User login
Baricitinib study highlights power of placebo effect in RA
CHICAGO – After 52 weeks of blinded adalimumab, a switch to 48 weeks of unblinded baricitinib without an adalimumab washout period resulted in an uptick in rheumatoid arthritis control with no flares and no increase in serious adverse events in the phase 3 RA-BEYOND baricitinib long-term extension study, Michael E. Weinblatt, MD, reported at the annual meeting of the American College of Rheumatology.
That’s information of practical clinical utility now that baricitinib, an oral inhibitor of Janus kinase subtypes 1 and 2, is approved as Olumiant for the treatment of moderate to severely active rheumatoid arthritis (RA). Perhaps even more interesting, however, is the way RA-BEYOND shined a spotlight on the high placebo response rate endemic to RA clinical trials, according to Dr. Weinblatt, professor of medicine at Harvard Medical School and codirector of clinical rheumatology at Brigham and Women’s Hospital, Boston.
He presented an analysis of two patient groups: 381 RA patients with moderately to severely active RA at baseline who were randomized to 52 weeks of double-blind baricitinib at 4 mg once daily in the previously reported phase 3 RA-BEAM trial (N Engl J Med. 2017 Feb 16;376[7]:652-62), after which they immediately enrolled in RA-BEYOND and were switched to 48 weeks of open-label, unblinded baricitinib; and 238 RA patients who were randomized to double-blind subcutaneous adalimumab (Humira) at 40 mg every 2 weeks in RA-BEAM before being switched to unblinded baricitinib in RA-BEYOND. All participants were on background oral methotrexate throughout.
Here’s the finding that captured Dr. Weinblatt’s attention: At the time of the switch, 28.2% of patients who’d been on blinded baricitinib for 52 weeks were nonresponders to the drug, meaning that after a full year of treatment they had a Clinical Disease Activity Index (CDAI) score greater than 10. Yet after a mere additional 4 weeks on the same drug in RA-BEYOND – this time unblinded as to their treatment – 23.4% of this group were transformed into responders, with low disease activity as defined by a CDAI of 10 or less. This finding speaks eloquently as to the power of the placebo effect. It’s a real issue for clinical trialists, the rheumatologist observed.
In the adalimumab-to-baricitinib group, 31.1% of patients were nonresponders to 52 weeks of blinded adalimumab. Four weeks after switching to open-label baricitinib, 29.7% of this group had a CDAI of 10 or less.
At week 48 of open-label baricitinib in RA-BEYOND, 54.2% of nonresponders to the 52 weeks of blinded baricitinib had become responders, as did 50% of nonresponders to a year of blinded adalimumab.
Taking a step back to describe the primary outcomes in RA-BEYOND, Dr. Weinblatt noted that at enrollment in RA-BEYOND, after 52 weeks on double-blind baricitinib, 71.8% of patients had a CDAI of 10 or less and 27% were in remission as defined by a CDAI of 2.8 or less. Subsequently, after 48 weeks on unblinded baricitinib, these rates climbed to 78.2% and 31.6%, respectively.
Similarly, in the adalimumab-to-baricitinib study arm, the low disease activity and remission rates at enrollment in RA-BEYOND were 68.9% and 24.4%, improving to 73.5% and 28.2% after 48 weeks on open-label baricitinib.
Scores on the SDAI (Simplified Disease Activity Index) and DAS28-ESR (Disease Activity Score based on 28 joints with erythrocyte sedimentation rate) followed suit in both groups.
During the 48 weeks of open-label baricitinib in RA-BEYOND, the incidence of herpes zoster was roughly 2.2% in both study arms, and the rate of adverse events leading to permanent drug discontinuation was 2.7%. During RA-BEYOND, serious infections occurred in 3.8% of the baricitinib-to-baricitinib group and 2.2% of the adalimumab-to-baricitinib group.
Dr. Weinblatt drew attention to the fact that the dose of baricitinib employed in RA-BEAM and RA-BEYOND was 4 mg/day, whereas the dose approved by the Food and Drug Administration is 2 mg/day.
The RA-BEAM and RA-BEYOND trials were sponsored by Eli Lilly. Dr. Weinblatt reported serving as a paid consultant to that pharmaceutical company and more than a dozen others.
SOURCE: Weinblatt ME et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 886.
CHICAGO – After 52 weeks of blinded adalimumab, a switch to 48 weeks of unblinded baricitinib without an adalimumab washout period resulted in an uptick in rheumatoid arthritis control with no flares and no increase in serious adverse events in the phase 3 RA-BEYOND baricitinib long-term extension study, Michael E. Weinblatt, MD, reported at the annual meeting of the American College of Rheumatology.
That’s information of practical clinical utility now that baricitinib, an oral inhibitor of Janus kinase subtypes 1 and 2, is approved as Olumiant for the treatment of moderate to severely active rheumatoid arthritis (RA). Perhaps even more interesting, however, is the way RA-BEYOND shined a spotlight on the high placebo response rate endemic to RA clinical trials, according to Dr. Weinblatt, professor of medicine at Harvard Medical School and codirector of clinical rheumatology at Brigham and Women’s Hospital, Boston.
He presented an analysis of two patient groups: 381 RA patients with moderately to severely active RA at baseline who were randomized to 52 weeks of double-blind baricitinib at 4 mg once daily in the previously reported phase 3 RA-BEAM trial (N Engl J Med. 2017 Feb 16;376[7]:652-62), after which they immediately enrolled in RA-BEYOND and were switched to 48 weeks of open-label, unblinded baricitinib; and 238 RA patients who were randomized to double-blind subcutaneous adalimumab (Humira) at 40 mg every 2 weeks in RA-BEAM before being switched to unblinded baricitinib in RA-BEYOND. All participants were on background oral methotrexate throughout.
Here’s the finding that captured Dr. Weinblatt’s attention: At the time of the switch, 28.2% of patients who’d been on blinded baricitinib for 52 weeks were nonresponders to the drug, meaning that after a full year of treatment they had a Clinical Disease Activity Index (CDAI) score greater than 10. Yet after a mere additional 4 weeks on the same drug in RA-BEYOND – this time unblinded as to their treatment – 23.4% of this group were transformed into responders, with low disease activity as defined by a CDAI of 10 or less. This finding speaks eloquently as to the power of the placebo effect. It’s a real issue for clinical trialists, the rheumatologist observed.
In the adalimumab-to-baricitinib group, 31.1% of patients were nonresponders to 52 weeks of blinded adalimumab. Four weeks after switching to open-label baricitinib, 29.7% of this group had a CDAI of 10 or less.
At week 48 of open-label baricitinib in RA-BEYOND, 54.2% of nonresponders to the 52 weeks of blinded baricitinib had become responders, as did 50% of nonresponders to a year of blinded adalimumab.
Taking a step back to describe the primary outcomes in RA-BEYOND, Dr. Weinblatt noted that at enrollment in RA-BEYOND, after 52 weeks on double-blind baricitinib, 71.8% of patients had a CDAI of 10 or less and 27% were in remission as defined by a CDAI of 2.8 or less. Subsequently, after 48 weeks on unblinded baricitinib, these rates climbed to 78.2% and 31.6%, respectively.
Similarly, in the adalimumab-to-baricitinib study arm, the low disease activity and remission rates at enrollment in RA-BEYOND were 68.9% and 24.4%, improving to 73.5% and 28.2% after 48 weeks on open-label baricitinib.
Scores on the SDAI (Simplified Disease Activity Index) and DAS28-ESR (Disease Activity Score based on 28 joints with erythrocyte sedimentation rate) followed suit in both groups.
During the 48 weeks of open-label baricitinib in RA-BEYOND, the incidence of herpes zoster was roughly 2.2% in both study arms, and the rate of adverse events leading to permanent drug discontinuation was 2.7%. During RA-BEYOND, serious infections occurred in 3.8% of the baricitinib-to-baricitinib group and 2.2% of the adalimumab-to-baricitinib group.
Dr. Weinblatt drew attention to the fact that the dose of baricitinib employed in RA-BEAM and RA-BEYOND was 4 mg/day, whereas the dose approved by the Food and Drug Administration is 2 mg/day.
The RA-BEAM and RA-BEYOND trials were sponsored by Eli Lilly. Dr. Weinblatt reported serving as a paid consultant to that pharmaceutical company and more than a dozen others.
SOURCE: Weinblatt ME et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 886.
CHICAGO – After 52 weeks of blinded adalimumab, a switch to 48 weeks of unblinded baricitinib without an adalimumab washout period resulted in an uptick in rheumatoid arthritis control with no flares and no increase in serious adverse events in the phase 3 RA-BEYOND baricitinib long-term extension study, Michael E. Weinblatt, MD, reported at the annual meeting of the American College of Rheumatology.
That’s information of practical clinical utility now that baricitinib, an oral inhibitor of Janus kinase subtypes 1 and 2, is approved as Olumiant for the treatment of moderate to severely active rheumatoid arthritis (RA). Perhaps even more interesting, however, is the way RA-BEYOND shined a spotlight on the high placebo response rate endemic to RA clinical trials, according to Dr. Weinblatt, professor of medicine at Harvard Medical School and codirector of clinical rheumatology at Brigham and Women’s Hospital, Boston.
He presented an analysis of two patient groups: 381 RA patients with moderately to severely active RA at baseline who were randomized to 52 weeks of double-blind baricitinib at 4 mg once daily in the previously reported phase 3 RA-BEAM trial (N Engl J Med. 2017 Feb 16;376[7]:652-62), after which they immediately enrolled in RA-BEYOND and were switched to 48 weeks of open-label, unblinded baricitinib; and 238 RA patients who were randomized to double-blind subcutaneous adalimumab (Humira) at 40 mg every 2 weeks in RA-BEAM before being switched to unblinded baricitinib in RA-BEYOND. All participants were on background oral methotrexate throughout.
Here’s the finding that captured Dr. Weinblatt’s attention: At the time of the switch, 28.2% of patients who’d been on blinded baricitinib for 52 weeks were nonresponders to the drug, meaning that after a full year of treatment they had a Clinical Disease Activity Index (CDAI) score greater than 10. Yet after a mere additional 4 weeks on the same drug in RA-BEYOND – this time unblinded as to their treatment – 23.4% of this group were transformed into responders, with low disease activity as defined by a CDAI of 10 or less. This finding speaks eloquently as to the power of the placebo effect. It’s a real issue for clinical trialists, the rheumatologist observed.
In the adalimumab-to-baricitinib group, 31.1% of patients were nonresponders to 52 weeks of blinded adalimumab. Four weeks after switching to open-label baricitinib, 29.7% of this group had a CDAI of 10 or less.
At week 48 of open-label baricitinib in RA-BEYOND, 54.2% of nonresponders to the 52 weeks of blinded baricitinib had become responders, as did 50% of nonresponders to a year of blinded adalimumab.
Taking a step back to describe the primary outcomes in RA-BEYOND, Dr. Weinblatt noted that at enrollment in RA-BEYOND, after 52 weeks on double-blind baricitinib, 71.8% of patients had a CDAI of 10 or less and 27% were in remission as defined by a CDAI of 2.8 or less. Subsequently, after 48 weeks on unblinded baricitinib, these rates climbed to 78.2% and 31.6%, respectively.
Similarly, in the adalimumab-to-baricitinib study arm, the low disease activity and remission rates at enrollment in RA-BEYOND were 68.9% and 24.4%, improving to 73.5% and 28.2% after 48 weeks on open-label baricitinib.
Scores on the SDAI (Simplified Disease Activity Index) and DAS28-ESR (Disease Activity Score based on 28 joints with erythrocyte sedimentation rate) followed suit in both groups.
During the 48 weeks of open-label baricitinib in RA-BEYOND, the incidence of herpes zoster was roughly 2.2% in both study arms, and the rate of adverse events leading to permanent drug discontinuation was 2.7%. During RA-BEYOND, serious infections occurred in 3.8% of the baricitinib-to-baricitinib group and 2.2% of the adalimumab-to-baricitinib group.
Dr. Weinblatt drew attention to the fact that the dose of baricitinib employed in RA-BEAM and RA-BEYOND was 4 mg/day, whereas the dose approved by the Food and Drug Administration is 2 mg/day.
The RA-BEAM and RA-BEYOND trials were sponsored by Eli Lilly. Dr. Weinblatt reported serving as a paid consultant to that pharmaceutical company and more than a dozen others.
SOURCE: Weinblatt ME et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 886.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Never underestimate the potency of the placebo effect in RA.
Major finding: After 100 weeks on oral baricitinib, 78.2% of RA patients had low disease activity and 31.6% were in remission.
Study details: This was an analysis of 619 rheumatoid arthritis patients who participated in RA-BEYOND, a phase 3, 48-week, long-term extension study built upon the earlier 52-week RA-BEAM trial.
Disclosures: Eli Lilly sponsored the study. The presenter serves as a paid consultant to that pharmaceutical company and more than a dozen others.
Source: Weinblatt ME et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 886.
New PTSD prevention guidelines released
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
New worldwide atopic dermatitis survey brings big surprises
PARIS – A major worldwide survey of the 12-month prevalence of atopic dermatitis (AD) across the course of life provides new insights into global disease trends, Jonathan I. Silverberg, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Among the most important takeaways from this Internet-based survey of more than 273,645 infants, children, and adults in 18 countries across five continents conducted in 2017 was that “global atopic dermatitis prevalence appears to be higher in adults, at 10%, than in younger cohorts, where it’s 4%-8%, which I think is quite provocative and requires further study and confirmation,” said Dr. Silverberg, a dermatologist at Northwestern University in Chicago.
“Let’s keep in mind that there’s this accepted dogma in the literature than atopic dermatitis is somehow only a childhood disorder – it doesn’t affect adults. Well, these data tell a very different story because we’re actually seeing overall highest prevalences throughout the world occurring in adulthood,” based on the U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis (Br J Dermatol. 1994 Sep;131[3]:383-96).
This is the biggest epidemiologic survey ever to examine the 12-month prevalence and severity of AD around the world for both adults and children. Survey respondents included 172,627 adults aged 18 years and older, 34,212 adolescents aged 12-17 years, 54,806 children aged 2-11 years, and more than 12,000 infants.
Key findings from the study include the following:
- AD prevalence rates varied widely from country to country around the world, as well as by age groups (see graphic).
- The highest rate in adults was observed in China. South Korea had the highest rates in both children and adolescents. The top AD rates in infancy occurred in France and the United Kingdom.
- Rates across the age spectrum were consistently lowest in Israel and Switzerland.

“These kinds of patterns raise fascinating questions about the potential risk factors or protective factors that happen in different countries. There are some startling differences in terms of the different regions,” Dr. Silverberg observed. “Certain regions of the world really stand out as having much higher prevalences, particularly China and South Korea, and then as you get into the adult years, Brazil and Mexico, which I think are areas that, at least in the global atopic dermatitis epidemiology community, are not quite as well recognized as being hot spots for atopic dermatitis.”
Indeed, the 12-month prevalence rate of AD among adults was 14% in Mexico and 12% in Brazil, as compared with 13% in Saudi Arabia, 11% in Australia and Spain, 10% in Canada and the United Kingdom, and 9% in the United States.
The prevalence was generally lowest in infants, then jumped substantially within countries during the childhood years, declined slightly in adolescents, and then peaked in adulthood.
AD severity was assessed using PO-SCORAD, the Patient-Oriented Scoring AD measure. Most affected individuals had moderate AD as defined by a PO-SCORAD score of 25-50. Across the age spectrum, the highest proportion of infants with AD who had moderate disease was in China, with 72%. In Taiwan, 63% of children with AD had moderate disease, as did 68% of adolescents and an equal proportion of adults.
In the United Kingdom, 49% percent of infants with AD had severe disease, making that country the world leader in the youngest age group. Severe AD was most common among Turkish children, where 30% of kids with the skin disease had a PO-SCORAD score greater than 50. In Brazil, 31% of adolescents with AD had severe disease, the world’s highest rate in that age group. Among adults with AD, the world’s highest rate of severe disease was 25%, which was seen in the United States, Brazil, and Saudi Arabia.
Across the age spectrum, Japan had a consistently lower-end, overall, 12-month AD prevalence rate of 5%. Germany, Italy, and France had overall rates of 6%, 7%, and 8%, respectively. The rate was 9% in the United States and Canada, and it was 10% in Australia.
Dr. Silverberg performed validation analyses using the Patient-Oriented Eczema Measure (POEM) and diagnostic criteria similar to the earlier landmark International Study of Asthma and Allergies in Childhood, or ISAAC (Lancet. 1998 Apr 25;351[9111]:1225-32). This was a huge study that excluded the United States, leaving a hole in the epidemiologic picture of the disease that the new survey fills. The validation analyses were supportive of the main findings based on the U.K. Working Party criteria.
Dr. Silverberg reported serving as a consultant to Pfizer, which sponsored the global epidemiologic survey, as well as to roughly a dozen other pharmaceutical companies.
bjancin@mdedge.com
SOURCE: Silverberg JI. EADV Congress, Abstract FC01.01.
PARIS – A major worldwide survey of the 12-month prevalence of atopic dermatitis (AD) across the course of life provides new insights into global disease trends, Jonathan I. Silverberg, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Among the most important takeaways from this Internet-based survey of more than 273,645 infants, children, and adults in 18 countries across five continents conducted in 2017 was that “global atopic dermatitis prevalence appears to be higher in adults, at 10%, than in younger cohorts, where it’s 4%-8%, which I think is quite provocative and requires further study and confirmation,” said Dr. Silverberg, a dermatologist at Northwestern University in Chicago.
“Let’s keep in mind that there’s this accepted dogma in the literature than atopic dermatitis is somehow only a childhood disorder – it doesn’t affect adults. Well, these data tell a very different story because we’re actually seeing overall highest prevalences throughout the world occurring in adulthood,” based on the U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis (Br J Dermatol. 1994 Sep;131[3]:383-96).
This is the biggest epidemiologic survey ever to examine the 12-month prevalence and severity of AD around the world for both adults and children. Survey respondents included 172,627 adults aged 18 years and older, 34,212 adolescents aged 12-17 years, 54,806 children aged 2-11 years, and more than 12,000 infants.
Key findings from the study include the following:
- AD prevalence rates varied widely from country to country around the world, as well as by age groups (see graphic).
- The highest rate in adults was observed in China. South Korea had the highest rates in both children and adolescents. The top AD rates in infancy occurred in France and the United Kingdom.
- Rates across the age spectrum were consistently lowest in Israel and Switzerland.

“These kinds of patterns raise fascinating questions about the potential risk factors or protective factors that happen in different countries. There are some startling differences in terms of the different regions,” Dr. Silverberg observed. “Certain regions of the world really stand out as having much higher prevalences, particularly China and South Korea, and then as you get into the adult years, Brazil and Mexico, which I think are areas that, at least in the global atopic dermatitis epidemiology community, are not quite as well recognized as being hot spots for atopic dermatitis.”
Indeed, the 12-month prevalence rate of AD among adults was 14% in Mexico and 12% in Brazil, as compared with 13% in Saudi Arabia, 11% in Australia and Spain, 10% in Canada and the United Kingdom, and 9% in the United States.
The prevalence was generally lowest in infants, then jumped substantially within countries during the childhood years, declined slightly in adolescents, and then peaked in adulthood.
AD severity was assessed using PO-SCORAD, the Patient-Oriented Scoring AD measure. Most affected individuals had moderate AD as defined by a PO-SCORAD score of 25-50. Across the age spectrum, the highest proportion of infants with AD who had moderate disease was in China, with 72%. In Taiwan, 63% of children with AD had moderate disease, as did 68% of adolescents and an equal proportion of adults.
In the United Kingdom, 49% percent of infants with AD had severe disease, making that country the world leader in the youngest age group. Severe AD was most common among Turkish children, where 30% of kids with the skin disease had a PO-SCORAD score greater than 50. In Brazil, 31% of adolescents with AD had severe disease, the world’s highest rate in that age group. Among adults with AD, the world’s highest rate of severe disease was 25%, which was seen in the United States, Brazil, and Saudi Arabia.
Across the age spectrum, Japan had a consistently lower-end, overall, 12-month AD prevalence rate of 5%. Germany, Italy, and France had overall rates of 6%, 7%, and 8%, respectively. The rate was 9% in the United States and Canada, and it was 10% in Australia.
Dr. Silverberg performed validation analyses using the Patient-Oriented Eczema Measure (POEM) and diagnostic criteria similar to the earlier landmark International Study of Asthma and Allergies in Childhood, or ISAAC (Lancet. 1998 Apr 25;351[9111]:1225-32). This was a huge study that excluded the United States, leaving a hole in the epidemiologic picture of the disease that the new survey fills. The validation analyses were supportive of the main findings based on the U.K. Working Party criteria.
Dr. Silverberg reported serving as a consultant to Pfizer, which sponsored the global epidemiologic survey, as well as to roughly a dozen other pharmaceutical companies.
bjancin@mdedge.com
SOURCE: Silverberg JI. EADV Congress, Abstract FC01.01.
PARIS – A major worldwide survey of the 12-month prevalence of atopic dermatitis (AD) across the course of life provides new insights into global disease trends, Jonathan I. Silverberg, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Among the most important takeaways from this Internet-based survey of more than 273,645 infants, children, and adults in 18 countries across five continents conducted in 2017 was that “global atopic dermatitis prevalence appears to be higher in adults, at 10%, than in younger cohorts, where it’s 4%-8%, which I think is quite provocative and requires further study and confirmation,” said Dr. Silverberg, a dermatologist at Northwestern University in Chicago.
“Let’s keep in mind that there’s this accepted dogma in the literature than atopic dermatitis is somehow only a childhood disorder – it doesn’t affect adults. Well, these data tell a very different story because we’re actually seeing overall highest prevalences throughout the world occurring in adulthood,” based on the U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis (Br J Dermatol. 1994 Sep;131[3]:383-96).
This is the biggest epidemiologic survey ever to examine the 12-month prevalence and severity of AD around the world for both adults and children. Survey respondents included 172,627 adults aged 18 years and older, 34,212 adolescents aged 12-17 years, 54,806 children aged 2-11 years, and more than 12,000 infants.
Key findings from the study include the following:
- AD prevalence rates varied widely from country to country around the world, as well as by age groups (see graphic).
- The highest rate in adults was observed in China. South Korea had the highest rates in both children and adolescents. The top AD rates in infancy occurred in France and the United Kingdom.
- Rates across the age spectrum were consistently lowest in Israel and Switzerland.

“These kinds of patterns raise fascinating questions about the potential risk factors or protective factors that happen in different countries. There are some startling differences in terms of the different regions,” Dr. Silverberg observed. “Certain regions of the world really stand out as having much higher prevalences, particularly China and South Korea, and then as you get into the adult years, Brazil and Mexico, which I think are areas that, at least in the global atopic dermatitis epidemiology community, are not quite as well recognized as being hot spots for atopic dermatitis.”
Indeed, the 12-month prevalence rate of AD among adults was 14% in Mexico and 12% in Brazil, as compared with 13% in Saudi Arabia, 11% in Australia and Spain, 10% in Canada and the United Kingdom, and 9% in the United States.
The prevalence was generally lowest in infants, then jumped substantially within countries during the childhood years, declined slightly in adolescents, and then peaked in adulthood.
AD severity was assessed using PO-SCORAD, the Patient-Oriented Scoring AD measure. Most affected individuals had moderate AD as defined by a PO-SCORAD score of 25-50. Across the age spectrum, the highest proportion of infants with AD who had moderate disease was in China, with 72%. In Taiwan, 63% of children with AD had moderate disease, as did 68% of adolescents and an equal proportion of adults.
In the United Kingdom, 49% percent of infants with AD had severe disease, making that country the world leader in the youngest age group. Severe AD was most common among Turkish children, where 30% of kids with the skin disease had a PO-SCORAD score greater than 50. In Brazil, 31% of adolescents with AD had severe disease, the world’s highest rate in that age group. Among adults with AD, the world’s highest rate of severe disease was 25%, which was seen in the United States, Brazil, and Saudi Arabia.
Across the age spectrum, Japan had a consistently lower-end, overall, 12-month AD prevalence rate of 5%. Germany, Italy, and France had overall rates of 6%, 7%, and 8%, respectively. The rate was 9% in the United States and Canada, and it was 10% in Australia.
Dr. Silverberg performed validation analyses using the Patient-Oriented Eczema Measure (POEM) and diagnostic criteria similar to the earlier landmark International Study of Asthma and Allergies in Childhood, or ISAAC (Lancet. 1998 Apr 25;351[9111]:1225-32). This was a huge study that excluded the United States, leaving a hole in the epidemiologic picture of the disease that the new survey fills. The validation analyses were supportive of the main findings based on the U.K. Working Party criteria.
Dr. Silverberg reported serving as a consultant to Pfizer, which sponsored the global epidemiologic survey, as well as to roughly a dozen other pharmaceutical companies.
bjancin@mdedge.com
SOURCE: Silverberg JI. EADV Congress, Abstract FC01.01.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Worldwide, the 12-month prevalence of atopic dermatitis (AD) varies substantially but is unexpectedly highest in adults.
Major finding: The global 12-month prevalence of AD in adults is 10%, substantially higher than in infants, children, or adolescents.
Study details: This was an Internet survey of 273,654 subjects conducted in 2017 in 18 countries on five continents.
Disclosures: The presenter reported serving as a consultant to Pfizer, the study sponsor, as well as to roughly a dozen other pharmaceutical companies.
Source: Silverberg JI. EADV Congress, Abstract FC01.01.
Home-based exercise for PAD tops supervised treadmill exercise
CHICAGO – Home-based exercise for peripheral arterial disease–related walking limitations works at least as well as – and arguably better than – the supervised outpatient hospital clinic-based treadmill exercise programs of the type approved for coverage by the Centers for Medicare and Medicaid Services in 2017, Mary M. McDermott, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
“The prevailing thinking is that supervised treadmill exercise is more effective than home-based exercise for PAD. And for the outcome of treadmill walking that is true. But for the outcome of 6-minute walking distance, which I would argue is more relevant to walking in daily life, home-based exercise programs appear to be better. Supervised treadmill exercise interventions preferentially improve treadmill walking performance, and that doesn’t translate as well to walking in daily life. Home-based exercise, where patients walk in a corridor or on the ground, is more relevant to the type of walking that they want to do,” explained Dr. McDermott, professor of medicine at the university as well as a leader in the field of research on exercise as a treatment for PAD.
However, she added a caveat regarding home-based exercise for symptomatic PAD: For it to be effective it must incorporate proven behavioral change techniques, including goal setting, monitoring progress, accountability to a coach, and face-to-face visits at least once per month.
“It seems you can’t just tell PAD patients to go home and walk because most of them won’t do it,” observed Dr. McDermott, who is a general internist and geriatrician.
Home-based exercise programs aren’t reimbursed by the CMS. But studies by Dr. McDermott and other investigators indicate that the results are more durable than for supervised treadmill exercise. For example, in the Group Oriented Arterial Leg Study (GOALS) – a 6-month group-mediated cognitive behavioral intervention in which PAD patients built up to walking at home for up to 50 minutes per session 5 days per week – 6-minute walking distance (6MWD) remained significantly better than in controls at follow-up after completion of the intervention. In fact, 6MWD actually increased further between 6 and 12 months in the home exercise group (J Am Heart Assoc. 2014 May 21;3(3):e000711. doi: 10.1161/JAHA.113.000711). Dr. McDermott was the lead author for this study.
In contrast, another study by Dr. McDermott now in press for the same journal found that the improvement in 6MWD achieved in PAD patients over the course of a 6-month supervised treadmill exercise program was not maintained during the next 6 months after completion of the intervention. Indeed, 6MWD showed a steady decline from its apex at the intervention’s conclusion, such that at the 12-month mark it was no longer significantly different from that of the control group, according to Dr. McDermott.
The Society for Vascular Surgery recommends a supervised exercise program as first-line therapy for PAD patients with intermittent claudication, with a Class I Level of Evidence A designation. Home-based exercise also gets a Class I recommendation, albeit with Level of Evidence B.
Dr. McDermott believes a home exercise program makes the most sense for PAD patients after their CMS benefit for a supervised clinic-based program has run out, or for patients – and there are a great many – who either can’t or don’t want to participate in a supervised program. She and others who’ve led randomized controlled trials of supervised exercise programs have found that close to 70% of eligible PAD patients decline to participate because of the inconvenience of going to the hospital outpatient facility at least three times per week or for other reasons.
“Also, it’s important to recognize that attendance can be a challenge, even when supervised exercise is covered by insurance. In our randomized trials, where we provide transportation, we still see only 65%-70% adherence to attendance,” she noted.
She stressed that it’s crucial for physicians and surgeons to educate their PAD patients about what to expect from an exercise program, be it supervised or home based.
“It’s not like revascularization, where they’re going to feel better in their walking immediately. It really takes a commitment. Four to six weeks is usually required before patients begin to experience a benefit, and I think it’s really important for patients to know that so they don’t get discouraged in the first couple of weeks,” Dr. McDermott said.
Turning to the key evidence-based behavioral change techniques shared by successful home-exercise programs for PAD, she noted that the GOALS trial intervention utilized weekly group sessions in which simple cognitive behavioral self-regulatory techniques were used to help patients set and stick to home-based walking goals. A similarly positive randomized controlled trial by investigators at the University of Oklahoma utilized once-monthly group meetings at the medical center (J Am Heart Assoc. 2014 Sep 18;3(5):e001107. doi: 10.1161/JAHA.114.001107).
In contrast, in the recent HONOR randomized clinical trial, where Dr. McDermott and her coinvestigators tested whether a home-based exercise intervention in which the active treatment group utilized a Fitbit wearable activity monitor and telephone coaching over the course of 9 months, the results proved disappointing. The intervention was no more effective than was usual care at improving 6MWD (JAMA. 2018 Apr 24;319(16):1665-76).
“One of the things I learned from doing this trial is that for a home-based exercise intervention in PAD to be successful, it’s not easy and there really needs to be some ongoing contact with a coach or nurse or a staff member that the patient feels accountable to. A wearable device is not a durably effective motivator for PAD patients. I think the reason this trial didn’t work so well is that most of it was by telephone and it was easy for patients to avoid our calls if they weren’t walking. Patients were initially really enthusiastic about the Fitbit, but we found that over time they stopped wearing it,” she said.
Dr. McDermott heartily endorses the Society for Vascular Surgery’s Class I recommendation that all PAD patients with intermittent claudication should exercise regularly, including those who’ve undergone revascularization procedures. Numerous clinical trials have demonstrated additive clinical benefits for opening the peripheral artery and strengthening skeletal muscles.
Uptake of supervised exercise programs for symptomatic PAD since the CMS coverage decision is quite variable regionally. Integrating new programs into existing cardiac rehabilitation facilities is a natural fit because staff members are very familiar with structured treadmill exercises already on site, but some freestanding programs are run by vascular surgery groups or cardiologists.
“I think part of the reason it hasn’t been taken up faster is that the reimbursement is such that you’re not going to make money on it,” Dr. McDermott said.
Asked if all patients with PAD should undergo an exercise treadmill test before embarking on an exercise program, Dr. McDermott replied, “I’m part of a writing group for the American Heart Association on how to implement these new guidelines. We’re not formally recommending a stress test. Some cardiologists on the panel suggested that it should be individualized based on patient history and symptoms. If they’re having symptoms of chest pain or they have a significant cardiac history, go ahead with a stress test. I don’t think it’s going to be recommended as a routine practice, but it’s safest to get a stress test.”
She reported having no financial conflicts regarding her presentation.
Requirements for CMS coverage of supervised exercise for symptomatic PAD
*The exercise program must consist of 12 weeks of thrice-weekly sessions.
*It has to be prescribed by a physician following a face-to-face meeting with the patient during which the physician provides education on cardiovascular risk prevention.
*An additional 36 sessions of supervised exercise can be obtained with a written note of justification by the physician following completion of the initial 12 weeks.
*The sessions must take place in a physician’s office or an outpatient hospital setting.
*The exercise has to be supervised by a physician, physician assistant, or nurse specialist.
*The exercise must be delivered by qualified personnel trained in basic and advanced cardiac life support as well as exercise therapy for PAD.
CHICAGO – Home-based exercise for peripheral arterial disease–related walking limitations works at least as well as – and arguably better than – the supervised outpatient hospital clinic-based treadmill exercise programs of the type approved for coverage by the Centers for Medicare and Medicaid Services in 2017, Mary M. McDermott, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
“The prevailing thinking is that supervised treadmill exercise is more effective than home-based exercise for PAD. And for the outcome of treadmill walking that is true. But for the outcome of 6-minute walking distance, which I would argue is more relevant to walking in daily life, home-based exercise programs appear to be better. Supervised treadmill exercise interventions preferentially improve treadmill walking performance, and that doesn’t translate as well to walking in daily life. Home-based exercise, where patients walk in a corridor or on the ground, is more relevant to the type of walking that they want to do,” explained Dr. McDermott, professor of medicine at the university as well as a leader in the field of research on exercise as a treatment for PAD.
However, she added a caveat regarding home-based exercise for symptomatic PAD: For it to be effective it must incorporate proven behavioral change techniques, including goal setting, monitoring progress, accountability to a coach, and face-to-face visits at least once per month.
“It seems you can’t just tell PAD patients to go home and walk because most of them won’t do it,” observed Dr. McDermott, who is a general internist and geriatrician.
Home-based exercise programs aren’t reimbursed by the CMS. But studies by Dr. McDermott and other investigators indicate that the results are more durable than for supervised treadmill exercise. For example, in the Group Oriented Arterial Leg Study (GOALS) – a 6-month group-mediated cognitive behavioral intervention in which PAD patients built up to walking at home for up to 50 minutes per session 5 days per week – 6-minute walking distance (6MWD) remained significantly better than in controls at follow-up after completion of the intervention. In fact, 6MWD actually increased further between 6 and 12 months in the home exercise group (J Am Heart Assoc. 2014 May 21;3(3):e000711. doi: 10.1161/JAHA.113.000711). Dr. McDermott was the lead author for this study.
In contrast, another study by Dr. McDermott now in press for the same journal found that the improvement in 6MWD achieved in PAD patients over the course of a 6-month supervised treadmill exercise program was not maintained during the next 6 months after completion of the intervention. Indeed, 6MWD showed a steady decline from its apex at the intervention’s conclusion, such that at the 12-month mark it was no longer significantly different from that of the control group, according to Dr. McDermott.
The Society for Vascular Surgery recommends a supervised exercise program as first-line therapy for PAD patients with intermittent claudication, with a Class I Level of Evidence A designation. Home-based exercise also gets a Class I recommendation, albeit with Level of Evidence B.
Dr. McDermott believes a home exercise program makes the most sense for PAD patients after their CMS benefit for a supervised clinic-based program has run out, or for patients – and there are a great many – who either can’t or don’t want to participate in a supervised program. She and others who’ve led randomized controlled trials of supervised exercise programs have found that close to 70% of eligible PAD patients decline to participate because of the inconvenience of going to the hospital outpatient facility at least three times per week or for other reasons.
“Also, it’s important to recognize that attendance can be a challenge, even when supervised exercise is covered by insurance. In our randomized trials, where we provide transportation, we still see only 65%-70% adherence to attendance,” she noted.
She stressed that it’s crucial for physicians and surgeons to educate their PAD patients about what to expect from an exercise program, be it supervised or home based.
“It’s not like revascularization, where they’re going to feel better in their walking immediately. It really takes a commitment. Four to six weeks is usually required before patients begin to experience a benefit, and I think it’s really important for patients to know that so they don’t get discouraged in the first couple of weeks,” Dr. McDermott said.
Turning to the key evidence-based behavioral change techniques shared by successful home-exercise programs for PAD, she noted that the GOALS trial intervention utilized weekly group sessions in which simple cognitive behavioral self-regulatory techniques were used to help patients set and stick to home-based walking goals. A similarly positive randomized controlled trial by investigators at the University of Oklahoma utilized once-monthly group meetings at the medical center (J Am Heart Assoc. 2014 Sep 18;3(5):e001107. doi: 10.1161/JAHA.114.001107).
In contrast, in the recent HONOR randomized clinical trial, where Dr. McDermott and her coinvestigators tested whether a home-based exercise intervention in which the active treatment group utilized a Fitbit wearable activity monitor and telephone coaching over the course of 9 months, the results proved disappointing. The intervention was no more effective than was usual care at improving 6MWD (JAMA. 2018 Apr 24;319(16):1665-76).
“One of the things I learned from doing this trial is that for a home-based exercise intervention in PAD to be successful, it’s not easy and there really needs to be some ongoing contact with a coach or nurse or a staff member that the patient feels accountable to. A wearable device is not a durably effective motivator for PAD patients. I think the reason this trial didn’t work so well is that most of it was by telephone and it was easy for patients to avoid our calls if they weren’t walking. Patients were initially really enthusiastic about the Fitbit, but we found that over time they stopped wearing it,” she said.
Dr. McDermott heartily endorses the Society for Vascular Surgery’s Class I recommendation that all PAD patients with intermittent claudication should exercise regularly, including those who’ve undergone revascularization procedures. Numerous clinical trials have demonstrated additive clinical benefits for opening the peripheral artery and strengthening skeletal muscles.
Uptake of supervised exercise programs for symptomatic PAD since the CMS coverage decision is quite variable regionally. Integrating new programs into existing cardiac rehabilitation facilities is a natural fit because staff members are very familiar with structured treadmill exercises already on site, but some freestanding programs are run by vascular surgery groups or cardiologists.
“I think part of the reason it hasn’t been taken up faster is that the reimbursement is such that you’re not going to make money on it,” Dr. McDermott said.
Asked if all patients with PAD should undergo an exercise treadmill test before embarking on an exercise program, Dr. McDermott replied, “I’m part of a writing group for the American Heart Association on how to implement these new guidelines. We’re not formally recommending a stress test. Some cardiologists on the panel suggested that it should be individualized based on patient history and symptoms. If they’re having symptoms of chest pain or they have a significant cardiac history, go ahead with a stress test. I don’t think it’s going to be recommended as a routine practice, but it’s safest to get a stress test.”
She reported having no financial conflicts regarding her presentation.
Requirements for CMS coverage of supervised exercise for symptomatic PAD
*The exercise program must consist of 12 weeks of thrice-weekly sessions.
*It has to be prescribed by a physician following a face-to-face meeting with the patient during which the physician provides education on cardiovascular risk prevention.
*An additional 36 sessions of supervised exercise can be obtained with a written note of justification by the physician following completion of the initial 12 weeks.
*The sessions must take place in a physician’s office or an outpatient hospital setting.
*The exercise has to be supervised by a physician, physician assistant, or nurse specialist.
*The exercise must be delivered by qualified personnel trained in basic and advanced cardiac life support as well as exercise therapy for PAD.
CHICAGO – Home-based exercise for peripheral arterial disease–related walking limitations works at least as well as – and arguably better than – the supervised outpatient hospital clinic-based treadmill exercise programs of the type approved for coverage by the Centers for Medicare and Medicaid Services in 2017, Mary M. McDermott, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
“The prevailing thinking is that supervised treadmill exercise is more effective than home-based exercise for PAD. And for the outcome of treadmill walking that is true. But for the outcome of 6-minute walking distance, which I would argue is more relevant to walking in daily life, home-based exercise programs appear to be better. Supervised treadmill exercise interventions preferentially improve treadmill walking performance, and that doesn’t translate as well to walking in daily life. Home-based exercise, where patients walk in a corridor or on the ground, is more relevant to the type of walking that they want to do,” explained Dr. McDermott, professor of medicine at the university as well as a leader in the field of research on exercise as a treatment for PAD.
However, she added a caveat regarding home-based exercise for symptomatic PAD: For it to be effective it must incorporate proven behavioral change techniques, including goal setting, monitoring progress, accountability to a coach, and face-to-face visits at least once per month.
“It seems you can’t just tell PAD patients to go home and walk because most of them won’t do it,” observed Dr. McDermott, who is a general internist and geriatrician.
Home-based exercise programs aren’t reimbursed by the CMS. But studies by Dr. McDermott and other investigators indicate that the results are more durable than for supervised treadmill exercise. For example, in the Group Oriented Arterial Leg Study (GOALS) – a 6-month group-mediated cognitive behavioral intervention in which PAD patients built up to walking at home for up to 50 minutes per session 5 days per week – 6-minute walking distance (6MWD) remained significantly better than in controls at follow-up after completion of the intervention. In fact, 6MWD actually increased further between 6 and 12 months in the home exercise group (J Am Heart Assoc. 2014 May 21;3(3):e000711. doi: 10.1161/JAHA.113.000711). Dr. McDermott was the lead author for this study.
In contrast, another study by Dr. McDermott now in press for the same journal found that the improvement in 6MWD achieved in PAD patients over the course of a 6-month supervised treadmill exercise program was not maintained during the next 6 months after completion of the intervention. Indeed, 6MWD showed a steady decline from its apex at the intervention’s conclusion, such that at the 12-month mark it was no longer significantly different from that of the control group, according to Dr. McDermott.
The Society for Vascular Surgery recommends a supervised exercise program as first-line therapy for PAD patients with intermittent claudication, with a Class I Level of Evidence A designation. Home-based exercise also gets a Class I recommendation, albeit with Level of Evidence B.
Dr. McDermott believes a home exercise program makes the most sense for PAD patients after their CMS benefit for a supervised clinic-based program has run out, or for patients – and there are a great many – who either can’t or don’t want to participate in a supervised program. She and others who’ve led randomized controlled trials of supervised exercise programs have found that close to 70% of eligible PAD patients decline to participate because of the inconvenience of going to the hospital outpatient facility at least three times per week or for other reasons.
“Also, it’s important to recognize that attendance can be a challenge, even when supervised exercise is covered by insurance. In our randomized trials, where we provide transportation, we still see only 65%-70% adherence to attendance,” she noted.
She stressed that it’s crucial for physicians and surgeons to educate their PAD patients about what to expect from an exercise program, be it supervised or home based.
“It’s not like revascularization, where they’re going to feel better in their walking immediately. It really takes a commitment. Four to six weeks is usually required before patients begin to experience a benefit, and I think it’s really important for patients to know that so they don’t get discouraged in the first couple of weeks,” Dr. McDermott said.
Turning to the key evidence-based behavioral change techniques shared by successful home-exercise programs for PAD, she noted that the GOALS trial intervention utilized weekly group sessions in which simple cognitive behavioral self-regulatory techniques were used to help patients set and stick to home-based walking goals. A similarly positive randomized controlled trial by investigators at the University of Oklahoma utilized once-monthly group meetings at the medical center (J Am Heart Assoc. 2014 Sep 18;3(5):e001107. doi: 10.1161/JAHA.114.001107).
In contrast, in the recent HONOR randomized clinical trial, where Dr. McDermott and her coinvestigators tested whether a home-based exercise intervention in which the active treatment group utilized a Fitbit wearable activity monitor and telephone coaching over the course of 9 months, the results proved disappointing. The intervention was no more effective than was usual care at improving 6MWD (JAMA. 2018 Apr 24;319(16):1665-76).
“One of the things I learned from doing this trial is that for a home-based exercise intervention in PAD to be successful, it’s not easy and there really needs to be some ongoing contact with a coach or nurse or a staff member that the patient feels accountable to. A wearable device is not a durably effective motivator for PAD patients. I think the reason this trial didn’t work so well is that most of it was by telephone and it was easy for patients to avoid our calls if they weren’t walking. Patients were initially really enthusiastic about the Fitbit, but we found that over time they stopped wearing it,” she said.
Dr. McDermott heartily endorses the Society for Vascular Surgery’s Class I recommendation that all PAD patients with intermittent claudication should exercise regularly, including those who’ve undergone revascularization procedures. Numerous clinical trials have demonstrated additive clinical benefits for opening the peripheral artery and strengthening skeletal muscles.
Uptake of supervised exercise programs for symptomatic PAD since the CMS coverage decision is quite variable regionally. Integrating new programs into existing cardiac rehabilitation facilities is a natural fit because staff members are very familiar with structured treadmill exercises already on site, but some freestanding programs are run by vascular surgery groups or cardiologists.
“I think part of the reason it hasn’t been taken up faster is that the reimbursement is such that you’re not going to make money on it,” Dr. McDermott said.
Asked if all patients with PAD should undergo an exercise treadmill test before embarking on an exercise program, Dr. McDermott replied, “I’m part of a writing group for the American Heart Association on how to implement these new guidelines. We’re not formally recommending a stress test. Some cardiologists on the panel suggested that it should be individualized based on patient history and symptoms. If they’re having symptoms of chest pain or they have a significant cardiac history, go ahead with a stress test. I don’t think it’s going to be recommended as a routine practice, but it’s safest to get a stress test.”
She reported having no financial conflicts regarding her presentation.
Requirements for CMS coverage of supervised exercise for symptomatic PAD
*The exercise program must consist of 12 weeks of thrice-weekly sessions.
*It has to be prescribed by a physician following a face-to-face meeting with the patient during which the physician provides education on cardiovascular risk prevention.
*An additional 36 sessions of supervised exercise can be obtained with a written note of justification by the physician following completion of the initial 12 weeks.
*The sessions must take place in a physician’s office or an outpatient hospital setting.
*The exercise has to be supervised by a physician, physician assistant, or nurse specialist.
*The exercise must be delivered by qualified personnel trained in basic and advanced cardiac life support as well as exercise therapy for PAD.
REPORTING FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Phone app diagnoses STEMI nearly as well as ECG
CHICAGO – A novel smartphone app performed nearly as well as a standard 12-lead ECG for diagnosis of ST-segment elevation MI (STEMI) in patients presenting with chest pain in ST LEUIS, an international, multicenter study.
“This study demonstrates that a 12-lead-equivalent ECG obtained using a smartphone coupled with a software application and inexpensive two-wire attachment can identify STEMI versus non-STEMI with an excellent correlation to a traditional 12-lead ECG. This technology holds substantial promise to improve outcomes in STEMI by enabling more rapid diagnosis and treatment anywhere in the world for inexpensive cost,” J. Brent Muhlestein, MD, said while presenting the ST LEUIS results at the American Heart Association scientific sessions.
This technology could provide a long-sought breakthrough in overcoming patient denial and motivating hard-headed individuals with a life-threatening MI to get to the hospital more quickly after symptom onset, instead of initially shrugging off the matter as indigestion or another nuisance. If individuals can use their handy cell phone or smartwatch to quickly obtain an ECG that shows they’re having a STEMI, they’re going to seek medical attention much sooner, with resultant greater salvage of heart muscle, noted Dr. Muhlestein of Intermountain Healthcare in Salt Lake City.
ST LEUIS tested whether a smartphone ECG app developed by AliveCor can accurately diagnose STEMI in patients with chest pain. The study, which took place at Intermountain Medical Center and a handful of other sites associated with the Duke University Cooperative Cardiovascular Society, included 204 patients who presented to EDs with chest pain. They simultaneously received both a standard 12-lead ECG and an ECG obtained using the AliveCor smartphone app. The matched ECG pairs were evaluated separately, both quantitatively and qualitatively, by a blinded panel of experienced cardiologists and classified as STEMI, left bundle branch block, non-STEMI, or uninterpretable. The study population included 92 patients with chest pain and activation of a STEMI protocol and 112 who came through the ED chest pain protocol.
Side-by-side ECG comparisons weren’t attempted in 14 pairs deemed not interpretable. In 13 cases this was because of technical problems with the smartphone ECG, and in the 14th because of ventricular pacing in the standard 12-lead ECG.
STEMI was diagnosed in 22.5% of the study population by 12-lead ECG and in 29.4% by smartphone app. The discrepancy was explained by small voltage differences in the ST-segment elevation which met criteria for STEMI by smartphone but not standard 12-lead ECG in 15 cases.
“It appears that the ST elevation was a little bit more obvious in the smartphone ECG,” Dr. Muhlestein observed.
Left bundle branch block was identified in 5.4% of patients by both methods.
The key performance numbers: The smartphone ECG had a sensitivity of 89%, specificity of 84%, positive predictive value of 70%, and negative predictive value of 95% for diagnosis of STEMI or left bundle branch block. The positive predictive value was diminished by the increased likelihood that the smartphone would call STEMI in discordant cases.
Dr. Muhlestein said that, despite the AliveCor device’s very good correlation with the standard 12-lead ECG, the system needs further tweaking.
“We definitely think this is not ready for prime time. Further refinements of the software and hardware may improve on our study results and broaden potential applications through increased ease of use and reliability. I’m sure smart engineers can make a much more simple, really user-friendly device now that we know it’s actually feasible. I envision a time when you turn it on and it speaks loud and tells you what to do and how to do it – like an AED [automated external defibrillator] – then uploads the ECG to the cloud, interprets it, and tells you whether you should go to the emergency department or not,” Dr. Muhlestein said.
This is a device that’s going to be a boon not only in the United States but also in developing countries, where even people living without electricity or running water often have cell phones, the cardiologist noted.
Dr. Muhlestein reported having no financial conflicts of interest regarding the study, which was sponsored by the participating medical institutions.
CHICAGO – A novel smartphone app performed nearly as well as a standard 12-lead ECG for diagnosis of ST-segment elevation MI (STEMI) in patients presenting with chest pain in ST LEUIS, an international, multicenter study.
“This study demonstrates that a 12-lead-equivalent ECG obtained using a smartphone coupled with a software application and inexpensive two-wire attachment can identify STEMI versus non-STEMI with an excellent correlation to a traditional 12-lead ECG. This technology holds substantial promise to improve outcomes in STEMI by enabling more rapid diagnosis and treatment anywhere in the world for inexpensive cost,” J. Brent Muhlestein, MD, said while presenting the ST LEUIS results at the American Heart Association scientific sessions.
This technology could provide a long-sought breakthrough in overcoming patient denial and motivating hard-headed individuals with a life-threatening MI to get to the hospital more quickly after symptom onset, instead of initially shrugging off the matter as indigestion or another nuisance. If individuals can use their handy cell phone or smartwatch to quickly obtain an ECG that shows they’re having a STEMI, they’re going to seek medical attention much sooner, with resultant greater salvage of heart muscle, noted Dr. Muhlestein of Intermountain Healthcare in Salt Lake City.
ST LEUIS tested whether a smartphone ECG app developed by AliveCor can accurately diagnose STEMI in patients with chest pain. The study, which took place at Intermountain Medical Center and a handful of other sites associated with the Duke University Cooperative Cardiovascular Society, included 204 patients who presented to EDs with chest pain. They simultaneously received both a standard 12-lead ECG and an ECG obtained using the AliveCor smartphone app. The matched ECG pairs were evaluated separately, both quantitatively and qualitatively, by a blinded panel of experienced cardiologists and classified as STEMI, left bundle branch block, non-STEMI, or uninterpretable. The study population included 92 patients with chest pain and activation of a STEMI protocol and 112 who came through the ED chest pain protocol.
Side-by-side ECG comparisons weren’t attempted in 14 pairs deemed not interpretable. In 13 cases this was because of technical problems with the smartphone ECG, and in the 14th because of ventricular pacing in the standard 12-lead ECG.
STEMI was diagnosed in 22.5% of the study population by 12-lead ECG and in 29.4% by smartphone app. The discrepancy was explained by small voltage differences in the ST-segment elevation which met criteria for STEMI by smartphone but not standard 12-lead ECG in 15 cases.
“It appears that the ST elevation was a little bit more obvious in the smartphone ECG,” Dr. Muhlestein observed.
Left bundle branch block was identified in 5.4% of patients by both methods.
The key performance numbers: The smartphone ECG had a sensitivity of 89%, specificity of 84%, positive predictive value of 70%, and negative predictive value of 95% for diagnosis of STEMI or left bundle branch block. The positive predictive value was diminished by the increased likelihood that the smartphone would call STEMI in discordant cases.
Dr. Muhlestein said that, despite the AliveCor device’s very good correlation with the standard 12-lead ECG, the system needs further tweaking.
“We definitely think this is not ready for prime time. Further refinements of the software and hardware may improve on our study results and broaden potential applications through increased ease of use and reliability. I’m sure smart engineers can make a much more simple, really user-friendly device now that we know it’s actually feasible. I envision a time when you turn it on and it speaks loud and tells you what to do and how to do it – like an AED [automated external defibrillator] – then uploads the ECG to the cloud, interprets it, and tells you whether you should go to the emergency department or not,” Dr. Muhlestein said.
This is a device that’s going to be a boon not only in the United States but also in developing countries, where even people living without electricity or running water often have cell phones, the cardiologist noted.
Dr. Muhlestein reported having no financial conflicts of interest regarding the study, which was sponsored by the participating medical institutions.
CHICAGO – A novel smartphone app performed nearly as well as a standard 12-lead ECG for diagnosis of ST-segment elevation MI (STEMI) in patients presenting with chest pain in ST LEUIS, an international, multicenter study.
“This study demonstrates that a 12-lead-equivalent ECG obtained using a smartphone coupled with a software application and inexpensive two-wire attachment can identify STEMI versus non-STEMI with an excellent correlation to a traditional 12-lead ECG. This technology holds substantial promise to improve outcomes in STEMI by enabling more rapid diagnosis and treatment anywhere in the world for inexpensive cost,” J. Brent Muhlestein, MD, said while presenting the ST LEUIS results at the American Heart Association scientific sessions.
This technology could provide a long-sought breakthrough in overcoming patient denial and motivating hard-headed individuals with a life-threatening MI to get to the hospital more quickly after symptom onset, instead of initially shrugging off the matter as indigestion or another nuisance. If individuals can use their handy cell phone or smartwatch to quickly obtain an ECG that shows they’re having a STEMI, they’re going to seek medical attention much sooner, with resultant greater salvage of heart muscle, noted Dr. Muhlestein of Intermountain Healthcare in Salt Lake City.
ST LEUIS tested whether a smartphone ECG app developed by AliveCor can accurately diagnose STEMI in patients with chest pain. The study, which took place at Intermountain Medical Center and a handful of other sites associated with the Duke University Cooperative Cardiovascular Society, included 204 patients who presented to EDs with chest pain. They simultaneously received both a standard 12-lead ECG and an ECG obtained using the AliveCor smartphone app. The matched ECG pairs were evaluated separately, both quantitatively and qualitatively, by a blinded panel of experienced cardiologists and classified as STEMI, left bundle branch block, non-STEMI, or uninterpretable. The study population included 92 patients with chest pain and activation of a STEMI protocol and 112 who came through the ED chest pain protocol.
Side-by-side ECG comparisons weren’t attempted in 14 pairs deemed not interpretable. In 13 cases this was because of technical problems with the smartphone ECG, and in the 14th because of ventricular pacing in the standard 12-lead ECG.
STEMI was diagnosed in 22.5% of the study population by 12-lead ECG and in 29.4% by smartphone app. The discrepancy was explained by small voltage differences in the ST-segment elevation which met criteria for STEMI by smartphone but not standard 12-lead ECG in 15 cases.
“It appears that the ST elevation was a little bit more obvious in the smartphone ECG,” Dr. Muhlestein observed.
Left bundle branch block was identified in 5.4% of patients by both methods.
The key performance numbers: The smartphone ECG had a sensitivity of 89%, specificity of 84%, positive predictive value of 70%, and negative predictive value of 95% for diagnosis of STEMI or left bundle branch block. The positive predictive value was diminished by the increased likelihood that the smartphone would call STEMI in discordant cases.
Dr. Muhlestein said that, despite the AliveCor device’s very good correlation with the standard 12-lead ECG, the system needs further tweaking.
“We definitely think this is not ready for prime time. Further refinements of the software and hardware may improve on our study results and broaden potential applications through increased ease of use and reliability. I’m sure smart engineers can make a much more simple, really user-friendly device now that we know it’s actually feasible. I envision a time when you turn it on and it speaks loud and tells you what to do and how to do it – like an AED [automated external defibrillator] – then uploads the ECG to the cloud, interprets it, and tells you whether you should go to the emergency department or not,” Dr. Muhlestein said.
This is a device that’s going to be a boon not only in the United States but also in developing countries, where even people living without electricity or running water often have cell phones, the cardiologist noted.
Dr. Muhlestein reported having no financial conflicts of interest regarding the study, which was sponsored by the participating medical institutions.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The app, designed to diagnose ST-segment elevation MI, had a sensitivity of 89%, specificity of 84%, and negative predictive value of 95% for this purpose.
Study details: This multicenter, international study featured blinded expert side-by-side comparisons of standard 12-lead ECGs and ECGs obtained via a smartphone app in 204 patients who presented with chest pain.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study, which was fully sponsored by the participating medical institutions.
TRED-HF: Despite recovery, dilated cardiomyopathy returns after halting HF drugs
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The heart failure relapse rate is high after medication withdrawal.
Major finding: Of patients who were seemingly recovered from dilated cardiomyopathy, 40% experienced early relapse following structured medication withdrawal.
Study details: This randomized crossover trial included 51 patients whose medications were withdrawn after their apparent recovery from dilated cardiomyopathy.
Disclosures: The study was funded by the British Heart Foundation. The presenter reported having no financial conflicts.
Source: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
DMARDs improve vascular abnormalities in early RA
, Maya H. Buch, PhD, said at the annual meeting of the American College of Rheumatology.
She presented a study of 71 treatment-naive patients with recently diagnosed RA and no history of cardiovascular disease. Patients were randomized to either etanercept plus methotrexate or methotrexate with treat-to-target escalation to triple nonbiologic disease-modifying antirheumatic drug therapy by week 12. Patients in the latter group were switched to etanercept plus methotrexate at week 24 if at that point they had a Disease Activity Score 28 of 2.6 or more.
All participants underwent cardiovascular MRI at baseline and again after 1 and 2 years in order to measure change in aortic stiffness, the primary study endpoint chosen because it is clinically meaningful and can reliably and reproducibly be measured by MRI.
The study was designed to shed light on possible mechanisms for the epidemiologic observation that effective treatment with methotrexate and/or tumor necrosis factor (TNF) inhibitors curbs the excess risk of cardiovascular events that accompanies RA. The thinking is that abnormal aortic distensibility represents an early harbinger of the increased cardiovascular risk associated with this form of inflammatory arthritis, explained Dr. Buch, professor of rheumatology at the University of Leeds (England) and section head of clinical and translational rheumatology at the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The mean aortic distensibility at baseline, when the study population was treatment naive, was 2.99 x 10–3 mm Hg–1. This value is abnormally low when compared with values seen in healthy age- and sex-matched controls. After 1 year of treatment, however, mean aortic distensibility improved significantly to 3.59 x 10–3 mm Hg–1. This improvement was maintained at year 2, when the mean value was 3.55.
Interestingly, neither treatment response status or disease activity was associated with the improvement in aortic stiffness. However, in a prespecified exploratory comparison between the 20 responders to etanercept plus methotrexate and the 10 responders to first-line methotrexate who never received etanercept, the group on biologic therapy had a 16% greater improvement in aortic distensibility at 1 year.
“It’s not a statistically significant difference. The study wasn’t powered for that,” she said in an interview. “But these data suggest that an anti-TNF treatment strategy may confer additional benefit. Of course, that will need to be confirmed in a larger trial. And if it is confirmed, it would suggest a role for etanercept plus methotrexate in personalizing tailored therapy in early RA patients at particularly high cardiovascular risk.”
Dr. Buch reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
SOURCE: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
, Maya H. Buch, PhD, said at the annual meeting of the American College of Rheumatology.
She presented a study of 71 treatment-naive patients with recently diagnosed RA and no history of cardiovascular disease. Patients were randomized to either etanercept plus methotrexate or methotrexate with treat-to-target escalation to triple nonbiologic disease-modifying antirheumatic drug therapy by week 12. Patients in the latter group were switched to etanercept plus methotrexate at week 24 if at that point they had a Disease Activity Score 28 of 2.6 or more.
All participants underwent cardiovascular MRI at baseline and again after 1 and 2 years in order to measure change in aortic stiffness, the primary study endpoint chosen because it is clinically meaningful and can reliably and reproducibly be measured by MRI.
The study was designed to shed light on possible mechanisms for the epidemiologic observation that effective treatment with methotrexate and/or tumor necrosis factor (TNF) inhibitors curbs the excess risk of cardiovascular events that accompanies RA. The thinking is that abnormal aortic distensibility represents an early harbinger of the increased cardiovascular risk associated with this form of inflammatory arthritis, explained Dr. Buch, professor of rheumatology at the University of Leeds (England) and section head of clinical and translational rheumatology at the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The mean aortic distensibility at baseline, when the study population was treatment naive, was 2.99 x 10–3 mm Hg–1. This value is abnormally low when compared with values seen in healthy age- and sex-matched controls. After 1 year of treatment, however, mean aortic distensibility improved significantly to 3.59 x 10–3 mm Hg–1. This improvement was maintained at year 2, when the mean value was 3.55.
Interestingly, neither treatment response status or disease activity was associated with the improvement in aortic stiffness. However, in a prespecified exploratory comparison between the 20 responders to etanercept plus methotrexate and the 10 responders to first-line methotrexate who never received etanercept, the group on biologic therapy had a 16% greater improvement in aortic distensibility at 1 year.
“It’s not a statistically significant difference. The study wasn’t powered for that,” she said in an interview. “But these data suggest that an anti-TNF treatment strategy may confer additional benefit. Of course, that will need to be confirmed in a larger trial. And if it is confirmed, it would suggest a role for etanercept plus methotrexate in personalizing tailored therapy in early RA patients at particularly high cardiovascular risk.”
Dr. Buch reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
SOURCE: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
, Maya H. Buch, PhD, said at the annual meeting of the American College of Rheumatology.
She presented a study of 71 treatment-naive patients with recently diagnosed RA and no history of cardiovascular disease. Patients were randomized to either etanercept plus methotrexate or methotrexate with treat-to-target escalation to triple nonbiologic disease-modifying antirheumatic drug therapy by week 12. Patients in the latter group were switched to etanercept plus methotrexate at week 24 if at that point they had a Disease Activity Score 28 of 2.6 or more.
All participants underwent cardiovascular MRI at baseline and again after 1 and 2 years in order to measure change in aortic stiffness, the primary study endpoint chosen because it is clinically meaningful and can reliably and reproducibly be measured by MRI.
The study was designed to shed light on possible mechanisms for the epidemiologic observation that effective treatment with methotrexate and/or tumor necrosis factor (TNF) inhibitors curbs the excess risk of cardiovascular events that accompanies RA. The thinking is that abnormal aortic distensibility represents an early harbinger of the increased cardiovascular risk associated with this form of inflammatory arthritis, explained Dr. Buch, professor of rheumatology at the University of Leeds (England) and section head of clinical and translational rheumatology at the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The mean aortic distensibility at baseline, when the study population was treatment naive, was 2.99 x 10–3 mm Hg–1. This value is abnormally low when compared with values seen in healthy age- and sex-matched controls. After 1 year of treatment, however, mean aortic distensibility improved significantly to 3.59 x 10–3 mm Hg–1. This improvement was maintained at year 2, when the mean value was 3.55.
Interestingly, neither treatment response status or disease activity was associated with the improvement in aortic stiffness. However, in a prespecified exploratory comparison between the 20 responders to etanercept plus methotrexate and the 10 responders to first-line methotrexate who never received etanercept, the group on biologic therapy had a 16% greater improvement in aortic distensibility at 1 year.
“It’s not a statistically significant difference. The study wasn’t powered for that,” she said in an interview. “But these data suggest that an anti-TNF treatment strategy may confer additional benefit. Of course, that will need to be confirmed in a larger trial. And if it is confirmed, it would suggest a role for etanercept plus methotrexate in personalizing tailored therapy in early RA patients at particularly high cardiovascular risk.”
Dr. Buch reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
SOURCE: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Abnormal aortic stiffness is present in patients with newly diagnosed RA but improves with disease-modifying antirheumatic drug therapy.
Major finding: Mean aortic distensibility in patients with early RA improved from an abnormally low 2.99 x 10–3 mm Hg–1 prior to treatment to a more robust 3.59 x 10–3 mm Hg–1 after 1 year of disease-modifying antirheumatic drug therapy.
Study details: This study included 71 patients with newly diagnosed RA whose aortic stiffness was measured serially via cardiovascular MRI over a 2-year period.
Disclosures: The presenter reported receiving funding from Pfizer, Roche, UCB, AbbVie, Sanofi, Eli Lilly, and Sandoz, but reported having no financial conflicts of interest regarding this study, which was sponsored by the U.K. National Institute for Healthcare Research.
Source: Buch MH et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L05.
Hamstring tendinopathy implicated in persistent Lyme arthritis
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Ultrasound evidence of hamstring calcific tendinopathy was found in 28 of 31 patients with persistent posttreatment Lyme arthritis, compared with 3 of 22 patients with knee osteoarthritis.
Study details: This was a retrospective imaging study of hamstring tendon status in 31 patients with persistent posttreatment Lyme arthritis, 22 patients with osteoarthritis, and 14 with inflammatory arthritis.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
Source: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
Weight loss cuts risk of psoriatic arthritis
CHICAGO – Overweight and obese psoriasis patients have it within their power to reduce their risk of developing psoriatic arthritis through weight loss, according to a large British longitudinal study.
Of the three modifiable lifestyle factors evaluated in the study as potential risk factors for the development of psoriatic arthritis in psoriasis patients – body mass index, smoking, and alcohol intake – reduction in BMI over time was clearly the winning strategy, Neil McHugh, MD, said at the annual meeting of the American College of Rheumatology.
The message from this study of 90,189 incident cases of psoriasis identified in the U.K. Clinical Practice Research Datalink was unequivocal: “If you’re overweight and have psoriasis and you lose weight, you reduce your chance of developing a nasty form of arthritis,” said Dr. McHugh, professor of pharmacoepidemiology and a rheumatologist at the University of Bath, England.
“As psoriatic arthritis affects around 20% of people with psoriasis, weight reduction amongst those who are obese may have the potential to greatly reduce their risk of psoriatic arthritis in addition to providing additional health benefits,” he added.
Among the more than 90,000 patients diagnosed with psoriasis, 1,409 subsequently developed psoriatic arthritis, with an overall incidence rate of 2.72 cases per 1,000 person-years. Baseline BMI was strongly associated in stepwise fashion with subsequent psoriatic arthritis. Psoriasis patients with a baseline BMI of 25-29.9 kg/m2 were at an adjusted 1.76-fold increased risk of later developing psoriatic arthritis, compared with psoriasis patients having a BMI of less than 25. For those with a BMI of 30-34.9 kg/m2, the risk of subsequent psoriatic arthritis was increased 2.04-fold. And for those with a baseline BMI of 35 kg/m2 or more, the risk was increased 2.42-fold in analyses adjusted for age, sex, psoriasis duration and severity, history of trauma, and diabetes.
In contrast, the risk of developing psoriatic arthritis wasn’t significantly different between psoriasis patients who were nonsmokers, ex-smokers, or current smokers. And while there was a significantly increased risk of developing psoriatic arthritis in psoriasis patients who were current drinkers, compared with nondrinkers, the risk in ex-drinkers and heavy drinkers was similar to that in nondrinkers, a counterintuitive finding Dr. McHugh suspects was a distortion due to small numbers.
While the observed relationship between baseline BMI and subsequent risk of psoriatic arthritis was informative, it only tells part of the story, since body weight so often changes over time. Dr. McHugh and his coinvestigators had data on change in BMI over the course of 10 years of follow-up in 15,627 psoriasis patients free of psoriatic arthritis at the time their psoriasis was diagnosed. The researchers developed a BMI risk calculator that expressed the effect of change in BMI over time on the cumulative risk of developing psoriatic arthritis.
“We were able to show that if, for instance, you started with a BMI of 25 at baseline and ended up with a BMI of 30, your risk of psoriatic arthritis goes up by 13%, whereas if you start at 30 and come down to 25, your risk decreases by 13%. And the more weight you lose, the greater you reduce your risk of developing psoriatic arthritis,” the rheumatologist explained in an interview.
Indeed, with more extreme changes in BMI over the course of a decade following diagnosis of psoriasis – for example, dropping from a baseline BMI of 36 kg/m2 to 23 kg/m2 – the risk of developing psoriatic arthritis fell by close to 30%.
Dr. McHugh reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
SOURCE: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.
CHICAGO – Overweight and obese psoriasis patients have it within their power to reduce their risk of developing psoriatic arthritis through weight loss, according to a large British longitudinal study.
Of the three modifiable lifestyle factors evaluated in the study as potential risk factors for the development of psoriatic arthritis in psoriasis patients – body mass index, smoking, and alcohol intake – reduction in BMI over time was clearly the winning strategy, Neil McHugh, MD, said at the annual meeting of the American College of Rheumatology.
The message from this study of 90,189 incident cases of psoriasis identified in the U.K. Clinical Practice Research Datalink was unequivocal: “If you’re overweight and have psoriasis and you lose weight, you reduce your chance of developing a nasty form of arthritis,” said Dr. McHugh, professor of pharmacoepidemiology and a rheumatologist at the University of Bath, England.
“As psoriatic arthritis affects around 20% of people with psoriasis, weight reduction amongst those who are obese may have the potential to greatly reduce their risk of psoriatic arthritis in addition to providing additional health benefits,” he added.
Among the more than 90,000 patients diagnosed with psoriasis, 1,409 subsequently developed psoriatic arthritis, with an overall incidence rate of 2.72 cases per 1,000 person-years. Baseline BMI was strongly associated in stepwise fashion with subsequent psoriatic arthritis. Psoriasis patients with a baseline BMI of 25-29.9 kg/m2 were at an adjusted 1.76-fold increased risk of later developing psoriatic arthritis, compared with psoriasis patients having a BMI of less than 25. For those with a BMI of 30-34.9 kg/m2, the risk of subsequent psoriatic arthritis was increased 2.04-fold. And for those with a baseline BMI of 35 kg/m2 or more, the risk was increased 2.42-fold in analyses adjusted for age, sex, psoriasis duration and severity, history of trauma, and diabetes.
In contrast, the risk of developing psoriatic arthritis wasn’t significantly different between psoriasis patients who were nonsmokers, ex-smokers, or current smokers. And while there was a significantly increased risk of developing psoriatic arthritis in psoriasis patients who were current drinkers, compared with nondrinkers, the risk in ex-drinkers and heavy drinkers was similar to that in nondrinkers, a counterintuitive finding Dr. McHugh suspects was a distortion due to small numbers.
While the observed relationship between baseline BMI and subsequent risk of psoriatic arthritis was informative, it only tells part of the story, since body weight so often changes over time. Dr. McHugh and his coinvestigators had data on change in BMI over the course of 10 years of follow-up in 15,627 psoriasis patients free of psoriatic arthritis at the time their psoriasis was diagnosed. The researchers developed a BMI risk calculator that expressed the effect of change in BMI over time on the cumulative risk of developing psoriatic arthritis.
“We were able to show that if, for instance, you started with a BMI of 25 at baseline and ended up with a BMI of 30, your risk of psoriatic arthritis goes up by 13%, whereas if you start at 30 and come down to 25, your risk decreases by 13%. And the more weight you lose, the greater you reduce your risk of developing psoriatic arthritis,” the rheumatologist explained in an interview.
Indeed, with more extreme changes in BMI over the course of a decade following diagnosis of psoriasis – for example, dropping from a baseline BMI of 36 kg/m2 to 23 kg/m2 – the risk of developing psoriatic arthritis fell by close to 30%.
Dr. McHugh reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
SOURCE: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.
CHICAGO – Overweight and obese psoriasis patients have it within their power to reduce their risk of developing psoriatic arthritis through weight loss, according to a large British longitudinal study.
Of the three modifiable lifestyle factors evaluated in the study as potential risk factors for the development of psoriatic arthritis in psoriasis patients – body mass index, smoking, and alcohol intake – reduction in BMI over time was clearly the winning strategy, Neil McHugh, MD, said at the annual meeting of the American College of Rheumatology.
The message from this study of 90,189 incident cases of psoriasis identified in the U.K. Clinical Practice Research Datalink was unequivocal: “If you’re overweight and have psoriasis and you lose weight, you reduce your chance of developing a nasty form of arthritis,” said Dr. McHugh, professor of pharmacoepidemiology and a rheumatologist at the University of Bath, England.
“As psoriatic arthritis affects around 20% of people with psoriasis, weight reduction amongst those who are obese may have the potential to greatly reduce their risk of psoriatic arthritis in addition to providing additional health benefits,” he added.
Among the more than 90,000 patients diagnosed with psoriasis, 1,409 subsequently developed psoriatic arthritis, with an overall incidence rate of 2.72 cases per 1,000 person-years. Baseline BMI was strongly associated in stepwise fashion with subsequent psoriatic arthritis. Psoriasis patients with a baseline BMI of 25-29.9 kg/m2 were at an adjusted 1.76-fold increased risk of later developing psoriatic arthritis, compared with psoriasis patients having a BMI of less than 25. For those with a BMI of 30-34.9 kg/m2, the risk of subsequent psoriatic arthritis was increased 2.04-fold. And for those with a baseline BMI of 35 kg/m2 or more, the risk was increased 2.42-fold in analyses adjusted for age, sex, psoriasis duration and severity, history of trauma, and diabetes.
In contrast, the risk of developing psoriatic arthritis wasn’t significantly different between psoriasis patients who were nonsmokers, ex-smokers, or current smokers. And while there was a significantly increased risk of developing psoriatic arthritis in psoriasis patients who were current drinkers, compared with nondrinkers, the risk in ex-drinkers and heavy drinkers was similar to that in nondrinkers, a counterintuitive finding Dr. McHugh suspects was a distortion due to small numbers.
While the observed relationship between baseline BMI and subsequent risk of psoriatic arthritis was informative, it only tells part of the story, since body weight so often changes over time. Dr. McHugh and his coinvestigators had data on change in BMI over the course of 10 years of follow-up in 15,627 psoriasis patients free of psoriatic arthritis at the time their psoriasis was diagnosed. The researchers developed a BMI risk calculator that expressed the effect of change in BMI over time on the cumulative risk of developing psoriatic arthritis.
“We were able to show that if, for instance, you started with a BMI of 25 at baseline and ended up with a BMI of 30, your risk of psoriatic arthritis goes up by 13%, whereas if you start at 30 and come down to 25, your risk decreases by 13%. And the more weight you lose, the greater you reduce your risk of developing psoriatic arthritis,” the rheumatologist explained in an interview.
Indeed, with more extreme changes in BMI over the course of a decade following diagnosis of psoriasis – for example, dropping from a baseline BMI of 36 kg/m2 to 23 kg/m2 – the risk of developing psoriatic arthritis fell by close to 30%.
Dr. McHugh reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
SOURCE: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: A psoriasis patient’s risk of developing psoriatic arthritis increases stepwise with greater body mass index, and the converse is true as well.
Study details: This study included more than 90,000 patients with a diagnosis of psoriasis in the U.K. Clinical Practice Research Datalink.
Disclosures: The presenter reported having no financial conflicts regarding this study, funded by the U.K. National Institute for Health Research.
Source: Green A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2134.
Intra-articular Wnt inhibitor for knee OA sails through phase 2
CHICAGO – A single intra-articular injection of a novel drug known for now as SMO4690 resulted in statistically significant and clinically meaningful improvements in pain and physical function through 6 months of follow-up in a phase 2b study including 695 patients with moderately to severely symptomatic knee OA, Yusuf Yazici, MD, reported at the annual meeting of the American College of Rheumatology.
Based on the encouraging results of this and another large phase 2 study, two pivotal phase 3, randomized clinical trials are due to start in the spring of 2019. If the results are positive, SMO4690 could become the first approved disease-modifying OA drug (DMOAD), something that’s been a long-sought, high-priority goal in rheumatology, noted Dr. Yazici, chief medical officer at San Diego–based Samumed, which is developing the drug, and a rheumatologist at New York University.
SMO4690 is a small molecule inhibitor of the Wnt signaling pathway. The drug has two distinct mechanisms of action for treatment of knee OA: It has an anti-inflammatory effect and it protects cartilage from degeneration, as demonstrated by a clinically significant improvement in joint space width by x-ray, compared with placebo at 12 months of follow-up after a single baseline injection in the earlier 455-patient, phase 2 study. Also, animal studies suggest SMO4690 generates cartilage, an exciting possibility now being evaluated in two ongoing serial MRI studies in knee OA patients.
Dr. Yazici presented patient-reported outcomes from the 695-patient, 24-week, multicenter, randomized, placebo-controlled, double-blind, phase 2b study, which evaluated four different concentrations of SMO4690. The 0.07- and 0.23-mg per 2-mL injection doses proved significantly better than placebo at 12 weeks – the primary endpoint – for all patient-reported outcomes.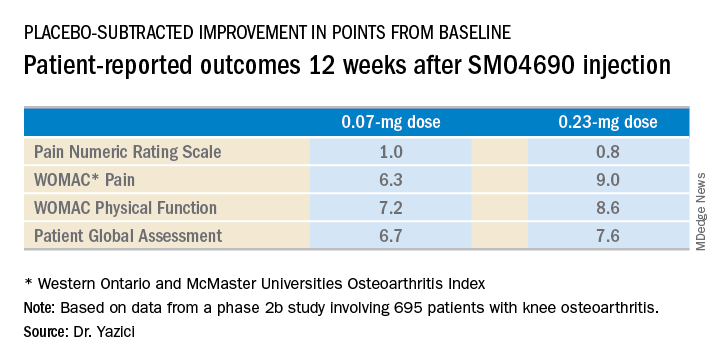
The 0.23-mg dose of SMO4690 remained superior to placebo for all four patient-reported outcomes at subsequent assessments at weeks 16, 20, and 24. The 0.07-mg dose remained significantly better than placebo through week 20.
As in the earlier phase 2 study, SMO4690 raised no significant safety concerns. Adverse events were similar in type and frequency in the active-treatment and placebo groups.
The study was sponsored by Samumed, where Dr. Yazici is employed as chief medical officer.
SOURCE: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.
CHICAGO – A single intra-articular injection of a novel drug known for now as SMO4690 resulted in statistically significant and clinically meaningful improvements in pain and physical function through 6 months of follow-up in a phase 2b study including 695 patients with moderately to severely symptomatic knee OA, Yusuf Yazici, MD, reported at the annual meeting of the American College of Rheumatology.
Based on the encouraging results of this and another large phase 2 study, two pivotal phase 3, randomized clinical trials are due to start in the spring of 2019. If the results are positive, SMO4690 could become the first approved disease-modifying OA drug (DMOAD), something that’s been a long-sought, high-priority goal in rheumatology, noted Dr. Yazici, chief medical officer at San Diego–based Samumed, which is developing the drug, and a rheumatologist at New York University.
SMO4690 is a small molecule inhibitor of the Wnt signaling pathway. The drug has two distinct mechanisms of action for treatment of knee OA: It has an anti-inflammatory effect and it protects cartilage from degeneration, as demonstrated by a clinically significant improvement in joint space width by x-ray, compared with placebo at 12 months of follow-up after a single baseline injection in the earlier 455-patient, phase 2 study. Also, animal studies suggest SMO4690 generates cartilage, an exciting possibility now being evaluated in two ongoing serial MRI studies in knee OA patients.
Dr. Yazici presented patient-reported outcomes from the 695-patient, 24-week, multicenter, randomized, placebo-controlled, double-blind, phase 2b study, which evaluated four different concentrations of SMO4690. The 0.07- and 0.23-mg per 2-mL injection doses proved significantly better than placebo at 12 weeks – the primary endpoint – for all patient-reported outcomes.
The 0.23-mg dose of SMO4690 remained superior to placebo for all four patient-reported outcomes at subsequent assessments at weeks 16, 20, and 24. The 0.07-mg dose remained significantly better than placebo through week 20.
As in the earlier phase 2 study, SMO4690 raised no significant safety concerns. Adverse events were similar in type and frequency in the active-treatment and placebo groups.
The study was sponsored by Samumed, where Dr. Yazici is employed as chief medical officer.
SOURCE: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.
CHICAGO – A single intra-articular injection of a novel drug known for now as SMO4690 resulted in statistically significant and clinically meaningful improvements in pain and physical function through 6 months of follow-up in a phase 2b study including 695 patients with moderately to severely symptomatic knee OA, Yusuf Yazici, MD, reported at the annual meeting of the American College of Rheumatology.
Based on the encouraging results of this and another large phase 2 study, two pivotal phase 3, randomized clinical trials are due to start in the spring of 2019. If the results are positive, SMO4690 could become the first approved disease-modifying OA drug (DMOAD), something that’s been a long-sought, high-priority goal in rheumatology, noted Dr. Yazici, chief medical officer at San Diego–based Samumed, which is developing the drug, and a rheumatologist at New York University.
SMO4690 is a small molecule inhibitor of the Wnt signaling pathway. The drug has two distinct mechanisms of action for treatment of knee OA: It has an anti-inflammatory effect and it protects cartilage from degeneration, as demonstrated by a clinically significant improvement in joint space width by x-ray, compared with placebo at 12 months of follow-up after a single baseline injection in the earlier 455-patient, phase 2 study. Also, animal studies suggest SMO4690 generates cartilage, an exciting possibility now being evaluated in two ongoing serial MRI studies in knee OA patients.
Dr. Yazici presented patient-reported outcomes from the 695-patient, 24-week, multicenter, randomized, placebo-controlled, double-blind, phase 2b study, which evaluated four different concentrations of SMO4690. The 0.07- and 0.23-mg per 2-mL injection doses proved significantly better than placebo at 12 weeks – the primary endpoint – for all patient-reported outcomes.
The 0.23-mg dose of SMO4690 remained superior to placebo for all four patient-reported outcomes at subsequent assessments at weeks 16, 20, and 24. The 0.07-mg dose remained significantly better than placebo through week 20.
As in the earlier phase 2 study, SMO4690 raised no significant safety concerns. Adverse events were similar in type and frequency in the active-treatment and placebo groups.
The study was sponsored by Samumed, where Dr. Yazici is employed as chief medical officer.
SOURCE: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The 0.23-mg dose of SMO4690 proved superior to placebo for four key patient-reported outcomes.
Study details: This was a 24-week, randomized, multicenter, placebo-controlled, double-blind, phase 2b study in 695 patients with moderately to severely symptomatic knee OA.
Disclosures: The presenter is chief medical officer at Samumed, the study sponsor.
Source: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.




















