User login
Second-Cancer Signal Affirmed After Lenalidomide for Myeloma
SAN DIEGO – The risk of a secondary malignancy doubled in patients with newly diagnosed multiple myeloma treated with melphalan plus thalidomide or lenalidomide in a retrospective, pooled analysis of 2,283 patients.
Incidence rates per 100 persons per year of follow-up were 0.95 with high-dose melphalan (Alkeran) followed by lenalidomide (Revlimid) maintenance and 1.05 with melphalan and thalidomide. In comparison, rates were 0.40 with cyclophosphamide, lenalidomide, and dexamethasone and 0.42 with melphalan and no immunomodulatory drugs, Dr. Antonio Palumbo reported at the annual meeting of the American Society of Hematology (ASH).
At 4 years of follow-up, second cancers were diagnosed in 48 (2.1%) of the 2,283 patients enrolled in nine experimental trials of the European Myeloma Network. There was consistent evidence of an increase in late events over time.
"I do not want to underestimate the issue," Dr. Palumbo said. "There is a signal, but the first conclusion is caution. When you come to 48 cancers versus 2,200 patients, by chance many things may happen."
He noted that the risk of multiple myeloma progression is between 10 and 15 times higher than the diagnosis of a second cancer, and suggested that the emphasis on second cancers may be overshadowing the risk of death due to toxic effects and infections.
Of the 48 secondary cancers, 8 of the 10 hematologic malignancies and 8 of the 38 solid tumors were fatal. In contrast, there were 124 toxic deaths (8.6%) and 49 infective deaths (3.4%) among 1,435 patients given the combination of melphalan-prednisone-thalidomide or bortezomib (Velcade)-melphalan-prednisone, said Dr. Palumbo, chief of the myeloma unit at the University of Torino (Italy).
"We take it for granted that with chemo we have some toxic effects," he said in an interview. "We should increase our alert of our combinations, and not focus solely on the second cancers."
Session co-moderator Dr. Meral Beksac, with Ankara (Turkey) University, said the Italian data suggest caution and greater vigilance regarding routine cancer screenings among multiple myeloma patients, but would not change her treatment approach.
"Dr. Palumbo has shown very beautifully that the benefits you achieve in terms of the long-term myeloma effect outweigh the risk of secondary malignancies," she said in an interview. "Personally, I think we must plan to avoid alkylating agents when we now have these better agents."
Preliminary data from three trials showing a fourfold increase in secondary cancers in multiple myeloma patients treated with lenalidomide as maintenance therapy or in combination with melphalan prompted investigations into the safety of lenalidomide in the United States and Europe in 2011.
The European Medicines Agency concluded in September that the benefits of lenalidomide continue to outweigh the risks within the approved setting of relapsed multiple myeloma, but recommended that a warning be added on the risk of second cancers. The U.S. Food and Drug Administration review is ongoing, and includes the risk for thalidomide, since lenalidomide is an analogue of thalidomide.
Although the development of acute myeloid leukemia (AML) following multiple myeloma was observed decades ago, the underlying mechanisms remain unclear. Swedish researchers recently reported that the risk of AML and myelodysplastic syndromes is 11.5-fold higher in multiple myeloma patients than in the general population, even before the introduction of novel agents (Blood 2011;118:4086-92). In addition, the risk of AML/MDS was eightfold higher in patients with monoclonal gammopathy of undetermined significance (MGUS), even though none of the MGUS patients developed multiple myeloma, according to session co-moderator Dr. Sigurdur Y. Kristinsson, who was a coauthor of the Swedish study.
"Even those people that never develop the disease have an increased risk of AML and MDS, so it shows that it’s not only the treatment that we’re giving, but it’s also an inherent susceptibility," Dr. Kristinsson, with the Karolinska Hospital and Institute in Stockholm, said in an interview.
Work is ongoing to identify multiple myeloma patients at an increased risk of second cancers, thereby allowing clinicians to tailor therapy to reduce risks. A separate poster presentation at the ASH meeting reported that higher risk of second cancers was associated with older age, male sex, and radiation and/or surgery among roughly 29,250 multiple myeloma patients in the Surveillance, Epidemiology, and End Results (SEER) database.
Subgroup analysis of the pooled Italian data did not identify specific subgroups at greater risk, Dr. Palumbo said. The incidence rate was higher at 1.13 per 100 person-years for patients given melphalan-lenalidomide vs. 0.76 per 100 person-years for patients treated with autologous stem cell transplantation and lenalidomide (median age 68 years vs. 59 years, respectively).
Speaking on behalf of the investigators, Dr. Palumbo reported employment with, serving as a consultant and on the speakers bureau of, having equity ownership in, and receiving research funding, patent royalties, and honoraria from Celgene, maker of lenalidomide. Dr. Beksac reported honoraria and speakers bureau activity with Celgene and Janssen Cilag. Dr. Kristinsson reported no conflicts of interest.
SAN DIEGO – The risk of a secondary malignancy doubled in patients with newly diagnosed multiple myeloma treated with melphalan plus thalidomide or lenalidomide in a retrospective, pooled analysis of 2,283 patients.
Incidence rates per 100 persons per year of follow-up were 0.95 with high-dose melphalan (Alkeran) followed by lenalidomide (Revlimid) maintenance and 1.05 with melphalan and thalidomide. In comparison, rates were 0.40 with cyclophosphamide, lenalidomide, and dexamethasone and 0.42 with melphalan and no immunomodulatory drugs, Dr. Antonio Palumbo reported at the annual meeting of the American Society of Hematology (ASH).
At 4 years of follow-up, second cancers were diagnosed in 48 (2.1%) of the 2,283 patients enrolled in nine experimental trials of the European Myeloma Network. There was consistent evidence of an increase in late events over time.
"I do not want to underestimate the issue," Dr. Palumbo said. "There is a signal, but the first conclusion is caution. When you come to 48 cancers versus 2,200 patients, by chance many things may happen."
He noted that the risk of multiple myeloma progression is between 10 and 15 times higher than the diagnosis of a second cancer, and suggested that the emphasis on second cancers may be overshadowing the risk of death due to toxic effects and infections.
Of the 48 secondary cancers, 8 of the 10 hematologic malignancies and 8 of the 38 solid tumors were fatal. In contrast, there were 124 toxic deaths (8.6%) and 49 infective deaths (3.4%) among 1,435 patients given the combination of melphalan-prednisone-thalidomide or bortezomib (Velcade)-melphalan-prednisone, said Dr. Palumbo, chief of the myeloma unit at the University of Torino (Italy).
"We take it for granted that with chemo we have some toxic effects," he said in an interview. "We should increase our alert of our combinations, and not focus solely on the second cancers."
Session co-moderator Dr. Meral Beksac, with Ankara (Turkey) University, said the Italian data suggest caution and greater vigilance regarding routine cancer screenings among multiple myeloma patients, but would not change her treatment approach.
"Dr. Palumbo has shown very beautifully that the benefits you achieve in terms of the long-term myeloma effect outweigh the risk of secondary malignancies," she said in an interview. "Personally, I think we must plan to avoid alkylating agents when we now have these better agents."
Preliminary data from three trials showing a fourfold increase in secondary cancers in multiple myeloma patients treated with lenalidomide as maintenance therapy or in combination with melphalan prompted investigations into the safety of lenalidomide in the United States and Europe in 2011.
The European Medicines Agency concluded in September that the benefits of lenalidomide continue to outweigh the risks within the approved setting of relapsed multiple myeloma, but recommended that a warning be added on the risk of second cancers. The U.S. Food and Drug Administration review is ongoing, and includes the risk for thalidomide, since lenalidomide is an analogue of thalidomide.
Although the development of acute myeloid leukemia (AML) following multiple myeloma was observed decades ago, the underlying mechanisms remain unclear. Swedish researchers recently reported that the risk of AML and myelodysplastic syndromes is 11.5-fold higher in multiple myeloma patients than in the general population, even before the introduction of novel agents (Blood 2011;118:4086-92). In addition, the risk of AML/MDS was eightfold higher in patients with monoclonal gammopathy of undetermined significance (MGUS), even though none of the MGUS patients developed multiple myeloma, according to session co-moderator Dr. Sigurdur Y. Kristinsson, who was a coauthor of the Swedish study.
"Even those people that never develop the disease have an increased risk of AML and MDS, so it shows that it’s not only the treatment that we’re giving, but it’s also an inherent susceptibility," Dr. Kristinsson, with the Karolinska Hospital and Institute in Stockholm, said in an interview.
Work is ongoing to identify multiple myeloma patients at an increased risk of second cancers, thereby allowing clinicians to tailor therapy to reduce risks. A separate poster presentation at the ASH meeting reported that higher risk of second cancers was associated with older age, male sex, and radiation and/or surgery among roughly 29,250 multiple myeloma patients in the Surveillance, Epidemiology, and End Results (SEER) database.
Subgroup analysis of the pooled Italian data did not identify specific subgroups at greater risk, Dr. Palumbo said. The incidence rate was higher at 1.13 per 100 person-years for patients given melphalan-lenalidomide vs. 0.76 per 100 person-years for patients treated with autologous stem cell transplantation and lenalidomide (median age 68 years vs. 59 years, respectively).
Speaking on behalf of the investigators, Dr. Palumbo reported employment with, serving as a consultant and on the speakers bureau of, having equity ownership in, and receiving research funding, patent royalties, and honoraria from Celgene, maker of lenalidomide. Dr. Beksac reported honoraria and speakers bureau activity with Celgene and Janssen Cilag. Dr. Kristinsson reported no conflicts of interest.
SAN DIEGO – The risk of a secondary malignancy doubled in patients with newly diagnosed multiple myeloma treated with melphalan plus thalidomide or lenalidomide in a retrospective, pooled analysis of 2,283 patients.
Incidence rates per 100 persons per year of follow-up were 0.95 with high-dose melphalan (Alkeran) followed by lenalidomide (Revlimid) maintenance and 1.05 with melphalan and thalidomide. In comparison, rates were 0.40 with cyclophosphamide, lenalidomide, and dexamethasone and 0.42 with melphalan and no immunomodulatory drugs, Dr. Antonio Palumbo reported at the annual meeting of the American Society of Hematology (ASH).
At 4 years of follow-up, second cancers were diagnosed in 48 (2.1%) of the 2,283 patients enrolled in nine experimental trials of the European Myeloma Network. There was consistent evidence of an increase in late events over time.
"I do not want to underestimate the issue," Dr. Palumbo said. "There is a signal, but the first conclusion is caution. When you come to 48 cancers versus 2,200 patients, by chance many things may happen."
He noted that the risk of multiple myeloma progression is between 10 and 15 times higher than the diagnosis of a second cancer, and suggested that the emphasis on second cancers may be overshadowing the risk of death due to toxic effects and infections.
Of the 48 secondary cancers, 8 of the 10 hematologic malignancies and 8 of the 38 solid tumors were fatal. In contrast, there were 124 toxic deaths (8.6%) and 49 infective deaths (3.4%) among 1,435 patients given the combination of melphalan-prednisone-thalidomide or bortezomib (Velcade)-melphalan-prednisone, said Dr. Palumbo, chief of the myeloma unit at the University of Torino (Italy).
"We take it for granted that with chemo we have some toxic effects," he said in an interview. "We should increase our alert of our combinations, and not focus solely on the second cancers."
Session co-moderator Dr. Meral Beksac, with Ankara (Turkey) University, said the Italian data suggest caution and greater vigilance regarding routine cancer screenings among multiple myeloma patients, but would not change her treatment approach.
"Dr. Palumbo has shown very beautifully that the benefits you achieve in terms of the long-term myeloma effect outweigh the risk of secondary malignancies," she said in an interview. "Personally, I think we must plan to avoid alkylating agents when we now have these better agents."
Preliminary data from three trials showing a fourfold increase in secondary cancers in multiple myeloma patients treated with lenalidomide as maintenance therapy or in combination with melphalan prompted investigations into the safety of lenalidomide in the United States and Europe in 2011.
The European Medicines Agency concluded in September that the benefits of lenalidomide continue to outweigh the risks within the approved setting of relapsed multiple myeloma, but recommended that a warning be added on the risk of second cancers. The U.S. Food and Drug Administration review is ongoing, and includes the risk for thalidomide, since lenalidomide is an analogue of thalidomide.
Although the development of acute myeloid leukemia (AML) following multiple myeloma was observed decades ago, the underlying mechanisms remain unclear. Swedish researchers recently reported that the risk of AML and myelodysplastic syndromes is 11.5-fold higher in multiple myeloma patients than in the general population, even before the introduction of novel agents (Blood 2011;118:4086-92). In addition, the risk of AML/MDS was eightfold higher in patients with monoclonal gammopathy of undetermined significance (MGUS), even though none of the MGUS patients developed multiple myeloma, according to session co-moderator Dr. Sigurdur Y. Kristinsson, who was a coauthor of the Swedish study.
"Even those people that never develop the disease have an increased risk of AML and MDS, so it shows that it’s not only the treatment that we’re giving, but it’s also an inherent susceptibility," Dr. Kristinsson, with the Karolinska Hospital and Institute in Stockholm, said in an interview.
Work is ongoing to identify multiple myeloma patients at an increased risk of second cancers, thereby allowing clinicians to tailor therapy to reduce risks. A separate poster presentation at the ASH meeting reported that higher risk of second cancers was associated with older age, male sex, and radiation and/or surgery among roughly 29,250 multiple myeloma patients in the Surveillance, Epidemiology, and End Results (SEER) database.
Subgroup analysis of the pooled Italian data did not identify specific subgroups at greater risk, Dr. Palumbo said. The incidence rate was higher at 1.13 per 100 person-years for patients given melphalan-lenalidomide vs. 0.76 per 100 person-years for patients treated with autologous stem cell transplantation and lenalidomide (median age 68 years vs. 59 years, respectively).
Speaking on behalf of the investigators, Dr. Palumbo reported employment with, serving as a consultant and on the speakers bureau of, having equity ownership in, and receiving research funding, patent royalties, and honoraria from Celgene, maker of lenalidomide. Dr. Beksac reported honoraria and speakers bureau activity with Celgene and Janssen Cilag. Dr. Kristinsson reported no conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: At 4 years of follow-up, second cancers were diagnosed in 2.1% of patients.
Data Source: Retrospective, pooled analysis of 2,283 patients who received lenalidomide for treatment of multiple myeloma in nine experimental trials.
Disclosures: Speaking on behalf of the investigators, Dr. Palumbo reported employment with, serving as a consultant and on the speakers bureau of, having equity ownership in, and receiving research funding, patent royalties, and honoraria from Celgene, maker of lenalidomide. Dr. Beksac reported honoraria and speakers bureau activity with Celgene and Janssen Cilag. Dr. Kristinsson reported no conflicts of interest.
Chemotherapy Alone Bests Radiation for Nonbulky Hodgkin's Lymphoma
SAN DIEGO – In the long run, standard chemotherapy alone is more effective than radiation in keeping patients with limited-stage nonbulky Hodgkin’s lymphoma alive, according to updated results from an intergroup trial in 405 patients.
At 12 years, 94% of patients receiving ABVD – doxorubicin (Adriamycin), bleomycin (Blenoxane), vinblastine (Velbe), and dacarbazine – chemotherapy were alive, compared with 87% receiving subtotal nodal radiation with or without chemotherapy (hazard ratio for death, 0.50; P = .04).
Although 5-year data showed that patients treated with radiation experienced greater disease control, the survival advantage with chemotherapy resulted from a lower rate of death from other causes, Dr. Ralph M. Meyer said at the annual meeting of the American Society of Hematology.
A total of 12 patients in the ABVD arm died: six due to Hodgkin’s, four to a second cancer, and two due to cardiac causes. In contrast, 10 of the 24 deaths in the radiation arm were due to a second cancer. There were two deaths due to cardiac events, four to Hodgkin’s, three fatal infections, and five other deaths.
Dr. Meyer acknowledged that interpretation of the results is bound to be controversial because subtotal nodal radiation is no longer current practice as it is considered excessive. Patients today with low-risk stage IA or IIA nonbulky disease typically receive two cycles of ABVD with 20 Gy of involved-field radiation therapy. Although modern technology has reduced radiation exposure, radiation therapy would still include the coronary artery, heart, and substantial areas of the subdiaphragmatic, he said at a press briefing.
What is clear from the National Cancer Institute of Canada Clinical Trials Group and Eastern Cooperative Oncology Group study is that measures of disease control, like freedom from progressive disease, are not accurate surrogates for long-term overall survival in patients with stage I-II nonbulky Hodgkin’s lymphoma, said Dr. Meyer, director of the National Cancer Institute of Canada Clinical Trials Group.
"New proxies that predict for risks of late-treatment effects are needed," he said.
Trials are testing the use of PET scanning during therapy to identify patients who may receive chemotherapy alone. It is hypothesized that if PET scans are negative and patients are in remission after two cycles of chemotherapy, the cure rate will be high with further chemotherapy alone, whereas radiation therapy or some other form of chemotherapy should be considered if there is residual PET activity.
How the use of PET will alter current treatment management will be another contentious issue since results from ongoing trials are not expected for 2-3 years. Moreover, the trials are limited because they are using disease control and progression-free survival as end points, which the current trial has shown are not good proxies for overall survival, Dr. Meyer said.
"Thus it will cause issues and interpretation as to how to proceed with these results," he said.
Dr. Meyer and his associates randomly assigned 405 patients with previously untreated stage IA or IIA nonbulky Hodgkin’s lymphoma to receive ABVD chemotherapy alone or subtotal nodal radiation at a dose of 35 Gy in 20 daily fractions. Patients in the radiation group with a favorable risk profile received radiation only, while those with an unfavorable risk received two cycles of ABVD followed by radiation therapy. Median follow-up was 11.3 years.
At 12 years, freedom from disease progression was 92% with radiation vs. 87% with ABVD chemotherapy (HR, 1.91; P = .05,), Dr. Meyer said. Event-free survival was similar at 80% with radiation therapy and 85% with ABVD (HR, 0.88; P = .60).
The results were simultaneously published in the New England Journal of Medicine (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1111961]).
Dr. Meyer reported honoraria from Lilly and Celgene.
chemotherapy for lymphoma, ABVD lymphoma, nodal radiation, nonbulky Hodgkin's,
SAN DIEGO – In the long run, standard chemotherapy alone is more effective than radiation in keeping patients with limited-stage nonbulky Hodgkin’s lymphoma alive, according to updated results from an intergroup trial in 405 patients.
At 12 years, 94% of patients receiving ABVD – doxorubicin (Adriamycin), bleomycin (Blenoxane), vinblastine (Velbe), and dacarbazine – chemotherapy were alive, compared with 87% receiving subtotal nodal radiation with or without chemotherapy (hazard ratio for death, 0.50; P = .04).
Although 5-year data showed that patients treated with radiation experienced greater disease control, the survival advantage with chemotherapy resulted from a lower rate of death from other causes, Dr. Ralph M. Meyer said at the annual meeting of the American Society of Hematology.
A total of 12 patients in the ABVD arm died: six due to Hodgkin’s, four to a second cancer, and two due to cardiac causes. In contrast, 10 of the 24 deaths in the radiation arm were due to a second cancer. There were two deaths due to cardiac events, four to Hodgkin’s, three fatal infections, and five other deaths.
Dr. Meyer acknowledged that interpretation of the results is bound to be controversial because subtotal nodal radiation is no longer current practice as it is considered excessive. Patients today with low-risk stage IA or IIA nonbulky disease typically receive two cycles of ABVD with 20 Gy of involved-field radiation therapy. Although modern technology has reduced radiation exposure, radiation therapy would still include the coronary artery, heart, and substantial areas of the subdiaphragmatic, he said at a press briefing.
What is clear from the National Cancer Institute of Canada Clinical Trials Group and Eastern Cooperative Oncology Group study is that measures of disease control, like freedom from progressive disease, are not accurate surrogates for long-term overall survival in patients with stage I-II nonbulky Hodgkin’s lymphoma, said Dr. Meyer, director of the National Cancer Institute of Canada Clinical Trials Group.
"New proxies that predict for risks of late-treatment effects are needed," he said.
Trials are testing the use of PET scanning during therapy to identify patients who may receive chemotherapy alone. It is hypothesized that if PET scans are negative and patients are in remission after two cycles of chemotherapy, the cure rate will be high with further chemotherapy alone, whereas radiation therapy or some other form of chemotherapy should be considered if there is residual PET activity.
How the use of PET will alter current treatment management will be another contentious issue since results from ongoing trials are not expected for 2-3 years. Moreover, the trials are limited because they are using disease control and progression-free survival as end points, which the current trial has shown are not good proxies for overall survival, Dr. Meyer said.
"Thus it will cause issues and interpretation as to how to proceed with these results," he said.
Dr. Meyer and his associates randomly assigned 405 patients with previously untreated stage IA or IIA nonbulky Hodgkin’s lymphoma to receive ABVD chemotherapy alone or subtotal nodal radiation at a dose of 35 Gy in 20 daily fractions. Patients in the radiation group with a favorable risk profile received radiation only, while those with an unfavorable risk received two cycles of ABVD followed by radiation therapy. Median follow-up was 11.3 years.
At 12 years, freedom from disease progression was 92% with radiation vs. 87% with ABVD chemotherapy (HR, 1.91; P = .05,), Dr. Meyer said. Event-free survival was similar at 80% with radiation therapy and 85% with ABVD (HR, 0.88; P = .60).
The results were simultaneously published in the New England Journal of Medicine (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1111961]).
Dr. Meyer reported honoraria from Lilly and Celgene.
SAN DIEGO – In the long run, standard chemotherapy alone is more effective than radiation in keeping patients with limited-stage nonbulky Hodgkin’s lymphoma alive, according to updated results from an intergroup trial in 405 patients.
At 12 years, 94% of patients receiving ABVD – doxorubicin (Adriamycin), bleomycin (Blenoxane), vinblastine (Velbe), and dacarbazine – chemotherapy were alive, compared with 87% receiving subtotal nodal radiation with or without chemotherapy (hazard ratio for death, 0.50; P = .04).
Although 5-year data showed that patients treated with radiation experienced greater disease control, the survival advantage with chemotherapy resulted from a lower rate of death from other causes, Dr. Ralph M. Meyer said at the annual meeting of the American Society of Hematology.
A total of 12 patients in the ABVD arm died: six due to Hodgkin’s, four to a second cancer, and two due to cardiac causes. In contrast, 10 of the 24 deaths in the radiation arm were due to a second cancer. There were two deaths due to cardiac events, four to Hodgkin’s, three fatal infections, and five other deaths.
Dr. Meyer acknowledged that interpretation of the results is bound to be controversial because subtotal nodal radiation is no longer current practice as it is considered excessive. Patients today with low-risk stage IA or IIA nonbulky disease typically receive two cycles of ABVD with 20 Gy of involved-field radiation therapy. Although modern technology has reduced radiation exposure, radiation therapy would still include the coronary artery, heart, and substantial areas of the subdiaphragmatic, he said at a press briefing.
What is clear from the National Cancer Institute of Canada Clinical Trials Group and Eastern Cooperative Oncology Group study is that measures of disease control, like freedom from progressive disease, are not accurate surrogates for long-term overall survival in patients with stage I-II nonbulky Hodgkin’s lymphoma, said Dr. Meyer, director of the National Cancer Institute of Canada Clinical Trials Group.
"New proxies that predict for risks of late-treatment effects are needed," he said.
Trials are testing the use of PET scanning during therapy to identify patients who may receive chemotherapy alone. It is hypothesized that if PET scans are negative and patients are in remission after two cycles of chemotherapy, the cure rate will be high with further chemotherapy alone, whereas radiation therapy or some other form of chemotherapy should be considered if there is residual PET activity.
How the use of PET will alter current treatment management will be another contentious issue since results from ongoing trials are not expected for 2-3 years. Moreover, the trials are limited because they are using disease control and progression-free survival as end points, which the current trial has shown are not good proxies for overall survival, Dr. Meyer said.
"Thus it will cause issues and interpretation as to how to proceed with these results," he said.
Dr. Meyer and his associates randomly assigned 405 patients with previously untreated stage IA or IIA nonbulky Hodgkin’s lymphoma to receive ABVD chemotherapy alone or subtotal nodal radiation at a dose of 35 Gy in 20 daily fractions. Patients in the radiation group with a favorable risk profile received radiation only, while those with an unfavorable risk received two cycles of ABVD followed by radiation therapy. Median follow-up was 11.3 years.
At 12 years, freedom from disease progression was 92% with radiation vs. 87% with ABVD chemotherapy (HR, 1.91; P = .05,), Dr. Meyer said. Event-free survival was similar at 80% with radiation therapy and 85% with ABVD (HR, 0.88; P = .60).
The results were simultaneously published in the New England Journal of Medicine (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1111961]).
Dr. Meyer reported honoraria from Lilly and Celgene.
chemotherapy for lymphoma, ABVD lymphoma, nodal radiation, nonbulky Hodgkin's,
chemotherapy for lymphoma, ABVD lymphoma, nodal radiation, nonbulky Hodgkin's,
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: At 12 years, the overall survival rate was 94% with chemotherapy alone and 87% with subtotal nodal radiation.
Data Source: Prospective, randomized trial in 405 patients with untreated nonbulky Hodgkin’s lymphoma.
Disclosures: Dr. Meyers reported honoraria from Lilly and Celgene.
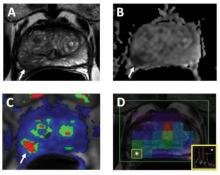
Combining MRI With Prostate Ultrasound Biopsy Bests Biopsy Alone
CHICAGO – Fusing MRI with real-time, three-dimensional ultrasound allows for more targeted prostate biopsies and finds additional cancers, compared with standard systematic biopsies.
"This may lead to fewer total biopsies, improved yield and improved confidence for active surveillance," Dr. Daniel J.A. Margolis said at the annual meeting of the Radiological Society of North America.
Direct MRI-guided biopsy is not universally available, leaving most centers to use two-dimensional ultrasound to systematically biopsy 12 areas of the prostate, whether they are all suspicious or not. Not surprising, roughly 30% of systematic core biopsies are false negative, explained radiologist Dr. Margolis of the University of California, Los Angeles.
Researchers at UCLA departments and the medical device company Eigen have been using external-array 3 Tesla MRI scans, including T2-weighted, diffusion-weighted, and dynamic contrast-enhanced images to identify suspicious areas in the prostate. The areas are scored on a 5-point scale by a radiologist based on cancer risk, and the data are used to create a 3-D contoured reconstruction that is fused with real-time, transrectal ultrasound during biopsy.
Early results were promising in the two groups of men most likely to benefit from the new imaging technology – those with a prior negative biopsy and elevated prostate-specific antigen (PSA) levels and those with low-risk prostate cancer under active surveillance. In 47 such men, the biopsy-positivity rate was 33% with MRI-fusion ultrasound vs. 7% for systematic, nontargeted biopsy (Urol. Oncol. 2011;29:334-42).
At the meeting, Dr. Margolis presented data from 57 consecutive men with a previous biopsy, in whom MRI-fusion ultrasound identified 101 suspicious areas. In all, 22 men had 28 positive MRI targets.
Positive biopsies were found in 12 patients on targets only. Nine patients had positive lesions on both MRI-fusion ultrasound and systematic biopsy. In one additional patient, the positive systematic core was changed from Gleason 3+3 to 3+4 disease with the targeted biopsy.
Seven patients had positive biopsies found on systematic biopsy only, although all were Gleason score 3+3, less than 4 mm in size and less than 25% of the core, Dr. Margolis said.
A separate study presented in the same session suggests that fusing MRI with transrectal ultrasound biopsy may also be useful in identifying aggressive tumors in men with no prior prostate biopsy or suspicious digital rectal exam and a PSA of 3-10 ng/mL (mean 8 ng/mL).
The overall cancer detection rate was 52% among 323 (168/323) such men, 73% within MRI targets (144/197) and 19% with sextant biopsy (24/126), reported Dr. François Cornud, a consultant radiologist at René Descartes University, Paris.
In 98 patients with both MRI targeted- and sextant-positive biopsies, targeted biopsies identified significantly more cancers with a Gleason score greater than 6 (44% vs. 25%), with a length in any core of more than 7 mm (50% vs. 25.5%) and with a longer mean length (5.3 mm vs. 0.8 mm).
Interestingly, performance was similar whether the multiparametric MRI data were fused with the real-time ultrasound images visually or by a computerized electromagnetic navigator system.
"Targeted biopsies definitely provide better evaluation of tumor burden and Gleason score," Dr. Cornud said, adding that "a negative MRI prior to biopsy may mean no cancer or indolent cancer and may suggest that in these patients biopsy may be deferred."
Both studies were well received, although one attendee questioned whether the researchers have been able to convince frequently reluctant urologists that targeted biopsies are worth it. Dr. Margolis said his project was actually instigated by an urologist. Dr. Cornud said the majority of his urologists are now requesting an MRI and its findings.
Dr. Margolis reported a research grant from Siemens AG and a coauthor reported conflicts with several pharmaceutical and device firms. Dr. Cornud and his coauthors reported no relevant disclosures.
Direct MRI-guided biopsy, prostate, Eigen, prior negative biopsy, elevated prostate-specific antigen (PSA) levels,
CHICAGO – Fusing MRI with real-time, three-dimensional ultrasound allows for more targeted prostate biopsies and finds additional cancers, compared with standard systematic biopsies.
"This may lead to fewer total biopsies, improved yield and improved confidence for active surveillance," Dr. Daniel J.A. Margolis said at the annual meeting of the Radiological Society of North America.
Direct MRI-guided biopsy is not universally available, leaving most centers to use two-dimensional ultrasound to systematically biopsy 12 areas of the prostate, whether they are all suspicious or not. Not surprising, roughly 30% of systematic core biopsies are false negative, explained radiologist Dr. Margolis of the University of California, Los Angeles.
Researchers at UCLA departments and the medical device company Eigen have been using external-array 3 Tesla MRI scans, including T2-weighted, diffusion-weighted, and dynamic contrast-enhanced images to identify suspicious areas in the prostate. The areas are scored on a 5-point scale by a radiologist based on cancer risk, and the data are used to create a 3-D contoured reconstruction that is fused with real-time, transrectal ultrasound during biopsy.
Early results were promising in the two groups of men most likely to benefit from the new imaging technology – those with a prior negative biopsy and elevated prostate-specific antigen (PSA) levels and those with low-risk prostate cancer under active surveillance. In 47 such men, the biopsy-positivity rate was 33% with MRI-fusion ultrasound vs. 7% for systematic, nontargeted biopsy (Urol. Oncol. 2011;29:334-42).
At the meeting, Dr. Margolis presented data from 57 consecutive men with a previous biopsy, in whom MRI-fusion ultrasound identified 101 suspicious areas. In all, 22 men had 28 positive MRI targets.
Positive biopsies were found in 12 patients on targets only. Nine patients had positive lesions on both MRI-fusion ultrasound and systematic biopsy. In one additional patient, the positive systematic core was changed from Gleason 3+3 to 3+4 disease with the targeted biopsy.
Seven patients had positive biopsies found on systematic biopsy only, although all were Gleason score 3+3, less than 4 mm in size and less than 25% of the core, Dr. Margolis said.
A separate study presented in the same session suggests that fusing MRI with transrectal ultrasound biopsy may also be useful in identifying aggressive tumors in men with no prior prostate biopsy or suspicious digital rectal exam and a PSA of 3-10 ng/mL (mean 8 ng/mL).
The overall cancer detection rate was 52% among 323 (168/323) such men, 73% within MRI targets (144/197) and 19% with sextant biopsy (24/126), reported Dr. François Cornud, a consultant radiologist at René Descartes University, Paris.
In 98 patients with both MRI targeted- and sextant-positive biopsies, targeted biopsies identified significantly more cancers with a Gleason score greater than 6 (44% vs. 25%), with a length in any core of more than 7 mm (50% vs. 25.5%) and with a longer mean length (5.3 mm vs. 0.8 mm).
Interestingly, performance was similar whether the multiparametric MRI data were fused with the real-time ultrasound images visually or by a computerized electromagnetic navigator system.
"Targeted biopsies definitely provide better evaluation of tumor burden and Gleason score," Dr. Cornud said, adding that "a negative MRI prior to biopsy may mean no cancer or indolent cancer and may suggest that in these patients biopsy may be deferred."
Both studies were well received, although one attendee questioned whether the researchers have been able to convince frequently reluctant urologists that targeted biopsies are worth it. Dr. Margolis said his project was actually instigated by an urologist. Dr. Cornud said the majority of his urologists are now requesting an MRI and its findings.
Dr. Margolis reported a research grant from Siemens AG and a coauthor reported conflicts with several pharmaceutical and device firms. Dr. Cornud and his coauthors reported no relevant disclosures.
CHICAGO – Fusing MRI with real-time, three-dimensional ultrasound allows for more targeted prostate biopsies and finds additional cancers, compared with standard systematic biopsies.
"This may lead to fewer total biopsies, improved yield and improved confidence for active surveillance," Dr. Daniel J.A. Margolis said at the annual meeting of the Radiological Society of North America.
Direct MRI-guided biopsy is not universally available, leaving most centers to use two-dimensional ultrasound to systematically biopsy 12 areas of the prostate, whether they are all suspicious or not. Not surprising, roughly 30% of systematic core biopsies are false negative, explained radiologist Dr. Margolis of the University of California, Los Angeles.
Researchers at UCLA departments and the medical device company Eigen have been using external-array 3 Tesla MRI scans, including T2-weighted, diffusion-weighted, and dynamic contrast-enhanced images to identify suspicious areas in the prostate. The areas are scored on a 5-point scale by a radiologist based on cancer risk, and the data are used to create a 3-D contoured reconstruction that is fused with real-time, transrectal ultrasound during biopsy.
Early results were promising in the two groups of men most likely to benefit from the new imaging technology – those with a prior negative biopsy and elevated prostate-specific antigen (PSA) levels and those with low-risk prostate cancer under active surveillance. In 47 such men, the biopsy-positivity rate was 33% with MRI-fusion ultrasound vs. 7% for systematic, nontargeted biopsy (Urol. Oncol. 2011;29:334-42).
At the meeting, Dr. Margolis presented data from 57 consecutive men with a previous biopsy, in whom MRI-fusion ultrasound identified 101 suspicious areas. In all, 22 men had 28 positive MRI targets.
Positive biopsies were found in 12 patients on targets only. Nine patients had positive lesions on both MRI-fusion ultrasound and systematic biopsy. In one additional patient, the positive systematic core was changed from Gleason 3+3 to 3+4 disease with the targeted biopsy.
Seven patients had positive biopsies found on systematic biopsy only, although all were Gleason score 3+3, less than 4 mm in size and less than 25% of the core, Dr. Margolis said.
A separate study presented in the same session suggests that fusing MRI with transrectal ultrasound biopsy may also be useful in identifying aggressive tumors in men with no prior prostate biopsy or suspicious digital rectal exam and a PSA of 3-10 ng/mL (mean 8 ng/mL).
The overall cancer detection rate was 52% among 323 (168/323) such men, 73% within MRI targets (144/197) and 19% with sextant biopsy (24/126), reported Dr. François Cornud, a consultant radiologist at René Descartes University, Paris.
In 98 patients with both MRI targeted- and sextant-positive biopsies, targeted biopsies identified significantly more cancers with a Gleason score greater than 6 (44% vs. 25%), with a length in any core of more than 7 mm (50% vs. 25.5%) and with a longer mean length (5.3 mm vs. 0.8 mm).
Interestingly, performance was similar whether the multiparametric MRI data were fused with the real-time ultrasound images visually or by a computerized electromagnetic navigator system.
"Targeted biopsies definitely provide better evaluation of tumor burden and Gleason score," Dr. Cornud said, adding that "a negative MRI prior to biopsy may mean no cancer or indolent cancer and may suggest that in these patients biopsy may be deferred."
Both studies were well received, although one attendee questioned whether the researchers have been able to convince frequently reluctant urologists that targeted biopsies are worth it. Dr. Margolis said his project was actually instigated by an urologist. Dr. Cornud said the majority of his urologists are now requesting an MRI and its findings.
Dr. Margolis reported a research grant from Siemens AG and a coauthor reported conflicts with several pharmaceutical and device firms. Dr. Cornud and his coauthors reported no relevant disclosures.
Direct MRI-guided biopsy, prostate, Eigen, prior negative biopsy, elevated prostate-specific antigen (PSA) levels,
Direct MRI-guided biopsy, prostate, Eigen, prior negative biopsy, elevated prostate-specific antigen (PSA) levels,
FROM THE ANNUAL MEETING OF THE RADIOLOGICAL SOCIETY OF NORTH AMERICA
Major Finding: Among 57 consecutive men with a previous biopsy in whom MRI-fusion ultrasound identified 101 suspicious areas, 22 men had 28 positive MRI targets. In a second study, fusing MRI with transrectal ultrasound biopsy was useful in identifying aggressive tumors in men with no prior prostate biopsy or suspicious digital rectal exam and a PSA of 3-10 ng/mL. The overall cancer detection rate was 52% among 323 (168/323) such men, 73% within MRI targets (144/197) and 19% with sextant biopsy (24/126).
Data Source: Prospective study in 57 men with a prior prostate biopsy, and a prospective study in 323 men with no prior biopsy and PSA levels of 3-10 ng/mL.
Disclosures: Dr. Margolis reported a research grant from Siemens AG, and a coauthor reported conflicts with several pharmaceutical and device firms. Dr. Cornud and his coauthors reported no relevant disclosures.
Gemtuzumab Extends Survival in Acute Myeloid Leukemia
SAN DIEGO – Adding low doses of gemtuzumab to standard chemotherapy extends survival in de novo acute myeloid leukemia without the toxicity that triggered the monoclonal antibody to be taken off the market in the United States last year, a study has shown.
Median event-free survival among 280 patients, aged 50-70 years, was increased from 11.9 months with standard daunorubicin (Cerubidine) and cytarabine (Ara-C) chemotherapy to 19.6 months with the addition of gemtuzumab ozogamicin (Mylotarg).
The 2-year estimate of event-free survival was 16.5% vs. 41.1% (log-rank P value = .00018; hazard ratio, 0.57).
This translated into an increase in median overall survival from 19.2 months to 34 months with the addition of gemtuzumab, Dr. Sylvie Castaigne reported on behalf of the Acute Leukemia French Association (ALFA) at the annual meeting of the American Society of Hematology. The 2-year overall survival estimate was 43.5% vs. 53.1% (log rank P = .046; HR, 0.70).
Notably, this improvement in survival was not present in patients with unfavorable cytogenetics, comprising 23% of the gemtuzumab group.
At the urging of the U.S. Food and Drug Administration, Pfizer voluntarily withdrew gemtuzumab from the market in June 2010, because of concerns about its toxicity and lack of clinical benefit in patients with acute myeloid leukemia (AML).
The drug remains available in Europe on a compassionate basis for relapsed AML, but not in the frontline setting, according to Dr. Castaigne, professor of hematology at Hôpital de Versailles (France).
When asked during a press briefing whether the current data could resurrect gemtuzumab in the United States or be parlayed into a new indication in Europe, she said, "I think many physicians will ask Pfizer to get the drug on the market."
Dr. Armand Keating, ASH president-elect, who moderated the presentation of the study, said in an interview that "I think it has the potential to change practice, but my concern is that there may be more side effects than are being reported in this particular study and certainly there were previous reports of veno-occlusive disease."
Dr. Castaigne reported three episodes of veno-occlusive disease, two of which were fatal. Prolonged grade 3 or greater thrombocytopenia occurred in 19 patients.
Dr. Martin Tallman, chief of the leukemia service at Memorial Sloan-Kettering Cancer Center in New York City, agreed that the data are impressive, but also expressed concern about the veno-occlusive disease events.
"Would I add gemtuzumab to my next patient Monday morning with favorable cytogenic risk, off study? No," he said in an interview. "Does it change standard of care? No.
"It is impressive, but I think we need more studies."
Still, Dr. Tallman said that he would advise Pfizer to bring gemtuzumab back on the market for clinical trials, and that a Pfizer executive told him during the presentation that Pfizer would see what it could do.
Dr. Keating, director of hematology at the University of Toronto, said the new data might lead to a consideration of putting gemtuzumab back on the market. "I think it would be very reasonable," he added.
Only 13,000 new patients are diagnosed each year in the United States with AML, but there are 9,000 deaths. Overall survival has improved among younger adults, but there is no evidence of improvement among older adults in 4 decades of investigation, Dr. Castaigne said.
The ALFA group opted to pursue gemtuzumab in AML based on phase I data suggesting that repeated lower-dose infusions would reduce the toxicity associated with the previous 9-mg/m2 dose given on days 1 and 14, while enhancing the efficacy of gemtuzumab.
From January 2008 to November 2010, 280 patients were randomized to chemotherapy with daunorubicin 60 mg/m2 on days 1-3 and cytarabine 200 mg/m2 on days 1-7 with or without gemtuzumab 3 mg/m2 on days 1, 4 and 7. Two patients withdrew consent, and were excluded from the analysis. Their median age was 62 years.
"Would I add gemtuzumab to my next patient Monday morning with favorable cytogenic risk, off study? No."
If bone marrow blasts were more than 10% at day 15, a second course of daunorubicin 60 mg/m2 on days 1 and 2 and cytarabine 1 g/m2 every 12 hours on days 1-3 was given.
Two rounds of consolidation chemotherapy were given to patients who experienced a complete response.
Median relapse-free survival among complete responders was 12.5 months in the control group and 28.1 months in the gemtuzumab group, with 21.7% vs. 50.8% alive at 2 years (log-rank P value = .00029; HR, 0.51), Dr. Castaigne said.
The rate of fatal events possibly related to treatment was similar at 6.7% in the chemotherapy group and 8.7% in the gemtuzumab group, she said.
The incidence of grade 3-4 sepsis was similar at 16% with chemotherapy and 20% with gemtuzumab, as was the rate of ICU admission (12% vs. 14%).
Additional data at the meeting also show a significant survival benefit with gemtuzumab among 806 older patients, with a median age of 67 years, according to Dr. Alan Burnett, head of hematology at Cardiff (Wales) University. Median overall survival increased from 39% to 47% with the addition of gemtuzumab to chemotherapy (P = .02). A lower benefit was observed in those with adverse cytogenetics or secondary disease, according to the study.
Dr. Castaigne reported financial relationships with Pfizer/Wyeth. Dr. Burnett reported financial relationships with Pfizer. Dr. Tallman reported consulting for Genzyme Oncology.
SAN DIEGO – Adding low doses of gemtuzumab to standard chemotherapy extends survival in de novo acute myeloid leukemia without the toxicity that triggered the monoclonal antibody to be taken off the market in the United States last year, a study has shown.
Median event-free survival among 280 patients, aged 50-70 years, was increased from 11.9 months with standard daunorubicin (Cerubidine) and cytarabine (Ara-C) chemotherapy to 19.6 months with the addition of gemtuzumab ozogamicin (Mylotarg).
The 2-year estimate of event-free survival was 16.5% vs. 41.1% (log-rank P value = .00018; hazard ratio, 0.57).
This translated into an increase in median overall survival from 19.2 months to 34 months with the addition of gemtuzumab, Dr. Sylvie Castaigne reported on behalf of the Acute Leukemia French Association (ALFA) at the annual meeting of the American Society of Hematology. The 2-year overall survival estimate was 43.5% vs. 53.1% (log rank P = .046; HR, 0.70).
Notably, this improvement in survival was not present in patients with unfavorable cytogenetics, comprising 23% of the gemtuzumab group.
At the urging of the U.S. Food and Drug Administration, Pfizer voluntarily withdrew gemtuzumab from the market in June 2010, because of concerns about its toxicity and lack of clinical benefit in patients with acute myeloid leukemia (AML).
The drug remains available in Europe on a compassionate basis for relapsed AML, but not in the frontline setting, according to Dr. Castaigne, professor of hematology at Hôpital de Versailles (France).
When asked during a press briefing whether the current data could resurrect gemtuzumab in the United States or be parlayed into a new indication in Europe, she said, "I think many physicians will ask Pfizer to get the drug on the market."
Dr. Armand Keating, ASH president-elect, who moderated the presentation of the study, said in an interview that "I think it has the potential to change practice, but my concern is that there may be more side effects than are being reported in this particular study and certainly there were previous reports of veno-occlusive disease."
Dr. Castaigne reported three episodes of veno-occlusive disease, two of which were fatal. Prolonged grade 3 or greater thrombocytopenia occurred in 19 patients.
Dr. Martin Tallman, chief of the leukemia service at Memorial Sloan-Kettering Cancer Center in New York City, agreed that the data are impressive, but also expressed concern about the veno-occlusive disease events.
"Would I add gemtuzumab to my next patient Monday morning with favorable cytogenic risk, off study? No," he said in an interview. "Does it change standard of care? No.
"It is impressive, but I think we need more studies."
Still, Dr. Tallman said that he would advise Pfizer to bring gemtuzumab back on the market for clinical trials, and that a Pfizer executive told him during the presentation that Pfizer would see what it could do.
Dr. Keating, director of hematology at the University of Toronto, said the new data might lead to a consideration of putting gemtuzumab back on the market. "I think it would be very reasonable," he added.
Only 13,000 new patients are diagnosed each year in the United States with AML, but there are 9,000 deaths. Overall survival has improved among younger adults, but there is no evidence of improvement among older adults in 4 decades of investigation, Dr. Castaigne said.
The ALFA group opted to pursue gemtuzumab in AML based on phase I data suggesting that repeated lower-dose infusions would reduce the toxicity associated with the previous 9-mg/m2 dose given on days 1 and 14, while enhancing the efficacy of gemtuzumab.
From January 2008 to November 2010, 280 patients were randomized to chemotherapy with daunorubicin 60 mg/m2 on days 1-3 and cytarabine 200 mg/m2 on days 1-7 with or without gemtuzumab 3 mg/m2 on days 1, 4 and 7. Two patients withdrew consent, and were excluded from the analysis. Their median age was 62 years.
"Would I add gemtuzumab to my next patient Monday morning with favorable cytogenic risk, off study? No."
If bone marrow blasts were more than 10% at day 15, a second course of daunorubicin 60 mg/m2 on days 1 and 2 and cytarabine 1 g/m2 every 12 hours on days 1-3 was given.
Two rounds of consolidation chemotherapy were given to patients who experienced a complete response.
Median relapse-free survival among complete responders was 12.5 months in the control group and 28.1 months in the gemtuzumab group, with 21.7% vs. 50.8% alive at 2 years (log-rank P value = .00029; HR, 0.51), Dr. Castaigne said.
The rate of fatal events possibly related to treatment was similar at 6.7% in the chemotherapy group and 8.7% in the gemtuzumab group, she said.
The incidence of grade 3-4 sepsis was similar at 16% with chemotherapy and 20% with gemtuzumab, as was the rate of ICU admission (12% vs. 14%).
Additional data at the meeting also show a significant survival benefit with gemtuzumab among 806 older patients, with a median age of 67 years, according to Dr. Alan Burnett, head of hematology at Cardiff (Wales) University. Median overall survival increased from 39% to 47% with the addition of gemtuzumab to chemotherapy (P = .02). A lower benefit was observed in those with adverse cytogenetics or secondary disease, according to the study.
Dr. Castaigne reported financial relationships with Pfizer/Wyeth. Dr. Burnett reported financial relationships with Pfizer. Dr. Tallman reported consulting for Genzyme Oncology.
SAN DIEGO – Adding low doses of gemtuzumab to standard chemotherapy extends survival in de novo acute myeloid leukemia without the toxicity that triggered the monoclonal antibody to be taken off the market in the United States last year, a study has shown.
Median event-free survival among 280 patients, aged 50-70 years, was increased from 11.9 months with standard daunorubicin (Cerubidine) and cytarabine (Ara-C) chemotherapy to 19.6 months with the addition of gemtuzumab ozogamicin (Mylotarg).
The 2-year estimate of event-free survival was 16.5% vs. 41.1% (log-rank P value = .00018; hazard ratio, 0.57).
This translated into an increase in median overall survival from 19.2 months to 34 months with the addition of gemtuzumab, Dr. Sylvie Castaigne reported on behalf of the Acute Leukemia French Association (ALFA) at the annual meeting of the American Society of Hematology. The 2-year overall survival estimate was 43.5% vs. 53.1% (log rank P = .046; HR, 0.70).
Notably, this improvement in survival was not present in patients with unfavorable cytogenetics, comprising 23% of the gemtuzumab group.
At the urging of the U.S. Food and Drug Administration, Pfizer voluntarily withdrew gemtuzumab from the market in June 2010, because of concerns about its toxicity and lack of clinical benefit in patients with acute myeloid leukemia (AML).
The drug remains available in Europe on a compassionate basis for relapsed AML, but not in the frontline setting, according to Dr. Castaigne, professor of hematology at Hôpital de Versailles (France).
When asked during a press briefing whether the current data could resurrect gemtuzumab in the United States or be parlayed into a new indication in Europe, she said, "I think many physicians will ask Pfizer to get the drug on the market."
Dr. Armand Keating, ASH president-elect, who moderated the presentation of the study, said in an interview that "I think it has the potential to change practice, but my concern is that there may be more side effects than are being reported in this particular study and certainly there were previous reports of veno-occlusive disease."
Dr. Castaigne reported three episodes of veno-occlusive disease, two of which were fatal. Prolonged grade 3 or greater thrombocytopenia occurred in 19 patients.
Dr. Martin Tallman, chief of the leukemia service at Memorial Sloan-Kettering Cancer Center in New York City, agreed that the data are impressive, but also expressed concern about the veno-occlusive disease events.
"Would I add gemtuzumab to my next patient Monday morning with favorable cytogenic risk, off study? No," he said in an interview. "Does it change standard of care? No.
"It is impressive, but I think we need more studies."
Still, Dr. Tallman said that he would advise Pfizer to bring gemtuzumab back on the market for clinical trials, and that a Pfizer executive told him during the presentation that Pfizer would see what it could do.
Dr. Keating, director of hematology at the University of Toronto, said the new data might lead to a consideration of putting gemtuzumab back on the market. "I think it would be very reasonable," he added.
Only 13,000 new patients are diagnosed each year in the United States with AML, but there are 9,000 deaths. Overall survival has improved among younger adults, but there is no evidence of improvement among older adults in 4 decades of investigation, Dr. Castaigne said.
The ALFA group opted to pursue gemtuzumab in AML based on phase I data suggesting that repeated lower-dose infusions would reduce the toxicity associated with the previous 9-mg/m2 dose given on days 1 and 14, while enhancing the efficacy of gemtuzumab.
From January 2008 to November 2010, 280 patients were randomized to chemotherapy with daunorubicin 60 mg/m2 on days 1-3 and cytarabine 200 mg/m2 on days 1-7 with or without gemtuzumab 3 mg/m2 on days 1, 4 and 7. Two patients withdrew consent, and were excluded from the analysis. Their median age was 62 years.
"Would I add gemtuzumab to my next patient Monday morning with favorable cytogenic risk, off study? No."
If bone marrow blasts were more than 10% at day 15, a second course of daunorubicin 60 mg/m2 on days 1 and 2 and cytarabine 1 g/m2 every 12 hours on days 1-3 was given.
Two rounds of consolidation chemotherapy were given to patients who experienced a complete response.
Median relapse-free survival among complete responders was 12.5 months in the control group and 28.1 months in the gemtuzumab group, with 21.7% vs. 50.8% alive at 2 years (log-rank P value = .00029; HR, 0.51), Dr. Castaigne said.
The rate of fatal events possibly related to treatment was similar at 6.7% in the chemotherapy group and 8.7% in the gemtuzumab group, she said.
The incidence of grade 3-4 sepsis was similar at 16% with chemotherapy and 20% with gemtuzumab, as was the rate of ICU admission (12% vs. 14%).
Additional data at the meeting also show a significant survival benefit with gemtuzumab among 806 older patients, with a median age of 67 years, according to Dr. Alan Burnett, head of hematology at Cardiff (Wales) University. Median overall survival increased from 39% to 47% with the addition of gemtuzumab to chemotherapy (P = .02). A lower benefit was observed in those with adverse cytogenetics or secondary disease, according to the study.
Dr. Castaigne reported financial relationships with Pfizer/Wyeth. Dr. Burnett reported financial relationships with Pfizer. Dr. Tallman reported consulting for Genzyme Oncology.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: Overall survival increased from 19 months in the standard treatment group to 34 months in patients given gemtuzumab ozogamicin.
Data Source: Prospective, randomized phase III trial in 280 patients, aged 50-70 years, with untreated acute myeloid leukemia.
Disclosures: Dr. Castaigne reported financial relationships with Pfizer/Wyeth. Dr. Burnett reported financial relationships with Pfizer. Dr. Tallman reported consulting for Genzyme Oncology.
Carfilzomib Analyses Provide New Insights in Multiple Myeloma
SAN DIEGO – Data mined from several phase II studies of carfilzomib monotherapy confirm previous observations, but also raise new questions and insights into the use of the investigational proteasome inhibitor in relapsed and/or refractory multiple myeloma.
The first of three posters simultaneously presented at the American Society of Hematology used multivariate modeling to confirm a dose-response relationship for carfilzomib in bortezomib (Velcade)-naive and bortezomib-treated patients.
After study effect was adjusted for, the odds of achieving a partial response or better for a patient treated with 27 mg/m2 were 4.08-fold higher than for a patient receiving only 20 mg/m2 (P less than .001).
When using the average dose as a continuous variable and again adjusting for study effect, the odds of a response increased by 1.28-fold for each 1-mg/m2 increase in average carfilzomib dose, equivalent to a 5.52-fold increase in the odds of a response if the average dose increased from 20 mg/m2 to 27 mg/m2 (P less than .001), reported Sunhee Ro, Ph.D., an employee of San Francisco–based Onyx Pharmaceuticals, which is developing the drug. Statistician Pierre Squifflet of the International Drug Development Institute in Belgium was the lead author.
The dose-effect relationship was observed not only on the primary end point of overall response rate, but also time to progression, progression-free survival, and overall survival.
The analysis included three study populations: 266 heavily pretreated patients who had received at least two prior therapies including bortezomib and either thalidomide or lenalidomide and who received carfilzomib 20 mg/m2 in cycle one with escalation to 27 mg/m2 if tolerable, 35 patients pretreated with one to three prior therapies including bortezomib who received carfilzomib 20 mg/m2, and 129 bortezomib-naive patients treated at 20 mg/m2 or the same dose with escalation to 27 mg/m2 if tolerable.
A corresponding toxicity analysis is underway, as are clinical trials assessing higher-dose regimens. The analysis raises the obvious question of whether the 20- to-27-mg/m2 dose schedule Onyx used in its recent accelerated review filing for carfilzomib is possibly too low.
Dr. Ro and other Onyx employees milling about the poster were hesitant to comment, with Ruben Sanchez, the associate director of publications at Onyx, saying only that the maximum tolerated dose of carfilzomib is 20-70 mg/m2 and that studies are ongoing at an "experimental dose" of 20-56 mg/m2.
Unfavorable Cytogenetics
The second poster confirmed that unfavorable cytogenetics did not significantly impact overall response rates or response duration, but found that median overall survival was shorter for those with cytogenetic abnormalities than for those with no detected abnormalities at 11.9 months vs. 19.2 months.
A similar trend was observed for median progression-free survival at 3.6 months vs. 4.6 months, reported Dr. Andrzej J. Jakubowiak of the University of Michigan Comprehensive Cancer Center in Ann Arbor.
When the researchers looked at specific cytogenetic abnormalities in the 234 evaluable patients dosed using the 20- to 27-mg/m2 schedule, median overall survival was the lowest among those with deletion 17p3 at 7 months vs. 10.6 months with deletion 13, 10.4 months with hypodiploidy, and 11.8 months with translocation t(4:14).
Progression-free survival and time to progression also tended to be longer for patients with hypoploidy and translocation t(4:14) than for patients with deletion 13 and deletion 17p13. This observation is compatible with current thinking highlighted in an educational multiple myeloma program at the meeting, that cytogenetic abnormalities are becoming something of a moving target, coauthor Dr. Sundar Jagannath, director of the multiple myeloma program at Mt. Sinai Medical Center in New York, said in an interview.
"One area, 17p13 deletion, is still a challenge, but t4;14 translocation, hypodiploidy, and deletion 13 ... are all no longer considered as high risk in the presence of protease inhibitors," he said. "It is nothing unique to carfilzomib; it’s just the class of protease inhibitors."
Dr. Jagannath said that even in patients with the problematic 17p13 deletion, researchers have used gene expression profiling to further delineate those at high and low risk, and that data clearly show that outcomes are improved in low-risk 17p13 deletion patients with the inclusion of the protease inhibitor bortezomib therapy.
Safety Data
The third poster features integrated safety data from 526 heavily pretreated patients receiving carfilzomib 20 mg/m2 or 20-27 mg/m2, showing that grade 3/4 treatment adverse events occurred in 80% of patients, but were characterized as mostly reversible and primarily hematologic in nature.
The most common grade 3 adverse events were thrombocytopenia (23%), anemia (22%), lymphopenia (18%), and pneumonia (11%). There were no fatal hematologic events and no grade 4 or 5 bleeding reactions associated with thrombocytopenia, reported Dr. Seema Singhal, professor of medicine and director of the multiple myeloma program at Northwestern Memorial Hospital in Chicago, and her associates.
Cardiac events, some of which resulted in discontinuation or death, occurred. Cardiac failure events were reported in 7% with discontinuation due to heart failure in 2%, cardiac arrest in 1%, and myocardial ischemia in less than 1%.
"The extent to which these [events] were due to patients’ baseline comorbidities, toxicity from prior treatment, effects of multiple myeloma, carfilzomib itself, or a combination of these factors cannot be determined," the authors wrote.
Dr. Jagannath said the cardiac events do not represent a new signal.
The studies in the analyses were supported by Onyx. Dr. Ro is an employee of Onyx. Dr. Jakubowiak, Dr. Singhal, and Dr Jagannath reported financial relationships with several firms including Onyx.
SAN DIEGO – Data mined from several phase II studies of carfilzomib monotherapy confirm previous observations, but also raise new questions and insights into the use of the investigational proteasome inhibitor in relapsed and/or refractory multiple myeloma.
The first of three posters simultaneously presented at the American Society of Hematology used multivariate modeling to confirm a dose-response relationship for carfilzomib in bortezomib (Velcade)-naive and bortezomib-treated patients.
After study effect was adjusted for, the odds of achieving a partial response or better for a patient treated with 27 mg/m2 were 4.08-fold higher than for a patient receiving only 20 mg/m2 (P less than .001).
When using the average dose as a continuous variable and again adjusting for study effect, the odds of a response increased by 1.28-fold for each 1-mg/m2 increase in average carfilzomib dose, equivalent to a 5.52-fold increase in the odds of a response if the average dose increased from 20 mg/m2 to 27 mg/m2 (P less than .001), reported Sunhee Ro, Ph.D., an employee of San Francisco–based Onyx Pharmaceuticals, which is developing the drug. Statistician Pierre Squifflet of the International Drug Development Institute in Belgium was the lead author.
The dose-effect relationship was observed not only on the primary end point of overall response rate, but also time to progression, progression-free survival, and overall survival.
The analysis included three study populations: 266 heavily pretreated patients who had received at least two prior therapies including bortezomib and either thalidomide or lenalidomide and who received carfilzomib 20 mg/m2 in cycle one with escalation to 27 mg/m2 if tolerable, 35 patients pretreated with one to three prior therapies including bortezomib who received carfilzomib 20 mg/m2, and 129 bortezomib-naive patients treated at 20 mg/m2 or the same dose with escalation to 27 mg/m2 if tolerable.
A corresponding toxicity analysis is underway, as are clinical trials assessing higher-dose regimens. The analysis raises the obvious question of whether the 20- to-27-mg/m2 dose schedule Onyx used in its recent accelerated review filing for carfilzomib is possibly too low.
Dr. Ro and other Onyx employees milling about the poster were hesitant to comment, with Ruben Sanchez, the associate director of publications at Onyx, saying only that the maximum tolerated dose of carfilzomib is 20-70 mg/m2 and that studies are ongoing at an "experimental dose" of 20-56 mg/m2.
Unfavorable Cytogenetics
The second poster confirmed that unfavorable cytogenetics did not significantly impact overall response rates or response duration, but found that median overall survival was shorter for those with cytogenetic abnormalities than for those with no detected abnormalities at 11.9 months vs. 19.2 months.
A similar trend was observed for median progression-free survival at 3.6 months vs. 4.6 months, reported Dr. Andrzej J. Jakubowiak of the University of Michigan Comprehensive Cancer Center in Ann Arbor.
When the researchers looked at specific cytogenetic abnormalities in the 234 evaluable patients dosed using the 20- to 27-mg/m2 schedule, median overall survival was the lowest among those with deletion 17p3 at 7 months vs. 10.6 months with deletion 13, 10.4 months with hypodiploidy, and 11.8 months with translocation t(4:14).
Progression-free survival and time to progression also tended to be longer for patients with hypoploidy and translocation t(4:14) than for patients with deletion 13 and deletion 17p13. This observation is compatible with current thinking highlighted in an educational multiple myeloma program at the meeting, that cytogenetic abnormalities are becoming something of a moving target, coauthor Dr. Sundar Jagannath, director of the multiple myeloma program at Mt. Sinai Medical Center in New York, said in an interview.
"One area, 17p13 deletion, is still a challenge, but t4;14 translocation, hypodiploidy, and deletion 13 ... are all no longer considered as high risk in the presence of protease inhibitors," he said. "It is nothing unique to carfilzomib; it’s just the class of protease inhibitors."
Dr. Jagannath said that even in patients with the problematic 17p13 deletion, researchers have used gene expression profiling to further delineate those at high and low risk, and that data clearly show that outcomes are improved in low-risk 17p13 deletion patients with the inclusion of the protease inhibitor bortezomib therapy.
Safety Data
The third poster features integrated safety data from 526 heavily pretreated patients receiving carfilzomib 20 mg/m2 or 20-27 mg/m2, showing that grade 3/4 treatment adverse events occurred in 80% of patients, but were characterized as mostly reversible and primarily hematologic in nature.
The most common grade 3 adverse events were thrombocytopenia (23%), anemia (22%), lymphopenia (18%), and pneumonia (11%). There were no fatal hematologic events and no grade 4 or 5 bleeding reactions associated with thrombocytopenia, reported Dr. Seema Singhal, professor of medicine and director of the multiple myeloma program at Northwestern Memorial Hospital in Chicago, and her associates.
Cardiac events, some of which resulted in discontinuation or death, occurred. Cardiac failure events were reported in 7% with discontinuation due to heart failure in 2%, cardiac arrest in 1%, and myocardial ischemia in less than 1%.
"The extent to which these [events] were due to patients’ baseline comorbidities, toxicity from prior treatment, effects of multiple myeloma, carfilzomib itself, or a combination of these factors cannot be determined," the authors wrote.
Dr. Jagannath said the cardiac events do not represent a new signal.
The studies in the analyses were supported by Onyx. Dr. Ro is an employee of Onyx. Dr. Jakubowiak, Dr. Singhal, and Dr Jagannath reported financial relationships with several firms including Onyx.
SAN DIEGO – Data mined from several phase II studies of carfilzomib monotherapy confirm previous observations, but also raise new questions and insights into the use of the investigational proteasome inhibitor in relapsed and/or refractory multiple myeloma.
The first of three posters simultaneously presented at the American Society of Hematology used multivariate modeling to confirm a dose-response relationship for carfilzomib in bortezomib (Velcade)-naive and bortezomib-treated patients.
After study effect was adjusted for, the odds of achieving a partial response or better for a patient treated with 27 mg/m2 were 4.08-fold higher than for a patient receiving only 20 mg/m2 (P less than .001).
When using the average dose as a continuous variable and again adjusting for study effect, the odds of a response increased by 1.28-fold for each 1-mg/m2 increase in average carfilzomib dose, equivalent to a 5.52-fold increase in the odds of a response if the average dose increased from 20 mg/m2 to 27 mg/m2 (P less than .001), reported Sunhee Ro, Ph.D., an employee of San Francisco–based Onyx Pharmaceuticals, which is developing the drug. Statistician Pierre Squifflet of the International Drug Development Institute in Belgium was the lead author.
The dose-effect relationship was observed not only on the primary end point of overall response rate, but also time to progression, progression-free survival, and overall survival.
The analysis included three study populations: 266 heavily pretreated patients who had received at least two prior therapies including bortezomib and either thalidomide or lenalidomide and who received carfilzomib 20 mg/m2 in cycle one with escalation to 27 mg/m2 if tolerable, 35 patients pretreated with one to three prior therapies including bortezomib who received carfilzomib 20 mg/m2, and 129 bortezomib-naive patients treated at 20 mg/m2 or the same dose with escalation to 27 mg/m2 if tolerable.
A corresponding toxicity analysis is underway, as are clinical trials assessing higher-dose regimens. The analysis raises the obvious question of whether the 20- to-27-mg/m2 dose schedule Onyx used in its recent accelerated review filing for carfilzomib is possibly too low.
Dr. Ro and other Onyx employees milling about the poster were hesitant to comment, with Ruben Sanchez, the associate director of publications at Onyx, saying only that the maximum tolerated dose of carfilzomib is 20-70 mg/m2 and that studies are ongoing at an "experimental dose" of 20-56 mg/m2.
Unfavorable Cytogenetics
The second poster confirmed that unfavorable cytogenetics did not significantly impact overall response rates or response duration, but found that median overall survival was shorter for those with cytogenetic abnormalities than for those with no detected abnormalities at 11.9 months vs. 19.2 months.
A similar trend was observed for median progression-free survival at 3.6 months vs. 4.6 months, reported Dr. Andrzej J. Jakubowiak of the University of Michigan Comprehensive Cancer Center in Ann Arbor.
When the researchers looked at specific cytogenetic abnormalities in the 234 evaluable patients dosed using the 20- to 27-mg/m2 schedule, median overall survival was the lowest among those with deletion 17p3 at 7 months vs. 10.6 months with deletion 13, 10.4 months with hypodiploidy, and 11.8 months with translocation t(4:14).
Progression-free survival and time to progression also tended to be longer for patients with hypoploidy and translocation t(4:14) than for patients with deletion 13 and deletion 17p13. This observation is compatible with current thinking highlighted in an educational multiple myeloma program at the meeting, that cytogenetic abnormalities are becoming something of a moving target, coauthor Dr. Sundar Jagannath, director of the multiple myeloma program at Mt. Sinai Medical Center in New York, said in an interview.
"One area, 17p13 deletion, is still a challenge, but t4;14 translocation, hypodiploidy, and deletion 13 ... are all no longer considered as high risk in the presence of protease inhibitors," he said. "It is nothing unique to carfilzomib; it’s just the class of protease inhibitors."
Dr. Jagannath said that even in patients with the problematic 17p13 deletion, researchers have used gene expression profiling to further delineate those at high and low risk, and that data clearly show that outcomes are improved in low-risk 17p13 deletion patients with the inclusion of the protease inhibitor bortezomib therapy.
Safety Data
The third poster features integrated safety data from 526 heavily pretreated patients receiving carfilzomib 20 mg/m2 or 20-27 mg/m2, showing that grade 3/4 treatment adverse events occurred in 80% of patients, but were characterized as mostly reversible and primarily hematologic in nature.
The most common grade 3 adverse events were thrombocytopenia (23%), anemia (22%), lymphopenia (18%), and pneumonia (11%). There were no fatal hematologic events and no grade 4 or 5 bleeding reactions associated with thrombocytopenia, reported Dr. Seema Singhal, professor of medicine and director of the multiple myeloma program at Northwestern Memorial Hospital in Chicago, and her associates.
Cardiac events, some of which resulted in discontinuation or death, occurred. Cardiac failure events were reported in 7% with discontinuation due to heart failure in 2%, cardiac arrest in 1%, and myocardial ischemia in less than 1%.
"The extent to which these [events] were due to patients’ baseline comorbidities, toxicity from prior treatment, effects of multiple myeloma, carfilzomib itself, or a combination of these factors cannot be determined," the authors wrote.
Dr. Jagannath said the cardiac events do not represent a new signal.
The studies in the analyses were supported by Onyx. Dr. Ro is an employee of Onyx. Dr. Jakubowiak, Dr. Singhal, and Dr Jagannath reported financial relationships with several firms including Onyx.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: After study effect was adjusted for, the odds of achieving a partial response or better for a patient treated with 27 mg/m2 of carfilzomib were 4.08-fold higher than for a patient receiving only 20 mg/m2 (P less than .001) in one of three studies.
Data Source: Analyses of 1,190 patients with relapsed/refractory multiple myeloma treated with carfilzomib.
Disclosures: The studies in the analyses were supported by Onyx. Dr. Ro is an employee of Onyx. Dr. Jakubowiak, Dr. Singhal, and Dr Jagannath reported financial relationships with several firms including Onyx.
Early Thrombolysis Improves Long-term Outcomes After DVT
SAN DIEGO – Catheter-directed thrombolysis added to standard therapy for deep vein thrombosis reduced the risk of post-thrombotic syndrome by 14.5%, but at an increased cost of bleeding among 209 patients in a randomized, controlled trial.
At 2 years, 55.6% of patients receiving standard treatment with anticoagulation and compression stockings developed post-thrombotic syndrome (PTS), compared with 41.1% receiving catheter-directed thrombolysis (CDT) plus standard therapy (P = .047) in the multicenter CaVenT study.
The number needed to treat to prevent one PTS was seven, Dr. Per Morten Sandset and his colleagues will report in a late-breaking abstract to be presented Dec. 13 at the annual meeting of the American Society of Hematology. About one in four patients is still at risk for developing PTS after adequate treatment with anticoagulation and compression stockings.
The CaVenT (Catheter-Directed Thrombolysis for Acute Iliofemoral Deep Vein Thrombosis) trial provides much-needed prospective, randomized data on CDT, and is unique in that it focuses on functional rather than surrogate outcomes used in previous trials and case series, Dr. Sandset said at a press briefing in which he discussed the findings.
Still, the study is small and unlikely to change practice or resolve the controversy that has surrounded the use of early fibrinolysis since systemic thrombolytic therapy was introduced decades ago.
"For the first time, we have the evidence to support this type of treatment for centers that have developed this methodology, but I also believe we need further study," said Dr. Sandset, a professor in the division of specialized medicine and surgery at Oslo University in Norway.
Briefing moderator Dr. Charles Abrams, associate chief of hematology-oncology at the University of Pennsylvania School of Medicine in Philadelphia, said CaVenT provides the best randomized data to date, but that many clinicians, particularly in the United States, have been hesitant to adopt early fibrinolysis because of the increased risk of a serious bleeding complication.
"This is a tantalizing trial, but I don’t think ... when I’m back at my own institution that the next patient I see with a deep vein thrombosis is probably going to get it," he said.
Both men said results are eagerly awaited from the ongoing phase III, randomized ATTRACT trial evaluating CDT with blood-thinning drugs in 692 patients with proximal DVT. The cohort is three times larger than that of the CaVenT trial, but results from the North American trial are not expected possibly until 2015.
CaVenT randomized 209 patients who presented at 20 hospitals in Norway with their first acute iliofemoral DVT and symptoms present for up to 21 days to CDT with alteplase (Activase) followed by standard treatment or standard treatment alone. In all, 189 patients were evaluable for analysis. Their average age was 51.5 years (range 18-75 years), and 36% were women.
CDT significantly increased the rate of iliofemoral patency at six months from 47.4% with standard therapy to 65.9% (P = .012), Dr. Sandset said.
Importantly, patients who regained iliofemoral patency at six months had significantly less PTS at 2 years than those who experienced insufficient recanalization (36.9% vs. 61.3%, P less than .001). In all, 80 of the 90 patients in the CDT arm had successful lysis.
Bleeding complications were reported in 20 patients in the CDT arm and none in the control arm. Five bleeding events were clinically relevant and three were major, including compartment syndrome of the calf requiring surgery, abdominal wall hematoma requiring transfusion, and an inguinal puncture site hematoma.
No deaths, pulmonary embolisms, strokes or other complications with a permanently reduced outcome were reported, Dr. Sandset said.
"CDT should be considered in patients with acute iliofemoral DVT and no apparent risk of bleeding," he said, adding that the results should be taken into account when guidelines are revised.
One of the problems for clinicians managing patients with DVT is that PTS can vary from simple heaviness in the leg to a constantly swollen leg that can impair the patient’s ability to walk or hold a steady job, Dr. Abrams said.
"Patients with bigger clots and clots higher up in their thigh get more persistent symptoms, but you really can’t predict all that well who will have a bad long-term complication," he said in an interview. "And the downside of this is that the administration of this drug in other trials has led to bleeding complications in 5% of patients, and 2% of that 5% are either strokes or retroperitoneal bleeding."
Dr. Abrams noted that older patients are also at greater risk of a bleeding complication than younger patients, and that clinicians will have to weigh the pros and cons of the current findings in this context with their patients.
Dr. Sandset and Dr. Abrams reported no conflicts of interest.
SAN DIEGO – Catheter-directed thrombolysis added to standard therapy for deep vein thrombosis reduced the risk of post-thrombotic syndrome by 14.5%, but at an increased cost of bleeding among 209 patients in a randomized, controlled trial.
At 2 years, 55.6% of patients receiving standard treatment with anticoagulation and compression stockings developed post-thrombotic syndrome (PTS), compared with 41.1% receiving catheter-directed thrombolysis (CDT) plus standard therapy (P = .047) in the multicenter CaVenT study.
The number needed to treat to prevent one PTS was seven, Dr. Per Morten Sandset and his colleagues will report in a late-breaking abstract to be presented Dec. 13 at the annual meeting of the American Society of Hematology. About one in four patients is still at risk for developing PTS after adequate treatment with anticoagulation and compression stockings.
The CaVenT (Catheter-Directed Thrombolysis for Acute Iliofemoral Deep Vein Thrombosis) trial provides much-needed prospective, randomized data on CDT, and is unique in that it focuses on functional rather than surrogate outcomes used in previous trials and case series, Dr. Sandset said at a press briefing in which he discussed the findings.
Still, the study is small and unlikely to change practice or resolve the controversy that has surrounded the use of early fibrinolysis since systemic thrombolytic therapy was introduced decades ago.
"For the first time, we have the evidence to support this type of treatment for centers that have developed this methodology, but I also believe we need further study," said Dr. Sandset, a professor in the division of specialized medicine and surgery at Oslo University in Norway.
Briefing moderator Dr. Charles Abrams, associate chief of hematology-oncology at the University of Pennsylvania School of Medicine in Philadelphia, said CaVenT provides the best randomized data to date, but that many clinicians, particularly in the United States, have been hesitant to adopt early fibrinolysis because of the increased risk of a serious bleeding complication.
"This is a tantalizing trial, but I don’t think ... when I’m back at my own institution that the next patient I see with a deep vein thrombosis is probably going to get it," he said.
Both men said results are eagerly awaited from the ongoing phase III, randomized ATTRACT trial evaluating CDT with blood-thinning drugs in 692 patients with proximal DVT. The cohort is three times larger than that of the CaVenT trial, but results from the North American trial are not expected possibly until 2015.
CaVenT randomized 209 patients who presented at 20 hospitals in Norway with their first acute iliofemoral DVT and symptoms present for up to 21 days to CDT with alteplase (Activase) followed by standard treatment or standard treatment alone. In all, 189 patients were evaluable for analysis. Their average age was 51.5 years (range 18-75 years), and 36% were women.
CDT significantly increased the rate of iliofemoral patency at six months from 47.4% with standard therapy to 65.9% (P = .012), Dr. Sandset said.
Importantly, patients who regained iliofemoral patency at six months had significantly less PTS at 2 years than those who experienced insufficient recanalization (36.9% vs. 61.3%, P less than .001). In all, 80 of the 90 patients in the CDT arm had successful lysis.
Bleeding complications were reported in 20 patients in the CDT arm and none in the control arm. Five bleeding events were clinically relevant and three were major, including compartment syndrome of the calf requiring surgery, abdominal wall hematoma requiring transfusion, and an inguinal puncture site hematoma.
No deaths, pulmonary embolisms, strokes or other complications with a permanently reduced outcome were reported, Dr. Sandset said.
"CDT should be considered in patients with acute iliofemoral DVT and no apparent risk of bleeding," he said, adding that the results should be taken into account when guidelines are revised.
One of the problems for clinicians managing patients with DVT is that PTS can vary from simple heaviness in the leg to a constantly swollen leg that can impair the patient’s ability to walk or hold a steady job, Dr. Abrams said.
"Patients with bigger clots and clots higher up in their thigh get more persistent symptoms, but you really can’t predict all that well who will have a bad long-term complication," he said in an interview. "And the downside of this is that the administration of this drug in other trials has led to bleeding complications in 5% of patients, and 2% of that 5% are either strokes or retroperitoneal bleeding."
Dr. Abrams noted that older patients are also at greater risk of a bleeding complication than younger patients, and that clinicians will have to weigh the pros and cons of the current findings in this context with their patients.
Dr. Sandset and Dr. Abrams reported no conflicts of interest.
SAN DIEGO – Catheter-directed thrombolysis added to standard therapy for deep vein thrombosis reduced the risk of post-thrombotic syndrome by 14.5%, but at an increased cost of bleeding among 209 patients in a randomized, controlled trial.
At 2 years, 55.6% of patients receiving standard treatment with anticoagulation and compression stockings developed post-thrombotic syndrome (PTS), compared with 41.1% receiving catheter-directed thrombolysis (CDT) plus standard therapy (P = .047) in the multicenter CaVenT study.
The number needed to treat to prevent one PTS was seven, Dr. Per Morten Sandset and his colleagues will report in a late-breaking abstract to be presented Dec. 13 at the annual meeting of the American Society of Hematology. About one in four patients is still at risk for developing PTS after adequate treatment with anticoagulation and compression stockings.
The CaVenT (Catheter-Directed Thrombolysis for Acute Iliofemoral Deep Vein Thrombosis) trial provides much-needed prospective, randomized data on CDT, and is unique in that it focuses on functional rather than surrogate outcomes used in previous trials and case series, Dr. Sandset said at a press briefing in which he discussed the findings.
Still, the study is small and unlikely to change practice or resolve the controversy that has surrounded the use of early fibrinolysis since systemic thrombolytic therapy was introduced decades ago.
"For the first time, we have the evidence to support this type of treatment for centers that have developed this methodology, but I also believe we need further study," said Dr. Sandset, a professor in the division of specialized medicine and surgery at Oslo University in Norway.
Briefing moderator Dr. Charles Abrams, associate chief of hematology-oncology at the University of Pennsylvania School of Medicine in Philadelphia, said CaVenT provides the best randomized data to date, but that many clinicians, particularly in the United States, have been hesitant to adopt early fibrinolysis because of the increased risk of a serious bleeding complication.
"This is a tantalizing trial, but I don’t think ... when I’m back at my own institution that the next patient I see with a deep vein thrombosis is probably going to get it," he said.
Both men said results are eagerly awaited from the ongoing phase III, randomized ATTRACT trial evaluating CDT with blood-thinning drugs in 692 patients with proximal DVT. The cohort is three times larger than that of the CaVenT trial, but results from the North American trial are not expected possibly until 2015.
CaVenT randomized 209 patients who presented at 20 hospitals in Norway with their first acute iliofemoral DVT and symptoms present for up to 21 days to CDT with alteplase (Activase) followed by standard treatment or standard treatment alone. In all, 189 patients were evaluable for analysis. Their average age was 51.5 years (range 18-75 years), and 36% were women.
CDT significantly increased the rate of iliofemoral patency at six months from 47.4% with standard therapy to 65.9% (P = .012), Dr. Sandset said.
Importantly, patients who regained iliofemoral patency at six months had significantly less PTS at 2 years than those who experienced insufficient recanalization (36.9% vs. 61.3%, P less than .001). In all, 80 of the 90 patients in the CDT arm had successful lysis.
Bleeding complications were reported in 20 patients in the CDT arm and none in the control arm. Five bleeding events were clinically relevant and three were major, including compartment syndrome of the calf requiring surgery, abdominal wall hematoma requiring transfusion, and an inguinal puncture site hematoma.
No deaths, pulmonary embolisms, strokes or other complications with a permanently reduced outcome were reported, Dr. Sandset said.
"CDT should be considered in patients with acute iliofemoral DVT and no apparent risk of bleeding," he said, adding that the results should be taken into account when guidelines are revised.
One of the problems for clinicians managing patients with DVT is that PTS can vary from simple heaviness in the leg to a constantly swollen leg that can impair the patient’s ability to walk or hold a steady job, Dr. Abrams said.
"Patients with bigger clots and clots higher up in their thigh get more persistent symptoms, but you really can’t predict all that well who will have a bad long-term complication," he said in an interview. "And the downside of this is that the administration of this drug in other trials has led to bleeding complications in 5% of patients, and 2% of that 5% are either strokes or retroperitoneal bleeding."
Dr. Abrams noted that older patients are also at greater risk of a bleeding complication than younger patients, and that clinicians will have to weigh the pros and cons of the current findings in this context with their patients.
Dr. Sandset and Dr. Abrams reported no conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: At 2 years, 55.6% of patients receiving standard DVT treatment developed post-thrombotic syndrome vs. 41.1% receiving catheter-directed thrombolysis plus standard therapy (P = .047).
Data Source: Prospective, randomized trial in 209 patients with an acute iliofemoral deep vein thrombosis.
Disclosures: Dr. Sandset and Dr. Abrams reported no conflicts of interest.
Multiparametric Magnetic Resonance Imaging Identifies Candidates for Prostate Cancer Surveillance
CHICAGO – Multiparametric magnetic resonance imaging trumped national guidelines in correctly classifying patients with prostate cancer as candidates for active surveillance in a retrospective study of 126 men.
National Comprehensive Cancer Network (NCCN) guidelines misclassified 22 of the 126 patients, compared with 12 using multiparametric magnetic resonance imaging (MP-MRI).
When MP-MRI was added to the NCCN criteria, only 5 patients were misclassified, Dr. Baris Turkbey reported in an award-winning paper at the annual meeting of the Radiological Society of North America.
"Presently, MRI is not in any urology guideline, but we want to change this," he said. "Our future goal is to create an NCI [National Cancer Institute] prostate cancer nomogram that includes multiparametric MRI, and our scientists are close to finishing it."
Dr. Turkbey, a fellow in the division of cancer treatment and diagnosis at the National Institutes of Health in Bethesda, Md., and his colleagues evaluated 126 men with biopsy-proven prostate cancer who underwent 3T MP-MRI of the prostate and subsequent radical prostatectomy at a median of 48 days. Their mean age was 59 years and mean prostate-specific antigen (PSA) level 6.67 ng/mL.
MP-MRI images were obtained of the largest and most aggressive lesion using T2-weighted MRI, diffusion-weighted MRI, MR spectroscopy, and dynamic contrast-enhanced MRI. Each dominant lesion was then assigned an MP-MRI score of low (at least two positive sequences), moderate (three positive sequences), or high (four positive sequences).
Patients were eligible for active surveillance on MP-MRI if they had a dominant tumor of less than 0.5 cm3 without extracapsular extension or seminal vesicle invasion and a low imaging score. The NCCN criteria for active surveillance are T1c disease, Gleason score of 6 or less, fewer than three positive biopsy cores, PSA less than 10 ng/mL, and PSA density less than 0.15 ng/mL/g.
Based on histopathological findings, 14 of 126 patents were eligible for active surveillance, with the remaining 112 candidates for active whole gland treatment.
NCCN guidelines wrongly classified 5 of 14 active surveillance patients and 17 of the 112 active treatment patients, whereas MP-MRI wrongly classified 1 active surveillance and 11 active treatment patients, Dr. Turkbey said.
The sensitivity, specificity, and overall accuracy of the NCCN guidelines were 64%, 35%, and 83%, respectively (P = .00002), compared with 93%, 54%, and 91% with MP-MRI (P less than .000001).
The study was limited by using a relatively simple, nonweighted MP-MRI scoring system and comparing MP-MRI with NCCN guidelines only, he acknowledged. Dr. Turkbey said the researchers are currently evaluating a system in which the various parameters are weighted to obtain better predictions.
When asked whether MP-MRI would be cost effective in routine clinical practice, Dr. Turkbey said that "compared to the costs of doing the wrong thing to a patient, an annual or semiannual MRI is well worth it."
Dr. Turkbey reported having no conflicts of interest. A coauthor reported serving as a researcher for Koninklijke Philips Electronics, General Electric, Siemens, Hoffman-La Roche, and iCAD.
National Comprehensive Cancer Network, NCCN, guidelines, misclassified, MP-MRI, Dr. Baris Turkbey, the Radiological Society of North America, NCI, National Cancer Institute, prostate cancer nomogram, multiparametric MRI, prostatectomy, MP-MRI images, MR spectroscopy, dynamic contrast-enhanced MRI,
CHICAGO – Multiparametric magnetic resonance imaging trumped national guidelines in correctly classifying patients with prostate cancer as candidates for active surveillance in a retrospective study of 126 men.
National Comprehensive Cancer Network (NCCN) guidelines misclassified 22 of the 126 patients, compared with 12 using multiparametric magnetic resonance imaging (MP-MRI).
When MP-MRI was added to the NCCN criteria, only 5 patients were misclassified, Dr. Baris Turkbey reported in an award-winning paper at the annual meeting of the Radiological Society of North America.
"Presently, MRI is not in any urology guideline, but we want to change this," he said. "Our future goal is to create an NCI [National Cancer Institute] prostate cancer nomogram that includes multiparametric MRI, and our scientists are close to finishing it."
Dr. Turkbey, a fellow in the division of cancer treatment and diagnosis at the National Institutes of Health in Bethesda, Md., and his colleagues evaluated 126 men with biopsy-proven prostate cancer who underwent 3T MP-MRI of the prostate and subsequent radical prostatectomy at a median of 48 days. Their mean age was 59 years and mean prostate-specific antigen (PSA) level 6.67 ng/mL.
MP-MRI images were obtained of the largest and most aggressive lesion using T2-weighted MRI, diffusion-weighted MRI, MR spectroscopy, and dynamic contrast-enhanced MRI. Each dominant lesion was then assigned an MP-MRI score of low (at least two positive sequences), moderate (three positive sequences), or high (four positive sequences).
Patients were eligible for active surveillance on MP-MRI if they had a dominant tumor of less than 0.5 cm3 without extracapsular extension or seminal vesicle invasion and a low imaging score. The NCCN criteria for active surveillance are T1c disease, Gleason score of 6 or less, fewer than three positive biopsy cores, PSA less than 10 ng/mL, and PSA density less than 0.15 ng/mL/g.
Based on histopathological findings, 14 of 126 patents were eligible for active surveillance, with the remaining 112 candidates for active whole gland treatment.
NCCN guidelines wrongly classified 5 of 14 active surveillance patients and 17 of the 112 active treatment patients, whereas MP-MRI wrongly classified 1 active surveillance and 11 active treatment patients, Dr. Turkbey said.
The sensitivity, specificity, and overall accuracy of the NCCN guidelines were 64%, 35%, and 83%, respectively (P = .00002), compared with 93%, 54%, and 91% with MP-MRI (P less than .000001).
The study was limited by using a relatively simple, nonweighted MP-MRI scoring system and comparing MP-MRI with NCCN guidelines only, he acknowledged. Dr. Turkbey said the researchers are currently evaluating a system in which the various parameters are weighted to obtain better predictions.
When asked whether MP-MRI would be cost effective in routine clinical practice, Dr. Turkbey said that "compared to the costs of doing the wrong thing to a patient, an annual or semiannual MRI is well worth it."
Dr. Turkbey reported having no conflicts of interest. A coauthor reported serving as a researcher for Koninklijke Philips Electronics, General Electric, Siemens, Hoffman-La Roche, and iCAD.
CHICAGO – Multiparametric magnetic resonance imaging trumped national guidelines in correctly classifying patients with prostate cancer as candidates for active surveillance in a retrospective study of 126 men.
National Comprehensive Cancer Network (NCCN) guidelines misclassified 22 of the 126 patients, compared with 12 using multiparametric magnetic resonance imaging (MP-MRI).
When MP-MRI was added to the NCCN criteria, only 5 patients were misclassified, Dr. Baris Turkbey reported in an award-winning paper at the annual meeting of the Radiological Society of North America.
"Presently, MRI is not in any urology guideline, but we want to change this," he said. "Our future goal is to create an NCI [National Cancer Institute] prostate cancer nomogram that includes multiparametric MRI, and our scientists are close to finishing it."
Dr. Turkbey, a fellow in the division of cancer treatment and diagnosis at the National Institutes of Health in Bethesda, Md., and his colleagues evaluated 126 men with biopsy-proven prostate cancer who underwent 3T MP-MRI of the prostate and subsequent radical prostatectomy at a median of 48 days. Their mean age was 59 years and mean prostate-specific antigen (PSA) level 6.67 ng/mL.
MP-MRI images were obtained of the largest and most aggressive lesion using T2-weighted MRI, diffusion-weighted MRI, MR spectroscopy, and dynamic contrast-enhanced MRI. Each dominant lesion was then assigned an MP-MRI score of low (at least two positive sequences), moderate (three positive sequences), or high (four positive sequences).
Patients were eligible for active surveillance on MP-MRI if they had a dominant tumor of less than 0.5 cm3 without extracapsular extension or seminal vesicle invasion and a low imaging score. The NCCN criteria for active surveillance are T1c disease, Gleason score of 6 or less, fewer than three positive biopsy cores, PSA less than 10 ng/mL, and PSA density less than 0.15 ng/mL/g.
Based on histopathological findings, 14 of 126 patents were eligible for active surveillance, with the remaining 112 candidates for active whole gland treatment.
NCCN guidelines wrongly classified 5 of 14 active surveillance patients and 17 of the 112 active treatment patients, whereas MP-MRI wrongly classified 1 active surveillance and 11 active treatment patients, Dr. Turkbey said.
The sensitivity, specificity, and overall accuracy of the NCCN guidelines were 64%, 35%, and 83%, respectively (P = .00002), compared with 93%, 54%, and 91% with MP-MRI (P less than .000001).
The study was limited by using a relatively simple, nonweighted MP-MRI scoring system and comparing MP-MRI with NCCN guidelines only, he acknowledged. Dr. Turkbey said the researchers are currently evaluating a system in which the various parameters are weighted to obtain better predictions.
When asked whether MP-MRI would be cost effective in routine clinical practice, Dr. Turkbey said that "compared to the costs of doing the wrong thing to a patient, an annual or semiannual MRI is well worth it."
Dr. Turkbey reported having no conflicts of interest. A coauthor reported serving as a researcher for Koninklijke Philips Electronics, General Electric, Siemens, Hoffman-La Roche, and iCAD.
National Comprehensive Cancer Network, NCCN, guidelines, misclassified, MP-MRI, Dr. Baris Turkbey, the Radiological Society of North America, NCI, National Cancer Institute, prostate cancer nomogram, multiparametric MRI, prostatectomy, MP-MRI images, MR spectroscopy, dynamic contrast-enhanced MRI,
National Comprehensive Cancer Network, NCCN, guidelines, misclassified, MP-MRI, Dr. Baris Turkbey, the Radiological Society of North America, NCI, National Cancer Institute, prostate cancer nomogram, multiparametric MRI, prostatectomy, MP-MRI images, MR spectroscopy, dynamic contrast-enhanced MRI,
FROM THE ANNUAL MEETING OF THE RADIOLOGICAL SOCIETY OF NORTH AMERICA
Major Finding: The sensitivity, specificity and overall accuracy of the NCCN guidelines were 64%, 35%, and 83% (P = .00002), compared with 93%, 54%, and 91% with MP-MRI (P less than .000001).
Data Source: Retrospective analysis of 126 patients with prostate cancer.
Disclosures: Dr. Turkbey reported no conflicts of interest. A coauthor reported serving as a researcher for Koninklijke Philips Electronics, General Electric, Siemens, Hoffman-La Roche, and iCAD.
Automated EMR Search Speeds ED Evaluations
CHICAGO – In as little as 15 seconds, an automated electronic medical record search application can retrieve vital past medical history for patients presenting to the emergency department.
"We believe this kind of innovative interface allows for a more efficient view of the entire patient history in the electronic health record," Dr. Arun Krishnaraj said at the annual meeting of the Radiological Society of North America.
The researchers also hope that the novel search tool, known as QPID (Queriable Patient Inference Dossier), will help reduce inappropriate imaging in the emergency department (ED), where incomplete awareness of past imaging studies often leads to duplication.
QPID is a programmable, ontology-driven semantic search application that extracts data from multiple data repositories and then indexes or prepares that information for a search, explained Dr. Krishnaraj of Massachusetts General Hospital and Harvard Medical School in Boston. It gathers data through Web services available in Harvard’s network and can be automated to run against a service schedule or care unit census. It is not a data repository nor does it store personal health information.
QPID goes beyond the simple key word search by allowing a clinician to use a variety of natural language expressions to perform Boolean searches for two terms such as "hepatitis and cirrhosis"; negate a target phrase to exclude unwanted hits, for example, "the patient does not have hepatitis"; and to find exact or partial matches for acronyms, synonyms, or misspellings such as "hepatitis/heputytus," he said.
The three distinct advantages of QPID over a traditional manual record search is that it can search for a concept such as "Does the patient have a malignancy?"; it enables complex structured queries to be run automatically; and it integrates the search output into a web browser or office application, Dr. Krishnaraj said.
The automated queries can be retrieved with a simple search string such as "*malignancy." QPID would then search for evidence of malignancy including synonyms such as tumor, mass, and neoplasm. It will exclude the term "mass" when used in other ways, as in "Mass." for Massachusetts, while also matching exact acronyms for types of malignancy such as NSCLC (non–small cell lung cancer).
QPID has been live for about 6 months at the Massachusetts General ED and about 1 month at the Brigham and Women’s Hospital ED, Dr. Krishnaraj said in an interview. It is available as a search platform to any clinician within Massachusetts General, with custom interfaces developed for several divisions including radiology and gastroenterology, among others.
The researchers validated QPID by performing an automated search for each of the 74 topics included in the application for 500 consecutive patients who presented to the hospital’s ED in 2010. The automated results were then compared with those from two clinicians who performed an untimed manual review for the same 74 search topics on 30 randomly selected patients in the cohort.
"We believe this kind of innovative interface allows for a more efficient view of the entire patient history in the electronic health record."
The average search time for QPID to research all 74 topics was 15 seconds, plus or minus 5 seconds, Dr. Krishnaraj said. To complete a thorough review of all available data, the manual review averaged 5-10 minutes per patient.
For finding laboratory results, QPID demonstrated a sensitivity of 97% and specificity of 99%, with a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.
Excellent results were also seen for free text searches, such as "is there a history of PE in the last 10 years?" For these, QPID had a sensitivity of 98%, specificity of 93%, PPV of 90%, and NPV of 98%.
Dr. Krishnaraj acknowledged that the overall results could be affected by data in the EMR not detected by either QPID or manual review. Other limitations include the potential for false positives, overestimation of low-prevalence conditions, and missing data for care received outside the hospital network and not recorded electronically.
The researchers are currently evaluating how best to measure the effect of QPID use on imaging use in the two hospitals’ large and busy EDs.
Dr. Krishnaraj and a coauthor reported research support/grants from General Electric.
CHICAGO – In as little as 15 seconds, an automated electronic medical record search application can retrieve vital past medical history for patients presenting to the emergency department.
"We believe this kind of innovative interface allows for a more efficient view of the entire patient history in the electronic health record," Dr. Arun Krishnaraj said at the annual meeting of the Radiological Society of North America.
The researchers also hope that the novel search tool, known as QPID (Queriable Patient Inference Dossier), will help reduce inappropriate imaging in the emergency department (ED), where incomplete awareness of past imaging studies often leads to duplication.
QPID is a programmable, ontology-driven semantic search application that extracts data from multiple data repositories and then indexes or prepares that information for a search, explained Dr. Krishnaraj of Massachusetts General Hospital and Harvard Medical School in Boston. It gathers data through Web services available in Harvard’s network and can be automated to run against a service schedule or care unit census. It is not a data repository nor does it store personal health information.
QPID goes beyond the simple key word search by allowing a clinician to use a variety of natural language expressions to perform Boolean searches for two terms such as "hepatitis and cirrhosis"; negate a target phrase to exclude unwanted hits, for example, "the patient does not have hepatitis"; and to find exact or partial matches for acronyms, synonyms, or misspellings such as "hepatitis/heputytus," he said.
The three distinct advantages of QPID over a traditional manual record search is that it can search for a concept such as "Does the patient have a malignancy?"; it enables complex structured queries to be run automatically; and it integrates the search output into a web browser or office application, Dr. Krishnaraj said.
The automated queries can be retrieved with a simple search string such as "*malignancy." QPID would then search for evidence of malignancy including synonyms such as tumor, mass, and neoplasm. It will exclude the term "mass" when used in other ways, as in "Mass." for Massachusetts, while also matching exact acronyms for types of malignancy such as NSCLC (non–small cell lung cancer).
QPID has been live for about 6 months at the Massachusetts General ED and about 1 month at the Brigham and Women’s Hospital ED, Dr. Krishnaraj said in an interview. It is available as a search platform to any clinician within Massachusetts General, with custom interfaces developed for several divisions including radiology and gastroenterology, among others.
The researchers validated QPID by performing an automated search for each of the 74 topics included in the application for 500 consecutive patients who presented to the hospital’s ED in 2010. The automated results were then compared with those from two clinicians who performed an untimed manual review for the same 74 search topics on 30 randomly selected patients in the cohort.
"We believe this kind of innovative interface allows for a more efficient view of the entire patient history in the electronic health record."
The average search time for QPID to research all 74 topics was 15 seconds, plus or minus 5 seconds, Dr. Krishnaraj said. To complete a thorough review of all available data, the manual review averaged 5-10 minutes per patient.
For finding laboratory results, QPID demonstrated a sensitivity of 97% and specificity of 99%, with a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.
Excellent results were also seen for free text searches, such as "is there a history of PE in the last 10 years?" For these, QPID had a sensitivity of 98%, specificity of 93%, PPV of 90%, and NPV of 98%.
Dr. Krishnaraj acknowledged that the overall results could be affected by data in the EMR not detected by either QPID or manual review. Other limitations include the potential for false positives, overestimation of low-prevalence conditions, and missing data for care received outside the hospital network and not recorded electronically.
The researchers are currently evaluating how best to measure the effect of QPID use on imaging use in the two hospitals’ large and busy EDs.
Dr. Krishnaraj and a coauthor reported research support/grants from General Electric.
CHICAGO – In as little as 15 seconds, an automated electronic medical record search application can retrieve vital past medical history for patients presenting to the emergency department.
"We believe this kind of innovative interface allows for a more efficient view of the entire patient history in the electronic health record," Dr. Arun Krishnaraj said at the annual meeting of the Radiological Society of North America.
The researchers also hope that the novel search tool, known as QPID (Queriable Patient Inference Dossier), will help reduce inappropriate imaging in the emergency department (ED), where incomplete awareness of past imaging studies often leads to duplication.
QPID is a programmable, ontology-driven semantic search application that extracts data from multiple data repositories and then indexes or prepares that information for a search, explained Dr. Krishnaraj of Massachusetts General Hospital and Harvard Medical School in Boston. It gathers data through Web services available in Harvard’s network and can be automated to run against a service schedule or care unit census. It is not a data repository nor does it store personal health information.
QPID goes beyond the simple key word search by allowing a clinician to use a variety of natural language expressions to perform Boolean searches for two terms such as "hepatitis and cirrhosis"; negate a target phrase to exclude unwanted hits, for example, "the patient does not have hepatitis"; and to find exact or partial matches for acronyms, synonyms, or misspellings such as "hepatitis/heputytus," he said.
The three distinct advantages of QPID over a traditional manual record search is that it can search for a concept such as "Does the patient have a malignancy?"; it enables complex structured queries to be run automatically; and it integrates the search output into a web browser or office application, Dr. Krishnaraj said.
The automated queries can be retrieved with a simple search string such as "*malignancy." QPID would then search for evidence of malignancy including synonyms such as tumor, mass, and neoplasm. It will exclude the term "mass" when used in other ways, as in "Mass." for Massachusetts, while also matching exact acronyms for types of malignancy such as NSCLC (non–small cell lung cancer).
QPID has been live for about 6 months at the Massachusetts General ED and about 1 month at the Brigham and Women’s Hospital ED, Dr. Krishnaraj said in an interview. It is available as a search platform to any clinician within Massachusetts General, with custom interfaces developed for several divisions including radiology and gastroenterology, among others.
The researchers validated QPID by performing an automated search for each of the 74 topics included in the application for 500 consecutive patients who presented to the hospital’s ED in 2010. The automated results were then compared with those from two clinicians who performed an untimed manual review for the same 74 search topics on 30 randomly selected patients in the cohort.
"We believe this kind of innovative interface allows for a more efficient view of the entire patient history in the electronic health record."
The average search time for QPID to research all 74 topics was 15 seconds, plus or minus 5 seconds, Dr. Krishnaraj said. To complete a thorough review of all available data, the manual review averaged 5-10 minutes per patient.
For finding laboratory results, QPID demonstrated a sensitivity of 97% and specificity of 99%, with a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.
Excellent results were also seen for free text searches, such as "is there a history of PE in the last 10 years?" For these, QPID had a sensitivity of 98%, specificity of 93%, PPV of 90%, and NPV of 98%.
Dr. Krishnaraj acknowledged that the overall results could be affected by data in the EMR not detected by either QPID or manual review. Other limitations include the potential for false positives, overestimation of low-prevalence conditions, and missing data for care received outside the hospital network and not recorded electronically.
The researchers are currently evaluating how best to measure the effect of QPID use on imaging use in the two hospitals’ large and busy EDs.
Dr. Krishnaraj and a coauthor reported research support/grants from General Electric.
FROM THE ANNUAL MEETING OF THE RADIOLOGICAL SOCIETY OF NORTH AMERICA
Tomosynthesis Shines in Dense Breast Cases
CHICAGO – Adding tomosynthesis to full-field digital mammography improved cancer detection and reduced recall rates in women with dense breasts in a study of 293 patients.
"Both clinically and in trials, we’ve seen that tomosynthesis offers benefit for all women, but I think there is a particular benefit, the increased gains are more, for women with dense breast tissue," Dr. Elizabeth Rafferty said at the annual meeting of the Radiological Society of North America. "I think that underscores where we may start our triage efforts with limited resources."
She reported on an enriched case set of 69 biopsy-proven cancers, 74 benign biopsies, 50 recalled screening cases, and 100 negative screening cases, all with a BI-RADS (Breast Imaging Reporting and Data System) density score of 3 (heterogeneously dense) or 4 (extremely dense). Calcification was present in 25% and noncalcification in 75% of cases.
Eight radiologists read the cases in two separate sessions separated by 1 month, with half of the cases read in each mode for each reading session. Identification of the lesion location and type and initial BI-RADS score (0, 1, 2) were used to determine the recall rate. A probability of malignancy score from 0% to 100% was used to calculate the receiver operating area under the curve (AUC).
The difference in the AUC between standard full-field digital mammography (FFDM) plus tomosynthesis and FFDM alone was significantly higher at 8.3% for all cases (AUC 0.940 vs. 0.857, P value less than .0001), 4.1% for calcification cases (0.818 vs. 0.777, P = .048), and 11% for noncalcification cases (0.977 vs. 0.867, P = .0001), reported Dr. Rafferty, director of breast imaging at Massachusetts General Hospital in Boston.
The recall rate for all cancer cases was 9.7% higher for FFDM plus tomosynthesis vs. FFDM alone. Specifically, it was 3.8% higher for calcification cases and 14.3% higher for noncalcification cases.
Seven of the eight readers increased their cancer detection rate using FFDM plus tomosynthesis, while one reader had the same detection rate on the two modalities. For six of the seven readers, the improvement in cancer detection was statistically significant, she said.
For noncancer screening cases, the recall rate for FFDM plus tomosynthesis was 23.3% vs. 33.9% for FFDM alone, representing a 31% reduction in the noncancer recall rate.
Six of the eight readers had significant decreases in their screening recall rate using the combined imaging modality, while the other two readers had no significant change.
"In women with dense breast tissue, tomosynthesis, when added to FFDM, appears to offer particular value both in terms of sensitivity as well as specificity of the examination," Dr. Rafferty said.
She noted that the numbers were too small to identify a difference in performance with FFDM plus tomosynthesis between dense and extremely dense breasts.
"In women with dense breast tissue, tomosynthesis ... appears to offer particular value."
An attendee also asked whether she would recommend using tomosynthesis in lieu of screening ultrasound.
"In terms of the positive predictive value of screening ultrasound, I think that screening mammography, or some form of screening mammography, is going to remain the mainstay," she said. "But in terms of our diagnostic evaluation, I think ultrasound has become an incredibly important tool in the diagnostic evaluation.
"Basically, tomosynthesis examination plus ultrasound has become for me, and I think for many other people, kind of the go-to regimen instead of using additional views. The two are very complementary."
Dr. Rafferty reported no relevant financial disclosures. A coauthor reported serving as a patent holder and employee of Hologic.
CHICAGO – Adding tomosynthesis to full-field digital mammography improved cancer detection and reduced recall rates in women with dense breasts in a study of 293 patients.
"Both clinically and in trials, we’ve seen that tomosynthesis offers benefit for all women, but I think there is a particular benefit, the increased gains are more, for women with dense breast tissue," Dr. Elizabeth Rafferty said at the annual meeting of the Radiological Society of North America. "I think that underscores where we may start our triage efforts with limited resources."
She reported on an enriched case set of 69 biopsy-proven cancers, 74 benign biopsies, 50 recalled screening cases, and 100 negative screening cases, all with a BI-RADS (Breast Imaging Reporting and Data System) density score of 3 (heterogeneously dense) or 4 (extremely dense). Calcification was present in 25% and noncalcification in 75% of cases.
Eight radiologists read the cases in two separate sessions separated by 1 month, with half of the cases read in each mode for each reading session. Identification of the lesion location and type and initial BI-RADS score (0, 1, 2) were used to determine the recall rate. A probability of malignancy score from 0% to 100% was used to calculate the receiver operating area under the curve (AUC).
The difference in the AUC between standard full-field digital mammography (FFDM) plus tomosynthesis and FFDM alone was significantly higher at 8.3% for all cases (AUC 0.940 vs. 0.857, P value less than .0001), 4.1% for calcification cases (0.818 vs. 0.777, P = .048), and 11% for noncalcification cases (0.977 vs. 0.867, P = .0001), reported Dr. Rafferty, director of breast imaging at Massachusetts General Hospital in Boston.
The recall rate for all cancer cases was 9.7% higher for FFDM plus tomosynthesis vs. FFDM alone. Specifically, it was 3.8% higher for calcification cases and 14.3% higher for noncalcification cases.
Seven of the eight readers increased their cancer detection rate using FFDM plus tomosynthesis, while one reader had the same detection rate on the two modalities. For six of the seven readers, the improvement in cancer detection was statistically significant, she said.
For noncancer screening cases, the recall rate for FFDM plus tomosynthesis was 23.3% vs. 33.9% for FFDM alone, representing a 31% reduction in the noncancer recall rate.
Six of the eight readers had significant decreases in their screening recall rate using the combined imaging modality, while the other two readers had no significant change.
"In women with dense breast tissue, tomosynthesis, when added to FFDM, appears to offer particular value both in terms of sensitivity as well as specificity of the examination," Dr. Rafferty said.
She noted that the numbers were too small to identify a difference in performance with FFDM plus tomosynthesis between dense and extremely dense breasts.
"In women with dense breast tissue, tomosynthesis ... appears to offer particular value."
An attendee also asked whether she would recommend using tomosynthesis in lieu of screening ultrasound.
"In terms of the positive predictive value of screening ultrasound, I think that screening mammography, or some form of screening mammography, is going to remain the mainstay," she said. "But in terms of our diagnostic evaluation, I think ultrasound has become an incredibly important tool in the diagnostic evaluation.
"Basically, tomosynthesis examination plus ultrasound has become for me, and I think for many other people, kind of the go-to regimen instead of using additional views. The two are very complementary."
Dr. Rafferty reported no relevant financial disclosures. A coauthor reported serving as a patent holder and employee of Hologic.
CHICAGO – Adding tomosynthesis to full-field digital mammography improved cancer detection and reduced recall rates in women with dense breasts in a study of 293 patients.
"Both clinically and in trials, we’ve seen that tomosynthesis offers benefit for all women, but I think there is a particular benefit, the increased gains are more, for women with dense breast tissue," Dr. Elizabeth Rafferty said at the annual meeting of the Radiological Society of North America. "I think that underscores where we may start our triage efforts with limited resources."
She reported on an enriched case set of 69 biopsy-proven cancers, 74 benign biopsies, 50 recalled screening cases, and 100 negative screening cases, all with a BI-RADS (Breast Imaging Reporting and Data System) density score of 3 (heterogeneously dense) or 4 (extremely dense). Calcification was present in 25% and noncalcification in 75% of cases.
Eight radiologists read the cases in two separate sessions separated by 1 month, with half of the cases read in each mode for each reading session. Identification of the lesion location and type and initial BI-RADS score (0, 1, 2) were used to determine the recall rate. A probability of malignancy score from 0% to 100% was used to calculate the receiver operating area under the curve (AUC).
The difference in the AUC between standard full-field digital mammography (FFDM) plus tomosynthesis and FFDM alone was significantly higher at 8.3% for all cases (AUC 0.940 vs. 0.857, P value less than .0001), 4.1% for calcification cases (0.818 vs. 0.777, P = .048), and 11% for noncalcification cases (0.977 vs. 0.867, P = .0001), reported Dr. Rafferty, director of breast imaging at Massachusetts General Hospital in Boston.
The recall rate for all cancer cases was 9.7% higher for FFDM plus tomosynthesis vs. FFDM alone. Specifically, it was 3.8% higher for calcification cases and 14.3% higher for noncalcification cases.
Seven of the eight readers increased their cancer detection rate using FFDM plus tomosynthesis, while one reader had the same detection rate on the two modalities. For six of the seven readers, the improvement in cancer detection was statistically significant, she said.
For noncancer screening cases, the recall rate for FFDM plus tomosynthesis was 23.3% vs. 33.9% for FFDM alone, representing a 31% reduction in the noncancer recall rate.
Six of the eight readers had significant decreases in their screening recall rate using the combined imaging modality, while the other two readers had no significant change.
"In women with dense breast tissue, tomosynthesis, when added to FFDM, appears to offer particular value both in terms of sensitivity as well as specificity of the examination," Dr. Rafferty said.
She noted that the numbers were too small to identify a difference in performance with FFDM plus tomosynthesis between dense and extremely dense breasts.
"In women with dense breast tissue, tomosynthesis ... appears to offer particular value."
An attendee also asked whether she would recommend using tomosynthesis in lieu of screening ultrasound.
"In terms of the positive predictive value of screening ultrasound, I think that screening mammography, or some form of screening mammography, is going to remain the mainstay," she said. "But in terms of our diagnostic evaluation, I think ultrasound has become an incredibly important tool in the diagnostic evaluation.
"Basically, tomosynthesis examination plus ultrasound has become for me, and I think for many other people, kind of the go-to regimen instead of using additional views. The two are very complementary."
Dr. Rafferty reported no relevant financial disclosures. A coauthor reported serving as a patent holder and employee of Hologic.
FROM THE ANNUAL MEETING OF THE RADIOLOGICAL SOCIETY OF NORTH AMERICA
Major Finding: The recall rate for cancer cases was 9.7% higher with tomosynthesis plus full-field digital mammography vs. FFDM alone, while the recall rate for noncancer screening cases was reduced 31% with tomosynthesis plus FFDM.
Data Source: Enriched case set of 293 cases with heterogeneously dense or extremely dense breast tissue.
Disclosures: Dr. Rafferty reported no relevant financial disclosures. A coauthor reported serving as a patent holder and employee of Hologic.
Breast-Mammogram Detector Mismatch Results in Excess Radiation
CHICAGO – A mismatch between breast size and detector size during mammography resulted in significantly higher doses of radiation for women with large breasts in a study of 886 patients.
On average, women with large breasts screened on a small detector received almost 5 milligray (mGy) of radiation, which exceeds the American College of Radiology guidelines of 3-4 mGy or less for a standard two-view mammogram.
When a mismatch occurs, women with large breasts receive significantly higher doses of radiation than women with small breasts or their counterparts with large breasts correctly matched to a large detector, Dr. Cathy Wells said when presenting the award-winning study at the annual meeting of the Radiological Society of North America.
"Women with large breasts should be imaged with a large detector to avoid an unnecessary increase in radiation dose," she urged.
The quality assurance study involved 886 women who presented for screening or diagnostic mammography during a 6-week period in late 2009. The exams were performed with a phosphor charge-coupled device detector, which is available in pre-set sizes (large or small) due to manufacturing constraints, she said. Insufficient data for 22 patients left 426 screening and 438 diagnostic patients evaluable for analysis.
A sizeable number, or almost 20% of patients, were affected by a mismatch between breast and detector size, said Dr. Wells, who completed the study at Beth Israel Deaconess Medical Center and is now a breast imaging fellow at Massachusetts General Hospital, both in Boston.
The percentage of mismatches varied from 10% of screening patients with large breasts, defined as a "C" cup or larger, to 27% of screening patients with small breasts imaged with a large detector.
A mismatch occurred in 22% of diagnostic mammography patients with large breasts and 17% of diagnostic patients with small breasts.
Despite the sizeable number of mismatches in the study, not all women will be faced with this problem when they arrive for their mammogram, Dr. Wells said in an interview. The phosphor charge-coupled device detector is one of four types of digital detectors currently available in the United States, and to her knowledge the only type that has such size constraints. In addition, not all imaging centers use this detector type.
Some centers, including her own, have both large- and small-size detectors available, although there can be a wait for the proper size, she noted. Women can choose to wait or be imaged with a different detector after a discussion with the technologist.
"The best option for women to ensure a correct match between breast size and detector size would be to talk with the technologist who performs the actual mammogram, [as] the scheduler or person at the check-in desk will likely not know the answer," Dr. Wells said.
"Women could ask the technologist whether the detector comes in different sizes, since not all do, and if so, whether they are correctly matched."
Screening mammogram patients with correctly matched breast and detector sizes received an average mean glandular dose per breast of 3.3 mGy, compared with 4.9 mGy for mismatched patients with large breasts (P value less than .05).
This was due to significantly more views obtained in mismatched patients with large breasts, compared with both the large-breast patients imaged on a large detector and small-breast patients imaged on a small detector (mean 5.9 views vs. 4.6 views vs. 4.7 views, P less than .05), Dr. Wells said. Interestingly, small-breast patients mismatched to a large detector underwent a similar number of views at a mean of 4.6, but actually received slightly less radiation at mean dose of 2.9 mGy (P less than .05).
During diagnostic mammograms, the radiation dose was again significantly higher among mismatched patients with large breasts, compared with the correctly matched large- and small-breast groups (8.2 mGy vs. 6.7 mGy, P less than .05), but it did not appear to be related to the number of views obtained, she said, adding that other factors must be at work. Several variables contribute to radiation dose, but in this case, the most likely culprit is compression thickness, Dr. Wells said.
"It may be more difficult to adequately compress a large breast with a small detector, resulting in a larger radiation dose," she said. "We hope to analyze the data again, to answer this question."
Dr. Wells and her coauthors reported having no conflicts of interest.
CHICAGO – A mismatch between breast size and detector size during mammography resulted in significantly higher doses of radiation for women with large breasts in a study of 886 patients.
On average, women with large breasts screened on a small detector received almost 5 milligray (mGy) of radiation, which exceeds the American College of Radiology guidelines of 3-4 mGy or less for a standard two-view mammogram.
When a mismatch occurs, women with large breasts receive significantly higher doses of radiation than women with small breasts or their counterparts with large breasts correctly matched to a large detector, Dr. Cathy Wells said when presenting the award-winning study at the annual meeting of the Radiological Society of North America.
"Women with large breasts should be imaged with a large detector to avoid an unnecessary increase in radiation dose," she urged.
The quality assurance study involved 886 women who presented for screening or diagnostic mammography during a 6-week period in late 2009. The exams were performed with a phosphor charge-coupled device detector, which is available in pre-set sizes (large or small) due to manufacturing constraints, she said. Insufficient data for 22 patients left 426 screening and 438 diagnostic patients evaluable for analysis.
A sizeable number, or almost 20% of patients, were affected by a mismatch between breast and detector size, said Dr. Wells, who completed the study at Beth Israel Deaconess Medical Center and is now a breast imaging fellow at Massachusetts General Hospital, both in Boston.
The percentage of mismatches varied from 10% of screening patients with large breasts, defined as a "C" cup or larger, to 27% of screening patients with small breasts imaged with a large detector.
A mismatch occurred in 22% of diagnostic mammography patients with large breasts and 17% of diagnostic patients with small breasts.
Despite the sizeable number of mismatches in the study, not all women will be faced with this problem when they arrive for their mammogram, Dr. Wells said in an interview. The phosphor charge-coupled device detector is one of four types of digital detectors currently available in the United States, and to her knowledge the only type that has such size constraints. In addition, not all imaging centers use this detector type.
Some centers, including her own, have both large- and small-size detectors available, although there can be a wait for the proper size, she noted. Women can choose to wait or be imaged with a different detector after a discussion with the technologist.
"The best option for women to ensure a correct match between breast size and detector size would be to talk with the technologist who performs the actual mammogram, [as] the scheduler or person at the check-in desk will likely not know the answer," Dr. Wells said.
"Women could ask the technologist whether the detector comes in different sizes, since not all do, and if so, whether they are correctly matched."
Screening mammogram patients with correctly matched breast and detector sizes received an average mean glandular dose per breast of 3.3 mGy, compared with 4.9 mGy for mismatched patients with large breasts (P value less than .05).
This was due to significantly more views obtained in mismatched patients with large breasts, compared with both the large-breast patients imaged on a large detector and small-breast patients imaged on a small detector (mean 5.9 views vs. 4.6 views vs. 4.7 views, P less than .05), Dr. Wells said. Interestingly, small-breast patients mismatched to a large detector underwent a similar number of views at a mean of 4.6, but actually received slightly less radiation at mean dose of 2.9 mGy (P less than .05).
During diagnostic mammograms, the radiation dose was again significantly higher among mismatched patients with large breasts, compared with the correctly matched large- and small-breast groups (8.2 mGy vs. 6.7 mGy, P less than .05), but it did not appear to be related to the number of views obtained, she said, adding that other factors must be at work. Several variables contribute to radiation dose, but in this case, the most likely culprit is compression thickness, Dr. Wells said.
"It may be more difficult to adequately compress a large breast with a small detector, resulting in a larger radiation dose," she said. "We hope to analyze the data again, to answer this question."
Dr. Wells and her coauthors reported having no conflicts of interest.
CHICAGO – A mismatch between breast size and detector size during mammography resulted in significantly higher doses of radiation for women with large breasts in a study of 886 patients.
On average, women with large breasts screened on a small detector received almost 5 milligray (mGy) of radiation, which exceeds the American College of Radiology guidelines of 3-4 mGy or less for a standard two-view mammogram.
When a mismatch occurs, women with large breasts receive significantly higher doses of radiation than women with small breasts or their counterparts with large breasts correctly matched to a large detector, Dr. Cathy Wells said when presenting the award-winning study at the annual meeting of the Radiological Society of North America.
"Women with large breasts should be imaged with a large detector to avoid an unnecessary increase in radiation dose," she urged.
The quality assurance study involved 886 women who presented for screening or diagnostic mammography during a 6-week period in late 2009. The exams were performed with a phosphor charge-coupled device detector, which is available in pre-set sizes (large or small) due to manufacturing constraints, she said. Insufficient data for 22 patients left 426 screening and 438 diagnostic patients evaluable for analysis.
A sizeable number, or almost 20% of patients, were affected by a mismatch between breast and detector size, said Dr. Wells, who completed the study at Beth Israel Deaconess Medical Center and is now a breast imaging fellow at Massachusetts General Hospital, both in Boston.
The percentage of mismatches varied from 10% of screening patients with large breasts, defined as a "C" cup or larger, to 27% of screening patients with small breasts imaged with a large detector.
A mismatch occurred in 22% of diagnostic mammography patients with large breasts and 17% of diagnostic patients with small breasts.
Despite the sizeable number of mismatches in the study, not all women will be faced with this problem when they arrive for their mammogram, Dr. Wells said in an interview. The phosphor charge-coupled device detector is one of four types of digital detectors currently available in the United States, and to her knowledge the only type that has such size constraints. In addition, not all imaging centers use this detector type.
Some centers, including her own, have both large- and small-size detectors available, although there can be a wait for the proper size, she noted. Women can choose to wait or be imaged with a different detector after a discussion with the technologist.
"The best option for women to ensure a correct match between breast size and detector size would be to talk with the technologist who performs the actual mammogram, [as] the scheduler or person at the check-in desk will likely not know the answer," Dr. Wells said.
"Women could ask the technologist whether the detector comes in different sizes, since not all do, and if so, whether they are correctly matched."
Screening mammogram patients with correctly matched breast and detector sizes received an average mean glandular dose per breast of 3.3 mGy, compared with 4.9 mGy for mismatched patients with large breasts (P value less than .05).
This was due to significantly more views obtained in mismatched patients with large breasts, compared with both the large-breast patients imaged on a large detector and small-breast patients imaged on a small detector (mean 5.9 views vs. 4.6 views vs. 4.7 views, P less than .05), Dr. Wells said. Interestingly, small-breast patients mismatched to a large detector underwent a similar number of views at a mean of 4.6, but actually received slightly less radiation at mean dose of 2.9 mGy (P less than .05).
During diagnostic mammograms, the radiation dose was again significantly higher among mismatched patients with large breasts, compared with the correctly matched large- and small-breast groups (8.2 mGy vs. 6.7 mGy, P less than .05), but it did not appear to be related to the number of views obtained, she said, adding that other factors must be at work. Several variables contribute to radiation dose, but in this case, the most likely culprit is compression thickness, Dr. Wells said.
"It may be more difficult to adequately compress a large breast with a small detector, resulting in a larger radiation dose," she said. "We hope to analyze the data again, to answer this question."
Dr. Wells and her coauthors reported having no conflicts of interest.
FROM THE ANNUAL MEETING OF THE RADIOLOGICAL SOCIETY OF NORTH AMERICA
Major Finding: Screening mammogram patients with correctly matched breast and detector sizes received an average mean glandular dose per breast of 3.3 mGy vs. 4.9 mGy for mismatched patients with large breasts (P value less than .05).
Data Source: Quality assurance study in 886 mammography patients.
Disclosures: Dr. Wells and her coauthors reported having no conflicts of interest.