User login
FDA urged to bring rheumatologists into gadolinium safety discussion
CHICAGO – The Food and Drug Administration’s updated safety warning about gadolinium-based contrast agents was dismissed as too little, too late during a postsession discussion at the annual meeting of the American College of Rheumatology.
The class warning that “gadolinium is retained for months or years in brain, bone, and other organs,” a directive that a new patient medication guide be given to everyone before receiving a gadolinium-based contrast agent, and a request that manufacturers conduct human and animal studies to further assess the safety of these products, was highlighted at the FDA update session on safety issues in the treatment of rheumatic disease.
The request for further studies grew out of the FDA Advisory Committee on Medical Imaging’s Fall 2017 meeting, which concluded that, “based on available data, there is insufficient evidence of a causal relationship between adverse events and gadolinium retention and recommended the need for additional studies to inform regulatory action,” explained Rachel L. Glaser, MD, a medical officer in the agency’s division of pulmonary, allergy, and rheumatology products and a practicing rheumatologist.
Jonathan Kay, MD, rose from the audience to take the FDA to task for delegating matters related to gadolinium-based contrast agent safety to the radiologists comprising the Advisory Committee on Medical Imaging. “I think the FDA ought to bring this matter to a group of physicians who actually care for patients to get our input on this. Maybe the Arthritis Advisory Committee,” he said.
“It’s been known for more than 15 years that gadolinium deposits in the bone, and for more than 5 years that gadolinium deposits in the brain of patients who’ve received multiple magnetic resonance studies. I’m concerned that the FDA has this issue in the purview of the radiologists because radiologists administer these contrast agents and then don’t see the patients back in follow-up, whereas rheumatologists do,” noted Dr. Kay, professor of medicine and director of clinical research in rheumatology at the University of Massachusetts, Worcester.
He blasted the FDA’s call for further human and animal studies, noting “nephrogenic systemic fibrosis was the human experiment that was done with gadolinium contrast, showing that these agents, when they deposit in tissue, are extremely toxic. I can’t see other human studies being done to determine the potential consequences of these agents when they deposit long term in brain, bone, and other tissues. And the animal studies have already been done demonstrating the danger of these compounds. Might you take this out of the realm of radiologists and involve physicians who take care of patients chronically and observe the long-term consequences of these toxic effects?”
Dr. Glaser responded that the deliberations of the agency’s Advisory Committee on Medical Imaging are reviewed under the purview of the FDA’s Center for Drug Evaluation and Research. “That does include radiologists, but also other clinical reviewers of various backgrounds, including pharmacologists and toxicologists.”
Dr. Kay did not relent, further commenting that he has personally seen “over 150 patients with the devastating consequences of nephrogenic systemic fibrosis.” He added that a prominent radiologist has stated in a public forum that the risk of getting injured from crossing the street is greater than that from getting an MRI with gadolinium. “That’s completely wrong,” he declared.
Dr. Glaser and Dr. Kay reported having no financial conflicts.
CHICAGO – The Food and Drug Administration’s updated safety warning about gadolinium-based contrast agents was dismissed as too little, too late during a postsession discussion at the annual meeting of the American College of Rheumatology.
The class warning that “gadolinium is retained for months or years in brain, bone, and other organs,” a directive that a new patient medication guide be given to everyone before receiving a gadolinium-based contrast agent, and a request that manufacturers conduct human and animal studies to further assess the safety of these products, was highlighted at the FDA update session on safety issues in the treatment of rheumatic disease.
The request for further studies grew out of the FDA Advisory Committee on Medical Imaging’s Fall 2017 meeting, which concluded that, “based on available data, there is insufficient evidence of a causal relationship between adverse events and gadolinium retention and recommended the need for additional studies to inform regulatory action,” explained Rachel L. Glaser, MD, a medical officer in the agency’s division of pulmonary, allergy, and rheumatology products and a practicing rheumatologist.
Jonathan Kay, MD, rose from the audience to take the FDA to task for delegating matters related to gadolinium-based contrast agent safety to the radiologists comprising the Advisory Committee on Medical Imaging. “I think the FDA ought to bring this matter to a group of physicians who actually care for patients to get our input on this. Maybe the Arthritis Advisory Committee,” he said.
“It’s been known for more than 15 years that gadolinium deposits in the bone, and for more than 5 years that gadolinium deposits in the brain of patients who’ve received multiple magnetic resonance studies. I’m concerned that the FDA has this issue in the purview of the radiologists because radiologists administer these contrast agents and then don’t see the patients back in follow-up, whereas rheumatologists do,” noted Dr. Kay, professor of medicine and director of clinical research in rheumatology at the University of Massachusetts, Worcester.
He blasted the FDA’s call for further human and animal studies, noting “nephrogenic systemic fibrosis was the human experiment that was done with gadolinium contrast, showing that these agents, when they deposit in tissue, are extremely toxic. I can’t see other human studies being done to determine the potential consequences of these agents when they deposit long term in brain, bone, and other tissues. And the animal studies have already been done demonstrating the danger of these compounds. Might you take this out of the realm of radiologists and involve physicians who take care of patients chronically and observe the long-term consequences of these toxic effects?”
Dr. Glaser responded that the deliberations of the agency’s Advisory Committee on Medical Imaging are reviewed under the purview of the FDA’s Center for Drug Evaluation and Research. “That does include radiologists, but also other clinical reviewers of various backgrounds, including pharmacologists and toxicologists.”
Dr. Kay did not relent, further commenting that he has personally seen “over 150 patients with the devastating consequences of nephrogenic systemic fibrosis.” He added that a prominent radiologist has stated in a public forum that the risk of getting injured from crossing the street is greater than that from getting an MRI with gadolinium. “That’s completely wrong,” he declared.
Dr. Glaser and Dr. Kay reported having no financial conflicts.
CHICAGO – The Food and Drug Administration’s updated safety warning about gadolinium-based contrast agents was dismissed as too little, too late during a postsession discussion at the annual meeting of the American College of Rheumatology.
The class warning that “gadolinium is retained for months or years in brain, bone, and other organs,” a directive that a new patient medication guide be given to everyone before receiving a gadolinium-based contrast agent, and a request that manufacturers conduct human and animal studies to further assess the safety of these products, was highlighted at the FDA update session on safety issues in the treatment of rheumatic disease.
The request for further studies grew out of the FDA Advisory Committee on Medical Imaging’s Fall 2017 meeting, which concluded that, “based on available data, there is insufficient evidence of a causal relationship between adverse events and gadolinium retention and recommended the need for additional studies to inform regulatory action,” explained Rachel L. Glaser, MD, a medical officer in the agency’s division of pulmonary, allergy, and rheumatology products and a practicing rheumatologist.
Jonathan Kay, MD, rose from the audience to take the FDA to task for delegating matters related to gadolinium-based contrast agent safety to the radiologists comprising the Advisory Committee on Medical Imaging. “I think the FDA ought to bring this matter to a group of physicians who actually care for patients to get our input on this. Maybe the Arthritis Advisory Committee,” he said.
“It’s been known for more than 15 years that gadolinium deposits in the bone, and for more than 5 years that gadolinium deposits in the brain of patients who’ve received multiple magnetic resonance studies. I’m concerned that the FDA has this issue in the purview of the radiologists because radiologists administer these contrast agents and then don’t see the patients back in follow-up, whereas rheumatologists do,” noted Dr. Kay, professor of medicine and director of clinical research in rheumatology at the University of Massachusetts, Worcester.
He blasted the FDA’s call for further human and animal studies, noting “nephrogenic systemic fibrosis was the human experiment that was done with gadolinium contrast, showing that these agents, when they deposit in tissue, are extremely toxic. I can’t see other human studies being done to determine the potential consequences of these agents when they deposit long term in brain, bone, and other tissues. And the animal studies have already been done demonstrating the danger of these compounds. Might you take this out of the realm of radiologists and involve physicians who take care of patients chronically and observe the long-term consequences of these toxic effects?”
Dr. Glaser responded that the deliberations of the agency’s Advisory Committee on Medical Imaging are reviewed under the purview of the FDA’s Center for Drug Evaluation and Research. “That does include radiologists, but also other clinical reviewers of various backgrounds, including pharmacologists and toxicologists.”
Dr. Kay did not relent, further commenting that he has personally seen “over 150 patients with the devastating consequences of nephrogenic systemic fibrosis.” He added that a prominent radiologist has stated in a public forum that the risk of getting injured from crossing the street is greater than that from getting an MRI with gadolinium. “That’s completely wrong,” he declared.
Dr. Glaser and Dr. Kay reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ACR ANNUAL MEETING
Montreal Cognitive Assessment fares well for rapid, reliable screening in SLE
CHICAGO – The Montreal Cognitive Assessment Test provides persuasive advantages over the standard neuropsychological test battery often recommended in guidelines as a screening tool for cognitive impairment in patients with systemic lupus erythematosus, Nicolas Paez-Venegas, MD, asserted at the annual meeting of the American College of Rheumatology.
The MoCA, as it’s known, offers brevity, simplicity, and none of the considerable expense and inconvenience of bringing in a trained specialist to administer a neuropsychological battery. Moreover, in a comparative efficacy study, the MoCA outperformed two other brief screening tools for cognitive impairment – the Mini-Mental State Examination and the Cognitive Symptom Inventory – and showed excellent correspondence with the results of the formal neuropsychological battery, reported Dr. Paez-Venegas, a psychiatrist at the Jalisco Institute of Mental Health, in Zapopan, Mexico.
He presented a cross-sectional study that pitted the three brief screening tests against a gold-standard neuropsychological battery in 44 patients with systemic lupus erythematosus (SLE) according to the 2012 Systemic Lupus International Collaborating Clinics Criteria, none of whom had any known medical or psychiatric comorbidities.
The MoCA proved to have the best congruence with the findings of the neuropsychological battery, with an area under the curve of 99.4%, 84% sensitivity, and 100% specificity for cognitive impairment. The Mini-Mental State Examination had 55% sensitivity and 100% specificity, while the Cognitive Symptom Inventory displayed 55% sensitivity and 31% specificity.
“We therefore encourage rheumatologists to apply the MoCA test as a valuable and easily implemented tool for detecting cognitive impairment as part of an integrated approach in SLE,” Dr. Paez-Venegas said.
Periodic screening for cognitive impairment in patients with SLE is an important aspect of patient management because cognitive impairment is a common manifestation of the disease, affecting up to two-thirds of patients, and it can have a serious impact upon quality of life and self-concept. Because such screening isn’t a one-time event, resort to a neuropsychological battery becomes particularly problematic. The battery employed in this study included the Wechsler Adult Intelligence Scale–Fourth Edition test, the Digit-Symbol test, the Finger-Tapping test of motor control, the Stroop test, Trail Making A and B, the Paced Auditory Serial Addition test, letter-number sequencing, the Wechsler Vocabulary test, the Rey-Osterrieth complex figure test, semantic and phonemic fluency tests, and a test of verbal Spanish comprehension. The battery is a comprehensive tool often employed in research studies but is not well suited for use in a busy clinical practice.
Overall, 70% of the SLE patients demonstrated cognitive impairment in one or more domains on the neuropsychological battery. Processing speed was the most frequently affected domain, involving 23 of the 44 patients. Only a single patient displayed abnormal motor control.
The MoCA test assesses attention, executive function, concentration, language, memory, abstraction, orientation, visuospatial cognitive capacity, and calculation.
Dr. Paez-Venegas’s study was published online earlier this year (J Clin Rheumatol 2018 Jul 18. doi: 10.1097/RHU.0000000000000876). He reported having no financial conflicts regarding his study.
SOURCE: Paez-Venegas N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 708.
CHICAGO – The Montreal Cognitive Assessment Test provides persuasive advantages over the standard neuropsychological test battery often recommended in guidelines as a screening tool for cognitive impairment in patients with systemic lupus erythematosus, Nicolas Paez-Venegas, MD, asserted at the annual meeting of the American College of Rheumatology.
The MoCA, as it’s known, offers brevity, simplicity, and none of the considerable expense and inconvenience of bringing in a trained specialist to administer a neuropsychological battery. Moreover, in a comparative efficacy study, the MoCA outperformed two other brief screening tools for cognitive impairment – the Mini-Mental State Examination and the Cognitive Symptom Inventory – and showed excellent correspondence with the results of the formal neuropsychological battery, reported Dr. Paez-Venegas, a psychiatrist at the Jalisco Institute of Mental Health, in Zapopan, Mexico.
He presented a cross-sectional study that pitted the three brief screening tests against a gold-standard neuropsychological battery in 44 patients with systemic lupus erythematosus (SLE) according to the 2012 Systemic Lupus International Collaborating Clinics Criteria, none of whom had any known medical or psychiatric comorbidities.
The MoCA proved to have the best congruence with the findings of the neuropsychological battery, with an area under the curve of 99.4%, 84% sensitivity, and 100% specificity for cognitive impairment. The Mini-Mental State Examination had 55% sensitivity and 100% specificity, while the Cognitive Symptom Inventory displayed 55% sensitivity and 31% specificity.
“We therefore encourage rheumatologists to apply the MoCA test as a valuable and easily implemented tool for detecting cognitive impairment as part of an integrated approach in SLE,” Dr. Paez-Venegas said.
Periodic screening for cognitive impairment in patients with SLE is an important aspect of patient management because cognitive impairment is a common manifestation of the disease, affecting up to two-thirds of patients, and it can have a serious impact upon quality of life and self-concept. Because such screening isn’t a one-time event, resort to a neuropsychological battery becomes particularly problematic. The battery employed in this study included the Wechsler Adult Intelligence Scale–Fourth Edition test, the Digit-Symbol test, the Finger-Tapping test of motor control, the Stroop test, Trail Making A and B, the Paced Auditory Serial Addition test, letter-number sequencing, the Wechsler Vocabulary test, the Rey-Osterrieth complex figure test, semantic and phonemic fluency tests, and a test of verbal Spanish comprehension. The battery is a comprehensive tool often employed in research studies but is not well suited for use in a busy clinical practice.
Overall, 70% of the SLE patients demonstrated cognitive impairment in one or more domains on the neuropsychological battery. Processing speed was the most frequently affected domain, involving 23 of the 44 patients. Only a single patient displayed abnormal motor control.
The MoCA test assesses attention, executive function, concentration, language, memory, abstraction, orientation, visuospatial cognitive capacity, and calculation.
Dr. Paez-Venegas’s study was published online earlier this year (J Clin Rheumatol 2018 Jul 18. doi: 10.1097/RHU.0000000000000876). He reported having no financial conflicts regarding his study.
SOURCE: Paez-Venegas N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 708.
CHICAGO – The Montreal Cognitive Assessment Test provides persuasive advantages over the standard neuropsychological test battery often recommended in guidelines as a screening tool for cognitive impairment in patients with systemic lupus erythematosus, Nicolas Paez-Venegas, MD, asserted at the annual meeting of the American College of Rheumatology.
The MoCA, as it’s known, offers brevity, simplicity, and none of the considerable expense and inconvenience of bringing in a trained specialist to administer a neuropsychological battery. Moreover, in a comparative efficacy study, the MoCA outperformed two other brief screening tools for cognitive impairment – the Mini-Mental State Examination and the Cognitive Symptom Inventory – and showed excellent correspondence with the results of the formal neuropsychological battery, reported Dr. Paez-Venegas, a psychiatrist at the Jalisco Institute of Mental Health, in Zapopan, Mexico.
He presented a cross-sectional study that pitted the three brief screening tests against a gold-standard neuropsychological battery in 44 patients with systemic lupus erythematosus (SLE) according to the 2012 Systemic Lupus International Collaborating Clinics Criteria, none of whom had any known medical or psychiatric comorbidities.
The MoCA proved to have the best congruence with the findings of the neuropsychological battery, with an area under the curve of 99.4%, 84% sensitivity, and 100% specificity for cognitive impairment. The Mini-Mental State Examination had 55% sensitivity and 100% specificity, while the Cognitive Symptom Inventory displayed 55% sensitivity and 31% specificity.
“We therefore encourage rheumatologists to apply the MoCA test as a valuable and easily implemented tool for detecting cognitive impairment as part of an integrated approach in SLE,” Dr. Paez-Venegas said.
Periodic screening for cognitive impairment in patients with SLE is an important aspect of patient management because cognitive impairment is a common manifestation of the disease, affecting up to two-thirds of patients, and it can have a serious impact upon quality of life and self-concept. Because such screening isn’t a one-time event, resort to a neuropsychological battery becomes particularly problematic. The battery employed in this study included the Wechsler Adult Intelligence Scale–Fourth Edition test, the Digit-Symbol test, the Finger-Tapping test of motor control, the Stroop test, Trail Making A and B, the Paced Auditory Serial Addition test, letter-number sequencing, the Wechsler Vocabulary test, the Rey-Osterrieth complex figure test, semantic and phonemic fluency tests, and a test of verbal Spanish comprehension. The battery is a comprehensive tool often employed in research studies but is not well suited for use in a busy clinical practice.
Overall, 70% of the SLE patients demonstrated cognitive impairment in one or more domains on the neuropsychological battery. Processing speed was the most frequently affected domain, involving 23 of the 44 patients. Only a single patient displayed abnormal motor control.
The MoCA test assesses attention, executive function, concentration, language, memory, abstraction, orientation, visuospatial cognitive capacity, and calculation.
Dr. Paez-Venegas’s study was published online earlier this year (J Clin Rheumatol 2018 Jul 18. doi: 10.1097/RHU.0000000000000876). He reported having no financial conflicts regarding his study.
SOURCE: Paez-Venegas N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 708.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The Montreal Cognitive Assessment Test showed 84% sensitivity and 100% specificity for cognitive impairment in systemic lupus erythematosus patients.
Study details: This comparative effectiveness study included 44 systemic lupus erythematosus patients assessed for cognitive impairment using three different tools.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
Source: Paez-Venegas N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 708.
Gout: new data support treat-to-target approach
CHICAGO – Failure to reach the therapeutic target of a serum urate level below 6 mg/dL in gout patients is an independent risk factor for all-cause mortality conferring a 139% increased risk, Fernando Perez-Ruiz, MD, PhD, said at the annual meeting of the American College of Rheumatology.
This new finding from a prospective cohort study of 1,193 gout patients constitutes a ringing endorsement that a treat-to-target approach should become the standard in the management of this disease, declared Dr. Perez-Ruiz, a rheumatologist at Hospital Universitario Cruces, Barakaldo, Spain.
“This is encouraging news. We can say to patients and clinicians that we should make every effort to reach the therapeutic target. This is a concept that’s not new in medicine. We do it for diabetes, for hypertension, for hyperlipidemia, and I think now for the first time we will do it for gout,” the rheumatologist said at a press conference highlighting the study findings.
“A lot of physicians including, unfortunately, rheumatologists don’t treat gout to target. They feel like if a patient is doing nicely, that’s good enough. But it’s like lowering cholesterol: If you’re at 400 mg/dL and you go to 300, does that mean it’s fine and you won’t get a myocardial infarction?” he asked rhetorically.
The study included 1,193 gout patients with a mean age at baseline of 60 years, 6.8 years disease duration, and an average of 3-4 flares during the previous year. Mean follow-up was 48 months, translating to 4,830 patient-years of prospective observation. Overall mortality was 13%, mostly from cardiovascular causes.
The mean baseline serum urate level was 9.1 mg/dL. Although both ACR and EULAR guidelines recommend a serum urate level below 6 mg/dL as a therapeutic target, 16.3% of subjects had a level of 6 mg/dL or more despite treatment. The crude mortality rate during follow-up was 80.9 deaths per 1,000 person-years in those with serum urate levels of 6 mg/dL or more, compared with 25.7 per 1,000 person-years in patients with serum urate levels below 6 mg/dL. In a multivariate analysis adjusted for age, prior cardiovascular events, other comorbid conditions, sex, baseline serum urate level, alcohol intake, and other potential confounders, a serum urate of 6 mg/dL or more was independently associated with a 139% increased risk of mortality during follow-up.
“I think the message we would like to give to clinicians is, ‘If you can do that [i.e., maintain the serum urate level below 6 mg/dL], do it. You have the knowledge, you have the means, make the effort. Your patient will benefit from that. Don’t take risks,’” Dr. Perez-Ruiz said.
Session moderator Shraddha Jatwani, MD, a rheumatologist at St. Vincent Hospital in Evansville, Ind., pronounced this a message she will take home to her clinical practice.
“What we usually see in clinical practice is that gout patients are among the most noncompliant. Once they stop hurting they just don’t see the need to take their medication daily. And now that we have this data, we can tell them that their gout medications are like statins, which help reduce the risk of heart attacks. Taking their gout medication will help them reduce their mortality risk. This information will help us to change patient perception,” she said.
Dr. Perez-Ruiz reported relationships with Amgen, Grünenthal, and Menarini.
SOURCE: Perez-Ruiz F et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 869.
CHICAGO – Failure to reach the therapeutic target of a serum urate level below 6 mg/dL in gout patients is an independent risk factor for all-cause mortality conferring a 139% increased risk, Fernando Perez-Ruiz, MD, PhD, said at the annual meeting of the American College of Rheumatology.
This new finding from a prospective cohort study of 1,193 gout patients constitutes a ringing endorsement that a treat-to-target approach should become the standard in the management of this disease, declared Dr. Perez-Ruiz, a rheumatologist at Hospital Universitario Cruces, Barakaldo, Spain.
“This is encouraging news. We can say to patients and clinicians that we should make every effort to reach the therapeutic target. This is a concept that’s not new in medicine. We do it for diabetes, for hypertension, for hyperlipidemia, and I think now for the first time we will do it for gout,” the rheumatologist said at a press conference highlighting the study findings.
“A lot of physicians including, unfortunately, rheumatologists don’t treat gout to target. They feel like if a patient is doing nicely, that’s good enough. But it’s like lowering cholesterol: If you’re at 400 mg/dL and you go to 300, does that mean it’s fine and you won’t get a myocardial infarction?” he asked rhetorically.
The study included 1,193 gout patients with a mean age at baseline of 60 years, 6.8 years disease duration, and an average of 3-4 flares during the previous year. Mean follow-up was 48 months, translating to 4,830 patient-years of prospective observation. Overall mortality was 13%, mostly from cardiovascular causes.
The mean baseline serum urate level was 9.1 mg/dL. Although both ACR and EULAR guidelines recommend a serum urate level below 6 mg/dL as a therapeutic target, 16.3% of subjects had a level of 6 mg/dL or more despite treatment. The crude mortality rate during follow-up was 80.9 deaths per 1,000 person-years in those with serum urate levels of 6 mg/dL or more, compared with 25.7 per 1,000 person-years in patients with serum urate levels below 6 mg/dL. In a multivariate analysis adjusted for age, prior cardiovascular events, other comorbid conditions, sex, baseline serum urate level, alcohol intake, and other potential confounders, a serum urate of 6 mg/dL or more was independently associated with a 139% increased risk of mortality during follow-up.
“I think the message we would like to give to clinicians is, ‘If you can do that [i.e., maintain the serum urate level below 6 mg/dL], do it. You have the knowledge, you have the means, make the effort. Your patient will benefit from that. Don’t take risks,’” Dr. Perez-Ruiz said.
Session moderator Shraddha Jatwani, MD, a rheumatologist at St. Vincent Hospital in Evansville, Ind., pronounced this a message she will take home to her clinical practice.
“What we usually see in clinical practice is that gout patients are among the most noncompliant. Once they stop hurting they just don’t see the need to take their medication daily. And now that we have this data, we can tell them that their gout medications are like statins, which help reduce the risk of heart attacks. Taking their gout medication will help them reduce their mortality risk. This information will help us to change patient perception,” she said.
Dr. Perez-Ruiz reported relationships with Amgen, Grünenthal, and Menarini.
SOURCE: Perez-Ruiz F et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 869.
CHICAGO – Failure to reach the therapeutic target of a serum urate level below 6 mg/dL in gout patients is an independent risk factor for all-cause mortality conferring a 139% increased risk, Fernando Perez-Ruiz, MD, PhD, said at the annual meeting of the American College of Rheumatology.
This new finding from a prospective cohort study of 1,193 gout patients constitutes a ringing endorsement that a treat-to-target approach should become the standard in the management of this disease, declared Dr. Perez-Ruiz, a rheumatologist at Hospital Universitario Cruces, Barakaldo, Spain.
“This is encouraging news. We can say to patients and clinicians that we should make every effort to reach the therapeutic target. This is a concept that’s not new in medicine. We do it for diabetes, for hypertension, for hyperlipidemia, and I think now for the first time we will do it for gout,” the rheumatologist said at a press conference highlighting the study findings.
“A lot of physicians including, unfortunately, rheumatologists don’t treat gout to target. They feel like if a patient is doing nicely, that’s good enough. But it’s like lowering cholesterol: If you’re at 400 mg/dL and you go to 300, does that mean it’s fine and you won’t get a myocardial infarction?” he asked rhetorically.
The study included 1,193 gout patients with a mean age at baseline of 60 years, 6.8 years disease duration, and an average of 3-4 flares during the previous year. Mean follow-up was 48 months, translating to 4,830 patient-years of prospective observation. Overall mortality was 13%, mostly from cardiovascular causes.
The mean baseline serum urate level was 9.1 mg/dL. Although both ACR and EULAR guidelines recommend a serum urate level below 6 mg/dL as a therapeutic target, 16.3% of subjects had a level of 6 mg/dL or more despite treatment. The crude mortality rate during follow-up was 80.9 deaths per 1,000 person-years in those with serum urate levels of 6 mg/dL or more, compared with 25.7 per 1,000 person-years in patients with serum urate levels below 6 mg/dL. In a multivariate analysis adjusted for age, prior cardiovascular events, other comorbid conditions, sex, baseline serum urate level, alcohol intake, and other potential confounders, a serum urate of 6 mg/dL or more was independently associated with a 139% increased risk of mortality during follow-up.
“I think the message we would like to give to clinicians is, ‘If you can do that [i.e., maintain the serum urate level below 6 mg/dL], do it. You have the knowledge, you have the means, make the effort. Your patient will benefit from that. Don’t take risks,’” Dr. Perez-Ruiz said.
Session moderator Shraddha Jatwani, MD, a rheumatologist at St. Vincent Hospital in Evansville, Ind., pronounced this a message she will take home to her clinical practice.
“What we usually see in clinical practice is that gout patients are among the most noncompliant. Once they stop hurting they just don’t see the need to take their medication daily. And now that we have this data, we can tell them that their gout medications are like statins, which help reduce the risk of heart attacks. Taking their gout medication will help them reduce their mortality risk. This information will help us to change patient perception,” she said.
Dr. Perez-Ruiz reported relationships with Amgen, Grünenthal, and Menarini.
SOURCE: Perez-Ruiz F et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 869.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Lowering serum urate in gout patients confers a survival advantage.
Major finding: A serum urate of 6 mg/dL or more in gout patients was independently associated with a 139% increased risk of all-cause mortality.
Study details: This was a prospective study of 1,193 gout patients followed for an average of 4 years.
Disclosures: Dr. Perez-Ruiz reported relationships with Amgen, Grünenthal, and Menarini.
Source: Perez-Ruiz F et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 869.
Novel topical JAK inhibitor shows promise for atopic dermatitis
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.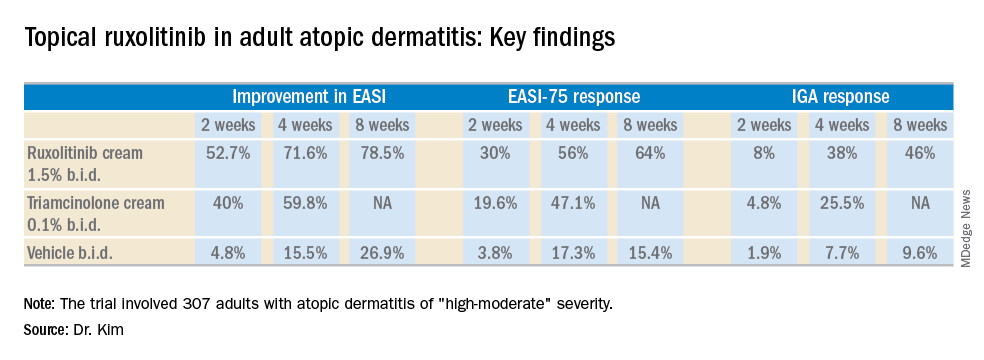
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A novel topical Janus kinase inhibitor may provide a valuable alternative to potent topical steroids in atopic dermatitis.
Major finding: At week 4, the mean improvement in Eczema Area and Severity Index score was 72% with ruxolitinib cream 1.5% twice a day, compared with 60% with triamcinolone cream 0.1% twice a day.
Study details: This 8-week, phase 2 clinical trial included 307 adult atopic dermatitis patients randomized to ruxolitinib cream, triamcinolone cream, or vehicle.
Disclosures: The study was sponsored by Incyte. The presenter reported serving as a consultant to and recipient of research funding from the company.
Total knee replacement risk soars after arthroscopic surgery for meniscal tear
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Risk of total knee replacement is five times higher after arthroscopic partial meniscectomy.
Major finding: Patients randomized to arthroscopic partial meniscectomy were 400% more likely to subsequently undergo total knee replacement than were those randomized to physical therapy alone.
Study details: This was a presentation of the 5-year follow-up results in 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy.
Disclosures: The presenter reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
Source: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
High incidence of treatment-resistant hypertension in SLE comes with high mortality
CHICAGO – Patients with systemic lupus erythematosus (SLE) have an incidence of treatment-resistant hypertension (TRH) twice the rate of matched controls, and all-cause mortality in affected SLE patients is sharply higher than in individuals whose SLE is not complicated by comorbid TRH, Annette Oeser reported at the annual meeting of the American College of Rheumatology.

TRH is thus an important yet underappreciated comorbidity for clinicians to recognize in patients with SLE, added Ms. Oeser of Vanderbilt University, Nashville, Tenn.
She presented a single-center, retrospective study of 1,044 SLE patients and 5,241 controls matched by age, race, and sex. During an average of 6 and maximum of 17 years of follow-up, 10% of SLE patients and 5% of controls developed TRH. The incidence was 14.7 cases per 1,000 person-years in the SLE population and 7.4 per 1,000 in controls. Of note, the incidence curves began to diverge within the first months following diagnosis of the autoimmune disease.
TRH was defined in the conventional way as an inability to achieve a blood pressure of 140/90 mm Hg or less while on three antihypertensive drugs having different mechanisms or as the simultaneous use of four or more antihypertensive agents, noted Ms. Oeser, the study coordinator, who presented the findings on behalf of senior investigator Cecilia P. Chung, MD, a rheumatologist at Vanderbilt.
The SLE patients with TRH were older than those without TRH by a margin of 47 versus 41 years of age. A total of 45% of SLE patients with TRH were black, compared with 21% of those without TRH. The group with SLE and TRH also had a higher C-reactive protein (10.2 versus 3.3 mg/L), a higher erythrocyte sedimentation rate (40 versus 24 mm/hr), a lower estimated glomerular filtration rate (65.0 versus 88.2 mL/min per 1.73 m2), and a higher creatinine (1.1 versus 0.8 mg/day).
Overall, 25% of SLE patients with TRH died during follow-up, as did 10% of those without resistant hypertension, for an unadjusted 289% increased risk of all-cause mortality. Upon adjustment for age, sex, calendar year, end-stage renal disease, and creatinine, the SLE patients with TRH still remained at a 78% increased risk of mortality.
Ms. Oeser and Dr. Chung reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
SOURCE: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
CHICAGO – Patients with systemic lupus erythematosus (SLE) have an incidence of treatment-resistant hypertension (TRH) twice the rate of matched controls, and all-cause mortality in affected SLE patients is sharply higher than in individuals whose SLE is not complicated by comorbid TRH, Annette Oeser reported at the annual meeting of the American College of Rheumatology.

TRH is thus an important yet underappreciated comorbidity for clinicians to recognize in patients with SLE, added Ms. Oeser of Vanderbilt University, Nashville, Tenn.
She presented a single-center, retrospective study of 1,044 SLE patients and 5,241 controls matched by age, race, and sex. During an average of 6 and maximum of 17 years of follow-up, 10% of SLE patients and 5% of controls developed TRH. The incidence was 14.7 cases per 1,000 person-years in the SLE population and 7.4 per 1,000 in controls. Of note, the incidence curves began to diverge within the first months following diagnosis of the autoimmune disease.
TRH was defined in the conventional way as an inability to achieve a blood pressure of 140/90 mm Hg or less while on three antihypertensive drugs having different mechanisms or as the simultaneous use of four or more antihypertensive agents, noted Ms. Oeser, the study coordinator, who presented the findings on behalf of senior investigator Cecilia P. Chung, MD, a rheumatologist at Vanderbilt.
The SLE patients with TRH were older than those without TRH by a margin of 47 versus 41 years of age. A total of 45% of SLE patients with TRH were black, compared with 21% of those without TRH. The group with SLE and TRH also had a higher C-reactive protein (10.2 versus 3.3 mg/L), a higher erythrocyte sedimentation rate (40 versus 24 mm/hr), a lower estimated glomerular filtration rate (65.0 versus 88.2 mL/min per 1.73 m2), and a higher creatinine (1.1 versus 0.8 mg/day).
Overall, 25% of SLE patients with TRH died during follow-up, as did 10% of those without resistant hypertension, for an unadjusted 289% increased risk of all-cause mortality. Upon adjustment for age, sex, calendar year, end-stage renal disease, and creatinine, the SLE patients with TRH still remained at a 78% increased risk of mortality.
Ms. Oeser and Dr. Chung reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
SOURCE: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
CHICAGO – Patients with systemic lupus erythematosus (SLE) have an incidence of treatment-resistant hypertension (TRH) twice the rate of matched controls, and all-cause mortality in affected SLE patients is sharply higher than in individuals whose SLE is not complicated by comorbid TRH, Annette Oeser reported at the annual meeting of the American College of Rheumatology.

TRH is thus an important yet underappreciated comorbidity for clinicians to recognize in patients with SLE, added Ms. Oeser of Vanderbilt University, Nashville, Tenn.
She presented a single-center, retrospective study of 1,044 SLE patients and 5,241 controls matched by age, race, and sex. During an average of 6 and maximum of 17 years of follow-up, 10% of SLE patients and 5% of controls developed TRH. The incidence was 14.7 cases per 1,000 person-years in the SLE population and 7.4 per 1,000 in controls. Of note, the incidence curves began to diverge within the first months following diagnosis of the autoimmune disease.
TRH was defined in the conventional way as an inability to achieve a blood pressure of 140/90 mm Hg or less while on three antihypertensive drugs having different mechanisms or as the simultaneous use of four or more antihypertensive agents, noted Ms. Oeser, the study coordinator, who presented the findings on behalf of senior investigator Cecilia P. Chung, MD, a rheumatologist at Vanderbilt.
The SLE patients with TRH were older than those without TRH by a margin of 47 versus 41 years of age. A total of 45% of SLE patients with TRH were black, compared with 21% of those without TRH. The group with SLE and TRH also had a higher C-reactive protein (10.2 versus 3.3 mg/L), a higher erythrocyte sedimentation rate (40 versus 24 mm/hr), a lower estimated glomerular filtration rate (65.0 versus 88.2 mL/min per 1.73 m2), and a higher creatinine (1.1 versus 0.8 mg/day).
Overall, 25% of SLE patients with TRH died during follow-up, as did 10% of those without resistant hypertension, for an unadjusted 289% increased risk of all-cause mortality. Upon adjustment for age, sex, calendar year, end-stage renal disease, and creatinine, the SLE patients with TRH still remained at a 78% increased risk of mortality.
Ms. Oeser and Dr. Chung reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
SOURCE: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Treatment-resistant hypertension is an important yet underappreciated comorbidity for clinicians to recognize in patients with systemic lupus erythematosus.
Major finding: The incidence rate of treatment-resistant hypertension was twice as great in patients with systemic lupus erythematosus compared with matched controls.
Study details: This retrospective, single-center study included 1,044 systemic lupus erythematosus patients and 5,241 matched controls.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was supported by Vanderbilt University, the Rheumatology Research Foundation, the National Institutes of Health, and the Lupus Research Alliance.
Source: Chung CP et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 706.
Empagliflozin reduces left ventricular mass
CHICAGO – Empagliflozin significantly reduced left ventricular mass compared with placebo over the course of 6 months in patients with type 2 diabetes and stable coronary artery disease in the randomized EMPA-HEART CardioLink-6 trial.
Use of empagliflozin (Jardiance), a sodium-glucose cotransporter 2 inhibitor (SGLT2i), also was associated with a clinically meaningful reduction in ambulatory systolic blood pressure and a boost in hematocrit in this population of normotensive patients with preserved left ventricular ejection fraction and high utilization of background guideline-directed medical therapy, Subodh Verma, MD, reported at the American Heart Association scientific sessions.
“Taken together, these data suggest that empagliflozin promotes early statistically and clinically significant reverse remodeling, which may contribute to the cardiovascular and heart failure benefits observed in the EMPA-REG OUTCOME trial and other SGLT2i studies,” added Dr. Verma, professor of surgery, pharmacology, and toxicology at the University of Toronto.
EMPA-REG OUTCOME was a landmark randomized trial that included 7,020 patients with type 2 diabetes and established ischemic cardiovascular disease in which the SGLT2i reduced all-cause mortality by 32%, compared with placebo over a median 3.1 years of follow-up, cardiovascular mortality by 38%, and hospitalizations for heart failure by 35% (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The mechanism responsible for these impressive clinical benefits has been unclear. The EMPA-HEART CardioLink-6 trial was a small study – 97 randomized patients – designed to shed light on this issue. The hypothesis was that SGLT2i therapy facilitates cardiac reverse remodeling. This indeed turned out to be the case when cardiac MRI findings at baseline and after 6 months were compared by blinded evaluators.
From a baseline mean left ventricular mass indexed to body surface area of 60 g/m2, which is within normal range, left ventricular mass decreased by a mean of 4.71 g in the empagliflozin group, compared with a mere 0.39-g reduction in placebo-treated controls.
Dr. Verma underscored the importance of this result: “Left ventricular mass is a strong and independent predictor of major cardiovascular events, including cardiovascular and all-cause mortality, myocardial infarction, and heart failure. Furthermore, the magnitude of left ventricular mass regression correlates with the extent of clinical outcome benefit seen with pharmacological and device therapies.”
In a prespecified subgroup analysis stratified by baseline LV mass index, patients with a baseline value greater than 60 g/m2 experienced a much greater benefit from empagliflozin, with a mean between-group difference in LV mass index reduction of 7.26 g/m2, compared with a 0.46-g/m2 difference between the SGLT2i and placebo among those with a baseline LV mass index of 60 g/m2 or less.
Ambulatory systolic blood pressure fell from a baseline of 139 mm Hg by a mean of 7.9 mm Hg in the empagliflozin group and 0.7 mm Hg with placebo. From a baseline hematocrit of 42%, hematocrit improved by an absolute 1.91% more with empagliflozin than placebo. However, there were no significant between-group differences in the secondary cardiac MRI outcomes of change in LV end-systolic volume index, LV end-diastolic volume index, or LV ejection fraction.
Discussant Elliott M. Antman, MD, hailed EMPA-HEART CardioLink-6 as “a very important mechanistic study.”
“As I leave Chicago for home, I plan to further increase the use of SGLT2 inhibitors in my patients with type 2 diabetes, especially if they have a history of heart failure, and especially if they have coronary artery disease. I would encourage you to think about doing the same, and I would also recommend that we urge our colleagues in general medicine, endocrinology, and nephrology to consider this information as well,” said Dr. Antman, professor of medicine and associate dean for clinical and translational research at Harvard Medical School, Boston, as well as an AHA past president.
He noted that EMPA-HEART CardioLink-6 provides “biologically plausible data” to explain the mechanism for the major clinical benefits of empagliflozin earlier documented in EMPA-REG OUTCOME. The likely driver of the reduction in left ventricular mass seen in EMPA-HEART CardioLink-6 was the combination of lower systolic blood pressure and higher hematocrit.
“These surrogates suggest that our traditional concepts of afterload and preload appear to be favorably affected by SGLT2 inhibition,” according to the cardiologist.
The EMPA-HEART CardioLink-6 study was funded by Boehringer Ingelheim. Dr. Verma reported receiving research support and/or speaker payments from that pharmaceutical company and roughly a dozen others. Dr. Antman had no disclosures.
SOURCE: Verma S. AHA 2018, Abstract 19332.
CHICAGO – Empagliflozin significantly reduced left ventricular mass compared with placebo over the course of 6 months in patients with type 2 diabetes and stable coronary artery disease in the randomized EMPA-HEART CardioLink-6 trial.
Use of empagliflozin (Jardiance), a sodium-glucose cotransporter 2 inhibitor (SGLT2i), also was associated with a clinically meaningful reduction in ambulatory systolic blood pressure and a boost in hematocrit in this population of normotensive patients with preserved left ventricular ejection fraction and high utilization of background guideline-directed medical therapy, Subodh Verma, MD, reported at the American Heart Association scientific sessions.
“Taken together, these data suggest that empagliflozin promotes early statistically and clinically significant reverse remodeling, which may contribute to the cardiovascular and heart failure benefits observed in the EMPA-REG OUTCOME trial and other SGLT2i studies,” added Dr. Verma, professor of surgery, pharmacology, and toxicology at the University of Toronto.
EMPA-REG OUTCOME was a landmark randomized trial that included 7,020 patients with type 2 diabetes and established ischemic cardiovascular disease in which the SGLT2i reduced all-cause mortality by 32%, compared with placebo over a median 3.1 years of follow-up, cardiovascular mortality by 38%, and hospitalizations for heart failure by 35% (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The mechanism responsible for these impressive clinical benefits has been unclear. The EMPA-HEART CardioLink-6 trial was a small study – 97 randomized patients – designed to shed light on this issue. The hypothesis was that SGLT2i therapy facilitates cardiac reverse remodeling. This indeed turned out to be the case when cardiac MRI findings at baseline and after 6 months were compared by blinded evaluators.
From a baseline mean left ventricular mass indexed to body surface area of 60 g/m2, which is within normal range, left ventricular mass decreased by a mean of 4.71 g in the empagliflozin group, compared with a mere 0.39-g reduction in placebo-treated controls.
Dr. Verma underscored the importance of this result: “Left ventricular mass is a strong and independent predictor of major cardiovascular events, including cardiovascular and all-cause mortality, myocardial infarction, and heart failure. Furthermore, the magnitude of left ventricular mass regression correlates with the extent of clinical outcome benefit seen with pharmacological and device therapies.”
In a prespecified subgroup analysis stratified by baseline LV mass index, patients with a baseline value greater than 60 g/m2 experienced a much greater benefit from empagliflozin, with a mean between-group difference in LV mass index reduction of 7.26 g/m2, compared with a 0.46-g/m2 difference between the SGLT2i and placebo among those with a baseline LV mass index of 60 g/m2 or less.
Ambulatory systolic blood pressure fell from a baseline of 139 mm Hg by a mean of 7.9 mm Hg in the empagliflozin group and 0.7 mm Hg with placebo. From a baseline hematocrit of 42%, hematocrit improved by an absolute 1.91% more with empagliflozin than placebo. However, there were no significant between-group differences in the secondary cardiac MRI outcomes of change in LV end-systolic volume index, LV end-diastolic volume index, or LV ejection fraction.
Discussant Elliott M. Antman, MD, hailed EMPA-HEART CardioLink-6 as “a very important mechanistic study.”
“As I leave Chicago for home, I plan to further increase the use of SGLT2 inhibitors in my patients with type 2 diabetes, especially if they have a history of heart failure, and especially if they have coronary artery disease. I would encourage you to think about doing the same, and I would also recommend that we urge our colleagues in general medicine, endocrinology, and nephrology to consider this information as well,” said Dr. Antman, professor of medicine and associate dean for clinical and translational research at Harvard Medical School, Boston, as well as an AHA past president.
He noted that EMPA-HEART CardioLink-6 provides “biologically plausible data” to explain the mechanism for the major clinical benefits of empagliflozin earlier documented in EMPA-REG OUTCOME. The likely driver of the reduction in left ventricular mass seen in EMPA-HEART CardioLink-6 was the combination of lower systolic blood pressure and higher hematocrit.
“These surrogates suggest that our traditional concepts of afterload and preload appear to be favorably affected by SGLT2 inhibition,” according to the cardiologist.
The EMPA-HEART CardioLink-6 study was funded by Boehringer Ingelheim. Dr. Verma reported receiving research support and/or speaker payments from that pharmaceutical company and roughly a dozen others. Dr. Antman had no disclosures.
SOURCE: Verma S. AHA 2018, Abstract 19332.
CHICAGO – Empagliflozin significantly reduced left ventricular mass compared with placebo over the course of 6 months in patients with type 2 diabetes and stable coronary artery disease in the randomized EMPA-HEART CardioLink-6 trial.
Use of empagliflozin (Jardiance), a sodium-glucose cotransporter 2 inhibitor (SGLT2i), also was associated with a clinically meaningful reduction in ambulatory systolic blood pressure and a boost in hematocrit in this population of normotensive patients with preserved left ventricular ejection fraction and high utilization of background guideline-directed medical therapy, Subodh Verma, MD, reported at the American Heart Association scientific sessions.
“Taken together, these data suggest that empagliflozin promotes early statistically and clinically significant reverse remodeling, which may contribute to the cardiovascular and heart failure benefits observed in the EMPA-REG OUTCOME trial and other SGLT2i studies,” added Dr. Verma, professor of surgery, pharmacology, and toxicology at the University of Toronto.
EMPA-REG OUTCOME was a landmark randomized trial that included 7,020 patients with type 2 diabetes and established ischemic cardiovascular disease in which the SGLT2i reduced all-cause mortality by 32%, compared with placebo over a median 3.1 years of follow-up, cardiovascular mortality by 38%, and hospitalizations for heart failure by 35% (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The mechanism responsible for these impressive clinical benefits has been unclear. The EMPA-HEART CardioLink-6 trial was a small study – 97 randomized patients – designed to shed light on this issue. The hypothesis was that SGLT2i therapy facilitates cardiac reverse remodeling. This indeed turned out to be the case when cardiac MRI findings at baseline and after 6 months were compared by blinded evaluators.
From a baseline mean left ventricular mass indexed to body surface area of 60 g/m2, which is within normal range, left ventricular mass decreased by a mean of 4.71 g in the empagliflozin group, compared with a mere 0.39-g reduction in placebo-treated controls.
Dr. Verma underscored the importance of this result: “Left ventricular mass is a strong and independent predictor of major cardiovascular events, including cardiovascular and all-cause mortality, myocardial infarction, and heart failure. Furthermore, the magnitude of left ventricular mass regression correlates with the extent of clinical outcome benefit seen with pharmacological and device therapies.”
In a prespecified subgroup analysis stratified by baseline LV mass index, patients with a baseline value greater than 60 g/m2 experienced a much greater benefit from empagliflozin, with a mean between-group difference in LV mass index reduction of 7.26 g/m2, compared with a 0.46-g/m2 difference between the SGLT2i and placebo among those with a baseline LV mass index of 60 g/m2 or less.
Ambulatory systolic blood pressure fell from a baseline of 139 mm Hg by a mean of 7.9 mm Hg in the empagliflozin group and 0.7 mm Hg with placebo. From a baseline hematocrit of 42%, hematocrit improved by an absolute 1.91% more with empagliflozin than placebo. However, there were no significant between-group differences in the secondary cardiac MRI outcomes of change in LV end-systolic volume index, LV end-diastolic volume index, or LV ejection fraction.
Discussant Elliott M. Antman, MD, hailed EMPA-HEART CardioLink-6 as “a very important mechanistic study.”
“As I leave Chicago for home, I plan to further increase the use of SGLT2 inhibitors in my patients with type 2 diabetes, especially if they have a history of heart failure, and especially if they have coronary artery disease. I would encourage you to think about doing the same, and I would also recommend that we urge our colleagues in general medicine, endocrinology, and nephrology to consider this information as well,” said Dr. Antman, professor of medicine and associate dean for clinical and translational research at Harvard Medical School, Boston, as well as an AHA past president.
He noted that EMPA-HEART CardioLink-6 provides “biologically plausible data” to explain the mechanism for the major clinical benefits of empagliflozin earlier documented in EMPA-REG OUTCOME. The likely driver of the reduction in left ventricular mass seen in EMPA-HEART CardioLink-6 was the combination of lower systolic blood pressure and higher hematocrit.
“These surrogates suggest that our traditional concepts of afterload and preload appear to be favorably affected by SGLT2 inhibition,” according to the cardiologist.
The EMPA-HEART CardioLink-6 study was funded by Boehringer Ingelheim. Dr. Verma reported receiving research support and/or speaker payments from that pharmaceutical company and roughly a dozen others. Dr. Antman had no disclosures.
SOURCE: Verma S. AHA 2018, Abstract 19332.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: .
Major finding: Six months of empagliflozin reduced left ventricular mass by a mean of 4.71 g, vs. 0.39 g with placebo.
Study details: This 97-patient, 6-month, randomized trial evaluated the impact of SGLT2 inhibition with empagliflozin on left ventricular remodeling.
Disclosures: The EMPA-HEART CardioLink-6 study was funded by Boehringer Ingelheim. The presenter reported receiving research support and/or speaker payments from that pharmaceutical company and roughly a dozen others. Source: Verma S. AHA 2018, Abstract 19332.
New HHS physical activity guidelines break fresh ground
CHICAGO – The newly released comprehensive second edition of the federal physical activity guidelines have a lofty goal.
Adm. Brett P. Giroir, MD, declared in introducing the recommendations at the American Heart Association scientific sessions.
“Physical activity is one of the most effective preventive health interventions available, and we need more emphasis on prevention as we transition to a value-based reimbursement structure that rewards better health maintenance and avoids chronic conditions,” added Adm. Giroir, assistant secretary for health at the U.S. Department of Health & Human Services.
Although the agency opted to unveil the new guidelines before an audience of cardiologists at the AHA scientific sessions, the report includes sections relevant for a wide range of medical specialists, including primary care physicians, pediatricians, psychiatrists, neurologists, endocrinologists, and geriatricians.
Before launching into a description of what’s new in the second edition, Adm. Giroir set the stage with blunt talk about the nation’s poor state of physical fitness.
“Inactivity causes 10% of premature mortality in the United States. That means if we can just get 25% of inactive people to be active and meet the recommendations, almost 75,000 deaths per year would be prevented in the United States. And on an even larger scale worldwide, if 25% of those same people who are inactive started moving and met the guidelines, more than 1.3 million deaths would be prevented,” according to Adm. Giroir.
At present, only 26% of men, 19% of women, and 20% of teenagers meet the physical activity recommendations.
Failure to meet the federal aerobic physical activity recommendations accounts for an estimated nearly $117 billion in annual health care costs. And it poses a national security threat, too: Nearly one-third of all 17- to 24-year-olds are disqualified from military service because of obesity. Even more eye-opening, he continued, is that fully 71% of all 17- to 24-year-olds are ineligible for military service because of obesity, lack of physical fitness, lack of education, or substance use.
The actual recommendations contained in the second edition of the Physical Activity Guidelines for Americans remain unchanged from those in the first, issued a decade earlier. That is, in order to gain substantial health benefits, adults and adolescents should engage in at least 150-300 min/week of moderate intensity aerobic physical activity or 75-150 min/week of vigorous intensity aerobic activity. Plus they should do muscle-strengthening exercises such as weight lifting or push-ups at moderate or greater intensity at least 2 days/week.
Asked why the guidelines are sticking with time-based physical activity recommendations in an era when popular smartwatches and other mobile devices can readily spit out number of steps walked, calories burned, and heart-rate data, cardiologist William E. Kraus, MD, one of the 17 members of the scientific advisory committee who reviewed and graded the scientific evidence on physical activity, sedentary behavior, and health, answered. He said the group’s careful review concluded that “there’s just not enough evidence at this time to make a recommendation” with regard to mobile device–based measurements of physical activity and their relationship with health benefits.
“We’re hoping to spur more research in this area, so that the next time we make recommendations, that can be incorporated,” added Dr. Kraus, a professor of medicine and cardiologist at Duke University, Durham, N.C., as well as president-elect of the American College of Sports Medicine.
What’s new in the guidelines
“This edition tells us that it’s easier to meet the recommendations in the physical activity guidelines,” according to Adm. Giroir. “The new guidelines demonstrate, based on the best science, everyone can dramatically improve their health just by moving: anytime, anywhere, and by any means that gets you active.” He broke the guidelines down as follows:
- We have new evidence about the risks of sedentary behavior, and new evidence that any amount – any amount – of moderate to vigorous physical activity, like walking, dancing, line dancing if you’re from Texas, and household chores is beneficial,” Adm. Giroir observed.
- While the first edition of the federal guidelines cited strong evidence that physical activity reduces the risk of two types of cancer, breast and colon, the intervening decade has brought forth strong evidence of a protective effect against an additional six types of cancer: bladder, endometrial, kidney, stomach, esophageal, and lung cancer.
- The guidelines formulate for the first time physical activity standards for children aged 3-5 years. The recommended target is at least 3 hr/day of varied physical activity, consistent with existing guidelines in Australia Canada, and the United Kingdom.
- Updated recommendations for children aged 6-17 years call for an hour or more/day of moderate- or vigorous-intensity physical activity on a daily basis, with that activity level falling within the vigorous category on at least 3 days/week. Plus, it recommends bone- and muscle-strengthening activity on at least 3 days.
- The pediatric guidelines are linked to a planned HHS national strategy to expand children’s participation in youth sports as part of an effort to curb childhood obesity, rates of which have more than tripled since the 1970s.
“We’ll soon announce funding opportunities for communities to increase participation in sports among children and teens through participation in affordable programs with trained coaches,” said Dr. Giroir, a pediatrician.
The new guidelines endorse what is called “the comprehensive school physical activity model.”
“I strongly believe our schools should take action to implement this approach. There is a lot of interest right now to affect change in the schools across our country. Part of the answer, I think, is to provide kids with high-quality physical education, but I think we recognize that alone will not be enough.” The comprehensive school activity model calls for not only enriching school PE programs but also incorporating active transport to school, classroom activity, active learning, and after school programs – activity in all settings during the school day. “I’m very hopeful that this model, which is endorsed in the guidelines document, will be widely adopted by schools in this country over the next decade,” Dr. Giroir said.
The first edition declared that only bouts of physical activity of at least 10 minutes duration should count toward meeting the guidelines. That requirement is gone in the second edition. It was an impediment to being active, and upon close examination it wasn’t based on scientific evidence. That means taking the stairs instead of the escalator or parking farther away from the store count toward the weekly physical activity goal, Dr. Kraus said.
“It makes it easier to achieve the guidelines and to encourage Americans to move more and sit less just by making a better choice at many times during the day,” observed Dr. Giroir, a four-star admiral in the U.S. Public Health Service Commissioned Corps.
The latest guidelines contain up-to-date information on the benefits of regular physical activity in terms of brain health, including reduced risk of developing Alzheimer’s disease, and improved cognition, including performance on academic achievement tests and measures of executive function, memory, and processing speed, in preadolescent children as well as older adults. Solid evidence also is cited for improved cognition in patients with MS, dementia, ADHD, and Parkinson’s disease.
The guidelines provide new recommendations for physical activity for women during pregnancy and post partum.
A section of the guidelines is devoted to proven evidence-based strategies to promote physical activity at the individual, small group, and community level.
Physicians now have a resource to aid them in prescribing an individualized physical activity prescription for their patients with existing health conditions, including osteoarthritis, type 2 diabetes, cancer survivors, and physical disabilities.
The new physical activity guidelines and related resources for health care professionals are available at the Health.gov website.
SOURCE: Giroir BP. AHA Scientific Sessions, Session ME.05.
CHICAGO – The newly released comprehensive second edition of the federal physical activity guidelines have a lofty goal.
Adm. Brett P. Giroir, MD, declared in introducing the recommendations at the American Heart Association scientific sessions.
“Physical activity is one of the most effective preventive health interventions available, and we need more emphasis on prevention as we transition to a value-based reimbursement structure that rewards better health maintenance and avoids chronic conditions,” added Adm. Giroir, assistant secretary for health at the U.S. Department of Health & Human Services.
Although the agency opted to unveil the new guidelines before an audience of cardiologists at the AHA scientific sessions, the report includes sections relevant for a wide range of medical specialists, including primary care physicians, pediatricians, psychiatrists, neurologists, endocrinologists, and geriatricians.
Before launching into a description of what’s new in the second edition, Adm. Giroir set the stage with blunt talk about the nation’s poor state of physical fitness.
“Inactivity causes 10% of premature mortality in the United States. That means if we can just get 25% of inactive people to be active and meet the recommendations, almost 75,000 deaths per year would be prevented in the United States. And on an even larger scale worldwide, if 25% of those same people who are inactive started moving and met the guidelines, more than 1.3 million deaths would be prevented,” according to Adm. Giroir.
At present, only 26% of men, 19% of women, and 20% of teenagers meet the physical activity recommendations.
Failure to meet the federal aerobic physical activity recommendations accounts for an estimated nearly $117 billion in annual health care costs. And it poses a national security threat, too: Nearly one-third of all 17- to 24-year-olds are disqualified from military service because of obesity. Even more eye-opening, he continued, is that fully 71% of all 17- to 24-year-olds are ineligible for military service because of obesity, lack of physical fitness, lack of education, or substance use.
The actual recommendations contained in the second edition of the Physical Activity Guidelines for Americans remain unchanged from those in the first, issued a decade earlier. That is, in order to gain substantial health benefits, adults and adolescents should engage in at least 150-300 min/week of moderate intensity aerobic physical activity or 75-150 min/week of vigorous intensity aerobic activity. Plus they should do muscle-strengthening exercises such as weight lifting or push-ups at moderate or greater intensity at least 2 days/week.
Asked why the guidelines are sticking with time-based physical activity recommendations in an era when popular smartwatches and other mobile devices can readily spit out number of steps walked, calories burned, and heart-rate data, cardiologist William E. Kraus, MD, one of the 17 members of the scientific advisory committee who reviewed and graded the scientific evidence on physical activity, sedentary behavior, and health, answered. He said the group’s careful review concluded that “there’s just not enough evidence at this time to make a recommendation” with regard to mobile device–based measurements of physical activity and their relationship with health benefits.
“We’re hoping to spur more research in this area, so that the next time we make recommendations, that can be incorporated,” added Dr. Kraus, a professor of medicine and cardiologist at Duke University, Durham, N.C., as well as president-elect of the American College of Sports Medicine.
What’s new in the guidelines
“This edition tells us that it’s easier to meet the recommendations in the physical activity guidelines,” according to Adm. Giroir. “The new guidelines demonstrate, based on the best science, everyone can dramatically improve their health just by moving: anytime, anywhere, and by any means that gets you active.” He broke the guidelines down as follows:
- We have new evidence about the risks of sedentary behavior, and new evidence that any amount – any amount – of moderate to vigorous physical activity, like walking, dancing, line dancing if you’re from Texas, and household chores is beneficial,” Adm. Giroir observed.
- While the first edition of the federal guidelines cited strong evidence that physical activity reduces the risk of two types of cancer, breast and colon, the intervening decade has brought forth strong evidence of a protective effect against an additional six types of cancer: bladder, endometrial, kidney, stomach, esophageal, and lung cancer.
- The guidelines formulate for the first time physical activity standards for children aged 3-5 years. The recommended target is at least 3 hr/day of varied physical activity, consistent with existing guidelines in Australia Canada, and the United Kingdom.
- Updated recommendations for children aged 6-17 years call for an hour or more/day of moderate- or vigorous-intensity physical activity on a daily basis, with that activity level falling within the vigorous category on at least 3 days/week. Plus, it recommends bone- and muscle-strengthening activity on at least 3 days.
- The pediatric guidelines are linked to a planned HHS national strategy to expand children’s participation in youth sports as part of an effort to curb childhood obesity, rates of which have more than tripled since the 1970s.
“We’ll soon announce funding opportunities for communities to increase participation in sports among children and teens through participation in affordable programs with trained coaches,” said Dr. Giroir, a pediatrician.
The new guidelines endorse what is called “the comprehensive school physical activity model.”
“I strongly believe our schools should take action to implement this approach. There is a lot of interest right now to affect change in the schools across our country. Part of the answer, I think, is to provide kids with high-quality physical education, but I think we recognize that alone will not be enough.” The comprehensive school activity model calls for not only enriching school PE programs but also incorporating active transport to school, classroom activity, active learning, and after school programs – activity in all settings during the school day. “I’m very hopeful that this model, which is endorsed in the guidelines document, will be widely adopted by schools in this country over the next decade,” Dr. Giroir said.
The first edition declared that only bouts of physical activity of at least 10 minutes duration should count toward meeting the guidelines. That requirement is gone in the second edition. It was an impediment to being active, and upon close examination it wasn’t based on scientific evidence. That means taking the stairs instead of the escalator or parking farther away from the store count toward the weekly physical activity goal, Dr. Kraus said.
“It makes it easier to achieve the guidelines and to encourage Americans to move more and sit less just by making a better choice at many times during the day,” observed Dr. Giroir, a four-star admiral in the U.S. Public Health Service Commissioned Corps.
The latest guidelines contain up-to-date information on the benefits of regular physical activity in terms of brain health, including reduced risk of developing Alzheimer’s disease, and improved cognition, including performance on academic achievement tests and measures of executive function, memory, and processing speed, in preadolescent children as well as older adults. Solid evidence also is cited for improved cognition in patients with MS, dementia, ADHD, and Parkinson’s disease.
The guidelines provide new recommendations for physical activity for women during pregnancy and post partum.
A section of the guidelines is devoted to proven evidence-based strategies to promote physical activity at the individual, small group, and community level.
Physicians now have a resource to aid them in prescribing an individualized physical activity prescription for their patients with existing health conditions, including osteoarthritis, type 2 diabetes, cancer survivors, and physical disabilities.
The new physical activity guidelines and related resources for health care professionals are available at the Health.gov website.
SOURCE: Giroir BP. AHA Scientific Sessions, Session ME.05.
CHICAGO – The newly released comprehensive second edition of the federal physical activity guidelines have a lofty goal.
Adm. Brett P. Giroir, MD, declared in introducing the recommendations at the American Heart Association scientific sessions.
“Physical activity is one of the most effective preventive health interventions available, and we need more emphasis on prevention as we transition to a value-based reimbursement structure that rewards better health maintenance and avoids chronic conditions,” added Adm. Giroir, assistant secretary for health at the U.S. Department of Health & Human Services.
Although the agency opted to unveil the new guidelines before an audience of cardiologists at the AHA scientific sessions, the report includes sections relevant for a wide range of medical specialists, including primary care physicians, pediatricians, psychiatrists, neurologists, endocrinologists, and geriatricians.
Before launching into a description of what’s new in the second edition, Adm. Giroir set the stage with blunt talk about the nation’s poor state of physical fitness.
“Inactivity causes 10% of premature mortality in the United States. That means if we can just get 25% of inactive people to be active and meet the recommendations, almost 75,000 deaths per year would be prevented in the United States. And on an even larger scale worldwide, if 25% of those same people who are inactive started moving and met the guidelines, more than 1.3 million deaths would be prevented,” according to Adm. Giroir.
At present, only 26% of men, 19% of women, and 20% of teenagers meet the physical activity recommendations.
Failure to meet the federal aerobic physical activity recommendations accounts for an estimated nearly $117 billion in annual health care costs. And it poses a national security threat, too: Nearly one-third of all 17- to 24-year-olds are disqualified from military service because of obesity. Even more eye-opening, he continued, is that fully 71% of all 17- to 24-year-olds are ineligible for military service because of obesity, lack of physical fitness, lack of education, or substance use.
The actual recommendations contained in the second edition of the Physical Activity Guidelines for Americans remain unchanged from those in the first, issued a decade earlier. That is, in order to gain substantial health benefits, adults and adolescents should engage in at least 150-300 min/week of moderate intensity aerobic physical activity or 75-150 min/week of vigorous intensity aerobic activity. Plus they should do muscle-strengthening exercises such as weight lifting or push-ups at moderate or greater intensity at least 2 days/week.
Asked why the guidelines are sticking with time-based physical activity recommendations in an era when popular smartwatches and other mobile devices can readily spit out number of steps walked, calories burned, and heart-rate data, cardiologist William E. Kraus, MD, one of the 17 members of the scientific advisory committee who reviewed and graded the scientific evidence on physical activity, sedentary behavior, and health, answered. He said the group’s careful review concluded that “there’s just not enough evidence at this time to make a recommendation” with regard to mobile device–based measurements of physical activity and their relationship with health benefits.
“We’re hoping to spur more research in this area, so that the next time we make recommendations, that can be incorporated,” added Dr. Kraus, a professor of medicine and cardiologist at Duke University, Durham, N.C., as well as president-elect of the American College of Sports Medicine.
What’s new in the guidelines
“This edition tells us that it’s easier to meet the recommendations in the physical activity guidelines,” according to Adm. Giroir. “The new guidelines demonstrate, based on the best science, everyone can dramatically improve their health just by moving: anytime, anywhere, and by any means that gets you active.” He broke the guidelines down as follows:
- We have new evidence about the risks of sedentary behavior, and new evidence that any amount – any amount – of moderate to vigorous physical activity, like walking, dancing, line dancing if you’re from Texas, and household chores is beneficial,” Adm. Giroir observed.
- While the first edition of the federal guidelines cited strong evidence that physical activity reduces the risk of two types of cancer, breast and colon, the intervening decade has brought forth strong evidence of a protective effect against an additional six types of cancer: bladder, endometrial, kidney, stomach, esophageal, and lung cancer.
- The guidelines formulate for the first time physical activity standards for children aged 3-5 years. The recommended target is at least 3 hr/day of varied physical activity, consistent with existing guidelines in Australia Canada, and the United Kingdom.
- Updated recommendations for children aged 6-17 years call for an hour or more/day of moderate- or vigorous-intensity physical activity on a daily basis, with that activity level falling within the vigorous category on at least 3 days/week. Plus, it recommends bone- and muscle-strengthening activity on at least 3 days.
- The pediatric guidelines are linked to a planned HHS national strategy to expand children’s participation in youth sports as part of an effort to curb childhood obesity, rates of which have more than tripled since the 1970s.
“We’ll soon announce funding opportunities for communities to increase participation in sports among children and teens through participation in affordable programs with trained coaches,” said Dr. Giroir, a pediatrician.
The new guidelines endorse what is called “the comprehensive school physical activity model.”
“I strongly believe our schools should take action to implement this approach. There is a lot of interest right now to affect change in the schools across our country. Part of the answer, I think, is to provide kids with high-quality physical education, but I think we recognize that alone will not be enough.” The comprehensive school activity model calls for not only enriching school PE programs but also incorporating active transport to school, classroom activity, active learning, and after school programs – activity in all settings during the school day. “I’m very hopeful that this model, which is endorsed in the guidelines document, will be widely adopted by schools in this country over the next decade,” Dr. Giroir said.
The first edition declared that only bouts of physical activity of at least 10 minutes duration should count toward meeting the guidelines. That requirement is gone in the second edition. It was an impediment to being active, and upon close examination it wasn’t based on scientific evidence. That means taking the stairs instead of the escalator or parking farther away from the store count toward the weekly physical activity goal, Dr. Kraus said.
“It makes it easier to achieve the guidelines and to encourage Americans to move more and sit less just by making a better choice at many times during the day,” observed Dr. Giroir, a four-star admiral in the U.S. Public Health Service Commissioned Corps.
The latest guidelines contain up-to-date information on the benefits of regular physical activity in terms of brain health, including reduced risk of developing Alzheimer’s disease, and improved cognition, including performance on academic achievement tests and measures of executive function, memory, and processing speed, in preadolescent children as well as older adults. Solid evidence also is cited for improved cognition in patients with MS, dementia, ADHD, and Parkinson’s disease.
The guidelines provide new recommendations for physical activity for women during pregnancy and post partum.
A section of the guidelines is devoted to proven evidence-based strategies to promote physical activity at the individual, small group, and community level.
Physicians now have a resource to aid them in prescribing an individualized physical activity prescription for their patients with existing health conditions, including osteoarthritis, type 2 diabetes, cancer survivors, and physical disabilities.
The new physical activity guidelines and related resources for health care professionals are available at the Health.gov website.
SOURCE: Giroir BP. AHA Scientific Sessions, Session ME.05.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Tofacitinib impresses in first trial for dermatomyositis
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Among patients with refractory dermatomyositis, the mean Cutaneous Dermatomyositis Disease Area and Severity Index activity score improved from 28 at baseline to 9.5 after 12 weeks on oral tofacitinib.
Study details: This first-in-class, open-label, 12-week study included 10 patients with refractory dermatomyositis placed on extended-release tofacitinib at 11 mg once daily.
Disclosures: The presenter reported having no financial conflicts regarding the study, sponsored by Pfizer.
Source: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02
Rituximab shines in pediatric vasculitis
CHICAGO – Rituximab demonstrated a high degree of effectiveness with no safety surprises in the first-ever major clinical trial of the potent B-cell inhibitor conducted in pediatric patients with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, Jennifer Cooper, MD, PharmD, said at the annual meeting of the American College of Rheumatology.
“This is exciting news. We know that rituximab is very effective in treating adults with GPA and MPA, both in terms of inducing remission and even for maintenance therapy for this rare and severe disease. A lot of pediatric rheumatologists would like to have access to rituximab. Some are even using it for this condition. But until now there were no data in children. I hope this study improves access to rituximab for pediatric patients with ANCA-associated vasculitis,” said Dr. Cooper, a pediatric rheumatologist at the University of Colorado, Denver.
She presented the results of the PePRS (Pediatric Polyangiitis and Rituximab Study), a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries. She anticipates the results will be practice changing, given that pediatric GPA and MPA are recognized as severe systemic autoimmune disorders with a high unmet need for new therapies.
“I don’t believe there are plans for a randomized, controlled trial. Since we now have the pharmacokinetic and safety data in children, we’ll hopefully be able to use extrapolation and our exploratory efficacy endpoints to gain a pediatric indication, or at least a label update for these patients based on this study,” Dr. Cooper continued.
A total of 19 patients had GPA and 6 had MPA, with a median disease duration of 6 months at study entry. All received three pulsed doses of methylprednisolone during the screening period. Then for induction remission they got four once-weekly intravenous infusions of rituximab (Rituxan) at 375 mg/m2 as well as oral corticosteroids, which were tapered from 1 mg/kg per day to 0.2 mg/kg per day over the course of the first 6 months. After that, two-thirds of patients received additional rituximab at their provider’s discretion.
The remission rate by 6 months as defined by Pediatric Vasculitis Activity Score (PVAS) criteria was 56%. At 12 months, the rate was 92%, and at 18 months the rate of remission and sustained disease control was 100%. The mean and median durations of remission were 72 and 56 weeks, respectively.
These results in pediatric patients are comparable to those seen in adults in the landmark RAVE (Rituximab in ANCA-Associated Vasculitis) trial (N Engl J Med. 2013 Aug 1;369[5]:417-27), where 64% of patients reached remission at 6 months, Dr. Cooper noted.
All 25 patients were able to tolerate the four rituximab infusions for remission induction. The main side effect was infusion-related reactions. Overall, 32% of patients experienced such reactions in response to their first infusion, 20% with the second, 12% with the third, and only 8% with the fourth.
Eight patients withdrew from the study after 18 months, mostly because of transfer to adult care. The remaining participants were followed for as long as 4.5 years.
F. Hoffmann-La Roche and Genentech sponsored the PePRS study. Dr. Cooper was a Genentech clinical research fellow at the time.
SOURCE: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.
CHICAGO – Rituximab demonstrated a high degree of effectiveness with no safety surprises in the first-ever major clinical trial of the potent B-cell inhibitor conducted in pediatric patients with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, Jennifer Cooper, MD, PharmD, said at the annual meeting of the American College of Rheumatology.
“This is exciting news. We know that rituximab is very effective in treating adults with GPA and MPA, both in terms of inducing remission and even for maintenance therapy for this rare and severe disease. A lot of pediatric rheumatologists would like to have access to rituximab. Some are even using it for this condition. But until now there were no data in children. I hope this study improves access to rituximab for pediatric patients with ANCA-associated vasculitis,” said Dr. Cooper, a pediatric rheumatologist at the University of Colorado, Denver.
She presented the results of the PePRS (Pediatric Polyangiitis and Rituximab Study), a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries. She anticipates the results will be practice changing, given that pediatric GPA and MPA are recognized as severe systemic autoimmune disorders with a high unmet need for new therapies.
“I don’t believe there are plans for a randomized, controlled trial. Since we now have the pharmacokinetic and safety data in children, we’ll hopefully be able to use extrapolation and our exploratory efficacy endpoints to gain a pediatric indication, or at least a label update for these patients based on this study,” Dr. Cooper continued.
A total of 19 patients had GPA and 6 had MPA, with a median disease duration of 6 months at study entry. All received three pulsed doses of methylprednisolone during the screening period. Then for induction remission they got four once-weekly intravenous infusions of rituximab (Rituxan) at 375 mg/m2 as well as oral corticosteroids, which were tapered from 1 mg/kg per day to 0.2 mg/kg per day over the course of the first 6 months. After that, two-thirds of patients received additional rituximab at their provider’s discretion.
The remission rate by 6 months as defined by Pediatric Vasculitis Activity Score (PVAS) criteria was 56%. At 12 months, the rate was 92%, and at 18 months the rate of remission and sustained disease control was 100%. The mean and median durations of remission were 72 and 56 weeks, respectively.
These results in pediatric patients are comparable to those seen in adults in the landmark RAVE (Rituximab in ANCA-Associated Vasculitis) trial (N Engl J Med. 2013 Aug 1;369[5]:417-27), where 64% of patients reached remission at 6 months, Dr. Cooper noted.
All 25 patients were able to tolerate the four rituximab infusions for remission induction. The main side effect was infusion-related reactions. Overall, 32% of patients experienced such reactions in response to their first infusion, 20% with the second, 12% with the third, and only 8% with the fourth.
Eight patients withdrew from the study after 18 months, mostly because of transfer to adult care. The remaining participants were followed for as long as 4.5 years.
F. Hoffmann-La Roche and Genentech sponsored the PePRS study. Dr. Cooper was a Genentech clinical research fellow at the time.
SOURCE: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.
CHICAGO – Rituximab demonstrated a high degree of effectiveness with no safety surprises in the first-ever major clinical trial of the potent B-cell inhibitor conducted in pediatric patients with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, Jennifer Cooper, MD, PharmD, said at the annual meeting of the American College of Rheumatology.
“This is exciting news. We know that rituximab is very effective in treating adults with GPA and MPA, both in terms of inducing remission and even for maintenance therapy for this rare and severe disease. A lot of pediatric rheumatologists would like to have access to rituximab. Some are even using it for this condition. But until now there were no data in children. I hope this study improves access to rituximab for pediatric patients with ANCA-associated vasculitis,” said Dr. Cooper, a pediatric rheumatologist at the University of Colorado, Denver.
She presented the results of the PePRS (Pediatric Polyangiitis and Rituximab Study), a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries. She anticipates the results will be practice changing, given that pediatric GPA and MPA are recognized as severe systemic autoimmune disorders with a high unmet need for new therapies.
“I don’t believe there are plans for a randomized, controlled trial. Since we now have the pharmacokinetic and safety data in children, we’ll hopefully be able to use extrapolation and our exploratory efficacy endpoints to gain a pediatric indication, or at least a label update for these patients based on this study,” Dr. Cooper continued.
A total of 19 patients had GPA and 6 had MPA, with a median disease duration of 6 months at study entry. All received three pulsed doses of methylprednisolone during the screening period. Then for induction remission they got four once-weekly intravenous infusions of rituximab (Rituxan) at 375 mg/m2 as well as oral corticosteroids, which were tapered from 1 mg/kg per day to 0.2 mg/kg per day over the course of the first 6 months. After that, two-thirds of patients received additional rituximab at their provider’s discretion.
The remission rate by 6 months as defined by Pediatric Vasculitis Activity Score (PVAS) criteria was 56%. At 12 months, the rate was 92%, and at 18 months the rate of remission and sustained disease control was 100%. The mean and median durations of remission were 72 and 56 weeks, respectively.
These results in pediatric patients are comparable to those seen in adults in the landmark RAVE (Rituximab in ANCA-Associated Vasculitis) trial (N Engl J Med. 2013 Aug 1;369[5]:417-27), where 64% of patients reached remission at 6 months, Dr. Cooper noted.
All 25 patients were able to tolerate the four rituximab infusions for remission induction. The main side effect was infusion-related reactions. Overall, 32% of patients experienced such reactions in response to their first infusion, 20% with the second, 12% with the third, and only 8% with the fourth.
Eight patients withdrew from the study after 18 months, mostly because of transfer to adult care. The remaining participants were followed for as long as 4.5 years.
F. Hoffmann-La Roche and Genentech sponsored the PePRS study. Dr. Cooper was a Genentech clinical research fellow at the time.
SOURCE: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The first clinical trial of rituximab for newly diagnosed or relapsing GPA or MPA in pediatric patients showed remission rates of 56%, 92%, and 100% by months 6, 12, and 18, respectively.
Study details: The PePRS study was a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries.
Disclosures: F. Hoffmann-La Roche and Genentech sponsored the study. The presenter was a Genentech clinical research fellow at the time.
Source: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.














