User login
Dabigatran vs Warfarin Before Cardioversion of Atrial Arrhythmias
Atrial fibrillation (AF) is the most common cardiac arrhythmia, followed by atrial flutter. Both arrhythmias may increase the risk of stroke. Atrial fibrillation affects about 1% to 2% of the population.1 Patients with atrial flutter often have episodes of AF.
Direct current cardioversion (DCCV) treats atrial arrhythmias by attempting to return the patient to a normal sinus rhythm. When sinus rhythm is restored, cardiac structural changes that might have occurred as a result of AF or atrial flutter may be reversed.2 However, patients undergoing cardioversion are at an increased risk of stroke if a thrombus is present in the left atria. This thrombus may become dislodged during the procedure. Although sinus rhythm may be restored during cardioversion, restoration of the atrial mechanical function may take several weeks, and new thrombi may form during that time. Stroke risk is significantly decreased with anticoagulation.3,4
Current guidelines on antithrombotic therapy for AF and atrial flutter recommend that patients who are appropriate candidates for electrical cardioversion need to be properly anticoagulated for 3 to 4 weeks before and after the procedure if the duration of AF or flutter is > 48 hours or is unknown.5 The practice of anticoagulating candidates needing cardioversion for 3 to 4 weeks before the procedure and 4 weeks after the procedure is based on the theory that it takes about 14 days for a new thrombus to firmly adhere to the atrial wall.6 Therefore 3 to 4 weeks of anticoagulation before cardioversion will prevent new thrombi from forming and theoretically allows enough time for older thrombi to adhere to the atrial wall. Anticoagulation for 4 weeks after cardioversion will prevent new thrombi from forming in the atria during the several weeks that atrial remodeling takes place.3,7 These practices are based on physiologic concepts and observational studies and have not been evaluated in randomized, controlled clinical trials.7
To receive an electrical cardioversion, patients at the VA Portland Health Care System (VAPORHCS) should maintain a therapeutic international normalized ratio (INR), defined as 2.0 to 3.0, for 4 consecutive weeks. The Anticoagulation Clinic monitors patients receiving warfarin for planned DCCV at least weekly. The estimated average time for cardioversion candidates at the VAPORHCS to achieve stability on warfarin is 2 months. Prolonging the time to DCCV may expose symptomatic patients to additional discomfort, lead to further cardiac remodeling, and result in poorer outcomes.
In response to the delays attributed to time needed to achieve INR stability, the VISN 20 Pharmacy and Therapeutics (P&T) committee approved the use of dabigatran prior to cardioversion of AF in October 2011. This quality improvement (QI) project evaluated the time elapsed between initiation of anticoagulation with dabigatran vs warfarin and DCCV and the associated costs of anticoagulation before DCCV.
Methods
A single site, retrospective chart review of patients scheduled for cardioversion from November 2011 to December 2013 was conducted. This QI project was considered exempt from institutional review board approval. VAPORHCS patients aged > 18 years who initiated dabigatran or
warfarin for planned cardioversion of AF or atrial flutter were included in the study. Exclusion criteria included use of dabigatran or warfarin within 3 months before the decision to cardiovert and emergency cardioversion performed within 48 hours of symptom onset. Patients were assigned to either the dabigatran or warfarin group, based on the prescribed anticoagulant. The primary objectives were to evaluate the time elapsed from initiation of anticoagulation to planned cardioversion of AF or atrial flutter and to evaluate treatment costs associated with dabigatran vs warfarin before planned cardioversion of AF or atrial flutter. The secondary objective was to identify reasons for rescheduled or cancelled cardioversions.
Data Collection
Potential patients were identified using the computerized patient record system and VistA. Demographics, including age, gender, indication for cardioversion, calculated CHADS2 score for thromboembolic risk, and calculated HAS-BLED score for bleeding risk were collected to evaluate the potential differences between the 2 groups. Anticoagulation time before cardioversion was evaluated by collecting the first fill date of dabigatran or warfarin and the date that cardioversion was performed. An internal cost analysis was completed. The cost analysis for dabigatran included medication and laboratory costs. The cost analysis for warfarin included costs associated with the medication, laboratory, and pharmacists’ monitoring time.
Statistical Analysis
Statistical analysis was performed using Sigma Plot, Version 12.5 for Windows (System Software, Inc., Chicago, Illinois). Demographic parameters and the primary objectives of time and cost were analyzed using the Mann-Whitney U test. The secondary objective of reasons for rescheduled or cancelled cardioversions was reported using descriptive statistics. A P value of ≥ .05 was considered statistically significant.
Results
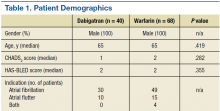
Forty dabigatran patients and 68 warfarin patients met inclusion criteria (Table 1). All patients were male with a median age of 65 years in both groups, which is representative of the VA patient population of mostly older adult males. The CHADS2 and HAS-BLED scores were similar between the groups.
Primary Objectives
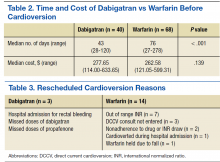
There was a difference in anticoagulation time before cardioversion between the 2 groups (Table 2). The median number of days that elapsed between initiation of dabigatran and cardioversion was 43 (range 28-120 days) vs 76 days (range 27-278 days) in the warfarin group (P < .001). Patients whose cardioversions were cancelled were not included in the time analysis. The difference in total cost per patient was not statistically significant. The median cost for dabigatran was $277.65 (range: $114.00-$633.65) per patient and $262.58 (range $121.0-$599.31) per patient in the warfarin group (P = .139). All patients, including those whose cardioversions were cancelled, were included in the cost analysis. Costs for cancellations were evaluated from the date of initiation to the date of the cardioversion cancellation decision.
Secondary Objective
In the dabigatran group, 3 patients rescheduled cardioversions and 5 patients cancelled cardioversions. Fourteen warfarin patients rescheduled cardioversions, and 10 patients cancelled cardioversions (Tables 3 and 4). Two dabigatran patients were rescheduled due to missed doses of dabigatran or propafenone and 7 warfarin patients were rescheduled due to out of range INRs (< 2.0) at their preprocedure appointment. Three dabigatran patients presented without symptoms at their preprocedure appointments and their cardioversions were cancelled. Similarly, 5 warfarin patients spontaneously returned to sinus rhythm, and their cardioversions were cancelled.
Discussion
Currently, there are 4 target-specific oral anticoagulants (TSOACs) approved by the FDA for nonvalvular AF: dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor, and rivaroxaban apixaban and edoxaban are factor Xa inhibitors.8-11 The American College of Chest Physicians (CHEST) 2012 guidelines on antithrombotic therapy for AF recommend anticoagulation with warfarin, low molecular weight heparin (LMWH) or dabigatran before cardioversion (grade 1B for all 3 options).5
Anticoagulation with warfarin (Class Ia, Level B), dabigatran, rivaroxaban, or apixaban (Class IIa, Level C) before and after cardioversion was also recommended by the recently published American College of Cardiology/ American Heart Association/ Heart Rhythm Society (ACC/AHA/ HRS) 2014 guidelines for the management of patients with atrial fibrillation.1 Edoxaban was not included since the guidelines were published prior to FDA approval. The main evidence supporting the inclusion of the 3 TSOACs in the 2014 ACC/AHA/HRS guidelines are based on post hoc analyses of the major landmark trials (RE-LY, ROCKET-AF, and ARISTOTLE) evaluating the use of dabigatran, rivaroxaban, and apixaban, respectively, before and after DCCV.12-15 Major adverse events (AEs) were similar between warfarin and the TSOAC comparator in all 3 post hoc analyses.
Low molecular weight heparin was not included as an option for anticoagulation before cardioversion in the ACC/AHA/HRS 2014 guidelines. This is likely due to lack of evidence, as most of the evidence supporting anticoagulation included warfarin and not heparin. The 2 guidelines did not differ in their recommendations on the duration of pre- and postprocedure anticoagulation of 3 and 4 weeks, respectively.1,5 Nor did they differ on the use of transesophageal echocardiogram (TEE) to rule out left atrial thrombus if a patient has not been anticoagulated for 3 weeks before cardioversion.
A recent nonrandomized cohort study by Choo and colleagues evaluated the timing, rescheduling, and cancellation of scheduled DCCV in 193 patients receiving warfarin or dabigatran.16 The study found that patients receiving dabigatran waited 22 fewer days until scheduled DCCV and had lower rates of rescheduled cardioversions than did patients receiving warfarin. The results of this study were similar to the findings at VAPORHCS. The most common reasons for rescheduled or cancelled DCCVs at VAPORHCS were out of range INRs and spontaneous return to sinus rhythm, respectively, which were the same reasons that Choo and colleagues found for rescheduling or cancellations in their study.
Dabigatran patients received drug therapy at VAPORHCS for fewer days before cardioversion than did the patients taking warfarin. The median total cost per patient was about $15 higher in the dabigatran group. Based on these findings and the recommendations of the 2 guidelines, both drugs remain reasonable and appropriate options for patients before cardioversion.
Reasons to Select Dabigatran
If warfarin or a TSOAC is clinically indicated for anticoagulation, then patient preference and nonclinical barriers to safe monitoring may also factor in the decision. Some patients from the surrounding states are referred to VAPORHCS for cardioversions and continue to receive primary care from their facility. Patients receiving primary care and anticoagulation management outside VAPORHCS were not included in this QI project. It may add an additional layer of difficulty to initiate warfarin on a remote patient if the patient does not have access to anticoagulation monitoring locally. Additionally, it may be difficult for the remote anticoagulation providers to communicate information efficiently with the cardiology team at VAPORHCS. It also may be challenging for VAPORHCS to safely manage warfarin in a remote patient without full access to laboratory results and the patient’s primary care provider. For these reasons, dabigatran may be a more favorable option in remote patients referred to VAPORHCS for their cardioversion.
Additionally, dabigatran may be a more appropriate anticoagulant in highly symptomatic AF patients in whom the potential for longer wait times may expose the patient to more symptoms and decreased quality of life. The longer the duration of AF or atrial flutter, the less likely that sinus rhythm will be restored in patients undergoing DCCV.2 A study of 157 patients with AF showed that the adjusted risk for return to AF after DCCV increased if the AF was present for > 2 months before the DCCV.17 If returning patients to sinus rhythm is the highest priority for reversal of cardiac restructuring and symptoms, then a shorter time to DCCV may be preferred, and a TSOAC may be the preferred agent in this case.
Reasons to Select Warfarin
Warfarin may be a more appropriate option in patients with a high bleeding risk due to the current lack of a reversal agent for dabigatran. Dabigatran is not recommended in patients with creatinine clearances < 30 mL/min; thus, warfarin may be a better choice in patients with impaired renal function. It may be reasonable to consider switching a current warfarin patient with a history of variable INRs to a TSOAC in preparation for cardioversion to potentially shorten the time to cardioversion if the patient is highly symptomatic. Low molecular weight heparin may be considered as a last resort for patients who may not be able to tolerate warfarin or TSOACs. However, if LMWH were to be used, it may be more reasonable to consider a TEE-guided DCCV rather than 3 full weeks of anticoagulation with LMWH.
Limitations
There were several limitations to this single site, retrospective, QI project with a small sample size. All patients were older, adult males. Results may not be relevant to other institutions and patient populations, including females and younger patients.
Standardized anticoagulation clinic encounter times (15 minutes for phone call and 5 minutes for letter) were used to calculate pharmacist’s monitoring time costs for warfarin patients. This standardized time did not account for the amount of time spent in monitoring and creating dosing plans that may vary drastically between patients. The time and cost analyses did not account for pharmacy technician reminder phone calls for missed or late INR draws or home health nurse INR draws and visits. Theoretically, patients with home health services have fewer missed or late INRs, and phone encounter times may be shorter between the pharmacist and the nurse vs the pharmacist and the patient.
Finally, it was difficult to capture administrative reasons for delayed DCCV in both groups. In the warfarin group, communication between the anticoagulation clinic and the cardiology team may have been delayed due to staff vacations, sick time, or differences in staff work schedules. In both groups, assessing how procedure scheduling affected wait times was difficult. Procedure room availability, clinic schedules, staff schedules, and preprocedure appointment availability likely impacted patient wait times for DCCV but were difficult to assess and quantify. Finally, power was not calculated for this project.
Conclusions
Based on the recommendations of the CHEST 2012 guidelines, the ACC/AHA/HRS 2014 guidelines, and recent literature, TSOACs are reasonable anticoagulants to consider before and after planned cardioversion of atrial arrhythmias. The findings of this QI project support the
use of either dabigatran or warfarin before a planned cardioversion at VAPORHCS. Several factors should be considered when choosing an oral anticoagulant before a planned DCCV, including indication, duration of anticoagulation, previous anticoagulant use, medication adherence, renal function, risk of thromboembolism vs bleeding risk, and potential need for a reversal agent.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(2):2071-2104.
2. Van Gelder IC, Crijns HJ, van Gilst WH, Hamer HP, Lie KI. Decrease of right and left atrial sizes after direct-current electrical cardioversion in chronic atrial fibrillation. Am J Cardiol. 1991;67(1):93-95.
3. Manning WJ, Leeman DE, Gotch PJ, Come PC. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13(3):617-623.
4. Design of a clinical trial for the assessment of cardioversion using transesophageal echocardiography (The ACUTE Multicenter Study). Steering and Publications Committees of the ACUTE Study. Am J Cardiol. 1998;81(7):877-883.
5. You JJ, Singer DE, Howard PA, et al; American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
6. Mancini GB, Goldberger AL. Cardioversion of atrial fibrillation: consideration of embolization, anticoagulation, prophylactic pacemaker, and long-term success. Am Heart J. 1982;104(3):617-621.
7. Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82(12):1545-1547, A8.
8. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2015.
9. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2015.
10. Eliquis [package insert]. Princeton, NJ: Bristol Myers Squibb Company; 2015.
11. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2015.
12. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131-136.
13. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
14. Piccini JP, Stevens SR, Lokhnygina Y, et al; ROCKET AF Steering Committee & Investigators. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61(19):1998-2006.
15. Flaker G, Lopes RD, Al-Khatib SM, et al; ARISTOTLE Committees and Investigators. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol. 2014;63(11):1082-1087.
16. Choo WK, Fraser S, Padfield G, et al. Dabigatran improves the efficiency of an elective direct current cardioversion service. Br J Cardiol. 2014;21(1):29-32.
17. Alt E, Ammer R, Lehmann G, et al. Patient characteristics and underlying heart disease as predictors of recurrent atrial fibrillation after internal and external cardioversion in patients treated with oral sotalol. Am Heart J. 1997;134(3):419-425.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, followed by atrial flutter. Both arrhythmias may increase the risk of stroke. Atrial fibrillation affects about 1% to 2% of the population.1 Patients with atrial flutter often have episodes of AF.
Direct current cardioversion (DCCV) treats atrial arrhythmias by attempting to return the patient to a normal sinus rhythm. When sinus rhythm is restored, cardiac structural changes that might have occurred as a result of AF or atrial flutter may be reversed.2 However, patients undergoing cardioversion are at an increased risk of stroke if a thrombus is present in the left atria. This thrombus may become dislodged during the procedure. Although sinus rhythm may be restored during cardioversion, restoration of the atrial mechanical function may take several weeks, and new thrombi may form during that time. Stroke risk is significantly decreased with anticoagulation.3,4
Current guidelines on antithrombotic therapy for AF and atrial flutter recommend that patients who are appropriate candidates for electrical cardioversion need to be properly anticoagulated for 3 to 4 weeks before and after the procedure if the duration of AF or flutter is > 48 hours or is unknown.5 The practice of anticoagulating candidates needing cardioversion for 3 to 4 weeks before the procedure and 4 weeks after the procedure is based on the theory that it takes about 14 days for a new thrombus to firmly adhere to the atrial wall.6 Therefore 3 to 4 weeks of anticoagulation before cardioversion will prevent new thrombi from forming and theoretically allows enough time for older thrombi to adhere to the atrial wall. Anticoagulation for 4 weeks after cardioversion will prevent new thrombi from forming in the atria during the several weeks that atrial remodeling takes place.3,7 These practices are based on physiologic concepts and observational studies and have not been evaluated in randomized, controlled clinical trials.7
To receive an electrical cardioversion, patients at the VA Portland Health Care System (VAPORHCS) should maintain a therapeutic international normalized ratio (INR), defined as 2.0 to 3.0, for 4 consecutive weeks. The Anticoagulation Clinic monitors patients receiving warfarin for planned DCCV at least weekly. The estimated average time for cardioversion candidates at the VAPORHCS to achieve stability on warfarin is 2 months. Prolonging the time to DCCV may expose symptomatic patients to additional discomfort, lead to further cardiac remodeling, and result in poorer outcomes.
In response to the delays attributed to time needed to achieve INR stability, the VISN 20 Pharmacy and Therapeutics (P&T) committee approved the use of dabigatran prior to cardioversion of AF in October 2011. This quality improvement (QI) project evaluated the time elapsed between initiation of anticoagulation with dabigatran vs warfarin and DCCV and the associated costs of anticoagulation before DCCV.
Methods
A single site, retrospective chart review of patients scheduled for cardioversion from November 2011 to December 2013 was conducted. This QI project was considered exempt from institutional review board approval. VAPORHCS patients aged > 18 years who initiated dabigatran or
warfarin for planned cardioversion of AF or atrial flutter were included in the study. Exclusion criteria included use of dabigatran or warfarin within 3 months before the decision to cardiovert and emergency cardioversion performed within 48 hours of symptom onset. Patients were assigned to either the dabigatran or warfarin group, based on the prescribed anticoagulant. The primary objectives were to evaluate the time elapsed from initiation of anticoagulation to planned cardioversion of AF or atrial flutter and to evaluate treatment costs associated with dabigatran vs warfarin before planned cardioversion of AF or atrial flutter. The secondary objective was to identify reasons for rescheduled or cancelled cardioversions.
Data Collection
Potential patients were identified using the computerized patient record system and VistA. Demographics, including age, gender, indication for cardioversion, calculated CHADS2 score for thromboembolic risk, and calculated HAS-BLED score for bleeding risk were collected to evaluate the potential differences between the 2 groups. Anticoagulation time before cardioversion was evaluated by collecting the first fill date of dabigatran or warfarin and the date that cardioversion was performed. An internal cost analysis was completed. The cost analysis for dabigatran included medication and laboratory costs. The cost analysis for warfarin included costs associated with the medication, laboratory, and pharmacists’ monitoring time.
Statistical Analysis
Statistical analysis was performed using Sigma Plot, Version 12.5 for Windows (System Software, Inc., Chicago, Illinois). Demographic parameters and the primary objectives of time and cost were analyzed using the Mann-Whitney U test. The secondary objective of reasons for rescheduled or cancelled cardioversions was reported using descriptive statistics. A P value of ≥ .05 was considered statistically significant.
Results
Forty dabigatran patients and 68 warfarin patients met inclusion criteria (Table 1). All patients were male with a median age of 65 years in both groups, which is representative of the VA patient population of mostly older adult males. The CHADS2 and HAS-BLED scores were similar between the groups.
Primary Objectives
There was a difference in anticoagulation time before cardioversion between the 2 groups (Table 2). The median number of days that elapsed between initiation of dabigatran and cardioversion was 43 (range 28-120 days) vs 76 days (range 27-278 days) in the warfarin group (P < .001). Patients whose cardioversions were cancelled were not included in the time analysis. The difference in total cost per patient was not statistically significant. The median cost for dabigatran was $277.65 (range: $114.00-$633.65) per patient and $262.58 (range $121.0-$599.31) per patient in the warfarin group (P = .139). All patients, including those whose cardioversions were cancelled, were included in the cost analysis. Costs for cancellations were evaluated from the date of initiation to the date of the cardioversion cancellation decision.
Secondary Objective
In the dabigatran group, 3 patients rescheduled cardioversions and 5 patients cancelled cardioversions. Fourteen warfarin patients rescheduled cardioversions, and 10 patients cancelled cardioversions (Tables 3 and 4). Two dabigatran patients were rescheduled due to missed doses of dabigatran or propafenone and 7 warfarin patients were rescheduled due to out of range INRs (< 2.0) at their preprocedure appointment. Three dabigatran patients presented without symptoms at their preprocedure appointments and their cardioversions were cancelled. Similarly, 5 warfarin patients spontaneously returned to sinus rhythm, and their cardioversions were cancelled.
Discussion
Currently, there are 4 target-specific oral anticoagulants (TSOACs) approved by the FDA for nonvalvular AF: dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor, and rivaroxaban apixaban and edoxaban are factor Xa inhibitors.8-11 The American College of Chest Physicians (CHEST) 2012 guidelines on antithrombotic therapy for AF recommend anticoagulation with warfarin, low molecular weight heparin (LMWH) or dabigatran before cardioversion (grade 1B for all 3 options).5
Anticoagulation with warfarin (Class Ia, Level B), dabigatran, rivaroxaban, or apixaban (Class IIa, Level C) before and after cardioversion was also recommended by the recently published American College of Cardiology/ American Heart Association/ Heart Rhythm Society (ACC/AHA/ HRS) 2014 guidelines for the management of patients with atrial fibrillation.1 Edoxaban was not included since the guidelines were published prior to FDA approval. The main evidence supporting the inclusion of the 3 TSOACs in the 2014 ACC/AHA/HRS guidelines are based on post hoc analyses of the major landmark trials (RE-LY, ROCKET-AF, and ARISTOTLE) evaluating the use of dabigatran, rivaroxaban, and apixaban, respectively, before and after DCCV.12-15 Major adverse events (AEs) were similar between warfarin and the TSOAC comparator in all 3 post hoc analyses.
Low molecular weight heparin was not included as an option for anticoagulation before cardioversion in the ACC/AHA/HRS 2014 guidelines. This is likely due to lack of evidence, as most of the evidence supporting anticoagulation included warfarin and not heparin. The 2 guidelines did not differ in their recommendations on the duration of pre- and postprocedure anticoagulation of 3 and 4 weeks, respectively.1,5 Nor did they differ on the use of transesophageal echocardiogram (TEE) to rule out left atrial thrombus if a patient has not been anticoagulated for 3 weeks before cardioversion.
A recent nonrandomized cohort study by Choo and colleagues evaluated the timing, rescheduling, and cancellation of scheduled DCCV in 193 patients receiving warfarin or dabigatran.16 The study found that patients receiving dabigatran waited 22 fewer days until scheduled DCCV and had lower rates of rescheduled cardioversions than did patients receiving warfarin. The results of this study were similar to the findings at VAPORHCS. The most common reasons for rescheduled or cancelled DCCVs at VAPORHCS were out of range INRs and spontaneous return to sinus rhythm, respectively, which were the same reasons that Choo and colleagues found for rescheduling or cancellations in their study.
Dabigatran patients received drug therapy at VAPORHCS for fewer days before cardioversion than did the patients taking warfarin. The median total cost per patient was about $15 higher in the dabigatran group. Based on these findings and the recommendations of the 2 guidelines, both drugs remain reasonable and appropriate options for patients before cardioversion.
Reasons to Select Dabigatran
If warfarin or a TSOAC is clinically indicated for anticoagulation, then patient preference and nonclinical barriers to safe monitoring may also factor in the decision. Some patients from the surrounding states are referred to VAPORHCS for cardioversions and continue to receive primary care from their facility. Patients receiving primary care and anticoagulation management outside VAPORHCS were not included in this QI project. It may add an additional layer of difficulty to initiate warfarin on a remote patient if the patient does not have access to anticoagulation monitoring locally. Additionally, it may be difficult for the remote anticoagulation providers to communicate information efficiently with the cardiology team at VAPORHCS. It also may be challenging for VAPORHCS to safely manage warfarin in a remote patient without full access to laboratory results and the patient’s primary care provider. For these reasons, dabigatran may be a more favorable option in remote patients referred to VAPORHCS for their cardioversion.
Additionally, dabigatran may be a more appropriate anticoagulant in highly symptomatic AF patients in whom the potential for longer wait times may expose the patient to more symptoms and decreased quality of life. The longer the duration of AF or atrial flutter, the less likely that sinus rhythm will be restored in patients undergoing DCCV.2 A study of 157 patients with AF showed that the adjusted risk for return to AF after DCCV increased if the AF was present for > 2 months before the DCCV.17 If returning patients to sinus rhythm is the highest priority for reversal of cardiac restructuring and symptoms, then a shorter time to DCCV may be preferred, and a TSOAC may be the preferred agent in this case.
Reasons to Select Warfarin
Warfarin may be a more appropriate option in patients with a high bleeding risk due to the current lack of a reversal agent for dabigatran. Dabigatran is not recommended in patients with creatinine clearances < 30 mL/min; thus, warfarin may be a better choice in patients with impaired renal function. It may be reasonable to consider switching a current warfarin patient with a history of variable INRs to a TSOAC in preparation for cardioversion to potentially shorten the time to cardioversion if the patient is highly symptomatic. Low molecular weight heparin may be considered as a last resort for patients who may not be able to tolerate warfarin or TSOACs. However, if LMWH were to be used, it may be more reasonable to consider a TEE-guided DCCV rather than 3 full weeks of anticoagulation with LMWH.
Limitations
There were several limitations to this single site, retrospective, QI project with a small sample size. All patients were older, adult males. Results may not be relevant to other institutions and patient populations, including females and younger patients.
Standardized anticoagulation clinic encounter times (15 minutes for phone call and 5 minutes for letter) were used to calculate pharmacist’s monitoring time costs for warfarin patients. This standardized time did not account for the amount of time spent in monitoring and creating dosing plans that may vary drastically between patients. The time and cost analyses did not account for pharmacy technician reminder phone calls for missed or late INR draws or home health nurse INR draws and visits. Theoretically, patients with home health services have fewer missed or late INRs, and phone encounter times may be shorter between the pharmacist and the nurse vs the pharmacist and the patient.
Finally, it was difficult to capture administrative reasons for delayed DCCV in both groups. In the warfarin group, communication between the anticoagulation clinic and the cardiology team may have been delayed due to staff vacations, sick time, or differences in staff work schedules. In both groups, assessing how procedure scheduling affected wait times was difficult. Procedure room availability, clinic schedules, staff schedules, and preprocedure appointment availability likely impacted patient wait times for DCCV but were difficult to assess and quantify. Finally, power was not calculated for this project.
Conclusions
Based on the recommendations of the CHEST 2012 guidelines, the ACC/AHA/HRS 2014 guidelines, and recent literature, TSOACs are reasonable anticoagulants to consider before and after planned cardioversion of atrial arrhythmias. The findings of this QI project support the
use of either dabigatran or warfarin before a planned cardioversion at VAPORHCS. Several factors should be considered when choosing an oral anticoagulant before a planned DCCV, including indication, duration of anticoagulation, previous anticoagulant use, medication adherence, renal function, risk of thromboembolism vs bleeding risk, and potential need for a reversal agent.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, followed by atrial flutter. Both arrhythmias may increase the risk of stroke. Atrial fibrillation affects about 1% to 2% of the population.1 Patients with atrial flutter often have episodes of AF.
Direct current cardioversion (DCCV) treats atrial arrhythmias by attempting to return the patient to a normal sinus rhythm. When sinus rhythm is restored, cardiac structural changes that might have occurred as a result of AF or atrial flutter may be reversed.2 However, patients undergoing cardioversion are at an increased risk of stroke if a thrombus is present in the left atria. This thrombus may become dislodged during the procedure. Although sinus rhythm may be restored during cardioversion, restoration of the atrial mechanical function may take several weeks, and new thrombi may form during that time. Stroke risk is significantly decreased with anticoagulation.3,4
Current guidelines on antithrombotic therapy for AF and atrial flutter recommend that patients who are appropriate candidates for electrical cardioversion need to be properly anticoagulated for 3 to 4 weeks before and after the procedure if the duration of AF or flutter is > 48 hours or is unknown.5 The practice of anticoagulating candidates needing cardioversion for 3 to 4 weeks before the procedure and 4 weeks after the procedure is based on the theory that it takes about 14 days for a new thrombus to firmly adhere to the atrial wall.6 Therefore 3 to 4 weeks of anticoagulation before cardioversion will prevent new thrombi from forming and theoretically allows enough time for older thrombi to adhere to the atrial wall. Anticoagulation for 4 weeks after cardioversion will prevent new thrombi from forming in the atria during the several weeks that atrial remodeling takes place.3,7 These practices are based on physiologic concepts and observational studies and have not been evaluated in randomized, controlled clinical trials.7
To receive an electrical cardioversion, patients at the VA Portland Health Care System (VAPORHCS) should maintain a therapeutic international normalized ratio (INR), defined as 2.0 to 3.0, for 4 consecutive weeks. The Anticoagulation Clinic monitors patients receiving warfarin for planned DCCV at least weekly. The estimated average time for cardioversion candidates at the VAPORHCS to achieve stability on warfarin is 2 months. Prolonging the time to DCCV may expose symptomatic patients to additional discomfort, lead to further cardiac remodeling, and result in poorer outcomes.
In response to the delays attributed to time needed to achieve INR stability, the VISN 20 Pharmacy and Therapeutics (P&T) committee approved the use of dabigatran prior to cardioversion of AF in October 2011. This quality improvement (QI) project evaluated the time elapsed between initiation of anticoagulation with dabigatran vs warfarin and DCCV and the associated costs of anticoagulation before DCCV.
Methods
A single site, retrospective chart review of patients scheduled for cardioversion from November 2011 to December 2013 was conducted. This QI project was considered exempt from institutional review board approval. VAPORHCS patients aged > 18 years who initiated dabigatran or
warfarin for planned cardioversion of AF or atrial flutter were included in the study. Exclusion criteria included use of dabigatran or warfarin within 3 months before the decision to cardiovert and emergency cardioversion performed within 48 hours of symptom onset. Patients were assigned to either the dabigatran or warfarin group, based on the prescribed anticoagulant. The primary objectives were to evaluate the time elapsed from initiation of anticoagulation to planned cardioversion of AF or atrial flutter and to evaluate treatment costs associated with dabigatran vs warfarin before planned cardioversion of AF or atrial flutter. The secondary objective was to identify reasons for rescheduled or cancelled cardioversions.
Data Collection
Potential patients were identified using the computerized patient record system and VistA. Demographics, including age, gender, indication for cardioversion, calculated CHADS2 score for thromboembolic risk, and calculated HAS-BLED score for bleeding risk were collected to evaluate the potential differences between the 2 groups. Anticoagulation time before cardioversion was evaluated by collecting the first fill date of dabigatran or warfarin and the date that cardioversion was performed. An internal cost analysis was completed. The cost analysis for dabigatran included medication and laboratory costs. The cost analysis for warfarin included costs associated with the medication, laboratory, and pharmacists’ monitoring time.
Statistical Analysis
Statistical analysis was performed using Sigma Plot, Version 12.5 for Windows (System Software, Inc., Chicago, Illinois). Demographic parameters and the primary objectives of time and cost were analyzed using the Mann-Whitney U test. The secondary objective of reasons for rescheduled or cancelled cardioversions was reported using descriptive statistics. A P value of ≥ .05 was considered statistically significant.
Results
Forty dabigatran patients and 68 warfarin patients met inclusion criteria (Table 1). All patients were male with a median age of 65 years in both groups, which is representative of the VA patient population of mostly older adult males. The CHADS2 and HAS-BLED scores were similar between the groups.
Primary Objectives
There was a difference in anticoagulation time before cardioversion between the 2 groups (Table 2). The median number of days that elapsed between initiation of dabigatran and cardioversion was 43 (range 28-120 days) vs 76 days (range 27-278 days) in the warfarin group (P < .001). Patients whose cardioversions were cancelled were not included in the time analysis. The difference in total cost per patient was not statistically significant. The median cost for dabigatran was $277.65 (range: $114.00-$633.65) per patient and $262.58 (range $121.0-$599.31) per patient in the warfarin group (P = .139). All patients, including those whose cardioversions were cancelled, were included in the cost analysis. Costs for cancellations were evaluated from the date of initiation to the date of the cardioversion cancellation decision.
Secondary Objective
In the dabigatran group, 3 patients rescheduled cardioversions and 5 patients cancelled cardioversions. Fourteen warfarin patients rescheduled cardioversions, and 10 patients cancelled cardioversions (Tables 3 and 4). Two dabigatran patients were rescheduled due to missed doses of dabigatran or propafenone and 7 warfarin patients were rescheduled due to out of range INRs (< 2.0) at their preprocedure appointment. Three dabigatran patients presented without symptoms at their preprocedure appointments and their cardioversions were cancelled. Similarly, 5 warfarin patients spontaneously returned to sinus rhythm, and their cardioversions were cancelled.
Discussion
Currently, there are 4 target-specific oral anticoagulants (TSOACs) approved by the FDA for nonvalvular AF: dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran is a direct thrombin inhibitor, and rivaroxaban apixaban and edoxaban are factor Xa inhibitors.8-11 The American College of Chest Physicians (CHEST) 2012 guidelines on antithrombotic therapy for AF recommend anticoagulation with warfarin, low molecular weight heparin (LMWH) or dabigatran before cardioversion (grade 1B for all 3 options).5
Anticoagulation with warfarin (Class Ia, Level B), dabigatran, rivaroxaban, or apixaban (Class IIa, Level C) before and after cardioversion was also recommended by the recently published American College of Cardiology/ American Heart Association/ Heart Rhythm Society (ACC/AHA/ HRS) 2014 guidelines for the management of patients with atrial fibrillation.1 Edoxaban was not included since the guidelines were published prior to FDA approval. The main evidence supporting the inclusion of the 3 TSOACs in the 2014 ACC/AHA/HRS guidelines are based on post hoc analyses of the major landmark trials (RE-LY, ROCKET-AF, and ARISTOTLE) evaluating the use of dabigatran, rivaroxaban, and apixaban, respectively, before and after DCCV.12-15 Major adverse events (AEs) were similar between warfarin and the TSOAC comparator in all 3 post hoc analyses.
Low molecular weight heparin was not included as an option for anticoagulation before cardioversion in the ACC/AHA/HRS 2014 guidelines. This is likely due to lack of evidence, as most of the evidence supporting anticoagulation included warfarin and not heparin. The 2 guidelines did not differ in their recommendations on the duration of pre- and postprocedure anticoagulation of 3 and 4 weeks, respectively.1,5 Nor did they differ on the use of transesophageal echocardiogram (TEE) to rule out left atrial thrombus if a patient has not been anticoagulated for 3 weeks before cardioversion.
A recent nonrandomized cohort study by Choo and colleagues evaluated the timing, rescheduling, and cancellation of scheduled DCCV in 193 patients receiving warfarin or dabigatran.16 The study found that patients receiving dabigatran waited 22 fewer days until scheduled DCCV and had lower rates of rescheduled cardioversions than did patients receiving warfarin. The results of this study were similar to the findings at VAPORHCS. The most common reasons for rescheduled or cancelled DCCVs at VAPORHCS were out of range INRs and spontaneous return to sinus rhythm, respectively, which were the same reasons that Choo and colleagues found for rescheduling or cancellations in their study.
Dabigatran patients received drug therapy at VAPORHCS for fewer days before cardioversion than did the patients taking warfarin. The median total cost per patient was about $15 higher in the dabigatran group. Based on these findings and the recommendations of the 2 guidelines, both drugs remain reasonable and appropriate options for patients before cardioversion.
Reasons to Select Dabigatran
If warfarin or a TSOAC is clinically indicated for anticoagulation, then patient preference and nonclinical barriers to safe monitoring may also factor in the decision. Some patients from the surrounding states are referred to VAPORHCS for cardioversions and continue to receive primary care from their facility. Patients receiving primary care and anticoagulation management outside VAPORHCS were not included in this QI project. It may add an additional layer of difficulty to initiate warfarin on a remote patient if the patient does not have access to anticoagulation monitoring locally. Additionally, it may be difficult for the remote anticoagulation providers to communicate information efficiently with the cardiology team at VAPORHCS. It also may be challenging for VAPORHCS to safely manage warfarin in a remote patient without full access to laboratory results and the patient’s primary care provider. For these reasons, dabigatran may be a more favorable option in remote patients referred to VAPORHCS for their cardioversion.
Additionally, dabigatran may be a more appropriate anticoagulant in highly symptomatic AF patients in whom the potential for longer wait times may expose the patient to more symptoms and decreased quality of life. The longer the duration of AF or atrial flutter, the less likely that sinus rhythm will be restored in patients undergoing DCCV.2 A study of 157 patients with AF showed that the adjusted risk for return to AF after DCCV increased if the AF was present for > 2 months before the DCCV.17 If returning patients to sinus rhythm is the highest priority for reversal of cardiac restructuring and symptoms, then a shorter time to DCCV may be preferred, and a TSOAC may be the preferred agent in this case.
Reasons to Select Warfarin
Warfarin may be a more appropriate option in patients with a high bleeding risk due to the current lack of a reversal agent for dabigatran. Dabigatran is not recommended in patients with creatinine clearances < 30 mL/min; thus, warfarin may be a better choice in patients with impaired renal function. It may be reasonable to consider switching a current warfarin patient with a history of variable INRs to a TSOAC in preparation for cardioversion to potentially shorten the time to cardioversion if the patient is highly symptomatic. Low molecular weight heparin may be considered as a last resort for patients who may not be able to tolerate warfarin or TSOACs. However, if LMWH were to be used, it may be more reasonable to consider a TEE-guided DCCV rather than 3 full weeks of anticoagulation with LMWH.
Limitations
There were several limitations to this single site, retrospective, QI project with a small sample size. All patients were older, adult males. Results may not be relevant to other institutions and patient populations, including females and younger patients.
Standardized anticoagulation clinic encounter times (15 minutes for phone call and 5 minutes for letter) were used to calculate pharmacist’s monitoring time costs for warfarin patients. This standardized time did not account for the amount of time spent in monitoring and creating dosing plans that may vary drastically between patients. The time and cost analyses did not account for pharmacy technician reminder phone calls for missed or late INR draws or home health nurse INR draws and visits. Theoretically, patients with home health services have fewer missed or late INRs, and phone encounter times may be shorter between the pharmacist and the nurse vs the pharmacist and the patient.
Finally, it was difficult to capture administrative reasons for delayed DCCV in both groups. In the warfarin group, communication between the anticoagulation clinic and the cardiology team may have been delayed due to staff vacations, sick time, or differences in staff work schedules. In both groups, assessing how procedure scheduling affected wait times was difficult. Procedure room availability, clinic schedules, staff schedules, and preprocedure appointment availability likely impacted patient wait times for DCCV but were difficult to assess and quantify. Finally, power was not calculated for this project.
Conclusions
Based on the recommendations of the CHEST 2012 guidelines, the ACC/AHA/HRS 2014 guidelines, and recent literature, TSOACs are reasonable anticoagulants to consider before and after planned cardioversion of atrial arrhythmias. The findings of this QI project support the
use of either dabigatran or warfarin before a planned cardioversion at VAPORHCS. Several factors should be considered when choosing an oral anticoagulant before a planned DCCV, including indication, duration of anticoagulation, previous anticoagulant use, medication adherence, renal function, risk of thromboembolism vs bleeding risk, and potential need for a reversal agent.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(2):2071-2104.
2. Van Gelder IC, Crijns HJ, van Gilst WH, Hamer HP, Lie KI. Decrease of right and left atrial sizes after direct-current electrical cardioversion in chronic atrial fibrillation. Am J Cardiol. 1991;67(1):93-95.
3. Manning WJ, Leeman DE, Gotch PJ, Come PC. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13(3):617-623.
4. Design of a clinical trial for the assessment of cardioversion using transesophageal echocardiography (The ACUTE Multicenter Study). Steering and Publications Committees of the ACUTE Study. Am J Cardiol. 1998;81(7):877-883.
5. You JJ, Singer DE, Howard PA, et al; American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
6. Mancini GB, Goldberger AL. Cardioversion of atrial fibrillation: consideration of embolization, anticoagulation, prophylactic pacemaker, and long-term success. Am Heart J. 1982;104(3):617-621.
7. Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82(12):1545-1547, A8.
8. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2015.
9. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2015.
10. Eliquis [package insert]. Princeton, NJ: Bristol Myers Squibb Company; 2015.
11. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2015.
12. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131-136.
13. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
14. Piccini JP, Stevens SR, Lokhnygina Y, et al; ROCKET AF Steering Committee & Investigators. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61(19):1998-2006.
15. Flaker G, Lopes RD, Al-Khatib SM, et al; ARISTOTLE Committees and Investigators. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol. 2014;63(11):1082-1087.
16. Choo WK, Fraser S, Padfield G, et al. Dabigatran improves the efficiency of an elective direct current cardioversion service. Br J Cardiol. 2014;21(1):29-32.
17. Alt E, Ammer R, Lehmann G, et al. Patient characteristics and underlying heart disease as predictors of recurrent atrial fibrillation after internal and external cardioversion in patients treated with oral sotalol. Am Heart J. 1997;134(3):419-425.
1. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(2):2071-2104.
2. Van Gelder IC, Crijns HJ, van Gilst WH, Hamer HP, Lie KI. Decrease of right and left atrial sizes after direct-current electrical cardioversion in chronic atrial fibrillation. Am J Cardiol. 1991;67(1):93-95.
3. Manning WJ, Leeman DE, Gotch PJ, Come PC. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13(3):617-623.
4. Design of a clinical trial for the assessment of cardioversion using transesophageal echocardiography (The ACUTE Multicenter Study). Steering and Publications Committees of the ACUTE Study. Am J Cardiol. 1998;81(7):877-883.
5. You JJ, Singer DE, Howard PA, et al; American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
6. Mancini GB, Goldberger AL. Cardioversion of atrial fibrillation: consideration of embolization, anticoagulation, prophylactic pacemaker, and long-term success. Am Heart J. 1982;104(3):617-621.
7. Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82(12):1545-1547, A8.
8. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2015.
9. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2015.
10. Eliquis [package insert]. Princeton, NJ: Bristol Myers Squibb Company; 2015.
11. Savaysa [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc; 2015.
12. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131-136.
13. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
14. Piccini JP, Stevens SR, Lokhnygina Y, et al; ROCKET AF Steering Committee & Investigators. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61(19):1998-2006.
15. Flaker G, Lopes RD, Al-Khatib SM, et al; ARISTOTLE Committees and Investigators. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J Am Coll Cardiol. 2014;63(11):1082-1087.
16. Choo WK, Fraser S, Padfield G, et al. Dabigatran improves the efficiency of an elective direct current cardioversion service. Br J Cardiol. 2014;21(1):29-32.
17. Alt E, Ammer R, Lehmann G, et al. Patient characteristics and underlying heart disease as predictors of recurrent atrial fibrillation after internal and external cardioversion in patients treated with oral sotalol. Am Heart J. 1997;134(3):419-425.