User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Skip vasoreactivity testing in connective tissue disease PAH work-up
LAS VEGAS – It’s okay to skip vasoreactivity testing when working up connective tissue disease patients for suspected pulmonary artery hypertension.
“If you are in a smaller town and your cardiologist doesn’t do vasoreactivity testing, don’t worry about it. You don’t really need it. It’s no longer recommended for patients with scleroderma and connective tissue disease” because they are not vasoreactive, said Dr. Kristin Highland, a pulmonologist and rheumatologist at the Cleveland Clinic.
Right heart catheterization, however, remains the standard for pulmonary artery hypertension (PAH) diagnosis, which requires a mean, resting pulmonary arterial pressure at or above 25 mm Hg with normal pulmonary capillary wedge pressure but increased pulmonary vascular resistance.
Treatment is generally based on functional class, but “I like to look at more data than just the functional class,” Dr. Highland said at the annual Perspectives in Rheumatic Diseases, held by the Global Academy for Medical Education.
Right ventricular heart failure, poor walk tests, and markedly elevated brain natriuretic peptide are among the signs of aggressive disease. Pericardial effusion in the setting of pulmonary hypertension and high right arterial pressure with low cardiac output suggest a dire situation. “If I have any [such findings], I am thinking parenteral and combination therapy;” stand-alone oral therapies are generally for the less sick, she said.
It’s important to rule out pulmonary venous occlusive disease (PVOD) before treating PAH. “These are patients who behave like PAH, but they have pulmonary venule involvement,” as well, and can be thrown into pulmonary edema by specific PAH therapies. Computed tomography can help identify PVOD in scleroderma (Arthritis Rheum. 2012;64[9]:2995-3005). “There are really no good options for these patients except transplant,” Dr. Highland said.
PAH can occur in all connective tissue diseases, but it’s most likely in scleroderma, with a prevalence of about 20%.
Survival improves if disease is caught early, so it’s generally recommended that scleroderma patients be screened by Doppler echocardiography at least yearly. It will capture right-sided hypertrophy, tricuspid regurgitation, pulmonary artery dilation, and other indications that further work-up is warranted.
Although echo is the most useful screening test, there are other noninvasive options. For instance, a decline in the diffusing capacity for carbon monoxide is perhaps one of the earliest signs of pending PAH in scleroderma. Meanwhile, an N-terminal brain natriuretic peptide level of 395 pg/mL has 56% sensitivity and 95% specificity for the presence of PAH. A ratio of forced vital capacity to diffusion capacity above 2 is 71% sensitive and 72% specific (J Rheumatol. 2008;35[3]:458-65).
“Their FVC [forced vital capacity] might be down a little bit, but if their diffusion is way down out of proportion to the FVC, they end up with an elevated ratio. Those patients probably have some pulmonary hypertension along with their interstitial lung disease,” Dr. Highland said.
Dr. Highland is a consultant for Boehringer Ingelheim, an adviser for Bayer Healthcare, and a speaker for Bayer, Actelion, Intermune, Gilead, Genentech, Lung Biotechnology, and United Therapeutics. The Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – It’s okay to skip vasoreactivity testing when working up connective tissue disease patients for suspected pulmonary artery hypertension.
“If you are in a smaller town and your cardiologist doesn’t do vasoreactivity testing, don’t worry about it. You don’t really need it. It’s no longer recommended for patients with scleroderma and connective tissue disease” because they are not vasoreactive, said Dr. Kristin Highland, a pulmonologist and rheumatologist at the Cleveland Clinic.
Right heart catheterization, however, remains the standard for pulmonary artery hypertension (PAH) diagnosis, which requires a mean, resting pulmonary arterial pressure at or above 25 mm Hg with normal pulmonary capillary wedge pressure but increased pulmonary vascular resistance.
Treatment is generally based on functional class, but “I like to look at more data than just the functional class,” Dr. Highland said at the annual Perspectives in Rheumatic Diseases, held by the Global Academy for Medical Education.
Right ventricular heart failure, poor walk tests, and markedly elevated brain natriuretic peptide are among the signs of aggressive disease. Pericardial effusion in the setting of pulmonary hypertension and high right arterial pressure with low cardiac output suggest a dire situation. “If I have any [such findings], I am thinking parenteral and combination therapy;” stand-alone oral therapies are generally for the less sick, she said.
It’s important to rule out pulmonary venous occlusive disease (PVOD) before treating PAH. “These are patients who behave like PAH, but they have pulmonary venule involvement,” as well, and can be thrown into pulmonary edema by specific PAH therapies. Computed tomography can help identify PVOD in scleroderma (Arthritis Rheum. 2012;64[9]:2995-3005). “There are really no good options for these patients except transplant,” Dr. Highland said.
PAH can occur in all connective tissue diseases, but it’s most likely in scleroderma, with a prevalence of about 20%.
Survival improves if disease is caught early, so it’s generally recommended that scleroderma patients be screened by Doppler echocardiography at least yearly. It will capture right-sided hypertrophy, tricuspid regurgitation, pulmonary artery dilation, and other indications that further work-up is warranted.
Although echo is the most useful screening test, there are other noninvasive options. For instance, a decline in the diffusing capacity for carbon monoxide is perhaps one of the earliest signs of pending PAH in scleroderma. Meanwhile, an N-terminal brain natriuretic peptide level of 395 pg/mL has 56% sensitivity and 95% specificity for the presence of PAH. A ratio of forced vital capacity to diffusion capacity above 2 is 71% sensitive and 72% specific (J Rheumatol. 2008;35[3]:458-65).
“Their FVC [forced vital capacity] might be down a little bit, but if their diffusion is way down out of proportion to the FVC, they end up with an elevated ratio. Those patients probably have some pulmonary hypertension along with their interstitial lung disease,” Dr. Highland said.
Dr. Highland is a consultant for Boehringer Ingelheim, an adviser for Bayer Healthcare, and a speaker for Bayer, Actelion, Intermune, Gilead, Genentech, Lung Biotechnology, and United Therapeutics. The Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – It’s okay to skip vasoreactivity testing when working up connective tissue disease patients for suspected pulmonary artery hypertension.
“If you are in a smaller town and your cardiologist doesn’t do vasoreactivity testing, don’t worry about it. You don’t really need it. It’s no longer recommended for patients with scleroderma and connective tissue disease” because they are not vasoreactive, said Dr. Kristin Highland, a pulmonologist and rheumatologist at the Cleveland Clinic.
Right heart catheterization, however, remains the standard for pulmonary artery hypertension (PAH) diagnosis, which requires a mean, resting pulmonary arterial pressure at or above 25 mm Hg with normal pulmonary capillary wedge pressure but increased pulmonary vascular resistance.
Treatment is generally based on functional class, but “I like to look at more data than just the functional class,” Dr. Highland said at the annual Perspectives in Rheumatic Diseases, held by the Global Academy for Medical Education.
Right ventricular heart failure, poor walk tests, and markedly elevated brain natriuretic peptide are among the signs of aggressive disease. Pericardial effusion in the setting of pulmonary hypertension and high right arterial pressure with low cardiac output suggest a dire situation. “If I have any [such findings], I am thinking parenteral and combination therapy;” stand-alone oral therapies are generally for the less sick, she said.
It’s important to rule out pulmonary venous occlusive disease (PVOD) before treating PAH. “These are patients who behave like PAH, but they have pulmonary venule involvement,” as well, and can be thrown into pulmonary edema by specific PAH therapies. Computed tomography can help identify PVOD in scleroderma (Arthritis Rheum. 2012;64[9]:2995-3005). “There are really no good options for these patients except transplant,” Dr. Highland said.
PAH can occur in all connective tissue diseases, but it’s most likely in scleroderma, with a prevalence of about 20%.
Survival improves if disease is caught early, so it’s generally recommended that scleroderma patients be screened by Doppler echocardiography at least yearly. It will capture right-sided hypertrophy, tricuspid regurgitation, pulmonary artery dilation, and other indications that further work-up is warranted.
Although echo is the most useful screening test, there are other noninvasive options. For instance, a decline in the diffusing capacity for carbon monoxide is perhaps one of the earliest signs of pending PAH in scleroderma. Meanwhile, an N-terminal brain natriuretic peptide level of 395 pg/mL has 56% sensitivity and 95% specificity for the presence of PAH. A ratio of forced vital capacity to diffusion capacity above 2 is 71% sensitive and 72% specific (J Rheumatol. 2008;35[3]:458-65).
“Their FVC [forced vital capacity] might be down a little bit, but if their diffusion is way down out of proportion to the FVC, they end up with an elevated ratio. Those patients probably have some pulmonary hypertension along with their interstitial lung disease,” Dr. Highland said.
Dr. Highland is a consultant for Boehringer Ingelheim, an adviser for Bayer Healthcare, and a speaker for Bayer, Actelion, Intermune, Gilead, Genentech, Lung Biotechnology, and United Therapeutics. The Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Gout treatment lore doesn’t hold up to evidence
LAS VEGAS – It’s okay to start urate lowering therapy during a gout attack, according to Dr. Brian Mandell, a rheumatology professor at the Cleveland Clinic.
“I was taught that you don’t treat a gout attack with urate lowering therapy” because it could trigger a subsequent mobilization attack. “This has been the paradigm for years,” he said at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
But a 2012 study casts doubt on that. In the study, 26 men hospitalized for gout attack were randomized to 300 mg allopurinol daily, while 25 were randomized to placebo. All of the patients were on indomethacin and colchicine. Urate levels dropped rapidly to below 6 mg/dL in the allopurinol group, but there were only two flares. Placebo subjects, meanwhile, had three flares (P= 0.60). There was no significant difference in daily pain scores (Am J Med. 2012 Nov;125(11):1126-1134.e7.).
“The bottom line is the groups did essentially the same. If you are using prophylaxis to treat an acute attack, you are probably perfectly fine to use allopurinol; you don’t need to wait,” Dr. Mandell said. “Does this mean you should start everybody in the hospital on full-dose allopurinol? No, but I think this really broadens our thought processes in terms of handling urate lowering therapy.”
It’s also time to rethink allopurinol dose adjustments in patients with chronic kidney disease (CKD). The thought has been that these patients should be on lower doses to prevent metabolite buildup in the kidneys and hypersensitivity reactions, but “if you follow those guidelines, less than 20% of patients will lower their serum urate, so you will be giving the drug for nothing,” Dr. Mandell said.
“It is true that allopurinol hypersensitivity is more common in patients with CKD,” but it happens in a few per thousand people, and hypersensitivity might have nothing to do with allopurinol dose. “There’s no repeated documentation between the levels and reactions, and in a number of small studies there is no evidence that dose adjustment decreases the frequency of hypersensitivity reactions. We have no data to say this is the right route to take,” he said.
In fact, a study that included 45 CKD patients found that increasing allopurinol beyond creatinine clearance-based dosing doesn’t cause problems. “Toxicity was not increased in patients receiving higher doses of allopurinol, including those with renal impairment,” the authors concluded (Arthritis Rheum. 2011 Feb;63(2):412-21.).
Given the rarity of hypersensitivity reactions, it’s not surprising they didn’t occur in the study’s small group of CKD patients, Dr. Mandell said. “If you are really ultra conservative, you are [still] not going to use allopurinol in CKD,” he said.
But Dr. Mandell said he starts patients on 50 mg of allopurinol. “I educate them about allergic reactions and increase the dose every 1-2 weeks until I hit” a serum uric acid below 6 mg/dL, which often takes more than 300 mg per day. “Alternatively, you can start febuxostat, and if I were to do that I would start at 20 mg” – breaking the lowest dose pill of 40 mg in half – “and then titrate up,” he said.
“It just doesn’t make sense,” Dr. Mandell said, that allopurinol hypersensitivity is related to dose. Instead, there seems to be a genetic predisposition for toxicity in, for instance, Asian patients with the HLA-B*5801 genotype, he noted.
Pegloticase is a bit more problematic than allopurinol. It’s highly effective – patients can drop their serum uric acid from 14 to 0.1 mg/dL within hours – but that comes at the cost of a high mobilization flair rate and infusion reactions in about a quarter of patients, Dr. Mandell said.
There’s a partial workaround. Dr. Mandell said he checks the uric acid level before the second infusion. If it’s not below 6 mg/dL after the first infusion, patients have antibodies against pegloticase. “The drug isn’t going to work, and patients are more likely to get an allergic reaction, so stop the drug,” he said.
Dr. Mandell is a consultant for AstraZeneca and Crealta Pharmaceuticals. The Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – It’s okay to start urate lowering therapy during a gout attack, according to Dr. Brian Mandell, a rheumatology professor at the Cleveland Clinic.
“I was taught that you don’t treat a gout attack with urate lowering therapy” because it could trigger a subsequent mobilization attack. “This has been the paradigm for years,” he said at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
But a 2012 study casts doubt on that. In the study, 26 men hospitalized for gout attack were randomized to 300 mg allopurinol daily, while 25 were randomized to placebo. All of the patients were on indomethacin and colchicine. Urate levels dropped rapidly to below 6 mg/dL in the allopurinol group, but there were only two flares. Placebo subjects, meanwhile, had three flares (P= 0.60). There was no significant difference in daily pain scores (Am J Med. 2012 Nov;125(11):1126-1134.e7.).
“The bottom line is the groups did essentially the same. If you are using prophylaxis to treat an acute attack, you are probably perfectly fine to use allopurinol; you don’t need to wait,” Dr. Mandell said. “Does this mean you should start everybody in the hospital on full-dose allopurinol? No, but I think this really broadens our thought processes in terms of handling urate lowering therapy.”
It’s also time to rethink allopurinol dose adjustments in patients with chronic kidney disease (CKD). The thought has been that these patients should be on lower doses to prevent metabolite buildup in the kidneys and hypersensitivity reactions, but “if you follow those guidelines, less than 20% of patients will lower their serum urate, so you will be giving the drug for nothing,” Dr. Mandell said.
“It is true that allopurinol hypersensitivity is more common in patients with CKD,” but it happens in a few per thousand people, and hypersensitivity might have nothing to do with allopurinol dose. “There’s no repeated documentation between the levels and reactions, and in a number of small studies there is no evidence that dose adjustment decreases the frequency of hypersensitivity reactions. We have no data to say this is the right route to take,” he said.
In fact, a study that included 45 CKD patients found that increasing allopurinol beyond creatinine clearance-based dosing doesn’t cause problems. “Toxicity was not increased in patients receiving higher doses of allopurinol, including those with renal impairment,” the authors concluded (Arthritis Rheum. 2011 Feb;63(2):412-21.).
Given the rarity of hypersensitivity reactions, it’s not surprising they didn’t occur in the study’s small group of CKD patients, Dr. Mandell said. “If you are really ultra conservative, you are [still] not going to use allopurinol in CKD,” he said.
But Dr. Mandell said he starts patients on 50 mg of allopurinol. “I educate them about allergic reactions and increase the dose every 1-2 weeks until I hit” a serum uric acid below 6 mg/dL, which often takes more than 300 mg per day. “Alternatively, you can start febuxostat, and if I were to do that I would start at 20 mg” – breaking the lowest dose pill of 40 mg in half – “and then titrate up,” he said.
“It just doesn’t make sense,” Dr. Mandell said, that allopurinol hypersensitivity is related to dose. Instead, there seems to be a genetic predisposition for toxicity in, for instance, Asian patients with the HLA-B*5801 genotype, he noted.
Pegloticase is a bit more problematic than allopurinol. It’s highly effective – patients can drop their serum uric acid from 14 to 0.1 mg/dL within hours – but that comes at the cost of a high mobilization flair rate and infusion reactions in about a quarter of patients, Dr. Mandell said.
There’s a partial workaround. Dr. Mandell said he checks the uric acid level before the second infusion. If it’s not below 6 mg/dL after the first infusion, patients have antibodies against pegloticase. “The drug isn’t going to work, and patients are more likely to get an allergic reaction, so stop the drug,” he said.
Dr. Mandell is a consultant for AstraZeneca and Crealta Pharmaceuticals. The Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – It’s okay to start urate lowering therapy during a gout attack, according to Dr. Brian Mandell, a rheumatology professor at the Cleveland Clinic.
“I was taught that you don’t treat a gout attack with urate lowering therapy” because it could trigger a subsequent mobilization attack. “This has been the paradigm for years,” he said at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
But a 2012 study casts doubt on that. In the study, 26 men hospitalized for gout attack were randomized to 300 mg allopurinol daily, while 25 were randomized to placebo. All of the patients were on indomethacin and colchicine. Urate levels dropped rapidly to below 6 mg/dL in the allopurinol group, but there were only two flares. Placebo subjects, meanwhile, had three flares (P= 0.60). There was no significant difference in daily pain scores (Am J Med. 2012 Nov;125(11):1126-1134.e7.).
“The bottom line is the groups did essentially the same. If you are using prophylaxis to treat an acute attack, you are probably perfectly fine to use allopurinol; you don’t need to wait,” Dr. Mandell said. “Does this mean you should start everybody in the hospital on full-dose allopurinol? No, but I think this really broadens our thought processes in terms of handling urate lowering therapy.”
It’s also time to rethink allopurinol dose adjustments in patients with chronic kidney disease (CKD). The thought has been that these patients should be on lower doses to prevent metabolite buildup in the kidneys and hypersensitivity reactions, but “if you follow those guidelines, less than 20% of patients will lower their serum urate, so you will be giving the drug for nothing,” Dr. Mandell said.
“It is true that allopurinol hypersensitivity is more common in patients with CKD,” but it happens in a few per thousand people, and hypersensitivity might have nothing to do with allopurinol dose. “There’s no repeated documentation between the levels and reactions, and in a number of small studies there is no evidence that dose adjustment decreases the frequency of hypersensitivity reactions. We have no data to say this is the right route to take,” he said.
In fact, a study that included 45 CKD patients found that increasing allopurinol beyond creatinine clearance-based dosing doesn’t cause problems. “Toxicity was not increased in patients receiving higher doses of allopurinol, including those with renal impairment,” the authors concluded (Arthritis Rheum. 2011 Feb;63(2):412-21.).
Given the rarity of hypersensitivity reactions, it’s not surprising they didn’t occur in the study’s small group of CKD patients, Dr. Mandell said. “If you are really ultra conservative, you are [still] not going to use allopurinol in CKD,” he said.
But Dr. Mandell said he starts patients on 50 mg of allopurinol. “I educate them about allergic reactions and increase the dose every 1-2 weeks until I hit” a serum uric acid below 6 mg/dL, which often takes more than 300 mg per day. “Alternatively, you can start febuxostat, and if I were to do that I would start at 20 mg” – breaking the lowest dose pill of 40 mg in half – “and then titrate up,” he said.
“It just doesn’t make sense,” Dr. Mandell said, that allopurinol hypersensitivity is related to dose. Instead, there seems to be a genetic predisposition for toxicity in, for instance, Asian patients with the HLA-B*5801 genotype, he noted.
Pegloticase is a bit more problematic than allopurinol. It’s highly effective – patients can drop their serum uric acid from 14 to 0.1 mg/dL within hours – but that comes at the cost of a high mobilization flair rate and infusion reactions in about a quarter of patients, Dr. Mandell said.
There’s a partial workaround. Dr. Mandell said he checks the uric acid level before the second infusion. If it’s not below 6 mg/dL after the first infusion, patients have antibodies against pegloticase. “The drug isn’t going to work, and patients are more likely to get an allergic reaction, so stop the drug,” he said.
Dr. Mandell is a consultant for AstraZeneca and Crealta Pharmaceuticals. The Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PERSPECTIVES IN RHEUMATIC DISEASES
VIDEO: Fibromyalgia doesn’t fit the disease model
LAS VEGAS – There’s no easy fix for fibromyalgia, a problem that seems to lurk in the gap between mind and body.
Pharmaceuticals don’t work too well, and patients usually stop taking them after a while. Physicians are often at a loss for what to try next.
Part of the problem, at least for now, is that fibromyalgia is a poor fit for the medical model; it may be time to reconsider it from a broader perspective, according to Dr. Brian Walitt, director of clinical pain research at the National Center for Complementary and Integrative Health, in Bethesda, Md. He shared his insights – and a few tips for helping sufferers – in a video interview at the annual Perspectives in Rheumatic Diseases, held by Global Academy for Medical Education.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – There’s no easy fix for fibromyalgia, a problem that seems to lurk in the gap between mind and body.
Pharmaceuticals don’t work too well, and patients usually stop taking them after a while. Physicians are often at a loss for what to try next.
Part of the problem, at least for now, is that fibromyalgia is a poor fit for the medical model; it may be time to reconsider it from a broader perspective, according to Dr. Brian Walitt, director of clinical pain research at the National Center for Complementary and Integrative Health, in Bethesda, Md. He shared his insights – and a few tips for helping sufferers – in a video interview at the annual Perspectives in Rheumatic Diseases, held by Global Academy for Medical Education.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – There’s no easy fix for fibromyalgia, a problem that seems to lurk in the gap between mind and body.
Pharmaceuticals don’t work too well, and patients usually stop taking them after a while. Physicians are often at a loss for what to try next.
Part of the problem, at least for now, is that fibromyalgia is a poor fit for the medical model; it may be time to reconsider it from a broader perspective, according to Dr. Brian Walitt, director of clinical pain research at the National Center for Complementary and Integrative Health, in Bethesda, Md. He shared his insights – and a few tips for helping sufferers – in a video interview at the annual Perspectives in Rheumatic Diseases, held by Global Academy for Medical Education.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
FDA panel considers contraindications, removal guidelines for Essure
FROM AN FDA ADVISORY COMMITTEE MEETING
Essure permanent contraceptive coils should probably be removed if they’re not positioned correctly and a woman is in pain or gets pregnant, or if a woman develops autoimmune or hypersensitivity symptoms after the device is placed, members of the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel concluded Sept. 24.
The panel met in Silver Spring, Md., to consider the device’s future in the wake of more than 5,000 complaints from patients about pain and other serious problems following implantation of Bayer’s fallopian tube permanent sterilization coil.
The 19-member advisory panel didn’t vote on any formal recommendations to the FDA, but reached a general consensus following a series of deliberations.
After dozens of Essure patients testified about apparent immune reactions after implantation, panel members seemed particularly concerned with the possibility that nickel, or some other element in Essure, can cause type IV hypersensitivity reactions, which many witnesses said were at least partly contributing to their problems.
The panel members also expressed concern with the difficulty women had finding help when they developed chronic pain and other “life-altering side effects that stop you from functioning like a person,” as Angie Firmalino, an administrator of the 21,508-member Facebook group Essure Problems, testified.
Many of those patients said implantation was the “worst mistake” of their lives, and called to have the device removed from the market. But their input was tempered by reviews from both the FDA and Essure-manufacturer Bayer, which concluded that most women do well with Essure.
The American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, and Planned Parenthood Federation of America issued a joint statement noting that Essure is the only permanent contraception that can be performed non-surgically and is an important option for some women.
"We are grateful to the Food and Drug Administration for hosting an important conversation about the safety of Essure and on the steps that the agency and the industry can take in order to ensure that Essure is used safely in the future. But we urge the FDA to recognize that restricting women's access to certain methods of birth control will limit their choices and, in some cases, expose them to additional risk," the groups wrote.
One of the main themes of the panel's deliberations was the lack of solid information about the risks and benefits of Essure. The device was approved by the FDA in 2002 without a randomized controlled trial under the agency’s “premarket approval” process.
In addition to possible removal scenarios, some panel members suggested that histories of hypersensitivity, autoimmune reactions, pelvic pain, or pelvic surgery should be contraindications to Essure. One panel member said that the only proper patient for the device is a woman who can’t undergo traditional tubal ligation or receive long-acting reversible contraception.
But such recommendations were all tentative, given the lack of data.
To fill in the gaps, panel members called for the establishment of a prospective patient registry for Essure, similar to the isotretinoin and vaginal mesh registries. Most thought that the other option – a new randomized, controlled trial – would have to be too large to capture what are probably rare adverse events, and take too long for a product that’s already on the market.
A registry is needed "so we can have an accurate assessment of what's going on," said panelist Dr. David B. Seifer, from the Oregon Health & Science University in Portland.
The purpose of an Essure registry would be primarily to get an idea of how well the device works to prevent pregnancy. Real-world data seemed to be somewhat at odds with the nearly 100% efficacy reported in Bayer’s studies.
Beyond that, the aim of launching the registry would be to track and analyze outcomes in order to get a sense of which women do well with the device, and who should be steered to other options.
The registry would capture the true incidence of chronic pelvic pain, device migration, allergic or autoimmune issues, and other problems. It could also track removal of the device. Overall, panelists agreed that there needs to be a standard protocol on how to remove the device.
Several panelists wanted the registry to capture pathology reports on removed devices, as well, to see what kind of adverse response Essure triggered in the body.
Education on Essure also needs to improve, panelists concluded, for both patients and physicians. Several panel members said that the current device labeling was inadequate. At least one panel member said that until more is known about what problems are actually related to the device, labeling should include a summary of the more than 100 types of adverse events reported to the FDA.
"Current labeling is very benign. You can't limit hypersensitivity warnings to nickel allergy," said panelist Donna D. Baird, Ph.D., of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C.
Several panelists said that there should be an official, check-box disclosure form for Essure, to make sure patients are fully aware of potential problems and other sterilization options.
On the physician side, panelists agreed that doctors should not insert the Essure device unless they have a plan already in place – and funded – to remove it. Device labeling should also tell doctors that if Essure doesn’t slip into the fallopian tube relatively easily, to abandon the procedure because difficult placement is likely a sign of trouble, the panelists concluded.
"If you don't have access to someone who can remove it, I don't think you should be implanting it," said panelist Cynthia Chauhan, a consumer representative from Wichita, Kan.
The FDA is accepting public comments on Essure until Oct. 24.
*This story was updated 10/2/2015.
FROM AN FDA ADVISORY COMMITTEE MEETING
Essure permanent contraceptive coils should probably be removed if they’re not positioned correctly and a woman is in pain or gets pregnant, or if a woman develops autoimmune or hypersensitivity symptoms after the device is placed, members of the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel concluded Sept. 24.
The panel met in Silver Spring, Md., to consider the device’s future in the wake of more than 5,000 complaints from patients about pain and other serious problems following implantation of Bayer’s fallopian tube permanent sterilization coil.
The 19-member advisory panel didn’t vote on any formal recommendations to the FDA, but reached a general consensus following a series of deliberations.
After dozens of Essure patients testified about apparent immune reactions after implantation, panel members seemed particularly concerned with the possibility that nickel, or some other element in Essure, can cause type IV hypersensitivity reactions, which many witnesses said were at least partly contributing to their problems.
The panel members also expressed concern with the difficulty women had finding help when they developed chronic pain and other “life-altering side effects that stop you from functioning like a person,” as Angie Firmalino, an administrator of the 21,508-member Facebook group Essure Problems, testified.
Many of those patients said implantation was the “worst mistake” of their lives, and called to have the device removed from the market. But their input was tempered by reviews from both the FDA and Essure-manufacturer Bayer, which concluded that most women do well with Essure.
The American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, and Planned Parenthood Federation of America issued a joint statement noting that Essure is the only permanent contraception that can be performed non-surgically and is an important option for some women.
"We are grateful to the Food and Drug Administration for hosting an important conversation about the safety of Essure and on the steps that the agency and the industry can take in order to ensure that Essure is used safely in the future. But we urge the FDA to recognize that restricting women's access to certain methods of birth control will limit their choices and, in some cases, expose them to additional risk," the groups wrote.
One of the main themes of the panel's deliberations was the lack of solid information about the risks and benefits of Essure. The device was approved by the FDA in 2002 without a randomized controlled trial under the agency’s “premarket approval” process.
In addition to possible removal scenarios, some panel members suggested that histories of hypersensitivity, autoimmune reactions, pelvic pain, or pelvic surgery should be contraindications to Essure. One panel member said that the only proper patient for the device is a woman who can’t undergo traditional tubal ligation or receive long-acting reversible contraception.
But such recommendations were all tentative, given the lack of data.
To fill in the gaps, panel members called for the establishment of a prospective patient registry for Essure, similar to the isotretinoin and vaginal mesh registries. Most thought that the other option – a new randomized, controlled trial – would have to be too large to capture what are probably rare adverse events, and take too long for a product that’s already on the market.
A registry is needed "so we can have an accurate assessment of what's going on," said panelist Dr. David B. Seifer, from the Oregon Health & Science University in Portland.
The purpose of an Essure registry would be primarily to get an idea of how well the device works to prevent pregnancy. Real-world data seemed to be somewhat at odds with the nearly 100% efficacy reported in Bayer’s studies.
Beyond that, the aim of launching the registry would be to track and analyze outcomes in order to get a sense of which women do well with the device, and who should be steered to other options.
The registry would capture the true incidence of chronic pelvic pain, device migration, allergic or autoimmune issues, and other problems. It could also track removal of the device. Overall, panelists agreed that there needs to be a standard protocol on how to remove the device.
Several panelists wanted the registry to capture pathology reports on removed devices, as well, to see what kind of adverse response Essure triggered in the body.
Education on Essure also needs to improve, panelists concluded, for both patients and physicians. Several panel members said that the current device labeling was inadequate. At least one panel member said that until more is known about what problems are actually related to the device, labeling should include a summary of the more than 100 types of adverse events reported to the FDA.
"Current labeling is very benign. You can't limit hypersensitivity warnings to nickel allergy," said panelist Donna D. Baird, Ph.D., of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C.
Several panelists said that there should be an official, check-box disclosure form for Essure, to make sure patients are fully aware of potential problems and other sterilization options.
On the physician side, panelists agreed that doctors should not insert the Essure device unless they have a plan already in place – and funded – to remove it. Device labeling should also tell doctors that if Essure doesn’t slip into the fallopian tube relatively easily, to abandon the procedure because difficult placement is likely a sign of trouble, the panelists concluded.
"If you don't have access to someone who can remove it, I don't think you should be implanting it," said panelist Cynthia Chauhan, a consumer representative from Wichita, Kan.
The FDA is accepting public comments on Essure until Oct. 24.
*This story was updated 10/2/2015.
FROM AN FDA ADVISORY COMMITTEE MEETING
Essure permanent contraceptive coils should probably be removed if they’re not positioned correctly and a woman is in pain or gets pregnant, or if a woman develops autoimmune or hypersensitivity symptoms after the device is placed, members of the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel concluded Sept. 24.
The panel met in Silver Spring, Md., to consider the device’s future in the wake of more than 5,000 complaints from patients about pain and other serious problems following implantation of Bayer’s fallopian tube permanent sterilization coil.
The 19-member advisory panel didn’t vote on any formal recommendations to the FDA, but reached a general consensus following a series of deliberations.
After dozens of Essure patients testified about apparent immune reactions after implantation, panel members seemed particularly concerned with the possibility that nickel, or some other element in Essure, can cause type IV hypersensitivity reactions, which many witnesses said were at least partly contributing to their problems.
The panel members also expressed concern with the difficulty women had finding help when they developed chronic pain and other “life-altering side effects that stop you from functioning like a person,” as Angie Firmalino, an administrator of the 21,508-member Facebook group Essure Problems, testified.
Many of those patients said implantation was the “worst mistake” of their lives, and called to have the device removed from the market. But their input was tempered by reviews from both the FDA and Essure-manufacturer Bayer, which concluded that most women do well with Essure.
The American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, and Planned Parenthood Federation of America issued a joint statement noting that Essure is the only permanent contraception that can be performed non-surgically and is an important option for some women.
"We are grateful to the Food and Drug Administration for hosting an important conversation about the safety of Essure and on the steps that the agency and the industry can take in order to ensure that Essure is used safely in the future. But we urge the FDA to recognize that restricting women's access to certain methods of birth control will limit their choices and, in some cases, expose them to additional risk," the groups wrote.
One of the main themes of the panel's deliberations was the lack of solid information about the risks and benefits of Essure. The device was approved by the FDA in 2002 without a randomized controlled trial under the agency’s “premarket approval” process.
In addition to possible removal scenarios, some panel members suggested that histories of hypersensitivity, autoimmune reactions, pelvic pain, or pelvic surgery should be contraindications to Essure. One panel member said that the only proper patient for the device is a woman who can’t undergo traditional tubal ligation or receive long-acting reversible contraception.
But such recommendations were all tentative, given the lack of data.
To fill in the gaps, panel members called for the establishment of a prospective patient registry for Essure, similar to the isotretinoin and vaginal mesh registries. Most thought that the other option – a new randomized, controlled trial – would have to be too large to capture what are probably rare adverse events, and take too long for a product that’s already on the market.
A registry is needed "so we can have an accurate assessment of what's going on," said panelist Dr. David B. Seifer, from the Oregon Health & Science University in Portland.
The purpose of an Essure registry would be primarily to get an idea of how well the device works to prevent pregnancy. Real-world data seemed to be somewhat at odds with the nearly 100% efficacy reported in Bayer’s studies.
Beyond that, the aim of launching the registry would be to track and analyze outcomes in order to get a sense of which women do well with the device, and who should be steered to other options.
The registry would capture the true incidence of chronic pelvic pain, device migration, allergic or autoimmune issues, and other problems. It could also track removal of the device. Overall, panelists agreed that there needs to be a standard protocol on how to remove the device.
Several panelists wanted the registry to capture pathology reports on removed devices, as well, to see what kind of adverse response Essure triggered in the body.
Education on Essure also needs to improve, panelists concluded, for both patients and physicians. Several panel members said that the current device labeling was inadequate. At least one panel member said that until more is known about what problems are actually related to the device, labeling should include a summary of the more than 100 types of adverse events reported to the FDA.
"Current labeling is very benign. You can't limit hypersensitivity warnings to nickel allergy," said panelist Donna D. Baird, Ph.D., of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C.
Several panelists said that there should be an official, check-box disclosure form for Essure, to make sure patients are fully aware of potential problems and other sterilization options.
On the physician side, panelists agreed that doctors should not insert the Essure device unless they have a plan already in place – and funded – to remove it. Device labeling should also tell doctors that if Essure doesn’t slip into the fallopian tube relatively easily, to abandon the procedure because difficult placement is likely a sign of trouble, the panelists concluded.
"If you don't have access to someone who can remove it, I don't think you should be implanting it," said panelist Cynthia Chauhan, a consumer representative from Wichita, Kan.
The FDA is accepting public comments on Essure until Oct. 24.
*This story was updated 10/2/2015.
VIDEO: How to handle pregnancy in lupus
LAS VEGAS – Pregnancy outcomes can be excellent in women with lupus, but they need help to make sure everything goes okay.
Rheumatologist Dr. Jennifer Grossman, director of lupus clinical investigational research at the University of California, Los Angeles, knows how to do it right. In a video interview at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education, she discussed tips on managing pregnancy in lupus patients, including the use of hydroxychloroquine to prevent flares, azathioprine to treat lupus nephritis, and aspirin to reduce the risk of preeclampsia.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pregnancy outcomes can be excellent in women with lupus, but they need help to make sure everything goes okay.
Rheumatologist Dr. Jennifer Grossman, director of lupus clinical investigational research at the University of California, Los Angeles, knows how to do it right. In a video interview at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education, she discussed tips on managing pregnancy in lupus patients, including the use of hydroxychloroquine to prevent flares, azathioprine to treat lupus nephritis, and aspirin to reduce the risk of preeclampsia.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Pregnancy outcomes can be excellent in women with lupus, but they need help to make sure everything goes okay.
Rheumatologist Dr. Jennifer Grossman, director of lupus clinical investigational research at the University of California, Los Angeles, knows how to do it right. In a video interview at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education, she discussed tips on managing pregnancy in lupus patients, including the use of hydroxychloroquine to prevent flares, azathioprine to treat lupus nephritis, and aspirin to reduce the risk of preeclampsia.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
VIDEO: Simple skin finding differentiates diffuse scleroderma from limited disease
LAS VEGAS – Scleroderma treatment is evolving quickly.
Newer classification criteria from the American College of Rheumatology and the European League Against Rheumatism are helping diagnose and treat the disease earlier, and ongoing investigations are defining the proper roles of mycophenolate mofetil, cyclophosphamide, stem cell transplants, and other treatments in the disease. Meanwhile, the Food and Drug Administration has granted Breakthrough Therapy Designation status to tocilizumab for its development as a potential treatment for scleroderma.
In an interview at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education, Dr. Daniel Furst, the Carl M. Pearson Professor in Rheumatology at the University of California, Los Angeles, gave a quick review of those and other recent developments and shared a few treatment pearls, including how he uses a simple skin finding to differentiate diffuse from limited disease.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Scleroderma treatment is evolving quickly.
Newer classification criteria from the American College of Rheumatology and the European League Against Rheumatism are helping diagnose and treat the disease earlier, and ongoing investigations are defining the proper roles of mycophenolate mofetil, cyclophosphamide, stem cell transplants, and other treatments in the disease. Meanwhile, the Food and Drug Administration has granted Breakthrough Therapy Designation status to tocilizumab for its development as a potential treatment for scleroderma.
In an interview at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education, Dr. Daniel Furst, the Carl M. Pearson Professor in Rheumatology at the University of California, Los Angeles, gave a quick review of those and other recent developments and shared a few treatment pearls, including how he uses a simple skin finding to differentiate diffuse from limited disease.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Scleroderma treatment is evolving quickly.
Newer classification criteria from the American College of Rheumatology and the European League Against Rheumatism are helping diagnose and treat the disease earlier, and ongoing investigations are defining the proper roles of mycophenolate mofetil, cyclophosphamide, stem cell transplants, and other treatments in the disease. Meanwhile, the Food and Drug Administration has granted Breakthrough Therapy Designation status to tocilizumab for its development as a potential treatment for scleroderma.
In an interview at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education, Dr. Daniel Furst, the Carl M. Pearson Professor in Rheumatology at the University of California, Los Angeles, gave a quick review of those and other recent developments and shared a few treatment pearls, including how he uses a simple skin finding to differentiate diffuse from limited disease.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
VIDEO: Don’t rely on ANCA to diagnose primary vasculitis
LAS VEGAS – Vasculitis isn’t always the final diagnosis, even with a positive ANCA.
A wide range of conditions – even bacterial endocarditis and adulterated cocaine – can set up secondary vasculitis, and it’s easy to mistake one of them for the main problem when it’s really only a sideshow. Clinicians are especially likely to get in trouble if they rely on ANCA (antineutrophil cytoplasmic antibody) as a screening test for vasculitis.
In an interview at a conference held by the Global Academy for Medical Education, Dr. Brian Mandell, a rheumatology professor at the Cleveland Clinic, explained how to avoid the trap. The Global Academy for Medical Education and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Vasculitis isn’t always the final diagnosis, even with a positive ANCA.
A wide range of conditions – even bacterial endocarditis and adulterated cocaine – can set up secondary vasculitis, and it’s easy to mistake one of them for the main problem when it’s really only a sideshow. Clinicians are especially likely to get in trouble if they rely on ANCA (antineutrophil cytoplasmic antibody) as a screening test for vasculitis.
In an interview at a conference held by the Global Academy for Medical Education, Dr. Brian Mandell, a rheumatology professor at the Cleveland Clinic, explained how to avoid the trap. The Global Academy for Medical Education and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Vasculitis isn’t always the final diagnosis, even with a positive ANCA.
A wide range of conditions – even bacterial endocarditis and adulterated cocaine – can set up secondary vasculitis, and it’s easy to mistake one of them for the main problem when it’s really only a sideshow. Clinicians are especially likely to get in trouble if they rely on ANCA (antineutrophil cytoplasmic antibody) as a screening test for vasculitis.
In an interview at a conference held by the Global Academy for Medical Education, Dr. Brian Mandell, a rheumatology professor at the Cleveland Clinic, explained how to avoid the trap. The Global Academy for Medical Education and this news organization are owned by Frontline Medical Communications.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
FDA panel set to vet Essure safety
On Sept. 24, the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel will meet in Silver Spring, Md., to discuss the future of Bayer’s Essure permanent female sterilization coil.
FDA officials have asked the 19-member advisory panel to evaluate the currently available scientific data on the safety and effectiveness of the Essure system and make recommendations on appropriate use, product labeling, and the potential need for additional postmarket clinical studies.
Since Essure’s approval in 2002, the FDA has received more than 5,000 complaints about Essure. Most concern chronic pain, perforation, dyspareunia, device migration, menstrual problems, and possible allergic reactions to the nickel in Essure, but more than 100 adverse symptoms have been reported to the agency, according to newly posted meeting materials.
The complaints have been filed mostly in the past 2 years by patients who have organized online to share their stories and lobby to have Essure taken off the market; some are involved in legal action against Bayer. Meanwhile, Bayer maintains that, for most of the approximately 750,000 women who have received Essure, the device is safe and effective.
Advisory panel members will have their work cut out for them as they sort through the issues. The FDA has released a new 89-page review of Essure, and Bayer has submitted its own lengthy review document. Testimony is expected from Bayer, FDA reviewers, patients, and others. The meeting is scheduled to run almost 12 hours, from 8 a.m. to 7:30 p.m. Eastern time, at the agency’s White Oak Campus in Silver Spring, Md., and will be broadcast live online.
FDA noted in its review that it has been monitoring social media with automated software that scours Twitter, Facebook, patient forums, and other websites for “posts with resemblance to adverse events” or “Proto-AEs.” The program picked up 350,000 references to Essure between September 2013 and July 2015 and classified more than 20,000 as Proto-AEs, mostly related to pain, hysterectomy, malaise, pregnancy, and device removal. The social media data, however, are preliminary since the FDA has yet to remove duplications and retweets.
On Sept. 24, the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel will meet in Silver Spring, Md., to discuss the future of Bayer’s Essure permanent female sterilization coil.
FDA officials have asked the 19-member advisory panel to evaluate the currently available scientific data on the safety and effectiveness of the Essure system and make recommendations on appropriate use, product labeling, and the potential need for additional postmarket clinical studies.
Since Essure’s approval in 2002, the FDA has received more than 5,000 complaints about Essure. Most concern chronic pain, perforation, dyspareunia, device migration, menstrual problems, and possible allergic reactions to the nickel in Essure, but more than 100 adverse symptoms have been reported to the agency, according to newly posted meeting materials.
The complaints have been filed mostly in the past 2 years by patients who have organized online to share their stories and lobby to have Essure taken off the market; some are involved in legal action against Bayer. Meanwhile, Bayer maintains that, for most of the approximately 750,000 women who have received Essure, the device is safe and effective.
Advisory panel members will have their work cut out for them as they sort through the issues. The FDA has released a new 89-page review of Essure, and Bayer has submitted its own lengthy review document. Testimony is expected from Bayer, FDA reviewers, patients, and others. The meeting is scheduled to run almost 12 hours, from 8 a.m. to 7:30 p.m. Eastern time, at the agency’s White Oak Campus in Silver Spring, Md., and will be broadcast live online.
FDA noted in its review that it has been monitoring social media with automated software that scours Twitter, Facebook, patient forums, and other websites for “posts with resemblance to adverse events” or “Proto-AEs.” The program picked up 350,000 references to Essure between September 2013 and July 2015 and classified more than 20,000 as Proto-AEs, mostly related to pain, hysterectomy, malaise, pregnancy, and device removal. The social media data, however, are preliminary since the FDA has yet to remove duplications and retweets.
On Sept. 24, the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel will meet in Silver Spring, Md., to discuss the future of Bayer’s Essure permanent female sterilization coil.
FDA officials have asked the 19-member advisory panel to evaluate the currently available scientific data on the safety and effectiveness of the Essure system and make recommendations on appropriate use, product labeling, and the potential need for additional postmarket clinical studies.
Since Essure’s approval in 2002, the FDA has received more than 5,000 complaints about Essure. Most concern chronic pain, perforation, dyspareunia, device migration, menstrual problems, and possible allergic reactions to the nickel in Essure, but more than 100 adverse symptoms have been reported to the agency, according to newly posted meeting materials.
The complaints have been filed mostly in the past 2 years by patients who have organized online to share their stories and lobby to have Essure taken off the market; some are involved in legal action against Bayer. Meanwhile, Bayer maintains that, for most of the approximately 750,000 women who have received Essure, the device is safe and effective.
Advisory panel members will have their work cut out for them as they sort through the issues. The FDA has released a new 89-page review of Essure, and Bayer has submitted its own lengthy review document. Testimony is expected from Bayer, FDA reviewers, patients, and others. The meeting is scheduled to run almost 12 hours, from 8 a.m. to 7:30 p.m. Eastern time, at the agency’s White Oak Campus in Silver Spring, Md., and will be broadcast live online.
FDA noted in its review that it has been monitoring social media with automated software that scours Twitter, Facebook, patient forums, and other websites for “posts with resemblance to adverse events” or “Proto-AEs.” The program picked up 350,000 references to Essure between September 2013 and July 2015 and classified more than 20,000 as Proto-AEs, mostly related to pain, hysterectomy, malaise, pregnancy, and device removal. The social media data, however, are preliminary since the FDA has yet to remove duplications and retweets.
Aspirin, hydrochlorothiazide okay in gout
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
New ACR/EULAR gout classification criteria offer better sensitivity, specificity
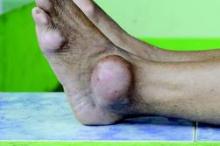
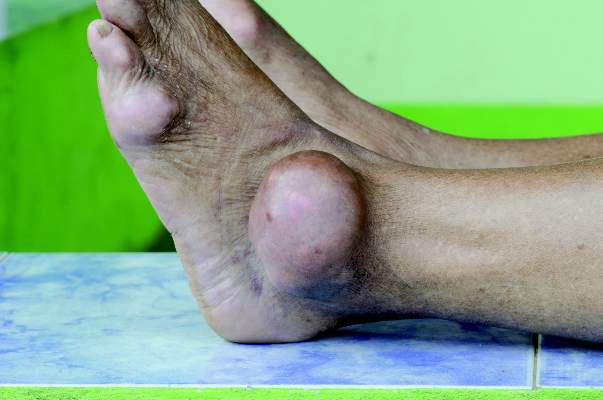
The presence of monosodium urate monohydrate crystals in a symptomatic joint, bursa, or tophus is sufficient to diagnose gout, according to new gout classification criteria from the American College of Rheumatology and the European League Against Rheumatism.
When symptomatic urate crystals are missing, other signs and symptoms are considered and scored; a score of 8 or more constitutes gout. “The threshold chosen for this classification criteria set yielded the best combination of sensitivity and specificity,” at 92% and 89%, respectively, and outperformed previous classification schemes, said the authors, led by Dr. Tuhina Neogi of Boston University.
To qualify for gout, patients must first have at least one episode of swelling, pain, or tenderness in a peripheral joint or bursa. They get a score of 1 if that happens in the ankle or midfoot, and a score of 2 if it involves a metatarsophalangeal joint. If the affected joint is red and too painful to touch and use, patients get an additional score of 3. Chalklike drainage from a subcutaneous nodule in a gout-prone area, and serum urate at or above 10 mg/dL, both get a score of 4. Imaging of one or more gout erosions in the hands or feet also gets a score of 4 (Arthritis Rheumatol. 2015 Oct;67[10]:2557-68. doi: 10.1002/art.39254).
Overall, the criteria incorporate clinical, laboratory, and imaging evidence. A web-based calculator makes the scoring easy.
“Although MSU [monosodium urate monohydrate] crystal results are extremely helpful when positive, they are not a feasible universal standard, particularly because many potential study subjects are likely to be recruited from nonrheumatology settings. We aimed to develop a new set of criteria that could be flexible enough to enable accurate classification of gout regardless of MSU status,” the authors said.
“This classification criteria set will enable a standardized approach to identifying a relatively homogeneous group of individuals who have the clinical entity of gout for enrollment into studies. The criteria permit characterization of an individual as having gout regardless of whether he or she is currently experiencing an acute symptomatic episode and regardless of any comorbidities,” they said.
The hope of the work is to facilitate a better understanding of gout and speed development of new trials and treatments. The criteria will “help to ensure that patients with the same disease are being evaluated, which will enhance our ability to study the disease, including performing outcomes studies and clinical trials,” Dr. Neogi said in a written statement.
Previous gout classification criteria were developed when advanced imaging was not available. “Additionally, the increasing prevalence of gout, advances in therapeutics, and the development of international research collaborations to understand the impact, mechanisms, and optimal treatment of this condition emphasize the need for accurate and uniform classification criteria for gout,” according to the statement.
The new criteria are based on a systematic review of the literature on advanced gout imaging; a diagnostic study in which the presence of MSU crystals in synovial fluid or tophi was the gold standard; a ranking exercise of paper patient cases; and a multicriterion decision analysis exercise. The criteria were then validated in 330 patients.
The work was supported in part by the National Institutes of Health, the Agency for Healthcare Research and Quality, and Arthritis New Zealand. Numerous authors reported receiving consulting fees, speaking fees, and/or honoraria from companies that market drugs or specialty foods for gout.
The presence of monosodium urate monohydrate crystals in a symptomatic joint, bursa, or tophus is sufficient to diagnose gout, according to new gout classification criteria from the American College of Rheumatology and the European League Against Rheumatism.
When symptomatic urate crystals are missing, other signs and symptoms are considered and scored; a score of 8 or more constitutes gout. “The threshold chosen for this classification criteria set yielded the best combination of sensitivity and specificity,” at 92% and 89%, respectively, and outperformed previous classification schemes, said the authors, led by Dr. Tuhina Neogi of Boston University.
To qualify for gout, patients must first have at least one episode of swelling, pain, or tenderness in a peripheral joint or bursa. They get a score of 1 if that happens in the ankle or midfoot, and a score of 2 if it involves a metatarsophalangeal joint. If the affected joint is red and too painful to touch and use, patients get an additional score of 3. Chalklike drainage from a subcutaneous nodule in a gout-prone area, and serum urate at or above 10 mg/dL, both get a score of 4. Imaging of one or more gout erosions in the hands or feet also gets a score of 4 (Arthritis Rheumatol. 2015 Oct;67[10]:2557-68. doi: 10.1002/art.39254).
Overall, the criteria incorporate clinical, laboratory, and imaging evidence. A web-based calculator makes the scoring easy.
“Although MSU [monosodium urate monohydrate] crystal results are extremely helpful when positive, they are not a feasible universal standard, particularly because many potential study subjects are likely to be recruited from nonrheumatology settings. We aimed to develop a new set of criteria that could be flexible enough to enable accurate classification of gout regardless of MSU status,” the authors said.
“This classification criteria set will enable a standardized approach to identifying a relatively homogeneous group of individuals who have the clinical entity of gout for enrollment into studies. The criteria permit characterization of an individual as having gout regardless of whether he or she is currently experiencing an acute symptomatic episode and regardless of any comorbidities,” they said.
The hope of the work is to facilitate a better understanding of gout and speed development of new trials and treatments. The criteria will “help to ensure that patients with the same disease are being evaluated, which will enhance our ability to study the disease, including performing outcomes studies and clinical trials,” Dr. Neogi said in a written statement.
Previous gout classification criteria were developed when advanced imaging was not available. “Additionally, the increasing prevalence of gout, advances in therapeutics, and the development of international research collaborations to understand the impact, mechanisms, and optimal treatment of this condition emphasize the need for accurate and uniform classification criteria for gout,” according to the statement.
The new criteria are based on a systematic review of the literature on advanced gout imaging; a diagnostic study in which the presence of MSU crystals in synovial fluid or tophi was the gold standard; a ranking exercise of paper patient cases; and a multicriterion decision analysis exercise. The criteria were then validated in 330 patients.
The work was supported in part by the National Institutes of Health, the Agency for Healthcare Research and Quality, and Arthritis New Zealand. Numerous authors reported receiving consulting fees, speaking fees, and/or honoraria from companies that market drugs or specialty foods for gout.
The presence of monosodium urate monohydrate crystals in a symptomatic joint, bursa, or tophus is sufficient to diagnose gout, according to new gout classification criteria from the American College of Rheumatology and the European League Against Rheumatism.
When symptomatic urate crystals are missing, other signs and symptoms are considered and scored; a score of 8 or more constitutes gout. “The threshold chosen for this classification criteria set yielded the best combination of sensitivity and specificity,” at 92% and 89%, respectively, and outperformed previous classification schemes, said the authors, led by Dr. Tuhina Neogi of Boston University.
To qualify for gout, patients must first have at least one episode of swelling, pain, or tenderness in a peripheral joint or bursa. They get a score of 1 if that happens in the ankle or midfoot, and a score of 2 if it involves a metatarsophalangeal joint. If the affected joint is red and too painful to touch and use, patients get an additional score of 3. Chalklike drainage from a subcutaneous nodule in a gout-prone area, and serum urate at or above 10 mg/dL, both get a score of 4. Imaging of one or more gout erosions in the hands or feet also gets a score of 4 (Arthritis Rheumatol. 2015 Oct;67[10]:2557-68. doi: 10.1002/art.39254).
Overall, the criteria incorporate clinical, laboratory, and imaging evidence. A web-based calculator makes the scoring easy.
“Although MSU [monosodium urate monohydrate] crystal results are extremely helpful when positive, they are not a feasible universal standard, particularly because many potential study subjects are likely to be recruited from nonrheumatology settings. We aimed to develop a new set of criteria that could be flexible enough to enable accurate classification of gout regardless of MSU status,” the authors said.
“This classification criteria set will enable a standardized approach to identifying a relatively homogeneous group of individuals who have the clinical entity of gout for enrollment into studies. The criteria permit characterization of an individual as having gout regardless of whether he or she is currently experiencing an acute symptomatic episode and regardless of any comorbidities,” they said.
The hope of the work is to facilitate a better understanding of gout and speed development of new trials and treatments. The criteria will “help to ensure that patients with the same disease are being evaluated, which will enhance our ability to study the disease, including performing outcomes studies and clinical trials,” Dr. Neogi said in a written statement.
Previous gout classification criteria were developed when advanced imaging was not available. “Additionally, the increasing prevalence of gout, advances in therapeutics, and the development of international research collaborations to understand the impact, mechanisms, and optimal treatment of this condition emphasize the need for accurate and uniform classification criteria for gout,” according to the statement.
The new criteria are based on a systematic review of the literature on advanced gout imaging; a diagnostic study in which the presence of MSU crystals in synovial fluid or tophi was the gold standard; a ranking exercise of paper patient cases; and a multicriterion decision analysis exercise. The criteria were then validated in 330 patients.
The work was supported in part by the National Institutes of Health, the Agency for Healthcare Research and Quality, and Arthritis New Zealand. Numerous authors reported receiving consulting fees, speaking fees, and/or honoraria from companies that market drugs or specialty foods for gout.
FROM ARTHRITIS & RHEUMATOLOGY