User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
New knee osteoarthritis guidelines differ slightly from some previous recommendations
New knee osteoarthritis guidelines from Osteoarthritis Research Society International, an update of the group’s 2010 guidelines, recommend weight loss, education, exercise, and much more that’s familiar to practicing rheumatologists.
However, the group’s advice varies a bit from recent osteoarthritis (OA) guidelines separately issued by the American College of Rheumatology (ACR) and the American Academy of Orthopaedic Surgeons (AAOS).
For instance, the AAOS was neutral on acetaminophen and corticosteroid knee shots in its 2013 recommendations, citing a lack of evidence, but Osteoarthritis Research Society International (OARSI) guidelines recommend both in the absence of relevant comorbidities. Recent studies of steroid knee shots demonstrate "clinically significant short-term decreases in pain" that are "significantly greater" than are those with intra-articular hyaluronic acid, according to guidelines lead author Dr. Timothy McAlindon, chief of rheumatology at Tufts University in Boston, and his colleagues (Osteoarthritis Cartilage 2014 Jan. 23 [doi:10.1016/j.joca.2014.01.003]).
Meanwhile, hyaluronic acid knee injections provided greater long-term relief in another study, one of the findings that led OARSI, like the ACR, to suggest that the jury’s still out on hyaluronic acid. The AAOS rejected hyaluronic acid because of a lack of efficacy.
OARSI’s advice is based on recent literature and the expert opinion of its 13-member review panel; most members were rheumatologists and most were European. They voted on 13 nonpharmaceutical and 16 pharmaceutical treatments, deciding if they were appropriate, inappropriate, or – when evidence was scanty – of uncertain value for knee OA.
Treatments that made the cut as appropriate for all knee OA patients included biomechanical interventions, corticosteroid knee injections, land and water-based exercise, self-management and education, strength training, and weight management. Other treatments approved by the panelists included acetaminophen, warm soaks in mineral-rich water (balneotherapy), topical capsaicin, walking canes, duloxetine (Cymbalta), and NSAIDs when comorbidities don’t rule them out.
A variety of therapies fell into the "uncertain" category: acupuncture, avocado-soybean unsaponfiable supplements, chondroitin, crutches, diacerein, glucosamine, intra-articular hyaluronic acid, opioids, rose hip, transcutaneous electrical nerve stimulation, and therapeutic ultrasound. The group voted risedronate (Actonel) and neuromuscular electrical stimulation as inappropriate for knee OA because of a lack of evidence.
Newer findings have "increased safety concerns regarding use of treatments such as acetaminophen and opioids ... while evidence for use of treatments such as duloxetine, balneotherapy, and land-based exercises such as t’ai chi has strengthened," the authors noted.
OARSI reviewed biomechanical interventions more favorably than did the other groups, mostly because research now suggests that knee braces and foot orthoses improve function and decrease pain, stiffness, and drug use. Another trial supported wedged insoles as an alternative to valgus bracing.
Similar to the ACR guidelines but unlike stronger recommendations from the AAOS, OARSI also supported oral NSAIDs for most patients but was unsure about them when patients have heart disease and other relevant problems. The group recommended concomitant proton-pump inhibitors when gastric bleeding is a worry.
They noted that naproxen seems safer on the cardiovascular system than COX-2 inhibitors. Diclofenac appears to carry the highest risk for abnormal liver values, while celecoxib (Celebrex) seems to cause fewer ulcers but more cardiovascular problems. Topical NSAIDs work as well as oral formulations for OA knee pain, with fewer problems.
Unlike the ACR, the group also considered topical capsaicin "appropriate in patients without relevant comorbidities" and duloxetine "appropriate for most clinical subphenotypes," although adverse events – nausea, fatigue, and others – and the "availability of more targeted therapies predicated uncertain appropriateness for individuals with knee-only OA and comorbidities," they said.
OARSI funded the work, and is supported by companies promoting arthritis products. Most of the panelists have financial ties to Sanofi, Pfizer, Merck, Don-Joy, and other companies, but recused themselves when needed from voting.
OARSI didn’t tell us a lot about how treatments work in combination. That’s a major fault with all the guidelines. There’s very little literature on multimodal therapy, so guidelines consider treatments in isolation, whereas in rheumatology, we combine treatments.
OARSI’s guidelines were also very much influenced by Europeans. That’s not a bad thing, but in the United States, we don’t always treat people the same way as they do in Europe. Diacerein isn’t available here, and avocado-soy unsaponfiables aren’t popular.
 |
|
Also, OARSI doesn’t separate glucosamine sulfate from glucosamine hydrochloride. Glucosamine HCl clearly is not effective, but the literature has some confusion about whether glucosamine sulfate works. I’m also not sure it’s appropriate to lump all the intra-articular hyaluronics together; there may be differences among them.
Guidelines, in general, have minimal impact. There are too many for practicing physicians to track, and sometimes they contradict each other.
Dr. Roy Altman is a professor of medicine in the division of rheumatology at the University of California, Los Angeles. He works with Ferring, Pfizer, Novartis, and other companies on arthritis research projects, and is an editorial advisory board member of Rheumatology News. He was also an author of the 2012 American College of Rheumatology hand, knee, and hip OA guidelines.
OARSI didn’t tell us a lot about how treatments work in combination. That’s a major fault with all the guidelines. There’s very little literature on multimodal therapy, so guidelines consider treatments in isolation, whereas in rheumatology, we combine treatments.
OARSI’s guidelines were also very much influenced by Europeans. That’s not a bad thing, but in the United States, we don’t always treat people the same way as they do in Europe. Diacerein isn’t available here, and avocado-soy unsaponfiables aren’t popular.
 |
|
Also, OARSI doesn’t separate glucosamine sulfate from glucosamine hydrochloride. Glucosamine HCl clearly is not effective, but the literature has some confusion about whether glucosamine sulfate works. I’m also not sure it’s appropriate to lump all the intra-articular hyaluronics together; there may be differences among them.
Guidelines, in general, have minimal impact. There are too many for practicing physicians to track, and sometimes they contradict each other.
Dr. Roy Altman is a professor of medicine in the division of rheumatology at the University of California, Los Angeles. He works with Ferring, Pfizer, Novartis, and other companies on arthritis research projects, and is an editorial advisory board member of Rheumatology News. He was also an author of the 2012 American College of Rheumatology hand, knee, and hip OA guidelines.
OARSI didn’t tell us a lot about how treatments work in combination. That’s a major fault with all the guidelines. There’s very little literature on multimodal therapy, so guidelines consider treatments in isolation, whereas in rheumatology, we combine treatments.
OARSI’s guidelines were also very much influenced by Europeans. That’s not a bad thing, but in the United States, we don’t always treat people the same way as they do in Europe. Diacerein isn’t available here, and avocado-soy unsaponfiables aren’t popular.
 |
|
Also, OARSI doesn’t separate glucosamine sulfate from glucosamine hydrochloride. Glucosamine HCl clearly is not effective, but the literature has some confusion about whether glucosamine sulfate works. I’m also not sure it’s appropriate to lump all the intra-articular hyaluronics together; there may be differences among them.
Guidelines, in general, have minimal impact. There are too many for practicing physicians to track, and sometimes they contradict each other.
Dr. Roy Altman is a professor of medicine in the division of rheumatology at the University of California, Los Angeles. He works with Ferring, Pfizer, Novartis, and other companies on arthritis research projects, and is an editorial advisory board member of Rheumatology News. He was also an author of the 2012 American College of Rheumatology hand, knee, and hip OA guidelines.
New knee osteoarthritis guidelines from Osteoarthritis Research Society International, an update of the group’s 2010 guidelines, recommend weight loss, education, exercise, and much more that’s familiar to practicing rheumatologists.
However, the group’s advice varies a bit from recent osteoarthritis (OA) guidelines separately issued by the American College of Rheumatology (ACR) and the American Academy of Orthopaedic Surgeons (AAOS).
For instance, the AAOS was neutral on acetaminophen and corticosteroid knee shots in its 2013 recommendations, citing a lack of evidence, but Osteoarthritis Research Society International (OARSI) guidelines recommend both in the absence of relevant comorbidities. Recent studies of steroid knee shots demonstrate "clinically significant short-term decreases in pain" that are "significantly greater" than are those with intra-articular hyaluronic acid, according to guidelines lead author Dr. Timothy McAlindon, chief of rheumatology at Tufts University in Boston, and his colleagues (Osteoarthritis Cartilage 2014 Jan. 23 [doi:10.1016/j.joca.2014.01.003]).
Meanwhile, hyaluronic acid knee injections provided greater long-term relief in another study, one of the findings that led OARSI, like the ACR, to suggest that the jury’s still out on hyaluronic acid. The AAOS rejected hyaluronic acid because of a lack of efficacy.
OARSI’s advice is based on recent literature and the expert opinion of its 13-member review panel; most members were rheumatologists and most were European. They voted on 13 nonpharmaceutical and 16 pharmaceutical treatments, deciding if they were appropriate, inappropriate, or – when evidence was scanty – of uncertain value for knee OA.
Treatments that made the cut as appropriate for all knee OA patients included biomechanical interventions, corticosteroid knee injections, land and water-based exercise, self-management and education, strength training, and weight management. Other treatments approved by the panelists included acetaminophen, warm soaks in mineral-rich water (balneotherapy), topical capsaicin, walking canes, duloxetine (Cymbalta), and NSAIDs when comorbidities don’t rule them out.
A variety of therapies fell into the "uncertain" category: acupuncture, avocado-soybean unsaponfiable supplements, chondroitin, crutches, diacerein, glucosamine, intra-articular hyaluronic acid, opioids, rose hip, transcutaneous electrical nerve stimulation, and therapeutic ultrasound. The group voted risedronate (Actonel) and neuromuscular electrical stimulation as inappropriate for knee OA because of a lack of evidence.
Newer findings have "increased safety concerns regarding use of treatments such as acetaminophen and opioids ... while evidence for use of treatments such as duloxetine, balneotherapy, and land-based exercises such as t’ai chi has strengthened," the authors noted.
OARSI reviewed biomechanical interventions more favorably than did the other groups, mostly because research now suggests that knee braces and foot orthoses improve function and decrease pain, stiffness, and drug use. Another trial supported wedged insoles as an alternative to valgus bracing.
Similar to the ACR guidelines but unlike stronger recommendations from the AAOS, OARSI also supported oral NSAIDs for most patients but was unsure about them when patients have heart disease and other relevant problems. The group recommended concomitant proton-pump inhibitors when gastric bleeding is a worry.
They noted that naproxen seems safer on the cardiovascular system than COX-2 inhibitors. Diclofenac appears to carry the highest risk for abnormal liver values, while celecoxib (Celebrex) seems to cause fewer ulcers but more cardiovascular problems. Topical NSAIDs work as well as oral formulations for OA knee pain, with fewer problems.
Unlike the ACR, the group also considered topical capsaicin "appropriate in patients without relevant comorbidities" and duloxetine "appropriate for most clinical subphenotypes," although adverse events – nausea, fatigue, and others – and the "availability of more targeted therapies predicated uncertain appropriateness for individuals with knee-only OA and comorbidities," they said.
OARSI funded the work, and is supported by companies promoting arthritis products. Most of the panelists have financial ties to Sanofi, Pfizer, Merck, Don-Joy, and other companies, but recused themselves when needed from voting.
New knee osteoarthritis guidelines from Osteoarthritis Research Society International, an update of the group’s 2010 guidelines, recommend weight loss, education, exercise, and much more that’s familiar to practicing rheumatologists.
However, the group’s advice varies a bit from recent osteoarthritis (OA) guidelines separately issued by the American College of Rheumatology (ACR) and the American Academy of Orthopaedic Surgeons (AAOS).
For instance, the AAOS was neutral on acetaminophen and corticosteroid knee shots in its 2013 recommendations, citing a lack of evidence, but Osteoarthritis Research Society International (OARSI) guidelines recommend both in the absence of relevant comorbidities. Recent studies of steroid knee shots demonstrate "clinically significant short-term decreases in pain" that are "significantly greater" than are those with intra-articular hyaluronic acid, according to guidelines lead author Dr. Timothy McAlindon, chief of rheumatology at Tufts University in Boston, and his colleagues (Osteoarthritis Cartilage 2014 Jan. 23 [doi:10.1016/j.joca.2014.01.003]).
Meanwhile, hyaluronic acid knee injections provided greater long-term relief in another study, one of the findings that led OARSI, like the ACR, to suggest that the jury’s still out on hyaluronic acid. The AAOS rejected hyaluronic acid because of a lack of efficacy.
OARSI’s advice is based on recent literature and the expert opinion of its 13-member review panel; most members were rheumatologists and most were European. They voted on 13 nonpharmaceutical and 16 pharmaceutical treatments, deciding if they were appropriate, inappropriate, or – when evidence was scanty – of uncertain value for knee OA.
Treatments that made the cut as appropriate for all knee OA patients included biomechanical interventions, corticosteroid knee injections, land and water-based exercise, self-management and education, strength training, and weight management. Other treatments approved by the panelists included acetaminophen, warm soaks in mineral-rich water (balneotherapy), topical capsaicin, walking canes, duloxetine (Cymbalta), and NSAIDs when comorbidities don’t rule them out.
A variety of therapies fell into the "uncertain" category: acupuncture, avocado-soybean unsaponfiable supplements, chondroitin, crutches, diacerein, glucosamine, intra-articular hyaluronic acid, opioids, rose hip, transcutaneous electrical nerve stimulation, and therapeutic ultrasound. The group voted risedronate (Actonel) and neuromuscular electrical stimulation as inappropriate for knee OA because of a lack of evidence.
Newer findings have "increased safety concerns regarding use of treatments such as acetaminophen and opioids ... while evidence for use of treatments such as duloxetine, balneotherapy, and land-based exercises such as t’ai chi has strengthened," the authors noted.
OARSI reviewed biomechanical interventions more favorably than did the other groups, mostly because research now suggests that knee braces and foot orthoses improve function and decrease pain, stiffness, and drug use. Another trial supported wedged insoles as an alternative to valgus bracing.
Similar to the ACR guidelines but unlike stronger recommendations from the AAOS, OARSI also supported oral NSAIDs for most patients but was unsure about them when patients have heart disease and other relevant problems. The group recommended concomitant proton-pump inhibitors when gastric bleeding is a worry.
They noted that naproxen seems safer on the cardiovascular system than COX-2 inhibitors. Diclofenac appears to carry the highest risk for abnormal liver values, while celecoxib (Celebrex) seems to cause fewer ulcers but more cardiovascular problems. Topical NSAIDs work as well as oral formulations for OA knee pain, with fewer problems.
Unlike the ACR, the group also considered topical capsaicin "appropriate in patients without relevant comorbidities" and duloxetine "appropriate for most clinical subphenotypes," although adverse events – nausea, fatigue, and others – and the "availability of more targeted therapies predicated uncertain appropriateness for individuals with knee-only OA and comorbidities," they said.
OARSI funded the work, and is supported by companies promoting arthritis products. Most of the panelists have financial ties to Sanofi, Pfizer, Merck, Don-Joy, and other companies, but recused themselves when needed from voting.
FROM OSTEOARTHRITIS AND CARTILAGE
Military’s top doc: Medical lessons from Iraq saved lives in Boston bombings
Dr. Jonathan Woodson fielded e-mails in his Pentagon office last April from nervous colleagues in Boston.
His fellow vascular surgeons there wanted to know if more attacks were coming in the Marathon bombings. "When you work at the Pentagon, everyone thinks you have total situational awareness," said Dr. Woodson, assistant secretary of defense for health affairs.
His former colleagues, however, also wanted tips on how to care for bombing victims, and knew that military surgeons like Dr. Woodson had answers. Before President Obama tapped him in 2010 to become the military’s top doctor and oversee the Pentagon’s $50 billion-plus medical budget, he’d scrubbed in on battlefield cases in Kosovo, Iraq, and Afghanistan, in addition to performing his vascular surgery and administrative duties at Boston University and Boston Medical Center.
"We’ve been dealing with [improvised explosive device] injuries for over a decade," said Dr. Woodson, a brigadier general in the U.S. Army Reserve.
"The good news is, I had trained some of the folks who worked" on the bombing victims, and "they knew what my techniques were." Still, there were discussions about particular fixes; hypertrophic bone growths, infections, and other wound complications; and ways to help young people recover from devastating injuries. Many of the lessons came from Dr. Woodson’s military service.
The back-and-forth about the Boston attacks highlights how important it is for military and civilian physicians to cross-pollinate. In the past, "there’s always been a cadre of usually academic surgeons and physicians who maintain contact with the military. I think [it] is very important to formalize these connections. One of my strategic imperatives is to make sure we invest in partnerships [with] academic medical centers and key professional organizations" so that war innovations make it into civilian practice, and, in turn, are remembered for future conflicts.
"There’s the potential for this expertise to be lost," said Dr. Woodson, who was finishing a paper with two colleagues on the history of military medical innovations (Semin. Vasc. Surg. 2010;23:235-42) when he was nominated to serve as the secretary of defense’s principal adviser on health issues.
There’ve been many innovations in recent years, including the recognition that extensive fasciotomies can save badly damaged limbs.
At the start of Operation Iraqi Freedom in 2003, Dr. Woodson was in northern Kuwait running a makeshift U.S. military hospital out of a commandeered Kuwaiti facility. "As the troops went over the berm, we were taking the first wave of casualties," performing surgery in full chemical-attack gear. "Luckily," the incoming Scud missiles weren’t very accurate. It was a humbling experience," he said.
A young soldier was helicoptered in with a crushed leg after a hit to his armored vehicle. "We did an interposition graft to repair the popliteal artery," and, because of severe swelling, extensive fasciotomies to prevent compartment syndrome; the soldier still had his leg when he was airlifted out. Although not standard practice at the time, that case and others like it quickly demonstrated "the benefit of doing four-compartment fasciotomies early on to preserve limbs. Fasciotomies are now [used routinely] to address these injuries," Dr. Woodson said.
Tourniquets – which "prior to this conflict had a very bad reputation" – have proved their worth in recent battles as well, a lesson that helped save lives in Boston. Likewise, the military has learned the value of temporary vascular shunts, narrow tubing – most often Argyle carotid artery shunts – to bypass tears or reconnect severed vessels when there’s no time for a definitive repair. "We [also] came to understand very early on the need for aggressive blood replacement. Previously, protocols called for lactated Ringer’s or saline infusions, but we realized they just lead to massive soft tissue edema, increased lung water," and other problems, he said.
Currently, Dr. Woodson and his military colleagues are helping coach the rehabilitation of the Boston bombing victims. Rehab has seen "major advances in the military that are now finding their way into civilian practice," including improved prosthetics; better understanding of rehabilitation psychology; and recognition of subtle traumatic brain, eye, ear, and other injuries that can derail recovery efforts, he said.
"Because soldiers were athletes before [their injury], they often have aggressive goals in mind. Some want to return to swimming, surfing, or skiing. We try to meet those goals ... to get them fully reincorporated into the lives they want to lead"; adaptive sports programs help, among other things. It’s a very appropriate strategy for the Boston bombing survivors, since many were athletes, he said.
Dr. Jonathan Woodson fielded e-mails in his Pentagon office last April from nervous colleagues in Boston.
His fellow vascular surgeons there wanted to know if more attacks were coming in the Marathon bombings. "When you work at the Pentagon, everyone thinks you have total situational awareness," said Dr. Woodson, assistant secretary of defense for health affairs.
His former colleagues, however, also wanted tips on how to care for bombing victims, and knew that military surgeons like Dr. Woodson had answers. Before President Obama tapped him in 2010 to become the military’s top doctor and oversee the Pentagon’s $50 billion-plus medical budget, he’d scrubbed in on battlefield cases in Kosovo, Iraq, and Afghanistan, in addition to performing his vascular surgery and administrative duties at Boston University and Boston Medical Center.
"We’ve been dealing with [improvised explosive device] injuries for over a decade," said Dr. Woodson, a brigadier general in the U.S. Army Reserve.
"The good news is, I had trained some of the folks who worked" on the bombing victims, and "they knew what my techniques were." Still, there were discussions about particular fixes; hypertrophic bone growths, infections, and other wound complications; and ways to help young people recover from devastating injuries. Many of the lessons came from Dr. Woodson’s military service.
The back-and-forth about the Boston attacks highlights how important it is for military and civilian physicians to cross-pollinate. In the past, "there’s always been a cadre of usually academic surgeons and physicians who maintain contact with the military. I think [it] is very important to formalize these connections. One of my strategic imperatives is to make sure we invest in partnerships [with] academic medical centers and key professional organizations" so that war innovations make it into civilian practice, and, in turn, are remembered for future conflicts.
"There’s the potential for this expertise to be lost," said Dr. Woodson, who was finishing a paper with two colleagues on the history of military medical innovations (Semin. Vasc. Surg. 2010;23:235-42) when he was nominated to serve as the secretary of defense’s principal adviser on health issues.
There’ve been many innovations in recent years, including the recognition that extensive fasciotomies can save badly damaged limbs.
At the start of Operation Iraqi Freedom in 2003, Dr. Woodson was in northern Kuwait running a makeshift U.S. military hospital out of a commandeered Kuwaiti facility. "As the troops went over the berm, we were taking the first wave of casualties," performing surgery in full chemical-attack gear. "Luckily," the incoming Scud missiles weren’t very accurate. It was a humbling experience," he said.
A young soldier was helicoptered in with a crushed leg after a hit to his armored vehicle. "We did an interposition graft to repair the popliteal artery," and, because of severe swelling, extensive fasciotomies to prevent compartment syndrome; the soldier still had his leg when he was airlifted out. Although not standard practice at the time, that case and others like it quickly demonstrated "the benefit of doing four-compartment fasciotomies early on to preserve limbs. Fasciotomies are now [used routinely] to address these injuries," Dr. Woodson said.
Tourniquets – which "prior to this conflict had a very bad reputation" – have proved their worth in recent battles as well, a lesson that helped save lives in Boston. Likewise, the military has learned the value of temporary vascular shunts, narrow tubing – most often Argyle carotid artery shunts – to bypass tears or reconnect severed vessels when there’s no time for a definitive repair. "We [also] came to understand very early on the need for aggressive blood replacement. Previously, protocols called for lactated Ringer’s or saline infusions, but we realized they just lead to massive soft tissue edema, increased lung water," and other problems, he said.
Currently, Dr. Woodson and his military colleagues are helping coach the rehabilitation of the Boston bombing victims. Rehab has seen "major advances in the military that are now finding their way into civilian practice," including improved prosthetics; better understanding of rehabilitation psychology; and recognition of subtle traumatic brain, eye, ear, and other injuries that can derail recovery efforts, he said.
"Because soldiers were athletes before [their injury], they often have aggressive goals in mind. Some want to return to swimming, surfing, or skiing. We try to meet those goals ... to get them fully reincorporated into the lives they want to lead"; adaptive sports programs help, among other things. It’s a very appropriate strategy for the Boston bombing survivors, since many were athletes, he said.
Dr. Jonathan Woodson fielded e-mails in his Pentagon office last April from nervous colleagues in Boston.
His fellow vascular surgeons there wanted to know if more attacks were coming in the Marathon bombings. "When you work at the Pentagon, everyone thinks you have total situational awareness," said Dr. Woodson, assistant secretary of defense for health affairs.
His former colleagues, however, also wanted tips on how to care for bombing victims, and knew that military surgeons like Dr. Woodson had answers. Before President Obama tapped him in 2010 to become the military’s top doctor and oversee the Pentagon’s $50 billion-plus medical budget, he’d scrubbed in on battlefield cases in Kosovo, Iraq, and Afghanistan, in addition to performing his vascular surgery and administrative duties at Boston University and Boston Medical Center.
"We’ve been dealing with [improvised explosive device] injuries for over a decade," said Dr. Woodson, a brigadier general in the U.S. Army Reserve.
"The good news is, I had trained some of the folks who worked" on the bombing victims, and "they knew what my techniques were." Still, there were discussions about particular fixes; hypertrophic bone growths, infections, and other wound complications; and ways to help young people recover from devastating injuries. Many of the lessons came from Dr. Woodson’s military service.
The back-and-forth about the Boston attacks highlights how important it is for military and civilian physicians to cross-pollinate. In the past, "there’s always been a cadre of usually academic surgeons and physicians who maintain contact with the military. I think [it] is very important to formalize these connections. One of my strategic imperatives is to make sure we invest in partnerships [with] academic medical centers and key professional organizations" so that war innovations make it into civilian practice, and, in turn, are remembered for future conflicts.
"There’s the potential for this expertise to be lost," said Dr. Woodson, who was finishing a paper with two colleagues on the history of military medical innovations (Semin. Vasc. Surg. 2010;23:235-42) when he was nominated to serve as the secretary of defense’s principal adviser on health issues.
There’ve been many innovations in recent years, including the recognition that extensive fasciotomies can save badly damaged limbs.
At the start of Operation Iraqi Freedom in 2003, Dr. Woodson was in northern Kuwait running a makeshift U.S. military hospital out of a commandeered Kuwaiti facility. "As the troops went over the berm, we were taking the first wave of casualties," performing surgery in full chemical-attack gear. "Luckily," the incoming Scud missiles weren’t very accurate. It was a humbling experience," he said.
A young soldier was helicoptered in with a crushed leg after a hit to his armored vehicle. "We did an interposition graft to repair the popliteal artery," and, because of severe swelling, extensive fasciotomies to prevent compartment syndrome; the soldier still had his leg when he was airlifted out. Although not standard practice at the time, that case and others like it quickly demonstrated "the benefit of doing four-compartment fasciotomies early on to preserve limbs. Fasciotomies are now [used routinely] to address these injuries," Dr. Woodson said.
Tourniquets – which "prior to this conflict had a very bad reputation" – have proved their worth in recent battles as well, a lesson that helped save lives in Boston. Likewise, the military has learned the value of temporary vascular shunts, narrow tubing – most often Argyle carotid artery shunts – to bypass tears or reconnect severed vessels when there’s no time for a definitive repair. "We [also] came to understand very early on the need for aggressive blood replacement. Previously, protocols called for lactated Ringer’s or saline infusions, but we realized they just lead to massive soft tissue edema, increased lung water," and other problems, he said.
Currently, Dr. Woodson and his military colleagues are helping coach the rehabilitation of the Boston bombing victims. Rehab has seen "major advances in the military that are now finding their way into civilian practice," including improved prosthetics; better understanding of rehabilitation psychology; and recognition of subtle traumatic brain, eye, ear, and other injuries that can derail recovery efforts, he said.
"Because soldiers were athletes before [their injury], they often have aggressive goals in mind. Some want to return to swimming, surfing, or skiing. We try to meet those goals ... to get them fully reincorporated into the lives they want to lead"; adaptive sports programs help, among other things. It’s a very appropriate strategy for the Boston bombing survivors, since many were athletes, he said.
Hyperventilation during EEG for suspected epilepsy poses little risk
Hyperventilation during EEG workup, a common practice to elicit interictal epileptiform discharges, helps to diagnose and classify seizure disorders, and is rarely associated with adverse events, according to a British investigation published online in Seizure – European Journal of Epilepsy.
The International League Against Epilepsy and other groups recommend hyperventilation (HV) as part of a standard EEG, but there has been uncertainty about the risks of triggering a seizure with HV and its diagnostic value, and about what to tell patients about the risk-benefit ratio.
Dr. Nick Kane, a neurophysiologist at Frenchay Hospital in Bristol (England), and his associates evaluated 3,475 patients hyperventilated for a median of 3 minutes during EEG. Among the patients, 3,170 (91%) were referred for epilepsy or possible epilepsy, 102 (3%) had possible psychogenic nonepileptic seizures, and 203 (6%) had other diagnoses. Patients ranged from infants to nonagenarians and were split evenly between the sexes.
Among the 3,170 suspected-epilepsy cases, HV during EEG elicited interictal epileptiform discharges (IEDs) in 95 patients (3%) who did not have them at rest and exacerbated IEDs in another 292 patients (9.2%). In other words, HV directly or indirectly diagnosed epilepsy and helped to classify seizure type in 12.2% of cases (Seizure 2014;23:129-34).
HV triggered seizures in 69 suspected-epilepsy cases (2.2%), but it’s "worth bearing in mind that in our survey, the vast majority [of provoked seizures] were generalized absences in children without any apparent sequelae. Indeed, provocation of this seizure type is the intention of HV," the team said.
Only one patient had a generalized tonic-clonic seizure.
The findings are in keeping with previous investigations and "confirm that HV in selected patients is a valid activation technique in diagnostic electroencephalography, where the potential benefits outweigh the risks, and also provide information that may assist the informed consent process," the investigators concluded.
The 69 seizures included 59 generalized attacks (86%) – 54 of them absences in children about 10 years old – plus 8 focal seizures (12%) mostly in adults and 2 unspecified seizures.
Twenty-five of the children with absence seizures did not have them at rest, meaning that "HV gives a nearly twofold increase in generalized absence seizures" for diagnostic purposes, Dr. Kane and his team noted.
In the overall group of 3,475 patients, there were no significant cardiovascular or respiratory events, just wheezing in 1 patient with asthma and tachycardia in 1 patient with psychogenic nonepileptic seizures. Both problems ended when the patients stopped hyperventilating.
Thirty-one patients (0.9%) had psychogenic nonepileptic seizures, which involved rhythmic jerks and twitches without EEG abnormalities.
"There are clinical situations where HV is best avoided altogether, particularly when there is a risk of cerebral vasospasm-induced transient ischemic attack or stroke, a recognized complication of HV in patients with sickle cell and moyamoya disease. ... [And] it is sensible to avoid HV in patients with known recent cerebrovascular events (including cerebral infarction, subarachnoid and intracerebral hemorrhage) and significant coronary artery disease," they wrote.
The investigators had no disclosures. The British Society for Clinical Neurophysiology and Association of Neurophysiological Scientists funded the work.
Hyperventilation during EEG workup, a common practice to elicit interictal epileptiform discharges, helps to diagnose and classify seizure disorders, and is rarely associated with adverse events, according to a British investigation published online in Seizure – European Journal of Epilepsy.
The International League Against Epilepsy and other groups recommend hyperventilation (HV) as part of a standard EEG, but there has been uncertainty about the risks of triggering a seizure with HV and its diagnostic value, and about what to tell patients about the risk-benefit ratio.
Dr. Nick Kane, a neurophysiologist at Frenchay Hospital in Bristol (England), and his associates evaluated 3,475 patients hyperventilated for a median of 3 minutes during EEG. Among the patients, 3,170 (91%) were referred for epilepsy or possible epilepsy, 102 (3%) had possible psychogenic nonepileptic seizures, and 203 (6%) had other diagnoses. Patients ranged from infants to nonagenarians and were split evenly between the sexes.
Among the 3,170 suspected-epilepsy cases, HV during EEG elicited interictal epileptiform discharges (IEDs) in 95 patients (3%) who did not have them at rest and exacerbated IEDs in another 292 patients (9.2%). In other words, HV directly or indirectly diagnosed epilepsy and helped to classify seizure type in 12.2% of cases (Seizure 2014;23:129-34).
HV triggered seizures in 69 suspected-epilepsy cases (2.2%), but it’s "worth bearing in mind that in our survey, the vast majority [of provoked seizures] were generalized absences in children without any apparent sequelae. Indeed, provocation of this seizure type is the intention of HV," the team said.
Only one patient had a generalized tonic-clonic seizure.
The findings are in keeping with previous investigations and "confirm that HV in selected patients is a valid activation technique in diagnostic electroencephalography, where the potential benefits outweigh the risks, and also provide information that may assist the informed consent process," the investigators concluded.
The 69 seizures included 59 generalized attacks (86%) – 54 of them absences in children about 10 years old – plus 8 focal seizures (12%) mostly in adults and 2 unspecified seizures.
Twenty-five of the children with absence seizures did not have them at rest, meaning that "HV gives a nearly twofold increase in generalized absence seizures" for diagnostic purposes, Dr. Kane and his team noted.
In the overall group of 3,475 patients, there were no significant cardiovascular or respiratory events, just wheezing in 1 patient with asthma and tachycardia in 1 patient with psychogenic nonepileptic seizures. Both problems ended when the patients stopped hyperventilating.
Thirty-one patients (0.9%) had psychogenic nonepileptic seizures, which involved rhythmic jerks and twitches without EEG abnormalities.
"There are clinical situations where HV is best avoided altogether, particularly when there is a risk of cerebral vasospasm-induced transient ischemic attack or stroke, a recognized complication of HV in patients with sickle cell and moyamoya disease. ... [And] it is sensible to avoid HV in patients with known recent cerebrovascular events (including cerebral infarction, subarachnoid and intracerebral hemorrhage) and significant coronary artery disease," they wrote.
The investigators had no disclosures. The British Society for Clinical Neurophysiology and Association of Neurophysiological Scientists funded the work.
Hyperventilation during EEG workup, a common practice to elicit interictal epileptiform discharges, helps to diagnose and classify seizure disorders, and is rarely associated with adverse events, according to a British investigation published online in Seizure – European Journal of Epilepsy.
The International League Against Epilepsy and other groups recommend hyperventilation (HV) as part of a standard EEG, but there has been uncertainty about the risks of triggering a seizure with HV and its diagnostic value, and about what to tell patients about the risk-benefit ratio.
Dr. Nick Kane, a neurophysiologist at Frenchay Hospital in Bristol (England), and his associates evaluated 3,475 patients hyperventilated for a median of 3 minutes during EEG. Among the patients, 3,170 (91%) were referred for epilepsy or possible epilepsy, 102 (3%) had possible psychogenic nonepileptic seizures, and 203 (6%) had other diagnoses. Patients ranged from infants to nonagenarians and were split evenly between the sexes.
Among the 3,170 suspected-epilepsy cases, HV during EEG elicited interictal epileptiform discharges (IEDs) in 95 patients (3%) who did not have them at rest and exacerbated IEDs in another 292 patients (9.2%). In other words, HV directly or indirectly diagnosed epilepsy and helped to classify seizure type in 12.2% of cases (Seizure 2014;23:129-34).
HV triggered seizures in 69 suspected-epilepsy cases (2.2%), but it’s "worth bearing in mind that in our survey, the vast majority [of provoked seizures] were generalized absences in children without any apparent sequelae. Indeed, provocation of this seizure type is the intention of HV," the team said.
Only one patient had a generalized tonic-clonic seizure.
The findings are in keeping with previous investigations and "confirm that HV in selected patients is a valid activation technique in diagnostic electroencephalography, where the potential benefits outweigh the risks, and also provide information that may assist the informed consent process," the investigators concluded.
The 69 seizures included 59 generalized attacks (86%) – 54 of them absences in children about 10 years old – plus 8 focal seizures (12%) mostly in adults and 2 unspecified seizures.
Twenty-five of the children with absence seizures did not have them at rest, meaning that "HV gives a nearly twofold increase in generalized absence seizures" for diagnostic purposes, Dr. Kane and his team noted.
In the overall group of 3,475 patients, there were no significant cardiovascular or respiratory events, just wheezing in 1 patient with asthma and tachycardia in 1 patient with psychogenic nonepileptic seizures. Both problems ended when the patients stopped hyperventilating.
Thirty-one patients (0.9%) had psychogenic nonepileptic seizures, which involved rhythmic jerks and twitches without EEG abnormalities.
"There are clinical situations where HV is best avoided altogether, particularly when there is a risk of cerebral vasospasm-induced transient ischemic attack or stroke, a recognized complication of HV in patients with sickle cell and moyamoya disease. ... [And] it is sensible to avoid HV in patients with known recent cerebrovascular events (including cerebral infarction, subarachnoid and intracerebral hemorrhage) and significant coronary artery disease," they wrote.
The investigators had no disclosures. The British Society for Clinical Neurophysiology and Association of Neurophysiological Scientists funded the work.
FROM SEIZURE
Major finding: Hyperventilation made or strengthened the diagnosis of epilepsy in 387 (12.2%) suspected epilepsy cases and induced just one generalized tonic-clonic seizure.
Data Source: A prospective study of outcomes from 3,475 patients hyperventilated during EEG.
Disclosures: The investigators had no disclosures. The British Society for Clinical Neurophysiology and Association of Neurophysiological Scientists funded the work.
Consider rituximab when RA patients fail their first TNF inhibitor
Rheumatoid arthritis patients who fail initial tumor necrosis factor–inhibitor treatment may do a bit better when switched to rituximab instead of another anti-tumor necrosis factor agent, according to findings from an open-label, prospective study.
In the SWITCH-RA trial, investigators enrolled 405 rheumatoid arthritis (RA) patients who switched to the anti-CD20 B-cell-depleting drug rituximab (Rituxan) after they didn’t respond to or couldn’t tolerate their first tumor necrosis factor inhibitor (TNFi) and 323 RA patients in the same situation who switched instead to a second TNFi (Ann. Rheum. Dis. 2014 Jan. 17 [doi:10.1136/annrheumdis-2013-203993]).
After 6 months, the rituximab group had a mean improvement of –1.5 points on the 10-point and 28-joint RA Disease Activity Score, calculated with erythrocyte sedimentation rate (DAS28-3–ESR), after controlling for baseline characteristics and disease activity.
The group that switched to a second TNFi improved by a mean of –1.1 points (P = .007). The patients who received rituximab were sicker at baseline than those who started a second TNFi (mean DAS28-3–ESR of 5.2 vs. 4.8 points, respectively). The investigators didn’t name the TNFi agents to which patients were switched.
The results "provide evidence from real-world practice" that patients "achieve significantly better clinical responses over 6 months if they receive rituximab rather than an alternative TNF inhibitor as their second biological therapy," said Dr. Paul Emery of the University of Leeds (England) and his associates.
The findings support rituximab’s current Food and Drug Administration RA indication for use with methotrexate in adults who don’t do well on TNF inhibitors.
In a subanalysis of the data, rituximab kept its statistical edge only when used in seropositive subjects, who made up about 80% of the study population. Although seronegative patients showed improvements at 6 months, "there was no significant difference between the rituximab and alternative TNF inhibitor groups." Due to the low number of seronegative patients, the project was "underpowered to detect small differences," but the findings also jibe with "recent studies reporting enhancement of clinical responsiveness to rituximab in seropositive" patients, the investigators said.
In a second subanalysis, rituximab kept its edge only in those who quit initial TNFi therapy because it didn’t work and not in those who couldn’t tolerate it.
That makes sense because, when the first TNFi doesn’t work, it’s probably because patients don’t have TNF-alpha–mediated disease. When its power fades over time, most likely it means that patients have developed antibodies to it. In both situations, a biologic with a different mechanism of action, like rituximab, is probably the best choice, Dr. Emery and his associates said.
Study subjects were from Europe, South America, and Canada. Most were middle-aged women with RA for about 8 years who had been on their first TNFi for about 2 years.
Pneumonia was diagnosed in 0.7% of rituximab and 0.2% of second-TNFi patients. Urinary tract infections were diagnosed in 0.7% of rituximab patients but no second-TNFi patients. One TNFi patient tested positive for tuberculosis but did fine with prophylactic treatment, they noted.
Two TNFi patients were diagnosed with new-onset squamous cell carcinomas. One rituximab patient developed prostate cancer and another Waldenstrom’s macroglobulinemia.
Hoffmann-La Roche makes rituximab and paid a third party to write up the results. Dr. Emery advises and runs studies for Roche and other companies marketing drugs for RA. Three of the other 14 investigators are Roche employees, and most of the rest have financial ties to the company.
Rheumatoid arthritis patients who fail initial tumor necrosis factor–inhibitor treatment may do a bit better when switched to rituximab instead of another anti-tumor necrosis factor agent, according to findings from an open-label, prospective study.
In the SWITCH-RA trial, investigators enrolled 405 rheumatoid arthritis (RA) patients who switched to the anti-CD20 B-cell-depleting drug rituximab (Rituxan) after they didn’t respond to or couldn’t tolerate their first tumor necrosis factor inhibitor (TNFi) and 323 RA patients in the same situation who switched instead to a second TNFi (Ann. Rheum. Dis. 2014 Jan. 17 [doi:10.1136/annrheumdis-2013-203993]).
After 6 months, the rituximab group had a mean improvement of –1.5 points on the 10-point and 28-joint RA Disease Activity Score, calculated with erythrocyte sedimentation rate (DAS28-3–ESR), after controlling for baseline characteristics and disease activity.
The group that switched to a second TNFi improved by a mean of –1.1 points (P = .007). The patients who received rituximab were sicker at baseline than those who started a second TNFi (mean DAS28-3–ESR of 5.2 vs. 4.8 points, respectively). The investigators didn’t name the TNFi agents to which patients were switched.
The results "provide evidence from real-world practice" that patients "achieve significantly better clinical responses over 6 months if they receive rituximab rather than an alternative TNF inhibitor as their second biological therapy," said Dr. Paul Emery of the University of Leeds (England) and his associates.
The findings support rituximab’s current Food and Drug Administration RA indication for use with methotrexate in adults who don’t do well on TNF inhibitors.
In a subanalysis of the data, rituximab kept its statistical edge only when used in seropositive subjects, who made up about 80% of the study population. Although seronegative patients showed improvements at 6 months, "there was no significant difference between the rituximab and alternative TNF inhibitor groups." Due to the low number of seronegative patients, the project was "underpowered to detect small differences," but the findings also jibe with "recent studies reporting enhancement of clinical responsiveness to rituximab in seropositive" patients, the investigators said.
In a second subanalysis, rituximab kept its edge only in those who quit initial TNFi therapy because it didn’t work and not in those who couldn’t tolerate it.
That makes sense because, when the first TNFi doesn’t work, it’s probably because patients don’t have TNF-alpha–mediated disease. When its power fades over time, most likely it means that patients have developed antibodies to it. In both situations, a biologic with a different mechanism of action, like rituximab, is probably the best choice, Dr. Emery and his associates said.
Study subjects were from Europe, South America, and Canada. Most were middle-aged women with RA for about 8 years who had been on their first TNFi for about 2 years.
Pneumonia was diagnosed in 0.7% of rituximab and 0.2% of second-TNFi patients. Urinary tract infections were diagnosed in 0.7% of rituximab patients but no second-TNFi patients. One TNFi patient tested positive for tuberculosis but did fine with prophylactic treatment, they noted.
Two TNFi patients were diagnosed with new-onset squamous cell carcinomas. One rituximab patient developed prostate cancer and another Waldenstrom’s macroglobulinemia.
Hoffmann-La Roche makes rituximab and paid a third party to write up the results. Dr. Emery advises and runs studies for Roche and other companies marketing drugs for RA. Three of the other 14 investigators are Roche employees, and most of the rest have financial ties to the company.
Rheumatoid arthritis patients who fail initial tumor necrosis factor–inhibitor treatment may do a bit better when switched to rituximab instead of another anti-tumor necrosis factor agent, according to findings from an open-label, prospective study.
In the SWITCH-RA trial, investigators enrolled 405 rheumatoid arthritis (RA) patients who switched to the anti-CD20 B-cell-depleting drug rituximab (Rituxan) after they didn’t respond to or couldn’t tolerate their first tumor necrosis factor inhibitor (TNFi) and 323 RA patients in the same situation who switched instead to a second TNFi (Ann. Rheum. Dis. 2014 Jan. 17 [doi:10.1136/annrheumdis-2013-203993]).
After 6 months, the rituximab group had a mean improvement of –1.5 points on the 10-point and 28-joint RA Disease Activity Score, calculated with erythrocyte sedimentation rate (DAS28-3–ESR), after controlling for baseline characteristics and disease activity.
The group that switched to a second TNFi improved by a mean of –1.1 points (P = .007). The patients who received rituximab were sicker at baseline than those who started a second TNFi (mean DAS28-3–ESR of 5.2 vs. 4.8 points, respectively). The investigators didn’t name the TNFi agents to which patients were switched.
The results "provide evidence from real-world practice" that patients "achieve significantly better clinical responses over 6 months if they receive rituximab rather than an alternative TNF inhibitor as their second biological therapy," said Dr. Paul Emery of the University of Leeds (England) and his associates.
The findings support rituximab’s current Food and Drug Administration RA indication for use with methotrexate in adults who don’t do well on TNF inhibitors.
In a subanalysis of the data, rituximab kept its statistical edge only when used in seropositive subjects, who made up about 80% of the study population. Although seronegative patients showed improvements at 6 months, "there was no significant difference between the rituximab and alternative TNF inhibitor groups." Due to the low number of seronegative patients, the project was "underpowered to detect small differences," but the findings also jibe with "recent studies reporting enhancement of clinical responsiveness to rituximab in seropositive" patients, the investigators said.
In a second subanalysis, rituximab kept its edge only in those who quit initial TNFi therapy because it didn’t work and not in those who couldn’t tolerate it.
That makes sense because, when the first TNFi doesn’t work, it’s probably because patients don’t have TNF-alpha–mediated disease. When its power fades over time, most likely it means that patients have developed antibodies to it. In both situations, a biologic with a different mechanism of action, like rituximab, is probably the best choice, Dr. Emery and his associates said.
Study subjects were from Europe, South America, and Canada. Most were middle-aged women with RA for about 8 years who had been on their first TNFi for about 2 years.
Pneumonia was diagnosed in 0.7% of rituximab and 0.2% of second-TNFi patients. Urinary tract infections were diagnosed in 0.7% of rituximab patients but no second-TNFi patients. One TNFi patient tested positive for tuberculosis but did fine with prophylactic treatment, they noted.
Two TNFi patients were diagnosed with new-onset squamous cell carcinomas. One rituximab patient developed prostate cancer and another Waldenstrom’s macroglobulinemia.
Hoffmann-La Roche makes rituximab and paid a third party to write up the results. Dr. Emery advises and runs studies for Roche and other companies marketing drugs for RA. Three of the other 14 investigators are Roche employees, and most of the rest have financial ties to the company.
FROM ANNALS OF THE RHEUMATIC DISEASES
Major finding: RA patients who failed their first TNF inhibitor dropped 1.5 points on the 10-point DAS28 after 6 months of treatment. Those who switched to another TNF inhibitor dropped 1.1 points.
Data Source: A prospective, open-label, observational study in over 700 RA patients.
Disclosures: Most of the investigators have financial ties to rituximab’s maker, Hoffmann-La Roche, which provided funding for third-party manuscript writing assistance.
Fatal U.S. dengue fever case misdiagnosed as West Nile virus
Keep dengue fever on the differential of acute flulike illness, investigators advised in the Jan. 24 Morbidity and Mortality Weekly Report following the death of a 63-year-old Texas woman from hemophagocytic lymphohistiocytosis that was triggered by dengue virus infection.
It was the third death from dengue virus infection apparently acquired in the United States. Americans usually pick up the virus from travel to tropical areas, where it’s endemic. All three cases were geographically associated with Texas, the investigators said (MMWR 2014;63:50-4).
"This case underscores the need for clinicians in the United States to be vigilant for dengue and request diagnostic testing for suspected cases, which should be reported to public health authorities. Because of nonspecific signs and symptoms, such cases can be misdiagnosed as influenza, WNV [West Nile virus] infection, or another common acute febrile illness. Competent DENV [dengue virus] vectors are present in most states, and importation of DENV via travelers has resulted in recent dengue outbreaks in Florida, Hawaii, and Texas," said investigators, led by Tyler Sharp, Ph.D., of the Centers for Disease Control and Prevention.
The woman was initially diagnosed with WNV after returning to Texas feverish with fatigue, headache, and other problems that started during an Aug. 2012 vacation to Santa Fe, N.M.
Her serology at the time was weakly positive for WNV infection probably, in retrospect, from a cross-reaction with dengue virus antibodies.
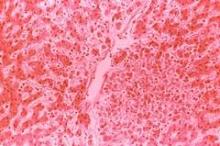
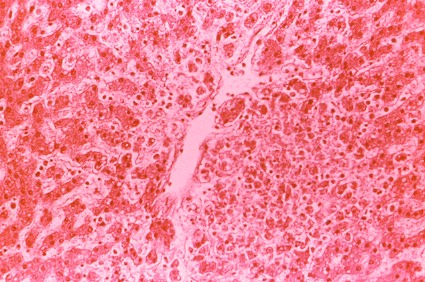
She grew worse and was hospitalized Sept. 22. Shortly thereafter, she was diagnosed with virus-induced hemophagocytic lymphohistiocytosis that necessitated a bone marrow biopsy. She developed pancytopenia, liver failure, and disseminated intravascular coagulopathy, and died Oct. 3.
About a month later, DENV-3 was detected in her bone marrow biopsy. "Erythrophagocytosis was evident," the investigators said.
Aedes aegypti and other DENV-carrying mosquitoes are rare in Santa Fe because of the elevation. It’s possible "an imported mosquito might have survived in the warmer August climate, fed on a DENV-infected person, and subsequently infected the patient," they said.
Eighteen people from Texas donated blood to her before the biopsy, so it’s also possible she picked up the virus from contaminated blood, a rare route of transmission.
However, none of the donors reported fever in the weeks before or the week after donating blood, and none of the 14 tested afterward had evidence of dengue infection.
The woman had Crohn’s disease and was taking an immunosuppressive, both of which increase the risk for HLH, a hyperinflammatory syndrome following strong immune system activation. Epstein-Barr virus is a common trigger, but dengue is not. There have been 27 documented cases since 1966, 8 of which were fatal. MMWR’s case is the first reported in the United States.
The woman attended a craft show in Santa Fe, went for regular walks, and spent most evenings on the patio. Her traveling companions remained healthy and tested negative for infection. The woman had been to France earlier in the year.
From 2001 to 2007, a total of 796 cases of dengue fever were reported in the United States, most of which were imported. About 95% of people develop acute febrile illness, but not more serious complications.
The investigation funding source and investigator disclosures were not included in the report.
Keep dengue fever on the differential of acute flulike illness, investigators advised in the Jan. 24 Morbidity and Mortality Weekly Report following the death of a 63-year-old Texas woman from hemophagocytic lymphohistiocytosis that was triggered by dengue virus infection.
It was the third death from dengue virus infection apparently acquired in the United States. Americans usually pick up the virus from travel to tropical areas, where it’s endemic. All three cases were geographically associated with Texas, the investigators said (MMWR 2014;63:50-4).
"This case underscores the need for clinicians in the United States to be vigilant for dengue and request diagnostic testing for suspected cases, which should be reported to public health authorities. Because of nonspecific signs and symptoms, such cases can be misdiagnosed as influenza, WNV [West Nile virus] infection, or another common acute febrile illness. Competent DENV [dengue virus] vectors are present in most states, and importation of DENV via travelers has resulted in recent dengue outbreaks in Florida, Hawaii, and Texas," said investigators, led by Tyler Sharp, Ph.D., of the Centers for Disease Control and Prevention.
The woman was initially diagnosed with WNV after returning to Texas feverish with fatigue, headache, and other problems that started during an Aug. 2012 vacation to Santa Fe, N.M.
Her serology at the time was weakly positive for WNV infection probably, in retrospect, from a cross-reaction with dengue virus antibodies.
She grew worse and was hospitalized Sept. 22. Shortly thereafter, she was diagnosed with virus-induced hemophagocytic lymphohistiocytosis that necessitated a bone marrow biopsy. She developed pancytopenia, liver failure, and disseminated intravascular coagulopathy, and died Oct. 3.
About a month later, DENV-3 was detected in her bone marrow biopsy. "Erythrophagocytosis was evident," the investigators said.
Aedes aegypti and other DENV-carrying mosquitoes are rare in Santa Fe because of the elevation. It’s possible "an imported mosquito might have survived in the warmer August climate, fed on a DENV-infected person, and subsequently infected the patient," they said.
Eighteen people from Texas donated blood to her before the biopsy, so it’s also possible she picked up the virus from contaminated blood, a rare route of transmission.
However, none of the donors reported fever in the weeks before or the week after donating blood, and none of the 14 tested afterward had evidence of dengue infection.
The woman had Crohn’s disease and was taking an immunosuppressive, both of which increase the risk for HLH, a hyperinflammatory syndrome following strong immune system activation. Epstein-Barr virus is a common trigger, but dengue is not. There have been 27 documented cases since 1966, 8 of which were fatal. MMWR’s case is the first reported in the United States.
The woman attended a craft show in Santa Fe, went for regular walks, and spent most evenings on the patio. Her traveling companions remained healthy and tested negative for infection. The woman had been to France earlier in the year.
From 2001 to 2007, a total of 796 cases of dengue fever were reported in the United States, most of which were imported. About 95% of people develop acute febrile illness, but not more serious complications.
The investigation funding source and investigator disclosures were not included in the report.
Keep dengue fever on the differential of acute flulike illness, investigators advised in the Jan. 24 Morbidity and Mortality Weekly Report following the death of a 63-year-old Texas woman from hemophagocytic lymphohistiocytosis that was triggered by dengue virus infection.
It was the third death from dengue virus infection apparently acquired in the United States. Americans usually pick up the virus from travel to tropical areas, where it’s endemic. All three cases were geographically associated with Texas, the investigators said (MMWR 2014;63:50-4).
"This case underscores the need for clinicians in the United States to be vigilant for dengue and request diagnostic testing for suspected cases, which should be reported to public health authorities. Because of nonspecific signs and symptoms, such cases can be misdiagnosed as influenza, WNV [West Nile virus] infection, or another common acute febrile illness. Competent DENV [dengue virus] vectors are present in most states, and importation of DENV via travelers has resulted in recent dengue outbreaks in Florida, Hawaii, and Texas," said investigators, led by Tyler Sharp, Ph.D., of the Centers for Disease Control and Prevention.
The woman was initially diagnosed with WNV after returning to Texas feverish with fatigue, headache, and other problems that started during an Aug. 2012 vacation to Santa Fe, N.M.
Her serology at the time was weakly positive for WNV infection probably, in retrospect, from a cross-reaction with dengue virus antibodies.
She grew worse and was hospitalized Sept. 22. Shortly thereafter, she was diagnosed with virus-induced hemophagocytic lymphohistiocytosis that necessitated a bone marrow biopsy. She developed pancytopenia, liver failure, and disseminated intravascular coagulopathy, and died Oct. 3.
About a month later, DENV-3 was detected in her bone marrow biopsy. "Erythrophagocytosis was evident," the investigators said.
Aedes aegypti and other DENV-carrying mosquitoes are rare in Santa Fe because of the elevation. It’s possible "an imported mosquito might have survived in the warmer August climate, fed on a DENV-infected person, and subsequently infected the patient," they said.
Eighteen people from Texas donated blood to her before the biopsy, so it’s also possible she picked up the virus from contaminated blood, a rare route of transmission.
However, none of the donors reported fever in the weeks before or the week after donating blood, and none of the 14 tested afterward had evidence of dengue infection.
The woman had Crohn’s disease and was taking an immunosuppressive, both of which increase the risk for HLH, a hyperinflammatory syndrome following strong immune system activation. Epstein-Barr virus is a common trigger, but dengue is not. There have been 27 documented cases since 1966, 8 of which were fatal. MMWR’s case is the first reported in the United States.
The woman attended a craft show in Santa Fe, went for regular walks, and spent most evenings on the patio. Her traveling companions remained healthy and tested negative for infection. The woman had been to France earlier in the year.
From 2001 to 2007, a total of 796 cases of dengue fever were reported in the United States, most of which were imported. About 95% of people develop acute febrile illness, but not more serious complications.
The investigation funding source and investigator disclosures were not included in the report.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Caffeine-laden drinks emit ‘absolute safety signals’ for teens
It’s unclear if and to what extent food, drinks, and supplements containing caffeine are dangerous for adolescents and young adults, according to a 166-page summary of an Institute of Medicine workshop released Jan. 17.
The 2-day workshop came about at the request of the Food and Drug Administration as an intelligence gathering exercise. The FDA is considering restrictions on such products because of safety concerns, with warning labels and limits on caffeine levels among the options.
The agency needs to "get the science right" before it acts, said Michael Taylor, deputy commissioner for foods and veterinary medicine.
There was no unanimous call at the workshop to ban the products, which are fizzy beverages that come in cans or syrups in small twist-top bottles. Instead, the speakers, who were academic researchers and physicians, expressed concern based on study results and their own experiences. Some said they’ve seen serious side effects from energy drinks, but others said they haven’t and blamed the concern on media hype.
Most of the presentations were about caffeine use in general, not energy drinks in particular. There simply hasn’t been much study of the products, a problem noted by many speakers, some of whom called for a national registry to systematically track adverse events.
Energy drinks have been associated with an increasing number of emergency department visits and several sudden cardiac deaths in teens and young people, but it is unclear what role the products played in these events.
Certain populations might be most at risk. The majority of deaths seem to have been in teen and young men with underlying cardiac problems who were engaged in sports or other physical activities. Genetic differences in caffeine sensitivity may be at work as well.
If there is a risk with energy drinks, it’s "probably very small," said Dr. Jeffrey J. Goldberger, professor of medicine and director of the Program in Cardiac Arrhythmias at Northwestern University in Chicago.
With an estimated 6 billion caffeinated energy drinks sold in the United States annually, a larger problem would have been apparent by now. Also, while there are data indicating a dose-dependent effect of caffeine on heart rate and blood pressure, the prevailing evidence suggests caffeine has no effect on arrhythmias, beyond anecdotal reports, he said.
Nonetheless, the deaths and emergency department visits "are absolute safety signals," said pediatric cardiologist Dr. Steven Lipshultz, chairman of the pediatrics department at Wayne State University in Detroit.
Until energy drinks are proven safe, it would be wise to ban their sales to kids and teens, he said.
U.S. poison centers received 6,724 energy product calls from June 2010 to July 2013. Outcomes were minimal in most cases, with no problems or temporary problems such as hyperactivity or agitation. Tachycardia and other moderate effects were reported in about 10% of calls, and "serious harms" reported in less than half of 1% (29 calls). One death was reported in 2011, according to a review by Dr. Alvin C. Bronstein, medical director of the Rocky Mountain Poison and Drug Center in Denver.
The number of calls to the poison center about energy drink has been about level since 2012.
Energy drinks contain about the same amount of caffeine as coffee, somewhere in the range of 200 mg per serving – more in some products and less in others. About 10% of 13- to 24-year-olds report using them, the highest of any age group, but far fewer than the number that report drinking coffee, soda, or tea. In a survey of 1,000 college seniors, more than 60% reported using energy drinks in addition to other caffeinated beverages.
The products typically come with more than just caffeine; glucuronolactone, taurine, vitamins, and herbal extracts are common ingredients. As with energy drinks themselves, the effects of those ingredients when mixed with caffeine is not well studied. The evidence that exists suggests that, if anything, most additional ingredients inhibit the adverse effects of caffeine, said Stephen W. Schaffer, Ph.D., a hypertension and diabetes researcher at the University of South Alabama in Mobile.
Energy drinks account for just 1%-4% of U.S. caffeine intake depending on age group, and have not led to an overall increase in the amount of caffeine Americans consume, said registered dietician Diane Mitchell, department of nutritional sciences at Pennsylvania State University in State College, Pa.
In general, 400 mg/day of caffeine intake is considered safe in nonpregnant adults, and 2.5 mg/kg per day in children up to age 17.
It’s unclear if and to what extent food, drinks, and supplements containing caffeine are dangerous for adolescents and young adults, according to a 166-page summary of an Institute of Medicine workshop released Jan. 17.
The 2-day workshop came about at the request of the Food and Drug Administration as an intelligence gathering exercise. The FDA is considering restrictions on such products because of safety concerns, with warning labels and limits on caffeine levels among the options.
The agency needs to "get the science right" before it acts, said Michael Taylor, deputy commissioner for foods and veterinary medicine.
There was no unanimous call at the workshop to ban the products, which are fizzy beverages that come in cans or syrups in small twist-top bottles. Instead, the speakers, who were academic researchers and physicians, expressed concern based on study results and their own experiences. Some said they’ve seen serious side effects from energy drinks, but others said they haven’t and blamed the concern on media hype.
Most of the presentations were about caffeine use in general, not energy drinks in particular. There simply hasn’t been much study of the products, a problem noted by many speakers, some of whom called for a national registry to systematically track adverse events.
Energy drinks have been associated with an increasing number of emergency department visits and several sudden cardiac deaths in teens and young people, but it is unclear what role the products played in these events.
Certain populations might be most at risk. The majority of deaths seem to have been in teen and young men with underlying cardiac problems who were engaged in sports or other physical activities. Genetic differences in caffeine sensitivity may be at work as well.
If there is a risk with energy drinks, it’s "probably very small," said Dr. Jeffrey J. Goldberger, professor of medicine and director of the Program in Cardiac Arrhythmias at Northwestern University in Chicago.
With an estimated 6 billion caffeinated energy drinks sold in the United States annually, a larger problem would have been apparent by now. Also, while there are data indicating a dose-dependent effect of caffeine on heart rate and blood pressure, the prevailing evidence suggests caffeine has no effect on arrhythmias, beyond anecdotal reports, he said.
Nonetheless, the deaths and emergency department visits "are absolute safety signals," said pediatric cardiologist Dr. Steven Lipshultz, chairman of the pediatrics department at Wayne State University in Detroit.
Until energy drinks are proven safe, it would be wise to ban their sales to kids and teens, he said.
U.S. poison centers received 6,724 energy product calls from June 2010 to July 2013. Outcomes were minimal in most cases, with no problems or temporary problems such as hyperactivity or agitation. Tachycardia and other moderate effects were reported in about 10% of calls, and "serious harms" reported in less than half of 1% (29 calls). One death was reported in 2011, according to a review by Dr. Alvin C. Bronstein, medical director of the Rocky Mountain Poison and Drug Center in Denver.
The number of calls to the poison center about energy drink has been about level since 2012.
Energy drinks contain about the same amount of caffeine as coffee, somewhere in the range of 200 mg per serving – more in some products and less in others. About 10% of 13- to 24-year-olds report using them, the highest of any age group, but far fewer than the number that report drinking coffee, soda, or tea. In a survey of 1,000 college seniors, more than 60% reported using energy drinks in addition to other caffeinated beverages.
The products typically come with more than just caffeine; glucuronolactone, taurine, vitamins, and herbal extracts are common ingredients. As with energy drinks themselves, the effects of those ingredients when mixed with caffeine is not well studied. The evidence that exists suggests that, if anything, most additional ingredients inhibit the adverse effects of caffeine, said Stephen W. Schaffer, Ph.D., a hypertension and diabetes researcher at the University of South Alabama in Mobile.
Energy drinks account for just 1%-4% of U.S. caffeine intake depending on age group, and have not led to an overall increase in the amount of caffeine Americans consume, said registered dietician Diane Mitchell, department of nutritional sciences at Pennsylvania State University in State College, Pa.
In general, 400 mg/day of caffeine intake is considered safe in nonpregnant adults, and 2.5 mg/kg per day in children up to age 17.
It’s unclear if and to what extent food, drinks, and supplements containing caffeine are dangerous for adolescents and young adults, according to a 166-page summary of an Institute of Medicine workshop released Jan. 17.
The 2-day workshop came about at the request of the Food and Drug Administration as an intelligence gathering exercise. The FDA is considering restrictions on such products because of safety concerns, with warning labels and limits on caffeine levels among the options.
The agency needs to "get the science right" before it acts, said Michael Taylor, deputy commissioner for foods and veterinary medicine.
There was no unanimous call at the workshop to ban the products, which are fizzy beverages that come in cans or syrups in small twist-top bottles. Instead, the speakers, who were academic researchers and physicians, expressed concern based on study results and their own experiences. Some said they’ve seen serious side effects from energy drinks, but others said they haven’t and blamed the concern on media hype.
Most of the presentations were about caffeine use in general, not energy drinks in particular. There simply hasn’t been much study of the products, a problem noted by many speakers, some of whom called for a national registry to systematically track adverse events.
Energy drinks have been associated with an increasing number of emergency department visits and several sudden cardiac deaths in teens and young people, but it is unclear what role the products played in these events.
Certain populations might be most at risk. The majority of deaths seem to have been in teen and young men with underlying cardiac problems who were engaged in sports or other physical activities. Genetic differences in caffeine sensitivity may be at work as well.
If there is a risk with energy drinks, it’s "probably very small," said Dr. Jeffrey J. Goldberger, professor of medicine and director of the Program in Cardiac Arrhythmias at Northwestern University in Chicago.
With an estimated 6 billion caffeinated energy drinks sold in the United States annually, a larger problem would have been apparent by now. Also, while there are data indicating a dose-dependent effect of caffeine on heart rate and blood pressure, the prevailing evidence suggests caffeine has no effect on arrhythmias, beyond anecdotal reports, he said.
Nonetheless, the deaths and emergency department visits "are absolute safety signals," said pediatric cardiologist Dr. Steven Lipshultz, chairman of the pediatrics department at Wayne State University in Detroit.
Until energy drinks are proven safe, it would be wise to ban their sales to kids and teens, he said.
U.S. poison centers received 6,724 energy product calls from June 2010 to July 2013. Outcomes were minimal in most cases, with no problems or temporary problems such as hyperactivity or agitation. Tachycardia and other moderate effects were reported in about 10% of calls, and "serious harms" reported in less than half of 1% (29 calls). One death was reported in 2011, according to a review by Dr. Alvin C. Bronstein, medical director of the Rocky Mountain Poison and Drug Center in Denver.
The number of calls to the poison center about energy drink has been about level since 2012.
Energy drinks contain about the same amount of caffeine as coffee, somewhere in the range of 200 mg per serving – more in some products and less in others. About 10% of 13- to 24-year-olds report using them, the highest of any age group, but far fewer than the number that report drinking coffee, soda, or tea. In a survey of 1,000 college seniors, more than 60% reported using energy drinks in addition to other caffeinated beverages.
The products typically come with more than just caffeine; glucuronolactone, taurine, vitamins, and herbal extracts are common ingredients. As with energy drinks themselves, the effects of those ingredients when mixed with caffeine is not well studied. The evidence that exists suggests that, if anything, most additional ingredients inhibit the adverse effects of caffeine, said Stephen W. Schaffer, Ph.D., a hypertension and diabetes researcher at the University of South Alabama in Mobile.
Energy drinks account for just 1%-4% of U.S. caffeine intake depending on age group, and have not led to an overall increase in the amount of caffeine Americans consume, said registered dietician Diane Mitchell, department of nutritional sciences at Pennsylvania State University in State College, Pa.
In general, 400 mg/day of caffeine intake is considered safe in nonpregnant adults, and 2.5 mg/kg per day in children up to age 17.
Questionable benefit from HPV vaccine after age 15
Quadrivalent human papillomavirus vaccine may not protect a significant percentage of women against squamous intraepithelial lesions and other cervical dysplasias, according to a study published in the Journal of Clinical Oncology.
The Canadian researchers linked vaccination and cervical screening databases, among others, from the province of Manitoba to compare the incidence of cervical dysplasia in 3,541 women at least 15 years old when they were vaccinated with 9,594 aged-matched women who were not vaccinated. About 87% of the vaccinated subjects had more than one shot recorded in the three-shot series.
Among those women vaccinated between 15 and 17 years of age and followed for a median of 3.1 years, adjusted vaccine effectiveness (VE) was a nonstatistically significant 35% against high-grade squamous intraepithelial lesions (HSILs) and 21% against low-grade squamous intraepithelial lesions (LSILs). No protective effect was found (VE –1%) against atypical squamous cells of undetermined significance (ASCUS) (J. Clin. Oncol. 2014 Jan. 6 [doi: 10.1200/JCO.2013.52.4645]).
Among women 18 years or older with normal cervical cytology when vaccinated, the team estimated VE at a nonsignificant 23% against HSILs, and found no protective effect against LSILs (VE 18 %) or ASCUS (VE 20%). They found no evidence of protection among women with abnormal cervical cytology before immunization (VE 8%).
Both immunized and nonimmunized women had a cumulative 3-year probability of 2.8% for ASCUS. The vaccinated group had a cumulative 3-year probability of 3.3% for LSILs and 2.3% for HSILs; the unvaccinated group had a cumulative 3-year probability of 3.7% for LSILs and 2.6% for HSILs. Carcinoma-in-situ was detected in 12 vaccinated females (0.3%) and 22 unvaccinated females (0.2%).
"These findings affirm the importance of vaccinating females at a young age before any significant exposure to HPV occurs and underscores the need for maintaining organized high-quality screening programs that cover all sexually active women, even if they were vaccinated," concluded Dr. Salaheddin M. Mahmud of the University of Manitoba, Winnipeg, and his associates.
Although in preapproval trials, the "vaccine was shown to be greater than 90% effective against HPV 16/18-associated dysplasia, VE in practice is likely much lower because these types are only responsible for approximately half of HSILs and a quarter of LSILs. Moreover, these high VE estimates were only observed in per-protocol analyses that were typically limited to HPV-naive women who received all three doses. In the intention-to-treat analyses, which were closer in design to our analysis, efficacy estimates were much lower, especially against HPV16-associated dysplasia. Efficacy was also lower among older women and among women with abnormal baseline Pap cytology; in one trial, VE was a mere 18.7% in the latter group," they said.
Vaccinated women in the study were more likely to have Pap smears, leading to a possible detection bias; VE was a bit higher when the analysis was limited to women in both groups who had at least one Pap smear after enrollment.
The researchers added that "vaccinated women were also more likely to have been screened before enrollment, [which] may reflect increased concern ... about the risk of sexually transmitted infections."
The work was funded in part by the government of Manitoba. Dr. Mahmud reported receiving research funding from GlaxoSmithKline, Sanofi-Aventis, and Pfizer. Another author, Erich V. Kliewer, is a paid adviser to GlaxoSmithKline and to Merck, maker of the vaccine. A third author, Alain A. Demers, reported other remunerations from Merck.
Quadrivalent human papillomavirus vaccine may not protect a significant percentage of women against squamous intraepithelial lesions and other cervical dysplasias, according to a study published in the Journal of Clinical Oncology.
The Canadian researchers linked vaccination and cervical screening databases, among others, from the province of Manitoba to compare the incidence of cervical dysplasia in 3,541 women at least 15 years old when they were vaccinated with 9,594 aged-matched women who were not vaccinated. About 87% of the vaccinated subjects had more than one shot recorded in the three-shot series.
Among those women vaccinated between 15 and 17 years of age and followed for a median of 3.1 years, adjusted vaccine effectiveness (VE) was a nonstatistically significant 35% against high-grade squamous intraepithelial lesions (HSILs) and 21% against low-grade squamous intraepithelial lesions (LSILs). No protective effect was found (VE –1%) against atypical squamous cells of undetermined significance (ASCUS) (J. Clin. Oncol. 2014 Jan. 6 [doi: 10.1200/JCO.2013.52.4645]).
Among women 18 years or older with normal cervical cytology when vaccinated, the team estimated VE at a nonsignificant 23% against HSILs, and found no protective effect against LSILs (VE 18 %) or ASCUS (VE 20%). They found no evidence of protection among women with abnormal cervical cytology before immunization (VE 8%).
Both immunized and nonimmunized women had a cumulative 3-year probability of 2.8% for ASCUS. The vaccinated group had a cumulative 3-year probability of 3.3% for LSILs and 2.3% for HSILs; the unvaccinated group had a cumulative 3-year probability of 3.7% for LSILs and 2.6% for HSILs. Carcinoma-in-situ was detected in 12 vaccinated females (0.3%) and 22 unvaccinated females (0.2%).
"These findings affirm the importance of vaccinating females at a young age before any significant exposure to HPV occurs and underscores the need for maintaining organized high-quality screening programs that cover all sexually active women, even if they were vaccinated," concluded Dr. Salaheddin M. Mahmud of the University of Manitoba, Winnipeg, and his associates.
Although in preapproval trials, the "vaccine was shown to be greater than 90% effective against HPV 16/18-associated dysplasia, VE in practice is likely much lower because these types are only responsible for approximately half of HSILs and a quarter of LSILs. Moreover, these high VE estimates were only observed in per-protocol analyses that were typically limited to HPV-naive women who received all three doses. In the intention-to-treat analyses, which were closer in design to our analysis, efficacy estimates were much lower, especially against HPV16-associated dysplasia. Efficacy was also lower among older women and among women with abnormal baseline Pap cytology; in one trial, VE was a mere 18.7% in the latter group," they said.
Vaccinated women in the study were more likely to have Pap smears, leading to a possible detection bias; VE was a bit higher when the analysis was limited to women in both groups who had at least one Pap smear after enrollment.
The researchers added that "vaccinated women were also more likely to have been screened before enrollment, [which] may reflect increased concern ... about the risk of sexually transmitted infections."
The work was funded in part by the government of Manitoba. Dr. Mahmud reported receiving research funding from GlaxoSmithKline, Sanofi-Aventis, and Pfizer. Another author, Erich V. Kliewer, is a paid adviser to GlaxoSmithKline and to Merck, maker of the vaccine. A third author, Alain A. Demers, reported other remunerations from Merck.
Quadrivalent human papillomavirus vaccine may not protect a significant percentage of women against squamous intraepithelial lesions and other cervical dysplasias, according to a study published in the Journal of Clinical Oncology.
The Canadian researchers linked vaccination and cervical screening databases, among others, from the province of Manitoba to compare the incidence of cervical dysplasia in 3,541 women at least 15 years old when they were vaccinated with 9,594 aged-matched women who were not vaccinated. About 87% of the vaccinated subjects had more than one shot recorded in the three-shot series.
Among those women vaccinated between 15 and 17 years of age and followed for a median of 3.1 years, adjusted vaccine effectiveness (VE) was a nonstatistically significant 35% against high-grade squamous intraepithelial lesions (HSILs) and 21% against low-grade squamous intraepithelial lesions (LSILs). No protective effect was found (VE –1%) against atypical squamous cells of undetermined significance (ASCUS) (J. Clin. Oncol. 2014 Jan. 6 [doi: 10.1200/JCO.2013.52.4645]).
Among women 18 years or older with normal cervical cytology when vaccinated, the team estimated VE at a nonsignificant 23% against HSILs, and found no protective effect against LSILs (VE 18 %) or ASCUS (VE 20%). They found no evidence of protection among women with abnormal cervical cytology before immunization (VE 8%).
Both immunized and nonimmunized women had a cumulative 3-year probability of 2.8% for ASCUS. The vaccinated group had a cumulative 3-year probability of 3.3% for LSILs and 2.3% for HSILs; the unvaccinated group had a cumulative 3-year probability of 3.7% for LSILs and 2.6% for HSILs. Carcinoma-in-situ was detected in 12 vaccinated females (0.3%) and 22 unvaccinated females (0.2%).
"These findings affirm the importance of vaccinating females at a young age before any significant exposure to HPV occurs and underscores the need for maintaining organized high-quality screening programs that cover all sexually active women, even if they were vaccinated," concluded Dr. Salaheddin M. Mahmud of the University of Manitoba, Winnipeg, and his associates.
Although in preapproval trials, the "vaccine was shown to be greater than 90% effective against HPV 16/18-associated dysplasia, VE in practice is likely much lower because these types are only responsible for approximately half of HSILs and a quarter of LSILs. Moreover, these high VE estimates were only observed in per-protocol analyses that were typically limited to HPV-naive women who received all three doses. In the intention-to-treat analyses, which were closer in design to our analysis, efficacy estimates were much lower, especially against HPV16-associated dysplasia. Efficacy was also lower among older women and among women with abnormal baseline Pap cytology; in one trial, VE was a mere 18.7% in the latter group," they said.
Vaccinated women in the study were more likely to have Pap smears, leading to a possible detection bias; VE was a bit higher when the analysis was limited to women in both groups who had at least one Pap smear after enrollment.
The researchers added that "vaccinated women were also more likely to have been screened before enrollment, [which] may reflect increased concern ... about the risk of sexually transmitted infections."
The work was funded in part by the government of Manitoba. Dr. Mahmud reported receiving research funding from GlaxoSmithKline, Sanofi-Aventis, and Pfizer. Another author, Erich V. Kliewer, is a paid adviser to GlaxoSmithKline and to Merck, maker of the vaccine. A third author, Alain A. Demers, reported other remunerations from Merck.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: Among women aged 15-17 years, the adjusted effectiveness of quadrivalent human papillomavirus vaccine is a nonstatistically significant 35% against HSILs and 21% against LSILs, with no protective effect against ASCUS.
Data Source: Provincial records of cervical dysplasia incidence in 3,541 women vaccinated at or after age 15 compared with 9,594 aged-matched, unvaccinated controls.
Disclosures: The work was funded in part by the province of Manitoba. Two of the five authors disclosed compensation from Merck, maker of the vaccine.
Give calcium before and after thyroidectomies in gastric bypass patients
Patients who’ve had roux-en-Y gastric bypasses have higher incidences of recalcitrant, symptomatic hypocalcemia after thyroidectomies and spend more time in the hospital afterward for intravenous calcium, according to investigators from the Massachusetts General Hospital in Boston.
Because of that, "it is necessary to ensure that all patients with prior RYGBPs [roux-en-Y gastric bypasses] [take] oral calcium supplements before thyroidectomy," and afterward "recalcitrant, postoperative hypocalcemia should be anticipated, calcium levels closely monitored, and early calcium and vitamin D supplementation initiated preemptively," said the researchers, led by endocrine surgery fellow Dr. Travis McKenzie (Surgery 2013;154:1300-6).
Based on the findings, "we recommend starting oral calcium as early as possible in the evaluation phase before thyroidectomy," supplementing with 1.5-2 gm/day of oral calcium, as per American Society for Metabolic and Bariatric Surgery guidelines. "We prefer calcium citrate, because it has better bioavailability when compared with calcium carbonate after RYGBP." Vitamin D–deficient patients should also get calcitriol (0.25 mcg orally twice daily) for 7 days before the operation, they said.
The team compared outcomes in 19 patients who underwent thyroidectomies an average of 53 months after gastric bypass to outcomes in 38 age-, sex-, and body mass index–matched controls, with pre-bypass BMI matched in the study group to pre-thyroidectomy BMI in the control group, about 45 kg/m2 in both cases. Almost all of the patients in both arms had total thyroidectomies, and thyroid malignancies were found in about half of each.
Overall, eight (42%) of the previous-bypass patients, but none of the controls, developed hypocalcemia after thyroid surgery (P less than .01) and four (21%) – but, again, no controls – required intravenous calcium (P less than .01). The need for intravenous calcium, in turn, led to longer hospital stays in the bypass group (2.2 vs. 1.2 days; P = .02). In the bypass group, about 4 patients, 20%, who were on calcium and vitamin D supplements before surgery developed postop symptomatic hypocalcemia, vs. 11 (58%) of those who were not on supplements (P = .18).
Among the 16 bypass patients and 33 controls who did not have intentional parathyroidectomies as part of their operations, both symptomatic postop hypocalcemia (6 patients; 38% vs 0%; P less than.01) and use of intravenous calcium (3 patients;19% vs. 0%; P = .03) were higher in the bypass group.
In addition to preop calcium supplements, all Mass General patients with previous RYGBPs are now started immediately on postop calcium citrate with vitamin D3 (200-mg elemental calcium/tablet), two tablets four times daily, which is continued indefinitely. "We do not feel that higher dosing will increase efficacy as absorption is limited beyond these doses." Patients also get post-op calcitriol (0.25 mcg orally twice daily) for at least a week, and stopped at that point only if there are no symptoms of hypocalcemia, the investigators said.
The problem "is likely multifactorial, including relative hypoparathyroidism after thyroidectomy in the setting of malabsorptive enteric anatomy and metabolic bone disease," they noted.
"An argument has been made that PTH [parathyroid hormone] testing may allow early identification of patients at risk for hypocalcemia after thyroidectomy, prompting early initiation of calcium and calcitriol when PTH is less than [10 mcg/L]. This finding would not change our immediate management since all patients after thyroidectomy in the setting of previous RYGBP are now maintained on both calcium and calcitriol postoperatively. Furthermore, following PTH levels in this unique patient population may not be accurate because secondary hyperparathyroidism is frequently present after RYGBP. In our study group, patients with symptomatic hypocalcemia had an average PTH of [34 mcg/L]," the investigators said.
Patients were, on average, about 50 years old in both study arms, and more than 90% of the subjects were women. No one developed permanent hypoparathyroidism in either arm.
The work was funded in part by the National Cancer Institute and the Massachusetts General Hospital Department of Surgery. Investigator disclosures were not available.
This article focuses on the challenges associated with the management of transient hypocalcemia after thyroidectomy in patients with a history of a gastric bypass. While for most patients, this is a transient problem, in a subset of patients, their hypoparathyroidism ends up being permanent. While, thankfully, permanent hypoparathyroidism is a rare complication after thyroidectomy, in this cohort of patients, it can be a disastrous situation. Because of the altered absorption of calcium and vitamin D, permanent hypoparathyroidism can be almost impossible to manage effectively in this population. This is something that both bariatric and endocrine surgeons need to know and think about.
 |
|
Our institution took care of a patient who had hypoparathyroidism following a thyroidectomy 10 years earlier. She was well managed on calcium and vitamin D, but then had a gastric bypass; the surgeon didn’t realize her past medical history made her a poor candidate. Afterward, she had to be repeatedly admitted for intravenous calcium, as her symptoms could not be controlled with even massive doses of calcium and vitamin D. Ultimately, she needed to have her gastric bypass reversed so she could absorb oral calcium more effectively.
For many patients with nodular thyroid disease, we need to decide whether to do a lobectomy or remove the whole thyroid. I think surgeons can get a bit cavalier about total thyroidectomies because the risk of hypoparathyroidism is very low in most patients, but the risk/benefit ratio is clearly different in this population. Because of this concern, we have a higher threshold to do total thyroidectomies in patients with a history of a gastric bypass. We specifically ask on our endocrine surgery in-take forms if patients have had a gastric bypass; we don’t assume patients will self-report, because it’s often something they want to keep private. When we see that have had a bypass, it frames our conversation about whether or not to have surgery, and the extent of surgery.
Dr. Rebecca Sippel is an associate professor and chief of the section of endocrine surgery at the University of Wisconsin (Madison). She is also an editorial adviser for Surgery News.
This article focuses on the challenges associated with the management of transient hypocalcemia after thyroidectomy in patients with a history of a gastric bypass. While for most patients, this is a transient problem, in a subset of patients, their hypoparathyroidism ends up being permanent. While, thankfully, permanent hypoparathyroidism is a rare complication after thyroidectomy, in this cohort of patients, it can be a disastrous situation. Because of the altered absorption of calcium and vitamin D, permanent hypoparathyroidism can be almost impossible to manage effectively in this population. This is something that both bariatric and endocrine surgeons need to know and think about.
 |
|
Our institution took care of a patient who had hypoparathyroidism following a thyroidectomy 10 years earlier. She was well managed on calcium and vitamin D, but then had a gastric bypass; the surgeon didn’t realize her past medical history made her a poor candidate. Afterward, she had to be repeatedly admitted for intravenous calcium, as her symptoms could not be controlled with even massive doses of calcium and vitamin D. Ultimately, she needed to have her gastric bypass reversed so she could absorb oral calcium more effectively.
For many patients with nodular thyroid disease, we need to decide whether to do a lobectomy or remove the whole thyroid. I think surgeons can get a bit cavalier about total thyroidectomies because the risk of hypoparathyroidism is very low in most patients, but the risk/benefit ratio is clearly different in this population. Because of this concern, we have a higher threshold to do total thyroidectomies in patients with a history of a gastric bypass. We specifically ask on our endocrine surgery in-take forms if patients have had a gastric bypass; we don’t assume patients will self-report, because it’s often something they want to keep private. When we see that have had a bypass, it frames our conversation about whether or not to have surgery, and the extent of surgery.
Dr. Rebecca Sippel is an associate professor and chief of the section of endocrine surgery at the University of Wisconsin (Madison). She is also an editorial adviser for Surgery News.
This article focuses on the challenges associated with the management of transient hypocalcemia after thyroidectomy in patients with a history of a gastric bypass. While for most patients, this is a transient problem, in a subset of patients, their hypoparathyroidism ends up being permanent. While, thankfully, permanent hypoparathyroidism is a rare complication after thyroidectomy, in this cohort of patients, it can be a disastrous situation. Because of the altered absorption of calcium and vitamin D, permanent hypoparathyroidism can be almost impossible to manage effectively in this population. This is something that both bariatric and endocrine surgeons need to know and think about.
 |
|
Our institution took care of a patient who had hypoparathyroidism following a thyroidectomy 10 years earlier. She was well managed on calcium and vitamin D, but then had a gastric bypass; the surgeon didn’t realize her past medical history made her a poor candidate. Afterward, she had to be repeatedly admitted for intravenous calcium, as her symptoms could not be controlled with even massive doses of calcium and vitamin D. Ultimately, she needed to have her gastric bypass reversed so she could absorb oral calcium more effectively.
For many patients with nodular thyroid disease, we need to decide whether to do a lobectomy or remove the whole thyroid. I think surgeons can get a bit cavalier about total thyroidectomies because the risk of hypoparathyroidism is very low in most patients, but the risk/benefit ratio is clearly different in this population. Because of this concern, we have a higher threshold to do total thyroidectomies in patients with a history of a gastric bypass. We specifically ask on our endocrine surgery in-take forms if patients have had a gastric bypass; we don’t assume patients will self-report, because it’s often something they want to keep private. When we see that have had a bypass, it frames our conversation about whether or not to have surgery, and the extent of surgery.
Dr. Rebecca Sippel is an associate professor and chief of the section of endocrine surgery at the University of Wisconsin (Madison). She is also an editorial adviser for Surgery News.
Patients who’ve had roux-en-Y gastric bypasses have higher incidences of recalcitrant, symptomatic hypocalcemia after thyroidectomies and spend more time in the hospital afterward for intravenous calcium, according to investigators from the Massachusetts General Hospital in Boston.
Because of that, "it is necessary to ensure that all patients with prior RYGBPs [roux-en-Y gastric bypasses] [take] oral calcium supplements before thyroidectomy," and afterward "recalcitrant, postoperative hypocalcemia should be anticipated, calcium levels closely monitored, and early calcium and vitamin D supplementation initiated preemptively," said the researchers, led by endocrine surgery fellow Dr. Travis McKenzie (Surgery 2013;154:1300-6).
Based on the findings, "we recommend starting oral calcium as early as possible in the evaluation phase before thyroidectomy," supplementing with 1.5-2 gm/day of oral calcium, as per American Society for Metabolic and Bariatric Surgery guidelines. "We prefer calcium citrate, because it has better bioavailability when compared with calcium carbonate after RYGBP." Vitamin D–deficient patients should also get calcitriol (0.25 mcg orally twice daily) for 7 days before the operation, they said.
The team compared outcomes in 19 patients who underwent thyroidectomies an average of 53 months after gastric bypass to outcomes in 38 age-, sex-, and body mass index–matched controls, with pre-bypass BMI matched in the study group to pre-thyroidectomy BMI in the control group, about 45 kg/m2 in both cases. Almost all of the patients in both arms had total thyroidectomies, and thyroid malignancies were found in about half of each.
Overall, eight (42%) of the previous-bypass patients, but none of the controls, developed hypocalcemia after thyroid surgery (P less than .01) and four (21%) – but, again, no controls – required intravenous calcium (P less than .01). The need for intravenous calcium, in turn, led to longer hospital stays in the bypass group (2.2 vs. 1.2 days; P = .02). In the bypass group, about 4 patients, 20%, who were on calcium and vitamin D supplements before surgery developed postop symptomatic hypocalcemia, vs. 11 (58%) of those who were not on supplements (P = .18).
Among the 16 bypass patients and 33 controls who did not have intentional parathyroidectomies as part of their operations, both symptomatic postop hypocalcemia (6 patients; 38% vs 0%; P less than.01) and use of intravenous calcium (3 patients;19% vs. 0%; P = .03) were higher in the bypass group.
In addition to preop calcium supplements, all Mass General patients with previous RYGBPs are now started immediately on postop calcium citrate with vitamin D3 (200-mg elemental calcium/tablet), two tablets four times daily, which is continued indefinitely. "We do not feel that higher dosing will increase efficacy as absorption is limited beyond these doses." Patients also get post-op calcitriol (0.25 mcg orally twice daily) for at least a week, and stopped at that point only if there are no symptoms of hypocalcemia, the investigators said.
The problem "is likely multifactorial, including relative hypoparathyroidism after thyroidectomy in the setting of malabsorptive enteric anatomy and metabolic bone disease," they noted.
"An argument has been made that PTH [parathyroid hormone] testing may allow early identification of patients at risk for hypocalcemia after thyroidectomy, prompting early initiation of calcium and calcitriol when PTH is less than [10 mcg/L]. This finding would not change our immediate management since all patients after thyroidectomy in the setting of previous RYGBP are now maintained on both calcium and calcitriol postoperatively. Furthermore, following PTH levels in this unique patient population may not be accurate because secondary hyperparathyroidism is frequently present after RYGBP. In our study group, patients with symptomatic hypocalcemia had an average PTH of [34 mcg/L]," the investigators said.
Patients were, on average, about 50 years old in both study arms, and more than 90% of the subjects were women. No one developed permanent hypoparathyroidism in either arm.
The work was funded in part by the National Cancer Institute and the Massachusetts General Hospital Department of Surgery. Investigator disclosures were not available.
Patients who’ve had roux-en-Y gastric bypasses have higher incidences of recalcitrant, symptomatic hypocalcemia after thyroidectomies and spend more time in the hospital afterward for intravenous calcium, according to investigators from the Massachusetts General Hospital in Boston.
Because of that, "it is necessary to ensure that all patients with prior RYGBPs [roux-en-Y gastric bypasses] [take] oral calcium supplements before thyroidectomy," and afterward "recalcitrant, postoperative hypocalcemia should be anticipated, calcium levels closely monitored, and early calcium and vitamin D supplementation initiated preemptively," said the researchers, led by endocrine surgery fellow Dr. Travis McKenzie (Surgery 2013;154:1300-6).
Based on the findings, "we recommend starting oral calcium as early as possible in the evaluation phase before thyroidectomy," supplementing with 1.5-2 gm/day of oral calcium, as per American Society for Metabolic and Bariatric Surgery guidelines. "We prefer calcium citrate, because it has better bioavailability when compared with calcium carbonate after RYGBP." Vitamin D–deficient patients should also get calcitriol (0.25 mcg orally twice daily) for 7 days before the operation, they said.
The team compared outcomes in 19 patients who underwent thyroidectomies an average of 53 months after gastric bypass to outcomes in 38 age-, sex-, and body mass index–matched controls, with pre-bypass BMI matched in the study group to pre-thyroidectomy BMI in the control group, about 45 kg/m2 in both cases. Almost all of the patients in both arms had total thyroidectomies, and thyroid malignancies were found in about half of each.
Overall, eight (42%) of the previous-bypass patients, but none of the controls, developed hypocalcemia after thyroid surgery (P less than .01) and four (21%) – but, again, no controls – required intravenous calcium (P less than .01). The need for intravenous calcium, in turn, led to longer hospital stays in the bypass group (2.2 vs. 1.2 days; P = .02). In the bypass group, about 4 patients, 20%, who were on calcium and vitamin D supplements before surgery developed postop symptomatic hypocalcemia, vs. 11 (58%) of those who were not on supplements (P = .18).
Among the 16 bypass patients and 33 controls who did not have intentional parathyroidectomies as part of their operations, both symptomatic postop hypocalcemia (6 patients; 38% vs 0%; P less than.01) and use of intravenous calcium (3 patients;19% vs. 0%; P = .03) were higher in the bypass group.
In addition to preop calcium supplements, all Mass General patients with previous RYGBPs are now started immediately on postop calcium citrate with vitamin D3 (200-mg elemental calcium/tablet), two tablets four times daily, which is continued indefinitely. "We do not feel that higher dosing will increase efficacy as absorption is limited beyond these doses." Patients also get post-op calcitriol (0.25 mcg orally twice daily) for at least a week, and stopped at that point only if there are no symptoms of hypocalcemia, the investigators said.
The problem "is likely multifactorial, including relative hypoparathyroidism after thyroidectomy in the setting of malabsorptive enteric anatomy and metabolic bone disease," they noted.
"An argument has been made that PTH [parathyroid hormone] testing may allow early identification of patients at risk for hypocalcemia after thyroidectomy, prompting early initiation of calcium and calcitriol when PTH is less than [10 mcg/L]. This finding would not change our immediate management since all patients after thyroidectomy in the setting of previous RYGBP are now maintained on both calcium and calcitriol postoperatively. Furthermore, following PTH levels in this unique patient population may not be accurate because secondary hyperparathyroidism is frequently present after RYGBP. In our study group, patients with symptomatic hypocalcemia had an average PTH of [34 mcg/L]," the investigators said.
Patients were, on average, about 50 years old in both study arms, and more than 90% of the subjects were women. No one developed permanent hypoparathyroidism in either arm.
The work was funded in part by the National Cancer Institute and the Massachusetts General Hospital Department of Surgery. Investigator disclosures were not available.
FROM SURGERY
Major finding: Almost half (42%) of patients who have had gastric bypasses develop recalcitrant, symptomatic hypocalcemia after thyroidectomies. Patients who have not had gastric bypasses do not become symptomatically hypocalcemic.
Data source: A retrospective study of 57 thyroidectomy patients
Disclosures: The work was funded in part by the National Cancer Institute and Massachusetts General Hospital. Investigator disclosures were not available.
Perception of safety spurs e-cigarette use in young adults
Debunking popular notions about electronic cigarettes – that they’re safer than real ones and help people quit smoking – might deter young adults from trying them, according to a study published online in the American Journal of Preventive Medicine.
They asked 1,379 20-somethings who had never tried e-cigarettes what they thought about the products, and then resurveyed the group a year later to see who had tried them.
More than 10% of those who thought e-cigarettes were less harmful than tobacco ones , but only 4.6% of those who did not, had tried e-cigarettes within a year (odds ratio, 2.34; 95% confidence interval, 1.49-3.69). Similarly, 10% who thought that e-cigarettes could help people quit smoking, but only 5.4% who did not, had tried them (OR, 1.98; 95% CI, 1.29-3.04). The results were adjusted for age, sex, education, and baseline smoking status (Am. J. Prev. Med. 2014;46:175-8).
The findings "suggest that messages about the lack of evidence on e-cigarettes being cessation aids, and the uncertainty of the risks associated with e-cigarette use" – addiction, pneumonia, heart failure, and so on – "may discourage young adults from experimenting with e-cigarettes," said investigators Kelvin Choi, Ph.D., of the National Institute on Minority Health and Health Disparities in Bethesda, Md., and Jean Forster, Ph.D., of the University of Minnesota, Minneapolis.
"Although a recent review of the literature on e-cigarettes suggests that [they] may be a viable reduced-harm alternative to cigarettes, previous studies have found that experimentation with e-cigarettes was not associated with intention to quit, making quit attempts, or smoking cessation," they said.
At the 1-year follow-up, 7.4% (102) of the sample reported trying e-cigarettes, including 2.9% (28) of baseline nonsmokers. Almost 22% (53) of smokers and 11.9% (21) of former smokers had, as well, meaning that almost 12% of people who said they had quit smoking at baseline were reintroduced to nicotine through e-cigarettes, the authors noted.
Participants were members of the Minnesota Adolescent Community Cohort. The study sample was split about evenly between men and women, and most of the subjects were white, which could limit the findings’ generalizability, the authors said. They also noted that since "smokers were more likely to drop out from the study, the prevalence of experimentation of e-cigarettes and potentially the associations between beliefs and subsequent e-cigarette experimentation could have been underestimated." A majority were enrolled in or graduated from a 4-year college.
E-cigarettes look like tobacco cigarettes but contain a device that heats liquid nicotine into a vapor, which is then inhaled.
The investigators have no disclosures. The work was funded by the National Cancer Institute.
Debunking popular notions about electronic cigarettes – that they’re safer than real ones and help people quit smoking – might deter young adults from trying them, according to a study published online in the American Journal of Preventive Medicine.
They asked 1,379 20-somethings who had never tried e-cigarettes what they thought about the products, and then resurveyed the group a year later to see who had tried them.
More than 10% of those who thought e-cigarettes were less harmful than tobacco ones , but only 4.6% of those who did not, had tried e-cigarettes within a year (odds ratio, 2.34; 95% confidence interval, 1.49-3.69). Similarly, 10% who thought that e-cigarettes could help people quit smoking, but only 5.4% who did not, had tried them (OR, 1.98; 95% CI, 1.29-3.04). The results were adjusted for age, sex, education, and baseline smoking status (Am. J. Prev. Med. 2014;46:175-8).
The findings "suggest that messages about the lack of evidence on e-cigarettes being cessation aids, and the uncertainty of the risks associated with e-cigarette use" – addiction, pneumonia, heart failure, and so on – "may discourage young adults from experimenting with e-cigarettes," said investigators Kelvin Choi, Ph.D., of the National Institute on Minority Health and Health Disparities in Bethesda, Md., and Jean Forster, Ph.D., of the University of Minnesota, Minneapolis.
"Although a recent review of the literature on e-cigarettes suggests that [they] may be a viable reduced-harm alternative to cigarettes, previous studies have found that experimentation with e-cigarettes was not associated with intention to quit, making quit attempts, or smoking cessation," they said.
At the 1-year follow-up, 7.4% (102) of the sample reported trying e-cigarettes, including 2.9% (28) of baseline nonsmokers. Almost 22% (53) of smokers and 11.9% (21) of former smokers had, as well, meaning that almost 12% of people who said they had quit smoking at baseline were reintroduced to nicotine through e-cigarettes, the authors noted.
Participants were members of the Minnesota Adolescent Community Cohort. The study sample was split about evenly between men and women, and most of the subjects were white, which could limit the findings’ generalizability, the authors said. They also noted that since "smokers were more likely to drop out from the study, the prevalence of experimentation of e-cigarettes and potentially the associations between beliefs and subsequent e-cigarette experimentation could have been underestimated." A majority were enrolled in or graduated from a 4-year college.
E-cigarettes look like tobacco cigarettes but contain a device that heats liquid nicotine into a vapor, which is then inhaled.
The investigators have no disclosures. The work was funded by the National Cancer Institute.
Debunking popular notions about electronic cigarettes – that they’re safer than real ones and help people quit smoking – might deter young adults from trying them, according to a study published online in the American Journal of Preventive Medicine.
They asked 1,379 20-somethings who had never tried e-cigarettes what they thought about the products, and then resurveyed the group a year later to see who had tried them.
More than 10% of those who thought e-cigarettes were less harmful than tobacco ones , but only 4.6% of those who did not, had tried e-cigarettes within a year (odds ratio, 2.34; 95% confidence interval, 1.49-3.69). Similarly, 10% who thought that e-cigarettes could help people quit smoking, but only 5.4% who did not, had tried them (OR, 1.98; 95% CI, 1.29-3.04). The results were adjusted for age, sex, education, and baseline smoking status (Am. J. Prev. Med. 2014;46:175-8).
The findings "suggest that messages about the lack of evidence on e-cigarettes being cessation aids, and the uncertainty of the risks associated with e-cigarette use" – addiction, pneumonia, heart failure, and so on – "may discourage young adults from experimenting with e-cigarettes," said investigators Kelvin Choi, Ph.D., of the National Institute on Minority Health and Health Disparities in Bethesda, Md., and Jean Forster, Ph.D., of the University of Minnesota, Minneapolis.
"Although a recent review of the literature on e-cigarettes suggests that [they] may be a viable reduced-harm alternative to cigarettes, previous studies have found that experimentation with e-cigarettes was not associated with intention to quit, making quit attempts, or smoking cessation," they said.
At the 1-year follow-up, 7.4% (102) of the sample reported trying e-cigarettes, including 2.9% (28) of baseline nonsmokers. Almost 22% (53) of smokers and 11.9% (21) of former smokers had, as well, meaning that almost 12% of people who said they had quit smoking at baseline were reintroduced to nicotine through e-cigarettes, the authors noted.
Participants were members of the Minnesota Adolescent Community Cohort. The study sample was split about evenly between men and women, and most of the subjects were white, which could limit the findings’ generalizability, the authors said. They also noted that since "smokers were more likely to drop out from the study, the prevalence of experimentation of e-cigarettes and potentially the associations between beliefs and subsequent e-cigarette experimentation could have been underestimated." A majority were enrolled in or graduated from a 4-year college.
E-cigarettes look like tobacco cigarettes but contain a device that heats liquid nicotine into a vapor, which is then inhaled.
The investigators have no disclosures. The work was funded by the National Cancer Institute.
THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Major finding: More than 10% of young adults who think e-cigarettes are safer than the usual kind, but only 4.6% of those who do not, will try an e-cigarette within a year (OR, 2.34; 95% CI, 1.49-3.69).
Data source: Telephone surveys of 1,379 people in their 20s.
Disclosures: The investigators have no disclosures. The work was funded by the National Cancer Institute.
Premature infants on parenteral nutrition among the casualties in zinc shortage
During the ongoing nationwide shortage of injectable zinc, hospitals should keep what stocks they have in reserve for patients who need them most, particularly infants on parenteral nutrition, investigators advised in the Jan. 17 Morbidity and Mortality Weekly Report.
Seven infants, five of them premature, developed severe zinc deficiencies in late 2012 while on parenteral nutrition (PN) secondary to extreme cholestasis. Zinc is normally added to PN for premature or medically compromised infants, but the hospitals – one in Washington, the other in Houston – had run out because of the shortage (MMWR 2014;63:35-7).
Six infants developed zinc-deficiency dermatitis – and three concomitant bacterial infections – after being on zinc-deficient PN from 4-34 weeks. Serum zinc levels in those tested were well below normal. One child died of causes possibly related to zinc deficiency. The other six children improved after the Food and Drug Administration helped arrange emergency shipments to the hospitals, but another child subsequently died of unrelated causes, said the investigators led by Dr. Duke J. Ruktanonchai, a Centers for Disease Control and Prevention epidemic intelligence service officer.
Injectable zinc has been scarce in the United States since late 2012. By mid-January 2013, 17 hospitals in 10 states had reported shortages.
The situation is slowly improving. FDA has allowed Baxter Healthcare to import injectable zinc from Laboratoire Aguettant, a foreign manufacturer. One U.S. manufacturer is ramping up production after addressing manufacturing problems, including particulate matter in injectable products. The other domestic supplier is already operating at maximum capacity. FDA is posting updates on the situation on its drug shortage website.
There might have been more zinc-deficiency cases in infants. Serum zinc levels aren’t routinely checked in infants; the amount of blood needed might pose a risk, especially to the premature. That "might explain, in part, why no other cases were reported," the investigators said.
Infants are particularly vulnerable to deficiencies because their systemic zinc reserves are not fully developed, so they depend totally on zinc from breast milk or formula.
Whenever administering PN without the standard micronutrients, it’s wise to monitor for signs and symptoms of deficiency. Dermatitis and growth impairment are the most common signs of zinc deficiency. The three zinc-deficient infants in Washington had erosive diaper-area dermatitis and blisters on their extremities, according to the report.
The funding source for the investigation and investigator disclosures were not reported.
During the ongoing nationwide shortage of injectable zinc, hospitals should keep what stocks they have in reserve for patients who need them most, particularly infants on parenteral nutrition, investigators advised in the Jan. 17 Morbidity and Mortality Weekly Report.
Seven infants, five of them premature, developed severe zinc deficiencies in late 2012 while on parenteral nutrition (PN) secondary to extreme cholestasis. Zinc is normally added to PN for premature or medically compromised infants, but the hospitals – one in Washington, the other in Houston – had run out because of the shortage (MMWR 2014;63:35-7).
Six infants developed zinc-deficiency dermatitis – and three concomitant bacterial infections – after being on zinc-deficient PN from 4-34 weeks. Serum zinc levels in those tested were well below normal. One child died of causes possibly related to zinc deficiency. The other six children improved after the Food and Drug Administration helped arrange emergency shipments to the hospitals, but another child subsequently died of unrelated causes, said the investigators led by Dr. Duke J. Ruktanonchai, a Centers for Disease Control and Prevention epidemic intelligence service officer.
Injectable zinc has been scarce in the United States since late 2012. By mid-January 2013, 17 hospitals in 10 states had reported shortages.
The situation is slowly improving. FDA has allowed Baxter Healthcare to import injectable zinc from Laboratoire Aguettant, a foreign manufacturer. One U.S. manufacturer is ramping up production after addressing manufacturing problems, including particulate matter in injectable products. The other domestic supplier is already operating at maximum capacity. FDA is posting updates on the situation on its drug shortage website.
There might have been more zinc-deficiency cases in infants. Serum zinc levels aren’t routinely checked in infants; the amount of blood needed might pose a risk, especially to the premature. That "might explain, in part, why no other cases were reported," the investigators said.
Infants are particularly vulnerable to deficiencies because their systemic zinc reserves are not fully developed, so they depend totally on zinc from breast milk or formula.
Whenever administering PN without the standard micronutrients, it’s wise to monitor for signs and symptoms of deficiency. Dermatitis and growth impairment are the most common signs of zinc deficiency. The three zinc-deficient infants in Washington had erosive diaper-area dermatitis and blisters on their extremities, according to the report.
The funding source for the investigation and investigator disclosures were not reported.
During the ongoing nationwide shortage of injectable zinc, hospitals should keep what stocks they have in reserve for patients who need them most, particularly infants on parenteral nutrition, investigators advised in the Jan. 17 Morbidity and Mortality Weekly Report.
Seven infants, five of them premature, developed severe zinc deficiencies in late 2012 while on parenteral nutrition (PN) secondary to extreme cholestasis. Zinc is normally added to PN for premature or medically compromised infants, but the hospitals – one in Washington, the other in Houston – had run out because of the shortage (MMWR 2014;63:35-7).
Six infants developed zinc-deficiency dermatitis – and three concomitant bacterial infections – after being on zinc-deficient PN from 4-34 weeks. Serum zinc levels in those tested were well below normal. One child died of causes possibly related to zinc deficiency. The other six children improved after the Food and Drug Administration helped arrange emergency shipments to the hospitals, but another child subsequently died of unrelated causes, said the investigators led by Dr. Duke J. Ruktanonchai, a Centers for Disease Control and Prevention epidemic intelligence service officer.
Injectable zinc has been scarce in the United States since late 2012. By mid-January 2013, 17 hospitals in 10 states had reported shortages.
The situation is slowly improving. FDA has allowed Baxter Healthcare to import injectable zinc from Laboratoire Aguettant, a foreign manufacturer. One U.S. manufacturer is ramping up production after addressing manufacturing problems, including particulate matter in injectable products. The other domestic supplier is already operating at maximum capacity. FDA is posting updates on the situation on its drug shortage website.
There might have been more zinc-deficiency cases in infants. Serum zinc levels aren’t routinely checked in infants; the amount of blood needed might pose a risk, especially to the premature. That "might explain, in part, why no other cases were reported," the investigators said.
Infants are particularly vulnerable to deficiencies because their systemic zinc reserves are not fully developed, so they depend totally on zinc from breast milk or formula.
Whenever administering PN without the standard micronutrients, it’s wise to monitor for signs and symptoms of deficiency. Dermatitis and growth impairment are the most common signs of zinc deficiency. The three zinc-deficient infants in Washington had erosive diaper-area dermatitis and blisters on their extremities, according to the report.
The funding source for the investigation and investigator disclosures were not reported.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major finding: Seven infants developed severe zinc deficiencies after receiving zinc-deficient parenteral nutrition as a result of the nationwide shortage of injectable zinc.
Data source: A CDC investigation
Disclosures: Funding source and investigator disclosures were not reported.