User login
Transcatheter aortic valve-in-ring for mitral disease a winner
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
REPORTING FROM TCT 2017
Key clinical point: .
Major finding: Thirty-day all-cause mortality following a transcatheter valve-in-ring procedure in unacceptably high surgical-risk patients with severe mitral valve disease due to a failing annuloplasty ring was 6.8%.
Study details: This prospective observational study included 60 patients who underwent transcatheter mitral valve replacement for severe mitral valve disease, half due to a failed annuloplasty ring and half secondary to mitral annular calcification.
Disclosures: The MITRAL trial was partially supported by Edwards Lifesciences. The study presenter reported receiving a research grant from the company.
Source: Guerrero M. No abstract.
Pendulum swings on mesenteric venous thrombosis treatment
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Carotid stenting isn’t safer than endarterectomy with contralateral carotid occlusion
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
bjancin@frontlinemedcom.com
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
New frontier in TAVR is bicuspid disease
DENVER – Thirty-day transcatheter aortic valve replacement (TAVR) outcomes in real-world clinical practice using the Evolut R self-expanding valve were as good in patients treated for bicuspid disease as for tricuspid disease, according to a retrospective analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) national registry.
“I’ve always been insecure about whether we have the right technology to be able to treat bicuspid disease. This registry data is reassuring to me that we might. I think it may be time to do a prospective registry for low-surgical-risk patients with bicuspid disease and see if we can emulate these kinds of results,” said Dr. Popma, the director of interventional cardiology at Beth Israel Deaconess Medical Center and a professor of medicine at Harvard Medical School, both in Boston.
“I think that the one limitation to recruitment in our low-risk TAVR trial is patients with bicuspid disease. Probably 25%-30% of low-risk patients are bicuspid, so we can’t include them right now in our low-risk trial,” he added at the meeting sponsored by the Cardiovascular Research Foundation.
Even though TAVR for patients with bicuspid disease is off-label, operators do perform the procedure. All of these cases are captured in the STS/ACC TVT registry. Dr. Popma reported on 6,717 patients who underwent TAVR with placement of the Evolut R valve at 305 U.S. centers during 2014-2016. The purpose of this retrospective study was to compare 30-day outcomes in the 191 TAVR patients with native valve bicuspid disease with the outcomes in the 6,526 with tricuspid disease.
The two groups were evenly matched in terms of key baseline characteristics, including aortic valve mean gradient, severity of aortic, mitral, and tricuspid regurgitation, and comorbid conditions – with the exception of coronary artery disease, which was present in 48% of the bicuspid group versus 65% of those with tricuspid disease. Also, the bicuspid disease group was younger by an average of nearly 9 years, and their mean baseline left ventricular ejection fraction of 52.5% was lower than the LVEF of 55.5% seen in the tricuspid group.
Procedure time averaged 126 minutes in the bicuspid group and 116 in the tricuspid group. Femoral access was utilized in 87% of the bicuspid patients and in 92% of tricuspid patients. The device was implanted successfully in 97% of the bicuspid group and in 99% of the tricuspid group. More than one valve was required in 3.7% of the bicuspid disease group, a rate similar to that in the tricuspid group. Total hospital length of stay was roughly 6 days in both groups.
Rates of symptomatic improvement at 30 days were closely similar in the two groups. Preprocedurally, two-thirds of patients in both groups had a New York Heart Association class III; at 30 days, however, that was true for a mere 2.4% of the bicuspid patients and 10.3% of the tricuspid patients. By day 30, 52% of the bicuspid group and 48% of the tricuspid group were NYHA class I.
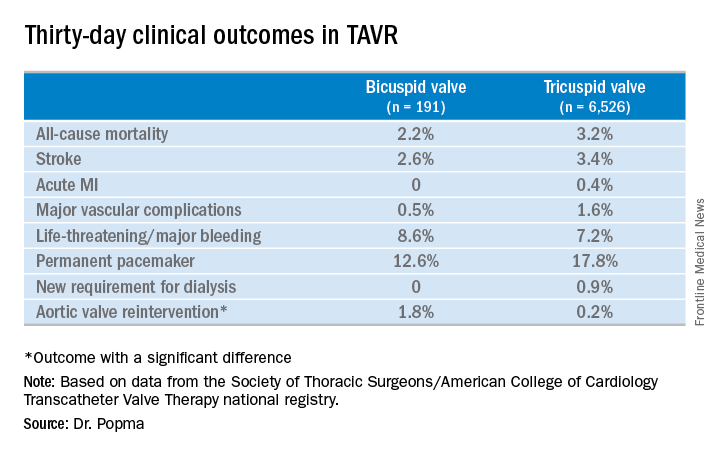
No or only trace aortic regurgitation was present at 30 days in 62% of the bicuspid group and in 61% of the tricuspid group, while mild aortic regurgitation was noted in 31% and 33%, respectively.
Thirty-day mean aortic valve gradient improved to a similar extent in the two groups: from a baseline of 47.2 mm Hg to 9.4 mm Hg in the bicuspid group and from 42.9 mm Hg to 7.5 mm Hg in the tricuspid group.
Dr. Popma noted that an earlier analysis he carried out comparing outcomes of TAVR using the earlier-generation CoreValve in bicuspid versus tricuspid disease showed suboptimal rates of paravalvular regurgitation and an increased need for multiple valves in the bicuspid group.
“The lesson is ‘Thank God we’ve got new technology!’ because the new technology has made a big difference for us,” the cardiologist observed. “We think that the advancement in the technique and the advancement in the valves is going to give us fairly comparable outcomes with Evolut in bicuspid and tricuspid patients.”
Discussant Hasan Jilaihawi, MD, a codirector of transcatheter valve therapy at New York University, pronounced the short-term outcomes in patients with bicuspid aortic valve disease “better than I would have expected,” adding that he, too, thinks it’s time for a prospective registry study of the Evolut valve in such patients.
Dr. Popma’s study was supported by Medtronic. He reported having received research grants from Medtronic and other medical device companies.
SOURCE: Popma JJ. TCT 2017.
DENVER – Thirty-day transcatheter aortic valve replacement (TAVR) outcomes in real-world clinical practice using the Evolut R self-expanding valve were as good in patients treated for bicuspid disease as for tricuspid disease, according to a retrospective analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) national registry.
“I’ve always been insecure about whether we have the right technology to be able to treat bicuspid disease. This registry data is reassuring to me that we might. I think it may be time to do a prospective registry for low-surgical-risk patients with bicuspid disease and see if we can emulate these kinds of results,” said Dr. Popma, the director of interventional cardiology at Beth Israel Deaconess Medical Center and a professor of medicine at Harvard Medical School, both in Boston.
“I think that the one limitation to recruitment in our low-risk TAVR trial is patients with bicuspid disease. Probably 25%-30% of low-risk patients are bicuspid, so we can’t include them right now in our low-risk trial,” he added at the meeting sponsored by the Cardiovascular Research Foundation.
Even though TAVR for patients with bicuspid disease is off-label, operators do perform the procedure. All of these cases are captured in the STS/ACC TVT registry. Dr. Popma reported on 6,717 patients who underwent TAVR with placement of the Evolut R valve at 305 U.S. centers during 2014-2016. The purpose of this retrospective study was to compare 30-day outcomes in the 191 TAVR patients with native valve bicuspid disease with the outcomes in the 6,526 with tricuspid disease.
The two groups were evenly matched in terms of key baseline characteristics, including aortic valve mean gradient, severity of aortic, mitral, and tricuspid regurgitation, and comorbid conditions – with the exception of coronary artery disease, which was present in 48% of the bicuspid group versus 65% of those with tricuspid disease. Also, the bicuspid disease group was younger by an average of nearly 9 years, and their mean baseline left ventricular ejection fraction of 52.5% was lower than the LVEF of 55.5% seen in the tricuspid group.
Procedure time averaged 126 minutes in the bicuspid group and 116 in the tricuspid group. Femoral access was utilized in 87% of the bicuspid patients and in 92% of tricuspid patients. The device was implanted successfully in 97% of the bicuspid group and in 99% of the tricuspid group. More than one valve was required in 3.7% of the bicuspid disease group, a rate similar to that in the tricuspid group. Total hospital length of stay was roughly 6 days in both groups.
Rates of symptomatic improvement at 30 days were closely similar in the two groups. Preprocedurally, two-thirds of patients in both groups had a New York Heart Association class III; at 30 days, however, that was true for a mere 2.4% of the bicuspid patients and 10.3% of the tricuspid patients. By day 30, 52% of the bicuspid group and 48% of the tricuspid group were NYHA class I.

No or only trace aortic regurgitation was present at 30 days in 62% of the bicuspid group and in 61% of the tricuspid group, while mild aortic regurgitation was noted in 31% and 33%, respectively.
Thirty-day mean aortic valve gradient improved to a similar extent in the two groups: from a baseline of 47.2 mm Hg to 9.4 mm Hg in the bicuspid group and from 42.9 mm Hg to 7.5 mm Hg in the tricuspid group.
Dr. Popma noted that an earlier analysis he carried out comparing outcomes of TAVR using the earlier-generation CoreValve in bicuspid versus tricuspid disease showed suboptimal rates of paravalvular regurgitation and an increased need for multiple valves in the bicuspid group.
“The lesson is ‘Thank God we’ve got new technology!’ because the new technology has made a big difference for us,” the cardiologist observed. “We think that the advancement in the technique and the advancement in the valves is going to give us fairly comparable outcomes with Evolut in bicuspid and tricuspid patients.”
Discussant Hasan Jilaihawi, MD, a codirector of transcatheter valve therapy at New York University, pronounced the short-term outcomes in patients with bicuspid aortic valve disease “better than I would have expected,” adding that he, too, thinks it’s time for a prospective registry study of the Evolut valve in such patients.
Dr. Popma’s study was supported by Medtronic. He reported having received research grants from Medtronic and other medical device companies.
SOURCE: Popma JJ. TCT 2017.
DENVER – Thirty-day transcatheter aortic valve replacement (TAVR) outcomes in real-world clinical practice using the Evolut R self-expanding valve were as good in patients treated for bicuspid disease as for tricuspid disease, according to a retrospective analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) national registry.
“I’ve always been insecure about whether we have the right technology to be able to treat bicuspid disease. This registry data is reassuring to me that we might. I think it may be time to do a prospective registry for low-surgical-risk patients with bicuspid disease and see if we can emulate these kinds of results,” said Dr. Popma, the director of interventional cardiology at Beth Israel Deaconess Medical Center and a professor of medicine at Harvard Medical School, both in Boston.
“I think that the one limitation to recruitment in our low-risk TAVR trial is patients with bicuspid disease. Probably 25%-30% of low-risk patients are bicuspid, so we can’t include them right now in our low-risk trial,” he added at the meeting sponsored by the Cardiovascular Research Foundation.
Even though TAVR for patients with bicuspid disease is off-label, operators do perform the procedure. All of these cases are captured in the STS/ACC TVT registry. Dr. Popma reported on 6,717 patients who underwent TAVR with placement of the Evolut R valve at 305 U.S. centers during 2014-2016. The purpose of this retrospective study was to compare 30-day outcomes in the 191 TAVR patients with native valve bicuspid disease with the outcomes in the 6,526 with tricuspid disease.
The two groups were evenly matched in terms of key baseline characteristics, including aortic valve mean gradient, severity of aortic, mitral, and tricuspid regurgitation, and comorbid conditions – with the exception of coronary artery disease, which was present in 48% of the bicuspid group versus 65% of those with tricuspid disease. Also, the bicuspid disease group was younger by an average of nearly 9 years, and their mean baseline left ventricular ejection fraction of 52.5% was lower than the LVEF of 55.5% seen in the tricuspid group.
Procedure time averaged 126 minutes in the bicuspid group and 116 in the tricuspid group. Femoral access was utilized in 87% of the bicuspid patients and in 92% of tricuspid patients. The device was implanted successfully in 97% of the bicuspid group and in 99% of the tricuspid group. More than one valve was required in 3.7% of the bicuspid disease group, a rate similar to that in the tricuspid group. Total hospital length of stay was roughly 6 days in both groups.
Rates of symptomatic improvement at 30 days were closely similar in the two groups. Preprocedurally, two-thirds of patients in both groups had a New York Heart Association class III; at 30 days, however, that was true for a mere 2.4% of the bicuspid patients and 10.3% of the tricuspid patients. By day 30, 52% of the bicuspid group and 48% of the tricuspid group were NYHA class I.

No or only trace aortic regurgitation was present at 30 days in 62% of the bicuspid group and in 61% of the tricuspid group, while mild aortic regurgitation was noted in 31% and 33%, respectively.
Thirty-day mean aortic valve gradient improved to a similar extent in the two groups: from a baseline of 47.2 mm Hg to 9.4 mm Hg in the bicuspid group and from 42.9 mm Hg to 7.5 mm Hg in the tricuspid group.
Dr. Popma noted that an earlier analysis he carried out comparing outcomes of TAVR using the earlier-generation CoreValve in bicuspid versus tricuspid disease showed suboptimal rates of paravalvular regurgitation and an increased need for multiple valves in the bicuspid group.
“The lesson is ‘Thank God we’ve got new technology!’ because the new technology has made a big difference for us,” the cardiologist observed. “We think that the advancement in the technique and the advancement in the valves is going to give us fairly comparable outcomes with Evolut in bicuspid and tricuspid patients.”
Discussant Hasan Jilaihawi, MD, a codirector of transcatheter valve therapy at New York University, pronounced the short-term outcomes in patients with bicuspid aortic valve disease “better than I would have expected,” adding that he, too, thinks it’s time for a prospective registry study of the Evolut valve in such patients.
Dr. Popma’s study was supported by Medtronic. He reported having received research grants from Medtronic and other medical device companies.
SOURCE: Popma JJ. TCT 2017.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: Thirty-day clinical outcomes and symptomatic improvement were reassuringly similar both in TAVR patients who received the Evolut R valve for tricuspid disease and off-label for bicuspid disease.
Study details: This was a retrospective U.S. national registry study comparing 30-day outcomes in 191 TAVR patients with native valve bicuspid disease and 6,526 with tricuspid disease, all of whom underwent TAVR with placement of the Evolut R valve.
Disclosures: The study presenter reported having received research grants from Medtronic, the study sponsor, as well as other medical device companies.
Source: Popma JJ. TCT 2017.
Vascular surgeons are top tier for burnout risk
CHICAGO – Vascular surgeons are solidly within the top tier of surgical subspecialists in terms of risk for burnout, Joan M. Anzia, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
Joining them in this unwelcome company with an elevated rate of lower quality of life are trauma surgeons, urologists, and otolaryngologists, according to the results of a 9-year-old national study of burnout and career satisfaction among American surgeons that has served as a wakeup call for the profession (Ann Surg. 2009 Sep;250[3]:463-71).
“This is where the biggest impact on burnout is going to be: institutional interventions to target the known drivers of burnout. Looking at nights on call, work compression, looking at the amount of time you guys spend in front of a computer documenting your EHR and your billing. Do you really need to do those things? You need help from midlevel professionals and others who can free you to practice at the top of your life, doing the work you love, which for most surgeons is being in the OR,” said Dr. Anzia, professor of psychiatry and behavioral sciences and the departmental vice chair of education at Northwestern University in Chicago.
The Society for Vascular Surgery is one of many professional specialty organizations that are focusing on the burnout problem. They are joined by the Association of American Medical Colleges, the Accreditation Council for Graduate Medical Education, the National Academy of Medicine, the Liaison Committee on Medical Education, the American Medical Association, and other interested groups.
“Since 2008, burnout rates in every specialty have increased by an average of an absolute 10%. That’s just remarkable, and it’s why people are very, very concerned,” noted the psychiatrist, who serves as the physician health liaison at Northwestern Memorial Hospital. In that capacity, she is frequently called upon to help physicians with the classic manifestations of burnout, including substance use disorders that arose as the practitioners tried to self-treat their burnout rather than seeking help.
“He reports only to the dean,” according to Dr. Anzia.
Why are vascular surgeons at such high risk for burnout? According to the Maslach Burnout Inventory, the leading psychological assessment tool for burnout, the syndrome has three main components: emotional exhaustion, a sense of loss of meaning in work, and feeling ineffective in one’s work. Studies show vascular surgeons often score high in all three domains.
Vascular surgeons’ work is extremely stressful. They average 20 hours per week in the OR, and almost 3 nights on call per week. They care for acutely ill patients and perform high-intensity, high-risk procedures in which unpredictable events are common.
“Work compression – not just workload, but facing multiple demands at once that you’re trying to balance – that’s one of the key drivers of burnout, and work compression is really common in vascular surgery,” Dr. Anzia noted.
In the national surgeon burnout study, younger surgeons and those with children still living at home were at increased risk for burnout. So were surgeons whose compensation was entirely based upon the Relative Value Unit system. The number of nights on call per week was another independent risk factor.
Dr. Shanafelt and his coinvestigators found that roughly 30% of respondent surgeons screened positive for depression, and 6.4% of the study population reported having suicidal thoughts within the past 12 months.
“We lose the equivalent of two to three medical school classes worth of physicians every year to suicide. And let me tell you: 98% of those folks, at the time they suicided, had major depression, which is eminently treatable. And the reason they weren’t treated was they, like most physicians, avoided treatment. They had difficulty accessing care. They were worried about stigma, life insurance, things like that. This is a huge problem which is mostly preventable, but we are not addressing it effectively,” Dr. Anzia said.
While institutional interventions aimed at the prevention of physician burnout such as spending less time on the electronic health record will have a major impact on the problem, thought leaders in medical education have come to realize that it also will be necessary to address the broader culture of medicine.
“There are so many implicit beliefs that every one of us grew up with, like ‘I work when I’m sick,’ or ‘I can work without sleep.’ All those things that we believe make us good physicians actually may not be entirely true,” the psychiatrist said.
She reported having no financial conflicts of interest regarding her presentation.
CHICAGO – Vascular surgeons are solidly within the top tier of surgical subspecialists in terms of risk for burnout, Joan M. Anzia, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
Joining them in this unwelcome company with an elevated rate of lower quality of life are trauma surgeons, urologists, and otolaryngologists, according to the results of a 9-year-old national study of burnout and career satisfaction among American surgeons that has served as a wakeup call for the profession (Ann Surg. 2009 Sep;250[3]:463-71).
“This is where the biggest impact on burnout is going to be: institutional interventions to target the known drivers of burnout. Looking at nights on call, work compression, looking at the amount of time you guys spend in front of a computer documenting your EHR and your billing. Do you really need to do those things? You need help from midlevel professionals and others who can free you to practice at the top of your life, doing the work you love, which for most surgeons is being in the OR,” said Dr. Anzia, professor of psychiatry and behavioral sciences and the departmental vice chair of education at Northwestern University in Chicago.
The Society for Vascular Surgery is one of many professional specialty organizations that are focusing on the burnout problem. They are joined by the Association of American Medical Colleges, the Accreditation Council for Graduate Medical Education, the National Academy of Medicine, the Liaison Committee on Medical Education, the American Medical Association, and other interested groups.
“Since 2008, burnout rates in every specialty have increased by an average of an absolute 10%. That’s just remarkable, and it’s why people are very, very concerned,” noted the psychiatrist, who serves as the physician health liaison at Northwestern Memorial Hospital. In that capacity, she is frequently called upon to help physicians with the classic manifestations of burnout, including substance use disorders that arose as the practitioners tried to self-treat their burnout rather than seeking help.
“He reports only to the dean,” according to Dr. Anzia.
Why are vascular surgeons at such high risk for burnout? According to the Maslach Burnout Inventory, the leading psychological assessment tool for burnout, the syndrome has three main components: emotional exhaustion, a sense of loss of meaning in work, and feeling ineffective in one’s work. Studies show vascular surgeons often score high in all three domains.
Vascular surgeons’ work is extremely stressful. They average 20 hours per week in the OR, and almost 3 nights on call per week. They care for acutely ill patients and perform high-intensity, high-risk procedures in which unpredictable events are common.
“Work compression – not just workload, but facing multiple demands at once that you’re trying to balance – that’s one of the key drivers of burnout, and work compression is really common in vascular surgery,” Dr. Anzia noted.
In the national surgeon burnout study, younger surgeons and those with children still living at home were at increased risk for burnout. So were surgeons whose compensation was entirely based upon the Relative Value Unit system. The number of nights on call per week was another independent risk factor.
Dr. Shanafelt and his coinvestigators found that roughly 30% of respondent surgeons screened positive for depression, and 6.4% of the study population reported having suicidal thoughts within the past 12 months.
“We lose the equivalent of two to three medical school classes worth of physicians every year to suicide. And let me tell you: 98% of those folks, at the time they suicided, had major depression, which is eminently treatable. And the reason they weren’t treated was they, like most physicians, avoided treatment. They had difficulty accessing care. They were worried about stigma, life insurance, things like that. This is a huge problem which is mostly preventable, but we are not addressing it effectively,” Dr. Anzia said.
While institutional interventions aimed at the prevention of physician burnout such as spending less time on the electronic health record will have a major impact on the problem, thought leaders in medical education have come to realize that it also will be necessary to address the broader culture of medicine.
“There are so many implicit beliefs that every one of us grew up with, like ‘I work when I’m sick,’ or ‘I can work without sleep.’ All those things that we believe make us good physicians actually may not be entirely true,” the psychiatrist said.
She reported having no financial conflicts of interest regarding her presentation.
CHICAGO – Vascular surgeons are solidly within the top tier of surgical subspecialists in terms of risk for burnout, Joan M. Anzia, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
Joining them in this unwelcome company with an elevated rate of lower quality of life are trauma surgeons, urologists, and otolaryngologists, according to the results of a 9-year-old national study of burnout and career satisfaction among American surgeons that has served as a wakeup call for the profession (Ann Surg. 2009 Sep;250[3]:463-71).
“This is where the biggest impact on burnout is going to be: institutional interventions to target the known drivers of burnout. Looking at nights on call, work compression, looking at the amount of time you guys spend in front of a computer documenting your EHR and your billing. Do you really need to do those things? You need help from midlevel professionals and others who can free you to practice at the top of your life, doing the work you love, which for most surgeons is being in the OR,” said Dr. Anzia, professor of psychiatry and behavioral sciences and the departmental vice chair of education at Northwestern University in Chicago.
The Society for Vascular Surgery is one of many professional specialty organizations that are focusing on the burnout problem. They are joined by the Association of American Medical Colleges, the Accreditation Council for Graduate Medical Education, the National Academy of Medicine, the Liaison Committee on Medical Education, the American Medical Association, and other interested groups.
“Since 2008, burnout rates in every specialty have increased by an average of an absolute 10%. That’s just remarkable, and it’s why people are very, very concerned,” noted the psychiatrist, who serves as the physician health liaison at Northwestern Memorial Hospital. In that capacity, she is frequently called upon to help physicians with the classic manifestations of burnout, including substance use disorders that arose as the practitioners tried to self-treat their burnout rather than seeking help.
“He reports only to the dean,” according to Dr. Anzia.
Why are vascular surgeons at such high risk for burnout? According to the Maslach Burnout Inventory, the leading psychological assessment tool for burnout, the syndrome has three main components: emotional exhaustion, a sense of loss of meaning in work, and feeling ineffective in one’s work. Studies show vascular surgeons often score high in all three domains.
Vascular surgeons’ work is extremely stressful. They average 20 hours per week in the OR, and almost 3 nights on call per week. They care for acutely ill patients and perform high-intensity, high-risk procedures in which unpredictable events are common.
“Work compression – not just workload, but facing multiple demands at once that you’re trying to balance – that’s one of the key drivers of burnout, and work compression is really common in vascular surgery,” Dr. Anzia noted.
In the national surgeon burnout study, younger surgeons and those with children still living at home were at increased risk for burnout. So were surgeons whose compensation was entirely based upon the Relative Value Unit system. The number of nights on call per week was another independent risk factor.
Dr. Shanafelt and his coinvestigators found that roughly 30% of respondent surgeons screened positive for depression, and 6.4% of the study population reported having suicidal thoughts within the past 12 months.
“We lose the equivalent of two to three medical school classes worth of physicians every year to suicide. And let me tell you: 98% of those folks, at the time they suicided, had major depression, which is eminently treatable. And the reason they weren’t treated was they, like most physicians, avoided treatment. They had difficulty accessing care. They were worried about stigma, life insurance, things like that. This is a huge problem which is mostly preventable, but we are not addressing it effectively,” Dr. Anzia said.
While institutional interventions aimed at the prevention of physician burnout such as spending less time on the electronic health record will have a major impact on the problem, thought leaders in medical education have come to realize that it also will be necessary to address the broader culture of medicine.
“There are so many implicit beliefs that every one of us grew up with, like ‘I work when I’m sick,’ or ‘I can work without sleep.’ All those things that we believe make us good physicians actually may not be entirely true,” the psychiatrist said.
She reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
DAPT duration: How low can you go?
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The composite endpoint of death, MI, stroke, revascularization, and major bleeding 24 months after primary PCI with a second-generation DES was 6.6% in patients who got the standard 12 months of DAPT and 4.8% in those who got 6.
Study details: A prospective randomized international study that enrolled 1,100 STEMI patients who underwent primary PCI.
Disclosures: The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. The presenter reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
Source: Kedhi, E. No abstract.
Who fares best after successful ECT?
PARIS – , Pascal Sienaert, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This conclusion is based on the results of two prospective studies by ResPECT – the Research in Psychiatry and ECT by the Flemish-Dutch Research Consortium – which, in turn, confirm the findings of an earlier metaanalysis of 32 studies including 702 patients conducted by investigators at Trinity College Dublin, noted Dr. Sienaert, a psychiatrist at the Catholic University of Leuven (Belgium) Academic Center for ECT and Neuromodulation.
Dr. Sienaert noted that the relapse rate in the ECT metaanalysis is nearly identical to that reported in the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial in real-world patients with major depression who achieved remission in response to second-step or later antidepressant medication.
“It’s a common misconception that relapse is higher after ECT than medication,” the psychiatrist said.
In the ECT metaanalysis, continuation ECT after induction of remission did not substantially affect the relapse risk. But that’s because the prevailing maintenance ECT strategy in the studies included in the 2013 metaanalysis relied upon a fixed-dose treatment schedule, according to Dr. Sienaert.
“In most studies, fixed-schedule maintenance ECT is used and with rather high relapse rates. Most clinicians have the experience that flexible, clinically driven on an as-needed-basis maintenance ECT has lower relapse rates,” he said. “Still, relapse remains the most pressing issue in the field, and it is very difficult for us as clinicians to predict which patients will relapse and which will not.”
That’s where the two ResPECT studies come into play.
In one of the studies, 116 patients with major depression at three tertiary psychiatric hospitals were randomized double blind to twice-weekly high-dose ultrabrief pulse (0.3-0.4 milliseconds) right unilateral or high-dose brief pulse (1.0 millisecond) right unilateral ECT. The dosing was at eight times the seizure threshold until remission as defined by a Montgomery-Åsberg Depression Rating Scale (MADRS) score below 10 or for a maximum of 6 weeks. Among the 87 completers, the remission rate was 68% in the brief pulse group, significantly higher than the 49% rate with ultrabrief ECT. Cognitive effects on semantic and lexical memory, and retrograde amnesia were the same in the two groups (J Clin Psychiatry. 2013 Nov;74[11]:e1029-36).
Dr. Sienaert and his coinvestigators then prospectively followed the 50 remitters for 6 months, during which all but one patient remained on antidepressant medication. The relapse rate, defined as rehospitalization for depression, restart of ECT, suicide, or a MADRS score above 15, was 25% at 3 months and about 40% at 6 months. The investigators found several predictors of a lower relapse rate. The strongest was early complete remission as defined by a Clinical Global Impressions Scale score of 1 out of a maximum of a possible 7 points within the first four ECT sessions: The 6-month relapse rate was 10% among those early complete remitters versus 63% in the other remitters (J Affect Disord. 2015 Sep 15;184:137-44).
“These are very small numbers in these groups, but the signal that emerges is the same as we have seen in the Irish metaanalysis: Early complete remitters were older, had shorter current episodes of depression, and showed more baseline psychotic features,” Dr. Sienaert said.
In a more recent ResPECT consortium study, the Mood Disorders in Elderly Treated With ECT (MODECT) study, 110 patients aged 55 and older with unipolar depression treated by ECT were followed with serial brain imaging studies prior to and for 6 months post treatment in an effort to gain insight into the mechanism of the particularly strong benefit of ECT in late-life depression. The response rate to ECT was significantly higher in those with onset of depression at age 55 or older than in those with disease onset before age 55, by a margin of 87% vs. 67%. The presence of baseline psychotic symptoms also was associated with a higher response rate.
In contrast, treatment response proved unrelated to changes in hippocampal volume, white matter hypersensitivities, amyloid load, or serum brain-derived neurotrophic factor, which is believed to be an important mediator of neuroplasticity. Thus, ECT’s mechanism of action in late-life depression remains elusive, the authors reported (Am J Geriatr Psychiatry. 2017 Feb;25[2]:178-89).
In a separate study, Dr. Sienaert and his colleagues found that ECT’s superior efficacy, compared with antidepressant medication in patients with late-life depression, was independent of their vascular disease burden. The study population was comprised of 81 patients in an antidepressant drug trial and 43 in an ECT trial, all of whom were inpatients with unipolar major depression. Their mean age was in the mid-70s.
The investigators gauged vascular burden by adding up each patient’s number of vascular risk factors, namely, diabetes, hypertension, smoking, hypercholesterolemia, known cardiovascular disease, and cerebrovascular disease. The depression remission rate was 80% in the ECT patients with no vascular risk factors, dropping to 58% in those with one or more. In the antidepressant drug trial participants, the remission rate was 38% in those with no vascular risk factors, compared with 32% in patients with one or more. Using different cutoffs for the number of vascular risk factors did not significantly alter the results (Int J Geriatr Psychiatry. 2017 Jun 28. doi: 10.1002/gps.4754).
At present, once a patient has achieved remission in response to ECT, most psychiatrists stop the therapy altogether. That’s often a mistake, according to session cochair Eduard Vieta, MD, PhD.
“. In many cases you need to continue ECT. Especially in patients who are refractory or treatment resistant, I don’t see a reason why maintenance ECT shouldn’t be the first choice. Yet in the guidelines, ECT is always the third- or fourth-line therapy,” said Dr. Vieta, professor of psychiatry at the University of Barcelona and scientific director of the Spanish Research Network on Mental Diseases.
Dr. Sienaert concurred, adding that he has patients who are on weekly maintenance ECT for as long as 16 years, with continued good results.
He reported having received honoraria from Mecta, a manufacturer of ECT equipment.
bjancin@frontlinemedcom.com
PARIS – , Pascal Sienaert, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This conclusion is based on the results of two prospective studies by ResPECT – the Research in Psychiatry and ECT by the Flemish-Dutch Research Consortium – which, in turn, confirm the findings of an earlier metaanalysis of 32 studies including 702 patients conducted by investigators at Trinity College Dublin, noted Dr. Sienaert, a psychiatrist at the Catholic University of Leuven (Belgium) Academic Center for ECT and Neuromodulation.
Dr. Sienaert noted that the relapse rate in the ECT metaanalysis is nearly identical to that reported in the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial in real-world patients with major depression who achieved remission in response to second-step or later antidepressant medication.
“It’s a common misconception that relapse is higher after ECT than medication,” the psychiatrist said.
In the ECT metaanalysis, continuation ECT after induction of remission did not substantially affect the relapse risk. But that’s because the prevailing maintenance ECT strategy in the studies included in the 2013 metaanalysis relied upon a fixed-dose treatment schedule, according to Dr. Sienaert.
“In most studies, fixed-schedule maintenance ECT is used and with rather high relapse rates. Most clinicians have the experience that flexible, clinically driven on an as-needed-basis maintenance ECT has lower relapse rates,” he said. “Still, relapse remains the most pressing issue in the field, and it is very difficult for us as clinicians to predict which patients will relapse and which will not.”
That’s where the two ResPECT studies come into play.
In one of the studies, 116 patients with major depression at three tertiary psychiatric hospitals were randomized double blind to twice-weekly high-dose ultrabrief pulse (0.3-0.4 milliseconds) right unilateral or high-dose brief pulse (1.0 millisecond) right unilateral ECT. The dosing was at eight times the seizure threshold until remission as defined by a Montgomery-Åsberg Depression Rating Scale (MADRS) score below 10 or for a maximum of 6 weeks. Among the 87 completers, the remission rate was 68% in the brief pulse group, significantly higher than the 49% rate with ultrabrief ECT. Cognitive effects on semantic and lexical memory, and retrograde amnesia were the same in the two groups (J Clin Psychiatry. 2013 Nov;74[11]:e1029-36).
Dr. Sienaert and his coinvestigators then prospectively followed the 50 remitters for 6 months, during which all but one patient remained on antidepressant medication. The relapse rate, defined as rehospitalization for depression, restart of ECT, suicide, or a MADRS score above 15, was 25% at 3 months and about 40% at 6 months. The investigators found several predictors of a lower relapse rate. The strongest was early complete remission as defined by a Clinical Global Impressions Scale score of 1 out of a maximum of a possible 7 points within the first four ECT sessions: The 6-month relapse rate was 10% among those early complete remitters versus 63% in the other remitters (J Affect Disord. 2015 Sep 15;184:137-44).
“These are very small numbers in these groups, but the signal that emerges is the same as we have seen in the Irish metaanalysis: Early complete remitters were older, had shorter current episodes of depression, and showed more baseline psychotic features,” Dr. Sienaert said.
In a more recent ResPECT consortium study, the Mood Disorders in Elderly Treated With ECT (MODECT) study, 110 patients aged 55 and older with unipolar depression treated by ECT were followed with serial brain imaging studies prior to and for 6 months post treatment in an effort to gain insight into the mechanism of the particularly strong benefit of ECT in late-life depression. The response rate to ECT was significantly higher in those with onset of depression at age 55 or older than in those with disease onset before age 55, by a margin of 87% vs. 67%. The presence of baseline psychotic symptoms also was associated with a higher response rate.
In contrast, treatment response proved unrelated to changes in hippocampal volume, white matter hypersensitivities, amyloid load, or serum brain-derived neurotrophic factor, which is believed to be an important mediator of neuroplasticity. Thus, ECT’s mechanism of action in late-life depression remains elusive, the authors reported (Am J Geriatr Psychiatry. 2017 Feb;25[2]:178-89).
In a separate study, Dr. Sienaert and his colleagues found that ECT’s superior efficacy, compared with antidepressant medication in patients with late-life depression, was independent of their vascular disease burden. The study population was comprised of 81 patients in an antidepressant drug trial and 43 in an ECT trial, all of whom were inpatients with unipolar major depression. Their mean age was in the mid-70s.
The investigators gauged vascular burden by adding up each patient’s number of vascular risk factors, namely, diabetes, hypertension, smoking, hypercholesterolemia, known cardiovascular disease, and cerebrovascular disease. The depression remission rate was 80% in the ECT patients with no vascular risk factors, dropping to 58% in those with one or more. In the antidepressant drug trial participants, the remission rate was 38% in those with no vascular risk factors, compared with 32% in patients with one or more. Using different cutoffs for the number of vascular risk factors did not significantly alter the results (Int J Geriatr Psychiatry. 2017 Jun 28. doi: 10.1002/gps.4754).
At present, once a patient has achieved remission in response to ECT, most psychiatrists stop the therapy altogether. That’s often a mistake, according to session cochair Eduard Vieta, MD, PhD.
“. In many cases you need to continue ECT. Especially in patients who are refractory or treatment resistant, I don’t see a reason why maintenance ECT shouldn’t be the first choice. Yet in the guidelines, ECT is always the third- or fourth-line therapy,” said Dr. Vieta, professor of psychiatry at the University of Barcelona and scientific director of the Spanish Research Network on Mental Diseases.
Dr. Sienaert concurred, adding that he has patients who are on weekly maintenance ECT for as long as 16 years, with continued good results.
He reported having received honoraria from Mecta, a manufacturer of ECT equipment.
bjancin@frontlinemedcom.com
PARIS – , Pascal Sienaert, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This conclusion is based on the results of two prospective studies by ResPECT – the Research in Psychiatry and ECT by the Flemish-Dutch Research Consortium – which, in turn, confirm the findings of an earlier metaanalysis of 32 studies including 702 patients conducted by investigators at Trinity College Dublin, noted Dr. Sienaert, a psychiatrist at the Catholic University of Leuven (Belgium) Academic Center for ECT and Neuromodulation.
Dr. Sienaert noted that the relapse rate in the ECT metaanalysis is nearly identical to that reported in the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial in real-world patients with major depression who achieved remission in response to second-step or later antidepressant medication.
“It’s a common misconception that relapse is higher after ECT than medication,” the psychiatrist said.
In the ECT metaanalysis, continuation ECT after induction of remission did not substantially affect the relapse risk. But that’s because the prevailing maintenance ECT strategy in the studies included in the 2013 metaanalysis relied upon a fixed-dose treatment schedule, according to Dr. Sienaert.
“In most studies, fixed-schedule maintenance ECT is used and with rather high relapse rates. Most clinicians have the experience that flexible, clinically driven on an as-needed-basis maintenance ECT has lower relapse rates,” he said. “Still, relapse remains the most pressing issue in the field, and it is very difficult for us as clinicians to predict which patients will relapse and which will not.”
That’s where the two ResPECT studies come into play.
In one of the studies, 116 patients with major depression at three tertiary psychiatric hospitals were randomized double blind to twice-weekly high-dose ultrabrief pulse (0.3-0.4 milliseconds) right unilateral or high-dose brief pulse (1.0 millisecond) right unilateral ECT. The dosing was at eight times the seizure threshold until remission as defined by a Montgomery-Åsberg Depression Rating Scale (MADRS) score below 10 or for a maximum of 6 weeks. Among the 87 completers, the remission rate was 68% in the brief pulse group, significantly higher than the 49% rate with ultrabrief ECT. Cognitive effects on semantic and lexical memory, and retrograde amnesia were the same in the two groups (J Clin Psychiatry. 2013 Nov;74[11]:e1029-36).
Dr. Sienaert and his coinvestigators then prospectively followed the 50 remitters for 6 months, during which all but one patient remained on antidepressant medication. The relapse rate, defined as rehospitalization for depression, restart of ECT, suicide, or a MADRS score above 15, was 25% at 3 months and about 40% at 6 months. The investigators found several predictors of a lower relapse rate. The strongest was early complete remission as defined by a Clinical Global Impressions Scale score of 1 out of a maximum of a possible 7 points within the first four ECT sessions: The 6-month relapse rate was 10% among those early complete remitters versus 63% in the other remitters (J Affect Disord. 2015 Sep 15;184:137-44).
“These are very small numbers in these groups, but the signal that emerges is the same as we have seen in the Irish metaanalysis: Early complete remitters were older, had shorter current episodes of depression, and showed more baseline psychotic features,” Dr. Sienaert said.
In a more recent ResPECT consortium study, the Mood Disorders in Elderly Treated With ECT (MODECT) study, 110 patients aged 55 and older with unipolar depression treated by ECT were followed with serial brain imaging studies prior to and for 6 months post treatment in an effort to gain insight into the mechanism of the particularly strong benefit of ECT in late-life depression. The response rate to ECT was significantly higher in those with onset of depression at age 55 or older than in those with disease onset before age 55, by a margin of 87% vs. 67%. The presence of baseline psychotic symptoms also was associated with a higher response rate.
In contrast, treatment response proved unrelated to changes in hippocampal volume, white matter hypersensitivities, amyloid load, or serum brain-derived neurotrophic factor, which is believed to be an important mediator of neuroplasticity. Thus, ECT’s mechanism of action in late-life depression remains elusive, the authors reported (Am J Geriatr Psychiatry. 2017 Feb;25[2]:178-89).
In a separate study, Dr. Sienaert and his colleagues found that ECT’s superior efficacy, compared with antidepressant medication in patients with late-life depression, was independent of their vascular disease burden. The study population was comprised of 81 patients in an antidepressant drug trial and 43 in an ECT trial, all of whom were inpatients with unipolar major depression. Their mean age was in the mid-70s.
The investigators gauged vascular burden by adding up each patient’s number of vascular risk factors, namely, diabetes, hypertension, smoking, hypercholesterolemia, known cardiovascular disease, and cerebrovascular disease. The depression remission rate was 80% in the ECT patients with no vascular risk factors, dropping to 58% in those with one or more. In the antidepressant drug trial participants, the remission rate was 38% in those with no vascular risk factors, compared with 32% in patients with one or more. Using different cutoffs for the number of vascular risk factors did not significantly alter the results (Int J Geriatr Psychiatry. 2017 Jun 28. doi: 10.1002/gps.4754).
At present, once a patient has achieved remission in response to ECT, most psychiatrists stop the therapy altogether. That’s often a mistake, according to session cochair Eduard Vieta, MD, PhD.
“. In many cases you need to continue ECT. Especially in patients who are refractory or treatment resistant, I don’t see a reason why maintenance ECT shouldn’t be the first choice. Yet in the guidelines, ECT is always the third- or fourth-line therapy,” said Dr. Vieta, professor of psychiatry at the University of Barcelona and scientific director of the Spanish Research Network on Mental Diseases.
Dr. Sienaert concurred, adding that he has patients who are on weekly maintenance ECT for as long as 16 years, with continued good results.
He reported having received honoraria from Mecta, a manufacturer of ECT equipment.
bjancin@frontlinemedcom.com
REPORTING FROM THE ECNP CONGRESS
Huge AWARE study shows chronic spontaneous urticaria is seriously undertreated
GENEVA – Partial results of the first worldwide observational study on the burden of chronic spontaneous urticaria (CSU) and its treatment in real-world clinical practice can be summarized succinctly: “It’s worse than we thought,” Marcus Maurer, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We knew it was bad, but what comes out of looking at the daily life and treatment patterns in patients with CSU is a nightmare,” said Dr. Maurer, professor of dermatology and allergy and director of research in the department of dermatology at Charite University Hospital in Berlin.
Dr. Maurer presented the year-1 results in 1,550 AWARE participants at 256 sites across Germany. The results, he said, are sobering and reflective of what is being found in the other participating countries. The findings document a low level of adherence to the published treatment guidelines, and a lot of miserable CSU patients.
“My talk here is a clear call to action,” he declared. “The AWARE study shows we have two problems. One, patients don’t get treatment – and we can solve that. And number two, patients don’t get the right treatment. And the right treatment is treatment that controls the disease. In the guidelines we say, ‘Treat the disease until it is gone.’ That’s a great sentence. It means we should give enough treatment to control the disease: as much as needed, as little as possible, and in that order. First we establish control, then we have all the time in the world to find a treatment that gets patients to stay that way in the long run.”
He is a coauthor of the guidelines, a joint initiative of the European Academy of Allergy and Clinical Immunology, the EU-funded Global Allergy and Asthma European Network of centers of excellence, the European Dermatology Forum, and the World Allergy Organization (Allergy. 2014 Jul;69[7]:868-87).
He asked a packed hall of dermatologists how many of them like taking care of patients with CSU. One lonely hand went up. Dr. Maurer was unsurprised.
“, and it’s one where we struggle. No question, we struggle with these patients, maybe more so than with our psoriasis and atopic dermatitis patients. But the good news is there are new concepts of this disease and new treatment approaches,” he said. “We know that in 98% of patients, using the guideline algorithm, we can establish control in CSU.”
He summarized the joint guideline algorithm as follows: Routinely use a simple validated assessment tool such as the patient-reported Urticaria Control Test (UCT) (J Allergy Clin Immunol. 2014 May;133[5]:1365-72) to determine a CSU patient’s burden of disease, prescribe second-generation, nonsedating H1 antihistamines as first-line therapy, updose those agents to up to four times standard dosing as second-line therapy if the follow-up UCT results show the disease is still uncontrolled, and if it remains uncontrolled then move on to third-line treatment with add-on omalizumab (Xolair) or a maximum of 10 days of montelukast (Singulair) or cyclosporine.
When he asked how many audience members use the UCT, which assesses disease control over the previous 4 weeks, a mere handful responded affirmatively. “Please,” he urged his colleagues, “this test is so simple. It’s four questions, it’s free, it’s available online in more than 30 languages. It allows us to identify patients with uncontrolled disease. We have the ability to control the disease now, so let use this test. Patients can fill it out quickly in your waiting room so you can monitor how good your treatment is.”
AWARE study bears bad news
At study enrollment, the 1,550 German participants in the AWARE study had been diagnosed with CSU a mean of 4.9 years previously. Yet fully 78% had uncontrolled CSU as defined by a score below 12 points on the 0-16 UCT. Forty-four percent of patients had experienced angioedema within the previous 6 months, and 56% percent of patients had a baseline Dermatology Life Quality Index (DLQI) score indicating CSU had a moderate, very large, or extremely large effect on their quality of life. Yet 36% of patients weren’t receiving any treatment at baseline, and only 6.5% of patients were receiving guideline-recommended add-on third-line therapy.
In particular, most physicians fail to appreciate the negative impact of angioedema in CSU patients.
“This is not a swollen lip just once a year, this is angioedema weekly or monthly that really impairs quality of life. It’s not just a swollen lip or a swollen eye for the day or for a couple of hours, it’s the fear of going to sleep because in the morning you may wake up looking like that and not be able to go to work,” Dr. Maurer pointed out.
During the first year of the study, the overall situation improved significantly over the course of office visits every 3 months. The prevalence of DLQI scores indicative of a moderate or worse quality of life impact gradually fell from 56% to 28%. The proportion of patients on third-line treatment rose stepwise to 29.5% at 1 year. The rate of uncontrolled CSU as defined by a UCT score below 12 dropped steadily over time to 42%. At 1 year, only 16% of patients reported having an angioedema episode within the prior 12 weeks.
Still, this leaves much room for further improvement, Dr. Maurer commented.
“We’re on the right track, but patients who are in our treatment for a year should have their disease controlled. That’s our job. We have the treatments, and we should provide them. Even after 1 year there are still patients with a DLQI score above 10, and that’s not good,” he said.
Dr. Maurer was first author of the recently published interim results of the German portion of the AWARE study (Clin Exp Allergy. 2017 May;47[5]:684-52). The AWARE study is sponsored by Novartis, manufacturer of omalizumab, which is approved by the Food and Drug Administration for treating CSU. Dr. Maurer reported receiving research grants from and serving as an advisor to and paid speaker for Novartis and numerous other pharmaceutical companies.
bjancin@frontlinemedcom.com
GENEVA – Partial results of the first worldwide observational study on the burden of chronic spontaneous urticaria (CSU) and its treatment in real-world clinical practice can be summarized succinctly: “It’s worse than we thought,” Marcus Maurer, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We knew it was bad, but what comes out of looking at the daily life and treatment patterns in patients with CSU is a nightmare,” said Dr. Maurer, professor of dermatology and allergy and director of research in the department of dermatology at Charite University Hospital in Berlin.
Dr. Maurer presented the year-1 results in 1,550 AWARE participants at 256 sites across Germany. The results, he said, are sobering and reflective of what is being found in the other participating countries. The findings document a low level of adherence to the published treatment guidelines, and a lot of miserable CSU patients.
“My talk here is a clear call to action,” he declared. “The AWARE study shows we have two problems. One, patients don’t get treatment – and we can solve that. And number two, patients don’t get the right treatment. And the right treatment is treatment that controls the disease. In the guidelines we say, ‘Treat the disease until it is gone.’ That’s a great sentence. It means we should give enough treatment to control the disease: as much as needed, as little as possible, and in that order. First we establish control, then we have all the time in the world to find a treatment that gets patients to stay that way in the long run.”
He is a coauthor of the guidelines, a joint initiative of the European Academy of Allergy and Clinical Immunology, the EU-funded Global Allergy and Asthma European Network of centers of excellence, the European Dermatology Forum, and the World Allergy Organization (Allergy. 2014 Jul;69[7]:868-87).
He asked a packed hall of dermatologists how many of them like taking care of patients with CSU. One lonely hand went up. Dr. Maurer was unsurprised.
“, and it’s one where we struggle. No question, we struggle with these patients, maybe more so than with our psoriasis and atopic dermatitis patients. But the good news is there are new concepts of this disease and new treatment approaches,” he said. “We know that in 98% of patients, using the guideline algorithm, we can establish control in CSU.”
He summarized the joint guideline algorithm as follows: Routinely use a simple validated assessment tool such as the patient-reported Urticaria Control Test (UCT) (J Allergy Clin Immunol. 2014 May;133[5]:1365-72) to determine a CSU patient’s burden of disease, prescribe second-generation, nonsedating H1 antihistamines as first-line therapy, updose those agents to up to four times standard dosing as second-line therapy if the follow-up UCT results show the disease is still uncontrolled, and if it remains uncontrolled then move on to third-line treatment with add-on omalizumab (Xolair) or a maximum of 10 days of montelukast (Singulair) or cyclosporine.
When he asked how many audience members use the UCT, which assesses disease control over the previous 4 weeks, a mere handful responded affirmatively. “Please,” he urged his colleagues, “this test is so simple. It’s four questions, it’s free, it’s available online in more than 30 languages. It allows us to identify patients with uncontrolled disease. We have the ability to control the disease now, so let use this test. Patients can fill it out quickly in your waiting room so you can monitor how good your treatment is.”
AWARE study bears bad news
At study enrollment, the 1,550 German participants in the AWARE study had been diagnosed with CSU a mean of 4.9 years previously. Yet fully 78% had uncontrolled CSU as defined by a score below 12 points on the 0-16 UCT. Forty-four percent of patients had experienced angioedema within the previous 6 months, and 56% percent of patients had a baseline Dermatology Life Quality Index (DLQI) score indicating CSU had a moderate, very large, or extremely large effect on their quality of life. Yet 36% of patients weren’t receiving any treatment at baseline, and only 6.5% of patients were receiving guideline-recommended add-on third-line therapy.
In particular, most physicians fail to appreciate the negative impact of angioedema in CSU patients.
“This is not a swollen lip just once a year, this is angioedema weekly or monthly that really impairs quality of life. It’s not just a swollen lip or a swollen eye for the day or for a couple of hours, it’s the fear of going to sleep because in the morning you may wake up looking like that and not be able to go to work,” Dr. Maurer pointed out.
During the first year of the study, the overall situation improved significantly over the course of office visits every 3 months. The prevalence of DLQI scores indicative of a moderate or worse quality of life impact gradually fell from 56% to 28%. The proportion of patients on third-line treatment rose stepwise to 29.5% at 1 year. The rate of uncontrolled CSU as defined by a UCT score below 12 dropped steadily over time to 42%. At 1 year, only 16% of patients reported having an angioedema episode within the prior 12 weeks.
Still, this leaves much room for further improvement, Dr. Maurer commented.
“We’re on the right track, but patients who are in our treatment for a year should have their disease controlled. That’s our job. We have the treatments, and we should provide them. Even after 1 year there are still patients with a DLQI score above 10, and that’s not good,” he said.
Dr. Maurer was first author of the recently published interim results of the German portion of the AWARE study (Clin Exp Allergy. 2017 May;47[5]:684-52). The AWARE study is sponsored by Novartis, manufacturer of omalizumab, which is approved by the Food and Drug Administration for treating CSU. Dr. Maurer reported receiving research grants from and serving as an advisor to and paid speaker for Novartis and numerous other pharmaceutical companies.
bjancin@frontlinemedcom.com
GENEVA – Partial results of the first worldwide observational study on the burden of chronic spontaneous urticaria (CSU) and its treatment in real-world clinical practice can be summarized succinctly: “It’s worse than we thought,” Marcus Maurer, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We knew it was bad, but what comes out of looking at the daily life and treatment patterns in patients with CSU is a nightmare,” said Dr. Maurer, professor of dermatology and allergy and director of research in the department of dermatology at Charite University Hospital in Berlin.
Dr. Maurer presented the year-1 results in 1,550 AWARE participants at 256 sites across Germany. The results, he said, are sobering and reflective of what is being found in the other participating countries. The findings document a low level of adherence to the published treatment guidelines, and a lot of miserable CSU patients.
“My talk here is a clear call to action,” he declared. “The AWARE study shows we have two problems. One, patients don’t get treatment – and we can solve that. And number two, patients don’t get the right treatment. And the right treatment is treatment that controls the disease. In the guidelines we say, ‘Treat the disease until it is gone.’ That’s a great sentence. It means we should give enough treatment to control the disease: as much as needed, as little as possible, and in that order. First we establish control, then we have all the time in the world to find a treatment that gets patients to stay that way in the long run.”
He is a coauthor of the guidelines, a joint initiative of the European Academy of Allergy and Clinical Immunology, the EU-funded Global Allergy and Asthma European Network of centers of excellence, the European Dermatology Forum, and the World Allergy Organization (Allergy. 2014 Jul;69[7]:868-87).
He asked a packed hall of dermatologists how many of them like taking care of patients with CSU. One lonely hand went up. Dr. Maurer was unsurprised.
“, and it’s one where we struggle. No question, we struggle with these patients, maybe more so than with our psoriasis and atopic dermatitis patients. But the good news is there are new concepts of this disease and new treatment approaches,” he said. “We know that in 98% of patients, using the guideline algorithm, we can establish control in CSU.”
He summarized the joint guideline algorithm as follows: Routinely use a simple validated assessment tool such as the patient-reported Urticaria Control Test (UCT) (J Allergy Clin Immunol. 2014 May;133[5]:1365-72) to determine a CSU patient’s burden of disease, prescribe second-generation, nonsedating H1 antihistamines as first-line therapy, updose those agents to up to four times standard dosing as second-line therapy if the follow-up UCT results show the disease is still uncontrolled, and if it remains uncontrolled then move on to third-line treatment with add-on omalizumab (Xolair) or a maximum of 10 days of montelukast (Singulair) or cyclosporine.
When he asked how many audience members use the UCT, which assesses disease control over the previous 4 weeks, a mere handful responded affirmatively. “Please,” he urged his colleagues, “this test is so simple. It’s four questions, it’s free, it’s available online in more than 30 languages. It allows us to identify patients with uncontrolled disease. We have the ability to control the disease now, so let use this test. Patients can fill it out quickly in your waiting room so you can monitor how good your treatment is.”
AWARE study bears bad news
At study enrollment, the 1,550 German participants in the AWARE study had been diagnosed with CSU a mean of 4.9 years previously. Yet fully 78% had uncontrolled CSU as defined by a score below 12 points on the 0-16 UCT. Forty-four percent of patients had experienced angioedema within the previous 6 months, and 56% percent of patients had a baseline Dermatology Life Quality Index (DLQI) score indicating CSU had a moderate, very large, or extremely large effect on their quality of life. Yet 36% of patients weren’t receiving any treatment at baseline, and only 6.5% of patients were receiving guideline-recommended add-on third-line therapy.
In particular, most physicians fail to appreciate the negative impact of angioedema in CSU patients.
“This is not a swollen lip just once a year, this is angioedema weekly or monthly that really impairs quality of life. It’s not just a swollen lip or a swollen eye for the day or for a couple of hours, it’s the fear of going to sleep because in the morning you may wake up looking like that and not be able to go to work,” Dr. Maurer pointed out.
During the first year of the study, the overall situation improved significantly over the course of office visits every 3 months. The prevalence of DLQI scores indicative of a moderate or worse quality of life impact gradually fell from 56% to 28%. The proportion of patients on third-line treatment rose stepwise to 29.5% at 1 year. The rate of uncontrolled CSU as defined by a UCT score below 12 dropped steadily over time to 42%. At 1 year, only 16% of patients reported having an angioedema episode within the prior 12 weeks.
Still, this leaves much room for further improvement, Dr. Maurer commented.
“We’re on the right track, but patients who are in our treatment for a year should have their disease controlled. That’s our job. We have the treatments, and we should provide them. Even after 1 year there are still patients with a DLQI score above 10, and that’s not good,” he said.
Dr. Maurer was first author of the recently published interim results of the German portion of the AWARE study (Clin Exp Allergy. 2017 May;47[5]:684-52). The AWARE study is sponsored by Novartis, manufacturer of omalizumab, which is approved by the Food and Drug Administration for treating CSU. Dr. Maurer reported receiving research grants from and serving as an advisor to and paid speaker for Novartis and numerous other pharmaceutical companies.
bjancin@frontlinemedcom.com
EXPERT ANALYSIS FROM THE EADV CONGRESS
Neutrophilic urticarial dermatosis is usually misdiagnosed
GENEVA – Neutrophilic urticarial dermatosis (NUD) in patients with systemic lupus erythematosus (SLE) is “almost always” initially misdiagnosed as a lupus flare and treated inappropriately, Dan Lipsker, MD, PhD, said in a plenary address at the annual congress of the European Academy of Dermatology and Venereology.
“This is a condition that is underdiagnosed and overtreated,” declared Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
NUD is not rare. Dr. Lipsker estimates it occurs in 1%-2% of patients with SLE. In a retrospective study of seven patients with NUD and SLE, he and his colleagues reported that NUD was initially misdiagnosed as a lupus flare in 4 patients, who were then treated with immunosuppressive drugs (Medicine. 2014 Dec;93[29]:e351]). “That’s quite logical because the patients had a rash, fever, and joint pain,” the dermatologist noted.
However, : It won’t alleviate the symptoms and needlessly exposes the patient to drug toxicities.
The treatment for NUD is not prednisone, mycophenolate mofetil, an antimalarial, or other drugs conventionally prescribed for SLE; it’s a drug that inhibits neutrophil migration, such as dapsone at 50-200 mg per day or colchicine at 0.5-1.0 mg per day. Typically, within just a few days after starting the appropriate therapy, the joint pain and rash of NUD are gone, according to Dr. Lipsker.
Making the diagnosis
The rash of NUD is distinctly different from a classic lupus rash. It consists of pale red macules or slightly raised nonpruritic papules. Individual lesions will disappear spontaneously within 24-48 hours.
The histopathology of NUD is characteristic of a neutrophilic dermatosis. On biopsy, an intense neutrophilic perivascular and interstitial infiltrate with leukocytoclasia is seen. There is no damage to the blood vessel walls, which readily distinguishes NUD from urticarial vasculitis.
Other neutrophilic dermatoses have also been reported with increased frequency in patients with SLE. These include Sweet syndrome, pyoderma gangrenosum, bullous SLE, amicrobial pustulosis of the folds, and palisaded neutrophilic granulomatous dermatitis. Dr. Lipsker lumps them, together with NUD, as neutrophilic cutaneous lupus erythematosus. Affected SLE patients have an exaggerated innate immune response. It is as yet unclear if these neutrophilic dermatoses have prognostic significance in the setting of SLE, he said.
Dr. Lipsker reported having no financial conflicts of interest regarding his presentation.
GENEVA – Neutrophilic urticarial dermatosis (NUD) in patients with systemic lupus erythematosus (SLE) is “almost always” initially misdiagnosed as a lupus flare and treated inappropriately, Dan Lipsker, MD, PhD, said in a plenary address at the annual congress of the European Academy of Dermatology and Venereology.
“This is a condition that is underdiagnosed and overtreated,” declared Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
NUD is not rare. Dr. Lipsker estimates it occurs in 1%-2% of patients with SLE. In a retrospective study of seven patients with NUD and SLE, he and his colleagues reported that NUD was initially misdiagnosed as a lupus flare in 4 patients, who were then treated with immunosuppressive drugs (Medicine. 2014 Dec;93[29]:e351]). “That’s quite logical because the patients had a rash, fever, and joint pain,” the dermatologist noted.
However, : It won’t alleviate the symptoms and needlessly exposes the patient to drug toxicities.
The treatment for NUD is not prednisone, mycophenolate mofetil, an antimalarial, or other drugs conventionally prescribed for SLE; it’s a drug that inhibits neutrophil migration, such as dapsone at 50-200 mg per day or colchicine at 0.5-1.0 mg per day. Typically, within just a few days after starting the appropriate therapy, the joint pain and rash of NUD are gone, according to Dr. Lipsker.
Making the diagnosis
The rash of NUD is distinctly different from a classic lupus rash. It consists of pale red macules or slightly raised nonpruritic papules. Individual lesions will disappear spontaneously within 24-48 hours.
The histopathology of NUD is characteristic of a neutrophilic dermatosis. On biopsy, an intense neutrophilic perivascular and interstitial infiltrate with leukocytoclasia is seen. There is no damage to the blood vessel walls, which readily distinguishes NUD from urticarial vasculitis.
Other neutrophilic dermatoses have also been reported with increased frequency in patients with SLE. These include Sweet syndrome, pyoderma gangrenosum, bullous SLE, amicrobial pustulosis of the folds, and palisaded neutrophilic granulomatous dermatitis. Dr. Lipsker lumps them, together with NUD, as neutrophilic cutaneous lupus erythematosus. Affected SLE patients have an exaggerated innate immune response. It is as yet unclear if these neutrophilic dermatoses have prognostic significance in the setting of SLE, he said.
Dr. Lipsker reported having no financial conflicts of interest regarding his presentation.
GENEVA – Neutrophilic urticarial dermatosis (NUD) in patients with systemic lupus erythematosus (SLE) is “almost always” initially misdiagnosed as a lupus flare and treated inappropriately, Dan Lipsker, MD, PhD, said in a plenary address at the annual congress of the European Academy of Dermatology and Venereology.
“This is a condition that is underdiagnosed and overtreated,” declared Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
NUD is not rare. Dr. Lipsker estimates it occurs in 1%-2% of patients with SLE. In a retrospective study of seven patients with NUD and SLE, he and his colleagues reported that NUD was initially misdiagnosed as a lupus flare in 4 patients, who were then treated with immunosuppressive drugs (Medicine. 2014 Dec;93[29]:e351]). “That’s quite logical because the patients had a rash, fever, and joint pain,” the dermatologist noted.
However, : It won’t alleviate the symptoms and needlessly exposes the patient to drug toxicities.
The treatment for NUD is not prednisone, mycophenolate mofetil, an antimalarial, or other drugs conventionally prescribed for SLE; it’s a drug that inhibits neutrophil migration, such as dapsone at 50-200 mg per day or colchicine at 0.5-1.0 mg per day. Typically, within just a few days after starting the appropriate therapy, the joint pain and rash of NUD are gone, according to Dr. Lipsker.
Making the diagnosis
The rash of NUD is distinctly different from a classic lupus rash. It consists of pale red macules or slightly raised nonpruritic papules. Individual lesions will disappear spontaneously within 24-48 hours.
The histopathology of NUD is characteristic of a neutrophilic dermatosis. On biopsy, an intense neutrophilic perivascular and interstitial infiltrate with leukocytoclasia is seen. There is no damage to the blood vessel walls, which readily distinguishes NUD from urticarial vasculitis.
Other neutrophilic dermatoses have also been reported with increased frequency in patients with SLE. These include Sweet syndrome, pyoderma gangrenosum, bullous SLE, amicrobial pustulosis of the folds, and palisaded neutrophilic granulomatous dermatitis. Dr. Lipsker lumps them, together with NUD, as neutrophilic cutaneous lupus erythematosus. Affected SLE patients have an exaggerated innate immune response. It is as yet unclear if these neutrophilic dermatoses have prognostic significance in the setting of SLE, he said.
Dr. Lipsker reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE EADV CONGRESS
VAMPIRE 3: Embolic filter for primary PCI reduces MACE
DENVER – Distal embolic protection using a filter device during primary PCI for acute MI significantly reduced the incidence of the no-reflow phenomenon and serious adverse events in the randomized VAMPIRE 3 trial.
The reason VAMPIRE 3 (Vacuum Aspiration Thrombus Removal) was a positive trial when other studies of distal embolic protection during coronary intervention have failed was that VAMPIRE 3 targeted a high-risk, high-benefit population: the subset of acute MI patients with attenuated, high-risk coronary plaque identified by intravascular ultrasound, Kiyoshi Hibi, MD, explained at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Also, the filter device used in the trial was easily deployed and problem free, which doesn’t appear to be true of distal protection devices used in some earlier trials.
Discussants agreed with his conclusion, but predicted distal protection during primary PCI for acute coronary syndrome is unlikely to catch on with U.S. interventional cardiologists anytime soon. That’s because, for the most part, they don’t perform advanced imaging in the setting of primary PCI for ACS.
VAMPIRE 3 was a multicenter Japanese study involving 200 patients undergoing primary PCI for acute MI who displayed intravascular ultrasound evidence of attenuated plaque at least 5 mm in length as defined by images showing backward signal attenuation of 180 degrees or more behind a noncalcified plaque. Participants were randomized to placement of the Filtrap distal embolic protection device manufactured by Nipro of Tokyo or to no filter during their procedure, which in all cases entailed the use of a thrombus aspiration catheter.
The primary endpoint was the incidence of the no-reflow phenomenon, an adverse event previously shown by German investigators to be associated with a 66% increase in 5-year mortality (J Am Coll Cardiol. 2010 May 25;55[21]:2383-9). In VAMPIRE 3, the no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally. The secondary endpoint – the corrected TIMI frame count – was 23.0 in the distal protection group, significantly better than the 30.5 in control patients, the cardiologist reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The rate of in-hospital adverse events – a composite of death, cardiac arrest, cardiogenic shock, and ischemic stroke adjudicated by an independent committee – was 1% in the distal protection group and significantly higher at 8.3% in conventionally managed controls. All five cases of cardiac arrest/cardiogenic shock occurred in patients who didn’t receive the prophylactic filter, he continued.
In a previous study, Dr. Hibi and his coworkers showed that the incidence of the no-reflow phenomenon in a series of 179 acute MI patients was 18%. More importantly, the incidence was 71% among patients with attenuated plaque of 5 mm or more, compared with 10%-11% among those with less or no attenuated plaque (JACC Cardiovasc Interv. 2010 May;3[5]:540-9).
“I am convinced by these results,” discussant Jonathan M. Hill, MD, said about VAMPIRE 3, “but the concern is the dependency on imaging. All of these cases were entirely dependent on an imaging diagnosis.
“Certainly in the acute setting, I think Japanese practice is the extreme, with greater than 80% penetration for intravascular imaging, compared to less than 10% in the United States. So to implement your protocol would be difficult unless there was acceptance of the requirement for intravascular imaging,” added Dr. Hill, an interventional cardiologist at King’s College London.
The prevailing philosophy among American cardiologists is that time is heart muscle. The priority is to open the blocked coronary vessel as quickly as possible.
“We don’t use that much advanced imaging,“ agreed David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“It seems like the difference between this trial and previous ones that have not used advanced imaging was the use of intravascular imaging to identify the optimal group of patients that might benefit from this. So I think until that practice changes in the United States, I suspect this is going to get very limited use. I don’t see these devices ramping up quickly for coronary applications,” he predicted.
VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. Dr. Hibi reported serving as a consultant to Nipro and Boston Scientific.
SOURCE: Hibi K. TCT 2017
DENVER – Distal embolic protection using a filter device during primary PCI for acute MI significantly reduced the incidence of the no-reflow phenomenon and serious adverse events in the randomized VAMPIRE 3 trial.
The reason VAMPIRE 3 (Vacuum Aspiration Thrombus Removal) was a positive trial when other studies of distal embolic protection during coronary intervention have failed was that VAMPIRE 3 targeted a high-risk, high-benefit population: the subset of acute MI patients with attenuated, high-risk coronary plaque identified by intravascular ultrasound, Kiyoshi Hibi, MD, explained at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Also, the filter device used in the trial was easily deployed and problem free, which doesn’t appear to be true of distal protection devices used in some earlier trials.
Discussants agreed with his conclusion, but predicted distal protection during primary PCI for acute coronary syndrome is unlikely to catch on with U.S. interventional cardiologists anytime soon. That’s because, for the most part, they don’t perform advanced imaging in the setting of primary PCI for ACS.
VAMPIRE 3 was a multicenter Japanese study involving 200 patients undergoing primary PCI for acute MI who displayed intravascular ultrasound evidence of attenuated plaque at least 5 mm in length as defined by images showing backward signal attenuation of 180 degrees or more behind a noncalcified plaque. Participants were randomized to placement of the Filtrap distal embolic protection device manufactured by Nipro of Tokyo or to no filter during their procedure, which in all cases entailed the use of a thrombus aspiration catheter.
The primary endpoint was the incidence of the no-reflow phenomenon, an adverse event previously shown by German investigators to be associated with a 66% increase in 5-year mortality (J Am Coll Cardiol. 2010 May 25;55[21]:2383-9). In VAMPIRE 3, the no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally. The secondary endpoint – the corrected TIMI frame count – was 23.0 in the distal protection group, significantly better than the 30.5 in control patients, the cardiologist reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The rate of in-hospital adverse events – a composite of death, cardiac arrest, cardiogenic shock, and ischemic stroke adjudicated by an independent committee – was 1% in the distal protection group and significantly higher at 8.3% in conventionally managed controls. All five cases of cardiac arrest/cardiogenic shock occurred in patients who didn’t receive the prophylactic filter, he continued.
In a previous study, Dr. Hibi and his coworkers showed that the incidence of the no-reflow phenomenon in a series of 179 acute MI patients was 18%. More importantly, the incidence was 71% among patients with attenuated plaque of 5 mm or more, compared with 10%-11% among those with less or no attenuated plaque (JACC Cardiovasc Interv. 2010 May;3[5]:540-9).
“I am convinced by these results,” discussant Jonathan M. Hill, MD, said about VAMPIRE 3, “but the concern is the dependency on imaging. All of these cases were entirely dependent on an imaging diagnosis.
“Certainly in the acute setting, I think Japanese practice is the extreme, with greater than 80% penetration for intravascular imaging, compared to less than 10% in the United States. So to implement your protocol would be difficult unless there was acceptance of the requirement for intravascular imaging,” added Dr. Hill, an interventional cardiologist at King’s College London.
The prevailing philosophy among American cardiologists is that time is heart muscle. The priority is to open the blocked coronary vessel as quickly as possible.
“We don’t use that much advanced imaging,“ agreed David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“It seems like the difference between this trial and previous ones that have not used advanced imaging was the use of intravascular imaging to identify the optimal group of patients that might benefit from this. So I think until that practice changes in the United States, I suspect this is going to get very limited use. I don’t see these devices ramping up quickly for coronary applications,” he predicted.
VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. Dr. Hibi reported serving as a consultant to Nipro and Boston Scientific.
SOURCE: Hibi K. TCT 2017
DENVER – Distal embolic protection using a filter device during primary PCI for acute MI significantly reduced the incidence of the no-reflow phenomenon and serious adverse events in the randomized VAMPIRE 3 trial.
The reason VAMPIRE 3 (Vacuum Aspiration Thrombus Removal) was a positive trial when other studies of distal embolic protection during coronary intervention have failed was that VAMPIRE 3 targeted a high-risk, high-benefit population: the subset of acute MI patients with attenuated, high-risk coronary plaque identified by intravascular ultrasound, Kiyoshi Hibi, MD, explained at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Also, the filter device used in the trial was easily deployed and problem free, which doesn’t appear to be true of distal protection devices used in some earlier trials.
Discussants agreed with his conclusion, but predicted distal protection during primary PCI for acute coronary syndrome is unlikely to catch on with U.S. interventional cardiologists anytime soon. That’s because, for the most part, they don’t perform advanced imaging in the setting of primary PCI for ACS.
VAMPIRE 3 was a multicenter Japanese study involving 200 patients undergoing primary PCI for acute MI who displayed intravascular ultrasound evidence of attenuated plaque at least 5 mm in length as defined by images showing backward signal attenuation of 180 degrees or more behind a noncalcified plaque. Participants were randomized to placement of the Filtrap distal embolic protection device manufactured by Nipro of Tokyo or to no filter during their procedure, which in all cases entailed the use of a thrombus aspiration catheter.
The primary endpoint was the incidence of the no-reflow phenomenon, an adverse event previously shown by German investigators to be associated with a 66% increase in 5-year mortality (J Am Coll Cardiol. 2010 May 25;55[21]:2383-9). In VAMPIRE 3, the no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally. The secondary endpoint – the corrected TIMI frame count – was 23.0 in the distal protection group, significantly better than the 30.5 in control patients, the cardiologist reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The rate of in-hospital adverse events – a composite of death, cardiac arrest, cardiogenic shock, and ischemic stroke adjudicated by an independent committee – was 1% in the distal protection group and significantly higher at 8.3% in conventionally managed controls. All five cases of cardiac arrest/cardiogenic shock occurred in patients who didn’t receive the prophylactic filter, he continued.
In a previous study, Dr. Hibi and his coworkers showed that the incidence of the no-reflow phenomenon in a series of 179 acute MI patients was 18%. More importantly, the incidence was 71% among patients with attenuated plaque of 5 mm or more, compared with 10%-11% among those with less or no attenuated plaque (JACC Cardiovasc Interv. 2010 May;3[5]:540-9).
“I am convinced by these results,” discussant Jonathan M. Hill, MD, said about VAMPIRE 3, “but the concern is the dependency on imaging. All of these cases were entirely dependent on an imaging diagnosis.
“Certainly in the acute setting, I think Japanese practice is the extreme, with greater than 80% penetration for intravascular imaging, compared to less than 10% in the United States. So to implement your protocol would be difficult unless there was acceptance of the requirement for intravascular imaging,” added Dr. Hill, an interventional cardiologist at King’s College London.
The prevailing philosophy among American cardiologists is that time is heart muscle. The priority is to open the blocked coronary vessel as quickly as possible.
“We don’t use that much advanced imaging,“ agreed David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“It seems like the difference between this trial and previous ones that have not used advanced imaging was the use of intravascular imaging to identify the optimal group of patients that might benefit from this. So I think until that practice changes in the United States, I suspect this is going to get very limited use. I don’t see these devices ramping up quickly for coronary applications,” he predicted.
VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. Dr. Hibi reported serving as a consultant to Nipro and Boston Scientific.
SOURCE: Hibi K. TCT 2017
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally.
Study details: This randomized multicenter trial included 200 acute MI patients.
Disclosures: VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. The presenter reported serving as a consultant to Nipro and Boston Scientific.






















