User login
Despite Prevention Efforts, HIV Infections Reach 50,000 Every Year
An estimated 50,000 people are infected with HIV every year in the United States, despite successful efforts to prevent HIV infections and a marked drop in deaths since the epidemic began 30 years ago, according to a report from the Centers for Disease Control and Prevention.
Moreover, about 20% of people infected with HIV are undiagnosed, according to the report on HIV surveillance in the United States between 1981 and 2008, which appeared in the June 3 issue of Morbidity and Mortality Weekly Report (MMWR 2011;60:689-93).
The report summarizes the findings of the CDC’s analysis of data from the National HIV Surveillance System of HIV and AIDS cases in people aged 13 years and older, between 1981, the beginning of the AIDS epidemic, and 2008.
The estimated number of people aged 13 years and older newly diagnosed with AIDS every year rapidly increased from 1981 to 1992, from 318 in 1981, peaking at 75,457 in 1992. The estimated number of AIDS-related deaths also increased, from 451 in 1981, peaking at 50,628 in 1995.
This was followed by a marked drop in AIDS diagnoses and deaths from 1995 to 1998, after highly-active antiretroviral drugs became available, and in 1999, the number of new cases and deaths started to stabilize. Between 1999 and 2008, there was an average of 38,279 diagnoses and 17,489 deaths per year. In addition, the estimated number of people aged 13 years and older who were living with AIDS increased from 219,318 in 1996 to 479,161 in 2008, more than a twofold increase.
Still, the report pointed out, an estimated 1,178,350 people at the end of 2008 were living with HIV, about 20% of whom were undiagnosed. These findings "underscore the importance of the national HIV/AIDS Strategy focus on reducing HIV risk behaviors, increasing opportunities for routine testing and enhancing use of care." Of those living with HIV at the end of 2008, 75% were men; of the men, almost 66% were men who have sex with men.
The report also finds disparities between races and ethnic groups: The prevalence of HIV was about eightfold higher among blacks and about 2.5 times higher among Hispanics than among whites. And Asians or Pacific Islanders, and American Indians or Alaska natives accounted for a higher proportion of those with undiagnosed HIV infections (as did men with high-risk heterosexual contacts) than among blacks, whites, or Hispanics.
An editorial note accompanying the report points out that HIV prevention efforts have prevented an estimated 350,000 HIV infections during 1991-2006, at a savings of $125 billion in medical costs.
More than half of the estimated 50,000 people who are infected every year in the United States are men who have sex with men, and almost half are black.
But the proportion of men who have sex with men who are diagnosed with HIV continues to increase, and has increased steadily since the early 1990s, and late diagnoses of HIV are common, according to the editorial note. Of the newly diagnosed HIV cases in 2008, 33% were diagnosed with AIDS within 1 year of the HIV diagnosis, and were "likely" infected an average of 10 years before being diagnosed – during which time, "they missed opportunities to obtain medical care and to prevent unwitting transmission of HIV to others," the editorial noted.
The report and editorial note ends by pointing out that the National HIV/AIDS Strategy is refocusing efforts to increase HIV prevention efforts in communities with the highest prevalence of HIV infection, "using a combination of effective strategies that seek to optimize entry into and retention in care and maintenance of viral suppression."
"Over the last three decades, prevention efforts have helped reduce new infections and treatment advances have allowed people with HIV to live longer, healthier lives," CDC Director Dr. Thomas Frieden said in a statement issued on June 2.. "But as these improvements have taken place, our nation’s collective sense of crisis has waned. Far too many Americans underestimate their risk of infection or believe HIV is no longer a serious health threat, but they must understand that HIV remains an incurable infection. We must increase our resolve to end this epidemic."
An estimated 50,000 people are infected with HIV every year in the United States, despite successful efforts to prevent HIV infections and a marked drop in deaths since the epidemic began 30 years ago, according to a report from the Centers for Disease Control and Prevention.
Moreover, about 20% of people infected with HIV are undiagnosed, according to the report on HIV surveillance in the United States between 1981 and 2008, which appeared in the June 3 issue of Morbidity and Mortality Weekly Report (MMWR 2011;60:689-93).
The report summarizes the findings of the CDC’s analysis of data from the National HIV Surveillance System of HIV and AIDS cases in people aged 13 years and older, between 1981, the beginning of the AIDS epidemic, and 2008.
The estimated number of people aged 13 years and older newly diagnosed with AIDS every year rapidly increased from 1981 to 1992, from 318 in 1981, peaking at 75,457 in 1992. The estimated number of AIDS-related deaths also increased, from 451 in 1981, peaking at 50,628 in 1995.
This was followed by a marked drop in AIDS diagnoses and deaths from 1995 to 1998, after highly-active antiretroviral drugs became available, and in 1999, the number of new cases and deaths started to stabilize. Between 1999 and 2008, there was an average of 38,279 diagnoses and 17,489 deaths per year. In addition, the estimated number of people aged 13 years and older who were living with AIDS increased from 219,318 in 1996 to 479,161 in 2008, more than a twofold increase.
Still, the report pointed out, an estimated 1,178,350 people at the end of 2008 were living with HIV, about 20% of whom were undiagnosed. These findings "underscore the importance of the national HIV/AIDS Strategy focus on reducing HIV risk behaviors, increasing opportunities for routine testing and enhancing use of care." Of those living with HIV at the end of 2008, 75% were men; of the men, almost 66% were men who have sex with men.
The report also finds disparities between races and ethnic groups: The prevalence of HIV was about eightfold higher among blacks and about 2.5 times higher among Hispanics than among whites. And Asians or Pacific Islanders, and American Indians or Alaska natives accounted for a higher proportion of those with undiagnosed HIV infections (as did men with high-risk heterosexual contacts) than among blacks, whites, or Hispanics.
An editorial note accompanying the report points out that HIV prevention efforts have prevented an estimated 350,000 HIV infections during 1991-2006, at a savings of $125 billion in medical costs.
More than half of the estimated 50,000 people who are infected every year in the United States are men who have sex with men, and almost half are black.
But the proportion of men who have sex with men who are diagnosed with HIV continues to increase, and has increased steadily since the early 1990s, and late diagnoses of HIV are common, according to the editorial note. Of the newly diagnosed HIV cases in 2008, 33% were diagnosed with AIDS within 1 year of the HIV diagnosis, and were "likely" infected an average of 10 years before being diagnosed – during which time, "they missed opportunities to obtain medical care and to prevent unwitting transmission of HIV to others," the editorial noted.
The report and editorial note ends by pointing out that the National HIV/AIDS Strategy is refocusing efforts to increase HIV prevention efforts in communities with the highest prevalence of HIV infection, "using a combination of effective strategies that seek to optimize entry into and retention in care and maintenance of viral suppression."
"Over the last three decades, prevention efforts have helped reduce new infections and treatment advances have allowed people with HIV to live longer, healthier lives," CDC Director Dr. Thomas Frieden said in a statement issued on June 2.. "But as these improvements have taken place, our nation’s collective sense of crisis has waned. Far too many Americans underestimate their risk of infection or believe HIV is no longer a serious health threat, but they must understand that HIV remains an incurable infection. We must increase our resolve to end this epidemic."
An estimated 50,000 people are infected with HIV every year in the United States, despite successful efforts to prevent HIV infections and a marked drop in deaths since the epidemic began 30 years ago, according to a report from the Centers for Disease Control and Prevention.
Moreover, about 20% of people infected with HIV are undiagnosed, according to the report on HIV surveillance in the United States between 1981 and 2008, which appeared in the June 3 issue of Morbidity and Mortality Weekly Report (MMWR 2011;60:689-93).
The report summarizes the findings of the CDC’s analysis of data from the National HIV Surveillance System of HIV and AIDS cases in people aged 13 years and older, between 1981, the beginning of the AIDS epidemic, and 2008.
The estimated number of people aged 13 years and older newly diagnosed with AIDS every year rapidly increased from 1981 to 1992, from 318 in 1981, peaking at 75,457 in 1992. The estimated number of AIDS-related deaths also increased, from 451 in 1981, peaking at 50,628 in 1995.
This was followed by a marked drop in AIDS diagnoses and deaths from 1995 to 1998, after highly-active antiretroviral drugs became available, and in 1999, the number of new cases and deaths started to stabilize. Between 1999 and 2008, there was an average of 38,279 diagnoses and 17,489 deaths per year. In addition, the estimated number of people aged 13 years and older who were living with AIDS increased from 219,318 in 1996 to 479,161 in 2008, more than a twofold increase.
Still, the report pointed out, an estimated 1,178,350 people at the end of 2008 were living with HIV, about 20% of whom were undiagnosed. These findings "underscore the importance of the national HIV/AIDS Strategy focus on reducing HIV risk behaviors, increasing opportunities for routine testing and enhancing use of care." Of those living with HIV at the end of 2008, 75% were men; of the men, almost 66% were men who have sex with men.
The report also finds disparities between races and ethnic groups: The prevalence of HIV was about eightfold higher among blacks and about 2.5 times higher among Hispanics than among whites. And Asians or Pacific Islanders, and American Indians or Alaska natives accounted for a higher proportion of those with undiagnosed HIV infections (as did men with high-risk heterosexual contacts) than among blacks, whites, or Hispanics.
An editorial note accompanying the report points out that HIV prevention efforts have prevented an estimated 350,000 HIV infections during 1991-2006, at a savings of $125 billion in medical costs.
More than half of the estimated 50,000 people who are infected every year in the United States are men who have sex with men, and almost half are black.
But the proportion of men who have sex with men who are diagnosed with HIV continues to increase, and has increased steadily since the early 1990s, and late diagnoses of HIV are common, according to the editorial note. Of the newly diagnosed HIV cases in 2008, 33% were diagnosed with AIDS within 1 year of the HIV diagnosis, and were "likely" infected an average of 10 years before being diagnosed – during which time, "they missed opportunities to obtain medical care and to prevent unwitting transmission of HIV to others," the editorial noted.
The report and editorial note ends by pointing out that the National HIV/AIDS Strategy is refocusing efforts to increase HIV prevention efforts in communities with the highest prevalence of HIV infection, "using a combination of effective strategies that seek to optimize entry into and retention in care and maintenance of viral suppression."
"Over the last three decades, prevention efforts have helped reduce new infections and treatment advances have allowed people with HIV to live longer, healthier lives," CDC Director Dr. Thomas Frieden said in a statement issued on June 2.. "But as these improvements have taken place, our nation’s collective sense of crisis has waned. Far too many Americans underestimate their risk of infection or believe HIV is no longer a serious health threat, but they must understand that HIV remains an incurable infection. We must increase our resolve to end this epidemic."
FROM THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Despite Prevention Efforts, HIV Infections Reach 50,000 Every Year
An estimated 50,000 people are infected with HIV every year in the United States, despite successful efforts to prevent HIV infections and a marked drop in deaths since the epidemic began 30 years ago, according to a report from the Centers for Disease Control and Prevention.
Moreover, about 20% of people infected with HIV are undiagnosed, according to the report on HIV surveillance in the United States between 1981 and 2008, which appeared in the June 3 issue of Morbidity and Mortality Weekly Report (MMWR 2011;60:689-93).
The report summarizes the findings of the CDC’s analysis of data from the National HIV Surveillance System of HIV and AIDS cases in people aged 13 years and older, between 1981, the beginning of the AIDS epidemic, and 2008.
The estimated number of people aged 13 years and older newly diagnosed with AIDS every year rapidly increased from 1981 to 1992, from 318 in 1981, peaking at 75,457 in 1992. The estimated number of AIDS-related deaths also increased, from 451 in 1981, peaking at 50,628 in 1995.
This was followed by a marked drop in AIDS diagnoses and deaths from 1995 to 1998, after highly-active antiretroviral drugs became available, and in 1999, the number of new cases and deaths started to stabilize. Between 1999 and 2008, there was an average of 38,279 diagnoses and 17,489 deaths per year. In addition, the estimated number of people aged 13 years and older who were living with AIDS increased from 219,318 in 1996 to 479,161 in 2008, more than a twofold increase.
Still, the report pointed out, an estimated 1,178,350 people at the end of 2008 were living with HIV, about 20% of whom were undiagnosed. These findings "underscore the importance of the national HIV/AIDS Strategy focus on reducing HIV risk behaviors, increasing opportunities for routine testing and enhancing use of care." Of those living with HIV at the end of 2008, 75% were men; of the men, almost 66% were men who have sex with men.
The report also finds disparities between races and ethnic groups: The prevalence of HIV was about eightfold higher among blacks and about 2.5 times higher among Hispanics than among whites. And Asians or Pacific Islanders, and American Indians or Alaska natives accounted for a higher proportion of those with undiagnosed HIV infections (as did men with high-risk heterosexual contacts) than among blacks, whites, or Hispanics.
An editorial note accompanying the report points out that HIV prevention efforts have prevented an estimated 350,000 HIV infections during 1991-2006, at a savings of $125 billion in medical costs.
More than half of the estimated 50,000 people who are infected every year in the United States are men who have sex with men, and almost half are black.
But the proportion of men who have sex with men who are diagnosed with HIV continues to increase, and has increased steadily since the early 1990s, and late diagnoses of HIV are common, according to the editorial note. Of the newly diagnosed HIV cases in 2008, 33% were diagnosed with AIDS within 1 year of the HIV diagnosis, and were "likely" infected an average of 10 years before being diagnosed – during which time, "they missed opportunities to obtain medical care and to prevent unwitting transmission of HIV to others," the editorial noted.
The report and editorial note ends by pointing out that the National HIV/AIDS Strategy is refocusing efforts to increase HIV prevention efforts in communities with the highest prevalence of HIV infection, "using a combination of effective strategies that seek to optimize entry into and retention in care and maintenance of viral suppression."
"Over the last three decades, prevention efforts have helped reduce new infections and treatment advances have allowed people with HIV to live longer, healthier lives," CDC Director Dr. Thomas Frieden said in a statement issued on June 2.. "But as these improvements have taken place, our nation’s collective sense of crisis has waned. Far too many Americans underestimate their risk of infection or believe HIV is no longer a serious health threat, but they must understand that HIV remains an incurable infection. We must increase our resolve to end this epidemic."
An estimated 50,000 people are infected with HIV every year in the United States, despite successful efforts to prevent HIV infections and a marked drop in deaths since the epidemic began 30 years ago, according to a report from the Centers for Disease Control and Prevention.
Moreover, about 20% of people infected with HIV are undiagnosed, according to the report on HIV surveillance in the United States between 1981 and 2008, which appeared in the June 3 issue of Morbidity and Mortality Weekly Report (MMWR 2011;60:689-93).
The report summarizes the findings of the CDC’s analysis of data from the National HIV Surveillance System of HIV and AIDS cases in people aged 13 years and older, between 1981, the beginning of the AIDS epidemic, and 2008.
The estimated number of people aged 13 years and older newly diagnosed with AIDS every year rapidly increased from 1981 to 1992, from 318 in 1981, peaking at 75,457 in 1992. The estimated number of AIDS-related deaths also increased, from 451 in 1981, peaking at 50,628 in 1995.
This was followed by a marked drop in AIDS diagnoses and deaths from 1995 to 1998, after highly-active antiretroviral drugs became available, and in 1999, the number of new cases and deaths started to stabilize. Between 1999 and 2008, there was an average of 38,279 diagnoses and 17,489 deaths per year. In addition, the estimated number of people aged 13 years and older who were living with AIDS increased from 219,318 in 1996 to 479,161 in 2008, more than a twofold increase.
Still, the report pointed out, an estimated 1,178,350 people at the end of 2008 were living with HIV, about 20% of whom were undiagnosed. These findings "underscore the importance of the national HIV/AIDS Strategy focus on reducing HIV risk behaviors, increasing opportunities for routine testing and enhancing use of care." Of those living with HIV at the end of 2008, 75% were men; of the men, almost 66% were men who have sex with men.
The report also finds disparities between races and ethnic groups: The prevalence of HIV was about eightfold higher among blacks and about 2.5 times higher among Hispanics than among whites. And Asians or Pacific Islanders, and American Indians or Alaska natives accounted for a higher proportion of those with undiagnosed HIV infections (as did men with high-risk heterosexual contacts) than among blacks, whites, or Hispanics.
An editorial note accompanying the report points out that HIV prevention efforts have prevented an estimated 350,000 HIV infections during 1991-2006, at a savings of $125 billion in medical costs.
More than half of the estimated 50,000 people who are infected every year in the United States are men who have sex with men, and almost half are black.
But the proportion of men who have sex with men who are diagnosed with HIV continues to increase, and has increased steadily since the early 1990s, and late diagnoses of HIV are common, according to the editorial note. Of the newly diagnosed HIV cases in 2008, 33% were diagnosed with AIDS within 1 year of the HIV diagnosis, and were "likely" infected an average of 10 years before being diagnosed – during which time, "they missed opportunities to obtain medical care and to prevent unwitting transmission of HIV to others," the editorial noted.
The report and editorial note ends by pointing out that the National HIV/AIDS Strategy is refocusing efforts to increase HIV prevention efforts in communities with the highest prevalence of HIV infection, "using a combination of effective strategies that seek to optimize entry into and retention in care and maintenance of viral suppression."
"Over the last three decades, prevention efforts have helped reduce new infections and treatment advances have allowed people with HIV to live longer, healthier lives," CDC Director Dr. Thomas Frieden said in a statement issued on June 2.. "But as these improvements have taken place, our nation’s collective sense of crisis has waned. Far too many Americans underestimate their risk of infection or believe HIV is no longer a serious health threat, but they must understand that HIV remains an incurable infection. We must increase our resolve to end this epidemic."
An estimated 50,000 people are infected with HIV every year in the United States, despite successful efforts to prevent HIV infections and a marked drop in deaths since the epidemic began 30 years ago, according to a report from the Centers for Disease Control and Prevention.
Moreover, about 20% of people infected with HIV are undiagnosed, according to the report on HIV surveillance in the United States between 1981 and 2008, which appeared in the June 3 issue of Morbidity and Mortality Weekly Report (MMWR 2011;60:689-93).
The report summarizes the findings of the CDC’s analysis of data from the National HIV Surveillance System of HIV and AIDS cases in people aged 13 years and older, between 1981, the beginning of the AIDS epidemic, and 2008.
The estimated number of people aged 13 years and older newly diagnosed with AIDS every year rapidly increased from 1981 to 1992, from 318 in 1981, peaking at 75,457 in 1992. The estimated number of AIDS-related deaths also increased, from 451 in 1981, peaking at 50,628 in 1995.
This was followed by a marked drop in AIDS diagnoses and deaths from 1995 to 1998, after highly-active antiretroviral drugs became available, and in 1999, the number of new cases and deaths started to stabilize. Between 1999 and 2008, there was an average of 38,279 diagnoses and 17,489 deaths per year. In addition, the estimated number of people aged 13 years and older who were living with AIDS increased from 219,318 in 1996 to 479,161 in 2008, more than a twofold increase.
Still, the report pointed out, an estimated 1,178,350 people at the end of 2008 were living with HIV, about 20% of whom were undiagnosed. These findings "underscore the importance of the national HIV/AIDS Strategy focus on reducing HIV risk behaviors, increasing opportunities for routine testing and enhancing use of care." Of those living with HIV at the end of 2008, 75% were men; of the men, almost 66% were men who have sex with men.
The report also finds disparities between races and ethnic groups: The prevalence of HIV was about eightfold higher among blacks and about 2.5 times higher among Hispanics than among whites. And Asians or Pacific Islanders, and American Indians or Alaska natives accounted for a higher proportion of those with undiagnosed HIV infections (as did men with high-risk heterosexual contacts) than among blacks, whites, or Hispanics.
An editorial note accompanying the report points out that HIV prevention efforts have prevented an estimated 350,000 HIV infections during 1991-2006, at a savings of $125 billion in medical costs.
More than half of the estimated 50,000 people who are infected every year in the United States are men who have sex with men, and almost half are black.
But the proportion of men who have sex with men who are diagnosed with HIV continues to increase, and has increased steadily since the early 1990s, and late diagnoses of HIV are common, according to the editorial note. Of the newly diagnosed HIV cases in 2008, 33% were diagnosed with AIDS within 1 year of the HIV diagnosis, and were "likely" infected an average of 10 years before being diagnosed – during which time, "they missed opportunities to obtain medical care and to prevent unwitting transmission of HIV to others," the editorial noted.
The report and editorial note ends by pointing out that the National HIV/AIDS Strategy is refocusing efforts to increase HIV prevention efforts in communities with the highest prevalence of HIV infection, "using a combination of effective strategies that seek to optimize entry into and retention in care and maintenance of viral suppression."
"Over the last three decades, prevention efforts have helped reduce new infections and treatment advances have allowed people with HIV to live longer, healthier lives," CDC Director Dr. Thomas Frieden said in a statement issued on June 2.. "But as these improvements have taken place, our nation’s collective sense of crisis has waned. Far too many Americans underestimate their risk of infection or believe HIV is no longer a serious health threat, but they must understand that HIV remains an incurable infection. We must increase our resolve to end this epidemic."
FROM THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Major Finding: An estimated 50,000 people are infected with HIV every year in the United States, despite successful efforts to prevent HIV infections and a decrease in the number of newly diagnosed cases and deaths since the AIDS epidemic began 30 years ago.
Data Source: The Centers for Disease Control and Prevention’s Morbidity and Mortality Report.
Disclosures: None.
Short-Term NSAID Use Raises Death/Recurrent MI Risk
For patients with a history of myo-cardial infarction, any length of treatment with nonsteroidal anti-inflammatory drugs poses an unacceptably high risk for death or recurrent heart attack, based on findings from a Danish study using hospital and pharmacy registry data.
The risk elevation began during the first week of therapy and continued throughout the course of treatment, with some differences in the magnitude of risk between NSAIDs.
“These results challenge the view that NSAIDs are not harmful during short-term [1-week] treatment and indicate that a revision of current recommendations regarding NSAID treatment in patients with established cardiovascular disease is required,” concluded Anne-Marie Schjerning of the department of cardiology, Copenhagen University Hospital in Gentofte, Denmark, and her coauthors. (Circulation 2011 May 9 [(doi: 0.1161/CIRCULATIONAHA.110.004671]).
The significant increase in death and recurrent myocardial infarction associated with the use of NSAIDs in a study of people with a history of myocardial infarction indicates that current recommendations regarding NSAID use in patients with cardiovascular disease need to be revised, the study authors concluded.
Although international guidelines state that NSAID use should be discouraged in people with established cardiovascular disease, they say that if such use is unavoidable, the duration of NSAID treatment “should be as short as possible,” the authors pointed out.
The investigators conducted the study to address the paucity of information on the association between the duration of treatment with NSAIDs and the risk of cardiovascular disease, in this population of patients. Of the 83,675 people aged 30 years and older who had had their first MI from 1997 through 2006 identified in the national registries (mean age, 68 years), 42% had received NSAIDs.
Overall, treatment with NSAIDs was associated with a 45% greater risk of death/recurrent MI during the first 7 days of treatment, which persisted and was increased by 65% over a 30- to 90-day period of treatment.
The greatest risk identified was with diclofenac (hazard ratio, 3.26; 95% confidence interval, 2.57–3.86 for death/MI at day 1–7 of treatment). Diclofenac is available over the counter in many countries, the authors noted.
A significant increase in risk was seen after 1 week of treatment with ibuprofen, in the first week of treatment with rofecoxib (which has been withdrawn from the market), and after 14–30 days with celecoxib.
The risk associated with ibuprofen was lower than the risk associated with the two cyclooxygenase-2 (COX-2) selective inhibitors, rofecoxib and celecoxib, and it was lower than the risk associated with the use of diclofenac.
There was no increased risk of death or recurrent MI associated with naproxen for the entire treatment duration, which exceeded 90 days in some cases. However, naproxen has been associated with an increased risk of GI bleeding, compared with rofecoxib, in one study, the authors noted.
The authors said they had no relevant financial disclosures.
For patients with a history of myo-cardial infarction, any length of treatment with nonsteroidal anti-inflammatory drugs poses an unacceptably high risk for death or recurrent heart attack, based on findings from a Danish study using hospital and pharmacy registry data.
The risk elevation began during the first week of therapy and continued throughout the course of treatment, with some differences in the magnitude of risk between NSAIDs.
“These results challenge the view that NSAIDs are not harmful during short-term [1-week] treatment and indicate that a revision of current recommendations regarding NSAID treatment in patients with established cardiovascular disease is required,” concluded Anne-Marie Schjerning of the department of cardiology, Copenhagen University Hospital in Gentofte, Denmark, and her coauthors. (Circulation 2011 May 9 [(doi: 0.1161/CIRCULATIONAHA.110.004671]).
The significant increase in death and recurrent myocardial infarction associated with the use of NSAIDs in a study of people with a history of myocardial infarction indicates that current recommendations regarding NSAID use in patients with cardiovascular disease need to be revised, the study authors concluded.
Although international guidelines state that NSAID use should be discouraged in people with established cardiovascular disease, they say that if such use is unavoidable, the duration of NSAID treatment “should be as short as possible,” the authors pointed out.
The investigators conducted the study to address the paucity of information on the association between the duration of treatment with NSAIDs and the risk of cardiovascular disease, in this population of patients. Of the 83,675 people aged 30 years and older who had had their first MI from 1997 through 2006 identified in the national registries (mean age, 68 years), 42% had received NSAIDs.
Overall, treatment with NSAIDs was associated with a 45% greater risk of death/recurrent MI during the first 7 days of treatment, which persisted and was increased by 65% over a 30- to 90-day period of treatment.
The greatest risk identified was with diclofenac (hazard ratio, 3.26; 95% confidence interval, 2.57–3.86 for death/MI at day 1–7 of treatment). Diclofenac is available over the counter in many countries, the authors noted.
A significant increase in risk was seen after 1 week of treatment with ibuprofen, in the first week of treatment with rofecoxib (which has been withdrawn from the market), and after 14–30 days with celecoxib.
The risk associated with ibuprofen was lower than the risk associated with the two cyclooxygenase-2 (COX-2) selective inhibitors, rofecoxib and celecoxib, and it was lower than the risk associated with the use of diclofenac.
There was no increased risk of death or recurrent MI associated with naproxen for the entire treatment duration, which exceeded 90 days in some cases. However, naproxen has been associated with an increased risk of GI bleeding, compared with rofecoxib, in one study, the authors noted.
The authors said they had no relevant financial disclosures.
For patients with a history of myo-cardial infarction, any length of treatment with nonsteroidal anti-inflammatory drugs poses an unacceptably high risk for death or recurrent heart attack, based on findings from a Danish study using hospital and pharmacy registry data.
The risk elevation began during the first week of therapy and continued throughout the course of treatment, with some differences in the magnitude of risk between NSAIDs.
“These results challenge the view that NSAIDs are not harmful during short-term [1-week] treatment and indicate that a revision of current recommendations regarding NSAID treatment in patients with established cardiovascular disease is required,” concluded Anne-Marie Schjerning of the department of cardiology, Copenhagen University Hospital in Gentofte, Denmark, and her coauthors. (Circulation 2011 May 9 [(doi: 0.1161/CIRCULATIONAHA.110.004671]).
The significant increase in death and recurrent myocardial infarction associated with the use of NSAIDs in a study of people with a history of myocardial infarction indicates that current recommendations regarding NSAID use in patients with cardiovascular disease need to be revised, the study authors concluded.
Although international guidelines state that NSAID use should be discouraged in people with established cardiovascular disease, they say that if such use is unavoidable, the duration of NSAID treatment “should be as short as possible,” the authors pointed out.
The investigators conducted the study to address the paucity of information on the association between the duration of treatment with NSAIDs and the risk of cardiovascular disease, in this population of patients. Of the 83,675 people aged 30 years and older who had had their first MI from 1997 through 2006 identified in the national registries (mean age, 68 years), 42% had received NSAIDs.
Overall, treatment with NSAIDs was associated with a 45% greater risk of death/recurrent MI during the first 7 days of treatment, which persisted and was increased by 65% over a 30- to 90-day period of treatment.
The greatest risk identified was with diclofenac (hazard ratio, 3.26; 95% confidence interval, 2.57–3.86 for death/MI at day 1–7 of treatment). Diclofenac is available over the counter in many countries, the authors noted.
A significant increase in risk was seen after 1 week of treatment with ibuprofen, in the first week of treatment with rofecoxib (which has been withdrawn from the market), and after 14–30 days with celecoxib.
The risk associated with ibuprofen was lower than the risk associated with the two cyclooxygenase-2 (COX-2) selective inhibitors, rofecoxib and celecoxib, and it was lower than the risk associated with the use of diclofenac.
There was no increased risk of death or recurrent MI associated with naproxen for the entire treatment duration, which exceeded 90 days in some cases. However, naproxen has been associated with an increased risk of GI bleeding, compared with rofecoxib, in one study, the authors noted.
The authors said they had no relevant financial disclosures.
Rituximab Seems Safe in RA Lung Disease
To date, no new significant safety signals associated with rituximab therapy in patients with severe rheumatoid arthritis who also have lung disease have been identified in an ongoing observational study of such patients, according to Dr. Shouvik Dass.
Overall, the incidence of serious infections has been low and is about what would be expected in these patients, who already have significant comorbidity because of longstanding RA and preexisting lung disease, said Dr. Dass, of the department of rheumatology, University of Leeds (England).
Because interstitial lung disease and various other lung diseases are more common among patients with RA, they may be considered contraindications to treatment with anti–tumor necrosis factor therapy, because of reports that the anti-TNF agents may cause, or at least may be associated with, major deteriorations in interstitial lung disease. Although these data are not definitive, as a result, the use of rituximab – a CD20-directed cytolytic antibody – may be increasing among patients with RA and pulmonary disease, said Dr. Dass.
“The effect of biologic therapies as a whole on lung disease is not completely clear,” he said in an interview. He also referred to data – mostly case reports– in the hematologic literature associating rituximab with worsening lung disease.
He and his associates reviewed the records of 67 patients with RA and concomitant lung disease who were treated with rituximab between 2004 and 2010 at their center, which he said has one of the largest single cohorts of RA patients treated with rituximab. The patients' mean age was 60 years and most (56) were female. The most common pulmonary diagnosis was interstitial lung disease (ILD) in 48 patients; 14 patients had chronic obstructive pulmonary disease (COPD), and 5 patients had bronchiectasis; 2 patients also had had a previous pulmonary empyema.
All patients received two infusions of 1,000 mg rituximab with methylprednisolone per course, which was repeated when RA became active again, at intervals of no less than 6 months. Half of the patients received at least two treatment cycles.
Over a median of about 2 years of follow-up (the range was about 8 months to 6.5 years) after treatment with rituximab, there were three deaths among these patients: 2 of the 48 patients with ILD and 1 of the 14 patients with COPD. The patient with COPD died of an infective COPD exacerbation 12 months after the third cycle of rituximab. One of the patients with ILD died of pneumonia and possible acute progression of ILD, Dr. Dass said, noting that clinical and CT changes attributable to either condition were observed 4 weeks after the patient had been treated with the first cycle of rituximab. Suicide was the cause of death in the second patient with ILD, 3 months after treatment with the first course of rituximab. Another three patients had a single episode of serious respiratory tract infection, which required hospital admission or treatment with intravenous antibiotics.
Based on these cases, there were no definite new significant safety signals that were observed “beyond that which might be expected in this group of patients with longstanding severe RA and concomitant lung disease,” Dr. Dass said.
However, he pointed out that the one death caused by respiratory deterioration was temporally related to rituximab therapy.
“B-cell depletion is now an important therapy for RA, and therefore this study aims to add insight into the safety and practical usage of rituximab,” he noted in the interview. “We hope to encourage ongoing review and follow-up of such patients.”
In the United States, rituximab is marketed as Rituxan by Genentech, a member of the Roche Group, and is a registered trademark of Biogen Idec.
Dr. Dass and two of his six coauthors disclosed that they are consultants for Roche. Dr. Dass received an award from EULAR for this research.
To date, no new significant safety signals associated with rituximab therapy in patients with severe rheumatoid arthritis who also have lung disease have been identified in an ongoing observational study of such patients, according to Dr. Shouvik Dass.
Overall, the incidence of serious infections has been low and is about what would be expected in these patients, who already have significant comorbidity because of longstanding RA and preexisting lung disease, said Dr. Dass, of the department of rheumatology, University of Leeds (England).
Because interstitial lung disease and various other lung diseases are more common among patients with RA, they may be considered contraindications to treatment with anti–tumor necrosis factor therapy, because of reports that the anti-TNF agents may cause, or at least may be associated with, major deteriorations in interstitial lung disease. Although these data are not definitive, as a result, the use of rituximab – a CD20-directed cytolytic antibody – may be increasing among patients with RA and pulmonary disease, said Dr. Dass.
“The effect of biologic therapies as a whole on lung disease is not completely clear,” he said in an interview. He also referred to data – mostly case reports– in the hematologic literature associating rituximab with worsening lung disease.
He and his associates reviewed the records of 67 patients with RA and concomitant lung disease who were treated with rituximab between 2004 and 2010 at their center, which he said has one of the largest single cohorts of RA patients treated with rituximab. The patients' mean age was 60 years and most (56) were female. The most common pulmonary diagnosis was interstitial lung disease (ILD) in 48 patients; 14 patients had chronic obstructive pulmonary disease (COPD), and 5 patients had bronchiectasis; 2 patients also had had a previous pulmonary empyema.
All patients received two infusions of 1,000 mg rituximab with methylprednisolone per course, which was repeated when RA became active again, at intervals of no less than 6 months. Half of the patients received at least two treatment cycles.
Over a median of about 2 years of follow-up (the range was about 8 months to 6.5 years) after treatment with rituximab, there were three deaths among these patients: 2 of the 48 patients with ILD and 1 of the 14 patients with COPD. The patient with COPD died of an infective COPD exacerbation 12 months after the third cycle of rituximab. One of the patients with ILD died of pneumonia and possible acute progression of ILD, Dr. Dass said, noting that clinical and CT changes attributable to either condition were observed 4 weeks after the patient had been treated with the first cycle of rituximab. Suicide was the cause of death in the second patient with ILD, 3 months after treatment with the first course of rituximab. Another three patients had a single episode of serious respiratory tract infection, which required hospital admission or treatment with intravenous antibiotics.
Based on these cases, there were no definite new significant safety signals that were observed “beyond that which might be expected in this group of patients with longstanding severe RA and concomitant lung disease,” Dr. Dass said.
However, he pointed out that the one death caused by respiratory deterioration was temporally related to rituximab therapy.
“B-cell depletion is now an important therapy for RA, and therefore this study aims to add insight into the safety and practical usage of rituximab,” he noted in the interview. “We hope to encourage ongoing review and follow-up of such patients.”
In the United States, rituximab is marketed as Rituxan by Genentech, a member of the Roche Group, and is a registered trademark of Biogen Idec.
Dr. Dass and two of his six coauthors disclosed that they are consultants for Roche. Dr. Dass received an award from EULAR for this research.
To date, no new significant safety signals associated with rituximab therapy in patients with severe rheumatoid arthritis who also have lung disease have been identified in an ongoing observational study of such patients, according to Dr. Shouvik Dass.
Overall, the incidence of serious infections has been low and is about what would be expected in these patients, who already have significant comorbidity because of longstanding RA and preexisting lung disease, said Dr. Dass, of the department of rheumatology, University of Leeds (England).
Because interstitial lung disease and various other lung diseases are more common among patients with RA, they may be considered contraindications to treatment with anti–tumor necrosis factor therapy, because of reports that the anti-TNF agents may cause, or at least may be associated with, major deteriorations in interstitial lung disease. Although these data are not definitive, as a result, the use of rituximab – a CD20-directed cytolytic antibody – may be increasing among patients with RA and pulmonary disease, said Dr. Dass.
“The effect of biologic therapies as a whole on lung disease is not completely clear,” he said in an interview. He also referred to data – mostly case reports– in the hematologic literature associating rituximab with worsening lung disease.
He and his associates reviewed the records of 67 patients with RA and concomitant lung disease who were treated with rituximab between 2004 and 2010 at their center, which he said has one of the largest single cohorts of RA patients treated with rituximab. The patients' mean age was 60 years and most (56) were female. The most common pulmonary diagnosis was interstitial lung disease (ILD) in 48 patients; 14 patients had chronic obstructive pulmonary disease (COPD), and 5 patients had bronchiectasis; 2 patients also had had a previous pulmonary empyema.
All patients received two infusions of 1,000 mg rituximab with methylprednisolone per course, which was repeated when RA became active again, at intervals of no less than 6 months. Half of the patients received at least two treatment cycles.
Over a median of about 2 years of follow-up (the range was about 8 months to 6.5 years) after treatment with rituximab, there were three deaths among these patients: 2 of the 48 patients with ILD and 1 of the 14 patients with COPD. The patient with COPD died of an infective COPD exacerbation 12 months after the third cycle of rituximab. One of the patients with ILD died of pneumonia and possible acute progression of ILD, Dr. Dass said, noting that clinical and CT changes attributable to either condition were observed 4 weeks after the patient had been treated with the first cycle of rituximab. Suicide was the cause of death in the second patient with ILD, 3 months after treatment with the first course of rituximab. Another three patients had a single episode of serious respiratory tract infection, which required hospital admission or treatment with intravenous antibiotics.
Based on these cases, there were no definite new significant safety signals that were observed “beyond that which might be expected in this group of patients with longstanding severe RA and concomitant lung disease,” Dr. Dass said.
However, he pointed out that the one death caused by respiratory deterioration was temporally related to rituximab therapy.
“B-cell depletion is now an important therapy for RA, and therefore this study aims to add insight into the safety and practical usage of rituximab,” he noted in the interview. “We hope to encourage ongoing review and follow-up of such patients.”
In the United States, rituximab is marketed as Rituxan by Genentech, a member of the Roche Group, and is a registered trademark of Biogen Idec.
Dr. Dass and two of his six coauthors disclosed that they are consultants for Roche. Dr. Dass received an award from EULAR for this research.
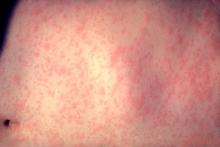
Measles Cases Spike: Importation the Cause
As of May 20, 118 cases of measles in people aged 3 months to 68 years had been reported in the United States this year, the highest number reported for this 19-week period in any year since 1996, according to the Centers for Disease Control and Prevention.
Most of the cases (105 or 89%) were associated with importation from other countries, an “unusually large number of importations” that was related to recent increases in measles in countries visited by U.S. travelers, according to the Morbidity and Mortality Weekly Report (2011;60:[Early Release]1–4). Most of the cases that were associated with importations from European countries were from France, where about 10,000 cases of measles have been reported from January through April this year. Between 2001 and 2008, the CDC received a median of 56 reports of measles annually.
Fifty-three (45%) of the cases were in people aged 20 years and older; of the rest, 18 (15%) were in children under age 12 months, 24 (20%) were children aged 1–4 years, and 23 (19%) were in individuals aged 5–19 years.
Of the 118 reported cases from 23 states and New York City, 105 (89%) had not been vaccinated. The cases included 45 U.S. residents aged 1–19 years, of whom 39 (87%) had not been vaccinated: In 24 cases, parents had claimed religious or personal exemption and 8 had missed opportunities to be vaccinated.
Of the 42 U.S. residents aged 20 and older who were among the cases, 35 (83%) had not been vaccinated, including 6 who declined vaccination because of “philosophical objections to vaccination.” Of the 33 U.S residents who were eligible to receive the vaccine and had traveled abroad, 30 (91%) were not vaccinated, and in one case (3%), the person had received one of the two recommended doses.
The size of the outbreaks ranged from 3 to 21 cases. The 21 cases were in a community in Minnesota where many children were not vaccinated because parents were concerned about the safety of the measles, mumps, and rubella (MMR) vaccine. That outbreak resulted in infection of at least seven infants who were too young to receive the MMR vaccine.
Nine outbreaks accounted for 58 (49%) of the 118 cases and, in 6 outbreaks, the index case acquired measles abroad; the source of the other 3 outbreaks could not be determined.
Of the 118 cases reported between Jan. 1, 2011, and May 20, 2011, there were no deaths or cases of encephalitis, but 40% (47) needed to be hospitalized, and there were nine cases of pneumonia.
Because cases of measles continue to be imported into the United States, clinicians should suspect measles in people with a febrile rash illness and “clinically compatible symptoms,” such as cough, coryza, and/or conjunctivitis, “who have recently traveled abroad or have had contact with travelers.” This is particularly true if the patient is unimmunized either by choice, by young age or a true contraindication to the vaccine. When measles is suspected, clinicians should use airborne isolation in a medical facility or home quarantine of the patient, along with known conatacts during the incubation period. Also, report the case immediately to the local health department, and obtain viral specimens for testing, according to the report.
As of May 20, 118 cases of measles in people aged 3 months to 68 years had been reported in the United States this year, the highest number reported for this 19-week period in any year since 1996, according to the Centers for Disease Control and Prevention.
Most of the cases (105 or 89%) were associated with importation from other countries, an “unusually large number of importations” that was related to recent increases in measles in countries visited by U.S. travelers, according to the Morbidity and Mortality Weekly Report (2011;60:[Early Release]1–4). Most of the cases that were associated with importations from European countries were from France, where about 10,000 cases of measles have been reported from January through April this year. Between 2001 and 2008, the CDC received a median of 56 reports of measles annually.
Fifty-three (45%) of the cases were in people aged 20 years and older; of the rest, 18 (15%) were in children under age 12 months, 24 (20%) were children aged 1–4 years, and 23 (19%) were in individuals aged 5–19 years.
Of the 118 reported cases from 23 states and New York City, 105 (89%) had not been vaccinated. The cases included 45 U.S. residents aged 1–19 years, of whom 39 (87%) had not been vaccinated: In 24 cases, parents had claimed religious or personal exemption and 8 had missed opportunities to be vaccinated.
Of the 42 U.S. residents aged 20 and older who were among the cases, 35 (83%) had not been vaccinated, including 6 who declined vaccination because of “philosophical objections to vaccination.” Of the 33 U.S residents who were eligible to receive the vaccine and had traveled abroad, 30 (91%) were not vaccinated, and in one case (3%), the person had received one of the two recommended doses.
The size of the outbreaks ranged from 3 to 21 cases. The 21 cases were in a community in Minnesota where many children were not vaccinated because parents were concerned about the safety of the measles, mumps, and rubella (MMR) vaccine. That outbreak resulted in infection of at least seven infants who were too young to receive the MMR vaccine.
Nine outbreaks accounted for 58 (49%) of the 118 cases and, in 6 outbreaks, the index case acquired measles abroad; the source of the other 3 outbreaks could not be determined.
Of the 118 cases reported between Jan. 1, 2011, and May 20, 2011, there were no deaths or cases of encephalitis, but 40% (47) needed to be hospitalized, and there were nine cases of pneumonia.
Because cases of measles continue to be imported into the United States, clinicians should suspect measles in people with a febrile rash illness and “clinically compatible symptoms,” such as cough, coryza, and/or conjunctivitis, “who have recently traveled abroad or have had contact with travelers.” This is particularly true if the patient is unimmunized either by choice, by young age or a true contraindication to the vaccine. When measles is suspected, clinicians should use airborne isolation in a medical facility or home quarantine of the patient, along with known conatacts during the incubation period. Also, report the case immediately to the local health department, and obtain viral specimens for testing, according to the report.
As of May 20, 118 cases of measles in people aged 3 months to 68 years had been reported in the United States this year, the highest number reported for this 19-week period in any year since 1996, according to the Centers for Disease Control and Prevention.
Most of the cases (105 or 89%) were associated with importation from other countries, an “unusually large number of importations” that was related to recent increases in measles in countries visited by U.S. travelers, according to the Morbidity and Mortality Weekly Report (2011;60:[Early Release]1–4). Most of the cases that were associated with importations from European countries were from France, where about 10,000 cases of measles have been reported from January through April this year. Between 2001 and 2008, the CDC received a median of 56 reports of measles annually.
Fifty-three (45%) of the cases were in people aged 20 years and older; of the rest, 18 (15%) were in children under age 12 months, 24 (20%) were children aged 1–4 years, and 23 (19%) were in individuals aged 5–19 years.
Of the 118 reported cases from 23 states and New York City, 105 (89%) had not been vaccinated. The cases included 45 U.S. residents aged 1–19 years, of whom 39 (87%) had not been vaccinated: In 24 cases, parents had claimed religious or personal exemption and 8 had missed opportunities to be vaccinated.
Of the 42 U.S. residents aged 20 and older who were among the cases, 35 (83%) had not been vaccinated, including 6 who declined vaccination because of “philosophical objections to vaccination.” Of the 33 U.S residents who were eligible to receive the vaccine and had traveled abroad, 30 (91%) were not vaccinated, and in one case (3%), the person had received one of the two recommended doses.
The size of the outbreaks ranged from 3 to 21 cases. The 21 cases were in a community in Minnesota where many children were not vaccinated because parents were concerned about the safety of the measles, mumps, and rubella (MMR) vaccine. That outbreak resulted in infection of at least seven infants who were too young to receive the MMR vaccine.
Nine outbreaks accounted for 58 (49%) of the 118 cases and, in 6 outbreaks, the index case acquired measles abroad; the source of the other 3 outbreaks could not be determined.
Of the 118 cases reported between Jan. 1, 2011, and May 20, 2011, there were no deaths or cases of encephalitis, but 40% (47) needed to be hospitalized, and there were nine cases of pneumonia.
Because cases of measles continue to be imported into the United States, clinicians should suspect measles in people with a febrile rash illness and “clinically compatible symptoms,” such as cough, coryza, and/or conjunctivitis, “who have recently traveled abroad or have had contact with travelers.” This is particularly true if the patient is unimmunized either by choice, by young age or a true contraindication to the vaccine. When measles is suspected, clinicians should use airborne isolation in a medical facility or home quarantine of the patient, along with known conatacts during the incubation period. Also, report the case immediately to the local health department, and obtain viral specimens for testing, according to the report.
Major Finding: Most of the measles cases – 105 of 118 – were
associated with importation from other countries; 105 (89%) had not been
vaccinated.
Data Source: Cases reported between Jan. 1, 2011, and May 20, 2011.
Disclosures: The authors had no relevant financial disclosures.
Rare Lymphoma Reports Continue in Young Patients on TNF Blockers
Cases of a rare, aggressive, and usually fatal lymphoma continue to be reported in people being treated with tumor necrosis factor blockers, azathioprine, and/or mercaptopurine, the Food and Drug Administration announced in an April 14 statement.
The reports of the lymphoma, hepatosplenic T-cell lymphoma (HSTCL), have primarily involved adolescents and young adults being treated with these agents for Crohn’s disease or ulcerative colitis. One patient, however, was being treated for psoriasis, and two others for rheumatoid arthritis.
Most patients were on a combination of treatments that are known to suppress the immune system, but there have been cases in patients taking azathioprine or mercaptopurine alone, the statement said.
"The risks and benefits of using TNF blockers, azathioprine, and/or mercaptopurine should be carefully weighed when prescribing these drugs to children and young adults, especially for the treatment of Crohn’s disease and ulcerative colitis," according to the FDA.
The statement recommends that health care professionals monitor patients on these treatments for malignancies and educate patients and their caregivers about the signs and symptoms of HSTCL, which can include splenomegaly, hepatomegaly, abdominal pain, persistent fever, night sweats, and weight loss.
The statement also notes that people with rheumatoid arthritis, Crohn’s, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis "may be more likely to develop lymphoma," compared with the general U.S. population, making it difficult to estimate the increased risk of malignancies associated with TNF blockers, azathioprine and/or mercaptopurine.
As of Dec. 31, 2010, the FDA’s Adverse Event Reporting System (AERS), the medical literature, and the Cancer Survivors Network had received the following unduplicated reports of HSTCL:
20 cases in patients taking infliximab (Remicade), including 18 patients also taking mercaptopurine or azathioprine.
• 1 case in a patient taking etanercept (Enbrel).
• 2 cases in patients taking adalimumab (Humira).
• 5 cases in patients taking a combination of infliximab and adalimumab (including 4 patients also taking mercaptopurine or azathioprine).
• 12 cases in patients taking azathioprine.
• 3 cases in patients taking mercaptopurine.
No cases have been reported in the TNF blockers certolizumab pegol (Cimzia) and golimumab (Simponi).
Reports of serious adverse events associated with these and other drugs should be reported online to the FDA’s MedWatch program or by phone to 800-332-1088.
Cases of a rare, aggressive, and usually fatal lymphoma continue to be reported in people being treated with tumor necrosis factor blockers, azathioprine, and/or mercaptopurine, the Food and Drug Administration announced in an April 14 statement.
The reports of the lymphoma, hepatosplenic T-cell lymphoma (HSTCL), have primarily involved adolescents and young adults being treated with these agents for Crohn’s disease or ulcerative colitis. One patient, however, was being treated for psoriasis, and two others for rheumatoid arthritis.
Most patients were on a combination of treatments that are known to suppress the immune system, but there have been cases in patients taking azathioprine or mercaptopurine alone, the statement said.
"The risks and benefits of using TNF blockers, azathioprine, and/or mercaptopurine should be carefully weighed when prescribing these drugs to children and young adults, especially for the treatment of Crohn’s disease and ulcerative colitis," according to the FDA.
The statement recommends that health care professionals monitor patients on these treatments for malignancies and educate patients and their caregivers about the signs and symptoms of HSTCL, which can include splenomegaly, hepatomegaly, abdominal pain, persistent fever, night sweats, and weight loss.
The statement also notes that people with rheumatoid arthritis, Crohn’s, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis "may be more likely to develop lymphoma," compared with the general U.S. population, making it difficult to estimate the increased risk of malignancies associated with TNF blockers, azathioprine and/or mercaptopurine.
As of Dec. 31, 2010, the FDA’s Adverse Event Reporting System (AERS), the medical literature, and the Cancer Survivors Network had received the following unduplicated reports of HSTCL:
20 cases in patients taking infliximab (Remicade), including 18 patients also taking mercaptopurine or azathioprine.
• 1 case in a patient taking etanercept (Enbrel).
• 2 cases in patients taking adalimumab (Humira).
• 5 cases in patients taking a combination of infliximab and adalimumab (including 4 patients also taking mercaptopurine or azathioprine).
• 12 cases in patients taking azathioprine.
• 3 cases in patients taking mercaptopurine.
No cases have been reported in the TNF blockers certolizumab pegol (Cimzia) and golimumab (Simponi).
Reports of serious adverse events associated with these and other drugs should be reported online to the FDA’s MedWatch program or by phone to 800-332-1088.
Cases of a rare, aggressive, and usually fatal lymphoma continue to be reported in people being treated with tumor necrosis factor blockers, azathioprine, and/or mercaptopurine, the Food and Drug Administration announced in an April 14 statement.
The reports of the lymphoma, hepatosplenic T-cell lymphoma (HSTCL), have primarily involved adolescents and young adults being treated with these agents for Crohn’s disease or ulcerative colitis. One patient, however, was being treated for psoriasis, and two others for rheumatoid arthritis.
Most patients were on a combination of treatments that are known to suppress the immune system, but there have been cases in patients taking azathioprine or mercaptopurine alone, the statement said.
"The risks and benefits of using TNF blockers, azathioprine, and/or mercaptopurine should be carefully weighed when prescribing these drugs to children and young adults, especially for the treatment of Crohn’s disease and ulcerative colitis," according to the FDA.
The statement recommends that health care professionals monitor patients on these treatments for malignancies and educate patients and their caregivers about the signs and symptoms of HSTCL, which can include splenomegaly, hepatomegaly, abdominal pain, persistent fever, night sweats, and weight loss.
The statement also notes that people with rheumatoid arthritis, Crohn’s, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis "may be more likely to develop lymphoma," compared with the general U.S. population, making it difficult to estimate the increased risk of malignancies associated with TNF blockers, azathioprine and/or mercaptopurine.
As of Dec. 31, 2010, the FDA’s Adverse Event Reporting System (AERS), the medical literature, and the Cancer Survivors Network had received the following unduplicated reports of HSTCL:
20 cases in patients taking infliximab (Remicade), including 18 patients also taking mercaptopurine or azathioprine.
• 1 case in a patient taking etanercept (Enbrel).
• 2 cases in patients taking adalimumab (Humira).
• 5 cases in patients taking a combination of infliximab and adalimumab (including 4 patients also taking mercaptopurine or azathioprine).
• 12 cases in patients taking azathioprine.
• 3 cases in patients taking mercaptopurine.
No cases have been reported in the TNF blockers certolizumab pegol (Cimzia) and golimumab (Simponi).
Reports of serious adverse events associated with these and other drugs should be reported online to the FDA’s MedWatch program or by phone to 800-332-1088.
FDA Approves Narrow-Spectrum Antibiotic for C. Difficile
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Approves Narrow-Spectrum Antibiotic for C. difficile
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Approves Narrow-Spectrum Antibiotic for C. Difficile
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
The Food and Drug Administration on May 27 approved the orally administered macrolide antibiotic fidaxomicin for the treatment of Clostridium difficile–associated diarrhea.
In two phase III studies of patients with mild to severe Clostridium difficile–associated diarrhea (CDAD), the clinical cure rates at the end of 10 days of treatment were similar among those treated with fidaxomicin (88%) and among those treated with oral vancomycin (86%-87%). The clinical cure rate was defined as having three or fewer unformed bowel movements for 2 consecutive days or a marked reduction in the number of unformed bowel movements at the end of treatment, plus no further treatment required within 2 days of stopping medication.
More patients treated with fidaxomicin had a lower recurrence rate after treatment ended, compared with those treated with vancomycin, according to the FDA.
Previously, vancomycin was the only other drug treatment approved specifically for treating C. difficile; oral metronidazole is used off label for this indication.
Fidaxomicin – which is taken twice a day for 10 days – has a narrow spectrum of activity, with bactericidal activity against C. difficile, and is poorly absorbed and locally active in the gastrointestinal tract, according to its manufacturer, Optimer Pharmaceuticals Inc. The company will market the drug as Dificid.
"To maintain the effectiveness of Dificid, and to reduce the development of drug-resistant bacteria, the drug should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile," the FDA statement said.
The most common side effects reported with fidaxomicin included nausea, vomiting, headache, abdominal pain, and diarrhea, according to the FDA statement.
At a meeting in April, an FDA advisory panel unanimously agreed that the trials indicated that fidaxomicin was a safe and effective treatment for CDAD. The panel recommended that leukopenia, neutropenia, and GI bleeding in patients treated with the antibiotic should be followed after approval, because the rates of these adverse events were slightly higher among fidaxomicin-treated patients in the studies.
At the meeting, company officials said the company was developing an oral suspension formulation and was planning pediatric studies of the drug.
FROM THE FOOD AND DRUG ADMINISTRATION
U.S. Measles Cases Highest Since 1996
As of May 20, 118 cases of measles in people aged 3 months to 68 years had been reported in the United States this year, the highest number reported for this 19-week period in any year since 1996, according to the Centers for Disease Control and Prevention.
Most of the cases (105 or 89%) were associated with importation from other countries, an "unusually large number of importations" that was related to recent increases in measles in countries visited by U.S. travelers, according to the May 24 Morbidity and Mortality Weekly Report (MMWR 60 [Early Release]; 1-4). Most of the cases that were associated with importations from European countries were from France, where about 10,000 cases of measles have been reported from January through April this year. Between 2001 and 2008, the CDC received a median of 56 reports of measles annually.
Fifty-three (45%) of the cases were in people aged 20 years and older; of the rest, 18 (15%) were in children under age 12 months, 24 (20%) were children aged 1-4 years, and 23 (19%) were in individuals aged 5-19 years.
Of the 118 reported cases from 23 states and New York City, 105 (89%) had not been vaccinated. The cases included 45 U.S. residents aged 1-19 years, of whom 39 (87%) had not been vaccinated: In 24 cases, parents had claimed religious or personal exemption and 8 had missed opportunities to be vaccinated.
Of the 42 U.S. residents aged 20 and older who were among the cases, 35 (83%) had not been vaccinated, including 6 who declined vaccination because of "philosophical objections to vaccination." Of the 33 U.S residents who were eligible to receive the vaccine and had traveled abroad, 30 (91%) were not vaccinated, and in one case (3%), the person had received one of the two recommended doses.
The size of the outbreaks ranged from 3 to 21 cases. The 21 cases were in a community in Minnesota where many children were not vaccinated because parents were concerned about the safety of the measles, mumps, and rubella vaccine (MMR). That outbreak resulted in infection of at least seven infants who were too young to receive the MMR vaccine.
Nine outbreaks accounted for 58 (49%) of the 118 cases and, in 6 outbreaks, the index case acquired measles abroad; the source of the other 3 outbreaks could not be determined.
Of the 118 cases reported between Jan. 1, 2011 and May 20, 2011, there were no deaths or cases of encephalitis, but 40% (47) needed to be hospitalized, and there were nine cases of pneumonia.
Because cases of measles continue to be imported into the United States, clinicians should suspect measles in people with a febrile rash illness and "clinically compatible symptoms," such as cough, coryza, and/or conjunctivitis, "who have recently traveled abroad or have had contact with travelers." When measles is suspected, clinicians should isolate the patient, report the case immediately to the local health department, and obtain viral specimens for testing, according to the report.
As of May 20, 118 cases of measles in people aged 3 months to 68 years had been reported in the United States this year, the highest number reported for this 19-week period in any year since 1996, according to the Centers for Disease Control and Prevention.
Most of the cases (105 or 89%) were associated with importation from other countries, an "unusually large number of importations" that was related to recent increases in measles in countries visited by U.S. travelers, according to the May 24 Morbidity and Mortality Weekly Report (MMWR 60 [Early Release]; 1-4). Most of the cases that were associated with importations from European countries were from France, where about 10,000 cases of measles have been reported from January through April this year. Between 2001 and 2008, the CDC received a median of 56 reports of measles annually.
Fifty-three (45%) of the cases were in people aged 20 years and older; of the rest, 18 (15%) were in children under age 12 months, 24 (20%) were children aged 1-4 years, and 23 (19%) were in individuals aged 5-19 years.
Of the 118 reported cases from 23 states and New York City, 105 (89%) had not been vaccinated. The cases included 45 U.S. residents aged 1-19 years, of whom 39 (87%) had not been vaccinated: In 24 cases, parents had claimed religious or personal exemption and 8 had missed opportunities to be vaccinated.
Of the 42 U.S. residents aged 20 and older who were among the cases, 35 (83%) had not been vaccinated, including 6 who declined vaccination because of "philosophical objections to vaccination." Of the 33 U.S residents who were eligible to receive the vaccine and had traveled abroad, 30 (91%) were not vaccinated, and in one case (3%), the person had received one of the two recommended doses.
The size of the outbreaks ranged from 3 to 21 cases. The 21 cases were in a community in Minnesota where many children were not vaccinated because parents were concerned about the safety of the measles, mumps, and rubella vaccine (MMR). That outbreak resulted in infection of at least seven infants who were too young to receive the MMR vaccine.
Nine outbreaks accounted for 58 (49%) of the 118 cases and, in 6 outbreaks, the index case acquired measles abroad; the source of the other 3 outbreaks could not be determined.
Of the 118 cases reported between Jan. 1, 2011 and May 20, 2011, there were no deaths or cases of encephalitis, but 40% (47) needed to be hospitalized, and there were nine cases of pneumonia.
Because cases of measles continue to be imported into the United States, clinicians should suspect measles in people with a febrile rash illness and "clinically compatible symptoms," such as cough, coryza, and/or conjunctivitis, "who have recently traveled abroad or have had contact with travelers." When measles is suspected, clinicians should isolate the patient, report the case immediately to the local health department, and obtain viral specimens for testing, according to the report.
As of May 20, 118 cases of measles in people aged 3 months to 68 years had been reported in the United States this year, the highest number reported for this 19-week period in any year since 1996, according to the Centers for Disease Control and Prevention.
Most of the cases (105 or 89%) were associated with importation from other countries, an "unusually large number of importations" that was related to recent increases in measles in countries visited by U.S. travelers, according to the May 24 Morbidity and Mortality Weekly Report (MMWR 60 [Early Release]; 1-4). Most of the cases that were associated with importations from European countries were from France, where about 10,000 cases of measles have been reported from January through April this year. Between 2001 and 2008, the CDC received a median of 56 reports of measles annually.
Fifty-three (45%) of the cases were in people aged 20 years and older; of the rest, 18 (15%) were in children under age 12 months, 24 (20%) were children aged 1-4 years, and 23 (19%) were in individuals aged 5-19 years.
Of the 118 reported cases from 23 states and New York City, 105 (89%) had not been vaccinated. The cases included 45 U.S. residents aged 1-19 years, of whom 39 (87%) had not been vaccinated: In 24 cases, parents had claimed religious or personal exemption and 8 had missed opportunities to be vaccinated.
Of the 42 U.S. residents aged 20 and older who were among the cases, 35 (83%) had not been vaccinated, including 6 who declined vaccination because of "philosophical objections to vaccination." Of the 33 U.S residents who were eligible to receive the vaccine and had traveled abroad, 30 (91%) were not vaccinated, and in one case (3%), the person had received one of the two recommended doses.
The size of the outbreaks ranged from 3 to 21 cases. The 21 cases were in a community in Minnesota where many children were not vaccinated because parents were concerned about the safety of the measles, mumps, and rubella vaccine (MMR). That outbreak resulted in infection of at least seven infants who were too young to receive the MMR vaccine.
Nine outbreaks accounted for 58 (49%) of the 118 cases and, in 6 outbreaks, the index case acquired measles abroad; the source of the other 3 outbreaks could not be determined.
Of the 118 cases reported between Jan. 1, 2011 and May 20, 2011, there were no deaths or cases of encephalitis, but 40% (47) needed to be hospitalized, and there were nine cases of pneumonia.
Because cases of measles continue to be imported into the United States, clinicians should suspect measles in people with a febrile rash illness and "clinically compatible symptoms," such as cough, coryza, and/or conjunctivitis, "who have recently traveled abroad or have had contact with travelers." When measles is suspected, clinicians should isolate the patient, report the case immediately to the local health department, and obtain viral specimens for testing, according to the report.
FROM MMWR
Major Finding: Most measles cases – 105 of 118 – were associated with importation from other countries; 105 (89%) had not been vaccinated.
Data Source: Cases reported between Jan. 1, 2011 and May 20, 2011
Disclosures: The authors had no relevant financial disclosures.