User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Apremilast response durable at 52 weeks
If apremilast (Otezla) works initially for psoriasis, it’s likely to keep on helping for at least a year, according to phase III results published by the drug’s maker, Celgene, in the Journal of the American Academy of Dermatology.
The trial randomized 562 patients with moderate to severe plaque psoriasis to apremilast 30 mg twice daily, and 282 to placebo (J Am Acad Dermatol. 2015 Jul;73[1]:37-49).
At week 16, 33% of apremilast patients, but only 5.3% of placebo patients, achieved a 75% or greater reduction from their baseline Psoriasis Area and Severity Index (PASI-75) scores (P less than .0001).
The placebo group was next switched to apremilast so that all the subjects were on the drug from weeks 16 to 32. By week 32, patients switched from placebo caught up with their apremilast peers on PASI-75 response rates and improved pruritus scores.
The study continued past week 32 with 154 patients who had been on apremilast since baseline and had reached PASI-75; half were rerandomized to placebo, half to the drug. At week 52, 47 (61%) of apremilast patients had maintained their PASI-75 response, versus 9 (12%) of placebo patients.
“PASI response was maintained over 52 weeks with continued apremilast treatment. In addition, apremilast demonstrated improvements in nail and scalp psoriasis, both difficult-to-treat forms of psoriasis. Most patients rerandomized to placebo who lost PASI-75 response regained it after apremilast reinitiation,” said the authors, led by Dr. Kim Papp of Probity Medical Research in Waterloo, Ont.
Apremilast, an oral phosphodiesterase 4 inhibitor, was approved by the Food and Drug Administration in 2014 for moderate to severe plaque psoriasis and psoriatic arthritis. The 16-week results are included in the drug’s label.
The label warns of weight loss and depression with apremilast. The new report doesn’t mention depression but does note a mean weight loss of 2.08 kg with the drug, and that 19% of patients lost more than 5% of their body weight. However, no one left the study because of it.
During the first 16 weeks of the trial, the most common side effects were diarrhea (7.1% placebo versus 19% apremilast); nausea (6.7% placebo versus 16% apremilast); upper respiratory tract infection (7.4% versus 10%); nasopharyngitis (8.2% versus 7.3%); and tension headache (4.3% versus 7.3%).
Side effects tended to present early with apremilast, and the incidence didn’t increase as treatment continued. To minimize side effects, the investigators titrated the drug in 10-mg increments over the first week of treatment. Discontinuations due to side effects were low.
Serious adverse events occurred in 2.8% of placebo and 2.1% of apremilast subjects. In the apremilast group, they included three cases of coronary artery disease and three cases of nephrolithiasis, plus two cases each of urinary tract infections, acute myocardial infarctions, and chronic obstructive pulmonary disease.
The investigators excluded patients with major uncontrolled comorbidities, significant infections, active or incompletely treated tuberculosis, biologic use within 12-24 months, use of active topical agents within 2 weeks, and prolonged sun or ultraviolet exposure. Most of the subjects were white, two-thirds were men, and the average age in the study was 45 years. Weak or low-potency topical corticosteroids, coal tar shampoo and salicylic acid for scalp lesions, and unmedicated moisturizers were allowed in the study.
Celgene Corporation, the maker of apremilast, funded the work. Three investigators are employees, and most of the rest reported financial relationships with the company.
If apremilast (Otezla) works initially for psoriasis, it’s likely to keep on helping for at least a year, according to phase III results published by the drug’s maker, Celgene, in the Journal of the American Academy of Dermatology.
The trial randomized 562 patients with moderate to severe plaque psoriasis to apremilast 30 mg twice daily, and 282 to placebo (J Am Acad Dermatol. 2015 Jul;73[1]:37-49).
At week 16, 33% of apremilast patients, but only 5.3% of placebo patients, achieved a 75% or greater reduction from their baseline Psoriasis Area and Severity Index (PASI-75) scores (P less than .0001).
The placebo group was next switched to apremilast so that all the subjects were on the drug from weeks 16 to 32. By week 32, patients switched from placebo caught up with their apremilast peers on PASI-75 response rates and improved pruritus scores.
The study continued past week 32 with 154 patients who had been on apremilast since baseline and had reached PASI-75; half were rerandomized to placebo, half to the drug. At week 52, 47 (61%) of apremilast patients had maintained their PASI-75 response, versus 9 (12%) of placebo patients.
“PASI response was maintained over 52 weeks with continued apremilast treatment. In addition, apremilast demonstrated improvements in nail and scalp psoriasis, both difficult-to-treat forms of psoriasis. Most patients rerandomized to placebo who lost PASI-75 response regained it after apremilast reinitiation,” said the authors, led by Dr. Kim Papp of Probity Medical Research in Waterloo, Ont.
Apremilast, an oral phosphodiesterase 4 inhibitor, was approved by the Food and Drug Administration in 2014 for moderate to severe plaque psoriasis and psoriatic arthritis. The 16-week results are included in the drug’s label.
The label warns of weight loss and depression with apremilast. The new report doesn’t mention depression but does note a mean weight loss of 2.08 kg with the drug, and that 19% of patients lost more than 5% of their body weight. However, no one left the study because of it.
During the first 16 weeks of the trial, the most common side effects were diarrhea (7.1% placebo versus 19% apremilast); nausea (6.7% placebo versus 16% apremilast); upper respiratory tract infection (7.4% versus 10%); nasopharyngitis (8.2% versus 7.3%); and tension headache (4.3% versus 7.3%).
Side effects tended to present early with apremilast, and the incidence didn’t increase as treatment continued. To minimize side effects, the investigators titrated the drug in 10-mg increments over the first week of treatment. Discontinuations due to side effects were low.
Serious adverse events occurred in 2.8% of placebo and 2.1% of apremilast subjects. In the apremilast group, they included three cases of coronary artery disease and three cases of nephrolithiasis, plus two cases each of urinary tract infections, acute myocardial infarctions, and chronic obstructive pulmonary disease.
The investigators excluded patients with major uncontrolled comorbidities, significant infections, active or incompletely treated tuberculosis, biologic use within 12-24 months, use of active topical agents within 2 weeks, and prolonged sun or ultraviolet exposure. Most of the subjects were white, two-thirds were men, and the average age in the study was 45 years. Weak or low-potency topical corticosteroids, coal tar shampoo and salicylic acid for scalp lesions, and unmedicated moisturizers were allowed in the study.
Celgene Corporation, the maker of apremilast, funded the work. Three investigators are employees, and most of the rest reported financial relationships with the company.
If apremilast (Otezla) works initially for psoriasis, it’s likely to keep on helping for at least a year, according to phase III results published by the drug’s maker, Celgene, in the Journal of the American Academy of Dermatology.
The trial randomized 562 patients with moderate to severe plaque psoriasis to apremilast 30 mg twice daily, and 282 to placebo (J Am Acad Dermatol. 2015 Jul;73[1]:37-49).
At week 16, 33% of apremilast patients, but only 5.3% of placebo patients, achieved a 75% or greater reduction from their baseline Psoriasis Area and Severity Index (PASI-75) scores (P less than .0001).
The placebo group was next switched to apremilast so that all the subjects were on the drug from weeks 16 to 32. By week 32, patients switched from placebo caught up with their apremilast peers on PASI-75 response rates and improved pruritus scores.
The study continued past week 32 with 154 patients who had been on apremilast since baseline and had reached PASI-75; half were rerandomized to placebo, half to the drug. At week 52, 47 (61%) of apremilast patients had maintained their PASI-75 response, versus 9 (12%) of placebo patients.
“PASI response was maintained over 52 weeks with continued apremilast treatment. In addition, apremilast demonstrated improvements in nail and scalp psoriasis, both difficult-to-treat forms of psoriasis. Most patients rerandomized to placebo who lost PASI-75 response regained it after apremilast reinitiation,” said the authors, led by Dr. Kim Papp of Probity Medical Research in Waterloo, Ont.
Apremilast, an oral phosphodiesterase 4 inhibitor, was approved by the Food and Drug Administration in 2014 for moderate to severe plaque psoriasis and psoriatic arthritis. The 16-week results are included in the drug’s label.
The label warns of weight loss and depression with apremilast. The new report doesn’t mention depression but does note a mean weight loss of 2.08 kg with the drug, and that 19% of patients lost more than 5% of their body weight. However, no one left the study because of it.
During the first 16 weeks of the trial, the most common side effects were diarrhea (7.1% placebo versus 19% apremilast); nausea (6.7% placebo versus 16% apremilast); upper respiratory tract infection (7.4% versus 10%); nasopharyngitis (8.2% versus 7.3%); and tension headache (4.3% versus 7.3%).
Side effects tended to present early with apremilast, and the incidence didn’t increase as treatment continued. To minimize side effects, the investigators titrated the drug in 10-mg increments over the first week of treatment. Discontinuations due to side effects were low.
Serious adverse events occurred in 2.8% of placebo and 2.1% of apremilast subjects. In the apremilast group, they included three cases of coronary artery disease and three cases of nephrolithiasis, plus two cases each of urinary tract infections, acute myocardial infarctions, and chronic obstructive pulmonary disease.
The investigators excluded patients with major uncontrolled comorbidities, significant infections, active or incompletely treated tuberculosis, biologic use within 12-24 months, use of active topical agents within 2 weeks, and prolonged sun or ultraviolet exposure. Most of the subjects were white, two-thirds were men, and the average age in the study was 45 years. Weak or low-potency topical corticosteroids, coal tar shampoo and salicylic acid for scalp lesions, and unmedicated moisturizers were allowed in the study.
Celgene Corporation, the maker of apremilast, funded the work. Three investigators are employees, and most of the rest reported financial relationships with the company.
FROM JAAD
Key clinical point: Apremilast doesn’t seem to lose effect over time.
Major finding: At week 52, 61% of apremilast patients had maintained their PASI-75 response, versus 12% of patients switched to placebo at week 32.
Data source: ESTEEM 1, a phase III trial of patients with moderate to severe plaque psoriasis.
Disclosures: Celgene, the maker of apremilast, funded the work. Three investigators are employees, and most of the rest reported financial ties with the company.
Malignancy risk persists into middle age for childhood cancer survivors
The increased risk of malignancy following childhood cancer persists past the age of 40 years, according to an analysis of 3,171 members of the Childhood Cancer Survivor Study published online Aug. 10 in the Journal of Clinical Oncology.
Survivors “have a substantial risk of a new malignancy in the fifth and sixth decades of life in excess of what is expected among the general U.S. population ... Being free of SN [subsequent neoplasms] before age 40 years does not preclude survivors from having an increased risk of future [neoplasms] ... These data suggest the need for life-long monitoring and should inform anticipatory guidance provided to survivors of childhood cancer,” said the investigators, led by Dr. Lucie Turcotte, a pediatric oncologist at the University of Minnesota, Minneapolis (J Clin Oncol. 2015 Aug 10. doi: 10.1200/JCO.2015.60.9487).
The Childhood Cancer Survivor Study (CCSS) is an ongoing project to gauge the late effects of childhood cancers. Previous reports found an increased risk of malignancy in early adulthood.
The 3,171 patients in the latest analysis were diagnosed from the period of 1970-1986, and had completed at least one follow-up questionnaire after age 40.
Among them, there were 679 subsequent neoplasms (SNs) over the age of 40, including 196 subsequent malignant neoplasms (SMNs) in 180 people, as well as 419 nonmelanoma skin cancers, 21 nonmalignant meningiomas, and 43 other benign neoplasms.
At age 55 years, the cumulative incidence after the age of 40 of new SNs was 34.6% and new SMNs 16.3%.
Survivors were twice as likely as was the general population to be diagnosed with an SMN after age 40 (standardized incidence ratio [SIR] 2.2; 95% CI 1.9-2.5). Among SMNs, risk was increased for breast cancer (SIR 5.5; 95% CI 4.5-6.7), renal cancer (SIR 3.9; 95% CI 2.0-7.5), soft tissue sarcoma (SIR 2.6; 95% CI 1.5- 4.4), and thyroid cancer (SIR 1.9; 95% CI 1.0-3.5).
On multivariate analysis, female sex (relative risk [RR] 1.9; 95% CI 1.3-2.6), platinum chemotherapy (RR 2.3; CI 1.0-5.2), and therapeutic radiation exposure (RR 2.2; 95% CI 1.4-3.3) increased the risk of SMN.
“Therapeutic radiation exposure continues to place survivors at increased risk for SMNs ... well into their fifth and sixth decades of life, indicating a need for ongoing monitoring of this at-risk subgroup,” the authors said.
Females and Hodgkin lymphoma survivors were at increased risk for breast cancer, driven by high-dose, chest-directed radiation as children, which was previously a central component of treatment.
Routine mammography is typically started around age 40-50, but “the risks experienced by the survivor population are unique,” and may warrant greater vigilance. “Survivors without an SN before age 40 may be particularly vulnerable because they have not had previous neoplasms that may have altered screening practices,” the investigators said.
They did not find an excess risk for subsequent head, neck, lung, colon, or female genital tract malignancies. “This contrasts with observations in previous CCSS publications showing an increased risk for these malignancies among [younger adult] survivors ... It is possible that the period of highest risk for these malignancies among survivors occurs before age 40 years and that, as the incidence of these cancers increases with age in the general population, the risk beyond what is experienced by the general population is diminished,” they said.
Male survivors aged older than 40 years who were not irradiated as children did not have an increased risk of SMNs, regardless of their SN history before age 40.
The work was funded in part by the National Institutes of Health and the Children’s Cancer Research Fund. Dr. Turcotte has no disclosures.
The correlation between therapeutic exposures and development of subsequent malignant neoplasms (SMNs) is well established in survivors observed for 2-3 decades; the latest study shows that the risk continues into the fourth and fifth decades.
It seems clear that female survivors of Hodgkin lymphoma remain at significant risk of developing breast cancer into their 40s and 50s, warranting vigilant surveillance well beyond this age.
It is also important to recognize the potential SMNs that were not significantly increased in this cohort. These survivors were at no statistically increased risk compared with the general population of developing subsequent head and neck, lung, or colon cancers, as had previously been described ... [It] seems that for certain types of SMNs, screening recommendations for survivors may return to those of the general population.
Because of the strong association between the intensity of treatment received and SMNs, attempts to decrease the intensity of therapy while retaining excellent outcomes whenever possible have been made during the past 3 decades. Significant reductions in late mortality [have been] observed across treatment areas for acute lymphoblastic leukemia and Wilms tumor. Importantly, significant reductions in the cumulative incidence of death at 15 years from subsequent neoplasm diagnosis [have been] reported.
Genomic variables that modify genes that regulate drug metabolism and/or disposition or those responsible for DNA repair may also influence SMN susceptibility. Learning more about how the interactions between genomic and treatment-related factors modify the risk for SMNs and other adverse health outcomes in individual survivors as they age will be critical for the development of more personalized strategies for screening, intervention, and prevention.
Dr. Mark Applebaum is a pediatric oncology fellow and Dr. Susan Cohn is professor of pediatrics at the University of Chicago. Dr. Cohen reported stocks or other ownership interests in a number of companies, including Gilead, AstraZeneca, Pfizer, and Abbott. They made their comments in an editorial that accompanied the study (J Clin Oncol. 2015 Aug 10. doi: 10.1200/JCO.2015.62.7703).
The correlation between therapeutic exposures and development of subsequent malignant neoplasms (SMNs) is well established in survivors observed for 2-3 decades; the latest study shows that the risk continues into the fourth and fifth decades.
It seems clear that female survivors of Hodgkin lymphoma remain at significant risk of developing breast cancer into their 40s and 50s, warranting vigilant surveillance well beyond this age.
It is also important to recognize the potential SMNs that were not significantly increased in this cohort. These survivors were at no statistically increased risk compared with the general population of developing subsequent head and neck, lung, or colon cancers, as had previously been described ... [It] seems that for certain types of SMNs, screening recommendations for survivors may return to those of the general population.
Because of the strong association between the intensity of treatment received and SMNs, attempts to decrease the intensity of therapy while retaining excellent outcomes whenever possible have been made during the past 3 decades. Significant reductions in late mortality [have been] observed across treatment areas for acute lymphoblastic leukemia and Wilms tumor. Importantly, significant reductions in the cumulative incidence of death at 15 years from subsequent neoplasm diagnosis [have been] reported.
Genomic variables that modify genes that regulate drug metabolism and/or disposition or those responsible for DNA repair may also influence SMN susceptibility. Learning more about how the interactions between genomic and treatment-related factors modify the risk for SMNs and other adverse health outcomes in individual survivors as they age will be critical for the development of more personalized strategies for screening, intervention, and prevention.
Dr. Mark Applebaum is a pediatric oncology fellow and Dr. Susan Cohn is professor of pediatrics at the University of Chicago. Dr. Cohen reported stocks or other ownership interests in a number of companies, including Gilead, AstraZeneca, Pfizer, and Abbott. They made their comments in an editorial that accompanied the study (J Clin Oncol. 2015 Aug 10. doi: 10.1200/JCO.2015.62.7703).
The correlation between therapeutic exposures and development of subsequent malignant neoplasms (SMNs) is well established in survivors observed for 2-3 decades; the latest study shows that the risk continues into the fourth and fifth decades.
It seems clear that female survivors of Hodgkin lymphoma remain at significant risk of developing breast cancer into their 40s and 50s, warranting vigilant surveillance well beyond this age.
It is also important to recognize the potential SMNs that were not significantly increased in this cohort. These survivors were at no statistically increased risk compared with the general population of developing subsequent head and neck, lung, or colon cancers, as had previously been described ... [It] seems that for certain types of SMNs, screening recommendations for survivors may return to those of the general population.
Because of the strong association between the intensity of treatment received and SMNs, attempts to decrease the intensity of therapy while retaining excellent outcomes whenever possible have been made during the past 3 decades. Significant reductions in late mortality [have been] observed across treatment areas for acute lymphoblastic leukemia and Wilms tumor. Importantly, significant reductions in the cumulative incidence of death at 15 years from subsequent neoplasm diagnosis [have been] reported.
Genomic variables that modify genes that regulate drug metabolism and/or disposition or those responsible for DNA repair may also influence SMN susceptibility. Learning more about how the interactions between genomic and treatment-related factors modify the risk for SMNs and other adverse health outcomes in individual survivors as they age will be critical for the development of more personalized strategies for screening, intervention, and prevention.
Dr. Mark Applebaum is a pediatric oncology fellow and Dr. Susan Cohn is professor of pediatrics at the University of Chicago. Dr. Cohen reported stocks or other ownership interests in a number of companies, including Gilead, AstraZeneca, Pfizer, and Abbott. They made their comments in an editorial that accompanied the study (J Clin Oncol. 2015 Aug 10. doi: 10.1200/JCO.2015.62.7703).
The increased risk of malignancy following childhood cancer persists past the age of 40 years, according to an analysis of 3,171 members of the Childhood Cancer Survivor Study published online Aug. 10 in the Journal of Clinical Oncology.
Survivors “have a substantial risk of a new malignancy in the fifth and sixth decades of life in excess of what is expected among the general U.S. population ... Being free of SN [subsequent neoplasms] before age 40 years does not preclude survivors from having an increased risk of future [neoplasms] ... These data suggest the need for life-long monitoring and should inform anticipatory guidance provided to survivors of childhood cancer,” said the investigators, led by Dr. Lucie Turcotte, a pediatric oncologist at the University of Minnesota, Minneapolis (J Clin Oncol. 2015 Aug 10. doi: 10.1200/JCO.2015.60.9487).
The Childhood Cancer Survivor Study (CCSS) is an ongoing project to gauge the late effects of childhood cancers. Previous reports found an increased risk of malignancy in early adulthood.
The 3,171 patients in the latest analysis were diagnosed from the period of 1970-1986, and had completed at least one follow-up questionnaire after age 40.
Among them, there were 679 subsequent neoplasms (SNs) over the age of 40, including 196 subsequent malignant neoplasms (SMNs) in 180 people, as well as 419 nonmelanoma skin cancers, 21 nonmalignant meningiomas, and 43 other benign neoplasms.
At age 55 years, the cumulative incidence after the age of 40 of new SNs was 34.6% and new SMNs 16.3%.
Survivors were twice as likely as was the general population to be diagnosed with an SMN after age 40 (standardized incidence ratio [SIR] 2.2; 95% CI 1.9-2.5). Among SMNs, risk was increased for breast cancer (SIR 5.5; 95% CI 4.5-6.7), renal cancer (SIR 3.9; 95% CI 2.0-7.5), soft tissue sarcoma (SIR 2.6; 95% CI 1.5- 4.4), and thyroid cancer (SIR 1.9; 95% CI 1.0-3.5).
On multivariate analysis, female sex (relative risk [RR] 1.9; 95% CI 1.3-2.6), platinum chemotherapy (RR 2.3; CI 1.0-5.2), and therapeutic radiation exposure (RR 2.2; 95% CI 1.4-3.3) increased the risk of SMN.
“Therapeutic radiation exposure continues to place survivors at increased risk for SMNs ... well into their fifth and sixth decades of life, indicating a need for ongoing monitoring of this at-risk subgroup,” the authors said.
Females and Hodgkin lymphoma survivors were at increased risk for breast cancer, driven by high-dose, chest-directed radiation as children, which was previously a central component of treatment.
Routine mammography is typically started around age 40-50, but “the risks experienced by the survivor population are unique,” and may warrant greater vigilance. “Survivors without an SN before age 40 may be particularly vulnerable because they have not had previous neoplasms that may have altered screening practices,” the investigators said.
They did not find an excess risk for subsequent head, neck, lung, colon, or female genital tract malignancies. “This contrasts with observations in previous CCSS publications showing an increased risk for these malignancies among [younger adult] survivors ... It is possible that the period of highest risk for these malignancies among survivors occurs before age 40 years and that, as the incidence of these cancers increases with age in the general population, the risk beyond what is experienced by the general population is diminished,” they said.
Male survivors aged older than 40 years who were not irradiated as children did not have an increased risk of SMNs, regardless of their SN history before age 40.
The work was funded in part by the National Institutes of Health and the Children’s Cancer Research Fund. Dr. Turcotte has no disclosures.
The increased risk of malignancy following childhood cancer persists past the age of 40 years, according to an analysis of 3,171 members of the Childhood Cancer Survivor Study published online Aug. 10 in the Journal of Clinical Oncology.
Survivors “have a substantial risk of a new malignancy in the fifth and sixth decades of life in excess of what is expected among the general U.S. population ... Being free of SN [subsequent neoplasms] before age 40 years does not preclude survivors from having an increased risk of future [neoplasms] ... These data suggest the need for life-long monitoring and should inform anticipatory guidance provided to survivors of childhood cancer,” said the investigators, led by Dr. Lucie Turcotte, a pediatric oncologist at the University of Minnesota, Minneapolis (J Clin Oncol. 2015 Aug 10. doi: 10.1200/JCO.2015.60.9487).
The Childhood Cancer Survivor Study (CCSS) is an ongoing project to gauge the late effects of childhood cancers. Previous reports found an increased risk of malignancy in early adulthood.
The 3,171 patients in the latest analysis were diagnosed from the period of 1970-1986, and had completed at least one follow-up questionnaire after age 40.
Among them, there were 679 subsequent neoplasms (SNs) over the age of 40, including 196 subsequent malignant neoplasms (SMNs) in 180 people, as well as 419 nonmelanoma skin cancers, 21 nonmalignant meningiomas, and 43 other benign neoplasms.
At age 55 years, the cumulative incidence after the age of 40 of new SNs was 34.6% and new SMNs 16.3%.
Survivors were twice as likely as was the general population to be diagnosed with an SMN after age 40 (standardized incidence ratio [SIR] 2.2; 95% CI 1.9-2.5). Among SMNs, risk was increased for breast cancer (SIR 5.5; 95% CI 4.5-6.7), renal cancer (SIR 3.9; 95% CI 2.0-7.5), soft tissue sarcoma (SIR 2.6; 95% CI 1.5- 4.4), and thyroid cancer (SIR 1.9; 95% CI 1.0-3.5).
On multivariate analysis, female sex (relative risk [RR] 1.9; 95% CI 1.3-2.6), platinum chemotherapy (RR 2.3; CI 1.0-5.2), and therapeutic radiation exposure (RR 2.2; 95% CI 1.4-3.3) increased the risk of SMN.
“Therapeutic radiation exposure continues to place survivors at increased risk for SMNs ... well into their fifth and sixth decades of life, indicating a need for ongoing monitoring of this at-risk subgroup,” the authors said.
Females and Hodgkin lymphoma survivors were at increased risk for breast cancer, driven by high-dose, chest-directed radiation as children, which was previously a central component of treatment.
Routine mammography is typically started around age 40-50, but “the risks experienced by the survivor population are unique,” and may warrant greater vigilance. “Survivors without an SN before age 40 may be particularly vulnerable because they have not had previous neoplasms that may have altered screening practices,” the investigators said.
They did not find an excess risk for subsequent head, neck, lung, colon, or female genital tract malignancies. “This contrasts with observations in previous CCSS publications showing an increased risk for these malignancies among [younger adult] survivors ... It is possible that the period of highest risk for these malignancies among survivors occurs before age 40 years and that, as the incidence of these cancers increases with age in the general population, the risk beyond what is experienced by the general population is diminished,” they said.
Male survivors aged older than 40 years who were not irradiated as children did not have an increased risk of SMNs, regardless of their SN history before age 40.
The work was funded in part by the National Institutes of Health and the Children’s Cancer Research Fund. Dr. Turcotte has no disclosures.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors aren’t out of the woods when they hit middle age.
Major finding: Survivors are twice as likely as the general population to be diagnosed with a malignant neoplasm after the age of 40 (standardized incidence ratio 2.2; 95% CI 1.9-2.5).
Data source: Analysis of 3,171 individuals, aged older than 40 years, in the Childhood Cancer Survivor Study.
Disclosures: The National Institutes of Health and the Children’s Cancer Research Fund provided funding for the work. The lead author has no disclosures.
Laparoscopic sleeve gastrectomy: Comorbidity benefits fade with time
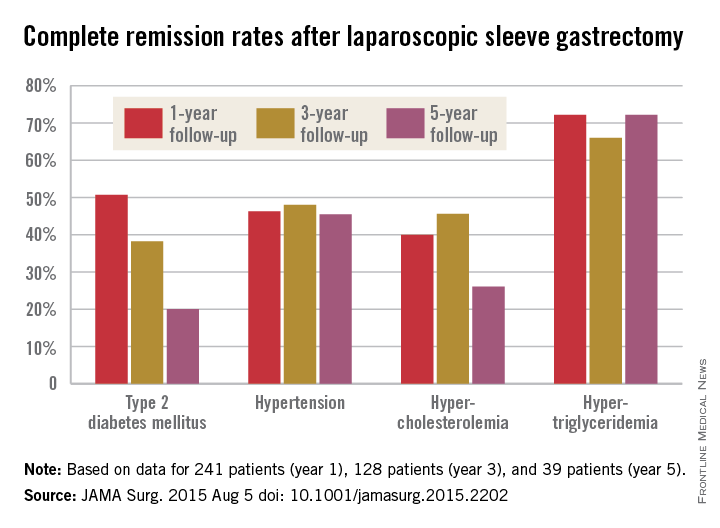
Five years after laparoscopic sleeve gastrectomy, patients will have regained, on average, about half of their preop excess weight, according to an Israeli investigation published online Aug. 5 in JAMA Surgery.
Things went better at first for the 443 patients in the study; at 1 year follow-up, they had lost, on average, 76.8% of their excess weight, but then it started to come back. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%. The failure rate – the number of patients with a percentage of excess weight loss less than 50% – increased from 13.3% at 1 year to 21.1% at 3 years and 38.5% at 5 years (JAMA Surg. 2015 Aug. 5. doi:10.1001/jamasurg.2015.2202).
The story was similar for obesity-related comorbidities; early gains eroded with time. Complete remission of diabetes, for instance, was maintained by 50.7% of patients at 1 year, 38.2% at 3 years, and just 20.0% at 5 years. Likewise, a drop in LDL cholesterol from baseline was significant at years 1 and 3, but not 5. Meanwhile, laparoscopic sleeve gastrectomy (LSG) didn’t significantly improve total cholesterol over baseline, and triglyceride improvements began to fade after year 1.
“In our opinion, the presence of obesity-related comorbidities should play a major role when choosing the appropriate procedure for a specific patient. For example, performing an operation that yields a low resolution rate of hyperlipidemia translates into lifelong medical treatment in a young patient with significant hyperlipidemia. In that case, a malabsorptive procedure might be more beneficial than LSG. If the recurrence of obesity is known to be followed by the remittance of an existing comorbidity in a specific procedure, an alternative procedure should be considered. The weight loss durability failure of almost 40% at 5 years of follow-up of LSG should be one of the deciding factors in such cases,” said senior investigator and bariatric surgeon Dr. Andrei Keidar of Beilinson Hospital in Petah Tikva, Israel, and his colleagues.
LSG is becoming more popular in part because it’s easier to learn and less disruptive than gastric bypass, but there are not enough data on long-term outcomes; the investigators sought to fill the gaps.
The average age in the study was 42.2 years; mean body mass index was 43.9 kg/m2, and mean preop excess weight was 51.2 kg. The majority of subjects were women. The operations were performed from 2006 to 2013, and there was considerable loss to follow-up during the project.
Baseline triglycerides followed overall trends with a drop from a mean of 155.2 mg/dL to 106.3 mg/dL at year 1, followed by a tick upward to 107.2 mg/dL at year 3 and 126.4 mg/dL at year 5.
The mean preop HDL cholesterol of 46.7 mg/dL rose to 52.8 mg/dL at year 1 and remained at about that level at 5 years. Improvements in hypertension were fairly durable, as well, with remission in 46.3% of patients at 1 year, 48.0% at 3 years, and 45.5% at 5 years.
“Surprisingly, our results showed that none of the changes in obesity-related comorbidity status correlated with” the amount of “excess weight prior to the surgery,” the investigators noted.
The authors didn’t compare LSG to other bariatric surgeries, but did note that in 2012, the American Society for Metabolic and Bariatric Surgery found that short-term weight loss and improvement in comorbidities was better with LSG than with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric bypass. On the other hand, RYGB has been found to beat LSG on lipid fraction improvements and short term control of type 2 diabetes.
The investigators had no disclosures.
Laparoscopic sleeve gastrectomy [has evolved] very quickly during the last several years into the dominant procedure in use despite a complete void of information about the longer-term effects. [The investigators try] to address this ... but clearly raise more issues than they can answer.
 |
Dr. Anita P. Courcoulas |
It is unclear whether current studies will address critical questions about the long-term outcomes of bariatric surgery, including the sustainability of weight loss and comorbidity control and long-term complication rates. The answers will likely be generated over time not only by ... large-scale efforts but also by thoughtful inference that will be made through pooled analyses of data like that from [this study] and from many other disparate randomized and nonrandomized studies of bariatric surgery. It will take time, patience, and a willingness to avoid a rush to judgment. In the meantime, clinicians and prospective patients will need to discuss and weigh the evidence in a dynamic exchange driven not always by final conclusions but by the most current available data.
Dr. Anita Courcoulas is professor of surgery and chief of the section of minimally invasive bariatric and general surgery at the University of Pittsburgh. She reported receiving grants from Nutrisystem, Ethicon, and Covidien and serving as a project consultant for Ethicon and Apollo Endosurgery. She made her comments in an editorial that accompanied the study.
Laparoscopic sleeve gastrectomy [has evolved] very quickly during the last several years into the dominant procedure in use despite a complete void of information about the longer-term effects. [The investigators try] to address this ... but clearly raise more issues than they can answer.
 |
Dr. Anita P. Courcoulas |
It is unclear whether current studies will address critical questions about the long-term outcomes of bariatric surgery, including the sustainability of weight loss and comorbidity control and long-term complication rates. The answers will likely be generated over time not only by ... large-scale efforts but also by thoughtful inference that will be made through pooled analyses of data like that from [this study] and from many other disparate randomized and nonrandomized studies of bariatric surgery. It will take time, patience, and a willingness to avoid a rush to judgment. In the meantime, clinicians and prospective patients will need to discuss and weigh the evidence in a dynamic exchange driven not always by final conclusions but by the most current available data.
Dr. Anita Courcoulas is professor of surgery and chief of the section of minimally invasive bariatric and general surgery at the University of Pittsburgh. She reported receiving grants from Nutrisystem, Ethicon, and Covidien and serving as a project consultant for Ethicon and Apollo Endosurgery. She made her comments in an editorial that accompanied the study.
Laparoscopic sleeve gastrectomy [has evolved] very quickly during the last several years into the dominant procedure in use despite a complete void of information about the longer-term effects. [The investigators try] to address this ... but clearly raise more issues than they can answer.
 |
Dr. Anita P. Courcoulas |
It is unclear whether current studies will address critical questions about the long-term outcomes of bariatric surgery, including the sustainability of weight loss and comorbidity control and long-term complication rates. The answers will likely be generated over time not only by ... large-scale efforts but also by thoughtful inference that will be made through pooled analyses of data like that from [this study] and from many other disparate randomized and nonrandomized studies of bariatric surgery. It will take time, patience, and a willingness to avoid a rush to judgment. In the meantime, clinicians and prospective patients will need to discuss and weigh the evidence in a dynamic exchange driven not always by final conclusions but by the most current available data.
Dr. Anita Courcoulas is professor of surgery and chief of the section of minimally invasive bariatric and general surgery at the University of Pittsburgh. She reported receiving grants from Nutrisystem, Ethicon, and Covidien and serving as a project consultant for Ethicon and Apollo Endosurgery. She made her comments in an editorial that accompanied the study.
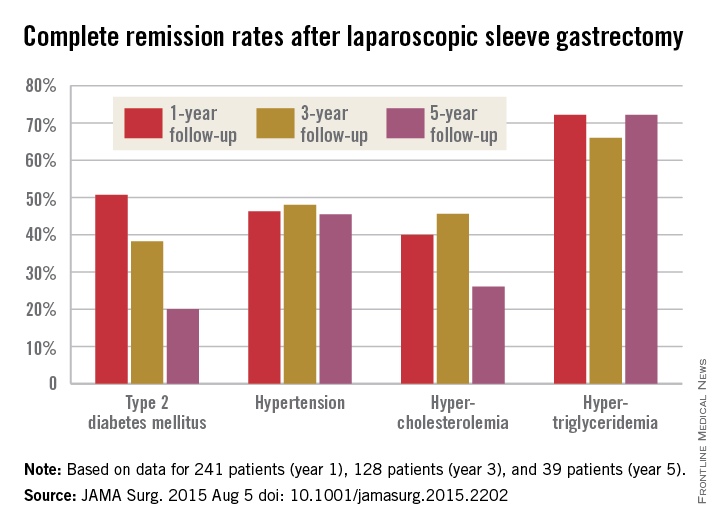
Five years after laparoscopic sleeve gastrectomy, patients will have regained, on average, about half of their preop excess weight, according to an Israeli investigation published online Aug. 5 in JAMA Surgery.
Things went better at first for the 443 patients in the study; at 1 year follow-up, they had lost, on average, 76.8% of their excess weight, but then it started to come back. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%. The failure rate – the number of patients with a percentage of excess weight loss less than 50% – increased from 13.3% at 1 year to 21.1% at 3 years and 38.5% at 5 years (JAMA Surg. 2015 Aug. 5. doi:10.1001/jamasurg.2015.2202).
The story was similar for obesity-related comorbidities; early gains eroded with time. Complete remission of diabetes, for instance, was maintained by 50.7% of patients at 1 year, 38.2% at 3 years, and just 20.0% at 5 years. Likewise, a drop in LDL cholesterol from baseline was significant at years 1 and 3, but not 5. Meanwhile, laparoscopic sleeve gastrectomy (LSG) didn’t significantly improve total cholesterol over baseline, and triglyceride improvements began to fade after year 1.

“In our opinion, the presence of obesity-related comorbidities should play a major role when choosing the appropriate procedure for a specific patient. For example, performing an operation that yields a low resolution rate of hyperlipidemia translates into lifelong medical treatment in a young patient with significant hyperlipidemia. In that case, a malabsorptive procedure might be more beneficial than LSG. If the recurrence of obesity is known to be followed by the remittance of an existing comorbidity in a specific procedure, an alternative procedure should be considered. The weight loss durability failure of almost 40% at 5 years of follow-up of LSG should be one of the deciding factors in such cases,” said senior investigator and bariatric surgeon Dr. Andrei Keidar of Beilinson Hospital in Petah Tikva, Israel, and his colleagues.
LSG is becoming more popular in part because it’s easier to learn and less disruptive than gastric bypass, but there are not enough data on long-term outcomes; the investigators sought to fill the gaps.
The average age in the study was 42.2 years; mean body mass index was 43.9 kg/m2, and mean preop excess weight was 51.2 kg. The majority of subjects were women. The operations were performed from 2006 to 2013, and there was considerable loss to follow-up during the project.
Baseline triglycerides followed overall trends with a drop from a mean of 155.2 mg/dL to 106.3 mg/dL at year 1, followed by a tick upward to 107.2 mg/dL at year 3 and 126.4 mg/dL at year 5.
The mean preop HDL cholesterol of 46.7 mg/dL rose to 52.8 mg/dL at year 1 and remained at about that level at 5 years. Improvements in hypertension were fairly durable, as well, with remission in 46.3% of patients at 1 year, 48.0% at 3 years, and 45.5% at 5 years.
“Surprisingly, our results showed that none of the changes in obesity-related comorbidity status correlated with” the amount of “excess weight prior to the surgery,” the investigators noted.
The authors didn’t compare LSG to other bariatric surgeries, but did note that in 2012, the American Society for Metabolic and Bariatric Surgery found that short-term weight loss and improvement in comorbidities was better with LSG than with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric bypass. On the other hand, RYGB has been found to beat LSG on lipid fraction improvements and short term control of type 2 diabetes.
The investigators had no disclosures.
Five years after laparoscopic sleeve gastrectomy, patients will have regained, on average, about half of their preop excess weight, according to an Israeli investigation published online Aug. 5 in JAMA Surgery.
Things went better at first for the 443 patients in the study; at 1 year follow-up, they had lost, on average, 76.8% of their excess weight, but then it started to come back. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%. The failure rate – the number of patients with a percentage of excess weight loss less than 50% – increased from 13.3% at 1 year to 21.1% at 3 years and 38.5% at 5 years (JAMA Surg. 2015 Aug. 5. doi:10.1001/jamasurg.2015.2202).
The story was similar for obesity-related comorbidities; early gains eroded with time. Complete remission of diabetes, for instance, was maintained by 50.7% of patients at 1 year, 38.2% at 3 years, and just 20.0% at 5 years. Likewise, a drop in LDL cholesterol from baseline was significant at years 1 and 3, but not 5. Meanwhile, laparoscopic sleeve gastrectomy (LSG) didn’t significantly improve total cholesterol over baseline, and triglyceride improvements began to fade after year 1.

“In our opinion, the presence of obesity-related comorbidities should play a major role when choosing the appropriate procedure for a specific patient. For example, performing an operation that yields a low resolution rate of hyperlipidemia translates into lifelong medical treatment in a young patient with significant hyperlipidemia. In that case, a malabsorptive procedure might be more beneficial than LSG. If the recurrence of obesity is known to be followed by the remittance of an existing comorbidity in a specific procedure, an alternative procedure should be considered. The weight loss durability failure of almost 40% at 5 years of follow-up of LSG should be one of the deciding factors in such cases,” said senior investigator and bariatric surgeon Dr. Andrei Keidar of Beilinson Hospital in Petah Tikva, Israel, and his colleagues.
LSG is becoming more popular in part because it’s easier to learn and less disruptive than gastric bypass, but there are not enough data on long-term outcomes; the investigators sought to fill the gaps.
The average age in the study was 42.2 years; mean body mass index was 43.9 kg/m2, and mean preop excess weight was 51.2 kg. The majority of subjects were women. The operations were performed from 2006 to 2013, and there was considerable loss to follow-up during the project.
Baseline triglycerides followed overall trends with a drop from a mean of 155.2 mg/dL to 106.3 mg/dL at year 1, followed by a tick upward to 107.2 mg/dL at year 3 and 126.4 mg/dL at year 5.
The mean preop HDL cholesterol of 46.7 mg/dL rose to 52.8 mg/dL at year 1 and remained at about that level at 5 years. Improvements in hypertension were fairly durable, as well, with remission in 46.3% of patients at 1 year, 48.0% at 3 years, and 45.5% at 5 years.
“Surprisingly, our results showed that none of the changes in obesity-related comorbidity status correlated with” the amount of “excess weight prior to the surgery,” the investigators noted.
The authors didn’t compare LSG to other bariatric surgeries, but did note that in 2012, the American Society for Metabolic and Bariatric Surgery found that short-term weight loss and improvement in comorbidities was better with LSG than with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric bypass. On the other hand, RYGB has been found to beat LSG on lipid fraction improvements and short term control of type 2 diabetes.
The investigators had no disclosures.
FROM JAMA SURGERY
Key clinical point: Laparoscopic sleeve gastrectomy (LSG) might not be the best surgical choice for bariatric patients with significant hyperlipidemia.
Major finding: One year after LSG, patients lost, on average, 76.8% of their excess weight. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%.
Data source: Retrospective study of 443 Israeli laparoscopic sleeve gastrectomies.
Disclosures: The investigators had no disclosures.
SVS: AAA surveillance comes at an emotional cost
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
AT The 2015 Vascular Annual Meeting
Key clinical point: Check with your AAA surveillance patients to make sure they know their rupture risk is low.
Major finding: Surveillance patients who thought it was “very likely” their aneurysm would rupture within a year had an emotional impact score of 45. Patients who thought rupture was unlikely had a sore of 12 (P less than .001).
Data source: Surveys of 1,008 AAA patients at six U.S. medical centers.
Disclosures: There was no outside funding for the work, and the lead investigator has no relevant disclosures.
SVS: AAA reimbursement needs to take anatomic complexity into account
CHICAGO – Anatomic complexity should be factored into reimbursements for abdominal aortic aneurysm repairs, University of Rochester (N.Y.) investigators concluded after they compared costs to complexity in 33 open and 107 endovascular repairs during 2007-2010.
They found that complex aneurysms – especially ones with Anatomic Severity Grade (ASG) scores above 15 – need more adjunctive procedures and cost more to repair, although at the moment, payers don’t usually take complexity directly into account.
It’s the first study to show a direct relationship between anatomic complexity and hospital cost. “Preoperative assessment with ASG scores can delineate patients at greater risk for increased resource utilization. A critical examination of the relationship between anatomic complexity and finances is required within the context of aggressive endovascular treatment strategies and shifts towards value-based reimbursement. Anatomy is related to cost. [Complexity] should be considered as a factor when calculating limited bundle reimbursements,” said investigator Dr. Khurram Rasheed, a vascular surgery resident in Rochester.
Developed by the Society for Vascular Surgery, the ASG is an assessment of the aortic neck, aneurysm body, iliac arteries, and pelvic perfusion for 16 parameters, including angles, calcifications, and tortuosity. Each parameter is scored from 0-3. Higher scores mean greater complexity, with 48 being the highest possible score (J. Vasc. Surg. 2002;35:1061-6).
An ASG of 15 proved to be a handy marker for when complexity starts to affect the bottom line. A score of 15 or higher correlated with increased costs and increased propensity for requiring intraoperative adjuncts such as renal artery stenting (odds ratio, 5.75; 95% confidence interval, 1.82-18.19). It also correlated with chronic kidney disease and end-stage renal disease, meaning that sicker patients were likely to have worse anatomy and cost more to repair, Dr. Rasheed reported at the meeting hosted by the Society for Special Surgery.
All the cases in the study were elective, and the majority of the patients were elderly white men.
The mean total-cost of endovascular aortic repair (EVAR) was $24,701, mean length of stay (LOS) of 3.0 days, and mean ASG score of 15.9. Cases below an ASG score of 15 cost a mean of $22,020 and had a mean LOS of 2.93 days. Above 15, the mean cost was $26,574 and mean LOS was 3.07 days.
About a quarter of EVAR patients required intraoperative adjuncts, most above an ASG score of 15; their cases cost a mean of $31,509, with a mean ASG score of 18.48 and LOS of 3.85 days.
For open repair, the mean total cost was $38,310, LOS of 13.5 days, and ASG score of 18.1. When five patients with unusually long hospital stays were excluded, open repair cost less than EVAR, which is consistent with previous reports. Just two open-repair patients (6%) needed adjunct procedures.
Open-repair cases with an ASG score below 15 cost a mean of $24,508 and had a mean LOS of 10 days. Cases with a higher score cost a mean of $41,071 and stayed in the hospital an average of 14.2 days. Despite trends, the ASG score differences in cost and LOS for open-repair cases did not reach statistical significance; type II error was probably to blame, Dr. Rasheed said.
The investigators have no disclosures.
CHICAGO – Anatomic complexity should be factored into reimbursements for abdominal aortic aneurysm repairs, University of Rochester (N.Y.) investigators concluded after they compared costs to complexity in 33 open and 107 endovascular repairs during 2007-2010.
They found that complex aneurysms – especially ones with Anatomic Severity Grade (ASG) scores above 15 – need more adjunctive procedures and cost more to repair, although at the moment, payers don’t usually take complexity directly into account.
It’s the first study to show a direct relationship between anatomic complexity and hospital cost. “Preoperative assessment with ASG scores can delineate patients at greater risk for increased resource utilization. A critical examination of the relationship between anatomic complexity and finances is required within the context of aggressive endovascular treatment strategies and shifts towards value-based reimbursement. Anatomy is related to cost. [Complexity] should be considered as a factor when calculating limited bundle reimbursements,” said investigator Dr. Khurram Rasheed, a vascular surgery resident in Rochester.
Developed by the Society for Vascular Surgery, the ASG is an assessment of the aortic neck, aneurysm body, iliac arteries, and pelvic perfusion for 16 parameters, including angles, calcifications, and tortuosity. Each parameter is scored from 0-3. Higher scores mean greater complexity, with 48 being the highest possible score (J. Vasc. Surg. 2002;35:1061-6).
An ASG of 15 proved to be a handy marker for when complexity starts to affect the bottom line. A score of 15 or higher correlated with increased costs and increased propensity for requiring intraoperative adjuncts such as renal artery stenting (odds ratio, 5.75; 95% confidence interval, 1.82-18.19). It also correlated with chronic kidney disease and end-stage renal disease, meaning that sicker patients were likely to have worse anatomy and cost more to repair, Dr. Rasheed reported at the meeting hosted by the Society for Special Surgery.
All the cases in the study were elective, and the majority of the patients were elderly white men.
The mean total-cost of endovascular aortic repair (EVAR) was $24,701, mean length of stay (LOS) of 3.0 days, and mean ASG score of 15.9. Cases below an ASG score of 15 cost a mean of $22,020 and had a mean LOS of 2.93 days. Above 15, the mean cost was $26,574 and mean LOS was 3.07 days.
About a quarter of EVAR patients required intraoperative adjuncts, most above an ASG score of 15; their cases cost a mean of $31,509, with a mean ASG score of 18.48 and LOS of 3.85 days.
For open repair, the mean total cost was $38,310, LOS of 13.5 days, and ASG score of 18.1. When five patients with unusually long hospital stays were excluded, open repair cost less than EVAR, which is consistent with previous reports. Just two open-repair patients (6%) needed adjunct procedures.
Open-repair cases with an ASG score below 15 cost a mean of $24,508 and had a mean LOS of 10 days. Cases with a higher score cost a mean of $41,071 and stayed in the hospital an average of 14.2 days. Despite trends, the ASG score differences in cost and LOS for open-repair cases did not reach statistical significance; type II error was probably to blame, Dr. Rasheed said.
The investigators have no disclosures.
CHICAGO – Anatomic complexity should be factored into reimbursements for abdominal aortic aneurysm repairs, University of Rochester (N.Y.) investigators concluded after they compared costs to complexity in 33 open and 107 endovascular repairs during 2007-2010.
They found that complex aneurysms – especially ones with Anatomic Severity Grade (ASG) scores above 15 – need more adjunctive procedures and cost more to repair, although at the moment, payers don’t usually take complexity directly into account.
It’s the first study to show a direct relationship between anatomic complexity and hospital cost. “Preoperative assessment with ASG scores can delineate patients at greater risk for increased resource utilization. A critical examination of the relationship between anatomic complexity and finances is required within the context of aggressive endovascular treatment strategies and shifts towards value-based reimbursement. Anatomy is related to cost. [Complexity] should be considered as a factor when calculating limited bundle reimbursements,” said investigator Dr. Khurram Rasheed, a vascular surgery resident in Rochester.
Developed by the Society for Vascular Surgery, the ASG is an assessment of the aortic neck, aneurysm body, iliac arteries, and pelvic perfusion for 16 parameters, including angles, calcifications, and tortuosity. Each parameter is scored from 0-3. Higher scores mean greater complexity, with 48 being the highest possible score (J. Vasc. Surg. 2002;35:1061-6).
An ASG of 15 proved to be a handy marker for when complexity starts to affect the bottom line. A score of 15 or higher correlated with increased costs and increased propensity for requiring intraoperative adjuncts such as renal artery stenting (odds ratio, 5.75; 95% confidence interval, 1.82-18.19). It also correlated with chronic kidney disease and end-stage renal disease, meaning that sicker patients were likely to have worse anatomy and cost more to repair, Dr. Rasheed reported at the meeting hosted by the Society for Special Surgery.
All the cases in the study were elective, and the majority of the patients were elderly white men.
The mean total-cost of endovascular aortic repair (EVAR) was $24,701, mean length of stay (LOS) of 3.0 days, and mean ASG score of 15.9. Cases below an ASG score of 15 cost a mean of $22,020 and had a mean LOS of 2.93 days. Above 15, the mean cost was $26,574 and mean LOS was 3.07 days.
About a quarter of EVAR patients required intraoperative adjuncts, most above an ASG score of 15; their cases cost a mean of $31,509, with a mean ASG score of 18.48 and LOS of 3.85 days.
For open repair, the mean total cost was $38,310, LOS of 13.5 days, and ASG score of 18.1. When five patients with unusually long hospital stays were excluded, open repair cost less than EVAR, which is consistent with previous reports. Just two open-repair patients (6%) needed adjunct procedures.
Open-repair cases with an ASG score below 15 cost a mean of $24,508 and had a mean LOS of 10 days. Cases with a higher score cost a mean of $41,071 and stayed in the hospital an average of 14.2 days. Despite trends, the ASG score differences in cost and LOS for open-repair cases did not reach statistical significance; type II error was probably to blame, Dr. Rasheed said.
The investigators have no disclosures.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Get the hang of calculating Anatomic Severity Grade scores on your triple A cases; it might one day increase your reimbursements.
Major finding: EVAR cases below an ASG score of 15 cost a mean of $22,020 and had a length of stay of 2.93 days. Above 15, the cost was $26,574 and mean LOS was 3.07 days.
Data source: Review of 33 open and 107 endovascular repairs at the University of Rochester in New York.
Disclosures: The investigators have no disclosures.
SVS: Cryoallografts, NAIS best to reconstruct infected aortic grafts
CHICAGO – Neoaortoiliac system reconstruction or cryopreserved allografts should be used first-line to replace infected aortic endografts, followed by antibiotic-soaked prosthetic grafts, according to a review of 206 cases from vascular surgery centers across the United States.
In the study, 75 patients had cryoallograft reconstruction, mostly cryoartery, or neoaortoiliac system (NAIS) reconstruction with femoral vein; 53 (71%) were alive at 5 years. Forty-nine of the 92 (53%) patients reconstructed with antibiotic-soaked prosthetic grafts also made it to 5 years, while two of 19 (11%) reconstructed with untreated prosthetic grafts survived that long.
The cases were reconstructed inline. The index procedure had been endovascular aortic repair (EVAR) in 165, and thoracic EVAR (TEVAR) in 21, with a better 5-year survival for the EVAR group. Another 11 patients had extra-anatomic reconstruction after initial EVAR, and their 5-year survival was comparable to inline reconstruction at about 50% overall. Nine other patients were managed medically; the majority died soon after being diagnosed with an infected graft.
“Clinicians should have a high index of suspicion to diagnose symptomatic postop EVAR and TEVAR patients with graft infection, especially in those patients with chronic infections or contaminated index procedures. NAIS and cryopreserved allografts require longer procedure times” – about 500 minutes versus about 350 minutes for prosthetic grafts – “but offer improved survival, while prosthetics soaked in antibiotic do better than prosthetic grafts alone,” said lead investigator Dr. Audra Duncan, professor of surgery at the Mayo Clinic in Rochester, Minn.
Patients were treated at the Mayo Clinic, the Cleveland Clinic in Ohio, Johns Hopkins University in Baltimore, the University of California, Los Angeles, and 15 other vascular centers around the country during 2004-2014. They were 68 years old on average, and 78% were men. Comorbidities included hypertension in 84%, smoking in 58%, and renal insufficiency in 30%.
On multivariate analysis, chronic infection, polymicrobial infection, and prosthetic reconstruction, among other things, predicted mortality after reconstruction.
Graft infections were primarily polymicrobial and fungal, and were diagnosed a mean of 716 days after the initial implant, generally by CT. Symptoms included pain in 66%, mostly in the back and abdomen, and fever and chills, also in 66%. Streptococcus, Escherichia coli, and both methicillin-sensitive and -resistant Staphylococcus aureus were among the most commonly isolated organisms. No particular type of graft seemed more likely to get infected.
The sources of infection are unknown, but index procedures were complicated by urinary tract, groin, and other infections in about one-third of patients. About one-third also had interval procedures, including endoleak intervention. About 14% of patients were thought to have had a contaminated index procedure.
Patients stayed in the hospital a mean of 24 days after reconstruction. Early complications included persistent sepsis in 27 patients, myocardial infarction in 9, recurrent infection in 9, and pneumonia in 8. Mortality at 30 days was 11%.
Nineteen replacement grafts – mostly unsoaked Dacron – were explanted after a mean of 540 days. Persistent sepsis after reconstruction was associated with unsoaked Dacron and polytetrafluoroethylene (PTFE) grafts.
To prevent graft infections, Mayo patients “take an antibiotic for any invasive procedure,” including dental work. “I am not sure we have data to support that, but it is something we do,” Dr. Duncan said.
Dr. Duncan has no relevant disclosures.
CHICAGO – Neoaortoiliac system reconstruction or cryopreserved allografts should be used first-line to replace infected aortic endografts, followed by antibiotic-soaked prosthetic grafts, according to a review of 206 cases from vascular surgery centers across the United States.
In the study, 75 patients had cryoallograft reconstruction, mostly cryoartery, or neoaortoiliac system (NAIS) reconstruction with femoral vein; 53 (71%) were alive at 5 years. Forty-nine of the 92 (53%) patients reconstructed with antibiotic-soaked prosthetic grafts also made it to 5 years, while two of 19 (11%) reconstructed with untreated prosthetic grafts survived that long.
The cases were reconstructed inline. The index procedure had been endovascular aortic repair (EVAR) in 165, and thoracic EVAR (TEVAR) in 21, with a better 5-year survival for the EVAR group. Another 11 patients had extra-anatomic reconstruction after initial EVAR, and their 5-year survival was comparable to inline reconstruction at about 50% overall. Nine other patients were managed medically; the majority died soon after being diagnosed with an infected graft.
“Clinicians should have a high index of suspicion to diagnose symptomatic postop EVAR and TEVAR patients with graft infection, especially in those patients with chronic infections or contaminated index procedures. NAIS and cryopreserved allografts require longer procedure times” – about 500 minutes versus about 350 minutes for prosthetic grafts – “but offer improved survival, while prosthetics soaked in antibiotic do better than prosthetic grafts alone,” said lead investigator Dr. Audra Duncan, professor of surgery at the Mayo Clinic in Rochester, Minn.
Patients were treated at the Mayo Clinic, the Cleveland Clinic in Ohio, Johns Hopkins University in Baltimore, the University of California, Los Angeles, and 15 other vascular centers around the country during 2004-2014. They were 68 years old on average, and 78% were men. Comorbidities included hypertension in 84%, smoking in 58%, and renal insufficiency in 30%.
On multivariate analysis, chronic infection, polymicrobial infection, and prosthetic reconstruction, among other things, predicted mortality after reconstruction.
Graft infections were primarily polymicrobial and fungal, and were diagnosed a mean of 716 days after the initial implant, generally by CT. Symptoms included pain in 66%, mostly in the back and abdomen, and fever and chills, also in 66%. Streptococcus, Escherichia coli, and both methicillin-sensitive and -resistant Staphylococcus aureus were among the most commonly isolated organisms. No particular type of graft seemed more likely to get infected.
The sources of infection are unknown, but index procedures were complicated by urinary tract, groin, and other infections in about one-third of patients. About one-third also had interval procedures, including endoleak intervention. About 14% of patients were thought to have had a contaminated index procedure.
Patients stayed in the hospital a mean of 24 days after reconstruction. Early complications included persistent sepsis in 27 patients, myocardial infarction in 9, recurrent infection in 9, and pneumonia in 8. Mortality at 30 days was 11%.
Nineteen replacement grafts – mostly unsoaked Dacron – were explanted after a mean of 540 days. Persistent sepsis after reconstruction was associated with unsoaked Dacron and polytetrafluoroethylene (PTFE) grafts.
To prevent graft infections, Mayo patients “take an antibiotic for any invasive procedure,” including dental work. “I am not sure we have data to support that, but it is something we do,” Dr. Duncan said.
Dr. Duncan has no relevant disclosures.
CHICAGO – Neoaortoiliac system reconstruction or cryopreserved allografts should be used first-line to replace infected aortic endografts, followed by antibiotic-soaked prosthetic grafts, according to a review of 206 cases from vascular surgery centers across the United States.
In the study, 75 patients had cryoallograft reconstruction, mostly cryoartery, or neoaortoiliac system (NAIS) reconstruction with femoral vein; 53 (71%) were alive at 5 years. Forty-nine of the 92 (53%) patients reconstructed with antibiotic-soaked prosthetic grafts also made it to 5 years, while two of 19 (11%) reconstructed with untreated prosthetic grafts survived that long.
The cases were reconstructed inline. The index procedure had been endovascular aortic repair (EVAR) in 165, and thoracic EVAR (TEVAR) in 21, with a better 5-year survival for the EVAR group. Another 11 patients had extra-anatomic reconstruction after initial EVAR, and their 5-year survival was comparable to inline reconstruction at about 50% overall. Nine other patients were managed medically; the majority died soon after being diagnosed with an infected graft.
“Clinicians should have a high index of suspicion to diagnose symptomatic postop EVAR and TEVAR patients with graft infection, especially in those patients with chronic infections or contaminated index procedures. NAIS and cryopreserved allografts require longer procedure times” – about 500 minutes versus about 350 minutes for prosthetic grafts – “but offer improved survival, while prosthetics soaked in antibiotic do better than prosthetic grafts alone,” said lead investigator Dr. Audra Duncan, professor of surgery at the Mayo Clinic in Rochester, Minn.
Patients were treated at the Mayo Clinic, the Cleveland Clinic in Ohio, Johns Hopkins University in Baltimore, the University of California, Los Angeles, and 15 other vascular centers around the country during 2004-2014. They were 68 years old on average, and 78% were men. Comorbidities included hypertension in 84%, smoking in 58%, and renal insufficiency in 30%.
On multivariate analysis, chronic infection, polymicrobial infection, and prosthetic reconstruction, among other things, predicted mortality after reconstruction.
Graft infections were primarily polymicrobial and fungal, and were diagnosed a mean of 716 days after the initial implant, generally by CT. Symptoms included pain in 66%, mostly in the back and abdomen, and fever and chills, also in 66%. Streptococcus, Escherichia coli, and both methicillin-sensitive and -resistant Staphylococcus aureus were among the most commonly isolated organisms. No particular type of graft seemed more likely to get infected.
The sources of infection are unknown, but index procedures were complicated by urinary tract, groin, and other infections in about one-third of patients. About one-third also had interval procedures, including endoleak intervention. About 14% of patients were thought to have had a contaminated index procedure.
Patients stayed in the hospital a mean of 24 days after reconstruction. Early complications included persistent sepsis in 27 patients, myocardial infarction in 9, recurrent infection in 9, and pneumonia in 8. Mortality at 30 days was 11%.
Nineteen replacement grafts – mostly unsoaked Dacron – were explanted after a mean of 540 days. Persistent sepsis after reconstruction was associated with unsoaked Dacron and polytetrafluoroethylene (PTFE) grafts.
To prevent graft infections, Mayo patients “take an antibiotic for any invasive procedure,” including dental work. “I am not sure we have data to support that, but it is something we do,” Dr. Duncan said.
Dr. Duncan has no relevant disclosures.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Neoaortoiliac system reconstruction or cryopreserved allografts perform best in replacing infected aortic endografts, but if you have to use prosthetics, use ones soaked in antibiotics.
Major finding: Seventy-five patients had cryoallograft reconstruction, mostly cryoartery, or neoaortoiliac system (NAIS) reconstruction with femoral vein; 53 (71%) were alive at 5 years. Two of 19 (11%) patients reconstructed with untreated prosthetic grafts survived that long.
Data source: Review of 206 patients at 19 vascular surgery centers in the United States.
Disclosures: The lead investigator has no relevant disclosures.
SVS: Beta-blockers cut stroke, death risk in carotid stenting
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to non-users, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005]. Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with post-procedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think” the benefits are due to “up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days. There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short term users were more symptomatic; those and other differences were controlled for on multivariate analysis. Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average age in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators have no disclosures.
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to non-users, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005]. Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with post-procedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think” the benefits are due to “up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days. There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short term users were more symptomatic; those and other differences were controlled for on multivariate analysis. Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average age in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators have no disclosures.
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to non-users, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005]. Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with post-procedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think” the benefits are due to “up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days. There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short term users were more symptomatic; those and other differences were controlled for on multivariate analysis. Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average age in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators have no disclosures.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand.
Major finding: The risk of stroke or death after carotid artery stenting is reduced by 34% when patients are on long-term beta-blockers preoperatively.
Data source: Review of 5,248 stent cases during 2005-2014
Disclosures: The investigators have no disclosures.
SVS: Stroke reduction outweighs bleeding risk of dual antiplatelet therapy in CEA
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Strokes are less likely after CEA if patients are on perioperative clopidogrel and aspirin.
Major finding: On multivariate analysis, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62, P = .04); and stroke death (OR, 0.65; P = .03).
Data source: Review of more than 28,000 carotid endarterectomy patients
Disclosures: The presenter has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
SVS: Choose endarterectomy over stenting for complex carotid lesions
CHICAGO – Carotid endarterectomy is safer than stenting when lesions have greater complexity based on length, location, and presence of sequential lesions, according to a subanalysis of the CREST study.
Previous studies have shown that carotid artery stenting (CAS) is also risky in patients with type 3 aortic arches; atherosclerotic aortic arches; internal carotid artery tortuosity; circumferential calcification; and ulcerated lesions.
Taken together, “we can now begin to populate a list of conditions that are high risk for carotid artery stenting. For patients with these factors, we would strongly recommend that carotid endarterectomy be employed rather than carotid artery stenting,” said CREST (Carotid Revascularization Endarterectomy versus Stent Trial) investigator Dr. Wesley Moore, professor and chief, emeritus, of the division of vascular surgery at the University of California, Los Angeles.
“However, in the absence of these higher risk characteristics, carotid artery stenting should yield results equivalent to carotid endarterectomy,” he said at a meeting hosted by the Society for Vascular Surgery.
CREST demonstrated that carotid artery stenting carries about twice the risk of stroke and death (4.4%) as carotid endarterectomy (2.3%); the investigators revisited their subjects’ preop angiograms to see if lesion characteristics were to blame in a subanalysis of the trial results.
In CREST, 438 patients had angiograms before carotid endarterectomies (CEA), about a third of the total number of CEA patients, while preop angiograms were done in all of the 1,262 CAS patients. There were no statistically significant differences in age, gender, stenosis symptoms, smoking history, arrhythmias, and left ventricular hypertrophy between CEA and CAS patients.
For lesions longer than 12.85 mm – the median length in CREST – the combined outcome of strokes and death occurred in 1.9% of CEA and 6.1% of CAS patients (CAS odds ratio, 3.45; 95% confidence interval, 1.21-9.83). For sequential lesions, strokes and death occurred in 0.7% of CEA and 5.8% of CAS patients (CAS OR, 9.21; 95% CI, 1.23-68.94).
With long, sequential lesions distal to the carotid bulb, stroke and death occurred in 6.3% of CAS patients but no CEA patients, giving an “infinite odds ratio in favor of CEA,” Dr. Moore said.
Two-thirds of all the patients in CREST had lesion risk factors for CAS, which might help explain the original findings.
CREST also found that stenting was riskier in older people and women, but it seems likely now that age and gender were surrogates for adverse lesion characteristics.
“The fact of the matter is that older patients have more complex lesions, so age tended to be a surrogate for lesion complexity. If I have an 80-year-old with a short, isolated lesion, I don’t think the fact that they are 80 represents higher risk. I think the lesion being short puts them in the same low risk [category] as other favorable CAS characteristics. I think that’s also true for gender,” Dr. Moore said.
Angiograms almost always underestimate the length of carotid lesions. CT and MRI do a better job, but “ultrasound may be even better than those two,” he noted.
CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. Dr. Moore has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
CHICAGO – Carotid endarterectomy is safer than stenting when lesions have greater complexity based on length, location, and presence of sequential lesions, according to a subanalysis of the CREST study.
Previous studies have shown that carotid artery stenting (CAS) is also risky in patients with type 3 aortic arches; atherosclerotic aortic arches; internal carotid artery tortuosity; circumferential calcification; and ulcerated lesions.
Taken together, “we can now begin to populate a list of conditions that are high risk for carotid artery stenting. For patients with these factors, we would strongly recommend that carotid endarterectomy be employed rather than carotid artery stenting,” said CREST (Carotid Revascularization Endarterectomy versus Stent Trial) investigator Dr. Wesley Moore, professor and chief, emeritus, of the division of vascular surgery at the University of California, Los Angeles.
“However, in the absence of these higher risk characteristics, carotid artery stenting should yield results equivalent to carotid endarterectomy,” he said at a meeting hosted by the Society for Vascular Surgery.
CREST demonstrated that carotid artery stenting carries about twice the risk of stroke and death (4.4%) as carotid endarterectomy (2.3%); the investigators revisited their subjects’ preop angiograms to see if lesion characteristics were to blame in a subanalysis of the trial results.
In CREST, 438 patients had angiograms before carotid endarterectomies (CEA), about a third of the total number of CEA patients, while preop angiograms were done in all of the 1,262 CAS patients. There were no statistically significant differences in age, gender, stenosis symptoms, smoking history, arrhythmias, and left ventricular hypertrophy between CEA and CAS patients.
For lesions longer than 12.85 mm – the median length in CREST – the combined outcome of strokes and death occurred in 1.9% of CEA and 6.1% of CAS patients (CAS odds ratio, 3.45; 95% confidence interval, 1.21-9.83). For sequential lesions, strokes and death occurred in 0.7% of CEA and 5.8% of CAS patients (CAS OR, 9.21; 95% CI, 1.23-68.94).
With long, sequential lesions distal to the carotid bulb, stroke and death occurred in 6.3% of CAS patients but no CEA patients, giving an “infinite odds ratio in favor of CEA,” Dr. Moore said.
Two-thirds of all the patients in CREST had lesion risk factors for CAS, which might help explain the original findings.
CREST also found that stenting was riskier in older people and women, but it seems likely now that age and gender were surrogates for adverse lesion characteristics.
“The fact of the matter is that older patients have more complex lesions, so age tended to be a surrogate for lesion complexity. If I have an 80-year-old with a short, isolated lesion, I don’t think the fact that they are 80 represents higher risk. I think the lesion being short puts them in the same low risk [category] as other favorable CAS characteristics. I think that’s also true for gender,” Dr. Moore said.
Angiograms almost always underestimate the length of carotid lesions. CT and MRI do a better job, but “ultrasound may be even better than those two,” he noted.
CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. Dr. Moore has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
CHICAGO – Carotid endarterectomy is safer than stenting when lesions have greater complexity based on length, location, and presence of sequential lesions, according to a subanalysis of the CREST study.
Previous studies have shown that carotid artery stenting (CAS) is also risky in patients with type 3 aortic arches; atherosclerotic aortic arches; internal carotid artery tortuosity; circumferential calcification; and ulcerated lesions.
Taken together, “we can now begin to populate a list of conditions that are high risk for carotid artery stenting. For patients with these factors, we would strongly recommend that carotid endarterectomy be employed rather than carotid artery stenting,” said CREST (Carotid Revascularization Endarterectomy versus Stent Trial) investigator Dr. Wesley Moore, professor and chief, emeritus, of the division of vascular surgery at the University of California, Los Angeles.
“However, in the absence of these higher risk characteristics, carotid artery stenting should yield results equivalent to carotid endarterectomy,” he said at a meeting hosted by the Society for Vascular Surgery.
CREST demonstrated that carotid artery stenting carries about twice the risk of stroke and death (4.4%) as carotid endarterectomy (2.3%); the investigators revisited their subjects’ preop angiograms to see if lesion characteristics were to blame in a subanalysis of the trial results.
In CREST, 438 patients had angiograms before carotid endarterectomies (CEA), about a third of the total number of CEA patients, while preop angiograms were done in all of the 1,262 CAS patients. There were no statistically significant differences in age, gender, stenosis symptoms, smoking history, arrhythmias, and left ventricular hypertrophy between CEA and CAS patients.
For lesions longer than 12.85 mm – the median length in CREST – the combined outcome of strokes and death occurred in 1.9% of CEA and 6.1% of CAS patients (CAS odds ratio, 3.45; 95% confidence interval, 1.21-9.83). For sequential lesions, strokes and death occurred in 0.7% of CEA and 5.8% of CAS patients (CAS OR, 9.21; 95% CI, 1.23-68.94).
With long, sequential lesions distal to the carotid bulb, stroke and death occurred in 6.3% of CAS patients but no CEA patients, giving an “infinite odds ratio in favor of CEA,” Dr. Moore said.
Two-thirds of all the patients in CREST had lesion risk factors for CAS, which might help explain the original findings.
CREST also found that stenting was riskier in older people and women, but it seems likely now that age and gender were surrogates for adverse lesion characteristics.
“The fact of the matter is that older patients have more complex lesions, so age tended to be a surrogate for lesion complexity. If I have an 80-year-old with a short, isolated lesion, I don’t think the fact that they are 80 represents higher risk. I think the lesion being short puts them in the same low risk [category] as other favorable CAS characteristics. I think that’s also true for gender,” Dr. Moore said.
Angiograms almost always underestimate the length of carotid lesions. CT and MRI do a better job, but “ultrasound may be even better than those two,” he noted.
CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. Dr. Moore has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Carotid endarterectomy had a lower rate of the combined outcome of stroke or death in patients with greater lesion complexity, which may be a better marker than age or gender for determining the best treatment modality.
Major finding: For lesions longer than 12.85 mm, the combined outcome of strokes and death occurred in 1.9% of carotid endarterectomy patients and 6.1% of carotid stent patients (CAS OR, 3.45; 95% CI, 1.21-9.83). Subsanalysis of the CREST study
Data source: Subsanalysis of the CREST study
Disclosures: CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. The presenter has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
WCD: EDTA wash eliminates toluidine blue staining artifacts in Mohs surgery
VANCOUVER – Investigators at the Memorial Sloan Kettering Skin Cancer Center in Hauppauge, N.Y. have found a simple solution for a vexing problem in Mohs surgery, histology sections that don’t fully stain with toluidine blue.
The problem, it turns out, is that aluminum chloride and Monsel’s solution (ferric subsulfate) – common topical hemostatics used during the procedure – block the stain from binding tissue. Washing sections in ethylenediaminetetraacetic acid (EDTA) before staining completely eliminates the problem.
“I think this will be very useful for Mohs surgeons. It’s a very simple step, a simple modification of routine staining protocol that [eradicates] this type of artifact. When technicians collect the specimen onto the slide, they wash it with EDTA solution for about 10 seconds.” Toluidine blue staining afterward “will be perfect. There won’t be any artifact,” said senior investigator Dr. Chih-Shan Jason Chen, director of dermatologic surgery at the Hauppauge center.
It’s an important finding (and one not yet published in the medical literature, according to Dr. Chen) because staining artifacts make it impossible to read toluidine blue sections with confidence, so Mohs surgeons have to go back and take more sections, creating a deeper wound.
Over a period of the past 2 years, the EDTA wash “has made a significant difference in my practice. I never see this type of artifact anymore. I can be very comfortable taking a very thin layer of tissue and don’t need to take extra stages. We don’t need to do fancy reconstructive surgery” for the wound to heal. “This process fits with the spirit of Mohs surgery, the concept of tissue conservation,” Dr. Chen said at the World Congress of Dermatology.
Perhaps about 20% of Mohs surgeons rely heavily on toluidine blue, especially to stain for basal cell carcinomas. “The nests of cancer cells are very small, so if you have an artifact, you are going to miss them,” he noted.
The first clue that hemostatic agents were to blame was that the artifacts didn’t occur with the first Mohs cut, but only in second and subsequent sections after hemostatic agents had been applied, and especially in thinner sections more permeable to those agents. Dr. Chen tried the EDTA wash, and it worked.
His research assistant, Curtis Chen, an incoming medical student at the University of Miami, figured out the chemistry. Aluminum and iron cations from the agents bind the tissue sample, inhibiting adherence of toluidine blue. EDTA, a chelating agent, scavenges the ions, releasing the tissue for staining.
Dr. Chen and Mr. Chen had no relevant financial disclosures, and there was no outside funding for the work.
VANCOUVER – Investigators at the Memorial Sloan Kettering Skin Cancer Center in Hauppauge, N.Y. have found a simple solution for a vexing problem in Mohs surgery, histology sections that don’t fully stain with toluidine blue.
The problem, it turns out, is that aluminum chloride and Monsel’s solution (ferric subsulfate) – common topical hemostatics used during the procedure – block the stain from binding tissue. Washing sections in ethylenediaminetetraacetic acid (EDTA) before staining completely eliminates the problem.
“I think this will be very useful for Mohs surgeons. It’s a very simple step, a simple modification of routine staining protocol that [eradicates] this type of artifact. When technicians collect the specimen onto the slide, they wash it with EDTA solution for about 10 seconds.” Toluidine blue staining afterward “will be perfect. There won’t be any artifact,” said senior investigator Dr. Chih-Shan Jason Chen, director of dermatologic surgery at the Hauppauge center.
It’s an important finding (and one not yet published in the medical literature, according to Dr. Chen) because staining artifacts make it impossible to read toluidine blue sections with confidence, so Mohs surgeons have to go back and take more sections, creating a deeper wound.
Over a period of the past 2 years, the EDTA wash “has made a significant difference in my practice. I never see this type of artifact anymore. I can be very comfortable taking a very thin layer of tissue and don’t need to take extra stages. We don’t need to do fancy reconstructive surgery” for the wound to heal. “This process fits with the spirit of Mohs surgery, the concept of tissue conservation,” Dr. Chen said at the World Congress of Dermatology.
Perhaps about 20% of Mohs surgeons rely heavily on toluidine blue, especially to stain for basal cell carcinomas. “The nests of cancer cells are very small, so if you have an artifact, you are going to miss them,” he noted.
The first clue that hemostatic agents were to blame was that the artifacts didn’t occur with the first Mohs cut, but only in second and subsequent sections after hemostatic agents had been applied, and especially in thinner sections more permeable to those agents. Dr. Chen tried the EDTA wash, and it worked.
His research assistant, Curtis Chen, an incoming medical student at the University of Miami, figured out the chemistry. Aluminum and iron cations from the agents bind the tissue sample, inhibiting adherence of toluidine blue. EDTA, a chelating agent, scavenges the ions, releasing the tissue for staining.
Dr. Chen and Mr. Chen had no relevant financial disclosures, and there was no outside funding for the work.
VANCOUVER – Investigators at the Memorial Sloan Kettering Skin Cancer Center in Hauppauge, N.Y. have found a simple solution for a vexing problem in Mohs surgery, histology sections that don’t fully stain with toluidine blue.
The problem, it turns out, is that aluminum chloride and Monsel’s solution (ferric subsulfate) – common topical hemostatics used during the procedure – block the stain from binding tissue. Washing sections in ethylenediaminetetraacetic acid (EDTA) before staining completely eliminates the problem.
“I think this will be very useful for Mohs surgeons. It’s a very simple step, a simple modification of routine staining protocol that [eradicates] this type of artifact. When technicians collect the specimen onto the slide, they wash it with EDTA solution for about 10 seconds.” Toluidine blue staining afterward “will be perfect. There won’t be any artifact,” said senior investigator Dr. Chih-Shan Jason Chen, director of dermatologic surgery at the Hauppauge center.
It’s an important finding (and one not yet published in the medical literature, according to Dr. Chen) because staining artifacts make it impossible to read toluidine blue sections with confidence, so Mohs surgeons have to go back and take more sections, creating a deeper wound.
Over a period of the past 2 years, the EDTA wash “has made a significant difference in my practice. I never see this type of artifact anymore. I can be very comfortable taking a very thin layer of tissue and don’t need to take extra stages. We don’t need to do fancy reconstructive surgery” for the wound to heal. “This process fits with the spirit of Mohs surgery, the concept of tissue conservation,” Dr. Chen said at the World Congress of Dermatology.
Perhaps about 20% of Mohs surgeons rely heavily on toluidine blue, especially to stain for basal cell carcinomas. “The nests of cancer cells are very small, so if you have an artifact, you are going to miss them,” he noted.
The first clue that hemostatic agents were to blame was that the artifacts didn’t occur with the first Mohs cut, but only in second and subsequent sections after hemostatic agents had been applied, and especially in thinner sections more permeable to those agents. Dr. Chen tried the EDTA wash, and it worked.
His research assistant, Curtis Chen, an incoming medical student at the University of Miami, figured out the chemistry. Aluminum and iron cations from the agents bind the tissue sample, inhibiting adherence of toluidine blue. EDTA, a chelating agent, scavenges the ions, releasing the tissue for staining.
Dr. Chen and Mr. Chen had no relevant financial disclosures, and there was no outside funding for the work.
EXPERT ANALYSIS FROM WCD 2015