User login
Psoriasis patients’ QOL improved with calcipotriene/betamethasone
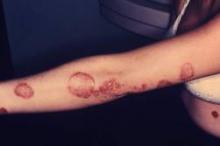
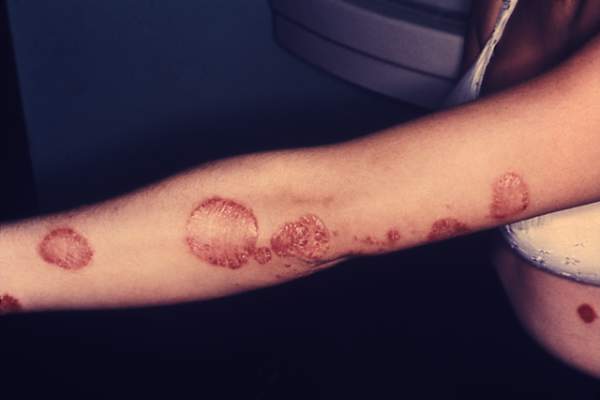
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Key clinical point: Calcipotriene 0.005% plus betamethasone diproprionate 0.064% was safe and effective and improved patients’ perceived quality of life.
Major finding: The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8.
Data source: Data from 147 adults aged 18 years and older with psoraisis vulgaris of an average of 11 years’ duration.
Disclosures: Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study.
VIDEO: How the latest topical antifungals are working for patients
LAS VEGAS – The latest topical treatments “are going to change the way we treat onychomycosis,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Pariser of Eastern Virginia Medical School, Norfolk, explained how the newly approved topical onychomycosis drugs work and shared some treatment strategies. Do they work even when patients are wearing nail polish? Tune in and find out.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – The latest topical treatments “are going to change the way we treat onychomycosis,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Pariser of Eastern Virginia Medical School, Norfolk, explained how the newly approved topical onychomycosis drugs work and shared some treatment strategies. Do they work even when patients are wearing nail polish? Tune in and find out.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – The latest topical treatments “are going to change the way we treat onychomycosis,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In an interview at the meeting, Dr. Pariser of Eastern Virginia Medical School, Norfolk, explained how the newly approved topical onychomycosis drugs work and shared some treatment strategies. Do they work even when patients are wearing nail polish? Tune in and find out.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
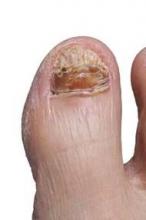
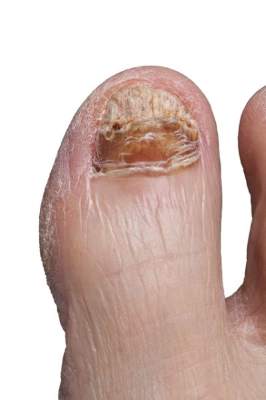
New onychomycosis drugs show similar success and side effects
LAS VEGAS – The latest topical therapies for onychomycosis are strong enough to penetrate nail polish, but patients report few side effects.
“New topical antifungals have improved the challenge of nail penetration and enhanced the efficacy of topical therapy,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Two drugs, efinaconazole and tavaborole, have shown effectiveness against onychomycosis in clinical trials since 2013, and both have been approved by the Food and Drug Administration, said Dr. Pariser, a dermatologist in private practice in Norfolk, Va., and a professor at Eastern Virginia Medical School, Norfolk.
Significantly more patients in studies of both medications showed improvement after 52 weeks of treatment, compared with control patients, but the clinical trials of the two drugs aren’t directly comparable for several reasons, Dr. Pariser noted. The studies differed in their entry criteria, including patient age and nail involvement, and, in some cases, nails were clipped prior to clinical evaluation. In addition, the investigators in the studies used different laboratories, and the definitions of treatment success and investigators’ interpretations of clinical cure may vary, he noted.
Reports of adverse events related to the drugs were low for both. Although 65% of 801 patients in a pooled analysis of efinaconazole data reported at least one adverse event, only 8% of these events were deemed related to the study drug. No serious drug-related adverse events were reported in an analysis of 1,186 patients treated with tavaborole who reported at least one treatment-related adverse event.
The most common adverse events in efinaconazole patients included ingrown toenails, application-site dermatitis, and application-site vesicles, reported in approximately 2% of patients, and application-site pain reported by 1%. Similarly, the most common adverse events in tavaborole patients included application site exfoliation and ingrown toenail in approximately 3% of patients, application site erythema in 2%, and application site dermatitis in 1%.
In addition, early findings from separate studies of both medications suggest that their penetration ability is not affected by nail polish, although more research is needed, Dr. Pariser said.
Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – The latest topical therapies for onychomycosis are strong enough to penetrate nail polish, but patients report few side effects.
“New topical antifungals have improved the challenge of nail penetration and enhanced the efficacy of topical therapy,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Two drugs, efinaconazole and tavaborole, have shown effectiveness against onychomycosis in clinical trials since 2013, and both have been approved by the Food and Drug Administration, said Dr. Pariser, a dermatologist in private practice in Norfolk, Va., and a professor at Eastern Virginia Medical School, Norfolk.
Significantly more patients in studies of both medications showed improvement after 52 weeks of treatment, compared with control patients, but the clinical trials of the two drugs aren’t directly comparable for several reasons, Dr. Pariser noted. The studies differed in their entry criteria, including patient age and nail involvement, and, in some cases, nails were clipped prior to clinical evaluation. In addition, the investigators in the studies used different laboratories, and the definitions of treatment success and investigators’ interpretations of clinical cure may vary, he noted.
Reports of adverse events related to the drugs were low for both. Although 65% of 801 patients in a pooled analysis of efinaconazole data reported at least one adverse event, only 8% of these events were deemed related to the study drug. No serious drug-related adverse events were reported in an analysis of 1,186 patients treated with tavaborole who reported at least one treatment-related adverse event.
The most common adverse events in efinaconazole patients included ingrown toenails, application-site dermatitis, and application-site vesicles, reported in approximately 2% of patients, and application-site pain reported by 1%. Similarly, the most common adverse events in tavaborole patients included application site exfoliation and ingrown toenail in approximately 3% of patients, application site erythema in 2%, and application site dermatitis in 1%.
In addition, early findings from separate studies of both medications suggest that their penetration ability is not affected by nail polish, although more research is needed, Dr. Pariser said.
Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – The latest topical therapies for onychomycosis are strong enough to penetrate nail polish, but patients report few side effects.
“New topical antifungals have improved the challenge of nail penetration and enhanced the efficacy of topical therapy,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Two drugs, efinaconazole and tavaborole, have shown effectiveness against onychomycosis in clinical trials since 2013, and both have been approved by the Food and Drug Administration, said Dr. Pariser, a dermatologist in private practice in Norfolk, Va., and a professor at Eastern Virginia Medical School, Norfolk.
Significantly more patients in studies of both medications showed improvement after 52 weeks of treatment, compared with control patients, but the clinical trials of the two drugs aren’t directly comparable for several reasons, Dr. Pariser noted. The studies differed in their entry criteria, including patient age and nail involvement, and, in some cases, nails were clipped prior to clinical evaluation. In addition, the investigators in the studies used different laboratories, and the definitions of treatment success and investigators’ interpretations of clinical cure may vary, he noted.
Reports of adverse events related to the drugs were low for both. Although 65% of 801 patients in a pooled analysis of efinaconazole data reported at least one adverse event, only 8% of these events were deemed related to the study drug. No serious drug-related adverse events were reported in an analysis of 1,186 patients treated with tavaborole who reported at least one treatment-related adverse event.
The most common adverse events in efinaconazole patients included ingrown toenails, application-site dermatitis, and application-site vesicles, reported in approximately 2% of patients, and application-site pain reported by 1%. Similarly, the most common adverse events in tavaborole patients included application site exfoliation and ingrown toenail in approximately 3% of patients, application site erythema in 2%, and application site dermatitis in 1%.
In addition, early findings from separate studies of both medications suggest that their penetration ability is not affected by nail polish, although more research is needed, Dr. Pariser said.
Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: Safe Use of Topical Cidofovir As a Weapon Against Stubborn Warts
NEWPORT BEACH, CALIF. – There is a role for topical cidofovir in the treatment of recalcitrant warts in children, Dr. James R. Treat said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The drug is not to be used as a first, second, or even third-line option, but can be effective in stubborn cases, said Dr. Treat of Children’s Hospital of Philadelphia.
In an interview at the meeting, Dr. Treat explained how and when he incorporates topical cidofovir and other strategies into managing verruca vulgaris when other treatments fail.
Dr. Treat had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – There is a role for topical cidofovir in the treatment of recalcitrant warts in children, Dr. James R. Treat said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The drug is not to be used as a first, second, or even third-line option, but can be effective in stubborn cases, said Dr. Treat of Children’s Hospital of Philadelphia.
In an interview at the meeting, Dr. Treat explained how and when he incorporates topical cidofovir and other strategies into managing verruca vulgaris when other treatments fail.
Dr. Treat had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – There is a role for topical cidofovir in the treatment of recalcitrant warts in children, Dr. James R. Treat said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The drug is not to be used as a first, second, or even third-line option, but can be effective in stubborn cases, said Dr. Treat of Children’s Hospital of Philadelphia.
In an interview at the meeting, Dr. Treat explained how and when he incorporates topical cidofovir and other strategies into managing verruca vulgaris when other treatments fail.
Dr. Treat had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
VIDEO: Safe use of topical cidofovir as a weapon against stubborn warts
NEWPORT BEACH, CALIF. – There is a role for topical cidofovir in the treatment of recalcitrant warts in children, Dr. James R. Treat said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The drug is not to be used as a first, second, or even third-line option, but can be effective in stubborn cases, said Dr. Treat of Children’s Hospital of Philadelphia.
In an interview at the meeting, Dr. Treat explained how and when he incorporates topical cidofovir and other strategies into managing verruca vulgaris when other treatments fail.
Dr. Treat had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – There is a role for topical cidofovir in the treatment of recalcitrant warts in children, Dr. James R. Treat said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The drug is not to be used as a first, second, or even third-line option, but can be effective in stubborn cases, said Dr. Treat of Children’s Hospital of Philadelphia.
In an interview at the meeting, Dr. Treat explained how and when he incorporates topical cidofovir and other strategies into managing verruca vulgaris when other treatments fail.
Dr. Treat had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – There is a role for topical cidofovir in the treatment of recalcitrant warts in children, Dr. James R. Treat said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The drug is not to be used as a first, second, or even third-line option, but can be effective in stubborn cases, said Dr. Treat of Children’s Hospital of Philadelphia.
In an interview at the meeting, Dr. Treat explained how and when he incorporates topical cidofovir and other strategies into managing verruca vulgaris when other treatments fail.
Dr. Treat had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
VIDEO: Dr. Sheila F. Friedlander discusses when and why to worry about acne in young children
NEWPORT BEACH, CALIF.– “The group we worry about are the 1- to 7-year-olds,” when it comes to new-onset acne, Dr. Sheila Fallon Friedlander said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Friedlander, a professor at the University of California, San Diego, explained the additional clinical signs that can indicate a serious problem, and what questions to ask parents.
Tune in for her tips on how to evaluate children aged 1-7 years with acne.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF.– “The group we worry about are the 1- to 7-year-olds,” when it comes to new-onset acne, Dr. Sheila Fallon Friedlander said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Friedlander, a professor at the University of California, San Diego, explained the additional clinical signs that can indicate a serious problem, and what questions to ask parents.
Tune in for her tips on how to evaluate children aged 1-7 years with acne.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF.– “The group we worry about are the 1- to 7-year-olds,” when it comes to new-onset acne, Dr. Sheila Fallon Friedlander said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Friedlander, a professor at the University of California, San Diego, explained the additional clinical signs that can indicate a serious problem, and what questions to ask parents.
Tune in for her tips on how to evaluate children aged 1-7 years with acne.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
VIDEO: A practical protocol for monitoring discoid lupus
NEWPORT BEACH, CALIF.– Patients with discoid lupus should be checked routinely for systemic disease, but complete autoantibody studies aren’t necessary for those patients with a prior work-up and a documented negative antinuclear antibodies, according to Dr. Ruth Ann Vleugels.
“The reason why we are doing these investigations is because between 5% and up to about 20% of our discoid lupus patients can develop systemic disease,” said Dr. Vleugels of Brigham and Women’s Hospital in Boston, Mass. Some patients don’t develop symptoms until years later, she noted.
In an interview at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Vleugels shared her protocol for managing discoid lupus patients. She had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF.– Patients with discoid lupus should be checked routinely for systemic disease, but complete autoantibody studies aren’t necessary for those patients with a prior work-up and a documented negative antinuclear antibodies, according to Dr. Ruth Ann Vleugels.
“The reason why we are doing these investigations is because between 5% and up to about 20% of our discoid lupus patients can develop systemic disease,” said Dr. Vleugels of Brigham and Women’s Hospital in Boston, Mass. Some patients don’t develop symptoms until years later, she noted.
In an interview at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Vleugels shared her protocol for managing discoid lupus patients. She had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF.– Patients with discoid lupus should be checked routinely for systemic disease, but complete autoantibody studies aren’t necessary for those patients with a prior work-up and a documented negative antinuclear antibodies, according to Dr. Ruth Ann Vleugels.
“The reason why we are doing these investigations is because between 5% and up to about 20% of our discoid lupus patients can develop systemic disease,” said Dr. Vleugels of Brigham and Women’s Hospital in Boston, Mass. Some patients don’t develop symptoms until years later, she noted.
In an interview at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Vleugels shared her protocol for managing discoid lupus patients. She had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
VIDEO: What’s on the horizon for headaches
BALTIMORE – Methods to prevent and treat migraines are lacking, but monoclonal antibodies and the calcitonin receptor system are showing promise, according to Dr. Peter Goadsby of the University of California, San Francisco.
“It’s an awful mishmash of things we have to offer,” Dr. Goadsby said at the annual meeting of the American Neurological Association. However, data presented at the meeting suggest that new options may be on the horizon.
In an interview at the meeting, Dr. Goadsby explained details of several studies that showed dramatic results, and the “tantalizing” implications for patients.
BALTIMORE – Methods to prevent and treat migraines are lacking, but monoclonal antibodies and the calcitonin receptor system are showing promise, according to Dr. Peter Goadsby of the University of California, San Francisco.
“It’s an awful mishmash of things we have to offer,” Dr. Goadsby said at the annual meeting of the American Neurological Association. However, data presented at the meeting suggest that new options may be on the horizon.
In an interview at the meeting, Dr. Goadsby explained details of several studies that showed dramatic results, and the “tantalizing” implications for patients.
BALTIMORE – Methods to prevent and treat migraines are lacking, but monoclonal antibodies and the calcitonin receptor system are showing promise, according to Dr. Peter Goadsby of the University of California, San Francisco.
“It’s an awful mishmash of things we have to offer,” Dr. Goadsby said at the annual meeting of the American Neurological Association. However, data presented at the meeting suggest that new options may be on the horizon.
In an interview at the meeting, Dr. Goadsby explained details of several studies that showed dramatic results, and the “tantalizing” implications for patients.
AT ANA 2014
VIDEO: Dr. Alan Menter discusses apremilast’s approval for psoriasis
NEWPORT BEACH, CALIF. – The approval of apremilast (Otezla) for the treatment of moderate to severe plaque psoriasis will make a significant impact on patient care, Dr. Alan Menter of Baylor University Medical Center, Dallas, Tex., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“I think where this drug is going to have a role is ... patients who are risk averse to needles, have contraindications to TNF-alpha agents ... patients who want a drug with minimal to no long-term side effects,” with the exception of the significant incidence of diarrhea within the early weeks of starting the drug, Dr. Menter explained. Clinicians should explain the risk of diarrhea to patients, and help them get through the first few months. The diarrhea “definitely does vanish with time,” he said. Overall, the risk of side effects is extremely low, he noted. “It is probably the safest drug we have approved for psoriasis today.”
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – The approval of apremilast (Otezla) for the treatment of moderate to severe plaque psoriasis will make a significant impact on patient care, Dr. Alan Menter of Baylor University Medical Center, Dallas, Tex., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“I think where this drug is going to have a role is ... patients who are risk averse to needles, have contraindications to TNF-alpha agents ... patients who want a drug with minimal to no long-term side effects,” with the exception of the significant incidence of diarrhea within the early weeks of starting the drug, Dr. Menter explained. Clinicians should explain the risk of diarrhea to patients, and help them get through the first few months. The diarrhea “definitely does vanish with time,” he said. Overall, the risk of side effects is extremely low, he noted. “It is probably the safest drug we have approved for psoriasis today.”
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – The approval of apremilast (Otezla) for the treatment of moderate to severe plaque psoriasis will make a significant impact on patient care, Dr. Alan Menter of Baylor University Medical Center, Dallas, Tex., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“I think where this drug is going to have a role is ... patients who are risk averse to needles, have contraindications to TNF-alpha agents ... patients who want a drug with minimal to no long-term side effects,” with the exception of the significant incidence of diarrhea within the early weeks of starting the drug, Dr. Menter explained. Clinicians should explain the risk of diarrhea to patients, and help them get through the first few months. The diarrhea “definitely does vanish with time,” he said. Overall, the risk of side effects is extremely low, he noted. “It is probably the safest drug we have approved for psoriasis today.”
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
VIDEO: What’s unique about treating acne in adult women
NEWPORT BEACH, CALIF. – Do more women today really have acne? Or are they simply more likely to seek help because they learn of new and better medications?
Acne often causes more psychosocial and psychological stress in adult women than in men or adolescents, Dr. Hilary Baldwin of SUNY Downstate Medical Center, Brooklyn, N.Y., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Baldwin explained what makes the treatment of acne in adult women distinct from acne treatment for men and adolescents, and what underused medications can yield success.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – Do more women today really have acne? Or are they simply more likely to seek help because they learn of new and better medications?
Acne often causes more psychosocial and psychological stress in adult women than in men or adolescents, Dr. Hilary Baldwin of SUNY Downstate Medical Center, Brooklyn, N.Y., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Baldwin explained what makes the treatment of acne in adult women distinct from acne treatment for men and adolescents, and what underused medications can yield success.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEWPORT BEACH, CALIF. – Do more women today really have acne? Or are they simply more likely to seek help because they learn of new and better medications?
Acne often causes more psychosocial and psychological stress in adult women than in men or adolescents, Dr. Hilary Baldwin of SUNY Downstate Medical Center, Brooklyn, N.Y., said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
In an interview at the meeting, Dr. Baldwin explained what makes the treatment of acne in adult women distinct from acne treatment for men and adolescents, and what underused medications can yield success.
SDEF and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR