User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Melatonin receptor agonist resets blind patients’ internal clocks
SAN FRANCISCO – Tasimelteon, an experimental melatonin receptor agonist, reset the internal clock of blind patients to a 24-hour cycle in a randomized trial by Vanda Pharmaceuticals, its maker.
The 84 totally blind subjects had non-24-hour sleep-wake disorder, meaning that their circadian rhythms were out of synch with the external world, causing sleep and daytime performance problems. That’s not uncommon in blindness; without the regulating effects of perceived light, internal clocks revert to their intrinsic rhythm of about 24.5 hours.
Forty-two patients were randomized to 20 mg of tasimelteon 1 hour before bedtime for 6 months, and 42 others to placebo. Entrainment to a 24-hour cycle was gauged by the timing of peak urinary excretions of cortisol and 6-sulfatoxymelatonin, a melatonin metabolite.
Eight patients on tasimelteon synched up to the 24-hour clock, and 10 both entrained and had improvements in various sleep and function measures. One placebo patient entrained during the trial, and none had improvements in sleep or function.
In a follow-up study, 10 entrained tasimelteon patients were randomized to continue the drug, and 10 others to switch to placebo. One tasimelteon patient, but eight placebo patients, reverted to a non-24-hour cycle. "Maintenance is required to maintain entrainment and clinical benefit," said lead investigator Steven Lockley, Ph.D., a neuroscientist in the division of sleep medicine at Brigham and Women’s Hospital in Boston.
"The medication is able to replace the time cue usually provided by light and synchronize the circadian clock in totally blind people. None of the traditional medications used to treat sleep disorders or sleepiness have this ability," he said at the Endocrine Society’s annual meeting.
Vanda filed with the Food and Drug Administration in May for an indication to treat non-24-hour sleep-wake disorder in totally blind patients. The drug might also prove useful for other circadian problems, Dr. Lockley said.
Side effects included nausea, headache, and sleepiness, but were uncommon and not significantly more frequent than with placebo. Subjects were aged 21-84 years, and 34 were women.
Dr. Lockley is an investigator for Vanda Pharmaceuticals, which funded the work. Another investigator is a consultant to the company, and the remaining six are employees.
SAN FRANCISCO – Tasimelteon, an experimental melatonin receptor agonist, reset the internal clock of blind patients to a 24-hour cycle in a randomized trial by Vanda Pharmaceuticals, its maker.
The 84 totally blind subjects had non-24-hour sleep-wake disorder, meaning that their circadian rhythms were out of synch with the external world, causing sleep and daytime performance problems. That’s not uncommon in blindness; without the regulating effects of perceived light, internal clocks revert to their intrinsic rhythm of about 24.5 hours.
Forty-two patients were randomized to 20 mg of tasimelteon 1 hour before bedtime for 6 months, and 42 others to placebo. Entrainment to a 24-hour cycle was gauged by the timing of peak urinary excretions of cortisol and 6-sulfatoxymelatonin, a melatonin metabolite.
Eight patients on tasimelteon synched up to the 24-hour clock, and 10 both entrained and had improvements in various sleep and function measures. One placebo patient entrained during the trial, and none had improvements in sleep or function.
In a follow-up study, 10 entrained tasimelteon patients were randomized to continue the drug, and 10 others to switch to placebo. One tasimelteon patient, but eight placebo patients, reverted to a non-24-hour cycle. "Maintenance is required to maintain entrainment and clinical benefit," said lead investigator Steven Lockley, Ph.D., a neuroscientist in the division of sleep medicine at Brigham and Women’s Hospital in Boston.
"The medication is able to replace the time cue usually provided by light and synchronize the circadian clock in totally blind people. None of the traditional medications used to treat sleep disorders or sleepiness have this ability," he said at the Endocrine Society’s annual meeting.
Vanda filed with the Food and Drug Administration in May for an indication to treat non-24-hour sleep-wake disorder in totally blind patients. The drug might also prove useful for other circadian problems, Dr. Lockley said.
Side effects included nausea, headache, and sleepiness, but were uncommon and not significantly more frequent than with placebo. Subjects were aged 21-84 years, and 34 were women.
Dr. Lockley is an investigator for Vanda Pharmaceuticals, which funded the work. Another investigator is a consultant to the company, and the remaining six are employees.
SAN FRANCISCO – Tasimelteon, an experimental melatonin receptor agonist, reset the internal clock of blind patients to a 24-hour cycle in a randomized trial by Vanda Pharmaceuticals, its maker.
The 84 totally blind subjects had non-24-hour sleep-wake disorder, meaning that their circadian rhythms were out of synch with the external world, causing sleep and daytime performance problems. That’s not uncommon in blindness; without the regulating effects of perceived light, internal clocks revert to their intrinsic rhythm of about 24.5 hours.
Forty-two patients were randomized to 20 mg of tasimelteon 1 hour before bedtime for 6 months, and 42 others to placebo. Entrainment to a 24-hour cycle was gauged by the timing of peak urinary excretions of cortisol and 6-sulfatoxymelatonin, a melatonin metabolite.
Eight patients on tasimelteon synched up to the 24-hour clock, and 10 both entrained and had improvements in various sleep and function measures. One placebo patient entrained during the trial, and none had improvements in sleep or function.
In a follow-up study, 10 entrained tasimelteon patients were randomized to continue the drug, and 10 others to switch to placebo. One tasimelteon patient, but eight placebo patients, reverted to a non-24-hour cycle. "Maintenance is required to maintain entrainment and clinical benefit," said lead investigator Steven Lockley, Ph.D., a neuroscientist in the division of sleep medicine at Brigham and Women’s Hospital in Boston.
"The medication is able to replace the time cue usually provided by light and synchronize the circadian clock in totally blind people. None of the traditional medications used to treat sleep disorders or sleepiness have this ability," he said at the Endocrine Society’s annual meeting.
Vanda filed with the Food and Drug Administration in May for an indication to treat non-24-hour sleep-wake disorder in totally blind patients. The drug might also prove useful for other circadian problems, Dr. Lockley said.
Side effects included nausea, headache, and sleepiness, but were uncommon and not significantly more frequent than with placebo. Subjects were aged 21-84 years, and 34 were women.
Dr. Lockley is an investigator for Vanda Pharmaceuticals, which funded the work. Another investigator is a consultant to the company, and the remaining six are employees.
AT ENDO 2013
Major finding: Tasimelteon, an experimental melatonin receptor agonist, entrains about half of totally blind patients to a 24-hour circadian rhythm.
Data Source: Randomized, blinded, placebo-controlled trial.
Disclosures: The lead researcher is an investigator for Vanda Pharmaceuticals, which makes the drug and funded the study. Another investigator is a consultant to the company, and the remaining six are employees.
RSV hospitalization risk greatest among children less than 1 month old
Being under a month old, living with children under 5 years old, and being born during peak respiratory syncytial virus season all increase the likelihood that young children will be hospitalized with the infection, according to a 5-year, prospective surveillance study of children up to 2 years old published online July 22 in Pediatrics.
Investigators found 559 (26%) RSV-infected children among the 2,149 hospitalized with acute respiratory infections during 2000-2005 in counties that included Nashville, Tenn.; Rochester, N.Y.; and Cincinnati. Most of the children had been previously healthy, and most were born full-term (Pediatrics 2013;132:e341–e348 [doi: 10.1542/peds.2013-0303]).
The findings suggest that preventive measures – including vaccines under development – should be aimed at all young children, not just premature infants and others traditionally thought of as being at high risk, according to Dr. Caroline Breese Hall of the University of Rochester (N.Y.) and her associates.
"These findings indicate that strategies for diminishing the health care burden from RSV infections should include appropriate prophylaxis and the development of vaccines that are effective in very young infants, even those within the first month of life. In addition, general infection control practices such as restrictions of visits from ill individuals and careful hand washing should be emphasized, especially during the peak months of RSV circulation," which were December and January in this study, the authors concluded.
The RSV hospitalization rate was 5.2 per 1,000 children less than 24 months old; infants in their first month of life had the highest rate at 25.9 per 1,000 children. Infection was confirmed in the study by reverse-transcriptase polymerase chain reaction.
Infants no older than 2 months had a hospitalization rate of 17.9 per 1,000 children and accounted for 44% of RSV hospitalizations.
Ten percent of hospitalized kids were born preterm, "but their risk of hospitalization was not significantly different from that for term infants." It was a different story with very premature infants, that is, those born at less than 30 weeks’ gestation. They accounted for only 3% of RSV cases but had an RSV hospitalization rate of 18.7 per 1,000 children, about three times that of term infants, the authors noted.
Previous attempts to characterize children hospitalized with RSV have tended to rely on retrospective data – often discharge diagnoses – and have stratified risk by 6-month blocks of time. To increase precision, the authors used birth certificates to quantify risk by months of age.
About 21% of the children less than 12 months old had a comorbid condition, most often cardiopulmonary disease; coexisting medical conditions were even more common among older children, occurring in 53% of those aged 12-23 months.
The proportion of hospitalized children less than 24 months old living with another child under 5 years old (57%) was more than twice that of children living with older children (19%).
Overall, gender and race did not affect hospitalization rates.
The team did not study the effects of palivizumab – a monoclonal antibody used to prevent RSV complications in premature infants and others generally thought to be at high risk – on hospitalization rates, which "was unlikely to be appreciable because only a small proportion (less than 5%) of our study population was eligible" to receive it, the team noted.
The Centers for Disease Control and prevention funded the work. Six of the 14 authors reported research funding from or speaking, advising, or consulting relationships with several companies, including GlaxoSmithKline, MedImmune, Merck, Sanofi Pasteur, Novartis, BD Diagnostics, Novavax, and Quidel. Some of those firms are involved with RSV vaccine development.
Being under a month old, living with children under 5 years old, and being born during peak respiratory syncytial virus season all increase the likelihood that young children will be hospitalized with the infection, according to a 5-year, prospective surveillance study of children up to 2 years old published online July 22 in Pediatrics.
Investigators found 559 (26%) RSV-infected children among the 2,149 hospitalized with acute respiratory infections during 2000-2005 in counties that included Nashville, Tenn.; Rochester, N.Y.; and Cincinnati. Most of the children had been previously healthy, and most were born full-term (Pediatrics 2013;132:e341–e348 [doi: 10.1542/peds.2013-0303]).
The findings suggest that preventive measures – including vaccines under development – should be aimed at all young children, not just premature infants and others traditionally thought of as being at high risk, according to Dr. Caroline Breese Hall of the University of Rochester (N.Y.) and her associates.
"These findings indicate that strategies for diminishing the health care burden from RSV infections should include appropriate prophylaxis and the development of vaccines that are effective in very young infants, even those within the first month of life. In addition, general infection control practices such as restrictions of visits from ill individuals and careful hand washing should be emphasized, especially during the peak months of RSV circulation," which were December and January in this study, the authors concluded.
The RSV hospitalization rate was 5.2 per 1,000 children less than 24 months old; infants in their first month of life had the highest rate at 25.9 per 1,000 children. Infection was confirmed in the study by reverse-transcriptase polymerase chain reaction.
Infants no older than 2 months had a hospitalization rate of 17.9 per 1,000 children and accounted for 44% of RSV hospitalizations.
Ten percent of hospitalized kids were born preterm, "but their risk of hospitalization was not significantly different from that for term infants." It was a different story with very premature infants, that is, those born at less than 30 weeks’ gestation. They accounted for only 3% of RSV cases but had an RSV hospitalization rate of 18.7 per 1,000 children, about three times that of term infants, the authors noted.
Previous attempts to characterize children hospitalized with RSV have tended to rely on retrospective data – often discharge diagnoses – and have stratified risk by 6-month blocks of time. To increase precision, the authors used birth certificates to quantify risk by months of age.
About 21% of the children less than 12 months old had a comorbid condition, most often cardiopulmonary disease; coexisting medical conditions were even more common among older children, occurring in 53% of those aged 12-23 months.
The proportion of hospitalized children less than 24 months old living with another child under 5 years old (57%) was more than twice that of children living with older children (19%).
Overall, gender and race did not affect hospitalization rates.
The team did not study the effects of palivizumab – a monoclonal antibody used to prevent RSV complications in premature infants and others generally thought to be at high risk – on hospitalization rates, which "was unlikely to be appreciable because only a small proportion (less than 5%) of our study population was eligible" to receive it, the team noted.
The Centers for Disease Control and prevention funded the work. Six of the 14 authors reported research funding from or speaking, advising, or consulting relationships with several companies, including GlaxoSmithKline, MedImmune, Merck, Sanofi Pasteur, Novartis, BD Diagnostics, Novavax, and Quidel. Some of those firms are involved with RSV vaccine development.
Being under a month old, living with children under 5 years old, and being born during peak respiratory syncytial virus season all increase the likelihood that young children will be hospitalized with the infection, according to a 5-year, prospective surveillance study of children up to 2 years old published online July 22 in Pediatrics.
Investigators found 559 (26%) RSV-infected children among the 2,149 hospitalized with acute respiratory infections during 2000-2005 in counties that included Nashville, Tenn.; Rochester, N.Y.; and Cincinnati. Most of the children had been previously healthy, and most were born full-term (Pediatrics 2013;132:e341–e348 [doi: 10.1542/peds.2013-0303]).
The findings suggest that preventive measures – including vaccines under development – should be aimed at all young children, not just premature infants and others traditionally thought of as being at high risk, according to Dr. Caroline Breese Hall of the University of Rochester (N.Y.) and her associates.
"These findings indicate that strategies for diminishing the health care burden from RSV infections should include appropriate prophylaxis and the development of vaccines that are effective in very young infants, even those within the first month of life. In addition, general infection control practices such as restrictions of visits from ill individuals and careful hand washing should be emphasized, especially during the peak months of RSV circulation," which were December and January in this study, the authors concluded.
The RSV hospitalization rate was 5.2 per 1,000 children less than 24 months old; infants in their first month of life had the highest rate at 25.9 per 1,000 children. Infection was confirmed in the study by reverse-transcriptase polymerase chain reaction.
Infants no older than 2 months had a hospitalization rate of 17.9 per 1,000 children and accounted for 44% of RSV hospitalizations.
Ten percent of hospitalized kids were born preterm, "but their risk of hospitalization was not significantly different from that for term infants." It was a different story with very premature infants, that is, those born at less than 30 weeks’ gestation. They accounted for only 3% of RSV cases but had an RSV hospitalization rate of 18.7 per 1,000 children, about three times that of term infants, the authors noted.
Previous attempts to characterize children hospitalized with RSV have tended to rely on retrospective data – often discharge diagnoses – and have stratified risk by 6-month blocks of time. To increase precision, the authors used birth certificates to quantify risk by months of age.
About 21% of the children less than 12 months old had a comorbid condition, most often cardiopulmonary disease; coexisting medical conditions were even more common among older children, occurring in 53% of those aged 12-23 months.
The proportion of hospitalized children less than 24 months old living with another child under 5 years old (57%) was more than twice that of children living with older children (19%).
Overall, gender and race did not affect hospitalization rates.
The team did not study the effects of palivizumab – a monoclonal antibody used to prevent RSV complications in premature infants and others generally thought to be at high risk – on hospitalization rates, which "was unlikely to be appreciable because only a small proportion (less than 5%) of our study population was eligible" to receive it, the team noted.
The Centers for Disease Control and prevention funded the work. Six of the 14 authors reported research funding from or speaking, advising, or consulting relationships with several companies, including GlaxoSmithKline, MedImmune, Merck, Sanofi Pasteur, Novartis, BD Diagnostics, Novavax, and Quidel. Some of those firms are involved with RSV vaccine development.
FROM PEDIATRICS
Major finding: The RSV hospitalization rate for children in their first month of life is 25.9 per 1,000.
Data source: Prospective study of children up to 2 years old hospitalized with acute respiratory infections during 2000-2005.
Disclosures: The Centers for Disease Control and prevention funded the work. Six of the 14 authors reported research funding from or speaking, advising, or consulting relationships with several companies, including GlaxoSmithKline, MedImmune, Merck, Sanofi Pasteur, Novartis, BD Diagnostics, Novavax, and Quidel. Some of those firms are involved with RSV vaccine development.
Vibration platform strengthened bones, improved walking in moderate cerebral palsy
SAN FRANCISCO – Regular sessions on vibration platforms improved bone density, muscle mass, and walking ability in children with cerebral palsy in a small study from New Zealand.
Five boys and eight girls aged 14-19 years with moderate cerebral palsy had four sessions per week in which they stood on the platforms for 3 minutes, took a 3-minute break, and repeated the cycle twice more.
After 5 months, their mean total leg bone mineral density (BMD) increased from 1.06 g/cm2 to 1.08 g/cm2, their total spine BMD increased from 1.01 g/cm2 to 1.02 g/cm2, and their total lower-tibia volumetric BMD increased from 607 mg/cm3 to 690 mm/cm3. These modest changes were similar to those seen with osteoporosis medications.
The subjects’ mean midtibia muscle cross-sectional area increased from 3,163 mm2 to 3,440 mm2, and their mean 6-minute walk distance increased from 286 to 314 m. The results were all significant. One girl was able to walk up stairs for the first time after the trial, and was still able to do so 6 months later, according to lead investigator Silmara Gusso, Ph.D., a research fellow at the University of Auckland, New Zealand.
Parents reported fewer falls at home, better balance, and more regular bowel function. Several said they wanted to buy one of the devices, a Galileo home vibration platform. Physiotherapists said they wanted to try them on children with other musculoskeletal challenges, Dr. Gusso said at the Endocrine Society’s Annual Meeting.
"We were quite surprised to see the changes over such a short period of time ... [and] very impressed with the feedback we got from families, physiotherapists, and the kids themselves," who "opened huge smiles" when they stepped on the platforms. It was all "much more than we expected. We are quite excited about it," she said.
Vibration platforms aren’t new; they’ve been used before to help with osteoporosis, surgery recoveries, and athletic injuries.
The trial may be the first, however, to try them for cerebral palsy. The idea was to see if they’d help with osteopenia and muscle mass, something that’s hard to address with standard physiotherapy, which focuses on strength, stretching, balance, and gentle walking, Dr. Gusso said.
The platforms were set to tip side to side at 1,200 oscillations per minute (20 Hz), "which is pretty hard exercise" even for someone without cerebral palsy. The children had a brace they could hold on to for balance. Their weekly physiotherapy sessions continued throughout the study.
The research will be expanded to include children with other disabilities. "It’s a small and simple device that can bring extra benefits" to standard approaches, Dr. Gusso said.
Dr. Gusso and her team said they had nothing to disclose. The work was funded by the Jubilee Trust, the Australasia Paediatric Endocrine Group, and others.
SAN FRANCISCO – Regular sessions on vibration platforms improved bone density, muscle mass, and walking ability in children with cerebral palsy in a small study from New Zealand.
Five boys and eight girls aged 14-19 years with moderate cerebral palsy had four sessions per week in which they stood on the platforms for 3 minutes, took a 3-minute break, and repeated the cycle twice more.
After 5 months, their mean total leg bone mineral density (BMD) increased from 1.06 g/cm2 to 1.08 g/cm2, their total spine BMD increased from 1.01 g/cm2 to 1.02 g/cm2, and their total lower-tibia volumetric BMD increased from 607 mg/cm3 to 690 mm/cm3. These modest changes were similar to those seen with osteoporosis medications.
The subjects’ mean midtibia muscle cross-sectional area increased from 3,163 mm2 to 3,440 mm2, and their mean 6-minute walk distance increased from 286 to 314 m. The results were all significant. One girl was able to walk up stairs for the first time after the trial, and was still able to do so 6 months later, according to lead investigator Silmara Gusso, Ph.D., a research fellow at the University of Auckland, New Zealand.
Parents reported fewer falls at home, better balance, and more regular bowel function. Several said they wanted to buy one of the devices, a Galileo home vibration platform. Physiotherapists said they wanted to try them on children with other musculoskeletal challenges, Dr. Gusso said at the Endocrine Society’s Annual Meeting.
"We were quite surprised to see the changes over such a short period of time ... [and] very impressed with the feedback we got from families, physiotherapists, and the kids themselves," who "opened huge smiles" when they stepped on the platforms. It was all "much more than we expected. We are quite excited about it," she said.
Vibration platforms aren’t new; they’ve been used before to help with osteoporosis, surgery recoveries, and athletic injuries.
The trial may be the first, however, to try them for cerebral palsy. The idea was to see if they’d help with osteopenia and muscle mass, something that’s hard to address with standard physiotherapy, which focuses on strength, stretching, balance, and gentle walking, Dr. Gusso said.
The platforms were set to tip side to side at 1,200 oscillations per minute (20 Hz), "which is pretty hard exercise" even for someone without cerebral palsy. The children had a brace they could hold on to for balance. Their weekly physiotherapy sessions continued throughout the study.
The research will be expanded to include children with other disabilities. "It’s a small and simple device that can bring extra benefits" to standard approaches, Dr. Gusso said.
Dr. Gusso and her team said they had nothing to disclose. The work was funded by the Jubilee Trust, the Australasia Paediatric Endocrine Group, and others.
SAN FRANCISCO – Regular sessions on vibration platforms improved bone density, muscle mass, and walking ability in children with cerebral palsy in a small study from New Zealand.
Five boys and eight girls aged 14-19 years with moderate cerebral palsy had four sessions per week in which they stood on the platforms for 3 minutes, took a 3-minute break, and repeated the cycle twice more.
After 5 months, their mean total leg bone mineral density (BMD) increased from 1.06 g/cm2 to 1.08 g/cm2, their total spine BMD increased from 1.01 g/cm2 to 1.02 g/cm2, and their total lower-tibia volumetric BMD increased from 607 mg/cm3 to 690 mm/cm3. These modest changes were similar to those seen with osteoporosis medications.
The subjects’ mean midtibia muscle cross-sectional area increased from 3,163 mm2 to 3,440 mm2, and their mean 6-minute walk distance increased from 286 to 314 m. The results were all significant. One girl was able to walk up stairs for the first time after the trial, and was still able to do so 6 months later, according to lead investigator Silmara Gusso, Ph.D., a research fellow at the University of Auckland, New Zealand.
Parents reported fewer falls at home, better balance, and more regular bowel function. Several said they wanted to buy one of the devices, a Galileo home vibration platform. Physiotherapists said they wanted to try them on children with other musculoskeletal challenges, Dr. Gusso said at the Endocrine Society’s Annual Meeting.
"We were quite surprised to see the changes over such a short period of time ... [and] very impressed with the feedback we got from families, physiotherapists, and the kids themselves," who "opened huge smiles" when they stepped on the platforms. It was all "much more than we expected. We are quite excited about it," she said.
Vibration platforms aren’t new; they’ve been used before to help with osteoporosis, surgery recoveries, and athletic injuries.
The trial may be the first, however, to try them for cerebral palsy. The idea was to see if they’d help with osteopenia and muscle mass, something that’s hard to address with standard physiotherapy, which focuses on strength, stretching, balance, and gentle walking, Dr. Gusso said.
The platforms were set to tip side to side at 1,200 oscillations per minute (20 Hz), "which is pretty hard exercise" even for someone without cerebral palsy. The children had a brace they could hold on to for balance. Their weekly physiotherapy sessions continued throughout the study.
The research will be expanded to include children with other disabilities. "It’s a small and simple device that can bring extra benefits" to standard approaches, Dr. Gusso said.
Dr. Gusso and her team said they had nothing to disclose. The work was funded by the Jubilee Trust, the Australasia Paediatric Endocrine Group, and others.
AT ENDO 2013
Major finding: After 5 months of vibration platform therapy, children with moderate cerebral palsy could walk about 30 m more in 6 minutes.
Data source: Uncontrolled trial involving 13 subjects.
Disclosures: Dr. Gusso and her team said they had nothing to disclose. The work was funded by the Jubilee Trust, the Australasia Paediatric Endocrine Group, and others.
Control of congenital adrenal hyperplasia improves with more frequent steroid dosing
SAN FRANCISCO – Administering daily hydrocortisone prescriptions in four divided doses, instead of three, can improve control of congenital adrenal hyperplasia and obviate the need for additional steroids, British researchers found in a retrospective cross-sectional study.
A decade ago, it was assumed that children with poorly controlled moderate-to-severe disease "needed higher doses rather than more frequent dosing; if patients were not controlled on three-times-a-day dosing, we’d increase the dose. Now, we go four times a day, and don’t necessarily have to increase the dose. We see better control with QID dosing," said lead investigator Dr. Anbezhil Subbarayan, a pediatric endocrinologist at Great Ormond Street Hospital for Children in London.
His team compared 107 children under treatment there with a similar cohort treated a decade ago and found that the approach led to a reduction in mean daily hydrocortisone doses from 17.5 mg/m2 to 13.3 mg/m2 and a drop in mean daily doses of 9 alpha-fludrocortisone, administered in one or two divided doses, from 112 mcg/m2 to 102 mcg/m2.
That led to a corresponding drop in the prevalence of steroid-induced systolic hypertension from 58% of patients to 21%; a drop in diastolic hypertension prevalence from 24% to 9%; and improvement in mean body mass index standard deviation scores (BMI-SDS) from 1.57 to 0.98. The results were also published online(Clin. Endocrinol [Oxf] 2013 [doi:10.1111/cen.12265]).
"Our message" is that "patients under poor control should [try] four-times-a-day" hydrocortisone dosing. With a half-life of just 4-6 hours, spacing out hydrocortisone administration "should improve control in patients with moderate-to-severe disease without increasing the need for steroids, which can worsen cardiovascular risk factors," Dr. Subbarayan said at the Endocrine Society’s annual meeting.
"Ideally, the first hydrocortisone dose should be given at 4:00 a.m. Some parents are motivated" to do that; for those who find it impractical, "we try to encourage giving hydrocortisone first thing in the morning, maybe at 6:00 a.m." For children with mild disease, divided doses three times a day might be enough, he said.
Despite reduced steroid doses and cardiovascular risk factors, obesity and systolic hypertension remain problematic in kids with congenital adrenal hyperplasia.
The team found that 24% of their children were obese (BMI-SDS greater than 2), and that BMI-SD scores were significantly higher, compared with British children overall. The mean systolic blood pressure of 108 mm Hg (SD 13.5) was higher, as well. Differences in diastolic pressure did not reach statistical significance.
The values were assessed during annual reviews when the children were admitted to the hospital for 24 hours for serial monitoring of cortisol levels and clinical parameters, and dose adjustments. The median age in the study was 9.2 years; 64% were girls.
The authors said they have no disclosures.
SAN FRANCISCO – Administering daily hydrocortisone prescriptions in four divided doses, instead of three, can improve control of congenital adrenal hyperplasia and obviate the need for additional steroids, British researchers found in a retrospective cross-sectional study.
A decade ago, it was assumed that children with poorly controlled moderate-to-severe disease "needed higher doses rather than more frequent dosing; if patients were not controlled on three-times-a-day dosing, we’d increase the dose. Now, we go four times a day, and don’t necessarily have to increase the dose. We see better control with QID dosing," said lead investigator Dr. Anbezhil Subbarayan, a pediatric endocrinologist at Great Ormond Street Hospital for Children in London.
His team compared 107 children under treatment there with a similar cohort treated a decade ago and found that the approach led to a reduction in mean daily hydrocortisone doses from 17.5 mg/m2 to 13.3 mg/m2 and a drop in mean daily doses of 9 alpha-fludrocortisone, administered in one or two divided doses, from 112 mcg/m2 to 102 mcg/m2.
That led to a corresponding drop in the prevalence of steroid-induced systolic hypertension from 58% of patients to 21%; a drop in diastolic hypertension prevalence from 24% to 9%; and improvement in mean body mass index standard deviation scores (BMI-SDS) from 1.57 to 0.98. The results were also published online(Clin. Endocrinol [Oxf] 2013 [doi:10.1111/cen.12265]).
"Our message" is that "patients under poor control should [try] four-times-a-day" hydrocortisone dosing. With a half-life of just 4-6 hours, spacing out hydrocortisone administration "should improve control in patients with moderate-to-severe disease without increasing the need for steroids, which can worsen cardiovascular risk factors," Dr. Subbarayan said at the Endocrine Society’s annual meeting.
"Ideally, the first hydrocortisone dose should be given at 4:00 a.m. Some parents are motivated" to do that; for those who find it impractical, "we try to encourage giving hydrocortisone first thing in the morning, maybe at 6:00 a.m." For children with mild disease, divided doses three times a day might be enough, he said.
Despite reduced steroid doses and cardiovascular risk factors, obesity and systolic hypertension remain problematic in kids with congenital adrenal hyperplasia.
The team found that 24% of their children were obese (BMI-SDS greater than 2), and that BMI-SD scores were significantly higher, compared with British children overall. The mean systolic blood pressure of 108 mm Hg (SD 13.5) was higher, as well. Differences in diastolic pressure did not reach statistical significance.
The values were assessed during annual reviews when the children were admitted to the hospital for 24 hours for serial monitoring of cortisol levels and clinical parameters, and dose adjustments. The median age in the study was 9.2 years; 64% were girls.
The authors said they have no disclosures.
SAN FRANCISCO – Administering daily hydrocortisone prescriptions in four divided doses, instead of three, can improve control of congenital adrenal hyperplasia and obviate the need for additional steroids, British researchers found in a retrospective cross-sectional study.
A decade ago, it was assumed that children with poorly controlled moderate-to-severe disease "needed higher doses rather than more frequent dosing; if patients were not controlled on three-times-a-day dosing, we’d increase the dose. Now, we go four times a day, and don’t necessarily have to increase the dose. We see better control with QID dosing," said lead investigator Dr. Anbezhil Subbarayan, a pediatric endocrinologist at Great Ormond Street Hospital for Children in London.
His team compared 107 children under treatment there with a similar cohort treated a decade ago and found that the approach led to a reduction in mean daily hydrocortisone doses from 17.5 mg/m2 to 13.3 mg/m2 and a drop in mean daily doses of 9 alpha-fludrocortisone, administered in one or two divided doses, from 112 mcg/m2 to 102 mcg/m2.
That led to a corresponding drop in the prevalence of steroid-induced systolic hypertension from 58% of patients to 21%; a drop in diastolic hypertension prevalence from 24% to 9%; and improvement in mean body mass index standard deviation scores (BMI-SDS) from 1.57 to 0.98. The results were also published online(Clin. Endocrinol [Oxf] 2013 [doi:10.1111/cen.12265]).
"Our message" is that "patients under poor control should [try] four-times-a-day" hydrocortisone dosing. With a half-life of just 4-6 hours, spacing out hydrocortisone administration "should improve control in patients with moderate-to-severe disease without increasing the need for steroids, which can worsen cardiovascular risk factors," Dr. Subbarayan said at the Endocrine Society’s annual meeting.
"Ideally, the first hydrocortisone dose should be given at 4:00 a.m. Some parents are motivated" to do that; for those who find it impractical, "we try to encourage giving hydrocortisone first thing in the morning, maybe at 6:00 a.m." For children with mild disease, divided doses three times a day might be enough, he said.
Despite reduced steroid doses and cardiovascular risk factors, obesity and systolic hypertension remain problematic in kids with congenital adrenal hyperplasia.
The team found that 24% of their children were obese (BMI-SDS greater than 2), and that BMI-SD scores were significantly higher, compared with British children overall. The mean systolic blood pressure of 108 mm Hg (SD 13.5) was higher, as well. Differences in diastolic pressure did not reach statistical significance.
The values were assessed during annual reviews when the children were admitted to the hospital for 24 hours for serial monitoring of cortisol levels and clinical parameters, and dose adjustments. The median age in the study was 9.2 years; 64% were girls.
The authors said they have no disclosures.
AT ENDO 2013
Major finding: Administering daily hydrocortisone in four divided doses, instead of increasing the prescription and sticking with three divided doses, reduced the prevalence of steroid-induced systolic hypertension in children with congenital adrenal hyperplasia from 58% to 21%.
Data source: Retrospective, cross-sectional study of 107 patients.
Disclosures: The authors said they have no disclosures.
Slight cognition benefit found for testosterone gel after menopause
SAN FRANCISCO – Postmenopausal women showed a modest improvement in verbal learning and memory after being on testosterone gel for 6 months, according to researchers from Monash University in Melbourne.
Ninety healthy, cognitively normal women aged 55-65 years were randomized in the trial to 0.22 g transdermal testosterone gel (LibiGel) daily, or to placebo.
At the end of 26 weeks, women on testosterone scored 1.6 points better on the 48-point International Shopping List Task, meaning that they recalled a total of 1.6 more words while being read three lists containing 16 words each. The placebo group improved a bit from the mean baseline score of 33.7 words, as well, but not quite as much. There were no other significant differences in well-being and cognitive function.
Lead investigator Susan Davis, chair of Women’s Health at Monash, said the finding, and a few similar ones from previous work, provide "evidence for the conduct of large-scale clinical studies" to see if testosterone can help preserve cognition as women age. "Possibly, there’s a beneficial effect, but it does need to be explored."
"Women complain of loss of memory after menopause. ... We hypothesize that women after menopause are not operating in their optimum cognitive range." That could have something to do with naturally declining levels of testosterone; supplementation could be more about "optimizing normality rather than treating a disease," she said at the Endocrine Society’s annual meeting.
There’s evidence, mostly from trials in men, that the hormone has "an important role in brain function," she said.
Mean serum total testosterone was at the lower limit of normal in both groups at baseline; it increased a mean of 1.9 nmol/L in the treated group and remained largely unchanged in the placebo group. Women on systemic hormone therapy were excluded from the study.
Dr. Davis did not mention any adverse events but noted that the literature on testosterone therapy in women "has not shown anything sinister" with physiologic doses.
She was the lead investigator in a trial of 814 women that found a modest benefit for testosterone patches on libido. Breast cancer was diagnosed in four women treated for up to 2 years in the study, but none in the placebo group; vaginal bleeding was more common in the treated group, as well (N. Engl. J. Med. 2008;359:2005-17).
Dr. Davis is the principal investigator and a scientific board member for BioSante Pharmaceuticals, makers of the testosterone gel used in the study. She is also an investigator for and consultant to Trimel, maker of an intranasal testosterone gel. The work was supported by BioSante.
BioSante Pharmaceuticals,
SAN FRANCISCO – Postmenopausal women showed a modest improvement in verbal learning and memory after being on testosterone gel for 6 months, according to researchers from Monash University in Melbourne.
Ninety healthy, cognitively normal women aged 55-65 years were randomized in the trial to 0.22 g transdermal testosterone gel (LibiGel) daily, or to placebo.
At the end of 26 weeks, women on testosterone scored 1.6 points better on the 48-point International Shopping List Task, meaning that they recalled a total of 1.6 more words while being read three lists containing 16 words each. The placebo group improved a bit from the mean baseline score of 33.7 words, as well, but not quite as much. There were no other significant differences in well-being and cognitive function.
Lead investigator Susan Davis, chair of Women’s Health at Monash, said the finding, and a few similar ones from previous work, provide "evidence for the conduct of large-scale clinical studies" to see if testosterone can help preserve cognition as women age. "Possibly, there’s a beneficial effect, but it does need to be explored."
"Women complain of loss of memory after menopause. ... We hypothesize that women after menopause are not operating in their optimum cognitive range." That could have something to do with naturally declining levels of testosterone; supplementation could be more about "optimizing normality rather than treating a disease," she said at the Endocrine Society’s annual meeting.
There’s evidence, mostly from trials in men, that the hormone has "an important role in brain function," she said.
Mean serum total testosterone was at the lower limit of normal in both groups at baseline; it increased a mean of 1.9 nmol/L in the treated group and remained largely unchanged in the placebo group. Women on systemic hormone therapy were excluded from the study.
Dr. Davis did not mention any adverse events but noted that the literature on testosterone therapy in women "has not shown anything sinister" with physiologic doses.
She was the lead investigator in a trial of 814 women that found a modest benefit for testosterone patches on libido. Breast cancer was diagnosed in four women treated for up to 2 years in the study, but none in the placebo group; vaginal bleeding was more common in the treated group, as well (N. Engl. J. Med. 2008;359:2005-17).
Dr. Davis is the principal investigator and a scientific board member for BioSante Pharmaceuticals, makers of the testosterone gel used in the study. She is also an investigator for and consultant to Trimel, maker of an intranasal testosterone gel. The work was supported by BioSante.
SAN FRANCISCO – Postmenopausal women showed a modest improvement in verbal learning and memory after being on testosterone gel for 6 months, according to researchers from Monash University in Melbourne.
Ninety healthy, cognitively normal women aged 55-65 years were randomized in the trial to 0.22 g transdermal testosterone gel (LibiGel) daily, or to placebo.
At the end of 26 weeks, women on testosterone scored 1.6 points better on the 48-point International Shopping List Task, meaning that they recalled a total of 1.6 more words while being read three lists containing 16 words each. The placebo group improved a bit from the mean baseline score of 33.7 words, as well, but not quite as much. There were no other significant differences in well-being and cognitive function.
Lead investigator Susan Davis, chair of Women’s Health at Monash, said the finding, and a few similar ones from previous work, provide "evidence for the conduct of large-scale clinical studies" to see if testosterone can help preserve cognition as women age. "Possibly, there’s a beneficial effect, but it does need to be explored."
"Women complain of loss of memory after menopause. ... We hypothesize that women after menopause are not operating in their optimum cognitive range." That could have something to do with naturally declining levels of testosterone; supplementation could be more about "optimizing normality rather than treating a disease," she said at the Endocrine Society’s annual meeting.
There’s evidence, mostly from trials in men, that the hormone has "an important role in brain function," she said.
Mean serum total testosterone was at the lower limit of normal in both groups at baseline; it increased a mean of 1.9 nmol/L in the treated group and remained largely unchanged in the placebo group. Women on systemic hormone therapy were excluded from the study.
Dr. Davis did not mention any adverse events but noted that the literature on testosterone therapy in women "has not shown anything sinister" with physiologic doses.
She was the lead investigator in a trial of 814 women that found a modest benefit for testosterone patches on libido. Breast cancer was diagnosed in four women treated for up to 2 years in the study, but none in the placebo group; vaginal bleeding was more common in the treated group, as well (N. Engl. J. Med. 2008;359:2005-17).
Dr. Davis is the principal investigator and a scientific board member for BioSante Pharmaceuticals, makers of the testosterone gel used in the study. She is also an investigator for and consultant to Trimel, maker of an intranasal testosterone gel. The work was supported by BioSante.
BioSante Pharmaceuticals,
BioSante Pharmaceuticals,
AT ENDO 2013
Major finding: After 6 months of daily testosterone gel, women scored 1.57 points better on the 48-point International Shopping List Task test.
Data Source: A randomized, placebo-controlled trial in 90 postmenopausal women.
Disclosures: The lead investigator is the principal investigator and a scientific board member for BioSante Pharmaceuticals, makers of the testosterone gel used in the study. She is also an investigator for and consultant to Trimel, maker of an intranasal testosterone gel.
Afatinib approved for non-small cell lung tumors with growth factor mutations
The tyrosine kinase inhibitor afatinib received Food and Drug Administration approval July 12 for the first-line treatment of metastatic non–small cell lung cancers that express epidermal growth factor exon 19 deletions or exon 21 L858R substitution gene mutations. Its brand name is Gilotrif, and it will be marketed by Boehringer Ingelheim.
The agency also approved a kit to detect those mutations, Qiagen’s therascreen EGFR RGQ PCR Kit; they occur in most of the 10% of non–small cell lung cancer (NSCLC) tumors that have epidermal growth factor receptor mutations.
Another kinase inhibitor, Genentech’s erlotinib (Tarceva), was approved for the same indication in May, along with its own mutation screening tool, Roche’s cobas EGFR Mutation Test.
In the study that won approval for afatinib, 230 NSCLC patients with the mutations were randomized to afatinib 40 mg orally; 115 others were randomized to up to six cycles of pemetrexed and cisplatin. Median progression-free survival was 11.1 months in the afatinib group and 6.9 months in the chemotherapy group. There was no statistically significant difference in overall survival. The therascreen kit was validated in that trial.
The side effects of Gilotrif include diarrhea, which can lead to kidney failure and severe dehydration; liver toxicity; lung inflammation; and severe rashes. Less serious side effects include acnelike skin eruptions, dry skin, pruritus, mouth inflammation, paronychia, decreased appetite, decreased weight, cystitis, nosebleed, runny nose, fever, eye inflammation, and hypokalemia.
In a phase II Japanese study published online by the Journal of Clinical Oncology July 1, 50 mg per day of afatinib demonstrated "modest but noteworthy efficacy" in NSCLC patients who progressed after being on erlotinib or gefitinib, or both, for 12 or more weeks.
Of 62 treated patients, 45 (72.6%) were EGFR mutation positive in their primary tumor; 51 (82.3%) patients had developed resistance to erlotinib or gefitinib. Median progression-free survival was 4.4 months and median overall survival 19.0 months (J. Clin. Oncol. 2013 July 1 [doi: 10.1200/JCO.2012.45.0981]).
The tyrosine kinase inhibitor afatinib received Food and Drug Administration approval July 12 for the first-line treatment of metastatic non–small cell lung cancers that express epidermal growth factor exon 19 deletions or exon 21 L858R substitution gene mutations. Its brand name is Gilotrif, and it will be marketed by Boehringer Ingelheim.
The agency also approved a kit to detect those mutations, Qiagen’s therascreen EGFR RGQ PCR Kit; they occur in most of the 10% of non–small cell lung cancer (NSCLC) tumors that have epidermal growth factor receptor mutations.
Another kinase inhibitor, Genentech’s erlotinib (Tarceva), was approved for the same indication in May, along with its own mutation screening tool, Roche’s cobas EGFR Mutation Test.
In the study that won approval for afatinib, 230 NSCLC patients with the mutations were randomized to afatinib 40 mg orally; 115 others were randomized to up to six cycles of pemetrexed and cisplatin. Median progression-free survival was 11.1 months in the afatinib group and 6.9 months in the chemotherapy group. There was no statistically significant difference in overall survival. The therascreen kit was validated in that trial.
The side effects of Gilotrif include diarrhea, which can lead to kidney failure and severe dehydration; liver toxicity; lung inflammation; and severe rashes. Less serious side effects include acnelike skin eruptions, dry skin, pruritus, mouth inflammation, paronychia, decreased appetite, decreased weight, cystitis, nosebleed, runny nose, fever, eye inflammation, and hypokalemia.
In a phase II Japanese study published online by the Journal of Clinical Oncology July 1, 50 mg per day of afatinib demonstrated "modest but noteworthy efficacy" in NSCLC patients who progressed after being on erlotinib or gefitinib, or both, for 12 or more weeks.
Of 62 treated patients, 45 (72.6%) were EGFR mutation positive in their primary tumor; 51 (82.3%) patients had developed resistance to erlotinib or gefitinib. Median progression-free survival was 4.4 months and median overall survival 19.0 months (J. Clin. Oncol. 2013 July 1 [doi: 10.1200/JCO.2012.45.0981]).
The tyrosine kinase inhibitor afatinib received Food and Drug Administration approval July 12 for the first-line treatment of metastatic non–small cell lung cancers that express epidermal growth factor exon 19 deletions or exon 21 L858R substitution gene mutations. Its brand name is Gilotrif, and it will be marketed by Boehringer Ingelheim.
The agency also approved a kit to detect those mutations, Qiagen’s therascreen EGFR RGQ PCR Kit; they occur in most of the 10% of non–small cell lung cancer (NSCLC) tumors that have epidermal growth factor receptor mutations.
Another kinase inhibitor, Genentech’s erlotinib (Tarceva), was approved for the same indication in May, along with its own mutation screening tool, Roche’s cobas EGFR Mutation Test.
In the study that won approval for afatinib, 230 NSCLC patients with the mutations were randomized to afatinib 40 mg orally; 115 others were randomized to up to six cycles of pemetrexed and cisplatin. Median progression-free survival was 11.1 months in the afatinib group and 6.9 months in the chemotherapy group. There was no statistically significant difference in overall survival. The therascreen kit was validated in that trial.
The side effects of Gilotrif include diarrhea, which can lead to kidney failure and severe dehydration; liver toxicity; lung inflammation; and severe rashes. Less serious side effects include acnelike skin eruptions, dry skin, pruritus, mouth inflammation, paronychia, decreased appetite, decreased weight, cystitis, nosebleed, runny nose, fever, eye inflammation, and hypokalemia.
In a phase II Japanese study published online by the Journal of Clinical Oncology July 1, 50 mg per day of afatinib demonstrated "modest but noteworthy efficacy" in NSCLC patients who progressed after being on erlotinib or gefitinib, or both, for 12 or more weeks.
Of 62 treated patients, 45 (72.6%) were EGFR mutation positive in their primary tumor; 51 (82.3%) patients had developed resistance to erlotinib or gefitinib. Median progression-free survival was 4.4 months and median overall survival 19.0 months (J. Clin. Oncol. 2013 July 1 [doi: 10.1200/JCO.2012.45.0981]).
Tight glycemic control normalized thyroid function after pediatric heart surgery
SAN FRANCISCO – Tight glycemic control helps normalize thyroid hormone concentrations following cardiac surgery in children, according to results from a randomized trial presented at the Endocrine Society’s annual meeting.
That could be important because "it’s well known that cardiac surgical procedures can induce nonthyroidal illness in children" – a brief free-hormone surge followed by low T3 (triiodothyronine) and T4 (thyroxine) and increased rT3 (reverse triiodothyronine). It’s associated with longer recoveries, said lead investigator Dr. Carmen Soto-Rivera, an endocrinologist at Boston Children’s Hospital.
But it’s difficult to say what the clinical implications of her team’s findings are, she noted, because "we still don’t know if nonthyroidal illness is adaptive or maladaptive."
The study involved 191 children who underwent cardiac surgery with cardiopulmonary bypass. After surgery, her team randomized 99 children who were 0-36 months old to tight glycemic control, with a blood glucose target of 80-110 mg/dL; and 92 others to the standard treatment, permissive hyperglycemia; their median blood glucose hovered around 120 mg/dL.
Two days after surgery, 98% of the children had T4 and T3 levels below age-specific normal ranges, consistent with nonthyroidal illness. The thyroid hormone binding ratio (THBR) was normal in most of the patients, so it did not explain the drop in T4 and T3.
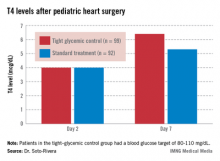
The groups had begun to separate by day 7. Tight-control patients had a higher median T4 than did patients on standard treatment (6.4 vs. 5.3 mcg/dL; P = .02) and a greater median T4 change from day 2 (2.4 vs. 1.3 mcg/dL, P = .02).
The trend continued among children still in the hospital on day 14. The 30 tight-control patients, compared with 37 on standard treatment, had a higher median T3 (104.5 vs. 74 ng/dL; P = .02) and a greater change from day 2 (26 vs. 5 ng/dL; P = .02). They had a higher T4 on day 14 too, but the difference was not significant.
"Interestingly, we found that [tight glucose control] resulted in lower [C-reactive protein] levels by day 7," about 3 mg/L, versus about 4 mg/L in the standard-treatment arm (P = .01). Perhaps "the faster increase in thyroid hormone levels may be mediated by decreased inhibition of deiodinases due to lowered inflammation," Dr. Soto-Rivera said.
The findings differ from previous reports of tight glucose control increasing peripheral inactivation of thyroid hormone, she said. "We believe that because our glucose targets were higher and we had [a low] rate of hypoglycemia in both arms, we potentially prevented a fasting response in these children," leading to decreased inhibition of the thyroid axis, she added.
"We believe that almost no one was treated with thyroid replacement during [the study], but we are in the process of confirming this," she said.
The project was a subgroup analysis of a larger trial that found no significant benefit for tight glycemic control on infection rates, mortality, length of stay, and organ failure following pediatric heart surgery (N. Engl. J. Med. 2012;367:1208-1219).
Dr. Soto-Rivera said she had no disclosures. The National Institutes of Health funded the project.
SAN FRANCISCO – Tight glycemic control helps normalize thyroid hormone concentrations following cardiac surgery in children, according to results from a randomized trial presented at the Endocrine Society’s annual meeting.
That could be important because "it’s well known that cardiac surgical procedures can induce nonthyroidal illness in children" – a brief free-hormone surge followed by low T3 (triiodothyronine) and T4 (thyroxine) and increased rT3 (reverse triiodothyronine). It’s associated with longer recoveries, said lead investigator Dr. Carmen Soto-Rivera, an endocrinologist at Boston Children’s Hospital.
But it’s difficult to say what the clinical implications of her team’s findings are, she noted, because "we still don’t know if nonthyroidal illness is adaptive or maladaptive."
The study involved 191 children who underwent cardiac surgery with cardiopulmonary bypass. After surgery, her team randomized 99 children who were 0-36 months old to tight glycemic control, with a blood glucose target of 80-110 mg/dL; and 92 others to the standard treatment, permissive hyperglycemia; their median blood glucose hovered around 120 mg/dL.
Two days after surgery, 98% of the children had T4 and T3 levels below age-specific normal ranges, consistent with nonthyroidal illness. The thyroid hormone binding ratio (THBR) was normal in most of the patients, so it did not explain the drop in T4 and T3.
The groups had begun to separate by day 7. Tight-control patients had a higher median T4 than did patients on standard treatment (6.4 vs. 5.3 mcg/dL; P = .02) and a greater median T4 change from day 2 (2.4 vs. 1.3 mcg/dL, P = .02).
The trend continued among children still in the hospital on day 14. The 30 tight-control patients, compared with 37 on standard treatment, had a higher median T3 (104.5 vs. 74 ng/dL; P = .02) and a greater change from day 2 (26 vs. 5 ng/dL; P = .02). They had a higher T4 on day 14 too, but the difference was not significant.
"Interestingly, we found that [tight glucose control] resulted in lower [C-reactive protein] levels by day 7," about 3 mg/L, versus about 4 mg/L in the standard-treatment arm (P = .01). Perhaps "the faster increase in thyroid hormone levels may be mediated by decreased inhibition of deiodinases due to lowered inflammation," Dr. Soto-Rivera said.
The findings differ from previous reports of tight glucose control increasing peripheral inactivation of thyroid hormone, she said. "We believe that because our glucose targets were higher and we had [a low] rate of hypoglycemia in both arms, we potentially prevented a fasting response in these children," leading to decreased inhibition of the thyroid axis, she added.
"We believe that almost no one was treated with thyroid replacement during [the study], but we are in the process of confirming this," she said.
The project was a subgroup analysis of a larger trial that found no significant benefit for tight glycemic control on infection rates, mortality, length of stay, and organ failure following pediatric heart surgery (N. Engl. J. Med. 2012;367:1208-1219).
Dr. Soto-Rivera said she had no disclosures. The National Institutes of Health funded the project.
SAN FRANCISCO – Tight glycemic control helps normalize thyroid hormone concentrations following cardiac surgery in children, according to results from a randomized trial presented at the Endocrine Society’s annual meeting.
That could be important because "it’s well known that cardiac surgical procedures can induce nonthyroidal illness in children" – a brief free-hormone surge followed by low T3 (triiodothyronine) and T4 (thyroxine) and increased rT3 (reverse triiodothyronine). It’s associated with longer recoveries, said lead investigator Dr. Carmen Soto-Rivera, an endocrinologist at Boston Children’s Hospital.
But it’s difficult to say what the clinical implications of her team’s findings are, she noted, because "we still don’t know if nonthyroidal illness is adaptive or maladaptive."
The study involved 191 children who underwent cardiac surgery with cardiopulmonary bypass. After surgery, her team randomized 99 children who were 0-36 months old to tight glycemic control, with a blood glucose target of 80-110 mg/dL; and 92 others to the standard treatment, permissive hyperglycemia; their median blood glucose hovered around 120 mg/dL.
Two days after surgery, 98% of the children had T4 and T3 levels below age-specific normal ranges, consistent with nonthyroidal illness. The thyroid hormone binding ratio (THBR) was normal in most of the patients, so it did not explain the drop in T4 and T3.
The groups had begun to separate by day 7. Tight-control patients had a higher median T4 than did patients on standard treatment (6.4 vs. 5.3 mcg/dL; P = .02) and a greater median T4 change from day 2 (2.4 vs. 1.3 mcg/dL, P = .02).
The trend continued among children still in the hospital on day 14. The 30 tight-control patients, compared with 37 on standard treatment, had a higher median T3 (104.5 vs. 74 ng/dL; P = .02) and a greater change from day 2 (26 vs. 5 ng/dL; P = .02). They had a higher T4 on day 14 too, but the difference was not significant.
"Interestingly, we found that [tight glucose control] resulted in lower [C-reactive protein] levels by day 7," about 3 mg/L, versus about 4 mg/L in the standard-treatment arm (P = .01). Perhaps "the faster increase in thyroid hormone levels may be mediated by decreased inhibition of deiodinases due to lowered inflammation," Dr. Soto-Rivera said.
The findings differ from previous reports of tight glucose control increasing peripheral inactivation of thyroid hormone, she said. "We believe that because our glucose targets were higher and we had [a low] rate of hypoglycemia in both arms, we potentially prevented a fasting response in these children," leading to decreased inhibition of the thyroid axis, she added.
"We believe that almost no one was treated with thyroid replacement during [the study], but we are in the process of confirming this," she said.
The project was a subgroup analysis of a larger trial that found no significant benefit for tight glycemic control on infection rates, mortality, length of stay, and organ failure following pediatric heart surgery (N. Engl. J. Med. 2012;367:1208-1219).
Dr. Soto-Rivera said she had no disclosures. The National Institutes of Health funded the project.
AT ENDO 2013
Major finding: Seven days after heart surgery, children on tight glycemic control had significantly higher median T4 levels than did children on standard treatment (6.4 vs. 5.3 mcg/dL).
Data source: Randomized trial of 191 children following heart surgery.
Disclosures: Dr. Soto-Rivera said she has no disclosures. The National Institutes of Health funded the project.
Testosterone gel's pain relief modest in opioid-induced androgen deficiency
SAN FRANCISCO – Men with opioid-induced androgen deficiency had a modest improvement in two laboratory measures of pain tolerance after 14 weeks of testosterone gel treatment but no significant improvements in subjective measures of pain perception, findings from a randomized trial showed.
Thirty-six men in the study received daily 5-g applications of 1% testosterone gel, while 29 others received a placebo gel. The patients were about 50 years old on average and had been taking opioids for at least a month, mostly for back pain.
There was no statistically significant difference in overall subjective Brief Pain Inventory scores at week 14, but there was a nonsignificant trend for less pain interference with daily life among treated men and a trend on the Short Form (SF)–36 for fewer emotional problems.
In the lab, testosterone subjects had about a 5-W improvement in their toleration of a blunted pressure pin applied to their forearms, from a baseline tolerance of 27 W. Men in the placebo group had a tolerance reduction of about 5 W, from a baseline score of 19 W. That difference, and a modest improvement in thumb-pressure tolerance in the treated group, were both significant. Other trends toward greater pain tolerance in the testosterone group were not.
"Objective pain perception showed improvement in the testosterone arm," said lead investigator Dr. Shehzad Basaria, director of Boston University’s androgen clinical research unit. But that improvement trend was not seen with subjective pain perception, he noted, and the magnitude of the change was "small."
Nonetheless, "when you start an analgesic drug, laboratory pain improves before a person says he is subjectively better," Dr. Basaria said at the Endocrine Society’s annual meeting. "We need to do a larger and longer-term study to see if [the lab findings] translate into improvements in subjective" perceptions.
For now, he noted, "I would initiate testosterone replacement only for [current] indications. I would not initiate it for pain control. If pain improves, that’s a bonus."
Testosterone did not affect opioid requirements; there was no significant difference in opioid use between the two groups at the end of the study. At baseline, patients had a mean total testosterone concentration of 228 ng/dL and a mean free testosterone concentration of 44 pg/mL; dosing was adjusted in the testosterone arm at 2 weeks to achieve total serum concentrations of 500-1,000 ng/dL.
Dr. Basaria said he had no relevant financial disclosures. The study was funded by AbbVie Pharmaceuticals, makers of the AndroGel testosterone gel used in the study.
SAN FRANCISCO – Men with opioid-induced androgen deficiency had a modest improvement in two laboratory measures of pain tolerance after 14 weeks of testosterone gel treatment but no significant improvements in subjective measures of pain perception, findings from a randomized trial showed.
Thirty-six men in the study received daily 5-g applications of 1% testosterone gel, while 29 others received a placebo gel. The patients were about 50 years old on average and had been taking opioids for at least a month, mostly for back pain.
There was no statistically significant difference in overall subjective Brief Pain Inventory scores at week 14, but there was a nonsignificant trend for less pain interference with daily life among treated men and a trend on the Short Form (SF)–36 for fewer emotional problems.
In the lab, testosterone subjects had about a 5-W improvement in their toleration of a blunted pressure pin applied to their forearms, from a baseline tolerance of 27 W. Men in the placebo group had a tolerance reduction of about 5 W, from a baseline score of 19 W. That difference, and a modest improvement in thumb-pressure tolerance in the treated group, were both significant. Other trends toward greater pain tolerance in the testosterone group were not.
"Objective pain perception showed improvement in the testosterone arm," said lead investigator Dr. Shehzad Basaria, director of Boston University’s androgen clinical research unit. But that improvement trend was not seen with subjective pain perception, he noted, and the magnitude of the change was "small."
Nonetheless, "when you start an analgesic drug, laboratory pain improves before a person says he is subjectively better," Dr. Basaria said at the Endocrine Society’s annual meeting. "We need to do a larger and longer-term study to see if [the lab findings] translate into improvements in subjective" perceptions.
For now, he noted, "I would initiate testosterone replacement only for [current] indications. I would not initiate it for pain control. If pain improves, that’s a bonus."
Testosterone did not affect opioid requirements; there was no significant difference in opioid use between the two groups at the end of the study. At baseline, patients had a mean total testosterone concentration of 228 ng/dL and a mean free testosterone concentration of 44 pg/mL; dosing was adjusted in the testosterone arm at 2 weeks to achieve total serum concentrations of 500-1,000 ng/dL.
Dr. Basaria said he had no relevant financial disclosures. The study was funded by AbbVie Pharmaceuticals, makers of the AndroGel testosterone gel used in the study.
SAN FRANCISCO – Men with opioid-induced androgen deficiency had a modest improvement in two laboratory measures of pain tolerance after 14 weeks of testosterone gel treatment but no significant improvements in subjective measures of pain perception, findings from a randomized trial showed.
Thirty-six men in the study received daily 5-g applications of 1% testosterone gel, while 29 others received a placebo gel. The patients were about 50 years old on average and had been taking opioids for at least a month, mostly for back pain.
There was no statistically significant difference in overall subjective Brief Pain Inventory scores at week 14, but there was a nonsignificant trend for less pain interference with daily life among treated men and a trend on the Short Form (SF)–36 for fewer emotional problems.
In the lab, testosterone subjects had about a 5-W improvement in their toleration of a blunted pressure pin applied to their forearms, from a baseline tolerance of 27 W. Men in the placebo group had a tolerance reduction of about 5 W, from a baseline score of 19 W. That difference, and a modest improvement in thumb-pressure tolerance in the treated group, were both significant. Other trends toward greater pain tolerance in the testosterone group were not.
"Objective pain perception showed improvement in the testosterone arm," said lead investigator Dr. Shehzad Basaria, director of Boston University’s androgen clinical research unit. But that improvement trend was not seen with subjective pain perception, he noted, and the magnitude of the change was "small."
Nonetheless, "when you start an analgesic drug, laboratory pain improves before a person says he is subjectively better," Dr. Basaria said at the Endocrine Society’s annual meeting. "We need to do a larger and longer-term study to see if [the lab findings] translate into improvements in subjective" perceptions.
For now, he noted, "I would initiate testosterone replacement only for [current] indications. I would not initiate it for pain control. If pain improves, that’s a bonus."
Testosterone did not affect opioid requirements; there was no significant difference in opioid use between the two groups at the end of the study. At baseline, patients had a mean total testosterone concentration of 228 ng/dL and a mean free testosterone concentration of 44 pg/mL; dosing was adjusted in the testosterone arm at 2 weeks to achieve total serum concentrations of 500-1,000 ng/dL.
Dr. Basaria said he had no relevant financial disclosures. The study was funded by AbbVie Pharmaceuticals, makers of the AndroGel testosterone gel used in the study.
AT ENDO 2013
Major finding: Men treated with testosterone gel for 14 weeks had about a 5-W improvement in their toleration of a blunted pressure pin applied to their forearms; men on placebo lost about 5 W of tolerance.
Data Source: A randomized, blinded controlled trial of testosterone gel in 65 men with opioid-induced androgen deficiency.
Disclosures: The lead investigator said he had no relevant financial disclosures. The study was funded by AbbVie Pharmaceuticals, makers of the AndroGel testosterone gel used in the study.
When prescribed by mail, vitamin D compliance is low
SAN FRANCISCO – Only one-quarter of patients who were informed by mail to take vitamin D because of insufficient levels found in a blood test met their target of at least 30 ng/mL 6 months later, according to a chart review.
This less-than-ideal compliance among 338 osteopenic patients in a University of Toronto osteoporosis program may be partly attributable to the fact that the importance of the treatment wasn’t adequately addressed while the patients were in the clinic.
After blood tests there, 265 patients (78%) were mailed a form letter telling them that they had insufficient vitamin D levels, defined as 20-29 ng/mL, and to take 4,000 IU/day for 3 months and then 2,000 IU/day thereafter.
Meanwhile, 73 (22%) received a letter telling them they were deficient, with levels of less than 20 ng/mL, and to take one 50,000 IU pill per week and 2,000 IU/day for 3 months, then switch to 2,000 IU/day.
"We were recommending fairly high doses of vitamin D, and that may have been intimidating," especially for a largely asymptomatic condition. "There was no chance really for them to discuss face-to-face with a clinician what their vitamin D status meant, and what treatment entailed," lead investigator Dr. Vithika Sivabalasundaram, an internal medicine resident at the university, said at the Endocrine Society’s annual meeting.
Perhaps not surprisingly, only half (169) of the patients followed through with repeat blood tests after 6 months, as instructed. Of those, just 56% (95) met the target of at least 30 ng/mL. The others (74) were largely unchanged or actually lost ground.
The lesson is probably that "we need to inform our patients of the benefits of vitamin D on bone health, and that there’s fairly low toxicity associated with taking vitamin D," Dr. Sivabalasundaram said.
To that end, when patients get their blood drawn in the clinic, they’re now "informed that they might be placed on this vitamin D protocol, so when they receive the letter, they are not surprised by it and they understand what it means. That wasn’t done before. We are [also] having a nurse phone [patients] to discuss their vitamin D status and answer their questions. We are developing an information pamphlet" as well, and plan to translate the letter into other languages, she said, adding that "right now, we are just sending [it] out in English," which may be a problem for the clinic’s multicultural population.
The only predictor of hitting the 30 ng/mL mark in the study was how soon after 6 months patients got their blood checked, which was probably a surrogate for how seriously they took the protocol, Dr. Sivabalasundaram said.
Baseline vitamin D status and use of glucocorticoids didn’t predict success, nor did past osteoporotic fractures. "We thought that would motivate patients, but we didn’t see that," she said.
Patients with malabsorption syndrome were less likely to hit the mark, as were obese patients, "which makes sense because vitamin D is sequestered into fat cells," she said.
Patients were about 60 years old on average, mostly women, and mostly white.
Dr. Sivabalasundaram said that she had no conflicts to disclose. One of her coinvestigators has been a speaker and advisory group member for several pharmaceutical companies.
SAN FRANCISCO – Only one-quarter of patients who were informed by mail to take vitamin D because of insufficient levels found in a blood test met their target of at least 30 ng/mL 6 months later, according to a chart review.
This less-than-ideal compliance among 338 osteopenic patients in a University of Toronto osteoporosis program may be partly attributable to the fact that the importance of the treatment wasn’t adequately addressed while the patients were in the clinic.
After blood tests there, 265 patients (78%) were mailed a form letter telling them that they had insufficient vitamin D levels, defined as 20-29 ng/mL, and to take 4,000 IU/day for 3 months and then 2,000 IU/day thereafter.
Meanwhile, 73 (22%) received a letter telling them they were deficient, with levels of less than 20 ng/mL, and to take one 50,000 IU pill per week and 2,000 IU/day for 3 months, then switch to 2,000 IU/day.
"We were recommending fairly high doses of vitamin D, and that may have been intimidating," especially for a largely asymptomatic condition. "There was no chance really for them to discuss face-to-face with a clinician what their vitamin D status meant, and what treatment entailed," lead investigator Dr. Vithika Sivabalasundaram, an internal medicine resident at the university, said at the Endocrine Society’s annual meeting.
Perhaps not surprisingly, only half (169) of the patients followed through with repeat blood tests after 6 months, as instructed. Of those, just 56% (95) met the target of at least 30 ng/mL. The others (74) were largely unchanged or actually lost ground.
The lesson is probably that "we need to inform our patients of the benefits of vitamin D on bone health, and that there’s fairly low toxicity associated with taking vitamin D," Dr. Sivabalasundaram said.
To that end, when patients get their blood drawn in the clinic, they’re now "informed that they might be placed on this vitamin D protocol, so when they receive the letter, they are not surprised by it and they understand what it means. That wasn’t done before. We are [also] having a nurse phone [patients] to discuss their vitamin D status and answer their questions. We are developing an information pamphlet" as well, and plan to translate the letter into other languages, she said, adding that "right now, we are just sending [it] out in English," which may be a problem for the clinic’s multicultural population.
The only predictor of hitting the 30 ng/mL mark in the study was how soon after 6 months patients got their blood checked, which was probably a surrogate for how seriously they took the protocol, Dr. Sivabalasundaram said.
Baseline vitamin D status and use of glucocorticoids didn’t predict success, nor did past osteoporotic fractures. "We thought that would motivate patients, but we didn’t see that," she said.
Patients with malabsorption syndrome were less likely to hit the mark, as were obese patients, "which makes sense because vitamin D is sequestered into fat cells," she said.
Patients were about 60 years old on average, mostly women, and mostly white.
Dr. Sivabalasundaram said that she had no conflicts to disclose. One of her coinvestigators has been a speaker and advisory group member for several pharmaceutical companies.
SAN FRANCISCO – Only one-quarter of patients who were informed by mail to take vitamin D because of insufficient levels found in a blood test met their target of at least 30 ng/mL 6 months later, according to a chart review.
This less-than-ideal compliance among 338 osteopenic patients in a University of Toronto osteoporosis program may be partly attributable to the fact that the importance of the treatment wasn’t adequately addressed while the patients were in the clinic.
After blood tests there, 265 patients (78%) were mailed a form letter telling them that they had insufficient vitamin D levels, defined as 20-29 ng/mL, and to take 4,000 IU/day for 3 months and then 2,000 IU/day thereafter.
Meanwhile, 73 (22%) received a letter telling them they were deficient, with levels of less than 20 ng/mL, and to take one 50,000 IU pill per week and 2,000 IU/day for 3 months, then switch to 2,000 IU/day.
"We were recommending fairly high doses of vitamin D, and that may have been intimidating," especially for a largely asymptomatic condition. "There was no chance really for them to discuss face-to-face with a clinician what their vitamin D status meant, and what treatment entailed," lead investigator Dr. Vithika Sivabalasundaram, an internal medicine resident at the university, said at the Endocrine Society’s annual meeting.
Perhaps not surprisingly, only half (169) of the patients followed through with repeat blood tests after 6 months, as instructed. Of those, just 56% (95) met the target of at least 30 ng/mL. The others (74) were largely unchanged or actually lost ground.
The lesson is probably that "we need to inform our patients of the benefits of vitamin D on bone health, and that there’s fairly low toxicity associated with taking vitamin D," Dr. Sivabalasundaram said.
To that end, when patients get their blood drawn in the clinic, they’re now "informed that they might be placed on this vitamin D protocol, so when they receive the letter, they are not surprised by it and they understand what it means. That wasn’t done before. We are [also] having a nurse phone [patients] to discuss their vitamin D status and answer their questions. We are developing an information pamphlet" as well, and plan to translate the letter into other languages, she said, adding that "right now, we are just sending [it] out in English," which may be a problem for the clinic’s multicultural population.
The only predictor of hitting the 30 ng/mL mark in the study was how soon after 6 months patients got their blood checked, which was probably a surrogate for how seriously they took the protocol, Dr. Sivabalasundaram said.
Baseline vitamin D status and use of glucocorticoids didn’t predict success, nor did past osteoporotic fractures. "We thought that would motivate patients, but we didn’t see that," she said.
Patients with malabsorption syndrome were less likely to hit the mark, as were obese patients, "which makes sense because vitamin D is sequestered into fat cells," she said.
Patients were about 60 years old on average, mostly women, and mostly white.
Dr. Sivabalasundaram said that she had no conflicts to disclose. One of her coinvestigators has been a speaker and advisory group member for several pharmaceutical companies.
AT ENDO 2013
Major finding: Roughly half of 338 osteopenic women completed a 6-month vitamin D protocol, and just over half of those who did hit the target of 30 ng/mL.
Data Source: Chart review.
Disclosures: Dr. Sivabalasundaram said that she had no disclosures. One of her coinvestigators has been a speaker and advisory group member for several pharmaceutical companies.
Anti-Müllerian hormone identified PCOS in small study
SAN FRANCISCO – An anti-Müllerian hormone level of 3.4 ng/mL or greater identified polycystic ovary syndrome in a study of 31 nonobese adolescents.
That cutoff was determined to best discriminate between PCOS and controls, with a positive predictive value of 75% and a negative predictive value of 61% in a study of 15 nonobese adolescents with PCOS aged 13-21 years, and 16 controls, reported Dr. Aviva Sopher at the Endocrine Society’s Annual Meeting.
The goal of the study wasn’t to define a definitive anti-Müllerian hormone (AMH) cutoff; that may come later as Dr. Sopher’s group and others continue to investigate the matter. Instead, the project was a preliminary proof-of-concept effort to gauge the utility of AMH in adolescent PCOS diagnosis.
For now, because there was "overlap in AMH values between PCOS and controls" and "a normal adolescent girl with polyfollicular ovaries and no other symptoms can have an AMH in the range that we think of [as signifying] PCOS, I wouldn’t use AMH on its own. I am suggesting the use of AMH in conjunction with clinical presentation and lab work," said Dr. Sopher, a pediatric endocrinologist at Columbia University in New York.
The hope, however, is that AMH will eventually replace the need for ultrasound; the transabdominal approach is "suboptimal" in adolescents, and transvaginal ultrasound is "overly invasive in this age group," she said.
The hormone is produced by growing follicles and is a marker of their number. Blood levels were assessed in the study by enzyme-linked immunosorbent assay (ELISA). PCOS was diagnosed by National Institutes of Health criteria (Fertil. Steril. 2010; 93:1938-41).
AMH was significantly higher in subjects with PCOS (4.4 ng/mL) than in controls (2.4 ng/mL), and correlated significantly with average ovarian size, the appearance of polycystic ovaries, free testosterone, and androstenedione.
The PCOS participants were 1.5-fold more likely to have an AMH level of more that 3.4 ng/mL than were the healthy controls, and that cutoff had a positive predictive value for PCOS of 75% and a negative predictive value of non-PCOS of 61%.
Mean ovarian size was similar in both groups (7.1 cc in subjects with PCOS versus 6.7 cc in controls), as were body mass index z-scores (0.45 vs. 0.19) and percent body fat (36.6% vs. 34.2%). The differences were not significant.
The subjects were at least 2 years post menarche. Exclusion criteria included premature birth; other potential causes of hirsutism and irregular menses; and birth control pill use within 3 months of enrollment. Normal-weight girls were selected "to exclude the confounding factor of obesity," Dr. Sopher said.
AMH is a useful adjunct to diagnose adolescent PCOS, and it has the potential to replace ultrasound as a marker of follicle count, she concluded.
The authors said they had nothing to disclose. The study was funded by the National Institutes of Health.
"I think AMH will diagnose women with an increased number of follicles, irrespective of their androgens. If in addition they have elevated androgen levels, they will be most probably have PCOS," said Dr. Rodolfo Rey.
But the value proposed in this study "is quite low. Many apparently normal girls have values over 3.4 ng/mL. The range of AMH is quite large" in teenagers and young women – perhaps up to 6-7 ng/mL. "This study needs more controls [to capture] the whole range of normal," he said.
Several investigators have proposed replacing ultrasound with AMH to diagnose PCOS in adult women, but "the problem is always the cutoff level. I haven’t seen studies that give cutoff values that could replace ultrasound. We don’t have enough data," he said.
Also, not all PCOS patients have polycystic ovaries. "AMH is reflecting the number of follicles rather than the levels of androgens." By relying on it too heavily, it’s possible to miss hyperandrogenism without polycystic ovaries.
Dr. Rey is a pediatric endocrinologist at Children’s Hospital in Buenos Aires, Argentina. He has received honoraria from Beckman Coulter for his work on AMH assays.
"I think AMH will diagnose women with an increased number of follicles, irrespective of their androgens. If in addition they have elevated androgen levels, they will be most probably have PCOS," said Dr. Rodolfo Rey.
But the value proposed in this study "is quite low. Many apparently normal girls have values over 3.4 ng/mL. The range of AMH is quite large" in teenagers and young women – perhaps up to 6-7 ng/mL. "This study needs more controls [to capture] the whole range of normal," he said.
Several investigators have proposed replacing ultrasound with AMH to diagnose PCOS in adult women, but "the problem is always the cutoff level. I haven’t seen studies that give cutoff values that could replace ultrasound. We don’t have enough data," he said.
Also, not all PCOS patients have polycystic ovaries. "AMH is reflecting the number of follicles rather than the levels of androgens." By relying on it too heavily, it’s possible to miss hyperandrogenism without polycystic ovaries.
Dr. Rey is a pediatric endocrinologist at Children’s Hospital in Buenos Aires, Argentina. He has received honoraria from Beckman Coulter for his work on AMH assays.
"I think AMH will diagnose women with an increased number of follicles, irrespective of their androgens. If in addition they have elevated androgen levels, they will be most probably have PCOS," said Dr. Rodolfo Rey.
But the value proposed in this study "is quite low. Many apparently normal girls have values over 3.4 ng/mL. The range of AMH is quite large" in teenagers and young women – perhaps up to 6-7 ng/mL. "This study needs more controls [to capture] the whole range of normal," he said.
Several investigators have proposed replacing ultrasound with AMH to diagnose PCOS in adult women, but "the problem is always the cutoff level. I haven’t seen studies that give cutoff values that could replace ultrasound. We don’t have enough data," he said.
Also, not all PCOS patients have polycystic ovaries. "AMH is reflecting the number of follicles rather than the levels of androgens." By relying on it too heavily, it’s possible to miss hyperandrogenism without polycystic ovaries.
Dr. Rey is a pediatric endocrinologist at Children’s Hospital in Buenos Aires, Argentina. He has received honoraria from Beckman Coulter for his work on AMH assays.
SAN FRANCISCO – An anti-Müllerian hormone level of 3.4 ng/mL or greater identified polycystic ovary syndrome in a study of 31 nonobese adolescents.
That cutoff was determined to best discriminate between PCOS and controls, with a positive predictive value of 75% and a negative predictive value of 61% in a study of 15 nonobese adolescents with PCOS aged 13-21 years, and 16 controls, reported Dr. Aviva Sopher at the Endocrine Society’s Annual Meeting.
The goal of the study wasn’t to define a definitive anti-Müllerian hormone (AMH) cutoff; that may come later as Dr. Sopher’s group and others continue to investigate the matter. Instead, the project was a preliminary proof-of-concept effort to gauge the utility of AMH in adolescent PCOS diagnosis.
For now, because there was "overlap in AMH values between PCOS and controls" and "a normal adolescent girl with polyfollicular ovaries and no other symptoms can have an AMH in the range that we think of [as signifying] PCOS, I wouldn’t use AMH on its own. I am suggesting the use of AMH in conjunction with clinical presentation and lab work," said Dr. Sopher, a pediatric endocrinologist at Columbia University in New York.
The hope, however, is that AMH will eventually replace the need for ultrasound; the transabdominal approach is "suboptimal" in adolescents, and transvaginal ultrasound is "overly invasive in this age group," she said.
The hormone is produced by growing follicles and is a marker of their number. Blood levels were assessed in the study by enzyme-linked immunosorbent assay (ELISA). PCOS was diagnosed by National Institutes of Health criteria (Fertil. Steril. 2010; 93:1938-41).
AMH was significantly higher in subjects with PCOS (4.4 ng/mL) than in controls (2.4 ng/mL), and correlated significantly with average ovarian size, the appearance of polycystic ovaries, free testosterone, and androstenedione.
The PCOS participants were 1.5-fold more likely to have an AMH level of more that 3.4 ng/mL than were the healthy controls, and that cutoff had a positive predictive value for PCOS of 75% and a negative predictive value of non-PCOS of 61%.
Mean ovarian size was similar in both groups (7.1 cc in subjects with PCOS versus 6.7 cc in controls), as were body mass index z-scores (0.45 vs. 0.19) and percent body fat (36.6% vs. 34.2%). The differences were not significant.
The subjects were at least 2 years post menarche. Exclusion criteria included premature birth; other potential causes of hirsutism and irregular menses; and birth control pill use within 3 months of enrollment. Normal-weight girls were selected "to exclude the confounding factor of obesity," Dr. Sopher said.
AMH is a useful adjunct to diagnose adolescent PCOS, and it has the potential to replace ultrasound as a marker of follicle count, she concluded.
The authors said they had nothing to disclose. The study was funded by the National Institutes of Health.
SAN FRANCISCO – An anti-Müllerian hormone level of 3.4 ng/mL or greater identified polycystic ovary syndrome in a study of 31 nonobese adolescents.
That cutoff was determined to best discriminate between PCOS and controls, with a positive predictive value of 75% and a negative predictive value of 61% in a study of 15 nonobese adolescents with PCOS aged 13-21 years, and 16 controls, reported Dr. Aviva Sopher at the Endocrine Society’s Annual Meeting.
The goal of the study wasn’t to define a definitive anti-Müllerian hormone (AMH) cutoff; that may come later as Dr. Sopher’s group and others continue to investigate the matter. Instead, the project was a preliminary proof-of-concept effort to gauge the utility of AMH in adolescent PCOS diagnosis.
For now, because there was "overlap in AMH values between PCOS and controls" and "a normal adolescent girl with polyfollicular ovaries and no other symptoms can have an AMH in the range that we think of [as signifying] PCOS, I wouldn’t use AMH on its own. I am suggesting the use of AMH in conjunction with clinical presentation and lab work," said Dr. Sopher, a pediatric endocrinologist at Columbia University in New York.
The hope, however, is that AMH will eventually replace the need for ultrasound; the transabdominal approach is "suboptimal" in adolescents, and transvaginal ultrasound is "overly invasive in this age group," she said.
The hormone is produced by growing follicles and is a marker of their number. Blood levels were assessed in the study by enzyme-linked immunosorbent assay (ELISA). PCOS was diagnosed by National Institutes of Health criteria (Fertil. Steril. 2010; 93:1938-41).
AMH was significantly higher in subjects with PCOS (4.4 ng/mL) than in controls (2.4 ng/mL), and correlated significantly with average ovarian size, the appearance of polycystic ovaries, free testosterone, and androstenedione.
The PCOS participants were 1.5-fold more likely to have an AMH level of more that 3.4 ng/mL than were the healthy controls, and that cutoff had a positive predictive value for PCOS of 75% and a negative predictive value of non-PCOS of 61%.
Mean ovarian size was similar in both groups (7.1 cc in subjects with PCOS versus 6.7 cc in controls), as were body mass index z-scores (0.45 vs. 0.19) and percent body fat (36.6% vs. 34.2%). The differences were not significant.
The subjects were at least 2 years post menarche. Exclusion criteria included premature birth; other potential causes of hirsutism and irregular menses; and birth control pill use within 3 months of enrollment. Normal-weight girls were selected "to exclude the confounding factor of obesity," Dr. Sopher said.
AMH is a useful adjunct to diagnose adolescent PCOS, and it has the potential to replace ultrasound as a marker of follicle count, she concluded.
The authors said they had nothing to disclose. The study was funded by the National Institutes of Health.
AT ENDO 2013
Major finding: An anti-Müllerian hormone cutoff level of 3.4 ng/mL discriminated between PCOS patients and controls.
Data source: Prospective, case-control trial with 31 subjects.
Disclosures: The investigators said they had nothing to disclose. The study was funded by the National Institutes of Health.