User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
YMCAs Branch Out to Offer Diabetes Prevention Programs
NEW YORK - Taking an "if we build it, they will come" approach, the YMCA is rolling out a diabetes-prevention program at a Y near you. And they need primary care physicians to refer patients to these programs.
The building started last year, when the YMCA of the USA began offering lifestyle training to people with prediabetes, and by February the program had taken root at 116 sites in more than 30 U.S. communities, David G. Marrero, Ph.D., said at the annual American Diabetes Association advanced postgraduate course.
The premise of the program is that people who lose 7% of their body weight and gradually increase their weekly exercise to 150 minutes can significantly lower their risk for developing frank type 2 diabetes. Candidates for the program must be overweight and either at high risk for diabetes or with a diagnosis of prediabetes.
The course that is offered at YMCAs involves an hour-long session once a week for 16 weeks. Among the session topics are the following:
• Ways to Eat Less Fat and Fewer Calories.
• Being Active: A Way of Life.
• Four Keys to Healthy Eating Out.
• Talk Back to Negative Thoughts.
• The Slippery Slope of Lifestyle Change.
• Ways to Stay Motivated.
In an effort to stay motivated, those enrolled in the course will attend monthly refresher courses for 8 months to discuss areas in which they are having trouble maintaining their new healthful habits, and reviewing the basic strategies presented during the original sessions.
Evidence of the prevention program’s efficacy, when it was offered as one-on-one training, was demonstrated nearly a decade ago by the landmark Diabetes Prevention Program, which recruited more than 3,000 Americans who did not have diabetes but who did have an elevated fasting blood glucose level. The findings showed that in a controlled, randomized study setting, teaching people to exercise more and change their diet to achieve significant weight loss led to a 58% cut in the rate of new diabetes cases during 3 years of follow-up, compared with control participants (N. Engl. J. Med. 2002;346:393-403).
"The DPP showed the efficacy of lifestyle modification, but the issue remained of how to translate this powerful finding to the public," said Dr. Marrero, professor of medicine and director of the Diabetes Translational Research Center at Indiana University in Indianapolis. "The DPP was expensive." By Dr. Marrero’s calculations, the original DPP lifestyle intervention cost nearly $1,500 per person. With about 70 million Americans estimated to have prediabetes, it was clear that adapting the one-on-one program for a group-based program for use at YMCAs was key.
His group reported on the success of a pilot group–based version in 2008 (Am. J. Prev. Med. 2008;35:357-63). Based on this evidence – and with the backing of the Centers for Disease Control and Prevention – the YMCA moved to expand the program nationally.
By the end of 2011, the program is expected to be available at 150 sites operated by about 50 different YMCAs, said Dr. Matt Longjohn, senior director of chronic disease prevention at the YMCA of the USA.
The CDC is supporting the rollout of the program, as mandated by the National Diabetes Prevention Program of the Patient Protection and Affordable Care Act.
For people without coverage, the year-long program costs about $300 per participant, noted Dr. Longjohn, but many Ys offer it at a reduced rate because they also receive support from the CDC. For example, YMCA of Delaware charges Y members $149 for the prevention-training course; nonmembers pay $199 for the program.
UnitedHealthcare has also signed on to cover the cost of program participation for its beneficiaries who are at risk for diabetes.
The 200 lifestyle coaches who have so far been trained to run the programs receive 3 days of instruction. Dr. Marrero emphasized that coach training is standardized so that it is consistent across all YMCAs nationwide.
Dr. Marrero said that the next step is encouraging primary care physicians to broadly screen and identify people who would benefit from a diabetes-prevention intervention. He and his associates recently began a pilot study to assess the benefits of conducting routine diabetes screening in primary care practices. YMCA branches that offer the diabetes prevention classes are promoting the program to local health care providers. People who sign up to take a set of classes are expected to provide some type of documentation that they have prediabetes, Dr. Longjohn said in an interview.
Dr. Marrero said that he had been an advisor to Eli Lilly, a consultant to Sanofi-Aventis and to YMCA of the USA, and a speaker for Taking Control of Your Diabetes. Dr. Longjohn reported having no disclosures.
NEW YORK - Taking an "if we build it, they will come" approach, the YMCA is rolling out a diabetes-prevention program at a Y near you. And they need primary care physicians to refer patients to these programs.
The building started last year, when the YMCA of the USA began offering lifestyle training to people with prediabetes, and by February the program had taken root at 116 sites in more than 30 U.S. communities, David G. Marrero, Ph.D., said at the annual American Diabetes Association advanced postgraduate course.
The premise of the program is that people who lose 7% of their body weight and gradually increase their weekly exercise to 150 minutes can significantly lower their risk for developing frank type 2 diabetes. Candidates for the program must be overweight and either at high risk for diabetes or with a diagnosis of prediabetes.
The course that is offered at YMCAs involves an hour-long session once a week for 16 weeks. Among the session topics are the following:
• Ways to Eat Less Fat and Fewer Calories.
• Being Active: A Way of Life.
• Four Keys to Healthy Eating Out.
• Talk Back to Negative Thoughts.
• The Slippery Slope of Lifestyle Change.
• Ways to Stay Motivated.
In an effort to stay motivated, those enrolled in the course will attend monthly refresher courses for 8 months to discuss areas in which they are having trouble maintaining their new healthful habits, and reviewing the basic strategies presented during the original sessions.
Evidence of the prevention program’s efficacy, when it was offered as one-on-one training, was demonstrated nearly a decade ago by the landmark Diabetes Prevention Program, which recruited more than 3,000 Americans who did not have diabetes but who did have an elevated fasting blood glucose level. The findings showed that in a controlled, randomized study setting, teaching people to exercise more and change their diet to achieve significant weight loss led to a 58% cut in the rate of new diabetes cases during 3 years of follow-up, compared with control participants (N. Engl. J. Med. 2002;346:393-403).
"The DPP showed the efficacy of lifestyle modification, but the issue remained of how to translate this powerful finding to the public," said Dr. Marrero, professor of medicine and director of the Diabetes Translational Research Center at Indiana University in Indianapolis. "The DPP was expensive." By Dr. Marrero’s calculations, the original DPP lifestyle intervention cost nearly $1,500 per person. With about 70 million Americans estimated to have prediabetes, it was clear that adapting the one-on-one program for a group-based program for use at YMCAs was key.
His group reported on the success of a pilot group–based version in 2008 (Am. J. Prev. Med. 2008;35:357-63). Based on this evidence – and with the backing of the Centers for Disease Control and Prevention – the YMCA moved to expand the program nationally.
By the end of 2011, the program is expected to be available at 150 sites operated by about 50 different YMCAs, said Dr. Matt Longjohn, senior director of chronic disease prevention at the YMCA of the USA.
The CDC is supporting the rollout of the program, as mandated by the National Diabetes Prevention Program of the Patient Protection and Affordable Care Act.
For people without coverage, the year-long program costs about $300 per participant, noted Dr. Longjohn, but many Ys offer it at a reduced rate because they also receive support from the CDC. For example, YMCA of Delaware charges Y members $149 for the prevention-training course; nonmembers pay $199 for the program.
UnitedHealthcare has also signed on to cover the cost of program participation for its beneficiaries who are at risk for diabetes.
The 200 lifestyle coaches who have so far been trained to run the programs receive 3 days of instruction. Dr. Marrero emphasized that coach training is standardized so that it is consistent across all YMCAs nationwide.
Dr. Marrero said that the next step is encouraging primary care physicians to broadly screen and identify people who would benefit from a diabetes-prevention intervention. He and his associates recently began a pilot study to assess the benefits of conducting routine diabetes screening in primary care practices. YMCA branches that offer the diabetes prevention classes are promoting the program to local health care providers. People who sign up to take a set of classes are expected to provide some type of documentation that they have prediabetes, Dr. Longjohn said in an interview.
Dr. Marrero said that he had been an advisor to Eli Lilly, a consultant to Sanofi-Aventis and to YMCA of the USA, and a speaker for Taking Control of Your Diabetes. Dr. Longjohn reported having no disclosures.
NEW YORK - Taking an "if we build it, they will come" approach, the YMCA is rolling out a diabetes-prevention program at a Y near you. And they need primary care physicians to refer patients to these programs.
The building started last year, when the YMCA of the USA began offering lifestyle training to people with prediabetes, and by February the program had taken root at 116 sites in more than 30 U.S. communities, David G. Marrero, Ph.D., said at the annual American Diabetes Association advanced postgraduate course.
The premise of the program is that people who lose 7% of their body weight and gradually increase their weekly exercise to 150 minutes can significantly lower their risk for developing frank type 2 diabetes. Candidates for the program must be overweight and either at high risk for diabetes or with a diagnosis of prediabetes.
The course that is offered at YMCAs involves an hour-long session once a week for 16 weeks. Among the session topics are the following:
• Ways to Eat Less Fat and Fewer Calories.
• Being Active: A Way of Life.
• Four Keys to Healthy Eating Out.
• Talk Back to Negative Thoughts.
• The Slippery Slope of Lifestyle Change.
• Ways to Stay Motivated.
In an effort to stay motivated, those enrolled in the course will attend monthly refresher courses for 8 months to discuss areas in which they are having trouble maintaining their new healthful habits, and reviewing the basic strategies presented during the original sessions.
Evidence of the prevention program’s efficacy, when it was offered as one-on-one training, was demonstrated nearly a decade ago by the landmark Diabetes Prevention Program, which recruited more than 3,000 Americans who did not have diabetes but who did have an elevated fasting blood glucose level. The findings showed that in a controlled, randomized study setting, teaching people to exercise more and change their diet to achieve significant weight loss led to a 58% cut in the rate of new diabetes cases during 3 years of follow-up, compared with control participants (N. Engl. J. Med. 2002;346:393-403).
"The DPP showed the efficacy of lifestyle modification, but the issue remained of how to translate this powerful finding to the public," said Dr. Marrero, professor of medicine and director of the Diabetes Translational Research Center at Indiana University in Indianapolis. "The DPP was expensive." By Dr. Marrero’s calculations, the original DPP lifestyle intervention cost nearly $1,500 per person. With about 70 million Americans estimated to have prediabetes, it was clear that adapting the one-on-one program for a group-based program for use at YMCAs was key.
His group reported on the success of a pilot group–based version in 2008 (Am. J. Prev. Med. 2008;35:357-63). Based on this evidence – and with the backing of the Centers for Disease Control and Prevention – the YMCA moved to expand the program nationally.
By the end of 2011, the program is expected to be available at 150 sites operated by about 50 different YMCAs, said Dr. Matt Longjohn, senior director of chronic disease prevention at the YMCA of the USA.
The CDC is supporting the rollout of the program, as mandated by the National Diabetes Prevention Program of the Patient Protection and Affordable Care Act.
For people without coverage, the year-long program costs about $300 per participant, noted Dr. Longjohn, but many Ys offer it at a reduced rate because they also receive support from the CDC. For example, YMCA of Delaware charges Y members $149 for the prevention-training course; nonmembers pay $199 for the program.
UnitedHealthcare has also signed on to cover the cost of program participation for its beneficiaries who are at risk for diabetes.
The 200 lifestyle coaches who have so far been trained to run the programs receive 3 days of instruction. Dr. Marrero emphasized that coach training is standardized so that it is consistent across all YMCAs nationwide.
Dr. Marrero said that the next step is encouraging primary care physicians to broadly screen and identify people who would benefit from a diabetes-prevention intervention. He and his associates recently began a pilot study to assess the benefits of conducting routine diabetes screening in primary care practices. YMCA branches that offer the diabetes prevention classes are promoting the program to local health care providers. People who sign up to take a set of classes are expected to provide some type of documentation that they have prediabetes, Dr. Longjohn said in an interview.
Dr. Marrero said that he had been an advisor to Eli Lilly, a consultant to Sanofi-Aventis and to YMCA of the USA, and a speaker for Taking Control of Your Diabetes. Dr. Longjohn reported having no disclosures.
YMCAs Branch Out to Offer Diabetes Prevention Programs
NEW YORK - Taking an "if we build it, they will come" approach, the YMCA is rolling out a diabetes-prevention program at a Y near you. And they need primary care physicians to refer patients to these programs.
The building started last year, when the YMCA of the USA began offering lifestyle training to people with prediabetes, and by February the program had taken root at 116 sites in more than 30 U.S. communities, David G. Marrero, Ph.D., said at the annual American Diabetes Association advanced postgraduate course.
The program puts into practice lessons learned nearly a decade ago in the landmark Diabetes Prevention Program, which recruited more than 3,000 Americans who did not have diabetes but who did have an elevated fasting blood glucose level.
The findings showed that in a controlled, randomized study setting, teaching people on a one-on-one basis to exercise more and change their diet to achieve significant weight loss led to a 58% cut in the rate of new diabetes cases during 3 years of follow-up, compared with control participants (N. Engl. J. Med. 2002;346:393-403).
“The DPP showed the efficacy of lifestyle modification, but the issue remained of how to translate this powerful finding to the public,” said Dr. Marrero, professor of medicine and director of the Diabetes Translational Research Center at Indiana University in Indianapolis. “The DPP was expensive.”
By Dr. Marrero’s calculations, the original DPP lifestyle intervention cost nearly $1,500 per person. With about 70 million Americans estimated to have prediabetes, it was clear that adapting the one-on-one program for a group-based program for use at YMCAs was key.
His group reported on the success of a pilot group–based version in 2008 (Am. J. Prev. Med. 2008;35:357-63). Based on this evidence – and with the backing of the Centers for Disease Control and Prevention – the YMCA moved to expand the program nationally.
By the end of 2011, the program is expected to be available at 150 sites operated by about 50 different YMCAs, said Dr. Matt Longjohn, senior director of chronic disease prevention at the YMCA of the USA.
The premise of the program is that people who lose 7% of their body weight and gradually increase their weekly exercise to 150 minutes can significantly lower their risk for developing frank type 2 diabetes. Candidates for the program must be overweight and either at high risk for diabetes or with a diagnosis of prediabetes.
The course that is offered at YMCAs involves an hour-long session once a week for 16 weeks. Among the session topics are the following:
– Ways to Eat Less Fat and Fewer Calories.
– Being Active: A Way of Life.
– Four Keys to Healthy Eating Out.
– Talk Back to Negative Thoughts.
– The Slippery Slope of Lifestyle Change.
– Ways to Stay Motivated.
In an effort to stay motivated, those enrolled in the course will attend monthly refresher courses for 8 months to discuss areas in which they are having trouble maintaining their new healthful habits, and reviewing the basic strategies presented during the original sessions.
The CDC is supporting the rollout of the program, as was mandated by the National Diabetes Prevention Program of the Patient Protection and Affordable Care Act.
For people without coverage, the year-long program costs about $300 per participant, noted Dr. Longjohn, but many Ys offer it at a reduced rate because they also receive support from the CDC. For example, YMCA of Delaware charges Y members $149 for the prevention-training course; nonmembers pay $199 for the program.
UnitedHealthcare has also signed on to cover the cost of program participation for its beneficiaries who are at risk for diabetes.
The 200 lifestyle coaches who have so far been trained to run the programs receive 3 days of instruction. Dr. Marrero emphasized that coach training is standardized so that it is consistent across all YMCAs nationwide.
Dr. Marrero said that the next step is encouraging primary care physicians to broadly screen and identify people who would benefit from a diabetes-prevention intervention. He and his associates recently began a pilot study to assess the benefits of conducting routine diabetes screening in primary care practices.
YMCA branches that offer the diabetes prevention classes are promoting the program to local health care providers. People who sign up to take a set of classes are expected to provide some type of documentation that they have prediabetes, Dr. Longjohn said in an interview.
Dr. Marrero said that he had been an advisor to Eli Lilly, a consultant to Sanofi-Aventis and to YMCA of the USA, and a speaker for Taking Control of Your Diabetes. Dr. Longjohn reported having no disclosures.
NEW YORK - Taking an "if we build it, they will come" approach, the YMCA is rolling out a diabetes-prevention program at a Y near you. And they need primary care physicians to refer patients to these programs.
The building started last year, when the YMCA of the USA began offering lifestyle training to people with prediabetes, and by February the program had taken root at 116 sites in more than 30 U.S. communities, David G. Marrero, Ph.D., said at the annual American Diabetes Association advanced postgraduate course.
The program puts into practice lessons learned nearly a decade ago in the landmark Diabetes Prevention Program, which recruited more than 3,000 Americans who did not have diabetes but who did have an elevated fasting blood glucose level.
The findings showed that in a controlled, randomized study setting, teaching people on a one-on-one basis to exercise more and change their diet to achieve significant weight loss led to a 58% cut in the rate of new diabetes cases during 3 years of follow-up, compared with control participants (N. Engl. J. Med. 2002;346:393-403).
“The DPP showed the efficacy of lifestyle modification, but the issue remained of how to translate this powerful finding to the public,” said Dr. Marrero, professor of medicine and director of the Diabetes Translational Research Center at Indiana University in Indianapolis. “The DPP was expensive.”
By Dr. Marrero’s calculations, the original DPP lifestyle intervention cost nearly $1,500 per person. With about 70 million Americans estimated to have prediabetes, it was clear that adapting the one-on-one program for a group-based program for use at YMCAs was key.
His group reported on the success of a pilot group–based version in 2008 (Am. J. Prev. Med. 2008;35:357-63). Based on this evidence – and with the backing of the Centers for Disease Control and Prevention – the YMCA moved to expand the program nationally.
By the end of 2011, the program is expected to be available at 150 sites operated by about 50 different YMCAs, said Dr. Matt Longjohn, senior director of chronic disease prevention at the YMCA of the USA.
The premise of the program is that people who lose 7% of their body weight and gradually increase their weekly exercise to 150 minutes can significantly lower their risk for developing frank type 2 diabetes. Candidates for the program must be overweight and either at high risk for diabetes or with a diagnosis of prediabetes.
The course that is offered at YMCAs involves an hour-long session once a week for 16 weeks. Among the session topics are the following:
– Ways to Eat Less Fat and Fewer Calories.
– Being Active: A Way of Life.
– Four Keys to Healthy Eating Out.
– Talk Back to Negative Thoughts.
– The Slippery Slope of Lifestyle Change.
– Ways to Stay Motivated.
In an effort to stay motivated, those enrolled in the course will attend monthly refresher courses for 8 months to discuss areas in which they are having trouble maintaining their new healthful habits, and reviewing the basic strategies presented during the original sessions.
The CDC is supporting the rollout of the program, as was mandated by the National Diabetes Prevention Program of the Patient Protection and Affordable Care Act.
For people without coverage, the year-long program costs about $300 per participant, noted Dr. Longjohn, but many Ys offer it at a reduced rate because they also receive support from the CDC. For example, YMCA of Delaware charges Y members $149 for the prevention-training course; nonmembers pay $199 for the program.
UnitedHealthcare has also signed on to cover the cost of program participation for its beneficiaries who are at risk for diabetes.
The 200 lifestyle coaches who have so far been trained to run the programs receive 3 days of instruction. Dr. Marrero emphasized that coach training is standardized so that it is consistent across all YMCAs nationwide.
Dr. Marrero said that the next step is encouraging primary care physicians to broadly screen and identify people who would benefit from a diabetes-prevention intervention. He and his associates recently began a pilot study to assess the benefits of conducting routine diabetes screening in primary care practices.
YMCA branches that offer the diabetes prevention classes are promoting the program to local health care providers. People who sign up to take a set of classes are expected to provide some type of documentation that they have prediabetes, Dr. Longjohn said in an interview.
Dr. Marrero said that he had been an advisor to Eli Lilly, a consultant to Sanofi-Aventis and to YMCA of the USA, and a speaker for Taking Control of Your Diabetes. Dr. Longjohn reported having no disclosures.
NEW YORK - Taking an "if we build it, they will come" approach, the YMCA is rolling out a diabetes-prevention program at a Y near you. And they need primary care physicians to refer patients to these programs.
The building started last year, when the YMCA of the USA began offering lifestyle training to people with prediabetes, and by February the program had taken root at 116 sites in more than 30 U.S. communities, David G. Marrero, Ph.D., said at the annual American Diabetes Association advanced postgraduate course.
The program puts into practice lessons learned nearly a decade ago in the landmark Diabetes Prevention Program, which recruited more than 3,000 Americans who did not have diabetes but who did have an elevated fasting blood glucose level.
The findings showed that in a controlled, randomized study setting, teaching people on a one-on-one basis to exercise more and change their diet to achieve significant weight loss led to a 58% cut in the rate of new diabetes cases during 3 years of follow-up, compared with control participants (N. Engl. J. Med. 2002;346:393-403).
“The DPP showed the efficacy of lifestyle modification, but the issue remained of how to translate this powerful finding to the public,” said Dr. Marrero, professor of medicine and director of the Diabetes Translational Research Center at Indiana University in Indianapolis. “The DPP was expensive.”
By Dr. Marrero’s calculations, the original DPP lifestyle intervention cost nearly $1,500 per person. With about 70 million Americans estimated to have prediabetes, it was clear that adapting the one-on-one program for a group-based program for use at YMCAs was key.
His group reported on the success of a pilot group–based version in 2008 (Am. J. Prev. Med. 2008;35:357-63). Based on this evidence – and with the backing of the Centers for Disease Control and Prevention – the YMCA moved to expand the program nationally.
By the end of 2011, the program is expected to be available at 150 sites operated by about 50 different YMCAs, said Dr. Matt Longjohn, senior director of chronic disease prevention at the YMCA of the USA.
The premise of the program is that people who lose 7% of their body weight and gradually increase their weekly exercise to 150 minutes can significantly lower their risk for developing frank type 2 diabetes. Candidates for the program must be overweight and either at high risk for diabetes or with a diagnosis of prediabetes.
The course that is offered at YMCAs involves an hour-long session once a week for 16 weeks. Among the session topics are the following:
– Ways to Eat Less Fat and Fewer Calories.
– Being Active: A Way of Life.
– Four Keys to Healthy Eating Out.
– Talk Back to Negative Thoughts.
– The Slippery Slope of Lifestyle Change.
– Ways to Stay Motivated.
In an effort to stay motivated, those enrolled in the course will attend monthly refresher courses for 8 months to discuss areas in which they are having trouble maintaining their new healthful habits, and reviewing the basic strategies presented during the original sessions.
The CDC is supporting the rollout of the program, as was mandated by the National Diabetes Prevention Program of the Patient Protection and Affordable Care Act.
For people without coverage, the year-long program costs about $300 per participant, noted Dr. Longjohn, but many Ys offer it at a reduced rate because they also receive support from the CDC. For example, YMCA of Delaware charges Y members $149 for the prevention-training course; nonmembers pay $199 for the program.
UnitedHealthcare has also signed on to cover the cost of program participation for its beneficiaries who are at risk for diabetes.
The 200 lifestyle coaches who have so far been trained to run the programs receive 3 days of instruction. Dr. Marrero emphasized that coach training is standardized so that it is consistent across all YMCAs nationwide.
Dr. Marrero said that the next step is encouraging primary care physicians to broadly screen and identify people who would benefit from a diabetes-prevention intervention. He and his associates recently began a pilot study to assess the benefits of conducting routine diabetes screening in primary care practices.
YMCA branches that offer the diabetes prevention classes are promoting the program to local health care providers. People who sign up to take a set of classes are expected to provide some type of documentation that they have prediabetes, Dr. Longjohn said in an interview.
Dr. Marrero said that he had been an advisor to Eli Lilly, a consultant to Sanofi-Aventis and to YMCA of the USA, and a speaker for Taking Control of Your Diabetes. Dr. Longjohn reported having no disclosures.
Nondiabetic Gastric Bypass Patients May Develop Hypoglycemia
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
FROM THE ANNUAL ADA ADVANCED POSTGRADUATE COURSE
Nondiabetic Gastric Bypass Patients May Develop Hypoglycemia
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
FROM THE ANNUAL ADA ADVANCED POSTGRADUATE COURSE
Nondiabetic Gastric Bypass Patients May Develop Hypoglycemia
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
NEW YORK – Obese patients without diabetes who underwent gastric bypass showed a surprisingly high prevalence of hyperinsulinemic hypoglycemia starting about a year following bariatric surgery, based on anecdotal experience at one U.S. center.
"We have no data on the prevalence, but it is turning out to be a very prevalent side effect of gastric bypass. All of a sudden at the University of Minnesota these patients are queued up to get into our clinic," Dr. John P. Bantle said at the annual ADA Advanced Postgraduate Course.
"I know of about 25 or 30 cases reported in the literature, but I’ve seen more than that many cases myself. They’re taking over my clinic," said Dr. Bantle, an endocrinologist and professor of medicine at the University of Minnesota in Minneapolis.
"The only thing that protects against patients developing this is preexisting diabetes. Patients who had diabetes [before undergoing gastric bypass surgery] don’t get this because there needs to be a strong insulin response" to ingestion of carbohydrates, something that patients with a history of inadequate insulin production don’t mount. "It happens in people who have healthy insulin-producing beta cells and can make a robust response to postprandial hyperglycemia," he said.
The likely cause is the rapid transit of food between the stomach and small intestine that gastric bypass creates. "Carbohydrates are absorbed much more quickly than nature intended," producing an acute hyperglycemic episode that provokes a strong hyperinsulinemia. That, in turn, brings on a sharp hypoglycemia that can cause the patient to pass out. It is a form of dumping syndrome that does not appear as quickly following bariatric surgery as other manifestations of dumping syndrome, he said.
An alternative explanation, which Dr. Bantle calls much less likely, is that gastric bypass changes the blood level of glucagonlike peptide–1, leading to beta-cell hyperplasia and hyperinsulinemia.
This type of hypoglycemia does not occur in patients who have their pylorus intact following bariatric surgery, such as those who undergo gastric banding or receive a gastric sleeve, he said.
In addition to a delay of more than a year following surgery before it appears, other features that characterize this postprandial hypoglycemia include normal fasting glucose and serum insulin levels, and a carbohydrate-triggered plasma glucose level of less than 50 mg/dL accompanied by a serum insulin level that exceeds 50 microU/mL.
The best treatment is carbohydrate avoidance or restriction, Dr. Bantle said. If that proves impossible, patients can try taking acarbose with a meal that contains carbohydrates. They can also have one or two glucose pills ready to take at the first sign of hypoglycemic symptoms, he said.
Dr. Bantle said that he is a consultant to Unilever, and serves as a speaker for Eli Lilly, Merck, and Novo Nordisk.
EXPERT ANALYSIS FROM THE ANNUAL ADA ADVANCED POSTGRADUATE COURSE
U.S. Military Succeeds With Family-Based Resiliency Training
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
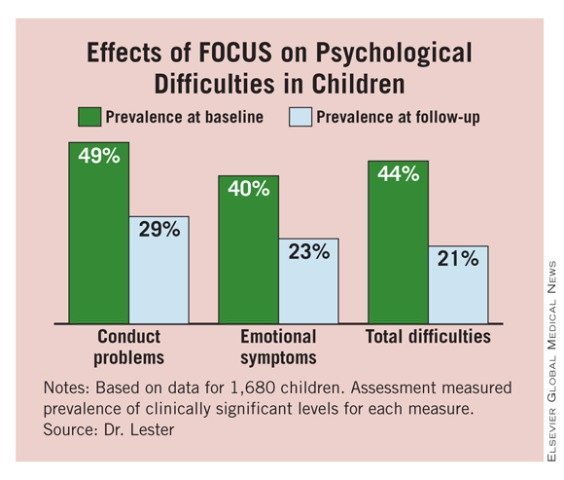
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
Major Finding: Starting in 2008, a family-based resiliency training program for U.S. military families led to improvements in several psychiatric measures. Among parents on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the intervention.
Data Source: Review of baseline and follow-up psychiatric assessments in 1,680 children and their families from July 2008 to July 2010.
Disclosures: Dr. Lester said she had no disclosures.
U.S. Military Succeeds With Family-Based Resiliency Training
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
Major Finding: Starting in 2008, a family-based resiliency training program for U.S. military families led to improvements in several psychiatric measures. Among parents on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the intervention.
Data Source: Review of baseline and follow-up psychiatric assessments in 1,680 children and their families from July 2008 to July 2010.
Disclosures: Dr. Lester said she had no disclosures.
U.S. Military Succeeds With Family-Based Resiliency Training
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
NEW YORK – A novel, family-centered, resiliency-training program involving U.S. military families produced substantial improvements in psychological measures among both children and their parents during the first 2 years of the program involving nearly 1,700 children and their families.
"Child psychological health assessment indicates significant reduction of emotional distress and behavioral problems, and increase in prosocial behaviors" after participation in the intervention program, Dr. Patricia E. Lester said at the annual meeting of the American Academy of Child & Adolescent Psychiatry. "Children also reported significantly increased positive coping skills."
Their parents derived benefits as well. "Parental psychological health measures and functional adjustment for active duty and nonactive duty indicate significant improvement following intervention. Family adjustment assessment indicated a greater prevalence of families with healthy functioning following intervention," said Dr. Lester, medical director of the child and family trauma psychiatry service at the University of California, Los Angeles.
The FOCUS (Families Overcoming Under Stress) program that was developed by Dr. Lester and her associates also has an initial assessment component, which found that both active duty and nonactive duty parents had baseline psychological symptom levels that were increased, relative to community norms, and that nearly half of their children had significant baseline emotional/behavioral symptoms.
The FOCUS program adapted evidence-based interventions for military families who are affected by wartime stress, and it combines both assessment and education about resiliency skills. Specific aims include teaching parents to appreciate the children’s experiences, to normalize distress, to promote perspective taking, to increase positive interactions within the family, and to encourage family-level problem solving and goal setting through family plans.
The program includes eight sessions, starting with two sessions for parents only, then two with the children only, a third session only for parents, and finally three sessions that include the entire family.
Implementation of FOCUS began at seven U.S. military sites for the Navy and Marine Corps in March 2008. In June 2009, the program expanded to an additional seven sites within these two services, and then in September 2009, it expanded to four sites for the Army and Air Force. The program included 1,680 children aged 3-18 years from July 2008 to July 2010.
Data collected so far show that both active duty and nonactive duty parents had significant reductions from baseline to postintervention follow-up in three measures that were made using the Brief Symptom Inventory (global severity index, anxiety, and depression). For example, among active duty parents, global severity dropped by an average of 4.42 points, anxiety fell by an average of 1.39 points, and average depression scores were reduced by 2.39 points. Similar statistically significant drops in all three scores also occurred among nonactive duty parents.
The prevalence of clinically significant anxiety and depression also fell significantly in both types of parents. Among those on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the FOCUS intervention, and clinically significant anxiety dropped from 22% to 6%. Again, similar significant cuts in prevalence rates also occurred in nonactive duty parents.
To assess children, Dr. Lester and her associates used the SDQ (Strengths and Difficulties Questionnaire). They saw significant declines in the children’s total difficulties scores among both girls and boys and in all age subgroups that were assessed (3-7 years, 8-10, and 11 and older). The prevalence of conduct problems, emotional symptoms, and total difficulties all dropped significantly from baseline to follow-up after participation in FOCUS. (See box.)
Dr. Lester said she had no disclosures.
 |
Major Finding: Starting in 2008, a family-based resiliency training program for U.S. military families led to improvements in several psychiatric measures. Among parents on active duty, clinically significant depression was reduced from 30% at baseline to 7% at follow-up after the intervention.
Data Source: Review of baseline and follow-up psychiatric assessments in 1,680 children and their families from July 2008 to July 2010.
Disclosures: Dr. Lester said she had no disclosures.
Doubt Cast on Major Role for PFO Closure in Cryptogenic Stroke
CHICAGO – Closure-device problems did not explain why endovascular closure of patent foramen ovale in patients with a history of cryptogenic stroke failed to produce better outcomes than those of medically-treated patients. The device performed well, Dr. Anthony J. Furlan said at the annual scientific sessions of the American Heart Association.
The problem was patient selection.
"For the vast majority of patients who have had a cryptogenic stroke and a patent foramen ovale [PFO] medical therapy is a good first-choice," said Dr. Furlan, professor and chairman of neurology at Case Western Reserve University in Cleveland. "The challenge now to the endovascular community is to refine the patient selection criteria and not close these holes so liberally."
Based on the results from A Prospective, Multicenter, Randomized Controlled Trial to Evaluate the Safety and Efficacy of the STARFlex Septal Closure System Versus Best Medical Therapy in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale (CLOSURE I), the first randomized study to compare anticoagulant therapy and endovascular PFO closure, "we should see a significant drop in the number of PFOs that get closed," Dr. Furlan said.
The CLOSURE I investigators selected patients "who are being closed worldwide," he said. "Many patients who have a cryptogenic stroke also have a hole and nothing else, but people don’t look that hard. Assessments tend to stop when they find a PFO, he said.
The CLOSURE I results "clearly showed that in patients with stroke of unknown cause and PFO there is no need for systematic PFO closure because it is not effective and may be associated with major vascular complications, atrial fibrillation, or bleeding," said Dr. Pierre Amarenco, professor and chairman of the department of neurology and stroke center at Bichat Hospital in Paris. "Cryptogenic stroke likely includes a broad range of underlying etiologies that may have diluted the true, causal effect of PFO. These results should now be translated into practice for the million patients with stroke of unknown cause and PFO. On a case-by-case basis, after careful evaluation by a vascular neurologist, there may be some patients for whom closing the PFO can be deemed useful, but these cases are likely very rare."
CLOSURE I enrolled 18- to 60-year-olds who had a cryptogenic stroke or transient ischemic attack (TIA) within the past 6 months, and a PFO documented by transesophageal echocardiography. Investigators at 87 centers in the United States and Canada randomized 909 patients during June 2003–October 2008 to endovascular closure with the STARFlex device or to medical therapy. Patients in the closure group also received aspirin and clopidogrel for 6 months following their procedure, followed by aspirin alone for an additional 18 months. Patients in the medical-treatment arm received warfarin alone, dosed to a target international normalized ratio of 2.0-3.0 (139 patients), warfarin plus aspirin (80 patients), or aspirin alone (243 patients) throughout their follow-up. The average age of the patients was 46; 52% were men. Nearly three-quarters had an index cryptogenic stroke.
"We believe the study population was representative of patients aged 60 or younger with cryptogenic stroke or TIA and a PFO," Dr. Furlan said.
The study’s primary end points were the individual rates of stroke and TIA and the composite rate after 2 years of follow-up on an intent-to-treat basis. The composite rates were 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant. The numeric difference was driven primarily by a difference in the TIA rate, 3.3% in the closure group and 4.6% in the medical therapy group, not significantly different. The stroke rates were nearly identical in the two groups, 3.1% and 3.4%. The composite end point showed no significant difference between the two treatment arms regardless of the shunt severity or whether or not the patients had an atrial septal aneurysm.
Roughly 80% of the strokes that occurred during follow-up in both arms of the study had no relationship to paradoxical embolism, suggesting that in many of these patients the PFO may be coincidental, Dr. Furlan said.
In the control arm, the outcomes did not significantly differ regardless of whether patients received warfarin plus aspirin, warfarin alone, or aspirin alone. Dr. Furlan noted that the study was not powered to find significant differences among these three subgroups.
The overall rate of major adverse events was similar, 17%, in both treatment arms (see table). Patients who underwent PFO closure had significantly more major vascular complications and atrial fibrillations. Vascular complications occurred completely secondary to the closures, with none in the medically-managed patients, Dr. Furlan said. The closure procedure achieved technical success in 90% of patients in that arm of the study, with 86% of the PFOs closed at 6 months after treatment based on follow-up echocardiography examinations.
The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.
I find 11 reasons why the CLOSURE I results are misleading and should not deter physicians from performing a closure procedure in patients who have had a cryptogenic stroke and have a patent foramen ovale.
1. The study tested whether PFO closure is superior to medical therapy. This was an inappropriate design because the efficacy of medical therapy had never previously been studied in a trial.
2. Patients in the PFO closure arm also received aspirin therapy for 24 months. This was a design mistake because if medical therapy is effective then PFO closure would not be expected to add any additional benefit.
3. The trial should not have excluded patients with deep vein thrombosis or hypercoagulopathy because these patients should benefit most from PFO closure.
4. The trial enrolled patients very slowly, at a rate of about two patients per year at each of the participating centers, a pace that must have introduced a selection bias.
5. The total number of about 900 enrolled patients and the study design’s presumption that the event rates would be 6% in the medical arm and 2% in the closure arm were too optimistic to produce a statistically significant difference between the two treatments tested. The study’s original design called for 1,600 patients.
6. The 2-year follow-up was too short. The average age of the patients in the study was 46. Patients like this choose to have PFO closure to avoid 30 years of anticoagulation therapy. The advantages of PFO closure would have become clearer during longer follow-up, as patients on warfarin therapy typically abandon it after several years.
7. The control, medical-therapy arm showed some strange outcomes. The event rates were higher in patients with smaller PFOs, and in those without a septal aneurysm.
8. Some of the operators performed PFO closures while still relatively inexperienced, early in their learning curve.
9. The STARFlex closure device used in the study is now outdated. It results in higher rates of atrial fibrillation and clot formation than do other closure devices now available.
10. Long-term anticoagulation is not a viable option for most patients. About 70% of patients on a warfarin regimen stop taking the drug after 5 years.
11. The 17% complication rate in patients undergoing PFO closure was unusually high. In the reported, worldwide experience with PFO closure the serious complication rate is about 3%.
Even if patients and physicians believe the CLOSURE I results despite all these problems, the question remains of which treatment option seems best for patients who have had a cryptogenic stroke and have a PFO. Doing nothing is not a good option, and closing the PFO by open surgery is not attractive because the periprocedural mortality rate is 0.5%-1.0%. The CLOSURE I results showed that medical therapy is no better and no safer than is endovascular PFO closure, and the annual risk for a major bleed on warfarin is 0.5%. For the long term, endovascular closure of a PFO is the most reliable way to prevent recurrent paradoxical embolism, and it has no downside; it was as safe as medical therapy. Patients with a PFO will usually prefer endovascular closure because they then won’t have to face the prospect of having to take anticoagulation therapy for the rest of their lives.
Dr. Horst Sievert is director of the CardioVascular Center in Frankfurt, Germany. He said that he has received consulting fees, travel expenses, or honoraria from Access Closure, AGA, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, Occlutech, Osprey, Ovalis, pfm, Medical Mepro GmbH, ReCor, Rox Medical, Sorin, Spectranetics, Square One, TriReme Medical, Trivascular, Veryan Medical, and Viacor. He said that he owns stock or has stock options in CardioKinetix, Access Closure, CoAptus, Lumen Biomedical, and Coherex. He made these comments during a talk in January at the annual International Symposium on Endovascular Therapy in Miami Beach.
I find 11 reasons why the CLOSURE I results are misleading and should not deter physicians from performing a closure procedure in patients who have had a cryptogenic stroke and have a patent foramen ovale.
1. The study tested whether PFO closure is superior to medical therapy. This was an inappropriate design because the efficacy of medical therapy had never previously been studied in a trial.
2. Patients in the PFO closure arm also received aspirin therapy for 24 months. This was a design mistake because if medical therapy is effective then PFO closure would not be expected to add any additional benefit.
3. The trial should not have excluded patients with deep vein thrombosis or hypercoagulopathy because these patients should benefit most from PFO closure.
4. The trial enrolled patients very slowly, at a rate of about two patients per year at each of the participating centers, a pace that must have introduced a selection bias.
5. The total number of about 900 enrolled patients and the study design’s presumption that the event rates would be 6% in the medical arm and 2% in the closure arm were too optimistic to produce a statistically significant difference between the two treatments tested. The study’s original design called for 1,600 patients.
6. The 2-year follow-up was too short. The average age of the patients in the study was 46. Patients like this choose to have PFO closure to avoid 30 years of anticoagulation therapy. The advantages of PFO closure would have become clearer during longer follow-up, as patients on warfarin therapy typically abandon it after several years.
7. The control, medical-therapy arm showed some strange outcomes. The event rates were higher in patients with smaller PFOs, and in those without a septal aneurysm.
8. Some of the operators performed PFO closures while still relatively inexperienced, early in their learning curve.
9. The STARFlex closure device used in the study is now outdated. It results in higher rates of atrial fibrillation and clot formation than do other closure devices now available.
10. Long-term anticoagulation is not a viable option for most patients. About 70% of patients on a warfarin regimen stop taking the drug after 5 years.
11. The 17% complication rate in patients undergoing PFO closure was unusually high. In the reported, worldwide experience with PFO closure the serious complication rate is about 3%.
Even if patients and physicians believe the CLOSURE I results despite all these problems, the question remains of which treatment option seems best for patients who have had a cryptogenic stroke and have a PFO. Doing nothing is not a good option, and closing the PFO by open surgery is not attractive because the periprocedural mortality rate is 0.5%-1.0%. The CLOSURE I results showed that medical therapy is no better and no safer than is endovascular PFO closure, and the annual risk for a major bleed on warfarin is 0.5%. For the long term, endovascular closure of a PFO is the most reliable way to prevent recurrent paradoxical embolism, and it has no downside; it was as safe as medical therapy. Patients with a PFO will usually prefer endovascular closure because they then won’t have to face the prospect of having to take anticoagulation therapy for the rest of their lives.
Dr. Horst Sievert is director of the CardioVascular Center in Frankfurt, Germany. He said that he has received consulting fees, travel expenses, or honoraria from Access Closure, AGA, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, Occlutech, Osprey, Ovalis, pfm, Medical Mepro GmbH, ReCor, Rox Medical, Sorin, Spectranetics, Square One, TriReme Medical, Trivascular, Veryan Medical, and Viacor. He said that he owns stock or has stock options in CardioKinetix, Access Closure, CoAptus, Lumen Biomedical, and Coherex. He made these comments during a talk in January at the annual International Symposium on Endovascular Therapy in Miami Beach.
I find 11 reasons why the CLOSURE I results are misleading and should not deter physicians from performing a closure procedure in patients who have had a cryptogenic stroke and have a patent foramen ovale.
1. The study tested whether PFO closure is superior to medical therapy. This was an inappropriate design because the efficacy of medical therapy had never previously been studied in a trial.
2. Patients in the PFO closure arm also received aspirin therapy for 24 months. This was a design mistake because if medical therapy is effective then PFO closure would not be expected to add any additional benefit.
3. The trial should not have excluded patients with deep vein thrombosis or hypercoagulopathy because these patients should benefit most from PFO closure.
4. The trial enrolled patients very slowly, at a rate of about two patients per year at each of the participating centers, a pace that must have introduced a selection bias.
5. The total number of about 900 enrolled patients and the study design’s presumption that the event rates would be 6% in the medical arm and 2% in the closure arm were too optimistic to produce a statistically significant difference between the two treatments tested. The study’s original design called for 1,600 patients.
6. The 2-year follow-up was too short. The average age of the patients in the study was 46. Patients like this choose to have PFO closure to avoid 30 years of anticoagulation therapy. The advantages of PFO closure would have become clearer during longer follow-up, as patients on warfarin therapy typically abandon it after several years.
7. The control, medical-therapy arm showed some strange outcomes. The event rates were higher in patients with smaller PFOs, and in those without a septal aneurysm.
8. Some of the operators performed PFO closures while still relatively inexperienced, early in their learning curve.
9. The STARFlex closure device used in the study is now outdated. It results in higher rates of atrial fibrillation and clot formation than do other closure devices now available.
10. Long-term anticoagulation is not a viable option for most patients. About 70% of patients on a warfarin regimen stop taking the drug after 5 years.
11. The 17% complication rate in patients undergoing PFO closure was unusually high. In the reported, worldwide experience with PFO closure the serious complication rate is about 3%.
Even if patients and physicians believe the CLOSURE I results despite all these problems, the question remains of which treatment option seems best for patients who have had a cryptogenic stroke and have a PFO. Doing nothing is not a good option, and closing the PFO by open surgery is not attractive because the periprocedural mortality rate is 0.5%-1.0%. The CLOSURE I results showed that medical therapy is no better and no safer than is endovascular PFO closure, and the annual risk for a major bleed on warfarin is 0.5%. For the long term, endovascular closure of a PFO is the most reliable way to prevent recurrent paradoxical embolism, and it has no downside; it was as safe as medical therapy. Patients with a PFO will usually prefer endovascular closure because they then won’t have to face the prospect of having to take anticoagulation therapy for the rest of their lives.
Dr. Horst Sievert is director of the CardioVascular Center in Frankfurt, Germany. He said that he has received consulting fees, travel expenses, or honoraria from Access Closure, AGA, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, Occlutech, Osprey, Ovalis, pfm, Medical Mepro GmbH, ReCor, Rox Medical, Sorin, Spectranetics, Square One, TriReme Medical, Trivascular, Veryan Medical, and Viacor. He said that he owns stock or has stock options in CardioKinetix, Access Closure, CoAptus, Lumen Biomedical, and Coherex. He made these comments during a talk in January at the annual International Symposium on Endovascular Therapy in Miami Beach.
CHICAGO – Closure-device problems did not explain why endovascular closure of patent foramen ovale in patients with a history of cryptogenic stroke failed to produce better outcomes than those of medically-treated patients. The device performed well, Dr. Anthony J. Furlan said at the annual scientific sessions of the American Heart Association.
The problem was patient selection.
"For the vast majority of patients who have had a cryptogenic stroke and a patent foramen ovale [PFO] medical therapy is a good first-choice," said Dr. Furlan, professor and chairman of neurology at Case Western Reserve University in Cleveland. "The challenge now to the endovascular community is to refine the patient selection criteria and not close these holes so liberally."
Based on the results from A Prospective, Multicenter, Randomized Controlled Trial to Evaluate the Safety and Efficacy of the STARFlex Septal Closure System Versus Best Medical Therapy in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale (CLOSURE I), the first randomized study to compare anticoagulant therapy and endovascular PFO closure, "we should see a significant drop in the number of PFOs that get closed," Dr. Furlan said.
The CLOSURE I investigators selected patients "who are being closed worldwide," he said. "Many patients who have a cryptogenic stroke also have a hole and nothing else, but people don’t look that hard. Assessments tend to stop when they find a PFO, he said.
The CLOSURE I results "clearly showed that in patients with stroke of unknown cause and PFO there is no need for systematic PFO closure because it is not effective and may be associated with major vascular complications, atrial fibrillation, or bleeding," said Dr. Pierre Amarenco, professor and chairman of the department of neurology and stroke center at Bichat Hospital in Paris. "Cryptogenic stroke likely includes a broad range of underlying etiologies that may have diluted the true, causal effect of PFO. These results should now be translated into practice for the million patients with stroke of unknown cause and PFO. On a case-by-case basis, after careful evaluation by a vascular neurologist, there may be some patients for whom closing the PFO can be deemed useful, but these cases are likely very rare."
CLOSURE I enrolled 18- to 60-year-olds who had a cryptogenic stroke or transient ischemic attack (TIA) within the past 6 months, and a PFO documented by transesophageal echocardiography. Investigators at 87 centers in the United States and Canada randomized 909 patients during June 2003–October 2008 to endovascular closure with the STARFlex device or to medical therapy. Patients in the closure group also received aspirin and clopidogrel for 6 months following their procedure, followed by aspirin alone for an additional 18 months. Patients in the medical-treatment arm received warfarin alone, dosed to a target international normalized ratio of 2.0-3.0 (139 patients), warfarin plus aspirin (80 patients), or aspirin alone (243 patients) throughout their follow-up. The average age of the patients was 46; 52% were men. Nearly three-quarters had an index cryptogenic stroke.
"We believe the study population was representative of patients aged 60 or younger with cryptogenic stroke or TIA and a PFO," Dr. Furlan said.
The study’s primary end points were the individual rates of stroke and TIA and the composite rate after 2 years of follow-up on an intent-to-treat basis. The composite rates were 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant. The numeric difference was driven primarily by a difference in the TIA rate, 3.3% in the closure group and 4.6% in the medical therapy group, not significantly different. The stroke rates were nearly identical in the two groups, 3.1% and 3.4%. The composite end point showed no significant difference between the two treatment arms regardless of the shunt severity or whether or not the patients had an atrial septal aneurysm.
Roughly 80% of the strokes that occurred during follow-up in both arms of the study had no relationship to paradoxical embolism, suggesting that in many of these patients the PFO may be coincidental, Dr. Furlan said.
In the control arm, the outcomes did not significantly differ regardless of whether patients received warfarin plus aspirin, warfarin alone, or aspirin alone. Dr. Furlan noted that the study was not powered to find significant differences among these three subgroups.
The overall rate of major adverse events was similar, 17%, in both treatment arms (see table). Patients who underwent PFO closure had significantly more major vascular complications and atrial fibrillations. Vascular complications occurred completely secondary to the closures, with none in the medically-managed patients, Dr. Furlan said. The closure procedure achieved technical success in 90% of patients in that arm of the study, with 86% of the PFOs closed at 6 months after treatment based on follow-up echocardiography examinations.
The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.
CHICAGO – Closure-device problems did not explain why endovascular closure of patent foramen ovale in patients with a history of cryptogenic stroke failed to produce better outcomes than those of medically-treated patients. The device performed well, Dr. Anthony J. Furlan said at the annual scientific sessions of the American Heart Association.
The problem was patient selection.
"For the vast majority of patients who have had a cryptogenic stroke and a patent foramen ovale [PFO] medical therapy is a good first-choice," said Dr. Furlan, professor and chairman of neurology at Case Western Reserve University in Cleveland. "The challenge now to the endovascular community is to refine the patient selection criteria and not close these holes so liberally."
Based on the results from A Prospective, Multicenter, Randomized Controlled Trial to Evaluate the Safety and Efficacy of the STARFlex Septal Closure System Versus Best Medical Therapy in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale (CLOSURE I), the first randomized study to compare anticoagulant therapy and endovascular PFO closure, "we should see a significant drop in the number of PFOs that get closed," Dr. Furlan said.
The CLOSURE I investigators selected patients "who are being closed worldwide," he said. "Many patients who have a cryptogenic stroke also have a hole and nothing else, but people don’t look that hard. Assessments tend to stop when they find a PFO, he said.
The CLOSURE I results "clearly showed that in patients with stroke of unknown cause and PFO there is no need for systematic PFO closure because it is not effective and may be associated with major vascular complications, atrial fibrillation, or bleeding," said Dr. Pierre Amarenco, professor and chairman of the department of neurology and stroke center at Bichat Hospital in Paris. "Cryptogenic stroke likely includes a broad range of underlying etiologies that may have diluted the true, causal effect of PFO. These results should now be translated into practice for the million patients with stroke of unknown cause and PFO. On a case-by-case basis, after careful evaluation by a vascular neurologist, there may be some patients for whom closing the PFO can be deemed useful, but these cases are likely very rare."
CLOSURE I enrolled 18- to 60-year-olds who had a cryptogenic stroke or transient ischemic attack (TIA) within the past 6 months, and a PFO documented by transesophageal echocardiography. Investigators at 87 centers in the United States and Canada randomized 909 patients during June 2003–October 2008 to endovascular closure with the STARFlex device or to medical therapy. Patients in the closure group also received aspirin and clopidogrel for 6 months following their procedure, followed by aspirin alone for an additional 18 months. Patients in the medical-treatment arm received warfarin alone, dosed to a target international normalized ratio of 2.0-3.0 (139 patients), warfarin plus aspirin (80 patients), or aspirin alone (243 patients) throughout their follow-up. The average age of the patients was 46; 52% were men. Nearly three-quarters had an index cryptogenic stroke.
"We believe the study population was representative of patients aged 60 or younger with cryptogenic stroke or TIA and a PFO," Dr. Furlan said.
The study’s primary end points were the individual rates of stroke and TIA and the composite rate after 2 years of follow-up on an intent-to-treat basis. The composite rates were 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant. The numeric difference was driven primarily by a difference in the TIA rate, 3.3% in the closure group and 4.6% in the medical therapy group, not significantly different. The stroke rates were nearly identical in the two groups, 3.1% and 3.4%. The composite end point showed no significant difference between the two treatment arms regardless of the shunt severity or whether or not the patients had an atrial septal aneurysm.
Roughly 80% of the strokes that occurred during follow-up in both arms of the study had no relationship to paradoxical embolism, suggesting that in many of these patients the PFO may be coincidental, Dr. Furlan said.
In the control arm, the outcomes did not significantly differ regardless of whether patients received warfarin plus aspirin, warfarin alone, or aspirin alone. Dr. Furlan noted that the study was not powered to find significant differences among these three subgroups.
The overall rate of major adverse events was similar, 17%, in both treatment arms (see table). Patients who underwent PFO closure had significantly more major vascular complications and atrial fibrillations. Vascular complications occurred completely secondary to the closures, with none in the medically-managed patients, Dr. Furlan said. The closure procedure achieved technical success in 90% of patients in that arm of the study, with 86% of the PFOs closed at 6 months after treatment based on follow-up echocardiography examinations.
The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Among patients with a recent history of cryptogenic stroke or transient ischemic attack and a patent foramen ovale, endovascular closure of the PFO led to no significant reduction in risk. After 2 years of follow-up on an intent-to-treat basis, the rate of stroke or TIA was 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant.
Data Source: CLOSURE I, a multicenter randomized trial with 909 patients in the United States and Canada.
Disclosures: The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.
Doubt Cast on Major Role for PFO Closure in Cryptogenic Stroke
CHICAGO – Closure-device problems did not explain why endovascular closure of patent foramen ovale in patients with a history of cryptogenic stroke failed to produce better outcomes than those of medically-treated patients. The device performed well, Dr. Anthony J. Furlan said at the annual scientific sessions of the American Heart Association.
The problem was patient selection.
"For the vast majority of patients who have had a cryptogenic stroke and a patent foramen ovale [PFO] medical therapy is a good first-choice," said Dr. Furlan, professor and chairman of neurology at Case Western Reserve University in Cleveland. "The challenge now to the endovascular community is to refine the patient selection criteria and not close these holes so liberally."
Based on the results from A Prospective, Multicenter, Randomized Controlled Trial to Evaluate the Safety and Efficacy of the STARFlex Septal Closure System Versus Best Medical Therapy in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale (CLOSURE I), the first randomized study to compare anticoagulant therapy and endovascular PFO closure, "we should see a significant drop in the number of PFOs that get closed," Dr. Furlan said.
The CLOSURE I investigators selected patients "who are being closed worldwide," he said. "Many patients who have a cryptogenic stroke also have a hole and nothing else, but people don’t look that hard. Assessments tend to stop when they find a PFO, he said.
The CLOSURE I results "clearly showed that in patients with stroke of unknown cause and PFO there is no need for systematic PFO closure because it is not effective and may be associated with major vascular complications, atrial fibrillation, or bleeding," said Dr. Pierre Amarenco, professor and chairman of the department of neurology and stroke center at Bichat Hospital in Paris. "Cryptogenic stroke likely includes a broad range of underlying etiologies that may have diluted the true, causal effect of PFO. These results should now be translated into practice for the million patients with stroke of unknown cause and PFO. On a case-by-case basis, after careful evaluation by a vascular neurologist, there may be some patients for whom closing the PFO can be deemed useful, but these cases are likely very rare."
CLOSURE I enrolled 18- to 60-year-olds who had a cryptogenic stroke or transient ischemic attack (TIA) within the past 6 months, and a PFO documented by transesophageal echocardiography. Investigators at 87 centers in the United States and Canada randomized 909 patients during June 2003–October 2008 to endovascular closure with the STARFlex device or to medical therapy. Patients in the closure group also received aspirin and clopidogrel for 6 months following their procedure, followed by aspirin alone for an additional 18 months. Patients in the medical-treatment arm received warfarin alone, dosed to a target international normalized ratio of 2.0-3.0 (139 patients), warfarin plus aspirin (80 patients), or aspirin alone (243 patients) throughout their follow-up. The average age of the patients was 46; 52% were men. Nearly three-quarters had an index cryptogenic stroke.
"We believe the study population was representative of patients aged 60 or younger with cryptogenic stroke or TIA and a PFO," Dr. Furlan said.
The study’s primary end points were the individual rates of stroke and TIA and the composite rate after 2 years of follow-up on an intent-to-treat basis. The composite rates were 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant. The numeric difference was driven primarily by a difference in the TIA rate, 3.3% in the closure group and 4.6% in the medical therapy group, not significantly different. The stroke rates were nearly identical in the two groups, 3.1% and 3.4%. The composite end point showed no significant difference between the two treatment arms regardless of the shunt severity or whether or not the patients had an atrial septal aneurysm.
Roughly 80% of the strokes that occurred during follow-up in both arms of the study had no relationship to paradoxical embolism, suggesting that in many of these patients the PFO may be coincidental, Dr. Furlan said.
In the control arm, the outcomes did not significantly differ regardless of whether patients received warfarin plus aspirin, warfarin alone, or aspirin alone. Dr. Furlan noted that the study was not powered to find significant differences among these three subgroups.
The overall rate of major adverse events was similar, 17%, in both treatment arms (see table). Patients who underwent PFO closure had significantly more major vascular complications and atrial fibrillations. Vascular complications occurred completely secondary to the closures, with none in the medically-managed patients, Dr. Furlan said. The closure procedure achieved technical success in 90% of patients in that arm of the study, with 86% of the PFOs closed at 6 months after treatment based on follow-up echocardiography examinations.
The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.
I find 11 reasons why the CLOSURE I results are misleading and should not deter physicians from performing a closure procedure in patients who have had a cryptogenic stroke and have a patent foramen ovale.
1. The study tested whether PFO closure is superior to medical therapy. This was an inappropriate design because the efficacy of medical therapy had never previously been studied in a trial.
2. Patients in the PFO closure arm also received aspirin therapy for 24 months. This was a design mistake because if medical therapy is effective then PFO closure would not be expected to add any additional benefit.
3. The trial should not have excluded patients with deep vein thrombosis or hypercoagulopathy because these patients should benefit most from PFO closure.
4. The trial enrolled patients very slowly, at a rate of about two patients per year at each of the participating centers, a pace that must have introduced a selection bias.
5. The total number of about 900 enrolled patients and the study design’s presumption that the event rates would be 6% in the medical arm and 2% in the closure arm were too optimistic to produce a statistically significant difference between the two treatments tested. The study’s original design called for 1,600 patients.
6. The 2-year follow-up was too short. The average age of the patients in the study was 46. Patients like this choose to have PFO closure to avoid 30 years of anticoagulation therapy. The advantages of PFO closure would have become clearer during longer follow-up, as patients on warfarin therapy typically abandon it after several years.
7. The control, medical-therapy arm showed some strange outcomes. The event rates were higher in patients with smaller PFOs, and in those without a septal aneurysm.
8. Some of the operators performed PFO closures while still relatively inexperienced, early in their learning curve.
9. The STARFlex closure device used in the study is now outdated. It results in higher rates of atrial fibrillation and clot formation than do other closure devices now available.
10. Long-term anticoagulation is not a viable option for most patients. About 70% of patients on a warfarin regimen stop taking the drug after 5 years.
11. The 17% complication rate in patients undergoing PFO closure was unusually high. In the reported, worldwide experience with PFO closure the serious complication rate is about 3%.
Even if patients and physicians believe the CLOSURE I results despite all these problems, the question remains of which treatment option seems best for patients who have had a cryptogenic stroke and have a PFO. Doing nothing is not a good option, and closing the PFO by open surgery is not attractive because the periprocedural mortality rate is 0.5%-1.0%. The CLOSURE I results showed that medical therapy is no better and no safer than is endovascular PFO closure, and the annual risk for a major bleed on warfarin is 0.5%. For the long term, endovascular closure of a PFO is the most reliable way to prevent recurrent paradoxical embolism, and it has no downside; it was as safe as medical therapy. Patients with a PFO will usually prefer endovascular closure because they then won’t have to face the prospect of having to take anticoagulation therapy for the rest of their lives.
Dr. Horst Sievert is director of the CardioVascular Center in Frankfurt, Germany. He said that he has received consulting fees, travel expenses, or honoraria from Access Closure, AGA, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, Occlutech, Osprey, Ovalis, pfm, Medical Mepro GmbH, ReCor, Rox Medical, Sorin, Spectranetics, Square One, TriReme Medical, Trivascular, Veryan Medical, and Viacor. He said that he owns stock or has stock options in CardioKinetix, Access Closure, CoAptus, Lumen Biomedical, and Coherex. He made these comments during a talk in January at the annual International Symposium on Endovascular Therapy in Miami Beach.
I find 11 reasons why the CLOSURE I results are misleading and should not deter physicians from performing a closure procedure in patients who have had a cryptogenic stroke and have a patent foramen ovale.
1. The study tested whether PFO closure is superior to medical therapy. This was an inappropriate design because the efficacy of medical therapy had never previously been studied in a trial.
2. Patients in the PFO closure arm also received aspirin therapy for 24 months. This was a design mistake because if medical therapy is effective then PFO closure would not be expected to add any additional benefit.
3. The trial should not have excluded patients with deep vein thrombosis or hypercoagulopathy because these patients should benefit most from PFO closure.
4. The trial enrolled patients very slowly, at a rate of about two patients per year at each of the participating centers, a pace that must have introduced a selection bias.
5. The total number of about 900 enrolled patients and the study design’s presumption that the event rates would be 6% in the medical arm and 2% in the closure arm were too optimistic to produce a statistically significant difference between the two treatments tested. The study’s original design called for 1,600 patients.
6. The 2-year follow-up was too short. The average age of the patients in the study was 46. Patients like this choose to have PFO closure to avoid 30 years of anticoagulation therapy. The advantages of PFO closure would have become clearer during longer follow-up, as patients on warfarin therapy typically abandon it after several years.
7. The control, medical-therapy arm showed some strange outcomes. The event rates were higher in patients with smaller PFOs, and in those without a septal aneurysm.
8. Some of the operators performed PFO closures while still relatively inexperienced, early in their learning curve.
9. The STARFlex closure device used in the study is now outdated. It results in higher rates of atrial fibrillation and clot formation than do other closure devices now available.
10. Long-term anticoagulation is not a viable option for most patients. About 70% of patients on a warfarin regimen stop taking the drug after 5 years.
11. The 17% complication rate in patients undergoing PFO closure was unusually high. In the reported, worldwide experience with PFO closure the serious complication rate is about 3%.
Even if patients and physicians believe the CLOSURE I results despite all these problems, the question remains of which treatment option seems best for patients who have had a cryptogenic stroke and have a PFO. Doing nothing is not a good option, and closing the PFO by open surgery is not attractive because the periprocedural mortality rate is 0.5%-1.0%. The CLOSURE I results showed that medical therapy is no better and no safer than is endovascular PFO closure, and the annual risk for a major bleed on warfarin is 0.5%. For the long term, endovascular closure of a PFO is the most reliable way to prevent recurrent paradoxical embolism, and it has no downside; it was as safe as medical therapy. Patients with a PFO will usually prefer endovascular closure because they then won’t have to face the prospect of having to take anticoagulation therapy for the rest of their lives.
Dr. Horst Sievert is director of the CardioVascular Center in Frankfurt, Germany. He said that he has received consulting fees, travel expenses, or honoraria from Access Closure, AGA, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, Occlutech, Osprey, Ovalis, pfm, Medical Mepro GmbH, ReCor, Rox Medical, Sorin, Spectranetics, Square One, TriReme Medical, Trivascular, Veryan Medical, and Viacor. He said that he owns stock or has stock options in CardioKinetix, Access Closure, CoAptus, Lumen Biomedical, and Coherex. He made these comments during a talk in January at the annual International Symposium on Endovascular Therapy in Miami Beach.
I find 11 reasons why the CLOSURE I results are misleading and should not deter physicians from performing a closure procedure in patients who have had a cryptogenic stroke and have a patent foramen ovale.
1. The study tested whether PFO closure is superior to medical therapy. This was an inappropriate design because the efficacy of medical therapy had never previously been studied in a trial.
2. Patients in the PFO closure arm also received aspirin therapy for 24 months. This was a design mistake because if medical therapy is effective then PFO closure would not be expected to add any additional benefit.
3. The trial should not have excluded patients with deep vein thrombosis or hypercoagulopathy because these patients should benefit most from PFO closure.
4. The trial enrolled patients very slowly, at a rate of about two patients per year at each of the participating centers, a pace that must have introduced a selection bias.
5. The total number of about 900 enrolled patients and the study design’s presumption that the event rates would be 6% in the medical arm and 2% in the closure arm were too optimistic to produce a statistically significant difference between the two treatments tested. The study’s original design called for 1,600 patients.
6. The 2-year follow-up was too short. The average age of the patients in the study was 46. Patients like this choose to have PFO closure to avoid 30 years of anticoagulation therapy. The advantages of PFO closure would have become clearer during longer follow-up, as patients on warfarin therapy typically abandon it after several years.
7. The control, medical-therapy arm showed some strange outcomes. The event rates were higher in patients with smaller PFOs, and in those without a septal aneurysm.
8. Some of the operators performed PFO closures while still relatively inexperienced, early in their learning curve.
9. The STARFlex closure device used in the study is now outdated. It results in higher rates of atrial fibrillation and clot formation than do other closure devices now available.
10. Long-term anticoagulation is not a viable option for most patients. About 70% of patients on a warfarin regimen stop taking the drug after 5 years.
11. The 17% complication rate in patients undergoing PFO closure was unusually high. In the reported, worldwide experience with PFO closure the serious complication rate is about 3%.
Even if patients and physicians believe the CLOSURE I results despite all these problems, the question remains of which treatment option seems best for patients who have had a cryptogenic stroke and have a PFO. Doing nothing is not a good option, and closing the PFO by open surgery is not attractive because the periprocedural mortality rate is 0.5%-1.0%. The CLOSURE I results showed that medical therapy is no better and no safer than is endovascular PFO closure, and the annual risk for a major bleed on warfarin is 0.5%. For the long term, endovascular closure of a PFO is the most reliable way to prevent recurrent paradoxical embolism, and it has no downside; it was as safe as medical therapy. Patients with a PFO will usually prefer endovascular closure because they then won’t have to face the prospect of having to take anticoagulation therapy for the rest of their lives.
Dr. Horst Sievert is director of the CardioVascular Center in Frankfurt, Germany. He said that he has received consulting fees, travel expenses, or honoraria from Access Closure, AGA, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, Occlutech, Osprey, Ovalis, pfm, Medical Mepro GmbH, ReCor, Rox Medical, Sorin, Spectranetics, Square One, TriReme Medical, Trivascular, Veryan Medical, and Viacor. He said that he owns stock or has stock options in CardioKinetix, Access Closure, CoAptus, Lumen Biomedical, and Coherex. He made these comments during a talk in January at the annual International Symposium on Endovascular Therapy in Miami Beach.
CHICAGO – Closure-device problems did not explain why endovascular closure of patent foramen ovale in patients with a history of cryptogenic stroke failed to produce better outcomes than those of medically-treated patients. The device performed well, Dr. Anthony J. Furlan said at the annual scientific sessions of the American Heart Association.
The problem was patient selection.
"For the vast majority of patients who have had a cryptogenic stroke and a patent foramen ovale [PFO] medical therapy is a good first-choice," said Dr. Furlan, professor and chairman of neurology at Case Western Reserve University in Cleveland. "The challenge now to the endovascular community is to refine the patient selection criteria and not close these holes so liberally."
Based on the results from A Prospective, Multicenter, Randomized Controlled Trial to Evaluate the Safety and Efficacy of the STARFlex Septal Closure System Versus Best Medical Therapy in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale (CLOSURE I), the first randomized study to compare anticoagulant therapy and endovascular PFO closure, "we should see a significant drop in the number of PFOs that get closed," Dr. Furlan said.
The CLOSURE I investigators selected patients "who are being closed worldwide," he said. "Many patients who have a cryptogenic stroke also have a hole and nothing else, but people don’t look that hard. Assessments tend to stop when they find a PFO, he said.
The CLOSURE I results "clearly showed that in patients with stroke of unknown cause and PFO there is no need for systematic PFO closure because it is not effective and may be associated with major vascular complications, atrial fibrillation, or bleeding," said Dr. Pierre Amarenco, professor and chairman of the department of neurology and stroke center at Bichat Hospital in Paris. "Cryptogenic stroke likely includes a broad range of underlying etiologies that may have diluted the true, causal effect of PFO. These results should now be translated into practice for the million patients with stroke of unknown cause and PFO. On a case-by-case basis, after careful evaluation by a vascular neurologist, there may be some patients for whom closing the PFO can be deemed useful, but these cases are likely very rare."
CLOSURE I enrolled 18- to 60-year-olds who had a cryptogenic stroke or transient ischemic attack (TIA) within the past 6 months, and a PFO documented by transesophageal echocardiography. Investigators at 87 centers in the United States and Canada randomized 909 patients during June 2003–October 2008 to endovascular closure with the STARFlex device or to medical therapy. Patients in the closure group also received aspirin and clopidogrel for 6 months following their procedure, followed by aspirin alone for an additional 18 months. Patients in the medical-treatment arm received warfarin alone, dosed to a target international normalized ratio of 2.0-3.0 (139 patients), warfarin plus aspirin (80 patients), or aspirin alone (243 patients) throughout their follow-up. The average age of the patients was 46; 52% were men. Nearly three-quarters had an index cryptogenic stroke.
"We believe the study population was representative of patients aged 60 or younger with cryptogenic stroke or TIA and a PFO," Dr. Furlan said.
The study’s primary end points were the individual rates of stroke and TIA and the composite rate after 2 years of follow-up on an intent-to-treat basis. The composite rates were 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant. The numeric difference was driven primarily by a difference in the TIA rate, 3.3% in the closure group and 4.6% in the medical therapy group, not significantly different. The stroke rates were nearly identical in the two groups, 3.1% and 3.4%. The composite end point showed no significant difference between the two treatment arms regardless of the shunt severity or whether or not the patients had an atrial septal aneurysm.
Roughly 80% of the strokes that occurred during follow-up in both arms of the study had no relationship to paradoxical embolism, suggesting that in many of these patients the PFO may be coincidental, Dr. Furlan said.
In the control arm, the outcomes did not significantly differ regardless of whether patients received warfarin plus aspirin, warfarin alone, or aspirin alone. Dr. Furlan noted that the study was not powered to find significant differences among these three subgroups.
The overall rate of major adverse events was similar, 17%, in both treatment arms (see table). Patients who underwent PFO closure had significantly more major vascular complications and atrial fibrillations. Vascular complications occurred completely secondary to the closures, with none in the medically-managed patients, Dr. Furlan said. The closure procedure achieved technical success in 90% of patients in that arm of the study, with 86% of the PFOs closed at 6 months after treatment based on follow-up echocardiography examinations.
The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.
CHICAGO – Closure-device problems did not explain why endovascular closure of patent foramen ovale in patients with a history of cryptogenic stroke failed to produce better outcomes than those of medically-treated patients. The device performed well, Dr. Anthony J. Furlan said at the annual scientific sessions of the American Heart Association.
The problem was patient selection.
"For the vast majority of patients who have had a cryptogenic stroke and a patent foramen ovale [PFO] medical therapy is a good first-choice," said Dr. Furlan, professor and chairman of neurology at Case Western Reserve University in Cleveland. "The challenge now to the endovascular community is to refine the patient selection criteria and not close these holes so liberally."
Based on the results from A Prospective, Multicenter, Randomized Controlled Trial to Evaluate the Safety and Efficacy of the STARFlex Septal Closure System Versus Best Medical Therapy in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale (CLOSURE I), the first randomized study to compare anticoagulant therapy and endovascular PFO closure, "we should see a significant drop in the number of PFOs that get closed," Dr. Furlan said.
The CLOSURE I investigators selected patients "who are being closed worldwide," he said. "Many patients who have a cryptogenic stroke also have a hole and nothing else, but people don’t look that hard. Assessments tend to stop when they find a PFO, he said.
The CLOSURE I results "clearly showed that in patients with stroke of unknown cause and PFO there is no need for systematic PFO closure because it is not effective and may be associated with major vascular complications, atrial fibrillation, or bleeding," said Dr. Pierre Amarenco, professor and chairman of the department of neurology and stroke center at Bichat Hospital in Paris. "Cryptogenic stroke likely includes a broad range of underlying etiologies that may have diluted the true, causal effect of PFO. These results should now be translated into practice for the million patients with stroke of unknown cause and PFO. On a case-by-case basis, after careful evaluation by a vascular neurologist, there may be some patients for whom closing the PFO can be deemed useful, but these cases are likely very rare."
CLOSURE I enrolled 18- to 60-year-olds who had a cryptogenic stroke or transient ischemic attack (TIA) within the past 6 months, and a PFO documented by transesophageal echocardiography. Investigators at 87 centers in the United States and Canada randomized 909 patients during June 2003–October 2008 to endovascular closure with the STARFlex device or to medical therapy. Patients in the closure group also received aspirin and clopidogrel for 6 months following their procedure, followed by aspirin alone for an additional 18 months. Patients in the medical-treatment arm received warfarin alone, dosed to a target international normalized ratio of 2.0-3.0 (139 patients), warfarin plus aspirin (80 patients), or aspirin alone (243 patients) throughout their follow-up. The average age of the patients was 46; 52% were men. Nearly three-quarters had an index cryptogenic stroke.
"We believe the study population was representative of patients aged 60 or younger with cryptogenic stroke or TIA and a PFO," Dr. Furlan said.
The study’s primary end points were the individual rates of stroke and TIA and the composite rate after 2 years of follow-up on an intent-to-treat basis. The composite rates were 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant. The numeric difference was driven primarily by a difference in the TIA rate, 3.3% in the closure group and 4.6% in the medical therapy group, not significantly different. The stroke rates were nearly identical in the two groups, 3.1% and 3.4%. The composite end point showed no significant difference between the two treatment arms regardless of the shunt severity or whether or not the patients had an atrial septal aneurysm.
Roughly 80% of the strokes that occurred during follow-up in both arms of the study had no relationship to paradoxical embolism, suggesting that in many of these patients the PFO may be coincidental, Dr. Furlan said.
In the control arm, the outcomes did not significantly differ regardless of whether patients received warfarin plus aspirin, warfarin alone, or aspirin alone. Dr. Furlan noted that the study was not powered to find significant differences among these three subgroups.
The overall rate of major adverse events was similar, 17%, in both treatment arms (see table). Patients who underwent PFO closure had significantly more major vascular complications and atrial fibrillations. Vascular complications occurred completely secondary to the closures, with none in the medically-managed patients, Dr. Furlan said. The closure procedure achieved technical success in 90% of patients in that arm of the study, with 86% of the PFOs closed at 6 months after treatment based on follow-up echocardiography examinations.
The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Among patients with a recent history of cryptogenic stroke or transient ischemic attack and a patent foramen ovale, endovascular closure of the PFO led to no significant reduction in risk. After 2 years of follow-up on an intent-to-treat basis, the rate of stroke or TIA was 5.9% among the 447 patients in the closure group and 7.7% among 462 patients in the medical-therapy group, a difference that was not statistically significant.
Data Source: CLOSURE I, a multicenter randomized trial with 909 patients in the United States and Canada.
Disclosures: The CLOSURE I study was sponsored by NMT Medical, which markets the STARFlex closure device. Dr. Furlan said that he is a consultant to NMT Medical and was the principal investigator for the trial. Dr. Amarenco said that he has received research grants from Sanofi-Aventis, Bristol-Myers Squibb, AstraZeneca, Merck, and Pfizer.



