User login
Scleroderma patients suffer from small bowel bacterial overgrowth
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
AT THE EULAR CONGRESS 2013
Scleroderma patients suffer from small bowel bacterial overgrowth
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
MADRID – More than a third of patients with systemic sclerosis and intestinal symptoms have an increase in gastrointestinal tract bacteria, an alteration in the type of gut microbes present, or both, based on data from a French study presented at the annual European Congress of Rheumatology.
Small intestinal bacterial overgrowth (SIBO) was found to affect 14 (38%) of 37 patients included in the study. These patients had been recruited from a larger group of 120 scleroderma patients who complained of gastrointestinal symptoms over a 2-year period.
"SIBO can compromise patients’ quality of life and be responsible for mortality," study investigator Dr. Marie Tauber said. SIBO can cause GI symptoms such as bloating, diarrhea, malabsorption, weight loss, and malnutrition, which may have a significant impact on patients’ overall prognosis, she noted.
Dr. Tauber, a dermatologist who took part in the research as part of an internship within the rheumatology department of the Hôpital Cochin in Paris, explained the three goals of the study. The first was to examine the prevalence of SIBO in patients with systemic sclerosis exhibiting GI symptoms, and the second was to identify subsets of patients who might be at increased risk. A third goal was to observe the impact of optimal SIBO treatment on the patients’ conditions.
The median age of participants was 59 years, and 79% were women. The median disease duration was approximately 10 years, and 49% of patients had diffuse cutaneous disease.
A diagnosis of SIBO was based on positive hydrogen and methane breath tests, and blood assays were used to assess the presence of malabsorption.
The researchers also administered two questionnaires – the generic SF-36 (Short Form 36) Health Survey and the disease-specific UCLA SCTC GIT (University of California, Los Angeles, Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument) – to patients at the time of their breath testing visits.
All patients with SIBO were treated with a rotating regimen of amoxicillin, ciprofloxacin, and metronidazole, each given for 1 month at a time. Breath tests, as well as the UCLA SCTC GIT, were repeated. Fewer than 50% of patients had a negative breath test after antibody treatment, which highlighted a need to repeat the test after antibiotic treatment to determine whether a second course is required, Dr. Tauber noted.
Three clinical parameters separated patients with and without SIBO: longer disease duration (11 vs. 7 years; P = .02), lower prevalence of anti-topoisomerase I antibodies (Anti-Scl-70 Ab, 7% vs. 39%; P = .04), and higher prevalence of definite pulmonary arterial hypertension (PAH, 21% vs. 0%; P = .04).
Total UCLA SCTC GIT scores were higher in patients with SIBO than in those without it (0.79 vs. 0.31; P = .03). SIBO-affected patients also were more likely to have weight loss of 5% or more (43% vs. 8%; P = .03).
Given the small number of patients, it is difficult to draw firm conclusions from these data, and larger studies are needed, Dr. Tauber observed. However, the findings suggest that there may be factors associated with SIBO that could be targeted in an intervention program.
Two patients with SIBO died despite antibiotic treatment, which "underscores, unfortunately, the association between SIBO and mortality," Dr. Tauber said.
"To our knowledge, this is the first study using the UCLA SCTC GIT to identify patients at risk of SIBO in systemic sclerosis," and the data support the systematic use of this score, together with regular weight evaluation in these patients, she concluded.
Dr. Tauber had no financial conflicts to disclose.
AT THE EULAR CONGRESS 2013
Major finding: SIBO affected 14 (38%) of 37 scleroderma patients with gastrointestinal symptoms.
Data source: Observational study of 120 adults with systemic sclerosis and intestinal symptoms.
Disclosures: Dr. Tauber had no financial conflicts to disclose.
Apremilast effects sustained at 1 year in psoriatic arthritis
MADRID – Apremilast improves the signs and symptoms of psoriatic arthritis in about 60% of patients at 1 year, according to long-term data from the PALACE 1 trial.
At week 52, a 20% improvement in disease symptoms according to American College of Rheumatology (ACR 20) response criteria was achieved by 57%-63% of patients treated with apremilast, providing evidence of sustained treatment effects.
The PALACE 1 trial’s primary endpoint of an ACR 20 at 16 weeks, already reported last year, was achieved by 31% of patients treated with apremilast 20 mg and 40% of those given apremilast 30 mg, compared with 19% of those given placebo.
"Oral apremilast demonstrated long-term efficacy, including improvement in signs and symptoms and physical function, and skin manifestations," Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual European Congress of Rheumatology.
Apremilast is an oral phosphodiesterase 4 inhibitor under investigation as a treatment for active psoriatic arthritis (PsA). It is being evaluated as a possible treatment for skin psoriasis, ankylosing spondylitis, rheumatoid arthritis, and Behçet’s disease.
PALACE 1 was a phase III, multicenter, double-blind, placebo-controlled study of apremilast for the treatment of active PsA. A total of 504 patients with a documented diagnosis of PsA for at least 6 months were recruited into the study (Ann. Rheum. Dis. 2013;72[Suppls3]:163).
Functional outcomes improved
Physical function improved according to measurements on the Health Assessment Questionnaire–Disability Index (HAQ-DI). At baseline, HAQ-DI scores were 1.21, and "we saw that patients improved by –0.35, which is certainly the level that patients can say, ‘I feel better and I can do my daily activities better,’ " Dr. Kavanaugh said.
Additionally, 25% and 37% of patients treated with apremilast 20 mg and 30 mg, respectively, achieved a 75% improvement in the Psoriasis Area and Severity Index at 52 weeks, which is a "very high bar" to achieve, he noted.
The main side effect seen was diarrhea, affecting 11%-19% of patients given apremilast and 2.4% given placebo. However, diarrhea occurred mainly in the first 6 months of therapy and could be managed by taking appropriate measures on an individual patient basis, Dr. Kavanaugh said. This might include prescription of an antidiarrheal agent.
"Prolonged exposure to apremilast did not result in any unexpected increased incidence of adverse events or laboratory abnormalities," he noted. The latter could mean that, if approved, apremilast might not need routine laboratory monitoring.
The PALACE development program
PALACE 1 is one of several clinical trials that have investigated the efficacy and safety of apremilast in active PsA. In these studies, 24 weeks’ treatment with one of two oral doses (20 mg or 30 mg twice daily) of apremilast was compared to placebo. The primary endpoint of the studies was the percentage of patients achieving ACR 20 at 16 weeks.
For inclusion in the trials, patients had to have active disease despite prior therapy with disease-modifying antirheumatic drugs (DMARDs), biologic agents, or both. Dr. Kavanaugh noted that the majority of patients had failed DMARD therapy in PALACE 1, with almost one-quarter receiving prior biologic therapy.
Patients who had a less than 20% reduction from baseline in swollen/tender joint counts at 16 weeks were re-randomized to receive apremilast 20 mg or 30 mg if they had originally been treated with placebo, while patients originally randomized to active treatment stayed on their initial dose if they failed to respond significantly. At the end of the planned 24-week treatment period, all remaining patients on placebo were re-randomized to apremilast 20 mg or 30 mg until 1 year of follow up.
PALACE 3 data also reported
The results of PALACE 3 and combined 6-month safety data from the PALACE 1, PALACE 2, and PALACE 3 trials were also reported at the congress.
In PALACE 3 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:685), significantly more patients achieved the primary endpoint of an ACR 20 at 16 weeks if they were treated with either the 20-mg dose (29.4%, P = .02) or 30-mg dose (42.8%, P less than .0001) of apremilast, compared with those given placebo (18.9%). There was also statistically significant and "clinically meaningful" improvement in physical function and pain. "The results of PALACE 3 support the efficacy and safety findings of the PALACE 1 study and help establish the profile of apremilast in PsA," the PALACE 3 investigators concluded.
The pooled safety findings revealed no new safety concerns and showed apremilast was generally well tolerated (Ann. Rheum. Dis. 2013;72[Suppl. 3]:85). Rates of diarrhea at 24 weeks were 12.6% and 16.5% for the 20-mg and 30-mg doses of apremilast, and 2.8% for placebo, respectively. Other side effects of note included nausea (10% and 16.1% vs. 4.6%), headache (8.4% and 11.5% vs. 4.6%), and upper respiratory tract infection (7% and 6% vs. 3%).
Time for regulatory approval
Based on the positive findings of the PALACE 1, 2, and 3 studies, apremilast’s developer, Celgene, is expected to file for regulatory approval in the treatment of active PsA. In doing so, apremilast will join another novel agent, ustekinumab, in the queue for approval for this indication.
Ustekinumab is a human interleukin-12 and -23 antagonist produced by Janssen that is already approved in Europe and in the United States for skin psoriasis. One-year data also show that it is effective and well tolerated for PsA. It is given subcutaneously, whereas apremilast is an oral agent.
Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
MADRID – Apremilast improves the signs and symptoms of psoriatic arthritis in about 60% of patients at 1 year, according to long-term data from the PALACE 1 trial.
At week 52, a 20% improvement in disease symptoms according to American College of Rheumatology (ACR 20) response criteria was achieved by 57%-63% of patients treated with apremilast, providing evidence of sustained treatment effects.
The PALACE 1 trial’s primary endpoint of an ACR 20 at 16 weeks, already reported last year, was achieved by 31% of patients treated with apremilast 20 mg and 40% of those given apremilast 30 mg, compared with 19% of those given placebo.
"Oral apremilast demonstrated long-term efficacy, including improvement in signs and symptoms and physical function, and skin manifestations," Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual European Congress of Rheumatology.
Apremilast is an oral phosphodiesterase 4 inhibitor under investigation as a treatment for active psoriatic arthritis (PsA). It is being evaluated as a possible treatment for skin psoriasis, ankylosing spondylitis, rheumatoid arthritis, and Behçet’s disease.
PALACE 1 was a phase III, multicenter, double-blind, placebo-controlled study of apremilast for the treatment of active PsA. A total of 504 patients with a documented diagnosis of PsA for at least 6 months were recruited into the study (Ann. Rheum. Dis. 2013;72[Suppls3]:163).
Functional outcomes improved
Physical function improved according to measurements on the Health Assessment Questionnaire–Disability Index (HAQ-DI). At baseline, HAQ-DI scores were 1.21, and "we saw that patients improved by –0.35, which is certainly the level that patients can say, ‘I feel better and I can do my daily activities better,’ " Dr. Kavanaugh said.
Additionally, 25% and 37% of patients treated with apremilast 20 mg and 30 mg, respectively, achieved a 75% improvement in the Psoriasis Area and Severity Index at 52 weeks, which is a "very high bar" to achieve, he noted.
The main side effect seen was diarrhea, affecting 11%-19% of patients given apremilast and 2.4% given placebo. However, diarrhea occurred mainly in the first 6 months of therapy and could be managed by taking appropriate measures on an individual patient basis, Dr. Kavanaugh said. This might include prescription of an antidiarrheal agent.
"Prolonged exposure to apremilast did not result in any unexpected increased incidence of adverse events or laboratory abnormalities," he noted. The latter could mean that, if approved, apremilast might not need routine laboratory monitoring.
The PALACE development program
PALACE 1 is one of several clinical trials that have investigated the efficacy and safety of apremilast in active PsA. In these studies, 24 weeks’ treatment with one of two oral doses (20 mg or 30 mg twice daily) of apremilast was compared to placebo. The primary endpoint of the studies was the percentage of patients achieving ACR 20 at 16 weeks.
For inclusion in the trials, patients had to have active disease despite prior therapy with disease-modifying antirheumatic drugs (DMARDs), biologic agents, or both. Dr. Kavanaugh noted that the majority of patients had failed DMARD therapy in PALACE 1, with almost one-quarter receiving prior biologic therapy.
Patients who had a less than 20% reduction from baseline in swollen/tender joint counts at 16 weeks were re-randomized to receive apremilast 20 mg or 30 mg if they had originally been treated with placebo, while patients originally randomized to active treatment stayed on their initial dose if they failed to respond significantly. At the end of the planned 24-week treatment period, all remaining patients on placebo were re-randomized to apremilast 20 mg or 30 mg until 1 year of follow up.
PALACE 3 data also reported
The results of PALACE 3 and combined 6-month safety data from the PALACE 1, PALACE 2, and PALACE 3 trials were also reported at the congress.
In PALACE 3 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:685), significantly more patients achieved the primary endpoint of an ACR 20 at 16 weeks if they were treated with either the 20-mg dose (29.4%, P = .02) or 30-mg dose (42.8%, P less than .0001) of apremilast, compared with those given placebo (18.9%). There was also statistically significant and "clinically meaningful" improvement in physical function and pain. "The results of PALACE 3 support the efficacy and safety findings of the PALACE 1 study and help establish the profile of apremilast in PsA," the PALACE 3 investigators concluded.
The pooled safety findings revealed no new safety concerns and showed apremilast was generally well tolerated (Ann. Rheum. Dis. 2013;72[Suppl. 3]:85). Rates of diarrhea at 24 weeks were 12.6% and 16.5% for the 20-mg and 30-mg doses of apremilast, and 2.8% for placebo, respectively. Other side effects of note included nausea (10% and 16.1% vs. 4.6%), headache (8.4% and 11.5% vs. 4.6%), and upper respiratory tract infection (7% and 6% vs. 3%).
Time for regulatory approval
Based on the positive findings of the PALACE 1, 2, and 3 studies, apremilast’s developer, Celgene, is expected to file for regulatory approval in the treatment of active PsA. In doing so, apremilast will join another novel agent, ustekinumab, in the queue for approval for this indication.
Ustekinumab is a human interleukin-12 and -23 antagonist produced by Janssen that is already approved in Europe and in the United States for skin psoriasis. One-year data also show that it is effective and well tolerated for PsA. It is given subcutaneously, whereas apremilast is an oral agent.
Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
MADRID – Apremilast improves the signs and symptoms of psoriatic arthritis in about 60% of patients at 1 year, according to long-term data from the PALACE 1 trial.
At week 52, a 20% improvement in disease symptoms according to American College of Rheumatology (ACR 20) response criteria was achieved by 57%-63% of patients treated with apremilast, providing evidence of sustained treatment effects.
The PALACE 1 trial’s primary endpoint of an ACR 20 at 16 weeks, already reported last year, was achieved by 31% of patients treated with apremilast 20 mg and 40% of those given apremilast 30 mg, compared with 19% of those given placebo.
"Oral apremilast demonstrated long-term efficacy, including improvement in signs and symptoms and physical function, and skin manifestations," Dr. Arthur Kavanaugh, professor of medicine at the University of California, San Diego, said at the annual European Congress of Rheumatology.
Apremilast is an oral phosphodiesterase 4 inhibitor under investigation as a treatment for active psoriatic arthritis (PsA). It is being evaluated as a possible treatment for skin psoriasis, ankylosing spondylitis, rheumatoid arthritis, and Behçet’s disease.
PALACE 1 was a phase III, multicenter, double-blind, placebo-controlled study of apremilast for the treatment of active PsA. A total of 504 patients with a documented diagnosis of PsA for at least 6 months were recruited into the study (Ann. Rheum. Dis. 2013;72[Suppls3]:163).
Functional outcomes improved
Physical function improved according to measurements on the Health Assessment Questionnaire–Disability Index (HAQ-DI). At baseline, HAQ-DI scores were 1.21, and "we saw that patients improved by –0.35, which is certainly the level that patients can say, ‘I feel better and I can do my daily activities better,’ " Dr. Kavanaugh said.
Additionally, 25% and 37% of patients treated with apremilast 20 mg and 30 mg, respectively, achieved a 75% improvement in the Psoriasis Area and Severity Index at 52 weeks, which is a "very high bar" to achieve, he noted.
The main side effect seen was diarrhea, affecting 11%-19% of patients given apremilast and 2.4% given placebo. However, diarrhea occurred mainly in the first 6 months of therapy and could be managed by taking appropriate measures on an individual patient basis, Dr. Kavanaugh said. This might include prescription of an antidiarrheal agent.
"Prolonged exposure to apremilast did not result in any unexpected increased incidence of adverse events or laboratory abnormalities," he noted. The latter could mean that, if approved, apremilast might not need routine laboratory monitoring.
The PALACE development program
PALACE 1 is one of several clinical trials that have investigated the efficacy and safety of apremilast in active PsA. In these studies, 24 weeks’ treatment with one of two oral doses (20 mg or 30 mg twice daily) of apremilast was compared to placebo. The primary endpoint of the studies was the percentage of patients achieving ACR 20 at 16 weeks.
For inclusion in the trials, patients had to have active disease despite prior therapy with disease-modifying antirheumatic drugs (DMARDs), biologic agents, or both. Dr. Kavanaugh noted that the majority of patients had failed DMARD therapy in PALACE 1, with almost one-quarter receiving prior biologic therapy.
Patients who had a less than 20% reduction from baseline in swollen/tender joint counts at 16 weeks were re-randomized to receive apremilast 20 mg or 30 mg if they had originally been treated with placebo, while patients originally randomized to active treatment stayed on their initial dose if they failed to respond significantly. At the end of the planned 24-week treatment period, all remaining patients on placebo were re-randomized to apremilast 20 mg or 30 mg until 1 year of follow up.
PALACE 3 data also reported
The results of PALACE 3 and combined 6-month safety data from the PALACE 1, PALACE 2, and PALACE 3 trials were also reported at the congress.
In PALACE 3 (Ann. Rheum. Dis. 2013;72[Suppl. 3]:685), significantly more patients achieved the primary endpoint of an ACR 20 at 16 weeks if they were treated with either the 20-mg dose (29.4%, P = .02) or 30-mg dose (42.8%, P less than .0001) of apremilast, compared with those given placebo (18.9%). There was also statistically significant and "clinically meaningful" improvement in physical function and pain. "The results of PALACE 3 support the efficacy and safety findings of the PALACE 1 study and help establish the profile of apremilast in PsA," the PALACE 3 investigators concluded.
The pooled safety findings revealed no new safety concerns and showed apremilast was generally well tolerated (Ann. Rheum. Dis. 2013;72[Suppl. 3]:85). Rates of diarrhea at 24 weeks were 12.6% and 16.5% for the 20-mg and 30-mg doses of apremilast, and 2.8% for placebo, respectively. Other side effects of note included nausea (10% and 16.1% vs. 4.6%), headache (8.4% and 11.5% vs. 4.6%), and upper respiratory tract infection (7% and 6% vs. 3%).
Time for regulatory approval
Based on the positive findings of the PALACE 1, 2, and 3 studies, apremilast’s developer, Celgene, is expected to file for regulatory approval in the treatment of active PsA. In doing so, apremilast will join another novel agent, ustekinumab, in the queue for approval for this indication.
Ustekinumab is a human interleukin-12 and -23 antagonist produced by Janssen that is already approved in Europe and in the United States for skin psoriasis. One-year data also show that it is effective and well tolerated for PsA. It is given subcutaneously, whereas apremilast is an oral agent.
Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
AT THE EULAR CONGRESS 2013
Major finding: ACR 20 response at 1 year was 57%-63% for patients given apremilast.
Data source: PALACE 1, a 504-patient, phase III, multicenter, double blind, placebo-controlled study of apremilast for the treatment of active psoriatic arthritis.
Disclosures: Celgene funded the study. Dr. Kavanaugh has provided expert advice to and/or received research grants from the following companies: AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB.
Algorithm helps to DETECT pulmonary hypertension in systemic sclerosis
MADRID – The use of a two-step algorithm significantly increased the rate at which pulmonary arterial hypertension was diagnosed in patients with systemic sclerosis in a prospective, observational, cross-sectional study.
The results of the DETECT study, presented at the annual European Congress of Rheumatology, showed that the two-step algorithm had a sensitivity of 96% for correctly identifying the condition, which was higher than the 71% sensitivity obtained using methods recommended currently by the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines. The ESC/ERS recommendations are mainly based on consensus rather than robust evidence, and focus on the use of transthoracic echocardiography.
"DETECT is unique because it shows that if you just do an echocardiogram that you miss 29% of people who subsequently have pulmonary arterial hypertension [PAH], whereas if you apply the DETECT algorithm you miss only 4% of the people," Dr. Dinesh Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor, said in an interview.
Dr. Khanna, who was a coinvestigator in the study, added: "PAH is a leading cause of mortality; it has high prevalence [and] it has a median survival of 2 to 3 years. ... You don’t want to miss these patients." Dr. Khanna presented recommendations for annual screening of PAH in systemic sclerosis patients at another session at the meeting.
Although 4% of patients are still being missed, this is a dramatic improvement over current clinical practice, said DETECT investigator Dr. Christopher Denton, who presented the findings of the international, multicenter trial. The study was also recently published online (Ann. Rheum. Dis. 2013 May 18 [doi: 10.1136/annrheumdis-2013-203301]).
"There is a general feeling that patients need to be screened so that diagnoses can be made and licensed therapies can be initiated," said Dr. Denton of the Royal Free Hospital in London. "The goal of the study was to rationalize a large number of potential variables into a small number that could be developed into a risk score," he added, and "ultimately to ensure that the most appropriate patients are referred for diagnostic right heart catheter studies." Right heart catheterization (RHC) remains the only method for confirming a diagnosis of PAH.
A total of 646 adult patients with established scleroderma (greater than 3 years) and reduced diffusing capacity of the lung for carbon monoxide (DLCO less than 60% of predicted) were screened and 466 enrolled in the study. All of them underwent RHC, and 145 (31%) were found to have PAH. This was defined as a mean pulmonary arterial pressure of 25 mm Hg or higher.
Of the 145 patients with PAH, 87 met World Health Organization (WHO) group 1 criteria for mild PAH, and this was the group of interest, as a diagnosis of PAH "had been robustly excluded" by normal methods, Dr. Denton said. This group of patients was compared with the group that did not have PAH (n = 321).
Patients with WHO group 1 PAH were slightly older than patients who did not have PAH (mean ages, 61 and 56 years). The PAH patients also tended to have a longer disease duration (163 vs. 130 months) and had slightly lower DLCO (43% vs. 48% of predicted).
The DETECT investigators examined 112 variables, including demographic and clinical parameters, serum tests, and electro- and echocardiogram results, that they thought might be able to help differentiate patients with PAH from those without it. After expert analysis and various types of statistical modeling, they ended up with eight items that were used to develop the two-step algorithm.
Step 1 of the algorithm involves testing for lung function, expressed as a ratio of the percentage predicted forced vital capacity and DLCO; the presence of current or past telangiectasia; serum anticentromere antibody positivity; serum levels of N-terminal prohormone brain natriuretic peptide (NT-proBNP); serum urate levels; and right axis deviation on an electrocardiogram. Step 2 involves measurement of two echocardiographic parameters: right atrium area and tricuspid regurgitation velocity.
"The aim is to make this a computer-based system," Dr. Denton explained. A trial electronic version of the tool is being tested, which involves the aforementioned clinical variables being entered first to determine if an echocardiogram is warranted, and then determining if the results of the echocardiogram warrant further referral for RHC.
The rates of referral for RHC were higher if the two-step algorithm was used, compared with the use of ESC/ERS guideline-recommended methods (62% and 40%). The specificity of the algorithm was 48%, with positive and negative predictive values of 35% and 98%, respectively. The values for guideline-recommended methods were 69%, 40%, and 89%.
The DETECT algorithm has the potential to revise standards of care in patients with systemic sclerosis. Dr. Denton noted that not only was it a sensitive, noninvasive screening tool, but that it also had the potential to reduce the number of missed diagnoses and to potentially identify PAH earlier in mildly symptomatic patients.
"The reason to use a two-step approach is that this potentially will improve the use of echocardiography as well as the more invasive test of right heart catheterization," he commented. "So we hope that this sort of approach will ultimately improve the approach and the standard of care for systemic sclerosis."
DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.
MADRID – The use of a two-step algorithm significantly increased the rate at which pulmonary arterial hypertension was diagnosed in patients with systemic sclerosis in a prospective, observational, cross-sectional study.
The results of the DETECT study, presented at the annual European Congress of Rheumatology, showed that the two-step algorithm had a sensitivity of 96% for correctly identifying the condition, which was higher than the 71% sensitivity obtained using methods recommended currently by the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines. The ESC/ERS recommendations are mainly based on consensus rather than robust evidence, and focus on the use of transthoracic echocardiography.
"DETECT is unique because it shows that if you just do an echocardiogram that you miss 29% of people who subsequently have pulmonary arterial hypertension [PAH], whereas if you apply the DETECT algorithm you miss only 4% of the people," Dr. Dinesh Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor, said in an interview.
Dr. Khanna, who was a coinvestigator in the study, added: "PAH is a leading cause of mortality; it has high prevalence [and] it has a median survival of 2 to 3 years. ... You don’t want to miss these patients." Dr. Khanna presented recommendations for annual screening of PAH in systemic sclerosis patients at another session at the meeting.
Although 4% of patients are still being missed, this is a dramatic improvement over current clinical practice, said DETECT investigator Dr. Christopher Denton, who presented the findings of the international, multicenter trial. The study was also recently published online (Ann. Rheum. Dis. 2013 May 18 [doi: 10.1136/annrheumdis-2013-203301]).
"There is a general feeling that patients need to be screened so that diagnoses can be made and licensed therapies can be initiated," said Dr. Denton of the Royal Free Hospital in London. "The goal of the study was to rationalize a large number of potential variables into a small number that could be developed into a risk score," he added, and "ultimately to ensure that the most appropriate patients are referred for diagnostic right heart catheter studies." Right heart catheterization (RHC) remains the only method for confirming a diagnosis of PAH.
A total of 646 adult patients with established scleroderma (greater than 3 years) and reduced diffusing capacity of the lung for carbon monoxide (DLCO less than 60% of predicted) were screened and 466 enrolled in the study. All of them underwent RHC, and 145 (31%) were found to have PAH. This was defined as a mean pulmonary arterial pressure of 25 mm Hg or higher.
Of the 145 patients with PAH, 87 met World Health Organization (WHO) group 1 criteria for mild PAH, and this was the group of interest, as a diagnosis of PAH "had been robustly excluded" by normal methods, Dr. Denton said. This group of patients was compared with the group that did not have PAH (n = 321).
Patients with WHO group 1 PAH were slightly older than patients who did not have PAH (mean ages, 61 and 56 years). The PAH patients also tended to have a longer disease duration (163 vs. 130 months) and had slightly lower DLCO (43% vs. 48% of predicted).
The DETECT investigators examined 112 variables, including demographic and clinical parameters, serum tests, and electro- and echocardiogram results, that they thought might be able to help differentiate patients with PAH from those without it. After expert analysis and various types of statistical modeling, they ended up with eight items that were used to develop the two-step algorithm.
Step 1 of the algorithm involves testing for lung function, expressed as a ratio of the percentage predicted forced vital capacity and DLCO; the presence of current or past telangiectasia; serum anticentromere antibody positivity; serum levels of N-terminal prohormone brain natriuretic peptide (NT-proBNP); serum urate levels; and right axis deviation on an electrocardiogram. Step 2 involves measurement of two echocardiographic parameters: right atrium area and tricuspid regurgitation velocity.
"The aim is to make this a computer-based system," Dr. Denton explained. A trial electronic version of the tool is being tested, which involves the aforementioned clinical variables being entered first to determine if an echocardiogram is warranted, and then determining if the results of the echocardiogram warrant further referral for RHC.
The rates of referral for RHC were higher if the two-step algorithm was used, compared with the use of ESC/ERS guideline-recommended methods (62% and 40%). The specificity of the algorithm was 48%, with positive and negative predictive values of 35% and 98%, respectively. The values for guideline-recommended methods were 69%, 40%, and 89%.
The DETECT algorithm has the potential to revise standards of care in patients with systemic sclerosis. Dr. Denton noted that not only was it a sensitive, noninvasive screening tool, but that it also had the potential to reduce the number of missed diagnoses and to potentially identify PAH earlier in mildly symptomatic patients.
"The reason to use a two-step approach is that this potentially will improve the use of echocardiography as well as the more invasive test of right heart catheterization," he commented. "So we hope that this sort of approach will ultimately improve the approach and the standard of care for systemic sclerosis."
DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.
MADRID – The use of a two-step algorithm significantly increased the rate at which pulmonary arterial hypertension was diagnosed in patients with systemic sclerosis in a prospective, observational, cross-sectional study.
The results of the DETECT study, presented at the annual European Congress of Rheumatology, showed that the two-step algorithm had a sensitivity of 96% for correctly identifying the condition, which was higher than the 71% sensitivity obtained using methods recommended currently by the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines. The ESC/ERS recommendations are mainly based on consensus rather than robust evidence, and focus on the use of transthoracic echocardiography.
"DETECT is unique because it shows that if you just do an echocardiogram that you miss 29% of people who subsequently have pulmonary arterial hypertension [PAH], whereas if you apply the DETECT algorithm you miss only 4% of the people," Dr. Dinesh Khanna, director of the scleroderma program at the University of Michigan, Ann Arbor, said in an interview.
Dr. Khanna, who was a coinvestigator in the study, added: "PAH is a leading cause of mortality; it has high prevalence [and] it has a median survival of 2 to 3 years. ... You don’t want to miss these patients." Dr. Khanna presented recommendations for annual screening of PAH in systemic sclerosis patients at another session at the meeting.
Although 4% of patients are still being missed, this is a dramatic improvement over current clinical practice, said DETECT investigator Dr. Christopher Denton, who presented the findings of the international, multicenter trial. The study was also recently published online (Ann. Rheum. Dis. 2013 May 18 [doi: 10.1136/annrheumdis-2013-203301]).
"There is a general feeling that patients need to be screened so that diagnoses can be made and licensed therapies can be initiated," said Dr. Denton of the Royal Free Hospital in London. "The goal of the study was to rationalize a large number of potential variables into a small number that could be developed into a risk score," he added, and "ultimately to ensure that the most appropriate patients are referred for diagnostic right heart catheter studies." Right heart catheterization (RHC) remains the only method for confirming a diagnosis of PAH.
A total of 646 adult patients with established scleroderma (greater than 3 years) and reduced diffusing capacity of the lung for carbon monoxide (DLCO less than 60% of predicted) were screened and 466 enrolled in the study. All of them underwent RHC, and 145 (31%) were found to have PAH. This was defined as a mean pulmonary arterial pressure of 25 mm Hg or higher.
Of the 145 patients with PAH, 87 met World Health Organization (WHO) group 1 criteria for mild PAH, and this was the group of interest, as a diagnosis of PAH "had been robustly excluded" by normal methods, Dr. Denton said. This group of patients was compared with the group that did not have PAH (n = 321).
Patients with WHO group 1 PAH were slightly older than patients who did not have PAH (mean ages, 61 and 56 years). The PAH patients also tended to have a longer disease duration (163 vs. 130 months) and had slightly lower DLCO (43% vs. 48% of predicted).
The DETECT investigators examined 112 variables, including demographic and clinical parameters, serum tests, and electro- and echocardiogram results, that they thought might be able to help differentiate patients with PAH from those without it. After expert analysis and various types of statistical modeling, they ended up with eight items that were used to develop the two-step algorithm.
Step 1 of the algorithm involves testing for lung function, expressed as a ratio of the percentage predicted forced vital capacity and DLCO; the presence of current or past telangiectasia; serum anticentromere antibody positivity; serum levels of N-terminal prohormone brain natriuretic peptide (NT-proBNP); serum urate levels; and right axis deviation on an electrocardiogram. Step 2 involves measurement of two echocardiographic parameters: right atrium area and tricuspid regurgitation velocity.
"The aim is to make this a computer-based system," Dr. Denton explained. A trial electronic version of the tool is being tested, which involves the aforementioned clinical variables being entered first to determine if an echocardiogram is warranted, and then determining if the results of the echocardiogram warrant further referral for RHC.
The rates of referral for RHC were higher if the two-step algorithm was used, compared with the use of ESC/ERS guideline-recommended methods (62% and 40%). The specificity of the algorithm was 48%, with positive and negative predictive values of 35% and 98%, respectively. The values for guideline-recommended methods were 69%, 40%, and 89%.
The DETECT algorithm has the potential to revise standards of care in patients with systemic sclerosis. Dr. Denton noted that not only was it a sensitive, noninvasive screening tool, but that it also had the potential to reduce the number of missed diagnoses and to potentially identify PAH earlier in mildly symptomatic patients.
"The reason to use a two-step approach is that this potentially will improve the use of echocardiography as well as the more invasive test of right heart catheterization," he commented. "So we hope that this sort of approach will ultimately improve the approach and the standard of care for systemic sclerosis."
DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.
AT THE EULAR CONGRESS 2013
Major finding: Only 4% of cases were missed using the two-step algorithm, compared with 29% for guideline-recommended detection.
Data source: DETECT is an international, multicenter, prospective, observational, cross-sectional study of 87 systemic sclerosis patients with and 321 without pulmonary arterial hypertension.
Disclosures: DETECT was an academic-led study funded by Actelion. Dr. Denton has received consulting and speaker fees and/or research funding from, or has been a clinical trial investigator for, several companies including Actelion, Boehringer Ingelheim, and CSL Behring. Dr. Khanna disclosed acting as a consultant for several companies including Actelion, Bayer, and Celgene.
MRI detects high level of subclinical small joint inflammation
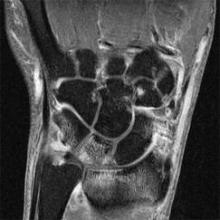
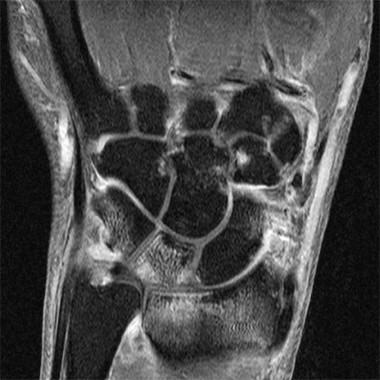
MADRID – A high percentage of patients with early arthritis have inflammation of the small joints that can be detected with MRI but not by physical examination.
Results of a cross-sectional study, presented by Dr. Annemarie Krabben at the annual European Congress of Rheumatology, found that 66% of wrist, 27% of metacarpophalangeal (MCP), and 13% of metatarsophalangeal (MTP) joints that were not clinically swollen showed signs of inflammation on MRI. However, inflammation on MRI was present in 92% of wrists, 86% of MCP, and 29% of MTP joints that were clinically swollen.
"You would expect that inflammation on MRI would be present in the clinically swollen joints, but we also saw inflammation in the non-swollen joints," explained Dr. Krabben of Leiden University Medical Center in the Netherlands.
Furthermore, "when you look at the joints with MRI-detected inflammation, a lot of these didn’t have clinical inflammation," she added.
Clinical joint swelling was absent but signs of bone marrow edema were detected on MRI in 60% of wrist, 53% of MCP, and 78% of MTP joints. If severe MRI-detected edema was considered, joint swelling was absent in 35%, 39%, and 58% of wrist, MCP, and MTP joints, respectively. Joints without clinical swelling showed signs of inflammation on MRI in 61% of wrist, 64% of MCP, and 77% of MTP joints.
The study involved patients with early arthritis who were part of the Leiden Early Arthritis Clinic cohort. This cohort was established in 1993 to detect and treat inflammatory disorders early in the disease state (Rheumatology [Oxford] 2011;50:93-100).
Upon entry into the cohort, patients underwent a physical examination that included 68 tender and 66 swollen joint counts and 1.5 Tesla MRI of the wrist, MCP, and MTP joints. The latter were used to determine the presence and extent of synovitis, bone marrow edema, and tenosynovitis.
In total, 1,790 small joints were examined in 179 patients who had a median duration of symptoms of 15 weeks. Overall, 30% of wrist, 15% of MCP, and 11% of MTP joints were swollen at physical examination and the majority also showed inflammation on MRI.
"There was a lot of subclinical inflammation, especially bone marrow edema, in the nonswollen joints," Dr. Krabben said. Bone marrow edema is linked to erosive disease progression, she observed and suggested that the next step is to see what happens to patients with subclinical inflammation at baseline, and whether this will eventually progress to erosive disease.
The study was supported by the Dutch Arthritis Foundation (Reumafonds), the Netherlands Organization for Health Research and Development, and the Center for Translational Molecular Medicine. Dr. Krabben has received research funding from Reumafonds.
MADRID – A high percentage of patients with early arthritis have inflammation of the small joints that can be detected with MRI but not by physical examination.
Results of a cross-sectional study, presented by Dr. Annemarie Krabben at the annual European Congress of Rheumatology, found that 66% of wrist, 27% of metacarpophalangeal (MCP), and 13% of metatarsophalangeal (MTP) joints that were not clinically swollen showed signs of inflammation on MRI. However, inflammation on MRI was present in 92% of wrists, 86% of MCP, and 29% of MTP joints that were clinically swollen.
"You would expect that inflammation on MRI would be present in the clinically swollen joints, but we also saw inflammation in the non-swollen joints," explained Dr. Krabben of Leiden University Medical Center in the Netherlands.
Furthermore, "when you look at the joints with MRI-detected inflammation, a lot of these didn’t have clinical inflammation," she added.
Clinical joint swelling was absent but signs of bone marrow edema were detected on MRI in 60% of wrist, 53% of MCP, and 78% of MTP joints. If severe MRI-detected edema was considered, joint swelling was absent in 35%, 39%, and 58% of wrist, MCP, and MTP joints, respectively. Joints without clinical swelling showed signs of inflammation on MRI in 61% of wrist, 64% of MCP, and 77% of MTP joints.
The study involved patients with early arthritis who were part of the Leiden Early Arthritis Clinic cohort. This cohort was established in 1993 to detect and treat inflammatory disorders early in the disease state (Rheumatology [Oxford] 2011;50:93-100).
Upon entry into the cohort, patients underwent a physical examination that included 68 tender and 66 swollen joint counts and 1.5 Tesla MRI of the wrist, MCP, and MTP joints. The latter were used to determine the presence and extent of synovitis, bone marrow edema, and tenosynovitis.
In total, 1,790 small joints were examined in 179 patients who had a median duration of symptoms of 15 weeks. Overall, 30% of wrist, 15% of MCP, and 11% of MTP joints were swollen at physical examination and the majority also showed inflammation on MRI.
"There was a lot of subclinical inflammation, especially bone marrow edema, in the nonswollen joints," Dr. Krabben said. Bone marrow edema is linked to erosive disease progression, she observed and suggested that the next step is to see what happens to patients with subclinical inflammation at baseline, and whether this will eventually progress to erosive disease.
The study was supported by the Dutch Arthritis Foundation (Reumafonds), the Netherlands Organization for Health Research and Development, and the Center for Translational Molecular Medicine. Dr. Krabben has received research funding from Reumafonds.
MADRID – A high percentage of patients with early arthritis have inflammation of the small joints that can be detected with MRI but not by physical examination.
Results of a cross-sectional study, presented by Dr. Annemarie Krabben at the annual European Congress of Rheumatology, found that 66% of wrist, 27% of metacarpophalangeal (MCP), and 13% of metatarsophalangeal (MTP) joints that were not clinically swollen showed signs of inflammation on MRI. However, inflammation on MRI was present in 92% of wrists, 86% of MCP, and 29% of MTP joints that were clinically swollen.
"You would expect that inflammation on MRI would be present in the clinically swollen joints, but we also saw inflammation in the non-swollen joints," explained Dr. Krabben of Leiden University Medical Center in the Netherlands.
Furthermore, "when you look at the joints with MRI-detected inflammation, a lot of these didn’t have clinical inflammation," she added.
Clinical joint swelling was absent but signs of bone marrow edema were detected on MRI in 60% of wrist, 53% of MCP, and 78% of MTP joints. If severe MRI-detected edema was considered, joint swelling was absent in 35%, 39%, and 58% of wrist, MCP, and MTP joints, respectively. Joints without clinical swelling showed signs of inflammation on MRI in 61% of wrist, 64% of MCP, and 77% of MTP joints.
The study involved patients with early arthritis who were part of the Leiden Early Arthritis Clinic cohort. This cohort was established in 1993 to detect and treat inflammatory disorders early in the disease state (Rheumatology [Oxford] 2011;50:93-100).
Upon entry into the cohort, patients underwent a physical examination that included 68 tender and 66 swollen joint counts and 1.5 Tesla MRI of the wrist, MCP, and MTP joints. The latter were used to determine the presence and extent of synovitis, bone marrow edema, and tenosynovitis.
In total, 1,790 small joints were examined in 179 patients who had a median duration of symptoms of 15 weeks. Overall, 30% of wrist, 15% of MCP, and 11% of MTP joints were swollen at physical examination and the majority also showed inflammation on MRI.
"There was a lot of subclinical inflammation, especially bone marrow edema, in the nonswollen joints," Dr. Krabben said. Bone marrow edema is linked to erosive disease progression, she observed and suggested that the next step is to see what happens to patients with subclinical inflammation at baseline, and whether this will eventually progress to erosive disease.
The study was supported by the Dutch Arthritis Foundation (Reumafonds), the Netherlands Organization for Health Research and Development, and the Center for Translational Molecular Medicine. Dr. Krabben has received research funding from Reumafonds.
AT THE EULAR CONGRESS 2013
Proposed ACR-EULAR scleroderma classification criteria 'more inclusive'
MADRID – New classification criteria for scleroderma presented at the annual European Congress of Rheumatology correctly identify more patients who could potentially be included in epidemiological studies and clinical trials than is possible with existing classification systems.
The new system is still a proposal and is under review by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), according to Dr. Frank van den Hoogen, who is the director of the rheumatology center at Sint Maartenskliniek in Nijmegen and head of the department of rheumatology at Radboud University in Nijmegen, both in the Netherlands.
In a validation cohort, the ACR-EULAR criteria had a sensitivity of 91% and a specificity of 92% to correctly identify patients with systemic sclerosis. By comparison, the 1980 Preliminary ARA Criteria had a sensitivity of 75% and a specificity of 72%.
The whole process of developing the ACR-EULAR criteria has taken about 5 years, Dr. van den Hoogen explained in an interview. "The ARA criteria were not as sensitive as we wanted because they excluded some patients with limited disease and also patients with newly diagnosed disease," he added.
"The purpose of classification criteria is to include similar patients in research," Dr. van den Hoogen said. "Classification criteria are not synonymous with diagnostic criteria," he explained, "[they] are generally more standardized and less inclusive." This is because physicians will see patients with multiple symptoms and it would not be possible to include every symptom seen in routine practice in a set of classification criteria. Nevertheless, diagnostic criteria do tend to mirror classification criteria.
The process of determining which items to include was driven by both data and consensus. Delphi exercises and a nominal group technique were used to create a set of potential items for the classification of systemic sclerosis.
Several patient cases were then reviewed by leading scleroderma experts based in Europe and North America. The cases represented the full spectrum of systemic sclerosis, including those with a low and those with a high probability of having the disease. Experts ranked the importance of the symptoms exhibited by each of these cases, and a whittled down list with a scoring system was obtained. Systemic sclerosis was present if a score of 9 or more was achieved.
Skin thickening of the fingers of both hands extending past the metacarpophalangeal (MCP) joints was considered to be indicative of scleroderma, and was given a score of 9. Conversely, patients with skin involvement likely to be due to another scleroderma-like disorder or skin thickening sparing the fingers were not likely to have systemic sclerosis.
Other items included skin thickening of the fingers, with subitems of puffy fingers (score = 2) and whole finger skin thickening, distal to the MCP (4); fingertip lesions, with subitems of digital tip ulcers (2) and pitting scars (3), telangiectasia (2), abnormal nailfold capillaries (2); pulmonary arterial hypertension, interstitial lung disease, or both (2); Raynaud’s phenomenon (3); and the presence of any scleroderma-related autoantibodies (3).
The ability of these criteria to correctly identify patients with and without systemic sclerosis was then prospectively tested in a random sample of 200 individuals and further validated in a cohort of 405 individuals that included both early and prevalent cases of scleroderma and its mimics who had been collected from several European and North American scleroderma centers.
"The proposed ACR-EULAR criteria for the classification of systemic sclerosis should allow more patients to be classified correctly as systemic sclerosis," Dr. van den Hoogen said. This includes those with early (less than 3 years) scleroderma and the 20% of patients who have limited disease but who do not meet current classification criteria.
"New ACR [EULAR] criteria show increased sensitivity in comparison to the old [ARA] criteria," concurred Dr. Suzana Jordan of University Hospital Zurich. She presented findings on the use of the proposed system in 317 patients from the Zurich scleroderma cohort. This cohort mainly consists of patients with early or mild disease.
Applying the criteria to this Swiss patient population, Dr. Jordan noted that "75% of systemic sclerosis patients were correctly identified compared to just over half of all patients (51%) using the ARA criteria." Furthermore, "50% of early scleroderma patients who did not fulfill the old criteria met the new," she concluded.
Dr. van den Hoogen said that he had no disclosures, except that this work was funded jointly by EULAR and the ACR.
MADRID – New classification criteria for scleroderma presented at the annual European Congress of Rheumatology correctly identify more patients who could potentially be included in epidemiological studies and clinical trials than is possible with existing classification systems.
The new system is still a proposal and is under review by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), according to Dr. Frank van den Hoogen, who is the director of the rheumatology center at Sint Maartenskliniek in Nijmegen and head of the department of rheumatology at Radboud University in Nijmegen, both in the Netherlands.
In a validation cohort, the ACR-EULAR criteria had a sensitivity of 91% and a specificity of 92% to correctly identify patients with systemic sclerosis. By comparison, the 1980 Preliminary ARA Criteria had a sensitivity of 75% and a specificity of 72%.
The whole process of developing the ACR-EULAR criteria has taken about 5 years, Dr. van den Hoogen explained in an interview. "The ARA criteria were not as sensitive as we wanted because they excluded some patients with limited disease and also patients with newly diagnosed disease," he added.
"The purpose of classification criteria is to include similar patients in research," Dr. van den Hoogen said. "Classification criteria are not synonymous with diagnostic criteria," he explained, "[they] are generally more standardized and less inclusive." This is because physicians will see patients with multiple symptoms and it would not be possible to include every symptom seen in routine practice in a set of classification criteria. Nevertheless, diagnostic criteria do tend to mirror classification criteria.
The process of determining which items to include was driven by both data and consensus. Delphi exercises and a nominal group technique were used to create a set of potential items for the classification of systemic sclerosis.
Several patient cases were then reviewed by leading scleroderma experts based in Europe and North America. The cases represented the full spectrum of systemic sclerosis, including those with a low and those with a high probability of having the disease. Experts ranked the importance of the symptoms exhibited by each of these cases, and a whittled down list with a scoring system was obtained. Systemic sclerosis was present if a score of 9 or more was achieved.
Skin thickening of the fingers of both hands extending past the metacarpophalangeal (MCP) joints was considered to be indicative of scleroderma, and was given a score of 9. Conversely, patients with skin involvement likely to be due to another scleroderma-like disorder or skin thickening sparing the fingers were not likely to have systemic sclerosis.
Other items included skin thickening of the fingers, with subitems of puffy fingers (score = 2) and whole finger skin thickening, distal to the MCP (4); fingertip lesions, with subitems of digital tip ulcers (2) and pitting scars (3), telangiectasia (2), abnormal nailfold capillaries (2); pulmonary arterial hypertension, interstitial lung disease, or both (2); Raynaud’s phenomenon (3); and the presence of any scleroderma-related autoantibodies (3).
The ability of these criteria to correctly identify patients with and without systemic sclerosis was then prospectively tested in a random sample of 200 individuals and further validated in a cohort of 405 individuals that included both early and prevalent cases of scleroderma and its mimics who had been collected from several European and North American scleroderma centers.
"The proposed ACR-EULAR criteria for the classification of systemic sclerosis should allow more patients to be classified correctly as systemic sclerosis," Dr. van den Hoogen said. This includes those with early (less than 3 years) scleroderma and the 20% of patients who have limited disease but who do not meet current classification criteria.
"New ACR [EULAR] criteria show increased sensitivity in comparison to the old [ARA] criteria," concurred Dr. Suzana Jordan of University Hospital Zurich. She presented findings on the use of the proposed system in 317 patients from the Zurich scleroderma cohort. This cohort mainly consists of patients with early or mild disease.
Applying the criteria to this Swiss patient population, Dr. Jordan noted that "75% of systemic sclerosis patients were correctly identified compared to just over half of all patients (51%) using the ARA criteria." Furthermore, "50% of early scleroderma patients who did not fulfill the old criteria met the new," she concluded.
Dr. van den Hoogen said that he had no disclosures, except that this work was funded jointly by EULAR and the ACR.
MADRID – New classification criteria for scleroderma presented at the annual European Congress of Rheumatology correctly identify more patients who could potentially be included in epidemiological studies and clinical trials than is possible with existing classification systems.
The new system is still a proposal and is under review by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), according to Dr. Frank van den Hoogen, who is the director of the rheumatology center at Sint Maartenskliniek in Nijmegen and head of the department of rheumatology at Radboud University in Nijmegen, both in the Netherlands.
In a validation cohort, the ACR-EULAR criteria had a sensitivity of 91% and a specificity of 92% to correctly identify patients with systemic sclerosis. By comparison, the 1980 Preliminary ARA Criteria had a sensitivity of 75% and a specificity of 72%.
The whole process of developing the ACR-EULAR criteria has taken about 5 years, Dr. van den Hoogen explained in an interview. "The ARA criteria were not as sensitive as we wanted because they excluded some patients with limited disease and also patients with newly diagnosed disease," he added.
"The purpose of classification criteria is to include similar patients in research," Dr. van den Hoogen said. "Classification criteria are not synonymous with diagnostic criteria," he explained, "[they] are generally more standardized and less inclusive." This is because physicians will see patients with multiple symptoms and it would not be possible to include every symptom seen in routine practice in a set of classification criteria. Nevertheless, diagnostic criteria do tend to mirror classification criteria.
The process of determining which items to include was driven by both data and consensus. Delphi exercises and a nominal group technique were used to create a set of potential items for the classification of systemic sclerosis.
Several patient cases were then reviewed by leading scleroderma experts based in Europe and North America. The cases represented the full spectrum of systemic sclerosis, including those with a low and those with a high probability of having the disease. Experts ranked the importance of the symptoms exhibited by each of these cases, and a whittled down list with a scoring system was obtained. Systemic sclerosis was present if a score of 9 or more was achieved.
Skin thickening of the fingers of both hands extending past the metacarpophalangeal (MCP) joints was considered to be indicative of scleroderma, and was given a score of 9. Conversely, patients with skin involvement likely to be due to another scleroderma-like disorder or skin thickening sparing the fingers were not likely to have systemic sclerosis.
Other items included skin thickening of the fingers, with subitems of puffy fingers (score = 2) and whole finger skin thickening, distal to the MCP (4); fingertip lesions, with subitems of digital tip ulcers (2) and pitting scars (3), telangiectasia (2), abnormal nailfold capillaries (2); pulmonary arterial hypertension, interstitial lung disease, or both (2); Raynaud’s phenomenon (3); and the presence of any scleroderma-related autoantibodies (3).
The ability of these criteria to correctly identify patients with and without systemic sclerosis was then prospectively tested in a random sample of 200 individuals and further validated in a cohort of 405 individuals that included both early and prevalent cases of scleroderma and its mimics who had been collected from several European and North American scleroderma centers.
"The proposed ACR-EULAR criteria for the classification of systemic sclerosis should allow more patients to be classified correctly as systemic sclerosis," Dr. van den Hoogen said. This includes those with early (less than 3 years) scleroderma and the 20% of patients who have limited disease but who do not meet current classification criteria.
"New ACR [EULAR] criteria show increased sensitivity in comparison to the old [ARA] criteria," concurred Dr. Suzana Jordan of University Hospital Zurich. She presented findings on the use of the proposed system in 317 patients from the Zurich scleroderma cohort. This cohort mainly consists of patients with early or mild disease.
Applying the criteria to this Swiss patient population, Dr. Jordan noted that "75% of systemic sclerosis patients were correctly identified compared to just over half of all patients (51%) using the ARA criteria." Furthermore, "50% of early scleroderma patients who did not fulfill the old criteria met the new," she concluded.
Dr. van den Hoogen said that he had no disclosures, except that this work was funded jointly by EULAR and the ACR.
AT THE EULAR CONGRESS 2013
Device cuts DVT risk, saves stroke patients' lives
LONDON – Intermittent pneumatic compression reduced the absolute risk of proximal deep vein thrombosis in patients who had suffered a stroke and were immobile by 3.6% in a large, randomized trial.
The incidence of proximal DVT at 30 days in the CLOTS 3 study was 8.5% with intermittent pneumatic compression (IPC) and 12.1% with routine poststroke care alone (P = .001). The adjusted odds ratio was 0.65.
There was also a 14% reduction in the risk of death seen at 6 months favoring IPC use over routine care alone (P = .042). This was a surprising finding, said principal investigator Dr. Martin Dennis of the University of Edinburgh’s clinical neurosciences division and Western General Hospital in Edinburgh, Scotland.
Importantly, IPC appears to be effective across a variety of prespecified subgroups, including both ischemic and hemorrhagic stroke.
Findings will change practice
The findings, which were published online in the Lancet (2013 May 31 [doi:10.1016/ S0140-6736(13)61050-8]) to coincide with their presentation at the annual European Stroke Conference, are practice changing and suggest that national stroke guidelines need to be updated.
"This study is a major breakthrough, showing how a simple and safe treatment can save lives," Dr. Tony Rudd, a consultant stroke physician at Guy’s and St. Thomas’ NHS Foundation Trust, London, said in a statement issued by the University of Edinburgh.
"The challenge now will be to ensure that all patients who might benefit are offered treatment," added Dr. Rudd, who chairs the Royal College of Physicians’ Intercollegiate Stroke Working Party. "It is one of the most important research studies to emerge in the field of stroke in recent years," he noted.
Dr. Christine Roffe, consultant in stroke medicine and professor of medicine at Keele University in Stoke-on-Trent, England, also praised the study’s results. "That something as simple as a compressive sleeve saves lives after stroke is fascinating," she said in an interview at the conference. Dr. Roffe was not involved in the study.
The CLOTS 3 study
CLOTS 3 follows on from two other trials performed by the CLOTS (Clots in Legs or Stockings after Stroke) Trials Collaboration, in which compression stockings were examined as a possible means of preventing thrombotic complications in patients who had suffered a stroke. Results of CLOTS 1 (Lancet 2009;373:1958-65) and CLOTS 2 were negative, however, and no benefit of compression stockings was seen in stroke patients.
Between December 2008 and September 2012, a total of 2,876 patients were enrolled in CLOTS 3. For inclusion, patients had to be immobile and randomized within 0-3 days of having had a stroke. Immobility was defined as being unable to walk to the bathroom without the help of another person.
Patients were randomized to receive either routine poststroke care alone or with additional IPC delivered by Covidien’s Kendall SCD Express Sequential Compression System. The latter involved wearing thigh-high, inflatable sleeves continuously for up to 30 days, during which time the device automatically provided IPC depending on the position of the patient. The mean and median durations of wear were 12.5 days and 9.0 days, respectively.
DVT was assessed using duplex ultrasound at 7-10 days and again at 25-30 days if possible. Both patient groups wore compression sleeves to ensure that the ultrasound technicians remained blinded to the treatment group. Follow-up was at 6 months via postal questionnaires sent to patients’ primary care physicians asking about vital status and the occurrence of venous thromboembolism since hospital discharge. Patients were also sent a postal questionnaire and telephoned if they did not respond.
DVT risk reduced
The effect on proximal DVT at 30 days was the primary outcome measure, but IPC also reduced the incidence of symptomatic DVT (4.6% vs. 6.3%; P = .0045) and any DVT (16.2% vs. 21.1%; P = .001) versus routine care. There was no significant difference in the incidence of pulmonary embolism between study arms (2.0% vs. 2.4%, respectively, P = .453).
In terms of safety, there was no difference between the treatment groups in the number of falls with injury or fractures as a result of constantly wearing the compression sleeves. There was a significant difference in skin ulcers (3.1% with IPC vs. 1.4% without, P = .002), but close inspection of the data suggested that only 10 (0.7%) cases were due to IPC.
"During the study, [the manufacturers of the IPC device] brought out a new comfort sleeve," Dr. Dennis noted in an interview.
"Normally these sleeves were being used for short periods in surgical patients, but we were using them for longer periods, so they brought out a softer sleeve," he observed. Anecdotally, he conceded that some people found the sleeves uncomfortable, too hot, or the system "noisy" to use.
The bottom line is that "intermittent pneumatic compression in people who are immobile with stroke reduces the risk of deep vein thrombosis," Dr. Dennis said.
He emphasized that "IPC is feasible in stroke patients, and it is relatively safe. It is an effective means of reducing venous thromboembolism after stroke, with a number needed to treat of about 28 for proximal DVT."
Intriguingly, it may also improve overall survival, "although we weren’t expecting to see that effect," Dr. Dennis said. The number needed to treat to prevent 1 death in 30 days was 43.
Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
Venous thromboembolism is a common cause of hospital-related morbidity. Anticoagulants (e.g., heparin, low-molecular-weight heparin [LMWH]) reduce VTE with acceptable safety. Stroke patients have a high risk of VTE, but also a heightened bleeding risk in the setting of anticoagulants. Mechanical devices (elastic stockings, sequential compression devices) are attractive alternatives, but efficacy is unproven in many settings. Unlike previous trials that demonstrated a lack of efficacy of elastic stockings, the CLOTS 3 study provides convincing evidence that SCDs reduce VTE and decrease mortality in patients with a stroke. Although encouraging, VTE event rates remained high, 8.5%, in contrast to VTE rates of 4.8% in a prior study of enoxaparin in stroke patients.
Together, these findings support recommendations by the American College of Chest Physicians: In patients with acute ischemic stroke and restricted mobility, use prophylactic-dose subcutaneous heparin or LMWH or intermittent SCDs as opposed to no prophylaxis.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
Venous thromboembolism is a common cause of hospital-related morbidity. Anticoagulants (e.g., heparin, low-molecular-weight heparin [LMWH]) reduce VTE with acceptable safety. Stroke patients have a high risk of VTE, but also a heightened bleeding risk in the setting of anticoagulants. Mechanical devices (elastic stockings, sequential compression devices) are attractive alternatives, but efficacy is unproven in many settings. Unlike previous trials that demonstrated a lack of efficacy of elastic stockings, the CLOTS 3 study provides convincing evidence that SCDs reduce VTE and decrease mortality in patients with a stroke. Although encouraging, VTE event rates remained high, 8.5%, in contrast to VTE rates of 4.8% in a prior study of enoxaparin in stroke patients.
Together, these findings support recommendations by the American College of Chest Physicians: In patients with acute ischemic stroke and restricted mobility, use prophylactic-dose subcutaneous heparin or LMWH or intermittent SCDs as opposed to no prophylaxis.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
Venous thromboembolism is a common cause of hospital-related morbidity. Anticoagulants (e.g., heparin, low-molecular-weight heparin [LMWH]) reduce VTE with acceptable safety. Stroke patients have a high risk of VTE, but also a heightened bleeding risk in the setting of anticoagulants. Mechanical devices (elastic stockings, sequential compression devices) are attractive alternatives, but efficacy is unproven in many settings. Unlike previous trials that demonstrated a lack of efficacy of elastic stockings, the CLOTS 3 study provides convincing evidence that SCDs reduce VTE and decrease mortality in patients with a stroke. Although encouraging, VTE event rates remained high, 8.5%, in contrast to VTE rates of 4.8% in a prior study of enoxaparin in stroke patients.
Together, these findings support recommendations by the American College of Chest Physicians: In patients with acute ischemic stroke and restricted mobility, use prophylactic-dose subcutaneous heparin or LMWH or intermittent SCDs as opposed to no prophylaxis.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
LONDON – Intermittent pneumatic compression reduced the absolute risk of proximal deep vein thrombosis in patients who had suffered a stroke and were immobile by 3.6% in a large, randomized trial.
The incidence of proximal DVT at 30 days in the CLOTS 3 study was 8.5% with intermittent pneumatic compression (IPC) and 12.1% with routine poststroke care alone (P = .001). The adjusted odds ratio was 0.65.
There was also a 14% reduction in the risk of death seen at 6 months favoring IPC use over routine care alone (P = .042). This was a surprising finding, said principal investigator Dr. Martin Dennis of the University of Edinburgh’s clinical neurosciences division and Western General Hospital in Edinburgh, Scotland.
Importantly, IPC appears to be effective across a variety of prespecified subgroups, including both ischemic and hemorrhagic stroke.
Findings will change practice
The findings, which were published online in the Lancet (2013 May 31 [doi:10.1016/ S0140-6736(13)61050-8]) to coincide with their presentation at the annual European Stroke Conference, are practice changing and suggest that national stroke guidelines need to be updated.
"This study is a major breakthrough, showing how a simple and safe treatment can save lives," Dr. Tony Rudd, a consultant stroke physician at Guy’s and St. Thomas’ NHS Foundation Trust, London, said in a statement issued by the University of Edinburgh.
"The challenge now will be to ensure that all patients who might benefit are offered treatment," added Dr. Rudd, who chairs the Royal College of Physicians’ Intercollegiate Stroke Working Party. "It is one of the most important research studies to emerge in the field of stroke in recent years," he noted.
Dr. Christine Roffe, consultant in stroke medicine and professor of medicine at Keele University in Stoke-on-Trent, England, also praised the study’s results. "That something as simple as a compressive sleeve saves lives after stroke is fascinating," she said in an interview at the conference. Dr. Roffe was not involved in the study.
The CLOTS 3 study
CLOTS 3 follows on from two other trials performed by the CLOTS (Clots in Legs or Stockings after Stroke) Trials Collaboration, in which compression stockings were examined as a possible means of preventing thrombotic complications in patients who had suffered a stroke. Results of CLOTS 1 (Lancet 2009;373:1958-65) and CLOTS 2 were negative, however, and no benefit of compression stockings was seen in stroke patients.
Between December 2008 and September 2012, a total of 2,876 patients were enrolled in CLOTS 3. For inclusion, patients had to be immobile and randomized within 0-3 days of having had a stroke. Immobility was defined as being unable to walk to the bathroom without the help of another person.
Patients were randomized to receive either routine poststroke care alone or with additional IPC delivered by Covidien’s Kendall SCD Express Sequential Compression System. The latter involved wearing thigh-high, inflatable sleeves continuously for up to 30 days, during which time the device automatically provided IPC depending on the position of the patient. The mean and median durations of wear were 12.5 days and 9.0 days, respectively.
DVT was assessed using duplex ultrasound at 7-10 days and again at 25-30 days if possible. Both patient groups wore compression sleeves to ensure that the ultrasound technicians remained blinded to the treatment group. Follow-up was at 6 months via postal questionnaires sent to patients’ primary care physicians asking about vital status and the occurrence of venous thromboembolism since hospital discharge. Patients were also sent a postal questionnaire and telephoned if they did not respond.
DVT risk reduced
The effect on proximal DVT at 30 days was the primary outcome measure, but IPC also reduced the incidence of symptomatic DVT (4.6% vs. 6.3%; P = .0045) and any DVT (16.2% vs. 21.1%; P = .001) versus routine care. There was no significant difference in the incidence of pulmonary embolism between study arms (2.0% vs. 2.4%, respectively, P = .453).
In terms of safety, there was no difference between the treatment groups in the number of falls with injury or fractures as a result of constantly wearing the compression sleeves. There was a significant difference in skin ulcers (3.1% with IPC vs. 1.4% without, P = .002), but close inspection of the data suggested that only 10 (0.7%) cases were due to IPC.
"During the study, [the manufacturers of the IPC device] brought out a new comfort sleeve," Dr. Dennis noted in an interview.
"Normally these sleeves were being used for short periods in surgical patients, but we were using them for longer periods, so they brought out a softer sleeve," he observed. Anecdotally, he conceded that some people found the sleeves uncomfortable, too hot, or the system "noisy" to use.
The bottom line is that "intermittent pneumatic compression in people who are immobile with stroke reduces the risk of deep vein thrombosis," Dr. Dennis said.
He emphasized that "IPC is feasible in stroke patients, and it is relatively safe. It is an effective means of reducing venous thromboembolism after stroke, with a number needed to treat of about 28 for proximal DVT."
Intriguingly, it may also improve overall survival, "although we weren’t expecting to see that effect," Dr. Dennis said. The number needed to treat to prevent 1 death in 30 days was 43.
Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
LONDON – Intermittent pneumatic compression reduced the absolute risk of proximal deep vein thrombosis in patients who had suffered a stroke and were immobile by 3.6% in a large, randomized trial.
The incidence of proximal DVT at 30 days in the CLOTS 3 study was 8.5% with intermittent pneumatic compression (IPC) and 12.1% with routine poststroke care alone (P = .001). The adjusted odds ratio was 0.65.
There was also a 14% reduction in the risk of death seen at 6 months favoring IPC use over routine care alone (P = .042). This was a surprising finding, said principal investigator Dr. Martin Dennis of the University of Edinburgh’s clinical neurosciences division and Western General Hospital in Edinburgh, Scotland.
Importantly, IPC appears to be effective across a variety of prespecified subgroups, including both ischemic and hemorrhagic stroke.
Findings will change practice
The findings, which were published online in the Lancet (2013 May 31 [doi:10.1016/ S0140-6736(13)61050-8]) to coincide with their presentation at the annual European Stroke Conference, are practice changing and suggest that national stroke guidelines need to be updated.
"This study is a major breakthrough, showing how a simple and safe treatment can save lives," Dr. Tony Rudd, a consultant stroke physician at Guy’s and St. Thomas’ NHS Foundation Trust, London, said in a statement issued by the University of Edinburgh.
"The challenge now will be to ensure that all patients who might benefit are offered treatment," added Dr. Rudd, who chairs the Royal College of Physicians’ Intercollegiate Stroke Working Party. "It is one of the most important research studies to emerge in the field of stroke in recent years," he noted.
Dr. Christine Roffe, consultant in stroke medicine and professor of medicine at Keele University in Stoke-on-Trent, England, also praised the study’s results. "That something as simple as a compressive sleeve saves lives after stroke is fascinating," she said in an interview at the conference. Dr. Roffe was not involved in the study.
The CLOTS 3 study
CLOTS 3 follows on from two other trials performed by the CLOTS (Clots in Legs or Stockings after Stroke) Trials Collaboration, in which compression stockings were examined as a possible means of preventing thrombotic complications in patients who had suffered a stroke. Results of CLOTS 1 (Lancet 2009;373:1958-65) and CLOTS 2 were negative, however, and no benefit of compression stockings was seen in stroke patients.
Between December 2008 and September 2012, a total of 2,876 patients were enrolled in CLOTS 3. For inclusion, patients had to be immobile and randomized within 0-3 days of having had a stroke. Immobility was defined as being unable to walk to the bathroom without the help of another person.
Patients were randomized to receive either routine poststroke care alone or with additional IPC delivered by Covidien’s Kendall SCD Express Sequential Compression System. The latter involved wearing thigh-high, inflatable sleeves continuously for up to 30 days, during which time the device automatically provided IPC depending on the position of the patient. The mean and median durations of wear were 12.5 days and 9.0 days, respectively.
DVT was assessed using duplex ultrasound at 7-10 days and again at 25-30 days if possible. Both patient groups wore compression sleeves to ensure that the ultrasound technicians remained blinded to the treatment group. Follow-up was at 6 months via postal questionnaires sent to patients’ primary care physicians asking about vital status and the occurrence of venous thromboembolism since hospital discharge. Patients were also sent a postal questionnaire and telephoned if they did not respond.
DVT risk reduced
The effect on proximal DVT at 30 days was the primary outcome measure, but IPC also reduced the incidence of symptomatic DVT (4.6% vs. 6.3%; P = .0045) and any DVT (16.2% vs. 21.1%; P = .001) versus routine care. There was no significant difference in the incidence of pulmonary embolism between study arms (2.0% vs. 2.4%, respectively, P = .453).
In terms of safety, there was no difference between the treatment groups in the number of falls with injury or fractures as a result of constantly wearing the compression sleeves. There was a significant difference in skin ulcers (3.1% with IPC vs. 1.4% without, P = .002), but close inspection of the data suggested that only 10 (0.7%) cases were due to IPC.
"During the study, [the manufacturers of the IPC device] brought out a new comfort sleeve," Dr. Dennis noted in an interview.
"Normally these sleeves were being used for short periods in surgical patients, but we were using them for longer periods, so they brought out a softer sleeve," he observed. Anecdotally, he conceded that some people found the sleeves uncomfortable, too hot, or the system "noisy" to use.
The bottom line is that "intermittent pneumatic compression in people who are immobile with stroke reduces the risk of deep vein thrombosis," Dr. Dennis said.
He emphasized that "IPC is feasible in stroke patients, and it is relatively safe. It is an effective means of reducing venous thromboembolism after stroke, with a number needed to treat of about 28 for proximal DVT."
Intriguingly, it may also improve overall survival, "although we weren’t expecting to see that effect," Dr. Dennis said. The number needed to treat to prevent 1 death in 30 days was 43.
Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
AT THE EUROPEAN STROKE CONFERENCE
Major finding: Fewer proximal DVTs occurred at 30 days with intermittent pneumatic compression than with usual care (8.5% vs. 12.1%, P = .001).
Data source: CLOTS 3, a multicenter trial of 2,876 immobile patients with stroke treated with or without intermittent pneumatic compression in addition to usual care.
Disclosures: Dr. Dennis, Dr. Rudd, and Dr. Roffe had no relevant disclosures. The University of Edinburgh and NHS Lothian sponsored the study with funding from the Chief Scientist Office of the Scottish Government, the National Institute of Heath Research Health Technology Assessment Programme, and the Scottish Stroke Research Network. Covidien provided the equipment used in the study free of charge.
Early, intensive BP drop benefits stroke patients
LONDON – Receipt of early, intensive blood pressure–lowering treatment improved functional outcomes in patients with acute intracerebral hemorrhage in a large, randomized trial.
There was a significant, favorable shift in the distribution of modified Rankin Scale (mRS) scores with a more intensive approach to blood pressuring lowering than guideline-recommended treatment, resulting in lower levels of disability (odds ratio, 0.87; P = .04).
However, the results of the eagerly anticipated INTERACT2 trial showed only a modest and nonsignificant reduction in the primary endpoint of death and disability (OR, 0.87; P less than .06).
The trial’s findings are still clinically relevant, the study’s investigators believe, and the approach now warrants implementation in routine clinical practice in most patients with acute intracerebral hemorrhage (ICH), particularly as there were no undue safety concerns when compared with the guideline-recommended approach.
"Blood pressure lowering in acute ICH is safe, so go early, go intensive, and go sustained in most of our patients because it improves the chances of a better recovery in survivors," said Dr. Craig Anderson, of the George Institute for Global Health in Sydney, Australia. "Ultimately, that’s what patients and families want us to do."
Dr. Anderson, who is the study’s chief investigator and is also professor of stroke medicine and clinical neuroscience at the University of Sydney, presented the study’s findings on May 29 at the annual European Stroke Conference; the results were also published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214609).
Design of INTERACT2
INTERACT2 (Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial) was an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients. Patients were recruited within 6 hours of onset of spontaneous ICH and had elevated systolic blood pressure (150-220 mm Hg). The mean age was 63.5 years, and most patients were men (63%), Chinese (68%), and hypertensive (72%).
Patients were centrally allocated to randomly receive either intensive (n = 1,399) or guideline-recommended (n = 1,430) blood pressure–lowering treatment. Intensive treatment aimed to lower the systolic blood pressure rapidly, within 1 hour, to a target of less than 140 mm Hg. By contrast, guideline-recommended treatment aimed to reduce systolic blood pressure below a target of 180 mm Hg with no time limit.
The antihypertensive agents used were not stipulated in the study protocol, and hence a variety of agents were used by the 141 hospitals that participated in the trial. Intravenous agents were used in 90% of patients in the intensive-treatment arm versus 43% in the conventionally treated arm. Intensively managed patients were more likely to receive an IV bolus dose and an infusion (30% vs. 18%) or multiple agents (27% vs. 8%). The most commonly used agent was urapidil, an alpha-blocker available in some parts of Europe, but not in the United States.
Key findings
The primary measure of a poor outcome, defined as an mRS score of 3-6 at 90 days, was observed in 719 (52%) of intensively treated patients versus 785 (55.6%) of guideline-treated patients. An mRS score of 6 indicates death, and 3-5 denotes major disability.
An ordinal analysis of the mRS showed lower scores with the intensive than with the guideline-recommended blood pressure–lowering approach, with more patients able to remain independent despite having some level of disability (mRS score 0-2 in 48% of intensively treated vs. 44% of guideline-treated patients).
Health-related quality of life was assessed using the European Quality of Life-5 Dimensions questionnaire, and intensively treated patients reported fewer problems in mobility (64% vs. 67%; P = .13), self-care (47% vs. 52%; P = .02), usual activities (61% vs. 66%; P = .006), pain or discomfort (40% vs. 45%; P = .01), and anxiety or depression (34% vs. 38%, P = .05) than did those in the standard-treatment arm.
"I’ve never seen this in a clinical trial before," Dr. Anderson observed. "It’s very hard to find a signal in clinical trials, and I guess I was surprised by the degree of significance in benefit on usual activities, which is a very high measure of functional recovery when the patient is home."
Nonfatal serious adverse events occurred in a similar proportion of intensively treated and guideline-treated patients (23.3% and 23.6%, respectively). "The important thing is that we tried to look for any hazard, and we found no excess harms any way we looked at it," Dr. Anderson reported.
Implications for current practice
"If the results of this study with respect to the primary outcome were not as robust as some may have hoped, practitioners should be reassured by the safety data," Dr. Jennifer Frontera of the Cleveland Clinic Foundation commented in an editorial accompanying the NEJM paper.
There were no significant differences between the two treatment approaches in terms of neurologic deterioration, expansion of the ICH, ischemic stroke, cardiovascular events, or severe symptomatic hypotension, she noted.
The ongoing ATACH II (Antihypertensive Treatment of Acute Cerebral Hemorrhage) trial should hopefully shed more light on the benefit of early blood pressure lowering after ICH onset. ATACH II has similar blood pressure–lowering targets and primary and secondary endpoints as INTERACT2. A key difference, however, is that nicardipine is being used as the sole blood pressure–lowering agent. Results should be available in 2016.
Dr. Philip Bath, professor of stroke medicine and chair of the division of stroke at the University of Nottingham, England, who chaired the session in which the INTERACT2 findings were revealed, said that it is still too early to change the guidelines, particularly in view of the fact that several other trials are also ongoing. These include the ENOS (Efficacy of Nitric Oxide on Stroke) trial, of which he is the principal investigator.
"I think randomized data that are near are probably more important than guidelines, so I would always favor more data. It’s not as if we are going to have to wait a very long time; they will be there in the next year or two," Dr. Bath argued.
Study investigator Dr. Christian Stapf, professor of neurology at Université Diderot–Sorbonne Paris Cité, France, commented that, as a clinical scientist, he agreed that more data would be preferable before changing practice.
As a clinician, however, he said, "At this stage I don’t see the risk of any harm. I have no reason not to do it, and if anything it will only help."
Dr. Anderson further commented at a press briefing: "We have presented, for the first time, a strategy that can improve recovery from ICH, the most devastating type of stroke." He emphasized that it was rapid and intensive blood pressure lowering, rather than the use of any specific treatment, that was important in the trial.
This is very good news for the stroke community, Dr. Anderson argued. "We would expect guidelines to change and clinical practice to change as a result of this strategy."
The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.
LONDON – Receipt of early, intensive blood pressure–lowering treatment improved functional outcomes in patients with acute intracerebral hemorrhage in a large, randomized trial.
There was a significant, favorable shift in the distribution of modified Rankin Scale (mRS) scores with a more intensive approach to blood pressuring lowering than guideline-recommended treatment, resulting in lower levels of disability (odds ratio, 0.87; P = .04).
However, the results of the eagerly anticipated INTERACT2 trial showed only a modest and nonsignificant reduction in the primary endpoint of death and disability (OR, 0.87; P less than .06).
The trial’s findings are still clinically relevant, the study’s investigators believe, and the approach now warrants implementation in routine clinical practice in most patients with acute intracerebral hemorrhage (ICH), particularly as there were no undue safety concerns when compared with the guideline-recommended approach.
"Blood pressure lowering in acute ICH is safe, so go early, go intensive, and go sustained in most of our patients because it improves the chances of a better recovery in survivors," said Dr. Craig Anderson, of the George Institute for Global Health in Sydney, Australia. "Ultimately, that’s what patients and families want us to do."
Dr. Anderson, who is the study’s chief investigator and is also professor of stroke medicine and clinical neuroscience at the University of Sydney, presented the study’s findings on May 29 at the annual European Stroke Conference; the results were also published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214609).
Design of INTERACT2
INTERACT2 (Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial) was an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients. Patients were recruited within 6 hours of onset of spontaneous ICH and had elevated systolic blood pressure (150-220 mm Hg). The mean age was 63.5 years, and most patients were men (63%), Chinese (68%), and hypertensive (72%).
Patients were centrally allocated to randomly receive either intensive (n = 1,399) or guideline-recommended (n = 1,430) blood pressure–lowering treatment. Intensive treatment aimed to lower the systolic blood pressure rapidly, within 1 hour, to a target of less than 140 mm Hg. By contrast, guideline-recommended treatment aimed to reduce systolic blood pressure below a target of 180 mm Hg with no time limit.
The antihypertensive agents used were not stipulated in the study protocol, and hence a variety of agents were used by the 141 hospitals that participated in the trial. Intravenous agents were used in 90% of patients in the intensive-treatment arm versus 43% in the conventionally treated arm. Intensively managed patients were more likely to receive an IV bolus dose and an infusion (30% vs. 18%) or multiple agents (27% vs. 8%). The most commonly used agent was urapidil, an alpha-blocker available in some parts of Europe, but not in the United States.
Key findings
The primary measure of a poor outcome, defined as an mRS score of 3-6 at 90 days, was observed in 719 (52%) of intensively treated patients versus 785 (55.6%) of guideline-treated patients. An mRS score of 6 indicates death, and 3-5 denotes major disability.
An ordinal analysis of the mRS showed lower scores with the intensive than with the guideline-recommended blood pressure–lowering approach, with more patients able to remain independent despite having some level of disability (mRS score 0-2 in 48% of intensively treated vs. 44% of guideline-treated patients).
Health-related quality of life was assessed using the European Quality of Life-5 Dimensions questionnaire, and intensively treated patients reported fewer problems in mobility (64% vs. 67%; P = .13), self-care (47% vs. 52%; P = .02), usual activities (61% vs. 66%; P = .006), pain or discomfort (40% vs. 45%; P = .01), and anxiety or depression (34% vs. 38%, P = .05) than did those in the standard-treatment arm.
"I’ve never seen this in a clinical trial before," Dr. Anderson observed. "It’s very hard to find a signal in clinical trials, and I guess I was surprised by the degree of significance in benefit on usual activities, which is a very high measure of functional recovery when the patient is home."
Nonfatal serious adverse events occurred in a similar proportion of intensively treated and guideline-treated patients (23.3% and 23.6%, respectively). "The important thing is that we tried to look for any hazard, and we found no excess harms any way we looked at it," Dr. Anderson reported.
Implications for current practice
"If the results of this study with respect to the primary outcome were not as robust as some may have hoped, practitioners should be reassured by the safety data," Dr. Jennifer Frontera of the Cleveland Clinic Foundation commented in an editorial accompanying the NEJM paper.
There were no significant differences between the two treatment approaches in terms of neurologic deterioration, expansion of the ICH, ischemic stroke, cardiovascular events, or severe symptomatic hypotension, she noted.
The ongoing ATACH II (Antihypertensive Treatment of Acute Cerebral Hemorrhage) trial should hopefully shed more light on the benefit of early blood pressure lowering after ICH onset. ATACH II has similar blood pressure–lowering targets and primary and secondary endpoints as INTERACT2. A key difference, however, is that nicardipine is being used as the sole blood pressure–lowering agent. Results should be available in 2016.
Dr. Philip Bath, professor of stroke medicine and chair of the division of stroke at the University of Nottingham, England, who chaired the session in which the INTERACT2 findings were revealed, said that it is still too early to change the guidelines, particularly in view of the fact that several other trials are also ongoing. These include the ENOS (Efficacy of Nitric Oxide on Stroke) trial, of which he is the principal investigator.
"I think randomized data that are near are probably more important than guidelines, so I would always favor more data. It’s not as if we are going to have to wait a very long time; they will be there in the next year or two," Dr. Bath argued.
Study investigator Dr. Christian Stapf, professor of neurology at Université Diderot–Sorbonne Paris Cité, France, commented that, as a clinical scientist, he agreed that more data would be preferable before changing practice.
As a clinician, however, he said, "At this stage I don’t see the risk of any harm. I have no reason not to do it, and if anything it will only help."
Dr. Anderson further commented at a press briefing: "We have presented, for the first time, a strategy that can improve recovery from ICH, the most devastating type of stroke." He emphasized that it was rapid and intensive blood pressure lowering, rather than the use of any specific treatment, that was important in the trial.
This is very good news for the stroke community, Dr. Anderson argued. "We would expect guidelines to change and clinical practice to change as a result of this strategy."
The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.
LONDON – Receipt of early, intensive blood pressure–lowering treatment improved functional outcomes in patients with acute intracerebral hemorrhage in a large, randomized trial.
There was a significant, favorable shift in the distribution of modified Rankin Scale (mRS) scores with a more intensive approach to blood pressuring lowering than guideline-recommended treatment, resulting in lower levels of disability (odds ratio, 0.87; P = .04).
However, the results of the eagerly anticipated INTERACT2 trial showed only a modest and nonsignificant reduction in the primary endpoint of death and disability (OR, 0.87; P less than .06).
The trial’s findings are still clinically relevant, the study’s investigators believe, and the approach now warrants implementation in routine clinical practice in most patients with acute intracerebral hemorrhage (ICH), particularly as there were no undue safety concerns when compared with the guideline-recommended approach.
"Blood pressure lowering in acute ICH is safe, so go early, go intensive, and go sustained in most of our patients because it improves the chances of a better recovery in survivors," said Dr. Craig Anderson, of the George Institute for Global Health in Sydney, Australia. "Ultimately, that’s what patients and families want us to do."
Dr. Anderson, who is the study’s chief investigator and is also professor of stroke medicine and clinical neuroscience at the University of Sydney, presented the study’s findings on May 29 at the annual European Stroke Conference; the results were also published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214609).
Design of INTERACT2
INTERACT2 (Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial) was an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients. Patients were recruited within 6 hours of onset of spontaneous ICH and had elevated systolic blood pressure (150-220 mm Hg). The mean age was 63.5 years, and most patients were men (63%), Chinese (68%), and hypertensive (72%).
Patients were centrally allocated to randomly receive either intensive (n = 1,399) or guideline-recommended (n = 1,430) blood pressure–lowering treatment. Intensive treatment aimed to lower the systolic blood pressure rapidly, within 1 hour, to a target of less than 140 mm Hg. By contrast, guideline-recommended treatment aimed to reduce systolic blood pressure below a target of 180 mm Hg with no time limit.
The antihypertensive agents used were not stipulated in the study protocol, and hence a variety of agents were used by the 141 hospitals that participated in the trial. Intravenous agents were used in 90% of patients in the intensive-treatment arm versus 43% in the conventionally treated arm. Intensively managed patients were more likely to receive an IV bolus dose and an infusion (30% vs. 18%) or multiple agents (27% vs. 8%). The most commonly used agent was urapidil, an alpha-blocker available in some parts of Europe, but not in the United States.
Key findings
The primary measure of a poor outcome, defined as an mRS score of 3-6 at 90 days, was observed in 719 (52%) of intensively treated patients versus 785 (55.6%) of guideline-treated patients. An mRS score of 6 indicates death, and 3-5 denotes major disability.
An ordinal analysis of the mRS showed lower scores with the intensive than with the guideline-recommended blood pressure–lowering approach, with more patients able to remain independent despite having some level of disability (mRS score 0-2 in 48% of intensively treated vs. 44% of guideline-treated patients).
Health-related quality of life was assessed using the European Quality of Life-5 Dimensions questionnaire, and intensively treated patients reported fewer problems in mobility (64% vs. 67%; P = .13), self-care (47% vs. 52%; P = .02), usual activities (61% vs. 66%; P = .006), pain or discomfort (40% vs. 45%; P = .01), and anxiety or depression (34% vs. 38%, P = .05) than did those in the standard-treatment arm.
"I’ve never seen this in a clinical trial before," Dr. Anderson observed. "It’s very hard to find a signal in clinical trials, and I guess I was surprised by the degree of significance in benefit on usual activities, which is a very high measure of functional recovery when the patient is home."
Nonfatal serious adverse events occurred in a similar proportion of intensively treated and guideline-treated patients (23.3% and 23.6%, respectively). "The important thing is that we tried to look for any hazard, and we found no excess harms any way we looked at it," Dr. Anderson reported.
Implications for current practice
"If the results of this study with respect to the primary outcome were not as robust as some may have hoped, practitioners should be reassured by the safety data," Dr. Jennifer Frontera of the Cleveland Clinic Foundation commented in an editorial accompanying the NEJM paper.
There were no significant differences between the two treatment approaches in terms of neurologic deterioration, expansion of the ICH, ischemic stroke, cardiovascular events, or severe symptomatic hypotension, she noted.
The ongoing ATACH II (Antihypertensive Treatment of Acute Cerebral Hemorrhage) trial should hopefully shed more light on the benefit of early blood pressure lowering after ICH onset. ATACH II has similar blood pressure–lowering targets and primary and secondary endpoints as INTERACT2. A key difference, however, is that nicardipine is being used as the sole blood pressure–lowering agent. Results should be available in 2016.
Dr. Philip Bath, professor of stroke medicine and chair of the division of stroke at the University of Nottingham, England, who chaired the session in which the INTERACT2 findings were revealed, said that it is still too early to change the guidelines, particularly in view of the fact that several other trials are also ongoing. These include the ENOS (Efficacy of Nitric Oxide on Stroke) trial, of which he is the principal investigator.
"I think randomized data that are near are probably more important than guidelines, so I would always favor more data. It’s not as if we are going to have to wait a very long time; they will be there in the next year or two," Dr. Bath argued.
Study investigator Dr. Christian Stapf, professor of neurology at Université Diderot–Sorbonne Paris Cité, France, commented that, as a clinical scientist, he agreed that more data would be preferable before changing practice.
As a clinician, however, he said, "At this stage I don’t see the risk of any harm. I have no reason not to do it, and if anything it will only help."
Dr. Anderson further commented at a press briefing: "We have presented, for the first time, a strategy that can improve recovery from ICH, the most devastating type of stroke." He emphasized that it was rapid and intensive blood pressure lowering, rather than the use of any specific treatment, that was important in the trial.
This is very good news for the stroke community, Dr. Anderson argued. "We would expect guidelines to change and clinical practice to change as a result of this strategy."
The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.
AT THE EUROPEAN STROKE CONFERENCE
Major finding: The odds ratio for intensive blood pressure lowering preventing death or disability, compared with guideline-recommended treatment, was 0.87 (P = .06).
Data source: INTERACT2, an international, multicenter, prospective, open-label, blinded-endpoint, randomized trial of 2,839 patients with elevated systolic blood pressure after spontaneous intracerebral hemorrhage, who were treated with an intensive or guideline-recommended blood pressure–lowering approach.
Disclosures: The National Health and Medical Research Council of Australia funded INTERACT2, which was an investigator-led trial. The experts cited in this report had no relevant disclosures.
Exercise program improved rheumatoid arthritis of the hands
BIRMINGHAM, ENGLAND – Patients with hand or wrist problems from rheumatoid arthritis can significantly benefit from a hand-specific exercise program beyond what is achieved with usual care.
"There was a significant difference in the primary outcome measure [the Michigan Hand Outcomes Questionnaire] in favor of the exercise program and this was actually maintained over the 12-month follow period as well," study investigator Dr. Mark Williams said at the annual meeting of the British Society for Rheumatology. Dr. Williams is a research fellow in the Clinical Trials Unit at the University of Warwick, England, where the trial is being coordinated.
In the randomized, controlled trial called SARAH (Strengthening and Stretching for Rheumatoid Arthritis of the Hand), mean Michigan Hand Outcomes Questionnaire (MHQ) scores at 4 months’ assessment improved from 52.1 at baseline in both groups to 61.1 in the exercise group (n = 246) and 56.6 in the usual care group (n = 244), giving a mean difference in hand function improvement of 4.5 (P less than .0002) in favor of the exercise program.
The effects of the 12-week and home-based intervention were sustained at 12 months’ follow-up, with mean MHQ scores of 60.7 (n = 216) and 56.4 (n = 222) in the exercise and usual care groups, respectively, giving a mean difference of 4.3 (P less than .002).
Clinical guidelines in England and Wales state that patients with RA should have access to specialist hand therapy to help increase strength, movement, and function. Prior to the results of the SARAH trial, however, the evidence upon which this recommendation is based was "rather weak," Dr. Williams observed.
The aims of the study were therefore to determine the clinical effectiveness of an exercise program developed to specifically target the hands and upper limbs in patients with RA hand dysfunction, and then to examine its cost-effectiveness.
The study was performed within 17 National Health Service Trusts in England and included RA patients with pain or dysfunction of the hands or wrist joints who were not taking disease-modifying antirheumatic therapy, or if they were, had been stable on treatment for at least 3 months. The 490 patients randomized in the trial had a median age of 63 years and 10 years’ RA disease duration.
Usual care consisted of one-on-one sessions with a hand therapist and information about joint protection and general exercise advice, with functional splinting if there was a clinical need. In addition to this, the patients who were randomized to the SARAH exercise program received a further five sessions of supervised exercises over a 12-week period; sessions included 11 exercises designed to help with stretching and strengthening of the hand, guidance on a daily exercise program to be performed by patients at home, and strategies to encourage adherence (Physiotherapy 2012;98:121-30).
"In order to get patients to adhere to this exercise plan, which is fairly key, we used behavioral strategies, including an exercise diary and a behavioral action plan," Dr. Williams explained. "This was like a contract between the patient and [his or her] therapist." A total of 438 (89%) patients were followed up at 12 months.
Patient-reported self-efficacy was higher with the exercise program, compared with usual care. Indeed, in a letter recently sent to all the patients who participated in the trial, Dr. Williams noted that, "43% of the patients receiving the SARAH exercise programme and 20% of patients receiving usual care reported some or much improvement."
There was no difference in pain scores between groups, with trends for all participants to report some improvement in pain over time. There was also no difference in the number of adverse events between the groups.
Importantly, the SARAH exercise program was shown to be cost effective, incurring only an additional £100 per patient (approximately US$150) to provide. This means existing health care staff could potentially deliver the program, and with relative ease, the SARAH trial team believes.
Their next step is to see if the intervention’s benefits are extended beyond 12 months. The researchers are also looking at how to best to train healthcare professionals to deliver the program in routine practice in the United Kingdom.
The U.K. National Institute for Health Research Health Technology Assessment Program funded the trial. Dr. Williams had no conflicts of interest.
BIRMINGHAM, ENGLAND – Patients with hand or wrist problems from rheumatoid arthritis can significantly benefit from a hand-specific exercise program beyond what is achieved with usual care.
"There was a significant difference in the primary outcome measure [the Michigan Hand Outcomes Questionnaire] in favor of the exercise program and this was actually maintained over the 12-month follow period as well," study investigator Dr. Mark Williams said at the annual meeting of the British Society for Rheumatology. Dr. Williams is a research fellow in the Clinical Trials Unit at the University of Warwick, England, where the trial is being coordinated.
In the randomized, controlled trial called SARAH (Strengthening and Stretching for Rheumatoid Arthritis of the Hand), mean Michigan Hand Outcomes Questionnaire (MHQ) scores at 4 months’ assessment improved from 52.1 at baseline in both groups to 61.1 in the exercise group (n = 246) and 56.6 in the usual care group (n = 244), giving a mean difference in hand function improvement of 4.5 (P less than .0002) in favor of the exercise program.
The effects of the 12-week and home-based intervention were sustained at 12 months’ follow-up, with mean MHQ scores of 60.7 (n = 216) and 56.4 (n = 222) in the exercise and usual care groups, respectively, giving a mean difference of 4.3 (P less than .002).
Clinical guidelines in England and Wales state that patients with RA should have access to specialist hand therapy to help increase strength, movement, and function. Prior to the results of the SARAH trial, however, the evidence upon which this recommendation is based was "rather weak," Dr. Williams observed.
The aims of the study were therefore to determine the clinical effectiveness of an exercise program developed to specifically target the hands and upper limbs in patients with RA hand dysfunction, and then to examine its cost-effectiveness.
The study was performed within 17 National Health Service Trusts in England and included RA patients with pain or dysfunction of the hands or wrist joints who were not taking disease-modifying antirheumatic therapy, or if they were, had been stable on treatment for at least 3 months. The 490 patients randomized in the trial had a median age of 63 years and 10 years’ RA disease duration.
Usual care consisted of one-on-one sessions with a hand therapist and information about joint protection and general exercise advice, with functional splinting if there was a clinical need. In addition to this, the patients who were randomized to the SARAH exercise program received a further five sessions of supervised exercises over a 12-week period; sessions included 11 exercises designed to help with stretching and strengthening of the hand, guidance on a daily exercise program to be performed by patients at home, and strategies to encourage adherence (Physiotherapy 2012;98:121-30).
"In order to get patients to adhere to this exercise plan, which is fairly key, we used behavioral strategies, including an exercise diary and a behavioral action plan," Dr. Williams explained. "This was like a contract between the patient and [his or her] therapist." A total of 438 (89%) patients were followed up at 12 months.
Patient-reported self-efficacy was higher with the exercise program, compared with usual care. Indeed, in a letter recently sent to all the patients who participated in the trial, Dr. Williams noted that, "43% of the patients receiving the SARAH exercise programme and 20% of patients receiving usual care reported some or much improvement."
There was no difference in pain scores between groups, with trends for all participants to report some improvement in pain over time. There was also no difference in the number of adverse events between the groups.
Importantly, the SARAH exercise program was shown to be cost effective, incurring only an additional £100 per patient (approximately US$150) to provide. This means existing health care staff could potentially deliver the program, and with relative ease, the SARAH trial team believes.
Their next step is to see if the intervention’s benefits are extended beyond 12 months. The researchers are also looking at how to best to train healthcare professionals to deliver the program in routine practice in the United Kingdom.
The U.K. National Institute for Health Research Health Technology Assessment Program funded the trial. Dr. Williams had no conflicts of interest.
BIRMINGHAM, ENGLAND – Patients with hand or wrist problems from rheumatoid arthritis can significantly benefit from a hand-specific exercise program beyond what is achieved with usual care.
"There was a significant difference in the primary outcome measure [the Michigan Hand Outcomes Questionnaire] in favor of the exercise program and this was actually maintained over the 12-month follow period as well," study investigator Dr. Mark Williams said at the annual meeting of the British Society for Rheumatology. Dr. Williams is a research fellow in the Clinical Trials Unit at the University of Warwick, England, where the trial is being coordinated.
In the randomized, controlled trial called SARAH (Strengthening and Stretching for Rheumatoid Arthritis of the Hand), mean Michigan Hand Outcomes Questionnaire (MHQ) scores at 4 months’ assessment improved from 52.1 at baseline in both groups to 61.1 in the exercise group (n = 246) and 56.6 in the usual care group (n = 244), giving a mean difference in hand function improvement of 4.5 (P less than .0002) in favor of the exercise program.
The effects of the 12-week and home-based intervention were sustained at 12 months’ follow-up, with mean MHQ scores of 60.7 (n = 216) and 56.4 (n = 222) in the exercise and usual care groups, respectively, giving a mean difference of 4.3 (P less than .002).
Clinical guidelines in England and Wales state that patients with RA should have access to specialist hand therapy to help increase strength, movement, and function. Prior to the results of the SARAH trial, however, the evidence upon which this recommendation is based was "rather weak," Dr. Williams observed.
The aims of the study were therefore to determine the clinical effectiveness of an exercise program developed to specifically target the hands and upper limbs in patients with RA hand dysfunction, and then to examine its cost-effectiveness.
The study was performed within 17 National Health Service Trusts in England and included RA patients with pain or dysfunction of the hands or wrist joints who were not taking disease-modifying antirheumatic therapy, or if they were, had been stable on treatment for at least 3 months. The 490 patients randomized in the trial had a median age of 63 years and 10 years’ RA disease duration.
Usual care consisted of one-on-one sessions with a hand therapist and information about joint protection and general exercise advice, with functional splinting if there was a clinical need. In addition to this, the patients who were randomized to the SARAH exercise program received a further five sessions of supervised exercises over a 12-week period; sessions included 11 exercises designed to help with stretching and strengthening of the hand, guidance on a daily exercise program to be performed by patients at home, and strategies to encourage adherence (Physiotherapy 2012;98:121-30).
"In order to get patients to adhere to this exercise plan, which is fairly key, we used behavioral strategies, including an exercise diary and a behavioral action plan," Dr. Williams explained. "This was like a contract between the patient and [his or her] therapist." A total of 438 (89%) patients were followed up at 12 months.
Patient-reported self-efficacy was higher with the exercise program, compared with usual care. Indeed, in a letter recently sent to all the patients who participated in the trial, Dr. Williams noted that, "43% of the patients receiving the SARAH exercise programme and 20% of patients receiving usual care reported some or much improvement."
There was no difference in pain scores between groups, with trends for all participants to report some improvement in pain over time. There was also no difference in the number of adverse events between the groups.
Importantly, the SARAH exercise program was shown to be cost effective, incurring only an additional £100 per patient (approximately US$150) to provide. This means existing health care staff could potentially deliver the program, and with relative ease, the SARAH trial team believes.
Their next step is to see if the intervention’s benefits are extended beyond 12 months. The researchers are also looking at how to best to train healthcare professionals to deliver the program in routine practice in the United Kingdom.
The U.K. National Institute for Health Research Health Technology Assessment Program funded the trial. Dr. Williams had no conflicts of interest.
AT RHEUMATOLOGY 2013
Major finding: There was a mean difference in Michigan Hand Outcomes Questionnaire scores of 4.5 (P less than .002) at 4 months and 4.3 (P less than .002) at 12 months.
Data source: Multicenter, randomized, controlled trial of 490 patients with hand or wrist problems from RA who were treated with usual care or a hand-specific exercise program, with follow-up at 4 and 12 months.
Disclosures: The U.K. National Institute for Health Research Health Technology Assessment Program funded the trial. Dr. Williams had no conflicts of interest.
SLE drug used in pregnancy does not up children’s infection, developmental risk
BIRMINGHAM, ENGLAND – Women with systemic lupus erythematous can be advised that azathioprine does not appear to adversely affect their children in the long term if they become pregnant.
In a U.K. cross-sectional survey that included children who were 17 years or younger and born to mothers with systemic lupus erythematosus (SLE), a univariate analysis showed that exposure to azathioprine did not increase the risk of major infections (odds ratio, 1.6; 95% confidence interval 0.8-3.2; P = .15) or developmental problems (OR, 1.22; 95% CI 0.44-3.42; P = .702).
Only the age of the child at assessment was associated with an increased risk of developmental problems, which were defined as attention–deficit/hyperactivity disorder, developmental delay, special needs, or special-education needs (OR, 1.15; P = .003).
The study provides "reassuring data to counsel women who are planning pregnancy in the future," Dr. Mary Gayed said the annual meeting of the British Society for Rheumatology.
"We know, from previous research, that certain immunosuppressant agents are used in pregnancy for lupus to prevent flare and to ensure optimum outcome for mother and child," Dr. Gayed said, but there have been few studies looking at long-term outcomes.
Dr. Gayed, a rheumatologist at Sandwell and West Birmingham (England) Hospitals, noted that the few, small studies that have been conducted have suggested a link between azathioprine use and an increased risk of infection, the presence of anticardiolipin antibodies, and developmental delay in the child.
To investigate the long-term consequences of SLE drugs on children’s outcomes further, Dr. Gayed and her associates performed a retrospective study involving eight English centers. The study involved a total of 287 children aged 17 years or younger who were born to 200 women who, before giving birth, had satisfied four or more SLE criteria as defined by the American College of Rheumatology.
The women had a mean age of 32 years when they gave birth to their first child and had a median disease duration of 7.1 years. The majority (65%) of women were white, with 15% of mothers being Asian, 10% Afro-Caribbean, and the remainder of "other" or unspecified ethnicity.
Maternal antibodies were detected in the children, including anti-Ro with or without lupus anticoagulant in 38%, any antiphospholipid antibody in 43%, lupus anticoagulant specifically in 33%, and anticardiolipin (IgG or IgM) in 23%.
Dr. Gayed reported that aspirin was the most commonly used drug during pregnancy and that 202 children had been exposed to it during their mothers’ pregnancy, followed by prednisolone exposure in 169 children, hydroxychloroquine in 152, azathioprine in 88, and heparin in 70. Another three children were exposed to cyclosporine, and one to mycophenolate.
In terms of obstetric outcomes, only 10% of mothers had experienced preeclampsia, of which 6% of cases were severe enough to warrant induction. Intrauterine growth restriction occurred in 11% of pregnancies (not known in a further 6%), with only 7% of cases being severe enough for the mother to be induced.
The mean gestation period was 36.3 weeks and most children were born by vaginal delivery (49%), with 39% born by cesarean section, and 11% with the aid of forceps. The mean birth weight of neonates was 2.7 kg.
The children were assessed at a mean age of 4.6 years. Infection requiring hospital assessment occurred in 40 (14%) of the 287 children studied. The age at which infections occurred reflected the age at which children were assessed in the study, with around half of all infections seen occurring in children less than 5 years of age.
Around 40% of infections were chest-related, Dr. Gayed reported: 17% were caused by pneumonia, 12% bronchiolitis, 5% upper respiratory tract infection, and 7% tonsillitis.
The age of children at assessment, the duration of pregnancy, and birth weight were also not associated with infection risk.
Dr. Gayed had no conflicts of interest. One coauthor disclosed receiving consultancy fees and honoraria from Genentech and Roche and an educational grant from Aspreva/Vifor.
BIRMINGHAM, ENGLAND – Women with systemic lupus erythematous can be advised that azathioprine does not appear to adversely affect their children in the long term if they become pregnant.
In a U.K. cross-sectional survey that included children who were 17 years or younger and born to mothers with systemic lupus erythematosus (SLE), a univariate analysis showed that exposure to azathioprine did not increase the risk of major infections (odds ratio, 1.6; 95% confidence interval 0.8-3.2; P = .15) or developmental problems (OR, 1.22; 95% CI 0.44-3.42; P = .702).
Only the age of the child at assessment was associated with an increased risk of developmental problems, which were defined as attention–deficit/hyperactivity disorder, developmental delay, special needs, or special-education needs (OR, 1.15; P = .003).
The study provides "reassuring data to counsel women who are planning pregnancy in the future," Dr. Mary Gayed said the annual meeting of the British Society for Rheumatology.
"We know, from previous research, that certain immunosuppressant agents are used in pregnancy for lupus to prevent flare and to ensure optimum outcome for mother and child," Dr. Gayed said, but there have been few studies looking at long-term outcomes.
Dr. Gayed, a rheumatologist at Sandwell and West Birmingham (England) Hospitals, noted that the few, small studies that have been conducted have suggested a link between azathioprine use and an increased risk of infection, the presence of anticardiolipin antibodies, and developmental delay in the child.
To investigate the long-term consequences of SLE drugs on children’s outcomes further, Dr. Gayed and her associates performed a retrospective study involving eight English centers. The study involved a total of 287 children aged 17 years or younger who were born to 200 women who, before giving birth, had satisfied four or more SLE criteria as defined by the American College of Rheumatology.
The women had a mean age of 32 years when they gave birth to their first child and had a median disease duration of 7.1 years. The majority (65%) of women were white, with 15% of mothers being Asian, 10% Afro-Caribbean, and the remainder of "other" or unspecified ethnicity.
Maternal antibodies were detected in the children, including anti-Ro with or without lupus anticoagulant in 38%, any antiphospholipid antibody in 43%, lupus anticoagulant specifically in 33%, and anticardiolipin (IgG or IgM) in 23%.
Dr. Gayed reported that aspirin was the most commonly used drug during pregnancy and that 202 children had been exposed to it during their mothers’ pregnancy, followed by prednisolone exposure in 169 children, hydroxychloroquine in 152, azathioprine in 88, and heparin in 70. Another three children were exposed to cyclosporine, and one to mycophenolate.
In terms of obstetric outcomes, only 10% of mothers had experienced preeclampsia, of which 6% of cases were severe enough to warrant induction. Intrauterine growth restriction occurred in 11% of pregnancies (not known in a further 6%), with only 7% of cases being severe enough for the mother to be induced.
The mean gestation period was 36.3 weeks and most children were born by vaginal delivery (49%), with 39% born by cesarean section, and 11% with the aid of forceps. The mean birth weight of neonates was 2.7 kg.
The children were assessed at a mean age of 4.6 years. Infection requiring hospital assessment occurred in 40 (14%) of the 287 children studied. The age at which infections occurred reflected the age at which children were assessed in the study, with around half of all infections seen occurring in children less than 5 years of age.
Around 40% of infections were chest-related, Dr. Gayed reported: 17% were caused by pneumonia, 12% bronchiolitis, 5% upper respiratory tract infection, and 7% tonsillitis.
The age of children at assessment, the duration of pregnancy, and birth weight were also not associated with infection risk.
Dr. Gayed had no conflicts of interest. One coauthor disclosed receiving consultancy fees and honoraria from Genentech and Roche and an educational grant from Aspreva/Vifor.
BIRMINGHAM, ENGLAND – Women with systemic lupus erythematous can be advised that azathioprine does not appear to adversely affect their children in the long term if they become pregnant.
In a U.K. cross-sectional survey that included children who were 17 years or younger and born to mothers with systemic lupus erythematosus (SLE), a univariate analysis showed that exposure to azathioprine did not increase the risk of major infections (odds ratio, 1.6; 95% confidence interval 0.8-3.2; P = .15) or developmental problems (OR, 1.22; 95% CI 0.44-3.42; P = .702).
Only the age of the child at assessment was associated with an increased risk of developmental problems, which were defined as attention–deficit/hyperactivity disorder, developmental delay, special needs, or special-education needs (OR, 1.15; P = .003).
The study provides "reassuring data to counsel women who are planning pregnancy in the future," Dr. Mary Gayed said the annual meeting of the British Society for Rheumatology.
"We know, from previous research, that certain immunosuppressant agents are used in pregnancy for lupus to prevent flare and to ensure optimum outcome for mother and child," Dr. Gayed said, but there have been few studies looking at long-term outcomes.
Dr. Gayed, a rheumatologist at Sandwell and West Birmingham (England) Hospitals, noted that the few, small studies that have been conducted have suggested a link between azathioprine use and an increased risk of infection, the presence of anticardiolipin antibodies, and developmental delay in the child.
To investigate the long-term consequences of SLE drugs on children’s outcomes further, Dr. Gayed and her associates performed a retrospective study involving eight English centers. The study involved a total of 287 children aged 17 years or younger who were born to 200 women who, before giving birth, had satisfied four or more SLE criteria as defined by the American College of Rheumatology.
The women had a mean age of 32 years when they gave birth to their first child and had a median disease duration of 7.1 years. The majority (65%) of women were white, with 15% of mothers being Asian, 10% Afro-Caribbean, and the remainder of "other" or unspecified ethnicity.
Maternal antibodies were detected in the children, including anti-Ro with or without lupus anticoagulant in 38%, any antiphospholipid antibody in 43%, lupus anticoagulant specifically in 33%, and anticardiolipin (IgG or IgM) in 23%.
Dr. Gayed reported that aspirin was the most commonly used drug during pregnancy and that 202 children had been exposed to it during their mothers’ pregnancy, followed by prednisolone exposure in 169 children, hydroxychloroquine in 152, azathioprine in 88, and heparin in 70. Another three children were exposed to cyclosporine, and one to mycophenolate.
In terms of obstetric outcomes, only 10% of mothers had experienced preeclampsia, of which 6% of cases were severe enough to warrant induction. Intrauterine growth restriction occurred in 11% of pregnancies (not known in a further 6%), with only 7% of cases being severe enough for the mother to be induced.
The mean gestation period was 36.3 weeks and most children were born by vaginal delivery (49%), with 39% born by cesarean section, and 11% with the aid of forceps. The mean birth weight of neonates was 2.7 kg.
The children were assessed at a mean age of 4.6 years. Infection requiring hospital assessment occurred in 40 (14%) of the 287 children studied. The age at which infections occurred reflected the age at which children were assessed in the study, with around half of all infections seen occurring in children less than 5 years of age.
Around 40% of infections were chest-related, Dr. Gayed reported: 17% were caused by pneumonia, 12% bronchiolitis, 5% upper respiratory tract infection, and 7% tonsillitis.
The age of children at assessment, the duration of pregnancy, and birth weight were also not associated with infection risk.
Dr. Gayed had no conflicts of interest. One coauthor disclosed receiving consultancy fees and honoraria from Genentech and Roche and an educational grant from Aspreva/Vifor.
AT RHEUMATOLOGY 2013
Major finding: Exposure to azathioprine during gestation did not increase the risk of infection in children (odds ratio, 1.6; 95% confidence interval 0.8-3.2; P = .15) or developmental problems (OR, 1.22; 95% CI 0.44-3.42; P = .702).
Data source: Multicenter, cross-sectional, retrospective survey of 287 children born to 200 women with systemic lupus erythematosus.
Disclosures: Dr. Gayed had no conflicts of interest. One coauthor disclosed receiving consultancy fees and honoraria from Genentech and Roche and an educational grant from Aspreva/Vitor.