User login
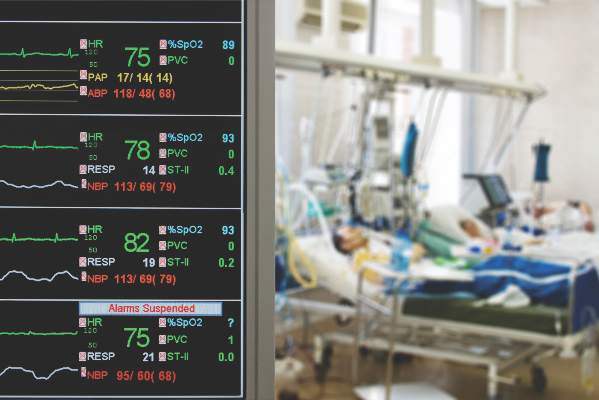
ICU care improves survival without raising costs
Compared with care on a general hospital ward, ICU care improved survival without raising costs significantly in a study of more than 1 million Medicare patients hospitalized with pneumonia, published online Sept. 22 in JAMA.
The retrospective cohort study involved older patients whose condition was considered “borderline” – not one that would clearly benefit from ICU admission but also not one for which ICU admission could clearly be ruled out. The decision of whether to admit these study participants to a general ward or an ICU was deemed discretionary. “Contrary to [our] prespecified hypothesis, [our] findings suggest that ICU admission for borderline patients … is associated with reduced mortality without a considerable increase in costs,” said Dr. Thomas S. Valley of the division of pulmonary and critical care medicine, University of Michigan, Ann Arbor, and his associates.
The investigators analyzed data from the American Hospital Association’s annual surveys and the Healthcare Cost Reporting Information Systems regarding 1,327,370 Medicare patients admitted to 2,988 hospitals across the country during a recent 2-year period. A total of 328,404 patients (29.5% of the study population) were admitted to ICUs and the remainder to general hospital wards.
After the data were adjusted to account for numerous patient, disease, and hospital variables, ICU admission was associated with significantly lower 30-day mortality (14.8%), compared with general ward admission (20.5%) – an absolute reduction of 5.7%. Yet the differences between the two groups were nonsignificant regarding payments by Medicare ($9,918 for ICU vs. $11,238 for general ward care) and hospital costs ($14,162 for ICU vs $11,320 for general ward care).
These findings were consistent across numerous sensitivity analyses, including some that compared urban against rural hospitals, white against nonwhite patients, small against large ICUs, and severely ill against less severely ill patients, Dr. Valley and his associates said (JAMA. 2015 Sep 22;314[12]:1272-79. doi: 10.1001.jama.2015.11068).
There are several reasons why ICU care might be beneficial for “borderline” patients with pneumonia. Greater attention from nurses and other clinicians could allow for more timely recognition of decompensation; more aggressive care is more likely to head off the development of sepsis; better adherence to guideline-based treatment is known to improve mortality; and a greater likelihood of being managed by a pulmonary or critical care specialist with greater expertise in pneumonia care should improve outcomes, the researchers noted.
Their study findings have important implications for health care reform. “In order to contain U.S. health care costs, it has been suggested that reducing critical care bed supply would result in more efficient admission decisions and cost savings with minimal mortality decrements.” This “presumes that ICU admission for discretionary patients provides minimal benefit but substantially increases costs.” The results of this study clearly refute that assumption, Dr. Valley and his associates said.
This study provides important empirical evidence that ICU admission can benefit “low-risk” patients. It demonstrates that the value of intensive care extends beyond mere life support for patients with an acutely failing organ and instead includes all the organizational and human resources that comprise an ICU.
It would be tempting to use these results to justify more liberal ICU admission, but that would be untenable in this era of constrained health care resources. Rather than increasing ICU use, we should make general wards function more like ICUs. The task at hand is to study why intensive care saves lives, then use that information to make hospital care safe and effective for all patients, regardless of where in the hospital they are cared for.
Dr. Ian J. Barbash is in the division of pulmonary, allergy, and critical care medicine at the University of Pittsburgh. Dr. Jeremy M. Kahn is in the department of health policy and management at the university’s Graduate School of Public Health. Both Dr. Barbash and Dr. Kahn are also at the university’s Clinical Research, Investigation, and Systems Modeling of Acute Illness Center. Both authors reported having no relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Valley’s report (JAMA. 2015;314:1240-41. doi: 10.1001/jama.2015.11171).
This study provides important empirical evidence that ICU admission can benefit “low-risk” patients. It demonstrates that the value of intensive care extends beyond mere life support for patients with an acutely failing organ and instead includes all the organizational and human resources that comprise an ICU.
It would be tempting to use these results to justify more liberal ICU admission, but that would be untenable in this era of constrained health care resources. Rather than increasing ICU use, we should make general wards function more like ICUs. The task at hand is to study why intensive care saves lives, then use that information to make hospital care safe and effective for all patients, regardless of where in the hospital they are cared for.
Dr. Ian J. Barbash is in the division of pulmonary, allergy, and critical care medicine at the University of Pittsburgh. Dr. Jeremy M. Kahn is in the department of health policy and management at the university’s Graduate School of Public Health. Both Dr. Barbash and Dr. Kahn are also at the university’s Clinical Research, Investigation, and Systems Modeling of Acute Illness Center. Both authors reported having no relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Valley’s report (JAMA. 2015;314:1240-41. doi: 10.1001/jama.2015.11171).
This study provides important empirical evidence that ICU admission can benefit “low-risk” patients. It demonstrates that the value of intensive care extends beyond mere life support for patients with an acutely failing organ and instead includes all the organizational and human resources that comprise an ICU.
It would be tempting to use these results to justify more liberal ICU admission, but that would be untenable in this era of constrained health care resources. Rather than increasing ICU use, we should make general wards function more like ICUs. The task at hand is to study why intensive care saves lives, then use that information to make hospital care safe and effective for all patients, regardless of where in the hospital they are cared for.
Dr. Ian J. Barbash is in the division of pulmonary, allergy, and critical care medicine at the University of Pittsburgh. Dr. Jeremy M. Kahn is in the department of health policy and management at the university’s Graduate School of Public Health. Both Dr. Barbash and Dr. Kahn are also at the university’s Clinical Research, Investigation, and Systems Modeling of Acute Illness Center. Both authors reported having no relevant financial disclosures. They made these remarks in an editorial accompanying Dr. Valley’s report (JAMA. 2015;314:1240-41. doi: 10.1001/jama.2015.11171).
Compared with care on a general hospital ward, ICU care improved survival without raising costs significantly in a study of more than 1 million Medicare patients hospitalized with pneumonia, published online Sept. 22 in JAMA.
The retrospective cohort study involved older patients whose condition was considered “borderline” – not one that would clearly benefit from ICU admission but also not one for which ICU admission could clearly be ruled out. The decision of whether to admit these study participants to a general ward or an ICU was deemed discretionary. “Contrary to [our] prespecified hypothesis, [our] findings suggest that ICU admission for borderline patients … is associated with reduced mortality without a considerable increase in costs,” said Dr. Thomas S. Valley of the division of pulmonary and critical care medicine, University of Michigan, Ann Arbor, and his associates.
The investigators analyzed data from the American Hospital Association’s annual surveys and the Healthcare Cost Reporting Information Systems regarding 1,327,370 Medicare patients admitted to 2,988 hospitals across the country during a recent 2-year period. A total of 328,404 patients (29.5% of the study population) were admitted to ICUs and the remainder to general hospital wards.
After the data were adjusted to account for numerous patient, disease, and hospital variables, ICU admission was associated with significantly lower 30-day mortality (14.8%), compared with general ward admission (20.5%) – an absolute reduction of 5.7%. Yet the differences between the two groups were nonsignificant regarding payments by Medicare ($9,918 for ICU vs. $11,238 for general ward care) and hospital costs ($14,162 for ICU vs $11,320 for general ward care).
These findings were consistent across numerous sensitivity analyses, including some that compared urban against rural hospitals, white against nonwhite patients, small against large ICUs, and severely ill against less severely ill patients, Dr. Valley and his associates said (JAMA. 2015 Sep 22;314[12]:1272-79. doi: 10.1001.jama.2015.11068).
There are several reasons why ICU care might be beneficial for “borderline” patients with pneumonia. Greater attention from nurses and other clinicians could allow for more timely recognition of decompensation; more aggressive care is more likely to head off the development of sepsis; better adherence to guideline-based treatment is known to improve mortality; and a greater likelihood of being managed by a pulmonary or critical care specialist with greater expertise in pneumonia care should improve outcomes, the researchers noted.
Their study findings have important implications for health care reform. “In order to contain U.S. health care costs, it has been suggested that reducing critical care bed supply would result in more efficient admission decisions and cost savings with minimal mortality decrements.” This “presumes that ICU admission for discretionary patients provides minimal benefit but substantially increases costs.” The results of this study clearly refute that assumption, Dr. Valley and his associates said.
Compared with care on a general hospital ward, ICU care improved survival without raising costs significantly in a study of more than 1 million Medicare patients hospitalized with pneumonia, published online Sept. 22 in JAMA.
The retrospective cohort study involved older patients whose condition was considered “borderline” – not one that would clearly benefit from ICU admission but also not one for which ICU admission could clearly be ruled out. The decision of whether to admit these study participants to a general ward or an ICU was deemed discretionary. “Contrary to [our] prespecified hypothesis, [our] findings suggest that ICU admission for borderline patients … is associated with reduced mortality without a considerable increase in costs,” said Dr. Thomas S. Valley of the division of pulmonary and critical care medicine, University of Michigan, Ann Arbor, and his associates.
The investigators analyzed data from the American Hospital Association’s annual surveys and the Healthcare Cost Reporting Information Systems regarding 1,327,370 Medicare patients admitted to 2,988 hospitals across the country during a recent 2-year period. A total of 328,404 patients (29.5% of the study population) were admitted to ICUs and the remainder to general hospital wards.
After the data were adjusted to account for numerous patient, disease, and hospital variables, ICU admission was associated with significantly lower 30-day mortality (14.8%), compared with general ward admission (20.5%) – an absolute reduction of 5.7%. Yet the differences between the two groups were nonsignificant regarding payments by Medicare ($9,918 for ICU vs. $11,238 for general ward care) and hospital costs ($14,162 for ICU vs $11,320 for general ward care).
These findings were consistent across numerous sensitivity analyses, including some that compared urban against rural hospitals, white against nonwhite patients, small against large ICUs, and severely ill against less severely ill patients, Dr. Valley and his associates said (JAMA. 2015 Sep 22;314[12]:1272-79. doi: 10.1001.jama.2015.11068).
There are several reasons why ICU care might be beneficial for “borderline” patients with pneumonia. Greater attention from nurses and other clinicians could allow for more timely recognition of decompensation; more aggressive care is more likely to head off the development of sepsis; better adherence to guideline-based treatment is known to improve mortality; and a greater likelihood of being managed by a pulmonary or critical care specialist with greater expertise in pneumonia care should improve outcomes, the researchers noted.
Their study findings have important implications for health care reform. “In order to contain U.S. health care costs, it has been suggested that reducing critical care bed supply would result in more efficient admission decisions and cost savings with minimal mortality decrements.” This “presumes that ICU admission for discretionary patients provides minimal benefit but substantially increases costs.” The results of this study clearly refute that assumption, Dr. Valley and his associates said.
FROM JAMA
Key clinical point: ICU care improved survival without raising costs in older patients with pneumonia, compared with care on a general hospital ward.
Major finding: ICU admission was associated with significantly lower 30-day mortality (14.8%), compared with general ward admission (20.5%).
Data source: A retrospective cohort study involving 1,112,394 Medicare patients treated for pneumonia at 2,988 U.S. hospitals during a 2-year period.
Disclosures: This study was supported by the National Institutes of Health, the Department of Veterans Affairs Health Services Research and Development Service, and the Agency for Healthcare Research and Quality. Dr. Valley and his associates reported having no relevant financial disclosures.
Prostate cancer: Men with comorbidity may be better off with no ADT
Forgoing androgen deprivation therapy cuts rather than raises overall and cardiac mortality in certain patients with prostate cancer, depending on their comorbidities, according to a Research Letter to the Editor published online Sept. 22 in JAMA.
Currently, 6 months of androgen deprivation therapy (ADT) plus radiotherapy is the standard treatment for unfavorable-risk localized prostate cancer. However, concerns have been raised as to whether men with moderate to severe comorbidity show a survival benefit with the added ADT, and even whether the treatment potentially raises their cardiac risk, said Dr. Anthony V. D’Amico of the department of radiation oncology, Brigham and Women’s Hospital, Boston, and his associates.
To examine this issue, the investigators analyzed survival outcomes among 206 men treated for unfavorable-risk prostate cancer at three academic and three community-based Massachusetts medical centers in 1995-2001. A total of 157 men had no or minimal comorbidity, while the remaining 49 had moderate or severe comorbidity. These study participants were randomly assigned to receive radiotherapy alone (104 patients) or radiotherapy plus ADT (102 patients).
After a median follow-up of 16.6 years, 156 of the men (76%) had died. Twenty-nine died of prostate cancer (19%), 39 of cardiac causes (25%), and 88 of other causes (56%). Survival did not differ between the group given radiotherapy alone and the group given additive ADT. However, survival did differ according to comorbidity profiles, the investigators said (JAMA. 2015 Sep 22;314:1291-3). http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.8577.
Among men with no or minimal comorbidity, adding ADT to radiotherapy actually increased overall mortality (hazard ratio, 1.51) and prostate cancer mortality (HR, 4.30), had no effect on cardiac mortality (HR, 1.72), and decreased mortality from other causes (HR, 0.60). Conversely, among men with moderate or severe comorbidity, forgoing rather than receiving additive ADT decreased overall mortality (HR, 0.36) and cardiac mortality (HR, 0.17), had no effect on prostate cancer mortality (HR, 2.41), and increased mortality from other causes (HR, 2.79). These findings indicate that adding ADT to radiotherapy “should be carefully considered” in patients who have moderate or severe comorbidity, Dr. D’Amico and his associates said.
Forgoing androgen deprivation therapy cuts rather than raises overall and cardiac mortality in certain patients with prostate cancer, depending on their comorbidities, according to a Research Letter to the Editor published online Sept. 22 in JAMA.
Currently, 6 months of androgen deprivation therapy (ADT) plus radiotherapy is the standard treatment for unfavorable-risk localized prostate cancer. However, concerns have been raised as to whether men with moderate to severe comorbidity show a survival benefit with the added ADT, and even whether the treatment potentially raises their cardiac risk, said Dr. Anthony V. D’Amico of the department of radiation oncology, Brigham and Women’s Hospital, Boston, and his associates.
To examine this issue, the investigators analyzed survival outcomes among 206 men treated for unfavorable-risk prostate cancer at three academic and three community-based Massachusetts medical centers in 1995-2001. A total of 157 men had no or minimal comorbidity, while the remaining 49 had moderate or severe comorbidity. These study participants were randomly assigned to receive radiotherapy alone (104 patients) or radiotherapy plus ADT (102 patients).
After a median follow-up of 16.6 years, 156 of the men (76%) had died. Twenty-nine died of prostate cancer (19%), 39 of cardiac causes (25%), and 88 of other causes (56%). Survival did not differ between the group given radiotherapy alone and the group given additive ADT. However, survival did differ according to comorbidity profiles, the investigators said (JAMA. 2015 Sep 22;314:1291-3). http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.8577.
Among men with no or minimal comorbidity, adding ADT to radiotherapy actually increased overall mortality (hazard ratio, 1.51) and prostate cancer mortality (HR, 4.30), had no effect on cardiac mortality (HR, 1.72), and decreased mortality from other causes (HR, 0.60). Conversely, among men with moderate or severe comorbidity, forgoing rather than receiving additive ADT decreased overall mortality (HR, 0.36) and cardiac mortality (HR, 0.17), had no effect on prostate cancer mortality (HR, 2.41), and increased mortality from other causes (HR, 2.79). These findings indicate that adding ADT to radiotherapy “should be carefully considered” in patients who have moderate or severe comorbidity, Dr. D’Amico and his associates said.
Forgoing androgen deprivation therapy cuts rather than raises overall and cardiac mortality in certain patients with prostate cancer, depending on their comorbidities, according to a Research Letter to the Editor published online Sept. 22 in JAMA.
Currently, 6 months of androgen deprivation therapy (ADT) plus radiotherapy is the standard treatment for unfavorable-risk localized prostate cancer. However, concerns have been raised as to whether men with moderate to severe comorbidity show a survival benefit with the added ADT, and even whether the treatment potentially raises their cardiac risk, said Dr. Anthony V. D’Amico of the department of radiation oncology, Brigham and Women’s Hospital, Boston, and his associates.
To examine this issue, the investigators analyzed survival outcomes among 206 men treated for unfavorable-risk prostate cancer at three academic and three community-based Massachusetts medical centers in 1995-2001. A total of 157 men had no or minimal comorbidity, while the remaining 49 had moderate or severe comorbidity. These study participants were randomly assigned to receive radiotherapy alone (104 patients) or radiotherapy plus ADT (102 patients).
After a median follow-up of 16.6 years, 156 of the men (76%) had died. Twenty-nine died of prostate cancer (19%), 39 of cardiac causes (25%), and 88 of other causes (56%). Survival did not differ between the group given radiotherapy alone and the group given additive ADT. However, survival did differ according to comorbidity profiles, the investigators said (JAMA. 2015 Sep 22;314:1291-3). http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.8577.
Among men with no or minimal comorbidity, adding ADT to radiotherapy actually increased overall mortality (hazard ratio, 1.51) and prostate cancer mortality (HR, 4.30), had no effect on cardiac mortality (HR, 1.72), and decreased mortality from other causes (HR, 0.60). Conversely, among men with moderate or severe comorbidity, forgoing rather than receiving additive ADT decreased overall mortality (HR, 0.36) and cardiac mortality (HR, 0.17), had no effect on prostate cancer mortality (HR, 2.41), and increased mortality from other causes (HR, 2.79). These findings indicate that adding ADT to radiotherapy “should be carefully considered” in patients who have moderate or severe comorbidity, Dr. D’Amico and his associates said.
FROM JAMA
Key clinical point: Forgoing androgen deprivation therapy cuts rather than raises overall and cardiac mortality in certain patients with prostate cancer, according to their comorbidities.
Major finding: Among men with moderate or severe comorbidity, forgoing rather than receiving additive ADT decreased overall mortality (HR, 0.36) and cardiac mortality (HR, 0.17), had no effect on prostate cancer mortality (HR, 2.41), and increased mortality from other causes (HR, 2.79).
Data source: A multicenter randomized cohort study involving 206 men with unfavorable-risk prostate cancer followed for a median of 16 years.
Disclosures: This financial supporter(s) of this study were not identified. Dr. D’Amico and his associates reported having no relevant financial disclosures.
Pregnancy complications predict mother’s premature CVD death
Certain combinations of complications during pregnancy raise the mother’s risk of premature cardiovascular disease–related death decades later by as much as sevenfold, according to a report published online Sept. 21 in Circulation.
These findings from a large observational cohort study with 50 years of follow-up indicate that the body’s extreme response to the challenges of pregnancy either expose or initiate an underlying vulnerability to CVD, researchers said.
Previously, a few individual complications of pregnancy have been linked to premature CVD death in the mother, but this is the first study to identify two unsuspected complications – glycosuria and an abnormal decline in hemoglobin – and to identify particular combinations of complications that are especially high risk. Now that this link is established, clinicians can identify patients who develop these complications in current pregnancies or experienced them in past pregnancies and target them for early CVD prevention, said Piera M. Cirillo, staff scientist, and Barbara A. Cohn, Ph.D., director of Child Health and Development Studies, Public Health Institute, Berkeley, Calif.
Given the substantial challenge pregnancy presents to a woman’s cardiovascular system – demanding a doubling of blood volume, increased cardiac output, marked elevation of coagulation factors, changes in blood pressure, temporary hyperlipidemia, increased insulin resistance, and the temporary accumulation of visceral fat – “pregnancy is really a stress test for the cardiovascular system,” Dr. Cohn said in a statement accompanying the report.
Dr. Cohn and Ms. Cirillo analyzed the records of 14,062 healthy women (67% white, 23% black, 3% Latina, 4% Asian, and 3% other races) enrolled in a study of prenatal health when they were pregnant in 1959-1967, following them up for CVD death in 2011. These study participants were a median of 26 years old at enrollment and a median of 66 years at follow-up. The complications of interest were early preeclampsia, late preeclampsia, preexisting hypertension, gestational hypertension, glycosuria (a surrogate for gestational diabetes, which wasn’t routinely assessed at the time of the original study), abnormal hemoglobin changes during pregnancy, hemorrhage, delivery of a small-for-gestational-age (SGA) baby, and irregular menses preceding pregnancy (a surrogate for polycystic ovarian syndrome).
A total of 9,059 mothers (64%) had none of these complications, 4,293 (31%) had a single complication, and 710 (5%) had at least two complications. There were 368 CVD deaths, which occurred at a median age of 66 years, the investigators said (Circulation. 2015 Sep 21. doi: 10.1161/circulationaha.113.003901).
The presence of one or more complications raised the risk of premature CVD death by roughly twofold to as high as sevenfold, depending on the complication. Preeclampsia, and particularly early-onset preeclampsia, was the strongest predictor of CVD death before age 60 years, followed by preexisting hypertension and the presence of glycosuria. Preterm and SGA delivery and abnormal decline in hemoglobin were less strong but still significant predictors. Hemorrhage and irregular menses showed no association with premature CVD death.
These risk factors were specific to CVD death and showed no association with all-cause mortality or cancer-related mortality, the investigators noted. Mothers who had both preexisting hypertension and a preterm delivery were at 7-fold-higher risk of premature CVD death, those who had preexisting hypertension plus preeclampsia were at 5.6-fold higher risk, those with preexisting hypertension plus an SGA baby were at 4.8-fold higher risk, and those with gestational hypertension plus preterm delivery were at fivefold higher risk.
The investigators discovered that an abnormal decline in hemoglobin during pregnancy also predicted premature CVD death. Most mothers show a steady decline in hemoglobin, beginning in early pregnancy and continuing through week 32. At 32-36 weeks hemoglobin increases, then it declines again until week 38, when it stabilizes until delivery at a level slightly below that seen in early pregnancy. The abnormal decline was clearly distinct from this established pattern, with higher-than-normal hemoglobin early in the second trimester and lower-than-normal levels in the third.
Early high hemoglobin is known to be a marker for poor fetal growth, placental infarction, and high blood viscosity, while later low hemoglobin “may indicate a failure to produce red cells in sufficient volume to ‘catch up’ to plasma volume increases, or could indicate a defect that results in late increases in blood volume. Thus, abnormal hemoglobin decline may be an early indicator of the lack of an adaptive response that ultimately results in increased CVD in these women,” Ms. Cirillo and Dr. Cohn said.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Ms. Cirillo and Dr. Cohn reported having no relevant financial disclosures.
Certain combinations of complications during pregnancy raise the mother’s risk of premature cardiovascular disease–related death decades later by as much as sevenfold, according to a report published online Sept. 21 in Circulation.
These findings from a large observational cohort study with 50 years of follow-up indicate that the body’s extreme response to the challenges of pregnancy either expose or initiate an underlying vulnerability to CVD, researchers said.
Previously, a few individual complications of pregnancy have been linked to premature CVD death in the mother, but this is the first study to identify two unsuspected complications – glycosuria and an abnormal decline in hemoglobin – and to identify particular combinations of complications that are especially high risk. Now that this link is established, clinicians can identify patients who develop these complications in current pregnancies or experienced them in past pregnancies and target them for early CVD prevention, said Piera M. Cirillo, staff scientist, and Barbara A. Cohn, Ph.D., director of Child Health and Development Studies, Public Health Institute, Berkeley, Calif.
Given the substantial challenge pregnancy presents to a woman’s cardiovascular system – demanding a doubling of blood volume, increased cardiac output, marked elevation of coagulation factors, changes in blood pressure, temporary hyperlipidemia, increased insulin resistance, and the temporary accumulation of visceral fat – “pregnancy is really a stress test for the cardiovascular system,” Dr. Cohn said in a statement accompanying the report.
Dr. Cohn and Ms. Cirillo analyzed the records of 14,062 healthy women (67% white, 23% black, 3% Latina, 4% Asian, and 3% other races) enrolled in a study of prenatal health when they were pregnant in 1959-1967, following them up for CVD death in 2011. These study participants were a median of 26 years old at enrollment and a median of 66 years at follow-up. The complications of interest were early preeclampsia, late preeclampsia, preexisting hypertension, gestational hypertension, glycosuria (a surrogate for gestational diabetes, which wasn’t routinely assessed at the time of the original study), abnormal hemoglobin changes during pregnancy, hemorrhage, delivery of a small-for-gestational-age (SGA) baby, and irregular menses preceding pregnancy (a surrogate for polycystic ovarian syndrome).
A total of 9,059 mothers (64%) had none of these complications, 4,293 (31%) had a single complication, and 710 (5%) had at least two complications. There were 368 CVD deaths, which occurred at a median age of 66 years, the investigators said (Circulation. 2015 Sep 21. doi: 10.1161/circulationaha.113.003901).
The presence of one or more complications raised the risk of premature CVD death by roughly twofold to as high as sevenfold, depending on the complication. Preeclampsia, and particularly early-onset preeclampsia, was the strongest predictor of CVD death before age 60 years, followed by preexisting hypertension and the presence of glycosuria. Preterm and SGA delivery and abnormal decline in hemoglobin were less strong but still significant predictors. Hemorrhage and irregular menses showed no association with premature CVD death.
These risk factors were specific to CVD death and showed no association with all-cause mortality or cancer-related mortality, the investigators noted. Mothers who had both preexisting hypertension and a preterm delivery were at 7-fold-higher risk of premature CVD death, those who had preexisting hypertension plus preeclampsia were at 5.6-fold higher risk, those with preexisting hypertension plus an SGA baby were at 4.8-fold higher risk, and those with gestational hypertension plus preterm delivery were at fivefold higher risk.
The investigators discovered that an abnormal decline in hemoglobin during pregnancy also predicted premature CVD death. Most mothers show a steady decline in hemoglobin, beginning in early pregnancy and continuing through week 32. At 32-36 weeks hemoglobin increases, then it declines again until week 38, when it stabilizes until delivery at a level slightly below that seen in early pregnancy. The abnormal decline was clearly distinct from this established pattern, with higher-than-normal hemoglobin early in the second trimester and lower-than-normal levels in the third.
Early high hemoglobin is known to be a marker for poor fetal growth, placental infarction, and high blood viscosity, while later low hemoglobin “may indicate a failure to produce red cells in sufficient volume to ‘catch up’ to plasma volume increases, or could indicate a defect that results in late increases in blood volume. Thus, abnormal hemoglobin decline may be an early indicator of the lack of an adaptive response that ultimately results in increased CVD in these women,” Ms. Cirillo and Dr. Cohn said.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Ms. Cirillo and Dr. Cohn reported having no relevant financial disclosures.
Certain combinations of complications during pregnancy raise the mother’s risk of premature cardiovascular disease–related death decades later by as much as sevenfold, according to a report published online Sept. 21 in Circulation.
These findings from a large observational cohort study with 50 years of follow-up indicate that the body’s extreme response to the challenges of pregnancy either expose or initiate an underlying vulnerability to CVD, researchers said.
Previously, a few individual complications of pregnancy have been linked to premature CVD death in the mother, but this is the first study to identify two unsuspected complications – glycosuria and an abnormal decline in hemoglobin – and to identify particular combinations of complications that are especially high risk. Now that this link is established, clinicians can identify patients who develop these complications in current pregnancies or experienced them in past pregnancies and target them for early CVD prevention, said Piera M. Cirillo, staff scientist, and Barbara A. Cohn, Ph.D., director of Child Health and Development Studies, Public Health Institute, Berkeley, Calif.
Given the substantial challenge pregnancy presents to a woman’s cardiovascular system – demanding a doubling of blood volume, increased cardiac output, marked elevation of coagulation factors, changes in blood pressure, temporary hyperlipidemia, increased insulin resistance, and the temporary accumulation of visceral fat – “pregnancy is really a stress test for the cardiovascular system,” Dr. Cohn said in a statement accompanying the report.
Dr. Cohn and Ms. Cirillo analyzed the records of 14,062 healthy women (67% white, 23% black, 3% Latina, 4% Asian, and 3% other races) enrolled in a study of prenatal health when they were pregnant in 1959-1967, following them up for CVD death in 2011. These study participants were a median of 26 years old at enrollment and a median of 66 years at follow-up. The complications of interest were early preeclampsia, late preeclampsia, preexisting hypertension, gestational hypertension, glycosuria (a surrogate for gestational diabetes, which wasn’t routinely assessed at the time of the original study), abnormal hemoglobin changes during pregnancy, hemorrhage, delivery of a small-for-gestational-age (SGA) baby, and irregular menses preceding pregnancy (a surrogate for polycystic ovarian syndrome).
A total of 9,059 mothers (64%) had none of these complications, 4,293 (31%) had a single complication, and 710 (5%) had at least two complications. There were 368 CVD deaths, which occurred at a median age of 66 years, the investigators said (Circulation. 2015 Sep 21. doi: 10.1161/circulationaha.113.003901).
The presence of one or more complications raised the risk of premature CVD death by roughly twofold to as high as sevenfold, depending on the complication. Preeclampsia, and particularly early-onset preeclampsia, was the strongest predictor of CVD death before age 60 years, followed by preexisting hypertension and the presence of glycosuria. Preterm and SGA delivery and abnormal decline in hemoglobin were less strong but still significant predictors. Hemorrhage and irregular menses showed no association with premature CVD death.
These risk factors were specific to CVD death and showed no association with all-cause mortality or cancer-related mortality, the investigators noted. Mothers who had both preexisting hypertension and a preterm delivery were at 7-fold-higher risk of premature CVD death, those who had preexisting hypertension plus preeclampsia were at 5.6-fold higher risk, those with preexisting hypertension plus an SGA baby were at 4.8-fold higher risk, and those with gestational hypertension plus preterm delivery were at fivefold higher risk.
The investigators discovered that an abnormal decline in hemoglobin during pregnancy also predicted premature CVD death. Most mothers show a steady decline in hemoglobin, beginning in early pregnancy and continuing through week 32. At 32-36 weeks hemoglobin increases, then it declines again until week 38, when it stabilizes until delivery at a level slightly below that seen in early pregnancy. The abnormal decline was clearly distinct from this established pattern, with higher-than-normal hemoglobin early in the second trimester and lower-than-normal levels in the third.
Early high hemoglobin is known to be a marker for poor fetal growth, placental infarction, and high blood viscosity, while later low hemoglobin “may indicate a failure to produce red cells in sufficient volume to ‘catch up’ to plasma volume increases, or could indicate a defect that results in late increases in blood volume. Thus, abnormal hemoglobin decline may be an early indicator of the lack of an adaptive response that ultimately results in increased CVD in these women,” Ms. Cirillo and Dr. Cohn said.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Ms. Cirillo and Dr. Cohn reported having no relevant financial disclosures.
FROM CIRCULATION
Key clinical point: Certain pregnancy complications predict premature CVD death in the mother.
Major finding: Mothers who had both preexisting hypertension and a preterm delivery were at sevenfold-higher risk of premature CVD death.
Data source: An observational cohort study in 14,062 California women who were pregnant in 1959-1967 and were followed up for CVD death in 2011.
Disclosures: The Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the study. Ms. Cirillo and Dr. Cohn reported having no relevant financial disclosures.
Trabectedin superior for advanced liposarcoma, leiomyosarcoma
Trabectedin proved superior to standard dacarbazine therapy by numerous measures but not by overall survival in an industry-sponsored phase III clinical trial reported online Sept. 14 in Journal of Clinical Oncology.
Trabectedin, which has been used extensively in Europe for a decade but has not been approved in the U.S., has a complex mechanism of action that affects several critical cell biology processes within and surrounding tumor cells. It exhibited activity against metastatic soft tissue sarcomas in several phase II trials, said Dr. George D. Demetri of the Ludwig Center at Harvard Medical School and the Center for Sarcoma and Bone Oncology, Dana-Farber Cancer Institute, both in Boston, and his associates.
In this study, trabectedin was assessed in 518 patients aged 15 years and older who had heavily pretreated and rapidly progressing advanced or metastatic liposarcoma or leiomyosarcoma and were treated at 85 sites in four countries. These participants were randomly assigned to receive either trabectedin (345 patients) or dacarbazine (173 patients) via central intravenous infusion in 3-week cycles.
Compared with dacarbazine, trabectedin reduced the risk of disease progression by 45% (hazard ratio, 0.55), and superior disease control was discernible at the first patient assessment at 6 weeks. Median progression-free survival was significantly longer with trabectedin (4.2 months) than with dacarbazine (1.5 months), a benefit that was consistent across all 19 subgroups of patients assessed in sensitivity analyses, regardless of disease histology, previous therapies, or any clinical characteristics. Trabectedin also bested dacarbazine with regard to objective response rate (9.9% vs. 6.9%), median duration of response (6.5 months vs. 4.2 months), achievement of stable disease (51% vs. 35%), and achievement of durable stable disease (34% vs. 19%).
Trabectedin showed only a nonsignificant 13% reduction in overall survival, which was the primary endpoint of this study. However, several previous studies have demonstrated that it can be extremely difficult to prolong overall survival despite robust improvements in progression-free survival in patients with advanced sarcomas. Given this “historical difficulty in demonstrating overall survival improvement,” the documentation of disease control such as that achieved in this study may be considered a measure of clinically relevant efficacy in this setting, Dr. Demetri and his associates wrote (J. Clin. Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4734]).
Adverse events in this study population “were consistent with the well-characterized safety and toxicity profiles of both study drugs.” Toxicity was more common with trabectedin, and deaths considered to be treatment-related occurred only in the trabectedin group: three cases of sepsis/septic shock and one each of rhabdomyolysis/sepsis, renal failure, cardiac arrest, and multiorgan failure, for a treatment-related mortality of 2.1%.
This study confirms what we originally heard a decade ago from European investigators: Trabectedin shows clinical activity against liposarcomas and leiomyosarcomas. But the benefits demonstrated here still seem small, and it is not yet clear whether the data are now sufficient to make trabectedin the standard of care for all patients who have these sarcomas.
The primary end point – improving overall survival to a greater degree than dacarbazine – was not met. However, in the investigators’ defense, this was partly because the dacarbazine group survived much longer than expected. This, in turn, may be because more patients who had disease progression with dacarbazine (56%) than with trabectedin (47%) crossed over to other anticancer drugs, including the receptor tyrosine kinase inhibitor pazopanib, which was approved during the course of this study.
Dr. Gary K. Schwartz is at the Herbert Irving Comprehensive Cancer Center and Columbia University, New York. He reported ties to Novartis, AstraZeneca, and Boehringer Ingelheim. Dr. Schwartz made these remarks in an editorial accompanying Dr. Demetri’s report (J. Clin. Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.63.5938]).
This study confirms what we originally heard a decade ago from European investigators: Trabectedin shows clinical activity against liposarcomas and leiomyosarcomas. But the benefits demonstrated here still seem small, and it is not yet clear whether the data are now sufficient to make trabectedin the standard of care for all patients who have these sarcomas.
The primary end point – improving overall survival to a greater degree than dacarbazine – was not met. However, in the investigators’ defense, this was partly because the dacarbazine group survived much longer than expected. This, in turn, may be because more patients who had disease progression with dacarbazine (56%) than with trabectedin (47%) crossed over to other anticancer drugs, including the receptor tyrosine kinase inhibitor pazopanib, which was approved during the course of this study.
Dr. Gary K. Schwartz is at the Herbert Irving Comprehensive Cancer Center and Columbia University, New York. He reported ties to Novartis, AstraZeneca, and Boehringer Ingelheim. Dr. Schwartz made these remarks in an editorial accompanying Dr. Demetri’s report (J. Clin. Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.63.5938]).
This study confirms what we originally heard a decade ago from European investigators: Trabectedin shows clinical activity against liposarcomas and leiomyosarcomas. But the benefits demonstrated here still seem small, and it is not yet clear whether the data are now sufficient to make trabectedin the standard of care for all patients who have these sarcomas.
The primary end point – improving overall survival to a greater degree than dacarbazine – was not met. However, in the investigators’ defense, this was partly because the dacarbazine group survived much longer than expected. This, in turn, may be because more patients who had disease progression with dacarbazine (56%) than with trabectedin (47%) crossed over to other anticancer drugs, including the receptor tyrosine kinase inhibitor pazopanib, which was approved during the course of this study.
Dr. Gary K. Schwartz is at the Herbert Irving Comprehensive Cancer Center and Columbia University, New York. He reported ties to Novartis, AstraZeneca, and Boehringer Ingelheim. Dr. Schwartz made these remarks in an editorial accompanying Dr. Demetri’s report (J. Clin. Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.63.5938]).
Trabectedin proved superior to standard dacarbazine therapy by numerous measures but not by overall survival in an industry-sponsored phase III clinical trial reported online Sept. 14 in Journal of Clinical Oncology.
Trabectedin, which has been used extensively in Europe for a decade but has not been approved in the U.S., has a complex mechanism of action that affects several critical cell biology processes within and surrounding tumor cells. It exhibited activity against metastatic soft tissue sarcomas in several phase II trials, said Dr. George D. Demetri of the Ludwig Center at Harvard Medical School and the Center for Sarcoma and Bone Oncology, Dana-Farber Cancer Institute, both in Boston, and his associates.
In this study, trabectedin was assessed in 518 patients aged 15 years and older who had heavily pretreated and rapidly progressing advanced or metastatic liposarcoma or leiomyosarcoma and were treated at 85 sites in four countries. These participants were randomly assigned to receive either trabectedin (345 patients) or dacarbazine (173 patients) via central intravenous infusion in 3-week cycles.
Compared with dacarbazine, trabectedin reduced the risk of disease progression by 45% (hazard ratio, 0.55), and superior disease control was discernible at the first patient assessment at 6 weeks. Median progression-free survival was significantly longer with trabectedin (4.2 months) than with dacarbazine (1.5 months), a benefit that was consistent across all 19 subgroups of patients assessed in sensitivity analyses, regardless of disease histology, previous therapies, or any clinical characteristics. Trabectedin also bested dacarbazine with regard to objective response rate (9.9% vs. 6.9%), median duration of response (6.5 months vs. 4.2 months), achievement of stable disease (51% vs. 35%), and achievement of durable stable disease (34% vs. 19%).
Trabectedin showed only a nonsignificant 13% reduction in overall survival, which was the primary endpoint of this study. However, several previous studies have demonstrated that it can be extremely difficult to prolong overall survival despite robust improvements in progression-free survival in patients with advanced sarcomas. Given this “historical difficulty in demonstrating overall survival improvement,” the documentation of disease control such as that achieved in this study may be considered a measure of clinically relevant efficacy in this setting, Dr. Demetri and his associates wrote (J. Clin. Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4734]).
Adverse events in this study population “were consistent with the well-characterized safety and toxicity profiles of both study drugs.” Toxicity was more common with trabectedin, and deaths considered to be treatment-related occurred only in the trabectedin group: three cases of sepsis/septic shock and one each of rhabdomyolysis/sepsis, renal failure, cardiac arrest, and multiorgan failure, for a treatment-related mortality of 2.1%.
Trabectedin proved superior to standard dacarbazine therapy by numerous measures but not by overall survival in an industry-sponsored phase III clinical trial reported online Sept. 14 in Journal of Clinical Oncology.
Trabectedin, which has been used extensively in Europe for a decade but has not been approved in the U.S., has a complex mechanism of action that affects several critical cell biology processes within and surrounding tumor cells. It exhibited activity against metastatic soft tissue sarcomas in several phase II trials, said Dr. George D. Demetri of the Ludwig Center at Harvard Medical School and the Center for Sarcoma and Bone Oncology, Dana-Farber Cancer Institute, both in Boston, and his associates.
In this study, trabectedin was assessed in 518 patients aged 15 years and older who had heavily pretreated and rapidly progressing advanced or metastatic liposarcoma or leiomyosarcoma and were treated at 85 sites in four countries. These participants were randomly assigned to receive either trabectedin (345 patients) or dacarbazine (173 patients) via central intravenous infusion in 3-week cycles.
Compared with dacarbazine, trabectedin reduced the risk of disease progression by 45% (hazard ratio, 0.55), and superior disease control was discernible at the first patient assessment at 6 weeks. Median progression-free survival was significantly longer with trabectedin (4.2 months) than with dacarbazine (1.5 months), a benefit that was consistent across all 19 subgroups of patients assessed in sensitivity analyses, regardless of disease histology, previous therapies, or any clinical characteristics. Trabectedin also bested dacarbazine with regard to objective response rate (9.9% vs. 6.9%), median duration of response (6.5 months vs. 4.2 months), achievement of stable disease (51% vs. 35%), and achievement of durable stable disease (34% vs. 19%).
Trabectedin showed only a nonsignificant 13% reduction in overall survival, which was the primary endpoint of this study. However, several previous studies have demonstrated that it can be extremely difficult to prolong overall survival despite robust improvements in progression-free survival in patients with advanced sarcomas. Given this “historical difficulty in demonstrating overall survival improvement,” the documentation of disease control such as that achieved in this study may be considered a measure of clinically relevant efficacy in this setting, Dr. Demetri and his associates wrote (J. Clin. Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4734]).
Adverse events in this study population “were consistent with the well-characterized safety and toxicity profiles of both study drugs.” Toxicity was more common with trabectedin, and deaths considered to be treatment-related occurred only in the trabectedin group: three cases of sepsis/septic shock and one each of rhabdomyolysis/sepsis, renal failure, cardiac arrest, and multiorgan failure, for a treatment-related mortality of 2.1%.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Trabectedin was superior to standard dacarbazine by most measures in advanced, refractory liposarcoma and leiomyosarcoma, though not for overall survival.
Major finding: Trabectedin reduced the risk of disease progression by 45% (HR, 0.55).
Data source: An international industry-sponsored open-label randomized phase III clinical trial involving 518 patients.
Disclosures: This study was supported by Janssen Pharmaceuticals and the Adelson Medical Research Foundation. Dr. Demetri and his associates reported ties to numerous industry sources.
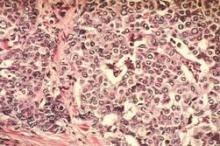
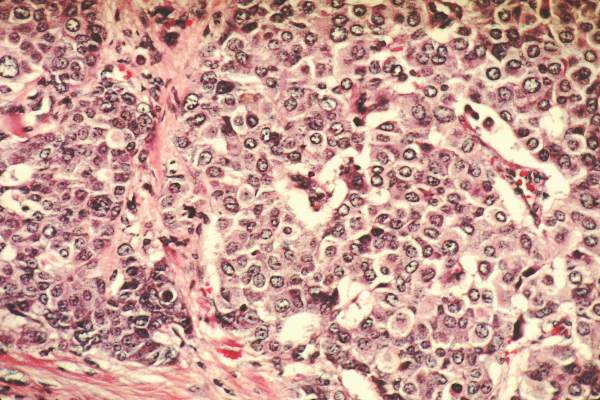
Excellent survival after ASCT for light-chain amyloidosis
Early mortality after autologous hematopoietic stem cell transplantation (ASCT) for light-chain amyloidosis has declined dramatically in recent years, and 5-year survival is now deemed “excellent” at 77%, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Light-chain amyloidosis results in deposition of insoluble amyloid fibers in many tissues, particularly the heart and kidneys, and can lead to organ failure and death. While medical therapies target the plasma cell clone that is the source of the amyloid, treatments have minimal effect on amyloid that has already accumulated in tissues. Alternatively, ASCT can produce durable hematologic responses and has resulted in improved function of affected organs, based on several single-center studies.
However, the only large, prospective randomized clinical trial to compare ASCT and medical therapy was done in 2007. That study showed autotransplantation carried a high (24%) early mortality, which contributed heavily to inferior overall survival, said Dr. Anita D’Souza of the division of hematology and oncology, Medical College of Wisconsin, Milwaukee, and her associates.
Since that study, supportive care during the peritransplantation period has greatly improved. Large U.S. transplant centers now report that early mortality is less than 5%.
To assess time trends in autotransplantation mortality, Dr. D’Souza and her associates analyzed data from the Center for International Blood & Marrow Transplant Research, a registry that collects transplant data from 320 centers worldwide and captures information concerning most U.S. procedures. They focused on 1,536 North American patients with light-chain amyloidosis who underwent ASCT during three successive 5-year periods: 1995-2000, 2001-2006, and 2007-2012. The median follow-up was 56 months.
Over time, 30-day mortality declined from 11% to 5% to 3%, respectively; and 100-day mortality declined from 20% to 11% to 5%. One-year overall survival rose from 75% to 85% to 90%, and 5-year overall survival improved from 55% to 61% to 77%.
Even among patients with renal amyloidosis, 3-year overall survival improved over time from 78% during the earliest study period to 89% during the most recent time period. However, mortality did not improve significantly over time among patients with cardiac involvement, which continues to be the single most-important variable associated with poor outcomes, the researchers said (J Clin Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4015]).
A transplant center’s experience with this procedure proved to be of crucial importance to early patient mortality. Centers that performed a low volume of ASCT for light-chain amyloidosis, defined as fewer than four transplants per year, had a 30-day mortality of 5% and a 100-day mortality of 7%, while centers that performed more than four procedures per year had significantly lower mortalities of 1% and 3%, respectively. There were no significant differences between low- and high-volume centers regarding patient, organ, or medication factors, which “led us to believe that high-volume centers do not necessarily select fitter patients for transplantation. Rather, they may be more experienced in supporting and treating these patients in the early posttransplantation period,” Dr. D’Souza and her associates said.
These “reassuring” findings, together with the recent development of novel plasma-cell–targeting agents such as bortezomib, suggest that it is time to consider performing a more current multicenter prospective study comparing autotransplantation against medical therapy, they added.
It is important to place these study findings in context, and note that approximately 20% of patients seen at most transplant centers are considered good candidates for autologous hematopoietic cell transplantation. The outcomes of the procedure remain unsatisfactory for most patients.
Delayed diagnosis appears to be a major obstacle to effective treatment. Many patients experience significant organ dysfunction before a diagnosis is established, even when the underlying plasma cell dyscrasia is being actively followed by a hematologist.
Dr. Noffar Bar, Dr. Terri L. Parker, and Dr. Madhav V. Dhodapkar are at Yale Cancer Center, New Haven Conn. They made these remarks in an editorial accompanying Dr. D’Souza’s report (J Clin Oncol. Sep 14 [doi:10.1200/HCO.2015.63.2224]). Their financial disclosures are available at www.jco.org.
It is important to place these study findings in context, and note that approximately 20% of patients seen at most transplant centers are considered good candidates for autologous hematopoietic cell transplantation. The outcomes of the procedure remain unsatisfactory for most patients.
Delayed diagnosis appears to be a major obstacle to effective treatment. Many patients experience significant organ dysfunction before a diagnosis is established, even when the underlying plasma cell dyscrasia is being actively followed by a hematologist.
Dr. Noffar Bar, Dr. Terri L. Parker, and Dr. Madhav V. Dhodapkar are at Yale Cancer Center, New Haven Conn. They made these remarks in an editorial accompanying Dr. D’Souza’s report (J Clin Oncol. Sep 14 [doi:10.1200/HCO.2015.63.2224]). Their financial disclosures are available at www.jco.org.
It is important to place these study findings in context, and note that approximately 20% of patients seen at most transplant centers are considered good candidates for autologous hematopoietic cell transplantation. The outcomes of the procedure remain unsatisfactory for most patients.
Delayed diagnosis appears to be a major obstacle to effective treatment. Many patients experience significant organ dysfunction before a diagnosis is established, even when the underlying plasma cell dyscrasia is being actively followed by a hematologist.
Dr. Noffar Bar, Dr. Terri L. Parker, and Dr. Madhav V. Dhodapkar are at Yale Cancer Center, New Haven Conn. They made these remarks in an editorial accompanying Dr. D’Souza’s report (J Clin Oncol. Sep 14 [doi:10.1200/HCO.2015.63.2224]). Their financial disclosures are available at www.jco.org.
Early mortality after autologous hematopoietic stem cell transplantation (ASCT) for light-chain amyloidosis has declined dramatically in recent years, and 5-year survival is now deemed “excellent” at 77%, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Light-chain amyloidosis results in deposition of insoluble amyloid fibers in many tissues, particularly the heart and kidneys, and can lead to organ failure and death. While medical therapies target the plasma cell clone that is the source of the amyloid, treatments have minimal effect on amyloid that has already accumulated in tissues. Alternatively, ASCT can produce durable hematologic responses and has resulted in improved function of affected organs, based on several single-center studies.
However, the only large, prospective randomized clinical trial to compare ASCT and medical therapy was done in 2007. That study showed autotransplantation carried a high (24%) early mortality, which contributed heavily to inferior overall survival, said Dr. Anita D’Souza of the division of hematology and oncology, Medical College of Wisconsin, Milwaukee, and her associates.
Since that study, supportive care during the peritransplantation period has greatly improved. Large U.S. transplant centers now report that early mortality is less than 5%.
To assess time trends in autotransplantation mortality, Dr. D’Souza and her associates analyzed data from the Center for International Blood & Marrow Transplant Research, a registry that collects transplant data from 320 centers worldwide and captures information concerning most U.S. procedures. They focused on 1,536 North American patients with light-chain amyloidosis who underwent ASCT during three successive 5-year periods: 1995-2000, 2001-2006, and 2007-2012. The median follow-up was 56 months.
Over time, 30-day mortality declined from 11% to 5% to 3%, respectively; and 100-day mortality declined from 20% to 11% to 5%. One-year overall survival rose from 75% to 85% to 90%, and 5-year overall survival improved from 55% to 61% to 77%.
Even among patients with renal amyloidosis, 3-year overall survival improved over time from 78% during the earliest study period to 89% during the most recent time period. However, mortality did not improve significantly over time among patients with cardiac involvement, which continues to be the single most-important variable associated with poor outcomes, the researchers said (J Clin Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4015]).
A transplant center’s experience with this procedure proved to be of crucial importance to early patient mortality. Centers that performed a low volume of ASCT for light-chain amyloidosis, defined as fewer than four transplants per year, had a 30-day mortality of 5% and a 100-day mortality of 7%, while centers that performed more than four procedures per year had significantly lower mortalities of 1% and 3%, respectively. There were no significant differences between low- and high-volume centers regarding patient, organ, or medication factors, which “led us to believe that high-volume centers do not necessarily select fitter patients for transplantation. Rather, they may be more experienced in supporting and treating these patients in the early posttransplantation period,” Dr. D’Souza and her associates said.
These “reassuring” findings, together with the recent development of novel plasma-cell–targeting agents such as bortezomib, suggest that it is time to consider performing a more current multicenter prospective study comparing autotransplantation against medical therapy, they added.
Early mortality after autologous hematopoietic stem cell transplantation (ASCT) for light-chain amyloidosis has declined dramatically in recent years, and 5-year survival is now deemed “excellent” at 77%, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Light-chain amyloidosis results in deposition of insoluble amyloid fibers in many tissues, particularly the heart and kidneys, and can lead to organ failure and death. While medical therapies target the plasma cell clone that is the source of the amyloid, treatments have minimal effect on amyloid that has already accumulated in tissues. Alternatively, ASCT can produce durable hematologic responses and has resulted in improved function of affected organs, based on several single-center studies.
However, the only large, prospective randomized clinical trial to compare ASCT and medical therapy was done in 2007. That study showed autotransplantation carried a high (24%) early mortality, which contributed heavily to inferior overall survival, said Dr. Anita D’Souza of the division of hematology and oncology, Medical College of Wisconsin, Milwaukee, and her associates.
Since that study, supportive care during the peritransplantation period has greatly improved. Large U.S. transplant centers now report that early mortality is less than 5%.
To assess time trends in autotransplantation mortality, Dr. D’Souza and her associates analyzed data from the Center for International Blood & Marrow Transplant Research, a registry that collects transplant data from 320 centers worldwide and captures information concerning most U.S. procedures. They focused on 1,536 North American patients with light-chain amyloidosis who underwent ASCT during three successive 5-year periods: 1995-2000, 2001-2006, and 2007-2012. The median follow-up was 56 months.
Over time, 30-day mortality declined from 11% to 5% to 3%, respectively; and 100-day mortality declined from 20% to 11% to 5%. One-year overall survival rose from 75% to 85% to 90%, and 5-year overall survival improved from 55% to 61% to 77%.
Even among patients with renal amyloidosis, 3-year overall survival improved over time from 78% during the earliest study period to 89% during the most recent time period. However, mortality did not improve significantly over time among patients with cardiac involvement, which continues to be the single most-important variable associated with poor outcomes, the researchers said (J Clin Oncol. 2015 Sep 14 [doi:10.1200/JCO.2015.62.4015]).
A transplant center’s experience with this procedure proved to be of crucial importance to early patient mortality. Centers that performed a low volume of ASCT for light-chain amyloidosis, defined as fewer than four transplants per year, had a 30-day mortality of 5% and a 100-day mortality of 7%, while centers that performed more than four procedures per year had significantly lower mortalities of 1% and 3%, respectively. There were no significant differences between low- and high-volume centers regarding patient, organ, or medication factors, which “led us to believe that high-volume centers do not necessarily select fitter patients for transplantation. Rather, they may be more experienced in supporting and treating these patients in the early posttransplantation period,” Dr. D’Souza and her associates said.
These “reassuring” findings, together with the recent development of novel plasma-cell–targeting agents such as bortezomib, suggest that it is time to consider performing a more current multicenter prospective study comparing autotransplantation against medical therapy, they added.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Early mortality after autologous hematopoietic stem cell transplantation for light-chain amyloidosis has declined dramatically in recent years.
Major finding: 30-day mortality declined from 11% to 5% to 3% in three successive time periods, and 100-day mortality declined from 20% to 11% to 5%.
Data source: A retrospective international cohort study of mortality outcomes in 1,536 patients with light-chain amyloidosis treated during 1995-2012 and followed for a median of 56 months.
Disclosures: This study was supported by the National Cancer Institute; the National Heart, Lung, and Blood Institute; the Health Resources and Services Administration; the Department of the Navy, the Department of Defense, other government groups, several private organizations; and numerous industry sources. Dr. D’Souza reported having no relevant financial disclosures; her associates reported ties to numerous pharmaceutical and biomedical companies.
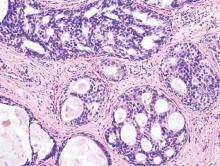
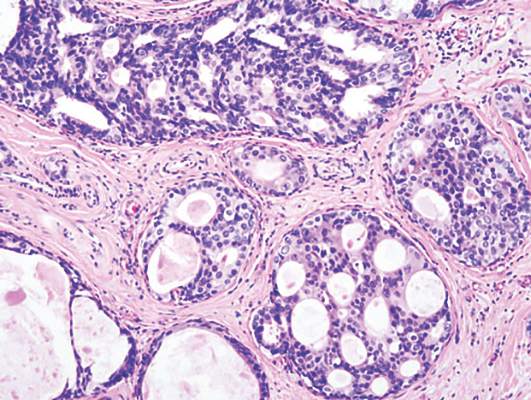
LCIS: 2% annual cancer risk, less than 1% with chemoprevention
Women with lobular carcinoma in situ (LCIS) showed a 2% annual risk of developing breast cancer, and women who chose chemoprevention reduced their annual cancer rate to less than 1%, in a single-center longitudinal study published online in Journal of Clinical Oncology.
LCIS is known to raise the risk of developing ductal carcinoma in situ or invasive carcinoma substantially, but estimates vary widely. In addition, no specific risk factors have been linked to disease progression. To determine the absolute risk of developing breast cancer over time and to explore the clinicopathological features that predispose patients to progression, researchers performed a longitudinal analysis of data concerning 1,060 patients diagnosed between 1980 and 2009 at Memorial Sloan Kettering Cancer Center, New York.
Most patients (831, 78%) chose to manage their condition with surveillance alone, 175 (17%) chose surveillance plus chemoprevention using a selective estrogen receptor modulator or an aromatase inhibitor, and the remaining 56 patients (5%) opted for bilateral prophylactic mastectomy. Overall, 150 of these women developed 168 breast cancers during a median follow-up of 81 months (range, 6-368 months), said Dr. Tari A. King and her associates at Memorial Sloan Kettering.
The annual incidence of breast cancer was 2% per year for the entire study population, with no indication that risk would plateau. Overall cumulative cancer incidence was 26%, similar to that reported in a National Surgical Adjuvant Breast and Bowel Project study. The use of chemoprevention was strongly associated with a reduction in breast cancer risk (HR, 0.27); the minority of women who chose this option reduced their annual cancer rate to less than 1%. This finding supports the current recommendation to use chemoprevention and highlights the significant impact of proper patient education and counseling, the researchers noted (J Clin Oncol. 2015 Sep 14. doi:10.1200/JCO.2015.61.4743).
Unexpectedly, none of the clinicopathological features commonly thought to influence cancer risk – age at diagnosis, family history, breast density, menopausal status, presence of synchronous atypical hyperplasia, or presence of bilateral LCIS – were risk factors. Only tumor volume, as measured by the ratio of the number of slides with LCIS to the total number of slides available, was significantly greater in women whose disease progressed (0.5 to 1) than in women whose disease did not (0.3 to 1).
The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
Women with lobular carcinoma in situ (LCIS) showed a 2% annual risk of developing breast cancer, and women who chose chemoprevention reduced their annual cancer rate to less than 1%, in a single-center longitudinal study published online in Journal of Clinical Oncology.
LCIS is known to raise the risk of developing ductal carcinoma in situ or invasive carcinoma substantially, but estimates vary widely. In addition, no specific risk factors have been linked to disease progression. To determine the absolute risk of developing breast cancer over time and to explore the clinicopathological features that predispose patients to progression, researchers performed a longitudinal analysis of data concerning 1,060 patients diagnosed between 1980 and 2009 at Memorial Sloan Kettering Cancer Center, New York.
Most patients (831, 78%) chose to manage their condition with surveillance alone, 175 (17%) chose surveillance plus chemoprevention using a selective estrogen receptor modulator or an aromatase inhibitor, and the remaining 56 patients (5%) opted for bilateral prophylactic mastectomy. Overall, 150 of these women developed 168 breast cancers during a median follow-up of 81 months (range, 6-368 months), said Dr. Tari A. King and her associates at Memorial Sloan Kettering.
The annual incidence of breast cancer was 2% per year for the entire study population, with no indication that risk would plateau. Overall cumulative cancer incidence was 26%, similar to that reported in a National Surgical Adjuvant Breast and Bowel Project study. The use of chemoprevention was strongly associated with a reduction in breast cancer risk (HR, 0.27); the minority of women who chose this option reduced their annual cancer rate to less than 1%. This finding supports the current recommendation to use chemoprevention and highlights the significant impact of proper patient education and counseling, the researchers noted (J Clin Oncol. 2015 Sep 14. doi:10.1200/JCO.2015.61.4743).
Unexpectedly, none of the clinicopathological features commonly thought to influence cancer risk – age at diagnosis, family history, breast density, menopausal status, presence of synchronous atypical hyperplasia, or presence of bilateral LCIS – were risk factors. Only tumor volume, as measured by the ratio of the number of slides with LCIS to the total number of slides available, was significantly greater in women whose disease progressed (0.5 to 1) than in women whose disease did not (0.3 to 1).
The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
Women with lobular carcinoma in situ (LCIS) showed a 2% annual risk of developing breast cancer, and women who chose chemoprevention reduced their annual cancer rate to less than 1%, in a single-center longitudinal study published online in Journal of Clinical Oncology.
LCIS is known to raise the risk of developing ductal carcinoma in situ or invasive carcinoma substantially, but estimates vary widely. In addition, no specific risk factors have been linked to disease progression. To determine the absolute risk of developing breast cancer over time and to explore the clinicopathological features that predispose patients to progression, researchers performed a longitudinal analysis of data concerning 1,060 patients diagnosed between 1980 and 2009 at Memorial Sloan Kettering Cancer Center, New York.
Most patients (831, 78%) chose to manage their condition with surveillance alone, 175 (17%) chose surveillance plus chemoprevention using a selective estrogen receptor modulator or an aromatase inhibitor, and the remaining 56 patients (5%) opted for bilateral prophylactic mastectomy. Overall, 150 of these women developed 168 breast cancers during a median follow-up of 81 months (range, 6-368 months), said Dr. Tari A. King and her associates at Memorial Sloan Kettering.
The annual incidence of breast cancer was 2% per year for the entire study population, with no indication that risk would plateau. Overall cumulative cancer incidence was 26%, similar to that reported in a National Surgical Adjuvant Breast and Bowel Project study. The use of chemoprevention was strongly associated with a reduction in breast cancer risk (HR, 0.27); the minority of women who chose this option reduced their annual cancer rate to less than 1%. This finding supports the current recommendation to use chemoprevention and highlights the significant impact of proper patient education and counseling, the researchers noted (J Clin Oncol. 2015 Sep 14. doi:10.1200/JCO.2015.61.4743).
Unexpectedly, none of the clinicopathological features commonly thought to influence cancer risk – age at diagnosis, family history, breast density, menopausal status, presence of synchronous atypical hyperplasia, or presence of bilateral LCIS – were risk factors. Only tumor volume, as measured by the ratio of the number of slides with LCIS to the total number of slides available, was significantly greater in women whose disease progressed (0.5 to 1) than in women whose disease did not (0.3 to 1).
The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Women with lobular carcinoma in situ showed a 2% annual risk of developing breast cancer and a cumulative risk of 26% at 15 years, and chemoprevention lowers cancer risk.
Major finding: Chemoprevention reduced breast cancer risk (HR, 0.27), so that women who chose this option reduced their annual cancer rate to less than 1%.
Data source: A single-center longitudinal cohort study involving 1,060 patients diagnosed during 1980-2009 and followed for a median of 81 months.
Disclosures: The National Cancer Institute, the Walsh Family Fund, and the Cary Grossman Breast Research Fellowship supported the study. Dr. King reported having no conflicts of interest; one of her associates reported receiving honoraria from Genomic Health.
More genomic instability found in breast tumors of black women
Several differences between black and white women in the genotypic traits of their breast tumors have been identified and may explain, at least in part, the greater aggressiveness of breast cancer in black women, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Researchers analyzed data from The Cancer Genome Atlas in what they described as the first study to systematically characterize the racial pattern of genomic and gene expression traits in primary breast tumors and to examine the association of these traits with tumor recurrence. They focused on 1,374 samples from white and 264 samples from black patients with stage I, II, or III breast cancer diagnosed in 1988-2013, reported Dr. Tanya Keenan of Massachusetts General Hospital and Harvard Medical School, Boston, and her associates.
The five most frequent mutations—in the TP53, PIK3CA, CDH1, GATA3, MLLT3, and MAP3K1 genes – were the same between black women and white women, but they occurred in different frequencies by race. Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology. These differences also “might have implications as genotype-driven targeted therapies are developed,” the investigators said.
In addition, the breast cancers in black women had significantly greater intratumor genetic heterogeneity, which “may reflect either greater underlying genomic instability or more exposure to epigenetic or environmental agents of DNA damage. Either way, the greater genomic diversity within African American tumors suggests a greater capacity for clonal evolution that may contribute to aggressive or therapy-resistant disease,” they noted (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.62.2126).
Black women had a higher risk of breast cancer recurrence than white women, which was greatly attenuated in multivariable analyses that controlled for their excess of TP53 mutations and PAM50 basal subtypes. This also suggests that differences in tumor genetics account, at least in part, for the known disparity in survival outcomes between the races, Dr. Keenan and her associates said.
Several differences between black and white women in the genotypic traits of their breast tumors have been identified and may explain, at least in part, the greater aggressiveness of breast cancer in black women, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Researchers analyzed data from The Cancer Genome Atlas in what they described as the first study to systematically characterize the racial pattern of genomic and gene expression traits in primary breast tumors and to examine the association of these traits with tumor recurrence. They focused on 1,374 samples from white and 264 samples from black patients with stage I, II, or III breast cancer diagnosed in 1988-2013, reported Dr. Tanya Keenan of Massachusetts General Hospital and Harvard Medical School, Boston, and her associates.
The five most frequent mutations—in the TP53, PIK3CA, CDH1, GATA3, MLLT3, and MAP3K1 genes – were the same between black women and white women, but they occurred in different frequencies by race. Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology. These differences also “might have implications as genotype-driven targeted therapies are developed,” the investigators said.
In addition, the breast cancers in black women had significantly greater intratumor genetic heterogeneity, which “may reflect either greater underlying genomic instability or more exposure to epigenetic or environmental agents of DNA damage. Either way, the greater genomic diversity within African American tumors suggests a greater capacity for clonal evolution that may contribute to aggressive or therapy-resistant disease,” they noted (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.62.2126).
Black women had a higher risk of breast cancer recurrence than white women, which was greatly attenuated in multivariable analyses that controlled for their excess of TP53 mutations and PAM50 basal subtypes. This also suggests that differences in tumor genetics account, at least in part, for the known disparity in survival outcomes between the races, Dr. Keenan and her associates said.
Several differences between black and white women in the genotypic traits of their breast tumors have been identified and may explain, at least in part, the greater aggressiveness of breast cancer in black women, according to a report published online Sept. 14 in Journal of Clinical Oncology.
Researchers analyzed data from The Cancer Genome Atlas in what they described as the first study to systematically characterize the racial pattern of genomic and gene expression traits in primary breast tumors and to examine the association of these traits with tumor recurrence. They focused on 1,374 samples from white and 264 samples from black patients with stage I, II, or III breast cancer diagnosed in 1988-2013, reported Dr. Tanya Keenan of Massachusetts General Hospital and Harvard Medical School, Boston, and her associates.
The five most frequent mutations—in the TP53, PIK3CA, CDH1, GATA3, MLLT3, and MAP3K1 genes – were the same between black women and white women, but they occurred in different frequencies by race. Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology. These differences also “might have implications as genotype-driven targeted therapies are developed,” the investigators said.
In addition, the breast cancers in black women had significantly greater intratumor genetic heterogeneity, which “may reflect either greater underlying genomic instability or more exposure to epigenetic or environmental agents of DNA damage. Either way, the greater genomic diversity within African American tumors suggests a greater capacity for clonal evolution that may contribute to aggressive or therapy-resistant disease,” they noted (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.62.2126).
Black women had a higher risk of breast cancer recurrence than white women, which was greatly attenuated in multivariable analyses that controlled for their excess of TP53 mutations and PAM50 basal subtypes. This also suggests that differences in tumor genetics account, at least in part, for the known disparity in survival outcomes between the races, Dr. Keenan and her associates said.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Several differences in breast tumor genetics may explain the greater aggressiveness of breast cancer in black vs. white women.
Major finding: Compared with white women, black women had significantly more TP53 mutations, more PAM50 basal tumors, and more TNBC basal-like 1 and mesenchymal stem–like tumors, all of which signal a more aggressive tumor biology.
Data source: An analysis of tumor-specific genotypic traits in 1,374 breast cancer samples from white and 264 samples from black patients, collected in The Cancer Genome Atlas.
Disclosures: The sponsor of this study was not identified. Dr. Keenan reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
Fulvestrant may best anastrozole as first-line therapy
Fulvestrant may extend survival longer than does anastrozole when used as first-line therapy for locally advanced or metastatic estrogen receptor–positive breast cancer, investigators reported online Sept. 14 in the Journal of Clinical Oncology.
Compared with anastrozole, fulvestrant reduced overall mortality risk by approximately 30% in a small international, open-label phase II clinical trial funded by the manufacturer. A double-blind phase III trial is now underway to confirm and extend these preliminary results, said Dr. Matthew J. Ellis of the Lester and Sue Smith Breast Center, Baylor University, Houston, and his associates.
In the phase II study, 102 postmenopausal women were randomly assigned to receive 500 mg fulvestrant and 103 to receive 1 mg anastrozole as first-line therapy at 62 medical centers in 9 countries. Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (hazard ratio, 0.70). This difference persisted across all subgroup analyses and in a sensitivity analysis, the investigators said (J Clin Oncol. 2015 Sept. 14. doi: 10.1200/JCO.2015.61.5831).
No new safety or tolerability problems occurred. Two serious adverse events that were considered to be treatment related developed in the fulvestrant group: one case of hypertension and one case of pulmonary embolism.
It is relatively uncommon in countries with advanced health care for patients to present with advanced breast cancer that has never been treated with endocrine therapy. Given the high prevalence of the disease, however, this still “represents a numerically substantial patient population” in the West, and it continues to comprise a significant proportion of patients in developing countries, Dr. Ellis and his associates added.
This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
Fulvestrant may extend survival longer than does anastrozole when used as first-line therapy for locally advanced or metastatic estrogen receptor–positive breast cancer, investigators reported online Sept. 14 in the Journal of Clinical Oncology.
Compared with anastrozole, fulvestrant reduced overall mortality risk by approximately 30% in a small international, open-label phase II clinical trial funded by the manufacturer. A double-blind phase III trial is now underway to confirm and extend these preliminary results, said Dr. Matthew J. Ellis of the Lester and Sue Smith Breast Center, Baylor University, Houston, and his associates.
In the phase II study, 102 postmenopausal women were randomly assigned to receive 500 mg fulvestrant and 103 to receive 1 mg anastrozole as first-line therapy at 62 medical centers in 9 countries. Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (hazard ratio, 0.70). This difference persisted across all subgroup analyses and in a sensitivity analysis, the investigators said (J Clin Oncol. 2015 Sept. 14. doi: 10.1200/JCO.2015.61.5831).
No new safety or tolerability problems occurred. Two serious adverse events that were considered to be treatment related developed in the fulvestrant group: one case of hypertension and one case of pulmonary embolism.
It is relatively uncommon in countries with advanced health care for patients to present with advanced breast cancer that has never been treated with endocrine therapy. Given the high prevalence of the disease, however, this still “represents a numerically substantial patient population” in the West, and it continues to comprise a significant proportion of patients in developing countries, Dr. Ellis and his associates added.
This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
Fulvestrant may extend survival longer than does anastrozole when used as first-line therapy for locally advanced or metastatic estrogen receptor–positive breast cancer, investigators reported online Sept. 14 in the Journal of Clinical Oncology.
Compared with anastrozole, fulvestrant reduced overall mortality risk by approximately 30% in a small international, open-label phase II clinical trial funded by the manufacturer. A double-blind phase III trial is now underway to confirm and extend these preliminary results, said Dr. Matthew J. Ellis of the Lester and Sue Smith Breast Center, Baylor University, Houston, and his associates.
In the phase II study, 102 postmenopausal women were randomly assigned to receive 500 mg fulvestrant and 103 to receive 1 mg anastrozole as first-line therapy at 62 medical centers in 9 countries. Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (hazard ratio, 0.70). This difference persisted across all subgroup analyses and in a sensitivity analysis, the investigators said (J Clin Oncol. 2015 Sept. 14. doi: 10.1200/JCO.2015.61.5831).
No new safety or tolerability problems occurred. Two serious adverse events that were considered to be treatment related developed in the fulvestrant group: one case of hypertension and one case of pulmonary embolism.
It is relatively uncommon in countries with advanced health care for patients to present with advanced breast cancer that has never been treated with endocrine therapy. Given the high prevalence of the disease, however, this still “represents a numerically substantial patient population” in the West, and it continues to comprise a significant proportion of patients in developing countries, Dr. Ellis and his associates added.
This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Fulvestrant may extend survival better than anastrozole does as first-line therapy for advanced ER-positive breast cancer.
Major finding: Median overall survival was 54.1 months with fulvestrant and 48.4 months with anastrozole, an extension of approximately 6 months (HR, 0.70).
Data source: An industry-sponsored, international, randomized, open-label phase-II trial involving 205 postmenopausal women with ER-positive locally advanced or metastatic breast cancer.
Disclosures: This study was supported by AstraZeneca. Dr. Ellis reported ties to AstraZeneca, Bioclassifier, Pfizer, Novartis, and Celgene; his associates reported ties to numerous industry sources.
DCIS: Recurrence risk rises after forgoing radiotherapy
The risk of recurrence continues to rise for at least 12 years among patients with low-risk DCIS who forgo radiotherapy after undergoing lumpectomy, investigators reported online Sept. 14 in Journal of Clinical Oncology.
These investigators performed an extended follow-up of 665 patients who participated in an Eastern Cooperative Oncology Group–American College of Radiology Imaging Network nonrandomized trial in 1997-2002. All the participants had either a single low- or intermediate-grade tumor sized 2.5 cm or smaller (cohort 1, 561 patients) or a high-grade tumor 1 cm or smaller (cohort 2, 104 patients), and lumpectomy without radiotherapy was considered a reasonable treatment option for all based on favorable clinical and pathological characteristics. The median patient age was 59 years, and 30% of the study population received tamoxifen during follow-up, said Dr. Lawrence J. Solin of Albert Einstein Healthcare Network, Philadelphia, and his associates.
During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast. The 12-year rates of developing any recurrence were 14.4% in cohort 1 and 24.6% in cohort 2, and the 12-year rates of developing an invasive cancer were 7.5% and 13.4%, respectively. Most important, the risk of any recurrence increased steadily over time, without any plateau, the investigators said (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.60.8588).
“Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and whether or not to forgo adding adjuvant treatment after surgical excision,” Dr. Solin and his associates said.
Most of the study participants were postmenopausal and had small, asymptomatic microcalcifications detected on routine screening mammography; most also had negative margins at resection. Since these traits are similar to those of patients with DCIS in population-based studies, the findings of this trial are relevant to contemporary practice, the researchers added.
“Not all patients and their physicians will agree on what is considered too high a risk of developing [a recurrence] or [an invasive recurrence] to recommend observation after surgical excision, or what risk is considered too low to justify adding radiation treatment. However, this study provides 12-year data to begin those discussions, and to help inform the treatment decision-making process,” Dr. Solin and his associates said.
The risk of recurrence continues to rise for at least 12 years among patients with low-risk DCIS who forgo radiotherapy after undergoing lumpectomy, investigators reported online Sept. 14 in Journal of Clinical Oncology.
These investigators performed an extended follow-up of 665 patients who participated in an Eastern Cooperative Oncology Group–American College of Radiology Imaging Network nonrandomized trial in 1997-2002. All the participants had either a single low- or intermediate-grade tumor sized 2.5 cm or smaller (cohort 1, 561 patients) or a high-grade tumor 1 cm or smaller (cohort 2, 104 patients), and lumpectomy without radiotherapy was considered a reasonable treatment option for all based on favorable clinical and pathological characteristics. The median patient age was 59 years, and 30% of the study population received tamoxifen during follow-up, said Dr. Lawrence J. Solin of Albert Einstein Healthcare Network, Philadelphia, and his associates.
During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast. The 12-year rates of developing any recurrence were 14.4% in cohort 1 and 24.6% in cohort 2, and the 12-year rates of developing an invasive cancer were 7.5% and 13.4%, respectively. Most important, the risk of any recurrence increased steadily over time, without any plateau, the investigators said (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.60.8588).
“Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and whether or not to forgo adding adjuvant treatment after surgical excision,” Dr. Solin and his associates said.
Most of the study participants were postmenopausal and had small, asymptomatic microcalcifications detected on routine screening mammography; most also had negative margins at resection. Since these traits are similar to those of patients with DCIS in population-based studies, the findings of this trial are relevant to contemporary practice, the researchers added.
“Not all patients and their physicians will agree on what is considered too high a risk of developing [a recurrence] or [an invasive recurrence] to recommend observation after surgical excision, or what risk is considered too low to justify adding radiation treatment. However, this study provides 12-year data to begin those discussions, and to help inform the treatment decision-making process,” Dr. Solin and his associates said.
The risk of recurrence continues to rise for at least 12 years among patients with low-risk DCIS who forgo radiotherapy after undergoing lumpectomy, investigators reported online Sept. 14 in Journal of Clinical Oncology.
These investigators performed an extended follow-up of 665 patients who participated in an Eastern Cooperative Oncology Group–American College of Radiology Imaging Network nonrandomized trial in 1997-2002. All the participants had either a single low- or intermediate-grade tumor sized 2.5 cm or smaller (cohort 1, 561 patients) or a high-grade tumor 1 cm or smaller (cohort 2, 104 patients), and lumpectomy without radiotherapy was considered a reasonable treatment option for all based on favorable clinical and pathological characteristics. The median patient age was 59 years, and 30% of the study population received tamoxifen during follow-up, said Dr. Lawrence J. Solin of Albert Einstein Healthcare Network, Philadelphia, and his associates.
During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast. The 12-year rates of developing any recurrence were 14.4% in cohort 1 and 24.6% in cohort 2, and the 12-year rates of developing an invasive cancer were 7.5% and 13.4%, respectively. Most important, the risk of any recurrence increased steadily over time, without any plateau, the investigators said (J Clin Oncol. 2015 Sep 14. doi: 10.1200/JCO.2015.60.8588).
“Individual patients and their physicians will need to decide if these 12-year risks are acceptable, and whether or not to forgo adding adjuvant treatment after surgical excision,” Dr. Solin and his associates said.
Most of the study participants were postmenopausal and had small, asymptomatic microcalcifications detected on routine screening mammography; most also had negative margins at resection. Since these traits are similar to those of patients with DCIS in population-based studies, the findings of this trial are relevant to contemporary practice, the researchers added.
“Not all patients and their physicians will agree on what is considered too high a risk of developing [a recurrence] or [an invasive recurrence] to recommend observation after surgical excision, or what risk is considered too low to justify adding radiation treatment. However, this study provides 12-year data to begin those discussions, and to help inform the treatment decision-making process,” Dr. Solin and his associates said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The risk of recurrence continues to rise for at least 12 years in patients with DCIS who had lumpectomy without radiotherapy.
Major finding: During a median of 12.3 years of follow-up, there were 99 recurrences: 48 cases of DCIS and 51 cases of invasive cancer in the ipsilateral breast.
Data source: Extended follow-up of a prospective clinical trial involving 665 patients with low-risk DCIS who underwent lumpectomy without radiotherapy.
Disclosures: This study was supported in part by the National Cancer Institute and the Breast Cancer Research Foundation. Dr. Solin reported having no financial disclosures; his associates reported ties to numerous industry sources.
For metastatic breast cancer, adding pertuzumab not cost-effective
Adding pertuzumab to standard combination therapy with docetaxel plus trastuzumab is not cost-effective in patients with metastatic HER2-positive breast cancer, according to a report published online Sept. 8 in Journal of Clinical Oncology.
Adding pertuzumab in this setting is highly effective at extending survival, although patients usually succumb to their disease eventually. And this approach is recommended as the first-line treatment of choice by the National Comprehensive Cancer Network. But its cost-effectiveness has never been studied in the United States until now, said Dr. Ben Y. Durkee of the department of radiation oncology at Stanford (Calif.) University.
Dr. Durkee and his associates assessed the cost-effectiveness of adding pertuzumab to standard treatment by incorporating into their statistical model such existing data as the annual incidence of metastatic HER2-positive breast cancer (17,450 patients per year), the direct and indirect costs of standard combination therapy alone ($347,627 per patient per year), and the direct and indirect costs of adding pertuzumab therapy ($805,449 per patient per year) until disease progressed or toxic effects became unmanageable. The model assigned hypothetical patients to four possible health states based on data in the literature – stable disease, progressing disease, hospice, and dead – and tracked each one weekly for their remaining lifetimes.
This model produced outcomes consistent with those reported in the literature regarding overall survival, progression-free survival, major toxicities, and time spent in each health state. Median overall survival was 39.4 months for standard treatment and 56.9 months with the addition of pertuzumab.
In this statistical model, adding pertuzumab yielded an extra 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821. “Taken together, the addition of pertuzumab to combined docetaxel plus trastuzumab cost $713,219 per QALY gained,” which is “well above any commonly used threshold and well above the de facto threshold of cost-effectiveness for interventions already in practice,” Dr. Durkee and his associates noted (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.9105).
Willingness-to-pay thresholds in most analyses of medical treatments range from $50,000 to $160,000 per QALY. A sensitivity analysis of the data demonstrated a 0% chance of cost-effectiveness at a willingness-to-pay level of $100,000 per QALY gained. Even when the willingness-to-pay level rose to $500,000 per QALY, this analysis showed a 0% chance of cost-effectiveness, the researchers noted.
“This analysis highlights the economic challenges of extending life for patients with noncurable disease. It also typifies the broader observation that half of our health care dollars are spent on 5% of the population,” the investigators said.
The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.
The high cost of treatment in the study by Durkee et al. is not entirely driven by the price of pertuzumab. Treatment overall is costly for patients with metastatic breast cancer; when it is highly effective and must be continued for the patient’s entire life span, it adds enormous costs in addition to its important benefits.
 |
Dr. Peter B. Bach |
We face this same problem with other cancer therapies for which the ongoing cost of life-maintaining treatment is substantial – far more than, for example, the average per-capita gross domestic product. By definition, we cannot economically afford a larger and larger pool of people receiving expensive maintenance treatments. Yet the last thing we want would be to deny lifesaving therapy to anyone who could benefit from it.
Dr. Peter B. Bach is at the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, New York. His financial disclosures are available at www.jco.org. Dr. Bach made these remarks in an editorial accompanying Dr. Durkee’s report (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.63.7397).
The high cost of treatment in the study by Durkee et al. is not entirely driven by the price of pertuzumab. Treatment overall is costly for patients with metastatic breast cancer; when it is highly effective and must be continued for the patient’s entire life span, it adds enormous costs in addition to its important benefits.
 |
Dr. Peter B. Bach |
We face this same problem with other cancer therapies for which the ongoing cost of life-maintaining treatment is substantial – far more than, for example, the average per-capita gross domestic product. By definition, we cannot economically afford a larger and larger pool of people receiving expensive maintenance treatments. Yet the last thing we want would be to deny lifesaving therapy to anyone who could benefit from it.
Dr. Peter B. Bach is at the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, New York. His financial disclosures are available at www.jco.org. Dr. Bach made these remarks in an editorial accompanying Dr. Durkee’s report (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.63.7397).
The high cost of treatment in the study by Durkee et al. is not entirely driven by the price of pertuzumab. Treatment overall is costly for patients with metastatic breast cancer; when it is highly effective and must be continued for the patient’s entire life span, it adds enormous costs in addition to its important benefits.
 |
Dr. Peter B. Bach |
We face this same problem with other cancer therapies for which the ongoing cost of life-maintaining treatment is substantial – far more than, for example, the average per-capita gross domestic product. By definition, we cannot economically afford a larger and larger pool of people receiving expensive maintenance treatments. Yet the last thing we want would be to deny lifesaving therapy to anyone who could benefit from it.
Dr. Peter B. Bach is at the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, New York. His financial disclosures are available at www.jco.org. Dr. Bach made these remarks in an editorial accompanying Dr. Durkee’s report (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.63.7397).
Adding pertuzumab to standard combination therapy with docetaxel plus trastuzumab is not cost-effective in patients with metastatic HER2-positive breast cancer, according to a report published online Sept. 8 in Journal of Clinical Oncology.
Adding pertuzumab in this setting is highly effective at extending survival, although patients usually succumb to their disease eventually. And this approach is recommended as the first-line treatment of choice by the National Comprehensive Cancer Network. But its cost-effectiveness has never been studied in the United States until now, said Dr. Ben Y. Durkee of the department of radiation oncology at Stanford (Calif.) University.
Dr. Durkee and his associates assessed the cost-effectiveness of adding pertuzumab to standard treatment by incorporating into their statistical model such existing data as the annual incidence of metastatic HER2-positive breast cancer (17,450 patients per year), the direct and indirect costs of standard combination therapy alone ($347,627 per patient per year), and the direct and indirect costs of adding pertuzumab therapy ($805,449 per patient per year) until disease progressed or toxic effects became unmanageable. The model assigned hypothetical patients to four possible health states based on data in the literature – stable disease, progressing disease, hospice, and dead – and tracked each one weekly for their remaining lifetimes.
This model produced outcomes consistent with those reported in the literature regarding overall survival, progression-free survival, major toxicities, and time spent in each health state. Median overall survival was 39.4 months for standard treatment and 56.9 months with the addition of pertuzumab.
In this statistical model, adding pertuzumab yielded an extra 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821. “Taken together, the addition of pertuzumab to combined docetaxel plus trastuzumab cost $713,219 per QALY gained,” which is “well above any commonly used threshold and well above the de facto threshold of cost-effectiveness for interventions already in practice,” Dr. Durkee and his associates noted (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.9105).
Willingness-to-pay thresholds in most analyses of medical treatments range from $50,000 to $160,000 per QALY. A sensitivity analysis of the data demonstrated a 0% chance of cost-effectiveness at a willingness-to-pay level of $100,000 per QALY gained. Even when the willingness-to-pay level rose to $500,000 per QALY, this analysis showed a 0% chance of cost-effectiveness, the researchers noted.
“This analysis highlights the economic challenges of extending life for patients with noncurable disease. It also typifies the broader observation that half of our health care dollars are spent on 5% of the population,” the investigators said.
The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.
Adding pertuzumab to standard combination therapy with docetaxel plus trastuzumab is not cost-effective in patients with metastatic HER2-positive breast cancer, according to a report published online Sept. 8 in Journal of Clinical Oncology.
Adding pertuzumab in this setting is highly effective at extending survival, although patients usually succumb to their disease eventually. And this approach is recommended as the first-line treatment of choice by the National Comprehensive Cancer Network. But its cost-effectiveness has never been studied in the United States until now, said Dr. Ben Y. Durkee of the department of radiation oncology at Stanford (Calif.) University.
Dr. Durkee and his associates assessed the cost-effectiveness of adding pertuzumab to standard treatment by incorporating into their statistical model such existing data as the annual incidence of metastatic HER2-positive breast cancer (17,450 patients per year), the direct and indirect costs of standard combination therapy alone ($347,627 per patient per year), and the direct and indirect costs of adding pertuzumab therapy ($805,449 per patient per year) until disease progressed or toxic effects became unmanageable. The model assigned hypothetical patients to four possible health states based on data in the literature – stable disease, progressing disease, hospice, and dead – and tracked each one weekly for their remaining lifetimes.
This model produced outcomes consistent with those reported in the literature regarding overall survival, progression-free survival, major toxicities, and time spent in each health state. Median overall survival was 39.4 months for standard treatment and 56.9 months with the addition of pertuzumab.
In this statistical model, adding pertuzumab yielded an extra 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821. “Taken together, the addition of pertuzumab to combined docetaxel plus trastuzumab cost $713,219 per QALY gained,” which is “well above any commonly used threshold and well above the de facto threshold of cost-effectiveness for interventions already in practice,” Dr. Durkee and his associates noted (J Clin Oncol. 2015 Sep 8. doi:10.1200/JCO.2015.62.9105).
Willingness-to-pay thresholds in most analyses of medical treatments range from $50,000 to $160,000 per QALY. A sensitivity analysis of the data demonstrated a 0% chance of cost-effectiveness at a willingness-to-pay level of $100,000 per QALY gained. Even when the willingness-to-pay level rose to $500,000 per QALY, this analysis showed a 0% chance of cost-effectiveness, the researchers noted.
“This analysis highlights the economic challenges of extending life for patients with noncurable disease. It also typifies the broader observation that half of our health care dollars are spent on 5% of the population,” the investigators said.
The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Adding pertuzumab to docetaxel plus trastuzumab combination therapy is not cost-effective for patients with metastatic HER2-positive breast cancer.
Major finding: Adding pertuzumab yielded an additional 1.82 life-years per patient and 0.64 QALYs at a per-patient cost of $457,821, for a total cost of $713,219 per QALY gained.
Data source: A cost-effectiveness analysis based on an incidence of 17,450 eligible patients per year in the United States, a $347,627 cost for trastuzumab plus docetaxel, and an $805,449 cost for adding pertuzumab to that combination.
Disclosures: The Henry S. Kaplan Research Fund, the department of radiation oncology at Stanford University, and the National Institute on Aging supported the study. Dr. Durkee reported having no conflicts of interest. An associate reported stock or other ownership with ViewRay.