User login
AMG 827 Trial Participants Show Improvement of Chronic Plaque Psoriasis
GOTHENBURG, SWEDEN – Blockade of the receptor for the proinflammatory cytokine interleukin-17 using a novel fully human IgG2 monoclonal antibody has shown promise as a treatment for chronic plaque psoriasis.
In a small, phase I, double-blind randomized study, a single dose of AMG 827 was well tolerated and led to rapid clinical improvement accompanied by reversal of the molecular, cellular, and histologic hallmarks of the disease, Dr. David A. Martin reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon these findings, a phase II study of AMG 827 in patients with plaque psoriasis is underway, added Dr. Martin, an employee of the drug’s manufacturer and study sponsor, Amgen (Seattle).
He reported on 20 psoriasis patients who were randomized 4:4:1 to a single IV dose of AMG 827 at either 350 mg or 700 mg or to placebo. Biopsies were obtained from lesional and nonlesional skin at baseline and from lesional skin on days 8, 15, and 43.
By day 15 the 16 patients who received AMG 827 showed roughly a mean 60% improvement over baseline in terms of Psoriasis Area and Severity Index (PASI) scores. By day 43 the group treated with the 700-mg dose showed continued gains, with an 85% improvement in PASI scores over baseline. A PASI 75, or a 75% improvement over baseline, was achieved by day 43 in five of eight patients in the 350-mg group, seven of eight who got 700 mg of AMG 827, and none of four placebo-treated controls.
Physician’s Global Assessment ratings of psoriasis as "clear" or "minimal" were attained by all eight patients in the 700-mg group, five of eight who received 350 mg, and none of four controls.
At baseline, roughly 10,000 gene expression sequences were abnormally up- or downregulated in lesional skin. By day 8, this was reduced to about 2,000 abnormally expressed gene sequences. By day 15, the abnormal gene expression signature in the 700-mg group had dropped to roughly 500 abnormal sequences, nearly the same as in nonlesional skin at baseline.
The low level of abnormal gene expression was maintained in the 700-mg group out to day 43. Meanwhile, the abnormal gene expression signature in the 350-mg group improved from 2,000 sequences on day 8 to about 1,000 on day 43, Dr. Martin said.
Histopathologic findings in lesional skin included significant reductions in epidermal thickness, keratin-16 mRNA levels, Ki-67 cell counts, and the number of infiltrating leukocyte subsets by day 15, with further improvements noted by day 43.
AMG 827 is also under study for the treatment of rheumatoid arthritis and other inflammatory diseases. Interleukin-17 is known to activate local expression of a range of cytokines, metalloproteinases, chemokines, and antimicrobial peptides.
GOTHENBURG, SWEDEN – Blockade of the receptor for the proinflammatory cytokine interleukin-17 using a novel fully human IgG2 monoclonal antibody has shown promise as a treatment for chronic plaque psoriasis.
In a small, phase I, double-blind randomized study, a single dose of AMG 827 was well tolerated and led to rapid clinical improvement accompanied by reversal of the molecular, cellular, and histologic hallmarks of the disease, Dr. David A. Martin reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon these findings, a phase II study of AMG 827 in patients with plaque psoriasis is underway, added Dr. Martin, an employee of the drug’s manufacturer and study sponsor, Amgen (Seattle).
He reported on 20 psoriasis patients who were randomized 4:4:1 to a single IV dose of AMG 827 at either 350 mg or 700 mg or to placebo. Biopsies were obtained from lesional and nonlesional skin at baseline and from lesional skin on days 8, 15, and 43.
By day 15 the 16 patients who received AMG 827 showed roughly a mean 60% improvement over baseline in terms of Psoriasis Area and Severity Index (PASI) scores. By day 43 the group treated with the 700-mg dose showed continued gains, with an 85% improvement in PASI scores over baseline. A PASI 75, or a 75% improvement over baseline, was achieved by day 43 in five of eight patients in the 350-mg group, seven of eight who got 700 mg of AMG 827, and none of four placebo-treated controls.
Physician’s Global Assessment ratings of psoriasis as "clear" or "minimal" were attained by all eight patients in the 700-mg group, five of eight who received 350 mg, and none of four controls.
At baseline, roughly 10,000 gene expression sequences were abnormally up- or downregulated in lesional skin. By day 8, this was reduced to about 2,000 abnormally expressed gene sequences. By day 15, the abnormal gene expression signature in the 700-mg group had dropped to roughly 500 abnormal sequences, nearly the same as in nonlesional skin at baseline.
The low level of abnormal gene expression was maintained in the 700-mg group out to day 43. Meanwhile, the abnormal gene expression signature in the 350-mg group improved from 2,000 sequences on day 8 to about 1,000 on day 43, Dr. Martin said.
Histopathologic findings in lesional skin included significant reductions in epidermal thickness, keratin-16 mRNA levels, Ki-67 cell counts, and the number of infiltrating leukocyte subsets by day 15, with further improvements noted by day 43.
AMG 827 is also under study for the treatment of rheumatoid arthritis and other inflammatory diseases. Interleukin-17 is known to activate local expression of a range of cytokines, metalloproteinases, chemokines, and antimicrobial peptides.
GOTHENBURG, SWEDEN – Blockade of the receptor for the proinflammatory cytokine interleukin-17 using a novel fully human IgG2 monoclonal antibody has shown promise as a treatment for chronic plaque psoriasis.
In a small, phase I, double-blind randomized study, a single dose of AMG 827 was well tolerated and led to rapid clinical improvement accompanied by reversal of the molecular, cellular, and histologic hallmarks of the disease, Dr. David A. Martin reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon these findings, a phase II study of AMG 827 in patients with plaque psoriasis is underway, added Dr. Martin, an employee of the drug’s manufacturer and study sponsor, Amgen (Seattle).
He reported on 20 psoriasis patients who were randomized 4:4:1 to a single IV dose of AMG 827 at either 350 mg or 700 mg or to placebo. Biopsies were obtained from lesional and nonlesional skin at baseline and from lesional skin on days 8, 15, and 43.
By day 15 the 16 patients who received AMG 827 showed roughly a mean 60% improvement over baseline in terms of Psoriasis Area and Severity Index (PASI) scores. By day 43 the group treated with the 700-mg dose showed continued gains, with an 85% improvement in PASI scores over baseline. A PASI 75, or a 75% improvement over baseline, was achieved by day 43 in five of eight patients in the 350-mg group, seven of eight who got 700 mg of AMG 827, and none of four placebo-treated controls.
Physician’s Global Assessment ratings of psoriasis as "clear" or "minimal" were attained by all eight patients in the 700-mg group, five of eight who received 350 mg, and none of four controls.
At baseline, roughly 10,000 gene expression sequences were abnormally up- or downregulated in lesional skin. By day 8, this was reduced to about 2,000 abnormally expressed gene sequences. By day 15, the abnormal gene expression signature in the 700-mg group had dropped to roughly 500 abnormal sequences, nearly the same as in nonlesional skin at baseline.
The low level of abnormal gene expression was maintained in the 700-mg group out to day 43. Meanwhile, the abnormal gene expression signature in the 350-mg group improved from 2,000 sequences on day 8 to about 1,000 on day 43, Dr. Martin said.
Histopathologic findings in lesional skin included significant reductions in epidermal thickness, keratin-16 mRNA levels, Ki-67 cell counts, and the number of infiltrating leukocyte subsets by day 15, with further improvements noted by day 43.
AMG 827 is also under study for the treatment of rheumatoid arthritis and other inflammatory diseases. Interleukin-17 is known to activate local expression of a range of cytokines, metalloproteinases, chemokines, and antimicrobial peptides.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: PASI 75 was achieved by day 43 in five of eight patients who received 350 mg AMG 827, in seven of eight who got 700 mg, and none of four placebo-treated controls.
Data Source: A phase II study of AMG 827 of 20 patients with plaque psoriasis who were randomized 4:4:1 to a single intravenous dose of AMG 827 at either 350 mg or 700 mg or to placebo.
Disclosures: Dr. Martin is an employee of the AMG 827 manufacturer and study sponsor, Amgen (Seattle).
Skin Infections From Mycobacteria Present Challenges
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions within a new tattoo.
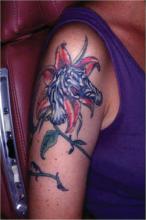
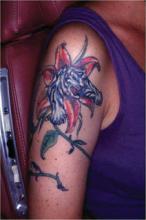
That’s the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that’s not responding to appropriate antibiotic therapy.
“RGMs are underrecognized, underreported, and we think they’re increasing in prevalence,” the dermatologist said.
“I think ‘staph’ first because it’s so much more common. But if an infection isn’t responding within a couple of weeks and tests show it’s not methicillin-resistant staph – and especially if there’s been a procedure – think RGM,” Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures. The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28?-30?C while others require 35?-37?C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
“Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won’t do the specific techniques for mycobacteria, and you’ll get a negative result. It’s important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I’m thinking and what to do,” she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. Indeed, M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues. In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. Indeed, the median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
“I’ve recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM,” Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it’s quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They’re eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
“In the United States, people who cut hair are more regulated than people who do tattoos,” Dr. Drage noted.
She reported having no relevant financial conflicts.
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions within a new tattoo.
That’s the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that’s not responding to appropriate antibiotic therapy.
“RGMs are underrecognized, underreported, and we think they’re increasing in prevalence,” the dermatologist said.
“I think ‘staph’ first because it’s so much more common. But if an infection isn’t responding within a couple of weeks and tests show it’s not methicillin-resistant staph – and especially if there’s been a procedure – think RGM,” Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures. The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28?-30?C while others require 35?-37?C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
“Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won’t do the specific techniques for mycobacteria, and you’ll get a negative result. It’s important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I’m thinking and what to do,” she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. Indeed, M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues. In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. Indeed, the median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
“I’ve recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM,” Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it’s quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They’re eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
“In the United States, people who cut hair are more regulated than people who do tattoos,” Dr. Drage noted.
She reported having no relevant financial conflicts.
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions within a new tattoo.
That’s the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that’s not responding to appropriate antibiotic therapy.
“RGMs are underrecognized, underreported, and we think they’re increasing in prevalence,” the dermatologist said.
“I think ‘staph’ first because it’s so much more common. But if an infection isn’t responding within a couple of weeks and tests show it’s not methicillin-resistant staph – and especially if there’s been a procedure – think RGM,” Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures. The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28?-30?C while others require 35?-37?C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
“Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won’t do the specific techniques for mycobacteria, and you’ll get a negative result. It’s important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I’m thinking and what to do,” she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. Indeed, M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues. In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. Indeed, the median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
“I’ve recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM,” Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it’s quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They’re eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
“In the United States, people who cut hair are more regulated than people who do tattoos,” Dr. Drage noted.
She reported having no relevant financial conflicts.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
EADV: Skin Infections From Mycobacteria Present Challenges
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions with a new tattoo.
That's the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that's not responding to appropriate antibiotic therapy.
Diagnosis
"RGMs are underrecognized, underreported, and we think they're increasing in prevalence," the dermatologist said.
"I think 'staph' first because it's so much more common. But if an infection isn’t responding within a couple of weeks and tests show it's not methicillin-resistant staph – and especially if there's been a procedure – think RGM," Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures.
The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28 degrees C to 30 degrees C while others require 35 degrees C to 37 degrees C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
"Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won't do the specific techniques for mycobacteria, and you'll get a negative result. It's important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I'm thinking and what to do," she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues.
In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. The median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
"I've recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM," Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it's quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They're eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
"In the United States, people who cut hair are more regulated than people who do tattoos," Dr. Drage noted.
She reported having no relevant financial conflicts.
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions with a new tattoo.
That's the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that's not responding to appropriate antibiotic therapy.
Diagnosis
"RGMs are underrecognized, underreported, and we think they're increasing in prevalence," the dermatologist said.
"I think 'staph' first because it's so much more common. But if an infection isn’t responding within a couple of weeks and tests show it's not methicillin-resistant staph – and especially if there's been a procedure – think RGM," Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures.
The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28 degrees C to 30 degrees C while others require 35 degrees C to 37 degrees C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
"Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won't do the specific techniques for mycobacteria, and you'll get a negative result. It's important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I'm thinking and what to do," she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues.
In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. The median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
"I've recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM," Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it's quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They're eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
"In the United States, people who cut hair are more regulated than people who do tattoos," Dr. Drage noted.
She reported having no relevant financial conflicts.
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions with a new tattoo.
That's the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that's not responding to appropriate antibiotic therapy.
Diagnosis
"RGMs are underrecognized, underreported, and we think they're increasing in prevalence," the dermatologist said.
"I think 'staph' first because it's so much more common. But if an infection isn’t responding within a couple of weeks and tests show it's not methicillin-resistant staph – and especially if there's been a procedure – think RGM," Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures.
The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28 degrees C to 30 degrees C while others require 35 degrees C to 37 degrees C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
"Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won't do the specific techniques for mycobacteria, and you'll get a negative result. It's important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I'm thinking and what to do," she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues.
In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. The median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
"I've recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM," Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it's quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They're eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
"In the United States, people who cut hair are more regulated than people who do tattoos," Dr. Drage noted.
She reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Skin Infections Caused By Mycobacteria Present Challenges
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions within a new tattoo.
That’s the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that’s not responding to appropriate antibiotic therapy.
“RGMs are underrecognized, underreported, and we think they’re increasing in prevalence,” the dermatologist said.
“I think ‘staph’ first because it’s so much more common. But if an infection isn’t responding within a couple of weeks and tests show it’s not methicillin-resistant staph – and especially if there’s been a procedure – think RGM,” Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures. The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28?-30?C while others require 35?-37?C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
“Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won’t do the specific techniques for mycobacteria, and you’ll get a negative result. It’s important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I’m thinking and what to do,” she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. Indeed, M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues. In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. Indeed, the median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
“I’ve recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM,” Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it’s quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They’re eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
“In the United States, people who cut hair are more regulated than people who do tattoos,” Dr. Drage noted.
She reported having no relevant financial conflicts.
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions within a new tattoo.
That’s the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that’s not responding to appropriate antibiotic therapy.
“RGMs are underrecognized, underreported, and we think they’re increasing in prevalence,” the dermatologist said.
“I think ‘staph’ first because it’s so much more common. But if an infection isn’t responding within a couple of weeks and tests show it’s not methicillin-resistant staph – and especially if there’s been a procedure – think RGM,” Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures. The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28?-30?C while others require 35?-37?C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
“Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won’t do the specific techniques for mycobacteria, and you’ll get a negative result. It’s important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I’m thinking and what to do,” she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. Indeed, M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues. In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. Indeed, the median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
“I’ve recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM,” Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it’s quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They’re eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
“In the United States, people who cut hair are more regulated than people who do tattoos,” Dr. Drage noted.
She reported having no relevant financial conflicts.
GOTHENBURG, Sweden – Consider rapidly growing nontuberculous mycobacteria infection as a distinct possibility in patients who present with a rash or other skin lesions within a new tattoo.
That’s the advice of Mayo Clinic dermatologist Dr. Lisa A. Drage, who encountered six affected patients in relatively short order, all with tattoos done by the same artist at a single Rochester, Minn., tattoo parlor.
The likely source of this outbreak of Mycobacterium chelonae infection was the tap water the tattoo artist used to dilute black ink to create shades of gray for the popular gray wash technique, which creates a subtle photographic effect, she explained at the annual congress of the European Academy of Dermatology and Venereology.
The tipoff that the likely contaminant was tap water was that the skin lesions, although quite varied in appearance, were concentrated in the gray areas of the tattoos, with the deep black areas and clear-skin uninked portions largely spared, Dr. Drage said.
More broadly, she suggested keeping M. chelonae and the other rapidly growing mycobacteria (RGM) in mind when diagnosing any patient with a postprocedure skin infection that’s not responding to appropriate antibiotic therapy.
“RGMs are underrecognized, underreported, and we think they’re increasing in prevalence,” the dermatologist said.
“I think ‘staph’ first because it’s so much more common. But if an infection isn’t responding within a couple of weeks and tests show it’s not methicillin-resistant staph – and especially if there’s been a procedure – think RGM,” Dr. Drage said.
The reason RGM infections are underdiagnosed is that the culture techniques required to confirm the diagnosis are completely different from those utilized in conventional bacterial cultures. The techniques for nontuberculous mycobacteria (NTM) are quite involved, with multiple cultures needing to be started at a variety of temperatures, since some RGM will grow only at 28?-30?C while others require 35?-37?C. Unlike the case in M. tuberculosis infections, where polymerase chain reaction or other rapid diagnostic systems can be applied directly to the clinical specimen, the NTM organisms must first be grown out on solid culture media before DNA sequencing can be utilized to identify the species.
“Most physicians who send the specimen to the laboratory fail to order the appropriate test. If you just send it for bacteriologic testing, they won’t do the specific techniques for mycobacteria, and you’ll get a negative result. It’s important to obtain cultures specifically for mycobacteria, along with susceptibility studies. I find it most helpful to speak with the lab so they know what I’m thinking and what to do,” she said.
The RGM, which include M. chelonae and M. fortuitum, are the most common NTM involved in community-acquired skin and soft-tissue infections. Indeed, M. fortuitum alone is believed to account for more than 60% of such infections.
These are environmental microorganisms; there is no person-to-person spread. Tap water is the most common source of contamination. The NTM reside in the biofilm. They are resistant to sterilizers, disinfectants, and antiseptics. Numerous infections have come from hospital water systems.
Cutaneous infections with RGM have been documented in association with a variety of procedures, including Mohs surgery, acupuncture, punch biopsy, liposuction, laser resurfacing and other cosmetic procedures, and tattooing. In one California study, 29 of 30 pedicure foot baths in 18 salons in five counties contained NTM.
The skin lesions typically appear within a couple of weeks after the procedure, but lengthy delay in diagnosis is common. Patients may initially shrug off the skin lesions as bug bites or something else not warranting medical attention. And once they visit a physician, further diagnostic delay often ensues. In the six Minnesota patients with M. chelonae infections in tattoos, for example, the skin lesions appeared 1-2 weeks after the tattoo was placed, and all patients sought medical care promptly, but they were treated for a diagnosis other than NTM infection. Indeed, the median time between tattoo placement and the correct diagnosis was 18 weeks.
Timely diagnosis of RGM infections is made more challenging by the highly variable clinical appearance of the skin lesions. Painful red nodules that ulcerate and drain are one of the classic pictures. Pink or purple papules, granulomatous papules, pustules, plaques, folliculitis, cellulitis, and nonhealing ulcers can also be encountered.
“I’ve recently seen three patients referred for nonresponsive pyoderma gangrenosum that was actually due to NTM,” Dr. Drage recalled.
The histopathologic findings can vary widely as well. A tuberculoid granulomatous infiltrate is most helpful, but some patients may instead have a lymphohistiocytic infiltrate, palisading or sarcoidal granulomas, a mixed inflammatory infiltrate, or other nonspecific findings. Acid-fast bacilli staining typically produces negative results.
Treatment of NTM skin infections is based entirely on susceptibility test results. There are no clearcut treatment guidelines. Many infections will be resistant to the more common antimicrobials, and it’s quite common for resistance to emerge during the required lengthy course of treatment. How lengthy? The typical treatment duration required for a localized infection is about 4 months, climbing to 6 months for more severe or disseminated infection.
Oral clarithromycin at 500 mg twice daily is a good option to start with while awaiting the susceptibility test results, as it covers some of the more common NTM. Combination therapy is often employed to prevent or treat resistance. Surgical removal of isolated foci of infection is often helpful, according to Dr. Drage.
As for the M. chelonae infection outbreak in tattoos, once Dr. Drage and her colleagues recognized the common denominator in the cases, she decided it was time to meet the artist.
She found the tattoo artists were dismayed at the rash of complications and highly receptive to her suggestion that they switch from tap water to sterile water for their work. They’re eager to learn how to have safer, better procedures, she said, but reliable information can be hard for them to come by because of the lack of professional standards.
“In the United States, people who cut hair are more regulated than people who do tattoos,” Dr. Drage noted.
She reported having no relevant financial conflicts.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
EADV: Exploring the Hapten Hypothesis of Atopic Disease
GOTHENBURG, Sweden – While the hygiene hypothesis is the most popular explanation offered for the increase in atopic disease in developed countries., it's not the only plausible explanation, according to Dr. John P. McFadden.
The hapten hypothesis holds that the 400% rise in atopic dermatitis, asthma, and hay fever during the past 50 years is caused at least in part by the revolutionary increase in exposure to chemical haptens in the personal environment during the same time frame, Dr. McFadden said at the annual congress of the European Academy of Dermatology and Venereology.
Haptens are low-molecular-weight organic chemicals that aren't allergenic on their own but can bind to a peptide or protein, thereby altering its configuration and rendering it foreign and allergenic. Examples of haptens include antibiotics and some other drugs, as well as chemicals present in toiletries, processed foods, powdered milk, preservatives used in vaccines, and metal jewelry, explained Dr. McFadden of St. John's Institute of Dermatology, St. Thomas' Hospital, London.
He noted that Scottish investigators have documented a relentless rise in cases of childhood asthma and eczema in that country from 1945 to 1997 occurring in parallel with an increasing prevalence of adult nickel allergy.
"Obviously, association doesn't prove causation. But there does seem to be a change, not just in nickel exposure, but in exposure to other haptens," he said.
Exposure to haptens has exploded in modern life. For example, global sales of toiletries quadrupled during 1959-76. Today more than 80% of baby skin care products contain chemical fragrances. Various brands of powdered milk contain a mean of 12 haptens each. In 1992, just 6% of young women living in Tokyo dyed their hair; by 2001, this figure had jumped to 89% – and meanwhile the incidence of atopic disease in the Tokyo area doubled. Antibiotics weren't in general use until the second half of the 20th century. And that’s when pierced earrings took off in popularity as well, Dr. McFadden noted.
Also, epidemiologic studies show that certain maternal occupations predispose to the birth of atopic children. Among these occupations are hairdresser, beautician, cleaner, electroplater, bar staff, dental assistant, confectionary maker, and book binder. What these diverse occupations have in common is increased environmental exposure to haptens.
The cornerstone of the hygiene hypothesis is that major improvements in public health have led to a cleaner home environment, resulting in less microbial stimulation of immune function and a consequent predisposition to atopic disease. Under audience questioning, Dr. McFadden conceded the hygiene hypothesis "may have some validity," but he added he finds it troubling that many adherents of the hypothesis have "a tendency to be slightly in lazy in explaining away discrepancies.
"When you go back home," Dr. McFadden continued, "I want you to ask your allergist colleagues three questions: One, the biggest reduction in infections came at the end of the 19th century, with improvements in sanitation and nutrition – not in the second half of the 20th century, when the greatest increase in atopic disease occurred – so why was there no reported increase in allergy back then? Two, they say our immune systems haven’t met infections, but actually the vaccination programs mean our immune systems think we've met polio, tetanus, diphtheria, and measles, all by the age of 1 year – how does that fit with the hygiene hypothesis? And three, studies have repeatedly shown that respiratory infections are associated with the development of atopic disease, and we've all seen cases of eczema that are triggered by cutaneous infections – how does that fit in?"
The hapten hypothesis holds that persistent low-grade exposure to environmental haptens via the skin and oral routes at key times of Th2 cytokine immune dominance – namely, pregnancy and the first year of life – can lead to atopy. Dietary hapten intake may interfere with oral immune tolerance mechanisms, while repeated cutaneous exposure to haptens could skew the innate immune system into promoting Th2 responses.
"We're postulating that all of this hapten exposure probably doesn’t matter the rest of the time, but during these vulnerable periods it may be important," Dr. McFadden said.
Last year he and his coworkers laid out in detail the proposed immunologic mechanisms driving the hapten-atopy hypothesis (Trends Immunol. 2009;30:67-74). Consistent with their hypothesis, mouse studies have shown that repeated low-grade exposure to haptens can result in two types of nontolerogenic response: the classic one, namely, allergic contact dermatitis, but also atopic dermatitis. In humans, it’s well established that repeated exposure to haptens can cause allergic contact dermatitis, but it is as yet unknown if hapten exposure contributes in any way to atopic dermatitis. But it’s an issue well worth pursuing, noted Dr. McFadden.
"The question as to whether increases in environmental hapten exposure are contributing to atopy is a legitimate one," he said.
Dr. McFadden disclosed that he has no relevant financial interests.
GOTHENBURG, Sweden – While the hygiene hypothesis is the most popular explanation offered for the increase in atopic disease in developed countries., it's not the only plausible explanation, according to Dr. John P. McFadden.
The hapten hypothesis holds that the 400% rise in atopic dermatitis, asthma, and hay fever during the past 50 years is caused at least in part by the revolutionary increase in exposure to chemical haptens in the personal environment during the same time frame, Dr. McFadden said at the annual congress of the European Academy of Dermatology and Venereology.
Haptens are low-molecular-weight organic chemicals that aren't allergenic on their own but can bind to a peptide or protein, thereby altering its configuration and rendering it foreign and allergenic. Examples of haptens include antibiotics and some other drugs, as well as chemicals present in toiletries, processed foods, powdered milk, preservatives used in vaccines, and metal jewelry, explained Dr. McFadden of St. John's Institute of Dermatology, St. Thomas' Hospital, London.
He noted that Scottish investigators have documented a relentless rise in cases of childhood asthma and eczema in that country from 1945 to 1997 occurring in parallel with an increasing prevalence of adult nickel allergy.
"Obviously, association doesn't prove causation. But there does seem to be a change, not just in nickel exposure, but in exposure to other haptens," he said.
Exposure to haptens has exploded in modern life. For example, global sales of toiletries quadrupled during 1959-76. Today more than 80% of baby skin care products contain chemical fragrances. Various brands of powdered milk contain a mean of 12 haptens each. In 1992, just 6% of young women living in Tokyo dyed their hair; by 2001, this figure had jumped to 89% – and meanwhile the incidence of atopic disease in the Tokyo area doubled. Antibiotics weren't in general use until the second half of the 20th century. And that’s when pierced earrings took off in popularity as well, Dr. McFadden noted.
Also, epidemiologic studies show that certain maternal occupations predispose to the birth of atopic children. Among these occupations are hairdresser, beautician, cleaner, electroplater, bar staff, dental assistant, confectionary maker, and book binder. What these diverse occupations have in common is increased environmental exposure to haptens.
The cornerstone of the hygiene hypothesis is that major improvements in public health have led to a cleaner home environment, resulting in less microbial stimulation of immune function and a consequent predisposition to atopic disease. Under audience questioning, Dr. McFadden conceded the hygiene hypothesis "may have some validity," but he added he finds it troubling that many adherents of the hypothesis have "a tendency to be slightly in lazy in explaining away discrepancies.
"When you go back home," Dr. McFadden continued, "I want you to ask your allergist colleagues three questions: One, the biggest reduction in infections came at the end of the 19th century, with improvements in sanitation and nutrition – not in the second half of the 20th century, when the greatest increase in atopic disease occurred – so why was there no reported increase in allergy back then? Two, they say our immune systems haven’t met infections, but actually the vaccination programs mean our immune systems think we've met polio, tetanus, diphtheria, and measles, all by the age of 1 year – how does that fit with the hygiene hypothesis? And three, studies have repeatedly shown that respiratory infections are associated with the development of atopic disease, and we've all seen cases of eczema that are triggered by cutaneous infections – how does that fit in?"
The hapten hypothesis holds that persistent low-grade exposure to environmental haptens via the skin and oral routes at key times of Th2 cytokine immune dominance – namely, pregnancy and the first year of life – can lead to atopy. Dietary hapten intake may interfere with oral immune tolerance mechanisms, while repeated cutaneous exposure to haptens could skew the innate immune system into promoting Th2 responses.
"We're postulating that all of this hapten exposure probably doesn’t matter the rest of the time, but during these vulnerable periods it may be important," Dr. McFadden said.
Last year he and his coworkers laid out in detail the proposed immunologic mechanisms driving the hapten-atopy hypothesis (Trends Immunol. 2009;30:67-74). Consistent with their hypothesis, mouse studies have shown that repeated low-grade exposure to haptens can result in two types of nontolerogenic response: the classic one, namely, allergic contact dermatitis, but also atopic dermatitis. In humans, it’s well established that repeated exposure to haptens can cause allergic contact dermatitis, but it is as yet unknown if hapten exposure contributes in any way to atopic dermatitis. But it’s an issue well worth pursuing, noted Dr. McFadden.
"The question as to whether increases in environmental hapten exposure are contributing to atopy is a legitimate one," he said.
Dr. McFadden disclosed that he has no relevant financial interests.
GOTHENBURG, Sweden – While the hygiene hypothesis is the most popular explanation offered for the increase in atopic disease in developed countries., it's not the only plausible explanation, according to Dr. John P. McFadden.
The hapten hypothesis holds that the 400% rise in atopic dermatitis, asthma, and hay fever during the past 50 years is caused at least in part by the revolutionary increase in exposure to chemical haptens in the personal environment during the same time frame, Dr. McFadden said at the annual congress of the European Academy of Dermatology and Venereology.
Haptens are low-molecular-weight organic chemicals that aren't allergenic on their own but can bind to a peptide or protein, thereby altering its configuration and rendering it foreign and allergenic. Examples of haptens include antibiotics and some other drugs, as well as chemicals present in toiletries, processed foods, powdered milk, preservatives used in vaccines, and metal jewelry, explained Dr. McFadden of St. John's Institute of Dermatology, St. Thomas' Hospital, London.
He noted that Scottish investigators have documented a relentless rise in cases of childhood asthma and eczema in that country from 1945 to 1997 occurring in parallel with an increasing prevalence of adult nickel allergy.
"Obviously, association doesn't prove causation. But there does seem to be a change, not just in nickel exposure, but in exposure to other haptens," he said.
Exposure to haptens has exploded in modern life. For example, global sales of toiletries quadrupled during 1959-76. Today more than 80% of baby skin care products contain chemical fragrances. Various brands of powdered milk contain a mean of 12 haptens each. In 1992, just 6% of young women living in Tokyo dyed their hair; by 2001, this figure had jumped to 89% – and meanwhile the incidence of atopic disease in the Tokyo area doubled. Antibiotics weren't in general use until the second half of the 20th century. And that’s when pierced earrings took off in popularity as well, Dr. McFadden noted.
Also, epidemiologic studies show that certain maternal occupations predispose to the birth of atopic children. Among these occupations are hairdresser, beautician, cleaner, electroplater, bar staff, dental assistant, confectionary maker, and book binder. What these diverse occupations have in common is increased environmental exposure to haptens.
The cornerstone of the hygiene hypothesis is that major improvements in public health have led to a cleaner home environment, resulting in less microbial stimulation of immune function and a consequent predisposition to atopic disease. Under audience questioning, Dr. McFadden conceded the hygiene hypothesis "may have some validity," but he added he finds it troubling that many adherents of the hypothesis have "a tendency to be slightly in lazy in explaining away discrepancies.
"When you go back home," Dr. McFadden continued, "I want you to ask your allergist colleagues three questions: One, the biggest reduction in infections came at the end of the 19th century, with improvements in sanitation and nutrition – not in the second half of the 20th century, when the greatest increase in atopic disease occurred – so why was there no reported increase in allergy back then? Two, they say our immune systems haven’t met infections, but actually the vaccination programs mean our immune systems think we've met polio, tetanus, diphtheria, and measles, all by the age of 1 year – how does that fit with the hygiene hypothesis? And three, studies have repeatedly shown that respiratory infections are associated with the development of atopic disease, and we've all seen cases of eczema that are triggered by cutaneous infections – how does that fit in?"
The hapten hypothesis holds that persistent low-grade exposure to environmental haptens via the skin and oral routes at key times of Th2 cytokine immune dominance – namely, pregnancy and the first year of life – can lead to atopy. Dietary hapten intake may interfere with oral immune tolerance mechanisms, while repeated cutaneous exposure to haptens could skew the innate immune system into promoting Th2 responses.
"We're postulating that all of this hapten exposure probably doesn’t matter the rest of the time, but during these vulnerable periods it may be important," Dr. McFadden said.
Last year he and his coworkers laid out in detail the proposed immunologic mechanisms driving the hapten-atopy hypothesis (Trends Immunol. 2009;30:67-74). Consistent with their hypothesis, mouse studies have shown that repeated low-grade exposure to haptens can result in two types of nontolerogenic response: the classic one, namely, allergic contact dermatitis, but also atopic dermatitis. In humans, it’s well established that repeated exposure to haptens can cause allergic contact dermatitis, but it is as yet unknown if hapten exposure contributes in any way to atopic dermatitis. But it’s an issue well worth pursuing, noted Dr. McFadden.
"The question as to whether increases in environmental hapten exposure are contributing to atopy is a legitimate one," he said.
Dr. McFadden disclosed that he has no relevant financial interests.
ANNUAL CONGRESS OF THE EUROPEAN ACADEMY
OF DERMATOLOGY AND VENEREOLOGY
EADV: Leukemia Patients Predisposed to Aggressive Melanoma
GOTHENBURG, Sweden - Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
"Melanoma and CLL is a dangerously common association with bad outcomes," said Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark's level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
"We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier," he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. "Now we know that’s true for melanoma, too," Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion's true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
"That's where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don't know yet," he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
"Maybe that’s something we should consider using in patients with CLL," Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
"Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers," he said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
GOTHENBURG, Sweden - Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
"Melanoma and CLL is a dangerously common association with bad outcomes," said Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark's level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
"We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier," he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. "Now we know that’s true for melanoma, too," Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion's true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
"That's where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don't know yet," he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
"Maybe that’s something we should consider using in patients with CLL," Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
"Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers," he said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
GOTHENBURG, Sweden - Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
"Melanoma and CLL is a dangerously common association with bad outcomes," said Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark's level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
"We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier," he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. "Now we know that’s true for melanoma, too," Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion's true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
"That's where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don't know yet," he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
"Maybe that’s something we should consider using in patients with CLL," Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
"Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers," he said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Individuals with a history of chronic lymphocytic leukemia are at
2.5-fold increased risk of subsequently developing melanoma.
Data Source: A population-based study that included 212,245 melanoma patients in the National Cancer Institute's Surveillance, Epidemiology, and End Results database in the years 1990-2006.
Disclosures: Dr. Brewer declared having no financial conflicts, noting that his research on
lymphoma-related skin cancer has been funded mainly by the Dermatology
Foundation.
Chronic Lymphocytic Leukemia Patients Predisposed to Aggressive Melanoma
GOTHENBURG, SWEDEN – Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
“Melanoma and CLL is a dangerously common association with bad outcomes,” declared Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark’s level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
“We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier,” he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. “Now we know that’s true for melanoma, too,” Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion’s true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
“That’s where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don’t know yet,” he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
“Maybe that’s something we should consider using in patients with CLL,” Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
“Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers,” the dermatologist said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
GOTHENBURG, SWEDEN – Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
“Melanoma and CLL is a dangerously common association with bad outcomes,” declared Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark’s level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
“We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier,” he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. “Now we know that’s true for melanoma, too,” Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion’s true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
“That’s where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don’t know yet,” he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
“Maybe that’s something we should consider using in patients with CLL,” Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
“Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers,” the dermatologist said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
GOTHENBURG, SWEDEN – Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
“Melanoma and CLL is a dangerously common association with bad outcomes,” declared Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark’s level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
“We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier,” he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. “Now we know that’s true for melanoma, too,” Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion’s true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
“That’s where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don’t know yet,” he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
“Maybe that’s something we should consider using in patients with CLL,” Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
“Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers,” the dermatologist said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
EADV: Cutaneous Lupus Linked to Increased Skin Cancer Risk
GOTHENBURG, SWEDEN – Patients with cutaneous lupus erythematosus appear to have an elevated overall risk of cancer, especially nonmelanoma skin cancer, lung cancer, and non-Hodgkin's lymphoma.
That's the preliminary conclusion from a Swedish national cohort study involving 3,788 Swedes with cutaneous LE (CLE), each matched to three controls and followed for an average of 4.1 years, said Dr. Carina M. Grönhagen at the annual congress of the European Academy of Dermatology and Venereology.
The take-home message from this first-ever look at the cancer risk associated with CLE is that patients with this skin disease need to be followed regularly for the emergence of malignancy. And they need to receive a strong antismoking message.
"Many of these cancers are connected to smoking, and patients with CLE are known to be smokers to a higher degree than in a normal population," observed Dr. Grönhagen, a dermatology resident at Danderyd Hospital and doctoral candidate in medical epidemiology at the Karolinska Institute, Stockholm.
She and her coworkers decided to look at cancer rates in patients with CLE because CLE is an autoimmune disease, and epidemiologic studies indicate other autoimmune diseases are associated with increased cancer risk.
The overall number of cases of cancer documented in the CLE group during the study period was 188, compared with an expected 112. This 67% increased incidence rate ratio remained significant after adjustment for comorbid SLE, which dropped the ratio only to 60%.
The greatest increase in cancer risk seen in the CLE cohort was for nonmelanoma skin cancer, with a 4.3-fold relative risk, compared with controls. The other strongest risk increases were the 2.9-fold increase in lung cancer, the 2.7-fold increase in non-Hodgkin’s lymphoma, and the 2.7-fold rise in buccal cancer.
Asked if she thinks the observed increase in cancer in association with CLE is caused by the skin disease itself, or instead perhaps the immunosuppressive therapies employed in its treatment, Dr. Grönhagen replied that the well-established high rate of smoking among CLE patients is probably a significant contributor. But the immunologic derangement inherent in CLE is also likely to play a role, especially with regard to the increase in nonmelanoma skin cancer.
Dr. Grönhagen said her presentation at the congress was an interim analysis. Assigning three controls per CLE patient is insufficient to draw ironclad conclusions. She reported that with her coworkers, she is in the process of comparing cancer rates in the CLE cohort to those in the entire Swedish population in order to generate standardized incidence rates rather than incidence rate ratios.
She declared having no relevant financial relationships.
GOTHENBURG, SWEDEN – Patients with cutaneous lupus erythematosus appear to have an elevated overall risk of cancer, especially nonmelanoma skin cancer, lung cancer, and non-Hodgkin's lymphoma.
That's the preliminary conclusion from a Swedish national cohort study involving 3,788 Swedes with cutaneous LE (CLE), each matched to three controls and followed for an average of 4.1 years, said Dr. Carina M. Grönhagen at the annual congress of the European Academy of Dermatology and Venereology.
The take-home message from this first-ever look at the cancer risk associated with CLE is that patients with this skin disease need to be followed regularly for the emergence of malignancy. And they need to receive a strong antismoking message.
"Many of these cancers are connected to smoking, and patients with CLE are known to be smokers to a higher degree than in a normal population," observed Dr. Grönhagen, a dermatology resident at Danderyd Hospital and doctoral candidate in medical epidemiology at the Karolinska Institute, Stockholm.
She and her coworkers decided to look at cancer rates in patients with CLE because CLE is an autoimmune disease, and epidemiologic studies indicate other autoimmune diseases are associated with increased cancer risk.
The overall number of cases of cancer documented in the CLE group during the study period was 188, compared with an expected 112. This 67% increased incidence rate ratio remained significant after adjustment for comorbid SLE, which dropped the ratio only to 60%.
The greatest increase in cancer risk seen in the CLE cohort was for nonmelanoma skin cancer, with a 4.3-fold relative risk, compared with controls. The other strongest risk increases were the 2.9-fold increase in lung cancer, the 2.7-fold increase in non-Hodgkin’s lymphoma, and the 2.7-fold rise in buccal cancer.
Asked if she thinks the observed increase in cancer in association with CLE is caused by the skin disease itself, or instead perhaps the immunosuppressive therapies employed in its treatment, Dr. Grönhagen replied that the well-established high rate of smoking among CLE patients is probably a significant contributor. But the immunologic derangement inherent in CLE is also likely to play a role, especially with regard to the increase in nonmelanoma skin cancer.
Dr. Grönhagen said her presentation at the congress was an interim analysis. Assigning three controls per CLE patient is insufficient to draw ironclad conclusions. She reported that with her coworkers, she is in the process of comparing cancer rates in the CLE cohort to those in the entire Swedish population in order to generate standardized incidence rates rather than incidence rate ratios.
She declared having no relevant financial relationships.
GOTHENBURG, SWEDEN – Patients with cutaneous lupus erythematosus appear to have an elevated overall risk of cancer, especially nonmelanoma skin cancer, lung cancer, and non-Hodgkin's lymphoma.
That's the preliminary conclusion from a Swedish national cohort study involving 3,788 Swedes with cutaneous LE (CLE), each matched to three controls and followed for an average of 4.1 years, said Dr. Carina M. Grönhagen at the annual congress of the European Academy of Dermatology and Venereology.
The take-home message from this first-ever look at the cancer risk associated with CLE is that patients with this skin disease need to be followed regularly for the emergence of malignancy. And they need to receive a strong antismoking message.
"Many of these cancers are connected to smoking, and patients with CLE are known to be smokers to a higher degree than in a normal population," observed Dr. Grönhagen, a dermatology resident at Danderyd Hospital and doctoral candidate in medical epidemiology at the Karolinska Institute, Stockholm.
She and her coworkers decided to look at cancer rates in patients with CLE because CLE is an autoimmune disease, and epidemiologic studies indicate other autoimmune diseases are associated with increased cancer risk.
The overall number of cases of cancer documented in the CLE group during the study period was 188, compared with an expected 112. This 67% increased incidence rate ratio remained significant after adjustment for comorbid SLE, which dropped the ratio only to 60%.
The greatest increase in cancer risk seen in the CLE cohort was for nonmelanoma skin cancer, with a 4.3-fold relative risk, compared with controls. The other strongest risk increases were the 2.9-fold increase in lung cancer, the 2.7-fold increase in non-Hodgkin’s lymphoma, and the 2.7-fold rise in buccal cancer.
Asked if she thinks the observed increase in cancer in association with CLE is caused by the skin disease itself, or instead perhaps the immunosuppressive therapies employed in its treatment, Dr. Grönhagen replied that the well-established high rate of smoking among CLE patients is probably a significant contributor. But the immunologic derangement inherent in CLE is also likely to play a role, especially with regard to the increase in nonmelanoma skin cancer.
Dr. Grönhagen said her presentation at the congress was an interim analysis. Assigning three controls per CLE patient is insufficient to draw ironclad conclusions. She reported that with her coworkers, she is in the process of comparing cancer rates in the CLE cohort to those in the entire Swedish population in order to generate standardized incidence rates rather than incidence rate ratios.
She declared having no relevant financial relationships.
ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Among patients with cutaneous lupus erythematosus, 188 cases of cancer were documented during
the study perio, compared with an expected 112. This 67%
increased incidence rate ratio remained significant after adjustment for
comorbid systemic LE, which dropped the ratio only to 60%.
Data Source: A Swedish national cohort study involving 3,788 Swedes with cutaneous LE, each matched to three controls and followed for an average of
4.1 years,.
Disclosures: Dr. Grönhagen declared having no relevant financial relationships.
Bone Hormone May Predict Mortality in HF
STOCKHOLM – Higher blood levels of the bone hormone osteoprotegerin are associated with an increased long-term risk of death in patients with chronic heart failure, according to Dr. Ragnhild R. Roysland.
The relationship between osteoprotegerin (OPG) and mortality in heart failure is independent of the conventional cardiovascular risk factors, based on a secondary analysis of results of the Italian GISSI-Heart Failure trial.
Osteoprotegerin, a cytokine that's a member of the tumor necrosis factor superfamily, is drawing increasing attention among cardiovascular researchers as a likely key player in what's known as “the calcification paradox.” In recent human studies, increased OPG levels have been linked to a greater atherosclerotic burden; an increased risk of death following acute MI; and now, in GISSI-HF, to increased mortality in the setting of chronic heart failure, Dr. Ragnhild R. Roysland said at the congress.
The GISSI-HF study was a randomized clinical trial in which 6,975 Italian patients with all kinds of chronic heart failure were assigned to rosuvastatin, fish oil, or placebo and followed up for a median of 3.9 years. The primary results have been published in Lancet (2008;372:1223-30;1231-9). Dr. Roysland presented a new substudy involving 1,229 GISSI-HF participants for whom baseline OPG levels were available.
The aim was to see if baseline OPG was predictive of all-cause mortality. This indeed proved to be the case. During follow-up 332 patients died. The mortality rate was 20% in those in the bottom tertile for baseline OPG with a value less than 1,210 ng/L, and more than twice as great at 45% among patients in the highest tertile, with an OPG of at least 1,923 ng/L. Patients with an intermediate-tertile baseline OPG of 1,210-1,922 ng/L split the difference in terms of mortality, said Dr. Roysland of Akershus University Hospital, Lørenskog, Norway.
Treatment assignment in GISSI-HF did not affect OPG levels. However, if a medication could be identified that reduces OPG levels and attenuates the risk associated with high OPG, then OPG could become an important marker to use in managing chronic heart failure in clinical practice.
Dr. Marco Metra commented that as a member of the European Society of Cardiology congress scientific program committee, he'd reviewed all the heart failure studies scheduled for presentation at the meeting.
Two other studies in addition to Dr. Roysland's consistently showed that heart failure patients with high levels of OPG have a poor prognosis.
“We know that patients with heart failure have low levels of vitamin D and increased rates of osteoporosis and bone fractures. So the bone is in some way a target of heart failure,” observed Dr. Metra of the University of Brescia (Italy).
Dr. Roysland said she had no financial conflicts.
Mortality for patients with high OPG levels, at 45%, was more than twice that in patients with low levels, at 20%.
Source DR. ROYSLAND
STOCKHOLM – Higher blood levels of the bone hormone osteoprotegerin are associated with an increased long-term risk of death in patients with chronic heart failure, according to Dr. Ragnhild R. Roysland.
The relationship between osteoprotegerin (OPG) and mortality in heart failure is independent of the conventional cardiovascular risk factors, based on a secondary analysis of results of the Italian GISSI-Heart Failure trial.
Osteoprotegerin, a cytokine that's a member of the tumor necrosis factor superfamily, is drawing increasing attention among cardiovascular researchers as a likely key player in what's known as “the calcification paradox.” In recent human studies, increased OPG levels have been linked to a greater atherosclerotic burden; an increased risk of death following acute MI; and now, in GISSI-HF, to increased mortality in the setting of chronic heart failure, Dr. Ragnhild R. Roysland said at the congress.
The GISSI-HF study was a randomized clinical trial in which 6,975 Italian patients with all kinds of chronic heart failure were assigned to rosuvastatin, fish oil, or placebo and followed up for a median of 3.9 years. The primary results have been published in Lancet (2008;372:1223-30;1231-9). Dr. Roysland presented a new substudy involving 1,229 GISSI-HF participants for whom baseline OPG levels were available.
The aim was to see if baseline OPG was predictive of all-cause mortality. This indeed proved to be the case. During follow-up 332 patients died. The mortality rate was 20% in those in the bottom tertile for baseline OPG with a value less than 1,210 ng/L, and more than twice as great at 45% among patients in the highest tertile, with an OPG of at least 1,923 ng/L. Patients with an intermediate-tertile baseline OPG of 1,210-1,922 ng/L split the difference in terms of mortality, said Dr. Roysland of Akershus University Hospital, Lørenskog, Norway.
Treatment assignment in GISSI-HF did not affect OPG levels. However, if a medication could be identified that reduces OPG levels and attenuates the risk associated with high OPG, then OPG could become an important marker to use in managing chronic heart failure in clinical practice.
Dr. Marco Metra commented that as a member of the European Society of Cardiology congress scientific program committee, he'd reviewed all the heart failure studies scheduled for presentation at the meeting.
Two other studies in addition to Dr. Roysland's consistently showed that heart failure patients with high levels of OPG have a poor prognosis.
“We know that patients with heart failure have low levels of vitamin D and increased rates of osteoporosis and bone fractures. So the bone is in some way a target of heart failure,” observed Dr. Metra of the University of Brescia (Italy).
Dr. Roysland said she had no financial conflicts.
Mortality for patients with high OPG levels, at 45%, was more than twice that in patients with low levels, at 20%.
Source DR. ROYSLAND
STOCKHOLM – Higher blood levels of the bone hormone osteoprotegerin are associated with an increased long-term risk of death in patients with chronic heart failure, according to Dr. Ragnhild R. Roysland.
The relationship between osteoprotegerin (OPG) and mortality in heart failure is independent of the conventional cardiovascular risk factors, based on a secondary analysis of results of the Italian GISSI-Heart Failure trial.
Osteoprotegerin, a cytokine that's a member of the tumor necrosis factor superfamily, is drawing increasing attention among cardiovascular researchers as a likely key player in what's known as “the calcification paradox.” In recent human studies, increased OPG levels have been linked to a greater atherosclerotic burden; an increased risk of death following acute MI; and now, in GISSI-HF, to increased mortality in the setting of chronic heart failure, Dr. Ragnhild R. Roysland said at the congress.
The GISSI-HF study was a randomized clinical trial in which 6,975 Italian patients with all kinds of chronic heart failure were assigned to rosuvastatin, fish oil, or placebo and followed up for a median of 3.9 years. The primary results have been published in Lancet (2008;372:1223-30;1231-9). Dr. Roysland presented a new substudy involving 1,229 GISSI-HF participants for whom baseline OPG levels were available.
The aim was to see if baseline OPG was predictive of all-cause mortality. This indeed proved to be the case. During follow-up 332 patients died. The mortality rate was 20% in those in the bottom tertile for baseline OPG with a value less than 1,210 ng/L, and more than twice as great at 45% among patients in the highest tertile, with an OPG of at least 1,923 ng/L. Patients with an intermediate-tertile baseline OPG of 1,210-1,922 ng/L split the difference in terms of mortality, said Dr. Roysland of Akershus University Hospital, Lørenskog, Norway.
Treatment assignment in GISSI-HF did not affect OPG levels. However, if a medication could be identified that reduces OPG levels and attenuates the risk associated with high OPG, then OPG could become an important marker to use in managing chronic heart failure in clinical practice.
Dr. Marco Metra commented that as a member of the European Society of Cardiology congress scientific program committee, he'd reviewed all the heart failure studies scheduled for presentation at the meeting.
Two other studies in addition to Dr. Roysland's consistently showed that heart failure patients with high levels of OPG have a poor prognosis.
“We know that patients with heart failure have low levels of vitamin D and increased rates of osteoporosis and bone fractures. So the bone is in some way a target of heart failure,” observed Dr. Metra of the University of Brescia (Italy).
Dr. Roysland said she had no financial conflicts.
Mortality for patients with high OPG levels, at 45%, was more than twice that in patients with low levels, at 20%.
Source DR. ROYSLAND
Genetic Test Predicts Response to ACE Inhibitors
STOCKHOLM – Genetic profiling can identify the roughly one-fourth of patients with stable coronary artery disease who will not obtain clinical benefit from ACE inhibitor therapy, as well as a far larger group of superresponders.
“Our findings open the route to individualize therapy by pharmacogenetic profiling. Such individualized therapy could revolutionize drug therapy by enabling physicians to prescribe drugs only to those patients most likely to benefit from the therapy. This would not only increase efficacy, but also decrease unnecessary treatment of patients and avoid unwanted side effects, thereby decreasing overall costs,” Dr. Jasper J. Brugts said at the congress.
Although developing the pharmacogenetic profile entailed a huge multinational research effort, the resultant gene test – which focuses on three single nucleotide polymorphisms on two genes – is simple. But, pharmacogenetic profiling to target ACE inhibitor therapy is not yet ready for use in clinical practice. Further confirmatory studies need to be done first, added Dr. Brugts of Erasmus University, Rotterdam, the Netherlands.
He and his coworkers developed their pharmacogenetic score for predicting clinical outcomes in perindopril-treated patients who have stable CAD by analyzing data from the landmark placebo-controlled, randomized EUROPA (European Trial on Reduction of Cardiac Events With Perindopril in Stable Coronary Artery Disease) trial. In that study, perindopril provided a 20% reduction in the relative risk of cardiac death or MI, compared with placebo, during 4.2 years of follow-up.
The investigators were able to isolate DNA from 9,454 of the 12,218 EUROPA participants. They analyzed 12 candidate genes and 52 haplotype-tagging single nucleotide polymorphisms (SNPs) lying within the pharmacogenetic pathway of ACE inhibitors.
In a multivariate analysis, three SNPs emerged as having a significant negative association with treatment benefit. Two are located on the angiotensin II type 1 receptor gene; the third is on the bradykinin type 1 receptor gene. The greater the number of unfavorable alleles in these SNPs that a patient possessed, the lesser the treatment benefit of perindopril.
In the overall EUROPA study, for example, the absolute risk of cardiac death or MI during follow-up was 9.9% with placebo, vs. 8.0% with perindopril, meaning that the ACE inhibitor provided a 20% relative risk reduction. But in patients with none of the unfavorable alleles, the event rate was 12.2% with placebo, compared with 6.3% with perindopril – a 55% relative risk reduction.
These data illustrate an easily overlooked key point about clinical trial data, according to Dr. Brugts: “The treatment effect that is observed for the total group in a clinical trial does not necessarily count for the patient sitting in front of you at your desk. The risk reduction varies from patient to patient.”
Altogether, 74% of EUROPA patients who were included in the pharmacogenetic study were identified as perindopril responders via blinded genetic profiling. They averaged a 33% relative risk reduction vs. placebo, compared with the 20% figure for the overall study.
In contrast, 26% of study participants had a “nonresponder” genetic profile based upon the three SNPs. They averaged a 26% higher event rate than did placebo-treated patients with the same pharmacogenetic score, although this increased risk did not reach statistical significance.
The pharmacogenetic profile has held up in a preliminary confirmatory analysis of 1,051 patients with cerebrovascular disease who were randomized to perindopril or placebo in the previously reported PROGRESS trial (Lancet 2001;358:1033-41), Dr. Brugts continued.
The pharmacogenetic research is funded by the Netherlands Heart Foundation. He said he had no conflicts of interest.
STOCKHOLM – Genetic profiling can identify the roughly one-fourth of patients with stable coronary artery disease who will not obtain clinical benefit from ACE inhibitor therapy, as well as a far larger group of superresponders.
“Our findings open the route to individualize therapy by pharmacogenetic profiling. Such individualized therapy could revolutionize drug therapy by enabling physicians to prescribe drugs only to those patients most likely to benefit from the therapy. This would not only increase efficacy, but also decrease unnecessary treatment of patients and avoid unwanted side effects, thereby decreasing overall costs,” Dr. Jasper J. Brugts said at the congress.
Although developing the pharmacogenetic profile entailed a huge multinational research effort, the resultant gene test – which focuses on three single nucleotide polymorphisms on two genes – is simple. But, pharmacogenetic profiling to target ACE inhibitor therapy is not yet ready for use in clinical practice. Further confirmatory studies need to be done first, added Dr. Brugts of Erasmus University, Rotterdam, the Netherlands.
He and his coworkers developed their pharmacogenetic score for predicting clinical outcomes in perindopril-treated patients who have stable CAD by analyzing data from the landmark placebo-controlled, randomized EUROPA (European Trial on Reduction of Cardiac Events With Perindopril in Stable Coronary Artery Disease) trial. In that study, perindopril provided a 20% reduction in the relative risk of cardiac death or MI, compared with placebo, during 4.2 years of follow-up.
The investigators were able to isolate DNA from 9,454 of the 12,218 EUROPA participants. They analyzed 12 candidate genes and 52 haplotype-tagging single nucleotide polymorphisms (SNPs) lying within the pharmacogenetic pathway of ACE inhibitors.
In a multivariate analysis, three SNPs emerged as having a significant negative association with treatment benefit. Two are located on the angiotensin II type 1 receptor gene; the third is on the bradykinin type 1 receptor gene. The greater the number of unfavorable alleles in these SNPs that a patient possessed, the lesser the treatment benefit of perindopril.
In the overall EUROPA study, for example, the absolute risk of cardiac death or MI during follow-up was 9.9% with placebo, vs. 8.0% with perindopril, meaning that the ACE inhibitor provided a 20% relative risk reduction. But in patients with none of the unfavorable alleles, the event rate was 12.2% with placebo, compared with 6.3% with perindopril – a 55% relative risk reduction.
These data illustrate an easily overlooked key point about clinical trial data, according to Dr. Brugts: “The treatment effect that is observed for the total group in a clinical trial does not necessarily count for the patient sitting in front of you at your desk. The risk reduction varies from patient to patient.”
Altogether, 74% of EUROPA patients who were included in the pharmacogenetic study were identified as perindopril responders via blinded genetic profiling. They averaged a 33% relative risk reduction vs. placebo, compared with the 20% figure for the overall study.
In contrast, 26% of study participants had a “nonresponder” genetic profile based upon the three SNPs. They averaged a 26% higher event rate than did placebo-treated patients with the same pharmacogenetic score, although this increased risk did not reach statistical significance.
The pharmacogenetic profile has held up in a preliminary confirmatory analysis of 1,051 patients with cerebrovascular disease who were randomized to perindopril or placebo in the previously reported PROGRESS trial (Lancet 2001;358:1033-41), Dr. Brugts continued.
The pharmacogenetic research is funded by the Netherlands Heart Foundation. He said he had no conflicts of interest.
STOCKHOLM – Genetic profiling can identify the roughly one-fourth of patients with stable coronary artery disease who will not obtain clinical benefit from ACE inhibitor therapy, as well as a far larger group of superresponders.
“Our findings open the route to individualize therapy by pharmacogenetic profiling. Such individualized therapy could revolutionize drug therapy by enabling physicians to prescribe drugs only to those patients most likely to benefit from the therapy. This would not only increase efficacy, but also decrease unnecessary treatment of patients and avoid unwanted side effects, thereby decreasing overall costs,” Dr. Jasper J. Brugts said at the congress.
Although developing the pharmacogenetic profile entailed a huge multinational research effort, the resultant gene test – which focuses on three single nucleotide polymorphisms on two genes – is simple. But, pharmacogenetic profiling to target ACE inhibitor therapy is not yet ready for use in clinical practice. Further confirmatory studies need to be done first, added Dr. Brugts of Erasmus University, Rotterdam, the Netherlands.
He and his coworkers developed their pharmacogenetic score for predicting clinical outcomes in perindopril-treated patients who have stable CAD by analyzing data from the landmark placebo-controlled, randomized EUROPA (European Trial on Reduction of Cardiac Events With Perindopril in Stable Coronary Artery Disease) trial. In that study, perindopril provided a 20% reduction in the relative risk of cardiac death or MI, compared with placebo, during 4.2 years of follow-up.
The investigators were able to isolate DNA from 9,454 of the 12,218 EUROPA participants. They analyzed 12 candidate genes and 52 haplotype-tagging single nucleotide polymorphisms (SNPs) lying within the pharmacogenetic pathway of ACE inhibitors.
In a multivariate analysis, three SNPs emerged as having a significant negative association with treatment benefit. Two are located on the angiotensin II type 1 receptor gene; the third is on the bradykinin type 1 receptor gene. The greater the number of unfavorable alleles in these SNPs that a patient possessed, the lesser the treatment benefit of perindopril.
In the overall EUROPA study, for example, the absolute risk of cardiac death or MI during follow-up was 9.9% with placebo, vs. 8.0% with perindopril, meaning that the ACE inhibitor provided a 20% relative risk reduction. But in patients with none of the unfavorable alleles, the event rate was 12.2% with placebo, compared with 6.3% with perindopril – a 55% relative risk reduction.
These data illustrate an easily overlooked key point about clinical trial data, according to Dr. Brugts: “The treatment effect that is observed for the total group in a clinical trial does not necessarily count for the patient sitting in front of you at your desk. The risk reduction varies from patient to patient.”
Altogether, 74% of EUROPA patients who were included in the pharmacogenetic study were identified as perindopril responders via blinded genetic profiling. They averaged a 33% relative risk reduction vs. placebo, compared with the 20% figure for the overall study.
In contrast, 26% of study participants had a “nonresponder” genetic profile based upon the three SNPs. They averaged a 26% higher event rate than did placebo-treated patients with the same pharmacogenetic score, although this increased risk did not reach statistical significance.
The pharmacogenetic profile has held up in a preliminary confirmatory analysis of 1,051 patients with cerebrovascular disease who were randomized to perindopril or placebo in the previously reported PROGRESS trial (Lancet 2001;358:1033-41), Dr. Brugts continued.
The pharmacogenetic research is funded by the Netherlands Heart Foundation. He said he had no conflicts of interest.