User login
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.
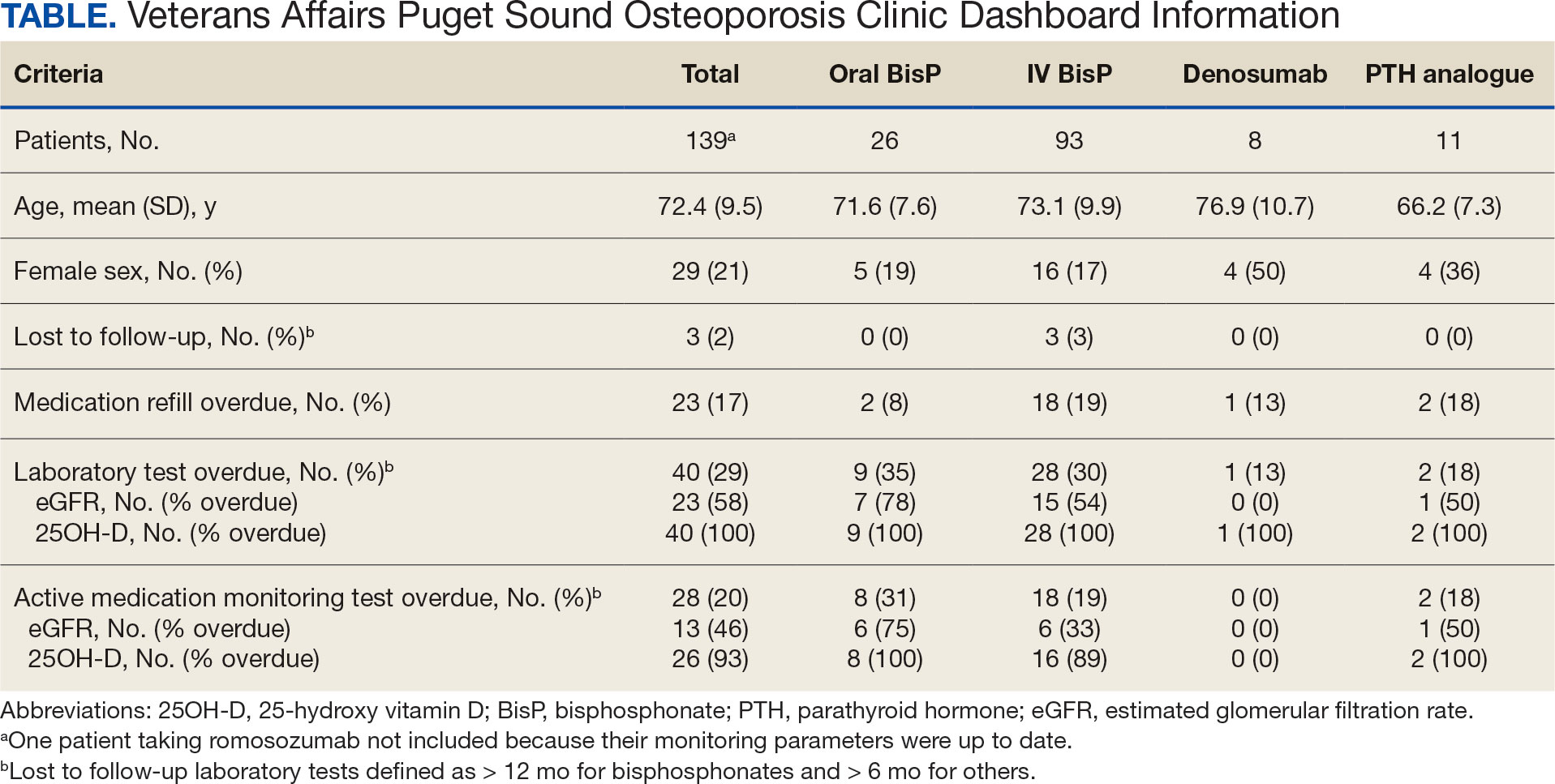
The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Using AI to ID Osteoporosis: A Medico-Legal Minefield?
Could an artificial intelligence (AI)–driven tool that mines medical records for suspected cases of osteoporosis be so successful that it becomes a potential liability? Yes, according to Christopher White, PhD, executive director of Maridulu Budyari Gumal, the Sydney Partnership for Health, Education, Research, and Enterprise, a research translation center in Liverpool, Australia.
In a thought-provoking presentation at the Endocrine Society’s AI in Healthcare Virtual Summit, White described the results after his fracture liaison team at Prince of Wales Hospital in Randwick, Australia, tried to plug the “osteoporosis treatment gap” by mining medical records to identify patients with the disorder.
‘Be Careful What You Wish For’
White and colleagues developed a robust standalone database over 20 years that informed fracture risk among patients with osteoporosis in Sydney. The database included all relevant clinical information, as well as bone density measurements, on about 30,000 patients and could be interrogated for randomized controlled trial recruitment.
However, a “crisis” occurred around 2011, when the team received a recruitment request for the first head-to-head comparison of alendronate with romosozumab. “We had numerous postmenopausal women in the age range with the required bone density, but we hadn’t captured the severity of their vertebral fracture or how many they actually had,” White told the this news organization. For recruitment into the study, participants must have had at least two moderate or severe vertebral fractures or a proximal vertebral fracture that was sustained between 3 and 24 months before recruitment.
White turned to his hospital’s mainframe, which had coding data and time intervals for patients who were admitted with vertebral or hip fractures. He calculated how many patients who met the study criteria had been discharged and how many of those he thought he’d be able to capture through the mainframe. He was confident he would have enough, but he was wrong. He underrecruited and could not participate in the trial.
Determined not to wind up in a similar situation in the future, he investigated and found that other centers were struggling with similar problems. This led to a collaboration with four investigators who were using AI and Advanced Encryption Standard (AES) coding to identify patients at risk for osteoporotic fractures. White, meanwhile, had developed a natural language processing tool called XRAIT that also identified patients at fracture risk. A study comparing the two electronic search programs, which screen medical records for fractures, found that both reliably identified patients who had had a fracture. White and his colleagues concluded that hybrid tools combining XRAIT and AES would likely improve the identification of patients with osteoporosis who would require follow-up or might participate in future trials.
Those patients were not being identified sooner for multiple reasons, White explained. Sometimes, the radiologist would report osteoporosis, but it wouldn’t get coded. Or, in the emergency department, a patient with a fracture would be treated and then sent home, and the possibility of osteoporosis wasn’t reported.
“As we went deeper and deeper with our tools into the medical record, we found more and more patients who hadn’t been coded or reported but who actually had osteoporosis,” White said. “It was incredibly prevalent.”
But the number of patients identified was more than the hospital could comfortably handle.
Ironically, he added, “To my relief and probably not to the benefit of the patients, there was a system upgrade of the radiology reporting system, which was incompatible with the natural language processing technology that I had installed. The AI was turned off at that point, but I had a look over the edge and into the mine pit.”
“The lesson learned,” White told this news organization, is “If you mine the medical record for unidentified patients before you know what to do with the output, you create a medico-legal minefield. You need to be careful what you wish for with technology, because it may actually come true.”
Grappling With the Treatment Gap
An (over)abundance of patients is likely contributing to the “osteoporosis treatment gap” that Australia’s fracture liaison services, which handle many of these patients, are grappling with. One recent meta-analysis showed that not all eligible patients are treated and that not all patients who are treated actually start treatment. Another study showed that only a minority of patients — anywhere between 20% and 40% — who start are still persisting at about 3 years, White said.
Various types of fracture liaison services exist, he noted. The model that has been shown to best promote adherence is the one requiring clinicians to “identify, educate [usually, the primary care physician], evaluate, start treatment, continue treatment, and follow-up at 12 months for to confirm that there is adherence.”
What’s happening now, he said, is that the technology is identifying a high number of vertebral crush fractures, and there’s no education or evaluation. “The radiologist just refers the patient to a primary care physician and hopes for the best. AI isn’t contributing to solving the treatment gap problem; it’s amplifying it. It’s ahead of the ability of organizations to accommodate the findings.”
Solutions, he said, would require support at the top of health systems and organizations, and funding to proceed; data surveys concentrating on vertical integration of the medical record to follow patients wherever they are — eg, hospital, primary care — in their health journeys; a workflow with synchronous diagnosis and treatment planning, delivery, monitoring, and payment; and clinical and community champions advocating and “leading the charge in health tech.”
Furthermore, he advised, organizations need to be “very, very careful with safety and security — that is, managing the digital risks.”
“Oscar Wilde said there are two tragedies in life: One is not getting what one wants, and the other is getting it,” White concluded. “In my career, we’ve moved on from not knowing how to treat osteoporosis to knowing how to treat it. And that is both an asset and a liability.”
A version of this article first appeared on Medscape.com.
Could an artificial intelligence (AI)–driven tool that mines medical records for suspected cases of osteoporosis be so successful that it becomes a potential liability? Yes, according to Christopher White, PhD, executive director of Maridulu Budyari Gumal, the Sydney Partnership for Health, Education, Research, and Enterprise, a research translation center in Liverpool, Australia.
In a thought-provoking presentation at the Endocrine Society’s AI in Healthcare Virtual Summit, White described the results after his fracture liaison team at Prince of Wales Hospital in Randwick, Australia, tried to plug the “osteoporosis treatment gap” by mining medical records to identify patients with the disorder.
‘Be Careful What You Wish For’
White and colleagues developed a robust standalone database over 20 years that informed fracture risk among patients with osteoporosis in Sydney. The database included all relevant clinical information, as well as bone density measurements, on about 30,000 patients and could be interrogated for randomized controlled trial recruitment.
However, a “crisis” occurred around 2011, when the team received a recruitment request for the first head-to-head comparison of alendronate with romosozumab. “We had numerous postmenopausal women in the age range with the required bone density, but we hadn’t captured the severity of their vertebral fracture or how many they actually had,” White told the this news organization. For recruitment into the study, participants must have had at least two moderate or severe vertebral fractures or a proximal vertebral fracture that was sustained between 3 and 24 months before recruitment.
White turned to his hospital’s mainframe, which had coding data and time intervals for patients who were admitted with vertebral or hip fractures. He calculated how many patients who met the study criteria had been discharged and how many of those he thought he’d be able to capture through the mainframe. He was confident he would have enough, but he was wrong. He underrecruited and could not participate in the trial.
Determined not to wind up in a similar situation in the future, he investigated and found that other centers were struggling with similar problems. This led to a collaboration with four investigators who were using AI and Advanced Encryption Standard (AES) coding to identify patients at risk for osteoporotic fractures. White, meanwhile, had developed a natural language processing tool called XRAIT that also identified patients at fracture risk. A study comparing the two electronic search programs, which screen medical records for fractures, found that both reliably identified patients who had had a fracture. White and his colleagues concluded that hybrid tools combining XRAIT and AES would likely improve the identification of patients with osteoporosis who would require follow-up or might participate in future trials.
Those patients were not being identified sooner for multiple reasons, White explained. Sometimes, the radiologist would report osteoporosis, but it wouldn’t get coded. Or, in the emergency department, a patient with a fracture would be treated and then sent home, and the possibility of osteoporosis wasn’t reported.
“As we went deeper and deeper with our tools into the medical record, we found more and more patients who hadn’t been coded or reported but who actually had osteoporosis,” White said. “It was incredibly prevalent.”
But the number of patients identified was more than the hospital could comfortably handle.
Ironically, he added, “To my relief and probably not to the benefit of the patients, there was a system upgrade of the radiology reporting system, which was incompatible with the natural language processing technology that I had installed. The AI was turned off at that point, but I had a look over the edge and into the mine pit.”
“The lesson learned,” White told this news organization, is “If you mine the medical record for unidentified patients before you know what to do with the output, you create a medico-legal minefield. You need to be careful what you wish for with technology, because it may actually come true.”
Grappling With the Treatment Gap
An (over)abundance of patients is likely contributing to the “osteoporosis treatment gap” that Australia’s fracture liaison services, which handle many of these patients, are grappling with. One recent meta-analysis showed that not all eligible patients are treated and that not all patients who are treated actually start treatment. Another study showed that only a minority of patients — anywhere between 20% and 40% — who start are still persisting at about 3 years, White said.
Various types of fracture liaison services exist, he noted. The model that has been shown to best promote adherence is the one requiring clinicians to “identify, educate [usually, the primary care physician], evaluate, start treatment, continue treatment, and follow-up at 12 months for to confirm that there is adherence.”
What’s happening now, he said, is that the technology is identifying a high number of vertebral crush fractures, and there’s no education or evaluation. “The radiologist just refers the patient to a primary care physician and hopes for the best. AI isn’t contributing to solving the treatment gap problem; it’s amplifying it. It’s ahead of the ability of organizations to accommodate the findings.”
Solutions, he said, would require support at the top of health systems and organizations, and funding to proceed; data surveys concentrating on vertical integration of the medical record to follow patients wherever they are — eg, hospital, primary care — in their health journeys; a workflow with synchronous diagnosis and treatment planning, delivery, monitoring, and payment; and clinical and community champions advocating and “leading the charge in health tech.”
Furthermore, he advised, organizations need to be “very, very careful with safety and security — that is, managing the digital risks.”
“Oscar Wilde said there are two tragedies in life: One is not getting what one wants, and the other is getting it,” White concluded. “In my career, we’ve moved on from not knowing how to treat osteoporosis to knowing how to treat it. And that is both an asset and a liability.”
A version of this article first appeared on Medscape.com.
Could an artificial intelligence (AI)–driven tool that mines medical records for suspected cases of osteoporosis be so successful that it becomes a potential liability? Yes, according to Christopher White, PhD, executive director of Maridulu Budyari Gumal, the Sydney Partnership for Health, Education, Research, and Enterprise, a research translation center in Liverpool, Australia.
In a thought-provoking presentation at the Endocrine Society’s AI in Healthcare Virtual Summit, White described the results after his fracture liaison team at Prince of Wales Hospital in Randwick, Australia, tried to plug the “osteoporosis treatment gap” by mining medical records to identify patients with the disorder.
‘Be Careful What You Wish For’
White and colleagues developed a robust standalone database over 20 years that informed fracture risk among patients with osteoporosis in Sydney. The database included all relevant clinical information, as well as bone density measurements, on about 30,000 patients and could be interrogated for randomized controlled trial recruitment.
However, a “crisis” occurred around 2011, when the team received a recruitment request for the first head-to-head comparison of alendronate with romosozumab. “We had numerous postmenopausal women in the age range with the required bone density, but we hadn’t captured the severity of their vertebral fracture or how many they actually had,” White told the this news organization. For recruitment into the study, participants must have had at least two moderate or severe vertebral fractures or a proximal vertebral fracture that was sustained between 3 and 24 months before recruitment.
White turned to his hospital’s mainframe, which had coding data and time intervals for patients who were admitted with vertebral or hip fractures. He calculated how many patients who met the study criteria had been discharged and how many of those he thought he’d be able to capture through the mainframe. He was confident he would have enough, but he was wrong. He underrecruited and could not participate in the trial.
Determined not to wind up in a similar situation in the future, he investigated and found that other centers were struggling with similar problems. This led to a collaboration with four investigators who were using AI and Advanced Encryption Standard (AES) coding to identify patients at risk for osteoporotic fractures. White, meanwhile, had developed a natural language processing tool called XRAIT that also identified patients at fracture risk. A study comparing the two electronic search programs, which screen medical records for fractures, found that both reliably identified patients who had had a fracture. White and his colleagues concluded that hybrid tools combining XRAIT and AES would likely improve the identification of patients with osteoporosis who would require follow-up or might participate in future trials.
Those patients were not being identified sooner for multiple reasons, White explained. Sometimes, the radiologist would report osteoporosis, but it wouldn’t get coded. Or, in the emergency department, a patient with a fracture would be treated and then sent home, and the possibility of osteoporosis wasn’t reported.
“As we went deeper and deeper with our tools into the medical record, we found more and more patients who hadn’t been coded or reported but who actually had osteoporosis,” White said. “It was incredibly prevalent.”
But the number of patients identified was more than the hospital could comfortably handle.
Ironically, he added, “To my relief and probably not to the benefit of the patients, there was a system upgrade of the radiology reporting system, which was incompatible with the natural language processing technology that I had installed. The AI was turned off at that point, but I had a look over the edge and into the mine pit.”
“The lesson learned,” White told this news organization, is “If you mine the medical record for unidentified patients before you know what to do with the output, you create a medico-legal minefield. You need to be careful what you wish for with technology, because it may actually come true.”
Grappling With the Treatment Gap
An (over)abundance of patients is likely contributing to the “osteoporosis treatment gap” that Australia’s fracture liaison services, which handle many of these patients, are grappling with. One recent meta-analysis showed that not all eligible patients are treated and that not all patients who are treated actually start treatment. Another study showed that only a minority of patients — anywhere between 20% and 40% — who start are still persisting at about 3 years, White said.
Various types of fracture liaison services exist, he noted. The model that has been shown to best promote adherence is the one requiring clinicians to “identify, educate [usually, the primary care physician], evaluate, start treatment, continue treatment, and follow-up at 12 months for to confirm that there is adherence.”
What’s happening now, he said, is that the technology is identifying a high number of vertebral crush fractures, and there’s no education or evaluation. “The radiologist just refers the patient to a primary care physician and hopes for the best. AI isn’t contributing to solving the treatment gap problem; it’s amplifying it. It’s ahead of the ability of organizations to accommodate the findings.”
Solutions, he said, would require support at the top of health systems and organizations, and funding to proceed; data surveys concentrating on vertical integration of the medical record to follow patients wherever they are — eg, hospital, primary care — in their health journeys; a workflow with synchronous diagnosis and treatment planning, delivery, monitoring, and payment; and clinical and community champions advocating and “leading the charge in health tech.”
Furthermore, he advised, organizations need to be “very, very careful with safety and security — that is, managing the digital risks.”
“Oscar Wilde said there are two tragedies in life: One is not getting what one wants, and the other is getting it,” White concluded. “In my career, we’ve moved on from not knowing how to treat osteoporosis to knowing how to treat it. And that is both an asset and a liability.”
A version of this article first appeared on Medscape.com.
Is Vitamin E Beneficial for Bone Health?
Vitamin E may be best known for boosting skin and eye health as well as immune function. In recent years, researchers have explored the potential benefits of vitamin E on bone loss, especially in women with menopause-related osteoporosis. While data are beginning to roll in from these studies, evidence supporting a positive impact of vitamin E on osteoporosis and hip fracture risk in perimenopausal women remains elusive.
For osteoporosis, the rationale for using vitamin E is based on its antioxidant activity, which can scavenge potentially damaging free radicals. Researchers have asked whether vitamin E can help maintain the integrity of bone matrix and stimulate bone formation while minimizing bone resorption, particularly in trabecular (spongy) bone, the bone compartment preferentially affected in perimenopausal bone loss.
Vitamin E mostly consists of two isomers: alpha-tocopherol and gamma-tocopherol. Alpha-tocopherol has higher antioxidant activity and is found in nuts, seeds, vegetable oils, green leafy vegetables, fortified cereals, and vitamin E supplements. Gamma-tocopherol is known for its superior anti-inflammatory properties and accounts for about 70% of the total vitamin E intake in a typical American diet, largely sourced from soybean and other vegetable oils.
Benefits and Risks in Bone Loss Studies
Perimenopausal bone loss is caused, to a great extent, by the decrease in sex hormones. Studies of vitamin E in ovariectomized rats have yielded mixed results. This animal model lacks sex hormones and has similar bone changes to those of postmenopausal women. Some animal studies have suggested a positive effect of vitamin E on bone while others have reported no effect.
Studies in humans also have produced conflicting reports of positive, neutral, and negative associations of vitamin E with bone health. For example, the Women’s Health Initiative examined the relationship between vitamin and mineral antioxidants and bone health in postmenopausal women and found no significant association between antioxidants and bone mineral density.
Another study examining data from children and adolescents enrolled in the National Health and Nutrition Examination Survey (NHANES) database found an inverse association between alpha-tocopherol and lumbar spine bone density, suggesting a deleterious effect on bone. Inverse associations also have been reported in certain studies of postmenopausal women.
High doses of alpha-tocopherol have been linked to a risk for impaired bone health through a variety of mechanisms, such as interference with vitamin K metabolism; competitive binding for alpha-tocopherol transfer protein, inhibiting the entry of beneficial vitamin E isomers, including gamma-tocopherol; and pro-oxidant effects that harm bone. Thus, postmenopausal women taking vitamin E supplements primarily as high doses of alpha-tocopherol might be hindering their bone health.
Data for gamma-tocopherol are more promising. Some studies hypothesize that gamma-tocopherol might uncouple bone turnover, leading to increased bone formation without affecting bone resorption. Further, a randomized controlled study of mixed tocopherols (rather than alpha-tocopherol) vs placebo reported a protective effect of this preparation on bone outcomes by suppressing bone resorption. This raises the importance of considering the specific forms of vitamin E when evaluating its role in bone health.
Limitations of Current Studies
Researchers acknowledge several limitations in studies to date. For example, there are very few randomized controlled trials assessing the impact of vitamin E on bone health. Most studies are cross-sectional or observational, even when longitudinal. Cross-sectional and observational designs prevent us from establishing a causal relationship between vitamin E and bone endpoints.
Such designs also run the risk of additional confounders that may affect associations between vitamin E and bone, or the lack thereof. These could include both known and unknown confounders. Of note, gamma-tocopherol intake data were not available for certain NHANES studies.
Further, people often consume multiple nutrients and supplements, complicating the identification of specific nutrient-disease associations. Most human studies estimate tocopherol intake by dietary questionnaires or measure serum tocopherol levels, which reflect short-term dietary intake, while bone mineral density is probably influenced by long-term dietary patterns.
Too Soon to Prescribe Vitamin E for Bone Health
Some nutrition experts advocate for vitamin E supplements containing mixed tocopherols, specifically suggesting a ratio of 50-100 IU of gamma-tocopherol per 400 IU of D-alpha-tocopherol. Additional research is essential to confirm and further clarify the role of gamma-tocopherol in bone formation and resorption. In fact, it is also important to explore the influence of other compounds in the vitamin E family on skeletal health.
Until more data are available, we would recommend following the Institute of Medicine’s guidelines for the recommended daily allowance (RDA) of vitamin E. This is age dependent, ranging from 4 to 11 mg/d between the ages of 0 and 13 years, and 15 mg/d thereafter.
Overall, evidence of vitamin E’s impact on osteoporosis and hip fracture risk in perimenopausal women remains inconclusive. Although some observational and interventional studies suggest potential benefits, more interventional studies, particularly randomized controlled trials, are necessary to explore the risks and benefits of vitamin E supplementation and serum vitamin E levels on bone density and fracture risk more thoroughly.
Dr. Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, both in Charlottesville, has disclosed no relevant financial relationships. Dr. Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia, and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma.
A version of this article appeared on Medscape.com.
Vitamin E may be best known for boosting skin and eye health as well as immune function. In recent years, researchers have explored the potential benefits of vitamin E on bone loss, especially in women with menopause-related osteoporosis. While data are beginning to roll in from these studies, evidence supporting a positive impact of vitamin E on osteoporosis and hip fracture risk in perimenopausal women remains elusive.
For osteoporosis, the rationale for using vitamin E is based on its antioxidant activity, which can scavenge potentially damaging free radicals. Researchers have asked whether vitamin E can help maintain the integrity of bone matrix and stimulate bone formation while minimizing bone resorption, particularly in trabecular (spongy) bone, the bone compartment preferentially affected in perimenopausal bone loss.
Vitamin E mostly consists of two isomers: alpha-tocopherol and gamma-tocopherol. Alpha-tocopherol has higher antioxidant activity and is found in nuts, seeds, vegetable oils, green leafy vegetables, fortified cereals, and vitamin E supplements. Gamma-tocopherol is known for its superior anti-inflammatory properties and accounts for about 70% of the total vitamin E intake in a typical American diet, largely sourced from soybean and other vegetable oils.
Benefits and Risks in Bone Loss Studies
Perimenopausal bone loss is caused, to a great extent, by the decrease in sex hormones. Studies of vitamin E in ovariectomized rats have yielded mixed results. This animal model lacks sex hormones and has similar bone changes to those of postmenopausal women. Some animal studies have suggested a positive effect of vitamin E on bone while others have reported no effect.
Studies in humans also have produced conflicting reports of positive, neutral, and negative associations of vitamin E with bone health. For example, the Women’s Health Initiative examined the relationship between vitamin and mineral antioxidants and bone health in postmenopausal women and found no significant association between antioxidants and bone mineral density.
Another study examining data from children and adolescents enrolled in the National Health and Nutrition Examination Survey (NHANES) database found an inverse association between alpha-tocopherol and lumbar spine bone density, suggesting a deleterious effect on bone. Inverse associations also have been reported in certain studies of postmenopausal women.
High doses of alpha-tocopherol have been linked to a risk for impaired bone health through a variety of mechanisms, such as interference with vitamin K metabolism; competitive binding for alpha-tocopherol transfer protein, inhibiting the entry of beneficial vitamin E isomers, including gamma-tocopherol; and pro-oxidant effects that harm bone. Thus, postmenopausal women taking vitamin E supplements primarily as high doses of alpha-tocopherol might be hindering their bone health.
Data for gamma-tocopherol are more promising. Some studies hypothesize that gamma-tocopherol might uncouple bone turnover, leading to increased bone formation without affecting bone resorption. Further, a randomized controlled study of mixed tocopherols (rather than alpha-tocopherol) vs placebo reported a protective effect of this preparation on bone outcomes by suppressing bone resorption. This raises the importance of considering the specific forms of vitamin E when evaluating its role in bone health.
Limitations of Current Studies
Researchers acknowledge several limitations in studies to date. For example, there are very few randomized controlled trials assessing the impact of vitamin E on bone health. Most studies are cross-sectional or observational, even when longitudinal. Cross-sectional and observational designs prevent us from establishing a causal relationship between vitamin E and bone endpoints.
Such designs also run the risk of additional confounders that may affect associations between vitamin E and bone, or the lack thereof. These could include both known and unknown confounders. Of note, gamma-tocopherol intake data were not available for certain NHANES studies.
Further, people often consume multiple nutrients and supplements, complicating the identification of specific nutrient-disease associations. Most human studies estimate tocopherol intake by dietary questionnaires or measure serum tocopherol levels, which reflect short-term dietary intake, while bone mineral density is probably influenced by long-term dietary patterns.
Too Soon to Prescribe Vitamin E for Bone Health
Some nutrition experts advocate for vitamin E supplements containing mixed tocopherols, specifically suggesting a ratio of 50-100 IU of gamma-tocopherol per 400 IU of D-alpha-tocopherol. Additional research is essential to confirm and further clarify the role of gamma-tocopherol in bone formation and resorption. In fact, it is also important to explore the influence of other compounds in the vitamin E family on skeletal health.
Until more data are available, we would recommend following the Institute of Medicine’s guidelines for the recommended daily allowance (RDA) of vitamin E. This is age dependent, ranging from 4 to 11 mg/d between the ages of 0 and 13 years, and 15 mg/d thereafter.
Overall, evidence of vitamin E’s impact on osteoporosis and hip fracture risk in perimenopausal women remains inconclusive. Although some observational and interventional studies suggest potential benefits, more interventional studies, particularly randomized controlled trials, are necessary to explore the risks and benefits of vitamin E supplementation and serum vitamin E levels on bone density and fracture risk more thoroughly.
Dr. Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, both in Charlottesville, has disclosed no relevant financial relationships. Dr. Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia, and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma.
A version of this article appeared on Medscape.com.
Vitamin E may be best known for boosting skin and eye health as well as immune function. In recent years, researchers have explored the potential benefits of vitamin E on bone loss, especially in women with menopause-related osteoporosis. While data are beginning to roll in from these studies, evidence supporting a positive impact of vitamin E on osteoporosis and hip fracture risk in perimenopausal women remains elusive.
For osteoporosis, the rationale for using vitamin E is based on its antioxidant activity, which can scavenge potentially damaging free radicals. Researchers have asked whether vitamin E can help maintain the integrity of bone matrix and stimulate bone formation while minimizing bone resorption, particularly in trabecular (spongy) bone, the bone compartment preferentially affected in perimenopausal bone loss.
Vitamin E mostly consists of two isomers: alpha-tocopherol and gamma-tocopherol. Alpha-tocopherol has higher antioxidant activity and is found in nuts, seeds, vegetable oils, green leafy vegetables, fortified cereals, and vitamin E supplements. Gamma-tocopherol is known for its superior anti-inflammatory properties and accounts for about 70% of the total vitamin E intake in a typical American diet, largely sourced from soybean and other vegetable oils.
Benefits and Risks in Bone Loss Studies
Perimenopausal bone loss is caused, to a great extent, by the decrease in sex hormones. Studies of vitamin E in ovariectomized rats have yielded mixed results. This animal model lacks sex hormones and has similar bone changes to those of postmenopausal women. Some animal studies have suggested a positive effect of vitamin E on bone while others have reported no effect.
Studies in humans also have produced conflicting reports of positive, neutral, and negative associations of vitamin E with bone health. For example, the Women’s Health Initiative examined the relationship between vitamin and mineral antioxidants and bone health in postmenopausal women and found no significant association between antioxidants and bone mineral density.
Another study examining data from children and adolescents enrolled in the National Health and Nutrition Examination Survey (NHANES) database found an inverse association between alpha-tocopherol and lumbar spine bone density, suggesting a deleterious effect on bone. Inverse associations also have been reported in certain studies of postmenopausal women.
High doses of alpha-tocopherol have been linked to a risk for impaired bone health through a variety of mechanisms, such as interference with vitamin K metabolism; competitive binding for alpha-tocopherol transfer protein, inhibiting the entry of beneficial vitamin E isomers, including gamma-tocopherol; and pro-oxidant effects that harm bone. Thus, postmenopausal women taking vitamin E supplements primarily as high doses of alpha-tocopherol might be hindering their bone health.
Data for gamma-tocopherol are more promising. Some studies hypothesize that gamma-tocopherol might uncouple bone turnover, leading to increased bone formation without affecting bone resorption. Further, a randomized controlled study of mixed tocopherols (rather than alpha-tocopherol) vs placebo reported a protective effect of this preparation on bone outcomes by suppressing bone resorption. This raises the importance of considering the specific forms of vitamin E when evaluating its role in bone health.
Limitations of Current Studies
Researchers acknowledge several limitations in studies to date. For example, there are very few randomized controlled trials assessing the impact of vitamin E on bone health. Most studies are cross-sectional or observational, even when longitudinal. Cross-sectional and observational designs prevent us from establishing a causal relationship between vitamin E and bone endpoints.
Such designs also run the risk of additional confounders that may affect associations between vitamin E and bone, or the lack thereof. These could include both known and unknown confounders. Of note, gamma-tocopherol intake data were not available for certain NHANES studies.
Further, people often consume multiple nutrients and supplements, complicating the identification of specific nutrient-disease associations. Most human studies estimate tocopherol intake by dietary questionnaires or measure serum tocopherol levels, which reflect short-term dietary intake, while bone mineral density is probably influenced by long-term dietary patterns.
Too Soon to Prescribe Vitamin E for Bone Health
Some nutrition experts advocate for vitamin E supplements containing mixed tocopherols, specifically suggesting a ratio of 50-100 IU of gamma-tocopherol per 400 IU of D-alpha-tocopherol. Additional research is essential to confirm and further clarify the role of gamma-tocopherol in bone formation and resorption. In fact, it is also important to explore the influence of other compounds in the vitamin E family on skeletal health.
Until more data are available, we would recommend following the Institute of Medicine’s guidelines for the recommended daily allowance (RDA) of vitamin E. This is age dependent, ranging from 4 to 11 mg/d between the ages of 0 and 13 years, and 15 mg/d thereafter.
Overall, evidence of vitamin E’s impact on osteoporosis and hip fracture risk in perimenopausal women remains inconclusive. Although some observational and interventional studies suggest potential benefits, more interventional studies, particularly randomized controlled trials, are necessary to explore the risks and benefits of vitamin E supplementation and serum vitamin E levels on bone density and fracture risk more thoroughly.
Dr. Pani, Assistant Professor, Department of Internal Medicine, UVA School of Medicine; Medical Director, Department of General Medicine, Same Day Care Clinic, both in Charlottesville, has disclosed no relevant financial relationships. Dr. Misra, Professor, Chair, Physician-in-Chief, Department of Pediatrics, University of Virginia, and UVA Health Children’s, Charlottesville, has disclosed being a key opinion leader for Lumos Pharma.
A version of this article appeared on Medscape.com.
Iron Overload: The Silent Bone Breaker
TOPLINE:
Patients with serum ferritin levels higher than 1000 μg/L show a 91% increased risk for any fracture, with a doubled risk for vertebral and humerus fractures compared with those without iron overload.
METHODOLOGY:
- Iron overload’s association with decreased bone mineral density is established, but its relationship to osteoporotic fracture risk has remained understudied and inconsistent across fracture sites.
- Researchers conducted a population-based cohort study using a UK general practice database to evaluate the fracture risk in 20,264 patients with iron overload and 192,956 matched controls without elevated ferritin (mean age, 57 years; about 40% women).
- Patients with iron overload were identified as those with laboratory-confirmed iron overload (serum ferritin levels > 1000 μg/L; n = 13,510) or a diagnosis of an iron overloading disorder, such as thalassemia major, sickle cell disease, or hemochromatosis (n = 6754).
- The primary outcome of interest was the first occurrence of an osteoporotic fracture after the diagnosis of iron overload or first record of high ferritin.
- A sensitivity analysis was conducted to check the impact of laboratory-confirmed iron overload on the risk for osteoporotic fracture compared with a diagnosis code without elevated ferritin.
TAKEAWAY:
- In the overall cohort, patients with iron overload had a 55% higher risk for any osteoporotic fracture than control individuals (adjusted hazard ratio [aHR], 1.55; 95% CI, 1.42-1.68), with the highest risk observed for vertebral fractures (aHR, 1.97; 95% CI, 1.63-2.37) and humerus fractures (aHR, 1.91; 95% CI, 1.61-2.26).
- Patients with laboratory-confirmed iron overload showed a 91% increased risk for any fracture (aHR, 1.91; 95% CI, 1.73-2.10), with a 2.5-fold higher risk observed for vertebral fractures (aHR, 2.51; 95% CI, 2.01-3.12), followed by humerus fractures (aHR, 2.41; 95% CI, 1.96-2.95).
- There was no increased risk for fracture at any site in patients with a diagnosis of an iron overloading disorder but no laboratory-confirmed iron overload.
- No sex-specific differences were identified in the association between iron overload and fracture risk.
IN PRACTICE:
“The main clinical message from our findings is that clinicians should consider iron overloading as a risk factor for fracture. Importantly, among high-risk patients presenting with serum ferritin values exceeding 1000 μg/L, osteoporosis screening and treatment strategies should be initiated in accordance with the guidelines for patients with hepatic disease,” the authors wrote.
SOURCE:
The study was led by Andrea Michelle Burden, PhD, Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zürich in Switzerland, and was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study could not assess the duration of iron overload on fracture risk, and thus, patients could enter the cohort with a single elevated serum ferritin value that may not have reflected systemic iron overload. The authors also acknowledged potential exposure misclassification among matched control individuals because only 2.9% had a serum ferritin value available at baseline. Also, researchers were unable to adjust for inflammation status due to the limited availability of C-reactive protein measurements and the lack of leukocyte count data in primary care settings.
DISCLOSURES:
This study received support through grants from the German Research Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with serum ferritin levels higher than 1000 μg/L show a 91% increased risk for any fracture, with a doubled risk for vertebral and humerus fractures compared with those without iron overload.
METHODOLOGY:
- Iron overload’s association with decreased bone mineral density is established, but its relationship to osteoporotic fracture risk has remained understudied and inconsistent across fracture sites.
- Researchers conducted a population-based cohort study using a UK general practice database to evaluate the fracture risk in 20,264 patients with iron overload and 192,956 matched controls without elevated ferritin (mean age, 57 years; about 40% women).
- Patients with iron overload were identified as those with laboratory-confirmed iron overload (serum ferritin levels > 1000 μg/L; n = 13,510) or a diagnosis of an iron overloading disorder, such as thalassemia major, sickle cell disease, or hemochromatosis (n = 6754).
- The primary outcome of interest was the first occurrence of an osteoporotic fracture after the diagnosis of iron overload or first record of high ferritin.
- A sensitivity analysis was conducted to check the impact of laboratory-confirmed iron overload on the risk for osteoporotic fracture compared with a diagnosis code without elevated ferritin.
TAKEAWAY:
- In the overall cohort, patients with iron overload had a 55% higher risk for any osteoporotic fracture than control individuals (adjusted hazard ratio [aHR], 1.55; 95% CI, 1.42-1.68), with the highest risk observed for vertebral fractures (aHR, 1.97; 95% CI, 1.63-2.37) and humerus fractures (aHR, 1.91; 95% CI, 1.61-2.26).
- Patients with laboratory-confirmed iron overload showed a 91% increased risk for any fracture (aHR, 1.91; 95% CI, 1.73-2.10), with a 2.5-fold higher risk observed for vertebral fractures (aHR, 2.51; 95% CI, 2.01-3.12), followed by humerus fractures (aHR, 2.41; 95% CI, 1.96-2.95).
- There was no increased risk for fracture at any site in patients with a diagnosis of an iron overloading disorder but no laboratory-confirmed iron overload.
- No sex-specific differences were identified in the association between iron overload and fracture risk.
IN PRACTICE:
“The main clinical message from our findings is that clinicians should consider iron overloading as a risk factor for fracture. Importantly, among high-risk patients presenting with serum ferritin values exceeding 1000 μg/L, osteoporosis screening and treatment strategies should be initiated in accordance with the guidelines for patients with hepatic disease,” the authors wrote.
SOURCE:
The study was led by Andrea Michelle Burden, PhD, Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zürich in Switzerland, and was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study could not assess the duration of iron overload on fracture risk, and thus, patients could enter the cohort with a single elevated serum ferritin value that may not have reflected systemic iron overload. The authors also acknowledged potential exposure misclassification among matched control individuals because only 2.9% had a serum ferritin value available at baseline. Also, researchers were unable to adjust for inflammation status due to the limited availability of C-reactive protein measurements and the lack of leukocyte count data in primary care settings.
DISCLOSURES:
This study received support through grants from the German Research Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with serum ferritin levels higher than 1000 μg/L show a 91% increased risk for any fracture, with a doubled risk for vertebral and humerus fractures compared with those without iron overload.
METHODOLOGY:
- Iron overload’s association with decreased bone mineral density is established, but its relationship to osteoporotic fracture risk has remained understudied and inconsistent across fracture sites.
- Researchers conducted a population-based cohort study using a UK general practice database to evaluate the fracture risk in 20,264 patients with iron overload and 192,956 matched controls without elevated ferritin (mean age, 57 years; about 40% women).
- Patients with iron overload were identified as those with laboratory-confirmed iron overload (serum ferritin levels > 1000 μg/L; n = 13,510) or a diagnosis of an iron overloading disorder, such as thalassemia major, sickle cell disease, or hemochromatosis (n = 6754).
- The primary outcome of interest was the first occurrence of an osteoporotic fracture after the diagnosis of iron overload or first record of high ferritin.
- A sensitivity analysis was conducted to check the impact of laboratory-confirmed iron overload on the risk for osteoporotic fracture compared with a diagnosis code without elevated ferritin.
TAKEAWAY:
- In the overall cohort, patients with iron overload had a 55% higher risk for any osteoporotic fracture than control individuals (adjusted hazard ratio [aHR], 1.55; 95% CI, 1.42-1.68), with the highest risk observed for vertebral fractures (aHR, 1.97; 95% CI, 1.63-2.37) and humerus fractures (aHR, 1.91; 95% CI, 1.61-2.26).
- Patients with laboratory-confirmed iron overload showed a 91% increased risk for any fracture (aHR, 1.91; 95% CI, 1.73-2.10), with a 2.5-fold higher risk observed for vertebral fractures (aHR, 2.51; 95% CI, 2.01-3.12), followed by humerus fractures (aHR, 2.41; 95% CI, 1.96-2.95).
- There was no increased risk for fracture at any site in patients with a diagnosis of an iron overloading disorder but no laboratory-confirmed iron overload.
- No sex-specific differences were identified in the association between iron overload and fracture risk.
IN PRACTICE:
“The main clinical message from our findings is that clinicians should consider iron overloading as a risk factor for fracture. Importantly, among high-risk patients presenting with serum ferritin values exceeding 1000 μg/L, osteoporosis screening and treatment strategies should be initiated in accordance with the guidelines for patients with hepatic disease,” the authors wrote.
SOURCE:
The study was led by Andrea Michelle Burden, PhD, Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zürich in Switzerland, and was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study could not assess the duration of iron overload on fracture risk, and thus, patients could enter the cohort with a single elevated serum ferritin value that may not have reflected systemic iron overload. The authors also acknowledged potential exposure misclassification among matched control individuals because only 2.9% had a serum ferritin value available at baseline. Also, researchers were unable to adjust for inflammation status due to the limited availability of C-reactive protein measurements and the lack of leukocyte count data in primary care settings.
DISCLOSURES:
This study received support through grants from the German Research Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New Approaches to Research Beyond Massive Clinical Trials
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
Can New Target Boost Bone Health in Older Women With T2D?
TOPLINE:
In older postmenopausal women with type 2 diabetes (T2D), pyridoxamine treatment has potential to prevent fractures and protect bone tissue by targeting advanced glycation end products and also lowers levels of A1c, an early glycation product.
METHODOLOGY:
- Despite greater bone density and low bone turnover, people with T2D have increased fractures risk and higher associated mortality, but previous research linking advanced glycation end products (AGEs) to bone fragility suggests an AGE inhibitor could be a novel therapeutic strategy to prevent the accumulation of AGE in bone tissue.
- This randomized clinical trial, conducted at the Metabolic Bone Disease Unit of Columbia University Irving Medical Center, New York City, from December 2017 to February 2021, assessed the efficacy of the vitamin B6 metabolite pyridoxamine, an AGE inhibitor, in promoting bone formation in 55 older postmenopausal women with T2D.
- The participants received either 200 mg of oral pyridoxamine dihydrochloride (n = 27; mean age, 75.6 years) or matching placebo tablets (n = 28; mean age, 73.1 years) twice daily for 1 year.
- The primary outcome was the change in the levels of the bone formation marker Procollagen Type I Intact N-terminal Propeptide (P1NP) from baseline to after 12 months of treatment.
- Other outcomes included changes in bone mineral density measured at the lumbar spine, total hip, femoral neck, and 1/3 radius using dual energy x-ray absorptiometry; A1c levels; and skin autofluorescence at 12 months, a surrogate for bone AGEs. The safety of pyridoxamine was evaluated by monitoring neurologic findings and adverse events because high doses of the parent vitamin B6 have been reported to cause neurotoxicity.
TAKEAWAY:
- At 12 months, pyridoxamine treatment increased P1NP levels by 23% (P = .028) compared with 4.1% with placebo (P = .576), a “nearly significant difference.”
- Bone mineral density at the femoral neck increased by 2.64% with pyridoxamine but decreased by 0.91% with placebo (P = .007), with no changes at the lumbar spine, total hip, or 1/3 radius. The levels of bone resorption markers or skin autofluorescence were not significantly different between the groups.
- A1c levels decreased by 0.38% in the pyridoxamine group and correlated with increased P1NP levels, compared with a 0.05% increase in the placebo group (P = .04).
- Pyridoxamine was well tolerated. Four serious adverse events were reported in the pyridoxamine group and seven in the placebo group; none of these were related to the trial treatment.
IN PRACTICE:
“[The study] findings suggest that AGE inhibition might clinically improve the low bone formation state of T2D, and that PM [pyridoxamine] might warrant further investigation as a potential disease mechanism-directed approach for the therapy of T2D bone fragility,” the authors wrote.
SOURCE:
The study was led by Aiden V. Brossfield, Metabolic Bone Disease Unit, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study findings were preliminary. The study’s small sample size and individual variability led to a lack of statistical significance. The exclusion of men may have limited the generalizability of the findings. The short duration of 1 year may have been insufficient for detecting changes in skin AGEs. The levels of circulating AGEs or pyridoxamine were not measured, which could have provided additional insights.
DISCLOSURES:
The study was supported by a grant from the US National Institute on Aging. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In older postmenopausal women with type 2 diabetes (T2D), pyridoxamine treatment has potential to prevent fractures and protect bone tissue by targeting advanced glycation end products and also lowers levels of A1c, an early glycation product.
METHODOLOGY:
- Despite greater bone density and low bone turnover, people with T2D have increased fractures risk and higher associated mortality, but previous research linking advanced glycation end products (AGEs) to bone fragility suggests an AGE inhibitor could be a novel therapeutic strategy to prevent the accumulation of AGE in bone tissue.
- This randomized clinical trial, conducted at the Metabolic Bone Disease Unit of Columbia University Irving Medical Center, New York City, from December 2017 to February 2021, assessed the efficacy of the vitamin B6 metabolite pyridoxamine, an AGE inhibitor, in promoting bone formation in 55 older postmenopausal women with T2D.
- The participants received either 200 mg of oral pyridoxamine dihydrochloride (n = 27; mean age, 75.6 years) or matching placebo tablets (n = 28; mean age, 73.1 years) twice daily for 1 year.
- The primary outcome was the change in the levels of the bone formation marker Procollagen Type I Intact N-terminal Propeptide (P1NP) from baseline to after 12 months of treatment.
- Other outcomes included changes in bone mineral density measured at the lumbar spine, total hip, femoral neck, and 1/3 radius using dual energy x-ray absorptiometry; A1c levels; and skin autofluorescence at 12 months, a surrogate for bone AGEs. The safety of pyridoxamine was evaluated by monitoring neurologic findings and adverse events because high doses of the parent vitamin B6 have been reported to cause neurotoxicity.
TAKEAWAY:
- At 12 months, pyridoxamine treatment increased P1NP levels by 23% (P = .028) compared with 4.1% with placebo (P = .576), a “nearly significant difference.”
- Bone mineral density at the femoral neck increased by 2.64% with pyridoxamine but decreased by 0.91% with placebo (P = .007), with no changes at the lumbar spine, total hip, or 1/3 radius. The levels of bone resorption markers or skin autofluorescence were not significantly different between the groups.
- A1c levels decreased by 0.38% in the pyridoxamine group and correlated with increased P1NP levels, compared with a 0.05% increase in the placebo group (P = .04).
- Pyridoxamine was well tolerated. Four serious adverse events were reported in the pyridoxamine group and seven in the placebo group; none of these were related to the trial treatment.
IN PRACTICE:
“[The study] findings suggest that AGE inhibition might clinically improve the low bone formation state of T2D, and that PM [pyridoxamine] might warrant further investigation as a potential disease mechanism-directed approach for the therapy of T2D bone fragility,” the authors wrote.
SOURCE:
The study was led by Aiden V. Brossfield, Metabolic Bone Disease Unit, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study findings were preliminary. The study’s small sample size and individual variability led to a lack of statistical significance. The exclusion of men may have limited the generalizability of the findings. The short duration of 1 year may have been insufficient for detecting changes in skin AGEs. The levels of circulating AGEs or pyridoxamine were not measured, which could have provided additional insights.
DISCLOSURES:
The study was supported by a grant from the US National Institute on Aging. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In older postmenopausal women with type 2 diabetes (T2D), pyridoxamine treatment has potential to prevent fractures and protect bone tissue by targeting advanced glycation end products and also lowers levels of A1c, an early glycation product.
METHODOLOGY:
- Despite greater bone density and low bone turnover, people with T2D have increased fractures risk and higher associated mortality, but previous research linking advanced glycation end products (AGEs) to bone fragility suggests an AGE inhibitor could be a novel therapeutic strategy to prevent the accumulation of AGE in bone tissue.
- This randomized clinical trial, conducted at the Metabolic Bone Disease Unit of Columbia University Irving Medical Center, New York City, from December 2017 to February 2021, assessed the efficacy of the vitamin B6 metabolite pyridoxamine, an AGE inhibitor, in promoting bone formation in 55 older postmenopausal women with T2D.
- The participants received either 200 mg of oral pyridoxamine dihydrochloride (n = 27; mean age, 75.6 years) or matching placebo tablets (n = 28; mean age, 73.1 years) twice daily for 1 year.
- The primary outcome was the change in the levels of the bone formation marker Procollagen Type I Intact N-terminal Propeptide (P1NP) from baseline to after 12 months of treatment.
- Other outcomes included changes in bone mineral density measured at the lumbar spine, total hip, femoral neck, and 1/3 radius using dual energy x-ray absorptiometry; A1c levels; and skin autofluorescence at 12 months, a surrogate for bone AGEs. The safety of pyridoxamine was evaluated by monitoring neurologic findings and adverse events because high doses of the parent vitamin B6 have been reported to cause neurotoxicity.
TAKEAWAY:
- At 12 months, pyridoxamine treatment increased P1NP levels by 23% (P = .028) compared with 4.1% with placebo (P = .576), a “nearly significant difference.”
- Bone mineral density at the femoral neck increased by 2.64% with pyridoxamine but decreased by 0.91% with placebo (P = .007), with no changes at the lumbar spine, total hip, or 1/3 radius. The levels of bone resorption markers or skin autofluorescence were not significantly different between the groups.
- A1c levels decreased by 0.38% in the pyridoxamine group and correlated with increased P1NP levels, compared with a 0.05% increase in the placebo group (P = .04).
- Pyridoxamine was well tolerated. Four serious adverse events were reported in the pyridoxamine group and seven in the placebo group; none of these were related to the trial treatment.
IN PRACTICE:
“[The study] findings suggest that AGE inhibition might clinically improve the low bone formation state of T2D, and that PM [pyridoxamine] might warrant further investigation as a potential disease mechanism-directed approach for the therapy of T2D bone fragility,” the authors wrote.
SOURCE:
The study was led by Aiden V. Brossfield, Metabolic Bone Disease Unit, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study findings were preliminary. The study’s small sample size and individual variability led to a lack of statistical significance. The exclusion of men may have limited the generalizability of the findings. The short duration of 1 year may have been insufficient for detecting changes in skin AGEs. The levels of circulating AGEs or pyridoxamine were not measured, which could have provided additional insights.
DISCLOSURES:
The study was supported by a grant from the US National Institute on Aging. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Oxidative Stress Marker May Signal Fracture Risk in T2D
TOPLINE:
Elevated levels of plasma F2-isoprostanes, a reliable marker of oxidative stress, are associated with an increased risk for fractures in older ambulatory patients with type 2 diabetes (T2D) independently of bone density.
METHODOLOGY:
- Patients with T2D face an increased risk for fractures at any given bone mineral density; oxidative stress levels (reflected in circulating F2-isoprostanes), which are elevated in T2D, are associated with other T2D complications, and may weaken bone integrity.
- Researchers analyzed data from an observational cohort study to investigate the association between the levels of circulating F2-isoprostanes and the risk for clinical fractures in older patients with T2D.
- The data included 703 older ambulatory adults (baseline age, 70-79 years; about half White individuals and half Black individuals ; about half men and half women) from the Health, Aging and Body Composition Study, of whom 132 had T2D.
- Plasma F2-isoprostane levels were measured using baseline serum samples; bone turnover markers were also measured including procollagen type 1 N-terminal propeptide, osteocalcin, and C-terminal telopeptide of type 1 collagen.
- Incident clinical fractures were tracked over a follow-up period of up to 17.3 years, with fractures verified through radiology reports.
TAKEAWAY:
- Overall, 25.8% patients in the T2D group and 23.5% adults in the non-diabetes group reported an incident clinical fracture during a mean follow-up period of 6.2 and 8.0 years, respectively.
- In patients with T2D, the risk for incident clinical fracture increased by 93% for every standard deviation increase in the log F2-isoprostane serum levels (hazard ratio [HR], 1.93; 95% CI, 1.26-2.95; P = .002) independently of baseline bone density, medication use, and other risk factors, with no such association reported in individuals without T2D (HR, 0.98; 95% CI, 0.81-1.18; P = .79).
- In the T2D group, elevated plasma F2-isoprostane levels were also associated with a decrease in total hip bone mineral density over 4 years (r = −0.28; P = .008), but not in the non-diabetes group.
- No correlation was found between plasma F2-isoprostane levels and circulating advanced glycoxidation end-products, bone turnover markers, or A1c levels in either group.
IN PRACTICE:
“Oxidative stress in T2D may play an important role in the decline of bone quality and not just bone quantity,” the authors wrote.
SOURCE:
This study was led by Bowen Wang, PhD, Rensselaer Polytechnic Institute, Troy, New York. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
This study was conducted in a well-functioning elderly population with only White and Black participants, which may limit the generalizability of the findings to other age groups or less healthy populations. Additionally, the study did not assess prevalent vertebral fracture risk due to the small sample size.
DISCLOSURES:
This study was supported by the US National Institute on Aging and the Intramural Research Program of the US National Institutes of Health and the Dr and Ms Sands and Sands Family for Orthopaedic Research. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of plasma F2-isoprostanes, a reliable marker of oxidative stress, are associated with an increased risk for fractures in older ambulatory patients with type 2 diabetes (T2D) independently of bone density.
METHODOLOGY:
- Patients with T2D face an increased risk for fractures at any given bone mineral density; oxidative stress levels (reflected in circulating F2-isoprostanes), which are elevated in T2D, are associated with other T2D complications, and may weaken bone integrity.
- Researchers analyzed data from an observational cohort study to investigate the association between the levels of circulating F2-isoprostanes and the risk for clinical fractures in older patients with T2D.
- The data included 703 older ambulatory adults (baseline age, 70-79 years; about half White individuals and half Black individuals ; about half men and half women) from the Health, Aging and Body Composition Study, of whom 132 had T2D.
- Plasma F2-isoprostane levels were measured using baseline serum samples; bone turnover markers were also measured including procollagen type 1 N-terminal propeptide, osteocalcin, and C-terminal telopeptide of type 1 collagen.
- Incident clinical fractures were tracked over a follow-up period of up to 17.3 years, with fractures verified through radiology reports.
TAKEAWAY:
- Overall, 25.8% patients in the T2D group and 23.5% adults in the non-diabetes group reported an incident clinical fracture during a mean follow-up period of 6.2 and 8.0 years, respectively.
- In patients with T2D, the risk for incident clinical fracture increased by 93% for every standard deviation increase in the log F2-isoprostane serum levels (hazard ratio [HR], 1.93; 95% CI, 1.26-2.95; P = .002) independently of baseline bone density, medication use, and other risk factors, with no such association reported in individuals without T2D (HR, 0.98; 95% CI, 0.81-1.18; P = .79).
- In the T2D group, elevated plasma F2-isoprostane levels were also associated with a decrease in total hip bone mineral density over 4 years (r = −0.28; P = .008), but not in the non-diabetes group.
- No correlation was found between plasma F2-isoprostane levels and circulating advanced glycoxidation end-products, bone turnover markers, or A1c levels in either group.
IN PRACTICE:
“Oxidative stress in T2D may play an important role in the decline of bone quality and not just bone quantity,” the authors wrote.
SOURCE:
This study was led by Bowen Wang, PhD, Rensselaer Polytechnic Institute, Troy, New York. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
This study was conducted in a well-functioning elderly population with only White and Black participants, which may limit the generalizability of the findings to other age groups or less healthy populations. Additionally, the study did not assess prevalent vertebral fracture risk due to the small sample size.
DISCLOSURES:
This study was supported by the US National Institute on Aging and the Intramural Research Program of the US National Institutes of Health and the Dr and Ms Sands and Sands Family for Orthopaedic Research. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of plasma F2-isoprostanes, a reliable marker of oxidative stress, are associated with an increased risk for fractures in older ambulatory patients with type 2 diabetes (T2D) independently of bone density.
METHODOLOGY:
- Patients with T2D face an increased risk for fractures at any given bone mineral density; oxidative stress levels (reflected in circulating F2-isoprostanes), which are elevated in T2D, are associated with other T2D complications, and may weaken bone integrity.
- Researchers analyzed data from an observational cohort study to investigate the association between the levels of circulating F2-isoprostanes and the risk for clinical fractures in older patients with T2D.
- The data included 703 older ambulatory adults (baseline age, 70-79 years; about half White individuals and half Black individuals ; about half men and half women) from the Health, Aging and Body Composition Study, of whom 132 had T2D.
- Plasma F2-isoprostane levels were measured using baseline serum samples; bone turnover markers were also measured including procollagen type 1 N-terminal propeptide, osteocalcin, and C-terminal telopeptide of type 1 collagen.
- Incident clinical fractures were tracked over a follow-up period of up to 17.3 years, with fractures verified through radiology reports.
TAKEAWAY:
- Overall, 25.8% patients in the T2D group and 23.5% adults in the non-diabetes group reported an incident clinical fracture during a mean follow-up period of 6.2 and 8.0 years, respectively.
- In patients with T2D, the risk for incident clinical fracture increased by 93% for every standard deviation increase in the log F2-isoprostane serum levels (hazard ratio [HR], 1.93; 95% CI, 1.26-2.95; P = .002) independently of baseline bone density, medication use, and other risk factors, with no such association reported in individuals without T2D (HR, 0.98; 95% CI, 0.81-1.18; P = .79).
- In the T2D group, elevated plasma F2-isoprostane levels were also associated with a decrease in total hip bone mineral density over 4 years (r = −0.28; P = .008), but not in the non-diabetes group.
- No correlation was found between plasma F2-isoprostane levels and circulating advanced glycoxidation end-products, bone turnover markers, or A1c levels in either group.
IN PRACTICE:
“Oxidative stress in T2D may play an important role in the decline of bone quality and not just bone quantity,” the authors wrote.
SOURCE:
This study was led by Bowen Wang, PhD, Rensselaer Polytechnic Institute, Troy, New York. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
This study was conducted in a well-functioning elderly population with only White and Black participants, which may limit the generalizability of the findings to other age groups or less healthy populations. Additionally, the study did not assess prevalent vertebral fracture risk due to the small sample size.
DISCLOSURES:
This study was supported by the US National Institute on Aging and the Intramural Research Program of the US National Institutes of Health and the Dr and Ms Sands and Sands Family for Orthopaedic Research. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
How to Stop Bone Loss After Denosumab? No Easy Answers
Patients who discontinue treatment with the osteoporosis drug denosumab, despite transitioning to zoledronate, show significant losses in lumbar spine bone mineral density (BMD) within a year, according to the latest findings to show that the rapid rebound of bone loss after denosumab discontinuation is not easily prevented with other therapies — even bisphosphonates.
“When initiating denosumab for osteoporosis treatment, it is recommended to engage in thorough shared decision-making with the patient to ensure they understand the potential risks associated with discontinuing the medication,” senior author Shau-Huai Fu, MD, PhD, Department of Orthopedics, National Taiwan University Hospital Yunlin Branch, Douliu, told this news organization.
Furthermore, “integrating a case manager system is crucial to support long-term adherence and compliance,” he added.
The results are from the Denosumab Sequential Therapy prospective, open-label, parallel-group randomized clinical trial, published online in JAMA Network Open.
In the study, 101 patients were recruited between April 2019 and May 2021 at a referral center and two hospitals in Taiwan. The patients, including postmenopausal women and men over the age of 50, had been treated with regular denosumab for at least 2 years and had no previous exposure to other anti-osteoporosis medication.
They were randomized to treatment either with continuous denosumab at the standard dose of 60 mg twice yearly or to discontinue denosumab and receive the standard intravenous dose of the bisphosphonate zoledronate at 5 mg at the time when the next dose of denosumab would have been administered.
There were no differences between the two groups in serum bone turnover markers at baseline.
The current results, reflecting the first year of the 2-year study, show that, overall, those receiving zoledronate (n = 76), had a significant decrease in lumbar spine BMD, compared with a slight increase in the denosumab continuation group (–0.68% vs 1.30%, respectively; P = .03).
No significant differences were observed between the groups in terms of the study’s other measures of total hip BMD (median, 0% vs 1.12%; P = .24), and femoral neck BMD (median, 0.18% vs 0.17%; P = .71).
Additional findings from multivariable analyses in the study also supported results from previous studies showing that a longer duration of denosumab use is associated with a more substantial rebound effect: Among 15 of the denosumab users in the study who had ≥ 3 prior years of the drug, the reduction in lumbar spine BMD was even greater with zoledronate compared with denosumab continuation (–3.20% vs 1.30%; P = .003).
Though the lack of losses in the other measures of total hip and femoral neck BMD may seem encouraging, evidence from the bulk of other studies suggests cautious interpretation of those findings, Fu said.
“Although our study did not observe a noticeable decline in total hip or femoral neck BMD, other randomized controlled trials with longer durations of denosumab use have reported significant reductions in these areas,” Fu said. “Therefore, it cannot be assumed that non-lumbar spine regions are entirely safe.”
Fracture Risk Is the Overriding Concern
Meanwhile, the loss of lumbar spine BMD is of particular concern because of its role in what amounts to the broader, overriding concern of denosumab discontinuation — the risk for fracture, Fu noted.
“Real-world observations indicate that fractures caused by or associated with discontinuation of denosumab primarily occur in the spine,” he explained.
Previous research underscores the risk for fracture with denosumab discontinuation — and the greater risk with longer-term denosumab use, showing an 11.8% annual incidence of vertebral fracture after discontinuation of denosumab used for less than 2 years, increasing to 16.0% upon discontinuation after more than 2 years of treatment.
Randomized trials have shown sequential zoledronate to have some benefit in offsetting that risk, reducing first-year fracture risk by 3%-4% in some studies.
In the current study, 3 of 76 participants experienced a vertebral fracture in the first year of discontinuation, all involving women, including 2 who had been receiving denosumab for ≥ 4 years before medication transition.
If a transition to a bisphosphonate is anticipated, the collective findings suggest doing it as early on in denosumab treatment as possible, Fu and his colleagues noted in the study.
“When medication transition from denosumab is expected or when long-term denosumab treatment may not be suitable, earlier medication transition with potent sequential therapy should be considered,” they wrote.
Dosing Adjustments?
The findings add to the evidence that “patients who gain the most with denosumab are likely to lose the most with zoledronate,” Nelson Watts, MD, who authored an editorial accompanying the study, told this news organization.
Furthermore, “denosumab and other medications seem to do more [and faster] for BMD in the spine, so we expect more loss in the spine than in the hip,” said Watts, who is director of Mercy Health Osteoporosis and Bone Health Services, Bon Secours Mercy Health in Cincinnati, Ohio.
“Studies are needed but not yet done to see if a higher dose or more frequent zoledronate would be better for BMD than the ‘usual’ yearly dose,” Watts added.
The only published clinical recommendations on the matter are discussed in a position paper from the European Calcified Tissue Society (ECTS).
“Pending additional robust data, a pragmatic approach is to begin treatment with zoledronate 6 months after the last denosumab injection and monitor the effect with bone turnover markers, for example, 3 and 6 months after the zoledronate infusion,” they recommended.
In cases of increased bone turnover markers, including above the mean found in age- and sex-matched cohorts, “repeated infusion of zoledronate should be considered,” the society added.
If bone turnover markers are not available for monitoring the patients, “a pragmatic approach could be administrating a second infusion of zoledronate 6 months after the first infusion,” they wrote.
Clinicians Need to Be Proactive From the Start
Bente Langdahl, MD, of the Medical Department of Endocrinology, Aarhus University Hospital in Denmark, who was a coauthor on the ECTS position statement, told this news organization that clinicians should also be proactive on the other side of treatment — before it begins — to prevent problems with discontinuation.
“I think denosumab is a very good treatment for some patients with high fracture risk and very low BMD, but both patients and clinicians should know that this treatment is either lifelong or there needs to be a plan for discontinuation,” Langdahl said.
Langdahl noted that denosumab is coming off patent soon; hence, issues with cost could become more manageable.
But until then, “I think [cost] should be considered before starting treatment because if patients cannot afford denosumab, they should have been started on zoledronate from the beginning.”
Discontinuation Reasons Vary
Research indicates that, broadly, adherence to denosumab ranges from about 45% to 72% at 2 years, with some reasons for discontinuation including the need for dental treatment or cost, Fu and colleagues reported.
Fu added, however, that other reasons for discontinuing denosumab “are not due to ‘need’ but rather factors such as relocating, missing follow-up appointments, or poor adherence.”
Lorenz Hofbauer, MD, who is head of the Division of Endocrinology, Diabetes, and Bone Diseases, Department of Medicine III at the Technical University Medical Center in Dresden, Germany, noted that another issue contributing to some hesitation by patients about remaining on, or even initiating denosumab, is the known risk for osteonecrosis of the jaw (ONJ).
Though reported as being rare, research continuing to stir concern for ONJ with denosumab use includes one recent study of patients with breast cancer showing those treated with denosumab had a fivefold higher risk for ONJ vs those on bisphosphonates.
“About 20% of my patients have ONJ concerns or other questions, which may delay treatment with denosumab or other therapies,” Hofbauer told this news organization.
“There is a high need to discuss risk versus benefits toward a shared decision-making,” he said.
Conversely, however, Hofbauer noted that adherence to denosumab at his center is fairly high — at 90%, which he says is largely credited to an electronically supported recall system in place at the center.
Denosumab maker Amgen also offers patient reminders via email, text, or phone through its Bone Matters patient support system, which also provides access to a call center for questions or to update treatment appointment information.
In terms of the ongoing question of how to best prevent fracture risk when patients do wind up discontinuing denosumab, Watts concluded in his editorial that more robust studies are needed.
“The dilemma is what to do with longer-term users who stop, and the real question is not what happens to BMD, but what happens to fracture risk,” he wrote.
“It is unlikely that the fracture risk question can be answered due to ethical limitations, but finding the best option, [whether it is] oral or intravenous bisphosphonate, timing, dose, and frequency, to minimize bone loss and the rebound increase in bone resorption after stopping long-term denosumab requires larger and longer studies of better design.”
The authors had no disclosures to report. Watts has been an investigator, consultant, and speaker for Amgen outside of the published editorial. Hofbauer is on advisory boards for Alexion Pharmaceuticals, Amolyt Pharma, Amgen, and UCB. Langdahl has been a primary investigator on previous and ongoing clinical trials involving denosumab.
A version of this article appeared on Medscape.com.
Patients who discontinue treatment with the osteoporosis drug denosumab, despite transitioning to zoledronate, show significant losses in lumbar spine bone mineral density (BMD) within a year, according to the latest findings to show that the rapid rebound of bone loss after denosumab discontinuation is not easily prevented with other therapies — even bisphosphonates.
“When initiating denosumab for osteoporosis treatment, it is recommended to engage in thorough shared decision-making with the patient to ensure they understand the potential risks associated with discontinuing the medication,” senior author Shau-Huai Fu, MD, PhD, Department of Orthopedics, National Taiwan University Hospital Yunlin Branch, Douliu, told this news organization.
Furthermore, “integrating a case manager system is crucial to support long-term adherence and compliance,” he added.
The results are from the Denosumab Sequential Therapy prospective, open-label, parallel-group randomized clinical trial, published online in JAMA Network Open.
In the study, 101 patients were recruited between April 2019 and May 2021 at a referral center and two hospitals in Taiwan. The patients, including postmenopausal women and men over the age of 50, had been treated with regular denosumab for at least 2 years and had no previous exposure to other anti-osteoporosis medication.
They were randomized to treatment either with continuous denosumab at the standard dose of 60 mg twice yearly or to discontinue denosumab and receive the standard intravenous dose of the bisphosphonate zoledronate at 5 mg at the time when the next dose of denosumab would have been administered.
There were no differences between the two groups in serum bone turnover markers at baseline.
The current results, reflecting the first year of the 2-year study, show that, overall, those receiving zoledronate (n = 76), had a significant decrease in lumbar spine BMD, compared with a slight increase in the denosumab continuation group (–0.68% vs 1.30%, respectively; P = .03).
No significant differences were observed between the groups in terms of the study’s other measures of total hip BMD (median, 0% vs 1.12%; P = .24), and femoral neck BMD (median, 0.18% vs 0.17%; P = .71).
Additional findings from multivariable analyses in the study also supported results from previous studies showing that a longer duration of denosumab use is associated with a more substantial rebound effect: Among 15 of the denosumab users in the study who had ≥ 3 prior years of the drug, the reduction in lumbar spine BMD was even greater with zoledronate compared with denosumab continuation (–3.20% vs 1.30%; P = .003).
Though the lack of losses in the other measures of total hip and femoral neck BMD may seem encouraging, evidence from the bulk of other studies suggests cautious interpretation of those findings, Fu said.
“Although our study did not observe a noticeable decline in total hip or femoral neck BMD, other randomized controlled trials with longer durations of denosumab use have reported significant reductions in these areas,” Fu said. “Therefore, it cannot be assumed that non-lumbar spine regions are entirely safe.”
Fracture Risk Is the Overriding Concern
Meanwhile, the loss of lumbar spine BMD is of particular concern because of its role in what amounts to the broader, overriding concern of denosumab discontinuation — the risk for fracture, Fu noted.
“Real-world observations indicate that fractures caused by or associated with discontinuation of denosumab primarily occur in the spine,” he explained.
Previous research underscores the risk for fracture with denosumab discontinuation — and the greater risk with longer-term denosumab use, showing an 11.8% annual incidence of vertebral fracture after discontinuation of denosumab used for less than 2 years, increasing to 16.0% upon discontinuation after more than 2 years of treatment.
Randomized trials have shown sequential zoledronate to have some benefit in offsetting that risk, reducing first-year fracture risk by 3%-4% in some studies.
In the current study, 3 of 76 participants experienced a vertebral fracture in the first year of discontinuation, all involving women, including 2 who had been receiving denosumab for ≥ 4 years before medication transition.
If a transition to a bisphosphonate is anticipated, the collective findings suggest doing it as early on in denosumab treatment as possible, Fu and his colleagues noted in the study.
“When medication transition from denosumab is expected or when long-term denosumab treatment may not be suitable, earlier medication transition with potent sequential therapy should be considered,” they wrote.
Dosing Adjustments?
The findings add to the evidence that “patients who gain the most with denosumab are likely to lose the most with zoledronate,” Nelson Watts, MD, who authored an editorial accompanying the study, told this news organization.
Furthermore, “denosumab and other medications seem to do more [and faster] for BMD in the spine, so we expect more loss in the spine than in the hip,” said Watts, who is director of Mercy Health Osteoporosis and Bone Health Services, Bon Secours Mercy Health in Cincinnati, Ohio.
“Studies are needed but not yet done to see if a higher dose or more frequent zoledronate would be better for BMD than the ‘usual’ yearly dose,” Watts added.
The only published clinical recommendations on the matter are discussed in a position paper from the European Calcified Tissue Society (ECTS).
“Pending additional robust data, a pragmatic approach is to begin treatment with zoledronate 6 months after the last denosumab injection and monitor the effect with bone turnover markers, for example, 3 and 6 months after the zoledronate infusion,” they recommended.
In cases of increased bone turnover markers, including above the mean found in age- and sex-matched cohorts, “repeated infusion of zoledronate should be considered,” the society added.
If bone turnover markers are not available for monitoring the patients, “a pragmatic approach could be administrating a second infusion of zoledronate 6 months after the first infusion,” they wrote.
Clinicians Need to Be Proactive From the Start
Bente Langdahl, MD, of the Medical Department of Endocrinology, Aarhus University Hospital in Denmark, who was a coauthor on the ECTS position statement, told this news organization that clinicians should also be proactive on the other side of treatment — before it begins — to prevent problems with discontinuation.
“I think denosumab is a very good treatment for some patients with high fracture risk and very low BMD, but both patients and clinicians should know that this treatment is either lifelong or there needs to be a plan for discontinuation,” Langdahl said.
Langdahl noted that denosumab is coming off patent soon; hence, issues with cost could become more manageable.
But until then, “I think [cost] should be considered before starting treatment because if patients cannot afford denosumab, they should have been started on zoledronate from the beginning.”
Discontinuation Reasons Vary
Research indicates that, broadly, adherence to denosumab ranges from about 45% to 72% at 2 years, with some reasons for discontinuation including the need for dental treatment or cost, Fu and colleagues reported.
Fu added, however, that other reasons for discontinuing denosumab “are not due to ‘need’ but rather factors such as relocating, missing follow-up appointments, or poor adherence.”
Lorenz Hofbauer, MD, who is head of the Division of Endocrinology, Diabetes, and Bone Diseases, Department of Medicine III at the Technical University Medical Center in Dresden, Germany, noted that another issue contributing to some hesitation by patients about remaining on, or even initiating denosumab, is the known risk for osteonecrosis of the jaw (ONJ).
Though reported as being rare, research continuing to stir concern for ONJ with denosumab use includes one recent study of patients with breast cancer showing those treated with denosumab had a fivefold higher risk for ONJ vs those on bisphosphonates.
“About 20% of my patients have ONJ concerns or other questions, which may delay treatment with denosumab or other therapies,” Hofbauer told this news organization.
“There is a high need to discuss risk versus benefits toward a shared decision-making,” he said.
Conversely, however, Hofbauer noted that adherence to denosumab at his center is fairly high — at 90%, which he says is largely credited to an electronically supported recall system in place at the center.
Denosumab maker Amgen also offers patient reminders via email, text, or phone through its Bone Matters patient support system, which also provides access to a call center for questions or to update treatment appointment information.
In terms of the ongoing question of how to best prevent fracture risk when patients do wind up discontinuing denosumab, Watts concluded in his editorial that more robust studies are needed.
“The dilemma is what to do with longer-term users who stop, and the real question is not what happens to BMD, but what happens to fracture risk,” he wrote.
“It is unlikely that the fracture risk question can be answered due to ethical limitations, but finding the best option, [whether it is] oral or intravenous bisphosphonate, timing, dose, and frequency, to minimize bone loss and the rebound increase in bone resorption after stopping long-term denosumab requires larger and longer studies of better design.”
The authors had no disclosures to report. Watts has been an investigator, consultant, and speaker for Amgen outside of the published editorial. Hofbauer is on advisory boards for Alexion Pharmaceuticals, Amolyt Pharma, Amgen, and UCB. Langdahl has been a primary investigator on previous and ongoing clinical trials involving denosumab.
A version of this article appeared on Medscape.com.
Patients who discontinue treatment with the osteoporosis drug denosumab, despite transitioning to zoledronate, show significant losses in lumbar spine bone mineral density (BMD) within a year, according to the latest findings to show that the rapid rebound of bone loss after denosumab discontinuation is not easily prevented with other therapies — even bisphosphonates.
“When initiating denosumab for osteoporosis treatment, it is recommended to engage in thorough shared decision-making with the patient to ensure they understand the potential risks associated with discontinuing the medication,” senior author Shau-Huai Fu, MD, PhD, Department of Orthopedics, National Taiwan University Hospital Yunlin Branch, Douliu, told this news organization.
Furthermore, “integrating a case manager system is crucial to support long-term adherence and compliance,” he added.
The results are from the Denosumab Sequential Therapy prospective, open-label, parallel-group randomized clinical trial, published online in JAMA Network Open.
In the study, 101 patients were recruited between April 2019 and May 2021 at a referral center and two hospitals in Taiwan. The patients, including postmenopausal women and men over the age of 50, had been treated with regular denosumab for at least 2 years and had no previous exposure to other anti-osteoporosis medication.
They were randomized to treatment either with continuous denosumab at the standard dose of 60 mg twice yearly or to discontinue denosumab and receive the standard intravenous dose of the bisphosphonate zoledronate at 5 mg at the time when the next dose of denosumab would have been administered.
There were no differences between the two groups in serum bone turnover markers at baseline.
The current results, reflecting the first year of the 2-year study, show that, overall, those receiving zoledronate (n = 76), had a significant decrease in lumbar spine BMD, compared with a slight increase in the denosumab continuation group (–0.68% vs 1.30%, respectively; P = .03).
No significant differences were observed between the groups in terms of the study’s other measures of total hip BMD (median, 0% vs 1.12%; P = .24), and femoral neck BMD (median, 0.18% vs 0.17%; P = .71).
Additional findings from multivariable analyses in the study also supported results from previous studies showing that a longer duration of denosumab use is associated with a more substantial rebound effect: Among 15 of the denosumab users in the study who had ≥ 3 prior years of the drug, the reduction in lumbar spine BMD was even greater with zoledronate compared with denosumab continuation (–3.20% vs 1.30%; P = .003).
Though the lack of losses in the other measures of total hip and femoral neck BMD may seem encouraging, evidence from the bulk of other studies suggests cautious interpretation of those findings, Fu said.
“Although our study did not observe a noticeable decline in total hip or femoral neck BMD, other randomized controlled trials with longer durations of denosumab use have reported significant reductions in these areas,” Fu said. “Therefore, it cannot be assumed that non-lumbar spine regions are entirely safe.”
Fracture Risk Is the Overriding Concern
Meanwhile, the loss of lumbar spine BMD is of particular concern because of its role in what amounts to the broader, overriding concern of denosumab discontinuation — the risk for fracture, Fu noted.
“Real-world observations indicate that fractures caused by or associated with discontinuation of denosumab primarily occur in the spine,” he explained.
Previous research underscores the risk for fracture with denosumab discontinuation — and the greater risk with longer-term denosumab use, showing an 11.8% annual incidence of vertebral fracture after discontinuation of denosumab used for less than 2 years, increasing to 16.0% upon discontinuation after more than 2 years of treatment.
Randomized trials have shown sequential zoledronate to have some benefit in offsetting that risk, reducing first-year fracture risk by 3%-4% in some studies.
In the current study, 3 of 76 participants experienced a vertebral fracture in the first year of discontinuation, all involving women, including 2 who had been receiving denosumab for ≥ 4 years before medication transition.
If a transition to a bisphosphonate is anticipated, the collective findings suggest doing it as early on in denosumab treatment as possible, Fu and his colleagues noted in the study.
“When medication transition from denosumab is expected or when long-term denosumab treatment may not be suitable, earlier medication transition with potent sequential therapy should be considered,” they wrote.
Dosing Adjustments?
The findings add to the evidence that “patients who gain the most with denosumab are likely to lose the most with zoledronate,” Nelson Watts, MD, who authored an editorial accompanying the study, told this news organization.
Furthermore, “denosumab and other medications seem to do more [and faster] for BMD in the spine, so we expect more loss in the spine than in the hip,” said Watts, who is director of Mercy Health Osteoporosis and Bone Health Services, Bon Secours Mercy Health in Cincinnati, Ohio.
“Studies are needed but not yet done to see if a higher dose or more frequent zoledronate would be better for BMD than the ‘usual’ yearly dose,” Watts added.
The only published clinical recommendations on the matter are discussed in a position paper from the European Calcified Tissue Society (ECTS).
“Pending additional robust data, a pragmatic approach is to begin treatment with zoledronate 6 months after the last denosumab injection and monitor the effect with bone turnover markers, for example, 3 and 6 months after the zoledronate infusion,” they recommended.
In cases of increased bone turnover markers, including above the mean found in age- and sex-matched cohorts, “repeated infusion of zoledronate should be considered,” the society added.
If bone turnover markers are not available for monitoring the patients, “a pragmatic approach could be administrating a second infusion of zoledronate 6 months after the first infusion,” they wrote.
Clinicians Need to Be Proactive From the Start
Bente Langdahl, MD, of the Medical Department of Endocrinology, Aarhus University Hospital in Denmark, who was a coauthor on the ECTS position statement, told this news organization that clinicians should also be proactive on the other side of treatment — before it begins — to prevent problems with discontinuation.
“I think denosumab is a very good treatment for some patients with high fracture risk and very low BMD, but both patients and clinicians should know that this treatment is either lifelong or there needs to be a plan for discontinuation,” Langdahl said.
Langdahl noted that denosumab is coming off patent soon; hence, issues with cost could become more manageable.
But until then, “I think [cost] should be considered before starting treatment because if patients cannot afford denosumab, they should have been started on zoledronate from the beginning.”
Discontinuation Reasons Vary
Research indicates that, broadly, adherence to denosumab ranges from about 45% to 72% at 2 years, with some reasons for discontinuation including the need for dental treatment or cost, Fu and colleagues reported.
Fu added, however, that other reasons for discontinuing denosumab “are not due to ‘need’ but rather factors such as relocating, missing follow-up appointments, or poor adherence.”
Lorenz Hofbauer, MD, who is head of the Division of Endocrinology, Diabetes, and Bone Diseases, Department of Medicine III at the Technical University Medical Center in Dresden, Germany, noted that another issue contributing to some hesitation by patients about remaining on, or even initiating denosumab, is the known risk for osteonecrosis of the jaw (ONJ).
Though reported as being rare, research continuing to stir concern for ONJ with denosumab use includes one recent study of patients with breast cancer showing those treated with denosumab had a fivefold higher risk for ONJ vs those on bisphosphonates.
“About 20% of my patients have ONJ concerns or other questions, which may delay treatment with denosumab or other therapies,” Hofbauer told this news organization.
“There is a high need to discuss risk versus benefits toward a shared decision-making,” he said.
Conversely, however, Hofbauer noted that adherence to denosumab at his center is fairly high — at 90%, which he says is largely credited to an electronically supported recall system in place at the center.
Denosumab maker Amgen also offers patient reminders via email, text, or phone through its Bone Matters patient support system, which also provides access to a call center for questions or to update treatment appointment information.
In terms of the ongoing question of how to best prevent fracture risk when patients do wind up discontinuing denosumab, Watts concluded in his editorial that more robust studies are needed.
“The dilemma is what to do with longer-term users who stop, and the real question is not what happens to BMD, but what happens to fracture risk,” he wrote.
“It is unlikely that the fracture risk question can be answered due to ethical limitations, but finding the best option, [whether it is] oral or intravenous bisphosphonate, timing, dose, and frequency, to minimize bone loss and the rebound increase in bone resorption after stopping long-term denosumab requires larger and longer studies of better design.”
The authors had no disclosures to report. Watts has been an investigator, consultant, and speaker for Amgen outside of the published editorial. Hofbauer is on advisory boards for Alexion Pharmaceuticals, Amolyt Pharma, Amgen, and UCB. Langdahl has been a primary investigator on previous and ongoing clinical trials involving denosumab.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Sustained Benefits With TransCon PTH in Hypoparathyroidism
TOPLINE:
Long-term treatment with TransCon parathyroid hormone (PTH), a replacement therapy for hypoparathyroidism, demonstrates sustained efficacy and safety in patients with hypoparathyroidism over 52 weeks, with 95% of participants able to discontinue conventional therapy.
METHODOLOGY:
- Conventional therapy for hypoparathyroidism (active vitamin D and elemental calcium) alleviates symptoms of hypocalcemia, but it does not improve insufficient PTH levels and is linked to long-term complications, such as nephrocalcinosis, nephrolithiasis, and renal dysfunction.
- This phase 3 (PaTHway) trial aimed to investigate the long-term efficacy, safety, and tolerability of TransCon PTH (palopegteriparatide) in adults with hypoparathyroidism.
- Overall, 82 patients with chronic hypoparathyroidism (mean age, 48.6 years; 78% women; 93% White) were randomly assigned to receive TransCon PTH or placebo, both coadministered with conventional therapy for 26 weeks.
- At the 26-week visit, patients who completed the blinded treatment (n = 79) were assigned to receive only TransCon PTH with conventional therapy in an ongoing 156-week open-label extension.
- For this analysis at week 52, the main efficacy endpoint was the proportion of patients (n = 78) with normal serum calcium levels (8.3-10.6 mg/dL) and independence from conventional therapy (active vitamin D and therapeutic doses of calcium); safety assessments included serum chemistries, 24-hour urine calcium excretion, and treatment-emergent adverse events.
TAKEAWAY:
- At week 52, the majority of the patients receiving TransCon PTH achieved normal serum calcium levels within the normal range (86%) and independence from conventional therapy (95%). None required active vitamin D.
- In secondary endpoints, patients receiving TransCon PTH showed sustained improvement in Hypoparathyroidism Patient Experience Scale scores, reflecting better symptom management, enhanced functioning, and overall well-being through week 52.
- At week 52, the mean 24-hour urine calcium excretion in patients first randomized to TransCon PTH was 185.1 mg/d, remaining well below the upper limit of normal (≤ 250 mg/d), while the placebo group mean fell to 223.1 mg/d during the open-label extension of TransCon PTH.
- TransCon PTH was well-tolerated, with most treatment-emergent adverse events being mild or moderate and none leading to treatment discontinuation.
IN PRACTICE:
“These results suggest that TransCon PTH may improve outcomes and advance the standard of care for adults living with hypoparathyroidism,” the authors wrote.
SOURCE:
The study was led by Bart L. Clarke, MD, Mayo Clinic, Rochester, Minnesota. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study’s limitations included the open-label design during the extension period, which may have introduced bias in patient-reported outcomes. Additionally, the study population was predominantly women and White, which may have limited the generalizability of the findings. Further research is needed to assess the long-term effects of TransCon PTH on renal complications. One patient died of fatal cardiac arrest deemed unrelated to the study drug.
DISCLOSURES:
The study was funded by Ascendis Pharma A/S. Seven authors declared being current or former employees of Ascendis Pharma. The other authors declared receiving grants, research funding, honoraria, serving as consultants, advisory board members, study investigators, and other ties with Ascendis Pharma and multiple other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term treatment with TransCon parathyroid hormone (PTH), a replacement therapy for hypoparathyroidism, demonstrates sustained efficacy and safety in patients with hypoparathyroidism over 52 weeks, with 95% of participants able to discontinue conventional therapy.
METHODOLOGY:
- Conventional therapy for hypoparathyroidism (active vitamin D and elemental calcium) alleviates symptoms of hypocalcemia, but it does not improve insufficient PTH levels and is linked to long-term complications, such as nephrocalcinosis, nephrolithiasis, and renal dysfunction.
- This phase 3 (PaTHway) trial aimed to investigate the long-term efficacy, safety, and tolerability of TransCon PTH (palopegteriparatide) in adults with hypoparathyroidism.
- Overall, 82 patients with chronic hypoparathyroidism (mean age, 48.6 years; 78% women; 93% White) were randomly assigned to receive TransCon PTH or placebo, both coadministered with conventional therapy for 26 weeks.
- At the 26-week visit, patients who completed the blinded treatment (n = 79) were assigned to receive only TransCon PTH with conventional therapy in an ongoing 156-week open-label extension.
- For this analysis at week 52, the main efficacy endpoint was the proportion of patients (n = 78) with normal serum calcium levels (8.3-10.6 mg/dL) and independence from conventional therapy (active vitamin D and therapeutic doses of calcium); safety assessments included serum chemistries, 24-hour urine calcium excretion, and treatment-emergent adverse events.
TAKEAWAY:
- At week 52, the majority of the patients receiving TransCon PTH achieved normal serum calcium levels within the normal range (86%) and independence from conventional therapy (95%). None required active vitamin D.
- In secondary endpoints, patients receiving TransCon PTH showed sustained improvement in Hypoparathyroidism Patient Experience Scale scores, reflecting better symptom management, enhanced functioning, and overall well-being through week 52.
- At week 52, the mean 24-hour urine calcium excretion in patients first randomized to TransCon PTH was 185.1 mg/d, remaining well below the upper limit of normal (≤ 250 mg/d), while the placebo group mean fell to 223.1 mg/d during the open-label extension of TransCon PTH.
- TransCon PTH was well-tolerated, with most treatment-emergent adverse events being mild or moderate and none leading to treatment discontinuation.
IN PRACTICE:
“These results suggest that TransCon PTH may improve outcomes and advance the standard of care for adults living with hypoparathyroidism,” the authors wrote.
SOURCE:
The study was led by Bart L. Clarke, MD, Mayo Clinic, Rochester, Minnesota. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study’s limitations included the open-label design during the extension period, which may have introduced bias in patient-reported outcomes. Additionally, the study population was predominantly women and White, which may have limited the generalizability of the findings. Further research is needed to assess the long-term effects of TransCon PTH on renal complications. One patient died of fatal cardiac arrest deemed unrelated to the study drug.
DISCLOSURES:
The study was funded by Ascendis Pharma A/S. Seven authors declared being current or former employees of Ascendis Pharma. The other authors declared receiving grants, research funding, honoraria, serving as consultants, advisory board members, study investigators, and other ties with Ascendis Pharma and multiple other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term treatment with TransCon parathyroid hormone (PTH), a replacement therapy for hypoparathyroidism, demonstrates sustained efficacy and safety in patients with hypoparathyroidism over 52 weeks, with 95% of participants able to discontinue conventional therapy.
METHODOLOGY:
- Conventional therapy for hypoparathyroidism (active vitamin D and elemental calcium) alleviates symptoms of hypocalcemia, but it does not improve insufficient PTH levels and is linked to long-term complications, such as nephrocalcinosis, nephrolithiasis, and renal dysfunction.
- This phase 3 (PaTHway) trial aimed to investigate the long-term efficacy, safety, and tolerability of TransCon PTH (palopegteriparatide) in adults with hypoparathyroidism.
- Overall, 82 patients with chronic hypoparathyroidism (mean age, 48.6 years; 78% women; 93% White) were randomly assigned to receive TransCon PTH or placebo, both coadministered with conventional therapy for 26 weeks.
- At the 26-week visit, patients who completed the blinded treatment (n = 79) were assigned to receive only TransCon PTH with conventional therapy in an ongoing 156-week open-label extension.
- For this analysis at week 52, the main efficacy endpoint was the proportion of patients (n = 78) with normal serum calcium levels (8.3-10.6 mg/dL) and independence from conventional therapy (active vitamin D and therapeutic doses of calcium); safety assessments included serum chemistries, 24-hour urine calcium excretion, and treatment-emergent adverse events.
TAKEAWAY:
- At week 52, the majority of the patients receiving TransCon PTH achieved normal serum calcium levels within the normal range (86%) and independence from conventional therapy (95%). None required active vitamin D.
- In secondary endpoints, patients receiving TransCon PTH showed sustained improvement in Hypoparathyroidism Patient Experience Scale scores, reflecting better symptom management, enhanced functioning, and overall well-being through week 52.
- At week 52, the mean 24-hour urine calcium excretion in patients first randomized to TransCon PTH was 185.1 mg/d, remaining well below the upper limit of normal (≤ 250 mg/d), while the placebo group mean fell to 223.1 mg/d during the open-label extension of TransCon PTH.
- TransCon PTH was well-tolerated, with most treatment-emergent adverse events being mild or moderate and none leading to treatment discontinuation.
IN PRACTICE:
“These results suggest that TransCon PTH may improve outcomes and advance the standard of care for adults living with hypoparathyroidism,” the authors wrote.
SOURCE:
The study was led by Bart L. Clarke, MD, Mayo Clinic, Rochester, Minnesota. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study’s limitations included the open-label design during the extension period, which may have introduced bias in patient-reported outcomes. Additionally, the study population was predominantly women and White, which may have limited the generalizability of the findings. Further research is needed to assess the long-term effects of TransCon PTH on renal complications. One patient died of fatal cardiac arrest deemed unrelated to the study drug.
DISCLOSURES:
The study was funded by Ascendis Pharma A/S. Seven authors declared being current or former employees of Ascendis Pharma. The other authors declared receiving grants, research funding, honoraria, serving as consultants, advisory board members, study investigators, and other ties with Ascendis Pharma and multiple other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Risk Assessment Tool Can Help Predict Fractures in Cancer
TOPLINE:
METHODOLOGY:
- Cancer-specific guidelines recommend using FRAX to assess fracture risk, but its applicability in patients with cancer remains unclear.
- This retrospective cohort study included 9877 patients with cancer (mean age, 67.1 years) and 45,875 matched control individuals without cancer (mean age, 66.2 years). All participants had dual-energy x-ray absorptiometry (DXA) scans.
- Researchers collected data on bone mineral density and fractures. The 10-year probabilities of major osteoporotic fractures and hip fractures were calculated using FRAX, and the observed 10-year probabilities of these fractures were compared with FRAX-derived probabilities.
- Compared with individuals without cancer, patients with cancer had a shorter mean follow-up duration (8.5 vs 7.6 years), a slightly higher mean body mass index, and a higher percentage of parental hip fractures (7.0% vs 8.2%); additionally, patients with cancer were more likely to have secondary causes of osteoporosis (10% vs 38.4%) and less likely to receive osteoporosis medication (9.9% vs 4.2%).
TAKEAWAY:
- Compared with individuals without cancer, patients with cancer had a significantly higher incidence rate of major fractures (12.9 vs 14.5 per 1000 person-years) and hip fractures (3.5 vs 4.2 per 1000 person-years).
- FRAX with bone mineral density exhibited excellent calibration for predicting major osteoporotic fractures (slope, 1.03) and hip fractures (0.97) in patients with cancer, regardless of the site of cancer diagnosis. FRAX without bone mineral density, however, underestimated the risk for both major (0.87) and hip fractures (0.72).
- In patients with cancer, FRAX with bone mineral density findings were associated with incident major osteoporotic fractures (hazard ratio [HR] per SD, 1.84) and hip fractures (HR per SD, 3.61).
- When models were adjusted for FRAX with bone mineral density, patients with cancer had an increased risk for both major osteoporotic fractures (HR, 1.17) and hip fractures (HR, 1.30). No difference was found in the risk for fracture between patients with and individuals without cancer when the models were adjusted for FRAX without bone mineral density, even when considering osteoporosis medication use.
IN PRACTICE:
“This retrospective cohort study demonstrates that individuals with cancer are at higher risk of fracture than individuals without cancer and that FRAX, particularly with BMD [bone mineral density], may accurately predict fracture risk in this population. These results, along with the known mortality risk of osteoporotic fractures among cancer survivors, further emphasize the clinical importance of closing the current osteoporosis care gap among cancer survivors,” the authors wrote.
SOURCE:
This study, led by Carrie Ye, MD, MPH, University of Alberta, Edmonton, Alberta, Canada, was published online in JAMA Oncology.
LIMITATIONS:
This study cohort included a selected group of cancer survivors who were referred for DXA scans and may not represent the general cancer population. The cohort consisted predominantly of women, limiting the generalizability to men with cancer. Given the heterogeneity of the population, the findings may not be applicable to all cancer subgroups. Information on cancer stage or the presence of bone metastases at the time of fracture risk assessment was lacking, which could have affected the findings.
DISCLOSURES:
This study was funded by the CancerCare Manitoba Foundation. Three authors reported having ties with various sources, including two who received grants from various organizations.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cancer-specific guidelines recommend using FRAX to assess fracture risk, but its applicability in patients with cancer remains unclear.
- This retrospective cohort study included 9877 patients with cancer (mean age, 67.1 years) and 45,875 matched control individuals without cancer (mean age, 66.2 years). All participants had dual-energy x-ray absorptiometry (DXA) scans.
- Researchers collected data on bone mineral density and fractures. The 10-year probabilities of major osteoporotic fractures and hip fractures were calculated using FRAX, and the observed 10-year probabilities of these fractures were compared with FRAX-derived probabilities.
- Compared with individuals without cancer, patients with cancer had a shorter mean follow-up duration (8.5 vs 7.6 years), a slightly higher mean body mass index, and a higher percentage of parental hip fractures (7.0% vs 8.2%); additionally, patients with cancer were more likely to have secondary causes of osteoporosis (10% vs 38.4%) and less likely to receive osteoporosis medication (9.9% vs 4.2%).
TAKEAWAY:
- Compared with individuals without cancer, patients with cancer had a significantly higher incidence rate of major fractures (12.9 vs 14.5 per 1000 person-years) and hip fractures (3.5 vs 4.2 per 1000 person-years).
- FRAX with bone mineral density exhibited excellent calibration for predicting major osteoporotic fractures (slope, 1.03) and hip fractures (0.97) in patients with cancer, regardless of the site of cancer diagnosis. FRAX without bone mineral density, however, underestimated the risk for both major (0.87) and hip fractures (0.72).
- In patients with cancer, FRAX with bone mineral density findings were associated with incident major osteoporotic fractures (hazard ratio [HR] per SD, 1.84) and hip fractures (HR per SD, 3.61).
- When models were adjusted for FRAX with bone mineral density, patients with cancer had an increased risk for both major osteoporotic fractures (HR, 1.17) and hip fractures (HR, 1.30). No difference was found in the risk for fracture between patients with and individuals without cancer when the models were adjusted for FRAX without bone mineral density, even when considering osteoporosis medication use.
IN PRACTICE:
“This retrospective cohort study demonstrates that individuals with cancer are at higher risk of fracture than individuals without cancer and that FRAX, particularly with BMD [bone mineral density], may accurately predict fracture risk in this population. These results, along with the known mortality risk of osteoporotic fractures among cancer survivors, further emphasize the clinical importance of closing the current osteoporosis care gap among cancer survivors,” the authors wrote.
SOURCE:
This study, led by Carrie Ye, MD, MPH, University of Alberta, Edmonton, Alberta, Canada, was published online in JAMA Oncology.
LIMITATIONS:
This study cohort included a selected group of cancer survivors who were referred for DXA scans and may not represent the general cancer population. The cohort consisted predominantly of women, limiting the generalizability to men with cancer. Given the heterogeneity of the population, the findings may not be applicable to all cancer subgroups. Information on cancer stage or the presence of bone metastases at the time of fracture risk assessment was lacking, which could have affected the findings.
DISCLOSURES:
This study was funded by the CancerCare Manitoba Foundation. Three authors reported having ties with various sources, including two who received grants from various organizations.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cancer-specific guidelines recommend using FRAX to assess fracture risk, but its applicability in patients with cancer remains unclear.
- This retrospective cohort study included 9877 patients with cancer (mean age, 67.1 years) and 45,875 matched control individuals without cancer (mean age, 66.2 years). All participants had dual-energy x-ray absorptiometry (DXA) scans.
- Researchers collected data on bone mineral density and fractures. The 10-year probabilities of major osteoporotic fractures and hip fractures were calculated using FRAX, and the observed 10-year probabilities of these fractures were compared with FRAX-derived probabilities.
- Compared with individuals without cancer, patients with cancer had a shorter mean follow-up duration (8.5 vs 7.6 years), a slightly higher mean body mass index, and a higher percentage of parental hip fractures (7.0% vs 8.2%); additionally, patients with cancer were more likely to have secondary causes of osteoporosis (10% vs 38.4%) and less likely to receive osteoporosis medication (9.9% vs 4.2%).
TAKEAWAY:
- Compared with individuals without cancer, patients with cancer had a significantly higher incidence rate of major fractures (12.9 vs 14.5 per 1000 person-years) and hip fractures (3.5 vs 4.2 per 1000 person-years).
- FRAX with bone mineral density exhibited excellent calibration for predicting major osteoporotic fractures (slope, 1.03) and hip fractures (0.97) in patients with cancer, regardless of the site of cancer diagnosis. FRAX without bone mineral density, however, underestimated the risk for both major (0.87) and hip fractures (0.72).
- In patients with cancer, FRAX with bone mineral density findings were associated with incident major osteoporotic fractures (hazard ratio [HR] per SD, 1.84) and hip fractures (HR per SD, 3.61).
- When models were adjusted for FRAX with bone mineral density, patients with cancer had an increased risk for both major osteoporotic fractures (HR, 1.17) and hip fractures (HR, 1.30). No difference was found in the risk for fracture between patients with and individuals without cancer when the models were adjusted for FRAX without bone mineral density, even when considering osteoporosis medication use.
IN PRACTICE:
“This retrospective cohort study demonstrates that individuals with cancer are at higher risk of fracture than individuals without cancer and that FRAX, particularly with BMD [bone mineral density], may accurately predict fracture risk in this population. These results, along with the known mortality risk of osteoporotic fractures among cancer survivors, further emphasize the clinical importance of closing the current osteoporosis care gap among cancer survivors,” the authors wrote.
SOURCE:
This study, led by Carrie Ye, MD, MPH, University of Alberta, Edmonton, Alberta, Canada, was published online in JAMA Oncology.
LIMITATIONS:
This study cohort included a selected group of cancer survivors who were referred for DXA scans and may not represent the general cancer population. The cohort consisted predominantly of women, limiting the generalizability to men with cancer. Given the heterogeneity of the population, the findings may not be applicable to all cancer subgroups. Information on cancer stage or the presence of bone metastases at the time of fracture risk assessment was lacking, which could have affected the findings.
DISCLOSURES:
This study was funded by the CancerCare Manitoba Foundation. Three authors reported having ties with various sources, including two who received grants from various organizations.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.