User login
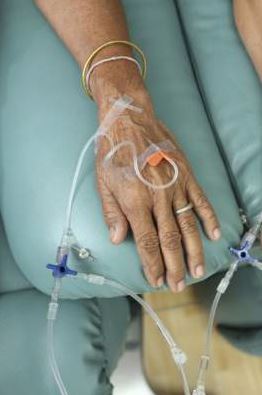
Low-volume surgeons have most complications with mesh slings
The 10-year incidence of serious complications after mesh-sling surgery for stress urinary incontinence is a relatively low 3.29, but patients treated by surgeons who perform a low volume of the procedures have a 37% higher relative risk of requiring further surgery for complications, compared with patients of experienced surgeons, according to a report published online Sept. 9 in JAMA Surgery.
“These findings support the regulatory statements that suggest that patients should be counseled regarding serious complications that can occur with mesh-based procedures for SUI and that surgeons should achieve expertise in their chosen procedure,” wrote Dr. Blayne Welk of the department of surgery and the department of epidemiology and biostatistics at Western University and St. Joseph’s Health Care, London (Ont.) and his associates.
The investigators performed a population-based retrospective cohort study to determine the incidence of surgical removal or revision after a mesh-sling procedure for SUI and to examine whether there are specific risk factors for mesh-related complications. They analyzed data for 59,887 women who underwent the procedure across Ontario during a 10-year period. Median follow-up was 4.4 years.
The procedures were performed by 1,068 surgeons: 293 urologists, 625 gynecologists, and 150 unspecified clinicians. Cases were classified according to whether the surgeon performed a high or low volume of mesh-sling procedures specifically for SUI. High volume was defined as a number at or above the 75th percentile for yearly volume in the province, or more than 16 procedures per year.
Overall, 1,307 women (2.2%) required mesh removal or revision a median of 1 year after the initial surgery. The cumulative incidence of the composite outcome of removal/revision of vaginal or urethral mesh, removal of a foreign body, endoscopic treatment of a urethral foreign body or mesh encrustation, uretrolysis, or repair of a urethrovaginal fistula was 3.29 at 10 years, Dr. Welk and his associates reported. Surgical specialty had no significant effect on complication risk.
This incidence is consistent with previous report from HMOs in the United States and a meta-analysis of clinical trial results, the investigators noted (JAMA Surg. 2015 Sept. 9. doi: 10.1001/jamasurg.2015.2590). Patients of low-volume surgeons had a 37% higher relative risk of complications requiring reoperation than did patients of high-volume surgeons. In a further analysis of the data, patients of low-volume surgeons were significantly more likely to experience the composite outcome than were patients of high-volume surgeons (hazard ratio, 1.37), and, again, the difference between surgical specialties was nonsignificant.
Urologists and gynecologists have very different surgical training and day-to-day practices, the researchers noted, and as a result they hypothesized that complication rates might differ between the two groups of clinicians. But that was not proven in the findings.
“This suggests that procedure-specific knowledge and experience is important for surgery to treat SUI, rather than general operative training,” Dr. Welk and his associates wrote.
Women who underwent multiple mesh-based procedures for SUI were at highest risk for the composite endpoint. Their absolute risk for later mesh removal or revision was 4.87%.
“This novel finding should temper the enthusiasm of case series that suggest that the use of multiple synthetic slings is safe and efficacious,” the researchers wrote.
This finding is also important in light of the fact that 1,307 of the study participants underwent more than one mesh implant procedure, presumably for recurrent SUI.
This study was supported by the Ontario Ministry of Health and Long-term Care and the Academic Medical Organization of Southwestern Ontario. Dr. Welk reported receiving grant funding from Astellas Canada.
The call to centralize complex, high-risk surgical procedures at expert centers is well known, but what about commonly performed, same-day procedures with low risks of complications, such as mesh-sling surgery for stress urinary incontinence? Must patients be referred only to high-volume surgeons?
This likely would be impractical if not impossible for a procedure that is performed so frequently. A more reasonable approach would be for low-volume surgeons to use structured proctoring and/or coaching models to develop expertise and mandatory outcomes reporting to assure high-quality care. Even though clinicians will not welcome this approach, it probably will become required in the near future and tied to reimbursements. Ultimately, surgeons should be the drivers for change and should not wait for payers or regulators to impose punitive measures.
Dr. Christian P. Meyer and Dr. Quoc-Dien Trinh are with the Center for Surgery and Public Health and the division of urologic surgery at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported having no relevant financial disclosures. These remarks are adapted from an invited commentary (JAMA Surg. 2015 Sept. 9. doi: 10.1001/jamasurg.2015.2596) accompanying Dr. Welk’s report.
The call to centralize complex, high-risk surgical procedures at expert centers is well known, but what about commonly performed, same-day procedures with low risks of complications, such as mesh-sling surgery for stress urinary incontinence? Must patients be referred only to high-volume surgeons?
This likely would be impractical if not impossible for a procedure that is performed so frequently. A more reasonable approach would be for low-volume surgeons to use structured proctoring and/or coaching models to develop expertise and mandatory outcomes reporting to assure high-quality care. Even though clinicians will not welcome this approach, it probably will become required in the near future and tied to reimbursements. Ultimately, surgeons should be the drivers for change and should not wait for payers or regulators to impose punitive measures.
Dr. Christian P. Meyer and Dr. Quoc-Dien Trinh are with the Center for Surgery and Public Health and the division of urologic surgery at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported having no relevant financial disclosures. These remarks are adapted from an invited commentary (JAMA Surg. 2015 Sept. 9. doi: 10.1001/jamasurg.2015.2596) accompanying Dr. Welk’s report.
The call to centralize complex, high-risk surgical procedures at expert centers is well known, but what about commonly performed, same-day procedures with low risks of complications, such as mesh-sling surgery for stress urinary incontinence? Must patients be referred only to high-volume surgeons?
This likely would be impractical if not impossible for a procedure that is performed so frequently. A more reasonable approach would be for low-volume surgeons to use structured proctoring and/or coaching models to develop expertise and mandatory outcomes reporting to assure high-quality care. Even though clinicians will not welcome this approach, it probably will become required in the near future and tied to reimbursements. Ultimately, surgeons should be the drivers for change and should not wait for payers or regulators to impose punitive measures.
Dr. Christian P. Meyer and Dr. Quoc-Dien Trinh are with the Center for Surgery and Public Health and the division of urologic surgery at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported having no relevant financial disclosures. These remarks are adapted from an invited commentary (JAMA Surg. 2015 Sept. 9. doi: 10.1001/jamasurg.2015.2596) accompanying Dr. Welk’s report.
The 10-year incidence of serious complications after mesh-sling surgery for stress urinary incontinence is a relatively low 3.29, but patients treated by surgeons who perform a low volume of the procedures have a 37% higher relative risk of requiring further surgery for complications, compared with patients of experienced surgeons, according to a report published online Sept. 9 in JAMA Surgery.
“These findings support the regulatory statements that suggest that patients should be counseled regarding serious complications that can occur with mesh-based procedures for SUI and that surgeons should achieve expertise in their chosen procedure,” wrote Dr. Blayne Welk of the department of surgery and the department of epidemiology and biostatistics at Western University and St. Joseph’s Health Care, London (Ont.) and his associates.
The investigators performed a population-based retrospective cohort study to determine the incidence of surgical removal or revision after a mesh-sling procedure for SUI and to examine whether there are specific risk factors for mesh-related complications. They analyzed data for 59,887 women who underwent the procedure across Ontario during a 10-year period. Median follow-up was 4.4 years.
The procedures were performed by 1,068 surgeons: 293 urologists, 625 gynecologists, and 150 unspecified clinicians. Cases were classified according to whether the surgeon performed a high or low volume of mesh-sling procedures specifically for SUI. High volume was defined as a number at or above the 75th percentile for yearly volume in the province, or more than 16 procedures per year.
Overall, 1,307 women (2.2%) required mesh removal or revision a median of 1 year after the initial surgery. The cumulative incidence of the composite outcome of removal/revision of vaginal or urethral mesh, removal of a foreign body, endoscopic treatment of a urethral foreign body or mesh encrustation, uretrolysis, or repair of a urethrovaginal fistula was 3.29 at 10 years, Dr. Welk and his associates reported. Surgical specialty had no significant effect on complication risk.
This incidence is consistent with previous report from HMOs in the United States and a meta-analysis of clinical trial results, the investigators noted (JAMA Surg. 2015 Sept. 9. doi: 10.1001/jamasurg.2015.2590). Patients of low-volume surgeons had a 37% higher relative risk of complications requiring reoperation than did patients of high-volume surgeons. In a further analysis of the data, patients of low-volume surgeons were significantly more likely to experience the composite outcome than were patients of high-volume surgeons (hazard ratio, 1.37), and, again, the difference between surgical specialties was nonsignificant.
Urologists and gynecologists have very different surgical training and day-to-day practices, the researchers noted, and as a result they hypothesized that complication rates might differ between the two groups of clinicians. But that was not proven in the findings.
“This suggests that procedure-specific knowledge and experience is important for surgery to treat SUI, rather than general operative training,” Dr. Welk and his associates wrote.
Women who underwent multiple mesh-based procedures for SUI were at highest risk for the composite endpoint. Their absolute risk for later mesh removal or revision was 4.87%.
“This novel finding should temper the enthusiasm of case series that suggest that the use of multiple synthetic slings is safe and efficacious,” the researchers wrote.
This finding is also important in light of the fact that 1,307 of the study participants underwent more than one mesh implant procedure, presumably for recurrent SUI.
This study was supported by the Ontario Ministry of Health and Long-term Care and the Academic Medical Organization of Southwestern Ontario. Dr. Welk reported receiving grant funding from Astellas Canada.
The 10-year incidence of serious complications after mesh-sling surgery for stress urinary incontinence is a relatively low 3.29, but patients treated by surgeons who perform a low volume of the procedures have a 37% higher relative risk of requiring further surgery for complications, compared with patients of experienced surgeons, according to a report published online Sept. 9 in JAMA Surgery.
“These findings support the regulatory statements that suggest that patients should be counseled regarding serious complications that can occur with mesh-based procedures for SUI and that surgeons should achieve expertise in their chosen procedure,” wrote Dr. Blayne Welk of the department of surgery and the department of epidemiology and biostatistics at Western University and St. Joseph’s Health Care, London (Ont.) and his associates.
The investigators performed a population-based retrospective cohort study to determine the incidence of surgical removal or revision after a mesh-sling procedure for SUI and to examine whether there are specific risk factors for mesh-related complications. They analyzed data for 59,887 women who underwent the procedure across Ontario during a 10-year period. Median follow-up was 4.4 years.
The procedures were performed by 1,068 surgeons: 293 urologists, 625 gynecologists, and 150 unspecified clinicians. Cases were classified according to whether the surgeon performed a high or low volume of mesh-sling procedures specifically for SUI. High volume was defined as a number at or above the 75th percentile for yearly volume in the province, or more than 16 procedures per year.
Overall, 1,307 women (2.2%) required mesh removal or revision a median of 1 year after the initial surgery. The cumulative incidence of the composite outcome of removal/revision of vaginal or urethral mesh, removal of a foreign body, endoscopic treatment of a urethral foreign body or mesh encrustation, uretrolysis, or repair of a urethrovaginal fistula was 3.29 at 10 years, Dr. Welk and his associates reported. Surgical specialty had no significant effect on complication risk.
This incidence is consistent with previous report from HMOs in the United States and a meta-analysis of clinical trial results, the investigators noted (JAMA Surg. 2015 Sept. 9. doi: 10.1001/jamasurg.2015.2590). Patients of low-volume surgeons had a 37% higher relative risk of complications requiring reoperation than did patients of high-volume surgeons. In a further analysis of the data, patients of low-volume surgeons were significantly more likely to experience the composite outcome than were patients of high-volume surgeons (hazard ratio, 1.37), and, again, the difference between surgical specialties was nonsignificant.
Urologists and gynecologists have very different surgical training and day-to-day practices, the researchers noted, and as a result they hypothesized that complication rates might differ between the two groups of clinicians. But that was not proven in the findings.
“This suggests that procedure-specific knowledge and experience is important for surgery to treat SUI, rather than general operative training,” Dr. Welk and his associates wrote.
Women who underwent multiple mesh-based procedures for SUI were at highest risk for the composite endpoint. Their absolute risk for later mesh removal or revision was 4.87%.
“This novel finding should temper the enthusiasm of case series that suggest that the use of multiple synthetic slings is safe and efficacious,” the researchers wrote.
This finding is also important in light of the fact that 1,307 of the study participants underwent more than one mesh implant procedure, presumably for recurrent SUI.
This study was supported by the Ontario Ministry of Health and Long-term Care and the Academic Medical Organization of Southwestern Ontario. Dr. Welk reported receiving grant funding from Astellas Canada.
FROM JAMA SURGERY
Key clinical point: The complication rate after mesh sling surgery for SUI is relatively low and highly correlated with the surgeon’s experience with the procedure.
Major finding: Patients of low-volume surgeons had a 37% higher relative risk of complications requiring reoperation than did patients of high-volume surgeons.
Data source: A population-based retrospective cohort study of 59,887 women who had SUI mesh surgery across Ontario during a 10-year period.
Disclosures: This study was supported by the Ontario Ministry of Health and Long-term Care and the Academic Medical Organization of Southwestern Ontario. Dr. Welk reported receiving grant funding from Astellas Canada.
Postop delirium heightens risk of other dangerous complications
Delirium is not only the most common major complication of elective surgery in older adults, it also markedly raises the risk of all adverse outcomes, including prolonged length of stay, discharge to an institution rather than home, and readmission within 30 days, according to a report published online Sept. 9 in JAMA Surgery.
“Given its high prevalence and negative effect, delirium should be considered as the leading postoperative complication contributing to adverse outcomes” in this patient population, wrote Dr. Lauren J. Gleason of the department of medicine at Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and her associates.
Delirium is typically considered a less serious event than other major postoperative complications, even though its association with higher in-hospital mortality, 6-month mortality, functional decline, and higher health care costs has been well documented. To assess whether delirium should be considered equivalent to other major life-altering or life-threatening complications, the investigators studied 566 patients aged 70 years and older who underwent elective orthopedic, general, or vascular procedures at Beth Israel Deaconess or Brigham and Women’s Hospital in a 3-year period. The data were collected for the Successful Aging after Elective Surgery (SAGES) study.
Operations included total hip or knee replacement, lumbar or cervical laminectomy, lower-extremity bypass, open abdominal aortic aneurysm repair, open colectomyt, and laparoscopic colectomy. In general, these patients were highly functional and highly educated. The mean age was 76.7 years, and 93% of the study participants were white.
Delirium developed in 135 patients (24%) – much more frequently than all other major complications combined (47 patients, or 8%). This is comparable with rates of delirium reported in other studies of elective noncardiac surgeries, Dr. Gleason and her associates noted. Other major complications included unstable arrhythmias, respiratory failure, abscess requiring incision and drainage, abdominal compartment syndrome, anastomotic leak, deep surgical site infection, and hernia repair.
Compared with patients who didn’t develop delirium, those who did had a twofold higher risk of prolonged length of stay, a 50% higher risk of discharge to an institution, and more than double the risk of 30-day readmission, the investigators reported (JAMA Surg. 2015 Sept 9. doi: 10.1001/jamasurg.2015.2606).
Since delirium is often preventable, clinicians should try harder to implement preventive strategies before surgery and continue them afterward. The Hospital Elder Life Program, proactive geriatric consultation, and comanagement services all have proved effective in this regard, they added.
This study was supported by the National Institute on Aging, the Health and Resources Services Administration, and the John A. Hartford Foundation. The authors reported no relevant disclosures.
Delirium is not only the most common major complication of elective surgery in older adults, it also markedly raises the risk of all adverse outcomes, including prolonged length of stay, discharge to an institution rather than home, and readmission within 30 days, according to a report published online Sept. 9 in JAMA Surgery.
“Given its high prevalence and negative effect, delirium should be considered as the leading postoperative complication contributing to adverse outcomes” in this patient population, wrote Dr. Lauren J. Gleason of the department of medicine at Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and her associates.
Delirium is typically considered a less serious event than other major postoperative complications, even though its association with higher in-hospital mortality, 6-month mortality, functional decline, and higher health care costs has been well documented. To assess whether delirium should be considered equivalent to other major life-altering or life-threatening complications, the investigators studied 566 patients aged 70 years and older who underwent elective orthopedic, general, or vascular procedures at Beth Israel Deaconess or Brigham and Women’s Hospital in a 3-year period. The data were collected for the Successful Aging after Elective Surgery (SAGES) study.
Operations included total hip or knee replacement, lumbar or cervical laminectomy, lower-extremity bypass, open abdominal aortic aneurysm repair, open colectomyt, and laparoscopic colectomy. In general, these patients were highly functional and highly educated. The mean age was 76.7 years, and 93% of the study participants were white.
Delirium developed in 135 patients (24%) – much more frequently than all other major complications combined (47 patients, or 8%). This is comparable with rates of delirium reported in other studies of elective noncardiac surgeries, Dr. Gleason and her associates noted. Other major complications included unstable arrhythmias, respiratory failure, abscess requiring incision and drainage, abdominal compartment syndrome, anastomotic leak, deep surgical site infection, and hernia repair.
Compared with patients who didn’t develop delirium, those who did had a twofold higher risk of prolonged length of stay, a 50% higher risk of discharge to an institution, and more than double the risk of 30-day readmission, the investigators reported (JAMA Surg. 2015 Sept 9. doi: 10.1001/jamasurg.2015.2606).
Since delirium is often preventable, clinicians should try harder to implement preventive strategies before surgery and continue them afterward. The Hospital Elder Life Program, proactive geriatric consultation, and comanagement services all have proved effective in this regard, they added.
This study was supported by the National Institute on Aging, the Health and Resources Services Administration, and the John A. Hartford Foundation. The authors reported no relevant disclosures.
Delirium is not only the most common major complication of elective surgery in older adults, it also markedly raises the risk of all adverse outcomes, including prolonged length of stay, discharge to an institution rather than home, and readmission within 30 days, according to a report published online Sept. 9 in JAMA Surgery.
“Given its high prevalence and negative effect, delirium should be considered as the leading postoperative complication contributing to adverse outcomes” in this patient population, wrote Dr. Lauren J. Gleason of the department of medicine at Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and her associates.
Delirium is typically considered a less serious event than other major postoperative complications, even though its association with higher in-hospital mortality, 6-month mortality, functional decline, and higher health care costs has been well documented. To assess whether delirium should be considered equivalent to other major life-altering or life-threatening complications, the investigators studied 566 patients aged 70 years and older who underwent elective orthopedic, general, or vascular procedures at Beth Israel Deaconess or Brigham and Women’s Hospital in a 3-year period. The data were collected for the Successful Aging after Elective Surgery (SAGES) study.
Operations included total hip or knee replacement, lumbar or cervical laminectomy, lower-extremity bypass, open abdominal aortic aneurysm repair, open colectomyt, and laparoscopic colectomy. In general, these patients were highly functional and highly educated. The mean age was 76.7 years, and 93% of the study participants were white.
Delirium developed in 135 patients (24%) – much more frequently than all other major complications combined (47 patients, or 8%). This is comparable with rates of delirium reported in other studies of elective noncardiac surgeries, Dr. Gleason and her associates noted. Other major complications included unstable arrhythmias, respiratory failure, abscess requiring incision and drainage, abdominal compartment syndrome, anastomotic leak, deep surgical site infection, and hernia repair.
Compared with patients who didn’t develop delirium, those who did had a twofold higher risk of prolonged length of stay, a 50% higher risk of discharge to an institution, and more than double the risk of 30-day readmission, the investigators reported (JAMA Surg. 2015 Sept 9. doi: 10.1001/jamasurg.2015.2606).
Since delirium is often preventable, clinicians should try harder to implement preventive strategies before surgery and continue them afterward. The Hospital Elder Life Program, proactive geriatric consultation, and comanagement services all have proved effective in this regard, they added.
This study was supported by the National Institute on Aging, the Health and Resources Services Administration, and the John A. Hartford Foundation. The authors reported no relevant disclosures.
FROM JAMA SURGERY
Key clinical point: Delirium is the most common and among the most harmful of complications of elective surgery in older adults.
Major finding: Elderly patients who developed delirium had a twofold higher risk of prolonged length of stay, a 50% higher risk of discharge to an institution, and more than double the risk of 30-day readmission.
Data source: A prospective cohort study involving 566 older adults undergoing elective surgery at two hospitals in a 3-year period.
Disclosures: This study was supported by the National Institute on Aging, the Health and Resources Services Administration, and the John A. Hartford Foundation.
Preop chemo feasible for high-risk soft-tissue sarcomas
Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas of the limbs or superficial trunk, even with concurrent radiotherapy and even in selected elderly patients, investigators reported online Sept. 7 in Journal of Clinical Oncology.
Full-dose preoperative chemotherapy now can be offered and an excellent chemotherapeutic dose intensity now can be achieved with concomitant radiotherapy in cases “when surgical reasons suggest that major preoperative shrinkage may help,” said Dr. Elena Palassini of Istituto Nazionale dei Tumori, Bologna (Italy), and her associates in the Italian Sarcoma Group and Spanish Sarcoma Group.
The investigators already reported the efficacy results of their international phase III randomized clinical trial assessing preoperative chemotherapy (with or without radiotherapy, at the treating physician’s discretion). In the current analysis, they focused on the toxicity data for 303 of the trial participants, all of whom had high-grade, deep, large, adult-type soft-tissue sarcomas arising from the extremities or trunk wall.
The median patient age was 48 years (range, 15-79 years), and 13% of the study population was aged 65 years or older. A total of 152 received preoperative radiotherapy along with epirubicin plus ifosfamide.
“The most interesting clinical finding was the tolerability of the combination of preoperative chemotherapy and radiotherapy,” which is particularly remarkable because patients chosen for combination treatment had the largest tumors and the most challenging presentations, the investigators wrote.
Participants who received combination preoperative therapy showed “only limited worsening of toxicities” compared with those who had preoperative chemotherapy alone, Dr. Palassini and her associates said (J Clin Oncol. 2015 Sept. 7. doi: 10.1200/JCO.2015.62.9394).
Grade 3 or 4 thrombocytopenia was more frequent with combined therapy than with chemotherapy alone, but grade 4 leukopenia and grade 3 or 4 anemia were not. There were no cases of fatal toxicity, and the rate of wound complications was not significantly higher when radiotherapy was added to chemotherapy (17.1%) than when it was not (9.9%).
Even though some patients failed to complete all planned cycles of chemotherapy or experienced dose reductions or interruptions because of toxic effects, the overall median chemotherapeutic dose index remained “excellent” at greater than 90%. This was true even in patients aged 65 years and older, which “clearly suggests the possibility of selecting and treating at least a proportion of [older] patients in the adjuvant setting with full-dose regimens.” This finding is especially important because older patients comprise 30% of those newly diagnosed as having soft-tissue sarcoma, the investigators noted.
The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas of the limbs or superficial trunk, even with concurrent radiotherapy and even in selected elderly patients, investigators reported online Sept. 7 in Journal of Clinical Oncology.
Full-dose preoperative chemotherapy now can be offered and an excellent chemotherapeutic dose intensity now can be achieved with concomitant radiotherapy in cases “when surgical reasons suggest that major preoperative shrinkage may help,” said Dr. Elena Palassini of Istituto Nazionale dei Tumori, Bologna (Italy), and her associates in the Italian Sarcoma Group and Spanish Sarcoma Group.
The investigators already reported the efficacy results of their international phase III randomized clinical trial assessing preoperative chemotherapy (with or without radiotherapy, at the treating physician’s discretion). In the current analysis, they focused on the toxicity data for 303 of the trial participants, all of whom had high-grade, deep, large, adult-type soft-tissue sarcomas arising from the extremities or trunk wall.
The median patient age was 48 years (range, 15-79 years), and 13% of the study population was aged 65 years or older. A total of 152 received preoperative radiotherapy along with epirubicin plus ifosfamide.
“The most interesting clinical finding was the tolerability of the combination of preoperative chemotherapy and radiotherapy,” which is particularly remarkable because patients chosen for combination treatment had the largest tumors and the most challenging presentations, the investigators wrote.
Participants who received combination preoperative therapy showed “only limited worsening of toxicities” compared with those who had preoperative chemotherapy alone, Dr. Palassini and her associates said (J Clin Oncol. 2015 Sept. 7. doi: 10.1200/JCO.2015.62.9394).
Grade 3 or 4 thrombocytopenia was more frequent with combined therapy than with chemotherapy alone, but grade 4 leukopenia and grade 3 or 4 anemia were not. There were no cases of fatal toxicity, and the rate of wound complications was not significantly higher when radiotherapy was added to chemotherapy (17.1%) than when it was not (9.9%).
Even though some patients failed to complete all planned cycles of chemotherapy or experienced dose reductions or interruptions because of toxic effects, the overall median chemotherapeutic dose index remained “excellent” at greater than 90%. This was true even in patients aged 65 years and older, which “clearly suggests the possibility of selecting and treating at least a proportion of [older] patients in the adjuvant setting with full-dose regimens.” This finding is especially important because older patients comprise 30% of those newly diagnosed as having soft-tissue sarcoma, the investigators noted.
The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas of the limbs or superficial trunk, even with concurrent radiotherapy and even in selected elderly patients, investigators reported online Sept. 7 in Journal of Clinical Oncology.
Full-dose preoperative chemotherapy now can be offered and an excellent chemotherapeutic dose intensity now can be achieved with concomitant radiotherapy in cases “when surgical reasons suggest that major preoperative shrinkage may help,” said Dr. Elena Palassini of Istituto Nazionale dei Tumori, Bologna (Italy), and her associates in the Italian Sarcoma Group and Spanish Sarcoma Group.
The investigators already reported the efficacy results of their international phase III randomized clinical trial assessing preoperative chemotherapy (with or without radiotherapy, at the treating physician’s discretion). In the current analysis, they focused on the toxicity data for 303 of the trial participants, all of whom had high-grade, deep, large, adult-type soft-tissue sarcomas arising from the extremities or trunk wall.
The median patient age was 48 years (range, 15-79 years), and 13% of the study population was aged 65 years or older. A total of 152 received preoperative radiotherapy along with epirubicin plus ifosfamide.
“The most interesting clinical finding was the tolerability of the combination of preoperative chemotherapy and radiotherapy,” which is particularly remarkable because patients chosen for combination treatment had the largest tumors and the most challenging presentations, the investigators wrote.
Participants who received combination preoperative therapy showed “only limited worsening of toxicities” compared with those who had preoperative chemotherapy alone, Dr. Palassini and her associates said (J Clin Oncol. 2015 Sept. 7. doi: 10.1200/JCO.2015.62.9394).
Grade 3 or 4 thrombocytopenia was more frequent with combined therapy than with chemotherapy alone, but grade 4 leukopenia and grade 3 or 4 anemia were not. There were no cases of fatal toxicity, and the rate of wound complications was not significantly higher when radiotherapy was added to chemotherapy (17.1%) than when it was not (9.9%).
Even though some patients failed to complete all planned cycles of chemotherapy or experienced dose reductions or interruptions because of toxic effects, the overall median chemotherapeutic dose index remained “excellent” at greater than 90%. This was true even in patients aged 65 years and older, which “clearly suggests the possibility of selecting and treating at least a proportion of [older] patients in the adjuvant setting with full-dose regimens.” This finding is especially important because older patients comprise 30% of those newly diagnosed as having soft-tissue sarcoma, the investigators noted.
The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas, even with concurrent radiotherapy.
Major finding: The overall median chemotherapeutic dose index was “excellent” at greater than 90%.
Data source: An analysis of toxicity data from a 5-year international phase III randomized trial involving 303 patients.
Disclosures: The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
Reference payment plan cuts colonoscopy costs by 20%
When a large state employer – California’s public employee system – switched to “reference payment” health insurance coverage of colonoscopy, patients shifted toward using lower-priced facilities and the price of the procedure dropped by 20%, according to a report published online Sept. 8 in JAMA Internal Medicine.
This reference payment plan provided full coverage for colonoscopy up to the reference payment limit of $1,500, requiring patients to pay the difference between this limit and the price they were actually charged if they chose a higher-priced facility. The $1,500 limit was set at the 80th percentile of the range of prices charged by all ambulatory surgical centers in the region; most hospital-based colonoscopy facilities charged more, said James C. Robinson, Ph.D., of the University of California-Berkeley School of Public Health.
He and his coinvestigators analyzed insurance claims data, comparing the costs of 21,644 colonoscopies performed during the 3 years preceding this type of coverage with those of 13,551 colonoscopies performed during the 2 years after reference payment coverage was implemented. They compared these findings with those for a control group: 258,616 people covered by a standard health insurance plan that remained the same during the 5-year study period.
During the earlier period, 69% of state enrollees underwent colonoscopies at the less-expensive ambulatory centers, while the remaining 31% had them at more expensive hospital-based outpatient departments. After reference payment was implemented, the proportion of enrollees who chose the less expensive option rose to 86% the first year and to 90% the second year. In comparison, the control group’s use of ambulatory centers remained steady at 71%-74% throughout the study period.
After the reference payment plan was implemented, the mean price paid per colonoscopy decreased by 20.3% in the first year and 21.0% the second year among the state employees, while it rose in the control group. With reference payment, each colonoscopy cost $476 less the first year and $576 less the second year than with standard health insurance, Dr. Robinson and his associates said (JAMA Intern. Med. 2015 Sep 8. doi:10.1001/jamainternmed.2015.4588).
At the same time that prices dropped, there were no significant changes in complication rates, suggesting that the quality of care did not suffer. The incidence of serious gastrointestinal complications (including perforation, lower GI bleeding, and infection), other gastrointestinal complications (such as paralytic ileus, nausea, vomiting, dehydration, pain, diverticulitis, and enterocolitis), and cardiovascular complications (arrhythmia, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension, and shock) remained steady throughout the study period.
During the first 2 years after implementation of the reference payment system, the state public employee system saved approximately $7.0 million, or 28%, compared with what it would have spent on colonoscopy without that system, the investigators noted.
Robinson et al. interpreted the steady complication rate throughout their study to be an indicator that the quality of colonoscopies didn’t decline when patients switched to less-expensive providers. However, they were unable to assess other important measures of quality, such as the adenoma detection rate. Such assessments are needed to ensure that patients who choose lower-priced practices still receive high-quality care.
David Lieberman, M.D., is in the division of gastroenterology and hepatology at Oregon Health and Science University, Portland. John Allen, M.D., AGAF, is at Yale University, New Haven, Conn. Dr. Lieberman reported serving on scientific advisory boards for Given Imaging, Ironwood Pharmaceuticals, Exact Sciences, and Motus GI. Dr. Allen reported serving as a consultant for Olympus, gMed, and Pentax. Dr. Lieberman and Dr. Allen made these remarks in a commentary (JAMA Intern Med. 2015 Sep 8 [doi:101001/jamainternmed.2015.4594]).
Robinson et al. interpreted the steady complication rate throughout their study to be an indicator that the quality of colonoscopies didn’t decline when patients switched to less-expensive providers. However, they were unable to assess other important measures of quality, such as the adenoma detection rate. Such assessments are needed to ensure that patients who choose lower-priced practices still receive high-quality care.
David Lieberman, M.D., is in the division of gastroenterology and hepatology at Oregon Health and Science University, Portland. John Allen, M.D., AGAF, is at Yale University, New Haven, Conn. Dr. Lieberman reported serving on scientific advisory boards for Given Imaging, Ironwood Pharmaceuticals, Exact Sciences, and Motus GI. Dr. Allen reported serving as a consultant for Olympus, gMed, and Pentax. Dr. Lieberman and Dr. Allen made these remarks in a commentary (JAMA Intern Med. 2015 Sep 8 [doi:101001/jamainternmed.2015.4594]).
Robinson et al. interpreted the steady complication rate throughout their study to be an indicator that the quality of colonoscopies didn’t decline when patients switched to less-expensive providers. However, they were unable to assess other important measures of quality, such as the adenoma detection rate. Such assessments are needed to ensure that patients who choose lower-priced practices still receive high-quality care.
David Lieberman, M.D., is in the division of gastroenterology and hepatology at Oregon Health and Science University, Portland. John Allen, M.D., AGAF, is at Yale University, New Haven, Conn. Dr. Lieberman reported serving on scientific advisory boards for Given Imaging, Ironwood Pharmaceuticals, Exact Sciences, and Motus GI. Dr. Allen reported serving as a consultant for Olympus, gMed, and Pentax. Dr. Lieberman and Dr. Allen made these remarks in a commentary (JAMA Intern Med. 2015 Sep 8 [doi:101001/jamainternmed.2015.4594]).
When a large state employer – California’s public employee system – switched to “reference payment” health insurance coverage of colonoscopy, patients shifted toward using lower-priced facilities and the price of the procedure dropped by 20%, according to a report published online Sept. 8 in JAMA Internal Medicine.
This reference payment plan provided full coverage for colonoscopy up to the reference payment limit of $1,500, requiring patients to pay the difference between this limit and the price they were actually charged if they chose a higher-priced facility. The $1,500 limit was set at the 80th percentile of the range of prices charged by all ambulatory surgical centers in the region; most hospital-based colonoscopy facilities charged more, said James C. Robinson, Ph.D., of the University of California-Berkeley School of Public Health.
He and his coinvestigators analyzed insurance claims data, comparing the costs of 21,644 colonoscopies performed during the 3 years preceding this type of coverage with those of 13,551 colonoscopies performed during the 2 years after reference payment coverage was implemented. They compared these findings with those for a control group: 258,616 people covered by a standard health insurance plan that remained the same during the 5-year study period.
During the earlier period, 69% of state enrollees underwent colonoscopies at the less-expensive ambulatory centers, while the remaining 31% had them at more expensive hospital-based outpatient departments. After reference payment was implemented, the proportion of enrollees who chose the less expensive option rose to 86% the first year and to 90% the second year. In comparison, the control group’s use of ambulatory centers remained steady at 71%-74% throughout the study period.
After the reference payment plan was implemented, the mean price paid per colonoscopy decreased by 20.3% in the first year and 21.0% the second year among the state employees, while it rose in the control group. With reference payment, each colonoscopy cost $476 less the first year and $576 less the second year than with standard health insurance, Dr. Robinson and his associates said (JAMA Intern. Med. 2015 Sep 8. doi:10.1001/jamainternmed.2015.4588).
At the same time that prices dropped, there were no significant changes in complication rates, suggesting that the quality of care did not suffer. The incidence of serious gastrointestinal complications (including perforation, lower GI bleeding, and infection), other gastrointestinal complications (such as paralytic ileus, nausea, vomiting, dehydration, pain, diverticulitis, and enterocolitis), and cardiovascular complications (arrhythmia, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension, and shock) remained steady throughout the study period.
During the first 2 years after implementation of the reference payment system, the state public employee system saved approximately $7.0 million, or 28%, compared with what it would have spent on colonoscopy without that system, the investigators noted.
When a large state employer – California’s public employee system – switched to “reference payment” health insurance coverage of colonoscopy, patients shifted toward using lower-priced facilities and the price of the procedure dropped by 20%, according to a report published online Sept. 8 in JAMA Internal Medicine.
This reference payment plan provided full coverage for colonoscopy up to the reference payment limit of $1,500, requiring patients to pay the difference between this limit and the price they were actually charged if they chose a higher-priced facility. The $1,500 limit was set at the 80th percentile of the range of prices charged by all ambulatory surgical centers in the region; most hospital-based colonoscopy facilities charged more, said James C. Robinson, Ph.D., of the University of California-Berkeley School of Public Health.
He and his coinvestigators analyzed insurance claims data, comparing the costs of 21,644 colonoscopies performed during the 3 years preceding this type of coverage with those of 13,551 colonoscopies performed during the 2 years after reference payment coverage was implemented. They compared these findings with those for a control group: 258,616 people covered by a standard health insurance plan that remained the same during the 5-year study period.
During the earlier period, 69% of state enrollees underwent colonoscopies at the less-expensive ambulatory centers, while the remaining 31% had them at more expensive hospital-based outpatient departments. After reference payment was implemented, the proportion of enrollees who chose the less expensive option rose to 86% the first year and to 90% the second year. In comparison, the control group’s use of ambulatory centers remained steady at 71%-74% throughout the study period.
After the reference payment plan was implemented, the mean price paid per colonoscopy decreased by 20.3% in the first year and 21.0% the second year among the state employees, while it rose in the control group. With reference payment, each colonoscopy cost $476 less the first year and $576 less the second year than with standard health insurance, Dr. Robinson and his associates said (JAMA Intern. Med. 2015 Sep 8. doi:10.1001/jamainternmed.2015.4588).
At the same time that prices dropped, there were no significant changes in complication rates, suggesting that the quality of care did not suffer. The incidence of serious gastrointestinal complications (including perforation, lower GI bleeding, and infection), other gastrointestinal complications (such as paralytic ileus, nausea, vomiting, dehydration, pain, diverticulitis, and enterocolitis), and cardiovascular complications (arrhythmia, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension, and shock) remained steady throughout the study period.
During the first 2 years after implementation of the reference payment system, the state public employee system saved approximately $7.0 million, or 28%, compared with what it would have spent on colonoscopy without that system, the investigators noted.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A state employer’s switch to “reference payment” insurance coverage of colonoscopy cut the cost of the procedure by 20%.
Major finding: With the reference payment plan, each colonoscopy cost $476 less the first year and $576 less the second year than with standard health insurance.
Data source: An analysis of insurance claims data for colonoscopies performed before (21,644 procedures) and after (13,551 procedures) implementation of a reference payment system for California public employees.
Disclosures: The California Public Employees’ Retirement System and the U.S. Agency for Healthcare Research and Quality supported the study. Dr. Robinson and his associates reported having no relevant disclosures.
Imetelstat elicits response in myelofibrosis, thrombocythemia
The telomerase inhibitor imetelstat showed promise against advanced myelofibrosis and essential thrombocythemia in two industry-funded preliminary studies, according to separate reports published online Sept. 3 in the New England Journal of Medicine.
In previous in vitro and animal studies, imetelstat inhibited the proliferation of various types of malignant cells but was not active in normal somatic tissue. Researchers assessed the agent for advanced myelofibrosis in part because, at present, only one available treatment – allogeneic stem-cell transplantation (ASCT) – sometimes induces long-term remission. ASCT carries a relatively high rate of treatment-related death and complications, and is contraindicated in many older patients.
In the first report, researchers conducted a small, single-center cohort study to collect preliminary data on the agent’s efficacy and safety in 33 patients with primary myelofibrosis (18 participants), myelofibrosis that was related to polycythemia (10 participants), or myelofibrosis associated with essential thrombocytopenia (10 participants). Imetelstat was administered in 2-hour intravenous infusions given in 3-week cycles, said Dr. Ayalew Tefferi of the division of hematology, Mayo Clinic, Rochester Minn.
The median duration of treatment was 8.6 months (range, 1.4-21.7 months). Seven patients (21%) had either a complete or partial response; the 4 patients with a complete response had documented complete reversal of bone marrow fibrosis. The time to onset of response was 3.5 months (range, 1.4-7.2 months), and the median duration of response was 18 months (range, 13-20 months).
These remissions “confirm selective anticlonal activity, which has not previously been documented in drug treatment of myelofibrosis,” noted Dr. Tefferi and his associates (N Engl J Med 2015 Sep 3. doi:10.1056/NEJMoa1310523). Three of the seven patients who responded to imetelstat “had been heavily dependent on red-cell transfusions at study entry and became transfusion-independent and sustained a hemoglobin level of more than 10 g/dL for a minimum of 3 months during therapy,” they noted.
In addition, 8 of 10 patients who had marked leukocytosis at baseline had either a complete resolution (3 patients) or a reduction of at least 50% in white-cell counts (5 patients). All 11 participants who had thrombocytosis at baseline had either complete resolution (10 patients) or a reduction in platelet count of at least 50% (1 patient). Of the 27 participants who had leukoerythroblastosis at baseline, 22 showed either complete resolution (13 patients) or a reduction of at least 50% in the percentage of immature myeloid cells and nucleated red cells (9 patients). Also, 17 of the 21 participants who had at least 1% circulating blasts at baseline had either a complete disappearance of circulating blasts (14 patients) or a reduction of at least 50% (3 patients).
The most clinically significant adverse effect of imetelstat, myelosuppression, occurred in 22 patients (67%) and often necessitated dose reductions. Low-grade elevations in liver enzymes also were a concern. One patient died from an intracranial hemorrhage that the treating physician attributed to drug-induced grade 4 thrombocytopenia. Other adverse events that may or may not have been treatment related included fever, epistaxis, bruising, hematoma, lung infection, skin infection, and upper-GI hemorrhage.
These findings not only identify imetelstat as a possible treatment for myelofibrosis, they also suggest that other telomerase-targeting strategies may be beneficial in this disease, Dr. Tefferi and his associates added.
In the second report, researchers performing a phase-II study at seven medical centers in the United States, Germany, and Switzerland found that imetelstat produced rapid and durable hematologic and molecular responses in all 18 patients in their study of essential thrombocythemia refractory to other treatments. This result is particularly encouraging because current standard therapies “induce nonspecific reductions in platelet counts but do not typically eliminate or alter the biologic characteristics of the disease,” said Dr. Gabriela M. Baerlocher of the department of hematology and the Stem Cell Molecular Diagnostics Laboratory, University of Bern, Switzerland.
These study participants had either failed to respond to hydroxyurea, anagrelide, and interferon therapy or were forced to discontinue these agents because of adverse effects. After weekly treatment with imetelstat at one of two doses, 100% of the patients achieved a hematologic response, attaining platelet counts of 250,000-300,000 per cc. Sixteen participants (89%) achieved a complete hematologic response. The median time to complete response was 6.1 weeks, Dr. Baerlocher and her associates said (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMoa1503479).
After a median follow-up of 17 months on a maintenance dose of imetelstat, 10 patients were still receiving treatment. The median duration of response had not been reached as of press time (range, 5-30 months).
The most important adverse events were neutropenia (15 patients) and abnormal results on liver-function tests (14 patients). The treating physicians attributed 18 adverse events of grade 3 or higher to the study drug, including headache, anemia, and one syncopal episode. Other adverse events included fatigue, nausea, diarrhea, infections, and rash.
The results of both of these studies are compelling and certainly warrant further research, given the limited treatment options for myeloproliferative disorders.
Although imetelstat’s mechanism of action remains to be elucidated, both studies hint at the possibility that the agent may actually change the natural history of these debilitating disorders.
More important, assessing imetelstat’s long-term safety profile is a vital next step for researchers.
Dr. Mary Armanios and Carol W. Greider, Ph.D., are at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore. Dr. Armanios reported having no relevant disclosures; Dr. Greider reported patents related to an RNA component of telomerase and telomerase-associated proteins. Dr. Armanios and Dr. Greider made these remarks in an editorial accompanying the two reports on imetelstat (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMe1508740).
The results of both of these studies are compelling and certainly warrant further research, given the limited treatment options for myeloproliferative disorders.
Although imetelstat’s mechanism of action remains to be elucidated, both studies hint at the possibility that the agent may actually change the natural history of these debilitating disorders.
More important, assessing imetelstat’s long-term safety profile is a vital next step for researchers.
Dr. Mary Armanios and Carol W. Greider, Ph.D., are at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore. Dr. Armanios reported having no relevant disclosures; Dr. Greider reported patents related to an RNA component of telomerase and telomerase-associated proteins. Dr. Armanios and Dr. Greider made these remarks in an editorial accompanying the two reports on imetelstat (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMe1508740).
The results of both of these studies are compelling and certainly warrant further research, given the limited treatment options for myeloproliferative disorders.
Although imetelstat’s mechanism of action remains to be elucidated, both studies hint at the possibility that the agent may actually change the natural history of these debilitating disorders.
More important, assessing imetelstat’s long-term safety profile is a vital next step for researchers.
Dr. Mary Armanios and Carol W. Greider, Ph.D., are at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore. Dr. Armanios reported having no relevant disclosures; Dr. Greider reported patents related to an RNA component of telomerase and telomerase-associated proteins. Dr. Armanios and Dr. Greider made these remarks in an editorial accompanying the two reports on imetelstat (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMe1508740).
The telomerase inhibitor imetelstat showed promise against advanced myelofibrosis and essential thrombocythemia in two industry-funded preliminary studies, according to separate reports published online Sept. 3 in the New England Journal of Medicine.
In previous in vitro and animal studies, imetelstat inhibited the proliferation of various types of malignant cells but was not active in normal somatic tissue. Researchers assessed the agent for advanced myelofibrosis in part because, at present, only one available treatment – allogeneic stem-cell transplantation (ASCT) – sometimes induces long-term remission. ASCT carries a relatively high rate of treatment-related death and complications, and is contraindicated in many older patients.
In the first report, researchers conducted a small, single-center cohort study to collect preliminary data on the agent’s efficacy and safety in 33 patients with primary myelofibrosis (18 participants), myelofibrosis that was related to polycythemia (10 participants), or myelofibrosis associated with essential thrombocytopenia (10 participants). Imetelstat was administered in 2-hour intravenous infusions given in 3-week cycles, said Dr. Ayalew Tefferi of the division of hematology, Mayo Clinic, Rochester Minn.
The median duration of treatment was 8.6 months (range, 1.4-21.7 months). Seven patients (21%) had either a complete or partial response; the 4 patients with a complete response had documented complete reversal of bone marrow fibrosis. The time to onset of response was 3.5 months (range, 1.4-7.2 months), and the median duration of response was 18 months (range, 13-20 months).
These remissions “confirm selective anticlonal activity, which has not previously been documented in drug treatment of myelofibrosis,” noted Dr. Tefferi and his associates (N Engl J Med 2015 Sep 3. doi:10.1056/NEJMoa1310523). Three of the seven patients who responded to imetelstat “had been heavily dependent on red-cell transfusions at study entry and became transfusion-independent and sustained a hemoglobin level of more than 10 g/dL for a minimum of 3 months during therapy,” they noted.
In addition, 8 of 10 patients who had marked leukocytosis at baseline had either a complete resolution (3 patients) or a reduction of at least 50% in white-cell counts (5 patients). All 11 participants who had thrombocytosis at baseline had either complete resolution (10 patients) or a reduction in platelet count of at least 50% (1 patient). Of the 27 participants who had leukoerythroblastosis at baseline, 22 showed either complete resolution (13 patients) or a reduction of at least 50% in the percentage of immature myeloid cells and nucleated red cells (9 patients). Also, 17 of the 21 participants who had at least 1% circulating blasts at baseline had either a complete disappearance of circulating blasts (14 patients) or a reduction of at least 50% (3 patients).
The most clinically significant adverse effect of imetelstat, myelosuppression, occurred in 22 patients (67%) and often necessitated dose reductions. Low-grade elevations in liver enzymes also were a concern. One patient died from an intracranial hemorrhage that the treating physician attributed to drug-induced grade 4 thrombocytopenia. Other adverse events that may or may not have been treatment related included fever, epistaxis, bruising, hematoma, lung infection, skin infection, and upper-GI hemorrhage.
These findings not only identify imetelstat as a possible treatment for myelofibrosis, they also suggest that other telomerase-targeting strategies may be beneficial in this disease, Dr. Tefferi and his associates added.
In the second report, researchers performing a phase-II study at seven medical centers in the United States, Germany, and Switzerland found that imetelstat produced rapid and durable hematologic and molecular responses in all 18 patients in their study of essential thrombocythemia refractory to other treatments. This result is particularly encouraging because current standard therapies “induce nonspecific reductions in platelet counts but do not typically eliminate or alter the biologic characteristics of the disease,” said Dr. Gabriela M. Baerlocher of the department of hematology and the Stem Cell Molecular Diagnostics Laboratory, University of Bern, Switzerland.
These study participants had either failed to respond to hydroxyurea, anagrelide, and interferon therapy or were forced to discontinue these agents because of adverse effects. After weekly treatment with imetelstat at one of two doses, 100% of the patients achieved a hematologic response, attaining platelet counts of 250,000-300,000 per cc. Sixteen participants (89%) achieved a complete hematologic response. The median time to complete response was 6.1 weeks, Dr. Baerlocher and her associates said (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMoa1503479).
After a median follow-up of 17 months on a maintenance dose of imetelstat, 10 patients were still receiving treatment. The median duration of response had not been reached as of press time (range, 5-30 months).
The most important adverse events were neutropenia (15 patients) and abnormal results on liver-function tests (14 patients). The treating physicians attributed 18 adverse events of grade 3 or higher to the study drug, including headache, anemia, and one syncopal episode. Other adverse events included fatigue, nausea, diarrhea, infections, and rash.
The telomerase inhibitor imetelstat showed promise against advanced myelofibrosis and essential thrombocythemia in two industry-funded preliminary studies, according to separate reports published online Sept. 3 in the New England Journal of Medicine.
In previous in vitro and animal studies, imetelstat inhibited the proliferation of various types of malignant cells but was not active in normal somatic tissue. Researchers assessed the agent for advanced myelofibrosis in part because, at present, only one available treatment – allogeneic stem-cell transplantation (ASCT) – sometimes induces long-term remission. ASCT carries a relatively high rate of treatment-related death and complications, and is contraindicated in many older patients.
In the first report, researchers conducted a small, single-center cohort study to collect preliminary data on the agent’s efficacy and safety in 33 patients with primary myelofibrosis (18 participants), myelofibrosis that was related to polycythemia (10 participants), or myelofibrosis associated with essential thrombocytopenia (10 participants). Imetelstat was administered in 2-hour intravenous infusions given in 3-week cycles, said Dr. Ayalew Tefferi of the division of hematology, Mayo Clinic, Rochester Minn.
The median duration of treatment was 8.6 months (range, 1.4-21.7 months). Seven patients (21%) had either a complete or partial response; the 4 patients with a complete response had documented complete reversal of bone marrow fibrosis. The time to onset of response was 3.5 months (range, 1.4-7.2 months), and the median duration of response was 18 months (range, 13-20 months).
These remissions “confirm selective anticlonal activity, which has not previously been documented in drug treatment of myelofibrosis,” noted Dr. Tefferi and his associates (N Engl J Med 2015 Sep 3. doi:10.1056/NEJMoa1310523). Three of the seven patients who responded to imetelstat “had been heavily dependent on red-cell transfusions at study entry and became transfusion-independent and sustained a hemoglobin level of more than 10 g/dL for a minimum of 3 months during therapy,” they noted.
In addition, 8 of 10 patients who had marked leukocytosis at baseline had either a complete resolution (3 patients) or a reduction of at least 50% in white-cell counts (5 patients). All 11 participants who had thrombocytosis at baseline had either complete resolution (10 patients) or a reduction in platelet count of at least 50% (1 patient). Of the 27 participants who had leukoerythroblastosis at baseline, 22 showed either complete resolution (13 patients) or a reduction of at least 50% in the percentage of immature myeloid cells and nucleated red cells (9 patients). Also, 17 of the 21 participants who had at least 1% circulating blasts at baseline had either a complete disappearance of circulating blasts (14 patients) or a reduction of at least 50% (3 patients).
The most clinically significant adverse effect of imetelstat, myelosuppression, occurred in 22 patients (67%) and often necessitated dose reductions. Low-grade elevations in liver enzymes also were a concern. One patient died from an intracranial hemorrhage that the treating physician attributed to drug-induced grade 4 thrombocytopenia. Other adverse events that may or may not have been treatment related included fever, epistaxis, bruising, hematoma, lung infection, skin infection, and upper-GI hemorrhage.
These findings not only identify imetelstat as a possible treatment for myelofibrosis, they also suggest that other telomerase-targeting strategies may be beneficial in this disease, Dr. Tefferi and his associates added.
In the second report, researchers performing a phase-II study at seven medical centers in the United States, Germany, and Switzerland found that imetelstat produced rapid and durable hematologic and molecular responses in all 18 patients in their study of essential thrombocythemia refractory to other treatments. This result is particularly encouraging because current standard therapies “induce nonspecific reductions in platelet counts but do not typically eliminate or alter the biologic characteristics of the disease,” said Dr. Gabriela M. Baerlocher of the department of hematology and the Stem Cell Molecular Diagnostics Laboratory, University of Bern, Switzerland.
These study participants had either failed to respond to hydroxyurea, anagrelide, and interferon therapy or were forced to discontinue these agents because of adverse effects. After weekly treatment with imetelstat at one of two doses, 100% of the patients achieved a hematologic response, attaining platelet counts of 250,000-300,000 per cc. Sixteen participants (89%) achieved a complete hematologic response. The median time to complete response was 6.1 weeks, Dr. Baerlocher and her associates said (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMoa1503479).
After a median follow-up of 17 months on a maintenance dose of imetelstat, 10 patients were still receiving treatment. The median duration of response had not been reached as of press time (range, 5-30 months).
The most important adverse events were neutropenia (15 patients) and abnormal results on liver-function tests (14 patients). The treating physicians attributed 18 adverse events of grade 3 or higher to the study drug, including headache, anemia, and one syncopal episode. Other adverse events included fatigue, nausea, diarrhea, infections, and rash.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The telomerase inhibitor imetelstat showed promise in separate preliminary studies for treatment of myelofibrosis and thrombocythemia.
Major finding: A complete or partial response to imetelstat was seen in 7 of 33 patients with advanced myelofibrosis and 18 of 18 with thrombocythemia.
Data source: An international phase-II open-label study involving 18 patients with essential thrombocythemia and a single-center observational cohort study involving 33 patients with myelofibrosis.
Disclosures: Both studies were funded by Geron.
ESC: Bivalirudin no better than unfractionated heparin in PCI
LONDON – Bivalirudin did not prove superior to unfractionated heparin in reducing the rate of major adverse cardiovascular events in two nested, open-label, randomized clinical trials involving patients presenting with acute coronary syndrome who were expected to undergo percutaneous coronary intervention, Dr. Marco Valgimigli reported.
In addition, post-PCI infusions of bivalirudin for 4 hours or longer did not reduce the rate of adverse bleeding events, compared with no infusion.
These findings add important data to the understanding of antithrombotic therapy in ACS patients undergoing invasive treatment, but they do not resolve the persistent question of which method is best for preventing thrombotic complications while limiting the risk of bleeding during and after such procedures, said Dr. Valgimigli of Erasmus University in Rotterdam.
Previous studies comparing bivalirudin, a direct thrombin inhibitor, against unfractionated heparin, an indirect thrombin inhibitor, have yielded conflicting results regarding ischemic and bleeding outcomes, so Dr. Valgimigli and his fellow investigators in the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial conducted two industry-sponsored superiority trials to try to settle the question.
The findings of one of these trials were reported by Dr. Valgimigli at the annual congress of the European Society of Cardiology on Sept. 1, when the results of both were simultaneously published online (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMoa1507854).
The MATRIX studies were conducted at 78 medical centers in Italy, the Netherlands, Spain, and Sweden. They involved 7,213 patients who presented with either ST-elevation MI or non-STEMI ACS and were expected to undergo PCI. The first trial, MATRIX Antithrombin, assessed outcomes in 3,610 of these participants who were randomly assigned to receive bivalirudin and 3,603 assigned to receive unfractionated heparin. In the second trial, MATRIX Treatment Duration, the bivalirudin group was further randomized to receive either a post-PCI bivalirudin infusion (1,799 patients) or no post-PCI infusion (1,811 patients).
MATRIX Antithrombin
At 1-month follow-up, the rate of major adverse cardiovascular events (MACEs) – a composite of death from any cause, myocardial infarction, or stroke – was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94. Similarly, the rate of net adverse clinical events was not significantly lower with bivalirudin (11.2%) than with heparin (12.4%), for a rate ratio of 0.89.
MATRIX Treatment Duration
The primary outcome in the MATRIX Treatment Duration study – a composite of urgent target-vessel revascularization, definite stent thrombosis, or net adverse clinical events at 30 days – occurred in 11.0% of patients who received post-PCI bivalirudin infusions and 11.9% of those who did not, a nonsignificant difference (rate ratio, 0.91). However, the rate of subacute definite stent thrombosis was significantly higher in the post-PCI infusion group, at 0.7%, compared with 0.2% in the group that didn’t receive post-PCI infusions (RR, 4.37).
“I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients as well as, perhaps, based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors,” Dr. Valgimigli said, noting that this is in keeping with the current labeling of the drug in Europe and the United States.
The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Mary Ann Moon contributed to this report.
The MATRIX investigators properly conclude that their studies did not produce a clear winner, either in the comparison of bivalirudin vs. heparin or in the comparison of post-PCI bivalirudin infusion vs. no infusion. But this should not diminish the credit due to Dr. Valgimigli and his associates for conducting two trials to address important and complex issues.
The second trial provides the best evidence to date on whether it is beneficial to prolong the infusion of bivalirudin after PCI is completed. The agent did not reduce rates of urgent target-vessel revascularization, definite stent thrombosis, and net adverse clinical events – either as a composite outcome or as individual components.
Dr. Peter B. Berger is with North Shore-Long Island Jewish Health System in Great Neck, N.Y. He reported receiving grants and personal fees from Boehringer Ingelheim, Medicure, Bristol-Myers Squibb/Sanofi, Novartis, Tethys, Thrombovision, Helena, Accumetrics, AstraAeneca, Haemoscope, the Medicines Company, and Corgenix/Aspirinworks. Dr. Berger made these remarks in an editorial accompanying the MATRIX report (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMe1509637).
The MATRIX investigators properly conclude that their studies did not produce a clear winner, either in the comparison of bivalirudin vs. heparin or in the comparison of post-PCI bivalirudin infusion vs. no infusion. But this should not diminish the credit due to Dr. Valgimigli and his associates for conducting two trials to address important and complex issues.
The second trial provides the best evidence to date on whether it is beneficial to prolong the infusion of bivalirudin after PCI is completed. The agent did not reduce rates of urgent target-vessel revascularization, definite stent thrombosis, and net adverse clinical events – either as a composite outcome or as individual components.
Dr. Peter B. Berger is with North Shore-Long Island Jewish Health System in Great Neck, N.Y. He reported receiving grants and personal fees from Boehringer Ingelheim, Medicure, Bristol-Myers Squibb/Sanofi, Novartis, Tethys, Thrombovision, Helena, Accumetrics, AstraAeneca, Haemoscope, the Medicines Company, and Corgenix/Aspirinworks. Dr. Berger made these remarks in an editorial accompanying the MATRIX report (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMe1509637).
The MATRIX investigators properly conclude that their studies did not produce a clear winner, either in the comparison of bivalirudin vs. heparin or in the comparison of post-PCI bivalirudin infusion vs. no infusion. But this should not diminish the credit due to Dr. Valgimigli and his associates for conducting two trials to address important and complex issues.
The second trial provides the best evidence to date on whether it is beneficial to prolong the infusion of bivalirudin after PCI is completed. The agent did not reduce rates of urgent target-vessel revascularization, definite stent thrombosis, and net adverse clinical events – either as a composite outcome or as individual components.
Dr. Peter B. Berger is with North Shore-Long Island Jewish Health System in Great Neck, N.Y. He reported receiving grants and personal fees from Boehringer Ingelheim, Medicure, Bristol-Myers Squibb/Sanofi, Novartis, Tethys, Thrombovision, Helena, Accumetrics, AstraAeneca, Haemoscope, the Medicines Company, and Corgenix/Aspirinworks. Dr. Berger made these remarks in an editorial accompanying the MATRIX report (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMe1509637).
LONDON – Bivalirudin did not prove superior to unfractionated heparin in reducing the rate of major adverse cardiovascular events in two nested, open-label, randomized clinical trials involving patients presenting with acute coronary syndrome who were expected to undergo percutaneous coronary intervention, Dr. Marco Valgimigli reported.
In addition, post-PCI infusions of bivalirudin for 4 hours or longer did not reduce the rate of adverse bleeding events, compared with no infusion.
These findings add important data to the understanding of antithrombotic therapy in ACS patients undergoing invasive treatment, but they do not resolve the persistent question of which method is best for preventing thrombotic complications while limiting the risk of bleeding during and after such procedures, said Dr. Valgimigli of Erasmus University in Rotterdam.
Previous studies comparing bivalirudin, a direct thrombin inhibitor, against unfractionated heparin, an indirect thrombin inhibitor, have yielded conflicting results regarding ischemic and bleeding outcomes, so Dr. Valgimigli and his fellow investigators in the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial conducted two industry-sponsored superiority trials to try to settle the question.
The findings of one of these trials were reported by Dr. Valgimigli at the annual congress of the European Society of Cardiology on Sept. 1, when the results of both were simultaneously published online (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMoa1507854).
The MATRIX studies were conducted at 78 medical centers in Italy, the Netherlands, Spain, and Sweden. They involved 7,213 patients who presented with either ST-elevation MI or non-STEMI ACS and were expected to undergo PCI. The first trial, MATRIX Antithrombin, assessed outcomes in 3,610 of these participants who were randomly assigned to receive bivalirudin and 3,603 assigned to receive unfractionated heparin. In the second trial, MATRIX Treatment Duration, the bivalirudin group was further randomized to receive either a post-PCI bivalirudin infusion (1,799 patients) or no post-PCI infusion (1,811 patients).
MATRIX Antithrombin
At 1-month follow-up, the rate of major adverse cardiovascular events (MACEs) – a composite of death from any cause, myocardial infarction, or stroke – was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94. Similarly, the rate of net adverse clinical events was not significantly lower with bivalirudin (11.2%) than with heparin (12.4%), for a rate ratio of 0.89.
MATRIX Treatment Duration
The primary outcome in the MATRIX Treatment Duration study – a composite of urgent target-vessel revascularization, definite stent thrombosis, or net adverse clinical events at 30 days – occurred in 11.0% of patients who received post-PCI bivalirudin infusions and 11.9% of those who did not, a nonsignificant difference (rate ratio, 0.91). However, the rate of subacute definite stent thrombosis was significantly higher in the post-PCI infusion group, at 0.7%, compared with 0.2% in the group that didn’t receive post-PCI infusions (RR, 4.37).
“I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients as well as, perhaps, based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors,” Dr. Valgimigli said, noting that this is in keeping with the current labeling of the drug in Europe and the United States.
The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Mary Ann Moon contributed to this report.
LONDON – Bivalirudin did not prove superior to unfractionated heparin in reducing the rate of major adverse cardiovascular events in two nested, open-label, randomized clinical trials involving patients presenting with acute coronary syndrome who were expected to undergo percutaneous coronary intervention, Dr. Marco Valgimigli reported.
In addition, post-PCI infusions of bivalirudin for 4 hours or longer did not reduce the rate of adverse bleeding events, compared with no infusion.
These findings add important data to the understanding of antithrombotic therapy in ACS patients undergoing invasive treatment, but they do not resolve the persistent question of which method is best for preventing thrombotic complications while limiting the risk of bleeding during and after such procedures, said Dr. Valgimigli of Erasmus University in Rotterdam.
Previous studies comparing bivalirudin, a direct thrombin inhibitor, against unfractionated heparin, an indirect thrombin inhibitor, have yielded conflicting results regarding ischemic and bleeding outcomes, so Dr. Valgimigli and his fellow investigators in the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial conducted two industry-sponsored superiority trials to try to settle the question.
The findings of one of these trials were reported by Dr. Valgimigli at the annual congress of the European Society of Cardiology on Sept. 1, when the results of both were simultaneously published online (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMoa1507854).
The MATRIX studies were conducted at 78 medical centers in Italy, the Netherlands, Spain, and Sweden. They involved 7,213 patients who presented with either ST-elevation MI or non-STEMI ACS and were expected to undergo PCI. The first trial, MATRIX Antithrombin, assessed outcomes in 3,610 of these participants who were randomly assigned to receive bivalirudin and 3,603 assigned to receive unfractionated heparin. In the second trial, MATRIX Treatment Duration, the bivalirudin group was further randomized to receive either a post-PCI bivalirudin infusion (1,799 patients) or no post-PCI infusion (1,811 patients).
MATRIX Antithrombin
At 1-month follow-up, the rate of major adverse cardiovascular events (MACEs) – a composite of death from any cause, myocardial infarction, or stroke – was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94. Similarly, the rate of net adverse clinical events was not significantly lower with bivalirudin (11.2%) than with heparin (12.4%), for a rate ratio of 0.89.
MATRIX Treatment Duration
The primary outcome in the MATRIX Treatment Duration study – a composite of urgent target-vessel revascularization, definite stent thrombosis, or net adverse clinical events at 30 days – occurred in 11.0% of patients who received post-PCI bivalirudin infusions and 11.9% of those who did not, a nonsignificant difference (rate ratio, 0.91). However, the rate of subacute definite stent thrombosis was significantly higher in the post-PCI infusion group, at 0.7%, compared with 0.2% in the group that didn’t receive post-PCI infusions (RR, 4.37).
“I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients as well as, perhaps, based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors,” Dr. Valgimigli said, noting that this is in keeping with the current labeling of the drug in Europe and the United States.
The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Mary Ann Moon contributed to this report.
AT THE ESC CONGRESS 2015
Key clinical point: Compared with unfractionated heparin, bivalirudin did not reduce the MACE rate in patients with ACS who were candidates for PCI.
Major finding: At the 1-month follow-up, the MACE rate was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94.
Data source: A randomized, multicenter, open-label superiority trial involving 7,213 ACS patients expected to undergo PCI.
Disclosures: The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Finerenone cuts albuminuria in diabetic nephropathy
Adding the mineralocorticoid receptor antagonist finerenone to standard renin-angiotensin system (RAS) blockade decreased albuminuria and improved the urinary albumin/creatinine ratio in an industry-sponsored phase IIB clinical trial of diabetic nephropathy, according to a report published online Sept. 1 in JAMA.
Finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist (MRA), had greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro. It also reduced proteinuria and end-organ damage to a greater degree in animal studies, and was less likely to induce hyperkalemia than either of those related drugs in preliminary human studies. “Thus, finerenone may be able to address the unmet medical need of safely managing albuminuria without adversely affecting serum potassium in patients with type 2 diabetes mellitus who have a clinical diagnosis of diabetic kidney disease,” said Dr. George L. Bakris of the ASH Comprehensive Hypertension Center, University of Chicago, and his associates.
To investigate this possibility, they performed the Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN), an international randomized, double-blind trial comparing seven oral doses of finerenone against matching placebo in 823 patients who had type 2 diabetes and persistent albuminuria and who were already taking an RAS blocker (an ACE inhibitor or ARB). These study participants (mean age 64 years) were treated for 90 days at 148 medical centers in 23 countries.
The primary outcome measure was the change in urinary albumin/creatinine ratio at the conclusion of treatment. Compared with placebo, the four highest doses of finerenone reduced this ratio in a dose-dependent manner: the ratio was 0.79 with the 7.5-mg dose, 0.76 with the 10-mg dose, 0.67 with the 15-mg dose, and 0.62 with the 20-mg dose. This represents reductions in urinary albumin/creatinine ratio ranging from 21% to 38%, compared with placebo. In addition, only 13.6% of the placebo group achieved a decrease of at least half in urinary albumin/creatinine ratio, compared with 17.2%, 17.2%, 33.6%, and 40.2%, respectively, of these four highest-dose groups, Dr. Bakris and his associates said (JAMA. 2015 Sept 1. doi: 10.1001/jama.2015.10081). The incidences of adverse events and of serious adverse events were similar across all the study groups. A total of 1.5% of patients had serious adverse events thought to be related to treatment, including 12 patients who discontinued finerenone because of increases in serum potassium.
Longer-term studies are needed to assess clinical rather than just laboratory endpoints, the longer-term effects of finerenone on kidney disease progression, and potential beneficial antifibrotic or anti-inflammatory effects, the investigators added.
The ARTS-DN study was funded by Bayer HealthCare AG. Dr. Bakris reported receiving research support from and serving as an advisor/consultant to Takeda, Bayer HealthCare AG, Medtronic, Relypsa, AbbVie, Bristol-Myers Squibb, CVRx, Elcelyx, Eli Lilly/Boehringer Ingelheim, Janssen, Novartis, GlaxoSmithKline, Tengion, and ZS Pharma. His associates reported ties to numerous industry sources.
Adding the mineralocorticoid receptor antagonist finerenone to standard renin-angiotensin system (RAS) blockade decreased albuminuria and improved the urinary albumin/creatinine ratio in an industry-sponsored phase IIB clinical trial of diabetic nephropathy, according to a report published online Sept. 1 in JAMA.
Finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist (MRA), had greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro. It also reduced proteinuria and end-organ damage to a greater degree in animal studies, and was less likely to induce hyperkalemia than either of those related drugs in preliminary human studies. “Thus, finerenone may be able to address the unmet medical need of safely managing albuminuria without adversely affecting serum potassium in patients with type 2 diabetes mellitus who have a clinical diagnosis of diabetic kidney disease,” said Dr. George L. Bakris of the ASH Comprehensive Hypertension Center, University of Chicago, and his associates.
To investigate this possibility, they performed the Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN), an international randomized, double-blind trial comparing seven oral doses of finerenone against matching placebo in 823 patients who had type 2 diabetes and persistent albuminuria and who were already taking an RAS blocker (an ACE inhibitor or ARB). These study participants (mean age 64 years) were treated for 90 days at 148 medical centers in 23 countries.
The primary outcome measure was the change in urinary albumin/creatinine ratio at the conclusion of treatment. Compared with placebo, the four highest doses of finerenone reduced this ratio in a dose-dependent manner: the ratio was 0.79 with the 7.5-mg dose, 0.76 with the 10-mg dose, 0.67 with the 15-mg dose, and 0.62 with the 20-mg dose. This represents reductions in urinary albumin/creatinine ratio ranging from 21% to 38%, compared with placebo. In addition, only 13.6% of the placebo group achieved a decrease of at least half in urinary albumin/creatinine ratio, compared with 17.2%, 17.2%, 33.6%, and 40.2%, respectively, of these four highest-dose groups, Dr. Bakris and his associates said (JAMA. 2015 Sept 1. doi: 10.1001/jama.2015.10081). The incidences of adverse events and of serious adverse events were similar across all the study groups. A total of 1.5% of patients had serious adverse events thought to be related to treatment, including 12 patients who discontinued finerenone because of increases in serum potassium.
Longer-term studies are needed to assess clinical rather than just laboratory endpoints, the longer-term effects of finerenone on kidney disease progression, and potential beneficial antifibrotic or anti-inflammatory effects, the investigators added.
The ARTS-DN study was funded by Bayer HealthCare AG. Dr. Bakris reported receiving research support from and serving as an advisor/consultant to Takeda, Bayer HealthCare AG, Medtronic, Relypsa, AbbVie, Bristol-Myers Squibb, CVRx, Elcelyx, Eli Lilly/Boehringer Ingelheim, Janssen, Novartis, GlaxoSmithKline, Tengion, and ZS Pharma. His associates reported ties to numerous industry sources.
Adding the mineralocorticoid receptor antagonist finerenone to standard renin-angiotensin system (RAS) blockade decreased albuminuria and improved the urinary albumin/creatinine ratio in an industry-sponsored phase IIB clinical trial of diabetic nephropathy, according to a report published online Sept. 1 in JAMA.
Finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist (MRA), had greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro. It also reduced proteinuria and end-organ damage to a greater degree in animal studies, and was less likely to induce hyperkalemia than either of those related drugs in preliminary human studies. “Thus, finerenone may be able to address the unmet medical need of safely managing albuminuria without adversely affecting serum potassium in patients with type 2 diabetes mellitus who have a clinical diagnosis of diabetic kidney disease,” said Dr. George L. Bakris of the ASH Comprehensive Hypertension Center, University of Chicago, and his associates.
To investigate this possibility, they performed the Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN), an international randomized, double-blind trial comparing seven oral doses of finerenone against matching placebo in 823 patients who had type 2 diabetes and persistent albuminuria and who were already taking an RAS blocker (an ACE inhibitor or ARB). These study participants (mean age 64 years) were treated for 90 days at 148 medical centers in 23 countries.
The primary outcome measure was the change in urinary albumin/creatinine ratio at the conclusion of treatment. Compared with placebo, the four highest doses of finerenone reduced this ratio in a dose-dependent manner: the ratio was 0.79 with the 7.5-mg dose, 0.76 with the 10-mg dose, 0.67 with the 15-mg dose, and 0.62 with the 20-mg dose. This represents reductions in urinary albumin/creatinine ratio ranging from 21% to 38%, compared with placebo. In addition, only 13.6% of the placebo group achieved a decrease of at least half in urinary albumin/creatinine ratio, compared with 17.2%, 17.2%, 33.6%, and 40.2%, respectively, of these four highest-dose groups, Dr. Bakris and his associates said (JAMA. 2015 Sept 1. doi: 10.1001/jama.2015.10081). The incidences of adverse events and of serious adverse events were similar across all the study groups. A total of 1.5% of patients had serious adverse events thought to be related to treatment, including 12 patients who discontinued finerenone because of increases in serum potassium.
Longer-term studies are needed to assess clinical rather than just laboratory endpoints, the longer-term effects of finerenone on kidney disease progression, and potential beneficial antifibrotic or anti-inflammatory effects, the investigators added.
The ARTS-DN study was funded by Bayer HealthCare AG. Dr. Bakris reported receiving research support from and serving as an advisor/consultant to Takeda, Bayer HealthCare AG, Medtronic, Relypsa, AbbVie, Bristol-Myers Squibb, CVRx, Elcelyx, Eli Lilly/Boehringer Ingelheim, Janssen, Novartis, GlaxoSmithKline, Tengion, and ZS Pharma. His associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Adding finerenone to renin-angiotensin system blockers decreases albuminuria and improves the urinary albumin/creatinine ratio in diabetic nephropathy.
Major finding: The four highest doses of finerenone reduced the urinary albumim/creatinine ratio by 21%-38%, compared with placebo.
Data source: An international randomized, double-blind, placebo-controlled phase IIB clinical trial involving 823 patients with diabetic nephropathy who were treated for 90 days.
Disclosures: The ARTS-DN study was funded by Bayer HealthCare AG. Dr. Bakris reported receiving research support from and serving as an advisor/consultant to Takeda, Bayer HealthCare AG, Medtronic, Relypsa, AbbVie, Bristol-Myers Squibb, CVRx, Elcelyx, Eli Lilly/Boehringer Ingelheim, Janssen, Novartis, GlaxoSmithKline, Tengion, and ZS Pharma. His associates reported ties to numerous industry sources.
ESC: Novel apnea treatment not helpful, possibly harmful in heart failure
Adaptive servo-ventilation is not beneficial and may even be harmful for patients who have predominantly central sleep apnea accompanying heart failure with reduced ejection fraction, Dr. Martin R. Cowie reported at the annual congress of the European Society of Cardiology.
The noninvasive therapy did control central sleep apnea in a large international randomized controlled trial, but nevertheless did not affect the composite end point of death from any cause, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening HF. Moreover, it unexpectedly raised the risk of cardiovascular death by 34%, and significantly increased all-cause mortality as well, said Dr. Cowie of Imperial College London.
Adaptive servo-ventilation delivers servo-controlled inspiratory pressure on top of expiratory positive airway pressure during sleep, to alleviate central sleep apnea. This form of sleep-disordered breathing, which may manifest as Cheyne-Stokes respiration in patients who have HF with reduced ejection fraction, is reported to affect up to 40% of this patient population. Its prevalence rises as the severity of HF increases, and it is an independent risk marker for poor prognosis and death in HF.
A recent trial showed that continuous positive airway pressure (CPAP) did not improve morbidity or mortality in patients who had HF with central sleep apnea, but suggested that a treatment that could reduce the apnea-hypopnea index (AHI) – the number of apnea or hypopnea events per hour of sleep – to below 15 might be effective. Adaptive servo-ventilation can accomplish this, and small studies and meta-analyses have shown that the treatment improves surrogate markers including plasma concentration of brain natriuretic peptide, left ventricular ejection fraction (LVEF), and functional outcomes in heart failure.
Dr. Cowie and his associates conducted the SERVE-HF trial, assessing the effect of adding adaptive servo-ventilation to guideline-based medical therapy on survival and cardiovascular outcomes. He presented the trial results at the meeting, and they were simultaneously published online (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMoa1506459).
The industry-sponsored study comprised 1,325 patients aged 22 and older treated and followed at 91 medical centers for a median of 31 months (range, 0-80 months). They were randomly assigned to receive medical therapy plus adaptive servo-ventilation delivered through a face mask for at least 5 hours every night (666 intervention subjects) or medical therapy alone (659 control subjects).
Central sleep apnea was well controlled only in the intervention group. At 1 year, their mean AHI was 6.6 events per hour, and the oxygen desaturation index – the number of times per hour that the blood oxygen level dropped by 3 or more percentage points from baseline level – was 8.6.
Yet the primary composite end point was not significantly different between the two study groups: The rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it. The treatment also had no significant effect on a broad spectrum of secondary measures such as symptoms and quality of life. Six-minute walk distance gradually declined in both groups, but that decline was significantly more pronounced in the intervention group, the investigator said.
Even more worrisome was the significant increase in mortality associated with adaptive servo-ventilation. Cardiovascular mortality was 29.9% with the treatment, compared with 24.0% without it, for a hazard ratio of 1.34. All-cause mortality was 34.8% with the treatment and 29.3% without it, for an HR of 1.28.
The reason for this unexpected result is not yet known. One explanation is that central sleep apnea may be a compensatory mechanism with potentially beneficial effects in patients who have HF. Attenuating those effects with adaptive servo-ventilation may then have been detrimental. For example, central sleep apnea, and particularly Cheyne-Stokes breathing, may beneficially activate the respiratory muscles, increase sympathetic nervous system activity, induce hypercapnic acidosis, increase end-expiratory lung volume, and raise intrinsic positive airway pressure.
Another possibility is that applying positive airway pressure with adaptive servo-ventilation may impair cardiac function in at least a portion of patients who have HF by decreasing cardiac output and stroke volume during treatment.
ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
Adaptive servo-ventilation should not be used outside of clinical trials in heart failure patients who have predominantly central sleep apnea, at least until the reason for the unexpected 34% increase in cardiovascular mortality is understood.
The issue is important because at least one new technique to abolish Cheyne-Stokes respiration that doesn’t use positive pressure therapy – phrenic-nerve stimulation – has already been developed and is being assessed in a clinical trial. If Cheyne-Stokes respiration is actually beneficial in HF, this strategy may prove harmful.
Dr. Ulysses J. Magalang is in the division of pulmonary, allergy, critical care, and sleep medicine at Ohio State University Wexner Medical Center, Columbus. Dr. Allan I. Pack is at the Center for Sleep and Circadian Neurobiology at the University of Pennsylvania, Philadelphia. Dr. Magalang reported grants support from the Rudi Schulte Family Foundation, Hill-Rom, and the Tzagournis Medical Research Endowment; Dr. Pack reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the SERVE-HF report (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMe1510397Th).
Adaptive servo-ventilation should not be used outside of clinical trials in heart failure patients who have predominantly central sleep apnea, at least until the reason for the unexpected 34% increase in cardiovascular mortality is understood.
The issue is important because at least one new technique to abolish Cheyne-Stokes respiration that doesn’t use positive pressure therapy – phrenic-nerve stimulation – has already been developed and is being assessed in a clinical trial. If Cheyne-Stokes respiration is actually beneficial in HF, this strategy may prove harmful.
Dr. Ulysses J. Magalang is in the division of pulmonary, allergy, critical care, and sleep medicine at Ohio State University Wexner Medical Center, Columbus. Dr. Allan I. Pack is at the Center for Sleep and Circadian Neurobiology at the University of Pennsylvania, Philadelphia. Dr. Magalang reported grants support from the Rudi Schulte Family Foundation, Hill-Rom, and the Tzagournis Medical Research Endowment; Dr. Pack reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the SERVE-HF report (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMe1510397Th).
Adaptive servo-ventilation should not be used outside of clinical trials in heart failure patients who have predominantly central sleep apnea, at least until the reason for the unexpected 34% increase in cardiovascular mortality is understood.
The issue is important because at least one new technique to abolish Cheyne-Stokes respiration that doesn’t use positive pressure therapy – phrenic-nerve stimulation – has already been developed and is being assessed in a clinical trial. If Cheyne-Stokes respiration is actually beneficial in HF, this strategy may prove harmful.
Dr. Ulysses J. Magalang is in the division of pulmonary, allergy, critical care, and sleep medicine at Ohio State University Wexner Medical Center, Columbus. Dr. Allan I. Pack is at the Center for Sleep and Circadian Neurobiology at the University of Pennsylvania, Philadelphia. Dr. Magalang reported grants support from the Rudi Schulte Family Foundation, Hill-Rom, and the Tzagournis Medical Research Endowment; Dr. Pack reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the SERVE-HF report (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMe1510397Th).
Adaptive servo-ventilation is not beneficial and may even be harmful for patients who have predominantly central sleep apnea accompanying heart failure with reduced ejection fraction, Dr. Martin R. Cowie reported at the annual congress of the European Society of Cardiology.
The noninvasive therapy did control central sleep apnea in a large international randomized controlled trial, but nevertheless did not affect the composite end point of death from any cause, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening HF. Moreover, it unexpectedly raised the risk of cardiovascular death by 34%, and significantly increased all-cause mortality as well, said Dr. Cowie of Imperial College London.
Adaptive servo-ventilation delivers servo-controlled inspiratory pressure on top of expiratory positive airway pressure during sleep, to alleviate central sleep apnea. This form of sleep-disordered breathing, which may manifest as Cheyne-Stokes respiration in patients who have HF with reduced ejection fraction, is reported to affect up to 40% of this patient population. Its prevalence rises as the severity of HF increases, and it is an independent risk marker for poor prognosis and death in HF.
A recent trial showed that continuous positive airway pressure (CPAP) did not improve morbidity or mortality in patients who had HF with central sleep apnea, but suggested that a treatment that could reduce the apnea-hypopnea index (AHI) – the number of apnea or hypopnea events per hour of sleep – to below 15 might be effective. Adaptive servo-ventilation can accomplish this, and small studies and meta-analyses have shown that the treatment improves surrogate markers including plasma concentration of brain natriuretic peptide, left ventricular ejection fraction (LVEF), and functional outcomes in heart failure.
Dr. Cowie and his associates conducted the SERVE-HF trial, assessing the effect of adding adaptive servo-ventilation to guideline-based medical therapy on survival and cardiovascular outcomes. He presented the trial results at the meeting, and they were simultaneously published online (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMoa1506459).
The industry-sponsored study comprised 1,325 patients aged 22 and older treated and followed at 91 medical centers for a median of 31 months (range, 0-80 months). They were randomly assigned to receive medical therapy plus adaptive servo-ventilation delivered through a face mask for at least 5 hours every night (666 intervention subjects) or medical therapy alone (659 control subjects).
Central sleep apnea was well controlled only in the intervention group. At 1 year, their mean AHI was 6.6 events per hour, and the oxygen desaturation index – the number of times per hour that the blood oxygen level dropped by 3 or more percentage points from baseline level – was 8.6.
Yet the primary composite end point was not significantly different between the two study groups: The rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it. The treatment also had no significant effect on a broad spectrum of secondary measures such as symptoms and quality of life. Six-minute walk distance gradually declined in both groups, but that decline was significantly more pronounced in the intervention group, the investigator said.
Even more worrisome was the significant increase in mortality associated with adaptive servo-ventilation. Cardiovascular mortality was 29.9% with the treatment, compared with 24.0% without it, for a hazard ratio of 1.34. All-cause mortality was 34.8% with the treatment and 29.3% without it, for an HR of 1.28.
The reason for this unexpected result is not yet known. One explanation is that central sleep apnea may be a compensatory mechanism with potentially beneficial effects in patients who have HF. Attenuating those effects with adaptive servo-ventilation may then have been detrimental. For example, central sleep apnea, and particularly Cheyne-Stokes breathing, may beneficially activate the respiratory muscles, increase sympathetic nervous system activity, induce hypercapnic acidosis, increase end-expiratory lung volume, and raise intrinsic positive airway pressure.
Another possibility is that applying positive airway pressure with adaptive servo-ventilation may impair cardiac function in at least a portion of patients who have HF by decreasing cardiac output and stroke volume during treatment.
ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
Adaptive servo-ventilation is not beneficial and may even be harmful for patients who have predominantly central sleep apnea accompanying heart failure with reduced ejection fraction, Dr. Martin R. Cowie reported at the annual congress of the European Society of Cardiology.
The noninvasive therapy did control central sleep apnea in a large international randomized controlled trial, but nevertheless did not affect the composite end point of death from any cause, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening HF. Moreover, it unexpectedly raised the risk of cardiovascular death by 34%, and significantly increased all-cause mortality as well, said Dr. Cowie of Imperial College London.
Adaptive servo-ventilation delivers servo-controlled inspiratory pressure on top of expiratory positive airway pressure during sleep, to alleviate central sleep apnea. This form of sleep-disordered breathing, which may manifest as Cheyne-Stokes respiration in patients who have HF with reduced ejection fraction, is reported to affect up to 40% of this patient population. Its prevalence rises as the severity of HF increases, and it is an independent risk marker for poor prognosis and death in HF.
A recent trial showed that continuous positive airway pressure (CPAP) did not improve morbidity or mortality in patients who had HF with central sleep apnea, but suggested that a treatment that could reduce the apnea-hypopnea index (AHI) – the number of apnea or hypopnea events per hour of sleep – to below 15 might be effective. Adaptive servo-ventilation can accomplish this, and small studies and meta-analyses have shown that the treatment improves surrogate markers including plasma concentration of brain natriuretic peptide, left ventricular ejection fraction (LVEF), and functional outcomes in heart failure.
Dr. Cowie and his associates conducted the SERVE-HF trial, assessing the effect of adding adaptive servo-ventilation to guideline-based medical therapy on survival and cardiovascular outcomes. He presented the trial results at the meeting, and they were simultaneously published online (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMoa1506459).
The industry-sponsored study comprised 1,325 patients aged 22 and older treated and followed at 91 medical centers for a median of 31 months (range, 0-80 months). They were randomly assigned to receive medical therapy plus adaptive servo-ventilation delivered through a face mask for at least 5 hours every night (666 intervention subjects) or medical therapy alone (659 control subjects).
Central sleep apnea was well controlled only in the intervention group. At 1 year, their mean AHI was 6.6 events per hour, and the oxygen desaturation index – the number of times per hour that the blood oxygen level dropped by 3 or more percentage points from baseline level – was 8.6.
Yet the primary composite end point was not significantly different between the two study groups: The rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it. The treatment also had no significant effect on a broad spectrum of secondary measures such as symptoms and quality of life. Six-minute walk distance gradually declined in both groups, but that decline was significantly more pronounced in the intervention group, the investigator said.
Even more worrisome was the significant increase in mortality associated with adaptive servo-ventilation. Cardiovascular mortality was 29.9% with the treatment, compared with 24.0% without it, for a hazard ratio of 1.34. All-cause mortality was 34.8% with the treatment and 29.3% without it, for an HR of 1.28.
The reason for this unexpected result is not yet known. One explanation is that central sleep apnea may be a compensatory mechanism with potentially beneficial effects in patients who have HF. Attenuating those effects with adaptive servo-ventilation may then have been detrimental. For example, central sleep apnea, and particularly Cheyne-Stokes breathing, may beneficially activate the respiratory muscles, increase sympathetic nervous system activity, induce hypercapnic acidosis, increase end-expiratory lung volume, and raise intrinsic positive airway pressure.
Another possibility is that applying positive airway pressure with adaptive servo-ventilation may impair cardiac function in at least a portion of patients who have HF by decreasing cardiac output and stroke volume during treatment.
ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
FROM THE ESC CONGRESS 2015
Key clinical point: Adaptive servo-ventilation is not beneficial and may even be harmful for central sleep apnea accompanying heart failure.
Major finding: The composite rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it, a nonsignificant difference.
Data source: An international randomized clinical trial involving 1,325 adults followed for a median of 31 months.
Disclosures: ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
First-line ambrisentan plus tadalafil halved PAH events
First-line combination therapy with ambrisentan and tadalafil cut the rate of clinical events in pulmonary arterial hypertension (PAH) by half, compared with monotherapy using either drug, in an international phase 3-4 clinical trial reported online Aug. 27 in the New England Journal of Medicine.
Ambrisentan, a selective endothelin-A-receptor antagonist, and tadalafil, a phosphodiesterase type 5 inhibitor, target different intracellular pathways known to have dysfunctional signaling in PAH, so researchers expected them to have an additive effect when combined. The study findings support the rationale of targeting multiple affected pathways early in the course of PAH, rather than following the traditional approach of sequentially adding newer agents to established background therapy, said Dr. Nazzareno Galie of the department of experimental, diagnostic, and specialty medicine, University of Bologna (Italy), and his associates.
The 4-year, industry-sponsored trial involved 500 adults treated at 120 medical centers in 14 countries for PAH with World Health Organization functional class II or III symptoms. It included patients whose disorder was idiopathic; hereditary; or associated with connective tissue disease, drugs or toxins, stable HIV infection, or repaired congenital heart defects.
The mean age of participants was 54.4 years, and 78% were women. The mean pulmonary artery pressure was 48.7 mm Hg, and mean 6-minute walk distance was 353 m at baseline. A total of 253 patients were randomly assigned to receive oral, once-daily combination therapy, 126 to receive ambrisentan plus placebo, and 121 to receive tadalafil plus placebo. They were assessed at monthly intervals during the 24-week treatment period and were allowed to continue therapy indefinitely. The mean duration of use of the study medication was 517 days. Patients were followed up a final time 1 month after taking their last dose of study medication.
The primary efficacy endpoint was the first event of clinical failure, which was a composite of death, hospitalization for worsening PAH, disease progression, or unsatisfactory long-term treatment response. Only 18% of the combination-therapy group reached this endpoint, compared with 34% of the ambrisentan group, 28% of the tadalafil group, and 31% of the pooled-monotherapy group. The hazard ratios for the primary endpoint were 0.50 for the combination therapy versus pooled monotherapy, 0.48 for combination therapy versus ambrisentan alone, and 0.53 for combination therapy versus tadalafil alone.
This treatment benefit was mainly driven by one component of the combined endpoint: The rate of hospitalization for worsening PAH was three times higher with the two monotherapies (12%) than with combination therapy (4%). Improvement in the secondary endpoints of change in N-terminal pro–brain natriuretic peptide level, the percentage of participants with a satisfactory treatment response, and change in 6-minute walk distance all significantly favored the combination therapy, Dr. Galie and his associates said (N Engl J Med. 2015 Aug 27. doi: 10.1056/NEJMoa1413687).It is important to note, however, that “despite improvements in a variety of factors with combination therapy, we found no significant difference in WHO functional class among the study groups at week 24,” they wrote.
The combination of ambrisentan and tadalafil produced more adverse effects than either monotherapy, but the rate of discontinuation of a study drug and the rate of serious adverse events were similar across the three study groups. The most frequent adverse effects were peripheral edema, headache, nasal congestion, and anemia.
The AMBITION study was funded by Gilead Sciences and GlaxoSmithKline, which designed the trial, collected and analyzed the data, and wrote the report in conjunction with the authors. Gilead Sciences, GlaxoSmithKline, and Eli Lilly provided the study drugs. Dr. Galie reported receiving grants and personal fees from GlaxoSmithKline, Actelion, Bayer, and Pfizer; his associates reported ties to numerous industry sources.
First-line combination therapy with ambrisentan and tadalafil cut the rate of clinical events in pulmonary arterial hypertension (PAH) by half, compared with monotherapy using either drug, in an international phase 3-4 clinical trial reported online Aug. 27 in the New England Journal of Medicine.
Ambrisentan, a selective endothelin-A-receptor antagonist, and tadalafil, a phosphodiesterase type 5 inhibitor, target different intracellular pathways known to have dysfunctional signaling in PAH, so researchers expected them to have an additive effect when combined. The study findings support the rationale of targeting multiple affected pathways early in the course of PAH, rather than following the traditional approach of sequentially adding newer agents to established background therapy, said Dr. Nazzareno Galie of the department of experimental, diagnostic, and specialty medicine, University of Bologna (Italy), and his associates.
The 4-year, industry-sponsored trial involved 500 adults treated at 120 medical centers in 14 countries for PAH with World Health Organization functional class II or III symptoms. It included patients whose disorder was idiopathic; hereditary; or associated with connective tissue disease, drugs or toxins, stable HIV infection, or repaired congenital heart defects.
The mean age of participants was 54.4 years, and 78% were women. The mean pulmonary artery pressure was 48.7 mm Hg, and mean 6-minute walk distance was 353 m at baseline. A total of 253 patients were randomly assigned to receive oral, once-daily combination therapy, 126 to receive ambrisentan plus placebo, and 121 to receive tadalafil plus placebo. They were assessed at monthly intervals during the 24-week treatment period and were allowed to continue therapy indefinitely. The mean duration of use of the study medication was 517 days. Patients were followed up a final time 1 month after taking their last dose of study medication.
The primary efficacy endpoint was the first event of clinical failure, which was a composite of death, hospitalization for worsening PAH, disease progression, or unsatisfactory long-term treatment response. Only 18% of the combination-therapy group reached this endpoint, compared with 34% of the ambrisentan group, 28% of the tadalafil group, and 31% of the pooled-monotherapy group. The hazard ratios for the primary endpoint were 0.50 for the combination therapy versus pooled monotherapy, 0.48 for combination therapy versus ambrisentan alone, and 0.53 for combination therapy versus tadalafil alone.
This treatment benefit was mainly driven by one component of the combined endpoint: The rate of hospitalization for worsening PAH was three times higher with the two monotherapies (12%) than with combination therapy (4%). Improvement in the secondary endpoints of change in N-terminal pro–brain natriuretic peptide level, the percentage of participants with a satisfactory treatment response, and change in 6-minute walk distance all significantly favored the combination therapy, Dr. Galie and his associates said (N Engl J Med. 2015 Aug 27. doi: 10.1056/NEJMoa1413687).It is important to note, however, that “despite improvements in a variety of factors with combination therapy, we found no significant difference in WHO functional class among the study groups at week 24,” they wrote.
The combination of ambrisentan and tadalafil produced more adverse effects than either monotherapy, but the rate of discontinuation of a study drug and the rate of serious adverse events were similar across the three study groups. The most frequent adverse effects were peripheral edema, headache, nasal congestion, and anemia.
The AMBITION study was funded by Gilead Sciences and GlaxoSmithKline, which designed the trial, collected and analyzed the data, and wrote the report in conjunction with the authors. Gilead Sciences, GlaxoSmithKline, and Eli Lilly provided the study drugs. Dr. Galie reported receiving grants and personal fees from GlaxoSmithKline, Actelion, Bayer, and Pfizer; his associates reported ties to numerous industry sources.
First-line combination therapy with ambrisentan and tadalafil cut the rate of clinical events in pulmonary arterial hypertension (PAH) by half, compared with monotherapy using either drug, in an international phase 3-4 clinical trial reported online Aug. 27 in the New England Journal of Medicine.
Ambrisentan, a selective endothelin-A-receptor antagonist, and tadalafil, a phosphodiesterase type 5 inhibitor, target different intracellular pathways known to have dysfunctional signaling in PAH, so researchers expected them to have an additive effect when combined. The study findings support the rationale of targeting multiple affected pathways early in the course of PAH, rather than following the traditional approach of sequentially adding newer agents to established background therapy, said Dr. Nazzareno Galie of the department of experimental, diagnostic, and specialty medicine, University of Bologna (Italy), and his associates.
The 4-year, industry-sponsored trial involved 500 adults treated at 120 medical centers in 14 countries for PAH with World Health Organization functional class II or III symptoms. It included patients whose disorder was idiopathic; hereditary; or associated with connective tissue disease, drugs or toxins, stable HIV infection, or repaired congenital heart defects.
The mean age of participants was 54.4 years, and 78% were women. The mean pulmonary artery pressure was 48.7 mm Hg, and mean 6-minute walk distance was 353 m at baseline. A total of 253 patients were randomly assigned to receive oral, once-daily combination therapy, 126 to receive ambrisentan plus placebo, and 121 to receive tadalafil plus placebo. They were assessed at monthly intervals during the 24-week treatment period and were allowed to continue therapy indefinitely. The mean duration of use of the study medication was 517 days. Patients were followed up a final time 1 month after taking their last dose of study medication.
The primary efficacy endpoint was the first event of clinical failure, which was a composite of death, hospitalization for worsening PAH, disease progression, or unsatisfactory long-term treatment response. Only 18% of the combination-therapy group reached this endpoint, compared with 34% of the ambrisentan group, 28% of the tadalafil group, and 31% of the pooled-monotherapy group. The hazard ratios for the primary endpoint were 0.50 for the combination therapy versus pooled monotherapy, 0.48 for combination therapy versus ambrisentan alone, and 0.53 for combination therapy versus tadalafil alone.
This treatment benefit was mainly driven by one component of the combined endpoint: The rate of hospitalization for worsening PAH was three times higher with the two monotherapies (12%) than with combination therapy (4%). Improvement in the secondary endpoints of change in N-terminal pro–brain natriuretic peptide level, the percentage of participants with a satisfactory treatment response, and change in 6-minute walk distance all significantly favored the combination therapy, Dr. Galie and his associates said (N Engl J Med. 2015 Aug 27. doi: 10.1056/NEJMoa1413687).It is important to note, however, that “despite improvements in a variety of factors with combination therapy, we found no significant difference in WHO functional class among the study groups at week 24,” they wrote.
The combination of ambrisentan and tadalafil produced more adverse effects than either monotherapy, but the rate of discontinuation of a study drug and the rate of serious adverse events were similar across the three study groups. The most frequent adverse effects were peripheral edema, headache, nasal congestion, and anemia.
The AMBITION study was funded by Gilead Sciences and GlaxoSmithKline, which designed the trial, collected and analyzed the data, and wrote the report in conjunction with the authors. Gilead Sciences, GlaxoSmithKline, and Eli Lilly provided the study drugs. Dr. Galie reported receiving grants and personal fees from GlaxoSmithKline, Actelion, Bayer, and Pfizer; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: First-line combination therapy with ambrisentan plus tadalafil cut the rate of clinical events in pulmonary arterial hypertension by half, compared with either monotherapy.
Major finding: Only 18% of the combination-therapy group reached the primary efficacy endpoint of clinical failure, compared with 34% of the ambrisentan group, 28% of the tadalafil group, and 31% of the pooled-monotherapy group.
Data source: An international, randomized, double-blind phase 3-4 clinical trial involving 500 men and women with previously untreated PAH.
Disclosures: The AMBITION study was funded by Gilead Sciences and GlaxoSmithKline, which designed the trial, collected and analyzed the data, and wrote the report in conjunction with the authors. Gilead Sciences, GlaxoSmithKline, and Eli Lilly provided the study drugs. Dr. Galie reported receiving grants and personal fees from GlaxoSmithKline, Actelion, Bayer, and Pfizer; his associates reported ties to numerous industry sources.
Physical Activity, Omega-3 Supplements Didn’t Improve Cognitive Outcomes
Neither a physical activity program nor omega-3 long-chain polyunsaturated fatty acid supplements improved cognitive function in two separate studies of high-risk elderly patients reported online Aug. 25 in JAMA.
Both studies were secondary analyses of large randomized clinical trials. Their findings contradict the results of many epidemiologic and observational studies attesting to the cognitive benefits of both lifestyle interventions.
The first study involved 1,635 sedentary men and women aged 70-89 years who had lower-extremity functional limitations and were participating in the LIFE (Lifestyle Interventions and Independence for Elders) trial at eight U.S. medical centers. These participants were randomly assigned to either a physical activity intervention (818 study subjects) or a health education program (817 control subjects) and were assessed with a comprehensive battery of neuropsychological tests every 6 months for 2 years, according to Dr. Kaycee M. Sink of the Sticht Center on Aging at Wake Forest University, Winston-Salem, N.C., and her associates.
The intervention comprised two weekly clinic visits plus three to four weekly home sessions focused on strength, flexibility, and balance training, as well as walking. The control situation consisted of weekly 60- to 90-minute workshops on topics such as travel safety, preventive services, legal and financial issues, and nutrition. As expected, the intervention group achieved a higher level of moderate to vigorous physical activity throughout follow-up (mean increase of 130.4 minutes/week), compared with the control group (mean increase of 30.5 minutes/week).
However, after 2 years, there were no significant differences between the two groups in either global cognitive scores or in individual scores on numerous measures of psychomotor speed, attention, concentration, working memory, word list learning, word recall, visuospatial function, figural memory, language, or executive function. There also were no differences in the rates of mild cognitive impairment, dementia, or both combined: 13.2% of the intervention group and 12.1% of the control group developed MCI or dementia by 2 years, a nonsignificant difference, the investigators said (JAMA. 2015;314[8]:781-90).
It is possible that the level of physical activity in this intervention may not have been sufficient to produce changes in cognitive measures, or that cognitive function improved in the short term but dissipated by the end of the second year of follow-up. Alternatively, the study population on the whole was well educated (more than two-thirds attended college), and high cognitive reserve may have protected against cognitive decline in both groups. It is also possible that the health education intervention provided enough cognitive and social stimulation to preserve cognitive function in the control group, Dr. Sink and her associates said.
The second report was an ancillary study of AREDS2 (Age-Related Eye Disease Study 2), a randomized clinical trial that assessed various dietary supplements’ effect on age-related macular degeneration and cataracts. This trial’s median 5-year follow-up of older patients (mean age, 73 years) gave researchers a chance to examine any possible cognitive benefits of treatment with omega-3 long-chain polyunsaturated fatty acids – docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and/or lutein/zeaxanthin, said Dr. Emily Y. Chew of the division of epidemiology and clinical applications at the National Eye Institute and National Institutes of Health, and her associates (JAMA 2015;314[8]:791-801).
The 3,073 study participants were assessed using eight tests of cognitive function after first “passing” a hearing handicap inventory, a depression scale, and the Telephone Interview of Cognitive Status to assure their functional status. The eight tests examined immediate and delayed recall, language, executive function, word fluency, memory, attention, and processing speed.
At 5-year follow-up, there were no significant differences between the two study groups in either a global assessment of cognitive function or in any of the individual component assessments. The yearly change in scores on the composite measure was –0.19 with supplements and –0.18 without supplements, a nonsignificant difference on their scale from –22 to +17, Dr. Chew and her associates said.
It is not yet known why abundant observational data support the use of these supplements to improve cognitive function but most randomized clinical trials, like this one, fail to show such beneficial effects. “It is possible that these supplements were started too late in the aging process” to exert an effect, or that a 5-year duration of treatment was insufficient, they noted.
These two high-quality, well-designed randomized clinical trials failed to demonstrate significant cognitive benefits with either physical activity or omega-3 supplements, but that shouldn’t lead to nihilism regarding lifestyle factors in older adults. There is abundant, clear evidence that both physical activity and a healthy diet improve a wide variety of health outcomes.
An active lifestyle throughout the lifespan would probably be more effective in preventing cognitive decline than [would] starting limited physical activity after the onset of aging. Similarly, adherence to a healthy diet throughout life would probably be more effective than initiating isolated nutritional supplements late in life.
Dr. Sudeep S. Gill is in the department of medicine and Dallas P. Seitz, Ph.D., is in the department of psychiatry at Queen’s University, Kingston (Ont.). Dr. Gill reported having no relevant financial disclosures and Dr. Seitz reported receiving an advisory board honorarium from Eli Lilly. Dr. Gill and Dr. Seitz made these remarks in an editorial accompanying the two reports on lifestyle interventions and cognitive health (JAMA 2015;314[8]:774-5).
These two high-quality, well-designed randomized clinical trials failed to demonstrate significant cognitive benefits with either physical activity or omega-3 supplements, but that shouldn’t lead to nihilism regarding lifestyle factors in older adults. There is abundant, clear evidence that both physical activity and a healthy diet improve a wide variety of health outcomes.
An active lifestyle throughout the lifespan would probably be more effective in preventing cognitive decline than [would] starting limited physical activity after the onset of aging. Similarly, adherence to a healthy diet throughout life would probably be more effective than initiating isolated nutritional supplements late in life.
Dr. Sudeep S. Gill is in the department of medicine and Dallas P. Seitz, Ph.D., is in the department of psychiatry at Queen’s University, Kingston (Ont.). Dr. Gill reported having no relevant financial disclosures and Dr. Seitz reported receiving an advisory board honorarium from Eli Lilly. Dr. Gill and Dr. Seitz made these remarks in an editorial accompanying the two reports on lifestyle interventions and cognitive health (JAMA 2015;314[8]:774-5).
These two high-quality, well-designed randomized clinical trials failed to demonstrate significant cognitive benefits with either physical activity or omega-3 supplements, but that shouldn’t lead to nihilism regarding lifestyle factors in older adults. There is abundant, clear evidence that both physical activity and a healthy diet improve a wide variety of health outcomes.
An active lifestyle throughout the lifespan would probably be more effective in preventing cognitive decline than [would] starting limited physical activity after the onset of aging. Similarly, adherence to a healthy diet throughout life would probably be more effective than initiating isolated nutritional supplements late in life.
Dr. Sudeep S. Gill is in the department of medicine and Dallas P. Seitz, Ph.D., is in the department of psychiatry at Queen’s University, Kingston (Ont.). Dr. Gill reported having no relevant financial disclosures and Dr. Seitz reported receiving an advisory board honorarium from Eli Lilly. Dr. Gill and Dr. Seitz made these remarks in an editorial accompanying the two reports on lifestyle interventions and cognitive health (JAMA 2015;314[8]:774-5).
Neither a physical activity program nor omega-3 long-chain polyunsaturated fatty acid supplements improved cognitive function in two separate studies of high-risk elderly patients reported online Aug. 25 in JAMA.
Both studies were secondary analyses of large randomized clinical trials. Their findings contradict the results of many epidemiologic and observational studies attesting to the cognitive benefits of both lifestyle interventions.
The first study involved 1,635 sedentary men and women aged 70-89 years who had lower-extremity functional limitations and were participating in the LIFE (Lifestyle Interventions and Independence for Elders) trial at eight U.S. medical centers. These participants were randomly assigned to either a physical activity intervention (818 study subjects) or a health education program (817 control subjects) and were assessed with a comprehensive battery of neuropsychological tests every 6 months for 2 years, according to Dr. Kaycee M. Sink of the Sticht Center on Aging at Wake Forest University, Winston-Salem, N.C., and her associates.
The intervention comprised two weekly clinic visits plus three to four weekly home sessions focused on strength, flexibility, and balance training, as well as walking. The control situation consisted of weekly 60- to 90-minute workshops on topics such as travel safety, preventive services, legal and financial issues, and nutrition. As expected, the intervention group achieved a higher level of moderate to vigorous physical activity throughout follow-up (mean increase of 130.4 minutes/week), compared with the control group (mean increase of 30.5 minutes/week).
However, after 2 years, there were no significant differences between the two groups in either global cognitive scores or in individual scores on numerous measures of psychomotor speed, attention, concentration, working memory, word list learning, word recall, visuospatial function, figural memory, language, or executive function. There also were no differences in the rates of mild cognitive impairment, dementia, or both combined: 13.2% of the intervention group and 12.1% of the control group developed MCI or dementia by 2 years, a nonsignificant difference, the investigators said (JAMA. 2015;314[8]:781-90).
It is possible that the level of physical activity in this intervention may not have been sufficient to produce changes in cognitive measures, or that cognitive function improved in the short term but dissipated by the end of the second year of follow-up. Alternatively, the study population on the whole was well educated (more than two-thirds attended college), and high cognitive reserve may have protected against cognitive decline in both groups. It is also possible that the health education intervention provided enough cognitive and social stimulation to preserve cognitive function in the control group, Dr. Sink and her associates said.
The second report was an ancillary study of AREDS2 (Age-Related Eye Disease Study 2), a randomized clinical trial that assessed various dietary supplements’ effect on age-related macular degeneration and cataracts. This trial’s median 5-year follow-up of older patients (mean age, 73 years) gave researchers a chance to examine any possible cognitive benefits of treatment with omega-3 long-chain polyunsaturated fatty acids – docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and/or lutein/zeaxanthin, said Dr. Emily Y. Chew of the division of epidemiology and clinical applications at the National Eye Institute and National Institutes of Health, and her associates (JAMA 2015;314[8]:791-801).
The 3,073 study participants were assessed using eight tests of cognitive function after first “passing” a hearing handicap inventory, a depression scale, and the Telephone Interview of Cognitive Status to assure their functional status. The eight tests examined immediate and delayed recall, language, executive function, word fluency, memory, attention, and processing speed.
At 5-year follow-up, there were no significant differences between the two study groups in either a global assessment of cognitive function or in any of the individual component assessments. The yearly change in scores on the composite measure was –0.19 with supplements and –0.18 without supplements, a nonsignificant difference on their scale from –22 to +17, Dr. Chew and her associates said.
It is not yet known why abundant observational data support the use of these supplements to improve cognitive function but most randomized clinical trials, like this one, fail to show such beneficial effects. “It is possible that these supplements were started too late in the aging process” to exert an effect, or that a 5-year duration of treatment was insufficient, they noted.
Neither a physical activity program nor omega-3 long-chain polyunsaturated fatty acid supplements improved cognitive function in two separate studies of high-risk elderly patients reported online Aug. 25 in JAMA.
Both studies were secondary analyses of large randomized clinical trials. Their findings contradict the results of many epidemiologic and observational studies attesting to the cognitive benefits of both lifestyle interventions.
The first study involved 1,635 sedentary men and women aged 70-89 years who had lower-extremity functional limitations and were participating in the LIFE (Lifestyle Interventions and Independence for Elders) trial at eight U.S. medical centers. These participants were randomly assigned to either a physical activity intervention (818 study subjects) or a health education program (817 control subjects) and were assessed with a comprehensive battery of neuropsychological tests every 6 months for 2 years, according to Dr. Kaycee M. Sink of the Sticht Center on Aging at Wake Forest University, Winston-Salem, N.C., and her associates.
The intervention comprised two weekly clinic visits plus three to four weekly home sessions focused on strength, flexibility, and balance training, as well as walking. The control situation consisted of weekly 60- to 90-minute workshops on topics such as travel safety, preventive services, legal and financial issues, and nutrition. As expected, the intervention group achieved a higher level of moderate to vigorous physical activity throughout follow-up (mean increase of 130.4 minutes/week), compared with the control group (mean increase of 30.5 minutes/week).
However, after 2 years, there were no significant differences between the two groups in either global cognitive scores or in individual scores on numerous measures of psychomotor speed, attention, concentration, working memory, word list learning, word recall, visuospatial function, figural memory, language, or executive function. There also were no differences in the rates of mild cognitive impairment, dementia, or both combined: 13.2% of the intervention group and 12.1% of the control group developed MCI or dementia by 2 years, a nonsignificant difference, the investigators said (JAMA. 2015;314[8]:781-90).
It is possible that the level of physical activity in this intervention may not have been sufficient to produce changes in cognitive measures, or that cognitive function improved in the short term but dissipated by the end of the second year of follow-up. Alternatively, the study population on the whole was well educated (more than two-thirds attended college), and high cognitive reserve may have protected against cognitive decline in both groups. It is also possible that the health education intervention provided enough cognitive and social stimulation to preserve cognitive function in the control group, Dr. Sink and her associates said.
The second report was an ancillary study of AREDS2 (Age-Related Eye Disease Study 2), a randomized clinical trial that assessed various dietary supplements’ effect on age-related macular degeneration and cataracts. This trial’s median 5-year follow-up of older patients (mean age, 73 years) gave researchers a chance to examine any possible cognitive benefits of treatment with omega-3 long-chain polyunsaturated fatty acids – docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and/or lutein/zeaxanthin, said Dr. Emily Y. Chew of the division of epidemiology and clinical applications at the National Eye Institute and National Institutes of Health, and her associates (JAMA 2015;314[8]:791-801).
The 3,073 study participants were assessed using eight tests of cognitive function after first “passing” a hearing handicap inventory, a depression scale, and the Telephone Interview of Cognitive Status to assure their functional status. The eight tests examined immediate and delayed recall, language, executive function, word fluency, memory, attention, and processing speed.
At 5-year follow-up, there were no significant differences between the two study groups in either a global assessment of cognitive function or in any of the individual component assessments. The yearly change in scores on the composite measure was –0.19 with supplements and –0.18 without supplements, a nonsignificant difference on their scale from –22 to +17, Dr. Chew and her associates said.
It is not yet known why abundant observational data support the use of these supplements to improve cognitive function but most randomized clinical trials, like this one, fail to show such beneficial effects. “It is possible that these supplements were started too late in the aging process” to exert an effect, or that a 5-year duration of treatment was insufficient, they noted.
FROM JAMA