User login
Rhymin’ pediatric dermatologist provides Demodex tips
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Ixekizumab psoriasis outcomes, sliced and diced
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
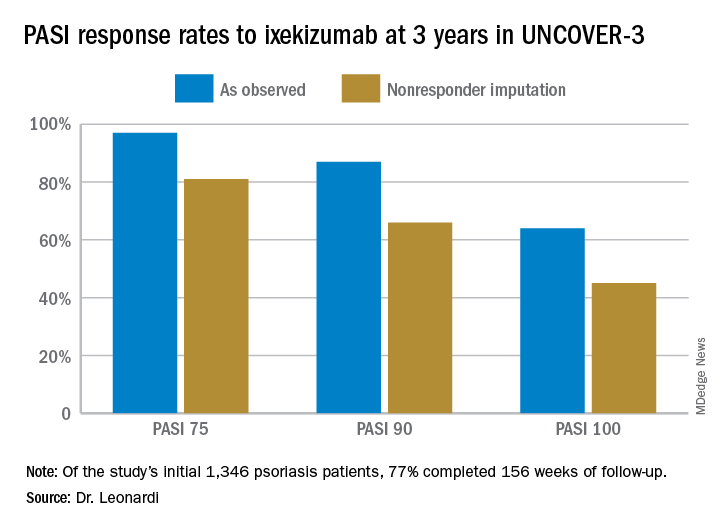
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
What’s new with adalimumab? Plenty
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Not all AF maze operations are aMAZE-ing
SNOWMASS, COLO. – The term “maze procedure” for surgical ablation of atrial fibrillation is bandied about rather loosely these days, but as far as Hartzell V. Schaff, MD, is concerned, the operation of choice remains the classic cut-and-sew maze III procedure developed by James L. Cox, MD, while at Washington University, St. Louis.
“The classic Cox maze III, the cut-and-sew maze, is the best procedure for getting rid of atrial fibrillation and is in my view the gold standard. Some people argue that transmurality isn’t important, but it can occur because of gap lesions,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Unlike modifications of the Cox maze III – such as the mini maze or the maze IV, which utilizes radiofrequency energy or cryoablation to create scars in an effort to achieve pulmonary vein isolation – the maze III cannot be done as a minimally invasive procedure. After all, it requires making incisions in both atria, along with aortic cross-clamping and cardiopulmonary bypass. But it has a significantly higher long-term rate of freedom from recurrent atrial fibrillation (AF) than the other operations. And crucially, it enables the surgeon to readily obliterate the left atrial appendage.
“The most important thing when you do any surgical procedure for atrial fibrillation, I think, is getting rid of the left atrial appendage. When you do cut-and-sew maze, that’s done 100% of the time,” explained Dr. Schaff, professor of surgery at the Mayo Clinic in Rochester, Minn.
“We really have a lot of work left to do as surgeons in improving the outcome of surgery for atrial fibrillation. One of the things we as surgeons don’t do well is getting rid of the left atrial appendage. This ought to be done in every patient that has surgical ablation for atrial fibrillation,” according to the cardiothoracic surgeon.
And yet, he continued, in a series of nearly 87,000 patients with AF who underwent nonemergent cardiac surgery in the Society of Thoracic Surgeons database, 48.0% of whom underwent surgical ablation for AF, only 63.9% of those who had standalone ablation for lone AF got their left atrial appendage dealt with, compared with 86%-89% of those who underwent concomitant cardiac surgery, such as mitral valve repair or replacement (Ann Thorac Surg. 2017 Aug;104[2]:493-500).
“That’s awful, really. And the reason for that low left atrial appendage obliteration rate is this: For many of those patients who had surgery for lone atrial fibrillation, the surgeons were trying to do minimally invasive surgery, where they do pulmonary vein isolation on the right side, so they don’t have access to the left atrial appendage,” Dr. Schaff said.
“In the past,” he recalled, “we would ligate the left atrial appendage. Nowadays because of echocardiographic studies that show there’s persistent patency in a sizable percentage of patients, we amputate the left atrial appendage in almost all of the patients.”
The terminology surrounding surgical ablation for AF, in his view, has become rather confusing. “Most of you, when you refer a patient for surgical ablation for AF, the surgeons will just say they do a maze procedure,” Dr. Schaff cautioned. “Somehow, all of that [maze IV, mini maze] today is lumped together as a classic maze procedure, but it’s really not. We have different lesion sets and energy sources.”
And different outcomes as well. In a series of 1,189 adults who underwent surgical ablation for AF at the Mayo Clinic, of whom 44% had a biatrial cut-and-sew maze while the rest had surgical cryotherapy, radiofrequency ablation, or a combination of the two, the rate of freedom from AF 1 year post surgery was 85% with the cut-and-sew maze versus 71% with the alternatives. At 5 years or more, the rates were 78% and 52%, respectively. In a multivariate analysis, freedom from AF was independently associated with preoperative paroxysmal rather than permanent AF, performance of the classic maze III procedure, concomitant treatment of associated mitral valve disease, and younger age.
Moreover, rates of the major early postoperative complications – stroke, bleeding, and renal failure – were similar in the cut-and-sew maze III and other groups.
“So a lesser procedure doesn’t necessarily mean fewer complications,” Dr. Schaff noted.
One of the criticisms levied against the maze III is that it’s too much surgery for AF. But it’s actually relatively inexpensive because the disposables – suture, needles, scalpel – are those used in the commonly performed concomitant cardiac surgical procedures. “The Cox maze III does take extra time, but with experience it’s not much extra time,” he asserted.
Indeed, in a series of 452 Mayo Clinic maze III patients, the cross-clamp and cardiopulmonary bypass times were 52 and 73 minutes, respectively, for those undergoing an isolated maze III, compared with 73 and 86 minutes for patients whose maze III was done in conjunction with other procedures, most commonly mitral valve repair or replacement.
An underrecognized group of patients who benefit from a standalone cut-and-sew maze are those with tachycardia-induced cardiomyopathy marked by AF or atrial flutter, rapid uncontrolled ventricular response, a decreased left ventricular ejection fraction, and no associated valvular or congenital heart disease. In a series of 37 such patients identified and treated with a maze III operation at the Mayo Clinic, their average preoperative left ventricular ejection fraction of 43% improved to about 55% at discharge, a benefit sustained at last follow-up a median of 63 months later. The outcome was particularly impressive in the 11 patients with a severely depressed left ventricular ejection fraction averaging 31% preoperatively, which jumped to 53% at discharge (Ann Thorac Surg. 2006 Aug;82[2]:494-500).
“Their ejection fraction goes up when you control the tachycardia-induced cardiomyopathy,” he observed. “So reduced left ventricular ejection fraction may be an indication for surgery rather than a contraindication.”
Dr. Schaff emphasized that it’s important for cardiologists and surgeons not to overpromise what surgical ablation of AF can accomplish. The only randomized trial of surgical ablation of AF versus no ablation during mitral valve surgery, sponsored by the National Institutes of Health and Canadian Institutes of Health Research and carried out by the Cardiothoracic Surgical Trials Network, showed no significant between-group differences at 1 year in any of numerous quality of life measures, nor was there a survival benefit for ablation (N Engl J Med. 2015 Apr 9;372(15):1399-409).
“We must point out that there’s no indication that controlling atrial fibrillation has anything to do with improving survival. It has to do with symptomatic benefit and perhaps reducing risk of stroke,” he said.
Dr. Schaff reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – The term “maze procedure” for surgical ablation of atrial fibrillation is bandied about rather loosely these days, but as far as Hartzell V. Schaff, MD, is concerned, the operation of choice remains the classic cut-and-sew maze III procedure developed by James L. Cox, MD, while at Washington University, St. Louis.
“The classic Cox maze III, the cut-and-sew maze, is the best procedure for getting rid of atrial fibrillation and is in my view the gold standard. Some people argue that transmurality isn’t important, but it can occur because of gap lesions,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Unlike modifications of the Cox maze III – such as the mini maze or the maze IV, which utilizes radiofrequency energy or cryoablation to create scars in an effort to achieve pulmonary vein isolation – the maze III cannot be done as a minimally invasive procedure. After all, it requires making incisions in both atria, along with aortic cross-clamping and cardiopulmonary bypass. But it has a significantly higher long-term rate of freedom from recurrent atrial fibrillation (AF) than the other operations. And crucially, it enables the surgeon to readily obliterate the left atrial appendage.
“The most important thing when you do any surgical procedure for atrial fibrillation, I think, is getting rid of the left atrial appendage. When you do cut-and-sew maze, that’s done 100% of the time,” explained Dr. Schaff, professor of surgery at the Mayo Clinic in Rochester, Minn.
“We really have a lot of work left to do as surgeons in improving the outcome of surgery for atrial fibrillation. One of the things we as surgeons don’t do well is getting rid of the left atrial appendage. This ought to be done in every patient that has surgical ablation for atrial fibrillation,” according to the cardiothoracic surgeon.
And yet, he continued, in a series of nearly 87,000 patients with AF who underwent nonemergent cardiac surgery in the Society of Thoracic Surgeons database, 48.0% of whom underwent surgical ablation for AF, only 63.9% of those who had standalone ablation for lone AF got their left atrial appendage dealt with, compared with 86%-89% of those who underwent concomitant cardiac surgery, such as mitral valve repair or replacement (Ann Thorac Surg. 2017 Aug;104[2]:493-500).
“That’s awful, really. And the reason for that low left atrial appendage obliteration rate is this: For many of those patients who had surgery for lone atrial fibrillation, the surgeons were trying to do minimally invasive surgery, where they do pulmonary vein isolation on the right side, so they don’t have access to the left atrial appendage,” Dr. Schaff said.
“In the past,” he recalled, “we would ligate the left atrial appendage. Nowadays because of echocardiographic studies that show there’s persistent patency in a sizable percentage of patients, we amputate the left atrial appendage in almost all of the patients.”
The terminology surrounding surgical ablation for AF, in his view, has become rather confusing. “Most of you, when you refer a patient for surgical ablation for AF, the surgeons will just say they do a maze procedure,” Dr. Schaff cautioned. “Somehow, all of that [maze IV, mini maze] today is lumped together as a classic maze procedure, but it’s really not. We have different lesion sets and energy sources.”
And different outcomes as well. In a series of 1,189 adults who underwent surgical ablation for AF at the Mayo Clinic, of whom 44% had a biatrial cut-and-sew maze while the rest had surgical cryotherapy, radiofrequency ablation, or a combination of the two, the rate of freedom from AF 1 year post surgery was 85% with the cut-and-sew maze versus 71% with the alternatives. At 5 years or more, the rates were 78% and 52%, respectively. In a multivariate analysis, freedom from AF was independently associated with preoperative paroxysmal rather than permanent AF, performance of the classic maze III procedure, concomitant treatment of associated mitral valve disease, and younger age.
Moreover, rates of the major early postoperative complications – stroke, bleeding, and renal failure – were similar in the cut-and-sew maze III and other groups.
“So a lesser procedure doesn’t necessarily mean fewer complications,” Dr. Schaff noted.
One of the criticisms levied against the maze III is that it’s too much surgery for AF. But it’s actually relatively inexpensive because the disposables – suture, needles, scalpel – are those used in the commonly performed concomitant cardiac surgical procedures. “The Cox maze III does take extra time, but with experience it’s not much extra time,” he asserted.
Indeed, in a series of 452 Mayo Clinic maze III patients, the cross-clamp and cardiopulmonary bypass times were 52 and 73 minutes, respectively, for those undergoing an isolated maze III, compared with 73 and 86 minutes for patients whose maze III was done in conjunction with other procedures, most commonly mitral valve repair or replacement.
An underrecognized group of patients who benefit from a standalone cut-and-sew maze are those with tachycardia-induced cardiomyopathy marked by AF or atrial flutter, rapid uncontrolled ventricular response, a decreased left ventricular ejection fraction, and no associated valvular or congenital heart disease. In a series of 37 such patients identified and treated with a maze III operation at the Mayo Clinic, their average preoperative left ventricular ejection fraction of 43% improved to about 55% at discharge, a benefit sustained at last follow-up a median of 63 months later. The outcome was particularly impressive in the 11 patients with a severely depressed left ventricular ejection fraction averaging 31% preoperatively, which jumped to 53% at discharge (Ann Thorac Surg. 2006 Aug;82[2]:494-500).
“Their ejection fraction goes up when you control the tachycardia-induced cardiomyopathy,” he observed. “So reduced left ventricular ejection fraction may be an indication for surgery rather than a contraindication.”
Dr. Schaff emphasized that it’s important for cardiologists and surgeons not to overpromise what surgical ablation of AF can accomplish. The only randomized trial of surgical ablation of AF versus no ablation during mitral valve surgery, sponsored by the National Institutes of Health and Canadian Institutes of Health Research and carried out by the Cardiothoracic Surgical Trials Network, showed no significant between-group differences at 1 year in any of numerous quality of life measures, nor was there a survival benefit for ablation (N Engl J Med. 2015 Apr 9;372(15):1399-409).
“We must point out that there’s no indication that controlling atrial fibrillation has anything to do with improving survival. It has to do with symptomatic benefit and perhaps reducing risk of stroke,” he said.
Dr. Schaff reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – The term “maze procedure” for surgical ablation of atrial fibrillation is bandied about rather loosely these days, but as far as Hartzell V. Schaff, MD, is concerned, the operation of choice remains the classic cut-and-sew maze III procedure developed by James L. Cox, MD, while at Washington University, St. Louis.
“The classic Cox maze III, the cut-and-sew maze, is the best procedure for getting rid of atrial fibrillation and is in my view the gold standard. Some people argue that transmurality isn’t important, but it can occur because of gap lesions,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Unlike modifications of the Cox maze III – such as the mini maze or the maze IV, which utilizes radiofrequency energy or cryoablation to create scars in an effort to achieve pulmonary vein isolation – the maze III cannot be done as a minimally invasive procedure. After all, it requires making incisions in both atria, along with aortic cross-clamping and cardiopulmonary bypass. But it has a significantly higher long-term rate of freedom from recurrent atrial fibrillation (AF) than the other operations. And crucially, it enables the surgeon to readily obliterate the left atrial appendage.
“The most important thing when you do any surgical procedure for atrial fibrillation, I think, is getting rid of the left atrial appendage. When you do cut-and-sew maze, that’s done 100% of the time,” explained Dr. Schaff, professor of surgery at the Mayo Clinic in Rochester, Minn.
“We really have a lot of work left to do as surgeons in improving the outcome of surgery for atrial fibrillation. One of the things we as surgeons don’t do well is getting rid of the left atrial appendage. This ought to be done in every patient that has surgical ablation for atrial fibrillation,” according to the cardiothoracic surgeon.
And yet, he continued, in a series of nearly 87,000 patients with AF who underwent nonemergent cardiac surgery in the Society of Thoracic Surgeons database, 48.0% of whom underwent surgical ablation for AF, only 63.9% of those who had standalone ablation for lone AF got their left atrial appendage dealt with, compared with 86%-89% of those who underwent concomitant cardiac surgery, such as mitral valve repair or replacement (Ann Thorac Surg. 2017 Aug;104[2]:493-500).
“That’s awful, really. And the reason for that low left atrial appendage obliteration rate is this: For many of those patients who had surgery for lone atrial fibrillation, the surgeons were trying to do minimally invasive surgery, where they do pulmonary vein isolation on the right side, so they don’t have access to the left atrial appendage,” Dr. Schaff said.
“In the past,” he recalled, “we would ligate the left atrial appendage. Nowadays because of echocardiographic studies that show there’s persistent patency in a sizable percentage of patients, we amputate the left atrial appendage in almost all of the patients.”
The terminology surrounding surgical ablation for AF, in his view, has become rather confusing. “Most of you, when you refer a patient for surgical ablation for AF, the surgeons will just say they do a maze procedure,” Dr. Schaff cautioned. “Somehow, all of that [maze IV, mini maze] today is lumped together as a classic maze procedure, but it’s really not. We have different lesion sets and energy sources.”
And different outcomes as well. In a series of 1,189 adults who underwent surgical ablation for AF at the Mayo Clinic, of whom 44% had a biatrial cut-and-sew maze while the rest had surgical cryotherapy, radiofrequency ablation, or a combination of the two, the rate of freedom from AF 1 year post surgery was 85% with the cut-and-sew maze versus 71% with the alternatives. At 5 years or more, the rates were 78% and 52%, respectively. In a multivariate analysis, freedom from AF was independently associated with preoperative paroxysmal rather than permanent AF, performance of the classic maze III procedure, concomitant treatment of associated mitral valve disease, and younger age.
Moreover, rates of the major early postoperative complications – stroke, bleeding, and renal failure – were similar in the cut-and-sew maze III and other groups.
“So a lesser procedure doesn’t necessarily mean fewer complications,” Dr. Schaff noted.
One of the criticisms levied against the maze III is that it’s too much surgery for AF. But it’s actually relatively inexpensive because the disposables – suture, needles, scalpel – are those used in the commonly performed concomitant cardiac surgical procedures. “The Cox maze III does take extra time, but with experience it’s not much extra time,” he asserted.
Indeed, in a series of 452 Mayo Clinic maze III patients, the cross-clamp and cardiopulmonary bypass times were 52 and 73 minutes, respectively, for those undergoing an isolated maze III, compared with 73 and 86 minutes for patients whose maze III was done in conjunction with other procedures, most commonly mitral valve repair or replacement.
An underrecognized group of patients who benefit from a standalone cut-and-sew maze are those with tachycardia-induced cardiomyopathy marked by AF or atrial flutter, rapid uncontrolled ventricular response, a decreased left ventricular ejection fraction, and no associated valvular or congenital heart disease. In a series of 37 such patients identified and treated with a maze III operation at the Mayo Clinic, their average preoperative left ventricular ejection fraction of 43% improved to about 55% at discharge, a benefit sustained at last follow-up a median of 63 months later. The outcome was particularly impressive in the 11 patients with a severely depressed left ventricular ejection fraction averaging 31% preoperatively, which jumped to 53% at discharge (Ann Thorac Surg. 2006 Aug;82[2]:494-500).
“Their ejection fraction goes up when you control the tachycardia-induced cardiomyopathy,” he observed. “So reduced left ventricular ejection fraction may be an indication for surgery rather than a contraindication.”
Dr. Schaff emphasized that it’s important for cardiologists and surgeons not to overpromise what surgical ablation of AF can accomplish. The only randomized trial of surgical ablation of AF versus no ablation during mitral valve surgery, sponsored by the National Institutes of Health and Canadian Institutes of Health Research and carried out by the Cardiothoracic Surgical Trials Network, showed no significant between-group differences at 1 year in any of numerous quality of life measures, nor was there a survival benefit for ablation (N Engl J Med. 2015 Apr 9;372(15):1399-409).
“We must point out that there’s no indication that controlling atrial fibrillation has anything to do with improving survival. It has to do with symptomatic benefit and perhaps reducing risk of stroke,” he said.
Dr. Schaff reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2019
Tropical travelers’ top dermatologic infestations
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans
This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
What cardiologists need to know about ARVC
SNOWMASS, COLO. – Electrophysiologist N.A. Mark Estes III, MD, has a piece of advice for his general cardiology colleagues regarding arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited arrhythmia syndrome that’s one of the most common causes of sudden death in athletes under the age of 40 years.
“When you have a patient with syncope and an ECG abnormality that suggests ARVC, you immediately restrict activity and immediately go on to imaging. And then it’s time to get that patient off to somebody who deals with these patients all the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
ARVC is thought to affect 1 in 5,000 people. It’s the cause of sudden death in roughly 5% of young athletes in the United States and 25% in Italy, with the disparity believed to be largely caused by underrecognition of the disease.
Dr. Estes was a coauthor of a 2015 international task force consensus statement on the treatment of ARVC (Eur Heart J. 2015 Dec 7;36[46]:3227-37). An updated report that incorporates much new information is due to be released in May 2019.
“The new guidelines are going to put some very clear limits on physical activity: Less than 7 METs [metabolic equivalents], which is about a brisk walk. Exercise is not good with ARVC,” said Dr. Estes, professor of medicine at the University of Pittsburgh.
Patients with ARVC absolutely must be restricted from participation in competitive sports, a step whose importance was demonstrated in the North American Multidisciplinary Study of ARVC, for which Dr. Estes was a coauthor. That study, too recent for inclusion in the 2015 task force consensus statement, showed that competitive sport was associated with a 100% increased risk of ventricular tachyarrhythmias and death, as well as earlier presentation with symptoms, compared with patients with ARVC who engaged in much less intense recreational athletic activity, who in turn didn’t have a risk level any different from inactive patients. That being said, however, the investigators also noted that the absolute risk of ventricular tachyarrhythmias or death remained high even in the inactive and recreational sports participants, at 23% by 2 years post diagnosis (Eur Heart J. 2015 Jul 14;36[27]:1735-43).
ARVC has three phases: An early concealed phase, with an associated high rate of syncope or sudden cardiac death; an intermediate phase marked by frequent arrhythmias; and a late phase involving cardiomyopathy and heart failure. Pathologically, the disease involves progressive loss of myocytes caused by abnormalities of plakoglobin and plakophilin, which Dr. Estes described as “essentially the glue that holds the sarcomeres together.” As myocytes are lost they are replaced by fibrofatty deposits, mostly in the right ventricle, creating a substrate for ventricular arrhythmias.
When to suspect ARVC?
“Anyone who has syncope with exertion automatically has a red flag because there is almost certainly something causing that event that’s potentially malignant,” according to the cardiologist.
An early, sensitive, and reasonably specific hallmark of ARVC on the 12-lead ECG is T wave inversion in right precordial leads V1, V2, and V3 or beyond. An epsilon wave – a small positive deflection at the end of the QRS complex – is present in about one in five patients with ARVC and is diagnostic of the condition.
“It’s an ECG you want to commit to memory. And when you see it, think ARVC,” Dr. Estes advised.
Abnormalities on MRI and 2D echocardiography include right ventricular enlargement and wall motion abnormalities, with left ventricular involvement becoming common in more advanced disease.
The detailed structural, electrographic, functional imaging, histologic, and clinical diagnostic criteria for ARVC, known as the 2010 Task Force Criteria, are readily available (Circulation. 2010 Apr 6;121[13]:1533-41). So are guidelines on genetic testing, which practitioners need to understand is now the standard of care for patients with ARVC and, in the event they are found to have a pathogenic mutation, in first-degree family members as well (Europace. 2011 Aug;13(8):1077-109).
“These days all you need to do is simply Google ‘syncope’ and ‘ARVC’ and you will get to the guidelines,” Dr. Estes advised. “It’s virtually impossible to keep up with all this information, but you should know how to access it and apply it in an individual patient. And learn the role of genetic testing. It’s very much an evolving area, quite mature for the long QT syndromes, but not a robust database for ARVC now. Yet there’s no doubt it’s going to be used more and more for diagnosis, risk stratification, and therapy of ARVC in the future.”
Once the diagnosis of ARVC is made in accordance with the 2010 Task Force Criteria, risk stratification and decision making about definitive therapy become paramount. The international task force consensus statement gives a Class I recommendation to implantable cardioverter defibrillator (ICD) therapy in high-risk ARVC patients, defined as those who’ve had a cardiac arrest, sustained ventricular tachycardia, or have severe right and/or left ventricular dysfunction. ICD therapy gets a Class IIa recommendation in those with at least one of the major risk factors: syncope, nonsustained ventricular tachycardia, and moderate ventricular dysfunction. For ARVC patients with one or more minor risk factors, which include frequent premature ventricular contractions and inducible ventricular tachycardia upon electrophysiological testing, the strength of the recommendation for ICD therapy drops to Class IIb, or “may be considered.” And for low-risk patients, those with confirmed ARVC but no phenotypic expressions of the disorder, the ICD recommendation is Class III, meaning nonindicated.
“There’s a very low threshold for putting in an ICD in patients with ARVC because if you eliminate the arrhythmic mortality, progression to heart failure needing advanced therapies or transplant is extremely rare as long as they’re restricted from athletics,” Dr. Estes said.
He reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.bjancin@mdedge.com
SNOWMASS, COLO. – Electrophysiologist N.A. Mark Estes III, MD, has a piece of advice for his general cardiology colleagues regarding arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited arrhythmia syndrome that’s one of the most common causes of sudden death in athletes under the age of 40 years.
“When you have a patient with syncope and an ECG abnormality that suggests ARVC, you immediately restrict activity and immediately go on to imaging. And then it’s time to get that patient off to somebody who deals with these patients all the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
ARVC is thought to affect 1 in 5,000 people. It’s the cause of sudden death in roughly 5% of young athletes in the United States and 25% in Italy, with the disparity believed to be largely caused by underrecognition of the disease.
Dr. Estes was a coauthor of a 2015 international task force consensus statement on the treatment of ARVC (Eur Heart J. 2015 Dec 7;36[46]:3227-37). An updated report that incorporates much new information is due to be released in May 2019.
“The new guidelines are going to put some very clear limits on physical activity: Less than 7 METs [metabolic equivalents], which is about a brisk walk. Exercise is not good with ARVC,” said Dr. Estes, professor of medicine at the University of Pittsburgh.
Patients with ARVC absolutely must be restricted from participation in competitive sports, a step whose importance was demonstrated in the North American Multidisciplinary Study of ARVC, for which Dr. Estes was a coauthor. That study, too recent for inclusion in the 2015 task force consensus statement, showed that competitive sport was associated with a 100% increased risk of ventricular tachyarrhythmias and death, as well as earlier presentation with symptoms, compared with patients with ARVC who engaged in much less intense recreational athletic activity, who in turn didn’t have a risk level any different from inactive patients. That being said, however, the investigators also noted that the absolute risk of ventricular tachyarrhythmias or death remained high even in the inactive and recreational sports participants, at 23% by 2 years post diagnosis (Eur Heart J. 2015 Jul 14;36[27]:1735-43).
ARVC has three phases: An early concealed phase, with an associated high rate of syncope or sudden cardiac death; an intermediate phase marked by frequent arrhythmias; and a late phase involving cardiomyopathy and heart failure. Pathologically, the disease involves progressive loss of myocytes caused by abnormalities of plakoglobin and plakophilin, which Dr. Estes described as “essentially the glue that holds the sarcomeres together.” As myocytes are lost they are replaced by fibrofatty deposits, mostly in the right ventricle, creating a substrate for ventricular arrhythmias.
When to suspect ARVC?
“Anyone who has syncope with exertion automatically has a red flag because there is almost certainly something causing that event that’s potentially malignant,” according to the cardiologist.
An early, sensitive, and reasonably specific hallmark of ARVC on the 12-lead ECG is T wave inversion in right precordial leads V1, V2, and V3 or beyond. An epsilon wave – a small positive deflection at the end of the QRS complex – is present in about one in five patients with ARVC and is diagnostic of the condition.
“It’s an ECG you want to commit to memory. And when you see it, think ARVC,” Dr. Estes advised.
Abnormalities on MRI and 2D echocardiography include right ventricular enlargement and wall motion abnormalities, with left ventricular involvement becoming common in more advanced disease.
The detailed structural, electrographic, functional imaging, histologic, and clinical diagnostic criteria for ARVC, known as the 2010 Task Force Criteria, are readily available (Circulation. 2010 Apr 6;121[13]:1533-41). So are guidelines on genetic testing, which practitioners need to understand is now the standard of care for patients with ARVC and, in the event they are found to have a pathogenic mutation, in first-degree family members as well (Europace. 2011 Aug;13(8):1077-109).
“These days all you need to do is simply Google ‘syncope’ and ‘ARVC’ and you will get to the guidelines,” Dr. Estes advised. “It’s virtually impossible to keep up with all this information, but you should know how to access it and apply it in an individual patient. And learn the role of genetic testing. It’s very much an evolving area, quite mature for the long QT syndromes, but not a robust database for ARVC now. Yet there’s no doubt it’s going to be used more and more for diagnosis, risk stratification, and therapy of ARVC in the future.”
Once the diagnosis of ARVC is made in accordance with the 2010 Task Force Criteria, risk stratification and decision making about definitive therapy become paramount. The international task force consensus statement gives a Class I recommendation to implantable cardioverter defibrillator (ICD) therapy in high-risk ARVC patients, defined as those who’ve had a cardiac arrest, sustained ventricular tachycardia, or have severe right and/or left ventricular dysfunction. ICD therapy gets a Class IIa recommendation in those with at least one of the major risk factors: syncope, nonsustained ventricular tachycardia, and moderate ventricular dysfunction. For ARVC patients with one or more minor risk factors, which include frequent premature ventricular contractions and inducible ventricular tachycardia upon electrophysiological testing, the strength of the recommendation for ICD therapy drops to Class IIb, or “may be considered.” And for low-risk patients, those with confirmed ARVC but no phenotypic expressions of the disorder, the ICD recommendation is Class III, meaning nonindicated.
“There’s a very low threshold for putting in an ICD in patients with ARVC because if you eliminate the arrhythmic mortality, progression to heart failure needing advanced therapies or transplant is extremely rare as long as they’re restricted from athletics,” Dr. Estes said.
He reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.bjancin@mdedge.com
SNOWMASS, COLO. – Electrophysiologist N.A. Mark Estes III, MD, has a piece of advice for his general cardiology colleagues regarding arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited arrhythmia syndrome that’s one of the most common causes of sudden death in athletes under the age of 40 years.
“When you have a patient with syncope and an ECG abnormality that suggests ARVC, you immediately restrict activity and immediately go on to imaging. And then it’s time to get that patient off to somebody who deals with these patients all the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
ARVC is thought to affect 1 in 5,000 people. It’s the cause of sudden death in roughly 5% of young athletes in the United States and 25% in Italy, with the disparity believed to be largely caused by underrecognition of the disease.
Dr. Estes was a coauthor of a 2015 international task force consensus statement on the treatment of ARVC (Eur Heart J. 2015 Dec 7;36[46]:3227-37). An updated report that incorporates much new information is due to be released in May 2019.
“The new guidelines are going to put some very clear limits on physical activity: Less than 7 METs [metabolic equivalents], which is about a brisk walk. Exercise is not good with ARVC,” said Dr. Estes, professor of medicine at the University of Pittsburgh.
Patients with ARVC absolutely must be restricted from participation in competitive sports, a step whose importance was demonstrated in the North American Multidisciplinary Study of ARVC, for which Dr. Estes was a coauthor. That study, too recent for inclusion in the 2015 task force consensus statement, showed that competitive sport was associated with a 100% increased risk of ventricular tachyarrhythmias and death, as well as earlier presentation with symptoms, compared with patients with ARVC who engaged in much less intense recreational athletic activity, who in turn didn’t have a risk level any different from inactive patients. That being said, however, the investigators also noted that the absolute risk of ventricular tachyarrhythmias or death remained high even in the inactive and recreational sports participants, at 23% by 2 years post diagnosis (Eur Heart J. 2015 Jul 14;36[27]:1735-43).
ARVC has three phases: An early concealed phase, with an associated high rate of syncope or sudden cardiac death; an intermediate phase marked by frequent arrhythmias; and a late phase involving cardiomyopathy and heart failure. Pathologically, the disease involves progressive loss of myocytes caused by abnormalities of plakoglobin and plakophilin, which Dr. Estes described as “essentially the glue that holds the sarcomeres together.” As myocytes are lost they are replaced by fibrofatty deposits, mostly in the right ventricle, creating a substrate for ventricular arrhythmias.
When to suspect ARVC?
“Anyone who has syncope with exertion automatically has a red flag because there is almost certainly something causing that event that’s potentially malignant,” according to the cardiologist.
An early, sensitive, and reasonably specific hallmark of ARVC on the 12-lead ECG is T wave inversion in right precordial leads V1, V2, and V3 or beyond. An epsilon wave – a small positive deflection at the end of the QRS complex – is present in about one in five patients with ARVC and is diagnostic of the condition.
“It’s an ECG you want to commit to memory. And when you see it, think ARVC,” Dr. Estes advised.
Abnormalities on MRI and 2D echocardiography include right ventricular enlargement and wall motion abnormalities, with left ventricular involvement becoming common in more advanced disease.
The detailed structural, electrographic, functional imaging, histologic, and clinical diagnostic criteria for ARVC, known as the 2010 Task Force Criteria, are readily available (Circulation. 2010 Apr 6;121[13]:1533-41). So are guidelines on genetic testing, which practitioners need to understand is now the standard of care for patients with ARVC and, in the event they are found to have a pathogenic mutation, in first-degree family members as well (Europace. 2011 Aug;13(8):1077-109).
“These days all you need to do is simply Google ‘syncope’ and ‘ARVC’ and you will get to the guidelines,” Dr. Estes advised. “It’s virtually impossible to keep up with all this information, but you should know how to access it and apply it in an individual patient. And learn the role of genetic testing. It’s very much an evolving area, quite mature for the long QT syndromes, but not a robust database for ARVC now. Yet there’s no doubt it’s going to be used more and more for diagnosis, risk stratification, and therapy of ARVC in the future.”
Once the diagnosis of ARVC is made in accordance with the 2010 Task Force Criteria, risk stratification and decision making about definitive therapy become paramount. The international task force consensus statement gives a Class I recommendation to implantable cardioverter defibrillator (ICD) therapy in high-risk ARVC patients, defined as those who’ve had a cardiac arrest, sustained ventricular tachycardia, or have severe right and/or left ventricular dysfunction. ICD therapy gets a Class IIa recommendation in those with at least one of the major risk factors: syncope, nonsustained ventricular tachycardia, and moderate ventricular dysfunction. For ARVC patients with one or more minor risk factors, which include frequent premature ventricular contractions and inducible ventricular tachycardia upon electrophysiological testing, the strength of the recommendation for ICD therapy drops to Class IIb, or “may be considered.” And for low-risk patients, those with confirmed ARVC but no phenotypic expressions of the disorder, the ICD recommendation is Class III, meaning nonindicated.
“There’s a very low threshold for putting in an ICD in patients with ARVC because if you eliminate the arrhythmic mortality, progression to heart failure needing advanced therapies or transplant is extremely rare as long as they’re restricted from athletics,” Dr. Estes said.
He reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.bjancin@mdedge.com
REPORTING FROM ACC SNOWMASS 2019
Physiological versus pathological cardiac remodeling in athletes
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – according to Matthew W. Martinez, MD, medical director of the Sports Cardiology and Hypertrophic Cardiomyopathy Center at the Lehigh Valley Health Network in Allentown, Pa.
“The MRI may be the single test that best helps you sort out when you’re not quite sure. If you think about a single study that’s going to help you identify cardiac arrest etiologies – hypertrophic cardiomyopathy, myocarditis, anomalous coronaries, left-sided disease, right-sided disease like arrhythmogenic right ventricular cardiomyopathy, valvular heart disease, aortic disease – MRI is a very powerful tool because it will help you evaluate all of those groups more than 90% of the time,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Martinez, who serves as lead cardiologist for U.S. Major League Soccer and is also heavily involved with the National Football League, spends a lot of time with elite professional or Olympic athletes who fall into what he calls “the gray zone,” with a left ventricular wall thickness of 12-15 mm as measured on echocardiography. While that would clearly be considered abnormal in a nonathlete or a recreational sports enthusiast, his experience as well as that of other sports cardiologists working with professional soccer, football, and basketball players, bicyclists, and high-level track and field competitors has been that wall thickness in the 12- to 15-mm range in elite athletes can represent physiological adaptation to their enormous cardiovascular workloads. For example, more than 10% of National Football League players have a maximum left ventricular wall thickness of 13 mm or more, as do more than 10% of National Basketball Association players.
But what if that echocardiographic measurement of wall thickness is off by a millimeter or two, as is often par for the course?
“It’s well described that MRI gives a better look at wall thickness than echocardiography, especially where there’s areas of hypertrophy next to normal wall. In that gray zone, where we have to know if it’s really 10-12 or 14-16 mm, the MRI better identifies the actual thickness,” he said.
In addition, cardiac MRI readily provides accurate, reproducible measurements of left and right ventricular chamber size. But the most important way in which cardiac MRI helps in evaluating the significance of cardiac remodeling in athletes is via the gadolinium study. Late gadolinium enhancement is a concerning finding. It indicates the presence of myocardial fibrosis and scar, which at least in the general population is a prognostic sign for worse outcome.
“If you detect fibrosis, the search for pathology has to start,” the cardiologist emphasized.
He noted that the most comprehensive review to date of myocardial fibrosis in endurance athletes identified the intraventricular septum and the junction of the right ventricle and septum as the most common sites of involvement. The investigators concluded that, while there is a lack of compelling data on the clinical impact and prognosis of myocardial fibrosis in athletes, potential mechanisms include exercise-induced repetitive microinjury, pulmonary artery pressure overload, genetic predisposition, and silent myocarditis (Mayo Clin Proc. 2016 Nov;91[11]:1617-31).
That being said about the value of cardiac MRI as a tiebreaker, Dr. Martinez asserted that “there’s no specific test that’s going to get you out of jail. ... I would submit to you that you have to load the boat. Be comprehensive and try to build a case for one side or the other. And I would encourage you to ask for help; we do it all the time.”
Dilated chambers outside the normal range are common in competitive athletes. Don’t accept the echocardiographic hard numeric cutoffs that have been established as “normal” in the general population. For example, 36% of National Basketball Association players have a left ventricular end diastolic dimension (LVEDD) greater than 60 mm.
“I’ve seen LVEDDs up to 70 mm in cyclists. And all but a handful have a normal left ventricular ejection fraction greater than 50%,” he noted.
Dilated chambers in elite athletes are reassuring, provided stroke volume is preserved or, as is more often the case, enhanced.
“One of the hallmarks of being an athlete is the ability to suck in blood and increase stroke volume as a result. A typical stroke volume in an athlete is well above normal, with 85-90 cc or more being common. On tissue Doppler assessment, you shouldn’t have a normal inflow pattern or normal relaxation. A septal E prime velocity of 11-14 cm/sec is what I typically expect in an athlete. A lower E prime velocity suggests early pathologic change. If you find an E prime velocity of less than 9 cm/sec on tissue Doppler, or an elevated filling pressure like 15 mm Hg, that correlates with a greater than 90% sensitivity for pathology, such as hypertrophic cardiomyopathy. The average E prime velocity in Major League Soccer players is about 13 cm/sec, so that’s an important number to keep in your head,” according to the cardiologist.
Cardiac remodeling in elite athletes tends towards one of two forms, depending upon their sport. Endurance athletes, such as marathon runners, are repetitively volume challenged, so expect a tendency towards aortic regurgitation. For pressure-challenged athletes, such as power weightlifters, the tendency is toward aortic stenosis.
“But also expect a blend. It’s rarely just one or the other. Understanding that can help you discern the gray zone athlete,” he said.
Dr. Martinez reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Consider adopting the MESA 10-year CHD risk calculator
SNOWMASS, COLO. – The Multi-Ethnic Study of Atherosclerosis–based 10-year coronary heart disease risk calculator offers significant advantages over the far more widely used Atherosclerotic Cardiovascular Disease risk estimator based upon the pooled cohort equations recommended in the American College of Cardiology/American Heart Association guidelines, Robert A. Vogel, MD, said at the Annual Cardiovascular Conference at Snowmass.
“I don’t use the PCE very much. I use the MESA [Multi-Ethnic Study of Atherosclerosis]. I like it because it gives me options I don’t have with the PCE [pooled cohort equations],” Dr. Vogel, a preventive cardiology expert at the University of Colorado at Denver, Aurora, said at the meeting sponsored by the American College of Cardiology.
Those added options include, importantly, the ability to plug in a patient’s coronary artery calcium score. MESA, a landmark longitudinal National Institutes of Health–sponsored study, generated a great deal of the data that established the prognostic value of measuring coronary artery calcium.
“You can do the MESA worksheet with or without coronary artery calcium, either way,” Dr. Vogel noted.
Another big advantage for the MESA risk calculator: It asks a yes/no question about family history of heart attack in first-degree relatives. That’s truly risk-altering information, and yet it’s absent from the ACC/AHA ASCVD (Atherosclerotic Cardiovascular Disease) risk calculator, the cardiologist continued.
Plus, the MESA risk calculator includes four ethnic options: Caucasian, African American, Chinese, or Hispanic, while the ACC/AHA’s PCE-based tool includes only the categories of white, African American, or other.
Dr. Vogel offered a case example for purposes of comparison and contrast of the two risk calculators: a 48-year-old white man with no history or symptoms of cardiovascular disease whose father had an MI at age 52. He wants to know whether he should start taking a statin. The patient is a former smoker who quit 3 years ago. His physical exam is normal. He has a body mass index of 29 kg/m2, an LDL cholesterol of 134 mg/dL, a total cholesterol of 194 mg/dL, a triglyceride level of 92 mg/dL, blood pressure of 128/78 mm Hg, an HDL cholesterol of 42 mg/dL, and a hemoglobin A1c of 5.9%.
Using the PCE-based risk calculator, the man’s 10-year risk of cardiovascular disease is only 3.3%, which falls below the ACC/AHA guideline-recommended threshold for statin therapy for primary prevention. But with the MESA score, as a result of the additional information regarding the family history of premature cardiovascular disease, the patient’s risk jumps to 4.9%, even without including a coronary artery calcium score.
“This shows you how big a factor the family history is. I think it’s unfortunate that the PCE doesn’t put it in,” Dr. Vogel said.
Indeed, in an analysis from MESA, a 60-year-old man with a lipid and blood pressure profile similar to that of Dr. Vogel’s patient would have a 10-year cardiovascular disease risk of 6% with a negative family history and a 9% risk with a positive history, he noted.
In the 48-year-old patient used as an example by Dr. Vogel, plugging into the MESA risk calculator a coronary artery calcium score of, say, 50, 100, or 150 Agatston units would boost the 10-year cardiovascular disease risk to 7.5%, 8.9%, and 9.9%, respectively.
Both the MESA and the ACC/AHA risk calculators incorporate diabetes as a simple yes/no item. It’s either present or absent. This is too crude a dichotomy for Dr. Vogel’s liking in light of data showing that individuals with impaired glucose tolerance have a cumulative risk of cardiovascular mortality that’s intermediate between diabetic and normoglycemic individuals. And of course, the patient in his case example had a HbA1c of 5.9%. So what is a physician to do?
“I fudge the numbers,” he said. “
He also adjusts a former smoker’s estimated 10-year cardiovascular risk based upon how long ago the smoker quit. An ex-smoker’s hazard ratio for coronary heart disease doesn’t drop to that of a never-smoker until 8 years after quitting. And since the patient in this example has been tobacco-free for only 3 years, Dr. Vogel bumps up that individual’s risk level once again. So at this point, even without the additional information that would be provided by a coronary calcium score, the patient’s adjusted MESA 10-year risk is in the 10% range, compared with the 3.3% figure derived using the PCE-based risk calculator.
He reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
SNOWMASS, COLO. – The Multi-Ethnic Study of Atherosclerosis–based 10-year coronary heart disease risk calculator offers significant advantages over the far more widely used Atherosclerotic Cardiovascular Disease risk estimator based upon the pooled cohort equations recommended in the American College of Cardiology/American Heart Association guidelines, Robert A. Vogel, MD, said at the Annual Cardiovascular Conference at Snowmass.
“I don’t use the PCE very much. I use the MESA [Multi-Ethnic Study of Atherosclerosis]. I like it because it gives me options I don’t have with the PCE [pooled cohort equations],” Dr. Vogel, a preventive cardiology expert at the University of Colorado at Denver, Aurora, said at the meeting sponsored by the American College of Cardiology.
Those added options include, importantly, the ability to plug in a patient’s coronary artery calcium score. MESA, a landmark longitudinal National Institutes of Health–sponsored study, generated a great deal of the data that established the prognostic value of measuring coronary artery calcium.
“You can do the MESA worksheet with or without coronary artery calcium, either way,” Dr. Vogel noted.
Another big advantage for the MESA risk calculator: It asks a yes/no question about family history of heart attack in first-degree relatives. That’s truly risk-altering information, and yet it’s absent from the ACC/AHA ASCVD (Atherosclerotic Cardiovascular Disease) risk calculator, the cardiologist continued.
Plus, the MESA risk calculator includes four ethnic options: Caucasian, African American, Chinese, or Hispanic, while the ACC/AHA’s PCE-based tool includes only the categories of white, African American, or other.
Dr. Vogel offered a case example for purposes of comparison and contrast of the two risk calculators: a 48-year-old white man with no history or symptoms of cardiovascular disease whose father had an MI at age 52. He wants to know whether he should start taking a statin. The patient is a former smoker who quit 3 years ago. His physical exam is normal. He has a body mass index of 29 kg/m2, an LDL cholesterol of 134 mg/dL, a total cholesterol of 194 mg/dL, a triglyceride level of 92 mg/dL, blood pressure of 128/78 mm Hg, an HDL cholesterol of 42 mg/dL, and a hemoglobin A1c of 5.9%.
Using the PCE-based risk calculator, the man’s 10-year risk of cardiovascular disease is only 3.3%, which falls below the ACC/AHA guideline-recommended threshold for statin therapy for primary prevention. But with the MESA score, as a result of the additional information regarding the family history of premature cardiovascular disease, the patient’s risk jumps to 4.9%, even without including a coronary artery calcium score.
“This shows you how big a factor the family history is. I think it’s unfortunate that the PCE doesn’t put it in,” Dr. Vogel said.
Indeed, in an analysis from MESA, a 60-year-old man with a lipid and blood pressure profile similar to that of Dr. Vogel’s patient would have a 10-year cardiovascular disease risk of 6% with a negative family history and a 9% risk with a positive history, he noted.
In the 48-year-old patient used as an example by Dr. Vogel, plugging into the MESA risk calculator a coronary artery calcium score of, say, 50, 100, or 150 Agatston units would boost the 10-year cardiovascular disease risk to 7.5%, 8.9%, and 9.9%, respectively.
Both the MESA and the ACC/AHA risk calculators incorporate diabetes as a simple yes/no item. It’s either present or absent. This is too crude a dichotomy for Dr. Vogel’s liking in light of data showing that individuals with impaired glucose tolerance have a cumulative risk of cardiovascular mortality that’s intermediate between diabetic and normoglycemic individuals. And of course, the patient in his case example had a HbA1c of 5.9%. So what is a physician to do?
“I fudge the numbers,” he said. “
He also adjusts a former smoker’s estimated 10-year cardiovascular risk based upon how long ago the smoker quit. An ex-smoker’s hazard ratio for coronary heart disease doesn’t drop to that of a never-smoker until 8 years after quitting. And since the patient in this example has been tobacco-free for only 3 years, Dr. Vogel bumps up that individual’s risk level once again. So at this point, even without the additional information that would be provided by a coronary calcium score, the patient’s adjusted MESA 10-year risk is in the 10% range, compared with the 3.3% figure derived using the PCE-based risk calculator.
He reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
SNOWMASS, COLO. – The Multi-Ethnic Study of Atherosclerosis–based 10-year coronary heart disease risk calculator offers significant advantages over the far more widely used Atherosclerotic Cardiovascular Disease risk estimator based upon the pooled cohort equations recommended in the American College of Cardiology/American Heart Association guidelines, Robert A. Vogel, MD, said at the Annual Cardiovascular Conference at Snowmass.
“I don’t use the PCE very much. I use the MESA [Multi-Ethnic Study of Atherosclerosis]. I like it because it gives me options I don’t have with the PCE [pooled cohort equations],” Dr. Vogel, a preventive cardiology expert at the University of Colorado at Denver, Aurora, said at the meeting sponsored by the American College of Cardiology.
Those added options include, importantly, the ability to plug in a patient’s coronary artery calcium score. MESA, a landmark longitudinal National Institutes of Health–sponsored study, generated a great deal of the data that established the prognostic value of measuring coronary artery calcium.
“You can do the MESA worksheet with or without coronary artery calcium, either way,” Dr. Vogel noted.
Another big advantage for the MESA risk calculator: It asks a yes/no question about family history of heart attack in first-degree relatives. That’s truly risk-altering information, and yet it’s absent from the ACC/AHA ASCVD (Atherosclerotic Cardiovascular Disease) risk calculator, the cardiologist continued.
Plus, the MESA risk calculator includes four ethnic options: Caucasian, African American, Chinese, or Hispanic, while the ACC/AHA’s PCE-based tool includes only the categories of white, African American, or other.
Dr. Vogel offered a case example for purposes of comparison and contrast of the two risk calculators: a 48-year-old white man with no history or symptoms of cardiovascular disease whose father had an MI at age 52. He wants to know whether he should start taking a statin. The patient is a former smoker who quit 3 years ago. His physical exam is normal. He has a body mass index of 29 kg/m2, an LDL cholesterol of 134 mg/dL, a total cholesterol of 194 mg/dL, a triglyceride level of 92 mg/dL, blood pressure of 128/78 mm Hg, an HDL cholesterol of 42 mg/dL, and a hemoglobin A1c of 5.9%.
Using the PCE-based risk calculator, the man’s 10-year risk of cardiovascular disease is only 3.3%, which falls below the ACC/AHA guideline-recommended threshold for statin therapy for primary prevention. But with the MESA score, as a result of the additional information regarding the family history of premature cardiovascular disease, the patient’s risk jumps to 4.9%, even without including a coronary artery calcium score.
“This shows you how big a factor the family history is. I think it’s unfortunate that the PCE doesn’t put it in,” Dr. Vogel said.
Indeed, in an analysis from MESA, a 60-year-old man with a lipid and blood pressure profile similar to that of Dr. Vogel’s patient would have a 10-year cardiovascular disease risk of 6% with a negative family history and a 9% risk with a positive history, he noted.
In the 48-year-old patient used as an example by Dr. Vogel, plugging into the MESA risk calculator a coronary artery calcium score of, say, 50, 100, or 150 Agatston units would boost the 10-year cardiovascular disease risk to 7.5%, 8.9%, and 9.9%, respectively.
Both the MESA and the ACC/AHA risk calculators incorporate diabetes as a simple yes/no item. It’s either present or absent. This is too crude a dichotomy for Dr. Vogel’s liking in light of data showing that individuals with impaired glucose tolerance have a cumulative risk of cardiovascular mortality that’s intermediate between diabetic and normoglycemic individuals. And of course, the patient in his case example had a HbA1c of 5.9%. So what is a physician to do?
“I fudge the numbers,” he said. “
He also adjusts a former smoker’s estimated 10-year cardiovascular risk based upon how long ago the smoker quit. An ex-smoker’s hazard ratio for coronary heart disease doesn’t drop to that of a never-smoker until 8 years after quitting. And since the patient in this example has been tobacco-free for only 3 years, Dr. Vogel bumps up that individual’s risk level once again. So at this point, even without the additional information that would be provided by a coronary calcium score, the patient’s adjusted MESA 10-year risk is in the 10% range, compared with the 3.3% figure derived using the PCE-based risk calculator.
He reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
REPORTING FROM ACC SNOWMASS 2019
How to get surgical-like results using MitraClip
SNOWMASS, COLO. – Achieving optimal surgical-like results via transcatheter repair of primary mitral regurgitation using the MitraClip in prohibitively high-surgical-risk patients becomes much more likely by taking into account key predictive anatomic and procedural features as well as the major comorbidities influencing outcome, Paul Sorajja, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
. Dr. Sorajja was first author of a study of 1-year outcomes in 2,952 patients with MR in the Society of Thoracic Surgery/American College of Cardiology Transcatheter Valve Therapy Registry (STS/ACC TVT) who were commercially treated with the MitraClip, at present the only transcatheter device approved for this indication. Sixty-two percent had a surgical-like result, and their 1-year mortality rate of 21.7% was significantly lower than the 29.2% rate for patients with residual grade 2 MR and the 48.9% mortality in patients left with grade 3 or 4 MR.
It’s noteworthy that even though the acute procedural success rate – defined as residual grade 2 or less MR – was impressively high at 91.8% in this group of nearly 3,000 patients, roughly one in five in the overall series was rehospitalized for heart failure within 1 year, and one in four was dead. So there remains considerable room for improvement in long-term outcomes of transcatheter repair of MR, said Dr. Sorajja, director of the Center of Valve and Structural Heart Disease at the Minneapolis Heart Institute.
Anatomic predictors of good short-term outcome
“There are specific anatomic criteria, but an easier way to think about whether your patient could get an optimal result is to remember the physical limits of this therapy. What I teach is 1-2-5-50: the MitraClip device is about 1 cm tall, about 2 cm wide, and you need about 5 mm of leaflet inside the clip to create coaptation for MR reduction. And if you do that, you will reduce the mitral valve area by about 50%, so you have to be very careful in patients with small valve areas because you can worsen mitral stenosis,” the cardiologist explained.
Only two transechocardiographic views are needed to know if a patient will have a good result with the MitraClip. A bicommissural view traversing the valve medial to lateral shows where the MR jet is; if it’s in the middle of the mitral valve, that’s favorable because it means the interventionalist has a lot of freedom to operate. Then, going orthogonal from the bicommissural view to get an anterior-posterior view allows the operator to get a good look at the valve leaflets and apply the 1-2-5 rule to determine if the leaflet approximation is favorable, he continued.
Some mitral valve anatomy variants make it challenging to get surgical-like results with a transcatheter repair. These include calcified leaflets, large gaps between leaflets, Barlow’s valves, and small leaflets. Be aware of key success-limiting comorbidities.
In the STS/ACC TVT registry study, severe tricuspid regurgitation, present in 10% of patients preprocedurally, virtually doubled the adjusted risk of 1-year mortality in multivariate analyses.
“Tricuspid regurgitation is one of the most common concomitant lesions. And the presence of tricuspid regurgitation is ominous,” Dr. Sorajja said. “Treatment of concomitant lesions such as this is going to be necessary for us to get a truly surgical-like result in outcomes.”
Toward this end, he put in a plug to consider referring patients with severe tricuspid regurgitation for enrollment in the TRILUMINATE II trial, the pivotal U.S. trial for the investigational Tri-Clip device for transcatheter tricuspid valve repair, for which he is coprincipal investigator. The trial, pitting the Tri-Clip against medical therapy, is due to start in the spring of 2019.
In multivariate analyses of the MitraClip registry study, other predictors of the combined endpoint of death and rehospitalization for heart failure at 1 year, in addition to severe tricuspid regurgitation, included dialysis, with an adjusted 2.09-fold increased risk; moderate or severe lung disease, with a 1.28-fold risk; postprocedural residual MR; diminished left ventricular ejection fraction; and advanced age (J Am Coll Cardiol. 2017 Nov 7;70[19]:2315-27).
Case experience counts
In a soon-to-be-published more up-to-date analysis of more than 12,000 MitraClip patients in the STS/ACC TVT registry, which captures all U.S. commercial use of the device for its approved indication, Dr. Sorajja and his coinvestigators documented a procedural truism: The more cases an interventionalist performs, the better the results. However, even inexperienced users of the MitraClip were able to obtain at least moderate results – that is, residual grade 2 or less MR – 90% of the time or more.
“There is some change with greater experience, but it’s actually quite small,” according to Dr. Sorajja.
However, optimal results – grade 0 or 1 MR – are another matter entirely.
“For grade 1 or less, the learning curve is much steeper. It plateaus somewhere between 50 and 75 cases. In other words, in most cases you can get to moderate MR, but getting to grade 1 requires more experience. That relationship between case experience and outcome also applies to complication rates and case time,” he said.
Although at present the MitraClip is the only Food and Drug Administration–approved transcatheter device for MR repair, there are many others in the developmental pipeline, the he noted.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
SNOWMASS, COLO. – Achieving optimal surgical-like results via transcatheter repair of primary mitral regurgitation using the MitraClip in prohibitively high-surgical-risk patients becomes much more likely by taking into account key predictive anatomic and procedural features as well as the major comorbidities influencing outcome, Paul Sorajja, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
. Dr. Sorajja was first author of a study of 1-year outcomes in 2,952 patients with MR in the Society of Thoracic Surgery/American College of Cardiology Transcatheter Valve Therapy Registry (STS/ACC TVT) who were commercially treated with the MitraClip, at present the only transcatheter device approved for this indication. Sixty-two percent had a surgical-like result, and their 1-year mortality rate of 21.7% was significantly lower than the 29.2% rate for patients with residual grade 2 MR and the 48.9% mortality in patients left with grade 3 or 4 MR.
It’s noteworthy that even though the acute procedural success rate – defined as residual grade 2 or less MR – was impressively high at 91.8% in this group of nearly 3,000 patients, roughly one in five in the overall series was rehospitalized for heart failure within 1 year, and one in four was dead. So there remains considerable room for improvement in long-term outcomes of transcatheter repair of MR, said Dr. Sorajja, director of the Center of Valve and Structural Heart Disease at the Minneapolis Heart Institute.
Anatomic predictors of good short-term outcome
“There are specific anatomic criteria, but an easier way to think about whether your patient could get an optimal result is to remember the physical limits of this therapy. What I teach is 1-2-5-50: the MitraClip device is about 1 cm tall, about 2 cm wide, and you need about 5 mm of leaflet inside the clip to create coaptation for MR reduction. And if you do that, you will reduce the mitral valve area by about 50%, so you have to be very careful in patients with small valve areas because you can worsen mitral stenosis,” the cardiologist explained.
Only two transechocardiographic views are needed to know if a patient will have a good result with the MitraClip. A bicommissural view traversing the valve medial to lateral shows where the MR jet is; if it’s in the middle of the mitral valve, that’s favorable because it means the interventionalist has a lot of freedom to operate. Then, going orthogonal from the bicommissural view to get an anterior-posterior view allows the operator to get a good look at the valve leaflets and apply the 1-2-5 rule to determine if the leaflet approximation is favorable, he continued.
Some mitral valve anatomy variants make it challenging to get surgical-like results with a transcatheter repair. These include calcified leaflets, large gaps between leaflets, Barlow’s valves, and small leaflets. Be aware of key success-limiting comorbidities.
In the STS/ACC TVT registry study, severe tricuspid regurgitation, present in 10% of patients preprocedurally, virtually doubled the adjusted risk of 1-year mortality in multivariate analyses.
“Tricuspid regurgitation is one of the most common concomitant lesions. And the presence of tricuspid regurgitation is ominous,” Dr. Sorajja said. “Treatment of concomitant lesions such as this is going to be necessary for us to get a truly surgical-like result in outcomes.”
Toward this end, he put in a plug to consider referring patients with severe tricuspid regurgitation for enrollment in the TRILUMINATE II trial, the pivotal U.S. trial for the investigational Tri-Clip device for transcatheter tricuspid valve repair, for which he is coprincipal investigator. The trial, pitting the Tri-Clip against medical therapy, is due to start in the spring of 2019.
In multivariate analyses of the MitraClip registry study, other predictors of the combined endpoint of death and rehospitalization for heart failure at 1 year, in addition to severe tricuspid regurgitation, included dialysis, with an adjusted 2.09-fold increased risk; moderate or severe lung disease, with a 1.28-fold risk; postprocedural residual MR; diminished left ventricular ejection fraction; and advanced age (J Am Coll Cardiol. 2017 Nov 7;70[19]:2315-27).
Case experience counts
In a soon-to-be-published more up-to-date analysis of more than 12,000 MitraClip patients in the STS/ACC TVT registry, which captures all U.S. commercial use of the device for its approved indication, Dr. Sorajja and his coinvestigators documented a procedural truism: The more cases an interventionalist performs, the better the results. However, even inexperienced users of the MitraClip were able to obtain at least moderate results – that is, residual grade 2 or less MR – 90% of the time or more.
“There is some change with greater experience, but it’s actually quite small,” according to Dr. Sorajja.
However, optimal results – grade 0 or 1 MR – are another matter entirely.
“For grade 1 or less, the learning curve is much steeper. It plateaus somewhere between 50 and 75 cases. In other words, in most cases you can get to moderate MR, but getting to grade 1 requires more experience. That relationship between case experience and outcome also applies to complication rates and case time,” he said.
Although at present the MitraClip is the only Food and Drug Administration–approved transcatheter device for MR repair, there are many others in the developmental pipeline, the he noted.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
SNOWMASS, COLO. – Achieving optimal surgical-like results via transcatheter repair of primary mitral regurgitation using the MitraClip in prohibitively high-surgical-risk patients becomes much more likely by taking into account key predictive anatomic and procedural features as well as the major comorbidities influencing outcome, Paul Sorajja, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
. Dr. Sorajja was first author of a study of 1-year outcomes in 2,952 patients with MR in the Society of Thoracic Surgery/American College of Cardiology Transcatheter Valve Therapy Registry (STS/ACC TVT) who were commercially treated with the MitraClip, at present the only transcatheter device approved for this indication. Sixty-two percent had a surgical-like result, and their 1-year mortality rate of 21.7% was significantly lower than the 29.2% rate for patients with residual grade 2 MR and the 48.9% mortality in patients left with grade 3 or 4 MR.
It’s noteworthy that even though the acute procedural success rate – defined as residual grade 2 or less MR – was impressively high at 91.8% in this group of nearly 3,000 patients, roughly one in five in the overall series was rehospitalized for heart failure within 1 year, and one in four was dead. So there remains considerable room for improvement in long-term outcomes of transcatheter repair of MR, said Dr. Sorajja, director of the Center of Valve and Structural Heart Disease at the Minneapolis Heart Institute.
Anatomic predictors of good short-term outcome
“There are specific anatomic criteria, but an easier way to think about whether your patient could get an optimal result is to remember the physical limits of this therapy. What I teach is 1-2-5-50: the MitraClip device is about 1 cm tall, about 2 cm wide, and you need about 5 mm of leaflet inside the clip to create coaptation for MR reduction. And if you do that, you will reduce the mitral valve area by about 50%, so you have to be very careful in patients with small valve areas because you can worsen mitral stenosis,” the cardiologist explained.
Only two transechocardiographic views are needed to know if a patient will have a good result with the MitraClip. A bicommissural view traversing the valve medial to lateral shows where the MR jet is; if it’s in the middle of the mitral valve, that’s favorable because it means the interventionalist has a lot of freedom to operate. Then, going orthogonal from the bicommissural view to get an anterior-posterior view allows the operator to get a good look at the valve leaflets and apply the 1-2-5 rule to determine if the leaflet approximation is favorable, he continued.
Some mitral valve anatomy variants make it challenging to get surgical-like results with a transcatheter repair. These include calcified leaflets, large gaps between leaflets, Barlow’s valves, and small leaflets. Be aware of key success-limiting comorbidities.
In the STS/ACC TVT registry study, severe tricuspid regurgitation, present in 10% of patients preprocedurally, virtually doubled the adjusted risk of 1-year mortality in multivariate analyses.
“Tricuspid regurgitation is one of the most common concomitant lesions. And the presence of tricuspid regurgitation is ominous,” Dr. Sorajja said. “Treatment of concomitant lesions such as this is going to be necessary for us to get a truly surgical-like result in outcomes.”
Toward this end, he put in a plug to consider referring patients with severe tricuspid regurgitation for enrollment in the TRILUMINATE II trial, the pivotal U.S. trial for the investigational Tri-Clip device for transcatheter tricuspid valve repair, for which he is coprincipal investigator. The trial, pitting the Tri-Clip against medical therapy, is due to start in the spring of 2019.
In multivariate analyses of the MitraClip registry study, other predictors of the combined endpoint of death and rehospitalization for heart failure at 1 year, in addition to severe tricuspid regurgitation, included dialysis, with an adjusted 2.09-fold increased risk; moderate or severe lung disease, with a 1.28-fold risk; postprocedural residual MR; diminished left ventricular ejection fraction; and advanced age (J Am Coll Cardiol. 2017 Nov 7;70[19]:2315-27).
Case experience counts
In a soon-to-be-published more up-to-date analysis of more than 12,000 MitraClip patients in the STS/ACC TVT registry, which captures all U.S. commercial use of the device for its approved indication, Dr. Sorajja and his coinvestigators documented a procedural truism: The more cases an interventionalist performs, the better the results. However, even inexperienced users of the MitraClip were able to obtain at least moderate results – that is, residual grade 2 or less MR – 90% of the time or more.
“There is some change with greater experience, but it’s actually quite small,” according to Dr. Sorajja.
However, optimal results – grade 0 or 1 MR – are another matter entirely.
“For grade 1 or less, the learning curve is much steeper. It plateaus somewhere between 50 and 75 cases. In other words, in most cases you can get to moderate MR, but getting to grade 1 requires more experience. That relationship between case experience and outcome also applies to complication rates and case time,” he said.
Although at present the MitraClip is the only Food and Drug Administration–approved transcatheter device for MR repair, there are many others in the developmental pipeline, the he noted.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
SGLT2 inhibitors morph into HF drugs
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019



















