User login
Greek-Style Coffee May Aid Arterial Elasticity
STOCKHOLM – The fountain of youth just might a basin filled with rich, strong coffee, a study of one of the world's longest-lived people indicates.
“Our results suggest that drinking coffee in moderation should be encouraged even in elderly hypertensive subjects, as it seems it may improve arterial aging. Maybe regular coffee consumption is one of the key elements of the longevity we have noticed in the Ikaria Islanders, Dr. Christina Chrysohoou mused.
The Aegean Sea island of Ikaria has one of the world's highest proportions of individuals who survive into their 90s and 100s. Residents of the isolated Greek island were featured in “The Blue Zones: Lessons for Living Longer From the People Who've Lived the Longest,” by Dan Buettner (National Geographic Books, 2008).
Seeking an explanation for the Ikarians' exceptional longevity, last year Dr. Chrysohoou led a 5-month University of Athens–sponsored in-depth study of 343 male and 330 female long-time residents aged 65-100 years. As a cardiologist, Dr. Chrysohoou said, one of the factors she was particularly eager to examine was coffee consumption, since it is a deeply embedded part of the Ikarian way of life, and also because coffee – especially Greek-style coffee – is a rich source of antioxidants and anti-inflammatory compounds, which could have a salutary effect on cardiovascular risk.
This indeed appeared to be the case. Among the 465 study participants being treated for hypertension, those who were moderate coffee drinkers – averaging 1-2 of the traditional small 50-mL cups daily – had a significantly lower prevalence of diabetes, dyslipidemia, and cardiovascular disease as well as a lower mean body mass index and higher creatinine clearance than did hypertensive non–coffee drinkers (see box, next page).
Of particular interest was the finding that moderate coffee drinkers with hypertension had significantly greater aortic distensibility as measured echocardiographically than did hypertensive subjects who consumed coffee rarely or never. Consumption of 1-2 cups/day remained an independent predictor of enhanced arterial elasticity after adjustment for potential confounders such as age, physical activity, body mass index, blood pressure, education, diabetes, smoking, and diet.
Islanders who drank less than 1 cup or at least 3 cups of coffee per day did not derive any benefit in terms of aortic distensibility compared with coffee teetotalers. This is probably because modest quaffers do not obtain adequate quantities of the beneficial polyphenolic compounds and other micronutrients, while people who consume 3 or more cups daily ingest so much caffeine that the pressor response outweighs the positive effects of the micronutrients, according to Dr. Chrysohoou.
Traditional Greek coffee is very strong and dark. It is made by boiling the beans for 2-3 minutes. The resultant beverage contains up to 50 times greater concentrations of cafestol, kahweol, and other diterpenes than those of filtered coffee. Greek coffee also is rich in flavonoids, niacin, magnesium, potassium, and vitamin E, she explained.
One caveat regarding the study findings is that coffee drinking on Ikaria is very much a social experience. The elderly study participants generally take their coffee while socializing in the morning or early afternoon with long-time friends in tavernas and cafés, or with family at home. Coffee consumption on the island is a relaxing, unhurried experience enjoyed while discussing daily events.
“The psychological and social circumstances play an important role,” she observed.
“I'm a clinical cardiologist, and most clinicians forbid coffee for their hypertensive patients,” noted Dr. Xavier Bosch of the University of Barcelona, who added he will reconsider his stance as a result of the Greek study.
The other key factor Dr. Chrysohoou and her coworkers identified as likely to contribute to the extended life expectancy of Ikaria Islanders is that these oldest residents are of a generation that tends to adhere most strictly to the traditional Mediterranean diet as popularized by the late University of Minnesota cardiovascular epidemiologist Dr. Ancel Keys, also known for formulating K-rations in World War II.
Dr. Chrysohoou declared having no financial conflicts.
Consumption of 1-2 cups/day was an independent predictor of enhanced arterial elasticity.
Source DR. CHRYSOHOOU
Source ©Robert Brown/Fotolia.com
Source Elsevier Global Medical News
STOCKHOLM – The fountain of youth just might a basin filled with rich, strong coffee, a study of one of the world's longest-lived people indicates.
“Our results suggest that drinking coffee in moderation should be encouraged even in elderly hypertensive subjects, as it seems it may improve arterial aging. Maybe regular coffee consumption is one of the key elements of the longevity we have noticed in the Ikaria Islanders, Dr. Christina Chrysohoou mused.
The Aegean Sea island of Ikaria has one of the world's highest proportions of individuals who survive into their 90s and 100s. Residents of the isolated Greek island were featured in “The Blue Zones: Lessons for Living Longer From the People Who've Lived the Longest,” by Dan Buettner (National Geographic Books, 2008).
Seeking an explanation for the Ikarians' exceptional longevity, last year Dr. Chrysohoou led a 5-month University of Athens–sponsored in-depth study of 343 male and 330 female long-time residents aged 65-100 years. As a cardiologist, Dr. Chrysohoou said, one of the factors she was particularly eager to examine was coffee consumption, since it is a deeply embedded part of the Ikarian way of life, and also because coffee – especially Greek-style coffee – is a rich source of antioxidants and anti-inflammatory compounds, which could have a salutary effect on cardiovascular risk.
This indeed appeared to be the case. Among the 465 study participants being treated for hypertension, those who were moderate coffee drinkers – averaging 1-2 of the traditional small 50-mL cups daily – had a significantly lower prevalence of diabetes, dyslipidemia, and cardiovascular disease as well as a lower mean body mass index and higher creatinine clearance than did hypertensive non–coffee drinkers (see box, next page).
Of particular interest was the finding that moderate coffee drinkers with hypertension had significantly greater aortic distensibility as measured echocardiographically than did hypertensive subjects who consumed coffee rarely or never. Consumption of 1-2 cups/day remained an independent predictor of enhanced arterial elasticity after adjustment for potential confounders such as age, physical activity, body mass index, blood pressure, education, diabetes, smoking, and diet.
Islanders who drank less than 1 cup or at least 3 cups of coffee per day did not derive any benefit in terms of aortic distensibility compared with coffee teetotalers. This is probably because modest quaffers do not obtain adequate quantities of the beneficial polyphenolic compounds and other micronutrients, while people who consume 3 or more cups daily ingest so much caffeine that the pressor response outweighs the positive effects of the micronutrients, according to Dr. Chrysohoou.
Traditional Greek coffee is very strong and dark. It is made by boiling the beans for 2-3 minutes. The resultant beverage contains up to 50 times greater concentrations of cafestol, kahweol, and other diterpenes than those of filtered coffee. Greek coffee also is rich in flavonoids, niacin, magnesium, potassium, and vitamin E, she explained.
One caveat regarding the study findings is that coffee drinking on Ikaria is very much a social experience. The elderly study participants generally take their coffee while socializing in the morning or early afternoon with long-time friends in tavernas and cafés, or with family at home. Coffee consumption on the island is a relaxing, unhurried experience enjoyed while discussing daily events.
“The psychological and social circumstances play an important role,” she observed.
“I'm a clinical cardiologist, and most clinicians forbid coffee for their hypertensive patients,” noted Dr. Xavier Bosch of the University of Barcelona, who added he will reconsider his stance as a result of the Greek study.
The other key factor Dr. Chrysohoou and her coworkers identified as likely to contribute to the extended life expectancy of Ikaria Islanders is that these oldest residents are of a generation that tends to adhere most strictly to the traditional Mediterranean diet as popularized by the late University of Minnesota cardiovascular epidemiologist Dr. Ancel Keys, also known for formulating K-rations in World War II.
Dr. Chrysohoou declared having no financial conflicts.
Consumption of 1-2 cups/day was an independent predictor of enhanced arterial elasticity.
Source DR. CHRYSOHOOU
Source ©Robert Brown/Fotolia.com
Source Elsevier Global Medical News
STOCKHOLM – The fountain of youth just might a basin filled with rich, strong coffee, a study of one of the world's longest-lived people indicates.
“Our results suggest that drinking coffee in moderation should be encouraged even in elderly hypertensive subjects, as it seems it may improve arterial aging. Maybe regular coffee consumption is one of the key elements of the longevity we have noticed in the Ikaria Islanders, Dr. Christina Chrysohoou mused.
The Aegean Sea island of Ikaria has one of the world's highest proportions of individuals who survive into their 90s and 100s. Residents of the isolated Greek island were featured in “The Blue Zones: Lessons for Living Longer From the People Who've Lived the Longest,” by Dan Buettner (National Geographic Books, 2008).
Seeking an explanation for the Ikarians' exceptional longevity, last year Dr. Chrysohoou led a 5-month University of Athens–sponsored in-depth study of 343 male and 330 female long-time residents aged 65-100 years. As a cardiologist, Dr. Chrysohoou said, one of the factors she was particularly eager to examine was coffee consumption, since it is a deeply embedded part of the Ikarian way of life, and also because coffee – especially Greek-style coffee – is a rich source of antioxidants and anti-inflammatory compounds, which could have a salutary effect on cardiovascular risk.
This indeed appeared to be the case. Among the 465 study participants being treated for hypertension, those who were moderate coffee drinkers – averaging 1-2 of the traditional small 50-mL cups daily – had a significantly lower prevalence of diabetes, dyslipidemia, and cardiovascular disease as well as a lower mean body mass index and higher creatinine clearance than did hypertensive non–coffee drinkers (see box, next page).
Of particular interest was the finding that moderate coffee drinkers with hypertension had significantly greater aortic distensibility as measured echocardiographically than did hypertensive subjects who consumed coffee rarely or never. Consumption of 1-2 cups/day remained an independent predictor of enhanced arterial elasticity after adjustment for potential confounders such as age, physical activity, body mass index, blood pressure, education, diabetes, smoking, and diet.
Islanders who drank less than 1 cup or at least 3 cups of coffee per day did not derive any benefit in terms of aortic distensibility compared with coffee teetotalers. This is probably because modest quaffers do not obtain adequate quantities of the beneficial polyphenolic compounds and other micronutrients, while people who consume 3 or more cups daily ingest so much caffeine that the pressor response outweighs the positive effects of the micronutrients, according to Dr. Chrysohoou.
Traditional Greek coffee is very strong and dark. It is made by boiling the beans for 2-3 minutes. The resultant beverage contains up to 50 times greater concentrations of cafestol, kahweol, and other diterpenes than those of filtered coffee. Greek coffee also is rich in flavonoids, niacin, magnesium, potassium, and vitamin E, she explained.
One caveat regarding the study findings is that coffee drinking on Ikaria is very much a social experience. The elderly study participants generally take their coffee while socializing in the morning or early afternoon with long-time friends in tavernas and cafés, or with family at home. Coffee consumption on the island is a relaxing, unhurried experience enjoyed while discussing daily events.
“The psychological and social circumstances play an important role,” she observed.
“I'm a clinical cardiologist, and most clinicians forbid coffee for their hypertensive patients,” noted Dr. Xavier Bosch of the University of Barcelona, who added he will reconsider his stance as a result of the Greek study.
The other key factor Dr. Chrysohoou and her coworkers identified as likely to contribute to the extended life expectancy of Ikaria Islanders is that these oldest residents are of a generation that tends to adhere most strictly to the traditional Mediterranean diet as popularized by the late University of Minnesota cardiovascular epidemiologist Dr. Ancel Keys, also known for formulating K-rations in World War II.
Dr. Chrysohoou declared having no financial conflicts.
Consumption of 1-2 cups/day was an independent predictor of enhanced arterial elasticity.
Source DR. CHRYSOHOOU
Source ©Robert Brown/Fotolia.com
Source Elsevier Global Medical News
EADV: Study Points to Optimal Etanercept Regimen for Psoriasis, PsA
GOTHENBURG, Sweden - In patients with both plaque psoriasis and psoriatic arthritis, initial treatment with etanercept at 50 mg twice weekly resulted in faster clearance of skin lesions than 50 mg once weekly.
The twice-weekly regimen, however, offered no advantage for treatment of joint and tendon symptoms. These were the key findings of the Psoriasis Randomized Etanercept Study in Subjects with Psoriatic Arthritis (PRESTA), a collaborative study that paired dermatologists and rheumatologists in an effort to determine how to optimize the use of etanercept to treat both skin and rheumatologic symptoms in these dual-diagnosis patients.
The PRESTA results will enable physicians to individualize treatment of such patients with a degree of confidence not before possible, Dr. Wolfram Sterry, one of the study investigators, said at the annual congress of the European Academy of Dermatology and Venereology.
PRESTA findings suggest that patients with more severe cutaneous disease are best off with an initial 12 weeks of etanercept (Enbrel) at 50 mg twice weekly before stepping down to 50 mg once weekly, while 50 mg/week from the outset is sufficient in patients with milder skin involvement, explained Dr. Sterry, professor and chair of the department of dermatology and allergy at Charité University Hospital, Berlin.
PRESTA also showed the ideal dosing regimen for psoriatic arthritis to be closer to that employed in rheumatoid arthritis than for that used in psoriasis, he added.
PRESTA was a 24-week, multicenter, randomized, double-blind clinical trial in 752 patients with both psoriasis evaluated by dermatologists and psoriatic arthritis evaluated by rheumatologists. Participants were assigned to subcutaneous etanercept at either 50 mg once or twice weekly for 12 weeks, after which everyone received open-label etanercept at 50 mg/week for a further 12 weeks.
The primary efficacy end point was the number of patients achieving “clear” or “almost clear” on the Physician’s Global Assessment of psoriasis at 12 weeks. This outcome was obtained in 46% (176 of 379) of patients on twice-weekly therapy, compared with 32% (119 of 373) of patients on once-weekly treatment, a significant difference, said Dr. Sterry. In addition, the twice-weekly etanercept group showed significantly greater improvement from baseline to week 12 in the Psoriasis Area and Severity Index: 71%, compared with 62% for once-weekly therapy.
In contrast, both groups were equally likely to meet American College of Rheumatology (ACR) criteria for treatment response. At week 12, an ACR 20% response was achieved by 66% of patients on twice-weekly and 61% on once-weekly etanercept. An ACR 50% response was documented in 45% of those on twice-weekly and 41% on once-weekly etanercept. An ACR 70% response occurred in 20% on twice-weekly and 22% on once-weekly therapy, he noted.
By week 24, 66% of patients who initially got twice-weekly etanercept had an ACR 20% response and 35% had an ACR 70% response, as did 61% and 37%, respectively, of patients on 50 mg once weekly for the full 24 weeks.
Of note, by 24 weeks both patient groups achieved comparable improvements in their skin disease. Patients who initially received twice-weekly etanercept had a 78% improvement from baseline in the psoriasis area and severity index, while those on once-weekly therapy all along had a 74% improvement.
Another noteworthy finding, said Dr. Sterry, is that the study showed definitively, for the first time, that etanercept significantly improves two clinically important extra-articular manifestations of psoriatic arthritis: dactylitis and enthesis. These disease manifestations were present at baseline in 42% and 38% of patients, respectively. By week 24, dactylitis scores had improved by a mean of 85%, compared with baseline in both study arms, while enthesis scores improved by 81%.
There were no significant differences in the safety profiles between the two study arms.
The prevalence of psoriatic arthritis among psoriasis patients is estimated to be up to 30%, noted Dr. Sterry. The arthritis component typically does not present until about a decade after onset of the skin disease. This was true in PRESTA: Participants had a mean 18.9-year duration of psoriasis, with psoriatic arthritis appearing 11.9 years after the skin disease.
Dr. Sterry disclosed that he has received consulting fees from Wyeth, which sponsored PRESTA, as well as from Abbott, Schering-Plough, and Janssen-Cilag.
GOTHENBURG, Sweden - In patients with both plaque psoriasis and psoriatic arthritis, initial treatment with etanercept at 50 mg twice weekly resulted in faster clearance of skin lesions than 50 mg once weekly.
The twice-weekly regimen, however, offered no advantage for treatment of joint and tendon symptoms. These were the key findings of the Psoriasis Randomized Etanercept Study in Subjects with Psoriatic Arthritis (PRESTA), a collaborative study that paired dermatologists and rheumatologists in an effort to determine how to optimize the use of etanercept to treat both skin and rheumatologic symptoms in these dual-diagnosis patients.
The PRESTA results will enable physicians to individualize treatment of such patients with a degree of confidence not before possible, Dr. Wolfram Sterry, one of the study investigators, said at the annual congress of the European Academy of Dermatology and Venereology.
PRESTA findings suggest that patients with more severe cutaneous disease are best off with an initial 12 weeks of etanercept (Enbrel) at 50 mg twice weekly before stepping down to 50 mg once weekly, while 50 mg/week from the outset is sufficient in patients with milder skin involvement, explained Dr. Sterry, professor and chair of the department of dermatology and allergy at Charité University Hospital, Berlin.
PRESTA also showed the ideal dosing regimen for psoriatic arthritis to be closer to that employed in rheumatoid arthritis than for that used in psoriasis, he added.
PRESTA was a 24-week, multicenter, randomized, double-blind clinical trial in 752 patients with both psoriasis evaluated by dermatologists and psoriatic arthritis evaluated by rheumatologists. Participants were assigned to subcutaneous etanercept at either 50 mg once or twice weekly for 12 weeks, after which everyone received open-label etanercept at 50 mg/week for a further 12 weeks.
The primary efficacy end point was the number of patients achieving “clear” or “almost clear” on the Physician’s Global Assessment of psoriasis at 12 weeks. This outcome was obtained in 46% (176 of 379) of patients on twice-weekly therapy, compared with 32% (119 of 373) of patients on once-weekly treatment, a significant difference, said Dr. Sterry. In addition, the twice-weekly etanercept group showed significantly greater improvement from baseline to week 12 in the Psoriasis Area and Severity Index: 71%, compared with 62% for once-weekly therapy.
In contrast, both groups were equally likely to meet American College of Rheumatology (ACR) criteria for treatment response. At week 12, an ACR 20% response was achieved by 66% of patients on twice-weekly and 61% on once-weekly etanercept. An ACR 50% response was documented in 45% of those on twice-weekly and 41% on once-weekly etanercept. An ACR 70% response occurred in 20% on twice-weekly and 22% on once-weekly therapy, he noted.
By week 24, 66% of patients who initially got twice-weekly etanercept had an ACR 20% response and 35% had an ACR 70% response, as did 61% and 37%, respectively, of patients on 50 mg once weekly for the full 24 weeks.
Of note, by 24 weeks both patient groups achieved comparable improvements in their skin disease. Patients who initially received twice-weekly etanercept had a 78% improvement from baseline in the psoriasis area and severity index, while those on once-weekly therapy all along had a 74% improvement.
Another noteworthy finding, said Dr. Sterry, is that the study showed definitively, for the first time, that etanercept significantly improves two clinically important extra-articular manifestations of psoriatic arthritis: dactylitis and enthesis. These disease manifestations were present at baseline in 42% and 38% of patients, respectively. By week 24, dactylitis scores had improved by a mean of 85%, compared with baseline in both study arms, while enthesis scores improved by 81%.
There were no significant differences in the safety profiles between the two study arms.
The prevalence of psoriatic arthritis among psoriasis patients is estimated to be up to 30%, noted Dr. Sterry. The arthritis component typically does not present until about a decade after onset of the skin disease. This was true in PRESTA: Participants had a mean 18.9-year duration of psoriasis, with psoriatic arthritis appearing 11.9 years after the skin disease.
Dr. Sterry disclosed that he has received consulting fees from Wyeth, which sponsored PRESTA, as well as from Abbott, Schering-Plough, and Janssen-Cilag.
GOTHENBURG, Sweden - In patients with both plaque psoriasis and psoriatic arthritis, initial treatment with etanercept at 50 mg twice weekly resulted in faster clearance of skin lesions than 50 mg once weekly.
The twice-weekly regimen, however, offered no advantage for treatment of joint and tendon symptoms. These were the key findings of the Psoriasis Randomized Etanercept Study in Subjects with Psoriatic Arthritis (PRESTA), a collaborative study that paired dermatologists and rheumatologists in an effort to determine how to optimize the use of etanercept to treat both skin and rheumatologic symptoms in these dual-diagnosis patients.
The PRESTA results will enable physicians to individualize treatment of such patients with a degree of confidence not before possible, Dr. Wolfram Sterry, one of the study investigators, said at the annual congress of the European Academy of Dermatology and Venereology.
PRESTA findings suggest that patients with more severe cutaneous disease are best off with an initial 12 weeks of etanercept (Enbrel) at 50 mg twice weekly before stepping down to 50 mg once weekly, while 50 mg/week from the outset is sufficient in patients with milder skin involvement, explained Dr. Sterry, professor and chair of the department of dermatology and allergy at Charité University Hospital, Berlin.
PRESTA also showed the ideal dosing regimen for psoriatic arthritis to be closer to that employed in rheumatoid arthritis than for that used in psoriasis, he added.
PRESTA was a 24-week, multicenter, randomized, double-blind clinical trial in 752 patients with both psoriasis evaluated by dermatologists and psoriatic arthritis evaluated by rheumatologists. Participants were assigned to subcutaneous etanercept at either 50 mg once or twice weekly for 12 weeks, after which everyone received open-label etanercept at 50 mg/week for a further 12 weeks.
The primary efficacy end point was the number of patients achieving “clear” or “almost clear” on the Physician’s Global Assessment of psoriasis at 12 weeks. This outcome was obtained in 46% (176 of 379) of patients on twice-weekly therapy, compared with 32% (119 of 373) of patients on once-weekly treatment, a significant difference, said Dr. Sterry. In addition, the twice-weekly etanercept group showed significantly greater improvement from baseline to week 12 in the Psoriasis Area and Severity Index: 71%, compared with 62% for once-weekly therapy.
In contrast, both groups were equally likely to meet American College of Rheumatology (ACR) criteria for treatment response. At week 12, an ACR 20% response was achieved by 66% of patients on twice-weekly and 61% on once-weekly etanercept. An ACR 50% response was documented in 45% of those on twice-weekly and 41% on once-weekly etanercept. An ACR 70% response occurred in 20% on twice-weekly and 22% on once-weekly therapy, he noted.
By week 24, 66% of patients who initially got twice-weekly etanercept had an ACR 20% response and 35% had an ACR 70% response, as did 61% and 37%, respectively, of patients on 50 mg once weekly for the full 24 weeks.
Of note, by 24 weeks both patient groups achieved comparable improvements in their skin disease. Patients who initially received twice-weekly etanercept had a 78% improvement from baseline in the psoriasis area and severity index, while those on once-weekly therapy all along had a 74% improvement.
Another noteworthy finding, said Dr. Sterry, is that the study showed definitively, for the first time, that etanercept significantly improves two clinically important extra-articular manifestations of psoriatic arthritis: dactylitis and enthesis. These disease manifestations were present at baseline in 42% and 38% of patients, respectively. By week 24, dactylitis scores had improved by a mean of 85%, compared with baseline in both study arms, while enthesis scores improved by 81%.
There were no significant differences in the safety profiles between the two study arms.
The prevalence of psoriatic arthritis among psoriasis patients is estimated to be up to 30%, noted Dr. Sterry. The arthritis component typically does not present until about a decade after onset of the skin disease. This was true in PRESTA: Participants had a mean 18.9-year duration of psoriasis, with psoriatic arthritis appearing 11.9 years after the skin disease.
Dr. Sterry disclosed that he has received consulting fees from Wyeth, which sponsored PRESTA, as well as from Abbott, Schering-Plough, and Janssen-Cilag.
Annual Congress of the European Academy of Dermatology and Venereology
Major Finding: After 24 weeks of etanercept therapy, dactylitis scores had improved by a mean of 85%, compared with baseline, while enthesis scores improved by 81%.
Data Source: A 24-week, multicenter, randomized, double-blind clinical trial of 752 patients with both psoriasis and psoriatic arthritis.
Disclosures: Dr. Sterry disclosed that he has received consulting fees from Wyeth, which sponsored PRESTA, as well as from Abbott, Schering-Plough, and Janssen-Cilag.
PRESTA: Defining Optimal Etanercept Regimen for Psoriasis and Psoriatic Arthritis
GOTHENBURG, SWEDEN – In patients with both plaque psoriasis and psoriatic arthritis, initial treatment with etanercept at 50 mg twice weekly resulted in faster clearance of skin lesions than 50 mg once weekly.
The twice-weekly regimen, however, offered no advantage for treatment of joint and tendon symptoms. These were the key findings of the Psoriasis Randomized Etanercept Study in Subjects with Psoriatic Arthritis (PRESTA), a collaborative study that paired dermatologists and rheumatologists in an effort to determine how to optimize the use of etanercept to treat both skin and rheumatologic symptoms in these dual-diagnosis patients.
The PRESTA results will enable physicians to individualize treatment of such patients with a degree of confidence not before possible, Dr. Wolfram Sterry, one of the study investigators, said at the annual congress of the European Academy of Dermatology and Venereology.
PRESTA findings suggest that patients with more severe cutaneous disease are best off with an initial 12 weeks of etanercept (Enbrel) at 50 mg twice weekly before stepping down to 50 mg once weekly, while 50 mg/week from the outset is sufficient in patients with milder skin involvement, explained Dr. Sterry, professor and chair of the department of dermatology and allergy at Charit? University Hospital, Berlin.
PRESTA also showed the ideal dosing regimen for psoriatic arthritis to be closer to that employed in rheumatoid arthritis than for that used in psoriasis, he added.
PRESTA was a 24-week, multicenter, randomized, double-blind clinical trial in 752 patients with both psoriasis evaluated by dermatologists and psoriatic arthritis evaluated by rheumatologists. Participants were assigned to subcutaneous etanercept at either 50 mg once or twice weekly for 12 weeks, after which everyone received open-label etanercept at 50 mg/week for a further 12 weeks.
The primary efficacy end point was the number of patients achieving “clear” or “almost clear” on the Physician’s Global Assessment of psoriasis at 12 weeks. This outcome was obtained in 46% (176 of 379) of patients on twice-weekly therapy, compared with 32% (119 of 373) of patients on once-weekly treatment, a significant difference, said Dr. Sterry. In addition, the twice-weekly etanercept group showed significantly greater improvement from baseline to week 12 in the Psoriasis Area and Severity Index: 71%, compared with 62% for once-weekly therapy.
In contrast, both groups were equally likely to meet American College of Rheumatology (ACR) criteria for treatment response. At week 12, an ACR 20% response was achieved by 66% of patients on twice-weekly and 61% on once-weekly etanercept. An ACR 50% response was documented in 45% of those on twice-weekly and 41% on once-weekly etanercept. An ACR 70% response occurred in 20% on twice-weekly and 22% on once-weekly therapy, he noted.
By week 24, 66% of patients who initially got twice-weekly etanercept had an ACR 20% response and 35% had an ACR 70% response, as did 61% and 37%, respectively, of patients on 50 mg once weekly for the full 24 weeks.
Of note, by 24 weeks both patient groups achieved comparable improvements in their skin disease. Patients who initially received twice-weekly etanercept had a 78% improvement from baseline in the psoriasis area and severity index, while those on once-weekly therapy all along had a 74% improvement.
Another noteworthy finding, said Dr. Sterry, is that the study showed definitively, for the first time, that etanercept significantly improves two clinically important extra-articular manifestations of psoriatic arthritis: dactylitis and enthesis. These disease manifestations were present at baseline in 42% and 38% of patients, respectively. By week 24, dactylitis scores had improved by a mean of 85%, compared with baseline in both study arms, while enthesis scores improved by 81%.
There were no significant differences in the safety profiles between the two study arms.
The prevalence of psoriatic arthritis among psoriasis patients is estimated to be up to 30%, noted Dr. Sterry. The arthritis component typically does not present until about a decade after onset of the skin disease. This was true in PRESTA: Participants had a mean 18.9-year duration of psoriasis, with psoriatic arthritis appearing 11.9 years after the skin disease.
Dr. Sterry disclosed that he has received consulting fees from Wyeth, which sponsored PRESTA, as well as from Abbott, Schering-Plough, and Janssen-Cilag.
GOTHENBURG, SWEDEN – In patients with both plaque psoriasis and psoriatic arthritis, initial treatment with etanercept at 50 mg twice weekly resulted in faster clearance of skin lesions than 50 mg once weekly.
The twice-weekly regimen, however, offered no advantage for treatment of joint and tendon symptoms. These were the key findings of the Psoriasis Randomized Etanercept Study in Subjects with Psoriatic Arthritis (PRESTA), a collaborative study that paired dermatologists and rheumatologists in an effort to determine how to optimize the use of etanercept to treat both skin and rheumatologic symptoms in these dual-diagnosis patients.
The PRESTA results will enable physicians to individualize treatment of such patients with a degree of confidence not before possible, Dr. Wolfram Sterry, one of the study investigators, said at the annual congress of the European Academy of Dermatology and Venereology.
PRESTA findings suggest that patients with more severe cutaneous disease are best off with an initial 12 weeks of etanercept (Enbrel) at 50 mg twice weekly before stepping down to 50 mg once weekly, while 50 mg/week from the outset is sufficient in patients with milder skin involvement, explained Dr. Sterry, professor and chair of the department of dermatology and allergy at Charit? University Hospital, Berlin.
PRESTA also showed the ideal dosing regimen for psoriatic arthritis to be closer to that employed in rheumatoid arthritis than for that used in psoriasis, he added.
PRESTA was a 24-week, multicenter, randomized, double-blind clinical trial in 752 patients with both psoriasis evaluated by dermatologists and psoriatic arthritis evaluated by rheumatologists. Participants were assigned to subcutaneous etanercept at either 50 mg once or twice weekly for 12 weeks, after which everyone received open-label etanercept at 50 mg/week for a further 12 weeks.
The primary efficacy end point was the number of patients achieving “clear” or “almost clear” on the Physician’s Global Assessment of psoriasis at 12 weeks. This outcome was obtained in 46% (176 of 379) of patients on twice-weekly therapy, compared with 32% (119 of 373) of patients on once-weekly treatment, a significant difference, said Dr. Sterry. In addition, the twice-weekly etanercept group showed significantly greater improvement from baseline to week 12 in the Psoriasis Area and Severity Index: 71%, compared with 62% for once-weekly therapy.
In contrast, both groups were equally likely to meet American College of Rheumatology (ACR) criteria for treatment response. At week 12, an ACR 20% response was achieved by 66% of patients on twice-weekly and 61% on once-weekly etanercept. An ACR 50% response was documented in 45% of those on twice-weekly and 41% on once-weekly etanercept. An ACR 70% response occurred in 20% on twice-weekly and 22% on once-weekly therapy, he noted.
By week 24, 66% of patients who initially got twice-weekly etanercept had an ACR 20% response and 35% had an ACR 70% response, as did 61% and 37%, respectively, of patients on 50 mg once weekly for the full 24 weeks.
Of note, by 24 weeks both patient groups achieved comparable improvements in their skin disease. Patients who initially received twice-weekly etanercept had a 78% improvement from baseline in the psoriasis area and severity index, while those on once-weekly therapy all along had a 74% improvement.
Another noteworthy finding, said Dr. Sterry, is that the study showed definitively, for the first time, that etanercept significantly improves two clinically important extra-articular manifestations of psoriatic arthritis: dactylitis and enthesis. These disease manifestations were present at baseline in 42% and 38% of patients, respectively. By week 24, dactylitis scores had improved by a mean of 85%, compared with baseline in both study arms, while enthesis scores improved by 81%.
There were no significant differences in the safety profiles between the two study arms.
The prevalence of psoriatic arthritis among psoriasis patients is estimated to be up to 30%, noted Dr. Sterry. The arthritis component typically does not present until about a decade after onset of the skin disease. This was true in PRESTA: Participants had a mean 18.9-year duration of psoriasis, with psoriatic arthritis appearing 11.9 years after the skin disease.
Dr. Sterry disclosed that he has received consulting fees from Wyeth, which sponsored PRESTA, as well as from Abbott, Schering-Plough, and Janssen-Cilag.
GOTHENBURG, SWEDEN – In patients with both plaque psoriasis and psoriatic arthritis, initial treatment with etanercept at 50 mg twice weekly resulted in faster clearance of skin lesions than 50 mg once weekly.
The twice-weekly regimen, however, offered no advantage for treatment of joint and tendon symptoms. These were the key findings of the Psoriasis Randomized Etanercept Study in Subjects with Psoriatic Arthritis (PRESTA), a collaborative study that paired dermatologists and rheumatologists in an effort to determine how to optimize the use of etanercept to treat both skin and rheumatologic symptoms in these dual-diagnosis patients.
The PRESTA results will enable physicians to individualize treatment of such patients with a degree of confidence not before possible, Dr. Wolfram Sterry, one of the study investigators, said at the annual congress of the European Academy of Dermatology and Venereology.
PRESTA findings suggest that patients with more severe cutaneous disease are best off with an initial 12 weeks of etanercept (Enbrel) at 50 mg twice weekly before stepping down to 50 mg once weekly, while 50 mg/week from the outset is sufficient in patients with milder skin involvement, explained Dr. Sterry, professor and chair of the department of dermatology and allergy at Charit? University Hospital, Berlin.
PRESTA also showed the ideal dosing regimen for psoriatic arthritis to be closer to that employed in rheumatoid arthritis than for that used in psoriasis, he added.
PRESTA was a 24-week, multicenter, randomized, double-blind clinical trial in 752 patients with both psoriasis evaluated by dermatologists and psoriatic arthritis evaluated by rheumatologists. Participants were assigned to subcutaneous etanercept at either 50 mg once or twice weekly for 12 weeks, after which everyone received open-label etanercept at 50 mg/week for a further 12 weeks.
The primary efficacy end point was the number of patients achieving “clear” or “almost clear” on the Physician’s Global Assessment of psoriasis at 12 weeks. This outcome was obtained in 46% (176 of 379) of patients on twice-weekly therapy, compared with 32% (119 of 373) of patients on once-weekly treatment, a significant difference, said Dr. Sterry. In addition, the twice-weekly etanercept group showed significantly greater improvement from baseline to week 12 in the Psoriasis Area and Severity Index: 71%, compared with 62% for once-weekly therapy.
In contrast, both groups were equally likely to meet American College of Rheumatology (ACR) criteria for treatment response. At week 12, an ACR 20% response was achieved by 66% of patients on twice-weekly and 61% on once-weekly etanercept. An ACR 50% response was documented in 45% of those on twice-weekly and 41% on once-weekly etanercept. An ACR 70% response occurred in 20% on twice-weekly and 22% on once-weekly therapy, he noted.
By week 24, 66% of patients who initially got twice-weekly etanercept had an ACR 20% response and 35% had an ACR 70% response, as did 61% and 37%, respectively, of patients on 50 mg once weekly for the full 24 weeks.
Of note, by 24 weeks both patient groups achieved comparable improvements in their skin disease. Patients who initially received twice-weekly etanercept had a 78% improvement from baseline in the psoriasis area and severity index, while those on once-weekly therapy all along had a 74% improvement.
Another noteworthy finding, said Dr. Sterry, is that the study showed definitively, for the first time, that etanercept significantly improves two clinically important extra-articular manifestations of psoriatic arthritis: dactylitis and enthesis. These disease manifestations were present at baseline in 42% and 38% of patients, respectively. By week 24, dactylitis scores had improved by a mean of 85%, compared with baseline in both study arms, while enthesis scores improved by 81%.
There were no significant differences in the safety profiles between the two study arms.
The prevalence of psoriatic arthritis among psoriasis patients is estimated to be up to 30%, noted Dr. Sterry. The arthritis component typically does not present until about a decade after onset of the skin disease. This was true in PRESTA: Participants had a mean 18.9-year duration of psoriasis, with psoriatic arthritis appearing 11.9 years after the skin disease.
Dr. Sterry disclosed that he has received consulting fees from Wyeth, which sponsored PRESTA, as well as from Abbott, Schering-Plough, and Janssen-Cilag.
EADV: Topical Smoothened Inhibitor Promising for Multiple BCCs
GOTHENBURG, Sweden - A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein "smoothened," a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. "Four weeks was probably too short a treatment," Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
"These are some very exciting results. I’m impressed," commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
GOTHENBURG, Sweden - A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein "smoothened," a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. "Four weeks was probably too short a treatment," Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
"These are some very exciting results. I’m impressed," commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
GOTHENBURG, Sweden - A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein "smoothened," a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. "Four weeks was probably too short a treatment," Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
"These are some very exciting results. I’m impressed," commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
Topical 'Smoothened' Inhibitor Promising for Multiple BCCs
GOTHENBURG, Sweden – A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein “smoothened,” a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. “Four weeks was probably too short a treatment,” Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
“These are some very exciting results. I’m impressed,” commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
GOTHENBURG, Sweden – A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein “smoothened,” a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. “Four weeks was probably too short a treatment,” Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
“These are some very exciting results. I’m impressed,” commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
GOTHENBURG, Sweden – A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein “smoothened,” a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. “Four weeks was probably too short a treatment,” Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
“These are some very exciting results. I’m impressed,” commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
EADV: Implant Reduced Phototoxic Attacks in Erythropoietic Protoporphyria
GOTHENBURG, Sweden - A resorbable subcutaneous implant of afamelanotide significantly reduced painful phototoxic attacks in patients with erythropoietic protoporphyria in a year-long multinational clinical trial.
Afamelanotide, a first-in-class investigational chemical analog of alpha-melanocyte-stimulating hormone, is a melanocortin-1 agonist that activates melanocytes and increases melanin levels in the skin.
Treatment with the controlled-release afamelanotide implants was life changing for study participants, Dr. Elisabeth I. Minder noted at the annual congress of the European Academy of Dermatology and Venereology.
"You have to understand that EPP [erythropoietic protoporphyria] patients are conditioned early in life that sunlight is harmful: no exposure to sunlight, no painful attacks. Afamelanotide, compared to placebo, not only reduced phototoxic attacks, it also enabled patients to spend significantly more time outdoors. Anecdotally, patients were quite excited to explore new activities which were never possible in their lives previously," explained Dr. Minder of Triemli Hospital, Zurich.
EPP is a rare inherited disease characterized by severely painful, often intolerable phototoxic attacks lasting for days. An estimated 10,000 individuals are affected worldwide she said.
The afamelanotide implant (Scenesse) is also in clinical trials for more common diseases, including vitiligo, solar urticaria, and polymorphous light eruption, as well as for prevention of actinic keratoses and squamous cell carcinomas in organ transplant recipients.
The European phase III EPP trial involved 100 affected patients who were randomized double blind to a year's worth of alternating treatment cycles consisting of a subcutaneous implant of 16 mg of afamelanotide lasting for 60 days followed by 60 days of a placebo implant.
A total of 91 patients completed the study. The low dropout rate was testimony to how grateful participants were to finally have access to an affective therapy for their disorder, even if for only half of the study period, Dr. Minder said.
Daily pain diaries demonstrated that patients experienced significantly fewer days of severe, moderate, and mild pain during the periods in which they were being treated with afamelanotide. For example, patients spent one-third fewer days in severe pain and less than half as many days in moderate pain during the afamelanotide phase, compared with the placebo phase.
During the active-treatment periods, patients reported spending significantly more time outdoors exposed to the sun. More than half of patients reported spending greater than 6 hours per day outdoors while they were on afamelanotide, something that was unthinkable at baseline.
Only two adverse events were more frequent during active treatment: nausea, which occurred 1-2 days post-implant in 33% of patients when being treated with afamelanotide, compared with 18% of patients when being treated with placebo; and hot flushes, which were noted 1-2 days after afamelanotide implantation in 11% of patients, compared with just 1% with placebo.
Session chair Dr. Maurice Van Steensel of Maastricht (the Netherlands) University commented that a truly double-blind study is impossible with afamelanotide because the alpha-melanocyte-stimulating hormone analog causes tanning. For this very reason, he added, the tanning industry may be interested in this agent as a sunless tanning product.
The U.S. Food and Drug Administration announced in March that Clinuvel (afamelanotide’s manufacturer), could move forward with plans to conduct a phase II clinical trial for treating EPP patients. The confirmatory study is currently enrolling participants. The FDA also granted orphan drug status for the treatment for solar urticaria in late 2009.
In an earlier open-label pilot study, Dr. Minder and her associates showed that treatment with afamelanotide increased tolerance to exposure to artificial light under controlled conditions by 11-fold (N. Engl. J. Med. 2009;360:306-7).
The study was sponsored by Clinuvel Pharmaceuticals. Dr. Minder declared having no financial conflicts.
Having cared for patients with erythropoietic protoporphyria, as well as other forms of photosensitivity, I understand the need for effective therapies in this area. The results of the treatment in the study are impressive. However, I think further work needs to be done with this drug.
Vincent DeLeo, M.D. |
As pointed out by others, it is very difficult to run a completely blinded study with the drug since it does induce a visible tan so that both patients and evaluators are aware of the treatment group.
In addition, the end points studied are very subjective reports of pain.
More importantly, we should be concerned about the possibility of adverse effects of a drug which stimulates melanocytes.
It is imperative that long-term safety studies be done to ascertain the risk, if any, of this drug on the development or progression of malignant melanoma.
Dr. DeLeo is chairman of the department of dermatology at St. Luke's-Roosevelt Hospital Center and Beth Israel Medical Center in New York
Having cared for patients with erythropoietic protoporphyria, as well as other forms of photosensitivity, I understand the need for effective therapies in this area. The results of the treatment in the study are impressive. However, I think further work needs to be done with this drug.
Vincent DeLeo, M.D. |
As pointed out by others, it is very difficult to run a completely blinded study with the drug since it does induce a visible tan so that both patients and evaluators are aware of the treatment group.
In addition, the end points studied are very subjective reports of pain.
More importantly, we should be concerned about the possibility of adverse effects of a drug which stimulates melanocytes.
It is imperative that long-term safety studies be done to ascertain the risk, if any, of this drug on the development or progression of malignant melanoma.
Dr. DeLeo is chairman of the department of dermatology at St. Luke's-Roosevelt Hospital Center and Beth Israel Medical Center in New York
Having cared for patients with erythropoietic protoporphyria, as well as other forms of photosensitivity, I understand the need for effective therapies in this area. The results of the treatment in the study are impressive. However, I think further work needs to be done with this drug.
Vincent DeLeo, M.D. |
As pointed out by others, it is very difficult to run a completely blinded study with the drug since it does induce a visible tan so that both patients and evaluators are aware of the treatment group.
In addition, the end points studied are very subjective reports of pain.
More importantly, we should be concerned about the possibility of adverse effects of a drug which stimulates melanocytes.
It is imperative that long-term safety studies be done to ascertain the risk, if any, of this drug on the development or progression of malignant melanoma.
Dr. DeLeo is chairman of the department of dermatology at St. Luke's-Roosevelt Hospital Center and Beth Israel Medical Center in New York
GOTHENBURG, Sweden - A resorbable subcutaneous implant of afamelanotide significantly reduced painful phototoxic attacks in patients with erythropoietic protoporphyria in a year-long multinational clinical trial.
Afamelanotide, a first-in-class investigational chemical analog of alpha-melanocyte-stimulating hormone, is a melanocortin-1 agonist that activates melanocytes and increases melanin levels in the skin.
Treatment with the controlled-release afamelanotide implants was life changing for study participants, Dr. Elisabeth I. Minder noted at the annual congress of the European Academy of Dermatology and Venereology.
"You have to understand that EPP [erythropoietic protoporphyria] patients are conditioned early in life that sunlight is harmful: no exposure to sunlight, no painful attacks. Afamelanotide, compared to placebo, not only reduced phototoxic attacks, it also enabled patients to spend significantly more time outdoors. Anecdotally, patients were quite excited to explore new activities which were never possible in their lives previously," explained Dr. Minder of Triemli Hospital, Zurich.
EPP is a rare inherited disease characterized by severely painful, often intolerable phototoxic attacks lasting for days. An estimated 10,000 individuals are affected worldwide she said.
The afamelanotide implant (Scenesse) is also in clinical trials for more common diseases, including vitiligo, solar urticaria, and polymorphous light eruption, as well as for prevention of actinic keratoses and squamous cell carcinomas in organ transplant recipients.
The European phase III EPP trial involved 100 affected patients who were randomized double blind to a year's worth of alternating treatment cycles consisting of a subcutaneous implant of 16 mg of afamelanotide lasting for 60 days followed by 60 days of a placebo implant.
A total of 91 patients completed the study. The low dropout rate was testimony to how grateful participants were to finally have access to an affective therapy for their disorder, even if for only half of the study period, Dr. Minder said.
Daily pain diaries demonstrated that patients experienced significantly fewer days of severe, moderate, and mild pain during the periods in which they were being treated with afamelanotide. For example, patients spent one-third fewer days in severe pain and less than half as many days in moderate pain during the afamelanotide phase, compared with the placebo phase.
During the active-treatment periods, patients reported spending significantly more time outdoors exposed to the sun. More than half of patients reported spending greater than 6 hours per day outdoors while they were on afamelanotide, something that was unthinkable at baseline.
Only two adverse events were more frequent during active treatment: nausea, which occurred 1-2 days post-implant in 33% of patients when being treated with afamelanotide, compared with 18% of patients when being treated with placebo; and hot flushes, which were noted 1-2 days after afamelanotide implantation in 11% of patients, compared with just 1% with placebo.
Session chair Dr. Maurice Van Steensel of Maastricht (the Netherlands) University commented that a truly double-blind study is impossible with afamelanotide because the alpha-melanocyte-stimulating hormone analog causes tanning. For this very reason, he added, the tanning industry may be interested in this agent as a sunless tanning product.
The U.S. Food and Drug Administration announced in March that Clinuvel (afamelanotide’s manufacturer), could move forward with plans to conduct a phase II clinical trial for treating EPP patients. The confirmatory study is currently enrolling participants. The FDA also granted orphan drug status for the treatment for solar urticaria in late 2009.
In an earlier open-label pilot study, Dr. Minder and her associates showed that treatment with afamelanotide increased tolerance to exposure to artificial light under controlled conditions by 11-fold (N. Engl. J. Med. 2009;360:306-7).
The study was sponsored by Clinuvel Pharmaceuticals. Dr. Minder declared having no financial conflicts.
GOTHENBURG, Sweden - A resorbable subcutaneous implant of afamelanotide significantly reduced painful phototoxic attacks in patients with erythropoietic protoporphyria in a year-long multinational clinical trial.
Afamelanotide, a first-in-class investigational chemical analog of alpha-melanocyte-stimulating hormone, is a melanocortin-1 agonist that activates melanocytes and increases melanin levels in the skin.
Treatment with the controlled-release afamelanotide implants was life changing for study participants, Dr. Elisabeth I. Minder noted at the annual congress of the European Academy of Dermatology and Venereology.
"You have to understand that EPP [erythropoietic protoporphyria] patients are conditioned early in life that sunlight is harmful: no exposure to sunlight, no painful attacks. Afamelanotide, compared to placebo, not only reduced phototoxic attacks, it also enabled patients to spend significantly more time outdoors. Anecdotally, patients were quite excited to explore new activities which were never possible in their lives previously," explained Dr. Minder of Triemli Hospital, Zurich.
EPP is a rare inherited disease characterized by severely painful, often intolerable phototoxic attacks lasting for days. An estimated 10,000 individuals are affected worldwide she said.
The afamelanotide implant (Scenesse) is also in clinical trials for more common diseases, including vitiligo, solar urticaria, and polymorphous light eruption, as well as for prevention of actinic keratoses and squamous cell carcinomas in organ transplant recipients.
The European phase III EPP trial involved 100 affected patients who were randomized double blind to a year's worth of alternating treatment cycles consisting of a subcutaneous implant of 16 mg of afamelanotide lasting for 60 days followed by 60 days of a placebo implant.
A total of 91 patients completed the study. The low dropout rate was testimony to how grateful participants were to finally have access to an affective therapy for their disorder, even if for only half of the study period, Dr. Minder said.
Daily pain diaries demonstrated that patients experienced significantly fewer days of severe, moderate, and mild pain during the periods in which they were being treated with afamelanotide. For example, patients spent one-third fewer days in severe pain and less than half as many days in moderate pain during the afamelanotide phase, compared with the placebo phase.
During the active-treatment periods, patients reported spending significantly more time outdoors exposed to the sun. More than half of patients reported spending greater than 6 hours per day outdoors while they were on afamelanotide, something that was unthinkable at baseline.
Only two adverse events were more frequent during active treatment: nausea, which occurred 1-2 days post-implant in 33% of patients when being treated with afamelanotide, compared with 18% of patients when being treated with placebo; and hot flushes, which were noted 1-2 days after afamelanotide implantation in 11% of patients, compared with just 1% with placebo.
Session chair Dr. Maurice Van Steensel of Maastricht (the Netherlands) University commented that a truly double-blind study is impossible with afamelanotide because the alpha-melanocyte-stimulating hormone analog causes tanning. For this very reason, he added, the tanning industry may be interested in this agent as a sunless tanning product.
The U.S. Food and Drug Administration announced in March that Clinuvel (afamelanotide’s manufacturer), could move forward with plans to conduct a phase II clinical trial for treating EPP patients. The confirmatory study is currently enrolling participants. The FDA also granted orphan drug status for the treatment for solar urticaria in late 2009.
In an earlier open-label pilot study, Dr. Minder and her associates showed that treatment with afamelanotide increased tolerance to exposure to artificial light under controlled conditions by 11-fold (N. Engl. J. Med. 2009;360:306-7).
The study was sponsored by Clinuvel Pharmaceuticals. Dr. Minder declared having no financial conflicts.
From the Annual Congress of the European Academy of Dermatology and Venereology
Major Finding: During the active-treatment periods, more than half of
patients reported spending greater than 6 hours per day outdoors while
they were on afamelanotide.
Data Source: A European trial that involved 100 affected patients who were
randomized double blind to a year’s worth of alternating treatment
cycles consisting of a subcutaneous implant of 16 mg of afamelanotide
lasting for 60 days followed by 60 days of a placebo implant..
Disclosures: The study was sponsored by Clinuvel Pharmaceuticals. Dr. Minder declared having no financial conflicts.
Afamelanotide Implants Effective For Erythropoietic Protoporphyria
GOTHENBURG, SWEDEN – A resorbable subcutaneous implant of afamelanotide significantly reduced painful phototoxic attacks in patients with erythropoietic protoporphyria in a year-long multinational clinical trial.
Afamelanotide, a first-in-class investigational chemical analog of alpha-melanocyte-stimulating hormone, is a melanocortin-1 agonist that activates melanocytes and increases melanin levels in the skin.
Treatment with the controlled-release afamelanotide implants was life changing for study participants, Dr. Elisabeth I. Minder noted at the annual congress of the European Academy of Dermatology and Venereology.
“You have to understand that EPP [erythropoietic protoporphyria] patients are conditioned early in life that sunlight is harmful: no exposure to sunlight, no painful attacks. Afamelanotide, compared to placebo, not only reduced phototoxic attacks, it also enabled patients to spend significantly more time outdoors. Anecdotally, patients were quite excited to explore new activities which were never possible in their lives previously,” explained Dr. Minder of Triemli Hospital, Zurich.
EPP is a rare inherited disease characterized by severely painful, often intolerable phototoxic attacks lasting for days. An estimated 10,000 individuals are affected worldwide she said.
The afamelanotide implant (Scenesse) is also in clinical trials for more common diseases, including vitiligo, solar urticaria, and polymorphous light eruption, as well as for prevention of actinic keratoses and squamous cell carcinomas in organ transplant recipients.
The European phase-III EPP trial involved 100 affected patients who were randomized double blind to a year’s worth of alternating treatment cycles consisting of a subcutaneous implant of 16 mg of afamelanotide lasting for 60 days followed by 60 days of a placebo implant.
A total of 91 patients completed the study. The low dropout rate was testimony to how grateful participants were to finally have access to an affective therapy for their disorder, even if for only half of the study period, Dr. Minder said.
Daily pain diaries demonstrated that patients experienced significantly fewer days of severe, moderate, and mild pain during the periods in which they were being treated with afamelanotide. For example, patients spent one-third fewer days in severe pain and less than half as many days in moderate pain during the afamelanotide phase, compared with the placebo phase.
During the active-treatment periods, patients reported spending significantly more time outdoors exposed to the sun. More than half of patients reported spending greater than 6 hours per day outdoors while they were on afamelanotide, something that was unthinkable at baseline.
Only two adverse events were more frequent during active treatment: nausea, which occurred 1-2 days post-implant in 33% of patients when being treated with afamelanotide, compared with 18% of patients when being treated with placebo; and hot flushes, which were noted 1-2 days after afamelanotide implantation in 11% of patients, compared with just 1% with placebo.
Session chair Dr. Maurice Van Steensel of Maastricht (the Netherlands) University commented that a truly double-blind study is impossible with afamelanotide because the alpha-melanocyte-stimulating hormone analog causes tanning. For this very reason, he added, the tanning industry may be interested in this agent as a sunless tanning product.
The U.S. Food and Drug Administration announced in March that Clinuvel (afamelanotide’s manufacturer), could move forward with plans to conduct a phase II clinical trial for treating EPP patients. The confirmatory study is currently enrolling participants. The FDA also granted orphan drug status for the treatment for solar urticaria in late 2009.
In an earlier open-label pilot study, Dr. Minder and her associates showed that treatment with afamelanotide increased tolerance to exposure to artificial light under controlled conditions by 11-fold (N. Engl. J. Med. 2009;360:306-7).
The study was sponsored by Clinuvel Pharmaceuticals. Dr. Minder declared having no financial conflicts.
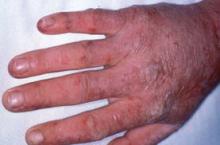
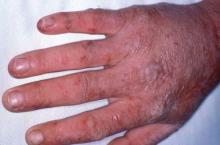
Acute photosensitivity reaction in EPP.
GOTHENBURG, SWEDEN – A resorbable subcutaneous implant of afamelanotide significantly reduced painful phototoxic attacks in patients with erythropoietic protoporphyria in a year-long multinational clinical trial.
Afamelanotide, a first-in-class investigational chemical analog of alpha-melanocyte-stimulating hormone, is a melanocortin-1 agonist that activates melanocytes and increases melanin levels in the skin.
Treatment with the controlled-release afamelanotide implants was life changing for study participants, Dr. Elisabeth I. Minder noted at the annual congress of the European Academy of Dermatology and Venereology.
“You have to understand that EPP [erythropoietic protoporphyria] patients are conditioned early in life that sunlight is harmful: no exposure to sunlight, no painful attacks. Afamelanotide, compared to placebo, not only reduced phototoxic attacks, it also enabled patients to spend significantly more time outdoors. Anecdotally, patients were quite excited to explore new activities which were never possible in their lives previously,” explained Dr. Minder of Triemli Hospital, Zurich.
EPP is a rare inherited disease characterized by severely painful, often intolerable phototoxic attacks lasting for days. An estimated 10,000 individuals are affected worldwide she said.
The afamelanotide implant (Scenesse) is also in clinical trials for more common diseases, including vitiligo, solar urticaria, and polymorphous light eruption, as well as for prevention of actinic keratoses and squamous cell carcinomas in organ transplant recipients.
The European phase-III EPP trial involved 100 affected patients who were randomized double blind to a year’s worth of alternating treatment cycles consisting of a subcutaneous implant of 16 mg of afamelanotide lasting for 60 days followed by 60 days of a placebo implant.
A total of 91 patients completed the study. The low dropout rate was testimony to how grateful participants were to finally have access to an affective therapy for their disorder, even if for only half of the study period, Dr. Minder said.
Daily pain diaries demonstrated that patients experienced significantly fewer days of severe, moderate, and mild pain during the periods in which they were being treated with afamelanotide. For example, patients spent one-third fewer days in severe pain and less than half as many days in moderate pain during the afamelanotide phase, compared with the placebo phase.
During the active-treatment periods, patients reported spending significantly more time outdoors exposed to the sun. More than half of patients reported spending greater than 6 hours per day outdoors while they were on afamelanotide, something that was unthinkable at baseline.
Only two adverse events were more frequent during active treatment: nausea, which occurred 1-2 days post-implant in 33% of patients when being treated with afamelanotide, compared with 18% of patients when being treated with placebo; and hot flushes, which were noted 1-2 days after afamelanotide implantation in 11% of patients, compared with just 1% with placebo.
Session chair Dr. Maurice Van Steensel of Maastricht (the Netherlands) University commented that a truly double-blind study is impossible with afamelanotide because the alpha-melanocyte-stimulating hormone analog causes tanning. For this very reason, he added, the tanning industry may be interested in this agent as a sunless tanning product.
The U.S. Food and Drug Administration announced in March that Clinuvel (afamelanotide’s manufacturer), could move forward with plans to conduct a phase II clinical trial for treating EPP patients. The confirmatory study is currently enrolling participants. The FDA also granted orphan drug status for the treatment for solar urticaria in late 2009.
In an earlier open-label pilot study, Dr. Minder and her associates showed that treatment with afamelanotide increased tolerance to exposure to artificial light under controlled conditions by 11-fold (N. Engl. J. Med. 2009;360:306-7).
The study was sponsored by Clinuvel Pharmaceuticals. Dr. Minder declared having no financial conflicts.
Acute photosensitivity reaction in EPP.
GOTHENBURG, SWEDEN – A resorbable subcutaneous implant of afamelanotide significantly reduced painful phototoxic attacks in patients with erythropoietic protoporphyria in a year-long multinational clinical trial.
Afamelanotide, a first-in-class investigational chemical analog of alpha-melanocyte-stimulating hormone, is a melanocortin-1 agonist that activates melanocytes and increases melanin levels in the skin.
Treatment with the controlled-release afamelanotide implants was life changing for study participants, Dr. Elisabeth I. Minder noted at the annual congress of the European Academy of Dermatology and Venereology.
“You have to understand that EPP [erythropoietic protoporphyria] patients are conditioned early in life that sunlight is harmful: no exposure to sunlight, no painful attacks. Afamelanotide, compared to placebo, not only reduced phototoxic attacks, it also enabled patients to spend significantly more time outdoors. Anecdotally, patients were quite excited to explore new activities which were never possible in their lives previously,” explained Dr. Minder of Triemli Hospital, Zurich.
EPP is a rare inherited disease characterized by severely painful, often intolerable phototoxic attacks lasting for days. An estimated 10,000 individuals are affected worldwide she said.
The afamelanotide implant (Scenesse) is also in clinical trials for more common diseases, including vitiligo, solar urticaria, and polymorphous light eruption, as well as for prevention of actinic keratoses and squamous cell carcinomas in organ transplant recipients.
The European phase-III EPP trial involved 100 affected patients who were randomized double blind to a year’s worth of alternating treatment cycles consisting of a subcutaneous implant of 16 mg of afamelanotide lasting for 60 days followed by 60 days of a placebo implant.
A total of 91 patients completed the study. The low dropout rate was testimony to how grateful participants were to finally have access to an affective therapy for their disorder, even if for only half of the study period, Dr. Minder said.
Daily pain diaries demonstrated that patients experienced significantly fewer days of severe, moderate, and mild pain during the periods in which they were being treated with afamelanotide. For example, patients spent one-third fewer days in severe pain and less than half as many days in moderate pain during the afamelanotide phase, compared with the placebo phase.
During the active-treatment periods, patients reported spending significantly more time outdoors exposed to the sun. More than half of patients reported spending greater than 6 hours per day outdoors while they were on afamelanotide, something that was unthinkable at baseline.
Only two adverse events were more frequent during active treatment: nausea, which occurred 1-2 days post-implant in 33% of patients when being treated with afamelanotide, compared with 18% of patients when being treated with placebo; and hot flushes, which were noted 1-2 days after afamelanotide implantation in 11% of patients, compared with just 1% with placebo.
Session chair Dr. Maurice Van Steensel of Maastricht (the Netherlands) University commented that a truly double-blind study is impossible with afamelanotide because the alpha-melanocyte-stimulating hormone analog causes tanning. For this very reason, he added, the tanning industry may be interested in this agent as a sunless tanning product.
The U.S. Food and Drug Administration announced in March that Clinuvel (afamelanotide’s manufacturer), could move forward with plans to conduct a phase II clinical trial for treating EPP patients. The confirmatory study is currently enrolling participants. The FDA also granted orphan drug status for the treatment for solar urticaria in late 2009.
In an earlier open-label pilot study, Dr. Minder and her associates showed that treatment with afamelanotide increased tolerance to exposure to artificial light under controlled conditions by 11-fold (N. Engl. J. Med. 2009;360:306-7).
The study was sponsored by Clinuvel Pharmaceuticals. Dr. Minder declared having no financial conflicts.
Acute photosensitivity reaction in EPP.
Avoiding MI During Ski Vacations
STOCKHOLM – Most acute myocardial infarctions that occur in winter visitors to mountain ski areas happen during the first 2 days of vacation, according to a study conducted in Austria’s Tyrolean Alps.
In all, 39% of MIs in the retrospective study occurred on the first day of physical activity, which was usually the day after arrival. Another 15% happened on day 2 of physical activity in what was planned, on average, to be an 8-day vacation, Dr. Gert Klug reported at the annual congress of the European Society of Cardiology.
Most affected individuals were not physically fit, having not regularly engaged beforehand in the minimum 2.5 hr/wk of physical activity recommended in ESC guidelines for cardiovascular risk reduction, noted Dr. Klug of the Medical University of Innsbruck (Austria).
“The fact that most of the infarcts happened in the very early phase of the vacation hints at a causal relationship between lack of preparation for the intense regime of physical exertion and exposure to high altitudes and low ambient temperatures,” he said.
Dr. Klug and coworkers reviewed the medical records of more than 1,500 patients admitted to Innsbruck Medical Center for acute MI in 2006-2010. They conducted more detailed follow-up questionnaire interviews with 110 survivors.
In addition to the early timing of the MIs and lack of physical preparation for the rigors of skiing and snowboarding, another common theme was the impact of altitude. The MIs occurred at a mean 1,350 m above sea level in patients who lived at an average altitude of 170 m.
Also notable was the finding that 70% of MI patients had two or more standard cardiovascular risk factors, such as dyslipidemia, diabetes, or smoking. Yet only 19% had previous cardiovascular symptoms before they arrived in the mountains.
“Strenuous exercise without proper preparation – in combination with cardiovascular risk factors, high altitude, and cold ambient temperatures during winter vacations – leads to an increased risk of acute MI in the very early phase of winter holidays. So our advice is to prepare properly for winter vacations by doing a stepwise increase in exercise beforehand,” Dr. Klug concluded.
He noted, however, that in 48% of cases, MI symptom onset occurred when mountain visitors were not skiing or doing other sports.
Dr. Klug declared having no financial conflicts.
STOCKHOLM – Most acute myocardial infarctions that occur in winter visitors to mountain ski areas happen during the first 2 days of vacation, according to a study conducted in Austria’s Tyrolean Alps.
In all, 39% of MIs in the retrospective study occurred on the first day of physical activity, which was usually the day after arrival. Another 15% happened on day 2 of physical activity in what was planned, on average, to be an 8-day vacation, Dr. Gert Klug reported at the annual congress of the European Society of Cardiology.
Most affected individuals were not physically fit, having not regularly engaged beforehand in the minimum 2.5 hr/wk of physical activity recommended in ESC guidelines for cardiovascular risk reduction, noted Dr. Klug of the Medical University of Innsbruck (Austria).
“The fact that most of the infarcts happened in the very early phase of the vacation hints at a causal relationship between lack of preparation for the intense regime of physical exertion and exposure to high altitudes and low ambient temperatures,” he said.
Dr. Klug and coworkers reviewed the medical records of more than 1,500 patients admitted to Innsbruck Medical Center for acute MI in 2006-2010. They conducted more detailed follow-up questionnaire interviews with 110 survivors.
In addition to the early timing of the MIs and lack of physical preparation for the rigors of skiing and snowboarding, another common theme was the impact of altitude. The MIs occurred at a mean 1,350 m above sea level in patients who lived at an average altitude of 170 m.
Also notable was the finding that 70% of MI patients had two or more standard cardiovascular risk factors, such as dyslipidemia, diabetes, or smoking. Yet only 19% had previous cardiovascular symptoms before they arrived in the mountains.
“Strenuous exercise without proper preparation – in combination with cardiovascular risk factors, high altitude, and cold ambient temperatures during winter vacations – leads to an increased risk of acute MI in the very early phase of winter holidays. So our advice is to prepare properly for winter vacations by doing a stepwise increase in exercise beforehand,” Dr. Klug concluded.
He noted, however, that in 48% of cases, MI symptom onset occurred when mountain visitors were not skiing or doing other sports.
Dr. Klug declared having no financial conflicts.
STOCKHOLM – Most acute myocardial infarctions that occur in winter visitors to mountain ski areas happen during the first 2 days of vacation, according to a study conducted in Austria’s Tyrolean Alps.
In all, 39% of MIs in the retrospective study occurred on the first day of physical activity, which was usually the day after arrival. Another 15% happened on day 2 of physical activity in what was planned, on average, to be an 8-day vacation, Dr. Gert Klug reported at the annual congress of the European Society of Cardiology.
Most affected individuals were not physically fit, having not regularly engaged beforehand in the minimum 2.5 hr/wk of physical activity recommended in ESC guidelines for cardiovascular risk reduction, noted Dr. Klug of the Medical University of Innsbruck (Austria).
“The fact that most of the infarcts happened in the very early phase of the vacation hints at a causal relationship between lack of preparation for the intense regime of physical exertion and exposure to high altitudes and low ambient temperatures,” he said.
Dr. Klug and coworkers reviewed the medical records of more than 1,500 patients admitted to Innsbruck Medical Center for acute MI in 2006-2010. They conducted more detailed follow-up questionnaire interviews with 110 survivors.
In addition to the early timing of the MIs and lack of physical preparation for the rigors of skiing and snowboarding, another common theme was the impact of altitude. The MIs occurred at a mean 1,350 m above sea level in patients who lived at an average altitude of 170 m.
Also notable was the finding that 70% of MI patients had two or more standard cardiovascular risk factors, such as dyslipidemia, diabetes, or smoking. Yet only 19% had previous cardiovascular symptoms before they arrived in the mountains.
“Strenuous exercise without proper preparation – in combination with cardiovascular risk factors, high altitude, and cold ambient temperatures during winter vacations – leads to an increased risk of acute MI in the very early phase of winter holidays. So our advice is to prepare properly for winter vacations by doing a stepwise increase in exercise beforehand,” Dr. Klug concluded.
He noted, however, that in 48% of cases, MI symptom onset occurred when mountain visitors were not skiing or doing other sports.
Dr. Klug declared having no financial conflicts.
Avoiding MI During Ski Vacations
STOCKHOLM – Most acute myocardial infarctions that occur in winter visitors to mountain ski areas happen during the first 2 days of vacation, according to a study conducted in Austria’s Tyrolean Alps.
In all, 39% of MIs in the retrospective study occurred on the first day of physical activity, which was usually the day after arrival. Another 15% happened on day 2 of physical activity in what was planned, on average, to be an 8-day vacation, Dr. Gert Klug reported at the annual congress of the European Society of Cardiology.
Most affected individuals were not physically fit, having not regularly engaged beforehand in the minimum 2.5 hr/wk of physical activity recommended in ESC guidelines for cardiovascular risk reduction, noted Dr. Klug of the Medical University of Innsbruck (Austria).
“The fact that most of the infarcts happened in the very early phase of the vacation hints at a causal relationship between lack of preparation for the intense regime of physical exertion and exposure to high altitudes and low ambient temperatures,” he said.
Dr. Klug and coworkers reviewed the medical records of more than 1,500 patients admitted to Innsbruck Medical Center for acute MI in 2006-2010. They conducted more detailed follow-up questionnaire interviews with 110 survivors.
In addition to the early timing of the MIs and lack of physical preparation for the rigors of skiing and snowboarding, another common theme was the impact of altitude. The MIs occurred at a mean 1,350 m above sea level in patients who lived at an average altitude of 170 m.
Also notable was the finding that 70% of MI patients had two or more standard cardiovascular risk factors, such as dyslipidemia, diabetes, or smoking. Yet only 19% had previous cardiovascular symptoms before they arrived in the mountains.
“Strenuous exercise without proper preparation – in combination with cardiovascular risk factors, high altitude, and cold ambient temperatures during winter vacations – leads to an increased risk of acute MI in the very early phase of winter holidays. So our advice is to prepare properly for winter vacations by doing a stepwise increase in exercise beforehand,” Dr. Klug concluded.
He noted, however, that in 48% of cases, MI symptom onset occurred when mountain visitors were not skiing or doing other sports.
Dr. Klug declared having no financial conflicts.
STOCKHOLM – Most acute myocardial infarctions that occur in winter visitors to mountain ski areas happen during the first 2 days of vacation, according to a study conducted in Austria’s Tyrolean Alps.
In all, 39% of MIs in the retrospective study occurred on the first day of physical activity, which was usually the day after arrival. Another 15% happened on day 2 of physical activity in what was planned, on average, to be an 8-day vacation, Dr. Gert Klug reported at the annual congress of the European Society of Cardiology.
Most affected individuals were not physically fit, having not regularly engaged beforehand in the minimum 2.5 hr/wk of physical activity recommended in ESC guidelines for cardiovascular risk reduction, noted Dr. Klug of the Medical University of Innsbruck (Austria).
“The fact that most of the infarcts happened in the very early phase of the vacation hints at a causal relationship between lack of preparation for the intense regime of physical exertion and exposure to high altitudes and low ambient temperatures,” he said.
Dr. Klug and coworkers reviewed the medical records of more than 1,500 patients admitted to Innsbruck Medical Center for acute MI in 2006-2010. They conducted more detailed follow-up questionnaire interviews with 110 survivors.
In addition to the early timing of the MIs and lack of physical preparation for the rigors of skiing and snowboarding, another common theme was the impact of altitude. The MIs occurred at a mean 1,350 m above sea level in patients who lived at an average altitude of 170 m.
Also notable was the finding that 70% of MI patients had two or more standard cardiovascular risk factors, such as dyslipidemia, diabetes, or smoking. Yet only 19% had previous cardiovascular symptoms before they arrived in the mountains.
“Strenuous exercise without proper preparation – in combination with cardiovascular risk factors, high altitude, and cold ambient temperatures during winter vacations – leads to an increased risk of acute MI in the very early phase of winter holidays. So our advice is to prepare properly for winter vacations by doing a stepwise increase in exercise beforehand,” Dr. Klug concluded.
He noted, however, that in 48% of cases, MI symptom onset occurred when mountain visitors were not skiing or doing other sports.
Dr. Klug declared having no financial conflicts.
STOCKHOLM – Most acute myocardial infarctions that occur in winter visitors to mountain ski areas happen during the first 2 days of vacation, according to a study conducted in Austria’s Tyrolean Alps.
In all, 39% of MIs in the retrospective study occurred on the first day of physical activity, which was usually the day after arrival. Another 15% happened on day 2 of physical activity in what was planned, on average, to be an 8-day vacation, Dr. Gert Klug reported at the annual congress of the European Society of Cardiology.
Most affected individuals were not physically fit, having not regularly engaged beforehand in the minimum 2.5 hr/wk of physical activity recommended in ESC guidelines for cardiovascular risk reduction, noted Dr. Klug of the Medical University of Innsbruck (Austria).
“The fact that most of the infarcts happened in the very early phase of the vacation hints at a causal relationship between lack of preparation for the intense regime of physical exertion and exposure to high altitudes and low ambient temperatures,” he said.
Dr. Klug and coworkers reviewed the medical records of more than 1,500 patients admitted to Innsbruck Medical Center for acute MI in 2006-2010. They conducted more detailed follow-up questionnaire interviews with 110 survivors.
In addition to the early timing of the MIs and lack of physical preparation for the rigors of skiing and snowboarding, another common theme was the impact of altitude. The MIs occurred at a mean 1,350 m above sea level in patients who lived at an average altitude of 170 m.
Also notable was the finding that 70% of MI patients had two or more standard cardiovascular risk factors, such as dyslipidemia, diabetes, or smoking. Yet only 19% had previous cardiovascular symptoms before they arrived in the mountains.
“Strenuous exercise without proper preparation – in combination with cardiovascular risk factors, high altitude, and cold ambient temperatures during winter vacations – leads to an increased risk of acute MI in the very early phase of winter holidays. So our advice is to prepare properly for winter vacations by doing a stepwise increase in exercise beforehand,” Dr. Klug concluded.
He noted, however, that in 48% of cases, MI symptom onset occurred when mountain visitors were not skiing or doing other sports.
Dr. Klug declared having no financial conflicts.
Psoriatic Arthritis Patients May Be Predisposed to Depression
EDINBURGH - Depression is common, underdiagnosed, and undertreated in patients with psoriatic arthritis.
Psychiatric evaluation of 50 consecutive patients at the University of Glasgow psoriatic arthritis clinic indicated that 15 patients, or 30%, were depressed. Three were rated as severely depressed based on their scores on the Hospital Anxiety and Depression Scale (HADS), while 12 others had moderate depression, Dr. Rajeev Krishnadas reported at the International Congress of the Royal College of Psychiatrists.
This high prevalence of depression in psoriatic arthritis patients is consistent with reports in the dermatologic literature (Br. J. Dermatol. 2008;159:704-10).
Of note, none of the depressed Scottish psoriatic arthritis patients was on a therapeutic dose of an antidepressant, added Dr. Krishnadas of the Sackler Institute of Psychobiological Research, Southern General Hospital, Glasgow.
This study is part of a larger ongoing investigation looking at the relationship between systemic inflammation and depression in patients with rheumatoid arthritis or psoriatic arthritis. In this portion of the study, a positive association was noted between HADS scores and C-reactive protein levels in the psoriatic arthritis cohort, although it must be noted that CRP scores accounted for only 7% of the overall variance in HADS scores.
Not surprisingly, higher HADS scores were associated with worse quality of life as assessed by the Dermatology Quality of Life Questionnaire as well as with higher scores on a self-rated pain scale.
Of greater interest psychodynamically, a negative correlation was found between HADS scores and emotional intelligence as measured by the Trait Emotional Intelligence Questionnaire – Short Form. High trait emotional intelligence reflects greater awareness of one's own feelings as well as the feelings of others'. Individuals with high emotional intelligence are far better able to regulate their emotions than are those with lower trait emotional intelligence.
Patients who scored high in trait emotional intelligence had higher quality of life scores, lower CRP levels, and lower scores on the pain scale.
These findings are consistent with the hypothesis that a poor ability to be aware of and regulate one's emotions predisposes to depression in the presence of a chronic medical condition or other major stressor, according to Dr. Krishnadas.
He declared having no conflicts of interest.
EDINBURGH - Depression is common, underdiagnosed, and undertreated in patients with psoriatic arthritis.
Psychiatric evaluation of 50 consecutive patients at the University of Glasgow psoriatic arthritis clinic indicated that 15 patients, or 30%, were depressed. Three were rated as severely depressed based on their scores on the Hospital Anxiety and Depression Scale (HADS), while 12 others had moderate depression, Dr. Rajeev Krishnadas reported at the International Congress of the Royal College of Psychiatrists.
This high prevalence of depression in psoriatic arthritis patients is consistent with reports in the dermatologic literature (Br. J. Dermatol. 2008;159:704-10).
Of note, none of the depressed Scottish psoriatic arthritis patients was on a therapeutic dose of an antidepressant, added Dr. Krishnadas of the Sackler Institute of Psychobiological Research, Southern General Hospital, Glasgow.
This study is part of a larger ongoing investigation looking at the relationship between systemic inflammation and depression in patients with rheumatoid arthritis or psoriatic arthritis. In this portion of the study, a positive association was noted between HADS scores and C-reactive protein levels in the psoriatic arthritis cohort, although it must be noted that CRP scores accounted for only 7% of the overall variance in HADS scores.
Not surprisingly, higher HADS scores were associated with worse quality of life as assessed by the Dermatology Quality of Life Questionnaire as well as with higher scores on a self-rated pain scale.
Of greater interest psychodynamically, a negative correlation was found between HADS scores and emotional intelligence as measured by the Trait Emotional Intelligence Questionnaire – Short Form. High trait emotional intelligence reflects greater awareness of one's own feelings as well as the feelings of others'. Individuals with high emotional intelligence are far better able to regulate their emotions than are those with lower trait emotional intelligence.
Patients who scored high in trait emotional intelligence had higher quality of life scores, lower CRP levels, and lower scores on the pain scale.
These findings are consistent with the hypothesis that a poor ability to be aware of and regulate one's emotions predisposes to depression in the presence of a chronic medical condition or other major stressor, according to Dr. Krishnadas.
He declared having no conflicts of interest.
EDINBURGH - Depression is common, underdiagnosed, and undertreated in patients with psoriatic arthritis.
Psychiatric evaluation of 50 consecutive patients at the University of Glasgow psoriatic arthritis clinic indicated that 15 patients, or 30%, were depressed. Three were rated as severely depressed based on their scores on the Hospital Anxiety and Depression Scale (HADS), while 12 others had moderate depression, Dr. Rajeev Krishnadas reported at the International Congress of the Royal College of Psychiatrists.
This high prevalence of depression in psoriatic arthritis patients is consistent with reports in the dermatologic literature (Br. J. Dermatol. 2008;159:704-10).
Of note, none of the depressed Scottish psoriatic arthritis patients was on a therapeutic dose of an antidepressant, added Dr. Krishnadas of the Sackler Institute of Psychobiological Research, Southern General Hospital, Glasgow.
This study is part of a larger ongoing investigation looking at the relationship between systemic inflammation and depression in patients with rheumatoid arthritis or psoriatic arthritis. In this portion of the study, a positive association was noted between HADS scores and C-reactive protein levels in the psoriatic arthritis cohort, although it must be noted that CRP scores accounted for only 7% of the overall variance in HADS scores.
Not surprisingly, higher HADS scores were associated with worse quality of life as assessed by the Dermatology Quality of Life Questionnaire as well as with higher scores on a self-rated pain scale.
Of greater interest psychodynamically, a negative correlation was found between HADS scores and emotional intelligence as measured by the Trait Emotional Intelligence Questionnaire – Short Form. High trait emotional intelligence reflects greater awareness of one's own feelings as well as the feelings of others'. Individuals with high emotional intelligence are far better able to regulate their emotions than are those with lower trait emotional intelligence.
Patients who scored high in trait emotional intelligence had higher quality of life scores, lower CRP levels, and lower scores on the pain scale.
These findings are consistent with the hypothesis that a poor ability to be aware of and regulate one's emotions predisposes to depression in the presence of a chronic medical condition or other major stressor, according to Dr. Krishnadas.
He declared having no conflicts of interest.
from the annual International Congress of the Royal College of Psychiatrists