User login
Simple Falls, Slips Proving Treacherous for Obese
MILWAUKEE – The obesity epidemic in the United States is bringing Americans to their knees, literally.
A growing number of obese patients are presenting to the emergency department with low-energy (LE) knee dislocations (KDs) caused by slips and falls simply from standing or from a single step.
Despite the isolated nature of their injuries, this new cohort of LE patients stayed in the hospital just as long as multisystem trauma patients with KDs resulting from high-energy injuries like car or motorcycle collisions and more than twice as long as nonobese patients with traditional low-energy knee dislocations from sports injuries.
The reason?
Obese patients with low-energy KDs are more likely to have vascular and nerve injuries and to require open arterial procedures than are patients with high-energy trauma or nonobese patients with LE knee dislocations, Dr. Andrew Georgiadis explained at the annual meeting of the Midwestern Vascular Surgical Society.
He noted that knee dislocation involves progressive hyperextension of the knee, and that, at 30 degrees of hyperextension, the posterior knee capsule is rent, and at 50 degrees, the popliteal artery actually fails.
Dr. Georgiadis and his surgical colleagues have been studying this phenomenon at Detroit’s Henry Ford Hospital, where, over a 17-year period, the proportion of low-energy KDs in the obese has risen from 17% in 1995-2000 to 33% in 2001-2006 and now represents the majority (53%) of all KDs in the hospital.
Among 53 KD patients treated between January 1995 and April 2012, 28 had high-energy injuries and 25 had low-energy injuries, of which 18 were obese and 7 nonobese. Five of the obese patients had a BMI of at least 30 kg/m2 or less than 40 kg/m2, while the remaining 13 had a BMI of more than 40 kg/m2.
When compared with the high-energy and LE non-obese patients, LE obese patients were significantly more likely to have a vascular injury (33% vs. 9%), vascular repair (28% vs. 6%), and nerve injury (50% vs. 6%), said Dr. Georgiadis, an orthopedic surgery resident at Henry Ford.
These rates were even higher in the morbidly obese (BMI over 40 kg/m2) at 39%, 39%, and 54%.
Although there were only seven arterial repairs in the entire series, five of these patients had "massive BMIs" between 42-69 kg/m2 and "they all had seemingly innocuous trauma, they all had transected arteries, they all had a vein graft bypass, and all of them had some early complication related to their procedure," he said.
Those complications included wound breakdown, early return to the operating room for a fasciotomy, graft occlusion requiring early thrombectomy/revision, and rhabdomyolysis/limb loss in a patient with a prolonged diagnosis.
When asked by the audience why the grafts thrombosed early, Dr. Georgiadis replied, "I think technical difficulty is really at the heart of all these things. And remember, these are patients who are probably being diagnosed later than someone who is crushed between two buses, so there are a lot of factors combining in these cases."
LE obese patients stayed in the hospital for an average of 8.1 days, which was comparable with the 11.4 days in the high-energy KD patients, of whom only 43% had isolated injuries, and significantly longer than the 3.7 days for non-obese LE patients, he said.
Given the increasing prevalence of obese low-energy KD patients, "we should probably have heightened awareness of this injury, especially at tertiary care centers, to avoid the morbidity of neurovascular injury and the consequences of delayed recognition," Dr. Georgiadis said.
A quick glimpse at the literature suggests that the ever-expanding American is not the only one at risk of obesity-related KDs.
Four cases of knee dislocation were recently reported by Morriston Hospital in Swansea, Wales – all in morbidly obese women (BMI range of 35-41) who experienced a simple mechanical fall from standing. The four cases occurred over the course of 1 year, and all had multiple knee ligament rupture on MRI. One case also had peroneal nerve palsy, according to the article, entitled "Dislocation of the Knee: An Epidemic in Waiting?"
Dr. Georgiadis reported no conflicts of interest.
MILWAUKEE – The obesity epidemic in the United States is bringing Americans to their knees, literally.
A growing number of obese patients are presenting to the emergency department with low-energy (LE) knee dislocations (KDs) caused by slips and falls simply from standing or from a single step.
Despite the isolated nature of their injuries, this new cohort of LE patients stayed in the hospital just as long as multisystem trauma patients with KDs resulting from high-energy injuries like car or motorcycle collisions and more than twice as long as nonobese patients with traditional low-energy knee dislocations from sports injuries.
The reason?
Obese patients with low-energy KDs are more likely to have vascular and nerve injuries and to require open arterial procedures than are patients with high-energy trauma or nonobese patients with LE knee dislocations, Dr. Andrew Georgiadis explained at the annual meeting of the Midwestern Vascular Surgical Society.
He noted that knee dislocation involves progressive hyperextension of the knee, and that, at 30 degrees of hyperextension, the posterior knee capsule is rent, and at 50 degrees, the popliteal artery actually fails.
Dr. Georgiadis and his surgical colleagues have been studying this phenomenon at Detroit’s Henry Ford Hospital, where, over a 17-year period, the proportion of low-energy KDs in the obese has risen from 17% in 1995-2000 to 33% in 2001-2006 and now represents the majority (53%) of all KDs in the hospital.
Among 53 KD patients treated between January 1995 and April 2012, 28 had high-energy injuries and 25 had low-energy injuries, of which 18 were obese and 7 nonobese. Five of the obese patients had a BMI of at least 30 kg/m2 or less than 40 kg/m2, while the remaining 13 had a BMI of more than 40 kg/m2.
When compared with the high-energy and LE non-obese patients, LE obese patients were significantly more likely to have a vascular injury (33% vs. 9%), vascular repair (28% vs. 6%), and nerve injury (50% vs. 6%), said Dr. Georgiadis, an orthopedic surgery resident at Henry Ford.
These rates were even higher in the morbidly obese (BMI over 40 kg/m2) at 39%, 39%, and 54%.
Although there were only seven arterial repairs in the entire series, five of these patients had "massive BMIs" between 42-69 kg/m2 and "they all had seemingly innocuous trauma, they all had transected arteries, they all had a vein graft bypass, and all of them had some early complication related to their procedure," he said.
Those complications included wound breakdown, early return to the operating room for a fasciotomy, graft occlusion requiring early thrombectomy/revision, and rhabdomyolysis/limb loss in a patient with a prolonged diagnosis.
When asked by the audience why the grafts thrombosed early, Dr. Georgiadis replied, "I think technical difficulty is really at the heart of all these things. And remember, these are patients who are probably being diagnosed later than someone who is crushed between two buses, so there are a lot of factors combining in these cases."
LE obese patients stayed in the hospital for an average of 8.1 days, which was comparable with the 11.4 days in the high-energy KD patients, of whom only 43% had isolated injuries, and significantly longer than the 3.7 days for non-obese LE patients, he said.
Given the increasing prevalence of obese low-energy KD patients, "we should probably have heightened awareness of this injury, especially at tertiary care centers, to avoid the morbidity of neurovascular injury and the consequences of delayed recognition," Dr. Georgiadis said.
A quick glimpse at the literature suggests that the ever-expanding American is not the only one at risk of obesity-related KDs.
Four cases of knee dislocation were recently reported by Morriston Hospital in Swansea, Wales – all in morbidly obese women (BMI range of 35-41) who experienced a simple mechanical fall from standing. The four cases occurred over the course of 1 year, and all had multiple knee ligament rupture on MRI. One case also had peroneal nerve palsy, according to the article, entitled "Dislocation of the Knee: An Epidemic in Waiting?"
Dr. Georgiadis reported no conflicts of interest.
MILWAUKEE – The obesity epidemic in the United States is bringing Americans to their knees, literally.
A growing number of obese patients are presenting to the emergency department with low-energy (LE) knee dislocations (KDs) caused by slips and falls simply from standing or from a single step.
Despite the isolated nature of their injuries, this new cohort of LE patients stayed in the hospital just as long as multisystem trauma patients with KDs resulting from high-energy injuries like car or motorcycle collisions and more than twice as long as nonobese patients with traditional low-energy knee dislocations from sports injuries.
The reason?
Obese patients with low-energy KDs are more likely to have vascular and nerve injuries and to require open arterial procedures than are patients with high-energy trauma or nonobese patients with LE knee dislocations, Dr. Andrew Georgiadis explained at the annual meeting of the Midwestern Vascular Surgical Society.
He noted that knee dislocation involves progressive hyperextension of the knee, and that, at 30 degrees of hyperextension, the posterior knee capsule is rent, and at 50 degrees, the popliteal artery actually fails.
Dr. Georgiadis and his surgical colleagues have been studying this phenomenon at Detroit’s Henry Ford Hospital, where, over a 17-year period, the proportion of low-energy KDs in the obese has risen from 17% in 1995-2000 to 33% in 2001-2006 and now represents the majority (53%) of all KDs in the hospital.
Among 53 KD patients treated between January 1995 and April 2012, 28 had high-energy injuries and 25 had low-energy injuries, of which 18 were obese and 7 nonobese. Five of the obese patients had a BMI of at least 30 kg/m2 or less than 40 kg/m2, while the remaining 13 had a BMI of more than 40 kg/m2.
When compared with the high-energy and LE non-obese patients, LE obese patients were significantly more likely to have a vascular injury (33% vs. 9%), vascular repair (28% vs. 6%), and nerve injury (50% vs. 6%), said Dr. Georgiadis, an orthopedic surgery resident at Henry Ford.
These rates were even higher in the morbidly obese (BMI over 40 kg/m2) at 39%, 39%, and 54%.
Although there were only seven arterial repairs in the entire series, five of these patients had "massive BMIs" between 42-69 kg/m2 and "they all had seemingly innocuous trauma, they all had transected arteries, they all had a vein graft bypass, and all of them had some early complication related to their procedure," he said.
Those complications included wound breakdown, early return to the operating room for a fasciotomy, graft occlusion requiring early thrombectomy/revision, and rhabdomyolysis/limb loss in a patient with a prolonged diagnosis.
When asked by the audience why the grafts thrombosed early, Dr. Georgiadis replied, "I think technical difficulty is really at the heart of all these things. And remember, these are patients who are probably being diagnosed later than someone who is crushed between two buses, so there are a lot of factors combining in these cases."
LE obese patients stayed in the hospital for an average of 8.1 days, which was comparable with the 11.4 days in the high-energy KD patients, of whom only 43% had isolated injuries, and significantly longer than the 3.7 days for non-obese LE patients, he said.
Given the increasing prevalence of obese low-energy KD patients, "we should probably have heightened awareness of this injury, especially at tertiary care centers, to avoid the morbidity of neurovascular injury and the consequences of delayed recognition," Dr. Georgiadis said.
A quick glimpse at the literature suggests that the ever-expanding American is not the only one at risk of obesity-related KDs.
Four cases of knee dislocation were recently reported by Morriston Hospital in Swansea, Wales – all in morbidly obese women (BMI range of 35-41) who experienced a simple mechanical fall from standing. The four cases occurred over the course of 1 year, and all had multiple knee ligament rupture on MRI. One case also had peroneal nerve palsy, according to the article, entitled "Dislocation of the Knee: An Epidemic in Waiting?"
Dr. Georgiadis reported no conflicts of interest.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGICAL SOCIETY
Company for Crizotinib? New ALK Inhibitors Attack Lung Cancer
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
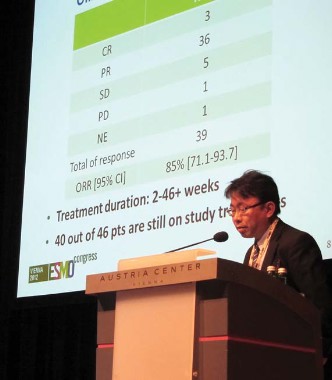
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
VIENNA – A wave of new anaplastic lymphoma kinase inhibitors and a second-generation heat shock protein 90 inhibitor are making headway as potential targeted treatments for non-small cell lung cancer.
Early reports on the experimental agents – none has a generic name as yet – suggest at least some may be active in patients not responding to crizotinib (Xalkori), a first-in-class anaplastic lymphoma kinase (ALK) inhibitor that is approved for ALK-driven non-small cell lung cancer (NSCLC) and is under investigation for other ALK-driven malignancies.
Some agents were also active in tumors with epidermal growth factor receptor (EGFR) mutations that were not responding to EGFR inhibitors.
CH5424802
Chugai Pharmaceutical’s selective ALK inhibitor CH5424802 produced an overall response rate of 85%, including 3 complete responses and 36 partial responses among 46 patients with advanced or metastatic ALK-positive NSCLC previously treated with one or more chemotherapies, but not with ALK inhibitors.
The oral agent has inhibitory activity against several kinases, including ALK L1196M and ALK C1156Y, two point mutations identified in patients resistant to the reigning ALK inhibitor crizotinib, Dr. Makoto Nishio reported during a developmental therapeutics session at the European Society for Medical Oncology Congress.
Twice-daily treatment at 300 mg exceeded 46 weeks in some patients, with 40 of the 46 patients still on therapy in the second phase of the phase I/II study, said Dr. Nishio, of the Cancer Institute Hospital, Japanese Foundation for Cancer Research in Tokyo.
Session cochair Dr. Sanjay Popat, of Royal Marsden Hospital in London, described the response waterfall plot as "really quite spectacular, with almost every patient having some evidence of benefit from this drug."
In addition, CH5424802 was very well tolerated, with the typical crizotinib-associated vision disturbances rare and gastrointestinal toxicity mild.
AP26113
A first-in-human, dose-finding study in advanced tumors provided preliminary evidence that Ariad Pharmaceuticals’ oral dual ALK-EGFR inhibitor AP26113 is active in ALK-positive as well as EGFR-mutant NSCLC.
Partial responses were achieved at doses of at least 60 mg/day in 6 of 9 evaluable crizotinib-resistant ALK-positive patients and in 2 of 2 crizotinib-naïve patients.
The longest response is 6 months in a crizotinib-resistant patient and 9 months in a crizotinib-naïve patient; both responses are ongoing, said Dr. Scott Gettinger, a thoracic oncologist with the Yale Medical Group in New Haven, Conn.
One ALK-positive crizotinib-resistant patient also showed a response in a preexisting brain metastasis.
Among 12 patients with EGFR-mutant lung cancer, one partial response that is ongoing occurred at 120 mg/day in a patient failed by prior erlotinib (Tarceva), bevacizumab (Avastin), and at least one round of chemotherapy.
Four of the 12 patients remain on study. Six have discontinued therapy due to progressive disease, 1 for non–drug-related adverse events and 1 due to sudden death that could be related to AP26113 and is being studied further, Dr. Gettinger said.
The most common adverse events of any grade were nausea (26%), diarrhea (18%), decreased appetite (12%), and vomiting (12%). Noticeably absent were visual disturbances and skin rash.
"What you don’t see on this list is rash, and ... this is very meaningful because this limits us when we treat patients with EGFR-mutant lung cancer with either erlotinib or gefitinib [Iressa]," he said.
Based on preclinical data, higher doses are expected for future EGFR cohorts in the ongoing study. The maximum tolerated dose (MTD) has not been identified.
LDK378
Dr. Alice Shaw provided the latest crizotinib news at the meeting, as well as further data from the first-in-human trial of Novartis’ selective oral ALK inhibitor LDK378 in advanced tumors, including crizotinib-refractory patients.
At doses of at least 400 mg/day, complete plus partial responses were reported in 41% of 59 NSCLC patients and in 47% of the 45 crizotinib-refractory NSCLC patients. When partial responses documented on only one occasion were also included, response rates rose to 71% and 80%, respectively.
More information is needed on the biology of these patients, particularly the crizotinib-refractory patients who failed to benefit from LDK378, to determine whether they might be better off being treated with a heat shock protein 90 (HSP90) inhibitor, Dr. Popat said.
Responses were only seen in NSCLC, said Dr. Shaw, a thoracic oncologist at Massachusetts General Hospital Cancer Center in Boston.
Since initial data were reported on 31 patients at this year’s American Society of Clinical Oncology meeting, the MTD has been identified as 750 mg/day.
LDK378 was well tolerated, with grade 3/4 reversible increased transaminases in 3% and diarrhea in 5% of patients. Grade 3/4 hyperglycemia in 10% merits further attention and likely reflects an off-target effect of LDK378, Dr. Popat said.
AUY922
Finally, a phase II study of Novartis/Vernalis’ second-generation HSP90 inhibitor AUY922 in previously treated stage IIIb or IV NSCLC showed responses in 50% of crizotinib-naïve and 32% of previously treated ALK-positive patients, Dr. Enriqueta Felip reported.
There was also a 26% response rate in patients with EGFR mutations who progressed following treatment with EGFR tyrosine kinase inhibitors.
"This compares relatively favorably with the second-generation EGFR inhibitors, specifically afatinib, which in the LUX-[Lung]1 study reported a radiologically confirmed 7% response rate," Dr. Popat observed.
As previously observed with HSP90 inhibitors, ocular toxicity was common, occurring in 74% across all grades and 7% at grade 3/4 among the 121 patients.
No new safety signals were observed at the previously identified dose of 70 mg/m2 IV once weekly, said Dr. Felip, of Vall d’Hebron University Hospital in Barcelona.
Expansion of the EGFR mutation group is ongoing, and further studies are planned to confirm these efficacy signals, she said. AUY922 is in broad development, with phase I/II trials ongoing in breast cancer, gastric cancer, and multiple myeloma.
Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Response rates reached as high as 85% in patients with advanced tumors that were predominantly non-small cell lung cancer.
Data Source: Investigators reported on early phase I/II trials of novel ALK inhibitors and a second-generation heat shock protein 90 inhibitor.
Disclosures: Dr. Nishio and his coauthors reported research funding from the study sponsor, Chugai Pharmaceutical, as well as Pfizer. Dr. Gettinger reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Ariad Pharmaceuticals. Dr. Shaw reported an advisory role with Pfizer, Novartis, Chugai, Ariad, and Daiichi, and her coauthors reported ties including employment and stock ownership with study sponsor Novartis. Dr. Felip reported no conflicts; several coauthors reported financial ties including employment with the study sponsor Novartis. Dr. Popat reported no conflicts of interest.
Antisclerostin Therapy AMG 785 Scores Big in Osteoporosis Arena*
MINNEAPOLIS – The investigational antisclerostin antibody AMG 785 produced rapid increases in bone mineral density roughly 50% to 60% higher than standard drugs in postmenopausal women with low bone mineral density in a phase II trial.
At 1 year, the increase in spine bone mineral density (BMD) was 4% with alendronate (Fosamax), 7% with teriparatide (Forteo), and 11.3% with AMG 785 given in a subcutaneous dose of 210 mg/mo.
Increases in BMD followed a similar, but slightly less dramatic pattern, at the total hip (2% vs. 1.5% vs. 4.1%) and femoral neck (1% vs. 1% vs. 3.7%), Dr. Michael McClung reported at the annual meeting of the American Society for Bone and Mineral Research (ASMBR) The differences in BMD at all three sites significantly favored AMG 785 over either active comparator.
AMG 785 is thought to increase bone formation on quiescent surfaces by inhibiting sclerostin, a protein encoded by the SOST gene in osteocytes that downregulates osteoblast-mediated bone formation.
The phase II results are the first longer-term response data presented for an antisclerostin antibody and build on preclinical work, suggesting that these bone-building drugs increase bone formation without the increase in bone resorption seen with some osteoanabolic agents. Data were also presented at the meeting from two phase I studies of Eli Lilly’s antisclerostin antibody, blosozumab.
The drug will be useful for that small proportion of patients who have had substantial bone loss and destruction of the architecture and strength of their bone over time, Dr. McClung said in an interview.
"There are some patients who are truly in need of skeletal reconstruction, and this would be the strategy to do that," he said. "None of our other drugs have that potential.
"The idea of being able to rebuild the skeletal architecture, the skeletal mass, and the skeletal strength back toward, or even to, normal is a really exciting prospect."
The phase II trial randomly assigned 419 postmenopausal women with low bone mineral density to one of five doses of subcutaneous AMG 785 (70 mg monthly, 140 mg monthly, 210 mg monthly, 140 mg every 3 months, or 210 mg every 3 months) or placebo, and one of two open-label active comparators: 70 mg weekly oral alendronate or 20 mcg daily subcutaneous teriparatide.
The women had average lumbar spine, total hip, and femoral neck T scores of –2.3, -1.5, and –1.9, respectively, but did not have severe osteoporosis. Their average age was 67 years.
At 1 year, all doses of AMG 785 significantly increased BMD at the hip, spine, and femoral neck compared with placebo (P less than .005). A clear dose-response relationship was observed, both in terms of the total dose and dosing interval favoring the higher and monthly doses, said Dr. McClung, director of the Oregon Osteoporosis Center in Portland.
Serum bone turnover marker analyses revealed that all doses of AMG 785 increased PINP (procollagen type I N-terminal propeptide) and reduced CTX (C-telopeptide of type I collagen) from baseline by week 1. As expected, researchers observed decreases in both markers with alendronate and increases in both markers with teriparatide.
Although some have characterized antisclerostin antibodies as a game-changer in osteoporosis, Dr. McClung cautioned that the results are just the first step and said the study produced some surprises in that the very dramatic changes in bone makers occurred within a week of beginning therapy, but the effects on stimulating bone formation were transient and the values returned to baseline between 6 and 12 months, despite patients continuing on therapy.
"There’s lots we need to learn about this," he said, noting that the blunting of the bone response has not been observed in animals. "It seems unlikely that we’ll simply identify patients in need of skeletal restoration and add a sclerostin therapy and treat them until they don’t have osteoporosis anymore.
"Likely we’ll use sclerostin therapy for a relatively short time – 6 months, 12 months – followed by probably another drug, like an antiresorptive drug, and then attempt to take advantage of that first burst of anabolic activity again."
Still, it was hard to miss the buzz over this new therapeutic target, with 20 or so sclerostin abstracts at the meeting and the AMG 785 study winning the 2012 ASBMR Most Outstanding Clinical Abstract Award.
Early positive signals from the phase II trial also prompted Amgen to initiate a phase III randomized, alendronate-controlled trial in more than 5,000 postmenopausal women with osteoporosis to determine whether AMG 785 can prevent fractures, the Holy Grail in osteoporosis management.
The most common adverse event with AMG 785 in the phase II trial was injection site reaction (9.8%). No fatal adverse events were reported. The maximum tolerated dose has not been identified, with the monthly 210-mg dose to be carried forward into subsequent phase III trials, Dr. McClung said.
The trial was funded by Amgen and UCB Pharma. Dr. McClung reported financial relationships with Amgen, Lilly, Merck, Novartis, and Warner Chilcott.
CORRECTION 10/19/12: The headline for this story misstated the name of the investigational drug. The headline should read "Antisclerostin Therapy AMG 785 Scores Big in Osteoporosis Arena."
MINNEAPOLIS – The investigational antisclerostin antibody AMG 785 produced rapid increases in bone mineral density roughly 50% to 60% higher than standard drugs in postmenopausal women with low bone mineral density in a phase II trial.
At 1 year, the increase in spine bone mineral density (BMD) was 4% with alendronate (Fosamax), 7% with teriparatide (Forteo), and 11.3% with AMG 785 given in a subcutaneous dose of 210 mg/mo.
Increases in BMD followed a similar, but slightly less dramatic pattern, at the total hip (2% vs. 1.5% vs. 4.1%) and femoral neck (1% vs. 1% vs. 3.7%), Dr. Michael McClung reported at the annual meeting of the American Society for Bone and Mineral Research (ASMBR) The differences in BMD at all three sites significantly favored AMG 785 over either active comparator.
AMG 785 is thought to increase bone formation on quiescent surfaces by inhibiting sclerostin, a protein encoded by the SOST gene in osteocytes that downregulates osteoblast-mediated bone formation.
The phase II results are the first longer-term response data presented for an antisclerostin antibody and build on preclinical work, suggesting that these bone-building drugs increase bone formation without the increase in bone resorption seen with some osteoanabolic agents. Data were also presented at the meeting from two phase I studies of Eli Lilly’s antisclerostin antibody, blosozumab.
The drug will be useful for that small proportion of patients who have had substantial bone loss and destruction of the architecture and strength of their bone over time, Dr. McClung said in an interview.
"There are some patients who are truly in need of skeletal reconstruction, and this would be the strategy to do that," he said. "None of our other drugs have that potential.
"The idea of being able to rebuild the skeletal architecture, the skeletal mass, and the skeletal strength back toward, or even to, normal is a really exciting prospect."
The phase II trial randomly assigned 419 postmenopausal women with low bone mineral density to one of five doses of subcutaneous AMG 785 (70 mg monthly, 140 mg monthly, 210 mg monthly, 140 mg every 3 months, or 210 mg every 3 months) or placebo, and one of two open-label active comparators: 70 mg weekly oral alendronate or 20 mcg daily subcutaneous teriparatide.
The women had average lumbar spine, total hip, and femoral neck T scores of –2.3, -1.5, and –1.9, respectively, but did not have severe osteoporosis. Their average age was 67 years.
At 1 year, all doses of AMG 785 significantly increased BMD at the hip, spine, and femoral neck compared with placebo (P less than .005). A clear dose-response relationship was observed, both in terms of the total dose and dosing interval favoring the higher and monthly doses, said Dr. McClung, director of the Oregon Osteoporosis Center in Portland.
Serum bone turnover marker analyses revealed that all doses of AMG 785 increased PINP (procollagen type I N-terminal propeptide) and reduced CTX (C-telopeptide of type I collagen) from baseline by week 1. As expected, researchers observed decreases in both markers with alendronate and increases in both markers with teriparatide.
Although some have characterized antisclerostin antibodies as a game-changer in osteoporosis, Dr. McClung cautioned that the results are just the first step and said the study produced some surprises in that the very dramatic changes in bone makers occurred within a week of beginning therapy, but the effects on stimulating bone formation were transient and the values returned to baseline between 6 and 12 months, despite patients continuing on therapy.
"There’s lots we need to learn about this," he said, noting that the blunting of the bone response has not been observed in animals. "It seems unlikely that we’ll simply identify patients in need of skeletal restoration and add a sclerostin therapy and treat them until they don’t have osteoporosis anymore.
"Likely we’ll use sclerostin therapy for a relatively short time – 6 months, 12 months – followed by probably another drug, like an antiresorptive drug, and then attempt to take advantage of that first burst of anabolic activity again."
Still, it was hard to miss the buzz over this new therapeutic target, with 20 or so sclerostin abstracts at the meeting and the AMG 785 study winning the 2012 ASBMR Most Outstanding Clinical Abstract Award.
Early positive signals from the phase II trial also prompted Amgen to initiate a phase III randomized, alendronate-controlled trial in more than 5,000 postmenopausal women with osteoporosis to determine whether AMG 785 can prevent fractures, the Holy Grail in osteoporosis management.
The most common adverse event with AMG 785 in the phase II trial was injection site reaction (9.8%). No fatal adverse events were reported. The maximum tolerated dose has not been identified, with the monthly 210-mg dose to be carried forward into subsequent phase III trials, Dr. McClung said.
The trial was funded by Amgen and UCB Pharma. Dr. McClung reported financial relationships with Amgen, Lilly, Merck, Novartis, and Warner Chilcott.
CORRECTION 10/19/12: The headline for this story misstated the name of the investigational drug. The headline should read "Antisclerostin Therapy AMG 785 Scores Big in Osteoporosis Arena."
MINNEAPOLIS – The investigational antisclerostin antibody AMG 785 produced rapid increases in bone mineral density roughly 50% to 60% higher than standard drugs in postmenopausal women with low bone mineral density in a phase II trial.
At 1 year, the increase in spine bone mineral density (BMD) was 4% with alendronate (Fosamax), 7% with teriparatide (Forteo), and 11.3% with AMG 785 given in a subcutaneous dose of 210 mg/mo.
Increases in BMD followed a similar, but slightly less dramatic pattern, at the total hip (2% vs. 1.5% vs. 4.1%) and femoral neck (1% vs. 1% vs. 3.7%), Dr. Michael McClung reported at the annual meeting of the American Society for Bone and Mineral Research (ASMBR) The differences in BMD at all three sites significantly favored AMG 785 over either active comparator.
AMG 785 is thought to increase bone formation on quiescent surfaces by inhibiting sclerostin, a protein encoded by the SOST gene in osteocytes that downregulates osteoblast-mediated bone formation.
The phase II results are the first longer-term response data presented for an antisclerostin antibody and build on preclinical work, suggesting that these bone-building drugs increase bone formation without the increase in bone resorption seen with some osteoanabolic agents. Data were also presented at the meeting from two phase I studies of Eli Lilly’s antisclerostin antibody, blosozumab.
The drug will be useful for that small proportion of patients who have had substantial bone loss and destruction of the architecture and strength of their bone over time, Dr. McClung said in an interview.
"There are some patients who are truly in need of skeletal reconstruction, and this would be the strategy to do that," he said. "None of our other drugs have that potential.
"The idea of being able to rebuild the skeletal architecture, the skeletal mass, and the skeletal strength back toward, or even to, normal is a really exciting prospect."
The phase II trial randomly assigned 419 postmenopausal women with low bone mineral density to one of five doses of subcutaneous AMG 785 (70 mg monthly, 140 mg monthly, 210 mg monthly, 140 mg every 3 months, or 210 mg every 3 months) or placebo, and one of two open-label active comparators: 70 mg weekly oral alendronate or 20 mcg daily subcutaneous teriparatide.
The women had average lumbar spine, total hip, and femoral neck T scores of –2.3, -1.5, and –1.9, respectively, but did not have severe osteoporosis. Their average age was 67 years.
At 1 year, all doses of AMG 785 significantly increased BMD at the hip, spine, and femoral neck compared with placebo (P less than .005). A clear dose-response relationship was observed, both in terms of the total dose and dosing interval favoring the higher and monthly doses, said Dr. McClung, director of the Oregon Osteoporosis Center in Portland.
Serum bone turnover marker analyses revealed that all doses of AMG 785 increased PINP (procollagen type I N-terminal propeptide) and reduced CTX (C-telopeptide of type I collagen) from baseline by week 1. As expected, researchers observed decreases in both markers with alendronate and increases in both markers with teriparatide.
Although some have characterized antisclerostin antibodies as a game-changer in osteoporosis, Dr. McClung cautioned that the results are just the first step and said the study produced some surprises in that the very dramatic changes in bone makers occurred within a week of beginning therapy, but the effects on stimulating bone formation were transient and the values returned to baseline between 6 and 12 months, despite patients continuing on therapy.
"There’s lots we need to learn about this," he said, noting that the blunting of the bone response has not been observed in animals. "It seems unlikely that we’ll simply identify patients in need of skeletal restoration and add a sclerostin therapy and treat them until they don’t have osteoporosis anymore.
"Likely we’ll use sclerostin therapy for a relatively short time – 6 months, 12 months – followed by probably another drug, like an antiresorptive drug, and then attempt to take advantage of that first burst of anabolic activity again."
Still, it was hard to miss the buzz over this new therapeutic target, with 20 or so sclerostin abstracts at the meeting and the AMG 785 study winning the 2012 ASBMR Most Outstanding Clinical Abstract Award.
Early positive signals from the phase II trial also prompted Amgen to initiate a phase III randomized, alendronate-controlled trial in more than 5,000 postmenopausal women with osteoporosis to determine whether AMG 785 can prevent fractures, the Holy Grail in osteoporosis management.
The most common adverse event with AMG 785 in the phase II trial was injection site reaction (9.8%). No fatal adverse events were reported. The maximum tolerated dose has not been identified, with the monthly 210-mg dose to be carried forward into subsequent phase III trials, Dr. McClung said.
The trial was funded by Amgen and UCB Pharma. Dr. McClung reported financial relationships with Amgen, Lilly, Merck, Novartis, and Warner Chilcott.
CORRECTION 10/19/12: The headline for this story misstated the name of the investigational drug. The headline should read "Antisclerostin Therapy AMG 785 Scores Big in Osteoporosis Arena."
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR BONE AND MINERAL RESEARCH
Major Finding: At 1 year, the increase in spine bone mineral density was 4% with alendronate, 7% with teriparatide, and 11.3% with AMG 785.
Data Source: The data come from a phase II trial involving 419 women with low bone mineral density.
Disclosures: The study was funded by Amgen and UCB Pharma. Dr. McClung reported financial relationships with Amgen, Lilly, Merck, Novartis, and Warner-Chilcott.
Doxorubicin Remains First-Line Standard for Metastatic Soft-Tissue Sarcomas
VIENNA – A more aggressive combination of doxorubicin and ifosfamide provided no significant overall survival advantage over single-agent doxorubicin as first-line chemotherapy in advanced soft-tissue sarcoma in a phase III trial.
The European Organisation for Research and Treatment of Cancer (EORTC) 62012 study’s primary end point of median overall survival reached 14.3 months with the combination and 12.8 months with doxorubicin alone (hazard ratio, 0.83; P = .076). One-year survival rates were 60% and 51%, respectively.
As previously observed, the combination of doxorubicin (Adriamycin) and ifosfamide (Ifex) increased response rates and progression-free survival, but at a cost of considerably more toxicity. "The standard treatment remains single-agent doxorubicin," Dr. Winette van der Graaf said during a Presidential Symposium at the European Society for Medical Oncology Congress.
Combination therapy, she added, can be considered if surgery for unresectable tumors or curative metastasectomy is foreseen, and may be an option for highly symptomatic patients without comorbidity, although the pros and cons should be discussed with the patient.
"The advantage of having the results of this study is that it’s easier now to have this discussion with the patient," said Dr. van der Graaf, of Radboud University Nijmegen (Netherlands) Medical Centre.
The EORTC Soft Tissue and Bone Sarcoma Group designed the trial to answer the long-standing question of whether single-agent doxorubicin or doxorubicin plus ifosfamide is the best first-line treatment for metastatic soft-tissue sarcomas. An earlier EORTC phase III trial (J. Clin. Oncol. 1995;13:1537-45) explored the combination at a lower dose of ifosfamide than is used today, with more recent phase II trials suggesting that a higher dose could increase response rates and progression-free survival.
EORTC 62012 investigators at 38 centers in nine countries randomly assigned 455 patients, aged 18-60 years, with locally advanced unresectable or metastatic soft-tissue sarcoma to receive doxorubicin 75 mg/m2 bolus on day 1 or as a 72-hour continuous IV infusion or doxorubicin 25 mg/m2 on days 1-3 plus ifosfamide 2.5 g/m2 on days 1-4 and pegfilgrastim 6 mg subcutaneous on day 5.
Patients were stratified by age, performance status (0 vs. 1), liver metastases, and histological grade. No prior chemotherapy was allowed for advanced disease.
After a median follow-up of 56 months, median progression-free survival increased significantly from 4.6 months with single-agent doxorubicin to 7.4 months with the addition of ifosfamide (HR 0.74, P = .003), Dr. van der Graaf reported on behalf of lead author Dr. Ian Judson of the Royal Marsden Hospital, London.
Subgroup analyses revealed significantly longer progression-free survival among patients aged 40-49 years, but this significance disappeared when all patients under age 50 were included in the analysis. No subgroups had significantly better overall survival.
The complete response rate was very low, at 0.4% in the doxorubicin arm and 1.8% in the combination arm. Remarkably, partial responses doubled with combination therapy from 13.2% to 24.7%, but overall response rates (13.6% vs. 26.5%) were far less than expected from phase II trials, where they ranged from 52% to 66%, she observed.
At first blush, the difference in progression-free survival with combination therapy may not appear that important, but from the perspective of patients with metastatic soft-tissue sarcoma, who typically have a median survival of only 1 year, this means that they’ll spend about 20% of their lifetime longer with disease control, said discussant Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology at the Dana-Farber Cancer Institute in Boston.
"The question for us as clinicians is can we target patients who truly would get more benefit from this kind of doubling of tumor response and control, despite the sizable toxicities of the more toxic combination chemotherapy," he said.
Dr. Demetri pointed out that significantly more patients discontinued combination therapy than single-agent doxorubicin due to toxicity (40 patients vs. 6 patients). The most common grade 3 or higher adverse events in the combination and doxorubicin arms were febrile neutropenia (46% vs. 13.5%), anemia (35% vs. 4.6%) and thrombocytopenia (33.5% vs. 0.4%).
Dr. Demetri cautioned that the results should not be blindly extrapolated to clinical practice because patients in the trial were younger and healthier than those typically seen in practice. However, the solid data can be used to make informed choices, he said.
If a patient is asymptomatic, as many metastatic sarcoma patients are, then single-agent doxorubicin, which he described as a strong, aggressive, "truck" of a therapy, "may be sufficient" and "more kind, more humane" to the patient, he said. If the patient is symptomatic or tumor shrinkage is required to improve quality of life because of tumor-related pain, "then perhaps combination therapy, although more toxic, may be more beneficial," he added.
The EORTC sponsored the trial. Dr. van der Graaf and Dr. Judson reported no conflicts of interest. Dr. Demetri reported financial relationships with several pharmaceutical companies.
VIENNA – A more aggressive combination of doxorubicin and ifosfamide provided no significant overall survival advantage over single-agent doxorubicin as first-line chemotherapy in advanced soft-tissue sarcoma in a phase III trial.
The European Organisation for Research and Treatment of Cancer (EORTC) 62012 study’s primary end point of median overall survival reached 14.3 months with the combination and 12.8 months with doxorubicin alone (hazard ratio, 0.83; P = .076). One-year survival rates were 60% and 51%, respectively.
As previously observed, the combination of doxorubicin (Adriamycin) and ifosfamide (Ifex) increased response rates and progression-free survival, but at a cost of considerably more toxicity. "The standard treatment remains single-agent doxorubicin," Dr. Winette van der Graaf said during a Presidential Symposium at the European Society for Medical Oncology Congress.
Combination therapy, she added, can be considered if surgery for unresectable tumors or curative metastasectomy is foreseen, and may be an option for highly symptomatic patients without comorbidity, although the pros and cons should be discussed with the patient.
"The advantage of having the results of this study is that it’s easier now to have this discussion with the patient," said Dr. van der Graaf, of Radboud University Nijmegen (Netherlands) Medical Centre.
The EORTC Soft Tissue and Bone Sarcoma Group designed the trial to answer the long-standing question of whether single-agent doxorubicin or doxorubicin plus ifosfamide is the best first-line treatment for metastatic soft-tissue sarcomas. An earlier EORTC phase III trial (J. Clin. Oncol. 1995;13:1537-45) explored the combination at a lower dose of ifosfamide than is used today, with more recent phase II trials suggesting that a higher dose could increase response rates and progression-free survival.
EORTC 62012 investigators at 38 centers in nine countries randomly assigned 455 patients, aged 18-60 years, with locally advanced unresectable or metastatic soft-tissue sarcoma to receive doxorubicin 75 mg/m2 bolus on day 1 or as a 72-hour continuous IV infusion or doxorubicin 25 mg/m2 on days 1-3 plus ifosfamide 2.5 g/m2 on days 1-4 and pegfilgrastim 6 mg subcutaneous on day 5.
Patients were stratified by age, performance status (0 vs. 1), liver metastases, and histological grade. No prior chemotherapy was allowed for advanced disease.
After a median follow-up of 56 months, median progression-free survival increased significantly from 4.6 months with single-agent doxorubicin to 7.4 months with the addition of ifosfamide (HR 0.74, P = .003), Dr. van der Graaf reported on behalf of lead author Dr. Ian Judson of the Royal Marsden Hospital, London.
Subgroup analyses revealed significantly longer progression-free survival among patients aged 40-49 years, but this significance disappeared when all patients under age 50 were included in the analysis. No subgroups had significantly better overall survival.
The complete response rate was very low, at 0.4% in the doxorubicin arm and 1.8% in the combination arm. Remarkably, partial responses doubled with combination therapy from 13.2% to 24.7%, but overall response rates (13.6% vs. 26.5%) were far less than expected from phase II trials, where they ranged from 52% to 66%, she observed.
At first blush, the difference in progression-free survival with combination therapy may not appear that important, but from the perspective of patients with metastatic soft-tissue sarcoma, who typically have a median survival of only 1 year, this means that they’ll spend about 20% of their lifetime longer with disease control, said discussant Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology at the Dana-Farber Cancer Institute in Boston.
"The question for us as clinicians is can we target patients who truly would get more benefit from this kind of doubling of tumor response and control, despite the sizable toxicities of the more toxic combination chemotherapy," he said.
Dr. Demetri pointed out that significantly more patients discontinued combination therapy than single-agent doxorubicin due to toxicity (40 patients vs. 6 patients). The most common grade 3 or higher adverse events in the combination and doxorubicin arms were febrile neutropenia (46% vs. 13.5%), anemia (35% vs. 4.6%) and thrombocytopenia (33.5% vs. 0.4%).
Dr. Demetri cautioned that the results should not be blindly extrapolated to clinical practice because patients in the trial were younger and healthier than those typically seen in practice. However, the solid data can be used to make informed choices, he said.
If a patient is asymptomatic, as many metastatic sarcoma patients are, then single-agent doxorubicin, which he described as a strong, aggressive, "truck" of a therapy, "may be sufficient" and "more kind, more humane" to the patient, he said. If the patient is symptomatic or tumor shrinkage is required to improve quality of life because of tumor-related pain, "then perhaps combination therapy, although more toxic, may be more beneficial," he added.
The EORTC sponsored the trial. Dr. van der Graaf and Dr. Judson reported no conflicts of interest. Dr. Demetri reported financial relationships with several pharmaceutical companies.
VIENNA – A more aggressive combination of doxorubicin and ifosfamide provided no significant overall survival advantage over single-agent doxorubicin as first-line chemotherapy in advanced soft-tissue sarcoma in a phase III trial.
The European Organisation for Research and Treatment of Cancer (EORTC) 62012 study’s primary end point of median overall survival reached 14.3 months with the combination and 12.8 months with doxorubicin alone (hazard ratio, 0.83; P = .076). One-year survival rates were 60% and 51%, respectively.
As previously observed, the combination of doxorubicin (Adriamycin) and ifosfamide (Ifex) increased response rates and progression-free survival, but at a cost of considerably more toxicity. "The standard treatment remains single-agent doxorubicin," Dr. Winette van der Graaf said during a Presidential Symposium at the European Society for Medical Oncology Congress.
Combination therapy, she added, can be considered if surgery for unresectable tumors or curative metastasectomy is foreseen, and may be an option for highly symptomatic patients without comorbidity, although the pros and cons should be discussed with the patient.
"The advantage of having the results of this study is that it’s easier now to have this discussion with the patient," said Dr. van der Graaf, of Radboud University Nijmegen (Netherlands) Medical Centre.
The EORTC Soft Tissue and Bone Sarcoma Group designed the trial to answer the long-standing question of whether single-agent doxorubicin or doxorubicin plus ifosfamide is the best first-line treatment for metastatic soft-tissue sarcomas. An earlier EORTC phase III trial (J. Clin. Oncol. 1995;13:1537-45) explored the combination at a lower dose of ifosfamide than is used today, with more recent phase II trials suggesting that a higher dose could increase response rates and progression-free survival.
EORTC 62012 investigators at 38 centers in nine countries randomly assigned 455 patients, aged 18-60 years, with locally advanced unresectable or metastatic soft-tissue sarcoma to receive doxorubicin 75 mg/m2 bolus on day 1 or as a 72-hour continuous IV infusion or doxorubicin 25 mg/m2 on days 1-3 plus ifosfamide 2.5 g/m2 on days 1-4 and pegfilgrastim 6 mg subcutaneous on day 5.
Patients were stratified by age, performance status (0 vs. 1), liver metastases, and histological grade. No prior chemotherapy was allowed for advanced disease.
After a median follow-up of 56 months, median progression-free survival increased significantly from 4.6 months with single-agent doxorubicin to 7.4 months with the addition of ifosfamide (HR 0.74, P = .003), Dr. van der Graaf reported on behalf of lead author Dr. Ian Judson of the Royal Marsden Hospital, London.
Subgroup analyses revealed significantly longer progression-free survival among patients aged 40-49 years, but this significance disappeared when all patients under age 50 were included in the analysis. No subgroups had significantly better overall survival.
The complete response rate was very low, at 0.4% in the doxorubicin arm and 1.8% in the combination arm. Remarkably, partial responses doubled with combination therapy from 13.2% to 24.7%, but overall response rates (13.6% vs. 26.5%) were far less than expected from phase II trials, where they ranged from 52% to 66%, she observed.
At first blush, the difference in progression-free survival with combination therapy may not appear that important, but from the perspective of patients with metastatic soft-tissue sarcoma, who typically have a median survival of only 1 year, this means that they’ll spend about 20% of their lifetime longer with disease control, said discussant Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology at the Dana-Farber Cancer Institute in Boston.
"The question for us as clinicians is can we target patients who truly would get more benefit from this kind of doubling of tumor response and control, despite the sizable toxicities of the more toxic combination chemotherapy," he said.
Dr. Demetri pointed out that significantly more patients discontinued combination therapy than single-agent doxorubicin due to toxicity (40 patients vs. 6 patients). The most common grade 3 or higher adverse events in the combination and doxorubicin arms were febrile neutropenia (46% vs. 13.5%), anemia (35% vs. 4.6%) and thrombocytopenia (33.5% vs. 0.4%).
Dr. Demetri cautioned that the results should not be blindly extrapolated to clinical practice because patients in the trial were younger and healthier than those typically seen in practice. However, the solid data can be used to make informed choices, he said.
If a patient is asymptomatic, as many metastatic sarcoma patients are, then single-agent doxorubicin, which he described as a strong, aggressive, "truck" of a therapy, "may be sufficient" and "more kind, more humane" to the patient, he said. If the patient is symptomatic or tumor shrinkage is required to improve quality of life because of tumor-related pain, "then perhaps combination therapy, although more toxic, may be more beneficial," he added.
The EORTC sponsored the trial. Dr. van der Graaf and Dr. Judson reported no conflicts of interest. Dr. Demetri reported financial relationships with several pharmaceutical companies.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Pazopanib Edges Sunitinib as First-Line Kidney Cancer Therapy
VIENNA – The first head-to-head comparison of pazopanib and sunitinib is likely to reshape first-line treatment for renal cell carcinoma, long dominated by sunitinib.
The phase III COMPARZ trial shows similar potency for the two angiogenesis inhibitors, but pazopanib (Votrient) had a better tolerance and safety profile, Dr. Robert Motzer reported at the European Society for Medical Oncology (ESMO) Congress.
As previously observed in indirect comparisons, hand-foot syndrome, fatigue, and mucositis were more common with sunitinib (Sutent), while pazopanib had a higher incidence of liver abnormalities. The adverse events associated with sunitinib are the ones that really affect patients’ day-to-day living, remarked Dr. Motzer of Memorial Sloan–Kettering Cancer Center in New York.
This was expressed in patient-reported outcomes on four quality of life instruments, which favored pazopanib in all but 1 of 14 domains – the exception was the emotional domain of the FACT Kidney Symptom Index (FKSI-19), but this was not statistically significant.
"This trial tips the scale for the preferred treatment, in my opinion, for most patients with kidney cancer from sunitinib, which has been the reference standard, to pazopanib," he said during a press briefing.
COMPARZ (Comparing the Efficacy, Safety and Tolerability of Pazopanib vs. Sunitinib) randomized 1,110 treatment-naive patients with locally advanced or metastatic clear cell renal cell carcinoma (RCC) to pazopanib 800 mg administered daily on a continuous dosing schedule or sunitinib 50 mg given daily in a 6-week cycle of 4 weeks on, 2 weeks off.
Disease assessments were at weeks 6, 12, 18, 24, and then every 12 weeks. Patient-reported outcomes were measured at day 28 of every cycle.
The primary end point of median progression-free survival was 8.4 months for pazopanib and 9.5 months for sunitinib by independent central review (hazard ratio, 1.047), and 10.5 months vs. 10.2 months by the investigators (HR, 0.998). The upper bound of the 95% confidence interval for the hazard ratio was below 1.25, demonstrating the noninferiority of pazopanib to sunitinib, Dr. Motzer said.
The overall response rate (complete plus partial responses) was 31% for pazopanib and 25% for sunitinib (P = .03).
Median overall survival was 28.4 months vs. 29.3 months (HR, 0.908; P = .27), according to an interim analysis. The final overall survival data is anticipated in 2013, Dr. Motzer said.
Treatment-emergent hypertension, diarrhea, and nausea of any grade occurred equally in both arms. Patients given pazopanib had more hair-color changes (30% vs. 10%) and increased alanine transaminase (ALT) levels (31% vs. 18%), reflecting known increased liver toxicity, he said. In contrast, those given sunitinib experienced more fatigue (63% vs. 55%), hand-foot problems (50% vs. 29%), taste alterations (36% vs. 26%), and thrombocytopenia (34% vs. 10%).
Serious adverse events occurring in 3% or more of patients were increased ALT and increased aspartate aminotransferase (AST) in the pazopanib arm, and pyrexia and thrombocytopenia in the sunitinib arm. Fatal adverse events were reported in 2% and 3%, respectively.
Discussant Dr. Tim Eisen, who was a subinvestigator on the trial and is an oncology professor at the University of Cambridge, England, said clinicians need to consider two crucial things when selecting an RCC treatment: whether the two drugs are equally effective, and if so, which one is "softer going" on the patient.
The data show the two drugs are "equally effective" and that pazopanib "can be considered a first-line standard of care along with sunitinib," he said.
Pazopanib is "easing ahead," however, in terms of tolerance and toxicity.
"Pazopanib does score in terms of having fewer side effects that matter to patients," Dr. Eisen said. "Don’t forget these are maintenance agents taken for as long as they are controlling disease, and even low-grade toxicities can be highly significant in this group."
Fatigue, stomatitis, and hand-foot syndrome all trouble patients badly, whereas elevated ALT and AST don’t usually trouble patients, if managed properly.
On the other hand, he observed that patients can feel a lot better during the 2 weeks off sunitinib, although the disease can grow, "even grow significantly, in a small proportion of patients."
Dr. Eisen cautioned that the timing of the assessments is a consideration in COMPARZ because they would tend to favor pazopanib. "For my money, this doesn’t really matter very much for the progression-free and overall survival curves as they mature ... I would not be so generous about the quality of life assessments," he said.
Patients do not feel "too chipper" after 4 weeks of sunitinib, but feel much better during the 2-week washout. Thus, "If you are looking at day 28 of each cycle, you are timing the quality of life assessments to favor pazopanib," he said.
Dr. Eisen said the hazard ratio of up to 1.25 "does cover quite a few ... scenarios, but I think it is acceptable and provides adequate power for a group of patients with a relatively uncommon disease."
During his presentation, Dr. Motzer highlighted the PISCES study, showing that patients with metastatic RCC preferred pazopanib over sunitinib by a three-to-one margin and had fewer dose reductions and interruptions.
In COMPARZ, dose reductions were reported in 44% of patients on pazopanib and 51% on sunitinib, and discontinuations because of adverse events in 24% and 19%.
Dr. Robin Wiltshire, global medical affairs lead for sunitinib for Pfizer Oncologysaid that, based on the trial design, it was possible to achieve a positive outcome, even if pazopanib had a 25% reduction in efficacy.
"Noninferiority is not the same as equal efficacy, and we’ve actually seen from the primary end point that the PFS is a month improved with sunitinib," he said in an interview.
In addition, progression-free survival has been even longer in real-world databases.
Dr. Wiltshire said COMPARZ is unlikely to cause a "major sea change in practice" and that physicians have not switched over en masse since pazopanib was approved in 2009. Physicians have treated more than 150,000 patients with sunitinib since its 2006 approval for RCC and have learned how to boost efficacy and manage its side effects.
The pharmaceutical research and advisory firm, Decision Resources, reports that angiogenesis inhibitors make up 76% of the RCC therapy market, with sunitinib accounting for more than half of sales in this class. It projects that sunitinib will account for only 14% of sales in this class by 2020, owing to high uptake of pazopanib and launch of the recently approved axitinib (Inlyta) and the investigational agent tivozanib – the first drug to break the 12-month PFS barrier in RCC.
GlaxoSmithKline sponsored the trial. Dr. Motzer and his coauthors report financial relationships with several firms, including Pfizer and GSK, the manufacturer of pazopanib. Dr. Eisen reported serving as a subinvestigator on the trial.
VIENNA – The first head-to-head comparison of pazopanib and sunitinib is likely to reshape first-line treatment for renal cell carcinoma, long dominated by sunitinib.
The phase III COMPARZ trial shows similar potency for the two angiogenesis inhibitors, but pazopanib (Votrient) had a better tolerance and safety profile, Dr. Robert Motzer reported at the European Society for Medical Oncology (ESMO) Congress.
As previously observed in indirect comparisons, hand-foot syndrome, fatigue, and mucositis were more common with sunitinib (Sutent), while pazopanib had a higher incidence of liver abnormalities. The adverse events associated with sunitinib are the ones that really affect patients’ day-to-day living, remarked Dr. Motzer of Memorial Sloan–Kettering Cancer Center in New York.
This was expressed in patient-reported outcomes on four quality of life instruments, which favored pazopanib in all but 1 of 14 domains – the exception was the emotional domain of the FACT Kidney Symptom Index (FKSI-19), but this was not statistically significant.
"This trial tips the scale for the preferred treatment, in my opinion, for most patients with kidney cancer from sunitinib, which has been the reference standard, to pazopanib," he said during a press briefing.
COMPARZ (Comparing the Efficacy, Safety and Tolerability of Pazopanib vs. Sunitinib) randomized 1,110 treatment-naive patients with locally advanced or metastatic clear cell renal cell carcinoma (RCC) to pazopanib 800 mg administered daily on a continuous dosing schedule or sunitinib 50 mg given daily in a 6-week cycle of 4 weeks on, 2 weeks off.
Disease assessments were at weeks 6, 12, 18, 24, and then every 12 weeks. Patient-reported outcomes were measured at day 28 of every cycle.
The primary end point of median progression-free survival was 8.4 months for pazopanib and 9.5 months for sunitinib by independent central review (hazard ratio, 1.047), and 10.5 months vs. 10.2 months by the investigators (HR, 0.998). The upper bound of the 95% confidence interval for the hazard ratio was below 1.25, demonstrating the noninferiority of pazopanib to sunitinib, Dr. Motzer said.
The overall response rate (complete plus partial responses) was 31% for pazopanib and 25% for sunitinib (P = .03).
Median overall survival was 28.4 months vs. 29.3 months (HR, 0.908; P = .27), according to an interim analysis. The final overall survival data is anticipated in 2013, Dr. Motzer said.
Treatment-emergent hypertension, diarrhea, and nausea of any grade occurred equally in both arms. Patients given pazopanib had more hair-color changes (30% vs. 10%) and increased alanine transaminase (ALT) levels (31% vs. 18%), reflecting known increased liver toxicity, he said. In contrast, those given sunitinib experienced more fatigue (63% vs. 55%), hand-foot problems (50% vs. 29%), taste alterations (36% vs. 26%), and thrombocytopenia (34% vs. 10%).
Serious adverse events occurring in 3% or more of patients were increased ALT and increased aspartate aminotransferase (AST) in the pazopanib arm, and pyrexia and thrombocytopenia in the sunitinib arm. Fatal adverse events were reported in 2% and 3%, respectively.
Discussant Dr. Tim Eisen, who was a subinvestigator on the trial and is an oncology professor at the University of Cambridge, England, said clinicians need to consider two crucial things when selecting an RCC treatment: whether the two drugs are equally effective, and if so, which one is "softer going" on the patient.
The data show the two drugs are "equally effective" and that pazopanib "can be considered a first-line standard of care along with sunitinib," he said.
Pazopanib is "easing ahead," however, in terms of tolerance and toxicity.
"Pazopanib does score in terms of having fewer side effects that matter to patients," Dr. Eisen said. "Don’t forget these are maintenance agents taken for as long as they are controlling disease, and even low-grade toxicities can be highly significant in this group."
Fatigue, stomatitis, and hand-foot syndrome all trouble patients badly, whereas elevated ALT and AST don’t usually trouble patients, if managed properly.
On the other hand, he observed that patients can feel a lot better during the 2 weeks off sunitinib, although the disease can grow, "even grow significantly, in a small proportion of patients."
Dr. Eisen cautioned that the timing of the assessments is a consideration in COMPARZ because they would tend to favor pazopanib. "For my money, this doesn’t really matter very much for the progression-free and overall survival curves as they mature ... I would not be so generous about the quality of life assessments," he said.
Patients do not feel "too chipper" after 4 weeks of sunitinib, but feel much better during the 2-week washout. Thus, "If you are looking at day 28 of each cycle, you are timing the quality of life assessments to favor pazopanib," he said.
Dr. Eisen said the hazard ratio of up to 1.25 "does cover quite a few ... scenarios, but I think it is acceptable and provides adequate power for a group of patients with a relatively uncommon disease."
During his presentation, Dr. Motzer highlighted the PISCES study, showing that patients with metastatic RCC preferred pazopanib over sunitinib by a three-to-one margin and had fewer dose reductions and interruptions.
In COMPARZ, dose reductions were reported in 44% of patients on pazopanib and 51% on sunitinib, and discontinuations because of adverse events in 24% and 19%.
Dr. Robin Wiltshire, global medical affairs lead for sunitinib for Pfizer Oncologysaid that, based on the trial design, it was possible to achieve a positive outcome, even if pazopanib had a 25% reduction in efficacy.
"Noninferiority is not the same as equal efficacy, and we’ve actually seen from the primary end point that the PFS is a month improved with sunitinib," he said in an interview.
In addition, progression-free survival has been even longer in real-world databases.
Dr. Wiltshire said COMPARZ is unlikely to cause a "major sea change in practice" and that physicians have not switched over en masse since pazopanib was approved in 2009. Physicians have treated more than 150,000 patients with sunitinib since its 2006 approval for RCC and have learned how to boost efficacy and manage its side effects.
The pharmaceutical research and advisory firm, Decision Resources, reports that angiogenesis inhibitors make up 76% of the RCC therapy market, with sunitinib accounting for more than half of sales in this class. It projects that sunitinib will account for only 14% of sales in this class by 2020, owing to high uptake of pazopanib and launch of the recently approved axitinib (Inlyta) and the investigational agent tivozanib – the first drug to break the 12-month PFS barrier in RCC.
GlaxoSmithKline sponsored the trial. Dr. Motzer and his coauthors report financial relationships with several firms, including Pfizer and GSK, the manufacturer of pazopanib. Dr. Eisen reported serving as a subinvestigator on the trial.
VIENNA – The first head-to-head comparison of pazopanib and sunitinib is likely to reshape first-line treatment for renal cell carcinoma, long dominated by sunitinib.
The phase III COMPARZ trial shows similar potency for the two angiogenesis inhibitors, but pazopanib (Votrient) had a better tolerance and safety profile, Dr. Robert Motzer reported at the European Society for Medical Oncology (ESMO) Congress.
As previously observed in indirect comparisons, hand-foot syndrome, fatigue, and mucositis were more common with sunitinib (Sutent), while pazopanib had a higher incidence of liver abnormalities. The adverse events associated with sunitinib are the ones that really affect patients’ day-to-day living, remarked Dr. Motzer of Memorial Sloan–Kettering Cancer Center in New York.
This was expressed in patient-reported outcomes on four quality of life instruments, which favored pazopanib in all but 1 of 14 domains – the exception was the emotional domain of the FACT Kidney Symptom Index (FKSI-19), but this was not statistically significant.
"This trial tips the scale for the preferred treatment, in my opinion, for most patients with kidney cancer from sunitinib, which has been the reference standard, to pazopanib," he said during a press briefing.
COMPARZ (Comparing the Efficacy, Safety and Tolerability of Pazopanib vs. Sunitinib) randomized 1,110 treatment-naive patients with locally advanced or metastatic clear cell renal cell carcinoma (RCC) to pazopanib 800 mg administered daily on a continuous dosing schedule or sunitinib 50 mg given daily in a 6-week cycle of 4 weeks on, 2 weeks off.
Disease assessments were at weeks 6, 12, 18, 24, and then every 12 weeks. Patient-reported outcomes were measured at day 28 of every cycle.
The primary end point of median progression-free survival was 8.4 months for pazopanib and 9.5 months for sunitinib by independent central review (hazard ratio, 1.047), and 10.5 months vs. 10.2 months by the investigators (HR, 0.998). The upper bound of the 95% confidence interval for the hazard ratio was below 1.25, demonstrating the noninferiority of pazopanib to sunitinib, Dr. Motzer said.
The overall response rate (complete plus partial responses) was 31% for pazopanib and 25% for sunitinib (P = .03).
Median overall survival was 28.4 months vs. 29.3 months (HR, 0.908; P = .27), according to an interim analysis. The final overall survival data is anticipated in 2013, Dr. Motzer said.
Treatment-emergent hypertension, diarrhea, and nausea of any grade occurred equally in both arms. Patients given pazopanib had more hair-color changes (30% vs. 10%) and increased alanine transaminase (ALT) levels (31% vs. 18%), reflecting known increased liver toxicity, he said. In contrast, those given sunitinib experienced more fatigue (63% vs. 55%), hand-foot problems (50% vs. 29%), taste alterations (36% vs. 26%), and thrombocytopenia (34% vs. 10%).
Serious adverse events occurring in 3% or more of patients were increased ALT and increased aspartate aminotransferase (AST) in the pazopanib arm, and pyrexia and thrombocytopenia in the sunitinib arm. Fatal adverse events were reported in 2% and 3%, respectively.
Discussant Dr. Tim Eisen, who was a subinvestigator on the trial and is an oncology professor at the University of Cambridge, England, said clinicians need to consider two crucial things when selecting an RCC treatment: whether the two drugs are equally effective, and if so, which one is "softer going" on the patient.
The data show the two drugs are "equally effective" and that pazopanib "can be considered a first-line standard of care along with sunitinib," he said.
Pazopanib is "easing ahead," however, in terms of tolerance and toxicity.
"Pazopanib does score in terms of having fewer side effects that matter to patients," Dr. Eisen said. "Don’t forget these are maintenance agents taken for as long as they are controlling disease, and even low-grade toxicities can be highly significant in this group."
Fatigue, stomatitis, and hand-foot syndrome all trouble patients badly, whereas elevated ALT and AST don’t usually trouble patients, if managed properly.
On the other hand, he observed that patients can feel a lot better during the 2 weeks off sunitinib, although the disease can grow, "even grow significantly, in a small proportion of patients."
Dr. Eisen cautioned that the timing of the assessments is a consideration in COMPARZ because they would tend to favor pazopanib. "For my money, this doesn’t really matter very much for the progression-free and overall survival curves as they mature ... I would not be so generous about the quality of life assessments," he said.
Patients do not feel "too chipper" after 4 weeks of sunitinib, but feel much better during the 2-week washout. Thus, "If you are looking at day 28 of each cycle, you are timing the quality of life assessments to favor pazopanib," he said.
Dr. Eisen said the hazard ratio of up to 1.25 "does cover quite a few ... scenarios, but I think it is acceptable and provides adequate power for a group of patients with a relatively uncommon disease."
During his presentation, Dr. Motzer highlighted the PISCES study, showing that patients with metastatic RCC preferred pazopanib over sunitinib by a three-to-one margin and had fewer dose reductions and interruptions.
In COMPARZ, dose reductions were reported in 44% of patients on pazopanib and 51% on sunitinib, and discontinuations because of adverse events in 24% and 19%.
Dr. Robin Wiltshire, global medical affairs lead for sunitinib for Pfizer Oncologysaid that, based on the trial design, it was possible to achieve a positive outcome, even if pazopanib had a 25% reduction in efficacy.
"Noninferiority is not the same as equal efficacy, and we’ve actually seen from the primary end point that the PFS is a month improved with sunitinib," he said in an interview.
In addition, progression-free survival has been even longer in real-world databases.
Dr. Wiltshire said COMPARZ is unlikely to cause a "major sea change in practice" and that physicians have not switched over en masse since pazopanib was approved in 2009. Physicians have treated more than 150,000 patients with sunitinib since its 2006 approval for RCC and have learned how to boost efficacy and manage its side effects.
The pharmaceutical research and advisory firm, Decision Resources, reports that angiogenesis inhibitors make up 76% of the RCC therapy market, with sunitinib accounting for more than half of sales in this class. It projects that sunitinib will account for only 14% of sales in this class by 2020, owing to high uptake of pazopanib and launch of the recently approved axitinib (Inlyta) and the investigational agent tivozanib – the first drug to break the 12-month PFS barrier in RCC.
GlaxoSmithKline sponsored the trial. Dr. Motzer and his coauthors report financial relationships with several firms, including Pfizer and GSK, the manufacturer of pazopanib. Dr. Eisen reported serving as a subinvestigator on the trial.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Median progression-free survival was 8.4 months for pazopanib vs. 9.5 months for sunitinib by independent central review (hazard ratio, 1.047), and 10.5 months vs. 10.2 months by the investigators (HR, 0.998).
Data Source: Investigators analyzed a phase III trial of 1,110 patients with locally advanced or metastatic renal cell carcinoma.
Disclosures: GlaxoSmithKline sponsored the trial. Dr. Motzer and his coauthors report financial relationships with several firms, including Pfizer and GSK, the manufacturer of pazopanib. Dr. Eisen reported serving as a subinvestigator on the trial.
Heart Disease Confers Higher, but Not Insurmountable Risks in Pregnancy
Most women with heart disease can safely go through pregnancy, although striking differences in outcome were found by type of heart disease and between countries, a novel international registry shows.
Maternal mortality was 1%, but this was still 100 times higher than the mortality of 0.007% in the normal pregnant population. Seven of the 13 maternal deaths were due to cardiac events, three to thromboembolic events, and three to sepsis.
Maternal mortality was highest in women with cardiomyopathy at 2.4%, compared with 2.1% for those with valvular heart disease, 0.5% with congenital heart disease, and 0% for ischemic heart disease.
In addition, women with cardiomyopathy experienced higher rates of heart failure (24% vs. 18%, 8%, and 8%) and ventricular arrhythmias (11% vs. 0.6%, 1.6%, and 0%) than did those with valvular heart disease, congenital heart disease, and ischemic heart disease, cardiologist Dr. Jolien Roos-Hesselink and her associates reported (Eur. Heart J. 2012 [doi:10.1093/eurheartj/ehs270]).
Although cardiomyopathy is uncommon during pregnancy, it is difficult to manage in the context of left ventricular dysfunction or peripartum cardiomyopathy, with a high risk of an adverse outcome for both the mother and the baby, observed Dr. Roos-Hesselink of Erasmus Medical Center, Rotterdam, the Netherlands, and associates.
The analysis was based on 1,321 pregnant patients with structural or ischemic heart disease enrolled from 60 hospitals in 28 countries from 2008 to June 2011 in the European Registry on Pregnancy and Heart Disease, the world’s first registry to focus on this challenging clinical scenario.
The patients’ median age was 30 (range 16-53), median duration of pregnancy 38 weeks, 56% had one or more previous pregnancy, and 54% had undergone at least one prior cardiac intervention. About 50 patients were from the United States.
Most patients were New York Heart Association class I (70%), and only 0.3% were class IV. More women with cardiomyopathy were NYHA class III (8%), while those with ischemic heart disease were older, more likely to suffer from hypertension and diabetes, and more likely to be smokers.
Outcomes in women with congenital heart disease, the largest subgroup in the registry at 872 patients, were relatively good compared with other subgroups. The authors attributed this to the high rate of successful prior cardiac corrective surgery (66%), favorable NYHA class (76% class I and 21% class II) and low use of any medication (20%).
Even in this group, however, the rate of cesarean delivery was higher (38% vs. 23%) and mean birth weight was lower than in the background population (3,056 grams vs. 3,190 grams), Dr. Roos-Hesselink and associates noted.
Overall, 41% of the cohort had a cesarean delivery, with the highest rate in women with ischemic heart disease (60%) or cardiomyopathy (58%).
Cesarean delivery rates also varied widely between countries, with the highest rate reported in Italy, not the United States, she said in an interview.
"C-section rates are the product both of the background rates of a particular country and the numbers of women with severe heart disease, so that if the background rate was high and there were more women with severe heart disease in that group, then that country’s rates were higher," she explained.
During pregnancy, 338 patients (26%) were hospitalized, most (76%) only once. In all, 162 patients (12%) "had at least one period of heart failure during or after pregnancy, while this is rarely, if ever, seen in the general population," the investigators said.
Fetal mortality was significantly higher in the cohort than in the normal population (1.7% vs. 0.35%). In the cohort, 62% of the deaths were intrauterine fetal deaths with no further information, 21% were obviously due to the maternal condition, and 17% were because of structural fetal abnormalities. Neonatal death rates did not differ significantly between the cohort and the normal population (0.6% vs. 0.4%).
As for whether certain women with heart disease should avoid pregnancy, the data show there are certain groups of women who have a very high chance of experiencing an adverse outcome during pregnancy, but ultimately it is an individual’s choice whether to undergo a pregnancy, coauthor Dr. Mark R. Johnson, clinical chair in obstetrics at Imperial College, London, said in an interview.
The doctor’s job is to give advice about the risks," he said. One of the reasons the registry is so important is that "it gives us a more robust source to base our advice on."
Dr. Roos-Hesselink pointed out that guidelines published in Europe last year do consider some groups to be contraindicated for pregnancy including patients with pulmonary hypertension, patients with severe cyanosis, and patients with earlier peripartum cardiomyopathy and still diminished ventricular function.
"Some of these women do get pregnant against advice," she added. "Especially, we have seen this happen in Egypt," she said, noting that women who wanted a pregnancy for cultural reasons might not tell their husbands that they had heart defects.
Significant differences were found between developed and developing countries in maternal mortality (0.6% vs. 3.9%) and fetal death (0.9% vs. 6.5%), although the authors acknowledged that any between-country comparisons were "very fragile" because the size of the populations was "grossly unbalanced."
"Most women with adequate counseling and optimal care should not be discouraged and can go safely through pregnancy," the authors concluded.
In terms of counseling, Dr. Johnson said it’s important to give as accurate a picture as possible of the risks to which the woman would be exposing herself and her baby.
"In terms of management, the early recognition of a problem and its prompt management is really important, from things as simple as anemia or a urinary tract infection to the more severe situation of a cardiac arrhythmia or the development of heart failure," he said.
Dr. Roos-Hesselink said the standard at her clinic is to perform an echocardiogram and exercise test prepregnancy, with other tests such as magnetic resonance imaging done if needed. Symptomatic patients must be treated and a dilated aorta corrected prior to pregnancy.
"If the woman has a bad exercise capacity, this gives you an idea she will do badly during pregnancy," she added.
The study was supported by the European Society of Cardiology. The authors reported they have no conflicts of interest.
Most women with heart disease can safely go through pregnancy, although striking differences in outcome were found by type of heart disease and between countries, a novel international registry shows.
Maternal mortality was 1%, but this was still 100 times higher than the mortality of 0.007% in the normal pregnant population. Seven of the 13 maternal deaths were due to cardiac events, three to thromboembolic events, and three to sepsis.
Maternal mortality was highest in women with cardiomyopathy at 2.4%, compared with 2.1% for those with valvular heart disease, 0.5% with congenital heart disease, and 0% for ischemic heart disease.
In addition, women with cardiomyopathy experienced higher rates of heart failure (24% vs. 18%, 8%, and 8%) and ventricular arrhythmias (11% vs. 0.6%, 1.6%, and 0%) than did those with valvular heart disease, congenital heart disease, and ischemic heart disease, cardiologist Dr. Jolien Roos-Hesselink and her associates reported (Eur. Heart J. 2012 [doi:10.1093/eurheartj/ehs270]).
Although cardiomyopathy is uncommon during pregnancy, it is difficult to manage in the context of left ventricular dysfunction or peripartum cardiomyopathy, with a high risk of an adverse outcome for both the mother and the baby, observed Dr. Roos-Hesselink of Erasmus Medical Center, Rotterdam, the Netherlands, and associates.
The analysis was based on 1,321 pregnant patients with structural or ischemic heart disease enrolled from 60 hospitals in 28 countries from 2008 to June 2011 in the European Registry on Pregnancy and Heart Disease, the world’s first registry to focus on this challenging clinical scenario.
The patients’ median age was 30 (range 16-53), median duration of pregnancy 38 weeks, 56% had one or more previous pregnancy, and 54% had undergone at least one prior cardiac intervention. About 50 patients were from the United States.
Most patients were New York Heart Association class I (70%), and only 0.3% were class IV. More women with cardiomyopathy were NYHA class III (8%), while those with ischemic heart disease were older, more likely to suffer from hypertension and diabetes, and more likely to be smokers.
Outcomes in women with congenital heart disease, the largest subgroup in the registry at 872 patients, were relatively good compared with other subgroups. The authors attributed this to the high rate of successful prior cardiac corrective surgery (66%), favorable NYHA class (76% class I and 21% class II) and low use of any medication (20%).
Even in this group, however, the rate of cesarean delivery was higher (38% vs. 23%) and mean birth weight was lower than in the background population (3,056 grams vs. 3,190 grams), Dr. Roos-Hesselink and associates noted.
Overall, 41% of the cohort had a cesarean delivery, with the highest rate in women with ischemic heart disease (60%) or cardiomyopathy (58%).
Cesarean delivery rates also varied widely between countries, with the highest rate reported in Italy, not the United States, she said in an interview.
"C-section rates are the product both of the background rates of a particular country and the numbers of women with severe heart disease, so that if the background rate was high and there were more women with severe heart disease in that group, then that country’s rates were higher," she explained.
During pregnancy, 338 patients (26%) were hospitalized, most (76%) only once. In all, 162 patients (12%) "had at least one period of heart failure during or after pregnancy, while this is rarely, if ever, seen in the general population," the investigators said.
Fetal mortality was significantly higher in the cohort than in the normal population (1.7% vs. 0.35%). In the cohort, 62% of the deaths were intrauterine fetal deaths with no further information, 21% were obviously due to the maternal condition, and 17% were because of structural fetal abnormalities. Neonatal death rates did not differ significantly between the cohort and the normal population (0.6% vs. 0.4%).
As for whether certain women with heart disease should avoid pregnancy, the data show there are certain groups of women who have a very high chance of experiencing an adverse outcome during pregnancy, but ultimately it is an individual’s choice whether to undergo a pregnancy, coauthor Dr. Mark R. Johnson, clinical chair in obstetrics at Imperial College, London, said in an interview.
The doctor’s job is to give advice about the risks," he said. One of the reasons the registry is so important is that "it gives us a more robust source to base our advice on."
Dr. Roos-Hesselink pointed out that guidelines published in Europe last year do consider some groups to be contraindicated for pregnancy including patients with pulmonary hypertension, patients with severe cyanosis, and patients with earlier peripartum cardiomyopathy and still diminished ventricular function.
"Some of these women do get pregnant against advice," she added. "Especially, we have seen this happen in Egypt," she said, noting that women who wanted a pregnancy for cultural reasons might not tell their husbands that they had heart defects.
Significant differences were found between developed and developing countries in maternal mortality (0.6% vs. 3.9%) and fetal death (0.9% vs. 6.5%), although the authors acknowledged that any between-country comparisons were "very fragile" because the size of the populations was "grossly unbalanced."
"Most women with adequate counseling and optimal care should not be discouraged and can go safely through pregnancy," the authors concluded.
In terms of counseling, Dr. Johnson said it’s important to give as accurate a picture as possible of the risks to which the woman would be exposing herself and her baby.
"In terms of management, the early recognition of a problem and its prompt management is really important, from things as simple as anemia or a urinary tract infection to the more severe situation of a cardiac arrhythmia or the development of heart failure," he said.
Dr. Roos-Hesselink said the standard at her clinic is to perform an echocardiogram and exercise test prepregnancy, with other tests such as magnetic resonance imaging done if needed. Symptomatic patients must be treated and a dilated aorta corrected prior to pregnancy.
"If the woman has a bad exercise capacity, this gives you an idea she will do badly during pregnancy," she added.
The study was supported by the European Society of Cardiology. The authors reported they have no conflicts of interest.
Most women with heart disease can safely go through pregnancy, although striking differences in outcome were found by type of heart disease and between countries, a novel international registry shows.
Maternal mortality was 1%, but this was still 100 times higher than the mortality of 0.007% in the normal pregnant population. Seven of the 13 maternal deaths were due to cardiac events, three to thromboembolic events, and three to sepsis.
Maternal mortality was highest in women with cardiomyopathy at 2.4%, compared with 2.1% for those with valvular heart disease, 0.5% with congenital heart disease, and 0% for ischemic heart disease.
In addition, women with cardiomyopathy experienced higher rates of heart failure (24% vs. 18%, 8%, and 8%) and ventricular arrhythmias (11% vs. 0.6%, 1.6%, and 0%) than did those with valvular heart disease, congenital heart disease, and ischemic heart disease, cardiologist Dr. Jolien Roos-Hesselink and her associates reported (Eur. Heart J. 2012 [doi:10.1093/eurheartj/ehs270]).
Although cardiomyopathy is uncommon during pregnancy, it is difficult to manage in the context of left ventricular dysfunction or peripartum cardiomyopathy, with a high risk of an adverse outcome for both the mother and the baby, observed Dr. Roos-Hesselink of Erasmus Medical Center, Rotterdam, the Netherlands, and associates.
The analysis was based on 1,321 pregnant patients with structural or ischemic heart disease enrolled from 60 hospitals in 28 countries from 2008 to June 2011 in the European Registry on Pregnancy and Heart Disease, the world’s first registry to focus on this challenging clinical scenario.
The patients’ median age was 30 (range 16-53), median duration of pregnancy 38 weeks, 56% had one or more previous pregnancy, and 54% had undergone at least one prior cardiac intervention. About 50 patients were from the United States.
Most patients were New York Heart Association class I (70%), and only 0.3% were class IV. More women with cardiomyopathy were NYHA class III (8%), while those with ischemic heart disease were older, more likely to suffer from hypertension and diabetes, and more likely to be smokers.
Outcomes in women with congenital heart disease, the largest subgroup in the registry at 872 patients, were relatively good compared with other subgroups. The authors attributed this to the high rate of successful prior cardiac corrective surgery (66%), favorable NYHA class (76% class I and 21% class II) and low use of any medication (20%).
Even in this group, however, the rate of cesarean delivery was higher (38% vs. 23%) and mean birth weight was lower than in the background population (3,056 grams vs. 3,190 grams), Dr. Roos-Hesselink and associates noted.
Overall, 41% of the cohort had a cesarean delivery, with the highest rate in women with ischemic heart disease (60%) or cardiomyopathy (58%).
Cesarean delivery rates also varied widely between countries, with the highest rate reported in Italy, not the United States, she said in an interview.
"C-section rates are the product both of the background rates of a particular country and the numbers of women with severe heart disease, so that if the background rate was high and there were more women with severe heart disease in that group, then that country’s rates were higher," she explained.
During pregnancy, 338 patients (26%) were hospitalized, most (76%) only once. In all, 162 patients (12%) "had at least one period of heart failure during or after pregnancy, while this is rarely, if ever, seen in the general population," the investigators said.
Fetal mortality was significantly higher in the cohort than in the normal population (1.7% vs. 0.35%). In the cohort, 62% of the deaths were intrauterine fetal deaths with no further information, 21% were obviously due to the maternal condition, and 17% were because of structural fetal abnormalities. Neonatal death rates did not differ significantly between the cohort and the normal population (0.6% vs. 0.4%).
As for whether certain women with heart disease should avoid pregnancy, the data show there are certain groups of women who have a very high chance of experiencing an adverse outcome during pregnancy, but ultimately it is an individual’s choice whether to undergo a pregnancy, coauthor Dr. Mark R. Johnson, clinical chair in obstetrics at Imperial College, London, said in an interview.
The doctor’s job is to give advice about the risks," he said. One of the reasons the registry is so important is that "it gives us a more robust source to base our advice on."
Dr. Roos-Hesselink pointed out that guidelines published in Europe last year do consider some groups to be contraindicated for pregnancy including patients with pulmonary hypertension, patients with severe cyanosis, and patients with earlier peripartum cardiomyopathy and still diminished ventricular function.
"Some of these women do get pregnant against advice," she added. "Especially, we have seen this happen in Egypt," she said, noting that women who wanted a pregnancy for cultural reasons might not tell their husbands that they had heart defects.
Significant differences were found between developed and developing countries in maternal mortality (0.6% vs. 3.9%) and fetal death (0.9% vs. 6.5%), although the authors acknowledged that any between-country comparisons were "very fragile" because the size of the populations was "grossly unbalanced."
"Most women with adequate counseling and optimal care should not be discouraged and can go safely through pregnancy," the authors concluded.
In terms of counseling, Dr. Johnson said it’s important to give as accurate a picture as possible of the risks to which the woman would be exposing herself and her baby.
"In terms of management, the early recognition of a problem and its prompt management is really important, from things as simple as anemia or a urinary tract infection to the more severe situation of a cardiac arrhythmia or the development of heart failure," he said.
Dr. Roos-Hesselink said the standard at her clinic is to perform an echocardiogram and exercise test prepregnancy, with other tests such as magnetic resonance imaging done if needed. Symptomatic patients must be treated and a dilated aorta corrected prior to pregnancy.
"If the woman has a bad exercise capacity, this gives you an idea she will do badly during pregnancy," she added.
The study was supported by the European Society of Cardiology. The authors reported they have no conflicts of interest.
FROM THE EUROPEAN HEART JOURNAL
Major Finding: Maternal death occurred in 1% of women with heart disease, vs. 0.007% in the normal population.
Data Source: The findings are from the ongoing European Registry on Pregnancy and Heart Disease.
Disclosures: The study was supported by the European Society of Cardiology. The authors reported they have no conflicts of interest.
Post-EVAR Survival for Women on Par with Men
MILWAUKEE – Although female gender is associated with a higher rate of complications, women did not have significantly lower long-term survival after endovascular abdominal aortic aneurysm repair in a review of the Mayo Clinic AAA Registry.
At 30 days, 24% of women experienced complications after EVAR, compared with 15% of men (P value = .003).
On the other hand, death at 30 days was similar (2.5% vs. 1.5%; P = .41), as was combined early or late death (hazard ratio 1.1 vs. 1.0; P = .36), Dr. Peter Gloviczki reported at the meeting.
He highlighted a recent prospective analysis from Albany (N.Y.) Medical College showing that women had significantly higher mortality than did men (3.2% vs. 0.96%, P less than .005) and more frequent colon ischemia, native arterial rupture, and type 1 endoleaks after elective EVAR. There were no gender differences, however, for any of these outcomes following elective open repair or emergency EVAR or surgery (Vasc. Surg. 2012;55:906-13. Epub 2012 Feb. 8).
In the Mayo Clinic analysis, urgent presentation, age over 70 years, and high comorbidity scores were all significantly associated with complications and higher mortality, said Dr. Gloviczki, president of the Society for Vascular Surgery (SVS) and chair emeritus vascular and endovascular surgery, Mayo Clinic, Rochester, Minn.
The retrospective analysis included 1,002 consecutive patients with abdominal aortic aneurysm (AAA) treated with EVAR at Mayo Clinic from January 1997 to June 30, 2011. Of these, 871 were male (87%) and 131 female (13%). The majority (919) of cases were elective (92%), 43 symptomatic (4%), and 40 ruptured AAA (4%). Patients’ average age was 76 years (range 51-99 years).
Thirty-day mortality was 1% in the elective group, compared with 2.3% in the symptomatic AAA group and 12.5% in the ruptured AAA group (both P less than .0001), he said.
In contrast to the Albany analysis, early mortality after elective repair was similar between men and women (0.75% vs. 2.61%; P = .09). This was further confirmed by multivariate analysis (hazard ratio for all-cause death 1.16; P = .40), despite an increased risk in women for complications (HR 1.67; P = .001) and reinterventions (HR 1.96; P = .002), Dr. Gloviczki said.
High-risk patients, defined by an SVS comorbidity score of more than 10, however, had significantly higher 30-day mortality after elective EVAR than did low-risk patients (2.33% vs. 0.18%; P = .004).
This was driven by a significantly higher rate of early complications in the high-risk group (19.3% vs. 11.4%), particularly myocardial infarction (1.6% vs. 0.18%) and acute renal failure requiring temporary dialysis (3.26% vs. 1.09%; P less than .05 for all), Dr. Gloviczki observed.
At an average follow-up of 3.2 years, overall survival was significantly higher in patients undergoing elective EVAR vs. symptomatic or ruptured repair (64% vs. 50% and 56%; P less than .001), and in low-risk vs. high-risk elective patients (72% vs. 51%; P less than .001).
Both 30-day mortality and complications significantly increased with age after elective repair, he said.
Overall, there were five late ruptures and nine late conversions, for a complication-free 5-year survival of 64% in the elective group.
Dr. Gloviczki noted that access-related difficulties are driving the higher early complication rate in women, but that other factors like age and comorbidities may be at play.
He noted that Mayo Clinic performed its first EVAR in 1996, and that today, 63% of patients with an aneurysm undergo endovascular repair.
When asked what’s changed in his patient selection and aneurysm size cutoff, Dr. Gloviczki said that in younger patients, surgeons may want to intervene earlier if the aneurysm appears likely to increase in size and is suitable for an endograft, but that overall, age alone should not drive patient selection.
"What this study showed me is that characterizing patients as high risk vs. low risk is important, in addition to age," he said. "As you could see, there was an increased mortality in age, but when we looked at high-risk and low-risk criteria, we only lost one patient in the low-risk group. So age alone does not put you into a high-risk category, it is your additional cardiac, pulmonary and renal disease that does."
Dr. Gloviczki and his coauthors reported no relevant conflicts of interest.
MILWAUKEE – Although female gender is associated with a higher rate of complications, women did not have significantly lower long-term survival after endovascular abdominal aortic aneurysm repair in a review of the Mayo Clinic AAA Registry.
At 30 days, 24% of women experienced complications after EVAR, compared with 15% of men (P value = .003).
On the other hand, death at 30 days was similar (2.5% vs. 1.5%; P = .41), as was combined early or late death (hazard ratio 1.1 vs. 1.0; P = .36), Dr. Peter Gloviczki reported at the meeting.
He highlighted a recent prospective analysis from Albany (N.Y.) Medical College showing that women had significantly higher mortality than did men (3.2% vs. 0.96%, P less than .005) and more frequent colon ischemia, native arterial rupture, and type 1 endoleaks after elective EVAR. There were no gender differences, however, for any of these outcomes following elective open repair or emergency EVAR or surgery (Vasc. Surg. 2012;55:906-13. Epub 2012 Feb. 8).
In the Mayo Clinic analysis, urgent presentation, age over 70 years, and high comorbidity scores were all significantly associated with complications and higher mortality, said Dr. Gloviczki, president of the Society for Vascular Surgery (SVS) and chair emeritus vascular and endovascular surgery, Mayo Clinic, Rochester, Minn.
The retrospective analysis included 1,002 consecutive patients with abdominal aortic aneurysm (AAA) treated with EVAR at Mayo Clinic from January 1997 to June 30, 2011. Of these, 871 were male (87%) and 131 female (13%). The majority (919) of cases were elective (92%), 43 symptomatic (4%), and 40 ruptured AAA (4%). Patients’ average age was 76 years (range 51-99 years).
Thirty-day mortality was 1% in the elective group, compared with 2.3% in the symptomatic AAA group and 12.5% in the ruptured AAA group (both P less than .0001), he said.
In contrast to the Albany analysis, early mortality after elective repair was similar between men and women (0.75% vs. 2.61%; P = .09). This was further confirmed by multivariate analysis (hazard ratio for all-cause death 1.16; P = .40), despite an increased risk in women for complications (HR 1.67; P = .001) and reinterventions (HR 1.96; P = .002), Dr. Gloviczki said.
High-risk patients, defined by an SVS comorbidity score of more than 10, however, had significantly higher 30-day mortality after elective EVAR than did low-risk patients (2.33% vs. 0.18%; P = .004).
This was driven by a significantly higher rate of early complications in the high-risk group (19.3% vs. 11.4%), particularly myocardial infarction (1.6% vs. 0.18%) and acute renal failure requiring temporary dialysis (3.26% vs. 1.09%; P less than .05 for all), Dr. Gloviczki observed.
At an average follow-up of 3.2 years, overall survival was significantly higher in patients undergoing elective EVAR vs. symptomatic or ruptured repair (64% vs. 50% and 56%; P less than .001), and in low-risk vs. high-risk elective patients (72% vs. 51%; P less than .001).
Both 30-day mortality and complications significantly increased with age after elective repair, he said.
Overall, there were five late ruptures and nine late conversions, for a complication-free 5-year survival of 64% in the elective group.
Dr. Gloviczki noted that access-related difficulties are driving the higher early complication rate in women, but that other factors like age and comorbidities may be at play.
He noted that Mayo Clinic performed its first EVAR in 1996, and that today, 63% of patients with an aneurysm undergo endovascular repair.
When asked what’s changed in his patient selection and aneurysm size cutoff, Dr. Gloviczki said that in younger patients, surgeons may want to intervene earlier if the aneurysm appears likely to increase in size and is suitable for an endograft, but that overall, age alone should not drive patient selection.
"What this study showed me is that characterizing patients as high risk vs. low risk is important, in addition to age," he said. "As you could see, there was an increased mortality in age, but when we looked at high-risk and low-risk criteria, we only lost one patient in the low-risk group. So age alone does not put you into a high-risk category, it is your additional cardiac, pulmonary and renal disease that does."
Dr. Gloviczki and his coauthors reported no relevant conflicts of interest.
MILWAUKEE – Although female gender is associated with a higher rate of complications, women did not have significantly lower long-term survival after endovascular abdominal aortic aneurysm repair in a review of the Mayo Clinic AAA Registry.
At 30 days, 24% of women experienced complications after EVAR, compared with 15% of men (P value = .003).
On the other hand, death at 30 days was similar (2.5% vs. 1.5%; P = .41), as was combined early or late death (hazard ratio 1.1 vs. 1.0; P = .36), Dr. Peter Gloviczki reported at the meeting.
He highlighted a recent prospective analysis from Albany (N.Y.) Medical College showing that women had significantly higher mortality than did men (3.2% vs. 0.96%, P less than .005) and more frequent colon ischemia, native arterial rupture, and type 1 endoleaks after elective EVAR. There were no gender differences, however, for any of these outcomes following elective open repair or emergency EVAR or surgery (Vasc. Surg. 2012;55:906-13. Epub 2012 Feb. 8).
In the Mayo Clinic analysis, urgent presentation, age over 70 years, and high comorbidity scores were all significantly associated with complications and higher mortality, said Dr. Gloviczki, president of the Society for Vascular Surgery (SVS) and chair emeritus vascular and endovascular surgery, Mayo Clinic, Rochester, Minn.
The retrospective analysis included 1,002 consecutive patients with abdominal aortic aneurysm (AAA) treated with EVAR at Mayo Clinic from January 1997 to June 30, 2011. Of these, 871 were male (87%) and 131 female (13%). The majority (919) of cases were elective (92%), 43 symptomatic (4%), and 40 ruptured AAA (4%). Patients’ average age was 76 years (range 51-99 years).
Thirty-day mortality was 1% in the elective group, compared with 2.3% in the symptomatic AAA group and 12.5% in the ruptured AAA group (both P less than .0001), he said.
In contrast to the Albany analysis, early mortality after elective repair was similar between men and women (0.75% vs. 2.61%; P = .09). This was further confirmed by multivariate analysis (hazard ratio for all-cause death 1.16; P = .40), despite an increased risk in women for complications (HR 1.67; P = .001) and reinterventions (HR 1.96; P = .002), Dr. Gloviczki said.
High-risk patients, defined by an SVS comorbidity score of more than 10, however, had significantly higher 30-day mortality after elective EVAR than did low-risk patients (2.33% vs. 0.18%; P = .004).
This was driven by a significantly higher rate of early complications in the high-risk group (19.3% vs. 11.4%), particularly myocardial infarction (1.6% vs. 0.18%) and acute renal failure requiring temporary dialysis (3.26% vs. 1.09%; P less than .05 for all), Dr. Gloviczki observed.
At an average follow-up of 3.2 years, overall survival was significantly higher in patients undergoing elective EVAR vs. symptomatic or ruptured repair (64% vs. 50% and 56%; P less than .001), and in low-risk vs. high-risk elective patients (72% vs. 51%; P less than .001).
Both 30-day mortality and complications significantly increased with age after elective repair, he said.
Overall, there were five late ruptures and nine late conversions, for a complication-free 5-year survival of 64% in the elective group.
Dr. Gloviczki noted that access-related difficulties are driving the higher early complication rate in women, but that other factors like age and comorbidities may be at play.
He noted that Mayo Clinic performed its first EVAR in 1996, and that today, 63% of patients with an aneurysm undergo endovascular repair.
When asked what’s changed in his patient selection and aneurysm size cutoff, Dr. Gloviczki said that in younger patients, surgeons may want to intervene earlier if the aneurysm appears likely to increase in size and is suitable for an endograft, but that overall, age alone should not drive patient selection.
"What this study showed me is that characterizing patients as high risk vs. low risk is important, in addition to age," he said. "As you could see, there was an increased mortality in age, but when we looked at high-risk and low-risk criteria, we only lost one patient in the low-risk group. So age alone does not put you into a high-risk category, it is your additional cardiac, pulmonary and renal disease that does."
Dr. Gloviczki and his coauthors reported no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGICAL SOCIETY
Major Finding: Death rates were similar between women and men at 30 days (2.5% vs. 1.5%) as were rates for combined early or late death (hazard ratio 1.1 vs. 1.0).
Data Source: The study is a database review of 1,002 consecutive patients in the Mayo Clinic AAA Registry.
Disclosures: Dr. Gloviczki and his coauthors reported no relevant conflicts of interest.
General Surgery Residents Seeing Far Fewer Open Aortic Surgeries
MILWAUKEE – General surgery residents in a community-based residency program experienced a significant 49% decline in open aortic surgeries over the last decade, an analysis showed.
In 2000-2001, residents were exposed to 20-25 open aortic cases per year, but now get in on 8-15 cases per year, said Dr. Adam Rothermel, a third-year general surgery resident at Mount Carmel Hospital in Columbus, Ohio, where the analysis was conducted.
"Open aortic cases are difficult to find, and our residents, as a whole, would agree that we’re not coming out with good enough experience with these cases," he said at the annual meeting of the Midwestern Vascular Surgical Society.
The results reflect the exponential shift from open vascular surgery to endovascular procedures over the last decade, as well as the more recent implementation of the 80-hour resident work week.
The total number of carotid endarterectomy, infrainguinal bypass, and open aortic cases for the entire hospital decreased by 55%, 30%, and 71%, respectively, over the study period of 2000 to 2011.
Total resident cases over the same period were unchanged for carotid endarterectomy (77 vs. 84 cases), trended downward for infrainguinal bypass (62 vs. 52 cases), and were significantly lower for open aortic cases (43 vs. 8 cases) according to a review of resident case logs, Dr. Rothermel said.
He pointed out that a significant portion of vascular surgery in the United States is still performed by general surgeons, citing surveys showing that general surgeons performed 59% of the vascular procedures in the United States in 1985 (J. Vasc. Surg. 1987;6:611-21) and 49% in 1992 (J. Vasc. Surg. 1996:23:172-81).
Session moderator Dr. Jean E. Starr, medical director of endovascular services at Ohio State University Medical Center in Columbus, said the current results parallel what’s found nationally. She went on to ask what the findings imply for general surgery residents when they’ve finished training, and how this will reflect on patient practice in light of general surgeons performing half of vascular surgeries in the United States.
"When you get out of your general surgery training from a community based program and are expected then, going into say a rural center, to perform these operations, you have to give pause," Dr. Rothermel replied. "I don’t think I have a good way to fix the problem at this point, but I think we need to be aware of the trend."
Audience member Dr. Joseph Giglia, principal investigator for the laparoscopic aortic surgery program at the University of Cincinnati Medical Center, countered by asking whether the findings really matter given that open aortic cases are decreasing significantly across the country. He pointed out that the latest survey data were 20 years old, and submitted that general surgeons no longer perform 50% of vascular surgeries in the United States.
"I think these cases are important for our primary vascular residents to participate in," Dr. Giglia said. "I think there has to be a sea change, a real shift in the paradigm about who’s doing these cases and what we’re going to do in the future."
Dr. Rothermel agreed that another survey should be conducted to better reflect current practice trends.
If vascular surgeons are to pick up the bulk of the caseload, however, efforts to recruit medical students to the specialty may need to be enhanced. A recent survey of 338 medical students showed that 236 first- and second-year students had no clinical exposure to vascular surgery, while only 38 of the 102 third-year students had been exposed to vascular surgery after completing a general surgery rotation (Ann. Vasc. Surg. 2012 July 25 [doi:10.1016/j.avsg.2012.02.012]). Nearly half (49%) of first- and second-year students said that they would consider vascular surgery, however, with another 19% willing to do so if the length of training were reduced.
Dr. Rothermel and Dr. Starr reported no conflicts of interest.
MILWAUKEE – General surgery residents in a community-based residency program experienced a significant 49% decline in open aortic surgeries over the last decade, an analysis showed.
In 2000-2001, residents were exposed to 20-25 open aortic cases per year, but now get in on 8-15 cases per year, said Dr. Adam Rothermel, a third-year general surgery resident at Mount Carmel Hospital in Columbus, Ohio, where the analysis was conducted.
"Open aortic cases are difficult to find, and our residents, as a whole, would agree that we’re not coming out with good enough experience with these cases," he said at the annual meeting of the Midwestern Vascular Surgical Society.
The results reflect the exponential shift from open vascular surgery to endovascular procedures over the last decade, as well as the more recent implementation of the 80-hour resident work week.
The total number of carotid endarterectomy, infrainguinal bypass, and open aortic cases for the entire hospital decreased by 55%, 30%, and 71%, respectively, over the study period of 2000 to 2011.
Total resident cases over the same period were unchanged for carotid endarterectomy (77 vs. 84 cases), trended downward for infrainguinal bypass (62 vs. 52 cases), and were significantly lower for open aortic cases (43 vs. 8 cases) according to a review of resident case logs, Dr. Rothermel said.
He pointed out that a significant portion of vascular surgery in the United States is still performed by general surgeons, citing surveys showing that general surgeons performed 59% of the vascular procedures in the United States in 1985 (J. Vasc. Surg. 1987;6:611-21) and 49% in 1992 (J. Vasc. Surg. 1996:23:172-81).
Session moderator Dr. Jean E. Starr, medical director of endovascular services at Ohio State University Medical Center in Columbus, said the current results parallel what’s found nationally. She went on to ask what the findings imply for general surgery residents when they’ve finished training, and how this will reflect on patient practice in light of general surgeons performing half of vascular surgeries in the United States.
"When you get out of your general surgery training from a community based program and are expected then, going into say a rural center, to perform these operations, you have to give pause," Dr. Rothermel replied. "I don’t think I have a good way to fix the problem at this point, but I think we need to be aware of the trend."
Audience member Dr. Joseph Giglia, principal investigator for the laparoscopic aortic surgery program at the University of Cincinnati Medical Center, countered by asking whether the findings really matter given that open aortic cases are decreasing significantly across the country. He pointed out that the latest survey data were 20 years old, and submitted that general surgeons no longer perform 50% of vascular surgeries in the United States.
"I think these cases are important for our primary vascular residents to participate in," Dr. Giglia said. "I think there has to be a sea change, a real shift in the paradigm about who’s doing these cases and what we’re going to do in the future."
Dr. Rothermel agreed that another survey should be conducted to better reflect current practice trends.
If vascular surgeons are to pick up the bulk of the caseload, however, efforts to recruit medical students to the specialty may need to be enhanced. A recent survey of 338 medical students showed that 236 first- and second-year students had no clinical exposure to vascular surgery, while only 38 of the 102 third-year students had been exposed to vascular surgery after completing a general surgery rotation (Ann. Vasc. Surg. 2012 July 25 [doi:10.1016/j.avsg.2012.02.012]). Nearly half (49%) of first- and second-year students said that they would consider vascular surgery, however, with another 19% willing to do so if the length of training were reduced.
Dr. Rothermel and Dr. Starr reported no conflicts of interest.
MILWAUKEE – General surgery residents in a community-based residency program experienced a significant 49% decline in open aortic surgeries over the last decade, an analysis showed.
In 2000-2001, residents were exposed to 20-25 open aortic cases per year, but now get in on 8-15 cases per year, said Dr. Adam Rothermel, a third-year general surgery resident at Mount Carmel Hospital in Columbus, Ohio, where the analysis was conducted.
"Open aortic cases are difficult to find, and our residents, as a whole, would agree that we’re not coming out with good enough experience with these cases," he said at the annual meeting of the Midwestern Vascular Surgical Society.
The results reflect the exponential shift from open vascular surgery to endovascular procedures over the last decade, as well as the more recent implementation of the 80-hour resident work week.
The total number of carotid endarterectomy, infrainguinal bypass, and open aortic cases for the entire hospital decreased by 55%, 30%, and 71%, respectively, over the study period of 2000 to 2011.
Total resident cases over the same period were unchanged for carotid endarterectomy (77 vs. 84 cases), trended downward for infrainguinal bypass (62 vs. 52 cases), and were significantly lower for open aortic cases (43 vs. 8 cases) according to a review of resident case logs, Dr. Rothermel said.
He pointed out that a significant portion of vascular surgery in the United States is still performed by general surgeons, citing surveys showing that general surgeons performed 59% of the vascular procedures in the United States in 1985 (J. Vasc. Surg. 1987;6:611-21) and 49% in 1992 (J. Vasc. Surg. 1996:23:172-81).
Session moderator Dr. Jean E. Starr, medical director of endovascular services at Ohio State University Medical Center in Columbus, said the current results parallel what’s found nationally. She went on to ask what the findings imply for general surgery residents when they’ve finished training, and how this will reflect on patient practice in light of general surgeons performing half of vascular surgeries in the United States.
"When you get out of your general surgery training from a community based program and are expected then, going into say a rural center, to perform these operations, you have to give pause," Dr. Rothermel replied. "I don’t think I have a good way to fix the problem at this point, but I think we need to be aware of the trend."
Audience member Dr. Joseph Giglia, principal investigator for the laparoscopic aortic surgery program at the University of Cincinnati Medical Center, countered by asking whether the findings really matter given that open aortic cases are decreasing significantly across the country. He pointed out that the latest survey data were 20 years old, and submitted that general surgeons no longer perform 50% of vascular surgeries in the United States.
"I think these cases are important for our primary vascular residents to participate in," Dr. Giglia said. "I think there has to be a sea change, a real shift in the paradigm about who’s doing these cases and what we’re going to do in the future."
Dr. Rothermel agreed that another survey should be conducted to better reflect current practice trends.
If vascular surgeons are to pick up the bulk of the caseload, however, efforts to recruit medical students to the specialty may need to be enhanced. A recent survey of 338 medical students showed that 236 first- and second-year students had no clinical exposure to vascular surgery, while only 38 of the 102 third-year students had been exposed to vascular surgery after completing a general surgery rotation (Ann. Vasc. Surg. 2012 July 25 [doi:10.1016/j.avsg.2012.02.012]). Nearly half (49%) of first- and second-year students said that they would consider vascular surgery, however, with another 19% willing to do so if the length of training were reduced.
Dr. Rothermel and Dr. Starr reported no conflicts of interest.
AT THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGICAL SOCIETY
Major Finding: General surgery residents in a community-based program experienced a significant 49% decline in open aortic surgeries from 2000 to 2011.
Data Source: Review of all carotid endarterectomy, femoro-popliteal bypass, and open aortic surgeries performed at a community hospital and by residents from 2000 to 2011.
Disclosures: Dr. Rothermel and Dr. Starr reported no conflicts of interest.
Health Care Professionals Tank in Cancer Survey
VIENNA – Nearly one in five persons believes that their lifetime risk of cancer is nonmodifiable, a survey found.
"That’s clearly worrying," medical oncologist Dr. Derek Power said at the European Society for Medical Oncology Congress.
Equally disconcerting was the finding that 52% of survey respondents had a college degree and that 17% were health care professionals.
The 48-question, online survey was distributed to 748 people through the Irish Cancer Society.
The majority of the public (80%) and health care professionals (78%) were concerned about developing cancer, but only a small minority (20% and 10%) knew that cancer risk increases with age.
Smoking was ranked as the No. 1 risk factor for cancer by 87% of the cohort. Many overestimated the risk of cancer attributable to genetics, environment, and stress, however, and underestimated the cancer risks associated with age, obesity, and sunlight, said Dr. Power of Mercy University Hospital in Cork, Ireland.
In all, 47% of respondents thought cancer was caused by genetics, specifically family history genetics.
"That’s a huge overestimate," Dr. Power said at a press briefing. "Only about 20% of cancers are hereditary and significant numbers less than that are known to be caused by specific genes, about 5%-8%."
The World Health Organization estimates that about one-third of cancers are preventable by following a sensible diet, maintaining a normal body weight, and exercising, Dr. Power observed.
When the respondents were probed on specific aspects of diet, large knowledge gaps emerged. A significant proportion of respondents reported that "detox" diets (35%) and organic foods (61%) could reduce their cancer risk, but only 46% were aware that salt is a risk factor for cancer.
Vitamin and mineral supplements were also thought to be protective by 51% of the public and 54% of health care professionals, with less than half (40% and 28%) aware that red meat is a risk factor.
Despite years of evidence, only 27% of respondents were unaware that breast-feeding can help reduce breast cancer risk – a finding that has clear implications for any national health promotion strategy, Dr. Power said.
Similarly, only 42% of the public and 46% of health care professionals identified alcohol as cancer risk factor, despite its association with a number of cancers including esophageal, mouth, breast, and colorectal.
Interestingly, 63% of the public and 82% of health care professionals thought certain drinks were more dangerous than others, when in reality it is the quantity of alcohol consumed that is important, said Dr. Power. In addition, 39% of the respondents believed red wine was protective, although there is no data to support this.
Only 32% of the public and 41% of health care professionals were aware that obesity is a cancer risk factor, with 33% and 24%, respectively, unaware that the location of body fat is important.
When given a list of potential behaviors relevant to cancer risk, a stunning 48% of respondents thought that a blow to the breast could increase a woman’s risk of cancer, whereas 29% said wearing a tight bra could do so.
Other "cancer myths" about risks for which there is little supportive data were cell phone use (68%), aerosol use (71%), eating genetically modified foods (81%), and the effects of stress (94%).
"What we found is that a sizeable proportion of the population is misinformed about cancer risk," Dr. Power said.
One explanation for the poor knowledge of cancer risk factors, particularly in a cohort of so many college graduates and health care professionals, is that the cancer prevention message has been too narrow.
"A lot of national programs are very good at disseminating information on cardiovascular risk and diet through healthy eating and exercise, but for cancer prevention, what gets out there is really ‘Don’t smoke,’ " he said. "That’s the biggest thing the public was aware of, as you saw from our figures, but – in terms of lifestyle choices like obesity, healthy eating, physical exercise – that just doesn’t get out there amongst cancer prevention strategies."
Dr. Power said he hopes that the results of the survey will be used to highlight cancer risk misperceptions and that there will be more emphasis in national cancer prevention campaigns on lifestyle choices, particularly in light of the obesity epidemic and increasingly sedentary lifestyle of children.
He highlighted a recent report by the World Cancer Research Fund/American Institute for Cancer Research that grades the current evidence on the relationship between food, nutrition, and physical exercise for 17 cancer sites and offers 10 recommendations to reduce the risk of developing cancer (J. Fam. Health Care 2010;20:100-2).
As to whether the survey results were unique to Ireland, Dr. Power said the data are relatively consistent among Western countries, but that the perception of cancer risk does vary among regions. For example, HPV-associated cancers and environmental factors weigh heavily in Japan, whereas obesity is more of a concern in Western countries.
The cohort included 648 women and 100 men. Their average age was 37 years (range, 18-74 years). The investigators did not ascertain how many of the health care professionals were physicians.
Dr. Power reported no conflicts of interest.
VIENNA – Nearly one in five persons believes that their lifetime risk of cancer is nonmodifiable, a survey found.
"That’s clearly worrying," medical oncologist Dr. Derek Power said at the European Society for Medical Oncology Congress.
Equally disconcerting was the finding that 52% of survey respondents had a college degree and that 17% were health care professionals.
The 48-question, online survey was distributed to 748 people through the Irish Cancer Society.
The majority of the public (80%) and health care professionals (78%) were concerned about developing cancer, but only a small minority (20% and 10%) knew that cancer risk increases with age.
Smoking was ranked as the No. 1 risk factor for cancer by 87% of the cohort. Many overestimated the risk of cancer attributable to genetics, environment, and stress, however, and underestimated the cancer risks associated with age, obesity, and sunlight, said Dr. Power of Mercy University Hospital in Cork, Ireland.
In all, 47% of respondents thought cancer was caused by genetics, specifically family history genetics.
"That’s a huge overestimate," Dr. Power said at a press briefing. "Only about 20% of cancers are hereditary and significant numbers less than that are known to be caused by specific genes, about 5%-8%."
The World Health Organization estimates that about one-third of cancers are preventable by following a sensible diet, maintaining a normal body weight, and exercising, Dr. Power observed.
When the respondents were probed on specific aspects of diet, large knowledge gaps emerged. A significant proportion of respondents reported that "detox" diets (35%) and organic foods (61%) could reduce their cancer risk, but only 46% were aware that salt is a risk factor for cancer.
Vitamin and mineral supplements were also thought to be protective by 51% of the public and 54% of health care professionals, with less than half (40% and 28%) aware that red meat is a risk factor.
Despite years of evidence, only 27% of respondents were unaware that breast-feeding can help reduce breast cancer risk – a finding that has clear implications for any national health promotion strategy, Dr. Power said.
Similarly, only 42% of the public and 46% of health care professionals identified alcohol as cancer risk factor, despite its association with a number of cancers including esophageal, mouth, breast, and colorectal.
Interestingly, 63% of the public and 82% of health care professionals thought certain drinks were more dangerous than others, when in reality it is the quantity of alcohol consumed that is important, said Dr. Power. In addition, 39% of the respondents believed red wine was protective, although there is no data to support this.
Only 32% of the public and 41% of health care professionals were aware that obesity is a cancer risk factor, with 33% and 24%, respectively, unaware that the location of body fat is important.
When given a list of potential behaviors relevant to cancer risk, a stunning 48% of respondents thought that a blow to the breast could increase a woman’s risk of cancer, whereas 29% said wearing a tight bra could do so.
Other "cancer myths" about risks for which there is little supportive data were cell phone use (68%), aerosol use (71%), eating genetically modified foods (81%), and the effects of stress (94%).
"What we found is that a sizeable proportion of the population is misinformed about cancer risk," Dr. Power said.
One explanation for the poor knowledge of cancer risk factors, particularly in a cohort of so many college graduates and health care professionals, is that the cancer prevention message has been too narrow.
"A lot of national programs are very good at disseminating information on cardiovascular risk and diet through healthy eating and exercise, but for cancer prevention, what gets out there is really ‘Don’t smoke,’ " he said. "That’s the biggest thing the public was aware of, as you saw from our figures, but – in terms of lifestyle choices like obesity, healthy eating, physical exercise – that just doesn’t get out there amongst cancer prevention strategies."
Dr. Power said he hopes that the results of the survey will be used to highlight cancer risk misperceptions and that there will be more emphasis in national cancer prevention campaigns on lifestyle choices, particularly in light of the obesity epidemic and increasingly sedentary lifestyle of children.
He highlighted a recent report by the World Cancer Research Fund/American Institute for Cancer Research that grades the current evidence on the relationship between food, nutrition, and physical exercise for 17 cancer sites and offers 10 recommendations to reduce the risk of developing cancer (J. Fam. Health Care 2010;20:100-2).
As to whether the survey results were unique to Ireland, Dr. Power said the data are relatively consistent among Western countries, but that the perception of cancer risk does vary among regions. For example, HPV-associated cancers and environmental factors weigh heavily in Japan, whereas obesity is more of a concern in Western countries.
The cohort included 648 women and 100 men. Their average age was 37 years (range, 18-74 years). The investigators did not ascertain how many of the health care professionals were physicians.
Dr. Power reported no conflicts of interest.
VIENNA – Nearly one in five persons believes that their lifetime risk of cancer is nonmodifiable, a survey found.
"That’s clearly worrying," medical oncologist Dr. Derek Power said at the European Society for Medical Oncology Congress.
Equally disconcerting was the finding that 52% of survey respondents had a college degree and that 17% were health care professionals.
The 48-question, online survey was distributed to 748 people through the Irish Cancer Society.
The majority of the public (80%) and health care professionals (78%) were concerned about developing cancer, but only a small minority (20% and 10%) knew that cancer risk increases with age.
Smoking was ranked as the No. 1 risk factor for cancer by 87% of the cohort. Many overestimated the risk of cancer attributable to genetics, environment, and stress, however, and underestimated the cancer risks associated with age, obesity, and sunlight, said Dr. Power of Mercy University Hospital in Cork, Ireland.
In all, 47% of respondents thought cancer was caused by genetics, specifically family history genetics.
"That’s a huge overestimate," Dr. Power said at a press briefing. "Only about 20% of cancers are hereditary and significant numbers less than that are known to be caused by specific genes, about 5%-8%."
The World Health Organization estimates that about one-third of cancers are preventable by following a sensible diet, maintaining a normal body weight, and exercising, Dr. Power observed.
When the respondents were probed on specific aspects of diet, large knowledge gaps emerged. A significant proportion of respondents reported that "detox" diets (35%) and organic foods (61%) could reduce their cancer risk, but only 46% were aware that salt is a risk factor for cancer.
Vitamin and mineral supplements were also thought to be protective by 51% of the public and 54% of health care professionals, with less than half (40% and 28%) aware that red meat is a risk factor.
Despite years of evidence, only 27% of respondents were unaware that breast-feeding can help reduce breast cancer risk – a finding that has clear implications for any national health promotion strategy, Dr. Power said.
Similarly, only 42% of the public and 46% of health care professionals identified alcohol as cancer risk factor, despite its association with a number of cancers including esophageal, mouth, breast, and colorectal.
Interestingly, 63% of the public and 82% of health care professionals thought certain drinks were more dangerous than others, when in reality it is the quantity of alcohol consumed that is important, said Dr. Power. In addition, 39% of the respondents believed red wine was protective, although there is no data to support this.
Only 32% of the public and 41% of health care professionals were aware that obesity is a cancer risk factor, with 33% and 24%, respectively, unaware that the location of body fat is important.
When given a list of potential behaviors relevant to cancer risk, a stunning 48% of respondents thought that a blow to the breast could increase a woman’s risk of cancer, whereas 29% said wearing a tight bra could do so.
Other "cancer myths" about risks for which there is little supportive data were cell phone use (68%), aerosol use (71%), eating genetically modified foods (81%), and the effects of stress (94%).
"What we found is that a sizeable proportion of the population is misinformed about cancer risk," Dr. Power said.
One explanation for the poor knowledge of cancer risk factors, particularly in a cohort of so many college graduates and health care professionals, is that the cancer prevention message has been too narrow.
"A lot of national programs are very good at disseminating information on cardiovascular risk and diet through healthy eating and exercise, but for cancer prevention, what gets out there is really ‘Don’t smoke,’ " he said. "That’s the biggest thing the public was aware of, as you saw from our figures, but – in terms of lifestyle choices like obesity, healthy eating, physical exercise – that just doesn’t get out there amongst cancer prevention strategies."
Dr. Power said he hopes that the results of the survey will be used to highlight cancer risk misperceptions and that there will be more emphasis in national cancer prevention campaigns on lifestyle choices, particularly in light of the obesity epidemic and increasingly sedentary lifestyle of children.
He highlighted a recent report by the World Cancer Research Fund/American Institute for Cancer Research that grades the current evidence on the relationship between food, nutrition, and physical exercise for 17 cancer sites and offers 10 recommendations to reduce the risk of developing cancer (J. Fam. Health Care 2010;20:100-2).
As to whether the survey results were unique to Ireland, Dr. Power said the data are relatively consistent among Western countries, but that the perception of cancer risk does vary among regions. For example, HPV-associated cancers and environmental factors weigh heavily in Japan, whereas obesity is more of a concern in Western countries.
The cohort included 648 women and 100 men. Their average age was 37 years (range, 18-74 years). The investigators did not ascertain how many of the health care professionals were physicians.
Dr. Power reported no conflicts of interest.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Only 10% of health professionals and 20% of the public knew that cancer risk increases with age, but while 41% and 32% knew that obesity is a cancer risk factor, only 24% and 33% were aware that the location of body fat is important.
Data Source: Results were taken from an online survey of 748 persons, of which 17% were health care professionals.
Disclosures: Dr. Power reported no conflicts of interest.
Gefitinib Offers Palliative Care in Advanced Esophageal Cancer
VIENNA – Gefitinib failed to improve overall survival in advanced esophageal cancer but could prove beneficial in a setting in which palliative care is the standard of care.
Gefitinib (Iressa) significantly improved progression-free survival and provided some dramatic and durable responses in palliation in the first-ever randomized trial of second-line therapy for esophageal cancer, Dr. David Ferry said at the European Society for Medical Oncology Congress.
In addition, the Cancer Oesophagus Gefitinib (COG) trial demonstrated a striking effect of performance status on survival in 450 patients treated with the epidermal growth factor receptor (EGFR) inhibitor.
Median overall survival increased significantly from 1.97 months in patients with a performance status of 2 to 3.93 months and 6.03 months, respectively, in those with PS 1 and PS 0 (P value less than .0001; hazard ratios, 1.00, 1.40, 2.98, respectively).
"We can now look at performance status as a prognostic factor for patients with this disease," said Dr. Ferry, of New Cross Hospital, Wolverhampton, England.
The next step lies in the ongoing, companion translational research project, TRANS-COG, analyzing predictive biomarkers in more than 300 of the 450 patients’ biopsies in an effort to identify a molecularly defined subgroup of patients most likely to benefit from gefitinib.
"If we can identify a subgroup of patients – and I think we’re fairly optimistic we can – then we will move on to another trial where placebo will be the control arm and a different targeted agent will be the experimental arm," Dr. Ferry said at a press briefing.
He would not say what targeted agent that might be and added that there’s not sufficient benefit in an unselected population for clinicians to begin using gefitinib in their patients with advanced esophageal cancer, "no matter how tempted you might be."
More Study Details
COG was prompted by a round of promising single-agent phase II trials involving the EGFR inhibitors gefitinib and erlotinib reporting responses in the second-line setting for esophageal cancer. Roughly 50%-70% of esophageal cancers overexpress EGFR.
At the moment, there is no systemic therapy that has significantly altered the natural history of metastatic esophageal cancer progressing after chemotherapy.
Investigators at 51 centers in the United Kingdom evenly randomized 450 patients with metastatic esophageal cancers and type I/II junctional tumors to once-daily oral gefitinib 500 mg or placebo until progression. Patients with stable brain metastases who had received prior cranial irradiation were not excluded.
Three-fourths of patients had adenocarcinoma, 80% had esophageal involvement, and one-third had received at least two prior treatments. Performance status (PS) was balanced between the placebo and gefitinib arms, with 25% and 26% at PS 0, 55% and 52% at PS 1 and 20% and 22% at PS 2. They were a median age of 64 years.
Progression-free survival was 1.17 months in the placebo arm and 1.60 months in the gefitinib arm, which was statistically significant (P = .017; HR, 0.79), Dr. Ferry said.
Exploratory subgroup analyses showed that all patients, regardless of cytology, disease site, age, sex, and time since diagnosis, benefited equally.
This positive effect on progression did not translate into an overall survival benefit, with a median overall survival of 3.60 months for placebo and 3.73 months for gefitinib (P = .285; HR, 0.90).
At 4 weeks, patients receiving gefitinib reported significant improvements in difficulty swallowing (P = .004), but not in global health quality of life, dysphagia, or difficulty eating, the three other prespecified health-related quality of life outcomes.
Disease control rates at 8 weeks, however, significantly favored gefitinib over placebo (26% vs. 16%, P = .014), he said. True partial responses occurred in 3.1% vs. 0.4%, respectively.
Dr. Ferry presented images illustrating rapid and durable radiologic responses that were associated with palliation in patients with adenocarcinoma and squamous cancers. Patient receiving gefitinib experienced weight gain, increased appetite, and reduced chest wall pain, with responses lasting 18 months in one patient.
There were no new safety signals with gefitinib, although there was an excess of diarrhea and skin toxicity, he said.
Discussant Arnaud D. Roth of Geneva University Hospital told the audience it would be easy to write COG off as another boring, negative study in esophageal cancer but pointed out that deeper analyses of molecular and clinical factors turned the initially negative PETACC-3 trial in colon cancer into a success story.
"A study of this size is a fantastic opportunity to learn more about esophageal cancer biology," he said, remarking that gefitinib in esophageal cancer is "just the beginning of a spicy story," said Dr. Roth.
Cancer Research UK sponsored the trial. Dr. Ferry reported grant support, honoraria, and an advisory relationship with AstraZeneca in the development of gefitinib.
VIENNA – Gefitinib failed to improve overall survival in advanced esophageal cancer but could prove beneficial in a setting in which palliative care is the standard of care.
Gefitinib (Iressa) significantly improved progression-free survival and provided some dramatic and durable responses in palliation in the first-ever randomized trial of second-line therapy for esophageal cancer, Dr. David Ferry said at the European Society for Medical Oncology Congress.
In addition, the Cancer Oesophagus Gefitinib (COG) trial demonstrated a striking effect of performance status on survival in 450 patients treated with the epidermal growth factor receptor (EGFR) inhibitor.
Median overall survival increased significantly from 1.97 months in patients with a performance status of 2 to 3.93 months and 6.03 months, respectively, in those with PS 1 and PS 0 (P value less than .0001; hazard ratios, 1.00, 1.40, 2.98, respectively).
"We can now look at performance status as a prognostic factor for patients with this disease," said Dr. Ferry, of New Cross Hospital, Wolverhampton, England.
The next step lies in the ongoing, companion translational research project, TRANS-COG, analyzing predictive biomarkers in more than 300 of the 450 patients’ biopsies in an effort to identify a molecularly defined subgroup of patients most likely to benefit from gefitinib.
"If we can identify a subgroup of patients – and I think we’re fairly optimistic we can – then we will move on to another trial where placebo will be the control arm and a different targeted agent will be the experimental arm," Dr. Ferry said at a press briefing.
He would not say what targeted agent that might be and added that there’s not sufficient benefit in an unselected population for clinicians to begin using gefitinib in their patients with advanced esophageal cancer, "no matter how tempted you might be."
More Study Details
COG was prompted by a round of promising single-agent phase II trials involving the EGFR inhibitors gefitinib and erlotinib reporting responses in the second-line setting for esophageal cancer. Roughly 50%-70% of esophageal cancers overexpress EGFR.
At the moment, there is no systemic therapy that has significantly altered the natural history of metastatic esophageal cancer progressing after chemotherapy.
Investigators at 51 centers in the United Kingdom evenly randomized 450 patients with metastatic esophageal cancers and type I/II junctional tumors to once-daily oral gefitinib 500 mg or placebo until progression. Patients with stable brain metastases who had received prior cranial irradiation were not excluded.
Three-fourths of patients had adenocarcinoma, 80% had esophageal involvement, and one-third had received at least two prior treatments. Performance status (PS) was balanced between the placebo and gefitinib arms, with 25% and 26% at PS 0, 55% and 52% at PS 1 and 20% and 22% at PS 2. They were a median age of 64 years.
Progression-free survival was 1.17 months in the placebo arm and 1.60 months in the gefitinib arm, which was statistically significant (P = .017; HR, 0.79), Dr. Ferry said.
Exploratory subgroup analyses showed that all patients, regardless of cytology, disease site, age, sex, and time since diagnosis, benefited equally.
This positive effect on progression did not translate into an overall survival benefit, with a median overall survival of 3.60 months for placebo and 3.73 months for gefitinib (P = .285; HR, 0.90).
At 4 weeks, patients receiving gefitinib reported significant improvements in difficulty swallowing (P = .004), but not in global health quality of life, dysphagia, or difficulty eating, the three other prespecified health-related quality of life outcomes.
Disease control rates at 8 weeks, however, significantly favored gefitinib over placebo (26% vs. 16%, P = .014), he said. True partial responses occurred in 3.1% vs. 0.4%, respectively.
Dr. Ferry presented images illustrating rapid and durable radiologic responses that were associated with palliation in patients with adenocarcinoma and squamous cancers. Patient receiving gefitinib experienced weight gain, increased appetite, and reduced chest wall pain, with responses lasting 18 months in one patient.
There were no new safety signals with gefitinib, although there was an excess of diarrhea and skin toxicity, he said.
Discussant Arnaud D. Roth of Geneva University Hospital told the audience it would be easy to write COG off as another boring, negative study in esophageal cancer but pointed out that deeper analyses of molecular and clinical factors turned the initially negative PETACC-3 trial in colon cancer into a success story.
"A study of this size is a fantastic opportunity to learn more about esophageal cancer biology," he said, remarking that gefitinib in esophageal cancer is "just the beginning of a spicy story," said Dr. Roth.
Cancer Research UK sponsored the trial. Dr. Ferry reported grant support, honoraria, and an advisory relationship with AstraZeneca in the development of gefitinib.
VIENNA – Gefitinib failed to improve overall survival in advanced esophageal cancer but could prove beneficial in a setting in which palliative care is the standard of care.
Gefitinib (Iressa) significantly improved progression-free survival and provided some dramatic and durable responses in palliation in the first-ever randomized trial of second-line therapy for esophageal cancer, Dr. David Ferry said at the European Society for Medical Oncology Congress.
In addition, the Cancer Oesophagus Gefitinib (COG) trial demonstrated a striking effect of performance status on survival in 450 patients treated with the epidermal growth factor receptor (EGFR) inhibitor.
Median overall survival increased significantly from 1.97 months in patients with a performance status of 2 to 3.93 months and 6.03 months, respectively, in those with PS 1 and PS 0 (P value less than .0001; hazard ratios, 1.00, 1.40, 2.98, respectively).
"We can now look at performance status as a prognostic factor for patients with this disease," said Dr. Ferry, of New Cross Hospital, Wolverhampton, England.
The next step lies in the ongoing, companion translational research project, TRANS-COG, analyzing predictive biomarkers in more than 300 of the 450 patients’ biopsies in an effort to identify a molecularly defined subgroup of patients most likely to benefit from gefitinib.
"If we can identify a subgroup of patients – and I think we’re fairly optimistic we can – then we will move on to another trial where placebo will be the control arm and a different targeted agent will be the experimental arm," Dr. Ferry said at a press briefing.
He would not say what targeted agent that might be and added that there’s not sufficient benefit in an unselected population for clinicians to begin using gefitinib in their patients with advanced esophageal cancer, "no matter how tempted you might be."
More Study Details
COG was prompted by a round of promising single-agent phase II trials involving the EGFR inhibitors gefitinib and erlotinib reporting responses in the second-line setting for esophageal cancer. Roughly 50%-70% of esophageal cancers overexpress EGFR.
At the moment, there is no systemic therapy that has significantly altered the natural history of metastatic esophageal cancer progressing after chemotherapy.
Investigators at 51 centers in the United Kingdom evenly randomized 450 patients with metastatic esophageal cancers and type I/II junctional tumors to once-daily oral gefitinib 500 mg or placebo until progression. Patients with stable brain metastases who had received prior cranial irradiation were not excluded.
Three-fourths of patients had adenocarcinoma, 80% had esophageal involvement, and one-third had received at least two prior treatments. Performance status (PS) was balanced between the placebo and gefitinib arms, with 25% and 26% at PS 0, 55% and 52% at PS 1 and 20% and 22% at PS 2. They were a median age of 64 years.
Progression-free survival was 1.17 months in the placebo arm and 1.60 months in the gefitinib arm, which was statistically significant (P = .017; HR, 0.79), Dr. Ferry said.
Exploratory subgroup analyses showed that all patients, regardless of cytology, disease site, age, sex, and time since diagnosis, benefited equally.
This positive effect on progression did not translate into an overall survival benefit, with a median overall survival of 3.60 months for placebo and 3.73 months for gefitinib (P = .285; HR, 0.90).
At 4 weeks, patients receiving gefitinib reported significant improvements in difficulty swallowing (P = .004), but not in global health quality of life, dysphagia, or difficulty eating, the three other prespecified health-related quality of life outcomes.
Disease control rates at 8 weeks, however, significantly favored gefitinib over placebo (26% vs. 16%, P = .014), he said. True partial responses occurred in 3.1% vs. 0.4%, respectively.
Dr. Ferry presented images illustrating rapid and durable radiologic responses that were associated with palliation in patients with adenocarcinoma and squamous cancers. Patient receiving gefitinib experienced weight gain, increased appetite, and reduced chest wall pain, with responses lasting 18 months in one patient.
There were no new safety signals with gefitinib, although there was an excess of diarrhea and skin toxicity, he said.
Discussant Arnaud D. Roth of Geneva University Hospital told the audience it would be easy to write COG off as another boring, negative study in esophageal cancer but pointed out that deeper analyses of molecular and clinical factors turned the initially negative PETACC-3 trial in colon cancer into a success story.
"A study of this size is a fantastic opportunity to learn more about esophageal cancer biology," he said, remarking that gefitinib in esophageal cancer is "just the beginning of a spicy story," said Dr. Roth.
Cancer Research UK sponsored the trial. Dr. Ferry reported grant support, honoraria, and an advisory relationship with AstraZeneca in the development of gefitinib.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Finding: Median overall survival was 3.73 months with gefitinib vs. 3.60 months with placebo (P = .285).
Data Source: Data are from a phase III randomized trial of gefitinib in patients with esophageal cancer progressing after chemotherapy.
Disclosures: Cancer Research U.K. sponsored the trial and AstraZeneca supplied gefitinib and the matched placebo. Dr. Ferry reported grant support, honoraria, and advising AstraZeneca in the development of gefitinib.