired by Pfizer Inc. Dr. Taylor declared that he has received research grants from and has been on the speakers bureau for the companies.
DENVER – A novel oral, once-daily, tissue-selective estrogen complex designed to treat postmenopausal symptoms more safely than traditional estrogen/progestin hormone therapy achieved an overall favorable effect on metabolic parameters in a large phase III clinical trial.
The combination of the selective estrogen receptor modulator (SERM) bazedoxifene and conjugated estrogens produced "very favorable" changes in lipid profiles and no clinically meaningful effects on coagulation parameters, fibrinolytic activity, or carbohydrate metabolism in the phase III SMART-1 trial, Dr. Hugh S. Taylor reported at the annual meeting of the American Society for Reproductive Medicine.
The concept underlying the tissue-selective estrogen complex (TSEC) is that the SERM will serve as an antagonist to estrogen’s adverse effects on the uterus and breast without interfering with its favorable CNS effects on vasomotor symptoms, explained Dr. Taylor of Yale University, New Haven, Conn.
The Selective Estrogens, Menopause, and Response to Therapy (SMART-1) trial was a 2-year, double-blind, multicenter, placebo- and active comparator-controlled study involving 7,492 postmenopausal women aged 40-72 years with an intact uterus and acceptable baseline endometrial biopsy results. They were randomized to one of eight treatment arms: bazedoxifene at 10, 20, or 40 mg, each combined with conjugated estrogens at 0.45 or 0.625 mg; raloxifene at 60 mg/day; or placebo.
There are three large randomized SMART trials in the bazedoxifene/conjugated estrogens development program. Prior reports from SMART-1 showed that the TSEC significantly increased bone mineral density while reducing hot flashes and vulvar/vaginal symptoms compared with placebo (Fertil. Steril. 2009;92:1025-38, 1045-52). TSEC also was associated with low rates of endometrial hyperplasia and cumulative amenorrhea rates comparable to those with placebo (Fertil. Steril. 2009;92:1018-24, 1039-44). In the overall SMART series, the thrombotic event rate in TSEC-treated patients was similar to that of estrogen alone. In other words, there was no synergistic effect on clotting events with the combined therapy. The TSEC had no effect on blood pressure, Dr. Taylor continued.
At the meeting, he presented for the first time the results of the SMART-1 metabolic substudy. Metabolic end points take on particular importance because menopause is often associated with unfavorable effects on the metabolic profile. Dr. Taylor focused on the subset of women treated with bazedoxifene 20 mg/conjugated estrogens 0.45 mg because that’s the regimen going forward in development under the trade name Aprela.
The metabolic substudy included 111 women randomized to Aprela and 108 on placebo. All were 1-5 years postmenopausal, with an average of 3 years since their last menstrual period.
Patients who took Aprela had a mean 11% reduction in LDL cholesterol over a 2-year period compared with baseline, a significantly greater change than with placebo. Other significant lipid changes in the Aprela group included a 4% decrease in total cholesterol, an 11% increase in HDL cholesterol level, a 28% rise in the cardioprotective HDL2 subfraction, an 11% increase in apolipoprotein A-I, and a 19% drop in lipoprotein (a). Most of these favorable changes were significantly greater than with placebo at all time points in the study, with testing done at 6, 12, 18, and 24 months.
The fly in the lipid ointment was the 23% increase in triglyceride levels in the Aprela group, which was significantly greater than the 6% increase in the placebo arm. Most hormonal therapies have this unwelcome effect of boosting triglycerides, Dr. Taylor observed.
In terms of coagulation parameters, the Aprela group showed favorable changes in fibrinogen, antithrombin III activity, and plasminogen activator inhibitor–1 activity that were statistically significantly greater than with placebo, but small in size and not clinically meaningful. Fasting insulin, fasting glucose, plasma homocysteine, C-reactive protein, and thyroid-stimulating hormone levels were unaffected by Aprela, he said.
The SMART-1 trial was funded by Wyeth, which has been acquired by Pfizer Inc. Dr. Taylor declared that he has received research grants from and has been on the speakers bureau for the companies.
DENVER – A novel oral, once-daily, tissue-selective estrogen complex designed to treat postmenopausal symptoms more safely than traditional estrogen/progestin hormone therapy achieved an overall favorable effect on metabolic parameters in a large phase III clinical trial.
The combination of the selective estrogen receptor modulator (SERM) bazedoxifene and conjugated estrogens produced "very favorable" changes in lipid profiles and no clinically meaningful effects on coagulation parameters, fibrinolytic activity, or carbohydrate metabolism in the phase III SMART-1 trial, Dr. Hugh S. Taylor reported at the annual meeting of the American Society for Reproductive Medicine.
The concept underlying the tissue-selective estrogen complex (TSEC) is that the SERM will serve as an antagonist to estrogen’s adverse effects on the uterus and breast without interfering with its favorable CNS effects on vasomotor symptoms, explained Dr. Taylor of Yale University, New Haven, Conn.
The Selective Estrogens, Menopause, and Response to Therapy (SMART-1) trial was a 2-year, double-blind, multicenter, placebo- and active comparator-controlled study involving 7,492 postmenopausal women aged 40-72 years with an intact uterus and acceptable baseline endometrial biopsy results. They were randomized to one of eight treatment arms: bazedoxifene at 10, 20, or 40 mg, each combined with conjugated estrogens at 0.45 or 0.625 mg; raloxifene at 60 mg/day; or placebo.
There are three large randomized SMART trials in the bazedoxifene/conjugated estrogens development program. Prior reports from SMART-1 showed that the TSEC significantly increased bone mineral density while reducing hot flashes and vulvar/vaginal symptoms compared with placebo (Fertil. Steril. 2009;92:1025-38, 1045-52). TSEC also was associated with low rates of endometrial hyperplasia and cumulative amenorrhea rates comparable to those with placebo (Fertil. Steril. 2009;92:1018-24, 1039-44). In the overall SMART series, the thrombotic event rate in TSEC-treated patients was similar to that of estrogen alone. In other words, there was no synergistic effect on clotting events with the combined therapy. The TSEC had no effect on blood pressure, Dr. Taylor continued.
At the meeting, he presented for the first time the results of the SMART-1 metabolic substudy. Metabolic end points take on particular importance because menopause is often associated with unfavorable effects on the metabolic profile. Dr. Taylor focused on the subset of women treated with bazedoxifene 20 mg/conjugated estrogens 0.45 mg because that’s the regimen going forward in development under the trade name Aprela.
The metabolic substudy included 111 women randomized to Aprela and 108 on placebo. All were 1-5 years postmenopausal, with an average of 3 years since their last menstrual period.
Patients who took Aprela had a mean 11% reduction in LDL cholesterol over a 2-year period compared with baseline, a significantly greater change than with placebo. Other significant lipid changes in the Aprela group included a 4% decrease in total cholesterol, an 11% increase in HDL cholesterol level, a 28% rise in the cardioprotective HDL2 subfraction, an 11% increase in apolipoprotein A-I, and a 19% drop in lipoprotein (a). Most of these favorable changes were significantly greater than with placebo at all time points in the study, with testing done at 6, 12, 18, and 24 months.
The fly in the lipid ointment was the 23% increase in triglyceride levels in the Aprela group, which was significantly greater than the 6% increase in the placebo arm. Most hormonal therapies have this unwelcome effect of boosting triglycerides, Dr. Taylor observed.
In terms of coagulation parameters, the Aprela group showed favorable changes in fibrinogen, antithrombin III activity, and plasminogen activator inhibitor–1 activity that were statistically significantly greater than with placebo, but small in size and not clinically meaningful. Fasting insulin, fasting glucose, plasma homocysteine, C-reactive protein, and thyroid-stimulating hormone levels were unaffected by Aprela, he said.
The SMART-1 trial was funded by Wyeth, which has been acquired by Pfizer Inc. Dr. Taylor declared that he has received research grants from and has been on the speakers bureau for the companies.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE
Major Finding: Patients who took bazedoxifene plus a conjugated estrogen had a mean 11% reduction in LDL cholesterol level over 2 years compared with baseline, a significantly greater change than with placebo. Other significant lipid changes in the Aprela group included a 4% decrease in total cholesterol and an 11% increase in HDL cholesterol.
Data Source: SMART-1 metabolic substudy including 111 women randomized to bazedoxifene and Aprela and 108 to placebo.
Disclosures: The SMART-1 trial was funded by Wyeth, which has been acquired by Pfizer. Dr. Taylor declared that he has received research grants from and has been on the speakers bureau for the companies.
EADV: Patients Often Unaware of Infliximab Infusion Risk
GOTHENBURG, SWEDEN - More than one-fifth of infliximab patients who had a temporary medical situation contraindicating administration of the biologic presented for their scheduled infusion unaware treatment would be inappropriate at that time.
The finding from the RemiTRAC Infusion Registry – a large, prospective, observational Canadian registry of patients on infliximab for any indication – underscores the importance of reviewing the full list of contraindications to infliximab with every patient prior to each infusion, said Dr. Warren J. Winkelman said at the annual congress of the European Academy of Dermatology and Venereology. This is the key to minimizing infusion reactions.
The most common medical conditions RemiTRAC participants mistakenly perceived as not being a problem were flulike symptoms and upper respiratory infections, despite the fact patients were informed that these symptoms increased the risk of adverse events as part of standard pretreatment education.
The lesson provided by RemiTRAC is that it is particularly important to inquire about flulike symptoms and colds before administering an infliximab infusion, according to Dr. Winkelman of the University of Toronto.
He reported on 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry. The indication for the antitumor necrosis factor (TNF)–alpha agent was rheumatoid arthritis in 51% of patients, ankylosing spondylitis in 17%, Crohn’s disease in 11%, psoriasis in 9%, psoriatic arthritis in 6%, and ulcerative colitis in 4%.
Participants in the registry received a mean of 15 infliximab infusions during more than 900 patient-years of exposure to the biologic agent. Of 8,051 scheduled infusions, 6% were not administered because of clinical reasons. Sixty-two percent of these cancelled infusions were scotched because of infections, mainly upper respiratory infections or flulike symptoms. Another 9% of cancelled treatments were the result of scheduled surgery.
In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy, according to Dr. Winkelman.
The overall incidence of infusion reactions was 2.1%. The rate was 2.6% in patients being treated for rheumatoid arthritis, 1.9% in those with psoriasis, 0.7% in those with psoriatic arthritis, and 1.3% in patients with ankylosing spondylitis.
The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
GOTHENBURG, SWEDEN - More than one-fifth of infliximab patients who had a temporary medical situation contraindicating administration of the biologic presented for their scheduled infusion unaware treatment would be inappropriate at that time.
The finding from the RemiTRAC Infusion Registry – a large, prospective, observational Canadian registry of patients on infliximab for any indication – underscores the importance of reviewing the full list of contraindications to infliximab with every patient prior to each infusion, said Dr. Warren J. Winkelman said at the annual congress of the European Academy of Dermatology and Venereology. This is the key to minimizing infusion reactions.
The most common medical conditions RemiTRAC participants mistakenly perceived as not being a problem were flulike symptoms and upper respiratory infections, despite the fact patients were informed that these symptoms increased the risk of adverse events as part of standard pretreatment education.
The lesson provided by RemiTRAC is that it is particularly important to inquire about flulike symptoms and colds before administering an infliximab infusion, according to Dr. Winkelman of the University of Toronto.
He reported on 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry. The indication for the antitumor necrosis factor (TNF)–alpha agent was rheumatoid arthritis in 51% of patients, ankylosing spondylitis in 17%, Crohn’s disease in 11%, psoriasis in 9%, psoriatic arthritis in 6%, and ulcerative colitis in 4%.
Participants in the registry received a mean of 15 infliximab infusions during more than 900 patient-years of exposure to the biologic agent. Of 8,051 scheduled infusions, 6% were not administered because of clinical reasons. Sixty-two percent of these cancelled infusions were scotched because of infections, mainly upper respiratory infections or flulike symptoms. Another 9% of cancelled treatments were the result of scheduled surgery.
In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy, according to Dr. Winkelman.
The overall incidence of infusion reactions was 2.1%. The rate was 2.6% in patients being treated for rheumatoid arthritis, 1.9% in those with psoriasis, 0.7% in those with psoriatic arthritis, and 1.3% in patients with ankylosing spondylitis.
The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
GOTHENBURG, SWEDEN - More than one-fifth of infliximab patients who had a temporary medical situation contraindicating administration of the biologic presented for their scheduled infusion unaware treatment would be inappropriate at that time.
The finding from the RemiTRAC Infusion Registry – a large, prospective, observational Canadian registry of patients on infliximab for any indication – underscores the importance of reviewing the full list of contraindications to infliximab with every patient prior to each infusion, said Dr. Warren J. Winkelman said at the annual congress of the European Academy of Dermatology and Venereology. This is the key to minimizing infusion reactions.
The most common medical conditions RemiTRAC participants mistakenly perceived as not being a problem were flulike symptoms and upper respiratory infections, despite the fact patients were informed that these symptoms increased the risk of adverse events as part of standard pretreatment education.
The lesson provided by RemiTRAC is that it is particularly important to inquire about flulike symptoms and colds before administering an infliximab infusion, according to Dr. Winkelman of the University of Toronto.
He reported on 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry. The indication for the antitumor necrosis factor (TNF)–alpha agent was rheumatoid arthritis in 51% of patients, ankylosing spondylitis in 17%, Crohn’s disease in 11%, psoriasis in 9%, psoriatic arthritis in 6%, and ulcerative colitis in 4%.
Participants in the registry received a mean of 15 infliximab infusions during more than 900 patient-years of exposure to the biologic agent. Of 8,051 scheduled infusions, 6% were not administered because of clinical reasons. Sixty-two percent of these cancelled infusions were scotched because of infections, mainly upper respiratory infections or flulike symptoms. Another 9% of cancelled treatments were the result of scheduled surgery.
In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy, according to Dr. Winkelman.
The overall incidence of infusion reactions was 2.1%. The rate was 2.6% in patients being treated for rheumatoid arthritis, 1.9% in those with psoriasis, 0.7% in those with psoriatic arthritis, and 1.3% in patients with ankylosing spondylitis.
The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy.
Data Source: 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry
Disclosures: The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
Patients Often Unaware of Infliximab Infusion Risk
GOTHENBURG, SWEDEN – More than one-fifth of infliximab patients who had a temporary medical situation contraindicating administration of the biologic presented for their scheduled infusion unaware treatment would be inappropriate at that time.
The finding from the RemiTRAC Infusion Registry – a large, prospective, observational Canadian registry of patients on infliximab for any indication – underscores the importance of reviewing the full list of contraindications to infliximab with every patient prior to each infusion, said Dr. Warren J. Winkelman said at the annual congress of the European Academy of Dermatology and Venereology. This is the key to minimizing infusion reactions.
The most common medical conditions RemiTRAC participants mistakenly perceived as not being a problem were flulike symptoms and upper respiratory infections, despite the fact patients were informed that these symptoms increased the risk of adverse events as part of standard pretreatment education.
The lesson provided by RemiTRAC is that it is particularly important to inquire about flulike symptoms and colds before administering an infliximab infusion, according to Dr. Winkelman of the University of Toronto.
He reported on 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry. The indication for the antitumor necrosis factor (TNF)–alpha agent was rheumatoid arthritis in 51% of patients, ankylosing spondylitis in 17%, Crohn’s disease in 11%, psoriasis in 9%, psoriatic arthritis in 6%, and ulcerative colitis in 4%.
Participants in the registry received a mean of 15 infliximab infusions during more than 900 patient-years of exposure to the biologic agent. Of 8,051 scheduled infusions, 6% were not administered because of clinical reasons. Sixty-two percent of these cancelled infusions were scotched because of infections, mainly upper respiratory infections or flulike symptoms. Another 9% of cancelled treatments were the result of scheduled surgery.
In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy, according to Dr. Winkelman.
The overall incidence of infusion reactions was 2.1%. The rate was 2.6% in patients being treated for rheumatoid arthritis, 1.9% in those with psoriasis, 0.7% in those with psoriatic arthritis, and 1.3% in patients with ankylosing spondylitis.
The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
GOTHENBURG, SWEDEN – More than one-fifth of infliximab patients who had a temporary medical situation contraindicating administration of the biologic presented for their scheduled infusion unaware treatment would be inappropriate at that time.
The finding from the RemiTRAC Infusion Registry – a large, prospective, observational Canadian registry of patients on infliximab for any indication – underscores the importance of reviewing the full list of contraindications to infliximab with every patient prior to each infusion, said Dr. Warren J. Winkelman said at the annual congress of the European Academy of Dermatology and Venereology. This is the key to minimizing infusion reactions.
The most common medical conditions RemiTRAC participants mistakenly perceived as not being a problem were flulike symptoms and upper respiratory infections, despite the fact patients were informed that these symptoms increased the risk of adverse events as part of standard pretreatment education.
The lesson provided by RemiTRAC is that it is particularly important to inquire about flulike symptoms and colds before administering an infliximab infusion, according to Dr. Winkelman of the University of Toronto.
He reported on 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry. The indication for the antitumor necrosis factor (TNF)–alpha agent was rheumatoid arthritis in 51% of patients, ankylosing spondylitis in 17%, Crohn’s disease in 11%, psoriasis in 9%, psoriatic arthritis in 6%, and ulcerative colitis in 4%.
Participants in the registry received a mean of 15 infliximab infusions during more than 900 patient-years of exposure to the biologic agent. Of 8,051 scheduled infusions, 6% were not administered because of clinical reasons. Sixty-two percent of these cancelled infusions were scotched because of infections, mainly upper respiratory infections or flulike symptoms. Another 9% of cancelled treatments were the result of scheduled surgery.
In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy, according to Dr. Winkelman.
The overall incidence of infusion reactions was 2.1%. The rate was 2.6% in patients being treated for rheumatoid arthritis, 1.9% in those with psoriasis, 0.7% in those with psoriatic arthritis, and 1.3% in patients with ankylosing spondylitis.
The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
GOTHENBURG, SWEDEN – More than one-fifth of infliximab patients who had a temporary medical situation contraindicating administration of the biologic presented for their scheduled infusion unaware treatment would be inappropriate at that time.
The finding from the RemiTRAC Infusion Registry – a large, prospective, observational Canadian registry of patients on infliximab for any indication – underscores the importance of reviewing the full list of contraindications to infliximab with every patient prior to each infusion, said Dr. Warren J. Winkelman said at the annual congress of the European Academy of Dermatology and Venereology. This is the key to minimizing infusion reactions.
The most common medical conditions RemiTRAC participants mistakenly perceived as not being a problem were flulike symptoms and upper respiratory infections, despite the fact patients were informed that these symptoms increased the risk of adverse events as part of standard pretreatment education.
The lesson provided by RemiTRAC is that it is particularly important to inquire about flulike symptoms and colds before administering an infliximab infusion, according to Dr. Winkelman of the University of Toronto.
He reported on 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry. The indication for the antitumor necrosis factor (TNF)–alpha agent was rheumatoid arthritis in 51% of patients, ankylosing spondylitis in 17%, Crohn’s disease in 11%, psoriasis in 9%, psoriatic arthritis in 6%, and ulcerative colitis in 4%.
Participants in the registry received a mean of 15 infliximab infusions during more than 900 patient-years of exposure to the biologic agent. Of 8,051 scheduled infusions, 6% were not administered because of clinical reasons. Sixty-two percent of these cancelled infusions were scotched because of infections, mainly upper respiratory infections or flulike symptoms. Another 9% of cancelled treatments were the result of scheduled surgery.
In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy, according to Dr. Winkelman.
The overall incidence of infusion reactions was 2.1%. The rate was 2.6% in patients being treated for rheumatoid arthritis, 1.9% in those with psoriasis, 0.7% in those with psoriatic arthritis, and 1.3% in patients with ankylosing spondylitis.
The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: In 22% of instances of cancelled infusion, patients came to the clinic for their infusion unaware that their health status at that particular time was inappropriate for administration of anti-TNF therapy.
Data Source: 718 patients on infliximab for any indication at more than a dozen Canadian sites participating in the RemiTRAC Infusion Registry
Disclosures: The RemiTRAC Infusion Registry was supported by Schering-Plough Canada, which is now part of Merck. Dr. Winkelman disclosed serving as a consultant to the company.
Legius Syndrome Easily Misdiagnosed as Neurofibromatosis Type 1
GOTHENBURG, SWEDEN - Legius syndrome, first described only 3 years ago, can be easily misdiagnosed as neurofibromatosis type 1.
The diagnostic confusion has important consequences for the peace of mind of patients and their families. While Legius syndrome and neurofibromatosis type 1 (NF1) share several phenotypic features, Legius syndrome, unlike NF1, does not carry an increased cancer risk. Nor do patients with Legius syndrome develop clusters of benign cutaneous neurofibromas, Dr. Sirkku Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
As first described by Dr. Eric Legius and coworkers (Nat. Genet. 2007;39:1120-6) at the Catholic University of Leuven (Belgium), the hallmarks of Legius syndrome include multiple café au lait macules, axillary freckling, and autosomal dominant transmission, all of which are also among the NF1 diagnostic criteria established by the National Institutes of Health (JAMA 1997;278:51-7). But patients with Legius syndrome do not develop any other characteristic findings of NF1, such as bone lesions, plexiform or cutaneous neurofibromas, Lisch nodules in the iris, and nervous system tumors.
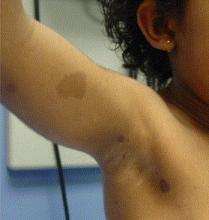
Legius syndrome is caused by mutations in the SPRED1 gene, while NF1 is caused by mutations in the gene encoding neurofibromin. Consider the possibility of Legius syndrome, which can be confirmed by molecular genetic analysis, in patients with six or more café au lait macules and axillary freckling but none of the other classic features of NF1, urged Dr. Peltonen of the University of Turku (Finland).
How often is Legius syndrome mistaken for NF1? A report by Dr. Legius and coworkers concluded half a cohort of 40 patients with Legius syndrome confirmed by genetic analysis fulfilled the NIH diagnostic criteria for NF1 based upon the presence of the requisite number of café au lait spots, axillary freckling, and/or a family history compatible with NF1. In the same report, the international group of investigators determined that 1.9% of a series of 1,318 patients with the clinical diagnosis of NF1 based upon the NIH criteria actually had Legius syndrome (JAMA 2009;302:2111-8).
Dr. Peltonen declared having no relevant financial interests.
GOTHENBURG, SWEDEN - Legius syndrome, first described only 3 years ago, can be easily misdiagnosed as neurofibromatosis type 1.
The diagnostic confusion has important consequences for the peace of mind of patients and their families. While Legius syndrome and neurofibromatosis type 1 (NF1) share several phenotypic features, Legius syndrome, unlike NF1, does not carry an increased cancer risk. Nor do patients with Legius syndrome develop clusters of benign cutaneous neurofibromas, Dr. Sirkku Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
As first described by Dr. Eric Legius and coworkers (Nat. Genet. 2007;39:1120-6) at the Catholic University of Leuven (Belgium), the hallmarks of Legius syndrome include multiple café au lait macules, axillary freckling, and autosomal dominant transmission, all of which are also among the NF1 diagnostic criteria established by the National Institutes of Health (JAMA 1997;278:51-7). But patients with Legius syndrome do not develop any other characteristic findings of NF1, such as bone lesions, plexiform or cutaneous neurofibromas, Lisch nodules in the iris, and nervous system tumors.
Legius syndrome is caused by mutations in the SPRED1 gene, while NF1 is caused by mutations in the gene encoding neurofibromin. Consider the possibility of Legius syndrome, which can be confirmed by molecular genetic analysis, in patients with six or more café au lait macules and axillary freckling but none of the other classic features of NF1, urged Dr. Peltonen of the University of Turku (Finland).
How often is Legius syndrome mistaken for NF1? A report by Dr. Legius and coworkers concluded half a cohort of 40 patients with Legius syndrome confirmed by genetic analysis fulfilled the NIH diagnostic criteria for NF1 based upon the presence of the requisite number of café au lait spots, axillary freckling, and/or a family history compatible with NF1. In the same report, the international group of investigators determined that 1.9% of a series of 1,318 patients with the clinical diagnosis of NF1 based upon the NIH criteria actually had Legius syndrome (JAMA 2009;302:2111-8).
Dr. Peltonen declared having no relevant financial interests.
GOTHENBURG, SWEDEN - Legius syndrome, first described only 3 years ago, can be easily misdiagnosed as neurofibromatosis type 1.
The diagnostic confusion has important consequences for the peace of mind of patients and their families. While Legius syndrome and neurofibromatosis type 1 (NF1) share several phenotypic features, Legius syndrome, unlike NF1, does not carry an increased cancer risk. Nor do patients with Legius syndrome develop clusters of benign cutaneous neurofibromas, Dr. Sirkku Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
As first described by Dr. Eric Legius and coworkers (Nat. Genet. 2007;39:1120-6) at the Catholic University of Leuven (Belgium), the hallmarks of Legius syndrome include multiple café au lait macules, axillary freckling, and autosomal dominant transmission, all of which are also among the NF1 diagnostic criteria established by the National Institutes of Health (JAMA 1997;278:51-7). But patients with Legius syndrome do not develop any other characteristic findings of NF1, such as bone lesions, plexiform or cutaneous neurofibromas, Lisch nodules in the iris, and nervous system tumors.
Legius syndrome is caused by mutations in the SPRED1 gene, while NF1 is caused by mutations in the gene encoding neurofibromin. Consider the possibility of Legius syndrome, which can be confirmed by molecular genetic analysis, in patients with six or more café au lait macules and axillary freckling but none of the other classic features of NF1, urged Dr. Peltonen of the University of Turku (Finland).
How often is Legius syndrome mistaken for NF1? A report by Dr. Legius and coworkers concluded half a cohort of 40 patients with Legius syndrome confirmed by genetic analysis fulfilled the NIH diagnostic criteria for NF1 based upon the presence of the requisite number of café au lait spots, axillary freckling, and/or a family history compatible with NF1. In the same report, the international group of investigators determined that 1.9% of a series of 1,318 patients with the clinical diagnosis of NF1 based upon the NIH criteria actually had Legius syndrome (JAMA 2009;302:2111-8).
Dr. Peltonen declared having no relevant financial interests.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
EADV: Diclofenac Gel Clears Actinic Keratoses in Transplant Recipients
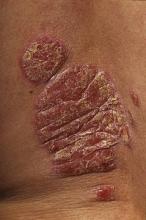
GOTHENBURG, SWEDEN – Topical diclofenac 3% gel proved effective for the treatment of actinic keratoses in organ transplant recipients, according to the results of a new study.
Sixteen weeks of twice-daily therapy with 3% diclofenac in 2.5% hyaluronic acid (Solaraze) not only proved effective and well tolerated for actinic keratoses (AK) clearance in a randomized, placebo-controlled trial, it also prevented invasive squamous cell carcinomas (SCCs) in organ transplant recipients, Dr. Eggert Stockfleth reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon an earlier favorable but uncontrolled six-patient series (B. J. Dermatol. 2007;156 Suppl. 3:40-2), Dr. Stockfleth and his coworkers conducted a follow-up randomized trial of topical diclofenace 3% gel in 32 organ transplant recipients.
Organ transplant recipients' immunocompromised status renders them highly vulnerable to multiple invasive SCCs, he said. As graft survival has improved over the years, the high incidence of aggressive cutaneous malignancies in organ transplant recipients has come to the fore as a major concern.
In Australia and New Zealand, cancer is now the No. 1 cause of death in organ transplant recipients, not heart disease, graft rejection, or infection. And the risk of aggressive skin cancers in these patients is far higher than the risk of other malignancies, according to Dr. Stockfleth of the director of the skin cancer center at Charité University Hospital, Berlin.
The 32 study participants were randomized 3-to-1 to diclofenac or a vehicle control. Study eligibility required a stable graft during the previous 12 months plus three or more AKs in a 50-cm2 area on the face, hands, or scalp.
Twenty-eight patients (88%) completed the 16-week, twice-daily treatment phase and presented for final evaluation 4 weeks later. Complete clearance of AKs was achieved in 9 of 22 (41%) of the diclofenac group, compared with 0 of 6 controls.
Side effects were limited to mild erythema and mild-to-moderate swelling of treated areas. No laboratory abnormalities or effects on graft function were noted.
With further follow-up, 55% of the patients who had previously cleared developed new AKs in the treated areas an average of 9.3 months after treatment ended. None of these patients developed invasive SCC in the study area within 24 months of follow-up, suggesting that topical diclofenac gel may also prevent invasive SCCs in this high-risk population.
Other treatments that have demonstrated efficacy for treatment of AKs and/or prevention of nonmelanoma skin cancer in high-risk organ transplant recipients include regular use of a sunscreen, imiquimod 5% cream, topical 5-fluorouracil, and photodynamic therapy.
Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Intendis, Meda, Abbott, and LEO.
GOTHENBURG, SWEDEN – Topical diclofenac 3% gel proved effective for the treatment of actinic keratoses in organ transplant recipients, according to the results of a new study.
Sixteen weeks of twice-daily therapy with 3% diclofenac in 2.5% hyaluronic acid (Solaraze) not only proved effective and well tolerated for actinic keratoses (AK) clearance in a randomized, placebo-controlled trial, it also prevented invasive squamous cell carcinomas (SCCs) in organ transplant recipients, Dr. Eggert Stockfleth reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon an earlier favorable but uncontrolled six-patient series (B. J. Dermatol. 2007;156 Suppl. 3:40-2), Dr. Stockfleth and his coworkers conducted a follow-up randomized trial of topical diclofenace 3% gel in 32 organ transplant recipients.
Organ transplant recipients' immunocompromised status renders them highly vulnerable to multiple invasive SCCs, he said. As graft survival has improved over the years, the high incidence of aggressive cutaneous malignancies in organ transplant recipients has come to the fore as a major concern.
In Australia and New Zealand, cancer is now the No. 1 cause of death in organ transplant recipients, not heart disease, graft rejection, or infection. And the risk of aggressive skin cancers in these patients is far higher than the risk of other malignancies, according to Dr. Stockfleth of the director of the skin cancer center at Charité University Hospital, Berlin.
The 32 study participants were randomized 3-to-1 to diclofenac or a vehicle control. Study eligibility required a stable graft during the previous 12 months plus three or more AKs in a 50-cm2 area on the face, hands, or scalp.
Twenty-eight patients (88%) completed the 16-week, twice-daily treatment phase and presented for final evaluation 4 weeks later. Complete clearance of AKs was achieved in 9 of 22 (41%) of the diclofenac group, compared with 0 of 6 controls.
Side effects were limited to mild erythema and mild-to-moderate swelling of treated areas. No laboratory abnormalities or effects on graft function were noted.
With further follow-up, 55% of the patients who had previously cleared developed new AKs in the treated areas an average of 9.3 months after treatment ended. None of these patients developed invasive SCC in the study area within 24 months of follow-up, suggesting that topical diclofenac gel may also prevent invasive SCCs in this high-risk population.
Other treatments that have demonstrated efficacy for treatment of AKs and/or prevention of nonmelanoma skin cancer in high-risk organ transplant recipients include regular use of a sunscreen, imiquimod 5% cream, topical 5-fluorouracil, and photodynamic therapy.
Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Intendis, Meda, Abbott, and LEO.
GOTHENBURG, SWEDEN – Topical diclofenac 3% gel proved effective for the treatment of actinic keratoses in organ transplant recipients, according to the results of a new study.
Sixteen weeks of twice-daily therapy with 3% diclofenac in 2.5% hyaluronic acid (Solaraze) not only proved effective and well tolerated for actinic keratoses (AK) clearance in a randomized, placebo-controlled trial, it also prevented invasive squamous cell carcinomas (SCCs) in organ transplant recipients, Dr. Eggert Stockfleth reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon an earlier favorable but uncontrolled six-patient series (B. J. Dermatol. 2007;156 Suppl. 3:40-2), Dr. Stockfleth and his coworkers conducted a follow-up randomized trial of topical diclofenace 3% gel in 32 organ transplant recipients.
Organ transplant recipients' immunocompromised status renders them highly vulnerable to multiple invasive SCCs, he said. As graft survival has improved over the years, the high incidence of aggressive cutaneous malignancies in organ transplant recipients has come to the fore as a major concern.
In Australia and New Zealand, cancer is now the No. 1 cause of death in organ transplant recipients, not heart disease, graft rejection, or infection. And the risk of aggressive skin cancers in these patients is far higher than the risk of other malignancies, according to Dr. Stockfleth of the director of the skin cancer center at Charité University Hospital, Berlin.
The 32 study participants were randomized 3-to-1 to diclofenac or a vehicle control. Study eligibility required a stable graft during the previous 12 months plus three or more AKs in a 50-cm2 area on the face, hands, or scalp.
Twenty-eight patients (88%) completed the 16-week, twice-daily treatment phase and presented for final evaluation 4 weeks later. Complete clearance of AKs was achieved in 9 of 22 (41%) of the diclofenac group, compared with 0 of 6 controls.
Side effects were limited to mild erythema and mild-to-moderate swelling of treated areas. No laboratory abnormalities or effects on graft function were noted.
With further follow-up, 55% of the patients who had previously cleared developed new AKs in the treated areas an average of 9.3 months after treatment ended. None of these patients developed invasive SCC in the study area within 24 months of follow-up, suggesting that topical diclofenac gel may also prevent invasive SCCs in this high-risk population.
Other treatments that have demonstrated efficacy for treatment of AKs and/or prevention of nonmelanoma skin cancer in high-risk organ transplant recipients include regular use of a sunscreen, imiquimod 5% cream, topical 5-fluorouracil, and photodynamic therapy.
Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Intendis, Meda, Abbott, and LEO.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Complete clearance of actinic keratoses was achieved in 41% of patients treated wtih diclofenac 3% gel, compared with 0% in the control group.
Data Source: The 32 organ transplant patients were randomized 3-to-1 to diclofenac or a vehicle control.
Disclosures: Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Itendis, Meda, Abbott, and Leo.
Expert Panel: Switch Methotrexate Nonresponders to Subcutaneous Form
GOTHENBURG, Sweden - A psoriasis patient should not be considered a methotrexate nonresponder before a subcutaneous dose is tried, an expert panel from the International Psoriasis Council said at the annual congress of the European Academy of Dermatology and Venereology.
"Improvement in psoriasis with methotrexate is dependent on the blood level. And we know that absorption of methotrexate in the gastrointestinal tract is incomplete and quite variable from person to person, especially at higher doses. You can turn a patient into a responder by switching from oral to subcutaneous administration," said Dr. Knud Kragballe, professor of dermatology at Aarhus (Denmark) University Hospital.
Methotrexate is a dermatologic tradition, and most dermatologists were trained to administer the drug orally, said Dr. Kragballe. The oral form is easier to use than injectable methotrexate, and a logical starting place; however, some patients who do not get sufficient benefit because oral methotrexate’s bioavailability is about 30% less than that of the subcutaneous form, he added.
The switch is a straightforward matter. The subcutaneous dose is equivalent to the oral dose. If a patient is not showing sufficient benefit, for example, on 20 mg of oral methotrexate once weekly, try 20 mg subcutaneously once weekly, he said.
The widespread dermatologic reluctance to use subcutaneous methotrexate is partly a matter of training, but is also a function of the plethora of biologic agents available, according to Dr. Kragballe.
"I think it’s important to utilize the full potential of any drug before switching to another drug. We have too many cases dismissed as methotrexate nonresponders when the problem is the drug is not being used at the proper dose and route of administration," he said.
An audience show of hands indicated only a minority of attendees at the International Psoriasis Council–sponsored meet-the-experts session now utilize subcutaneous methotrexate. But the subcutaneous route is not the hassle many dermatologists imagine, according to Dr. Wolfram Sterry.
"We switched 3 or 4 years ago in my department, and now we do only subcutaneous methotrexate. That way you know 10 mg is 10 mg, and that’s it. I haven’t yet found a patient who is uncomfortable with subcutaneous administration," said Dr. Sterry, professor and chairman of the department of dermatology and allergy at Charité University Hospital, Berlin.
Dr. Craig Leonardi said that he, like Dr. Kragballe, typically starts patients on oral methotrexate because it is easier, and that’s how he was trained, but he doesn’t hesitate to shift to the subcutaneous form.
In the United States, another reason to favor subcutaneous methotrexate, besides better bioavailability, is that the cost is only about one-tenth that of tablets.
"If a patient comes in who doesn’t have insurance and really can’t afford to pay for medications, subcutaneous methotrexate is the most cost-effective way to get the job done," said Dr. Leonardi a dermatologist and psoriasis specialist at St. Louis University.
International Psoriasis Council President, Dr. Alan Menter, commented that even though dermatologists have been using methotrexate for 40 years, the pharmacokinetics of the drug is still unclear.
Dr. Menter and his coworkers at Baylor University Medical Center, Dallas, where he is chief of dermatology, have been investigating a novel form of aminopterin – an antineoplastic drug closely related to methotrexate – which in preliminary phase I and II studies offered significant advantages over methotrexate: more predictable oral absorption, less uptake in cerebrospinal fluid, and less hepatotoxicity.
Speaking of hepatotoxicity, Dr. Menter said he and his colleagues have not conducted a liver biopsy in years, even though they have patients who have been on methotrexate for 2 decades.
"I think our gastroenterologists can now scan livers and look at early fibrosis much better than we can," Dr. Menter said.
Panelists noted there is no consensus on how to give folic acid to patients on methotrexate. Dr. Leonardi has patients take 1 mg daily. He thinks it is easier for patients to follow a daily routine.
Dr. Kragballe, in contrast, gives folic acid once per week 2 days after the methotrexate. "But there’s no science behind it," he noted. The important thing is that every psoriasis patient on methotrexate be on folic acid.
All of the International Psoriasis Council panelists disclosed serving as consultants, speakers, and investigators for numerous companies that market psoriasis therapies.
GOTHENBURG, Sweden - A psoriasis patient should not be considered a methotrexate nonresponder before a subcutaneous dose is tried, an expert panel from the International Psoriasis Council said at the annual congress of the European Academy of Dermatology and Venereology.
"Improvement in psoriasis with methotrexate is dependent on the blood level. And we know that absorption of methotrexate in the gastrointestinal tract is incomplete and quite variable from person to person, especially at higher doses. You can turn a patient into a responder by switching from oral to subcutaneous administration," said Dr. Knud Kragballe, professor of dermatology at Aarhus (Denmark) University Hospital.
Methotrexate is a dermatologic tradition, and most dermatologists were trained to administer the drug orally, said Dr. Kragballe. The oral form is easier to use than injectable methotrexate, and a logical starting place; however, some patients who do not get sufficient benefit because oral methotrexate’s bioavailability is about 30% less than that of the subcutaneous form, he added.
The switch is a straightforward matter. The subcutaneous dose is equivalent to the oral dose. If a patient is not showing sufficient benefit, for example, on 20 mg of oral methotrexate once weekly, try 20 mg subcutaneously once weekly, he said.
The widespread dermatologic reluctance to use subcutaneous methotrexate is partly a matter of training, but is also a function of the plethora of biologic agents available, according to Dr. Kragballe.
"I think it’s important to utilize the full potential of any drug before switching to another drug. We have too many cases dismissed as methotrexate nonresponders when the problem is the drug is not being used at the proper dose and route of administration," he said.
An audience show of hands indicated only a minority of attendees at the International Psoriasis Council–sponsored meet-the-experts session now utilize subcutaneous methotrexate. But the subcutaneous route is not the hassle many dermatologists imagine, according to Dr. Wolfram Sterry.
"We switched 3 or 4 years ago in my department, and now we do only subcutaneous methotrexate. That way you know 10 mg is 10 mg, and that’s it. I haven’t yet found a patient who is uncomfortable with subcutaneous administration," said Dr. Sterry, professor and chairman of the department of dermatology and allergy at Charité University Hospital, Berlin.
Dr. Craig Leonardi said that he, like Dr. Kragballe, typically starts patients on oral methotrexate because it is easier, and that’s how he was trained, but he doesn’t hesitate to shift to the subcutaneous form.
In the United States, another reason to favor subcutaneous methotrexate, besides better bioavailability, is that the cost is only about one-tenth that of tablets.
"If a patient comes in who doesn’t have insurance and really can’t afford to pay for medications, subcutaneous methotrexate is the most cost-effective way to get the job done," said Dr. Leonardi a dermatologist and psoriasis specialist at St. Louis University.
International Psoriasis Council President, Dr. Alan Menter, commented that even though dermatologists have been using methotrexate for 40 years, the pharmacokinetics of the drug is still unclear.
Dr. Menter and his coworkers at Baylor University Medical Center, Dallas, where he is chief of dermatology, have been investigating a novel form of aminopterin – an antineoplastic drug closely related to methotrexate – which in preliminary phase I and II studies offered significant advantages over methotrexate: more predictable oral absorption, less uptake in cerebrospinal fluid, and less hepatotoxicity.
Speaking of hepatotoxicity, Dr. Menter said he and his colleagues have not conducted a liver biopsy in years, even though they have patients who have been on methotrexate for 2 decades.
"I think our gastroenterologists can now scan livers and look at early fibrosis much better than we can," Dr. Menter said.
Panelists noted there is no consensus on how to give folic acid to patients on methotrexate. Dr. Leonardi has patients take 1 mg daily. He thinks it is easier for patients to follow a daily routine.
Dr. Kragballe, in contrast, gives folic acid once per week 2 days after the methotrexate. "But there’s no science behind it," he noted. The important thing is that every psoriasis patient on methotrexate be on folic acid.
All of the International Psoriasis Council panelists disclosed serving as consultants, speakers, and investigators for numerous companies that market psoriasis therapies.
GOTHENBURG, Sweden - A psoriasis patient should not be considered a methotrexate nonresponder before a subcutaneous dose is tried, an expert panel from the International Psoriasis Council said at the annual congress of the European Academy of Dermatology and Venereology.
"Improvement in psoriasis with methotrexate is dependent on the blood level. And we know that absorption of methotrexate in the gastrointestinal tract is incomplete and quite variable from person to person, especially at higher doses. You can turn a patient into a responder by switching from oral to subcutaneous administration," said Dr. Knud Kragballe, professor of dermatology at Aarhus (Denmark) University Hospital.
Methotrexate is a dermatologic tradition, and most dermatologists were trained to administer the drug orally, said Dr. Kragballe. The oral form is easier to use than injectable methotrexate, and a logical starting place; however, some patients who do not get sufficient benefit because oral methotrexate’s bioavailability is about 30% less than that of the subcutaneous form, he added.
The switch is a straightforward matter. The subcutaneous dose is equivalent to the oral dose. If a patient is not showing sufficient benefit, for example, on 20 mg of oral methotrexate once weekly, try 20 mg subcutaneously once weekly, he said.
The widespread dermatologic reluctance to use subcutaneous methotrexate is partly a matter of training, but is also a function of the plethora of biologic agents available, according to Dr. Kragballe.
"I think it’s important to utilize the full potential of any drug before switching to another drug. We have too many cases dismissed as methotrexate nonresponders when the problem is the drug is not being used at the proper dose and route of administration," he said.
An audience show of hands indicated only a minority of attendees at the International Psoriasis Council–sponsored meet-the-experts session now utilize subcutaneous methotrexate. But the subcutaneous route is not the hassle many dermatologists imagine, according to Dr. Wolfram Sterry.
"We switched 3 or 4 years ago in my department, and now we do only subcutaneous methotrexate. That way you know 10 mg is 10 mg, and that’s it. I haven’t yet found a patient who is uncomfortable with subcutaneous administration," said Dr. Sterry, professor and chairman of the department of dermatology and allergy at Charité University Hospital, Berlin.
Dr. Craig Leonardi said that he, like Dr. Kragballe, typically starts patients on oral methotrexate because it is easier, and that’s how he was trained, but he doesn’t hesitate to shift to the subcutaneous form.
In the United States, another reason to favor subcutaneous methotrexate, besides better bioavailability, is that the cost is only about one-tenth that of tablets.
"If a patient comes in who doesn’t have insurance and really can’t afford to pay for medications, subcutaneous methotrexate is the most cost-effective way to get the job done," said Dr. Leonardi a dermatologist and psoriasis specialist at St. Louis University.
International Psoriasis Council President, Dr. Alan Menter, commented that even though dermatologists have been using methotrexate for 40 years, the pharmacokinetics of the drug is still unclear.
Dr. Menter and his coworkers at Baylor University Medical Center, Dallas, where he is chief of dermatology, have been investigating a novel form of aminopterin – an antineoplastic drug closely related to methotrexate – which in preliminary phase I and II studies offered significant advantages over methotrexate: more predictable oral absorption, less uptake in cerebrospinal fluid, and less hepatotoxicity.
Speaking of hepatotoxicity, Dr. Menter said he and his colleagues have not conducted a liver biopsy in years, even though they have patients who have been on methotrexate for 2 decades.
"I think our gastroenterologists can now scan livers and look at early fibrosis much better than we can," Dr. Menter said.
Panelists noted there is no consensus on how to give folic acid to patients on methotrexate. Dr. Leonardi has patients take 1 mg daily. He thinks it is easier for patients to follow a daily routine.
Dr. Kragballe, in contrast, gives folic acid once per week 2 days after the methotrexate. "But there’s no science behind it," he noted. The important thing is that every psoriasis patient on methotrexate be on folic acid.
All of the International Psoriasis Council panelists disclosed serving as consultants, speakers, and investigators for numerous companies that market psoriasis therapies.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Expert Panel Recommends "SubQ Switch" for Oral Methotrexate Nonresponders
GOTHENBURG, Sweden - A psoriasis patient should not be considered a methotrexate nonresponder before a subcutaneous dose is tried, an expert panel from the International Psoriasis Council said at the annual congress of the European Academy of Dermatology and Venereology.
"Improvement in psoriasis with methotrexate is dependent on the blood level. And we know that absorption of methotrexate in the gastrointestinal tract is incomplete and quite variable from person to person, especially at higher doses. You can turn a patient into a responder by switching from oral to subcutaneous administration," said Dr. Knud Kragballe, professor of dermatology at Aarhus (Denmark) University Hospital.
Methotrexate is a dermatologic tradition, and most dermatologists were trained to administer the drug orally, said Dr. Kragballe. The oral form is easier to use than injectable methotrexate, and a logical starting place; however, some patients who do not get sufficient benefit because oral methotrexate’s bioavailability is about 30% less than that of the subcutaneous form, he added.
The switch is a straightforward matter. The subcutaneous dose is equivalent to the oral dose. If a patient is not showing sufficient benefit, for example, on 20 mg of oral methotrexate once weekly, try 20 mg subcutaneously once weekly, he said.
The widespread dermatologic reluctance to use subcutaneous methotrexate is partly a matter of training, but is also a function of the plethora of biologic agents available, according to Dr. Kragballe.
"I think it’s important to utilize the full potential of any drug before switching to another drug. We have too many cases dismissed as methotrexate nonresponders when the problem is the drug is not being used at the proper dose and route of administration," he said.
An audience show of hands indicated only a minority of attendees at the International Psoriasis Council–sponsored meet-the-experts session now utilize subcutaneous methotrexate. But the subcutaneous route is not the hassle many dermatologists imagine, according to Dr. Wolfram Sterry.
"We switched 3 or 4 years ago in my department, and now we do only subcutaneous methotrexate. That way you know 10 mg is 10 mg, and that’s it. I haven’t yet found a patient who is uncomfortable with subcutaneous administration," said Dr. Sterry, professor and chairman of the department of dermatology and allergy at Charité University Hospital, Berlin.
Dr. Craig Leonardi said that he, like Dr. Kragballe, typically starts patients on oral methotrexate because it is easier, and that’s how he was trained, but he doesn’t hesitate to shift to the subcutaneous form.
In the United States, another reason to favor subcutaneous methotrexate, besides better bioavailability, is that the cost is only about one-tenth that of tablets.
"If a patient comes in who doesn’t have insurance and really can’t afford to pay for medications, subcutaneous methotrexate is the most cost-effective way to get the job done," said Dr. Leonardi a dermatologist and psoriasis specialist at St. Louis University.
International Psoriasis Council President, Dr. Alan Menter, commented that even though dermatologists have been using methotrexate for 40 years, the pharmacokinetics of the drug is still unclear.
Dr. Menter and his coworkers at Baylor University Medical Center, Dallas, where he is chief of dermatology, have been investigating a novel form of aminopterin – an antineoplastic drug closely related to methotrexate – which in preliminary phase I and II studies offered significant advantages over methotrexate: more predictable oral absorption, less uptake in cerebrospinal fluid, and less hepatotoxicity.
Speaking of hepatotoxicity, Dr. Menter said he and his colleagues have not conducted a liver biopsy in years, even though they have patients who have been on methotrexate for 2 decades.
"I think our gastroenterologists can now scan livers and look at early fibrosis much better than we can," Dr. Menter said.
Panelists noted there is no consensus on how to give folic acid to patients on methotrexate. Dr. Leonardi has patients take 1 mg daily. He thinks it is easier for patients to follow a daily routine.
Dr. Kragballe, in contrast, gives folic acid once per week 2 days after the methotrexate. "But there’s no science behind it," he noted. The important thing is that every psoriasis patient on methotrexate be on folic acid.
All of the International Psoriasis Council panelists disclosed serving as consultants, speakers, and investigators for numerous companies that market psoriasis therapies.
GOTHENBURG, Sweden - A psoriasis patient should not be considered a methotrexate nonresponder before a subcutaneous dose is tried, an expert panel from the International Psoriasis Council said at the annual congress of the European Academy of Dermatology and Venereology.
"Improvement in psoriasis with methotrexate is dependent on the blood level. And we know that absorption of methotrexate in the gastrointestinal tract is incomplete and quite variable from person to person, especially at higher doses. You can turn a patient into a responder by switching from oral to subcutaneous administration," said Dr. Knud Kragballe, professor of dermatology at Aarhus (Denmark) University Hospital.
Methotrexate is a dermatologic tradition, and most dermatologists were trained to administer the drug orally, said Dr. Kragballe. The oral form is easier to use than injectable methotrexate, and a logical starting place; however, some patients who do not get sufficient benefit because oral methotrexate’s bioavailability is about 30% less than that of the subcutaneous form, he added.
The switch is a straightforward matter. The subcutaneous dose is equivalent to the oral dose. If a patient is not showing sufficient benefit, for example, on 20 mg of oral methotrexate once weekly, try 20 mg subcutaneously once weekly, he said.
The widespread dermatologic reluctance to use subcutaneous methotrexate is partly a matter of training, but is also a function of the plethora of biologic agents available, according to Dr. Kragballe.
"I think it’s important to utilize the full potential of any drug before switching to another drug. We have too many cases dismissed as methotrexate nonresponders when the problem is the drug is not being used at the proper dose and route of administration," he said.
An audience show of hands indicated only a minority of attendees at the International Psoriasis Council–sponsored meet-the-experts session now utilize subcutaneous methotrexate. But the subcutaneous route is not the hassle many dermatologists imagine, according to Dr. Wolfram Sterry.
"We switched 3 or 4 years ago in my department, and now we do only subcutaneous methotrexate. That way you know 10 mg is 10 mg, and that’s it. I haven’t yet found a patient who is uncomfortable with subcutaneous administration," said Dr. Sterry, professor and chairman of the department of dermatology and allergy at Charité University Hospital, Berlin.
Dr. Craig Leonardi said that he, like Dr. Kragballe, typically starts patients on oral methotrexate because it is easier, and that’s how he was trained, but he doesn’t hesitate to shift to the subcutaneous form.
In the United States, another reason to favor subcutaneous methotrexate, besides better bioavailability, is that the cost is only about one-tenth that of tablets.
"If a patient comes in who doesn’t have insurance and really can’t afford to pay for medications, subcutaneous methotrexate is the most cost-effective way to get the job done," said Dr. Leonardi a dermatologist and psoriasis specialist at St. Louis University.
International Psoriasis Council President, Dr. Alan Menter, commented that even though dermatologists have been using methotrexate for 40 years, the pharmacokinetics of the drug is still unclear.
Dr. Menter and his coworkers at Baylor University Medical Center, Dallas, where he is chief of dermatology, have been investigating a novel form of aminopterin – an antineoplastic drug closely related to methotrexate – which in preliminary phase I and II studies offered significant advantages over methotrexate: more predictable oral absorption, less uptake in cerebrospinal fluid, and less hepatotoxicity.
Speaking of hepatotoxicity, Dr. Menter said he and his colleagues have not conducted a liver biopsy in years, even though they have patients who have been on methotrexate for 2 decades.
"I think our gastroenterologists can now scan livers and look at early fibrosis much better than we can," Dr. Menter said.
Panelists noted there is no consensus on how to give folic acid to patients on methotrexate. Dr. Leonardi has patients take 1 mg daily. He thinks it is easier for patients to follow a daily routine.
Dr. Kragballe, in contrast, gives folic acid once per week 2 days after the methotrexate. "But there’s no science behind it," he noted. The important thing is that every psoriasis patient on methotrexate be on folic acid.
All of the International Psoriasis Council panelists disclosed serving as consultants, speakers, and investigators for numerous companies that market psoriasis therapies.
GOTHENBURG, Sweden - A psoriasis patient should not be considered a methotrexate nonresponder before a subcutaneous dose is tried, an expert panel from the International Psoriasis Council said at the annual congress of the European Academy of Dermatology and Venereology.
"Improvement in psoriasis with methotrexate is dependent on the blood level. And we know that absorption of methotrexate in the gastrointestinal tract is incomplete and quite variable from person to person, especially at higher doses. You can turn a patient into a responder by switching from oral to subcutaneous administration," said Dr. Knud Kragballe, professor of dermatology at Aarhus (Denmark) University Hospital.
Methotrexate is a dermatologic tradition, and most dermatologists were trained to administer the drug orally, said Dr. Kragballe. The oral form is easier to use than injectable methotrexate, and a logical starting place; however, some patients who do not get sufficient benefit because oral methotrexate’s bioavailability is about 30% less than that of the subcutaneous form, he added.
The switch is a straightforward matter. The subcutaneous dose is equivalent to the oral dose. If a patient is not showing sufficient benefit, for example, on 20 mg of oral methotrexate once weekly, try 20 mg subcutaneously once weekly, he said.
The widespread dermatologic reluctance to use subcutaneous methotrexate is partly a matter of training, but is also a function of the plethora of biologic agents available, according to Dr. Kragballe.
"I think it’s important to utilize the full potential of any drug before switching to another drug. We have too many cases dismissed as methotrexate nonresponders when the problem is the drug is not being used at the proper dose and route of administration," he said.
An audience show of hands indicated only a minority of attendees at the International Psoriasis Council–sponsored meet-the-experts session now utilize subcutaneous methotrexate. But the subcutaneous route is not the hassle many dermatologists imagine, according to Dr. Wolfram Sterry.
"We switched 3 or 4 years ago in my department, and now we do only subcutaneous methotrexate. That way you know 10 mg is 10 mg, and that’s it. I haven’t yet found a patient who is uncomfortable with subcutaneous administration," said Dr. Sterry, professor and chairman of the department of dermatology and allergy at Charité University Hospital, Berlin.
Dr. Craig Leonardi said that he, like Dr. Kragballe, typically starts patients on oral methotrexate because it is easier, and that’s how he was trained, but he doesn’t hesitate to shift to the subcutaneous form.
In the United States, another reason to favor subcutaneous methotrexate, besides better bioavailability, is that the cost is only about one-tenth that of tablets.
"If a patient comes in who doesn’t have insurance and really can’t afford to pay for medications, subcutaneous methotrexate is the most cost-effective way to get the job done," said Dr. Leonardi a dermatologist and psoriasis specialist at St. Louis University.
International Psoriasis Council President, Dr. Alan Menter, commented that even though dermatologists have been using methotrexate for 40 years, the pharmacokinetics of the drug is still unclear.
Dr. Menter and his coworkers at Baylor University Medical Center, Dallas, where he is chief of dermatology, have been investigating a novel form of aminopterin – an antineoplastic drug closely related to methotrexate – which in preliminary phase I and II studies offered significant advantages over methotrexate: more predictable oral absorption, less uptake in cerebrospinal fluid, and less hepatotoxicity.
Speaking of hepatotoxicity, Dr. Menter said he and his colleagues have not conducted a liver biopsy in years, even though they have patients who have been on methotrexate for 2 decades.
"I think our gastroenterologists can now scan livers and look at early fibrosis much better than we can," Dr. Menter said.
Panelists noted there is no consensus on how to give folic acid to patients on methotrexate. Dr. Leonardi has patients take 1 mg daily. He thinks it is easier for patients to follow a daily routine.
Dr. Kragballe, in contrast, gives folic acid once per week 2 days after the methotrexate. "But there’s no science behind it," he noted. The important thing is that every psoriasis patient on methotrexate be on folic acid.
All of the International Psoriasis Council panelists disclosed serving as consultants, speakers, and investigators for numerous companies that market psoriasis therapies.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
AMG 827 Trial Participants Show Improvement of Chronic Plaque Psoriasis
GOTHENBURG, SWEDEN – Blockade of the receptor for the proinflammatory cytokine interleukin-17 using a novel fully human IgG2 monoclonal antibody has shown promise as a treatment for chronic plaque psoriasis.
In a small, phase I, double-blind randomized study, a single dose of AMG 827 was well tolerated and led to rapid clinical improvement accompanied by reversal of the molecular, cellular, and histologic hallmarks of the disease, Dr. David A. Martin reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon these findings, a phase II study of AMG 827 in patients with plaque psoriasis is underway, added Dr. Martin, an employee of the drug’s manufacturer and study sponsor, Amgen (Seattle).
He reported on 20 psoriasis patients who were randomized 4:4:1 to a single IV dose of AMG 827 at either 350 mg or 700 mg or to placebo. Biopsies were obtained from lesional and nonlesional skin at baseline and from lesional skin on days 8, 15, and 43.
By day 15 the 16 patients who received AMG 827 showed roughly a mean 60% improvement over baseline in terms of Psoriasis Area and Severity Index (PASI) scores. By day 43 the group treated with the 700-mg dose showed continued gains, with an 85% improvement in PASI scores over baseline. A PASI 75, or a 75% improvement over baseline, was achieved by day 43 in five of eight patients in the 350-mg group, seven of eight who got 700 mg of AMG 827, and none of four placebo-treated controls.
Physician’s Global Assessment ratings of psoriasis as "clear" or "minimal" were attained by all eight patients in the 700-mg group, five of eight who received 350 mg, and none of four controls.
At baseline, roughly 10,000 gene expression sequences were abnormally up- or downregulated in lesional skin. By day 8, this was reduced to about 2,000 abnormally expressed gene sequences. By day 15, the abnormal gene expression signature in the 700-mg group had dropped to roughly 500 abnormal sequences, nearly the same as in nonlesional skin at baseline.
The low level of abnormal gene expression was maintained in the 700-mg group out to day 43. Meanwhile, the abnormal gene expression signature in the 350-mg group improved from 2,000 sequences on day 8 to about 1,000 on day 43, Dr. Martin said.
Histopathologic findings in lesional skin included significant reductions in epidermal thickness, keratin-16 mRNA levels, Ki-67 cell counts, and the number of infiltrating leukocyte subsets by day 15, with further improvements noted by day 43.
AMG 827 is also under study for the treatment of rheumatoid arthritis and other inflammatory diseases. Interleukin-17 is known to activate local expression of a range of cytokines, metalloproteinases, chemokines, and antimicrobial peptides.
GOTHENBURG, SWEDEN – Blockade of the receptor for the proinflammatory cytokine interleukin-17 using a novel fully human IgG2 monoclonal antibody has shown promise as a treatment for chronic plaque psoriasis.
In a small, phase I, double-blind randomized study, a single dose of AMG 827 was well tolerated and led to rapid clinical improvement accompanied by reversal of the molecular, cellular, and histologic hallmarks of the disease, Dr. David A. Martin reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon these findings, a phase II study of AMG 827 in patients with plaque psoriasis is underway, added Dr. Martin, an employee of the drug’s manufacturer and study sponsor, Amgen (Seattle).
He reported on 20 psoriasis patients who were randomized 4:4:1 to a single IV dose of AMG 827 at either 350 mg or 700 mg or to placebo. Biopsies were obtained from lesional and nonlesional skin at baseline and from lesional skin on days 8, 15, and 43.
By day 15 the 16 patients who received AMG 827 showed roughly a mean 60% improvement over baseline in terms of Psoriasis Area and Severity Index (PASI) scores. By day 43 the group treated with the 700-mg dose showed continued gains, with an 85% improvement in PASI scores over baseline. A PASI 75, or a 75% improvement over baseline, was achieved by day 43 in five of eight patients in the 350-mg group, seven of eight who got 700 mg of AMG 827, and none of four placebo-treated controls.
Physician’s Global Assessment ratings of psoriasis as "clear" or "minimal" were attained by all eight patients in the 700-mg group, five of eight who received 350 mg, and none of four controls.
At baseline, roughly 10,000 gene expression sequences were abnormally up- or downregulated in lesional skin. By day 8, this was reduced to about 2,000 abnormally expressed gene sequences. By day 15, the abnormal gene expression signature in the 700-mg group had dropped to roughly 500 abnormal sequences, nearly the same as in nonlesional skin at baseline.
The low level of abnormal gene expression was maintained in the 700-mg group out to day 43. Meanwhile, the abnormal gene expression signature in the 350-mg group improved from 2,000 sequences on day 8 to about 1,000 on day 43, Dr. Martin said.
Histopathologic findings in lesional skin included significant reductions in epidermal thickness, keratin-16 mRNA levels, Ki-67 cell counts, and the number of infiltrating leukocyte subsets by day 15, with further improvements noted by day 43.
AMG 827 is also under study for the treatment of rheumatoid arthritis and other inflammatory diseases. Interleukin-17 is known to activate local expression of a range of cytokines, metalloproteinases, chemokines, and antimicrobial peptides.
GOTHENBURG, SWEDEN – Blockade of the receptor for the proinflammatory cytokine interleukin-17 using a novel fully human IgG2 monoclonal antibody has shown promise as a treatment for chronic plaque psoriasis.
In a small, phase I, double-blind randomized study, a single dose of AMG 827 was well tolerated and led to rapid clinical improvement accompanied by reversal of the molecular, cellular, and histologic hallmarks of the disease, Dr. David A. Martin reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon these findings, a phase II study of AMG 827 in patients with plaque psoriasis is underway, added Dr. Martin, an employee of the drug’s manufacturer and study sponsor, Amgen (Seattle).
He reported on 20 psoriasis patients who were randomized 4:4:1 to a single IV dose of AMG 827 at either 350 mg or 700 mg or to placebo. Biopsies were obtained from lesional and nonlesional skin at baseline and from lesional skin on days 8, 15, and 43.
By day 15 the 16 patients who received AMG 827 showed roughly a mean 60% improvement over baseline in terms of Psoriasis Area and Severity Index (PASI) scores. By day 43 the group treated with the 700-mg dose showed continued gains, with an 85% improvement in PASI scores over baseline. A PASI 75, or a 75% improvement over baseline, was achieved by day 43 in five of eight patients in the 350-mg group, seven of eight who got 700 mg of AMG 827, and none of four placebo-treated controls.
Physician’s Global Assessment ratings of psoriasis as "clear" or "minimal" were attained by all eight patients in the 700-mg group, five of eight who received 350 mg, and none of four controls.
At baseline, roughly 10,000 gene expression sequences were abnormally up- or downregulated in lesional skin. By day 8, this was reduced to about 2,000 abnormally expressed gene sequences. By day 15, the abnormal gene expression signature in the 700-mg group had dropped to roughly 500 abnormal sequences, nearly the same as in nonlesional skin at baseline.
The low level of abnormal gene expression was maintained in the 700-mg group out to day 43. Meanwhile, the abnormal gene expression signature in the 350-mg group improved from 2,000 sequences on day 8 to about 1,000 on day 43, Dr. Martin said.
Histopathologic findings in lesional skin included significant reductions in epidermal thickness, keratin-16 mRNA levels, Ki-67 cell counts, and the number of infiltrating leukocyte subsets by day 15, with further improvements noted by day 43.
AMG 827 is also under study for the treatment of rheumatoid arthritis and other inflammatory diseases. Interleukin-17 is known to activate local expression of a range of cytokines, metalloproteinases, chemokines, and antimicrobial peptides.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: PASI 75 was achieved by day 43 in five of eight patients who received 350 mg AMG 827, in seven of eight who got 700 mg, and none of four placebo-treated controls.
Data Source: A phase II study of AMG 827 of 20 patients with plaque psoriasis who were randomized 4:4:1 to a single intravenous dose of AMG 827 at either 350 mg or 700 mg or to placebo.
Disclosures: Dr. Martin is an employee of the AMG 827 manufacturer and study sponsor, Amgen (Seattle).