User login
Secukinumab Earns FDA Approval for Plaque Psoriasis
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
Secukinumab earns FDA approval for plaque psoriasis
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
Consider phototherapy for certain psoriasis patients
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
LAS VEGAS – Psoriasis patients who are unresponsive to topical therapy, do not have psoriatic arthritis, and can make regular office visits may be candidates for successful treatment with phototherapy.
A patient with type II skin and extensive plaque psoriasis could be treated with a targeted NB-UVB laser, Dr. Kenneth B. Gordon said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, it’s important to evaluate each patient individually on phototherapy decisions, rather than relying on published protocols, noted Dr. Gordon of Northwestern University Feinberg School of Medicine in Chicago.
The advantages of the NB-UVB laser for psoriasis include a high rate of response (data show more than 75% of patients achieve PASI 75), the ability to avoid treating uninvolved skin, and potential long-term clearing of lesions, he said.
For someone with extensive plaque psoriasis, Dr. Gordon said he would start with 3 treatments per week, at 200 mJ, and increase the fluence by 25 mJ if necessary. “A good response can be expected in 4-6 weeks,” he said.
As for long-term treatment, “I leave it up to the patient but offer long-term maintenance,” said Dr. Gordon. Long-term efficacy of NB-UVB for plaque psoriasis has not been well studied, and safety data are unknown. “It is unclear whether there is a skin cancer risk,” he said. Reducing the number of exposures for a patient on maintenance therapy may be an option, he added.
Short-term treatment with NB-UVB can be effective in some psoriasis patients, such as in cases of an acute flare of guttate psoriasis, Dr. Gordon noted. Psoralen and UV light therapy (PUVA) is another option, he said.
Phototherapy also has a role in managing psoriasis in conjunction with retinoids, Dr. Gordon said. He cited an example of a 59-year-old woman with palmar psoriasis. For this patient, he said he would recommend starting with acitretin for approximately 1 month, if the patient tolerates it. “If the acitretin works by itself, no need to add phototherapy,” he said. However, if UVB is added, account for the photosensitizing agent, he emphasized. “Decrease the starting dose by 25-50 mJ or decrease the skin type by one,” he said.
Some psoriasis patients will not benefit from phototherapy, particularly those with erythroderma, said Dr. Gordon. “If skin is highly inflamed, phototherapy could induce easy burning, Koebnerization,” or other complications, he noted.
Dr. Gordon disclosed that his department at Northwestern derives income from phototherapy, but he personally does not. He also disclosed receiving research grants from and/or serving as a consultant to multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, and Janssen.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Dermatologists counsel psoriasis patients on obesity, less on alcohol and tobacco
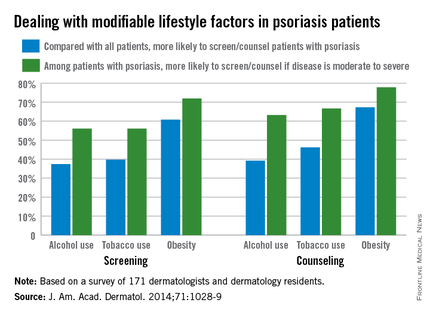
Less than half of dermatologists screen or counsel psoriasis patients about alcohol or tobacco use, compared with all patients, but approximately 60% screen or counsel psoriasis patients about obesity, based on data from an online survey of 171 dermatologists and dermatology residents.
The findings were published in a letter to the editor in the November issue of the Journal of the American Academy of Dermatology (2014;71:1028-9).
However, among patients with psoriasis, more than two-thirds of the respondents said they were more likely to both screen and counsel patients with moderate to severe psoriasis about alcohol, tobacco, and obesity, compared with psoriasis patients overall.
Previous research suggests that if psoriasis patients’ issues with alcohol, tobacco, and obesity are addressed, “patients may experience disease improvement, emphasizing the importance of screening and counseling,” wrote Brandon L. Adler and Aimee E. Krausz, medical students at Albert Einstein College of Medicine, Bronx, N.Y., and their colleagues.
Overall, 87% of respondents believed themselves responsible for screening for the three risk factors, but 56% believed themselves responsible for counseling.
Although the results were limited by the focus on academic dermatologists, the findings suggest a need to improve dermatologists’ confidence in counseling patients, the researchers noted. “Systematic training and effective counseling instruments would empower practitioners to translate this knowledge into clinical practice.”
The study was supported by the Dermatology Foundation Career Development Awards Program.
The researchers had no financial conflicts to disclose.
|
Less than half of dermatologists screen or counsel psoriasis patients about alcohol or tobacco use, compared with all patients, but approximately 60% screen or counsel psoriasis patients about obesity, based on data from an online survey of 171 dermatologists and dermatology residents.
The findings were published in a letter to the editor in the November issue of the Journal of the American Academy of Dermatology (2014;71:1028-9).
However, among patients with psoriasis, more than two-thirds of the respondents said they were more likely to both screen and counsel patients with moderate to severe psoriasis about alcohol, tobacco, and obesity, compared with psoriasis patients overall.
Previous research suggests that if psoriasis patients’ issues with alcohol, tobacco, and obesity are addressed, “patients may experience disease improvement, emphasizing the importance of screening and counseling,” wrote Brandon L. Adler and Aimee E. Krausz, medical students at Albert Einstein College of Medicine, Bronx, N.Y., and their colleagues.
Overall, 87% of respondents believed themselves responsible for screening for the three risk factors, but 56% believed themselves responsible for counseling.
Although the results were limited by the focus on academic dermatologists, the findings suggest a need to improve dermatologists’ confidence in counseling patients, the researchers noted. “Systematic training and effective counseling instruments would empower practitioners to translate this knowledge into clinical practice.”
The study was supported by the Dermatology Foundation Career Development Awards Program.
The researchers had no financial conflicts to disclose.
|

Less than half of dermatologists screen or counsel psoriasis patients about alcohol or tobacco use, compared with all patients, but approximately 60% screen or counsel psoriasis patients about obesity, based on data from an online survey of 171 dermatologists and dermatology residents.
The findings were published in a letter to the editor in the November issue of the Journal of the American Academy of Dermatology (2014;71:1028-9).
However, among patients with psoriasis, more than two-thirds of the respondents said they were more likely to both screen and counsel patients with moderate to severe psoriasis about alcohol, tobacco, and obesity, compared with psoriasis patients overall.
Previous research suggests that if psoriasis patients’ issues with alcohol, tobacco, and obesity are addressed, “patients may experience disease improvement, emphasizing the importance of screening and counseling,” wrote Brandon L. Adler and Aimee E. Krausz, medical students at Albert Einstein College of Medicine, Bronx, N.Y., and their colleagues.
Overall, 87% of respondents believed themselves responsible for screening for the three risk factors, but 56% believed themselves responsible for counseling.
Although the results were limited by the focus on academic dermatologists, the findings suggest a need to improve dermatologists’ confidence in counseling patients, the researchers noted. “Systematic training and effective counseling instruments would empower practitioners to translate this knowledge into clinical practice.”
The study was supported by the Dermatology Foundation Career Development Awards Program.
The researchers had no financial conflicts to disclose.
|

FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
VIDEO: What Works Now and What’s Up Next for Laser Treatments of Vascular Malformations
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: What works now and what’s up next for laser treatments of vascular malformations
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pulsed dye lasers have stood the test of time for treating vascular malformations, rosacea, and telangectases, Dr. Christopher Zachary said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
However, Dr. Zachary noted in an interview at the meeting that the field of laser therapy is on the cusp of new innovation, not only in devices, but also in how the devices are used. Different wavelengths and combination treatments will provide more options for patients, he explained. “These are great times for those who are interested in treating vascular problems,” he said.
Dr. Zachary disclosed serving as a consultant for Cutera and Zeltiq, on the speakers bureau for Cynosure and Solta, and on the advisory board for Zeltiq.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: Benzoyl peroxide hits the shower
LAS VEGAS – Benzoyl peroxide can be particularly effective on truncal acne when used as short contact therapy, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Stein Gold explained how she has changed her practice with regard to benzoyl peroxide for some of her acne patients.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Benzoyl peroxide can be particularly effective on truncal acne when used as short contact therapy, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Stein Gold explained how she has changed her practice with regard to benzoyl peroxide for some of her acne patients.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Benzoyl peroxide can be particularly effective on truncal acne when used as short contact therapy, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Stein Gold explained how she has changed her practice with regard to benzoyl peroxide for some of her acne patients.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Apremilast succeeds against nail, scalp, palmoplantar psoriasis
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Key clinical point: Apremilast significantly improved psoriasis in a subset of patient with nail, scalp, and palmoplantar involvement.
Major finding: After 16 weeks of twice-daily apremilast, significantly more patients with nail psoriasis achieved NAPSI-50, compared with placebo patients (45% vs. 19%, respectively).
Data source: Analysis of data from ESTEEM 2.
Disclosures:Dr. Crowley reported receiving fees from Celgene during the study and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
VIDEO: Don’t discount topical therapy for psoriasis
LAS VEGAS– Don’t underestimate the value of topical therapy for treating psoriasis patients, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Stein Gold explained how to overcome some of the compliance issues associated with topical therapies for psoriasis, and offered her suggestions for managing side effects in an interview at the meeting.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Don’t underestimate the value of topical therapy for treating psoriasis patients, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Stein Gold explained how to overcome some of the compliance issues associated with topical therapies for psoriasis, and offered her suggestions for managing side effects in an interview at the meeting.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Don’t underestimate the value of topical therapy for treating psoriasis patients, Dr. Linda Stein Gold said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Stein Gold explained how to overcome some of the compliance issues associated with topical therapies for psoriasis, and offered her suggestions for managing side effects in an interview at the meeting.
Dr. Stein Gold disclosed relationships with multiple pharmaceutical companies including Galderma, Stiefel, Allergan, Valeant, Ranbaxy, Promius, and Actavis.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: When atopic dermatitis and contact dermatitis collide
LAS VEGAS– Atopic dermatitis patients may be at increased risk for contact as well, Dr. Joseph Fowler said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Fowler reviewed several emerging allergens, explained how contact dermatitis and atopic dermatitis interact, and shared some tips for identifying and managing both conditions when they occur simultaneously.
Dr. Fowler disclosed relationships with multiple pharmaceutical companies, none of which were relevant to the content of this video. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Atopic dermatitis patients may be at increased risk for contact as well, Dr. Joseph Fowler said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Fowler reviewed several emerging allergens, explained how contact dermatitis and atopic dermatitis interact, and shared some tips for identifying and managing both conditions when they occur simultaneously.
Dr. Fowler disclosed relationships with multiple pharmaceutical companies, none of which were relevant to the content of this video. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS– Atopic dermatitis patients may be at increased risk for contact as well, Dr. Joseph Fowler said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Fowler reviewed several emerging allergens, explained how contact dermatitis and atopic dermatitis interact, and shared some tips for identifying and managing both conditions when they occur simultaneously.
Dr. Fowler disclosed relationships with multiple pharmaceutical companies, none of which were relevant to the content of this video. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR