User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
RA Incidence Rises With Age, Peaking During 70s
Major Finding: The incidence rate of newly diagnosed rheumatoid arthritis rises with age, peaking among people in their 70s with an incidence of 73 new cases per 100,000 population annually.
Data Source: Review of Swedish national patient registers for 2006-2008, which identified about 10,000 newly diagnosed cases of RA.
Disclosures: Mr. Eriksson said he had no disclosures.
LONDON – The incidence of rheumatoid arthritis rises with age in both men and women and shows the biggest jump during the sixth decade of life, when the incidence among adults in their 50s nearly doubles compared with those in their 40s, based on findings from an analysis of about 10,000 Swedish patients diagnosed for the first time during 2006-2008.
The nationwide data also showed that peak RA incidence occurs among men and women in their 70s, with a new-onset rate of at least 73 cases per 100,000 population annually, Jonas Eriksson said. h
The nationwide Swedish data that allowed analysis of about 10,000 cases far exceeded the scope of previous incidence estimates, enabling Mr. Eriksson and his associates in the clinical epidemiology unit at the Karolinska Institute, Stockholm, to estimate incidence rates by age and sex. They said they used data collected by the Swedish Rheumatology Quality Register, the National Patient Register, and the Prescribed Drug Register.
To assess incident RA cases, the investigators used three different definitions of new-onset disease. The most restrictive definition involved patients who met all of five separate defining criteria:
▸ A first-ever inpatient visit, a specialist outpatient visit, or inclusion in the Swedish Rheumatology Quality Register with a RA diagnosis during 2006-2008;
▸ At least one visit to a rheumatology or internal medicine department;
▸ At least two visits with a diagnosis of RA during 2006-2008;
▸ A second visit with a RA diagnosis within 1 year after a first visit; and
▸ Exclusion of patients treated with a disease-modifying antirheumatic drug more than 6 months before the first visit with a RA diagnosis, pain in joints, or an unspecified diagnosis.
Applying these criteria to the databases for 2006-2008 identified 7,953 patients with a presumed first-time diagnosis of RA, which resulted in a calculated incidence rate of 35 cases/100,000 population per year. Broken down by gender, the rates were 22 cases/100,000 in men and 48 cases/100,000 in women. Mr. Eriksson also reported incidence rates among men and women broken down by age. (See table.) The peak new-onset rates occurred in people aged 70-79 years, with rates of 60 cases/100,000 per year among men and 86 cases/100,000 per year in women.
To further broaden the analysis, the researchers calculated incidence rates using two less stringent definitions. They applied a “medium” definition that eliminated the exclusion portion of their initial, strict definition. This identified 9,133 new-onset cases during the 3 years studied, with an overall incidence rate of 41 cases/100,000 per year and rates of 25 cases/100,000 per year in men and 55 cases/100,000 per year in women.
A third, “liberal” definition of RA limited the identifying criteria to the first two elements from the original list of five: a first-ever inpatient visit, a specialist outpatient visit, or inclusion in the Swedish Rheumatology Quality Register during 2006-2008; and at least one visit to a rheumatology or internal medicine department. This identified 11,715 new-onset cases in 2006-2008, an overall rate of 52/100,000 per year, with rates of 33/100,000 per year in men and 71/100,000 per year in women, he said.
Vitals
Source Elsevier Global Medical News
Major Finding: The incidence rate of newly diagnosed rheumatoid arthritis rises with age, peaking among people in their 70s with an incidence of 73 new cases per 100,000 population annually.
Data Source: Review of Swedish national patient registers for 2006-2008, which identified about 10,000 newly diagnosed cases of RA.
Disclosures: Mr. Eriksson said he had no disclosures.
LONDON – The incidence of rheumatoid arthritis rises with age in both men and women and shows the biggest jump during the sixth decade of life, when the incidence among adults in their 50s nearly doubles compared with those in their 40s, based on findings from an analysis of about 10,000 Swedish patients diagnosed for the first time during 2006-2008.
The nationwide data also showed that peak RA incidence occurs among men and women in their 70s, with a new-onset rate of at least 73 cases per 100,000 population annually, Jonas Eriksson said. h
The nationwide Swedish data that allowed analysis of about 10,000 cases far exceeded the scope of previous incidence estimates, enabling Mr. Eriksson and his associates in the clinical epidemiology unit at the Karolinska Institute, Stockholm, to estimate incidence rates by age and sex. They said they used data collected by the Swedish Rheumatology Quality Register, the National Patient Register, and the Prescribed Drug Register.
To assess incident RA cases, the investigators used three different definitions of new-onset disease. The most restrictive definition involved patients who met all of five separate defining criteria:
▸ A first-ever inpatient visit, a specialist outpatient visit, or inclusion in the Swedish Rheumatology Quality Register with a RA diagnosis during 2006-2008;
▸ At least one visit to a rheumatology or internal medicine department;
▸ At least two visits with a diagnosis of RA during 2006-2008;
▸ A second visit with a RA diagnosis within 1 year after a first visit; and
▸ Exclusion of patients treated with a disease-modifying antirheumatic drug more than 6 months before the first visit with a RA diagnosis, pain in joints, or an unspecified diagnosis.
Applying these criteria to the databases for 2006-2008 identified 7,953 patients with a presumed first-time diagnosis of RA, which resulted in a calculated incidence rate of 35 cases/100,000 population per year. Broken down by gender, the rates were 22 cases/100,000 in men and 48 cases/100,000 in women. Mr. Eriksson also reported incidence rates among men and women broken down by age. (See table.) The peak new-onset rates occurred in people aged 70-79 years, with rates of 60 cases/100,000 per year among men and 86 cases/100,000 per year in women.
To further broaden the analysis, the researchers calculated incidence rates using two less stringent definitions. They applied a “medium” definition that eliminated the exclusion portion of their initial, strict definition. This identified 9,133 new-onset cases during the 3 years studied, with an overall incidence rate of 41 cases/100,000 per year and rates of 25 cases/100,000 per year in men and 55 cases/100,000 per year in women.
A third, “liberal” definition of RA limited the identifying criteria to the first two elements from the original list of five: a first-ever inpatient visit, a specialist outpatient visit, or inclusion in the Swedish Rheumatology Quality Register during 2006-2008; and at least one visit to a rheumatology or internal medicine department. This identified 11,715 new-onset cases in 2006-2008, an overall rate of 52/100,000 per year, with rates of 33/100,000 per year in men and 71/100,000 per year in women, he said.
Vitals
Source Elsevier Global Medical News
Major Finding: The incidence rate of newly diagnosed rheumatoid arthritis rises with age, peaking among people in their 70s with an incidence of 73 new cases per 100,000 population annually.
Data Source: Review of Swedish national patient registers for 2006-2008, which identified about 10,000 newly diagnosed cases of RA.
Disclosures: Mr. Eriksson said he had no disclosures.
LONDON – The incidence of rheumatoid arthritis rises with age in both men and women and shows the biggest jump during the sixth decade of life, when the incidence among adults in their 50s nearly doubles compared with those in their 40s, based on findings from an analysis of about 10,000 Swedish patients diagnosed for the first time during 2006-2008.
The nationwide data also showed that peak RA incidence occurs among men and women in their 70s, with a new-onset rate of at least 73 cases per 100,000 population annually, Jonas Eriksson said. h
The nationwide Swedish data that allowed analysis of about 10,000 cases far exceeded the scope of previous incidence estimates, enabling Mr. Eriksson and his associates in the clinical epidemiology unit at the Karolinska Institute, Stockholm, to estimate incidence rates by age and sex. They said they used data collected by the Swedish Rheumatology Quality Register, the National Patient Register, and the Prescribed Drug Register.
To assess incident RA cases, the investigators used three different definitions of new-onset disease. The most restrictive definition involved patients who met all of five separate defining criteria:
▸ A first-ever inpatient visit, a specialist outpatient visit, or inclusion in the Swedish Rheumatology Quality Register with a RA diagnosis during 2006-2008;
▸ At least one visit to a rheumatology or internal medicine department;
▸ At least two visits with a diagnosis of RA during 2006-2008;
▸ A second visit with a RA diagnosis within 1 year after a first visit; and
▸ Exclusion of patients treated with a disease-modifying antirheumatic drug more than 6 months before the first visit with a RA diagnosis, pain in joints, or an unspecified diagnosis.
Applying these criteria to the databases for 2006-2008 identified 7,953 patients with a presumed first-time diagnosis of RA, which resulted in a calculated incidence rate of 35 cases/100,000 population per year. Broken down by gender, the rates were 22 cases/100,000 in men and 48 cases/100,000 in women. Mr. Eriksson also reported incidence rates among men and women broken down by age. (See table.) The peak new-onset rates occurred in people aged 70-79 years, with rates of 60 cases/100,000 per year among men and 86 cases/100,000 per year in women.
To further broaden the analysis, the researchers calculated incidence rates using two less stringent definitions. They applied a “medium” definition that eliminated the exclusion portion of their initial, strict definition. This identified 9,133 new-onset cases during the 3 years studied, with an overall incidence rate of 41 cases/100,000 per year and rates of 25 cases/100,000 per year in men and 55 cases/100,000 per year in women.
A third, “liberal” definition of RA limited the identifying criteria to the first two elements from the original list of five: a first-ever inpatient visit, a specialist outpatient visit, or inclusion in the Swedish Rheumatology Quality Register during 2006-2008; and at least one visit to a rheumatology or internal medicine department. This identified 11,715 new-onset cases in 2006-2008, an overall rate of 52/100,000 per year, with rates of 33/100,000 per year in men and 71/100,000 per year in women, he said.
Vitals
Source Elsevier Global Medical News
Lesinurad Cuts Uric Acid in Refractory Gout : Ninety percent of patients who remained on treatment for 28 weeks met the study's target level.
Major Finding: After 4 weeks of treatment with a combination of 200-600 mg/day lesinurad plus allopurinol and colchicine, 71%-87% of patients had serum uric acid levels lower than 6 mg/dL, compared with 28% of patients who were treated with allopurinol, colchicine, and placebo.
Data Source: A randomized, phase II study with 208 patients who were diagnosed with gout and whose serum uric acid levels remained above 6 mg/dL despite at least 6 weeks on steady treatment with 200-600 mg/day of allo-purinol.
Disclosures: The study was funded by Ardea Biosciences, which is developing lesinurad. Dr. Perez-Ruiz said that he has had financial relationships with Ardea, Menarini, and Novartis. Dr. Dougados said he has been a consultant for and received research support from Roche.
LONDON – An investigational drug that boosts uric acid secretion led to significant cuts in serum uric acid levels during 4 weeks of treatment in a phase II study of 208 patients with allopurinol-refractory gout.
In addition, among 30 patients who remained on the uricosuric agent lesinurad for 28 weeks in an extension phase, 27 patients (90%) reached the study's target level of serum uric acid (lower than 6 mg/dL), according to Dr. Fernando Perez-Ruiz.
Treatment with dosages of 200-600 mg/day of lesinurad also appeared safe, with no adverse effects or other safety concerns seen during the limited treatment period reported, said Dr. Perez-Ruiz, a rheumatologist at Hospital de Cruces in Barakaldo, Spain.
The drug worked equally well in patients with normal renal function (a glomerular filtration rate of at least 90 mL/min per 1.73 m
Phase III Trial to Start This Year
Based on these promising early results, a phase III trial of lesinurad will start before the end of this year, said Barry D. Quart, Pharm.D., president of Ardea Biosciences, the San Diego–based company that is developing the drug.
The phase III study will likely focus on the 200-mg and 400-mg/day dosages, as over time most patients appeared to respond to these lower dosages.
“Almost no one needs 600 mg/day to get a response,” Dr. Quart said in an interview.
Although the current study used patients' serum uric acid levels of lower than 6 mg/dL as the end point and did not assess clinical features of gout, this end point is highly meaningful, said Dr. Maxime Dougados, professor of rheumatology at René Descartes University in Paris.
Gout treatment guidelines developed by the European League Against Rheumatism (EULAR) emphasize “the importance of treating patients to a target serum uric acid of less than 6 mg/dL,” commented Dr. Dougados.
New Drugs Increase Excretion
“Until now, we have drugs that decrease uric acid synthesis, but we have not recently had any drugs to increase excretion,” Dr. Dougados observed, adding that “Now we have a new drug to increase excretion, and that is very important because allopurinol is often not sufficient to achieve the target serum uric acid level.”
Lesinurad works by inhibiting URAT1, a uric acid transporter molecule in the kidney that takes uric acid out of urine and places it back into the blood.
The study led by Dr. Perez-Ruiz enrolled patients who met the 1977 gout diagnosis criteria of the American Rheumatism Association (now the American College of Rheumatology) and who had a serum uric acid level greater than 6 mg/dL despite being on a stable dose of 200-600 mg allopurinol for at least 6 weeks.
Patients were randomized to daily treatment with 200 mg, 400 mg, or 600 mg of oral lesinurad or placebo.
All patients also received a 0.5-mg or 0.6-mg daily dose of colchicine, and they all also remained on the dosage of allopurinol that they were taking at entry into the study.
After 4 weeks on treatment, the percentage of patients whose serum uric acid level had fallen below 6 mg/dL was 28% in the 72 placebo patients, and 71%, 76%, and 87% in the three lesinurad treatment groups, which each contained 42-48 patients.
The difference in the percentage of responding patients in each of the three treatment arms was statistically significant, compared with the placebo group.
“Lesinurad produced rapid and sustained reductions in uric acid levels when added on to allopurinol in patients who were not adequately responding to allopurinol alone,” Dr. Perez-Ruiz concluded.
Major Finding: After 4 weeks of treatment with a combination of 200-600 mg/day lesinurad plus allopurinol and colchicine, 71%-87% of patients had serum uric acid levels lower than 6 mg/dL, compared with 28% of patients who were treated with allopurinol, colchicine, and placebo.
Data Source: A randomized, phase II study with 208 patients who were diagnosed with gout and whose serum uric acid levels remained above 6 mg/dL despite at least 6 weeks on steady treatment with 200-600 mg/day of allo-purinol.
Disclosures: The study was funded by Ardea Biosciences, which is developing lesinurad. Dr. Perez-Ruiz said that he has had financial relationships with Ardea, Menarini, and Novartis. Dr. Dougados said he has been a consultant for and received research support from Roche.
LONDON – An investigational drug that boosts uric acid secretion led to significant cuts in serum uric acid levels during 4 weeks of treatment in a phase II study of 208 patients with allopurinol-refractory gout.
In addition, among 30 patients who remained on the uricosuric agent lesinurad for 28 weeks in an extension phase, 27 patients (90%) reached the study's target level of serum uric acid (lower than 6 mg/dL), according to Dr. Fernando Perez-Ruiz.
Treatment with dosages of 200-600 mg/day of lesinurad also appeared safe, with no adverse effects or other safety concerns seen during the limited treatment period reported, said Dr. Perez-Ruiz, a rheumatologist at Hospital de Cruces in Barakaldo, Spain.
The drug worked equally well in patients with normal renal function (a glomerular filtration rate of at least 90 mL/min per 1.73 m
Phase III Trial to Start This Year
Based on these promising early results, a phase III trial of lesinurad will start before the end of this year, said Barry D. Quart, Pharm.D., president of Ardea Biosciences, the San Diego–based company that is developing the drug.
The phase III study will likely focus on the 200-mg and 400-mg/day dosages, as over time most patients appeared to respond to these lower dosages.
“Almost no one needs 600 mg/day to get a response,” Dr. Quart said in an interview.
Although the current study used patients' serum uric acid levels of lower than 6 mg/dL as the end point and did not assess clinical features of gout, this end point is highly meaningful, said Dr. Maxime Dougados, professor of rheumatology at René Descartes University in Paris.
Gout treatment guidelines developed by the European League Against Rheumatism (EULAR) emphasize “the importance of treating patients to a target serum uric acid of less than 6 mg/dL,” commented Dr. Dougados.
New Drugs Increase Excretion
“Until now, we have drugs that decrease uric acid synthesis, but we have not recently had any drugs to increase excretion,” Dr. Dougados observed, adding that “Now we have a new drug to increase excretion, and that is very important because allopurinol is often not sufficient to achieve the target serum uric acid level.”
Lesinurad works by inhibiting URAT1, a uric acid transporter molecule in the kidney that takes uric acid out of urine and places it back into the blood.
The study led by Dr. Perez-Ruiz enrolled patients who met the 1977 gout diagnosis criteria of the American Rheumatism Association (now the American College of Rheumatology) and who had a serum uric acid level greater than 6 mg/dL despite being on a stable dose of 200-600 mg allopurinol for at least 6 weeks.
Patients were randomized to daily treatment with 200 mg, 400 mg, or 600 mg of oral lesinurad or placebo.
All patients also received a 0.5-mg or 0.6-mg daily dose of colchicine, and they all also remained on the dosage of allopurinol that they were taking at entry into the study.
After 4 weeks on treatment, the percentage of patients whose serum uric acid level had fallen below 6 mg/dL was 28% in the 72 placebo patients, and 71%, 76%, and 87% in the three lesinurad treatment groups, which each contained 42-48 patients.
The difference in the percentage of responding patients in each of the three treatment arms was statistically significant, compared with the placebo group.
“Lesinurad produced rapid and sustained reductions in uric acid levels when added on to allopurinol in patients who were not adequately responding to allopurinol alone,” Dr. Perez-Ruiz concluded.
Major Finding: After 4 weeks of treatment with a combination of 200-600 mg/day lesinurad plus allopurinol and colchicine, 71%-87% of patients had serum uric acid levels lower than 6 mg/dL, compared with 28% of patients who were treated with allopurinol, colchicine, and placebo.
Data Source: A randomized, phase II study with 208 patients who were diagnosed with gout and whose serum uric acid levels remained above 6 mg/dL despite at least 6 weeks on steady treatment with 200-600 mg/day of allo-purinol.
Disclosures: The study was funded by Ardea Biosciences, which is developing lesinurad. Dr. Perez-Ruiz said that he has had financial relationships with Ardea, Menarini, and Novartis. Dr. Dougados said he has been a consultant for and received research support from Roche.
LONDON – An investigational drug that boosts uric acid secretion led to significant cuts in serum uric acid levels during 4 weeks of treatment in a phase II study of 208 patients with allopurinol-refractory gout.
In addition, among 30 patients who remained on the uricosuric agent lesinurad for 28 weeks in an extension phase, 27 patients (90%) reached the study's target level of serum uric acid (lower than 6 mg/dL), according to Dr. Fernando Perez-Ruiz.
Treatment with dosages of 200-600 mg/day of lesinurad also appeared safe, with no adverse effects or other safety concerns seen during the limited treatment period reported, said Dr. Perez-Ruiz, a rheumatologist at Hospital de Cruces in Barakaldo, Spain.
The drug worked equally well in patients with normal renal function (a glomerular filtration rate of at least 90 mL/min per 1.73 m
Phase III Trial to Start This Year
Based on these promising early results, a phase III trial of lesinurad will start before the end of this year, said Barry D. Quart, Pharm.D., president of Ardea Biosciences, the San Diego–based company that is developing the drug.
The phase III study will likely focus on the 200-mg and 400-mg/day dosages, as over time most patients appeared to respond to these lower dosages.
“Almost no one needs 600 mg/day to get a response,” Dr. Quart said in an interview.
Although the current study used patients' serum uric acid levels of lower than 6 mg/dL as the end point and did not assess clinical features of gout, this end point is highly meaningful, said Dr. Maxime Dougados, professor of rheumatology at René Descartes University in Paris.
Gout treatment guidelines developed by the European League Against Rheumatism (EULAR) emphasize “the importance of treating patients to a target serum uric acid of less than 6 mg/dL,” commented Dr. Dougados.
New Drugs Increase Excretion
“Until now, we have drugs that decrease uric acid synthesis, but we have not recently had any drugs to increase excretion,” Dr. Dougados observed, adding that “Now we have a new drug to increase excretion, and that is very important because allopurinol is often not sufficient to achieve the target serum uric acid level.”
Lesinurad works by inhibiting URAT1, a uric acid transporter molecule in the kidney that takes uric acid out of urine and places it back into the blood.
The study led by Dr. Perez-Ruiz enrolled patients who met the 1977 gout diagnosis criteria of the American Rheumatism Association (now the American College of Rheumatology) and who had a serum uric acid level greater than 6 mg/dL despite being on a stable dose of 200-600 mg allopurinol for at least 6 weeks.
Patients were randomized to daily treatment with 200 mg, 400 mg, or 600 mg of oral lesinurad or placebo.
All patients also received a 0.5-mg or 0.6-mg daily dose of colchicine, and they all also remained on the dosage of allopurinol that they were taking at entry into the study.
After 4 weeks on treatment, the percentage of patients whose serum uric acid level had fallen below 6 mg/dL was 28% in the 72 placebo patients, and 71%, 76%, and 87% in the three lesinurad treatment groups, which each contained 42-48 patients.
The difference in the percentage of responding patients in each of the three treatment arms was statistically significant, compared with the placebo group.
“Lesinurad produced rapid and sustained reductions in uric acid levels when added on to allopurinol in patients who were not adequately responding to allopurinol alone,” Dr. Perez-Ruiz concluded.
Hyperuricemia Boosts Risk of Hypertension in Young Adults
Young adults with hyperuricemia faced a significantly increased risk for later developing hypertension, based on follow-up of more than 4,900 Americans.
This link between hyperuricemia and the later appearance of hypertension did not involve a confounding role by metabolic syndrome.
And although the analysis could not establish a causal link between hyperuricemia and hypertension, the results indicated that an elevated serum level of uric acid marks people with an increased risk for later having hypertension, according to Dr. Eswar Krishnan.
Dr. Krishnan and his associates conducted a multivariate analysis that adjusted for baseline differences in subject age, gender, race, serum creatinine clearance, and waist circumferences.
The investigators found that people in the highest quartile of serum uric acid level at baseline had a significant, 76% increased risk for later developing hypertension, compared with the quartile of people with the lowest baseline serum uric acid level, reported Dr. Krishnan, a rheumatologist at Stanford (Calif.) University.
The study used data from the 5,115 people enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA), which entered people between the ages of 18-33 years at four U.S. sites in 1986.
The investigators followed them for up to 20 years.
Excluding people who at baseline had hypertension or any other component of metabolic syndrome (abdominal obesity, elevated triglycerides, depressed high-density lipoprotein cholesterol, elevated fasting glucose) left 4,918 people for the analysis.
The researchers used serum uric acid levels as the basis for dividing the study group into quartile.
They found that in men serum uric acid levels ranged from 0.4-5.3 mg/dL uric acid in the lowest quartile to 6.8 mg/dL or greater in the highest quartile, and in women ranged from 0.6-3.7 mg/dL in the lowest quartile to 5.0 mg/dL or greater in the highest quartile.
During the 20 years of follow-up, 7% of the men in the lowest quartile for serum uric acid developed incident hypertension. In contrast, 16% of the men in the highest uric acid quartile developed new-onset hypertension. The difference was statistically significant, Dr. Krishnan and his associates reported at the annual European Congress of Rheumatology in London.
When the researchers subdivided the CARDIA subjects by race and sex, elevated serum uric acid levels linked with a significantly increased risk of later developing hypertension among black men and women and among white men.
The link did not reach statistical significance among white women because of the small number of incident cases of hypertension during follow-up.
The analysis was sponsored by Takeda, which markets febuxostat (Uloric), a drug approved to lower serum uric acid levels in patients with gout.
Dr. Krishnan said that he has been a consultant to Takeda, Savient, and Ardea. Three coauthors on the study are Takeda employees.
Young adults with hyperuricemia faced a significantly increased risk for later developing hypertension, based on follow-up of more than 4,900 Americans.
This link between hyperuricemia and the later appearance of hypertension did not involve a confounding role by metabolic syndrome.
And although the analysis could not establish a causal link between hyperuricemia and hypertension, the results indicated that an elevated serum level of uric acid marks people with an increased risk for later having hypertension, according to Dr. Eswar Krishnan.
Dr. Krishnan and his associates conducted a multivariate analysis that adjusted for baseline differences in subject age, gender, race, serum creatinine clearance, and waist circumferences.
The investigators found that people in the highest quartile of serum uric acid level at baseline had a significant, 76% increased risk for later developing hypertension, compared with the quartile of people with the lowest baseline serum uric acid level, reported Dr. Krishnan, a rheumatologist at Stanford (Calif.) University.
The study used data from the 5,115 people enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA), which entered people between the ages of 18-33 years at four U.S. sites in 1986.
The investigators followed them for up to 20 years.
Excluding people who at baseline had hypertension or any other component of metabolic syndrome (abdominal obesity, elevated triglycerides, depressed high-density lipoprotein cholesterol, elevated fasting glucose) left 4,918 people for the analysis.
The researchers used serum uric acid levels as the basis for dividing the study group into quartile.
They found that in men serum uric acid levels ranged from 0.4-5.3 mg/dL uric acid in the lowest quartile to 6.8 mg/dL or greater in the highest quartile, and in women ranged from 0.6-3.7 mg/dL in the lowest quartile to 5.0 mg/dL or greater in the highest quartile.
During the 20 years of follow-up, 7% of the men in the lowest quartile for serum uric acid developed incident hypertension. In contrast, 16% of the men in the highest uric acid quartile developed new-onset hypertension. The difference was statistically significant, Dr. Krishnan and his associates reported at the annual European Congress of Rheumatology in London.
When the researchers subdivided the CARDIA subjects by race and sex, elevated serum uric acid levels linked with a significantly increased risk of later developing hypertension among black men and women and among white men.
The link did not reach statistical significance among white women because of the small number of incident cases of hypertension during follow-up.
The analysis was sponsored by Takeda, which markets febuxostat (Uloric), a drug approved to lower serum uric acid levels in patients with gout.
Dr. Krishnan said that he has been a consultant to Takeda, Savient, and Ardea. Three coauthors on the study are Takeda employees.
Young adults with hyperuricemia faced a significantly increased risk for later developing hypertension, based on follow-up of more than 4,900 Americans.
This link between hyperuricemia and the later appearance of hypertension did not involve a confounding role by metabolic syndrome.
And although the analysis could not establish a causal link between hyperuricemia and hypertension, the results indicated that an elevated serum level of uric acid marks people with an increased risk for later having hypertension, according to Dr. Eswar Krishnan.
Dr. Krishnan and his associates conducted a multivariate analysis that adjusted for baseline differences in subject age, gender, race, serum creatinine clearance, and waist circumferences.
The investigators found that people in the highest quartile of serum uric acid level at baseline had a significant, 76% increased risk for later developing hypertension, compared with the quartile of people with the lowest baseline serum uric acid level, reported Dr. Krishnan, a rheumatologist at Stanford (Calif.) University.
The study used data from the 5,115 people enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA), which entered people between the ages of 18-33 years at four U.S. sites in 1986.
The investigators followed them for up to 20 years.
Excluding people who at baseline had hypertension or any other component of metabolic syndrome (abdominal obesity, elevated triglycerides, depressed high-density lipoprotein cholesterol, elevated fasting glucose) left 4,918 people for the analysis.
The researchers used serum uric acid levels as the basis for dividing the study group into quartile.
They found that in men serum uric acid levels ranged from 0.4-5.3 mg/dL uric acid in the lowest quartile to 6.8 mg/dL or greater in the highest quartile, and in women ranged from 0.6-3.7 mg/dL in the lowest quartile to 5.0 mg/dL or greater in the highest quartile.
During the 20 years of follow-up, 7% of the men in the lowest quartile for serum uric acid developed incident hypertension. In contrast, 16% of the men in the highest uric acid quartile developed new-onset hypertension. The difference was statistically significant, Dr. Krishnan and his associates reported at the annual European Congress of Rheumatology in London.
When the researchers subdivided the CARDIA subjects by race and sex, elevated serum uric acid levels linked with a significantly increased risk of later developing hypertension among black men and women and among white men.
The link did not reach statistical significance among white women because of the small number of incident cases of hypertension during follow-up.
The analysis was sponsored by Takeda, which markets febuxostat (Uloric), a drug approved to lower serum uric acid levels in patients with gout.
Dr. Krishnan said that he has been a consultant to Takeda, Savient, and Ardea. Three coauthors on the study are Takeda employees.
Transfusions Raise Risk of Death in CABG
PHILADELPHIA – Blood transfusions can kill surgery patients, a finding that puts the onus on surgeons to administer transfusions only when absolutely necessary, according to Dr. Gaetano Paone.
An analysis of more than 31,000 patients who had isolated coronary artery bypass grafting surgery in Michigan during January 2006–June 2010 showed that receiving one or more transfusion conferred a nearly threefold higher risk of operative mortality than did not receiving blood, Dr. Paone reported at the meeting.
“There is great variability in the rates of transfusions across institutions,” noted Dr. Paone, a cardiac surgeon at Henry Ford Hospital in Detroit, in an interview. In some places, the transfusion rates of isolated CABG patients are 15%, and other places have rates of more than 90%. “That suggests it's quite discretionary.”
Dr. Paone and his associates examined data on 31,818 patients who underwent isolated CABG during the study period at any one of the 33 Michigan hospitals that perform cardiac surgery. The data came from records maintained by the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
The researchers calculated the mortality risk faced by each patient using the STS-PROM (Society of Thoracic Surgeons Predicted Risk of Mortality) model, which takes into account 30 preoperative patient variables. They stratified the patients into four risk groups based on their scores, which represent the percent risk for 30-day perioperative mortality (less than 2%, 2%–5%, 6%–10%, and more than 10%), and divided patients into the 55% who received transfusions and the 45% who did not receive any blood. Overall operative mortality in the patients studied was 2%. As expected, operative mortality was higher in patients who received a transfusion (3.3%) than in those who did not (0.6%) – a significant sixfold difference.
The analysis also showed that the significant link between increased mortality and transfusion remained fairly constant across all four risk strata in the study, ranging from a twofold increased risk in patients with an STS-PROM score of 2%–5%, to a fourfold increased risk in patients with a score of more than 10%, said Dr. Paone, who had no disclosures.
To see a video interview with Dr. Paone, scan this QR code using your smartphone.
PHILADELPHIA – Blood transfusions can kill surgery patients, a finding that puts the onus on surgeons to administer transfusions only when absolutely necessary, according to Dr. Gaetano Paone.
An analysis of more than 31,000 patients who had isolated coronary artery bypass grafting surgery in Michigan during January 2006–June 2010 showed that receiving one or more transfusion conferred a nearly threefold higher risk of operative mortality than did not receiving blood, Dr. Paone reported at the meeting.
“There is great variability in the rates of transfusions across institutions,” noted Dr. Paone, a cardiac surgeon at Henry Ford Hospital in Detroit, in an interview. In some places, the transfusion rates of isolated CABG patients are 15%, and other places have rates of more than 90%. “That suggests it's quite discretionary.”
Dr. Paone and his associates examined data on 31,818 patients who underwent isolated CABG during the study period at any one of the 33 Michigan hospitals that perform cardiac surgery. The data came from records maintained by the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
The researchers calculated the mortality risk faced by each patient using the STS-PROM (Society of Thoracic Surgeons Predicted Risk of Mortality) model, which takes into account 30 preoperative patient variables. They stratified the patients into four risk groups based on their scores, which represent the percent risk for 30-day perioperative mortality (less than 2%, 2%–5%, 6%–10%, and more than 10%), and divided patients into the 55% who received transfusions and the 45% who did not receive any blood. Overall operative mortality in the patients studied was 2%. As expected, operative mortality was higher in patients who received a transfusion (3.3%) than in those who did not (0.6%) – a significant sixfold difference.
The analysis also showed that the significant link between increased mortality and transfusion remained fairly constant across all four risk strata in the study, ranging from a twofold increased risk in patients with an STS-PROM score of 2%–5%, to a fourfold increased risk in patients with a score of more than 10%, said Dr. Paone, who had no disclosures.
To see a video interview with Dr. Paone, scan this QR code using your smartphone.
PHILADELPHIA – Blood transfusions can kill surgery patients, a finding that puts the onus on surgeons to administer transfusions only when absolutely necessary, according to Dr. Gaetano Paone.
An analysis of more than 31,000 patients who had isolated coronary artery bypass grafting surgery in Michigan during January 2006–June 2010 showed that receiving one or more transfusion conferred a nearly threefold higher risk of operative mortality than did not receiving blood, Dr. Paone reported at the meeting.
“There is great variability in the rates of transfusions across institutions,” noted Dr. Paone, a cardiac surgeon at Henry Ford Hospital in Detroit, in an interview. In some places, the transfusion rates of isolated CABG patients are 15%, and other places have rates of more than 90%. “That suggests it's quite discretionary.”
Dr. Paone and his associates examined data on 31,818 patients who underwent isolated CABG during the study period at any one of the 33 Michigan hospitals that perform cardiac surgery. The data came from records maintained by the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
The researchers calculated the mortality risk faced by each patient using the STS-PROM (Society of Thoracic Surgeons Predicted Risk of Mortality) model, which takes into account 30 preoperative patient variables. They stratified the patients into four risk groups based on their scores, which represent the percent risk for 30-day perioperative mortality (less than 2%, 2%–5%, 6%–10%, and more than 10%), and divided patients into the 55% who received transfusions and the 45% who did not receive any blood. Overall operative mortality in the patients studied was 2%. As expected, operative mortality was higher in patients who received a transfusion (3.3%) than in those who did not (0.6%) – a significant sixfold difference.
The analysis also showed that the significant link between increased mortality and transfusion remained fairly constant across all four risk strata in the study, ranging from a twofold increased risk in patients with an STS-PROM score of 2%–5%, to a fourfold increased risk in patients with a score of more than 10%, said Dr. Paone, who had no disclosures.
To see a video interview with Dr. Paone, scan this QR code using your smartphone.
High EGFR Expression Flags Best Responders to Cetuximab
AMSTERDAM – Quantifying the amount of epidermal growth factor receptor in a patient’s advanced nonsmall cell lung cancer tumor appeared to identify the tumors that best responded to treatment with cetuximab in an analysis of 1,125 patients enrolled in a key multicenter treatment trial.
The analysis was a follow-up to the positive Cetuximab Plus Chemotherapy in Patients with Non–Small Cell Lung Cancer (FLEX) study, which showed a benefit from the addition of cetuximab (Erbitux) to platinum-based therapy (Lancet 2009;373:1525-31).
Division of the study population into 31% with a high level of epidermal growth factor receptor (EGFR) expression on the surface of tumor cells and 69% with lower expression identified a patient subgroup, the high expressors, that responded best to cetuximab with no increased toxicity, Dr. Robert Pirker reported at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
"The data clearly show that EGFR is a predictive biomarker because in high-EGFR cancers cetuximab changed survival, reduced the hazard ratio to 0.73 [compared with patients not getting cetuximab], but in patients with lower EGFR, cetuximab added nothing to survival," said Dr. Pirker, professor and program director for lung cancer at the Medical University of Vienna.
"We increase efficacy without increasing toxicity, improving the benefit-to-risk ratio. This is a major step forward toward personalized medicine, especially for patients with high EGFR, about a third of patients with advanced disease," he said.
Because the FLEX study’s prespecified goals included analysis of the link between EGFR density and cetuximab response, the new findings warrant immediate application to practice, according to Dr. Pirker. "I believe this is such a significant improvement in survival that we’ve never seen before that we should be able to provide it to our patients," he said.
Future work should examine the potential role of other drugs that affect EGFR activity (the tyrosine kinase inhibitors such as erlotinib and gefitinib) and also the potential role of cetuximab in high EGFR expressors with earlier-stage lung cancer, he added.
But while other lung cancer specialists saw this finding as an exciting new opportunity for directing EGFR-based treatment to the patients who stand to benefit most, they also called for caution in using EGFR quantification in routine practice.
"It needs prospective validation before it can be standard of care," commented Dr. Roy S. Herbst, chief of medical oncology at the Yale Cancer Center in New Haven, Conn. "It’s very intriguing and makes sense that the amount of receptor will make a difference for cetuximab, but it needs to be tested prospectively because they determined the cutoff retrospectively. It’s always dangerous to use a cutoff determined post hoc, because it becomes a self-fulfilling prophesy. It needs to be studied in a validation set. But it gives new hope for the potential use" of cetuximab and other drugs aimed at EGFR function, Dr. Herbst said in an interview.
"These findings from a post hoc analysis for the selected cutoff are extremely interesting and important, but it needs prospective validation," agreed Dr. Giorgio V. Scagliotti, professor of respiratory medicine at the University of Torino, Italy.
The EGFR scoring formula that Dr. Pirker and his associates developed used a commercially available immunohistochemistry staining kit, made by DAKO. Scoring involved rating the staining intensity of each stained cell in a tumor specimen on a scale of 0-3, where 0 is no staining and 3 is the highest level of staining, and then multiplying each score level by the percent of cells in the specimen with that score. This results in a potential score range of 0 (100% of cells have a score of 0) to 300 (100% of cells have a score of 3).
In their review of the FLEX data, the researchers found that 345 (31%) of patients had tumors with a score of at least 200, the cutoff they selected post hoc for a high level of EGFR expression, and the other 776 (69%) had a low score, less than 200.
With this method distinguishing tumors with high and low levels of EGFR expression, the 345 patients in the high category had a 0.73 hazard ratio for mortality when treated with cetuximab plus chemotherapy (cisplatin plus vinorelbine), compared with patients treated with chemotherapy only (P = .011). This appeared to improve on the reduced mortality seen with cetuximab treatment in the entire study of 1,125 patients, which produced a hazard ratio of 0.87 (P = .044). In the 776 patients with lower EGFR expression, the addition of cetuximab led to no change in survival compared with patients who received the control treatment regimen.
Cetuximab’s enhanced efficacy in high EGFR expressors occurred in both the 135 patients with an adenocarcinoma, and in the 144 patients with a squamous cell carcinoma. Among the high expressors, cetuximab also had significantly better performance measures by tumor response rate, with a twofold higher response rate with cetuximab compared with controls (P = .002), and by time-to-treatment-failure, with a statistically significant, average 22% drop in this time (P = .026) with cetuximab compared with controls.
But focusing on high expressors did not result in a significantly improved duration of progression-free survival. The analysis also showed no increased rate of safety issues in the high expressors who received cetuximab, including no increased rate of skin or subcutaneous disorders.
The FLEX study was funded by Merck, which markets cetuximab (Erbitux) outside the United States. Dr. Pirker disclosed relationships with seven companies, including Merck and Eli Lilly, which markets cetuximab in the United States. Dr. Herbst also cited ties to numerous companies, including ImClone, a Lilly subsidiary that developed cetuximab. Dr. Scagliotti had relationships with four companies, including Lilly.
AMSTERDAM – Quantifying the amount of epidermal growth factor receptor in a patient’s advanced nonsmall cell lung cancer tumor appeared to identify the tumors that best responded to treatment with cetuximab in an analysis of 1,125 patients enrolled in a key multicenter treatment trial.
The analysis was a follow-up to the positive Cetuximab Plus Chemotherapy in Patients with Non–Small Cell Lung Cancer (FLEX) study, which showed a benefit from the addition of cetuximab (Erbitux) to platinum-based therapy (Lancet 2009;373:1525-31).
Division of the study population into 31% with a high level of epidermal growth factor receptor (EGFR) expression on the surface of tumor cells and 69% with lower expression identified a patient subgroup, the high expressors, that responded best to cetuximab with no increased toxicity, Dr. Robert Pirker reported at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
"The data clearly show that EGFR is a predictive biomarker because in high-EGFR cancers cetuximab changed survival, reduced the hazard ratio to 0.73 [compared with patients not getting cetuximab], but in patients with lower EGFR, cetuximab added nothing to survival," said Dr. Pirker, professor and program director for lung cancer at the Medical University of Vienna.
"We increase efficacy without increasing toxicity, improving the benefit-to-risk ratio. This is a major step forward toward personalized medicine, especially for patients with high EGFR, about a third of patients with advanced disease," he said.
Because the FLEX study’s prespecified goals included analysis of the link between EGFR density and cetuximab response, the new findings warrant immediate application to practice, according to Dr. Pirker. "I believe this is such a significant improvement in survival that we’ve never seen before that we should be able to provide it to our patients," he said.
Future work should examine the potential role of other drugs that affect EGFR activity (the tyrosine kinase inhibitors such as erlotinib and gefitinib) and also the potential role of cetuximab in high EGFR expressors with earlier-stage lung cancer, he added.
But while other lung cancer specialists saw this finding as an exciting new opportunity for directing EGFR-based treatment to the patients who stand to benefit most, they also called for caution in using EGFR quantification in routine practice.
"It needs prospective validation before it can be standard of care," commented Dr. Roy S. Herbst, chief of medical oncology at the Yale Cancer Center in New Haven, Conn. "It’s very intriguing and makes sense that the amount of receptor will make a difference for cetuximab, but it needs to be tested prospectively because they determined the cutoff retrospectively. It’s always dangerous to use a cutoff determined post hoc, because it becomes a self-fulfilling prophesy. It needs to be studied in a validation set. But it gives new hope for the potential use" of cetuximab and other drugs aimed at EGFR function, Dr. Herbst said in an interview.
"These findings from a post hoc analysis for the selected cutoff are extremely interesting and important, but it needs prospective validation," agreed Dr. Giorgio V. Scagliotti, professor of respiratory medicine at the University of Torino, Italy.
The EGFR scoring formula that Dr. Pirker and his associates developed used a commercially available immunohistochemistry staining kit, made by DAKO. Scoring involved rating the staining intensity of each stained cell in a tumor specimen on a scale of 0-3, where 0 is no staining and 3 is the highest level of staining, and then multiplying each score level by the percent of cells in the specimen with that score. This results in a potential score range of 0 (100% of cells have a score of 0) to 300 (100% of cells have a score of 3).
In their review of the FLEX data, the researchers found that 345 (31%) of patients had tumors with a score of at least 200, the cutoff they selected post hoc for a high level of EGFR expression, and the other 776 (69%) had a low score, less than 200.
With this method distinguishing tumors with high and low levels of EGFR expression, the 345 patients in the high category had a 0.73 hazard ratio for mortality when treated with cetuximab plus chemotherapy (cisplatin plus vinorelbine), compared with patients treated with chemotherapy only (P = .011). This appeared to improve on the reduced mortality seen with cetuximab treatment in the entire study of 1,125 patients, which produced a hazard ratio of 0.87 (P = .044). In the 776 patients with lower EGFR expression, the addition of cetuximab led to no change in survival compared with patients who received the control treatment regimen.
Cetuximab’s enhanced efficacy in high EGFR expressors occurred in both the 135 patients with an adenocarcinoma, and in the 144 patients with a squamous cell carcinoma. Among the high expressors, cetuximab also had significantly better performance measures by tumor response rate, with a twofold higher response rate with cetuximab compared with controls (P = .002), and by time-to-treatment-failure, with a statistically significant, average 22% drop in this time (P = .026) with cetuximab compared with controls.
But focusing on high expressors did not result in a significantly improved duration of progression-free survival. The analysis also showed no increased rate of safety issues in the high expressors who received cetuximab, including no increased rate of skin or subcutaneous disorders.
The FLEX study was funded by Merck, which markets cetuximab (Erbitux) outside the United States. Dr. Pirker disclosed relationships with seven companies, including Merck and Eli Lilly, which markets cetuximab in the United States. Dr. Herbst also cited ties to numerous companies, including ImClone, a Lilly subsidiary that developed cetuximab. Dr. Scagliotti had relationships with four companies, including Lilly.
AMSTERDAM – Quantifying the amount of epidermal growth factor receptor in a patient’s advanced nonsmall cell lung cancer tumor appeared to identify the tumors that best responded to treatment with cetuximab in an analysis of 1,125 patients enrolled in a key multicenter treatment trial.
The analysis was a follow-up to the positive Cetuximab Plus Chemotherapy in Patients with Non–Small Cell Lung Cancer (FLEX) study, which showed a benefit from the addition of cetuximab (Erbitux) to platinum-based therapy (Lancet 2009;373:1525-31).
Division of the study population into 31% with a high level of epidermal growth factor receptor (EGFR) expression on the surface of tumor cells and 69% with lower expression identified a patient subgroup, the high expressors, that responded best to cetuximab with no increased toxicity, Dr. Robert Pirker reported at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
"The data clearly show that EGFR is a predictive biomarker because in high-EGFR cancers cetuximab changed survival, reduced the hazard ratio to 0.73 [compared with patients not getting cetuximab], but in patients with lower EGFR, cetuximab added nothing to survival," said Dr. Pirker, professor and program director for lung cancer at the Medical University of Vienna.
"We increase efficacy without increasing toxicity, improving the benefit-to-risk ratio. This is a major step forward toward personalized medicine, especially for patients with high EGFR, about a third of patients with advanced disease," he said.
Because the FLEX study’s prespecified goals included analysis of the link between EGFR density and cetuximab response, the new findings warrant immediate application to practice, according to Dr. Pirker. "I believe this is such a significant improvement in survival that we’ve never seen before that we should be able to provide it to our patients," he said.
Future work should examine the potential role of other drugs that affect EGFR activity (the tyrosine kinase inhibitors such as erlotinib and gefitinib) and also the potential role of cetuximab in high EGFR expressors with earlier-stage lung cancer, he added.
But while other lung cancer specialists saw this finding as an exciting new opportunity for directing EGFR-based treatment to the patients who stand to benefit most, they also called for caution in using EGFR quantification in routine practice.
"It needs prospective validation before it can be standard of care," commented Dr. Roy S. Herbst, chief of medical oncology at the Yale Cancer Center in New Haven, Conn. "It’s very intriguing and makes sense that the amount of receptor will make a difference for cetuximab, but it needs to be tested prospectively because they determined the cutoff retrospectively. It’s always dangerous to use a cutoff determined post hoc, because it becomes a self-fulfilling prophesy. It needs to be studied in a validation set. But it gives new hope for the potential use" of cetuximab and other drugs aimed at EGFR function, Dr. Herbst said in an interview.
"These findings from a post hoc analysis for the selected cutoff are extremely interesting and important, but it needs prospective validation," agreed Dr. Giorgio V. Scagliotti, professor of respiratory medicine at the University of Torino, Italy.
The EGFR scoring formula that Dr. Pirker and his associates developed used a commercially available immunohistochemistry staining kit, made by DAKO. Scoring involved rating the staining intensity of each stained cell in a tumor specimen on a scale of 0-3, where 0 is no staining and 3 is the highest level of staining, and then multiplying each score level by the percent of cells in the specimen with that score. This results in a potential score range of 0 (100% of cells have a score of 0) to 300 (100% of cells have a score of 3).
In their review of the FLEX data, the researchers found that 345 (31%) of patients had tumors with a score of at least 200, the cutoff they selected post hoc for a high level of EGFR expression, and the other 776 (69%) had a low score, less than 200.
With this method distinguishing tumors with high and low levels of EGFR expression, the 345 patients in the high category had a 0.73 hazard ratio for mortality when treated with cetuximab plus chemotherapy (cisplatin plus vinorelbine), compared with patients treated with chemotherapy only (P = .011). This appeared to improve on the reduced mortality seen with cetuximab treatment in the entire study of 1,125 patients, which produced a hazard ratio of 0.87 (P = .044). In the 776 patients with lower EGFR expression, the addition of cetuximab led to no change in survival compared with patients who received the control treatment regimen.
Cetuximab’s enhanced efficacy in high EGFR expressors occurred in both the 135 patients with an adenocarcinoma, and in the 144 patients with a squamous cell carcinoma. Among the high expressors, cetuximab also had significantly better performance measures by tumor response rate, with a twofold higher response rate with cetuximab compared with controls (P = .002), and by time-to-treatment-failure, with a statistically significant, average 22% drop in this time (P = .026) with cetuximab compared with controls.
But focusing on high expressors did not result in a significantly improved duration of progression-free survival. The analysis also showed no increased rate of safety issues in the high expressors who received cetuximab, including no increased rate of skin or subcutaneous disorders.
The FLEX study was funded by Merck, which markets cetuximab (Erbitux) outside the United States. Dr. Pirker disclosed relationships with seven companies, including Merck and Eli Lilly, which markets cetuximab in the United States. Dr. Herbst also cited ties to numerous companies, including ImClone, a Lilly subsidiary that developed cetuximab. Dr. Scagliotti had relationships with four companies, including Lilly.
FROM THE WORLD CONFERENCE ON LUNG CANCER
Major Finding: Patients with high EGFR scores had significantly improved survival with the addition of cetuximab to chemotherapy (hazard ratio, 0.73; P = .011), but those with low scores saw no difference (hazard ratio 0.99).
Data Source: Prespecified secondary analysis of data collected in the, multicenter Cetuximab Plus Chemotherapy in Patients with Advanced Non–Small Cell Lung Cancer (FLEX) study.
Disclosures: The FLEX study was funded by Merck, which markets cetuximab (Erbitux) outside the United States. Dr. Pirker disclosed relationships with seven companies, including Merck and Eli Lilly, which markets cetuximab in the U.S. Dr. Herbst also cited ties to numerous companies, including ImClone, a Lilly subsidiary that developed cetuximab. Dr. Scagliotti had relationships with four companies, including Lilly.
Staging: EBUS Equals Mediastinoscopy?
PHILADELPHIA - Endobronchial ultrasound-guided biopsy of mediastinal lymph nodes in patients with operable non-small cell lung cancer worked as effectively for staging as did the standard approach - mediastinoscopy in a head-to-head comparison of the two methods.
"Our results have shown that EBUS-TBNA [endobronchial ultrasound-guided transbronchial needle aspiration], when performed as in this study, can replace mediastinoscopy for accurate staging of the mediastinum in potentially resectable lung cancer," Dr. Kazuhiro Yasufuku said at the annual meeting of the American Association for Thoracic Surgery.
Based on these results, which were obtained in 153 patients treated by any one of seven surgeons working at Toronto General Hospital, Dr. Yasufuku and his colleagues now routinely use EBUS-TBNA as their initial approach for staging patients with inoperable non?small cell lung cancer (NSCLC), who account for about 70% of all NSCLC patients they treat. As long as they can collect adequate cell specimens for cytologic analysis from the lymph node stations they routinely assess, they rely exclusively on EBUS-TBNA for staging, which allows them to avoid mediastinoscopy for most of their patients, Dr. Yasufuku said in an interview.
"We knew that EBUS-TBNA was good, but [until now] we never knew how it compared with the gold standard, mediastinoscopy," he said. The major limiting factor is lymph node size, he noted. Surgeons find it challenging to routinely obtain an adequate cell specimen from nodes smaller than 5 mm in diameter, Dr. Yasufuku said. "The smaller the node, the harder it is to put a needle into it."
The Toronto group uses rapid, onsite cytologic evaluation, which means that a cytologist attends the procedure in the combined surgical and endoscopy suite. In the study, and also in routine practice, "we can make repeated needle passes until we obtain good specimens. The surgeon can learn how to place the needle by getting immediate feedback" on the specimens, he said.
The specimens obtained allow for a tissue diagnosis, and typically provide enough material to assess cells for the presence of epidermal growth factor receptor mutations, he added.
EBUS-TBNA uses local rather than general anesthesia, is less invasive, and has fewer complications compared with mediastinoscopy, said Dr. Yasufuku, a thoracic surgeon and director of the interventional thoracic surgery program at Toronto General and the University of Toronto.
The study enrolled adults with NSCLC who required mediastinoscopy as part of their staging to determine their suitability for lung cancer resection. The study excluded patients who were not fit for definitive surgical resection, because the researchers used the status of the surgically excised lymph nodes as the basis for judging the diagnostic accuracy of both techniques.
During July 2006?August 2010, they enrolled 153 patients with an average age of 69 years. The most common NSCLC histologic subtype was adenocarcinoma (59%), followed by squamous cell carcinoma (25%). Staging by ultrasound imaging identified 57% of the patients with stage I or II disease, and 39% with stage IIIA disease. The remaining 4% had stage IIIB or stage IV disease.
All patients underwent general anesthesia. A surgeon first performed EBUS-TBNA on each patient, followed immediately by mediastinoscopy. All patients then underwent surgical lymph node resection to definitively assess their nodes if EBUS-TBNA, mediastinoscopy, or both did not show signs of metastatic disease.
The surgeons attempted biopsies at five lymph node stations in each patient: stations 2R, 2L, 4R, 4L, and 7. They successfully biopsied an average of three stations per patient using EBUS-TBNA, with an inadequate specimen obtained on an average of one station per patient. Average lymph node diameter on the short axis was 7 mm, and the procedure averaged a total of 20 minutes per patient. Overall, EBUS-TBNA identified 78 biopsies as malignant.
During mediastinoscopy, surgeons successfully biopsied an average of 3.8 nodes per patient, with inadequate specimens obtained from 10 nodes, an average of fewer than 0.1 inadequate specimen per patient.
Mediastinoscopy retrieved 79 biopsies that were identified as malignant.
Despite any sampling differences, the surgeons reached an identical and correct diagnosis using both modalities in 136 patients (89%). Neither modality produced the correct diagnosis in four patients (3%), which meant that overall EBUS-TBNA and mediastinoscopy agreed 92% of the time. EBUS-TBNA was correct and mediastinoscopy incorrect in seven patients, and mediastinoscopy was correct and EBUS-TBNA incorrect in six patients.
These outcomes meant that EBUS-TBNA had 81% sensitivity, 91% negative predictive value, and 93% diagnostic accuracy. Mediastinoscopy led to 79% sensitivity, 90% negative predictive value, and 93% accuracy. Both methods had a specificity and positive predictive value of 100%, Dr. Yasufuku said.
No complications occurred after EBUS-TBNA, but there were four minor complications following subsequent mediastinoscopy: Two patients had a hematoma, one had a recurrent nerve injury, and one had a wound infection.
"It was a very clean study, showing that in the hands of a trained surgeon in our setting, EBUS-TBNA works very well. We clearly showed that the diagnostic yield is similar, and that patients who require mediastinoscopy as part of their staging can undergo EBUS-TBNA as their initial modality. Depending on what you find, you want to also do mediastinoscopy," he added.
"I?m convinced that [Dr. Yasufuku has] demonstrated equivalent ability to stage the mediastinum with EBUS-TBNA and with mediastinoscopy," commented Dr. Joel D. Cooper, professor of surgery and chief of thoracic surgery at the University of Pennsylvania in Philadelphia.
The study was supported by Olympus Medical Systems, a company that markets an EBUS-TBNA system. Dr. Yasufuku said that he has received research support from Olympus. Dr. Cooper said that he had no relevant disclosures.
PHILADELPHIA - Endobronchial ultrasound-guided biopsy of mediastinal lymph nodes in patients with operable non-small cell lung cancer worked as effectively for staging as did the standard approach - mediastinoscopy in a head-to-head comparison of the two methods.
"Our results have shown that EBUS-TBNA [endobronchial ultrasound-guided transbronchial needle aspiration], when performed as in this study, can replace mediastinoscopy for accurate staging of the mediastinum in potentially resectable lung cancer," Dr. Kazuhiro Yasufuku said at the annual meeting of the American Association for Thoracic Surgery.
Based on these results, which were obtained in 153 patients treated by any one of seven surgeons working at Toronto General Hospital, Dr. Yasufuku and his colleagues now routinely use EBUS-TBNA as their initial approach for staging patients with inoperable non?small cell lung cancer (NSCLC), who account for about 70% of all NSCLC patients they treat. As long as they can collect adequate cell specimens for cytologic analysis from the lymph node stations they routinely assess, they rely exclusively on EBUS-TBNA for staging, which allows them to avoid mediastinoscopy for most of their patients, Dr. Yasufuku said in an interview.
"We knew that EBUS-TBNA was good, but [until now] we never knew how it compared with the gold standard, mediastinoscopy," he said. The major limiting factor is lymph node size, he noted. Surgeons find it challenging to routinely obtain an adequate cell specimen from nodes smaller than 5 mm in diameter, Dr. Yasufuku said. "The smaller the node, the harder it is to put a needle into it."
The Toronto group uses rapid, onsite cytologic evaluation, which means that a cytologist attends the procedure in the combined surgical and endoscopy suite. In the study, and also in routine practice, "we can make repeated needle passes until we obtain good specimens. The surgeon can learn how to place the needle by getting immediate feedback" on the specimens, he said.
The specimens obtained allow for a tissue diagnosis, and typically provide enough material to assess cells for the presence of epidermal growth factor receptor mutations, he added.
EBUS-TBNA uses local rather than general anesthesia, is less invasive, and has fewer complications compared with mediastinoscopy, said Dr. Yasufuku, a thoracic surgeon and director of the interventional thoracic surgery program at Toronto General and the University of Toronto.
The study enrolled adults with NSCLC who required mediastinoscopy as part of their staging to determine their suitability for lung cancer resection. The study excluded patients who were not fit for definitive surgical resection, because the researchers used the status of the surgically excised lymph nodes as the basis for judging the diagnostic accuracy of both techniques.
During July 2006?August 2010, they enrolled 153 patients with an average age of 69 years. The most common NSCLC histologic subtype was adenocarcinoma (59%), followed by squamous cell carcinoma (25%). Staging by ultrasound imaging identified 57% of the patients with stage I or II disease, and 39% with stage IIIA disease. The remaining 4% had stage IIIB or stage IV disease.
All patients underwent general anesthesia. A surgeon first performed EBUS-TBNA on each patient, followed immediately by mediastinoscopy. All patients then underwent surgical lymph node resection to definitively assess their nodes if EBUS-TBNA, mediastinoscopy, or both did not show signs of metastatic disease.
The surgeons attempted biopsies at five lymph node stations in each patient: stations 2R, 2L, 4R, 4L, and 7. They successfully biopsied an average of three stations per patient using EBUS-TBNA, with an inadequate specimen obtained on an average of one station per patient. Average lymph node diameter on the short axis was 7 mm, and the procedure averaged a total of 20 minutes per patient. Overall, EBUS-TBNA identified 78 biopsies as malignant.
During mediastinoscopy, surgeons successfully biopsied an average of 3.8 nodes per patient, with inadequate specimens obtained from 10 nodes, an average of fewer than 0.1 inadequate specimen per patient.
Mediastinoscopy retrieved 79 biopsies that were identified as malignant.
Despite any sampling differences, the surgeons reached an identical and correct diagnosis using both modalities in 136 patients (89%). Neither modality produced the correct diagnosis in four patients (3%), which meant that overall EBUS-TBNA and mediastinoscopy agreed 92% of the time. EBUS-TBNA was correct and mediastinoscopy incorrect in seven patients, and mediastinoscopy was correct and EBUS-TBNA incorrect in six patients.
These outcomes meant that EBUS-TBNA had 81% sensitivity, 91% negative predictive value, and 93% diagnostic accuracy. Mediastinoscopy led to 79% sensitivity, 90% negative predictive value, and 93% accuracy. Both methods had a specificity and positive predictive value of 100%, Dr. Yasufuku said.
No complications occurred after EBUS-TBNA, but there were four minor complications following subsequent mediastinoscopy: Two patients had a hematoma, one had a recurrent nerve injury, and one had a wound infection.
"It was a very clean study, showing that in the hands of a trained surgeon in our setting, EBUS-TBNA works very well. We clearly showed that the diagnostic yield is similar, and that patients who require mediastinoscopy as part of their staging can undergo EBUS-TBNA as their initial modality. Depending on what you find, you want to also do mediastinoscopy," he added.
"I?m convinced that [Dr. Yasufuku has] demonstrated equivalent ability to stage the mediastinum with EBUS-TBNA and with mediastinoscopy," commented Dr. Joel D. Cooper, professor of surgery and chief of thoracic surgery at the University of Pennsylvania in Philadelphia.
The study was supported by Olympus Medical Systems, a company that markets an EBUS-TBNA system. Dr. Yasufuku said that he has received research support from Olympus. Dr. Cooper said that he had no relevant disclosures.
PHILADELPHIA - Endobronchial ultrasound-guided biopsy of mediastinal lymph nodes in patients with operable non-small cell lung cancer worked as effectively for staging as did the standard approach - mediastinoscopy in a head-to-head comparison of the two methods.
"Our results have shown that EBUS-TBNA [endobronchial ultrasound-guided transbronchial needle aspiration], when performed as in this study, can replace mediastinoscopy for accurate staging of the mediastinum in potentially resectable lung cancer," Dr. Kazuhiro Yasufuku said at the annual meeting of the American Association for Thoracic Surgery.
Based on these results, which were obtained in 153 patients treated by any one of seven surgeons working at Toronto General Hospital, Dr. Yasufuku and his colleagues now routinely use EBUS-TBNA as their initial approach for staging patients with inoperable non?small cell lung cancer (NSCLC), who account for about 70% of all NSCLC patients they treat. As long as they can collect adequate cell specimens for cytologic analysis from the lymph node stations they routinely assess, they rely exclusively on EBUS-TBNA for staging, which allows them to avoid mediastinoscopy for most of their patients, Dr. Yasufuku said in an interview.
"We knew that EBUS-TBNA was good, but [until now] we never knew how it compared with the gold standard, mediastinoscopy," he said. The major limiting factor is lymph node size, he noted. Surgeons find it challenging to routinely obtain an adequate cell specimen from nodes smaller than 5 mm in diameter, Dr. Yasufuku said. "The smaller the node, the harder it is to put a needle into it."
The Toronto group uses rapid, onsite cytologic evaluation, which means that a cytologist attends the procedure in the combined surgical and endoscopy suite. In the study, and also in routine practice, "we can make repeated needle passes until we obtain good specimens. The surgeon can learn how to place the needle by getting immediate feedback" on the specimens, he said.
The specimens obtained allow for a tissue diagnosis, and typically provide enough material to assess cells for the presence of epidermal growth factor receptor mutations, he added.
EBUS-TBNA uses local rather than general anesthesia, is less invasive, and has fewer complications compared with mediastinoscopy, said Dr. Yasufuku, a thoracic surgeon and director of the interventional thoracic surgery program at Toronto General and the University of Toronto.
The study enrolled adults with NSCLC who required mediastinoscopy as part of their staging to determine their suitability for lung cancer resection. The study excluded patients who were not fit for definitive surgical resection, because the researchers used the status of the surgically excised lymph nodes as the basis for judging the diagnostic accuracy of both techniques.
During July 2006?August 2010, they enrolled 153 patients with an average age of 69 years. The most common NSCLC histologic subtype was adenocarcinoma (59%), followed by squamous cell carcinoma (25%). Staging by ultrasound imaging identified 57% of the patients with stage I or II disease, and 39% with stage IIIA disease. The remaining 4% had stage IIIB or stage IV disease.
All patients underwent general anesthesia. A surgeon first performed EBUS-TBNA on each patient, followed immediately by mediastinoscopy. All patients then underwent surgical lymph node resection to definitively assess their nodes if EBUS-TBNA, mediastinoscopy, or both did not show signs of metastatic disease.
The surgeons attempted biopsies at five lymph node stations in each patient: stations 2R, 2L, 4R, 4L, and 7. They successfully biopsied an average of three stations per patient using EBUS-TBNA, with an inadequate specimen obtained on an average of one station per patient. Average lymph node diameter on the short axis was 7 mm, and the procedure averaged a total of 20 minutes per patient. Overall, EBUS-TBNA identified 78 biopsies as malignant.
During mediastinoscopy, surgeons successfully biopsied an average of 3.8 nodes per patient, with inadequate specimens obtained from 10 nodes, an average of fewer than 0.1 inadequate specimen per patient.
Mediastinoscopy retrieved 79 biopsies that were identified as malignant.
Despite any sampling differences, the surgeons reached an identical and correct diagnosis using both modalities in 136 patients (89%). Neither modality produced the correct diagnosis in four patients (3%), which meant that overall EBUS-TBNA and mediastinoscopy agreed 92% of the time. EBUS-TBNA was correct and mediastinoscopy incorrect in seven patients, and mediastinoscopy was correct and EBUS-TBNA incorrect in six patients.
These outcomes meant that EBUS-TBNA had 81% sensitivity, 91% negative predictive value, and 93% diagnostic accuracy. Mediastinoscopy led to 79% sensitivity, 90% negative predictive value, and 93% accuracy. Both methods had a specificity and positive predictive value of 100%, Dr. Yasufuku said.
No complications occurred after EBUS-TBNA, but there were four minor complications following subsequent mediastinoscopy: Two patients had a hematoma, one had a recurrent nerve injury, and one had a wound infection.
"It was a very clean study, showing that in the hands of a trained surgeon in our setting, EBUS-TBNA works very well. We clearly showed that the diagnostic yield is similar, and that patients who require mediastinoscopy as part of their staging can undergo EBUS-TBNA as their initial modality. Depending on what you find, you want to also do mediastinoscopy," he added.
"I?m convinced that [Dr. Yasufuku has] demonstrated equivalent ability to stage the mediastinum with EBUS-TBNA and with mediastinoscopy," commented Dr. Joel D. Cooper, professor of surgery and chief of thoracic surgery at the University of Pennsylvania in Philadelphia.
The study was supported by Olympus Medical Systems, a company that markets an EBUS-TBNA system. Dr. Yasufuku said that he has received research support from Olympus. Dr. Cooper said that he had no relevant disclosures.
Bariatric Surgery Now 'Safer Than Appendectomy'
ORLANDO – Bariatric surgery achieved an unprecedented level of safety through 2009, as U.S. surgeons mastered the laparoscopic gastric bypass approach and offered patients gastric banding or gastroplasty, based on data collected on more than 100,000 U.S. patients treated at academic medical centers during 2002-2009.
This recent era also ushered in a new list of risk factors for in-hospital mortality in patients undergoing bariatric surgery, including two modifiable risk factors: diabetes and the type of surgery used, Dr. Brian R. Smith said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
"We find six preoperative factors that predict mortality. We can’t change patient age, sex, or insurance type, but we can better manage their diabetes preoperatively, and we can change the type of surgery they receive" to minimize their risk, said Dr. Smith, a surgeon at the University of California, Irvine, and chief of general surgery at the Veterans Affairs Healthcare System in Long Beach, Calif.
"Bariatric surgery is now statistically safer than appendectomy. Probably the most significant factor is that [surgeons] have gotten better with the laparoscopic approach; we got over the learning curve," he said in an interview.
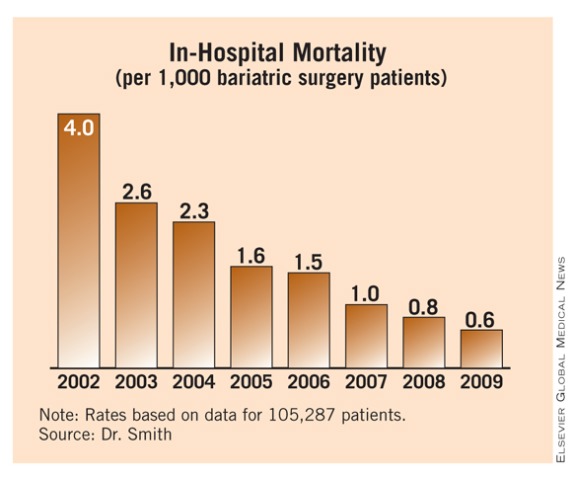
Dr. Smith and his associates reviewed 105,287 patients who underwent bariatric surgery during 2002-2009 at hospitals that contribute data to the University HealthSystem Consortium, a database of about 360 U.S. academic medical centers and affiliated hospitals. During that period, bariatric surgery volume ranged from about 10,000 cases in 2002 to about 16,000 in 2009.
Through 2003, open gastric bypass was used exclusively but, starting in 2004, surgeons began performing laparoscopic gastric bypass and gastric banding. By 2005, about 60% of the roughly 13,000 bariatric procedures that year involved laparoscopic bypass, with open bypass reduced to less than 20% of the total. During 2009, nearly 70% of bariatric procedures done at hospitals in the consortium were laparoscopic bypasses, about a quarter were banding or gastroplasty, and only about 6% of cases involved open bypass.
Concurrent with this shift in type of bariatric surgery performed came a striking drop in in-hospital mortality. In 2002, the rate was 4 deaths/1,000 patients. Over the following 7 years, mortality steadily fell and reached a new low of 0.6 deaths/1,000 patients in 2009, Dr. Smith reported.
"It’s a remarkable achievement – an American surgical success story," commented Dr. John M. Morton, director of bariatric surgery at Stanford (Calif.) University. Dr. Morton attributed the sharp decline in mortality to the rapid switch from open to laparoscopic gastric bypass, the focus starting in 2004 on treating bariatric surgery patients at designated centers of excellence, improved clinical pathways, and better patient selection. "We consistently see mortality rates of 0.1%, 0.3%, tops. That makes bariatric surgery as safe as laparoscopic cholecystectomy and hip replacement," he said in an interview.
Dr. Smith agreed that the rapid drop in the number of open gastric bypass procedures starting in 2005 and their replacement by laparoscopic procedures played a major role in the fall in patient mortality during the mid-2000s.
The more than 100,000 patients reviewed by Dr. Smith included 17% who were older than 60 years. About 80% were women and about 73% were white. The prevalence of hypertension was 56%, 30% had diabetes, and 22% had hyperlipidemia. Two-thirds of the patients had private medical insurance coverage.
A multivariate analysis identified six factors linked with an increased mortality risk: age older than 60 years, male sex, Medicare coverage, diabetes, open surgery, and gastric bypass surgery. Diabetes had not previously been identified as a mortality risk in published analyses, and the new list did not include hypertension, which had been a risk factor in prior analyses.
On the basis of these factors, Dr. Smith and his associates developed a mortality risk–scoring formula that assigned 1 point for each of four risk factors – male sex, Medicare insurance, open surgery, and gastric bypass – and 0.5 points for each of the other two factors, age 60 years or older and diabetes. After assigning these point values to the patients in the database, they found that patients with a risk score of 3.5 or greater had a sevenfold increased risk of in-hospital mortality, compared with patients with a score of zero or 0.5.
Dr. Smith said he had no disclosures. Dr. Morton said that he has received an educational grant from Ethicon Endo-Surgery, and he has received honoraria from and served on the scientific advisory board of Vibrynt.
ORLANDO – Bariatric surgery achieved an unprecedented level of safety through 2009, as U.S. surgeons mastered the laparoscopic gastric bypass approach and offered patients gastric banding or gastroplasty, based on data collected on more than 100,000 U.S. patients treated at academic medical centers during 2002-2009.
This recent era also ushered in a new list of risk factors for in-hospital mortality in patients undergoing bariatric surgery, including two modifiable risk factors: diabetes and the type of surgery used, Dr. Brian R. Smith said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
"We find six preoperative factors that predict mortality. We can’t change patient age, sex, or insurance type, but we can better manage their diabetes preoperatively, and we can change the type of surgery they receive" to minimize their risk, said Dr. Smith, a surgeon at the University of California, Irvine, and chief of general surgery at the Veterans Affairs Healthcare System in Long Beach, Calif.
"Bariatric surgery is now statistically safer than appendectomy. Probably the most significant factor is that [surgeons] have gotten better with the laparoscopic approach; we got over the learning curve," he said in an interview.

Dr. Smith and his associates reviewed 105,287 patients who underwent bariatric surgery during 2002-2009 at hospitals that contribute data to the University HealthSystem Consortium, a database of about 360 U.S. academic medical centers and affiliated hospitals. During that period, bariatric surgery volume ranged from about 10,000 cases in 2002 to about 16,000 in 2009.
Through 2003, open gastric bypass was used exclusively but, starting in 2004, surgeons began performing laparoscopic gastric bypass and gastric banding. By 2005, about 60% of the roughly 13,000 bariatric procedures that year involved laparoscopic bypass, with open bypass reduced to less than 20% of the total. During 2009, nearly 70% of bariatric procedures done at hospitals in the consortium were laparoscopic bypasses, about a quarter were banding or gastroplasty, and only about 6% of cases involved open bypass.
Concurrent with this shift in type of bariatric surgery performed came a striking drop in in-hospital mortality. In 2002, the rate was 4 deaths/1,000 patients. Over the following 7 years, mortality steadily fell and reached a new low of 0.6 deaths/1,000 patients in 2009, Dr. Smith reported.
"It’s a remarkable achievement – an American surgical success story," commented Dr. John M. Morton, director of bariatric surgery at Stanford (Calif.) University. Dr. Morton attributed the sharp decline in mortality to the rapid switch from open to laparoscopic gastric bypass, the focus starting in 2004 on treating bariatric surgery patients at designated centers of excellence, improved clinical pathways, and better patient selection. "We consistently see mortality rates of 0.1%, 0.3%, tops. That makes bariatric surgery as safe as laparoscopic cholecystectomy and hip replacement," he said in an interview.
Dr. Smith agreed that the rapid drop in the number of open gastric bypass procedures starting in 2005 and their replacement by laparoscopic procedures played a major role in the fall in patient mortality during the mid-2000s.
The more than 100,000 patients reviewed by Dr. Smith included 17% who were older than 60 years. About 80% were women and about 73% were white. The prevalence of hypertension was 56%, 30% had diabetes, and 22% had hyperlipidemia. Two-thirds of the patients had private medical insurance coverage.
A multivariate analysis identified six factors linked with an increased mortality risk: age older than 60 years, male sex, Medicare coverage, diabetes, open surgery, and gastric bypass surgery. Diabetes had not previously been identified as a mortality risk in published analyses, and the new list did not include hypertension, which had been a risk factor in prior analyses.
On the basis of these factors, Dr. Smith and his associates developed a mortality risk–scoring formula that assigned 1 point for each of four risk factors – male sex, Medicare insurance, open surgery, and gastric bypass – and 0.5 points for each of the other two factors, age 60 years or older and diabetes. After assigning these point values to the patients in the database, they found that patients with a risk score of 3.5 or greater had a sevenfold increased risk of in-hospital mortality, compared with patients with a score of zero or 0.5.
Dr. Smith said he had no disclosures. Dr. Morton said that he has received an educational grant from Ethicon Endo-Surgery, and he has received honoraria from and served on the scientific advisory board of Vibrynt.
ORLANDO – Bariatric surgery achieved an unprecedented level of safety through 2009, as U.S. surgeons mastered the laparoscopic gastric bypass approach and offered patients gastric banding or gastroplasty, based on data collected on more than 100,000 U.S. patients treated at academic medical centers during 2002-2009.
This recent era also ushered in a new list of risk factors for in-hospital mortality in patients undergoing bariatric surgery, including two modifiable risk factors: diabetes and the type of surgery used, Dr. Brian R. Smith said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
"We find six preoperative factors that predict mortality. We can’t change patient age, sex, or insurance type, but we can better manage their diabetes preoperatively, and we can change the type of surgery they receive" to minimize their risk, said Dr. Smith, a surgeon at the University of California, Irvine, and chief of general surgery at the Veterans Affairs Healthcare System in Long Beach, Calif.
"Bariatric surgery is now statistically safer than appendectomy. Probably the most significant factor is that [surgeons] have gotten better with the laparoscopic approach; we got over the learning curve," he said in an interview.

Dr. Smith and his associates reviewed 105,287 patients who underwent bariatric surgery during 2002-2009 at hospitals that contribute data to the University HealthSystem Consortium, a database of about 360 U.S. academic medical centers and affiliated hospitals. During that period, bariatric surgery volume ranged from about 10,000 cases in 2002 to about 16,000 in 2009.
Through 2003, open gastric bypass was used exclusively but, starting in 2004, surgeons began performing laparoscopic gastric bypass and gastric banding. By 2005, about 60% of the roughly 13,000 bariatric procedures that year involved laparoscopic bypass, with open bypass reduced to less than 20% of the total. During 2009, nearly 70% of bariatric procedures done at hospitals in the consortium were laparoscopic bypasses, about a quarter were banding or gastroplasty, and only about 6% of cases involved open bypass.
Concurrent with this shift in type of bariatric surgery performed came a striking drop in in-hospital mortality. In 2002, the rate was 4 deaths/1,000 patients. Over the following 7 years, mortality steadily fell and reached a new low of 0.6 deaths/1,000 patients in 2009, Dr. Smith reported.
"It’s a remarkable achievement – an American surgical success story," commented Dr. John M. Morton, director of bariatric surgery at Stanford (Calif.) University. Dr. Morton attributed the sharp decline in mortality to the rapid switch from open to laparoscopic gastric bypass, the focus starting in 2004 on treating bariatric surgery patients at designated centers of excellence, improved clinical pathways, and better patient selection. "We consistently see mortality rates of 0.1%, 0.3%, tops. That makes bariatric surgery as safe as laparoscopic cholecystectomy and hip replacement," he said in an interview.
Dr. Smith agreed that the rapid drop in the number of open gastric bypass procedures starting in 2005 and their replacement by laparoscopic procedures played a major role in the fall in patient mortality during the mid-2000s.
The more than 100,000 patients reviewed by Dr. Smith included 17% who were older than 60 years. About 80% were women and about 73% were white. The prevalence of hypertension was 56%, 30% had diabetes, and 22% had hyperlipidemia. Two-thirds of the patients had private medical insurance coverage.
A multivariate analysis identified six factors linked with an increased mortality risk: age older than 60 years, male sex, Medicare coverage, diabetes, open surgery, and gastric bypass surgery. Diabetes had not previously been identified as a mortality risk in published analyses, and the new list did not include hypertension, which had been a risk factor in prior analyses.
On the basis of these factors, Dr. Smith and his associates developed a mortality risk–scoring formula that assigned 1 point for each of four risk factors – male sex, Medicare insurance, open surgery, and gastric bypass – and 0.5 points for each of the other two factors, age 60 years or older and diabetes. After assigning these point values to the patients in the database, they found that patients with a risk score of 3.5 or greater had a sevenfold increased risk of in-hospital mortality, compared with patients with a score of zero or 0.5.
Dr. Smith said he had no disclosures. Dr. Morton said that he has received an educational grant from Ethicon Endo-Surgery, and he has received honoraria from and served on the scientific advisory board of Vibrynt.
FROM THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY ANNUAL MEETING
Major Finding: During 2009, bariatric surgery done at U.S. academic hospitals had an in-hospital mortality rate of 0.6/1,000 patients, compared with a rate of 4/1,000 cases in 2002.
Data Source: Review of 105,287 patients undergoing bariatric surgery at U.S. hospitals participating in the University Health Consortium.
Disclosures: Dr. Smith said he had no disclosures. Dr. Morton said that he has received an educational grant from Ethicon Endo-Surgery, and he has received honoraria from and served on the scientific advisory board of Vibrynt.
TAVR Showed Increased Neurologic Events
PHILADELPHIA - The percutaneous, transcatheter replacement of stenotic aortic valves has captured attention as an option for patients who are either too sick to undergo aortic valve replacement by conventional open surgery, or who are surgical candidates but would prefer to avoid sternotomy.
Despite early success with the use of transcatheter aortic valve repair (TAVR) in the two parts of a recent pivotal trial, Dr. D. Craig Miller said the approach has two important limitations: the poorly defined long-term durability of aortic valves placed percutaneously (which thus far have track records of less than 3 years) and the significantly increased risk of a neurologic event from TAVR, compared with conventional open aortic valve repair (AVR).
A summary analysis of neurologic events following TAVR in the Placement of Aortic Transcatheter Valve (PARTNER) trial showed a total, 1-year event rate of 6% in the as-treated TAVR patients who received their valves via the transfemoral route, compared with a 2% rate in the open AVR patients, a statistically significant difference, Dr. Miller said at the annual meeting of the American Association for Thoracic Surgery.
As-treated patients who received a TAVR via the transapical route had a 1-year neurologic event rate of 14%, compared with a 10% rate in patients treated with open AVR, also a statistically significant difference. The substantially higher rate of events in patients assigned to the transapical arm of the study related to the higher atherosclerotic burden in these patients, both those who underwent TAVR and those who had open AVR.
More than half of the neurologic event risk seen with TAVR occurred during the first 2 weeks after treatment, suggesting a periprocedural cause, said Dr. Miller, FACS, professor of cardiovascular surgery at Stanford (Calif.) University.
"The early neurologic events are undoubtedly due to particulate embolization, although we can't prove it," he said. "A cerebral protection device, such as a deflector across the ostium, may reduce the event rate." He cited a report on initial clinical experience using a percutaneously deployed deflector in three patients undergoing TAVR (J. Am. Coll. Cardiol. Intv. 2010;3:1133-8). Other changes to TAVR that might reduce neurologic event rates include improved antiplatelet and antithrombotic therapy with clopidogrel, aspirin, warfarin, and dabigatran. Development of smaller TAVR devices might also further reduce neurologic events, he said.
Analysis of the correlates of the early neurologic events showed that they significantly linked with a lower aortic valve area index. In other words, patients with smaller, tighter aortic valves were more likely to experience an early event.
During the late, nonperiprocedural phase, the risk for neurologic events was increased for patients with a higher NYHA heart failure stage, those who had had a stroke or transient ischemic attack within the prior 12 months, or those who were not candidates for TAVR via the transfemoral route.
Patients enrolled in the randomized portion of the trial had a low rate of major strokes, with a total of 29 events (18 in the TAVR patients and 11 in those undergoing open AVR), a difference that was not statistically significant. The analysis therefore also included minor strokes and transient ischemic attacks to total an adequate number of events to potentially show a statistical significant difference between the TAVR and open AVR subgroups, Dr. Miller said.
The data Dr. Miller reported came from cohort A of the PARTNER trial, the cohort that focused on patients who could be randomized to either TAVR or open AVR. The primary end point of all-cause mortality in this cohort, reported in April at the American College of Cardiology Scientific Sessions, showed that 1-year survival following TAVR was not inferior to open AVR. A prior report, for cohort B (patients considered too sick to undergo open AVR), had shown that TAVR produced superior outcomes, compared with conven- tional medical management ( N. Engl. J. Med. 2010; 363:1597-607).
The considerable interest in transcatheter aortic valve replacement among patients and physicians alike suggests that it may fall on payers to set limits on which patients undergo this procedure, and on heart-valve teams to ensure that procedures are done appropriately and safely.
"How might we prevent a runaway train [of transcatheter aortic valve replacement], as seen now in Germany, where 20%-25% of all aortic valve replacements are done percutaneously? This will be up to payers," Dr. D. Craig Miller said as he presented new data on the neurologic adverse events in the Placement of Aortic Transcatheter Valves (PARTNER) trial. "I'm personally disappointed with what's happened in Europe. There are no restrictions [on the use of transcatheter aortic valve replacement], and the results are not as good as in PARTNER.
"It behooves us to work with a functional heart-valve team to make sure these complementary techniques [transcatheter aortic valve replacement and open valve replacement] are used appropriately. I don't think that open aortic valve replacement is an endangered species."
Deciding which patients should undergo transcatheter aortic valve replacement (TAVR) will require "defining the line between utility and futility," he said. "You don't want to empty every nursing home in California of patients with aortic stenosis, and on the young side, you don't want the percutaneous option used in patients at low surgical risk." Concern about using TAVR on patients who are good open surgery candidates focuses on the unknown long-term durability of TAVR, and the "high price to pay in neurologic events, at least in the current version of TAVR," he said.
"Patients will always flock to the least invasive approach. That's where the heart-valve team will be very important. This will only work well if surgeons and cardiologists work together to decide whether something should be done about aortic stenosis in a patient and, if so, which treatment is best. TAVR and open replacement are complementary, not competitive.
"You can't let this technology go everywhere," Dr. Miller warned. TAVR is "tricky, and the learning curve is steep and unforgiving. We [at Stanford] still insist on having two surgeons and two interventionalists on every case, because when a case goes south it goes in a hurry. We have done 100 cases, and problems still come up and are, to some extent, unpredictable."
The PARTNER trial was sponsored by Edwards Lifesciences. Dr. Miller said that he has been the Stanford Principal Investigator for PARTNER and has served as an unpaid consultant to Edwards. He has also been a consultant to Abbott Vascular, Medtronic Cardiovascular, and St. Jude Medical.
PHILADELPHIA - The percutaneous, transcatheter replacement of stenotic aortic valves has captured attention as an option for patients who are either too sick to undergo aortic valve replacement by conventional open surgery, or who are surgical candidates but would prefer to avoid sternotomy.
Despite early success with the use of transcatheter aortic valve repair (TAVR) in the two parts of a recent pivotal trial, Dr. D. Craig Miller said the approach has two important limitations: the poorly defined long-term durability of aortic valves placed percutaneously (which thus far have track records of less than 3 years) and the significantly increased risk of a neurologic event from TAVR, compared with conventional open aortic valve repair (AVR).
A summary analysis of neurologic events following TAVR in the Placement of Aortic Transcatheter Valve (PARTNER) trial showed a total, 1-year event rate of 6% in the as-treated TAVR patients who received their valves via the transfemoral route, compared with a 2% rate in the open AVR patients, a statistically significant difference, Dr. Miller said at the annual meeting of the American Association for Thoracic Surgery.
As-treated patients who received a TAVR via the transapical route had a 1-year neurologic event rate of 14%, compared with a 10% rate in patients treated with open AVR, also a statistically significant difference. The substantially higher rate of events in patients assigned to the transapical arm of the study related to the higher atherosclerotic burden in these patients, both those who underwent TAVR and those who had open AVR.
More than half of the neurologic event risk seen with TAVR occurred during the first 2 weeks after treatment, suggesting a periprocedural cause, said Dr. Miller, FACS, professor of cardiovascular surgery at Stanford (Calif.) University.
"The early neurologic events are undoubtedly due to particulate embolization, although we can't prove it," he said. "A cerebral protection device, such as a deflector across the ostium, may reduce the event rate." He cited a report on initial clinical experience using a percutaneously deployed deflector in three patients undergoing TAVR (J. Am. Coll. Cardiol. Intv. 2010;3:1133-8). Other changes to TAVR that might reduce neurologic event rates include improved antiplatelet and antithrombotic therapy with clopidogrel, aspirin, warfarin, and dabigatran. Development of smaller TAVR devices might also further reduce neurologic events, he said.
Analysis of the correlates of the early neurologic events showed that they significantly linked with a lower aortic valve area index. In other words, patients with smaller, tighter aortic valves were more likely to experience an early event.
During the late, nonperiprocedural phase, the risk for neurologic events was increased for patients with a higher NYHA heart failure stage, those who had had a stroke or transient ischemic attack within the prior 12 months, or those who were not candidates for TAVR via the transfemoral route.
Patients enrolled in the randomized portion of the trial had a low rate of major strokes, with a total of 29 events (18 in the TAVR patients and 11 in those undergoing open AVR), a difference that was not statistically significant. The analysis therefore also included minor strokes and transient ischemic attacks to total an adequate number of events to potentially show a statistical significant difference between the TAVR and open AVR subgroups, Dr. Miller said.
The data Dr. Miller reported came from cohort A of the PARTNER trial, the cohort that focused on patients who could be randomized to either TAVR or open AVR. The primary end point of all-cause mortality in this cohort, reported in April at the American College of Cardiology Scientific Sessions, showed that 1-year survival following TAVR was not inferior to open AVR. A prior report, for cohort B (patients considered too sick to undergo open AVR), had shown that TAVR produced superior outcomes, compared with conven- tional medical management ( N. Engl. J. Med. 2010; 363:1597-607).
The considerable interest in transcatheter aortic valve replacement among patients and physicians alike suggests that it may fall on payers to set limits on which patients undergo this procedure, and on heart-valve teams to ensure that procedures are done appropriately and safely.
"How might we prevent a runaway train [of transcatheter aortic valve replacement], as seen now in Germany, where 20%-25% of all aortic valve replacements are done percutaneously? This will be up to payers," Dr. D. Craig Miller said as he presented new data on the neurologic adverse events in the Placement of Aortic Transcatheter Valves (PARTNER) trial. "I'm personally disappointed with what's happened in Europe. There are no restrictions [on the use of transcatheter aortic valve replacement], and the results are not as good as in PARTNER.
"It behooves us to work with a functional heart-valve team to make sure these complementary techniques [transcatheter aortic valve replacement and open valve replacement] are used appropriately. I don't think that open aortic valve replacement is an endangered species."
Deciding which patients should undergo transcatheter aortic valve replacement (TAVR) will require "defining the line between utility and futility," he said. "You don't want to empty every nursing home in California of patients with aortic stenosis, and on the young side, you don't want the percutaneous option used in patients at low surgical risk." Concern about using TAVR on patients who are good open surgery candidates focuses on the unknown long-term durability of TAVR, and the "high price to pay in neurologic events, at least in the current version of TAVR," he said.
"Patients will always flock to the least invasive approach. That's where the heart-valve team will be very important. This will only work well if surgeons and cardiologists work together to decide whether something should be done about aortic stenosis in a patient and, if so, which treatment is best. TAVR and open replacement are complementary, not competitive.
"You can't let this technology go everywhere," Dr. Miller warned. TAVR is "tricky, and the learning curve is steep and unforgiving. We [at Stanford] still insist on having two surgeons and two interventionalists on every case, because when a case goes south it goes in a hurry. We have done 100 cases, and problems still come up and are, to some extent, unpredictable."
The PARTNER trial was sponsored by Edwards Lifesciences. Dr. Miller said that he has been the Stanford Principal Investigator for PARTNER and has served as an unpaid consultant to Edwards. He has also been a consultant to Abbott Vascular, Medtronic Cardiovascular, and St. Jude Medical.
PHILADELPHIA - The percutaneous, transcatheter replacement of stenotic aortic valves has captured attention as an option for patients who are either too sick to undergo aortic valve replacement by conventional open surgery, or who are surgical candidates but would prefer to avoid sternotomy.
Despite early success with the use of transcatheter aortic valve repair (TAVR) in the two parts of a recent pivotal trial, Dr. D. Craig Miller said the approach has two important limitations: the poorly defined long-term durability of aortic valves placed percutaneously (which thus far have track records of less than 3 years) and the significantly increased risk of a neurologic event from TAVR, compared with conventional open aortic valve repair (AVR).
A summary analysis of neurologic events following TAVR in the Placement of Aortic Transcatheter Valve (PARTNER) trial showed a total, 1-year event rate of 6% in the as-treated TAVR patients who received their valves via the transfemoral route, compared with a 2% rate in the open AVR patients, a statistically significant difference, Dr. Miller said at the annual meeting of the American Association for Thoracic Surgery.
As-treated patients who received a TAVR via the transapical route had a 1-year neurologic event rate of 14%, compared with a 10% rate in patients treated with open AVR, also a statistically significant difference. The substantially higher rate of events in patients assigned to the transapical arm of the study related to the higher atherosclerotic burden in these patients, both those who underwent TAVR and those who had open AVR.
More than half of the neurologic event risk seen with TAVR occurred during the first 2 weeks after treatment, suggesting a periprocedural cause, said Dr. Miller, FACS, professor of cardiovascular surgery at Stanford (Calif.) University.
"The early neurologic events are undoubtedly due to particulate embolization, although we can't prove it," he said. "A cerebral protection device, such as a deflector across the ostium, may reduce the event rate." He cited a report on initial clinical experience using a percutaneously deployed deflector in three patients undergoing TAVR (J. Am. Coll. Cardiol. Intv. 2010;3:1133-8). Other changes to TAVR that might reduce neurologic event rates include improved antiplatelet and antithrombotic therapy with clopidogrel, aspirin, warfarin, and dabigatran. Development of smaller TAVR devices might also further reduce neurologic events, he said.
Analysis of the correlates of the early neurologic events showed that they significantly linked with a lower aortic valve area index. In other words, patients with smaller, tighter aortic valves were more likely to experience an early event.
During the late, nonperiprocedural phase, the risk for neurologic events was increased for patients with a higher NYHA heart failure stage, those who had had a stroke or transient ischemic attack within the prior 12 months, or those who were not candidates for TAVR via the transfemoral route.
Patients enrolled in the randomized portion of the trial had a low rate of major strokes, with a total of 29 events (18 in the TAVR patients and 11 in those undergoing open AVR), a difference that was not statistically significant. The analysis therefore also included minor strokes and transient ischemic attacks to total an adequate number of events to potentially show a statistical significant difference between the TAVR and open AVR subgroups, Dr. Miller said.
The data Dr. Miller reported came from cohort A of the PARTNER trial, the cohort that focused on patients who could be randomized to either TAVR or open AVR. The primary end point of all-cause mortality in this cohort, reported in April at the American College of Cardiology Scientific Sessions, showed that 1-year survival following TAVR was not inferior to open AVR. A prior report, for cohort B (patients considered too sick to undergo open AVR), had shown that TAVR produced superior outcomes, compared with conven- tional medical management ( N. Engl. J. Med. 2010; 363:1597-607).
The considerable interest in transcatheter aortic valve replacement among patients and physicians alike suggests that it may fall on payers to set limits on which patients undergo this procedure, and on heart-valve teams to ensure that procedures are done appropriately and safely.
"How might we prevent a runaway train [of transcatheter aortic valve replacement], as seen now in Germany, where 20%-25% of all aortic valve replacements are done percutaneously? This will be up to payers," Dr. D. Craig Miller said as he presented new data on the neurologic adverse events in the Placement of Aortic Transcatheter Valves (PARTNER) trial. "I'm personally disappointed with what's happened in Europe. There are no restrictions [on the use of transcatheter aortic valve replacement], and the results are not as good as in PARTNER.
"It behooves us to work with a functional heart-valve team to make sure these complementary techniques [transcatheter aortic valve replacement and open valve replacement] are used appropriately. I don't think that open aortic valve replacement is an endangered species."
Deciding which patients should undergo transcatheter aortic valve replacement (TAVR) will require "defining the line between utility and futility," he said. "You don't want to empty every nursing home in California of patients with aortic stenosis, and on the young side, you don't want the percutaneous option used in patients at low surgical risk." Concern about using TAVR on patients who are good open surgery candidates focuses on the unknown long-term durability of TAVR, and the "high price to pay in neurologic events, at least in the current version of TAVR," he said.
"Patients will always flock to the least invasive approach. That's where the heart-valve team will be very important. This will only work well if surgeons and cardiologists work together to decide whether something should be done about aortic stenosis in a patient and, if so, which treatment is best. TAVR and open replacement are complementary, not competitive.
"You can't let this technology go everywhere," Dr. Miller warned. TAVR is "tricky, and the learning curve is steep and unforgiving. We [at Stanford] still insist on having two surgeons and two interventionalists on every case, because when a case goes south it goes in a hurry. We have done 100 cases, and problems still come up and are, to some extent, unpredictable."
The PARTNER trial was sponsored by Edwards Lifesciences. Dr. Miller said that he has been the Stanford Principal Investigator for PARTNER and has served as an unpaid consultant to Edwards. He has also been a consultant to Abbott Vascular, Medtronic Cardiovascular, and St. Jude Medical.
Elective PCI: 12% of Cases Found Inappropriate
NEW ORLEANS – About 12% of the more than 140,000 Americans who underwent elective coronary artery stenting during 2009-2010 had an inappropriate procedure, based on an analysis of data from a national registry maintained by the American College of Cardiology.
In contrast, the rate of inappropriate procedures was 1% in the larger group of more than 355,000 patients who had an acute need for percutaneous coronary interventions (PCI) during the period studied, Dr. Paul S. Chan said at the annual meeting of the ACC.
The analysis also showed a striking hospital-to-hospital variation in the rate of elective PCI cases flagged as inappropriate. About 25% of the hospitals doing elective cases had a rate below 6%; another quarter had a rate of 17% or higher. Yet some hospitals had inappropriate rates that exceeded 30%.
Starting in May 2011, the ACC will start reporting data from this analysis to each of the more than 1,000 participating U.S. hospitals. By carefully reviewing cases that have been flagged as inappropriate, it is hoped that hospitals will learn from their mistakes and drive down the inappropriate rate, especially for elective PCIs, said Dr. Chan of Saint Luke’s Hospital in Kansas City, Mo.
The ACC collects the PCI data through the CathPCI portion of its National Cardiovascular Data Registry (NCDR). Dr. Chan and his associates rated each procedure they reviewed as appropriate, inappropriate, or uncertain based on comprehensive criteria established by an ACC expert panel (J. Am. Coll. Card. 2009;53:530-53).
"About 25% of the interventional cases in my practice would be categorized as inappropriate," commented Dr. Edward J. McNulty, an interventional cardiologist at Kaiser Permanente in San Francisco. "I get most of my patients from surgeons or other interventional cardiologists who feel the patients are too high risk. I do a lot of left main and multivessel PCI. There is no way that I can explain on the NCDR form why I consider these patients appropriate. The NCDR data don’t allow you to appreciate nuances. The criteria tend to penalize physicians who deviate from the average [by performing] complex cases."
Additionally, the way patients’ drug use gets recorded on the day of hospitalization is problematic, as patients may stop a chronically used drug on the day before entering the hospital and be erroneously recorded as not being on a therapy. Another issue is misclassification of symptoms, such as a patient who perceives ischemic chest pain as shortness of breath.
"I believe that inappropriate PCIs occur, and these results can certainly show signals. But within the ‘inappropriate’ procedures are some cases with mitigating circumstances," Dr. McNulty said in an interview.
The ACC expert panel published appropriateness criteria for 198 different clinical scenarios based on six separate clinical elements in February 2009. They applied the criteria to 500,154 U.S. cases treated with PCI during July 2009 through the end of September 2010. Inappropriate cases were defined as situations where expected negative consequences exceeded expected benefits.
PCI performed for an acute problem (such as high-risk unstable angina or MI) occurred in 71% of the cases. In this group, 99% of the cases were rated as appropriate, 1% as inappropriate, and fewer than 1% as uncertain. The remaining 29% of PCI procedures occurred in elective cases, of which 50% were rated as appropriate, 12% as inappropriate, and 38% as uncertain, Dr. Chan reported.
The three most common reasons for rating a case as inappropriate included patients with no ischemia, patients with mild ischemia, and asymptomatic patients, he said in an interview.
To address this issue, the ACC should develop a "real-time decision aid" to encourage interventionalists to "take a step back" during a catheterization to review a patient’s history and make a more informed decision on the need for PCI. The interventional cardiologist should review the degree of symptoms a patient has had and the evidence of ischemia, and take those findings into account when deciding whether the patient needs PCI.
Dr. Chan and Dr. McNulty said they had no disclosures.
The incidence of inappropriate PCIs will never reach zero. There will always be cases that the clinician knows are appropriate but that are impossible to define adequately using the NCDR data forms. There probably are cases in which patients are not properly worked up, but I’m not sure you can consistently evaluate cases adequately using the NCDR database.
We must be careful in what we say about institutions that seem to have "inappropriate" cases. Part of the problem may be the documentation. In my hospital, we found patients who initially seemed inappropriate, but when we looked harder we found that the problem lay in data recording. Rather than judging whether hospitals are doing a good or bad job, the focus should be on helping hospitals do better by helping them improve their patient selection and their case documentation. But hospitals also need to look at which patients are undergoing coronary procedures and defer the ones that are truly inappropriate.
The goal is to help hospitals do a better job. When you give hospitals and physicians performance data they inevitably improve. It happened with our program at Christiana. When I arrived 5 years ago, we first entered the NCDR, and in our first report back we looked terrible. But – no surprise – a lot of the problem turned out to be getting the documentation of cases right, and giving people an opportunity to think carefully about case selection. We use the information we get back from the NCDR to fix what is fixable in our decision making.
It’s possible that data like these from the NCDR will eventually be released to the general public, a step that the Society of Thoracic Surgeons (STS) has already taken for their registry of cardiothoracic surgery programs. The NCDR is under pressure from insurers, public interest groups, the media, and other stakeholders to make its registry data public. It is great that the STS has made its data publicly available. The ACC has followed a lot of what the STS has pioneered, and I think it’s inevitable that the NCDR data will be made public. I don’t like the one- to three-star rating system that the STS uses, but I’m not sure there is any really good way to present the information to the general public.
William S. Weintraub, M.D., is chief of cardiology at Christiana Care Health System in Newark, Del. He serves on the management board of the NCDR, and previously chaired the NCDR’s CathPCI registry. He said that he has received consulting fees or honoraria from Eli Lilly, Sanofi-Aventis, Shinogi, Cardionet, and Bristol-Myers Squibb. He has also received research grants from AstraZeneca, Abbott, BMS, Sanofi-Aventis, and Otsuka.
The incidence of inappropriate PCIs will never reach zero. There will always be cases that the clinician knows are appropriate but that are impossible to define adequately using the NCDR data forms. There probably are cases in which patients are not properly worked up, but I’m not sure you can consistently evaluate cases adequately using the NCDR database.
We must be careful in what we say about institutions that seem to have "inappropriate" cases. Part of the problem may be the documentation. In my hospital, we found patients who initially seemed inappropriate, but when we looked harder we found that the problem lay in data recording. Rather than judging whether hospitals are doing a good or bad job, the focus should be on helping hospitals do better by helping them improve their patient selection and their case documentation. But hospitals also need to look at which patients are undergoing coronary procedures and defer the ones that are truly inappropriate.
The goal is to help hospitals do a better job. When you give hospitals and physicians performance data they inevitably improve. It happened with our program at Christiana. When I arrived 5 years ago, we first entered the NCDR, and in our first report back we looked terrible. But – no surprise – a lot of the problem turned out to be getting the documentation of cases right, and giving people an opportunity to think carefully about case selection. We use the information we get back from the NCDR to fix what is fixable in our decision making.
It’s possible that data like these from the NCDR will eventually be released to the general public, a step that the Society of Thoracic Surgeons (STS) has already taken for their registry of cardiothoracic surgery programs. The NCDR is under pressure from insurers, public interest groups, the media, and other stakeholders to make its registry data public. It is great that the STS has made its data publicly available. The ACC has followed a lot of what the STS has pioneered, and I think it’s inevitable that the NCDR data will be made public. I don’t like the one- to three-star rating system that the STS uses, but I’m not sure there is any really good way to present the information to the general public.
William S. Weintraub, M.D., is chief of cardiology at Christiana Care Health System in Newark, Del. He serves on the management board of the NCDR, and previously chaired the NCDR’s CathPCI registry. He said that he has received consulting fees or honoraria from Eli Lilly, Sanofi-Aventis, Shinogi, Cardionet, and Bristol-Myers Squibb. He has also received research grants from AstraZeneca, Abbott, BMS, Sanofi-Aventis, and Otsuka.
The incidence of inappropriate PCIs will never reach zero. There will always be cases that the clinician knows are appropriate but that are impossible to define adequately using the NCDR data forms. There probably are cases in which patients are not properly worked up, but I’m not sure you can consistently evaluate cases adequately using the NCDR database.
We must be careful in what we say about institutions that seem to have "inappropriate" cases. Part of the problem may be the documentation. In my hospital, we found patients who initially seemed inappropriate, but when we looked harder we found that the problem lay in data recording. Rather than judging whether hospitals are doing a good or bad job, the focus should be on helping hospitals do better by helping them improve their patient selection and their case documentation. But hospitals also need to look at which patients are undergoing coronary procedures and defer the ones that are truly inappropriate.
The goal is to help hospitals do a better job. When you give hospitals and physicians performance data they inevitably improve. It happened with our program at Christiana. When I arrived 5 years ago, we first entered the NCDR, and in our first report back we looked terrible. But – no surprise – a lot of the problem turned out to be getting the documentation of cases right, and giving people an opportunity to think carefully about case selection. We use the information we get back from the NCDR to fix what is fixable in our decision making.
It’s possible that data like these from the NCDR will eventually be released to the general public, a step that the Society of Thoracic Surgeons (STS) has already taken for their registry of cardiothoracic surgery programs. The NCDR is under pressure from insurers, public interest groups, the media, and other stakeholders to make its registry data public. It is great that the STS has made its data publicly available. The ACC has followed a lot of what the STS has pioneered, and I think it’s inevitable that the NCDR data will be made public. I don’t like the one- to three-star rating system that the STS uses, but I’m not sure there is any really good way to present the information to the general public.
William S. Weintraub, M.D., is chief of cardiology at Christiana Care Health System in Newark, Del. He serves on the management board of the NCDR, and previously chaired the NCDR’s CathPCI registry. He said that he has received consulting fees or honoraria from Eli Lilly, Sanofi-Aventis, Shinogi, Cardionet, and Bristol-Myers Squibb. He has also received research grants from AstraZeneca, Abbott, BMS, Sanofi-Aventis, and Otsuka.
NEW ORLEANS – About 12% of the more than 140,000 Americans who underwent elective coronary artery stenting during 2009-2010 had an inappropriate procedure, based on an analysis of data from a national registry maintained by the American College of Cardiology.
In contrast, the rate of inappropriate procedures was 1% in the larger group of more than 355,000 patients who had an acute need for percutaneous coronary interventions (PCI) during the period studied, Dr. Paul S. Chan said at the annual meeting of the ACC.
The analysis also showed a striking hospital-to-hospital variation in the rate of elective PCI cases flagged as inappropriate. About 25% of the hospitals doing elective cases had a rate below 6%; another quarter had a rate of 17% or higher. Yet some hospitals had inappropriate rates that exceeded 30%.
Starting in May 2011, the ACC will start reporting data from this analysis to each of the more than 1,000 participating U.S. hospitals. By carefully reviewing cases that have been flagged as inappropriate, it is hoped that hospitals will learn from their mistakes and drive down the inappropriate rate, especially for elective PCIs, said Dr. Chan of Saint Luke’s Hospital in Kansas City, Mo.
The ACC collects the PCI data through the CathPCI portion of its National Cardiovascular Data Registry (NCDR). Dr. Chan and his associates rated each procedure they reviewed as appropriate, inappropriate, or uncertain based on comprehensive criteria established by an ACC expert panel (J. Am. Coll. Card. 2009;53:530-53).
"About 25% of the interventional cases in my practice would be categorized as inappropriate," commented Dr. Edward J. McNulty, an interventional cardiologist at Kaiser Permanente in San Francisco. "I get most of my patients from surgeons or other interventional cardiologists who feel the patients are too high risk. I do a lot of left main and multivessel PCI. There is no way that I can explain on the NCDR form why I consider these patients appropriate. The NCDR data don’t allow you to appreciate nuances. The criteria tend to penalize physicians who deviate from the average [by performing] complex cases."
Additionally, the way patients’ drug use gets recorded on the day of hospitalization is problematic, as patients may stop a chronically used drug on the day before entering the hospital and be erroneously recorded as not being on a therapy. Another issue is misclassification of symptoms, such as a patient who perceives ischemic chest pain as shortness of breath.
"I believe that inappropriate PCIs occur, and these results can certainly show signals. But within the ‘inappropriate’ procedures are some cases with mitigating circumstances," Dr. McNulty said in an interview.
The ACC expert panel published appropriateness criteria for 198 different clinical scenarios based on six separate clinical elements in February 2009. They applied the criteria to 500,154 U.S. cases treated with PCI during July 2009 through the end of September 2010. Inappropriate cases were defined as situations where expected negative consequences exceeded expected benefits.
PCI performed for an acute problem (such as high-risk unstable angina or MI) occurred in 71% of the cases. In this group, 99% of the cases were rated as appropriate, 1% as inappropriate, and fewer than 1% as uncertain. The remaining 29% of PCI procedures occurred in elective cases, of which 50% were rated as appropriate, 12% as inappropriate, and 38% as uncertain, Dr. Chan reported.
The three most common reasons for rating a case as inappropriate included patients with no ischemia, patients with mild ischemia, and asymptomatic patients, he said in an interview.
To address this issue, the ACC should develop a "real-time decision aid" to encourage interventionalists to "take a step back" during a catheterization to review a patient’s history and make a more informed decision on the need for PCI. The interventional cardiologist should review the degree of symptoms a patient has had and the evidence of ischemia, and take those findings into account when deciding whether the patient needs PCI.
Dr. Chan and Dr. McNulty said they had no disclosures.
NEW ORLEANS – About 12% of the more than 140,000 Americans who underwent elective coronary artery stenting during 2009-2010 had an inappropriate procedure, based on an analysis of data from a national registry maintained by the American College of Cardiology.
In contrast, the rate of inappropriate procedures was 1% in the larger group of more than 355,000 patients who had an acute need for percutaneous coronary interventions (PCI) during the period studied, Dr. Paul S. Chan said at the annual meeting of the ACC.
The analysis also showed a striking hospital-to-hospital variation in the rate of elective PCI cases flagged as inappropriate. About 25% of the hospitals doing elective cases had a rate below 6%; another quarter had a rate of 17% or higher. Yet some hospitals had inappropriate rates that exceeded 30%.
Starting in May 2011, the ACC will start reporting data from this analysis to each of the more than 1,000 participating U.S. hospitals. By carefully reviewing cases that have been flagged as inappropriate, it is hoped that hospitals will learn from their mistakes and drive down the inappropriate rate, especially for elective PCIs, said Dr. Chan of Saint Luke’s Hospital in Kansas City, Mo.
The ACC collects the PCI data through the CathPCI portion of its National Cardiovascular Data Registry (NCDR). Dr. Chan and his associates rated each procedure they reviewed as appropriate, inappropriate, or uncertain based on comprehensive criteria established by an ACC expert panel (J. Am. Coll. Card. 2009;53:530-53).
"About 25% of the interventional cases in my practice would be categorized as inappropriate," commented Dr. Edward J. McNulty, an interventional cardiologist at Kaiser Permanente in San Francisco. "I get most of my patients from surgeons or other interventional cardiologists who feel the patients are too high risk. I do a lot of left main and multivessel PCI. There is no way that I can explain on the NCDR form why I consider these patients appropriate. The NCDR data don’t allow you to appreciate nuances. The criteria tend to penalize physicians who deviate from the average [by performing] complex cases."
Additionally, the way patients’ drug use gets recorded on the day of hospitalization is problematic, as patients may stop a chronically used drug on the day before entering the hospital and be erroneously recorded as not being on a therapy. Another issue is misclassification of symptoms, such as a patient who perceives ischemic chest pain as shortness of breath.
"I believe that inappropriate PCIs occur, and these results can certainly show signals. But within the ‘inappropriate’ procedures are some cases with mitigating circumstances," Dr. McNulty said in an interview.
The ACC expert panel published appropriateness criteria for 198 different clinical scenarios based on six separate clinical elements in February 2009. They applied the criteria to 500,154 U.S. cases treated with PCI during July 2009 through the end of September 2010. Inappropriate cases were defined as situations where expected negative consequences exceeded expected benefits.
PCI performed for an acute problem (such as high-risk unstable angina or MI) occurred in 71% of the cases. In this group, 99% of the cases were rated as appropriate, 1% as inappropriate, and fewer than 1% as uncertain. The remaining 29% of PCI procedures occurred in elective cases, of which 50% were rated as appropriate, 12% as inappropriate, and 38% as uncertain, Dr. Chan reported.
The three most common reasons for rating a case as inappropriate included patients with no ischemia, patients with mild ischemia, and asymptomatic patients, he said in an interview.
To address this issue, the ACC should develop a "real-time decision aid" to encourage interventionalists to "take a step back" during a catheterization to review a patient’s history and make a more informed decision on the need for PCI. The interventional cardiologist should review the degree of symptoms a patient has had and the evidence of ischemia, and take those findings into account when deciding whether the patient needs PCI.
Dr. Chan and Dr. McNulty said they had no disclosures.
Inner-City Families Struggle with Treating Asthma in Children
It’s challenging enough to control chronic asthma in children, but youngsters who live in low-income, inner-city households face some special barriers to optimal asthma management, including their family’s difficulty paying for medication, lack of family understanding about optimal treatment, and denial by the family about treatment compliance.
The best way to deal with at least some of these issues may be a new approach to educating families about their child’s persistent asthma, said Dr. Marina Reznik, a pediatrician at the Children’s Hospital at Montefiore Medical Center in New York. She has launched a study to test the ability of community health workers to improve family awareness and understanding of optimal asthma education and to see if this results in improved patient outcomes.
The study involves randomizing Bronx families who have a child with persistent asthma to receive either standard education materials or six visits from a community health worker, every other week over the course of 10 weeks. The health workers will instruct parents on optimal asthma control therapy, teach them how to administer an inhaled corticosteroid to their young child, and then continue to monitor the therapy over a 10-week period to make sure correct treatment delivery continues. Dr. Reznik plans to compare the outcome results between the intervention and control groups over the subsequent year.
She and her associates gained additional insight into the problems that parents face with administering correct asthma treatment to their children from the results of a pair of studies that they reported in March at the annual meeting of the Eastern Society for Pediatric Research in Philadelphia.
Comparing Perceived Asthma Compliance and Reality
In one study, Dr. Reznik and her associates tested the way that parents of children with asthma perceive their compliance with an inhaled corticosteroid regimen, compared with their actual compliance. They recruited 40 parents of a child aged 2-9 years with persistent asthma who required twice-daily therapy with an inhaled corticosteroid, a total of four puffs per day. All children were patients of the community health care center run by Montefiore. The participating parents averaged 33 years old, two-thirds were Hispanic, and 29% had not graduated high school.
Each parent received an inhaled corticosteroid actuator with an attached dose counter that recorded the number of puffs delivered. Thirty days later, the researchers surveyed the parents about their adherence to the two-puffs twice-daily regimen and also checked the dose counter on the family’s actuator.
Sixteen of the 40 parents (40%) claimed they had been 100% compliant with the regimen, while the dose counters revealed that only two families (5%) had achieved complete compliance. In addition, only one parent (3%) owned up in an interview to being completely nonadherent, while the dose counters showed that four parents (10%) had actually failed to administer any treatment during the study.
The results showed that parental self-reporting is "nonreliable" for assessing compliance with an asthma regimen, Dr. Reznik said. "The results may have implications for physicians using parental self-reports in managing children with persistent asthma."
The disparity between perceived and actual adherence may derive in part from parents’ concerns about the safety of this treatment, she suggested. "They see improved symptoms [in their children], but they are terrified of the drugs. They have misconceptions." Other social factors that make life difficult and complicated for these low-income parents may play a role as well, she said.
Delivering Asthma Medication Appropriately
The second set of results that her group reported at the meeting came from a study that focused on caregiver knowledge of the appropriate way to deliver an inhaled corticosteroid. Again, the study used parents of children aged 2-9 years old seen at the hospital’s community outpatient pediatric clinic. This time, they enrolled 66 caregivers, who averaged 32 years old, with 96% of the study group comprising mothers; 27% of the parents had not finished high school, 59% were unemployed, 59% were Hispanic and 26% were black.
Among the 66 participants, 92% said that they had used a spacer when delivering the inhaled corticosteroid to their child, with 78% saying they used the spacer for every treatment, and 5% saying they never used a spacer. In addition, 97% of the caregivers said that a physician or nurse had explained to them how to use the metered dose inhaler and spacer, 91% said that a physician or nurse had demonstrated the correct treatment technique, and 49% said that at some point a physician or nurse had watched their technique for administering the drug.
A researcher then watched each caregiver deliver two puffs of the inhaled corticosteroid to a doll. Only one of the participants (2%) correctly performed every step of drug administration with the metered dose inhaler and spacer. Although 97% correctly formed a tight seal with the inhaler, the most problematic steps involved waiting the appropriate interval between puffs, done by 27%, and instructing the recipient to exhale before the treatment inhalation, done by 24%. Other steps scored on the assessment involved shaking the inhaler for at least 5 seconds before administering a puff, pressing the inhaler just once for each puff, and administering the correct number of puffs.
Dr. Reznik and her associates concluded that the results highlighted the need for repeated training of caregivers to ensure ongoing, proper delivery of inhaled corticosteroids.
Most physicians don’t have the time to properly teach parents on the correct delivery of inhaled corticosteroids, Dr. Reznik said. In addition, many parents favor treatment with an inhaled, short-acting beta-agonist, such as albuterol, because of the immediate symptom relief it provides. "They don’t see the role of preventive treatment, compared with acute treatment," she said in an interview.
"There is a discrepancy between what physicians say and what parents hear, and there is more to this than education." Parents face the financial challenge of paying for the medications, and they fear the side effects of inhaled corticosteroids. "Physicians try to educate the family as much as possible, but with limited time, that may not be possible." The community health worker approach under development by Dr. Reznik features a user-friendly format in which the health worker goes to the family’s home, a format that she hopes will lead to improved caregiver education and reinforcement, improved drug delivery, and better outcomes.
Dr. Reznik said that she had no disclosures.
It’s challenging enough to control chronic asthma in children, but youngsters who live in low-income, inner-city households face some special barriers to optimal asthma management, including their family’s difficulty paying for medication, lack of family understanding about optimal treatment, and denial by the family about treatment compliance.
The best way to deal with at least some of these issues may be a new approach to educating families about their child’s persistent asthma, said Dr. Marina Reznik, a pediatrician at the Children’s Hospital at Montefiore Medical Center in New York. She has launched a study to test the ability of community health workers to improve family awareness and understanding of optimal asthma education and to see if this results in improved patient outcomes.
The study involves randomizing Bronx families who have a child with persistent asthma to receive either standard education materials or six visits from a community health worker, every other week over the course of 10 weeks. The health workers will instruct parents on optimal asthma control therapy, teach them how to administer an inhaled corticosteroid to their young child, and then continue to monitor the therapy over a 10-week period to make sure correct treatment delivery continues. Dr. Reznik plans to compare the outcome results between the intervention and control groups over the subsequent year.
She and her associates gained additional insight into the problems that parents face with administering correct asthma treatment to their children from the results of a pair of studies that they reported in March at the annual meeting of the Eastern Society for Pediatric Research in Philadelphia.
Comparing Perceived Asthma Compliance and Reality
In one study, Dr. Reznik and her associates tested the way that parents of children with asthma perceive their compliance with an inhaled corticosteroid regimen, compared with their actual compliance. They recruited 40 parents of a child aged 2-9 years with persistent asthma who required twice-daily therapy with an inhaled corticosteroid, a total of four puffs per day. All children were patients of the community health care center run by Montefiore. The participating parents averaged 33 years old, two-thirds were Hispanic, and 29% had not graduated high school.
Each parent received an inhaled corticosteroid actuator with an attached dose counter that recorded the number of puffs delivered. Thirty days later, the researchers surveyed the parents about their adherence to the two-puffs twice-daily regimen and also checked the dose counter on the family’s actuator.
Sixteen of the 40 parents (40%) claimed they had been 100% compliant with the regimen, while the dose counters revealed that only two families (5%) had achieved complete compliance. In addition, only one parent (3%) owned up in an interview to being completely nonadherent, while the dose counters showed that four parents (10%) had actually failed to administer any treatment during the study.
The results showed that parental self-reporting is "nonreliable" for assessing compliance with an asthma regimen, Dr. Reznik said. "The results may have implications for physicians using parental self-reports in managing children with persistent asthma."
The disparity between perceived and actual adherence may derive in part from parents’ concerns about the safety of this treatment, she suggested. "They see improved symptoms [in their children], but they are terrified of the drugs. They have misconceptions." Other social factors that make life difficult and complicated for these low-income parents may play a role as well, she said.
Delivering Asthma Medication Appropriately
The second set of results that her group reported at the meeting came from a study that focused on caregiver knowledge of the appropriate way to deliver an inhaled corticosteroid. Again, the study used parents of children aged 2-9 years old seen at the hospital’s community outpatient pediatric clinic. This time, they enrolled 66 caregivers, who averaged 32 years old, with 96% of the study group comprising mothers; 27% of the parents had not finished high school, 59% were unemployed, 59% were Hispanic and 26% were black.
Among the 66 participants, 92% said that they had used a spacer when delivering the inhaled corticosteroid to their child, with 78% saying they used the spacer for every treatment, and 5% saying they never used a spacer. In addition, 97% of the caregivers said that a physician or nurse had explained to them how to use the metered dose inhaler and spacer, 91% said that a physician or nurse had demonstrated the correct treatment technique, and 49% said that at some point a physician or nurse had watched their technique for administering the drug.
A researcher then watched each caregiver deliver two puffs of the inhaled corticosteroid to a doll. Only one of the participants (2%) correctly performed every step of drug administration with the metered dose inhaler and spacer. Although 97% correctly formed a tight seal with the inhaler, the most problematic steps involved waiting the appropriate interval between puffs, done by 27%, and instructing the recipient to exhale before the treatment inhalation, done by 24%. Other steps scored on the assessment involved shaking the inhaler for at least 5 seconds before administering a puff, pressing the inhaler just once for each puff, and administering the correct number of puffs.
Dr. Reznik and her associates concluded that the results highlighted the need for repeated training of caregivers to ensure ongoing, proper delivery of inhaled corticosteroids.
Most physicians don’t have the time to properly teach parents on the correct delivery of inhaled corticosteroids, Dr. Reznik said. In addition, many parents favor treatment with an inhaled, short-acting beta-agonist, such as albuterol, because of the immediate symptom relief it provides. "They don’t see the role of preventive treatment, compared with acute treatment," she said in an interview.
"There is a discrepancy between what physicians say and what parents hear, and there is more to this than education." Parents face the financial challenge of paying for the medications, and they fear the side effects of inhaled corticosteroids. "Physicians try to educate the family as much as possible, but with limited time, that may not be possible." The community health worker approach under development by Dr. Reznik features a user-friendly format in which the health worker goes to the family’s home, a format that she hopes will lead to improved caregiver education and reinforcement, improved drug delivery, and better outcomes.
Dr. Reznik said that she had no disclosures.
It’s challenging enough to control chronic asthma in children, but youngsters who live in low-income, inner-city households face some special barriers to optimal asthma management, including their family’s difficulty paying for medication, lack of family understanding about optimal treatment, and denial by the family about treatment compliance.
The best way to deal with at least some of these issues may be a new approach to educating families about their child’s persistent asthma, said Dr. Marina Reznik, a pediatrician at the Children’s Hospital at Montefiore Medical Center in New York. She has launched a study to test the ability of community health workers to improve family awareness and understanding of optimal asthma education and to see if this results in improved patient outcomes.
The study involves randomizing Bronx families who have a child with persistent asthma to receive either standard education materials or six visits from a community health worker, every other week over the course of 10 weeks. The health workers will instruct parents on optimal asthma control therapy, teach them how to administer an inhaled corticosteroid to their young child, and then continue to monitor the therapy over a 10-week period to make sure correct treatment delivery continues. Dr. Reznik plans to compare the outcome results between the intervention and control groups over the subsequent year.
She and her associates gained additional insight into the problems that parents face with administering correct asthma treatment to their children from the results of a pair of studies that they reported in March at the annual meeting of the Eastern Society for Pediatric Research in Philadelphia.
Comparing Perceived Asthma Compliance and Reality
In one study, Dr. Reznik and her associates tested the way that parents of children with asthma perceive their compliance with an inhaled corticosteroid regimen, compared with their actual compliance. They recruited 40 parents of a child aged 2-9 years with persistent asthma who required twice-daily therapy with an inhaled corticosteroid, a total of four puffs per day. All children were patients of the community health care center run by Montefiore. The participating parents averaged 33 years old, two-thirds were Hispanic, and 29% had not graduated high school.
Each parent received an inhaled corticosteroid actuator with an attached dose counter that recorded the number of puffs delivered. Thirty days later, the researchers surveyed the parents about their adherence to the two-puffs twice-daily regimen and also checked the dose counter on the family’s actuator.
Sixteen of the 40 parents (40%) claimed they had been 100% compliant with the regimen, while the dose counters revealed that only two families (5%) had achieved complete compliance. In addition, only one parent (3%) owned up in an interview to being completely nonadherent, while the dose counters showed that four parents (10%) had actually failed to administer any treatment during the study.
The results showed that parental self-reporting is "nonreliable" for assessing compliance with an asthma regimen, Dr. Reznik said. "The results may have implications for physicians using parental self-reports in managing children with persistent asthma."
The disparity between perceived and actual adherence may derive in part from parents’ concerns about the safety of this treatment, she suggested. "They see improved symptoms [in their children], but they are terrified of the drugs. They have misconceptions." Other social factors that make life difficult and complicated for these low-income parents may play a role as well, she said.
Delivering Asthma Medication Appropriately
The second set of results that her group reported at the meeting came from a study that focused on caregiver knowledge of the appropriate way to deliver an inhaled corticosteroid. Again, the study used parents of children aged 2-9 years old seen at the hospital’s community outpatient pediatric clinic. This time, they enrolled 66 caregivers, who averaged 32 years old, with 96% of the study group comprising mothers; 27% of the parents had not finished high school, 59% were unemployed, 59% were Hispanic and 26% were black.
Among the 66 participants, 92% said that they had used a spacer when delivering the inhaled corticosteroid to their child, with 78% saying they used the spacer for every treatment, and 5% saying they never used a spacer. In addition, 97% of the caregivers said that a physician or nurse had explained to them how to use the metered dose inhaler and spacer, 91% said that a physician or nurse had demonstrated the correct treatment technique, and 49% said that at some point a physician or nurse had watched their technique for administering the drug.
A researcher then watched each caregiver deliver two puffs of the inhaled corticosteroid to a doll. Only one of the participants (2%) correctly performed every step of drug administration with the metered dose inhaler and spacer. Although 97% correctly formed a tight seal with the inhaler, the most problematic steps involved waiting the appropriate interval between puffs, done by 27%, and instructing the recipient to exhale before the treatment inhalation, done by 24%. Other steps scored on the assessment involved shaking the inhaler for at least 5 seconds before administering a puff, pressing the inhaler just once for each puff, and administering the correct number of puffs.
Dr. Reznik and her associates concluded that the results highlighted the need for repeated training of caregivers to ensure ongoing, proper delivery of inhaled corticosteroids.
Most physicians don’t have the time to properly teach parents on the correct delivery of inhaled corticosteroids, Dr. Reznik said. In addition, many parents favor treatment with an inhaled, short-acting beta-agonist, such as albuterol, because of the immediate symptom relief it provides. "They don’t see the role of preventive treatment, compared with acute treatment," she said in an interview.
"There is a discrepancy between what physicians say and what parents hear, and there is more to this than education." Parents face the financial challenge of paying for the medications, and they fear the side effects of inhaled corticosteroids. "Physicians try to educate the family as much as possible, but with limited time, that may not be possible." The community health worker approach under development by Dr. Reznik features a user-friendly format in which the health worker goes to the family’s home, a format that she hopes will lead to improved caregiver education and reinforcement, improved drug delivery, and better outcomes.
Dr. Reznik said that she had no disclosures.





