User login
Transcription factor loss linked to worse colorectal cancer outcomes
BOSTON– Colorectal cancers arising in the hindgut are more likely to have loss or mutation of the transcription factor SMAD4 than are tumors arising in the proximal two-thirds of the colon, and that loss is associated with significantly worse patient outcomes, investigators reported.
A study of data on nearly 600 patients with colorectal cancer in the Cancer Genome Atlas (TCGA) database showed that loss of SMAD4, which codes for a cell-signaling protein in the transforming growth factor beta pathway, was detected in 87% of tumors originating in the hindgut (including the splenic flexure, descending colon, sigmoid colon, and rectum), compared with 50% of tumors arising in the midgut (small intestine, ascending colon, and transverse colon), reported Dr. Jesse Joshua Smith from Memorial Sloan Kettering Cancer Center, New York.
In a separate analysis of 388 patients with colorectal cancer treated at Dr. Smith’s center, loss of SMAD4 in patients with cancers in the hindgut was associated with significantly shorter recurrence-free survival: 47 months, compared with 231 months for patients with hindgut cancers that retained SMAD4.
“These data indicate that embryologic origin related to SMAD4 status in colorectal cancer may be important in patient outcomes, and could potentially inform individualized treatment options for patients,” Dr. Smith said at the AACR/NCI)/EORTC International Conference on Molecular Targets and Cancer Therapeutics.
Previous studies have suggested that alteration (mutation or loss) of SMAD4 may be more common in cancers of the rectum and other portions of the hindgut, he said.
A recent analysis of 113 patients with locally advanced rectal cancer treated at Memorial Sloan showed that 66% of the tumors evaluated by the MSK-IMPACT (Memorial Sloan Kettering-Integrated Mutational Profile of Actionable Targets) platform had SMAD4 copy number losses, he noted.
The investigators hypothesized that SMAD4 loss is more common in hindgut vs. midgut tumors. To test that, they looked at TCGA data on 595 patients with colorectal cancer to determine copy number loss and diploid status, and compared SMAD4 copy number loss to the mutational status of genes encoding for adenomatous polyposis coli (APC) and tumor protein p53 (TP53), as well as mismatch repair status and KRAS amplification.
They found that SMAD4 copy number loss was significantly more common in hindgut tumors (295 vs. 44 diploid, P < .0001) than in midgut tumors (127 vs. 129 diploid, P = nonsignificant). In addition, mutations in both APC and TP53 were significantly associated with SMAD4 copy number loss – and when adjusting for mismatch repair status, SMDA4 copy number loss remained significantly associated with tumors originating in the hindgut and with TP53 mutations.
Similarly, immunohistochemical analysis showed that SMAD4 staining was lower in hindgut vs. midgut tumors, and lower levels of staining were associated with more advanced tumor and nodal stage (P < .05).
As noted, SMAD4 copy number loss in patients with hindgut-derived tumors was associated with worse recurrence-free survival, compared with similar patients with retained SMAD4: median, 47 months, compared with 231 months.
A similar pattern was seen among patients with hindgut tumors with SMAD4 loss but no mutations in DNA mismatch repair genes, with respective median recurrence-free survival of 36 months vs. not yet reached at 140 months.
Dr. Smith and his colleagues plan to enroll 100 additional patients and prospectively collect tumor samples to help determine whether there is an association between embryologic origins of tumors according to SMAD4 status and poor treatment response. They also hope to determine whether there is a similar association in tumors that metastasize to the liver or the lungs.
There is evidence from mouse models to suggest that loss of SMAD4 may confer to tumors resistance to therapeutic regimens based on 5-fluorouracil, Dr. Smith said.
In an interview, he noted that there are also preliminary data to suggest that when SMAD4 is lost or mutated, other pathways, such as the vascular endothelial growth factor (VEGF) pathway, may be upregulated, suggesting that this subset of patients might respond to a VEGF inhibitor.
The findings suggest that in addition to considering somatic mutations in the development of colorectal cancer, investigators may wish to explore whether lineage-dependent mechanisms or embryologic origin play a role in disease severity, observed Dr. Levi A. Garraway, of the Dana-Farber Cancer Institute, Boston, who was not involved in the study.
Dr. Garraway moderated the briefing at which Dr. Smith presented his data.
The Howard Hughes Medical Institute supported the study. Dr. Smith and Dr. Garraway reported no relevant disclosures.
BOSTON– Colorectal cancers arising in the hindgut are more likely to have loss or mutation of the transcription factor SMAD4 than are tumors arising in the proximal two-thirds of the colon, and that loss is associated with significantly worse patient outcomes, investigators reported.
A study of data on nearly 600 patients with colorectal cancer in the Cancer Genome Atlas (TCGA) database showed that loss of SMAD4, which codes for a cell-signaling protein in the transforming growth factor beta pathway, was detected in 87% of tumors originating in the hindgut (including the splenic flexure, descending colon, sigmoid colon, and rectum), compared with 50% of tumors arising in the midgut (small intestine, ascending colon, and transverse colon), reported Dr. Jesse Joshua Smith from Memorial Sloan Kettering Cancer Center, New York.
In a separate analysis of 388 patients with colorectal cancer treated at Dr. Smith’s center, loss of SMAD4 in patients with cancers in the hindgut was associated with significantly shorter recurrence-free survival: 47 months, compared with 231 months for patients with hindgut cancers that retained SMAD4.
“These data indicate that embryologic origin related to SMAD4 status in colorectal cancer may be important in patient outcomes, and could potentially inform individualized treatment options for patients,” Dr. Smith said at the AACR/NCI)/EORTC International Conference on Molecular Targets and Cancer Therapeutics.
Previous studies have suggested that alteration (mutation or loss) of SMAD4 may be more common in cancers of the rectum and other portions of the hindgut, he said.
A recent analysis of 113 patients with locally advanced rectal cancer treated at Memorial Sloan showed that 66% of the tumors evaluated by the MSK-IMPACT (Memorial Sloan Kettering-Integrated Mutational Profile of Actionable Targets) platform had SMAD4 copy number losses, he noted.
The investigators hypothesized that SMAD4 loss is more common in hindgut vs. midgut tumors. To test that, they looked at TCGA data on 595 patients with colorectal cancer to determine copy number loss and diploid status, and compared SMAD4 copy number loss to the mutational status of genes encoding for adenomatous polyposis coli (APC) and tumor protein p53 (TP53), as well as mismatch repair status and KRAS amplification.
They found that SMAD4 copy number loss was significantly more common in hindgut tumors (295 vs. 44 diploid, P < .0001) than in midgut tumors (127 vs. 129 diploid, P = nonsignificant). In addition, mutations in both APC and TP53 were significantly associated with SMAD4 copy number loss – and when adjusting for mismatch repair status, SMDA4 copy number loss remained significantly associated with tumors originating in the hindgut and with TP53 mutations.
Similarly, immunohistochemical analysis showed that SMAD4 staining was lower in hindgut vs. midgut tumors, and lower levels of staining were associated with more advanced tumor and nodal stage (P < .05).
As noted, SMAD4 copy number loss in patients with hindgut-derived tumors was associated with worse recurrence-free survival, compared with similar patients with retained SMAD4: median, 47 months, compared with 231 months.
A similar pattern was seen among patients with hindgut tumors with SMAD4 loss but no mutations in DNA mismatch repair genes, with respective median recurrence-free survival of 36 months vs. not yet reached at 140 months.
Dr. Smith and his colleagues plan to enroll 100 additional patients and prospectively collect tumor samples to help determine whether there is an association between embryologic origins of tumors according to SMAD4 status and poor treatment response. They also hope to determine whether there is a similar association in tumors that metastasize to the liver or the lungs.
There is evidence from mouse models to suggest that loss of SMAD4 may confer to tumors resistance to therapeutic regimens based on 5-fluorouracil, Dr. Smith said.
In an interview, he noted that there are also preliminary data to suggest that when SMAD4 is lost or mutated, other pathways, such as the vascular endothelial growth factor (VEGF) pathway, may be upregulated, suggesting that this subset of patients might respond to a VEGF inhibitor.
The findings suggest that in addition to considering somatic mutations in the development of colorectal cancer, investigators may wish to explore whether lineage-dependent mechanisms or embryologic origin play a role in disease severity, observed Dr. Levi A. Garraway, of the Dana-Farber Cancer Institute, Boston, who was not involved in the study.
Dr. Garraway moderated the briefing at which Dr. Smith presented his data.
The Howard Hughes Medical Institute supported the study. Dr. Smith and Dr. Garraway reported no relevant disclosures.
BOSTON– Colorectal cancers arising in the hindgut are more likely to have loss or mutation of the transcription factor SMAD4 than are tumors arising in the proximal two-thirds of the colon, and that loss is associated with significantly worse patient outcomes, investigators reported.
A study of data on nearly 600 patients with colorectal cancer in the Cancer Genome Atlas (TCGA) database showed that loss of SMAD4, which codes for a cell-signaling protein in the transforming growth factor beta pathway, was detected in 87% of tumors originating in the hindgut (including the splenic flexure, descending colon, sigmoid colon, and rectum), compared with 50% of tumors arising in the midgut (small intestine, ascending colon, and transverse colon), reported Dr. Jesse Joshua Smith from Memorial Sloan Kettering Cancer Center, New York.
In a separate analysis of 388 patients with colorectal cancer treated at Dr. Smith’s center, loss of SMAD4 in patients with cancers in the hindgut was associated with significantly shorter recurrence-free survival: 47 months, compared with 231 months for patients with hindgut cancers that retained SMAD4.
“These data indicate that embryologic origin related to SMAD4 status in colorectal cancer may be important in patient outcomes, and could potentially inform individualized treatment options for patients,” Dr. Smith said at the AACR/NCI)/EORTC International Conference on Molecular Targets and Cancer Therapeutics.
Previous studies have suggested that alteration (mutation or loss) of SMAD4 may be more common in cancers of the rectum and other portions of the hindgut, he said.
A recent analysis of 113 patients with locally advanced rectal cancer treated at Memorial Sloan showed that 66% of the tumors evaluated by the MSK-IMPACT (Memorial Sloan Kettering-Integrated Mutational Profile of Actionable Targets) platform had SMAD4 copy number losses, he noted.
The investigators hypothesized that SMAD4 loss is more common in hindgut vs. midgut tumors. To test that, they looked at TCGA data on 595 patients with colorectal cancer to determine copy number loss and diploid status, and compared SMAD4 copy number loss to the mutational status of genes encoding for adenomatous polyposis coli (APC) and tumor protein p53 (TP53), as well as mismatch repair status and KRAS amplification.
They found that SMAD4 copy number loss was significantly more common in hindgut tumors (295 vs. 44 diploid, P < .0001) than in midgut tumors (127 vs. 129 diploid, P = nonsignificant). In addition, mutations in both APC and TP53 were significantly associated with SMAD4 copy number loss – and when adjusting for mismatch repair status, SMDA4 copy number loss remained significantly associated with tumors originating in the hindgut and with TP53 mutations.
Similarly, immunohistochemical analysis showed that SMAD4 staining was lower in hindgut vs. midgut tumors, and lower levels of staining were associated with more advanced tumor and nodal stage (P < .05).
As noted, SMAD4 copy number loss in patients with hindgut-derived tumors was associated with worse recurrence-free survival, compared with similar patients with retained SMAD4: median, 47 months, compared with 231 months.
A similar pattern was seen among patients with hindgut tumors with SMAD4 loss but no mutations in DNA mismatch repair genes, with respective median recurrence-free survival of 36 months vs. not yet reached at 140 months.
Dr. Smith and his colleagues plan to enroll 100 additional patients and prospectively collect tumor samples to help determine whether there is an association between embryologic origins of tumors according to SMAD4 status and poor treatment response. They also hope to determine whether there is a similar association in tumors that metastasize to the liver or the lungs.
There is evidence from mouse models to suggest that loss of SMAD4 may confer to tumors resistance to therapeutic regimens based on 5-fluorouracil, Dr. Smith said.
In an interview, he noted that there are also preliminary data to suggest that when SMAD4 is lost or mutated, other pathways, such as the vascular endothelial growth factor (VEGF) pathway, may be upregulated, suggesting that this subset of patients might respond to a VEGF inhibitor.
The findings suggest that in addition to considering somatic mutations in the development of colorectal cancer, investigators may wish to explore whether lineage-dependent mechanisms or embryologic origin play a role in disease severity, observed Dr. Levi A. Garraway, of the Dana-Farber Cancer Institute, Boston, who was not involved in the study.
Dr. Garraway moderated the briefing at which Dr. Smith presented his data.
The Howard Hughes Medical Institute supported the study. Dr. Smith and Dr. Garraway reported no relevant disclosures.
AT AACR/NCI/EORTC
Key clinical point: In colorectal cancer arising in the hindgut, loss of SMAD4 is associated with worse recurrence-free survival.
Major finding: Loss of SMAD4 in patients with cancers in the hindgut was associated with a median of 47 months recurrence-free survival, compared with 231 months for patients with hindgut cancers that retained SMAD4.
Data source: Analysis of genomic registry data on 595 patients, and of 388 patients with colorectal cancer in a case series.
Disclosures: The Howard Hughes Medical Institute supported the study. Dr. Smith and Dr. Garraway reported no relevant disclosures.
Rapid, cheap test prognostic in multiple myeloma
A rapid, low-cost assay measuring DNA and immunoglobulin in multiple myeloma cells is both a powerful predictor of prognosis and a tool for identifying new, previously unidentified therapeutic targets, its developers say.
The test, labeled DNA/CIG, uses two-color flow cytometry to evaluate DNA in the nucleus and immunoglobulin in the cytoplasm of bone marrow taken from patients with multiple myeloma (MM) at baseline, before the start of the aggressive “Total Therapy 3b” (TT3b) protocol developed at the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences in Little Rock.
The light chain cytoplasmic immunoglobulin (CIg) index generated by the test, combined with an associated 12-gene panel, serves as an independent prognostic factor in patients with myeloma. The CIg index is a marker for immunoglobulin production in myeloma cells, with lower index scores suggesting ineffective immunologic responses.
“We show that the presence of low CIg as detected by DNA/CIG is a major adverse prognostic factor in TT3b, even when other GEP [gene expression profiling]–derived prognostic factors were accounted for,” Dr. Xenon Papanikolaou of the University of Arkansas and colleagues wrote in the journal Leukemia (2015;29:1713-20).
The investigators performed DNA/CIG as part of the routine work-up of patients with newly diagnosed MM who were scheduled to undergo the current iteration of the Total Therapy protocol, consisting of two induction chemotherapy cycles of VTD-PACE (bortezomib, thalidomide, dexamethasone and 4-day continuous infusions of cisplatin, doxorubicin, cyclophosphamide and etoposide), two planned hematopoietic stem cell transplants, and 3 years of planned maintenance with bortezomib, lenalidomide and dexamethasone.
The authors looked at the association of the CIg index, gene-expression profiling, and standard prognostic measures with both overall and progression-free survival (OS and PFS).
They found that both the percentage of light chain–restricted cells and the CIg as determined by DNA/CIG were associated with poor clinical outcomes. Additionally, a CIg lower than 2.8, albumin lower than 3.5 g/dL, and age 65 and older were significantly associated with worse PFS and OS, independent of gene-expression profiling data.
Any CIg lower than 2.8 was associated with a twofold risk for worse OS (hazard ratio, 2.08; P = .012) and a nearly doubling of risk for worse PFS (HR, 1.84; P = .001).
In multivariate models, CIg remained an independent predictor for prognosis when GEP-defined high-risk and low albumin were added in. CIg was also linked to other prognostic factors, including beta2-microglobulin greater than 5.5 mg/L, percentage of LCR cells exceeding 50%, C-reactive protein equal to or greater than 8 mg/L, and GEP-derived high centrosome index.
Low CIg was also associated with an experimental group of 12 gene probes believed to be involved in cell cycle regulation, cell differentiation, and cellular drug transport. The investigators used the gene signatures to develop a risk score, called GEP12, that offered important prognostic information for patients in TT3b and in earlier clinical studies.
The finding that a low CIg index was linked to a high centrosome index as defined by GEP points to a possible role for CIg index as a treatment selection tool.
“Interestingly, a centrosome inhibitor has shown promising activity in preclinical models of MM, thus potentially providing a selective drug for patients with low CIg myeloma,” the authors write.
The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
A rapid, low-cost assay measuring DNA and immunoglobulin in multiple myeloma cells is both a powerful predictor of prognosis and a tool for identifying new, previously unidentified therapeutic targets, its developers say.
The test, labeled DNA/CIG, uses two-color flow cytometry to evaluate DNA in the nucleus and immunoglobulin in the cytoplasm of bone marrow taken from patients with multiple myeloma (MM) at baseline, before the start of the aggressive “Total Therapy 3b” (TT3b) protocol developed at the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences in Little Rock.
The light chain cytoplasmic immunoglobulin (CIg) index generated by the test, combined with an associated 12-gene panel, serves as an independent prognostic factor in patients with myeloma. The CIg index is a marker for immunoglobulin production in myeloma cells, with lower index scores suggesting ineffective immunologic responses.
“We show that the presence of low CIg as detected by DNA/CIG is a major adverse prognostic factor in TT3b, even when other GEP [gene expression profiling]–derived prognostic factors were accounted for,” Dr. Xenon Papanikolaou of the University of Arkansas and colleagues wrote in the journal Leukemia (2015;29:1713-20).
The investigators performed DNA/CIG as part of the routine work-up of patients with newly diagnosed MM who were scheduled to undergo the current iteration of the Total Therapy protocol, consisting of two induction chemotherapy cycles of VTD-PACE (bortezomib, thalidomide, dexamethasone and 4-day continuous infusions of cisplatin, doxorubicin, cyclophosphamide and etoposide), two planned hematopoietic stem cell transplants, and 3 years of planned maintenance with bortezomib, lenalidomide and dexamethasone.
The authors looked at the association of the CIg index, gene-expression profiling, and standard prognostic measures with both overall and progression-free survival (OS and PFS).
They found that both the percentage of light chain–restricted cells and the CIg as determined by DNA/CIG were associated with poor clinical outcomes. Additionally, a CIg lower than 2.8, albumin lower than 3.5 g/dL, and age 65 and older were significantly associated with worse PFS and OS, independent of gene-expression profiling data.
Any CIg lower than 2.8 was associated with a twofold risk for worse OS (hazard ratio, 2.08; P = .012) and a nearly doubling of risk for worse PFS (HR, 1.84; P = .001).
In multivariate models, CIg remained an independent predictor for prognosis when GEP-defined high-risk and low albumin were added in. CIg was also linked to other prognostic factors, including beta2-microglobulin greater than 5.5 mg/L, percentage of LCR cells exceeding 50%, C-reactive protein equal to or greater than 8 mg/L, and GEP-derived high centrosome index.
Low CIg was also associated with an experimental group of 12 gene probes believed to be involved in cell cycle regulation, cell differentiation, and cellular drug transport. The investigators used the gene signatures to develop a risk score, called GEP12, that offered important prognostic information for patients in TT3b and in earlier clinical studies.
The finding that a low CIg index was linked to a high centrosome index as defined by GEP points to a possible role for CIg index as a treatment selection tool.
“Interestingly, a centrosome inhibitor has shown promising activity in preclinical models of MM, thus potentially providing a selective drug for patients with low CIg myeloma,” the authors write.
The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
A rapid, low-cost assay measuring DNA and immunoglobulin in multiple myeloma cells is both a powerful predictor of prognosis and a tool for identifying new, previously unidentified therapeutic targets, its developers say.
The test, labeled DNA/CIG, uses two-color flow cytometry to evaluate DNA in the nucleus and immunoglobulin in the cytoplasm of bone marrow taken from patients with multiple myeloma (MM) at baseline, before the start of the aggressive “Total Therapy 3b” (TT3b) protocol developed at the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences in Little Rock.
The light chain cytoplasmic immunoglobulin (CIg) index generated by the test, combined with an associated 12-gene panel, serves as an independent prognostic factor in patients with myeloma. The CIg index is a marker for immunoglobulin production in myeloma cells, with lower index scores suggesting ineffective immunologic responses.
“We show that the presence of low CIg as detected by DNA/CIG is a major adverse prognostic factor in TT3b, even when other GEP [gene expression profiling]–derived prognostic factors were accounted for,” Dr. Xenon Papanikolaou of the University of Arkansas and colleagues wrote in the journal Leukemia (2015;29:1713-20).
The investigators performed DNA/CIG as part of the routine work-up of patients with newly diagnosed MM who were scheduled to undergo the current iteration of the Total Therapy protocol, consisting of two induction chemotherapy cycles of VTD-PACE (bortezomib, thalidomide, dexamethasone and 4-day continuous infusions of cisplatin, doxorubicin, cyclophosphamide and etoposide), two planned hematopoietic stem cell transplants, and 3 years of planned maintenance with bortezomib, lenalidomide and dexamethasone.
The authors looked at the association of the CIg index, gene-expression profiling, and standard prognostic measures with both overall and progression-free survival (OS and PFS).
They found that both the percentage of light chain–restricted cells and the CIg as determined by DNA/CIG were associated with poor clinical outcomes. Additionally, a CIg lower than 2.8, albumin lower than 3.5 g/dL, and age 65 and older were significantly associated with worse PFS and OS, independent of gene-expression profiling data.
Any CIg lower than 2.8 was associated with a twofold risk for worse OS (hazard ratio, 2.08; P = .012) and a nearly doubling of risk for worse PFS (HR, 1.84; P = .001).
In multivariate models, CIg remained an independent predictor for prognosis when GEP-defined high-risk and low albumin were added in. CIg was also linked to other prognostic factors, including beta2-microglobulin greater than 5.5 mg/L, percentage of LCR cells exceeding 50%, C-reactive protein equal to or greater than 8 mg/L, and GEP-derived high centrosome index.
Low CIg was also associated with an experimental group of 12 gene probes believed to be involved in cell cycle regulation, cell differentiation, and cellular drug transport. The investigators used the gene signatures to develop a risk score, called GEP12, that offered important prognostic information for patients in TT3b and in earlier clinical studies.
The finding that a low CIg index was linked to a high centrosome index as defined by GEP points to a possible role for CIg index as a treatment selection tool.
“Interestingly, a centrosome inhibitor has shown promising activity in preclinical models of MM, thus potentially providing a selective drug for patients with low CIg myeloma,” the authors write.
The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
FROM LEUKEMIA
Key clinical point: Cytoplasmic immunoglobulin index (CIg) is prognostic of clinical outcomes in patients with multiple myeloma.
Major finding: A CIg lower than 2.8 was prognostic for poor overall and progression-free survival, with hazard ratios of 2.08 and 1.84, respectively.
Data source: Prospective study in 177 patients with newly diagnosed multiple myeloma.
Disclosures: The study was supported in part by a grant from the National Cancer Institute. Senior author Dr. Bart Barlogies reports research funding from Celgene Corp and Millennium Pharmaceuticals, and consulting with those companies as well as Onyx Pharmaceuticals and Amgen. He is a coinventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, but has no financial interests in this company. The remaining authors reported having no conflicts of interest.
Ponatinib’s toxicity limits use for upfront chronic phase CML
The tyrosine kinase inhibitor ponatinib produced a high degree of complete cytogenetic responses when used in first-line treatment of patients with chronic myeloid leukemia in chronic phase. But the drug’s toxicities, especially its propensity for causing thromboembolic events, mitigate against its upfront use, investigators say.
In a small phase II study, 90% of evaluable patients with chronic-phase chronic myeloid leukemia (CML) treated with the tyrosine kinase inhibitor (TKI) had a complete cytogenetic response (CCyR) after 3 months, and 94% had a CCyR after 6 months, but that efficacy came at the cost of significant adverse events requiring dose reductions, treatment interruptions, and early termination of the trial for safety reasons at the recommendation of the Food and Drug Administration, reported Dr. Preetesh Jain of MD Anderson Cancer Center in Houston, Tex., and colleagues.
“[O]ur study shows that ponatinib is a very potent TKI with high clinical activity in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population who have other treatment options with high efficacy,” the investigators wrote (Lancet Haematol. 2015;2[9]:e376-e383).
Ponatinib (Iclusig) is a third-generation TKI with action against malignancies with mutations in the ABL kinase domain that make the cancers resistant to first- and second-generation TKIs such as imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna).
Ponatinib is approved in the United States for treatment of adult patients with CML positive for the T3151 mutation in chronic phase, accelerated phase, or blast phase, or T3151-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL). It is also approved for treatment of adults with chronic phase, accelerated phase, or blast phase CML or Ph+ALL for whom no other TKI is indicated.
The drug labeling carries a boxed warning about increased risk for vascular occlusion, heart failure, and hepatotoxicity. The warning notes that “[a]rterial and venous thrombosis and occlusions have occurred in at least 27% of Iclusig-treated patients, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures.”
In a single-arm, open label trial, the investigators enrolled 51 patients with recently diagnosed CML in chronic phase, starting them on doses of oral ponatinib 45 mg daily. The protocol was later amended to a starting dose of 30 mg daily because of the high frequency of dose reductions required among patients started at 45 mg. Following a warning from the FDA about vascular complications with the drug, patients were started on daily low-dose aspirin (81 mg), and all drug doses were reduced to either 30 mg or 15 mg daily.
One of the patients discontinued therapy prior to assessment because of the FDA warning, leaving 50 for assessment at 6 months.
Of 46 patients evaluable for the primary outcome of CCyR by 6 months, 43 (94%) achieved it. Major molecular responses, defined as a BCR-ABL to ABL transcript ratio of 0.1% or lower, occurred in 40 of 50 patients (80%) evaluable for this secondary endpoint, and deep molecular response (MR4.5), defined as a ratio less than 0.0032% or lower, occurred in 28 of 50 (55%).
Cardiovascular events, primarily hypertension, occurred in 25 patients (49%), 15 (29%) had grade 3-4 myelosuppression, and 5 patients (10%) developed cerebrovascular or vaso-occlusive disease.
In all, 43 patients (85%) needed treatment interruptions at some time and 45 (88%) needed dose reductions. The study was terminated in June 2014, a little more than a year after recruitment began.
The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
The tyrosine kinase inhibitor ponatinib produced a high degree of complete cytogenetic responses when used in first-line treatment of patients with chronic myeloid leukemia in chronic phase. But the drug’s toxicities, especially its propensity for causing thromboembolic events, mitigate against its upfront use, investigators say.
In a small phase II study, 90% of evaluable patients with chronic-phase chronic myeloid leukemia (CML) treated with the tyrosine kinase inhibitor (TKI) had a complete cytogenetic response (CCyR) after 3 months, and 94% had a CCyR after 6 months, but that efficacy came at the cost of significant adverse events requiring dose reductions, treatment interruptions, and early termination of the trial for safety reasons at the recommendation of the Food and Drug Administration, reported Dr. Preetesh Jain of MD Anderson Cancer Center in Houston, Tex., and colleagues.
“[O]ur study shows that ponatinib is a very potent TKI with high clinical activity in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population who have other treatment options with high efficacy,” the investigators wrote (Lancet Haematol. 2015;2[9]:e376-e383).
Ponatinib (Iclusig) is a third-generation TKI with action against malignancies with mutations in the ABL kinase domain that make the cancers resistant to first- and second-generation TKIs such as imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna).
Ponatinib is approved in the United States for treatment of adult patients with CML positive for the T3151 mutation in chronic phase, accelerated phase, or blast phase, or T3151-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL). It is also approved for treatment of adults with chronic phase, accelerated phase, or blast phase CML or Ph+ALL for whom no other TKI is indicated.
The drug labeling carries a boxed warning about increased risk for vascular occlusion, heart failure, and hepatotoxicity. The warning notes that “[a]rterial and venous thrombosis and occlusions have occurred in at least 27% of Iclusig-treated patients, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures.”
In a single-arm, open label trial, the investigators enrolled 51 patients with recently diagnosed CML in chronic phase, starting them on doses of oral ponatinib 45 mg daily. The protocol was later amended to a starting dose of 30 mg daily because of the high frequency of dose reductions required among patients started at 45 mg. Following a warning from the FDA about vascular complications with the drug, patients were started on daily low-dose aspirin (81 mg), and all drug doses were reduced to either 30 mg or 15 mg daily.
One of the patients discontinued therapy prior to assessment because of the FDA warning, leaving 50 for assessment at 6 months.
Of 46 patients evaluable for the primary outcome of CCyR by 6 months, 43 (94%) achieved it. Major molecular responses, defined as a BCR-ABL to ABL transcript ratio of 0.1% or lower, occurred in 40 of 50 patients (80%) evaluable for this secondary endpoint, and deep molecular response (MR4.5), defined as a ratio less than 0.0032% or lower, occurred in 28 of 50 (55%).
Cardiovascular events, primarily hypertension, occurred in 25 patients (49%), 15 (29%) had grade 3-4 myelosuppression, and 5 patients (10%) developed cerebrovascular or vaso-occlusive disease.
In all, 43 patients (85%) needed treatment interruptions at some time and 45 (88%) needed dose reductions. The study was terminated in June 2014, a little more than a year after recruitment began.
The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
The tyrosine kinase inhibitor ponatinib produced a high degree of complete cytogenetic responses when used in first-line treatment of patients with chronic myeloid leukemia in chronic phase. But the drug’s toxicities, especially its propensity for causing thromboembolic events, mitigate against its upfront use, investigators say.
In a small phase II study, 90% of evaluable patients with chronic-phase chronic myeloid leukemia (CML) treated with the tyrosine kinase inhibitor (TKI) had a complete cytogenetic response (CCyR) after 3 months, and 94% had a CCyR after 6 months, but that efficacy came at the cost of significant adverse events requiring dose reductions, treatment interruptions, and early termination of the trial for safety reasons at the recommendation of the Food and Drug Administration, reported Dr. Preetesh Jain of MD Anderson Cancer Center in Houston, Tex., and colleagues.
“[O]ur study shows that ponatinib is a very potent TKI with high clinical activity in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population who have other treatment options with high efficacy,” the investigators wrote (Lancet Haematol. 2015;2[9]:e376-e383).
Ponatinib (Iclusig) is a third-generation TKI with action against malignancies with mutations in the ABL kinase domain that make the cancers resistant to first- and second-generation TKIs such as imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna).
Ponatinib is approved in the United States for treatment of adult patients with CML positive for the T3151 mutation in chronic phase, accelerated phase, or blast phase, or T3151-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL). It is also approved for treatment of adults with chronic phase, accelerated phase, or blast phase CML or Ph+ALL for whom no other TKI is indicated.
The drug labeling carries a boxed warning about increased risk for vascular occlusion, heart failure, and hepatotoxicity. The warning notes that “[a]rterial and venous thrombosis and occlusions have occurred in at least 27% of Iclusig-treated patients, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures.”
In a single-arm, open label trial, the investigators enrolled 51 patients with recently diagnosed CML in chronic phase, starting them on doses of oral ponatinib 45 mg daily. The protocol was later amended to a starting dose of 30 mg daily because of the high frequency of dose reductions required among patients started at 45 mg. Following a warning from the FDA about vascular complications with the drug, patients were started on daily low-dose aspirin (81 mg), and all drug doses were reduced to either 30 mg or 15 mg daily.
One of the patients discontinued therapy prior to assessment because of the FDA warning, leaving 50 for assessment at 6 months.
Of 46 patients evaluable for the primary outcome of CCyR by 6 months, 43 (94%) achieved it. Major molecular responses, defined as a BCR-ABL to ABL transcript ratio of 0.1% or lower, occurred in 40 of 50 patients (80%) evaluable for this secondary endpoint, and deep molecular response (MR4.5), defined as a ratio less than 0.0032% or lower, occurred in 28 of 50 (55%).
Cardiovascular events, primarily hypertension, occurred in 25 patients (49%), 15 (29%) had grade 3-4 myelosuppression, and 5 patients (10%) developed cerebrovascular or vaso-occlusive disease.
In all, 43 patients (85%) needed treatment interruptions at some time and 45 (88%) needed dose reductions. The study was terminated in June 2014, a little more than a year after recruitment began.
The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
FROM LANCET HAEMATOLOGY
Key clinical point: The multi-tyrosine kinase inhibitor ponatinib (Iclusig) may not be suitable as first-line therapy for CML in chronic phase.
Major finding: Among evaluable patients, 94% had a complete cytogenic response by 6 months, but most also required treatment interruptions or dose reductions because of toxicities.
Data source: Single-arm phase II trial of 51 patients with recently diagnosed chronic myeloid leukemia in chronic phase.
Disclosures: The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
Nipple-sparing mastectomy feasible in N+ early breast cancer
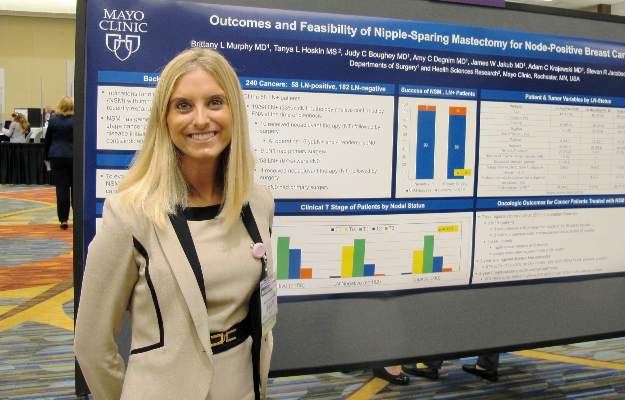
SAN FRANCISCO – Nipple-sparing mastectomy can be a safe surgical option for carefully selected patients with node-positive breast cancers, investigators reported at the 2015 ASCO Breast Cancer Symposium.
In a series of 226 patients with a total of 240 breast tumors, there was no significant difference between patients with node-positive or node-negative disease in the rate of conversion from a planned nipple-sparing procedure to a skin-sparing procedure, reported Dr. Brittany L. Murphy, a general surgery resident, and her colleagues at the Mayo Clinic in Rochester, Minn.
“Among women with node-positive breast cancer, nipple-sparing surgery may be appropriate for patients who do not have T4 or inflammatory carcinoma, patients that do not have multifocal disease, and who clinically and on imaging modalities do not appear to have nipple involvement,” Dr. Murphy said in an interview at the symposium.
In general, surgeons consider nipple-sparing mastectomies to be most appropriate for patients with early-stage disease; nodal involvement is often considered a contraindication, she said.
To see whether nipple-sparing surgery could be safely performed in patients with node-positive disease, the researchers took a retrospective look at data on 226 patients (14 with bilateral cancers) scheduled for nipple-sparing mastectomy at their center from 2009 through 2014.
In all, 182 of the cancers were lymph-node negative, and 58 were positive. Of the 58 node-positive cases, 27 (47%) were T2/T3 tumors, compared with 31 of 182 (17%) node-negative cases (P less than .0001). There were no significant differences between the groups in either estrogen receptor or HER2 receptor positivity, however.
Of the node-positive cases, 19 (33%) had cN1 (clinical) nodal involvement with pathology confirmed by fine-needle aspiration at the time of diagnosis, and 10 of these patients underwent neoadjuvant therapy followed by surgery. At the time of surgery, 6 of the 10 had pathologically confirmed positivity, and 4 were found to have ypN0 status. The remaining 9 patients in this group went on to primary surgery without neoadjuvant therapy.
Of the 39 patients who were clinically node negative, 4 had neoadjuvant therapy followed by surgery, and 35 went on to primary surgery.
The nipple-sparing procedure was successfully performed in 13 of the 14 node-positive patients who received neoadjuvant therapy and in 39 of 44 node-positive patients who went on to primary surgery. Six of the node-positive patients required conversion to a skin-sparing technique, either at the time of initial surgery based on frozen section pathology (five patients) or at a second procedure (one patient).
There were a total of seven locoregional recurrences among all patients treated with nipple-sparing mastectomy, including five in node-positive patients and two in node-negative patients. This difference was not significant.
Among the node-positive patients, three of the recurrences were in subcutaneous flaps away from the nipple at 13, 30, and 46 months of follow-up. In two cases, involved ipsilateral supraclavicular and mediastinal lymph nodes were detected at 24 and 32 months.
In the node-negative patients, one recurrence was in the nipple-areolar complex at 82 months, and one was in axillary nodes after negative sentinel lymph node biopsy at 20 months.
The 3-year locoregional disease-free estimates were 87% for lymph node positive patients compared with 99% for node negative patients (P = .007).
There were no differences between the groups regarding 3-year breast cancer–specific survival estimates, at 97% for lymph-node positive patients, and 99% for node-negative patients.
“Short-term oncologic outcomes were satisfactory. These data suggest that nipple-sparing mastectomy may be appropriate for carefully selected lymph node-positive breast cancer patients,” Dr, Murphy and colleagues wrote in a poster presented at the symposium.
SAN FRANCISCO – Nipple-sparing mastectomy can be a safe surgical option for carefully selected patients with node-positive breast cancers, investigators reported at the 2015 ASCO Breast Cancer Symposium.
In a series of 226 patients with a total of 240 breast tumors, there was no significant difference between patients with node-positive or node-negative disease in the rate of conversion from a planned nipple-sparing procedure to a skin-sparing procedure, reported Dr. Brittany L. Murphy, a general surgery resident, and her colleagues at the Mayo Clinic in Rochester, Minn.
“Among women with node-positive breast cancer, nipple-sparing surgery may be appropriate for patients who do not have T4 or inflammatory carcinoma, patients that do not have multifocal disease, and who clinically and on imaging modalities do not appear to have nipple involvement,” Dr. Murphy said in an interview at the symposium.
In general, surgeons consider nipple-sparing mastectomies to be most appropriate for patients with early-stage disease; nodal involvement is often considered a contraindication, she said.
To see whether nipple-sparing surgery could be safely performed in patients with node-positive disease, the researchers took a retrospective look at data on 226 patients (14 with bilateral cancers) scheduled for nipple-sparing mastectomy at their center from 2009 through 2014.
In all, 182 of the cancers were lymph-node negative, and 58 were positive. Of the 58 node-positive cases, 27 (47%) were T2/T3 tumors, compared with 31 of 182 (17%) node-negative cases (P less than .0001). There were no significant differences between the groups in either estrogen receptor or HER2 receptor positivity, however.
Of the node-positive cases, 19 (33%) had cN1 (clinical) nodal involvement with pathology confirmed by fine-needle aspiration at the time of diagnosis, and 10 of these patients underwent neoadjuvant therapy followed by surgery. At the time of surgery, 6 of the 10 had pathologically confirmed positivity, and 4 were found to have ypN0 status. The remaining 9 patients in this group went on to primary surgery without neoadjuvant therapy.
Of the 39 patients who were clinically node negative, 4 had neoadjuvant therapy followed by surgery, and 35 went on to primary surgery.
The nipple-sparing procedure was successfully performed in 13 of the 14 node-positive patients who received neoadjuvant therapy and in 39 of 44 node-positive patients who went on to primary surgery. Six of the node-positive patients required conversion to a skin-sparing technique, either at the time of initial surgery based on frozen section pathology (five patients) or at a second procedure (one patient).
There were a total of seven locoregional recurrences among all patients treated with nipple-sparing mastectomy, including five in node-positive patients and two in node-negative patients. This difference was not significant.
Among the node-positive patients, three of the recurrences were in subcutaneous flaps away from the nipple at 13, 30, and 46 months of follow-up. In two cases, involved ipsilateral supraclavicular and mediastinal lymph nodes were detected at 24 and 32 months.
In the node-negative patients, one recurrence was in the nipple-areolar complex at 82 months, and one was in axillary nodes after negative sentinel lymph node biopsy at 20 months.
The 3-year locoregional disease-free estimates were 87% for lymph node positive patients compared with 99% for node negative patients (P = .007).
There were no differences between the groups regarding 3-year breast cancer–specific survival estimates, at 97% for lymph-node positive patients, and 99% for node-negative patients.
“Short-term oncologic outcomes were satisfactory. These data suggest that nipple-sparing mastectomy may be appropriate for carefully selected lymph node-positive breast cancer patients,” Dr, Murphy and colleagues wrote in a poster presented at the symposium.
SAN FRANCISCO – Nipple-sparing mastectomy can be a safe surgical option for carefully selected patients with node-positive breast cancers, investigators reported at the 2015 ASCO Breast Cancer Symposium.
In a series of 226 patients with a total of 240 breast tumors, there was no significant difference between patients with node-positive or node-negative disease in the rate of conversion from a planned nipple-sparing procedure to a skin-sparing procedure, reported Dr. Brittany L. Murphy, a general surgery resident, and her colleagues at the Mayo Clinic in Rochester, Minn.
“Among women with node-positive breast cancer, nipple-sparing surgery may be appropriate for patients who do not have T4 or inflammatory carcinoma, patients that do not have multifocal disease, and who clinically and on imaging modalities do not appear to have nipple involvement,” Dr. Murphy said in an interview at the symposium.
In general, surgeons consider nipple-sparing mastectomies to be most appropriate for patients with early-stage disease; nodal involvement is often considered a contraindication, she said.
To see whether nipple-sparing surgery could be safely performed in patients with node-positive disease, the researchers took a retrospective look at data on 226 patients (14 with bilateral cancers) scheduled for nipple-sparing mastectomy at their center from 2009 through 2014.
In all, 182 of the cancers were lymph-node negative, and 58 were positive. Of the 58 node-positive cases, 27 (47%) were T2/T3 tumors, compared with 31 of 182 (17%) node-negative cases (P less than .0001). There were no significant differences between the groups in either estrogen receptor or HER2 receptor positivity, however.
Of the node-positive cases, 19 (33%) had cN1 (clinical) nodal involvement with pathology confirmed by fine-needle aspiration at the time of diagnosis, and 10 of these patients underwent neoadjuvant therapy followed by surgery. At the time of surgery, 6 of the 10 had pathologically confirmed positivity, and 4 were found to have ypN0 status. The remaining 9 patients in this group went on to primary surgery without neoadjuvant therapy.
Of the 39 patients who were clinically node negative, 4 had neoadjuvant therapy followed by surgery, and 35 went on to primary surgery.
The nipple-sparing procedure was successfully performed in 13 of the 14 node-positive patients who received neoadjuvant therapy and in 39 of 44 node-positive patients who went on to primary surgery. Six of the node-positive patients required conversion to a skin-sparing technique, either at the time of initial surgery based on frozen section pathology (five patients) or at a second procedure (one patient).
There were a total of seven locoregional recurrences among all patients treated with nipple-sparing mastectomy, including five in node-positive patients and two in node-negative patients. This difference was not significant.
Among the node-positive patients, three of the recurrences were in subcutaneous flaps away from the nipple at 13, 30, and 46 months of follow-up. In two cases, involved ipsilateral supraclavicular and mediastinal lymph nodes were detected at 24 and 32 months.
In the node-negative patients, one recurrence was in the nipple-areolar complex at 82 months, and one was in axillary nodes after negative sentinel lymph node biopsy at 20 months.
The 3-year locoregional disease-free estimates were 87% for lymph node positive patients compared with 99% for node negative patients (P = .007).
There were no differences between the groups regarding 3-year breast cancer–specific survival estimates, at 97% for lymph-node positive patients, and 99% for node-negative patients.
“Short-term oncologic outcomes were satisfactory. These data suggest that nipple-sparing mastectomy may be appropriate for carefully selected lymph node-positive breast cancer patients,” Dr, Murphy and colleagues wrote in a poster presented at the symposium.
AT THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Some women with lymph-node positive early breast cancer may safely undergo nipple-sparing mastectomy.
Major finding: Three-year breast cancer-specific survival estimates were 97% for node positive patients and 99% for node-negative patients.
Data source: Retrospective review of data on 226 women with 240 early breast cancers.
Disclosures: The study was institutionally supported. Dr. Murphy reported no conflicts of interest.
Positive lumpectomy margin risk rises with breast density
SAN FRANCISCO – Breast density is an independent risk factor for positive lumpectomy margins, pointing to a need for better methods of intraoperative margin assessment, researchers contend.
Data from a randomized clinical trial evaluating intraoperative tumor margin detection techniques indicate that for every increase in breast density category, the risk of positive margins on the main lumpectomy specimen increases by 46%, reported Dr. Tanir Allweis of Hebrew University Medical Center in Jerusalem, Israel.
“The use of newer technology and advanced techniques for intraoperative margin assessment, more extensive preop evaluation of these patients with MRI, or more liberal reshaving during the time of initial lumpectomy might be able to decrease the rate of reoperations in women with dense breasts,” Dr. Allweis said in an interview at the 2015 ASCO Breast Cancer Symposium.
She and colleague Dr. Freya Schnabel of New York University Langone Medical Center in New York City reviewed data on women enrolled in a clinical trial in which patients were randomized to lumpectomy with standard margin assessment or the use of an intraoperative radiofrequency spectroscopy device (MarginProbe). Of the 664 women enrolled in the trial, information on breast density was available for 450, and these women were included in the current study.The authors looked at data on breast density, patient and tumor characteristics, and the margin status of the primary lumpectomy specimen prior to randomization (that is, before the use of the device or the surgeon’s customary margin assessment technique).
They defined positive margins as ink on tumor. Breast density was rated on a scale of 1 (mostly fatty) to 4 (extremely dense) according to the American College of Radiology BI-RADS breast density descriptors.
Higher breast density was associated with younger age at diagnosis, lower body mass index, smaller breasts, and smaller specimen volume. Women with dense breasts were more likely to have had preoperative MRI (odds ratio [OR] 2. P less than .0001).
Each increase in breast density category was associated with an OR of 1.46 for positive margins in the main lumpectomy specimen. Thus, while women with mostly fatty breasts had a 14% risk for positive margins, women with extremely dense breasts had a 40% risk for positive margins.
The association between breast density and margin positivity remained significant after the researchers controlled for age, BMI, breast volume, and specimen volume (adjusted OR 1.39-1.52, P less than .036).
The investigators plan to explore whether the tumors in denser breasts may be larger than initially suspected because of the documented difficulties in imaging extremely dense tissues.
“These results suggest that the use of adjunctive methods for intraoperative margin assessment may be particularly helpful in this patient population. Further research will be important to clarify the benefit of various methods to decrease the rate for reexcision procedures in patients with increased breast density,” the investigators wrote in a poster presented at the symposium.
SAN FRANCISCO – Breast density is an independent risk factor for positive lumpectomy margins, pointing to a need for better methods of intraoperative margin assessment, researchers contend.
Data from a randomized clinical trial evaluating intraoperative tumor margin detection techniques indicate that for every increase in breast density category, the risk of positive margins on the main lumpectomy specimen increases by 46%, reported Dr. Tanir Allweis of Hebrew University Medical Center in Jerusalem, Israel.
“The use of newer technology and advanced techniques for intraoperative margin assessment, more extensive preop evaluation of these patients with MRI, or more liberal reshaving during the time of initial lumpectomy might be able to decrease the rate of reoperations in women with dense breasts,” Dr. Allweis said in an interview at the 2015 ASCO Breast Cancer Symposium.
She and colleague Dr. Freya Schnabel of New York University Langone Medical Center in New York City reviewed data on women enrolled in a clinical trial in which patients were randomized to lumpectomy with standard margin assessment or the use of an intraoperative radiofrequency spectroscopy device (MarginProbe). Of the 664 women enrolled in the trial, information on breast density was available for 450, and these women were included in the current study.The authors looked at data on breast density, patient and tumor characteristics, and the margin status of the primary lumpectomy specimen prior to randomization (that is, before the use of the device or the surgeon’s customary margin assessment technique).
They defined positive margins as ink on tumor. Breast density was rated on a scale of 1 (mostly fatty) to 4 (extremely dense) according to the American College of Radiology BI-RADS breast density descriptors.
Higher breast density was associated with younger age at diagnosis, lower body mass index, smaller breasts, and smaller specimen volume. Women with dense breasts were more likely to have had preoperative MRI (odds ratio [OR] 2. P less than .0001).
Each increase in breast density category was associated with an OR of 1.46 for positive margins in the main lumpectomy specimen. Thus, while women with mostly fatty breasts had a 14% risk for positive margins, women with extremely dense breasts had a 40% risk for positive margins.
The association between breast density and margin positivity remained significant after the researchers controlled for age, BMI, breast volume, and specimen volume (adjusted OR 1.39-1.52, P less than .036).
The investigators plan to explore whether the tumors in denser breasts may be larger than initially suspected because of the documented difficulties in imaging extremely dense tissues.
“These results suggest that the use of adjunctive methods for intraoperative margin assessment may be particularly helpful in this patient population. Further research will be important to clarify the benefit of various methods to decrease the rate for reexcision procedures in patients with increased breast density,” the investigators wrote in a poster presented at the symposium.
SAN FRANCISCO – Breast density is an independent risk factor for positive lumpectomy margins, pointing to a need for better methods of intraoperative margin assessment, researchers contend.
Data from a randomized clinical trial evaluating intraoperative tumor margin detection techniques indicate that for every increase in breast density category, the risk of positive margins on the main lumpectomy specimen increases by 46%, reported Dr. Tanir Allweis of Hebrew University Medical Center in Jerusalem, Israel.
“The use of newer technology and advanced techniques for intraoperative margin assessment, more extensive preop evaluation of these patients with MRI, or more liberal reshaving during the time of initial lumpectomy might be able to decrease the rate of reoperations in women with dense breasts,” Dr. Allweis said in an interview at the 2015 ASCO Breast Cancer Symposium.
She and colleague Dr. Freya Schnabel of New York University Langone Medical Center in New York City reviewed data on women enrolled in a clinical trial in which patients were randomized to lumpectomy with standard margin assessment or the use of an intraoperative radiofrequency spectroscopy device (MarginProbe). Of the 664 women enrolled in the trial, information on breast density was available for 450, and these women were included in the current study.The authors looked at data on breast density, patient and tumor characteristics, and the margin status of the primary lumpectomy specimen prior to randomization (that is, before the use of the device or the surgeon’s customary margin assessment technique).
They defined positive margins as ink on tumor. Breast density was rated on a scale of 1 (mostly fatty) to 4 (extremely dense) according to the American College of Radiology BI-RADS breast density descriptors.
Higher breast density was associated with younger age at diagnosis, lower body mass index, smaller breasts, and smaller specimen volume. Women with dense breasts were more likely to have had preoperative MRI (odds ratio [OR] 2. P less than .0001).
Each increase in breast density category was associated with an OR of 1.46 for positive margins in the main lumpectomy specimen. Thus, while women with mostly fatty breasts had a 14% risk for positive margins, women with extremely dense breasts had a 40% risk for positive margins.
The association between breast density and margin positivity remained significant after the researchers controlled for age, BMI, breast volume, and specimen volume (adjusted OR 1.39-1.52, P less than .036).
The investigators plan to explore whether the tumors in denser breasts may be larger than initially suspected because of the documented difficulties in imaging extremely dense tissues.
“These results suggest that the use of adjunctive methods for intraoperative margin assessment may be particularly helpful in this patient population. Further research will be important to clarify the benefit of various methods to decrease the rate for reexcision procedures in patients with increased breast density,” the investigators wrote in a poster presented at the symposium.
AT THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: The risk for positive lumpectomy margins rises as breast density increases.
Major finding: .Women with extremely dense breasts had a 40% risk for ink on tumor in the main lumpectomy specimen.
Data source: Review of data on 450 of 664 women enrolled in a randomized clinical trial.
Disclosures: Dune Medical Devices supported the trial. Dr. Allweis and Dr. Schnabel reported no conflicts of interest.
Dose-dense breast chemotherapy is also cost dense
SAN FRANCISCO – Despite the shorter course of care, dose-dense adjuvant chemotherapy for breast cancer may be considerably more costly than standard-dose chemotherapy, authors of a small study caution.
Among patients treated with four cycles of doxorubicin and cyclophosphamide (the AC regimen), the total cost for those treated with one cycle every 2 weeks (dose-dense schedule) was 77% higher than for patients treated once every 3 weeks, reported Helen O’Donovan, a medical student from Bon Secours Hospital and University College Cork, Ireland, and her associates.
The difference in cost was largely accounted for by the necessity for neutropenia prophylaxis with granulocytic colony stimulating factor (GCSF) among patients who received the dose-dense schedule, Ms. O’Donovan said in an interview at the 2015 ASCO Breast Cancer Symposium.
The mean cost of four doses of GCSF at her center was € 4,176 ($4,703). In the United States, a single syringe (one dose) of the long-acting GCSF pegfilgrastim (Neulasta) costs approximately $5,000 at retail pharmacies.
“We wanted to see whether GCSF in the long run might be cheaper if it could reduce admissions. It turns out that it wasn’t,” said Ms. O’Donovan.
To determine this, the investigators conducted a retrospective study comparing costs for patients treated with dose-dense chemotherapy with those treated once every 3 weeks for the same number of cycles. Because the hospital’s standard of care for several years has been the dose-dense regimen, the researchers were able to identify only 13 patients who had received the 3-week regimen. They matched these patients by age and year of diagnosis to 13 controls treated with dose-dense therapy.
All patients had early-stage breast cancer with no evidence of metastases and all were treated with AC-based chemotherapy with or without a taxane.
In all, six patients in the dose-dense group and five in the 3-week group required hospitalization for complications during chemotherapy. The mean duration of hospitalization was similar, at 4.7 and 4.6 days, respectively.
All patients in the dose-dense group received GCSF, compared with seven patients in the 3-week group. As noted, the mean costs for GCSF among patients with dose-dense chemotherapy were € 4,176 ($4,703), compared with € 1,900 ($2,139) for patients treated in the longer protocol (P = .0005).
Although the mean costs of hospitalization were not significantly different between the groups, adding in the cost of GCSF boosted the total costs to €6,163 ($6,937) vs. €3,486 ($3,923), and this difference was significant (P = .048).
Despite the GCSF prophylaxis and 100% compliance, febrile neutropenia was the most common reason for hospitalization in each group, with two patients in the dose-dense requiring hospitalization for 3 and 9 days, and three patients in the 3-week group needing admission, one for 4 days, and two for 8 days.
The authors acknowledged that the sample size was small, and that there may have been selection bias in the assignment of patients to the 3-week group.
SAN FRANCISCO – Despite the shorter course of care, dose-dense adjuvant chemotherapy for breast cancer may be considerably more costly than standard-dose chemotherapy, authors of a small study caution.
Among patients treated with four cycles of doxorubicin and cyclophosphamide (the AC regimen), the total cost for those treated with one cycle every 2 weeks (dose-dense schedule) was 77% higher than for patients treated once every 3 weeks, reported Helen O’Donovan, a medical student from Bon Secours Hospital and University College Cork, Ireland, and her associates.
The difference in cost was largely accounted for by the necessity for neutropenia prophylaxis with granulocytic colony stimulating factor (GCSF) among patients who received the dose-dense schedule, Ms. O’Donovan said in an interview at the 2015 ASCO Breast Cancer Symposium.
The mean cost of four doses of GCSF at her center was € 4,176 ($4,703). In the United States, a single syringe (one dose) of the long-acting GCSF pegfilgrastim (Neulasta) costs approximately $5,000 at retail pharmacies.
“We wanted to see whether GCSF in the long run might be cheaper if it could reduce admissions. It turns out that it wasn’t,” said Ms. O’Donovan.
To determine this, the investigators conducted a retrospective study comparing costs for patients treated with dose-dense chemotherapy with those treated once every 3 weeks for the same number of cycles. Because the hospital’s standard of care for several years has been the dose-dense regimen, the researchers were able to identify only 13 patients who had received the 3-week regimen. They matched these patients by age and year of diagnosis to 13 controls treated with dose-dense therapy.
All patients had early-stage breast cancer with no evidence of metastases and all were treated with AC-based chemotherapy with or without a taxane.
In all, six patients in the dose-dense group and five in the 3-week group required hospitalization for complications during chemotherapy. The mean duration of hospitalization was similar, at 4.7 and 4.6 days, respectively.
All patients in the dose-dense group received GCSF, compared with seven patients in the 3-week group. As noted, the mean costs for GCSF among patients with dose-dense chemotherapy were € 4,176 ($4,703), compared with € 1,900 ($2,139) for patients treated in the longer protocol (P = .0005).
Although the mean costs of hospitalization were not significantly different between the groups, adding in the cost of GCSF boosted the total costs to €6,163 ($6,937) vs. €3,486 ($3,923), and this difference was significant (P = .048).
Despite the GCSF prophylaxis and 100% compliance, febrile neutropenia was the most common reason for hospitalization in each group, with two patients in the dose-dense requiring hospitalization for 3 and 9 days, and three patients in the 3-week group needing admission, one for 4 days, and two for 8 days.
The authors acknowledged that the sample size was small, and that there may have been selection bias in the assignment of patients to the 3-week group.
SAN FRANCISCO – Despite the shorter course of care, dose-dense adjuvant chemotherapy for breast cancer may be considerably more costly than standard-dose chemotherapy, authors of a small study caution.
Among patients treated with four cycles of doxorubicin and cyclophosphamide (the AC regimen), the total cost for those treated with one cycle every 2 weeks (dose-dense schedule) was 77% higher than for patients treated once every 3 weeks, reported Helen O’Donovan, a medical student from Bon Secours Hospital and University College Cork, Ireland, and her associates.
The difference in cost was largely accounted for by the necessity for neutropenia prophylaxis with granulocytic colony stimulating factor (GCSF) among patients who received the dose-dense schedule, Ms. O’Donovan said in an interview at the 2015 ASCO Breast Cancer Symposium.
The mean cost of four doses of GCSF at her center was € 4,176 ($4,703). In the United States, a single syringe (one dose) of the long-acting GCSF pegfilgrastim (Neulasta) costs approximately $5,000 at retail pharmacies.
“We wanted to see whether GCSF in the long run might be cheaper if it could reduce admissions. It turns out that it wasn’t,” said Ms. O’Donovan.
To determine this, the investigators conducted a retrospective study comparing costs for patients treated with dose-dense chemotherapy with those treated once every 3 weeks for the same number of cycles. Because the hospital’s standard of care for several years has been the dose-dense regimen, the researchers were able to identify only 13 patients who had received the 3-week regimen. They matched these patients by age and year of diagnosis to 13 controls treated with dose-dense therapy.
All patients had early-stage breast cancer with no evidence of metastases and all were treated with AC-based chemotherapy with or without a taxane.
In all, six patients in the dose-dense group and five in the 3-week group required hospitalization for complications during chemotherapy. The mean duration of hospitalization was similar, at 4.7 and 4.6 days, respectively.
All patients in the dose-dense group received GCSF, compared with seven patients in the 3-week group. As noted, the mean costs for GCSF among patients with dose-dense chemotherapy were € 4,176 ($4,703), compared with € 1,900 ($2,139) for patients treated in the longer protocol (P = .0005).
Although the mean costs of hospitalization were not significantly different between the groups, adding in the cost of GCSF boosted the total costs to €6,163 ($6,937) vs. €3,486 ($3,923), and this difference was significant (P = .048).
Despite the GCSF prophylaxis and 100% compliance, febrile neutropenia was the most common reason for hospitalization in each group, with two patients in the dose-dense requiring hospitalization for 3 and 9 days, and three patients in the 3-week group needing admission, one for 4 days, and two for 8 days.
The authors acknowledged that the sample size was small, and that there may have been selection bias in the assignment of patients to the 3-week group.
AT THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Total costs for hospitalization and GCSF are higher with dose-dense chemotherapy compared with the same regimen delivered every 3 weeks.
Major finding: The total costs of hospitalization plus GCSF were $6,937 for the dose-dense schedule vs. $3,923 for chemotherapy every 3 weeks.
Data source: Retrospective single-center study of 26 patients scheduled for adjuvant chemotherapy for early-stage breast cancer.
Disclosures: Funding for the study was not disclosed. Ms. O’Donovan reported no conflicts of interest.
Two heads plus four hands equal safe, shorter bilateral mastectomies
SAN FRANCISCO – Two surgeons working in tandem, one on each side of the patient, can significantly reduce the total operative time for bilateral mastectomies without compromising safety or quality of results, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
A review of records on consecutive cases of bilateral mastectomy with tissue expander reconstruction (BMTR) showed that mean overall surgery time (start of incision to closure) was about 23 minutes shorter when two surgeons were working at once, and mean general surgery time (start of incision to end of mastectomy procedure) was 41 minutes shorter with two surgeons vs. a solo operator, reported Dr. Suniti Nimbkar of the department of surgery at Brigham and Women’s Hospital, Boston.
“We did find that there was a significant reduction in time when two surgeons work together, and that reduction is particularly emphasized when you have a patient who is larger, perhaps a heavier patient, and when you have to do more extensive surgery,” she said in an interview at the symposium.
Dual surgeons did take slightly longer to perform the reconstructive surgery portion of the procedure, however.
Effective cosurgery is like a healthy marriage or a successful restaurant kitchen, with partners learning how to anticipate each other’s needs, knowing when to lend a hand, and intuitively grasping when to get out of the way, Dr. Nimbkar suggested.
“As two surgeons work together more and more, they just inevitably grow together in how they operate. I know for example that my direct partner and I operate very similarly, whereas sometimes if I do a cosurgery with a second surgeon that I don’t always operate with we have to work together to understand each other’s approach,” she said.
The investigators scanned the charts of 116 consecutive women who underwent BMTR done by eight breast surgeons at their center, looking for potential differences in operative time and 30-day postoperative complications. In all, 67 of the procedures were cosurgeries, and 49 were done by a single surgeon.
They found that in bivariate analysis, mean general surgery time was 75.8 minutes for the surgical duos, compared with 116.8 minutes for the solo surgeons (P less than .0001). Overall surgery time was also shorter, at 255.2 minutes vs. 278.3 minutes, respectively (P = .005).
Although there were numerically more complications among patients of cosurgeons, there was no statistically significant difference in postoperative complications rates between the twin and singleton surgeons.
A linear regression model showed that factors significantly associated with general surgery time were cosurgeries (P less than .0001), total breast weight (P =.03) and axillary dissection (P = .0003).
The authors noted that although two surgeons cut operative times significantly, the amount of time savings was not proportional to what they expected.
“We also want to know if the plastic surgeons are happy with the results if there are two different surgeons. Do they feel that there is too much of a difference in the way the mastectomy comes out, or is it something they’re fine with? So one of the next steps we’re going to take is to survey the plastic surgeons as to whether they’re comfortable with what we’re doing or if they have ideas about how we can be more uniform,” Dr. Nimbkar said.
SAN FRANCISCO – Two surgeons working in tandem, one on each side of the patient, can significantly reduce the total operative time for bilateral mastectomies without compromising safety or quality of results, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
A review of records on consecutive cases of bilateral mastectomy with tissue expander reconstruction (BMTR) showed that mean overall surgery time (start of incision to closure) was about 23 minutes shorter when two surgeons were working at once, and mean general surgery time (start of incision to end of mastectomy procedure) was 41 minutes shorter with two surgeons vs. a solo operator, reported Dr. Suniti Nimbkar of the department of surgery at Brigham and Women’s Hospital, Boston.
“We did find that there was a significant reduction in time when two surgeons work together, and that reduction is particularly emphasized when you have a patient who is larger, perhaps a heavier patient, and when you have to do more extensive surgery,” she said in an interview at the symposium.
Dual surgeons did take slightly longer to perform the reconstructive surgery portion of the procedure, however.
Effective cosurgery is like a healthy marriage or a successful restaurant kitchen, with partners learning how to anticipate each other’s needs, knowing when to lend a hand, and intuitively grasping when to get out of the way, Dr. Nimbkar suggested.
“As two surgeons work together more and more, they just inevitably grow together in how they operate. I know for example that my direct partner and I operate very similarly, whereas sometimes if I do a cosurgery with a second surgeon that I don’t always operate with we have to work together to understand each other’s approach,” she said.
The investigators scanned the charts of 116 consecutive women who underwent BMTR done by eight breast surgeons at their center, looking for potential differences in operative time and 30-day postoperative complications. In all, 67 of the procedures were cosurgeries, and 49 were done by a single surgeon.
They found that in bivariate analysis, mean general surgery time was 75.8 minutes for the surgical duos, compared with 116.8 minutes for the solo surgeons (P less than .0001). Overall surgery time was also shorter, at 255.2 minutes vs. 278.3 minutes, respectively (P = .005).
Although there were numerically more complications among patients of cosurgeons, there was no statistically significant difference in postoperative complications rates between the twin and singleton surgeons.
A linear regression model showed that factors significantly associated with general surgery time were cosurgeries (P less than .0001), total breast weight (P =.03) and axillary dissection (P = .0003).
The authors noted that although two surgeons cut operative times significantly, the amount of time savings was not proportional to what they expected.
“We also want to know if the plastic surgeons are happy with the results if there are two different surgeons. Do they feel that there is too much of a difference in the way the mastectomy comes out, or is it something they’re fine with? So one of the next steps we’re going to take is to survey the plastic surgeons as to whether they’re comfortable with what we’re doing or if they have ideas about how we can be more uniform,” Dr. Nimbkar said.
SAN FRANCISCO – Two surgeons working in tandem, one on each side of the patient, can significantly reduce the total operative time for bilateral mastectomies without compromising safety or quality of results, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
A review of records on consecutive cases of bilateral mastectomy with tissue expander reconstruction (BMTR) showed that mean overall surgery time (start of incision to closure) was about 23 minutes shorter when two surgeons were working at once, and mean general surgery time (start of incision to end of mastectomy procedure) was 41 minutes shorter with two surgeons vs. a solo operator, reported Dr. Suniti Nimbkar of the department of surgery at Brigham and Women’s Hospital, Boston.
“We did find that there was a significant reduction in time when two surgeons work together, and that reduction is particularly emphasized when you have a patient who is larger, perhaps a heavier patient, and when you have to do more extensive surgery,” she said in an interview at the symposium.
Dual surgeons did take slightly longer to perform the reconstructive surgery portion of the procedure, however.
Effective cosurgery is like a healthy marriage or a successful restaurant kitchen, with partners learning how to anticipate each other’s needs, knowing when to lend a hand, and intuitively grasping when to get out of the way, Dr. Nimbkar suggested.
“As two surgeons work together more and more, they just inevitably grow together in how they operate. I know for example that my direct partner and I operate very similarly, whereas sometimes if I do a cosurgery with a second surgeon that I don’t always operate with we have to work together to understand each other’s approach,” she said.
The investigators scanned the charts of 116 consecutive women who underwent BMTR done by eight breast surgeons at their center, looking for potential differences in operative time and 30-day postoperative complications. In all, 67 of the procedures were cosurgeries, and 49 were done by a single surgeon.
They found that in bivariate analysis, mean general surgery time was 75.8 minutes for the surgical duos, compared with 116.8 minutes for the solo surgeons (P less than .0001). Overall surgery time was also shorter, at 255.2 minutes vs. 278.3 minutes, respectively (P = .005).
Although there were numerically more complications among patients of cosurgeons, there was no statistically significant difference in postoperative complications rates between the twin and singleton surgeons.
A linear regression model showed that factors significantly associated with general surgery time were cosurgeries (P less than .0001), total breast weight (P =.03) and axillary dissection (P = .0003).
The authors noted that although two surgeons cut operative times significantly, the amount of time savings was not proportional to what they expected.
“We also want to know if the plastic surgeons are happy with the results if there are two different surgeons. Do they feel that there is too much of a difference in the way the mastectomy comes out, or is it something they’re fine with? So one of the next steps we’re going to take is to survey the plastic surgeons as to whether they’re comfortable with what we’re doing or if they have ideas about how we can be more uniform,” Dr. Nimbkar said.
AT THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point:Bilateral mastectomy performed by two surgeons shortens operative time without increasing complications.
Major finding: Mean general surgery time was 75.8 minutes for two surgeons vs. 116.8 minutes for solo surgeons (P less than .0001).
Data source: Retrospective review of records on 116 consecutive patients undergoing bilateral mastectomies.
Disclosures: The study was internally funded. The authors reported no conflicts of interest.
ASCO: Neoadjuvant chemo does not increase breast cancer surgery complications
SAN FRANCISCO – Breast cancer surgery within 30 days of neoadjuvant chemotherapy appears to be safe, with no significant increase in risk of postoperative complications compared with surgery performed in women who did not receive chemotherapy.
An analysis of data on more than 3,500 patients who underwent surgery for invasive breast cancer after receiving neoadjuvant chemotherapy showed that in an analysis adjusted to better balance patient characteristics, there were no significant differences in postoperative complication rates for patients who received chemotherapy and those who did not, reported Dr. Erin Cordeiro from the Ottawa Hospital in Ontario, Canada, and associates, at a breast cancer symposium sponsored jointly by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“I really think this should put to rest the concern that surgeons might have about neoadjuvant chemotherapy particularly because this [study] compressed people within 30 days of the surgery date,” commented Dr. Charles E. Geyer, Jr., from the Virginia Commonwealth University School of Medicine in Richmond. Dr. Geyer was the invited discussant.
The use of neoadjuvant chemotherapy in the treatment of patients with breast cancer continues to increase, growing from 14% in 2006, to 20% in 2011, Dr. Cordeiro said.
But because myelosuppression, particularly neutropenia, is a common side of chemotherapy, surgeons are often concerned that operating too soon after a patient completes a course of chemotherapy could result in increased post-operative complications, she said.
To determine whether neoadjuvant chemotherapy would increase the proportion of overall 30-day postoperative complications among patients undergoing surgery for invasive breast cancer, Dr. Cordeiro drew on the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which contains validated, risk-adjusted surgical outcomes data from more than 600 hospitals in 49 states, Canada, and Europe.
She identified data on 67,685 patients who underwent surgery for invasive breast cancer from 2005 through 2012. Of this group, 3,624 (5.5%) had received neoadjuvant chemotherapy. Investigators excludedpPatients with high-risk comorbidities and those who underwent other surgery concurrent with breast surgery (e.g., oophorectomy).
In unadjusted analysis, the rate of a composite of overall 30-day post-operative complications, the primary outcome, was 4.9% for patients who had undergone neoadjuvant chemotherapy, compared with 3.7% for patients who did not and this difference was significant (P= .0003). The higher rate among patient who had received chemotherapy recently was largely due to infections, Dr. Cordeiro said.
However, in a multivariate analysis adjusted for propensity score – a measure of the probability of receiving neoeadjuvant chemotherapy by age, diabetes, chronic obstructive pulmonary disease, hypertension, bilateral surgery, and/or year of surgery – the differences between patients who underwent neoadjuvant chemo and those who did not vanished (odds ratio 1.16; 95% confidence interval, 0.98-1.36).
Dr. Cordeiro acknowledged that the study was limited by the use of retrospective data, a lack of knowledge of exactly when chemotherapy was stopped in relation to the surgery data, lack of data on which regimens were used, and no information about prophylatic antibiotics or thromboprophylaxis.
The investigators plan to perform additional subgroup analyses to determine whether there might be differences in complication rates in patients who did or did not receive neoadjuvant chemo by type of breast surgery (breast-conserving vs. mastectomy), type of axillary surgery (sentinel node biopsy vs. axillary lymph node dissection) or among patients who undergo immediate reconstruction.
The study was presented as a poster and in an oral abstract session.
SAN FRANCISCO – Breast cancer surgery within 30 days of neoadjuvant chemotherapy appears to be safe, with no significant increase in risk of postoperative complications compared with surgery performed in women who did not receive chemotherapy.
An analysis of data on more than 3,500 patients who underwent surgery for invasive breast cancer after receiving neoadjuvant chemotherapy showed that in an analysis adjusted to better balance patient characteristics, there were no significant differences in postoperative complication rates for patients who received chemotherapy and those who did not, reported Dr. Erin Cordeiro from the Ottawa Hospital in Ontario, Canada, and associates, at a breast cancer symposium sponsored jointly by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“I really think this should put to rest the concern that surgeons might have about neoadjuvant chemotherapy particularly because this [study] compressed people within 30 days of the surgery date,” commented Dr. Charles E. Geyer, Jr., from the Virginia Commonwealth University School of Medicine in Richmond. Dr. Geyer was the invited discussant.
The use of neoadjuvant chemotherapy in the treatment of patients with breast cancer continues to increase, growing from 14% in 2006, to 20% in 2011, Dr. Cordeiro said.
But because myelosuppression, particularly neutropenia, is a common side of chemotherapy, surgeons are often concerned that operating too soon after a patient completes a course of chemotherapy could result in increased post-operative complications, she said.
To determine whether neoadjuvant chemotherapy would increase the proportion of overall 30-day postoperative complications among patients undergoing surgery for invasive breast cancer, Dr. Cordeiro drew on the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which contains validated, risk-adjusted surgical outcomes data from more than 600 hospitals in 49 states, Canada, and Europe.
She identified data on 67,685 patients who underwent surgery for invasive breast cancer from 2005 through 2012. Of this group, 3,624 (5.5%) had received neoadjuvant chemotherapy. Investigators excludedpPatients with high-risk comorbidities and those who underwent other surgery concurrent with breast surgery (e.g., oophorectomy).
In unadjusted analysis, the rate of a composite of overall 30-day post-operative complications, the primary outcome, was 4.9% for patients who had undergone neoadjuvant chemotherapy, compared with 3.7% for patients who did not and this difference was significant (P= .0003). The higher rate among patient who had received chemotherapy recently was largely due to infections, Dr. Cordeiro said.
However, in a multivariate analysis adjusted for propensity score – a measure of the probability of receiving neoeadjuvant chemotherapy by age, diabetes, chronic obstructive pulmonary disease, hypertension, bilateral surgery, and/or year of surgery – the differences between patients who underwent neoadjuvant chemo and those who did not vanished (odds ratio 1.16; 95% confidence interval, 0.98-1.36).
Dr. Cordeiro acknowledged that the study was limited by the use of retrospective data, a lack of knowledge of exactly when chemotherapy was stopped in relation to the surgery data, lack of data on which regimens were used, and no information about prophylatic antibiotics or thromboprophylaxis.
The investigators plan to perform additional subgroup analyses to determine whether there might be differences in complication rates in patients who did or did not receive neoadjuvant chemo by type of breast surgery (breast-conserving vs. mastectomy), type of axillary surgery (sentinel node biopsy vs. axillary lymph node dissection) or among patients who undergo immediate reconstruction.
The study was presented as a poster and in an oral abstract session.
SAN FRANCISCO – Breast cancer surgery within 30 days of neoadjuvant chemotherapy appears to be safe, with no significant increase in risk of postoperative complications compared with surgery performed in women who did not receive chemotherapy.
An analysis of data on more than 3,500 patients who underwent surgery for invasive breast cancer after receiving neoadjuvant chemotherapy showed that in an analysis adjusted to better balance patient characteristics, there were no significant differences in postoperative complication rates for patients who received chemotherapy and those who did not, reported Dr. Erin Cordeiro from the Ottawa Hospital in Ontario, Canada, and associates, at a breast cancer symposium sponsored jointly by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“I really think this should put to rest the concern that surgeons might have about neoadjuvant chemotherapy particularly because this [study] compressed people within 30 days of the surgery date,” commented Dr. Charles E. Geyer, Jr., from the Virginia Commonwealth University School of Medicine in Richmond. Dr. Geyer was the invited discussant.
The use of neoadjuvant chemotherapy in the treatment of patients with breast cancer continues to increase, growing from 14% in 2006, to 20% in 2011, Dr. Cordeiro said.
But because myelosuppression, particularly neutropenia, is a common side of chemotherapy, surgeons are often concerned that operating too soon after a patient completes a course of chemotherapy could result in increased post-operative complications, she said.
To determine whether neoadjuvant chemotherapy would increase the proportion of overall 30-day postoperative complications among patients undergoing surgery for invasive breast cancer, Dr. Cordeiro drew on the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which contains validated, risk-adjusted surgical outcomes data from more than 600 hospitals in 49 states, Canada, and Europe.
She identified data on 67,685 patients who underwent surgery for invasive breast cancer from 2005 through 2012. Of this group, 3,624 (5.5%) had received neoadjuvant chemotherapy. Investigators excludedpPatients with high-risk comorbidities and those who underwent other surgery concurrent with breast surgery (e.g., oophorectomy).
In unadjusted analysis, the rate of a composite of overall 30-day post-operative complications, the primary outcome, was 4.9% for patients who had undergone neoadjuvant chemotherapy, compared with 3.7% for patients who did not and this difference was significant (P= .0003). The higher rate among patient who had received chemotherapy recently was largely due to infections, Dr. Cordeiro said.
However, in a multivariate analysis adjusted for propensity score – a measure of the probability of receiving neoeadjuvant chemotherapy by age, diabetes, chronic obstructive pulmonary disease, hypertension, bilateral surgery, and/or year of surgery – the differences between patients who underwent neoadjuvant chemo and those who did not vanished (odds ratio 1.16; 95% confidence interval, 0.98-1.36).
Dr. Cordeiro acknowledged that the study was limited by the use of retrospective data, a lack of knowledge of exactly when chemotherapy was stopped in relation to the surgery data, lack of data on which regimens were used, and no information about prophylatic antibiotics or thromboprophylaxis.
The investigators plan to perform additional subgroup analyses to determine whether there might be differences in complication rates in patients who did or did not receive neoadjuvant chemo by type of breast surgery (breast-conserving vs. mastectomy), type of axillary surgery (sentinel node biopsy vs. axillary lymph node dissection) or among patients who undergo immediate reconstruction.
The study was presented as a poster and in an oral abstract session.
AT THE ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Neoadjuvant chemotherapy does not appear to increase the risk for 30-day post-operative complications following surgery for invasive breast cancer.
Major finding: The odds ratio for postoperative complications among patients who underwent chemotherapy was 1.16 but was not statistically significant.
Data source: Retrospective review from a prospective surgical outcomes database on 67,685 patients, 3,624 of whom had neoadjuvant chemotherapy.
Disclosures: Funding for the study was not disclosed. Dr. Cordeiro reported having no conflicts of interest. Dr. Geyer reported a consulting or advisory role with Stennion, research funding from Incyte, and travel expenses from Abbvie, AstraZeneca, and Genetech/Roche.
Many Women With Triple-negative Breast Cancer Aren’t Screened for BRCA
SAN FRANCISCO – Many younger women diagnosed with triple-negative breast cancers do not get tested for BRCA, despite guideline recommendations, investigators report.
Among 173 women with triple-negative tumors -- lacking the HER2, estrogen and progesterone receptors –17% of those who should have been tested for BRCA according to National Comprehensive Cancer Network (NCCN) guidelines, were not tested.
Women less likely to be tested were those 55 years or older, African Americans, those who list Medicaid as their primary form of insurance, and those with stage 3 disease, reported Staci Aubry, a 4th-year medical student at Rush University Medical Center in Chicago, and her associates.
In an interview, Ms. Aubry said that some of the women who were eligible for genetic testing under the guidelines simply declined it.
“Often patients, if they didn’t have a daughter or if they were older and were in the 50 to 60 [year-old] range, even though they’re still included in the NCCN guidelines to be screened, still refused to be tested,” she said.
NCCN guidelines on genetic and familial high-risk assessment for breast and ovarian cancer susceptibility recommend BRCA genetic testing for all women below age 60 years who are diagnosed with triple-negative breast cancer.
“Some histopathologic features have reported to occur more frequently in breast cancers characterized by a BRCA1 or BRCA2 mutation. For example, several studies have shown that BRCA1 breast cancer is more likely to be characterized as ER-/PR-negative and HER2-negative (i.e., “triple negative”), the guidelines note.
To see whether clinicians were adhering to this recommendation, the investigators searched the Commission on Cancer registry tumor database for cases of triple-negative breast cancers diagnosed from 2006 through 2013.
They identified 173 patients, 105 of whom were younger than 60 and thus recommended for screening. Of this group, 87 (83%) were tested for BRCA, and 15 were found to be BRCA positive, but the remaining 18 patients (17%) were not tested.
When the authors looked at demographic and clinical factors that might have accounted for the differences between women who were tested for the gene and those who were not, four factors stood out. Women who did not get tested were more likely to be 55 or older (P = .002), African American (P = .001), Medicaid insured (P = .021) or to have American Joint Commission on Cancer stage 3 disease (P = .014).
“The main message for clinicians is to be aware that these disparities exist, and keep in mind that these four groups – people who are under Medicaid insurance, between 55 and 60 years of age, African American, or who have higher disease stage – should be targeted for screening,” Ms. Aubry said.
SAN FRANCISCO – Many younger women diagnosed with triple-negative breast cancers do not get tested for BRCA, despite guideline recommendations, investigators report.
Among 173 women with triple-negative tumors -- lacking the HER2, estrogen and progesterone receptors –17% of those who should have been tested for BRCA according to National Comprehensive Cancer Network (NCCN) guidelines, were not tested.
Women less likely to be tested were those 55 years or older, African Americans, those who list Medicaid as their primary form of insurance, and those with stage 3 disease, reported Staci Aubry, a 4th-year medical student at Rush University Medical Center in Chicago, and her associates.
In an interview, Ms. Aubry said that some of the women who were eligible for genetic testing under the guidelines simply declined it.
“Often patients, if they didn’t have a daughter or if they were older and were in the 50 to 60 [year-old] range, even though they’re still included in the NCCN guidelines to be screened, still refused to be tested,” she said.
NCCN guidelines on genetic and familial high-risk assessment for breast and ovarian cancer susceptibility recommend BRCA genetic testing for all women below age 60 years who are diagnosed with triple-negative breast cancer.
“Some histopathologic features have reported to occur more frequently in breast cancers characterized by a BRCA1 or BRCA2 mutation. For example, several studies have shown that BRCA1 breast cancer is more likely to be characterized as ER-/PR-negative and HER2-negative (i.e., “triple negative”), the guidelines note.
To see whether clinicians were adhering to this recommendation, the investigators searched the Commission on Cancer registry tumor database for cases of triple-negative breast cancers diagnosed from 2006 through 2013.
They identified 173 patients, 105 of whom were younger than 60 and thus recommended for screening. Of this group, 87 (83%) were tested for BRCA, and 15 were found to be BRCA positive, but the remaining 18 patients (17%) were not tested.
When the authors looked at demographic and clinical factors that might have accounted for the differences between women who were tested for the gene and those who were not, four factors stood out. Women who did not get tested were more likely to be 55 or older (P = .002), African American (P = .001), Medicaid insured (P = .021) or to have American Joint Commission on Cancer stage 3 disease (P = .014).
“The main message for clinicians is to be aware that these disparities exist, and keep in mind that these four groups – people who are under Medicaid insurance, between 55 and 60 years of age, African American, or who have higher disease stage – should be targeted for screening,” Ms. Aubry said.
SAN FRANCISCO – Many younger women diagnosed with triple-negative breast cancers do not get tested for BRCA, despite guideline recommendations, investigators report.
Among 173 women with triple-negative tumors -- lacking the HER2, estrogen and progesterone receptors –17% of those who should have been tested for BRCA according to National Comprehensive Cancer Network (NCCN) guidelines, were not tested.
Women less likely to be tested were those 55 years or older, African Americans, those who list Medicaid as their primary form of insurance, and those with stage 3 disease, reported Staci Aubry, a 4th-year medical student at Rush University Medical Center in Chicago, and her associates.
In an interview, Ms. Aubry said that some of the women who were eligible for genetic testing under the guidelines simply declined it.
“Often patients, if they didn’t have a daughter or if they were older and were in the 50 to 60 [year-old] range, even though they’re still included in the NCCN guidelines to be screened, still refused to be tested,” she said.
NCCN guidelines on genetic and familial high-risk assessment for breast and ovarian cancer susceptibility recommend BRCA genetic testing for all women below age 60 years who are diagnosed with triple-negative breast cancer.
“Some histopathologic features have reported to occur more frequently in breast cancers characterized by a BRCA1 or BRCA2 mutation. For example, several studies have shown that BRCA1 breast cancer is more likely to be characterized as ER-/PR-negative and HER2-negative (i.e., “triple negative”), the guidelines note.
To see whether clinicians were adhering to this recommendation, the investigators searched the Commission on Cancer registry tumor database for cases of triple-negative breast cancers diagnosed from 2006 through 2013.
They identified 173 patients, 105 of whom were younger than 60 and thus recommended for screening. Of this group, 87 (83%) were tested for BRCA, and 15 were found to be BRCA positive, but the remaining 18 patients (17%) were not tested.
When the authors looked at demographic and clinical factors that might have accounted for the differences between women who were tested for the gene and those who were not, four factors stood out. Women who did not get tested were more likely to be 55 or older (P = .002), African American (P = .001), Medicaid insured (P = .021) or to have American Joint Commission on Cancer stage 3 disease (P = .014).
“The main message for clinicians is to be aware that these disparities exist, and keep in mind that these four groups – people who are under Medicaid insurance, between 55 and 60 years of age, African American, or who have higher disease stage – should be targeted for screening,” Ms. Aubry said.
AT THE ASCO BREAST CANCER SYMPOSIUM
Many Women With Triple-negative Breast Cancer Aren’t Screened for BRCA
SAN FRANCISCO – Many younger women diagnosed with triple-negative breast cancers do not get tested for BRCA, despite guideline recommendations, investigators report.
Among 173 women with triple-negative tumors -- lacking the HER2, estrogen and progesterone receptors –17% of those who should have been tested for BRCA according to National Comprehensive Cancer Network (NCCN) guidelines, were not tested.
Women less likely to be tested were those 55 years or older, African Americans, those who list Medicaid as their primary form of insurance, and those with stage 3 disease, reported Staci Aubry, a 4th-year medical student at Rush University Medical Center in Chicago, and her associates.
In an interview, Ms. Aubry said that some of the women who were eligible for genetic testing under the guidelines simply declined it.
“Often patients, if they didn’t have a daughter or if they were older and were in the 50 to 60 [year-old] range, even though they’re still included in the NCCN guidelines to be screened, still refused to be tested,” she said.
NCCN guidelines on genetic and familial high-risk assessment for breast and ovarian cancer susceptibility recommend BRCA genetic testing for all women below age 60 years who are diagnosed with triple-negative breast cancer.
“Some histopathologic features have reported to occur more frequently in breast cancers characterized by a BRCA1 or BRCA2 mutation. For example, several studies have shown that BRCA1 breast cancer is more likely to be characterized as ER-/PR-negative and HER2-negative (i.e., “triple negative”), the guidelines note.
To see whether clinicians were adhering to this recommendation, the investigators searched the Commission on Cancer registry tumor database for cases of triple-negative breast cancers diagnosed from 2006 through 2013.
They identified 173 patients, 105 of whom were younger than 60 and thus recommended for screening. Of this group, 87 (83%) were tested for BRCA, and 15 were found to be BRCA positive, but the remaining 18 patients (17%) were not tested.
When the authors looked at demographic and clinical factors that might have accounted for the differences between women who were tested for the gene and those who were not, four factors stood out. Women who did not get tested were more likely to be 55 or older (P = .002), African American (P = .001), Medicaid insured (P = .021) or to have American Joint Commission on Cancer stage 3 disease (P = .014).
“The main message for clinicians is to be aware that these disparities exist, and keep in mind that these four groups – people who are under Medicaid insurance, between 55 and 60 years of age, African American, or who have higher disease stage – should be targeted for screening,” Ms. Aubry said.
SAN FRANCISCO – Many younger women diagnosed with triple-negative breast cancers do not get tested for BRCA, despite guideline recommendations, investigators report.
Among 173 women with triple-negative tumors -- lacking the HER2, estrogen and progesterone receptors –17% of those who should have been tested for BRCA according to National Comprehensive Cancer Network (NCCN) guidelines, were not tested.
Women less likely to be tested were those 55 years or older, African Americans, those who list Medicaid as their primary form of insurance, and those with stage 3 disease, reported Staci Aubry, a 4th-year medical student at Rush University Medical Center in Chicago, and her associates.
In an interview, Ms. Aubry said that some of the women who were eligible for genetic testing under the guidelines simply declined it.
“Often patients, if they didn’t have a daughter or if they were older and were in the 50 to 60 [year-old] range, even though they’re still included in the NCCN guidelines to be screened, still refused to be tested,” she said.
NCCN guidelines on genetic and familial high-risk assessment for breast and ovarian cancer susceptibility recommend BRCA genetic testing for all women below age 60 years who are diagnosed with triple-negative breast cancer.
“Some histopathologic features have reported to occur more frequently in breast cancers characterized by a BRCA1 or BRCA2 mutation. For example, several studies have shown that BRCA1 breast cancer is more likely to be characterized as ER-/PR-negative and HER2-negative (i.e., “triple negative”), the guidelines note.
To see whether clinicians were adhering to this recommendation, the investigators searched the Commission on Cancer registry tumor database for cases of triple-negative breast cancers diagnosed from 2006 through 2013.
They identified 173 patients, 105 of whom were younger than 60 and thus recommended for screening. Of this group, 87 (83%) were tested for BRCA, and 15 were found to be BRCA positive, but the remaining 18 patients (17%) were not tested.
When the authors looked at demographic and clinical factors that might have accounted for the differences between women who were tested for the gene and those who were not, four factors stood out. Women who did not get tested were more likely to be 55 or older (P = .002), African American (P = .001), Medicaid insured (P = .021) or to have American Joint Commission on Cancer stage 3 disease (P = .014).
“The main message for clinicians is to be aware that these disparities exist, and keep in mind that these four groups – people who are under Medicaid insurance, between 55 and 60 years of age, African American, or who have higher disease stage – should be targeted for screening,” Ms. Aubry said.
SAN FRANCISCO – Many younger women diagnosed with triple-negative breast cancers do not get tested for BRCA, despite guideline recommendations, investigators report.
Among 173 women with triple-negative tumors -- lacking the HER2, estrogen and progesterone receptors –17% of those who should have been tested for BRCA according to National Comprehensive Cancer Network (NCCN) guidelines, were not tested.
Women less likely to be tested were those 55 years or older, African Americans, those who list Medicaid as their primary form of insurance, and those with stage 3 disease, reported Staci Aubry, a 4th-year medical student at Rush University Medical Center in Chicago, and her associates.
In an interview, Ms. Aubry said that some of the women who were eligible for genetic testing under the guidelines simply declined it.
“Often patients, if they didn’t have a daughter or if they were older and were in the 50 to 60 [year-old] range, even though they’re still included in the NCCN guidelines to be screened, still refused to be tested,” she said.
NCCN guidelines on genetic and familial high-risk assessment for breast and ovarian cancer susceptibility recommend BRCA genetic testing for all women below age 60 years who are diagnosed with triple-negative breast cancer.
“Some histopathologic features have reported to occur more frequently in breast cancers characterized by a BRCA1 or BRCA2 mutation. For example, several studies have shown that BRCA1 breast cancer is more likely to be characterized as ER-/PR-negative and HER2-negative (i.e., “triple negative”), the guidelines note.
To see whether clinicians were adhering to this recommendation, the investigators searched the Commission on Cancer registry tumor database for cases of triple-negative breast cancers diagnosed from 2006 through 2013.
They identified 173 patients, 105 of whom were younger than 60 and thus recommended for screening. Of this group, 87 (83%) were tested for BRCA, and 15 were found to be BRCA positive, but the remaining 18 patients (17%) were not tested.
When the authors looked at demographic and clinical factors that might have accounted for the differences between women who were tested for the gene and those who were not, four factors stood out. Women who did not get tested were more likely to be 55 or older (P = .002), African American (P = .001), Medicaid insured (P = .021) or to have American Joint Commission on Cancer stage 3 disease (P = .014).
“The main message for clinicians is to be aware that these disparities exist, and keep in mind that these four groups – people who are under Medicaid insurance, between 55 and 60 years of age, African American, or who have higher disease stage – should be targeted for screening,” Ms. Aubry said.
AT THE ASCO BREAST CANCER SYMPOSIUM