User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Communication key to helping kids after disasters
Posttraumatic stress disorder can be hard to spot in kids after natural or manmade disasters.
They may not understand that intrusive thoughts, panic attacks, and other symptoms are problems that can be addressed, and are unlikely to mention them.
As a result, parents, teachers, and others often underestimate children’s distress levels and overestimate their resilience. One way around the problem is to ask children how they’re doing, and probe for signs of trouble. It helps to let them know that PTSD and adjustment problems are normal after a frightening event, and to teach them how to anticipate and cope with PTSD triggers.
That’s just a small fraction of the useful advice in new guidance from the American Academy of Pediatrics on the psychosocial support of children and families after disasters, published online Sept. 14 (Pediatrics. 2015 Sept. 14. doi:10.1542/peds.2015-2861).
“Children are particularly vulnerable to the effects of disasters and other traumatic events because of a lack of experience, skills, and resources to be able to independently meet their developmental, socioemotional, mental, and behavioral health needs,” said the authors, led by Dr. David Schonfeld of St. Christopher’s Hospital for Children, and Thomas Demaria, Ph.D., of Long Island (N.Y.) University.
Mental health triage should come right after medical stabilization. Dissociative symptoms; extreme confusion or inability to concentrate or make even simple decisions; intense fear, anxiety, panic, helplessness, or horror; depression at the time of the event; uncontrollable and intense grief; suicidal ideation; and marked somatization are among the warning signs that kids are in trouble.
Psychiatric medications to blunt such reactions are usually the wrong call. “Children need to develop an understanding of the event and learn to express and cope with their reactions.” If medication does seem necessary, its best to let an expert in childhood trauma make the decision, the authors said.
Dismissing children’s concerns is a mistake. “In reality, if children feel worried, then they are worried. Telling them that they should not be worried is usually ineffective.” It’s also a mistake to avoid talking about grief for fear of making it worse. Children’s “distress is caused by the reaction to the death itself, rather than any question or invitation to talk. Talking may provide some relief if not coerced. Avoiding discussion is rarely helpful and often isolates children at a time when they are most in need of support and assistance,” they said.
Simple, basic facts about the event – as long as they’re not graphic or overwhelming – will help children make sense of what they’ve been through, and reassurance that things will eventually be okay can be healing. Kids also have to know that the situation isn’t their fault, and how to cope with it.
Parents can share how they’re upset about losing their home, for instance, but then discuss how talking to another trusted adult, getting exercise, meditating, and helping others makes them feel better. Pediatricians can boost spirits by saying something like “the tornado created a big mess, but we are pulling together as a community” or “living in a shelter with all the other children in the neighborhood must have been a real adventure,” the authors said.
Having children contribute to food drives or draw hopeful pictures for victims in the hospital can help them regain a sense of control and usefulness. Resuming their routines as soon as possible will also help bring back a sense of normalcy.
Bereavement counseling is in order when children are struggling with the loss of a loved one, and cognitive behavioral therapy for kids with PTSD.
Posttraumatic stress disorder can be hard to spot in kids after natural or manmade disasters.
They may not understand that intrusive thoughts, panic attacks, and other symptoms are problems that can be addressed, and are unlikely to mention them.
As a result, parents, teachers, and others often underestimate children’s distress levels and overestimate their resilience. One way around the problem is to ask children how they’re doing, and probe for signs of trouble. It helps to let them know that PTSD and adjustment problems are normal after a frightening event, and to teach them how to anticipate and cope with PTSD triggers.
That’s just a small fraction of the useful advice in new guidance from the American Academy of Pediatrics on the psychosocial support of children and families after disasters, published online Sept. 14 (Pediatrics. 2015 Sept. 14. doi:10.1542/peds.2015-2861).
“Children are particularly vulnerable to the effects of disasters and other traumatic events because of a lack of experience, skills, and resources to be able to independently meet their developmental, socioemotional, mental, and behavioral health needs,” said the authors, led by Dr. David Schonfeld of St. Christopher’s Hospital for Children, and Thomas Demaria, Ph.D., of Long Island (N.Y.) University.
Mental health triage should come right after medical stabilization. Dissociative symptoms; extreme confusion or inability to concentrate or make even simple decisions; intense fear, anxiety, panic, helplessness, or horror; depression at the time of the event; uncontrollable and intense grief; suicidal ideation; and marked somatization are among the warning signs that kids are in trouble.
Psychiatric medications to blunt such reactions are usually the wrong call. “Children need to develop an understanding of the event and learn to express and cope with their reactions.” If medication does seem necessary, its best to let an expert in childhood trauma make the decision, the authors said.
Dismissing children’s concerns is a mistake. “In reality, if children feel worried, then they are worried. Telling them that they should not be worried is usually ineffective.” It’s also a mistake to avoid talking about grief for fear of making it worse. Children’s “distress is caused by the reaction to the death itself, rather than any question or invitation to talk. Talking may provide some relief if not coerced. Avoiding discussion is rarely helpful and often isolates children at a time when they are most in need of support and assistance,” they said.
Simple, basic facts about the event – as long as they’re not graphic or overwhelming – will help children make sense of what they’ve been through, and reassurance that things will eventually be okay can be healing. Kids also have to know that the situation isn’t their fault, and how to cope with it.
Parents can share how they’re upset about losing their home, for instance, but then discuss how talking to another trusted adult, getting exercise, meditating, and helping others makes them feel better. Pediatricians can boost spirits by saying something like “the tornado created a big mess, but we are pulling together as a community” or “living in a shelter with all the other children in the neighborhood must have been a real adventure,” the authors said.
Having children contribute to food drives or draw hopeful pictures for victims in the hospital can help them regain a sense of control and usefulness. Resuming their routines as soon as possible will also help bring back a sense of normalcy.
Bereavement counseling is in order when children are struggling with the loss of a loved one, and cognitive behavioral therapy for kids with PTSD.
Posttraumatic stress disorder can be hard to spot in kids after natural or manmade disasters.
They may not understand that intrusive thoughts, panic attacks, and other symptoms are problems that can be addressed, and are unlikely to mention them.
As a result, parents, teachers, and others often underestimate children’s distress levels and overestimate their resilience. One way around the problem is to ask children how they’re doing, and probe for signs of trouble. It helps to let them know that PTSD and adjustment problems are normal after a frightening event, and to teach them how to anticipate and cope with PTSD triggers.
That’s just a small fraction of the useful advice in new guidance from the American Academy of Pediatrics on the psychosocial support of children and families after disasters, published online Sept. 14 (Pediatrics. 2015 Sept. 14. doi:10.1542/peds.2015-2861).
“Children are particularly vulnerable to the effects of disasters and other traumatic events because of a lack of experience, skills, and resources to be able to independently meet their developmental, socioemotional, mental, and behavioral health needs,” said the authors, led by Dr. David Schonfeld of St. Christopher’s Hospital for Children, and Thomas Demaria, Ph.D., of Long Island (N.Y.) University.
Mental health triage should come right after medical stabilization. Dissociative symptoms; extreme confusion or inability to concentrate or make even simple decisions; intense fear, anxiety, panic, helplessness, or horror; depression at the time of the event; uncontrollable and intense grief; suicidal ideation; and marked somatization are among the warning signs that kids are in trouble.
Psychiatric medications to blunt such reactions are usually the wrong call. “Children need to develop an understanding of the event and learn to express and cope with their reactions.” If medication does seem necessary, its best to let an expert in childhood trauma make the decision, the authors said.
Dismissing children’s concerns is a mistake. “In reality, if children feel worried, then they are worried. Telling them that they should not be worried is usually ineffective.” It’s also a mistake to avoid talking about grief for fear of making it worse. Children’s “distress is caused by the reaction to the death itself, rather than any question or invitation to talk. Talking may provide some relief if not coerced. Avoiding discussion is rarely helpful and often isolates children at a time when they are most in need of support and assistance,” they said.
Simple, basic facts about the event – as long as they’re not graphic or overwhelming – will help children make sense of what they’ve been through, and reassurance that things will eventually be okay can be healing. Kids also have to know that the situation isn’t their fault, and how to cope with it.
Parents can share how they’re upset about losing their home, for instance, but then discuss how talking to another trusted adult, getting exercise, meditating, and helping others makes them feel better. Pediatricians can boost spirits by saying something like “the tornado created a big mess, but we are pulling together as a community” or “living in a shelter with all the other children in the neighborhood must have been a real adventure,” the authors said.
Having children contribute to food drives or draw hopeful pictures for victims in the hospital can help them regain a sense of control and usefulness. Resuming their routines as soon as possible will also help bring back a sense of normalcy.
Bereavement counseling is in order when children are struggling with the loss of a loved one, and cognitive behavioral therapy for kids with PTSD.
FROM PEDIATRICS
Acne scars improved with topical epidermal growth factor serum
Topical synthetic epidermal growth factor serum moderately improved the appearance of atrophic acne scars in a small pilot study.
At the end of 12 weeks of twice-daily application, scar appearance improved from 2.875 to 2.38 points on a 5-point investigator global assessment scale. Mean Goodman and Baron acne scar grade fell from 3.00 to 2.75, with 3 representing moderate disease and 2 mild disease. Of eight pairs of before and after photographs given to a blinded investigator, posttreatment images were correctly identified in five. Two were assessed as 76%-100% improved, and three were assessed as 50%-75% improved (J Drugs Dermatol. 2015;14[9]:1005-1010).
The patients were an average of 38 years old, split about equally between the sexes, and racially diverse. They used a basic facial cleanser during the study, but were banned from using tretinoin and other topicals.
Previously studied topicals don’t do much for acne scars, so the usual go-to treatments are chemical peels, dermabrasion, resurfacing lasers, and percutaneous collagen needling. They all work in part by promoting collagen synthesis, but at the cost of pain and side effects. Epidermal growth factor (EGF) also promotes collagen synthesis, so the investigators thought it might help. The EGF used in the study – DNA Regeneration Serum, derived from barley – was supplied by its maker, DNA EGF Renewal in Los Angeles.
“The findings suggest EGF serum has the potential to be a modern, noninvasive treatment for an otherwise highly refractory condition. Whereas resurfacing procedures rely on skin injury to trigger [EGF] release, direct topical application offers the effects of EGF without the associated discomfort and recovery time,” said Dr. Ronald L. Moy of the University of Southern California, Los Angeles, and Rachel Seidel, a medical student at Georgetown University in Washington, D.C.
“All subjects in this study [also] noted improvements in skin texture, fine lines, and wrinkles, while the vast majority also saw a reduction in brown and age spots,” they said.
The investigators said they are interested next in seeing if topical EGF prevents scars in active acne. “We believe that, by counteracting collagen degradation during the course of the inflammatory response, significant tissue atrophy capable of causing visible scarring may be prevented,” they said.
Dr. Moy owns stock in DNA EGF Renewal and is the company’s scientific adviser.
Topical synthetic epidermal growth factor serum moderately improved the appearance of atrophic acne scars in a small pilot study.
At the end of 12 weeks of twice-daily application, scar appearance improved from 2.875 to 2.38 points on a 5-point investigator global assessment scale. Mean Goodman and Baron acne scar grade fell from 3.00 to 2.75, with 3 representing moderate disease and 2 mild disease. Of eight pairs of before and after photographs given to a blinded investigator, posttreatment images were correctly identified in five. Two were assessed as 76%-100% improved, and three were assessed as 50%-75% improved (J Drugs Dermatol. 2015;14[9]:1005-1010).
The patients were an average of 38 years old, split about equally between the sexes, and racially diverse. They used a basic facial cleanser during the study, but were banned from using tretinoin and other topicals.
Previously studied topicals don’t do much for acne scars, so the usual go-to treatments are chemical peels, dermabrasion, resurfacing lasers, and percutaneous collagen needling. They all work in part by promoting collagen synthesis, but at the cost of pain and side effects. Epidermal growth factor (EGF) also promotes collagen synthesis, so the investigators thought it might help. The EGF used in the study – DNA Regeneration Serum, derived from barley – was supplied by its maker, DNA EGF Renewal in Los Angeles.
“The findings suggest EGF serum has the potential to be a modern, noninvasive treatment for an otherwise highly refractory condition. Whereas resurfacing procedures rely on skin injury to trigger [EGF] release, direct topical application offers the effects of EGF without the associated discomfort and recovery time,” said Dr. Ronald L. Moy of the University of Southern California, Los Angeles, and Rachel Seidel, a medical student at Georgetown University in Washington, D.C.
“All subjects in this study [also] noted improvements in skin texture, fine lines, and wrinkles, while the vast majority also saw a reduction in brown and age spots,” they said.
The investigators said they are interested next in seeing if topical EGF prevents scars in active acne. “We believe that, by counteracting collagen degradation during the course of the inflammatory response, significant tissue atrophy capable of causing visible scarring may be prevented,” they said.
Dr. Moy owns stock in DNA EGF Renewal and is the company’s scientific adviser.
Topical synthetic epidermal growth factor serum moderately improved the appearance of atrophic acne scars in a small pilot study.
At the end of 12 weeks of twice-daily application, scar appearance improved from 2.875 to 2.38 points on a 5-point investigator global assessment scale. Mean Goodman and Baron acne scar grade fell from 3.00 to 2.75, with 3 representing moderate disease and 2 mild disease. Of eight pairs of before and after photographs given to a blinded investigator, posttreatment images were correctly identified in five. Two were assessed as 76%-100% improved, and three were assessed as 50%-75% improved (J Drugs Dermatol. 2015;14[9]:1005-1010).
The patients were an average of 38 years old, split about equally between the sexes, and racially diverse. They used a basic facial cleanser during the study, but were banned from using tretinoin and other topicals.
Previously studied topicals don’t do much for acne scars, so the usual go-to treatments are chemical peels, dermabrasion, resurfacing lasers, and percutaneous collagen needling. They all work in part by promoting collagen synthesis, but at the cost of pain and side effects. Epidermal growth factor (EGF) also promotes collagen synthesis, so the investigators thought it might help. The EGF used in the study – DNA Regeneration Serum, derived from barley – was supplied by its maker, DNA EGF Renewal in Los Angeles.
“The findings suggest EGF serum has the potential to be a modern, noninvasive treatment for an otherwise highly refractory condition. Whereas resurfacing procedures rely on skin injury to trigger [EGF] release, direct topical application offers the effects of EGF without the associated discomfort and recovery time,” said Dr. Ronald L. Moy of the University of Southern California, Los Angeles, and Rachel Seidel, a medical student at Georgetown University in Washington, D.C.
“All subjects in this study [also] noted improvements in skin texture, fine lines, and wrinkles, while the vast majority also saw a reduction in brown and age spots,” they said.
The investigators said they are interested next in seeing if topical EGF prevents scars in active acne. “We believe that, by counteracting collagen degradation during the course of the inflammatory response, significant tissue atrophy capable of causing visible scarring may be prevented,” they said.
Dr. Moy owns stock in DNA EGF Renewal and is the company’s scientific adviser.
FROM THE JOURNAL OF DRUGS AND DERMATOLOGY
Key clinical point: Topical synthetic epidermal growth factor serum may be a noninvasive way to improve the appearance of atrophic acne scars.
Major finding: Five of the 8 pairs of before and after photographs given to a blinded investigator were correctly identified as the posttreatment image.
Data source: A pilot study of eight patients with atrophic acne scars.
Disclosures: The EGF serum used in the study was supplied by its maker, DNA EGF Renewal. Dr. Moy owns stock in the company and is its scientific adviser.
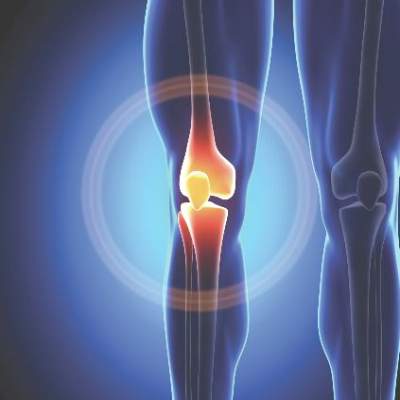
New cartilage, better function with stem cells for knee OA
Mesenchymal stem cell transplants appeared to regenerate cartilage and improve clinical outcomes after 2 years in patients with knee osteoarthritis in a small South Korean study.
There were 24 treated knees among the 11 men and 9 women in the study, whose average age was 58 years and mean body mass index was 26.6 kg/m2.
At baseline, 21 lesions (87.5%) were grade 2 or 3 on the MRI Osteoarthritis Knee Score scale for size of cartilage-loss area, and 23 lesions (95.9%) were grade 2 or 3 for percentage of full-thickness cartilage loss. A grade of 2-3 indicates moderate to severe disease.
At MRI follow-up 2 years after transplant, only five lesions (20.8%) were grade 2 or 3 for cartilage-loss area and five were grade 2 or 3 for full-thickness cartilage loss, reported Dr. Yong Sang Kim of the Yonsei Sarang Hospital in Seoul and his associates (Osteoarthritis Cartilage. 2015. doi: 10.1016/j.joca.2015.08.009).
There were clinical improvements, too. At baseline, the mean International Knee Documentation Committee score improved from 38.7 at baseline to 67.3 at follow-up and the mean Tegner activity scale score improved from 2.5 at baseline to 3.9 at follow-up. The results were all statistically significant and independent of age, sex, body mass index, and size and location of the cartilage lesions.
“This study showed encouraging clinical outcomes of [mesenchymal stem cell] implantation with fibrin glue as a scaffold in OA knees,” and it adds to the growing body of literature supporting mesenchymal stem cells (MSCs) in osteoarthritis. “MSC implantation with fibrin glue as a scaffold seems to be effective for repairing cartilage lesions in OA knees,” the investigators wrote.
The cells were derived from fat liposuctioned from the subjects’ buttocks and delivered to their damaged knees in a fibrinogen-thrombin gel under arthroscopic guidance. The cartilage lesions, which were most often on the medial femoral condyle, were debrided beforehand. Patients’ knees were immobilized for 2 weeks after transplant. Weight bearing was allowed at 4 weeks, and sports at 3 months.
It’s not fully known why MSCs help knee OA. Perhaps they release factors that stimulate cartilage formation by resident chondrocytes or other cells in the joint, and inhibit joint inflammation. The investigators derived their cells from fat – instead of bone marrow – because stem cells from fat are easier to get, easy to purify, and may be more chondrogenic. Fat is also richer in stem cells than marrow.
The next step is to identify predictors of good outcomes. “Patient characteristics or cartilage lesion variables may serve as important selection criteria for stem cell–based repair strategies,” Dr. Kim and his associates said.
The researchers had no disclosures.
Mesenchymal stem cell transplants appeared to regenerate cartilage and improve clinical outcomes after 2 years in patients with knee osteoarthritis in a small South Korean study.
There were 24 treated knees among the 11 men and 9 women in the study, whose average age was 58 years and mean body mass index was 26.6 kg/m2.
At baseline, 21 lesions (87.5%) were grade 2 or 3 on the MRI Osteoarthritis Knee Score scale for size of cartilage-loss area, and 23 lesions (95.9%) were grade 2 or 3 for percentage of full-thickness cartilage loss. A grade of 2-3 indicates moderate to severe disease.
At MRI follow-up 2 years after transplant, only five lesions (20.8%) were grade 2 or 3 for cartilage-loss area and five were grade 2 or 3 for full-thickness cartilage loss, reported Dr. Yong Sang Kim of the Yonsei Sarang Hospital in Seoul and his associates (Osteoarthritis Cartilage. 2015. doi: 10.1016/j.joca.2015.08.009).
There were clinical improvements, too. At baseline, the mean International Knee Documentation Committee score improved from 38.7 at baseline to 67.3 at follow-up and the mean Tegner activity scale score improved from 2.5 at baseline to 3.9 at follow-up. The results were all statistically significant and independent of age, sex, body mass index, and size and location of the cartilage lesions.
“This study showed encouraging clinical outcomes of [mesenchymal stem cell] implantation with fibrin glue as a scaffold in OA knees,” and it adds to the growing body of literature supporting mesenchymal stem cells (MSCs) in osteoarthritis. “MSC implantation with fibrin glue as a scaffold seems to be effective for repairing cartilage lesions in OA knees,” the investigators wrote.
The cells were derived from fat liposuctioned from the subjects’ buttocks and delivered to their damaged knees in a fibrinogen-thrombin gel under arthroscopic guidance. The cartilage lesions, which were most often on the medial femoral condyle, were debrided beforehand. Patients’ knees were immobilized for 2 weeks after transplant. Weight bearing was allowed at 4 weeks, and sports at 3 months.
It’s not fully known why MSCs help knee OA. Perhaps they release factors that stimulate cartilage formation by resident chondrocytes or other cells in the joint, and inhibit joint inflammation. The investigators derived their cells from fat – instead of bone marrow – because stem cells from fat are easier to get, easy to purify, and may be more chondrogenic. Fat is also richer in stem cells than marrow.
The next step is to identify predictors of good outcomes. “Patient characteristics or cartilage lesion variables may serve as important selection criteria for stem cell–based repair strategies,” Dr. Kim and his associates said.
The researchers had no disclosures.
Mesenchymal stem cell transplants appeared to regenerate cartilage and improve clinical outcomes after 2 years in patients with knee osteoarthritis in a small South Korean study.
There were 24 treated knees among the 11 men and 9 women in the study, whose average age was 58 years and mean body mass index was 26.6 kg/m2.
At baseline, 21 lesions (87.5%) were grade 2 or 3 on the MRI Osteoarthritis Knee Score scale for size of cartilage-loss area, and 23 lesions (95.9%) were grade 2 or 3 for percentage of full-thickness cartilage loss. A grade of 2-3 indicates moderate to severe disease.
At MRI follow-up 2 years after transplant, only five lesions (20.8%) were grade 2 or 3 for cartilage-loss area and five were grade 2 or 3 for full-thickness cartilage loss, reported Dr. Yong Sang Kim of the Yonsei Sarang Hospital in Seoul and his associates (Osteoarthritis Cartilage. 2015. doi: 10.1016/j.joca.2015.08.009).
There were clinical improvements, too. At baseline, the mean International Knee Documentation Committee score improved from 38.7 at baseline to 67.3 at follow-up and the mean Tegner activity scale score improved from 2.5 at baseline to 3.9 at follow-up. The results were all statistically significant and independent of age, sex, body mass index, and size and location of the cartilage lesions.
“This study showed encouraging clinical outcomes of [mesenchymal stem cell] implantation with fibrin glue as a scaffold in OA knees,” and it adds to the growing body of literature supporting mesenchymal stem cells (MSCs) in osteoarthritis. “MSC implantation with fibrin glue as a scaffold seems to be effective for repairing cartilage lesions in OA knees,” the investigators wrote.
The cells were derived from fat liposuctioned from the subjects’ buttocks and delivered to their damaged knees in a fibrinogen-thrombin gel under arthroscopic guidance. The cartilage lesions, which were most often on the medial femoral condyle, were debrided beforehand. Patients’ knees were immobilized for 2 weeks after transplant. Weight bearing was allowed at 4 weeks, and sports at 3 months.
It’s not fully known why MSCs help knee OA. Perhaps they release factors that stimulate cartilage formation by resident chondrocytes or other cells in the joint, and inhibit joint inflammation. The investigators derived their cells from fat – instead of bone marrow – because stem cells from fat are easier to get, easy to purify, and may be more chondrogenic. Fat is also richer in stem cells than marrow.
The next step is to identify predictors of good outcomes. “Patient characteristics or cartilage lesion variables may serve as important selection criteria for stem cell–based repair strategies,” Dr. Kim and his associates said.
The researchers had no disclosures.
FROM OSTEOARTHRITIS AND CARTILAGE
Key clinical point: Mesenchymal stem cell transplants give promising 2-year results in knee osteoarthritis, but larger studies with longer follow-up periods are needed.
Major finding: At baseline, 23 lesions (95.9%) were grade 2 or 3 for percentage of full-thickness cartilage loss; at MRI follow-up 2 years after transplant, only five lesions (20.8%) were grade 2 or 3.
Data source: A 2-year follow-up of 24 treated knees.
Disclosures: The investigators had no disclosures.
Muscle power tops strength for knee OA assessment
Leg extensor muscle power is an independent determinant of pain and quality of life in knee osteoarthritis, according to a cross-sectional analysis of data from a trial.
“Compared to strength, muscle power may be a more clinically important measure of muscle function within this population. New trials to systematically examine the impact of muscle power training interventions on disease severity in knee OA are particularly warranted,” concluded the investigators, led by Kieran Reid, Ph.D., of Tufts University in Boston (Arthritis Rheumatol. 2015 Aug 28. doi: 10.1002/art.39336).
The conclusion comes from their analysis of baseline data for 190 subjects participating in a trial comparing Tai Chi to standard physical therapy for knee OA. Their mean age was 60 years, and they had a mean body mass index of 32.7 kg/m2. The majority of participants were women.
On univariate analysis, greater muscle power – defined as the product of dynamic muscular force and muscle contraction velocity – was significantly associated with pain (r = –0.17, P less than .02), as assessed by the Western Ontario and McMaster Osteoarthritis Index. It was also significantly and positively associated with Short Form 36 physical component scores (r = 0.16, P less than .05), a measure of health-related quality of life.
After adjusting for multiple covariates, muscle power was a significant independent predictor of pain (P less than or equal to .05) and physical component scores (P less than or equal to .04). Strength, a far more common assessment in knee OA, was not an independent determinant of either pain or quality of life (P greater than or equal to .06).
To measure leg extension muscle power, subjects were asked to do two sets of five bilateral leg press repetitions, one set at 40% of their maximum strength and one set at 70%. Each repetition within a set was performed as quickly as possible, with 30 seconds rest between each repetition.
Knee OA patients “have substantial and accelerated impairments in muscle power,” and might benefit from training to improve it. Power generated at 70% resistance in the study seemed to be “a marginally greater determinant of pain and quality of life in knee OA” than power at 40%, suggesting that higher intensity training might be more helpful.
The investigators had several ideas about how power relates to knee pain. Power, rather than strength alone, helps keep the knee stable when in motion. Impairment could contribute to “inadequate control of tibial translation during ambulation, leading to damage, and ultimately knee pain.” Also, impairment probably limits the ability to dissipate knee joint loads, increasing “the risk of articular contact stress, which leads to pain,” they said.
“Muscle strength, the maximal force generating capacity of skeletal muscle, has been the focus of numerous investigations. However, studies examining muscle strength in the etiology of knee OA have presented inconsistent and controversial findings.” Dynamic leg extensor power “represents a more functionally relevant assessment of muscle performance and ... a more clinically important correlate of disease burden in knee OA,” they said.
The study was supported by grants from the National Institutes of Health. The investigators have no disclosures.
Leg extensor muscle power is an independent determinant of pain and quality of life in knee osteoarthritis, according to a cross-sectional analysis of data from a trial.
“Compared to strength, muscle power may be a more clinically important measure of muscle function within this population. New trials to systematically examine the impact of muscle power training interventions on disease severity in knee OA are particularly warranted,” concluded the investigators, led by Kieran Reid, Ph.D., of Tufts University in Boston (Arthritis Rheumatol. 2015 Aug 28. doi: 10.1002/art.39336).
The conclusion comes from their analysis of baseline data for 190 subjects participating in a trial comparing Tai Chi to standard physical therapy for knee OA. Their mean age was 60 years, and they had a mean body mass index of 32.7 kg/m2. The majority of participants were women.
On univariate analysis, greater muscle power – defined as the product of dynamic muscular force and muscle contraction velocity – was significantly associated with pain (r = –0.17, P less than .02), as assessed by the Western Ontario and McMaster Osteoarthritis Index. It was also significantly and positively associated with Short Form 36 physical component scores (r = 0.16, P less than .05), a measure of health-related quality of life.
After adjusting for multiple covariates, muscle power was a significant independent predictor of pain (P less than or equal to .05) and physical component scores (P less than or equal to .04). Strength, a far more common assessment in knee OA, was not an independent determinant of either pain or quality of life (P greater than or equal to .06).
To measure leg extension muscle power, subjects were asked to do two sets of five bilateral leg press repetitions, one set at 40% of their maximum strength and one set at 70%. Each repetition within a set was performed as quickly as possible, with 30 seconds rest between each repetition.
Knee OA patients “have substantial and accelerated impairments in muscle power,” and might benefit from training to improve it. Power generated at 70% resistance in the study seemed to be “a marginally greater determinant of pain and quality of life in knee OA” than power at 40%, suggesting that higher intensity training might be more helpful.
The investigators had several ideas about how power relates to knee pain. Power, rather than strength alone, helps keep the knee stable when in motion. Impairment could contribute to “inadequate control of tibial translation during ambulation, leading to damage, and ultimately knee pain.” Also, impairment probably limits the ability to dissipate knee joint loads, increasing “the risk of articular contact stress, which leads to pain,” they said.
“Muscle strength, the maximal force generating capacity of skeletal muscle, has been the focus of numerous investigations. However, studies examining muscle strength in the etiology of knee OA have presented inconsistent and controversial findings.” Dynamic leg extensor power “represents a more functionally relevant assessment of muscle performance and ... a more clinically important correlate of disease burden in knee OA,” they said.
The study was supported by grants from the National Institutes of Health. The investigators have no disclosures.
Leg extensor muscle power is an independent determinant of pain and quality of life in knee osteoarthritis, according to a cross-sectional analysis of data from a trial.
“Compared to strength, muscle power may be a more clinically important measure of muscle function within this population. New trials to systematically examine the impact of muscle power training interventions on disease severity in knee OA are particularly warranted,” concluded the investigators, led by Kieran Reid, Ph.D., of Tufts University in Boston (Arthritis Rheumatol. 2015 Aug 28. doi: 10.1002/art.39336).
The conclusion comes from their analysis of baseline data for 190 subjects participating in a trial comparing Tai Chi to standard physical therapy for knee OA. Their mean age was 60 years, and they had a mean body mass index of 32.7 kg/m2. The majority of participants were women.
On univariate analysis, greater muscle power – defined as the product of dynamic muscular force and muscle contraction velocity – was significantly associated with pain (r = –0.17, P less than .02), as assessed by the Western Ontario and McMaster Osteoarthritis Index. It was also significantly and positively associated with Short Form 36 physical component scores (r = 0.16, P less than .05), a measure of health-related quality of life.
After adjusting for multiple covariates, muscle power was a significant independent predictor of pain (P less than or equal to .05) and physical component scores (P less than or equal to .04). Strength, a far more common assessment in knee OA, was not an independent determinant of either pain or quality of life (P greater than or equal to .06).
To measure leg extension muscle power, subjects were asked to do two sets of five bilateral leg press repetitions, one set at 40% of their maximum strength and one set at 70%. Each repetition within a set was performed as quickly as possible, with 30 seconds rest between each repetition.
Knee OA patients “have substantial and accelerated impairments in muscle power,” and might benefit from training to improve it. Power generated at 70% resistance in the study seemed to be “a marginally greater determinant of pain and quality of life in knee OA” than power at 40%, suggesting that higher intensity training might be more helpful.
The investigators had several ideas about how power relates to knee pain. Power, rather than strength alone, helps keep the knee stable when in motion. Impairment could contribute to “inadequate control of tibial translation during ambulation, leading to damage, and ultimately knee pain.” Also, impairment probably limits the ability to dissipate knee joint loads, increasing “the risk of articular contact stress, which leads to pain,” they said.
“Muscle strength, the maximal force generating capacity of skeletal muscle, has been the focus of numerous investigations. However, studies examining muscle strength in the etiology of knee OA have presented inconsistent and controversial findings.” Dynamic leg extensor power “represents a more functionally relevant assessment of muscle performance and ... a more clinically important correlate of disease burden in knee OA,” they said.
The study was supported by grants from the National Institutes of Health. The investigators have no disclosures.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: It might be time to learn how to assess muscle power in your knee OA patients.
Major finding: After adjusting for multiple covariates, muscle power was a significant independent predictor of pain (P less than or equal to .05) and physical component scores (P less than or equal to .04).
Data source: Cross-sectional analysis of 190 knee OA patients.
Disclosures: The study was supported by grants from the National Institutes of Health. The investigators have no disclosures.
Breastfeeding protects against postpartum MS relapse
New mothers with MS are less likely to have a relapse within 6 months of delivery if they breastfeed their babies exclusively for at least 2 months, according to the findings of a prospective study of 201 women published online Aug. 31 in JAMA Neurology.
“Our findings indicate that women with MS should be supported if they choose to breastfeed exclusively since it clearly does not increase the risk of postpartum relapse. Relapse in the first 6 months post partum may be diminished by exclusive breastfeeding, but once regular feedings are introduced, disease activity is likely to return,” wrote Dr. Kerstin Hellwig of Ruhr-University Bochum (Germany) and her colleagues (JAMA Neurol. 2015 Aug 31. doi: 10.1001/jamaneurol.2015.1806).
The effect of breastfeeding on postpartum MS relapse has been controversial. Some studies have found that exclusive breastfeeding for at least the first 2 months might be beneficial, while others – studies that “defined breastfeeding crudely and/or measured breastfeeding retrospectively,” the authors said – found no protective effect.
The 201 women in the study had relapsing-remitting MS for a median of about 4.5 years and had voluntarily enrolled while pregnant in the German MS and pregnancy registry. They completed a series of questionnaires during pregnancy and the first post partum year.
Overall, 120 women (59.7%) intended to breastfeed exclusively for at least 2 months; 42 (20.9%) combined breastfeeding with supplemental feedings within the first 2 months; and 39 (19.4%) did not breastfeed; 178 (88.6%) reported using disease-modifying therapies before pregnancy, most often glatiramer acetate or interferon beta.
Among the 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 post partum months, compared with 29 women (24.2%) among the 120 who breastfed their children exclusively (adjusted hazard ratio, 1.70; 95% confidence interval, 1.02-2.85; P = 0.04).
The researchers described exclusively breastfeeding as acting like a “modestly effective treatment with a natural end date.”
“During exclusive breastfeeding, the pulsatile release of gonadotropin-releasing hormone and luteinizing hormone is suppressed with a corresponding suppression of the growth of ovarian follicles resulting in lactational amenorrhea and anovulation. Shortly after the breastfeeding frequency is reduced (1 or 2 regularly replaced breastfeeding meals are sufficient to interrupt this cycle), the ovarian activity resumes with the return of menses,” Dr. Hellwig and her associates wrote.
They also speculated that the “hormonal changes leading to anovulation might play a key role since women with MS are less likely to receive the diagnosis during their anovulatory years (childhood or after menopause) and women with MS were found to be more likely to experience relapse shortly before menstruation.”
The mean age in the study was about 31 years, but women who breastfed exclusively tended to be older than their peers and less likely to have received disease-modifying therapies before or at the time of conception. The first postpartum menses among exclusive breastfeeders came at a median of 185 days vs. 64 days for other women.
“In the present study, we observed that an earlier return of menses was associated with a higher risk of relapse in the first 6 months post-partum,” the authors wrote.
The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
New mothers with MS are less likely to have a relapse within 6 months of delivery if they breastfeed their babies exclusively for at least 2 months, according to the findings of a prospective study of 201 women published online Aug. 31 in JAMA Neurology.
“Our findings indicate that women with MS should be supported if they choose to breastfeed exclusively since it clearly does not increase the risk of postpartum relapse. Relapse in the first 6 months post partum may be diminished by exclusive breastfeeding, but once regular feedings are introduced, disease activity is likely to return,” wrote Dr. Kerstin Hellwig of Ruhr-University Bochum (Germany) and her colleagues (JAMA Neurol. 2015 Aug 31. doi: 10.1001/jamaneurol.2015.1806).
The effect of breastfeeding on postpartum MS relapse has been controversial. Some studies have found that exclusive breastfeeding for at least the first 2 months might be beneficial, while others – studies that “defined breastfeeding crudely and/or measured breastfeeding retrospectively,” the authors said – found no protective effect.
The 201 women in the study had relapsing-remitting MS for a median of about 4.5 years and had voluntarily enrolled while pregnant in the German MS and pregnancy registry. They completed a series of questionnaires during pregnancy and the first post partum year.
Overall, 120 women (59.7%) intended to breastfeed exclusively for at least 2 months; 42 (20.9%) combined breastfeeding with supplemental feedings within the first 2 months; and 39 (19.4%) did not breastfeed; 178 (88.6%) reported using disease-modifying therapies before pregnancy, most often glatiramer acetate or interferon beta.
Among the 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 post partum months, compared with 29 women (24.2%) among the 120 who breastfed their children exclusively (adjusted hazard ratio, 1.70; 95% confidence interval, 1.02-2.85; P = 0.04).
The researchers described exclusively breastfeeding as acting like a “modestly effective treatment with a natural end date.”
“During exclusive breastfeeding, the pulsatile release of gonadotropin-releasing hormone and luteinizing hormone is suppressed with a corresponding suppression of the growth of ovarian follicles resulting in lactational amenorrhea and anovulation. Shortly after the breastfeeding frequency is reduced (1 or 2 regularly replaced breastfeeding meals are sufficient to interrupt this cycle), the ovarian activity resumes with the return of menses,” Dr. Hellwig and her associates wrote.
They also speculated that the “hormonal changes leading to anovulation might play a key role since women with MS are less likely to receive the diagnosis during their anovulatory years (childhood or after menopause) and women with MS were found to be more likely to experience relapse shortly before menstruation.”
The mean age in the study was about 31 years, but women who breastfed exclusively tended to be older than their peers and less likely to have received disease-modifying therapies before or at the time of conception. The first postpartum menses among exclusive breastfeeders came at a median of 185 days vs. 64 days for other women.
“In the present study, we observed that an earlier return of menses was associated with a higher risk of relapse in the first 6 months post-partum,” the authors wrote.
The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
New mothers with MS are less likely to have a relapse within 6 months of delivery if they breastfeed their babies exclusively for at least 2 months, according to the findings of a prospective study of 201 women published online Aug. 31 in JAMA Neurology.
“Our findings indicate that women with MS should be supported if they choose to breastfeed exclusively since it clearly does not increase the risk of postpartum relapse. Relapse in the first 6 months post partum may be diminished by exclusive breastfeeding, but once regular feedings are introduced, disease activity is likely to return,” wrote Dr. Kerstin Hellwig of Ruhr-University Bochum (Germany) and her colleagues (JAMA Neurol. 2015 Aug 31. doi: 10.1001/jamaneurol.2015.1806).
The effect of breastfeeding on postpartum MS relapse has been controversial. Some studies have found that exclusive breastfeeding for at least the first 2 months might be beneficial, while others – studies that “defined breastfeeding crudely and/or measured breastfeeding retrospectively,” the authors said – found no protective effect.
The 201 women in the study had relapsing-remitting MS for a median of about 4.5 years and had voluntarily enrolled while pregnant in the German MS and pregnancy registry. They completed a series of questionnaires during pregnancy and the first post partum year.
Overall, 120 women (59.7%) intended to breastfeed exclusively for at least 2 months; 42 (20.9%) combined breastfeeding with supplemental feedings within the first 2 months; and 39 (19.4%) did not breastfeed; 178 (88.6%) reported using disease-modifying therapies before pregnancy, most often glatiramer acetate or interferon beta.
Among the 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 post partum months, compared with 29 women (24.2%) among the 120 who breastfed their children exclusively (adjusted hazard ratio, 1.70; 95% confidence interval, 1.02-2.85; P = 0.04).
The researchers described exclusively breastfeeding as acting like a “modestly effective treatment with a natural end date.”
“During exclusive breastfeeding, the pulsatile release of gonadotropin-releasing hormone and luteinizing hormone is suppressed with a corresponding suppression of the growth of ovarian follicles resulting in lactational amenorrhea and anovulation. Shortly after the breastfeeding frequency is reduced (1 or 2 regularly replaced breastfeeding meals are sufficient to interrupt this cycle), the ovarian activity resumes with the return of menses,” Dr. Hellwig and her associates wrote.
They also speculated that the “hormonal changes leading to anovulation might play a key role since women with MS are less likely to receive the diagnosis during their anovulatory years (childhood or after menopause) and women with MS were found to be more likely to experience relapse shortly before menstruation.”
The mean age in the study was about 31 years, but women who breastfed exclusively tended to be older than their peers and less likely to have received disease-modifying therapies before or at the time of conception. The first postpartum menses among exclusive breastfeeders came at a median of 185 days vs. 64 days for other women.
“In the present study, we observed that an earlier return of menses was associated with a higher risk of relapse in the first 6 months post-partum,” the authors wrote.
The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
FROM JAMA NEUROLOGY
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first 6 postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least 2 months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for 1 year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
PCV13 boosts pediatric pneumococcal meningitis antibiotic susceptibility
The 2010 replacement of 7-valent pneumococcal conjugate vaccine with 13-valent pneumococcal conjugate vaccine (Prevnar 13) has not reduced pneumococcal meningitis incidence, morbidity, or mortality in children, but the serology has shifted, according to a review of 173 cases of the illness at eight U.S. children’s hospitals published in Clinical Infectious Diseases.
Seventy-six (44%) of those cases occurred during 2007-2009 – before the switch was made – and 69 (40%) occurred afterward during 2011-2013. The remaining 28 cases were in the transition year of 2010 (Clin Infect Dis. 2015 Sep 1;61[5]:767-75).
“Our study revealed that after the introduction of PCV13 [pneumococcal conjugate vaccine 13], the number of cases of PM [pneumococcal meningitis] per year remained unchanged,” said the investigators led by Dr. Liset Olarte of the Baylor College of Medicine in Houston.
However, serology has shifted; the proportion of PCV13 serotype cases decreased from 54% during 2007-2009 to 27% during 2011-2013, meaning that non–PCV13 serotype cases represented 73% of all isolates during 2011-2013.
Antibiotic resistance also has decreased significantly. “After the introduction of PCV13, we found a significant decline in ceftriaxone-nonsusceptible isolates (MIC at or above 1 mcg/mL) from 13% to 3%. No ceftriaxone-resistant isolates (minimum inhibitory concentrations [MIC] at or above 2 mcg/mL) were identified in 2011-2013. ... These changes were closely related to the decline of serotype 19A.” Serotype 19A – which is covered by PCV13 – decreased 44% from 2007-2009 to 2011-2013, but remained the most common serotype in 2011-2013, the investigators said.
“If this trend continues, vancomycin may no longer be required as empiric therapy in addition to ceftriaxone or cefotaxime for children who are fully immunized with PCV13,” but “nonsusceptibility to penicillin is still relatively high; therefore, ceftriaxone or cefotaxime should continue to be the antibiotic of choice for empiric therapy. An increasing predominance of penicillin-nonsusceptible, non-PCV13 serotypes, such as 35B in the United States, warrants continuous monitoring of pneumococcal isolates,” they said.
There were slightly more boys than girls among the 173 cases, and about half the cases were in white children. The median patient age was about 1-2 years, and about a third of the children had an underlying disorder, with CNS problems such as head trauma or hydrocephalus being the most common. About half the children were thought to have been adequately immunized.
There were three (4%) deaths from 2007-2009; two (7%) in 2010; and seven (10%) from 2011-2013; the differences were not statistically significant.
The investigators are part of the U.S. Pediatric Multicenter Pneumococcal Surveillance Study, which includes Baylor, the University of Pittsburgh, and other universities and affiliated children’s hospitals.
The work was funded in part by Pfizer, Prevnar’s maker. The investigators said they had no financial conflicts.
The 2010 replacement of 7-valent pneumococcal conjugate vaccine with 13-valent pneumococcal conjugate vaccine (Prevnar 13) has not reduced pneumococcal meningitis incidence, morbidity, or mortality in children, but the serology has shifted, according to a review of 173 cases of the illness at eight U.S. children’s hospitals published in Clinical Infectious Diseases.
Seventy-six (44%) of those cases occurred during 2007-2009 – before the switch was made – and 69 (40%) occurred afterward during 2011-2013. The remaining 28 cases were in the transition year of 2010 (Clin Infect Dis. 2015 Sep 1;61[5]:767-75).
“Our study revealed that after the introduction of PCV13 [pneumococcal conjugate vaccine 13], the number of cases of PM [pneumococcal meningitis] per year remained unchanged,” said the investigators led by Dr. Liset Olarte of the Baylor College of Medicine in Houston.
However, serology has shifted; the proportion of PCV13 serotype cases decreased from 54% during 2007-2009 to 27% during 2011-2013, meaning that non–PCV13 serotype cases represented 73% of all isolates during 2011-2013.
Antibiotic resistance also has decreased significantly. “After the introduction of PCV13, we found a significant decline in ceftriaxone-nonsusceptible isolates (MIC at or above 1 mcg/mL) from 13% to 3%. No ceftriaxone-resistant isolates (minimum inhibitory concentrations [MIC] at or above 2 mcg/mL) were identified in 2011-2013. ... These changes were closely related to the decline of serotype 19A.” Serotype 19A – which is covered by PCV13 – decreased 44% from 2007-2009 to 2011-2013, but remained the most common serotype in 2011-2013, the investigators said.
“If this trend continues, vancomycin may no longer be required as empiric therapy in addition to ceftriaxone or cefotaxime for children who are fully immunized with PCV13,” but “nonsusceptibility to penicillin is still relatively high; therefore, ceftriaxone or cefotaxime should continue to be the antibiotic of choice for empiric therapy. An increasing predominance of penicillin-nonsusceptible, non-PCV13 serotypes, such as 35B in the United States, warrants continuous monitoring of pneumococcal isolates,” they said.
There were slightly more boys than girls among the 173 cases, and about half the cases were in white children. The median patient age was about 1-2 years, and about a third of the children had an underlying disorder, with CNS problems such as head trauma or hydrocephalus being the most common. About half the children were thought to have been adequately immunized.
There were three (4%) deaths from 2007-2009; two (7%) in 2010; and seven (10%) from 2011-2013; the differences were not statistically significant.
The investigators are part of the U.S. Pediatric Multicenter Pneumococcal Surveillance Study, which includes Baylor, the University of Pittsburgh, and other universities and affiliated children’s hospitals.
The work was funded in part by Pfizer, Prevnar’s maker. The investigators said they had no financial conflicts.
The 2010 replacement of 7-valent pneumococcal conjugate vaccine with 13-valent pneumococcal conjugate vaccine (Prevnar 13) has not reduced pneumococcal meningitis incidence, morbidity, or mortality in children, but the serology has shifted, according to a review of 173 cases of the illness at eight U.S. children’s hospitals published in Clinical Infectious Diseases.
Seventy-six (44%) of those cases occurred during 2007-2009 – before the switch was made – and 69 (40%) occurred afterward during 2011-2013. The remaining 28 cases were in the transition year of 2010 (Clin Infect Dis. 2015 Sep 1;61[5]:767-75).
“Our study revealed that after the introduction of PCV13 [pneumococcal conjugate vaccine 13], the number of cases of PM [pneumococcal meningitis] per year remained unchanged,” said the investigators led by Dr. Liset Olarte of the Baylor College of Medicine in Houston.
However, serology has shifted; the proportion of PCV13 serotype cases decreased from 54% during 2007-2009 to 27% during 2011-2013, meaning that non–PCV13 serotype cases represented 73% of all isolates during 2011-2013.
Antibiotic resistance also has decreased significantly. “After the introduction of PCV13, we found a significant decline in ceftriaxone-nonsusceptible isolates (MIC at or above 1 mcg/mL) from 13% to 3%. No ceftriaxone-resistant isolates (minimum inhibitory concentrations [MIC] at or above 2 mcg/mL) were identified in 2011-2013. ... These changes were closely related to the decline of serotype 19A.” Serotype 19A – which is covered by PCV13 – decreased 44% from 2007-2009 to 2011-2013, but remained the most common serotype in 2011-2013, the investigators said.
“If this trend continues, vancomycin may no longer be required as empiric therapy in addition to ceftriaxone or cefotaxime for children who are fully immunized with PCV13,” but “nonsusceptibility to penicillin is still relatively high; therefore, ceftriaxone or cefotaxime should continue to be the antibiotic of choice for empiric therapy. An increasing predominance of penicillin-nonsusceptible, non-PCV13 serotypes, such as 35B in the United States, warrants continuous monitoring of pneumococcal isolates,” they said.
There were slightly more boys than girls among the 173 cases, and about half the cases were in white children. The median patient age was about 1-2 years, and about a third of the children had an underlying disorder, with CNS problems such as head trauma or hydrocephalus being the most common. About half the children were thought to have been adequately immunized.
There were three (4%) deaths from 2007-2009; two (7%) in 2010; and seven (10%) from 2011-2013; the differences were not statistically significant.
The investigators are part of the U.S. Pediatric Multicenter Pneumococcal Surveillance Study, which includes Baylor, the University of Pittsburgh, and other universities and affiliated children’s hospitals.
The work was funded in part by Pfizer, Prevnar’s maker. The investigators said they had no financial conflicts.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: For children fully immunized with PCV13, empiric vancomycin may soon no longer be needed for pneumococcal meningitis.
Major finding: Following the 2010 introduction of PCV13, ceftriaxone-nonsusceptible isolates decreased significantly, with no isolates detected with a ceftriaxone minimum inhibitory concentration at or above 2 mcg/mL.
Data source: 173 pneumococcal meningitis cases at eight U.S. children’s hospitals
Disclosures: The work was funded in part by Pfizer, maker of the vaccine. The investigators said they have no financial conflicts.
As psoriasis maintenance therapy, step-down matched full-dose etanercept for quality of life measures
At 6 months, quality of life is largely the same whether psoriasis patients stay on their initial etanercept (Enbrel) dose of 50 mg twice weekly or step down to 50 mg once weekly with topical corticosteroids, according to a Canadian trial published in the Journal of The European Academy of Dermatology and Venereology.
“The opportunity to use a topical agent seemed to improve [patients’] overall satisfaction,” and weekly “etanercept and topical may be a less costly option compared with” etanercept twice weekly, said the investigators, led by Dr. Kim Papp, of Probity Medical Research in Waterloo, Ont.
Previously, the investigators showed that the step-down strategy works as well as full-dose maintenance in clearing the skin. They wanted to check if that also held true for quality of life, as assessed by the Dermatology Life Quality Index (DLQI) and the Treatment Satisfaction Questionnaire for Medication (TSQM). (J Eur Acad Dermatol Venereol. 2015 Aug;29(8):1555-61).
The 287 patients, all with moderate-to-severe plaque psoriasis for at least half a year, received etanercept 50 mg twice weekly for 12 weeks; 144 were then randomized to stay on that dose, and 143 others to drop to 50 mg weekly with the addition of topicals as required through week 24. The mean age of patients in the trial was 45 years; 88% of the subjects were white and 65% were men.
Topicals included hydrocortisone 2.5%, beta methasone valerate 0.1%, betamethasone dipropionate 0.05%, clobetasol 0.05%, calcitriol, or calcipotriol plus betamethasone dipropionate 0.05%; the topicals were selected by the researchers, who were allowed to change them as needed.
The mean change in DLQI from baseline to week 24 was 10.7 points in the etanercept group and 9.9 points in the etanercept-plus-topical group. Mean change in TSQM effectiveness, convenience, side-effects, and global satisfaction was 27.1, 14.8, -0.7 and 26.7 points in the etanercept group, and 32.5, 18.5, 1.3, and 28.4 points in the etanercept-plus-topical group. Healthcare visits, employment status, work productivity, and ability to perform daily activities were similar between the treatment arms.
The study was descriptive; the investigators didn’t run statistical analyses. Even so, measures of quality of life “were numerically similar in patients who stayed on etanercept 50 mg [twice weekly] and patients who received etanercept 50 mg [weekly] plus topical therapies. No notable differences between treatment arms ... were observed. Additionally, improvements in [Psoriasis Area and Severity Index] scores appeared to correlate with improvements in patient reported outcomes,” the investigators said.
“The duration of treatment with topical therapies was only 12 weeks; a longer duration may result in loss of efficacy as adherence to topical therapies may decrease with time,” they noted.
The work was funded by etanercept’s maker, Amgen. Dr. Papp reported consulting for the company. Two other investigators have been Amgen advisors and reported research grants, speakers fees, or other payments.
At 6 months, quality of life is largely the same whether psoriasis patients stay on their initial etanercept (Enbrel) dose of 50 mg twice weekly or step down to 50 mg once weekly with topical corticosteroids, according to a Canadian trial published in the Journal of The European Academy of Dermatology and Venereology.
“The opportunity to use a topical agent seemed to improve [patients’] overall satisfaction,” and weekly “etanercept and topical may be a less costly option compared with” etanercept twice weekly, said the investigators, led by Dr. Kim Papp, of Probity Medical Research in Waterloo, Ont.
Previously, the investigators showed that the step-down strategy works as well as full-dose maintenance in clearing the skin. They wanted to check if that also held true for quality of life, as assessed by the Dermatology Life Quality Index (DLQI) and the Treatment Satisfaction Questionnaire for Medication (TSQM). (J Eur Acad Dermatol Venereol. 2015 Aug;29(8):1555-61).
The 287 patients, all with moderate-to-severe plaque psoriasis for at least half a year, received etanercept 50 mg twice weekly for 12 weeks; 144 were then randomized to stay on that dose, and 143 others to drop to 50 mg weekly with the addition of topicals as required through week 24. The mean age of patients in the trial was 45 years; 88% of the subjects were white and 65% were men.
Topicals included hydrocortisone 2.5%, beta methasone valerate 0.1%, betamethasone dipropionate 0.05%, clobetasol 0.05%, calcitriol, or calcipotriol plus betamethasone dipropionate 0.05%; the topicals were selected by the researchers, who were allowed to change them as needed.
The mean change in DLQI from baseline to week 24 was 10.7 points in the etanercept group and 9.9 points in the etanercept-plus-topical group. Mean change in TSQM effectiveness, convenience, side-effects, and global satisfaction was 27.1, 14.8, -0.7 and 26.7 points in the etanercept group, and 32.5, 18.5, 1.3, and 28.4 points in the etanercept-plus-topical group. Healthcare visits, employment status, work productivity, and ability to perform daily activities were similar between the treatment arms.
The study was descriptive; the investigators didn’t run statistical analyses. Even so, measures of quality of life “were numerically similar in patients who stayed on etanercept 50 mg [twice weekly] and patients who received etanercept 50 mg [weekly] plus topical therapies. No notable differences between treatment arms ... were observed. Additionally, improvements in [Psoriasis Area and Severity Index] scores appeared to correlate with improvements in patient reported outcomes,” the investigators said.
“The duration of treatment with topical therapies was only 12 weeks; a longer duration may result in loss of efficacy as adherence to topical therapies may decrease with time,” they noted.
The work was funded by etanercept’s maker, Amgen. Dr. Papp reported consulting for the company. Two other investigators have been Amgen advisors and reported research grants, speakers fees, or other payments.
At 6 months, quality of life is largely the same whether psoriasis patients stay on their initial etanercept (Enbrel) dose of 50 mg twice weekly or step down to 50 mg once weekly with topical corticosteroids, according to a Canadian trial published in the Journal of The European Academy of Dermatology and Venereology.
“The opportunity to use a topical agent seemed to improve [patients’] overall satisfaction,” and weekly “etanercept and topical may be a less costly option compared with” etanercept twice weekly, said the investigators, led by Dr. Kim Papp, of Probity Medical Research in Waterloo, Ont.
Previously, the investigators showed that the step-down strategy works as well as full-dose maintenance in clearing the skin. They wanted to check if that also held true for quality of life, as assessed by the Dermatology Life Quality Index (DLQI) and the Treatment Satisfaction Questionnaire for Medication (TSQM). (J Eur Acad Dermatol Venereol. 2015 Aug;29(8):1555-61).
The 287 patients, all with moderate-to-severe plaque psoriasis for at least half a year, received etanercept 50 mg twice weekly for 12 weeks; 144 were then randomized to stay on that dose, and 143 others to drop to 50 mg weekly with the addition of topicals as required through week 24. The mean age of patients in the trial was 45 years; 88% of the subjects were white and 65% were men.
Topicals included hydrocortisone 2.5%, beta methasone valerate 0.1%, betamethasone dipropionate 0.05%, clobetasol 0.05%, calcitriol, or calcipotriol plus betamethasone dipropionate 0.05%; the topicals were selected by the researchers, who were allowed to change them as needed.
The mean change in DLQI from baseline to week 24 was 10.7 points in the etanercept group and 9.9 points in the etanercept-plus-topical group. Mean change in TSQM effectiveness, convenience, side-effects, and global satisfaction was 27.1, 14.8, -0.7 and 26.7 points in the etanercept group, and 32.5, 18.5, 1.3, and 28.4 points in the etanercept-plus-topical group. Healthcare visits, employment status, work productivity, and ability to perform daily activities were similar between the treatment arms.
The study was descriptive; the investigators didn’t run statistical analyses. Even so, measures of quality of life “were numerically similar in patients who stayed on etanercept 50 mg [twice weekly] and patients who received etanercept 50 mg [weekly] plus topical therapies. No notable differences between treatment arms ... were observed. Additionally, improvements in [Psoriasis Area and Severity Index] scores appeared to correlate with improvements in patient reported outcomes,” the investigators said.
“The duration of treatment with topical therapies was only 12 weeks; a longer duration may result in loss of efficacy as adherence to topical therapies may decrease with time,” they noted.
The work was funded by etanercept’s maker, Amgen. Dr. Papp reported consulting for the company. Two other investigators have been Amgen advisors and reported research grants, speakers fees, or other payments.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEROLOGY
Surgeons tout Essure fix without hysterectomy
On Sept. 24, the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel will meet in Silver Spring, Md., to consider the future of the Essure permanent contraceptive device.
Essure consists of an inner stainless steel coil wrapped in polyethylene terephthalate fibers and encased in an outer nickel-titanium coil. It’s delivered hysteroscopically for partial placement into the fallopian tube.
Once in place, the fibers trigger scar formation to block the tube.
When approved in 2002, “it finally looked like we had the answer that we were looking for,” an effective, quick, and inexpensive sterilization procedure that could be done in-office without anesthesia, said Dr. James Presthus, an early adopter at Minnesota Gynecology & Surgery in Edina, Minn., and a former Essure adviser.
But through May 2015, the FDA has received 5,093 reports of sometimes-severe problems with Essure, including chronic pelvic pain, dysmenorrhea, constitutional symptoms, and a handful of deaths that may, or may not, be associated with the device.
The reports and the upcoming FDA meeting are being driven by women who say they have been hurt by Essure. They’ve organized online to share their stories; the Facebook page Essure Problems is probably their largest meeting space, with almost 20,000 members.
Patients on the page have been encouraging each other to report problems to the FDA, and some are involved in legal action against manufacturer Bayer HealthCare.
Complaints ignored
There’s anger behind those efforts, and part of it is due to how women say they have been treated by their physicians. Social media is full of stories from patients who say their concerns were dismissed by physicians who told them that Essure couldn’t be the cause of their problems, or that hysterectomy was the only way to get the coils out.
Both of those assertions are false, said Dr. Edio Zampaglione, vice president of Bayer’s Women’s Health Division and an ob.gyn.
According to company estimates, Essure causes chronic pain in about 4% of women, especially if it’s not placed correctly or migrates and punctures surrounding tissue. Constitutional symptoms – for example, rashes, joint pain, hair loss, and weight changes – could be related to the nickel in Essure’s outer coil, if patients are allergic.
“We do believe these women” and take their reports seriously, Dr. Zampaglione said.
Bayer is reaching out to them for more information, and beefing up the physician-training programs it inherited when it bought Essure’s original maker, Conceptus, in 2013, he said.
Current labeling recommends salpingotomy or salpingectomy as first-line options for removal, and after a request from the FDA, Bayer is updating Essure’s label to give more information on how to use those procedures to remove the device.
Doctors who recommend hysterectomy first are probably just staying in their comfort zone, Dr, Zampaglione said.
It’s difficult to pinpoint the exact adverse event rate for Essure, but Bayer asserts – and physicians with no connections to the company said – that complications are rare.
The cases reported to the FDA represent less than 1% of the 750,000 women Bayer estimates have received the device, most of them in the U.S. Even so, adverse event reporting to the agency is notoriously low for drugs and devices, 10% or less by most estimates.
“I’ve implanted hundreds of these over the years. A lot of those patients come back to me for annual examinations, and they are not reporting problems,” Dr. Presthus said, but “after almost 13 years, we are finding out that Essure is not perfect; nothing is.”
It’s also difficult to separate out coincidence from true problems with the device. Outside of uterine anomalies, nickel allergy seems to be the only definitive indicator that patients will have problems with Essure. Until more is known, timing is the key to figuring out if Essure needs to come out, Dr. Presthus said.
“If somebody has it put in and then has all kinds of pelvic pain, it’s pretty easy to make the connection that it could be the device. Certainly, when patients have allergic reactions that started after they had it implanted,” Essure needs to be addressed, he said.
Removal without hysterectomy
His first step in such cases is to remove the device to see if it helps. Over the past year, Dr. Presthus has done two or more cases a week laparoscopically and said he hasn’t run into problems. The cases take about a half-hour each, and 80% or more of women report symptom relief.
“I do a linear salpingotomy over the proximal portion of the fallopian tube where the device is, and use fine-tip forceps to tease the tissue apart and identify the device. You tease it out, regrasp it, tease it out, and regrasp it. It’s really important to pull in a straight line” so Essure doesn’t break. “It’s not necessary to cut deep into the cornea of the uterus. I get an x-ray on the table to ensure there’s no metal left,” Dr. Presthus said.
Women have to know that there are alternatives before they opt for Essure, and that it can be taken out if needed. “They don’t have to [just] live with their problems or have a hysterectomy. The discussion you have before the procedure is important,” he said.
Dr. Charles Monteith in Raleigh, N.C., and Dr. Mark Sanchez in Clearwater, Fla., prefer an open approach, operating through a small incision above the bikini line.
Both are long-time experts in reversing tubal ligations, and are in high demand lately to take out Essure, and sometimes even restore fertility. Fertility can be restored in about a third of patients, they said.
“If the device is removed within 3 months, it’ll come out fairly easily with traction,” Dr. Monteith said. After that, scarring locks it into place, “so we start dissection over the device and follow it down into the uterine cavity, and remove it intact” – without traction – “with the surrounding tubal muscularis. That’s worked well for us,” he said.
Dr. Monteith said he has removed or reversed Essure in about 200 women.
“Avoid electrocautery because it causes the nickel in the outer coil to burn, spark, and fracture. Also, have an appreciation for variable insertions of the device. We’ve seen the coils pushed too deeply” into the fallopian tube, and “we’ve seen them barely going in. You have to palpate them to know where they are” before taking them out, so as not to cut into them by accident, he said.
Dr. Sanchez, also a high-volume Essure surgeon, cuts down into the corneal segment of the uterus and slowly dissects out the coil. “You have to pay close attention to make sure you get all the bits of the coil out as much as possible.” From there, restoring fertility usually means reanastomosing the fallopian tubes and corneal reimplantation, he said.
Other surgeons have developed similar techniques (Contraception. 2013 Sep;88[3]:334-6.).
Dr. Presthus, Dr. Monteith, and Dr. Sanchez reported having no current connections to Bayer, and are not involved in legal action against the company.
On Sept. 24, the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel will meet in Silver Spring, Md., to consider the future of the Essure permanent contraceptive device.
Essure consists of an inner stainless steel coil wrapped in polyethylene terephthalate fibers and encased in an outer nickel-titanium coil. It’s delivered hysteroscopically for partial placement into the fallopian tube.
Once in place, the fibers trigger scar formation to block the tube.
When approved in 2002, “it finally looked like we had the answer that we were looking for,” an effective, quick, and inexpensive sterilization procedure that could be done in-office without anesthesia, said Dr. James Presthus, an early adopter at Minnesota Gynecology & Surgery in Edina, Minn., and a former Essure adviser.
But through May 2015, the FDA has received 5,093 reports of sometimes-severe problems with Essure, including chronic pelvic pain, dysmenorrhea, constitutional symptoms, and a handful of deaths that may, or may not, be associated with the device.
The reports and the upcoming FDA meeting are being driven by women who say they have been hurt by Essure. They’ve organized online to share their stories; the Facebook page Essure Problems is probably their largest meeting space, with almost 20,000 members.
Patients on the page have been encouraging each other to report problems to the FDA, and some are involved in legal action against manufacturer Bayer HealthCare.
Complaints ignored
There’s anger behind those efforts, and part of it is due to how women say they have been treated by their physicians. Social media is full of stories from patients who say their concerns were dismissed by physicians who told them that Essure couldn’t be the cause of their problems, or that hysterectomy was the only way to get the coils out.
Both of those assertions are false, said Dr. Edio Zampaglione, vice president of Bayer’s Women’s Health Division and an ob.gyn.
According to company estimates, Essure causes chronic pain in about 4% of women, especially if it’s not placed correctly or migrates and punctures surrounding tissue. Constitutional symptoms – for example, rashes, joint pain, hair loss, and weight changes – could be related to the nickel in Essure’s outer coil, if patients are allergic.
“We do believe these women” and take their reports seriously, Dr. Zampaglione said.
Bayer is reaching out to them for more information, and beefing up the physician-training programs it inherited when it bought Essure’s original maker, Conceptus, in 2013, he said.
Current labeling recommends salpingotomy or salpingectomy as first-line options for removal, and after a request from the FDA, Bayer is updating Essure’s label to give more information on how to use those procedures to remove the device.
Doctors who recommend hysterectomy first are probably just staying in their comfort zone, Dr, Zampaglione said.
It’s difficult to pinpoint the exact adverse event rate for Essure, but Bayer asserts – and physicians with no connections to the company said – that complications are rare.
The cases reported to the FDA represent less than 1% of the 750,000 women Bayer estimates have received the device, most of them in the U.S. Even so, adverse event reporting to the agency is notoriously low for drugs and devices, 10% or less by most estimates.
“I’ve implanted hundreds of these over the years. A lot of those patients come back to me for annual examinations, and they are not reporting problems,” Dr. Presthus said, but “after almost 13 years, we are finding out that Essure is not perfect; nothing is.”
It’s also difficult to separate out coincidence from true problems with the device. Outside of uterine anomalies, nickel allergy seems to be the only definitive indicator that patients will have problems with Essure. Until more is known, timing is the key to figuring out if Essure needs to come out, Dr. Presthus said.
“If somebody has it put in and then has all kinds of pelvic pain, it’s pretty easy to make the connection that it could be the device. Certainly, when patients have allergic reactions that started after they had it implanted,” Essure needs to be addressed, he said.
Removal without hysterectomy
His first step in such cases is to remove the device to see if it helps. Over the past year, Dr. Presthus has done two or more cases a week laparoscopically and said he hasn’t run into problems. The cases take about a half-hour each, and 80% or more of women report symptom relief.
“I do a linear salpingotomy over the proximal portion of the fallopian tube where the device is, and use fine-tip forceps to tease the tissue apart and identify the device. You tease it out, regrasp it, tease it out, and regrasp it. It’s really important to pull in a straight line” so Essure doesn’t break. “It’s not necessary to cut deep into the cornea of the uterus. I get an x-ray on the table to ensure there’s no metal left,” Dr. Presthus said.
Women have to know that there are alternatives before they opt for Essure, and that it can be taken out if needed. “They don’t have to [just] live with their problems or have a hysterectomy. The discussion you have before the procedure is important,” he said.
Dr. Charles Monteith in Raleigh, N.C., and Dr. Mark Sanchez in Clearwater, Fla., prefer an open approach, operating through a small incision above the bikini line.
Both are long-time experts in reversing tubal ligations, and are in high demand lately to take out Essure, and sometimes even restore fertility. Fertility can be restored in about a third of patients, they said.
“If the device is removed within 3 months, it’ll come out fairly easily with traction,” Dr. Monteith said. After that, scarring locks it into place, “so we start dissection over the device and follow it down into the uterine cavity, and remove it intact” – without traction – “with the surrounding tubal muscularis. That’s worked well for us,” he said.
Dr. Monteith said he has removed or reversed Essure in about 200 women.
“Avoid electrocautery because it causes the nickel in the outer coil to burn, spark, and fracture. Also, have an appreciation for variable insertions of the device. We’ve seen the coils pushed too deeply” into the fallopian tube, and “we’ve seen them barely going in. You have to palpate them to know where they are” before taking them out, so as not to cut into them by accident, he said.
Dr. Sanchez, also a high-volume Essure surgeon, cuts down into the corneal segment of the uterus and slowly dissects out the coil. “You have to pay close attention to make sure you get all the bits of the coil out as much as possible.” From there, restoring fertility usually means reanastomosing the fallopian tubes and corneal reimplantation, he said.
Other surgeons have developed similar techniques (Contraception. 2013 Sep;88[3]:334-6.).
Dr. Presthus, Dr. Monteith, and Dr. Sanchez reported having no current connections to Bayer, and are not involved in legal action against the company.
On Sept. 24, the Food and Drug Administration’s Obstetrics and Gynecology Devices Panel will meet in Silver Spring, Md., to consider the future of the Essure permanent contraceptive device.
Essure consists of an inner stainless steel coil wrapped in polyethylene terephthalate fibers and encased in an outer nickel-titanium coil. It’s delivered hysteroscopically for partial placement into the fallopian tube.
Once in place, the fibers trigger scar formation to block the tube.
When approved in 2002, “it finally looked like we had the answer that we were looking for,” an effective, quick, and inexpensive sterilization procedure that could be done in-office without anesthesia, said Dr. James Presthus, an early adopter at Minnesota Gynecology & Surgery in Edina, Minn., and a former Essure adviser.
But through May 2015, the FDA has received 5,093 reports of sometimes-severe problems with Essure, including chronic pelvic pain, dysmenorrhea, constitutional symptoms, and a handful of deaths that may, or may not, be associated with the device.
The reports and the upcoming FDA meeting are being driven by women who say they have been hurt by Essure. They’ve organized online to share their stories; the Facebook page Essure Problems is probably their largest meeting space, with almost 20,000 members.
Patients on the page have been encouraging each other to report problems to the FDA, and some are involved in legal action against manufacturer Bayer HealthCare.
Complaints ignored
There’s anger behind those efforts, and part of it is due to how women say they have been treated by their physicians. Social media is full of stories from patients who say their concerns were dismissed by physicians who told them that Essure couldn’t be the cause of their problems, or that hysterectomy was the only way to get the coils out.
Both of those assertions are false, said Dr. Edio Zampaglione, vice president of Bayer’s Women’s Health Division and an ob.gyn.
According to company estimates, Essure causes chronic pain in about 4% of women, especially if it’s not placed correctly or migrates and punctures surrounding tissue. Constitutional symptoms – for example, rashes, joint pain, hair loss, and weight changes – could be related to the nickel in Essure’s outer coil, if patients are allergic.
“We do believe these women” and take their reports seriously, Dr. Zampaglione said.
Bayer is reaching out to them for more information, and beefing up the physician-training programs it inherited when it bought Essure’s original maker, Conceptus, in 2013, he said.
Current labeling recommends salpingotomy or salpingectomy as first-line options for removal, and after a request from the FDA, Bayer is updating Essure’s label to give more information on how to use those procedures to remove the device.
Doctors who recommend hysterectomy first are probably just staying in their comfort zone, Dr, Zampaglione said.
It’s difficult to pinpoint the exact adverse event rate for Essure, but Bayer asserts – and physicians with no connections to the company said – that complications are rare.
The cases reported to the FDA represent less than 1% of the 750,000 women Bayer estimates have received the device, most of them in the U.S. Even so, adverse event reporting to the agency is notoriously low for drugs and devices, 10% or less by most estimates.
“I’ve implanted hundreds of these over the years. A lot of those patients come back to me for annual examinations, and they are not reporting problems,” Dr. Presthus said, but “after almost 13 years, we are finding out that Essure is not perfect; nothing is.”
It’s also difficult to separate out coincidence from true problems with the device. Outside of uterine anomalies, nickel allergy seems to be the only definitive indicator that patients will have problems with Essure. Until more is known, timing is the key to figuring out if Essure needs to come out, Dr. Presthus said.
“If somebody has it put in and then has all kinds of pelvic pain, it’s pretty easy to make the connection that it could be the device. Certainly, when patients have allergic reactions that started after they had it implanted,” Essure needs to be addressed, he said.
Removal without hysterectomy
His first step in such cases is to remove the device to see if it helps. Over the past year, Dr. Presthus has done two or more cases a week laparoscopically and said he hasn’t run into problems. The cases take about a half-hour each, and 80% or more of women report symptom relief.
“I do a linear salpingotomy over the proximal portion of the fallopian tube where the device is, and use fine-tip forceps to tease the tissue apart and identify the device. You tease it out, regrasp it, tease it out, and regrasp it. It’s really important to pull in a straight line” so Essure doesn’t break. “It’s not necessary to cut deep into the cornea of the uterus. I get an x-ray on the table to ensure there’s no metal left,” Dr. Presthus said.
Women have to know that there are alternatives before they opt for Essure, and that it can be taken out if needed. “They don’t have to [just] live with their problems or have a hysterectomy. The discussion you have before the procedure is important,” he said.
Dr. Charles Monteith in Raleigh, N.C., and Dr. Mark Sanchez in Clearwater, Fla., prefer an open approach, operating through a small incision above the bikini line.
Both are long-time experts in reversing tubal ligations, and are in high demand lately to take out Essure, and sometimes even restore fertility. Fertility can be restored in about a third of patients, they said.
“If the device is removed within 3 months, it’ll come out fairly easily with traction,” Dr. Monteith said. After that, scarring locks it into place, “so we start dissection over the device and follow it down into the uterine cavity, and remove it intact” – without traction – “with the surrounding tubal muscularis. That’s worked well for us,” he said.
Dr. Monteith said he has removed or reversed Essure in about 200 women.
“Avoid electrocautery because it causes the nickel in the outer coil to burn, spark, and fracture. Also, have an appreciation for variable insertions of the device. We’ve seen the coils pushed too deeply” into the fallopian tube, and “we’ve seen them barely going in. You have to palpate them to know where they are” before taking them out, so as not to cut into them by accident, he said.
Dr. Sanchez, also a high-volume Essure surgeon, cuts down into the corneal segment of the uterus and slowly dissects out the coil. “You have to pay close attention to make sure you get all the bits of the coil out as much as possible.” From there, restoring fertility usually means reanastomosing the fallopian tubes and corneal reimplantation, he said.
Other surgeons have developed similar techniques (Contraception. 2013 Sep;88[3]:334-6.).
Dr. Presthus, Dr. Monteith, and Dr. Sanchez reported having no current connections to Bayer, and are not involved in legal action against the company.
Aspirin, weight loss cut colorectal cancer risk in Lynch syndrome
Losing weight and taking aspirin protect people who are genetically predisposed to colorectal cancer from developing the disease, according to a randomized study of 937 Lynch syndrome patients published online Aug. 17 in the Journal of Clinical Oncology.
In the general population, obesity is a known risk factor for colorectal cancer (CRC), especially in men, and aspirin has been shown to have protective benefits. The investigators wondered if that also held true in Lynch syndrome (LS), the most common cause of hereditary bowel cancer (J Clin Oncol. 2015 Aug 17. doi. 10.1200/JCO.2014.58.9952).
This “study showed some evidence that the excess CRC risk in patients with LS is abrogated by regular aspirin consumption. … Such patients are likely to benefit from obesity prevention and/or regular aspirin,” wrote Mohammad Movahedi, Ph.D., of Beheshti University of Medical Sciences in Tehran, Iran, and associates.
In a 2 × 2 factorial design, the LS subjects were randomly assigned to aspirin 600 mg/day or placebo, plus resistant starch 30 g/day or placebo, for a mean of about 2 years. Resistant starch is a dietary fiber.
After a mean follow-up of 4.6 years, CRC was diagnosed in 55 (6%) subjects. The risk of disease was 2.41 times greater for LS patients with a body mass index (BMI) of 25 kg/m2 or higher than for subjects below that mark (95% confidence interval, 1.22-4.85). Each 1-kg/m2 increase in BMI was associated with a 7% increase in CRC risk, about twice the excess risk obesity causes in the general population.
Obesity increased the risk of CRC only in LS subjects who did not get aspirin (adjusted hazard ratio, 2.75; 95% CI, 1.12-6.79; P = .03).
The obesity-CRC link was statistically significant only in men. Increasing BMI also seemed to pose a greater threat to men than to women, but the difference was not significant, Dr. Movahedi and associates said.
LS is the result of several mutations in DNA repair genes, but only one seemed to matter in the trial: Obesity was associated with a 3.72-fold greater risk for CRC in LS patients with the MLH1 mutation but did not increase risk in those with MSH2 or MSH6 mutations.
Obesity sets patients up for chronic inflammation, which is thought to contribute to cancer risk. That dynamic might be amplified in LS patients with stem cells that accumulate DNA damage because of a faulty repair system, the investigators said. Aspirin’s anti-inflammatory properties might counter the effect.
The “observation of sex-dependent greater CRC risk among obese men in the general population accords with our current findings in patients with LS and with reports from other observational studies,” they noted.
There were slightly more women in the trial than men, and the mean age in the study was 45.2 years.
Dr. Movahedi had no disclosures. The work was funded by the British Medical Research Council, Cancer Research UK, and the European Union, among others.
The new AGA guideline on Lynch syndrome was published in the September issue of Gastroenterology (2015 Jul 28 [doi: 10.1053/j.gastro.2015.07.036]) and summarized in the September issue of GI & Hepatology News.
Losing weight and taking aspirin protect people who are genetically predisposed to colorectal cancer from developing the disease, according to a randomized study of 937 Lynch syndrome patients published online Aug. 17 in the Journal of Clinical Oncology.
In the general population, obesity is a known risk factor for colorectal cancer (CRC), especially in men, and aspirin has been shown to have protective benefits. The investigators wondered if that also held true in Lynch syndrome (LS), the most common cause of hereditary bowel cancer (J Clin Oncol. 2015 Aug 17. doi. 10.1200/JCO.2014.58.9952).
This “study showed some evidence that the excess CRC risk in patients with LS is abrogated by regular aspirin consumption. … Such patients are likely to benefit from obesity prevention and/or regular aspirin,” wrote Mohammad Movahedi, Ph.D., of Beheshti University of Medical Sciences in Tehran, Iran, and associates.
In a 2 × 2 factorial design, the LS subjects were randomly assigned to aspirin 600 mg/day or placebo, plus resistant starch 30 g/day or placebo, for a mean of about 2 years. Resistant starch is a dietary fiber.
After a mean follow-up of 4.6 years, CRC was diagnosed in 55 (6%) subjects. The risk of disease was 2.41 times greater for LS patients with a body mass index (BMI) of 25 kg/m2 or higher than for subjects below that mark (95% confidence interval, 1.22-4.85). Each 1-kg/m2 increase in BMI was associated with a 7% increase in CRC risk, about twice the excess risk obesity causes in the general population.
Obesity increased the risk of CRC only in LS subjects who did not get aspirin (adjusted hazard ratio, 2.75; 95% CI, 1.12-6.79; P = .03).
The obesity-CRC link was statistically significant only in men. Increasing BMI also seemed to pose a greater threat to men than to women, but the difference was not significant, Dr. Movahedi and associates said.
LS is the result of several mutations in DNA repair genes, but only one seemed to matter in the trial: Obesity was associated with a 3.72-fold greater risk for CRC in LS patients with the MLH1 mutation but did not increase risk in those with MSH2 or MSH6 mutations.
Obesity sets patients up for chronic inflammation, which is thought to contribute to cancer risk. That dynamic might be amplified in LS patients with stem cells that accumulate DNA damage because of a faulty repair system, the investigators said. Aspirin’s anti-inflammatory properties might counter the effect.
The “observation of sex-dependent greater CRC risk among obese men in the general population accords with our current findings in patients with LS and with reports from other observational studies,” they noted.
There were slightly more women in the trial than men, and the mean age in the study was 45.2 years.
Dr. Movahedi had no disclosures. The work was funded by the British Medical Research Council, Cancer Research UK, and the European Union, among others.
The new AGA guideline on Lynch syndrome was published in the September issue of Gastroenterology (2015 Jul 28 [doi: 10.1053/j.gastro.2015.07.036]) and summarized in the September issue of GI & Hepatology News.
Losing weight and taking aspirin protect people who are genetically predisposed to colorectal cancer from developing the disease, according to a randomized study of 937 Lynch syndrome patients published online Aug. 17 in the Journal of Clinical Oncology.
In the general population, obesity is a known risk factor for colorectal cancer (CRC), especially in men, and aspirin has been shown to have protective benefits. The investigators wondered if that also held true in Lynch syndrome (LS), the most common cause of hereditary bowel cancer (J Clin Oncol. 2015 Aug 17. doi. 10.1200/JCO.2014.58.9952).
This “study showed some evidence that the excess CRC risk in patients with LS is abrogated by regular aspirin consumption. … Such patients are likely to benefit from obesity prevention and/or regular aspirin,” wrote Mohammad Movahedi, Ph.D., of Beheshti University of Medical Sciences in Tehran, Iran, and associates.
In a 2 × 2 factorial design, the LS subjects were randomly assigned to aspirin 600 mg/day or placebo, plus resistant starch 30 g/day or placebo, for a mean of about 2 years. Resistant starch is a dietary fiber.
After a mean follow-up of 4.6 years, CRC was diagnosed in 55 (6%) subjects. The risk of disease was 2.41 times greater for LS patients with a body mass index (BMI) of 25 kg/m2 or higher than for subjects below that mark (95% confidence interval, 1.22-4.85). Each 1-kg/m2 increase in BMI was associated with a 7% increase in CRC risk, about twice the excess risk obesity causes in the general population.
Obesity increased the risk of CRC only in LS subjects who did not get aspirin (adjusted hazard ratio, 2.75; 95% CI, 1.12-6.79; P = .03).
The obesity-CRC link was statistically significant only in men. Increasing BMI also seemed to pose a greater threat to men than to women, but the difference was not significant, Dr. Movahedi and associates said.
LS is the result of several mutations in DNA repair genes, but only one seemed to matter in the trial: Obesity was associated with a 3.72-fold greater risk for CRC in LS patients with the MLH1 mutation but did not increase risk in those with MSH2 or MSH6 mutations.
Obesity sets patients up for chronic inflammation, which is thought to contribute to cancer risk. That dynamic might be amplified in LS patients with stem cells that accumulate DNA damage because of a faulty repair system, the investigators said. Aspirin’s anti-inflammatory properties might counter the effect.
The “observation of sex-dependent greater CRC risk among obese men in the general population accords with our current findings in patients with LS and with reports from other observational studies,” they noted.
There were slightly more women in the trial than men, and the mean age in the study was 45.2 years.
Dr. Movahedi had no disclosures. The work was funded by the British Medical Research Council, Cancer Research UK, and the European Union, among others.
The new AGA guideline on Lynch syndrome was published in the September issue of Gastroenterology (2015 Jul 28 [doi: 10.1053/j.gastro.2015.07.036]) and summarized in the September issue of GI & Hepatology News.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: If obese Lynch syndrome patients aren’t already on daily aspirin, they probably should be.
Major finding: Obesity increased the risk of CRC only in Lynch syndrome subjects who were not on 600 mg of aspirin per day (adjusted HR, 2.75; 95% CI, 1.12-6.79; P = .03).
Data source: Randomized, placebo-controlled trial with 937 Lynch syndrome patients.
Disclosures: The lead investigator has no financial disclosures. The work was funded by the British Medical Research Council, Cancer Research UK, and the European Union, among others.
Warfarin as effective as LMWH to prevent recurrent VTEs in cancer patients
In cancer patients with acute, symptomatic venous thromboembolisms, daily tinzaparin does not significantly reduce recurrent venous thromboembolisms, overall mortality, or major bleeding compared with warfarin, but does reduce clinically-relevant nonmajor bleeding, according to a 900-patient randomized trial published in JAMA.
Tinzaparin (Innohep) is a low-molecular-weight heparin (LMWH) marketed outside the United States. LMWHs are generally recommended over warfarin to treat acute venous thromboembolism (VTE) in cancer patients, but the recommendation is based mostly on a single trial over a decade old; that might explain why, worldwide, vitamin K antagonists are still heavily used in patients with cancer-associated thrombosis, said the authors, led by Dr. Agnes Lee, of the University of British Columbia (Vancouver).
To revisit the issue in the modern treatment era, Dr. Lee and her colleagues randomized 449 adults with active cancer and documented deep vein thrombosis or pulmonary embolism to tinzaparin (175 IU/kg) once daily for 6 months, and 451 others to 6 months of conventional therapy with tinzaparin (175 IU/kg) once daily for 5-10 days followed by warfarin dose adjusted to maintain therapeutic range (JAMA. 2015;314[7]:677-86).
The subjects were recruited from 164 centers in Asia, Africa, Europe, Canada, and Central and South America; all had a life expectancy greater than 6 months. The mean age in the study was 59 years, and 60% of the subjects were women. Gynecologic and colorectal cancers were the most common in the study.
Recurrent VTE occurred in 31 patients in the tinzaparin group and 45 in the warfarin group, giving a 6-month cumulative incidence of 7.2% for tinzaparin versus 10.5% for warfarin (hazard ratio [HR] 0.65; 95% CI 0.41-1.03; P = .07).
There was major bleeding in 12 (2.7%) tinzaparin patients and 11 (2.4%) warfarin patients, an insignificant difference (HR 0.89; 95% CI 0.40-1.99; P = .77); 150 tinzaparin patients (33.4%) and 138 warfarin patients (30.6%) died in the trial, also an insignificant difference (HR, 1.08; 95% CI 0.85-1.36; P = .54).
There were 49 (10.9%) clinically relevant but nonmajor bleeds in the tinzaparin group versus 69 (15.3%) among warfarin patients, a difference that was significant (HR 0.58; 95% CI 0.40-0.84; P = .004). Clinically relevant nonmajor bleeding meant nonfatal bleeding outside of a critical area or organ that still required intervention but did not cause a fall in hemoglobin greater than 2 g/dL.
There were fewer than anticipated thrombotic events in the warfarin group. “We had expected a recurrence rate of 12.6% with warfarin; the observed rate was only 10.5%. This potentially affected the power of the trial to detect a benefit associated with tinzaparin.” Also, “a significant reduction in recurrent [VTEs] might be observed with tinzaparin” among patients at higher risk for them than were in the study, the investigators said.
Even so, the trial did demonstrate “that tinzaparin, even when given at a full therapeutic dose for up to 6 months, is safe in a broad oncology population,” they said.
LEO Pharma, makers of tinzaparin, funded the study. The company was involved throughout the trial, including data analysis and approval of the manuscript. Dr. Lee reported honoraria, consulting fees, and research funding from the company. Other authors reported payments from LEO for those or other reasons.
In cancer patients with acute, symptomatic venous thromboembolisms, daily tinzaparin does not significantly reduce recurrent venous thromboembolisms, overall mortality, or major bleeding compared with warfarin, but does reduce clinically-relevant nonmajor bleeding, according to a 900-patient randomized trial published in JAMA.
Tinzaparin (Innohep) is a low-molecular-weight heparin (LMWH) marketed outside the United States. LMWHs are generally recommended over warfarin to treat acute venous thromboembolism (VTE) in cancer patients, but the recommendation is based mostly on a single trial over a decade old; that might explain why, worldwide, vitamin K antagonists are still heavily used in patients with cancer-associated thrombosis, said the authors, led by Dr. Agnes Lee, of the University of British Columbia (Vancouver).
To revisit the issue in the modern treatment era, Dr. Lee and her colleagues randomized 449 adults with active cancer and documented deep vein thrombosis or pulmonary embolism to tinzaparin (175 IU/kg) once daily for 6 months, and 451 others to 6 months of conventional therapy with tinzaparin (175 IU/kg) once daily for 5-10 days followed by warfarin dose adjusted to maintain therapeutic range (JAMA. 2015;314[7]:677-86).
The subjects were recruited from 164 centers in Asia, Africa, Europe, Canada, and Central and South America; all had a life expectancy greater than 6 months. The mean age in the study was 59 years, and 60% of the subjects were women. Gynecologic and colorectal cancers were the most common in the study.
Recurrent VTE occurred in 31 patients in the tinzaparin group and 45 in the warfarin group, giving a 6-month cumulative incidence of 7.2% for tinzaparin versus 10.5% for warfarin (hazard ratio [HR] 0.65; 95% CI 0.41-1.03; P = .07).
There was major bleeding in 12 (2.7%) tinzaparin patients and 11 (2.4%) warfarin patients, an insignificant difference (HR 0.89; 95% CI 0.40-1.99; P = .77); 150 tinzaparin patients (33.4%) and 138 warfarin patients (30.6%) died in the trial, also an insignificant difference (HR, 1.08; 95% CI 0.85-1.36; P = .54).
There were 49 (10.9%) clinically relevant but nonmajor bleeds in the tinzaparin group versus 69 (15.3%) among warfarin patients, a difference that was significant (HR 0.58; 95% CI 0.40-0.84; P = .004). Clinically relevant nonmajor bleeding meant nonfatal bleeding outside of a critical area or organ that still required intervention but did not cause a fall in hemoglobin greater than 2 g/dL.
There were fewer than anticipated thrombotic events in the warfarin group. “We had expected a recurrence rate of 12.6% with warfarin; the observed rate was only 10.5%. This potentially affected the power of the trial to detect a benefit associated with tinzaparin.” Also, “a significant reduction in recurrent [VTEs] might be observed with tinzaparin” among patients at higher risk for them than were in the study, the investigators said.
Even so, the trial did demonstrate “that tinzaparin, even when given at a full therapeutic dose for up to 6 months, is safe in a broad oncology population,” they said.
LEO Pharma, makers of tinzaparin, funded the study. The company was involved throughout the trial, including data analysis and approval of the manuscript. Dr. Lee reported honoraria, consulting fees, and research funding from the company. Other authors reported payments from LEO for those or other reasons.
In cancer patients with acute, symptomatic venous thromboembolisms, daily tinzaparin does not significantly reduce recurrent venous thromboembolisms, overall mortality, or major bleeding compared with warfarin, but does reduce clinically-relevant nonmajor bleeding, according to a 900-patient randomized trial published in JAMA.
Tinzaparin (Innohep) is a low-molecular-weight heparin (LMWH) marketed outside the United States. LMWHs are generally recommended over warfarin to treat acute venous thromboembolism (VTE) in cancer patients, but the recommendation is based mostly on a single trial over a decade old; that might explain why, worldwide, vitamin K antagonists are still heavily used in patients with cancer-associated thrombosis, said the authors, led by Dr. Agnes Lee, of the University of British Columbia (Vancouver).
To revisit the issue in the modern treatment era, Dr. Lee and her colleagues randomized 449 adults with active cancer and documented deep vein thrombosis or pulmonary embolism to tinzaparin (175 IU/kg) once daily for 6 months, and 451 others to 6 months of conventional therapy with tinzaparin (175 IU/kg) once daily for 5-10 days followed by warfarin dose adjusted to maintain therapeutic range (JAMA. 2015;314[7]:677-86).
The subjects were recruited from 164 centers in Asia, Africa, Europe, Canada, and Central and South America; all had a life expectancy greater than 6 months. The mean age in the study was 59 years, and 60% of the subjects were women. Gynecologic and colorectal cancers were the most common in the study.
Recurrent VTE occurred in 31 patients in the tinzaparin group and 45 in the warfarin group, giving a 6-month cumulative incidence of 7.2% for tinzaparin versus 10.5% for warfarin (hazard ratio [HR] 0.65; 95% CI 0.41-1.03; P = .07).
There was major bleeding in 12 (2.7%) tinzaparin patients and 11 (2.4%) warfarin patients, an insignificant difference (HR 0.89; 95% CI 0.40-1.99; P = .77); 150 tinzaparin patients (33.4%) and 138 warfarin patients (30.6%) died in the trial, also an insignificant difference (HR, 1.08; 95% CI 0.85-1.36; P = .54).
There were 49 (10.9%) clinically relevant but nonmajor bleeds in the tinzaparin group versus 69 (15.3%) among warfarin patients, a difference that was significant (HR 0.58; 95% CI 0.40-0.84; P = .004). Clinically relevant nonmajor bleeding meant nonfatal bleeding outside of a critical area or organ that still required intervention but did not cause a fall in hemoglobin greater than 2 g/dL.
There were fewer than anticipated thrombotic events in the warfarin group. “We had expected a recurrence rate of 12.6% with warfarin; the observed rate was only 10.5%. This potentially affected the power of the trial to detect a benefit associated with tinzaparin.” Also, “a significant reduction in recurrent [VTEs] might be observed with tinzaparin” among patients at higher risk for them than were in the study, the investigators said.
Even so, the trial did demonstrate “that tinzaparin, even when given at a full therapeutic dose for up to 6 months, is safe in a broad oncology population,” they said.
LEO Pharma, makers of tinzaparin, funded the study. The company was involved throughout the trial, including data analysis and approval of the manuscript. Dr. Lee reported honoraria, consulting fees, and research funding from the company. Other authors reported payments from LEO for those or other reasons.
FROM JAMA
Key clinical point: In cancer patients, tinzaparin (Innohep) does not significantly reduce recurrent venous thromboembolisms, overall mortality, or major bleeding when compared to warfarin.
Major finding: Recurrent VTE occurred in 31 patients in the tinzaparin group and 45 in the warfarin group, giving a 6-month cumulative incidence of 7.2% for tinzaparin versus 10.5% for warfarin (HR 0.65; 95% CI 0.41-1.03; P = .07).
Data source: Randomized trial with 900 subjects.
Disclosures: LEO Pharma, maker of tinzaparin, funded the study. The company was involved throughout the trial, including data analysis and approval of the manuscript. The investigators reported honoraria, consulting fees, and other payments from the company.