User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
CAP shifting to viral disease as vaccines knock out bacterial causes
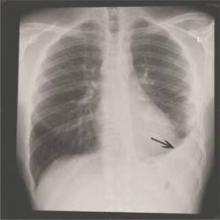
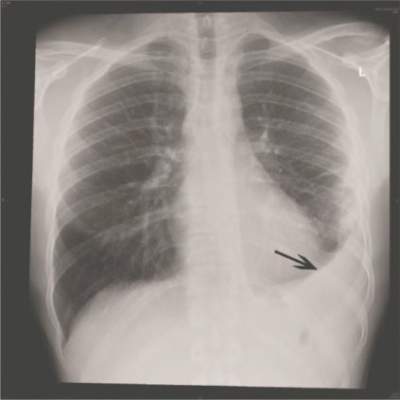
Viral infections appear to be the primary cause of pneumonia that results in hospitalization, according to a Centers for Disease Control and Prevention study of five urban hospitals in Chicago and Nashville, Tenn.
The study was published online July 14 in the New England Journal of Medicine.
From January 2010 through June 2012, 2,259 patients hospitalized for community-acquired pneumonia (CAP) had both radiographic evidence of disease and specimens for bacterial and viral testing. A pathogen was detected in just 853 (38%). One or more viruses were detected in 530 (23%), bacteria in 247 (11%), bacterial and viral pathogens in 59 (3%), and fungal or mycobacterial pathogens in 17 (1%). The findings indicate the annual incidence of community-acquired pneumonia requiring hospitalization is about 25 cases per 10,000 adults, with the highest rates among adults 65-79 years old (63 cases per 10,000) and 80 years or older (164 cases per 10,000).
The most common pathogens were rhinovirus in 9% of patients, influenza virus in 6%, and Streptococcus pneumoniae in 5%, which was the most commonly detected bacterium (N. Engl. J. Med. 14 July 2015 [doi:10.1056/NEJMoa1500245]).
CAP is thought to be caused most often by S. pneumoniae and other bacteria; the fact that viruses were more frequently detected “probably reflects the direct and indirect benefit of bacterial vaccines,” but also “relatively insensitive diagnostic tests,” said the investigators, led by CDC medical epidemiologist Dr. Seema Jain.
The project, dubbed the Etiology of Pneumonia in the Community (EPIC) study, “adds to the growing evidence of the contribution of viruses to hospitalizations of adults.” It also suggests “that improving the coverage and effectiveness of recommended influenza and pneumococcal vaccines and developing effective vaccines and treatments for human metapneumovirus, respiratory syncytial virus, and parainfluenza virus” – also found in study patients – “could reduce the burden of pneumonia among adults,” the researchers said.
The work matters because “the last U.S. population–based incidence estimates of hospitalization due to community-acquired pneumonia were made in the 1990s, before the availability of the pneumococcal conjugate vaccine and more sensitive molecular and antigen-based laboratory diagnostic tests. Thus, contemporary population-based etiologic studies involving U.S. adults with pneumonia are needed,” they noted.
Blood, urine, and respiratory cultures; serologic testing; antigen detection; molecular diagnostics, and chest x-rays were all used to find the cause of disease. The fact that infections were found in just 38% of the patients could have something to do with the team’s “inability to obtain lower respiratory tract specimens, antibiotic use before specimen collection, [and] insensitive diagnostic tests for known pathogens,” among other problems, they said.
There was a low prevalence of Enterobacteriaceae (1%) and other gram-negative bacteria, probably because patients with recent hospitalizations and severe immunosuppression were excluded. Subjects were on average 60 years old.
The CDC’s National Center for Immunizations and Respiratory Diseases funded the work. Several of the authors disclosed ties to industry, including GlaxoSmithKline, Abbvie, and Pfizer.
Viral infections appear to be the primary cause of pneumonia that results in hospitalization, according to a Centers for Disease Control and Prevention study of five urban hospitals in Chicago and Nashville, Tenn.
The study was published online July 14 in the New England Journal of Medicine.
From January 2010 through June 2012, 2,259 patients hospitalized for community-acquired pneumonia (CAP) had both radiographic evidence of disease and specimens for bacterial and viral testing. A pathogen was detected in just 853 (38%). One or more viruses were detected in 530 (23%), bacteria in 247 (11%), bacterial and viral pathogens in 59 (3%), and fungal or mycobacterial pathogens in 17 (1%). The findings indicate the annual incidence of community-acquired pneumonia requiring hospitalization is about 25 cases per 10,000 adults, with the highest rates among adults 65-79 years old (63 cases per 10,000) and 80 years or older (164 cases per 10,000).
The most common pathogens were rhinovirus in 9% of patients, influenza virus in 6%, and Streptococcus pneumoniae in 5%, which was the most commonly detected bacterium (N. Engl. J. Med. 14 July 2015 [doi:10.1056/NEJMoa1500245]).
CAP is thought to be caused most often by S. pneumoniae and other bacteria; the fact that viruses were more frequently detected “probably reflects the direct and indirect benefit of bacterial vaccines,” but also “relatively insensitive diagnostic tests,” said the investigators, led by CDC medical epidemiologist Dr. Seema Jain.
The project, dubbed the Etiology of Pneumonia in the Community (EPIC) study, “adds to the growing evidence of the contribution of viruses to hospitalizations of adults.” It also suggests “that improving the coverage and effectiveness of recommended influenza and pneumococcal vaccines and developing effective vaccines and treatments for human metapneumovirus, respiratory syncytial virus, and parainfluenza virus” – also found in study patients – “could reduce the burden of pneumonia among adults,” the researchers said.
The work matters because “the last U.S. population–based incidence estimates of hospitalization due to community-acquired pneumonia were made in the 1990s, before the availability of the pneumococcal conjugate vaccine and more sensitive molecular and antigen-based laboratory diagnostic tests. Thus, contemporary population-based etiologic studies involving U.S. adults with pneumonia are needed,” they noted.
Blood, urine, and respiratory cultures; serologic testing; antigen detection; molecular diagnostics, and chest x-rays were all used to find the cause of disease. The fact that infections were found in just 38% of the patients could have something to do with the team’s “inability to obtain lower respiratory tract specimens, antibiotic use before specimen collection, [and] insensitive diagnostic tests for known pathogens,” among other problems, they said.
There was a low prevalence of Enterobacteriaceae (1%) and other gram-negative bacteria, probably because patients with recent hospitalizations and severe immunosuppression were excluded. Subjects were on average 60 years old.
The CDC’s National Center for Immunizations and Respiratory Diseases funded the work. Several of the authors disclosed ties to industry, including GlaxoSmithKline, Abbvie, and Pfizer.
Viral infections appear to be the primary cause of pneumonia that results in hospitalization, according to a Centers for Disease Control and Prevention study of five urban hospitals in Chicago and Nashville, Tenn.
The study was published online July 14 in the New England Journal of Medicine.
From January 2010 through June 2012, 2,259 patients hospitalized for community-acquired pneumonia (CAP) had both radiographic evidence of disease and specimens for bacterial and viral testing. A pathogen was detected in just 853 (38%). One or more viruses were detected in 530 (23%), bacteria in 247 (11%), bacterial and viral pathogens in 59 (3%), and fungal or mycobacterial pathogens in 17 (1%). The findings indicate the annual incidence of community-acquired pneumonia requiring hospitalization is about 25 cases per 10,000 adults, with the highest rates among adults 65-79 years old (63 cases per 10,000) and 80 years or older (164 cases per 10,000).
The most common pathogens were rhinovirus in 9% of patients, influenza virus in 6%, and Streptococcus pneumoniae in 5%, which was the most commonly detected bacterium (N. Engl. J. Med. 14 July 2015 [doi:10.1056/NEJMoa1500245]).
CAP is thought to be caused most often by S. pneumoniae and other bacteria; the fact that viruses were more frequently detected “probably reflects the direct and indirect benefit of bacterial vaccines,” but also “relatively insensitive diagnostic tests,” said the investigators, led by CDC medical epidemiologist Dr. Seema Jain.
The project, dubbed the Etiology of Pneumonia in the Community (EPIC) study, “adds to the growing evidence of the contribution of viruses to hospitalizations of adults.” It also suggests “that improving the coverage and effectiveness of recommended influenza and pneumococcal vaccines and developing effective vaccines and treatments for human metapneumovirus, respiratory syncytial virus, and parainfluenza virus” – also found in study patients – “could reduce the burden of pneumonia among adults,” the researchers said.
The work matters because “the last U.S. population–based incidence estimates of hospitalization due to community-acquired pneumonia were made in the 1990s, before the availability of the pneumococcal conjugate vaccine and more sensitive molecular and antigen-based laboratory diagnostic tests. Thus, contemporary population-based etiologic studies involving U.S. adults with pneumonia are needed,” they noted.
Blood, urine, and respiratory cultures; serologic testing; antigen detection; molecular diagnostics, and chest x-rays were all used to find the cause of disease. The fact that infections were found in just 38% of the patients could have something to do with the team’s “inability to obtain lower respiratory tract specimens, antibiotic use before specimen collection, [and] insensitive diagnostic tests for known pathogens,” among other problems, they said.
There was a low prevalence of Enterobacteriaceae (1%) and other gram-negative bacteria, probably because patients with recent hospitalizations and severe immunosuppression were excluded. Subjects were on average 60 years old.
The CDC’s National Center for Immunizations and Respiratory Diseases funded the work. Several of the authors disclosed ties to industry, including GlaxoSmithKline, Abbvie, and Pfizer.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Viral infections may be on the rise as a cause of community-acquired pneumonia.
Major finding: Among more than 2,000 patients hospitalized for CAP, one or more viruses were detected in 23%, and bacteria in 11%.
Data source: Prospective surveillance study at five urban hospitals.
Disclosures: The CDC’s National Center for Immunizations and Respiratory Diseases funded the work. Several of the authors disclosed ties to industry.
SVS: Opt for early repair of PDA/GDA splanchnic aneurysms
CHICAGO – Pancreaticoduodenal and gastroduodenal artery aneurysms should be repaired at diagnosis, according to Dr. Michael Corey, a vascular surgeon at Massachusetts General Hospital in Boston.
The reason is “they rupture at small sizes. Most other small splanchnic artery aneurysms” – below 25 mm – “do not grow or rupture over time and can safely undergo surveillance imaging every 3 years,” he said at a meeting hosted by the Society for Vascular Surgery.
The insights come from Dr. Corey’s review of 264 splanchnic artery aneurysms (SAAs) treated at Massachusetts General Hospital from 1994 to 2014 .
Pancreaticoduodenal (PDA) and gastroduodenal (GDA) artery aneurysms were the most likely to cause trouble. Almost all of the 36 in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm, range 15-48 mm.
Those 7 accounted for more than half of the 13 ruptures in the study. There were also five ruptures among 95 splenic artery aneurysms – the most common aneurysm type in the study – at a mean of 42 mm, and one among 34 hepatic artery aneurysms at 40 mm. Thirty-day morbidity after rupture repair was 54% and mortality 8%.
Pancreaticoduodenal (odds ratio, 14.41; 95% confidence interval, 3.5-59.9; P = .0002) and gastroduodenal artery aneurysms (OR, 6.95; 95% CI, 1.1-45.1; P = .042) were far more predictive of rupture than aneurysm size (OR, 1.04; 95% CI, 1.01-1.08; P = .0042). The strongest predictor was type 4 Ehlers-Danlos syndrome (OR, 34.09; 95% CI, 2.4-479.8; P = .0089). Calcification, meanwhile, did not predict rupture, growth, or thrombus burden.
Dr. Corey and his colleagues reviewed Massachusetts General’s experience with SAAs because “no strong consensus exists in the literature concerning the indications for treatment; 2 cm is currently the indication for surgical treatment of asymptomatic lesions,” he said.
Two centimeters might be too aggressive in some cases. Among 176 aneurysms put under surveillance for a mean of 36.1 months, the mean aneurysm size was 16.3 mm but ranged up to 40 mm. Even so, none of them ruptured. Just 12 aneurysms grew during surveillance, and only 8 eventually needed intervention. Perhaps most “small asymptomatic lesions do not affect longevity,” Dr. Corey said. The mean aneurysm size was 31.1 mm in the 88 patients repaired within 6 months of diagnosis. Splenic, pancreaticoduodenal, gastroduodenal, and hepatic aneurysms were the most likely to be repaired early, the majority by coil embolization and other endovascular techniques. Thirty-day morbidity for intact repair was 13% and mortality 3%.
Most of the splenic artery aneurysms were asymptomatic at presentation. In the half that were watched, just six grew.
Similarly, 78 celiac artery aneurysms – the second most common in the study – all presented without symptoms. Just 3 of the 60 under surveillance grew over a mean of 43.6 months. “These aneurysms rarely change,” Dr. Corey said.
Most of the 34 hepatic artery aneurysms and 17 superior mesenteric artery (SMA) aneurysms were asymptomatic. Between both groups, 20 aneurysms were put under surveillance; growth was noted in 1, an SMA lesion.
Although there was a shift from open to endovascular repair during the study period, there were no statistically significant differences in morbidity or mortality between the two approaches.
Dr. Corey has no disclosures.
CHICAGO – Pancreaticoduodenal and gastroduodenal artery aneurysms should be repaired at diagnosis, according to Dr. Michael Corey, a vascular surgeon at Massachusetts General Hospital in Boston.
The reason is “they rupture at small sizes. Most other small splanchnic artery aneurysms” – below 25 mm – “do not grow or rupture over time and can safely undergo surveillance imaging every 3 years,” he said at a meeting hosted by the Society for Vascular Surgery.
The insights come from Dr. Corey’s review of 264 splanchnic artery aneurysms (SAAs) treated at Massachusetts General Hospital from 1994 to 2014 .
Pancreaticoduodenal (PDA) and gastroduodenal (GDA) artery aneurysms were the most likely to cause trouble. Almost all of the 36 in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm, range 15-48 mm.
Those 7 accounted for more than half of the 13 ruptures in the study. There were also five ruptures among 95 splenic artery aneurysms – the most common aneurysm type in the study – at a mean of 42 mm, and one among 34 hepatic artery aneurysms at 40 mm. Thirty-day morbidity after rupture repair was 54% and mortality 8%.
Pancreaticoduodenal (odds ratio, 14.41; 95% confidence interval, 3.5-59.9; P = .0002) and gastroduodenal artery aneurysms (OR, 6.95; 95% CI, 1.1-45.1; P = .042) were far more predictive of rupture than aneurysm size (OR, 1.04; 95% CI, 1.01-1.08; P = .0042). The strongest predictor was type 4 Ehlers-Danlos syndrome (OR, 34.09; 95% CI, 2.4-479.8; P = .0089). Calcification, meanwhile, did not predict rupture, growth, or thrombus burden.
Dr. Corey and his colleagues reviewed Massachusetts General’s experience with SAAs because “no strong consensus exists in the literature concerning the indications for treatment; 2 cm is currently the indication for surgical treatment of asymptomatic lesions,” he said.
Two centimeters might be too aggressive in some cases. Among 176 aneurysms put under surveillance for a mean of 36.1 months, the mean aneurysm size was 16.3 mm but ranged up to 40 mm. Even so, none of them ruptured. Just 12 aneurysms grew during surveillance, and only 8 eventually needed intervention. Perhaps most “small asymptomatic lesions do not affect longevity,” Dr. Corey said. The mean aneurysm size was 31.1 mm in the 88 patients repaired within 6 months of diagnosis. Splenic, pancreaticoduodenal, gastroduodenal, and hepatic aneurysms were the most likely to be repaired early, the majority by coil embolization and other endovascular techniques. Thirty-day morbidity for intact repair was 13% and mortality 3%.
Most of the splenic artery aneurysms were asymptomatic at presentation. In the half that were watched, just six grew.
Similarly, 78 celiac artery aneurysms – the second most common in the study – all presented without symptoms. Just 3 of the 60 under surveillance grew over a mean of 43.6 months. “These aneurysms rarely change,” Dr. Corey said.
Most of the 34 hepatic artery aneurysms and 17 superior mesenteric artery (SMA) aneurysms were asymptomatic. Between both groups, 20 aneurysms were put under surveillance; growth was noted in 1, an SMA lesion.
Although there was a shift from open to endovascular repair during the study period, there were no statistically significant differences in morbidity or mortality between the two approaches.
Dr. Corey has no disclosures.
CHICAGO – Pancreaticoduodenal and gastroduodenal artery aneurysms should be repaired at diagnosis, according to Dr. Michael Corey, a vascular surgeon at Massachusetts General Hospital in Boston.
The reason is “they rupture at small sizes. Most other small splanchnic artery aneurysms” – below 25 mm – “do not grow or rupture over time and can safely undergo surveillance imaging every 3 years,” he said at a meeting hosted by the Society for Vascular Surgery.
The insights come from Dr. Corey’s review of 264 splanchnic artery aneurysms (SAAs) treated at Massachusetts General Hospital from 1994 to 2014 .
Pancreaticoduodenal (PDA) and gastroduodenal (GDA) artery aneurysms were the most likely to cause trouble. Almost all of the 36 in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm, range 15-48 mm.
Those 7 accounted for more than half of the 13 ruptures in the study. There were also five ruptures among 95 splenic artery aneurysms – the most common aneurysm type in the study – at a mean of 42 mm, and one among 34 hepatic artery aneurysms at 40 mm. Thirty-day morbidity after rupture repair was 54% and mortality 8%.
Pancreaticoduodenal (odds ratio, 14.41; 95% confidence interval, 3.5-59.9; P = .0002) and gastroduodenal artery aneurysms (OR, 6.95; 95% CI, 1.1-45.1; P = .042) were far more predictive of rupture than aneurysm size (OR, 1.04; 95% CI, 1.01-1.08; P = .0042). The strongest predictor was type 4 Ehlers-Danlos syndrome (OR, 34.09; 95% CI, 2.4-479.8; P = .0089). Calcification, meanwhile, did not predict rupture, growth, or thrombus burden.
Dr. Corey and his colleagues reviewed Massachusetts General’s experience with SAAs because “no strong consensus exists in the literature concerning the indications for treatment; 2 cm is currently the indication for surgical treatment of asymptomatic lesions,” he said.
Two centimeters might be too aggressive in some cases. Among 176 aneurysms put under surveillance for a mean of 36.1 months, the mean aneurysm size was 16.3 mm but ranged up to 40 mm. Even so, none of them ruptured. Just 12 aneurysms grew during surveillance, and only 8 eventually needed intervention. Perhaps most “small asymptomatic lesions do not affect longevity,” Dr. Corey said. The mean aneurysm size was 31.1 mm in the 88 patients repaired within 6 months of diagnosis. Splenic, pancreaticoduodenal, gastroduodenal, and hepatic aneurysms were the most likely to be repaired early, the majority by coil embolization and other endovascular techniques. Thirty-day morbidity for intact repair was 13% and mortality 3%.
Most of the splenic artery aneurysms were asymptomatic at presentation. In the half that were watched, just six grew.
Similarly, 78 celiac artery aneurysms – the second most common in the study – all presented without symptoms. Just 3 of the 60 under surveillance grew over a mean of 43.6 months. “These aneurysms rarely change,” Dr. Corey said.
Most of the 34 hepatic artery aneurysms and 17 superior mesenteric artery (SMA) aneurysms were asymptomatic. Between both groups, 20 aneurysms were put under surveillance; growth was noted in 1, an SMA lesion.
Although there was a shift from open to endovascular repair during the study period, there were no statistically significant differences in morbidity or mortality between the two approaches.
Dr. Corey has no disclosures.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Pancreaticoduodenal and gastroduodenal artery aneurysms rupture at smaller sizes than do other visceral aneurysms.
Major finding: Almost all of the 36 aneurysms in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm (range, 15-48 mm).
Data source: Review of 264 splanchnic artery aneurysms treated at Massachusetts General Hospital from 1994 to 2014.
Disclosures: The lead investigator has no relevant disclosures.
SVS: Don’t let TPA delay urgent carotid interventions for mild and moderate strokes
CHICAGO – Urgent carotid interventions were safe after thrombolysis for acute mild to moderate strokes, according to a review of 165 patients at the Ochsner Clinic in New Orleans.
“Our data support the practice of not denying a patient an urgent carotid intervention simply because of TPA [tissue plasminogen activator] administration during the acute stroke period,” said lead investigator Dr. Nicolas Zea, an Ochsner vascular surgeon.
“Urgent carotid endarterectomy [CEA] or coronary artery stenting [CAS] can be safely undertaken in minor to moderate strokes with NIH stroke scale scores less than 10; TPA itself does not appear to be a contraindication, even within 72 hours,” he added.
Urgent carotid interventions are becoming more common after ischemic strokes to prevent recurrences. The approach is most effective within 2 weeks of the index event, but there have been concerns that intracranial hemorrhages (ICH) and other complications might be more likely if patients have had TPA.
Dr. Ochsner and his colleagues conducted their review because, “as vascular surgeons, we are going to encounter a lot more of these patients in the very near future,” Dr. Zea said a meeting hosted by the Society for Vascular Surgery.
From January 2009 to January 2015, 31 patients at Ochsner had carotid interventions – 25 CEA, 6 CAS – a mean of 2.1 days after receiving TPA for transient ischemic attacks (TIA) or ischemic strokes. The patients’ mean National Institutes of Health Stroke Scale (NIHSS) score was 6.6.
Over the same period, 134 patients who had not received TPA had urgent carotid interventions – 110 CEA, 24 CAS – a mean of 2.6 days after TIA or ischemic stroke presentation. Their mean NIHSS score was 6.1.
There were no statistically significant demographic or comorbidity differences between the TPA and no-TPA groups; patients were about 70 years old, on average, and the majority were men. Most had ipsilateral carotid stenosis greater than 70%, or acute occlusions.
The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the non-TPA group, a nonsignificant difference (P = 0.35).
In the TPA group, there was one (3.2%) ICH, one (3.2%) neck hematoma, and two (6.4%) deaths. In the no-TPA group, there were two (1.5%) ICHs, two (1.5%) neck hematomas, one (0.7%) ischemic stroke, two (1.5%) myocardial infarctions, and two (1.5%) deaths.
In both groups, ICH patients had stroke scores greater than 10. Also, although the rate of death was higher in the TPA group, the deaths “were not necessarily related to thrombolysis,” Dr. Zea noted. One death was from pulmonary embolism, the second from unknown causes. Deaths were due to acute mesenteric ischemia and ICH in the no-TPA group, Dr. Zea said.
There was one (3.2%) hemorrhagic conversion in the TPA group and two (1.5%) in the no-TPA group. Similarly, one (3.2%) TPA patient and two (1.5%) no-TPA patients had complications from access site bleeding. The differences were not statistically significant.
In the TPA group, it didn’t seem to matter if intervention came within 72 hours of administration – as in about half the cases – or afterward, when TPA risks have largely passed. There was one death and one ICH in patients in the earlier group, and one death in the later group, a nonsignificant difference.
There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
aotto@frontlinemedcom.com
CHICAGO – Urgent carotid interventions were safe after thrombolysis for acute mild to moderate strokes, according to a review of 165 patients at the Ochsner Clinic in New Orleans.
“Our data support the practice of not denying a patient an urgent carotid intervention simply because of TPA [tissue plasminogen activator] administration during the acute stroke period,” said lead investigator Dr. Nicolas Zea, an Ochsner vascular surgeon.
“Urgent carotid endarterectomy [CEA] or coronary artery stenting [CAS] can be safely undertaken in minor to moderate strokes with NIH stroke scale scores less than 10; TPA itself does not appear to be a contraindication, even within 72 hours,” he added.
Urgent carotid interventions are becoming more common after ischemic strokes to prevent recurrences. The approach is most effective within 2 weeks of the index event, but there have been concerns that intracranial hemorrhages (ICH) and other complications might be more likely if patients have had TPA.
Dr. Ochsner and his colleagues conducted their review because, “as vascular surgeons, we are going to encounter a lot more of these patients in the very near future,” Dr. Zea said a meeting hosted by the Society for Vascular Surgery.
From January 2009 to January 2015, 31 patients at Ochsner had carotid interventions – 25 CEA, 6 CAS – a mean of 2.1 days after receiving TPA for transient ischemic attacks (TIA) or ischemic strokes. The patients’ mean National Institutes of Health Stroke Scale (NIHSS) score was 6.6.
Over the same period, 134 patients who had not received TPA had urgent carotid interventions – 110 CEA, 24 CAS – a mean of 2.6 days after TIA or ischemic stroke presentation. Their mean NIHSS score was 6.1.
There were no statistically significant demographic or comorbidity differences between the TPA and no-TPA groups; patients were about 70 years old, on average, and the majority were men. Most had ipsilateral carotid stenosis greater than 70%, or acute occlusions.
The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the non-TPA group, a nonsignificant difference (P = 0.35).
In the TPA group, there was one (3.2%) ICH, one (3.2%) neck hematoma, and two (6.4%) deaths. In the no-TPA group, there were two (1.5%) ICHs, two (1.5%) neck hematomas, one (0.7%) ischemic stroke, two (1.5%) myocardial infarctions, and two (1.5%) deaths.
In both groups, ICH patients had stroke scores greater than 10. Also, although the rate of death was higher in the TPA group, the deaths “were not necessarily related to thrombolysis,” Dr. Zea noted. One death was from pulmonary embolism, the second from unknown causes. Deaths were due to acute mesenteric ischemia and ICH in the no-TPA group, Dr. Zea said.
There was one (3.2%) hemorrhagic conversion in the TPA group and two (1.5%) in the no-TPA group. Similarly, one (3.2%) TPA patient and two (1.5%) no-TPA patients had complications from access site bleeding. The differences were not statistically significant.
In the TPA group, it didn’t seem to matter if intervention came within 72 hours of administration – as in about half the cases – or afterward, when TPA risks have largely passed. There was one death and one ICH in patients in the earlier group, and one death in the later group, a nonsignificant difference.
There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
aotto@frontlinemedcom.com
CHICAGO – Urgent carotid interventions were safe after thrombolysis for acute mild to moderate strokes, according to a review of 165 patients at the Ochsner Clinic in New Orleans.
“Our data support the practice of not denying a patient an urgent carotid intervention simply because of TPA [tissue plasminogen activator] administration during the acute stroke period,” said lead investigator Dr. Nicolas Zea, an Ochsner vascular surgeon.
“Urgent carotid endarterectomy [CEA] or coronary artery stenting [CAS] can be safely undertaken in minor to moderate strokes with NIH stroke scale scores less than 10; TPA itself does not appear to be a contraindication, even within 72 hours,” he added.
Urgent carotid interventions are becoming more common after ischemic strokes to prevent recurrences. The approach is most effective within 2 weeks of the index event, but there have been concerns that intracranial hemorrhages (ICH) and other complications might be more likely if patients have had TPA.
Dr. Ochsner and his colleagues conducted their review because, “as vascular surgeons, we are going to encounter a lot more of these patients in the very near future,” Dr. Zea said a meeting hosted by the Society for Vascular Surgery.
From January 2009 to January 2015, 31 patients at Ochsner had carotid interventions – 25 CEA, 6 CAS – a mean of 2.1 days after receiving TPA for transient ischemic attacks (TIA) or ischemic strokes. The patients’ mean National Institutes of Health Stroke Scale (NIHSS) score was 6.6.
Over the same period, 134 patients who had not received TPA had urgent carotid interventions – 110 CEA, 24 CAS – a mean of 2.6 days after TIA or ischemic stroke presentation. Their mean NIHSS score was 6.1.
There were no statistically significant demographic or comorbidity differences between the TPA and no-TPA groups; patients were about 70 years old, on average, and the majority were men. Most had ipsilateral carotid stenosis greater than 70%, or acute occlusions.
The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the non-TPA group, a nonsignificant difference (P = 0.35).
In the TPA group, there was one (3.2%) ICH, one (3.2%) neck hematoma, and two (6.4%) deaths. In the no-TPA group, there were two (1.5%) ICHs, two (1.5%) neck hematomas, one (0.7%) ischemic stroke, two (1.5%) myocardial infarctions, and two (1.5%) deaths.
In both groups, ICH patients had stroke scores greater than 10. Also, although the rate of death was higher in the TPA group, the deaths “were not necessarily related to thrombolysis,” Dr. Zea noted. One death was from pulmonary embolism, the second from unknown causes. Deaths were due to acute mesenteric ischemia and ICH in the no-TPA group, Dr. Zea said.
There was one (3.2%) hemorrhagic conversion in the TPA group and two (1.5%) in the no-TPA group. Similarly, one (3.2%) TPA patient and two (1.5%) no-TPA patients had complications from access site bleeding. The differences were not statistically significant.
In the TPA group, it didn’t seem to matter if intervention came within 72 hours of administration – as in about half the cases – or afterward, when TPA risks have largely passed. There was one death and one ICH in patients in the earlier group, and one death in the later group, a nonsignificant difference.
There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
aotto@frontlinemedcom.com
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Tissue plasminogen activator does not contraindicate urgent carotid endarterectomies or stenting.
Major finding: The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the no-TPA group, a nonsignificant difference (P = 0.35).
Data source: A retrospective study of 165 patients.
Disclosures: There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
SVS: Five-year data support Endurant stent durability
CHICAGO – During 5 years of follow-up, there was one aneurysm-related death among 150 patients who received Endurant stent grafts for abdominal aortic aneurysms, according to outcome data from Endurant’s manufacturer, Medtronic.
The single death was in a man who developed a type I endoleak 2 years after implant. He declined further intervention, presented at about 3 years with a ruptured aneurysm, and died shortly thereafter. If that patient had been treated, it’s possible that Endurant’s freedom from aneurysm-related death would have been 100% at 5 years, instead of 99%.
Endurant “has proven to be safe and effective through 5 years of follow-up. Long-term outcomes indicate that it’s a durable repair with very low aneurysm-related mortality and a limited need for secondary interventions,” said Dr. Michael Singh, associate professor of surgery at the University of Pittsburgh, who presented the findings at a meeting hosted by the Society for Vascular Surgery.
The presentation completed Medtronic’s U.S. regulatory trial. The Food and Drug Administration approved the device in 2010 based on 30-day safety and 12-month efficacy data. Endurant is currently used in about half of endovascular triple A repairs worldwide, Medtronic said in a press release announcing the 5 year results.
The 150 subjects in the trial had aneurysms of at least 50 mm, with neck lengths of at least 10 mm, neck angulations no more than 60 degrees, and iliac fixation lengths of at least 15 mm. They were 73 years old, on average, and most were men. Endurant deployment was successful in all but one.
Twenty-five patients (17.7%) died during follow-up, all but the type I endoleak patient from causes expected in a triple A cohort, including stroke and lung disease. Six additional patients were lost to follow-up, and 18 withdrew for a variety of reasons, including physician advice.
At 5 years, aneurysm sacks had shrunk in about two-thirds of patients, and remained stable in most of the rest. Endurant didn’t fracture, migrate, or disconnect during follow-up.
Fifteen patients required 18 secondary endovascular interventions, including two for type I endoleaks, 10 for type II endoleaks, three for limb occlusion before 2 months, one for limb occlusion after 2 months, one for limb stenosis, and one for thromboembolism.
When asked to explain the robust outcomes, Dr. Singh noted that “we are dealing with trial patients ... with very selective inclusion and exclusion criteria” and favorable anatomy, “so you expect optimal results. In real-world experience, we might see something slightly” less optimal.
The type I endoleak patient who declined treatment might have had “the device placed in a degenerated aorta. We have come across this in our own practice regardless of the device that is utilized. I think the foundation” of endograft placement “is making sure that you are deploying the device in normal aorta,” at least as much as possible, he said.
The trial was funded by Medtronic. Dr. Singh said he has no relevant disclosures. The principal investigator is a Medtronic consultant.
What’s refreshing with this particular trial is that they showed us what happens in 5 years. There’s no question that EVAR has lower mortality than open repair immediately postop, but there have been arguments whether long-term survival is as good. The answer is ‘yes.’ The thing that impressed me the most was the freedom from aneurysm-related mortality at 99%. That’s phenomenal. It means that this device is very solid and very durable. At 5 years, they had a few type II endoleaks. I don’t think that’s too high compared to other trials. We are getting into the era when [these grafts] are very comparable. Selection comes down to anatomy, what physicians are comfortable with, and, to be honest, representative availability.
Dr. Mahmoud Malas is chief of endovascular surgery at Johns Hopkins Bayview Medical Center in Baltimore. He said he has no financial or other relationships with Medtronic.
What’s refreshing with this particular trial is that they showed us what happens in 5 years. There’s no question that EVAR has lower mortality than open repair immediately postop, but there have been arguments whether long-term survival is as good. The answer is ‘yes.’ The thing that impressed me the most was the freedom from aneurysm-related mortality at 99%. That’s phenomenal. It means that this device is very solid and very durable. At 5 years, they had a few type II endoleaks. I don’t think that’s too high compared to other trials. We are getting into the era when [these grafts] are very comparable. Selection comes down to anatomy, what physicians are comfortable with, and, to be honest, representative availability.
Dr. Mahmoud Malas is chief of endovascular surgery at Johns Hopkins Bayview Medical Center in Baltimore. He said he has no financial or other relationships with Medtronic.
What’s refreshing with this particular trial is that they showed us what happens in 5 years. There’s no question that EVAR has lower mortality than open repair immediately postop, but there have been arguments whether long-term survival is as good. The answer is ‘yes.’ The thing that impressed me the most was the freedom from aneurysm-related mortality at 99%. That’s phenomenal. It means that this device is very solid and very durable. At 5 years, they had a few type II endoleaks. I don’t think that’s too high compared to other trials. We are getting into the era when [these grafts] are very comparable. Selection comes down to anatomy, what physicians are comfortable with, and, to be honest, representative availability.
Dr. Mahmoud Malas is chief of endovascular surgery at Johns Hopkins Bayview Medical Center in Baltimore. He said he has no financial or other relationships with Medtronic.
CHICAGO – During 5 years of follow-up, there was one aneurysm-related death among 150 patients who received Endurant stent grafts for abdominal aortic aneurysms, according to outcome data from Endurant’s manufacturer, Medtronic.
The single death was in a man who developed a type I endoleak 2 years after implant. He declined further intervention, presented at about 3 years with a ruptured aneurysm, and died shortly thereafter. If that patient had been treated, it’s possible that Endurant’s freedom from aneurysm-related death would have been 100% at 5 years, instead of 99%.
Endurant “has proven to be safe and effective through 5 years of follow-up. Long-term outcomes indicate that it’s a durable repair with very low aneurysm-related mortality and a limited need for secondary interventions,” said Dr. Michael Singh, associate professor of surgery at the University of Pittsburgh, who presented the findings at a meeting hosted by the Society for Vascular Surgery.
The presentation completed Medtronic’s U.S. regulatory trial. The Food and Drug Administration approved the device in 2010 based on 30-day safety and 12-month efficacy data. Endurant is currently used in about half of endovascular triple A repairs worldwide, Medtronic said in a press release announcing the 5 year results.
The 150 subjects in the trial had aneurysms of at least 50 mm, with neck lengths of at least 10 mm, neck angulations no more than 60 degrees, and iliac fixation lengths of at least 15 mm. They were 73 years old, on average, and most were men. Endurant deployment was successful in all but one.
Twenty-five patients (17.7%) died during follow-up, all but the type I endoleak patient from causes expected in a triple A cohort, including stroke and lung disease. Six additional patients were lost to follow-up, and 18 withdrew for a variety of reasons, including physician advice.
At 5 years, aneurysm sacks had shrunk in about two-thirds of patients, and remained stable in most of the rest. Endurant didn’t fracture, migrate, or disconnect during follow-up.
Fifteen patients required 18 secondary endovascular interventions, including two for type I endoleaks, 10 for type II endoleaks, three for limb occlusion before 2 months, one for limb occlusion after 2 months, one for limb stenosis, and one for thromboembolism.
When asked to explain the robust outcomes, Dr. Singh noted that “we are dealing with trial patients ... with very selective inclusion and exclusion criteria” and favorable anatomy, “so you expect optimal results. In real-world experience, we might see something slightly” less optimal.
The type I endoleak patient who declined treatment might have had “the device placed in a degenerated aorta. We have come across this in our own practice regardless of the device that is utilized. I think the foundation” of endograft placement “is making sure that you are deploying the device in normal aorta,” at least as much as possible, he said.
The trial was funded by Medtronic. Dr. Singh said he has no relevant disclosures. The principal investigator is a Medtronic consultant.
CHICAGO – During 5 years of follow-up, there was one aneurysm-related death among 150 patients who received Endurant stent grafts for abdominal aortic aneurysms, according to outcome data from Endurant’s manufacturer, Medtronic.
The single death was in a man who developed a type I endoleak 2 years after implant. He declined further intervention, presented at about 3 years with a ruptured aneurysm, and died shortly thereafter. If that patient had been treated, it’s possible that Endurant’s freedom from aneurysm-related death would have been 100% at 5 years, instead of 99%.
Endurant “has proven to be safe and effective through 5 years of follow-up. Long-term outcomes indicate that it’s a durable repair with very low aneurysm-related mortality and a limited need for secondary interventions,” said Dr. Michael Singh, associate professor of surgery at the University of Pittsburgh, who presented the findings at a meeting hosted by the Society for Vascular Surgery.
The presentation completed Medtronic’s U.S. regulatory trial. The Food and Drug Administration approved the device in 2010 based on 30-day safety and 12-month efficacy data. Endurant is currently used in about half of endovascular triple A repairs worldwide, Medtronic said in a press release announcing the 5 year results.
The 150 subjects in the trial had aneurysms of at least 50 mm, with neck lengths of at least 10 mm, neck angulations no more than 60 degrees, and iliac fixation lengths of at least 15 mm. They were 73 years old, on average, and most were men. Endurant deployment was successful in all but one.
Twenty-five patients (17.7%) died during follow-up, all but the type I endoleak patient from causes expected in a triple A cohort, including stroke and lung disease. Six additional patients were lost to follow-up, and 18 withdrew for a variety of reasons, including physician advice.
At 5 years, aneurysm sacks had shrunk in about two-thirds of patients, and remained stable in most of the rest. Endurant didn’t fracture, migrate, or disconnect during follow-up.
Fifteen patients required 18 secondary endovascular interventions, including two for type I endoleaks, 10 for type II endoleaks, three for limb occlusion before 2 months, one for limb occlusion after 2 months, one for limb stenosis, and one for thromboembolism.
When asked to explain the robust outcomes, Dr. Singh noted that “we are dealing with trial patients ... with very selective inclusion and exclusion criteria” and favorable anatomy, “so you expect optimal results. In real-world experience, we might see something slightly” less optimal.
The type I endoleak patient who declined treatment might have had “the device placed in a degenerated aorta. We have come across this in our own practice regardless of the device that is utilized. I think the foundation” of endograft placement “is making sure that you are deploying the device in normal aorta,” at least as much as possible, he said.
The trial was funded by Medtronic. Dr. Singh said he has no relevant disclosures. The principal investigator is a Medtronic consultant.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: With careful selection and good follow-up, your triple A patients will do well with an Endurant stent.
Major finding: During 5 years of follow-up, there was one aneurysm-related death among 150 patients who received Endurant stent grafts.
Data source: Prospective, nonrandomized trial of 150 triple A patients at 26 U.S. medical centers.
Disclosures: The trial was funded by Medtronic. The presenter said he has no relevant disclosures. The principal investigator is a Medtronic consultant.
SVS: Four easy preop variables predict mortality in ruptured AAAs
CHICAGO – Age greater than 76 years, plus preoperative creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg collectively predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms, according to a new mortality risk score from Harborview Medical Center in Seattle.
Meeting all four criteria gives the maximum score of 4. Any one of the factors alone – a score of 1 – predicted 30% mortality with open repair and 9% with endovascular aneurysm repair (EVAR); a 2 predicted 80% mortality with open repair and 37% with EVAR; and a 3 predicted 82% mortality with open repair and 70% with EVAR.
Vascular surgeons at Harborview developed the system so they’d know whether to recommend transport or comfort care for ruptured abdominal aortic aneurysms (AAAs). The Level 1 trauma center serves more than a quarter of the U.S. landmass, and handles about 30-40 ruptured AAA’s annually. It’s not uncommon for patients to be flown in from Alaska.
Existing risk scores haven’t been validated for EVAR or rely on intraoperative variables, so they aren’t much help when counseling patients and referring physicians on what to do.
“Our ruptured AAA mortality risk score is based on four variables readily assessed preoperatively, allows accurate prediction of in-hospital mortality after repair of ruptured AAAs in the EVAR-first era, and does so better than any score thus published. It’s clinically relevant to the decision to transport and helps guide difficult discussions with patients and their families,” said investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, Seattle.
When using the new risk score. “we don’t ever block transfer, but we have discussions with referring providers and in several cases with patients and their families over the telephone.” When the situation is hopeless, “we explain the data.” Twice in the past 6 months, patients have opted to spend their last hours at home with their families, Dr. Garland said at the Society for Vascular Surgery’s annual meeting.
To develop their system, the investigators culled through 37,000 variables from 303 ruptured AAA patients treated at Harborview from 2002-2013. Fifteen patients died in the emergency department, en route to surgery, or after choosing comfort care. Overall, 30-day mortality was 54% for open repair and 22% for EVAR.
On multivariate analysis, the team isolated the four preoperative variables most predictive of death. Preoperative creatinine greater than 2 mg/dL almost quadrupled the risk (odds ratio 3.7; P < .001); systolic blood pressure less than 70 mm Hg nearly tripled it (OR 2.7; P = .002); and pH less than 7.2 (OR 2.6; P = .009) and age greater than 76 years (OR 2.1; P = .011) more than doubled it.
The investigators then checked their results against the Vascular Study Group of New England Cardiac Index, Glasgow Aneurysm Score, and Edinburgh Ruptured Aneurysm Score. “Our preoperative risk score was most predictive of death, with an area under the curve of 0.76,” Dr. Garland said.
There was no outside funding for the work and Dr. Garland has no disclosures.
CHICAGO – Age greater than 76 years, plus preoperative creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg collectively predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms, according to a new mortality risk score from Harborview Medical Center in Seattle.
Meeting all four criteria gives the maximum score of 4. Any one of the factors alone – a score of 1 – predicted 30% mortality with open repair and 9% with endovascular aneurysm repair (EVAR); a 2 predicted 80% mortality with open repair and 37% with EVAR; and a 3 predicted 82% mortality with open repair and 70% with EVAR.
Vascular surgeons at Harborview developed the system so they’d know whether to recommend transport or comfort care for ruptured abdominal aortic aneurysms (AAAs). The Level 1 trauma center serves more than a quarter of the U.S. landmass, and handles about 30-40 ruptured AAA’s annually. It’s not uncommon for patients to be flown in from Alaska.
Existing risk scores haven’t been validated for EVAR or rely on intraoperative variables, so they aren’t much help when counseling patients and referring physicians on what to do.
“Our ruptured AAA mortality risk score is based on four variables readily assessed preoperatively, allows accurate prediction of in-hospital mortality after repair of ruptured AAAs in the EVAR-first era, and does so better than any score thus published. It’s clinically relevant to the decision to transport and helps guide difficult discussions with patients and their families,” said investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, Seattle.
When using the new risk score. “we don’t ever block transfer, but we have discussions with referring providers and in several cases with patients and their families over the telephone.” When the situation is hopeless, “we explain the data.” Twice in the past 6 months, patients have opted to spend their last hours at home with their families, Dr. Garland said at the Society for Vascular Surgery’s annual meeting.
To develop their system, the investigators culled through 37,000 variables from 303 ruptured AAA patients treated at Harborview from 2002-2013. Fifteen patients died in the emergency department, en route to surgery, or after choosing comfort care. Overall, 30-day mortality was 54% for open repair and 22% for EVAR.
On multivariate analysis, the team isolated the four preoperative variables most predictive of death. Preoperative creatinine greater than 2 mg/dL almost quadrupled the risk (odds ratio 3.7; P < .001); systolic blood pressure less than 70 mm Hg nearly tripled it (OR 2.7; P = .002); and pH less than 7.2 (OR 2.6; P = .009) and age greater than 76 years (OR 2.1; P = .011) more than doubled it.
The investigators then checked their results against the Vascular Study Group of New England Cardiac Index, Glasgow Aneurysm Score, and Edinburgh Ruptured Aneurysm Score. “Our preoperative risk score was most predictive of death, with an area under the curve of 0.76,” Dr. Garland said.
There was no outside funding for the work and Dr. Garland has no disclosures.
CHICAGO – Age greater than 76 years, plus preoperative creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg collectively predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms, according to a new mortality risk score from Harborview Medical Center in Seattle.
Meeting all four criteria gives the maximum score of 4. Any one of the factors alone – a score of 1 – predicted 30% mortality with open repair and 9% with endovascular aneurysm repair (EVAR); a 2 predicted 80% mortality with open repair and 37% with EVAR; and a 3 predicted 82% mortality with open repair and 70% with EVAR.
Vascular surgeons at Harborview developed the system so they’d know whether to recommend transport or comfort care for ruptured abdominal aortic aneurysms (AAAs). The Level 1 trauma center serves more than a quarter of the U.S. landmass, and handles about 30-40 ruptured AAA’s annually. It’s not uncommon for patients to be flown in from Alaska.
Existing risk scores haven’t been validated for EVAR or rely on intraoperative variables, so they aren’t much help when counseling patients and referring physicians on what to do.
“Our ruptured AAA mortality risk score is based on four variables readily assessed preoperatively, allows accurate prediction of in-hospital mortality after repair of ruptured AAAs in the EVAR-first era, and does so better than any score thus published. It’s clinically relevant to the decision to transport and helps guide difficult discussions with patients and their families,” said investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, Seattle.
When using the new risk score. “we don’t ever block transfer, but we have discussions with referring providers and in several cases with patients and their families over the telephone.” When the situation is hopeless, “we explain the data.” Twice in the past 6 months, patients have opted to spend their last hours at home with their families, Dr. Garland said at the Society for Vascular Surgery’s annual meeting.
To develop their system, the investigators culled through 37,000 variables from 303 ruptured AAA patients treated at Harborview from 2002-2013. Fifteen patients died in the emergency department, en route to surgery, or after choosing comfort care. Overall, 30-day mortality was 54% for open repair and 22% for EVAR.
On multivariate analysis, the team isolated the four preoperative variables most predictive of death. Preoperative creatinine greater than 2 mg/dL almost quadrupled the risk (odds ratio 3.7; P < .001); systolic blood pressure less than 70 mm Hg nearly tripled it (OR 2.7; P = .002); and pH less than 7.2 (OR 2.6; P = .009) and age greater than 76 years (OR 2.1; P = .011) more than doubled it.
The investigators then checked their results against the Vascular Study Group of New England Cardiac Index, Glasgow Aneurysm Score, and Edinburgh Ruptured Aneurysm Score. “Our preoperative risk score was most predictive of death, with an area under the curve of 0.76,” Dr. Garland said.
There was no outside funding for the work and Dr. Garland has no disclosures.
AT SVS 2015
Key clinical point: You can rely on preoperative variables to recommend surgery or comfort care for ruptured AAAs.
Major finding: Age greater than 76 years, plus preop creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms.
Data source: More than 300 ruptured AAA patients treated at Harborview Medical Center in Seattle from 2002-2013
Disclosures: There was no outside funding for the work, and the presenting investigator has no relevant disclosures.
VIDEO: Sometimes, comfort care is best for ruptured AAAs
CHICAGO – Four preoperative variables predict whether or not patients will survive ruptured abdominal aortic aneurysm repairs, according to investigators from Harborview Medical Center in Seattle.
It’s an important finding because until now, it’s been hard to know how they’ll do. Previous risk scores also rely on intraoperative variables, or haven’t been validated for endovascular repair.
Investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, explained in a video interview what the four variables are at a meeting hosted by the Society for Vascular Surgery, and why it was so important for a level 1 trauma center like Harborview to identify them.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Four preoperative variables predict whether or not patients will survive ruptured abdominal aortic aneurysm repairs, according to investigators from Harborview Medical Center in Seattle.
It’s an important finding because until now, it’s been hard to know how they’ll do. Previous risk scores also rely on intraoperative variables, or haven’t been validated for endovascular repair.
Investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, explained in a video interview what the four variables are at a meeting hosted by the Society for Vascular Surgery, and why it was so important for a level 1 trauma center like Harborview to identify them.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Four preoperative variables predict whether or not patients will survive ruptured abdominal aortic aneurysm repairs, according to investigators from Harborview Medical Center in Seattle.
It’s an important finding because until now, it’s been hard to know how they’ll do. Previous risk scores also rely on intraoperative variables, or haven’t been validated for endovascular repair.
Investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, explained in a video interview what the four variables are at a meeting hosted by the Society for Vascular Surgery, and why it was so important for a level 1 trauma center like Harborview to identify them.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE 2015 VASCULAR ANNUAL MEETING
AATS: Metformin linked to better progression-free survival in early-stage NSCLC
SEATTLE – Metformin use before surgery for stage I and II non–small cell lung cancer (NSCLC) was associated with improved progression-free survival at 5 years in a retrospective database study of 138 patients who also had type 2 diabetes.
The patients were treated for stage I and II NSCLC at Rush University Medical Center, Chicago. They also had type 2 diabetes; 81 (59%) were on metformin in the 6 months before pulmonary resection, and 57 (41%) were not, reported Rush medical student Robert Medairos, who is one of the study researchers.
At 5 years follow-up, progression-free survival was 60% in the metformin group, but about 35% in the no-metformin group (P = .01). Overall survival was about 90% at 5 years in both study arms.
A larger study or longer follow-up may show overall survival benefits for metformin users, Mr. Medairos said at the annual meeting of the American Association for Thoracic Surgery.
Patients were about 70 years old on average in both groups, with 35 pack-year smoking histories, and an average body mass index of about 30 kg/m2. Both study arms had slightly more men than women, and were otherwise balanced for ethnicity and comorbidities.
About 12% of patients in the metformin group and 40% in the no-metformin group, were on insulin prior to surgery. Mean preoperative creatinine was 1 mg/dL in the metformin group, and 1.7 mg/dL in the no-metformin group. There were trends towards higher-stage disease and more lymph node involvement in the no-metformin group. About half the patients in both arms had adenocarcinomas, but a greater proportion of patients in the no-metformin group had squamous cell carcinomas.
The Rush study might be the first to look into metformin for early NSCLC, but there have been several studies in advanced disease. A recent retrospective analysis of 750 diabetes patients with stage IV NSCLC found a median survival of 5 months for the 61% on metformin at diagnosis, but 3 months for those who were not (Am. J. Respir. Crit. Care. Med. 2015;191:448-54).
Currently, there are about a dozen ongoing trials of the drug for lung cancer, and scores more for prostate, breast, brain, uterine, colorectal, thyroid, and other cancers.
Metformin’s metabolic effects might reduce the ability of cancers to grow and metabolize, or the drug might somehow boost the antineoplastic effects of chemotherapeutics. “Our next step is to look at histological and tissues samples to see if metformin changes gene transcription in lung cancer cells,” Mr. Medairos said.
Mr. Medairos has no disclosures.
SEATTLE – Metformin use before surgery for stage I and II non–small cell lung cancer (NSCLC) was associated with improved progression-free survival at 5 years in a retrospective database study of 138 patients who also had type 2 diabetes.
The patients were treated for stage I and II NSCLC at Rush University Medical Center, Chicago. They also had type 2 diabetes; 81 (59%) were on metformin in the 6 months before pulmonary resection, and 57 (41%) were not, reported Rush medical student Robert Medairos, who is one of the study researchers.
At 5 years follow-up, progression-free survival was 60% in the metformin group, but about 35% in the no-metformin group (P = .01). Overall survival was about 90% at 5 years in both study arms.
A larger study or longer follow-up may show overall survival benefits for metformin users, Mr. Medairos said at the annual meeting of the American Association for Thoracic Surgery.
Patients were about 70 years old on average in both groups, with 35 pack-year smoking histories, and an average body mass index of about 30 kg/m2. Both study arms had slightly more men than women, and were otherwise balanced for ethnicity and comorbidities.
About 12% of patients in the metformin group and 40% in the no-metformin group, were on insulin prior to surgery. Mean preoperative creatinine was 1 mg/dL in the metformin group, and 1.7 mg/dL in the no-metformin group. There were trends towards higher-stage disease and more lymph node involvement in the no-metformin group. About half the patients in both arms had adenocarcinomas, but a greater proportion of patients in the no-metformin group had squamous cell carcinomas.
The Rush study might be the first to look into metformin for early NSCLC, but there have been several studies in advanced disease. A recent retrospective analysis of 750 diabetes patients with stage IV NSCLC found a median survival of 5 months for the 61% on metformin at diagnosis, but 3 months for those who were not (Am. J. Respir. Crit. Care. Med. 2015;191:448-54).
Currently, there are about a dozen ongoing trials of the drug for lung cancer, and scores more for prostate, breast, brain, uterine, colorectal, thyroid, and other cancers.
Metformin’s metabolic effects might reduce the ability of cancers to grow and metabolize, or the drug might somehow boost the antineoplastic effects of chemotherapeutics. “Our next step is to look at histological and tissues samples to see if metformin changes gene transcription in lung cancer cells,” Mr. Medairos said.
Mr. Medairos has no disclosures.
SEATTLE – Metformin use before surgery for stage I and II non–small cell lung cancer (NSCLC) was associated with improved progression-free survival at 5 years in a retrospective database study of 138 patients who also had type 2 diabetes.
The patients were treated for stage I and II NSCLC at Rush University Medical Center, Chicago. They also had type 2 diabetes; 81 (59%) were on metformin in the 6 months before pulmonary resection, and 57 (41%) were not, reported Rush medical student Robert Medairos, who is one of the study researchers.
At 5 years follow-up, progression-free survival was 60% in the metformin group, but about 35% in the no-metformin group (P = .01). Overall survival was about 90% at 5 years in both study arms.
A larger study or longer follow-up may show overall survival benefits for metformin users, Mr. Medairos said at the annual meeting of the American Association for Thoracic Surgery.
Patients were about 70 years old on average in both groups, with 35 pack-year smoking histories, and an average body mass index of about 30 kg/m2. Both study arms had slightly more men than women, and were otherwise balanced for ethnicity and comorbidities.
About 12% of patients in the metformin group and 40% in the no-metformin group, were on insulin prior to surgery. Mean preoperative creatinine was 1 mg/dL in the metformin group, and 1.7 mg/dL in the no-metformin group. There were trends towards higher-stage disease and more lymph node involvement in the no-metformin group. About half the patients in both arms had adenocarcinomas, but a greater proportion of patients in the no-metformin group had squamous cell carcinomas.
The Rush study might be the first to look into metformin for early NSCLC, but there have been several studies in advanced disease. A recent retrospective analysis of 750 diabetes patients with stage IV NSCLC found a median survival of 5 months for the 61% on metformin at diagnosis, but 3 months for those who were not (Am. J. Respir. Crit. Care. Med. 2015;191:448-54).
Currently, there are about a dozen ongoing trials of the drug for lung cancer, and scores more for prostate, breast, brain, uterine, colorectal, thyroid, and other cancers.
Metformin’s metabolic effects might reduce the ability of cancers to grow and metabolize, or the drug might somehow boost the antineoplastic effects of chemotherapeutics. “Our next step is to look at histological and tissues samples to see if metformin changes gene transcription in lung cancer cells,” Mr. Medairos said.
Mr. Medairos has no disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Metformin might one day be part of routine lung cancer care.
Major finding: Five years after pulmonary resection for non–small cell lung cancer, progression-free survival was 60% in patients who were on metformin before surgery, but 35% in patients who were not (P = .01).
Data source: Retrospective study of 138 patients with type 2 diabetes and stage I and II non–small cell lung cancer.
Disclosures: The lead investigator has no relevant disclosures.
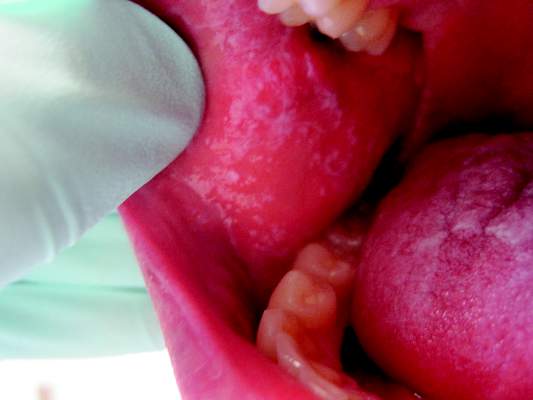
WCD: Red gums raise red flag in oral lichen planus
VANCOUVER – Gum involvement in oral lichen planus raises the risk of multisite or systemic disease, according to Dr. Roy Rogers, professor of dermatology at the Mayo Clinic in Rochester, Minn.
“When you see gingival involvement, this should stimulate you to look very carefully” for disease elsewhere, he said at the World Congress of Dermatology.
About 5% of oral lichen planus patients have involvement in three or more sites, and about 16% have cutaneous lesions, according to Dr. Rogers. Skin, eyes, ears, and nails are all potential targets, as is the esophagus. In fact, a complete oral exam may reveal erosive esophagitis to be an extension of oral lichen planus, which in some patients is painless and overlooked, he said.
Genital lesions are one of the most frequent extraoral manifestations of disease, and are easy to misdiagnose, especially in women. Dr. Rogers recalled a case of a woman who had several vulvovaginal surgeries before her lesions were recognized as lichen planus, and surgical trauma probably made her lesions worse. “A surgical approach is inappropriate until the disease is brought under control,” he said.
Lichen planus and its cutaneous manifestations are associated with hepatitis C, especially the Middle East, Southeast Asia, and the Mediterranean. Screening patients from those areas – as well as those with other risk factors – is appropriate.
Potentially 1% of lesions turn cancerous. Although the risk is low, “this requires regular follow-up either by you, or the patient’s dentist, or both” to monitor lesions, Dr. Rogers emphasized. “I encourage patients to see their dentist twice yearly and me at least yearly, if not more frequently, to renew their prescriptions and make sure everything is going well and not evolving into something else. If something’s not healing, a biopsy is indicated,” he said.
Good dental hygiene and care is a must for patients with oral lichen planus, because any inflammation in the mouth can exacerbate the condition. “Gingivitis, and certainly periodontal disease, should be controlled,” he added.
Fluorinated topical corticosteroids remain the standard of care, applied several times a day to lesions in the mouth or elsewhere to induce a remission, and then less often to maintain remission. Once the condition is under control, some patients need topical steroids only a few times a week.
When used in the mouth, topical corticosteroids can trigger secondary oral candidiasis, which patients might mistake for a flare. If their disease is under control, it almost always turns out to be thrush, said Dr. Rogers.
As an alternative to topical steroids, “I am an enthusiastic user of topical calcineurin inhibitors in oral lichen planus,” such as tacrolimus ointment, he said.
When an audience member asked about the risk of cancer from topical calcineurin inhibitors, Dr. Rogers responded, “it’s very, very low” because little is absorbed systemically. “It’s much more important to control the disease than allow it to continue” because the disease itself can lead to cancer, and treatment reduces the risk, he said.
Calcineurin inhibitors may, however, bump a precancerous lesion into a cancerous one, which is another reason for regular follow-up visits, and systemic treatment is called for in cases extensive disease, he added.
Dr. Rogers had no relevant disclosures.
VANCOUVER – Gum involvement in oral lichen planus raises the risk of multisite or systemic disease, according to Dr. Roy Rogers, professor of dermatology at the Mayo Clinic in Rochester, Minn.
“When you see gingival involvement, this should stimulate you to look very carefully” for disease elsewhere, he said at the World Congress of Dermatology.
About 5% of oral lichen planus patients have involvement in three or more sites, and about 16% have cutaneous lesions, according to Dr. Rogers. Skin, eyes, ears, and nails are all potential targets, as is the esophagus. In fact, a complete oral exam may reveal erosive esophagitis to be an extension of oral lichen planus, which in some patients is painless and overlooked, he said.
Genital lesions are one of the most frequent extraoral manifestations of disease, and are easy to misdiagnose, especially in women. Dr. Rogers recalled a case of a woman who had several vulvovaginal surgeries before her lesions were recognized as lichen planus, and surgical trauma probably made her lesions worse. “A surgical approach is inappropriate until the disease is brought under control,” he said.
Lichen planus and its cutaneous manifestations are associated with hepatitis C, especially the Middle East, Southeast Asia, and the Mediterranean. Screening patients from those areas – as well as those with other risk factors – is appropriate.
Potentially 1% of lesions turn cancerous. Although the risk is low, “this requires regular follow-up either by you, or the patient’s dentist, or both” to monitor lesions, Dr. Rogers emphasized. “I encourage patients to see their dentist twice yearly and me at least yearly, if not more frequently, to renew their prescriptions and make sure everything is going well and not evolving into something else. If something’s not healing, a biopsy is indicated,” he said.
Good dental hygiene and care is a must for patients with oral lichen planus, because any inflammation in the mouth can exacerbate the condition. “Gingivitis, and certainly periodontal disease, should be controlled,” he added.
Fluorinated topical corticosteroids remain the standard of care, applied several times a day to lesions in the mouth or elsewhere to induce a remission, and then less often to maintain remission. Once the condition is under control, some patients need topical steroids only a few times a week.
When used in the mouth, topical corticosteroids can trigger secondary oral candidiasis, which patients might mistake for a flare. If their disease is under control, it almost always turns out to be thrush, said Dr. Rogers.
As an alternative to topical steroids, “I am an enthusiastic user of topical calcineurin inhibitors in oral lichen planus,” such as tacrolimus ointment, he said.
When an audience member asked about the risk of cancer from topical calcineurin inhibitors, Dr. Rogers responded, “it’s very, very low” because little is absorbed systemically. “It’s much more important to control the disease than allow it to continue” because the disease itself can lead to cancer, and treatment reduces the risk, he said.
Calcineurin inhibitors may, however, bump a precancerous lesion into a cancerous one, which is another reason for regular follow-up visits, and systemic treatment is called for in cases extensive disease, he added.
Dr. Rogers had no relevant disclosures.
VANCOUVER – Gum involvement in oral lichen planus raises the risk of multisite or systemic disease, according to Dr. Roy Rogers, professor of dermatology at the Mayo Clinic in Rochester, Minn.
“When you see gingival involvement, this should stimulate you to look very carefully” for disease elsewhere, he said at the World Congress of Dermatology.
About 5% of oral lichen planus patients have involvement in three or more sites, and about 16% have cutaneous lesions, according to Dr. Rogers. Skin, eyes, ears, and nails are all potential targets, as is the esophagus. In fact, a complete oral exam may reveal erosive esophagitis to be an extension of oral lichen planus, which in some patients is painless and overlooked, he said.
Genital lesions are one of the most frequent extraoral manifestations of disease, and are easy to misdiagnose, especially in women. Dr. Rogers recalled a case of a woman who had several vulvovaginal surgeries before her lesions were recognized as lichen planus, and surgical trauma probably made her lesions worse. “A surgical approach is inappropriate until the disease is brought under control,” he said.
Lichen planus and its cutaneous manifestations are associated with hepatitis C, especially the Middle East, Southeast Asia, and the Mediterranean. Screening patients from those areas – as well as those with other risk factors – is appropriate.
Potentially 1% of lesions turn cancerous. Although the risk is low, “this requires regular follow-up either by you, or the patient’s dentist, or both” to monitor lesions, Dr. Rogers emphasized. “I encourage patients to see their dentist twice yearly and me at least yearly, if not more frequently, to renew their prescriptions and make sure everything is going well and not evolving into something else. If something’s not healing, a biopsy is indicated,” he said.
Good dental hygiene and care is a must for patients with oral lichen planus, because any inflammation in the mouth can exacerbate the condition. “Gingivitis, and certainly periodontal disease, should be controlled,” he added.
Fluorinated topical corticosteroids remain the standard of care, applied several times a day to lesions in the mouth or elsewhere to induce a remission, and then less often to maintain remission. Once the condition is under control, some patients need topical steroids only a few times a week.
When used in the mouth, topical corticosteroids can trigger secondary oral candidiasis, which patients might mistake for a flare. If their disease is under control, it almost always turns out to be thrush, said Dr. Rogers.
As an alternative to topical steroids, “I am an enthusiastic user of topical calcineurin inhibitors in oral lichen planus,” such as tacrolimus ointment, he said.
When an audience member asked about the risk of cancer from topical calcineurin inhibitors, Dr. Rogers responded, “it’s very, very low” because little is absorbed systemically. “It’s much more important to control the disease than allow it to continue” because the disease itself can lead to cancer, and treatment reduces the risk, he said.
Calcineurin inhibitors may, however, bump a precancerous lesion into a cancerous one, which is another reason for regular follow-up visits, and systemic treatment is called for in cases extensive disease, he added.
Dr. Rogers had no relevant disclosures.
AT WCD 2015
WCD: Topical squaric Acid May Help Alopecia Areata in Kids
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
AT WCD 2015
WCD: Topical squaric acid may help alopecia areata in kids
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
AT WCD 2015
Key clinical point: Squaric acid may work when other treatments fail in kids with alopecia areata.
Major finding: Fifty percent of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome with squaric acid.
Data source: A study of 10 children with alopecia aged 3-12 years.
Disclosures: There was no outside funding for the work, and Dr. Vatanchi reported having no relevant financial disclosures.